Abstract
Purpose:
Long-acting reversible contraceptive (LARC) methods do not require annual clinic visits for continuation, potentially impacting receipt of recommended sexually transmitted infection (STI)/human immunodeficiency virus (HIV) services for young women. We assess service receipt among new and continuing LARC users versus moderately and less effective method users and non-contraceptors.
Methods:
Using 2011–2015 National Survey of Family Growth data from sexually active women aged 15–24 years (n = 2,018), we conducted logistic comparisons of chlamydia, any STI and HIV testing, and sexual risk assessment in the past year by current contraceptive type.
Results:
Less than half of respondents were tested for chlamydia (40.9%), any STI (47.3%), or HIV (25.9%); 66.5% had their sexual risk assessed. Differences in service receipt between new and continuing LARC users as compared with moderately effective method users were not detected in multivariable models, except that continuing LARC users were less likely to be tested for HIV (adjusted prevalence ratio [aPR] = .52, 95% confidence interval [CI] = .32–.85). New, but not continuing, LARC users were more likely than less effective method users (aPR = 1.35, 95% CI = 1.03–1.76) and non-contraceptors (aPR = 1.43, 95% CI = 1.11–1.85) to have their sexual risk assessed, although both groups were more likely than non-contraceptors to be tested for chlamydia (new: aPR = 1.52, 95% CI = 1.08–2.15; continuing: aPR = 1.69, 95% CI = 1.24–2.29).
Conclusions:
We found little evidence that LARC use was associated with lower prevalence of STI testing. However, new, but not continuing, LARC users, as compared with those not using a method requiring a clinic visit, were more likely to have had their risk assessed, suggesting that initiating LARC may offer an opportunity to receive services that does not persist.
Keywords: Long-acting reversible contraception, STI testing, HIV testing
Unintended pregnancy, human immunodeficiency virus (HIV), and other sexually transmitted infections (STIs) are distinct, but interrelated health concerns. Given that each occurs in the context of sexual behavior, many have argued for an integrated prevention approach [1,2]. National guidelines for providing quality family planning services recommend comprehensive delivery of sexual and reproductive health prevention and care services, including STI/HIV testing and counseling [3]. Integration is especially salient for adolescent and young adult women: nearly half of the 20 million new STIs reported each year, including HIV, are among young people aged 15–24 years, and the proportion of pregnancies that are unintended is higher among adolescents (75%) and young adults (59%) compared with older women (31%–42%) [4,5].
Increasing use of long-acting reversible contraceptive (LARC) methods [6,7], namely intrauterine devices and implants, has renewed attention to the challenge of integrating unintended pregnancy and STI prevention, particularly among young people. Professional medical organizations recommend LARC methods as a highly effective pregnancy prevention option for all women of reproductive age, including adolescents [8–10]. Yet because they confer no STI/HIV prevention benefits, family planning guidelines suggest LARC users should also use condoms if they are not in a mutually monogamous relationship [3]. However, recent evidence shows condom use is low among adolescent LARC users and suggests they may be less likely to use condoms than users of moderately effective methods (e.g., birth control pills, injectables, patch, and ring) [11,12].
The implications of LARC use for STI/HIV prevention may also extend beyond condom use to health services. Specifically, LARC users may be less likely to receive recommended STI/HIV-related services, given the long-acting nature of these methods and young women’s care-seeking patterns and preferences. Whereas moderately effective methods must be refilled or administered by a provider at least annually, LARC methods remain effective for up to 3–10 years, depending on the method, and require less clinical interaction for continuation. Fewer family planning visits may mean fewer opportunities for testing, given that 75% of women receiving STI-related services report receiving them from obstetricians/gynecologists or family planning providers [13]. Moreover, many women intend to be tested for STIs at family planning clinics [14], which are the only source of care for a substantial proportion of women [15].
Limited research has explored associations between LARC use and receipt of health services. Although two prior studies examined use of clinical services, including STI testing, among young female LARC users, neither was conducted in the context of the current health-care system—one took place in the early 1990s and the other used home-based STI testing as part of study follow-up [16,17]. Using nationally representative data, we assess whether sexually active adolescent and young adult LARC users are less likely to receive STI/HIV services compared with users of moderately effective contraceptive methods that typically involve annual clinic visits. Given that new LARC users are inherently interacting with the health-care system at the time of insertion, we distinguish new and continuing LARC users. We also compare new and continuing LARC users to users of less effective methods that do not require regular clinical interactions for continuation and non-contraceptors. A more nuanced understanding of whether LARC use is related to receipt of STI/HIV services can inform strategies for integrating STI/HIV prevention with efforts to increase awareness of and access to LARC methods.
Methods
Data source and procedures
We used data from the 2011–2015 National Survey of Family Growth (NSFG) implemented by the National Center for Health Statistics at the Centers for Disease Control and Prevention (CDC). Details of the survey methodology are documented elsewhere [18]. Briefly, this continuously administered survey (with interviews conducted over 48 weeks each year) employs a multistage probability design that yields a nationally representative sample of women and men aged 15–44 years in the U.S. household population. Computer-assisted personal interviews (CAPI) are used to collect self-reported information about family life, marriage and divorce, pregnancy, fertility, contraceptive use, health behaviors, and outcomes. Additional sexual health-related indicators are assessed via audio computer-assisted self-interviews (ACASI).
