Abstract
Autoimmune or infectious diseases often instigate the undesirable damages to tissues or organs to trigger immune-related diseases, which involve plenty of immune cells, pathogens and autoantibodies. Nanomedicine has a great potential in modulating immune system. Particularly, biomimetic nanomodulators can be designed for prevention, diagnosis and therapy to achieve a better targeted immunotherapy. With the development of materials science and bioengineering, a wide range of membrane-coated nanomodulators are available. Herein, we summarize recent advancements of bioinspired membrane-coated nanoplatform for systemic protection against immune-related diseases including autoimmune and infectious diseases. We also rethink the challenges or limitations in the progress of the therapeutic nanoplatform, and discuss the further application of the nanomodulators in the view of translational medicine for combating immune-related diseases.
KEY WORDS: Biomimetic membrane, Nanomodulators, Immune system, Bioengineering, Immunotherapy, Autoimmune, Infectious diseases
Graphical abstract
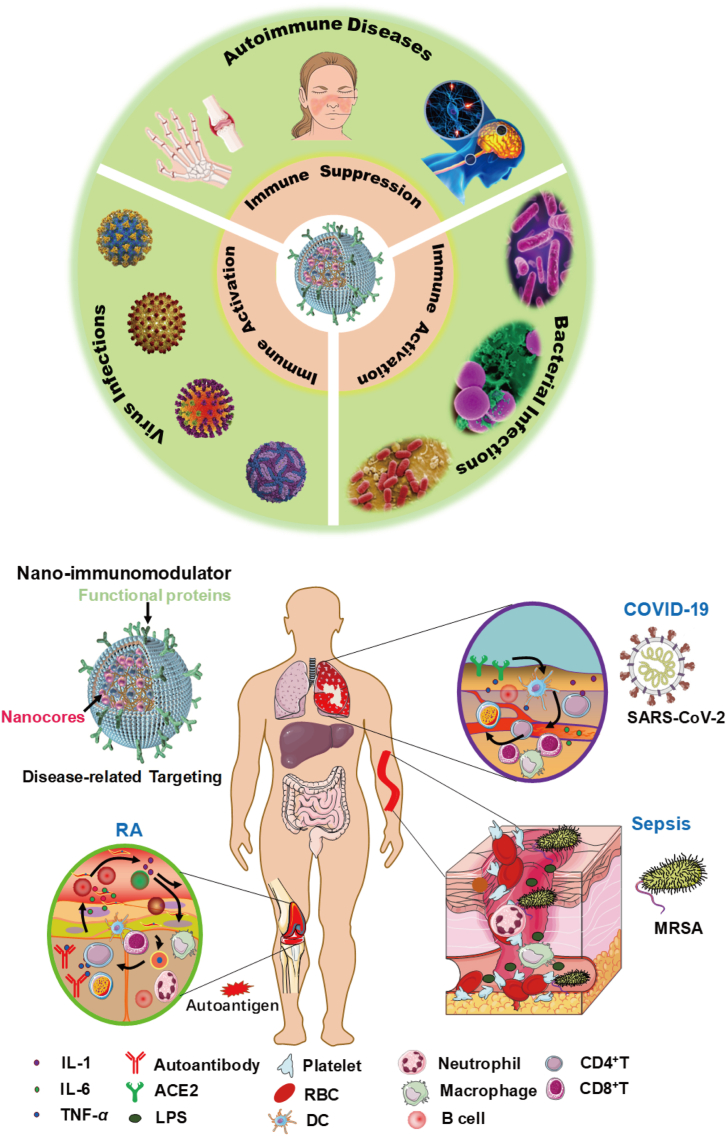
Biomimetic nanomodulators can be designed for prevention, diagnosis and therapy to achieve a better targeted immunotherapy in autoimmune and infectious diseases.
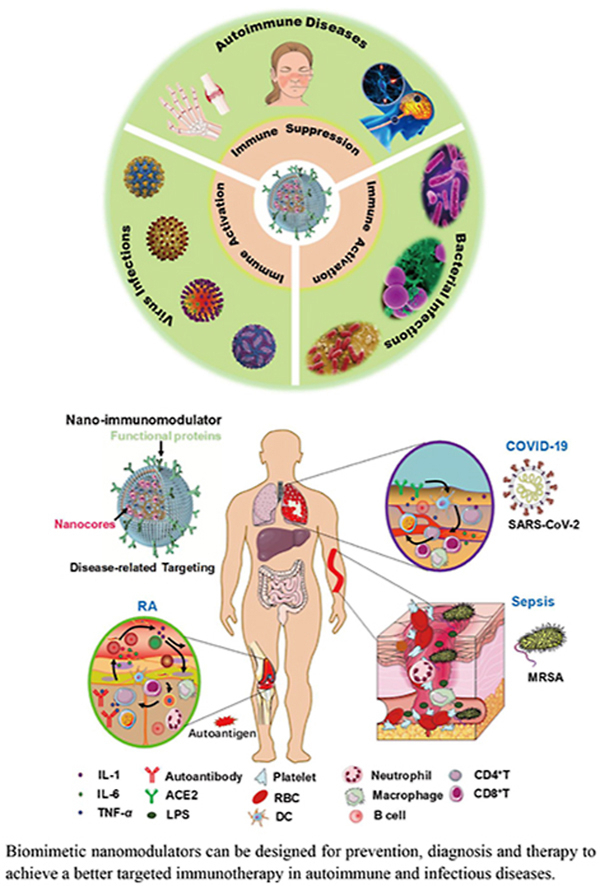
1. Introduction
Autoimmune diseases [e.g., rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and multiple sclerosis (MS)] and infectious diseases [e.g., corona virus disease 2019 (COVID-19), acquired immune deficiency syndrome (AIDS) and various bacterial infections] have emerged as serious threats to human health1, 2, 3. These diseases are often progressed with immune system dysfunction or hyperactivation, causing tremendous suffering to patients with a high fatality rate4, 5, 6. For example, due to the emergence of severe acute respiratory syndrome corona virus 2 (SARS-CoV-2), human immune system is invaded and attacked, especially in restraining immune cell responses and antibodies secretion, resulting in the failure of virus clearance and thousands of death6, 7, 8. Autoimmune diseases are frequently associated with the loss of B cell or T cell tolerance, which result in the pathology that immune cells actively infiltrate or attack normal tissues and organs9,10. Nowadays, numerous drugs including small chemicals or therapeutic antibodies have been developed to overcome the immune diseases, the clinical response remains unsatisfactory largely due to non-diseases specificity, heterogeneity or awkward drug delivery11, 12, 13. And worse, tremendous adverse effects including autoimmunity, overused drug resistance and system inflammation caused by the long-term administration have aroused many concerns. Even though the symptom and severity of the three major immune-related diseases are different, they exhibit some similar denominators in the dysfunction of immune homeostasis13. Thus, there is an urgent need to develop innovative, versatile and practical therapeutic strategies to reshape the immune microenvironment and normalize immune homeostasis.
Immunotherapy has been chosen as a classical clinical strategy for treating immune diseases. Amounts of immune modulating drugs are being approved in clinical or preclinical research. Nonetheless, one key limitation of current immunotherapies is the multi-faceted immune network, as it often involves intricate immune microenvironment communications between pathogens, cytokines and infiltrated immune cells, and conventional immunotherapy cannot achieve the desired outcomes14. The progress of nanomedicine provides an incomparable approach to exploiting novel solutions to the predicaments. For the autoimmune diseases, various inflammatory factors or autoantigens can pathologically activate immune cells including T cells, macrophages and neutrophils to release cytokines (e.g., TNF-α, IL-1 and IL-6) and stimulate downstream effector cells to attack normal tissues or organs through the ligand-receptors interactions in the immune microenvironment14, 15, 16. Cell membrane derived from the involved immune cells may be fabricated as a broad-spectrum anti-inflammation nanoplatform by leveraging the existed receptors to neutralize multiple cytokines instead of one17,18. In the infectious diseases, such as COVID-19, the virus can evade from CD4+ or CD8+ T cells and attach itself to lung epithelial cell membrane and then the spike protein (S protein) binds to the angiotensin-converting enzyme 2 (ACE2) to inject its viral genomes into the infected cell prior to replication, then cause the dysfunction of lung7,19. Using ACE2 anchored membrane as a nanodecoy to neutralize the virus would ameliorate immune cell stress and enhance immune response. Considering the fact that multiple immune cells play a key role in the progression of these diseases, employing the membrane derived from the relevant cells-coated nanoparticle as an immunomodulator would be feasible by mimicking the properties of the source cells.
Briefly, the membrane-coated nanoparticles are characterized by the typical core‒shell structure20,21, with a synthetic inner core and outer layer bioinspired membrane including cell membrane22, 23, 24, bacterial membrane25, 26, 27 or exosome28, 29, 30. Moreover, bioinspired membrane-camouflaged nanoparticles completely inherit the properties of the source cells and thus can bind inflammatory cytokines, participate in immune interactions and regulate immune response. It has been elucidated that due to the existence of membrane proteins such as CD47, PD-L1 and other engineering ligands, the bioinspired membrane-based nanoplatform can avoid being systemically cleared by macrophages or improve tissue biodistribution and half-life by targeting the disease-related sites15,31,32. Compared with conventional immunotherapy, bioinspired membrane-based nanomodulator has multifunctions not only by integrating the functions of outer membrane and the drugs in the inner cores which have the controlled release effect, but also can produce synergistic effects with the outer membrane in the immune environment33,34.
Cell membrane-based nanomodulator has shown unprecedented efficacy in treating various autoimmune diseases, virus and bacterial infections31. However, there is still much space to extend cell membrane-coated nanoplatform to address broad-spectrum diseases and benefit more patients. In this review, we briefly retrospect the burgeoning nanotechnology, summarize the sources of cell membrane and introduce the methods of coating. We discuss the different types of bioinspired membrane-coated nanoparticles to function as nanodecoy-immunotherapy, vaccine-immunotherapy, sono-immunotherapy, chemo-immunotherapy and other novel immunotherapies. We also summarize their applications in immune-related diseases including autoimmune diseases and infectious diseases, along with their special features and advantages (Scheme 1). Moreover, we rethink the current inadequacies and challenges in translation, and provide a translational perspective on the field of cell membrane-coated nanoplatform. Undoubtedly, with the development of nanotechnology and biomedicine, the application of the cell membrane-coated nanoparticles will carry forward as time progresses, and we also conclude with discussion on the directions of clinical translation in future.
Scheme 1.
Bioinspired membrane-based nanomodulators for targeted therapy in autoimmune diseases, virus infections and bacterial infections. After rational engineering, various bioinspired membranes can be wrapped over the inner cores, which successfully inherit the biofunctions (e.g., adhesion molecules: CD47, LFA-1 and ICAM-1; key receptors: IL-1R, TNF-αR and DR5; engineering proteins: TRAIL and other specific antibodies) of the source cells. Meanwhile the multifarious inner cores can be designed for delivering various immune modulating drugs or adjuvants. According to the desired application or the type of the diseases, specific membrane and the inner nanoparticles can be integrated for immune activation in antiviral or antibacterial therapy, and for immunosuppression in autoimmune diseases.
2. Advancement of membrane coating nanoplatform
In 2011, the first study about the red cell membrane camouflaged PLGA nanoparticle (RBC-NPs) was reported, and the RBC-NPs faultlessly inherit the properties of source cells including surface marker proteins CD47 and other receptor proteins21. Due to the proteins, compared with conventional liposomes or other synthetic carriers, membrane-coated nanoparticles can significantly prolong the circulation time by evading systemic clearance mediated by macrophages. Moreover, the outer layer can protect the drug loaded inner core from poor stability by avoiding being degraded by the low-pH gastric acid in some cases. Importantly, it can achieve personalized treatment through gene engineering-modified membrane, and enhance disease-targeting accumulation and reduce non-targeting tissue accumulation. With the development of materials engineering, miscellaneous types of membrane have been derived to fabricate 20–500 nm-sized membrane-coated nanoparticles through the processes of sonication, physical extrusion or microfluidic fabrication35, 36, 37, 38. Encouraged by the success, more researchers have devoted their attention to the study of bioinspired membrane-coated nanoplatform.
2.1. The sources of bioinspired membrane and nanomaterials
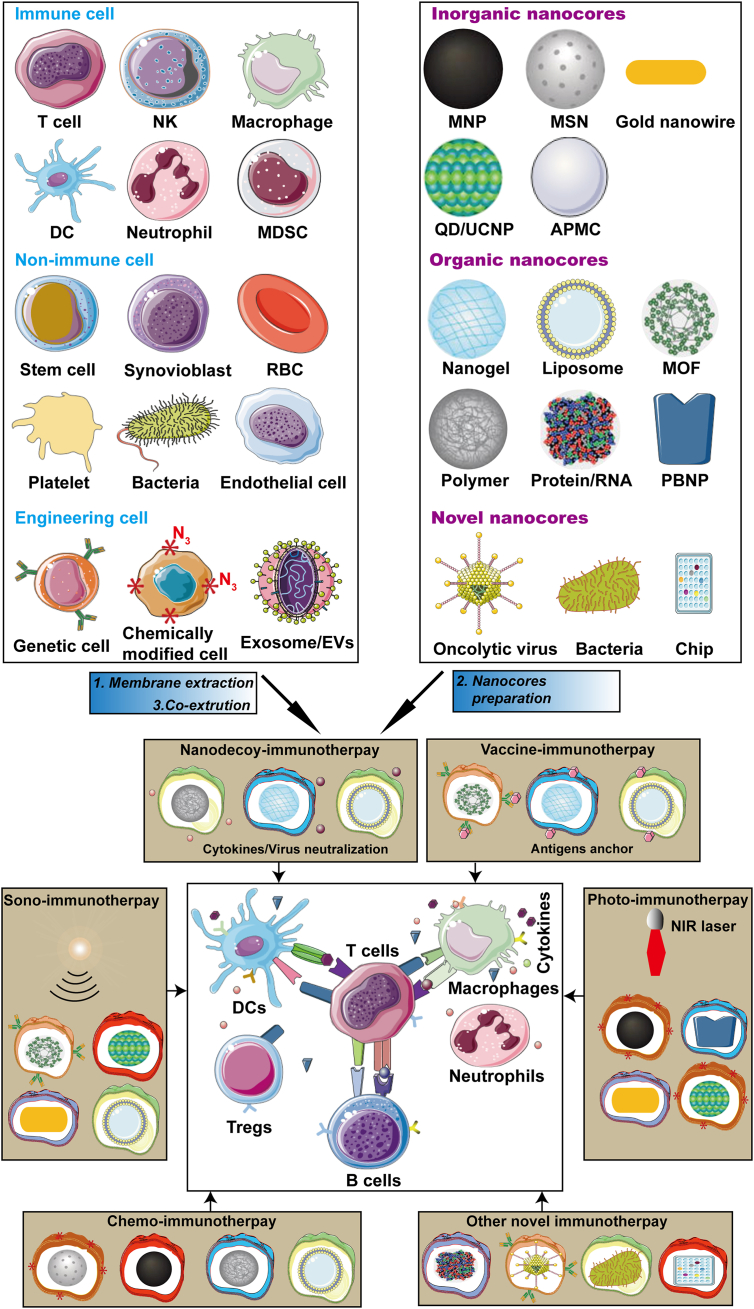
Bioinspired membrane wrapped onto the synthetic nanoparticles may derive from different cultured cells, extracted exosome and even bacteria. Especially, more than thirty kinds of membrane have been developed in immune-related diseases due to their membrane functions, including RBC, platelet, immune cells, cancer cells, stem cells, exosomes, bacteria, hybrid cell membrane, and genetic/chemical engineering cell membrane27,28,31. Meanwhile, various nanomaterials including polymer, silica, gold, iron oxide, nanogel, quantum dot/up-conversion material (QD/UC), metal-organic frameworks (MOF), protein complexes, oncolytic virus and chips have been prepared31,39. According to the different diseases or the treatments, we can choose the suitable sources of membrane and nanomaterial to construct the resultant cell membrane-coated nanoparticles for immune activation in infectious diseases, and immunosuppression in autoimmune diseases (Fig. 1).
