Abstract
Background:
Beyond perinatal folic acid supplementation, the need for additional prenatal prophylaxis of iron with or without other micronutrients remains unclear. We aim to investigate the maternal and infant health effects of iron plus folic acid and multiple micronutrient supplements vs folic acid alone when provided to pregnant women with no or mild anemia.
Methods:
In this randomized double-blind controlled trial, 18 775 nulliparous pregnant women with mild or no anemia were enrolled from 5 counties of northern China from May 2006 through April 2009. Women were randomly assigned to daily folicacid (400 μg)(control), folic acid–iron (30 mg), or folic acid, iron, and 13 additional vitamins and minerals provided before 20 weeks gestation to delivery. Primary outcome was perinatal mortality. Secondary outcomes included neonatal and infant mortality, preterm delivery, birthweight, birth length, gestational duration, and maternal hemoglobin concentration and anemia.
Results:
A total of 92.7% of women consumed 80% to 100% of supplements as instructed. On average, women consumed 177 supplements. Compared with daily prenatal folic acid, supplementation with iron–folic acid with or without other micronutrients did not affect the rate of perinatal mortality (8.8, 8.7, and 8.3, respectively) per 1000 births, and relative risks (RRs) were 1.00 (95% CI, 0.68–1.46; P=.99) and 0.94 (95% CI, 0.64–1.39; P=.76), respectively. Risk of other adverse maternal and infant outcomes also did not differ, except that RRs for third-trimester maternal anemia were 0.72 (95% CI, 0.63–0.83; P < .001) and 0.71 (95% CI, 0.62–0.82; P < .001), respectively.
Conclusion:
Prenatal iron–folic acid and other micronutrient supplements provided to Chinese women with no or mild anemia prevented later pregnancy anemia beyond any benefit conferred by folic acid alone but did not affect perinatal mortality or other infant outcomes.
Trial Registration:
clinicaltrials.gov Identifier: NCT00133744
About 3 million stillbirths and 3 million early neonatal deaths occurred world wide in 2004, 98% of which were in less developed countries.1 A high perinatal mortality rate was reported in rural China in 2000.2 Potential preventable causes of stillbirths and early neonatal deaths are maternal anemia and related micronutrient deficiencies.3 Aside from perinatal folic acid supplements to prevent neural tube defects, multiple health organizations recommend pregnant women routinely receive iron–folic acid supplements to prevent anemia.4 Meta-analyses of randomized controlled trials (RCTs) indicate that daily prenatal iron–folic acid supplementation prevents iron deficiency and anemia.5,6 The effect of prenatal iron–folic acid supplementation on perinatal mortality and other maternal and infant outcomes remains unclear, particularly among nonanemic women. Thus, some question the added benefit of prophylactic iron supplementation.7
In some settings, other micronutrient deficiencies may limit the effectiveness of prenatal iron–folic acid supplementation,8,9 leading to trials to determine the maternal and infant health effects conferred from additional multiple micronutrients (MMN) vsiron–folic acid alone.10,11 In 2005, only 2 trials of iron-folic acid vs MMN supplements had been published, but neither was adequately powered to examine perinatal morality. A study in Xian, China,12 used cluster randomization to examine birth weight as the primary outcome; the amount of iron in the iron–folic acid supplement was 60 mg vs 30 mg in the MMN supplement. It was unknown if this higher dose of iron would affect the comparison owing to either increased gastrointestinal effects or interactions with other nutrients. In recent meta-analyses, provision of MMN supplements vs iron–folic acid improved mean birth weight and reduced the risk of low birth weight but did not benefit birth length, head circumference, or the duration of gestation.13,14 The effects of MMN supplements on fetal and infant deaths were inconsistent15–22 and may have been modified by maternal baseline nutrition.23
China is a country in nutrition transition.24 In China, 29% of pregnant women were anemic in 2002.25 In southeast China, prenatal anemia prevalence declined by more than 50% between 1993 and 2005.26 With the exception of periconceptional folic acid, prenatal iron and other micronutrients are not routinely prescribed or used by nonanemic women. Similarly, in the United States and other industrialized countries, there are conflicting recommendations about use of prophylactic iron with or without other nutrients.7
The objective of the current study is to investigate the maternal and infant health effects of iron–folic acid and MMN supplements vs iron–folic acid alone when provided to pregnant women with no or mild anemia. We hypothesized that prenataliron–folic acid supplements with or without other micronutrients, given from the first prenatal visit through delivery, would reduce perinatal mortality beyond any reductions conferred by iron–folic acid alone.
METHODS
STUDY SETTING AND DESIGN
The trial took place nearby Beijing in 5 rural counties in Hebei Province, China, where basic health services were provided through 3-tier (county, township, and village) health care networks. Village clinics and township hospitals provided basic prenatal health care services, and county hospitals provided delivery service. Health facility and service capacity were similar in 5 counties, and the same guidelines or procedures for prenatal health care and delivery were used. The population at the time of the study was about 2.5 million, and gross domestic product per capita about $4700.
Eligible pregnant women were enrolled from May 2006 through April 2009 and individually randomized in a 1:1:1 ratio to receive a daily supplement containing folic acid, iron–folic acid, or MMN from early pregnancy through delivery. The study protocol was approved by the institutional review boards of the Centers for Disease Control and Prevention, Atlanta, Georgia, and Peking University, Beijing, China, and renewed annually.
