Abstract
We have discovered and synthesized a series of indole-based derivatives as novel sigma-2 (σ2) receptor ligands. Two ligands with high σ2 receptor affinity and subtype selectivity were then radiolabeled with F-18 in good radiochemical yields and purities, and evaluated in rodents. In biodistribution studies in male ICR mice, radioligand [18F]9, or 1-(4-(5,6-dimethoxyisoindolin-2-yl)butyl)-4-(2-[18F]fluoroethoxy)-1H-indole, was found to display high brain uptake and high brain-to-blood ratio. Pretreatment of animals with the selective σ2 receptor ligand CM398 led to significant reductions in both brain uptake (29%–54%) and brain-to-blood ratio (60%–88%) of the radioligand in a dose-dependent manner, indicating high and saturable specific binding of [18F]9 to σ2 receptors in the brain. Further, ex vivo autoradiography in male ICR mice demonstrated regionally heterogeneous specific binding of [18F]9 in the brain that is consistent with the distribution pattern of σ2 receptors. Dynamic positron emission tomography imaging confirmed regionally distinct distribution and high levels of specific binding for [18F]9 in the rat brain, along with appropriate tissue kinetics. Taken together, results from our current study indicated the novel radioligand [18F]9 as the first highly specific and promising imaging agent for σ2 receptors in the brain.
KEY WORDS: Indole-based derivatives, σ2 receptor, Fluorine-18, Positron emission tomography, Neuroimaging
Graphical abstract
Radioligand [18F]9 possesses favorable characteristics including nanomolar affinity, high selectivity, appropriate kinetics, and the highest levels of brain uptake, brain-to-blood ratio, and specific binding for neuroimaging of the σ2 receptors.
1. Introduction
Malfunctions in cholesterol homeostasis and lipid metabolism are involved in various human diseases1, 2, 3, 4, 5, 6, 7. Recently, the sigma-2 (σ2) receptor has been identified as transmembrane protein 97 (TMEM97)8, which is also named meningioma-associated protein (MAC30) and suggested to be a cholesterol-regulating gene9. Accordingly, much attention has been paid to the mechanism and role of the σ2 receptor/TMEM97 in regulating cholesterol homeostasis and its implications in diseases2,10,11. The σ2 receptor/TMEM97 was shown to have approximately 10-fold higher expression in proliferating than quiescent tumors12, 13, 14, indicating the σ2 receptor/TMEM97 as a biomarker for the proliferative status of solid tumors. TMEM97, progesterone receptor membrane component 1 (PGRMC1) and low-density lipoprotein receptor (LDLR) were reported to form a trimeric complex in HeLa cells. This complex accelerates the internalization of LDL in proliferating versus quiescent tumor cells15, which is in good agreement with the up- and down-regulation of the σ2 receptor/TMEM97 in 66P and 66Q cells14. Moreover, the TMEM97/PGRMC1/LDLR complex is a binding site for monomeric and oligomeric amyloid β-peptide (1–42) (Aβ1‒42)16. Inhibition of one of these proteins results in the disruption of the TMEM97/PGRMC1/LDLR complex, leading to decreased uptake of Aβ1‒42 in primary neurons16. Hence the σ2 receptor/TMEM97 is also considered a therapeutic target for Alzheimer's disease (AD). For example, CT1812, a σ2 receptor antagonist, is reported to prevent the binding of Aβ oligomers to neuronal receptors and thus holds potential as a novel drug for the treatment of AD17, 18, 19, 20. The availability of a radioligand for neuroimaging of the σ2 receptor/TMEM97 in the human brain will make it possible to investigate this target in AD progression, and to elucidate the treatment mechanism of CT1812 in clinical trials.
During the last decades, efforts in the development of σ2 receptor radioligands have been largely directed toward in vivo imaging of tumors, where upregulation of σ2 receptor is found21, 22, 23. Currently, N-(4-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)butyl)-2-(2-fluoroethyl)-5-methylbenzamide ([18F]ISO-1) is the only σ2 receptor radiotracer used in humans for tumor imaging24, 25, 26. However, it is not suitable to investigate σ2 receptors in AD due to its low brain uptake27. With the aim to develop radioligands with appropriate affinity for the σ2 receptor, high selectivity, high uptake, favorable kinetics and high specific binding in the brain, we chose compound 1 with nanomolar affinity and favorable selectivity for σ2 receptor as the lead compound and introduced an methoxy or fluoroethoxy group at the different positions of the indole ring to enable the incorporation of 11C or 18F for PET imaging (Fig. 1)28. Previous structure–activity relationship studies showed that ligands with a four-carbon chain between the indole ring and the 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline or 5,6-dimethoxyisoindoline moiety possessed high σ2 receptor affinity and selectivity29. Two radioligands, [18F]8 and [18F]11, were synthesized and evaluated in rodents, which showed [18F]11, with a 6-[18F]fluoroethoxy group at the indole ring and a 5,6-dimethoxyisoindoline moiety, displayed relatively good brain uptake but only moderate specific binding to the σ2 receptors in vivo. In an effort to search for radioligands with optimized pharmacokinetic and in vivo binding properties, we further investigated the effects of the [18F]fluoroethoxy group at different positions of the indole ring. Herein we report the synthesis of these indole-based derivatives and evaluation of two new 18F-labeled radiotracers, [18F]9 and [18F]10, for their potential to image σ2 receptors in the brain.
Figure 1.
Design concept of indole-based derivatives as new σ2 ligands.
2. Results
2.1. Chemical synthesis
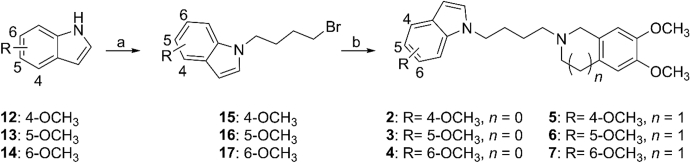
The synthetic routes for target compounds 2–7 are illustrated in Scheme 1. Based on the method reported previously28, N-alkylation of compounds 12–14 with 1,4-dibromobutane led to intermediates 15–17. Reaction with 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline or 5,6-dimethoxyisoindoline then provided target compounds 2–7. Compounds 8–11 has been reported previously29.
Scheme 1.
Synthetic route for target compounds with a methoxy group at the indole ring. Reagents and conditions: (a) DMF, 1,4-dibromobutane, KOH, TBAF (1 mol/L in THF), r.t., 2 h, 52%–72%; (b) acetonitrile, triethylamine, K2CO3, 5,6-dimethoxyisoindoline/6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline, 90 °C, 5 h, 55%–77%.
