Abstract
The European Commission requested the EFSA Panel on Plant Health to prepare and deliver risk assessments for commodities listed in Commission Implementing Regulation (EU) 2018/2019 as ‘High risk plants, plant products and other objects’. This Scientific Opinion covers plant health risks posed by dormant grafted plants, rootstocks, budwood and scions of Malus domestica imported from Turkey, taking into account the available scientific information, including the technical information provided by Turkey. All pests associated with the commodities were evaluated against specific criteria for their relevance for this opinion. Three quarantine pests (Anoplophora chinensis, Lopholeucaspis japonica and tomato ringspot virus), one protected zone quarantine pest (Erwinia amylovora) and eight non‐regulated pests (Calepitrimerus baileyi, Cenopalpus irani, Cicadatra persica, Diplodia bulgarica, Hoplolaimus galeatus, Malacosoma parallela, Pratylenchus loosi and Pyrolachnus pyri) that fulfilled all relevant criteria were selected for further evaluation. For E. amylovora, special requirements are specified in Commission Implementing Regulation (EU) 2019/2072. Based on the information provided in the dossier, the specific requirements for E. amylovora were not met. For Anoplophora chinensis, special measures are specified in Commission Implementing Decision (EU) 2012/138. The exporting country does meet the requirement for a certificate regarding plants for planting that originate from Turkish provinces other than Istanbul. For the 10 remaining selected pests, the risk mitigation measures proposed in the technical dossier from Turkey were evaluated taking into account the possible limiting factors. For the selected pests an expert judgement is given on the likelihood of pest freedom taking into consideration the risk mitigation measures acting on the pest, including uncertainties associated with the assessment. The degree of pest freedom varies among the pests evaluated, with D. bulgarica being the pest most frequently expected on the imported plants. The expert knowledge elicitation indicated with 95% certainty that between 9,863 and 10,000 bundles (consisting of 10 or 25 plants each) per 10,000 would be free from D. bulgarica.
Keywords: Apple, European Union, pathway risk assessment, plant health, plant pest, quarantine
1. Introduction
1.1. Background and Terms of Reference as provided by European Commission
1.1.1. Background
The new Plant Health Regulation (EU) 2016/2031 1 , on the protective measures against pests of plants, has been applied from December 2019. Provisions within the above Regulation are in place for the listing of ‘high risk plants, plant products and other objects’ (Article 42) on the basis of a preliminary assessment, and to be followed by a commodity risk assessment. A list of ‘high risk plants, plant products and other objects’ has been published in Regulation (EU) 2018/2019 2 . Scientific opinions are therefore needed to support the European Commission and the Member States in the work connected to Article 42 of Regulation (EU) 2016/2031, as stipulated in the terms of reference.
1.1.2. Terms of Reference
In view of the above and in accordance with Article 29 of Regulation (EC) No 178/2002 3 , the Commission asks EFSA to provide scientific opinions in the field of plant health.
In particular, EFSA is expected to prepare and deliver risk assessments for commodities listed in the relevant Implementing Act as "High risk plants, plant products and other\objects". Article 42, paragraphs 4 and 5, establishes that a risk assessment is needed as a follow‐up to evaluate whether the commodities will remain prohibited, removed from the list and additional measures will be applied or removed from the list without any additional measures. This task is expected to be on‐going, with a regular flow of dossiers being sent by the applicant required for the risk assessment.
Therefore, to facilitate the correct handling of the dossiers and the acquisition of the required data for the commodity risk assessment, a format for the submission of the required data for each dossier is needed.
Furthermore, a standard methodology for the performance of "commodity risk assessment" based on the work already done by Member States and other international organizations needs to be set.
In view of the above and in accordance with Article 29 of Regulation (EC) No 178/2002, the Commission asks EFSA to provide scientific opinion in the field of plant health for M. domestica from Turkey taking into account the available scientific information, including the technical dossier provided by the Ministry of Agriculture and Forestry of the Republic of Turkey.
1.2. Interpretation of the Terms of Reference
The EFSA Panel on Plant Health (hereafter referred to as ‘the Panel’) was requested to conduct a commodity risk assessment of Malus domestica from Turkey following the Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019a).
The EU quarantine pests that are regulated as a group in the Commission Implementing Regulation (EU) 2019/2072 were considered and evaluated separately at species level.
Annex II of Implementing Regulation (EU) 2019/2072 lists certain pests as non‐European populations or isolates or species. These pests are regulated quarantine pests. Consequently, the respective European populations, or isolates, or species are non‐regulated pests.
Annex VII of the same Regulation, in certain cases (e.g. point 32) makes reference to the following countries that are excluded from the obligation to comply with specific import requirements for those non‐European populations, or isolates, or species: Albania, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Faeroe Islands, Georgia, Iceland, Liechtenstein, Moldova, Monaco, Montenegro, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (Severo Zapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug), San Marino, Serbia, Switzerland, Turkey, Ukraine and the United Kingdom (except Northern Ireland 4 )). Those countries are historically linked to the reference to ‘non‐European countries’ existing in the previous legal framework, Directive 2000/29/EC.
Consequently, for those countries,
any pests identified, which are listed as non‐European species in Annex II of Implementing Regulation (EU) 2019/2072 should be investigated as any other non‐regulated pest.
any pest found in a European country that belongs to the same denomination as the pests listed as non‐European populations or isolates in Annex II of Implementing Regulation (EU) 2019/2072, should be considered as European populations or isolates and should not be considered in the assessment of those countries.
Pests listed as ‘Regulated Non‐Quarantine Pest' (RNQP)’ in Annex IV of the Commission Implementing Regulation (EU) 2019/2072, and deregulated pests (i.e. pest which were listed as quarantine pests in the Council Directive 2000/29/EC and were deregulated by Commission Implementing Regulation (EU) 2019/2072) were not considered for further evaluation.
In its evaluation the Panel:
Checked whether the information provided by the applicant (Ministry of Agriculture and Forestry of the Republic of Turkey) in the technical dossier (hereafter referred to as ‘the Dossier’) was sufficient to conduct a commodity risk assessment. When necessary, additional information was requested to the applicant.
Selected the relevant union EU‐regulated quarantine pests and protected zone quarantine pests (as specified in Commission Implementing Regulation (EU) 2019/2072 5 , hereafter referred to as ‘EU quarantine pests’) and other relevant pests present in Turkey and associated with the commodity.
Assessed whether or not the applicant country implements specific measures for Union quarantine pests for which specific measures are in place for the import of the commodity from the specific country in the relevant legislative texts for emergency measures (https://ec.europa.eu/food/plant/plant_health_biosecurity/legislation/emergency_measures_en); the assessment was restricted to whether or not the applicant country applies those measures. The effectiveness of those measures was not assessed.
Assessed whether the applicant country implements the special requirements specified in Annex VII (points 1–101) and Annex X of the Commission Implementing Regulation (EU) 2019/2072 targeting Union quarantine pests for the commodity in question from the specific country.
Assessed the effectiveness of the measures described in the dossier for those Union quarantine pests for which no specific measures are in place for the import of the commodity from the specific applicant country and other relevant pests present in applicant country and associated with the commodity.
Risk management decisions are not within EFSA’s remit. Therefore, the Panel provided a rating based on expert judgement regarding the likelihood of pest freedom for each relevant pest given the risk mitigation measures claimed to be implemented by the Ministry of Agriculture and Forestry of the Republic of Turkey.
2. Data and methodologies
2.1. Data provided by the Ministry of Agriculture and Forestry of the Republic of Turkey
The Panel considered all the data and information (hereafter called ‘the Dossier’) provided by Ministry of Agriculture and Forestry of the Republic of Turkey in November 2019, including the additional information provided by the Ministry of Agriculture and Forestry of the Republic of Turkey in December 2020 and in August 2021, after EFSA’s request. The Dossier is managed by EFSA.
The structure and overview of the Dossier is shown in Table 1. The number of the relevant section is indicated in the opinion when referring to a specific part of the Dossier.
Table 1.
Structure and overview of the Dossier
| Dossier section | Overview of contents | Filename |
|---|---|---|
| 1.0 | Technical dossier | Apple Technical Report‐TR‐05.10.2019.pdf |
| 2.0 | Updated Technical Dossier | Apple Technical Report‐V2‐11.12.2020.pdf |
| 3.0 | Additional information provided by the NPPO of Turkey in August 2021 | Answers‐Malus‐Q‐2019‐00790_0012‐Turkey.pdf |
The data and supporting information provided by the Ministry of Agriculture and Forestry of the Republic of Turkey formed the basis of the commodity risk assessment.
Table 2 shows the main data sources used by the Ministry of Agriculture and Forestry of the Republic of Turkey to compile the Dossier (details on literature searches can be found in the Dossier Section 2.0).
Table 2.
Database sources used in the literature searches by the Ministry of Agriculture and Forestry of the Republic of Turkey
| Acronym/short title | Database name and service provider | URL of database | Justification for choosing database |
|---|---|---|---|
| EPPO | Name: EPPO Global Database Provider: European and Mediterranean Plant Protection Organization | https://gd.eppo.int/ |
This database provides all pest‐specific information that has been produced or collected by EPPO. This database provides all pest‐specific information on host range, distribution ranges and pest status. |
| CABI | CABI: Invasive Species Compendium | https://www.cabi.org/isc/ | Encyclopaedic resource including science‐based information, comprising detailed data sheets on pests, diseases, weeds, host crops and natural enemies on trustable sources. |
| Plant Protection Bulletin | https://dergipark.org.tr/en/pub/bitkorb | Provides original research articles in English or Turkish languages on plant protection and health. | |
| Fauna Europaea | https://fauna‐eu.org/ | Main zoological taxonomic index in Europe, used to verify the taxonomic position of the insects. | |
| Plant Protection Products Database Application | https://bku.tarim.gov.tr/ | List of Registered Plant Protection Products in Turkey. |
2.2. Literature searches performed by EFSA
Literature searches in different databases were undertaken by EFSA to complete a list of pests potentially associated with M. domestica. The following searches were combined: (i) a general search to identify pests of M. domestica in different databases and (ii) a tailored search to identify whether these pests are present or not in Turkey and the EU. The searches were run between 24 January 2021 and 22 April 2021. No language, date or document type restrictions were applied in the search strategy.
The search strategy and search syntax were adapted to each of the databases listed in Table 3, according to the options and functionalities of the different databases and CABI keyword thesaurus.
Table 3.
Databases used by EFSA for the compilation of the pest list associated to M. domestica
As for Web of Science, the literature search was performed using a specific, ad hoc established search string (see Appendix B). The string was run in ‘All Databases’ with no range limits for time or language filters. This is further explained in Section 2.3.2.
Additional searches, limited to retrieve documents, were run when developing the opinion. The available scientific information, including previous EFSA opinions on the relevant pests and diseases (see pest data sheets in Appendix A) and the relevant literature and legislation (e.g. Regulation (EU) 2016/2031; Commission Implementing Regulations (EU) 2018/2019; (EU) 2018/2018 and (EU) 2019/2072) were taken into account.
2.3. Methodology
When developing the opinion, the Panel followed the EFSA Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019a).
In the first step, pests potentially associated with the commodity in the country of origin (EU‐quarantine pests and other pests) that may require risk mitigation measures were identified. The EU non‐quarantine pests not known to occur in the EU were selected based on evidence of their potential impact in the EU. After the first step, all the relevant pests that may need risk mitigation measures were identified.
In the second step, the proposed risk mitigation measures for each relevant pest were evaluated in terms of efficacy or compliance with EU requirements as explained in Section 1.2.
A conclusion on the likelihood of the commodity being free from each of the relevant pest was determined and uncertainties identified using expert judgements.
Pest freedom was assessed by estimating the number of infested/infected bundles out of 10,000 exported bundles of 10–25 plants each.
2.3.1. Commodity data
Based on the information provided by Turkey the characteristics of the commodity were summarised.
2.3.2. Identification of pests potentially associated with the commodity
To evaluate the pest risk associated with the importation of M. domestica from Turkey a pest list was compiled. The pest list is a compilation of all identified plant pests associated with M. domestica based on information provided in the Dossier Sections 1, 2, 3 and on searches performed by the Panel. The search strategy and search syntax were adapted to each of the databases listed in Table 3, according to the options and functionalities of the different databases and CABI keyword thesaurus.
The scientific names of the host plants (i.e. Malus domestica) were used when searching in the EPPO Global database and CABI Crop Protection Compendium. The same strategy was applied to the other databases excluding EUROPHYT and Web of Science.
EUROPHYT was consulted by searching for the interceptions associated to commodities imported from Turkey, at the species level, from 1994 to May 2020 and TRACES for interceptions from May 2020 to March 2022. For the pests selected for further evaluation a search in the EUROPHYT and/or TRACES was performed for the interceptions from the whole world, at the species level.
The search strategy used for Web of Science Databases was designed combining common names for pests and diseases, terms describing symptoms of plant diseases and the scientific and common names of the commodity. All the pests already retrieved using the other databases were removed from the search terms in order to be able to reduce the number of records to be screened.
The established search string is detailed in Appendix B and was run on 12 April 2021.
The titles and abstracts of the scientific papers retrieved were screened and the pests associated with M. domestica were included in the pest list. The pest list was eventually further compiled with other relevant information (e.g. EPPO code per pest, taxonomic information, categorisation, distribution) useful for the selection of the pests relevant for the purposes of this opinion.
The compiled pest list (see Microsoft Excel® filename in Appendix D) includes all identified pests that use M. domestica as host. According to the Interpretation of Terms of Reference.
The evaluation of the compiled pest list was done in two steps: first, the relevance of the EU‐quarantine pests was evaluated (Section 4.1); second, the relevance of any other plant pest was evaluated (Section 4.2).
Pests for which limited information was available on one or more criteria used to identify them as relevant for this opinion, e.g. on potential impact, are listed in Appendix C (List of pests that can potentially cause an effect not further assessed).
2.3.3. Listing and evaluation of risk mitigation measures
All proposed risk mitigation measures were listed and evaluated. When evaluating the likelihood of pest freedom at origin, the following types of potential infection sources for M. domestica in nurseries were considered (see also Figure 1):
pest entry from surrounding areas,
pest entry with new plants/seeds,
pest spread within the nursery.
Figure 1.
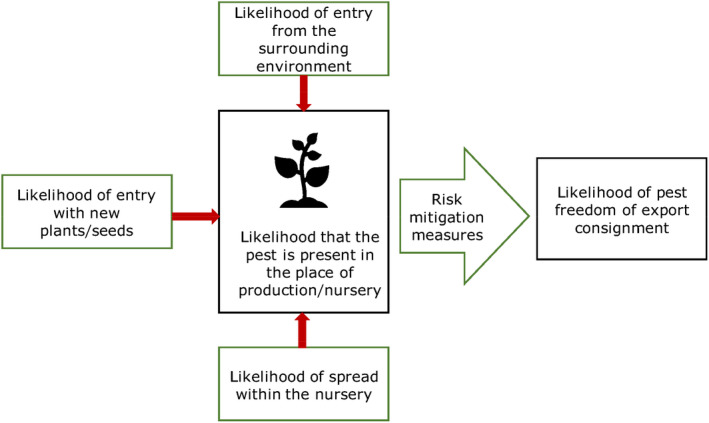
Conceptual framework to assess likelihood that plants are exported free from relevant pests. Source: EFSA PLH Panel (2019b)
The risk mitigation measures adopted in the plant nurseries (as communicated by Turkey) were evaluated with expert knowledge elicitation (EKE) according to the Guidance on uncertainty analysis in scientific assessment (EFSA Scientific Committee, 2018).
Information on the biology, estimates of likelihood of entry of the pest to the nursery and spread within the nursery, and the effect of the measures on a specific pest were summarised in pest data sheets compiled for each pest selected for further evaluation (see Appendix A).
2.3.4. Expert knowledge elicitation
To estimate the pest freedom of the commodity an EKE was performed following EFSA guidance (Annex B.8 of EFSA Scientific Committee, 2018).
The specific question for EKE was: ‘Taking into account (i) the risk mitigation measures in place in the nurseries, and (ii) other relevant information, how many of 10,000 bundles of M. domestica will be infested/infected with the relevant pest/pathogen when arriving in the EU?’. A bundle can contain from 10 to 25 plants.
The risk assessment uses bundles of 10–25 bare‐rooted plants or scions/budwood, as the most suitable unit. The following reasoning is given:
There is no quantitative information available regarding clustering of plants during production;
Plants are grouped in bundles of 10–25 after sorting;
For the pests under consideration, a cross‐contamination during transport is possible.
The EKE question was common to all pests for which the pest freedom of the commodity was estimated.
The uncertainties associated with the EKE were taken into account and quantified in the probability distribution applying the semi‐formal method described in Section 3.5.2 of the EFSA‐PLH Guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Finally, the results were reported in terms of the likelihood of pest freedom. The lower 5% percentile of the uncertainty distribution reflects the opinion that pest freedom is with 95% certainty above this limit.
3. Commodity data
3.1. Description of the commodity
The commodities to be imported are grafted plants, rootstocks, budwood and scions of M. domestica Borkh (common name: apple; family: Rosaceae). There are several apple rootstocks and varieties i.e. M7, M9, M26, M27, MM104, MM106, MM109, MM111, B9, G41, G935, Erva, Regalstar, Regalyou and Vita. The growing conditions are both field grown and grown in containers outside (pots, tubs). There are two types of grafts for the apple plants for propagation, clonal rootstocks planted at the nursery in February and bud‐grafted in August, and clonal rootstocks bench grafted (bare‐rooted grafted) and then planted in March. Grafted plants and rootstocks are bare rooted and without leaves. Budwood and scions are without leaves.
The commodities for export are the following types of M. domestica plants:
If whip and tongue grafting is used, the plants are grown for an additional 7‐ to 12‐month period. If T‐budding is used, the plants are grown for an additional 17‐ to 19‐month‐period.
Rootstocks are 8‐month‐old.
Budwood are 4‐ to 5‐month‐old.
Scions are 10‐ to 12‐month‐old.
The diameter of the exported grafted plants is 2.5–3 cm (Dossier, Section 3).
The assessment performed assumes that the characteristics of the commodity are as described above. According to ISPM 36 (FAO, 2019), the commodity can be classified as bare‐rooted plants for planting and budwood/graftwood.
3.2. Description of the production areas
The plants designated for export are grown in 30 different provinces in Turkey. The production is mainly concentrated in Isparta, Nigde, Bursa, Izmir and Konya provinces (Figure 2). Based on the global Köppen–Geiger climate zone classification (Kottek et al., 2006), the climate of these main production areas of M. domestica in Turkey, in particular Bursa, Isparta and Izmir provinces, is classified as Csa, main climate (C): temperate; (s): dry Summer;(a): hot Summer (Mediterranean climate). For Konya and Nigde provinces, the climate type is classified as Bsk, main climate (B): arid; (s: steppe;(k): cold.
Figure 2.
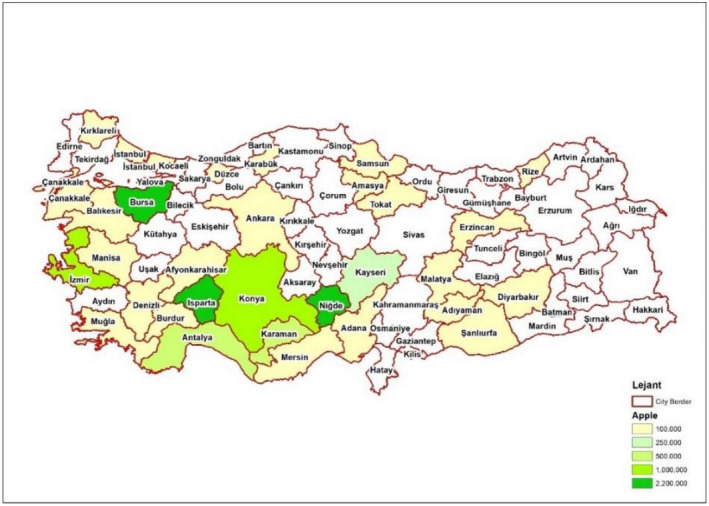
- Source: NPPO of Turkey.
3.3. Production and handling processes
3.3.1. Growing conditions
Prior to the establishment of the production sites, soil samples are taken and examined for the presence of quarantine organisms (e.g. root knot nematodes, etc.). Mother plants are subject to official control each year in spring, summer and autumn in terms of phytosanitary status. Phytosanitary inspectors check mother plants for the presence of harmful organisms. The production of plants is carried out in open field area.
Scions and budwood are taken from mother plants undergone control and supervision of the Ministry Provincial Directorate. This phytosanitary control is carried out on mother plants in spring, summer and autumn.
There are two different types of grafting for apple young plant propagation.
Clonal rootstocks are planted at the nursery in February and then bud‐grafted in August of the same year. Young plants are taken from the soil in November of the next year. Young plants are ready for delivery in 21 months from the planting of rootstock (Figure 3).
Clonal rootstocks are bench‐grafted and then planted at the nursery in March. Young plants are removed from the soil in November of the same year. Young plants are ready for delivery in 8 months.
Figure 3.

- Source: NPPO of Turkey.
3.3.2. Source of planting material
The propagation material (budwood, rootstocks, buds and scions) is obtained from the producer's own or another producer’s mother block. The mother blocks are under the control and supervision of the Ministry Provincial Directorate experts. The inspection and certification of the sapling and the propagation material is made by the Ministry experts. Before the establishment of mother block, soil sample is taken by the official inspector from the area subjected to official analysis in terms of quarantine organisms. The mother block can be established in the area determined to be free from quarantine organisms as a result of the analysis and basic certified saplings are planted in the area.
3.3.3. Production cycle
Before sapling production, an officer takes soil samples from the parcel for analysis for nematodes by the Ministry quarantine agency. If it is free from nematodes, production may begin. Before the rootstock planting, burnt animal manure, ammonium sulfate and urea fertiliser are applied to the growing area or mortar. In February, apple clonal rootstocks are planted in the sapling production parcel. During planting, Nogall application is made to protect against crown gall and rootstocks are planted. NPK fertilisers, humic acid, fulvic acid, organic fertilisers and plant growth regulators are applied to rootstocks and grafted plants through foliar or irrigation water. Plants are also sprayed against aphids, thrips, whiteflies, red spider pests, black spot, powdery mildew, root rot diseases and, depending on the situation, to control weeds. Grafting takes place in August or September. Bare‐rooted saplings are pulled out from the soil in dormant season.
For scions and budwood destined to the export, plant material is taken at the appropriate age (see Section 3.1). Apple fruit trees propagating material are produced under a certification scheme.
3.3.4. Pest monitoring during production
Official visual inspection is conducted at least once or twice a year during production or during uprooting of the plants. Visual inspection can be supported by the use of microscope or laboratory analysis if pests are suspected to be present; no further details were provided.
3.3.5. Post‐harvest processes and export procedure
Before the export, the plants are washed with water and their roots are cleaned from soil. Washed plants are labelled by making bundles of 10 or 25. In order to prevent water loss from the roots, before loading, the bundles are immersed in a solution of fosetyl‐al and then loaded. Official controls before export are carried out by the Ministry quarantine inspector. A phytosanitary certificate is issued to the saplings that are found suitable. Apple saplings are kept in cold storage at 98% humidity ± 2–4°C until the day they are marketed. Rootstocks to be exported are handled in a similar manner.
Scions and budwood are taken from the same mother plants that are used to produce the grafted plants, bundled and exported. The size of the bundles of scions and budwood was not specified, but we assume the same number of units per bundle as for rootstocks and grafted plants. No further details were available on handling and packing.
4. Identification of pests potentially associated with the commodity
The search for potential pests associated to M. domestica rendered 1,118 species (see Microsoft Excel® file in Appendix D).
4.1. Selection of relevant EU‐quarantine pests associated with the commodity
The EU listing of union quarantine pests and protected zone quarantine pests (Commission Implementing Regulation (EU) 2019/2072) is based on assessments concluding that the pests can enter, establish, spread and have potential impact in the EU.
Forty‐two EU‐quarantine species that are reported to use M. domestica as a host plant were evaluated (Table 5) for their relevance of being included in this opinion.
Table 5.
List of relevant pests selected for further evaluation
| Number | Current scientific name | EPPO code | Name used in the EU legislation | Taxonomic information | Group | Regulatory status |
|---|---|---|---|---|---|---|
| 1 | Calepitrimerus baileyi | CALEBA | Calepitrimerus baileyi | Acarida, Eriophyidae | Mite | Not regulated in the EU |
| 2 | Cenopalpus irani | – | Cenopalpus irani | Acarida, Tenuipalpidae | Mite | Not regulated in the EU |
| 3 | Cicadatra persica | – | Cicadatra persica | Hemiptera, Cicadidae | Insect | Not regulated in the EU |
| 4 | Diplodia bulgarica | – | Diplodia bulgarica | Ascomycota, Botryosphaeriaceae | Fungi | Not regulated in the EU |
| 5 | Hoplolaimus galeatus | HOLLGA | Hoplolaimus galeatus | Rhabditida, Hoplolaimidae | Nematode | Not regulated in the EU |
| 6 | Lopholeucaspis japonica | LOPLJA | Lopholeucaspis japonica | Hemiptera, Diaspididae | Insect | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 7 | Malacosoma parallela | MALAPA | Malocosoma parallela | Lepidoptera, Lasiocampidae | Insect |
Not regulated in the EU |
| 8 | Pratylenchus loosi | PRATLO | Pratylenchus loosi | Rhabditida, Pratylenchidae | Nematode | Not regulated in the EU |
| 9 | Pyrolachnus pyri | – | Pyrolachnus pyri | Hemiptera, Aphididae | Insect | Not regulated in the EU |
| 10 | Tomato ringspot virus | TORSV0 | Tomato ringspot virus | Picornavirales, Secoviridae | Virus | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072 |
The relevance of an EU‐quarantine pest for this opinion was based on evidence that:
the pest is present in Turkey.
M. domestica is a host of the pest.
one or more life stages of the pest can be associated with the specified commodity.
Pests that fulfilled all criteria were selected for further evaluation.
Table 4 presents an overview of the evaluation of the 42 EU‐quarantine pest species that are reported to use M. domestica as a host in regards of their relevance for this Opinion.
Table 4.
Overview of the evaluation of the 42 EU‐quarantine pest species known to use M. domestica as a host plant for their relevance for this opinion
| No. | Pest name according to EU legislation( a ) | EPPO code | Group | Pest present in Turkey | Malus domestica confirmed as a host (reference) | Pest can be associated with the commodity | Pest relevant for the opinion |
|---|---|---|---|---|---|---|---|
| 1 | Acleris minuta | ACLRMI | INS | No | Yes (CABI, online) | NA | No |
| 2 | Anastrepha fraterculus | ANSTFR | INS | No | Yes (CABI, online) | NA | No |
| 3 | Anastrepha ludens | ANSTLU | INS | No | Yes (CABI, online) | NA | No |
| 4 | Anastrepha suspensa | ANSTSU | INS | No | Yes (CABI, online) | NA | No |
| 5 | Anoplophora chinensis | ANOLCN | INS | Yes | Yes (CABI, online) | Yes | Yes |
| 6 | Anoplophora glabripennis | ANOLGL | INS | No | Yes (EPPO, online) | NA | No |
| 7 | Anthonomus quadrigibbus | TACYQU | INS | No | Yes (EPPO, online) | NA | No |
| 8 | Apple fruit crinkle viroid | AFCVD0 | VIR | No | Yes (EPPO, online) | NA | No |
| 9 | Apple necrotic mosaic virus | APNMV0 | VIR | No | Yes (EPPO, online) | NA | No |
| 10 | Apriona cinerea | APRICI | INS | No | Yes (EPPO, online) | NA | No |
| 11 | Apriona germari | APRIGE | INS | No | Yes (EPPO, online) | NA | No |
| 12 | Bactrocera dorsalis | DACUDO | INS | No | Yes (CABI, online) | NA | No |
| 13 | Bactrocera tryoni | DACUTR | INS | No | Yes (CABI, online) | NA | No |
| 14 | Bactrocera zonata | DACUZO | INS | No | Yes (EPPO, online) | NA | No |
| 15 | Bactrocera cucurbitae | DACUCU | INS | No | WOS Follet et al. 2019 | NA | No |
| 16 | Botryosphaeria kuwatsukai | PHYOPI | FUN | No | Yes (EPPO, online) | NA | No |
| 17 | Candidatus Phytoplasma aurantifolia | PHYPAF | BAC | No | Yes (CABI, online) | NA | No |
| 18 | Carposina sasakii | CARSSA | INS | No | Yes (CABI, online) | NA | No |
| 19 | Cherry rasp leaf virus | CRLV00 | VIR | No | Yes (EPPO, online) | NA | No |
| 20 | Choristoneura rosaceana | CHONRO | INS | No | Yes (EPPO, online) | NA | No |
| 21 | Conotrachelus nenuphar | CONHNE | INS | No | Yes (EPPO, online) | NA | No |
| 22 | Erwinia amylovora | ERWIAM | BAC | Yes | Yes (EPPO, online) | Yes | Yes |
| 23 | Grapholita inopinata | CYDIIN | INS | No | Yes (EPPO, online) | NA | No |
| 24 | Grapholita packardi | LASPPA | INS | No | Yes (EPPO, online) | NA | No |
| 25 | Grapholita prunivora | LASPPR | INS | No | Yes (EPPO, online) | NA | No |
| 26 | Gymnosporangium juniperi | FUN | Yes | CABI CPC online | No | No | |
| 27 | Lopholeucaspis japonica | LOPLIA | INS | Yes | Yes (EPPO, online) | Yes | Yes |
| 28 | Oemona hirta | OEMOHI | INS | No | Yes (EPPO, online) | NA | No |
| 29 | Phyllosticta solitaria | PHYSSL | FUN | No | Yes (PC https://doi.org/10.2903/j.efsa.2018.5510) | NA | No |
| 30 | Popillia japonica | POPIJA | INS | No | Yes (EPPO, online) | NA | No |
| 31 | Rhagoletis pomonella | RHAGPO | INS | No | Yes (EPPO, online) | NA | No |
| 32 | Saperda candida | SAPECN | INS | No | Yes (EPPO, online) | NA | No |
| 33 | Spodoptera eridania | PRODER | INS | No | Yes (CABI, online) | NA | No |
| 34 | Spodoptera frugiperda | LAPHFR | INS | No | Yes (CABI, online) | NA | No |
| 35 | Spodoptera litura | PRODLI | INS | No | Yes (CABI, online) | NA | No |
| 36 | Temperate fruit decay‐associated virus |
TFD AV0 |
VIR | No | Yes (Basso et al., 2015) | NA | No |
| 37 | Tobacco ringspot virus | TRSV00 | VIR | No | Yes (CABI, online) | NA | No |
| 38 | Tomato ringspot virus | TORSV0 | VIR | Yes | Yes (CABI, online) | Yes | Yes |
| 39 | Xiphinema americanum sensu stricto6 | XIPHAA | Nem | No | Yes (CABI, online) | NA | No |
| 40 | Xiphinema bricolense | XIPHBC | Nem | No | Yes (WoS Xu and Zhao, 2019) | NA | No |
| 41 | Xiphinema californicum | XIPHCA | Nem | No | Yes (WoS Xu and Zhao, 2019) | NA | No |
| 42 | Xiphinema rivesi (non‐EU populations) | XIPHRI | NEM | No | Yes (WoS Xu and Zhao, 2019) | NA | No |
: Commission Implementing Regulation (EU) 2019/2072.
: BAC: Bacteria and phytoplasmas; FUN: Fungi and oomycetes; INS: Insects and mites; NEM: Nematodes; VIR: Viruses and viroids.
Two species, known to use M. domestica as host, associated with the commodity and present in Turkey (Lopholeucaspis japonica, tomato ringspot virus) were selected for further evaluation.
Since special requirements or emergency measures are specified for Malus domestica with regards to Erwinia amylovora and Anoplophora chinensis, in Appendix X, item 9 of Commission Implementing Regulation (EU) 2019/2072 and Commission Implementing Regulation 2012/138/EU, respectively, the evaluation for these pests consisted of checking whether or not the exporting country applies these measures.
4.2. Selection of other relevant pests (non‐regulated in the EU) associated with the commodity
The information provided by Turkey, integrated with the search EFSA performed, was evaluated in order to assess whether there are other potentially relevant pests of M. domestica present in the country of export. For these potential pests that are non‐regulated in the EU, pest risk assessment information on the probability of entry, establishment, spread and impact is usually lacking. Therefore, these pests were also evaluated to determine their relevance for this opinion based on evidence that:
the pest is present in Turkey;
the pest is (i) absent or (ii) has a limited distribution in the EU;
M. domestica is a host of the pest;
one or more life stages of the pest can be associated with the specified commodity;
the pest may have an impact in the EU.
Pests that fulfilled the above listed criteria were selected for further evaluation.
Pest species were excluded from further evaluation when at least one of the conditions listed above (a‐e) was not met. Details can be found in the Appendix D (Microsoft Excel® file).
Of the evaluated pests not regulated in the EU, Calepitrimerus baileyi, Cenopalpus irani, Cicadatra persica, Diplodia bulgarica, Hoplomaimus galeatus, Malocosoma parallela, Pratylenchus loosi, Pyrolachnus pyri, were selected for further evaluation because these met all the selection criteria. More information on these pests can be found in the pest datasheets (Appendix A).
4.3. Overview of interceptions
Data on the interception of harmful organisms on plants of M. domestica can provide information on some of the organisms that can be present on M. domestica despite the current measures taken. According to EUROPHYT, online (accessed on March 2022) and TRACES‐NT, online (accessed on March 2022), there were no interceptions of plants for planting of M. domestica from Turkey destined to the EU Member States due to presence of harmful organisms between the years 1994 and March 2022.
4.4. List of potential pests not further assessed
The Panel highlighted one species (Phytophthora rosacearum) for which the distribution within the EU is uncertain, since it may be identified as Phytophthora megasperma in the past. The Panel also identified Colletotrichum siamense as a potential pest, but this was based on a single report of the fungus from banana in a ripening room (Appendix C).
4.5. Summary of pests selected for further evaluation
The 10 pests identified to be present in Turkey while having potential for association with the commodities destined for export are listed in Table 5. The effectiveness of the risk mitigation measures applied to the commodity was evaluated for 10 of these selected pests (Calepitrimerus baileyi, Cenopalpus irani, Cicadatra persica, Diplodia bulgarica, Hoplomaimus galeatus, Lopholeucaspis japonica, Malocosoma parallela, Pratylenchus loosi, Pyrolachnus pyri and tomato ringspot virus).
5. Risk mitigation measures
For the 10 selected pests (Table 5), the Panel assessed the possibility that they could be present in a M. domestica nursery and assessed the probability that pest freedom of a consignment is achieved by the proposed risk mitigation measures acting on the pest under evaluation.
The information used in the evaluation of the effectiveness of the risk mitigation measures is summarised in a pest data sheet (see Appendix A).
5.1. Possibility of pest presence in the export nurseries
For these 10 pests (Table 5), the Panel evaluated the likelihood that the pest could be present in a M. domestica nursery by evaluating the possibility that M. domestica in the export nursery are infested either by:
introduction of the pest from the environment surrounding the nursery;
introduction of the pest with new plants/seeds;
spread of the pest within the nursery.
5.2. Risk mitigation measures applied in Turkey
With the information provided by Turkey (Dossier sections 1.0, 2.0 and 3.0), the Panel summarised the risk mitigation measures (see Table 6) that are proposed in the production nurseries.
Table 6.
Overview of proposed risk mitigation measures for Malus domestica plants designated for export to the EU from Turkey
| No. | Risk mitigation measure (name) | Implementation in Turkey |
|---|---|---|
| 1 | Certified material |
The Ministerial experts and inspectors carry out the phytosanitary control on mother plants in spring, summer and autumn for harmful organisms, and the amount of propagation materials (buds, budwoods, rootstocks, scions, etc.) that can be obtained from mother plants is determined. For the saplings, the phytosanitary control is also carried out at the same time, regarding harmful organisms specified in quarantine and plant passports, and certification regulations. Rootstocks from certified plants are grafted with certified budwood or scions in a certified nursery. If free from the harmful organisms, the Ministry issues certificates and labels for the propagation material to be taken from plants in the mother blocks. |
| 2 | Phytosanitary certificates |
Export nurseries must obtain special certification from Turkish Authorities before they begin producing plants for planting. Nurseries must notify technical staff members responsible for production to obtain this certificate, which is then used for registration in the Turkish plant certification system. The phytosanitary inspections are done macroscopically. However, if there are signs of disease in the plants or in the immediate vicinity, the inspections are carried out by laboratory analysis. During the production period, official inspection is carried out. After the official approval that the sapling is free from the quarantine factor and true to type, its certificate‐passport label is issued by the Ministry. The Phytosanitary Certificates/Re‐Export Phytosanitary Certificates are issued in exportation of plants and plant products with respect to plant health. In issuing such certificates, the phytosanitary requirements of the importer country are taken into account, in compliance with the ISPM No: 7 and ISPM No: 12 rules. |
| 3 | Cleaning and disinfection of facilities, tools and machinery | Tools are disinfested with chemical compounds containing 10% chlorine prior to use. |
| 4 | Rouging and pruning | Applied in case of infections/infestations. No further details are available. |
| 5 | Biological and mechanical control |
Weeds are controlled mechanically in the nurseries and in the surrounding areas. During rootstocks planting, Nogall (biological control agent) is applied to protect against crown gall. |
| 6 | Pesticide application |
The saplings are sprayed against aphids, thrips, whiteflies, red spider pests, black spot, powdery mildew, root rot diseases and, depending on the situation, to control weeds. Before loading the plants on the trucks for transport, the roots of seedlings are sprayed with fungicide (Thiram). No specific details were available. |
| 7 | Surveillance and monitoring |
Both processes are conducted by Turkish inspectors according to Turkish phytosanitary regulations. According to the dossier, necessary precautions are taken to ensure that there are no plants other than certified saplings in the production plot and application areas. Plants within and around the production areas are annually inspected to check the presence of quarantine organisms. Visual inspection at least once or twice a year during production or during uprooting of the plants. Visual inspection can be supported by the use of microscope or laboratory analysis if pests are suspected to be present. In the event that these plants are infected/infested with harmful organisms subject to quarantine, in Turkey these plants are destroyed. |
| 8 | Sampling and laboratory testing |
For the identification of viruses, bacteria, fungi and nematodes in the seedlings to be exported, min. 5 to max. 25 seedlings are randomly taken from the plantation in the nursery garden and sealed by the inspector and sent to the laboratory for analysis. Soil samples are taken for laboratory analysis in terms of quarantine organisms, particularly to check if it is free from nematodes. If it is found that the soil is free from nematodes and other quarantine organisms, the production of saplings is started. |
| 9 | Root washing | Roots are washed to remove the soil |
| 10 | Refrigeration | The temperature of the storage tanks is between 2°C and 4°C and the humidity is 85–95%. Transportation is made with refrigerated trucks with the same conditions. |
| 11 | Pre‐consignment inspection | Prior to export, planting material for which a Phytosanitary Certificate is to be issued shall be subjected to phytosanitary inspection. Only certified plants for planting may be exported. Phytosanitary inspectors are responsible for export controls, sampling and issuing certificates. |
5.3. Evaluation of the current measures for the selected relevant pests including uncertainties
For each evaluated pest the relevant risk mitigation measures acting on the pest were identified. Any limiting factors on the effectiveness of the measures were documented.
All the relevant information including the related uncertainties deriving from the limiting factors used in the evaluation are summarised in a pest data sheet provided in Appendix A.
Based on this information, for each selected relevant pest, an expert judgement is given for the likelihood of pest freedom taking into consideration the risk mitigation measures and their combination acting on the pest.
An overview of the evaluation of each relevant pest is given in the sections below (Sections 5.3.1–5.3.10). The outcome of the EKE regarding pest freedom after the evaluation of the proposed risk mitigation measures is summarised in the Section 5.3.11.
