Abstract
We encountered an 11‐day‐old male neonate with vitamin K deficiency‐induced intracranial hemorrhage, despite receiving oral vitamin K2 (menaquinone‐4) prophylaxis according to Japanese guidelines. This case suggests that the current vitamin K deficiency‐bleeding prophylaxis programs cannot prevent bleeding completely. Better prophylaxis programs using both intramuscular and oral administration should be considered.
Keywords: intracranial hemorrhage, prophylaxis, vitamin K deficiency bleeding
Short abstract
The current vitamin K deficiency‐bleeding prophylaxis programs cannot prevent bleeding shortly after birth. We should establish an optimal global standard of prophylaxis using both intramuscular and oral administration of vitamin K promptly.
1. INTRODUCTION
Newborns are at risk of vitamin K deficiency bleeding (VKDB) caused by inadequate prenatal storage of vitamin K, and its deficiency in breast milk and impaired ability to perform the gamma‐carboxylation of vitamin K‐dependent proteins because of lower concentrations of the enzymes that drive the vitamin K cycle at birth. Children with VKDB are likely to develop severe sequelae and have high risk of mortality. 1 Therefore, prevention of VKDB by appropriate administration of vitamin K is crucial. Currently, various administration methods have been proposed in different countries, and varying results have been achieved. 2 However, VKDB continues to occur, albeit in small numbers. We encountered a case of a neonate with vitamin K deficiency (VKD)‐induced intracranial hemorrhage. This case is significant in that it has been blind spotted in current vitamin K prophylaxis in infants.
2. CASE HISTORY
An 11‐day‐old male neonate presented with afebrile left‐sided tonic–clonic seizures. The patient was delivered at 37 weeks and 6 days’ gestation to a healthy mother via cesarean section and weighed 2674 g at birth. The pregnancy was uneventful. On postnatal days one and seven, 2 mg of oral vitamin K2 (menaquinone‐4) was administered to the neonate as recommended by the Japanese VKDB prophylaxis program. The patient was fed both breast and artificial milk from Day 1 after birth and did not show prolonged jaundice with pale stool and dark urine. The commercial artificial milk fed to the patient contains 34 μg/L of vitamin K2 but no vitamin K1. Upon admission, the patient had intermittent convulsions. The weight was 2800 g (126 g gain after birth). There was no hepatomegaly. No bleeding spots or traumatic scars were observed. There were no gastrointestinal symptoms such as vomiting and diarrhea. Serum albumin, total bilirubin, direct bilirubin, aspartate aminotransferase, and alanine aminotransferase were 3.5 g/dL, 5.58 mg/dL, 0.24 mg/dL, 30 IU/L, and 16 IU/L, respectively. These findings indicated that there was no evidence of cholestatic disease, gastrointestinal diseases, or abuse. Serum proteins induced by vitamin K absence II (PIVKA‐II) were 23 mAU/mL. There was no family history of bleeding disorders. Computed tomography of the brain revealed intracranial hemorrhage in the left ventricle and the surrounding parenchyma (Figure 1). Because of the findings of high value of PIVKA‐II compared with those of the same age and no obvious cause of VKD, we judged the patient to have idiopathic VKDB.
FIGURE 1.
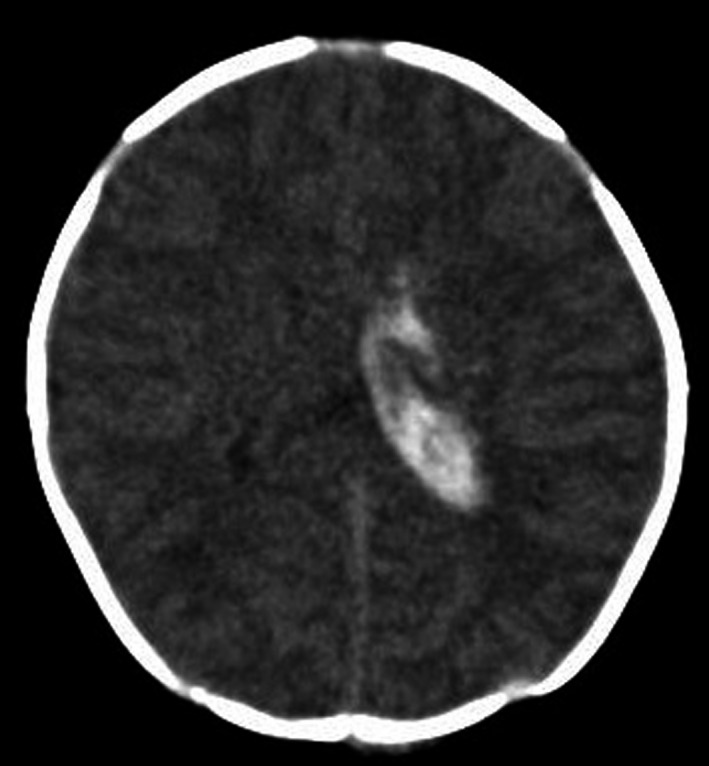
Brain computed tomography shows intracranial hemorrhage in the left ventricle and the surrounding parenchyma
3. DIFFERENTIAL DIAGNOSIS, INVESTIGATIONS, AND TREATMENT
Vitamin K was administered at 0.5 mg/kg intravenously, followed by short‐course anticonvulsants. Peripheral platelet counts were 85.0 × 104/μL. Both prothrombin time‐international normalized ratio (PT‐INR) and activated partial thromboplastin time (APTT) were prolonged, at 1.54 and exceeded the measurement range, respectively. Fibrinogen level was 227 mg/dL. These values were measured 4 h following intravenous vitamin K administration because of the initial difficulty in blood sample collection. Afterward, the patient became stable and the convulsions ceased. The following day, PT‐INR, and APTT had normalized, at 1.10 and 37.6 s, respectively. No further bleeding occurred subsequently. We also conducted cranial magnetic resonance imaging and magnetic resonance angiography to rule out malformation of the arteries in the brain, which revealed no abnormality. Accordingly, we made a diagnosis of idiopathic VKD‐induced intracranial hemorrhage. The patient completed 13 weekly oral doses of vitamin K2 supplements (2 mg at birth, and weekly for 3 months).