2.2. In vitro binding assays
Compounds 2–7 were assayed for their in vitro binding properties as previously reported30,31. The results are provided in Table 1. Introduction of methoxy group at the indole ring retained the nanomolar affinity for σ2 receptors [Ki (σ2) = 4.40–9.46 nmol/L] and moderate subtype selectivity [Ki (σ1)/Ki (σ2) = 7–102]. Compounds with 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline displayed higher subtype selectivity towards σ2 receptors than those with a 5,6-dimethoxyisoindoline moiety (compare 2–4 to 5–7).
Table 1.
In vitro binding properties of the indole-based ligandsa.
| Compd. | Ki (σ1) (nmol/L) | Ki (σ2) (nmol/L) | Ki (σ1)/Ki (σ2) |
|---|---|---|---|
| 2 | 149 ± 67 | 6.30 ± 2.00c | 24 |
| 3 | 190 ± 97 | 8.91 ± 1.40 | 21 |
| 4 | 70 ± 14 | 9.46 ± 1.20c | 7 |
| 5 | 259 ± 23 | 7.73 ± 4.49c | 33 |
| 6 | 450 ± 89 | 4.40 ± 1.43c | 102 |
| 7 | 357 ± 94 | 8.68 ± 1.99 | 41 |
| 8b | 1698 ± 548 | 2.40 ± 0.58 | 708 |
| 9b | 371 ± 105 | 1.79 ± 0.86 | 207 |
| 10b | 187 ± 1.41 | 3.27 ± 0.19 | 57 |
| 11b | 376 ± 351 | 2.63 ± 0.48 | 143 |
Values are the means ± standard deviation (SD) of at least two independent experiments, each done in triplicate.
From Ref. 29.
n = 2.
In general, compounds with a fluoroethoxy substituent (8–11) possessed higher affinity and subtype selectivity than those with a methoxy group (2–7). Therefore, compounds with the methoxy substituent were not pursued for further studies. Instead, we radiofluorinated the two compounds in the fluoroethoxy substituted series, i.e., [18F]9 and [18F]10 with high affinity and selectivity for σ2 receptors, to investigate the effects of the [18F]fluoroethoxy position at the indole ring on in vivo properties and evaluate their potential as σ2 receptor neuroimaging agents.
2.3. Radiochemistry
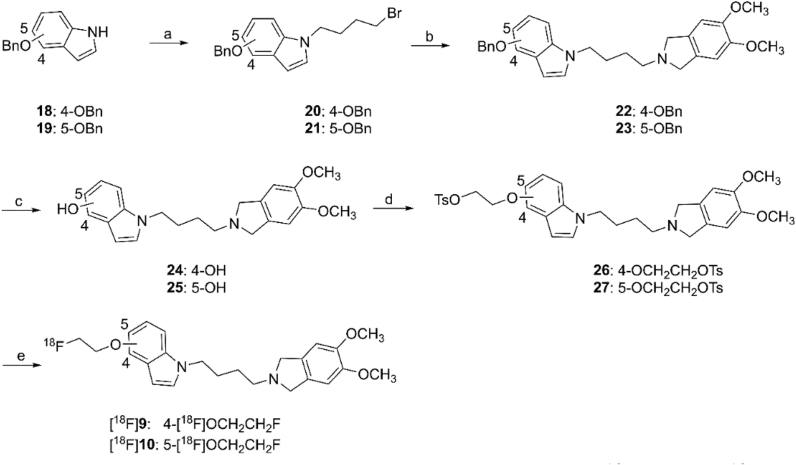
Synthesis of the radiolabeling precursors and radiosynthesis of radioligands [18F]9 and [18F]10 are depicted in Scheme 2. Intermediates 20 and 21 were obtained by reaction of 4-(benzyloxy)-1H-indole (18) or 5-(benzyloxy)-1H-indole (19) with 1,4-dibromobutane under basic conditions. N-Alkylation of 5,6-dimethoxyisoindoline with 20 and 21 provided 22 and 23, respectively. Debenzylation in the presence of H2 and 10% Pd on activated carbon as catalyst provided compounds 24 and 25. Further reaction with ethane-1,2-diyl bis(4-methylbenzenesulfonate) afforded compounds 26 and 27 as radiolabeling precursors.
Scheme 2.
Synthesis of precursors 26 and 27 and radiosynthesis of [18F]9 and [18F]10. Reagents and conditions: (a) DMF, 1,4-dibromobutane, KOH, 0 °C–r.t., 2 h, 47%–64%; (b) 5,6-dimethoxyisoindoline, acetonitrile, K2CO3, triethylamine, 90 °C, 2.5 h, 51%–79%; (c) H2, Pd/C, CH3OH, 50 °C, 63%–74%; (d) ethane-1,2-diyl bis(4-methylbenzenesulfonate), acetonitrile, KOH, 90 °C, 20 min, for 26, 59%; for 27, 17%; (e) [18F]F−, Kryptofix 2.2.2, K2CO3, CH3CN, 100 °C, 5 min.
Different from the synthetic methods previously described for [18F]8 and [18F]11, we employed for the radiosynthesis of [18F]9 and [18F]10 an efficient one-step SN2 reaction with [18F]fluoride as reported in the literature32, with retention times of 16.06 and 16.16 min on the chromatograms from semipreparative radio-high performance liquid chromatography (radio-HPLC, Supporting Information Fig. S1), radiochemical yields (RCYs) of 36%–50% (n = 10, decay-corrected) and 20%–29% (n = 5, decay-corrected), molar activity of 29–151 GBq/μmol (n = 10) and 55–72 GBq/μmol (n = 5), respectively, for [18F]9 and [18F]10, and radiochemical purity (RCP) of >99%. The total synthesis time was about 60 min. The RCYs for [18F]9 and [18F]10 from the one-step radiosynthesis were considerably higher than those for [18F]8 (10%–15%) and [18F]11 (16%–21%) prepared from a two-step procedure reported previously29.
Identity of the radioligand [18F]9 or [18F]10 was confirmed by co-injection with the corresponding reference compound 9 or 10 into an analytical HPLC system. The chromatograms are shown in Supporting Information Fig. S2, indicating co-elution of the labeled and unlabeled compounds. The retention times were 8.53 and 8.61 min for 9 and [18F]9, and 8.19 and 8.31 min for 10 and [18F]10, respectively, with the slight differences in retention times accounted for the distance between the UV and gamma detectors of the HPLC system.
