This nonrandomized controlled trial assessed the safety and therapeutic benefits of nomacopan in older adults with bullous pemphigoid.
Key Points
Question
Do older adults with bullous pemphigoid tolerate the administration of the C5 and leukotriene B4 inhibitor nomacopan, and does nomacopan provide a therapeutic benefit in bullous pemphigoid?
Findings
In this nonrandomized controlled trial of 9 patients with bullous pemphigoid, nomacopan was well tolerated in all patients for 42 days. In 7 of 9 patients, treatment with nomacopan was associated with a significant decrease in disease activity, including near complete remission in 3 patients.
Meaning
Findings of this study suggest that a larger randomized clinical trial is warranted to confirm the safety profile of nomacopan and to possibly establish nomacopan as a novel therapeutic option for bullous pemphigoid.
Abstract
Importance
Bullous pemphigoid is a difficult-to-treat autoimmune blistering skin disease that predominantly affects older adults and is associated with an increased mortality rate.
Objective
To examine the safety and therapeutic potential of nomacopan, an inhibitor of leukotriene B4 and complement C5, in patients with bullous pemphigoid.
Design, Setting, and Participants
This multicenter, single-group, phase 2a nonrandomized controlled trial was conducted in the dermatology departments of universities in the Netherlands and Germany. Participants were enrolled between September 2018 and April 2020. Older adult patients (aged ≥55 years) with mild to moderate, new-onset or relapsing bullous pemphigoid were recruited into the study.
Interventions
Patients received nomacopan, 90 mg, subcutaneously on day 1 and 30 mg subcutaneously daily until day 42.
Main Outcomes and Measures
The primary end point was the proportion of patients with grade 3 to 5 (severe) adverse events associated or possibly associated with nomacopan. Secondary end points included mean absolute and percentage changes in the Bullous Pemphigoid Disease Area Index (BPDAI) activity score, the BPDAI pruritus score, and the patient-reported outcome measures Dermatology Life Quality Index (DLQI) and Treatment of Autoimmune Bullous Disease Quality of Life (TABQOL).
Results
A total of 9 patients (median [range] age, 75 [55-85] years) with bullous pemphigoid were included in the trial, of whom 5 were women (55.6%). No serious adverse events associated with nomacopan were found. The mean (90% CI) BPDAI activity score decreased from 32.0 (8.7) points on day 1 to 19.6 (9.0) points on day 42. Seven of 9 patients (77.8%) responded to nomacopan with a reduction in the BPDAI activity score of at least 8 points between days 1 and 42; in 3 responders, the reduction was 80% or greater. On day 42, the mean (90% CI) BPDAI pruritus score had decreased by 6.8 (4.6) points from 17.6 (4.0) points on day 1. The mean (90% CI) DLQI score decreased from 11.3 (4.2) points at baseline to 6.4 (3.8) points by day 42, and the mean (90% CI) TABQOL score decreased from 14.6 (5.4) points at baseline to 10.3 (5.0) points on day 42.
Conclusions and Relevance
Results of this nonrandomized controlled trial suggest that nomacopan can be well tolerated in older patients with bullous pemphigoid and may have therapeutic benefits for suppressing acute flares of this disease. A larger, placebo-controlled randomized clinical trial is warranted to confirm this safety profile and to establish nomacopan as a new therapeutic option for bullous pemphigoid.
Trial Registration
ClinicalTrials.gov Identifier: NCT04035733
Introduction
Pemphigoid diseases constitute a group of autoimmune, blistering skin diseases featuring an autoantibody-directed immune response against proteins of the dermal-epidermal adhesion complex.1,2 Bullous pemphigoid is the most common pemphigoid disease. It is directed by IgG autoantibodies against the protein BP180.1
Therapies for bullous pemphigoid predominantly resort to systemic immunosuppression.3 The first-line therapy for bullous pemphigoid consists of either twice daily whole-body treatment with superpotent topical corticosteroids, such as clobetasol propionate, or systemic treatment with oral corticosteroids over several months. Often, corticosteroids alone cannot control disease, requiring the use of adjuvant immunosuppressants.3,4,5,6 Although vigorous immunosuppression can usually suppress disease, the 1-year mortality of patients with bullous pemphigoid is markedly increased.5,6,7,8,9,10 The reasons for this increase are probably in great part attributable to the adverse effects of this vigorous immunosuppression.9,11 Novel therapeutic strategies that target key pathways of the pathogenesis of bullous pemphigoid more specifically may be factors in improved patient outcomes.
In preclinical studies, investigators have highlighted the nonredundant roles of the anaphylatoxin C5a, an activation product of C5, and the eicosanoid leukotriene B4 (LTB4) in the development of inflammatory skin lesions in pemphigoid diseases.12,13,14,15,16,17,18 Both C5a and LTB4 are also abundant in skin lesions of patients with bullous pemphigoid,14,19,20 suggesting that both may play a similar role in human disease. Hence, the inhibition of LTB4 and C5a may ameliorate bullous pemphigoid. We aimed to examine the safety and therapeutic potential of nomacopan. Specifically, we investigated inhibiting LTB4 and C5 activation in bullous pemphigoid by treatment with nomacopan. Nomacopan is a recombinant protein that binds C5 and LTB4 and abrogates their activity.21,22,23,24,25 The systemic and topical treatment with nomacopan was previously tested in 19 patients with paroxysmal nocturnal hemoglobinuria (PNH) and atopic keratoconjunctivitis, respectively, and was well tolerated.26,27
Methods
This multicenter, single-group, phase 2a nonrandomized controlled trial included 9 patients with mild to moderate bullous pemphigoid and was conducted at 6 dermatology departments of universities in the Netherlands and Germany (Department of Dermatology, Allergy, and Venereology, University of Lübeck, Lübeck, Germany; Department of Dermatology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands; Department of Dermatology and Allergy, University of Kiel, Kiel, Germany; Department of Dermatology, University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany; Department of Dermatology, Maastricht University, Maastricht, the Netherlands; and Department of Dermatology, Venerology and Allergology, Julius Maximilians University Würzburg, Würzburg, Germany). This trial was conducted in accordance with the Declaration of Helsinki28 and Good Clinical Practice.29 It was approved by the ethics committee at each participating university, including the Medicine Institutional Review Board of the University Medical Center Groningen. Written informed consent was received from all participants before entering the study. We followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline.
Participants were enrolled between September 2018 and April 2020. Demographic data were collected, including date and year of birth, age, sex, and race and ethnicity. Race and ethnicity data were self-identified by participants and provided as free text without predefined categories.