4. OUTCOME AND FOLLOW‐UP
Although he had no obvious sequelae at the four‐month follow‐up, careful observation of his mental and motor developments is required with a focus on complications, such as hydrocephalus.
5. DISCUSSION
VKDB in infancy is classified according to the time of presentation: early (within 24 h), classic (within 1 week after birth), and late (between 2 weeks and 6 months of age). Our patient had late‐onset VKDB, which can be life‐threatening. Most reported cases of late‐onset VKDB present with intracranial hemorrhage as seen in our case. The fifth nationwide survey in Japan, that took place between 1999 and 2004, reported the incidence of late VKDB was estimated to be 1.9 cases per 100,000 births (95% confidence interval: 1.2–3.0) and, that of the 71 reported cases of late VKDB, 88.7% had received vitamin K2 at least once either during or after the neonatal period. The further breakdown of these figures revealed that the percentage of cases who had received each dose was 38%, 21.2%, and 15.5% for 1, 2, and 3 doses, respectively. Also, 29.5% of etiology of VKDB was idiopathic. 3 Therefore, this current case is representative of the 21.2% of VKDB cases in the 5th nationwide survey who had received two vitamin K2 doses and demonstrates that VKD can occur with severe consequences despite the current recommendation for oral administration of vitamin K to healthy infants. Currently, there is no globally standardized method for vitamin K administration. Vitamin K can be administered to neonates via different routes. For example, in the United States, Canada, Denmark, and New Zealand, it is administered intramuscularly on the first day of life and reportedly results in fewer bleeding cases than in countries where it is administered orally. Despite this assertion, no comparative study has been performed. 4 However, some countries oppose intramuscular injection of vitamin K or allow the option to refuse intramuscular injection of this agent, because intramuscularly administered vitamin K is reportedly a risk factor for childhood leukemia. 5 However, many follow‐up studies on whether intramuscular vitamin K injections increase the incidence of childhood cancer have shown negative results. Another reason the prophylactic strategy has been inclined toward oral administration is that it has the advantage of being easier, safer, and cheaper to administer compared with intramuscular injections. Especially in Japan, it became difficult for the public to accept intramuscular injections for children since the 1970s when it had been reported that intramuscular injections for children led to muscle contracture, resulting in many suing for malpractice. Furthermore, several epidemiological studies have shown that oral administration of vitamin K is equally effective in the prevention of VKDB in infancy. However, the success of oral prophylaxis depends on the protocol regimen and parent compliance. Above all, the fact that VKDB is not eradicated by both methods is a significant problem.
Currently, in Japan and some countries, instead of the conventional administration of three doses of oral vitamin K2 supplements (2 mg on Day 1, Week 1, and Week 4), a regimen of 13 weekly oral doses of vitamin K2 supplements (2 mg at birth, and, weekly for 3 months) is recommended based on the reduced frequency of VKD‐induced bleeding disorders. 2 The latter method is effective, particularly for infants who have underlying disorders that affect vitamin K metabolism and malabsorption, such as cholestatic liver disease, which sometimes is not recognized until patients develop bleeding. However, our case makes it clear that neither method can prevent bleeding that develops before the third dose. This also indicates that prophylaxis using only oral administration has limitations in preventing VKDB. Our case may be typical for an idiopathic VKDB, but cases of VKDB will persist unless efforts are made to eliminate these unfortunate cases.
