Abstract
Esophageal cancer is one of the most lethal cancers worldwide because of its rapid progression and poor prognosis. Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) are two major subtypes of esophageal cancer. ESCC predominantly affects African and Asian populations, which is closely related to chronic smoking and alcohol consumption. EAC typically arises in Barrett's esophagus with a predilection for Western countries. While surgical operation and chemoradiotherapy have been applied to combat this deadly cancer, molecularly targeted therapy is still at the early stages. With the development of large-scale next-generation sequencing, various genomic alterations in ESCC and EAC have been revealed and their potential roles in the initiation and progression of esophageal cancer have been studied. Potential therapeutic targets have been identified and novel approaches have been developed to combat esophageal cancer. In this review, we comprehensively analyze the genomic alterations in EAC and ESCC and summarize the potential role of the genetic alterations in the development of esophageal cancer. Progresses in the therapeutics based on the different tissue types and molecular signatures have also been reviewed and discussed.
KEY WORDS: Esophageal cancer, Esophageal squamous cell carcinoma, Esophageal adenocarcinoma, Next-generation sequencing, Genomic alteration, Somatic mutation, Copy number variation, Molecularly targeted therapy
Graphical abstract
Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) are two subtypes of esophageal cancer with diverse epidemiology and pathogenesis as well as different molecular profiles. This review analyzes the genomic alterations in ESCC and EAC and summarizes the potential therapeutic targets of ESCC and EAC.
1. Introduction
Carcinoma of the esophagus is the seventh most common cancer and the sixth leading cause of cancer-related mortality in the world1. Esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) are two major subtypes of esophageal cancer. ESCC represents the majority of esophageal cancer worldwide, especially in Asian and African populations and is thought to be associated with diet habit and exposure to carcinogens2. In contrast, EAC which develops from intestinal metaplasia of the esophageal epithelium due to chronic gastroesophageal reflux disease is the predominant subtype of esophageal cancer in Western countries3. Esophageal cancer is known for its rapid progression and poor outcomes. As esophageal cancer is often asymptomatic in its early stages, the disease is usually at an advanced stage at the time of diagnosis and the prognosis of esophageal cancer is poor with the overall five-year survival rate from 15% to 25%4. However, the options for the treatment of esophageal cancer are limited at present. Endoscopic or surgical treatment is applied to early-stage patients, while radiotherapy or/and chemotherapy is predominant for patients with advanced or metastatic cancer5. Therefore, it is urgent to develop new therapies targeting the essential factors involving in the initiation and progression of esophageal carcinoma.
Although emerging evidence shows that ESCC and EAC are two different diseases, molecular mechanism of tumorigenesis for each subtype remains unclear. Massively parallel, high-throughput next-generation DNA sequencing (NGS) has enabled comprehensive characterization of somatic mutations and copy number variations (CNVs) in a large scale of samples, which provides new clues and targets for the precise therapy of ESCC and EAC.
In this review, we summarize the recent progress in genomic and molecular characterization of ESCC and EAC. We also propose the opportunities to target the functional alterations for the therapy of ESCC and EAC.
2. Genetic alterations in ESCC and EAC
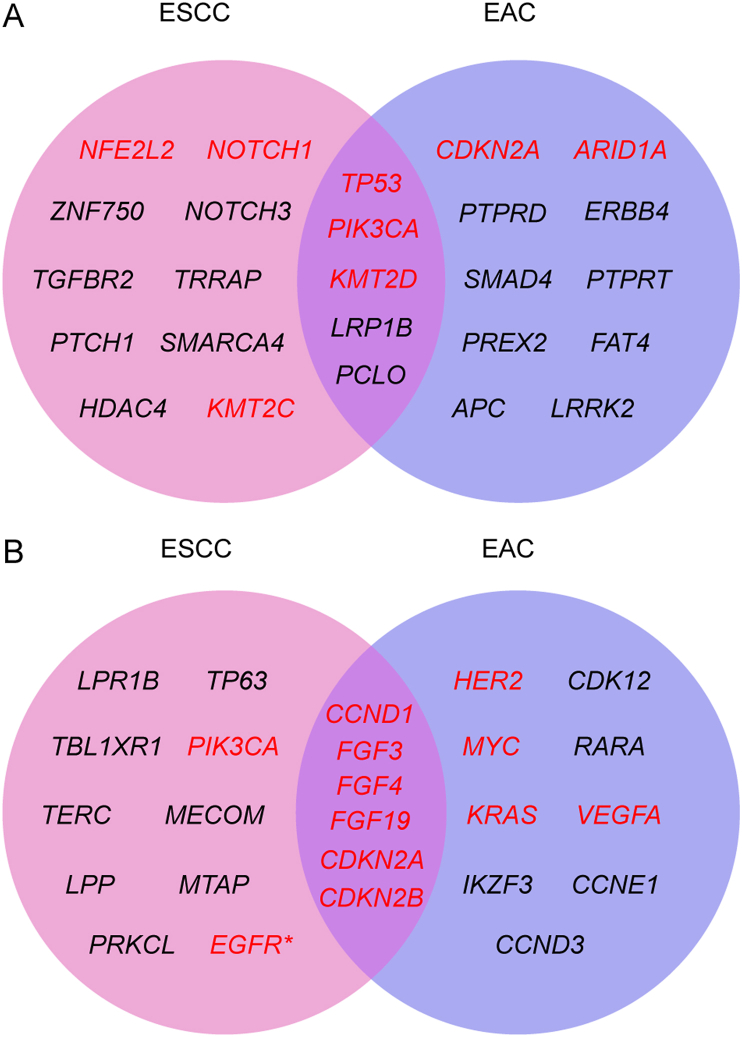
In light of the molecular events in the tumorigenesis, molecularly targeted therapies have been successfully employed for the treatment of tumors with identified driver genes, including non-small cell lung cancer (EGFR, ALK), melanoma (BRAF), renal cancer (VEGFR), hepatocellular cancer (PDGFRB), breast cancer (HER2, CDK4, CDK6), chronic lymphocytic leukemia (BTK), ovarian cancer (PARP) and gastrointestinal cancer (VEGFR, HER2)6. Esophageal cancer has been benefitted little from the targeted therapies due to the unclear pathogenesis. Therefore, defining genomic landscape of esophageal cancer would gain insights into the initiation and development of esophageal cancer and facilitate to develop molecularly targeted therapies. NGS has revolutionized cancer genomic research by providing a comprehensive profiling of cancer genome, including point mutations, insertions, deletions and copy number variations, making it a powerful tool to identify potential driver genes or pathways of human cancer7. In recent years, several NGS studies have been performed in esophageal cancer patients across the world. The cBioPortal for cancer genomics (https://www.cbioportal.org) is an open platform integrating cancer genomics data from multiple resources with high credibility. We obtained the genomic data of ESCC and EAC samples from cBioPortal (Supporting Information Tables S1–S4) and compared their genomic profile in an effort to identify potential therapeutic targets. The top 15 cancer-related genes with highest rate in mutation or copy number variation in ESCC or EAC were presented in Fig. 1. The genes in red have been reported to be associated with esophageal cancer and are or potentially are therapeutic targets for esophageal cancer. As shown in Fig. 1, TP53, PIK3CA and KMT2D are commonly mutated (Fig. 1A), and copy number variations in CCND1, FGF3, FGF4, FGF19, CDKN2A and CDKN2B are frequently found (Fig. 1B) in both subtypes of esophageal cancer, while distinct genetic alterations were also found in ESCC and EAC. Here, we summarize these cancer-related genes and outlined their potential functions in ESCC and EAC.
Figure 1.
Frequent genetic alterations in human esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). The data of genetic alterations in human ESCC and EAC were collected from cBioPortal, www.cbioportal.org, accessed in April 2021 (Tables S1–S4). The top 15 cancer-related genes with highest rate in mutation (A) or copy number variation (B) in ESCC and EAC were presented as a Venn diagram. Genes in red have been reported to be associated with esophageal cancer and are or potentially are therapeutic targets. ∗EGFR has been verified as a promising target to treat ESCC though it is not among the top 15 altered genes.
2.1. Genetic alterations with similar frequency in ESCC and EAC
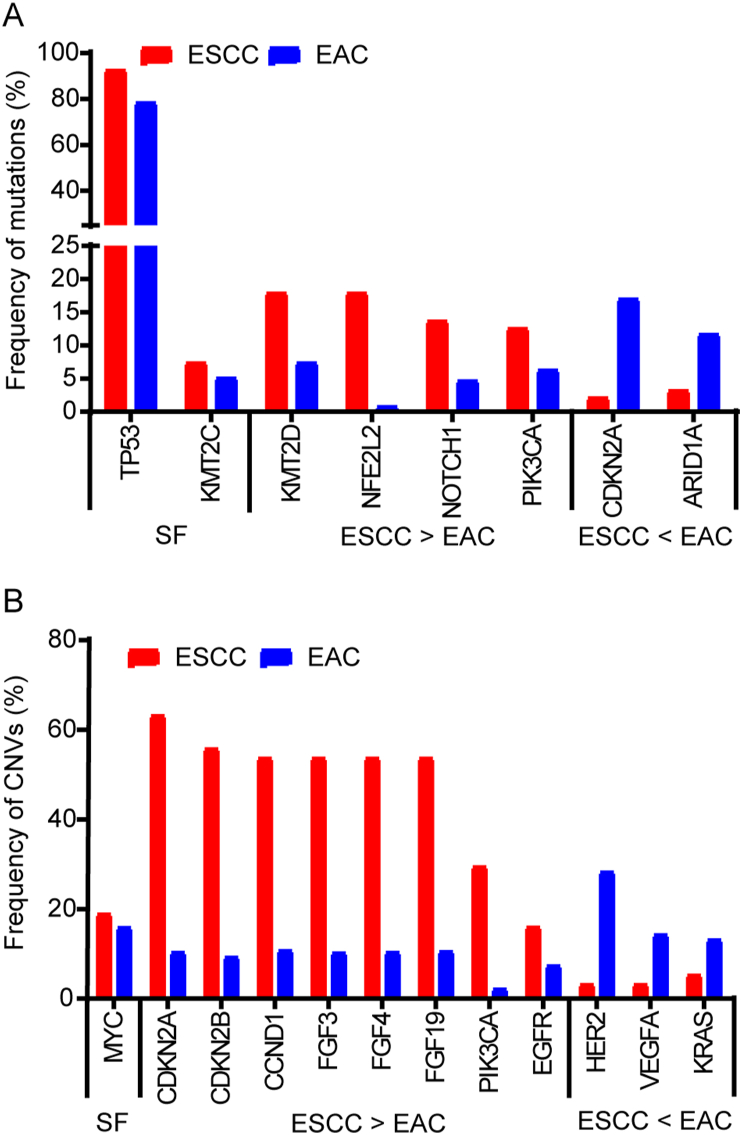
By comparing the frequency of genetic alterations between ESCC and EAC, we found that TP53, CDKN2A, MYC and KMT2C are commonly altered with similar frequency in both subtypes of esophageal cancer (Fig. 2), reflecting deregulation in genomic stability and cell cycle progression in both ESCC and EAC (Fig. 3).
Figure 2.
Comparison of genetic alterations between ESCC and EAC. The frequency of alteration of the genes in red presented in Fig. 1 was compared between ESCC and EAC according to the data from cBioPortal. SF: similar frequency. If the frequency of alteration in a gene is greater than 2-fold (≥2) in ESCC compared to that in EAC, the gene is assigned to ‘ESCC > EAC’. If the frequency of alteration in a gene is greater than 2-fold (≥2) in EAC compared to that in ESCC, the gene is assigned to ‘ESCC < EAC’. If the difference in the frequency of alteration in a gene is less than 2-fold, the gene was considered as ‘similar frequency’ in both ESCC and EAC. (A) Frequency of mutations. (B) Frequency of copy number variations.