Inclusion criteria were adult (aged ≥18 years) male and female patients with new-onset or relapsing bullous pemphigoid, a Karnofsky Performance Status score of 60% or greater, and a Bullous Pemphigoid Disease Area Index (BPDAI) activity score between 10 and 56 points. A BPDAI activity score lower than 20 points was considered to be mild, a score between 20 and 56 points was considered to be moderate, and a score higher than 56 points was considered to be severe, as previously defined.30,31 Bullous pemphigoid was diagnosed according to the following criteria: clinical presentation of cutaneous lesions and/or pruritus; direct immunofluorescence microscopy on a skin biopsy showing linear, n-serrated deposition of IgG, IgA, and/or C3 along the epidermal basement membrane zone; and/or indirect immunofluorescence microscopy performed with patient serum on 1.0 M sodium chloride human salt-split skin detecting IgG along the blister roof. Clobetasol propionate and systemic immunosuppressants had to be discontinued at least 5 days before baseline (day 1 of treatment). Main exclusion criteria were severe bullous pemphigoid; refractory bullous pemphigoid (defined as nonresponse or loss of response to maximal doses of topical or systemic corticosteroids); suspected drug-induced bullous pemphigoid (eg, by gliptins); and inability to discontinue systemic corticosteroids, immunosuppressants, or immunomodulators (eg, tetracyclines, dapsone, and niacinamide).
On day 1, a complement-ablating subcutaneous 60-mg dose of nomacopan followed by 30 mg 12 hours later was administered. From day 2 to 42, 30 mg of nomacopan was administered subcutaneously once daily. The drug was given by the study physicians, by a home nurse service, or by the patient after being trained (if the patient rejected the home nurse service). All patients received meningococcal vaccination and prophylactic oral penicillin for 10 days at the initiation of therapy to prevent Neisseria meningitidis infections, which are more likely to occur under complement C5 inhibition.32,33 Systemic or topical corticosteroids were prohibited except for up to 30 g of mometasone furoate cream, 0.1%, per week that was exclusively applied to lesions during the first 3 weeks of nomacopan treatment.
Study visits were scheduled on days 1, 7, 14, 21, 28, and 42, and there was a follow-up visit on day 72. At each visit, new adverse events (AEs) or changes to AEs and to concomitant medications were recorded. The Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 (US National Cancer Institute) was used to grade AEs. For the treatment of AEs, therapies known to modulate bullous pemphigoid were avoided.
To monitor the pharmacological activity of nomacopan, we measured terminal complement activity and free nomacopan in the serum by a CH50 enzyme-linked immunosorbent assay (ELISA; MicroVue, Quidel Corp) and a bespoke, validated ELISA, respectively. For these measurements, serum was taken before the first administration of nomacopan on day 1 and on days 7, 14, 21, and 42 immediately before the administration of the drug; at 3, 6, and 9 hours after nomacopan administration on day 14; and at the follow-up visit. In addition, total C5 and LTB4 levels in the serum were assayed at baseline before the first dose of nomacopan and on days 7, 14, 21, 42, and 72 by a commercial ELISA and by mass spectrophotometry, respectively.
The therapeutic potential of nomacopan was assessed at each study visit using the BPDAI activity score and the BPDAI pruritus score. The Dermatology Life Quality Index (DLQI) and the Treatment of Autoimmune Bullous Disease Quality of Life (TABQOL) were used as patient-reported outcome measures on days 1, 21, and 42, as previously described.6
End Points
The primary end point was the proportion of patients reporting CTCAE grades 3, 4, and 5 AEs associated or possibly associated with nomacopan during treatment. A clinical event was considered to be treatment related if it had plausible time association with the investigational medical product administration and was not explained by concurrent disease or other drugs or chemicals. A clinical event was considered to be possibly treatment related if it had reasonable time association with the investigational medical product administration and was unlikely to be attributed to concurrent disease or other drugs or chemicals.
The secondary end points, all comparing the situation on days 1 and 42, were mean absolute change and percentage change in BPDAI activity score; the proportion of patients with a decrease in BPDAI activity score of at least 4 points, which is the minimum clinically important difference for the BPDAI activity score34; the mean absolute change in BPDAI pruritus score; and the mean change in DLQI and TABQOL scores. The BPDAI activity score was originally not planned to be recorded on day 72, but after a protocol amendment it was recorded for 4 patients (Supplement 1).
The treatment response, including disease control, was also assessed by the clinician at each visit to ascertain whether new lesions had erupted over the past 3 days and whether existing lesions had extended or healed. Disease control was defined as the absence of new lesions in the past 3 days.35,36 Complete remission was defined as absence of inflammatory lesions or pruritus. By post hoc classification, complete responders, partial responders, and nonresponders (ie, patients with recalcitrant disease) were distinguished to better illustrate how changes in the BPDAI activity scores translated into clinical responses.
Statistical Analysis
Continuous variables were summarized using the number of patients, number of missing values, mean, median, SD, SE, minimum, maximum, and 90% CI as applicable. Categorical variables were summarized using the number of patients, number of missing values, frequency count, percentage, and 90% CI as applicable. The proportion of patients reporting safety events were analyzed as a primary end point. SAS statistical software version 9.3 (SAS Institute Inc) was used for data analysis. All available data were included in the safety and therapeutic potential analyses. No data were excluded or imputed. Data were analyzed from June to September 2020.
Results
A total of 9 patients with bullous pemphigoid were enrolled in the trial (eFigure 1 in Supplement 2). These patients had a median (range) age of 75 (55-85) years and included 5 (55.6%) women and 4 (44.4%) men. Their comorbidities and concomitant medications are provided in Table 1. Five patients (55.6%) presented with their first episode of bullous pemphigoid, and 4 (44.4%) presented with relapsing disease. Two patients (22.2%) exhibited mild bullous pemphigoid, and 7 (77.8%) had moderate bullous pemphigoid.
