Abstract
Without cesarean delivery, obstructed labor due to a disproportion of the fetus and the maternal birth canal can result in maternal and fetal injuries or even death. The precise frequency of obstructed labor is difficult to estimate because of the widespread use of cesarean delivery for indications other than proven cephalopelvic disproportion, but it has been estimated that at least one million mothers per year are affected by this disorder worldwide. Why is the fit between the fetus and the maternal pelvis so tight? Why did evolution not lead to a greater safety margin, as in other primates? Here, we review current research and suggest new hypotheses on the evolution of human childbirth and pelvic morphology. In 1960, Washburn suggested that this “obstetrical dilemma” arose because the human pelvis is an evolutionary compromise between two functions, bipedal gait and childbirth. However, recent biomechanical and kinematic studies indicate that pelvic width does not considerably affect the efficiency of bipedal gait and thus is unlikely to have constrained the evolution of a wider birth canal. Instead, bipedalism may have primarily constrained the flexibility of the pubic symphysis during pregnancy, which opens much wider in most mammals with larger fetuses than in humans. We argue that the birth canal is mainly constrained by the trade-off between two pregnancy-related functions: while a narrow pelvis is disadvantageous for childbirth, it offers better support for the weight exerted by the viscera and the large human fetus during the long gestation period. We discuss the implications of this hypothesis for understanding pelvic floor dysfunction. Furthermore, we propose that selection for a narrow pelvis has also acted in males due to the role of pelvic floor musculature in erectile function. Finally, we review the cliff-edge model of obstetric selection to explain why evolution cannot completely eliminate cephalopelvic disproportion. This model also predicts that the regular application of life-saving cesarean has evolutionary increased rates of cephalopelvic disproportion already. We address how evolutionary models contribute to understanding and decision-making in obstetrics and gynecology as well as in devising health care policies.
Keywords: arrest of descent, arrest of dilatation, bipedalism, cephalopelvic disproportion, cesarean delivery, cesarean section, cliff-edge model, encephalization, erectile dysfunction, evolution, failure to progress in labor, fecal incontinence, fetal head, fistula, Homo erectus, mismatch, parturition, pelvic dimensions, pelvic dimorphism, pelvic floor disorder, prolapse, pelvic inlet, starvation during pregnancy, symphysis pubis, urinary incontinence, uterine prolapse, uterine rupture, vaginal prolapse
Cephalopelvic disproportion and obstructed labor
Human childbirth is substantially more difficult than that of most other primate species, owing primarily to the close match between the maternal birth canal and the fetal head1 (Figure 1). As a consequence of this close fit, even small variation of maternal and fetal dimensions in human populations leads to a considerable rate of cephalo-pelvic disproportion (CPD) and obstructed labor. Where cesarean delivery is not easily available, particularly in developing regions of the Global South (Africa, S Asia and Latin America), obstructed and prolonged labor are frequent causes of maternal morbidity and mortality. Complications can be short-term, such as uterine rupture and chorioamnionitis, or long-term, such as fistulas and incontinence2–6. Despite rich epidemiological data on obstructed labor and caesarean delivery, precise frequency of CPD is difficult to estimate. Current methods of pelvimetry are unreliable predictors of anatomical disproportion7. Apart from an arrest of descent, which is often a sign of CPD8, cesarean deliveries are frequently performed for other indications, some of which take place before an arrest due to CPD would manifest, including fetal distress, failed induction, arrest of dilatation, repeat cesarean delivery, and maternal request9,10. With this complexity in mind, reported CPD rates range from 1% to 8% of childbirths across different geographic regions11 (Source: WHO 2003). Hence, even the most conservative estimate entails about 40,000 affected births in the US and about 1.3 million worldwide every year. It is intriguing that childbirth – a process so fundamental to our species’ existence – exhibits such significant complication rates.
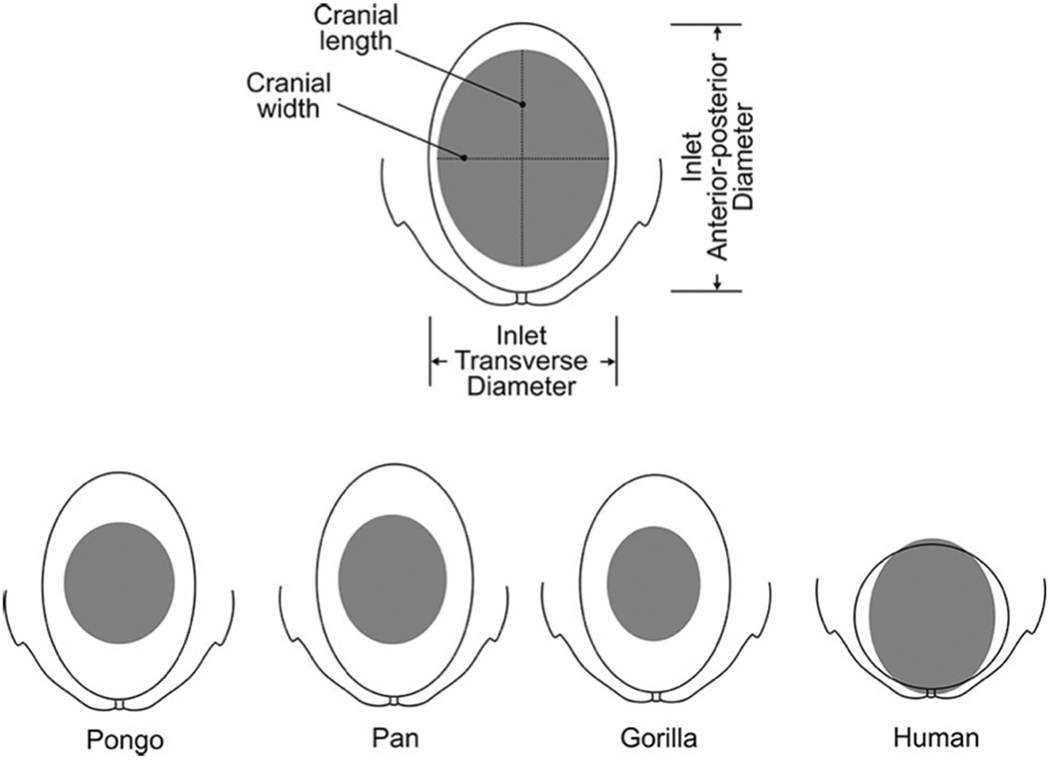
Figure 1.
Comparison of cephalopelvic proportions at the level of the pelvic inlet across closely related primates: Orangutan (Pongo), Chimpanzee (Pan), Gorilla (Gorilla) and modern humans (Homo sapiens). Humans show a very close fit between the pelvic inlet and the fetal cranium. Figure from Wittman & Wall107.
Human anatomical traits have been subject to natural selection for millions of years, and are therefore often considered to be the best fit available for a given function. Hence, evolutionary anthropologists have long asked the questions: Why is the human fetus so tightly matched to the maternal birth canal and thus so prone to birth complications? Why is there not a greater “safety margin,” as in most other primate species (Figure 1)? These long-standing evolutionary puzzles, which are of immediate relevance to obstetrics, gynecology, and public health, have received renewed attention in recent years. Here we review current theoretical and empirical research on the evolution of human pelvic form and childbirth. As several recent reviews highlighted the manifold social, cultural, psychological, and legal components underlying obstructed labor and surgical delivery12–19, we focus here on biological aspects and their evolution since the split of the human lineage from the great apes.
The conflicting effects of bipedalism and encephalization on pelvic architecture
Bipedalism (see Glossary) evolved in the human lineage 5–7 million years (myr) ago and coincided with a major remodeling of the pelvis. Approximately 2 myr later, it was followed by the massive increase in brain size in the fully bipedal genus Homo, accompanied by an increase in the size of the fetal head20.
Studying pelvic evolution throughout this time is difficult for several reasons. First, the pelvis is prone to fragmentation and thus poorly represented in the paleontological record21. Second, the pelvis is a highly integrated structure, in which selection on any one part results in changes to many others in order to preserve anatomical and functional integrity22,23. Finally, the human pelvis encountered multiple episodes of different selection pressures, each one leaving traces in the shape of the pelvis that are contingent on previous changes; this challenges the separate reconstruction of these selective forces24.
Despite these limitations, there is no doubt that the evolution of bipedalism coincided with major anatomical restructurings of the primate pelvis, as can be seen by comparing pelves of early bipedal hominids, such as australopithecines, to human-like apes (Figure 2). These modifications include the shortening and widening of the illium, the alignment of the sacrum and the pubic symphysis in a dorso-ventral plane, the broadening of the sacrum, the development of more prominent ischial spines, and a reduction of the distance between the hip joints and the vertebral column. Many of these pelvic changes relate to the muscular requirements for efficient upright locomotion and balance of the upright body as well as for support of the viscera. Pelvic changes during the transition to bipedalism also sculpted a very specific birth canal. In australopithecines, the birth canal is mediolaterally broad both at the level of the inlet and at lower levels25. Obstetric aspects likely played a minor role in the evolution of the australopithecine pelvis as their skull was relatively small24.
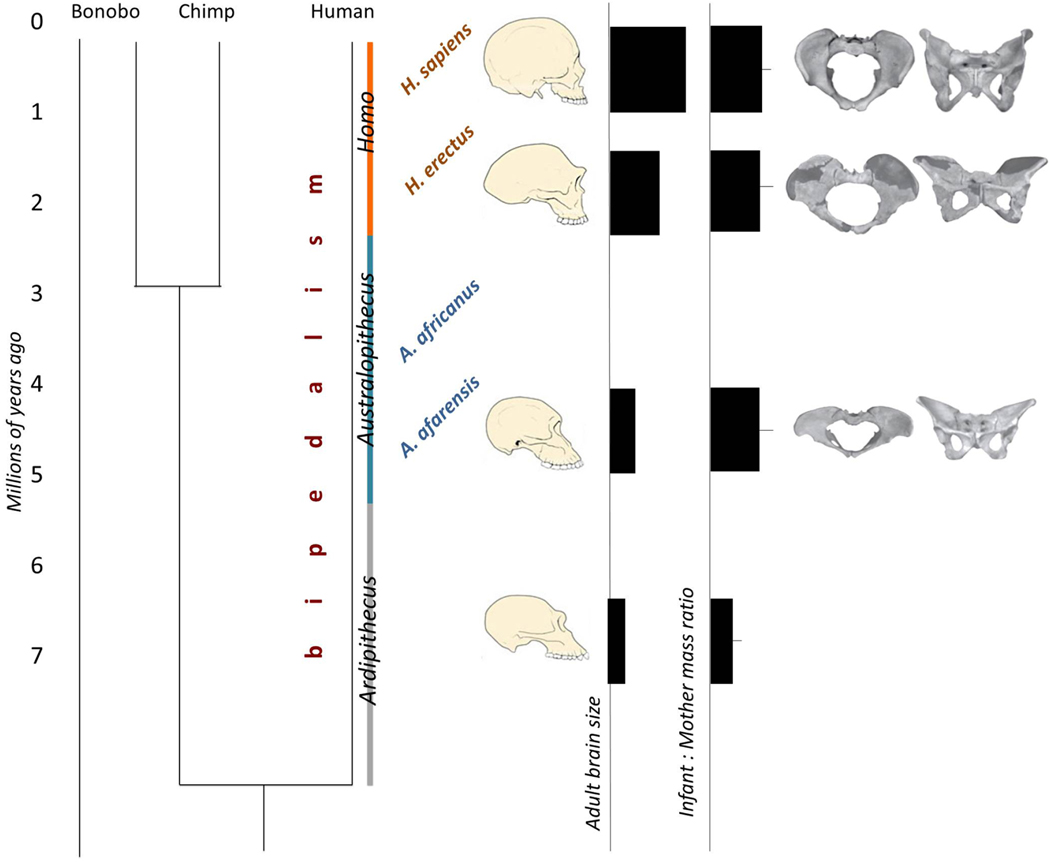
Figure 2. Timeline of human evolution, along with obstetrically relevant traits.
