Abstract
Isoprenylation is an essential protein modification in eukaryotic cells. Herein, we report that in Plasmodium falciparum, a number of proteins were labeled upon incubation of intraerythrocytic forms with either [3H]farnesyl pyrophosphate or [3H]geranylgeranyl pyrophosphate. By thin-layer chromatography, we showed that attached isoprenoids are partially modified to dolichol and other, uncharacterized, residues, confirming active isoprenoid metabolism in this parasite. Incubation of blood-stage P. falciparum treated with the isoprenylation inhibitor limonene significantly decreased the parasites' progression from the ring stage to the trophozoite stage and at 1.22 mM, 50% of the parasites died after the first cycle. Using Ras- and Rap-specific monoclonal antibodies, putative Rap and Ras proteins of P. falciparum were immunoprecipitated. Upon treatment with 0.5 mM limonene, isoprenylation of these proteins was significantly decreased, possibly explaining the observed arrest of parasite development.
Malaria, a major tropical disease caused by protozoa of the genus Plasmodium, affects 300 to 500 million people and causes the deaths of over 1 million individuals per year, mostly African children under 10 years of age. Plasmodium falciparum, the most virulent of the four species which infect humans, is associated with potentially fatal disease (37). The major clinical symptoms of the disease stem from the destruction of red blood cells during the multiplication of asexual parasites, leading to anemia, or cytoadherence of parasitized red blood cells to endothelial receptors, resulting in severe forms of malaria (26). Because of the expanding resistance of these parasites to virtually all of the reagents used in malaria therapy, new approaches to drug design are urgently needed. The identification of potential targets that are essential to the parasite's life cycle is a prerequisite for rational drug development.
Protein prenylation is a general phenomenon in eukaryotic cells and was recently detected in parasites like Giardia lamblia (22), Trypanosoma brucei (12), and Schistosoma mansoni (6). In P. falciparum, Chakrabarti et al. detected protein prenyl transferase activities (5). Additionally, Rab GTP-binding proteins (Rab6) cloned from P. falciparum (21, 34) have the carboxyl-terminal motif Cys-AAX (where the letter A initially signified an aliphatic amino acid and the letter X denoted an undefined amino acid) and Cys-Cys residues, which suggests that they may be prenylated. Finally, Jomaa et al. demonstrated an alternative isopentenyl synthesis pathway so far described only in algae or cyanobacteria, which could be efficiently inhibited by fosmidomycin (17).
The aims of this work were to characterize protein geranylgeranylation and farnesylation in the protozoan parasite P. falciparum and to test whether the monoterpene limonene, a nontoxic inhibitor of the prenyl protein transferase enzyme and initially used against tumor cells (1, 36), is also active against the fast-growing malaria parasite P. falciparum.
MATERIALS AND METHODS
Parasite culture.
The experiments were performed with an isolate (S20) of P. falciparum obtained from a patient living in Porto Velho (State of Rondônia, Brazil) (18). Parasites were cultivated by the method of Trager and Jensen (33) as modified by Kimura et al. (19).
Parasite development and multiplication were monitored by microscopic evaluation of Giemsa-stained thin smears. Synchronization was obtained by two treatments with a 6% (wt/vol) Plasmagel (Laboratoire Roger Bellon, Neuilly sur Seine, France) solution in physiological saline (28). Starting with asynchronous cultures, schizonts were concentrated by flotation in Plasmagel and subcultured with fresh erythrocytes at 48-h intervals. Ring (1 to 20 h after reinvasion), trophozoite (20 to 30 h after reinvasion), and schizont (30 to 45 h after reinvasion) forms were purified on a 40 to 70 to 80% discontinuous Percoll (Pharmacia LKB, Uppsala, Sweden) gradient (2).
Inhibition tests.
(+)-Limonene, diluted in methanol (both from Sigma Chemicals, St. Louis, Mo.), was used at concentrations of 0.05 to 5.0 mM in different experiments. Controls with methanol were performed in parallel.
The method of Desjardins et al. (10) was used to determine the 50 and 90% inhibitory concentrations (IC50 and IC90) of limonene. Briefly, ring-stage parasite cultures (5% hematocrit, 2% parasitemia) were exposed to increasing drug concentrations (0.05, 0.1, 0.2, 0.5, 1.0, 2.0, and 5.0 mM). After 24 h in culture, [G-3H]hypoxanthine (270 GBq/mmol, 7.3 Ci/mmol; Amersham Life Sciences, Buckinghamshire, United Kingdom) was added (5 μCi/ml, final radioactivity level), and after an additional 24-h incubation period, cells were harvested. All tests were done in triplicate. Suspensions of uninfected erythrocytes similarly treated were used for background subtraction. Parasitemia and parasite morphology were determined by examining Giemsa-stained smears immediately before the start of the assay and at the end of it. The IC50 was calculated by probit analysis (Minitab Statistical Software 13.30; Minitab Inc.).
