Abstract
Importance
Many patients with severe stroke have impaired airway protective reflexes, resulting in prolonged invasive mechanical ventilation.
Objective
To test whether early vs standard tracheostomy improved functional outcome among patients with stroke receiving mechanical ventilation.
Design, Setting, and Participants
In this randomized clinical trial, 382 patients with severe acute ischemic or hemorrhagic stroke receiving invasive ventilation were randomly assigned (1:1) to early tracheostomy (≤5 days of intubation) or ongoing ventilator weaning with standard tracheostomy if needed from day 10. Patients were randomized between July 28, 2015, and January 24, 2020, at 26 US and German neurocritical care centers. The final date of follow-up was August 9, 2020.
Interventions
Patients were assigned to an early tracheostomy strategy (n = 188) or to a standard tracheostomy (control group) strategy (n = 194).
Main Outcomes and Measures
The primary outcome was functional outcome at 6 months, based on the modified Rankin Scale score (range, 0 [best] to 6 [worst]) dichotomized to a score of 0 (no disability) to 4 (moderately severe disability) vs 5 (severe disability) or 6 (death).
Results
Among 382 patients randomized (median age, 59 years; 49.8% women), 366 (95.8%) completed the trial with available follow-up data on the primary outcome (177 patients [94.1%] in the early group; 189 patients [97.4%] in the standard group). A tracheostomy (predominantly percutaneously) was performed in 95.2% of the early tracheostomy group in a median of 4 days after intubation (IQR, 3-4 days) and in 67% of the control group in a median of 11 days after intubation (IQR, 10-12 days). The proportion without severe disability (modified Rankin Scale score, 0-4) at 6 months was not significantly different in the early tracheostomy vs the control group (43.5% vs 47.1%; difference, −3.6% [95% CI, −14.3% to 7.2%]; adjusted odds ratio, 0.93 [95% CI, 0.60-1.42]; P = .73). Of the serious adverse events, 5.0% (6 of 121 reported events) in the early tracheostomy group vs 3.4% (4 of 118 reported events) were related to tracheostomy.
Conclusions and Relevance
Among patients with severe stroke receiving mechanical ventilation, a strategy of early tracheostomy, compared with a standard approach to tracheostomy, did not significantly improve the rate of survival without severe disability at 6 months. However, the wide confidence intervals around the effect estimate may include a clinically important difference, so a clinically relevant benefit or harm from a strategy of early tracheostomy cannot be excluded.
Trial Registration
ClinicalTrials.gov Identifier: NCT02377167
This multicenter clinical trial investigated potential benefits of early vs standard tracheostomy in patients with severe stroke receiving mechanical ventilation.
Key Points
Question
Among patients with severe ischemic or hemorrhagic stroke receiving mechanical ventilation, does a strategy of early tracheostomy (ie, tracheostomy within 5 days of intubation) result in better long-term functional outcome than a standard approach to ventilator weaning for extubation or tracheostomy (ie, tracheostomy if needed after 10 days)?
Findings
In this randomized clinical trial that included 382 patients, the proportion who survived without severe disability (defined as a score of 0-4 on the modified Rankin Scale at 6 months) was 43.5% in the early tracheostomy group and 47.1% in the group that received a standard approach to ventilatory weaning and tracheostomy, a difference that was not statistically significant.
Meaning
Among patients with severe stroke receiving mechanical ventilation, a strategy of early tracheostomy, compared with a standard approach to tracheostomy, did not significantly improve functional outcome at 6 months.
Introduction
Patients with severe ischemic or hemorrhagic stroke who require invasive mechanical ventilation are at high risk of death and poor functional outcome.1,2 Although these patients may have the capacity to breathe independently, extubation was often reported to be delayed due to a weak cough, impaired swallowing, inability to maintain a patent upper airway, or awaiting procedures to treat or prevent secondary brain damage. Although up to 15% of the general critical care population eventually require a tracheostomy, the rate of tracheostomy was as high as 35% in studies of patients with acute brain injury, such as severe stroke.3,4
In a general population of patients in the intensive care unit (ICU) receiving mechanical ventilation, the optimal timing to exchange the endotracheal tube for a tracheostomy cannula is unknown, and tracheostomy is commonly deferred for 2 weeks or longer.5,6 Earlier tracheostomy was suggested to confer benefits, such as reduced need for analgesia and sedation, accelerated ventilator weaning, earlier mobilization and transfer to rehabilitation, fewer ICU complications, and even improved survival and functional outcome.4,7,8,9 However, existing data were largely based on retrospective studies or clinical trials that primarily included patients in the ICU without stroke.
The only prior randomized trial to evaluate potential benefits of early tracheostomy in mechanically ventilated patients with stroke was the single-center pilot Stroke-Related Early Tracheostomy vs Prolonged Orotracheal Intubation in Neurocritical Care Trial (SETPOINT). Sixty patients with stroke who were receiving mechanical ventilation were randomized to either early tracheostomy within 3 days of intubation or to prolonged intubation and tracheostomy performed between days 7 and 14 if they remained intubated. Although ICU length of stay, the primary end point, was not different between the groups, sedation use, duration of mechanical ventilation, and mortality were lower with early tracheostomy.10 Based on these pilot data, this multicenter randomized trial was designed to investigate potential benefits of early vs standard tracheostomy in patients with severe stroke receiving mechanical ventilation.11
Methods
Trial Design
The SETPOINT2 study was an investigator-initiated multicenter, randomized, outcome assessor–blinded trial conducted between July 2015 and August 2020 at 26 neurocritical care units in Germany and the US. The trial was designed and conducted by a steering committee consisting of 3 senior investigators (J.B., W.-D.N., and D.B.S.); the study design has been previously published.11 An independent data and safety monitoring board consisting of 2 clinician-researchers and 1 statistician provided oversight for the trial. Data were managed and the primary analysis performed by the Institute of Medical Biometry at the University of Heidelberg, Germany.
The trial protocol was approved by the ethics committee of the coordinating center University Hospital Heidelberg (Ethikkommission Medizinische Fakultät Heidelberg, code S-489/2014) and by the ethics committee or institutional review board of each participating center. The trial was performed in accordance with the principles of the Declaration of Helsinki. For patients who were unable to provide consent because of the effects of their stroke, written informed consent was provided by the patient’s legally authorized representative no later than the fourth day of intubation. The trial protocol and summary changes are available in Supplement 1 and the statistical analysis plan in Supplement 2. Study procedures and documentation are displayed in eTable 10 and study flow in eFigure 1 in Supplement 3.
Patients
Patients requiring invasive mechanical ventilation after acute, nontraumatic ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage were eligible if the stroke-related early tracheostomy score (SETscore) was more than 10 (Table 1). The SETscore is a 15-item score of clinical and radiographic criteria that predicts the need for tracheostomy after stroke, and has been independently validated in patients with stroke receiving mechanical ventilation12,13 (see eTable 8 in Supplement 3 for the detailed score). The SETscore was calculated on the day of enrollment, and a score of more than 10 was used as a marker of anticipated need for mechanical ventilation for at least 2 weeks. Additionally, the clinical judgment of the treating neurointensivist had to agree that tracheostomy was clinically indicated due to a high likelihood of prolonged mechanical ventilation and eventual need for the procedure. Race and ethnicity were assessed to detect potentially related differences in study access, treatment access, and outcome. At the time of admission, patients or their legally authorized representatives self-identified their race on a check box form that did not include a blank line to specify “other.” The determination was made by the enrolling subinvestigators and based on fixed categories.
