Abstract
Early life adversity (ELA) has been linked with increased arousal responses to threat, including increased amygdala reactivity. Effects of ELA on brain function are well recognized, and emerging evidence suggests that caregivers may influence how environmental stressors impact children’s brain function. We investigated the hypothesis that positive interaction between mother and child can buffer against ELA effects on children’s neural responses to threat, and related symptoms. N = 53 mother–child pairs (children ages 8–14 years) were recruited from an urban population at high risk for violence exposure. Maternal caregiving was measured using the Parenting Questionnaire and in a cooperation challenge task. Children viewed fearful and neutral face stimuli during functional magnetic resonance imaging. Children who experienced greater violence at home showed amygdala sensitization, whereas children experiencing more school and community violence showed amygdala habituation. Sensitization was in turn linked with externalizing symptoms. However, maternal warmth was associated with a normalization of amygdala sensitization in children, and fewer externalizing behaviors prospectively up to 1 year later. Findings suggested that the effects of violence exposure on threat-related neural circuitry depend on trauma context (inside or outside the home) and that primary caregivers can increase resilience.
Keywords: amygdala, habituation, maternal buffering, resilience, violence exposure
Introduction
Childhood trauma and other forms of early life adversity (ELA) are known to impact brain structure and function, particularly within networks that regulate fear and mobilize responses to threat (Hein & Monk, 2017; Herringa, 2017; Stevens, van Rooij, & Jovanovic, 2018). However, such changes cannot be considered uniformly negative or maladaptive. Impacts of ELA on brain function often appear to be initially adaptive in the specific environmental and developmental context of the child, with increased risk for psychopathology and other negative consequences appearing years later in adolescence and adulthood (Goff et al., 2013; McCrory, Gerin, & Viding, 2017; Raineki et al., 2012; Sumner Colich et al., 2019). Recent scientific and public health efforts focus on identifying factors that may protect against the negative consequences of ELA. Emerging findings suggest that social relationships, especially between the child and mother, can tightly regulate the function of fear neurocircuitry during early development. For example, among rodents (Moriceau & Sullivan, 2006), non-human primates (Sanchez, McCormack, & Howell, 2015), and humans (Tottenham et al., 2019), the simple presence of the mother during fear learning causes the child to approach rather than avoid cues that indicate threat. This is mediated by glucocorticoid-induced changes in the function of the amygdala (Moriceau et al., 2004), a brain region that regulates threat responses and the formation of conditioned fear memory (LeDoux, 2000; McGaugh, 2002). The tight dependency between threat learning and social interaction during early development suggests that mothers may play a key role in determining the early “wiring” of their children’s fear neurocircuitry, providing potential protection from the effects of ELA. Indeed, maternal presence can reduce children’s glucocorticoid release during stress exposure, a phenomenon referred to as social buffering (Gunnar et al., 2015). Here, we tested the possibility that mothers may buffer against the effects of ELA on threat neurocircuitry, focusing on the amygdala, in school-aged children at risk for violence exposure. Exposure to interpersonal violence is a common form of ELA among children living in U.S. cities, with up to 70% of children reporting witnessing a shooting (Buka et al., 2001; Cross et al., 2018) and 59% of all violent incidents estimated to involve a child (Harpaz-Rotem et al., 2007).
ELA has been linked with broad upregulation of fear and salience detection neurocircuitry responses to threat. For example, children exposed to ELA show larger amygdala volume (Tottenham et al., 2010), greater amygdala reactivity to threat (Tottenham et al., 2011; White et al., 2019), and early emergence of a mature pattern of connectivity between the amygdala and regulatory prefrontal regions (Gee, Gabard-Durnam et al., 2013). Hyper-reactivity persists into adulthood, with ELA-exposed adults also showing heightened amygdala responses to threat (Dannlowski et al., 2013; Dannlowski et al., 2012; Fonzo et al., 2016; van Harmelen et al., 2012). Notably, effects on brain function can vary depending on the type of stress exposure. A recent influential theory proposes that experiences of deprivation such as poverty, neglect, or institutional rearing have different impacts on the brain than experiences of threat, such as abuse or violence exposure (McLaughlin, Sheridan, & Lambert, 2014). Extending existing models that characterize stress as a force that exerts allostatic load on the brain and body (e.g., McEwen, 2000), such theories posit specific neurodevelopmental adaptations to environmental demands. The framework has since received robust empirical support, showing that deprivation, through reduced sensory input and cognitive enrichment, particularly impacts prefrontal and cortical associative areas (Machlin et al., 2019; Puetz et al., 2019; Sheridan, Peverill, Finn, & McLaughlin, 2017). In contrast, threat, through increased fear and chronic physiological arousal, particularly impacts the amygdala and hippocampus (Herringa et al., 2013; Keding & Herringa, 2015; Machlin et al., 2019; McLaughlin et al., 2016; Puetz et al., 2019; White et al., 2019). In particular, amygdala reactivity to social threat cues may appear to be upregulated when violence-exposed children show less habituation of the amygdala (a natural decrease with repeated presentations of similar stimuli) over repeated presentations of threat cues (Hein et al., 2020). While this lack of amygdala habituation has been linked with internalizing symptoms, there is a contrasting pattern for the emergence of posttraumatic stress disorder (PTSD) symptoms after childhood trauma, which have been associated with faster amygdala habituation to social threat cues (van den Bulk et al., 2016). These contrasting findings may indicate different neurocognitive contributors to internalizing versus PTSD, or there may be differences in children’s neural adaptation to different types of threat-related experiences.
Given evidence that neural adaptations to stress vary with environmental demands, it is plausible that even within the dimension of threat, exposures in different environmental contexts may differentially influence brain function. Studies distinguishing community from family-related violence in primary school-aged children have shown independent effects on mood and anxiety symptoms (Kliewer et al., 1998) and externalizing behaviors (Malik, 2008). In contrast, parent-reported internalizing behaviors and child-reported depression symptoms have been linked with community but not domestic violence exposure (Malik, 2008). Violence exposure in the home may exert different demands than violence exposure in external contexts, along a variety of dimensions such as chronicity, escapability, and the nature of affected social relationships (e.g., caregivers vs. peers vs. strangers). However, little research has been conducted to disentangle the mechanisms by which different forms of violence exposure may influence children’s risk for psychopathology. Here, we investigated the differential effects of violence in home versus community contexts on children’s neural responses to threat cues.
Several recent studies indicate that the presence of or relationship with caregivers can alter neural responses to threat in young children. In healthy children, pictures of mothers’ versus other faces elicit lower amygdala reactivity and more adult-like connectivity with the prefrontal cortex (Gee et al., 2014). Parenting traits have similar modulating effects on children’s neural responses to threat. In children with greater attachment security and lower separation anxiety, maternal cues suppress amygdala reactivity more effectively (Gee et al., 2014). Similarly, healthy adolescents reporting more warmth and support from their mothers show dampened amygdala reactivity to threat cues (Romund et al., 2016). In fact, a longitudinal study found that higher parental warmth predicted less anxiety and depression in children, even beyond other parenting variables (Santesteban-Echarri et al., 2017). Such protective effects may be powerful enough to buffer against abnormal neural threat responses in children exposed to ELA. In children who experienced early institutional care (Callaghan et al., 2019) and those with significant maltreatment history (Wymbs et al., 2020), the amygdala reactivity appeared to be buffered by higher feelings of security with current caregiver, and greater social support more broadly. In our prior research with urban families exposed to high levels of community violence, children’s ability to appropriately inhibit fear-potentiated startle to safety cues was enhanced when mothers were just outside the testing room, relative to peers whose mothers were less accessible (down the hall completing a set of questionnaires) (van Rooij et al., 2017). In the current study, we sought to extend these findings, using neuroimaging among children to better understand aspects of neural function that may be influenced by violence exposure, and to test the extent to which positive parenting behaviors can buffer against such effects. We posited that maternal buffering effects may be particularly relevant in the context of violence experienced in the caregiving environment (at home) relative to violence experienced in the community. We focused on maternal warmth as a particularly important component of maternal buffering effects, given the strong literature on warmth as a protective factor above and beyond other aspects of maternal care (Chen et al., 2011; Luecken et al., 2016; McCabe & Clark, 1999; Santesteban-Echarri et al., 2017).
We recruited mother–child dyads from an ongoing study of trauma and related psychopathology in an urban medical center serving primarily low-income Black American patients. Children were 8–14 years old, a developmental period when the impacts of trauma and violence exposure on brain function have been shown to be highest (Pechtel et al., 2014). Children participated in a functional magnetic resonance imaging (fMRI) task probing neural responses to fearful and neutral face stimuli and reported on their exposure to interpersonal violence across three contexts: in the community, at school, and at home. Warm parenting behaviors in the mother were measured using a maternal self-report scale focusing on parenting behaviors (McCabe & Clark, 1999), and validated in independent analyses of a cooperation challenge task that required the mother and child to interact to complete a challenging puzzle (Ginsburg, Grover, Cord, & Ialongo, 2006). We hypothesized that (a) violence exposure in the home would have different effects on amygdala function than violence in external contexts and (b) children whose mothers employed warm parenting behaviors would have reduced violence-related effects on amygdala function, indicative of a protective effect.