Figure 1.
Bioinspired membrane-based nanoparticles. Various cell types or exosomes have been applied as sources of membranes to wrap onto nanoparticles. Each kind of membrane can possess unique properties to provide functionalities to inner nanocores, the resultant nanoparticles can function as nanodecoy-, vaccine-, photo-, sono-, chemo-immunotherapy and the other novel immunotherapy to regulate immune response for virus or bacteria clearance, and autoimmune normalization.
2.2. Methods of coating technology
Currently, there are three main different methods used to manufacture cell membrane-coated nanoparticles, including physical extrusion, sonication, or microfluidic electroporation. Moreover, amounts of novel technologies, such as viscoelastic flows, have sprung up to facilitate the development of cell membrane-coated nanoparticles. Here, we briefly introduce these methods and discuss its advantages or disadvantages.
Initially, bioinspired membrane-coated nanoparticles were fabricated by mechanical extrusion in which the derived vehicles and inner nanocores were co-extruded successively through different apertures (400, 200, 100 or 50 nm) of porous polycarbonate membrane by using an Avanti mini extruder40. Overall, mechanical extrusion is a convenient and effective method, but it is hard to control mechanical forces during the extrusion process, which often leads to the destruction of the porous membrane. Very recently, sonication has also been applied in the field of cell membrane-coated nanoparticles. By using the ultrasonic energy, the obtained cell membranes are breaking into pieces, and then cocultured with nanocores under sonication41. Consequently, the approach can largely generate cell membrane-coated nanoparticles, but the method of sonication cannot ensure the diameter uniformity of resultant NPs. Moreover, the power and time of sonication have to be controlled seriously, otherwise the membrane proteins on the surface would be carbonized to lose its biofunctions. Microfluidic electroporation has been developed for fabricating cell membrane-coated nanoparticles recently37. Viscoelastic flow has also been applied in separating and obtaining different size of exosomes or vesicles35. In a small volume of samples, it is challengeable to isolate small exosomes from large membrane vesicles. Moreover, the method can be also used to directly isolate exosomes from cell culture media, serum and urine in a size-dependent and label-free manner. Overall, these methods may also help construct a versatile nanoplatform to promote exosome or extracellular vesicle applications in various immune diseases.
2.3. Characterization of bioinspired membrane-coated nanoparticles
To determine whether cell membrane is successfully coated onto the nanoparticle, physicochemical and biological properties are evaluated by different methods, including the diameter of core‒shell NPs, surface charge and protein composition. At present, the most intuitive approach to confirm the success of cell membrane coating on bare nanoparticle is to use transmission electron microscopy (TEM) or scanning electron microscopy (SEM) image. The diameter of cell membrane is about 8–10 nm, and thus we can see that the 16‒25-nm increase in the coated nanoparticle compared with bare nanoparticle21. TEM/SEM images can also be used to observe the morphology of membrane-coated NP. The size distribution of nanoparticle can further be evaluated by dynamic light scattering (DLS) instrument, which can intuitively exhibit the intensity of diameter. Another important parameter of the coated nanoparticles is zeta potential (ζ, mV), which can reflect the change of surface potential regarding the particles before and after the coating process14. Normally, the potential of bare cell membrane vesicles is about −30 mV, and will have an effect on the integral charge, which illustrates whether the vesicles are coated on the nanomaterials core. Importantly, the membrane biofunction has to be confirmed, and thus marker proteins and receptors would be detected by Western blotting42. In short, due to the existence of membrane proteins, it can protect the nanoparticles from being eliminated by immune system, and thus enhance the ability of immune regulation as shown in Table 114,30,42,48,49,57,67,68,75,78,81,87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100.
Table 1.
Applications of bioinspired membrane-based nanomodulator.
| Sources | Application | Diseases | Key molecules | Ref. |
|---|---|---|---|---|
| Neutrophil | Cytokines neutralization | RA | IL-1R; TNF-αR | 14 |
| HUVECs-TRAIL | Active targeting; drug delivery; cytokines neutralization | RA | TRAIL; DR5; IL-1R; TNF-αR | 42 |
| RBCs | Autoantibodies neutralization | AIHA; DIA | RBC antibody receptors | 48 |
| Pathological antibodies neutralization | pSS; SLE; autoimmune- thyroiditis | RBC-BCR | 57 | |
| Virus clearance | Influenza | HA; sialic acids | 75 | |
| Toxins neutralization | MRSA infections | α-toxin; wSP | 88,90 | |
| Toxins vaccine | Bacterial infection | PFTs; Hla | 89,96 | |
| MSCs-exosome | Drug delivery | MS | IDO; mRNAs; HSP70 | 30 |
| Platelet | Autoantibodies neutralization | ITP | Antibodies receptors | 49,98 |
| Drug delivery; bacteria binding | Bacterial infection | Vancomycin | 99,100 | |
| T cells | HIV neutralization | HIV/AID | CD4 receptor; CCR4/5 receptors | 68 |
| C6/36 | ZIKV neutralization | ZIKV infection | ZIKV receptors | 78 |
| PHH-hNTCP | HBV neutralization | HBV infection | hNTCP; HBVpreS/2 | 81 |
| THP-1/293T | SARS-CoV-2 neutralization | COVID-19 | ACE2; IL-6R | 67 |
| HEK-293T-L2 | Vaccine; immune activation | HPV | HPV16-L2 | 87 |
| HEK-293T | Drug delivery; sono-immunotherapy | Bacterial infection | TPPS, ROS | 97 |
| Macrophage | Endotoxin/cytokines neutralization | Bacterial infection | Virulence factors | 90,91 |
| Toxins vaccine | Bacterial infection | Anti-Pas IgG | 92,95 | |
| E. coli | Anti-bacterial vaccine | Bacterial infection | CD40; CD80; CD86 | 93,94 |
RA: rheumatoid arthritis; IL-1R: IL-1 receptor; DR5: death receptor 5; AIHA: autoimmune hemolytic anemia; DIA: drug-induced anemia; pSS: Sjögren's syndrome; SLE: systemic lupus erythematosus; HA: hyaluronic acid; MRSA: methicillin-resistant Staphylococcus aureus; wSP: whole secreted proteins; PFTs: pore forming toxins; Hla: α-haemolysin; MS: multiple sclerosis; IDO: indoleamine 2,3-dioxygenase; ITP: immune thrombocytopenia purpura; CCR: C-C chemokine receptor; PHH: primary human hepatocytes; hNTCP: human sodium taurocholate co-transporting polypeptide; ACE2: angiotensin-converting enzyme 2; HPV: human papillomavirus; TPPS: meso-tetrakis (4-sulfonatophenyl) porphyrin; ROS: reactive oxygen species.
3. Bioinspired membrane coating nanoplatform in autoimmune disease therapy
3.1. Nanodecoy-immunotherapy in autoimmune diseases
Nowadays, autoimmune diseases include a series of more than 80 illnesses and affect millions of people worldwide42, 43, 44, exhibiting a common pathogenesis: immune system is disordered and results in attacks on normal tissues or organs. Unfortunately, the incidence rate of these diseases is still increasing with the changes of lifestyle or environment44. Even though current treatments of autoimmune diseases improve greatly with the use of novel biologicals or small molecular chemicals, the therapeutic outcome has still been limited by the facts that the diseases often progress before a clinical diagnosis and the complexity of cytokine or immune system interactions14,42. What's worse, traditional small chemicals are difficult to be penetrated into the inflammatory tissues or organs. Whereas, bioinspired membrane-based nanoparticles can be as nanodecoys to absorb various cytokines and thus can be as a broad-spectrum therapeutic nanoplatform because of its targeted immune regulation and drug delivery, which has showed a promising potential in various autoimmune diseases treatments.
3.1.1. Rheumatoid arthritis
Rheumatoid arthritis is one of the most common chronic autoimmune diseases, which often involves various immune cells (e.g., autoantigen specific CD8+ T cells, neutrophils and macrophages) infiltration and cytokines secretion in the joint45,46. The microenvironment of RA is like tumor microenvironment (TME), which has hypoxia and high pressure making traditional drugs hard to penetrate into inflammatory tissues in depth15. Moreover, inhibiting one or a few cytokines cannot be enough to reverse the RA progression. Thus, immune cell or engineering-modified cell membrane-based NPs were developed to modulate the immune system and overcome these limitations for a better RA treatment.
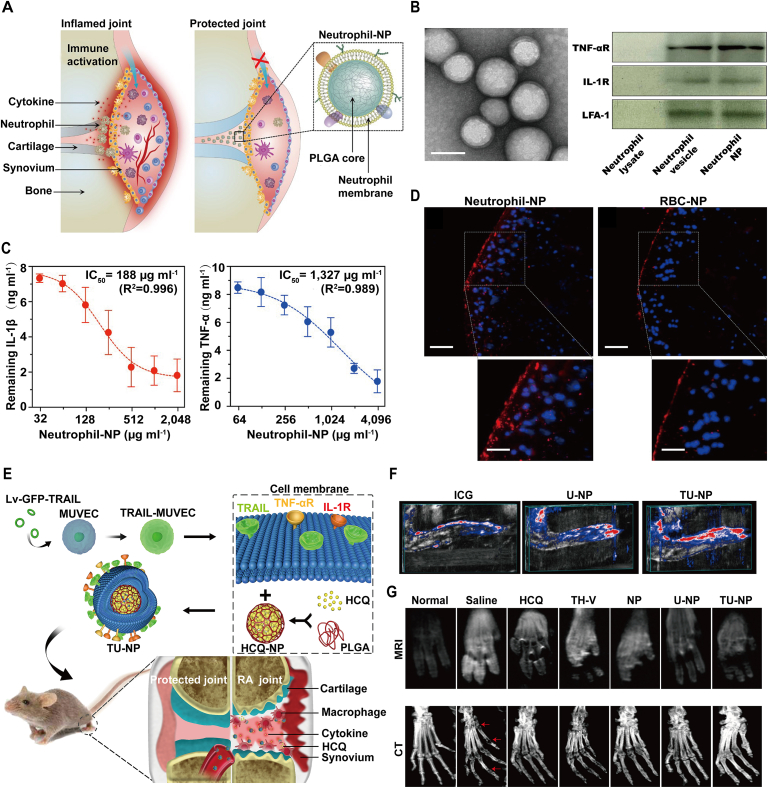
Bioinspired membrane-coated nanoplatform can be used as a nanodecoy to neutralize inflammatory cytokines and thus decrease immune cells infiltration. Neutrophil plays a key role in cartilage destruction and bone erosion in RA progression14,47. Thereby, suppressing neutrophil activation may be a promising approach for RA management. In one study, neutrophil membrane was extracted and coated onto the PLGA core to format neutrophil-NP as a nanodecoy for joint protection (Fig. 2A and D)14. Firstly, the membrane receptors including TNF-αR, IL-1R and the functional membrane protein lymphocyte function-associated antigen-1 (LFA-1) were detected on the core‒shell NPs by Western blotting. Due to the existence of surface receptors, the neutrophil-NPs exhibited a strong affinity to cytokines including IL-1β (IC50 = 188 μg/mL) and TNF-α (IC50 = 1327 μg/mL), which can act as a decoy to neutralize these inflammatory cytokines, decrease neutrophil activation and provide the possibility for further application in vivo. In collagen-induced arthritis (CIA) and human transgenic mouse model, neutrophil-NP, PBS, anti-IL-1β and anti-TNF-α were separately administrated into the mice by knee injection according to the schedule, and the results showed that neutrophil-NP-treated group had a stronger cartilage protection. It is confirmed by the lower knee diameter change compared with control group, even comparable to the treatments of clinical approved drugs anti-IL-1β and anti-TNF-α. Moreover, the histological results also confirmed that neutrophil-NP can inhibit immune cell infiltration and thus alleviate joint damage in the three RA mice models.
Figure 2.
Bioinspired membraned coated nanoplatform as nanodecoy in autoimmune diseases therapy. (A) Illustration of neutrophil-NPs designed for neutralizing cytokines, inhibiting inflammation and protecting joint. (B) Characterization of neutrophil-NPs including TEM image (left) and western blotting (right). Scale bar, 100 nm. (C) Binding capacity assay of neutrophil-NPs with IL-1β and TNF-α. (D) Fluorescence images of a cross-section of mouse joints stimulated with recombinant mouse IL-1β and incubated with neutrophil-NPs or RBC-NPs. Scale bar, 100 μm. Reprinted with the permission from Ref. 14. Copyright © 2018, Nature Publishing Group. (E) Schematic illustration of TU-NPs designed for targeting RA immunotherapy by coating TRAIL-expressing membrane onto HCQ-loaded PLGA cores. (F) Reconstructed 3D images of PA showed that TU-NPs could penetrate deeper into the inflamed tissues than that of ICG or U-NPs at 12 h post injection. (G) MRI and CT images of arthritic paws in different groups. The red arrow represents the erosion or destruction of joints. Reprinted with the permission from Ref. 42. Copyright © 2020, Elsevier.
