Abstract
Purpose
Six to 19% of critically ill COVID-19 patients display circulating auto-antibodies against type I interferons (IFN-AABs). Here, we establish a clinically applicable strategy for early identification of IFN-AAB-positive patients for potential subsequent clinical interventions.
Methods
We analyzed sera of 430 COVID-19 patients from four hospitals for presence of IFN-AABs by ELISA. Binding specificity and neutralizing activity were evaluated via competition assay and virus-infection-based neutralization assay. We defined clinical parameters associated with IFN-AAB positivity. In a subgroup of critically ill patients, we analyzed effects of therapeutic plasma exchange (TPE) on the levels of IFN-AABs, SARS-CoV-2 antibodies and clinical outcome.
Results
The prevalence of neutralizing AABs to IFN-α and IFN-ω in COVID-19 patients from all cohorts was 4.2% (18/430), while being undetectable in an uninfected control cohort. Neutralizing IFN-AABs were detectable exclusively in critically affected (max. WHO score 6–8), predominantly male (83%) patients (7.6%, 18/237 for IFN-α-AABs and 4.6%, 11/237 for IFN-ω-AABs in 237 patients with critical COVID-19). IFN-AABs were present early post-symptom onset and at the peak of disease. Fever and oxygen requirement at hospital admission co-presented with neutralizing IFN-AAB positivity. IFN-AABs were associated with lower probability of survival (7.7% versus 80.9% in patients without IFN-AABs). TPE reduced levels of IFN-AABs in three of five patients and may increase survival of IFN-AAB-positive patients compared to those not undergoing TPE.
Conclusion
IFN-AABs may serve as early biomarker for the development of severe COVID-19. We propose to implement routine screening of hospitalized COVID-19 patients for rapid identification of patients with IFN-AABs who most likely benefit from specific therapies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-022-01252-2.
Keywords: COVID-19, SARS-CoV-2, Type I interferon, Autoantibodies
Introduction
Since its first detection in Wuhan, China, in 2019, severe-acute-respiratory syndrome coronavirus 2 (SARS-CoV-2) has placed an unprecedented burden on health care systems worldwide. The clinical spectrum of the associated disease, COVID-19, ranges from asymptomatic infection to severe disease with hypoxemia, acute respiratory distress syndrome (ARDS), multiorgan failure, and death [1]. Approximately 35% of patients remain asymptomatic, 55% develop upper respiratory tract infections, whereas 15% develop severe pneumonia (defined as SpO2 < 90% at room air) and 5% critical pneumonia (defined as acute respiratory distress syndrome (ARDS), requiring mechanical ventilation or extra-corporeal membrane oxygenation (ECMO) [2].
Scores containing clinical and laboratory parameters support risk stratification and resource allocation in clinical practice worldwide [3]. Demographic and clinical risk factors for a severe disease course include advanced age, male sex, and pre-existing comorbidities [4]. Moreover, genetic polymorphisms are associated with progression to severe disease [5]. Cell-intrinsic innate viral sensors and antiviral cytokines, including type I and type III interferons (IFNs), orchestrate the control of SARS-CoV-2 infection [6]. Inherited mutations of genes involved in IFN induction and signaling and circulating auto-antibodies (AABs) that neutralize type I IFNs have been found to predispose infected individuals to severe COVID-19 [7, 8], presumably by contributing to an ineffective immune response with delayed or abolished type I IFN signaling. Neutralizing type I IFN-AABs are present in 6–17% of hospitalized COVID-19 patients with severe pneumonia [7, 9, 10] and 11–19% in critically ill COVID-19 patients [7, 11, 12], greatly exceeding estimated prevalences of around 0.33% [7] in uninfected individuals. Intriguingly, while neutralizing IFN-AABs in patients with autoimmune polyendocrine syndrome type 1 (APS-1) can associate with a severe course of SARS-CoV-2 infection [13–17], their mere presence does not inevitably lead to severe disease [18]. A recent global multi-cohort study reports prevalence of neutralizing IFN-AABs in 4% of uninfected individuals over 70 years of age, suggesting that IFN-AABs may pre-exist in some individuals that develop a critical course of COVID-19 [19]. Thus, we reasoned that IFN-AABs may serve as biomarkers that could, in conjunction with other clinical parameters, help to predict risk for developing severe COVID-19 and to stratify patients for specific therapies.
Specific therapies may comprise the administration of recombinant IFN-β or therapeutic plasma exchange (TPE). However, the clinical benefit of TPE and other approaches remains to be defined and requires studies involving large numbers of patients. With IFN-AABs present in up to 18% of deceased COVID-19 patients [19] and given the limited therapeutic options for severely affected COVID-19 patients, testing specific therapeutic approaches is of high urgency, yet clinically implementable strategies for rapid and early identification of IFN-AAB-positive patients upfront are missing.
Methods
Study Cohorts and Data Collection
Patients were recruited and data and sample collection was performed within one of four prospective observational studies conducted at Charité—Universitätsmedizin Berlin, Germany (Cohort A, [20]), Inselspital Universitätsspital Bern, Switzerland (Cohort B), Universitätsklinikum Freiburg, Germany (Cohort C), and Universitätsklinikum Heidelberg, Germany (Cohort D). For this analysis, all patients with a maximum WHO score of 3–8 (see supplementary methods) were included from Cohorts A–C (henceforth summarized as cross-sectional cohorts, CSC). For cohort D (therapeutic plasma exchange cohort, TPEC), only patients who underwent therapeutic plasma exchange for treatment of COVID-19-associated hyperinflammatory syndrome at the Department of Internal Medicine IV of Heidelberg University Hospital, Germany ([21] and supplementary methods), were retrospectively selected. All TPE procedures were performed in accordance with the German Medical Devices Act (“Medizinproduktegesetz”). Healthy controls were recruited from a study on SARS-CoV-2 exposition in health care workers (HC cohort). Samples from APS-1 patients were obtained from a published study [18] and published values are shown here for reference. All studies were conducted according to the Declaration of Helsinki and Good Clinical Practice principles.
Detection of IFN-AABs by Reverse ELISA
IFN-AABs were detected using an electrochemiluminescence immunoassay (ECLIA)-platform (MSD, Rockville, USA), as described recently [18]. Briefly, MSD GOLD 96-well small spot streptavidin SECTOR Plates (MSD) were washed with wash buffer (MSD) and blocked with 150 µl blocking buffer (Thermo Fisher, Waltham, USA) per well at 4 °C overnight. All further incubations were performed for 60 min at room temperature. After blocking, plates were incubated with IFN-α2 (Merck Sharp & Dohme, Kenilworth, USA) or IFN-⍵ (Peprotech, Rocky Hill, USA) linked to biotin (Thermo Scientific, Waltham, USA). Next, plates were incubated with patients’ sera following dilution at 1:100 in blocking buffer. Cytokine AABs were detected using a monoclonal mouse antibody to human IgG (D20JL-6, MSD). After incubation and washing, 150 µl of read buffer (ReadBufferT (4x), MSD) was added, incubated for 10 min at room temperature, and plates were analyzed using the MESO QuickPlex SQ 120 analyzer (MSD). Data are shown as light signal counts (LSC).
Competition Assays
All sera whose IFN-α2-AABs and/or IFN-⍵-AABs levels exceeded the 97.5th percentile of AAB levels of the analyzed health-care workers’ sera and samples that scored close to, but below this cut-off were assessed by competition assay using unbiotinylated IFN-α2 or IFN-⍵. The sera of interest were diluted 1:100 with blocking buffer and incubated overnight at 4 °C with 2.5 mg/ml, 0.025 mg/ml, and 0.00025 mg/ml unbiotinylated IFN-α2 or IFN-⍵. After incubation, reverse ELISA was performed, as described above. IFN-AABs in a given serum scored specific when preincubation with the highest concentration of IFN-α2 or IFN-⍵ resulted in an at least four-fold reduction of LSC in comparison to analysis of the identical serum without IFN-α2 or IFN-⍵ pre-incubation.
Virus Infection-Based Neutralization Assays
Calu-3 cells were pre-incubated with 1% human serum in the presence or absence of 200–400 IU/ml IFN-α2a (Roferon®-A, Roche) or 20–50 ng/ml IFN-ω (PeproTech). After 24 h, IFN and serum were removed and cells were infected with SARS-CoV-2 at a multiplicity of infection 0.01. Virus inoculum was removed after 1 h, cells were washed with PBS, and 100 µl medium was added per well. Twenty-four hours post-infection, cell culture supernatant was collected for viral RNA quantification by RT-PCR and infectious titer determination by plaque assay.
Cytokine and Chemokine Measurements
Cytokines and chemokines from a subset of patients from cohort A were analyzed using Quanterix’ single molecule array technology or multiplex ECLIA.
Results
We analyzed 430 serum samples collected within four independent observational clinical studies on COVID-19 for IFN-AAB positivity (Table 1), comprising 237 patients with critical COVID-19 (max. WHO score 6–8). Median age of patients in the CSC (cohorts A–C, 403 patients) was 61 years (IQR 52–71) and 72.2% (291/403) were male. Median Charlson Comorbidity Index (CCI) was 3 (IQR 1–4). Twenty-seven patients with critical disease course (median max. WHO score 7 (IQR 7–8)) who underwent TPE as compassionate use were selected retrospectively from center D (TPEC). Median age of patients in the TPEC was 65 years (IQR 56–72), 74.1% (20/27) were male, and median CCI was 4 (IQR 3–5). All patients from the TPEC required invasive mechanical ventilation (IMV), 77.8% (21/27) renal replacement therapy, one patient was treated with ECMO, and 13 out of 27 (48.2%) patients died despite maximum care. Six hundred sixty-seven serum samples from a healthy cohort (HC) consisting of health-care workers (Table S1) were screened for the presence of neutralizing IFN-AABs to set the cut-off for IFN-AAB positivity.