Materials and methods
Participants
Sixty-five children (33 girls, 32 boys) and their mothers were recruited from an ongoing study of mother–child pairs recruited from the general medical clinics of a large publicly funded hospital serving a patient population that is primarily low-income, and at high risk for trauma exposure and related psychiatric disorders (Gillespie et al., 2009). One child fell asleep during the task, two found the scanner noise too loud and did not complete the task, and nine had head motion exceeding quality control thresholds [described further in magnetic resonance imaging (MRI) acquisition and analysis] and their data were excluded from analyses. N = 53 [27 girls, 26 boys, age M (SD) = 10.9 (1.7), range = 8.2–14.8] were included in the final analyses.
Children and mothers were excluded if they reported a history of bipolar disorder or schizophrenia, active psychotic symptoms, or cognitive disability. For the purposes of the study, “mothers” were either biological mothers with full-time care of the child (N = 50), or grandmothers who were verified as legal guardian with full-time care (N = 3). For the neuroimaging component of the study, additional exclusion criteria were a history of head injury with loss of consciousness, stroke, epilepsy or other neurological disorder, brain tumor, autism spectrum disorder, contraindication for MRI, or hearing or vision impairment unable to be corrected by glasses. Testing took place at Grady Memorial Hospital, and the Facility for Education and Research in Neuroscience at Emory University. All study procedures were approved by the Institutional Review Boards of Emory University and the Grady Research Oversight Committee. Children younger than 11 provided oral assent to participate in the study and the mother or legal guardian provided written consent. Those older than 11 provided written consent. Children received a study t-shirt, a small toy, and a $10 gift certificate, and mothers received $50 for each study visit.
Trauma and symptom assessment
Children reported their exposure to potentially traumatic events and current PTSD symptoms in a semistructured interview format. Mothers also reported on children’s current PTSD symptoms, and problem behaviors, as well as their own parenting behaviors. Violence exposure was assessed using the Violence Exposure Scale for Children-Revised (VEX-R; Cross et al., 2018; Fox & Leavitt, 1995), a 12-item scale measuring children’s exposure to different types of violence at home, at school, and in the community, which includes line drawings to facilitate children’s understanding of the questions and a frequency rating (0: never, 1: one time, 2: a few times, 3: many times). Witnessing and experiencing each type of violence were included as separate event types, such that 24 items related to violence exposure were queried. Frequency ratings from each item were summed to assess total exposure frequency/chronicity. PTSD symptoms were assessed using the UCLA PTSD Reaction Index (UCLA-RI; Steinberg et al., 2004). The BASC-PRS was administered to mothers, to index children’s observable behaviors related to internalizing and externalizing problems (Reynolds & Kamphaus, 2004). Internalizing problems were measured using age- and sex-normed t-scores for anxiety, depression, and somatization, and externalizing problems were measured using t-scores for hyperactivity, aggression, and conduct problems. Maternal warmth was assessed using the Parenting Questionnaire (PQ; McCabe & Clark, 1999). The PQ is a 50-item parent self-report of parenting behaviors, including warmth. The 22-item warmth subscale has demonstrated good internal consistency (.90) in a sample of African American participants (McCabe & Clark, 1999) and in prior research with this cohort (.83; Cross et al., 2016; van Rooij et al., 2017). Pubertal development was queried using the Pubertal Development Scale (PDS; Petersen et al., 1988), an interview-based scale querying major physical changes during puberty, based on child’s report. A mean of the 5 items relevant to each gender was calculated as a summary index of pubertal development.
Procedure
Children completed a mock scan protocol 24 h to 2 weeks prior to MRI scanning. The mock scanner included the shell of the bore from a decommissioned Siemens Trio scanner (decorated as a space ship) and models of the head coil and bed. Participants were acclimated to the scanning environment, and practiced being inserted into the scanner bore, listening to recordings of scanner noise, and holding still. Participants then completed practice versions of the study tasks inside and outside of the mock scanner. They also viewed the real scanner at this time. On the day of the MRI scan, participants completed short practice versions of the study tasks to familiarize themselves with the button box and task procedures. Children completed a single-item mood rating before and after the MRI scan (range = −2 (very bad feelings)–2 (very good feelings)), and mean mood was not adversely impacted during the scanning session (Mpre = 1.4, Mpost = 1.5). Mothers observed the scan in a waiting room with a window allowing them to view their child in the MRI scanner.
The emotional faces task was a modified version of the task used by Gee, Tottenham and colleagues (2013). The event-related design included 24 fearful and 24 neutral female faces selected from the Karolinska Directed Emotional Faces database (Lundqvist et al., 1998), presented in a pseudo-random order. Each trial consisted of a 500 ms presentation of a face stimulus, followed by a variable intertrial interval (3,500–9,000 ms) during which a fixation cross was shown. To avoid biasing or decreasing participants’ emotional responses to the stimuli, the task only involved passive viewing of the stimuli.
During the laboratory assessment outside the scanner, mother–child dyads completed a cooperation challenge task which has been previously validated in African American mothers and children (Ginsburg et al., 2006; Ginsburg, Grover, & Ialongo, 2004). Each pair was provided with an Etch-a-sketch toy, and a set of three drawings to attempt to copy using the Etch-a-sketch. The mother was asked to control the right dial of the Etch-a-sketch, while the child controlled the left dial. The pairs worked together to create drawings of increasing complexity. The experimenter exited the room while the pairs worked on each drawing, returning to provide instructions for the next drawing. The task was videotaped, and later coded by two raters following the Johns Hopkins Coding Manual for Parent–Child Interactions (Ginsburg & Grover, 2014). Raters coded mothers’ behavior using a dimensional rating of behavior on a 0–4 scale (1 = never, 1 = very rarely, 2 = a little, 3 = some of the time, 4 = most of the time). The rater assigned a 0–4 rating to each minute of the video recording, during segments when the pairs were completing the third (most complex) drawing. The mean rating from the two independent raters was used in analyses. For the current study, ratings of mothers’ warmth behaviors were considered, although a variety of additional behavior scales were included in the coding protocol (including overcontrol, hostility, unresponsiveness, anxious behavior, self-criticism and others). For the warmth rating, raters considered mothers’ expressions of positive emotions toward the child, including praise, encouragement, compliments, words and gestures of endearment, and affectionate gestures.
MRI acquisition and analysis
Data acquisition
Functional and structural MRI scans were acquired on two 3.0-T Siemens Trio scanners, using a 32-channel head coil. Three children were scanned at the first facility, and the remainder of scanning moved to a second facility when the first scanner upgraded to a new system. A T1-weighted image using the Siemens tfl 3D MP-RAGE sequence (176 slices, TR = 2250 ms, TE = 4.18 ms, voxel size 1 mm3) was used for within-subject registration. Echo planar imaging (EPI) images were composed of 44 slices of 2.5 mm thickness acquired in a descending sequential slice order parallel to the anterior–posterior commissure line, with a .5 mm slice gap. Images were collected with TR = 2330 ms, TE = 30 ms, FA = 90°, 3 × 3 × 2.5 mm voxel size, and GRAPPA with an acceleration factor of 2.
MRI preprocessing and quality assurance
File conversion, image pre-processing, and statistical analyses were conducted in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Functional images were then corrected for slice timing and spatially realigned to the first image in the session. Additional motion correction was performed in the ArtRepair toolbox (https://www.nitrc.org/projects/art_repair/). Any volume with frame-wise displacement exceeding 2 mm was interpolated. N = 9 participants were fully excluded from the analyses as they had motion requiring >10% of volumes or >3 consecutive volumes to be interpolated. Remaining participants had an average of 3.1% of volumes replaced. The T1 was then co-registered to the mean of the realigned functional images and spatially normalized to Montreal Neurological Institute (MNI) space. The normalization parameters were then applied to the functional volumes and the images were smoothed with a 6 mm FWHM Gaussian kernel.
Fearful and neutral face stimuli were modeled using an event-related design. A first-level, fixed-effects analysis was used to estimate blood-oxygen-level dependent (BOLD) signal change to task stimuli. Onset times for fearful and neutral face stimuli were divided into sets (early, middle, and late), yielding six conditions each consisting of eight trials. Onset times for each task condition were entered into a general linear model and convolved with a hemodynamic response function. Subject-specific motion parameters were also included in the model as effects of non-interest. Whole-brain voxel-wise responses to the face stimuli were quantified using linear contrasts for fearful > neutral trials, fearful trials > implicit baseline, and neutral trials > implicit baseline. Individual participants’ contrast images were entered in group-level random effects models. Group-level whole-brain analyses used height-extent correction for multiple comparisons (Eklund, Nichols, & Knutsson, 2016; Woo, Krishnan, & Wager, 2014), with an initial cluster-forming threshold of p < .005, and cluster-wise false discovery rate (FDR) correction to p < .05 for each contrast.
A region of interest (ROI) for the amygdala was defined using a probabilistic atlas based on cytoarchitectonic mapping of human postmortem tissue (Amunts et al., 2005), thresholded at 50% probability. The mean of all voxels within the amygdala mask was extracted from early (first 8 trials), middle (middle 8 trials), and late (last 8 trials) portions of the task to quantify changing amygdala responses over time, separately for fearful and neutral face stimuli.
