Abstract
Background:
We retrospectively evaluated the composition of retrieved clots from ischemic stroke patients to study the association between histological composition and stroke etiology
Methods:
Consecutive patients enrolled in the Stroke Thromboembolism Registry of Imaging and Pathology (STRIP) were included in this study. All patients underwent mechanical thrombectomy and retrieved clots were sent to a central core lab for processing. Histological analysis was performed using Martius Scarlett Blue (MSB) staining and quantification for red blood cells (RBCs), white blood cells (WBCs), fibrin and platelets was performed using Orbit Image Software. A Wilcoxon test was used for continuous variables and chi-squared test for categorical variables.
Results:
1350 patients were included in this study. Overall rate of TICI 2c/3 was 68%. 501 patients received tPA (37%). 267 patients (20%) had a large artery atherosclerosis source (LAA), 662 (49%) a cardiac source (CE), 301 (22%) were cryptogenic and the remainder had other identifiable sources including hypercoagulable state or dissection. LAA thrombi had a higher mean RBC density (46%±23% versus 42%±22%, P=0.01) and a lower platelet density (24%±18% versus 27±18%, P=0.03) than CE thrombi. Clots from dissection patients had the highest mean RBC density (50%±24% while clots from patients with a hypercoagulable state had the lowest mean RBC density (26%±21%).
Conclusions:
Our study found statistically significant but clinically insignificant differences between clots of cardioembolic and large artery atherosclerosis etiologies. Future studies should emphasize molecular, proteomic and immunohistochemical characteristics to determine links between clot composition and etiology.
Introduction
Given the increased utilization of mechanical thrombectomy for treatment of acute ischemic stroke secondary to large vessel occlusion, there has been growing interest in analysis of retrieved thrombi (1–7). A number of studies have suggested that histopathological analysis can provide some valuable insights into stroke etiology (8–11). Many of these studies have relied on traditional H&E and Martius Scarlett Blue (MSB) stains to quantify the proportion of clot components including red blood cells (RBCs) fibrin, white blood cells (WBCs) and platelets. Other studies have sought to examine the role that immunohistochemistry and more advanced proteomic analyses can play in differentiating between various clot etiologies.
Understanding if there is a reliable histological signature of retrieved thrombi is important as, we could then potentially help in discerning between emboli which are more likely to be cardiac in etiology versus those of a large artery atherosclerosis etiology and perhaps influence secondary prevention strategies accordingly. If a large study were to find no such link, then it would suggest that the field should focus on more advanced analyses of clot composition including proteomic signatures, molecular analyses and immunohistochemistry. In order to study the association between stroke etiology and clot histological composition, we studied patients included the Stroke Thromboembolism Registry of Imaging and Pathology (STRIP). We hypothesized that clots retrieved from patients who had a large artery atherosclerosis source would have a higher proportion of platelets and white blood cells than those from a cardiac source.
Materials and Methods
Patient Population
Consecutive patients enrolled in the STRIP Registry from September 2016 to December 2019 were included. The study was institutional review board approved and waiver of consent was granted. Patients were included if they were >18 years of age, had undergone mechanical thrombectomy treatment for acute ischemic stroke and clot material was retrieved.
Clot Processing and Histological Characterization
Each embolus was immediately fixed in 10% phosphate buffered formalin. Emboli were shipped to a central core laboratory for standard tissue processing and embedded in paraffin. The formalin-fixed paraffin-embedded clot material was cut into 3- to 5-μm sections. Representative slides from each clot were stained with hematoxylin and eosin and Martius Scarlett Blue. Representative Martius Scarlett Blue–stained slides were sent for whole slide scanning (Aperio ScansScope AT-Turbo, Leica Biosystems). Histological quantification was performed using Orbit Image Analysis Software (www.Orbit.bio) as per the standard operating procedure(12). Details of the methodology for Orbit image analysis has been previously described.
Data Collection
Data regarding patient demographics, clinical presentation, treatment strategies, outcome, imaging findings, and stroke pathogenesis were collected using a data abstraction form. This is provided in the supplement. For the purpose of this study, we collected data on demographics, the use of tPA, location of the occluded vessel, number of passes and final TICI score. Stroke work-up was performed depending on each institution’s protocol. In general, this consisted of a form of carotid vascular imaging for detection of stenosis (i.e. CT angiography of the neck or ultrasound of the neck) as well as echocardiography (transesophageal or transthoracic) and cardiac monitoring. All stroke etiology work-ups were performed by vascular neurologists at the treating institution.