Study sample
For this analysis, the sample was restricted to sexually active female adolescents (15–19 years) and young adult women (20–24 years) at risk of unintended pregnancy (n = 2,018). Participants were considered to be sexually active and at risk of unintended pregnancy if they had vaginal sex with at least one male sex partner in the prior year and were not currently pregnant, seeking pregnancy, postpartum (completed pregnancy ≤2.5 months before interview), infecund, or using sterilization as their current method of contraception.
Measures
The independent variable of interest was type of contraceptive method currently used. We first used a recoded variable to determine the most effective method used during the month of the interview (if any), based on estimates of contraceptive effectiveness with typical use [19]. For users of highly effective LARC methods, we then used a calendar history of contraceptive use to assess whether the method was initiated within or before the past 12 months. The final categorical indicator distinguished (1) new LARC users (initiated ≤12 months prior); (2) continuing LARC users (initiated > 12 months prior); (3) current users of moderately effective methods, including oral contraceptives, Depo-Provera, the patch, and ring; (4) current users of less effective methods, including condoms, diaphragm, withdrawal, morning-after pill, foam, sponge, suppository, jelly or cream, periodic abstinence, or other methods (not specified); and (5) non-contraceptors.
Outcomes included dichotomous variables for chlamydia testing, any STI testing, HIV testing, and STI-related risk assessment in the past year, given existing recommendations for these services. The U.S. Preventive Services Task Force, CDC, and American College of Obstetricians and Gynecologists recommend annual chlamydia and gonorrhea screening for all sexually active women <25 years of age, and routine HIV screening starting at age 15, with recommended screening intervals for HIV varying based on risk [20]. The American Academy of Pediatrics also recommends risk assessment for sexually active individuals based on condom use, partner characteristics, participation in transactional sex, and prior STI treatment as part of quality, annual preventive care visits for young people [21].
Outcomes were measured using both the ACASI and the CAPI components of the survey. STI testing was based on two ACASI items—one that assessed receipt of chlamydia testing in the past year specifically, and another that asked about testing in the past year for other STIs “like gonorrhea, herpes, or syphilis.” We used the chlamydia testing item as one outcome variable, and also created a second composite outcome variable based on both items for any STI testing, as adolescents may not accurately report receipt of specific STI tests [22]. HIV testing in the past year (outside of blood donation) was based on CAPI items about ever being tested and the date of the last (or most recent) test. Finally, a proxy for STI-related risk assessment was based on four dichotomous ACASI items that assessed whether a doctor or a health-care provider asked about condom use, number of partners, type of sex (vaginal, oral, or anal), and sex of sex partners during the past year. These risk assessment items were added to the female survey beginning in 2013–2015. Participants answering “yes” to any of these items were coded as having had their STI/HIV-related risk assessed.
Socio-demographic covariates included age in years; race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic, other/multi-racial); mother’s highest level of education (<high school, high school or equivalent, some college+); and current insurance status, coded as private, public (Medicaid, Medicare, Children’s Health Insurance Program, other government health care), and other or uninsured (single-service plan, Indian Health Service, not covered). Additional covariates included past year live birth (yes vs. no), given recommendations related to postpartum LARC insertion as well as STI/HIV testing recommendations specific to pregnant women [20,23]; number of sexual partners (1 vs. 2+), given the possibility of risk-based service delivery despite guidelines for screening regardless of risk; and usual source of care (no usual source, family planning or community health clinic, private practice, or other), which is associated with contraceptive type and receipt of preventive services [24,25].
Analyses
We conducted all analyses using SAS-Callable SUDAAN to account for the complex sampling design and yield nationally representative estimates. To describe the analytic sample, we examined characteristics for respondents in each contraceptive category and used chi-square statistics to test for overall differences between groups. Our primary analyses compared prevalence of receiving each service among new and continuing LARC users as compared with respondents in each of the other contraceptive categories. Bivariate chi-square statistics were used to identify overall differences. We conducted unadjusted and adjusted comparisons using logistic regression, with separate models for each outcome. For each logistic model, we compared new LARC users and continuing LARC users to (1) moderately effective method users; (2) less effective method users; and (3) non-contraceptors. We report prevalence ratios because the prevalence of each outcome in this analysis is more than 10%, so odds ratios may overestimate differences in prevalence [26]. Multivariable models controlled for all covariates previously described given theoretical justification and the absence of multicollinearity. For STI and HIV testing outcomes, we also included an indicator distinguishing participants from 2011 to 2013 and from 2013 to 2015 to adjust for potential temporal effects. For the sexual risk assessment outcome assessed only in 2013 to 2015, listwise deletion removed incomplete cases from 2011 to 2013. We tested whether associations varied based on number of partners, yet interaction analyses were not significant so the results are not stratified by this factor.