PARTICIPANTS
To be eligible, pregnant women (1) recorded dates of their menstruation for 2 or more months before they became pregnant, (2) were nulliparous, (3) were at least 20 years old, (4) were not more than 20 weeks gestation, (5) were legally competent, (6) had not consumed micronutrient supplements other than folic acid in the prior 6 months, (7) had a hemoglobin (Hb) level greater than 10.0 g/dL, (8) resided in and received prenatal care in 1 of 5 counties, and (9) consented to participate. (To convert Hb to grams per liter, multiply by 10.0.)
ENROLLMENT AND INFORMED CONSENT
Before the study started, the project was publicized in each participating county using pamphlets, posters, radio, and television. During the first prenatal visit, the consent form was read to each woman by a physician trained in informed consent procedures. After the woman provided verbal consent, the form was signed by the physician plus a witness.
RANDOMIZATION AND MASKING
Randomization was stratified by county, and random block sizes of 3, 6, and 9 were used to ensure geographical balance with an approximately equal distribution of treatments within and across study counties. A statistician external to the study randomly assigned ten 4-digit lot numbers to each of the 3 supplement types (masked to the formulation and allocation) and generated the assignment list for each county proportional to the expected number of participants; within each county and block, lot numbers were randomly assigned using RANUNI in SAS statistics software (SAS Institute Inc). Aside from a pharmaceutical engineer who ensured allocation of lot numbers to the correct supplement formulations, all others (ie, participants, local physicians, study personnel, and investigators) were masked to the identity of the supplements. Treatment codes were broken after completion of the study and main analyses.
INTERVENTION
At enrollment, the physician assigned women the next lot number on the randomization schedule. Each woman received 2 bottles of supplements at enrollment and 1 at monthly follow-up visits. Each bottle contained 31 supplements, including 1 of the supplements (see eMethods 1 for supplement formulations; http://www.jamainternalmed.com) according to lot number. The capsules were of the same size, color, and shape, and bottles were identical except for the lot number. Study participants were instructed to take 1 supplement daily from enrollment until delivery. Supplements were provided at no cost; otherwise, no compensation was given for participation.
STUDY OUTCOMES
Trained county or township physicians completed relevant measurements and collected data based on a perinatal and child health care surveillance system (see eMethods 2 for detailed data collection procedures). To ensure data quality, Peking University staff conducted various training programs, performed monthly site supervisions, and cross-checked all death events. The primary outcome was perinatal mortality. Secondary outcomes included neonatal deaths, infant deaths, maternal Hb concentration and anemia at 24 to 28 weeks of gestation, birth weight, birth length, duration of gestation, and preterm delivery. (Outcome definitions are provided in eMethods 3.)
SAMPLE SIZE
The estimated perinatal mortality rate was 16 per 1000 births in the folic acid group based on our previous study.27 A 40% reduction in the iron–folic acid group compared with the folic acid group and a 50% reduction in the MMN group were hypothesized based on various observational studies.28–32 With a 2-sided significance level of P=.05, β-error specification of 0.20, and correction for continuity, 5272 per group were needed to detect a 40% reduction in the iron–folic acid group compared with the folic acid group and a 50% reduction in the MMN group compared with the iron–folic acid group. To account for the estimated 4.7% of women lost to follow-up, 4.2% of dropouts, and 4.0% of nonadherence,27 6280 women per group were needed. The statistical power was expected to be adequate for outcomes with a higher baseline prevalence (ie, low birth weight, preterm birth, and maternal anemia).
STATISTICAL ANALYSES
Multiple pregnancies were excluded from analyses. The denominator for perinatal mortality was live births plus stillbirths. The denominator for other study outcomes was live births. We defined mean compliance as the number of supplements consumed divided by the number of days from enrollment to birth; the number of supplements consumed was estimated from the number of supplements remaining in the bottle at each return visit.
All analyses were based on an intent-to-treat principle. All randomized women with a known birth outcome were included in analyses irrespective of compliance. We examined the distribution of key baseline characteristics among the 3 groups using χ2 tests for categorical variables and analysis of variance for continuous. χ2 Tests and independent sample t tests were used to test the differences in dichotomous and continuous outcomes between supplement groups (iron–folic acid vs folic acid, MMN vs folic acid, and MMN vs iron–folic acid), respectively. Unadjusted relative risks (RRs) with 95% confidence intervals (CIs) were calculated for mortality, low birth weight, preterm birth, and maternal anemia. Rate differences with 95% CIs were also calculated for mortality; a number needed to treat with 95% CIs was calculated for outcomes with statistical significance. Unadjusted mean differences with 95% CIs were calculated for continuous outcomes. The Data Safety Monitoring Board met 4 times during the study and reviewed 3 interim analyses by supplement group masked to allocation, in which the significance levels began lower and ended at the final analysis close to the prespecified overall significance level.33 All P values were 2-sided, and statistical significance was set at P=.05 for the final analysis without adjustment for multiple comparisons. We used SAS statistical software (version 9; SAS Institute Inc) for all analyses.
RESULTS
Among the 18 963 pregnant women considered for enrollment, 120 refused to participate in the study, and 68 were not eligible (Figure). Of the 18 775 women randomized, 28 moved, 33 dropped out, 2 died during pregnancy (1 from a traffic accident and 1 from autoimmune disease), and 815 had spontaneous or induced abortions. Of the 17 897 remaining women with known pregnancy outcomes (95.3%), 17 830 women (99.6%) had singleton pregnancies; 17 748 ended their pregnancy with live births, 82 with stillbirths. Among the live births, 71 early neonatal deaths, 87 neonatal deaths, and 134 infant deaths were identified. Of the 87 neonatal deaths, 24 died of asphyxia, 23 of premature birth, 15 had birth defects, 9 had pneumonia, and 16 died of other or unknown causes. The number of women having multiple pregnancies were not statistically different across the treatment groups (P=.30) (Figure).