Linear mixed-effects models (LMEMs) were estimated in IBM SPSS version 24 to examine amygdala responses, with emotion condition (fearful, neutral) and timepoint (early, middle, late trials) modeled within-subjects. Between-subjects variables included total violence exposure frequency, and maternal warmth. The primary maternal warmth indicator was based on the warmth subscale of the PQ, which all mothers completed (median split at total score = 91; n = 26 in higher-warmth and n = 27 in lower-warmth group). A secondary analysis was conducted with maternal warmth behavior modeled between-subjects in the subset of pairs who completed the cooperation challenge task (n = 34), comparing mothers who showed at least 1 warmth behavior, to those who did not. Although potential age effects on amygdala function were of a priori interest, there was no significant main effect or interaction with age in any model, and this factor was removed for parsimony. To assess the need for additional covariates in the models, we conducted bivariate correlations between amygdala reactivity and habituation, and potential moderators including pubertal development (PDS total score), scanner site, and gender. None of these factors were correlated with amygdala responses and were not included in the final LMEMs.
Results
Violence exposure and clinical features of the sample
Demographic and clinical features of the sample are reported in Table 1. In the sample as a whole, children reported a high frequency of violence exposure (VEX-R), with a total frequency score M (SD) = 14.7 (8.2) reflecting approximately 15 individual exposure events on average. There was no difference in exposure between girls and boys, p = .71. Events reported most frequently included witnessing someone being arrested, being yelled at or spanked, and witnessing someone being beat up. Age was positively associated with VEX-R total, r (52) = .36, p = .008. PDS score was similarly associated with violence exposure frequency (r = .34), but did not predict significant variance above and beyond the effect of age (full model including age, sex, PDS: F (3, 44) = 3.70, p = .02; R2ΔPDS = .02, p = .28). VEX-R was not associated with internalizing (p = .93) or externalizing (p = .82) scores on the BASC. 16.7% of the sample (5 girls, 4 boys) met PTSD diagnostic criteria on the UCLA-RI, child report. One child was taking a psychiatric medication (Adderall), and three reported using asthma or allergy medications (albuterol, Flovent). Mothers reported current cohabitation with the child’s father for n = 9 (17%).
Table 1.
Demographic and clinical features of the sample
| Characteristic | M (SD) or % | Association with VEX-R | Association with PQ Warmth |
|---|---|---|---|
| Age | 10.9(1.7) | r = .36, p = .008* | r = −.27, p = .05 |
| Pubertal development (PDS) | 2.0(0.5) | r = .34, p = .02* | r = −.18, p = .23 |
| Gender (% female) | 50.9% | t (51) = −.12, p = .91 | t (51) = −76, p = .45 |
| Handedness (% right) | 90.6% | t (51) = 2.28, p = .03* | t (51) = .16, p = .87 |
| Household income (% <2019 federal poverty level) | 76.5% | t (51) = .57, p = .57 | t (51) = .61, p = .54 |
| Families with disability support | 25.5% | t (51) = 1.13, p = .27 | t (51) = −1.31, p = .20 |
| Mothers’ education | Kruskal-Wallis H = 10.92, p = .09 | Kruskal-Wallis H = 7.01, p = .32 | |
| <12th grade | 15.7% | ||
| High school or GED | 37.3% | ||
| Some college | 23.5% | ||
| College degree | 19.6% | ||
| Graduate school degree | 3.9% | ||
| PTSD diagnosis (UCLA child report) | 17.0% | t (51) = −.15, p = .88 | t (51) = .76, p = .45 |
| Internalizing (BASC) | 50.5 (12.0) | r = −.11, p = .45 | r = −47, p = .0004** |
| Externalizing (BASC) | 53.2 (12.1) | r = −.03, p = .80 | r = −.64, p = 4.81 × 10−7** |
| Maternal warmth (PQ) | 90.3 (10.5) | r = −.04, p = .76 | — |
| Etch-a-sketch task (% mothers displaying warmth behavior) | 32.4% | t (32) = .63, p = .53 | t (32) = 1.06, p = .29 |
| Violence exposure frequency (VEX-R) | 14.7 (8.2) | — | r = −.04, p = .75 |
| School | 5.6 (7.7) | — | r = −.07, p = .59 |
| Community | 4.2 (3.7) | — | r = −.10, p = .49 |
| Home | 2.6 (2.2) | — | r = −.04, p = .77 |
p < .05
p < .001.
fMRI responses to fearful and neutral faces
The task engaged amygdala and ventral visual regions for fearful faces relative to baseline (Figure 1(a), Table S1), and neutral faces (Figure 1(b), Table S1) relative to baseline, in a whole-brain family-wise-error corrected analysis of the full sample, irrespective of violence exposure and age. There were no significant differences in the whole-brain comparison of fearful > neutral faces. Brain activation of similar magnitude for fearful and neutral face stimuli was expected for this developmental stage, as prior research with healthy children indicates that children in this age range do not yet show differential amygdala responses to fearful versus neutral faces (Thomas et al., 2001).
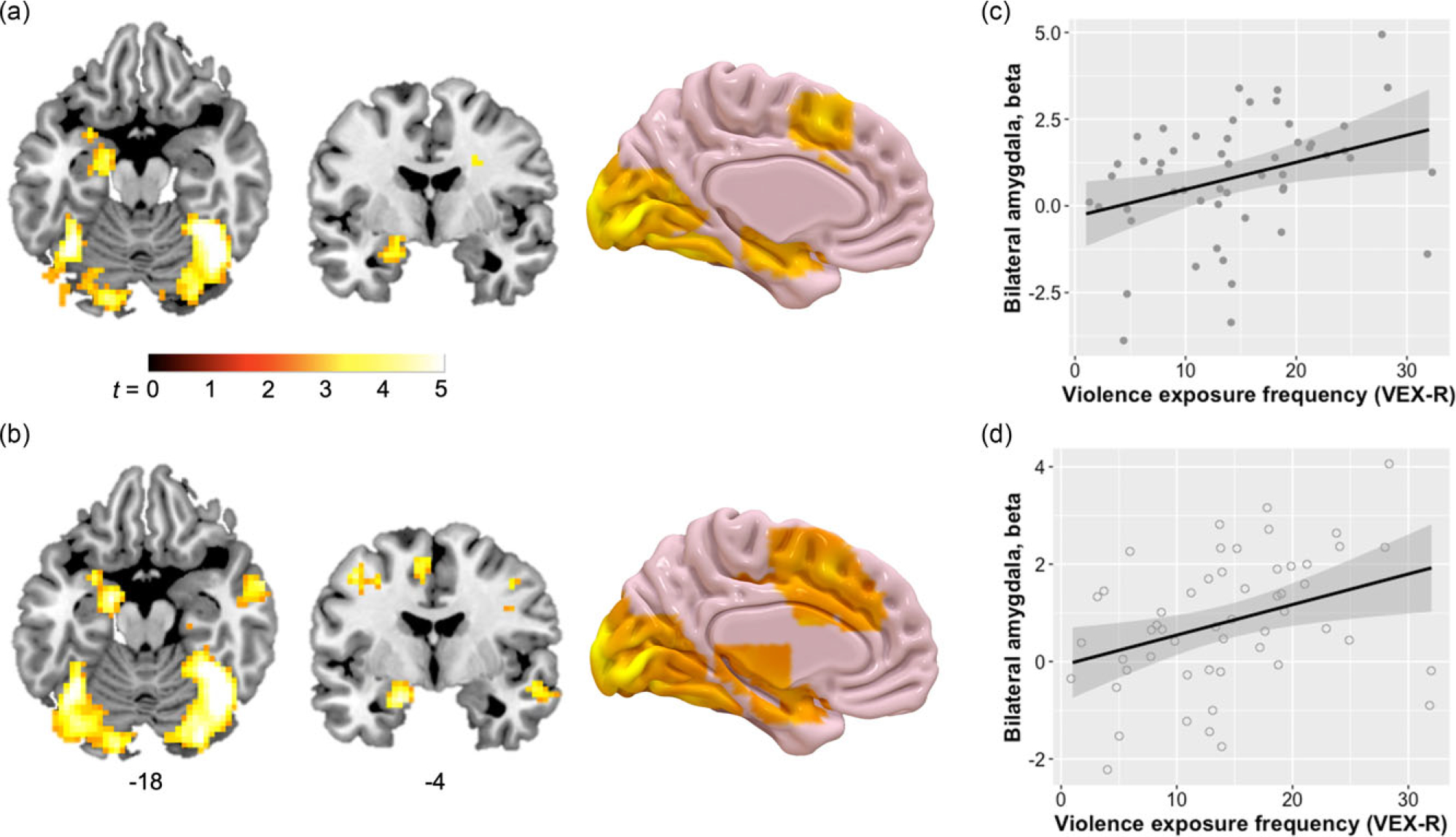
Figure 1. Functional magnetic resonance imaging (fMRI) responses to the fearful and neutral face stimuli, and associations with violence exposure. Whole-brain analyses of the full sample showed that for both fearful (a) and neutral faces (b) relative to baseline, children had fMRI activation in left amygdala and hippocampus, as well as ventral visual regions, pFDR < .05. Children did not have a differential response to fearful versus neutral pictures in any brain region (Fearful > Neutral contrast). Significant clusters are overlaid on a representative single-subject anatomical image in ICBM152 space. 3D rendering of the medial surface of the left hemisphere generated in Surf Ice (https://www.nitrc.org/projects/surfice). Violence exposure frequency was positively associated with children’s amygdala responses to (c) fearful face stimuli, r = .34, p = .01, and (d) neutral face stimuli, r = .35, p = .01. Beta values for the bilateral amygdala ROI are plotted on the y-axis, and error bars represent ± SE.