Table 1. Patient Demographic Characteristics, Comorbidities, Concomitant Medications, and Previous Therapies .
| Variable | No. (%) |
|---|---|
| No. of patients | 9 (100) |
| Age, median (range), y | 75 (55-85) |
| Sex | |
| Female | 5 (55.6) |
| Male | 4 (44.4) |
| Baseline disease | |
| New-onset disease | 5 (55.6) |
| Relapsing disease | 4 (44.4) |
| Disease severity | |
| Mild: BPDAI activity score <20 | 2 (22.2) |
| Moderate: BPDAI activity score 20-56 | 7 (77.8) |
| Comorbidities in >33% of patients | |
| Hypertension | 7 (77.8) |
| Gastroesophageal reflux disease | 3 (33.3) |
| Type 2 diabetes | 3 (33.3) |
| COPD | 3 (33.3) |
| Concomitant medications in >33% of patients | |
| Proton pump inhibitors | 6 (66.7) |
| β-Blockers | 5 (55.6) |
| Metformin | 3 (33.3) |
| ACE inhibitors | 3 (33.3) |
| Loop diuretics | 3 (33.3) |
| Calcium channel blockers | 3 (33.3) |
| Platelet aggregation inhibitors | 3 (33.3) |
| Antihistamines | 3 (33.3) |
| Vitamin D analogues | 3 (33.3) |
| Previous therapies for bullous pemphigoid within past 2 mo | |
| Topical corticosteroids | 4 (44.4) |
| Topical tacrolimus | 1 (11.1) |
| Dapsone | 1 (11.1) |
Abbreviations: ACE, angiotensin-converting enzyme; BPDAI, bullous pemphigoid disease area index; COPD, chronic obstructive pulmonary disease.
The mean (90% CI) total BPDAI activity score on day 1 was 32.0 (8.7) points. The immunopathological features of the patients are provided in eTable 1 in Supplement 2. The therapies for bullous pemphigoid before entering the trial were topical corticosteroids (n = 4 [44.4%]). Except for 1 patient (11.1%) using dapsone, none of the patients had received any systemic immunosuppressive or immunomodulatory treatments for bullous pemphigoid (Table 1).
Safety
All patients completed the 6-week nomacopan treatment period. All AEs are summarized in Table 2 and detailed in eTable 2 in Supplement 2. No patients reported a CTCAE grade 3 to 5 AE that was associated or possibly associated with nomacopan. Three serious AEs were reported in 2 patients, whose skin condition initially improved before worsening between days 28 and 42 (Figure 1; eFigure 2 in Supplement 2). Although in both patients the disease severity assessed by the BPDAI was lower on day 42 than on day 1, the site investigator declared this situation as a serious AE of “condition aggravated.” The other serious AE occurred in another patient who developed a bacterial knee infection 4 weeks after nomacopan was discontinued.
Table 2. Serious, Special Interest, and Most Frequent AEsa .
| AEs in individual patients | Severity | Day of start/end of study | AE association with nomacopan | Outcome |
|---|---|---|---|---|
| Serious AEs | ||||
| Localized infection (knee) | Severe | 69/NA | No association | Not resolved |
| Condition reaggravated (bullous pemphigoid) | Severe | 73/122 | No association | Recovered |
| Condition reaggravated (bullous pemphigoid) | Moderate | 67/85 | No association | Recovered |
| AEs of special interest | ||||
| Influenza | Mild | 18/43 | No association | Recovered |
| Respiratory tract infection | Moderate | 66/72 | No association | Recovered |
| UTI | Mild | 1/16 | No association | Recovered |
| UTI | Mild | 22/29 | No association | Recovered |
| Localized infection (knee) | Severe | 69/NA | No association | Not resolved |
| UTI | Mild | 1/29 | No association | Recovered |
| UTI | Mild | 43/58 | No association | Recovered |
| Condition reaggravated (bullous pemphigoid) | Severe | 73/122 | No association | Recovered |
| UTI | Mild | 43/NA | No association | Not resolved |
| URTI | Mild | 5/22 | No association | Recovered |
Abbreviations: AEs, adverse events; NA, not applicable; URTI, upper respiratory tract infection; UTI, urinary tract infection.
AEs in more than 1 patient included UTI (n = 3 [33.3%]), reaggravated bullous pemphigoid (2 [22.2%]), peripheral edema (2 [22.2%]), pruritus (2 [22.2%]), and headache (2 [22.2%]).
Figure 1. Bullous Pemphigoid Disease Area Index (BPDAI) Activity Scores Over Time for Patients in the Trial.
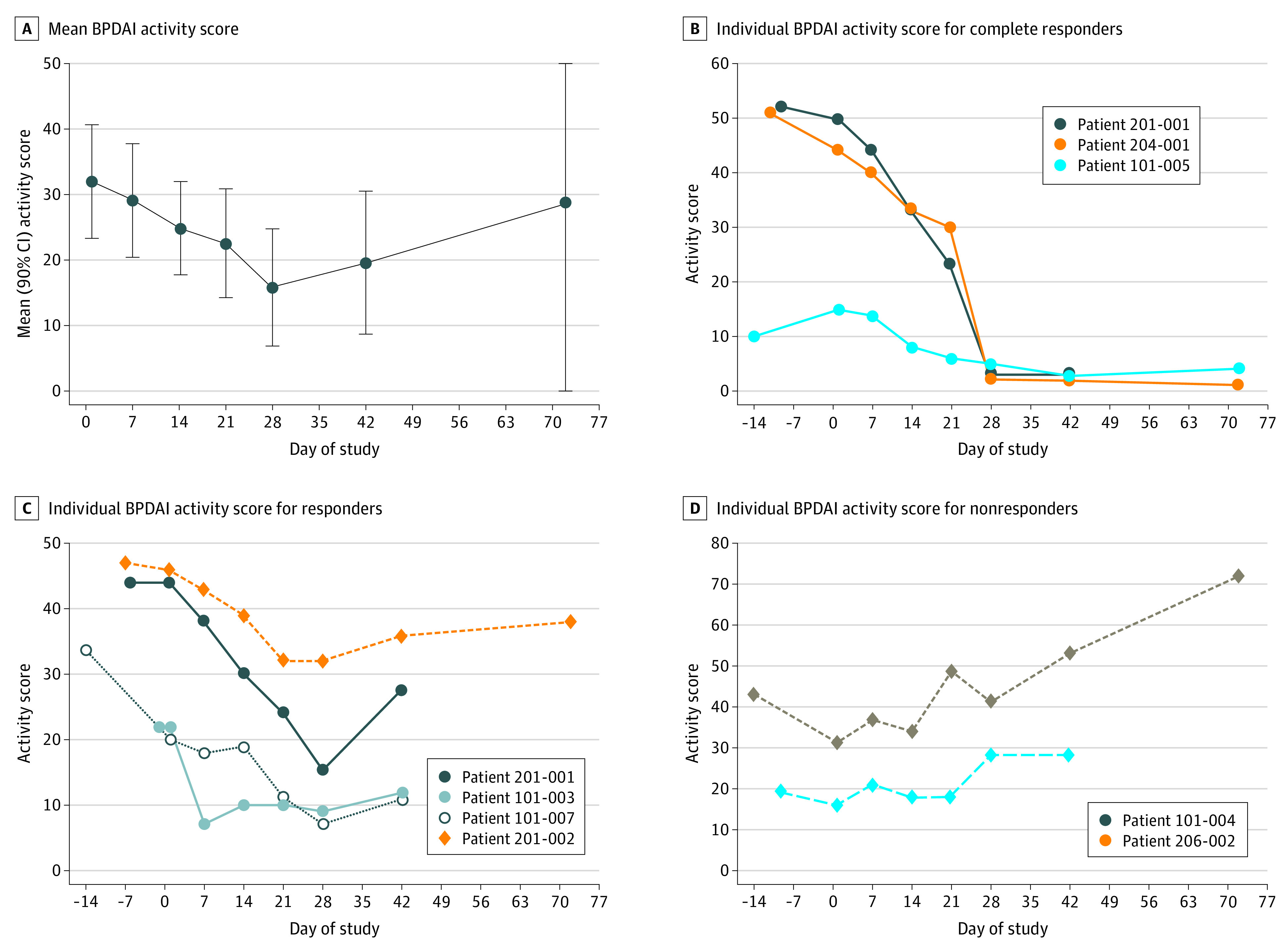
Mean BPDAI activity scores were from initiation of nomacopan treatment (day 1, baseline) to end of treatment (day 42) and at follow-up (day 72). Individual BPDAI activity scores were from time of screening to end of the study.
