Abstract
ABT-773 is a novel ketolide effective against antibacterial-resistant respiratory tract pathogens. The pharmacokinetic profile of ABT-773 was studied in rats and consisted of a mean peak concentration in plasma of 1.07 μg/ml and an area under the concentration-time curve (AUC) of 12.03 μg · h/ml when the compound was delivered at a dose of 25 mg/kg of body weight. It concentrated in rat lung tissue, with a lung tissue-to-plasma ratio of 29 based on the AUC. In acute systemic infections in mice, ABT-773 showed efficacy against macrolide-susceptible strains of Staphylococcus aureus, Streptococcus pneumoniae, S. pyogenes, and Listeria monocytogenes. Additionally, ABT-773 improved the survival of mice infected with resistant S. pneumoniae containing either the ermB gene, the mefE gene, or altered penicillin binding protein genes. In a rat lung model of infection, ABT-773 demonstrated 50% effective doses lower than those of comparator macrolides when evaluated against the following strains of S. pneumoniae: a macrolide-lincosamide-streptogramin B-susceptible strain, an ermB strain, and an mefE strain. ABT-773 was also effective against Haemophilus influenzae lung infections in rats. Thus, ABT-773 may prove to be a useful new antibacterial agent for the treatment of respiratory tract infections.
Multidrug-resistant Streptococcus pneumoniae is a major public health concern (7, 11, 14, 15, 17, 30, 31, 34). Significant morbidity and mortality occur from community-acquired pneumonia, meningitis, otitis media, and sinusitis. S. pneumoniae isolates are macrolide resistant due to the presence of either ermB or mefE determinants (20, 21, 26). The streptococcal ermB gene encodes an enzyme which methylates the ribosome, thus blocking macrolide binding. This leads to high-level resistance to macrolides-lincosamides-streptogramin B (MLSB resistance) (erythromycin MIC ≥ 64 μg/ml). The mefE gene encodes an active macrolide efflux pump which is associated with low-level resistance to 14- and 15-membered-ring macrolides (erythromycin MIC range, 1 to 32 μg/ml) (35). Macrolide resistance rates in S. pneumoniae have been reported to vary in different epidemiological settings (21, 30).
Resistance is not limited to streptococci. Thirty to forty percent of nontypeable Haemophilus influenzae isolates produce β-lactamase and are resistant to penicillins (16, 33). However, specific resistance mechanisms for macrolide resistance in H. influenzae, other than factors related to intrinsic activity, are rare.
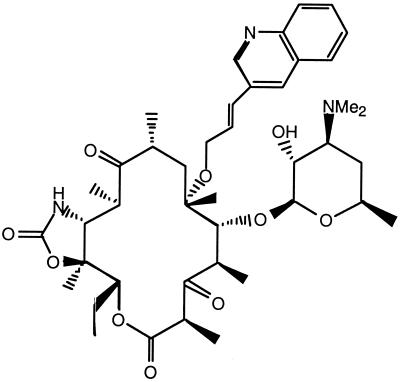
As bacterial resistance increases, there is need for new effective agents. Ketolides may provide physicians an additional therapeutic option (1, 5, 6, 8, 9, 10, 27). ABT-773, a novel ketolide synthesized at Abbott Laboratories, was designed to have activity against major resistant respiratory pathogens. The key chemical modifications to the erythromycin A core are a quinolylally moiety attached to the 6-O position, a keto group at the 3 position, and a cyclic carbamate group residing at the 11,12 position (Fig. 1). These structural changes have led to increased antimicrobial activity and improvements in the pharmacokinetic profile (25; Z. Ma, R. Clark, S. Wang, A. Nilius, R. Flamm, and Y. Or, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2133, 1999).
FIG. 1.
Structure of ABT-773 (molecular weight, 765,953).
The purpose of this paper is to report the therapeutic profile of ABT-773 in animal models. The value of these models is in providing insight through an overall assessment of factors influencing drug efficacy, such as potency, pharmacokinetics, and tissue penetration.
(Portions of this work were presented at the 9th European Congress of Clinical Microbiology and Infectious Diseases, Berlin, Germany, 1999, and at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 1999.)
MATERIALS AND METHODS
Antimicrobial agents.
ABT-773 and clarithromycin were synthesized at Abbott Laboratories, Abbott Park, Ill. Azithromycin was obtained from Pfizer Labs (New York, N.Y.), and penicillin G was obtained from Wyeth Laboratories Inc. (Philadelphia, Pa.).
Bacterial strains.
All bacterial strains were clinical isolates maintained in the culture collection of Abbott Laboratories or were obtained from the American Type Culture Collection (Manassas, Va.).
In vitro susceptibility test.
MICs for Staphylococcus aureus, S. pneumoniae, Streptococcus pyogenes, and H. influenzae were determined by broth microdilution methods described by the National Committee for Clinical Laboratory Standards (NCCLS). MICs for Listeria monocytogenes were determined by the agar dilution method on Mueller-Hinton agar supplemented with 5% sheep blood after incubation for 16 to 18 h at 35°C in ambient air (22).
In vivo tests.