The phylogenetic tree (left) shows the split between the humans and the closest living relatives of the genus Pan (Bonobo and Chimpanzee). Bipedalism arose early in the human lineage and is common to the three genera, Ardipithecus, Australopithecus, and Homo, of which relatively good fossil remains have been studied. Overall body size, adult brain size, and also the neonatal body mass increased in the human lineage (middle panel). Relative size and shape of the skull reflect the encephalization in genus Homo throughout the last 2 myr. The pelvic birth canal increased in the human lineage and shifted to a gynecoid shape. Figure combined from Pontzer25,108 and Gruss & Schmitt25,108.
Several myr after bipedalism evolved, the pelvis encountered a new evolutionary challenge in the genus Homo: the gradual increase of relative brain size (encephalization, see Glossary) and, hence, also of fetal size. Evolutionary effects of encephalization on the pelvis usually are inferred from comparisons of modern humans with early representatives of the genus Homo, mostly Homo erectus. Even though the cranium of H. erectus is already substantially larger than that of Australopithecus, pelvic differences between australopithecines and H. erectus are likely confounded by cooccurring ecological and behavioral changes that are independent of encephalization, such as changes in body size, ranging behavior, and thermoregulation24. It is currently unknown when the increase in brain size started to affect pelvic form, but it is clear that this process continued after the last common ancestor (see Glossary) with H. erectus.
Relative to modern humans, H. erectus has flaring illiac blades and a medio-laterally broad (platypelloid) birth canal (Figure 2). The prevailing view is that birth-related pelvic changes within the human lineage accounted for the anterio-posterior broadening of the birth canal. Compared to H. erectus, the modern human pelvis is medio-laterally narrower, but this narrowing is relatively recent and appears to have occurred after the adaptation to changed obstetric demands. Finally, modern humans also became taller, which adds another source of strain to the pelvis by increasing the requirements for locomotory musculature and support of the inner organs.
Despite the common evolutionary history and functional demands, pelvic shape varies considerably within and across modern human populations (for the classic types see26,27; Figure 3). Studying this variation, along with its functional consequences and complications, can help to determine the manifold roles of the pelvis in locomotion, pelvic floor support, and childbirth28, as detailed below.
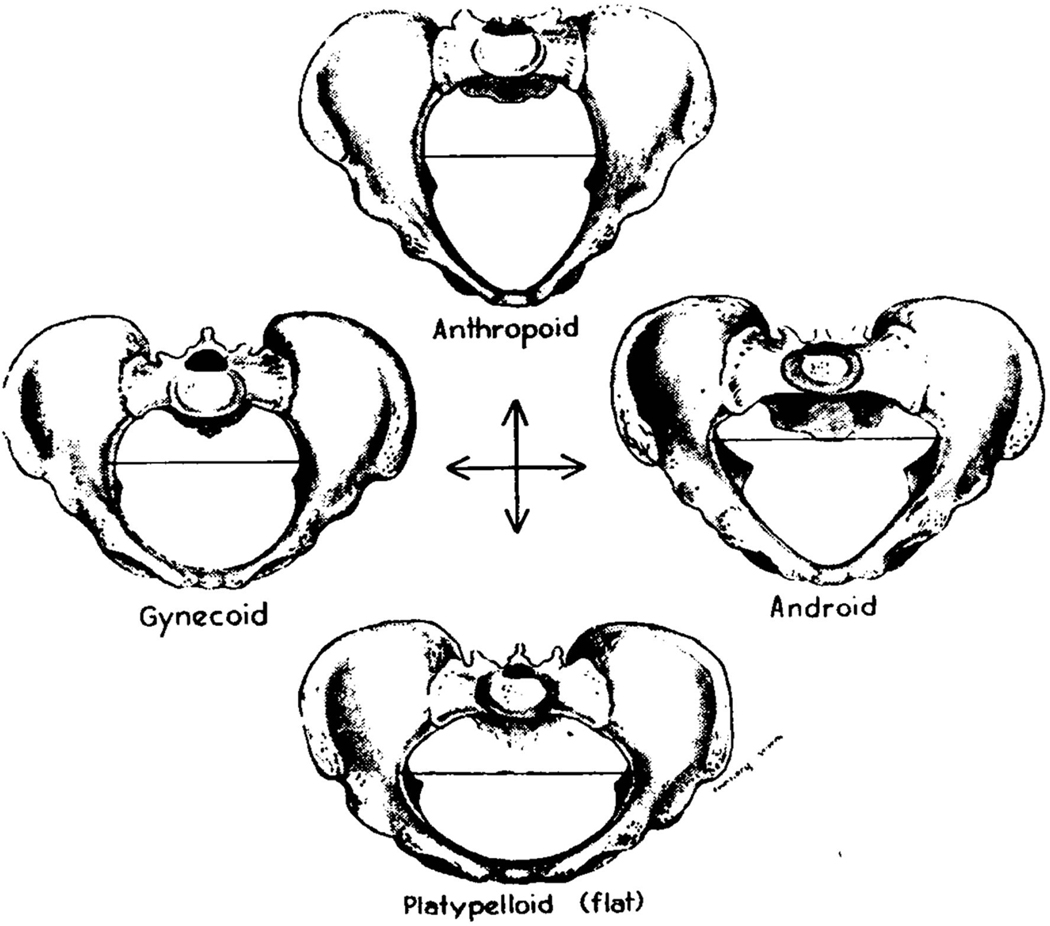
Figure 3.
The classic pelvic types with respect to the shape of pelvic canal109. The predominant type is gynecoid. Figure from Caldwell-Moloy26.
The concept of an obstetrical dilemma
In 1960, Sherwood L. Washburn introduced the term “obstetrical dilemma” in a review of the effect of tool use on human evolution29. Washburn proposed that bipedalism, while freeing hands for tool use, also resulted in the selection for a larger brain because of the increasing tool use. Eventually, this led to conflicting selection pressures on the pelvis: concurrent with the evolution of bipedalism, the size of the birth canal had been reduced relative to that of other primates, but the subsequent increase in brain size required a large birth canal. Such an opposition of selective forces is able to bring net evolutionary change in a trait to a halt; hence the term “obstetrical dilemma.” Washburn proposed that advantages gained by increased pelvic width are outweighed by biomechanical disadvantages for bipedal gait. For example, individuals with a wide pelvis would have an easier labor and delivery process; however, they would be less successful in gathering food and providing for offspring. Moreover, he reemphasized an idea suggested earlier by Portmann, that the problem of large fetuses was partially ameliorated in human evolution by giving birth at an earlier – more altricial – developmental stage, at which cranial size is smaller30,31. Whereas primates, in general, are born at an advanced (precocious) developmental stage relative to, e.g., mice, this trend secondarily reversed in the human lineage (secondary altriciality, see Glossary). Hence, human neonates are more developed than those of truly altricial animals, but less developed than those of other primates.
Washburn’s proposal has been highly influential and gained wide acceptance in the anthropological community. The details of this idea, however, have undergone further scrutiny. For simplicity, we will refer to generally “wide/broad” and “narrow” pelves in the discussion below, referring to a composite of multiple characteristics, including the width of birth canal, that make the female pelvis more or less suitable for childbirth (see Figure 4). More specific terms will be used when referring to detailed assessments of pelvic shape.
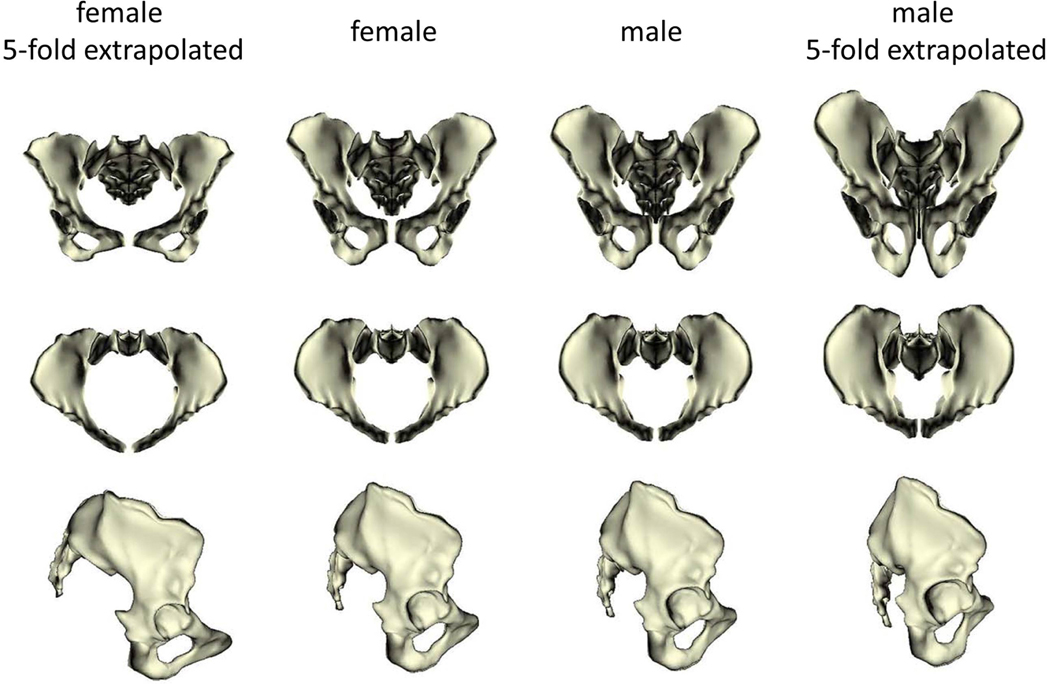
Figure 4. Pelvic sexual dimorphism.