Table 1. Baseline Demographics and Clinical Characteristics.
| Tracheostomy, No. (%) | ||
|---|---|---|
| Early (n = 186) | Standard (n = 194) | |
| Age, mean (SD), y | 59.3 (11.7) | 57.6 (12.0) |
| <50 | 36 (19.4) | 47 (24.2) |
| 50-70 | 118 (63.4) | 116 (59.8) |
| >70 | 32 (17.2) | 31 (16.0) |
| Sex | ||
| Women | 96 (51.6) | 93 (47.9) |
| Men | 90 (48.4) | 101 (52.1) |
| US center | 94 (50.5) | 95 (49.0) |
| Stroke type and severity | ||
| Prehospital mRS scorea | ||
| 0 | 160 (86.0) | 169 (87.1) |
| 1 | 26 (14.0) | 25 (12.9) |
| Admission, median (IQR) | ||
| NIHSSb | 21 (14-28) | 21 (14-28) |
| GCSc | 7 (4-9) | 6 (3-9) |
| SETscored | 14 (13-18) | 14 (13-18) |
| Diagnosis | ||
| Acute ischemic stroke | 49 (26.3) | 59 (30.4) |
| Hemorrhage | ||
| Intracerebral | 78 (41.9) | 78 (40.2) |
| Subarachnoid | 59 (31.7) | 57 (29.4) |
| Score, median (IQR) | ||
| NIHSS, acute ischemic stroke onlyb | 20 (15-24) | 19 (14-25) |
| ICH, intracerebral hemorrhage onlye | 3 (2-3) | 3 (2-3) |
| WFNS, subarachnoid hemorrhage onlyf | 5 (4-5) | 5 (4-5) |
| Tracheostomy elements | ||
| Performed | 177 (95.2) | 130 (67.0) |
| Percutaneous, No./total No. (%) | 158/177 (89.3) | 108/130 (83.1) |
| Time from intubation, median (IQR), d | 4 (3-4) | 11 (10-12) |
Abbreviations: GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SET, stroke-related early tracheostomy; WFNS, World Federation of Neurosurgical Societies.
Scores on the modified Rankin Scale (mRS) range from 0 to 6, with 0 indicating no symptoms; 1, no substantial disability despite symptoms; 2, slight disability; 3, moderate disability necessitating some help; 4, moderately severe disability without ability to walk unassisted; 5, severe disability requiring constant nursing care; and 6, death. Patients with a score of 0, 1, or 2 are considered functionally independent.
Range, 0 to 42 (higher scores, severe neurologic impairment).
Range, 3 to 15 points (lower scores, reduced consciousness).
A 15-item score of clinical and radiographic criteria that predicts the need for tracheostomy after stroke; range, 0 to 37 (≥10 estimates ≥2 weeks of ventilatory support).
Range, 0 to 6 (higher score, higher 30-day mortality).
Range, 1 to 5 (higher scores, higher mortality).
Exclusion criteria included a premorbid modified Rankin Scale score of more than 1, reflecting at least slight disability, duration of invasive mechanical ventilation for more than 4 days, clinical conditions either prohibiting early tracheostomy or mandating a surgical tracheostomy, pregnancy, life expectancy of fewer than 3 weeks due to a medical condition or an anticipated withdrawal of life-sustaining therapies, participation in any other interventional trial, or inability to obtain informed consent.
Randomization and Interventions
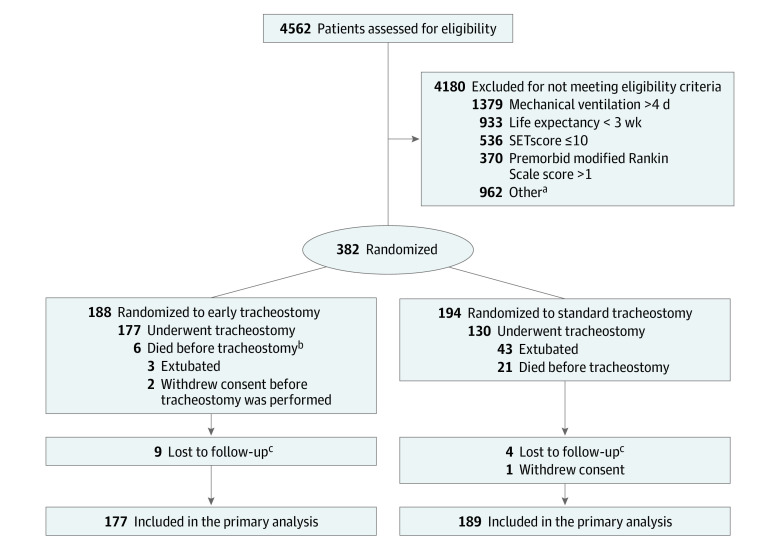
After written informed consent from their legal representatives, patients were randomly assigned in a 1:1 ratio to either tracheostomy within 5 days of intubation (early tracheostomy group), or to tracheostomy not before day 10, if the ongoing application of local standard weaning did not lead to successful extubation (standard tracheostomy group; Figure 1). Randomization was performed using a central web-based randomization tool to achieve comparable treatment groups. Block randomization was stratified for the participating centers to achieve equal group size per center (block size, 4).
Figure 1. Randomization and Follow-up of Patients in the SETPOINT2 Trial.
aTwo hundred ninety-four were not considered appropriate study candidates, 257 did not consent or had no legal proxy, 156 were participating in another trial, 96 had critical illness such that early tracheostomy could have compromised well-being, 64 were expected to need a permanent surgical tracheostomy, 14 had language barriers, 13 did not pursue maximal intensive care therapy, 9 required ventilation unrelated to stroke, 4 were admitted when tracheostomy interventionalists were unavailable, and 55 patients had other reasons.
bDied before planned tracheostomy or deterioration of clinical condition prevented tracheostomy at the planned time and ultimately died.
cCould not be reached or found for the telephone follow-up.
SET indicates stroke-related early tracheostomy (range, 0-37; ≥10 estimates ≥2 weeks of ventilatory support).