The efficacy of ABT-773 was compared to that of penicillin and/or clarithromycin in murine models of acute bacterial infections as described previously (3, 12). In brief, CF1 female mice (Charles River, Wilmington, Mass.) weighing 20 to 25 g were inoculated intraperitoneally with overnight culture dilutions in normal saline of S. pyogenes C203, L. monocytogenes PIU 2526, S. pneumoniae 6303, and S. pneumoniae 6396. The remaining organisms with low virulence, S. aureus NTCC 10649, S. pneumoniae 6502, S. pneumoniae 5649, S. pneumoniae 5654, S. pneumoniae 5669, and S. pneumoniae 5979, were enhanced by diluting in 5% hog gastric mucin (American Laboratories, Omaha, Nebr.). All cultures were adjusted to yield approximately 100 times the 50% lethal dose (LD50). Concurrently with each trial, the LD50 was confirmed by inoculation of untreated mice with log10 dilutions of bacterial inoculum over a 5-log10 dilution range (10 mice per log10 dilution). ABT-773 was formulated in 2% ethanol with 1 mol equivalent of HCl (vol/vol) in 5% sterile dextrose water and was dosed orally. Clarithromycin was formulated in 2% ethanol (vol/vol) in phosphate-buffered saline (PBS), pH 6.8, and was dosed orally. Penicillin G was formulated in normal saline for injection and was administered by the subcutaneous route. For trials with S. aureus, S. pyogenes, and S. pneumoniae, mice were treated 1 and 5 h postchallenge. For the L. monocytogenes infection, therapy was delayed for 24 h to allow establishment of an intracellular infection, and compounds were administered 24 and 28 h postinoculation. Mortality and/or morbidity was recorded for 7 days, and the mean effective dose sufficient to protect 50% of the mice (ED50) was calculated from the cumulative mortality by trimmed-logit analysis (19, 32). There were 10 mice per dosage group. To evaluate drug efficacy in an organ-specific model of infection, ABT-773 was tested with clarithromycin and azithromycin in pulmonary infection models in rats (4, 18). Sprague-Dawley CD male rats (Charles River) weighing 190 to 230 g were inoculated intratracheally with 0.5 ml containing 105 to 108 CFU of S. pneumoniae or H. influenzae in 5% hog gastric mucin. A stainless steel feeding needle (20-gauge by 3 in., with a 2.25-mm-diameter ball) with the last 1 cm bent to a 45° angle was used to deliver the inoculum. Groups of 10 infected rats were treated with vehicle and served as the infected controls. Clarithromycin and ABT-773 were formulated as previously mentioned, while azithromycin was formulated in 2% ethanol (vol/vol) in PBS, pH 6.8. Three consecutive days of therapy with either ABT-773, azithromycin, or clarithromycin was initiated 18 h postinoculation with S. pneumoniae and 5 h postinoculation with H. influenzae. Oral treatments with ABT-773 or azithromycin were administered once daily (q.d.), while clarithromycin was administered twice daily (12 h apart). Delivering the total daily dose of clarithromycin 12 h apart gives a more accurate representation of its efficacy and has potential to increase the time at which the concentrations remain above the MIC. Twice-daily dosing or dosing with extended-release formulation of clarithromycin and once-a-day dosing with azithromycin are methods representative of how these drugs are administered in humans. Preliminary evaluations of ABT-773 suggested only a slight difference in efficacy between twice daily and once-a-day dosing. Drug efficacy was determined by measuring the reduction of bacterial burden compared to that observed in vehicle-treated controls. Lungs were removed from the animals 12 h after the last clarithromycin treatment or 24 h after the last ABT-773 or azithromycin treatment on day 4 for S. pneumoniae and day 3 for H. influenzae infections. Lungs were aseptically removed and homogenized in 5 ml of sterile PBS with a sterile tissue homogenizer (Tekmar, Cincinnati, Ohio). Bacterial burden was assessed from log dilution plating (1/50 to 1/500,000) of the homogenates on chocolate agar plates for H. influenzae and Columbia agar-nalidixic acid plates for S. pneumoniae. Agar plates were incubated at 37°C in 5% CO2 for 24 h to allow the visualization and counting of colonies. The minimum number of bacteria detectable by this method is 50 CFU/lung pair. Calculation of asymptotic standard errors and analysis of variance were conducted for the dosage groups, and 95% confidence intervals were generated for ED50s for producing a 2- or 3-log10 reduction in bacteria compared to the mean burden observed in the vehicle-treated control (2, 28). The analysis of bacterial reduction is an acceptable interpretation of results obtained from an organ burden trial. An analysis based only on bacterial clearance or cures focuses on the maximum effective doses while excluding the rest of the dosage-response curve. Determining the drug dose required to show a 2- or 3-log reduction allows more influence by individual animals which show multiple-log reductions but are not cured.
Pharmacokinetics.
Single- and multiple-oral-dose pharmacokinetic studies were conducted with 190- to 230-g Sprague-Dawley CD male rats (Charles River). ABT-773 was solution formulated in 2% ethanol with 1 mol equivalent of HCl (vol/vol) in 5% sterile dextrose water. Clarithromycin and azithromycin were formulated in 2% ethanol (vol/vol) in PBS, pH 6.8. The dosages of each compound were selected as those which generally produce a 2-log reduction of H. influenzae using the pulmonary infection model in rats. In the multidose trial, ABT-773 and azithromycin were administered every 24 h, for a total of three dosings at 25 and 50 mg/kg of body weight, respectively. Clarithromycin was administered at 12-h intervals, with a total of five dosings at 50 mg/kg. Plasma and lung samples were collected at 0.5, 1, 2, 3, 6, 9, 12, and 24 h after the last dose. Three rats were used for each time point. The entire lung tissues were removed, weighed, rinsed free of blood with 0.9% NaCl, diluted 1:2 (wt/vol) in distilled water, and homogenized thoroughly. A 0.5-ml aliquot is extracted using the same methods used for plasma (see below). A hemoglobin count was determined to account for blood contribution to drug tissue levels. Blood samples were chilled immediately and centrifuged under refrigeration, and the plasma, along with lung tissue homogenates, was frozen within 1 h of collection. Drug concentrations in lung homogenate and plasma were determined by high-pressure liquid chromatography. Samples of 0.2 to 0.5 ml of plasma plus 50 μl of internal standard (proprietary ketolide) plus 0.5 ml of 0.5 M Na2CO3 were extracted with 6.0 ml of ethyl acetate-hexane (1:1, vol/vol). The organic layer was evaporated to dryness with nitrogen at room temperature while protected from light. Samples were reconstituted with acetonitrile–0.05 M phosphate buffer, pH 6.0 (1:2, vol/vol). Chromatographic conditions consisted of a YMC basic column (250 mm by 4.6 mm by 5 μm) with an acetonitrile:methanol-buffer-mobile phase (45:5:60, by volume) at a flow rate of 1.0 ml/min. The mobile phase buffer (pH 6.0) contained 0.01 M tetramethylammonium hydroxide in 0.05 M KH2PO4. Detection of analytes was accomplished by fluorescence at an excitation wavelength of 324 nm and an emission wavelength of 364 nm. The analytical method was linear over the concentration range 0.005 to 5.18 μg/ml. The precision expressed, as relative standard deviation, was <2.9% for triplicate analysis. The efficiency of extraction averaged 89.5%.
RESULTS
Efficacies of ABT-773 against infections caused by macrolide-susceptible respiratory and intracellular pathogens in mice.
In these studies, a mortality rate of 100% for each infection was produced in all groups of untreated mice. The ED50s of ABT-773 were consistently lower than those of clarithromycin against infections caused by macrolide-susceptible respiratory or intracellular pathogens (Table 1). Against S. aureus, the oral ED50 of ABT-773 was 9.4 mg/kg, compared to 30.2 mg/kg for clarithromycin. The ED50s of ABT-773 were approximately threefold lower than those of clarithromycin against S. pneumoniae (10.0 and 37.3 mg/kg, respectively). The ED50s generated for ABT-773 and clarithromycin against S. pyogenes were not significantly different, and the 95% confidence limits overlapped (1.9 and 3.7 mg/kg, respectively). The ED50 of ABT-773 was 100.1 mg/kg for intracellular infections with L. monocytogenes, while no efficacy was observed at the highest clarithromycin dose (200 mg/kg).
TABLE 1.
Oral efficacy of ABT-773 against important respiratory and intracellular bacterial infections in miceb
| Bacterium and compound | MIC (μg/ml) | ED50a (95% confidence limits) |
|---|---|---|
| S. aureus 10649c | ||
| ABT-773 | 0.03 | 9.4 (6.0–14.8) |
| Clarithromycin | 0.25 | 30.2 (20.9–43.8) |
| S. pneumoniae 6303d | ||
| ABT-773 | 0.001 | 10.0 (5.5–18.2) |
| Clarithromycin | 0.008 | 37.3 (22.8–60.8) |
| S. pyogenes C203e | ||
| ABT-773 | 0.002 | 1.9 (1.1–3.3) |
| Clarithromycin | 0.03 | 3.7 (2.3–6.1) |
| L. monocytogenes 2526f | ||
| ABT-773 | 0.03 | 100.1 (63.2–158.4) |
| Clarithromycin | 0.12 | >200 (NCg) |
Dosage (in milligrams per kilogram per day) required to protect 50% of the mice against inoculation with 100 times the LD50.
