Abstract
Many fluoroquinolone antibiotics are inhibitors of cytochrome P450 enzyme systems and may produce potentially important drug interactions when administered with other drugs. Studies were conducted to determine the effect of clinafloxacin on the pharmacokinetics of theophylline, caffeine, warfarin, and phenytoin, as well as the effect of phenytoin on the pharmacokinetics of clinafloxacin. Concomitant administration of 200 or 400 mg of clinafloxacin reduces mean theophylline clearance by approximately 50 and 70%, respectively, and reduces mean caffeine clearance by 84%. (R)-Warfarin concentrations in plasma during clinafloxacin administration are 32% higher and (S)-warfarin concentrations do not change during clinafloxacin treatment. An observed late pharmacodynamic effect was most likely due to gut flora changes. Phenytoin has no effect on clinafloxacin pharmacokinetics, while phenytoin clearance is 15% lower during clinafloxacin administration.
Clinafloxacin is a potent quinolone antibacterial with a broad range of activity that is of potential clinical importance in the management of serious infections, including those caused by many bacteria resistant to a wide variety of other antibiotics (5, 6, 7, 11, 16, 27). It has been studied primarily in adults hospitalized for the treatment of serious and potentially life-threatening infections, including nosocomial pneumonia, community-acquired pneumonia, febrile neutropenia, complicated intra-abdominal infections, complicated skin and soft tissue infections, endocarditis, and acute gynecologic infections.
Clinafloxacin pharmacokinetics in healthy subjects have been reported previously (31). Following administration of 200- and 400-mg twice-daily intravenous doses, the mean values for total body clearance and volume of distribution are approximately 320 ml/min and 156 liters, respectively, and the mean terminal elimination half-life (t1/2) is approximately 5.8 h. Clinafloxacin is approximately 50% bound to plasma protein, independent of concentration. Approximately 50 to 70% of a clinafloxacin dose is excreted unchanged in urine, indicating that renal clearance is the primary route of elimination. Clinafloxacin clearance is correlated to degree of renal function as measured by creatinine clearance (CLCR). Based on the relationship between clinafloxacin clearance and CLCR, patients with CLCR values of <40 ml/min should have their daily clinafloxacin dose halved (34).
The ability of clinafloxacin to inhibit seven major cytochrome P450 enzymes, CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4, was investigated using isoform-selective marker substrates and human liver microsomal preparations (T. F. Woolf and W. F. Trager, personal communication). Clinafloxacin was most effective in inhibiting CYP1A2 with nearly 50% inhibition observed at the 5 μM concentration. CYP2A6, CYP2E1, and CYP3A4 were not inhibited even at a clinafloxacin concentration of 125 μM. CYP2C19 was 15 and 75% inhibited at 5 and 125 μM clinafloxacin concentrations, respectively. A weaker interaction was seen with both CYP2C9 (approximately 30% at 125 μM) and CYP2D6 (approximately 25% at 125 μM). Based on these results, inhibition of theophylline and caffeine metabolism, metabolized primarily by CYP1A2, may be expected during clinafloxacin therapy. Similarly, inhibition of (R)- and (S)-warfarin metabolism, mediated by CYP3A4-CYP1A2 and CYP2C9, respectively, may also be expected. Clearance of phenytoin, while primarily dependent on CYP2C9, is also dependent upon CYP2C19. Thus, it is expected that an interaction between phenytoin and clinafloxacin may occur. Therefore, studies of drug interactions between clinafloxacin and theophylline, caffeine, warfarin, and phenytoin were conducted.
Pharmacokinetic interactions between many fluoroquinolone antibiotics and theophylline as well as caffeine are well documented (2, 8, 9, 10, 11, 36, 39, 40). Some quinolone antibiotics, such as levofloxacin, lomefloxacin, moxifloxacin, sparfloxacin, temafloxacin, and trovafloxacin, do not interact with xanthines (2, 9, 10, 12, 22, 36, 39, 40). In contrast, ciprofloxacin, enoxacin, grepafloxacin, ofloxacin, and norfloxacin inhibit the cytochrome P450-1A2 mixed-function oxidase system, and it is recommended that plasma theophylline concentrations be monitored and appropriate theophylline dosage adjustments be made during therapy with these quinolones (2, 36). In vitro studies of cytochrome P450 inhibition as well as in vivo studies in the rat suggest that clinafloxacin has a potential for xanthine interactions. Theophylline concentrations in plasma rats were approximately 50% higher following coadministration with clinafloxacin than following administration of theophylline alone (B. Ryerson, unpublished data). Structure activity relationships suggest that small, nonbulky substituents at the position 7 side chain of the fluoroquinolone may have a major influence on inhibition of xanthine metabolism (8). Inhibition is also influenced by position 1 substituents and to a lesser extent substituents at position 8. Comparison of the structure of clinafloxacin with those of several marketed and experimental quinolone antibiotics suggests a “very high” relative potential for a theophylline interaction. Inhibition of theophylline metabolism was reported in a clinafloxacin clinical study (25). Theophylline concentrations in plasma increased nearly twofold during clinafloxacin therapy compared to those observed prior to clinafloxacin therapy. Theophylline dosage adjustment and careful patient monitoring resulted in restoration of therapeutic theophylline concentrations in plasma. Therefore, formal studies were conducted with healthy subjects to evaluate the potential for interaction between clinafloxacin and theophylline and caffeine and to recommend dose adjustments for theophylline concomitant therapy.
Prolongation of prothrombin time due to a possible interaction between many fluoroquinolones and warfarin have been reported (10, 15, 17, 39; J. Leor and S. Matetzki, Letter, Ann. Intern. Med. 109:761, 1988; D. Linville, C. Emory, and L. Graves, Letter, Am. J. Med. 90:765, 1991; T. Linville and D. Matanin, Letter, Ann. Intern. Med. 110:751–752, 1989; F. E. Mott, S. Murphy, and V. Hunt, Letter, Ann. Intern. Med. 111:542–543, 1989). Claims for possible interaction were based on temporal association but otherwise lacked clear evidence of a causal effect. Evaluation of such reports is made difficult by the potential influence of factors such as the dose of antibiotic and duration of treatment, the infection being treated, concurrently administered drugs, illnesses, and medical conditions, as well as intensity of warfarin therapy. (R)- and (S)-warfarins are substrates of the CYP3A4-CYP1A2 and CYP2C9 isozymes, respectively, with the (S)-isomer having approximately fivefold greater anticoagulant activity (18, 43). No pharmacokinetic or pharmacodynamic interactions have been observed between grepafloxacin, levofloxacin, moxifloxacin, and norfloxacin and warfarin (10, 20, 37, 39). Enoxacin has no effect on the anticoagulant effect of warfarin. Clearance of (R)-warfarin was decreased due to inhibition of the (R)-6-hydroxywarfarin metabolic pathway (43). Temafloxacin does not alter prothrombin time (26). Three studies of the effects of ciprofloxacin on warfarin pharmacokinetics and pharmacodynamics in patients receiving warfarin therapy also fail to provide pharmacodynamic evidence of a possible interaction (1, 14, 35). None of these studies demonstrated an effect of ciprofloxacin on the anticoagulant effects of warfarin. However, one study showed a 15% increase from the control in mean (R)-warfarin concentration (14). In summary, it appears that clinically significant quinolone-warfarin interactions seldom occur. Interactions may be mediated in sensitive individuals by inhibition of cytochrome P450-1A2 responsible for (R)-warfarin metabolism or changes in intestinal bacterial flora (21). This suggests that the possibility of an interaction may occur only in a small percentage of patients. It is anticipated that a number of patients administered clinafloxacin will also be receiving warfarin. Therefore, a study was conducted to evaluate the potential for an interaction of clinafloxacin on warfarin pharmacokinetics and pharmacodynamics.