Figure.
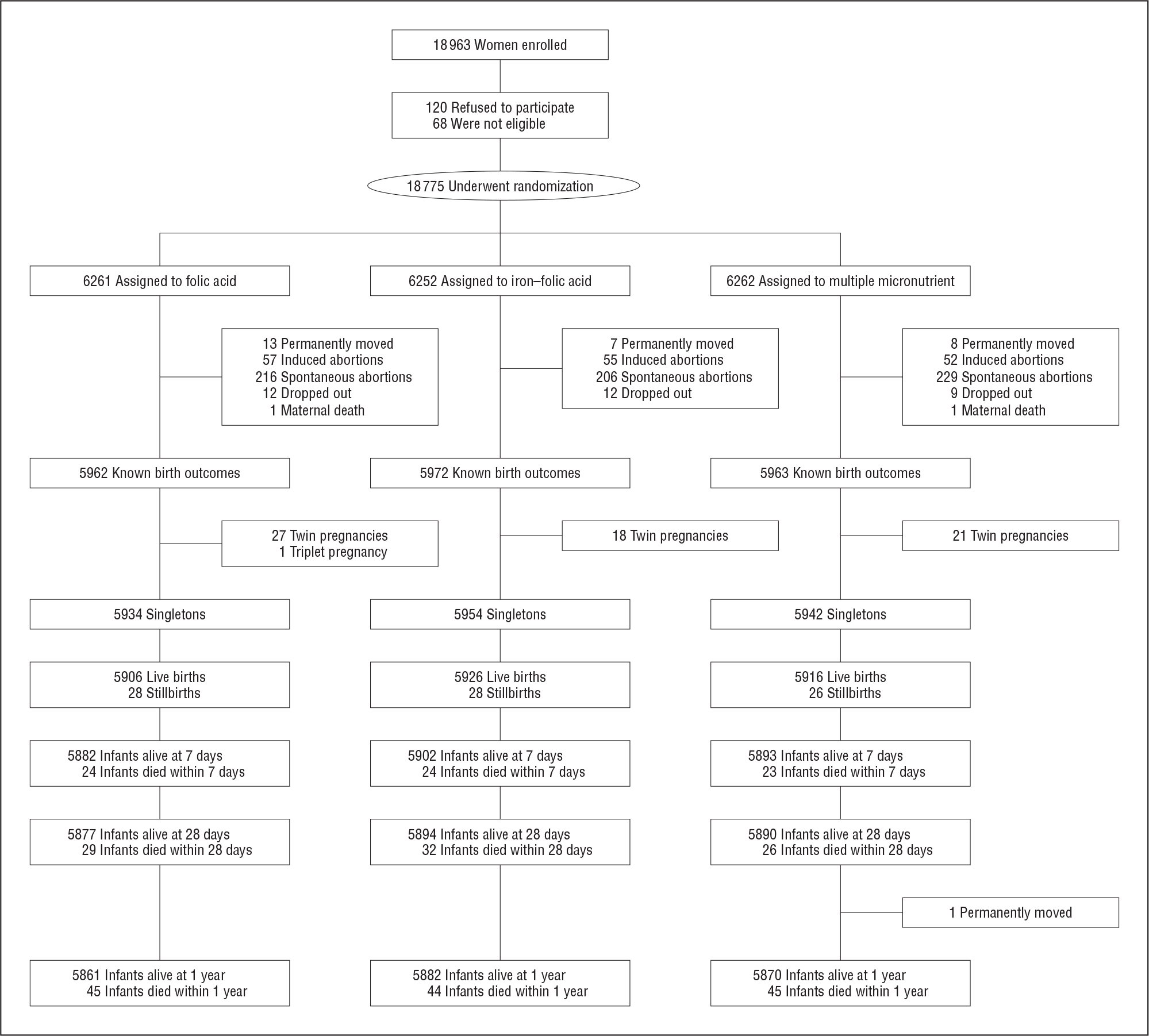
Flowchart of participants.
The baseline characteristics were balanced across supplement groups (Table 1). The mean (SD) maternal age, gestation at enrollment, and Hb level were 23.7 (2.9) years, 11.9 (4.6) weeks, and 12.5 (1.0) g/dL, respectively. Almost all participants were of Han ethnicity, 1.6% had primary or less education, and 90.8% were farmers. About 5% to 6% of women had Hb concentrations of 10.0 to 10.9 g/dL.
Table 1.