Groups of untreated mice showed 100% mortality.
Challenge inoculum of log10 5.1.
Challenge inoculum of log10 2.3.
Challenge inoculum of log10 5.4.
Challenge inoculum of log10 6.6.
NC, not calculated.
Efficacies of ABT-773 against systemic infections caused by resistant S. pneumoniae in mice.
The relative efficacies of ABT-773 and the reference standards, clarithromycin and penicillin, against acute infections in mice caused by resistant S. pneumoniae are reported in Table 2. Against S. pneumoniae strain 6502, which is MLSB susceptible and penicillin resistant, the ED50s of ABT-773, clarithromycin, and penicillin were 12.4, 16.6, and 167.4 mg/kg, respectively. Against S. pneumoniae strain 5649, which is resistant to macrolides via the mefE gene and is penicillin intermediate, the ED50s of ABT-773, clarithromycin, and penicillin were 50.1, >100, and <37.5 mg/kg, respectively. Against S. pneumoniae strain 5654, which also has an mefE gene and is penicillin resistant, the ED50s of ABT-773, clarithromycin, and penicillin were 52.8, >100, and 108.9 mg/kg, respectively. Against S. pneumoniae strain 6396, which shows constitutive macrolide resistance due to the presence of an ermB gene and is penicillin susceptible, the ED50s generated for ABT-773, clarithromycin, and penicillin were 50.1, >200, and 22.2 mg/kg. Against S. pneumoniae strain 5669, which also has an ermB gene and is penicillin intermediate, the ED50s of ABT-773, clarithromycin, and penicillin were 14.7, 123.8, and 24.1 mg/kg, respectively. Against S. pneumoniae strain 5979, which has an ermB gene and is penicillin resistant, ABT-773 demonstrated 50% protection of mice at 10.3 mg/kg while clarithromycin and penicillin showed no efficacy at the highest dosages given (200 and 800 mg/kg, respectively).
TABLE 2.
Oral efficacy of ABT-773 against bacterial infections caused by resistant S. pneumoniaeb in mice
| Strain (characteristics) and compound | MIC (μg/ml) | ED50a (95% confidence range) |
|---|---|---|
| 6502c (MLSs Penr) | ||
| ABT-773 | 0.001 | 12.4 (7.3–21.0) |
| Clarithromycin | 0.004 | 16.5 (9.0–30.3) |
| Penicillin | 2 | 167.4 (99.0–283.0) |
| 5649d (mefE, Peni) | ||
| ABT-773 | 0.06 | 50.1 (31.6–79.2) |
| Clarithromycin | 4 | >100 (NCi) |
| Penicillin | 0.5 | <37.5 (NC) |
| 5654e (mefE, Penr) | ||
| ABT-773 | 0.06 | 52.8 (27.2–102.5) |
| Clarithromycin | 4 | >100 (NC) |
| Penicillin | 2 | 108.9 (71.5–165.8) |
| 6396f (ermB, Pens) | ||
| ABT-773 | 0.015 | 50.1 (31.6–79.2) |
| Clarithromycin | >128 | >200 (NC) |
| Penicillin | 0.06 | 22.2 (14.1–35.0) |
| 5669g (ermB, Peni) | ||
| ABT-773 | 0.015 | 14.7 (6.0–36.0) |
| Clarithromycin | >128 | 123.8 (70.2–218.4) |
| Penicillin | 1 | 24.1 (12.5–46.4) |
| 5979h (ermB, Penr) | ||
| ABT-773 | 0.015 | 10.3 (7.1–15.0) |
| Clarithromycin | >128 | >200 (NC) |
| Penicillin | 4 | >800 (NC) |
Dosage (in milligrams per kilogram per day) required to protect 50% of the mice against inoculation with 100 times the LD50.
Groups of untreated mice showed 100% mortality.
Challenge inoculum of log10 7.1.
Challenge inoculum of log10 6.4.
Challenge inoculum of log10 6.2.
Challenge inoculum of log10 2.4.
Challenge inoculum of log10 5.6.
Challenge inoculum of log10 5.4.
NC, not calculated.
Efficacy of ABT-773 against S. pneumoniae-associated lung infections in rats.
The efficacy of ABT-773 was consistently better than those of the comparative macrolides, azithromycin, and clarithromycin against pulmonary infection in rats caused by susceptible or resistant (mefE or ermB) strains of S. pneumoniae (Table 3). After three consecutive days of oral dosing at 2.5 mg/kg/day, ABT-773 produced a mean 4.68-log10 reduction in bacterial lung burden compared to the vehicle-treated control group (P < 0.001) when an MLSB-susceptible stain (ATCC 6303) was tested. The ED50s that produced a 3-log10 reduction in viable bacterial count compared to the vehicle-treated controls for ABT-773, azithromycin, and clarithromycin were 1.2, 6.0, and 6.2 mg/kg/day, respectively, for this strain. Against the macrolide-resistant S. pneumoniae strain 5649, which has an efflux pump encoded by the mefE gene, ABT-773 produced a mean 4.45-log10 reduction in lung burden compared to the vehicle-treated control group when administered at 20 mg/kg/day (P < 0.001). The 3-log10 reduction ED50 of ABT-773 was 15.1 mg/kg/day, while the ED50s of azithromycin and clarithromycin were 78.7 and 69.7 mg/kg/day, respectively. S. pneumoniae strain 6396 has an ermB gene that causes constitutive MLSB resistance. The MICs of azithromycin and clarithromycin were >128 μg/ml. There was no significant reduction in bacterial lung burden in rats inoculated with S. pneumoniae strain 6396 after treatment with either azithromycin or clarithromycin at 100 mg/kg/day. ABT-773 (MIC, 0.015 μg/ml) produced a significant reduction in lung burden when administered at 10 mg/kg/day (P < 0.001). The 3-log10 reduction ED50 of ABT-773 was 2.3 mg/kg/day.
TABLE 3.