Statistical Analysis
The mean and standard deviation of RBCs, fibrin, platelets and WBCs for each stroke etiology subgroup was calculated. In order to model clot composition as a categorical variable, we also performed analyses based off the dominant component in a clot. If the density of a component was 50% or higher, then the clot was considered “rich” in that component (i.e. RBC rich, platelet rich, fibrin rich, etc).
Categorical variables were compared using the χ2 test. Continuous variables were compared using an ANOVA test across all groups as well as student’s t-test for each pair. We also performed an ROC analysis to determine if any threshold of RBC, WBC, fibrin and platelet density could be used to differentiate CE versus LAA clots. All statistical correlations were assessed using JMP 14.0 (www.jmp.com, Cary, NC)
A machine learning classification algorithms were estimated using the histological characterizations as the features in the algorithm. The classification algorithm was to differentiate cardioembolic versus large artery atherosclerosis subgroups. The stacked ensemble framework(13) was used in the H2o.ai R package(14). The algorithm included in the ensemble included regularized logistic regression, gradient boosted machines, deep neural networks, and extremely randomized forests. 5-fold cross-validation estimates of the area under the ROC curve and area under the precision-recall curve where estimated to evaluate classification performance.
Results
Patient Population
A total of 1350 patients were included. Mean age was 68.5±13.5 years. Overall rate of TICI 2c/3 was 68% (919/1350). A total of 501 patients received tPA (37%) and there was no difference in the proportion of RBCs, fibrin or platelets between clots which were and were-not treated with tPA (P=0.89, P=0.98 and P=0.75 respectively) Regarding stroke etiology, 267 patients (20%) had a large artery atherosclerosis source (LAA), 662 (49%) a cardiac course (CE), 301 (23%) were cryptogenic, 26 patients (2%) had dissection and 23 patients (2%) had a known hypercoagulable state. Mean number of passes was 2±2.
Histology Results
Histological results are summarized in Table 1. There were significant differences in RBC density across groups ranging from 26%±21% for patients with hypercoagulable state to 50%±24% for patients with dissection (P=0.0004). Patients with LAA clots had a higher mean RBC density than those with CE clots (46%±23% versus 42%±22%, P=0.01). LAA clots also had a significantly higher RBC density than ESUS clots (46%±23% versus 41%±24%, P=0.02). All groups had a significantly higher RBC composition than clots from patients with hypercoagulable states (P<.001). All other differences in RBC density were insignificant across groups. Figure 1 depicts the distribution of each component across different stroke etiologies
Table 1.
Clot Composition between Groups
| Cardioembolic | Large Artery Atherosclerosis | Other | ESUS | Dissection | Hypercoagualable State | P value Across Groups | |
|---|---|---|---|---|---|---|---|
| Number of cases | 662 | 267 | 71 | 301 | 26 | 23 | |
| Percentage of Clot Components | |||||||
| Mean (SD) RBC | 42 (22) | 46 (23) | 45 (23) | 41 (24) | 50 (24) | 26 (21) | 0.0004 |
| Mean (SD) WBC | 4 (2) | 4 (2) | 3 (2) | 4 (2) | 4 (3) | 3 (3) | 0.223 |
| Mean (SD) Fibrin | 27 (16) | 26 (15) | 25 (15) | 25 (14) | 27 (16) | 36 (17) | 0.01 |
| Mean (SD) Platelets | 27 (18) | 24 (18) | 26 (22) | 29 (21) | 18 (13) | 35 (27) | 0.002 |
| Dominant Clot Component | |||||||
| RBC N (%) | 252 (38) | 117 (44) | 30 (42) | 117 (39) | 11 (44) | 3 (13) | 0.08 |
| Fibrin N (%) | 73 (11) | 22 (8) | 5 (7) | 22 (7) | 3 (12) | 4 (17) | 0.28 |
| Platelets N (%) | 79 (12) | 26 (10) | 9 (13) | 49 (16) | 0 (0) | 6 (26) | 0.02 |
Figure 1).