5.3.1. Overview of the evaluation of Calepitrimerus baileyi (in bundles of all the commodity types)
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest free bundles |
9,956 out of 10,000 bundles |
9,969 out of 10,000 bundles |
9,981 out of 10,000 bundles |
9,990 out of 10,000 bundles |
9,997 out of 10,000 bundles |
| Proportion of infested bundles |
3 out of 10,000 bundles |
10 out of 10,000 bundles |
19 out of 10,000 bundles |
31 out of 10,000 bundles |
44 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associate with the commodity C. baileyi deutogynes hibernate mainly in small, permanently dormant buds and under the loose bark of spurs and around buds on 1‐year‐old shoots. Malus domestica is a host of the pest and the species can complete its life cycle on this host, however sometimes this species is vagrant surviving on the leaves. The most possible way to spread is through the introduction of plant materials, as the mite can be found in buds, even in resting ones. There is no reference in the literature regarding the possibility of fruit being a pathway. There are no data on the active dispersal capacity of the pest. It is present in Turkey with some details on its distribution, however there is no C. baileyi pest‐free area in Turkey. Measures taken against the pest and their efficacy The relevant proposed measures are: (i) Inspection, certification and surveillance, (ii) Roguing and pruning, (iii) Pesticide application, (iv) Natural biological control, (v) Refrigeration and (vi) Pre‐consignment inspection. Interception records There are no records of interceptions from Turkey. Shortcomings of current measures/procedures Visual inspection especially in the case of low infestations without using an adequate magnification considering the tiny size of the individuals both adults and juveniles. Phytoseiid species are reported preying on this species. They can be present in the environment though no details are provided in the dossier. Chemical applications can affect biological control agents. Some of the pesticides listed in the dossier might be effective against the mite, specifically acrinathrin and abamectin. However, no details are given on the pesticide application schedule and on the application methods. Low storage temperature can prevent or slow down the development of the pest but will not eliminate it. Main uncertainties – It is unclear whether the pesticides are applied on a calendar basis or following ad hoc application as function of pest presence, or both – Screening of certified material for this pest could not ensure pest absence because of the tiny size of the individuals both adults and juveniles |
||||
5.3.2. Overview of the evaluation of Cenopalpus irani (in bundles of all the commodity types)
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest free bundles |
9,952 out of 10,000 bundles |
9,968 out of 10,000 bundles |
9,980 out of 10,000 bundles |
9,990 out of 10,000 bundles |
9,999 out of 10,000 bundles |
| Proportion of infested bundles |
1 out of 10,000 bundles |
10 out of 10,000 bundles |
20 out of 10,000 bundles |
32 out of 10,000 bundles |
48 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associate with the commodity Cenopalpus irani is phytophagous, and has been reported on apple, pear, olive, walnut, quince, grapevine, sour cherry, plum, peach, fig and pistachio. It is widely distributed in apple orchards and one of the most important tenuipalpid pests on apple in Iran. C. irani feeds on stems, fruits, flowers, and leaves, often on the lower surface. Possible pathways of entry for C. irani are plants for planting since these mites overwinter in branches. It can spread by wind currents and longer distance dispersion can occur by transportation of planting material. It is reported as present in Turkey with no further details on its distribution. Measures taken against the pest and their efficacy The relevant proposed measures are: (i) Inspection, certification and surveillance, (ii) Cleaning and disinfection of facilities, tools and machinery, (iii) Roguing and pruning, (iv) Pesticide application, (v) Natural biological control, (vi) Refrigeration and (vii) Pre‐consignment inspection. Interception records There are no records of interceptions from Turkey. Shortcomings of current measures/procedures Potential C. irani infestations might be overlooked by visual inspection especially in the case of low infestations without using an adequate magnification considering the tiny size of the individuals both adults (ca. 0.3 mm length) and juveniles (ca. 0.2 mm length). The main predators in apple orchards belong to the families Phytoseiidae and Stigmaeidae. They can be present in the environment though no details are provided in the dossier. Some of the pesticides listed in the dossier might be effective against the mite, specifically acrinathrin and abamectin. However, no details are given on the pesticide application schedule and on the application methods. Low storage temperature can prevent or slow down the development of the pest but will not eliminate it. Main uncertainties • It is unclear whether the pesticides are applied on a calendar basis or following ad hoc application as function of pest presence, or both • Screening of certified material for this pest could not ensure pest absence because of the tiny size of the individuals both adults and juveniles |
||||
5.3.3. Overview of the evaluation of Cicadatra persica (in bundles of all the commodity types)
| Rating of the likelihood of pest freedom | Almost always pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest free plants |
9,999 out of 10,000 bundles |
9,999.3 out of 10,000 bundles |
9,999.5 out of 10,000 bundles |
9,999.8 out of 10,000 plants |
9,999.9 out of 10,000 bundles |
| Proportion of infested plants |
0.1 out of 10,000 bundles |
0.2 out of 10,000 bundles |
0.5 out of 10,000 bundles |
0.7 out of 10,000 bundles |
1 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associate with the commodity The only host reported is Malus domestica. Eggs are laid in small twigs and nymphs feed on the roots. Measures taken against the pest and their efficacy The relevant proposed measures are: (i) Inspection, certification and surveillance, (ii) Roguing and pruning, (iii) Pesticide application, (iv) Refrigeration and (v) Pre‐consignment inspection. Interception records In the EUROPHYT/TRACES NT database, there are /no interceptions of C. persica on plants for planting from Turkey. Shortcomings of current measures/procedures Visual detection of pest presence is difficult, due to egg laying inside stems and small branches. This causes twigs to split and die, causing a symptom called flagging which is also due to other pests. To confirm that a plant is infested by C. persica and not by another pests, it is essential to identify the species by morphological or molecular analyses. Chemical control of eggs and nymphs is usually not very effective because the eggs are laid inside tissue and the nymphs stay in the soil. No details are given on which pesticides are applied from those listed in Dossier, Section 2.0 on the pesticide application schedule and on the application methods. Low temperatures can slow down its development but not kill the insect. Main uncertainties – Eggs can be overlooked – Symptoms may be misclassified with other pests – The insecticide applications are not targeted to C. persica and may not be effective |
||||
5.3.4. Overview of the evaluation of Diplodia bulgarica (in bundles of all the commodity types)
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest free plants | 9,863 out of 10,000 bundles | 9,900 out of 10,000 bundles | 9,935 out of 10,000 bundles | 9,965 out of 10,000 bundles | 9,991 out of 10,000 bundles |
| Proportion of infested plants | 9 out of 10,000 bundles | 35 out of 10,000 bundles | 65 out of 10,000 bundles | 100 out of 10,000 bundles | 137 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associate with the commodity D. bulgarica was detected for the first time in 2021 in M. domestica in Turkey. It causes a severe canker disease on M. domestica in several other countries. It is possible that local populations of D. bulgarica are present in the neighbouring environment of the nursery with plants destined for export. Measures taken against the pest and their efficacy The primary measures taken in Turkey that would be effective against D. bulgarica include the use of certified material, regular inspections, and the use of pesticides. Interception records There are no records of interceptions from Turkey. Shortcomings of current measures/procedures There are no main shortcomings. Main uncertainties Pest pressure and the proximity of population sources in the surrounding environment is unknown. Efficacy of surveillance of the nursery and mother plants is not known. |
||||
5.3.5. Overview of the evaluation of Hoplolaimus galeatus (in bundles of rooted plants)
| Rating of the likelihood of pest freedom | Pest free with few exceptional cases (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest free bundles |
9,982 out of 10,000 bundles |
9,988 out of 10,000 bundles |
9,992 out of 10,000 bundles |
9,996 out of 10,000 bundles |
9,999 out of 10,000 bundles |
| Proportion of infested bundles |
1 out of 10,000 bundles |
4 out of 10,000 bundles |
8 out of 10,000 bundles |
12 out of 10,000 bundles |
18 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest/pathogen could enter exporting nurseries Hoplolaimus galeatus is a polyphagous, migratory endoparasite that occurs in both soil and roots and feeds on the cortical and vascular tissue of host plants. It can also be found as an ectoparasite. The nematode is widely distributed in the USA and parasitises various crops, grasses and woody plants. It has also been found in Canada, Sumatra, India, Tanzania, Central and South America, Pakistan, Australia, Spain and Turkey. H. galeatus is a serious pest in native lawns and golf courses and can also be very damaging to many crops, such as cotton, soybean, alfalfa, and corn. It has also been reported as a problem in some orchards (apple, cherry and peach trees) in Michigan, USA. In Turkey, H. galeatus has been found on sweet chestnut, cowpea, sesame, vegetable, kidney bean, plum, peach, olive, sunflower and apple. According to data available, the nematode has been reported in four regions (Antalya, Isparta, Sinop, Eskisehir) (Kepenekci, 2001, 2002; Kepenekci & Zeki, 2002). So far, no epidemics or economic losses have been reported in Turkey. The main pathways of this nematode are infested plants for planting, contaminated water, soil and growing media as such or attached to plants, agricultural machinery, tools and shoes. This nematode can be found in the roots of apple plants or other host plants in the environment and infest the commodity mainly through human‐assisted dispersal. Measures taken against the pest/pathogen and their efficacy The relevant proposed measures are: (i) Inspection, certification and surveillance, (ii) Sampling and laboratory testing, (iii) Selection of production sites, (iv) Removal of soil from roots (washing), and (v) Pre‐consignment inspection. Interception records There are no records of interceptions from Turkey. Shortcomings of current measures/procedures Lance nematodes (Hoplolaimus spp.) are not on the list of harmful organisms systematically monitored or tested for their presence on plants intended for planting in Turkey. Soil and plants are tested in the laboratory only for the presence of root‐knot and virus vector nematodes, but not for the presence of Hoplolaimus spp. The undetected presence of this nematode during inspections may contribute to the spread of H. galeatus infection. In addition, pre‐export root washing does not reduce the risk of nematode infestation in plants intended for planting that are infested with lance nematodes (migratory endoparasites). Main uncertainties • Soil is laboratory tested only for the presence of root‐knot and virus vector nematodes, but not for the presence of Hoplolaimus spp. • Symptoms caused by H. galeatus may be overlooked. • Presence of H. galeatus cannot be detected. • Root washing does not reduce the risk of nematodes (migratory endoparasites) infestation in plants intended for planting. |
||||
5.3.6. Overview of the evaluation of Lopholeucaspis japonica
| Rating of the likelihood of pest freedom |
Pest free with some exceptional cases – rooted plants (based on the Median) Pest free with few exceptional cases – scions and budwoods (based on the Median) |
||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest free bundles (rooted plants) |
9,956 out of 10,000 bundles |
9,971 out of 10,000 bundles |
9,985 out of 10,000 bundles |
9,993 out of 10,000 bundles |
9,999 out of 10,000 bundles |
| Proportion of infested bundles (rooted plants) |
1 out of 10,000 bundles |
7 out of 10,000 bundles |
15 out of 10,000 bundles |
29 out of 10,000 bundles |
44 out of 10,000 bundles |
| Proportion of pest free bundles (scions and budwood) |
9,978 out of 10,000 bundles |
9,986 out of 10,000 bundles |
9,982 out of 10,000 bundles |
9,996.5 out of 10,000 bundles |
9,999.5 out of 10,000 bundles |
| Proportion of infested bundles (scions and budwood) |
0.5 out of 10,000 bundles |
3.5 out of 10,000 bundles |
8 out of 10,000 bundles |
14 out of 10,000 bundles |
22 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Lopholeucaspis japonica is a polyphagous armoured scale that feeds on plants belonging to 38 families, with Malus domestica being reported as a host. Crawlers can be dispersed by wind or insects (ants, flies and ladybirds), occasionally also by human transport. Plants for planting and cut branches are reported as possible pathways. It is present in Turkey. It was recorded on Citrus spp. Up to date, there is no record on apple in Turkey. It was detected in the Black Sea region (Artvin, Giresun, Ordu, Samsun, Trabzon, Rize provinces) (Kaydan et. al., 2013); however, there is no L. japonica pest‐free area in Turkey. Measures taken against the pest and their efficacy The relevant proposed measures are: (i) Inspection, certification and surveillance, (ii) Roguing and pruning, (iii) Pesticide application, (iv) Natural biological control, (v) Refrigeration and (vi) Pre‐consignment inspection. Interception records There are no records of interceptions from Turkey. Shortcomings of current measures/procedures Low initial infestations might be overlooked and macroscopic misidentification is possible. Chemical applications can affect biological control agents. Chemicals are applied targeting mainly crawlers, however, no details are given on which pesticides are applied from those listed in Dossier, Section 2.0, on the pesticide application schedule and on the application methods. Low storage temperature can prevent or slow down the development of the pest but will not eliminate it. Main uncertainties – No records of L. japonica on Malus are available. – It is unclear whether the pesticides are applied on a calendar basis or following ad hoc application as function of pest presence, or both – Screening of certified material for this pest could not ensure pest absence because young stages can be difficult to detect. – The pest was detected in the Black Sea region, however no pest‐free area is determined in Turkey. |
||||
5.3.7. Overview of the evaluation of Malacosoma parallela (in bundles of all the commodity types)
| Rating of the likelihood of pest freedom | Almost always pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest free bundles |
9,991 out of 10,000 bundles |
9,994 out of 10,000 bundles |
9,996 out of 10,000 bundles |
9,998 out of 10,000 bundles |
10,000 out of 10,000 bundles |
| Proportion of infested bundles |
0 out of 10,000 bundles |
2 out of 10,000 bundles |
4 out of 10,000 bundles |
6 out of 10,000 bundles |
9 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associate with the commodity M. parallela is extremely polyphagous and causes most damage in its native range to Quercus spp., Prunus spp., and Malus spp. Significant damage also occurs on various other woody species, including many native species of Central Asia. Malacosoma parallela is present in Turkey, with no further details on its distribution. M. parallela can spread by flights of adult moths. All stages of the life cycle can be transported on host plants moving in trade, particularly plants for planting and cut branches. Eggs, larvae and pupae (cocoons) may be associated with wood carrying bark and may be present as contaminants on other commodities. Measures taken against the pest and their efficacy The relevant proposed measures are: (i) Inspection, certification and surveillance, (ii) Roguing and pruning, (iii) Pesticide application, (iv) Natural biological control, (v) Refrigeration and (vi) Pre‐consignment inspection. Interception records There are no records of interceptions of M. domestica plants for planting from Turkey Shortcomings of current measures/procedures Egg masses might be overlooked by non‐trained personnel. Some of the pesticides listed in the dossier might be effective against the moth. However, no details are given on which pesticides are applied from those listed in Dossier, Section 2.0, on the pesticide application schedule and on the application methods. Low temperatures can slow down its development but not kill the insect. Main uncertainties – The pest is reported in Turkey with no details on its distribution – Egg masses might be overlooked by non‐trained personnel – The insecticide applications are not targeted to M. parallela and may not be effective |
||||
5.3.8. Overview of the evaluation of Pratylenchus loosi (in bundles of rooted plants)
| Rating of the likelihood of pest freedom | Almost always pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest free bundles |
9,996 out of 10,000 bundles |
9,997 out of 10,000 bundles |
9,998 out of 10,000 bundles |
9,999 out of 10,000 bundles |
10,000 out of 10,000 bundles |
| Proportion of infested bundles |
0 out of 10,000 bundles |
1 out of 10,000 bundles |
2 out of 10,000 bundles |
3 out of 10,000 bundles |
4 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest/pathogen could enter exporting nurseries Pratylenchus loosi is a polyphagous, migratory endoparasite found in both soil and roots. It is considered the most serious pest of tea in Sri Lanka and many other tea‐producing countries including India, Japan, Korea, Taiwan, Iran and Russia. Yield reduction can range from 4 to 40%. Damage is greater in young infested tea plantations and nurseries where damage of 60 to 100% may occur if adequate control measures are not taken. This nematode has also been found on several important crops such as apples, oranges, pears, potatoes, eggplants, wheat, lentils, pasture grasses, coffee, cabbage and bananas. In Turkey, P. loosi has been reported from limited areas in very low populations in potato, eggplant, wheat and lentils but has not been found on apples. According to the available information, the nematode has been reported on cultivated plants in Turkey in two regions (Sanliurfa, Ankara). So far, no epidemics or economic losses have been reported in Turkey, but uncertainties exist due to lack of data from official monitoring surveys and reports of problems caused by this nematode in Turkish apple production. The main pathways of this nematode are infested plants for planting, contaminated water, soil and growing media as such or attached to plants, agricultural machinery, tools and shoes. This nematode may be present in the roots of apple plants or other host plants found in the environment and may infest the commodity mainly through human‐assisted dispersal. Measures taken against the pest/pathogen and their efficacy The relevant proposed measures are: (i) Inspection, certification and surveillance, (ii) Sampling and laboratory testing, (iii) Selection of production sites, (iv) Removal of soil from roots (washing), and (v) Pre‐consignment inspection. Interception records There are no records of interceptions from Turkey. Shortcomings of current measures/procedures Root‐lesion nematodes (Pratylenchus spp.) are not on the list of harmful organisms systematically monitored or tested for their presence on plants intended for planting in Turkey. Soil and plants are tested in the laboratory only for the presence of root‐knot and virus vector nematodes, but not for the presence of Pratylenchus spp. The undetected presence of this nematode during inspections may contribute to the spread of P. loosi infection. In addition, pre‐export root washing does not reduce the risk of nematode infestation in plants intended for planting that are infested with root lesion nematodes (migratory endoparasites). Main uncertainties • Soil is laboratory tested only for the presence of root‐knot and virus vector nematodes, but not for the presence of Pratylenchus spp. • Symptoms caused by P. loosi may be overlooked. • Presence of P. loosi is not easy to be detected. • Root washing does not reduce the risk of nematodes (migratory endoparasites) infestation in plants intended for planting. |
||||
5.3.9. Overview of the evaluation of Pyrolachnus pyri (in bundles of all the commodity types)
| Rating of the likelihood of pest freedom | Pest free with some exceptions (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest free plants |
9,964 out of 10,000 bundles |
9,975 out of 10,000 bundles |
9,985 out of 10,000 bundles |
9,992 out of 10,000 bundles |
9,998 out of 10,000 bundles |
| Proportion of infested plants |
2 out of 10,000 bundles |
8 out of 10,000 bundles |
15 out of 10,000 bundles |
25 out of 10,000 bundles |
36 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associate with the commodity The pest is reported on Malus domestica. Eggs are laid on branches where nymphs and adults also feed. Measures taken against the pest and their efficacy The relevant proposed measures are: (i) Inspection, certification and surveillance, (ii) Roguing and pruning, (iii) Pesticide application, (iv) Refrigeration and (v) Pre‐consignment inspection. Interception records There are no records of interceptions from Turkey. Shortcomings of current measures/procedures Visual detection of pest adults and nymphs is not difficult, though eggs laid on branches can be overlooked. No details are given on which pesticides are applied from those listed in Dossier, Section 2.0, on the pesticide application schedule and on the application methods. Low temperatures can slow down its development but not kill the insect. Main uncertainties • Eggs can be overlooked • Symptoms (i.e. honeydew and sooty moulds) may be misclassified with other pests • The insecticide applications are not targeted to P. pyri and may not be effective |
||||
5.3.10. Overview of the evaluation of Tomato ringspot virus (in bundles of all the commodity types)
| Rating of the likelihood of pest freedom | Almost always pest free (based on the Median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
|
Proportion of pest free bundles |
9,991 out of 10,000 bundles |
9,994 out of 10,000 bundles |
9,996 out of 10,000 bundles |
9,999 out of 10,000 bundles |
10,000 out of 10,000 bundles |
| Proportion of infested bundles |
0 out of 10,000 bundles |
1 out of 10,000 bundles |
4 out of 10,000 bundles |
6 out of 10,000 bundles |
9 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest/pathogen could enter exporting nurseries ToRSV has a wide host range, including herbaceous and woody plant species. Its occurrence in Turkey is restricted to four provinces/regions, where ToRSV has been found in some cultivated plant species. The dispersal range of ToRSV infection by natural processes appear to be constrained, as the nematode‐vector species of the Xiphinema americanum group have not been reported recently in Turkey. Measures taken against the pest/pathogen and their efficacy Only certified class plant material is used at the production areas, and quarantine practices are carried out in accordance with the ‘Seedling Certification Regulation’ and ‘Regulation on the Registration of Plant Passports and Operators’. Interception records There are no records of interceptions of M. domestica plants for planting from Turkey due to the presence of ToRSV. Shortcomings of current measures/procedures Details on the inspections and surveillance to detect ToRSV. Main uncertainties The certification process/status of the material. ToRSV dispersal by other nematode species is unknown and by other means (seeds or pollen to the mother plant) are unclear in woody plants. The extent of the inspections to detect ToRSV infections is unknown. |
||||
5.3.11. Outcome of expert knowledge elicitation
Table 7 and Figure 4 show the outcome of the EKE regarding pest freedom after the evaluation of the proposed risk mitigation measures for all the evaluated pests.
Table 7.
Assessment of the likelihood of pest freedom following evaluation of current risk mitigation measures against Calepitrimerus baileyi, Cenopalpus irani, Cicadatra persica, Diplodia bulgarica, Hoplomaimus galeatus, Lopholeucaspis japonica, Malocosoma parallela, Pratylenchus loosi, Pyrolachnus pyri and tomato ringspot virus on Malus domestica plants designated for export to the EU. In panel A, the median value for the assessed level of pest freedom for each pest is indicated by ‘M’, the 5% percentile is indicated by L, and the 95% percentile is indicated by U. The percentiles together span the 90% uncertainty range regarding pest freedom. The pest freedom categories are defined in panel B of the table
| Number | Group* | Pest species |
Sometimes pest free |
More often than not pest free |
Frequently pest free |
Very frequently pest free |
Extremely frequently pest free |
Pest free with some exceptional cases | Pest free with few exceptional cases |
Almost always pest free |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mite | Calepitrimerus baileyi | LM | U | ||||||
| 2 | Mite | Cenopalpus irani | LM | U | ||||||
| 3 | Insect | Cicadatra persica | LMU | |||||||
| 4 | Fungi | Diplodia bulgarica | L | M | U | |||||
| 5 | Nematode | Hoplolaimus galeatus | L | M | U | |||||
| 6 | Insect | Lopholeucaspis japonica – rooted plants | LM | U | ||||||
| 7 | Insect | Lopholeucaspis japonica – scions and budwood | L | M | U | |||||
| 8 | Insect | Malacosoma parallela | L | MU | ||||||
| 9 | Nematode | Pratylenchus loosi | LMU | |||||||
| 10 | Mite | Pyrolachnus pyri | LM | U | ||||||
| 11 | Virus | Tomato ringspot virus | L | MU | ||||||
| PANEL A | ||||||||||
Figure 4.
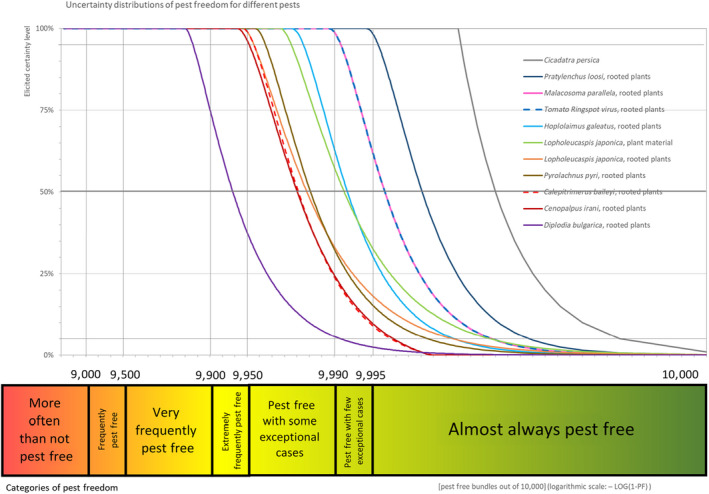
Elicited certainty (y‐axis) of the number of pest‐free Malus domestica bundles (x‐axis; log‐scaled) out of 10,000 plants designated for export to the EU from Turkey for all evaluated pests visualised as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%). The Panel is 95% confident that 9,956, 9,952, 9,999, 9,863, 9,982, 9,956, 9,991, 9,996, 9,964 and 9,991 or more bundles per 10,000 will be free from Calepitrimerus baileyi, Cenopalpus irani, Cicadatra persica, Diplodia bulgarica, Hoplomaimus galeatus, Lopholeucaspis japonica, Malacosoma parallela, Pratylenchus loosi, Pyrolachnus pyri and tomato ringspot virus, respectively
Figure 5 provides an explanation of the descending distribution function describing the likelihood of pest freedom after the evaluation of the proposed risk mitigation measures for Malus domestica trees designated for export to the EU for Diplodia bulgarica.
Figure 5.
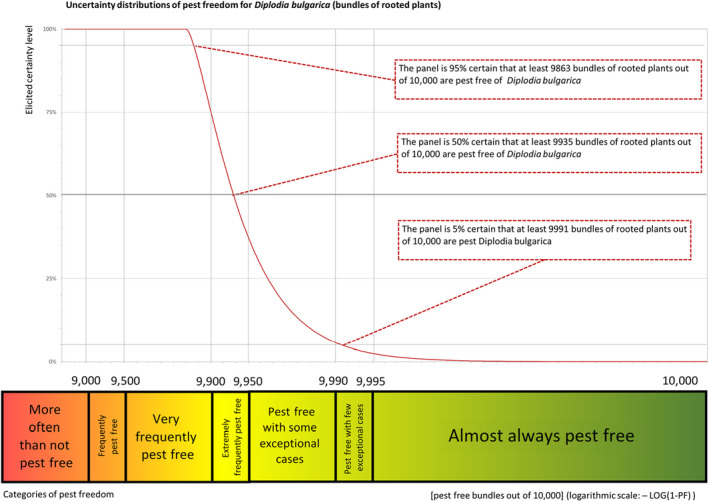
Explanation of the descending distribution function describing the likelihood of pest freedom after the evaluation of the proposed risk mitigation measures for plants designated for export to the EU based on based on the example of Diplodia bulgarica
| Pest freedom category |
Pest fee plants out of 10,000 |
|
|---|---|---|
|
Sometimes pest free |
≤ 5,000 | |
|
More often than not pest free |
5,000–≤ 9,000 | |
|
Frequently pest free |
9,000–≤ 9,500 | |
|
Very frequently pest free |
9,500–≤ 9,900 | |
|
Extremely frequently pest free |
9,900–≤ 9,950 | |
|
Pest free with some exceptional cases |
9,950–≤ 9,990 | |
|
Pest free with few exceptional cases |
9,990–≤ 9,995 | |
|
Almost always pest free |
9,995–≤ 10,000 |
| Legend of pest freedom categories | ||
|---|---|---|
| L |
Pest freedom category includes the elicited lower bound of the 90% uncertainty range |
|
| M |
Pest freedom category includes the elicited median |
|
| U |
Pest freedom category includes the elicited upper bound of the 90% uncertainty range |
|
| PANEL B | ||
5.4. Evaluation of the application of specific measures in the Turkey
Annex X of the Commission Implementing Regulation (EU) 2019/2072 specifies a list of plants, plant products and other objects, originating from third countries and the corresponding special requirements for their introduction into the Union territory or Protected Zones. According to the above‐mentioned annexes special measures are required for the import of the commodity from Turkey related to Erwinia amylovora.
According to Commission Implementing Decision (EU) 2012/138, special measures are in place for import of Malus domestica plants with respect to Anoplophora chinensis.
The evaluation of the specific measures is specified in Table 8.
Table 8.
Evaluation of specific measures regarding Erwinia amylovora and Anoplophora chinensis which are in place for the import of the commodities from Turkey
| Pest name | Point | Evaluation of Specific measure to be implemented |
|---|---|---|
| Erwinia amylovora | Commission Implementing Regulation (EU) 2019/2072, Annex X, item 9 | Based on the information provided in the dossier, including the supplementary information, the exporting country does not meet the specific requirements for a certificate regarding Erwinia amylovora. There is no official pest free area nor is there a buffer zone as specified in the legislation. |
| Anoplophora chinensis | Commission Implementing Decision (EU) 2012/138, Annex I | Based on the information provided in the dossier, including the supplementary information, the exporting country does meet the requirement for a certificate regarding Anoplophora chinensis for plant for planting that originates from Turkish provinces other than Istanbul. This insect has a limited distribution, and it is present and under eradication only in Istanbul province. |
6. Conclusions
There are 12 pests identified to be present in Turkey and considered to be potentially associated with bare‐rooted rootstocks and grafted plants of Malus domestica imported from Turkey and relevant for the EU.
For Erwinia amylovora, the exporting country does not meet the specific requirements for a certificate regarding this pest.
For Anoplophora chinensis, the exporting country does meet the requirement for a certificate regarding for plant for planting that originates from Turkish provinces other than Istanbul.
For the remaining pests (Calepitrimerus baileyi, Cenopalpus irani, Cicadatra persica, Diplodia bulgarica, Lopholeucaspis japonica, Malacosoma parallela, Pyrolachnus pyri and tomato ringspot virus), the likelihood of pest freedom after the evaluation of the proposed risk mitigation measures for bare‐rooted rootstocks, grafted plants, budwood and scions of Malus domestica designated for export to the EU was estimated. For Hoplomaimus galeatus and Pratylenchus loosi, the likelihood of pest freedom after the evaluation of the proposed risk mitigation measures for bare‐rooted rootstocks of Malus domestica designated for export to the EU was estimated.
For Calepitrimerus baileyi, the likelihood of pest freedom following evaluation of current risk mitigation measures was estimated as ‘Pest free with some exceptional cases’ with the 90% uncertainty range reaching from ‘Pest free with some exceptional cases’ to ‘Almost always pest free’. The EKE indicated, with 95% certainty, that between 9,956 and 10,000 units per 10,000 will be free from Calepitrimerus baileyi.
For Cenopalpus irani, the likelihood of pest freedom following evaluation of current risk mitigation measures was estimated as ‘Pest free with some exceptional cases’ with the 90% uncertainty range reaching from ‘Pest free with some exceptional cases’ to ‘Almost always pest free’. The EKE indicated, with 95% certainty, that between 9,952 and 10,000 units per 10,000 will be free from Cenopalpus irani.
For Cicadatra persica, the likelihood of pest freedom following evaluation of current risk mitigation measures was estimated as ‘Almost always pest free’ with the 90% uncertainty range reaching from ‘Almost always pest free’ to ‘Almost always pest free’. The EKE indicated, with 95% certainty, that between 9,999 and 10,000 units per 10,000 will be free from Cicadatra persica.
For Diplodia bulgarica, the likelihood of pest freedom following evaluation of current risk mitigation measures was estimated as ‘Pest free with some exceptional cases’ with the 90% uncertainty range reaching from ‘Pest free with some exceptional cases’ to ‘Almost always pest free. The EKE indicated, with 95% certainty, that between 9,863 and 10,000 units per 10,000 will be free from Diplodia bulgarica.
For Hoplomaimus galeatus, the likelihood of pest freedom following evaluation of current risk mitigation measures was estimated as ‘Pest free with some exceptional cases’ with the 90% uncertainty range reaching from ‘Pest free with some exceptional cases’ to ‘Almost always pest free’. The EKE indicated, with 95% certainty, that between 9,982 and 10,000 units per 10,000 will be free from Hoplomaimus galeatus.
For Lopholeucaspis japonica (rooted plants), the likelihood of pest freedom following evaluation of current risk mitigation measures was estimated as ‘Pest free with some exceptional cases’ with the 90% uncertainty range reaching from ‘Pest free with some exceptional cases’ to ‘Almost always pest free’. The EKE indicated, with 95% certainty, that between 9,956 and 10,000 units per 10,000 will be free from Lopholeucaspis japonica.
For Lopholeucaspis japonica (scions and budwoods), the likelihood of pest freedom following evaluation of current risk mitigation measures was estimated as ‘Pest free with some exceptional cases’ with the 90% uncertainty range reaching from ‘Pest free with some exceptional cases’ to ‘Almost always pest free’. The EKE indicated, with 95% certainty, that between 9,978 and 10,000 units per 10,000 will be free from Lopholeucaspis japonica.
For Malacosoma parallela, the likelihood of pest freedom following evaluation of current risk mitigation measures was estimated as ‘Almost always pest free’ with the 90% uncertainty range reaching from ‘Pest free with few exceptional cases’ to ‘Almost always pest free’. The EKE indicated, with 95% certainty, that between 9,991 and 10,000 units per 10,000 will be free from Malacosoma parallela.
For Pratylenchus loosi, the likelihood of pest freedom following evaluation of current risk mitigation measures was estimated as ‘Almost always pest free’ with the 90% uncertainty range reaching from ‘Almost always pest free’ to ‘Almost always pest free’. The EKE indicated, with 95% certainty, that between 9,996 and 10,000 units per 10,000 will be free from Pratylenchus loosi.
For Pyrolachnus pyri, the likelihood of pest freedom following evaluation of current risk mitigation measures was estimated as ‘Pest free with some exceptional cases’ with the 90% uncertainty range reaching from ‘Pest free with some exceptional cases’ to ‘Almost always pest free’. The EKE indicated, with 95% certainty, that between 9,964 and 10,000 units per 10,000 will be free from Pyrolachnus pyri.
For tomato ringspot virus, the likelihood of pest freedom following evaluation of current risk mitigation measures was estimated as ‘Almost always pest free’ with the 90% uncertainty range reaching from ‘Pest free with few exceptional cases’ to ‘Almost always pest free’. The EKE indicated, with 95% certainty, that between 9,991 and 10,000 units per 10,000 will be free from tomato ringspot virus.
Abbreviations
- CABI
Centre for Agriculture and Bioscience International
- EKE
expert knowledge elicitation
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- FUN
Fungi
- INS
Insect
- ISPM
International Standards for Phytosanitary Measures
- NEM
nematode
- PLH
plant health
- PRA
pest risk assessment
- RNQPs
regulated non‐quarantine pests
Glossary
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 1995, 2017)
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2017)
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2017)
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2017)
- Measures
Control (of a pest) is defined in ISPM 5 (FAO, 2017) as “Suppression, containment or eradication of a pest population” (FAO, 1995). Control measures are measures that have a direct effect on pest abundance. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk mitigation measures that do not directly affect pest abundance.
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2017)
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2017)
- Protected zone
A Protected zone is an area recognised at EU level to be free from a harmful organism, which is established in one or more other parts of the Union.
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2017)
- Regulated non‐quarantine pest
A non‐quarantine pest whose presence in plants for planting affects the intended use of those plants with an economically unacceptable impact and which is therefore regulated within the territory of the importing contracting party (FAO, 2017)
- Risk mitigation measure
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A risk mitigation measure may become a phytosanitary measure, action or procedure according to the decision of the risk manager
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2017)
Appendix A – Data sheets of pests selected for further evaluation via expert knowledge elicitation
A.1. Calepitrimerus baileyi
A.1.1. Organism information
| Taxonomic information |
Current valid scientific name: Calepitrimerus baileyi (Keifer, 1938) Synonyms: Phyllocoptes aphrastus (Keifer, 1940) Name used in the EU legislation: – Order: Acarina Family: Eriophyidae Common name: Bailey's rust mite, apple rust mite Name used in the Dossier: – |
|
| Group | Mites | |
| EPPO code | CALEBA | |
| Regulated status |
The pest is not regulated in the EU, neither is on any EPPO list, but it is present in EPPO database. |
|
| Pest status in Turkey | The pest is reported in Turkey in Erzurum (Alaoglu, 1984), Tokat (Yanar and Ecevit, 2005), Van Lake Basin: Iskele, Gürpinar, Edremit on Malus pumila Mill., M. sylvestris Mill., M. communis L. (Rosaceae). New records: Ankara, Van‐Ahlat, Iskele on Malus pumila Mill., M. sylvestris Mill., M. communis L. (Denizhan and Çobanoǧlu, 2010), Yalova, Armutlu (Denizhan, 2018). | |
| Pest status in the EU | Present in Poland and Greece (Fauna Europaea; GBIF; De Lillo, Amrine. 1998). | |
| Host status on Malus domestica | Malus domestica is a host of the pest and the species can complete its life cycle on this host (Abou‐Awad et al., 2011); however, another author wrote that this species is vagrant and that the mites survive on the leaves (Denizhan, 2018). The pest status is confirmed also by others authors (Abou‐Awad et al., 2011; Jeppson et al., 1975; Momen & Lamlom, 2021). | |
| PRA information | No PRA is available for C. baileyi. | |
| Other relevant information for the assessment | ||
| Biology | C. baileyi is able to develop successfully from egg to adult at temperatures between 23°C and 35°C, and 65% RH. It has two nymphal stages, each followed by a resting stage, before reaching adulthood. The duration of egg (incubation period), first instar nymph, nymphochrysalis, second instar nymph, imagochrysalis, pre‐oviposition and post‐oviposition decreases as temperature increases. The oviposition duration decreases with increasing temperature, specifically it goes from an average of 24–22 days with temperatures ranging from 23°C to 35°C. Most of the eggs are laid alongside the midrib or veins of the leaf. Females deposit between 12 and 23 eggs with temperatures ranging from 23°C to 35°C. The total life cycle is completed after 9.7 to 5.3 days depending on sex (i.e. males develop faster) and temperature (23–35°C) (Abou‐Awad et al., 2011). In Egypt, population dynamics of C. baileyi was affected by climatic conditions and about 11 generations were recorded per year (Abou‐Awad et al., 2011). | |
| Symptoms | Main type of symptoms | Mite feeding causes browning on the underside of apple leaves, partial defoliation, rolled and distorted leaves russet on fruit and delays or inhibits plant apical growth (Creelman, 1971; Abou‐Awad et al., 2011). |
| Presence of asymptomatic plants |
In early September, C. baileyi deutogynes hibernate mainly in small, permanently dormant buds and under the loose bark of spurs and around buds on 1‐year‐old shoots, and move into fruiting buds between the shoot and the pink bud stages and in vegetative buds when the buds have started to swell (Abou‐Awad et al., 2011). The deutogyne will seek refuge for aestivating and/or overwintering (e.g. under tree bark scales) until early spring when it begins laying eggs that develop into protogynes (Beaulieu and Knee, 2014). |
|
| Confusion with other pests |
The two conspecific morphs (deutogyne and protogynes) may be wrongly assigned to different species or even genera (Jeppson et al., 1975), although the forms can generally be correctly associated with each other with experience and good sample sizes (Beaulieu and Knee, 2014). There is a single sequence in GenBank of a specimen collected on Malus domestica (Calepitrimerus baileyi voucher MAL91.3 large subunit ribosomal RNA gene, partial sequence, ACCESSION MW633874) (visited on 10.29.21, can help with diagnosis. Calepitrimerus mathiasrexi is similar in morphology to Calepitrimerus baileyi and Calepitrimerus cariniferus Keifer (Keifer, 1938; Baker et al., 1996; Amrine et al., 2003), with microscopic differences (Ripka, 2010). |
|
| Host plant range | Malus pumila Mill., M. sylvestris Mill., M. communis L. (Rosaceae) (Denizhan & Çobanoǧlu, 2010; Creelman, 1971). | |
| Reported evidence of impact | Partial defoliation can reduce the productivity of the plants (Abou‐Awad et al., 2011; Creelman, 1971). | |
| Pathways and evidence that the commodity is a pathway | The most possible way to spread is through the introduction of plant materials, as the mite can be found in buds, even in resting ones. There is no reference in the literature regarding the possibility fruit being a pathway. There are no data on the active dispersal capacity of the pest. | |
| Surveillance information | No surveillance information is currently available from the Turkey NPPO for Calepitrimerus baileyi | |
A.1.2. Possibility of pest presence in the nursery
A.1.2.1. Possibility of entry from the surrounding environment
If present in the surroundings, the pest can enter the nursery (as Turkey is producing these plants for planting outdoors). The most likely pathway to enter the nursery is by infested plant material or by nursery workers and machinery, though the mite can also be transported by wind.
Uncertainties
-
–
No data are available on the population densities of the pest in the areas of production.
-
–
The main uncertainty is whether the pest is present in the production areas in Turkey.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery
A.1.2.2. Possibility of entry with new plants/seeds
The pest can be found on the trunk, stem, branches, leaves of plants for planting and it is difficult to be spotted during visual inspections. The pest can be hidden inside buds.
Uncertainties
-
–
Uncertain if certified material is screened for this pest
-
–
Pest present in Turkey and part of the certified mother material comes from same country, it is unclear if material is inspected for presence of this pest
Taking into consideration the above evidence and uncertainties, the Panel considers it possible that the pest could enter the nursery.
A.1.2.3. Possibility of spread within the nursery
If the pest enters the nursery from the surroundings, it could spread within the nursery either by passive dispersal (e.g. wind), infested plant material, or by nursery workers and machinery. Active dispersal is possible although very short range or transferred from plant to plant if plants are touching with each other.
Taking into consideration the above evidence, the Panel considers that the transfer of the pest within the nursery is possible.
A.1.3. Information from interceptions
There are no records of interceptions of M. domestica plants for planting from Turkey due to the presence of C. baileyi between 1994 and March 2022 (EUROPHYT and TRACES‐NT, online).
A.1.4. Evaluation of the risk mitigation options
In the table below, all risk mitigation measures currently applied in Turkey are listed and an indication of their effectiveness on C. baileyi is provided. The description of the risk mitigation measures currently applied in Turkey is provided in the Table 6.
| No. | Risk mitigation measure (name) | Description | Effective | Evaluation/Uncertainties |
|---|---|---|---|---|
| 1 | Certified material |
The Ministerial experts and inspectors carry out the phytosanitary control on mother plants in spring, summer and autumn for harmful organisms, and the amount of propagation materials (grafted plants, budwoods, rootstocks, scions) that can be obtained from mother plants is determined. For the saplings, the phytosanitary control is also carried out at the same time, regarding harmful organisms specified in quarantine and plant passports, and certification regulations. If free from the harmful organisms, the Ministry issues certificates and labels for the propagation material to be taken from plants in the mother blocks. Certified seed or certified seedling is grafted with certified budwood in a certified nursery. Certificate and combined certification‐passport labels are issued by the Ministerial Organization and sent to the producer for the saplings that meet the requirements in the Regulations. |
Yes |
Potential C. baileyi infestations might be overlooked by visual inspection especially in the case of low infestations without using an adequate magnification considering the tiny size of the individuals both adults and juveniles. Uncertainties: The details of the certification process are not given (e.g. number of plants, intensity of surveys and inspections, etc.). Specific figures on the intensity of survey (sampling effort) are not provided. |
| 2 | Phytosanitary certificates |
Export nurseries must obtain special certification from Turkish Authorities before they begin producing plants for planting. Nurseries must notify technical staff members responsible for production to obtain this certificate, which is then used for registration in the plant passport system. The phytosanitary inspections are done macroscopically. However, if there are signs of disease in the plants or in the immediate vicinity, the inspections are carried out by laboratory analysis. During the production period, official inspection is carried out. After the official approval that the sapling is free from the quarantine factor and true to type, its certificate‐passport label is issued by the Ministry. The Phytosanitary Certificates/Re‐Export Phytosanitary Certificates are issued in exportation of plants and plant products with respect to plant health. In issuing such certificates, the phytosanitary requirements of the importer country are taken into account, in compliance with the ISPM No: 7 and ISPM No: 12 rules. |
Yes |
The procedures applied could be effective in detecting C. baileyi infestations though low densities might be overlooked by visual inspection without using an adequate magnification considering the tiny size of the individuals both adults and juveniles. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
| 3 | Cleaning and disinfection of facilities, tools and machinery | Tools are disinfected with chemical compounds containing 10% chlorine prior to using in sapling and mother plants | Yes |
Cleaning of tools and machineries can lower the possibility of entry and spread. Uncertainties: No details are provided. |
| 4 | Roguing and pruning | Removal of infested branches | Yes |
Pruning can reduce infestation. |
| 5 | Biological and mechanical control |
Biological control with different natural enemies (predators and parasitoids) can reduce the pest populations. Nogall (biological control agent) is applied to protect against crown gall. |
Yes |
Phytoseiid species are reported preying on this species. They can be present in the environment though no details are provided in the dossier. Uncertainties: No details are provided on abundance and efficacy of the natural enemies. |
| 6 | Pesticide application |
The saplings are sprayed against aphids, thrips, whiteflies, red spider pests, black spot, powdery mildew, root rot diseases and, depending on the situation, to fight or protect against weeds. Before loading the plants on the trucks for transport, the roots of seedlings are sprayed with fungicide (Thiram). |
Yes |
Some of the pesticides listed in the dossier might be effective against the mite, specifically acrinathrin and abamectin. Uncertainties: No details are given on the pesticide application schedule and on the application methods. |
| 7 | Surveillance and monitoring | Necessary precautions are taken to ensure that there are no plants other than certified saplings in the production plot and application areas. Plants closer than 15 m from the plot are not usually available. Plants around the production areas are also annually inspected by the Ministry expert in terms of quarantine organisms. In the event that these plants are contaminated with harmful organisms subject to quarantine, these plants and saplings in this area are destroyed. | Yes |
It can be effective. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided and considering the tiny size of the individuals both adults and juveniles. |
| 8 | Sampling and laboratory testing |
For the identification of viruses, bacteria, fungi and nematodes in the seedlings to be exported, min. 5 to max. 25 seedlings are randomly taken from the plantation in the nursery garden and sealed by the inspector and sent to the laboratory for analysis. Soil samples are taken for laboratory analysis in terms of quarantine organisms, particularly to check if it is free from nematodes. If it is found that the growing medium is free from nematodes, the production of saplings is started. |
Yes |
It can be effective Uncertainties: The modalities and intensity of survey is not known. |
| 9 | Root Washing | Roots are washed in the washing areas, near the warehouses. | No | |
| 10 | Refrigeration | The temperature of the storage tanks is between 2°C and 4°C and the humidity is 85‐95%. Transportation is made with refrigerated trucks with the same conditions. | Yes | Low temperatures can slow down its development but not kill the mite. |
| 11 | Pre‐consignment inspection | Prior to export, planting material for which a Phytosanitary Certificate is to be issued shall be subjected to phytosanitary inspection. Only certified plants for planting may be exported. Phytosanitary inspectors are responsible for export controls, sampling and issuing certificates. | Yes |
The procedures applied could be effective in detecting C. baileyi though the mite presence could be overlooked by visual inspection especially in the case of low infestations without using an adequate magnification considering the tiny size of the individuals both adults and juveniles. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
A.1.5. Overall likelihood of pest freedom
A.1.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested consignments
Limited distribution of the pest.
All propagation material is produced within the nurseries.
The natural spread is limited.
Pesticides are effective against eggs, larvae and adults.
Pruning reduces infestation levels, increase sunlight exposure.
Biological enemies are present.
Presence of clear symptoms during the vegetative season.
Careful inspections by trained personnel using proper tools identify infestations.
Control of mother plants by educated experts.
Bundles are composed by 10 plants.
Mainly young plants, e.g. rootstocks, are exported.
A.1.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested consignments
Malus domestica is a preferred host.
Spread to more area in Turkey/no climatic restrictions.
Most of the propagation material is produced in other nurseries.
Wind and human assisted dispersal play a role in spreading the pest.
Pesticides are not effective against eggs, larvae and adults.
Biological enemies are not present or affected by pesticide treatments.
Inspections are not effective in identifying pest presence.
Control of mother plants is not effective.
Bundles are composed by 25 plants.
Mainly older plants, e.g. grafted trees, are exported.
Low density infestation can be overlooked due to the absence of symptoms.
A.1.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested consignments (Median)
The median is slightly closer to the lower values in relation to the uncertainties on pest pressure in the production areas of Malus domestica plants for planting.
A.1.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The values reflect a high uncertainty due to the lack of information on pest pressure, effectiveness of sampling and laboratory testing and the difficulty to detect the pest by visual inspection. Moreover, no details are given on the pesticide application schedule and on the application methods.
A.1.5.5. Elicitation outcomes of the assessment of the pest freedom for Calepitrimerus baileyi
The following Tables show the elicited and fitted values for pest infestation (Table A.1) and pest freedom (Table A.2).
Table A.1.
Elicited and fitted values of the uncertainty distribution of pest infestation by Calepitrimerus baileyi per 10,000 bundles of rooted plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 2 | 10 | 20 | 30 | 50 | ||||||||||
| EKE | 2.01 | 2.58 | 3.47 | 5.20 | 7.47 | 10.3 | 13.2 | 19.4 | 26.5 | 30.5 | 35.2 | 39.8 | 44.3 | 47.3 | 50.0 |
The EKE results is the BetaGeneral (1.0939, 1.8465, 1.58, 54) distribution fitted with @Risk version 7.6.
Table A.2.
The uncertainty distribution of plants free of Calepitrimerus baileyi per 10,000 bundles of rooted plants calculated by Table A.1
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,950 | 9,970 | 9,980 | 9,990 | 9,998 | ||||||||||
| EKE results | 9,950 | 9,953 | 9,956 | 9,960 | 9,965 | 9,969 | 9,974 | 9,981 | 9,987 | 9,990 | 9,993 | 9,995 | 9,997 | 9,997 | 9,998 |
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.2.
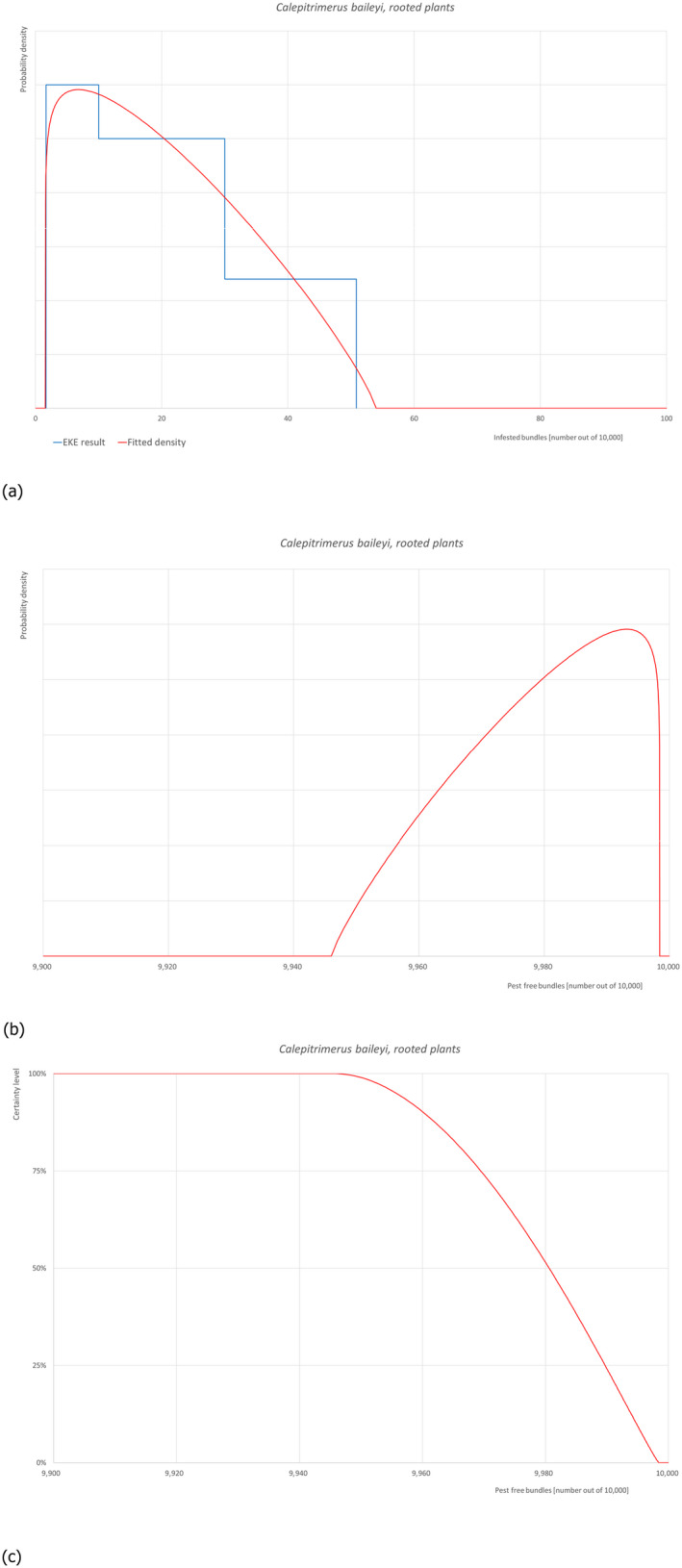
Figure A.1 (a) Elicited uncertainty of pest infestation per 10,000 bundles (histogram in blue– vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest free bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 bundles
A.1.6. References List
Abou‐Awad BA, Afia SI and Al‐Azzazy MM, 2011. The life‐history and bionomics of the apple rust mite Calepitrimerus baileyi (Acari: Eriophyidae). Acarines: Journal of the Egyptian Society of Acarology, 5, 57–63.