Efficacies of ABT-773 and comparative agents in experimental S. pneumoniae-associated lung infections in rats
| S. pneumoniae strain (characteristic) and compound | Daily dosee (mg/kg) | MIC (μg/ml) | Log10 CFU/lung (mean ± SE) | Pg | ED50h (95% confidence limits)
|
|
|---|---|---|---|---|---|---|
| 2-log10 reduction | 3-log10 reduction | |||||
| 6303 (MLSBs)a | ||||||
| Vehicle controld | NAi | NA | 8.13 ± 0.08 | NA | NA | |
| ABT-773 | 0.6 | 0.001 | 5.89 ± 0.67 | 0.029 | <0.6 (NC)j | 1.2 (1.2–1.3) |
| 2.5 | 3.45 ± 0.25 | <0.001 | ||||
| 10 | 3.27 ± 0.19 | <0.001 | ||||
| Azithromycin | 3.1 | 0.03 | 6.38 ± 0.52 | 0.027 | 3.7 (2.3–5.9) | 6.0 (3.8–9.6) |
| 12.5 | 2.33 ± 0.82 | 0.002 | ||||
| 50 | 1.14 ± 0.47 | <0.001 | ||||
| Clarithromycinf | 3.1 | 0.008 | 6.38 ± 0.85 | 0.109 | 3.7 (2.3–6.2) | 6.2 (3.8–10.3) |
| 12.5 | 2.60 ± 0.82 | 0.002 | ||||
| 50 | 2.33 ± 0.15 | <0.001 | ||||
| 5649 (mefE)b | ||||||
| Vehicle control | NA | NA | 5.37 ± 0.47 | NA | NA | |
| ABT-773 | 1.25 | 0.06 | 4.99 ± 0.70 | 0.65 | 11.8 (6.6–21.1) | 15.1 (8.4–27.1) |
| 5 | 5.39 ± 0.97 | 0.98 | ||||
| 20 | 0.92 ± 0.57 | <0.001 | ||||
| Azithromycin | 6.3 | 8 | 6.20 ± 0.70 | 0.26 | 24.9 (20.7–30.1) | 78.7 (62.2–99.5) |
| 25 | 3.37 ± 0.46 | 0.019 | ||||
| 100 | 1.98 ± 0.53 | <0.001 | ||||
| Clarithromycin | 6.3 | 4 | 4.66 ± 0.22 | 0.37 | 41.9 (39.3–44.7) | 69.7 (65.3–74.3) |
| 25 | 3.98 ± 0.35 | 0.083 | ||||
| 100 | 1.28 ± 0.55 | <0.001 | ||||
| 6396 (ermB)c | ||||||
| Vehicle control | NA | NA | 6.48 ± 0.31 | NA | NA | |
| ABT-773 | 0.6 | 0.015 | 4.75 ± 0.39 | 0.006 | 1.0 (0.6–1.6) | 2.3 (1.4–3.7) |
| 2.5 | 3.29 ± 0.07 | <0.001 | ||||
| 10 | 2.81 ± 0.28 | <0.001 | ||||
| Azithromycin | 10 | >128 | 6.75 ± 0.59 | 0.66 | >100 (NC) | >100 (NC) |
| 30 | 5.55 ± 0.59 | 0.14 | ||||
| 100 | 7.39 ± 0.63 | 0.17 | ||||
| Clarithromycin | 10 | >128 | 5.78 ± 0.64 | 0.28 | >100 (NC) | >100 (NC) |
| 30 | 6.61 ± 0.29 | 0.79 | ||||
| 100 | 7.76 ± 0.13 | 0.003 | ||||
Intratracheal inoculum of log10 5.8.
Intratracheal inoculum of log10 7.2.
Intratracheal inoculum of log10 5.9.
Vehicle-treated infected control rats were dosed orally for 3 days.
Rats were orally dosed daily for 3 days, starting 18 h postinfection.
Clarithromycin total daily dose given half strength 12 h apart.
Statistical difference for comparison with result for untreated control, determined by the t test or Mann-Whitney U test.
The ED50 (in milligrams per kilogram per day) to produce a 2- or 3-log10 reduction in bacterial count compared to the vehicle-treated controls using linear regression of the group means.
NA, not applicable.
NC, not calculated.
Efficacy of ABT-773 against H. influenzae-associated lung infections in rats.
The efficacy of ABT-773 was consistently improved over those of the comparative macrolides, azithromycin, and clarithromycin against pulmonary infection in rats caused by H. influenzae (Table 4). Against H. influenzae strain 1435, ABT-773 produced a mean 3.32-log10 reduction in lung burden compared to the vehicle-treated control group when administered at 60 mg/kg/day for 3 consecutive days (P < 0.001). The 2-log10 reduction in ED50 of ABT-773 was 19.8 mg/kg/day, while the ED50s of azithromycin and clarithromycin were 55.4 and 109.8 mg/kg/day, respectively. The antibacterial effect of ABT-773 was demonstrated against H. influenzae strain 3643 as a 4.00-log10 reduction in lung burden compared to that observed with the vehicle-treated control group when administered at 60 mg/kg/day (P = 0.002). The 2-log10 reductions in ED50s of ABT-773, azithromycin, and clarithromycin were 29.0, 44.2, and 67.8 mg/kg/day, respectively.
TABLE 4.
Efficacies of ABT-773 and comparative agents in experimental H. influenzae-associated lung infections in rats
| H. influenzae strain and compound | Daily dosed (mg/kg) | MIC (μg/ml) | Log10 CFU/lung (mean ± SEM) | Pf | ED50g (95% confidence limits)
|
|
|---|---|---|---|---|---|---|
| 2-log10 reduction | 3-log10 reduction | |||||
| 1435a | ||||||
| Vehicle controlc | NAh | NA | 5.23 ± 0.40 | NA | NA | |
| ABT-773 | 5 | 0.5 | 6.38 ± 0.66 | 0.14 | 19.8 (15.8–24.8) | 50.0 (35.6–70.3) |
| 20 | 3.19 ± 0.36 | 0.006 | ||||
| 60 | 1.91 ± 0.51 | <0.001 | ||||
| Azithromycin | 5 | 1 | 6.17 ± 0.61 | 0.21 | 55.4 (33.0–92.8) | 70.3 (41.9–117.8) |
| 25 | 5.27 ± 1.08 | 0.97 | ||||
| 80 | 1.58 ± 0.65 | <0.001 | ||||
| Clarithromycine | 10 | 2 | 4.94 ± 0.35 | 0.65 | 109.8 (87.8–137.4) | >120 (NCi) |
| 40 | 6.15 ± 0.64 | 0.23 | ||||
| 120 | 2.81 ± 0.16 | <0.001 | ||||
| 3643b | ||||||
| Vehicle control | NA | NA | 4.50 ± 0.38 | NA | NA | |
| ABT-773 | 5 | 2 | 4.86 ± 0.19 | 0.20 | 29.0 (28.7–29.2) | 44.5 (44.1–44.8) |
| 20 | 3.08 ± 0.33 | 0.02 | ||||
| 60 | 0.50 ± 0.50 | 0.002 | ||||
| Azithromycin | 5 | 1 | 4.57 ± 0.30 | 0.55 | 44.2 (44.0–44.5) | 67.3 (67.0–67.7) |
| 25 | 3.33 ± 0.35 | 0.06 | ||||
| 80 | 0.95 ± 0.59 | 0.002 | ||||
| Clarithromycin | 10 | 4 | 4.44 ± 0.30 | 0.89 | 67.8 (66.1–69.6) | 101.4 (98.8–104.0) |
| 40 | 3.33 ± 0.43 | 0.06 | ||||
| 120 | 0.94 ± 0.58 | 0.002 | ||||
Intratracheal inoculum of log10 7.8.