Phenytoin is an anticonvulsant drug metabolized through the 2C9 and 2C19 isoforms of the cytochrome P450 enzyme system (24). Therefore, phenytoin metabolism may be affected by concomitant administration of drugs that induce or inhibit the cytochrome P450 system (19). A sharp decrease in phenytoin concentrations was observed during ciprofloxacin therapy, with a return to therapeutic concentrations when ciprofloxacin was stopped (4, 29, 30). Enoxacin was also shown to decrease phenytoin steady-state concentrations by induction of metabolism (41). Phenytoin has been shown to induce several metabolic pathways, including glucuronidation (3, 42). Because patients treated with phenytoin may be administered clinafloxacin, studies were conducted to determine if a clinically important interaction exists between the two drugs.
(Preliminary results of these studies have been presented elsewhere [E. J. Randinitis, J. R. Koup, G. Rausch, and A. B. Vassos, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-019, 1998; E. J. Randinitis, J. R. Koup, N. J. Bron, N. J. Hounslow, G. Rausch, R. Abel, A. B. Vassos, and A. J. Sedman, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-020, 1998; E. J. Randinitis, C. W. Alvey, J. R. Koup, A. B. Vassos, and A. J. Sedman, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr A-008, 1999], and brief reports of portions of this work have been published [32, 33].)
MATERIALS AND METHODS
Subjects and study conduct.
Study protocols were approved by institutional review boards associated with the study sites, and all studies were conducted according to the ethical principles stated in the Declaration of Helsinki. All subjects provided written informed consent before entering the study and were free to withdraw at any time at their own discretion. Eligible subjects were men and women in good to excellent health as evaluated by medical history, physical examination, and clinical laboratory measurements.
Design of studies.
Subjects in the theophylline interaction study were administered single 200-mg aminophylline tablets (aminophylline tablets, containing 158 mg of theophylline; Roxane Laboratories) either alone or during steady-state clinafloxacin administration. The study utilized a nonblind, parallel-group design whereby all subjects received the first aminophylline dose, began twice-daily dosing with 200 or 400 mg of clinafloxacin (administered as 200-mg clinafloxacin capsules) after a 1-week washout period, and received the second aminophylline dose after 5 days of clinafloxacin dosing. The last clinafloxacin dose was at 36 h following the second aminophylline dose. Previous studies demonstrated that clinafloxacin steady state was achieved by the third day of twice-daily clinafloxacin dosing (31). Aminophylline tablets were administered following an overnight fast, with the fast continuing for 4 h postdose. All doses were administered at the clinic to ensure compliance, and subjects were confined to the clinic for 12 h following each aminophylline dose. Clinafloxacin doses were administered either 2 h before or 2 h following a meal at approximately the same time each day. Caffeine and caffeine-containing drinks and foods were prohibited from screening to closeout.
The caffeine interaction study design was identical to that described for the theophylline interaction study, except that the subjects received single 200-mg caffeine doses (Vivarin 200-mg caplets) either alone or after 5 days of twice-daily dosing with 400 mg of oral clinafloxacin (200-mg capsules). The last clinafloxacin dose was at 36 h following the caffeine dose.
For the warfarin interaction study, healthy subjects were maintained on warfarin (1-, 3-, and/or 5-mg tablets once daily) for at least 2 weeks to a stabilized international normalized ratio (INR) of 1.5 to 2. Subjects received 200-mg clinafloxacin capsules twice daily for 2 weeks while continuing to receive the same warfarin dose. Clinafloxacin doses were administered either 2 h before or 2 h following a meal at approximately the same time each day. A single 10-mg vitamin K tablet was administered to all subjects on the day after the last warfarin-clinafloxacin dose to return the subjects to a normal blood coagulation state. Subjects were confined to the clinic from 24 h prior to the first clinafloxacin dose to 24 h following the last dose.
In the study of the effect of clinafloxacin on phenytoin pharmacokinetics, subjects received 300 mg of phenytoin (three 100-mg Dilantin Kapseals) once daily for 10 days followed by 300 mg of phenytoin once daily plus 200 mg of clinafloxacin (200-mg capsules) twice daily for 14 days. Clinafloxacin doses were administered either 2 h before or 2 h following a meal at approximately the same time each day. Phenytoin doses were administered at the clinic. Phenytoin doses on pharmacokinetic sampling days were administered following an overnight fast, with the fast continuing for 4 h postdose.
In studying the effect of phenytoin on clinafloxacin, subjects were randomized to receive either 200 or 400 mg of clinafloxacin (administered as 200-mg capsules) twice daily for 5 days followed by 21 days of 200 or 400 mg of clinafloxacin twice daily plus 300 mg of once-daily phenytoin (three 100-mg Dilantin Kapseals). Phenytoin and clinafloxacin doses were administered at approximately the same time each day. Phenytoin doses on the pharmacokinetic sampling days were administered at the clinic following an overnight fast, with the fast continuing for 4 h postdose.
Safety.
Safety was evaluated by observation, a physical examination that included the taking of vital signs, and clinical laboratory tests.
Sampling.
In the theophylline study, venous blood samples (5 ml) were collected in tubes containing heparin before the aminophylline dose and at 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 9, 12, 18, 24, 36, and 48 h following the dose. In the caffeine study, venous blood samples (7 ml) were collected in tubes containing heparin before the caffeine dose and at 0.5, 1, 1.5, 2, 3, 4, 6, 9, 12, 24, 36, 48, 72 and 96 h following the dose. Blood samples for INR determinations were collected in the morning at screening, throughout the stabilization period, before the morning clinafloxacin doses, and at closeout. Blood samples for warfarin assay (6 ml) were collected in tubes containing EDTA prior to the warfarin dose and daily during clinafloxacin administration. In the clinafloxacin/phenytoin studies, blood samples (5 ml) were collected in tubes containing heparin before and 0.5, 1, 1.5, 2, 3, 4, 6, 9, 12, and 24 h following the dose. In each case, plasma was separated and stored frozen at −20°C until assayed. In the study of the effect of phenytoin on clinafloxacin pharmacokinetics, urine was quantitatively collected during the 0- to 12-h time interval following the last clinafloxacin dose. A 20-ml sample was retained and stored frozen at −20°C until assayed.
Sample assays. (i) Theophylline.