Alaoglu Ö, 1984. Studies on the systematics and their relation to hosts of eriophyoid mites (Acarina: Actinedida) on some plants in Erzurum and Erzincan regions, in Turkey. University of Atatürk. Journal of Agricultural Faculty, OMU, 15, 1–16.
Yanar D and Ecevit O, 2005. Plant injurious and predatory mite species in apple (Malus communis L.) orchards in Tokat Province. Journal of Agricultural Faculty, OMU, 20, 18–23.
Amrine JW, Stasny TAH and Flechtmann CHW, 2003. Revised keys to world genera of Eriophyoidea (Acari: Prostigmata). Indira Publishing House, West Bloomfield, USA. pp. iv+244.
Attiah HH, 1970. New records of eriophyid mites from Egypt (Acarina). Bulletin of the Entomological Society of Egypt, 54, 43–47.
Baker EW, Kono T, Amrine JW, Delfinado‐Baker M and Stasny TA, 1996. Eriophyoid Mites of the United States. Indira Publishing House, West Bloomfield, USA. pp. ix + 394.
Beaulieu F and Knee W, 2014. Plant‐feeding mites of the Canadian Prairies. Arthropods of Canadian Grasslands, 3.
Creelman IS, 1971. Insects of special interest. The Canadian agricultural insect Pest Review, 49, 1–2.
De Lillo E and Amrine JW, 1998. Eriophyoidea (Acari) on a computer database. Entomologica (Bari), 32, 2–7.
Denizhan E, 2011. Eriophyid mites (Acari: Eriophyidae) from Turkey. Zoosymposia, 6, 51–55.
Denizhan E and Çobanoǧlu S, 2010. Eriophyoid mites (Acari: Prostigmata: Eriophyoidea) in Van Lake Basin from Turkey. International Journal of Acarology, 36, 503–510. https://doi.org/10.1080/01647954.2010.491486
Denizhan E, 2018. Eriophyoid mites (Acari: Eriophyoidea) on fruit trees in Yalova, Turkey. Yuzuncu Yil University Journal of Agricultural Sciences, 28, 285–288. https://doi.org/10.29133/yyutbd.398096
FAUNA EUROPEA. Available online: https://fauna-eu.org/cdm_dataportal/taxon/32a7d368-eb69-406b-a22c-8667965c3a54#distribution
Jeppson LR, Keifer HH and Baker EW, 1975. Mites injurious to economic plants. Berkeley: University of California Press. 614 pp.
Keifer HH, 1938. Eriophyid studies II. The Bulletin Department of Agriculture State of California, 27, 301–323.
Momen FM and Lamlom M, 2021. Life history traits and demographic parameters of Typhlodromus transvaalensis reared on three eriophyid species (Acari: Phytoseiidae: Eriophyidae). International Journal of Acarology, 47, 346–351. https://doi.org/10.1080/01647954.2021.1912176
Ripka G, 2010. A new Calepitrimerus species and new gall mite records from Hungary (Acari: Prostigmata: Eriophyoidea). Acta Phytopathologica et Entomologica Hungarica, 45, 383–389. https://doi.org/10.1556/APhyt.45.2010.2.16
A.2. Cenopalpus irani
A.2.1. Organism information
| Taxonomic information |
Current valid scientific name: Cenopalpus irani Synonyms: Brevipalpus irani (Meyer, 1979) Name used in the EU legislation: – Order: Trombidiformes Family: Tenuipalpidae Common name: Iranian false spider mite Name used in the Dossier: – |
|
| Group | Mites | |
| EPPO code | – | |
| Regulated status | Not regulated | |
| Pest status in Turkey | C. irani is present in Turkey (Çobanoğlu et al., 2019). | |
| Pest status in the EU | C. irani is not present in the EU. | |
| Host status on Malus domestica | M. domestica is reported as a host of C. irani (Rashki et al., 2004). | |
| PRA information | No PRA is available for C. irani. | |
| Other relevant information for the assessment | ||
| Biology |
Females and males of C. irani are about 0.3 mm long, red, oval shaped and dorsoventrally flattened. These mites hibernate in branches, between October and March. C. irani is one of the most important tenuipalpid pests on apple and it completes three generations per year in Iran (Rashki et al., 2002). Fertilised females appear in April, at an average daily air temperature of +15°C. The first generation occurs at the end of April and May, the second at the end of June and the third near the end of August. Larvae and nymphal stages are about 0.2 mm long and red. The population of this mite rapidly increases to a high density during the summer with increasing temperature and dryness. Female populations peak in September and October and by the mid of this month they start to hibernate (Darbemamieh et al., 2009; Khanjani et al., 2012, 2013; Rashki et al., 2004). Both reproductive parameters such as fecundity and fertility, and survival parameters of C. irani are influenced by temperature. An increase in temperature, from 15 to 30°C, leads to increases in fecundity and fertility rates and to a decrease in mortality percentage (Bazgir et al., 2015). C. irani is phytophagous, and has been reported on apple, pear, olive, walnut, quince, grapevine, sour cherry, plum, peach, fig and pistachio (Mehrnejad and Ueckermann, 2001; Gholamzera et al., 2013). C. irani is widely distributed in apple orchards and is one of the most important tenuipalpid pests on apple in Iran (Darbemamieh et al., 2009; Rashki et al., 2002). It is reported as present in Turkey and widespread in Iran (Khanjani et al., 2012, 2013; Sultan et al., 2019). |
|
| Symptoms | Main type of symptoms |
C. irani feeds on stems, fruits, flowers and leaves, often on the lower surface, sometimes causing serious damage to various crops. It is difficult to detect spider mites at low densities, since these are invisible to the naked eye. To confirm their presence an examination with a stereomicroscope of the undersides of leaves is necessary. The presence of spider mites is usually associated with the presence of white exuviae and webbing; however, C . irani and other Tenuipalpidae are considered false spider mites as they do not produce silk webbings on plants (Fathipour et al., 2016). |
| Presence of asymptomatic plants |
The absence of leaves does not allow to detect symptoms. Resting stages of mites on the bark are not associated with symptoms. No information |
|
| Confusion with other pests | It can be confused with other tenuipalpid mites, such as for example Cenopalpus pulcher. | |
| Host plant range | The hosts of C. irani are: Chaenomeles sp., Cydonia oblonga, Ficus carica, Malus domestica, Olea sp., Pistacia mutica, Pistacia vera, Prunus cerasus, Prunus domestica, Pyrus persica, Pyrus communis, Populus alba, Vitis vinifera (Rashki et al., 2004; Mehrnejad and Ueckermann, 2001; Khanjani et al., 2012). | |
| Reported evidence of impact | This mite infests several rosaceous species and is reported as one of the most important tenuipalpid pests on apple in Iran. | |
| Pathways and evidence that the commodity is a pathway |
Possible pathways of entry for C. irani are plants for planting since these mites overwinter in branches. Spider mites can spread by wind currents and longer distance dispersion can occur by transportation of planting material (EPPO, online). |
|
| Surveillance information | No surveillance information is currently available from the Turkey NPPO. | |
A.2.2. Possibility of pest presence in the nursery
A.2.2.1. Possibility of entry from the surrounding environment
If present in the surroundings, the pest can enter the nursery (as Turkey is producing these plants for planting outdoors). The pest could enter the nursery either by passive dispersal (e.g. wind), infested plant material by nursery workers and machinery.
Uncertainties
No data are available on the population densities of the pest in the areas of production.
The main uncertainty is whether the pest is present in the production areas in Turkey.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery
A.2.2.2. Possibility of entry with new plants/seeds
The pest can be found on the trunk, stem, branches, leaves of plants for planting and it is difficult to be spotted during visual inspections. The pest can be hidden inside bark cracks.
Uncertainties
Uncertain if certified material is screened for this pest
Pest present in Turkey and part of the certified mother material comes from the same country, it is unclear if the material is inspected for presence of this pest
Taking into consideration the above evidence and uncertainties, the Panel considers it possible that the pest could enter the nursery.
A.2.2.3. Possibility of spread within the nursery
If the pest enters the nursery from the surroundings, it could spread within the nursery either by passive dispersal (e.g. wind), infested plant material, or by nursery workers and machinery. Active dispersal is possible although very short range or transferred from plant to plant if they are touching with each other. Given that the pest is polyphagous, it could be associated with other fruit crops.
Taking into consideration the above evidence, the Panel considers that the transfer of the pest within the nursery is possible.
A.2.3. Information from interceptions
There are no records of interceptions of M. domestica plants for planting from Turkey due to the presence of C. irani between 1994 and March 2022 (EUROPHYT and TRACES‐NT, online).
A.2.4. Evaluation of the risk mitigation options
In the table below, all risk mitigation measures currently applied in Turkey are listed and an indication of their effectiveness on C. irani is provided. The description of the risk mitigation measures currently applied in Turkey is provided in the Table 6.
| No. |
Risk mitigation measure (name) |
Description |
Effective | Evaluation/Uncertainties |
|---|---|---|---|---|
| 1 | Certified material |
The Ministerial experts and inspectors carry out the phytosanitary control on mother plants in spring, summer and autumn for harmful organisms, and the amount of propagation materials (grafted plants, budwoods, rootstocks, scions) that can be obtained from mother plants is determined. For the saplings, the phytosanitary control is also carried out at the same time, regarding harmful organisms specified in quarantine and plant passports, and certification regulations. If free from the harmful organisms, the Ministry issues certificates and labels for the propagation material to be taken from plants in the mother blocks. Certified seed or certified seedling is grafted with certified budwood in a certified nursery. Certificate and combined certification‐passport labels are issued by the Ministerial Organization and sent to the producer for the saplings that meet the requirements in the Regulations. |
Yes |
Potential C. irani infestations might be overlooked by visual inspection especially in the case of low infestations without using an adequate magnification considering the tiny size of both adults (ca. 0.3 mm length) and juveniles (ca. 0.2 mm length). Uncertainties: The details of the certification process are not given (e.g. number of plants, intensity of surveys and inspections, etc.). Specific figures on the intensity of survey (sampling effort) are not provided. |
| 2 | Phytosanitary certificates |
Export nurseries must obtain special certification from Turkish Authorities before they begin producing plants for planting. Nurseries must notify technical staff members responsible for production to obtain this certificate, which is then used for registration in the plant passport system. The phytosanitary inspections are done macroscopically. However, if there are signs of disease in the plants or in the immediate vicinity, the inspections are carried out by laboratory analysis. During the production period, official inspection is carried out. After the official approval that the sapling is free from the quarantine factor and true to type, its certificate‐passport label is issued by the Ministry. |
Yes |
The procedures applied could be effective in detecting C. irani infestations though low densities might be overlooked by visual inspection without using an adequate magnification considering the tiny size of both adults (ca. 0.3 mm length) and juveniles (ca. 0.2 mm length). Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
|
The Phytosanitary Certificates/Re‐Export Phytosanitary Certificates are issued in exportation of plants and plant products with respect to plant health. In issuing such certificates, the phytosanitary requirements of the importer country are taken into account, in compliance with the ISPM No: 7 and ISPM No: 12 rules. |
||||
| 3 | Cleaning and disinfection of facilities, tools and machinery | Tools are disinfected with chemical compounds containing 10% chlorine prior to using in sapling and mother plants | Yes |
Cleaning of tools and machineries can lower the possibility of entry and spread. Uncertainties: No details are provided |
| 4 | Roguing and pruning | Removal of infested branches | Yes | Pruning can reduce infestation. |
| 5 | Biological and mechanical control |
Biological control with different natural enemies (predators and parasitoids) can reduce the pest populations. During rootstocks planting, Nogall (biological control agent) is applied to protect against crown gall. |
Yes |
The main predators in apple orchards belong to the families Phytoseiidae and Stigmaeidae. They can be present in the environment though no details are provided in the dossier. Uncertainties: No details are provided on abundance and efficacy of the natural enemies. |
| 6 | Pesticide application |
The saplings are sprayed against aphids, thrips, whiteflies, red spider pests, black spot, powdery mildew, root rot diseases and, depending on the situation, to fight or protect against weeds. Before loading the plants on the trucks for transport, the roots of seedlings are sprayed with fungicide (Thiram). |
Yes |
Some of the pesticides listed in the dossier might be effective against the mite, specifically acrinathrin and abamectin. Uncertainties: No details are given on the pesticide application schedule and on the application methods. |
| 7 | Surveillance and monitoring | Necessary precautions are taken to ensure that there are no plants other than certified saplings in the production plot and application areas. Plants closer than 15 m from the plot are not usually available. Plants around the production areas are also annually inspected by the Ministry expert in terms of quarantine organisms. In the event that these plants are contaminated with harmful organisms subject to quarantine, these plants and saplings in this area are destroyed. | Yes |
It can be effective. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided and considering the tiny size of the individuals both adults (ca. 0.3 mm length) and juveniles (ca. 0.2 mm length). |
| 8 | Sampling and laboratory testing |
For the identification of viruses, bacteria, fungi and nematodes in the seedlings to be exported, min. 5 to max. 25 seedlings are randomly taken from the plantation in the nursery garden and sealed by the inspector and sent to the laboratory for analysis. Soil samples are taken for laboratory analysis in terms of quarantine organisms, particularly to check if it is free from nematodes. If it is found that the growing medium is free from nematodes, the production of saplings is started. |
Yes |
It can be effective. Uncertainties: The modalities and intensity of survey are not known. |
| 9 | Root Washing | Roots are washed in the washing areas, near the warehouses. | No | |
| 10 | Refrigeration | The temperature of the storage tanks is between 2°C and 4°C and the humidity is 85‐95%. Transportation is made with refrigerated trucks with the same conditions. | Yes | Low temperatures can slow down its development but not kill the mite. |
| 11 | Pre‐consignment inspection | Prior to export, planting material for which a Phytosanitary Certificate is to be issued shall be subjected to phytosanitary inspection. Only certified plants for planting may be exported. Phytosanitary inspectors are responsible for export controls, sampling and issuing certificates. | Yes |
The procedures applied could be effective in detecting C. irani though the mite presence could be overlooked by visual inspection especially in the case of low infestations without using an adequate magnification considering the tiny size of the individuals both adults (ca. 0.3 mm length) and juveniles (ca. 0.2 mm length). Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
A.2.5. Overall likelihood of pest freedom
A.2.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested consignments
Malus domestica is not the preferred host.
Limited distribution.
All material is produced within the nurseries.
The natural spread is limited.
Pesticides are effective against eggs, larvae and adults.
Pruning reduces infestation levels and increases sunlight exposure.
Biological enemies are present.
Careful inspections by trained personnel using proper tools identify infestations.
Mother plants are controlled by educated experts.
Bundles are composed by 10 plants.
Mainly young plants, e.g. rootstocks, are exported.
A.2.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested consignments
Malus domestica is a preferred host.
Spread to more area in Turkey/no climatic restrictions.
Most of the propagation material is produced in other nurseries.
Wind and human assisted dispersal play a role in spreading the pest.
Pesticides are not effective against eggs, larvae and adults.
Biological enemies are not present or affected by pesticide treatments.
Inspections are not effective in identifying pest presence.
Control of mother plants is not effective.
Bundles are composed by 25 plants.
Mainly older plants, e.g. grafted trees, are exported.
A.2.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested consignments (Median)
Due to the limited information available about pest presence and pressure in the nursery area, the panel considers lower values as likely as higher values.
A.2.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The values reflect a high uncertainty due to the lack of information on pest pressure and the difficulty to detect the pest by visual inspection.
A.2.5.5. Elicitation outcomes of the assessment of the pest freedom for Cenopalpus irani on crop
The following Tables show the elicited and fitted values for pest infestation (Table A.3) and pest freedom (Table A.4).
Table A.3.
Elicited and fitted values of the uncertainty distribution of pest infestation by Cenopalpus irani per 10,000 bundles of rooted plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 2 | 10 | 20 | 32 | 55 | ||||||||||
| EKE | 2.01 | 2.50 | 3.32 | 4.96 | 7.22 | 10.1 | 13.1 | 19.8 | 27.6 | 32.2 | 37.5 | 42.9 | 48.2 | 51.9 | 55.2 |
The EKE results is the BetaGeneral (1.0206, 1.9271, 1.67, 60.5) distribution fitted with @Risk version 7.6.
Table A.4.
The uncertainty distribution of plants free of Cenopalpus irani per 10,000 bundles of rooted plants calculated by Table A.3
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,945 | 9,968 | 9,980 | 9,990 | 9,998 | ||||||||||
| EKE results | 9,945 | 9,948 | 9,952 | 9,957 | 9,962 | 9,968 | 9,972 | 9,980 | 9,987 | 9,990 | 9,993 | 9,995 | 9,997 | 9,997 | 9,998 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.4.
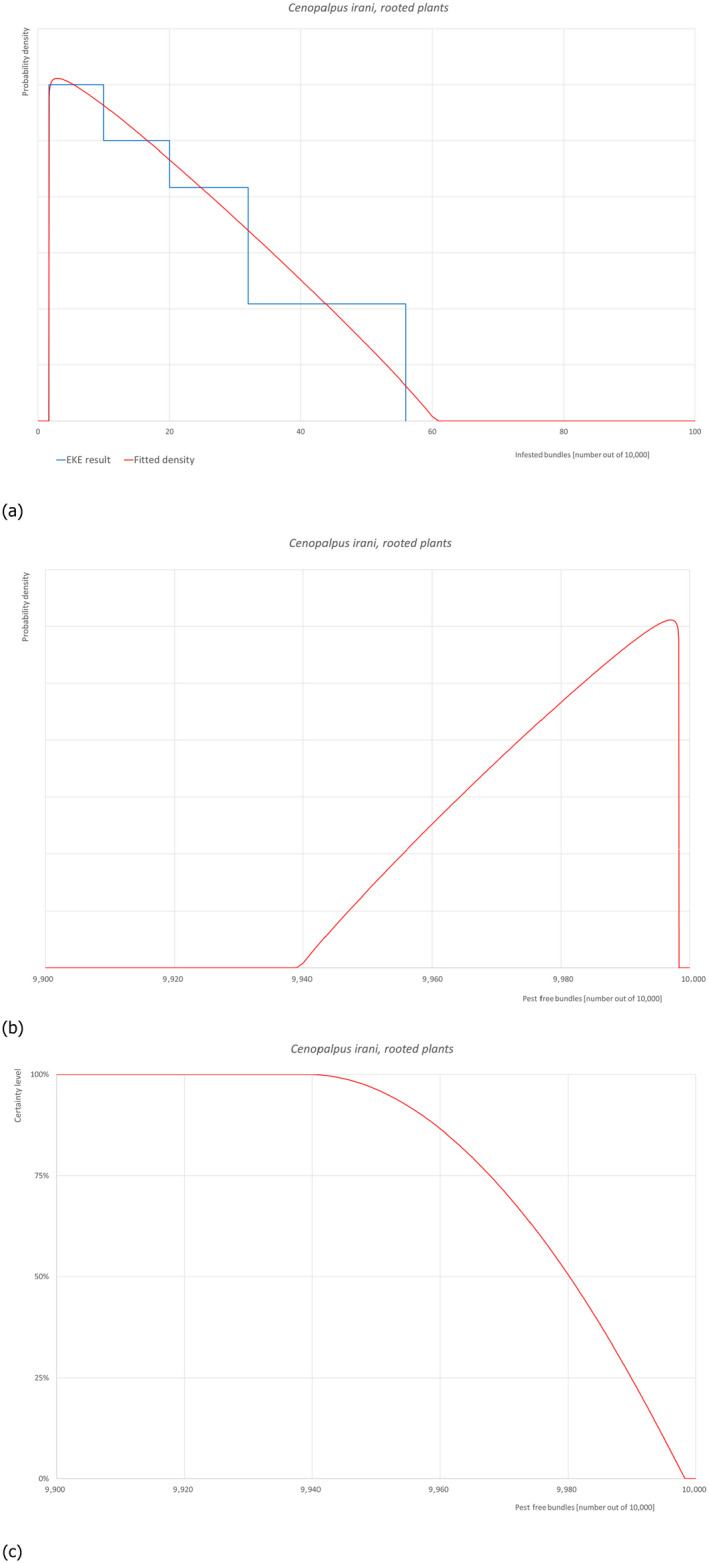
Figure A.2 (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue– vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest free plants per 10,000 (i.e. =1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.2.6. References List
Bazgir F, Jafari S, Shakarami J and Bahirae F, 2015. Effect of temperature on the reproductive parameters and survival of Cenopalpus irani Dosse (Tenuipalpidae). Acarina, 23, 181–187.
Beyzavi G, Ueckermann E, Faraji F and Ostovan H 2013. A catalog of Iranian prostigmatic mites of superfamilies Raphignathoidea & Tetranychoidea (Acari). Persian Journal of Acarology, 2, 389–474.
Çobanoğlu S, Erdoğan T and Kılıç N, 2019. Four new flat mite records for the mite fauna of Turkey (Acari: Tenuipalpidae), International Journal of Acarology, 45, 159–175. https://doi.org/10.1080/01647954.2018.1561751
Darbemamieh M, Kamali K and Fathipour Y, 2009. Bionomics of Cenopalpus irani, Bryobia rubrioculus and their egg predator Zetzellia mali (Acari: Tenuipalpidae, Tetranychidae, Stigmaeidae) in natural conditions. Munis Entomology and Zoology, 4, 341–354.
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm
Fathipour Y and Maleknia B, 2016. Mite predators. In: Omkar (Ed.) Ecofriendly Pest Management for Food Security. Elsevier, San Diego, USA, pp. 329–366. https://doi.org/10.1016/B978‐0‐12‐803265‐7.00011‐7
Khanjani M, Farzan S, Asadi M and Khanjani M, 2013. Checklist of the flat mites (Acari: Trombidiformes: Tenuipalpidae) of Iran. Persian Journal of Acarology, 2, 235–251. https://doi.org/10.22073/pja.v2i2.9957
Khanjani M, Khanjani M, Saboori AR and Seeman OD, 2012. The false spider mites of the genus Cenopalpus Pritchard & Baker (Acari: Tenuipalpidae) from Iran. Zootaxa, 3433, 1–59. https://doi.org/10.11646/zootaxa.3433.1.1
Mehrnejad MR and Ueckermann EA, 2001. Mites (Arthropoda: Acari) associated with pistachio tree (Anacardiaceae) in Iran (I). Systematic and Applied Acarology Special Publications, 6, 1–12. https://doi.org/10.11158/saasp.6.1.1
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt
Rashki M, Saboori A, Nowzari J and Zenouz BE, 2004. Biology of Cenopalpus irani (Acari: Tenuipalpidae), in Mahdasht region, Karaj. Systematic and Applied Acarology 9, 23. https://doi.org/10.11158/saa.9.1.3
A.3. Cicadatra persica
A.3.1. Organism information
| Taxonomic information |
Current valid scientific name: Cicadatra persica (Kirkaldy, 1909) Synonyms: Cicada lineola Hagen, 1856; Cicadatra lineola (Hagen, 1856); Tettigia (Cicadatra) lineola (Hagen, 1856), (Kirkaldy 1909). Name used in the EU legislation: – Order: Hemiptera Family: Cycadidae Common name: – Name used in the Dossier: Cicadatra persica (Kirkaldy, 1909) |
| Group | Insects |
| EPPO code | Not Available |
| Regulated status | The species is not included in any EPPO list and it is not regulated elsewhere in the world. |
| Pest status in Turkey | Present, widely distributed (Kartal and Zeybekoglu, 1999; Demir, 2008, 2019; Kemal and Koçak, 2014; Kaplan and Tezcan, 2016; Gbif). |
| Pest status in the EU |
Present: Italy (Sicily) (Gogala & Trilar 1998) but D’Urso and Sabella (2011) wrote that the presence of C. persica in Sicily is of uncertain validity or reported only once in remote times for which the presence is to be verified; Monaco (Demir 2008); Gogala and Trilar (1998) wrote that this species is unknown in Greece; however, the same authors suggested that there is the possibility that this species is present in Greece and Albania. Moreover, starting from 2007, there were some records of C. persica in Gbif site in Bulgaria (Háva, 2016; Trilar et al., 2020) |
| Pest status in other countries | Present in Azerbaijan, Georgia, North Macedonia, (Gogala and Trilar, 1998); Syria (Dardar et al., 2013), Iran, (Mozaffarian and Sanborn 2010); Pakistan (Ahmed and Sanborn, 2010; Ahmed, et al., 2012, 2013); Israel, Syria, Turkey, European part of Russia (Gogala and Trilar, 1998, Sanborn, 2014); Iran (Mozaffarian, 2013). |
| Host status on Malus domestica |
Malus domestica is a host of the pest and the species could complete its life cycle on this host (Dardar et al., 2012, 2013). However, in spite of the wide distribution of the species in Iran, large populations and the activity of species as pest have never been recorded. There is no host data in Iran (Mozaffarian, 2018). The species was also collected on herbaceous plants under Pyrus spp. and Prunus spp. (Demir, 2019). Cicadas often cause damage in orchards and olive groves but this usually occurs when they are close to woods. The Syrian authors have not described the habitat surrounding the damaged plants nor have specified the proximity of the damaged trees to woods. |
| PRA information | There is no PRA available |
| Other relevant information for the assessment | |
| Biology |
The egg‐nest of C. persica contains a number of slits, and each slit includes numerous eggs. The medium number of eggs per slit was 11. In a study conducted in Syria, the mean number of eggs per nest was about 155; dissection of the larger nests showed that it may attain at least 400 eggs; in almost 50% of the cases the number of egg‐nests per tree was between 1 and 2 (Dardar et al., 2013). Eggs are covered by a layer of macerated epidermal tissue (Logan and Maher, 2009) that may prevent penetration by contact insecticides, such as mineral oil (Dardar et al., 2013) In Syria, the first observation of the adults in the orchards was on 7 June, and the first observation of egg‐laying was on 14 June. The time between the two observations showed that the females become ready to mate and lay eggs, and the males start to sing and mate a few days after emergence (Dardar, et al., 2012). The emergence peak was recorded in the fourth week of June. Egg development lasted approximately 40 days, with the first eggs hatching on 1 August and the final hatch on 17 August (Dardar, et al., 2012). The length of the cycle is unknown (Dardar, et al., 2012, 2013). |
|
Symptoms |
Main type of symptoms |
The damage done during oviposition does not cause the death of the branches (Dardar et al., 2012). This damage leads to leaf fall and reduced growth (Dardar et al., 2013). However, the most obvious damage is that caused by oviposition in small twigs. This damage causes twigs to split and die, causing a symptom called flagging which is also caused by other pests (Dardar et al., 2012). The damage caused by this species is also due to the nymphs, which attack the roots of M. domestica underground (Dardar et al., 2013). The symptoms are easy to detect. |
| Presence of asymptomatic plants | No data available. | |
| Confusion with other pests |
Identification of species of Cicadatra is challenging due to the variation of species within the genus and the similar general appearance of many species (Ahmed et al., 2013). Specimens of C. persica can show different morphological patterns (Dardar and Belal, 2013). There is a systematic key to distinguishing the Iranian species of the genus Cicadatra (two) (Mozaffarian, 2018). There is a systematic key to distinguishing the species of the genus Cicadatra from Pakistan (9) (Ahmed et al., 2012, 2013). The species is very close to Cicadatra hyaline, C. hyalinata and C. atra but can be morphologically identified (Gogala & Trilar, 1998). Cicadatra persica can be differentiated by C. karachiensis by the black colour of pronotal collar; moreover, the specimens of C. persica are much larger with body lengths greater than 24 mm (Ahmed et al., 2010). Species can be also distinguished by analysing the songs (Gogala and Trilar, 1998; Dardar et al., 2013). There is a mitochondrial fragment of COI sequence of C. persica deposited in the Bold database that could permit the molecular identification. |
|
| Host plant range | The only host reported is Malus domestica (Dardar et al., 2012, 2013). However, C. persica was also collected on herbaceous plants under Pyrus and Prunus (Demir, 2019). | |
| Reported evidence of impact | In spite of the wide distribution of the species in Iran, large populations and the activity of species as pest have never been recorded. However, no sufficient data are available. | |
| Pathways and evidence that the commodity is a pathway | The most possible way to spread is through the introduction of plant materials, as eggs can be found in the branches or sprouts of plants. There are no data on the active dispersal capacity and flight capacity of the pest. | |
| Surveillance information | There is no surveillance for C. persica in Turkey (based on the apple technical report). | |
A.3.2. Possibility of pest presence in the nursery
A.3.2.1. Possibility of entry from the surrounding environment
If present in the surroundings, the pest can enter the nursery as Turkey is producing these plants for planting outdoors. The pest could enter the nursery mainly by active dispersal (flight). The only host reported is M. domestica. However, C. persica was also collected on herbaceous plants under Pyrus and Prunus. No surveillance for C. persica is performed in Turkey.
Uncertainties
-
–
The pest is reported to be widely distributed in Turkey however, no data are available on the distribution of the pest or population densities in the areas of production.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery.
A.3.2.2. Possibility of entry with new plants/seeds
The pest can be transported on host plants, particularly plants for planting and cut branches, as eggs can be found in the branches or sprouts of plants. This causes twigs to split and die, causing a symptom called flagging which is also due to other pests. Besides, the nymphs attack the roots of M. domestica underground, therefore they can be accidentally transported through plants for planting with soil or soil movement.
Uncertainties
-
–
Uncertain if certified material is screened for this pest. Although the symptoms are easy to detect, the eggs can be overlooked because they are laid inside tissues.
Taking into consideration the above evidence and uncertainties, the Panel considers it possible that the pest could enter the nursery.
A.3.2.3. Possibility of spread within the nursery
If the pest enters the nursery from the surroundings, it could spread either by adult flight, soil movement or infested plant material. The only host reported is M. domestica. However, C. persica was also collected on herbaceous plants.
Taking into consideration the above evidence, the Panel considers that the transfer of the pest within the nursery is possible.
A.3.3. Information from interceptions
There are no records of interceptions of M. domestica plants for planting from Turkey due to the presence of C. persica between 1994 and March 2022 (EUROPHYT and TRACES‐NT, online).
A.3.4. Evaluation of the risk mitigation options
In the table below, all risk mitigation measures currently applied in Turkey are listed and an indication of their effectiveness on C. persica is provided. The description of the risk mitigation measures currently applied in Turkey is provided in the Table 8.
| No. | Risk mitigation measure (name) |
Description |
Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|---|
| 1 | Certified material |
The Ministerial experts and inspectors carry out the phytosanitary control on mother plants in spring, summer and autumn for harmful organisms, and the amount of propagation materials (grafted plants, budwoods, rootstocks, scions) that can be obtained from mother plants is determined. For the saplings, the phytosanitary control is also carried out at the same time, regarding harmful organisms specified in quarantine and plant passports, and certification regulations. If free from the harmful organisms, the Ministry issues certificates and labels for the propagation material to be taken from plants in the mother blocks. Certified seed or certified seedling is grafted with certified budwood in a certified nursery. Certificate and combined certification‐passport labels are issued by the Ministerial Organization and sent to the producer for the saplings that meet the requirements in the Regulations. |
Yes |
The procedures applied could be effective in detecting C. persica infestations though visual inspections may fail to detect the eggs. The details of the certification process are not given (e.g. number of plants, intensity of surveys and inspections, etc.). Uncertainties: The details of the certification process are not given (e.g. number of plants, intensity of surveys and inspections, etc.). Specific figures on the intensity of survey (sampling effort) are not provided. |
| 2 | Phytosanitary certificates and plant passport |
Export nurseries must obtain special certification from Turkish Authorities before they begin producing plants for planting. Nurseries must notify technical staff members responsible for production to obtain this certificate, which is then used for registration in the plant passport system. The phytosanitary inspections are done macroscopically. However, if there are signs of disease in the plants or in the immediate vicinity, the inspections are carried out by laboratory analysis. During the production period, official inspection is carried out. After the official approval that the sapling is free from the quarantine factor and true to type, its certificate‐passport label is issued by the Ministry. The Phytosanitary Certificates/Re‐Export Phytosanitary Certificates are issued in exportation of plants and plant products with respect to plant health. In issuing such certificates, the phytosanitary requirements of the importer country are taken into account, in compliance with the ISPM No: 7 and ISPM No: 12 rules. |
Yes |
The procedures applied could be effective in detecting C. persica infestations though visual inspections may fail to detect the eggs. The details of the certification process are not given (e.g. number of plants, intensity of surveys and inspections, etc.). Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
| 3 | Cleaning and disinfection of facilities, tools and machinery | Tools are disinfected with chemical compounds containing 10% chlorine prior to using in sapling and mother plants | No | |
| 4 | Roguing and pruning | Applied in case of infections/infestations. | Yes | It could be useful in removing infested plant parts and identifying pest presence. |
| 5 | Biological control and mechanical control |
Weeds are controlled mechanically in the nurseries and in the surrounding areas. Nogall (biological control agent) is applied to protect against crown gall. |
No | |
| 6 | Pesticide application |
The saplings are sprayed against aphids, thrips, whiteflies, red spider pests, black spot, powdery mildew, root rot diseases and, depending on the situation, to fight or protect against weeds. Before loading the plants on the trucks for transport, the roots of seedlings are sprayed with fungicide (Thiram). |
Yes |
Although no specific insecticides targeting this pest are mentioned in the dossier, the active ingredients used for other insects would be somewhat effective against the pest. Vegetable oil can have an effect on adults or nymphs if directly sprayed. Uncertainties: No details are given on which pesticides are applied from those listed in the Dossier, on the pesticide application, schedule and on the application methods. |
| 7 | Surveillance and monitoring | Necessary precautions are taken to ensure that there are no plants other than certified saplings in the production plot and application areas. Plants closer than 15 m from the plot are not usually available. Plants around the production areas are also annually inspected by the Ministry expert in terms of quarantine organisms. In the event that these plants are contaminated with harmful organisms subject to quarantine, these plants and saplings in this area are destroyed. | Yes |
It can be effective. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
| 8 | Sampling and laboratory testing |
For the identification of viruses, bacteria, fungi and nematodes in the seedlings to be exported, min. 5 to max. 25 seedlings are randomly taken from the plantation in the nursery garden and sealed by the inspector and sent to the laboratory for analysis. Soil samples are taken for laboratory analysis in terms of quarantine organisms, particularly to check if it is free from nematodes. If it is found that the growing medium is free from nematodes, the production of saplings is started. |
No | |
| 9 | Root Washing | Roots are washed in the washing areas, near the warehouses. | Yes | It could be effective in removing nymphs from roots. |
| 10 | Refrigeration | The temperature of the storage tanks is between 2°C and 4°C and the humidity is 85‐95%. Transportation is made with refrigerated trucks with the same conditions. | Yes | Low temperatures can slow down its development but not kill the insect. |
| 11 | Pre‐consignment inspection | Prior to export, planting material for which a Phytosanitary Certificate is to be issued shall be subjected to phytosanitary inspection. Only certified plants for planting may be exported. Phytosanitary inspectors are responsible for export controls, sampling and issuing certificates. | Yes |
The procedures applied could be effective in detecting C. persica infestations though visual inspections may fail to detect the eggs. Uncertainties: No specific figures on the intensity of survey (sampling effort) are provided. |
A.3.5. Overall likelihood of pest freedom
A.3.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested consignments
Malus is not a preferred host.
There are only nymphs during winter (export time).
No other host plants in the surroundings.
Adults are large and visible on the twigs, besides egg laying causes evident leaf drop.
Adults fly and can be detected.
Detection by sound.
Pesticides are applied and are effective against emerging nymphs and adults.
Absence of soil prevents nymph development.
Washing is effective to remove nymphs from the roots.
Bundles are composed by 10 plants.
Mainly young plants, e.g. rootstocks, are exported.
A.3.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested consignments
Malus is a preferred host favourable (only) host.
Adults may appear earlier in the season (spring).
Other host plants are present in the nurseries and in the surroundings.
Eggs can be overlooked, only wounds are visible.
Not specific symptoms.
Eggs are resistant to contact pesticides.
Soil is not reached.
Soil remaining attached to the roots may be infested.
Bundles are composed by 25 plants.
Mainly older plants, e.g. grafted trees, are exported.
A.3.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested consignments (Median)
Due to the limited information available about pest presence and pressure in the nursery area, the panel considers lower values as likely as higher values.
A.3.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The values reflect a high uncertainty due to the lack of information on pest pressure.
A.3.5.5. Elicitation outcomes of the assessment of the pest freedom for Cicadatra persica
The following Tables show the elicited and fitted values for pest infestation/infection (Table A.5) and pest freedom (Table A.6).
Table A.5.
Elicited and fitted values of the uncertainty distribution of pest infestation by Cicadatra persica per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0.00 | 0.25 | 0.50 | 0.75 | 1.00 | ||||||||||
| EKE | 0.01 | 0.03 | 0.05 | 0.10 | 0.17 | 0.25 | 0.33 | 0.50 | 0.67 | 0.75 | 0.83 | 0.90 | 0.95 | 0.98 | 0.99 |
The EKE results BetaGeneral (1, 1, 0, 1) distribution fitted with @Risk version 7.6.
Table A.6.
The uncertainty distribution of plants free of Cicadatra persica per 10,000 bundles calculated by Table A.5
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,999.0 | 9,999.3 | 9,999.5 | 9,999.8 | 10,000 | ||||||||||
| EKE results | 9,999.0 | 9,999.0 | 9,999.1 | 9,999.1 | 9,999.2 | 9,999.3 | 9,999.3 | 9,999.5 | 9,999.7 | 9,999.8 | 9,999.8 | 9,999.9 | 9,999.95 | 9,999.98 | 10,000 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. =10,000 – the number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.6.
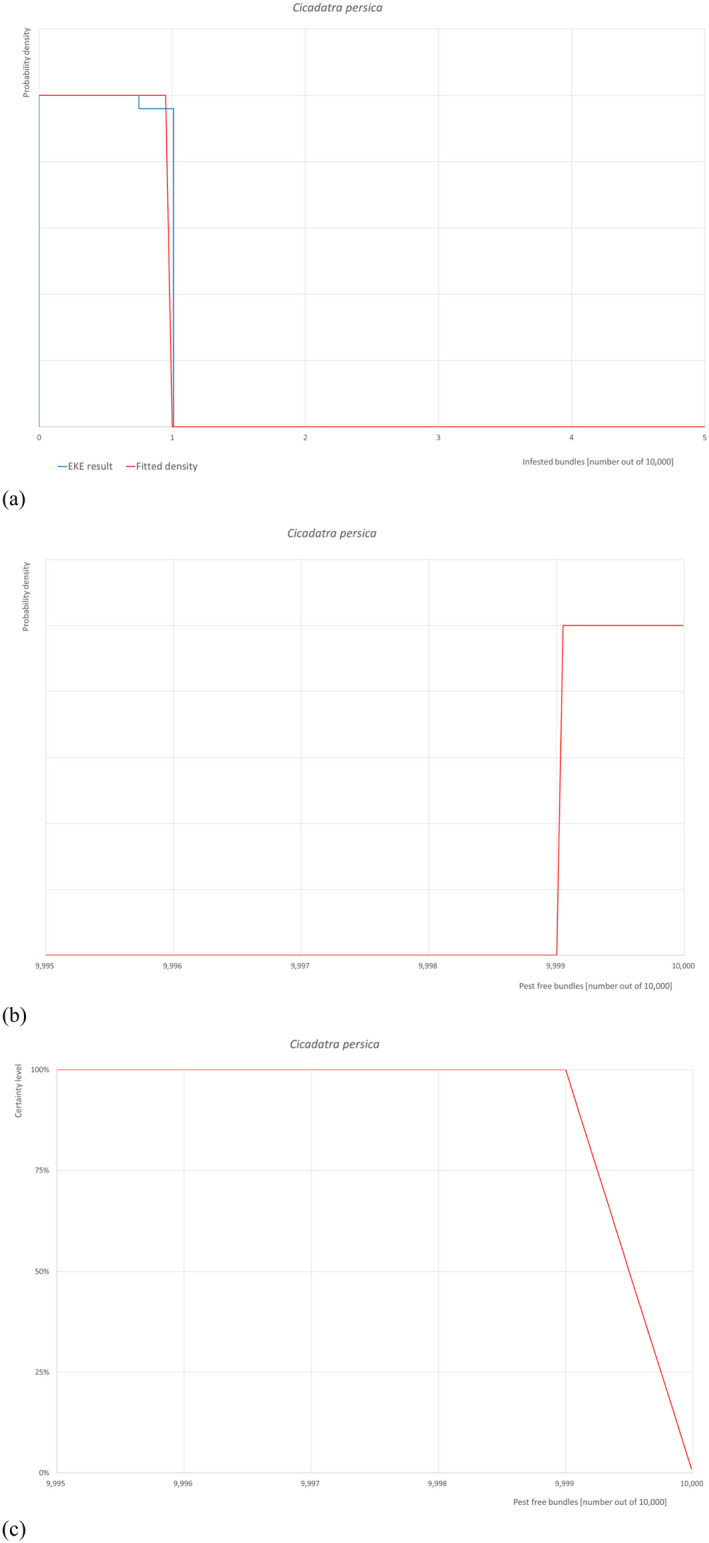
Figure A.3 (a) Elicited uncertainty of pest infestation per 10,000 bundles (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest free bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 bundles
A.3.6. References List
Ahmed Z, Sanborn AF and Hill KBR, 2010. A new species of the cicada genus Cicadatra from Pakistan: (Hemiptera: Cicadoidea: Cicadidae). Zoology in the Middle East, 51, 75–81. https://doi.org/10.1080/09397140.2010.10638443
Ahmed Z, Sanborn AF and Akhter MA, 2012. A new species of the cicada genus Cicadatra Kolenati, 1857 (Hemiptera, Cicadidae) from Pakistan with a key to the known species of Pakistani Cicadatra. ZooKeys, 174, 41–48. https://doi.org/10.3897/zookeys.174.2299
Ahmed Z, Sanborn AF and Akhter MA, 2013. Two new species of the genus Cicadatra Kolenati (Hemiptera: Cicadidae) from Pakistan. Zootaxa, 3750, 176–184. https://doi.org/10.11646/zootaxa.3750.2.5
Ahmed Z, Sanborn AF and Khatri I, 2015. A key to the cicada fauna of Pakistan based on structural variation in the timbals (Hemiptera: Cicadoidea). Pakistan Journal of Zoology, 47, 589–591.
Dardar MA and Belal HMR, 2013. Morphological differences among egg nests and adult individuals of Cicadatra persica (Hemiptera, Cicadidae), distributed in Erneh, Syria. ZooKeys, 319, 11–25. https://doi.org/10.3897/zookeys.319.4189
Dardar MA, Belal HMR and Basheer AM, 2012. Observations on some biological aspects of Cicadatra persica (Cicadidae: Hemiptera) in apple fruit orchards in Erneh, Syria. Journal of Entomological and Acarological Research, 44, 12. https://doi.org/10.4081/jear.2012.e12
Dardar MA, Belal HMR and Basheer AM, 2013. The occurrence of the cicada Cicadatra persica on apple trees, Malus domestica, in Erneh, Syria. Journal of Insect Science, 13, 3–7. https://doi.org/10.1673/031.013.4201
Demir E, 2008. The fulgoromorpha and Cicadomorpha of Turkey. Part I: Mediterranean region (Hemiptera). Munis Entomology and Zoology, 3, 447–522.
Demir E, 2019. Biodiversity and zoogeography of Cicadomorpha (Excl. Deltocephalinae) species from Southwestern Turkey (Insecta: Hemiptera). Munis Entomology and Zoology, 14, 236–243.
D’Urso V and Sabella G, 2011. Zoogeografia degli Auchenorrinchi di Sicilia (Insecta, Hemiptera). Biogeographia – The Journal of Integrative Biogeography, 30. https://doi.org/10.21426/b630110598
Gogala M and Trilar T, 1998. First record of Cicadatra persica Kirkaldy, 1909 from Macedonia, with description of its song. Acta Entomologica Slovenica, 6, 5–15.
Háva JJ, 2016. Cicadatra persica (Kirkaldy, 1909) new for Bulgaria (Hemiptera: Auchenorrhyncha: Cicadidae). Arquivos Entomolóxicos, 16, 137–138.
Kirkaldy GW, 1909. Hemiptera, old and new. The Canadian Entomologist, 2, 388–392.
Kartal V and Zeybekoglu U, 1999. An investigation on the morphology of genital organs and oviposition capacit of cicadatra persica kirkaldy, 1909 (Cicadidae, Homoptera). Turkish Journal of Zoology, 23.