Bioinspired membrane-coated nanoplatform can also be used as a targeted delivery carrier for RA treatment. During the progression of RA, inflammatory macrophages overexpress death receptor 5 (DR5), which provides an unparalleled possibility to develop a novel method by targeting inflamed M1 macrophage. Compared with bare cell membrane vesicle, gene engineering-modified cell membrane not only can bind to the corresponding receptors on the immune cells, but also can be used for specific targeting, treatment and imaging. In a recent study, gene engineering-modified cell membrane expressing TNF-related apoptosis-inducing ligand (TRAIL) was extracted and coated onto the HCQ-loaded PLGA nanoparticles (TU-NPs) to specifically induce inflammatory M1 macrophages apoptosis and simultaneously deliver anti-RA drugs for a targeted therapy (Fig. 2E and G)42. One highlight of the research is that TU-NPs can bind to DR5 of M1 macrophages to induce its apoptosis and then decrease inflammatory cytokines secretion. In vivo targeting experiment, CIA mice were injected intravenously with free ICG, ICG-labeled U-NP and TU-NP, and obviously TU-NP-treated mice showed significant fluorescence intensity and photoacoustic signal, which means that TU-NP had a superior RA targeting ability than U-NP. In the following therapeutic and prophylactic study, TU-NP can powerfully reverse the RA progression from the MRI facts that TU-NPs-treated mice showed lowest T1 weight signals and CT results that TU-NPs-treated mice exhibited least bone erosion. In short, displaying functional proteins or antibody on cell membrane to regulate immune system may be superior than that of bare cell membrane, which proposing a potential strategy for immunotherapy.
3.1.2. Type II immune hypersensitivities
Type II immune hypersensitivities diseases such as autoimmune hemolytic anemia (AIHA), drug-induced anemia (DIA) and immune thrombocytopenia purpura (ITP) are characterized by the production of pathological autoantibodies, which result in the damage of platelets, red blood cells or other immune cells48,49. The diseases can lead to the destruction of normal tissues or uncontrolled bleeding that even can cause the death of patients49. However, current treatments for these autoimmune blood diseases remain relatively nontargeting, even wide use of including systemic glucocorticoids, cytotoxic drugs, and B cell depleting monoclonal antibodies50,51. Even though much progress has been made with the use of present therapies, its efficacy remains unsatisfied and along with serious adverse effects, highlighting the desideratum for a better targeted immunotherapy.
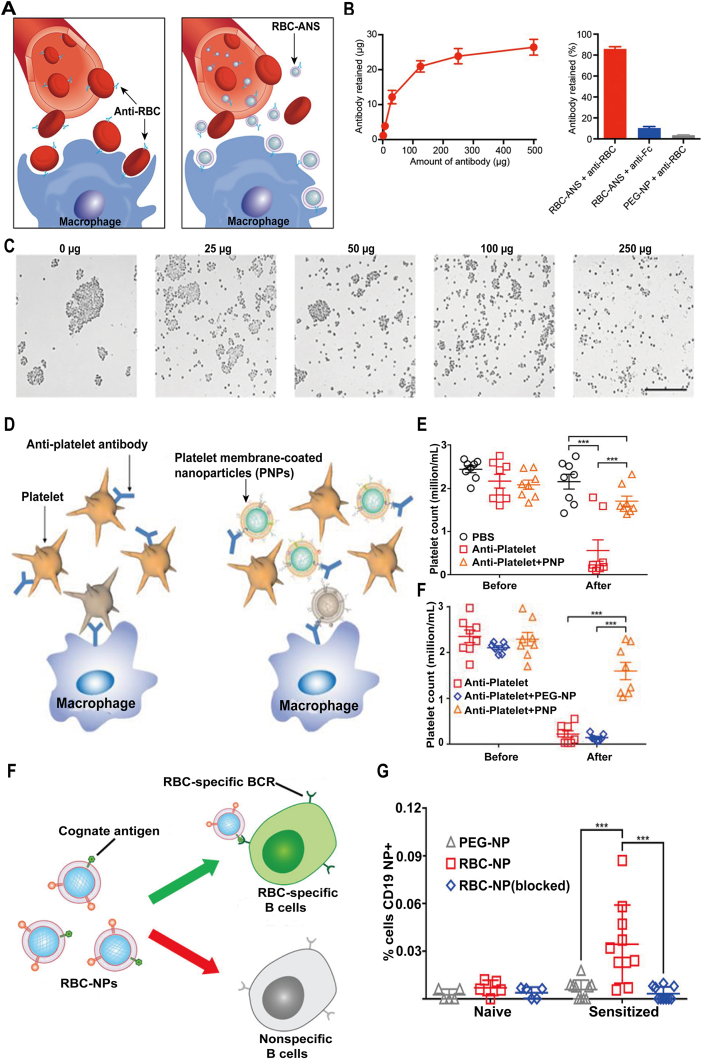
Based on the pathomechanism that autoantibodies mainly attack RBCs, how to clear the autoantibodies is the priority. In a recent study, a core‒shell nanoparticle as antibody nanosponge (ANS) by fusing RBCs membrane onto the PLGA cores was designed (Fig. 3A and C)48. With inheriting the properties of RBCs membrane, these autoantibodies can be effectively absorbed to the surface of ANS, disrupting AIHA advancement in a low-toxic manner. The results showed that RBC-ANS can reduce RBC-bound autoantibodies in a dose-dependent manner, for example, ∼60% and ∼95% reduction can be separately achieved with 100 μg and 1 mg of RBC-ANS. To know whether RBC-ANS can target the antibodies in vivo, anemia disease model was induced by i.p. injection of 500 μg anti-RBCs. Meanwhile, the equivalent anti-RBCs preincubated with 5 mg of RBC-ANS for 5 min was subsequently injected into the treated group mice as a contrast. It demonstrated that anti-RBCs were neutralized by the RBC-ANS and then the circulating RBCs were protected from being trapped. To further verify the potential application of RBC-ANS in clinics, the authors established a clinically relevant anemia mouse model by daily injection (i.p.) anti-RBCs. In the following treatment, RBC-ANS-treated group showed a more enhanced efficacy for neutralizing autoantibodies and postponing anemia progress compared with other treatments.
Figure 3.
Bioinspired membraned coated nanoplatform as nanodecoy in autoimmune diseases therapy. (A) Schematic representation of RBC-ANS neutralizing anti-RBC antibodies in AIHA. (B) RBC-ANS incubated with anti-RBCs demonstrated its particle saturation (250 μg versus 27 μg) and its specific binding capacity. (C) Different concentrations of RBC-ANS coincubated with anti-RBCs illustrated dose-related inhibition of RBC agglutination by RBC-ANS. Reprinted with the permission from Ref. 48. Copyright © 2014, PNAS. (D) Schematic of platelet membrane-coated nanoparticles (PNPs) for the treatment of ITP. (E) In vivo neutralization of anti-platelet antibody activity by PNPs. (F) In vivo treatment of antibody-induced thrombocytopenia by PNPs (n = 8). Reprinted with the permission from Ref. 49. Copyright © 2016, Elsevier. (G) Schematic of RBC-NPs for the targeting of RBC-specific B cells. (H) Targeting of autoimmune B cells using mRBC-NPs. Reprinted with the permission from Ref. 57. Copyright © 2018, American Chemical Society.
As described previously, enormous autoantibodies for deleting platelets are responsible for immune thrombocytopenia purpura (ITP), which can make people suffer the coagulation disorders. Inspired by previous works, leverage the biological membrane as a sponge to neutralize the antiantibodies may also produce unexpected outcomes in ITP therapy. In a recent study, platelet membrane-coated nanoparticles (PNPs) for specially clearing anti-platelet antibodies were manufactured (Fig. 3D and F)49. Because the PNPs outer layer was intact platelet membrane, it completely inherited the surface receptors or adhesion proteins of native platelet, which endow them with nanodecoy for binding autoantibodies. Both in vitro or in vivo, PNPs exhibited a superior pathological autoantibody binding capacity, thus protecting model mice from ITP.
3.1.3. B cells-related autoimmune diseases
Hyperactivation of B cells is often involved in autoimmunity and a large number of autoimmune diseases are of B cell origin, such as primary Sjögren's syndrome (pSS), SLE and autoimmune thyroiditis52,53. The activated B cells secrete larger amounts of pathological autoantibodies leading to the progression of the diseases54. Thus, specifically depleting the active B cells or plasmocyte may represent a promising approach to slowing diseases progress. Current monoclonal antibodies such as Rituxan and Belimumab have achieved some positive effects in clinics55,56, whereas the drug response remains unsatisfied because of heterogeneity of autoantigens in patients. Subsequently, more broad-spectrum strategies that can neutralize these autoantibodies should be developed. In one study, a method for the specific targeting of activated B cell populations by utilizing the nanoparticles covered with RBCs membrane expressing their cognate antigens was designed (Fig. 3G and H)57. Meanwhile, the researchers successfully implemented multi-functionalization of nanoparticles (RBC-NPs) with the homogeneous antigens expressed on the cells attacked by the immune B cells in the disease progression. In vitro experiments, B cell hybridomas were firstly used to confirm that RBC-NPs can be specially bound to an RBC antigen-specific hybridoma. In vivo experiments, two murine models were established to test the targeting ability. The results showed that alloimmune B cells exhibited a more enhanced affinity to hRBC-NPs than naïve RBC or PEG-NPs. To further evaluate whether hRBC-NPs can distinguish antigen-specific B cells generated in vivo, autoimmune murine model was established through sensitizing with rRBCs and anti-CD25 for 3 weeks. It was demonstrated that nanoparticles fused with RBC membrane decreased autoactivated B cells and could be applied to verify autoantigen specific B cells. Collectively, the bioinspired strategy would provide a practical approach to improving immunotherapy of a wide range of autoimmune diseases that are presently difficult to manage in clinics.
3.2. Bio-immunotherapy in autoimmune diseases
3.2.1. Multiple sclerosis
Multiple sclerosis (MS) is a T cell-mediated autoimmune disease that affects the brain and central nervous system, which often leads to inflammation and a wide range of symptoms throughout the body including paralysis, vision loss, and mobility problems58,59. The disease progresses with enormous migration of autoreactive lymphocytes across the blood–brain barrier (BBB)59. Increased active T and B lymphocytes, plasma cells, macrophages, and especially T-helper 1 cells (Th1) are recruited into brain where they can kill human neurons60. Currently, there is no cure for MS other than the drug that can slow the progression and relieve symptoms, and most of the drugs are found to hardly across the BBB, which proposes a great challenge for MS treatment58.
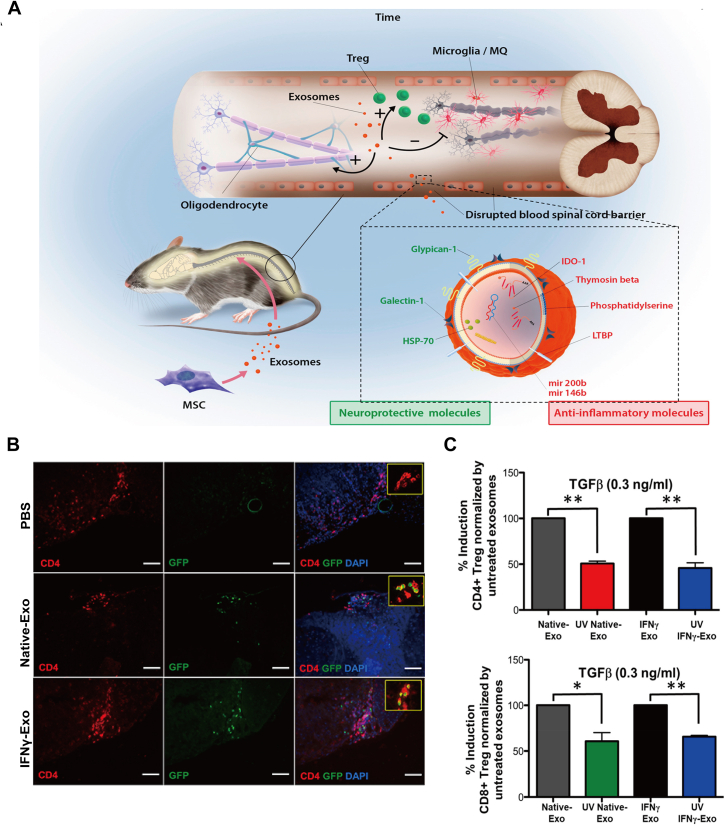
Exosomes are a kind of membrane enclosed vesicles with a diameter range from 30 to 200 nm, which play an important role on intercellular communications especially in physiological and pathological conditions61. Recently, exosomes have attracted much attention from clinicians and researchers because of their special characteristics including long circulation time, low immunogenicity compared to that of source cells and large cargo of various nucleic acids, lipids or proteins, which involves in immune system modulation61. Thereby, many studies have focused on the therapeutic function of exosomes in MS therapy. In a recent study, the extracted mesenchymal stem cell-derived exosomes (MSCs-exosomes) were found to be as a nanoplatform for MS treatment through intravenous injection of the exosomes (Fig. 4A and C)30. Compared with native MSC exosome (Native-Exo), exosome from MSCs induced by IFN-γ (IFNγ-Exo) can relieve the basic symptom, reduce demyelination and neuroinflammation, and largely upregulate the proportion of CD4+CD25+FOXP3+ regulatory T cells (Tregs) in the experimental autoimmune encephalomyelitis (EAE) mouse model. Moreover, the study also verified that IFNγ-Exo can suppress peripheral blood mononuclear cells (PBMCs) activation and inhibit pro-inflammatory cells Th1 and Th17 secrete cytokines in the spinal cord. On the contrary, IFNγ-Exo even can increase the levels of immunosuppressive cytokines indoleamine 2,3-dioxygenase (IDO), which is responsible for inhibiting T cell function. To further investigate the mechanisms of the inhibiting effect of IFNγ-Exo, deep RNA sequencing and proteomic analysis were performed to illustrate the enrichment of anti-inflammatory RNAs (mRNAs and noncoding RNAs) and neuroprotective proteins (MIC-1, Gal-1 and HSP70). Similar to these findings, researchers also manipulated exosomes derived from bone marrow mesenchymal stem cells (BMSCs) to treat EAE rat models and the results showed that it can significantly attenuate inflammation and demyelination of the CNS in EAE rats by regulating the polarization of microglia. Further analysis revealed that the exosomes can enter into the spinal cord and induce microglia toward M2 phenotype polarization (anti-inflammatory cytokines IL-10 and TGF-β) rather than M1 phenotype (pro-inflammatory cytokines TNF-α and IL-12). To target delivery anti-inflammatory cytokines to the recipient microglia or inflammatory macrophages, genetic engineering has been applied to create a novel and multifunctional exosome and also achieved positive effects62. Overall, engineered exosomes-based immunomodulator may be used as a promising therapeutic nanoplatform for CNS-related autoimmune diseases.