Following solvent extraction, theophylline concentration in plasma samples was assayed using a validated high-performance liquid chromatography (HPLC) method with UV detection using 7-(2-hydroxypropyl)-theophylline as the internal standard. The minimum quantitation limit for theophylline was 0.05 μg/ml. Detector responses were linear over the calibration range of 0.05 to 25 μg/ml. The precision of quality control samples assayed with study samples, expressed as percent coefficient of variation (%CV), was ≤7.3%. Inter- and intrarun %CV values for quality control samples were ≤3.3%.
(ii) Caffeine.
Caffeine concentration in plasma samples was assayed by a validated HPLC method following extraction with methylene chloride-isopropyl alcohol (90:10). UV detection was used for analysis. The minimum quantitation limit for caffeine was 0.025 μg/ml. The precision of quality control samples assayed with study samples, expressed as %CV, was ≤5.3%. Inter- and intrarun %CV values for quality control samples were ≤2.1%. Detector responses were linear over the calibration range of 0.05 to 25 μg/ml.
(iii) Warfarin.
Following extraction with ethyl ether and reaction with carbobenzyloxy-l-proline in the presence of 1,3-dicyclohexylcarbodimide and imidazole, the (R)- and (S)-warfarin diastereoisomers were separated on a silica column using a gradient mixture of hexane, ethyl acetate, and acetic acid. The eluent was monitored by fluorescence detection at excitation and emission wavelengths of 313 and 370 nm, respectively. The minimum quantitation limit for each isomer was 0.16 ng/ml. Assay precision, expressed as %CV of quality control samples assayed during sample analysis, was <21%. Assay precision for calibration standards observed during sample analysis was <15%. Standard curves were linear over the calibration range of 0.1 to 2.5 ng/ml.
(iv) Clinafloxacin.
Following precipitation with acetonitrile-perchloric acid, plasma and urine samples were assayed for clinafloxacin by liquid chromatographic methods described previously (31). Inter- and intrarun %CV values for plasma quality control samples were ≤3.8%. In addition, clinafloxacin was assayed in urine samples following treatment with bacterial β-glucuronidase at 37°C for 18 h. Inter- and intrarun %CV values for urine quality control samples were ≤13%. Assay of untreated and enzyme-treated urine samples enabled determination of unchanged clinafloxacin and total clinafloxacin (unchanged clinafloxacin plus clinafloxacin glucuronide), respectively.
(v) Phenytoin.
Plasma samples were assayed for phenytoin by a validated HPLC method following addition of an internal standard, 5-(p-methylphenyl)-5-phenylhydantoin. Phenytoin and the internal standard were extracted from plasma into diethyl ether and separated on a reverse-phase C18 column with a mobile phase of methanol and water. Detection was by UV absorbance at 220 nm, and quantitation was by peak-height ratios. The minimum quantitation limit for phenytoin was 0.09 μg/ml. Inter- and intrarun %CV values for quality control samples assayed with study samples was ≤7.5%. Detector responses were linear over the calibration range of 0.09 to 6.0 μg/ml.
Pharmacokinetic analysis.
Pharmacokinetic parameter values were calculated using noncompartmental analysis of concentration-time data. Maximum concentrations in plasma (Cmax) and the time to reach Cmax (Tmax) were recorded as observed. Areas under the concentration-time curve (AUC) were estimated using the linear trapezoidal rule. The AUC from 0 h to the time of the last quantifiable concentration (AUCLQC) was determined. Terminal-phase elimination rate constants (λz) were estimated as the absolute value of the slopes of a linear regression of the natural logarithm (ln) of concentration-time profiles during the terminal phase. t1/2 was calculated as ln(2)/λz. The AUC from 0 h to infinity (AUC∞) values was calculated by summing the AUCLQC and LQC/λz values. Caffeine AUC∞ values following the second dose were calculated as the sum of the AUC48 and C48/λz values, where C48 was the caffeine concentration at 48 h postdose. Values of total body clearance (CLoral) and volume of distribution after oral administration (Voral) were calculated as dose/AUC∞ and CLoral /λz, respectively. AUC24 values were determined for phenytoin, and AUC12 and values were determined for clinafloxacin. Minimum concentrations in plasma (Cmin) were calculated as the average of concentration in predose samples at steady state. The amount of unchanged clinafloxacin excreted in urine at 12 h (Ae) was calculated as the product of urine volume and clinafloxacin concentration in urine. The percentage of dose excreted into urine as unchanged clinafloxacin at 12 h (Ae%) was calculated as 100% · Ae/dose. Renal clearance of clinafloxacin from the plasma (CLR) was calculated as Ae/AUC12. The amount of clinafloxacin eliminated as glucuronide in urine at 12 h during steady state (Am) was calculated as the product of urine volume and the difference between total (unchanged plus glucuronide) and unchanged clinafloxacin. The percentage of dose excreted into urine as glucuronide at 12 h (Am%) was also calculated.
Statistical methods.
Pharmacokinetic parameter values obtained with the interactant drug were compared with those following administration alone for trends of possible clinical importance. Parameter values, as well as log-transformed Cmax and AUC values, were evaluated by analysis of variance (ANOVA) using a model incorporating clinafloxacin dose and subject within clinafloxacin dose, where appropriate, and treatment effects were evaluated by using WinNonlin Professional (version 2.1; Pharsight Corp., Moutain View, Calif.) and SAS (release 6.08; SAS Institute Inc., Cary, N.C.) software. Using ANOVA results, 90% confidence intervals for the ratio of test/reference mean values were determined. (R)- and (S)-warfarin concentrations in plasma over the course of the study were evaluated by ANOVA using a model consisting of subject, treatment, and day within treatment effects. INR values were analyzed using a repeated-measures ANOVA model consisting of subject and time effects. Absence of an interaction would be established if the 90% confidence intervals for the ratio of test to reference exposure, based on log-transformed data, was within the range of 80 to 125% (44).
RESULTS
Subject demographics and safety.
Subject demographics and disposition are summarized in Table 1. In general, clinafloxacin, theophylline, caffeine, warfarin, and phenytoin were well tolerated during these studies. Clinical laboratory abnormalities were sporadic, transient, and unrelated to drug administration. The most common events considered possibly or probably related to clinafloxacin and/or interactant drugs included photosensitivity, diarrhea, dizziness, headache, nervousness, and pruritus. These were mild in intensity.
TABLE 1.
Subject demographics and disposition
| Study | No. enrolled
|
Enrollment mean (range)
|
Withdrawals
|
|||||
|---|---|---|---|---|---|---|---|---|
| Sex
|
Smokers | Age (yr) | Wt (kg) | Completed | AEa | Other | ||
| Male | Female | |||||||
| Theophylline | 8 | 4 | 1 | 41 (21–68) | 78 (56–97) | 10 | 1b | 1c |
| Caffeine | 8 | 4 | 2 | 43 (24–66) | 82 (62–104) | 11 | 0 | 1c |
| Warfarin | 12 | 0 | 0 | 25 (21–31) | 77 (62–90) | 10 | 0 | 2d |
| Clinafloxacin on phenytoin | 2 | 13 | 3 | 38 (25–59) | 90 (64–124) | 11 | 4e | 0 |
| Phenytoin on clinafloxacin | 6 | 10 | 3 | 39 (28–52) | 83 (53–113) | 14 | 2f | 0 |
AE, adverse event.