Baseline Maternal Characteristics at Enrollment According to Study Interventiona
| Group, No. (%) |
|||
|---|---|---|---|
| Characteristic | FA (n = 6261) |
IFA (n = 6252) |
MMN (n = 6262) |
|
| |||
| Maternal age, mean (SD), y | 23.7 (2.9) | 23.7 (2.9) | 23.6 (2.8) |
| Education | |||
| ≤Primary | 94 (1.5) | 110 (1.8) | 94 (1.5) |
| Secondary | 5011 (80.0) | 5017 (80.2) | 5025 (80.2) |
| ≥High school | 1156 (18.5) | 1125 (18.0) | 1143 (18.3) |
| Ethnicity | |||
| Han | 6180 (98.7) | 6184 (98.9) | 6192 (98.9) |
| Other | 81 (1.3) | 68 (1.1) | 70 (1.1) |
| Occupation | |||
| Farmer | 5674 (90.6) | 5691 (91.0) | 5682 (90.7) |
| Other | 587 (9.4) | 561 (9.0) | 580 (9.3) |
| BMI | |||
| <18.5 | 365 (5.8) | 371 (5.9) | 364 (5.8) |
| 18.5–24.9 | 5008 (80.0) | 5029 (80.4) | 5009 (80.0) |
| 25.0–29.9 | 759 (12.1) | 735 (11.8) | 762 (12.2) |
| ≥30.0 | 129 (2.1) | 117 (1.9) | 127 (2.0) |
| Height, mean (SD), cm | 160.1 (4.5) | 160.2 (4.5) | 160.2 (4.5) |
| Gestational week | |||
| <12 | 3359 (53.6) | 3317 (53.1) | 3364 (53.7) |
| ≥12 | 2902 (46.4) | 2935 (46.9) | 2898 (46.3) |
| Gestational week, mean (SD) | 11.9 (4.6) | 12.0 (4.5) | 11.9 (4.6) |
| Hemoglobin level, g/dL | |||
| 10.0–10.9 | 369 (5.9) | 374 (6.0) | 317 (5.1) |
| 11.0–11.9 | 1408 (22.5) | 1457 (23.3) | 1433 (22.9) |
| 12.0–12.9 | 2640 (42.2) | 2628 (42.0) | 2662 (42.5) |
| ≥13.0 | 1844 (29.4) | 1793 (28.7) | 1850 (29.5) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); FA, folic acid; IFA, iron–folic acid; MMN, multiple micronutrient.
SI conversion factor: To convert hemoglobin to grams per liter, multiply by 10.
Data are expressed as number (percentage) of participants except where noted. χ2 Tests were used to examine statistical differences in categorical variables and analysis of variance to examine differences in means. P > .05 for all comparisons.
Adherence to the assigned supplements was high, and only 20 women reported having taken additional micronutrient supplements including calcium, iron, zinc, or MMNs. A total of 92.7% women consumed 80% to 100% of supplements as instructed; the mean number of supplements consumed was 177 capsules (Table 2). No serious adverse effects were reported. The adverse effects were nausea, vomiting, or other mild gastrointestinal discomfort: 133 women in the folic acid group (2.2%) reported gastrointestinal discomfort, 212 (3.6%) in the iron–folic acid group, and 355 (6.0%) in the MMN group (P < .001).
Table 2.
Compliance and Number of Supplement Consumed According to Study Interventiona
| Compliance and Distribution | Group, No. (%) |
||
|---|---|---|---|
| FA (n = 5934) |
IFA (n = 5954) |
MMN (n = 5942) |
|
|
| |||
| Mean compliance, % Compliance distribution, % | 93.3 (12.6) | 92.8 (13.3) | 91.5 (16.0) |
| 0–19 | 54 (0.9) | 64 (1.1) | 108 (1.8) |
| 20–39 | 46 (0.8) | 49 (0.8) | 71 (1.2) |
| 40–59 | 67 (1.1) | 66 (1.1) | 94 (1.6) |
| 60–79 | 201 (3.4) | 226 (3.8) | 252 (4.2) |
| 80–100 | 5566 (93.8) | 5549 (93.2) | 5417 (91.2) |
| Supplement (capsules) consumed, mean (SD), No. | 179 (41) | 177 (42) | 176 (45) |
| Supplement (capsules) consumed, range | |||
| <90 | 152 (2.6) | 179 (3.0) | 253 (4.3) |
| 90–119 | 256 (4.3) | 243 (4.1) | 284 (4.8) |
| 120–149 | 941 (15.9) | 990 (16.6) | 976 (16.4) |
| 150–179 | 1388 (23.4) | 1409 (23.7) | 1306 (22.0) |
| ≥180 | 3197 (53.9) | 3133 (52.6) | 3123 (52.6) |
Abbreviations: FA, folic acid; IFA, iron–folic acid; MMN, multiple micronutrient.
Data are expressed as number (percentage) of participants except where noted. Compliance calculated by number of actual supplements consumed divided by number of supplements expected to be consumed.
The perinatal mortality rate was 8.76 of 1000 births for the folic acid group, 8.73 of 1000 for the iron–folic acid group, and 8.25 of 1000 for the MMN group. Compared with prenatal folic acid alone, neither iron–folic acid (RR, 1.00; 95% CI, 0.68–1.46; P = .99) nor MMN supplements (RR, 0.94; 95% CI, 0.64–1.39; P = .76) affected the risk of perinatal mortality (Table 3). Compared with iron folic acid, MMN did not affect the risk of perinatal mortality (RR, 0.94; 95% CI, 0.64–1.39; P = .77). Similarly, risk of stillbirths, early neonatal deaths, neonatal deaths, or infant deaths did not differ by supplement group.
Table 3.