Average male and female pelves (the two middle columns) in anterior, superior and lateral views, along with five-fold linear extrapolations of the sex differences in pelvic shape (leftmost and rightmost columns). In females the pelvic canal is more spacious, the illiac blades are shorter and reach further laterally, the subpubic angle is broader, and the sacral bone is shorter and more outward projecting than in males. Figure from Fischer & Mitteroecker32.
Clearly, a broader birth canal is beneficial for childbirth. This female-specific selection for childbirth is manifested in the strong sexual dimorphism of pelvic anatomy, specifically in the size and shape of the pelvic outlet (Figure 4). In fact, the pelvis is the most dimorphic element of the human skeleton (e.g.,32). The source of selection for a narrow pelvis, however, is less clear. Washburn proposed that further widening the pelvis would impede bipedal gait, but recent biomechanical studies did not support this hypothesis (Figure 5). Warrener et al. showed that females and males do not differ in efficiency in either walking or running on a treadmill (Figure 5)33,34. The kinematic study of Whitcome and collegaues35 showed that during walking at higher speed females translate more of the pelvic rotation into strides than males, which leads to similar efficiency of locomotion despite wider hips. Thus, instead of decreasing efficiency, a wide pelvis contributes to stride length and, particularly in individuals with shorter legs, may even be beneficial for walking36.
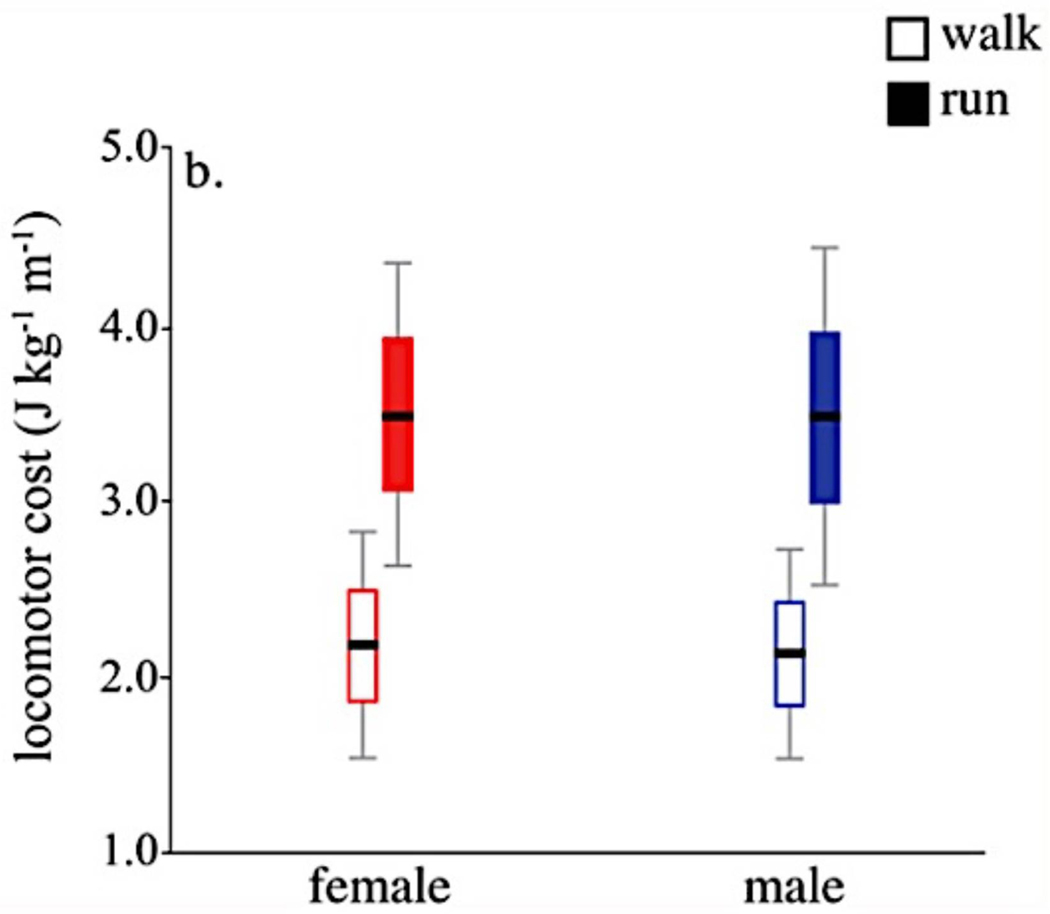
Figure 5.
The results of Warrener and colleagues34, who tested Washburn’s hypothesis that pelvis width negatively affects gait efficiency. Mass-specific locomotor costs (net volume of oxygen consumed during exercise) during walking and running on a treadmill was measured for 8 males and 7 females. Results showed no significant difference between the sexes despite wider hips in women than in men. These results suggest that bipedal gait is not be the source of selection for a narrow pelvis. Figure from Warrener & colleagues34.
These findings do not contradict the assumption that human pelvic architecture evolved in response to bipedal gait; they only show that, in modern humans, pelvic width does not considerably affect walking efficiency. This implies that walking efficiency is unlikely to be the cause for the evolutionary retention of our modern narrow pelvis; a broader pelvis, which would clearly ease childbirth, must have other functional disadvantages than only locomotion.
The importance of the pelvic floor
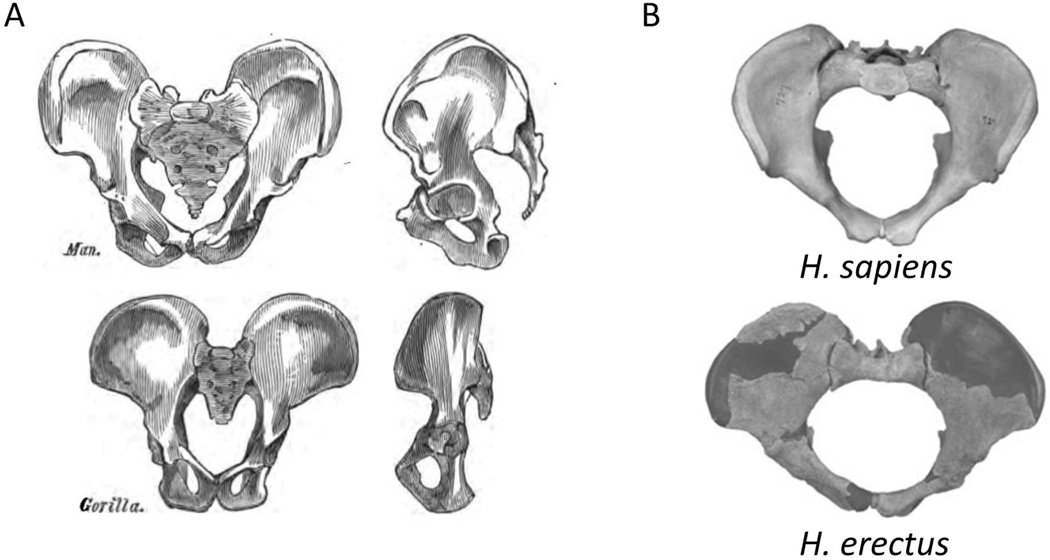
In primates, the pelvic floor musculature evolved primarily for moving the tail37, whereas in upright humans, the muscles and fasciae of the pelvic diaphragm create a horizontal pelvic floor that supports the abdominopelvic organs. It has thus been suggested that maintaining a relatively small pelvic outlet enhances this support function37. Furthermore, the ischial spines, which severely narrow the birth canal in modern humans, serve as attachments of the muscles and fasciae of the pelvic floor (Figure 6). These spines are considerably smaller and located more dorsally in quadrupeds, in which the pelvic floor is a vertical structure, and the weight of the fetus rests predominantly on the backbone37. Similarly, the sacral bone protrudes anteriorly into the birth canal in modern humans, whereas it is much shorter and posteriorly oriented in apes. After the evolution of bipedalism, the importance of pelvic support further intensified with the increase of fetal weight in the genus Homo. Indeed, the ischial spines are less prominent in early hominoids (such as H. erectus, Figure 6) than they are in the modern human pelvis37, and the modern narrow pelvis likely provides more support for the pelvic floor than the broader archaic one. This evidence suggests that a broad pelvis is more suitable for childbirth but less suitable for carrying the heavy fetus throughout a long pregnancy.
Figure 6. Evolution of pelvic floor support.
A: In the transition from quadruped to biped, the weight of the inner organs came to lie on the pelvic floor. The comparison of pelves between quadruped gorilla and bipedal human shows an increase in the size of pelvic floor-supporting structures: the ischial spines and the sacrum. B: The comparison between H. erectus and modern human pelvis shows a further enlargement of the ischial spines, concomitantly with an increase in mass and cranial size of the fetus. Figures from Huxley110 (A); and Gruss & Schmitt25 (B).