Results
Among sexually active young women at risk of unintended pregnancy, use of moderately effective methods was most common (43.5%), followed by use of less effective methods (24.3%) and then non-use of contraception (20.9%). LARC use was less common; 4.4% had initiated a LARC method in the past year and 6.9% were continuing users, for a total of 11.3% (Table 1). Overall, mean age of respondents was 20.7 years and the majority were non-Hispanic white (54.6%), had a mother with some college education or higher (55.4%), were privately insured (55.3%), and had a private usual source of care (67.7%). Nearly three-quarters (72.8%) had only one sex partner in the prior year; less than one-tenth (7.5%) had a live birth in the past year.
Table 1.
Sample characteristics by contraceptive type among sexually active adolescent and young adult women 15–24 years, 2011–2015 National Survey of Family Growth
| Overall (n = 2,018) | Continuing LARC (n = 155) %(95%CI) |
New LARC (n = 95) % (95% CI) |
Moderately effective (n = 798) %(95%CI) |
Less effective (n = 489) %(95%CI) |
No contraception (n = 480) %(95%CI) |
p-Valuea | |
|---|---|---|---|---|---|---|---|
| Total | 6.9 (5.5–8.5) | 4.4 (3.3–5.9) | 43.5 (40.4–46.7) | 24.3 (21.6–27.2) | 20.9 (18.6–23.5) | ||
| Age (mean, SE) | 20.7 (.1) | 21.9 (.2) | 21.0 (.2) | 20.7 (.1) | 20.8 (.2) | 19.9 (.2) | p < .001 |
| Race/ethnicity | p < .001 | ||||||
| Whiteb | 54.6 (50.6–58.5) | 46.4 (37.1–55.9) | 52.4 (38.3–66.2) | 64.0 (58.5–69.1) | 50.3 (43.3–57.3) | 43.4 (36.6–50.5) | |
| Blackb | 14.9 (12.7–17.3) | 12.2 (6.7–21.1) | 19.3 (11.6–30.4) | 11.9 (9.1–15.3) | 16.5 (12.9–21.0) | 19.1 (14.8–24.2) | |
| Hispanicc | 21.4 (18.4–24.6) | 33.8 (24.6–44.4) | 18.4 (9.7–32.4) | 14.9 (11.2–19.6) | 23.3 (18.3–29.2) | 29.0 (22.6–36.2) | |
| Other | 9.2 (6.7–12.5) | 7.6 (3.6–15.4) | 9.8 (4.0–22.2) | 9.2 (6.3–13.4) | 9.9 (6.1–15.7) | 8.6 (5.3–13.6) | |
| Mother’s level of education | p = .015 | ||||||
| <High school | 16.5 (14.1–19.2) | 19.0 (12.4–27.9) | 12.6 (7.1–21.5) | 11.9 (9.1–15.3) | 22.3 (16.5–29.4) | 19.4 (14.6–25.3) | |
| High school or equivalent | 28.1 (25.3–31.2) | 34.7 (25.2–45.6) | 31.0 (18.2–47.6) | 25.4 (21.2–30.0) | 29.0 (22.9–36.0) | 30.1 (24.5–36.4) | |
| Some college or higher | 55.4 (51.9–58.8) | 46.3 (36.0–56.9) | 56.4 (41.6–70.1) | 62.8 (57.8–67.4) | 48.7 (41.6–55.9) | 50.5 (43.3–57.6) | |
| Insurance status | p < .001 | ||||||
| No insurance | 17.6 (14.8–20.8) | 26.0 (17.1–37.4) | 15.4 (7.9–27.8) | 10.1 (7.9–13.0) | 23.9 (18.6–30.3) | 23.4 (17.2–31.0) | |
| Public | 27.1 (23.7–30.9) | 29.7 (23.1–37.2) | 46.8 (31.9–62.3) | 22.5 (18.3–27.4) | 28.1 (22.8–34.0) | 30.6 (25.3–36.4) | |
| Private | 55.3 (51.3–59.2) | 44.3 (33.8–55.3) | 37.8 (24.8–52.8) | 67.3 (62.0–72.2) | 47.9 (41.3–54.7) | 46.1 (39.6–52.7) | |
| Usual source of care | p = .002 | ||||||
| No usual source | 19.7 (17.3–22.4) | 20.4 (12.2–32.2) | 15.8 (8.3–28.0) | 14.4 (11.2–18.3) | 24.7 (19.4–30.8) | 25.7 (20.5–31.8) | |
| Usual source FP/community health clinic | 12.5 (10.4–14.9) | 18.7 (10.3–31.5) | 13.6 (8.1–21.7) | 10.6 (7.4–15.0) | 12.6 (9.5–16.4) | 14.1 (11.1–17.9) | |
| Usual source of care private clinic or other | 67.7 (64.4–70.9) | 60.9 (49.2–71.4) | 70.7 (58.9–80.3) | 75.0 (70.3–79.3) | 62.7 (56.8–68.3) | 60.1 (53.8–66.1) | |
| Number of partners, past 12 mo | p = .577 | ||||||
| 1 partner | 72.8 (69.8–75.6) | 74.0 (58.9–85.0) | 79.4 (62.0–90.1) | 73.5 (68.8–77.8) | 69.7 (64.3–74.5) | 73.0 (66.4–78.8) | |
| 2 + partners | 27.2 (24.4–30.2) | 26.0 (15.0–41.1) | 20.6 (9.9–38.0) | 26.5 (22.2–31.2) | 30.3 (25.5–35.7) | 27.0 (21.2–33.6) | |
| Live birth, past year | p < .001 | ||||||
| Yes | 7.5 (5.9–9.4) | 0 (N/A) | 36.7 (22.8–53.2) | 6.0 (4.2–8.4) | 7.4 (5.2–10.4) | 7.1 (4.5–11.2) | |
| No | 92.5 (90.6–94.1) | 100.0 (N/A) | 63.3 (46.8–77.2) | 94.0 (91.6–95.8) | 92.6 (89.6–94.8) | 92.9 (88.8–95.5) |
% = weighted percentages; CI = confidence interval; FP = family planning; LARC = long-acting reversible contraception; mo = months; n = unweighted number; SE = standard error.