Diarrhea considered severe in intensity and probably related to clinafloxacin administration.
Withdrew for personal reasons.
Failure to maintain INR of <2.5 before receiving clinafloxacin.
Two subjects for elevated liver function values, one subject for pruritis and rash, and one subject for nervousness—all considered by the clinician to be probably related to phenytoin.
For rash considered by the clinician to be related to phenytoin.
Pharmacokinetics and pharmacodynamics. (i) Effect of clinafloxacin on theophylline pharmacokinetics.
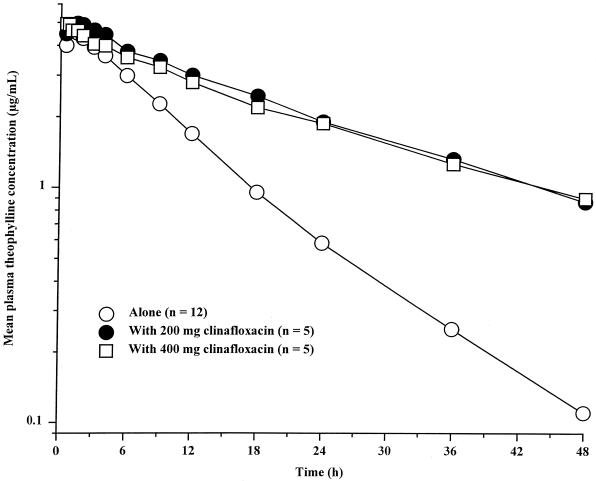
Mean theophylline concentration-time profiles are shown in Fig. 1, and theophylline pharmacokinetic parameters are summarized in Table 2. Mean theophylline Cmax and Tmax values following administration of aminophylline tablets during steady-state administration of clinafloxacin were similar to those observed for aminophylline tablets alone, indicating that clinafloxacin had no effect on absorption rate. Mean theophylline t1/2 and AUC∞ values following administration of aminophylline tablets with clinafloxacin were two- to threefold greater than those following administration of aminophylline tablets alone. Mean CLoral values were 46 and 69% lower following administration of aminophylline with 200 and 400 mg of clinafloxacin, respectively.
FIG. 1.
Mean plasma theophylline concentrations following administration of aminophylline tablets alone and with 200 and 400 mg of clinafloxacin every 12 h. The last clinafloxacin dose was administered 36 h following the aminophylline dose.
TABLE 2.
Summary of theophylline pharmacokinetic parameter values following administration of aminophylline tablets alone or with twice-daily clinafloxacin
| Aminophylline tablet administration | Mean (%CV) for parametera
|
|||||
|---|---|---|---|---|---|---|
| Cmaxf (μg/ml) | Tmax (h) | t1/2 (h) | AUC∞f (μg · h/ml) | CLoral (ml/min) | Voral (liters) | |
| Aloneb | 4.82 (29) | 0.75 (9) | 8.42 (21) | 55.3 (28) | 49.4 (28) | 34.9 (23) |
| With 200 mg of clinafloxacinc | 4.95 (28) | 1.20 (47) | 19.0 (9) | 11.4 (27) | 26.7 (26) | 36.6 (24) |
| Ratiod | 103 | 160 | 226 | 207 | 54.1 | 105 |
| 90% CIe | 94–112 | 93.8–226 | 200–252 | 181–236 | 36.3–71.7 | 101–109 |
| With 400 mg of clinafloxacinc | 5.48 (33) | 0.95 (56) | 22.3 (23) | 145 (19) | 15.5 (21) | 36.6 (22) |
| Ratiod | 114 | 127 | 264 | 262 | 31.4 | 105 |
| 90% CIe | 104–124 | 60.5–193 | 238–290 | 229–299 | 13.8–49c | 100–109 |
Mean, least squares mean value; %CV, percent coefficient of variation.
n = 12.
n = 5 (one subject receiving 200 mg of clinafloxacin was withdrawn prior to theophylline pharmacokinetic sampling due to severe diarrhea, and one subject withdrew for personal reasons prior to receiving 400 mg of clinafloxacin).
Calculated as 100% · with clinafloxacin/alone.
90% CI, 90% confidence interval estimate for the ratio of with clinafloxacin/alone mean values.
Based on log-transformed parameter estimates.
(ii) Effect of clinafloxacin on caffeine pharmacokinetics.
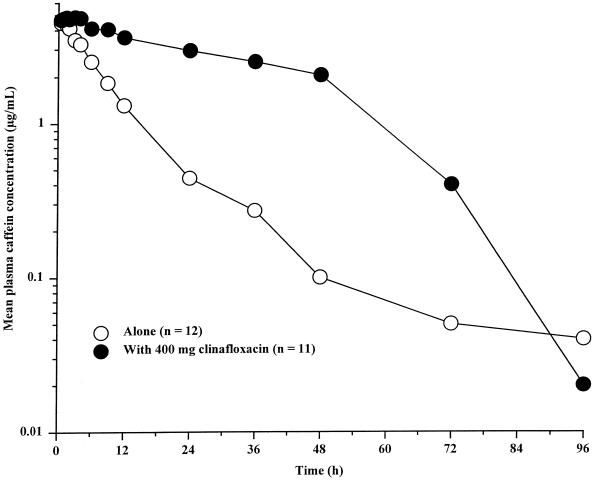
Mean plasma caffeine concentration-time profiles are shown in Fig. 2 and caffeine pharmacokinetic parameter values are summarized in Table 3. Caffeine Cmax following administration of caffeine tablets during clinafloxacin administration were similar to those observed for caffeine tablets alone. Differences in caffeine t1/2, AUC∞, and CLoral values indicated a substantial effect of clinafloxacin on caffeine pharmacokinetics. Mean caffeine AUC∞ and t1/2 values increased approximately four- and fivefold, respectively, following administration of caffeine with clinafloxacin, and the mean caffeine CLoral value was approximately 84% lower. Caffeine elimination t1/2 values decreased dramatically 12 h after the last clinafloxacin dose in all subjects. Individual elimination t1/2 values based on the concentration-time profile from 48 to 96 h following the dose during clinafloxacin dosing were similar to those observed when caffeine was administered alone. Clinafloxacin concentrations apparently decreased to less than the enzyme inhibitory concentration, and caffeine was cleared at a rate similar to that observed without clinafloxacin present.
FIG. 2.
Mean plasma caffeine concentrations following administration of caffeine tablets alone and with 400 mg of clinafloxacin twice daily. The last clinafloxacin dose was administered 36 h following the caffeine dose.
TABLE 3.