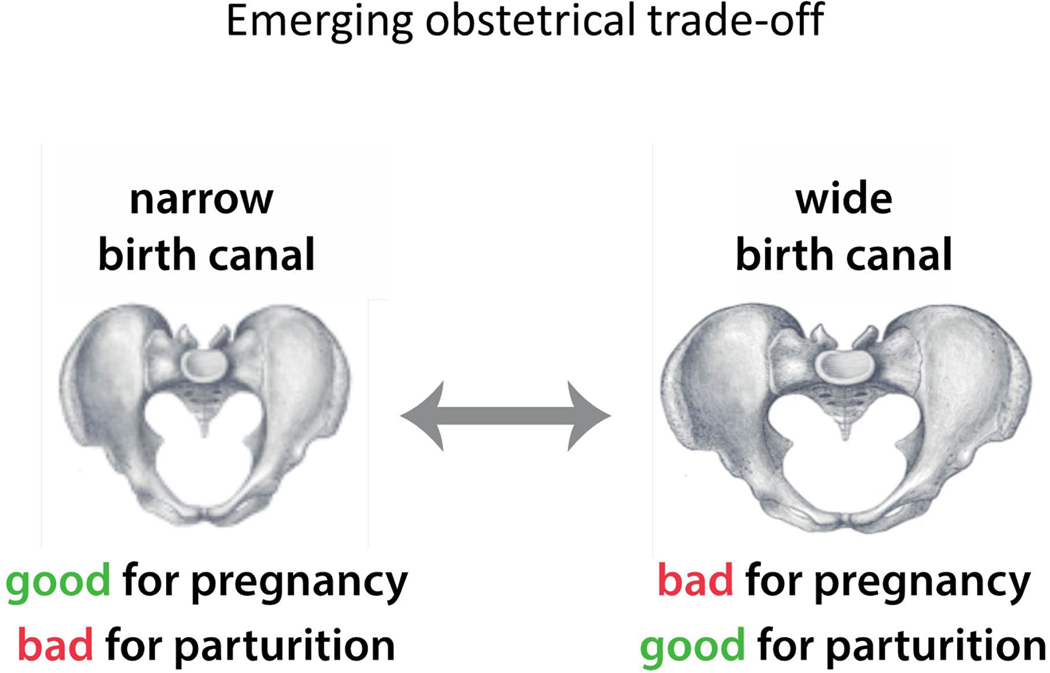
Evolutionarily, this situation intensifies the obstetric dilemma, because both carrying and childbirth are part of the same process – pregnancy (Figure 7). If gait was the major selective force for a narrow pelvis, walking efficiency might have been compensated independently by changes in leg musculature or behavior. But if pelvic floor support was the crucial driver for a narrow pelvis, such a compensation would not be possible: an evolutionary change that affects one function – either carrying or delivering the fetus – would always affect the other function, too.
Figure 7. The model of obstetric selection trade-off.
According to this model, advantages and disadvantages of a broad pelvis do not pertain to different functions, such as pregnancy and walking. Rather, they both pertain to pregnancy: while a broad pelvis is advantageous for childbirth, it is disadvantageous for supporting the large fetus. We propose that this disadvantage is limiting the broadening of the pelvis.
Pelvic floor disorders and the obstetrical dilemma
Pelvic floor disorders (PFD) are common complications associated with pregnancy in general and obstructed labor in particular. The pelvic floor is a complex structure comprised of ligaments, muscles, and fascia, arranged in several layers38. The term “pelvic floor disorders” refers to a wide range of complications, affecting different parts of pelvic floor architecture and reflecting disruptions of distinct functions39. One group of PFD arises at birth as a direct consequence of injuries to the muscles of the lower part of the pelvic floor, such as fistulas, uterine rupture, and injury to the sphincter muscles40. These injuries are frequent outcomes of prolonged labor due to CPD and thus reflect disadvantages of a narrow birth canal, specifically for passing the large fetus during parturition. According to the pelvic floor hypothesis, another group of disorders is expected to originate from strain to the muscles and connective tissue while carrying the heavy fetus. This strain is more likely to result in complications such as late-onset weakness of ligaments, associated with pelvic floor prolapse and incontinence, as a long-term consequence of multiple pregnancies (although acute injury can also cause these disorders). The strain due to carrying is exacerbated when the bony pelvis offers little support and the pelvic floor is weak. Thus, both a wide pelvis during carrying the fetus as well as a narrow pelvis during birthing can result in the same general group of disorders (PFD).
Studies addressing the association between PFD and pelvic anatomy often pool different PFD to obtain sufficient sample size, but this challenges the detection of associations between specific complications and specific pelvic features. Nonetheless, several studies detected associations of PFD with both a narrow and a wide bony pelvis. For example, in a study on 59 women with PFD and 39 controls, Handa and colleagues41 found that the transverse diameter of the pelvic inlet is greater in women with pelvic floor disorders than in those without. Interestingly, they also showed that prolapse is associated with a narrow obstetrical conjugate (the shortest pelvic diameter of the birth canal, see Glossary), presumably due to a predisposition to injury. Similarly, in a study on 34 patients and 34 controls, Sze et al.42 found that women with vaginal prolapse have a wider transverse diameter of the pelvic inlet than the control group. Two other large studies reported associations of a broad birth canal with urinary incontinence. Stav and coauthors26 found that women with wide transverse and anterio-posterior inlet diameters as well as with a wider pelvic outlet diameter are more frequently affected by incontinence than women with a narrower inlet. Similarly, Berger et al.43 reported that stress urinary incontinence is associated with a wide subpubic angle and a wide pelvic outlet (large interspinous and intertuberous diameters)43,44. Because of the relatively small effect sizes, large samples may be critical, as some smaller studies did not find differences in bony dimensions45,46. But even weak statistical associations between pelvic shape and pelvic floor function, which may be of limited clinical relevance today, can drive evolution: any consistent association between a morphological trait and survival rate or reproductive success imposes a selective pressure on this trait.
To understand the conflicting selection pressures acting on pregnancy, it will be important for future studies to distinguish between the different disorders and their associations with specific pelvic traits. Such studies may help to predict the risk of certain PFD based on pelvic anatomy and to develop preventive strategies for women with wider a pelvis, such as strengthening specific aspects of the pelvic floor, introducing external support for carrying, or encouraging behavioral changes that ameliorate unnecessary strain.
The human pubic symphysis is particularly inflexible
Softening and widening of the pubic symphysis as well as relaxation of the sacroiliac joints during pregnancy widens the birth canal and thus eases childbirth; it may appear as a human-specific adaptation to the tight feto-pelvic fit. However, in several other mammals with relatively large neonates, flexibility of the pubic symphysis is much larger than in humans47. In guinea pigs, for instance, the mean diameter of the fetal head is 20 mm, whereas the pelvic canal in early pregnancy is just 11 mm wide. To accommodate the fetal head, the pubic bones separate up to 23 mm during late pregnancy in response to estrogen and relaxin, and a ligament appears in the middle of the joint48,49. This flexibility is crucial, as calcification of the symphysis, which occurs if the first pregnancy is delayed, frequently leads to CPD in guinea pigs50. The emergence of an interpubic gap, bridged by a flexible ligament, is a prerequisite for successful delivery also in other species, including mice, bats, deer mice, and macaques49,51. In mice, for instance, the gap measures 4–10 mm at delivery52, whereas in humans the mean increase of the interpubic gap is only 3 mm53,54. In other mammals, by contrast, e.g. in many odd- and even-toed ungulates and in big cats, the pubic symphysis is completely fused by ossification47,49.
Why is the flexibility of the pubic symphysis so variable across mammals, and why is it so inflexible in humans despite the difficult childbirth? A widened pubic symphysis increases the birth canal, and thus also the vulnerability of the pelvic floor. Most species with a wide pubic gap are either very small and hence experience little pressure on the pelvic floor, engage in postures that reduce force on the pelvis and the pelvic floor (e.g., roosting head down in bats), or they reduce the pressure of the viscera and the fetus on the pelvic floor by living in water. An ossified symphysis, by contrast, allows for a higher net force applied by the muscles to the rest of the body and thus may facilitate more energetically efficient locomotion47. Apart from many fast running species, pubic flexibility is also strongly reduced in other large-bodied bipedal species, such as kangaroos.
In humans, a wide symphysis during pregnancy and birth is associated with severe pelvic girdle pain55,56, which is common among athletes and patients with traumatic pelvic injuries53,57. It is aggravated by weight-bearing and associated with difficulty in walking58. Evolutionarily, the larger birth canal that would result from increased symphysial flexibility apparently was outweighed by the increased risk of injuries to the pelvis and the pelvic floor. Grunstra and colleagues47 even hypothesized that bipedal locomotion in humans has not primarily constrained pelvic width – as classically suggested – but the flexibility of the symphysis.
Selection for a narrow pelvis may act via males: a role in erectile function
Not only the obstetric demands, but also the additional strain exerted on the pelvic floor by the heavy fetus, are specific to females. The evolutionary trade-off thus differs between the sexes and gave rise to the sexual dimorphism in pelvic morphology and pelvic canal size observable today. Similar dimorphism patterns are also seen in other primate species with a large fetal head relative to the size of the pelvic canal (cephalo-pelvic index, see Glossary), such as in lar gibbons, rhesus macaques, and squirrel monkeys, suggesting that pelvic dimorphism is, at least in part, a consequence of fetal size59,60.
It is important to consider the specific conditions required for sexual dimorphism to arise in the evolution of traits that are present in both sexes. Divergent evolution (see Glossary) of such traits requires opposing selection pressures in the two sexes; selection acting in only one sex is not sufficient because the developmental genes and processes underlying a trait, in this case the pelvis, are mostly the same whether they find themselves in a male or a female organism (“genetic correlation between the sexes”, see Glossary). This means that selection for a broader pelvis in females must have been counteracted by selection for a narrow pelvis in males. If there is no selection in males against the female adaptation, selection in females will also change – at least to a great extent – male shape, and no dimorphism will arise.
The fact that pelvic sexual dimorphism has nevertheless evolved implies that there has been selection opposing the widening of the pelvis in males. Studying the male pelvis may thus provide insights into the advantages of a narrower pelvis independent of disadvantages due to birthing complications25. Two relevant selection regimes are plausible: males can either share the same selective pressure for a narrow pelvis with females (as was suggested for gait or for visceral support) but lack the pressure for widening. Alternatively, selection in males may be for a male-specific function, perhaps also indirectly affecting the pelvis in females via a correlated effect.