Figure 4.
Bioinspired membraned coated nanoplatform as chemo-immunotherapy in autoimmune diseases therapy. (A) Scheme of MSC derived exosome in the treatment of EAE. (B) Immunohistochemistry of CD4+FOXP3+ cells and DAPI in spinal cord sections of EAE mice after injection of PBS, Native-Exo, and IFNγ-Exo. Scale bar, 100 μm. (C) Quantification of CD4+FOXP3+ or CD8+FOXP3+ in the FOXP3-eGFP mice splenocytes after stimulated by anti-CD3+ IL-2 with or without TGFβ. Reprinted with the permission from Ref. 30. Copyright © 2019, American Chemical Society.
4. Bioinspired membrane coating nanoplatform in virus infections
4.1. Nanodecoy-immunotherapy in virus infection
Infectious diseases including virus and bacterial infection are often accompanied with an abnormal immune response and have been a major cause of lethality worldwide63,91. As for infectious diseases, they share a common pathological feature that the pathogen must attach itself to cellular surfaces for initiating disease further progress64, 65, 66, 67. Considering the specific binding affinity between infectious pathogens and cells, nanoparticles covered with desired cell membrane as a nanodecoy are herein developed for virus/bacteria neutralization or clearance.
4.1.1. Unmodified membrane used for virus clearance
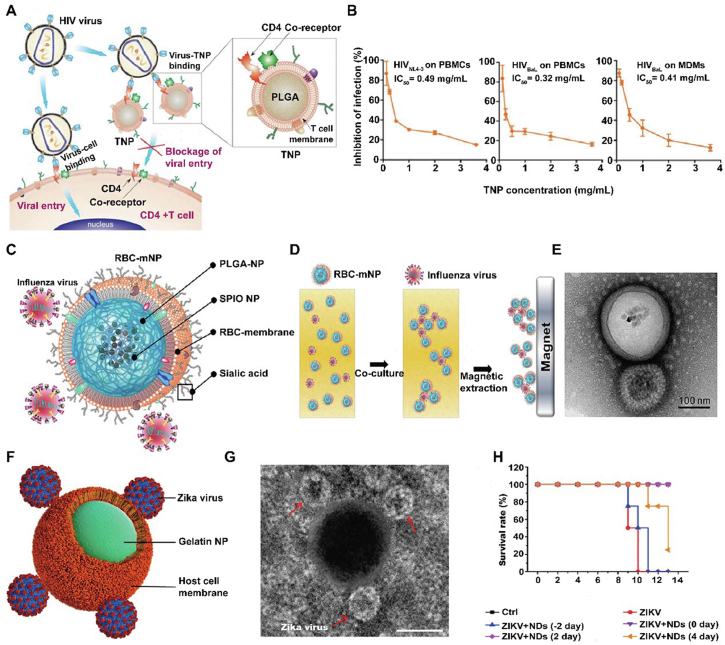
HIV continues to be one of the most dangerous viruses with nearly two million people newly infected every year, which has caused a great global health challenge68. HIV actively attacks the immune cells expressing CD4 and thus cause immune system dysfunctions or deficiency, which leads to the acquired immunodeficiency syndrome (AIDS) and aggrandizes a risk of other infectious diseases and cancers69. When the virus initially binds to target cells, its envelope glycoproteins, mainly gp120 would interact with CD4, and also can bind to the coreceptors C–C chemokine receptor type 5 (CCR5) or C-X-C chemokine receptor type 4 (CXCR4) on the infected cells70,71. However, current antiretroviral therapy can inhibit plasma virus to some extent, the residual HIV persists in latent cells, which poses a large barrier for viral extermination68. In a recent study, the constructed T-cell-membrane covered nanoparticles (TNPs) can effectively inherit parental CD4+ T cells properties and functions to target and fuse with the viruses, and subsequently inhibit viral entry into and damage of the target cells (Fig. 5A and B)68. TNPs have a high affinity and binding capacity to HIV gp120, and this was confirmed by the immunobinding assays. Recombinant HIV-1 gp120IIIB (gp120 of X4 strain) and HIV-1 gp120BaL (gp120 of R5 strain) were separately incubated with DiD labeled TNPs, and the results showed that the binding effect was dose-dependent. Compared with PEG-NP or RBC-NP, which had little affinity to gp120, TNP showed an enhanced HIV envelope protein binding ability. Moreover, TNPs can be as T cells decoys to bind with HIV gp120 and thus protect CD4+ T cells from gp120-mediated cell apoptosis with the confirmation of decreased CD4+ T cells death, whereas RBC-NP or PEG-NP had little effect even comparable to free gp120. To further test the neutralization effect of the virus on PBMCs, 200 TCID50 virus was incubated with TNPs with various concentrations and the neutralization effect was authenticated through checking HIV p24 antigen production. Surprisingly, TNPs can largely decrease antigen p24 production in the medium of PBMCs after incubation with HIVNL4-3 or HIVBaL in a dose-dependent manner. It showed that IC50 value for TNPs toward inhibiting X4 tropic HIVNL4-3 and R5 tropic HIVBaL strain on PBMCs were separately 0.49 and 0.32 mg/mL. Similar to that of PBMCs, TNPs also exhibited an enhanced ability of inhibiting HIV infection (IC50 = 0.41 mg/mL) on human-monocyte derived macrophages (MDMs) with a negligible toxicity. Overall, the researchers utilized the extracted CD4+ T cell membranes to cover onto PLGA cores and thus constructed biomimetic TNPs as HIV nanodecoys, which highlighted the potential of TNPs in substantially attenuating viral infectivity.
Figure 5.
Nanodecoy-immunotherapy in virus infection. (A) Scheme of TNPs designed for HIV clearance. (B) Different concentrations and strains of TNPs on the inhibition of HIV infection. Reprinted with the permission from Ref. 68. Copyright © 2018, Wiley. (C) Scheme of RBC-mNPs for influenza virus clearance and (D) isolation. (E) TEM images of influenza virus samples captured by RBC-mNPs. Reprinted with the permission from Ref. 75. Copyright © 2017, American Chemical Society. (F) Scheme of NDs adsorbing ZIKV. (G) TEM image of a single ND that adsorbs ZIKV. Red arrows indicate ZIKV. Scale bar, 100 nm. (H) NDs inhibit ZIKV infection and improve the survival rates. Reprinted with the permission from Ref. 78. Copyright © 2019, American Chemical Society.
Influenza can occur annually, which leads to the death and complications especially in the elderly and children with a huge social economic cost72. For the influenza viruses, they usually are negative-sense, single-stranded RNA viruses, indicating its high frequency mutations and thus make it hard to exploit special drugs or vaccines for the clear target73. Even though influenza viruses have various types or subtypes, they enter into the host cells all by the interactions between the viral HA proteins and sialic acids, which are abundantly expressed on the surfaces of RBCs74. Considering that, Chen et al.75 designed RBCs membrane-coated magnetic nanoparticles (RBC-mNP) for influenza virus targeting and isolation (Fig. 5C and E). Through the single emulsion process, 10-nm iron oxide nanoparticles were loaded into the PLGA cores, which endows them with proper magnetic properties. Then resultant nanoparticles were further fused with RBC membrane, completely inheriting the surface sialic acids with confirmation by DLS data, TEM images and immunogold staining. Whereafter, influenza viruses were separately mixed with RBC-NP and PEG-NP at a 1:1 ratio, and TEM or cryo-EM images showed that the viruses can be absorbed or attached on the RBC-NP through monovalent and polyvalent manner. In contrast, it did not observe PEG-NP induce the adhesion interactions with viruses. This can be explained by the special target binding due to the existence of viral HA proteins and RBC sialic acids.
Zika virus (ZIKV) is a single-stranded RNA virus and from the Flaviviridae family and the consequences of ZIKV infection are devastating76. ZIKV infection is observed to be involved in devastating neurological complications through infecting neural stem cells, and mainly includes microcephaly in mice or human fetuses77. To date, no vaccine or specific treatment is approved to address the issues. Based on the fact that ZIKV invades the host cells firstly through its envelope proteins (E protein) to bind the membrane receptors of host cell and trigger the following replication and thus cell membrane-based nanoparticle can be as host-mimicking nanodecoy for ZIKV absorption77. Rao et al.78 designed Aedes albopictus (C6/36) membrane covered gelatin nanoparticles (GNP) as nanodecoy (ND) for ZIKV elimination (Fig. 5F and H). The results showed that NDs-treated group can significantly block ZIKA pass the BBB and placental blood barrier (PBB), and thus improve the survival rate. In short, NDs can be as an effective nanoplatform for trapping and distancing ZIKV from the fetal brain and attenuated the ZIKV-caused fetal microcephaly in pregnant A129 mice. More importantly, the outer wrapping cell membranes endow NDs with advanced biocompatibility, long half-time and immune evasion, which guarantee the safety and availability of NDs in intricate microenvironment in vivo.
4.1.2. Gene engineering-modified membrane used for virus clearance
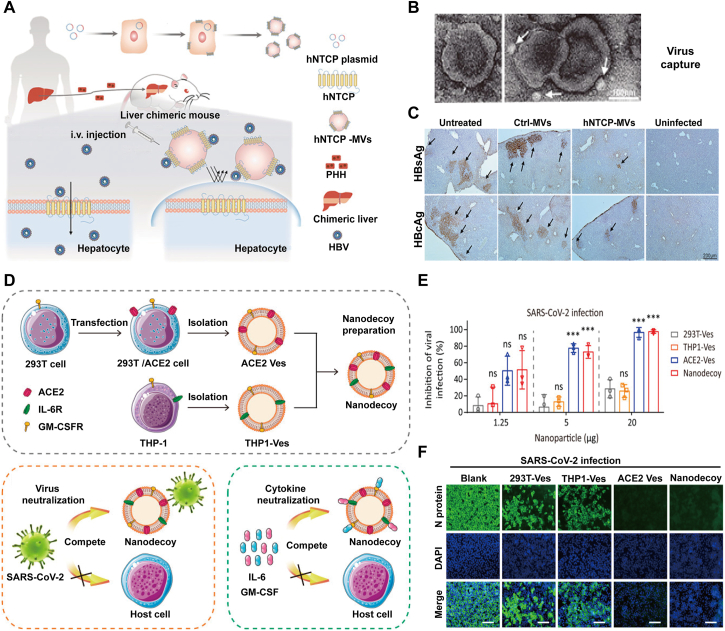
Chronic hepatitis B virus (HBV) infection is a serious public health issue and approximately 350 million people are chronically infected worldwide79. Particularly in China, HBV imperils patients and 25% patients with HBV infection would die suffering from hepatitis, cirrhosis, or hepatocellular carcinoma. Nevertheless, current clinical treatments or therapeutics cannot completely clear HBV in chronic infected patients. This is because that HBV is too crafty to be detected by the innate immune system and thus can escape immune recognition and subsequent elimination80. More urgently, functional cure is scarcely achieved in the infected patients with use of present approved anti-HBV drugs. Therefore, it is of crucial importance to develop a novel anti-HBV strategy. In a new study, human sodium taurocholate co-transporting polypeptide (hNTCP), the HBV specific receptor, was co-expressed on the parental cell through gene engineering and the resultant membrane was applied for further HBV neutralization research (Fig. 6A and C)81. The derived vesicles hNTCP-MVs exhibited a high affinity to HBV-PreS2 and HBV virion and it can effectively inhibit HBV virion binding and invading to host cells, with the confirmation of reduced HBV DNA and surface antigen (HBsAg) in the medium when incubated with hNTCP overexpressing cells after HBV infection. Moreover, 0.1 mg/mL hNTCP-MVs can significantly decrease about 76% HBsAg and 73% HBV DNA in the medium 3 days post infection. Whereas, equivalent control-MVs had a negligible effect on HBV infection and it was further confirmed in the HBV-Ae/Ba stable cells (genotype A/B with replication-competent HBV genome stably integrated). Through vein injection of 500 μg hNTCP-MVs in the Hu-FRGS mice upon the infection, it can obviously inhibit serum HBsAg and HBV DNA replication at 20 dpi while control-MVs cannot achieve it in human-liver-chimeric mouse model (Hu-FRGS). After the treatment, IHC staining revealed that hNTCP-MVs may also prevent the extended expression of HBsAg and HBV core antigen (HBcAg) in the model mice liver lobes with a negligible system toxicity. Overall, taking advantaging of gene engineering to anchor specific receptor on the parental cells and the subsequent MVs against virus infection, which can specifically recognize and neutralize pathogens, would be a promising strategy in future infectious diseases.
Figure 6.
Bioinspired membraned coated nanoplatform as nanodecoy immunotherapy for virus clearance. (A) Scheme of the generation of hNTCP-MVs and its antiviral mechanism. (B) TEM images of HBV hNTCP-MVs complexes and (C) its virus inhibition on Hu-FRGS mice with HBV infection. Scale bar, 200 μm. Reprinted with the permission from Ref. 81. Copyright © 2018, Wiley. (D) Scheme of the preparation of nanodecoys against COVID-19 by fusing the vesicles of gene engineering modified 293T/ACE2 and THP-1 cells, and its application in virus capture and cytokines neutralization. (E) Antiviral capacity against SARS-CoV-2 and (F) its immunofluorescence images of SARS-CoV-2−infected Vero-E6 cells. Scale bar, 100 μm. Reprinted with the permission from Ref. 67. Copyright © 2020, PNAS.