Relationship between violence exposure and amygdala response to face stimuli
LMEM analysis of the bilateral amygdala ROI across facial emotion condition (fearful, neutral) and timepoint (early, middle, late trials) showed a main effect of violence exposure frequency on amygdala activation, F (1, 51) = 6.14, p = .02. Post-hoc analysis indicated a positive association between violence exposure and amygdala reactivity (Figure 1(c and d)). Consistent with the whole-brain findings, this model showed no main effects of facial emotion (p = .29) or timepoint (p = .67), nor an interaction of emotion by timepoint (p = .29). Violence exposure frequency did not interact with timepoint or emotion.
The VEX-R items addressed violence exposure in three different contexts: at home, at school, and in the community. Interestingly, the frequency of violence experienced in these contexts was not intercorrelated: violence at home was not associated with school (r =−.19, p = .18) or community violence exposure (r = .05, p = .70), and school and community violence were not associated (r = .18, p = .19). This suggested that each child experienced violence mostly within one of the three contexts. To quantify the primary violence context for each child, focusing on exposure within the home, we calculated a violence context score using: [VEX-R Home–M(VEX-R School, VEX-R Community)]/VEX-R Total. This reflected the proportion of violence at home relative to violence elsewhere, such that a maximum score of 1 represented children who only experienced violence at home, and a minimum score of −1 represented children who only experienced violence elsewhere.
The violence context score had a significant effect on amygdala habituation/sensitization (context × timepoint: F (2,102) = 3.64, p = .03), such that greater violence in the home versus elsewhere was associated with greater amygdala sensitization over the course of the task, unrelated to the emotion of the face stimuli. Conversely, this indicated that children who experienced more violence in the external school and community contexts showed greater habituation over the task. The proportion of in-home violence was not associated with amygdala reactivity to fearful or neutral faces (context main effect: p > .05). Post-hoc assessment of violence exposure frequency in each of the three contexts (home, school, community) and amygdala habituation/sensitization (Figure S1) showed a positive association for frequency of home violence, a negative association for school violence, and no association for community violence.
Relationship between amygdala habituation and internalizing and externalizing problems
To determine whether alterations in amygdala habituation/sensitization might relate to children’s mental health, an additional LMEM included BASC age-normalized internalizing and externalizing scores, along with the within-subjects effects of emotion and timepoint for the amygdala ROI. Children with higher externalizing scores showed amygdala sensitization to neutral faces by the late trials (Figure 2; externalizing × emotion × timepoint: F (2, 96) = 4.08, p = .02). There were no main effects or interactions with internalizing scores.
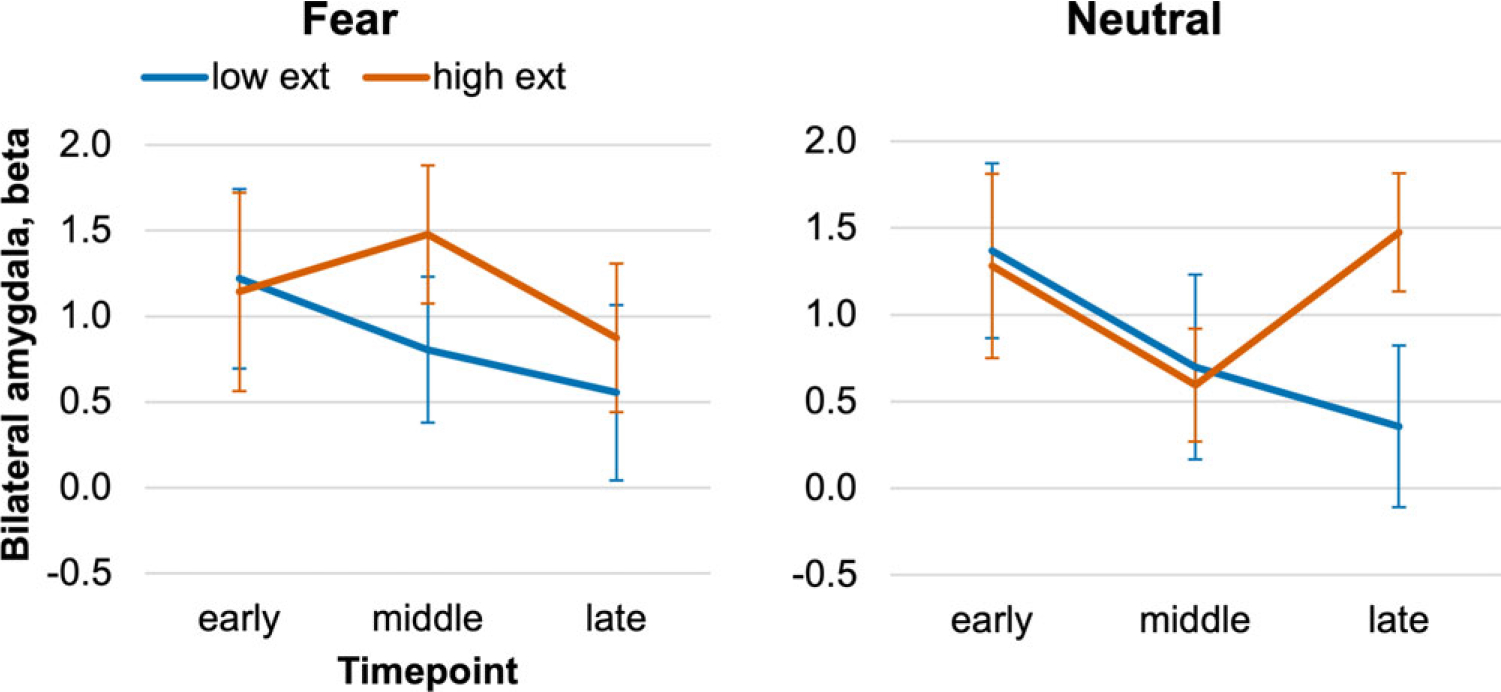
Figure 2. Amygdala sensitization co-varies with externalizing problems. Beta values for the bilateral amygdala ROI are plotted over the early, middle, and late windows of the Faces task. The continuous BASC scores for externalizing showed an interaction with stimulus emotion and timepoint, such that greater externalizing was associated with late sensitization to the neutral face stimuli, p = .02. For illustrative purposes, the charts show amygdala time-courses among children with externalizing scores ≤50th percentile relative to age norms (“low exter”), or >50th percentile (“high exter”). Error bars ± 1 SE.
Maternal buffering effects on amygdala response to face stimuli
Mothers’ self-report of warmth
The primary analyses of maternal buffering defined maternal warmth using mothers’ reports of their own parenting behavior on the PQ. Maternal warmth did not normalize the effect of overall violence exposure frequency on amygdala reactivity (warmth × violence F (1, 49) = 1.12, p = .30). However, maternal warmth did show a significant interaction with violence context score to influence amygdala sensitization (warmth × context × timepoint F (2, 98) = 5.01, p = .008). In children with less warm mothers, a greater proportion of violence at home versus elsewhere was associated with amygdala sensitization (Figure 3). In contrast, in children with warmer mothers, greater violence at home vs elsewhere was not associated amygdala sensitization, consistent with a maternal buffering effect.