Inhibition tests with 0.5 mM limonene were carried out in flat-bottom microtiter plates (Falcon). Freshly synchronized cultures of 5% hematocrit and 1% parasitemia (ring-stage parasites) were exposed to several dilutions of the compound to be tested in normal culture medium. After 24, 48, and 72 h (if not otherwise stated), the percentage of each form was determined. After counting, the value for each form was expressed as a percentage of the total number of parasites (multinuclear schizont-infected red blood cells were counted as single cells). The results of three independent tests were evaluated for significant discrepancies of each form per time point in treated versus untreated parasites by Student's t test.
Metabolic labeling.
Mixed cultures of P. falciparum with parasitemias of around 10% were left untreated or treated with 0.5 mM (+)-limonene for 20 h and labeled in the presence or absence of the drug for 18 h with [1-(n)-3H]geranylgeranyl pyrophosphate triammonium salt ([3H]GGPP; 16.5 Ci/mmol, 6.25 μCi/ml; Amersham) or with [1-(n)-3H]farnesyl pyrophosphate triammonium salt ([3H]FPP; 16.5 Ci/mmol, 6.25 μCi/ml; Amersham) in RPMI 1640 normal medium. The same protocol was used when parasites were labeled with 25 μCi of l-[35S]methionine (>1,000Ci/mmol; Amersham) per ml in 10 mM methionine-deficient RPMI medium. Each stage (the ring, trophozoite, or schizont form) was then purified as described above, followed by lysis of cells in twice their volume of ice-cold 10 mM Tris-HCl (pH 7.2)–150 mM NaCl–2% (vol/vol) Triton X-100–1 mM phenylmethylsulfonyl fluoride–5 mM iodoacetamide–1 mM Nα-p-tosyl-l-lysine chloromethyl ketone–1 μg of leupeptin per ml. After incubation for 15 min at 4°C, lysates were centrifuged at 10,000 × g for 30 min and supernatants were stored in liquid N2 for subsequent sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) analysis (7, 19).
Thin-layer chromatography (TLC).
After SDS-PAGE analysis, gel bands corresponding to labeled proteins of 21 to 24 kDa (labeled with [3H]GGPP or [3H]FPP) were excised, resuspended separately in 0.6 ml of 0.5% (vol/vol) formic acid, and centrifuged to remove insoluble components. The supernatant was transferred to a new tube and evaporated. Cleavage reactions were performed with 0.1 ml of ICH3 at room temperature for 48 h in the dark, 0.03 ml of 35% (wt/vol) Na2CO3 was added, and the samples were kept in the dark for an additional 12 h. Samples were further extracted with chloroform-methanol (9:1, vol/vol), and the organic phases were separated and dried by evaporation (11, 30).
Reverse-phase TLC (RP-TLC) was performed on C18 RP-precoated plates (Merck, Darmstadt, Germany) using acetonitrile-methanol (1:1, vol/vol) as the developing solvent. Authentic standards of farnesol, geranylgeraniol, and dolichol were used as previously described (7).
Gel electrophoresis.
SDS-PAGE was performed in 12.5% gels as described elsewhere (20). The same number of drug-treated or untreated parasites as mentioned above were solubilized in SDS sample buffer and applied to each well for analysis. All gels were treated with Amplify (Amersham), dried, and exposed to Kodak X-Omat film with intensifying screen sets at −70°C.
Immunoprecipitation assays.
Samples stored in liquid N2 were resuspended in immunoprecipitation buffer (1% [vol/vol] Triton X-100, 150 mM NaCl, 0.5% [wt/vol] sodium deoxycholate, 0.1% [wt/vol] SDS, 50 mM Tris-HCl [pH 8.0], and a protease inhibitor cocktail [0.2 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 2 mM β-mercaptoethanol, 5 mg of chymostatin per ml, 1 μg each of leupeptin, antipain, and pepstatin A per ml]) and then precleared with protein A-Sepharose beads (Pharmacia) (27). Parasites purified at the schizont stage were then incubated with anti-human Ras or anti-Rap/Krev-1 monoclonal immunoglobulins (1:50 dilution; Santa Cruz Biotechnology, Inc.) for 2 h at 4°C. The antigen-antibody complex was precipitated by using 100 μl of a 10% protein A–Sepharose slurry. After five washes with phosphate-buffered saline, the bound antigen was released in SDS sample buffer and analyzed by SDS-PAGE and autoradiography.