Table 1.
Baseline patient characteristics
| Individual cohorts | All patients of cross-sectional cohort | ||||||
|---|---|---|---|---|---|---|---|
| Center A, Berlin cohort | Center B, Bern cohort | Center C, Freiburg cohort | Center D, Heidelberg cohort (TPEC) | IFN-AAB Neutralizing | IFN-AAB Non-neutralizing | p-value | |
| Number of patients | 266 | 50 | 87 | 27 | 13 | 390 | / |
| IFN-AAB | 2.6 (7/266) | 6.0 (3/50) | .5 (3/87) | 18.5 (5/27) | 100 (13/13) | / | / |
| Neutralizing IFN-alpha | 2.6 (7/266) | 6.0 (3/50) | 3.5 (3/87) | 18.5 (5/27) | 100 (13/13) | ||
| Neutralizing IFN-omega | 1.1 (3/266) | 6.0 (3/50) | 2.3 (2/87) | 11.1 (3/27) | 61.5 (8/13) | ||
| Age (Median, IQR, available n) | 61 (50–71), 266 | 67.3 (56.8–74.5), 50 | 59 (53–67), 87 | 65 (56–72), 27 | 69.4 (52.5–75.6), 13 | 61.0 (52–70.2), 390 | 0.19 |
| Sex | Male: | ||||||
| Female | 27.8 (74/266) | 18.0 (9/50) | 33.3 (29/87) | 25.9 (7/27) | 15.4 (2/13) | 28.2 (110/390) | 0.31 |
| Male | 72.2 (193/266) | 82.0 (41/50) | 66.7 (58/87) | 74.1 (20/27) | 84.6 (11/13) | 71.8 (280/390) | OR = 2.16 (0.47–9.91) |
| BMI (kg/m2, Median, IQR, available n) | 28.4 (24,9–32,5), 245 | 27 (26–31), 47 | 27.7 (25.2–32.2), 56 | 31.5 (25.8–40.1), 27 | 27.4 (25.5–29.5), 10 | 28 (24.9–32.4), 338 | 0.73 |
| Comorbidities | |||||||
| CCI (Median, IQR, available n) | 2 (1–3.75), 265 | 4 (2–6.5), 33 | 3 (2–5), 83 | 4 (3–5), 27 | 3 (1.5–4), 13 | 3 (1–4), 367 | 0.54 |
| Chronic heart disease (%) | 58.9 (155/263) | 30.3 (10/33) | 23.0 (20/87) | 77.8 (21/27) | 46.2 (6/13) | 48.4 (179/370) | 0.74, OR = 0.83 (0.27–2.53) |
| Chronic pulmonary disease (%) | 18.5 (47/254) | 27.3 (9/33) | 10.4 (9/87) | 3.7 (1/27) | 23.1 (3/13) | 17.2 (62/361) | 0.58, OR = 1.45 (0.39–5.41) |
| Diabetes (%) | 26.7 (70/262) | 42.4 (14/33) | 26.4 (23/87) | 44.4 (12/27) | 23.1 (3/13) | 28.2 (104/369) | 0.54, OR = 0.70 (0.19–2.58) |
| Obesity (%) | 39.2 (96/245) | 31.9 (15/47) | 28.6 (16/56) | 55.6 (15/27) | 20.0 (2/10) | 37.0 (125/338) | 0.27, OR = 0.43 (0.08–2.04) |
| Autoimmune disease (%) | 2.8 (7/251) | 2.0 (1/50) | 5.7 (5/87) | 3.7 (1/27) | 0 (0/13) | 3.5 (13/375) | 0.72 |
| Symptoms: Fever | 57.0 (151/265) | 80.0 (40/50) | 77.5 (55/71) | 85.2 (23/27) | 100 (12/12) | 62.6 (234/374) | 0.0079 |
| Days between symptom onset and admission (median, IQR, available n) | 6 (2–9), 233 | 4 (2–8), 50 | 6 (3–9), 66 | 6 (3–8), 27 | 4 (3–8), 11 | 5 (2–9), 338 | 0.81 |
| Need for supplementary oxygen within first 72 h after admission | 78.4 (189/241) | 74.0 (37/50) | 72 (36/50) | 100 (12/12) | 100 (12/12) | 76.0 (250/329) | 0.0528 |
| IMV | 46.2 (117/253) | 60,0 (30/50) | 39,0 (32/82) | 100 (27/27) | 100 (13/13) | 44.5 (166/372) | 0.0001 |
| Length of ventilation in days (median, IQR, available n) | 32,5 (18,25–56,5), 116 | 10,5 (5–18,5), 30 | 16 (6–21), 23 | 24 (14–37), 27 | 20 (10.75–29.25), 12 | 24 (11–47,5), 157 | 0.40 |
| Length of hospital stay in days (median, IQR, available n) | 20 (10–44), 262 | 13,5 (5–23,25), 50 | 16 (7–33), 85 | 41 (24–63), 27 | 25 (16–47), 13 | 17 (9–37,8), 384 | 0.045 |
| Medication/treatment | |||||||
| Dexamethasone | 46.9 (123/262) | 48.0 (24/50) | 21.0 (17/87) | 74,1 (20/27) | 46.2 (6/13) | 41.6 (158/380) | 0.74 |
| Remdesivir | 9,2 (16/174) | 0 (0/50) | 12.6 (11/87) | 29.6 (8/27) | 0 (0/13) | 9.0 (27/300) | 0.52 |
| Renal replacement therapy | 29.3 (74/253) | 26.0 (13/50) | 25.3 (20/79) | 77.8 (21/27) | 69.2 (9/13) | 26.6 (98/369) | 0.0008 |
| ECMO | 17.4 (44/253) | 0 (0/50) | 24.4 (20/82) | 3.7 (1/27) | 46.2 (6/13) | 15.6 (58/372) | 0.0036 |
| Plasmapheresis | 0 (0/266) | 0 (0/50) | 0 (0/87) | 100 (27/27) | 0 (0/13) | 0 (0/390) | / |
| max. WHO score (Median, IQR, available n) | 5 (4–7), 266 | 7 (4–8), 50 | 6 (4–8), 83 | 7 (7–8), 27 | 8 (8–8), 13 | 6 (4–7), 386 | 0.0001 |
| Outcome | |||||||
| Discharged or transferred | 81.0 (205/253) | 72.0 (36/50) | 72.0 (59/82) | 51.8 (14/27) | 7.7 (1/13) | 80.4 (299/372) | |
| Deceased | 19.0 (48/253) | 28.0 (14/50) | 25.6 (21/82) | 48.2 (13/27) | 92.3 (12/13) | 19.1 (71/372) | 0.0001 |
| Unknown | / | / | 2.4 (2/82) | / | / | 0.5 (2/372) | |
Data are shown in % (N/n) unless otherwise indicated. IMV invasive mechanical ventilation, IQR interquartile range, CCI Charlson’s comorbidity index. Patients with DNI/DNR were excluded for IMV, RRT, ECMO, and Outcome (N = 13 Center A, N = 0 Center B, N = 5 Center C, and N = 0 Center D)
Prevalence of AABs Against IFN-α2 and IFN-ω in Patients with COVID-19
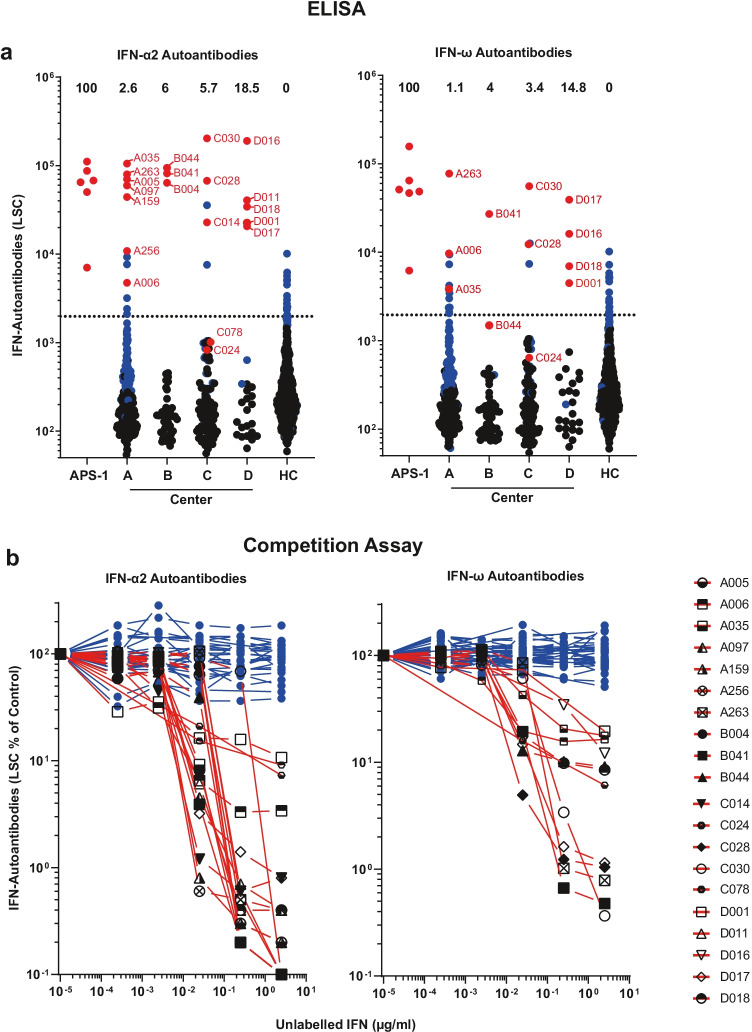
We first aimed to establish a sensitive screening assay for type I IFN-AABs. To this end, we first screened samples of our HC for prevalence of AABs against IFN-ɑ and/or IFN-⍵ by ELISA. Samples were considered positive when the respective LSC value exceeded the 97.5th percentile of AAB levels of the analyzed sera from the HC (cut-off for IFN-ɑ = 1980 LSC, IFN-⍵ = 1961 LSC). We then screened sera obtained at the peak of the disease (i.e., during the hospitalization period with highest individual WHO score) from patients of cohorts A-C (CSC) and cohort D (TPEC). The proportion of ELISA-positive patients in the CSC was 5.0% (20/403) for IFN-ɑ AABs and 4.2% (17/403) for IFN-⍵ AABs. It was significantly higher in cohort D (TPEC) (IFN-ɑ AABs 18.5%, 5/27, p = 0.0035 and IFN-⍵ AABs 14.8%, 4/27, p = 0.0132), as expected (Fig. 1a, Fig. S1, Table S2). Some sera displayed values approaching or equaling those detected in sera from patients with autoimmune polyendocrine syndrome type 1 (APS-1), a genetic disease involving the generation of high titer neutralizing type I IFN-AABs (Fig. 1a, [18]).
