Abstract
Cancer immunotherapy has become a new generation of anti-tumor treatment, but its indications still focus on several types of tumors that are sensitive to the immune system. Therefore, effective strategies that can expand its indications and enhance its efficiency become the key element for the further development of cancer immunotherapy. Natural products are reported to have this effect on cancer immunotherapy, including cancer vaccines, immune-check points inhibitors, and adoptive immune-cells therapy. And the mechanism of that is mainly attributed to the remodeling of the tumor-immunosuppressive microenvironment, which is the key factor that assists tumor to avoid the recognition and attack from immune system and cancer immunotherapy. Therefore, this review summarizes and concludes the natural products that reportedly improve cancer immunotherapy and investigates the mechanism. And we found that saponins, polysaccharides, and flavonoids are mainly three categories of natural products, which reflected significant effects combined with cancer immunotherapy through reversing the tumor-immunosuppressive microenvironment. Besides, this review also collected the studies about nano-technology used to improve the disadvantages of natural products. All of these studies showed the great potential of natural products in cancer immunotherapy.
KEY WORDS: Natural products, Cancer immunotherapy, Immunosuppressive microenvironment, Cancer vaccines, Immuno-check points, Adoptive immune-cells transfer immunotherapy
Abbreviations: AKT, alpha-serine/threonine-specific protein kinase; B2M, beta-2-microglobulin; bFGF, basic fibroblast growth factor; BMDCs, bone marrow dendritic cells; BPS, basil polysaccharide; BTLA, B- and T-lymphocyte attenuator; CAFs, cancer-associated fibroblasts; CCL22, C–C motif chemokine 22; CIKs, cytokine-induced killer cells; COX-2, cyclooxygenase-2; CRC, colorectal cancer; CTL, cytotoxic T cell; CTLA-4, cytotoxic T lymphocyte antigen-4; DAMPs, damage-associated molecular patterns; DCs, dendritic cells; FDA, US Food and Drug Administration; HCC, hepatocellular carcinoma; HER-2, human epidermal growth factor receptor-2; HIF-1α, hypoxia-inducible factor-1α; HMGB1, high-mobility group box 1; HSPs, heat shock proteins; ICD, Immunogenic cell death; ICTs, immunological checkpoints; IFN-γ, interferon γ; IL-10, interleukin-10; LLC, Lewis lung cancer; Mcl-1, myeloid leukemia cell differentiation protein 1; MDSCs, myeloid-derived suppressor cells; MHC, major histocompatibility complex class; MITF, melanogenesis associated transcription factor; MMP-9, matrix metalloprotein-9; mTOR, mechanistic target of rapamycin; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NKTs, natural killer T cells; NSCLC, non-small cell lung cancer; OVA, ovalbumin; PD-1, programmed death-1; PD-L1, programmed death receptor ligand 1; PGE-2, prostaglandin E2; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; STAT3, signal transducer and activator of transcription 3; TAMs, tumor-associated macrophages; TAP, transporters related with antigen processing; Teffs, effective T cells; TGF-β, transforming growth factor-β; Th1, T helper type 1; TILs, tumor infiltration lymphocytes; TLR, Toll-like receptor; TNF-α, tumor necrosis factor α; Tregs, regulatory T cells; TSA, tumor specific antigens; VEGF, vascular endothelial growth factor
Graphical abstract
Natural products can effectively enhance the therapeutic outcome and expand indications of cancer immunotherapies, including cancer vaccines, immune-checkpoint inhibitors, and adoptive immune-cells transfer therapy, through remodeling tumor immunosuppressive microenvironment.
1. Introduction
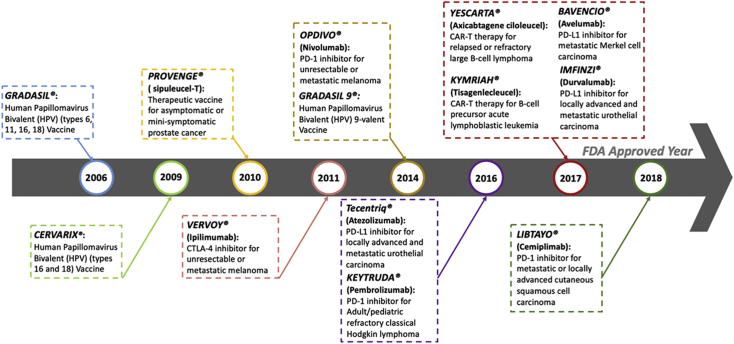
Cancer immunotherapy has become an irreversible trend herald in the field of cancer therapy and is regarded as the fourth type of anti-tumor treatment after surgery, radiotherapy, and chemotherapy due to the obvious efficacy and low side effects1,2. This kind of breakthrough therapy is defined that regulates immunological response through activating the organism's immune defense system or action of biological compounds to suppress and prevent tumor growth2. Up to now, 7 types of immune-checkpoint inhibitors, 2 kinds of adoptive immune-cell immunotherapies, and 4 types of cancer vaccines for anti-cancer treatments have been approved by the US Food and Drug Administration (FDA, Fig. 1)3, 4, 5, 6, 7, 8, 9.
Figure 1.
Cancer immunotherapies approved by the FDA.
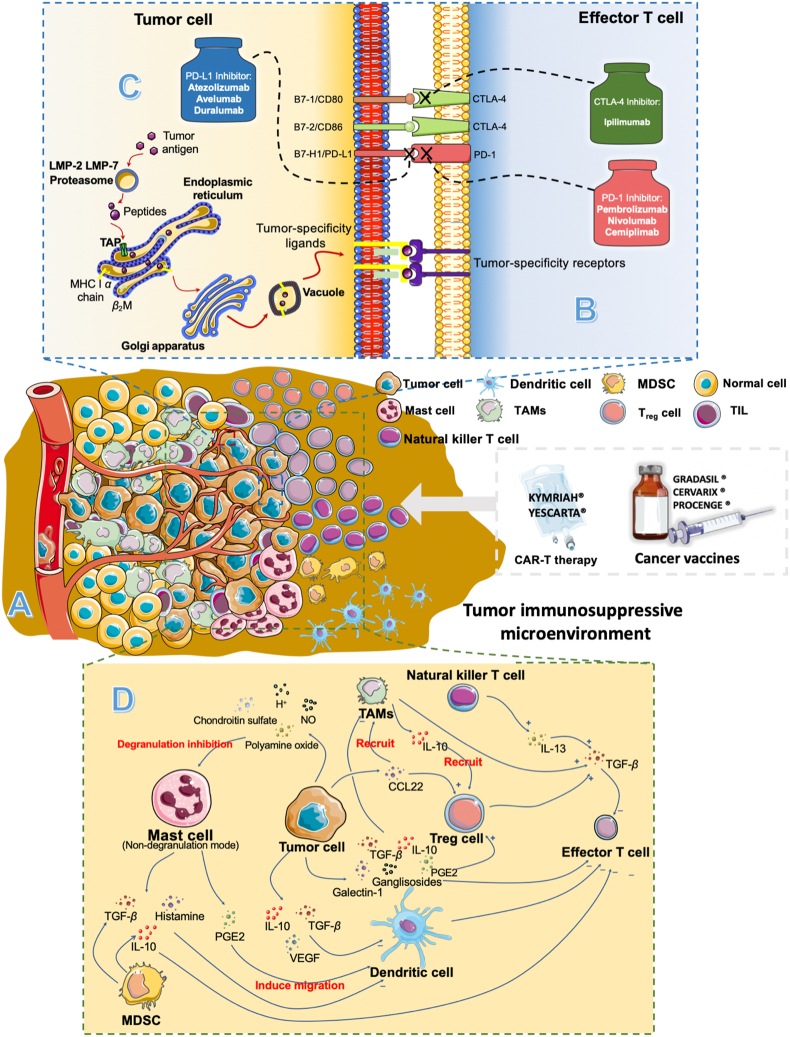
Despite cancer immunotherapy has achieved numerous remarkable advances so far, its indications still focus on several types of tumors that are sensitive to the immune system (also named as “hot tumor”), such as metastatic melanoma, urothelial carcinoma, and prostate cancer10, 11, 12. For the tumor with poor immunogenicities (also named as “cold tumor”) like triple-negative breast cancer, colorectal cancer, and lung cancer, there is almost no significant efficiency of cancer immunotherapy13. And the main reason resulting in this limitation is the immunosuppressive microenvironment within tumor sites (Fig. 2A), which can assist tumor cells in immune escape and even promote its growth14,15. As shown in Fig. 2B, the hot tumors can normally express tumor-specific antigens and the immune system can distinguish these antigens and then effectively kill tumor cells with the assistance of immunotherapy16. But for cold tumors, they will establish their unique immunosuppressive microenvironment against the recognition and attack from the immune system13,14,17, 18, 19. And this microenvironment can effectively passivate cancer immunotherapy. For example, although the number and activity of effective T cells (Teffs) can be increased and improved by cancer vaccines or immune-checkpoint inhibitors, the cold tumor cells still can weaken Teffs’ activities through immunosuppressive cells like regulatory T cells (Tregs) and immunosuppressive factors, such as interleukin-10 (IL-10), IL-13, IL-16, prostaglandin E2 (PGE-2) and so on16,19. Therefore, effective strategies that can reverse and remodel the complex immunosuppressive microenvironment within tumors become the key element for cancer immunotherapy to expand its indications.
Figure 2.
Tumor-associated immunosuppressive microenvironments and immunotherapy: (A) tumor immunosuppressive microenvironment. (B) tumor associated-agents presenting process. (C) Immunological checkpoints and (D) immunosuppressive factors and cells.
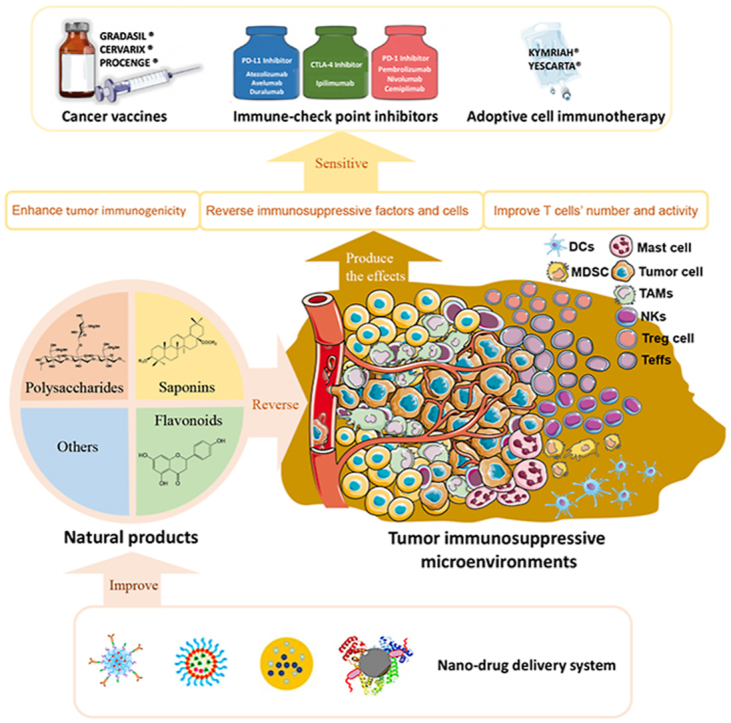
Natural products, which refer to the compounds extracted and/or optimized from nature, have shown great potential in the field of cancer immunotherapy due to their multi-target regulated abilities in recent years20, 21, 22, 23. It has been found that they can effectively enhance the therapeutic outcome and expand indications of all kinds of cancer immunotherapies, including cancer vaccines, immune-checkpoint inhibitors, and adoptive immune-cells transfer therapy24, 25, 26. In addition, there are also some clinical trials about natural products combined with immunotherapy that have been approved by the FDA (Table 1). Besides, natural products have achieved huge success in the discovery of anti-tumor agents27, 28, 29, 30, 31, 32. About 47% of anti-tumor agents are derived from naturally occurring compounds33, 34, 35. Because of the multi-benefits of natural products on anti-tumor and immunomodulatory fields, it has shown great potential to become qualified adjuvants for tumor immunotherapy.
Table 1.
List of clinical trials about natural products combined with cancer immunotherapy.
| NCT number | Natural product | Caner immunotherapy | Disease | Status | Phase |
|---|---|---|---|---|---|
| NCT03192059 | Curcumin | Pembrolizumab | Cervical cancer/Endometrial cancer/Uterine cancer | Recruiting | II |
| NCT00470574 | QS-21 | Sialyl lewisa-keyhole limpet hemocyanin conjugate vaccine | Breast cancer | Completed | – |
| NCT00036933 | QS-21 | MUC-2-Globo H-KLH conjugate vaccine | Prostate cancer | Completed | I |
| NCT00004929 | QS-21 | MUC-2-KLH vaccine | Prostate cancer | Completed | I |
| NCT00005632 | QS-21 | MUC1-KLH vaccine | Prostate cancer | Completed | I |
| NCT00003357 | OS-21 | GM2-KLH vaccine | Breast cancer | Completed | I |
| NCT00003819 | QS-21 | TF(c)-KLH conjugate vaccine | Prostate cancer | Completed | I |
| NCT00004156 | QS-21 | MUC1-KLH vaccine | Breast cancer | Completed | I |
| NCT00030823 | QS-21 | Globo-H-GM2-Lewis-y-MUC1-32(aa)-sTn(c)-Tn(c)-KLH conjugate vaccine | Breast cancer | Completed | – |
| NCT00006041 | QS-21 | MUC1-KLH vaccine | Fallopian tube cancer/Ovarian cancer/Primary peritoneal cavity cancer | Completed | I |
| NCT00006387 | QS-21 | Ras-peptide cancer vaccine | Colorectal cancer/Pancreatic cancer | Completed | I |
| NCT00001572 | QS-21 | Id-KLH vaccine | B cell lymphoma/Follicular lymphoma/Neoplasm | Completed | I |
| NCT00004052 | QS-21 | BCR‒ABL peptide vaccine | Leukemia | Completed | II |
| NCT00857545 | Saponin-based immunoadjuvant OBI-821 | Polyvalent antigen-KLH conjugate vaccine | Ovarian epithelial cancer/Fallopian tube cancer/Peritoneal cancer | Completed | II |
‒Not applicable.
This review collects and generalizes the newly obtained studies about how natural products expand indications and enhance the efficiency of cancer immunotherapies through remodeling tumor immunosuppressive microenvironment. Firstly, the antagonistic relationship between tumor immunosuppressive microenvironment and current cancer immunotherapies has been elaborated. Next, as for the three types of tumor immunotherapies approved by the FDA, the information about how natural products improve these three treatments through multi-cells and multi-pathways moderating effects have been provided in this part. Then, the effective nano-drug delivery system designed to overcome the shortages of natural products also are exhibited and discussed. And this multi-targeted immune sensitized effect of natural products is summarized in the last part.
2. Tumor immunosuppressive microenvironment
For the current cancer immunotherapies, their efficiencies mainly depend on the function of Teffs, which play the central role in the anti-tumor effect of the immune system. However, the existence of the tumor immunosuppressive microenvironment weakens the Teffs’ activities. Therefore, a deep understanding of the immunosuppressive microenvironment will help to find the key elements for improving the efficiency of cancer immunotherapy. And this inhibition of Teffs induced by the immunosuppressive microenvironment in tumors can be concluded into three main aspects: poor immunogenicity, Immunological checkpoints, and immunosuppressive factors and cells12.
2.1. Poor immunogenicity
The most significant basis for the elimination of tumor cells by the immune system is enough immunogenicity, which mainly depends on the number of tumor-specific antigens (TSA) that express on the surface of tumor cells (Fig. 2B). It is also the straightforward reason that results in limited clinical benefits of cancer immunotherapy for poorly immunogenic (cold) tumors compares to immunogenic (hot) tumors36,37. TSAs are usually induced by tumor-specific mutations. The lack of antigenic mutations will cause the lack of recognition by T cells for tumor cells38.