Summary of caffeine pharmacokinetic parameter values following administration of caffeine alone and with twice-daily administration of 400 mg of clinafloxacin
| Caffeine administration | Mean (%CV) for parametera
|
|||||
|---|---|---|---|---|---|---|
| Cmaxf (μg/ml) | Tmax (h) | t1/2 (h) | AUC∞f (μg · h/ml) | CLoral (ml/min) | Voral (liters) | |
| Aloneb | 4.97 (25) | 0.83 (53) | 6.16 (35) | 47.6 (22) | 78.2 (55) | 36.9 (30) |
| With clinafloxacinc | 5.35 (42) | 1.70 (101) | 36.3 (30) | 250 (50) | 12.5 (41) | 42.5 (23) |
| Ratiod | 108 | 204 | 589 | 525 | 16.0 | 115 |
| 90% CIe | 99.5–116 | 92.8–314 | 500–679 | 121–655 | 0–44.2 | 105–125 |
Mean, least squares mean value; %CV, percent coefficient of variation.
n = 12.
n = 11 (one subject withdrew for personal reasons prior to receiving the second caffeine dose).
Calculated as 100% · with clinafloxacin/alone.
90% CI, 90% confidence interval estimate for the ratio of with clinafloxacin/alone treatment mean values.
Based on log-transformed parameter estimates.
(iii) Effect of clinafloxacin on warfarin pharmacokinetics and pharmacodynamics.
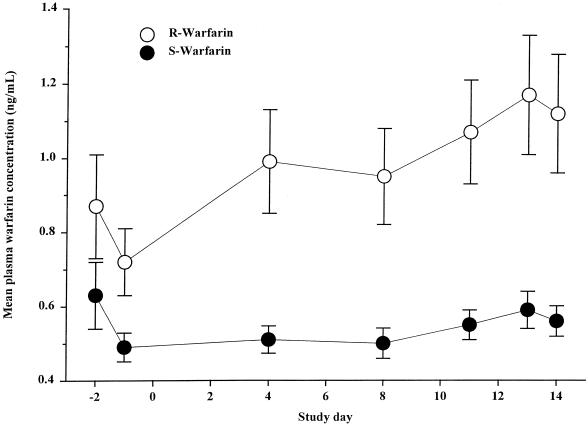
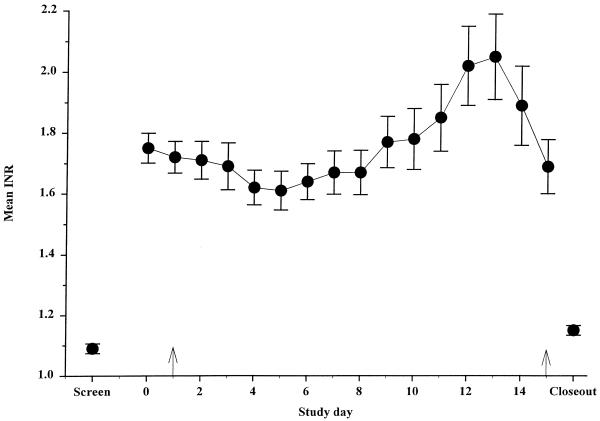
Mean (R)- and (S)-warfarin concentrations in plasma are shown in Fig. 3. (R)-Warfarin concentrations during clinafloxacin administration were significantly higher (32%) than those observed before clinafloxacin administration. (R)-Warfarin concentrations on the last day of clinafloxacin treatment were not significantly different from those throughout the clinafloxacin dosing period. Mean (S)-warfarin concentrations in plasma during twice-daily administration of clinafloxacin were similar to those observed before clinafloxacin administration. Mean INR values are given in Fig. 4. After 12 to 14 days of steady-state clinafloxacin treatment, the mean INR was significantly greater than baseline, showing an approximately 9 to 17% increase. Vitamin K administration on the last day reversed the effects of warfarin in these healthy subjects.
FIG. 3.
Mean plasma (R)- and (S)-warfarin concentrations before and during 200 mg clinafloxacin. Clinafloxacin dosing began on study day 1. Bars illustrate standard errors.
FIG. 4.
Mean INR before and during clinafloxacin administration. Warfarin dosing to a stable INR began 2 weeks prior to clinafloxacin. Arrows mark the beginning and end of 200-mg twice-daily clinafloxacin dosing. Warfarin and clinafloxacin administration were discontinued, and vitamin K was administered on day 15 to return healthy subjects to their original coagulation state. Bars illustrate standard errors.
(iv) Effect of clinafloxacin on phenytoin pharmacokinetics.
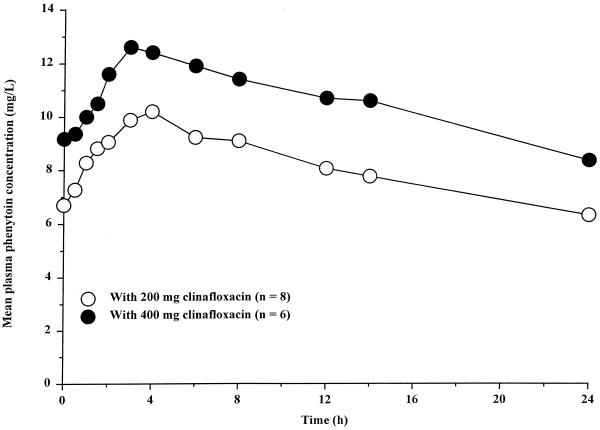
Mean plasma phenytoin concentration-time profiles are shown in Fig. 5, and pharmacokinetic parameter values are summarized in Table 4. Based on mean Tmax values, the rate of phenytoin absorption during steady-state clinafloxacin administration was similar to that when phenytoin was administered alone. Mean phenytoin Cmax and Cmin during clinafloxacin administration were 18 and 29% higher, respectively, than those observed before clinafloxacin administration. The mean CLoral of phenytoin was 15% lower during clinafloxacin administration than during phenytoin administration alone.
FIG. 5.
Mean steady-state plasma phenytoin concentrations following administration of 300 mg once daily alone and during administration of 400 mg of clinafloxacin twice daily.
TABLE 4.
Summary of phenytoin pharmacokinetic parameter values before and during administration of 400 mg clinafloxacin twice daily
| Phenytoin administration | Mean (%CV) for parametera
|
||||
|---|---|---|---|---|---|
| Cmaxf (μg/ml) | Tmax (h) | AUC∞f (μg · h/ml) | CLoral (ml/min) | Voral (liters) | |
| Aloneb | 6.74 (60) | 3.23 (67) | 123 (67) | 3.89 (90) | 47.5 (52) |
| With clinafloxacinc | 7.95 (63) | 3.82 (69) | 147 (67) | 4.87 (81) | 40.3 (61) |
| Ratiod | 118 | 118 | 120 | 125 | 84.9 |
| 90% CIe | 106–131 | 48.0–189 | 107–133 | 107–146 | 73.2–96.6 |
Mean, least squares mean values; %CV, percentage coefficient of variation.
n = 13 (two subjects were withdrawn for elevated liver function tests prior to receiving clinafloxacin; phenytoin pharmacokinetic sampling was completed).
n = 11 (two additional subjects were withdrawn prior to sampling in the phenytoin plus clinafloxacin treatment, one for a severe rash and one for nervousness, both attributed to phenytoin).
Calculated as 100% · with clinafloxacin/alone.
90% CI, 90% confidence interval estimate for the ratio of with clinafloxacin/alone mean values.
Based on log-transformed parameter estimates.