Fig. 1.
Prevalence of AABs against IFN-α2 and IFN-ω in patients with COVID-19. a ECLIA-based assay for detection of IgG AABs against IFN-α2 and IFN-ω in sera from hospitalized patients with COVID-19 from four different university hospital cohorts (Center A, n = 266; Center B, n = 50; Center C, n = 87; Center D, n = 27), in patients with APS-1 (n = 6), and healthy health care workers (HC) without documented SARS-CoV-2 infection (n = 667). Dotted lines indicate the 97.5th percentile of the ECLIA assay LSC in sera from the HC cohort. Dots indicate samples containing AABs scoring specific (red) or unspecific (blue) for IFN-α2 and IFN-ω binding in the competition assay (see b), respectively. Samples depicted as black dots were not tested in the competition assay. The prevalence of sera with specifically binding type I IFN-AABs in each cohort is given in percent. b Specificity of the ECLIA assay signal for IFN-α2- and IFN-ω-AABs was tested in an competition assay by preincubation of sera with increasing concentrations of unlabeled IFN-α2 and IFN-ω protein (0–2.5 µg/ml) before analysis. Samples showing a decrease in assay signal by at least 75% in the presence of the highest competitor concentration were defined as specific for type I IFN antibody reactivity and are indicated with red lines (IFN-α2 n = 20, IFN-ω n = 12). Samples showing no decrease in the presence of excess unlabeled type I IFN protein were regarded as unspecific for type I IFN antibody reactivity and are indicated with blue lines (IFN-α2 n = 62, IFN-ω n = 39)
Nonspecific binding is a common phenomenon in immunoassays for the detection of AABs, and high levels of inflammatory parameters such as C-reactive protein (CRP) correlate with non-specific binding of (auto-)antibodies [22]. Therefore, we probed the specificity of all samples exceeding the 97.5th percentile of the HC sera in the IFN-AAB ELISAs and 117 and 118 samples that scored below this cut-off, respectively, from all five cohorts in a competition assay (Fig. 1b, Table S2). As expected, sera that scored below the 97.5th percentile of the ELISA had a low chance of scoring positive in the competition assay (2/117 for IFN-α: C024, C078; 2/118 for IFN-ω: C024, B044). A substantial part, but not all, ELISA-positive samples of the five cohorts scored positive in the competition assay (18/34 alpha; 10/34 omega), indicating specific binding of IFN in those. Overall, we established a prevalence of specific IFN-ɑ-AAB of 3.7% (15/403) and of specific IFN-⍵-AAB of 2% (8/403) in the CSC (Fig. 1a, b). Cohort D (TPEC) showed 18.5% of sera specifically binding IFN-ɑ (5/27) and 14.8% for IFN-⍵ (4/27) or both (14.8%, 4/27) (Fig. 1a, b). Importantly, none of the tested sera from the HC displayed antibodies that specifically bound IFN-ɑ or IFN-⍵.
IFN-AABs Neutralize Exogenous IFN in a Virus Infection-Based Assay
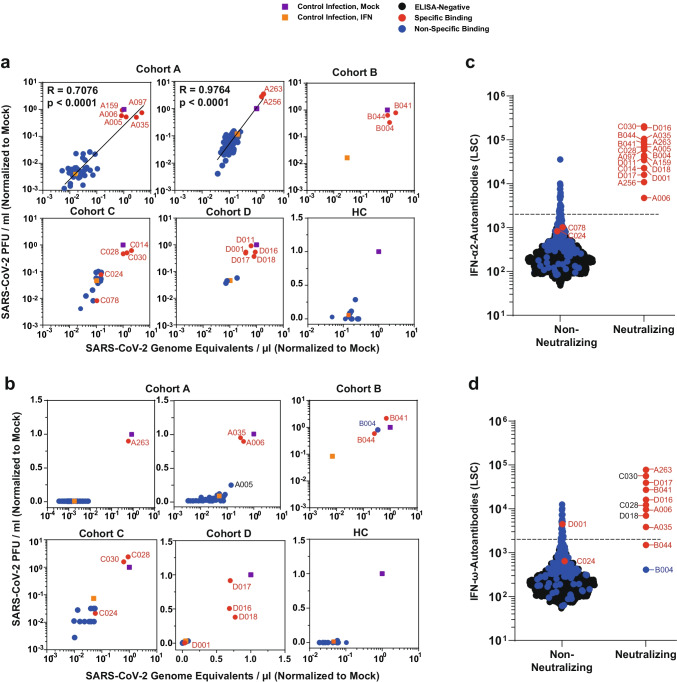
We next analyzed whether the presence of detectable and specifically IFN-binding AABs corresponded to a functional neutralization of IFN during infection. To this end, we applied a previously established assay of IFN-based inhibition of SARS-CoV-2 infection of the immortalized lung cell line Calu-3 [18], which we consider the gold standard for analysis of IFN neutralization. We tested the extent to which sera neutralize the antiviral activity of type I IFNs, resulting in efficient infection despite presence of IFNs. We tested all ELISA-positive sera as well as 102 IFN-ɑ-AAB- and 106 IFN-⍵-AAB ELISA-negative sera as a reference. 3.2% (13/403) and 2% (8/403) of the sera of the CSC specifically neutralized exogenous IFN-ɑ and IFN-ɷ, respectively, as judged by PCR-based quantification of SARS-CoV-2 genomic RNA in the supernatant and plaque assays that quantify infectivity of virus progeny (Fig. 2a, b, Fig. S1, Table S2). In cohort D (TPEC), 18.5% (5/27) and 11.1% (3/27) sera neutralized IFN-ɑ and IFN-ɷ activity, respectively (Fig. 2a, b, Fig. S1).
Fig. 2.
IFN-AABs neutralize exogenous IFN in a virus infection-based assay. a, b Selected sera were analyzed for IFN neutralization activity in a SARS-CoV-2 infection-based assay. The ability of individual sera to neutralize exogenous IFN-α2 (a) and IFN-ω (b) is shown by the rescue of susceptibility to infection as judged by quantification of viral RNA (x-axis) and infectivity (y-axis) in the supernatant. The infection condition in the absence of serum and IFN is set to 1. c, d The LSC value for individual sera, grouped into non-neutralizing and neutralizing sera, for the four COVID-19 cohorts. Dots indicate sera containing AABs scoring specific (red) or unspecific (blue) for IFN-α2 and IFN-ω binding in the competition assay (see b), respectively. Black dots indicate samples that scored below the threshold of the ELISA. Black dotted lines indicate the 97.5th percentile of the ECLIA assay LSC in sera from the healthy health care workers (HC) cohort (see Fig. 1). Neutralization ability of IFN-α and IFN-ω can be predicted at 100% for sera displaying LSCs above the respective red dotted lines (IFN-α: 35,639; IFN-ω: 12,603)
Strikingly, among all competition assay-positive samples of the four COVID-19 cohorts, 90% (18/20) and 83% (10/12) sera displayed IFN-ɑ and IFN-ɷ-neutralizing activity, respectively, indicating that a positive result in the competition assay associates with neutralization activity with a high likelihood (Fig. 1c, d). Examples for sera potentially containing low quantities of binding-competent, but non-neutralizing sera were derived from patients C078 (IFN-α) and C024 (IFN-ω). Conversely, a negative result in the competition assay was predictive of absence of neutralization ability. Specifically, among ELISA-positive, but competition assay-negative sera, 0 (0%) of 23 and 0 (0%) of 29 sera were able to neutralize IFN-ɑ and IFN-ɷ, respectively. Among 102 IFN-α-AAB-ELISA-negative sera, we observed two sera (C024 and C078) which scored negative in our standard IFN neutralization assay but that may weakly neutralize lower amounts of IFN-α (Fig. S2). Interestingly, we identified two samples that neutralized IFN-ω despite scoring negative in the IFN-ω-AAB ELISA (i.e., having LSC counts below the 97.5th percentile cut-off, B004 and B044) (Fig. 2b, d). The pronounced ability of these exact two sera to neutralize IFN-α (Fig. 2a, c) was the reason why we included them in the IFN-ω test, and suggests a potential cross-reactivity of IFN-α-AAB with IFN-ω.