Moreover, tumor cells can also prevent the TSAs-presenting process to the surface of tumor cells by the restriction of major histocompatibility complex class (MHC) through altering some elements in the TSAs presenting procedure, including proteasomes and transporters related to antigen processing (TAP), beta-2-microglobulin (B2M) or MHC39. Either lack of antigenic mutations or alternations in the antigen-presenting process may result in the “off-target” effect of T cell-based cancer immunotherapy.
2.2. Immunological checkpoints
Even though Teffs can recognize and bind to tumor cells, tumor cells still can utilize the immunological checkpoints (ICTs) to avoid the attack of Teffs (Fig. 2C). The ICT is the other important aspect of tumor cells for interrupting Teffs’ function40. The cytotoxic T lymphocyte antigen-4 (CTLA-4) is the first explored immunological checkpoint in previous studies and named as the “brake” of Teffs responses. The affinity between it and B7 costimulatory molecules is much higher than that between CD28 and Teffs, and thus it can effectively terminate the activation of Teffs41, 42, 43, 44. The other immunological checkpoints, programmed death-1 (PD-1) and programmed death receptor ligand 1 (PD-L1), also can inhibit the activity of Teffs for tumor cells40. The PD-L1 overexpression in tumor cells will also passivate the therapeutic outcomes of immunotherapy45. And there are increasing the number of immune-check points, such as T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), B- and T-lymphocyte attenuator (BTLA), and Killer-cell immunoglobulin-like receptors (KIRs) have been reported in these years.
2.3. Immunosuppressive cells and factors
Besides poor immunogenicity and the inhibition of ICTs, the immunosuppressive cells and factors also limit the efficiency of cancer immunotherapy (Fig. 2D). Immunosuppressive cells mainly consist of tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), dendritic cells (DCs), natural killer T cells (NKTs), mast cells, and tumor invading lymphocytes (Fig. 4). And these cells can weave an immunosuppressive network through the secretion of immunosuppressive factors, such as IL-10, PGE2, and transforming growth factor-β (TGF-β). Besides, some compounds in TME also can induce immunosuppression, such as H+, NO, and polyamine oxide.
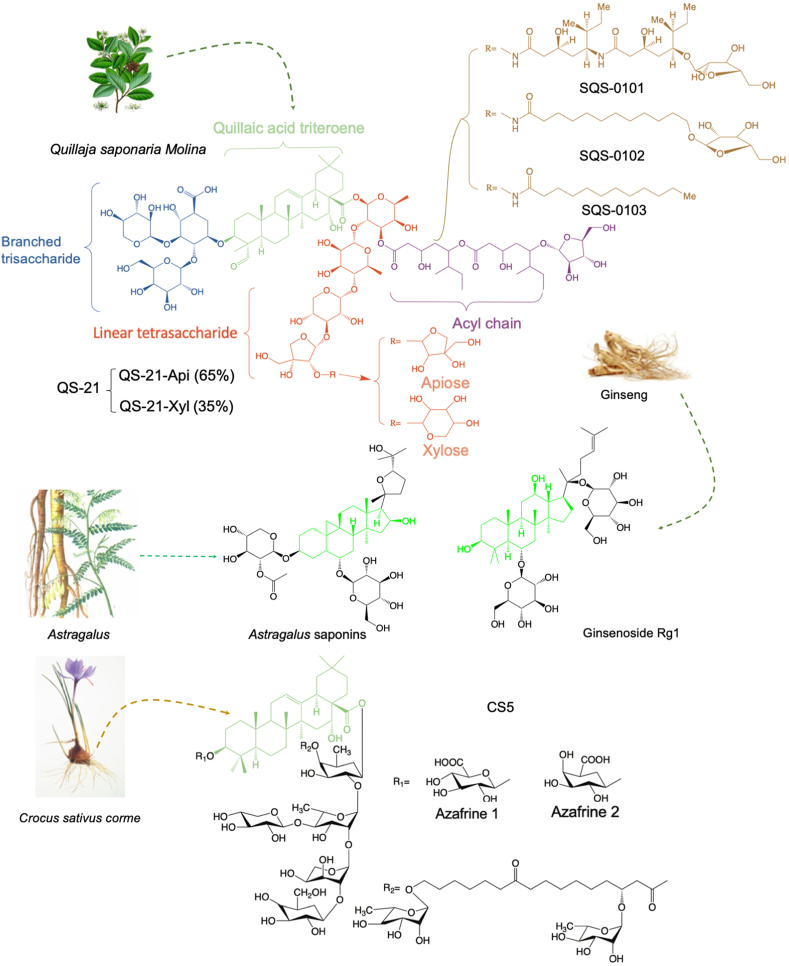
Figure 4.
Saponins improve the therapeutic effect of cancer vaccines as adjuvants.
Tumor cells can induce the non-degranulated mode of mast cells through secretion of H+, NO, chondroitin sulfate, and oxidized polyamines46, 47, 48, 49. And this mode can make mast cells promote the growth of the tumor and induce immunosuppression50. In addition, the histamine, TGF-β, and IL-10 secreted from mast cells will inhibit activities of Teffs51, 52, 53. Besides, mast cells also can affect the migration and function of DCs through secreting PGE2, indirectly enhancing the immunosuppression of tumors54. In addition, MDSCs in tumor also will secret TGF-β and IL-10 to inhibit Teffs’ activities51,53.
Moreover, tumor cells also secret macrophage-derived chemokines, C–C motif chemokine 22 (CCL22), to recruit monocytes into tumors and these monocytes will finally differentiate into TAMs under the condition of immunosuppressive factors55,56. TAMs are also an important part of solid tumors and almost 40% of non-malignant cells are TAMs in some kind of tumors55. These cells will transform into M2 immunosuppressive phenotype under the function of tumor-derived cytokines, including vascular endothelial growth factor (VEGF), galectin-1, gangliosides, TGF-β, PGE2, and IL-1057,58. Meanwhile, IL-10, TGF-β, and VEGF will inhibit the antigen presentation process of DCs. Then, the IL-10 also can recruit Tregs, which is a key factor to maintain immune tolerance, and Tregs will induce Teffs lymphocyte lysis through several ways like secreting TGF-β59. In addition, IL-13 secreted from NKTs within TME will also promote the secretion of TGF-β, thereby inhibiting the function of Teffs60.
As mentioned above, it can be concluded that the tumor immunosuppressive microenvironment is a complex network within tumor sites to help tumor cells escape from the recognition and attack of the immune system, especially for the Teffs. Therefore, finding effective agents to remodel this unique microenvironment and reactivate Teffs’ function become the key element for cancer immunotherapy. Natural products have shown great potential in remodeling tumor immunosuppressive microenvironment to improve the therapeutic outcome of cancer immunotherapy.
3. Natural products expand indications and enhance the efficiency of cancer vaccines
The cancer vaccines, including cancer-preventive vaccines and cancer-therapeutic vaccines, belong to one of the cancer-specific active immunotherapies. Cancer-preventive vaccines mainly fight virus infection through the induction of specific antibodies and long-lived memory B lymphocyte cells. Some of them, especially for those containing attenuated pathogens, also can trigger cellular immunity61. Except for HPV vaccines (GRADASIL® and CERVARIX®) are cancer-preventive vaccines, most cancer vaccines belong to therapeutic vaccines, such as PROVENGE®62. These vaccines kill malignant cells mainly through inducing CD8+ cytotoxic T lymphocytes induced by TSAs63,64.
The presenting processes of TSAs in cancer vaccines is a complex process, and the effect of cancer vaccines depends on the optimized combination of antigens, adjuvant, carrier, and vaccination routes. In addition to effectively activating CD8+ cytotoxic T lymphocytes, cancer vaccines must face the following two challenges: poor immunogenicity and tumor-associated immunosuppression65,66.
3.1. Natural products enhance the tumor immunogenicity by inducing the immunogenic cell death (ICD) effect
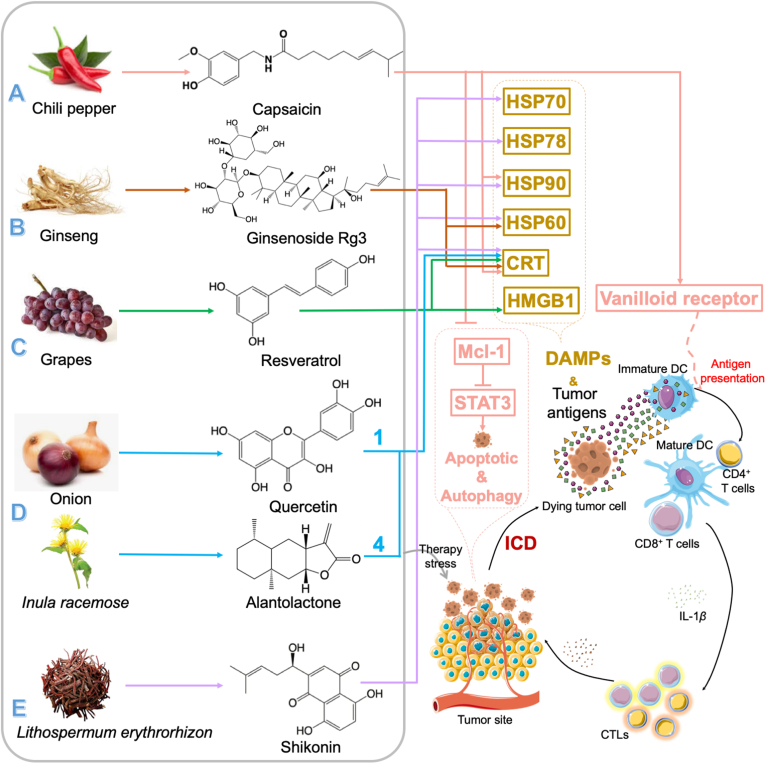
Immunogenic cell death (ICD) effect is a kind of cell apoptotic pathways, which can induce cancer cell death routine mainly depend on damage-associated molecular patterns (DAMPs), such as calreticulin (CRT), heat shock proteins (HSPs), and high-mobility group box 1 (HMGB1), and markedly enhance the immunogenicity of these dying tumor cells67,68 (Fig. 3). ICD effect can make cancer cells into “therapeutic vaccines” that can induce anti-tumor immunity without any additional adjuvants69.
Figure 3.
Immunogenic cell death (ICD) effect induced by natural products. Natural products (A. Capsaicin; B. Ginsenoside Rg3; C. Resveratrol; D. Quercetin: Alantolactone=1:4; E. Shikonin) induce immunogenic cell death (ICD) effect through damage-associated molecular patterns (DAMPs), including calreticulin (CRT), heat shock proteins (HSPs), and high-mobility group box 1 (HMGB1), to increase the tumor immunogenicity and make the tumor cells into “therapeutic vaccines”.
Up to now, only a limited number of agents can induce ICD70. Capsaicin, the pungent alkaloid of chili pepper, reportedly could trigger the ICD effect in primary effusion lymphoma (PEL) cells through the induction of DAMPs exposure, including HSP90 and calreticulin71 (Fig. 3A). Besides, it also could activate the DCs through binding to vanilloid receptor 1 (VR1)72. And this counteraction effect of immune suppression on DCs in PEL microenvironments has been proved in this study. Moreover, capsaicin could induce apoptotic and pro-survival autophagy through reducing signal transducer and activator of transcription 3 (STAT3) phosphorylation, which depends on down-regulating the expression of Induced myeloid leukemia cell differentiation protein 1 (MCL-1), a kind of anti-apoptosis molecules. And similar results were also be found in several solid and hematological tumors73, 74, 75, 76.
Ginsenoside Rg3, a saponin from ginseng, is reported that could induce the significance of ICD both in immunogenic tumor-like melanoma (B16F10) and non-immunogenic tumor-like Lewis lung cancer (LLC, Fig. 3B)77. The ICD effect induced by Rg3 could increase the expression of CRT and HSP60, which are chaperone proteins and ICD markers, on the surface of B16F10 and LLC cells. In addition, within the “eat me” signal of CRT, DCs took up the decries of dead tumor cells treated by Rg3 more easily than normal tumor cells. Furthermore, the Rg3-induced ICD effect could enhance interferon γ (IFN-γ) secretion to inhibit tumor growth.
Resveratrol, a non-flavonoid polyphenol widely distributed in the leaves and skins of grapes, also be verified that could induce ICD effect in ovarian carcinoma cells78 (Fig. 3C). And related studies showed that the cell surface exposure of CRT and HMGB1 were markedly increased in this kind of cell line. Besides, the effect of cancer vaccines of resveratrol has also been found in tumor rat models, in which subcutaneous injection of ID8 cells pre-treated by resveratrol could dramatically decrease the volume of tumors. Moreover, resveratrol also significantly inhibited the secretion of TGF-β while promotes the numbers of IL12 and IFN-γ.
Zhang et al.79 found the injection of quercetin and alantolactone at a molar ratio of 1:4 could induce ICD in the microsatellite-stable colorectal cancer (CRC) model (Fig. 3D). Their results showed this combination could induce the number of CRT exposed on the tumor cells member and HMGB1 release. In this combination, they found the injection of alantolactone alone could induce the ICD effect and quercetin could enhance this effect through modulating the production of reactive oxygen species (ROS) and interfering with protein kinase (also known as “AKT”) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways80. Moreover, it also could inhibit the secretion of IL-10, TGF-β, IL-1β, and CCL2. And the long-term anti-tumor immune-memory effects of this combination also were found in this study.
Besides making tumor cells be “therapeutic cancer vaccines”, the ICD effect also can enhance the curative effect of DCs-based tumor vaccines. Shikonin, a natural tea quinone pigment from Lithospermum erythrorhizon, has been reported to be an adjuvant for DCs-based cancer vaccines via the induction of ICD effect81 (Fig. 3E). It can effectively trigger mitochondria-associated apoptosis and promote the release of HSP70, HSP78, HSP90, CRT, and HMGB1 in melanoma cells. Besides, shikonin also improves the expressions of CD86 and MHC II and finally enhanced tumor immunogenicity to promote the efficacy of shikonin-treated B16 tumor cells- or doxorubicin-treated B16 tumor cells-loaded DCs vaccines82. In addition, Lin et al.82 also reported shikonin would enhance tumor-immunogenicity of tumor vaccines by ICD.
Therefore, the ICD effect induced by natural products not only can enhance the immunogenicity of tumor cells, which makes tumor cells into “therapeutic vaccines”, but also improve the curative effect of DCs-based tumor vaccines. Meanwhile, these natural products also can downregulate the secretion of immunosuppressive factors, such as IL-10, TGF-β, IL-1β, and CCL2, and upregulate the secretion of anti-tumor factors, like IFN-γ and IL12.
3.2. Natural products improve the therapeutic effect of cancer vaccines as adjuvants
Most the cancer vaccines, especially peptide-based and gene-based vaccines, have poor immune-stimulate effects and should combine with rational adjuvants to achieve ideal effects. And immunosuppressive factors and cells will also inhibit the therapeutic effects of cancer vaccines. However, in previous studies, natural products as adjuvants for vaccines could effectively enhance the immune-stimulate effect and reverse the immunosuppression induced by associated factors and cells.