Tracheostomy from day 10 was determined to be common standard care, based on prior trials, such as the UK TracMan14 (Tracheostomy Management) study, the largest tracheostomy randomized clinical trial to date, the results from an analysis of tracheostomy practices in the US National Inpatient Sample,8,15,16 and prestudy surveys of more than 250 US and German practicing neurointensivists.17
In the percutaneous dilatational tracheostomy procedure, the trachea was punctured through an anterior neck incision and a blunt-tipped guidewire inserted. Sequential or continuous dilation was performed over the guidewire, and a tracheostomy tube inserted on the final pass. To minimize variability and ensure procedural quality, only centers that routinely used the percutaneous dilatational tracheostomy procedure as a local standard were recruited. Most procedures were performed at bedside in the ICU, but transition to an “open” surgical tracheostomy procedure, in which the tracheal mucosa is sutured to the skin to create an epithelialized stoma, was permitted if clinically warranted.18 The procedure was closely monitored for detection of related adverse events (see eTable 9 in Supplement 3, for prespecified contraindications to percutaneous tracheostomy). Only the timing of the tracheostomy procedure varied between the groups. Critical care management of ventilator settings and weaning, analgesia and sedation, blood product transfusion, cerebral monitoring, and other elements of ICU management were center-specific but followed guidelines from the American Heart Association/American Stroke Association 19,20,21,22 and Neurocritical Care Society.23,24
The intention at the time of informed consent was that the patient would have at least 3 weeks of unrestricted ICU care prior to the consideration of withdrawal of life support measures. However, in such medically complex patients, clinical conditions may change from day to day, and discussions between the clinicians providing patient care and family members regarding the goals of care, including the provision of life support, were standard elements of therapy, and for ethical reasons could not be regulated by the trial.
Outcomes
The primary outcome was the modified Rankin Scale score, which assesses the degree of disability or dependence in daily activities, with scores ranging from 0 (no symptoms) to 6 (death), dichotomized at 0 to 4 (indicating survival with moderately severe disability, requiring assistance with most bodily needs, or better), vs 5 to 6 (indicating severe disability with complete dependence or death, respectively) at the 6-month follow-up. This dichotomization was determined in part through structured conversations with patients and their families, who felt strongly that modified Rankin Scale score category 4 (moderately severe disability) should not be grouped with severe disability or death.
Forty-three secondary outcomes were predefined in total. These included the following, which are reported either in this article or in Supplement 3: a categorical shift in the modified Rankin Scale score at 6 months; alternative dichotomization of the modified Rankin Scale using scores of 0 to 3 vs scores of 4 to 6 at 6 months; death in the ICU; death at 6 months; causes of death; deaths related to withdrawal of life-sustaining therapy; ICU length of stay; hospital length of stay; discharge destination; place of stay at 6 months; time to discontinuation of sedation; time to start of respirator weaning; weaning success; duration of ventilation; time to and success of extubation; number of extubation attempts; recovery of consciousness; time to first day out of coma; and days and fractions of days during the ICU stay without sedatives, opioids, or vasopressors. Other prespecified secondary outcomes regarding patient and caregiver burden, quality of life, postdischarge details, and other outcomes were regarded beyond the scope of this report and are not included in this article. Prespecified and nonprespecified short-term and long-term adverse events were differentiated by whether they related to tracheostomy and were recorded, along with serious adverse events, during the ICU stay and for up to 6 months after the stroke.
Masking treatment assignments was not possible for attending physicians, patients, their legal representatives, and most investigators. However, the long-term functional outcome (primary outcome) and the cause of mortality (secondary outcome) were assessed by independent adjudicators at each center, masked to the timing of tracheostomy. Screening, inclusion, randomization, and documentation during the ICU-stay were followed by a telephone interview with the patient, caregiver, and/or primary care physician at 6 months.
Sample Size Calculation
Sample size considerations were based on the primary outcome of treatment success defined as a modified Rankin Scale score of 0 to 4 after 6 months. Success rates of 30% in the control group and 45% in the early tracheostomy group were assumed. These rates are slightly more conservative than the effect observed in the pilot trial10 (30% vs 48%). A difference in effect of at least 15% was considered clinically meaningful by the study groups in the planning phase based on previous neurocritical care trials involving similar study populations,25 particularly when testing an invasive procedure that may carry the risk of adverse events in an already very compromised patient population. With these assumed rates, a 2-sided type I error rate of .05, a power of 80%, and an assumed dropout rate of 15%, a maximum overall sample size of 380 (190 per group) was chosen. The sample size was determined for the implemented 2-stage group sequential design according to O’Brien and Fleming26 with 1 interim analysis after 127 patients. To control the overall type I error rate at the .05 level in the 2-stage procedure, a 2-sided significance level of .0498 was used in the final analysis.
Statistical Analysis
The primary outcome was evaluated using a logistic regression model adjusting for age, country, and Glasgow Coma Scale score at admission. If the primary null hypothesis was successfully rejected, a hierarchical testing procedure was to be applied comparing mortality between treatment groups. Subgroup-specific treatment effects were analyzed in prespecified subgroups defined by age, sex, country, recruitment effort of the center (<30 vs ≥30 patients), primary diagnosis (acute ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage), and Glasgow Coma Scale score (<6 vs ≥6) and were visualized using a forest plot. The alternative dichotomization of the modified Rankin Scale score at 0 to 3 vs 4 to 6 was analyzed using the same model as for the primary outcome. In a sensitivity analysis, center as a random effect was added in the model for the primary outcome.
Mortality was analyzed by a Cox proportional hazards model adjusting for age, country, and Glasgow Coma Scale score at admission. The proportionality assumption was confirmed using a score test based on scaled Schoenfeld residuals. Time-to-event outcomes were analyzed using the Kaplan-Meier method. For effect estimates, hazard ratios and 95% CIs were calculated from unadjusted Cox proportional hazards models.
Secondary end points regarding ICU medications were defined post hoc with slight differences from the initial definitions in the protocol and statistical analysis plan. Instead of considering the number of days of a specific infusion, the number of ICU days without that respective infusion and the fraction of the ICU stay were chosen. Hodges-Lehmann estimates and respective 95% CIs were calculated post hoc for these variables. Categorical variables were described using absolute and relative frequencies. For rate differences, 95% CIs were calculated using the Wald method with continuity correction.
CIs for subgroup analyses and secondary end points were not adjusted for multiplicity. Because of the potential for type I error due to multiple comparisons, findings of analyses of secondary end points should be interpreted as exploratory. The safety analysis included a comparison of frequencies of adverse events and frequencies stratified by intensity and causality. Missing values for the modified Rankin Scale score at 6 months were handled by a multiple imputation approach,27,28 using the fully conditional specification by means of an ordinal regression model. The variables included in the imputation model were the modified Rankin Scale score, group, age, and Glasgow Coma Scale. Missing values for other end points were not imputed. The analyses of all end points were based on all randomized patients who did not withdraw consent immediately after randomization (full analysis set). Patients were analyzed according to their randomization group. All analyses were performed using SAS version 9.4 (SAS Institute Inc) and visualizations were created using R software version 4.0.5. A detailed description of the analysis is provided in the statistical analysis plan (Supplement 2).
Results
Patient Characteristics
Site-specific block randomization led to a slight imbalance of the 188 patients initially assigned to the early tracheostomy group and the 194 to the control group (Figure 1). The legal representatives of 2 patients in the early tracheostomy group withdrew consent immediately after randomization and before the procedure. Because follow-up of these patients was not possible, 2 additional patients were enrolled (overall, 382) to compensate for this and to reach the planned sample size of 380 (median age, 59 years; 49.8% women).
The full analysis set therefore included 380 patients (186 in the early tracheostomy group; 194 in the control group). These groups had similar prerandomization baseline demographic and clinical characteristics (5 Asian [1.3%], 50 Black [13.2%], 1 Hawaiian or Pacific Islander [0.3%], 223 White [58.7%], 6 other [1.6%], and 95 [25%] missing; eTable 1 in Supplement 3).