RESULTS
P. falciparum proteins are labeled during incubation with [3H]FPP or [3H]GGPP
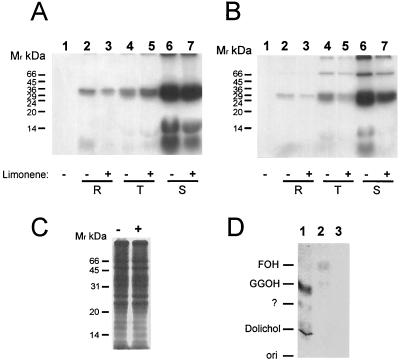
First, we examined the spectrum of isoprenylated polypeptides in different stages of P. falciparum. Parasites were incubated with [3H]FPP or [3H]GGPP for 18 h, purified on a Percoll gradient, lysed, and analyzed by SDS-PAGE and autoradiography. As shown in Fig. 1A and B, a number of putative isoprene-labeled polypeptides were observed. [3H]GGPP-labeled proteins with molecular masses of approximately 7 kDa, approximately 10 kDa, and 21 to 24 kDa appeared in the ring, trophozoite, and schizont stages, with most of the radioactivity incorporated into the cluster of 21- to 24-kDa proteins (Fig. 1A). When parasites were incubated with [3H]FPP, a similar pattern was detected but an additional 50-kDa protein was labeled (Fig. 1B). In our analysis, we detected stronger labeling in schizonts than in the ring and trophozoite stages. Noninfected red blood cells showed no incorporation of radioactivity under these conditions (Fig. 1A and B, lanes 1). Importantly, the signal observed at the top of the gel is not composed of high-molecular-weight proteins since the material did not enter the gel even after extended electrophoresis in low-percentage gels (data not shown).
FIG. 1.
Treatment of P. falciparum in vitro cultures with 0.5 mM limonene and metabolic labeling with [3H]GGPP and [3H]FPP. Parasites were treated for 24 h in the presence (+) or absence (−) of 0.5 mM limonene and labeled for 18 h with 6.25 μCi of [3H]GGPP (A) or [3H]FPP (B) per ml, lysed, and analyzed by SDS-PAGE (12.5% acrylamide) and fluorography. R, ring forms; T, trophozoites; S, schizonts. Lanes 1 in panels A and B show identically processed material from noninfected erythrocytes. Molecular mass (Mr) standards are indicated on the left. (C) Parasites were treated for 20 h with 0.5 mM limonene (+) or left untreated (−) and labeled with [35S]methionine for 18 h, harvested, washed, lysed, and analyzed by SDS-PAGE (12.5% acrylamide). (D) Radiochromatogram of chloroform-methanol (9:1)-extractable P. falciparum proteins and products derived from [3H]FPP- or [3H]GGPP-labeled P. falciparum proteins resolved by SDS-PAGE that were resolved by RP-TLC. Lanes: 1, moieties cleaved from 21- to 24-kDa proteins labeled with [3H]GGPP; 2, moieties cleaved from 21- to 24-kDa proteins labeled with [3H]FPP; 3, control containing cleaved material from the 21- to 24-kDa region of noninfected, [3H]GGPP-labeled red blood cells. Standards run in parallel are indicated on the left. FOH, farnesol; GGOH, geranylgeraniol; ori, origin.
RP-TLC of dephosphorylated isoprenoids reveals different attached isoprenoid moieties.
In order to identify isoprenoid moieties attached to the P. falciparum proteins, the bands of 21 to 24 kDa (labeled with [3H]GGPP or [3H]FPP) were excised, cleaved with ICH3, and then submitted to RP-TLC with isoprene standards. As shown in Fig. 1D, [3H]GGPP-labeled parasites contained proteins with attached [3H]geranylgeranyl and [3H]dolichol moieties and an undefined isoprenoid rest (lane 1), while [3H]FPP labeling of parasites led to 20- to 24-kDa proteins containing mostly [3H]farnesyl moieties and a faint signal for [3H]geranylgeranyl (lane 2). This indicates that at least four differently labeled and isoprenylated proteins were present in the 20- to 24-kDa cluster and that [3H]GGPP was transformed by P. falciparum into [3H]dolichol and another, undefined, isoprenoid intermediate.
Treatment of parasites with limonene decreases the incorporation of isoprenyl precursors in proteins.
To assess the effect of limonene treatment on P. falciparum, we compared the incorporation of labeled isoprenoid precursors in identical amounts of protein extracts obtained from different purified stages of P. falciparum left untreated or treated with 0.5 mM limonene. Treatment of parasites with 0.5 mM limonene during [3H]FPP labeling significantly decreased the incorporation of [3H]FPP into proteins. The effect was most dramatic for the ≈10-kDa band seen in trophozoite and schizont extracts (Fig. 1B, lane 4 versus lane 5 and lane 6 versus lane 7). Labeling of parasites with [3H]GGPP did not result in such differences in labeling patterns (Fig. 1A), although the ≈10-kDa band intensity appeared to be lower in the schizont-stage parasites (Fig. 1A, lane 7). Importantly, labeled proteins originated from P. falciparum parasite extracts and not from accidentally contaminating leukocytes concentrated by Percoll gradient purification of schizonts (leukocytes colocalize with schizont-infected erythrocytes), since the control sample containing red blood cells used for a culture run in parallel showed no incorporation of radioactivity at all (lanes 1 in Fig. 1A and B). Additionally, the primary effect of limonene seemed to be specifically on isoprenyl protein transferases, since no difference in bulk protein synthesis was seen when running whole [35S]methionine-labeled extracts of identical numbers of parasites that were left untreated or treated with 0.5 mM limonene (Fig. 1C).