Kaplan C and Tezcan S, 2016. Investigations on the istribution, Morphology and Some Bioecological Aspects of Cicadatra hyalina (Fabricius, 1798) (Hemiptera: Cicadidae) Occurring in Cherry Orchards in İzmir Province of Turkey. Türkiye Tarımsal Araştırmalar Dergisi, 3, 145–151. https://doi.org/10.19159/tutad.34726
Kemal M and Koçak AO, 2014. Illustrated and annotated list on the Entomofauna of Gören Mount (Van Province, East Turkey), with ecological remarks. Priamus, 33, 5–206.
Mozaffarian F, 2018. An Identification key to the species of Auchenorrhyncha of Iranian fauna recorded as pests in orchards and a review on the pest status of the species. Zootaxa, 4420, 475–501. https://doi.org/10.11646/zootaxa.4420.4.2
Mozaffarian F and Sanborn AF, 2010. The cicadas of Iran with the description of two new species (Hemiptera, Cicadidae). Deutsche Entomologische Zeitschrift 57, 69–84.
Mozaffarian F and Sanborn AF, 2013. A new species of the genus Cicadatra from Iran (Hemiptera: Auchenorrhyncha: Cicadidae). Acta Entomologica Musei Nationalis Pragae, 53, 39–48. https://doi.org/10.5281/zenodo.4468203
Sanborn AF, 2014. Catalogue of the Cicadoidea (Hemiptera: Auchenorrhyncha). Academic Press/Elsevier, London, UK, viii + 1001 pp.
Trilar T, Gjonov I and Gogala M, 2020. Checklist and provisional atlas of singing cicadas (Hemiptera: Cicadidae) of Bulgaria, based on bioacoustics. Biodiversity Data Journal, 8, 1–80. https://doi.org/10.3897/BDJ.8.E54424
A.4. Diplodia bulgarica
A.4.1. Organism information
| Taxonomic information |
Current valid scientific name: Diplodia bulgarica A.J.L. Phillips, J. Lopes & Bobev (Phillips, Lopes, Abdollahzadeh, Bobev & Alves, Persoonia 29: 33, 2012) (source: Index Fungorum) Phylum: Ascomycota Order: Botryosphaeriales Family: Botryosphaeriaceae Common name: N/A Name used in the Dossier: N/A |
|
| Group | Fungi | |
| EPPO code | N/A | |
| Regulated status | Diplodia bulgarica is not regulated in the EU and any other part of the world. | |
| Pest status in Turkey | Diplodia bulgarica has been recently reported from Turkey (Eken, 2021). | |
| Pest status in the EU | Present in Bulgaria (Phillips et al., 2012; Phillips et al., 2013; Giambra et al., 2016) and Germany (Hinrichs‐Berger et al., 2021) (U.S. National Fungus Collections Database). There is a possibility that the pest is present in other EU MSs, but not detected yet. | |
| Host status on Malus domestica | Diplodia bulgarica has been reported on Malus domestica (U.S. National Fungus Collections Database) (Phillips et al., 2012; Abdollahzadeh, 2015; Hanifeh et al., 2017; Nabi et al., 2020; Bari et al., 2021; Eken, 2021; Hinrichs‐Berger et al., 2021; Nourian et al., 2021). | |
| PRA information |
Commodity risk assessment of Malus domestica plants from Serbia (EFSA Panel on Plant Health et al., 2020) Express‐PRA zu Diplodia bulgarica – Auftreten (https://pra.eppo.int/pra/4ccb04b2‐3180‐4d08‐9be9‐cf0bee4cf5ab) |
|
| Other relevant information for the assessment | ||
| Biology |
Diplodia bulgarica was found for the first time in Bulgaria on Malus sylvestris (Phillips et al., 2012). Microscopic characteristics of D. bulgarica were first described for a specimen obtained from Malus sylvestris in Bulgaria (CBS H‐20189 holotype, culture ex‐type CBS 124254) (Phillips et al., 2012). Conidiomata pycnidial, produced on pine needles on water agar after 7–21 days, solitary, immersed, partially erumpent when mature, dark brown to black, globose to ovoid, up to 600 μm in diameter and 700 μm high, mostly unilocular; wall composed of an outer layer of dark brown, thick‐walled textura angularis, a middle layer of dark brown thin‐walled cells, an inner layer of thin‐walled hyaline cells. Ostiole central, circular, papillate. Conidiophores absent. Conidiogenous cells 9–18 × 2–5 μm, hyaline, smooth, thin‐walled, cylindrical, slightly swollen at the base, holoblastic, forming a single conidium at the tip, discrete, indeterminate, proliferating internally giving rise to periclinal thickenings or proliferating percurrently to form 1‐5 annellations. Conidia aseptate, externally smooth, internally verruculose, thick‐walled, oblong to ovoid, straight, both ends broadly rounded, (22.5–)24–27(−28) × (14.5–)15.5–18(−18.5) μm, 95% confidence limits = 25‐25.7 × 16.6‐17 μm (mean ± standard deviation of 50 conidia = 25.4 ± 1.2 × 16.8 ± 0.7 μm, length/width ratio = 1.5 ± 0.1), initially hyaline, soon becoming pale brown, later darkening and becoming 1‐septate (Phillips et al., 2012). No information on the biology and epidemiology of this fungus is available. Nevertheless, it is likely that its life cycle will be similar to other species of the genus. Indeed, several species in the Botryosphaeriaceae family cause similar symptoms on different plant hosts. Diplodia seriata, for example, is a widely studied pathogen that causes cankers and dieback of several hosts, including apple and grapevine. Its life cycle could be taken into account as an initial reference for D. bulgarica. Diplodia seriata overwinters in fruiting bodies (pycnidia and perithecia) on dead bark, dead twigs or mummified fruit. In the spring, pycnidia and perithecia release conidia and ascospores, respectively, under conditions of high humidity and during wet periods throughout the growing season. The spores are dispersed by splashing rains, wind and insects. The pathogen invades the tissue primarily through wounds, although in some hosts entry through natural openings, such as lenticels and stomata, is possible as well as direct penetration. Depending upon the host, the conidia can infect a variety of organs including leaves, the calyxes of blossoms, tiny fruits, and wounds in twigs and limbs. Infections of fruit and wood may not become visible for several weeks. The spores germinate at temperatures between 15 and 37°C and grow between 5 and 37°C. Infection is favoured by conditions that can stress the plant such as drought, frost damage, hail damage, poor nutrition, and poor pruning practices (CABI CPC). In Iran, D. bulgarica‐induced disease has been reported often prevalent in apple trees more than 15 years old that had been suffering from environmental stresses such as drought and nutrient deficiency (Hanifeh et al., 2017). In vitro, the optimal temperature for D. bulgarica growth is 25°C; the fungus still grows at 10°C but not at 35°C (Nourian et al., 2021). A study on vegetative compatibility and aggressiveness diversity has been done on 101 D. bulgarica isolates recovered from apple trees displaying symptoms of canker and decline in the West Azarbaijan province of Iran (Bari et al., 2021). Inter‐simple sequence repeat (ISSR) marker analyses revealed high within‐population diversity, low genetic differentiation, high gene flow and sharing of multilocus genotypes (MLGs) among geographic populations. Vegetative compatibility analyses revealed the occurrence of anastomosis between nonself pairings and high vegetative compatibility group diversity within populations. All studied MLGs produced necrotic lesions on detached shoots of the ‘Red Delicious’ apple but differed in their aggressiveness levels (Bari et al., 2021). A wide range of resistance/susceptibility levels has been found in the apple germplasm, ranging from highly susceptible to moderately resistant (Hanifeh et al., 2017). |
|
|
Symptoms |
Main type of symptoms | Diplodia bulgarica causes canker, gummosis, dieback, twig blight, and vascular discoloration of infected shoots (Abdollahzadeh, 2015). Sunken brown elliptical lesions having a series of concentric rings can also be observed (Nabi et al., 2020). These oval, sunken, brown lesions often develop next to bark injuries such as cracks, pruning wounds or sun damage. In older cankers, black pycnidia sometimes broke through the bark near the canker. As the infection develops the bark separated from the underlying wood and fell to the ground. The wood beneath was blackened and looked like charcoal. Some of the trees can be girdled by the canker and die (Hinrichs‐Berger et al., 2021). This pathogen has been reported to cause fruit rot in the west and northwest apple orchards of Iran (Hanifeh et al., 2017). |
| Presence of asymptomatic plants |
Little information is available. Diplodia bulgarica has been reported to be highly aggressive on apples (Hanifeh et al., 2017; Eken, 2021; Nourian et al., 2021); therefore, the occurrence of asymptomatic plants should be negligible. On the other hand, it should be taken into account that at least another species in the genus, i.e. Diplodia seriata, can survive endophytically inside some hosts, where it can invade almost any dead, woody tissues (CABI CPC). Further studies could unveil if D. bulgarica can be present within apple tissues as an endophyte while causing no disease symptoms. |
|
| Confusion with other pests | Species identification is done upon morphological and molecular features. Multilocus sequence analysis with concatenated sequences of internal transcribed spacer (ITS) region and elongation factor 1‐α (EF1‐α) was used to identify the species (Phillips et al., 2012). Phillips et al. (2013) stated that morphological characters alone are inadequate to define genera or identify species within Botryosphaeriaceae. Nevertheless, they provide taxonomic keys for the identification of several Botryosphaeriaceae species, including D. bulgarica. This species can be recognised by three characteristics: (a) conidia hyaline and aseptate, becoming brown and 1‐septate only with age; (b) average conidial length less than 29 μm; (c) on Malus, conidia pale brown Phillips et al., 2013. Diplodia bulgarica is morphologically distinct from other Diplodia species reported from apples. Conidia are shorter and wider than both D. intermedia and D. malorum. Iranian isolates of D. bulgarica have also rosulate colonies, but the conidia of D. rosulata (28 × 14.5 μm, length/width ratio = 1.93) are longer and narrower than those of D. bulgarica (25.4 × 16.8 μm, length/width ratio = 1.5). Furthermore, the conidia are distinctive in that they become pale brown soon after they are formed. Phylogenetically, this species is closely related to D. cupressi and D. tsugae (Phillips et al., 2012). | |
| Host plant range | Diplodia bulgarica has been reported on Malus domestica (Phillips et al., 2012; Abdollahzadeh, 2015; Hanifeh et al., 2017; Nabi et al., 2020; Bari et al., 2021; Eken, 2021; Hinrichs‐Berger et al., 2021; Nourian et al., 2021), M. sylvestris (Phillips et al., 2012; Phillips et al., 2013), and Pyrus communis (Hinrichs‐Berger et al., 2021) (U.S. National Fungus Collections Database) | |
| Reported evidence of impact | Diplodia bulgarica is reported as causing severe cankers in Iran (Abdollahzadeh, 2015), India (Nabi et al., 2020) and Germany (Hinrichs‐Berger et al., 2021). | |
| Pathways and evidence that the commodity is a pathway | Diplodia bulgarica can be present as a pathogen on trunks, twigs (Phillips et al., 2012), and fruits (Hanifeh et al., 2017). Presumably, according to the biology of other Diplodia species, D. bulgarica could be also present on leaves. | |
| Surveillance information |
No surveillance information for this pest is currently available from Turkey. There is no information available to assess whether the pest has ever been found in the nurseries or the surrounding environment of the nurseries. |
|
A.4.2. Possibility of pest presence in the nursery
A.4.2.1. Possibility of entry from the surrounding environment
Information on the epidemiology of Diplodia bulgarica is scarse, but other species of Diplodia that infect Malus domestica overwinter as fruiting bodies on dead bark, dead twigs or mummified fruit. Conidia and ascospores are released under conditions of high humidity and during wet periods throughout the growing season. The spores are dispersed by splashing rains, wind and insects and the pathogen invades the tissue primarily through wounds, although in some hosts entry through natural openings, such as lenticels and stomata, is possible as well as direct penetration.
Uncertainties
Specific details as to the epidemiology of D. bulgarica are lacking.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible that inoculum of D. bulgarica can enter nursery from the surrounding area.
A.4.2.2. Possibility of entry with new plants/seeds
Some species of Diplodia have reported asymptomatic infection and the pathogen could also enter via latent infections on planting material
Uncertainties
The possible existence and length of asymptomatic or epiphytic phases that would affect the detection of infected plants in the officially approved nurseries is not known.
A.4.2.3. Possibility of spread within the nursery
Sporulation and subsequent spread of inoculum, along with wounds caused either by insects or management practices cannot be ruled out.
Uncertainties
Specific details as to the epidemiology of D. bulgarica are lacking.
A.4.3. Information from interceptions
In the Europhyt and Traces databases (1994 to March 2022), there are no records of interception of D. bulgarica (all origins, all commodities).
A.4.4. Evaluation of the risk mitigation options
| No. |
Risk mitigation measure (name) |
Description |
Effective | Evaluation/Uncertainties |
|---|---|---|---|---|
| 1 | Certified material |
The Ministerial experts and inspectors carry out the phytosanitary control on mother plants in spring, summer and autumn for harmful organisms, and the amount of propagation materials (grafted plants, budwoods, rootstocks, scions) that can be obtained from mother plants is determined. For the saplings, the phytosanitary control is also carried out at the same time, regarding harmful organisms specified in quarantine and plant passports, and certification regulations. If free from the harmful organisms, the Ministry issues certificates and labels for the propagation material to be taken from plants in the mother blocks. Certified seed or certified seedling is grafted with certified budwood in a certified nursery. Certificate and combined certification‐passport labels are issued by the Ministerial Organization and sent to the producer for the saplings that meet the requirements in the Regulations. |
Yes |
Potential D. bulgarica infections could be detected, though visual detection is difficult due to possible latent infections. Uncertainties: The details of the certification process are not given (e.g. number of plants, intensity of surveys and inspections, etc.). Specific figures on the intensity of survey (sampling effort) are not provided. |
| 2 | Phytosanitary certificates and plant passport |
Export nurseries must obtain special certification from Turkish Authorities before they begin producing plants for planting. Nurseries must notify technical staff members responsible for production to obtain this certificate, which is then used for registration in the plant passport system. The phytosanitary inspections are done macroscopically. However, if there are signs of disease in the plants or in the immediate vicinity, the inspections are carried out by laboratory analysis. During the production period, official inspection is carried out. After the official approval that the sapling is free from the quarantine factor and true to type, its certificate‐passport label is issued by the Ministry. The Phytosanitary Certificates/Re‐Export Phytosanitary Certificates are issued in exportation of plants and plant products with respect to plant health. In issuing such certificates, the phytosanitary requirements of the importer country are taken into account, in compliance with the ISPM No: 7 and ISPM No: 12 rules. |
Yes |
The procedures applied could be effective in detecting D. bulgarica infections, but not on recent infections. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
| 3 | Cleaning and disinfection of facilities, tools and machinery | Tools are disinfected with chemical compounds containing 10% chlorine prior to using in sapling and mother plants | Yes |
The effect of these chemicals on limiting infections is not known. Uncertainties: No details are provided. |
| 4 | Roguing and pruning | Applied in case of infections/infestations. | Yes | It could be useful in removing infested plant parts and identifying pest presence. |
| 5 | Biological control and mechanical control | Weeds are controlled mechanically in the nurseries and in the surrounding areas. | No | |
| 6 | Pesticide application |
Before the rootstock planting, burnt animal manure, ammonium sulfate and urea fertiliser are applied to the growing area or mortar. During rootstocks planting, Nogall (biopesticide) is applied to protect against crown gall. The saplings are sprayed against aphids, thrips, whiteflies, red spider pests, black spot, powdery mildew, root rot diseases and, depending on the situation, to fight or protect against weeds. Before loading the plants on the trucks for transport, the roots of seedlings are sprayed with fungicide (Thiram). |
Yes | Thiram applications could be effective against the presence of fungal inoculum on the surface of the plants. |
| 7 | Surveillance and monitoring |
Both processes are conducted according to Turkish phytosanitary regulations. Necessary precautions are taken to ensure that there are no plants other than certified saplings in the production plot and application areas. Plants within and around the production areas are annually inspected to check the presence of quarantine organisms. Visual inspection at least once or twice a year during production or during uprooting of the plants. Visual inspection can be supported by the use of microscope or laboratory analysis if pests are suspected to be present. In the event that these plants are infected/infested with harmful organisms subject to quarantine, these plants are destroyed. |
Yes |
It can be effective but initial infections are very difficult to detect. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
| 8 | Sampling and laboratory testing |
For the identification of viruses, bacteria, fungi and nematodes in the seedlings to be exported, min. 5 to max. 25 seedlings are randomly taken from the plantation in the nursery garden and sealed by the inspector and sent to the laboratory for analysis. Soil samples are taken for laboratory analysis in terms of quarantine organisms, particularly to check if it is free from nematodes. If it is found that the growing medium is free from nematodes, the production of saplings is started. |
Yes | Uncertainties: if the sampling is sufficiently intense to detect the fungus. |
| 9 | Root Washing | Roots are washed in the washing areas, near the warehouses. | No | |
| 10 | Refrigeration | The temperature of the storage tanks is between 2°C and 4°C and the humidity is 85‐95%. Transportation is made with refrigerated trucks with the same conditions. | Yes | Low temperatures can slow down its development but not kill the fungus. The spread within the bundle can be reduced. |
| 11 | Pre‐consignment inspection | Prior to export, planting material for which a Phytosanitary Certificate is to be issued shall be subjected to phytosanitary inspection. Only certified plants for planting may be exported. Phytosanitary inspectors are responsible for export controls, sampling and issuing certificates. | Yes |
The procedures applied could be effective in detecting D. bulgarica infections though visual detection at the beginning of infestation is difficult. Uncertainties: No specific figures on the intensity of survey (sampling effort) are provided. |
A.4.5. Overall likelihood of pest freedom
A.4.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested consignments
The pathogen has been recently discovered in Turkey, there is no/low pest pressure in the area where the nurseries are located.
Symptomatic plants are easy to be detected.
If asymptomatic mother plants are introduced in the nursery, they are expected to show symptoms.
Irrigation system does not facilitate the splash dispersal of the spores.
The pathogen has limited (passive) dispersal capacity.
The varieties of Malus used are more resistant to the pathogen.
A.4.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested consignments
Since its first detection Diplodia bulgarica has spread in the country and it is likely that host plants are present in the surrounding environment.
The pathogen is widespread in Turkey and there is high pest pressure in the area.
The environmental conditions in the production area are favourable for the population built‐up.
Some latent infection may escape detection The irrigation system facilitates the splash dispersal of the spores in the greenhouse.
There are no fungicide treatments that are effective.
The varieties of Malus used are susceptible to the pathogen.
A.4.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested consignments (Median)
Uncertainties about pest pressure in Turkey.
The information on infections of D. bulgarica on apple plants in Turkey is missing.
The lack reported problems within the apple production area in Turkey.
The likelihood of introduction into apple production sites by natural means and human activities.
A.4.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
-
·
• The main uncertainty is the absence of basic knowledge of the biology of this pathogen, due to the relatively recent description of the species.
A.4.5.5. Elicitation outcomes of the assessment of the pest freedom for Diplodia bulgarica
The following Tables show the elicited and fitted values for pest infestation/infection (Table A.7) and pest freedom (Table A.8).
Table A.7.
Elicited and fitted values of the uncertainty distribution of pest infestation by Diplodia bulgarica per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 35 | 65 | 100 | 150 | ||||||||||
| EKE | 2.33 | 4.97 | 8.84 | 15.8 | 24.4 | 34.7 | 44.8 | 65.5 | 87.5 | 99.6 | 113 | 126 | 137 | 145 | 150 |
The EKE results is BetaGeneral (1.2194, 1.6018, 0, 158) distribution fitted with @Risk version 7.6.
Table A.8.
The uncertainty distribution of plants free of Diplodia bulgarica per 10,000 bundles calculated by Table A.7
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,850 | 9,900 | 9,935 | 9,965 | 10,000 | ||||||||||
| EKE results | 9,850 | 9,855 | 9,863 | 9,874 | 9,887 | 9,900 | 9,912 | 9,935 | 9,955 | 9,965 | 9,976 | 9,984 | 9,991 | 9,995 | 9,998 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. = 10,000 – the number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.8.
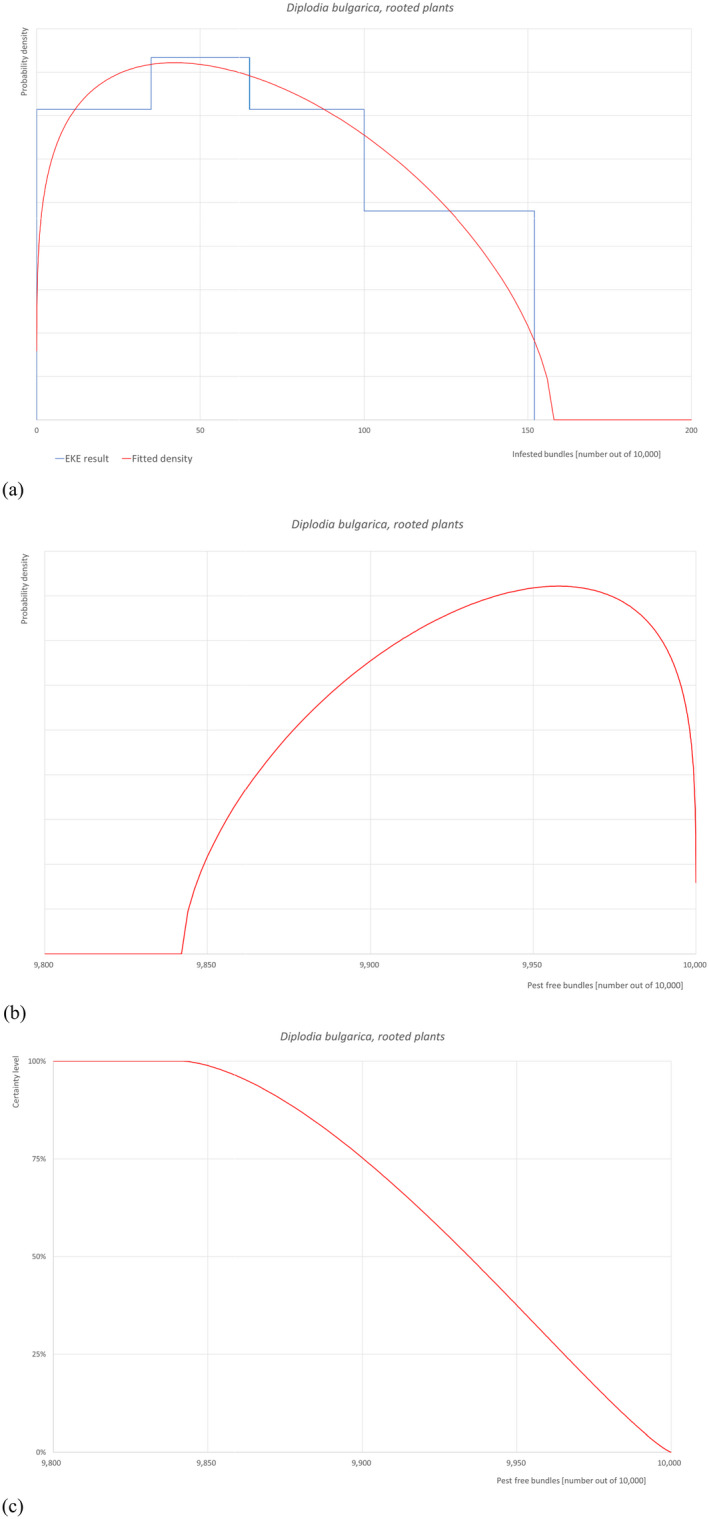
Figure A.3 (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.4.6. References List
Abdollahzadeh J, 2015. Diplodia bulgarica as a new pathogen and potential threat to the apple industry in Iran. Phytopathologia Mediterranea, 54, 128–132. https://doi.org/10.14601/Phytopathol_Mediterr‐14686
Bari RZ, Abrinbana M and Ghosta Y, 2021. Genetic variation, vegetative compatibility, and aggressiveness diversity of Diplodia bulgarica isolates from apple orchards in West Azarbaijan province of Iran. Plant Pathology, 70, 1326–1341. https://doi.org/10.1111/ppa.13374
CABI Crop Protection Compendium. Diplodia bulgarica. Available online: https://www.cabi.org/cpc/datasheet/13064342 [Accessed: 25 January 2022].
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Civera AV, Potting R, Zappalà L, Urek G, Gómez P, Lucchi A, Gardi C, de la Peña E and Yuen J, 2020. Commodity risk assessment of Malus domestica plants from Serbia. EFSA Journal 2020;18(2):6109, 32 pp. https://doi.org/10.2903/j.efsa.2020.6109
Eken C, 2021. Diplodia bulgarica, a new record for Turkey. Mycotaxon, 136, 669–673. https://doi.org/10.5248/136.669
EPPO Global Database. Available online: https://gd.eppo.int/ [Accessed: 25 January 2022].
GBIF. Available online: https://www.gbif.org/ [Accessed: 25 January 2022].
Giambra S, Piazza G, Alves A, Mondello V, Berbegal M, Armengol J and Burruano S, 2016. Botryosphaeriaceae species associated with diseased loquat trees in Italy and description of Diplodia rosacearum sp. nov. Mycosphere, 7, 978–989. https://doi.org/10.5943/mycosphere/si/1b/9
Hanifeh S, Zafari D and Soleimani MJ, 2017. Reaction of some apple cultivars to Diplodia bulgarica in Iran. Mycosphere, 8, 1253–1260. https://doi.org/10.5943/mycosphere/8/2/9
Hinrichs‐Berger J, Zegermacher K and Zgraja G, 2021. First report of Diplodia bulgarica causing black canker on apple (Malus domestica) and pear (Pyrus communis) in Germany. New Disease Report, 43, e12004. https://doi.org/10.1002/ndr2.12004
IndexFungorum. Diplodia bulgarica. Available online: https://www.indexfungorum.org/Names/NamesRecord.asp?RecordID=519632 [Accessed: 25 January 2022].
Nabi SU, Raja WH, Mir JI, Sharma OC, Singh DB, Sheikh MA, Yousuf N and Kamil D, 2020. First report of Diplodia bulgarica a new species causing canker disease of apple (Malus domestica Borkh) in India. Journal of Plant Pathology, 102, 555–556. https://doi.org/10.1007/s42161‐019‐00445‐w
Nourian A, Salehi M, Safaie N, Khelghatibana F and Abdollahzadeh J, 2021. Fungal canker agents in apple production hubs of Iran. Scientific Reports, 11, 16. https://doi.org/10.1038/s41598‐021‐02245‐8
Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ and Crous PW, 2013. The Botryosphaeriaceae: genera and species known from culture. Studies in Mycology 76, 51–167. https://doi.org/10.3114/sim0021
Phillips AJL, Lopes J, Abdollahzadeh J, Bobev S and Alves A, 2012. Resolving the Diplodia complex on apple and other Rosaceae hosts. Persoonia, 29, 29–38. https://doi.org/10.3767/003158512X658899
U.S. National Fungus Collections Database. Diplodia bulgarica. Available online: https://nt.ars‐grin.gov/fungaldatabases/new_allView.cfm?whichone=all&thisName=Diplodia%20bulgarica&organismtype=Fungus&fromAllCount=yes [Accessed: 25 January 2022].
A.5. Hoplolaimus galeatus (Lance nematode)
A.5.1. Organism information
| Taxonomic information |
Current valid scientific name: Hoplolaimus galeatus (Cobb, 1913) Thorne, 1935 Synonyms: – Name used in the EU legislation: not regulated in the EU Name used in the Dossier: Hoplolaimus galeatus (Cobb, 1913) Thorne, 1935 Order: Rhabditida Family: Hoplolaimidae |
|
| Group | Nematoda | |
| EPPO code | HOLLGA | |
| Regulated status |
EU status: – Non‐ EU: A1 list: Argentina (2019) (EPPO, online) |
|
| Pest status in Turkey | Present (Turkish dossier) | |
| Pest status in the EU | Present in Spain (Fauna Europea, online) | |
| Host status on Malus domestica | Apple, Malus domestica is recorded as a host of lance nematode Hoplolaimus galeatus (Pokharel, 2001; Crow & Brammer, 2001). | |
| PRA information | There is no PRA available. | |
| Other relevant information for the assessment | ||
| Biology |
Hoplolaimus galeatus belongs to the group of lance nematodes, Hoplolaimus spp. It is a polyphagous, migratory endoparasite that occurs in both soil and roots and feeds on the cortical and vascular tissue of host plants. It can also be found feeding ectoparasitically. This nematode is widely distributed in the USA parasitising various field crops, grasses and woody plants (Siddiqi, 2000). It is also found in Canada, Sumatra, India, Tanzania, Central and South America (Pokharel, 2011), Pakistan (CABI online), Australia (Nambiar et al., 2008), Spain (Fauna Europea online) and Turkey (Kepenekci, 2001; Kepenekci, 2002). In Turkey, H. galeatus has been found on sweet chestnut, cowpea, sesame, vegetable, kidney bean, plum, peach, olive, sunflower and apple. According to the available information, the nematode has been reported in four regions (Antalya, Isparta, Sinop, Eskisehir) (Kepenekci, 2001, 2002; Kepenekci and Zeki, 2002). So far, no epidemics or economic losses have been reported in Turkey. |
|
| Symptoms | Main type of symptoms |
Above‐ground symptoms caused by H. galeatus on turfgrasses are manifested by slow growth, turf thinning, wilting, poor response to adequate fertilisation and irrigation, and premature decay. These symptoms typically occur in irregular patterns throughout the turf stand. By the time above‐ground symptoms of a nematode infestation appear, the root system has already suffered significant damage. Infested roots show typical nematode damage. By moving and feeding, H. galeatus causes large necrotic lesions in the roots. The root system is reduced and there are hardly any small feeder roots left. The root tips appear to be dead and new roots are growing behind the injured tips. These new roots are usually damaged as well. |
| Presence of asymptomatic plants |
Symptoms caused by plant parasitic nematodes are often not very obvious because the population in the rhizosphere is usually small. Damage by plant parasitic nematodes (including H. galeatus) is usually more pronounced when plants are under stress due to lack of water or nutrients or are damaged by other diseases or insects. Above‐ground symptoms depend on the severity of the infestation. In general, symptoms caused by Hoplolaimus spp. on plants are inconspicuous when the nematode population is low and can be easily overlooked. In Turkey (see Turkish dossier), roots are examined macroscopically only for the presence of root galls caused by root‐knot nematodes (Meloidogyne spp.). Necrotic lesions caused by other nematodes are not monitored. |
|
| Confusion with other pathogens/pests |
Above‐ground symptoms depend on the severity of the infestation. If the nematode population is high, plants may be stunted, yellowing and unthrifty in appearance. Plants may wilt during the heat of the day and recover at night. Crop yields are reduced. These symptoms result from reduced water and nutrient availability due to impaired root function. Symptoms may be confused with mineral deficiencies, drought, or other soil‐dwelling pests and diseases, such as root‐knot nematodes and other root rot pathogens. More informative is damage to the root system. Parasitised roots may darken and develop poorly. Small feeder roots are gone, and root tips appear dead. If new roots have begun to grow, they are usually damaged as well. This damage to the root system is responsible for the yellow or dying areas in the grass. H. galeatus can easily be confused with other organisms living in the soil. |
|
| Host plant range | Alfalfa, apple, bananas, beans, Bermuda grass, boxwood, cabbage, carnation, Chinese holly, chrysanthemums, clover, corn, cotton, cranberry, grape, grasses, creeping bentgrass, creeping grasses, oak, peach, peanuts, peas, pine, slash pine, soybean, sweet potatoes, sugarcane, sycamore, tall fescue, vetch, wheat, white clover, etc. (Nemaplex; Mac Gowan and Dunn, 1989; Ye, 2018). | |
| Reported evidence of impact |
H. galeatus is a serious pest in native lawns and golf courses. It is considered an economically important pest of turfgrasses in Florida (Mac Gowan and Dunn, 1998; Nemaplex; Crow and Brammer, 2001) where it is ranked immediately after sting nematode (Belonolaimus longicaudatus), which is considered the most damaging nematode species to turfgrasses (Crow and Brammer, 2001; Crow, 2015). H. galeatus can also be very damaging to many crops, such as cotton, soybeans, alfalfa, and corn (Ye, 2018; Siddiqi, 2000). By feeding on the roots of grasses, H. galeatus destroys the root system. The damaged roots are dark, necrotic and have dead root tips; small feeder roots are not present. Destruction of the root system results in yellowing and drying of the grass. In cotton, it can cause significant damage to cortex and vascular tissue; without adequate moisture, cotton plants are susceptible to stunting, yellowing, and defoliation. In pines, cortex of infested roots may be destroyed; pine seedlings may die by up to 50%. In sycamores, this nematode can cause extensive root necrosis and a marked decrease in fresh weight (Fortuner, 1991; Nemaplex). According to Bird and Melakeberhan (1993), H. galeatus is also a problem in some orchards (apple, cherry and peach) in Michigan, USA. By feeding on the roots, H. galeatus not only causes damage individually, but also forms disease complexes with other soil‐dwelling microorganisms (bacteria and fungi). |
|
| Pathways and evidence that the commodity is a pathway |
|
|
| Surveillance information |
In order to identify plant pests and diseases in the planting material to be exported from Turkey, a minimum of 5 and a maximum of 25 saplings are taken at random from the planting in the nursery, sealed by the inspector and sent to the laboratory for analysis. The saplings in the growing area are examined macroscopically for pests. If pest infestation is suspected, samples are again taken and sent to the laboratory for analysis. |
|
A.5.2. Possibility of pest presence in the nursery
A.5.2.1. Possibility of entry from the surrounding environment
When H. galeatus is present in the environment, it can enter Malus production sites with planting material, water, soil, and growing media attached to agricultural machinery, tools, and shoes. Agricultural machinery is a very important means of spreading the nematode within and between different plantations.
Active dispersal of Hoplolaimus species, including H. galeatus, is limited to short distances. Transmission from the environment to the production field is mainly passive through the spread of infected plants, contaminated soil and run‐off rain water.
Uncertainties
Hoplolaimus galeatus occurs in Turkey. It has been reported from apple orchards, but there is no clear information on its distribution and abundance in the Malus domestica growing area.
The lack of data from official monitoring surveys and reports on problems caused by this nematode in apple production in Turkey leads to uncertainty. This is related to the fact that the nematode is either absent or has not been detected in apple orchards.
It is uncertain how many orchards in apple production areas in Turkey are infested with H. galetus. There is uncertainty about the possible occurrence of other host plants (cultivated or not cultivated) in the surrounding area, which are also considered hosts for this nematode.
Given the above evidence and uncertainties, the Panel considers it possible that the nematode is present in the environment and could enter Malus domestica nurseries with new plants for planting or other human activities.
A.5.2.2. Possibility of entry with new plants/seeds
Plants for planting (roots) are important pathway.
Plants for planting originating from production sites where the nematode is present may be infested. However, if the infestation is low to moderate, the nematode can be easily overlooked.
Uncertainties
Uncertainties exist regarding the lack of data to monitor the presence of H. galeatus in nurseries where M. domestica intended for planting originates.
Symptoms caused by H. galeatus often go undetected initially because the nematodes are microscopic root parasites and when nematode infestations in the roots of host plants are low, symptoms are not very pronounced. In addition, above‐ground symptoms are often general signs of root stress in the plant. Therefore, the presence of H. galeatus in apple roots may not be detected by visual inspection.
Given the above evidence and uncertainties, the Panel considers it possible that the infestation could be overlooked and that the nematode could be introduced into apple nurseries/orchards with new plants.
A.5.2.3. Possibility of spread within the nursery
Hoplolaimus spp. (including H. galeatus) actively move only short distances. Therefore, the main route of spread of this nematode within the nursery/production field is usually human impact. The nematode can be spread with plants for planting from infested production sites and by soil movement ‐ with soil as such or with soil associated with tools and machinery, and with contaminated runoff rainwater and irrigation water.
Uncertainties
If present, it is very likely that the nematode will spread within the production field.
Given the above evidence and uncertainties, the Panel considers that the nematode, if present in the field, can be transferred from one host plant to another.
A.5.3. Information from interceptions
No interceptions of Hoplolaimus galeatus from Turkey to the EU have been reported in EUROPHYT/TRACES so far (until March 2022).
A.5.4. Evaluation of the risk reduction options
In the table below, all risk mitigation measures currently applied in Turkey are listed and an indication of their effectiveness on H. galeatus is provided. The description of the risk mitigation measures currently applied in Turkey is provided in the Table 6.
| No. | Risk mitigation measure (name) | Description | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|---|
| 1 | Certified material |
The experts and inspectors of the Ministry carry out the phytosanitary control on mother plants in spring, summer and autumn for harmful organisms, and the amount of propagation materials (buds, budwoods, rootstocks, scions, etc.) that can be obtained from mother plants is determined. For the saplings, the phytosanitary control is also carried out at the same time, regarding harmful organisms specified in quarantine and plant passports, and certification regulations. Certified seed or certified seedling is grafted with certified budwood in a certified nursery. If free from the harmful organisms, the Ministry issues certificates and labels for the propagation material to be taken from plants in the mother blocks. |
No | |
|
2 |
Phytosanitary certificates and plant passport |
Export nurseries must obtain special certification from Turkish Autorities before they begin producing plants for planting. Nurseries must notify technical staff members responsible for production to obtain this certificate, which is then used for registration in the plant passport system. The phytosanitary inspections are done macroscopically. However, if there are signs of disease in the plants or in the immediate vicinity, the inspections are carried out by laboratory analysis. During the production period, official inspection is carried out. After the official approval that the sapling is free from the quarantine factor and true to type, its certificate‐passport label is issued by the Ministry. The Phytosanitary Certificates/Re‐Export Phytosanitary Certificates are issued in exportation of plants and plant products with respect to plant health. In issuing such certificates, the phytosanitary requirements of the importer country are taken into account, in compliance with the ISPM No: 7 and ISPM No: 12 rules. |
Yes |
Evaluation: Hoplolaimus spp. is not on the list of harmful organisms systematically monitored or tested for the presence on plants intended for planting in Turkey. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. Information on the distribution and abundance of H. galeatus in the Malus domestica growing area is unreliable. |
| 3 | Cleaning and disinfection of facilities, tools and machinery | Tools are disinfected with chemical compounds containing 10% chlorine prior to using in sapling and mother plants | No | |
| 4 | Rouging and pruning | Applied in case of infections/infestations. | No | |
| 5 | Biological and mechanical control |
Nogall (biological control agent) is applied to protect against crown gall. Weeds are controlled mechanically in the nurseries and in the surrounding areas. |
No | |
| 6 | Pesticide application |
The saplings are sprayed against aphids, thrips, whiteflies, red spider pests, black spot, powdery mildew, root rot diseases and, depending on the situation, to fight or protect against weeds. Before loading the plants on the trucks for transport, the roots of seedlings are sprayed with fungicide (Thiram). |
No | |
|
7 |
Surveillance and monitoring |
Both processes are conducted according to Turkish phytosanitary regulations. Necessary precautions are taken to ensure that there are no plants other than certified saplings in the production plot and application areas. Plants within and around the production areas are annually inspected to check the presence of quarantine organisms. Visual inspection at least once or twice a year during production or during uprooting of the plants. Visual inspection can be supported by the use of microscope or laboratory analysis if pests are suspected to be present. In the event that these plants are infected/infested with harmful organisms subject to quarantine, these plants are destroyed. |
Yes |
Evaluation: Details of the surveillance and monitoring during the production cycle are not provided. H. galeatus is not on the list of harmful organisms systematically monitored or tested for the presence on plants intended for planting in Turkey. Uncertainties: Details of the surveillance and monitoring have not been described. Information on the distribution and abundance of H. galeatus in the Malus domestica growing area is unreliable. |
| 8 | Sampling and laboratory testing |
For the identification of viruses, bacteria, fungi and nematodes in the seedlings to be exported, min. 5 to max. 25 seedlings are randomly taken from the plantation in the nursery garden and sealed by the inspector and sent to the laboratory for analysis. Soil samples are taken for laboratory analysis in terms of quarantine organisms, particularly to check if it is free from nematodes. If it is found that the growing medium is free from nematodes, the production of saplings is started. |
Yes |
Evaluation: Soil and plants are tested in the laboratory only for the presence of root‐knot and virus vector nematodes, but not for the presence of H. galeatus Uncertainties: Presence of H. galeatus cannot be detected. |
| 9 | Root washing | Roots are washed in the washing areas, near the warehouses. | Yes |
Evaluation: Root washing does not reduce the risk of nematode infestation in plants intended for planting that are infested with lance nematodes (migratory endoparasites). Uncertainties: Because H. galeatus occurs in both soil and roots, root washing does not reduce the risk of nematodes infestation in plants intended for planting. |
| 10 | Refrigeration | The temperature of the storage tanks is between 2°C and 4°C and the humidity is 85–95%. Transportation is made with refrigerated trucks with the same conditions. | No | |
| 11 | Pre‐consignment inspection | Prior to export, planting material for which a Phytosanitary Certificate is to be issued shall be subjected to phytosanitary inspection. Only certified plants for planting may be exported. Phytosanitary inspectors are responsible for export controls, sampling and issuing certificates. | Yes |
Evaluation: As for nematodes, inspectors pay particular attention to the presence of galls caused by root‐knot nematodes. Symptoms caused by H. galeatus cannot be detected
Uncertainties:
|
A.5.5. Overall likelihood of pest freedom
A.5.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested consignments
Apple is considered to be a minor host.
Apple growing areas are mainly in the part of the country, where H. galeatus has not been reported.
Effective weed control, crop rotation and field hygiene limit apple infestation.
Regular inspections by crop protection authorities are effective and further help to reduce the infection pressure of this nematode.
Washing the roots is effective against this nematode.
A.5.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested consignments
Apple is considered to be an important host.
Apple growing areas are mainly in the part of the country, where H. galeatus is widely distributed.
A similar pest pressure exists throughout the country and most apple plants are expected to be infested with nematodes.
Weed control, crop rotation and field sanitation are ineffective and do not help to reduce infestation of apples by this nematode.
Visual selection of apple plants for planting and visual inspections prior to export without laboratory testing are not effective and result in high infestation.
Postharvest root washing is not effective against this pest because it is endoparasitic.
A.5.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested consignments
Uncertainties about pest pressure in Turkey.
The information on infections of H. galeatus on apple plants in Turkey is missing.
The lack reported problems within the apple production area in Turkey.
The likelihood of introduction into apple production sites by natural means and human activities.
A.5.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The main uncertainty is the absence of nematode‐induced symptoms, so that the presence of the nematode in the apple roots can be overlooked; cannot be detected by visual inspection.
A.5.5.5. Elicitation outcomes of the assessment of the pest freedom for Hoplolaimus galeatus
The following Tables show the elicited and fitted values for pest infestation (Table A.9) and pest freedom (Table A.10).
Table A.9.
Elicited and fitted values of the uncertainty distribution of pest infestation by Hoplolaimus galeatus per 10,000 bundles of rooted plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 4 | 8 | 12 | 20 | ||||||||||
| EKE | 0.293 | 0.611 | 1.07 | 1.89 | 2.90 | 4.11 | 5.31 | 7.80 | 10.6 | 12.2 | 14.0 | 15.8 | 17.6 | 18.9 | 20.0 |
The EKE results is the BetaGeneral (1.2604, 2.0485, 0,22) distribution fitted with @Risk version 7.6.
Table A.10.
The uncertainty distribution of plants free of Hoplolaimus galeatus per 10,000 bundles of rooted plants calculated by Table A.9
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,980 | 9,988 | 9,992 | 9,996 | 10,000 | ||||||||||
| EKE results | 9,980 | 9,981 | 9,982 | 9,984 | 9,986 | 9,988 | 9,989 | 9,992 | 9,995 | 9,996 | 9,997 | 9,998 | 9,999 | 9,999.4 | 9,999.7 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.10.
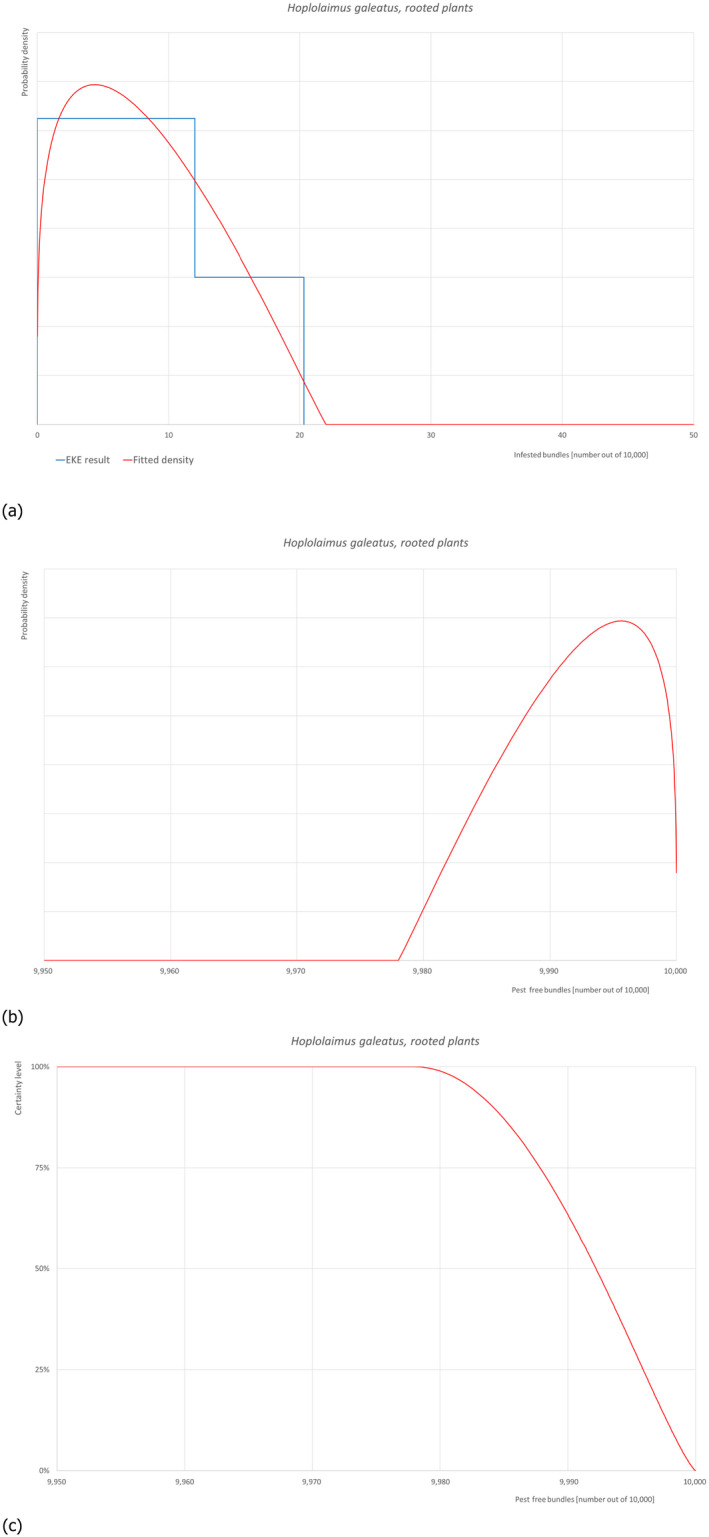
Figure A.5 (a) Elicited uncertainty of pest infestation per 10,000 bundles (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest free bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 bundles
A.5.6. References List
Bird GW and Melakeberhan H, 1993. Avoidance and Management of Nematode Problems in Tree Fruit Production in Michigan. Cooperative Extension Service, Michigan State University, Extension Bulletin E‐2419. 20 pp.