Overall chi-square;
non-Hispanic, single race;
includes non-Hispanic other and those reporting multiple race categories.
Respondents in each contraceptive use category differed by socio-demographic factors (Table 1). Continuing LARC users had the highest proportion of young women who were Hispanic (33.8%) and uninsured (26.0%) and lowest proportion who had mothers with at least some college education (46.3%). New LARC users had the highest proportion of publically insured participants (46.8%). Moderately effective method users were predominately non-Hispanic white (64.0%), had a mother with some college education or higher (62.8%), were privately insured (67.3%), and used a private clinic or some type of facility that was not a family planning or community health clinic (75.0%) as their usual source of care. About one-quarter of less effective method users (24.7%) and non-contraceptors (25.7%) did not have a usual source of care. Across the contraceptive methods, most respondents had only one partner in the prior year. Nearly two-fifths of new LARC users (36.7%) had a live birth in the past year compared with less than one-tenth of moderately (6.0%) and less effective method users (7.4%) and non-contraceptors (7.1%).
Receipt of most sexual health-related services was low (Figure 1). Overall, only 40.9% of all respondents reported being tested for chlamydia, 47.3% had any STI test, and 25.9% had an HIV test in the prior year. About two-thirds (66.5%) received any sexual risk assessment. Receipt of each service varied across the categories of contraceptive method users (p < .05 for each service) and was generally highest among new LARC users and moderately effective method users and lowest among less effective method users and non-contraceptors.
Figure 1.
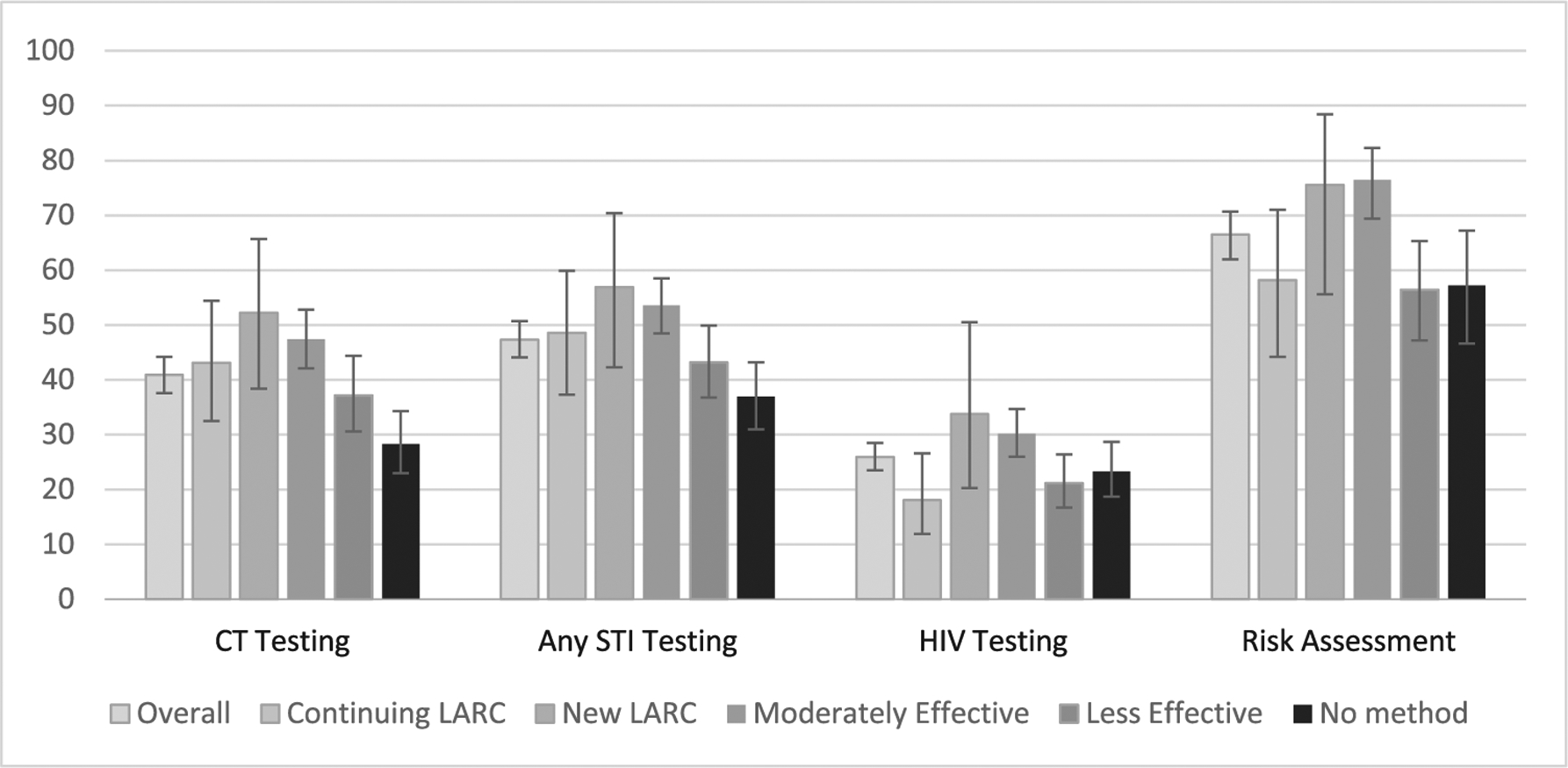
Sexual health services by contraceptive type among sexually active adolescent and young adult women 15–24 years, 2011–2015 National Survey of Family Growth.