Perinatal Deaths, Stillbirths, and Neonatal and Infant Deathsa
| Outcomes | No./No. (Cases/1 000) |
IFA vs FA |
MMN vs FA |
MMN vs IFA |
|||||
|---|---|---|---|---|---|---|---|---|---|
| FA | IFA | MMN | RR (95% CI) | RD (95% CI) | RR (95% CI) | RD (95% CI) | RR (95% CI) | RD (95% CI) | |
|
| |||||||||
| Perinatal deathb | 52/5934 | 52/5954 | 49/5942 | 1.00 | 0.02 | 0.94 | 0.02 | 0.94 | 0.02 |
| (8.76) | (8.73) | (8.25) | (0.68 to 1.46) | (−0.03 to 0.03) | (0.64 to 1.39) | (−0.04 to 0.03) | (0.64 to 1.39) | (−0.04 to 0.03) | |
| Stillbirthc | 28/5934 | 28/5954 | 26/5942 | 1.00 | 0.01 | 0.93 | 0.01 | 0.93 | 0.01 |
| (4.72) | (4.70) | (4.38) | (0.59 to 1.68) | (−0.03 to 0.02) | (0.54 to 1.58) | (−0.03 to 0.02) | (0.55 to 1.58) | (−0.03 to 0.02) | |
| Early neonatal deathd | 24/5906 | 24/5926 | 23/5916 | 1.00 | 0.01 | 0.96 | 0.01 | 0.96 | 0.01 |
| (4.06) | (4.05) | (3.89) | (0.57 to 1.75) | (−0.02 to 0.02) | (0.54 to 1.69) | (−0.02 to 0.02) | (0.54 to 1.70) | (−0.02 to 0.02) | |
| Neonatal deathe | 29/5906 | 32/5926 | 26/5916 | 1.10 | 0.01 | 0.90 | 0.01 | 0.81 | 0.01 |
| (4.91) | (5.40) | (4.39) | (0.67 to 1.82) | (−0.02 to 0.03) | (0.53 to 1.52) | (−0.03 to 0.02) | (0.49 to 1.36) | (−0.04 to 0.02) | |
| Infant deathf | 45/5906 | 44/5926 | 45/5915 | 0.97 | 0.02 | 1.00 | 0.02 | 1.02 | 0.02 |
| (7.62) | (7.42) | (7.61) | (0.64 to 1.48) | (−0.03 to 0.03) | (0.66 to 1.50) | (−0.03 to 0.03) | (0.68 to 1.55) | (−0.03 to 0.03) | |
Abbreviations: FA, folic acid; IFA, iron–folic acid; MMN, multiple micronutrient; RD, risk difference; RR, relative risk.
χ2 Tests were used to examine statistical differences in death rates between each of the 2 groups. P > .05 for all comparisons.
Stillbirth and early neonatal death. Perinatal death is primary outcome.
From 28 weeks of gestation to delivery.
From birth to 6 days after delivery.
From birth to 28 days after delivery.
During the first year of life.
Compared with folic acid alone, iron–folic acid and MMN increased third-trimester maternal Hb concentration by 0.04 and 0.06 g/dL, respectively, and decreased the anemia prevalence by 28% and 29% (Table 4), respectively, with a number needed to treat of 47 (95% CI, 33–81) for the iron–folic acid group and 46 (95% CI, 32–77) for the MMN group. Neither gestation duration nor birth weight or length differed significantly by supplement group.
Table 4.
Birth Outcomes and Maternal Hemoglobin Level in the Study Groupsa
| IFA vs FA |
MMN vs FA |
MMN vs IFA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | FA (n = 6261) |
IFA (n = 6252) |
MMN (n = 6262) |
MD/RR (95% CI) | P Value | MD/RR (95% CI) | P Value | MD/RR (95% CI) | P Value |
|
| |||||||||
| Birth weight, g | |||||||||
| Infants, No. | 5905 | 5922 | 5913 | ||||||
| Mean (SD) | 3290.6 (391.5) | 3292.5 (389.0) | 3299.0 (392.4) | 1.91 (−12.16 to 15.98) | .79 | 8.34 (−5.79 to 22.48) | .25 | 6.43 (−7.65 to 20.51) | .37 |
| Low birth weight, <2500 g | 125 (2.1) | 129 (2.2) | 116 (2.0) | 1.03 (0.81 to 1.31) | .82 | 0.93 (0.72 to 1.19) | .55 | 0.90 (0.70 to 1.15) | .10 |
| Birth length, cm | |||||||||
| Infants, No. | 5905 | 5922 | 5913 | ||||||
| Mean (SD) | 50.0 (1.1) | 50.0 (1.1) | 50.0 (1.0) | 0.01 (−0.03 to 0.05) | .60 | 0.03 (−0.01 to 0.07) | .14 | 0.02 (−0.02 to 0.06) | .34 |
| Gestational age, wk | |||||||||
| Infants, No. | 5906 | 5926 | 5916 | ||||||
| Mean (SD) | 39.6 (1.7) | 39.6 (1.7) | 39.6 (1.6) | −0.03 (−0.09 to 0.03) | .35 | 0.02 (−0.04 to 0.09) | .43 | 0.05 (−0.01 to 0.11) | .08 |
| Preterm birth, <37 wk | 353 (6.0) | 340 (5.7) | 308 (5.2) | 0.96 (0.83 to 1.11) | .58 | 0.87 (0.75 to 1.01) | .07 | 0.91 (0.78 to 1.05) | .20 |
| Maternal Hb level at 24–28 wk of gestation | |||||||||
| Women, No. | 5896 | 5913 | 5904 | ||||||
| Hb level, g/dL | 12.2 (0.9) | 12.2 (0.9) | 12.3 (0.9) | 0.04 (0.01 to 0.07) | .01 | 0.06 (0.03 to 0.09) | <.001 | 0.02 (−0.02 to 0.05) | .34 |
| Anemia, Hb level <11.0g/dL | 452 (7.7) | 327 (5.5) | 323 (5.5) | 0.72 (0.63 to 0.83) | <.001 | 0.71 (0.62 to 0.82) | <.001 | 0.99 (0.85 to 1.15) | .89 |
Abbreviations: FA, folic acid; IFA, iron–folic acid; Hb, hemoglobin; MD, mean difference; MMN, multiple micronutrient; RR, relative risk.