Therapeutic Potential
The mean (90% CI) BPDAI activity score decreased from 32.0 (8.7) points on day 1 to 19.6 (9.0) points (n = 9) on day 42, when nomacopan was discontinued (Figure 1A). In this period, the BPDAI activity score decreased by at least 8 points in 7 patients (77.8%), and 6 of these 7 patients had moderate bullous pemphigoid at baseline. Considering only the 7 patients with moderate bullous pemphigoid (mean [90% CI] BPDAI activity score at baseline, 36.7 [9.0] points), the mean (90% CI) decrease in the BPDAI activity score was approximately 50% over the 42 days of treatment (16 [17.0] points). Of the 7 responders, 3 showed an 80% or greater decrease, 3 had an approximately 40% decrease, and 1 had an approximately 30% decrease in BPDAI activity scores by day 42. In 6 of the 7 responders, new lesions ceased to erupt (eTable 3 in Supplement 2), and their BDPAI activity score started decreasing within a week of nomacopan therapy. Details of the BPDAI subcomponent scores of the individual patients are provided in eFigure 1 in Supplement 2. There was no change in the BPDAI damage score, which was designed to distinguish postinflammatory alterations from ongoing inflammation.
From day 7 onward, disease was under control in 4 or 5 patients during treatment with nomacopan (eTable 3 in Supplement 2). In 3 patients (33.3%), disease was in complete remission over the past 14 days of the 42-day treatment period (Figure 1B; eTable 3 in Supplement 2). In 2 of these 3 patients, both with more than 80% lower BPDAI activity score, disease was still in remission by day 72. Both patients received no further treatment for bullous pemphigoid after nomacopan treatment was discontinued on day 42.
In 2 of 9 patients (22.2%), the BPDAI activity score was higher on day 42 than on day 1 (Figure 1D). One of these patients received oral prednisolone as rescue therapy from day 39 through day 72 and beyond. The other patient received oral prednisolone from day 61 because disease substantially worsened after nomacopan was discontinued. Both patients entered the trial with relapsing disease.
The mean (90% CI) BPDAI pruritus score decreased from day 1 to day 42 by 6.8 (4.6) points, coming from 17.6 (4.0) points (Figure 2A). The pruritus score declined in 6 of 7 patients (77.8%) who responded to nomacopan with a decrease in the BPDAI score. The mean (90% CI) decrease in this patient subgroup was 7.3 (6.1) points. The delayed reduction of BPDAI pruritus score was associated with the decline of the total BPDAI activity score, which might be explained by the composition of the BPDAI pruritus score equally factoring in the intensity of pruritus today, in the past week, and in the past month34; therefore, by design, the BPDAI pruritus score lagged behind changes in the BPDAI activity score if changes occurred abruptly, as they appeared to do in this trial.
Figure 2. Bullous Pemphigoid Disease Area Index (BPDAI) Pruritus and Dermatology Life Quality Index (DLQI) Scores for Patients in the Trial.
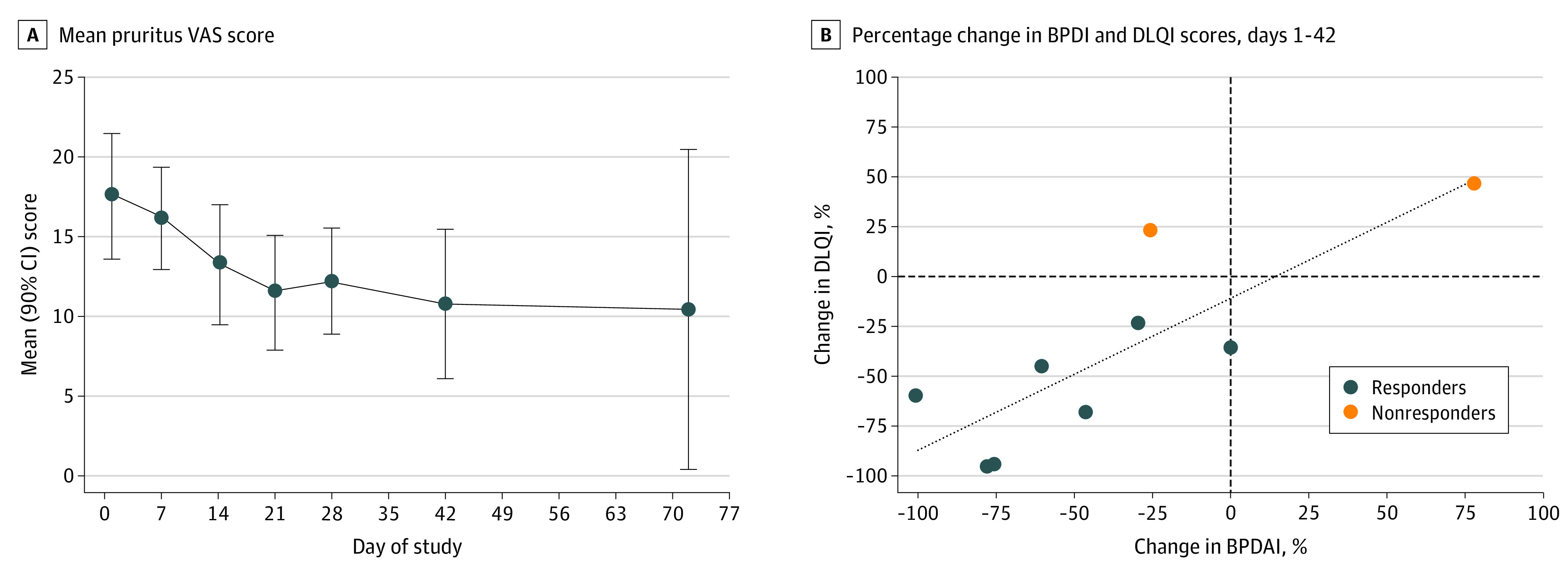
A, Mean BPDAI pruritus scores were from initiation of nomacopan treatment (day 1, baseline) to end of treatment (day 42) and at follow-up day (day 72). B, Association was found between improvement in DLQI score and decrease in BPDAI activity score from day 1 to 42. VAS indicates visual analog scale.