2.4. In vitro and in vivo assessments
2.4.1. In vitro stability
Radioligands [18F]9 and [18F]10 were incubated in saline at ambient temperature or in mouse serum at 37 °C for 2 h to determine their stability in vitro. The RCP remained at >99% as shown in Supporting Information Fig. S3, suggesting high stability of [18F]9 and [18F]10 in vitro.
2.4.2. Lipophilicity
Radioligands [18F]9 and [18F]10 were partitioned between 1-octanol and 0.05 mol/L potassium phosphate buffer (pH 7.4) to measure their logD7.4, which were 2.43 ± 0.01 (n = 3) and 2.29 ± 0.01 (n = 3), respectively, for [18F]9 and [18F]10, well within the optimal range for good blood‒brain barrier (BBB) permeability33.
2.4.3. Biodistribution and blocking experiments in mice
Biodistribution experiments were performed in male ICR mice. The results are presented in Table 2, Table 3. Initial uptake in the brain, at 2 min post-injection (p.i.), was observed to be higher for [18F]9 (4.44 ± 0.59% ID/g) than [18F]10 (3.76 ± 0.28% ID/g). Ligand [18F]9 showed the highest uptake in the brain (5.04 ± 0.67% ID/g) at 30 min, indicating a slow clearance. On the other hand, [18F]10 showed relatively fast brain washout, with level of uptake at 120 min only 42% of that at 2 min. The blood level of [18F]9 was low with 0.48 ± 0.08% ID/g at 30 min and 0.42 ± 0.05% ID/g at 60 min p.i., which led to high brain-to-blood ratios of 10.6 at both time points. The highest brain-to-blood ratio (3.13) was seen at 15 min for [18F]10.
Table 2.
Biodistribution of [18F]9 in micea.
| Organ | 2 min | 15 min | 30 min | 60 min | 120 min |
|---|---|---|---|---|---|
| Blood | 2.39 ± 0.36 | 0.70 ± 0.08 | 0.48 ± 0.08 | 0.42 ± 0.05 | 0.42 ± 0.03 |
| Brain | 4.44 ± 0.59 | 4.95 ± 0.36 | 5.04 ± 0.67 | 4.43 ± 0.39 | 3.41 ± 0.13 |
| Heart | 21.57 ± 1.58 | 11.23 ± 0.91 | 7.55 ± 1.30 | 3.49 ± 0.62 | 2.76 ± 0.41 |
| Liver | 3.24 ± 0.67 | 6.13 ± 0.94 | 8.69 ± 0.44 | 10.97 ± 0.95 | 11.9 ± 1.52 |
| Spleen | 5.01 ± 1.80 | 7.51 ± 1.17 | 9.93 ± 1.43 | 11.35 ± 2.85 | 10.51 ± 0.37 |
| Lung | 108.47 ± 20.11 | 36.37 ± 6.47 | 17.93 ± 2.42 | 9.95 ± 1.62 | 7.53 ± 1.07 |
| Kidney | 18.89 ± 2.48 | 18.40 ± 2.45 | 20.61 ± 1.80 | 17.40 ± 3.97 | 12.36 ± 0.81 |
| Pancreas | 8.74 ± 0.29 | 12.75 ± 2.12 | 18.93 ± 2.95 | 22.66 ± 3.36 | 24.21 ± 1.57 |
| Small intestineb | 6.21 ± 0.81 | 7.27 ± 0.88 | 10.80 ± 0.36 | 10.73 ± 1.27 | 10.88 ± 0.38 |
| Stomachb | 1.21 ± 0.14 | 1.52 ± 0.19 | 1.54 ± 0.20 | 1.39 ± 0.27 | 1.22 ± 0.13 |
| Muscle | 4.38 ± 0.99 | 3.74 ± 0.33 | 3.90 ± 0.23 | 3.01 ± 0.51 | 3.01 ± 0.27 |
| Bone | 2.59 ± 0.66 | 3.02 ± 0.45 | 4.50 ± 0.38 | 4.64 ± 1.57 | 5.57 ± 0.53 |
| Brain/Blood | 1.87 ± 0.13 | 7.21 ± 1.36 | 10.57 ± 1.86 | 10.56 ± 0.83 | 8.11 ± 0.37 |
Data shown are percentage of injected dose per gram of tissue (% ID/g), means ± SD, n = 5.
% ID/organ.
Table 3.
Biodistribution of [18F]10 in micea.
| Organ | 2 min | 15 min | 30 min | 60 min | 120 min |
|---|---|---|---|---|---|
| Blood | 2.77 ± 0.10 | 1.19 ± 0.09 | 1.19 ± 0.17 | 1.46 ± 0.12 | 1.89 ± 0.18 |
| Brain | 3.76 ± 0.28 | 3.70 ± 0.26 | 2.68 ± 0.27 | 1.69 ± 0.16 | 1.58 ± 0.16 |
| Heart | 15.47 ± 1.37 | 3.54 ± 0.13 | 2.36 ± 0.26 | 2.03 ± 0.21 | 2.31 ± 0.16 |
| Liver | 7.42 ± 1.66 | 14.65 ± 2.01 | 18.09 ± 1.16 | 18.32 ± 1.60 | 14.57 ± 2.33 |
| Spleen | 6.18 ± 1.09 | 11.32 ± 1.27 | 8.69 ± 0.95 | 6.13 ± 0.29 | 4.31 ± 0.50 |
| Lung | 61.17 ± 6.95 | 14.05 ± 3.06 | 7.10 ± 1.12 | 5.18 ± 0.83 | 3.41 ± 0.51 |
| Kidney | 19.00 ± 1.41 | 14.96 ± 1.49 | 8.75 ± 0.97 | 6.18 ± 1.02 | 3.94 ± 0.57 |
| Pancreas | 11.70 ± 2.14 | 23.72 ± 2.35 | 34.10 ± 3.60 | 39.66 ± 2.95 | 43.39 ± 6.87 |
| Small intestineb | 7.47 ± 0.93 | 12.37 ± 0.25 | 15.81 ± 1.40 | 18.14 ± 1.20 | 20.49 ± 1.33 |
| Stomachb | 1.62 ± 0.54 | 2.87 ± 0.32 | 2.83 ± 0.54 | 2.54 ± 0.43 | 3.54 ± 0.74 |
| Muscle | 5.60 ± 0.91 | 2.72 ± 0.96 | 2.48 ± 0.18 | 1.93 ± 0.17 | 1.86 ± 0.20 |
| Bone | 3.40 ± 1.14 | 4.40 ± 0.88 | 5.11 ± 0.68 | 5.19 ± 0.84 | 6.49 ± 1.23 |
| Brain/Blood | 1.36 ± 0.08 | 3.13 ± 0.35 | 2.29 ± 0.40 | 1.16 ± 0.16 | 0.85 ± 0.16 |
Data shown are percentage of injected dose per gram of tissue (% ID/g), means ± SD, n = 5.