Intratracheal inoculum of log10 8.6.
Vehicle-treated infected control rats were dosed orally for 3 days.
Rats were orally dosed daily for 3 days, starting 18 h postinfection.
Clarithromycin total daily dose given half strength 12 h apart.
Statistical difference for comparison with result for untreated control, determined by the t test or Mann-Whitney U test.
The ED50 (in milligrams per kilogram per day) to produce a 2- or 3-log10 reduction in bacterial count compared to the vehicle-treated controls using linear regression of the group means.
NA, not applicable.
NC, not calculated.
Pharmacokinetics.
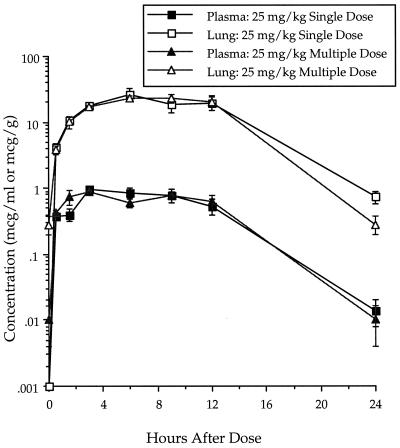
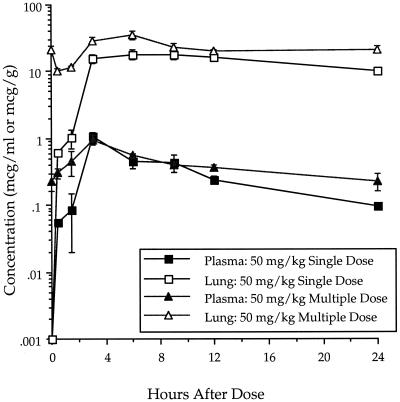
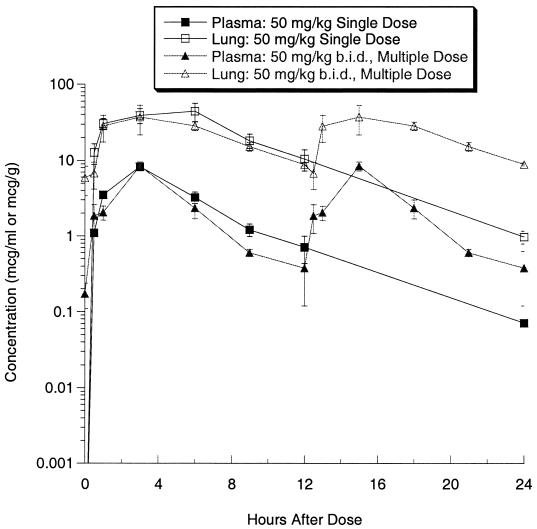
The concentrations of ABT-773 in plasma and lung were determined after single and multiple (once daily [q.d.] for 3 days) oral dosing of 25 mg/kg in rats (Fig. 2). The single-dose maximum concentration of drug (Cmax) and area under the concentration-time curve (AUC) in plasma were 1.07 μg/ml and 12.03 μg · h/ml, respectively, while the Cmax and AUC in lung were 32.11 μg/ml and 350.5 μg · h/ml, respectively (Table 5). After multiple dosing, the Cmax and AUC in plasma were 0.94 μg/ml and 12.13 μg · h/ml, respectively, while the Cmax and AUC in lung were 27.10 μg/ml and 351.3 μg · h/ml, respectively. The pharmacokinetics were, therefore, similar between single and multiple dosing. ABT-773 also accumulated to higher concentrations in lung tissue relative to the concentrations in plasma with both single and multiple doses. After multiple dosing (q.d. for 3 days) of azithromycin at 50 mg/kg, the Cmax and AUC in plasma were 0.97 μg/ml and 14.0 μg · h/ml, respectively, while the Cmax and AUC in lung were 37.5 μg/ml and 765.9 μg · h/ml, respectively (Table 5; Fig. 3). When clarithromycin was delivered twice a day with five dosages delivered over 48 h, the Cmax and AUC in plasma were 8.4 μg/ml and 68.1 μg · h/ml, respectively, while the Cmax and AUC in lung were 51.1 μg/ml and 554.9 μg · h/ml, respectively (Table 5; Fig. 4).
FIG. 2.
ABT-773 concentrations in plasma and lung after single and multiple 25-mg/kg oral dosing in rats. Error bars, standard errors.
TABLE 5.
Drug concentrations in plasma and lung after single and multiple oral dosing in rats
| Compound (dosea) and compartment | Single dose
|
Multiple doseb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmaxc | C24c | Tmaxd (h) | t1/2 (h) | AUC24e | Cmaxc | C24c | Tmax (h) | t1/2 (h) | AUC24e | |
| ABT-773 (25) | ||||||||||
| Plasma | 11.07 | 0.014 | 6.0 | 2.8 | 12.0 | 0.94 | 0.010 | 8.0 | 2.3 | 12.1 |
| Lung | 32.11 | 0.746 | 9.0 | 3.3 | 350.6 | 27.10 | 0.284 | 9.0 | 2.6 | 351.3 |
| T/Pf | 29.2 | 29.0 | ||||||||
| Azithromycin (50) | ||||||||||
| Plasma | 1.08 | 0.096 | 3.0 | 6.5 | 8.6 | 0.97 | 0.233 | 3.0 | 11.6 | 14.0 |
| Lung | 21.41 | 10.30 | 9.0 | 21.4 | 644.8 | 37.5 | 21.17 | 5.0 | 7.3 | 765.9 |
| T/P | 75.4 | 54.7 | ||||||||
| Clarithromycin (50) | ||||||||||
| Plasma | 8.20 | 0.165 | 3.0 | 3.15 | 44.8 | 8.4 | 0.168 | 3.0 | 8.9 | 68.1 |
| Lung | 55.27 | 1.105 | 5.0 | 3.53 | 413.8 | 51.13 | 1.023 | 3.3 | 12.5 | 554.9 |
| T/P | 9.2 | 8.1 | ||||||||
Dose in milligrams per kilogram.
Administered q.d. for 3 days or twice daily for 5 days.
Measured in micrograms per milliliter (plasma) or micrograms per gram (lung).
Time to Cmax.
Measured in micrograms · hours per milliliter (plasma) or micrograms · hours per gram (lung).
T/P, ratio of tissue AUC24 to plasma AUC24.
FIG. 3.
Azithromycin concentrations in plasma and lung after single and multiple 50-mg/kg oral dosing in rats. Error bars, standard errors.
FIG. 4.
Clarithromycin concentrations in plasma and lung after single and multiple 50-mg/kg oral dosing in rats. Error bars, standard errors.