(v) Effect of phenytoin on clinafloxacin pharmacokinetics.
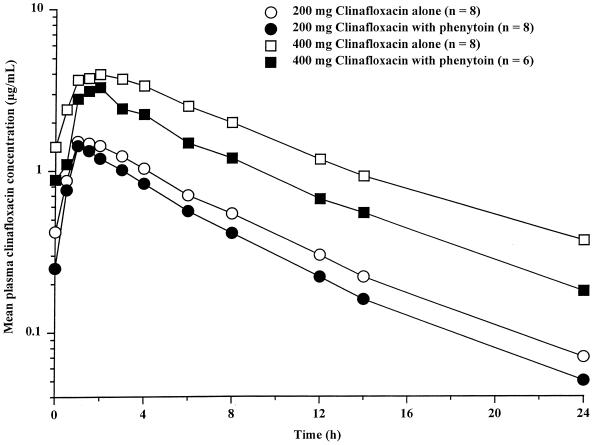
Mean plasma clinafloxacin concentration-time profiles are shown in Fig. 6 and pharmacokinetic parameter values are summarized Table 5. Based on Tmax and Cmax, rates of clinafloxacin absorption during 200- and 400-mg twice daily doses in the presence of steady-state once-daily 300 mg phenytoin doses were similar to those observed without phenytoin. The mean clinafloxacin CLoral values determined during phenytoin dosing were approximately 20 and 8% higher for 200- and 400-mg clinafloxacin doses, respectively, than those observed without phenytoin. Mean AUC12 values were 17 and 10% lower, respectively, during phenytoin dosing. Mean t1/2 values during periods of 200- and 400-mg clinafloxacin dosing were similar to those without phenytoin. Mean clinafloxacin Ae% values during phenytoin dosing were approximately 27 and 20% lower than those observed for 200- and 400-mg clinafloxacin dosing, respectively, without phenytoin. Mean clinafloxacin Am% values were approximately 38 and 114% higher for 200- and 400-mg clinafloxacin doses, respectively, than those observed without phenytoin.
FIG. 6.
Mean steady-state plasma clinafloxacin concentrations following administration of 200 and 400 mg of clinafloxacin twice daily alone and during steady-state 300 mg of phenytoin once daily.
TABLE 5.
Summary of clinafloxacin pharmacokinetic parameter values during administration of 200 and 400 mg twice daily alone and during steady-state administration of 300 mg of phenytoin
| Clinafloxacin dose (mg) | Mean (%CV) for parametera
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cmaxg (μg/ml) | Tmax (h) | t1/2 (h) | AUC12g (μg · h/ml) | CLoral (ml/min) | Cming (μg/ml) | Ae% | CLR (ml/min) | Am% | |
| 200 | |||||||||
| Aloneb | 1.63 (28) | 1.56 (43) | 5.34 (22) | 9.23 (26) | 372 (25) | 0.38 (41) | 63.7 (25) | 179 (18) | 9.33 (42) |
| With phenytoinb | 1.46 (32) | 1.56 (29) | 4.69 (21) | 7.60 (21) | 447 (19) | 0.21 (38) | 46.6 (25) | 169 (30) | 12.9 (49) |
| Ratioe | 89.4 | 100 | 87.9 | 82.3 | 120 | 59.1 | 73.1 | 94.0 | 138 |
| 90% CIf | 81.2–98.5 | 50.0–150 | 81.0–94.9 | 75.5–89.7 | 111–129 | 38.8–90.0 | 55.5–90.7 | 77.7–110 | 78.1–198 |
| 400 | |||||||||
| Aloneb | 4.01 (41) | 2.19 (34) | 5.94 (17) | 27.5 (47) | 265 (46) | 1.22 (57) | 50.3 (30) | 98.0 (46) | 6.1 (44) |
| With phenytoinc | 4.10 (27) | 1.49 (33) | 5.81 (16) | 24.8 (19) | 285 (9) | 1.12 (22) | 40.5 (37)d | 92.0 (34)d | 13.0 (38)d |
| Ratioe | 102 | 68.0 | 97.9 | 90.3 | 108 | 91.5 | 80.4 | 93.3 | 214 |
| 90% CIf | 89.6–117 | 31.5–104 | 90.1–106 | 72.6–112 | 75.8–140 | 71.0–118 | 38.0–123 | 45.9–141 | 135–293 |
Mean, least-squares mean value; %CV, percent coefficient of variation.
n = 8.
n = 6 (two subjects were withdrawn due to rash attributed to phenytoin prior to pharmacokinetic sampling for clinafloxacin).
n = 5 (incomplete urine collection in one subject).
Calculated as 100% · with phenytoin/alone.
90% CI, 90% confidence interval estimate for the ratio of with phenytoin/alone treatment mean values.
Based on log-transformed parameter estimates.
DISCUSSION
Enoxacin is reported to be the most potent of the quinolone antibiotics in inhibiting theophylline metabolism (38). Theophylline concentrations in plasma are more than doubled and clearance is reduced more than 60% when administered with enoxacin. Based on the reduction in theophylline clearance observed in this study, clinafloxacin is similar in potency to enoxacin in inhibiting the cytochrome P450-1A2 system. The observed high degree of metabolic interaction for clinafloxacin on xanthines confirms in vitro inhibition and in vivo studies the rat, as well as proposed structure-activity relationships, which suggested that clinafloxacin has a high potential for xanthine metabolic interaction. Changes in theophylline CLoral values are related to clinafloxacin dose. Concomitant administration of 200- or 400-mg twice-daily clinafloxacin doses with 200 mg of aminophylline reduced the mean theophylline clearance by approximately 50 and 70%, respectively. Based on these findings, the theophylline dose should be reduced by at least one-half in patients requiring clinafloxacin during theophylline therapy. Patients should be carefully monitored to determine whether further theophylline dose adjustment is warranted.
Concurrent administration of clinafloxacin with caffeine had minimal effects on caffeine absorption rate and volume of distribution. However, the mean caffeine CLoral value was approximately 84% lower during 400-mg twice-daily clinafloxacin dosing. Therefore, patients receiving clinafloxacin therapy should avoid caffeine-containing foods and beverages.
Multiple-dose clinafloxacin treatment in healthy subjects maintained on warfarin was associated with an elevation of (R)-warfarin concentrations and a late increase in INR. The 32% increase in (R)-warfarin concentrations is probably not clinically important given the lower activity of this isomer, relative to that of (S)-warfarin (18, 43). The more potent (S)-isomer of warfarin was unaffected by clinafloxacin administration. The increased INR did not correspond to pharmacokinetic changes and may suggest that the late pharmacodynamic interaction observed was likely due to changes in gut flora (13, 21). Changes in gut microflora have been reported with clinafloxacin (28). Therefore, as a precaution, prothrombin time should be monitored in patients on warfarin therapy, and appropriate warfarin dose adjustments made during clinafloxacin administration. The late pharmacodynamic interaction observed in this study demonstrates the advantage of a multiple-dose study design in which both warfarin and the test anti-infective are administered as in the clinical setting.