Limonene inhibits P. falciparum growth in vitro by decreasing the progression from the ring stage to the trophozoite stage
Limonene is a monoterpene found in the essential oils of citrus fruits and other plants. d-Limonene, which comprises >90% of orange peel oil, has a chemopreventive activity against rodent mammary, skin, liver, lung, and forestomach cancers (1), possibly by interfering with the isoprenoid metabolism of these tumor cells, specifically by inhibition of the isoprenoid protein transferases (8). To observe an inhibitory effect of limonene on the growth of P. falciparum parasites, we cultured defined numbers of parasites in the absence or presence of increasing concentrations of limonene. Three independent determinations of the IC50 for limonene demonstrated the precision of the test system. The results of parasitemia and the uptake of [G-3H]hypoxanthine by parasitized erythrocytes in the microtiter plates were similar. The IC50 of limonene was 1.22 mM, with a 95% confidence interval of 1.07 to 1.42 mM. In the same way, the IC90 was determined to be 2.27 mM (95% confidence interval, 1.99 to 2.70 mM). At the IC90, parasites died after 3 h of treatment.
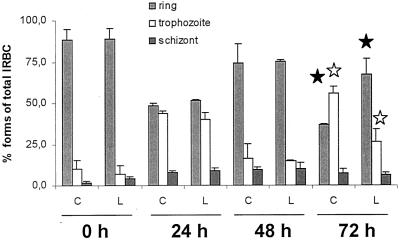
To determine the concentration of limonene that interferes with the incorporation of isoprenyl precursors in proteins, we chose 0.5 mM because under these conditions only a small percentage of parasites died and overall protein synthesis was not affected. As demonstrated in Fig. 2, after 72 h of culture in the presence of limonene, the shift from the ring stage to the trophozoite stage was significantly inhibited versus that of control parasites (P < 0.05) and ring forms accumulated in limonene-treated parasites (P < 0.05). The results shown were obtained in three independent trials starting with different initial parasitemias ranging from 1.3 to 3%. This indicates that at a concentration of 0.5 mM, limonene exerted a partially inhibitory effect on P. falciparum intraerythrocytic development, namely, during progression from the ring stage to the trophozoite stage. The observed interference with development, however, was delayed and a significant effect was only visible after one complete cell cycle (ring, trophozoite, and schizont stages and reinvasion), indicating a slow turnover of factors affected by limonene at this concentration. After a 72-h interval, the final parasite numbers were approximately 10% lower in limonene-treated cells (data not shown).
FIG. 2.
Monoterpene treatment partially arrests P. falciparum development at the ring stage. Parasite forms from cultures treated with 0.5 mM limonene and from untreated cultures were plotted against time of culture. The value for each form is given as a percentage of the sum of all forms at each time interval from three independent experiments. C, control cultures; L, limonene-treated cultures. The error bars indicate standard deviations. Significantly different values (P < 0.05) for treated versus untreated cultures are labeled with stars. Black stars indicate significant differences in treated or untreated ring-stage parasites, and white stars indicate significant differences in treated or untreated trophozoite-stage parasites. IRBC, infected red blood cells.
Immunoprecipitation of isoprenylated proteins.
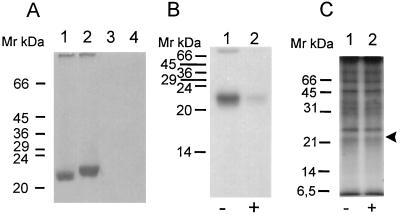
p21ras and p21rap have previously been shown to be modified by isoprenylation. In order to verify if P. falciparum forms of these proteins are recognized by anti-human Ras and anti-human Rap/Krev-1 antibodies, we immunoprecipitated radiolabeled schizont extracts. Since the p21h-ras protein is farnesylated (4), while the -CAAX motif of p21rap, a small GTP-binding protein encoded by the rap/krev-1 gene, is geranylgeranylated (3, 24), we immunoprecipitated lysates from schizont forms labeled with [3H]FPP or [3H]GGPP with p21h-ras or anti-p21rap antibodies, respectively. Figure 3A, lane 1, shows that p21rap antibodies immunoprecipitated a single [3H]GGPP-labeled band from P. falciparum lysates. Accordingly, anti-Ras antibodies immunoprecipitated a [3H]FPP-labeled protein (Fig. 3A, lane 2). However, when parasites were labeled with [3H]FPP, no signal was seen after immunoprecipitation with anti-Rap. The same result was obtained when labeling was performed with [3H]GGPP, followed by immunoprecipitation with anti-Ras (data not shown). Again, immunoprecipitation of identically treated noninfected red blood cells did not result in any signal after analysis by SDS-PAGE and autoradiography (Fig. 3A, lanes 3 and 4). This result adds further proof of the presence of Ras- or Rap-related proteins in P. falciparum, as described by others (16, 23, 32).
FIG. 3.