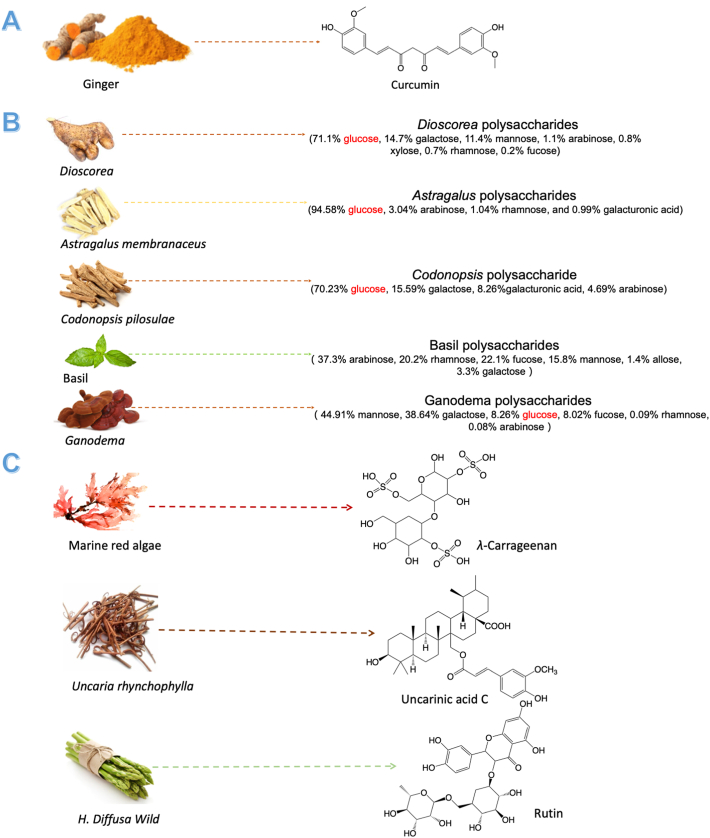
Saponins may be the most extensively reporting vaccine-adjuvants (Fig. 4), which could punch pores on cell membranes that allow antigens to access into cells, presented by MHC I, and finally increase the number of Teffs83. QS-21, an active compound from Quillaja Saponaria Molina, is the most promising saponin immunological adjuvant for cancer vaccines. It can promote the antigen presentation process and enhance the production of Teffs. It also can remodel the immunosuppression through regulating Th1 cytokines, including IL-2 and IFN-γ. Up to now, a series of phase I‒III clinical trials investigate the therapeutic outcomes of utilizing QS-21 as immunological adjuvants of cancer vaccines designed for lymphoma, leukemia, carcinoma, and cancers of breast, prostate, ovary, or lung84, 85, 86, 87, 88, 89, 90, 91, 92. As Fig. 4A shown, QS-21 consists of two principal isomeric molecular constituents and the immunological effect of it mainly attributed to its aldehyde groups and acyl chains. Its aldehyde groups can stimulate the adjuvant activity because they may format Schiff bases with free amino groups on the cell membrane of related immune cells93. And the acyl chains can induce cytotoxic T-cell proliferation94. Besides, three new QS-21 derivatives (SQS-0101, SQS-0102, and SQS-0103) also been reported to improve the stability and potent adjuvant activity of natural QS-2195, 96, 97. According to the advantages of saponins in tumor-vaccines adjuvant, more natural saponins had been investigated for the adjuvant of cancer vaccines. Castro-Díaz et al.98 demonstrated CS5, a new kind of saponins from Crocus sativus corms, which could enhance the production of specific antibodies of protein-based cancer vaccines. They also found the co-stimulatory immunity of CS5 is mainly attributed to its unique acyl group, which also could form Schiff bases with amino groups of immune cells membrane. Zhang et al.99 used Astragalus saponins as an adjuvant for protein-based vaccines and they found it was efficient in stimulating both humoral and cellular immune responses, especially for cellular immune. And the levels of IFN-γ and IL-4 were significantly enhanced by these saponins to improve the antitumor immunity. Ginsenoside Rg1, the well-known saponins from ginseng, was reported to activate the PBMC-derived DCs through promoting the tumor necrosis factor α (TNF-α), IL-1β, IL-8, and IL-6100. And the mechanism of Rg1 in promoting the activation of DCs was probably through modulating the NF-κB pathway in DCs. This activation is often correlated with the vaccine adjuvants' action. Wu et al.101 reported that RelB‒/‒ mice could not develop DC cells due to the genetic defect of NF-κB, which revealed the importance of this pathway for DCs. Then, O'Sullivan et al.102 indicated the importance of NF-κB activation in Teffs activation by DCs and this function would be enhanced if NF-κB activation in DCs is prolonged. Therefore, the regulation of NF-κB plays a critical role in the induction of mature DCs and that is the key point for the adjuvant activation of Rg1.
Besides, some natural products, which can down-regulate the NF-κB signaling pathway, have also been reported that can sensitize cancer vaccines, but the target issues mainly focus on tumor cells. Kamat et al.103 found curcumin has the potential to improve the therapeutic outcome of bacillus Calmette-Guerin vaccines, which is the golden standard immune therapy of bladder cancer, through inhibiting NF-κB and TRAIL pathways in cancer cells (Fig. 5A). Lu et al.104 also reported that curcumin could significantly enhance the effect of the TRP2 peptide vaccine for melanoma. Besides, they found curcumin would decrease the number of immune-suppressive cells (MDSCs and Tregs) in TME by inhibiting the Janus kinase (JAK)-STAT3 signal pathway. In addition, curcumin has been reported could suppress the expression of IDO by blocking the JAK‒STAT1 signaling pathway and it sensitizes the FAPαc vaccine for melanoma through this mechanism105,106. Wei et al.107 found the adjuvant effect of low dosages (1 μg/mL) of Dioscorea polysaccharides for DNA vaccines. Their results showed Dioscorea. polysaccharides could effectively enhance the anti-tumor effect of DNA vaccines for melanoma through the NF-κB inhibition of tumor cells.
Figure 5.
Natural products improve the therapeutic effect of cancer vaccines as adjuvants. (A) Curcumin and dioscorea polysaccharides sensitize cancer vaccines by down-regulating NF-κB signaling pathway in tumor cells. (B) Polysaccharides enhance the efficiency of cancer vaccines. (C) λ-Carrageenan, rutin, and uncarinic acid C sensitive cancer vaccines through TLR4 pathway.
Polysaccharides is the other category of natural products that reportedly sensitize cancer vaccines (Fig. 5B). β-Glucans, the glucose polymers linked together by a 1→3 linear β-glycosidic chain core, is an active compound of polysaccharides for the immune sensitized effect108. It could bind to some immune receptors, such as dectin-1, complement receptor 3, and Toll-like receptor (TLR) -2/6, and activate various immune cells. Some polysaccharides, which rich in β-glucans like Dioscorea polysaccharides, have been reported as potential adjuvants for cancer vaccines. Chang et al.109 evaluated two polysaccharides extracted from Astragalus membranaceus and Codonopsis pilosella for the adjuvant effect of DC-based cancer vaccines for 4T1 mammary carcinoma in mice. They found this combination could significantly enhance the numbers of CD40, CD80, and CD86 markers in DCs110. And their data also showed it could up-regulate the secretion of IL-6, TNF-α, and IL-1β. More importantly, this combination would not generate the “cytokine storm”, which is an excessive expression of vaccine adjuvant. Lv et al.111 investigated the adjuvant effect of basil polysaccharide on DC vaccines for SKOV3 cells. And the results showed that BPS could significantly inhibit the growth of SKOV3 cells and promote the mature of DCs through modulating the expressions of osteopontin, CD44, and matrix metalloprotein-9 (MMP-9). Therefore, it may be a suitable candidate for DC-based vaccines. Mannose and galactose also are two types of polysaccharides that can induce an immune response, but the mechanism is not clear. Ganoderma polysaccharides, which rich in mannose and galactose, can stimulate the maturation of bone marrow dendritic cells (BMDCs) and enhance the secretion of IFN-γ to produce Teffs. Besides, it also could induce DCs maturation. And Ganoderma polysaccharides-adjuvanted ovalbumin (OVA) immunization promoted the production of specific antibodies and enhanced the OVA-specific T helper type 1 (Th1) cells and cytotoxic T cell (CTL) responses, which could both protect mice from OVA-expressing tumor cells. Their data also showed that the immunization of Ganoderma polysaccharides mainly contribute to engaging pattern-recognition receptors families like TLR4112.
TLR4 may be the most important member of the TLR protein for adjuvants of vaccines (Fig. 5C). And it is the only TLR that could activate both MyD88-dependent and -independent (TRIF-dependent) pathways113. As one of the TLR4 agonists, monophosphoryl lipid A is approved as a kind of vaccine adjuvant and has shown immunogenic properties without severe side effects. λ-Carrageenan, which is extracted from marine red algae, could effectively enhance E7-specific immune responses induced by E7-peptide vaccines via TLR4 pathway114, and thus enhance its therapeutic outcomes. In addition, the injection of λ-carrageenan significantly up-regulated the proportion of M1 macrophages and DCs in TME and it also increased the numbers of activated lymphocytes and Th17 cells in mice spleens115. Uncarinic acid C (URC), an active compound from Uncaria hynchophylla, has been demonstrated could activate DCs in a fashion that favors Th1 polarization via the TLR4 pathway, and may be suited for DC-based vaccines116. Rutin, extracted from Hedyotis diffusa Willd, could induce cytokines production of immune cells through the TLR4 signaling pathway and enhance the antitumor effect of peptide-based cancer vaccines in HPV-related tumor models117.
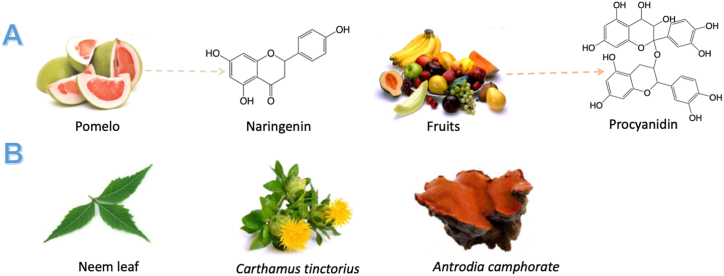
Flavonoid is another type of natural product that reportedly adjuvants potential for cancer vaccines (Fig. 6A). Procyanidin, the biological flavonoid widely distributed in fruit, was evaluated adjuvant effects with B16F10 cancer vaccine for melanoma cancer118. The results showed it could significantly enhance T cell-mediated immune responses and promote the secretion of various molecules like perforin, IFN-γ, and TNF-α. They also showed procyanidin could enhance the production of memory T cells induced by cancer vaccines, and then prolonged the survival rate of model rats. Naringenin, one of the most abundant flavonoids in diets, can improve the efficacy of OVA antitumor therapeutic vaccines by increasing intracellular ROS119. ROS has been reported to enhance MHC-I antigen cross-presentation through either regulation of the pH in the internalization compartments or antigen oxidation for the MHC-I antigen processing120,121. Naringenin-enhanced antigen cross-presentation is dependent on a moderate level of lipid peroxidation that increases antigen leakage from endosomes/lysosomes without impairing DC activation.
Figure 6.
Natural products improve the therapeutic effect of cancer vaccines as adjuvants. (A) Flavonoids; (B) other natural products.
Moreover, there are some other types of natural products that have been explored the adjuvants’ potential for cancer vaccines (Fig. 6B). Neem leaf glycoprotein could effectively up-regulate the expression of CD40, CD80, CD83, CD86, and MHCs on the surface of DCs and these matured DCs also could induce a high amount secretion of IFN-γ by T cells122. Besides, neem leaf glycoprotein would help to induce specific immune responses of TSAs through the antigen presentation modulated by macrophage123. More importantly, it also could increase the numbers of both central and effector memory CD8+ T cells to generate anti-tumor immunity of cancer vaccines124. Carthamus tinctorius could promote the production of IFN-γ and IL-10 of T cells in the spleen and stimulate DCs maturation through the up-regulation of immunological molecule expressions125. When DC-based vaccines were injected with antigens and C. tinctorius, this combination effectively enhances the numbers of TNF-α and IL-1β and stimulate many immunologic molecules that were mainly expressed on the cell membrane of DCs. In addition, C. tinctorius also could increase Teffs production and enhance the therapeutic outcome of cancer vaccines. Antrodia camphorate, a kind of promising adjuvants of human epidermal growth factor receptor-2 (HER-2)/neu DNA vaccines for MBT-2 tumor therapy126, could significantly enhance the Th1-like cellular immune responses and then promoted the production of IFN-γ and HER-2/neu-specific Teffs. Therefore, antrodia camphorate could enhance the antitumor effect of the HER-2/neu DNA vaccine.
As mentioned above, saponins, polysaccharides, and flavonoids are three main categories that showed the potential of being qualified adjuvants for cancer vaccines. And the mechanisms of these three categories are different. Saponins mainly rely on its unique chemical structure to punch pores on cell membranes that allow antigens to access into cells. Polysaccharides mainly depend on its compositions that have the immune-stimulating ability. For flavonoid, its effect on cancer vaccines mainly contributes to the unique regulating effect on immune-associated processes, such as ROS and lipid peroxidation. In addition, the NF-κB and TLR4 signaling pathways are two important pathways that are modulated by natural products to sensitize the efficiencies of cancer vaccines.
4. Natural products enhance the therapeutic effect of immune-check points inhibitors
Immune-check point is the other important way for tumor cells to inhibit the Teffs’ activities. Seven types of immune-checkpoint antibodies have been applied to clinical anti-tumor therapy. And the PD-1/PD-L1 pathway has stood out among ICTs due to the excellent therapeutic outcomes in many studies and clinical trials127. However, the existence of immunosuppressive microenvironments in tumors limits the application of anti-ICTs antibodies. And natural products have been reported to show the ability to regulate the expression of PD-1 and PD-L1 and reverse the immunosuppression.
4.1. Natural products inhibit the expressions of PD-1 and PD-L1 and reverse the immunosuppression
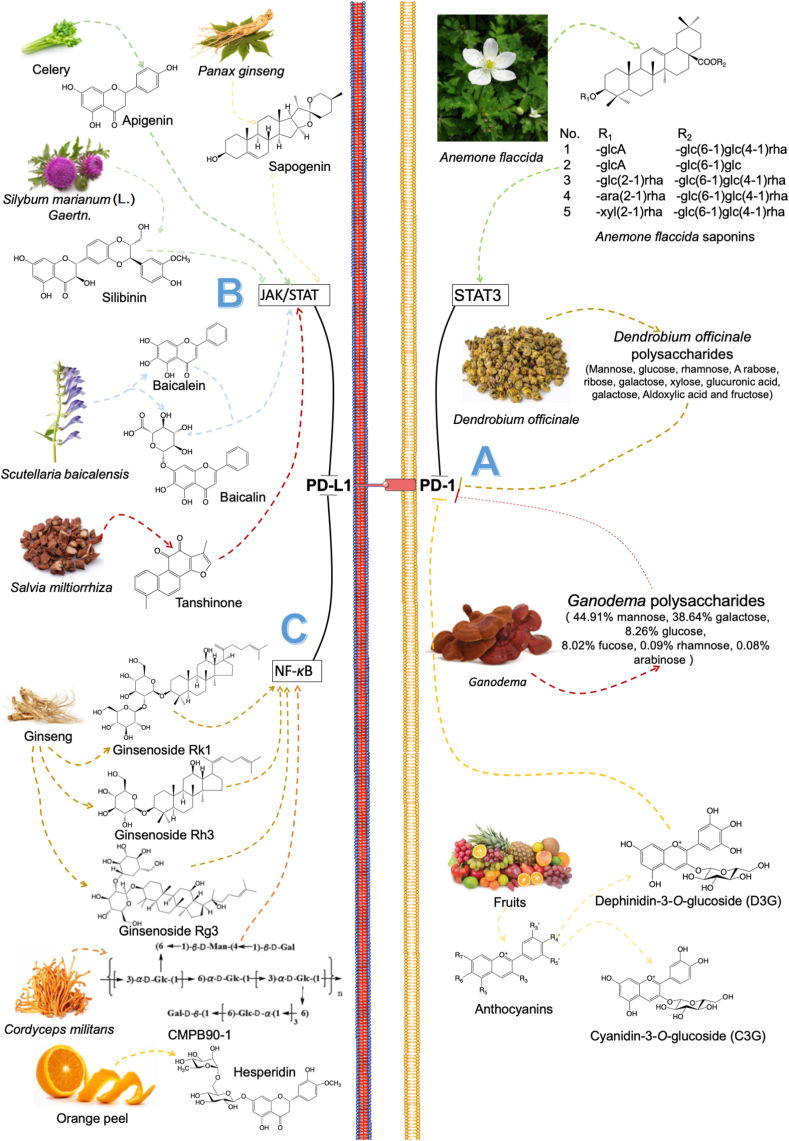
For PD-1 ligand, there are several natural products have been reported to inhibit its expression (Fig. 7A). A kind of triterpenoid saponin isolated from Anemone flaccida inhibited hepatocellular carcinoma (HCC) cell growth by blocking the activation of PD-1 and PD-L1 through the downregulation of STAT3 signaling pathways128. And these triterpenoid saponins also could reduce Tregs production and increased the number of splenic immune cells in H22 tumor-bearing mice. Liang et al.129 investigated the pharmacodynamic of Dendrobium officinale polysaccharides (DOPS) in colorectal cancer, which showed it could effectively improve infiltrating CD8+ cytotoxicity T lymphocyte activity through reducing PD-1 expression and enhance tumor immune response. Ganoderma lucidum polysaccharide combined with paclitaxel (PTX) could reserve the exhausted state of tumor infiltration lymphocytes (TILs) via down-regulating the expressions of PD-1130. Besides, G. lucidum polysaccharide also sensitized the therapeutic effect of PTX and restored the gut dysbiosis induced by PTX. Delphinidin-3-O-glucoside (D3G) and cyanidin-3-O-glucoside (C3G), which active metabolites of anthocyanins (ANC), both can inhibit the expression of PD-1 and PD-L1 in tumor microenvironments of colon cancer cells and induce cancer cell death131.