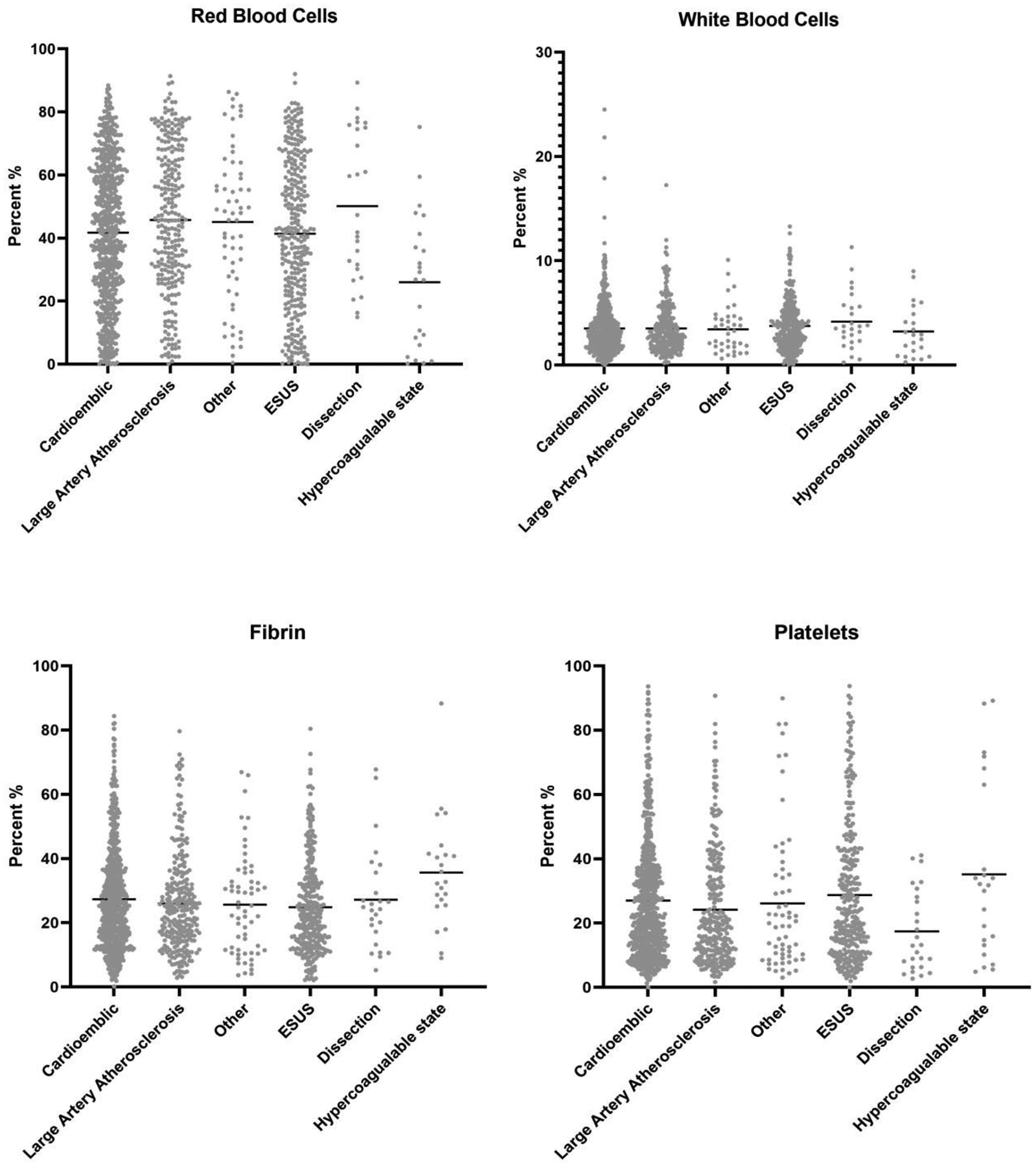
Scatter Plot showing each component of clot for various stroke etiologies
A total of 530 clots were considered RBC rich (39%). Hypercoagulable state clots were the least likely to be RBC rich (13%) while large artery atherosclerosis and dissection clots were the most likely to be RBC rich (44%). There was no difference in the proportion of patients with RBC rich clots between the LAA and CE groups (44% versus 38%, P=0.11). Hypercoagulable state clots were less likely to be RBC rich than all other groups (P<0.01).