Immunoprecipitation of p21ras from P. falciparum at the schizont stage. (A) Infected or noninfected red blood cells were labeled with [3H]FPP or [3H]GGPP and immunoprecipitated with anti-p20h-rap/krev (lanes 1 and 3) or anti-p21h-ras antibody (lanes 2 and 4). Lanes 3 and 4 show immunoprecipitation of noninfected red blood cells with the respective antibodies. (B and C) Limonene at 0.5 mM specifically inhibits protein p21h-ras prenylation but not protein synthesis. (B) Identical numbers of purified lysed schizonts were labeled with [3H]FPP, treated (+) with limonene (0.5 mM) or left untreated (−), and then immunoprecipitated with protein A-Sepharose-bound monoclonal anti-Ras immunoglobulins and analyzed by SDS-PAGE and autoradiography. Lanes: 1, untreated parasites; 2, treated parasites. (C) Identical numbers of purified lysed schizonts labeled with [35S]methionine and treated (+) with limonene (0.5 mM) or left untreated (−) were then immunoprecipitated with protein A-Sepharose-bound monoclonal anti-Ras immunoglobulins and analyzed by SDS-PAGE. Lanes: 1, untreated parasites; 2, treated parasites. The arrowhead indicates the molecular size range where the Ras-like protein is expected.
Ras-like protein levels are decreased upon treatment of P. falciparum parasites with limonene
Gelb et al. suggested that the antitumorigenic effect of limonene is based on the inhibition of Ras protein prenylation and its subsequent incorrect cytoplasmic (and not membrane-associated) localization (13). This effect has also been demonstrated for the human-derived myeloid THP-1 and lymphoid RPMI-8402 cell lines (15). Given the interference with the progression of limonene-treated parasites from the ring stage to the trophozoite stage, as demonstrated in Fig. 2, we asked if levels of cell cycle control-associated proteins, namely, Ras derivatives, are influenced by limonene treatment. In order to visualize if limonene treatment inhibits the isoprenylation of the putative plasmodial Ras analogue, the immunoprecipitation experiment was repeated by using parasites subjected to 0.5 mM limonene treatment. Immunoprecipitation of identical quantities of purified schizont extract protein showed a significant decrease in the band labeling intensities for the treated sample versus the untreated sample (Fig. 3B, lane 2 versus lane 1). When a [35S]methionine-labeled extract was immunoprecipitated with the anti-p21h-ras, no decrease in band intensity was found for any immunoprecipitated protein species, confirming that treatment with 0.5 mM limonene only inhibited the incorporation of farnesyl moieties (Fig. 3C). The various observed bands in Fig. 3C result from the low-stringency washes of the immunoprecipitated material.
DISCUSSION
Upon metabolic labeling with the isoprenoid precursor geranylgeranyl pyrophosphate or farnesyl pyrophosphate, we could detect several isoprenylated proteins in P. falciparum. Incorporation of [3H]FPP led to the labeling of proteins in the range of approximately 7 kDa, approximately 10 kDa, 21 to 24 kDa, and 50 kDa, whereas [3H]GGPP incubation failed to label the 50-kDa peptide. Similar results were obtained when Giardia lamblia (22) trophozoites were labeled with [3H]mevalonate. The authors also found a cluster of 21- to 26-kDa proteins and a 50-kDa protein. In Trypanosoma brucei (12), metabolic incorporation of [3H]mevalonate also resulted in the appearance of a 14-kDa and an ≈46-kDa prenylated protein and a 21- to 24-kDa protein cluster. However, additional, uncharacterized, proteins in the range of ≈30 kDa and ≈69 kDa were also found. Protein prenylation is usually demonstrated by metabolic labeling with [3H]mevalonic acid; however, this precursor is not incorporated into P. falciparum (5, 7, 25). Danesi et al. demonstrated specific labeling of either farnesylated or geranylgeranylated proteins in the human prostate cancer cell line PC-3 (9). Despite the fact that eukaryotic cells incorporate external precursors of isoprenoid metabolism only in the presence of hydroxymethylglutaryl-coenzyme A reductase inhibitors (9), we had observed efficient labeling without previous depletion of the parasites' intracellular prenyl diphosphate pool (7), possibly pointing to receptor-mediated uptake of these compounds from the medium. Additionally, the failure to take up mevalonate may also be interpreted as inability of the parasite to metabolize this compound since the mevalonate pathway is probably absent in P. falciparum (35).
Several studies have previously hypothesized the existence of protein prenylation in P. falciparum. First, it was shown that cell extracts of P. falciparum convert mevalonate into farnesyl pyrophosphate, a precursor of protein prenylation (25), although the extracts used in these trials may not have been free of erythrocyte components, explaining the mevalonate utilization in these tests despite the absence of mevalonate-processing enzymes in P. falciparum (17, 35). Second, by using commercially available antibodies against the Ras and Rap proteins, which are known to be farnesylated or geranylgeranylated in other cell types, Ras- and Rap-like proteins were identified in Plasmodium parasites (32). Additionally, others demonstrated the existence of Rab-like proteins (16). Later, the rab6 and rab11 genes were cloned and both showed typical prenylation sites (21, 34). A recent study identified protein farnesyl transferase and protein geranylgeranyl transferase I activities in P. falciparum (5).