SI conversion factor: To convert hemoglobin to grams per liter, multiply by 10.
Data are expressed as number (percentage) of participants or mean (SD) unless otherwise indicated. P values were calculated based on χ2 tests or independent sample t tests as appropriate.
COMMENT
In this primarily nonanemic rural population, prenatal supplementation with iron–folic acid or MMN vs folic acid alone did not decrease the risk of perinatal mortality or other infant or maternal outcomes except maternal anemia. Compared with folic acid alone, iron–folic acid and MMN increased Hb concentration and reduced anemia by about 30%. Our study population was rural (91% were farmers); nearly all women had at least a secondary education, and few were undernourished or anemic. In addition, having monitored their menstrual cycle for 2 or more months before enrollment, women were cognizant of their last menstrual period and prenatal health. Once enrolled, women were followed monthly and delivered in the hospital. The lack of differences in infant outcomes with prenatal supplementation may underline the importance of secondary factors, such as nutritional status, prenatal care, and economic status in assessing the degree to which our findings can be generalized.
Our findings are consistent with 2 previous meta-analyses of RCTs in developing countries comparing the effects of iron–folic acid with MMN on neonatal and perinatal mortality.34,35 Unlike one meta-analysis36 that found a trend toward higher early neonatal mortality in the group receiving MMN vs iron–folic acid alone, we found no difference between these groups. A recent meta-analysis34 showed an increased risk of neonatal mortality with MMN compared with iron–folic acid in settings where less than 60% of births occurred in facilities; however, consistent with our trial, there was no increased risk in settings where most births occurred in facilities. Thus, our results should be interpreted in the context of the existing burden of micronutrient deficiencies, adverse pregnancy, and fetal outcomes, and the setting. Our study population had low prevalence rates of all the outcomes assessed in the study, including a low prevalence of anemia (5%–7%) in late pregnancy and a low birth weight prevalence (approximately 2%), which were even lower than those seen in many industrialized countries. At such low rates of adverse outcomes, it is not surprising that the intervention had no impact. As such, this trial is not comparable with many of the studies included in the meta-analyses. Unlike many trials conducted in poor populations in developing countries, our trial is generalizable to a well-nourished population of nonanemic or mildly anemic women with good access to health care.
In this primarily nonanemic population, the relative reduction of anemia (approximately 30%) was substantial. Although iron status was not assessed in the entire population, this reduction suggests about 30% of anemia was due to iron deficiency, and only one-third of anemic women in our population could be expected to respond to iron supplementation. Anemic women are likely to be iron deficient and would benefit from supplements containing iron. However, overall improvement in Hb concentration was limited, owing to low anemia prevalence at baseline (<6%). Because 21.7% of the population had high Hb levels (≥13.2 g/dL)37,38 at enrollment and high Hb level has been hypothesized as a risk factor, we conducted post hoc analyses to test for possible interaction between supplement groups and maternal baseline Hb status (<13.2 g/dL vs ≥13.2 g/dL) on perinatal mortality by using Breslow-Day tests. All P values for interaction tests were not significant (P > .90).
Although we did not find a significant difference in low birth weight with prenatal MMN vs iron–folic acid supplementation, the point estimate (10% reduction) was similar to that estimated (11%–14%) in the previous meta-analyses.13,35 In our study population, we excluded women with moderate anemia at enrollment, and the low birth weight incidence was smaller than that observed in previous studies, diminishing the possibility of observing a significant effect. Our findings with regard to the effect of iron differ with those of 2 trials conducted in the United States. One trial compared the effects of iron alone vs placebo and the other compared prenatal vitamins with and without iron, both in iron-replete, nonanemic pregnant women.39,40 In both US trials, the iron-supplemented group had a significantly higher mean birth weight compared with the control group; the trial comparing iron with placebo showed a significantly lower incidence of infants with low birth weight39 and increased gestational age at delivery. Although it is difficult to compare these trials owing to differences in the populations and control supplements, the low birth weight incidence among the control groups in the US studies (9.5–16.7%) was much higher than our study (approximately 2%).
The inconsistent results between our study and those of other studies may relate to the modifying effects of maternal socioeconomic and nutritional status in response to micronutrient supplementation. In an RCT in rural western China,41 effects of micronutrient supplementation were observed in the poorest one-third of households but not in wealthier households. Compared with folic acid alone, women from the poorest households who received iron–folic acid had significant reductions in both preterm birth and early neonatal mortality; women who received MMN had a significant reduction in preterm births and low birth weight. In the large-scale SUMMIT trial in Indonesia,22 effects on early infant mortality (0–90 days) were observed among undernourished or anemic women; compared with women receiving iron–folic acid, women who received MMN showed significant reductions in early infant mortality (25% in undernourished women and 38% in anemic women, respectively).
Aside from the lack of a placebo group and information on the micronutrient status, the major limitation of the study was that the actual power was not adequate to detect a difference in perinatal mortality by supplement group, primarily because the observed perinatal mortality rate dramatically decreased during the past decade with the rapid socioeconomic development in China. Although there were no evident differences in perinatal mortality, we cannot rule out small differences because of inadequate sample size. Our study has several strengths. Supplements were randomized by individual. Capsule-taking was confirmed by independent household visits, and the compliance was greater than 90%. All pregnancy outcomes, including stillbirths, abortions, and early neonatal deaths, were ascertained by active community surveillance, review of vital registration certificates, and review of hospital records. Assessment of birth weight was conducted using standardized and accurate equipment and trained staff. Finally, less than 1% of those enrolled were lost to follow-up.