Stroke type distribution in the early tracheostomy vs standard tracheostomy group were acute ischemic stroke, 49 (26.3%) vs 59 (30.4%); intracerebral hemorrhage, 78 (41.9%) vs 78 (40.2%); and subarachnoid hemorrhage, 59 (31.7%) vs 57 (29.4%). Disease-specific severity metrics were also similar between the groups. The median of the National Institutes of Health Stroke Scale (NIHSS) scores in patients with ischemic stroke were 20 (IQR, 15-24) in the early tracheostomy group and 19 (IQR, 14-25) in the standard tracheostomy group. Median intracerebral hemorrhage scores were 3 (IQR, 2-3) in both groups, and the median World Federation of Neurosurgical Societies scores in patients with subarachnoid hemorrhage were 5 (IQR, 4-5) in both groups (Table 1).
Of 186 patients in the early tracheostomy group, 177 (95.2%) underwent tracheostomy a median of 4 days (IQR, 3-4 days) after intubation. Three patients (1.6%) were successfully extubated before tracheostomy for unknown reasons, and 6 (3.2%) died before the procedure or deteriorated clinically to render the procedure impossible and died later. Of the 194 patients in the standard tracheostomy group, 130 (67%) underwent tracheostomy a median of 11 days (IQR, 10-12 days) after intubation. Forty-three (22%) were successfully extubated and 21 (10.8%) died prior to the procedure being performed. Among patients who underwent tracheostomy, the procedure was performed percutaneously in 158 (89.3%) of early vs 108 (83.1%) of standard cases, with the remainder performed operatively as previously described (Table 1).
Primary Outcome
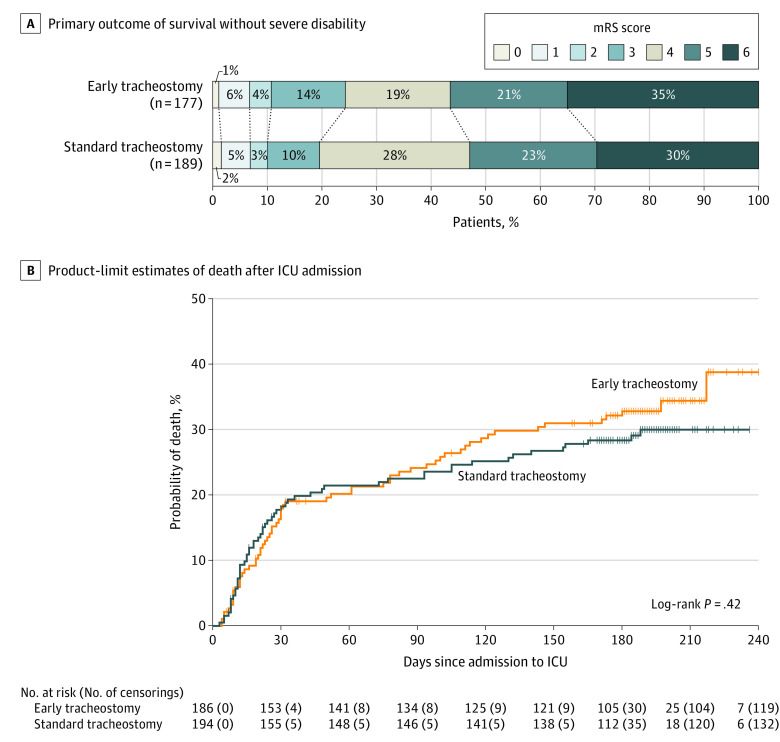
Within the full analysis set, the primary outcome was recorded for 366 patients; 13 were lost to follow-up and 1 withdrew consent. There was no significant difference in the primary outcome between treatment groups: 77 of 177 patients (43.5%) in the early tracheostomy group had a 6-month modified Rankin Scale score of 4 or less vs 89 of 189 patients (47.1%) in the standard tracheostomy group (difference, −3.6% [95% CI, −14.3% to 7.2%]; adjusted OR for favorable outcome 0.93 [95% CI, 0.60-1.42; P = .73], Table 2). Additionally, there were no significant differences in functional outcomes between early or standard tracheostomy within the predefined subgroups of age, sex, German vs US treatment center, high vs low-volume recruiting center, stroke subtype, or presenting Glasgow Coma Scale score (Figure 2). The sensitivity analysis, using center as random effect in the model confirmed the analysis (compare eTable 3 in Supplement 3). The primary and secondary outcome results are shown in Table 2, and results of the regression analysis of the primary outcome are shown in eTable 2 in Supplement 3.
Table 2. Primary and Secondary Outcome Results.
| Tracheostomy, No./total (%) | Absolute difference, % (95% CI) | Effect measure (95% CI) | ||
|---|---|---|---|---|
| Early (n =186) | Standard (n = 194) | |||
| Primary outcome, 6 mo | ||||
| Modified Rankin Scale score, 0-4a | 77/177 (43.5) | 89/189 (47.1) | −3.6 (−14.3 to 7.2) | aOR, 0.93 (0.60 to 1.42)b,c,d |
| Secondary outcomes | ||||
| Modified Rankin Scale score, 0-3 at 6 moa | 43/177 (24.3) | 37/189 (19.6) | 4.7 (−4.3 to 13.7) | aOR, 1.48 (0.89 to 2.48)b,c |
| Death | ||||
| at 6 mo | 62/177 (35.0) | 56/189 (29.6) | 5.4 (−4.7 to 15.5) | aHR, 1.06 (0.74 to 1.53)b,e |
| in ICUf | 26/186 (14.0) | 29/194 (14.9) | −1.0 (−8.6 to 6.6) | |
| Due to withdrawal of life-sustaining therapy | 24/186 (12.9) | 21/194 (10.8) | 2.1 (−5.0 to 9.1) | |
| Time to discharge, dg | ||||
| ICU, median (IQR) | 17 (12 to 26) | 19 (14 to 26) | HR, 1.12 (0.90 to 1.39) | |
| Hospital | 24 (15 to 41) | 26 (17 to 44) | HR, 1.06 (0.85 to 1.32) | |
| Discharge destination | ||||
| Home | 1/144 (0.7) | 5/156 (3.2) | ||
| Rehabilitation center | 101/144 (70.1) | 110/156 (70.5) | ||
| Long-term care facility | 26/144 (18.1) | 25/156 (16.0) | ||
| Otherh | 16/144 (11.1) | 16/156 (10.3) | ||