% ID/organ.
To verify the binding specificity of [18F]9 and [18F]10 to σ2 receptors in vivo, blocking experiments in male ICR mice were conducted by pre-administration (5 min prior to ∼280 kBq of radiotracer) of 9 (0.1 mL, 3 μmol/kg), 10 (0.1 mL, 3 μmol/kg), and various concentrations of CM398 (0.1 mL; 3, 5, 10 or 20 μmol/kg), a selective σ2 receptor ligand34. The blocking effects on [18F]9 and [18F]10 at 30 min p.i. are summarized in Fig. 2. For [18F]9, animals pre-treated with CM398 showed a dose-dependent reductions in brain uptake levels (29%–54%), and brain-to-blood ratios (60%–88%). Activity levels were also reduced in the heart (66%–77%), spleen (55%–65%), lungs (73%–82%) and kidneys (73%–76%). Similarly, self-blocking led to remarkable reductions of radioactivity concentrations in the above organs.
Figure 2.
Effects of pre-administration with compound 9 (0.1 mL, 3 μmol/kg), compound 10 (0.1 mL, 3 μmol/kg), or ascending doses of CM398 (3, 5, 10 or 20 μmol/kg) on biodistribution of (A) [18F]9 and (B) [18F]10 at 30 min p.i. All values are means ± SD (n = 5–6 for each group). Student's t test (independent, two-tailed), P < 0.001 for [18F]9 and P < 0.05 for [18F]10 (except for CM398 effect in the brain).
For [18F]10, pretreatment with compound 10 or CM398 led to slightly reduced activity uptake in the brain (8%–18%). The brain-to-blood ratio also decreased (37%–47%). In addition, radioactivity levels were reduced in the liver (30%–42%), spleen (47%–54%), and kidneys (31%–33%). Overall the blocking effect was smaller for [18F]10 than [18F]9, indicating lower levels of specific binding for [18F]10.
2.4.4. Effect of P-gp on radioligand uptake in the brain
P-glycoprotein (P-gp), which is highly expressed on the BBB, can restrict the brain penetration of molecules35. Male ICR mice were treated with cyclosporine A, a P-gp inhibitor, at 60 min before radioligand administration to assess its effect on brain uptake of [18F]9 and [18F]10. The results are presented in Fig. 3. A slight increase without significance in blood uptake was observed for [18F]9 and [18F]10 (P > 0.05). The initial brain uptake levels at 2 min p.i were significantly increased by 49% and 67% (P < 0.05) for [18F]9 and [18F]10, respectively. Moreover, the brain-to-blood ratios were significantly increased by 35% and 50% for [18F]9 and [18F]10, respectively, in the groups treated with cyclosporine A (P < 0.05), suggesting that [18F]9 and [18F]10 are moderate P-gp substrates.
Figure 3.
Effects of cyclosporine A on radioactivity levels of (A) [18F]9 and (B) [18F]10 in the mouse brain and blood (n = 5–8 per group). Student's t test (independent, two-tailed), for brain uptake and brain-to-blood ratio, P < 0.05 for both [18F]9 and [18F]10. Data presented as means ± SD.
2.4.5. Ex vivo autoradiography in mice
Given the more favorable binding property observed for [18F]9 in biodistribution studies, ex vivo autoradiography study was conducted to investigate its levels of uptake and specific binding in mouse brain regions. The results are presented in Figure 4, Figure 5, Figure 6, Figure 7. Radioactivity was widely and heterogeneously distributed in the mouse brain. High levels of accumulation were observed in the cortex, cerebellum, olfactory bulb, midbrain and lateral ventricle, similar to the results reported in the literature36. Uptake level was moderate in the striatum, and low in the thalamus and hippocampus. The pattern of distribution in the brain for [18F]9 is clearly different from that we recently obtained with a σ1 receptor radioligand37.
Figure 4.
Ex vivo autoradiograms of [18F]9 in the mouse brain under baseline (upper row) and CM398 blocking (lower row) conditions: (A) Cortex; (B) Hippocampus; (C) Midbrain; (D) Cerebellum; (E) Lateral ventricle; (F) Striatum; (G) Thalamus; (H) Olfactory bulb.
Figure 5.
Ex vivo autoradiography results of [18F]9 in the mouse brain under baseline and CM398 blocking conditions. Student's t test (independent, two-tailed), P < 0.001 for all brain regions listed. Data presented as means ± SD, n = 5.
Figure 6.
Ex vivo autoradiograms of [18F]9 in the mouse brain under baseline (upper row) and self-blocking (with compound 9 at 3 μmol/kg, lower row) conditions: (A) Cortex; (B) Hippocampus; (C) Midbrain; (D) Cerebellum; (E) Lateral ventricle; (F) Striatum; (G) Thalamus; (H) Olfactory bulb.
Figure 7.
Ex vivo autoradiography results of [18F]9 in the mouse brain under baseline and self-blocking (with compound 9 at 3 μmol/kg) conditions. Student's t test (independent, two-tailed), P < 0.001 for all brain regions listed. Data presented as means ± SD, n = 5.
When the mice were injected with CM398 (5 μmol/kg) or compound 9 (3 μmol/kg) at 5 min before radiotracer injection and sacrificed at 30 min p.i. for autoradiography, levels of radiotracer accumulation were dramatically reduced in all brain regions (Figure 4, Figure 5, Figure 6, Figure 7), with significant differences (P < 0.001) found in the cortex, hippocampus, midbrain, cerebellum, striatum, thalamus and olfactory bulb. The blocking effect averaged across brain regions was similar to that seen for the whole brain in the biodistribution study, reaffirming the high level of specific binding for [18F]9 in the brain.