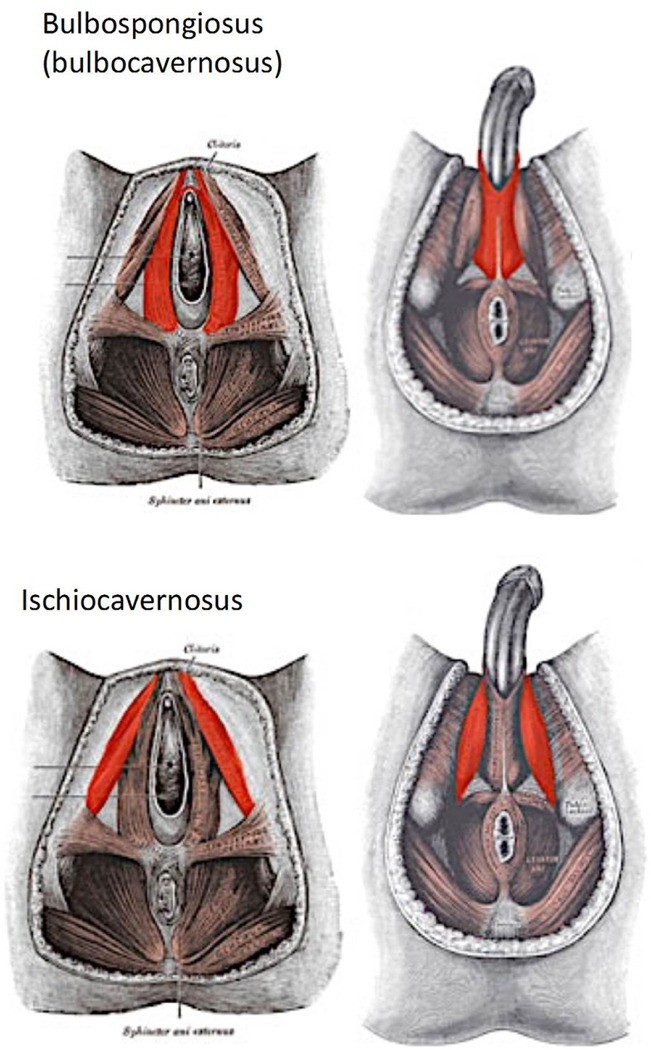
If pelvic width is associated with pelvic floor strength in males, as is the case in females, male-specific pelvic floor disorders may offer interesting insights. In addition to general effects such as pelvic pain and sphincter dysfunction, one of the disorders associated with a weak pelvic floor in males is erectile dysfunction (ED)61. The pelvic floor contributes to erectile function by the muscles that form the penile basis, in particular the ischiocavernosus and bulbocavernosus muscle (Figure 8). These muscles act as part of the complex involuntary muscular interplay establishing and maintaining the pressure during erection and ejaculation. The stability of these muscles may have become particularly important for erection in the human lineage because human males have evolved relatively large penises without a penile bone – a situation uncommon among primates62. A summary of US epidemiological studies, including the large Massachusetts Male Aging Study63–65, reports severe erectile dysfunction in 10% of men aged 40–70, with another 25% experiencing intermittent erectile difficulties, and 17% minimal difficulties (http://www.bumc.bu.edu/sexualmedicine/physicianinformation/epidemiology-of-ed/). The prevalence of erectile dysfunction increases steeply with age, but even 5–10% of men below 40 are affected. Vascular diseases and aging are the most important proximate factors underlying erectile dysfunction, but it is unclear to what extent they are conditional on pelvic floor strength, which has long been associated with male and female reproductive functioning66,67. Recent studies reported that improving pelvic floor strength considerably alleviated erectile problems in a substantial percentage of men61, even though direct estimates of how frequently a weak pelvic floor is the main cause of erectile dysfunction are, to our knowledge, lacking. For example, a study on 55 men showed large improvement of erectile function after six months of exercise of the pelvic floor musculature, with 40% regaining normal erectile function and further 35.5% improving68. Large randomized trials similarly revealed considerable positive effects of exercise on erectile dysfunction 69–71.
Figure 8. Comparison of the male and female pelvic floors.
The two muscles shown in red, bulbospongiosus and ischiocavernosus, are involved in the female pelvic floor and also crucial in maintaining the erection in males. Figures from https://www.qrsthai.com/erectile-dysfunction/.
In short, indirect evidence indicates that the strength of the pelvic floor affects erectile function and thus, likely, male reproduction. Furthermore, the fact that the evolutionary widening of the female pelvis did not “spill over” to widen the male pelvis, implies a selective advantage of a narrow pelvis in men. The missing information to link these two lines of evidence is whether the pelvic width is indeed associated with pelvic floor strength in males. Data in women suggest that this is the case, but we are not aware of studies that have addressed this question in men.
One way to address this question would be an individual-based study examining the association between pelvic measurements and erectile dysfunction. Such data may be readily available to urologists. An alternative, but less direct way would be a comparison of sex-specific pelvic morphology with the prevalence of erectile dysfunction across different populations. Literature suggests that inter-population variation in pelvic shape and erectile dysfunction prevalence is large72–76; a study combining these data, accounting for confounding factors such as overall health status, may be able to address the influence of pelvic morphology on erectile dysfunction.
The cliff-edge model
Despite the multiple sources of selection for a narrow pelvis in females and males, it still appears puzzling that a population’s fitness loss resulting from maternal and fetal mortality has been evolutionarily outweighed by the – presumably small – advantages for locomotion and pelvic floor stability. The recently developed cliff-edge model of obstetrical selection77–79 revealed why natural selection, however strong, is not able to fully optimize the match between fetal and maternal dimensions, thus avoiding CPD.
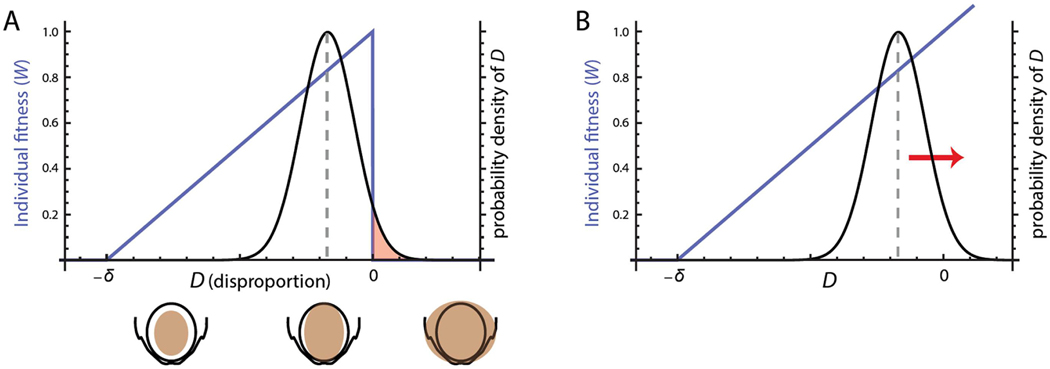
The cliff-edge model is based on an idealized variable D (disproportion), which represents the difference between the size of the fetus and that of the maternal birth canal. When the fetus is smaller than the birth canal and encounters no obstruction, D is negative, whereas when the size of the fetus exceeds that of the birth canal, thus leading to obstructed labor, D is positive (Figure 9A). In order to study the evolutionary dynamics of feto-pelvic disproportion, as expressed by D, an “evolutionary fitness” value is assigned to each value of D (y- and x-axes in Figure 9A, respectively). In evolutionary theory, fitness refers to the average number of offspring of individuals with a particular genotype or phenotype (in this case D). The offspring number is affected both by survival rate and fecundity; what do we know about survival and fecundity with respect to childbirth that enables us to attribute fitness to values of D? Clearly, without cesarean delivery, individuals with D>0 do not survive, so their fitness equals zero. Epidemiological data document that larger neonates have higher survival rates80,81 and thus higher fitness than smaller neonates. Furthermore, mothers with a wide pelvis have higher rates of PFD, which can lead to serious infections and social ostracization, as is common in sub-Saharan Africa40,82,83. These relationships translate into the blue line in Figure 9A: fitness increases approximately linearly as a function of D, as long as the fetus is smaller than the birth canal. When fetal size exceeds the size of the birth canal (D>0), survival and thus fitness drop sharply in the absence of medical care. This highly asymmetric fitness curve resembles a cliff edge (hence the name of the model).
Figure 9. Cliff-edge model.
(A) The x-axis represents the variable D (disproportion), which captures the difference between cranial size and pelvic size. This value is negative when the fetal cranium is smaller than the pelvic canal (D<0) and positive when fetal size exceeds the size of the pelvic canal, leading to cephalopelvic disproportion (CPD). The y-axis on the left represents individual fitness. As long as birth canal can encompass the fetus (D<0), individual fitness (blue curve) increases linearly with D (both a larger fetus and a narrower pelvis lead to higher fitness). For D>0, fitness is zero because of CPD. The black curve represents the probability density function (pdf) of D, that is, the distribution of pregnancies across different values of D. Evolution by natural selection maximizes the average fitness in a population, which leads to an evolutionary “optimum” that entails an average value of D (grey dashed line) smaller than the individual optimum (i.e., on the left side of the cliff edge) and a fraction of individuals with CPD (the red area beyond the cliff edge, at D>0). This evolutionary stable state trades off the high fitness for the pregnancies with D<0 (large fetuses that still fit through the birth canal) against the fraction of pregnancies with CPD. Figure modified from Mitteroecker & coleagues77.
(B) Caesarean delivery removes the cliff at the fitness threshold at D = 0, thus leading to a further evolutionary increase of D and the resulting CPD rate. Note that only cesarean sections performed due to actual disproportion have this effect. Figure modified from Mitteroecker & coleagues77.
The association of fitness with D imposes natural selection on the corresponding fetal and maternal dimensions. Only heritable traits can evolve by natural selection, and indeed, a number of studies reported considerable heritability (see Glossary) for fetal size and pelvic dimensions84–87. Hence, the disproportion D is expected to respond to selection and increase in the population. In other words, the population becomes enriched for those trait values that confer high fitness so that the trait distribution gradually approximates the fitness curve. In the case of our trait D, one would expect many pregnancies to be just below 0, that is, with fetuses that are as large as possible while still being able to be born.
Yet here lies the inherent limitation of evolving biological systems: many phenotypic traits in a population, including fetal size and pelvic dimensions, have approximately symmetrical (“normal”) distributions and thus cannot match the asymmetrical fitness curve specific of obstetrics. Symmetrical distributions arise because most traits are affected by many genes with small additive effects along with symmetrically distributed environmental influences. This mismatch limits the adaptive evolution of cephalo-pelvic fit: evolution by natural selection maximizes average fitness in the population (i.e., the average number of offspring per individual), which results in an average value of D below the value with maximal individual fitness (at D=0) a certain proportion with D<0, and a certain proportion of cases exhibiting CPD (D>0; Figure 9A). The persistent rates of CPD in human populations therefore do not suggest that natural selection has not worked, but rather that evolution is limited by the inherent biology of human reproductive traits. The model also shows that because of the cliff-edged fitness function, only weak selection towards larger D is necessary to explain the observed rates of CPD83. In other words, against the common intuition, only weak fitness advantages for large newborns, or for a narrow female pelvis, or both, are necessary to evolve the relatively high CPD rates.
The heritability of fetal and pelvic dimensions implies that women born by the Cesarean due to CPD are more likely to experience CPD when giving birth to their own offspring, compared with women who were born without CPD. Large multigenerational studies on well-documented populations (Scandinavia, US) have found extensive evidence for inheritance of CPD88–91, primarily via the maternal genome. Despite different time periods, geographical regions, and methodical details, these studies report relatively homogenous odds ratios for CPD ranging from 1.55 to 2.06, which likely are underestimates (non-anatomical reasons for obstructed labor are not or only weakly heritable but may be misdiagnosed as CPD). The expected odds ratio inferred from the cliff edge model is at 2.7779 (using an estimated heritability of 0.5 for D), a value remarkably close to the empirical observations, which supports the cliff edge model and its assumptions.