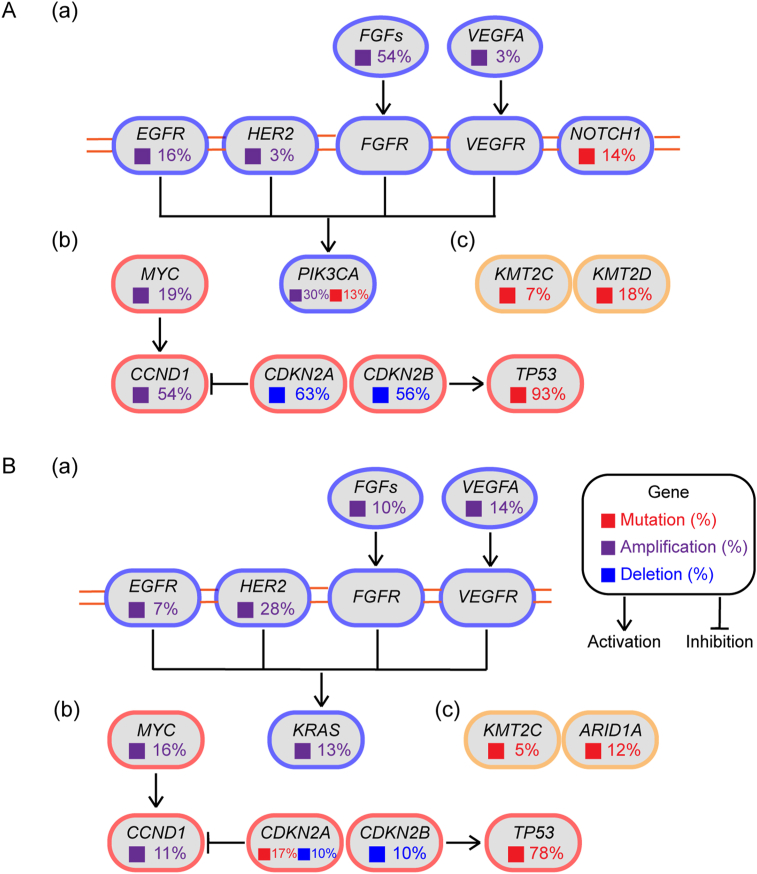
Figure 3.
Significantly dysregulated pathways in human ESCC (A) and EAC (B). The most altered genes were classified into three signaling pathways. The frequency of genetic alterations in ESCC and EAC were retrieved from cBioPortal (www.cbioportal.org, accessed in April 2021.). (a) RTK–MAPK–PI3K signaling. (b) Cell cycle regulation. (c) Epigenetic modulation.
2.1.1. TP53
The TP53 gene, localizing on chromosome 17p13.1, encodes a tumor suppressor protein responding to diverse cellular stresses to ensure cell and tissue homeostasis8. Somatic mutations of TP53 are one of the most common alterations in human cancer, and about 80% of esophageal cancer patients carry TP53 mutations (Figure 2, Figure 3). Most of them are hotspot mutations in DNA-binding domain9 (codons 175, 245, 248, 273, and 282), and truncated mutation at R342X in P53 tetramerization motif. These mutations abrogate the tumor suppressive function of P53, resulting in unstable genome. A recent study indicated that G245C and R273H mutants played important roles in tumorigenesis of ESCC, which might be exploited for diagnosis and therapy of patients carrying the mutants10. In a multivariable analysis in 161 patients with resectable ESCC, researchers revealed that the non-disruptive mutation in TP53 DNA-binding domain is a potential independent prognostic factor indicating prolonged survival and patients with elevated P53 expression showed a better outcome11. A genomic analysis of Barrett's esophagus (BE) patients found significantly higher numbers of TP53 mutations in BE patients with progression to EAC12. Taken together, these results suggest TP53 mutations are closely linked to the pathogenesis of esophageal cancer, which could be used as potential prognosis biomarker dependent on disease contexts.
2.1.2. CDKN2A
CDKN2A encodes a cyclin-dependent kinase inhibitor p16 (Ink4a), a tumor suppressor that regulates the transition of cell cycle from G1 to S phase. The CDKN2A gene is located within the frequently deleted region of chromosomal 913. Loss of p16 is common in variant cancers. Inactive or decreased p16 fails to bind to cyclin D, which assists cyclin-dependent kinase 4/6 (CDK4/6) in phosphorylating Rb protein, and results in loss of brake in cell cycle progression. CDKN2A deletion is found in about 63% ESCC (Figure 2, Figure 3A). A comprehensive analysis of whole-genome sequencing (WGS) of 31 ESCC tumors and paired normal samples found that CDKN2A was deleted in 29 out of 31 ESCCs14. Homozygous deletion and promotor methylation of CDKN2A were also found to occur in 86% of a cohort of 21 cases of ESCC15. In addition, loss-of-function mutations of CDKN2A are detected in about 17% EAC patients (Figure 2, Figure 3B). A whole exome sequencing from 25 pairs of EAC and BE revealed that the progression of BE often emerged with the inactivation of CDKN2A16. Furthermore, researchers have verified that methylation of the CDKN2A promotor is the predominant mechanism of p16 inactivation, which is a very common event in EAC and occurs as early as metaplasia17.
2.1.3. MYC
The MYC gene, located on chromosome 8q24.21, encodes a nuclear phosphoprotein that plays important roles in cell cycle progression, apoptosis and cellular transformation. The amplification of MYC occurs frequently in a variety of human cancers, contributing to tumor progression and indicating poor outcome18. According to the data from cBioPortal, MYC amplification occurs in nearly 20% esophageal cancer patients (Figure 2, Figure 3). MYC amplification was detected in the normal mucosa adjacent to the tumor in 23% of a group of esophageal cancer patients from high-risk region of China19. High expression level of MYC was significantly correlated with poor prognosis and also associated with invasion and lymph node metastasis in ESCC patients20. The amplification of MYC is also frequently detected in EAC, which is likely to be an early event during EAC carcinogenesis21.
2.1.4. KMT2C
KMT2C, mapped on chromosome 7q36.1, also known as myeloid/lymphoid or mixed-lineage leukemia protein 3 (MLL3), encodes a nuclear protein which possesses histone methylation activity and participates in transcriptional coactivation. The lysine methyltransferase 2 (KMT2) family members play essential roles in regulating cell growth and development, and mutations in these genes are frequently found in blood and solid cancers22. KMT2C has been reported to be frequently mutated in a variety of tumors. There are about 5% esophageal cancer patients carrying mutations in KMT2C (Figure 2, Figure 3), which have also been identified in other studies23,24. However, the function of KMT2C in esophageal cancer and the relationship between KMT2C mutations and clinicopathological characteristics of esophageal cancer remain poorly understood.
2.2. Differential genetic alterations in ESCC and EAC
Though ESCC and EAC share a few common genetic alterations, they exhibit distinct molecular profile as two subtypes of esophageal cancer (Fig. 2). In terms of genetic mutations, significantly higher mutational rates in NFE2L2, KMT2D, NOTCH1 and PIK3CA are found in ESCC, whereas more frequent mutations in CDKN2A and ARID1A are observed in EAC (Fig. 2A). There are also distinctions in CNVs between ESCC and EAC. HER2, VEGFA and KRAS are significantly more amplified in EAC, while ESCC exhibits higher CNVs of CDKN2A, CDKN2B, CCND1, FGFs, PIK3CA and EGFR (Fig. 2B). These molecular differences suggest ESCC and EAC should not be considered as one disease and the therapeutic targets might be different.
2.2.1. Genetic alterations with higher frequency in ESCC
2.2.1.1. FGFs
The family of fibroblast growth factors (FGFs) regulates a wide range of biological functions, including cellular proliferation, survival, migration and differentiation25. There is mounting evidence for the importance of FGF signaling in the pathogenesis of various tumor types, and clinical inhibitors targeting FGFs or their receptors are being rapidly developed26. FGF3/4/19, located at 11q13-14, are found amplified in more than 50% ESCC patients (Figure 2, Figure 3A), which often coincided with CCND1 amplification. Amplification of FGF3/4 was detected by DNA microarrays in 9 of 20 surgically resected primary ESCC tumors27. In a cohort of 3342 gastroesophageal cancers, amplification of FGF3, FGF4, FGF19 and FGFR1 were significantly more frequent in ESCC compared with EAC28, which indicated the preferential activation of FGF pathway in ESCC. Fibroblast growth factor receptor 1 (FGFR1), FGFR4 and fibroblast growth factor receptor like 1 (FGFRL1) was implicated in the progression of ESCC29, and genetic depletion of FGFRL1 was able to attenuate the motility and invasion in ESCC cells30. Moreover, several studies have demonstrated that FGFR1 amplification was an independent factor of poor prognosis in ESCC patients31,32. Given that the defined roles of abnormal FGF/FGFR signaling in tumorigenesis, agents targeting FGF/FGFR may be a potential opportunity for ESCC treatment.
2.2.1.2. EGFR
EGFR, located on chromosome 7p11.2, encodes a transmembrane glycoprotein that belongs to the ERBB family of receptor tyrosine kinases (RTK). Binding of epidermal growth factor receptor (EGFR) to epidermal growth factors triggers its autophosphorylation and subsequent activation of signal transduction pathways leading to cell proliferation, differentiation and survival33. Abnormal activation of EGFR is often found in epithelial tumors and related to poor prognosis and decreased survival34. Amplification of EGFR occurs in about 16% ESCC patients (Figure 2, Figure 3A), which was significantly associated with overexpression of EGFR35. The survival rate of patients with high EGFR expression was significantly lower than that of patients with lower expression in a study consisting of 441 ESCC patients, suggesting that expression of EGFR could be a prognostic predictor for ESCC36. Furthermore, a molecular prognostic model have showcased that comprised expression of EGFR, p-Sp1 and Fascin proteins was significantly associated with poor clinical outcome of ESCC patients37.
2.2.1.3. NOTCH1
The NOTCH1 gene, located on chromosome 9q34.3, encodes a member of the NOTCH family proteins. NOTCH signaling is an evolutionarily conserved intercellular signaling pathway that regulates interactions between physically adjacent cells through binding of NOTCH family receptors to their cognate ligands. There is emerging evidence that mutations in NOTCH genes and dysregulated NOTCH signaling are important factors in tumor initiation and development38, 39, 40. NOTCH1 mutation is found in 14% ESCC according to the data from cBioPortal (Figure 2, Figure 3A). The mutations of NOTCH1 in ESCC tended to cluster in the EGF-like repeats and resulted in loss of function41. Loss of NOTCH1 was found to predispose the esophagus to precancer and squamous cell carcinoma partially via accelerated telomere erosion42. Constitutively active NOTCH signaling pathway, regardless of transient or stable expressions of NOTCH1, was able to inhibit the proliferation of ESCC EC9706 cells via arresting cells at G1 phase43. Analysis of NGS data from 104 ESCCs from China found that patients with loss of function NOTCH1 mutations failed to respond to chemotherapy and had shorter survival times than patients without mutations in this gene44. Therefore, NOTCH seems to act as a tumor suppressor in the development of ESCC.