Mean WBC density was similar across all groups ranging from 3%±3% for hypercoagulable state clots to 4%±2% for other groups (P=0.223). There were no significant differences in any of the pairwise comparisons.
There were significant differences in mean fibrin density across groups ranging from 25%±14% for cryptogenic stroke patients to 36%±17% from those with hypercoagulable states (P=0.01). Patients with CE clots had a slightly higher mean fibrin density than those with ESUS (P=0.02). Patients with a hypercoagulable state had a significantly higher fibrin density than all other groups (P<0.05 for each pair-wise comparison). All other differences in fibrin density were insignificant across groups.
A total of 129 clots were considered fibrin rich (9.6%). Hypercoagulable state clots were the most likely to be fibrin rich (17%) while ESUS clots were the least likely (7%). There was no difference in the proportion of patients with fibrin rich clots between the LAA and CE groups (8% versus 11%, P=0.23). There was significant difference in the proportion of patients with fibrin rich clots across groups (P=0.28)
There were significant differences in mean platelet density across groups ranging from 18%±13% for dissection patients to 35%±27% for hypercoagulable state patients (P=0.002). CE patients had a higher platelet density than LAA patients (27%±18% versus 24%±18%, P=0.03) and a higher platelet density than dissection patients (27%±18% versus 18%±13%). ESUS patients had a higher platelet density than LAA patients (29%±21% versus 24%±18%, P=0.005). Hypercoagulable state patients had a higher platelet density than all other groups (P<0.05 for all comparisons).
A total of 169 clots were platelet rich (13%). The proportion of platelet rich clots ranged from 10% for LAA clots to 26% for hypercoagulable state clots (P=0.02). There was no difference in the proportion of platelet rich clots between CE and LAA patients (12% versus 10%, P=0.36).
On ROC analysis, the AUC for RBC density in differentiating CE from LAA clots was 0.55; the AUC for WBC density was 0.50; the AUC for fibrin density was 0.52; the AUC for platelet density was 0.55.
Machine Learning Classification Algorithm Results
The stacked ensemble for the classification algorithm differentiating CE from LAA had a 5-fold cross-validated AUC of 0.55 (Area under the precision-recall curve of 0.33).
Other Clinical Results
On ANOVA analysis, there was no difference in the mean number of passes based off stroke etiology. The mean number of passes was 1.9±1.4 for cardioembolic, 2.0±1.5 for LAA, 2.0±1.7 for embolic stroke of undetermined source, 2.7±2.3 for hypercoagulable source and 2.1±1.4 for dissection (P=0.19). First pass TICI 2c/3 for these groups respectively were 42.2%, 37.9%, 39.3%, 26.9% and 43.5% (P=0.18). Overall TICI 2c/3 rates were 70.5%, 65.9%, 66.5%, 61.5% and 69.6% respectively (P=0.07). Embolization to previously non-affected territories was significantly higher for dissection patients (13.0%) when compared to cardioembolic (2.1%), LAA (3.0%, ESUS (2.7%) and hypercoagulable state (4%) patients (P=0.02).
Discussion
Our study examining clot composition in a large cohort of ischemic stroke patients and its association with stroke etiology demonstrated a number of interesting findings. First, LAA clots had a higher mean RBC density and a lower mean platelet density than CE clots.On ROC analysis, it seems that identification of a reliable threshold with a high AUC for differentiating clots from these two etiologies based on composition analysis alone is not possible. The lack of a reliable histological biomarker on MSB between these two stroke etiologies suggests that conventional histological analyses looking at cellular composition does not provide insights into stroke etiology in cryptogenic cases. One interesting association that we found in our study was that thrombi from patients with a known hypercoagulable state had statistically and clinically significant differences in the mean RBC density and higher fibrin and platelet density than clots from any other known source. Ultimately, we feel these findings are important because they suggest that future investigations into what clues clots can provide for stroke etiology should be focused on more advanced testing such as proteomic composition, structural analyses and immunohistochemical markers rather than conventional histology.