Future dynamics of cephalopelvic disproportion
Based on the cliff-edge model, we predicted that CPD rates would increase – and may continue to increase – as a consequence of cesarean delivery (Fig. 9B). The regular and safe use of cesarean delivery in cases of CPD removes the fitness threshold at D=0; if the fetus is larger than the birth canal, fitness no longer drops to zero, it may even continue to increase with increasing D. Thus, cesarean delivery has changed the selective pressure on maternal and fetal dimensions. As a consequence, evolutionary dynamics will move the population distribution towards greater D, leading to increasing rates of CPD (Fig. 9B). In other words, the evolved equilibrium between the opposed selective forces of childbirth, pelvic floor stability, and infant survival has been disrupted by surgical delivery, giving rise to a new evolutionary trend. Assuming an average heritability of 0.5 for fetal and maternal traits, as well as a duration of two generations since caesarean deliveries have been regularly and safely performed, we estimated that the original frequency of CPD would increase by about 10–20% (depending on assumptions about male fitness and the genetic correlation between sexes), which is roughly half a percentage point of CPD incidence. Note, however, that only cesarean deliveries due to CPD are contributing to this effect, not those performed for other indications9,92.
This rough estimate is based on several assumptions (such as the representation of feto-pelvic disproportion by a single quantity). Nonetheless, it shows that evolution by natural selection, triggered through medical intervention, can affect human anatomy over decades, and not only within thousands or millions of years93. Yet, the model does not specify whether the increased rate of CPD results primarily from a change in fetal or maternal dimensions. Historical data on pelvic dimensions are, to our knowledge, not available, and changes in neonatal dimensions are mainly driven by changes in nutrition and obstetric practices78,92. In fact, the average neonatal weight has been decreasing in several industrialized countries because of shorter gestation length and a higher frequency of preterm birth, often due to medically-indicated preterm birth, coupled with a higher survival rate due to advances in neonatal care.
It is worthwhile to note that Berg-Lekås et al.90 reported an increase of primiparous dystocia from 2.2% to 12.5% between the 1950s and 1990s, an estimate that is probably attributed, at least in part, to changes in the reporting of this diagnosis, and in short-term changes of economic development and nutritional status (such as postwar recovery). In this case, women developed in a period of poor nourishment, resulting in smaller body size, whereas when they became pregnant, their fetuses developed in an environment without nutritional deprivation, which fostered fetal growth. Such environmental mismatch between maternal and fetal development adds to the increased frequency of cephalopelvic disproportion in countries recovering from famine or experiencing rapid socioeconomic development94.
The evolutionary pressures that had increased the frequency of cephalopelvic disproportion are now reduced by increasing access to neonatal care and treatment of pelvic floor disorders, promoting survival of neonates, and a higher rate of preterm neonates with a lower birthweight and women with a weak pelvic floor. This, in turn, reduces selection towards larger fetuses and narrower pelves. Fetal size is further constrained by maternal factors other than birth canal size, such as metabolic capacity95, that are not removed by caesarean delivery, and will set limits on an evolutionary increase in cephalopelvic disproportion.
What are the benefits for obstetrics and gynecology of understanding the evolutionary history of human traits and the selective pressures that have shaped them?
Evolutionary explanations for frequent obstetrical challenges (obstructed or prolonged labor, etc.) can facilitate research on medical disorders, including developing models for their prediction and strategies for prevention96–99. For example, the idea that distinct pelvic disorders can result from a pelvis that is either too broad for some functions or too narrow for others can refine research questions and help to predict and interpret research outcomes. Contingency on pelvic architecture may also explain some of the variation associated with mutations affecting connective tissue and associated pelvic floor disorders. Furthermore, evolutionary understanding can guide targeted interventions for women who need support for carrying, or who are particularly prone to injury at childbirth.
Finally, the insight that the male pelvis is likely subject to selective pressures for lower width raises questions about which functions mediate this selection. We discussed erectile function in this article, but other reasons may exist. Studying the male pelvis and its relationship with other disorders or traits is a promising area of investigation for understanding the conundrum of human childbirth and pelvic architecture.
Specific evolutionary models, such as the cliff edge model, allow for predictions of how regular medical treatment may affect ongoing evolutionary dynamics93. The evidence presented herein shows that medical interventions can sometimes lead to evolutionary changes that can take place in several generations, rather than across millennia. Importantly, the reduction of natural selection by medical treatment does not necessarily reduce evolutionary change; indeed, the disruption of evolutionary equilibriums can even induce new biological trends. Clearly, the goal of evolutionary reasoning is neither to criticize medical interventions, nor to impose naturalistic ideals on modern society, but to understand the transition of complex biosocial interactions and to inform public health and research policies.
Acknowledgements
MP acknowledges support by March of Dimes Prematurity Research Centre Collaborative Grant (#22-FY14-470). The work of RR is supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HSN275201300006C. PM is supported by Austrian Science Fund (FWF #P29397).
Funding:
MP: March of Dimes Prematurity Research Centre Collaborative Grant (#22-FY14-470) RR: This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HSN275201300006C.
PM: Austrian Science Fund (FWF #P29397)
Glossary
- Altriciality (secondary)
Characterizes species in which the offspring are born at an early developmental stage; the newborns thus are relatively immobile and helpless with a need for intensive parental care. Typical altricial species are rodents, such as mouse. and rat, or bears; their fetuses depend on the mother for many functions, e.g. food, protection, warmth. At the opposite end are precoccial neonates, which are highly developed and functional at birth, such as antelopes. Human neonates develop long and are, compared with mammals in general, precoccial, as are other primates. However, when compared to other primate species, human neonates are underdeveloped and more dependent, hence the term “secondary altricial”.
- Australopithecus
An early genus of the human lineage, from which the genus Homo arose. According to the fossil record, the genus originated in Africa and covered a period from 4 to 2 myr ago. Most species belonging to this genus were considerably smaller (1–1.5m height, ca. 35% of the cranial volume of modern human), bipedal, and strongly sexually dimorphic. Some of the best-known species are A. afarensis (to which the famous Lucy skeleton belongs), A. africanus, A. sediba and A. anamensis.
- Bipedalism
A form of terrestrial locomotion by means of two rear limbs or legs. In the human lineage, it evolved 7–5 myr ago. The wide time range is due to the difficulty to draw a line between various degrees of evolving bipedalism and due to scarce conclusive fossils.
- Cephalopelvic index (also fetopelvic index)
A broadly applied measure of the match between maternal and fetal dimensions 100–104. In humans it is usually estimated by the ratio between the biparietal diameter of the fetal head at term and the smallest pelvic diameter (either anteroposterior diameter of the inlet or bispinal diameter of the midpelvis). In comparative biology, the cephalopelvic index refers to neonatal head breadth, divided through the transverse diameter of the maternal pelvic inlet.
- Divergent evolution
Refers to the relatively strong accumulation of phenotypic differences between closely related populations, mostly due to differing selection pressure, leading to phenotypic divergence.
- Encephalization
An evolutionary increase in relative brain size and complexity. Increase of relative brain size in the genus Homo involved prenatal as well as postnatal growth acceleration of the brain, relative to closest primate relatives105.
- Genetic correlation between sexes
Because most of the genes expressed in one sex are also expressed in the other sex, selection on a trait in one sex will also lead to a correlated evolutionary response in the other sex. The magnitude of genetic correlation can be estimated by quantitative genetic methods106.
- Heritability
The fraction of observed phenotypic variation in a population that can be attributed to genetic variation within this population. Heritability measures the degree to which trait values are inherited and thus also the degree to which a trait responds to natural selection. E.g., a heritability of 0.5 indicates that half of the observed variation among the studied individuals is due to genetic variation, whereas the other half is due largely to environmental differences. It also implies only half of the effect of natural selection within a generation is inherited to the next generation.
- Homo
The genus of modern humans, the Homo sapiens. The genus originated about 2 myr ago, and most of its species are characterized by taller stature, greater cranial capacity, and less size dimorphism than Australopithecus. Species of this genus include, among others, H. habilis, H. erectus, H. neandertalensis, and H. naledi. H. sapiens originated in this genus about 300,000 years ago.
- Last common ancestor
A concept used by evolutionary biologists when inferring how traits have evolved during the species’ evolution. For example, when multiple closely related species possess a certain trait, it is more likely that they all inherited it from their common ancestor than the alternative scenario that all evolved the same trait independently.
- Obstetrical conjugate
The distance between the closest bony points of the sacral promontory and the pubic bone next to the symphysis (approximately 10–12 cm). It represents the shortest pelvic diameter through which the fetal head must pass during birth.
- Obstetrical dilemma
A concept originally proposed by the anthropologist Sherwood L. Washburn (1960) to explain the tight human feto-pelvic fit. It suggests that human pelvic form results from a compromise between the ability to walk upright and the ability to give birth to large neonates.
Footnotes
The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mihaela PAVLIČEV, Division of Human Genetics, Cincinnati Children`s Hospital Medical Center, University of Cincinnati; Department of Pediatrics, University of Cincinnati College of Medicine, and Department of Philosophy, University of Cincinnati.
Roberto ROMERO, Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Ann Arbor, MI; Center for Molecular Medicine and Genetics, Wayne State University, Ann Arbor, MI; Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI; Department of Epidemiology and Biostatistics, Michigan State University, East Lansing, MI.
Philipp MITTEROECKER, Department of Theoretical Biology, University of Vienna, Austria.