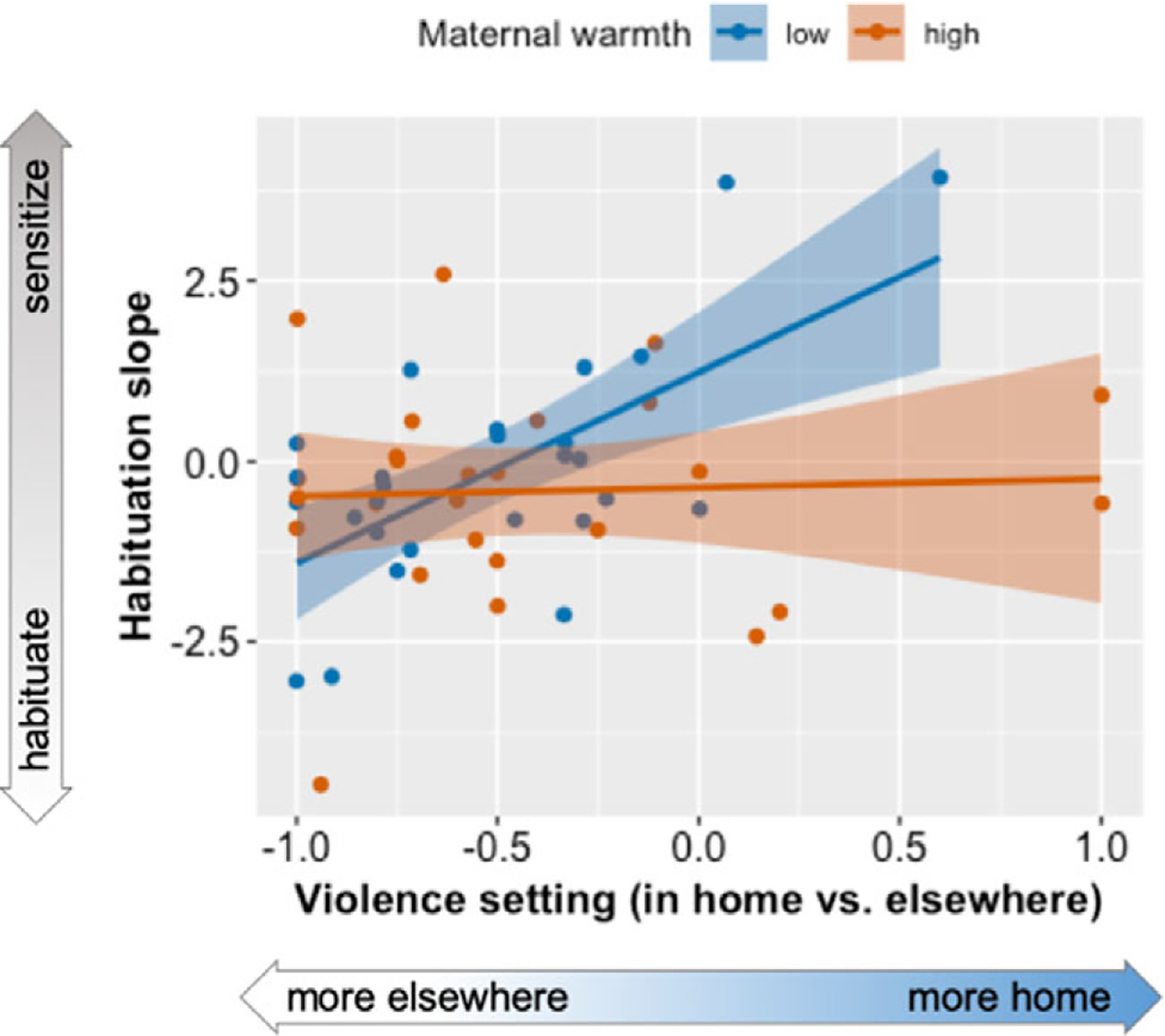
Figure 3. Maternal buffering of violence exposure. In the group of children whose mothers reported fewer warm parenting behaviors (blue), the violence context score (proportion of violence in the home vs. elsewhere) was associated with amygdala sensitization to fearful and neutral face stimuli over the course of the task, post-hoc r = .65, p < .001. Conversely, among children whose mothers reported greater warm parenting behaviors (red), the proportion of violence experienced in the home was unrelated to amygdala habituation, post-hoc r = .05, p = .83. This pointed to a maternal buffering effect. In the primary statistical model, habituation was quantified using a within-subjects timepoint factor, but for illustrative purposes, amygdala habituation is plotted on the y-axis as the linear slope of change across the three time windows.
Mothers’ behavior in cooperation challenge task
To investigate whether findings would hold when taking into account an objective measure of mothers’ warmth with their children, a secondary analysis used maternal warmth behavior expressed during the Etch-a-sketch task. This was a cooperation challenge task that was completed by a subset of n = 34 mother–child dyads. Among mothers who showed warmth behavior during the Etch-a-sketch task, relative to those who did not, self-reported warm parenting on the PQ was numerically higher but this was not significant [M ± SDwarm = 91.82(9.12), M ± SDnot warm 88.26(9.10), t (32) = 1.10, p = .29] suggesting that each captured different components of mothers’ interactions with their children. Despite this, the effects on amygdala activation paralleled the effect observed for self-report of warmth behaviors: primary violence exposure context (home vs. outside) was associated with amygdala sensitization in children whose mothers did not show warmth, but not in children whose mothers showed warmth during the cooperation challenge task (warmth × context × timepoint F (2, 60) = 4.12, p = .02).
Maternal buffering effects on current and future externalizing
Given the observed associations between amygdala habituation and externalizing symptoms, we then tested whether children of warm mothers also showed corresponding decrements in externalizing problems. Mothers’ report of warm parenting was strongly negatively associated with both internalizing and externalizing scores on the BASC (Table 1), indicating a protective effect. A subset of n = 28 mother–child pairs returned to the laboratory approximately 1 year later (11.7 ± 10.1 months later) and completed an update interview including questionnaires about children’s trauma exposure and related symptoms. Children whose mothers reported greater warm parenting behaviors at baseline showed fewer future externalizing symptoms 1 year later, r =−.59, p = .001.
Discussion
The current findings underscore the importance of social relationships throughout the lifespan as critical determinants of the response to stress and trauma exposure. In models of mental health outcomes after trauma and other forms of stress, social support is a resilience factor whose effect size outweighs even the negative impacts of childhood maltreatment (Arnberg et al., 2012; Betancourt et al., 2013; Evans, Steel, & DiLillo, 2013; McGuire et al., 2018; Stevens & Jovanovic, 2019). We found that the caregiver–child relationship may indeed be central to shaping threat responses in children, even in the context of violence exposure. Violence exposure influenced the amygdala response to threat but varied by the context. Cumulative violence was associated with increased amygdala reactivity to both fearful and neutral face stimuli; children who primarily experienced violence in the home showed amygdala sensitization over repeated trials whereas violence elsewhere was associated with amygdala habituation. However, we also observed a maternal buffering effect, such that the proportion of home violence was not linked with amygdala sensitization in children whose mothers used warm parenting behaviors. Strikingly, the effect was similar for mothers’ self-report of warm parenting, as well as for warm parenting behaviors observed in a mother–child interaction task conducted in the laboratory. These novel results suggest that (a) violence experienced in the caregiving environment appears to promote a pattern of amygdala response to threat that is consistent with hypervigilance and (b) the presence of a warm caregiving figure in the home can protect against violence-related alterations in brain function.
Effects of violence exposure on brain function in children
The findings in the current study were comparable to those of numerous prior studies showing a positive association between early life stress and children’s amygdala reactivity to negative facial expressions or other negative stimuli (Marusak et al., 2015; McLaughlin et al., 2015; Suzuki et al., 2014; Tottenham et al., 2011; White et al., 2012), especially those involving a physical threat such as an accident, witnessing or experiencing interpersonal violence, or experiencing abuse. We found that cumulative violence exposure, across all contexts, was positively associated with the amygdala response to both fearful and neutral face stimuli. This task was designed to elicit neural processing of overt social threat cues (fearful faces) and a neutral/non-threat baseline condition. However, several studies in healthy children show comparable activation magnitude to neutral and negative face stimuli in emotion-related brain regions including the amygdala, primary sensory cortex, dACC, and insula (Gee, Humphreys et al., 2013; Marusak, Carré, & Thomason, 2013; Thomas et al., 2001). It is possible that children may not interpret neutral expressions as affectively neutral, and that this may be exaggerated in some children. For example, neutral faces elicit left amygdala hyper-reactivity in children with pediatric bipolar disorder, who interpret neutral faces as hostile, relative to control participants (Rich et al., 2006). Further study is needed to assess whether violence exposure may predict greater attribution of hostility or other negative emotionality to ambiguous social cues such as neutral face stimuli.
Prior studies have not distinguished between effects of violence in different environmental contexts on the neural correlates of threat processing. Here, school and community violence were linked with greater amygdala habituation, while home violence was linked with greater amygdala sensitization. This may reflect different, likely helpful, initial adaptations to the unique demands of each context. Habituation is typically interpreted as a very simple learning process, by which the neural response to repeated stimuli decreases over repeated presentations (also termed “repetition suppression”), and has been shown to be a basic feature of very simple neural circuits (Bailey & Chen, 1988; Groves & Thompson, 1970). In the context of habituation to repeated trials of fearful and neutral face stimuli, there is an additional affective overlay which may compete with the learning function, such that threatening or salient stimuli may be prioritized (Ishai et al., 2004). In the current study, greater habituation may therefore reflect facilitated learning or plasticity, or lesser salience or arousal elicited by the facial expressions. Greater sensitization, on the other hand, may reflect a blunted learning function, or a heightened vigilance response such that attentional resources continue to be deployed even after many repetitions of similar stimuli. In the context of the current study, children who primarily experience violence at school may quickly learn to sift through the many incoming social cues from peers at school to decide which situations represent threat or safety (greater habituation). Prior neuropsychiatric work supports this idea, showing that anxious children living in communities with greater police-reported violence showed greater avoidance of threat in an attentional task (McCoy, Raver, & Sharkey, 2015). In contrast, children who primarily experience violence at home may not easily be able to escape or avoid a violent event, potentially developing hyper-vigilance for threat detection (greater sensitization). Again, existing neuropsychiatric findings support this idea, showing that children exposed to family violence show greater attention to threat in an attentional task (Briggs-Gowan et al., 2015). However, this interpretation requires further testing with greater control over the stress exposure context. Studies in animal models that manipulate whether threats are experienced inside or outside the nest, and the complexity of the social situation, may be informative.