2.2.1.4. PIK3CA
The PIK3CA gene, mapped on chromosome 3q26.32, encodes the catalytic subunit of phosphoinositide 3-kinase (PI3K), p110α. Class Ⅰ PI3K is a lipid kinase composed of an 85 kDa regulatory subunit and a 110 kDa catalytic subunit, which is responsible for phosphorylating phosphatidylinositol-4,5-bisphosphate (PIP2) to generate the second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3). The PI3K signaling pathway regulates various cellular processes, such as proliferation, cell cycle, apoptosis and cytoskeletal rearrangement45. PI3K pathway is frequently disturbed in many human cancers, mostly PIK3CA alterations46. The mutation and amplification of PIK3CA occur in 13% and 30% ESCC patients respectively (Figure 2, Figure 3A), which have been verified in a number of studies23,24,47. A comprehensive analysis of PI3Kα expression in ESCC patients indicated that overexpression of PI3Kα was associated with lymph node metastasis and PI3Kα may play a crucial role in the development of ESCC48. PIK3CA amplification was also associated with shorter survival in an investigation of 534 curatively resected ESCC patients, suggesting its role as a poor prognostic factor in resected ESCC49. The alteration of PIK3CA is common in ESCC, making it a promising target for ESCC treatment and potential biomarkers for individualized molecularly targeted therapy for ESCC50.
2.2.1.5. CDKN2B
The gene of CDKN2B encodes a cyclin-dependent kinase inhibitor p15, which interacts with CDK4 or CDK6, and prevents the activation of CDKs. Thus, the function of p15 is to regulate cell cycle progression by restraining G1/S transition. CDKN2B is located on chromosome 9p21.3, a region often implicated in the pathogenesis of multiple cancers. Genetic variants at 9p21.3 may modulate the expression of p15 and contribute to ESCC susceptibility51,52, highlighting the importance of 9p21.3 genetic variants in tumorigenesis. Among the genetic variants found in this region, deletion of CDKN2B is the most common alteration in ESCC, which occurs in 56% ESCC patients (Figure 2, Figure 3A). In a genetic analysis of 33 human esophageal carcinomas and their adjacent normal tissues, deletion of CDKN2B was observed in a large proportion of ESCC samples53. Additionally, the homozygous deletion of CDKN2B was notably associated with lymph node metastasis in ESCC54. Lately, a study revealed CDKN2A/2B loss could be a biomarker predictive of CDK4/6 inhibitor efficacy in both patient-derived cell and patient-derived xenograft models55, which is consistent with disorder of cell cycle in ESCC due to CDKN2A/2B deletion.
2.2.1.6. CCND1
CCND1, located on chromosome 11q13.3, encodes a highly conserved cyclin family member cyclin D1, which forms a complex with CDK4/6 and participates in cell cycle G1/S transition. Mutation, amplification and overexpression of this gene are able to release a cell from its controlled cell cycle and cause transformation to a malignant phenotype56. The amplification of CCND1 is detected in 54% ESCC patients (Figure 2, Figure 3A). In a whole exome sequencing analysis of tissues collected from 144 Japanese ESCC patients, cell cycle regulators constituted the most frequently disrupted category, including TP53 mutations (93.1%), CCND1 amplification (46.5%) and CDKN2A deletion (53.5%)57. Another study with 55 ESCC patients also found that ratios of CCND1 amplification or cyclin D1 overexpression were 42% or 58%, respectively58. High expression of cyclin D1 was able to increase distant metastasis, decrease overall survival and distant metastasis-free survival in resectable ESCC, which may help to identify patients with high risk of postoperative metastases59. Collectively, the alterations of CCND1 exist in almost half of ESCC patients, manifesting its critical character in the development and progression of ESCC.
2.2.1.7. KMT2D
KMT2D, localized to chromosome 12q13.12, encodes a histone methyltransferase that is responsible for methylating histone 3 at lysine 4. KMT2D plays critical roles in regulation of development, differentiation, metabolism and tumor suppression60. Frequent mutations in genes involving in histone modification including KMT2D have been found in ESCC. KMT2D mutations occur in 18% of ESCC (Figure 2, Figure 3A), the majority of which are truncated mutations resulting in proteins lacking the key methyltransferase domain, indicating a tumor suppressor role of KMT2D in ESCC23,24,28,47,57. Notably, KMT2D was mutated in 60% of the metastatic ESCC and in only 15.3% of the primary ESCC, proposing an important role of KMT2D in ESCC metastasis61. However, the clinical significance and prognostic value of KMT2D mutations in ESCC have not been fully elucidated, which deserves further investigation.
2.2.1.8. NFE2L2
The NFE2L2 gene, mapped on chromosome 2q31.3, encodes a transcription factor which is a member of small family of basic leucine zipper proteins. NFE2L2 is the master regulator of cellular antioxidant responses. Mutations of NFE2L2 frequently occur in human cancers, which drive continuous NFE2L2 activation and correlate with poor prognosis62. NFE2L2 mutations is found in 18% ESCC according to the data from cBioPortal (Fig. 2A). An analysis of 1145 tumor samples detected that NFE2L2 mutations existed in 11.4% samples, accompanied with increased NFE2L2 expression in the nuclei63. Recently, a WGS of 508 Chinese ESCC patients identified that NFE2L2 mutations were significantly associated with worse prognosis of ESCC64. Mutant NFE2L2 conferred attachment-independent cell survival, which was correlated with lymph node metastasis and tumor progression65. Down-regulation of mutant NFE2L2 by short hairpin RNA increased the sensitivity of ESCC cells to chemotherapy65. These results indicate the oncogenic function of NFE2L2 mutation in ESCC and strategies are proposed to target the NFE2L2 for future therapy66.
2.2.2. Genetic alterations with higher frequency in EAC
2.2.2.1. HER2
HER2, located on chromosome 17q12, encodes a member of EGFR family of RTKs. Although HER2 possesses an active tyrosine kinase domain, no direct ligand has been identified. HER2 exists in the extended active conformation, rendering HER2 constitutively available for dimerization67,68. HER2 is often amplified in human cancers such as breast cancer, gastric and esophageal cancer69, 70, 71. According to the data from cBioPortal, HER2 amplification occurs in 28% of EAC (Figure 2, Figure 3B). The HER2 expression in EAC patients is highly concordant with its copy number72. The overexpression of HER2 was also a typical feature in an animal model of BE-related EAC73, indicating a crucial role of HER2 in EAC pathogenesis. HER2 expression is likely to indicate a positive prognosis in EAC. Several studies have reported that EAC patients with high HER2 expression showed a superior overall survival compared to HER2-negative patients72,74,75. In consideration of the vital role in EAC, HER2 has become a therapeutic target for EAC patients.
2.2.2.2. VEGFA
VEGFA, located on chromosome 6p21.1, encodes a heparin-binding protein, which exists as a disulfide-linked homodimer. This growth factor stimulated proliferation and migration of vascular endothelial cells, which is essential for angiogenesis under both physiological and pathological circumstances. As angiogenesis is important to provide nutrients for tumor growth, vascular endothelial growth factor A (VEGFA) is highly expressed in cancer tissues and associated with more aggressive features. Data from cBioPortal shows that VEGFA amplification occurs in 14% of EAC (Figure 2, Figure 3B). In consistency with the high frequency of amplification, significant increase in VEGFA expression was found in tumor and peritumoral adipose tissue during the progression of normal squamous epithelium to EAC76,77. Elevated expression of VEGFA was also observed in lymph node metastases of EAC, indicating an important role in tumor metastasis78. Furthermore, VEGFA expression was significantly associated with poor prognosis, which supports the targeted-VEGFR therapy for EAC patients79.
2.2.2.3. KRAS
The KRAS gene located on chromosome 12p12.1 encodes the small GTPase KRAS. KRAS acts as a molecular switch, regulating diverse signal transduction pathways80. As one of the most well-known proto-oncogenes, KRAS has been intensively studied in the past years. The activating mutations of KRAS occur in approximately 30% of all human cancers, indicating its pivotal role in driving cancer progression81. Nevertheless, the amplification of KRAS is more common in EAC. In a WGS analysis of 22 fresh-frozen EAC samples, the amplification of KRAS by breakage-fusion-bridge was found in 6 samples, proposing a potential role in fueling EAC progression through KRAS amplification82. Additionally, KRAS amplification significantly related with nodal positive patients and poorer survival in the subgroup without neoadjuvant treatment. Co-existing of amplification in KRAS, HER2 as well as TP53 mutations has been reported to indicate an unfavorable prognosis in EAC83.
2.2.2.4. ARID1A
ARID1A, mapped on chromosome 1p36.11, encodes a member of SWI/SNF family, which is thought to regulate transcription of specific genes by modulating the chromatin structure. Inactivating mutations in ARID1A have been identified in a broad spectrum of cancers, indicating its action as a tumor suppressor84. There are about 12% of EAC harboring ARID1A mutations (Figure 2, Figure 3B). In a WGS study of endoscopic biopsies, ARID1A mutations were detected in 15% of high-grade dysplasia or EAC and loss of ARID1A was also identified in BE/high-grade dysplasia/EAC tissues. ARID1A-deficient EAC was reported to be accompanied with high microsatellite instability85, suggesting its role in genomic stability. Knock down of ARID1A in EAC OE33 cells was able to enhance cell growth, proliferation and invasion, suggesting its suppressive activity in EAC pathogenesis86. The overall survival time of patients with loss of ARID1A is apparently shorter than those with wild-type ARID1A (26.2 months vs. 60.1 months)87. Collectively, ARID1A acts as a tumor suppressor in the initiation and progression of EAC.
3. Emerging therapeutic targets in esophageal cancer
With the development of molecularly targeted drugs and revelation of genetic alterations in esophageal cancer, molecularly targeted therapy has emerged as a promising opportunity for the treatment of esophageal cancer. Here, we summarized the recent progress in the treatment of ESCC and EAC by exploiting their distinct genetic alterations.