2.4.6. Micro-PET/CT imaging in rats
Encouraged by the favorable properties of [18F]9 in mice, imaging experiments in Sprague–Dawley (SD) rats were performed on a micro-positron emission tomography (PET)/computed tomography (CT) scanner to further investigate the pharmacokinetic and binding characteristics of [18F]9 in the brain. After administration of [18F]9, dynamic PET scan was acquired for 120 min. PET images are shown in Fig. 8. Presented in Fig. 9 are the whole brain and regional time–activity curves (TACs) from both the baseline and CM398 blocking scans. The highest levels of uptake were found in the cerebellum and cortex, followed by the hippocampus, midbrain, striatum and thalamus. Pretreatment with CM398 (10 μmol/kg) at 5 min prior to the injection of [18F]9 resulted in markedly decreased uptake in the above brain regions with faster washout. Radiotracer accumulation in the whole-brain was remarkably reduced (>60%) after 30 min. These findings confirmed regionally distinct distribution of [18F]9 in the rat brain, and its high levels of specific binding to σ2 receptors.
Figure 8.
(A) Coronal, (B) axial and (C) sagittal rat brain micro-PET/CT fusion images summed from 30 to 45 min after injection of [18F]9 under baseline and CM398 (10 μmol/kg) blocking conditions.
Figure 9.
Time‒activity curves of [18F]9 (A) in the whole-brain from baseline (n = 3) and CM398 (10 μmol/kg) blocking scans (n = 3), and (B) in selected brain regions from one baseline and one CM398 blocking scans in two separate rats. SUV = standardized uptake value.
2.4.7. In vivo metabolic stability
The brain and pancreas of ICR mice were dissected at 30 min after injection of [18F]9 (0.2 mL, 33.3 MBq), processed, and analyzed by HPLC to examine the presence (or absence) of radioactive metabolites in these tissues. Representative radio-HPLC chromatograms are presented in Supporting Information Fig. S4. In the brain samples, the parent compound [18F]9 accounted for ≥95% of the radioactivity signal, indicating negligible entry of radio-metabolites into the brain. In contrast, only about ∼10% of the signal in pancreas was attributable to radiotracer [18F]9 (n = 3), with the rest from radio-metabolites.
3. Discussion
The σ2 receptor has been considered as a putative therapeutic target for neurological diseases19,20,38,39. However, to date there has been no suitable radioligand for imaging this receptor subtype in the brain, as previous efforts in σ2 radioligand development were primarily targeted for tumor imaging. Therefore, we set out to develop brain-penetrant radioligands with high σ2 affinity and subtype selectivity for neuroimaging applications. Indole-based derivatives with the 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline or 5,6-dimethoxyisoindoline pharmacophore as reported here and previously exhibited nanomolar affinity for σ2 receptors. Replacement of the methoxy substituent in the indole ring with fluoroethoxy group appeared to increase both σ2 affinity and subtype selectivity (2–7 vs. 8–11). As a result, we focused on radioligands with the [18F]fluoroethoxyindole moiety for development and evaluation as potential σ2 neuroimaging agents.
In our previous work, we synthesized and evaluated two radioligands [18F]8 and [18F]11, and found that they indeed entered the brain well29. Nonetheless, the level of specific binding was only moderate, and radioligand [18F]8, with the 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline pharmacophore, demonstrated less favorable in vivo kinetic and binding characteristics. In a continued effort to search for σ2 radioligands with improved kinetic and imaging properties, we further synthesized radioligands [18F]9 and [18F]10 with the [18F]fluoroethoxy group at the 4- and 5-position of the indole ring, respectively, and the less lipophilic 5,6-dimethoxyisoindoline pharmacophore.
As indicated by the results from biodistribution studies reported previously29, compared to [18F]9, radiotracer [18F]8, with the 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline pharmacophore exhibited lower initial brain uptake (3.29% ID/g at 2 min vs. 4.44% ID/g for [18F]9) and lower brain-to-blood ratio (3.0 at 15 min vs. 7.2 for [18F]9). Moreover, blocking with compound 11 led to only 24% reduction in brain uptake, suggesting low level of specific binding to σ2 receptors in the brain. So we focused on ligands with the 5,6-dimethoxyisoindoline pharmacophore to further investigate the effects of [18F]fluoroethoxy at the different positions of the indole ring on brain kinetics and specific binding.
Listed in Table 4 for comparison are the in vitro and in vivo characteristics of radioligands [18F]9, [18F]10 and [18F]11, with the [18F]fluoroethoxy moiety at the 4-, 5-, or 6-position of the indole ring, respectively. Similar to [18F]11, the two new radioligands [18F]9 and [18F]10 displayed the appropriate lipophilicity required for BBB permeability of neuroimaging agents, and good in vitro stability. Both in vitro binding affinity for σ2 receptor and σ2/σ1 subtype selectivity followed the order of [18F]9 > [18F]11 > [18F]10. Radioligands [18F]9 and [18F]11 displayed initial brain uptake at 2 min after injection (4.44% and 4.55% ID/g) higher than [18F]10 (3.76% ID/g), which are all much higher than that of the established σ2 radioligand [18F]ISO-1 (0.76% ID/g)27. Brain uptake at 30 min was similar for [18F]10 and [18F]11 (2.68% and 2.28% ID/g), but much higher for [18F]9 (5.04% ID/g), which is consistent with its highest σ2 affinity [Ki (σ2) = 1.79 ± 0.86 nmol/L] in this series. Radioligand [18F]9 also showed the highest brain-to-blood ratios, peaking at >10 between 30 and 60 min, compared with 3.0 and 5.0 for [18F]10 and [18F]11, respectively, at 15 min after injection. Further, in self-blocking or CM-398 pretreatment experiments, [18F]9 displayed the most remarkable reduction of radioactivity levels in the brain, heart, spleen, lungs and kidneys, indicating that [18F]9 possessed the highest specific binding to σ2 receptors in vivo. Most importantly, a dose-dependent blocking effect of CM398 on brain uptake and brain-to-blood ratios was observed, indicating highly specific and saturable binding of [18F]9 to σ2 receptors in the brain. Finally, all three radiotracers showed relatively low levels of radioactivity uptake in the bone at 120 min, suggesting little defluorination of these radiotracers in vivo.
Table 4.