While violence inside the home was associated with greater amygdala sensitization to social threat cues, this pattern may not persist into adulthood. In prior work with adult women recruited from the same sample, a group who reported experiencing significant childhood abuse (primarily experienced in the home environment) showed greater amygdala habituation to repeated fearful face stimuli, and habituation partially mediated the association between childhood abuse and post-traumatic stress disorder (Kim et al., 2019). One possibility may be that high levels of vigilance in childhood may tax the arousal/alerting system regulated by the amygdala, such that by adulthood only highly threatening inputs are detected as high-priority stimuli that require attentional and physiological mobilization. Another interpretation may be that the VEX-R captures different forms of violence exposure taking place in the home, outside of abuse of the child herself or himself. Many children in the present study reported physical altercations between siblings and cousins. Some reported experiencing violence themselves, involving incidents with siblings or extended family members rather than the primary caregivers. Very few mothers or children reported any abuse of the child in the home, although the self-report nature of the study design limits our ability to detect potential abuse.
An additional interesting reason why violence exposure in the home may differ from exposure in external contexts is that the presence of the mother in the home may alter the nature of the associations that are formed by the child. In rodent studies, if a mother is present during an aversive experience, this can alter the way that the experience shapes brain function in the offspring. For example, infant rat pups that were conditioned to associate mothers’ odor with an aversive shock stimulus showed abnormal amygdala function, relative to those who had aversive experiences without maternal odor cues, and the effects were identical to pups who had an abusive mother (Raineki, Moriceau, & Sullivan, 2010). In human children, the presence of their mother during the aversive experience of violence exposure may have similar effects on amygdala function in children exposed to violence in the home. This may be observed even when mothers are not involved in the violence but are merely present.
Maternal buffering of the effects of violence exposure
Findings were broadly consistent with previous studies of the role of caregivers in modulating children’s neural responses to threat. Reduced amygdala or threat reactivity in the presence of the mother or maternal cues has been observed in healthy (Gee et al., 2014) and ELA-exposed children (Callaghan et al., 2019; van Rooij et al., 2017). Additionally, warm and supportive parenting behaviors appear to similarly protect against the effects of ELA on brain function, or strengthen the protective effects of maternal cues (Brody et al., 2019; Callaghan et al., 2019; Gee et al., 2014; Romund et al., 2016), although some evidence suggests that familial affective responsiveness (appropriate affect either positive or negative) can predict heightened amygdala reactivity in children (Farber et al., 2019). On the other side of the coin, negative parenting behaviors such as hostility predict lower functional connectivity between children’s amygdala and prefrontal cortex (Kopala-Sibley et al., 2020). However, the findings of the current study were distinctive in that we did not observe an association between maternal warmth and amygdala reactivity per se to the fearful or neutral faces. We instead observed an effect on the time-course of the amygdala responses such that warm parenting protected against the pattern of amygdala sensitization that we had observed in children exposed primarily to violence in the home. These results were encouraging, suggesting that even within the home, the effects of violence exposure on amygdala function were buffered by the presence of a warm caregiver. A warm and stable caregiver may function as a “safe haven” (Kerns et al., 2015) in a tumultuous home environment. This may be a critical determinant of whether negative outcomes will follow ELA, given that amygdala hyper-reactivity and habituation have been shown to predict risk for future psychopathology following stress exposure (Admon et al., 2009; Fox & Kalin, 2014; Mattson et al., 2016; McLaughlin, Busso et al., 2014; Stevens et al., 2017; Swartz et al., 2015). Adult social support may have similar buffering effects, as amygdala reactivity is associated with anxiety symptoms in adults with low social support but this effect is absent in adults with high social support (Hyde et al., 2011).
Maternal warmth also showed a striking protective effect on current externalizing symptoms in children, which persisted up to a year later. This effect was large, predicting 34% of the variance for future externalizing symptoms. Similar long-term protective effects of parental warmth against psychopathology have been reported in a large sample (n = 2,491) of Puerto Rican children (Santesteban-Echarri et al., 2017), and among African American children (McCabe & Clark, 1999). Although violence exposure was not linked with externalizing symptoms in our sample of children ages 8–14, prior studies in somewhat older aged cohorts of adolescents show that externalizing symptoms begin to emerge by the later teen years. For example, in n = 169 children at M = 15 years of age, violence exposure (but not poverty) was associated with externalizing psychopathology along with blunted cortisol responses to a social stress task (Busso, McLaughlin, & Sheridan, 2017). Violence exposure also predicted future externalizing and internalizing problems in a large longitudinal cohort of adolescents (Miller et al., 2018). Taken together, the literature suggests that warm parenting behaviors expressed by mothers may be protective against the emergence of externalizing-related psychopathology in trauma-exposed adolescents. Although mothers were tested here, it is anticipated that the findings would be likely to extend to any primary caregiver, given that social buffering effects on stress and arousal are not limited to maternal relationships (Hennessy, Kaiser, & Sachser, 2009).
Limitations
There are a number of limitations that should be noted. First, the moderate sample size meant that tests of interaction effects between violence exposure and maternal warmth may be underpowered. Further investigation is needed to provide a strong test of whether maternal buffering effects are greatest for traumas experienced in the home environment. Second, although we were interested in developmental change over this window of child development, we did not observe any age effects on amygdala reactivity. It may be that there is significant development in the amygdala response to social threat cues, but that there are major individual differences in these trajectories that are masked when imaging is gathered at only 1 timepoint. Longitudinal investigation can better track such changes within individual children. Lastly, experimental studies manipulating parental engagement, such as studies involving interventions with parents to impact child anxiety or depressive symptoms (Lebowitz et al., 2020), will provide the strongest tests of maternal buffering effects. The current findings support the translational importance of such interventions in children exposed to violence.
Conclusion
The current study provided further evidence that early life stress, and particularly violence exposure, influence the function of threat neurocircuitry in school-aged children. Interestingly, violence experienced in the home environment was linked with a pattern of amygdala sensitization to repeated social cues indicating threat (fearful faces) or ambiguity (neutral faces), whereas violence experienced at school was associated with amygdala habituation to repeated cues. Finally, we identified a feature of mothers’ parenting—warm parenting behaviors—which was associated with a normalization of amygdala function in children exposed to greater in-home violence, as well as predicting fewer future externalizing behaviors. The findings are encouraging, suggesting that parenting behaviors can be protective even in the presence of highly impactful forms of ELA. A clear next step for translation might be to conduct early intervention studies involving parent-centered approaches to prevent the mental health impacts of ELA on children, with children’s amygdala responses to threat stimuli as an intermediate target.
Supplementary Material
Acknowledgements.
We would like to acknowledge L. Alexander Vance, Minhnguyen Cao, Dorthie Cross, Bekh Bradley, Allen W. Graham, and the staff and volunteers of the Grady Trauma Project for their support of the study, and the participants and their families for generously giving their time and effort. This study was funded by the National Institutes of Health, including R01MH100122 and R01MH111682 to TJ, 2R01MH091864 to NT, T32HL130025 to YJK, K23AT009713 to AP, and K12 HD085850 to JSS.
Footnotes
Conflicts of interest. None.