3.1. Common therapeutic targets in esophageal cancer
3.1.1. c-MET
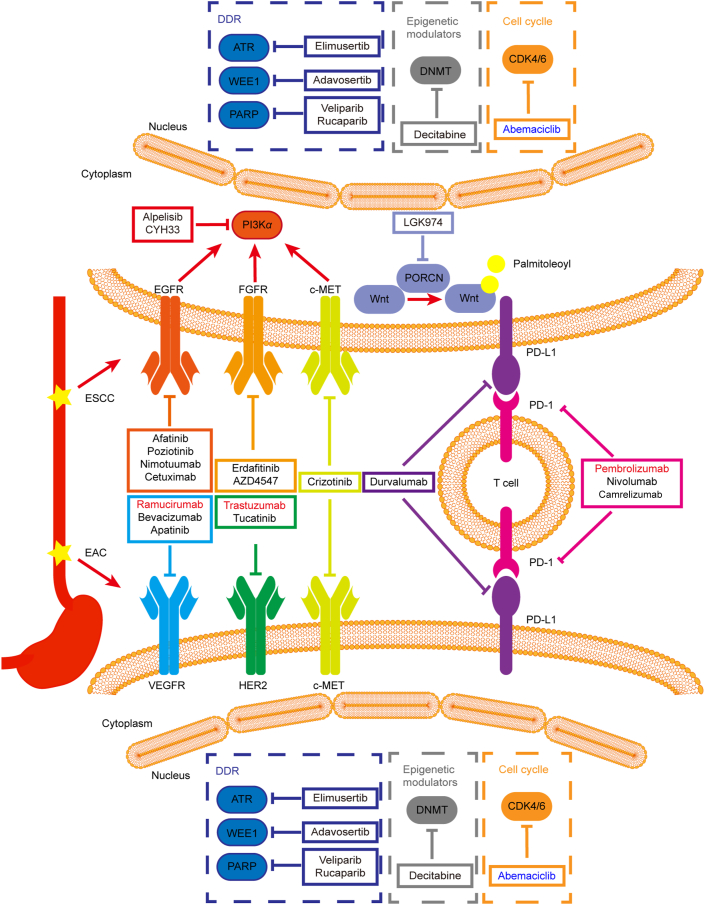
c-MET is a transmembrane receptor tyrosine kinase, which is activated by hepatocyte growth factor (HGF) via autophosphorylation at the activation loop of the kinase domain. The signal mediated by c-MET is involved in cell proliferation, motility, invasion and survival. The amplification and overexpression of c-MET is implicated in a wide variety of human cancers including esophageal cancer88,89. MET amplification was an independent prognosis factor in surgical ESCC and negatively affected the survival of ESCC patients90. The esophagogastric adenocarcinoma patients with MET amplification showed a substantially shorter median survival time and more sensitive to the c-MET inhibitor crizotinib91. These findings suggest c-MET may be a potential therapeutic target for esophageal cancer and crizotinib is being tested in clinical trials (Fig. 4 and Table 1). Anti-c-MET antibody telisotuzumab showed acceptable safety profile and clinical activity in esophageal cancer patients carrying MET amplification. Among four gastroesophageal cancer patients, three achieved a partial response and one had progressive disease as best response92. The completed phase Ⅰ and Ⅱ clinical studies with c-MET inhibitor AMG337 found that EAC patients with MET amplification responded well to AMG337 with manageable toxicities, and an objective response rate of 18% was observed in gastric/gastroesophageal junction/EAC patients with MET amplification93,94. Collectively, c-MET is a promising target for esophageal cancer patients with MET amplification.
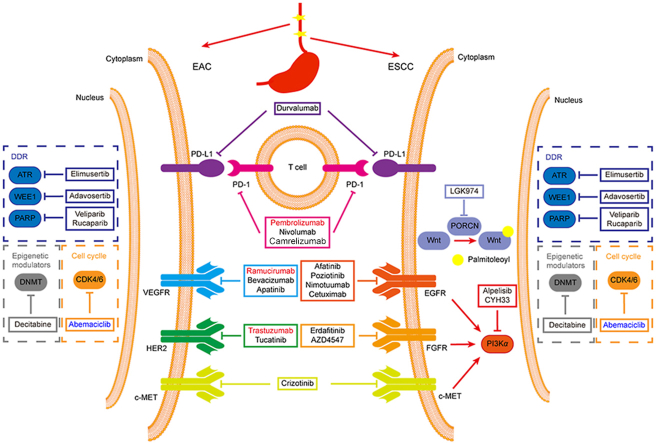
Figure 4.
Potential therapeutic targets and drugs for the treatment of ESCC and EAC. Drugs in red have been approved by FDA, drugs in black are at different stages of clinical trials, while drugs in blue are in preclinical studies.
Table 1.
Ongoing clinical trials of molecularly targeted drugs in esophageal cancer.
| NCT No. | Drug | Target | Disease | Phase |
|---|---|---|---|---|
| NCT02465060 | Crizotinib | c-MET | Esophageal cancer | Ⅱ |
| NCT04491942 | Elimusertib + chemotherapy | ATR | Esophageal cancer | Ⅰ |
| NCT04460937 | Adavosertib + radiotherapy | WEE1 | Esophageal cancer | Ⅰ |
| NCT04171700 | Rucaparib | PARP | Esophageal cancer | Ⅱ |
| NCT01366144 | Veliparib + chemotherapy | PARP | Esophageal cancer | Ⅰ |
| NCT03233724 | Decitabine | DNMT | Esophageal cancer | Ⅰ/Ⅱ |
| NCT03544736 | Nivolumab + radiotherapy | PD-1 | Esophageal cancer | Ⅰ/Ⅱ |
| NCT03691090 | Camrelizumab + chemotherapy | PD-1 | Esophageal cancer | Ⅲ |
| NCT02639065 | Durvalumab | PD-L1 | Esophageal cancer | Ⅱ |
| NCT03940976 | Afatinib | EGFR | ESCC | Ⅱ |
| NCT02409186 | Nimotuzumab + chemoradiotherapy | EGFR | ESCC | Ⅲ |
| NCT03770988 | Poziotinib | EGFR | ESCC | Ⅱ |
| NCT03126708 | Cetuximab | EGFR | ESCC | Ⅱ |
| NCT02699606 | Erdafitinib | FGFR | Esophageal cancer | Ⅱ |
| NCT01795768 | AZD4547 | FGFR | Esophageal cancer | Ⅱ |
| NCT03292250 | Alpelisib | PI3Kα | ESCC | Ⅱ |
| NCT03544905 | CYH33 | PI3Kα | ESCC | Ⅰ |
| NCT01351103 | LGK974 | Wnt | ESCC | Ⅰ |
| NCT04430738 | Tucatinib | HER2 | EAC | Ⅰ/Ⅱ |
| NCT03783936 | Trastuzumab + avelumab | HER2 | EAC | Ⅱ |
| NCT04499924 | Tucatinib | HER2 | EAC | Ⅱ/Ⅲ |
| NCT01359397 | Bevacizumab + chemotherapy | VEGFR | EAC | Ⅱ |
| NCT02898077 | Apatinib + chemotherapy | VEGFR | Esophageal cancer | Ⅱ |
3.1.2. CDK4/6
The cell cycle-related genes including TP53, CDKN2A, CDKN2B and CCND1 frequently altered in esophageal cancer (Fig. 3), indicating a highly disordered cell cycle progression. In recent years, therapies targeting cell cycle progression has made great progress. Three CDK4/6 inhibitors, abemaciclib, palbociclib and ribociclib, have emerged for the therapy of breast cancer. In light of the dysregulation of cell cycle, targeting CDK4/6 might be a promising approach for treatment of esophageal cancer. Abemaciclib was able to induce apoptosis and inhibit proliferation in EAC cells and significantly inhibited the tumor growth in vivo95. Clinical trials to test the efficacy of CDK4/6 inhibitors in esophageal cancer are warranted.
3.1.3. Genes involving DNA damage response
Esophageal cancer is characterized by a high mutation in TP53, which plays pivotal roles in the DNA damage response96. In response to DNA damages, activation of P53 initiates pathways involved in cell cycle arrest, DNA repair, and apoptosis in order to avoid propagation of damaged cells. Mutations in TP53 cause defective and weakened G1 and G2 checkpoints, rendering cells highly dependent on activated WEE1 to achieve cell cycle arrest in response to DNA damage. WEE1 was identified as a synthetic lethal target in TP53-mutated cancer. WEE1 inhibitor AZD1775 in combination with chemotherapy showed superior response rate in TP53 mutated patients (21%) compared with those with wild-type TP53 (12%)97. Combination DNA damage response inhibitors such as inhibitors targeting ATR, CHK1/2 with chemotherapy/radiotherapy also displayed potential for the treatment of esophageal cancer98. The combination of an ATR inhibitor, elimusertib, or a WEE1 inhibitor, adavosertib, with chemotherapy/radiotherapy for the treatment of advanced solid tumors including esophageal carcinoma is being tested in clinical trials (Fig. 4 and Table 1). PARP inhibitors alone or in combination with paclitaxel are being evaluated in esophageal cancer patients carrying deleterious mutation in homologous recombination repair genes (Fig. 4 and Table 1).
3.1.4. Epigenetic modulators
Epigenetic alterations such as KMT2C, KMT2D and ARID1A are common in esophageal cancer (Fig. 3). Though the role of these alterations has not been clearly elucidated, drug candidates targeting epigenetic modulators have displayed potential activity against esophageal cancer. Pretreatment of the DNA methyltransferase inhibitor azacitidine enhanced the efficacy of chemotherapy in advanced EAC patients. All 12 patients achieved R0 section after treatment of azacitidine plus chemotherapy. 3 patients (25%) had a complete response, 5 patients (42%) had a partial response, and 4 (33%) had stable disease99. Combination of a histone deacetylase inhibitor MS-275 and azacytidine was efficient to inhibit esophageal cancer cells, indicating a potential targeted therapy for the esophageal cancer100. Moreover, the DNA methyltransferase (DNMT) inhibitor decitabine is being tested in clinical trials (Fig. 4 and Table 1). As the role of epigenetic alterations in esophageal cancer is gradually revealed, epigenetic-targeted therapy might be a possible option for esophageal cancer patients.
3.1.5. Immune checkpoint
For the past few years, remarkable breakthrough of immunotherapy has been made for the treatment of solid tumors. Immune checkpoint inhibitors such as anti-CTLA4 or anti-PD-1/PD-L1 antibodies have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of multiple solid tumors. PD-1 inhibitor pembrolizumab exhibits desired activity in esophageal cancer patients, which has been used to treat ESCC with high expression of PD-L1 (Fig. 4 and Table 2)101. Immune checkpoint inhibitors alone or combined with chemoradiotherapy are being assessed in clinical trials (Fig. 4 and Table 1) and encouraging results have been obtained in esophageal cancer. In a single-arm phase Ⅱ trial, PD-1 inhibitor camrelizumab combined with apatinib and chemotherapy demonstrated significant efficacy and manageable safety in advanced ESCC patients. The objective response rate and disease control rate were 80% and 96.7% respectively. All adverse events were handled with appropriate medical care, which suggested a new combination strategy for ESCC treatment102. The level of serum lactate dehydrogenase may serve as a potential marker for the response of camrelizumab in ESCC patients103. Recently, the efficacy and safety of pembrolizumab in combination with chemoradiotherapy are being evaluated in both ESCC and EAC patients, which may help define the role of immunotherapy as a first-line treatment option for patients with esophageal carcinoma104.
Table 2.
Approved molecularly targeted drugs for esophageal cancer.
| Drug | Target | Company | Indication |
|---|---|---|---|
| Trastuzumab | HER2 | Pfizer | EAC |
| Ramucirumab | VEGFR | Lilly | EAC |
| Pembrolizumab | PD-1 | Merck Sharp & Dohme | ESCC |
3.2. Therapeutic targets in ESCC
3.2.1. EGFR
Amplification and overexpression of EGFR occurred frequently in ESCC and significantly correlated with tumor progression and invasion35,58,105, which indicates that EGFR is a potential molecular target in ESCC. The selective EGFR inhibitor gefitinib showed potent antitumor activity against ESCC in vitro and in vivo and augmented the efficacy of tumor necrosis factor-related death apoptosis inducing ligand in human ESCC cells106,107. Lapatinib, a dual inhibitor of EGFR and HER2, possessed significant activity in ESCC cells over-expressing EGFR and/or HER2 and synergistically inhibited the growth of ESCC patient-derived xenograft in combination with 5-fluorouracil108,109. Monoclonal antibodies against EGFR also exhibited favorable efficacy against ESCC. Nimotuzumab was able to promote radiosensitivity in EGFR-overexpressing ESCC cells by upregulating insulin like growth factor binding protein 3 (IGFBP-3)110. Cetuximab significantly inhibited the growth of ESCC cells overexpressing EGFR111. Clinical trials of small molecule inhibitors or monoclonal antibodies targeting EGFR for the treatment of ESCC are undergoing (Fig. 4 and Table 1) and clinical efficacy has been observed. Combination of chemoradiation with erlotinib has been shown to be effective and tolerable in ESCC patients in phase Ⅱ studies112,113. Among the 21 patients treated, 8 (38%) achieved a complete response, 10 (47.6.%) a partial response and 3 (14.4%) stable disease. No patient showed progressive disease113. In a randomized phase Ⅲ trial for locally advanced ESCC patients, the addition of erlotinib significantly improved overall survival compared with chemoradiotherapy alone (median, 39.4 vs. 27.4 months)114. Icotinib alone or combined with radiotherapy also showed favorable activity in ESCC patients with EGFR overexpression or amplification. The objective response rate and disease control rate was 16.7% or 46.3%, respectively, in a phase Ⅱ trial of icotinib monotherapy in ESCC patients with EGFR overexpression115. In another randomized phase Ⅱ trial with 127 ESCC patients enrolled, icotinib plus radiotherapy improved the median overall survival compared with radiotherapy alone (24.0 months vs. 16.3 months)116. Complete or partial responses were observed in 18 of 20 elderly ESCC patients in a phase Ⅱ trial of combined radiotherapy and gefitinib117. In summary, targeting EGFR is a promising therapeutic option for ESCC patients and EGFR amplification may serve as a biomarker indicating favorable response.