Similar to the known coronaviruses, SARS-CoV-2 is a spherical virion with a diameter ranging from 80 to 120 nm82. The outer layer of the virus is the structural protein, including spike (S) protein, envelope (E) protein and membrane (M) protein. Especially, S protein consisting of S1 and S2 subunit, has been reported to be the most important protein during the infection83, which can bind to the human angiotensin-converting enzyme 2 (ACE2) on the surface of pulmonary or other gastrointestinal epithelial cells and then invade the host cells mediated by the subsequent fusion. Moreover, the virus binding to the receptor human ACE2 mainly depends on its receptor-binding domain (RBD) and catalytical activation by proteases82, 83, 84. Based on previous works, a cell membrane based nanosponge as a bioinspired countermeasure to the SARS-CoV-2 was fabricated (Fig. 6D and F)67. In the design, ACE2 was anchored onto the membrane of 293T cells through gene engineering and then the derived vesicles were fused with human monocytes (THP-1) vesicles to prepare the novel virus nanodecoy. The strategy against SARS-CoV-2 has a powerful two-step approach by inheriting the properties of the two source cells, the first step is to capture the virus through harnessing the ACE2 receptors, and the following step is to neutralize the boosted inflammatory cytokines induced by the virus infection. To test its antiviral ability, pseudotyped SARS-CoV-2, SARS-CoV, strains of WIV1 and Rs3367 were incubated with the different nanoparticles including 293T-Ves, THP1-Ves, ACE2-Ves and the nanodecoy at the indicated concentrations. The results showed that the nanodecoys can significantly inhibit pseudotyped virus infection. Authentic SARS-CoV-2 was also assayed and showed similar results. Furthermore, due to the coexistence of cytokines receptors such as IL-6R and GM-CSFR (granulocyte−macrophage colony-stimulating factor), it can largely suppress inflammatory cytokines and ameliorate lung injury in the acute pneumonia mouse model. In short, the novel nanodecoy platform provides a promising and broad-spectrum antiviral approach against virus infection, which is superior than other conventional therapies presently in terms of the progress for COVID-19 due to the fact that the nanodecoy can target different variant virus.
4.2. Vaccine-immunotherapy for virus clearance
Human papillomavirus (HPV) is a double-stranded circular DNA virus, which can transmit through skin-to-skin or mucosa-to-mucosa contact and enter the body via cutaneous or mucosal trauma, and ultimately cause infectious diseases85. HPV infection is considered to be a causative factor for various cancers of the anogenital region and oropharynx, such as skin carcinogenesis and cervical cancer86. However, HPV can evade from the immunosurveillance by various mechanisms, and herein vaccine-immunotherapy would be a promising technique for protecting body from HPV infection.
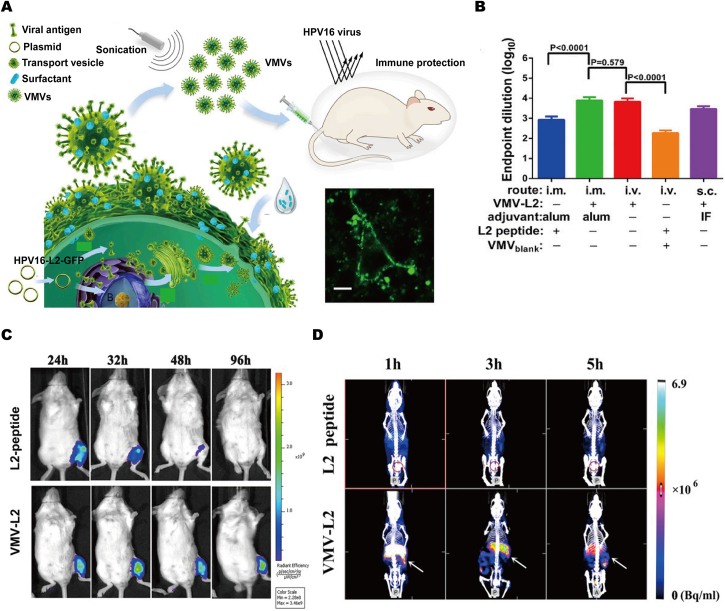
Inspired by the fact that most enveloped viruses hijack a host cell membrane and then release through the budding process that requires cell membrane participation, genetically engineered HPV16 viral antigen L2 to anchor onto cell membrane, then produced large amounts of virus-mimetic nanovesicles (VMVs-16L2) inheriting the properties of natural virus in size, shape and immunogenicity (Fig. 7A and D)87. In vivo experiments, when vaccinated mice with VMVs-16L2, the high level of antibody titer targets L2 was detected and estimated about 7–8 times higher total antibody titers and ∼10.5-fold higher neutralizing antibody titers than that of free L2 peptide, and thus can produce a superior protective effect. More importantly, the established vaccine nanoplatform can be further extended in other virus infections. Overall, due to the advantages of functionally displaying antigen epitopes or viral envelope glycoproteins onto cell membrane and the resultant VMVs-antigens, the straightforward, multifunctional and controlled nanobiotechnology platform can be used to fabricate various antigen delivery system against plenty of enveloped viruses.
Figure 7.
Bioinspired membraned coated nanoplatform as vaccine-immunotherapy for virus clearance. (A) Illustration showing the generation of virus-mimetic nanovesicles (VMV) for anchoring anti-tumor epitopes or enveloped-virus glycoprotein to VMV surface. (B) The results of mice (n = 5) immunized three times with 100 μg of VMV-l2 or 1.33 μg of L2 peptide with incomplete Freund's adjuvant (IFA) or alum adjuvant via i.v., i.m., or s.c. administration. (C) The change in fluorescence intensity at different time points post-injection suggested longer exposure time of VMV-L2 over free L2 peptide to immune cell. (D) SPET/CT images at 1, 3, and 5 h after i.v. injection of 99mTc labeled L2 peptide and VMV-L2. The spleen and bladder are indicated by white arrow and red circle. Reprinted with the permission from Ref. 87. Copyright © 2015, PNAS.
5. Bioinspired membrane coating nanoplatform in bacterial infection
5.1. Nanodecoy-immunotherapy in bacterial infection
Bacterial infection has become a serious threat to human health worldwide, and closely related to the host immune system. In addition to causing increased antibiotic resistance, the bacteria are good at adapting to its host by evading almost every aspect of the immune system88. Most of the bacteria initially adhere themselves to the host cells and tissues, improving their infectious rate and mediating the immune escape89. Therefore, taking advantage of the underlying infection mechanism and developing a new approach to overcoming it may help inhibit bacterial infection. Recently, cell membrane based nanotherapeutics have displayed its unique advantages in the field of anti-bacteria.
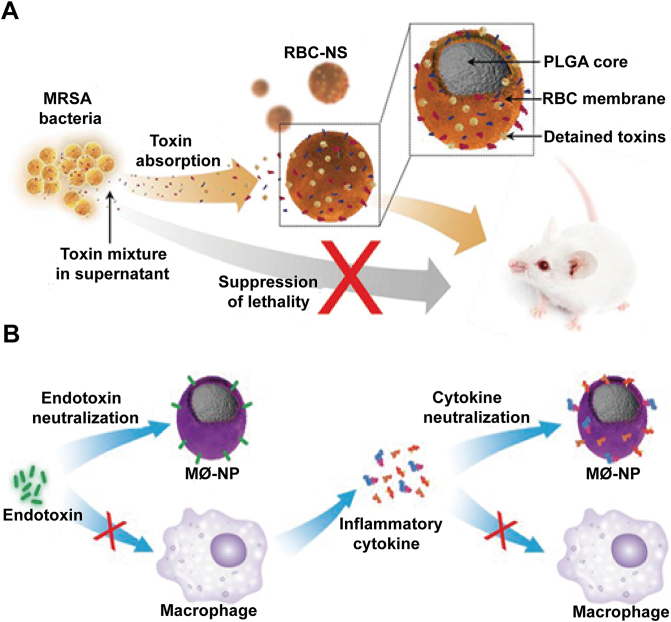
Sepsis is one of the most devastating diseases caused by bacterial infections with an uncontrolled systemic inflammatory response. Patients with sepsis may suffer from multiple organ dysfunctions or failures if not treated properly. In a recent study, RBC membranes-coated PLGA cores as detoxication nanosponges (RBC-NS) have been manufactured, which can neutralize membrane damaging toxins and divert them away from their cellular targets (Fig. 8A)90. Particularly in toxin-challenged mice model, the nanosponges significantly reduce the toxicity of staphylococcal alpha-haemolysin (α-toxin) and herein improve the survival rate. Extensively, the nanosponges also can be used in the infection caused by methicillin-resistant Staphylococcus aureus (MRSA). In MRSA whole secreted proteins (wSP) challenged mice model, the RBC membrane-based nanosponges bestow significant survival benefits against wSP-induced lethality. Moreover, in the MRSA sublethal dosage mice models, the RBC-NS obviously alleviate lung damages and suppress the activation of nuclear factor kappa B in the spleen. In short, these results can identify that RBC-NS can be used as the treatment strategy for severe MRSA infections and its derived diseases.
Figure 8.
Bioinspired membrane-based nanotherapeutics as nanodecoy-immunotherapy in bacterial infection. (A) Illustration of preparing RBC-NS to target MRSA toxin-induced toxic shock in mice. RBC-NS can act as nanodecoys to absorb and detain hemolytic toxins in MRSA culture and therefore protect mice from toxic shock induced by these toxins. Reprinted with the permission from Ref. 90. Copyright © 2019, Wiley. (B) Schematic representation of using MΦ-NPs to neutralize endotoxins and proinflammatory cytokines as a two-step process for sepsis management. Reprinted with the permission from Ref. 92. Copyright © 2017, PNAS.
Moreover, the endotoxin (mainly referred to as LPS) released from dead or lytic bacteria can be as a pathogen-associated molecular pattern (PAMP) and then captured or trapped by immune cells (monocytes and macrophages), immune cells membrane inherited receptors or proteins thus can be as a target for endotoxins91. In a recent study, macrophage membrane was extracted and wrapped onto PLGA cores to be as endotoxins decoys (MΦ-NPs), and MΦ-NPs have been verified to effectively inhibit LPS and the progress of sepsis (Fig. 8B)92. Because of the outer layer of source cell membrane, MΦ-NPs can specifically bind to LPS through its surface LPS-binding proteins and thus divert LPS away from targeting macrophages that would otherwise trigger the engagement of pattern recognition receptor (PRR) CD14. Meanwhile, due to the inhibition, MΦ-NPs can further avert sepsis cascade, including decrease LPS-induced nitric oxide (NO) production, PRR Toll-like receptor 4 (TLR4) and the subsequent nuclear factor-κB (NF-κB) transcription factor activation. In vitro studies, when incubated with MΦ-NPs, the inflammatory cytokines (IL-6, TNF-α, and IFN-γ) in the medium stimulated by LPS were significantly decreased. Compared with RBC-NPs or bare PEG-NPs, MΦ-NPs demonstrated a more enhanced protective efficacy, revealing its superior functions of macrophage membranes for endotoxins/LPS neutralization. In Escherichia coli challenged mouse model, mice received MΦ-NPs injection showed a lower proinflammatory cytokines levels through inhibiting the bacterial dissemination, and subsequently achieved an improved survival rate, whereas RBC-NPs and PEG-NPs treatments were not observed similar results.
5.2. Vaccine-immunotherapy in bacterial infection
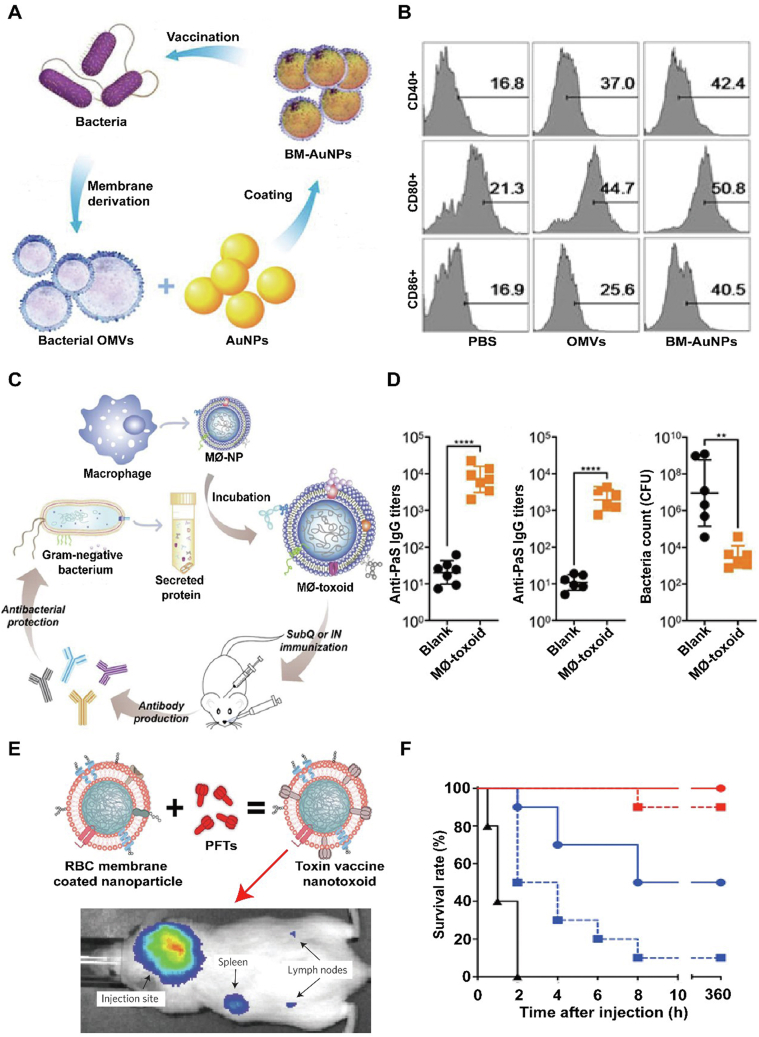
Particularly, bacterial outer membranes are ideal antigen derived materials because of the enrichment properties of immunogenicity and unique adjuvant, which can extremely active innate immunity and elicit adaptive immune responses93. Inspired by the advancement of nanotechnology, bacterial membranes were collected and coated onto gold nanoparticles to be as an antibacterial vaccine (Fig. 9A and B)94. In the study, E. coli outer membrane vesicles (OMVs) were extracted and distinctly wrapped onto small gold nanoparticles (AuNPs) with a diameter about 30–50 nm. This is because that the AuNPs have unique size and shape and would be precisely tailored to improve immune response rate. Other than the obviously intensive stability, the subsequent bacterial membrane-coated AuNPs (BM-AuNPs) can trigger a quick activation and maturation of DCs (indicated by the overexpression CD40, CD80 and CD86) in vivo when injected subcutaneously. Moreover, the BM-AuNPs also stimulate accelerated secretion of interferon gamma (INF-γ) and interleukin-17 (IL-17), but not interleukin-4 (IL-4) in vivo, which means that it can specifically induce Th1 and Th17 preferential cell responses to eradicate the source bacteria. Collectively, the study novelly utilized bacterial outer membranes to wrap synthetic inner cores, showing a significant potential for antibacterial vaccines in translation.
Figure 9.