| Location of survivors at 6 mo | ||||
| Home | 54/115 (47.0) | 65/133 (48.9) | ||
| Hospital | 6/115 (5.2) | 2/133 (1.5) | ||
| Rehabilitation center | 29/115 (25.2) | 29/133 (21.8) | ||
| Long-term care facility | 23/115 (20.0) | 36/133 (27.1) | ||
| Otheri | 3/115 (2.6) | 1/133 (0.8) | ||
| Respirator weaning started | 138/186 (74.2) | 130/194 (67.0) | 7.2 (−2.5 to 16.8) | |
| Time to start weaning, dj | 6 (4 to 11) | 6 (3 to 13) | HR, 1.24 (0.98 to 1.58) | |
| Successful weaningk | 87/186 (46.8) | 91/194 (46.9) | −0.1 (−10.7 to 10.4) | |
| Time to end mechanical ventilation, median (IQR), dj | 14 (8 to 20) | 11 (8 to 18) | HR, 0.97 (0.73 to 1.31) | |
| Extubation attempts | ||||
| At least 1 | 18/186 (9.7) | 75/194 (38.7) | ||
| More than 1 | 4/186 (2.2) | 21/194 (10.8) | ||
| First attempt successfull | 2/18 (11.1) | 35/75 (46.7) | ||
| Extubated after weaningl | 3/18 (16.7) | 43/75 (57.3) | ||
| Recovery of consciousnessm | 100/141 (70.9) | 97/144 (67.4) | 3.6 (−7.9 to 15.0) | |
| Time to first day out of coma, median (IQR), dj | 4 (1.5 to 7.5) | 5 (2 to 10) | HR, 1.15 (0.87 to 1.52) | |
| ICU stay, median (IQR), dm | ||||
| Without sedatives | 9 (5 to 14) | 9 (3 to 15) | HL, 0 (−1 to 2) | |
| Without opioids | 8 (1 to 14) | 8 (1 to 14) | HL, 0 (−2 to 1) | |
| Without vasopressors | 11 (6 to 16) | 12 (4.5 to 17) | HL, 0 (−1 to 2) | |
| Fraction of total days in ICU, median (IQR)m | ||||
| Without sedatives | 0.6 (0.3 to 0.8) | 0.5 (0.2 to 0.8) | HL, 0.02 (−0.04 to 0.1) | |
| Without opioids | 0.5 (0.1 to 0.8) | 0.5 (0.1 to 0.8) | HL, 0 (−0.08 to 0.04) | |
| Without vasopressors | 0.8 (0.4 to 1) | 0.8 (0.3 to 1) | HL, 0 (0 to 0.07) | |
Abbreviations: aHR, adjusted hazard ratio; aOR, adjusted odds ratio; GCS, Glasgow Coma Scale; HL, Hodges-Lehmann estimate; ICU, intensive care unit; mRS, modified Rankin Scale.
See Table 1 footnotes for score definitions.
Adjusted for age, baseline GCS, and country.
Used multiple imputation to replace missing values.
P = .73 for the primary end point. See eTable 2 in Supplement 3 for other variables.
Patients lost to follow-up were censored: 1 patient was excluded from this Cox regression due to missing GCS status at baseline.
Therapy withdrawals during ICU were counted as ICU deaths.
Median (IQR) by Kaplan-Meier method. Patients who died during ICU or hospital stay were censored after the last patient was discharged.
Other destinations: acute care hospital, palliative care facility, hospice, skilled nursing, and step-down unit.
Other locations: monastery, assisted living, skilled nursing, and nursing home.
Median (IQR) for patients who reached the end point. HR from a Cox proportional hazards model for which deaths before reaching the end point were censored after the last event in the cohort.
End of mechanical ventilation the same day as death or treatment withdrawal were not counted; 94 more decannulations noted in the follow-up (early tracheostomy, 41; standard tracheostomy, 54).
Nonterminal extubations not leading to reintubation or tracheotomy are counted as successful.
Fewer patients because variables were added after a protocol amendment (early group, 141; standard group, 144).
Figure 2. Functional Outcome Without Severe Disability 6 Months After Admission in Predesignated Subgroups According to Early or Standard Tracheostomy Timing.
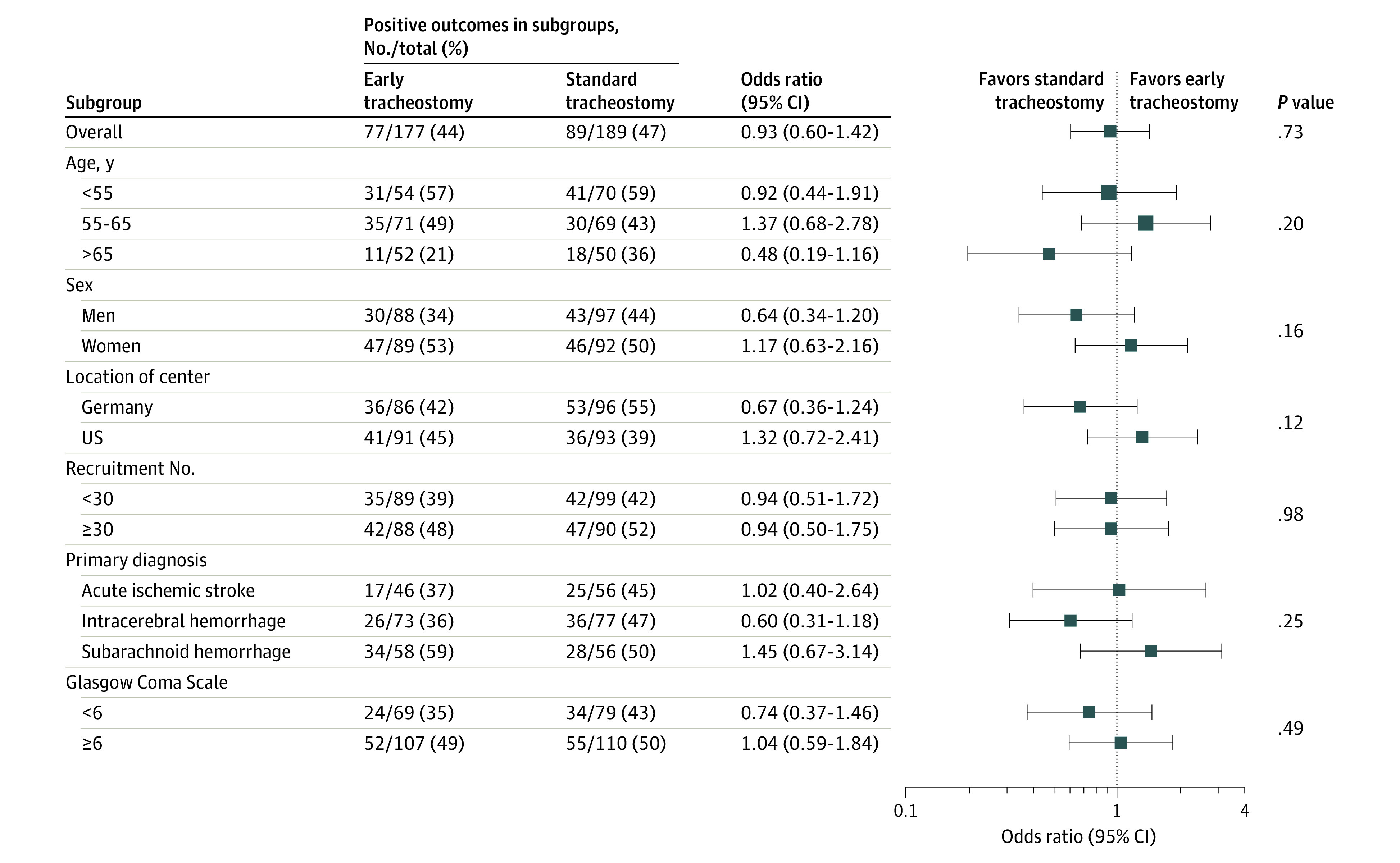
Odds ratios in prespecified subgroups for reaching an acceptable modified Rankin Scale score of 0 to 4 at 6 months in the early vs standard tracheostomy groups (see Table 1 footnotes for score definitions). The dashed vertical line indicates the point of no difference between the groups. The overall P value is the result of testing the treatment group effect in the primary analysis model. Other P values were obtained by testing an interaction between treatment group and the respective variables of interest in slightly modified versions of the primary analysis model.