By RP-TLC analysis, we confirmed the nature of isoprenoid moieties attached to P. falciparum proteins. Our finding that very little [3H]FPP was transformed into [3H]GGPP was reported earlier by Danesi et al. for human PC-3 cells. In their study, neither [3H]FPP nor [3H]GGPP was modified to any other compound (9). In our experiments, when parasites were labeled with [3H]GGPP, we detected attached [3H]geranylgeraniol and, surprisingly, [3H]dolichol moieties. The presence of [3H]dolichol in our TLC may account for the existence of a dolichylated protein with a molecular mass similar to that of the 27-kDa polypeptide described by Hjertman et al. in human colon carcinoma cells (14).
Limonene, the principal component of orange peel oil, has been identified as a nontoxic agent with potential for cancer chemotherapy (8). In several model systems, limonene prevents the formation of chemically induced tumors and displays significant antitumor effects (36). On this basis, limonene and its metabolites have been tested in clinical trials (36). Limonene and its metabolites have been demonstrated to selectively inhibit the isoprenylation of 21- to 26-kDa proteins, including the Ras protein (13, 15). Due to its activity on fast-growing cells such as tumor cells, on the one hand, and the presence of protein prenyl transferase activities in P. falciparum (5), on the other hand, we assessed the effects of limonene treatment on parasite cultures.
In order to analyze effects on cell metabolism which depend on isoprenylation, we opted for a limonene concentration 2.4 times lower than the IC50. This concentration allowed us to analyze proteins with attachment of isoprenyl precursors, since there was no inhibition of overall protein synthesis. Generally, the labeling of isoprenylated proteins decreased, most significantly after labeling with [3H]FPP. Other groups demonstrated equal effects of limonene on both farnesyl transferases and geranylgeranyl transferases (13). It may be proposed that the corresponding transferases in P. falciparum are different from their mammalian relatives, explaining the differential inhibition by limonene. Morphologically, after 72 h of treatment with 0.5 mM limonene, progression from the ring stage to the trophozoite stage was decreased, which led us to search for specific factors involved in this process. We focused on members of the Ras superfamily, which are related to cell cycle control and signaling in other cell types (14). For example, depletion of farnesylated Ras proteins leads to arrest of the cell cycle, possibly due to subsequently incorrect localization of the proteins (31). Therefore, we immunoprecipitated parasite lysates with anti-Ras and anti-Rap antibodies after incorporation of [3H]FPP or [3H]GGPP, respectively. Based on our results, we suggest that the anti-Ras-immunoprecipitable protein is the P. falciparum Ras-like protein, as found previously by Western blot analysis and immunofluorescence assay (32). Accordingly, the protein immunoprecipitated with anti-Rap immunoglobulins could be termed the P. falciparum Rap-like protein.
We then utilized the monoterpene inhibitor of protein prenyl transferases limonene to show that incorporation of radioactivity into P. falciparum Ras-like protein specifically decreases after treatment. This effect had been demonstrated for the human-derived myeloid THP-1 and lymphoid RPMI-8402 cell lines (4). Similarly, we found significant [3H]FPP labeling inhibition of the Ras-like protein by limonene but no decrease in the overall amount of precipitated proteins after labeling with [35S]methionine.
When limonene was evaluated in phase 1 and 2 clinical trials, applications at doses of 8 g/m2 showed no apparent toxic effect (36). Considering that the average corporal surface area is 1.8 m2, corresponding to 7 liters of liquid components (29), the dose used in clinical trials corresponds to a concentration of 15.5 mM. Thus, the IC90 for P. falciparum calculated herein, which was found to be 2.27 mM in vitro, lies significantly below the value of 15.5 mM used in vivo. Additionally, comparing the inhibition intensities in immunoprecipitation experiments detecting isoprenylated proteins, we concluded that the concentration of limonene that inhibits isoprenylation in Plasmodium to the same rate as in human cells is approximately 10 times lower, indicating that limonene is more active against Plasmodium than against human cells (8).
Taken together, these findings led us to conclude that limonene or other nontoxic monoterpenes currently being tested in cancer therapy may prove as efficient as the recently described compound phosmidomycin, which efficiently inhibited the non-mevalonate pathway of isoprenoid synthesis in P. falciparum (17).
ACKNOWLEDGMENTS
This work was supported by grants from FAPESP, CNPq, PRONEX, and UNDP/World Bank/WHO (TDR). I.C.M. is supported by a doctoral fellowship from CAPES/CNPq. G.W., E.A.K., and A.M.K are supported by CNPq fellowships. A.S.C. is a member of the Research Council of Argentina (CONICET).
We thank Julio Pudles and Carlos Eduardo Winter for valuable comments.