CT = chlamydia; LARC = long-acting reversible contraception; STI = sexually transmitted infection
Unadjusted and adjusted analyses are presented in Table 2. Despite the pattern across most outcomes for continuing LARC users to have lower receipt of services than moderately effective method users, we detected few differences. In unadjusted models, continuing LARC users were less likely than moderately effective method users to receive HIV testing and sexual risk assessment, but in multivariable models only the association with HIV testing remained (adjusted prevalence ratio [aPR] = .52, 95% confidence interval [CI] = .32–.85). Conversely, new, but not continuing, LARC users were more likely than less effective method users to be tested for chlamydia and have their sexual risk assessed, although only the association with sexual risk assessment remained in multivariable analyses (aPR = 1.35, 95% CI = 1.03–1.76). Similarly, new, but not continuing, LARC users were more likely than non-contraceptors to have received sexual risk assessment (aPR = 1.43, 95% CI = 1.11–1.85). However, both new and continuing LARC users were more likely than non-contraceptors to be tested for chlamydia (new: aPR = 1.52, 95% CI = 1.08–2.15; continuing: aPR = 1.69, 95% CI = 1.24–2.29).
Table 2.
Logistic regression models of STI/HIV services by contraceptive type among sexually active adolescent and young adult women 15–24 years, 2011–2015 National Survey of Family Growth
| Chlamydia testing | Any STI testing | HIV testing | Sexual risk assessment (2013–2015 only) | |||||
|---|---|---|---|---|---|---|---|---|
| PR (95% CI) (n = 2,005) |
aPR (95% CI) (n = 1,976) |
PR (95% CI) (n = 2,007) |
aPR (95% CI) (n = 1,978) |
PR (95% CI) (n = 2,017) |
aPR (95% CI) (n = 1,988) |
PR (95% CI) (n = 971) |
aPR (95% CI) (n = 950) |
|
| LARC versus moderately effective | ||||||||
| Continuing LARC | .91 (.68–1.21) | .88 (.65–1.20) | .91 (.70–1.17) | .84 (.64–1.12) | .60 (.39–.93) | .52 (.32–.85) | .76 (.60–.97) | .78 (.61–1.01) |
| New LARC | 1.10 (.82–1.47) | .98 (.73–1.30) | 1.06 (.81–1.39) | .89 (.67–1.20) | 1.12 (.69–1.82) | .88 (.50–1.54) | .99 (.79–1.24) | 1.01 (.80–1.26) |
| LARC versus less effective | ||||||||
| Continuing LARC | 1.16 (.85–1.57) | 1.17 (.87–1.59) | 1.12 (.85–1.48) | 1.11 (.83–1.48) | .86 (.54–1.36) | .84 (.50–1.40) | 1.03 (.76–1.40) | 1.05 (.78–1.41) |
| New LARC | 1.40 (1.04–1.90) | 1.30 (.95–1.79) | 1.32 (.99–1.75) | 1.18 (.86–1.61) | 1.60 (.96–2.64) | 1.41 (.81–2.46) | 1.34 (1.01–1.78) | 1.35 (1.03–1.76) |
| LARC versus no contraception | ||||||||
| Continuing LARC | 1.52 (1.09–2.12) | 1.52 (1.08–2.15) | 1.32 (.99–1.74) | 1.29 (.95–1.75) | .78 (.48–1.25) | .77 (.45–1.31) | 1.02 (.74–1.41) | 1.11 (.80–1.55) |
| New LARC | 1.84 (1.34–2.53) | 1.69 (1.24–2.29) | 1.54 (1.16–2.06) | 1.37 (1.00–1.87) | 1.45 (.86–2.43) | 1.30 (.73–2.32) | 1.32 (1.02–1.72) | 1.43 (1.11–1.85) |
Adjusted models include age, race/ethnicity, insurance status, mother’s highest level of education, usual source of care, live birth in the past year, number of partners, and data collection period. Bold findings indicate confidence interval does not overlap with 1.
aPR = adjusted prevalence ratio; CI = confidence interval; LARC = long-acting reversible contraception; PR = prevalence ratio.