References
- 1.Schultz A. The Life of Primates. The Universe Books; 1969. [Google Scholar]
- 2.Kearney R, Fitzpatrick M, Brennan S, et al. Levator ani injury in primiparous women with forceps delivery for fetal distress, forceps for second stage arrest, and spontaneous delivery. Int J Gynaecol Obstet. 2010;111(1):19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heilbrun ME, Nygaard IE, Lockhart ME, et al. Correlation between levator ani muscle injuries on magnetic resonance imaging and fecal incontinence, pelvic organ prolapse, and urinary incontinence in primiparous women. Am J Obstet Gynecol. 2010;202(5):488 e481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delancey JO, Toglia MR, Perucchini D. Internal and external anal sphincter anatomy as it relates to midline obstetric lacerations. Obstet Gynecol. 1997;90(6):924–927. [DOI] [PubMed] [Google Scholar]
- 5.Dolea C, AbuZahr C. Global burden of obstructed labour in the year 2000. WHO;2000. [Google Scholar]
- 6.Philpott RH. Obstructed labour. Clin Obstet Gynaecol. 1982;9(3):625–640. [PubMed] [Google Scholar]
- 7.Fine EA, Bracken M, Berkowitz RL. An Evaluation of the Usefulness of X-Ray Pelvimetry - Comparison of the Thoms and Modified Ball Methods with Manual Pelvimetry. American Journal of Obstetrics and Gynecology. 1980;137(1):15–20. [DOI] [PubMed] [Google Scholar]
- 8.Friedman EA, Sachtleben MR. Station of the fetal presenting part. VI. Arrest of descent in nulliparas. Obstet Gynecol. 1976;47(2):129–136. [PubMed] [Google Scholar]
- 9.MacDorman MF, Menacker F, Declercq E. Cesarean birth in the United States: epidemiology, trends, and outcomes. Clin Perinatol. 2008;35(2):293–307, v. [DOI] [PubMed] [Google Scholar]
- 10.Romero R, Tarca AL, Tromp G. Insights into the physiology of childbirth using transcriptomics. PLoS Med. 2006;3(6):e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neilson JP, Lavender T, Quenby S, Wray S. Obstructed labour. Br Med Bull. 2003;67:191–204. [DOI] [PubMed] [Google Scholar]
- 12.Betran AP, Temmerman M, Kingdon C, et al. Interventions to reduce unnecessary caesarean sections in healthy women and babies. Lancet. 2018;392(10155):1358–1368. [DOI] [PubMed] [Google Scholar]
- 13.Boerma T, Ronsmans C, Melesse DY, et al. Global epidemiology of use of and disparities in caesarean sections. Lancet. 2018;392(10155):1341–1348. [DOI] [PubMed] [Google Scholar]
- 14.Long Q, Kingdon C, Yang F, et al. Prevalence of and reasons for women’s, family members’, and health professionals’ preferences for cesarean section in China: A mixed-methods systematic review. PLoS Med. 2018;15(10):e1002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni L, Elsaharty A, McConachie I. Cesarean birth - What’s in a name? Int J Obstet Anesth. 2018;34:5–9. [DOI] [PubMed] [Google Scholar]
- 16.O’Donovan C, O’Donovan J. Why do women request an elective cesarean delivery for non-medical reasons? A systematic review of the qualitative literature. Birth. 2018;45(2):109–119. [DOI] [PubMed] [Google Scholar]
- 17.Rogers AJG, Harper LM, Mari G. A conceptual framework for the impact of obesity on risk of cesarean delivery. Am J Obstet Gynecol. 2018;219(4):356–363. [DOI] [PubMed] [Google Scholar]
- 18.Venturella R, Quaresima P, Micieli M, et al. Non-obstetrical indications for cesarean section: a state-of-the-art review. Arch Gynecol Obstet. 2018;298(1):9–16. [DOI] [PubMed] [Google Scholar]
- 19.Walker KF, Thornton JG. Delivery at Term: When, How, and Why. Clin Perinatol. 2018;45(2):199–211. [DOI] [PubMed] [Google Scholar]
- 20.Lovejoy CO, Heiple KG, Burstein AH. The gait of Australopithecus. American journal of physical anthropology. 1973;38(3):757–779. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg KR, DeSilva JM. Evolution of the Human Pelvis. Anatomical record. 2017;300(5):789–797. [DOI] [PubMed] [Google Scholar]
- 22.Grabowski M, Roseman CC. Complex and changing patterns of natural selection explain the evolution of the human hip. Journal of human evolution. 2015;85:94–110. [DOI] [PubMed] [Google Scholar]
- 23.Grabowski MW, Polk JD, Roseman CC. Divergent patterns of integration and reduced constraint in the human hip and the origins of bipedalism. Evolution; international journal of organic evolution. 2011;65(5):1336–1356. [DOI] [PubMed] [Google Scholar]
- 24.Churchill SE, Vansickle C. Pelvic Morphology in Homo erectus and Early Homo. Anatomical record. 2017;300(5):964–977. [DOI] [PubMed] [Google Scholar]
- 25.Gruss LT, Schmitt D. The evolution of the human pelvis: changing adaptations to bipedalism, obstetrics and thermoregulation. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2015;370(1663):20140063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caldwell WE, Moloy HC. Anatomical Variations in the Female Pelvis: Their Classification and Obstetrical Significance: (Section of Obstetrics and Gynaecology). Proc R Soc Med. 1938;32(1):1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caldwell WE, Moloy HC. Anatomical variations in the female pelvis and their effect in labor with a suggested classification. Am J Obstet Gynecol. 1933;26(4):479–505. [Google Scholar]
- 28.Betti L. Human Variation in Pelvic Shape and the Effects of Climate and Past Population History. Anatomical record. 2017;300(4):687–697. [DOI] [PubMed] [Google Scholar]
- 29.Washburn SL. Tools and human evolution. Sci Am. 1960;203:63–75. [PubMed] [Google Scholar]
- 30.Portmann A. Biologische Fragmente zu einer Lehre vom Menschen Basel: Schwabe Verlag; 1944. [Google Scholar]
- 31.Montagu A Neonatal and infant immaturity in man. Jama. 1961;178:56–57. [DOI] [PubMed] [Google Scholar]
- 32.Fischer B, Mitteroecker P. Allometry and Sexual Dimorphism in the Human Pelvis Anatomical record. 2017;300(4):698–705. [DOI] [PubMed] [Google Scholar]
- 33.Warrener AG. Hominin Hip Biomechanics: Changing Perspectives. Anatomical record. 2017;300(5):932–945. [DOI] [PubMed] [Google Scholar]
- 34.Warrener AG, Lewton KL, Pontzer H, Lieberman DE. A wider pelvis does not increase locomotor cost in humans, with implications for the evolution of childbirth. PloS one. 2015;10(3):e0118903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitcome KK, Miller EE, Burns JL. Pelvic Rotation Effect on Human Stride Length: Releasing the Constraint of Obstetric Selection. Anatomical record. 2017;300(4):752763. [DOI] [PubMed] [Google Scholar]
- 36.Gruss LT, Gruss R, Schmitt D. Pelvic Breadth and Locomotor Kinematics in Human Evolution. Anatomical record. 2017;300(4):739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abitbol MM. Evolution of the ischial spine and of the pelvic floor in the Hominoidea. American journal of physical anthropology. 1988;75(1):53–67. [DOI] [PubMed] [Google Scholar]
- 38.Ashton-Miller JA, DeLancey JO. Functional anatomy of the female pelvic floor. Annals of the New York Academy of Sciences. 2007;1101:266–296. [DOI] [PubMed] [Google Scholar]
- 39.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26. [DOI] [PubMed] [Google Scholar]
- 40.Arrowsmith S, Hamlin EC, Wall LL. Obstructed labor injury complex: obstetric fistula formation and the multifaceted morbidity of maternal birth trauma in the developing world. Obstet Gynecol Surv. 1996;51(9):568–574. [DOI] [PubMed] [Google Scholar]
- 41.Handa VL, Pannu HK, Siddique S, Gutman R, VanRooyen J, Cundiff G. Architectural differences in the bony pelvis of women with and without pelvic floor disorders. Obstet Gynecol. 2003;102(6):1283–1290. [DOI] [PubMed] [Google Scholar]
- 42.Sze EH, Kohli N, Miklos JR, Roat T, Karram MM. Computed tomography comparison of bony pelvis dimensions between women with and without genital prolapse. Obstet Gynecol. 1999;93(2):229–232. [DOI] [PubMed] [Google Scholar]
- 43.Berger MB, Doumouchtsis SK, DeLancey JO. Bony pelvis dimensions in women with and without stress urinary incontinence. Neurourol Urodyn. 2013;32(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stav K, Alcalay M, Peleg S, Lindner A, Gayer G, Hershkovitz I. Pelvis architecture and urinary incontinence in women. Eur Urol. 2007;52(1):239–244. [DOI] [PubMed] [Google Scholar]
- 45.Stein TA, Kaur G, Summers A, Larson KA, DeLancey JO. Comparison of bony dimensions at the level of the pelvic floor in women with and without pelvic organ prolapse. Am J Obstet Gynecol. 2009;200(3):241 e241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li R, Song Y, Ma M. Relationship between levator ani and bony pelvis morphology and clinical grade of prolapse in women. Clinical anatomy. 2015;28(6):813–819. [DOI] [PubMed] [Google Scholar]
- 47.Grunstra N, Zachos F, erdina AN, Fischer B, Pavlicev M, Mitteroecker P. Humans as inverted bats: A comparative approach to the obstetric cnundrum. American Journa of Human Biology. 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwabe C, Bullesbach EE. Relaxin. Comp Biochem Physiol B. 1990;96(1):15–21. [DOI] [PubMed] [Google Scholar]
- 49.Todd TW. Age changes in the pubic bone. American journal of physical anthropology. 1921;4(1):1–70. [Google Scholar]
- 50.Resources IoLA. Laboratory animal management: Rodents. ational Academies Press; 1996. [Google Scholar]
- 51.Schwabe C, Steinetz B, Weiss G, et al. Relaxin. Recent Prog Horm Res. 1978;34:123211. [DOI] [PubMed] [Google Scholar]
- 52.Ortega HH, Munoz-de-Toro MM, Luque EH, Montes GS. Morphological characteristics of the interpubic joint (Symphysis pubica) of rats, guinea pigs and mice in different physiological situations. A comparative study. Cells Tissues Organs. 2003;173(2):105114. [DOI] [PubMed] [Google Scholar]
- 53.Becker I, Woodley SJ, Stringer MD. The adult human pubic symphysis: a systematic review. Journal of anatomy. 2010;217(5):475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hisaw FL, Zarrow MX. The physiology of relaxin. Vitam Horm. 1950;8:151–178. [DOI] [PubMed] [Google Scholar]
- 55.Bjorklund K, Nordstrom ML, Bergstrom S. Sonographic assessment of symphyseal joint distention during pregnancy and post partum with special reference to pelvic pain. Acta Obstet Gynecol Scand. 1999;78(2):125–130. [DOI] [PubMed] [Google Scholar]