Patient-Reported Outcome Measures
In all 9 patients, the mean (90% CI) DLQI score was 11.3 (4.2) points at baseline and decreased to 6.2 (3.6) points by day 21 and 6.4 (3.8) points by day 42. There was an association between the BPDAI activity score and the DLQI score (Figure 2B). The mean (90% CI) TABQOL score was 14.6 (5.4) points (n = 8) at baseline and decreased to 12.4 (5.0) points (n = 8) by day 21 and 10.3 (5.0) points (n = 9) by day 42 of treatment.
Pharmacokinetic and Pharmacodynamic Activity of Nomacopan
The terminal complement activity (TCA) in the serum was determined at selected time points. Nomacopan treatment ablated the TCA (<10% residual TCA compared with baseline) in most patients (6 of 9 [66.7%]) (Figure 3A and B; eTable 4 in Supplement 2). Among patients who did not have their TCA ablated, 1 patient, who was a complete responder, did not receive the entire complement ablating dose of nomacopan on day 1, which led to only low serum levels of unbound nomacopan on day 7 followed by complete inhibition of the TCA by day 14 (Figure 3A and B). Another patient, a responder, likely did not self-inject with nomacopan shortly before day 41, as suggested by the minute serum concentration of unbound nomacopan that was detectable on day 42 (Figure 3A and B). One patient did not have full TCA ablation on days 7 and 14 but had full ablation on days 21 and 42. Total C5 level increased by approximately 40%, and LTB4 level increased by more than 40-fold. These levels reached new, stable, patient-specific equilibriums within a week of nomacopan treatment (Figure 3C and D). These increases reflected the longer half-life of C5 in the C5 to nomacopan complex and the sequestration of LTB4 by nomacopan preventing its rapid degradation. There is no assay that can distinguish between nomacopan-bound and nomacopan-unbound LTB4, but the total LTB4 levels in patients with bullous pemphigoid who were receiving nomacopan were higher than in patients with PNH or in healthy volunteers who were receiving nomacopan. This finding indicated that LTB4 was biosynthesized abundantly in bullous pemphigoid (eFigure 3 in Supplement 2).
Figure 3. Pharmacokinetic and Pharmacodynamic Measurements From Initiation to End of Treatment and at Follow-up for Patients in the Trial .
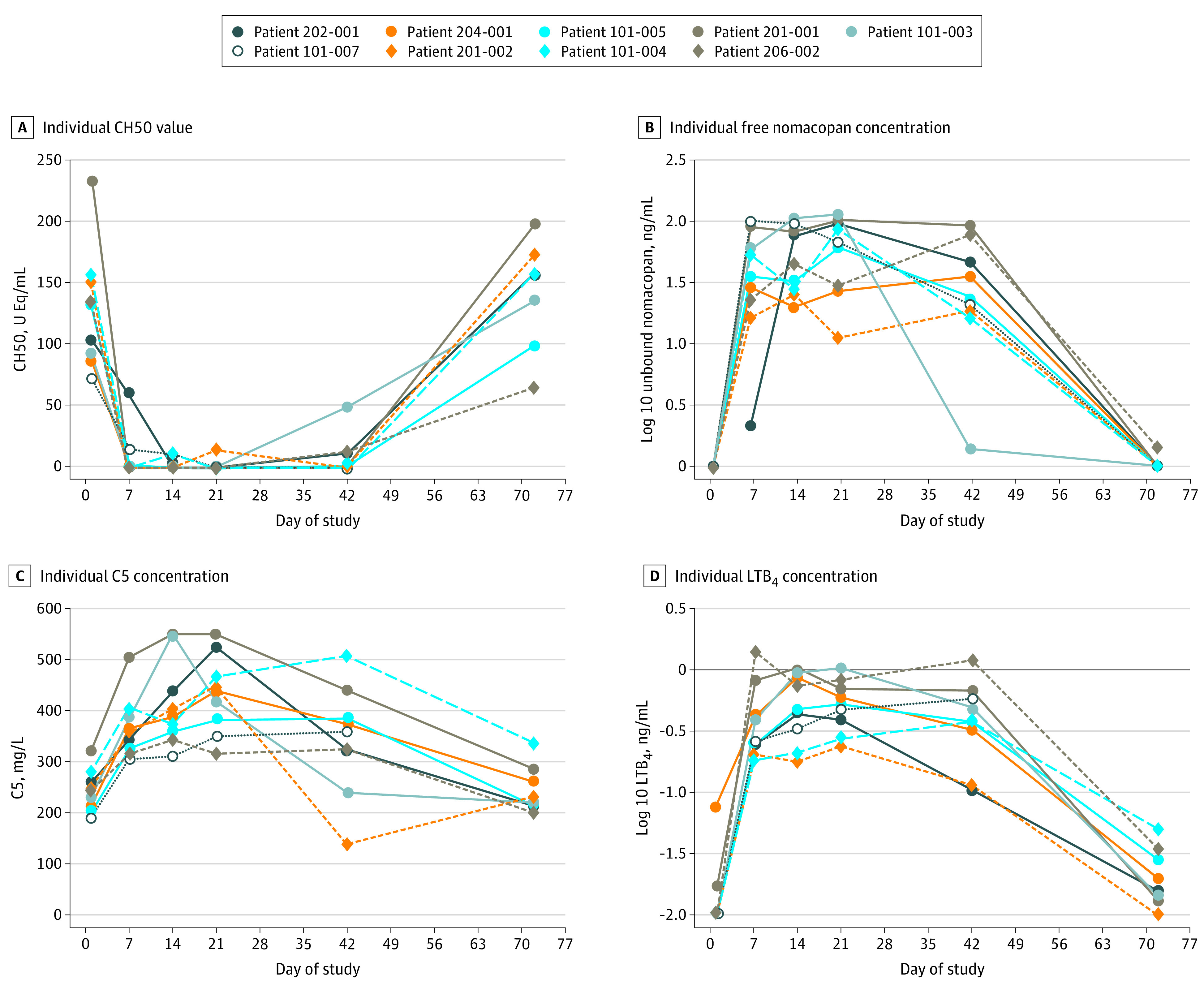
Values below the lower limit of quantification (LLOQ) for leukotriene B4 (LTB4) concentration and free nomacopan were plotted as equal to the LLOQ, which were 10 pg/mL for LTB4 and 1 ng/mL for free nomacopan.