Merging results from all three assays (Fig. 2c, d) revealed that an LSC value in the screening ELISA of > 35.639 (IFN-ɑ) and > 12.603 (IFN-ɷ) predicted specific binding in the competition assay and neutralization ability in the functional assay. Finally, the prevalence of ten individual antiphospholipid-ABs did not differ between patients with and without neutralizing IFN-AABs from cohort A (Fig. S3), suggesting that the presence of AABs is not generally increased in IFN-AAB-positive patients.
Laboratory Parameters of COVID-19 Patients Displaying Type I IFN-AABs
We next aimed to characterize the clinical phenotype of IFN-neutralizing AAB-positive COVID-19 patients in the CSC at hospital admission and to identify discriminatory markers that may serve as pre-selection criteria for their early identification and stratification. Interestingly, there were no statistically significant differences regarding clinical baseline characteristics, including demographic criteria and pre-existing comorbidities between patients with and without IFN-neutralizing AABs in the CSC using univariate analyses (Table 1). Yet, of all patients with available symptom records (cohorts A and C), the proportion of patients who reported fever and required supplemental oxygen therapy within 72 h from admission was higher in patients with neutralizing IFN-AABs than in those without (fever: 100%, 12/12 versus 62.6% (234/374), p = 0.0079 and oxygen: 100% (12/12) versus 76.0% (250/329), p = 0.0528).
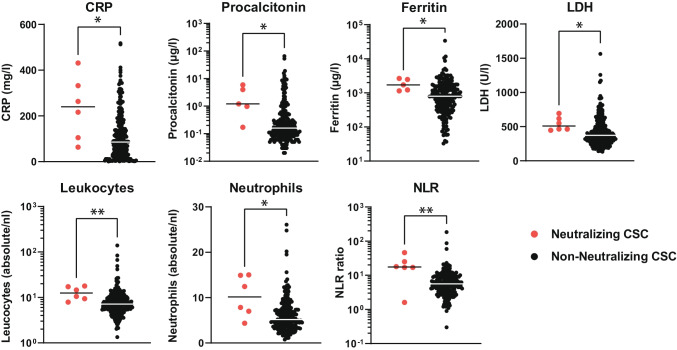
Furthermore, patients with IFN-AABs for which respective data were available displayed higher median values of C-reactive protein (CRP), procalcitonin, lactate dehydrogenase (LDH), ferritin, total leukocyte and neutrophil count, and neutrophil-to-lymphocyte ratio within the first 3 days of hospital admission compared to patients without IFN-AABs (Fig. 3, Fig. S4). In addition, patients with neutralizing IFN-AABs showed low levels of CD169/Siglec-1 expression on monocytes, a well-known type I IFN-response marker (Fig. S5). Interestingly, there was a tendency toward a negative correlation between CRP levels and CD169/Siglec-1 within 72 h from hospital admission.
Fig. 3.
Laboratory parameters of COVID-19 patients displaying type I IFN-AABs. Values of C-reactive protein (CRP), procalcitonin, ferritin, lactate dehydrogenase (LDH), absolute leukocyte and neutrophil count, and neutrophil-to-lymphocyte ratio (NLR) of patients with (N = 5–6) and without neutralizing IFN-AABs (N = 200–265) from the cross-sectional cohort (CSC, all WHO scores). For each patient, the first available parameter within 72 h of hospital admission is shown. Statistical testing was performed with Mann–Whitney U test
In IFN-AAB-Positive Patients, High Quantities of Neutralizing IFN-α2-AABs Were Present Both Soon Post-symptom Onset and at the Peak of Disease
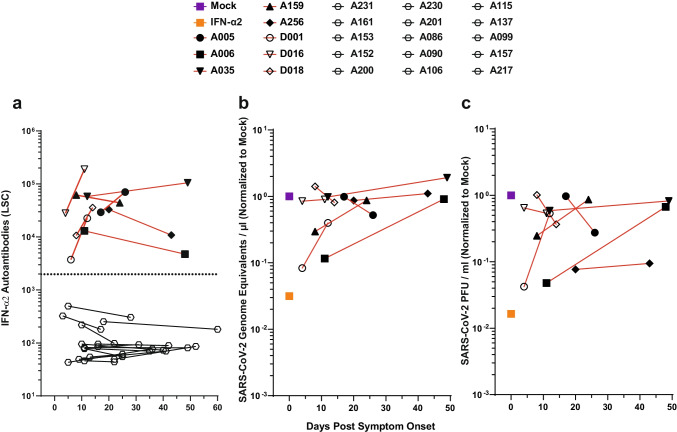
Next, we evaluated the temporal dynamics of IFN-AAB levels in sera from COVID-19 patients soon after symptom onset as compared to the peak of the disease. Available samples obtained in cohort A and in cohort D (TPEC) prior to TPE were analyzed (Fig. 4, Fig. S6). In all patient sera with detectable neutralizing IFN-AABs at the peak of the disease, early sera corresponding to ten (min. 4 to max. 20) days post-symptom onset contained abundant and neutralizing (Fig. 4, Fig. S5) IFN-AABs, suggesting that IFN-AABs either existed prior to the infection or were generated very early post-symptom onset. In contrast, sera collected early post symptom onset from patients that were IFN-AAB-negative at the peak of disease were negative, arguing against a transient induction of IFN-AABs.
Fig. 4.
In IFN-AAB-positive patients, high quantities of neutralizing IFN-α2-AABs were present both soon post-symptom onset and at the peak of disease. a Time course of antibody quantities in patient sera that scored IFN-AAB-positive at the peak of disease (N = 8, red lines). Additionally, time course of antibody quantities in patient sera that scored IFN-AAB-negative of the peak of disease is plotted (N = 15, black lines). The dotted line indicates the 97.5th percentile of the ECLIA assay LSC in sera from the HC cohort (see Fig. 1). b, c The ability of the sera to neutralize exogenous IFN-α2 is shown by the rescue of susceptibility to infection as judged by quantification of viral RNA (b) and infectivity (c) in the supernatant. The infection condition in the absence of serum and IFN is set to 1
Cytokine and Humoral Responses to SARS-CoV-2 Infection in IFN-AAB-Positive Patients
We next aimed to identify potential quantitative and/or qualitative differences in cytokine responses, viral load, and seroconversion kinetics in IFN-AAB-positive as opposed to IFN-AAB-negative patients. We analyzed serum cytokine levels in a subset of critical patients (WHO max. 6–8) from cohort A. Patients with neutralizing IFN-AABs demonstrated significantly higher levels of IFN-γ, and IFN-γ-induced protein 10 (IP-10) 1 to 2 weeks post-symptom onset while monocyte chemoattractant protein-1 (MCP-1) and TNF-α concentrations were similar compared to sera from patients without neutralizing IFN-AABs (Fig. S7). However, levels equalized among the two groups at 3 to 4 weeks post-symptom onset. As expected, patients with IFN-AABs had undetectable serum IFN-ɑ levels. Of note, by comparing upper-respiratory tract swabs and sera from patients with and without IFN-AABs from all infected cohorts, we failed to identify detectable differences in viral load level or decay over time (Fig. S8a) and we found no evidence for a difference in duration until seroconversion post-symptom onset (Fig. S8b). In conclusion, some cytokine responses were aberrantly elevated in patients with IFN-AABs within the first 2 weeks post-symptom onset. However, they normalized at weeks 3 and 4, and viral RNA production and time to seroconversion remained indistinguishable from patients without IFN-AABs.