Comparison of in vitro and in vivo characteristics for radioligands [18F]9, [18F]10 and [18F]11.
| Property | Radioligand |
|||
|---|---|---|---|---|
| [18F]9 | [18F]10 | [18F]1129 | ||
| Ki (σ2) (nmol/L) | 1.79 ± 0.86 | 3.27 ± 0.19 | 2.63 ± 0.48 | |
| Ki (σ1)/Ki (σ2) | 207 | 57 | 143 | |
| LogD7.4 | 2.43 ± 0.01 | 2.29 ± 0.01 | 2.17 ± 0.13 | |
| Brain uptake at 2 min (% ID/g) | 4.44 ± 0.59 | 3.76 ± 0.25 | 4.55 ± 0.43 | |
| Brain uptake at 30 min (% ID/g) | 5.04 ± 0.67 | 2.68 ± 0.25 | 2.28 ± 0.16 | |
| Brain/Blood ratio at 30 min | 10.57 | 2.29 | 3.62 | |
| Self-blocking effect | Brain | 58% | 14% | 21% |
| Brain/Blood | 90% | 37% | 68% | |
| Blocking effect of CM398 (5 μmol/kg) | Brain | 39% | 18% | – |
| Brain/Blood | 75% | 45% | – | |
| P-gp substrate | Yes | Yes | Yes | |
–Not applicable.
To elucidate the interaction between P-gp and [18F]9, the effect of the P-gp inhibitor cyclosporine A on brain uptake of [18F]9 was tested in ICR mice. Pretreatment with cyclosporine A increased uptake of [18F]9 in both the brain and blood. The differences in brain uptake and brain-to-blood ratio between control and cyclosporine A-treated groups were significant (P < 0.05), indicating that [18F]9 is a moderate P-gp substrate. Results from the same set of experiments for [18F]10 and [18F]11 also indicated that they might be substrates for P-gp.
Since [18F]9 has the highest σ2 specific binding in the brain in this series, ex vivo autoradiography experiments were conducted to further examine its distribution in brain regions. High levels of accumulation were found in the cortex, cerebellum and olfactory bulb, in line with results found with other σ2 radioligands in the literature36,40. On the other hand, higher levels of accumulation in the midbrain and lateral ventricle were also observed, with lower levels in the hippocampus and thalamus, which are clearly different from the regional expression pattern of σ1 receptors in the brain32,37,41,42, and thus establishing that radioligand [18F]9 is evidently binding to the σ2 receptors. These results verify that σ1 and σ2 receptors are distinctly and differently expressed in brain regions43. Pretreatment of animals with CM398 and compound 9 led to significant reductions in radiotracer uptake in different brain areas, further indicating the highly specific nature of [18F]9 binding to σ2 receptors in the mouse brain and reaffirming the results from biodistribution studies.
Micro-PET/CT imaging was carried out in SD rats to further investigate the pharmacokinetic and binding properties of [18F]9 in vivo. After intravenous injection, [18F]9 entered the brain rapidly, then slowly washed out from different rat brain regions, consistent with the findings from biodistribution experiments in mice. Highest levels of uptake were observed in the cerebellum and cortex, followed by striatum, hippocampus, midbrain, and thalamus, in line with those found from ex vivo autoradiography study. Blocking with CM398 led to significant reductions in activity uptake to almost homogeneous levels in different brain regions, and much faster washout rates, demonstrating high specific binding of [18F]9 to σ2 receptors in the rat brain.
Metabolism study in mice indicated that [18F]9 was the predominant radioactive species in the brain, with negligible amount of radio-metabolites, which is in good agreement with the high specific binding of [18F]9 in the mouse brain. However, only about 10% of the radioactivity was attributed to [18F]9 in the pancreas. As a result, there appeared to be little specific binding of [18F]9 in the mouse pancreas, although high activity level was observed in this organ.
4. Conclusions
Through structure–activity relationship studies, indole-based compounds with the 5,6-dimethoxyisoindoline pharmacophore, a four-carbon linker, and a fluoroethoxy substituent in the indole moiety were discovered to confer favorable properties as selective σ2 receptor ligands. Three radioligands with the [18F]fluoroethoxy group at different positions of the indole ring were prepared and found to readily enter the brain, thus fulfilling the first requirement as neuroimaging agents. Further ex vivo and in vivo evaluations identified radiotracer [18F]9, with the highest in vitro binding affinity and subtype selectivity for σ2 receptor, as the most favorable agent for neuroimaging. In rodents it exhibited the highest levels of brain uptake, brain-to-blood ratio, and specific binding among the σ2 receptor radioligands reported to date. Combined with its appropriate kinetics in the brain, the novel radioligand [18F]9 from the current study is undoubtedly the most promising radioligand for neuroimaging of the σ2 receptors, and thus warrants further evaluation in primates for its potential to image and quantitate cerebral σ2 receptors in various brain diseases.
5. Experimental
5.1. General information
The reagents and solvents, thin-layer chromatography (TLC),1H NMR and 13C NMR spectra, mass spectrometry (MS), high-resolution mass spectrometry (HRMS), HPLC, mice were obtained and handled in a manner similar to that reported in previous work29,41. Details are provided in the Supporting Information. Chemical synthesis and characterization data of intermediate and final compounds were provided in the Supporting Information. All the final compounds were characterized by 1H NMR, 13C NMR and HRMS. Chemical purities of ≥95% were indicated by NMR spectra and analysis with HPLC (see Supporting Information).
Normal male ICR mice (20 ± 2 g, 4–5 weeks) and male SD rats (200 ± 10 g, 6–7 weeks) were purchased from Vital River Experimental Animal Technical Co., Ltd. Experiments in animals were carried out in compliance with relevant regulations and institutional guidelines, and approved by the Institutional Animal Care and Use Committee of Beijing Normal University.
5.2. Radiochemistry
Synthesis of radiotracers32, determination of logD value42 and in vitro stability studies42 followed the procedures reported previously. Detailed procedures are provided in the Supporting Information.
5.3. Biological evaluations
In vitro radioligand competition studies30,31, in vivo biodistribution and blocking studies42, effect of P-gp on brain uptake29, ex vivo autoradiography41, in vivo micro-PET/CT imaging in rats32 and in vivo metabolism studies29 followed the procedures reported previously. Detailed procedures are provided in the Supporting Information.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21876013) and Beijing Natural Science Foundation (7212203, China).
Author contributions
All authors contributed to this manuscript and have approved the final article. Ying Zhang, Yiyun Huang and Hongmei Jia designed the study, participated in data analysis and interpretation and wrote the manuscript. Ying Zhang, Tao Wang and Xiaojun Zhang performed experiments. Winnie Deuther-Conrad and Peter Brust performed in vitro radioligand competition binding studies and participated in data analysis and interpretation. Hualong Fu, Mengchao Cui and Jinming Zhang designed and participated in experiments.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2021.08.029.