Crow WT and Brammer AS, 2001. Lance Nematode, Hoplolaimus galeatus (Cobb, 1913) Thorne, 1935 (Nematoda: Secernentea: Tylenchida: Tylenchoidea: Hoplolaimidae). University of Florida IFAS Extennsion. Availabl online: https://entnemdept.ufl.edu/creatures/nematode/lance_nematode.htm
Crow WT, 2015. Sting nematode, Belonolaimus longicaudatus Rau (Nematoda: Tylenchida: Belonolaimidae). University of Florida IFAS Extennsion. Available online: https://entnemdept.ufl.edu/creatures/nematode/sting_nematode.htm
EPPO (European and Mediterranean Plant Protection Organization), online. EPPO Global Database. Available online: https://www.eppo.int/ [Accessed: August 2021].
FAUNA EUROPEA. Available online: https://fauna‐eu.org/cdm_dataportal/taxon/30abcc32‐67cf‐4b06‐ac6e‐1392119a5bde
Fortuner R, 1991. The Hoplolaiminae. In: Nickle WR (ed.). Manual of Agricultural Nematology. Marcel Dekker Inc., 669–720.
Kepenekci I, 2001. Plant parasitic Nematodes of Tylenchida (Nematoda) associated with walnuts (Juglans regia L.) and chestnuts (Castanea sativa Miller) orchards in the black sea region. Tarim Bilimleri Dergisi, 7, 101–105.
Kepenekci I, 2002. Plant Parasitic Nematode Species of Tylenchida (Nematoda) Associated with Sesame (Sesamum indicum L.) Growing in the Mediterranean Region of Turkey. Turkish Journal of Agriculture and Forestry, 26, 323–330.
Kepenekci I and Zeki C, 2002. Nematodes of Tylenchida (Nematoda) associated with Apple in Turkey. Pakistan Journal of Nematology, 20, 61–63.
Mac Gowan JB and Dunn RA, 1989. Hoplolaimus galeatus: Lance nematode on St. Augustine grass from Florida. Nematology Circular No. 161, Florida Department of agriculture & Consumer Services, Division of Plant Industry, Contribution No. 371, Bureau of Nematology, 4 p.
Nambiar L, Quader M and Nobbs JM, 2008. First record of Hoplolaimus galeatus in Australia. Australasian Plant Disease Notes, 3, 145–146.
Nemaplex, online. Available online: http://nemaplex.ucdavis.edu/Taxadata/G063S2.aspx
Pokharel R, 2011. Importance of Plant Parasitic Nematodes in Colorado. Fact Sheet No. 2.952. Crops Colorado State University, Western Colorado Research Center. 5/2011.
Siddiqi MR, 2000. Tylenchida: Parasites of Plants and Insects, 2nd Edition. Cabi Publishing, Wallingford, UK, 833 pp.
Ye W, 2018. Nematodes of Agricultural Importance in North and South Carolina. In. Subbotin SA and Chitambar JJ (eds.). Plant Parasitic Nematodes in Sustainable Agriculture of North America. Springer, 247–276.
A.6. Lopholeucaspis japonica
A.6.1. Organism information
| Taxonomic information |
Current valid scientific name: Lopholeucaspis japonica Cockerell Synonyms: Leucaspis japonica (Fernald, 1903), Leucaspis japonica var. darwinensis (Green, 1916), Leucodiaspis hydrangeae (Takahashi, 1934), Leucodiaspis japonica (Takahashi, 1934), Leucodiaspis japonica darwiniensis (Takahashi, 1934), Leucaspis hydrangeae (Takahashi, 1934), Lopholeucaspis japonica (Balachowsky, 1953), Lopholeucaspis japonica darwiniensis (Balachowsky, 1953), Lopholeucaspis menoni (Borchsenius, 1964); Lopholeucaspis darwinienis (Borchsenius, 1966), Leucaspis menoni (Takagi, 1969) Name used in the EU legislation: Lopholeucaspis japonica Cockerell [LOPLJA] Order: Hemiptera Family: Diaspididae Common name: Japanese long scale, Japanese maple scale, Japanese pear white scale Name used in the Dossier: Lopholeucaspis japonica |
|
| Group | Insects | |
| EPPO code | LOPLJA | |
| Regulated status |
The pest is listed in Annex II of Commission Implementing Regulation (EU) 2019/2072 as Lopholeucaspis japonica Cockerell [LOPLJA] The pest is included in the EPPO A2 list (EPPO, online_a). Lopholeucaspis japonica is quarantine in Belarus, Israel, Mexico, Morocco and Tunisia (EPPO, online_b). |
|
| Pest status in Turkey | Lopholeucaspis japonica is present in Turkey. It was recorded on Citrus spp. Up to date there is no record on apple in Turkey. It was detected in the Black Sea region (Artvin, Giresun, Ordu, Samsun, Trabzon, Rize provinces) (Kaydan et. al., 2013). | |
| Pest status in the EU | Lopholeucaspis japonica is absent in the EU. It was intercepted in Croatia, Greece, Italy and Slovak Republic, but never found again (EFSA PLH Panel, 2018; EPPO, online_c). | |
| Host status on Malus domestica | M. domestica is reported as a host of Lopholeucaspis japonica (EPPO, online_d). | |
| PRA information |
Pest Risk Assessments available:
|
|
| Other relevant information for the assessment | ||
| Biology |
Lopholeucaspis japonica is oyster shell‐shaped armored scale, originating from Far East and it spread to tropical and semitropical areas (CABI, online). |
|
|
Females and males have different life cycle. The life stages of female are egg, two larval instars and adult, while male has additional two stages called pre‐pupa and pupa (CABI, online). Males are small and have wings (Bienkowski, 1993), while females are sessile enclosed in chitinous ‘puparium’ (Tabatadze and Yasnosh, 1999). The colour of females, eggs and crawlers is lavender. The wax which is covering the body of scales is white (Fulcher et al., 2011). Each female lay on average 25 eggs, which are laid underneath the female bodies (Addesso et al., 2016; Fulcher et al., 2011). Crawlers can be dispersed by wind or other insects (ants, flies and ladybirds), occasionally also by human transport (Magsig‐Castillo et al., 2010). Lopholeucaspis japonica has one or two overlapping generations per year (Addesso et al., 2016). It was reported that occasionally there can be a third generation in Georgia (Tabatadze and Yasnosh, 1999). In India, first generation crawlers were observed from late Mach until the end of April. Females and male pupae were present from June till the end of August. Second generation crawlers occurred in September and matured females in October (Harsur et al., 2018). Lopholeucaspis japonica overwinters as an immature stage on trunks and branches in Tennessee (Fulcher et al., 2011) and second instar males and females in Maryland (Gill et al., 2012). In addition, it has been reported to overwinter as fertilised females in Japan (Murakami, 1970) and in Pennsylvania (Stimmel, 1995). They can endure temperatures of −20 to −25°C (EPPO, 1997). |
||
| Symptoms | Main type of symptoms |
Lopholeucaspis japonica is usually on bark of branches and trunk but can be found also on leaves (Gill et al., 2012) and sometimes on fruits (EPPO, 1997). The scale feeds on plant storage cells, which causes them to collapse (Fulcher et al., 2011). When the population is high, the main symptoms on plants are premature leaf drop, dieback of branches and death of plants (Fulcher et al., 2011; Gill et al., 2012). Symptoms observed on pomegranate in India were yellowing of leaves, poor fruit set and stunted plant growth (Harsur et al., 2018). |
| Presence of asymptomatic plants | No information. | |
| Confusion with other pests |
Lopholeucaspis japonica can be confused with other armored scales. Lopholeucaspis japonica is similar to L. cockerelli but can be differentiated by the number of macroducts (García Morales et al., online). Other very similar scale is Pseudaulacaspis pentagona (Fulcher et al., 2011). |
|
| Host plant range |
Lopholeucaspis japonica is polyphagous armoured scale and feeds on plants belonging to 38 families (García Morales et al., online). Some of the many hosts of Lopholeucaspis japonica are Acer palmatum, Acer pictum, Acer ukurunduense, Citrus junos, Citrus unshiu, Diospyros kaki, Distylium racemosum, Elaeagnus umbellata, Euonymus alatus, Euonymus japonicus, Gleditsia japonica, Ilex crenata, Magnolia denudata, Magnolia kobus, Malus pumila, Malus domestica, Paeonia lactiflora, Poncirus trifoliata, Prunus × yedoensis, Pyrus pyrifolia, Robinia pseudoacacia, Rosa chinensis, Rosa multiflora, Salix sp., Staphylea bumalda, Syringa oblata and Ziziphus jujuba (Suh, 2020). Lopholeucaspis japonica is a pest of tea in China (Li et al., 1997). It is a serious pest of many crops (citrus, fruit trees, tea, tung) and ornamental plants in the area around the Black Sea (Tabbatadze and Yasnosh, 1999). In the US, it is known to damage Acer and Pyracantha (Davidson and Miller, 1990). |
|
| Reported evidence of impact | Not relevant, listed as EU Quarantine pest (Annex II, part A). | |
| Pathways and evidence that the commodity is a pathway | Possible pathways of entry for Lopholeucaspis japonica are plants for planting (excluding seeds), bonsai, cut flowers and cut branches (EFSA PLH Panel, 2018). | |
| Surveillance information | No surveillance information is currently available from the Turkish NPPO. | |
A.6.2. Possibility of pest presence in the nursery
A.6.2.1. Possibility of entry from the surrounding environment
If present in the surroundings, the pest can enter the nursery (as Turkey is producing these plants for planting outdoors). However, the scale was recorded on Citrus spp. in the Black Sea region and up to date there is no record on other plant species including apple. The pest could enter the nursery either by passive dispersal (e.g. wind) especially young instars than can be easily uplifted by wind, infested plant material by nursery workers and machinery. Given that the pest is very polyphagous the pest could be associated with several crops and wild hosts in the surrounding.
Uncertainties
The main apple production areas are located far away from the area where the pest was reported (Black Sea region).
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery especially if the nurseries are located close to the area where the scale was reported.
A.6.2.2. Possibility of entry with new plants/seeds
The pest can be found on the trunk, stem, branches, leaves of plants for planting (scions, grafted rootstocks). Although adults can be relatively easily spotted during visual inspections, young stages can be difficult to detect. The pest can be hidden inside bark cracks. In case of low populations, the species can be overlooked regarded as trunk spots. Introduction of the pest with certified material is very unlikely.
Uncertainties
-
–
Uncertain if certified material is screened for this pest
Taking into consideration the above evidence and uncertainties, the Panel considers it possible that the pest could enter the nursery although very unlikely.
A.6.2.3. Possibility of spread within the nursery
If the scale enters the nursery from the surroundings, the pest could spread within the nursery either by passive dispersal (e.g. wind), especially young instars than can be easily uplifted by wind, infested plant material, or by nursery workers and machinery. Active dispersal is possible and movement from plant to plant by mobile young instars is possible. Given that the pest is very polyphagous the pest could be associated with other crops in the nursery. During the growing season, visual inspection at least twice during vegetation period is performed, with microscopic observations if needed. Chemical control targeting crawlers is applied together with pruning which can affect diaspidid populations either directly by removal of infested branches and indirectly exposing the pest to biotic and abiotic control agents.
Taking into consideration the above evidence and uncertainties, the Panel considers that the transfer of the pest within the nursery is possible.
A.6.3. Information from interceptions
There are no records of interceptions of M. domestica plants for planting from Turkey due to the presence of L. japonica between 1994 and March 2022 (EUROPHYT and TRACES‐NT, online)
A.6.4. Evaluation of the risk mitigation options
In the table below, all risk mitigation measures currently applied in Turkey are listed and an indication of their effectiveness on L. japonica is provided. The description of the risk mitigation measures currently applied in Turkey is provided in the Table 6.
| No. |
Risk mitigation measure (name) |
Description |
Effective | Evaluation/Uncertainties |
|---|---|---|---|---|
| 1 | Certified material |
The Ministerial experts and inspectors carry out the phytosanitary control on mother plants in spring, summer and autumn for harmful organisms, and the amount of propagation materials (grafted plants, budwoods, rootstocks, scions) that can be obtained from mother plants is determined. For the saplings, the phytosanitary control is also carried out at the same time, regarding harmful organisms specified in quarantine and plant passports, and certification regulations. If free from the harmful organisms, the Ministry issues certificates and labels for the propagation material to be taken from plants in the mother blocks. Certified seed or certified seedling is grafted with certified budwood in a certified nursery. Certificate and combined certification‐passport labels are issued by the Ministerial Organization and sent to the producer for the saplings that meet the requirements in the Regulations. |
Yes |
Potential L. japonica infestations could be detected, though low initial infestations might be overlooked and macroscopic misidentification is possible. Uncertainties: The details of the certification process are not given (e.g. number of plants, intensity of surveys and inspections, etc.). Specific figures on the intensity of survey (sampling effort) are not provided. |
| 2 | Phytosanitary certificates and plant passport |
Export nurseries must obtain special certification from Turkish Authorities before they begin producing plants for planting. Nurseries must notify technical staff members responsible for production to obtain this certificate, which is then used for registration in the plant passport system. The phytosanitary inspections are done macroscopically. However, if there are signs of disease in the plants or in the immediate vicinity, the inspections are carried out by laboratory analysis. During the production period, official inspection is carried out. After the official approval that the sapling is free from the quarantine factor and true to type, its certificate‐passport label is issued by the Ministry. The Phytosanitary Certificates/Re‐Export Phytosanitary Certificates are issued in exportation of plants and plant products with respect to plant health. In issuing such certificates, the phytosanitary requirements of the importer country are taken into account, in compliance with the ISPM No: 7 and ISPM No: 12 rules. |
Yes |
The procedures applied could be effective in detecting L. japonica infestations though visual detection at the beginning of infestation is difficult as well as specific identification without morphological or molecular analyses. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
| 3 | Cleaning and disinfection of facilities, tools and machinery | Tools are disinfected with chemical compounds containing 10% chlorine prior to using in sapling and mother plants | No | |
| 4 | Roguing and pruning | Removal of infested branches | Yes | Pruning can affect diaspidid populations either directly by removal of infested branches and indirectly exposing the pest to biotic and abiotic control agents. |
| 5 | Biological control and mechanical control |
Biological control with different natural enemies (predators and parasitoids) can keep many potential diaspidid pests under economic injury densities. Nogall (biological control agent) is applied to protect against crown gall. |
Yes |
Chemical applications can affect biological control agents Uncertainties: No details are provided on abundance and efficacy of the natural enemies. |
| 6 | Pesticide application |
The saplings are sprayed against aphids, thrips, whiteflies, red spider pests, black spot, powdery mildew, root rot diseases and, depending on the situation, to fight or protect against weeds. Before loading the plants on the trucks for transport, the roots of seedlings are sprayed with fungicide (Thiram). |
Yes |
Chemicals are applied targeting mainly crawlers. Uncertainties: No details are given on which pesticides are applied from those listed in the Dossier, on the pesticide application schedule and on the application methods. |
| 7 | Surveillance and monitoring | Necessary precautions are taken to ensure that there are no plants other than certified saplings in the production plot and application areas. Plants closer than 15 m from the plot are not usually available. Plants around the production areas are also annually inspected by the Ministry expert in terms of quarantine organisms. In the event that these plants are contaminated with harmful organisms subject to quarantine, these plants and saplings in this area are destroyed. | Yes |
It can be effective. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
| 8 | Sampling and laboratory testing |
For the identification of viruses, bacteria, fungi and nematodes in the seedlings to be exported, min. 5 to max. 25 seedlings are randomly taken from the plantation in the nursery garden and sealed by the inspector and sent to the laboratory for analysis. Soil samples are taken for laboratory analysis in terms of quarantine organisms, particularly to check if it is free from nematodes. If it is found that the growing medium is free from nematodes, the production of saplings is started. |
No | |
| 9 | Root Washing | Roots are washed in the washing areas, near the warehouses. | No | |
| 10 | Refrigeration | The temperature of the storage tanks is between 2°C and 4°C and the humidity is 85–95%. Transportation is made with refrigerated trucks with the same conditions. | Yes | Low temperatures can slow down its development but not kill the insect. |
| 11 | Pre‐consignment inspection | Prior to export, planting material for which a Phytosanitary Certificate is to be issued shall be subjected to phytosanitary inspection. Only certified plants for planting may be exported. Phytosanitary inspectors are responsible for export controls, sampling and issuing certificates. | Yes |
The procedures applied could be effective in detecting L. japonica infestation though visual detection at the beginning of infestation is difficult as well as specific identification without morphological or molecular analyses. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
A.6.5. Overall likelihood of pest freedom
A.6.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested consignments
Malus domestica is not a preferred host.
Limited to Black Sea coastal area.
Adults and symptoms can be easily detected.
All material is produced within the nurseries.
Only crawlers are moving from the near environment.
Pesticides are effective against crawlers.
Pruning reduces infestation levels, increases sunlight exposure, new shoots are less attractive than older branches.
Natural enemies are present.
Inspections are effective.
Bundles are composed by 10 plants.
Mainly young plants, e.g. rootstocks, are exported.
A.6.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested consignments
Malus domestica is host.
Distribution of the pest is not limited to the Black sea costal area.
Pests ans symptoms are difficult to be detected.
Small infestations could be overlooked.
Certification may not look specifically for this pest, not.
Other hosts are widely distributed in Turkey, e.g. pomegranate.
Spread via grafting material, worker, with plant movement.
Pesticides are only effective for short periods on crawlers.
Biological control is not effective and pesticide treatments reduce the natural enemies
Inspections are not effective.
Bundles are composed by 25 plants.
Mainly older plants, e.g. grafted trees, are exported.
A.6.5.3 Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested consignments (Median)
Due to lack of information on the pest, the Panel judge lower values for being as likely as higher values. The median was placed closer to the lower scenario because:
-
–
Pesticides reported in the dossier are effective in the control of the pest.
-
–
There are no alternative hosts in the nursery surroundings.
A.6.5.4 Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
-
–
Data on efficacy of inspections are limited.
-
–
Timing of insecticide applications is unclear.
-
–
Pest pressure in the nursery areas is not known.
A.6.5.5. Elicitation outcomes of the assessment of the pest freedom for Lopholeucaspis japonica
The following Tables show the elicited and fitted values for pest infestation (Table A.11) and pest freedom (Table A.12).
Table A.11.
Elicited and fitted values of the uncertainty distribution of pest infestation by Lopholeucaspis japonica per 10,000 bundles of bare‐rooted plant material
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 8 | 15 | 30 | 50 | ||||||||||
| EKE | 0.177 | 0.501 | 1.10 | 2.43 | 4.40 | 7.07 | 9.97 | 16.6 | 24.4 | 29.0 | 34.2 | 39.4 | 44.3 | 47.5 | 50.1 |
The EKE results is the BetaGeneral (0.8838, 1.6206, 0, 53.5) distribution fitted with @Risk version 7.6.
Table A.12.
The uncertainty distribution of plants free of Lopholeucaspis japonica per 10,000 bundles of bare‐rooted plant material calculated by Table A.11
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,950 | 9,970 | 9,985 | 9,992 | 10,000 | ||||||||||
| EKE results | 9,949.9 | 9,952.5 | 9,955.7 | 9,960.6 | 9,965.8 | 9,971.0 | 9,975.6 | 9,983.4 | 9,990.0 | 9,992.9 | 9,995.6 | 9,997.6 | 9,998.9 | 9,999.5 | 9,999.8 |
The EKE results are the fitted values.
Based on the numbers of estimated infested bundles of bare‐rooted plant material the pest freedom was calculated (i.e. = 10,000 – number of infested bundles of bare‐rooted plant material per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.12.
Based on the numbers of estimated infested bundles of bare‐rooted plant material the pest freedom was calculated (i.e. = 10,000 – number of infested bundles of scions and budwoods per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.12.
Table A.14 The uncertainty distribution of plants free of Lopholeucaspis japonica per 10,000 bundles of scions and budwoods calculated by Table A.13
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,975 | 9,985 | 9,992 | 9,996 | 10,000 | ||||||||||
| EKE results | 9,975.0 |
9,976.3 |
9,977.9 |
9,980.3 |
9,982.9 |
9,985.5 |
9,987.8 |
9,991.7 |
9,995.0 |
9,996.5 |
9,997.8 |
9,998.8 |
9,999.5 |
9,999.8 |
9,999.9 |
The EKE results are the fitted values.
Table A.13.
Elicited and fitted values of the uncertainty distribution of pest infestation by Lopholeucaspis japonica per 10,000 bundles of scions and budwoods
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 4 | 8 | 15 | 25 | ||||||||||
| EKE | 0.088 | 0.250 | 0.550 | 1.22 | 2.20 | 3.53 | 4.99 | 8.28 | 12.2 | 14.5 | 17.1 | 19.7 | 22.1 | 23.7 | 25.0 |
The EKE results is the BetaGeneral (0.8828, 1.6145, 0, 26.7) distribution fitted with @Risk version 7.6.
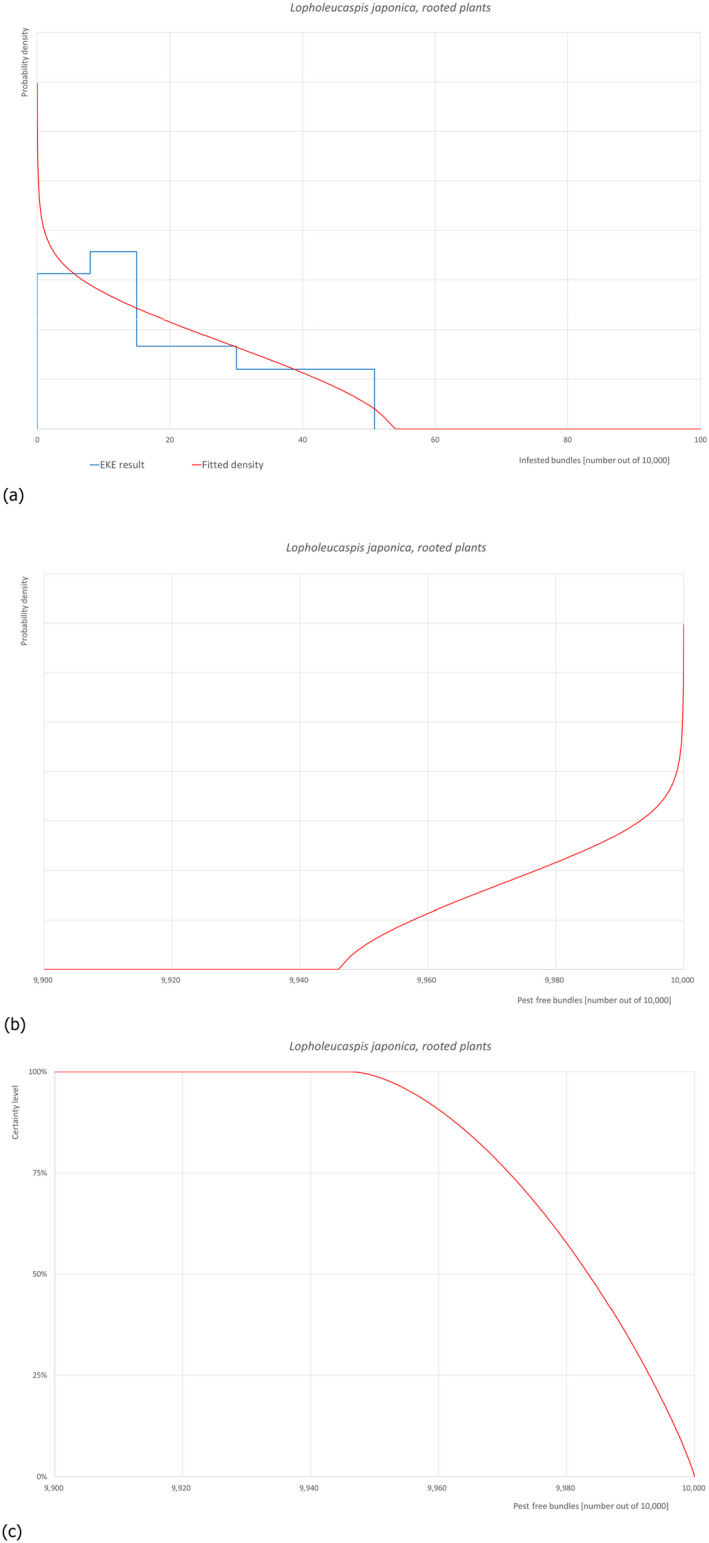
Figure A.6 (a) Elicited uncertainty of pest infestation per 10,000 bundles (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest free bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 bundles
A.6.6. References List
Addesso KM, Blalock A and O’Neal PA, 2016. Japanese Maple Scale Activity and Management in Field Nursery Production. Journal of Environmental Horticulture, 34, 41–46. https://doi.org/10.24266/0738‐2898‐34.2.41
Bienkowski AO, 1993. Morphology and systematics of the adult male of Lopholeucaspis japonica (Cockerell) (Coccinea Diaspididae). Russian Entomological Journal, 2, 25–29.
Biosecurity Australia, 2010. Final import risk analysis report for fresh apple fruit from the People’s Republic of China. Biosecurity Australia, Canberra.
CABI (Centre for Agriculture and Bioscience International), online. Lopholeucaspis japonica (Japanese baton shaped scale). Available online: https://www.cabi.org/cpc/datasheet/31328 [Accessed: 4 February 2021].
EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Gregoire J‐C, Jaques Miret JA, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Kertesz V and MacLeod A, 2018. Scientific Opinion on the pest categorisation of Lopholeucaspis japonica. EFSA Journal 2018;16(7):5353, 23 pp. https://doi.org/10.2903/j.efsa.2018.5353
EPPO (European and Mediterranean Plant Protection Organization), 1997. Lopholeucaspis japonica. In: Quarantine pests for Europe: data sheets on quarantine pests for the European Union and for the European and Mediterranean Plant Protection Organization. pp. 384–387. CAB International, Wallingford, UK.
EPPO (European and Mediterranean Plant Protection Organization), online_a. EPPO A2 List of pests recommended for regulation as quarantine pests, version 2019‐09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list [Accessed: 4 February 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Lopholeucaspis japonica (LOPLJA), Categorization. Available online: https://gd.eppo.int/taxon/LOPLJA/categorization [Accessed: 4 February 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Lopholeucaspis japonica (LOPLJA), Distribution. Available online: https://gd.eppo.int/taxon/LOPLJA/distribution [Accessed: 4 February 2021].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Lopholeucaspis japonica (LOPLJA), Host plants. Available online: https://gd.eppo.int/taxon/LOPLJA/hosts [Accessed: 31 March 2021].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 4 February 2021].
Fulcher A, Hale F and Halcomb M, 2011. Japanese maple scale: An important new insect pest in the nursery and landscape. University of Tennessee, Extension Publications.
García Morales M, Denno BD, Miller DR, Miller GL, Ben‐Dov Y and Hardy NB, online. ScaleNet: A literature‐based model of scale insect biology and systematics, Lopholeucaspis japonica. Available online: http://scalenet.info/catalogue/Lopholeucaspis%20japonica/ [Accessed: 4 February 2021].
Gill S, Shrewsbury P and Davidson J, 2012. Japanese maple scale (Lopholeucaspis japonica): a pest of nursery and landscape trees and shrubs. University of Maryland Extension fact sheet.
Harsur MM, Joshi S and Pal RN, 2018. Pomegranate: a new host for the invasive scale insect Lopholeucaspis japonica (Cockerell, 1897) (Hemiptera: Diaspididae) from Gujarat, India. Oriental Insects. 1080/00305316.2018.1451783
Li L, Wang R and Waterhouse DF, 1997. The distribution and importance of arthropod pests and weeds of agriculture and forestry plantations in southern China. Australian Centre for International Agricultural Research (ACIAR). https://doi.org/10.22004/ag.econ.117177
Magsig‐Castillo J, Morse JG, Walker GP, Bi JL, Rugman‐Jones PF and Stouthamer R, 2010. Phoretic dispersal of armored scale crawlers (Hemiptera: Diaspididae). Journal of Economic Entomology, 103, 1172–1179. https://doi.org/10.1603/ec10030
Miller DR and Davidson JA. 1990. A list of armoured scale pests. In: Rosen D (ed.). Armoured scale insects. Vol. 4B. Amsterdam, Elsevier, pp. 299–306.
Murakami Y, 1970. A review of biology and ecology of Diaspine scales in Japan (Homoptera, Coccoidea). Mushi, 43, 65–114.
Stimmel JF, 1995. “Japanese maple scale”, Lopholeucaspis japonica (Cockerell). Regulatory horticulture, entomology circular No. 176, Pennsylvania Department of Agriculture, Bureau of Plant Industry, 21, 33–34.
Suh SJ, 2020. Host plant list of the scale insects (Hemiptera: Coccomorpha) in South Korea. Insecta Mundi.
Tabatadze ES and Yasnosh VA, 2016. Population dynamics and biocontrol of the Japanese scale, Lopholeucaspis japonica (Cockerell) in Georgia. Entomologica, 33, 429–434.
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 4 February 2021].
A.7. Malacosoma parallela
A.7.1. Organism information
| Taxonomic information |
Current valid scientific name: Malacosoma parallela Staudinger Synonyms: Bombyx neustria var. parallela Staudinger, 1887 (Zolotuhin & Zahiri, 2008) Name used in the EU legislation: ‐ Order: Lepidoptera Family: Lasiocampidae Common name: mountain ring silk moth Name used in the Dossier: Malacosoma parallela |
|
| Group | Insects | |
| EPPO code | MALAPA | |
| Regulated status | The pest is included in the EPPO A2 list (EPPO, online). | |
| Pest status in Turkey | Malacosoma parallela is present in Turkey, with no further details on its distribution (EPPO, online; CABI). | |
| Pest status in the EU | Malacosoma parallela is absent in the EU. | |
| Host status on Malus domestica | Malus domestica is reported as a host of Malacosoma parallela (EPPO, online). | |
| PRA information |
EPPO Pest Risk Assessments available (EPPO online):
|
|
| Other relevant information for the assessment | ||
| Biology | The main outbreaks of M. parallela occur in mountain forests at an altitude of 1,000–1,800 m where the pest finds optimal conditions for its development. It can occur up to 2,400 m. Flight peaks of M. parallela usually occur between June and July, depending on altitude. The moth completes one generation per year. Adults have a crepuscular behaviour. Copulation occurs 2–3 h after emergence of the adults. Eggs are laid in groups; egg masses usually contain from 100 to 400 eggs covered by a thick layer of special female secretion (spumaline), which is shining whitish grey and silvery when fresh and then turns dark. Egg masses are laid around thin branches of host plants. The layer of secretion protects eggs against unfavourable conditions during overwintering. One female usually makes one egg mass, but sometimes two or three. Neonate caterpillars appear from the end of March at the same time as young leaves of host plants. They usually all hatch during 1–2 days and begin to make a web nest on branches. They feed on young leaves around the nest. The nest is usually constructed by the group of individuals hatched from one egg mass. It can be up to 25 cm long and 17 cm wide. When caterpillars reach third or fourth instar, the group usually leaves the first nest and constructs new ones (2 or 3) in places where there is more food. Caterpillars moult inside nests and feed on leaves around the nest. They leave the nests at the fifth or sixth instar and then continue to live individually. The length of their development time depends much on the altitude and host plant. Caterpillars moult five times before making cocoons on leaves and in other different places at the end of May and in June (Grechkin, 1956; Degtyareva, 1964; Sarkissyan, 1972; Romanenko, 1981; Maslov, 1988). | |
| Symptoms | Main type of symptoms | Defoliation of host plants is usually very spectacular. The presence of egg masses, nests and individual caterpillars is easily detected. Moths are attracted by sources of light |
| Presence of asymptomatic plants | No information | |
| Confusion with other pests | Egg masses encircle thin branches of host plants similar to the egg masses of the closely related European species Malacosoma neustria. | |
| Host plant range | M. parallela is extremely polyphagous and causes most damage in its native range to Quercus spp., Prunus spp., and Malus spp.. Significant damage also occurs on various other woody species, including many native species of Central Asia: Berberis integerrima, Chaenomeles japonica, Cotoneaster insignis, Cotoneaster suavis, Crataegus hissarica, Crataegus pontica, Crataegus turkestanica, Cydonia oblonga, Prunus armeniaca, Prunus avium, Prunus cerasus, Prunus divaricata, Prunus mahaleb, Prunus padus, Prunus persica, Pyrus communis, Rosa canina, Rosa corymbifera, Rosa kokanica, Rosa maracandica, Salix excelsa, Salix tenuijulis, Sorbus persica, Sorbus turkestanica. Other native and planted deciduous trees and shrubs are damaged occasionally: Atraphaxis pyrifolia, Elaeagnus angustifolia, Fraxinus sogdiana, Hippophae rhamnoides, Juglans regia, Lonicera korolkowii, Lonicera nummulariifolia, Myricaria bracteata, Populus alba, Populus tremula, Ribes nigrum, Ribes rubrum, Rubus idaeus, Rubus turkestanicus and Ulmus minor (Pavlovskii and Shtakelberg, 1955; Grechkin, 1956; Degtyareva, 1964; Sarkissyan, 1972; Romanenko, 1981; Maslov, 1988). | |
| Reported evidence of impact | M. parallela is an important defoliator of many deciduous trees in different countries of the former USSR. Outbreaks often last for two consecutive years. It was especially noted as a very dangerous pest of oak in the mountains of Armenia (Sarkissyan, 1972) and of forests, fruit trees and shrubs of Rosaceae, Fagaceae and Elaeagnaceae in the mountains of Tajikistan (Grechkin, 1956; Degtyareva, 1964). It attacks both stressed and healthy trees of different ages. Outbreaks occur throughout large mountain areas, often resulting in 100% defoliation and sometimes leading to the death of trees and forests. Damage may be caused by this species alone, or in association with Yponomeuta padellus, Euproctis kargalica, Erschoviella musculana, Lymantria dispar or other defoliators. Attacks may result in serious changes in the environment over large areas, including problems of erosion. | |
| Pathways and evidence that the commodity is a pathway | M. parallela can spread by flights of adult moths. All stages of the life cycle can be transported on host plants moving in trade, particularly plants for planting and cut branches. Eggs, larvae and pupae (cocoons) may be associated with wood carrying bark and may be present as contaminants on other commodities. | |
| Surveillance information | No surveillance information is currently available from the Turkey NPPO. | |
A.7.2. Possibility of pest presence in the nursery
A.7.2.1. Possibility of entry from the surrounding environment
If present in the surroundings, the pest can enter the nursery as Turkey is producing Malus domestica plants for planting outdoors. The pest could enter the nursery mainly by active dispersal (flight). Being highly polyphagous, the pest could be associated with many host plants occurring in the surroundings.
Uncertainties:
-
–
No data available on the distribution of the pest or population densities in the areas of production in Turkey.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery.
A.7.2.2. Possibility of entry with new plants/seeds
The pest (larvae, pupae and mainly eggs) can be transported on host plants, particularly plants for planting and cut branches. The presence of the pest can be easily detected by visual inspection, however, eggs masses can be overlooked by non‐trained personnel.
Uncertainties:
-
–
Uncertain if certified material is screened for this pest.
Taking into consideration the above evidence and uncertainties, the Panel considers it possible that the pest could enter the nursery, though unlikely because all stages can be detected by visual inspection.
A.7.2.3. Possibility of spread within the nursery
If the pest enters the nursery from the surroundings, it could spread either by adult flight, larval movement or infested plant material. Active dispersal of larvae is possible especially if plants are touching with each other (as in stoolbeds). Given that the pest is polyphagous the pest could be associated with other host plants produced in the nursery (e.g. Prunus spp.).
Taking into consideration the above evidence, the Panel considers that the transfer of the pest within the nursery is possible.
A.7.3. Information from interceptions
There are no records of interceptions of Malus domestica plants for planting from Turkey due to the presence of M. parallela between 1994 and March 2022 (EUROPHYT and TRACES‐NT, online).
A.7.4. Evaluation of the risk mitigation options
In the table below, all risk mitigation measures currently applied in Turkey are listed and an indication of their effectiveness on M. parallela is provided. The description of the risk mitigation measures currently applied in Turkey is provided in the Table 6.
| No. |
Risk mitigation measure (name) |
Description |
Effective | Evaluation/Uncertainties |
|---|---|---|---|---|
| 1 | Certified material |
The Ministerial experts and inspectors carry out the phytosanitary control on mother plants in spring, summer and autumn for harmful organisms, and the amount of propagation materials (grafted plants, budwoods, rootstocks, scions) that can be obtained from mother plants is determined. For the saplings, the phytosanitary control is also carried out at the same time, regarding harmful organisms specified in quarantine and plant passports, and certification regulations. If free from the harmful organisms, the Ministry issues certificates and labels for the propagation material to be taken from plants in the mother blocks. Certified seed or certified seedling is grafted with certified budwood in a certified nursery. Certificate and combined certification‐passport labels are issued by the Ministerial Organization and sent to the producer for the saplings that meet the requirements in the Regulations. |
Yes |
Potential M. parallela infestations could be easily detected, though egg masses might be overlooked by non‐trained personnel. Uncertainties: The details of the certification process are not given (e.g. number of plants, intensity of surveys and inspections, etc.). Specific figures on the intensity of survey (sampling effort) are not provided. |
| 2 | Phytosanitary certificates and plant passport |
Export nurseries must obtain special certification from Turkish Authorities before they begin producing plants for planting. Nurseries must notify technical staff members responsible for production to obtain this certificate, which is then used for registration in the plant passport system. The phytosanitary inspections are done macroscopically. However, if there are signs of disease in the plants or in the immediate vicinity, the inspections are carried out by laboratory analysis. During the production period, official inspection is carried out. After the official approval that the sapling is free from the quarantine factor and true to type, its certificate‐passport label is issued by the Ministry. The Phytosanitary Certificates/Re‐Export Phytosanitary Certificates are issued in exportation of plants and plant products with respect to plant health. In issuing such certificates, the phytosanitary requirements of the importer country are taken into account, in compliance with the ISPM No: 7 and ISPM No: 12 rules. |
Yes |
The procedures applied could be effective in detecting M. parallela infestations though egg masses might be overlooked by non‐trained personnel. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
| 3 | Cleaning and disinfection of facilities, tools and machinery | Tools are disinfected with chemical compounds containing 10% chlorine prior to using in sapling and mother plants | No | |
| 4 | Roguing and pruning | Removal of infested branches | Yes | Pruning can remove M. parallela egg masses and nests. |
| 5 | Biological and mechanical control |
Biological control with different natural enemies (predators and parasitoids) can reduce the pest populations. Nogall (biological control agent) is applied to protect against crown gall. |
Yes |
Natural enemies can be present in the environment. Uncertainties: No details are provided on abundance and efficacy of the natural enemies. |
| 6 | Pesticide application |
The saplings are sprayed against aphids, thrips, whiteflies, red spider pests, black spot, powdery mildew, root rot diseases and, depending on the situation, to fight or protect against weeds. Before loading the plants on the trucks for transport, the roots of seedlings are sprayed with fungicide (Thiram). |
Yes | Some of the pesticides listed in the dossier might be effective against the moth. Uncertainties: No details are given on which pesticides are applied from those listed in the Dossier, on the pesticide application schedule and on the application methods. |
| 7 | Surveillance and monitoring | Necessary precautions are taken to ensure that there are no plants other than certified saplings in the production plot and application areas. Plants closer than 15 m from the plot are not usually available. Plants around the production areas are also annually inspected by the Ministry expert in terms of quarantine organisms. In the event that these plants are contaminated with harmful organisms subject to quarantine, these plants and saplings in this area are destroyed. | Yes | It can be effective. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
| 8 | Sampling and laboratory testing |
For the identification of viruses, bacteria, fungi and nematodes in the seedlings to be exported, min. 5 to max. 25 seedlings are randomly taken from the plantation in the nursery garden and sealed by the inspector and sent to the laboratory for analysis. Soil samples are taken for laboratory analysis in terms of quarantine organisms, particularly to check if it is free from nematodes. If it is found that the growing medium is free from nematodes, the production of saplings is started. |
Yes |
It can be effective, however, the intensity of survey is not known. |
| 9 | Root Washing | Roots are washed in the washing areas, near the warehouses. | No | |
| 10 | Refrigeration | The temperature of the storage tanks is between 2°C and 4°C and the humidity is 85–95%. Transportation is made with refrigerated trucks with the same conditions. | Yes | Low temperatures can slow down its development but not kill the insect. |
| 11 | Pre‐consignment inspection | Prior to export, planting material for which a Phytosanitary Certificate is to be issued shall be subjected to phytosanitary inspection. Only certified plants for planting may be exported. Phytosanitary inspectors are responsible for export controls, sampling and issuing certificates. | Yes |
The procedures applied could be effective in detecting M. parallela infestation. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
A.7.5. Overall likelihood of pest freedom
A.7.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested consignments
Limited distribution/climatic restrictions.
All material is produced within the nurseries.
Pesticides are effective against eggs, larvae and adults.
Pruning reduces infestation levels.
Biological enemies are present in the environment.
Defoliation and nests presence facilitate the detection of the pest.
Visual inspection is performed by trained personnel.
Control of mother plants.
Bundles are composed by 10 plants.
Mainly young plants, e.g. rootstocks, are exported.
A.7.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested consignments
Malus is a preferred host.
Spread to more area in Turkey/no climatic restrictions.
Most of the propagation material is produced in other nurseries.
Wind and human assisted dispersal play a role in spreading the pest.
Pesticides are not effective against eggs, larvae and adults.
Biological enemies are not present or affected by pesticide treatments.
Inspections are not effective in identifying pest presence.
Control of mother plants is not effective.
Bundles are composed by 25 plants.
Mainly older plants, e.g. grafted trees, are exported.
A.7.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested consignments (Median)
Due to the limited information available about pest presence and pressure in the nursery area, the panel considers lower values for being as likely as higher values.
A.7.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Main uncertainties:
-
–
Data on efficacy of inspections are limited.
-
–
Timing of insecticide applications is unclear.
-
–
Pest pressure in the nursery areas is not known.
A.7.5.5. Elicitation outcomes of the assessment of the pest freedom for Malacosoma parallela
The following Tables show the elicited and fitted values for pest infestation (Table A.15) and pest freedom (Table A.16).
Table A.15.
Elicited and fitted values of the uncertainty distribution of pest infestation by Malacosoma parallela per 10,000 bundles of rooted plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 2 | 4 | 6 | 10 | ||||||||||
| EKE | 0.147 | 0.306 | 0.535 | 0.944 | 1.45 | 2.05 | 2.65 | 3.90 | 5.29 | 6.08 | 7.00 | 7.92 | 8.82 | 9.46 | 10.0 |
The EKE results is the BetaGeneral (1.2604, 2.0485, 0, 11) distribution fitted with @Risk version 7.6.
Table A.16.
The uncertainty distribution of plants free of Malacosoma parallela per 10,000 bundles of rooted plants calculated by Table A.15
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,990 | 9,994 | 9,996 | 9,998 | 10,000 | ||||||||||
| EKE results | 9,990 | 9,991 | 9,991 | 9,992 | 9,993 | 9,994 | 9,995 | 9,996 | 9,997 | 9,998 | 9,999 | 9,999.1 | 9,999.5 | 9,999.7 | 9,999.9 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.16.