In summary, our study is, to our knowledge, the first large-scale, double-blind, individual RCT to directly assess the impact of prenatal supplementation on perinatal mortality in a well-nourished Chinese population with good access to healthcare. Given perinatal and infant mortality were similar across supplement groups, the slight improvement in Hb status with iron–folic acid and MMN supplements among nonanemic or mildly anemic women provides some limited additional support for prophylactic iron supplementation to prevent third-trimester maternal anemia.
Supplementary Material
Funding/Support:
The study is supported by a cooperative agreement between Peking University Health Science Center and the Centers for Disease Control and Prevention.
Role of the Sponsor:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of Centers for Disease Control and Prevention. The sponsor had a role in study design, data collection, data analysis and interpretation, and writing of the report.
Footnotes
Conflict of Interest Disclosures: None reported.
Online-Only Material: The eMethods are available at http://www.jamainternalmed.com.
Contributor Information
Jian-meng Liu, Institute of Reproductive and Child Health/Ministry of Health Key Laboratory of Reproductive Health and Department of Epidemiology and Biostatistics, School of Public Health, Peking University Health Science Center, Beijing, China.
Zuguo Mei, Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia.
Rongwei Ye, Institute of Reproductive and Child Health/Ministry of Health Key Laboratory of Reproductive Health and Department of Epidemiology and Biostatistics, School of Public Health, Peking University Health Science Center, Beijing, China.
Mary K. Serdula, Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia.
Aiguo Ren, Institute of Reproductive and Child Health/Ministry of Health Key Laboratory of Reproductive Health and Department of Epidemiology and Biostatistics, School of Public Health, Peking University Health Science Center, Beijing, China.
Mary E. Cogswell, Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia.
REFERENCES
- 1.World Health Organization. Neonatal and perinatal mortality: country, regional and global estimates 2004. http://whqlibdoc.who.int/publications/2007/9789241596145_eng.pdf. Accessed December 30, 2010.
- 2.Wu Z, Viisainen K, Wang Y, Hemminki E. Perinatal mortality in rural China: retrospective cohort study. BMJ. 2003;327(7427):1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black RE, Allen LH, Bhutta ZA, et al. ; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. [DOI] [PubMed] [Google Scholar]
- 4.International Nutritional Anemia Consultative Group. Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/1-57881-020-5/en/index.html. Accessed January 25, 2011.
- 5.Pena-Rosas JP, Viteri FE. Effects of routine oral iron supplementation with or without folic acid for women during pregnancy. Cochrane Database Syst Rev. 2006;(3):CD004736. [DOI] [PubMed] [Google Scholar]
- 6.Peña-Rosas JP, Viteri FE. Effects and safety of preventive oral iron or iron folic acid supplementation for women during pregnancy. Cochrane Database Syst Rev. 2009;(4):CD004736. [DOI] [PubMed] [Google Scholar]
- 7.US Preventive Services Task Force. Routine iron supplementation during pregnancy. Policy statement. JAMA. 1993;270(23):2846–2848. [PubMed] [Google Scholar]
- 8.Shrimpton R, Shrimpton R, Schultink W. Can supplements help meet the micronutrient needs of the developing world? Proc Nutr Soc. 2002;61(2):223–229. [DOI] [PubMed] [Google Scholar]
- 9.Sloan NL, Jordan E, Winikoff B. Effects of iron supplementation on maternal hematologic status in pregnancy. Am J Public Health. 2002;92(2):288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UNICEF/UNU/WHO Study Team. Multiple Micronutrient Supplementation During Pregnancy (MMSDP): Efficacy Trials. London, England: Institute of Child Health, UCL; 2002. [Google Scholar]
- 11.UNICEF/UNU/WHO Study Team. Composition of Multiple Micronutrient Supplement to be Used in Pilot Programmes Among Pregnant Women in Developing Countries: Report of a Workshop Held at UNICEF Headquarters. New York, NY: UNICEF. [Google Scholar]
- 12.Zeng L, Dibley MJ, Cheng Y, et al. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ. 2008;337:a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fall CH, Fisher DJ, Osmond C, Margetts BM; Maternal Micronutrient Supplementation Study Group. Multiple micronutrient supplementation during pregnancy in low-income countries: a meta-analysis of effects on birth size and length of gestation. Food Nutr Bull. 2009;30(4)(suppl):S533–S546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah PS, Ohlsson A; Knowledge Synthesis Group on Determinants of Low Birth Weight and Preterm Births. Effects of prenatal multimicronutrient supplementation on pregnancy outcomes: a meta-analysis. CMAJ. 2009;180(12):E99–E108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christian P, Osrin D, Manandhar DS, Khatry SK, de L Costello AM, West KP Jr. Antenatal micronutrient supplements in Nepal. Lancet. 2005;366(9487):711–712. [DOI] [PubMed] [Google Scholar]
- 16.Christian P, West KP, Khatry SK, et al. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster-randomized trial in Nepal. Am J Clin Nutr. 2003;78(6):1194–1202. [DOI] [PubMed] [Google Scholar]