Contributor Information
Jinming Zhang, Email: zhangjm301@163.com.
Yiyun Huang, Email: henry.huang@yale.edu.
Hongmei Jia, Email: hmjia@bnu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gabitova L., Gorin A., Astsaturov I. Molecular pathways: sterols and receptor signaling in cancer. Clin Cancer Res. 2014;20:28–34. doi: 10.1158/1078-0432.CCR-13-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang K., Zeng C., Wang C., Sun M., Yin D., Sun T. Sigma-2 receptor—a potential target for cancer/Alzheimer's disease treatment via its regulation of cholesterol homeostasis. Molecules. 2020;25:5439. doi: 10.3390/molecules25225439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang T.Y., Yamauchi Y., Hasan M.T., Chang C. Cellular cholesterol homeostasis and Alzheimer's disease. J Lipid Res. 2017;58:2239–2254. doi: 10.1194/jlr.R075630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsushita Y., Nakagawa H., Koike K. Lipid metabolism in oncology: why it matters, how to research, and how to treat. Cancers. 2021;13:474. doi: 10.3390/cancers13030474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayengbam S.S., Singh A., Pillai A.D., Bhat M.K. Influence of cholesterol on cancer progression and therapy. Transl Oncol. 2021;14:101043. doi: 10.1016/j.tranon.2021.101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wellington C.L. Cholesterol at the crossroads: Alzheimer's disease and lipid metabolism. Clin Genet. 2004;66:1–16. doi: 10.1111/j.0009-9163.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- 7.Lukiw W.J., Pappolla M., Pelaez R.P., Bazan N.G. Alzheimer's disease—a dysfunction in cholesterol and lipid metabolism. Cell Mol Neurobiol. 2005;25:475–483. doi: 10.1007/s10571-005-4010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alon A., Schmidt H.R., Wood M.D., Sahn J.J., Martin S.F., Kruse A.C. Identification of the gene that codes for the σ2 receptor. Proc Natl Acad Sci U S A. 2017;114:7160–7165. doi: 10.1073/pnas.1705154114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartz F., Kern L., Erz D., Zhu M., Gilbert D., Meinhof T., et al. Identification of cholesterol-regulating genes by targeted RNAi screening. Cell Metabol. 2009;10:63–75. doi: 10.1016/j.cmet.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Shen H., Li J., Xie X., Yang H., Zhang M., Wang B., et al. BRD2 regulation of sigma-2 receptor upon cholesterol deprivation. Life Sci Alliance. 2020;4 doi: 10.26508/lsa.201900540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng C., Riad A., Mach R.H. The biological function of sigma-2 receptor/TMEM97 and its utility in PET imaging studies in cancer. Cancers. 2020;12:1877. doi: 10.3390/cancers12071877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mach R.H., Smith C.R., Al-Nabulsi I., Whirrett B.R., Childers S.R., Wheeler K.T. σ2 receptors as potential biomarkers of proliferation in breast cancer. Cancer Res. 1997;57:156–161. [PubMed] [Google Scholar]
- 13.Al-Nabulsi I., Mach R.H., Wang L.M., Wallen C.A., Keng P.C., Sten K., et al. Effect of ploidy, recruitment, environmental factors, and tamoxifen treatment on the expression of sigma-2 receptors in proliferating and quiescent tumour cells. Br J Cancer. 1999;81:925–933. doi: 10.1038/sj.bjc.6690789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheeler K.T., Wang L.M., Wallen C.A., Childers S.R., Cline J.M., Keng P.C., et al. Sigma-2 receptors as a biomaker of proliferaion in solid tumours. Br J Cancer. 2000;82:1223–1232. doi: 10.1054/bjoc.1999.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riad A., Zeng C., Weng C.C., Winters H., Xu K., Makvandi M., et al. Sigma-2 receptor/TMEM97 and PGRMC-1 increase the rate of internalization of LDL by LDL receptor through the formation of a ternary complex. Sci Rep. 2018;8:16845. doi: 10.1038/s41598-018-35430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riad A., Lengyel-Zhand Z., Zeng C., Weng C.C., Lee V.M., Trojanowski J.Q., et al. The sigma-2 receptor/TMEM97, PGRMC1, and LDL receptor complex are responsible for the cellular uptake of Abeta42 and its protein aggregates. Mol Neurobiol. 2020;57:3803–3813. doi: 10.1007/s12035-020-01988-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izzo N.J., Xu J., Zeng C., Kirk M.J., Mozzoni K., Silky C., et al. Alzheimer's therapeutics targeting amyloid beta 1-42 oligomers II: sigma-2/PGRMC1 receptors mediate Abeta 42 oligomer binding and synaptotoxicity. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izzo N.J., Staniszewski A., To L., Fa M., Teich A.F., Saeed F., et al. Alzheimer's therapeutics targeting amyloid beta 1-42 oligomers I: Abeta 42 oligomer binding to specific neuronal receptors is displaced by drug candidates that improve cognitive deficits. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grundman M., Morgan R., Lickliter J.D., Schneider L.S., DeKosky S., Izzo N.J., et al. A phase 1 clinical trial of the sigma-2 receptor complex allosteric antagonist CT1812, a novel therapeutic candidate for Alzheimer's disease. Alzheimers Dement. 2019;5:20–26. doi: 10.1016/j.trci.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izzo N.J., Yuede C.M., LaBarbera K.M., Limegrover C.S., Rehak C., Yurko R., et al. Preclinical and clinical biomarker studies of CT1812: a novel approach to Alzheimer's disease modification. Alzheimers Dement. 2021;17:1365–1382. doi: 10.1002/alz.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y.S., Lu H.L., Zhang L.J., Wu Z. Sigma-2 receptor ligands and their perspectives in cancer diagnosis and therapy. Med Res Rev. 2014;34:532–566. doi: 10.1002/med.21297. [DOI] [PubMed] [Google Scholar]
- 22.Mach R.H., Zeng C., Hawkins W.G. The σ2 receptor: a novel protein for the imaging and treatment of cancer. J Med Chem. 2013;56:7137–7160. doi: 10.1021/jm301545c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng C., McDonald E.S., Mach R.H. Molecular probes for imaging the sigma-2 receptor: in vitro and in vivo imaging studies. Handb Exp Pharmacol. 2017;244:309–330. doi: 10.1007/164_2016_96. [DOI] [PubMed] [Google Scholar]