Clinical Outcome of COVID-19 Patients with Neutralizing IFN-AABs
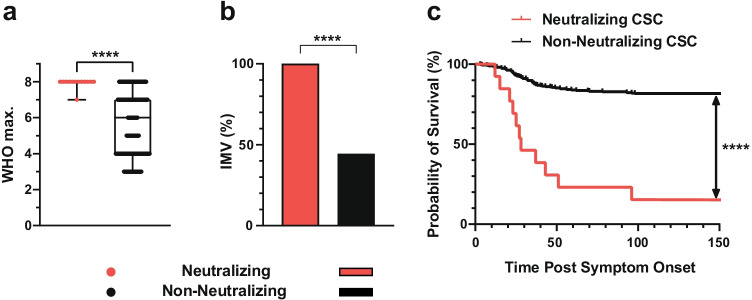
Neutralizing IFN-AAB-positive patients developed significantly higher max. WHO scores than patients without neutralizing IFN-AABs (median max. WHO score 8 (IQR 8–8) vs 6 (IQR 4–7), respectively; p < 0.0001, Fig. 5a). All patients with neutralizing IFN-AABs in the CSC required IMV (13/13, 100%), compared to 44.5% (166/372) in patients without IFN-AABs (p < 0.0001, Fig. 5b). Similarly, the proportion of neutralizing IFN-AAB-positive patients requiring renal replacement therapy and/or ECMO was markedly higher than in those without IFN-AABs (renal replacement therapy: 69.2%, 9/13 versus 26.6%, 98/369, p = 0.0008, ECMO: 46.2%, 6/13 versus 15.6%, 58/372, p = 0.0036, Table 1). Twelve out of thirteen neutralizing IFN-AAB-positive patients (92.3%) died in hospital compared to 19.1% (71/372) of patients without IFN-AABs (p < 0.0001) in the CSC (Table 1). Median survival of patients with neutralizing IFN-AABs was 28 days (IQR 22–65 days). Irrespective of the disease severity, the probability of surviving to 150 days post-symptom onset is 81.3% (300/369) for the patients from the non-neutralizing group, as opposed to 7.7% (1/13) for the patients of the neutralizing IFN-AAB-positive group (Fig. 5c). Conclusively, IFN-AAB positivity was associated with severe disease trajectories of COVID-19 and a worse clinical outcome in our cohorts.
Fig. 5.
Clinical outcome of COVID-19 patients with neutralizing IFN-AABs. a Median max. WHO score in hospital. Statistical testing was performed using the Mann–Whitney U test. b Proportion of patients requiring invasive mechanical ventilation (IMV) after hospital admission. Statistical testing was performed using the chi-square test. c Probability of survival of patients with and without neutralizing IFN-AABs from the cross-sectional cohort (CSC) from symptom onset until discharge (up to 150 days), death or transferral (p < 0.0001). Statistical testing was performed using a log-rank test. Neutralizing (N = 13), non-neutralizing (panels a and b: N = 372, panel c: N=369)
Inter-individual Effect of Therapeutic Plasma Exchange on IFN-AABs and SARS-CoV-2 Antibodies
Cohort D (TPEC) allowed us to compare trajectories of IFN-AAB-positive patients undergoing TPE to those not undergoing TPE. Criteria for initiation of TPE were presence of ARDS requiring IMV and/or vasopressor-dependent circulatory shock, clinical and laboratory features of a COVID-19-associated immunopathology with elevated D-dimers and ferritin levels, and persistent and refractory fever ≥ 38.5 °C without conclusive pathogenic evidence and despite anti-infectious treatment. TPE was initiated without prior screening for IFN-AABs within a median of 6 days (IQR 1–10) after hospital admission and the median number of TPE sessions per patient was 3 (IQR 2–5). TPE was performed using a continuous-flow centrifugation blood cell separator. Plasma with enclosed cytokines and immunoglobulins are separated from blood cells by gravity due to different densities of the respective blood components [21].
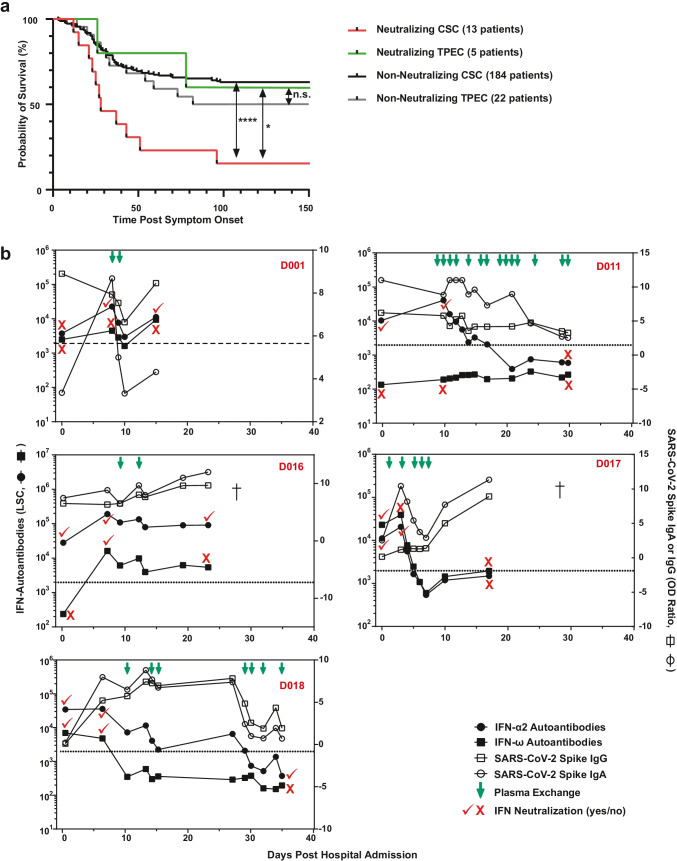
Focusing on severely ill patients (WHO group 6–8), survival of IFN-AAB-negative patients in the CSC cohorts and TPEC was similar (p = 0.34). Importantly, the proportion of neutralizing IFN-AAB-positive patients from the TPEC that survived in hospital was higher than of those patients from the CSC who did not undergo TPE (60%, 3/5 patients from the TPEC survived versus 7.7%, 1/13 patients from the CSC, p = 0.0412) (Fig. 6a), despite similar disease severity. The two groups (IFN-AAB-positive, CSC with N = 13 vs. TPEC, N = 5) displayed no differences regarding basic demographic characteristics and share similar median age, sex distribution, BMI, and comorbidities (not significant, Table S3). The five patients from the TPEC showed a longer median length of ventilation and a longer median stay in hospital compared to the 13 patients in the CSC. In both groups, patients were treated with dexamethasone but only the IFN-AAB-positive TPEC patients partly received remdesivir (3/5 patients, 60.0% vs. 0/13, 0%, p = 0.0044). No ECMO treatment was used in the IFN-AAB-positive TPEC patients. Regarding both groups, the four survivors in both groups (one in the CSC vs. three in the TPEC) showed no distinct demographic characteristics, comorbidities, or treatments in hospital (except TPE for the TPEC). They were older than 50 years and predominantly male (3/4, 75%). One had a BMI above 40 kg/m2 and CCI ranged from 1–3. Three out of 4 patients (75%) received renal replacement therapy and none of the patients was undergoing ECMO. Three out of 4 patients (75%) received dexamethasone and two patients (2/4, 50%) remdesivir.
Fig. 6.
Inter-individual effect of therapeutic plasma exchange on IFN-AABs and SARS-CoV-2 antibodies. a Probability of survival of neutralizing IFN-AAB-positive and -negative patients with critical COVID-19 (max. WHO score 6–8) with and without plasma exchange (CSC and TPEC) from symptom onset until discharge, death or transferral (p = 0.04, neutralizing CSC versus neutralizing TPEC; p < 0.0001, neutralizing CSC versus non-neutralizing CSC). Statistical testing was performed using a log-rank test. Neutralizing CSC (N = 13), non-neutralizing CSC (N = 184), neutralizing TEPC (N = 5), and non-neutralizing TPEC (N = 22). b Antibody profile in serum from individual COVID-19 patients of the TPEC subjected to plasma exchange. The quantity of IFN-α2- and IFN-ω-AABs, SARS-CoV-2-IgG and -IgA, and the IFN-α2 and IFN-ω neutralization status are given for various time points. The patient identifier is given in red. Viral load profiles were only available for patients D011 and D018 and are shown in Supplementary Fig. 7
Longitudinal analysis of sera revealed that three (D011, D017, D018) out of five patients responded to TPE with decreasing IFN-AAB levels below the cut-off and to a level that coincided with absence of neutralizing activity. In addition, a sustained reduction of IFN-AAB quantities was achieved only by repetitive TPE (Fig. 5b, Fig. S9). In contrast to IFN-AABs, quantities of SARS-CoV-2 Spike IgG and IgA were less, if at all, affected by TPE (note the logarithmic scale for IFN-AABs versus the linear scale for SARS-CoV-2-IgG/IgA). Overall, our findings in a limited number of patients suggest that TPE could positively affect the survival of critically ill IFN-AAB-positive patients. This needs to be corroborated in future, adequately powered clinical investigations. Potentially, a sustained and significant reduction of peripheral IFN-AAB levels must be achieved to prevent death.
Discussion
IFN-AABs strongly associate with adverse clinical outcome of SARS-CoV-2 infection [7, 9–11, 13, 17, 23].
In several studies, detection and quantification of IFN-AABs in sera from COVID-19 patients relies on ELISA and multiplex particle-based assay. While these assays are amenable to high-throughput and are highly sensitive, they result in a small proportion of false-positive results [22, 24], highlighting the ongoing need to reanalyze positive-tested patient material in functional assays demonstrating the neutralization activity. However, such assays are sophisticated and time-consuming. They include luciferase-based interferon-stimulated response element (ISRE) promoter reporter assays [10, 11], flow cytometry-based analyses of STAT phosphorylation [7, 11, 18], and virus infection-based assays [18, 23]. The latter allows probing the activity of the IFN-AABs in the context of infection-inhibitory concentrations of IFN-α and IFN-ω. Here, we applied and cross-validated previously established assays comprising an ELISA for sensitive identification, a specificity-validating competition assay, and a functional neutralization assay [18] using a large collection of serum samples obtained from three cross-sectional cohorts.