Figure 7.
Natural products down-regulate the expressions of PD-1 and PD-L1.
For PD-L1 ligand, its expression is controlled by several pathways, such as JAK-STAT pathway132 and NF-κB pathway133 (Fig. 7B and C), and several reviews have summarized the functions of these signaling pathways in regulating the expressions of PD-L1 ligand134, 135, 136. Some natural products have been explored the capacity for the down-regulation of PD-L1 through these pathways.
The JAK-STAT signaling pathway is related to the PD-L1 mRNA expression. It has been reported that increasing the activities of JAK and STAT signal transducers will upregulate the expression of PD-L1137, and thus the agents that can inhibit the JAK-STAT pathway have the ability to downregulate the PD-L1 expression. Zhang et al.138 found that berberine, an isoquinoline alkaloid extracted from Coptidis rhizomes, can reduce the expressions of PD-L1 through the JAK‒STAT signaling pathway and reverse the resistance induced by doxorubicin in the ovarian cancer cell. Silibinin, a type of flavonoid, has been shown to significantly downregulated PD-L1 expression through the JAK‒STAT pathway and increased the immunogenicity of non-small cell lung cancer (NSCLC)139. Baicalein and its conjugate baicalin, which are active flavonoids of Scutellaria baicalensis Georgi, could downregulate the PD-L1 expression through suppressing STAT3 activity and then reactivate T cells sensitivity to kill tumor cells140. A triterpenoid sapogenin monomeric compound extracted from Panax ginseng, panaxadiol reportedly enhanced the activity of CTLs of tumor-cells killing capacity by the suppression of STAT3 activity through JAK1 and JAK2 pathway. And their results also showed panaxadiol downregulated hypoxia-inducible factor-1α (HIF-1α), which also could affect PD-L1 expression141. Han et al.142 investigated tanshinone in hepatocellular carcinoma showed that it can induce apoptosis through the JAK2‒STAT3 signaling pathway and simultaneously down-regulate the PD-1 expression caused by TNF-γ, thereby enhancing the immunotherapy effect. Apigenin, a bioavailable flavonoid found in nature, has been reported that could inhibit the high-level expression of PD-L1 induced by IFN-γ through inhibiting phosphorylation of STAT1 in breast cancer cells and melanoma cells143,144.
The NF-κB pathway is the other pathway that can effectively modulate the expression of PD-L1. The mutated or hyperactivated NF-κB can significantly promote the PD-L1 expression145. Ginsenoside Rk1, an active compound extracted from ginseng, was reported that could inhibit PD-L1 expression in human lung adenocarcinoma (A549 and PC9) cells through inhibiting the NF-κB pathway146. In addition, inhibiting NF-κB transcription also could directly induce the apoptosis of A549 and PC9 cancer cells. Ginsenoside Rg3, the other active compound from ginseng, was identified that could reduce the expression of PD-L1 induced by cisplatin through inhibiting the activation of NF-κB in A549 cells147. And it also reported that Rg3 could evaluate the proportion of T cells in NSCLC patients148. A novel natural polysaccharide from Cordyceps militaris, CMPB90-1, reportedly restrained the PD-L1-PD-1 axis by downregulating AKT/NF-κB pathway149. And then, it also could reverse the inhibitory effects of TAMs on T cells by regulating the secretion of cytokines from M2 macrophages through this pathway. Moreover, Hsu et al.150 also found that C. militaris could reduce the expression of PD-L1 through modulating the secretions of IFN-γ and TNF-α in oral cancer cells and decrease the secretion of IL-17A. Hesperidin, a flavonoid compound from orange peel, has been reported that could strongly inhibit the mRNA expression and protein activity of PD-L1 through the inhibition of AKT/NF-κB signaling in triple-negative breast cancer151.
Besides the natural products mentioned above, there are still some natural products that have been shown could regulate PD-L1 expressions. Buzhong Yiqi Decoction (BYD) is a kind of compound preparation made from Astragali (Radix Astragali), Codonopsis (Radix Codonopsis Pilosella), Trigonal (Rhizoma Sparganii), and other main drugs. Xu et al.152 found this traditional Chinese decoction could down-regulate the expression of PD-1 and PD-L1 in gastric cancer cells to promote CTLs activities through the phosphoinositide 3-kinase (PI3K)/AKT/mechanistic target of rapamycin (mTOR) pathway. Besides, this downregulation effect is further enhanced after the combination with 5-fluorouracil152. SA-49, an active compound extracted from Sophora alopecuroides L., was found could induce melanogenesis associated transcription factor by activating protein kinase Cα and subsequently suppressing glycogen synthase kinase 3β, accordingly triggered lysosome-based degradation of PD-L1153. Yao et al.154 found that chaenomeles speciose nakai has the anti-tumor activity of down-regulating the expression of PD-L1 in a dose-dependent manner, thereby inhibiting tumor growth and enhancing its immune activity.
Therefore, it can be found that the mechanism of inhibition of PD-1/PD-L1 by natural products are mainly attributed to the regulation of the related signaling pathways, which have a common modulated effect of ICTs on almost all kinds of tumors. This mechanism can also remodel the immunosuppression in tumors through these signaling pathways, such as CMPB90-1 reversing the inhibition of TAMs and decreasing the secretion of IL-17A, which is the main difference from that of anti-ICTs antibodies. And this is a unique advantage of natural products in the modulation of ICTs.
4.2. Combination of natural products with anti-PD-1/PD-L1 antibodies
Besides the down-regulation of PD-1 and PD-L1 expressions, natural products also showed outstanding therapeutic outcomes of the combination with anti-PD-1/PD-L1 antibodies (Fig. 8).
Figure 8.
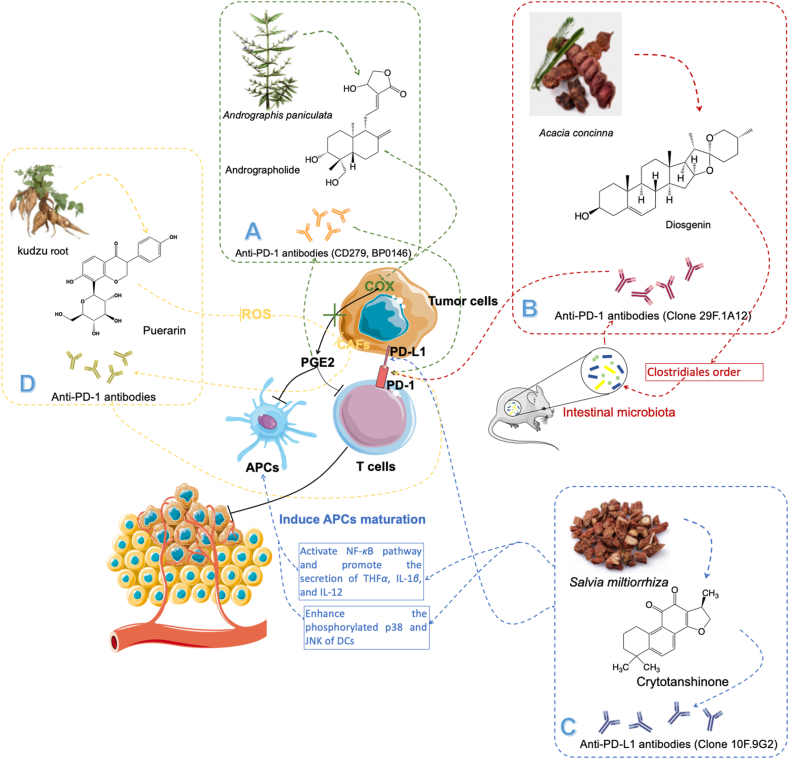
Natural products combine with anti-PD-1 and anti-PD-L1 antibodies to enhance the therapeutic outcomes of these antibodies. (A) Andrographolide improve the efficiency of anti-PD-1 antibodies (CD279, BP0146) by reducing PGE2 secretion; (B)Diosgenin enhance the therapeutic outcomes of anti-PD-1 antibodies (Clone 29F.1A12) by modulating intestinal microbiota; (C) Crytotanshinone improve the efficiency of anti-PD-L1 antibodies (Clone 10F.9G2) through activation of NF-κB pathway; (D) Puerarin improve the efficiency of anti-PD-L1 antibodies through inhibiting the CAFs activities.
Andrographolide, a compound extracted from Andrographis paniculata, plays an important role in Chinese traditional medicine for nearly 100 years (Fig. 8A). Liu et al.155 found it combined with anti-PD-1 antibody (CD279, BP0146) reflected better therapeutic outcomes than single-drug treatment for CT26 colon cancer. And this combination could enhance the activities of Teffs, increase IFN-γ secretion, and promote the expressions of FASL, perforin, and granzyme B, which are all Teffs-associated molecules. And the mechanism of this combined therapy is to inhibit the activity of cyclooxygenase-2 (COX-2) and release of PGE2, which is a key element for keeping the immunosuppression in tumors155.
Diosgenin, a natural steroidal saponin from Acacia concinna, can enhance the activity of anti-PD-1 antibodies by increasing the number of T infiltrated cells156 (Fig. 8B). Dong et al.157 proved that the combination of diosgenin with anti-PD-1 antibody (clone 29F.1A12) could effectively promote necrosis and apoptosis of melanoma cells. And they also found that this combined administration promoted the Teffs infiltration and expression of IFN-γ. Besides, their results showed the mechanism of diosgenin sensitizes the anti-PD-1 antibody mainly contributes to the regulation function of intestinal microbiota. It could increase the number of clostridiales orders, which represents the better sensitivity of anti-PD-1 antibodies therapy.
Cryptotanshinone, an agent purified from Salvia miltiorrhiza, has extremely high medicinal value in China. Liu et al.158 discovered the therapeutic effect of cryptotanshinone at low doses (10 μg/mouse) combined with the anti-PD-L1 antibody (clone 10F.9G2) in LLC-bearing mice (Fig. 8C). The results showed that LLC tumors were cued by this novel combination. And it also reflected the resultant tumor-free mice had resistance to LLC or not B16 melanoma, which illustrated that this combination enhanced the generation of antitumor immune responses and immunological memory of LLC. Besides, they also explore the mechanism of cryptotanshinone-induced DC maturation. Cryptotanshinone could effectively downregulate I-κBα expression and upregulate phosphorylated P65 in DCs, which activate NF-κB pathway and promote the secretion of cytokine genes including TNFα, IL-1β, and IL-12159,160. In addition, cryptotanshinone also enhanced phosphorylated p38 and c-Jun N-terminal kinase (JNK) of DCs that could upregulate the expression of surface costimulatory and MHC molecules159,161.
The cancer-associated fibroblasts (CAFs) is also an important factor that induces the immunosuppression in tumors162. Puerarin, a flavonoid extracted from the kudzu root, is widely used in China as the anti-fibrosis agent in multiple organs, such as the lung, heart, and liver. Xu et al.162 reported that puerarin could sensitize anti-PD-L1 antibody therapy through regulating the ROS level to decrease the number of CAFs (Fig. 8D). ROS is an important factor that can induce immunosuppression and enhance the CAFs proliferation163, 164, 165. Xu et al.162 found that puerarin could significantly inhibits ROS production, which results in the reversing of immunosuppression. The low ROS level also decreases the number of CAFs and this can remove the physical barrier in the 4T1 tumor model, which increases the infiltration of T cells by 2-fold compare to the control group. And the high level of infiltrated T cells enhances the therapeutic outcomes of anti-PD-L1 antibodies.
Although there is no clear conclusion about how to efficiently enhance the therapeutic outcomes of anti-ICTs antibodies, reversing immunosuppression and increasing tumor-infiltrated T cells' number are two important points that have been commonly accepted. As mention above, the inhibition of COX2 and PGE2 induced by andrographolide and activation of NF-κB in immune cells induced by cryptotanshinone both reverse the immunosuppression in tumor and finally improve the anti-ICTs therapy. In addition, the gut microbiome is also an important factor that modulates responses to anti-PD-L1 therapy166. And its mechanism also focuses on the modulation of immunosuppression and enhancement of Teffs’ function, and thus the regulation function of intestinal microbiota induced by diosgenin enhances the outcome of anti-PD-L1 therapy. For increasing the infiltrated T cells in tumors, Xu et al.162 found that puerarin could achieve it through downregulation of CAFs, which open the physical barrier of these T cells. In addition, the low ROS level induced by puerarin also reversing the immunosuppression in the tumor. Therefore, natural products have their unique advantages in the combination of anti-ICTs therapy.
5. Natural products sensitize the adoptive cell immunotherapy
Adoptive cell immunotherapy, especially T-cell-based adoptive immunotherapy, is a promising anti-cancer immunotherapy and has shown excellent therapeutic outcomes in the patients (Fig. 9A)167. Briefly, the process of this immunotherapy consists of three steps: 1) tumor fragments extracted from patient are cultured in vitro under the exaction of IL-2; 2) lymphocytes are overgrown under this condition and tumor cells will be killed after 2–3 weeks; 3) the pure lymphocytes are expanded to about 1011 cells and then adoptive back into patients. Therefore, the number and activity of the target lymphocytes cultured in vitro is the key point for adoptive cell immunotherapy. However, this approach remains challenging due to a series of reasons, including the restriction of antitumor effect by immunosuppressive mechanism, the insufficient anti-tumor effect of T-cells cultured in vitro, and expensive therapeutic cost. As the in-depth investigation of the immune-mediated function of natural products, these obstacles reportedly could be conquered by some natural agents.
Figure 9.