- 24.Dehdashti F., Laforest R., Gao F., Shoghi K.I., Aft R.L., Nussenbaum B., et al. Assessment of cellular proliferation in tumors by PET using 18F-ISO-1. J Nucl Med. 2013;54:350–357. doi: 10.2967/jnumed.112.111948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald E.S., Doot R.K., Young A.J., Schubert E.K., Tchou J., Pryma D.A., et al. Breast cancer 18F-ISO-1 uptake as a marker of proliferation status. J Nucl Med. 2020;61:665–670. doi: 10.2967/jnumed.119.232363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abramson Cancer Center of the University of Pennsylvania Imaging of in vivo sigma-2 receptor expression with 18F-ISO-1 positron emission tomography in metastatic breast cancer. ClinicalTrials.gov Identifier. 2017 NCT03057743. [Google Scholar]
- 27.Tu Z., Xu J., Jones L.A., Li S., Dumstorff C., Vangveravong S., et al. Fluorine-18-labeled benzamide analogues for imaging the σ2 receptor status of solid tumors with positron emission tomography. J Med Chem. 2007;50:3194–3204. doi: 10.1021/jm0614883. [DOI] [PubMed] [Google Scholar]
- 28.Mesangeau C., Amata E., Alsharif W., Seminerio M.J., Robson M.J., Matsumoto R.R., et al. Synthesis and pharmacological evaluation of indole-based sigma receptor ligands. Eur J Med Chem. 2011;46:5154v61. doi: 10.1016/j.ejmech.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L., Ye J., He Y., Deuther-Conrad W., Zhang J., Zhang X., et al. 18F-Labeled indole-based analogs as highly selective radioligands for imaging sigma-2 receptors in the brain. Bioorg Med Chem. 2017;25:3792–3802. doi: 10.1016/j.bmc.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Fan C., Jia H., Deuther-Conrad W., Brust P., Steinbach J., Liu B. Novel 99mTc labeled σ receptor ligand as a potential tumor imaging agent. Sci China Ser B Chem. 2006;49:169–176. [Google Scholar]
- 31.Bautista-Aguilera O.M., Budni J., Mina F., Medeiros E.B., Deuther-Conrad W., Entrena J.M., et al. Contilisant, a tetratarget small molecule for Alzheimer's disease therapy combining cholinesterase, monoamine oxidase inhibition, and H3R antagonism with S1R agonism profile. J Med Chem. 2018;61:6937–6943. doi: 10.1021/acs.jmedchem.8b00848. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Wang X., Zhang J., Deuther-Conrad W., Xie F., Zhang X., et al. Synthesis and evaluation of novel 18F-labeled spirocyclic piperidine derivatives as σ1 receptor ligands for positron emission tomography imaging. J Med Chem. 2013;56:3478–3491. doi: 10.1021/jm301734g. [DOI] [PubMed] [Google Scholar]
- 33.Patel S., Gibson R. In vivo site-directed radiotracers: a mini-review. Nucl Med Biol. 2008;35:805–815. doi: 10.1016/j.nucmedbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Intagliata S., Sharma A., King T.I., Mesangeau C., Seminerio M., Chin F.T., et al. Discovery of a highly selective sigma-2 receptor ligand, 1-(4-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)butyl)-3-methyl-1H-benzo[d]imidazole-2(3H)-one (CM398), with drug-like properties and antinociceptive effects in vivo. AAPS J. 2020;22:94. doi: 10.1208/s12248-020-00472-x. [DOI] [PubMed] [Google Scholar]
- 35.Kreisl W.C., Liow J.S., Kimura N., Seneca N., Zoghbi S.S., Morse C.L., et al. P-glycoprotein function at the blood–brain barrier in humans can be quantified with the substrate radiotracer 11C-N-desmethyl-loperamide. J Nucl Med. 2010;51:559–566. doi: 10.2967/jnumed.109.070151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abate C., Selivanova S.V., Muller A., Kramer S.D., Schibli R., Marottoli R., et al. Development of 3,4-dihydroisoquinolin-1(2H)-one derivatives for the Positron Emission Tomography (PET) imaging of σ2 receptors. Eur J Med Chem. 2013;69:920–930. doi: 10.1016/j.ejmech.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Tian J., He Y., Deuther-Conrad W., Fu H., Xie F., Zhang Y., et al. Synthesis and evaluation of new 1-oxa-8-azaspiro[4.5]decane derivatives as candidate radioligands for sigma-1 receptors. Bioorg Med Chem. 2020;28:115560. doi: 10.1016/j.bmc.2020.115560. [DOI] [PubMed] [Google Scholar]
- 38.Sahn J.J., Mejia G.L., Ray P.R., Martin S.F., Price T.J. Sigma 2 receptor/Tmem97 agonists produce long lasting antineuropathic pain effects in mice. ACS Chem Neurosci. 2017;8:1801–1811. doi: 10.1021/acschemneuro.7b00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lever J.R., Miller D.K., Green C.L., Fergason-Cantrell E.A., Watkinson L.D., Carmack T.L., et al. A selective sigma-2 receptor ligand antagonizes cocaine-induced hyperlocomotion in mice. Synapse. 2014;68:73–84. doi: 10.1002/syn.21717. [DOI] [PubMed] [Google Scholar]
- 40.Søby K.K., Mikkelsen J.D., Meier E., Thomsen C. Lu 28-179 labels a σ2-site in rat and human brain. Neuropharmacology. 2002;43:95–100. doi: 10.1016/s0028-3908(02)00071-0. [DOI] [PubMed] [Google Scholar]
- 41.He Y., Xie F., Ye J., Deuther-Conrad W., Cui B., Wang L., et al. 1-(4-[18F]Fluorobenzyl)-4-[(tetrahydrofuran-2-yl)methyl]piperazine: a novel suitable radioligand with low lipophilicity for imaging σ1 receptors in the brain. J Med Chem. 2017;60:4161–4172. doi: 10.1021/acs.jmedchem.6b01723. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y.Y., Wang X., Zhang J.M., Deuther-Conrad W., Zhang X.J., Huang Y., et al. Synthesis and evaluation of a 18F-labeled spirocyclic piperidine derivative as promising σ1 receptor imaging agent. Bioorg Med Chem. 2014;22:5270–5278. doi: 10.1016/j.bmc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Leitner M.L., Hohmann A.G., Patrick S.L., Walker J.M. Regional variation in the ratio of σ1 to σ2 binding in rat brain. Eur J Pharmacol. 1994;259:65–69. doi: 10.1016/0014-2999(94)90158-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.