Protocol Deviations
One responder used 47 g of mometasone furoate in week 1 and 37 g in week 2, instead of the permitted maximum weekly amount of 30 g (eTable 5 in Supplement 2). Two patients received only 30 mg, instead of 60 mg, of nomacopan for their first injection.
Discussion
In the current study, we applied nomacopan to treat patients with bullous pemphigoid, most of whom were older adults with diverse comorbidities. We evaluated the tolerability of nomacopan in this fragile patient population. Previously, nomacopan had been applied only to patients with PNH who were younger and had fewer comorbidities than the patients in this trial. It is, therefore, of great importance that no major AEs associated with nomacopan occurred in this study. If this safety profile is confirmed in larger clinical trials and nomacopan is proven to be therapeutically beneficial in bullous pemphigoid, the high mortality rate of patients with bullous pemphigoid might finally be lowered.
In addition, we sought to provide proof of concept that the dual inhibition of LTB4 and C5 by using nomacopan may be beneficial in treating bullous pemphigoid. We believe the clinical response in 7 of 9 patients with bullous pemphigoid indicated that nomacopan has the potential to decrease disease activity in bullous pemphigoid. Although the lesional application of up to 30 g of mometasone furoate per week was allowed over the first 21 days of the study, 8 of the 9 patients (and 6 of the 7 responders) used only a fraction of the permitted amount (eTable 5 in Supplement 2). The maximum permitted dose of 30 g mometasone furoate per week already was designed to be substantially below the standard of care for bullous pemphigoid.37 This observation, therefore, supports the therapeutic benefit of nomacopan and its potential therapeutic synergy with topical corticosteroids.
Clinical improvement was also reflected in the patient-reported outcome measures. The DLQI score showed an approximately 50% decrease from baseline to day 21 that was maintained until the end of treatment. The mean DLQI scores of 11.3 points at baseline and 6.4 points on day 42 suggested that the patients experienced an improvement in quality of life after receiving nomacopan, as indicated by a decrease in DLQI score (from a mean of 11-20 points to 6-10 points).38
It is not yet clear how the therapeutic benefit of nomacopan compares with established treatments for bullous pemphigoid. To address this question, we recently started a placebo-controlled, phase 3 randomized clinical trial in moderate and severe bullous pemphigoid to compare nomacopan plus oral corticosteroid with placebo plus oral corticosteroid.28 This future trial aims to ascertain whether corticosteroids can be tapered more rapidly and whether a higher proportion of patients can achieve disease remission under treatment with nomacopan plus corticosteroid than with placebo plus corticosteroid.
The potential of nomacopan to swiftly attenuate ongoing skin inflammation indicates that C5 and/or LTB4 are continually required to maintain skin inflammation. Because nomacopan inhibits both C5 and/or LTB4, we cannot discern the therapeutic role of the 2 pharmacological activities in bullous pemphigoid. However, in a preclinical study, inhibition of LTB4 alone by PASylated-L-nomacopan, a biotechnologically engineered variant of nomacopan-only binding LTB4, was found to be associated with reduced skin inflammation but was less effective than nomacopan.17 This result suggests an additive, nonredundant pharmacological benefit of C5 and LTB4 inhibition in this preclinical model.17 PASylated-L-nomacopan has not been tested in humans, complicating its application in patients with bullous pemphigoid in the foreseeable future. Although there is currently no licensed drug that specifically inhibits the biological activities of LTB4, eculizumab, a complement C5-neutralizing therapeutic monoclonal antibody, has been licensed for use in PNH and atypical hemolytic uremic syndrome for more than a decade. Eculizumab is one of the world’s most expensive drugs, with an annual treatment cost of $500 000 in the US.39,40,41 It also increases the risk of meningococcal infections and has a long half-life, which means that, after receiving a dose of eculizumab complement, C5 is inhibited for a minimum of 2 weeks (compared with <2 days using nomacopan).
These observations and its additional mode of action against LTB4 seem to suggest that nomacopan is a better candidate drug than eculizumab in treating older patients with bullous pemphigoid. Novel therapeutics targeting the generation of LTB4 or C5a or their biological activity are under development for other indications.39,40,41 In the future, these drugs could also be considered for the treatment of bullous pemphigoid and might clarify the individual contributions of C5a and LTB4 to bullous pemphigoid in humans.
Limitations
This trial has several limitations. It was not randomized and placebo-controlled, and the number of patients included was small. Therefore, it was not possible to distinguish the outcome of nomacopan from the outcome of placebo as well as from the natural course of disease. Furthermore, we could not ascertain the effectiveness of nomacopan vs that of other treatments for bullous pemphigoid. Moreover, because of the small sample size and the relatively short administration of nomacopan (only 6 weeks), we could not detect the adverse effects emerging under longer treatment time and could only detect frequent adverse effects. Given that a placebo group was missing, the adverse effects of nomacopan and their frequency could not be distinguished from spontaneous events in this patient population.
Conclusions
This nonrandomized controlled trial suggested that treatment with nomacopan for 6 weeks can be well tolerated by patients with bullous pemphigoid and that nomacopan might have a pronounced therapeutic benefit for bullous pemphigoid. Larger placebo-controlled, randomized clinical trials are warranted to confirm this safety profile of nomacopan and to possibly establish nomacopan as a new therapeutic option for bullous pemphigoid.