Surprisingly, sera from two patients were found to neutralize exogenous IFN-ω despite negative ELISA results. Presence of neutralization activity in the absence of detectable IFN-AABs has been reported [19]. Explanations for this phenomenon could include technical aspects of the detection method, including the possibility that IFN-AABs may be concealed by the binding of the cytokine to the plate or biotinylation of the cytokine [25].
Here, we calibrated our ELISA cut-off based on the 97.5th percentile in a cohort of uninfected individuals. Although this strategy may be inexact, the absence of prevalence IFN-AABs in a cohort of younger and predominantly female healthcare workers supports an age-dependent increase of IFN-AAB prevalence in uninfected individuals [19]. Furthermore, the prevalence of 3.2% (13/403) of patients with neutralizing AABs against IFN-α and/or IFN-ω in our cross-sectional patient cohort (median max. WHO score 6) and 18.5% (5/27) in critically affected patients (median max. WHO-Score 7) is in line with reported prevalences of 6–17% in severely [7, 9, 10, 12, 19] and 11–19% in critically ill [7, 11, 12, 19] individuals with COVID-19. Moreover, IFN-AAB positivity is associated with a worse clinical outcome and a decreased survival probability of hospitalized patients in our cohorts, confirming previous reports [7, 9–11, 13, 17, 19, 23]. Interestingly, a single study to date [26] suggested that survival was not adversely affected by the presence of type I IFN-AABs, while confirming the widely accepted association with an increased risk of admission to the intensive care unit.
We failed to identify a clear association of IFN-AABs with previously described demographic parameters in our cross-sectional cohort, including male sex or advanced age [7, 13], probably due to the relatively limited sample size of our cohorts. However, the presence of neutralizing IFN-AABs was associated with fever and need for supplementary oxygen within 72 h post hospital admission, as well as with elevated soluble and cellular markers of acute-phase reaction including elevated levels of CRP, procalcitonin, LDH and ferritin, and elevated total neutrophil and leukocyte counts within the first 3 days of admission in our CSC. Higher CRP values constitute a biomarker for a severe disease course, are included in a widely-used clinical risk score for mortality of COVID-19 [3], and associate with neutralizing IFN-AABs along with lower lymphocyte counts in severely affected patients [10]. As hospital admission and thus clinical deterioration occurred at a median of 5 days post-symptom onset in the CSC, fever and need for supplemental oxygen therapy up to 72 h post hospital admission may serve as suitable and simple clinical criteria to identify patients at risk for a severe disease course.
Our ability to detect IFN-AABs as early as 4 days post-symptom onset in sera from most patients that present with IFN-AABs at the peak of their disease suggest that they were present prior to the infection, or alternatively, but less likely, were induced very early post infection. Our data are in agreement with recently demonstrated presence of IFN-AABs at the day of hospital admission [23] and in 4% of uninfected individuals > 70 years old [19], underlining the idea that they can serve as biomarkers for predisposition for a severe course of SARS-CoV-2 infection. Future studies are required to elucidate the biological mechanisms that lead to elicitation of IFN-AABs in an age-dependent manner.
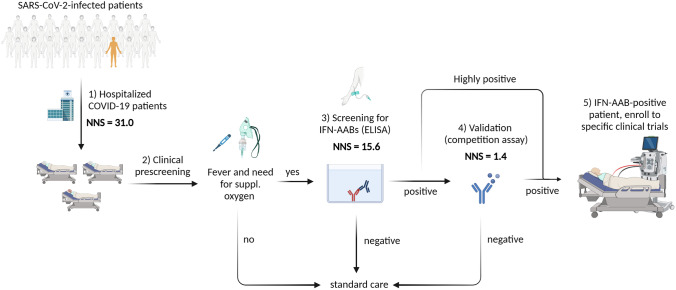
Given that IFN-AABs are risk factors for a worse clinical outcome in hospitalized patients with COVID-19, future rapid identification of IFN-AAB-positive patients after hospital admission seems key for the potential implementation and success of specific interventions such as antivirals and/or monoclonal antibodies and/or TPE. Mass screening of all hospitalized COVID-19 patients may be the ideal goal. However, in the context of limited resources, combination of clinical parameters and targeted diagnostic testing may serve to facilitate early, sensitive, and specific identification of IFN-AAB-positive patients. In the CSC cohort, the patient number needed to screen (NNS) without preselection in order to identify one patient with neutralizing IFN-AAB was 31.0 (403/13). We hypothesized that applying clinical pre-selection criteria which co-present with the neutralizing IFN-AAB positivity diminishes the NNS. Due to the limited number of IFN-AAB-positive patients (13), multiple testing correction was not feasible. Using univariate analyses, we established that temperature (> 38.5 °C or self-reported fever) before or upon hospital admission and the need for supplemental oxygen within the first 72 h after admission correlated best with presence of IFN-AAB positivity in the screening assay in all hospitalized COVID-19 patients (fever: p = 0.0079; supplemental oxygen p = 0.0528). In order to prevent early exclusion of IFN-AAB-positive patients by mere pre-selection on statistically significant parameters, we included fever and the need for supplemental oxygen which nominally associated with IFN-AAB positivity. Importantly for clinical implementation, both parameters are easily measurable and clinically reasonable and reduce the NNS to 15.6 (172/11). Selection of patients exceeding the cut-off for ELISA positivity (in our cohort 97.5th percentile of the HC) for further testing by competition assay would adjust the NNS in the competition assay to 1.4 (15/11). Therefore, in order to increase sensitivity, we propose to consider the need for supplemental oxygen within 72 h after admission and fever as pre-selection criteria for patients that undergo ELISA screening (Fig. 7).
Fig. 7.
Proposed diagnostic algorithm for rapid identification of neutralizing IFN-AAB-positive patients. The number needed to screen (NNS) is based on results from the cross-sectional cohort (CSC). ELISA for IFN-AAB detection was considered to be positive if it exceeded the 97.5th percentile of the healthy control cohort. (1) NNS of all hospitalized COVID-19 patients without preselection was 31.0 (403 patients in total, 13 patients with neutralizing IFN-AABs). (2) Prescreening of patients using the clinical criteria of fever at admission and need for supplemental oxygen within the first 72 h after hospitalization diminished the NNS in the IFN-AAB ELISA (3) by half, to 15.6 (172/11). For patients identified as positive in the screening ELISA, the NNS in the competition assay to confirm the presence of IFN-specific AABs is reduced to 1.4 (15/11) (4). For patients with high-titer IFN-AABs (light signal count > 35.639), the competition assay can be omitted. Patients highly positive in the IFN-AAB ELISA and those with specific results in the competition assay may be included in clinical studies that aim testing specific therapies, including therapeutic plasma exchange (5). Figure created with BioRender.com
Sera from all patients exceeding the LSC value of 35,639 (13/13) in the screening ELISA assay demonstrated neutralizing activity against IFN-α (Fig. 2c). We therefore propose to conduct the IFN neutralization assay only in case of an LSC value lower than 35,639, whereas patients with sera exceeding this value can be considered positive for neutralizing IFN-AABs without further testing (Fig. 7). Taken together, we identified clinical parameters that co-present with IFN-AAB positivity at hospital admission, which may serve as preselection in a yet-to-be-verified diagnostic algorithm. Due to the low number of IFN-AAB-positive patients in our study, the usefulness of these parameters and their statistical robustness require assessment and verification in prospective clinical studies.
Treatment with antiviral compounds and monoclonal antibodies is recommended in the early phase of SARS-CoV-2 infection for patients at high risk for progression to severe disease and may therefore also serve as therapeutic options for IFN-AAB-positive COVID-19 patients in addition to removal of autoantibodies by TPE and substitution of type I IFN by IFN-β administration. TPE in the context of COVID-19 has been analyzed in individual case reports and case–control studies, including IFN-AAB-positive and negative patients [17, 21, 23, 27] and might efficiently remove soluble circulating FcγReceptor-activating immune complexes [28]. TPE effectively decreased circulating IFN-AAB, but not SARS-CoV-2 antibody concentrations in four IFN-AAB-positive, severely ill patients [23] and in a child with APS-1 suffering from severe COVID-19 [17]. In our study, TPE was offered to patients in one center. Here, it reduced circulating IFN-AABs with patient-specific efficiency and appeared to increase the chances of in-hospital survival. Although clinical characteristics in both groups were similar, we cannot rule out confounding factors due to different clinical settings between centers contributing to different survival rates, such as the differences regarding administration of remdesivir described above. However, our data underline the rationale to initiate large-scale, adequately powered clinical trials in order to corroborate the potential benefit of TPE in a general cohort of adult, critically ill COVID-19 patients. Interestingly, SARS-CoV-2-IgG and IgA quantities were less affected by TPE for unknown reasons, which may include their rapid replenishment by highly abundant plasmablasts or an extravascular-to-intravascular rebound since immunoglobulins have a substantial extravascular distribution.
Given the low prevalence of detectable IFN-β-AABs (up to 1.3% in patients with critical COVID-19 [19]), IFN-β administration may substitute for neutralized IFN-α and -ω. While IFN-β therapy failed to result in a detectable clinical benefit in the SOLIDARITY trial [29], specifically IFN-AAB-positive patients may benefit from IFN-β therapy, a patient group that might have been under-represented in this study. Furthermore, the benefit of IFN-β administered by different routes should be systematically explored in this patient group.