Scores on the Glasgow Coma Scale range between 3 and 15 points, with lower scores indicating reduced levels of consciousness. It is composed of best eye response, best verbal response, and best motor response.
Secondary Outcomes
Randomization to receive early vs standard tracheostomy also did not influence the modified Rankin Scale score category shift (Figure 3A), or mortality (Figure 3B). The log-rank test for comparison of mortality yielded a P value of .42 and the adjusted hazard ratio was 1.06 (95% CI, 0.74-1.53; Table 2).
Figure 3. Functional Outcome 6 Months After Admission and Kaplan-Meier Estimates of Survival According to the Timing of Tracheostomy.
A, The primary outcome was survival without severe disability, defined as a score of 0 to 4 in the modified Rankin Scale (mRS) score at 6 months after admission in both treatment groups. For definition of the scores, see the Table 1 footnotes.
B, Product-limit survival estimates after admission to intensive care unit (ICU) during follow-up for both groups in the full analysis set. For early tracheostomy, the median observation time was 181 days (IQR, 61-194 days) and standard tracheostomy, 183.5 days (IQR, 93-194 days). Censored patients are indicated on the Kaplan-Meier curve by a vertical line.
There were no significant differences between the treatment groups in discharge destination, cause of death, median duration of mechanical ventilation, and most other ICU management parameters (Table 2). Additional secondary outcomes data on cessation of sedation, start of respirator weaning, and discharge from the ICU are shown in eFigures 2 through 4 in Supplement 3. The cumulative probability of cessation of sedation and initiation of respirator weaning are displayed eFigures 2 and 3 in Supplement 3. There were no significant differences in the total duration of mechanical ventilation or ICU length of stay (Table 2; eFigure 4 in Supplement 3).
Adverse Events
Regarding safety, 1 or more serious adverse event occurred in 88 of 186 patients (47.3%) in the early tracheostomy group and in 85 of 194 patients (43.8%) in the standard tracheostomy group; this corresponded to 121 serious adverse events in the early tracheostomy group and 118 in the standard tracheostomy group; 5.0% (6 of 121) were tracheostomy-related events in the early tracheostomy group and 3.4% (4 of 118) in the standard tracheostomy group (eTable 6 in Supplement 3). The most common adverse events of percutaneous tracheostomy were venous bleeding at the tracheostomy site (2.6%) and aspiration pneumonia diagnosed within 48 hours of tracheostomy (2.6%). A full list of serious adverse events, prespecified adverse events, their timing and causal relation to tracheostomy, and causes and time of death are provided in eTables 5, 6, and 7 in Supplement 3.
Discussion
In this multicenter, randomized clinical trial of patients with severe stroke requiring prolonged mechanical ventilation, randomization to receive tracheostomy within 5 days of intubation compared with tracheostomy performed 10 or more days after intubation if needed did not affect the functional outcome of patients, described as the proportion with a modified Rankin Scale score 0 to 4, and measured 6 months after randomization. However, the wide CIs around the effect estimate may include a clinically important difference; hence, a clinically relevant benefit or harm from a strategy of early tracheostomy cannot be excluded.
The findings of this study differ from those of the smaller, single-center pilot trial, in which patients had no significant difference in ICU length of stay but reduced mortality with an earlier tracheostomy, and from retrospective data that suggested various potential benefits of early tracheostomy after stroke. The current clinical trial suggests that earlier tracheostomy may have led to earlier discontinuation of sedation and initiation of ventilator weaning, although the differences did not achieve statistical significance, and any potential benefits may have been offset by a longer duration of weaning, and did not translate into shorter duration of mechanical ventilation, or ICU length of stay, and did not affect mortality or functional outcomes. One potential explanation for the discrepancy between the trials is the role of chance in the pilot study, which was not powered for mortality or functional outcomes. The delayed tracheostomy group in that study had a higher rate of poor outcome than the current, larger, multicenter trial, which may more accurately reflect the clinical course of patients with respiratory failure after severe stroke characterized by a of more than 10.
Retrospective design, small sample sizes, population imbalances, treatment heterogeneity, and uncontrolled confounders in previous tracheostomy studies may have contributed to an overestimation of the effect of tracheostomy timing on ICU de-escalating measures such as the weaning of sedation and mechanical ventilation to accelerate rehabilitation in neurocritical care patients. Furthermore, the effect of tracheostomy timing may be particularly difficult to detect in a trial with a well-treated control group; in the current study, both treatment groups demonstrated modified Rankin Scale scores of 0 to 4 at rates comparable with the early tracheostomy group in the pilot study, suggesting that the higher death rate in the control group of the pilot study may have been due to random chance in that smaller trial, or to less aggressive care.
In the current clinical trial, among patients in the group randomized for the standard approach to tracheostomy timing, 67% patients underwent tracheostomy, 11% died prior to tracheostomy, and 22% were successfully extubated and never required the tracheostomy procedure. This confirms prior studies of the SETscore that suggested only about 20% of patients with stroke and a SETscore more than 10 are successfully extubated.12,13 This finding contrasts with the largest prior randomized trial to evaluate early tracheostomy in a mixed ICU population (n = 909 patients), in which many patients in the “delayed” tracheostomy group who were thought by their physicians to need tracheostomy did not ultimately require one,14 suggesting that the eventual need for tracheostomy may be more reliably predicted among patients with stroke using a prediction score than in a general medical-surgical ICU population based only on the physicians’ estimate.
The percutaneous tracheostomy procedure, performed in 87% of 307 procedures, demonstrated a periprocedural adverse event rate of 15.8%. The most common adverse events were venous bleeding at the tracheostomy site (2.6%) and aspiration pneumonia diagnosed within 48 hours of tracheostomy (2.6%). Cerebral compromise was rare, even in the early tracheostomy group (0.6%), and no deaths were attributed to the procedure. There were no relevant differences in the occurrence of adverse or serious adverse events and no relevant differences in any cerebral compromise during the procedure between the early and standard treatment groups. This suggests that cautious, standardized percutaneous tracheostomy is a reasonable option for airway management of individual cerebrovascular ICU patients, independent of timing.