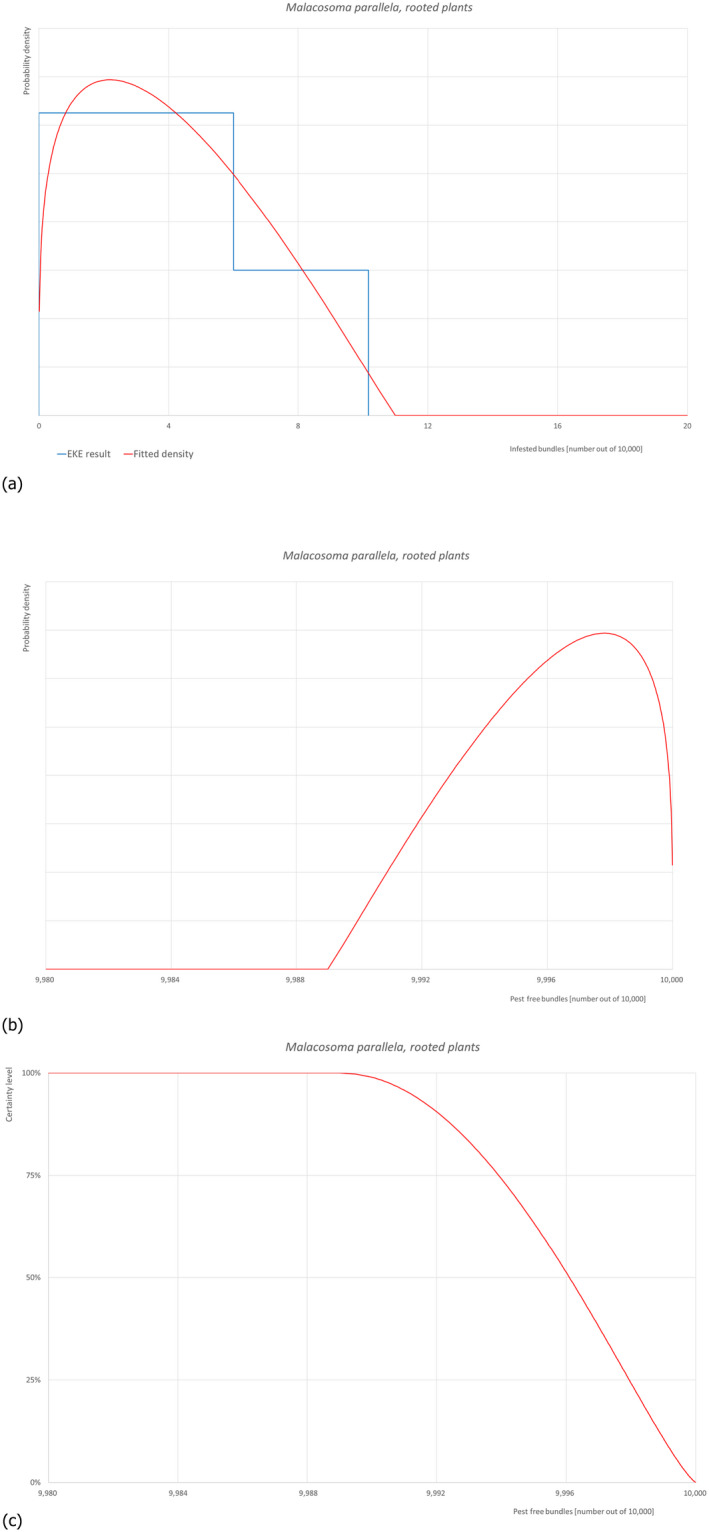
Figure A.7 (a) Elicited uncertainty of pest infestation per 10,000 bundles (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest free bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 bundles
A.7.6. References List
CABI (Centre for Agriculture and Bioscience International), online. Datasheet Malacosoma parallela (mountain ring silk moth). Available online: https://www.cabi.org/isc/datasheet/32330 [Accessed: 27 April 2022].
EPPO (European and Mediterranean Plant Protection Organization), 2005. Data sheets on quarantine pests, Malacosoma parallela. OEPP/EPPO, Bulletin OEPP/EPPO Bulletin 35, 431–433.
EPPO (European and Mediterranean Plant Protection Organization), online_b. Malacosoma parallela (MALAPA). Available online: https://https://gd.eppo.int/taxon/MALAPA [Accessed: 27 April 2022].
Degtyareva VI, 1964. The Main Lepidopteran Pests of Trees and Shrubs of the Central Part of Gissar Mountain Ridge and Gissar valley. Izdatel'stvoAkademii Nauk Tadzhikskoi SSR, Dushanbe (TJ) (in Russian).
Grechkin VP, 1956. Important species of pests of mountain forests of Tajikistan. Zoologicheskii Zhurnal 35, 1476–1492 (in Russian).
Maslov AD, 1988. Guide to Forest Protection against Pests and Diseases. Agropromizdat, Moscow (RU) (in Russian).
Pavlovskii EN and Shtakelberg AA, 1955. Guide to Forest Pests. Izdatel’stvo Akademii Nauk SSSR, Moscow‐Leningrad (RU) (in Russian).
Romanenko KE, 1981. Pests of Field Shelter Belts in Kirgizia. Ilim, Frunze (KG) (in Russian).
Sarkissyan RA, 1972. Population Dynamics of Euproctis chrysorrhoea and Malacosoma parallela in the Zangezur Mountains of the Armenian SSR. Candidate thesis, Erevan (AM) (in Russian).
A.8. Pratylenchus loosi (Root lesion nematode)
A.8.1. Organism information
| Taxonomic information |
Current valid scientific name: Pratylenchus loosi Loof, 1960 Synonyms: – Name used in the EU legislation: not regulated in the EU Name used in the Dossier: Pratylenchus loosi Loof Order: Rhabditida Family: Pratylenchidae |
|
| Group | Nematoda | |
| EPPO code | PRATLO | |
| Regulated status |
EU status: – Non‐EU: A1 list: Argentina (2019) (EPPO, Global Database) |
|
| Pest status in Turkey |
Present, (CABI, online) |
|
| Pest status in the EU | Present in Bulgaria (CABI, online) | |
| Host status on Malus domestica | In CABI Plantwise Knowledge Bank (online) apple, Malus domestica is recorded as a host of Pratylenchus loosi (https://www.plantwise.org/KnowledgeBank/datasheet/43898). | |
| PRA information | There is no PRA available. | |
| Other relevant information for the assessment | ||
| Biology |
Pratylenchus loosi belongs to the group of root lesion nematodes, Pratylenchus spp., with over 60 named species. Root lesion nematodes are the third most important group of nematodes after root‐knot and cyst nematodes, which have significant economic impacts on crops worldwide (Castillo and Vovlas, 2007; Jones et al, 2013). Like other root lesion nematodes, P. loosi is polyphagous, migratory endoparasite that occurs in both soil and roots. Although root lesion nematodes are polyphagous, there are distinct differences in host preferences among species in this nematode group (Castillo and Vovlas, 2007). P. loosi is a serious pest of tea (Camellia sinensis). Besides tea, it has also been found in association with several important crops such as apples in Sri Lanka, Japan and China, citrus in Japan, India and Iran, pears, Convallaria and natural grasses in Japan, coffee in Java, fruit trees in China, breadfruit (Artocarpus altilis) in Gualdeloupe, pasture grasses in Florida, cabbage in Kenya and bananas in American Samoa (Seinhorst, 1977; Inserra et al, 1996; Ekanayake and Toida, 1997; Brooks, 2004; Castillo and Vovlas, 2007; Waceke, 2007; Divsalar et al, 2012). P. loosi invades the roots where it reproduces, feeds and moves freely in the tissues. When the nematodes invade the roots, they cause thickening of the cell walls, dark brown or black necrotic lesions, and cavities. When the nematodes are searching for fresh feeding roots, or when the parasitised roots are severely damaged or overparasitised, or when the plants are old, stressed or diseased, the nematodes leave the roots and move into the soil. P. loosi has been known to survive for up to three years in host‐free soil in the lesions of the larger old storage roots of tea that are not removed after clearing old tea fields (Gnanapragasam and Mohotti, 2005). The optimum temperature for P. loosi development is 18–20°C; it requires 45–48 days to complete its life cycle (Seinhorst, 1977). The presence of plants such as Tephrosia vogelii, Sesbania cinerascens, Cassia elata and Acacia spp., as well as certain weeds increases the occurrence of this nematode species in the tea field. On the other hand, plants like Eragrostis curvula, Tagetes spp., Arachis pintoi, Tithonia diversifolia, Wedeliya trilobata, Vetiveria ziazanoides, Adhathoda vasica, Ricinis communis, Azadirachta indica, Madhuca indica, Sambucus javanica, Plectranthus zeylanicus, Indigofera teysamanii, Eupatorium inuliformes, Calliandra calothyrsus and Crotalaria anagyroides reduce the population density of this nematode (Gnanapragasam and Mohotti, 2005). Turkey’s replies to the questions posed by the Working Group state that P. loosi has been detected in limited areas in very low populations in potatoes, eggplants, wheat and lentils. So far, this species has not been found on apples in Turkey and no damage by it or other Pratylenchus species to fruit crops has been observed. According to the available information, the nematode has been reported on cultivated plants in Turkey in two regions (Sanliurfa, Ankara) (Yavuszlangolu et al., 2012; Kasapoglu Uludamar et al, 2018). No epidemics or economic losses have been reported in Turkey so far. |
|
| Symptoms | Main type of symptoms |
The above‐ground symptoms of Pratylenchus spp. infestation are not very specific. They appear as irregular, patchy areas while the plants wilt, become stunted, chlorotic, and often die. Symptoms caused by root lesion nematode infestation are more obvious on the roots, where dark brown or black necrotic lesions are observed on the root surface. |
| Presence of asymptomatic plants | In general, symptoms caused by Pratylenchus spp. on plants are inconspicuous and can be easily overlooked. P. loosi may also go undetected if the nematode infestation in the roots of host plants is low (symptoms are not very pronounced). The nematode may therefore not be detected by existing phytosanitary procedures and export controls, including laboratory tests. In Turkey (see Turkish dossier), roots are examined macroscopically only for the presence of root galls caused by root‐knot nematodes (Meloidogyne spp.). Necrotic lesions caused by root‐lesion nematodes are not monitored. | |
| Confusion with other pathogens/pests |
Symptoms of host plant infestation by P. loosi are expressed as reduced plant growth and vigour with moderate root necrosis. Typical above‐ground symptoms such as stunting, chlorosis and wilting result from reduced water and nutrient availability due to impaired root function. Therefore, these symptoms are similar to those of other soil‐borne diseases, insect damage, nutrient deficiency, or cultural and/or environmental stress. The most characteristic sign of a nematode problem in the field is often an irregularity or inconsistency of symptoms. However, yield losses can also occur without noticeable above‐ground symptoms. Symptoms on the underground parts of the plant can be more informative, but care must be taken to diagnose the cause of the symptoms. Many common symptoms, such as necrotic lesions, are also characteristic of damage caused by other root lesion nematodes. P. loosi can easily be confused with other Pratylenchus species. |
|
| Host plant range |
Camellia sinensis (tea) is the main host of Pratylenchus loosi (CABI Plantwise Knowledge Bank, online). Other hosts that may be affected (CABI Plantwise Knowledge Bank, online): Abelmoschus esculentus (okra), Acacia decurrens (green wattle), Alternanthera sessilis (sessile joyweed), Artemisia vulgaris (mugwort), Cassia alata (Ringworm senna), Catharanthus roseus (Madagascar periwinkle), Cestrum (jessamine), Cinnamomum camphora (camphor laurel), Citrus, Coffea (coffee), Convallaria, Cymbopogon citratus (lemongrass), Cyperus (flatsedge), Cyperus rotundus (purple nutsedge), Dioscorea (yam), Dioscorea rotundata, Diospyros kaki (persimmon), Dipteryx odorata (tonka bean), Fragaria ananassa (strawberry), Grevillea robusta (silky oak), Hibiscus rosa‐sinensis (China‐rose), Imperata cylindrica (cogon grass), Malus domestica (apple), Mangifera indica (mango), Musa x paradisiaca (plantain), Oplismenus compositus, Panicum hemitomon, Panicum repens (torpedo grass), Paspalum notatum (Bahia grass), Pisum sativum (pea), Poncirus trifoliata (Trifoliate orange), Prunus avium (sweet cherry), Pyrus communis (European pear), Saccharum officinarum (sugarcane), Sesbania cannabina (corkwood tree), Solanum nigrum (black nightshade), Solanum tuberosum (potato), Sorghum bicolor (sorghum), Tagetes (marigold), Tecoma stans (yellow bells), Tephrosia (hoary‐pea), Tithonia diversifolia (Mexican sunflower), Vigna unguiculata (cowpea), Zea mays (maize). |
|
| Reported evidence of impact |
P. loosi is known as a major pest of tea (Camellia sinensis) in Sri Lanka and many other tea‐producing countries including India, Japan, Korea, Taiwan, Iran and Russia (Luc et al. 2005; Castillo & Vovlas, 2007; Handoo et al., 2008). It is considered an important pest of tea grown at altitudes from 900 to 1800 m in Sri Lanka and from 0 to 300 m in Japan. P. loosi can seriously damage tea plantations by attacking not only the existing feeder roots and causing their slow decline, but also the main roots (storage roots) of tea plants, limiting nutrient and water uptake from the soil as well as |
|
|
carbohydrate reserves and subsequent recovery after pruning (Gnanapragasam, 2002; Castillo and Vovlas, 2007). Tea plants become weaker and chlorotic, have lower yields and may also die. Yield reduction can vary from 4 to 40% depending on the variety planted, prevailing climatic conditions, population density of nematodes, age and vigour of plants, soil type and pH. The extent of damage is greater in young infested tea plantations and nurseries where damage of 60 to 100% may occur if proper control measures are not taken (Gnanapragasam and Mohotti, 2005). This nematode has also been reported as a pest of pasture grasses and oranges (Singh et al., 2013; Disvalar et al., 2012). Poorer growth of Unshiu oranges in Japan (Ushiyana & Ogaki, 1970) and yellowing and reduction of leaves and necrotic lesions on parasitised roots of citrus trees in the southwest of Caspian Sea in Iran (Divsalar et al., 2012) have been reported. Unfortunately, no detailed information is available on the economic impact on grasses, oranges and other host plants except tea. Since P. loosi causes necrotic lesions on the roots, secondary infections by bacteria and fungi that further damage the root system are very common. The synergistic effect of interaction between P. loosi and soil‐borne root fungi (e.g. Rhizoctonia solani, Fusarium proliferatum, F. pallidoroseum, Sclerotium rolfsii) was reported in 2010 by Hoseini et al. The occurrence of soft root rot on mature tea roots was also reported, leading to death of affected plants in dry weather. The disease complex caused by P. loosi and a group of fungi Paecilomyces lilacinus, Paecilomyces sp. and Absidia corymbifera was also reported (Gnanapragasam and Mohotti, 2005). |
||
| Pathways and evidence that the commodity is a pathway |
|
|
| Surveillance information |
In order to identify plant pests and diseases in the planting material to be exported from Turkey, a minimum of 5 and a maximum of 25 saplings are taken at random from the planting in the nursery, sealed by the inspector and sent to the laboratory for analysis. The saplings in the growing area are examined macroscopically for pests. If pest infestation is suspected, samples are again taken and sent to the laboratory for analysis. |
|
A.8.2. Possibility of pest presence in the nursery
A.8.2.1. Possibility of entry from the surrounding environment
When P. loosi is present in the environment, it can enter Malus production sites with planting material, water, soil and growing media attached to agricultural machinery, tools and footwear. Agricultural machinery is a very important means of spreading the nematode within and between different plantations.
Root lesion nematodes, Pratylenchus spp. can migrate from plant to plant through the roots (Castillo and Vovlas, 2007). However, active dispersal of Pratylenchus species, including P. loosi, is limited to short distances (no more than a few metres). Transmission from the surrounding area to the production field is mainly passive through the spread of infected plants, contaminated soil and run‐off rain water.
Uncertainties
Pratylenchus loosi occurs in Turkey, but there is no information on its distribution and abundance in the Malus domestica growing area.
There are uncertainties regarding the lack of data from official monitoring surveys and reports of problems caused by this nematode in apple production in Turkey. This is related to the fact that the nematode is either actually absent or has not been detected in apple orchards.
It is uncertain how many orchards in apple production areas in Turkey are infested with P. loosi. There is uncertainty about the possible infestation of weeds in the surrounding area, which are also considered hosts for this nematode.
In view of the above evidence and uncertainties, the Panel considers that it is possible that the nematode is present in the environment and could enter Malus domestica nurseries with new plants for planting or other human activities.
A.8.2.2. Possibility of entry with new plants/seeds
Plants for planting (roots) are important pathway. P. loosi attacks the roots of host plants in which it lives, feeds, and reproduces
Plants for planting that originate from production sites where the nematode is present may be infested. However, infestation of such plants can be easily overlooked.
Uncertainties
There are uncertainties regarding the lack of data to monitor the presence of P. loosi in nurseries from which M. domestica intended for planting originate.
Symptoms caused by P. loosi often go undetected at first because the nematodes are microscopic root parasites and when nematode infestations in the roots of host plants are low, symptoms are not very pronounced. In addition, above‐ground symptoms are often general signs of root stress in the plant. Therefore, the presence of P. loosi in apple roots may not be detected by visual inspection.
Taking into consideration the above evidence and uncertainties, the Panel considers it is possible that the infestation could be overlooked and that the nematode could be introduced into apple nurseries/orchards with new plants.
A.8.2.3. Possibility of spread within the nursery
Pratylenchus spp. (including P. loosi) actively move only short distances (they can move from plant to plant through the roots), no more than a few (1‐2) meters from the root zone they infect (Castillo & Vovlas, 2007). Therefore, the main route of spread of this nematode within the nursery/production field is generally human assisted. The nematode can be spread with plants for planting from infested production sites and by soil movement activities – with soil as such or with soil associated with tools and machinery, and with contaminated runoff rainwater and irrigation water.
Uncertainties
If present, it is very likely that the nematode will spread within the production field.
Taking into consideration the above evidence and uncertainties, the Panel considers that the nematode, if present in the field, may be transferred from one host plant to another.
A.8.3. Information from interceptions
No interceptions of Pratylenchus loosi from Turkey to EU have been reported in EUROPHYT‐TRACES so far (until March 2022).
A.8.4. Evaluation of the risk reduction options
In the table below, all risk mitigation measures currently applied in Turkey are listed and an indication of their effectiveness on P. loosi is provided. The description of the risk mitigation measures currently applied in Turkey is provided in the Table 6.
| No. |
Risk mitigation measure (name) |
Description |
Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|---|
| 1 | Certified material |
The experts and inspectors of the Ministry carry out the phytosanitary control on mother plants in spring, summer and autumn for harmful organisms, and the amount of propagation materials (buds, budwoods, rootstocks, scions, etc.) that can be obtained from mother plants is determined. For the saplings, the phytosanitary control is also carried out at the same time, regarding harmful organisms specified in quarantine and plant passports, and certification regulations. Certified seed or certified seedling is grafted with certified budwood in a certified nursery. If free from the harmful organisms, the Ministry issues certificates and labels for the propagation material to be taken from plants in the mother blocks. |
No | |
|
2 |
Phytosanitary certificates and plant passport |
Export nurseries must obtain special certification from Turkish Autorities before they begin producing plants for planting. Nurseries must notify technical staff members responsible for production to obtain this certificate, which is then used for registration in the plant passport system. The phytosanitary inspections are done macroscopically. However, if there are signs of disease in the plants or in the immediate vicinity, the inspections are carried out by laboratory analysis. During the production period, official inspection is carried out. After the official approval that the sapling is free from the quarantine factor and true to type, its certificate‐passport label is issued by the Ministry. The Phytosanitary Certificates/Re‐Export Phytosanitary Certificates are issued in exportation of plants and plant products with respect to plant health. In issuing such certificates, the phytosanitary requirements of the importer country are taken into account, in compliance with the ISPM No: 7 and ISPM No: 12 rules. |
Yes |
Evaluation: Pratylenchus spp. is not on the list of harmful organisms systematically monitored or tested for the presence on plants intended for planting in Turkey. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. Information on thedistribution and abundance of P. loosi in the Malus domestica growing area is unreliable. |
| 3 | Cleaning and disinfection of facilities, tools and machinery | Tools are disinfested with chemical compounds containing 10% chlorine prior to using in sapling and mother plants | No | |
| 4 | Rouging and pruning | Applied in case of infections/infestations. | No | |
| 5 | Biological and mechanical control |
‘Nogall’ is applied to protect against crown gall. Weeds are controlled mechanically in the nurseries and in the surrounding areas. |
No | |
| 6 | Pesticide application |
The saplings are sprayed against aphids, thrips, whiteflies, red spider pests, black spot, powdery mildew, root rot diseases and, depending on the situation, to fight or protect against weeds. Before loading the plants on the trucks for transport, the roots of seedlings are sprayed with fungicide (Thiram). |
No | |
| 7 | Surveillance and monitoring |
Both processes are conducted according to Turkish phytosanitary regulations. Necessary precautions are taken to ensure that there are no plants other than certified saplings in the production plot and application areas. Plants within and around the production areas are annually inspected to check the presence of quarantine organisms. Visual inspection at least once or twice a year during production or during uprooting of the plants. Visual inspection can be supported by the use of microscope or laboratory analysis if pests are suspected to be present. In the event that these plants are infected/infested with harmful organisms subject to quarantine, these plants are destroyed. |
Yes |
Evaluation: Details of the surveillance and monitoring during the production cycle are not provided. Pratylenchus spp. is not on the list of harmful organisms systematically monitored or tested for the presence on plants intended for planting in Turkey. Uncertainties: Details of the surveillance and monitoring have not been described. Information on the distribution and abundance of P. loosi in the Malus domestica growing area is unreliable. |
| 8 |
Sampling and laboratory testing |
For the identification of viruses, bacteria, fungi and nematodes in the seedlings to be exported, min. 5 to max. 25 seedlings are randomly taken from the plantation in the nursery garden and sealed by the inspector and sent to the laboratory for analysis. Soil samples are taken for laboratory analysis in terms of quarantine organisms, particularly to check if it is free from nematodes. If it is found that the growing medium is free from nematodes, the production of saplings is started. |
Yes |
Evaluation: Soil and plants are tested in the laboratory only for the presence of root‐knot and virus vector nematodes, but not for the presence of Pratylenchus spp. Uncertainties: Soil is tested in the laboratory only for the presence of root‐knot and virus vector nematodes, but not for the presence of Pratylenchus spp. Therefore, P. loosi cannot be detected. |
| 9 | Root washing | Roots are washed in the washing areas, near the warehouses. | Yes |
Evaluation: Root washing does not reduce the risk of nematode infestation in plants intended for planting that are infested with root lesion nematodes (migratory endoparasites). Uncertainties: Because P. loosi is migratory endoparasite, root washing does not reduce the risk of nematodes infestation in plants intended for planting. |
| 10 | Refrigeration | The temperature of the storage tanks is between 2°C and 4°C and the humidity is 85–95%. Transportation is made with refrigerated trucks with the same conditions. | No | |
| 11 | Pre‐consignment inspection | Prior to export, planting material for which a Phytosanitary Certificate is to be issued shall be subjected to phytosanitary inspection. Only certified plants for planting may be exported. Phytosanitary inspectors are responsible for export controls, sampling and issuing certificates. | Yes |
Evaluation: As for nematodes, inspectors pay particular attention to the presence of galls caused by root‐knot nematodes. Symptoms caused by P. loosi cannot be detected Uncertainties: Even if inspectors examined plants for the presence of P. loosi, it might initially go undetected because the nematodes are microscopic root parasites and symptoms are not very pronounced when there is a little nematode infestation in the roots of host plants. |
A.8.5. Overall likelihood of pest freedom
A.8.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested consignments
Malus domestica is considered to be a minor host and its growing areas are mainly in the part of the country, where P. loosi has not been reported.
Effective weed control, crop rotation and field hygiene limit apple infestation.
Regular inspections by crop protection authorities are effective and further help to reduce the infection pressure of this nematode.
Washing the roots is effective against this nematode.
A.8.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested consignments
A similar pest pressure exists throughout the country.
The nematode is widespread in apple‐growing areas and its infestation is homogeneous.
Weed control, crop rotation and field sanitation are ineffective and do not help to reduce infestation of apples by this nematode.
Most apple plants are expected to be infested with nematodes.
Visual selection of apple plants for planting and visual inspections prior to export without laboratory testing are not effective and result in high infestation.
Postharvest root washing is not effective against this pest because it is endoparasitic.
A.8.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested consignments
The value of the median is estimated based on:
Uncertainties about pest pressure in Turkey.
The information on infections of P. loosi on apple plants in Turkey is missing.
The lack reported problems within the apple production area in Turkey.
The likelihood of introduction into apple production sites by natural means and human activities.
A.8.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The main uncertainty is the absence of nematode‐induced symptoms, so that the presence of the nematode in the apple roots can be overlooked; cannot be detected by visual inspection.
A.8.5.5. Elicitation outcomes of the assessment of the pest freedom for Pratylenchus loosi
The following Tables show the elicited and fitted values for pest infestation (Table A.17) and pest freedom (Table A.18).
Table A.17.
Elicited and fitted values of the uncertainty distribution of pest infestation by Pratylenchus loosi per 10,000 bundles of rooted plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 1 | 2 | 3 | 5 | ||||||||||
| EKE | 0.073 | 0.153 | 0.267 | 0.472 | 0.725 | 1.03 | 1.33 | 1.95 | 2.65 | 3.04 | 3.50 | 3.96 | 4.41 | 4.73 | 5.01 |
The EKE results is the BetaGeneral (1.2604, 2.0485, 0, 5.5) distribution fitted with @Risk version 7.6.
Table A.18.
The uncertainty distribution of plants free of Pratylenchus loosi per 10,000 bundles of rooted plants calculated by Table A.17
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,995 | 9,997 | 9,998 | 9,999 | 10,000 | ||||||||||
| EKE results | 9,995 | 9,995 | 9,996 | 9,996 | 9,996 | 9,997 | 9,997 | 9,998 | 9,999 | 9,999 | 9,999.3 | 9,999.5 | 9,999.7 | 9,999.8 | 9,999.9 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.18.
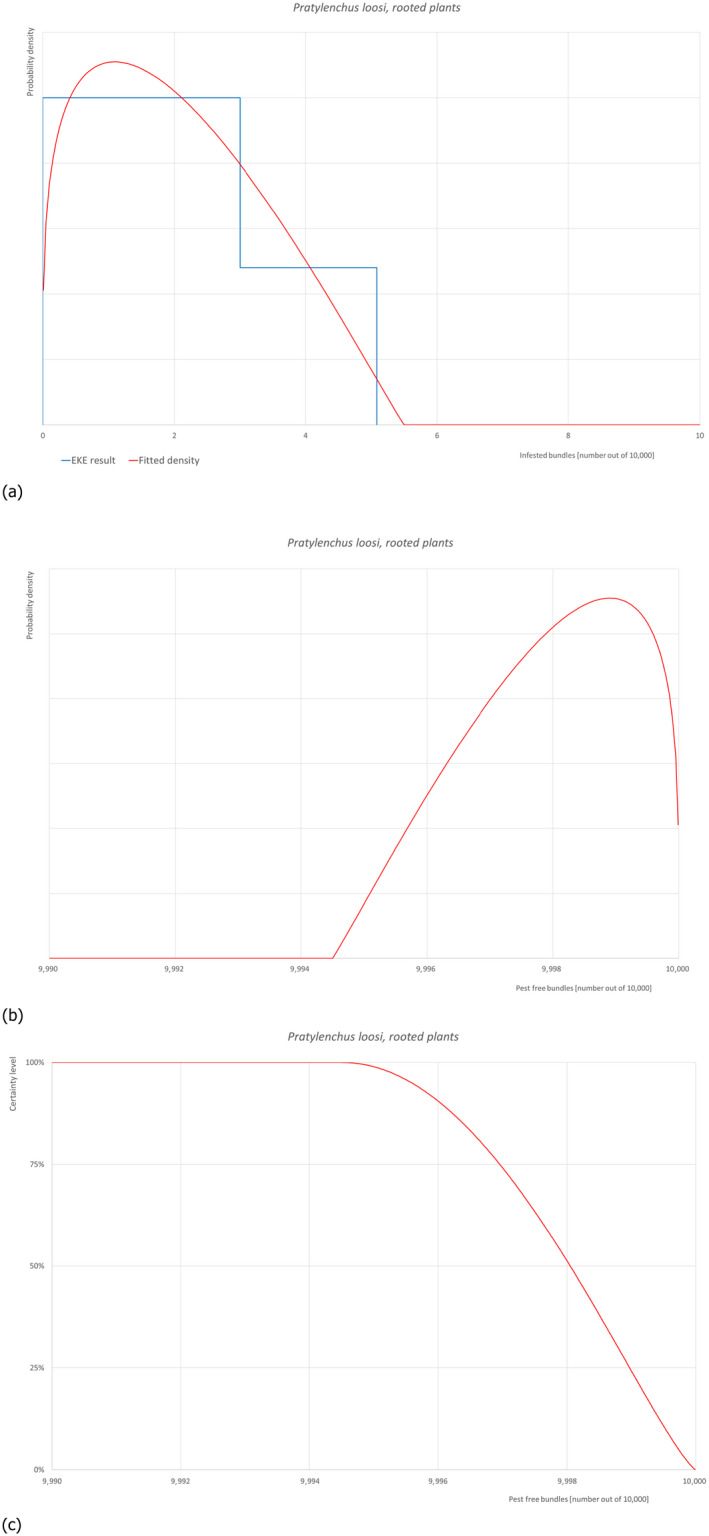
Figure A.8 (a) Elicited uncertainty of pest infestation per 10,000 bundles (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest free bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 bundles
A.8.6. References List
Brooks FE, 2004. Plant parasitic nematodes of banana in American Samoa. Nematropica, 34, 65–72.
CABI Plantwise Knowledge Bank, online. Available online: https://www.plantwise.org/KnowledgeBank/datasheet/43898 [Accessed: 6 December 2021].
Castillo P and Vovlas N, 2007. Pratylenchus (Nematoda: Pratylenchidae): diagnosis, biology, pathogenicity and management. Nematology monographs and perspectives, Vol 6. Leiden, Koninklijke Brill, 529 pp.
Divsalar N, Jamali S, Pedramfar H and Taheri H, 2012. Root lesion nematodes (Pratylenchus spp.) on citrus in south‐west of Caspian Sea. Journal of Agricultural Technology, 8, 2227–2238.
Gnanapragasam NC and Mohotti KM, 2005. Nematode Parasites of Tea. In: Luc M, Sikora RA and Bridge J (eds.). Plant Parasitic Nematodes in Subtropical and Tropical Agricultute, 2nd Edition, CABI International, Wallingford, UK, pp. 581–610.
Handoo ZA, Carta LK and Skantar AM, 2008. Taxonomy, Morphology and Phylogenetics of Coffee‐Associated Root‐Lesion Nematodes, Pratylenchus spp. In: Souza RM (ed.). Plant Parasitic Nematodes of Coffee. Springer Science + Business Media B. V., pp. 29–50.
Hoseini SMN, Pourjam E and Goltapeh EM, 2010. Synergistic studies on interaction of nematode‐fungal system of tea plant in Iran. Journal of Agricultural Technology, 6, 487–496.
Inserra RN, Duncan LW, Vovlas N and Loof PAA, 1996. Pratylenchus loosi feom pasture grasses in central Florida. Nematologica, 42, 159–172.
Ekanayake HMR and Toida Y, 1997. Nematode parasites of agricultural crops and their distribution in Sri Lanka. JIRCAS Journal, 4, 29–39.
Jones JT, Haegeman A, Danchin EGJ, Gaur HS, Helder J, Jones MGK, Kikuchi T, Manzanilla‐López R, Palomares‐Rius JE, Wesemael WML and Perry RN, 2013. Top 10 plant‐parasitic nematodes in molecular plant pathology. Molecular Plant Pathology, BSPP and John Wiley & Sons LTD, 1–16. https://doi.org/10.1111/mpp.12057
Kasapoglu Uldumar EB, Yildiz S, Imren M, Öcal A and Elekcioglu IH, 2018. Occurrence of plant parasitic nematode species in important crops in the Southeast Anatolia Region of Turkey. Türkiye Entomoloji Dergisi, 42, 63–74.
Seinhorst JW, 1977. Pratylenchus loosi. C.I.H. Descriptions of plant parasitic nematodes, Set 7, No. 98.
Singh SK, Hodda M and Ash GJ, 2013. Plant‐parasitic nematodes of potential phytosanitary importance, their main hosts and reported yield losses. EPPO Bulletin, 43, 334–374.
Ushiyama K and Ogaki C, 1970. Studies on the replant problem in Unshiu orange orchards. II. (I) The effects of citrus root nematodes, Tylenchulus semi‐pénétrons, and root lesion nematodes, Pratylenchus loosi, on citrus trees and fruits. (II) Effects of grass fallow and fumigation with nematicides on the growth of trees replanted in old citrus soils. Bulletin of the Horticultural Branch (Section), Kanagawa Agricultural Experiment Station, 18, 46–56.
Yavuszlangolu E, Elekcioglu HI, Nicol JM, Yorgancilar O, Hodson D, Yildirim AF, Yorgancilar A and Bolat N, 2012. Distribution, frequency and occurrence of cereal nematodes on the Central Anatolian Plateau in Turkey and their relationship with soil physicochemical properties. Nematology, 14, 839–854.
Waceke JW, 2007. Plant parasitic nematodes associated with cabbages in Kenya. African Crop Science Conference Proceedings, 8, 1071–1074.
A.9. Pyrolachnus pyri
A.9.1. Organism information
| Taxonomic information |
Current valid scientific name: Pyrolachnus pyri (Buckton) Synonyms: Cinara krishni, Dilachnus krishni, Lachnus pyri, Pyrolachnus krishni, Pyrolachnus macroconus Name used in the EU legislation: ‐‐‐‐‐ Order: Hemiptera Family: Aphididae Common name: Pear Aphid Name used in the Dossier: Pyrolachnus pyri |
|
| Group | Insects | |
| EPPO code | ‐‐‐‐‐‐‐‐‐‐‐ | |
| Regulated status |
The pest is not regulated in the EU, neither is on any EPPO list nor database. |
|
| Pest status in Turkey |
Present in Turkey. Fifteen aphid species were reported on pome fruit trees in Nigde province (central south Turkey). Among these, P. pyri represents a new record for the Turkish aphid fauna and is considered a rare species collected on Pyrus communis (Görür, 2004). |
|
| Pest status in the EU | Absent | |
| Host status on Malus domestica | Pyrolachnus pyri was observed on Malus domestica (Apple) and Prunus armeniaca (Apricot) in fruit orchards of Kashmir (Khan et al., 2017). | |
| PRA information | No PRA is available for Pyrolachnus pyri. | |
| Other relevant information for the assessment | ||
| Biology |
Apterae of Pyrolachnus pyri are dull yellow to dark brown, often dusted with wax, antennae and legs blackish (3.5–6.0 mm). Alatae have wings dark at their bases. Recorded from bark of branches of Pyrus communis, and from Malus domestica and Eriobotrya japonica as well (Ali Khan et al., 2017). It has been recorded from Iran, Bahrain, Pakistan, India, Nepal, Sri Lanka, Korea and China where it is one of the main pests feeding on pear trees completing six generations per year in Jingchuan district, Sichuan province (Blackman and Eastop, 1994). Holocyclic in China, where regular spring and autumn migrations, suggesting host alternation, were observed (Long and Chen 1988). Apparently anholocyclic elsewhere. In China, it overwinters as egg laid on the pear branches. Hatching of nymphs occurs from the last ten days of March to the second decade of April. In the adult stage it reproduces parthenogenetically giving birth to a generation of viviparous female nymphs on pear trees. In the last ten days of April, when the mean temperature for ten days reaches 10°C, the aphid develops its wings and migrates to the mountains, at an altitude of 3,000–3,400 m, on Populus szechuanica or Salix caprea. After two‐three generations carried out only by viviparous females, in the last ten days of August, winged forms appear and go back to pear trees. Both male and female aphid do not appear until the second or the last ten days of October, then the overwintering eggs are laid. The life time of adult averages 7–9 days. The preimaginal development includes five |
|
|
young instars, and the development of a generation needed about 30 days when the mean temperature of ten days was 18.9–19.1°C. The average number of progeny/life time is 43.2 nymphs. The critical period for chemical control should fall in the last stage of incubation of the overwintering eggs or in the second 10 days of October when both females and males appear. Predators recorded to feed on Pyrolachnus pyri were Harmonia dimidiata (F.), Hippodamia variegata (Goe.), and Adalia tetraspilota (Hope), Chrysoperla z. Sillemi E. & P, spiders and syrphid flies (Khan et al., 2017) P. macroconus Zhang, described from Eriobotrya in China (Zhang and Zhong, 1982d) is closely related and possibly a synonym (https://www.aphidsonworldsplants.info/). |
||
| Symptoms | Main type of symptoms | No data available, though species of this family produce abundant honeydew on which sooty molds develop. |
| Presence of asymptomatic plants | No data available | |
| Host plant range | The species is reported on Pyrus communis, Malus domestica and Eriobotrya japonica. Besides, Populus szechuanica and Salix caprea are secondary hosts. | |
| Reported evidence of impact | It is considered a major pest on pear in Asia (Blackman and Eastop, 1994). | |
| Pathways and evidence that the commodity is a pathway | The assessed commodities consist of grafted plants, rootstocks, budwood and scions. Since Pyrolachnus pyri was observed on Malus domestica (Apple) and Prunus armeniaca (Apricot), and because this species overwinters as eggs laid on the branches, M. domestica plants for planting can be considered a pathway. | |
| Surveillance information | No surveillance information is available for this species in Turkey. | |
A.9.2. Possibility of pest presence in the nursery
A.9.2.1. Possibility of entry from the surrounding environment
If present in the surroundings, the pest can enter the nursery as Turkey is producing the M. domestica plants for planting outdoors. The pest could enter the nursery by active dispersal (flight) and passive dispersal (air currents or human assisted movements). The pest is reported on M. domestica, as well as on Prunus armeniaca and Eriobotrya japonica which can be also present in the surroundings of the nursery. No surveillance for P. pyri is performed in Turkey.
Uncertainties
No further data are available on the distribution of the pest or population densities in Turkey, other than Nidge province.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nursery.
A.9.2.2. Possibility of entry with new plants/seeds
The pest can be transported on host plants, particularly plants for planting and cut branches, as eggs, nymphs and adults can be found on plant branches.
Uncertainties
Uncertain if certified material is screened for this pest. Although the colonies and the symptoms may be easy to detect, the eggs can be overlooked because of their small size.
Taking into consideration the above evidence and uncertainties, the Panel considers it possible that the pest could enter the nursery.
A.9.2.3. Possibility of spread within the nursery
If the pest enters the nursery from the surroundings, it could spread either by adult flight, by human assisted or infested plant material movement.
Taking into consideration the above evidence, the Panel considers that the transfer of the pest within the nursery is possible.
A.9.3. Information from interceptions
There are no records of interceptions of M. domestica plants for planting from Turkey due to the presence of P. pyri between 1994 and March 2022 (EUROPHYT and TRACES‐NT, online).
A.9.4. Evaluation of the risk mitigation options
In the table below, all risk mitigation measures currently applied in Turkey are listed and an indication of their effectiveness on P. pyri is provided. The description of the risk mitigation measures currently applied in Turkey is provided in the Table 8.
| No. | Risk mitigation measure (name) |
Description |
Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|---|
| 1 | Certified material |
The Ministerial experts and inspectors carry out the phytosanitary control on mother plants in spring, summer and autumn for harmful organisms, and the amount of propagation materials (grafted plants, budwoods, rootstocks, scions) that can be obtained from mother plants is determined. For the saplings, the phytosanitary control is also carried out at the same time, regarding harmful organisms specified in quarantine and plant passports, and certification regulations. If free from the harmful organisms, the Ministry issues certificates and labels for the propagation material to be taken from plants in the mother blocks. Certified seed or certified seedling is grafted with certified budwood in a certified nursery. Certificate and combined certification‐passport labels are issued by the Ministerial Organization and sent to the producer for the saplings that meet the requirements in the Regulations. |
Yes |
Potential P. pyri infestations could be detected, though eggs might be overlooked. Uncertainties: The details of the certification process are not given (e.g. number of plants, intensity of surveys and inspections, etc.). Specific figures on the intensity of survey (sampling effort) are not provided. |
| 2 | Phytosanitary certificates and plant passport |
Export nurseries must obtain special certification from Turkish Authorities before they begin producing plants for planting. Nurseries must notify technical staff members responsible for production to obtain this certificate, which is then used for registration in the plant passport system. The phytosanitary inspections are done macroscopically. However, if there are signs of disease in the plants or in the immediate vicinity, the inspections are carried out by laboratory analysis. During the production period, official inspection is carried out. After the official approval that the sapling is free from the quarantine factor and true to type, its certificate‐passport label is issued by the Ministry. The Phytosanitary Certificates/Re‐Export Phytosanitary Certificates are issued in exportation of plants and plant products with respect to plant health. In issuing such certificates, the phytosanitary requirements of the importer country are taken into account, in compliance with the ISPM No: 7 and ISPM No: 12 rules. |
Yes |
The procedures applied could be effective in detecting P. pyri infestations though visual detection might fail to detect eggs. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. |
| 3 | Cleaning and disinfection of facilities, tools and machinery | Tools are disinfested with chemical compounds containing 10% chlorine prior to using in sapling and mother plants. | No | |
| 4 | Roguing and pruning | Applied in case of infections/infestations. | Yes | It could be useful in removing infested plant parts. |
| 5 | Biological control and mechanical control |
Harmonia dimidiata (F.), Hippodamia variegata (Goe.), and Adalia tetraspilota (Hope), Chrysoperla z. Sillemi E. & P, spiders and syrphid flies are reported preying on P. pyri in China. Nogall (biological control agent) is applied to protect against crown gall. |
Yes | Natural enemies can play a role, though no data on species present and predation levels are available in Turkey. |
| 6 | Pesticide application |
The saplings are sprayed against aphids, thrips, whiteflies, red spider pests, black spot, powdery mildew, root rot diseases and, depending on the situation, to fight or protect against weeds. Before loading the plants on the trucks for transport, the roots of seedlings are sprayed with fungicide (Thiram). |
Yes |
Although no specific insecticides targeting this pest are mentioned in the dossier, the active ingredients used for other insects would be somewhat effective against the pest. Uncertainties: No details are given on which pesticides are applied from those listed in the Dossier on the pesticide application schedule and on the application methods. |
| 7 | Surveillance and monitoring | Necessary precautions are taken to ensure that there are no plants other than certified saplings in the production plot and application areas. Plants closer than 15 m from the plot are not usually available. Plants around the production areas are also annually inspected by the Ministry expert in terms of quarantine organisms. In the event that these plants are contaminated with harmful organisms subject to quarantine, these plants and saplings in this area are destroyed. | Yes | It can be effective, however specific figures on the intensity of survey (sampling effort) are not provided. |
| 8 | Sampling and laboratory testing |
For the identification of viruses, bacteria, fungi and nematodes in the seedlings to be exported, min. 5 to max. 25 seedlings are randomly taken from the plantation in the nursery garden and sealed by the inspector and sent to the laboratory for analysis. Soil samples are taken for laboratory analysis in terms of quarantine organisms, particularly to check if it is free from nematodes. If it is found that the growing medium is free from nematodes, the production of saplings is started. |
No | |
| 9 | Root Washing | Roots are washed in the washing areas, near the warehouses. | No | |
| 10 | Refrigeration | The temperature of the storage tanks is between 2°C and 4°C and the humidity is 85–95%. Transportation is made with refrigerated trucks with the same conditions. | Yes | Low temperatures can slow down its development but not kill the insect. |
| 11 | Pre‐consignment inspection | Prior to export, planting material for which a Phytosanitary Certificate is to be issued shall be subjected to phytosanitary inspection. Only certified plants for planting may be exported. Phytosanitary inspectors are responsible for export controls, sampling and issuing certificates. | Yes |
The procedures applied could be effective in detecting P. pyri infestations Uncertainties: Visual inspections may fail to detect the eggs. No specific figures on the intensity of survey (sampling effort) are provided. |
A.9.5. Overall likelihood of pest freedom
A.9.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested consignments
Reported only in few provinces.
Malus is not a preferred host.
The species is anolocyclic on the host and does not migrate to another host.
Honeydew and sooty moulds are visible as well as plants decay.
Lower number of eggs laid.
Presence of natural enemies.
Application of effective insecticides.
The pest mainly stays on the plant trunk.
Bundles are composed by 10 plants.
Mainly young plants, e.g. rootstocks, are exported.
A.9.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested consignments
More widely spread in Turkey.
Present in province of high apple production.
Malus, is a preferred host.
Other hosts are present.
Eggs are difficult to detect, especially overwintering eggs.
Possible misclassification.
Insecticides not fully effective.
No effective resident natural enemies.
Life cycle not know in detail.
Bundles are composed by 25 plants.
Mainly older plants, e.g. grafted trees, are exported.
A.9.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested consignments (Median)
Due to the limited information available about pest presence and pressure in the nursery area, the panel considers lower values as likely as higher values.
A.9.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The values reflect a high uncertainty due to the lack of information on pest pressure.
A.9.5.5. Elicitation outcomes of the assessment of the pest freedom for Pyrolachnus pyri
The following Tables show the elicited and fitted values for pest infestation/infection (Table A.19) and pest freedom (Table A.20).
Table A.19.
Elicited and fitted values of the uncertainty distribution of pest infestation by Pyrolachnus pyri per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 8 | 15 | 25 | 40 | ||||||||||
| EKE | 0.414 | 0.939 | 1.75 | 3.29 | 5.28 | 7.73 | 10.2 | 15.5 | 21.3 | 24.7 | 28.5 | 32.2 | 35.7 | 38.1 | 40.1 |
The EKE results is the Weibull (1.1254, 1.7753, 0, 43) distribution fitted with @Risk version 7.6.
Table A.20.