DISCUSSION
To gain a better understanding of the therapeutic potential of ABT-773, evaluations have been undertaken in rodent models of infection. The lethal systemic murine model gives an initial insight into a compound's properties of oral absorption, adequacy of serum concentration, and inhibition of bacterial growth. Mice were inoculated with a bacterial challenge designed to overwhelm the host's capacity to clear the infection without therapy. The net antibacterial effect as measured by increased survival is therefore presumed to be due to the effects of the antimicrobial agent.
The analysis of bacterial reduction is an acceptable interpretation of results obtained from an organ burden trial. An analysis based only on bacterial clearance or cures focuses on the maximum effective doses while excluding the rest of the dosage-response curve. Determining the drug dose required to show 99.0 or 99.9% reduction allows more influence by individual animals which show multiple-log reductions in bacterial burden but are not cured. In an effort to evaluate efficacies of ABT-773 against respiratory pathogens, the rat pulmonary infection model was used. In experimental rat lung infections caused by S. pneumoniae and H. influenzae, both a ≥2- and a ≥3-log10 reduction in lung bacterial burden were evaluated. Both ≥2- and ≥3-log10 reductions in bacterial burden have traditionally been considered to be significant reductions in experimental studies (2, 4).
ABT-773 showed improved efficacy over clarithromycin against macrolide-susceptible strains of S. aureus, S. pneumoniae, and L. monocytogenes and equivalent efficacy compared to clarithromycin against S. pyogenes in the lethal systemic mouse model. The range in ED50s of ABT-773 against susceptible respiratory pathogens was 1.9 to 10 mg/kg. ABT-773 was also effective in increasing the survival of mice inoculated with the intracellular bacterium, L. monocytogenes (Table 1). The improved efficacy of ABT-773 in comparison to clarithromycin against these gram-positive species correlates with the improved antibacterial activity of the compound in vitro (Table 6) (24).
TABLE 6.
In vitro susceptibilities of bacterial strains used in rodent modelsa
| Bacterium | MIC (μg/ml) of:
|
|||
|---|---|---|---|---|
| ABT-773 | Clarithromycin | Azithromycin | Penicillin | |
| S. aureus ATCC 10649 (MLSBs) | 0.03 | 0.25 | ||
| S. pyogenes C203 (MLSBs) | 0.002 | 0.03 | ||
| L. monocytogenes 2526 | 0.03 | 0.12 | ||
| S. pneumoniae 6303 (MLSBs) | 0.001 | 0.008 | 0.03 | |
| S. pneumoniae 6502 (MLSBs Penr) | 0.001 | 0.004 | 2 | |
| S. pneumoniae 5649 (mefE, Peni) | 0.06 | 4 | 8 | 0.5 |
| S. pneumoniae 5654 (mefE, Penr) | 0.06 | 4 | 2 | |
| S. pneumoniae 6396 (ermB, Pens) | 0.015 | >128 | >128 | 0.06 |
| S. pneumoniae 5669 (ermB, Peni) | 0.015 | >128 | 1 | |
| S. pneumoniae 5979 (ermB, Penr) | 0.015 | >128 | 4 | |
| H. influenzae 1435 | 0.5 | 2 | 1 | |
| H. influenzae 3643 | 2 | 4 | 1 | |
MICs were determined by the broth microdilution method for all isolates except for L. monocytogenes for which the NCCLS agar dilution method was used.
In addition to its efficacy for treatment of susceptible S. pneumoniae in a systemic infection model, ABT-773 also demonstrated a fivefold improvement in ED50 compared to other macrolides when evaluated against the susceptible strain of S. pneumoniae in the lung infection model in rats. ABT-773 also effectively reduced the bacterial lung burden in rats inoculated with H. influenzae. The in vivo potency of ABT-773 was greater than that of azithromycin against H. influenzae strain 3643; however, statistical differences were not demonstrated against the H. influenzae strain 1435 after comparing ED50s of 3-log10 reductions (Table 4).
Establishing an experimental H. influenzae infection in the rat lung is difficult as the natural host defenses of the rat are capable of clearing the infection. As shown in this study, the initial inoculum in the rat lung was 107 to 108 CFU, yet at the end of the study the mean number of CFU in the vehicle control animals was only in the range of 4.5 to 5.2 log10/lung. As bacterial reduction is being measured in an experimental model which has a decreasing background of organisms, it is difficult to predict how the effective dose in the rodent will correlate with the effective dose in humans. For this reason both the 2- and 3-log10 reductions in CFU were measured. These will ultimately be compared to the dose that demonstrates efficacy in the phase II and III clinical trials. Until then, we must be careful in extrapolation of these data for predicting the effective dose in humans.
Successful treatment of pulmonary infections, particularly those caused by H. influenzae, can typically be explained by the significant levels in tissue which are obtained with macrolides. The intrapulmonary pharmacokinetics of orally administered clarithromycin (500 mg every 12 h) in humans revealed a range in lung epithelial lining fluid-to-plasma ratios of 13.3 to 27.6 based on clarithromycin concentrations obtained at 4 and 8 h post-final dosing (13). The actual concentrations of clarithromycin in the epithelial lining fluid were 29.3 and 72.1 μg/ml, respectively. The range in alveolar cell-to-plasma ratio was even higher at 98.7 to 229.9. The actual alveolar macrophage concentrations were 505.8 and 256.7 μg/ml, respectively (13). Healthy volunteers receiving five oral doses of azithromycin beginning with a dose of 500 mg on the first day and then 250 mg once daily for the next 4 days revealed lung epithelial lining fluid-to-plasma ratios of 12.6 and 24.2 based on assessments at 4 and 8 h post-final dosing, respectively (29). The actual concentrations of azithromycin in the epithelial lining fluid were 1.01 and 2.18 μg/ml, respectively. The alveolar cell-to-plasma ratios were 533.8 and 635.6. The actual alveolar macrophage concentrations were 42.7 and 57.2 μg/ml at 4 and 8 h, respectively (29).
Determining the drug levels in plasma and lung tissue may be useful for understanding efficacy studies in rats. The pharmacokinetic profile of ABT-773, after single or multiple dosing at or near the dosage resulting in a 2-log reduction in lung bacterial burden for H. influenzae (25 mg/kg), produced a lung tissue-to-plasma AUC ratio of 29 (Table 5). The plasma and lung tissue AUCs were 12.03 and 350.5 μg · h/ml, respectively. This preliminary study demonstrated that the pharmacokinetics of ABT-773 includes a significant accumulation in lung tissue which is similar to those observed with other macrolides (23). Determining the free drug concentrations in lung epithelial lining fluid and intracellular concentrations extrapolated from alveolar macrophages in rats may be beneficial since these levels can be assessed in humans and may be important predictors of successful therapeutic outcomes (29). There is a need for sufficient drug levels in intracellular as well as extracellular compartments since some pathogens can be found in both (4). Studies have been planned to determine these levels for ABT-773 in both humans and rats.