Discussion
Prior research suggests that condom use may be lower in adolescents using LARC methods than in adolescents using oral contraceptives [11]. Given the possibility that LARC use could also affect receipt of STI/HIV services, we used nationally representative data to consider associations between contraceptive method type and receipt of STI/HIV-related services among sexually active adolescents and young women. Receipt of STI/HIV-related services for both new and continuing LARC users was largely similar to receipt of these services among adolescents and young women using moderately effective contraceptive methods that typically involve annual clinic visits. Compared with less effective method users that do not require routine visits and non-contraceptors, there were some differences. New, but not continuing, LARC users were more likely than less effective method users and non-contraceptors to have their sexual risk assessed, although both groups were more likely than non-contraceptors to be tested for chlamydia.
Overall, self-reported receipt of STI/HIV services appears suboptimal given recommendations for annual STI screening and sexual risk assessment and routine HIV testing among young women [20,21]. Prevalence was low even among new LARC and moderately effective method users, suggesting a missed opportunity for providing STI services to sexually active young women who presumably are accessing care for contraception. A study using 2002 NSFG data reached a similar conclusion, as only 35% of young women who had received a contraceptive service in the past year also received any STI-related service [27].
We found little evidence that LARC users as compared with moderately effective method users were less likely to receive STI testing or sexual risk assessment. These results align with the studies previously mentioned that considered associations between contraceptive type and service utilization/receipt. One study from the 1990s found no difference in frequency of clinic visits between a small sample of implant and oral contraceptive users [17]. A more recent analysis of data from the Contraceptive CHOICE Project found that receipt of STI screening among sexually active young women did not differ between LARC users and non-LARC users, although the authors acknowledge that the availability of home-based testing in the study could have minimized differences [16]. Future monitoring will be important if LARC use continues to increase among young women [6,7]. Our finding that continuing LARC users were less likely than moderately effective method users to have received HIV testing in the past year is of some concern, although given that screening for HIV is not necessarily recommended annually [20], it is possible that HIV testing was not indicated. Larger samples of LARC users may reveal differences in the receipt of other STI/HIV services that warrant attention.
Differences between new and continuing LARC users and individuals who were not using a method requiring a clinic visit also revealed a pattern that may warrant attention. Both new and continuing LARC users were more likely than non-contraceptors to have had chlamydia testing. However, only new LARC users were more likely than non-contraceptors to be tested for any STI and more likely than both non-contraceptors and less effective method users to have received sexual risk assessment. We did not detect differences in any STI testing and sexual risk assessment among continuing LARC users relative to less effective method users or non-contraceptors. These findings suggest that initiating LARC, like returning to a clinic for annual prescription renewal, may offer an opportunity to receive STI-related services, yet this potential benefit may not persist as LARC use continues.
Several more general yet notable findings regarding adolescent and young adult use of LARC emerged from our analysis. As documented previously [6,7], LARC use among adolescents and young women is still low, reinforcing the importance of efforts to increase awareness of and access to these highly effective contraceptive methods (e.g., provider training, provision at no cost) [28,29]. Additionally, we found the sociodemographic profile of young LARC users aligns with prior research, in that LARC use was more common among Hispanic women and those who are publically insured or uninsured [30,31]. The latter finding may reflect receipt of contraceptive care at Title X clinics where concerted efforts to reduce barriers to LARC have been made [32]. We also found that nearly one-third of new LARC users had a live birth in the past year compared with only 7.5% of young sexually active women overall, likely reflecting postpartum LARC insertion to avoid rapid repeat pregnancy and highlighting the importance of initiatives to reduce barriers to accessing LARC immediately postpartum [23,33,34]. Finally, it is promising that we observed no differences in STI testing (or other services) between new LARC users and moderately effective method users who likely accessed care in the prior year. Recommendations from the American College of Obstetricians and Gynecologists and CDC advise providers to insert intrauterine devices without routine STI screening prior [35,36], and increased likelihood of testing among new LARC users relative to other contraceptive users accessing care would suggest poor adherence to these guidelines.
This study has limitations and strengths. Because LARC use remains low among young women we may have had insufficient power to detect differences. Nonetheless, this study highlights the importance of this issue and provides a starting point for future monitoring. With larger samples of LARC users, it may be feasible to explore nuances of the associations, including effect modification by usual source of care and sexual risk. Research to understand whether continuing LARC use is associated with decreased likelihood of any clinical visit, a measure not currently assessed by NSFG, would also be useful. The data are also self-reported, and adolescents’ knowledge of STI testing history may be limited or they may be unwilling to disclose, although measurement via ACASI may minimize social desirability bias and improve validity of these measures [37]. Because the data are cross sectional, we cannot make causal inference about the impact of LARC use on receipt of services. Additionally, the cross-sectional design, along with our reliance on current contraceptive use, did not allow us to account for method switching; current users of less effective methods or no method may have been using methods that require clinic visits within the prior year. That said, we were able to use the calendar history of contraceptive use to distinguish new and continuing LARC users, which was particularly important for addressing our research question. Additionally, the data are from a nationally representative sample; the findings are thus generalizable to sexually active U.S. adolescent and young adult women 15–24 years of age at risk of unintended pregnancy.