- 17.Czeizel AE, Dudás I, Métneki J. Pregnancy outcomes in a randomised controlled trial of periconceptional multivitamin supplementation: final report. Arch Gynecol Obstet. 1994;255(3):131–139. [DOI] [PubMed] [Google Scholar]
- 18.Fawzi WW, Msamanga GI, Spiegelman D, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet. 1998;351(9114):1477–1482. [DOI] [PubMed] [Google Scholar]
- 19.Fawzi WW, Msamanga GI, Urassa W, et al. Vitamins and perinatal outcomes among HIV-negative women in Tanzania. N Engl J Med. 2007;356(14):1423–1431. [DOI] [PubMed] [Google Scholar]
- 20.Kaestel P, Michaelsen KF, Aaby P, Friis H. Effects of prenatal multimicronutrient supplements on birth weight and perinatal mortality: a randomised, controlled trial in Guinea-Bissau. Eur J Clin Nutr. 2005;59(9):1081–1089. [DOI] [PubMed] [Google Scholar]
- 21.Roberfroid D, Huybregts L, Lanou H, et al. ; MISAME Study Group. Effects of maternal multiple micronutrient supplementation on fetal growth: a double-blind randomized controlled trial in rural Burkina Faso. Am J Clin Nutr. 2008;88(5): 1330–1340. [DOI] [PubMed] [Google Scholar]
- 22.Shankar AH, Jahari AB, Sebayang SK, et al. ; Supplementation With Multiple Micronutrients Intervention Trial (SUMMIT) Study Group. Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double-blind cluster-randomised trial. Lancet. 2008;371(9608):215–227. [DOI] [PubMed] [Google Scholar]
- 23.Abu-Saad K, Fraser D. Maternal nutrition and birth outcomes. Epidemiol Rev. 2010;32(1):5–25. [DOI] [PubMed] [Google Scholar]
- 24.Zhai F, Wang H, Du S, et al. Prospective study on nutrition transition in China. Nutr Rev. 2009;67(suppl 1):S56–S61. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Worldwide prevalence of anaemia 1993–2005: WHO Global Database on Anaemia. http://whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf. Accessed October 30, 2011.
- 26.Jin L, Yeung LF, Cogswell ME, et al. Prevalence of anaemia among pregnant women in South-East China, 1993–2005. Public Health Nutr. 2010;13(10):1511–1518. [DOI] [PubMed] [Google Scholar]
- 27.Berry RJ, Li Z, Erickson JD, et al. Prevention of neural-tube defects with folic acid in China: China-US Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341(20):1485–1490. [DOI] [PubMed] [Google Scholar]
- 28.Garn SM, Ridella SA, Petzold AS, Falkner F. Maternal hematologic levels and pregnancy outcomes. Semin Perinatol. 1981;5(2):155–162. [PubMed] [Google Scholar]
- 29.Lister UG, Rossiter CE, Chong H. Perinatal mortality. BJOG. 1985;92(suppl s5): 86–99. [Google Scholar]
- 30.Malhotra M, Sharma JB, Batra S, Sharma S, Murthy NS, Arora R. Maternal and perinatal outcome in varying degrees of anemia. Int J Gynaecol Obstet. 2002; 79(2):93–100. [DOI] [PubMed] [Google Scholar]
- 31.Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal hemoglobin concentration during pregnancy and risk of stillbirth. JAMA. 2000;284(20): 2611–2617. [DOI] [PubMed] [Google Scholar]
- 32.Xiong X, Buekens P, Alexander S, Demianczuk N, Wollast E. Anemia during pregnancy and birth outcome: a meta-analysis. Am J Perinatol. 2000;17(3):137–146. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–556. [PubMed] [Google Scholar]
- 34.Haider BA, Yakoob MY, Bhutta ZA. Effect of multiple micronutrient supplementation during pregnancy on maternal and birth outcomes. BMC Public Health. 2011;11(suppl 3):S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawai K, Spiegelman D, Shankar AH, Fawzi WW. Maternal multiple micronutrient supplementation and pregnancy outcomes in developing countries: meta-analysis and meta-regression. Bull World Health Organ. 2011;89(6):402–411B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronsmans C, Fisher DJ, Osmond C, Margetts BM, Fall CH; Maternal Micronutrient Supplementation Study Group. Multiple micronutrient supplementation during pregnancy in low-income countries: a meta-analysis of effects on stillbirths and on early and late neonatal mortality. Food Nutr Bull. 2009;30(4)(suppl):S547–S555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy JF, O’Riordan J, Newcombe RG, Coles EC, Pearson JF. Relation of haemoglobin levels in first and second trimesters to outcome of pregnancy. Lancet. 1986;1(8488):992–995. [DOI] [PubMed] [Google Scholar]
- 38.Ziaei S, Norrozi M, Faghihzadeh S, Jafarbegloo E. A randomised placebocontrolled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin ≥ 13.2 g/dl [published correction appears in BJOG. 2007;114(10):1311]. BJOG. 2007;114(6):684–688. [DOI] [PubMed] [Google Scholar]
- 39.Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM. Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. Am J Clin Nutr. 2003;78(4):773–781. [DOI] [PubMed] [Google Scholar]
- 40.Siega-Riz AM, Hartzema AG, Turnbull C, Thorp J, McDonald T, Cogswell ME. The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: a randomized controlled trial. Am J Obstet Gynecol. 2006; 194(2):512–519. [DOI] [PubMed] [Google Scholar]
- 41.Zeng L, Yan H, Cheng Y, Dibley MJ. Modifying effects of wealth on the response to nutrient supplementation in pregnancy on birth weight, duration of gestation and perinatal mortality in rural western China: double-blind cluster randomized controlled trial. Int J Epidemiol. 2011;40(2):350–362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