Because phenytoin is nearly completely absorbed, changes in AUC values reflect changes in phenytoin clearance (23). Therefore, the observed 20% higher mean AUC24 value during clinafloxacin administration reflects a 15% lower clearance. Presumably, inhibition by clinafloxacin of the P450–2C19 isozyme was responsible for this interaction. In most patients, such a modest change would not be expected to be of clinical relevance. However, due to the nonlinear behavior of phenytoin pharmacokinetics, it is recommended that phenytoin concentrations in plasma be monitored during clinafloxacin therapy and that the phenytoin dose be adjusted accordingly (23). Decreased clinafloxacin Ae% and increased Am% values during administration with phenytoin suggest that phenytoin induces glucuronidation. This supports the previous observation of induction of glucuronidation by phenytoin (42). The modest differences between clinafloxacin CLoral, AUC12, and t1/2 values during administration with phenytoin and clinafloxacin administered alone are clinically unimportant, and clinafloxacin dose adjustments are unnecessary during concurrent therapy.
ACKNOWLEDGMENTS
We thank S. J. Warrington of Central Middlesex Hospital, London, England, for conducting the clinical portion of the warfarin interaction study and the staff of the Community Research Clinic, Ann Arbor, Mich., under the direction of A. J. Sedman, for conducting the clinical portions of the remaining studies. We also thank Analytical Development Corporation, Colorado Springs, Colo., for clinafloxacin analysis of biological samples; PPD Pharmaco International, Richmond, Va., for assay of theophylline and caffeine in biological samples; Medeval Ltd., University of Manchester, Manchester, United Kingdom, for analysis of warfarin enantiomers in biological samples; and AvTech Laboratories, Kalamazoo, Mich., for phenytoin as well as clinafloxacin analysis of biological samples.
REFERENCES
- 1.Bianco T M, Bussey H I, Farnett L E, Linn W D, Roush M K, Wong Y W J. Potential warfarin-ciprofloxacin interaction in patients receiving long-term anticoagulation. Pharmacotherapy. 1992;12:435–439. [PubMed] [Google Scholar]
- 2.Blondeau J M. Expanded activity and utility of the new fluoroquinolones: a review. Clin Ther. 1999;21:3–40. doi: 10.1016/s0149-2918(00)88266-1. [DOI] [PubMed] [Google Scholar]
- 3.Bock K W, Wiltfang J, Blume R, Ullrich D, Bircher J. Paracetamol as a test drug to determine glucuronide formation in man: effects of inducers and of smoking. Eur J Clin Pharmacol. 1987;31:677–683. doi: 10.1007/BF00541295. [DOI] [PubMed] [Google Scholar]
- 4.Brouwers P J, BeBoer L E, Guchelaar H J. Ciprofloxacin-phenytoin interaction. Ann Pharmacother. 1997;31:498. [PubMed] [Google Scholar]
- 5.Cohen M A, Huband M D, Gage J W, Yoder S L, Roland G E, Gracheck S J. In-vitro activity of clinafloxacin, trovafloxacin, and ciprofloxacin. J Antimicrob Chemother. 1997;40:205–211. doi: 10.1093/jac/40.2.205. [DOI] [PubMed] [Google Scholar]
- 6.Cohen M A, Huband M D, Gage J W, Yode S L, Roland G E. Bacterial eradication by clinafloxacin, CI-990, and ciprofloxacin employing MBC test, in-vitro time-kill and in-vivo time-kill studies. J Antimicrob Chemother. 1998;41:605–614. doi: 10.1093/jac/41.6.605. [DOI] [PubMed] [Google Scholar]
- 7.Cohen M A, Huband M D. Activity of clinafloxacin, trovafloxacin, quinupristin/dalfopristin, and other antimicrobial agents versus Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Diagn Microbiol Infect Dis. 1999;33:43–46. doi: 10.1016/s0732-8893(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 8.Domagala J M. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J Antimicrob Chemother. 1994;33:685–706. doi: 10.1093/jac/33.4.685. [DOI] [PubMed] [Google Scholar]
- 9.Edwards D J, Bowles S K, Svensson C K, Rybak M J. Inhibition of drug metabolism by quinolone antibiotics. Clin Pharmacokinet. 1988;13:194–204. doi: 10.2165/00003088-198815030-00004. [DOI] [PubMed] [Google Scholar]
- 10.Efthymiopoulos C, Bramer S L, Maroli A, Blum B. Theophylline and warfarin interaction studies with grepafloxacin. Clin Pharmacokinet. 1997;33(Suppl. 1):39–46. doi: 10.2165/00003088-199700331-00008. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs P C, Barry A L, Brown S D. In vitro activities of clinafloxacin against contemporary clinical bacterial isolates from 10 North American centers. Antimicrob Agents Chemother. 1998;42:1274–1277. doi: 10.1128/aac.42.5.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuhr U, Anders E M, Mahr G, Sorgel P, Staib A H. Inhibitory potency of quinolone antibacterial agents against cytochrome P4501A2 activity in vivo and in vitro. Antimicrob Agents Chemother. 1992;36:942–948. doi: 10.1128/aac.36.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gullov A L, Koefoed B G, Peterson P. Interaktion mellem warfarin og nalidixinsyre. Ugeskr Laeger. 1996;158:5174–5175. [PubMed] [Google Scholar]
- 14.Israel D S, Stotka J, Rock W, Sintek C D, Kamada A K, Klein C, Swaim W R, Pluhar R E, Toscano J P, Lettieri J T, Heller A H, Polk R E. Effect of ciprofloxacin on the pharmacokinetics and pharmacodynamics of warfarin. Clin Infect Dis. 1996;22:251–256. doi: 10.1093/clinids/22.2.251. [DOI] [PubMed] [Google Scholar]
- 15.Jolson H M, Tanner L A, Green, L. L, Grasela T J., Jr Adverse reaction reporting of interaction between warfarin and fluoroquinolones. Arch Intern Med. 1991;151:1003–1004. [PubMed] [Google Scholar]
- 16.Jorgensen J H, Weigel L M, Ferraro M J, Swenson J M, Tenover F C. Activities of newer fluoroquinolones against Streptococcus pneumoniae clinical isolates including those with mutations in the gyrA, parC, and parE loci. Antimicrob Agents Chemother. 1999;43:329–334. doi: 10.1128/aac.43.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamada A K. Possible interaction between ciprofloxacin and warfarin. Drug Intel Clin Pharm. 1990;24:27–28. doi: 10.1177/106002809002400106. [DOI] [PubMed] [Google Scholar]