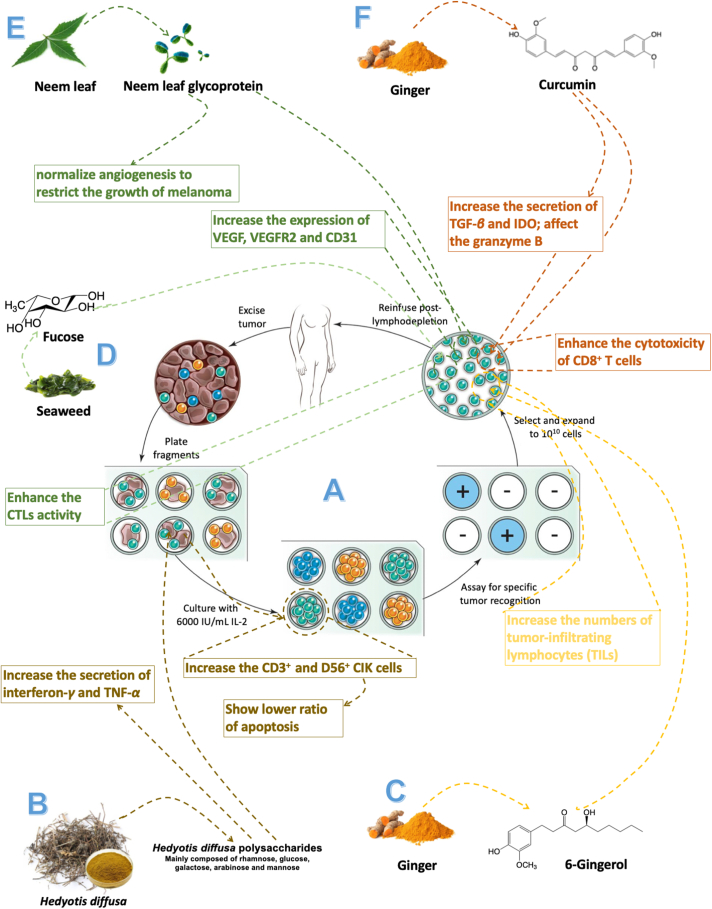
Natural products enhance the therapeutic effect of adoptive cell transfer therapy. (A) The generation of anti-tumor immune cells used for adoptive cell therapy. Reprinted with the permission from Ref. 170. Copyright © 2008, nature publishing group. (B) Hedyotis diffusa polysaccharides improve the efficiency of adoptive treatment of cytokine-induced killer (CIK) cells; (C) 6-gingeral expand the number of T cells in vitro for adoptive therapy; (D) neem leaf glycoprotein (NLGP) can significantly enhance the activity of immune cells in spleen; (E) Fucosylation can enhance the anti-tumor activity of T cells of adoptive therapy; (F) Curcumin improve the efficiency of adoptive T cells treatment.
First of all, natural products can efficiently expand the target lymphocytes in vitro and it also can keep the activities of these cells. Ma et al.168 reported the therapeutic effect of H. diffusa polysaccharides (HDP) on the adoptive treatment of cytokine-induced killer cells (CIKs), which is a kind of safety and feasibility adoptive cells therapy in anti-tumor treatment (Fig. 9B). Their results showed that HDP could up-regulate the percentage of CD3+ and CD56+ CIK cells. And it also had great anti-tumor ability due to the promotion of IFN-γ and TNF-α productions. Besides, there is a lower ratio of apoptosis in CIK cells treated by HDP. Ju et al.169 proved that 6-gingerol, the major active compound of ginger (Zingiber officinale), could effectively enhance the Teffs production in tumors (Fig. 9C). After the treatment with 3.0 μg/mL of 6-gingerol, they found it could effectively inhibit tumor growth due to the increased number of Teffs. Then, they inject CD8+ T cells, which were purified from the 6-gingerol pretreated tumor-bearing mice, into EG7 tumor-bearing mice. And they found more CD8+ T-cells infiltrated in tumors and more cells undergoing division in the 6-gingerol-treated group compared to the control group. Moreover, they treated CD8 T cells with 6-gingerol in vitro and transferred them into EGE7 tumor-bearing RAG2−/− mice. And these 6-gingerol-treated CD8 T-cells also showed outstanding anti-tumor activity. These results showed the great potential of natural products in adoptive cell therapy for cell expansion in vitro and this may reduce the cost of this immunotherapy.
Then, natural products also can enhance the therapeutic outcomes of adoptive cell therapy by improving the Teffs’ activities, enhancing the infiltration of lymphocytes, and remodeling tumor immunosuppressive microenvironments. Alatrash et al.170 reported fucosylation could enhance the homing of antigen-specific CTLs to malignant niches and increase its anti-tumor activity (Fig. 9D). Besides, fucosylation would not increase the homing of CTL in normal tissues. Their results showed fucosylation could alter CTL trafficking, cytolytic machinery, synapse formation, and expression of distinct activating surface molecules. And their method could effectively enhance the therapeutic effect of T-cells-based adoptive immunotherapy. Banerjee et al.171 found that neem leaf glycoprotein (NLGP) could significantly restrict the growth of melanoma through vascular normalization by regulating cell-mediated immunity to enhance the infiltration of immune cells (Fig. 9E). Their results showed the adoptive splenic immune cells therapy treated by NLGP could effectively normalize the angiogenesis, which is mainly induced by tumor-associated microenvironments. Besides, they also analyzed the expression of VEGF, VEGFR2, and CD31 in adoptive transfer immune cells pretreated by NLGP and their expressions all were down-regulated. Thus, NLGP may be suitable sensitize for adoptive immune cells therapy. In addition, Chang et al.172 investigated that curcumin could enhance adoptive T cells therapy through reversing the immunosuppression in tumors (Fig. 9F). They found that the curcumin could enhance the activity of Teffs, which could alter the tumor immunosuppression environment. And curcumin could increase the accumulation and function of T-cells by blocking various immunosuppressive factors and cells, including TGF-β, indoleamine-pyrrole 2,3-dioxygenase (IDO), and Tregs. In addition, the treatment of curcumin also would affect granzyme B promoter-conjugated optical reporter to improve the cytotoxicity of Teffs. And these results suggested that adoptive therapy with curcumin, a kind of multitargeting drug, has the potential for clinical application.
As mention above, natural products not only can expand the target immune cells in vitro, but also enhance the therapeutic effects of this immunotherapy, which shows great potential in adoptive immune-cells therapy.
6. Nano-drug delivery system enhance the therapeutic outcomes of natural products in cancer immunotherapies
After so many advantages have been explored in natural products in cancer immunotherapies, the shortages of natural products were also been emphasized in these studies and limited its further application, including low solubility, low bioavailability, and low tumor targeting. Because of these limitations, most the natural products have to apply to patients with a huge dosage, which may increase the risk of inducing side effects. Therefore, advanced drug-delivery systems should be designed for natural products to overcome these limitations. And the nano-drug delivery system, such as liposomes, micelles, and nano-particles, stands out due to the better outcomes of tumor-targeting, anti-tumor activities, immune-sensitization, stability, and safety.
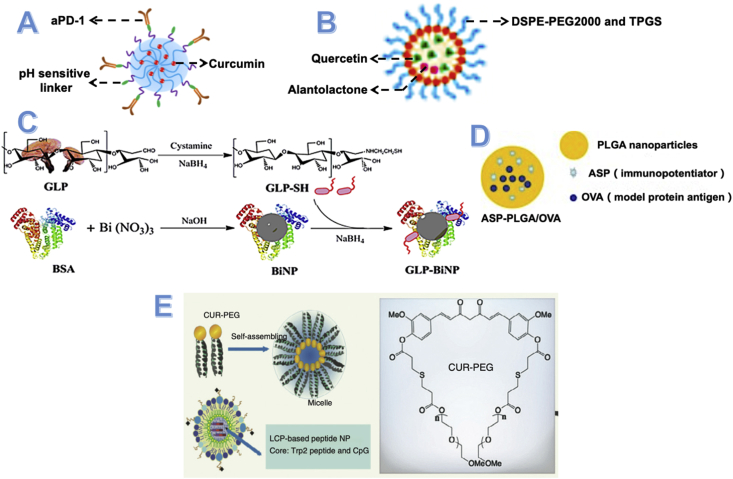
Xiao et al.173 reported a novel nanoparticle-linked anti-PD-1 antibodies on its surface and encapsulating curcumin. And this kind of nanoparticle was designed with dual pH sensitivity (Fig. 10A). Compare to curcumin solution, this kind of nanoparticles enhances the cellular uptake of curcumin. Besides the enhanced permeability and retention effect (EPR) effect, the decoration of anti-PD-1 antibodies will also help nanoparticles binding to PD-1+ T cells. Owing to the pH sensitivity, these nanoparticles showed higher targeting for tumor cells. Then, more curcumin was delivered into the tumor area to inhibit the NF-κB pathway, which significantly inhibits the production of CCL-22, TGF-β, and IL-10. And it will decrease the number of Treg cells and enhance the activities of Teffs. This nanoparticle effectively co-delivery anti-PD-1 antibodies and curcumin into tumors and showed excellent therapeutic outcomes in vivo and in vitro.
Figure 10.
Nano-drug delivery system design for natural products. (A) Scheme image of CUR@PPC-aPD-1. This novel nanoparticle linked anti-PD-1 antibodies on its surface through pH sensitivity linker and encapsulating curcumin. Reprinted with the permission of Ref. 173. Copyright © 2020, American Association for the Advancement of Science. (B) Nano-formulated codelivery of quercetin and alantolactone with DSPE-PEG2000 and TPGS. Reprinted with the permission of Ref. 79. Copyright © 2019, American Chemical Society. (C) Synthesized bismuth sulfide nanoparticles (BiNP) and conjugated with immunoactive Ganoderma lucidum polysaccharide (GLP) to form Ganoderma lucidum polysaccharide-conjugated bismuth sulfide nanoparticles. Reprinted with the permission of Ref. 174. Copyright © 2019, American Chemical Society. (D) Structure of angelica sinensis polysaccharide PLGA nanoparticles encapsulating ASP (immunopotentiator) and OVA (model protein antigen). Reprinted with the permission of Ref. 175. Copyright © 2018, Elsevier B.V. (E) Curcumin–polyethylene glycol conjugate (CUR–PEG), which can self-assemble to nanoparticles, showed combination effect with LCP-based peptide nanoparticles. Reprinted with the permission of Ref. 174. Copyright © 2016, The American Society of Gene & Cell Therapy.
Zhang et al.79 designed a novel nanoparticle encapsulating quercetin (Q) and alantolactone (A), which promoted antitumor responses through the ICD effect for microsatellite-stable colorectal cancer (Fig. 10B). They found the synergistic effect of quercetin and alantolactone at ICD effect and the anti-tumor activities were maximized at the molar ratio of 1:4. To deliver this effective molar ratio into the target area, they developed a new micelle encapsulated with Q and A, which was prepared with DSPE-PEG2000 and d-α-tocopherol polyethylene glycol succinate (TPGS) through the ethanol injection method. And they also found the drug release ratio of these Q-A micelles was also approximately 1:4, which is the optimal molar ratio. After various treatments of these Q-A micelles, a dramatic increase of CRT+ cells induced by the ICD effect and a significant decrease of Tregs and MDSCs also were reported. Moreover, the tumor-promoting inflammation was also inhibited by the G-M micelles treatment.
Yu et al.174 developed a kind of gold nanoparticle (Au-NP) containing Ganoderma lucidum polysaccharide (GLP) to overcome the drawbacks of GLP solutions, such as the short half-life, instability, and low tumor targeting (Fig. 10C). And the results also showed the good characteristics of the GLP-AuNP including increased instability, enhanced tumor targeting, and long-circulating time. Meanwhile, these GLP-AuNPs also kept the immunomodulated function of GLP like inducing dendritic cell (DC) activation and promoting the production of Teffs in the spleen. Besides, GLP-AuNPs exhibited strong inhibitory effects on 4T1 tumor growth and pulmonary metastasis when combined with doxorubicin. This work suggests that polysaccharides from natural herbs can be incorporated into nanocomposites with immunoregulatory characteristics for enhanced efficacy in tumor therapy.
Gu et al.175 successfully encapsulated the immunopotentiator Angelica Sinensis polysaccharide (ASP) and OVA into poly (lactic-co-glycolic acid, PLGA) to formulate the novel NPs-based vaccine delivery system (Fig. 10D). This kind of NPs showed good stability at 4 °C. And the OVA in the core of NPs could effectively release at 37 °C. Besides, these NPs also could enhance the cellular immune response through improve the activity and proliferation of Teffs. Furthermore, a strong specific antibody response induced by OVA and ASP is observed in this study. All of these results proved that the NPs vaccine delivery system could induce longer immune responses compared to the traditional vaccine preparations.
The Liposome is another vesicle to deliver antigen protein into the target area. Zhang et al.99 developed a liposome delivery system of A. saponins and basic fibroblast growth factor (bFGF) antigen to maximize the anti-tumor immune responses induced by these antigens. Through the adjuvant function of A. saponins and bFGF antigen protein, this liposome delivery system could induce lots of specific antibodies and enhance the production of IFN-γ in BALB/c mice. They also found this system could effectively inhibit the angiogenesis in TME, which is an additional anti-tumor function of this novel-designed liposome.
Lu et al.104 reported a kind of curcumin micelles that can remodel the suppressive immune microenvironment to improve the activity of a lipid-based the TRP2 peptide vaccine (Fig. 10E). The combination of curcumin-polyethylene glycol conjugate (CUR-PEG) micelles and TRP2 peptide vaccine showed an outstanding therapeutic effect compared to individual treatments. Moreover, it significantly boosted cytotoxic T-lymphocyte responses and IFN-γ secretion. Besides, this combination therapy also inhibited the production of immunosuppressive factors (such as IL-6 and chemokine ligand 2) and enhanced the number of proinflammatory cytokines (such as TNF-α and IFN-γ). A phenotype switch of M2 to M1 of this kind of micelles has also been reported in this study. These results all showed the great potential of CUR-PEG micelle as a novel adjuvant for peptide cancer vaccines.
Above all, there are some unique advantages of nano-drug delivery systems about improving the therapeutic effect of natural products. First of all, it can efficiently keep the activities and sensitized effect of cancer therapy and increase the circulation time of natural products. Then, it also enhances the tumor-targeting through the modification of some tumor-specific species and decreases the dosage of natural products. Thirdly, the nano-sized particles of natural products reported by previous studies also showed good stability and safety. Therefore, the nano-drug delivery system is an ideal strategy for natural products.
7. Summary and conclusions
After concluding and investigating the previous studies about natural products sensitizing cancer immunotherapy, we find that natural products can effectively enhance the therapeutic effects of all these immunotherapies, including cancer vaccines, immune-check points inhibitors antibodies, and adoptive cells immunotherapy. In addition, natural products successfully expand the indications of immunotherapy into “cold tumor”, such as triple-negative breast tumor, Lewis lung tumor, colorectal tumor model, and so on.
As the review is shown, natural products can enhance the specific cancer immunotherapy through modification of the corresponding mechanisms, such as the induction of ICD effect for cancer vaccines, the increase of infiltrated T cells for anti-ICDs antibodies, and the expansion of the target immune cells in vitro for adoptive immune-cells transfer therapy. More importantly, as for the immunosuppressive factors and cells in tumors, which is also the key element for passivating all kinds of cancer immunotherapies, natural products also have shown outstanding effects on it, such as inhibition of secretion of immunosuppressive factors (TGF-β, PGE2, IL-10 and so on), promotion of secretion of anti-tumor immune factors (IFN-γ, TNF-α, IL-1β and so on), downregulation of the number of immunosuppressive cells (Tregs, MDSCs, M1 macrophage cells, and so on) and enhancement of the activities and number of Teffs. All of these effects of natural products can contribute to the reversion of tumor-associated immunosuppressive microenvironment.
According to Table 2, saponins, polysaccharides, and flavonoids are three main categories that can improve immunotherapy. Some of them even could sensitize two or more kinds of immunotherapy, such as ginsenoside Rg3, ganoderma polysaccharides, and curcumin. And the mechanism mainly depends on the regulation of associated signaling pathways. Some pathways even can improve the therapeutic effects of different immunotherapies, such as the NF-κB pathway for cancer vaccines and anti-PD-L1 antibodies. The reason for that mainly contributes to the connection between these pathways and immunosuppression with tumors. As mentioned in part 1, tumor cells can “hijack” (upregulate or downregulate) some signaling pathways, which associate with the immune system, to induce immunosuppression. Therefore, the agents that can regulate these pathways to a normal level will reverse the immunosuppression and sensitize cancer immunotherapy. Natural products are such agents. And this is also their biggest advantage compared to other agents. According to this unique mechanism, as mentioned above, some natural products even can sensitize two or more kinds of cancer immunotherapies.
Table 2.