A number of previous studies have examined the histological characteristics of LAA and CE clots to see if they could provide any insight into the source of cryptogenic strokes. Prior studies examining correlations between thrombus composition and stroke pathogenesis have focused specifically on red blood cells, white blood cells and fibrin/platelet compositions with inconclusive results. In a study of 187 patients et al found that cardioembolic emboli had few RBCs and more fibrin/platelets than noncardioembolic emboli however they did not account for fibrin and platelet compositions(11). Meanwhile, a study by Kim et al of 37 patients found that cardioembolic clots were actually more likely to have a high RBC composition compared to those related to large artery atherosclerosis(15). A similar study by Boeckh-Behrens et al of 145 patients found that cardioembolic emboli had higher proportions of fibrin and platelets and less RBCs than non-cardioembolic emboli(9). Lastly, a prior study from our group found that LAA clots were more likely to be platelet rich and had a slightly lower RBC density than cardioembolic strokes(10). Our study of over 1300 patients showed no findings which allowed us to reliably distinguish CE and LAA clots.
Overall, the findings from our study and the disparate results from multiple prior studies suggest that routine histological staining with H&E or MSB probably will not allow us to differentiate clots of different etiologies and will likely play no role in determining stroke etiology in embolic stroke of undetermined source. However, some groups have looked into more sophisticated analyses of clot composition to help identify and differentiate stroke etiologies. Recently, Munoz et al have demonstrated the feasibility of mass spectrometry-based proteomic profiling of thrombotic material obtained by embolectomy in ischemic stroke and have help characterize the clot proteome(16). Meanwhile, other groups are examining the role of immunohistochemistry markers staining for proteins such as vWF and ADAMTS13 to point towards stroke etiology(17). Based on the findings from our study, we feel that histological analyses to determine stroke etiology are likely insufficient and future research should focus on more sophisticated analyses focused on immunohistochemistry, proteomics and molecular analyses.
One other feature that we did not specifically examine is the association between clot organization and structure and stroke etiology. While the relative proportion of each cellular component may not provide clues into stroke etiology, the way in which the components are organized might help in differentiation of embolic source. For example, in a study using scanning electron microscopy by Di Meglio et al, the authors found that ischemic stroke thrombi had an outer shell of densely compacted fibrin, von willebrand factor and platelets that made them refractory to thrombolysis(18). Perhaps we may find that clots found from cardioembolic sources and those from large artery atherosclerosis sources are organized differently from one another with different surface components.
Limitations
Our study has limitations. While the MSB stain is more accurate than the H&E stain in identifying major clot components, it still does not specifically identify other potentially key clot components such as von Willebrand factor and calcification. Therefore, the authors represent this subgroup as ‘platelet and other’ components as we acknowledge that there are potentially other components in addition to platelets in these regions. Immunohistochemical analysis using specific antibodies is the only way to accurately distinguish between platelets and platelet-related factors such as von Willebrand factor. Also, the determination of stroke pathogenesis and etiology was self-reported at each site, and therefore there may have been some site-to-site variability in the interpretation and implementation of the TOAST criteria. For example, different sites likely had different thresholds to perform TEE versus TTE or ultrasound bubble tests for cardioembolic sources. Lastly, there was no data on clinical outcome (i.e. mRS) of patients included in the registry.
Conclusions
Our study of over 1300 retrieved emboli in ischemic stroke patients shows no consistent or reliable means to differentiate cardioembolic and large artery atherosclerotic origin clots as determined by the MSB stain. Further research in this field should focus on more advanced techniques including immunohistochemistry and proteomic research.