Bioinspired membrane-based nanotherapeutics as nanodecoy-and vaccine-immunotherapy in bacterial infection. (A) Scheme of modulating antibacterial immunity via bacterial membrane-coated gold nanoparticles (BM-AuNPs). (B) BM-AuNPs activating DCs in the draining lymph nodes in vivo. Reprinted with the permission from Ref. 94. Copyright © 2015, American Chemical Society. (C) Schematic illustration of using macrophage-mimicking nanoparticles (MΦ-NPs) to prepare multiantigenic nanotoxoid against Gram-negative bacterial infection. (D) Immune response to subcutaneous MΦ-toxoid immunization including Anti-PaS IgG titers in the serum of mice on Day 21 following vaccinations, Anti-PaS titers in the lungs of mice 24 h after an intratracheal P. aeruginosa challenge on Day 35 following subcutaneous vaccinations and bacterial load in the lungs of mice vaccinated and infected. Reprinted with the permission from Ref. 95. Copyright © 2019, American Chemical Society. (E) Schematic preparation of nanoparticle-detained toxins (nanotoxoid) fabricated by RBC membranes covering onto PLGA cores and then integrating PFTs. (F) Survival rates of mice over a 15-day period following intravenous injections of 120 mg/kg Hla on Day 21 via the tail vein. Unvaccinated mice were used as negative control and mice vaccinated with heat-treated Hla served as positive controls. Reprinted with the permission from Ref. 96. Copyright © 2013, Nature Publishing Group.
Similar to that of previous work, cell membrane-coated nanoparticle integrated bacteria-related antigens may be used as vaccine to prevent bacterial infection by activating the immune system to target pathogen-associated antigens. Recently, macrophage membrane anchored various cytolysins or proteins secreted by gram-negative bacteria was covered onto PLGA cores as multiantigen nanotoxoid vaccine, which can enhance potent immunity against antibiotic-resistant bacteria (Fig. 9C and D)95. In the design, biomimetic nanovaccine take full advantages of the specific function of macrophages in eradicating pathogens and their natural binding for various virulence factors secreted by the bacteria. Both in in vitro and in vivo tests, MΦ-toxoid vaccine showed that it can effectively display various Pseudomonas aeruginosa antigens without toxicity to major tissue or organs. When used in mice pneumonia models, MΦ-toxoid vaccine demonstrates an enhanced protection efficacy through triggering strong humoral immune responses against live bacterial infection even immunized by the subcutaneous (subQ) or intranasal (IN) route. Collectively, macrophage membrane-based nanoparticles either as endotoxins decoys or vaccine, both can be as a promising solution to bacterial infections, and the nanoplatform has a favorable potential translational application in clinics.
Moreover, vaccine is also an effective approach to active immune response to eradicate toxins. Current toxin vaccines are mainly manufactured by using inactivated bacterial toxins through routinely chemical or heat denaturation, which would disrupt toxins' spatial conformation and the subsequent reduction of immunogenicity.
To better boost immune response to toxin without damages to body, cell membrane-based toxin-integrate strategy that can completely retain PFTs immunogenicity was developed (Fig. 9E and F)96. In the design, RBCs membrane anchored staphylococcal a-haemolysin (Hla), a model toxin, was wrapped onto PLGA cores (nanotoxoid) Surprisingly, through the spontaneous trap interaction between Hla and RBCs membrane, nanotoxoid can neutralize Hla virulence without compromised immunogenicity. On the other hand, nanotoxoid showed a high accumulation in the lymphatic drainage with a time-dependent manner when injected intravenously, meaning that it can be as a high-efficiency Hla deliver system to active immune response. Compared with traditional heat-denatured vaccine, mice immunized with nanotoxoid showed an enhanced protective immune response against Hla-induced virulence. In Hla-challenged mice models, nanotoxoid significantly improved the survival rate nearly 100% while other treatments cannot achieve it. Looking to the future, the nanovaccine platform can broaden its application field for different toxins' invasion by warping the nanoparticle core with desired cell membrane.
5.3. Sono-immunotherapy in bacterial infection
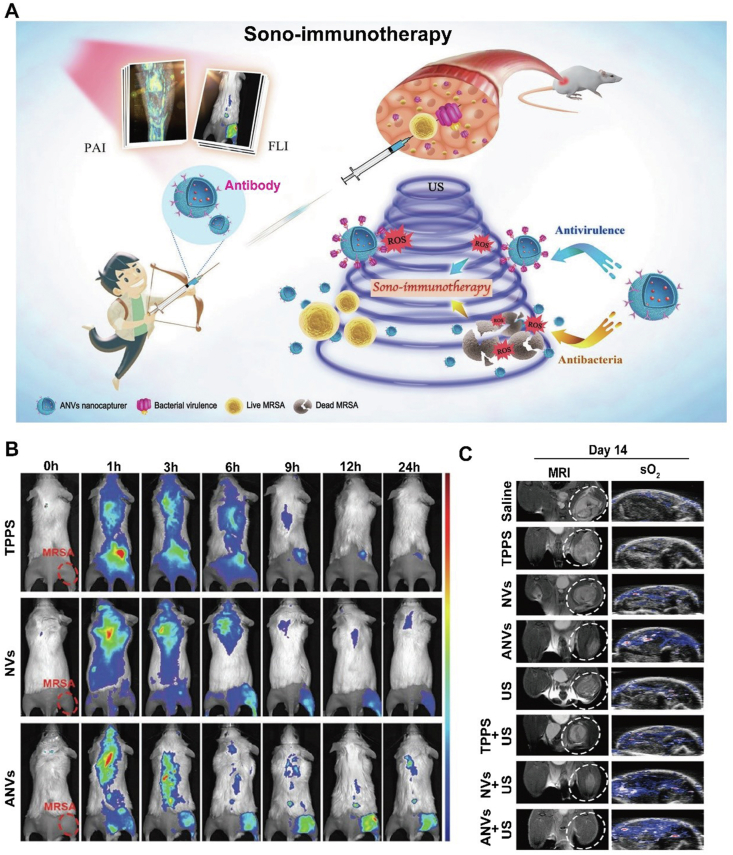
Sono-immunotherapy may be also a feasible solution to bacterial infection. Recently, a novel sonodynamic therapy (SDT) based on target nanovesicles against multidrug-resistant (MDR) bacteria was exploited (Fig. 10A and C)97. In the study, α-toxin antibody against MRSA was genetically displayed on the surface of cell that endowed the resultant nanovesicles (ANVs) with the ability of bacteria target and virulence capture. More importantly, a common sonosensitizer meso-tetrakis (4-sulfonatophenyl) porphyrin (TPPS) was encapsulated into the vesicles, in order to take advantage of the reactive oxygen species (ROS) produced under ultrasound activation to kill MRSA. Compared with traditional passive toxins absorption or competitively block the binding of pathogens to host cells by source bacteria or cell membrane, ANVs-TPPS therapy can actively recognize and eradicate virulence. Furthermore, due to the properties of TPPS, FL and PA images can be distinctly acquired to illustrate ANVs-TPPS target the MRSA infectious sites and prolong circulation time of TPPs, and hence guide the following therapeutic regiment. In vivo treatment, ANVs-TPPS combined with US can more obviously inhibit MRSA myositis when compared with that of bare NVs-TPPS, and this was confirmed by the obtained MRI, PA (oxyhemoglobin saturation) and HE images. Overall, the work firstly achieved a dual-mode therapy with combining antibacterial SDT and antivirulence immunotherapy, meanwhile guided the visualization therapy, which proposed a train of thought for developing antibiotic-free nanotheranostics.
Figure 10.
Bioinspired membrane-based nanotherapeutics as sono-immunotherapy in bacterial infection. (A) Schematic illustration of ANVs nanocapturer which combines antibacterial sonodynamic therapy and antivirulence immunotherapy. (B) Targeting analysis of FL images of MRSA-infected mice model after tail vein injection of TPPS solution, NVs, or ANVs. (C) Representative magnetic resonance images and PA images of oxyhemoglobin saturation of the MRSA-infected mice within 14 days post-injection in different groups. Reprinted with the permission from Ref. 97. Copyright © 2019, Wiley.
5.4. Chemo-immunotherapy in bacterial infection
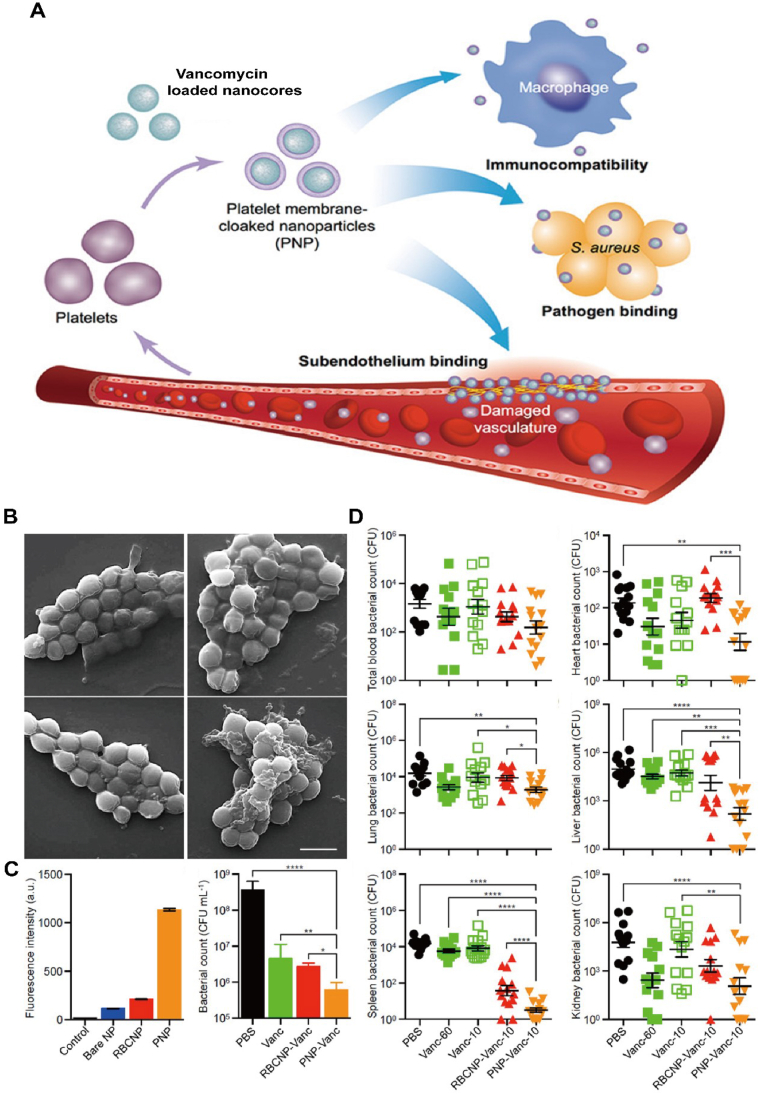
Pathogenic bacteria can also attach themselves to platelet and then release various toxins to damage platelets and aggravate bacterial infection98. Especially, MRSA can release α-toxin to make platelet aggression and the resultant inflammation, which in turn help MRSA colonization and avoid being cleared by immune system99. Considering the facts, researchers successfully constructed platelet membrane-based drug delivery system (PNPs), in which platelet membrane was wrapped onto the vancomycin loaded nanocores to target MRSA (Fig. 11A and D)100. PNPs showed a superior binding capacity with MRSA than red blood cell membrane-coated nanoparticles (RBCNP) or bare nanoparticles, when cocultured with MRSA. In the MRSA challenged mouse model, vancomycin-loaded PNPs-treated mice exhibited an enhanced bacterial inhibition, even in the major organs than that of free vancomycin or bare vancomycin-loaded nanocores. In short, the novel antibacterial strategy extended antibiotic potency through integrating targeted drug delivery and the biofunctions of platelet membrane.
Figure 11.
Bioinspired membrane-based nanotherapeutics as chemo-immunotherapy in bacterial infection. (A) Scheme of platelet membrane coated vancomycin-loaded nanoparticles (PNPs) to target MRSA. (B) SEM images of MRSA252 bacteria after incubation with PBS (top left), bare NPs (top right), RBCNPs (bottom left), and PNPs (bottom right). Scale bar, 1 μm. (C) Normalized fluorescence intensity of dye-loaded nanoformulation retained on MRSA252 based on flow cytometric analysis, and in vitro antimicrobial efficacy of the indicated treatments. (D) In vivo antimicrobial efficacy of the indicated treatments at the same concentration of vancomycin 10 mg/kg and its bacterial loads in the major organs. Reprinted with the permission from Ref. 100. Copyright © 2015, Nature Publishing Group.
6. Challenges for clinical translation
Immune suppression or activation-based bioinspired membrane coating nanoplatform have achieved much progress in targeted immunotherapy, however, technical limitations hindering the translation are still remained to be resolved. Statistically analyzing the previous researches, most studies have only been performed in mice, indicating that the following attentions have to be paid to promote its clinical translation. However, much attention should also be paid to its potential side effects, including the overuse of membrane-coated NPs caused cytotoxicity after the chemical or other modifications or produce pernicious inflammation via the unknown conjunctions with the various immune cells, leading to pathological cytokines or antibodies release.
6.1. Broadening sources of cell membrane
Presently, the source of cell membranes is narrow and broadening the membrane origin is necessary. As described previously, in the diseases of virus infection, bacterial infection and autoimmune diseases, immune cells or other disease-related cells are markedly involved in the progression. It broadens the application of bioinspired membrane-based nano-immunomodulator to target these diseases. However, currently used membranes are mainly from cell culture system in laboratory, not reflecting the real cell phenotypes changes or membrane proteins expression in the disease condition, and these may weaken its targeting ability, and thus it is important to regulate the culture conditions to conform to the actual situation. On the other hand, patient-derived cells also can be tailored to improve immunotherapy and enhance disease targeting ability101, 102, 103. In some cases, certain kinds of cells are hard to acquire directly, and stem cells or induced pluripotent stem cells (iPSCs) can be induced to differentiate into the desired cell types. For example, BMSCs have already been induced to chondrocyte and its derivates have been applied in RA or OA studies104, 105, 106. Therefore, we should pay more attention on the diseases derived membrane vesicles or exosomes for its further clinical translations.
6.2. Improving the methods of membrane extraction and coating
To acquire high purity and quality membrane, the undesired intracellular proteins or organelles should be removed clearly. However, current methods of membrane extraction or coating mainly including sonication, gradient centrifugation and mechanical extrusion are difficult to achieve it.
These may largely limit the application and novel method or technology has to be developed. For example, microfluidic sonication or microfluidic viscoelastic flows technology has displayed its unique role in high purity of extracellular vesicles sorting dependent on the size. Moreover, to meet clinical translational need, large-scale and quick production of functional membrane under a standardized operation protocol is necessary. Similar to that of previous study, present technology cannot achieve it effectively. Whereas, GMP standards for clinical use of stem cells, DCs and T cells have been built, and this would help promote high quality membrane-coated NPs production ultimately.