Trial Protocol
eFigure 1. Consort Flowchart of the AK801 Trial
eFigure 2. BPDAI Subcomponent Scores Over Time for the 9 Patients in the Trial
eFigure 3. Total LTB4 Levels (nM) in the Plasma of Healthy Volunteers (HV), Bullous Pemphigoid (BP) Patients and Paroxysmal Nocturnal Hemoglobinuria (PNH) Patients While Being Treated With Nomacopan
eTable 1. Immunopathological Features of the Study Subjects at Screening
eTable 2. Summary of Adverse Events (N = 9)
eTable 3. Summary of Disease Control and Number of New Lesions by Days of Nomacopan Treatment
eTable 4. Individual CH50 Values and Free Nomacopan Concentrations Immediately Before a Dose of Nomacopan
eTable 5. Amount of Mometasone (g) Used Each Week by Each Patient Over the First 3 Weeks of Nomacopan Treatment
Data Sharing Statement
References
- 1.Sadik CD, Schmidt E, Zillikens D, Hashimoto T. Recent progresses and perspectives in autoimmune bullous diseases. J Allergy Clin Immunol. 2020;145(4):1145-1147. doi: 10.1016/j.jaci.2020.02.020 [DOI] [PubMed] [Google Scholar]
- 2.Egami S, Yamagami J, Amagai M. Autoimmune bullous skin diseases, pemphigus and pemphigoid. J Allergy Clin Immunol. 2020;145(4):1031-1047. doi: 10.1016/j.jaci.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 3.Sadik CD, Zillikens D. Current treatments and developments in pemphigoid diseases as paradigm diseases for autoantibody-driven, organ-specific autoimmune diseases. Semin Hematol. 2016;53(suppl 1):S51-S53. doi: 10.1053/j.seminhematol.2016.04.015 [DOI] [PubMed] [Google Scholar]
- 4.Kibsgaard L, Bay B, Deleuran M, Vestergaard C. A retrospective consecutive case-series study on the effect of systemic treatment, length of admission time, and co-morbidities in 98 bullous pemphigoid patients admitted to a tertiary centre. Acta Derm Venereol. 2015;95(3):307-311. doi: 10.2340/00015555-1925 [DOI] [PubMed] [Google Scholar]
- 5.Terra JB, Potze WJ, Jonkman MF. Whole body application of a potent topical corticosteroid for bullous pemphigoid. J Eur Acad Dermatol Venereol. 2014;28(6):712-718. doi: 10.1111/jdv.12153 [DOI] [PubMed] [Google Scholar]
- 6.Tjokrowidjaja A, Daniel BS, Frew JW, et al. The development and validation of the treatment of autoimmune bullous disease quality of life questionnaire, a tool to measure the quality of life impacts of treatments used in patients with autoimmune blistering disease. Br J Dermatol. 2013;169(5):1000-1006. doi: 10.1111/bjd.12623 [DOI] [PubMed] [Google Scholar]
- 7.Joly P, Roujeau JC, Benichou J, et al. A comparison of two regimens of topical corticosteroids in the treatment of patients with bullous pemphigoid: a multicenter randomized study. J Invest Dermatol. 2009;129(7):1681-1687. doi: 10.1038/jid.2008.412 [DOI] [PubMed] [Google Scholar]
- 8.Joly P, Roujeau JC, Benichou J, et al. ; Bullous Diseases French Study Group . A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med. 2002;346(5):321-327. doi: 10.1056/NEJMoa011592 [DOI] [PubMed] [Google Scholar]
- 9.Kibsgaard L, Rasmussen M, Lamberg A, Deleuran M, Olesen AB, Vestergaard C. Increased frequency of multiple sclerosis among patients with bullous pemphigoid: a population-based cohort study on comorbidities anchored around the diagnosis of bullous pemphigoid. Br J Dermatol. 2017;176(6):1486-1491. doi: 10.1111/bjd.15405 [DOI] [PubMed] [Google Scholar]
- 10.Kridin K, Schwartz N, Cohen AD, Zelber-Sagi S. Mortality in bullous pemphigoid: a systematic review and meta-analysis of standardized mortality ratios. J Dermatol. 2018;45(9):1094-1100. doi: 10.1111/1346-8138.14503 [DOI] [PubMed] [Google Scholar]
- 11.Bech R, Kibsgaard L, Vestergaard C. Comorbidities and treatment strategies in bullous pemphigoid: an appraisal of the existing litterature. Front Med (Lausanne). 2018;5:238. doi: 10.3389/fmed.2018.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karsten CM, Beckmann T, Holtsche MM, et al. Tissue destruction in bullous pemphigoid can be complement independent and may be mitigated by C5aR2. Front Immunol. 2018;9:488. doi: 10.3389/fimmu.2018.00488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karsten CM, Pandey MK, Figge J, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat Med. 2012;18(9):1401-1406. doi: 10.1038/nm.2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Giudice GJ, Swartz SJ, et al. The role of complement in experimental bullous pemphigoid. J Clin Invest. 1995;95(4):1539-1544. doi: 10.1172/JCI117826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadik CD, Miyabe Y, Sezin T, Luster AD. The critical role of C5a as an initiator of neutrophil-mediated autoimmune inflammation of the joint and skin. Semin Immunol. 2018;37:21-29. doi: 10.1016/j.smim.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 16.Sezin T, Krajewski M, Wutkowski A, et al. The leukotriene B4 and its receptor BLT1 act as critical drivers of neutrophil recruitment in murine bullous pemphigoid-like epidermolysis bullosa acquisita. J Invest Dermatol. 2017;137(5):1104-1113. doi: 10.1016/j.jid.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 17.Sezin T, Murthy S, Attah C, et al. Dual inhibition of complement factor 5 and leukotriene B4 synergistically suppresses murine pemphigoid disease. JCI Insight. 2019;4(15):128239. doi: 10.1172/jci.insight.128239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murthy S, Schilf P, Patzelt S, et al. Dapsone suppresses disease in preclinical murine models of pemphigoid diseases. J Invest Dermatol. 2021;141(11):2587-2595.e2. doi: 10.1016/j.jid.2021.04.009 [DOI] [PubMed] [Google Scholar]
- 19.Grando SA, Glukhenky BT, Drannik GN, Epshtein EV, Kostromin AP, Korostash TA. Mediators of inflammation in blister fluids from patients with pemphigus vulgaris and bullous pemphigoid. Arch Dermatol. 1989;125(7):925-930. doi: 10.1001/archderm.1989.01670190059006 [DOI] [PubMed] [Google Scholar]