REFERENCES
- 1.Bardon S, Picard K, Martel P. Monoterpenes inhibit cell growth, cell cycle progression, and cyclin D1 gene expression in human breast cancer cell lines. Nutr Cancer. 1998;32:1–7. doi: 10.1080/01635589809514708. [DOI] [PubMed] [Google Scholar]
- 2.Braun-Breton C, Jendoubi M, Brunet E, Perrin L, Scaife J, Pereira da Silva L. In vivo time course of synthesis and processing of major schizont membrane polypeptides in Plasmodium falciparum. Mol Biochem Parasitol. 1986;20:33–43. doi: 10.1016/0166-6851(86)90140-4. [DOI] [PubMed] [Google Scholar]
- 3.Buss J E, Quilliam L A, Kato K, Casey P J, Solski P A, Wong G, Clark R, McCormick F, Bokoch G M, Der C J. The COOH-terminal domain of the Rap1A (Krev-1) protein is isoprenylated and supports transformation by an H-Ras:Rap1A chimeric protein. Mol Cell Biol. 1991;11:1523–1530. doi: 10.1128/mcb.11.3.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey P J, Solski P A, Der C J, Buss J E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci USA. 1989;86:8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakrabarti D, Azam T, DelVecchio C, Qiu L, Park Y I, Allen C M. Protein prenyl transferase activities of Plasmodium falciparum. Mol Biochem Parasitol. 1998;94:175–184. doi: 10.1016/s0166-6851(98)00065-6. [DOI] [PubMed] [Google Scholar]
- 6.Chen G Z, Bennett J L. Characterization of mevalonate-labeled lipids isolated from parasite proteins in Schistosoma mansoni. Mol Biochem Parasitol. 1993;59:287–292. doi: 10.1016/0166-6851(93)90226-n. [DOI] [PubMed] [Google Scholar]
- 7.Couto A S, Kimura E A, Peres V J, Uhrig M L, Katzin A M. Active isoprenoid pathway in the intra-erythrocytic stages of Plasmodium falciparum: presence of dolichols of 11 and 12 isoprene units. Biochem J. 1999;341:629–637. [PMC free article] [PubMed] [Google Scholar]
- 8.Crowell P L, Chang R R, Ren Z B, Elson C E, Gould M N. Selective inhibition of isoprenylation of 21- to 26-kDa proteins by the anticarcinogen d-limonene and its metabolites. J Biol Chem. 1991;266:17679–17685. [PubMed] [Google Scholar]
- 9.Danesi R, McLellan C A, Myers C E. Specific labeling of isoprenylated proteins: application to study inhibitors of the posttranslational farnesylation and geranylgeranylation. Biochem Biophys Res Commun. 1995;206:637–643. doi: 10.1006/bbrc.1995.1090. [DOI] [PubMed] [Google Scholar]
- 10.Desjardins R E, Canfield C J, Haynes J D, Chulay J D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ericsson J, Runquist M, Thelin A, Andersson M, Chojnacki T, Dallner G. Distribution of prenyltransferases in rat tissues. Evidence for a cytosolic all-trans-geranylgeranyl diphosphate synthase. J Biol Chem. 1993;268:832–838. [PubMed] [Google Scholar]
- 12.Field H, Blench I, Croft S, Field M C. Characterisation of protein isoprenylation in procyclic form Trypanosoma brucei. Mol Biochem Parasitol. 1996;82:67–80. doi: 10.1016/0166-6851(96)02723-5. [DOI] [PubMed] [Google Scholar]
- 13.Gelb M H, Tamanoi F, Yokoyama K, Ghomashchi F, Esson K, Gould M N. The inhibition of protein prenyltransferases by oxygenated metabolites of limonene and perillyl alcohol. Cancer Lett. 1995;91:169–175. doi: 10.1016/0304-3835(95)03747-k. [DOI] [PubMed] [Google Scholar]
- 14.Hjertman M, Wejde J, Dricu A, Carlberg M, Griffiths W J, Sjovall J, Larsson O. Evidence for protein dolichylation. FEBS Lett. 1997;416:235–238. doi: 10.1016/s0014-5793(97)01208-8. [DOI] [PubMed] [Google Scholar]
- 15.Hohl R J, Lewis K. Differential effects of monoterpenes and lovastatin on RAS processing. J Biol Chem. 1995;270:17508–17512. doi: 10.1074/jbc.270.29.17508. [DOI] [PubMed] [Google Scholar]
- 16.Jambou R, Zahraoui A, Olofsson B, Tavitian A, Jaureguiberry G. Small GTP-binding proteins in Plasmodium falciparum. Biol Cell. 1996;88:113–121. [PubMed] [Google Scholar]
- 17.Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Turbachova I, Eberl M, Zeidler J, Lichtenthaler H K, Soldati D, Beck E. Inhibitors of the non-mevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 18.Katzin A M, Kimura E S, Alexandre C O, Ramos A M. Detection of antigens in urine of patients with acute falciparum and vivax malaria infections. Am J Trop Med Hyg. 1991;45:453–462. doi: 10.4269/ajtmh.1991.45.453. [DOI] [PubMed] [Google Scholar]