The recruitment of patients in this trial was almost equal in Germany and the US, and patient demographics closely reflected the racial and ethnic compositions of those nations. Randomization groups were balanced in terms of stroke subtypes and severity: acute ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage were similarly represented, and stroke severity was high, reflected in the disease-specific grading scores (eTable 1 in Supplement 3). In this cohort overall, ICU mortality was 14%, 6-month mortality was 32%, and an acceptable functional outcome (modified Rankin Scale of 0 to 4) occurred in 45% patients at 6 months; these outcomes are better than in most previously reported studies of patients with severe stroke and respiratory failure,1,29,30 although the patient population in the current study was self-selected for those desiring aggressive treatment. As such, these findings may represent a reasonable benchmark for likely outcomes of patients with severe stroke with respiratory failure that pursue full supportive measures.
Limitations
This study has several limitations. First, the sample size was inadequate to detect an absolute difference in outcomes smaller than 15% in the rate of acceptable neurological outcome. Second, the time point ranges of within 5 days for early and from day 10 for late tracheostomy were chosen based on earlier studies, a formal survey among German and US neurointensivists, and as to what was considered reasonable standards of care, yet with enough difference between the groups to detect a treatment effect. The difference between performing tracheostomy on days 4 vs 11 of intubation is clinically meaningful, but the findings cannot be generalized to settings in which tracheostomy timing is customarily performed outside of the range studied in this trial. Third, neurointensivists and ICU staff could not be masked to the treatment assignments, making them susceptible to bias, especially around the withdrawal of life support and introduction of limitations to treat. Attempts to minimize this bias included providing detailed, guideline-based treatment recommendations for both groups and performing robust outcome assessments and masked assessment of the primary outcome, although these measures may be inadequate. However, no significant differences in deaths related to withdrawal of life support between the groups were observed, and a numerically slightly higher rate of withdrawal of life support measures in the early tracheostomy group (the opposite of what might be expected) argues against treatment bias.
Fourth, the trial combined 3 different stroke subtypes with important differences in outcomes and hospital courses, introducing heterogeneity to the study population. Yet the hypothesis held by most of the neurocritical care community surveyed17 was that unlike most other patients in a medical, surgical, or cardiovascular ICU, patients with stroke share an impaired ability to maintain a secure and patent airway, and have much less tendency to cardiopulmonary respiratory failure than other patients in the ICU who may require tracheostomy. The need for tracheostomy among patients in this study was considered to be dominated by the location and extent of their vascular brain lesion rather than its exact pathology. However, patients with different stroke subtypes may have different clinical trajectories, particularly subarachnoid hemorrhage when compared with space-occupying ischemic or hemorrhagic parenchymal strokes, and it is possible that study results might have been different in a narrower population involving only 1 stroke subtype.
Fifth, in this study, there was not a single standardized weaning protocol across centers. Because of the pragmatic nature of the trial, sites were asked to wean sedation and ventilation aggressively following tracheostomy, but detailed weaning data were not collected. It is likely, however, that this trial reflected clinical practice settings, and its results should be interpreted in that light.
Conclusions
Among patients with severe stroke receiving mechanical ventilation, a strategy of early tracheostomy, compared with a standard approach to tracheostomy, did not significantly improve the rate of survival without severe disability at 6 months. However, the wide confidence intervals around the effect estimate may include a clinically important difference, so a clinically relevant benefit or harm from a strategy of early tracheostomy cannot be excluded.
Trial Protocol
Statistical Analysis Plan
eTable 1. Additional demographics and clinical baseline characteristics
eTable 2. Logistic regression model for the primary outcome mRS 0-4 at 6 month follow-up
eTable 3. Mixed model for the primary outcome mRS 0-4 at 6 month follow-up including center as random effect (sensitivity analysis)
eTable 4. Enrolling Centers
eTable 5. (Potentially) Tracheostomy-related Adverse Events
eTable 6. Serious Adverse Events
eTable 7. Causes and time of death
eTable 8. SETscore for estimation of 2-week ventilation need
eTable 9. SETPOINT2’s Contraindications to Percutaneous Dilatation Tracheostomy
eTable 10. Study protocol: Procedures and documentation in SEPOINT2
eFigure 1. Study flow chart of SETPOINT2
eFigure 2. Kaplan-Meier Estimate of Time to Start of Respirator Weaning
eFigure 3. Kaplan-Meier Estimate of Time to Cessation of Sedation
eFigure 4. Kaplan-Meier Estimate of Time to Discharge from Intensive Care Unit
(Stroke-related Early Tracheostomy in Neurocritical Care Trial 2) and IGNITE (Initiative of German Neuro-Intensive Trial Engagement)
Data Sharing Statement
References
- 1.Lahiri S, Mayer SA, Fink ME, et al. Mechanical ventilation for acute stroke: a multi-state population-based study. Neurocrit Care. 2015;23(1):28-32. doi: 10.1007/s12028-014-0082-9 [DOI] [PubMed] [Google Scholar]
- 2.Robba C, Poole D, McNett M, et al. Mechanical ventilation in patients with acute brain injury: recommendations of the European Society of Intensive Care Medicine consensus. Intensive Care Med. 2020;46(12):2397-2410. doi: 10.1007/s00134-020-06283-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurtz P, Fitts V, Sumer Z, et al. How does care differ for neurological patients admitted to a neurocritical care unit versus a general ICU? Neurocrit Care. 2011;15(3):477-480. doi: 10.1007/s12028-011-9539-2 [DOI] [PubMed] [Google Scholar]
- 4.Bösel J. Use and timing of tracheostomy after severe stroke. Stroke. 2017;48(9):2638-2643. doi: 10.1161/STROKEAHA.117.017794 [DOI] [PubMed] [Google Scholar]
- 5.Siempos II, Ntaidou TK, Filippidis FT, Choi AMK. Effect of early versus late or no tracheostomy on mortality and pneumonia of critically ill patients receiving mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(2):150-158. doi: 10.1016/S2213-2600(15)00007-7 [DOI] [PubMed] [Google Scholar]
- 6.Chorath K, Hoang A, Rajasekaran K, Moreira A. Association of early vs late tracheostomy placement with pneumonia and ventilator days in critically ill patients: a meta-analysis. JAMA Otolaryngol Head Neck Surg. 2021;147(5):450-459. doi: 10.1001/jamaoto.2021.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCredie VA, Alali AS, Scales DC, et al. Effect of early versus late tracheostomy or prolonged intubation in critically ill patients with acute brain injury: a systematic review and meta-analysis. Neurocrit Care. 2017;26(1):14-25. doi: 10.1007/s12028-016-0297-z [DOI] [PubMed] [Google Scholar]