- 20.Kawana S, Ueno A, Nishiyama S. Increased levels of immunoreactive leukotriene B4 in blister fluids of bullous pemphigoid patients and effects of a selective 5-lipoxygenase inhibitor on experimental skin lesions. Acta Derm Venereol. 1990;70(4):281-285. [PubMed] [Google Scholar]
- 21.Barratt-Due A, Thorgersen EB, Lindstad JK, et al. Ornithodoros moubata complement inhibitor is an equally effective C5 inhibitor in pigs and humans. J Immunol. 2011;187(9):4913-4919. doi: 10.4049/jimmunol.1101000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hepburn NJ, Williams AS, Nunn MA, et al. In vivo characterization and therapeutic efficacy of a C5-specific inhibitor from the soft tick Ornithodoros moubata. J Biol Chem. 2007;282(11):8292-8299. doi: 10.1074/jbc.M609858200 [DOI] [PubMed] [Google Scholar]
- 23.Jore MM, Johnson S, Sheppard D, et al. Structural basis for therapeutic inhibition of complement C5. Nat Struct Mol Biol. 2016;23(5):378-386. doi: 10.1038/nsmb.3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunn MA, Sharma A, Paesen GC, et al. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. J Immunol. 2005;174(4):2084-2091. doi: 10.4049/jimmunol.174.4.2084 [DOI] [PubMed] [Google Scholar]
- 25.Roversi P, Ryffel B, Togbe D, et al. Bifunctional lipocalin ameliorates murine immune complex-induced acute lung injury. J Biol Chem. 2013;288(26):18789-18802. doi: 10.1074/jbc.M112.420331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sánchez-Tabernero S, Fajardo-Sanchez J, Weston-Davies W, et al. Dual inhibition of complement component 5 and leukotriene B4 by topical rVA576 in atopic keratoconjunctivis: TRACKER phase 1 clinical trial results. Orphanet J Rare Dis. 2021;16(1):270. doi: 10.1186/s13023-021-01890-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schols S, Nunn MA, Mackie I, et al. Successful treatment of a PNH patient non-responsive to eculizumab with the novel complement C5 inhibitor coversin (nomacopan). Br J Haematol. 2020;188(2):334-337. doi: 10.1111/bjh.16305 [DOI] [PubMed] [Google Scholar]
- 28.Nomacopan therapy in adult patients with bullous pemphigoid receiving adjunct oral corticosteriod therapy (ARREST-BP). ClinicalTrials.gov indentifier: NCT05061771. Accessed March 29, 2022. https://www.clinicaltrials.gov/ct2/show/NCT05061771
- 29.Dixon JR Jr. The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur. 1998;6(2):65-74. doi: 10.1080/105294199277860 [DOI] [PubMed] [Google Scholar]
- 30.Lévy-Sitbon C, Barbe C, Plee J, et al. Assessment of bullous pemphigoid disease area index during treatment: a prospective study of 30 patients. Dermatology. 2014;229(2):116-122. doi: 10.1159/000362717 [DOI] [PubMed] [Google Scholar]
- 31.Masmoudi W, Vaillant M, Vassileva S, et al. ; EADV Autoimmune Bullous Skin Disease Task Force . International validation of the Bullous Pemphigoid Disease Area Index severity score and calculation of cut-off values for defining mild, moderate and severe types of bullous pemphigoid. Br J Dermatol. 2021;184(6):1106-1112. doi: 10.1111/bjd.19611 [DOI] [PubMed] [Google Scholar]
- 32.Avasarala J, Sokola BS, Mullins S. Eculizumab package insert recommendations for meningococcal vaccinations: call for clarity and a targeted approach for use of the drug in neuromyelitis optica spectrum disorder. CNS Spectr. 2021;26(3):185-187. doi: 10.1017/S1092852919001627 [DOI] [PubMed] [Google Scholar]
- 33.Parker C. Eculizumab for paroxysmal nocturnal haemoglobinuria. Lancet. 2009;373(9665):759-767. doi: 10.1016/S0140-6736(09)60001-5 [DOI] [PubMed] [Google Scholar]
- 34.Wijayanti A, Zhao CY, Boettiger D, et al. The reliability, validity and responsiveness of two disease scores (BPDAI and ABSIS) for bullous pemphigoid: which one to use? Acta Derm Venereol. 2017;97(1):24-31. doi: 10.2340/00015555-2473 [DOI] [PubMed] [Google Scholar]
- 35.Feliciani C, Joly P, Jonkman MF, et al. Management of bullous pemphigoid: the European Dermatology Forum consensus in collaboration with the European Academy of Dermatology and Venereology. Br J Dermatol. 2015;172(4):867-877. doi: 10.1111/bjd.13717 [DOI] [PubMed] [Google Scholar]
- 36.Murrell DF, Daniel BS, Joly P, et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol. 2012;66(3):479-485. doi: 10.1016/j.jaad.2011.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320-332. doi: 10.1016/S0140-6736(12)61140-4 [DOI] [PubMed] [Google Scholar]
- 38.Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659-664. doi: 10.1111/j.0022-202X.2005.23621.x [DOI] [PubMed] [Google Scholar]
- 39.Bhatt L, Roinestad K, Van T, Springman EB. Recent advances in clinical development of leukotriene B4 pathway drugs. Semin Immunol. 2017;33:65-73. doi: 10.1016/j.smim.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 40.Jayne DRW, Bruchfeld AN, Harper L, et al. ; CLEAR Study Group . Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol. 2017;28(9):2756-2767. doi: 10.1681/ASN.2016111179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tesar V, Hruskova Z. Avacopan in the treatment of ANCA-associated vasculitis. Expert Opin Investig Drugs. 2018;27(5):491-496. doi: 10.1080/13543784.2018.1472234 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Consort Flowchart of the AK801 Trial
eFigure 2. BPDAI Subcomponent Scores Over Time for the 9 Patients in the Trial
eFigure 3. Total LTB4 Levels (nM) in the Plasma of Healthy Volunteers (HV), Bullous Pemphigoid (BP) Patients and Paroxysmal Nocturnal Hemoglobinuria (PNH) Patients While Being Treated With Nomacopan
eTable 1. Immunopathological Features of the Study Subjects at Screening
eTable 2. Summary of Adverse Events (N = 9)
eTable 3. Summary of Disease Control and Number of New Lesions by Days of Nomacopan Treatment
eTable 4. Individual CH50 Values and Free Nomacopan Concentrations Immediately Before a Dose of Nomacopan
eTable 5. Amount of Mometasone (g) Used Each Week by Each Patient Over the First 3 Weeks of Nomacopan Treatment
Data Sharing Statement