3.2.2. FGFR
FGF/FGFR participate in multiple cellular processes, whereas deregulation of FGF/FGFR signaling pathway is correlated with developmental disorders and cancers118. FGFR inhibitors are developed in recent years and erdafitinib is the first FGFR inhibitor approved by FDA for the therapy of urothelial carcinoma in 2019. Several other FGFR inhibitors also exhibit favorable activity against multiple solid tumors. The study of FGFR inhibitor on ESCC patients is at the early stage. The selective FGFR4 inhibitor H3B-6527 significantly inhibited the growth of ESCC cells in vitro and in vivo by blocking PI3K and mitogen-activated protein kinase (MAPK) signaling pathways119. FGFR inhibitor AZD4245 could enhance the sensitivity of ESCC cells with high level of active FGFR1 to gefitinib, proposing a potential combination for ESCC treatment120. Currently, several clinical trials with FGFR inhibitors for esophageal cancer are underway (Fig. 4 and Table 1).
3.2.3. PI3Kα
The mutations and amplification of PIK3CA are frequent and associated with poor prognosis in ESCC, indicating PI3Kα is a promising target for ESCC treatment. As the first PI3Kα-specific inhibitor approved by FDA, alpelisib has been proved to be effective in several solid tumors including head and neck squamous cell carcinoma and ESCC121. Our previous study has reported that CYH33, a novel PI3Kα-selective inhibitor, displayed significant activity against the proliferation of ESCC cells and the growth of xenografts derived from ESCC cells122. CYH33 also sensitized ESCC cells to radiation by abrogating survival signals in tumor cells and tumor microenvironment123. The combination of PI3Kα inhibitors with other targeted drugs also demonstrated potent anti-ESCC activity. Combination of alpelisib with a JNK inhibitor, SP600125, displayed a synergistic activity against ESCC in vitro and in vivo, proposing a new therapeutic strategy for ESCC patients124. Concurrent inhibition of PI3Kα and RAS/MEK synergistically inhibited the growth of xenografts harboring HRASG12S mutation, which was worthwhile to be considered in the treatment of ESCC patients harboring mutation or amplification in KRAS or HRAS125. Alpelisib and CYH33 are currently in clinical trials for the treatment of solid tumors including advanced ESCC (Fig. 4 and Table 1).
3.2.4. WNT signaling pathway
The WNT signal transduction cascade is a main regulator of development and a key driver of most types of tissue stem cells126. The alterations in WNT signaling pathway possess a fundamental role in cancer development and drug resistance127. WNT signaling pathway was reported to be hyperactivated in ESCC24,57. The biomarker signature score based on a panel of WNT-related markers (cytoplasmic β-catenin, nuclear c-MYC, nuclear disheveled segment polarity protein 1 and membrane α-catenin) was associated with ESCC recurrence/death128. WNT signaling was also found to be involved in radioresistance by promoting DNA damage repair mediated by a chromatin-associated protein trans-activating high-mobility group box 1129. These studies indicated that WNT signaling might be a potential therapeutic target for ESCC. LGK974 is a porcupine inhibitor, which inhibits the secretion of WNT and its signaling by blocking the palmitoylation of WNT130. LGK974 is currently in clinical trials for the treatment of solid tumors including ESCC (Fig. 4 and Table 1).
3.3. Therapeutic targets in EAC
3.3.1. HER2
HER2 amplification and overexpression have been found in a number of human cancers, such as breast cancer, gastric cancer and EAC. Currently, HER2-targeted therapy has been applied to the treatment of a few types of cancer overexpressing HER2. The anti-HER2 monoclonal antibody trastuzumab exhibited promising therapeutic effect in HER2-positive EAC patients. In a phase Ⅲ randomized trial for HER2-positive gastric or gastric-esophageal junction adenocarcinoma patients, the median overall survival was 13.8 months in trastuzumab plus chemotherapy group compared with 11.1 months in chemotherapy alone group131. Considering the favorable clinical efficacy, trastuzumab has been approved by FDA for the treatment of HER2-positive EAC patients in combination of chemotherapy (Fig. 4 and Table 2). Other HER2-targeted drugs are being tested in clinical trials (Fig. 4 and Table 1) and favorable activity against HER2-positive EAC has been observed. Lapatinib, a small-molecule inhibitor of HER-2 and EGFR, has been approved to treat HER2-positive breast cancer. Combined lapatinib and chemotherapy was effective in HER2-positive EAC patients with manageable toxicity132. In a phase Ⅱ study of trastuzumab and pertuzumab with chemoradiotherapy in EAC, complete response rate and overall survival was 34% and 71%, respectively. No unexpected adverse events were observed and patients with HER2 overexpression showed better response, indicating that such combination was promising in HER2-positive patients133. Based on the positive results of clinical trials, HER2-targetd drugs displayed great promising in the therapy of HER2-positive EAC.
3.3.2. VEGFR
VEGF plays an essential role in the regulation of angiogenesis and lymphangiogenesis, which is vital for tumor angiogenesis and metastasis. Targeting VEGFR has been proven effective in diverse solid tumors including EAC. Ramucirumab is a monoclonal antibody targeting VEGFR and has displayed efficacy in EAC patients. In a phase Ⅲ trial of ramucirumab monotherapy for gastric or gastric–esophageal junction adenocarcinoma patients, the median overall survival was 5.2 months in ramucirumab group and 3.8 months in placebo group134. Besides, ramucirumab in combination with paclitaxel significantly improved overall survival compared with placebo plus paclitaxel in gastric or gastric-esophageal junction adenocarcinoma patients (9.6 months vs. 7.4 months)135. Ramucirumab has been approved by FDA for treatment for EAC patients in view of the promising clinical results (Fig. 4 and Table 2). Bevacizumab is also a monoclonal antibody against VEGFR being tested in clinical trials (Fig. 4 and Table 1). In a phase Ⅱ study of bevacizumab combined with chemotherapy in metastatic gastric or gastro–esophageal junction adenocarcinoma patients, the overall response rate was 65% and time to disease progression improved over historical controls by 75%136. Small molecule VEGFR inhibitors apatinib also showed favorable antitumor activity in EAC patients and has been approved in China as the third-line treatment for gastro–esophageal junction adenocarcinoma137. The clinical trial of apatinib combined with chemotherapy in esophageal cancer patients is now undergoing (Fig. 4 and Table 1).
4. Conclusions and perspectives
Esophageal cancer is one of the most lethal malignancies worldwide, whereas the options of molecularly targeted therapy are very limited. At present, there are only three targeted drugs have been approved for clinical applications (Table 2). With the evolution of DNA sequencing technology, the landscape of esophageal cancer genome is being revealed in recent years. We analyzed the genomic data and found that these recurrently altered genes are enriched in regulators of cell cycle (TP53, CDKN2A, CDKN2B, CCND1 and MYC), members in the RTK-MAPK/PI3K pathway (FGFs, EGFR, HER2, VEGFA, PIK3CA, and KRAS), and epigenetic modulators (KMT2C, KMT2D and ARID1A) (Fig. 3). These alterations could disrupt normal epithelia homeostasis and contribute to tumorigenesis and promotion of esophageal cancer.
Though current sequencing technology have provided bulk genomic data to guide the treatment of esophageal cancer, how to select the genomic data due to the intratumoral heterogeneity is still a considerable challenge in clinical practice. Emerging strategies such as multiregional sequencing, single-cell sequencing and analysis of liquid biopsy samples have been introduced. These strategies may help better understand the tumor heterogeneity and provide more accurate data for precise medicine. However, bulk genomic data are usually utilized in the clinical practice due to the cost and efficiency. With further development of the sequencing technology, more precise genomic data will be available for the molecularly targeted therapy in esophageal cancer.
ESCC and EAC are two subtypes of esophageal cancer with diverse epidemiology and pathogenesis as well as different molecular profiles, which provide distinctive targets for the treatment. Further preclinical studies and clinical trials are in progress and some encouraging clinical efficacy has been observed (Fig. 4). Moreover, the combination of targeted drugs and chemotherapy/radiotherapy displayed great potential for this deadly disease. Combination regimens with improved efficacy and reduced toxicity are being tested in clinical trials (Table 1). With the increasing knowledge of molecular characteristics in ESCC and EAC, more molecularly targeted therapy will be developed to benefit the esophageal cancer patients in the near future.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81973345 and 82173832) and “Personalized Medicines-Molecular Signature-based Drug Discovery and Development”, Strategic Priority Research Program of the Chinese Academy of Sciences (XDA12020111, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2021.09.028.