- 8.Villwock JA, Villwock MR, Deshaies EM. Tracheostomy timing affects stroke recovery. J Stroke Cerebrovasc Dis. 2014;23(5):1069-1072. doi: 10.1016/j.jstrokecerebrovasdis.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 9.Araujo de Franca S, Tavares WM, Salinet ASM, Paiva WS, Teixeira MJ. Early tracheostomy in stroke patients: a meta-analysis and comparison with late tracheostomy. Clin Neurol Neurosurg. 2021;203:106554. doi: 10.1016/j.clineuro.2021.106554 [DOI] [PubMed] [Google Scholar]
- 10.Bösel J, Schiller P, Hook Y, et al. Stroke-related Early Tracheostomy versus Prolonged Orotracheal Intubation in Neurocritical Care Trial (SETPOINT): a randomized pilot trial. Stroke. 2013;44(1):21-28. doi: 10.1161/STROKEAHA.112.669895 [DOI] [PubMed] [Google Scholar]
- 11.Schönenberger S, Niesen WD, Fuhrer H, et al. ; SETPOINT2-Study Group; IGNITE-Study Group . Early tracheostomy in ventilated stroke patients: study protocol of the international multicentre randomized trial SETPOINT2 (Stroke-related Early Tracheostomy vs Prolonged Orotracheal Intubation in Neurocritical care Trial 2). Int J Stroke. 2016;11(3):368-379. doi: 10.1177/1747493015616638 [DOI] [PubMed] [Google Scholar]
- 12.Schönenberger S, Al-Suwaidan F, Kieser M, Uhlmann L, Bösel J. The SETscore to predict tracheostomy need in cerebrovascular neurocritical care patients. Neurocrit Care. 2016;25(1):94-104. doi: 10.1007/s12028-015-0235-5 [DOI] [PubMed] [Google Scholar]
- 13.Alsherbini K, Goyal N, Metter EJ, et al. Predictors for tracheostomy with external validation of the Stroke-Related Early Tracheostomy Score (SETscore). Neurocrit Care. 2019;30(1):185-192. doi: 10.1007/s12028-018-0596-7 [DOI] [PubMed] [Google Scholar]
- 14.Young D, Harrison DA, Cuthbertson BH, Rowan K; TracMan Collaborators . Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121-2129. doi: 10.1001/jama.2013.5154 [DOI] [PubMed] [Google Scholar]
- 15.Qureshi AI, Suarez JI, Parekh PD, Bhardwaj A. Prediction and timing of tracheostomy in patients with infratentorial lesions requiring mechanical ventilatory support. Crit Care Med. 2000;28(5):1383-1387. doi: 10.1097/00003246-200005000-00020 [DOI] [PubMed] [Google Scholar]
- 16.Walcott BP, Kamel H, Castro B, Kimberly WT, Sheth KN. Tracheostomy after severe ischemic stroke: a population-based study. J Stroke Cerebrovasc Dis. 2014;23(5):1024-1029. doi: 10.1016/j.jstrokecerebrovasdis.2013.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao CW, Hwang DY; and the SETPOINT2 Survey Investigators . US practitioner attitudes toward tracheostomy timing, benefits, risks, and techniques for severe stroke patients: a national survey and national inpatient sample analysis. Neurocrit Care. 2021;34(2):669-673. doi: 10.1007/s12028-020-01127-7 [DOI] [PubMed] [Google Scholar]
- 18.Bösel J, Schiller P, Hacke W, Steiner T. Benefits of early tracheostomy in ventilated stroke patients? current evidence and study protocol of the randomized pilot trial SETPOINT (Stroke-related Early Tracheostomy vs Prolonged Orotracheal Intubation in Neurocritical care Trial). Int J Stroke. 2012;7(2):173-182. doi: 10.1111/j.1747-4949.2011.00703.x [DOI] [PubMed] [Google Scholar]
- 19.Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. ; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology . Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711-1737. doi: 10.1161/STR.0b013e3182587839 [DOI] [PubMed] [Google Scholar]
- 20.Morgenstern LB, Hemphill JC III, Anderson C, et al. ; American Heart Association Stroke Council and Council on Cardiovascular Nursing . Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108-2129. doi: 10.1161/STR.0b013e3181ec611b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wijdicks EF, Sheth KN, Carter BS, et al. ; American Heart Association Stroke Council . Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(4):1222-1238. doi: 10.1161/01.str.0000441965.15164.d6 [DOI] [PubMed] [Google Scholar]
- 22.Adams HP Jr, del Zoppo G, Alberts MJ, et al. ; American Heart Association/American Stroke Association Stroke Council; American Heart Association/American Stroke Association Clinical Cardiology Council; American Heart Association/American Stroke Association Cardiovascular Radiology and Intervention Council; Atherosclerotic Peripheral Vascular Disease Working Group; Quality of Care Outcomes in Research Interdisciplinary Working Group . Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007;115(20):e478-e534. doi: 10.1161/CIRCULATIONAHA.107.181486 [DOI] [PubMed] [Google Scholar]
- 23.Diringer MN, Bleck TP, Claude Hemphill J III, et al. ; Neurocritical Care Society . Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15(2):211-240. doi: 10.1007/s12028-011-9605-9 [DOI] [PubMed] [Google Scholar]
- 24.Torbey MT, Bösel J, Rhoney DH, et al. Evidence-based guidelines for the management of large hemispheric infarction: a statement for health care professionals from the Neurocritical Care Society and the German Society for Neuro-intensive Care and Emergency Medicine. Neurocrit Care. 2015;22(1):146-164. doi: 10.1007/s12028-014-0085-6 [DOI] [PubMed] [Google Scholar]
- 25.Mayer SA, Brun NC, Begtrup K, et al. ; FAST Trial Investigators . Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127-2137. doi: 10.1056/NEJMoa0707534 [DOI] [PubMed] [Google Scholar]
- 26.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549-556. doi: 10.2307/2530245 [DOI] [PubMed] [Google Scholar]
- 27.Allison PD. Missing Data. Sage Publications; 2002. doi: 10.4135/9781412985079 [DOI] [Google Scholar]
- 28.van Buren S. Flexible Imputation of Missing Data. 2nd ed. CRC Press; 2018. doi: 10.1201/9780429492259 [DOI] [Google Scholar]
- 29.de Montmollin E, Terzi N, Dupuis C, et al. ; OUTCOMEREA Study Group . One-year survival in acute stroke patients requiring mechanical ventilation: a multicenter cohort study. Ann Intensive Care. 2020;10(1):53. doi: 10.1186/s13613-020-00669-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider H, Hertel F, Kuhn M, et al. Decannulation and Functional Outcome After Tracheostomy in Patients with Severe Stroke (DECAST): a prospective observational study. Neurocrit Care. 2017;27(1):26-34. doi: 10.1007/s12028-017-0390-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. Additional demographics and clinical baseline characteristics
eTable 2. Logistic regression model for the primary outcome mRS 0-4 at 6 month follow-up
eTable 3. Mixed model for the primary outcome mRS 0-4 at 6 month follow-up including center as random effect (sensitivity analysis)
eTable 4. Enrolling Centers
eTable 5. (Potentially) Tracheostomy-related Adverse Events
eTable 6. Serious Adverse Events
eTable 7. Causes and time of death
eTable 8. SETscore for estimation of 2-week ventilation need
eTable 9. SETPOINT2’s Contraindications to Percutaneous Dilatation Tracheostomy
eTable 10. Study protocol: Procedures and documentation in SEPOINT2
eFigure 1. Study flow chart of SETPOINT2
eFigure 2. Kaplan-Meier Estimate of Time to Start of Respirator Weaning
eFigure 3. Kaplan-Meier Estimate of Time to Cessation of Sedation
eFigure 4. Kaplan-Meier Estimate of Time to Discharge from Intensive Care Unit
(Stroke-related Early Tracheostomy in Neurocritical Care Trial 2) and IGNITE (Initiative of German Neuro-Intensive Trial Engagement)
Data Sharing Statement