Conclusions
Rapid and early identification of COVID-19 patients with circulating IFN-AABs at hospital admission is key to provide them with yet-to-be-established specific therapies before they clinically deteriorate. A high-throughput-amenable assay pipeline, composed of an ELISA-based assay for IFN-AABs in serum and a consecutive ELISA-based validation assay, can substitute methodologically complex gold-standard assays that quantify functional neutralization of IFNs. Future, large-scale prospective observational studies are required to verify if this pipeline may be stratified to a preselected group of patients based on clinical parameters that appeared to associate with IFN-AAB positivity, including presentation with fever and need for supplemental oxygen therapy within 72 h after admission. Identification of at-risk patients will enable clinicians to directly allocate them to larger clinical trials which are urgently required to determine clinical effectiveness of targeted therapies in this particularly vulnerable patient group.
Pa-COVID study Group (Consortium representative: Florian Kurth)
Set up study platform: Stefan Hippenstiel4, Martin Witzenrath4, Elisa T. Helbig4, Lena J. Lippert4, Pinkus Tober-Lau4, David Hillus4, Sarah Steinbrecher4, Sascha S. Haenel4, Alexandra Horn4, Willi M4. Koch, Nadine Olk4, Rosa C. Schuhmacher4, Katrin K. Stoyanova4, Lisa Ruby4, Claudia Zensen4, Mirja Mittermaier4, Fridolin Steinbeis4, Tilman Lingscheid4, Bettina Temmesfeld-Wollbrück4, Thomas Zoller4, Holger Müller-Redetzky4, Alexander Uhrig4, Daniel Grund4, Christoph Ruwwe-Glösenkamp4, Miriam S. Stegemann4, Katrin M. Heim4, Bastian Opitz4, Kai-Uwe Eckardt26, Martin Möckel27, Felix Balzer28, Claudia Spies28, Steffen Weber-Carstens28, Frank Tacke29, Chantip Dang-Heine2, Michael Hummel30, Georg Schwanitz31, Uwe D. Behrens31, Maria Rönnefarth2, Sein Schmidt2, Alexander Krannich2, Saskia Zvorc2, Jenny Kollek2 and Christof von Kalle2.
Inclusion of patients and clinical data curation: Linda Jürgens4, Malte Kleinschmidt4, Sophy Denker32, Moritz Pfeiffer4, Belén Millet Pascual-Leone4, Luisa Mrziglod4, Felix Machleidt4, Sebastian Albus4, Felix Bremer4, Tim Andermann4, Carmen Garcia4, Philipp Knape4, Philipp M. Krause4, Liron Lechtenberg4, Yaosi Li4, Panagiotis Pergantis4, Till Jacobi4, Teresa Ritter26, Berna Yedikat4, Lennart Pfannkuch4, Ute Kellermann4, Susanne Fieberg4, Laure Bosquillon de Jarcy1, Anne Wetzel4, Markus C. Brack4, Moritz Müller-Plathe4, Jan M. Kruse26, Daniel Zickler26, Andreas Edel28, Britta Stier26, Roland Körner26, Nils B. Müller26, Philipp Enghard26, Lucie Kretzler2, Lil A. Meyer-Arndt33, Linna Li2, and Isabelle Wirsching2.
Biobanking and -sampling: Denise Treue30, Dana Briesemeister30, Jenny Schlesinger30, Birgit Sawitzki25, Lara Bardtke4, Kai Pohl4, Philipp Georg4, Daniel Wendisch4, Anna L. Hiller4, Sophie Brumhard4, Marie Luisa Schmidt1, Leonie Meiners1, and Patricia Tscheak1.
Study Group-Specific Affiliations
26Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Institute of Nephrology and Internal Intensive Care Medicine, Berlin, Germany.
27Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Division of Emergency Medicine and Department of Cardiology, Berlin, Germany.
28Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Department of Anesthesiology and Intensive Care Medicine, Berlin, Germany.
29Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Department of Hepatology and Gastroenterology, Berlin, Germany.
30Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Central Biobank Charité (ZeBanC), Institute of Pathology, Berlin, Germany.
31Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Clinical Study Center, Berlin, Germany.
32Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Department of Hematology, Oncology and Tumor Immunology, Berlin, Germany.
33Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Department of Neurology, Berlin, Germany.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Mahtab Maleki for support with IFN-AAB ELISAs and competition assays. We thank Patricia Tscheak, Marie Luisa Schmidt, and Tatjana Schwarz for support with sample logistics and SARS-CoV-2 antibody testing. We thank Leif Hanitsch for advice on patient recruitment and evaluation. CT and VMC are participants of the Charité Clinician Scientist program funded by Charité – Universitätsmedizin Berlin and the Berlin Institute of Health. We thank Karin Reiter for the anti-phospholipid antibody measurements. We thank the patients for their ongoing trust and collaboration. We thank the involved biobanks (ZeBanC Berlin, FREEZE-Biobank Freiburg) for their support. DD and AL are members of SFB1425, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project #422681845. NS is partly funded by the Deutsche Forschungsgemeinschaft (DFG, Research Grant No. SCHA1838/4-2).
Author Contribution
Conceptualization: FK; HvB; CM; CG
Methodology: BA; TM; OS
Formal analysis and investigation: BA; TM; PS; Charlotte T; OS; JJ; BM; J-MD; Christoph T; CN, CH; DSS; DD, AL; NS; JNL; DA; SR; VF; UK; NU; TS; JS; TD; VMC; UM
Writing—original draft preparation: PS; Charlotte T; CG
Writing—review and editing: BA; TM; PS; Charlotte T; OS; DN; BM; CN; DD, AL; TCJ; KW; TS; JS; LS; VMC; UM; FK; HvB; CM; CG
Funding acquisition: JS; KW; UM; FK; HvB; CM; CG
Resources: DN, AN; CD; LES; FK; HvB; CM; CG
Supervision: HH; R-HH; NS; KW; VMC; FK; HvB; CM; CG
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Innovationsfond of Labor Berlin (to H.V.B, C.M., and C.G.); by funding from the Deutsche Forschungsgemeinschaft (DFG) Collaborative Research Centre CRC900 “Microbial Persistence and its Control,” project number 158989968, project C8 (awarded to C.G.); and by funding from the Berlin Institute of Health (BIH) (to C.G.). T.C.J. is in part funded through NIAID-NIH CEIRS contract HHSN272201400008C. Parts of the work were funded by the European Union’s Horizon 2020 research and innovation program through project RECOVER (GA101003589) to C.D.; the German Ministry of Research through the projects VARIPath (01KI2021) to V.M.C., and NaFoUniMedCovid19—COVIM, FKZ: 01KX2021 to C.D., V.M.C., L.E.S., F.K. and H.H. The Pa-COVID-19 Study is funded by BIH and the German Ministry of Research NaFoUniMedCovid19 NUM-NAPKON FKZ: 01KX2021. The COV-IMMUN study is supported by the Berlin University Alliance and the BIH Clinical Study Center (BIH-CSC). KW has received funding by the Deutsche Forschungsgemeinschaft (DFG, Research Grant No. WA 1597/6–1 and WA 1597/7–1).
Data Availability
The datasets and materials generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Code Availability
Not applicable. No data were coded in this study.
Declarations
Research Involving Human Participants
All studies were conducted according to the Declaration of Helsinki and Good Clinical Practice principles.
The Pa-COVID-19 study was approved by the ethics committee of Charité – Universitätsmedizin Berlin (EA2/066/20) and is registered in the German and WHO international clinical trials registry (DRKS00021688).
The Covimmun-study was approved by the ethics committee of Charité – Universitätsmedizin Berlin (EA1/068/20).
The study “Evaluation der humoralen und zellulären Immunantwort und der thromboembolischen Komplikationen bei Infektion mit dem neuartigen Coronavirus (nCoV19)” was approved by the Kantonale Ethikkommission KEK, Bern, Switzerland, Nr. 2020–00877, and is registered under the name “Characterizing the Immune Response and Neuronal Damage in COVID-19” at http://clinicaltrials.gov (NCT04510012).
The three studies (COVID-19 Register Freiburg, FREEZE-Covid19 cohort, and Biomarker COVID-19) were approved by the ethics committee of the University of Freiburg (EK-FR 153/20; FREEZE-Biobank EK-FR 383/19; EK-FR 225/20) and are registered in the German and WHO international clinical trials registry (DRKS00021522 for Biomarker COVID-19).
The Covid-Immun study was approved by the local Ethics Committee of the Medical Faculty of Heidelberg (S148/2020) and is registered in the German clinical trials registry (DRKS00021810).
Informed Consent to Participate and to Publish
Written informed consent to participate was obtained from all individual participants included in the studies. Furthermore, the authors affirm that human research participants provided written informed consent for publication of their demographic, clinical and laboratory data.
Conflict of Interest
V.M.C is named together with Euroimmun GmbH on a patent application filed recently regarding the diagnostic of SARS-CoV-2 by antibody testing. Technische Universität Berlin, Freie Universität Berlin, and Charité—Universitätsmedizin have filed a patent application for siRNAs inhibiting SARS-CoV-2 replication with D.N. as co-author. J.C.S, T.S. (full departmental disclosure): the department of Intensive Care Medicine has/had research and/or development/consulting contracts with (full disclosure) Orion Corporation, Abbott Nutrition International, B. Braun Medical AG, CSEM SA, Edwards Lifesciences Services GmbH/SA, Kenta Biotech Ltd, Maquet Critical Care AB, Omnicare Clinical Research AG, Phagenesis Ltd, Cytel, and Nestlé. No personal financial gains resulted from respective development/consulting contracts and/or educational grants.