Author contributions
Linghua Meng conceived the project and revised the manuscript. Xu Zhang and Yuxiang Wang summarized the literature and composed the manuscript. All authors proofread the manuscript and approved to submit the final manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Murphy G., McCormack V., Abedi-Ardekani B., Arnold M., Camargo M.C., Dar N.A., et al. International cancer seminars: a focus on esophageal squamous cell carcinoma. Ann Oncol. 2017;28:2086–2093. doi: 10.1093/annonc/mdx279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubenstein J.H., Shaheen N.J. Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology. 2015;149:302–317 e1. doi: 10.1053/j.gastro.2015.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pennathur A., Gibson M.K., Jobe B.A., Luketich J.D. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 5.He S., Xu J., Liu X., Zhen Y. Advances and challenges in the treatment of esophageal cancer. Acta Pharm Sin B. 2021;11:3379–3392. doi: 10.1016/j.apsb.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee Y.T., Tan Y.J., Oon C.E. Molecular targeted therapy: treating cancer with specificity. Eur J Pharmacol. 2018;834:188–196. doi: 10.1016/j.ejphar.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Alekseyev Y.O., Fazeli R., Yang S., Basran R., Maher T., Miller N.S., et al. A next-generation sequencing primer-how does it work and what can it do?. Acad Pathol. 2018;5 doi: 10.1177/2374289518766521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leroy B., Anderson M., Soussi T. TP53 mutations in human cancer: database reassessment and prospects for the next decade. Hum Mutat. 2014;35:672–688. doi: 10.1002/humu.22552. [DOI] [PubMed] [Google Scholar]
- 9.England B., Huang T.G., Karsy M. Current understanding of the role and targeting of tumor suppressor p53 in glioblastoma multiforme. Tumor Biol. 2013;34:2063–2074. doi: 10.1007/s13277-013-0871-3. [DOI] [PubMed] [Google Scholar]
- 10.Kang N., Wang Y., Guo S.C., Ou Y.W., Wang G.C., Chen J., et al. Mutant TP53 G245C and R273H promote cellular malignancy in esophageal squamous cell carcinoma. BMC Cell Biol. 2018;19:16. doi: 10.1186/s12860-018-0167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang M.R., Jin J.Y., Zhang F.R., Wu Y.X., Xu C.Y., Ying L.S., et al. Non-disruptive mutation in TP53 DNA-binding domain is a beneficial factor of esophageal squamous cell carcinoma. Ann Transl Med. 2020;8:316. doi: 10.21037/atm.2020.02.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stachler M.D., Camarda N.D., Deitrick C., Kim A., Agoston A.T., Odze R.D., et al. Detection of mutations in Barrett's esophagus before progression to high-grade dysplasia or adenocarcinoma. Gastroenterology. 2018;155:156–167. doi: 10.1053/j.gastro.2018.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witcher M., Emerson B.M. Epigenetic silencing of the p16INK4a tumor suppressor is associated with loss of CTCF binding and a chromatin boundary. Mol Cell. 2009;34:271–284. doi: 10.1016/j.molcel.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng C.X., Zhou Y., Li H.Y., Xiong T., Li S.C., Bi Y.H., et al. Whole-genome sequencing reveals diverse models of structural variations in esophageal squamous cell carcinoma. Am J Hum Genet. 2016;98:256–274. doi: 10.1016/j.ajhg.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smeds J., Berggren P., Ma X., Xu Z., Hemminki K., Kumar R. Genetic status of cell cycle regulators in squamous cell carcinoma of the oesophagus: the CDKN2A (p16INK4a and p14ARF) and p53 genes are major targets for inactivation. Carcinogenesis. 2002;23:645–655. doi: 10.1093/carcin/23.4.645. [DOI] [PubMed] [Google Scholar]
- 16.Stachler M.D., Taylor-Weiner A., Peng S.Y., McKenna A., Agoston A.T., Odze R.D., et al. Paired exome analysis of Barrett's esophagus and adenocarcinoma. Nat Genet. 2015;47:1047–1055. doi: 10.1038/ng.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bian Y.S., Osterheld M.C., Fontolliet C., Bosman F.T., Benhattar J. p16 inactivation by methylation of the CDKN2A promoter occurs early during neoplastic progression in Barrett's esophagus. Gastroenterology. 2002;122:1113–1121. doi: 10.1053/gast.2002.32370. [DOI] [PubMed] [Google Scholar]
- 18.Lin C.Y., Loven J., Rahl P.B., Paranal R.M., Burge C.B., Bradner J.E., et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu S.H., Hsieh L.L., Luo F.C., Weinstein I.B. Amplification of the EGF receptor and c-myc genes in human esophageal cancers. Int J Cancer. 1988;42:502–505. doi: 10.1002/ijc.2910420406. [DOI] [PubMed] [Google Scholar]
- 20.Wang W., Xue L., Wang P. Prognostic value of beta-catenin, c-myc, and cyclin D1 expressions in patients with esophageal squamous cell carcinoma. Med Oncol. 2011;28:163–169. doi: 10.1007/s12032-010-9436-0. [DOI] [PubMed] [Google Scholar]
- 21.von Rahden B.H., Stein H.J., Puhringer-Oppermann F., Sarbia M. c-myc amplification is frequent in esophageal adenocarcinoma and correlated with the upregulation of VEGF-A expression. Neoplasia. 2006;8:702–707. doi: 10.1593/neo.06277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagan R.J., Dingwall A.K. COMPASS ascending: emerging clues regarding the roles of MLL3/KMT2C and MLL2/KMT2D proteins in cancer. Cancer Lett. 2019;458:56–65. doi: 10.1016/j.canlet.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao Y.B., Chen Z.L., Li J.G., Hu X.D., Shi X.J., Sun Z.M., et al. Genetic landscape of esophageal squamous cell carcinoma. Nat Genet. 2014;46:1097–1102. doi: 10.1038/ng.3076. [DOI] [PubMed] [Google Scholar]
- 24.Song Y., Li L., Ou Y., Gao Z., Li E., Li X., et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91–95. doi: 10.1038/nature13176. [DOI] [PubMed] [Google Scholar]
- 25.Eswarakumar V.P., Lax I., Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Turner N., Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 27.Arai H., Ueno T., Tangoku A., Yoshino S., Abe T., Kawauchi S., et al. Detection of amplified oncogenes by genome DNA microarrays in human primary esophageal squamous cell carcinoma: comparison with conventional comparative genomic hybridization analysis. Cancer Genet Cytogen. 2003;146:16–21. doi: 10.1016/s0165-4608(03)00106-7. [DOI] [PubMed] [Google Scholar]
- 28.Salem M.E., Puccini A., Xiu J., Raghavan D., Lenz H.J., Korn W.M., et al. Comparative molecular analyses of esophageal squamous cell carcinoma, esophageal adenocarcinoma, and gastric adenocarcinoma. Oncologist. 2018;23:1319–1327. doi: 10.1634/theoncologist.2018-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimada Y., Okumura T., Takei Y., Watanabe K., Nagata T., Hori T., et al. Role of fibroblast growth factor receptors in esophageal squamous cell carcinoma. Esophagus. 2015;13:30–41. [Google Scholar]
- 30.Takei Y., Matsumura T., Watanabe K., Nakamine H., Sudo T., Shimizu K., et al. FGFRL1 deficiency reduces motility and tumorigenic potential of cells derived from oesophageal squamous cell carcinomas. Oncol Lett. 2018;16:809–814. doi: 10.3892/ol.2018.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Q., Liu Y., Jiang D., Wang H., Huang J., Xu Y., et al. High amplification of FGFR1 gene is a delayed poor prognostic factor in early stage ESCC patients. Oncotarget. 2017;8:74539–74553. doi: 10.18632/oncotarget.20215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D., Du L., Wang Z., Liu X., Qin Y., Wang Q., et al. Association of fibroblast growth factor receptor 1 gene amplification with poor survival in patients with esophageal squamous cell carcinoma. Oncotarget. 2017;8:88857–88869. doi: 10.18632/oncotarget.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbst R.S. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 34.Baselga J., Arteaga C.L. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–2459. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- 35.Hanawa M., Suzuki S., Dobashi Y., Yamane T., Kono K., Enomoto N., et al. EGFR protein overexpression and gene amplification in squamous cell carcinomas of the esophagus. Int J Cancer. 2006;118:1173–1180. doi: 10.1002/ijc.21454. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W., Zhu H., Liu X., Wang Q., Zhang X., He J., et al. Epidermal growth factor receptor is a prognosis predictor in patients with esophageal squamous cell carcinoma. Ann Thorac Surg. 2014;98:513–519. doi: 10.1016/j.athoracsur.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Cao H.H., Zheng C.P., Wang S.H., Wu J.Y., Shen J.H., Xu X.E., et al. A molecular prognostic model predicts esophageal squamous cell carcinoma prognosis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo L.X., Zhang T., Xiong Y., Yang Y.A. Roles of NOTCH1 as a therapeutic target and a biomarker for lung cancer: controversies and perspectives. Dis Markers. 2015;2015:520590. doi: 10.1155/2015/520590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Girardi T., Vicente C., Cools J., De Keersmaecker K. The genetics and molecular biology of T-ALL. Blood. 2017;129:1113–1123. doi: 10.1182/blood-2016-10-706465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Efstratiadis A., Szabolcs M., Klinakis A. Notch, Myc and breast cancer. Cell Cycle. 2007;6:418–429. doi: 10.4161/cc.6.4.3838. [DOI] [PubMed] [Google Scholar]
- 41.Li Y., Li Y., Chen X. NOTCH and esophageal squamous cell carcinoma. Adv Exp Med Biol. 2021;1287:59–68. doi: 10.1007/978-3-030-55031-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawangarun W., Mandasari M., Aida J., Morita K.I., Kayamori K., Ikeda T., et al. Loss of Notch1 predisposes oro-esophageal epithelium to tumorigenesis. Exp Cell Res. 2018;372:129–140. doi: 10.1016/j.yexcr.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 43.Lu Z.M., Liu H.T., Xue L.X., Xu P.R., Gong T.X., Hou G.Q. An activated Notch1 signaling pathway inhibits cell proliferation and induces apoptosis in human esophageal squamous cell carcinoma cell line EC9706. Int J Oncol. 2008;32:643–651. [PubMed] [Google Scholar]
- 44.Song B., Cui H.Y., Li Y.P., Cheng C.X., Yang B., Wang F., et al. Mutually exclusive mutations in NOTCH1 and PIK3CA associated with clinical prognosis and chemotherapy responses of esophageal squamous cell carcinoma in China. Oncotarget. 2016;7:3599–3613. doi: 10.18632/oncotarget.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vivanco I., Sawyers C.L. The phosphatidylinositol 3-kinase–AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 46.Fresno Vara J.A., Casado E., de Castro J., Cejas P., Belda-Iniesta C., Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Lin D.C., Hao J.J., Nagata Y., Xu L., Shang L., Meng X., et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet. 2014;46:467–473. doi: 10.1038/ng.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akagi I. Overexpression of PIK3CA is associated with lymph node metastasis in esophageal squamous cell carcinoma. Int J Oncology. 2009;34:767–775. doi: 10.3892/ijo_00000202. [DOI] [PubMed] [Google Scholar]
- 49.Kim H.S., Lee S.E., Bae Y.S., Kim D.J., Lee C.G., Hur J., et al. PIK3CA amplification is associated with poor prognosis among patients with curatively resected esophageal squamous cell carcinoma. Oncotarget. 2016;7:30691–30701. doi: 10.18632/oncotarget.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L., Shan L., Zhang S., Ying J., Xue L., Yuan Y., et al. PIK3CA gene mutations and overexpression: implications for prognostic biomarker and therapeutic target in Chinese esophageal squamous cell carcinoma. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu F., Pfeiffer R.M., Bhattacharjee S., Han S.S., Taylor P.R., Berndt S., et al. Common genetic variants in the 9p21 region and their associations with multiple tumours. Br J Cancer. 2013;108:1378–1386. doi: 10.1038/bjc.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin X., Yan C., Gao Y., Du J., Zhu X., Yu F., et al. Genetic variants at 9p21.3 are associated with risk of esophageal squamous cell carcinoma in a Chinese population. Cancer Sci. 2017;108:250–255. doi: 10.1111/cas.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J., Guo L., Peiffer D.A., Zhou L., Chan O.T., Bibikova M., et al. Genomic profiling of 766 cancer-related genes in archived esophageal normal and carcinoma tissues. Int J Cancer. 2008;122:2249–2254. doi: 10.1002/ijc.23397. [DOI] [PubMed] [Google Scholar]