The 50-mg/kg dose of azithromycin will generally produce a 2-log10 reduction in lung Haemophilus burden in rats. The single-dose Cmax and AUC at 24 h (AUC24) in plasma were 1.08 μg/ml and 8.6 μg · h/ml, respectively (Table 5). These values are approximately twofold higher than the reported Cmax of 0.4 μg/ml and AUC24 of 3.4 μg · h/ml following a single 500-mg dose of azithromycin in humans (36). The clarithromycin concentrations in plasma following a 50-mg/kg single dose in rats (Cmax of 8.2 μg/ml and AUC24 of 44.8 μg · h/ml) are also approximately twofold higher than the combined concentrations in plasma of clarithromycin and its bioactive metabolite (14-hydroxyclarithromycin not found in rodents), resulting in a Cmax of 3.4 μg/ml and an AUC24 of 25 μg · h/ml (4, 36). The plasma elimination half-lives (t1/2s) of both azithromycin and clarithromycin are shorter in rats than in humans.
When macrolide-susceptible strains of S. pneumoniae were tested, the ABT-773 MICs at which 90% of the strains are inhibited (MIC90s) of 0.002 μg/ml were identical for penicillin-susceptible and penicillin-nonsusceptible strains (24). When evaluated against multidrug-resistant strains of S. pneumoniae, the ABT-773 MIC90s are ≤0.008, 0.03, and 0.12 μg/ml for penicillin-susceptible, penicillin-intermediate, and penicillin-resistant strains, respectively (8). Reduced susceptibility to penicillin is an emerging clinical problem. In a recent surveillance study, 29.5% of S. pneumoniae isolates were not susceptible to penicillin (15). ABT-773 was compared to penicillin against S. pneumoniae using the lethal systemic mouse model. Penicillin had an ED50 of 22.2 mg/kg against a penicillin-susceptible strain (MIC = 0.06 μg/ml), but the range in ED50s increased to 108.9 to >800 mg/kg for resistant strains with altered penicillin binding proteins (penicillin MIC ≥ 2 μg/ml). In contrast, ABT-773 was equally effective at treating the penicillin-susceptible and penicillin-resistant isolates. The efficacy results correlate with the in vitro antibacterial activity.
The efficacy of ABT-773 was consistently better than those of the comparative macrolide antibiotics against S. pneumoniae-induced infections in rodents caused by resistant (mefE or ermB) strains. Higher macrolide doses were required in mice and rats to achieve a similar therapeutic response against resistant strains than were required against susceptible strains. The susceptibilities to ABT-773 of the S. pneumoniae strains used in infections in experimental animal models were comparable to those of isolates evaluated in the 1997-to-1998 national surveillance study in which the MIC90 of ABT-773 for 1,601 strains was 0.03 μg/ml (8). Using the lethal systemic infection model caused by S. pneumoniae in mice, clarithromycin had an ED50 of 16.5 mg/kg against an MLSB-susceptible strain, but the range in ED50s increased to 123.8 to >200 mg/kg against ermB or mefE strains (clarithromycin MIC ≥ 4 μg/ml). ABT-773 was more effective at prolonging survival. It showed a range in ED50s of 10.3 to 52.8 mg/kg against macrolide-susceptible, ermB, or mefE strains of S. pneumoniae. In rat lung infections, ABT-773 demonstrated >4-fold improved efficacy over azithromycin and clarithromycin, as measured by organ bacterial burden reduction, against the mefE-bearing S. pneumoniae (azithromycin, clarithromycin, and ABT-773 MICs = 8, 4, and 0.06 μg/ml, respectively). In lung infections associated with MLSB-resistant S. pneumoniae (ermB), ABT-773 showed superior efficacy compared to the macrolide comparators (azithromycin, clarithromycin, and ABT-773 MICs = >128, >128, and 0.015 μg/ml, respectively). Thus, the efficacies of ABT-773 correlated well with the improved antibacterial activities of ABT-773 against macrolide-susceptible and -resistant strains in comparison with those of other macrolides.
The data presented in this paper demonstrate the utility of the new ketolide ABT-773 for treatment of susceptible and resistant respiratory tract pathogens. The efficacy achieved in systemic or pulmonary infections in rodents can be used as a baseline for future pharmacodynamic investigations in animals. Such studies can provide guidance in evaluating potential clinical doses and efficacy in humans.
ACKNOWLEDGMENTS
We acknowledge Lenette Paige, Weiguo Qing, Yi-Chun Wang, and Sean Wilson for technical assistance with animal experiments. We also thank Alan Dutkiewicz, Todd Howard, Gina Augustine, and Guadalupe Medina for excellent animal husbandry and care.
REFERENCES
- 1.Agouridas C, Bonnefoy A, Chantot J F. Antibacterial activity of RU 64004 (HMR 3004), a novel ketolide derivative active against respiratory pathogens. Antimicrob Agents Chemother. 1997;41:2149–2158. doi: 10.1128/aac.41.10.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alder J, Clement J. Comparative chemotherapeutic activity of temafloxacin, cefoxitin, clindamycin, imipenem and ampicillin/sulbactam against Bacteroides fragilis in a mouse subcutaneous abscess model. J Antimicrob Chemother. 1993;31:303–311. doi: 10.1093/jac/31.2.303. [DOI] [PubMed] [Google Scholar]
- 3.Alder J, Clement J, Meulbroek J, Shipkowitz N, Mitten M, Jarvis K, Oleksijew A, Hutch T, Paige L, Flamm B, Chu D, Tanaka K. Efficacies of ABT-719 and related 2-pyridone, members of a new class of antibacterial agents, against experimental bacterial infections. Antimicrob Agents Chemother. 1995;39:971–975. doi: 10.1128/aac.39.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alder J D, Ewing P, Nilius A, Mitten M, Tovcimak A, Oleksijew A, Jarvis K, Paige L, Tanaka S K. Dynamics of clarithromycin and azithromycin efficacies against experimental Haemophilus influenzae pulmonary infection. Antimicrob Agents Chemother. 1998;42:2385–2390. doi: 10.1128/aac.42.9.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry A L, Fuchs P C, Brown S D. In vitro activity of the new ketolide HMR 3004 compared to an azalide and macrolides against Streptococcus pneumoniae and Haemophilus influenzae. Eur J Clin Microbiol Infect Dis. 1997;16:767–769. doi: 10.1007/BF01709263. [DOI] [PubMed] [Google Scholar]
- 6.Biedenbach D J, Barrett M S, Jones R N. Comparative antimicrobial activity and kill-curve investigations of novel ketolide antimicrobial drugs (HMR 3004 and HMR 3647) tested against Haemophilus influenzae and Moraxella catarrhalis strains. Diagn Microbiol Infect Dis. 1998;31:349–353. doi: 10.1016/s0732-8893(98)00022-4. [DOI] [PubMed] [Google Scholar]