6.3. Displaying multifunctional protein on the cell membrane
Membrane proteins are of crucial importance to maintain cells signaling communications and adhesion, and mediate the targeting and drug delivery efficacy of bioinspired membrane-coated nanoparticles. Nonetheless, during the process of cell lysis, sonication, centrifugation and extrusion, some key proteins or membrane uniformity would be damaged. To better target the disease-related sites, the functional proteins may help it improve targeting ability or enhance therapeutic efficacy, and surface engineering or modifying cell membrane is indispensable. Chemical modification and gene engineering are the two common protocols, but it often brings about unknown risks or worries for clinic translation. Therefore, how to produce quality controllable, cost-effective and reproducible is meaningful. At present, the production of the approved immunotherapies including DC-based vaccines107,108 and CAR-T cells109,110 may give us a hint to improve or develop novel modification methods for achieving clinical requirements.
7. Conclusions
Bioinspired membrane coating nanoplatform, as a nano-immunomodulator, which inherits both cell membrane properties and nanomaterial functional versatilities for targeted immunotherapy, offers new therapeutic strategy for immune-related diseases. Compared with liposomes or other synthetic vehicles, cell membrane systems hold superior disease-targeting ability through modulating the interactions between the nanoparticle surface membrane proteins and the infiltrating immune cells, hence we could take advantage of the payload release at the immune microenvironment. Due to the unique biocompatibility, cell membranes have been extended in immunologic adjuvant delivery (e.g., CpG and alum nanoparticles), antivirus, antibacterial infection, and autoimmune diseases therapy. Because of the fact that the entire cell membrane is translocated onto the surface of synthetic inner cores, it dutifully preserves the membrane biofunctions, including cytokines binding, immune evasion and disease targeting. Moreover, cell membrane can be further engineered to express or display our desired receptors or proteins to enhance immune modulation and acquire a better immunotherapy. There is still much space for improving understanding the mechanisms of complexed immune microenvironment, and advancing techniques to precisely manipulate bioinspired membrane-based system for eliciting multiantigen antiviral and antibacterial immune response, and alleviating autoimmune response. Considering the intricacy and heterogeneity of the patient-personalized immune microenvironment, and its resultant immune cellular interactions, it would be encouragingly rewarding yet challenging for the translation of bioinspired membrane-coated nano-immunoregulators into clinics in the future. Hopefully, increasing attention will be paid to the development of bioinspired membrane-coated nanoplatform, and these can largely promote present techniques and would provide significant changes for clinical management of infectious diseases and autoimmune diseases.
Acknowledgments
This work was supported by the Major State Basic Research Development Program of China (2017YFA0205201), the National Natural Science Foundation of China (81925019, 81901876, 81801817, 81603015 and U1705281), the Fundamental Research Funds for the Central Universities (20720190138, 20720190088 and 20720200019, China), Medical and Health Key project of Xiamen (3502Z20191106 and 2020Y4003, China), the Program for New Century Excellent Talents in University, China (NCET-13-0502) and China Postdoctoral Science Foundation Funded Project (K6419001, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Yuan Liu, Email: liuyuan@xmu.edu.cn.
Gang Liu, Email: gangliu.cmitm@xmu.edu.cn.
Zhongning Lin, Email: linzhn@xmu.edu.cn.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Fugger L., Jensen L.T., Rossjohn J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell. 2020;181:63–80. doi: 10.1016/j.cell.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makabenta J.M.V., Nabawy A., Li C.H., Schmidt-Malan S., Patel R., Rotello V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol. 2021;19:23–36. doi: 10.1038/s41579-020-0420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy M., Kolodziejczyk A.A., Thaiss C.A., Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17:219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 5.Carapetis J.R., Beaton A., Cunningham M.W., Guilherme L., Karthikeyan G., Mayosi B.M., et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. 2016;2:15084. doi: 10.1038/nrdp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q.H., Zhang Y.F., Wu L.L., Niu S., Song C.L., Zhang Z.Y., et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., et al. Targets of T Cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiskopf D., Schmitz K.S., Raadsen M.P., Grifoni A., Okba N.M.A., Endeman H., et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5:eabd2071. doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Handel A.E., Irani S.R., Holländer G.A. The role of thymic tolerance in CNS autoimmune disease. Nat Rev Neurol. 2018;14:723–734. doi: 10.1038/s41582-018-0095-7. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y.H., Yu M., Zheng Y.W., Fu G.P., Xin G., Zhu W., et al. CXCR5+PD-1+ follicular helper CD8 T cells control B cell tolerance. Nat Commun. 2019;10:4415. doi: 10.1038/s41467-019-12446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin K., Luo Z.M., Zhang B., Pang Z.Q. Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sin B. 2018;8:23–33. doi: 10.1016/j.apsb.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peacock S.J., Paterson G.K. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu Rev Biochem. 2015;84:577–601. doi: 10.1146/annurev-biochem-060614-034516. [DOI] [PubMed] [Google Scholar]
- 13.Buch M.H. Defining refractory rheumatoid arthritis. Ann Rheum Dis. 2018;77:966–969. doi: 10.1136/annrheumdis-2017-212862. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q.Z., Dehaini D., Zhang Y., Zhou J., Chen X.Y., Zhang L.F., et al. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat Nanotechnol. 2018;13:1182–1190. doi: 10.1038/s41565-018-0254-4. [DOI] [PubMed] [Google Scholar]
- 15.Li R.X., He Y.W., Zhu Y., Jiang L.X., Zhang S.Y., Qin J., et al. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 2019;19:124–134. doi: 10.1021/acs.nanolett.8b03439. [DOI] [PubMed] [Google Scholar]
- 16.Hirota K., Hashimoto M., Ito Y., Matsuura M., Ito H., Tanaka M., et al. Autoimmune Th17 cells induced synovial stromal and innate lymphoid cell secretion of the cytokine GM-CSF to initiate and augment autoimmune arthritis. Immunity. 2018;48:1220–1232. doi: 10.1016/j.immuni.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J.Y., Hall J.A., Kroehling L., Wu L., Najar T., Nguyen H.H., et al. Serum amyloid proteins induce pathogenic Th17 cells and promote inflammatory disease. Cell. 2020;180:79–91. doi: 10.1016/j.cell.2019.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nehar-Belaid D., Hong S., Marches R., Chen G., Bolisetty M., Baisch J., et al. Mapping systemic lupus erythematosus heterogeneity at the single-cell level. Nat Immunol. 2020;21:1094–1106. doi: 10.1038/s41590-020-0743-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L.H., Wang P.F., Nair M.S., Yu J., Rapp M., Wang Q., et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 20.Luk B.T., Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release. 2015;220:600–607. doi: 10.1016/j.jconrel.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu C.M., Zhang L., Aryal S., Cheung C., Fang R.H., Zhang L. Erythrocyte membrane camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z., Zhao P.F., Luo Z.Y., Zheng M.B., Tian H., Gong P., et al. Cancer cell membrane biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10:10049–10057. doi: 10.1021/acsnano.6b04695. [DOI] [PubMed] [Google Scholar]
- 23.Zou M.Z., Liu W.L., Gao F., Bai X.F., Chen H.S., Zeng X., et al. Artificial natural killer cells for specific tumor inhibition and renegade macrophage re-education. Adv Mater. 2019;31:e1904495. doi: 10.1002/adma.201904495. [DOI] [PubMed] [Google Scholar]
- 24.Lv P., Liu X., Chen X.M., Liu C., Zhang Y., Chu C.C., et al. Genetically engineered cell membrane nanovesicles for oncolytic adenovirus delivery: a versatile platform for cancer virotherapy. Nano Lett. 2019;19:2993–3001. doi: 10.1021/acs.nanolett.9b00145. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Chen Y.J., Lo C., Zhuang J., Angsantikul P., Zhang Q.Z., et al. Inhibition of pathogen adhesion by bacterial outer membrane-coated nanoparticles. Angew Chem Int Ed Engl. 2019;58:11404–11408. doi: 10.1002/anie.201906280. [DOI] [PubMed] [Google Scholar]
- 26.Cao Z.P., Cheng S.S., Wang X.Y., Pang Y., Liu J.Y. Camouflaging bacteria by wrapping with cell membranes. Nat Commun. 2019;10:3452. doi: 10.1038/s41467-019-11390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M., Li S.Y., Zhou H., Tang X.F., Wu Y., Jiang W., et al. Chemotaxis-driven delivery of nano-pathogenoids for complete eradication of tumors post-phototherapy. Nat Commun. 2020;11:1126. doi: 10.1038/s41467-020-14963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yong T.Y., Zhang X.Q., Bie N.N., Zhang H.B., Zhang X.T., Li F.Y., et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. 2019;10:3838. doi: 10.1038/s41467-019-11718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ha D., Yang N.N., Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riazifar M., Mohammadi M.R., Pone E.J., Yeri A., Lässer C., Segaliny A.I., et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. 2019;13:6670–6688. doi: 10.1021/acsnano.9b01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang R.H., Kroll A.V., Gao W.W., Zhang L.F. Cell membrane coating nanotechnology. Adv Mater. 2018;30:e1706759. doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan H.Z., Shao D., Lao Y.H., Li M.Q., Hu H.Z., Leong K.W. Engineering cell membrane-based nanotherapeutics to target inflammation. Adv Sci. 2019;6:1900605. doi: 10.1002/advs.201900605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu H.H., Jiang X.C., Li Y.S., Zhou Y., Zhang T.Y., Zhi P., et al. Engineering stem cell derived biomimetic vesicles for versatility and effective targeted delivery. Adv Funct Mater. 2020;30:2006169. [Google Scholar]
- 34.Zhang P.F., Zhang L., Qin Z.N., Hua S.H., Guo Z.D., Chu C.C., et al. Genetically engineered liposome-like nanovesicles as active targeted transport platform. Adv Mater. 2018;30:1705350. doi: 10.1002/adma.201705350. [DOI] [PubMed] [Google Scholar]
- 35.Liu C., Guo J.Y., Tian F., Yang N., Yan F.S., Ding Y.P., et al. Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano. 2017;11:6968–6976. doi: 10.1021/acsnano.7b02277. [DOI] [PubMed] [Google Scholar]
- 36.Liu C., Zhang W., Li Y.K., Chang J.Q., Tian F., Zhao F.H., et al. Microfluidic sonication to assemble exosome membrane-coated nanoparticles for immune evasion-mediated targeting. Nano Lett. 2019;19:7836–7844. doi: 10.1021/acs.nanolett.9b02841. [DOI] [PubMed] [Google Scholar]
- 37.Rao L., Cai B., Bu L.L., Liao Q.Q., Guo S.S., Zhao X.Z., et al. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano. 2017;11:3496–3505. doi: 10.1021/acsnano.7b00133. [DOI] [PubMed] [Google Scholar]
- 38.Xu J., Saklatvala R., Mittal S., Deshmukh S., Procopio A. Recent progress of potentiating immune checkpoint blockade with external stimuli—an industry perspective. Adv Sci. 2020;7:1903394. doi: 10.1002/advs.201903394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuerban K., Gao X.W., Zhang H., Liu J.Y., Dong M.X., Wu L., et al. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm Sin B. 2020;10:1534–1548. doi: 10.1016/j.apsb.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu G.N., Zhao X., Zhang Y.L., Xu J.C., Xu J.Q., Li Y., et al. Engineering biomimetic platesomes for pH-responsive drug delivery and enhanced antitumor activity. Adv Mater. 2019;31:e1900795. doi: 10.1002/adma.201900795. [DOI] [PubMed] [Google Scholar]
- 41.Zanella I., König E., Tomasi M., Gagliardi A., Frattini L., Fantappiè L., et al. Proteome-minimized outer membrane vesicles from Escherichia coli as a generalized vaccine platform. J Extracell Vesicles. 2021;10:e12066. doi: 10.1002/jev2.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi Y., Xie F., Rao P., Qian H., Chen R., Chen H., et al. TRAIL-expressing cell membrane nanovesicles as an anti-inflammatory platform for rheumatoid arthritis therapy. J Control Release. 2020;320:304–313. doi: 10.1016/j.jconrel.2020.01.054. [DOI] [PubMed] [Google Scholar]
- 43.Rosenblum M.D., Gratz I.K., Paw J.S., Abbas A.K. Treating human autoimmunity: current practice and future prospects. Sci Transl Med. 2012;4:125sr1. doi: 10.1126/scitranslmed.3003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller S.D., Turley D.M., Podojil J.R. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 45.Guo J., Zhang T., Cao H., Li X., Liang H., Liu M., et al. Sequencing of the MHC region defines HLA-DQA1 as the major genetic risk for seropositive rheumatoid arthritis in Han Chinese population. Ann Rheum Dis. 2019;78:773–780. doi: 10.1136/annrheumdis-2018-214725. [DOI] [PubMed] [Google Scholar]
- 46.Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 47.Wright H.L., Moots R.J., Edwards S.W. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10:593–601. doi: 10.1038/nrrheum.2014.80. [DOI] [PubMed] [Google Scholar]
- 48.Copp J.A., Fang R.H., Luk B.T., Hu C.M., Gao W., Zhang K., et al. Clearance of pathological antibodies using biomimetic nanoparticles. Proc Natl Acad Sci U S A. 2014;111:13481–13486. doi: 10.1073/pnas.1412420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei X.L., Gao J., Fang R.H., Luk B.T., Kroll A.V., Dehaini D., et al. Nanoparticles camouflaged in platelet membrane coating as an antibody decoy for the treatment of immune thrombocytopenia. Biomaterials. 2016;111:116–123. doi: 10.1016/j.biomaterials.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McClure M., Gopaluni S., Jayne D., Jones R. B cell therapy in ANCA-associated vasculitis: current and emerging treatment options. Nat Rev Rheumatol. 2018;14:580–591. doi: 10.1038/s41584-018-0065-x. [DOI] [PubMed] [Google Scholar]