The patient was properly administered the recommended doses of vitamin K2 twice. Given that the patient had VKDB quite early at 11 days, there are two main likely causes; the neonate either had an extremely low dietary intake of vitamin K or had malabsorption due to an underlying disease effecting normal hepatic or gastrointestinal function. From the former point of view, the patient was both breast and formulated fed shortly after birth and had good weight gain. Although Japanese authority has no regulations on a minimum vitamin K content of artificial milk as the western countries do, the commercial artificial milk fed to the patient contained 34 μg/L of vitamin K2. Although this is less than half the amount of vitamin K1 contained in the artificial milk in Western countries, it is considered to be sufficient for the intake of vitamin K, because of the high vitamin K content of the formula milk (about 17 times as much as 2 μg/L of breast milk). From the latter point of view, there are no findings of liver damage, cholestasis, and gastrointestinal dysfunction on laboratory tests on admission. Furthermore, there were rapid corrections of the PT, APTT, and cessation of bleeding after vitamin K administration, and, at the time of subsequent follow‐up, the patient has been quite healthy and had no jaundice. Accordingly, the existence of any diseases that affect vitamin K absorption and biosynthesis is negative. Therefore, VKDB in this patient is idiopathic. However, some studies have reported mild abnormalities of liver function, which may be transient, mild, and self‐correcting but sufficient to create a temporary cholestasis and impairment of vitamin K absorption. 6 In this patient, too, there might be temporary subclinical short‐term cholestasis that affects vitamin K absorption at onset of VKDB. Sutor et al. observed that, in European surveys, the proportion of cases deemed idiopathic declines with increasing thoroughness of investigation with as many as 60% having undiagnosed hepatobiliary disease, predominantly cholestasis. 7 We must always be cautious about the possible existence of an underlying disorder of VKDB.
As already discussed, the previous experience of side effects of intramuscular injection in the 1970s in Japan had made public acceptance of intramuscular injection to children difficult. However, in recent years, intramuscular injection of immunoglobulin to prevent mother‐to‐child transmission of the Hepatitis B virus, palivizumab for respiratory syncytial virus, and the vaccine for the coronavirus in 2019 have been performed in many children, including neonates without any problems. Therefore, the routine administration of vitamin K to all newborn infants by intramuscular injection might also be generally accepted. Considering this, we believe it is necessary to introduce intramuscular administration of vitamin K to ensure that children can absorb a sufficient amount of vitamin K in the early stages after birth. Additionally, a portion of the intramuscular dose is stored in the liver, which can help maintain vitamin K levels for several months. In the United Kingdom, both intramuscular and oral administration of vitamin K have been introduced. However, there is still room for improvement in the oral regimen because of the small number of VKDB cases. As one of our suggestions, intramuscular injection at birth followed by weekly oral administration for three months may reduce or eliminate cases of VKDB better than any method currently used worldwide.
With an optimized administration program, VKDB could be prevented entirely. We should no longer have to experience cases of children with VKDB. Therefore, an optimal global standard of prophylaxis using both oral and intramuscular administration of vitamin K should be established promptly.
AUTHOR CONTRIBUTIONS
M. Miyahara conceived the idea and drafted the manuscript. K. Osaki supervised, held discussions, and provided additional input. All authors approved the final version of the manuscript and were involved in the management of the case. We two are dual first authors.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVAL
This study was approved by the Ethics Committee of Okanami General Hospital.
CONSENT
The parents of the patient provided written informed consent for the publication of data.
ACKNOWLEDGMENT
We would like to thank Editage (www.editage.com) for English language editing.
Miyahara M, Osaki K. No child should suffer from vitamin K deficiency‐induced bleeding disorders. Clin Case Rep. 2022;10:e05829. doi: 10.1002/ccr3.5829
Funding information
There is no funding source
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, [M.M.], upon reasonable request.
REFERENCES
- 1. Loughnan PM, McDougall PN. Epidemiology of late onset haemorrhagic disease: a pooled data analysis. J Paediatr Child Health. 1993;29(3):177‐181. doi: 10.1111/j.1440-1754.1993.tb00480.x [DOI] [PubMed] [Google Scholar]
- 2. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12(3):780. doi: 10.3390/nu12030780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takahashi D, Shirahata A, Itoh S, Takahashi Y, Nishiguchi T, Matsuda Y. Vitamin K prophylaxis and late vitamin K deficiency bleeding in infants: fifth nationwide survey in Japan. Pediatr Int. 2011;53(6):897‐901. doi: 10.1111/j.1442-200X.2011.03392.x [DOI] [PubMed] [Google Scholar]
- 4. von Kries R, Shearer MJ, Göbel U. Vitamin K in infancy. Eur J Pediatr. 1988;147(2):106‐112. doi: 10.1007/BF00442204 [DOI] [PubMed] [Google Scholar]
- 5. Golding J, Greenwood R, Birmingham K, Mott M. Childhood cancer, intramuscular vitamin K, and pethidine given during labour. BMJ. 1992;305(6849):341‐346. doi: 10.1136/bmj.305.6849.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shearer MJ. Vitamin K metabolism and nutriture. Blood Rev. 1992;6(2):92‐104. doi: 10.1016/0268-960x(92)90011-e [DOI] [PubMed] [Google Scholar]
- 7. Sutor AH, von Kries R, Cornelissen EA, McNinch AW, Andrew M. Vitamin K deficiency bleeding (VKDB) in infancy. ISTH pediatric/perinatal subcommittee. international society on thrombosis and haemostasis. Thromb Haemost. 1999;81(3):456‐461. doi: 10.1055/s-0037-1614494 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [M.M.], upon reasonable request.