List of natural products that improve cancer immunotherapy, including cancer vaccines, immune-check points inhibitors and adoptive cell transfer therapy.
| Category | Source | Natural product | Key points of sensitizing immunotherapy | Ref. |
|---|---|---|---|---|
| Saponins | Ginseng | Ginsenoside Rg3 |
|
74,141,142 |
| Ginsenoside Rg1 |
|
97 | ||
| Ginsenoside Rk1 |
|
140 | ||
| Astragalus | Astragalus saponins |
|
96 | |
| Quillaja saponaria Molina | QS-21 |
|
165 | |
| Crocus sativus corme | CS5 |
|
95 | |
| Anemone flaccida | Anemone flaccida saponins |
|
125 | |
| Panax ginseng | Sapogenin |
|
136 | |
| Acacia concinna | Diosgenin |
|
151 | |
| Polysaccharides | Dioscorea | Dioscrorea polysaccharides |
|
104 |
| Astragalus membranaceus | Astragalus polysaccharides |
|
106 | |
| Codonopsis pilosulae | Codonopsis polysaccharides |
|
106 | |
| Basil | Basil polysaccharides |
|
108 | |
| Ganodema | Ganodema polysaccharides |
|
109 | |
| Dendrobium officinale | Dendrobium officinale polysaccharides |
|
126 | |
| Cordyceps militaris | CMPB90-1 |
|
143 | |
| Hedyotis diffusa | Hedyotis diffusa polysaccharides |
|
157 | |
| Flavonoids | Onion | Quercetin |
|
76 |
| H. Diffusa Wild | Rutin |
|
114 | |
| Fruits | Procyanidin |
|
115 | |
| Pomelo | Naringenin |
|
116 | |
| Silybum marianum (L.) Gaertn. | Silibinin |
|
134 | |
| Scutellaria baicalensis | Baicalein Baicalin |
|
135 | |
| Celery | Apigenin |
|
138 | |
| Orange peel | Hesperidin |
|
145 | |
| Kudzu root | Puerarin |
|
165 | |
| Others | Chili pepper | Capsaicin |
|
68 |
| Grapes | Resveratrol |
|
75 | |
| Ginger | Curcumin |
|
101,158 | |
| 6-Gingerol |
|
161 | ||
| Inula racemosa | Alantolactone |
|
76 | |
| Lithospermum erythrothizon | Shikonin |
|
79 | |
| Marine red algae | λ-Carrageenan |
|
166 | |
| Anthocyanin | Dephinidin-3-O-glucoside (D3G) Cyanidin-3-O-glucoside (C3G) |
|
128 | |
| Coptidis rhizomes | Berberine |
|
133 | |
| Salvia miltiorrhiza | Tanshinone |
|
152 | |
| Crytotanshinone |
|
152 | ||
| Sophora alopecuroides L. | SA-49 |
|
147 | |
| Andrographis paniculata | Andrographolide |
|
149 | |
| Seaweed | Fucose |
|
159 | |
| Neem leaf | Neem leaf glycoprotein |
|
160 |
Besides the significant sensitized effect of natural products into immunotherapy, there are many advantages of application natural products as sensitizers compared to the investigation of new agents. Firstly, most of them mentioned in this review have been approved by the FDA, the cost of increasing indications to them is much lower than developing whole new agents. Then, most the natural products have wide treatment windows and high security. More important, natural products still have other functions like anti-viral, anti-bacterial, anti-inflammatory, anti-fungal, and anti-cancer properties. And these functions could further help the anti-tumor treatments.
Moreover, there are several studies that investigated how to use nanotechnology to overcome the shortages of natural products, such as low solubility, low bioavailability, and low tumor targeting. And these new nanoparticles of natural products not only showed higher tumor targeting and long-term circulation, but also further enhance the anti-tumor effect of immunotherapy. Nano-technology promotes natural products to be the better sensitizer of cancer immunotherapy.
However, there are still some problems that should be solved to make natural products apply to cancer immunotherapy better. First of all, as for so many natural products combined with cancer immunotherapies, it should be screened for the most suitable one based on the specific cancer immunotherapy. For example, for peptide vaccines or mRNA vaccines with poor immune-stimulate effects, the suitable natural products should have the ability to improve this aspect, such as enhancing the antigen-presenting process, but for anti-ICTs therapy, the effect of increasing T infiltrated cells’ number is the most important character for the suitable natural products. Secondly, the signaling pathways related to the immune system and cancer immunotherapy should be explored more deeply and comprehensively. It can be found that the sensitization of natural products on cancer immunotherapy mainly depends on the specific signaling pathways associated with the immune system, and thus a comprehensive understanding of these signaling pathways will be a beneficial to selecting the more effective natural products. Thirdly, there should be more data to provide information about the function of natural products in cancer immunotherapy. Although various natural products that can sensitize cancer immunotherapy have been discussed in this review and some of them even have been applied into clinical trials, the data of this field is still too little compare to that of natural products used in chemotherapy and radiotherapy.
Above all, this review collects and concludes the emerging studies about natural products sensitizing cancer immunotherapies. And there is a clear conclusion that natural products have positive effects on cancer immunotherapies, which mainly depends on their unique modification of tumor immunosuppressive microenvironment. The emerging role of natural products in cancer immunotherapy still has more possible and is worthy to be paid more attention.
Acknowledgments
This work was supported by Science and Technology Plan Project of Shenyang (RC200406, China) and the Career Development Program for Young and Middle-aged Teachers in Shenyang Pharmaceutical University (Shenyang, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Fei Han, Email: hanfei_spu@163.com.
Zhonggui He, Email: hezhonggui@syphu.edu.cn.
Yongjun Wang, Email: wangyongjun@syphu.edu.cn.
Author contributions
Songtao Dong: conceptualization, collecting references and writing; Xiangnan Guo: collecting references; Fei Han: conceptualization, methodology, writing—review & editing; Zhonggui He: conceptualization, supervision, project administration; Yongjun Wang: conceptualization, methodology, writing—review & editing, supervision, project administration, funding acquisition.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Mellman I., Coukos G., Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finck A., Gill S.I., June C.H. Cancer immunotherapy comes of age and looks for maturity. Nat Commun. 2020;11:3325. doi: 10.1038/s41467-020-17140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hargadon K.M., Johnoson C.E., Williams C.J. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 4.O'Leary M.C., Lu X., Huang Y., Lin X., Mahmood I., Przepiorka D., et al. FDA approval summary: tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin Cancer Res. 2019;25:1142–1146. doi: 10.1158/1078-0432.CCR-18-2035. [DOI] [PubMed] [Google Scholar]
- 5.Bouchkouj N., Kasamon Y.L., de Claro R.A., George B., Lin X., Lee S., et al. FDA approval summary: axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma. Clin Cancer Res. 2019;25:1702–1708. doi: 10.1158/1078-0432.CCR-18-2743. [DOI] [PubMed] [Google Scholar]
- 6.Cheever M.A., Hignao C.S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 7.FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Rep. 2010;59:630–632. [PubMed] [Google Scholar]
- 8.Kirby T. FDA approves new upgraded Gardasil 9. Lancet Oncol. 2015;16 doi: 10.1016/S1470-2045(14)71191-X. [DOI] [PubMed] [Google Scholar]
- 9.FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Rep. 2010;59:626–629. [PubMed] [Google Scholar]
- 10.York A. Overcoming hurdles in cancer immunotherapy. Nat Rev Microbiol. 2021;19:222–223. doi: 10.1038/s41579-021-00526-7. [DOI] [PubMed] [Google Scholar]
- 11.Ochoa de Olza M., Navarro Rodrigo B., Zimmermann S., Coukos G. Turning up the heat on non-immunoreactive tumours: opportunities for clinical development. Lancet Oncol. 2020;21:e419–e430. doi: 10.1016/S1470-2045(20)30234-5. [DOI] [PubMed] [Google Scholar]
- 12.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 14.O'Donnell J.S., Teng M.W.L., Smyth M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 15.Tang T., Huang X., Zhang G., Hong Z., Bai X., Liang T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct Target Ther. 2021;6:72. doi: 10.1038/s41392-020-00449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garner H., de Visser K.E. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat Rev Immunol. 2020;20:483–497. doi: 10.1038/s41577-019-0271-z. [DOI] [PubMed] [Google Scholar]
- 17.Rabinovich G.A., Gabrilovich D., Sotomayor E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogg S.J., Beavis P.A., Dawson M.A., Johnstone R.W. Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov. 2020;19:776–800. doi: 10.1038/s41573-020-0077-5. [DOI] [PubMed] [Google Scholar]
- 19.Togashi Y., Shitara K., Nishikawa H. Regulatory T cells in cancer immunosuppression-implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16:356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 20.Mokbel K., Wazir U., Mokbel K. Chemoprevention of prostate cancer by natural agents: evidence from molecular and epidemiological studies. Anticancer Res. 2019;39:5231–5259. doi: 10.21873/anticanres.13720. [DOI] [PubMed] [Google Scholar]
- 21.Prakash Mishra A., Sharifi-Rad M., Shariati M.A., Mabkhot Y.N., Al-Showiman S.S., Rauf A., et al. Bioactive compounds and health benefits of edible Rumex species—a review. Cell Mol Biol. 2018;64:27–34. [PubMed] [Google Scholar]
- 22.Sun B., Yu S., Zhao D., Guo S., Wang X., Zhao K. Polysaccharides as vaccine adjuvants. Vaccine. 2018;36:5226–5234. doi: 10.1016/j.vaccine.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 23.Bahrami A., Fereidouni M., Pirro M., Bianconi V., Sahebkar A. Modulation of regulatory T cells by natural products in cancer. Cancer Lett. 2019;459:72–85. doi: 10.1016/j.canlet.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Zhang Q., Chen Y., Liang C.-L., Liu H., Qiu F., et al. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109570. 109570-70. [DOI] [PubMed] [Google Scholar]
- 25.He J., Yin P., Xu K. Effect and molecular mechanisms of traditional Chinese medicine on tumor targeting tumor-associated macrophages. Drug Des Dev Ther. 2020;14:907–919. doi: 10.2147/DDDT.S223646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudhakaran M., Sardesai S., Doseff A.I. Flavonoids: new frontier for immuno-regulation and breast cancer control. Antioxidants. 2019;8:4. doi: 10.3390/antiox8040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mignani S., Rodrigues J., Tomas H., Zablocka M., Shi X., Caminade A.M., et al. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem Soc Rev. 2018;47:514–532. doi: 10.1039/c7cs00550d. [DOI] [PubMed] [Google Scholar]
- 28.Braicu C., Mehterov N., Vladimirov B., Sarafian V., Nabavi S.M., Atanasov A.G., et al. Nutrigenomics in cancer: revisiting the effects of natural compounds. Semin Cancer Biol. 2017;46:84–106. doi: 10.1016/j.semcancer.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Efferth T., Saeed M.E.M., Kadioglu O., Seo E.J., Shirooie S., Mbaveng A.T., et al. Collateral sensitivity of natural products in drug-resistant cancer cells. Biotechnol Adv. 2020;38:107342. doi: 10.1016/j.biotechadv.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Fontana F., Raimondi M., Di Domizio A., Moretti R.M., Montagnani Marelli M., Limonta P. Unraveling the molecular mechanisms and the potential chemopreventive/therapeutic properties of natural compounds in melanoma. Semin Cancer Biol. 2019;59:266–282. doi: 10.1016/j.semcancer.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Ye J., Zhang R., Wu F., Zhai L., Wang K., Xiao M., et al. Non-apoptotic cell death in malignant tumor cells and natural compounds. Cancer Lett. 2018;420:210–227. doi: 10.1016/j.canlet.2018.01.061. [DOI] [PubMed] [Google Scholar]
- 32.Naysmith B.J., Hume P.A., Sperry J., Brimble M.A. Pyranonaphthoquinones- isolation, biology and synthesis: an update. Nat Prod Rep. 2017;34:25–61. doi: 10.1039/c6np00080k. [DOI] [PubMed] [Google Scholar]
- 33.Fontana F., Raimondi M., Marzagalli M., Di Domizio A., Limonta P. The emerging role of paraptosis in tumor cell biology: perspectives for cancer prevention and therapy with natural compounds. Biochim Biophys Acta Rev Cancer. 2020;1873:188338. doi: 10.1016/j.bbcan.2020.188338. [DOI] [PubMed] [Google Scholar]
- 34.Chhabra G., Singh C.K., Ndiaye M.A., Fedorowicz S., Molot A., Ahmad N. Prostate cancer chemoprevention by natural agents: clinical evidence and potential implications. Cancer Lett. 2018;422:9–18. doi: 10.1016/j.canlet.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garg N., Luzzatto-Knaan T., Melnik A.V., Caraballo-Rodríguez A.M., Floros D.J., Petras D., et al. Natural products as mediators of disease. Nat Prod Rep. 2017;34:194–219. doi: 10.1039/c6np00063k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Branca M.A. Rekindling cancer vaccines. Nat Biotechnol. 2016;34:1019–1024. doi: 10.1038/nbt.3690. [DOI] [PubMed] [Google Scholar]
- 37.Ott P.A., Hodi F.S. Talimogene laherparepvec for the treatment of advanced melanoma. Clin Cancer Res. 2016;22:3127–3131. doi: 10.1158/1078-0432.CCR-15-2709. [DOI] [PubMed] [Google Scholar]
- 38.Gubin M.M., Zhang X., Schuster H., Caron E., Ward J.P., Noguchi T., et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marincola F.M., Jaffee E.M., Hicklin D.J., Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 40.Blank C., Gajewski T.F., Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sotomayor E.M., Borrello I., Tubb E., Allison J.P., Levitsky H.I. In vivo blockade of CTLA-4 enhances the priming of responsive T cells but fails to prevent the induction of tumor antigen-specific tolerance. Proc Natl Acad Sci U S A. 1999;96:11476–11481. doi: 10.1073/pnas.96.20.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Elsas A., Hurwitz A.A., Allison J.P. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phan G.Q., Yang J.C., Sherry R.M., Hwu P., Topalian S.L., Schwartzentruber D.J., et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 45.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 46.Coleman J.W. Nitric oxide: a regulator of mast cell activation and mast cell-mediated inflammation. Clin Exp Immunol. 2002;129:4–10. doi: 10.1046/j.1365-2249.2002.01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vliagoftis H., Boucher W.S., Mak L.L., Theoharides T.C. Inhibition of mast cell secretion by oxidation products of natural polyamines. Biochem Pharmacol. 1992;43:2237–2245. doi: 10.1016/0006-2952(92)90183-j. [DOI] [PubMed] [Google Scholar]
- 48.Takanami I., Takeuchi K., Naruke M. Mast cell density is associated with angiogenesis and poor prognosis in pulmonary adenocarcinoma. Cancer. 2000;88:2686–2692. [PubMed] [Google Scholar]
- 49.Frossi B., De Carli M., Daniel K.C., Rivera J., Pucillo C. Oxidative stress stimulates IL-4 and IL-6 production in mast cells by an APE/Ref-1-dependent pathway. Eur J Immunol. 2003;33:2168–2177. doi: 10.1002/eji.200323995. [DOI] [PubMed] [Google Scholar]
- 50.Theoharides T.C., Kempuraj D., Tagen M., Conti P., Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 51.Gutzmer R., Diestel C., Mommert S., Köther B., Stark H., Wittmann M., et al. Histamine H4 receptor stimulation suppresses IL-12p70 production and mediates chemotaxis in human monocyte-derived dendritic cells. J Immunol. 2005;174:5224–5232. doi: 10.4049/jimmunol.174.9.5224. [DOI] [PubMed] [Google Scholar]