As LARC use increases among adolescents and young women, it will be important to monitor receipt of STI/HIV services. Further, studies that examine incident STI diagnoses by contraceptive type are needed to fully understand the broader impact of LARC use. The current study and these future directions address STI/HIV-related outcomes in addition to condom use, which has typically been the focus of research on contraceptive use and STI prevention. In general, a broader set of outcomes, including STI/HIV-related services, should be considered when exploring the STI prevention implications of other innovations related to increasing access to contraception (e.g., provision of oral contraception without a prescription).
Examining potential unintended consequences of providing LARC should not deter public health and clinical efforts to increase access to these highly effective methods. Understanding how LARC and STI/HIV service use are related can potentially inform practice strategies to prevent or minimize any adverse effects. Although we did not see differences in STI testing and risk assessment between continuing LARC and moderately effective method users, it may still be useful to address the need for routine preventive care, including STI/HIV services, in counseling and health education about LARC, whether clinic-based or via broader health promotion efforts.
More broadly, low receipt of services overall suggests a need to integrate STI/HIV prevention with efforts to provide quality family planning services. Provider training related to effective implementation of relevant clinical guidelines may be particularly useful. Beyond training, systems-level innovations will likely be important given limited time and competing priorities during family planning and health maintenance visits. A multi-component intervention involving patient education and provider training to integrate unintended pregnancy and STI prevention is currently being evaluated and could serve as a programmatic model if effective [38]. Electronic medical record reminders have also been shown to facilitate integrated delivery of family planning and STI services [39]. Implementing such strategies to provide comprehensive service delivery could help ensure that the advent and implementation of new prevention approaches for one outcome, in this case LARC methods for pregnancy prevention, promote overall sexual and reproductive health.
IMPLICATIONS AND CONTRIBUTIONS.
This study extends what is known about the STI/HIV prevention implications of LARC use among young women. Ongoing monitoring of differences in service receipt by contraceptive type may inform strategies for integrating STI/HIV prevention with efforts to increase awareness of and access to LARC methods.
Acknowledgments
We would like to thank Gladys Martinez, Ph.D., with the National Center for Health Statistics at the Centers for Disease Control and Prevention, for providing technical assistance.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- [1].Bearinger LH, Resnick MD. Dual method use in adolescents: A review and framework for research on use of STD and pregnancy protection. J Adolesc Health 2003;32:340–9. [DOI] [PubMed] [Google Scholar]
- [2].Cates W Jr. Sexually transmitted diseases and family planning. Strange or natural bedfellows, revisited. Sex Transm Dis 1993;20:174–8. [DOI] [PubMed] [Google Scholar]
- [3].Gavin L, Moskosky S, Carter M, et al. Providing quality family planning services: Recommendations of CDC and the U.S. Office of Population Affairs. MMWR Recomm Rep 2014;63:1–54. [PubMed] [Google Scholar]
- [4].Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med 2016;374:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2008. Sex Transm Dis 2013;40:187–93. [DOI] [PubMed] [Google Scholar]
- [6].Pazol K, Daniels K, Romero L, et al. Trends in long-acting reversible contraception use in adolescents and young adults: New estimates accounting for sexual experience. J Adolesc Health 2016;59:438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Abma JC, Martinez GM. Sexual activity and contraceptive use among teenagers in the United States, 2011–2015. Natl Health Stat Report 2017;(104):1–23. Hyattsville, MD: National Center for Health Statistics. 2017. [PubMed] [Google Scholar]
- [8].Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group, The American College of Obstetricians and Gynecologists. Committee Opinion No. 39: Adolescents and long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol 2012;120:983–8. [DOI] [PubMed] [Google Scholar]
- [9].Committee on Adolescence. Contraception for adolescents. Pediatrics 2014;134:e1244–56. [DOI] [PubMed] [Google Scholar]
- [10].Society for Adolescent Health and Medicine. Improving knowledge about, access to, and utilization of long-acting reversible contraception among adolescents and young adults. J Adolesc Health 2017;60:472–4. [DOI] [PubMed] [Google Scholar]
- [11].Steiner RJ, Liddon N, Swartzendruber AL, et al. Long-acting reversible contraception and condom use among female U.S. high school students: Implications for sexually transmitted infection prevention. JAMA Pediatr 2016;170:428–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Warner L, Williams L, Le B, et al. Dual use of condoms with long-acting reversible contraception (LARC) versus moderately effective methods among teen mother participating in the pregnancy risk assessment monitoring system (PRAMS). National STD Prevention Conference. Atlanta, GA. 2016. [Google Scholar]
- [13].Hall KS, Patton EW, Crissman HP, et al. A population-based study of US women’s preferred versus usual sources of reproductive health care. Am J Obstet Gynecol 2015;213:352e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Crissman HP, Hall KS, Patton EW, et al. U.S. women’s intended sources for reproductive health care. J Womens Health (Larchmt) 2016;25:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Frost JJ, Gold RB, Bucek A. Specialized family planning clinics in the United States: Why women choose them and their role in meeting women’s health care needs. Womens Health Issues 2012;22:e519–25. [DOI] [PubMed] [Google Scholar]
- [16].Skala SL, Secura GM, Peipert JF. Factors associated with screening for sexually transmitted infections. Am J Obstet Gynecol 2012;206:e321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Polaneczky M, Slap G, Forke C, et al. The use of levonorgestrel implants (Norplant) for contraception in adolescent mothers. N Engl J Med 1994;331:1201–6. [DOI] [PubMed] [Google Scholar]
- [18].Centers for Disease Control and Prevention. Use Data File Documentation, 2011–2013 National Survey of Family Growth: User’s Guide. Available at: http://www.cdc.gov/nchs/data/nsfg/NSFG_2011-2013_UserGuide_MainText.pdf.