Supplementary material. For supplementary material accompanying this paper visit https://doi.org/10.1017/S0954579421001085
References
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, & Hendler T (2009). Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences, 106(33), 14120–14125. 10.1073/pnas.0903183106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Scheider F, & Zilles K (2005). Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anatomy and Embryology, 210(5), 343–352. 10.1007/s00429-005-0025-5 [DOI] [PubMed] [Google Scholar]
- Arnberg FK, Hultman CM, Michel P-O, & Lundin T (2012). Social support moderates posttraumatic stress and general distress after disaster. Journal of Traumatic Stress, 25(6), 721–727. 10.1002/jts.21758 [DOI] [PubMed] [Google Scholar]
- Bailey C, & Chen M (1988). Morphological basis of short-term habituation in Aplysia. The Journal of Neuroscience, 8(7), 2452–2459. 10.1523/jneurosci.08-07-02452.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt TS, Borisova I, Williams TP, Meyers-Ohki SE, Rubin-Smith JE, Annan J, & Kohrt BA (2013). Psychosocial adjustment and mental health in former child soldiers – A systematic review of the literature and recommendations for future research. Journal of Child Psychology and Psychiatry, 54(1), 17–36. 10.1111/j.1469-7610.2012.02620.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Pollak SD, Grasso D, Voss J, Mian ND, Zobel E, McCarthy KJ, Wakschlag LS, & Pine DS (2015). Attention bias and anxiety in young children exposed to family violence. Journal of Child Psychology and Psychiatry, 56(11), 1194–1201. 10.1111/jcpp.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Nusslock R, Barton AW, Miller GE, Chen E, Holmes C, McCormick M, & Sweet LH (2019). The protective effects of supportive parenting on the relationship between adolescent poverty and resting-state functional brain connectivity during adulthood. Psychological Science, 30(7), 1040–1049. 10.1177/0956797619847989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Stichick TL, Birdthistle I, & Earls FJ (2001). Youth exposure to violence: Prevalence, risks, and consequences. American Journal of Orthopsychiatry, 71(3), 298–310. 10.1037/0002-9432.71.3.298 [DOI] [PubMed] [Google Scholar]
- Busso DS, McLaughlin KA, & Sheridan MA (2017). Dimensions of adversity, physiological reactivity, and externalizing psychopathology in adolescence: Deprivation and threat. Psychosomatic Medicine, 79(2), 162–171. 10.1097/psy.0000000000000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri DS, Caldera C, & Tottenham N (2019). Decreased amygdala reactivity to parent cues protects against anxiety following early adversity: An examination across 3 years. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(7), 664–671. 10.1016/j.bpsc.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, & Cole SW (2011). Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry, 16(7), 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross D, Kim YJ, Vance LA, Robinson G, Jovanovic T, & Bradley B (2016). Maternal child sexual abuse is associated with lower maternal warmth toward daughters but not sons. Journal of Child Sexual Abuse, 25(8), 813–826. 10.1080/10538712.2016.1234532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross D, Vance LA, Kim YJ, Ruchard AL, Fox N, Jovanovic T, & Bradley B (2018). Trauma exposure, PTSD, and parenting in a community sample of low-income, predominantly African American mothers and children. Psychological Trauma, 10(3), 327–335. 10.1037/tra0000264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D, Dohm K, Sehlmeyer C, Konrad C, Baune BT, Arolt V, Heindel W, Zwitserlood P, & Suslow T (2013). Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Human Brain Mapping, 34(11), 2899–2909. 10.1002/hbm.22112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow H, & Kugel H (2012). Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry, 71(4), 286–293. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SE, Steel AL, & DiLillo D (2013). Child maltreatment severity and adult trauma symptoms: Does perceived social support play a buffering role? Child Abuse & Neglect, 37(11), 934–943. 10.1016/j.chiabu.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber MJ, Romer AL, Kim MJ, Knodt AR, Elsayed NM, Williamson DE, & Hariri AR (2019). Paradoxical associations between familial affective responsiveness, stress, and amygdala reactivity. Emotion, 19(4), 645–654. 10.1037/emo0000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Ramsawh HJ, Flagan TM, Simmons AN, Sullivan SG, Allard CB, Paulus MP, & Stein MB (2016). Early life stress and the anxious brain: Evidence for a neural mechanism linking childhood emotional maltreatment to anxiety in adulthood. Psychological Medicine, 46(5), 1037–1054. 10.1017/S0033291715002603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, & Kalin NH (2014). A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. American Journal of Psychiatry, 171(11), 1162–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N, & Leavitt L (1995). The violence exposure scale for children-VEX (preschool version). College Park: Department of Human Development, University of Maryland. [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri D, Caldera C, & Tottenham N (2014). Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science, 25(11), 2067–2078. 10.1177/0956797614550878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY,& Tottenham N (2013). Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences, 110(39), 15638–15643. 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M,Hare TA,Bookheimer SY,& Tottenham N (2013). A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. The Journal of Neuroscience, 33(10), 4584–4593. 10.1523/jneurosci.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, & Ressler KJ (2009). Trauma exposure and stress-related disorders in inner city primary care patients. General Hospital Psychiatry, 31(6), 505–514. 10.1016/j.genhosppsych.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg GS, & Grover RL (2014). Coding manual for parent-child interactions. Baltimore: Johns Hopkins University School of Medicine. [Google Scholar]
- Ginsburg GS, Grover RL, Cord JJ, & Ialongo N (2006). Observational measures of parenting in anxious and non-anxious mothers: Does type of task matter? Journal of Clinical Child and Adolescent Psychology, 35(2), 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg GS, Grover RL, & Ialongo N (2004). Parenting behaviors among anxious and non-anxious mothers: Relation with concurrent and long-term child outcomes. Child and Family Behavior Therapy, 26(4), 23–41. 10.1300/J019v26n04_02 [DOI] [Google Scholar]
- Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, & Tottenham N (2013). Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience, 249, 129–138. 10.1016/j.neuroscience.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, & Thompson RF (1970). Habituation: A dual-process theory. Psychological Review, 77(5), 419–450. 10.1037/h0029810 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Hostinar CE, Sanchez MM, Tottenham N, & Sullivan RM (2015). Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Social Neuroscience, 10(5), 474–478. 10.1080/17470919.2015.1070198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz-Rotem I, Murphy RA, Berkowitz S, Marans S, & Rosenheck RA (2007). Clinical epidemiology of urban violence: Responding to children exposed to violence in ten communities. Journal of Interpersonal Violence, 22(11), 1479–1490. 10.1177/0886260507305571 [DOI] [PubMed] [Google Scholar]
- Hein TC, Goetschius LG, McLoyd VC, Brooks-Gunn J, McLanahan SS, Mitchell C, Lopez-Duran NL, Hyde LW, & Monk CS (2020). Childhood violence exposure and social deprivation are linked to adolescent threat and reward neural function. Social Cognitive and Affective Neuroscience, 15(11), 1252–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TC, & Monk CS (2017). Research review: Neural response to threat in children, adolescents, and adults after child maltreatment – a quantitative meta-analysis. Journal of Child Psychology and Psychiatry, 58(3), 222–230. 10.1111/jcpp.12651 [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, & Sachser N (2009). Social buffering of the stress response: Diversity, mechanisms, and functions. Frontiers in Neuroendocrinology, 30(4), 470–482. [DOI] [PubMed] [Google Scholar]
- Herringa RJ (2017). Trauma, PTSD, and the developing brain. Current Psychiatry Reports, 19(10), 69. 10.1007/s11920-017-0825-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, & Essex MJ (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences, 110(47), 19119. 10.1073/pnas.1310766110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Gorka A, Manuck SB, & Hariri AR (2011). Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia, 49(4), 651–656. 10.1016/j.neuropsychologia.2010.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Pessoa L, Bikle PC, & Ungerleider LG (2004). Repetition suppression of faces is modulated by emotion. Proceedings of the National Academy of Sciences, 101(26), 9827–9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keding TJ, & Herringa RJ (2015). Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacology, 40(3), 537–545. 10.1038/npp.2014.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns KA, Mathews BL, Koehn AJ, Williams CT, & Siener-Ciesla S (2015). Assessing both safe haven and secure base support in parent–child relationships. Attachment & Human Development, 17(4), 337–353. [DOI] [PubMed] [Google Scholar]
- Kim YJ, van Rooij SJH, Ely TD, Fani N, Ressler KJ, Jovanovic T, & Stevens JS (2019). Association between posttraumatic stress disorder severity and amygdala habituation to fearful stimuli. Depression & Anxiety, 36(7), 647–658. 10.1002/da.22928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer W, Lepore SJ, Oskin D, & Johnson PD (1998). The role of social and cognitive processes in children’s adjustment to community violence. Journal of Consulting and Clinical Psychology, 66(1), 199–209. 10.1037/0022-006X.66.1.199 [DOI] [PubMed] [Google Scholar]
- Kopala-Sibley DC, Cyr M, Finsaas MC, Orawe J, Huang A, Tottenham N, & Klein DN (2020). Early childhood parenting predicts late childhood brain functional connectivity during emotion perception and reward processing. Child Development, 91(1), 110–128. 10.1111/cdev.13126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz ER, Marin C, Martino A, Shimshoni Y, & Silverman WK (2020). Parent-based treatment as efficacious as cognitive-behavioral therapy for childhood anxiety: A randomized noninferiority study of supportive parenting for anxious childhood emotions. Journal of the American Academy of Child & Adolescent Psychiatry, 59(3), 362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23(1), 155–184. 10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Hagan MJ, Wolchik SA, Sandler IN, & Tein J-Y (2016). A longitudinal study of the effects of child-reported maternal warmth on cortisol stress response 15 years after parental divorce. Psychosomatic Medicine, 78(2), 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, & Öhman A (1998). The Karolinska Directed Emotional Faces – KDEF, CD ROM from Department of Clinical Neuroscience, Psychology section, Karolinska Institutet, ISBN 91–630-7164–9. [Google Scholar]