- 18.Kaminsky L S, Zhang Z Y. Human P450 metabolism of warfarin. Pharmacol Ther. 1997;73:67–74. doi: 10.1016/s0163-7258(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 19.Kutt H. Interactions between anticonvulsants and other commonly prescribed drugs. Epilepsia. 1984;25(Suppl. 2):S118–S131. doi: 10.1111/j.1528-1157.1984.tb05644.x. [DOI] [PubMed] [Google Scholar]
- 20.Liao S, Palmer M, Fowler C, Nayak R K. Absence of an effect of levofloxacin on warfarin pharmacokinetics and anticoagulation in male volunteers. J Clin Pharmacol. 1996;36:1072–1077. doi: 10.1177/009127009603601111. [DOI] [PubMed] [Google Scholar]
- 21.Lipsky J J. Antibiotic-associated hypoprothrombinaemia. J Antimicrob Chemother. 1988;21:281–300. doi: 10.1093/jac/21.3.281. [DOI] [PubMed] [Google Scholar]
- 22.Lode H. Potential interactions of the extended-spectrum fluoroquinolones with the CNS. Drug Safety. 1999;21:123–135. doi: 10.2165/00002018-199921020-00005. [DOI] [PubMed] [Google Scholar]
- 23.Ludden T M, Allerheiligen S R B, Browne T R, Koup J R. Sensitivity analysis of the effect of bioavailability or dosage form content on mean steady-state phenytoin concentration. Ther Drug Monit. 1991;13:120–125. doi: 10.1097/00007691-199103000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Mamiya K, Ieiri I, Shimamoto J, Yukawa E, Imai J, Nimomiya H, Yamada H, Otsubo K, Higuchi S, Tashiro N. The effects of genetic polymorphisms of CYP2C9 and CYP2C19 on phenytoin metabolism in Japanese adult patients with epilepsy: studies in stereoselective hydroxylation and population pharmacokinetics. Epilepsia. 1998;39:1317–1323. doi: 10.1111/j.1528-1157.1998.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 25.Matuschka P R, Vissing R S. Clinafloxacin-theophylline drug interaction. Ann Pharmacother. 1995;29:378–380. doi: 10.1177/106002809502900407. [DOI] [PubMed] [Google Scholar]
- 26.Millar E, Coles S, Wyld P, Nimmo W. Temafloxacin does not potentiate the anticoagulant effect of warfarin in healthy subjects. Clin Pharmacokinet. 1992;22(Suppl. 1):102–106. doi: 10.2165/00003088-199200221-00017. [DOI] [PubMed] [Google Scholar]
- 27.Moellering R C., Jr The emergence of bacterial resistance to antibiotics: achieving optimum outcomes with clinafloxacin, a seminar-in-print. Clin Drug Investig. 1998;15(Suppl. 1):1–48. [Google Scholar]
- 28.Oh H, Nord C E, Barkholt L, Hedberd M, Edlund C. Ecological disturbances in intestinal microflora caused by clinafloxacin, an extended-spectrum quinolone. Infection. 2000;28:272–277. doi: 10.1007/s150100070018. [DOI] [PubMed] [Google Scholar]
- 29.Pollak P T, Slayter K L. Hazards of doubling phenytoin dose in the face of an unrecognized interaction with ciprofloxacin. Ann Pharmacother. 1997;31:61–63. doi: 10.1177/106002809703100111. [DOI] [PubMed] [Google Scholar]
- 30.Pollak P T, Slayter K L. Comment: ciprofloxacin-phenytoin interaction. Ann Pharmacother. 1997;31:1549–1550. doi: 10.1177/106002809703101222. [DOI] [PubMed] [Google Scholar]
- 31.Randinitis E J, Brodfuehrer J I, Eiseman I, Vassos A B. Pharmacokinetics of clinafloxacin after single and multiple doses. Antimicrob Agents Chemother. 2001;45:2529–2535. doi: 10.1128/AAC.45.9.2529-2535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randinitis E J, Koup J R, Bron N J, Hounslow N J, Rausch G, Abel R, Vassos A B, Sedman A J. Drug interaction studies with clinafloxacin and probenecid, cimetidine, phenytoin, and warfarin. Drugs. 1999;58(Suppl. 2):254–255. [Google Scholar]
- 33.Randinitis E J, Koup J R, Rausch G, Vassos A B. Effect of clinafloxacin on the pharmacokinetics of theophylline and caffeine. Drugs. 1999;58(Suppl. 2):248–249. [Google Scholar]
- 34.Randinitis E J, Koup J R, Rausch G, Abel R, Bron N J, Hounslow N J, Vassos A B, Sedman A J. Clinafloxacin pharmacokinetics in subjects with various degrees of renal function. Antimicrob Agents Chemother. 2001;45:2536–2542. doi: 10.1128/AAC.45.9.2536-2542.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rindone J P, Keuey C L, Jones W N, Garewal H S. Hypoprothrombinemic effect of warfarin not influenced by ciprofloxacin. Clin Pharm. 1991;10:136–138. [PubMed] [Google Scholar]
- 36.Robson R A. The effects of quinolones on xanthine pharmacokinetics. Am J Med. 1992;92(Suppl. 4A):22S–25S. doi: 10.1016/0002-9343(92)90303-s. [DOI] [PubMed] [Google Scholar]
- 37.Rocci M L, Vlasses P H, Distlerath L M, Gregg M H, Wheeler S C, Zing W, Bjornsson T D. Norfloxacin does not alter warfarin's disposition or anticoagulant effect. J Clin Pharmacol. 1990;30:728–732. doi: 10.1002/j.1552-4604.1990.tb03634.x. [DOI] [PubMed] [Google Scholar]
- 38.Ruff F R, Santias M C, Callens E, Chauvin J P, Hazebroucq J. Effect of temafloxacin on the pharmacokinetics of theophylline. Am J Med. 1991;91(Suppl. 6A):64A–76A. doi: 10.1016/0002-9343(91)90315-o. [DOI] [PubMed] [Google Scholar]
- 39.Stass H, Kubitza D. Interaction profile of moxifloxacin. Drugs. 1999;58(Suppl. 2):235–236. [Google Scholar]
- 40.Stass H, Kubitaz D. Lack of pharmacokinetic interaction between moxifloxacin, a novel 8-methoxyfluoroquinolone, and theophylline. Clin Pharmacokinet. 2001;40(Suppl. 1):63–70. doi: 10.2165/00003088-200140001-00009. [DOI] [PubMed] [Google Scholar]
- 41.Thomas D, Humphrey G, Kinkel A W, Sedman A, Rowland M, Toon S. A study to evaluate the potential pharmacokinetic interaction between oral enoxacin and oral phenytoin. Pharm Res. 1986;3(Suppl.):99S. [Google Scholar]
- 42.Tomlinson B, Young R P, Ng M C Y, Anderson P J, Kay R, Critchley J A. Selective liver enzyme induction by carbamazepine and phenytoin in Chinese epileptics. Eur J Clin Pharmacol. 1996;50:411–415. doi: 10.1007/s002280050132. [DOI] [PubMed] [Google Scholar]
- 43.Toon S, Hopkins K J, Garstang F M, Aarons L, Sedman A, Rowland M. Enoxacin-warfarin interaction: pharmacokinetic and stereochemical aspects. Clin Pharmacol Ther. 1987;42:33–34. doi: 10.1038/clpt.1987.104. [DOI] [PubMed] [Google Scholar]
- 44.U.S. Food and Drug Administration. Guidance for industry: in vivo drug metabolism/drug interaction studies—study design, data analysis, and recommendations for dosing and labeling. U.S. Rockville, Md: Food and Drug Administration; 1999. [Google Scholar]