- [19].Sundaram A, Vaughan B, Kost K, et al. Contraceptive failure in the United States: Estimates from the 2006–2010 National Survey of Family Growth. Perspect Sex Reprod Health 2017;49:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee KC, Ngo-Metzger Q, Wolff T, et al. Sexually transmitted infections: Recommendations from the U.S. Preventive Services Task Force. Am Fam Physician 2016;94:907–15. [PubMed] [Google Scholar]
- [21].Hagan JF, Shaw JS, Duncan PM. Bright futures: Guidelines for health supervision of infants, children, and adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017. [Google Scholar]
- [22].Goodman KBC, Perasud P, Delnevo C. Patient knowledge of sexually transmitted disease (STD) testing in an urban clinic: A comparison of patient perceived testing and actual tests performed. National STD Prevention Conference. Minneapolis, MN. 2012. [Google Scholar]
- [23].Committee on Obstetric Practice, The American College of Obstetricians and Gynecologists. Committee opinion no. 670: Immediate postpartum long-acting reversible contraception. Obstet Gynecol 2016;128:e32–7. [DOI] [PubMed] [Google Scholar]
- [24].Blewett LA, Johnson PJ, Lee B, et al. When a usual source of care and usual provider matter: Adult prevention and screening services. J Gen Intern Med 2008;23:1354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Groskaufmanis L, Masho SW. Source of care and variation in long-acting reversible contraception use. Fertil Steril 2016;105:401–9. [DOI] [PubMed] [Google Scholar]
- [26].Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690–1. [DOI] [PubMed] [Google Scholar]
- [27].Farr SL, Kraft JM, Warner L, et al. The integration of STD/HIV services with contraceptive services for young women in the United States. Am J Obstet Gynecol 2009;201:e141–8. [DOI] [PubMed] [Google Scholar]
- [28].Harper CC, Rocca CH, Thompson KM, et al. Reductions in pregnancy rates in the USA with long-acting reversible contraception: A cluster randomised trial. Lancet 2015;386:562–8. [DOI] [PubMed] [Google Scholar]
- [29].Secura GM, Madden T, McNicholas C, et al. Provision of no-cost, long-acting contraception and teenage pregnancy. N Engl J Med 2014;371:1316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among U.S. women, 2009–2012. Obstet Gynecol 2015;126:917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Daniels K, Daugherty J, Jones J, Mosher W. Current contraceptive use and variation by selected characteristics among women aged 15–44: United States, 2011–2013. Natl Health Stat Report 2015;86:1–14. [PubMed] [Google Scholar]
- [32].Romero L, Pazol K, Warner L, et al. Vital signs: Trends in use of long-acting reversible contraception among teens aged 15–19 years seeking contraceptive services-United States, 2005–2013. MMWR Morb Mortal Wkly Rep 2015;64:363–9. [PMC free article] [PubMed] [Google Scholar]
- [33].American Congress of Obstetricians and Gynecologists. Medicaid Reimbursement for Postpartum LARC by State. Available at: https://www.acog.org/About-ACOG/ACOG-Departments/Long-Acting-Reversible-Contraception/Immediate-Postpartum-LARC-Medicaid-Reimbursement. Accessed July 29, 2017.
- [34].Association of State and Territorial Health Officials. Increasing Access to Contraception: LARC Immediately Postpartum Learning Community. Available at: http://www.astho.org/Programs/Maternal-and-Child-Health/Increasing-Access-to-Contraception/. Accessed July 29, 2017.
- [35].Committee on Practice Bulletins–Gynecology, Long-Acting Reversible Contraception Workgroup. The American College of Obstetricians and Gynecologists. Practice bulletin no. 186: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol 2017;130:e251–69. [DOI] [PubMed] [Google Scholar]
- [36].Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep 2016;65:1–66. [DOI] [PubMed] [Google Scholar]
- [37].Turner CF, Ku L, Rogers SM, et al. Adolescent sexual behavior, drug use, and violence: Increased reporting with computer survey technology. Science 1998;280:867–73. [DOI] [PubMed] [Google Scholar]
- [38].Ewing AC, Kottke MJ, Kraft JM, et al. 2GETHER—the dual protection project: Design and rationale of a randomized controlled trial to increase dual protection strategy selection and adherence among African American adolescent females. Contemp Clin Trials 2017;54:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shlay JC, McEwen D, Bell D, et al. Integration of family planning services into a sexually transmitted disease clinic setting. Sex Transm Dis 2013;40:669–74. [DOI] [PubMed] [Google Scholar]