- Machlin L, Miller AB, Snyder J, McLaughlin KA, & Sheridan MA (2019). Differential associations of deprivation and threat with cognitive control and fear conditioning in early childhood. Frontiers in Behavioral Neuroscience, 13(80). 10.3389/fnbeh.2019.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik NM (2008). Exposure to domestic and community violence in a non-risk sample: Associations with child functioning. Journal of Interpersonal Violence, 23(4), 490–504. 10.1177/0886260507312945 [DOI] [PubMed] [Google Scholar]
- Marusak HA, Carré JM, & Thomason ME (2013). The stimuli drive the response: An fMRI study of youth processing adult or child emotional face stimuli. Neuroimage, 83, 679–689. 10.1016/j.neuroimage.2013.07.002 [DOI] [PubMed] [Google Scholar]
- Marusak HA, Martin KR, Etkin A, & Thomason ME (2015). Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology, 40(5), 1250–1258. 10.1038/npp.2014.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson WI, Hyde LW, Shaw DS, Forbes EE, & Monk CS (2016). Clinical neuroprediction: Amygdala reactivity predicts depressive symptoms 2 years later. Social Cognitive and Affective Neuroscience, 11(6), 892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe KM, & Clark R (1999). Family protective factors among urban African American youth. Journal of Clinical Child Psychology, 28(2), 137–150. [DOI] [PubMed] [Google Scholar]
- McCoy DC, Raver CC, & Sharkey P (2015). Children’s cognitive performance and selective attention following recent community violence. Journal of Health and Social Behavior, 56(1), 19–36. 10.1177/0022146514567576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory EJ, Gerin MI, & Viding E (2017). Annual research review: Childhood maltreatment, latent vulnerability and the shift to preventative psychiatry – The contribution of functional brain imaging. Journal of Child Psychology and Psychiatry, 58(4), 338–357. 10.1111/jcpp.12713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2000). The neurobiology of stress: From serendipity to clinical relevance. Brain Research, 886(1–2), 172–189. [DOI] [PubMed] [Google Scholar]
- McGaugh JL (2002). Memory consolidation and the amygdala: A systems perspective. Trends in Neurosciences, 25(9), 456–461. [DOI] [PubMed] [Google Scholar]
- McGuire AP, Gauthier JM, Anderson LM, Hollingsworth DW, Tracy M, Galea S, & Coffey SF (2018). Social support moderates effects of natural disaster exposure on depression and posttraumatic stress disorder symptoms: Effects for displaced and nondisplaced residents. Journal of Traumatic Stress, 31(2), 223–233. 10.1002/jts.22270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, & Sheridan MA (2014). Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depression & Anxiety, 31(10), 834–842. 10.1002/da.22284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan MA (2015). Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child & Adolescent Psychiatry, 54(9), 753–762. 10.1016/j.jaac.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Gold AL, Duys A, Lambert HK, Peverill M, Heleniak C, Shechner T, Wojcieszak Z, & Pine DS (2016). Maltreatment exposure, brain structure, and fear conditioning in children and adolescents. Neuropsychopharmacology, 41(8), 1956–1964. 10.1038/npp.2015.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. 10.1016/j.neubiorev.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AB, Sheridan MA, Hanson JL, McLaughlin KA, Bates JE, Lansford JE, Petit GS, & Dodge KA (2018). Dimensions of deprivation and threat, psychopathology, and potential mediators: A multi-year longitudinal analysis. Journal of Abnormal Psychology, 127(2), 160–170. 10.1037/abn0000331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, & Sullivan RM (2004). Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. International Journal of Developmental Neuroscience, 22(5), 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, & Sullivan RM (2006). Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neuroscience, 9(8), 1004–1006. 10.1038/nn1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Lyons-Ruth K, Anderson CM, & Teicher MH (2014). Sensitive periods of amygdala development: The role of maltreatment in preadolescence. Neuroimage, 97, 236–244. 10.1016/j.neuroimage.2014.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Puetz VB, Viding E, Gerin MI, Pingault JB, Sethi A, Knodt AR, Radtke SR, Brigidi BD, Hariri AR, & McCrory E (2019). Investigating patterns of neural response associated with childhood abuse v. childhood neglect. Psychological Medicine, 1–10. 10.1017/s003329171900134x [DOI] [PubMed] [Google Scholar]
- Raineki C, Cortés MR, Belnoue L, & Sullivan RM (2012). Effects of early-life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. The Journal of Neuroscience, 32(22), 7758–7765. 10.1523/jneurosci.5843-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, & Sullivan RM (2010). Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biological Psychiatry, 67(12), 1137–1145. 10.1016/j.biopsych.2009.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, & Kamphaus RW (2004). Behavior Assessment System for Children (2nd Ed.). Circle Pines, MN: AGS. [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, & Leibenluft E (2006). Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceedings of the National Academy of Sciences, 103(23), 8900–8905. 10.1073/pnas.0603246103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romund L, Raufelder D, Flemming E, Lorenz RC, Pelz P, Gleich T, Heinz A, & Beck A (2016). Maternal parenting behavior and emotion processing in adolescents—An fMRI study. Biological Psychology, 120, 120–125. 10.1016/j.biopsycho.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Sanchez MM, McCormack KM, & Howell BR (2015). Social buffering of stress responses in nonhuman primates: Maternal regulation of the development of emotional regulatory brain circuits. Social Neuroscience, 10(5), 512–526. 10.1080/17470919.2015.1087426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesteban-Echarri O, Ramos-Olazagasti MA, Eisenberg RE, Wei C, Bird HR, Canino G, & Duarte CS (2017). Parental warmth and psychiatric disorders among Puerto Rican children in two different socio-cultural contexts. Journal of Psychiatric Research, 87, 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Peverill M, Finn AS, & McLaughlin KA (2017). Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning. Development and Psychopathology, 29(5), 1777–1794. 10.1017/S0954579417001390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Decker KB, & Pynoos RS (2004). The University of California at Los Angeles post-traumatic stress disorder reaction index. Current Psychiatry Reports, 6(2), 96–100. [DOI] [PubMed] [Google Scholar]
- Stevens JS, & Jovanovic T (2019). Role of social cognition in post-traumatic stress disorder: A review and meta-analysis. Genes, Brain and Behavior, 18(1), e12518. 10.1111/gbb.12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, Hudak LA, Jovanovic T, Rothbaum BO, & Ressler KJ (2017). Amygdala reactivity and anterior cingulate habituation predict PTSD symptom maintenance after acute civilian trauma. Biological Psychiatry, 81(12), 1023–1029. 10.1016/j.biopsych.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, van Rooij SJH, & Jovanovic T (2018). Developmental contributors to trauma response: The importance of sensitive periods, early environment, and sex differences. In Vermetten E, Baker DG, & Risbrough VB (Eds.), Behavioral neurobiology of PTSD (pp. 1–22). Cham: Springer International Publishing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Colich NL, Uddin M, Armstrong D, & McLaughlin KA (2019). Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biological Psychiatry, 85(3), 268–278. 10.1016/j.biopsych.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H,Luby JL, Botteron KN, Dietrich R, McAvoy MP, & Barch DM (2014). Early life stress and trauma and enhanced limbic activation to emotionally valenced faces in depressed and healthy children. Journal of the American Academy of Child & Adolescent Psychiatry, 53(7), 800–813.e810. 10.1016/j.jaac.2014.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, & Hariri AR (2015). A neural biomarker of psychological vulnerability to future life stress. Neuron, 85(3), 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, & Casey BJ (2001). Amygdala response to facial expressions in children and adults. Biological Psychiatry, 49(4), 309–316. 10.1016/S0006-3223(00)01066-0 [DOI] [PubMed] [Google Scholar]
- Tottenham N,Hare TA,Millner A, Gilhooly T, Zevin JD,& Casey BJ (2011). Elevated amygdala response to faces following early deprivation. Developmental Science, 14(2), 190–204. 10.1111/j.1467-7687.2010.00971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, & Eigsti IM (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science, 13(1), 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Shapiro M, Flannery J, Caldera C, & Sullivan RM (2019). Parental presence switches avoidance to attraction learning in children. Nature Human Behavior, 3(10), 1070–1077. 10.1038/s41562-019-0656-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bulk BG, Somerville LH, van Hoof M-J, van Lang NDJ, van der Wee NJA, Crone EA, & Vermeiren RRJM (2016). Amygdala habituation to emotional faces in adolescents with internalizing disorders, adolescents with childhood sexual abuse related PTSD and healthy adolescents. Developmental Cognitive Neuroscience, 21, 15–25. 10.1016/j.dcn.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen A-L, van Tol M-J, Demenescu LR, van der Wee NJA, Veltman DJ, Aleman A, van Buchem MA, Spinhoven P, Penninx BWJH, & Elzinga BM (2012). Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Social Cognitive and Affective Neuroscience, 8(4), 362–369. 10.1093/scan/nss007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJ, Cross D, Stevens JS, Vance LA, Kim YJ, Bradley B, Tottenham N, & Jovanovic T (2017). Maternal buffering of fear-potentiated startle in children and adolescents with trauma exposure. Social Neuroscience, 12(1), 22–31. 10.1080/17470919.2016.1164244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MG, Bogdan R, Fisher PM, Munoz KE, Williamson DE, & Hariri AR (2012). FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes, Brain and Behavior, 11(7), 869–878. 10.1111/j.1601-183X.2012.00837.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Voss JL, Chiang JJ, Wang L, McLaughlin KA, & Miller GE (2019). Exposure to violence and low family income are associated with heightened amygdala responsiveness to threat among adolescents. Developmental Cognitive Neuroscience, 40, 100709. 10.1016/j.dcn.2019.100709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C-W, Krishnan A, & Wager TD (2014). Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. Neuroimage, 91, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymbs NF, Orr C, Albaugh MD, Althoff RR, O’Loughlin K, Holbrook H, Garavan H, Montalvo-Ortiz JL, Mostofsky S, & Hudziak J (2020). Social supports moderate the effects of child adversity on neural correlates of threat processing. Child Abuse & Neglect, 102, 104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