Source of Funding
This work was supported by the National Institutes of Health grant number (R01 NS105853)
Footnotes
Disclosures
All authors have nothing to disclose
References
- 1.Brinjikji W, Duffy S, Burrows A, Hacke W, Liebeskind D, Majoie C, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerv Surg. 2017;9(6):529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brouwer PA, Brinjikji W, De Meyer SF. Clot Pathophysiology: Why Is It Clinically Important? Neuroimaging Clin N Am. 2018;28(4):611–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Meyer SF, Andersson T, Baxter B, Bendszus M, Brouwer P, Brinjikji W, et al. Analyses of thrombi in acute ischemic stroke: A consensus statement on current knowledge and future directions. Int J Stroke. 2017;12(6):606–14. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald S, Mereuta OM, Doyle KM, Dai D, Kadirvel R, Kallmes DF, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome. J Neurosurg Sci. 2019;63(3):292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson JC, Fitzgerald ST, Kadirvel R, Johnson C, Dai D, Karen D, et al. Clot permeability and histopathology: is a clot’s perviousness on CT imaging correlated with its histologic composition? Journal of neurointerventional surgery. 2020;12(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald S, Rossi R, Mereuta OM, Jabrah D, Okolo A, Douglas A, et al. Per-pass analysis of acute ischemic stroke clots: impact of stroke etiology on extracted clot area and histological composition. Journal of NeuroInterventional Surgery. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mereuta OM, Fitzgerald S, Christensen TA, Jaspersen AL, Dai D, Abbasi M, et al. High-resolution scanning electron microscopy for the analysis of three-dimensional ultrastructure of clots in acute ischemic stroke. Journal of NeuroInterventional Surgery. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berndt M, Friedrich B, Maegerlein C, Moench S, Hedderich D, Lehm M, et al. Thrombus Permeability in Admission Computed Tomographic Imaging Indicates Stroke Pathogenesis Based on Thrombus Histology. Stroke. 2018;49(11):2674–82. [DOI] [PubMed] [Google Scholar]
- 9.Boeckh-Behrens T, Kleine JF, Zimmer C, Neff F, Scheipl F, Pelisek J, et al. Thrombus Histology Suggests Cardioembolic Cause in Cryptogenic Stroke. Stroke. 2016;47(7):1864–71. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald S, Dai D, Wang S, Douglas A, Kadirvel R, Layton KF, et al. Platelet-Rich Emboli in Cerebral Large Vessel Occlusion Are Associated With a Large Artery Atherosclerosis Source. Stroke. 2019;50(7):1907–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sporns PB, Hanning U, Schwindt W, Velasco A, Minnerup J, Zoubi T, et al. Ischemic Stroke: What Does the Histological Composition Tell Us About the Origin of the Thrombus? Stroke. 2017;48(8):2206–10. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald S, Wang S, Dai D, Murphree DH Jr., Pandit A, Douglas A, et al. Orbit image analysis machine learning software can be used for the histological quantification of acute ischemic stroke blood clots. PLoS One. 2019;14(12):e0225841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van der Laan MJ, Polley EC, Hubbard AE. Super learner. Statistical applications in genetics and molecular biology. 2007;6(1). [DOI] [PubMed] [Google Scholar]
- 14.LeDell E, Gill N, Aiello S, Fu A, Candel A, Click C, et al. h2o: R Interface for the’H2O’Scalable Machine Learning Platform. R package version. 2020;3(0.4). [Google Scholar]
- 15.Kim SK, Yoon W, Kim TS, Kim HS, Heo TW, Park MS. Histologic Analysis of Retrieved Clots in Acute Ischemic Stroke: Correlation with Stroke Etiology and Gradient-Echo MRI. AJNR Am J Neuroradiol. 2015;36(9):1756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munoz R, Santamaria E, Rubio I, Ausin K, Ostolaza A, Labarga A, et al. Mass Spectrometry-Based Proteomic Profiling of Thrombotic Material Obtained by Endovascular Thrombectomy in Patients with Ischemic Stroke. Int J Mol Sci. 2018;19(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prochazka V, Jonszta T, Czerny D, Krajca J, Roubec M, Macak J, et al. The Role of von Willebrand Factor, ADAMTS13, and Cerebral Artery Thrombus Composition in Patient Outcome Following Mechanical Thrombectomy for Acute Ischemic Stroke. Med Sci Monit. 2018;24:3929–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Meglio L, Desilles JP, Ollivier V, Nomenjanahary MS, Di Meglio S, Deschildre C, et al. Acute ischemic stroke thrombi have an outer shell that impairs fibrinolysis. Neurology. 2019;93(18):e1686–e98. [DOI] [PMC free article] [PubMed] [Google Scholar]