- 54.Shen T.Y., Mei L.L., Qiu Y.T., Shi Z.Z. Identification of candidate target genes of genomic aberrations in esophageal squamous cell carcinoma. Oncol Lett. 2016;12:2956–2961. doi: 10.3892/ol.2016.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su D., Zhang D., Jin J., Ying L., Han M., Chen K., et al. Identification of predictors of drug sensitivity using patient-derived models of esophageal squamous cell carcinoma. Nat Commun. 2019;10:5076. doi: 10.1038/s41467-019-12846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donnellan R., Chetty R. Cyclin D1 and human neoplasia. J Clinical Pathol-Mol Pathol. 1998;51:1–7. doi: 10.1136/mp.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sawada G., Niida A., Uchi R., Hirata H., Shimamura T., Suzuki Y., et al. Genomic landscape of esophageal squamous cell carcinoma in a Japanese population. Gastroenterology. 2016;150:1171–1182. doi: 10.1053/j.gastro.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 58.Sunpaweravong P., Sunpaweravong S., Puttawibul P., Mitarnun W., Zeng C., Baron A.E., et al. Epidermal growth factor receptor and cyclin D1 are independently amplified and overexpressed in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131:111–119. doi: 10.1007/s00432-004-0610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hou X., Liang R.B., Wei J.C., Xu Y., Fu J.H., Luo R.Z., et al. Cyclin D1 expression predicts postoperative distant metastasis and survival in resectable esophageal squamous cell carcinoma. Oncotarget. 2016;7:31088–31096. doi: 10.18632/oncotarget.9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Froimchuk E., Jang Y., Ge K. Histone H3 lysine 4 methyltransferase KMT2D. Gene. 2017;627:337–342. doi: 10.1016/j.gene.2017.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dai W., Ko J.M.Y., Choi S.S.A., Yu Z., Ning L., Zheng H., et al. Whole-exome sequencing reveals critical genes underlying metastasis in oesophageal squamous cell carcinoma. J Pathol. 2017;242:500–510. doi: 10.1002/path.4925. [DOI] [PubMed] [Google Scholar]
- 62.Kerins M.J., Ooi A. A catalogue of somatic NRF2 gain-of-function mutations in cancer. Sci Rep. 2018;8:12846. doi: 10.1038/s41598-018-31281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim Y.R., Oh J.E., Kim M.S., Kang M.R., Park S.W., Han J.Y., et al. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol. 2010;220:446–451. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 64.Cui Y., Chen H., Xi R., Cui H., Zhao Y., Xu E., et al. Whole-genome sequencing of 508 patients identifies key molecular features associated with poor prognosis in esophageal squamous cell carcinoma. Cell Res. 2020;30:902–913. doi: 10.1038/s41422-020-0333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shibata T., Kokubu A., Saito S., Narisawa-Saito M., Sasaki H., Aoyagi K., et al. NRF2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia. 2011;13:864–873. doi: 10.1593/neo.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma S., Paiboonrungruan C., Yan T., Williams K.P., Major M.B., Chen X.L. Targeted therapy of esophageal squamous cell carcinoma: the NRF2 signaling pathway as target. Ann N Y Acad Sci. 2018;1434:164–172. doi: 10.1111/nyas.13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garrett T.P.J., McKern N.M., Lou M.Z., Elleman T.C., Adams T.E., Lovrecz G.O., et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell. 2003;11:495–505. doi: 10.1016/s1097-2765(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 68.GrausPorta D., Beerli R.R., Daly J.M., Hynes N.E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koboldt D.C., Fulton R.S., McLellan M.D., Schmidt H., Kalicki-Veizer J., McMichael J.F., et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oh D.Y., Bang Y.J. HER2-targeted therapies—a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17:33–48. doi: 10.1038/s41571-019-0268-3. [DOI] [PubMed] [Google Scholar]
- 71.Wang K., Johnson A., Au S.M., Klempner S.J., Bekaii-Saab T., Vacirca J.L., et al. Comprehensive genomic profiling of advanced esophageal squamous cell carcinomas and esophageal adenocarcinomas reveals similarities and differences. Oncologist. 2015;20:1132–1139. doi: 10.1634/theoncologist.2015-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plum P.S., Gebauer F., Kramer M., Alakus H., Berlth F., Chon S.H., et al. HER2/neu (ERBB2) expression and gene amplification correlates with better survival in esophageal adenocarcinoma. BMC Cancer. 2019;19:38. doi: 10.1186/s12885-018-5242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Realdon S., Dassie E., Fassan M., Dall'Olmo L., Hatem G., Buda A., et al. In vivo molecular imaging of HER2 expression in a rat model of Barrett's esophagus adenocarcinoma. Dis Esophagus. 2015;28:394–403. doi: 10.1111/dote.12210. [DOI] [PubMed] [Google Scholar]
- 74.Yoon H.H., Shi Q., Sukov W.R., Wiktor A.E., Khan M., Sattler C.A., et al. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin Cancer Res. 2012;18:546–554. doi: 10.1158/1078-0432.CCR-11-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoon H.H., Sukov W.R., Shi Q., Sattler C.A., Wiktor A.E., Diasio R.B., et al. HER-2/neu gene amplification in relation to expression of HER2 and HER3 proteins in patients with esophageal adenocarcinoma. Cancer. 2014;120:415–424. doi: 10.1002/cncr.28435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Griffiths E.A., Pritchard S.A., McGrath S.M., Valentine H.R., Price P.M., Welch I.M., et al. Increasing expression of hypoxia-inducible proteins in the Barrett's metaplasia-dysplasia-adenocarcinoma sequence. Br J Cancer. 2007;96:1377–1383. doi: 10.1038/sj.bjc.6603744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carraro A., Trevellin E., Fassan M., Kotsafti A., Lunardi F., Porzionato A., et al. Esophageal adenocarcinoma microenvironment: peritumoral adipose tissue effects associated with chemoresistance. Cancer Sci. 2017;108:2393–2404. doi: 10.1111/cas.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Gouw D., Rijpkema M., de Bitter T.J.J., Baart V.M., Sier C.F.M., Hernot S., et al. Identifying biomarkers in lymph node metastases of esophageal adenocarcinoma for tumor-targeted imaging. Mol Diagn Ther. 2020;24:191–200. doi: 10.1007/s40291-020-00448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prins M.J., Verhage R.J., ten Kate F.J., van Hillegersberg R. Cyclooxygenase isoenzyme-2 and vascular endothelial growth factor are associated with poor prognosis in esophageal adenocarcinoma. J Gastrointest Surg. 2012;16:956–966. doi: 10.1007/s11605-011-1814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jancik S., Drabek J., Radzioch D., Hajduch M. Clinical relevance of KRAS in human cancers. J Biomed Biotechnol. 2010;2010:150960. doi: 10.1155/2010/150960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu P., Wang Y., Li X. Targeting the untargetable KRAS in cancer therapy. Acta Pharm Sin B. 2019;9:871–879. doi: 10.1016/j.apsb.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nones K., Waddell N., Wayte N., Patch A.M., Bailey P., Newell F., et al. Genomic catastrophes frequently arise in esophageal adenocarcinoma and drive tumorigenesis. Nat Commun. 2014;5:5224. doi: 10.1038/ncomms6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Essakly A., Loeser H., Kraemer M., Alakus H., Chon S.H., Zander T., et al. PIK3CA and KRAS amplification in esophageal adenocarcinoma and their impact on the inflammatory tumor microenvironment and prognosis. Transl Oncol. 2020;13:157–164. doi: 10.1016/j.tranon.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu R.C., Wang T.L., Shih Ie M. The emerging roles of ARID1A in tumor suppression. Cancer Biol Ther. 2014;15:655–664. doi: 10.4161/cbt.28411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Drage M.G., Tippayawong M., Agoston A.T., Zheng Y., Bueno R., Hornick J.L., et al. Morphological features and prognostic significance of ARID1A-deficient esophageal adenocarcinomas. Arch Pathol Lab Med. 2017;141:970–977. doi: 10.5858/arpa.2016-0318-OA. [DOI] [PubMed] [Google Scholar]
- 86.Streppel M.M., Lata S., DelaBastide M., Montgomery E.A., Wang J.S., Canto M.I., et al. Next-generation sequencing of endoscopic biopsies identifies ARID1A as a tumor-suppressor gene in Barrett's esophagus. Oncogene. 2014;33:347–357. doi: 10.1038/onc.2012.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schallenberg S., Bork J., Essakly A., Alakus H., Buettner R., Hillmer A.M., et al. Loss of the SWI/SNF-ATPase subunit members SMARCF1 (ARID1A), SMARCA2 (BRM), SMARCA4 (BRG1) and SMARCB1 (INI1) in oesophageal adenocarcinoma. BMC Cancer. 2020;20:12. doi: 10.1186/s12885-019-6425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grugan K.D., Miller C.G., Yao Y., Michaylira C.Z., Ohashi S., Klein-Szanto A.J., et al. Fibroblast-secreted hepatocyte growth factor plays a functional role in esophageal squamous cell carcinoma invasion. Proc Natl Acad Sci U S A. 2010;107:11026–11031. doi: 10.1073/pnas.0914295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Herrera L.J., El-Hefnawy T., Queiroz de Oliveira P.E., Raja S., Finkelstein S., Gooding W., et al. The HGF receptor c-Met is overexpressed in esophageal adenocarcinoma. Neoplasia. 2005;7:75–84. doi: 10.1593/neo.04367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y., Jiang Z., Xu C., Wang H., Tan L., Su J., et al. Increased MET gene copy number negatively affects the survival of esophageal squamous cell carcinoma patients. BMC Cancer. 2019;19:240. doi: 10.1186/s12885-019-5450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lennerz J.K., Kwak E.L., Ackerman A., Michael M., Fox S.B., Bergethon K., et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. 2011;29:4803–4810. doi: 10.1200/JCO.2011.35.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Strickler J.H., LoRusso P., Salgia R., Kang Y.K., Yen C.J., Lin C.C., et al. Phase I dose-escalation and -expansion study of telisotuzumab (ABT-700), an anti-c-Met antibody, in patients with advanced solid tumors. Mol Cancer Ther. 2020;19:1210–1217. doi: 10.1158/1535-7163.MCT-19-0529. [DOI] [PubMed] [Google Scholar]
- 93.Hong D.S., LoRusso P., Hamid O., Janku F., Kittaneh M., Catenacci D.V.T., et al. Phase I study of AMG 337, a highly selective small-molecule MET inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2019;25:2403–2413. doi: 10.1158/1078-0432.CCR-18-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Cutsem E., Karaszewska B., Kang Y.K., Chung H.C., Shankaran V., Siena S., et al. A multicenter phase II study of AMG 337 in patients with MET-amplified gastric/gastroesophageal junction/esophageal adenocarcinoma and other MET-amplified solid tumors. Clin Cancer Res. 2019;25:2414–2423. doi: 10.1158/1078-0432.CCR-18-1337. [DOI] [PubMed] [Google Scholar]