- 52.Worm J., Falkenberg K., Olesen J. Histamine and migraine revisited: mechanisms and possible drug targets. J Headache Pain. 2019;20:30. doi: 10.1186/s10194-019-0984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conti P., Kempuraj D., Kandere K., Di Gioacchino M., Barbacane R.C., Castellani M.L., et al. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol Lett. 2003;86:123–129. doi: 10.1016/s0165-2478(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 54.Wasiuk A., de Vries V.C., Hartmann K., Roers A., Noelle R.J. Mast cells as regulators of adaptive immunity to tumours. Clin Exp Immunol. 2009;155:140–146. doi: 10.1111/j.1365-2249.2008.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pollard J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 56.Bingle L., Brown N.J., Lewis C.E. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 57.Kim R., Emi M., Tanabe K., Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 58.Lewis C.E., Pollard J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 59.Khazaie K., von Boehmer H. The impact of CD4+CD25+ Treg on tumor specific CD8+ T cell cytotoxicity and cancer. Semin Cancer Biol. 2006;16:124–136. doi: 10.1016/j.semcancer.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 61.Finn O.J. The dawn of vaccines for cancer prevention. Nat Rev Immunol. 2018;18:183–194. doi: 10.1038/nri.2017.140. [DOI] [PubMed] [Google Scholar]
- 62.Butterfield L.H. Cancer vaccines. BMJ. 2015;350:h988. doi: 10.1136/bmj.h988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palucka K., Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melero I., Gustav G., Gerritsen W., Huber C., Parmiani G., Scholl S., et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 65.Sahin U., Tureci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359:1355–1360. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 66.Hu Z., Ott P.A., Wu C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18:168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kroemer G., Galluzzi L., Kepp O., Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 68.Kepp O., Senovilla L., Vitale I., Vacchelli E., Adjemian S., Agostinis P., et al. Consensus guidelines for the detection of immunogenic cell death. OncoImmunology. 2014;3 doi: 10.4161/21624011.2014.955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galluzzi L., Vacchelli E., Bravo-San Pedro J.M., Buqué A., Senovilla L., Baracco E.E., et al. Classification of current anticancer immunotherapies. Oncotarget. 2014;5:12472–12508. doi: 10.18632/oncotarget.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radogna F., Dicato M., Diederich M. Natural modulators of the hallmarks of immunogenic cell death. Biochem Pharmacol. 2019;162:55–70. doi: 10.1016/j.bcp.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 71.Granato M., Gilardini Montani M.S., Filardi M., Faggioni A., Cirone M. Capsaicin triggers immunogenic PEL cell death, stimulates DCs and reverts PEL-induced immune suppression. Oncotarget. 2015;6:29543–29554. doi: 10.18632/oncotarget.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Basu S., Srivastava P. Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells. Proc Natl Acad Sci U S A. 2005;102:5120–5125. doi: 10.1073/pnas.0407780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Díaz-Laviada I., Rodríguez-Henche N. The potential antitumor effects of capsaicin. Prog Drug Res. 2014;68:181–208. doi: 10.1007/978-3-0348-0828-6_8. [DOI] [PubMed] [Google Scholar]
- 74.Jung M.Y., Kang H.J., Moon A. Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells involves Bcl-2 downregulation and caspase-3 activation. Cancer Lett. 2001;165:139–145. doi: 10.1016/s0304-3835(01)00426-8. [DOI] [PubMed] [Google Scholar]
- 75.Lee S.H., Krisanapun C., Baek S.J. NSAID-activated gene-1 as a molecular target for capsaicin-induced apoptosis through a novel molecular mechanism involving GSK3beta, C/EBPbeta and ATF3. Carcinogenesis. 2010;31:719–728. doi: 10.1093/carcin/bgq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ito K., Nakazato T., Yamato K., Miyakawa Y., Yamada T., Hozumi N., et al. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004;64:1071–1078. doi: 10.1158/0008-5472.can-03-1670. [DOI] [PubMed] [Google Scholar]
- 77.Son K.J., Choi K.R., Lee S.J., Lee H. Immunogenic cell death induced by ginsenoside Rg3: significance in dendritic cell-based anti-tumor immunotherapy. Immune Netw. 2016;16:75–84. doi: 10.4110/in.2016.16.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y., Yang S., Yang Y., Liu T. Resveratrol induces immunogenic cell death of human and murine ovarian carcinoma cells. Infect Agent Cancer. 2019;14:27. doi: 10.1186/s13027-019-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang J., Shen L., Li X., Song W., Liu Y., Huang L. Nanoformulated codelivery of Quercetin and Alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano. 2019;13:12511–12524. doi: 10.1021/acsnano.9b02875. [DOI] [PubMed] [Google Scholar]
- 80.Ward A.B., Mir H., Kapur N., Gales D.N., Carriere P.P., Singh S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J Surg Oncol. 2018;16:108. doi: 10.1186/s12957-018-1400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen H.M., Wang P.H., Chen S.S., Wen C.C., Chen Y.H., Yang W.C., et al. Shikonin induces immunogenic cell death in tumor cells and enhances dendritic cell-based cancer vaccine. Cancer Immunol Immunother. 2012;61:1989–2002. doi: 10.1007/s00262-012-1258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin T.J., Lin H.T., Chang W.T., Mitapalli S.P., Hsiao P.W., Yin S.Y., et al. Shikonin-enhanced cell immunogenicity of tumor vaccine is mediated by the differential effects of DAMP components. Mol Cancer. 2015;14:174. doi: 10.1186/s12943-015-0435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun H.X., Xie Y., Ye Y.P. Advances in saponin-based adjuvants. Vaccine. 2009;27:1787–1796. doi: 10.1016/j.vaccine.2009.01.091. [DOI] [PubMed] [Google Scholar]
- 84.Livingston P.O., Adluri S., Helling F., Yao T.J., Kensil C.R., Newman M.J., et al. Phase 1 trial of immunological adjuvant QS-21 with a GM2 ganglioside-keyhole limpet haemocyanin conjugate vaccine in patients with malignant melanoma. Vaccine. 1994;12:1275–1280. doi: 10.1016/s0264-410x(94)80052-2. [DOI] [PubMed] [Google Scholar]
- 85.Ragupathi G., Livingston P.O., Hood C., Gathuru J., Krown S.E., Chapman P.B., et al. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21. Clin Cancer Res. 2003;9:5214–5220. [PubMed] [Google Scholar]
- 86.Ragupathi G., Meyers M., Adluri S., Howard L., Musselli C., Livingston P.O. Induction of antibodies against GD3 ganglioside in melanoma patients by vaccination with GD3-lactone-KLH conjugate plus immunological adjuvant QS-21. Int J Cancer. 2000;85:659–666. doi: 10.1002/(sici)1097-0215(20000301)85:5<659::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 87.Gilewski T., Adluri S., Ragupathi G., Zhang S., Yao T.J., Panageas K., et al. Vaccination of high-risk breast cancer patients with mucin-1 (MUC1) keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res. 2000;6:1693–1701. [PubMed] [Google Scholar]
- 88.Slovin S.F., Ragupathi G., Fernandez C., Diani M., Jefferson M.P., Wilton A., et al. A polyvalent vaccine for high-risk prostate patients: "are more antigens better? Cancer Immunol Immunother. 2007;56:1921–1930. doi: 10.1007/s00262-007-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Slovin S.F., Ragupathi G., Musselli C., Fernandez C., Diani M., Verbel D., et al. Thomsen-Friedenreich (TF) antigen as a target for prostate cancer vaccine: clinical trial results with TF cluster (c)-KLH plus QS21 conjugate vaccine in patients with biochemically relapsed prostate cancer. Cancer Immunol Immunother. 2005;54:694–702. doi: 10.1007/s00262-004-0598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sabbatini P.J., Ragupathi G., Hood C., Aghajanian C.A., Juretzka M., Iasonos A., et al. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin Cancer Res. 2007;13:4170–4177. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- 91.Krug L.M., Ragupathi G., Hood C., Kris M.G., Miller V.A., Allen J.R., et al. Vaccination of patients with small-cell lung cancer with synthetic fucosyl GM-1 conjugated to keyhole limpet hemocyanin. Clin Cancer Res. 2004;10:6094–6100. doi: 10.1158/1078-0432.CCR-04-0482. [DOI] [PubMed] [Google Scholar]
- 92.Kirkwood J.M., Manola J., Ibrahim J., Sondak V., Ernstoff M.S., Rao U. A pooled analysis of Eastern Cooperative Oncology Group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670–1677. doi: 10.1158/1078-0432.ccr-1103-3. [DOI] [PubMed] [Google Scholar]
- 93.Rhodes J. Evidence for an intercellular covalent reaction essential in antigen-specific T cell activation. J Immunol. 1989;143:1482–1489. [PubMed] [Google Scholar]
- 94.Marciani D.J., Press J.B., Reynolds R.C., Pathak A.K., Pathak V., Gundy L.E., et al. Development of semisynthetic triterpenoid saponin derivatives with immune stimulating activity. Vaccine. 2000;18:3141–3151. doi: 10.1016/s0264-410x(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 95.Lacaille-Dubois M.A., Wagner H. New perspectives for natural triterpene glycosides as potential adjuvants. Phytomedicine. 2017;37:49–57. doi: 10.1016/j.phymed.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 96.Fernández-Tejada A., Tan D.S., Gin D.Y. Development of improved vaccine adjuvants based on the saponin natural product QS-21 through chemical synthesis. Acc Chem Res. 2016;49:1741–1756. doi: 10.1021/acs.accounts.6b00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adams M.M., Damani P., Perl N.R., Won A., Hong F., Livingston P.O., et al. Design and synthesis of potent Quillaja saponin vaccine adjuvants. J Am Chem Soc. 2010;132:1939–1945. doi: 10.1021/ja9082842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Castro-Díaz N., Salaun B., Perret R., Sierro S., Romero J.F., Fernández J.A., et al. Saponins from the Spanish saffron Crocus sativus are efficient adjuvants for protein-based vaccines. Vaccine. 2012;30:388–397. doi: 10.1016/j.vaccine.2011.10.080. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X.P., Li Y.D., Luo L.L., Liu Y.Q., Li Y., Guo C., et al. Astragalus saponins and liposome constitute an efficacious adjuvant formulation for cancer vaccines. Cancer Biother Radiopharm. 2018;33:25–31. doi: 10.1089/cbr.2017.2369. [DOI] [PubMed] [Google Scholar]
- 100.Huang Y., Zou Y., Lin L., Zheng R. Ginsenoside Rg1 activates dendritic cells and acts as a vaccine adjuvant inducing protective cellular responses against lymphomas. DNA Cell Biol. 2017;36:1168–1177. doi: 10.1089/dna.2017.3923. [DOI] [PubMed] [Google Scholar]
- 101.Wu L., D'Amico A., Winkel K.D., Suter M., Lo D., Shortman K. RelB is essential for the development of myeloid-related CD8alpha‒ dendritic cells but not of lymphoid-related CD8alpha+ dendritic cells. Immunity. 1998;9:839–847. doi: 10.1016/s1074-7613(00)80649-4. [DOI] [PubMed] [Google Scholar]
- 102.O'Sullivan B.J., Thomas R. CD40 ligation conditions dendritic cell antigen-presenting function through sustained activation of NF-kappaB. J Immunol. 2002;168:5491–5498. doi: 10.4049/jimmunol.168.11.5491. [DOI] [PubMed] [Google Scholar]
- 103.Kamat A.M., Tharakan S.T., Sung B., Aggarwal B.B. Curcumin potentiates the antitumor effects of Bacillus Calmette-Guerin against bladder cancer through the downregulation of NF-kappaB and upregulation of TRAIL receptors. Cancer Res. 2009;69:8958–8966. doi: 10.1158/0008-5472.CAN-09-2045. [DOI] [PubMed] [Google Scholar]
- 104.Lu Y., Miao L., Wang Y., Xu Z., Zhao Y., Shen Y., et al. Curcumin micelles remodel tumor microenvironment and enhance vaccine activity in an advanced melanoma model. Mol Ther. 2016;24:364–374. doi: 10.1038/mt.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jeong Y.I., Kim S.W., Jung I.D., Lee J.S., Chang J.H., Lee C.M., et al. Curcumin suppresses the induction of indoleamine 2,3-dioxygenase by blocking the Janus-activated kinase-protein kinase Cdelta-STAT1 signaling pathway in interferon-gamma-stimulated murine dendritic cells. J Biol Chem. 2009;284:3700–3708. doi: 10.1074/jbc.M807328200. [DOI] [PubMed] [Google Scholar]
- 106.Huang T., Chen Z., Fang L. Curcumin inhibits LPS-induced EMT through downregulation of NF-κB‒Snail signaling in breast cancer cells. Oncol Rep. 2013;29:117–124. doi: 10.3892/or.2012.2080. [DOI] [PubMed] [Google Scholar]
- 107.Wei W.C., Wang J.H., Aravindaram K., Wang S.J., Hsu C.C., Li C.J., et al. Polysaccharides from dioscorea (shān yào) and other phytochemicals enhance antitumor effects induced by DNA vaccine against melanoma. J Tradit Complement Med. 2014;4:42–48. doi: 10.4103/2225-4110.124342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chan G.C., Chan W.K., Sze D.M. The effects of beta-glucan on human immune and cancer cells. J Hematol Oncol. 2009;2:25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chang W.T., Lai T.H., Chyan Y.J., Yin S.Y., Chen Y.H., Wei W.C., et al. Specific medicinal plant polysaccharides effectively enhance the potency of a DC-based vaccine against mouse mammary tumor metastasis. PloS One. 2015;10 doi: 10.1371/journal.pone.0122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jonuleit H., Kühn U., Müller G., Steinbrink K., Paragnik L., Schmitt E., et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 111.Lv J., Shao Q., Wang H., Shi H., Wang T., Gao W., et al. Effects and mechanisms of curcumin and basil polysaccharide on the invasion of SKOV3 cells and dendritic cells. Mol Med Rep. 2013;8:1580–1586. doi: 10.3892/mmr.2013.1695. [DOI] [PubMed] [Google Scholar]
- 112.Pi C.C., Chu C.L., Lu C.Y., Zhuang Y.J., Wang C.L., Yu Y.H., et al. Polysaccharides from Ganoderma formosanum function as a Th1 adjuvant and stimulate cytotoxic T cell response in vivo. Vaccine. 2014;32:401–408. doi: 10.1016/j.vaccine.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 113.Fitzpatrick J.M., Minogue E., Curham L., Tyrrell H., Gavigan P., Hind W., et al. MyD88-dependent and -independent signalling via TLR3 and TLR4 are differentially modulated by Δ9-tetrahydrocannabinol and cannabidiol in human macrophages. J Neuroimmunol. 2020;343:577217. doi: 10.1016/j.jneuroim.2020.577217. [DOI] [PubMed] [Google Scholar]