- 51.Serra P., Santamaria P. Antigen-specific therapeutic approaches for autoimmunity. Nat Biotechnol. 2019;37:238–251. doi: 10.1038/s41587-019-0015-4. [DOI] [PubMed] [Google Scholar]
- 52.Knier B., Hiltensperger M., Sie C., Aly L., Lepennetier G., Engleitner T., et al. Myeloid-derived suppressor cells control B cell accumulation in the central nervous system during autoimmunity. Nat Immunol. 2018;19:1341–1351. doi: 10.1038/s41590-018-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnas J.L., Looney R.J., Anolik J.H. B cell targeted therapies in autoimmune disease. Curr Opin Immunol. 2019;61:92–99. doi: 10.1016/j.coi.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dörner T., Posch M.G., Li Y., Petricoul O., Cabanski M., Milojevic J.M., et al. Treatment of primary Sjögren’s syndrome with ianalumab (VAY736) targeting B cells by BAFF receptor blockade coupled with enhanced, antibody-dependent cellular cytotoxicity. Ann Rheum Dis. 2019;78:641–647. doi: 10.1136/annrheumdis-2018-214720. [DOI] [PubMed] [Google Scholar]
- 55.Haacke E.A., Bootsma H., Spijkervet F.K.L., Visser A., Vissink A., Kluin P.M., et al. FcRL4+ B-cells in salivary glands of primary Sjögren's syndrome patients. J Autoimmun. 2017;81:90–98. doi: 10.1016/j.jaut.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 56.Kadavath S., Bobic S., Efthimiou P. Use of B lymphocyte stimulator inhibitor belimumab may be associated with a decrease in the serum concentration of epidermal growth factor in patients with primary Sjögren's syndrome. Clin Rheumatol. 2015;34:1651–1652. doi: 10.1007/s10067-015-2872-7. [DOI] [PubMed] [Google Scholar]
- 57.Luk B.T., Jiang Y., Copp J.A., Hu C.J., Krishnan N., Gao W., et al. Biomimetic targeting of nanoparticles to immune cell subsets via cognate antigen interactions. Mol Pharm. 2018;15:3723–3728. doi: 10.1021/acs.molpharmaceut.8b00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li R., Patterson K.R., Bar-Or A. Reassessing B cell contributions in multiple sclerosis. Nat Immunol. 2018;19:696–707. doi: 10.1038/s41590-018-0135-x. [DOI] [PubMed] [Google Scholar]
- 59.Dong Y., Yong V.W. When encephalitogenic T cells collaborate with microglia in multiple sclerosis. Nat Rev Neurol. 2019;15:704–717. doi: 10.1038/s41582-019-0253-6. [DOI] [PubMed] [Google Scholar]
- 60.Lodygin D., Hermann M., Schweingruber N., Flügel-Koch C., Watanabe T., Schlosser C., et al. β-Synuclein-reactive T cells induce autoimmune CNS grey matter degeneration. Nature. 2019;566:503–508. doi: 10.1038/s41586-019-0964-2. [DOI] [PubMed] [Google Scholar]
- 61.Li Z.J., Liu F., He X., Yang X., Shan F.P., Feng J. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int Immunopharmacol. 2019;67:268–280. doi: 10.1016/j.intimp.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Casella G., Colombo F., Finardi A., Descamps H., Ill-Raga G., Spinelli A., et al. Extracellular vesicles containing IL-4 modulate neuroinflammation in a mouse model of multiple sclerosis. Mol Ther. 2018;26:2107–2118. doi: 10.1016/j.ymthe.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lundgren J.D., Babiker A.G., Gordin F., Emery S., Grund B., Sharma S., et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan R.H., Zhang Y.Y., Li Y.N., Xia L., Guo Y.Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 67.Rao L., Xia S., Xu W., Tian R., Yu G.C., Gu C.J., et al. Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines. Proc Natl Acad Sci U S A. 2020;117:27141–27147. doi: 10.1073/pnas.2014352117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei X.L., Zhang G., Ran D.N., Krishnan N., Fang R.H., Gao W., et al. T-cell-mimicking nanoparticles can neutralize HIV infectivity. Adv Mater. 2018;30:e1802233. doi: 10.1002/adma.201802233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barnett D., Walker B., Landay A., Denny T.N. CD4 immunophenotyping in HIV infection. Nat Rev Microbiol. 2008;6:S7–S15. doi: 10.1038/nrmicro1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doitsh G., Greene W.C. Dissecting how CD4 T cells are lost during HIV infection. Cell Host Microbe. 2016;19:280–291. doi: 10.1016/j.chom.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bronshtein T., Toledano N., Danino D., Pollack S., Machluf M. Cell derived liposomes expressing CCR5 as a new targeted drug-delivery system for HIV infected cells. J Control Release. 2011;151:139–148. doi: 10.1016/j.jconrel.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 72.Nie C.X., Parshad B., Bhatia S., Cheng C., Stadtmüller M., Oehrl A., et al. Topology matching design of an influenza-neutralizing spiky nanoparticle-based inhibitor with a dual mode of action. Angew Chem Int Ed Engl. 2020;59:15532–15536. doi: 10.1002/anie.202004832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nie C., Stadtmüller M., Parshad B., Wallert M., Ahmadi V., Kerkhoff Y., et al. Heteromultivalent topology-matched nanostructures as potent and broad-spectrum influenza A virus inhibitors. Sci Adv. 2021;7:eabd3803. doi: 10.1126/sciadv.abd3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quan L., Ji C., Ding X., Peng Y., Liu M., Sun J., et al. Cluster-transition determining sites underlying the antigenic evolution of seasonal influenza viruses. Mol Biol Evol. 2019;36:1172–1186. doi: 10.1093/molbev/msz050. [DOI] [PubMed] [Google Scholar]
- 75.Chen H.W., Fang Z.S., Chen Y.T., Chen Y.I., Yao B.Y., Cheng J.Y., et al. Targeting and enrichment of viral pathogen by cell membrane cloaked magnetic nanoparticles for enhanced detection. ACS Appl Mater Interfaces. 2017;9:39953–39961. doi: 10.1021/acsami.7b09931. [DOI] [PubMed] [Google Scholar]
- 76.Katzelnick L.C., Narvaez C., Arguello S., Lopez Mercado B., Collado D., Ampie O., et al. Zika virus infection enhances future risk of severe dengue disease. Science. 2020;369:1123–1128. doi: 10.1126/science.abb6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giraldo M.I., Xia H., Aguilera-Aguirre L., Hage A., van Tol S., Shan C., et al. Envelope protein ubiquitination drives entry and pathogenesis of Zika virus. Nature. 2020;585:414–419. doi: 10.1038/s41586-020-2457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rao L., Wang W.B., Meng Q.F., Tian M.F., Cai B., Wang Y.C., et al. A biomimetic nanodecoy traps Zika virus to prevent viral infection and fetal microcephaly development. Nano Lett. 2019;19:2215–2222. doi: 10.1021/acs.nanolett.8b03913. [DOI] [PubMed] [Google Scholar]
- 79.Shi Y., Zheng M. Hepatitis B virus persistence and reactivation. BMJ. 2020;370:m2200. doi: 10.1136/bmj.m2200. [DOI] [PubMed] [Google Scholar]
- 80.Revill P.A., Tu T., Netter H.J., Yuen L.K.W., Locarnini S.A., Littlejohn M. The evolution and clinical impact of hepatitis B virus genome diversity. Nat Rev Gastroenterol Hepatol. 2020;17:618–634. doi: 10.1038/s41575-020-0296-6. [DOI] [PubMed] [Google Scholar]
- 81.Liu X., Yuan L.Z., Zhang L., Mu Y.L., Li X.L., Liu C., et al. Bioinspired artificial nanodecoys for Hepatitis B virus. Angew Chem Int Ed Engl. 2018;57:12499–12503. doi: 10.1002/anie.201807212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang C., Wang S.B., Chen Y., Zhao J.Q., Han S.L., Zhao G.M., et al. Membrane nanoparticles derived from ACE2-rich cells block SARS-CoV-2 infection. ACS Nano. 2021;15:6340–6351. doi: 10.1021/acsnano.0c06836. [DOI] [PubMed] [Google Scholar]
- 83.Bonam S.R., Kotla N.G., Bohara R.A., Rochev Y., Webster T.J., Bayry J. Potential immuno-nanomedicine strategies to fight COVID-19 like pulmonary infections. Nano Today. 2021;36:101051. doi: 10.1016/j.nantod.2020.101051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Q.Z., Honko A., Zhou J.R., Gong H., Downs S.N., Vasquez J.H., et al. Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett. 2020;20:5570–5574. doi: 10.1021/acs.nanolett.0c02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Strickley J.D., Messerschmidt J.L., Awad M.E., Li T., Hasegawa T., Ha D.T., et al. Immunity to commensal papillomaviruses protects against skin cancer. Nature. 2019;575:519–522. doi: 10.1038/s41586-019-1719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roden R.B.S., Stern P.L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer. 2018;18:240–254. doi: 10.1038/nrc.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang P.F., Chen Y.X., Zeng Y., Shen C.G., Li R., Guo Z.D., et al. Virus-mimetic nanovesicles as a versatile antigen-delivery system. Proc Natl Acad Sci U S A. 2015;112:E6129–E6138. doi: 10.1073/pnas.1505799112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He Y.W., Li R.X., Li H.C., Zhang S.Y., Dai W.T., Wu Q., et al. Erythroliposomes: integrated hybrid nanovesicles composed of erythrocyte membranes and artificial lipid membranes for pore-forming toxin clearance. ACS Nano. 2019;13:4148–4159. doi: 10.1021/acsnano.8b08964. [DOI] [PubMed] [Google Scholar]
- 89.Palmela C., Chevarin C., Xu Z., Torres J., Sevrin G., Hirten R., et al. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut. 2018;67:574–587. doi: 10.1136/gutjnl-2017-314903. [DOI] [PubMed] [Google Scholar]
- 90.Chen Y.J., Zhang Y., Chen M.C., Zhuang J., Fang R.H., Gao W.W., et al. Biomimetic nanosponges suppress in vivo lethality induced by the whole secreted proteins of pathogenic bacteria. Small. 2019;15:e1804994. doi: 10.1002/smll.201804994. [DOI] [PubMed] [Google Scholar]
- 91.David M.Z., Daum R.S. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thamphiwatana S., Angsantikul P., Escajadillo T., Zhang Q., Olson J., Luk B.T., et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc Natl Acad Sci U S A. 2017;114:11488–11493. doi: 10.1073/pnas.1714267114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Irene C., Fantappiè L., Caproni E., Zerbini F., Anesi A., Tomasi M., et al. Bacterial outer membrane vesicles engineered with lipidated antigens as a platform for Staphylococcus aureus vaccine. Proc Natl Acad Sci U S A. 2019;116:21780–21788. doi: 10.1073/pnas.1905112116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao W.W., Fang R.H., Thamphiwatana S., Luk B.T., Li J.M., Angsantikul P., et al. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015;15:1403–1409. doi: 10.1021/nl504798g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wei X.L., Ran D.N., Campeau A., Xiao C., Zhou J.R., Dehaini D., et al. Multiantigenic nanotoxoids for antivirulence vaccination against antibiotic-resistant gram-negative bacteria. Nano Lett. 2019;19:4760–4769. doi: 10.1021/acs.nanolett.9b01844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu C.M., Fang R.H., Luk B.T., Zhang L.F. Nanoparticle-detained toxins for safe and effective vaccination. Nat Nanotechnol. 2013;8:933–938. doi: 10.1038/nnano.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pang X., Liu X., Cheng Y., Zhang C., Ren E., Liu C., et al. Sono-immunotherapeutic nanocapturer to combat multidrug-resistant bacterial infections. Adv Mater. 2019;31:e1902530. doi: 10.1002/adma.201902530. [DOI] [PubMed] [Google Scholar]
- 98.Surewaard B.G.J., Thanabalasuriar A., Zeng Z., Tkaczyk C., Cohen T.S., Bardoel B.W., et al. α-Toxin induces platelet aggregation and liver injury during Staphylococcus aureus sepsis. Cell Host Microbe. 2018;24:271–284. doi: 10.1016/j.chom.2018.06.017. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Claushuis T.A.M., de Vos A.F., Nieswandt B., Boon L., Roelofs J., de Boer O.J., et al. Platelet glycoprotein VI aids in local immunity during pneumonia-derived sepsis caused by gram-negative bacteria. Blood. 2018;131:864–876. doi: 10.1182/blood-2017-06-788067. [DOI] [PubMed] [Google Scholar]
- 100.Hu C.M., Fang R.H., Wang K.C., Luk B.T., Thamphiwatana S., Dehaini D., et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526:118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rao L., Yu G.T., Meng Q.F., Bu L.L., Tian R., Lin L.S., et al. Cancer cell membrane-coated nanoparticles for personalized therapy in patient-derived xenograft models. Adv Funct Mater. 2019;29:1905671. [Google Scholar]
- 102.Sun Y.X., Shi H., Yin S.Q., Ji C., Zhang X., Zhang B., et al. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano. 2018;12:7613–7628. doi: 10.1021/acsnano.7b07643. [DOI] [PubMed] [Google Scholar]
- 103.Gao Y.F., Zhang H., Zhou N.N., Xu P.W., Wang J.X., Gao Y., et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat Biomed Eng. 2020;4:743–753. doi: 10.1038/s41551-020-0583-0. [DOI] [PubMed] [Google Scholar]
- 104.Zhao Y., Wang Z.H., Jiang Y.N., Liu H., Song S.L., Wang C.Y., et al. Biomimetic composite scaffolds to manipulate stem cells for aiding rheumatoid arthritis management. Adv Funct Mater. 2019;29:1807860. [Google Scholar]
- 105.Song H.Y., Li X.Q., Zhao Z.C., Qian J., Wang Y., Cui J., et al. Reversal of osteoporotic activity by endothelial cell-secreted bone targeting and biocompatible exosomes. Nano Lett. 2019;19:3040–3048. doi: 10.1021/acs.nanolett.9b00287. [DOI] [PubMed] [Google Scholar]
- 106.Khan M., Nickoloff E., Abramova T., Johnson J., Verma S.K., Krishnamurthy P., et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117:52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hackstein H., Morelli A.E., Thomson A.W. Designer dendritic cells for tolerance induction: guided not misguided missiles. Trends Immunol. 2001;22:437–442. doi: 10.1016/s1471-4906(01)01959-7. [DOI] [PubMed] [Google Scholar]
- 108.Fu Y.L., Zhan X.X., Wang Y.C., Jiang X.B., Liu M., Yang Y.L., et al. NLRC3 expression in dendritic cells attenuates CD4(+) T cell response and autoimmunity. Embo J. 2019;38:e101397. doi: 10.15252/embj.2018101397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang B., Wang Y., Yuan Y.S., Sun J.Q., Liu L.L., Huang D., et al. In vitro elimination of autoreactive B cells from rheumatoid arthritis patients by universal chimeric antigen receptor T cells. Ann Rheum Dis. 2021;80:176–184. doi: 10.1136/annrheumdis-2020-217844. [DOI] [PubMed] [Google Scholar]
- 110.Ellebrecht C.T., Bhoj V.G., Nace A., Choi E.J., Mao X., Cho M.J., et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]