- 56.Ersdal HL, Verkuyl DA, Bjorklund K, Bergstrom S. Symphysiotomy in Zimbabwe; postoperative outcome, width of the symphysis joint, and knowledge, attitudes and practice among doctors and midwives. PloS one. 2008;3(10):e3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ronchetti I, Vleeming A, van Wingerden JP. Physical characteristics of women with severe pelvic girdle pain after pregnancy: a descriptive cohort study. Spine (Phila Pa 1976). 2008;33(5):E145–151. [DOI] [PubMed] [Google Scholar]
- 58.Jain S, Eedarapalli P, Jamjute P, Sawdy R. Review: Symphysis pubis dysfunction: a practical approach to management. The Obstetrician and Gynecologist. 2006;8:153–158. [Google Scholar]
- 59.Moffett EA. Dimorphism in the Size and Shape of the Birth Canal Across Anthropoid Primates. Anatomical record. 2017;300(5):870–889. [DOI] [PubMed] [Google Scholar]
- 60.Zollikofer CP, Scherrer M, Ponce de Leon MS. Development of Pelvic Sexual Dimorphism in Hylobatids: Testing the Obstetric Constraints Hypothesis. Anatomical record. 2017;300(5):859–869. [DOI] [PubMed] [Google Scholar]
- 61.Cohen D, Gonzalez J, Goldstein I. The Role of Pelvic Floor Muscles in Male Sexual Dysfunction and Pelvic Pain. Sex Med Rev. 2016;4(1):53–62. [DOI] [PubMed] [Google Scholar]
- 62.Dixson AF. Primate sexuality : comparative studies of the prosimians, monkeys, apes, and humans. 2nd ed. Oxford: Oxford University Press; 2012. [Google Scholar]
- 63.Feldman HA, Johannes CB, Derby CA, et al. Erectile dysfunction and coronary risk factors: prospective results from the Massachusetts male aging study. Prev Med. 2000;30(4):328–338. [DOI] [PubMed] [Google Scholar]
- 64.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000;163(2):460–463. [PubMed] [Google Scholar]
- 65.Travison TG, Shabsigh R, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The natural progression and remission of erectile dysfunction: results from the Massachusetts Male Aging Study. J Urol. 2007;177(1):241–246; discussion 246. [DOI] [PubMed] [Google Scholar]
- 66.Kegel AH. Progressive resistance exercise in the functional restoration of the perineal muscles. Am J Obstet Gynecol. 1948;56(2):238–248. [DOI] [PubMed] [Google Scholar]
- 67.Kegel AH. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60(10):521–524. [PubMed] [Google Scholar]
- 68.Dorey G, Speakman MJ, Feneley RC, Swinkels A, Dunn CD. Pelvic floor exercises for erectile dysfunction. BJU Int. 2005;96(4):595–597. [DOI] [PubMed] [Google Scholar]
- 69.Prota C, Gomes CM, Ribeiro LH, et al. Early postoperative pelvic-floor biofeedback improves erectile function in men undergoing radical prostatectomy: a prospective, randomized, controlled trial. Int J Impot Res. 2012;24(5):174–178. [DOI] [PubMed] [Google Scholar]
- 70.Siegel AL. Pelvic floor muscle training in males: practical applications. Urology. 2014;84(1):1–7. [DOI] [PubMed] [Google Scholar]
- 71.Lavoisier P, Roy P, Dantony E, Watrelot A, Ruggeri J, Dumoulin S. Pelvic-floor muscle rehabilitation in erectile dysfunction and premature ejaculation. Phys Ther. 2014;94(12):1731–1743. [DOI] [PubMed] [Google Scholar]
- 72.Nicolosi A, Moreira ED, Jr., Shirai M, Bin Mohd Tambi MI, Glasser DB. Epidemiology of erectile dysfunction in four countries: cross-national study of the prevalence and correlates of erectile dysfunction. Urology. 2003;61(1):201–206. [DOI] [PubMed] [Google Scholar]
- 73.Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ, Urologic Diseases in America P. Predictors and prevalence of erectile dysfunction in a racially diverse population. Archives of internal medicine. 2006;166(2):207–212. [DOI] [PubMed] [Google Scholar]
- 74.Laumann EO, West S, Glasser D, Carson C, Rosen R, Kang JH. Prevalence and correlates of erectile dysfunction by race and ethnicity among men aged 40 or older in the United States: from the male attitudes regarding sexual health survey. J Sex Med. 2007;4(1):57–65. [DOI] [PubMed] [Google Scholar]
- 75.Wu CJ, Hsieh JT, Lin JS, et al. Comparison of prevalence between self-reported erectile dysfunction and erectile dysfunction as defined by five-item International Index of Erectile Function in Taiwanese men older than 40 years. Urology. 2007;69(4):743–747. [DOI] [PubMed] [Google Scholar]
- 76.Cheng JY, Ng EM, Chen RY, Ko JS. Prevalence of erectile dysfunction in Asian populations: a meta-analysis. Int J Impot Res. 2007;19(3):229–244. [DOI] [PubMed] [Google Scholar]
- 77.Mitteroecker P, Huttegger SM, Fischer B, Pavlicev M. Cliff-edge model of obstetric selection in humans. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(51):14680–14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mitteroecker P, Huttegger SM, Fischer B, Pavlicev M. Reply to Grossman: The role of natural selection for the increase of Caesarean section rates. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(8):E1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitteroecker P, Windhager S, Pavlicev M. Cliff-edge model predicts intergenerational predisposition to dystocia and Caesarean delivery. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(44):11669–11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilcox AJ. On the importance--and the unimportance--of birthweight. Int J Epidemiol. 2001;30(6):1233–1241. [DOI] [PubMed] [Google Scholar]
- 81.Alberman E. Are our babies becoming bigger? J R Soc Med. 1991;84(5):257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wall LL. Obstetric vesicovaginal fistula as an international public-health problem. Lancet. 2006;368(9542):1201–1209. [DOI] [PubMed] [Google Scholar]
- 83.Wall LL. Birth trauma and the pelvic floor: lessons from the developing world. J Womens Health. 1999;8(2):149–155. [DOI] [PubMed] [Google Scholar]
- 84.Clausson B, Lichtenstein P, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG. 2000;107(3):375381. [DOI] [PubMed] [Google Scholar]
- 85.Sharma K. Genetic basis of human female pelvic morphology: a twin study. American journal of physical anthropology. 2002;117(4):327–333. [DOI] [PubMed] [Google Scholar]
- 86.Lunde A, Melve KK, Gjessing HK, Skjaerven R, Irgens LM. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. Am J Epidemiol. 2007;165(7):734–741. [DOI] [PubMed] [Google Scholar]
- 87.Gilmore JH, Schmitt JE, Knickmeyer RC, et al. Genetic and environmental contributions to neonatal brain structure: A twin study. Hum Brain Mapp. 2010;31(8):1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tollanes MC, Rasmussen S, Irgens LM. Caesarean section among relatives. Int J Epidemiol. 2008;37(6):1341–1348. [DOI] [PubMed] [Google Scholar]
- 89.Algovik M, Nilsson E, Cnattingius S, Lichtenstein P, Nordenskjold A, Westgren M. Genetic influence on dystocia. Acta Obstet Gynecol Scand. 2004;83(9):832–837. [DOI] [PubMed] [Google Scholar]
- 90.Berg-Lekas ML, Hogberg U, Winkvist A. Familial occurrence of dystocia. Am J Obstet Gynecol. 1998;179(1):117–121. [DOI] [PubMed] [Google Scholar]
- 91.Varner MW, Fraser AM, Hunter CY, Corneli PS, Ward RH. The intergenerational predisposition to operative delivery. Obstet Gynecol. 1996;87(6):905–911. [DOI] [PubMed] [Google Scholar]
- 92.Grossman R. Are human heads getting larger? Proceedings of the National Academy of Sciences of the United States of America. 2017;114(8):E1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mitteroecker P. How human bodies are evolving in modern societies. Nat Ecol Evol. 2019;3(3):324–326. [DOI] [PubMed] [Google Scholar]
- 94.Zaffarini E, Mitteroecker P. Secular changes in body height predict global rates of cesarean section. P Roy Soc B-Biol Sci. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dunsworth HM, Warrener AG, Deacon T, Ellison PT, Pontzer H. Metabolic hypothesis for human altriciality. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(38):15212–15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stearns SC, Medzhitov R. Evolutionary medicine. Sunderland, Massachussetts: Sinauer Associates, Inc., Publishers; 2016. [Google Scholar]
- 97.Brune M, Hochberg Z. Evolutionary medicine--the quest for a better understanding of health, disease and prevention. BMC medicine. 2013;11:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ruhli F, van Schaik K, Henneberg M. Evolutionary Medicine: The Ongoing Evolution of Human Physiology and Metabolism. Physiology (Bethesda). 2016;31(6):392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wagner G, Pavlicev M, Romero R. Evolutionary Approaches in Reproductive Biology I: Inferences from the tree of life and the comparative method. American Journal of Obstetrics and Gynecology. 2019. [Google Scholar]
- 100.Chen G, Uryasev S, Young TK. On prediction of the cesarean delivery risk in a large private practice. Am J Obstet Gynecol. 2004;191(2):616–624; discussion 624–615. [DOI] [PubMed] [Google Scholar]
- 101.Elkousy MA, Sammel M, Stevens E, Peipert JF, Macones G. The effect of birth weight on vaginal birth after cesarean delivery success rates. Am J Obstet Gynecol. 2003;188(3):824–830. [DOI] [PubMed] [Google Scholar]
- 102.Fox LK, Huerta-Enochian GS, Hamlin JA, Katz VL. The magnetic resonance imagingbased fetal-pelvic index: a pilot study in the community hospital. Am J Obstet Gynecol. 2004;190(6):1679–1685; discussion 1685–1678. [DOI] [PubMed] [Google Scholar]
- 103.Kawakita T, Reddy UM, Landy HJ, Iqbal SN, Huang CC, Grantz KL. Indications for primary cesarean delivery relative to body mass index. Am J Obstet Gynecol. 2016;215(4):515 e511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sukalich S, Mingione MJ, Glantz JC. Obstetric outcomes in overweight and obese adolescents. Am J Obstet Gynecol. 2006;195(3):851–855. [DOI] [PubMed] [Google Scholar]
- 105.Sakai T, Hirata S, Fuwa K, et al. Fetal brain development in chimpanzees versus humans. Current biology : CB. 2012;22(18):R791–792. [DOI] [PubMed] [Google Scholar]
- 106.Lande R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. . Evolution; international journal of organic evolution. 1980;34(2):292–305. [DOI] [PubMed] [Google Scholar]
- 107.Wittman AB, Wall LL. The evolutionary origins of obstructed labor: bipedalism, encephalization, and the human obstetric dilemma. Obstet Gynecol Surv. 2007;62(11):739–748. [DOI] [PubMed] [Google Scholar]
- 108.Pontzer H. Overview of hominin evolution. . Nature Education knowledge. 2012;3(10):8. [Google Scholar]
- 109.Caldwell WE, Moloy HC, D’Esopo DA. Further studies on the pelvic architecture. Am J Obstet Gynecol. 1934;28:482. [Google Scholar]
- 110.Huxley TH. On the Relations of Man to Lower Animals. 1961. [Google Scholar]