- 19.Kimura E A, Couto A S, Peres V J, Casal O L, Katzin A M. N-linked glycoproteins are related to schizogony of the intraerythrocytic stage in Plasmodium falciparum. J Biol Chem. 1996;271:14452–14461. doi: 10.1074/jbc.271.24.14452. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Langsley G, Chakrabarti D. Plasmodium falciparum: the small GTPase rab11. Exp Parasitol. 1996;83:250–251. doi: 10.1006/expr.1996.0071. [DOI] [PubMed] [Google Scholar]
- 22.Lujan H D, Mowatt M R, Chen G Z, Nash T E. Isoprenylation of proteins in the protozoan Giardia lamblia. Mol Biochem Parasitol. 1995;72:121–127. doi: 10.1016/0166-6851(94)00070-4. [DOI] [PubMed] [Google Scholar]
- 23.Macary C, Chauvin C, Thelu J, Oury B, Santoro F, Ambroise-Thomas P. Detection of homologous oncogene sequences in the genome of Plasmodium falciparum. C R Acad Sci Ser III. 1991;312:37–42. [PubMed] [Google Scholar]
- 24.Macchia M, Jannitti N, Gervasi G, Danesi R. Geranylgeranyl diphosphate-based inhibitors of post-translational geranylgeranylation of cellular proteins. J Med Chem. 1996;39:1352–1356. doi: 10.1021/jm960127s. [DOI] [PubMed] [Google Scholar]
- 25.Mbaya B, Rigomier D, Edorh G G, Karst F, Schrevel J. Isoprenoid metabolism in Plasmodium falciparum during the intraerythrocytic phase of malaria. Biochem Biophys Res Commun. 1990;173:849–854. doi: 10.1016/s0006-291x(05)80864-2. [DOI] [PubMed] [Google Scholar]
- 26.Miller L H, Good M F, Milon G. Malaria pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- 27.Moura I C, Pudles J. A Plasmodium chabaudi chabaudi high molecular mass glycoprotein translocated to the host cell membrane by a non-classical secretory pathway. Eur J Cell Biol. 1999;78:186–193. doi: 10.1016/s0171-9335(99)80097-1. [DOI] [PubMed] [Google Scholar]
- 28.Pasvol G, Wilson R J, Smalley M E, Brown J. Separation of viable schizont-infected red cells of Plasmodium falciparum from human blood. Ann Trop Med Parasitol. 1978;72:87–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- 29.Petersdorf R G, Adams R D, Braunwald E, Isselbacher K J M J B, Wilson J D, editors. Harrison's principles of internal medicine. 10th ed. New York, N.Y: McGraw-Hill, Inc.; 1983. pp. A1–A5. [Google Scholar]
- 30.Sagami H, Korenaga T, Ogura K. Geranylgeranyl diphosphate synthase catalyzing the single condensation between isopentenyl diphosphate and farnesyl diphosphate. J Biochem (Tokyo) 1993;114:118–121. doi: 10.1093/oxfordjournals.jbchem.a124125. [DOI] [PubMed] [Google Scholar]
- 31.Schulz S, Buhling F, Ansorge S. Prenylated proteins and lymphocyte proliferation: inhibition by d-limonene related monoterpenes. Eur J Immunol. 1994;24:301–307. doi: 10.1002/eji.1830240204. [DOI] [PubMed] [Google Scholar]
- 32.Thelu J, Bracchi V, Burnod J, Ambroise-Thomas P. Evidence for expression of a Ras-like and a stage specific GTP binding homologous protein by Plasmodium falciparum. Cell Signal. 1994;6:777–782. doi: 10.1016/0898-6568(94)00038-7. [DOI] [PubMed] [Google Scholar]
- 33.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 34.Van Wye J, Ghori N, Webster P, Mitschler R R, Elmendorf H G, Haldar K. Identification and localization of rab6, separation of rab6 from ERD2 and implications for an 'unstacked' Golgi, in Plasmodium falciparum. Mol Biochem Parasitol. 1996;83:107–120. doi: 10.1016/s0166-6851(96)02759-4. [DOI] [PubMed] [Google Scholar]
- 35.Vial H J. Isoprenoid biosynthesis and drug targeting in the Apicomplexa. Parasitol Today. 2000;16:140–141. doi: 10.1016/s0169-4758(00)01638-0. [DOI] [PubMed] [Google Scholar]
- 36.Vigushin D M, Poon G K, Boddy A, English J, Halbert G W, Pagonis C, Jarman M, Coombes R C. Phase I and pharmacokinetic study of d-limonene in patients with advanced cancer. Cancer Research Campaign Phase I/II Clinical Trials Committee. Cancer Chemother Pharmacol. 1998;42:111–117. doi: 10.1007/s002800050793. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. World malaria situation in 1994. Part I. Population at risk. Wkly Epidemiol Rec. 1997;72:269–274. [PubMed] [Google Scholar]