The uncertainty distribution of plants free of Pyrolachnus pyri per 10,000 bundles calculated by Table A.19
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,960 | 9,975 | 9,985 | 9,992 | 10,000 | ||||||||||
| EKE results | 9,959.9 | 9,961.9 | 9,964.3 | 9,967.8 | 9,971.5 | 9,975.3 | 9,978.7 | 9,984.5 | 9,989.8 | 9,992.3 | 9,994.7 | 9,996.7 | 9,998.2 | 9,999.1 | 9,999.6 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. 10000 – number of infested bundles per 10000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.20.
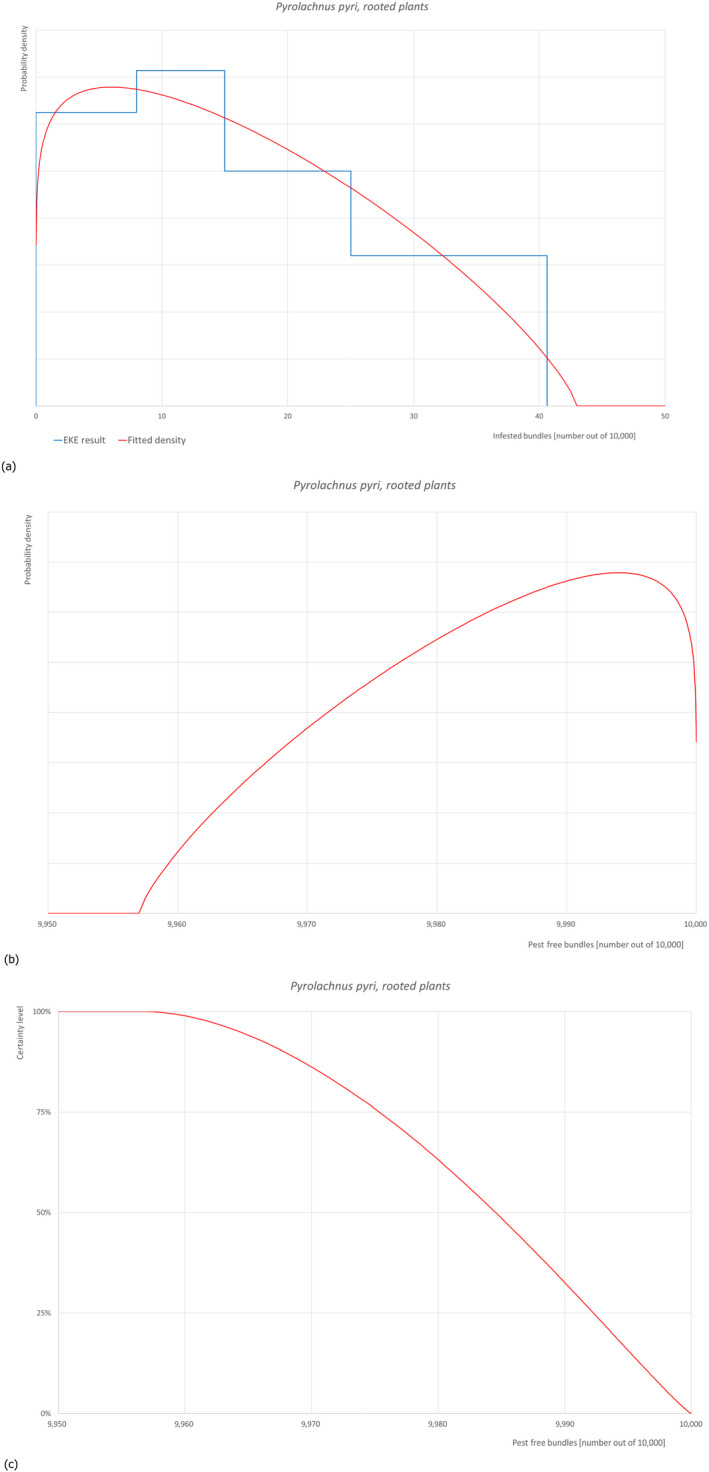
Figure A.9 (a) Comparison of judged values for the uncertainty distribution of pest infestation per 10,000 bundles (histogram in blue) and fitted distribution (red line); (b) density function to describe the uncertainties of the likelihood of pest freedom; (c) descending distribution function of the likelihood of pest freedom
A.9.6. References List
Aphids on World Plants. Available online: https://www.aphidsonworldsplants.info/d_APHIDS_P.htm#Pyrolachnus
Ghosh AK, 1974. New species and new records of aphids (Homoptera: Aphididae) from Northeast India. Oriental Insects, 8, 161–175.
Gorur G, 2004. Aphid (Homoptera: Aphididae) species on pome fruit trees in Nigde Province of Turkey. Turkiye Entomoloji Dergisi, 28, 21–26.
Khan A, Shah M and Riyaz S, 2017. Records of aphid and their natural enemies in agro‐ecosystem with special reference to horticultural ecosystem of Kashmir. Journal of Entomology and Zoology Studies, 5, 189–203.
Yamamoto T, Hattori M and Itino T, 2020. Seasonal migration in the Aphid Genus Stomaphis (Hemiptera: Aphididae): discovery of host alternation between Woody Plants in Subfamily Lachninae. Journal of Insect Science, 20, 1–10.
A.10. Tomato ringspot virus (ToRSV)
A.10.1. Organism information
| Taxonomic information |
Current valid scientific name: Tomato ringspot virus Synonyms: ToRSV, Tomato ringspot, Tomato ringspot nepovirus. Name used in the EU legislation: Tomato ringspot virus [ToRSV] Category: Virus Order: Picornavirales Family: Secoviridae Common name: ringspot of tomato, union necrosis of apple, chlorosis mosaic of raspberry, chlorosis of pelargonium, stem pitting of prunus, yellow vein of grapevine. Name used in the Dossier: Tomato ringspot virus (ToRSV) |
|
| Group | Virus and Viroids | |
| EPPO code | ToRSV0 | |
| Regulated status |
ToRSV is listed as EU Quarantine pest (Annex II, Part A of Commission Implementing Regulation (EU) 2019/2072); Pests not known to occur in the EU Union territory (2019). Quarantine pest: Morocco (2018), Tunisia (2012), Canada (2019), Mexico (2018), Israel (2009), Moldova (2017), Norway (2012). A1 list: Egypt (2018), Argentina (2019), Brazil (2018), Paraguay (1995), Uruguay (1995), Bahrain (2003), China (1993), Kazakhstan (2017), Georgia (2018), Ukraine (2019), APPPC (1993). A2 list: Jordan (2013), Russia (2014), Turkey (2016), EAEU (2016), EPPO (1975) |
|
| Pest status in Turkey | Present, restricted distribution (EPPO, 2010) or few occurrences (CABI, 2015). According to the additional information provided by Turkey, ToRSV has been reported on cultivated plants in four (Hakkari, Mugla, Hatay and west Anatolia) regions. | |
| Pest status in the EU | Present, no details (France, Lithuania, Poland). Few occurrences (Croatia). Transient under eradication (Germany and Netherlands) (EPPO, Online). | |
| Host status on Malus domestica | Malus domestica is reported as a host for ToRSV in the EPPO Global Database (EPPO, Online). | |
| PRA information |
Scientific Opinion on the pest categorisation of non‐EU viruses and viroids of Cydonia Mill., Malus Mill. and Pyrus L. (EFSA PLH Panel, 2019). Rapid Pest Risk Analysis for ToRSV in UK (EPPO, 2017). |
|
| Other relevant information for the assessment | ||
| Biology |
ToRSV is a bipartite positive‐sense RNA virus with isometric particles in Secoviridae family, Nepovirus genus (Sanfaçon et al., 2006). ToRSV has a wide range of hosts, infecting primarily perennial plants such as tomato, tobacco, cucumber, pepper, peach, apple, grape, cherry, strawberry, raspberry, plum, geranium, walnut, and ornamental plants (Stace‐Smith, 1984). Experimentally, its host diversity is also very high and about 35 families are susceptible to this virus (Zindovic et al., 2014). The most common symptom of ToRSV infection is the presence of annular spots on the leaves. ToRSV is transmitted in several ways, such as sap inoculation (under experimental conditions), seeds, vegetative propagation, pollen and different species of Xiphinema (Bitterlin et al., 1987; Pinkerton et al., 2008). |
|
| Symptoms | Main type of symptoms |
ToRSV mostly does not cause striking symptoms, and symptom expression varies according to the plant species, virus isolate, the age of the plant at the time of infection and environmental conditions. In general, infected plants show typical symptoms as a shock reaction. Plants can be seen as pale yellow and showing pale green spots on the leaves that develop along the major side veins, causing systemic chlorotic or necrotic ring stains, as well as deformation of the fruit growth. Chronically infected plants usually exhibit no obvious symptoms but show a general decline in productivity (Stace‐smith, 1984; Gonsalves, 1988; EPPO, 2013). Major diseases caused by ToRSV on fruit crops include vein yellowing in grapevines, and yellow bud mosaic in peach and almond which cause pale‐ green to pale‐yellow blotches to develop along the main vein or large lateral veins of leaves (EPPO, 2005). In apple plants, ToRSV causes a delay in foliation, the leaves are small and sparse, showing a vein yellowing and pale green colour. Terminal shoot growth is reduced and the stem internodes are short. And commonly, there is a partial or complete separation of the graft union on severely affected trees (EPPO, 2013). |
| Presence of asymptomatic plants | In certain cases, ToRSV disease could be asymptomatic. | |
| Confusion with other pathogens/pests | Note that geographical distribution, natural host range and vector relations of ToRSV are closely parallel to Tobacco ringspot virus (TRSV) (EPPO/CABI, 1996). | |
| Host plant range |
In nature, ToRSV occurs mostly in vegetable and perennial crops, including ornamental and woody plants, such as Lycopersicon esculentum Mill. (tomato), Cucumis sativus (cucumber), Nicotiana tabacum (tobacco), Solanum tuberosum (potato), Vitis vinifera (grapevine), Vaccinium corymbosum (blueberry), Fragaria vesca (strawberry), Pelargonium domesticum (geranium), raspberry (Rubus idaeus), Rubus fruticosus, Rubus sp. (blackberry), Malus sp. (apple), Hosta sp., Aquilegia vulgaris, Delphinium sp., Fragaria ananassa, Fraxina americana, Gladiolus sp., Heleborus foetidus, Hydrangea macrophylla, Iris sp., Punica granatum, Phaseolus vulgaris, Prunus persica, Prunus sp., Rosa sp., Trifolium sp., Vigna unguiculate, Viola cornuta (Samuitienė and Navalinskienė, 2001; Sanfaçon et al., 2006; EPPO, 2013). Additionally, other uncultivated hosts, such as Taraxacum officinale, Rumex acetosella, Stellaria spp., among other 21 species can be infected by ToRSV (Mountain et al., 1983; Powell et al., 1984). |
|
| Reported evidence of impact |
Not relevant, ToRSV is listed as EU Quarantine pest (Annex II, Part A of Commission Implementing Regulation (EU) 2019/2072). |
|
| Pathways and evidence that the commodity is a pathway | Plants for planting of Malus, Pelargonium, Prunus and Rubus are potential host commodities for ToRSV (EPPO, online). Thus, plants for planting coming from a country where ToRSV occurs can be the main pathway of entry (EFSA PLH Panel, 2013). | |
| Surveillance information |
According to the EPPO and CABI, ToRSV has a restricted presence in Turkey, with few occurrences, based on information dated on 2010 and 2015 (EPPO/CABI, online). This is in accordance with the information provided by the Ministry of Agriculture and Forestry (MAF) of Turkey in the requested additional information (Dossier section 3), where ToRSV has been reported on different cultivated plants in four Turkish regions. In particular, ToRSV was detected on tomato, pepper, cucumber and grapevine symptomatic samples in Hakkari province in 2014 and 2015 (Akdura and Şevik, 2021), also on tomato, pepper and cucumber in Muğla (Fidan, 1995), including strawberry in Aegean region (Yeşilçöllü et al., 2011; Yorganci and Sekin, 1984), on blackberry in Hatay (Sertkaya, 2010), and on almond nursery trees in west Anatolia (Azeri and Çiçek, 1997). To date, ToRSV has not been detected on apple in Turkey. ToRSV is included in Annex‐1/B list of the Regulation on Plant Quarantine, there is official sampling strategy for the detection of ToRSV, which information is provided in ‘Regulation on Plant Quarantine’ and ‘Plant Quarantine Sampling Instruction by Republic of Turkey Ministry of Agriculture and Forestry General Directorate of Food and Control’ (Anonymous, 2014; Anonymous, 2019). The inspection and monitoring are performed according to the information provided in ‘Instruction for Phytosanitary Standards in Production Materials of Fruit and Grapevine (Anonymous, 2006). From the information provided by the MAF of Turkey in the requested additional information (Dossier section 3) and also provided in the apple technical report, for the identification of ToRSV in the seedlings to be exported, among 5 and 25 seedlings are randomly taken from the plantation in the nursery and sealed by the inspector, and then, sent to the laboratory for analysis (Anonymous, 2014). The seedlings in the production area are examined macroscopically aspect pests. In case of suspected the virus detection, samples are taken again for analysis. It is sent to the laboratory for diagnosis. When the seedlings are exported in a different province, they are transported to the export point by plant passport. At the control stage, the plant passport is given to the inspector. Once all processes have been completed, the EU have requested that ‘Consignment complies with Annex VII points 3 a, 3 b 4 a, 45, 46 a(i), 46 b Option of Annex VII of Commission Implementing Regulation (EU) 2019/2072. That no symptoms of diseases caused by non‐European viruses been observed on the plants at the place of production since the beginning of the last complete cycle of vegetation. The plants have been: (a) Officially certified under a certification scheme requiring them to be derived in direct line from material which has been maintained under appropriate conditions and subjected to official testing for at least Tomato ringspot virus using appropriate indicators or equivalent methods and has been found free, in these tests, from those pests. (b) No symptoms of diseases caused by Tomato ringspot virus have been observed on plants at the place of production, or on susceptible plants in its immediate vicinity, since the beginning of the last complete cycle of vegetation (Anonymous, 2019). |
|
A.10.2. Possibility of pest presence in the nursery
A.10.2.1. Possibility of entry from the surrounding environment
ToRSV has a wide natural host range. Its occurrence in Turkey is restricted to four provinces/regions, where ToRSV has been found in some cultivated plant species. The production area of Izmir is placed in Aegean region, where ToRSV was detected in tomato, pepper, cucumber and strawberry (Fidan, 1995; Yeşilçöllü et al., 2011; Yorganci and Sekin, 1984). There is no specific information on the cultivated and non‐cultivated plant species in the nursery surroundings. According to the additional information requested to the Turkish MAF, the production area is surrounded by wire or stone wall or left empty, and also, there is a set of standard precautions to ensure that no plants other than certified saplings are present in the production plot and application areas. Weeds are controlled and wild plants around the production areas are also annually inspected by the Ministry expert in terms of quarantine organisms.
The dispersal range of ToRSV infection by natural processes appear to be constrained, as ToRSV is apparently limited to nematode transmission, in particular to the nematode‐vector species of the Xiphinema americanum group, which appears appear not to be established in Turkey. While ToRSV is primarily soil‐borne, seed transmission have been also reported in a range of test species (soybean, strawberry, raspberry and pelargonium) and pollen transmission in pelargonium (Kahn, 1956; Mellor and Stace‐Smith, 1963; Braun and Keplinger, 1973; Scarborough and Smith, 1977), with unknown factors associated to its transmission. ToRSV has not been detected in apple trees in Turkey.
Uncertainties
There is a lack of information about the particular plant species in the surrounding of nurseries.
It is unclear the extent of seed and pollen transmission in apple trees.
It is unknown whether there are other mechanisms of spread.
Taking into consideration the above evidence and uncertainties, the Panel considers that the possibility of entry into the nursery infecting apple plants from surrounding orchards may be unlikely.
A.10.2.2. Possibility of entry with new plants/seeds
Only certified class plant material is used at the production areas, and quarantine practices are carried out in accordance with the ‘Seedling Certification Regulation’ and ‘Regulation on the Registration of Plant Passports and Operators’. ToRSV disease can be symptomless, but usually apple trees show a symptom expression that would be easy to visualise during the surveys (Stace‐smith, 1984; Gonsalves, 1988; EPPO, 2013). ToRSV is capable of establishing via seed/pollen transmission in soybean, strawberry, raspberry and pelargonium plants (Kahn, 1956; Mellor and Stace‐Smith, 1963; Braun and Keplinger, 1973; Scarborough and Smith, 1977).
Uncertainties
There is a lack of information related to the virus‐free material certification, including the presence and sanitary status of alternative plant species for ToRSV that are grown in the nursery.
It is unclear to what extent the detection and sampling strategies are effective to detect asymptomatic infections.
It is unclear the extent of seed and pollen transmission in apple trees and mother plants.
Taking into consideration the above evidence and uncertainties, the Panel considers that the possibility of entry with other cultivated plants and ornamental material must be considered.
A.10.2.3. Possibility of spread within the nursery
According to the additional information requested to the Turkish MAF, apple fruit‐tree propagating materials are produced under the certification scheme in nurseries (Anonymous, 2010), and the apple plants are monitored and inspected during the vegetation period. ToRSV is readily transmissible by inoculation of sap in laboratory conditions (Stace‐Smith, 1984). ToRSV could be transmitted via clonal propagation of infected mother plants. Grafting and seed transmission has not been investigated in apple trees.
Uncertainties
It is unknown whether ToRSV could be transmitted by grafting and pruning processes.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pathogen within the nursery is very unlikely.
A.10.3. Information from interceptions
There are no records of interceptions of M. domestica plants for planting from Turkey due to the presence of ToRSV between 1994 and March 2022 (EUROPHYT and TRACES‐NT, online).
A.10.4. Evaluation of the risk reduction options
In the table below, all risk mitigation measures currently applied in Turkey are listed and an indication of their effectiveness on ToRSV is provided. The description of the risk mitigation measures currently applied in Turkey is provided in the Table 6.
| No. | Risk mitigation measure | Implementation in Turkey | Effect on pest | Evaluation and uncertainties |
|---|---|---|---|---|
| 1 | Certified material |
The Ministrial experts and inspectors carry out the phytosanitary control on mother plants in spring, summer and autumn for harmful organisms, and the amount of propagation materials (buds, budwoods, rootstocks, scions, etc.) that can be obtained from mother plants is determined. For the saplings, the phytosanitary control is also carried out at the same time, regarding harmful organisms specified in quarantine and plant passports, and certification regulations. Certified seed or certified seedling is grafted with certified budwood in a certified nursery. If free from the harmful organisms, the Ministry issues certificates and labels for the propagation material to be taken from plants in the mother blocks. |
Yes |
Practices for inspections and detections are applied according to the Turkish regulations and guidelines.
Uncertainties:
|
| 2 | Phytosanitary certificates and plant passport |
Export nurseries must obtain special certification from Turkish Authorities before they begin producing plants for planting. Nurseries must notify technical staff members responsible for production to obtain this certificate, which is then used for registration in the plant passport system. The phytosanitary inspections are done macroscopically. However, if there are signs of disease in the plants or in the immediate vicinity, the inspections are carried out by laboratory analysis. During the production period, official inspection is carried out. After the official approval that the sapling is free from the quarantine factor and true to type, its certificate‐passport label is issued by the Ministry. The Phytosanitary Certificates/Re‐Export Phytosanitary Certificates are issued in exportation of plants and plant products with respect to plant health. In issuing such certificates, the phytosanitary requirements of the importer country are taken into account, in compliance with the ISPM No: 7 and ISPM No: 12 rules. |
Yes |
The certificates relate to the compliance of material specified by the Turkish Authorities. Uncertainties: Specific figures on the intensity of survey (sampling effort) are not provided. There is a lack of details for the certification process, in addition to the surveillance and monitoring during production cycle. |
| 3 | Cleaning and disinfection of facilities, tools and machinery | Tools are disinfested with chemical compounds containing 10% chlorine prior to using in sapling and mother plants | No | |
| 4 | Rouging and pruning | Applied in case of infections/infestations. | Yes |
Identifying and removing suspicious plants could be effective to prevent viral infections. Uncertainties: The presence of latent infections |
| 5 | Biological and mechanical control |
‘Nogall’ is applied to protect against crown gall. Weeds are controlled mechanically in the nurseries and in the surrounding areas. |
Yes | Weeds control has benefit to prevent and reduce the source of viral inoculum. |
| 6 | Pesticide application |
The saplings are sprayed against aphids, thrips, whiteflies, red spider mites, black spot, powdery mildew, root rot diseases and, depending on the situation, to fight or protect against weeds. Before loading the plants on the trucks for transport, the roots of seedlings are sprayed with fungicide (Thiram). |
No | |
| 7 | Surveillance and monitoring |
Both processes are conducted according to Turkish phytosanitary regulations. Necessary precautions are taken to ensure that there are no plants other than certified saplings in the production plot and application areas. Plants within and around the production areas are annually inspected to check the presence of quarantine organisms. Visual inspection at least once or twice a year during production or during uprooting of the plants. Visual inspection can be supported by the use of microscope or laboratory analysis if pests are suspected to be present. In the event that these plants are infected/infested with harmful organisms subject to quarantine, these plants are destroyed. |
Yes |
Visual inspections may be effective to prevent viral infections. Uncertainties: It is unclear the effectivity of visual inspections to detect early infections, including the presence of latent infections. |
| 8 | Sampling and laboratory testing |
For the identification of viruses, bacteria, fungi and nematodes in the plants to be exported, min. 5 to max. 25 seedlings are randomly taken from the plantation in the nursery garden and sealed by the inspector and sent to the laboratory for analysis. Soil samples are taken for laboratory analysis in terms of quarantine organisms, particularly to check if it is free from nematodes. If it is found that the growing medium is free from nematodes, the production of saplings is started. |
Yes |
Laboratory analysis is convenient, and there is a monitoring of seedlings (5 to 25) randomly selected. Uncertainties: There is a lack of details for the analysis methodology and it is uncertain to what extent the inspection of this number of plants is effective to detect infected plants. |
| 9 | Root washing | Roots are washed to remove the soil. | No | |
| 10 | Refrigeration | The temperature of the storage tanks is between 2°C and 4°C and the humidity is 85–95%. Transportation is made with refrigerated trucks with the same conditions. | Yes | Not relevant, but low temperatures may ameliorate the multiplication of the virus, but will not eliminate it. |
| 11 | Pre‐consignment inspection | Prior to export, planting material for which a Phytosanitary Certificate is to be issued shall be subjected to phytosanitary inspection. Only certified plants for planting may be exported. Phytosanitary inspectors are responsible for export controls, sampling and issuing certificates. | Yes |
The inspection and provision of certified material are appropriated. Uncertainties: There is a lack of details for the phytosanitary inspections at this stage. |
A.10.5. Overall likelihood of pest freedom
A.10.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested consignments
Registration and certification of propagation material ensure virus‐free production.
Most of nurseries are placed in areas where the virus has not been reported.
ToRSV has not been reported in apple.
Nematode vectors are the only efficient way to get within the nurseries, and they are absent in the production areas.
No other vectors, human activities or plant material may spread the virus.
Visual inspections are under official regulation, and virus symptoms seems easy to detect in diseased plants.
A.10.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested consignments
The adherence to registration and certification criteria of propagation material for this pest is inappropriate and may increase the risk of entry.
Unidentified virus outbreaks are present in the surrounding of Malus production areas or the nurseries are places in areas close to places where the ToRSV is present.
Nematode vectors may be unidentified and present in the production areas.
Pest can enter by unknown vectors, or human activities or related plant material.
Visual inspection will not detect early stages of infections or asymptomatic plants.
A.10.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested consignments (median)
ToRSV has been reported in other plant host species from one region (Izmir) where apple is produced.
Presence of the primary vectors is very unlikely.
Introduction of the virus from the surrounding areas or from propagation material within the nurseries is very unlikely.
A.10.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Transmission efficiency by other potential nematode vectors species is not well documented.
Status of the virus in the surrounding areas is unknown.
A.10.5.5. Elicitation outcomes of the assessment of the pest freedom for tomato ringspot virus
The following Tables show the elicited and fitted values for pest infestation (Table A.21) and pest freedom (Table A.22).
Table A.21.
Elicited and fitted values of the uncertainty distribution of pest infestation by tomato ringspot virus per 10,000 bundles of rooted plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 2 | 4 | 6 | 10 | ||||||||||
| EKE | 0.147 | 0.306 | 0.535 | 0.944 | 1.45 | 2.05 | 2.65 | 3.90 | 5.29 | 6.08 | 7.00 | 7.92 | 8.82 | 9.46 | 10.0 |
The EKE results is the BetaGeneral (1.2604, 2.0485, 0, 11) distribution fitted with @Risk version 7.6.
Table A.22.
The uncertainty distribution of plants free of tomato ringspot virus per 10,000 bundles of rooted plants calculated by Table A.21
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,990 | 9,994 | 9,996 | 9,998 | 10,000 | ||||||||||
| EKE results | 9,990 | 9,991 | 9,991 | 9,992 | 9,993 | 9,994 | 9,995 | 9,996 | 9,997 | 9,998 | 9,999 | 9,999.1 | 9,999.5 | 9,999.7 | 9,999.9 |
The EKE results are the fitted values.
Based on the numbers of estimated infested bundles the pest freedom was calculated (i.e. = 10,000 – number of infested bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.22.
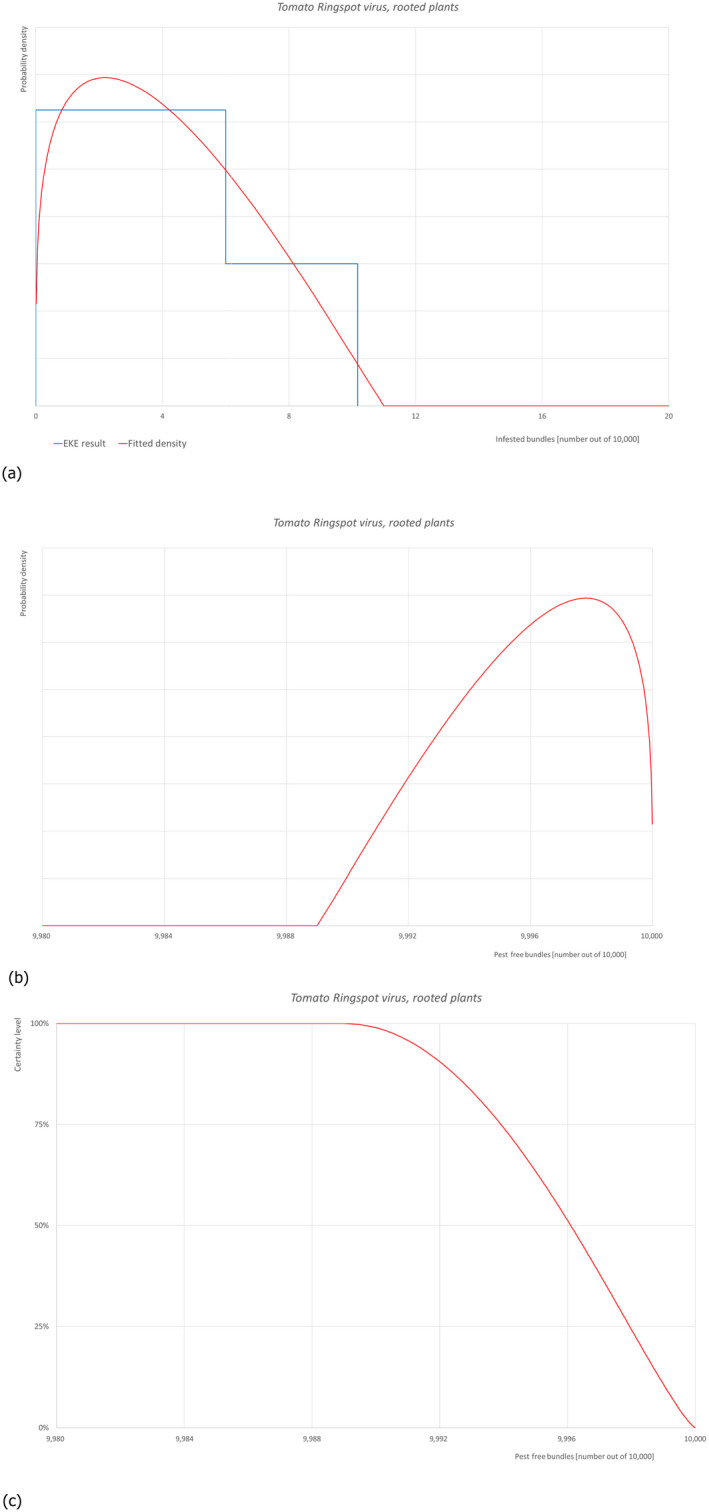
Figure A.10 (a) Elicited uncertainty of pest infestation per 10,000 bundles (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest free bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.10.6. References List
Akdura N and Şevik M, 2021. Molecular Characterization of Partial RdRp Genes of Tomato Ringspot Virus Isolates from Turkey. Avrupa Bilim ve Teknoloji Dergisi, 21, 74–82.
Anonymous, 2006. Meyve/Asma Fidan Ve Üretim Materyali Sertifikasyonu Ve Pazarlaması Yönetmeliği. (Instruction for Phytosanitary Standards in Production Materials of Fruit and Grapevine). Available online: https://www.tarimorman.gov.tr/Belgeler/Mevzuat/Yonetmelikler/Meyve.pdf
Anonymous, 2014. Bitki Karantinası Numune Alma Talimatı. Availabl online: https://zkm.tarimorman.gov.tr/antalya/Sayfalar/Detay.aspx?SayfaId=6 [Accessed: 25 June 2021].
Anonymous, 2018. Available online: https://www.tarimorman.gov.tr/GKGM/Belgeler/Bitki%20Sa%C4%9Fl%C4%B1%C4%9F%C4%B1%20Hizmetleri/bitki_bitkisel_urun/tasra/Zararli_Organizma_Teshis_Protokolleri/Viroloji.pdf. [Accessed: 25 June 2021].
Anonymous, 2019. Regulation on Plant Quarantine. Available online: https://www.tarimorman.gov.tr/Sayfalar/EN/Mevzuat.aspx?OgeId=15 [Accessed: 25 June 2021].
Azeri T and Çiçek Y, 1997. Detection of virus diseases affecting almond nursery trees in Western Anatolia (Turkey).
Bitterlin MW, Gonsalves D and Scorza R, 1987. Improved mechanical transmission of tomato ringspot virus to Prunus seedlings. Phytopathology, 77, 560–563.
Braun AJ and Keplinger JA, 1973. Seed transmission of tomato ringspot virus in raspberry. Plant Disease Reporter, 57, 431–432.
Bulletin OEPP/EPPO Bulletin, 274, 547–550.
Çağlayan K and Gazel M, 2016. Current Status of Virus Diseases in Highbush Blueberries in Turkey. Advanced Biochemical and Biotechnology, 1, 109. https://doi.org/10.29011/2574‐7258.000009
EFSA PLH Panel (EFSA Plant Health Panel), 2019. Scientific Opinion on the pest categorisation of non‐EU viruses and viroids of Cydonia Mill., Malus Mill. and Pyrus L. EFSA Journal 2019;17(9):5590, 81 pp. 10.2903/j.efsa.2019.5590
EPPO, 2005. Tomato ringspot nepovirus. Bulletin OEPP/EPPO Bulletin, 35, 313–318.
EPPO, 2013. PM3/32 (2). Tomato ringspot virus in fruit trees and grapevine: inspection. Phytosanitary procedures. Bulletin OEPP/EPPO Bulletin, 43, 397.
EPPO, Online.
Fidan Ü, 1995. Virus diseases of vegetables in greenhouses in İzmir and Muğla. Journal of Turkish Phytopathology, 24, 7–14.
Gonsalves D, 1988. Tomato ringspot virus decline; tobacco ringspot virus decline. In: Compendium of grape diseases, pp. 49–51. American Phytopathological Society, St. Paul, USA.
Kahn RP, 1956. Seed transmission of the tomato ringspot virus in the Lincoln variety of soybeans. Phytopathology, 46, 295.
Mellor FC and Stace‐Smith R, 1963. Reaction of strawberry to a ringspot virus from raspberry. Canadian Journal of Botany, 41, 865–870.
Mountain W, Powell C, Forer L and Stouffer R, 1983. Transmission of Tomato ringspot virus from dandelion via seed and dagger nematodes. Plant Disease, 67, 867–868.
Pinkerton JN, Kraus J, Martin RR and Schreiner RP, 2008. Epidemiology of Xiphinema americanum and Tomato ringspot virus on red raspberry, Rubus idaeus. Plant Disease, 92, 364–371.
Powell C, Forer L, Stouffer R, Cummins J, Gonsalves D, Rosenberger D, Hoffman J and Lister R, 1984. Orchard weeds as hosts of Tomato ringspot and Tobacco ringspot viruses. Plant Disease, 68, 242–244.
Samuitienė M and Navalinskienė M, 2001. Nepoviruses and their influence on field floriculture. Biologija, 4, 43–45.
Sanfaçon H, Zhang G, Chisholm J, Jafarpour B and Jovel J, 2006. In: Teixcira da Silva J (ed.), Molecular Biology of Tomato Ringspot Nepovirus, a Pathogen of Ornamentals, Small Fruits and Fruit Trees. Global Science Books, London, pp. 540–546.
Scarborough BA and Smith SH, 1977. Effects of tobacco‐ and tomato ringspot viruses on the reproductive tissues of Pelargonium X hortorum. Phytopathology, 67, 292–297.
Sertkaya G, 2010. Tomato ringspot nepovirus (ToRSV) in wild blackberry (Rubus fruticosus L.) in Hatay province of Turkey. 21st International Conference on Virus and other Graft Transmissible Diseases of Fruit Crops.
Stace‐Smith R, 1984. “Tomato ringspot virus”, CMI/AAB Descriptions of Plant Viruses, No. 290, AAB, Wellesbourne (GB).
Yeşilçöllü S, Gümüş M and Paylan IC, 2011. Studies on the detection of viruses in strawberry growing areas in Aegean region. Journal of Turkish Phytopathology, 40, 13–20.
Yorganci U and Sekin S, 1984. Spread of virus diseases of tobacco in the agean region. Biological serological and electron microscopic studies. Journal of Turkish Phytopathology, 13, 91–101.
Zindovic J, Marn VM and Plesko IM, 2014. Phytosanitary status of grapevine in Montenegro. EPPO Bulletin, 44, 60–64.
Appendix B – Web of Science All Databases Search String
In the table below the search string used in Web of Science is reported. In total, 184 papers were retrieved. Titles and abstracts were screened, and 13 pests were added to the list of pests (see Appendix D).