- 95.Kosovec J.E., Zaidi A.H., Omstead A.N., Matsui D., Biedka M.J., Cox E.J., et al. CDK4/6 dual inhibitor abemaciclib demonstrates compelling preclinical activity against esophageal adenocarcinoma: a novel therapeutic option for a deadly disease. Oncotarget. 2017;8:100421–100432. doi: 10.18632/oncotarget.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ou H.L., Schumacher B. DNA damage responses and p53 in the aging process. Blood. 2018;131:488–495. doi: 10.1182/blood-2017-07-746396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leijen S., van Geel R.M., Pavlick A.C., Tibes R., Rosen L., Razak A.R., et al. Phase I study evaluating WEE1 inhibitor AZD1775 as monotherapy and in combination with gemcitabine, cisplatin, or carboplatin in patients with advanced solid tumors. J Clin Oncol. 2016;34:4371–4380. doi: 10.1200/JCO.2016.67.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohashi S., Kikuchi O., Nakai Y., Ida T., Saito T., Kondo Y., et al. Synthetic lethality with trifluridine/tipiracil and checkpoint kinase 1 inhibitor for esophageal squamous cell carcinoma. Mol Cancer Ther. 2020;19:1363–1372. doi: 10.1158/1535-7163.MCT-19-0918. [DOI] [PubMed] [Google Scholar]
- 99.Schneider B.J., Shah M.A., Klute K., Ocean A., Popa E., Altorki N., et al. Phase I study of epigenetic priming with azacitidine prior to standard neoadjuvant chemotherapy for patients with resectable gastric and esophageal adenocarcinoma: evidence of tumor hypomethylation as an indicator of major histopathologic response. Clin Cancer Res. 2017;23:2673–2680. doi: 10.1158/1078-0432.CCR-16-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahrens T.D., Timme S., Hoeppner J., Ostendorp J., Hembach S., Follo M., et al. Selective inhibition of esophageal cancer cells by combination of HDAC inhibitors and azacytidine. Epigenetics. 2015;10:431–445. doi: 10.1080/15592294.2015.1039216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shah M.A., Kojima T., Hochhauser D., Enzinger P., Raimbourg J., Hollebecque A., et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol. 2019;5:546–550. doi: 10.1001/jamaoncol.2018.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang B., Qi L., Wang X., Xu J., Liu Y., Mu L., et al. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun. 2020;40:711–720. doi: 10.1002/cac2.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang X., Zhang B., Chen X., Mo H., Wu D., Lan B., et al. Lactate dehydrogenase and baseline markers associated with clinical outcomes of advanced esophageal squamous cell carcinoma patients treated with camrelizumab (SHR-1210), a novel anti-PD-1 antibody. Thorac Cancer. 2019;10:1395–1401. doi: 10.1111/1759-7714.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shah M.A., Bennouna J., Doi T., Shen L., Kato K., Adenis A., et al. KEYNOTE-975 study design: a phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with esophageal carcinoma. Future Oncol. 2021;17:1143–1153. doi: 10.2217/fon-2020-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kato H., Arao T., Matsumoto K., Fujita Y., Kimura H., Hayashi H., et al. Gene amplification of EGFR, HER2, FGFR2 and MET in esophageal squamous cell carcinoma. Int J Oncol. 2013;42:1151–1158. doi: 10.3892/ijo.2013.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hara F., Aoe M., Doihara H., Taira N., Shien T., Takahashi H., et al. Antitumor effect of gefitinib ('Iressa') on esophageal squamous cell carcinoma cell lines in vitro and in vivo. Cancer Lett. 2005;226:37–47. doi: 10.1016/j.canlet.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 107.Teraishi F., Kagawa S., Watanabe T., Tango Y., Kawashima T., Umeoka T., et al. ZD1839 (gefitinib, ‘Iressa’), an epidermal growth factor receptor-tyrosine kinase inhibitor, enhances the anti-cancer effects of TRAIL in human esophageal squamous cell carcinoma. FEBS Lett. 2005;579:4069–4075. doi: 10.1016/j.febslet.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 108.Mimura K., Kono K., Maruyama T., Watanabe M., Izawa S., Shiba S., et al. Lapatinib inhibits receptor phosphorylation and cell growth and enhances antibody-dependent cellular cytotoxicity of EGFR- and HER2-overexpressing esophageal cancer cell lines. Int J Cancer. 2011;129:2408–2416. doi: 10.1002/ijc.25896. [DOI] [PubMed] [Google Scholar]
- 109.Hou W., Qin X., Zhu X., Fei M., Liu P., Liu L., et al. Lapatinib inhibits the growth of esophageal squamous cell carcinoma and synergistically interacts with 5-fluorouracil in patient-derived xenograft models. Oncol Rep. 2013;30:707–714. doi: 10.3892/or.2013.2500. [DOI] [PubMed] [Google Scholar]
- 110.Zhao L., He L.R., Xi M.A., Cai M.Y., Shen J.X., Li Q.Q., et al. Nimotuzumab promotes radiosensitivity of EGFR-overexpression esophageal squamous cell carcinoma cells by upregulating IGFBP-3. J Transl Med. 2012;10:249. doi: 10.1186/1479-5876-10-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhu H., Wang C., Wang J., Chen D., Deng J., Deng J., et al. A subset of esophageal squamous cell carcinoma patient-derived xenografts respond to cetuximab, which is predicted by high EGFR expression and amplification. J Thorac Dis. 2018;10:5328–5338. doi: 10.21037/jtd.2018.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li G., Hu W., Wang J., Deng X., Zhang P., Zhang X., et al. Phase II study of concurrent chemoradiation in combination with erlotinib for locally advanced esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2010;78:1407–1412. doi: 10.1016/j.ijrobp.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 113.Zhao C.H., Lin L., Liu J.Z., Liu R.R., Chen Y.L., Ge F.J., et al. A phase II study of concurrent chemoradiotherapy and erlotinib for inoperable esophageal squamous cell carcinoma. Oncotarget. 2016;7:57310–57316. doi: 10.18632/oncotarget.9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xie C., Jing Z., Luo H., Jiang W., Ma L., Hu W., et al. Chemoradiotherapy with extended nodal irradiation and/or erlotinib in locally advanced oesophageal squamous cell cancer: long-term update of a randomised phase 3 trial. Br J Cancer. 2020;123:1616–1624. doi: 10.1038/s41416-020-01054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang J., Fan Q., Lu P., Ying J., Ma C., Liu W., et al. Icotinib in patients with pretreated advanced esophageal squamous cell carcinoma with EGFR overexpression or EGFR gene amplification: a single-arm, multicenter phase 2 study. J Thorac Oncol. 2016;11:910–917. doi: 10.1016/j.jtho.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 116.Luo H., Jiang W., Ma L., Chen P., Fang M., Ding L., et al. Icotinib with concurrent radiotherapy vs radiotherapy alone in older adults with unresectable esophageal squamous cell carcinoma: a phase II randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu Y.P., Zheng Y.D., Sun X.J., Yu X.M., Gu J.L., Wu W., et al. Concurrent radiotherapy with gefitinib in elderly patients with esophageal squamous cell carcinoma: preliminary results of a phase II study. Oncotarget. 2015;6:38429–38439. doi: 10.18632/oncotarget.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brooks A.N., Kilgour E., Smith P.D. Molecular pathways: fibroblast growth factor signaling: a new therapeutic opportunity in cancer. Clin Cancer Res. 2012;18:1855–1862. doi: 10.1158/1078-0432.CCR-11-0699. [DOI] [PubMed] [Google Scholar]
- 119.Xin Z., Song X., Jiang B., Gongsun X., Song L., Qin Q., et al. Blocking FGFR4 exerts distinct anti-tumorigenic effects in esophageal squamous cell carcinoma. Thorac Cancer. 2018;9:1687–1698. doi: 10.1111/1759-7714.12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Luo H., Quan J., Xiao H., Luo J., Zhang Q., Pi G., et al. FGFR inhibitor AZD4547 can enhance sensitivity of esophageal squamous cell carcinoma cells with epithelial-mesenchymal transition to gefitinib. Oncol Rep. 2018;39:2270–2278. doi: 10.3892/or.2018.6304. [DOI] [PubMed] [Google Scholar]
- 121.Elkabets M., Pazarentzos E., Juric D., Sheng Q., Pelossof R.A., Brook S., et al. AXL mediates resistance to PI3K alpha inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell. 2015;27:533–546. doi: 10.1016/j.ccell.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xiang H.Y., Wang X., Chen Y.H., Zhang X., Tan C., Wang Y., et al. Identification of methyl (5-(6-((4-(methylsulfonyl)piperazin-1-yl)methyl)-4-morpholinopyrrolo[2,1-f][1,2,4]triazin-2-yl)-4-(trifluoromethyl)pyridin-2-yl)carbamate (CYH33) as an orally bioavailable, highly potent, PI3K alpha inhibitor for the treatment of advanced solid tumors. Eur J Med Chem. 2021;209:112913. doi: 10.1016/j.ejmech.2020.112913. [DOI] [PubMed] [Google Scholar]
- 123.Shi J.J., Xing H., Wang Y.X., Zhang X., Zhan Q.M., Geng M.Y., et al. PI3Kalpha inhibitors sensitize esophageal squamous cell carcinoma to radiation by abrogating survival signals in tumor cells and tumor microenvironment. Cancer Lett. 2019;459:145–155. doi: 10.1016/j.canlet.2019.05.040. [DOI] [PubMed] [Google Scholar]
- 124.Badarni M., Prasad M., Balaban N., Zorea J., Yegodayev K.M., Joshua B.Z., et al. Repression of AXL expression by AP-1/JNK blockage overcomes resistance to PI3Ka therapy. JCI Insight. 2019;5 doi: 10.1172/jci.insight.125341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y.X., Zhang X., Ma Q.Y., Hu L.D., Zhang X., Wang Y., et al. Adaptive resistance to PI3Kalpha-selective inhibitor CYH33 is mediated by genomic and transcriptomic alterations in ESCC cells. Cell Death Dis. 2021;12:85. doi: 10.1038/s41419-020-03370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nusse R., Clevers H. Wnt/beta-Catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 127.Bugter J.M., Fenderico N., Maurice M.M. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat Rev Cancer. 2021;21:5–21. doi: 10.1038/s41568-020-00307-z. [DOI] [PubMed] [Google Scholar]
- 128.Hasan M.R., Saraya A., Gupta S.D., Chauhan S.S., Ralhan R. Wnt signaling protein expression based pridiction of recurrent free survival in esophageal cancer. Gastroenterology. 2017;152:S1030–S1031. [Google Scholar]
- 129.Zhao Y., Yi J., Tao L., Huang G., Chu X., Song H., et al. Wnt signaling induces radioresistance through upregulating HMGB1 in esophageal squamous cell carcinoma. Cell Death Dis. 2018;9:433. doi: 10.1038/s41419-018-0466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu Z., Xu X., O'Laoi R., Ma H., Zheng J., Chen S., et al. Design, synthesis, and evaluation of novel porcupine inhibitors featuring a fused 3-ring system based on the ‘reversed’ amide scaffold. Bioorg Med Chem. 2016;24:5861–5872. doi: 10.1016/j.bmc.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 131.Bang Y.J., Van Cutsem E., Feyereislova A., To G.A.T.I. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (TOGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 132.Smyth E.C., Rowley S., Cafferty F.H., Allum W., Grabsch H.I., Stenning S., et al. Safety and efficacy of the addition of lapatinib to perioperative chemotherapy for resectable HER2-positive gastroesophageal adenocarcinoma: a randomized phase 2 clinical trial. JAMA Oncol. 2019;5:1181–1187. doi: 10.1001/jamaoncol.2019.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stroes C.I., Schokker S., Creemers A., Molenaar R.J., Hulshof M., van der Woude S.O., et al. Phase II feasibility and biomarker study of neoadjuvant trastuzumab and pertuzumab with chemoradiotherapy for resectable human epidermal growth factor receptor 2-positive esophageal adenocarcinoma: TRAP study. J Clin Oncol. 2020;38:462–471. doi: 10.1200/JCO.19.01814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fuchs C.S., Tomasek J., Yong C.J., Dumitru F., Passalacqua R., Goswami C., et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 135.Wilke H., Muro K., Van Cutsem E., Oh S.C., Bodoky G., Shimada Y., et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 136.Shah M.A., Ramanathan R.K., Ilson D.H., Levnor A., D'Adamo D., O'Reilly E., et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201–5206. doi: 10.1200/JCO.2006.08.0887. [DOI] [PubMed] [Google Scholar]
- 137.Yanwei L., Feng H., Ren P., Yue J., Zhang W., Tang P., et al. Safety and efficacy of apatinib monotherapy for unresectable, metastatic esophageal cancer: a single-arm, open-label, phase II study. Oncologist. 2020;25:e1464–e1472. doi: 10.1634/theoncologist.2020-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.