- 114.Jung T.Y., Pham T.N., Umeyama A., Shoji N., Hashimoto T., Lee J.J., et al. Ursolic acid isolated from Uncaria rhynchophylla activates human dendritic cells via TLR2 and/or TLR4 and induces the production of IFN-gamma by CD4+ naïve T cells. Eur J Pharmacol. 2010;643:297–303. doi: 10.1016/j.ejphar.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 115.Lin C.C., Pan I.H., Li Y.R., Pan Y.G., Lin M.K., Lu Y.H., et al. The adjuvant effects of high-molecule-weight polysaccharides purified from Antrodia cinnamomea on dendritic cell function and DNA vaccines. PloS One. 2015;10 doi: 10.1371/journal.pone.0116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim K.S., Pham T.N., Jin C.J., Umeyama A., Shoji N., Hashimoto T., et al. Uncarinic Acid C isolated from Uncaria rhynchophylla induces differentiation of Th1-promoting dendritic cells through TLR4 signaling. Biomark Insights. 2011;6:27–38. doi: 10.4137/BMI.S6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Song Y.C., Huang H.C., Chang C.Y., Lee H.J., Liu C.T., Lo H.Y., et al. A potential herbal adjuvant combined with a peptide-based vaccine acts against HPV-related tumors through enhancing effector and memory T-cell immune responses. Front Immunol. 2020;11:62. doi: 10.3389/fimmu.2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang P.M., Du J.L., Wang G.N., Chia J.S., Hsu W.B., Pu P.C., et al. The Chinese herbal mixture Tien-Hsien liquid augments the anticancer immunity in tumor cell-vaccinated mice. Integr Cancer Ther. 2017;16:319–328. doi: 10.1177/1534735416651492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang L., Zeng W., Wang L., Wang Z., Yin X., Qin Y., et al. Naringenin enhances the antitumor effect of therapeutic vaccines by promoting antigen cross-presentation. J Immunol. 2020;204:622–631. doi: 10.4049/jimmunol.1900278. [DOI] [PubMed] [Google Scholar]
- 120.Teoh C.Y., Davies K.J. Potential roles of protein oxidation and the immunoproteasome in MHC class I antigen presentation: the 'PrOxI' hypothesis. Arch Biochem Biophys. 2004;423:88–96. doi: 10.1016/j.abb.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 121.Amigorena S., Savina A. Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr Opin Immunol. 2010;22:109–117. doi: 10.1016/j.coi.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 122.Goswami S., Bose A., Sarkar K., Roy S., Chakraborty T., Sanyal U., et al. Neem leaf glycoprotein matures myeloid derived dendritic cells and optimizes anti-tumor T cell functions. Vaccine. 2010;28:1241–1252. doi: 10.1016/j.vaccine.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 123.Guo L., Shi G., Gao Z., Shen J., Xing R., Qian Z. Response to hepatocarcinoma Hca-F of mice immunized with heat shock protein 70 from elemene combo tumor cell vaccine. Cell Mol Immunol. 2006;3:291–295. [PubMed] [Google Scholar]
- 124.Ghosh S., Sarkar M., Ghosh T., Guha I., Bhuniya A., Saha A., et al. Neem leaf glycoprotein promotes dual generation of central and effector memory CD8+ T cells against sarcoma antigen vaccine to induce protective anti-tumor immunity. Mol Immunol. 2016;71:42–53. doi: 10.1016/j.molimm.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 125.Chang J.M., Hung L.M., Chyan Y.J., Cheng C.M., Wu R.Y. Carthamus tinctorius enhances the antitumor activity of dendritic cell vaccines via polarization toward Th1 cytokines and increase of cytotoxic T lymphocytes. Evid Based Complement Alternat Med. 2011;2011:274858. doi: 10.1093/ecam/nen068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang C.H., Chang C.C., Lin C.M., Wang S.T., Wu M.T., Li E.I., et al. Promoting effect of Antrodia camphorata as an immunomodulating adjuvant on the antitumor efficacy of HER-2/neu DNA vaccine. Cancer Immunol Immunother. 2010;59:1259–1272. doi: 10.1007/s00262-010-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Han L., Yao S., Cao S., Mo G., Li J., Cao Y., et al. Triterpenoid saponins from Anemone flaccida suppress tumor cell proliferation by regulating MAPK, PD1/PDL1, and STAT3 signaling pathways and altering cancer metabolism. OncoTargets Ther. 2019;12:10917–10930. doi: 10.2147/OTT.S212666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liang J., Li H., Chen J., He L., Du X., Zhou L., et al. Dendrobium officinale polysaccharides alleviate colon tumorigenesis via restoring intestinal barrier function and enhancing anti-tumor immune response. Pharmacol Res. 2019;148:104417. doi: 10.1016/j.phrs.2019.104417. [DOI] [PubMed] [Google Scholar]
- 130.Chen Y., Wang Y., Xu L., Zhu W., Xu C., Xu M., et al. Influence of total glucosides of paeony on PD-1/PD-L1 expression in primary Sjögren's syndrome. Int J Rheum Dis. 2019;22:200–206. doi: 10.1111/1756-185X.13391. [DOI] [PubMed] [Google Scholar]
- 131.Mazewski C., Kim M.S., Gonzalez de Mejia E. Anthocyanins, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside, inhibit immune checkpoints in human colorectal cancer cells in vitro and in silico. Sci Rep. 2019;9:11560. doi: 10.1038/s41598-019-47903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Platanias L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 133.Lu Y.C., Yeh W.C., Ohashi P.S. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 134.Cha J.H., Chan L.C., Li C.W., Hsu J.L., Hung M.C. Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 2019;76:359–370. doi: 10.1016/j.molcel.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kakavand H., Rawson R.V., Pupo G.M., Yang J.Y.H., Menzies A.M., Carlino M.S., et al. PD-L1 expression and immune escape in melanoma resistance to MAPK inhibitors. Clin Cancer Res. 2017;23:6054–6061. doi: 10.1158/1078-0432.CCR-16-1688. [DOI] [PubMed] [Google Scholar]
- 136.Ju X., Zhang H., Zhou Z., Wang Q. Regulation of PD-L1 expression in cancer and clinical implications in immunotherapy. Am J Cancer Res. 2020;10:1–11. [PMC free article] [PubMed] [Google Scholar]
- 137.Prestipino A., Emhardt A.J., Aumann K., O'Sullivan D., Gorantla S.P., Duquesne S., et al. Oncogenic JAK2(V617F) causes PD-L1 expression, mediating immune escape in myeloproliferative neoplasms. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aam7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang S., Zhou L., Zhang M., Wang Y., Wang M., Du J., et al. Berberine maintains the neutrophil N1 phenotype to reverse cancer cell resistance to doxorubicin. Front Pharmacol. 2019;10:1658. doi: 10.3389/fphar.2019.01658. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 139.Cuyàs E., Pérez-Sánchez A., Micol V., Menendez J.A., Bosch-Barrera J. STAT3-targeted treatment with silibinin overcomes the acquired resistance to crizotinib in ALK-rearranged lung cancer. Cell Cycle. 2016;15:3413–3418. doi: 10.1080/15384101.2016.1245249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ke M., Zhang Z., Xu B., Zhao S., Ding Y., Wu X., et al. Baicalein and baicalin promote antitumor immunity by suppressing PD-L1 expression in hepatocellular carcinoma cells. Int Immunopharmacol. 2019;75:105824. doi: 10.1016/j.intimp.2019.105824. [DOI] [PubMed] [Google Scholar]
- 141.Wang Z., Li M.Y., Zhang Z.H., Zuo H.X., Wang J.Y., Xing Y., et al. Panaxadiol inhibits programmed cell death-ligand 1 expression and tumour proliferation via hypoxia-inducible factor (HIF)-1α and STAT3 in human colon cancer cells. Pharmacol Res. 2020;155:104727. doi: 10.1016/j.phrs.2020.104727. [DOI] [PubMed] [Google Scholar]
- 142.Han Z., Liu S., Lin H., Trivett A.L., Hannifin S., Yang D., et al. Inhibition of murine hepatoma tumor growth by cryptotanshinone involves TLR7-dependent activation of macrophages and induction of adaptive antitumor immune defenses. Cancer Immunol Immunother. 2019;68:1073–1085. doi: 10.1007/s00262-019-02338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu L., Zhang Y., Tian K., Chen X., Zhang R., Mu X., et al. Apigenin suppresses PD-L1 expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects. J Exp Clin Cancer Res. 2018;37:261. doi: 10.1186/s13046-018-0929-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Coombs M.R., Harrison M.E., Hoskin D.W. Apigenin inhibits the inducible expression of programmed death ligand 1 by human and mouse mammary carcinoma cells. Cancer Lett. 2016;380:424–433. doi: 10.1016/j.canlet.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 145.Peng J., Hamanishi J., Matsumura N., Abiko K., Murat K., Baba T., et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75:5034–5045. doi: 10.1158/0008-5472.CAN-14-3098. [DOI] [PubMed] [Google Scholar]
- 146.Hu M., Yang J., Qu L., Deng X., Duan Z., Fu R., et al. Ginsenoside Rk1 induces apoptosis and downregulates the expression of PD-L1 by targeting the NF-κB pathway in lung adenocarcinoma. Food Funct. 2020;11:456–471. doi: 10.1039/c9fo02166c. [DOI] [PubMed] [Google Scholar]
- 147.Jiang Z., Yang Y., Yang Y., Zhang Y., Yue Z., Pan Z., et al. Ginsenoside Rg3 attenuates cisplatin resistance in lung cancer by downregulating PD-L1 and resuming immune. Biomed Pharmacother. 2017;96:378–383. doi: 10.1016/j.biopha.2017.09.129. [DOI] [PubMed] [Google Scholar]
- 148.Lu P., Su W., Miao Z.H., Niu H.R., Liu J., Hua Q.L. Effect and mechanism of ginsenoside Rg3 on postoperative life span of patients with non-small cell lung cancer. Chin J Integr Med. 2008;14:33–36. doi: 10.1007/s11655-007-9002-6. [DOI] [PubMed] [Google Scholar]
- 149.Bi S., Huang W., Chen S., Huang C., Li C., Guo Z., et al. Cordyceps militaris polysaccharide converts immunosuppressive macrophages into M1-like phenotype and activates T lymphocytes by inhibiting the PD-L1/PD-1 axis between TAMs and T lymphocytes. Int J Biol Macromol. 2020;150:261–280. doi: 10.1016/j.ijbiomac.2020.02.050. [DOI] [PubMed] [Google Scholar]
- 150.Hsu P.Y., Lin Y.H., Yeh E.L., Lo H.C., Hsu T.H., Su C.C. Cordycepin and a preparation from Cordyceps militaris inhibit malignant transformation and proliferation by decreasing EGFR and IL-17RA signaling in a murine oral cancer model. Oncotarget. 2017;8:93712–93728. doi: 10.18632/oncotarget.21477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kongtawelert P., Wudtiwai B., Shwe T.H., Pothacharoen P., Phitak T. Inhibitory effect of hesperidin on the expression of programmed death ligand (PD-L1) in breast cancer. Molecules. 2020;25 doi: 10.3390/molecules25020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xu R., Wu J., Zhang X., Zou X., Li C., Wang H., et al. Modified Bu-zhong-yi-qi decoction synergies with 5 fluorouracile to inhibits gastric cancer progress via PD-1/PD- L1-dependent T cell immunization. Pharmacol Res. 2020;152:104623. doi: 10.1016/j.phrs.2019.104623. [DOI] [PubMed] [Google Scholar]
- 153.Zhang N., Dou Y., Liu L., Zhang X., Liu X., Zeng Q., et al. SA-49, a novel aloperine derivative, induces MITF-dependent lysosomal degradation of PD-L1. EBioMedicine. 2019;40:151–162. doi: 10.1016/j.ebiom.2019.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yao G., Liu C., Huo H., Liu A., Lv B., Zhang C., et al. Ethanol extract of Chaenomeles speciosa Nakai induces apoptosis in cancer cells and suppresses tumor growth in mice. Oncol Lett. 2013;6:256–260. doi: 10.3892/ol.2013.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu W., Fan T., Li M., Zhang G., Guo W., Yang X., et al. Andrographolide potentiates PD-1 blockade immunotherapy by inhibiting COX2-mediated PGE2 release. Int Immunopharmacol. 2020;81:106206. doi: 10.1016/j.intimp.2020.106206. [DOI] [PubMed] [Google Scholar]
- 156.Kukhetpitakwong R., Hahnvajanawong C., Homchampa P., Leelavatcharamas V., Satra J., Khunkitti W. Immunological adjuvant activities of saponin extracts from the pods of Acacia concinna. Int Immunopharmacol. 2006;6:1729–1735. doi: 10.1016/j.intimp.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 157.Dong M., Meng Z., Kuerban K., Qi F., Liu J., Wei Y., et al. Diosgenin promotes antitumor immunity and PD-1 antibody efficacy against melanoma by regulating intestinal microbiota. Cell Death Dis. 2018;9:1039. doi: 10.1038/s41419-018-1099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu S., Han Z., Trivett A.L., Lin H., Hannifin S., Yang D., et al. Cryptotanshinone has curative dual anti-proliferative and immunotherapeutic effects on mouse Lewis lung carcinoma. Cancer Immunol Immunother. 2019;68:1059–1071. doi: 10.1007/s00262-019-02326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 160.Liu Y.J. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/s0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 161.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 162.Xu H., Hu M., Liu M., An S., Guan K., Wang M., et al. Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials. 2020;235:119769. doi: 10.1016/j.biomaterials.2020.119769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hu K., Miao L., Goodwin T.J., Li J., Liu Q., Huang L. Quercetin remodels the tumor microenvironment to improve the permeation, retention, and antitumor effects of nanoparticles. ACS Nano. 2017;11:4916–4925. doi: 10.1021/acsnano.7b01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pei Y., Chen L., Huang Y., Wang J., Feng J., Xu M., et al. Sequential targeting TGF-β signaling and KRAS mutation increases therapeutic efficacy in pancreatic cancer. Small. 2019;15 doi: 10.1002/smll.201900631. [DOI] [PubMed] [Google Scholar]
- 165.Huang Y., Chen Y., Zhou S., Chen L., Wang J., Pei Y., et al. Dual-mechanism based CTLs infiltration enhancement initiated by nano-sapper potentiates immunotherapy against immune-excluded tumors. Nat Commun. 2020;11:622. doi: 10.1038/s41467-020-14425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rosenberg S.A., Restifo N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ma C., Wei Y., Liu Q., Xin Y., Cao G., Wang X., et al. Polysaccharides from Hedyotis diffusa enhance the antitumor activities of cytokine-induced killer cells. Biomed Pharmacother. 2019;117:109167. doi: 10.1016/j.biopha.2019.109167. [DOI] [PubMed] [Google Scholar]
- 169.Ju S.A., Park S.M., Lee Y.S., Bae J.H., Yu R., An W.G., et al. Administration of 6-gingerol greatly enhances the number of tumor-infiltrating lymphocytes in murine tumors. Int J Cancer. 2012;130:2618–2628. doi: 10.1002/ijc.26316. [DOI] [PubMed] [Google Scholar]
- 170.Alatrash G., Qiao N., Zhang M., Zope M., Perakis A.A., Sukhumalchandra P., et al. Fucosylation enhances the efficacy of adoptively transferred antigen-specific cytotoxic T lymphocytes. Clin Cancer Res. 2019;25:2610–2620. doi: 10.1158/1078-0432.CCR-18-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Banerjee S., Ghosh T., Barik S., Das A., Ghosh S., Bhuniya A., et al. Neem leaf glycoprotein prophylaxis transduces immune dependent stop signal for tumor angiogenic switch within tumor microenvironment. PloS One. 2014;9 doi: 10.1371/journal.pone.0110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chang Y.F., Chuang H.Y., Hsu C.H., Liu R.S., Gambhir S.S., Hwang J.J. Immunomodulation of curcumin on adoptive therapy with T cell functional imaging in mice. Cancer Prev Res. 2012;5:444–452. doi: 10.1158/1940-6207.CAPR-11-0308. (Phila) [DOI] [PubMed] [Google Scholar]
- 173.Xiao Z., Su Z., Han S., Huang J., Lin L., Shuai X. Dual pH-sensitive nanodrug blocks PD-1 immune checkpoint and uses T cells to deliver NF-κB inhibitor for antitumor immunotherapy. Sci Adv. 2020;6 doi: 10.1126/sciadv.aay7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yu H., Yang Y., Jiang T., Zhang X., Zhao Y., Pang G., et al. Effective radiotherapy in tumor assisted by ganoderma iucidum polysaccharide-conjugated bismuth sulfide nanoparticles through radiosensitization and dendritic cell activation. ACS Appl Mater Interfaces. 2019;11:27536–27547. doi: 10.1021/acsami.9b07804. [DOI] [PubMed] [Google Scholar]
- 175.Gu P., Liu Z., Sun Y., Ou N., Hu Y., Liu J., et al. Angelica sinensis polysaccharide encapsulated into PLGA nanoparticles as a vaccine delivery and adjuvant system for ovalbumin to promote immune responses. Int J Pharm. 2019;554:72–80. doi: 10.1016/j.ijpharm.2018.11.008. [DOI] [PubMed] [Google Scholar]