| Web of Science All databases |
TOPIC: (“Malus domestica” OR “M. Domestica” OR “apple tree$”) AND TOPIC: (“pathogen* OR pathogenic bacteria OR fung* OR oomycet* OR myce* OR bacteri* OR virus* OR viroid* OR insect$ OR mite$ OR phytoplasm* OR arthropod* OR nematod* OR disease$ OR infecti* OR damag* OR symptom* OR pest$ OR vector OR hostplant$ OR “host plant$” OR host OR “root lesion$” OR decline$ OR infestation$ OR damage$ OR symptom$ OR dieback* OR “die back*” OR malaise OR aphid$ OR curculio OR thrip$ OR cicad$ OR miner$ OR borer$ OR weevil$ OR “plant bug$” OR spittlebug$ OR moth$ OR mealybug$ OR cutworm$ OR pillbug$ OR “root feeder$” OR caterpillar$ OR “foliar feeder$” OR virosis OR viruses OR blight$ OR wilt$ OR wilted OR canker OR scab$ OR rot OR rots OR “rotten” OR “damping off” OR “damping‐off” OR blister$ OR smut OR “mould” OR “mold” OR “damping syndrome$” OR mildew OR scald$ OR “root knot” OR “root‐knot” OR rootkit OR cyst$ OR “dagger” OR “plant parasitic” OR “parasitic plant” OR “plant$parasitic” OR “root feeding” OR “root$feeding”) NOT TOPIC: (“heavy metal$” OR “pollut*” OR “weather” OR “propert*” OR probes OR “spectr*” OR “antioxidant$” OR “transformation” OR musca OR RNA OR “musca domestica” OR peel OR resistance OR gene OR DNA OR “Secondary plant metabolite$” OR metabolite$ OR Catechin OR “Epicatechin” OR “Rutin” OR “Phloridzin” OR “Chlorogenic acid” OR “Caffeic acid” OR “Phenolic compounds” OR “Quality” OR “Appearance” OR Postharvest OR Antibacterial OR Abiotic OR Storage OR Pollin* OR Ethylene OR Thinning OR fertil* OR Mulching OR Nutrient$ OR Pruning OR “human virus” OR “animal disease$” OR “plant extracts” OR “immunological” OR “purified fraction” OR “traditional medicine” OR “medicine” OR mammal$ OR bird$ OR “human disease$”) NOT TOPIC: (“Abortiporus biennis” OR “Acetobacter aceti” OR “Acetobacter pasteurianus” OR “Acetobacter persici” OR “Acleris comariana” OR “Acleris fimbriana” OR “Acleris minuta” OR “Acleris rhombana” OR “Acleris sparsana” OR “Acremonium mali” OR “Acremonium sclerotigenum” OR “Acremonium sp.” OR “Acronicta psi” OR “Acronicta rumicis” OR “Aculus malivagrans” OR “Aculus malus” OR “Aculus schlechtendali” OR “Adoretus versutus” OR “Adoxophyes orana” OR “Adoxophyes orana fasciata” OR “Aenetus virescens” OR “Aeolesthes holosericea” OR “Aeolesthes sarta” OR “Agapeta hamana” OR “Agrilus mali” OR “Agriopis bajaria” OR “Agrobacterium rhizogenes” OR “Agrobacterium sp.” OR “Agrobacterium tumefaciens” OR “Agrotis ipsilon” OR “Agrotis ipsilon aneituma” OR “Allocotaphis quaestionis” OR “Alternaria alternata” OR “Alternaria alternata f. sp. mali” OR “Alternaria arborescens” OR “Alternaria dumosa” OR “Alternaria eureka” OR “Alternaria frumenti” OR “Alternaria infectoria” OR “Alternaria kordkuyana” OR “Alternaria mali” OR “Alternaria malicola” OR “Alternaria sp.” OR “Alternaria tenuis” OR “Alternaria tenuissima” OR “Amara eurynota” OR “Amblyseius andersoni” OR “American plum line pattern virus” OR “Ametastegia” OR “Amitermes wahrmani” OR “Amphipyra pyramidea” OR “Amphitetranychus viennensis” OR “Amylostereum sacratum” OR “Anagyrus fusciventris” OR “Anarsia lineatella” OR “Anastrepha fraterculus” OR “Anastrepha ludens” OR “Anastrepha serpentina” OR “Anastrepha sp.” OR “Anastrepha suspensa” OR “Anoplophora chinensis” OR “Anoplophora glabripennis” OR “Anthonomus piri” OR “Anthonomus pomorum” OR “Anthonomus pyri” OR “Anthonomus quadrigibbus” OR “Antrodia serialis” OR “Anuraphis farfarae” OR “Anystis baccarum” OR “Aonidiella aurantii” OR “Apate monachus” OR “Aphelinus mali” OR “Aphidounguis mali” OR “Aphis craccivora” OR “Aphis eugeniae” OR “Aphis fabae” OR “Aphis gossypii” OR “Aphis odinae” OR “Aphis pomi” OR “Aphis spiraecola” OR “Aphis spiraephaga” OR “Aphis aurantii” OR “Aploneura ampelina” OR “Apocheima cinerarium” OR “Apocheima pilosaria” OR “Aporia crataegi” OR “Apple associated luteovirus” OR “Apple chat fruit agent” OR “Apple chat fruit disease” OR “Apple chlorotic leaf spot virus” OR “Apple chlorotic leafspot virus” OR “Apple dimple fruit viroid” OR “Apple fruit crinkle viroid” OR “Apple geminivirus” OR “Apple green crinkle agent” OR “Apple green crinkle associated virus” OR “Apple green crinkle disease” OR “Apple hammerhead viroid RNA” OR “Apple latent spherical virus” OR “Apple mosaic ilarvirus” OR “Apple mosaic virus” OR “Apple necrotic mosaic virus” OR “Apple proliferation phytoplasma” OR “Apple ringspot agent” OR “Apple ringspot disease” OR “Apple rough skin agent” OR “Apple rubbery wood agent” OR “Apple rubbery wood phytoplasma” OR “Apple rubbery wood‐associated virus 1” OR “Apple rubbery wood‐associated virus 2” OR “Apple scar skin viroid” OR “Apple sessile leaf phytoplasma” OR “Apple star crack agent” OR “Apple stem grooving virus” OR “Apple stem pitting virus” OR “Apriona cinerea” OR “Apriona germari” OR “Apterygothrips collyerae” OR “Archips argyrospilus” OR “Archips breviplicanus” OR “Archips crataegana” OR “Archips crataeganus” OR “Archips fuscocupreanus” OR “Archips podana” OR “Archips podanus” OR “Archips rosana” OR “Archips rosanus” OR “Archips subsidiaria” OR “Archips termias” OR “Archips xylosteanus” OR “Arcyria oerstedtii” OR “Argolamprotes micella” OR “Argyresthia conjugella” OR “Argyresthia cornella” OR “Argyroploce umbrosana” OR “Argyrotaenia citrana” OR “Argyrotaenia ljungiana” OR “Argyrotaenia velutinana” OR “Aridius nodifer” OR “Armillaria limonea” OR “Armillaria luteobubalina” OR “Armillaria mellea” OR “Armillaria novae‐zelandiae” OR “Armillaria sp.” OR “Armillaria tabescens” OR “Arrenoseius wainstein” OR “Ascochyta piricola” OR “Ascochyta pirina” OR “Ascochyta pyricola” OR “Aspergillus clavatus” OR “Aspergillus flavus” OR “Aspergillus niger” OR “Aspergillus ustus” OR “Aspergillus versicolor” OR “Asteromella mali” OR “Asymmetrasca decedens” OR “Asynonychus cervinus” OR “Athelia bombacina” OR “Athelia rolfsii” OR “Atractotomus mali” OR “Atrichatus aeneicollis” OR “Aulacorthum solani” OR “Aureobasidium pullulans” OR “Auriculariopsis ampla” OR “Automeris io” OR “Automeris zephyria” OR “Bacchisa fortunei” OR “Bacillus cereus” OR “Bacillus subtilis” OR “Bactrocera aquilonis” OR “Bactrocera dorsalis” OR “Bactrocera tryoni” OR “Bactrocera zonata” OR “Bdellodes sp.” OR “Bionectria ochroleuca” OR “Bispora antennata” OR “Bituberculate scale” OR “Bjerkandera adusta” OR “Blackberry chlorotic ringspot virus” OR “Blastobasis decolorella” OR “Blastobasis sp. nr. tarda” OR “Blattella germanica” OR “Boeremia exigua var. exigua” OR “Bohemannia pulverosella” OR “Bonagota cranaodes” OR “Bonagota salubricola” OR “Botryodiplodia malorum” OR “Botryodiplodia theobromae” OR “Botryosphaeria berengeriana” OR “Botryosphaeria berengeriana f. sp. pyricola” OR “Botryosphaeria dothidea” OR “Botryosphaeria kuwatsukai” OR “Botryosphaeria lutea” OR “Botryosphaeria obtusa” OR “Botryosphaeria parva” OR “Botryosphaeria quercuum” OR “Botryosphaeria ribis” OR “Botryosphaeria sinensis” OR “Botryosphaeria sp.” OR “Botryosphaeria stevensii” OR “Botryotinia fuckeliana” OR “Botrytis cinerea” OR “Botrytis mali” OR “Brachycaudus cardui” OR “Brachycaudus helichrysi” OR “Brahmina coriacea” OR “Brevipalpus noranae” OR “Brevipalpus obovatus” OR “Brevipalpus phoenicis” OR “Bryobia cristata” OR “Bryobia giannitsensis” OR “Bryobia graminum” OR “Bryobia macedonica” OR “Bryobia piliensis” OR “Bryobia praetiosa” OR “Bryobia rubrioculus” OR “Bryobia vasiljevi” OR “Burkholderia cepacia” OR “Byturus tomentosus” OR “Cacoecimorpha pronubana” OR “Cacopsylla costalis” OR “Cacopsylla mali” OR “Cacopsylla melanoneura” OR “Cacopsylla picta” OR “Cacopsylla pulchella” OR “Cacopsylla pulchra” OR “Cactodera chaubattia” OR “Caecilius flavus” OR “Caenorhabditis briggsae” OR “Caenorhabditis elegans” OR “Caenorhabditis remanei” OR “Calepitrimerus aphrastus” OR “Calepitrimerus baileyi” OR “Caliroa cerasi” OR “Callisto coffeella” OR “Calliteara horsfieldii” OR “Calocoris norvegicus” OR “Calonectria kyotensis” OR “Calosphaeria sp.” OR “Camarosporium karstenii” OR “Camarosporium multiforme” OR “Campylomma verbasci” OR “Candidatus Phytoplasma asteris” OR “Candidatus Phytoplasma aurantifolia” OR “Candidatus phytoplasma mali” OR “Candidatus Phytoplasma pruni” OR “Candidatus Phytoplasma solani” OR “Candidatus Phytoplasma mali” OR “Candidatus Phytoplasma pruni” OR “Candidatus Phytoplasma solani” OR “Candidatus Phytoplasma ziziphi” OR “Candidula intersecta” OR “Capnodium citri” OR “Capua semiferana” OR “Carabidae sp.” OR “Carcina quercana” OR “Carnation ringspot virus” OR “Carpophilus gaveni” OR “Carpophilus mutilatus” OR “Carposina sasakii” OR “Catoptes coronatus” OR “Cecidophyes malifoliae” OR “Cenopalpus irani” OR “Cenopalpus pulcher” OR “Cerambyx dux” OR “Ceratitis capitata” OR “Ceratitis quilicii” OR “Ceratitis rosa” OR “Ceratostomella mali” OR “Ceresa alta” OR “Ceroplastes ceriferus” OR “Ceroplastes sinensis” OR “Chaetocnema confinis” OR “Chaetomium sp.” OR “Chalastospora gossypii” OR “Cheiroseius samani” OR “Cherry leaf roll virus” OR “Cherry necrotic rusty mottle virus” OR “Cherry rasp leaf virus” OR “Chinavia hilaris” OR “Chloroclystis v‐ata” OR “Chondrostereum purpureum” OR “Choreutis pariana” OR “Choristoneura diversana” OR “Choristoneura hebenstreitella” OR “Choristoneura rosaceana” OR “Chrysobothris mali” OR “Chrysomphalus aonidum” OR “Chymomyza amoena” OR “Cicadatra persica” OR “Cicinobolus humuli” OR “Cilix glaucata” OR “Cirsium arvense” OR “Citrus concave gum‐associated virus” OR “Cladophialophora sp.” OR “Cladosporium cladosporioides” OR “Cladosporium fumago” OR “Cladosporium herbarum” OR “Cladosporium sp.” OR “Clarkeulia bourquini” OR “Clavibacter michiganensis” OR “Clepsis spectrana” OR “Clonostachys rosea” OR “Clover yellow mosaic virus” OR “Cnephasia asseclana” OR “Cnephasia stephensiana” OR “Cochlicopa lubrica” OR “Cochliobolus cynodontis” OR “Colaspis brunnea” OR “Coleophora prunifoliae” OR “Coleophora serratella” OR “Colletogloeum sp.” OR “Colletotrichum acerbum” OR “Colletotrichum acutatum” OR “Colletotrichum aenigma” OR “Colletotrichum alienum” OR “Colletotrichum clavatum” OR “Colletotrichum fioriniae” OR “Colletotrichum fragariae” OR “Colletotrichum fructicola” OR “Colletotrichum gloeosporioides” OR “Colletotrichum godetiae” OR “Colletotrichum kahawae” OR “Colletotrichum kahawae subsp. ciggaro” OR “Colletotrichum karsti” OR “Colletotrichum karstii” OR “Colletotrichum limetticola” OR “Colletotrichum melonis” OR “Colletotrichum noveboracense” OR “Colletotrichum nymphaeae” OR “Colletotrichum paranaense” OR “Colletotrichum rhombiforme” OR “Colletotrichum salicis” OR “Colletotrichum siamense” OR “Colletotrichum sp.” OR “Colletotrichum theobromicola” OR “Colletotrichum tropicale” OR “Colletotrichum gloeosporioides” OR “Collybia drucei” OR “Colocasia coryli” OR “Comstockaspis perniciosa” OR “Coniothecium chomatosporum” OR “Coniothyrium armeniacae” OR “Coniothyrium sp.” OR “Conistra rubiginosa” OR “Conogethes punctiferalis” OR “Conotrachelus nenuphar” OR “Conyza bonariensis” OR “Conyza canadensis” OR “Coprinus” OR “Coprinus atramentarius” OR “Cordana musae” OR “Coriolus velutinus” OR “Coriolus versicolor” OR “Coriolus zonatus” OR “Cornu aspersum” OR “Corticium centrifugum” OR “Corticium koleroga” OR “Corticium salmonicolor” OR “Corticium utriculicum” OR “Coryneum foliicola” OR “Corynoptera sp.” OR “Cosmia trapezina” OR “Cossus cossus” OR “Cossus insularis” OR “Costelytra zealandica” OR “Cotinis nitida” OR “Croesia holmiana” OR “Cryphonectria parasitica” OR “Cryptocoryneum condensatum” OR “Cryptosporiopsis curvispora” OR “Cryptosporiopsis malicorticis” OR “Cryptosporiopsis perennans” OR “Ctenopseustis obliquana” OR “Cucumber mosaic virus” OR “Cydia funebrana” OR “Cydia inopinata” OR “Cydia janthinana” OR “Cydia lobarzewskii” OR “Cydia molesta” OR “Cydia packardi” OR “Cydia pomonella” OR “Cydia prunivora” OR “Cydia pyrivora” OR “Cylindrocarpon candidum” OR “Cylindrocarpon destructans” OR “Cylindrocarpon didymum” OR “Cylindrocarpon heteronemum” OR “Cylindrocarpon liriodendri” OR “Cylindrocarpon macrodidymum” OR “Cylindrocarpon mali” OR “Cylindrocarpon obtusiusculum” OR “Cylindrocarpon pauciseptatum” OR “Cylindrocarpon sp.” OR “Cylindrocladium floridanum” OR “Cyphellophora sessilis” OR “Cytospora calvillae” OR “Cytospora carphosperma” OR “Cytospora chrysosperma” OR “Cytospora cincta” OR “Cytospora leucostoma” OR “Cytospora mali” OR “Cytospora melnikii” OR “Cytospora nivea” OR “Cytospora parasitica” OR “Cytospora rubescens” OR “Cytospora schulzeri” OR “Cytospora sp.” OR “Dactylonectria pauciseptata” OR “Daldinia concentrica” OR “Daldinia vernicosa” OR “Dasineura mali” OR “Deltinea bourquini” OR “Dematophora sp.” OR “Dendrothele tetracornis” OR “Dendryphiella vinosa” OR “Dermestes laniarius” OR “Devriesia pseudoamericana” OR “Diabrotica speciosa” OR “Diaphora mendica” OR “Diaporthe actinidiae” OR “Diaporthe ambigua” OR “Diaporthe cotoneastri” OR “Diaporthe dothidea” OR “Diaporthe eres” OR “Diaporthe foeniculina” OR “Diaporthe infecunda” OR “Diaporthe malorum” OR “Diaporthe oxe” OR “Diaporthe perniciosa” OR “Diaporthe serafiniae” OR “Diaporthe sp.” OR “Diaspidiotus ancylus” OR “Diaspidiotus perniciosus” OR “Diatrype sp.” OR “Dickeya dadantii” OR “Dictyosporium toruloides” OR “Diderma asteroides” OR “Didymella aliena” OR “Diloba caeruleocephala” OR “Diplocarpon mali” OR “Diplocarpon mespili” OR “Diplococcium asperum” OR “Diplodia bulgarica” OR “Diplodia intermedia” OR “Diplodia mutila” OR “Diplodia pseudoseriata” OR “Diplodia seriata” OR “Diplodia sp.” OR “Diptacus gigantorhynchus” OR “Diptacus sp.” OR “Discotylenchus” OR “Dissoconium aciculare” OR “Dissoconium eucalypti” OR “Dissoconium proteae” OR “Dissoconium sp.” OR “Diurnea fagella” OR “Dorysthenes huegelii” OR “Dothiorella sarmentorum” OR “Drosophila immigrans” OR “Drosophila lativittata” OR “Drosophila simulans” OR “Drosophila suzukii” OR “Dysaphis affinis” OR “Dysaphis anthrisci” OR “Dysaphis anthrisci majkopica” OR “Dysaphis armeniaca” OR “Dysaphis brachycyclica” OR “Dysaphis brancoi” OR “Dysaphis brancoi spp. malina” OR “Dysaphis brancoi spp. rogersoni” OR “Dysaphis brunii” OR “Dysaphis chaerophylli” OR “Dysaphis chaerophyllina” OR “Dysaphis devecta” OR “Dysaphis gallica” OR “Dysaphis malidauci” OR “Dysaphis meridialis” OR “Dysaphis mordvilkoi” OR “Dysaphis orientalis” OR “Dysaphis physocaulis” OR “Dysaphis plantaginea” OR “Dysaphis pyri” OR “Dysaphis radicola” OR “Dysaphis sibirica” OR “Dysaphis zini” OR “Dysaphys flava” OR “Dysmicoccus brevipes” OR “Eccopisa effractella” OR “Edwardsiana crataegi” OR “Edwardsiana lamellaris” OR “Edwardsiana rosae” OR “Elsinoe piri” OR “Elsinoe pyri” OR “Ematurga atomaria” OR “Emex australis” OR “Emex spinosa” OR “Empoasca decipiens” OR “Empoasca fabae” OR “Enarmonia formosana” OR “Eotetranychus ancora” OR “Eotetranychus carpini” OR “Eotetranychus clitus” OR “Eotetranychus frosti” OR “Eotetranychus pruni” OR “Eotetranychus prunicola” OR “Eotetranychus sexmaculatus” OR “Eotetranychus smithi” OR “Eotetranychus uncatus” OR “Eotetranychus willamettei” OR “Epiblema foenella” OR “Epicoccum nigrum” OR “Epicoccum sp.” OR “Epidiaspis leperii” OR “Epiphyas postvittana” OR “Epitrimerus pyri” OR “Epuraea imperialis” OR “Erannis defoliaria” OR “Eriococcus coccineus” OR “Eriogaster lanestris” OR “Eriophyes mali” OR “Eriophyes pyri” OR “Eriophyoidea sp.” OR “Eriosoma lanigerum” OR “Eriosoma lanuginosum” OR “Erwinia amylovora” OR “Erysiphe heraclei” OR “Erythricium salmonicolor” OR “Eucolaspis brunnea” OR “Eucolaspis sp.” OR “Eulecanium mali” OR “Eulecanium tiliae” OR “Eupalopsis vandergeesti” OR “Eupithecia insigniata” OR “Euproctis chrysorrhoea” OR “Eurhizococcus brasiliensis” OR “Eurytetranychus ulmi” OR “Eurytoma schreineri” OR “Eutetranychus africanus” OR “Eutetranychus orientalis” OR “Eutypa lata” OR “Euzophera bigella” OR “Euzophera pinguis” OR “Exophiala sp.” OR “Falagria sp.” OR “Fibulorhizoctonia psychrophila” OR “Fieberiella florii” OR “Flammulina velutipes” OR “Fomitopsis pinicola” OR “Forficula auricularia” OR “Fracchiaea sp.” OR “Frankliniella” OR “Frankliniella occidentalis” OR “Fusarium acuminatum” OR “Fusarium apiogenum” OR “Fusarium avenaceum” OR “Fusarium compactum” OR “Fusarium crookwellense” OR “Fusarium culmorum” OR “Fusarium equiseti” OR “Fusarium lateritium” OR “Fusarium oxysporum” OR “Fusarium proliferatum” OR “Fusarium pseudograminearum” OR “Fusarium semitectum” OR “Fusarium solani” OR “Fusarium stilboides” OR “Fusarium tricinctum” OR “Fusicladium dendriticum” OR “Fusicladium pomi” OR “Fusicladium pyrorum” OR “Fusicoccum luteum” OR “Fusicoccum parvum” OR “Galinsoga parviflora” OR “Galinsoga quadriradiata” OR “Ganoderma applanatum” OR “Geastrumia polystigmatis” OR “Gelechia rhombella” OR “Geniculosporium sp.” OR “Geosmithia sp.” OR “Geotrichum candidum” OR “Gibberella acuminata” OR “Gibberella avenacea” OR “Gibberella baccata” OR “Gibberella intricans” OR “Gibberella tricincta” OR “Globisporangium echinulatum” OR “Globisporangium heterothallicum” OR “Globisporangium irregulare” OR “Globisporangium paroecandrum” OR “Globisporangium rostratum” OR “Globisporangium ultimum” OR “Globodera pallida” OR “Globodera rostochiensis” OR “Gloeocystidiellum sacratum” OR “Gloeodes pomigena” OR “Gloeopeniophorella sacrata” OR “Gloeosporium album” OR “Gloeosporium fructigenum” OR “Gloeosporium perennans” OR “Gloeosporium sp.” OR “Glomerella cingulata” OR “Glomerella miyabeana” OR “Glomus constrictum” OR “Glomus deserticola” OR “Glomus etunicatum” OR “Glomus fasciculatum” OR “Glomus geosporum” OR “Glomus mosseae” OR “Glonium parvulum” OR “Gluconobacter oxydans” OR “Gonipterus scutellatus” OR “Gracilacus peperpotti” OR “Graphania mutans” OR “Graphiphora augur” OR “Grapholita dimorpha” OR “Grapholita funebrana” OR “Grapholita inopinata” OR “Grapholita molesta” OR “Grapholita packardi” OR “Grapholita prunivora” OR “Gryllotalpa gryllotalpa” OR “Gymnobathra parca” OR “Gymnosporangium clavipes” OR “Gymnosporangium confusum” OR “Gymnosporangium globosum” OR “Gymnosporangium juniperi” OR “Gymnosporangium juniperi‐virginiae” OR “Gymnosporangium juniperi‐virginianae” OR “Gymnosporangium tremelloides” OR “Gymnosporangium yamadae” OR “Gypsonoma minutana” OR “Hadrotrichum populi” OR “Halyomorpha halys” OR “Halyomorpha mista” OR “Haplothrips kurdjumovi” OR “Haplothrips niger” OR “Haptoncus luteolus” OR “Harmonia axyridis” OR “Harpalus calceatus” OR “Harpalus distinguendus” OR “Hedya dimidioalba” OR “Hedya nubiferana” OR “Helicobasidium mompa” OR “Helicotylenchus dihystera” OR “Helicoverpa armigera” OR “Heliothrips haemorrhoidalis” OR “Hemiberlesia cyanophylli” OR “Hemiberlesia lataniae” OR “Hemiberlesia rapax” OR “Hemicycliophora theinemanni” OR “Hendersonia lignicola” OR “Hendersonia mali” OR “Hendersonia piricola” OR “Hesperophanes sericeus” OR “Heteroporus biennis” OR “Heterorhabditis indica” OR “Hirneola auricula‐judae” OR “Holcocerus arenicolus” OR “Holotrichia longipennis” OR “Homeopronematus cf. staercki” OR “Homona coffearia” OR “Homona magnanima” OR “Hop stunt viroid” OR “Hop stut viroid” OR “Hoplocampa” OR “Hoplocampa minuta” OR “Hoplocampa testudinea” OR “Houjia sp.” OR “Houjia yanglingensis” OR “Hyalomyzus eriobotryae” OR “Hyalophora cecropia” OR “Hyalopterus pruni” OR “Hylastes ater” OR “Hymenobacter marinus” OR “Hymenobacter metalli” OR “Hymenobacter pomorum” OR “Hyphantria cunea” OR “Hyphodontia gossypina” OR “Hypholoma incertum” OR “Hypoaspis myrmophila” OR “Hypocrea sp.” OR “Hypoxylon serpens” OR “Hypsicera femoralis” OR “Icerya aegyptiaca” OR “Icerya purchasi” OR “Ilyonectria liriodendri” OR “Ilyonectria radicicola” OR “Janus compressus” OR “Lacanobia oleracea” OR “Lacanobia subjuncta” OR “Lachnella anomala” OR “Lambertella corni‐maris” OR “Lasiodiplodia brasiliense” OR “Lasiodiplodia brasiliensis” OR “Lasiodiplodia theobromae” OR “Lepidium draba” OR “Lepidosaphes ulmi” OR “Lepidosaphes ussuriensis” OR “Lepiota naucina” OR “Leptodontidium elatius” OR “Leptodontium elatius” OR “Leptosphaeria coniothyrium” OR “Leptothyrium pomi” OR “Leucoptera malifoliella” OR “Leucostoma cinctum” OR “Leucostoma personii” OR “Leucostoma persoonii” OR “Leucothyreus marginicollis” OR “Liberibacter europaeus” OR “Libertella blepharis” OR “Libertella sp.” OR “Limothrips cerealium” OR “Liothula omnivora” OR “Little cherry virus 2” OR “Longidorus caespiticola” OR “Longidorus danuvii” OR “Longidorus elongatus” OR “Longidorus euonymus” OR “Longidorus iranicus” OR “Longidorus leptocephalus” OR “Longidorus nanus” OR “Longidorus pisi” OR “Longidorus profundorum” OR “Longidorus rubi” OR “Longidorus sturhani” OR “Longistigma xizangensis” OR “Longitarsus fuliginosus” OR “Lonicera japonica” OR “Lophiostoma compressum” OR “Lophiostoma holmiorum” OR “Lophiostoma subcorticale” OR “Lophiostoma vicinum” OR “Lophium mytilinum” OR “Lopholeucaspis japonica” OR “Lorryia cristata” OR “Lorryia palpsetosa” OR “Lycorma delicatula” OR “Lygocoris communis” OR “Lygocoris pabulinus” OR “Lygus lineolaris” OR “Lymantria dispar” OR “Lymantria mathura” OR “Lymantria monacha” OR “Lymantria obfuscata” OR “Lyonetia clerkella” OR “Lyonetia prunifoliella” OR “Lyonetia prunifoliella malinella” OR “Lyonetia speculella” OR “Maconellicoccus hirsutus” OR “Macrodactylus subspinosus” OR “Macrolabis mali” OR “Macrophthalmothrips argus” OR “Macrosiphum chukotense” OR “Macrosiphum euphorbiae” OR “Macrosiphum rosae” OR “Macrosporium sp.” OR “Macrothylacia rubi” OR “Malacosoma americana” OR “Malacosoma americanum” OR “Malacosoma disstria” OR “Malacosoma indicum” OR “Malacosoma neustria” OR “Malacosoma parallela” OR “Mamestra brassicae” OR “Margarodes vitis” OR “Marssonina coronaria” OR “Marssonina sp.” OR “Medicago lupulina” OR “Megalometis chilensis” OR “Megaplatypus mutatus” OR “Megaselia sp.” OR “Melanopsamma pomiformis” OR “Meloidogyne arenaria” OR “Meloidogyne ethiopica” OR “Meloidogyne incognita” OR “Meloidogyne javanica” OR “Meloidogyne mali” OR “Meloidogyne nataliei” OR “Melolontha melolontha” OR “Merothrips brunneus” OR “Merulius sp.” OR “Metaseiulus muma” OR “Metaseiulus occidentalis” OR “Metcalfa pruinosa” OR “Meyernychus emeticae” OR “Micrambina rutila” OR “Microcerotermes diversus” OR “Microcyclospora malicola” OR “Microcyclospora pomicola” OR “Microcyclospora sp.” OR “Microcyclospora tardicrescens” OR “Microcyclosporella mali” OR “Microcyclosporella sp.” OR “Microdiplodia microsporella” OR “Micromus tasmaniae” OR “Microsphaeropsis ochracea” OR “Microthyriella rubi” OR “Monilia fructigena” OR “Monilia polystroma” OR “Monilia yunnanensis” OR “Monilinia fructicola” OR “Monilinia fructigena” OR “Monilinia laxa” OR “Monilinia laxa f.sp. mali” OR “Monilinia mali” OR “Monilinia mumeicola” OR “Monilinia polystroma” OR “Monilinia yunnanensis” OR “Mucor piriformis” OR “Mycosphaerella pomi” OR “Mycosphaerella punctiformis” OR “Mycosphaerella sentina” OR “Mycosphaerella tassiana” OR “Myzus ornatus” OR “Myzus persicae” OR “Nanidorus minor” OR “Nattrassia mangiferae” OR “Naupactus xanthographus” OR “Nearctaphis bakeri” OR “Nectria cinnabarina” OR “Nectria discophora” OR “Nectria ditissima” OR “Nectria galligena” OR “Nectria haematococca” OR “Nectria ochroleuca” OR “Nectria peziza” OR “Nectria pseudotrichia” OR “Nectria radicicola” OR “Nectria sp.” OR “Nectriaceae” OR “Nematoloma fasciculare” OR “Neodelphax fuscoterminata” OR “Neofabraea actinidiae” OR “Neofabraea alba” OR “Neofabraea brasiliensis” OR “Neofabraea kienholzii” OR “Neofabraea malicorticis” OR “Neofabraea perennans” OR “Neofabraea sp.” OR “Neofabraea vagabunda” OR “Neofusicoccum algeriense” OR “Neofusicoccum australe” OR “Neofusicoccum italicum” OR “Neofusicoccum luteum” OR “Neofusicoccum nonquaesitum” OR “Neofusicoccum parvum” OR “Neofusicoccum ribis” OR “Neonectria ditissima” OR “Neonectria galligena” OR “Neonectria macrodidyma” OR “Neonectria radicicola” OR “Nesothrips propinquus” OR “Nezara viridula” OR “Niesslia sp.” OR “Nigrospora sp.” OR “Nippolachnus piri” OR “Nitschkia parasitans” OR “Nyctemera annulata” OR “Nysius huttoni” OR “Ochroporus ossatus” OR “Oemona hirta” OR “Oidium farinosum” OR “Oligonychus biharensis” OR “Oligonychus litchii” OR “Oligonychus newcomeri” OR “Oligonychus sayedi” OR “Oligonychus yothersi” OR “Oncopodiella robusta” OR “Opatrum sabulosum” OR “Operophtera bruceata” OR “Operophtera brumata” OR “Ophiostoma quercus” OR “Ophiostoma roboris” OR “Opodiphthera eucalypti” OR “Opogona omoscopa” OR “Orchestes fagi” OR “Orgyia antiqua” OR “Orgyia leucostigma” OR “Orgyia recens” OR “Oribius destructor” OR “Oribius inimicus” OR “Orthosia cerasi” OR “Orthosia cruda” OR “Orthosia hibisci” OR “Orthosia incerta” OR “Orthosia stabilis” OR “Orthotydeus californicus” OR “Orthotylus marginalis” OR “Osmia cornifrons” OR “Osmoderma eremita” OR “Ostrinia nubilalis” OR “Otiorhynchus cribricollis” OR “Otiorhynchus meridionalis” OR “Otthia spiraeae” OR “Ovatus crataegarius” OR “Ovatus insitus” OR “Ovatus malisuctus” OR “Oxalis latifolia” OR “Oxalis pes‐caprae” OR “Pachyseius humeralis” OR “Pachysphinx modesta” OR “Paecilomyces niveus” OR “Paecilomyces sp.” OR “Palaeolecanium bituberculatum” OR “Pammene argyrana” OR “Pammene rhediella” OR “Panaeolus” OR “Pandemis cerasana” OR “Pandemis cinnamomeana” OR “Pandemis heparana” OR “Pandemis pyrusana” OR “Panonychus citri” OR “Panonychus inca” OR “Panonychus lishanensis” OR “Panonychus ulmi” OR “Pantoea agglomerans” OR “Pantomorus cervinus” OR “Pappia fissilis” OR “Paracoccus marginatus” OR “Paradevriesia pseudoamericana” OR “Paraphloeostiba gayndahensis” OR “Paratrichodorus allius” OR “Paratrichodorus porosus” OR “Paratrichodorus tunisiensis” OR “Paratylenchus” OR “Paratylenchus curvitatus” OR “Parlatoria crypta” OR “Parlatoria oleae” OR “Parlatoria pergandii” OR “Parlatoria pittospori” OR “Paropsis charybdis” OR “Parornix geminatella” OR “Parthenolecanium corni” OR “Parthenolecanium persicae” OR “Pasiphila rectangulata” OR “Paspalum urvillei” OR “Patellaria atrata” OR “Peach latent mosaic viroid” OR “Pear blister canker viroid” OR “Pellicularia koleroga” OR “Peltaster cerophilus” OR “Peltaster fructicola” OR “Peltaster gemmifer” OR “Peltaster sp.” OR “Peltosphaeria pustulans” OR “Penicillium aurantiogriseum” OR “Penicillium biourgeianum” OR “Penicillium brevicompactum” OR “Penicillium carneum” OR “Penicillium chrysogenum” OR “Penicillium commune” OR “Penicillium crustosum” OR “Penicillium digitatum” OR “Penicillium expansum” OR “Penicillium glabrum” OR “Penicillium glaucum” OR “Penicillium griseofulvum” OR “Penicillium novae‐zelandiae” OR “Penicillium paneum” OR “Penicillium polonicum” OR “Penicillium ramulosum” OR “Penicillium rugulosum” OR “Penicillium solitum” OR “Penicillium sp.” OR “Penicillium viridicatum” OR “Peniophora lycii” OR “Pennisetum clandestinum” OR “Pentatoma rufipes” OR “Perichaena corticalis” OR “Perichaena depressa” OR “Peridroma saucia” OR “Peritelus sphaeroides” OR “Pestalotia hartigii” OR “Pestalotia sp.” OR “Pestalotiopsis maculans” OR “Pestalotiopsis sp.” OR “Petiveria alliacea” OR “Petrobia harti” OR “Petrobia latens” OR “Petunia asteroid mosaic virus” OR “Pezicula alba” OR “Pezicula corticola” OR “Pezicula malicorticis” OR “Phacidiopycnis washingtonensis” OR “Phacidium lacerum” OR “Phaeoacremonium aleophilum” OR “Phaeoacremonium australiense” OR “Phaeoacremonium fraxinopennsylvanicum” OR “Phaeoacremonium geminum” OR “Phaeoacremonium inflatipes” OR “Phaeoacremonium iranianum” OR “Phaeoacremonium italicum” OR “Phaeoacremonium minimum” OR “Phaeoacremonium mortoniae” OR “Phaeoacremonium parasiticum” OR “Phaeoacremonium proliferatum” OR “Phaeoacremonium scolyti” OR “Phaeoacremonium subulatum” OR “Phanerochaete salmonicolor” OR “Phellinus alni” OR “Phellinus igniarius” OR “Phenacoccus aceris” OR “Phialophora sessilis” OR “Phigalia pilosaria” OR “Phlyctema vagabunda” OR “Phlyctinus callosus” OR “Pholiota aurivella” OR “Pholiota squarrosa” OR “Phoma cava” OR “Phoma enteroleuca” OR “Phoma exigua var. exigua” OR “Phoma glomerata” OR “Phoma herbarum” OR “Phoma macrostoma” OR “Phoma macrostoma var. macrostoma” OR “Phoma pirinia” OR “Phoma pomorum” OR “Phoma pomorum var. pomorum” OR “Phoma pyrina” OR “Phoma sp.” OR “Phomopsis” OR “Phomopsis cotoneastri” OR “Phomopsis mali” OR “Phomopsis oblonga” OR “Phomopsis perniciosa” OR “Phomopsis sp.” OR “Phorodon humuli” OR “Phyllachora pomigena” OR “Phyllactinia mali” OR “Phyllobius oblongus” OR “Phyllocoptes mali” OR “Phyllocoptes malinus” OR “Phyllonorycter blancardella” OR “Phyllonorycter corylifoliella” OR “Phyllonorycter crataegella” OR “Phyllonorycter cydoniella” OR “Phyllonorycter elmaella” OR “Phyllonorycter gerasimowi” OR “Phyllonorycter hostis” OR “Phyllonorycter mespilella” OR “Phyllonorycter oxyacanthae” OR “Phyllonorycter ringoniella” OR “Phyllosticta briardi” OR “Phyllosticta briardii” OR “Phyllosticta solitaria” OR “Phyllosticta sp.” OR “Phyllotreta nemorum” OR “Phyllotreta nigripes” OR “Phymatotrichopsis omnivora” OR “Physalospora malorum” OR “Physarum sp.” OR “Physocleora dimidiaria” OR “Phytomyza heringiana” OR “Phytophthora cactorum” OR “Phytophthora cambivora” OR “Phytophthora citricola” OR “Phytophthora cryptogea” OR “Phytophthora drechsleri” OR “Phytophthora fragariae” OR “Phytophthora gonapodyides” OR “Phytophthora megasperma” OR “Phytophthora megasperma var. megasperma” OR “Phytophthora nicotianae” OR “Phytophthora plurivora” OR “Phytophthora rosacearum” OR “Phytophthora sp.” OR “Phytophthora syringae” OR “Phytoplasma aurantifolia” OR “Phytoplasma mali” OR “Phytoplasma pruni” OR “Phytoplasma pyri” OR “Phytopythium vexans” OR “Phytoseiidae sp.” OR “Piezodorus guildinii” OR “Planococcus citri” OR “Planotortrix excessana” OR “Platynota flavedana” OR “Platynota idaeusalis” OR “Platynota stultana” OR “Pleochaeta mali” OR “Pleomassaria mali” OR “Pleospora allii” OR “Pleospora herbarum” OR “Pleospora mali” OR “Pleospora scrophulariae” OR “Pleospora sp.” OR “Pleospora tarda” OR “Plesiocoris rugicollis” OR “Pleurophoma cava” OR “Pleurotus sp.” OR “Plocamaphis gyirongensis” OR “Plum pox potyvirus” OR “Plutella xylostella” OR “Poa annua” OR “Podosphaera leucotricha” OR “Podosphaera leucotricha” OR “Podosphaera pannosa” OR “Poecilopachys australasia” OR “Polygonum aviculare” OR “Polyopeus pomi” OR “Polyphylla fullo” OR “Polyporus admirabilis” OR “Polyporus badius” OR “Polyporus ciliatus” OR “Polyporus leptocephalus” OR “Popillia japonica” OR “Poria ferruginosa” OR “Potebniamyces pyri” OR “Pratylenchus coffeae” OR “Pratylenchus curviatus” OR “Pratylenchus hippeastrum” OR “Pratylenchus laticaudata” OR “Pratylenchus loosi” OR “Pratylenchus neglectus” OR “Pratylenchus penetrans” OR “Pratylenchus scribneri” OR “Pratylenchus thornei” OR “Pratylenchus vulnus” OR “Prociphilus caryae ssp. fitchii” OR “Prociphilus kuwanai” OR “Prociphilus oriens” OR “Prociphilus pini” OR “Prociphilus sasakii” OR “Prodiplosis longifila” OR “Proeulia auraria” OR “Proeulia chrysopteris” OR “Prunus necrotic ringspot virus” OR “Psallus ambiguus” OR “Pseudaulacaspis pentagona” OR “Pseudexentera mali” OR “Pseudocamarosporium sp.” OR “Pseudocercospora mali” OR “Pseudocercospora sp.” OR “Pseudocercosporella sp.” OR “Pseudococcus calceolariae” OR “Pseudococcus comstocki” OR “Pseudococcus longispinus” OR “Pseudococcus maritimus” OR “Pseudococcus viburni” OR “Pseudocoremia suavis” OR “Pseudomonas cichorii” OR “Pseudomonas fluorescens” OR “Pseudomonas syringae” OR “Pseudomonas syringae pv. papulans” OR “Pseudomonas syringae pv. syringae” OR “Pseudomonas syringae pv. tomato” OR “Pseudomonas viridiflava” OR “Pseudoveronaea ellipsoidea” OR “Pseudoveronaea obclavata” OR “Pseudozyma fusiformata” OR “Psychoda surcoufi” OR “Psylla mali” OR “Psylla melanoneura” OR “Pterochloroides persicae” OR “Ptycholoma lecheanum” OR “Pycnoporus cinnabarinus” OR “Pyrenochaeta furfuracea” OR “Pyrolachnus pyri” OR “Pythium abappressorium” OR “Pythium arrhenomanes” OR “Pythium debaryanum” OR “Pythium echinulatum” OR “Pythium heterothallicum” OR “Pythium irregulare” OR “Pythium paroecandrum” OR “Pythium rostratum” OR “Pythium sp.” OR “Pythium sylvaticum” OR “Pythium ultimum” OR “Pythium vexans” OR “Quadraspidiotus ostreaeformis” OR “Quadraspidiotus perniciosus” OR “Quadraspidiotus pyri” OR “Ramichloridium apiculatum” OR “Ramichloridium luteum” OR “Ramichloridium sp.” OR “Ramularia eucalypti” OR “Ramularia mali” OR “Ramularia sp.” OR “Recurvaria nanella” OR “Recurvaria leucatella” OR “Recurvaria nanella” OR “Resseliella oculiperda” OR “Reticulitermes lucifugus” OR “Retithrips syriacus” OR “Rhagoletis pomonella” OR “Rhagoletis tabellaria” OR “Rhinocladiella” OR “Rhinotergum schestovici” OR “Rhizobium radiobacter” OR “Rhizobium rhizogenes” OR “Rhizoctonia” OR “Rhizoctonia solani” OR “Rhizopus sp.” OR “Rhizopus stolonifer” OR “Rhodocollybia purpurata” OR “Rhodosporidium babjevae” OR “Rhodotorula” OR “Rhopalosiphum insertum” OR “Rhopalosiphum oxyacanthae” OR “Rhopalosiphum padi” OR “Rhopobota naevana” OR “Rhopobota unipunctana” OR “Rhynchaenus pallicornis” OR “Rhynchites aequatus” OR “Rhynchites bacchus” OR “Ribautiana tenerrima” OR “Ricania speculum” OR “Richardia brasiliensis” OR “Rosellinia necatrix” OR “Rosellinia radiciperda” OR “Rosellinia sp.” OR “Rotylenchus quartus” OR “Rubus ellipticus” OR “Saperda candida” OR “Sarcodontia crocea” OR “Sarocladium liquanensis” OR “Sarocladium mali” OR “Saturnia pavonia” OR “Saturnia pyri” OR “Scelodonta strigicolis” OR “Schizoneurella indica” OR “Schizophyllum alneum” OR “Schizophyllum commune” OR “Schizotetranychus smirnovi” OR “Schizothyrium pomi” OR “Scleroramularia abundans” OR “Sclerotinia fruticola” OR “Sclerotinia sclerotiorum” OR “Sclerotium delphinii” OR “Sclerotium rolfsii” OR “Sclerotium rolfsii var. delphinii” OR “Scolypopa australis” OR “Scolytus amygdali” OR “Scolytus mali” OR “Scolytus nitidus” OR “Scolytus rugulosus” OR “Scutellospora pellucida” OR “Seimatosporium fusisporum” OR “Seimatosporium lichenicola” OR “Selenosporella” OR “Senecio vulgaris” OR “Septocylindrium aderholdii” OR “Septocylindrium radicola” OR “Septoria sp.” OR “Sigmothrips aotearoana” OR “Siphanta acuta” OR “Sitobion avenae” OR “Solanum carolinense” OR “Somena scintillans” OR “Spencermartinsia plurivora” OR “Sperchia intractana” OR “Sphaeria microtheca” OR “Sphaeropsis mali” OR “Sphaeropsis malorum” OR “Sphaeropsis pyriputrescens” OR “Sphaeropsis sapinea” OR “Sphaerotheca pannosa” OR “Sphinx perelegans” OR “Spilocaea pomi” OR “Spilonota ocellana” OR “Spodoptera eridania” OR “Spodoptera frugiperda” OR “Spodoptera littoralis” OR “Spodoptera litura” OR “Sporidesmajora pennsylvaniensis” OR “Sporidesmium asperum” OR “Sporidesmium sp.” OR “Sporobolomyces roseus” OR “Sporormiella sp.” OR “Stellaria media” OR “Stemphylium botryosum” OR “Stemphylium ilicis” OR “Stemphylium vesicarium” OR “Stenostola ferrea” OR “Stenotrophomonas maltophilia” OR “Stereum hirsutum” OR “Stethorus bifidus” OR “Stigmella magdalenae” OR “Stigmella malella” OR “Stigmella sorbi” OR “Stigmina carpophila” OR “Stomiopeltis sp.” OR “Strelitziana mali” OR “Strickeria kochii” OR “Strickeria obducens” OR “Swammerdamia pyrella” OR “Synanthedon hector” OR “Synanthedon myopaeformis” OR “Synanthedon scitula” OR “Syndemis musculana” OR “Tachypterellus quadrigibbus” OR “Tapinoma nigerrimum” OR “Tarsonemus nodosus” OR “Tatianaerhynchites aequatus” OR “Tebenna micalis” OR “Technomyrmex albipes” OR “Teichospora cruentula” OR “Teichospora seminuda” OR “Teleiodes vulgella” OR “Temperate fruit decay associated virus” OR “Tetranychus arabicus” OR “Tetranychus canadensis” OR “Tetranychus cinnabarinus” OR “Tetranychus desertorum” OR “Tetranychus frater” OR “Tetranychus kanzawai” OR “Tetranychus lambi” OR “Tetranychus ludeni” OR “Tetranychus mcdanieli” OR “Tetranychus mexicanus” OR “Tetranychus neocaledonicus” OR “Tetranychus pacificus” OR “Tetranychus schoenei” OR “Tetranychus turkestani” OR “Tetranychus urticae” OR “Tetranychus viennensis” OR “Thelonectria lucida” OR “Theocolax formiciformis” OR “Thielavia sp.” OR “Thrips australis” OR “Thrips hawaiiensis” OR “Thrips imaginis” OR “Thrips italicus” OR “Thrips obscuratus” OR “Thrips tabaci” OR “Tilletiopsis pallescens” OR “Tiracola grandirena” OR “Tischeria malifoliella” OR “Tobacco bushy stunt virus” OR “Tobacco mosaic virus” OR “Tobacco necrosis virus” OR “Tobacco ringspot virus” OR “Tomato bushy stunt virus” OR “Tomato ringspot virus” OR “Torula herbarum” OR “Torymus druparum” OR “Toxoptera aurantii” OR “Trametes hispida” OR “Trametes pubescens” OR “Trametes sp.” OR “Trametes versicolor” OR “Trametes zonata” OR “Trematosphaeria communis” OR “Trichia botrytis” OR “Trichoderma” OR “Trichoderma harzianum” OR “Trichoderma sp.” OR “Trichodorus” OR “Trichodorus cedarus” OR “Trichodorus nanjingensis” OR “Trichodorus persicus” OR “Trichodorus similis” OR “Trichodorus viruliferus” OR “Trichoferus campestris” OR “Trichoseptoria fructigena” OR “Trichothecium roseum” OR “Trioza urticae” OR “Tripospermum acerinum” OR “Tripospermum camelopardus” OR “Tripospermum myrti” OR “Tropinota hirta” OR “Tropinota squalida” OR “Truncatella angustata” OR “Tryblidiella rufula” OR “Trypodendron signatum” OR “Tubercularia vulgaris” OR “Tulare apple mosaic virus” OR “Tumularia” OR “Turanoclytus namanganensis” OR “Tydeus ancorarius” OR “Tydeus dorothyae” OR “Tydeus magnanus” OR “Tydeus plumosus” OR “Tydeus shabestariensis” OR “Tydeus unguis” OR “Tylenchorhynchus mashhood” OR “Typhlocyba pomaria” OR “Typhlodromus khosrovensis” OR “Typhlodromus pyri” OR “Typhlodromus vulgaris” OR “Tyrophagus curvipenis” OR “Urophorus humeralis” OR “Uwebraunia commune” OR “Uwebraunia dekkeri” OR “Valsa ambiens” OR “Valsa amphibola” OR “Valsa ceratosperma” OR “Valsa cincta” OR “Valsa leucostoma” OR “Valsa mali” OR “Valsa mali var. mali” OR “Valsa mali var. pyri” OR “Valsa malicola” OR “Valsa nivea” OR “Valsa persoonii” OR “Valsaria insitiva” OR “Valsella melastoma” OR “Venturia asperata” OR “Venturia inaequalis” OR “Venturia pyrina” OR “Verticillium albo‐atrum” OR “Verticillium dahliae” OR “Watabura nishiyae” OR “Xenotemna pallorana” OR “Xestia c‐nigrum” OR “Xiphinema americanum” OR “Xiphinema belmontense” OR “Xiphinema bricolense” OR “Xiphinema browni” OR “Xiphinema californicum” OR “Xiphinema diversicaudatum” OR “Xiphinema index” OR “Xiphinema mali” OR “Xiphinema meridianum” OR “Xiphinema mluci” OR “Xiphinema paramonovi” OR “Xiphinema parvistilus” OR “Xiphinema radicicola” OR “Xiphinema rivesi” OR “Xiphinema vuittenezi” OR “Xylaria sp.” OR “Xyleborinus saxesenii” OR “Xyleborus dispar” OR “Xylinophorus strigifrons” OR “Xylosandrus crassiusculus” OR “Xylosandrus germanus” OR “Xylotoles laetus” OR “Xylotrechus namanganensis” OR “Yponomeuta malinella” OR “Yponomeuta malinellus” OR “Zasmidium angulare” OR “Zetiasplozna thuemenii” OR “Zeugodacus cucurbitae” OR “Zeuzera coffeae” OR “Zeuzera pyrina” OR “Zygina zealandica” OR “Zygophiala cryptogama” OR “Zygophiala cylindrica” OR “Zygophiala emperorae” OR “Zygophiala qianensis” OR “Zygophiala sp.” OR “Zygophiala tardicrescens” OR “Zygophiala jamaicensis” OR “Zygophiala wisconsinensis”) |
Appendix C – List of pests that can potentially cause an effect not further assessed
Table C.1 List of potential pests not further assessed
| Pest name | EPPO Code | Group | Pest present in Turkey | Present in the EU |
Malus domestica confirmed as a host (reference) |
Pest can be associated with the commodity | Impact | Justification for inclusion in this list | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Phytophthora rosacearum | – | Fungi | Yes | Uncertain | Yes | Yes | Yes | Insuficient information Uncertain distribution |
Appendix D – Excel file with the pest list of Malus domestica
Appendix D can be found in the online version of this output (in the ‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7301
Supporting information
Excel file with the pest list of Malus domestica
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Baptista P, Chatzivassiliou E, Gonthier P, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Civera AV, Zappalà L, Lucchi A, Gómez P, Urek G, Bernardo U, Bubici G, Carluccio AV, Chiumenti M, Di Serio F, Fanelli E, Gardi C, Marzachì C, Mosbach‐Schulz O and Yuen J, 2022. Scientific Opinion on the commodity risk assessment of Malus domestica plants from Turkey. EFSA Journal 2022;20(5):7301, 142 pp. 10.2903/j.efsa.2022.7301
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00790
Panel members: Claude Bragard, Paula Baptista, Elisavet Chatzivassiliou, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Emilio Stefani, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen and Lucia Zappalà.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: EFSA wishes to acknowledge the contribution of Oresteia Sfyra and Patricia Nascimento to this opinion.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 2 and 3: Provided by NPPO of Turkey
Adopted: 31 March 2022
Notes
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants, amending Regulations (EU) 228/2013, (EU) 652/2014 and (EU) 1143/2014 of the European Parliament and of the Council and repealing Council Directives 69/464/EEC, 74/647/EEC, 93/85/EEC, 98/57/EC, 2000/29/EC, 2006/91/EC and 2007/33/EC. OJ L 317, 23.11.2016, pp. 4–104.
Commission Implementing Regulation (EU) 2018/2019 of 18 December 2018 establishing a provisional list of high risk plants, plant products or other objects, within the meaning of Article 42 of Regulation (EU) 2016/2031 and a list of plants for which phytosanitary certificates are not required for introduction into the Union, within the meaning of Article 73 of that Regulation C/2018/8877. OJ L 323, 19.12.2018, pp. 10–15.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, pp. 1–24.
In accordance with the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community, and in particular Article 5(4) of the Protocol on Ireland/Northern Ireland in conjunction with Annex 2 to that Protocol, for the purposes of this Annex, references to Member States include the United Kingdom in respect of Northern Ireland.
Commission Implementing Regulation (EU) 2019/2072 of 28 November 2019 establishing uniform conditions for the implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as regards protective measures against pests of plants, and repealing Commission Regulation (EC) No 690/2008 and amending Commission Implementing Regulation (EU) 2018/2019, OJ L 319, 10.12.2019, p. 1–279.
References
- Basso MF, da Silva JCF, Fajardo TVM, Fontes EPB and Zerbini FM, 2015. A novel, highly divergent ssDNA virus identified in Brazil infecting apple, pear and grapevine. Virus Research, 210, 27–33. [DOI] [PubMed] [Google Scholar]
- CABI (Centre for Agriculture and Bioscience International) , online. CABI. Crop Protection Compendium. Available online: https://www.cabi.org/cpc/
- EFSA PLH Panel (EFSA Panel on Plant Health) , 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , 2019a. Guidance on commodity risk assessment for the evaluation of high risk plants dossiers. EFSA Journal 2019;17(4):5668, 20 pp. 10.2903/j.efsa.2019.5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , 2019b. Commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan. EFSA Journal 2019;17(5):5667, 184 pp. 10.2903/j.efsa.2019.5668 [DOI] [PMC free article] [PubMed]
- EFSA Scientific Committee , 2018. Scientific Opinion on the principles and methods behind EFSA’s Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) , online. EPPO Global Database. Available online: https://www.eppo.int/ [Accessed: August 2021].
- EUROPHYT , online. European Union Notification System for Plant Health Interceptions – EUROPHYT. Available online: https://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 13 January 2022].
- FAO (Food and Agriculture Organization of the United Nations) , 1995. ISPM (International standardsforphytosanitary measures) No 4. Requirements for the establishment of pest free areas. Available online: https://www.ippc.int/en/publications/614/
- FAO (Food and Agriculture Organization of the United Nations) , 2016. ISPM (International standards for phytosanitary measures) No. 36. Integrated measures for plants for planting. FAO, Rome, 22 pp. Available online: https://www.ippc.int/en/publications/636/
- FAO (Food and Agriculture Organization of the United Nations) , 2017. ISPM (International standardsforphytosanitary measures) No. 5. Glossary of phytosanitary terms. FAO, Rome, 22 pp. Available online: https://www.ippc.int/en/publications/622
- FAO (Food and Agriculture Organization of the United Nations) , 2019. ISPM (International standards for phytosanitary measures) No. 36, Integrated measures for plants for planting. FAO, Rome. Available online: https://www.fao.org/3/k8114e/K8114E.pdf
- Follett PA, Jamieson L, Hamilton L and Wall M, 2019. New associations and host status: Infestability of kiwifruit by the fruit fly species Bactrocera dorsalis, Zeugodacus cucurbitae, and Ceratitis capitata (Diptera: Tephritidae). Crop Protection, 115, 113–121. [Google Scholar]
- Kottek M, Grieser J, Beck C, Rudolf B and Rubel F, 2006. World map of Köppen‐Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. [Google Scholar]
- TRACES‐NT , online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 13 January 2022].
- Xu YM and Zhao ZQ, 2019. Longidoridae and Trichodoridae (Nematoda: Dorylaimida and Triplonchida). Fauna of New Zealand, 79. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Excel file with the pest list of Malus domestica