Footnotes
Collaborators listed at the end of the article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bengisu Akbil, Tim Meyer, Paula Stubbemann and Charlotte Thibeault and Kurth, von Bernuth, Meisel, Goffinet. These authors contributed equally.
Contributor Information
Florian Kurth, Email: florian.kurth@charite.de.
Horst von Bernuth, Email: horst.von-bernuth@charite.de.
Christian Meisel, Email: christian.meisel@laborberlin.com.
Christine Goffinet, Email: christine.goffinet@charite.de.
References
- 1.Pfortmueller CA, Spinetti T, Urman RD, Luedi MM, Schefold JC. COVID-19-associated acute respiratory distress syndrome (CARDS): current knowledge on pathophysiology and ICU treatment–a narrative review. Best Pract Res Clin Anaesthesiol. 2021;35(3):351–368. doi: 10.1016/j.bpa.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sah P, Fitzpatrick MC, Zimmer CF, Abdollahi E, Juden-Kelly L, Moghadas SM, et al. Asymptomatic SARS-CoV-2 infection: A systematic review and meta-analysis. Proceedings of the National Academy of Sciences. 2021;118(34):e2109229118. doi: 10.1073/pnas.2109229118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knight SR, Ho A, Pius R, Buchan I, Carson G, Drake TM, et al. Risk stratification of patients admitted to hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 Host Genetics Initiative. Mapping the human genetic architecture of COVID-19. Nature. 2021;600(7889):472–7. 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed]
- 6.Kim Y-M, Shin E-C. Type I and III interferon responses in SARS-CoV-2 infection. Exp Mol Med. 2021;53(5):750–760. doi: 10.1038/s12276-021-00592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann H-H, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koning R, Bastard P, Casanova J-L, Brouwer MC, van de Beek D. Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensive Care Med. 2021;47(6):704–706. doi: 10.1007/s00134-021-06392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troya J, Bastard P, Planas-Serra L, Ryan P, Ruiz M, De Carranza M, et al. Neutralizing autoantibodies to type I IFNs in> 10% of patients with severe COVID-19 pneumonia hospitalized in Madrid, Spain. J Clin Immunol. 2021;41(5):914–922. doi: 10.1007/s10875-021-01036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goncalves D, Mezidi M, Bastard P, Perret M, Saker K, Fabien N, et al. Antibodies against type I interferon: detection and association with severe clinical outcome in COVID-19 patients. Clin Transl Immunol. 2021;10(8):e1327. doi: 10.1002/cti2.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Wijst MG, Vazquez SE, Hartoularos GC, Bastard P, Grant T, Bueno R, et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci Transl Med. 2021;13(612):eabh2624. doi: 10.1126/scitranslmed.abh2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastard P, Orlova E, Sozaeva L, Lévy R, James A, Schmitt MM, et al. Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J Exp Med. 2021;218(7):e20210554. doi: 10.1084/jem.20210554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beccuti G, Ghizzoni L, Cambria V, Codullo V, Sacchi P, Lovati E, et al. A COVID-19 pneumonia case report of autoimmune polyendocrine syndrome type 1 in Lombardy Italy. J Endocrinol Invest. 2020;43(8):1175–1177. doi: 10.1007/s40618-020-01323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpino A, Buganza R, Matarazzo P, Tuli G, Pinon M, Calvo PL, et al. Autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy in two siblings: same mutations but very different phenotypes. Genes. 2021;12(2):169. doi: 10.3390/genes12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferré EM, Schmitt MM, Ochoa S, Rosen LB, Shaw ER, Burbelo PD, et al. SARS-CoV-2 spike protein-directed monoclonal antibodies may ameliorate COVID-19 complications in APECED patients. Front Immunol. 2021;12:720205. doi: 10.3389/fimmu.2021.720205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemarquis A, Campbell T, Aranda-Guillén M, Hennings V, Brodin P, Kämpe O, et al. Severe COVID-19 in an APS1 patient with interferon autoantibodies treated with plasmapheresis. J Allergy Clin Immunol. 2021;148(1):96–98. doi: 10.1016/j.jaci.2021.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meisel C, Akbil B, Meyer T, Lankes E, Corman VM, Staudacher O, et al. Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1. J Clin Investig. 2021;131(14):e150867. doi: 10.1172/JCI150867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastard P, Gervais A, Le Voyer T, Rosain J, Philippot Q, Manry J, et al. Autoantibodies neutralizing type I IFNs are present in~ 4% of uninfected individuals over 70 years old and account for~ 20% of COVID-19 deaths. Sci Immunol. 2021;6(62):eabl4340. doi: 10.1126/sciimmunol.abl4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurth F, Roennefarth M, Thibeault C, Corman VM, Müller-Redetzky H, Mittermaier M, et al. Studying the pathophysiology of coronavirus disease 2019: a protocol for the Berlin prospective COVID-19 patient cohort (Pa-COVID-19) Infection. 2020;48(4):619–626. doi: 10.1007/s15010-020-01464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nusshag C, Morath C, Speer C, Kaelble F, Zeier M, Boxberger M, et al. Plasma exchange in patients with severe coronavirus disease 2019: a single-center experience. Crit Care Explor. 2021;3(8):e0517. doi: 10.1097/CCE.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Güven E, Duus K, Lydolph MC, Jørgensen CS, Laursen I, Houen G. Non-specific binding in solid phase immunoassays for autoantibodies correlates with inflammation markers. J Immunol Methods. 2014;403(1-2):26–36. doi: 10.1016/j.jim.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 23.de Prost N, Bastard P, Arrestier R, Fourati S, Mahévas M, Burrel S, et al. Plasma exchange to rescue patients with autoantibodies against type I interferons and life-threatening COVID-19 pneumonia. J Clin Immunol. 2021;41(3):536–544. doi: 10.1007/s10875-021-00994-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahud M, Zhukov O, Mo K, Popov J, Dlott J. False-positive results in ELISA-based anti FVIII antibody assay may occur with lupus anticoagulant and phospholipid antibodies. Haemophilia. 2012;18(5):777–781. doi: 10.1111/j.1365-2516.2012.02781.x. [DOI] [PubMed] [Google Scholar]
- 25.Meyer S, Woodward M, Hertel C, Vlaicu P, Haque Y, Kärner J, et al. AIRE-deficient patients harbor unique high-affinity disease-ameliorating autoantibodies. Cell. 2016;166(3):582–595. doi: 10.1016/j.cell.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abers MS, Rosen LB, Delmonte OM, Shaw E, Bastard P, Imberti L, et al. Neutralizing type-I interferon autoantibodies are associated with delayed viral clearance and intensive care unit admission in patients with COVID-19. Immunol Cell Biol. 2021;99(9):917–921. doi: 10.1111/imcb.12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez J, Gratacos-Ginès J, Olivas P, Costa M, Nieto S, Mateo D, et al. Plasma exchange: an effective rescue therapy in critically ill patients with coronavirus disease 2019 infection. Crit Care Med. 2020;48(12):e1350. doi: 10.1097/CCM.0000000000004613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ankerhold J, Giese S, Kolb P, Maul-Pavicic A, Göppert N, Ciminski K, et al. Circulating immune complexes drive immunopathology in COVID-19. bioRxiv. 2021:06.25.449893. 10.1101/2021.06.25.449893.
- 29.Pan H, Peto R, Henao-Restrepo A, Preziosi M, Sathiyamoorthy V, Abdool Karim Q, Consortium WST et al. Repurposed antiviral drugs for COVID-19-interim who solidarity trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.“Information on COVID-19 Treatment, Prevention and Research.” n.d. https://www.covid19treatmentguidelines.nih.gov.
- 32.Jones TC, Biele G, Mühlemann B, Veith T, Schneider J, Beheim-Schwarzbach J, et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science. 2021;373(6551):eabi5273. doi: 10.1126/science.abi5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okba NM, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, et al. Severe acute respiratory syndrome coronavirus 2− specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26(7):1478. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarz T, Tober-Lau P, Hillus D, Helbig ET, Lippert LJ, Thibeault C, et al. Delayed antibody and T-cell response to BNT162b2 vaccination in the elderly, Germany. Emerg Infect Dis. 2021;27(8):2174. doi: 10.3201/eid2708.211145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spinetti T, Hirzel C, Fux M, Walti LN, Schober P, Stueber F, et al. Reduced monocytic human leukocyte antigen-DR expression indicates immunosuppression in critically ill COVID-19 patients. Anesth Analg. 2020;131(4):993. doi: 10.1213/ANE.0000000000005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Stuckrad SL, Klotsche J, Biesen R, Lieber M, Thumfart J, Meisel C, et al. SIGLEC1 (CD169) is a sensitive biomarker for the deterioration of the clinical course in childhood systemic lupus erythematosus. Lupus. 2020;29(14):1914–1925. doi: 10.1177/0961203320965699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets and materials generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Not applicable. No data were coded in this study.