- 7.Breiman R F, Butler J C, Tenover F C, Eliott J A, Facklam R R. Emergence of drug-resistance pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 8.Brueggeman A B, Doern G, Huynh H, Wingert E, Rhomberg P. In vivo activity of ABT-773, a new ketolide, against recent clinical isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob Agents Chemother. 2000;44:447–449. doi: 10.1128/aac.44.2.447-449.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capobianco J O, Cao Z S, Shortridge V D, Ma Z, Flamm R K, Zhong P. Studies of the novel ketolide ABT-773: transport, binding to ribosomes, and inhibition of protein synthesis in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2000;44:1562–1567. doi: 10.1128/aac.44.6.1562-1567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu D T. Recent developments in macrolides and ketolides. Curr Opin Microbiol. 1999;2:467–474. doi: 10.1016/s1369-5274(99)00002-8. [DOI] [PubMed] [Google Scholar]
- 11.Cizman M, Pokorn M, Seme K, Paragi M, Orazem A. Influence of increased macrolide consumption on macrolide resistance of common respiratory pathogens. Eur J Clin Microbiol Infect Dis. 1999;18:522–524. doi: 10.1007/s100960050337. [DOI] [PubMed] [Google Scholar]
- 12.Clement J C, Tanaka S K, Alder J, Vojtko C, Beyer J, Hensey D, Ramer N, McDaniel D, Chu D. In vitro and in vivo evaluations of A-80556, a new fluoroquinolone. Antimicrob Agents Chemother. 1994;38:1071–1078. doi: 10.1128/aac.38.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conte J E, Golden J A, Duncan S, McKenna E, Zurlinden E. Intrapulmonary pharmacokinetics of clarithromycin and of erythromycin. Antimicrob Agents Chemother. 1995;39:334–338. doi: 10.1128/aac.39.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Azavedo J C, Yeung R, Bast D, Duncan C, Borgia S, Low D. Prevalence and mechanism of macrolide resistance in clinical isolates of group A streptococci from Ontario, Canada. Antimicrob Agents Chemother. 1999;43:2144–2147. doi: 10.1128/aac.43.9.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doern G V, Brueggemann A B, Huynh H, Wingert E, Rhomberg P. Antimicrobial resistance with Streptococcus pneumoniae in the United States, 1997–1998. Emerg Infect Dis. 1999;5:757–765. doi: 10.3201/eid0506.990603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doern G V, Brueggemann A, Pierce G, Holley H, Jr, Rauch A. Antibiotic resistance among clinical isolates of Haemophilus influenzae in the United States in 1994 and 1995 and detection of β-lactamase-positive strains resistant to amoxicillin-clavulanate: results of a national multicenter surveillance study. Antimicrob Agents Chemother. 1997;41:292–297. doi: 10.1128/aac.41.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doern G V, Brueggeman A, Pierce G, Hogan T, Holley H, Jr, Rauch A. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208–1213. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavalda J, Capdelva J, Almirante B, Otero J, Ruiz I, Laguarda M, Allende H, Crespo E, Pigrau C, Pahissa A. Treatment of experimental pneumonia due to penicillin-resistant Streptococcus pneumoniae in immunocompetent rats. Antimicrob Agents Chemother. 1997;41:795–801. doi: 10.1128/aac.41.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy D J, Swanson R, Hensey D, Ramer R, Bower R, Hansen C, Chu D, Fernandez P. Comparative antibacterial activities of temafloxacin hydrochloride (A-62254) and two reference fluoroquinolones. Antimicrob Agents Chemother. 1987;31:1768–1774. doi: 10.1128/aac.31.11.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston N J, De Azavedo J C, Kellner J D, Low D E. Prevalence and characterization of mechanisms of macrolide, lincosamide, and streptogramin resistance in isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2425–2426. doi: 10.1128/aac.42.9.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchese A, Tonoli E, Debbia E, Schito G. Macrolide resistance mechanisms and expression of phenotypes among Streptococcus pneumonia circulating in Italy. J Antimicrob Chemother. 1999;44:461–465. doi: 10.1093/jac/44.4.461. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7A4. Wayne, Pa: National Committee for Clinical Laboratory Stanards; 1997. [Google Scholar]
- 23.Nightingale C H. Pharmacokinetics and pharmacodynamics of newer macrolides. Pediatr Infect Dis J. 1997;16:438–443. doi: 10.1097/00006454-199704000-00027. [DOI] [PubMed] [Google Scholar]
- 24.Nilius A M, Bui M H, Almer L, Hensey-Rudloff D, Beyer J, Ma Z, Or Y S, Flamm R K. Comparative in vitro activity of ABT-773, a novel antibacterial ketolide. Antimicrob Agents Chemother. 2001;45:2163–2168. doi: 10.1128/AAC.45.7.2163-2168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Or Y S, Clark R, Wang S, Chu D T, Nilius A, Flamm R, Mitten M, Ewing P, Alder J, Ma Z. Design, synthesis, and antimicrobial activity of 6-O-substituted ketolides active against respiratory tract pathogens. J Med Chem. 2000;43:1045–1049. doi: 10.1021/jm990618n. [DOI] [PubMed] [Google Scholar]
- 26.Oster P, Zanchi A, Cresti S, Lattanzi M, Montagnani F, Cellesi C, Rossolini G M. Patterns of macrolide resistance determinants among community-acquired Streptococcus pneumoniae isolates over a 5-year period of decreased macrolide susceptibility rates. Antimicrob Agents Chemother. 1999;43:2510–2512. doi: 10.1128/aac.43.10.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piper K E, Rouse M, Steckelberg J, Wilson W, Patel R. Ketolide treatment of Haemophilus influenzae experimental pneumonia. Antimicrob Agents Chemother. 1999;43:708–710. doi: 10.1128/aac.43.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao C R. Linear statistical inference and its applications. New York, N.Y: Wiley; 1973. [Google Scholar]
- 29.Rodvold K A, Gothfried M H, Danziger L H, Servi R J. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob Agents Chemother. 1997;41:1399–1402. doi: 10.1128/aac.41.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sessegolo J F, Levin A S S, Levy C E, Asensi M, Facklam R R, Teixeira L M. Distribution of serotypes and antimicrobial resistance of Streptococcus pneumoniae strains isolated in Brazil from 1988 to 1992. J Clin Microbiol. 1994;32:906–911. doi: 10.1128/jcm.32.4.906-911.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shortridge V D, Doern G, Brueggeman A, Beyer J, Flamm R. Prevalence of macrolide resistance mechanisms in Streptococcus pneumoniae isolates from multicenter antibiotic resistance surveillance study conducted in the United States in 1994–1995. Clin Infect Dis. 1999;29:1186–1189. doi: 10.1086/313452. [DOI] [PubMed] [Google Scholar]
- 32.Streiner D L. Maintaining standards: differences between the standard deviation and standard error, and when to use each. Can J Psychiatr. 1996;41:498–502. doi: 10.1177/070674379604100805. [DOI] [PubMed] [Google Scholar]
- 33.Thornsberry C, Ogilvie P, Kahn J, Mauriz Y the Laboratory Investigator Group. Surveillance of antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States in 1996–1997 respiratory season. Diagn Microbiol Infect Dis. 1997;29:249–257. doi: 10.1016/s0732-8893(97)00195-8. [DOI] [PubMed] [Google Scholar]
- 34.Zhanel G G, Karlowsky J, Palatnick L, Vercaigne L, Low D, Hoban D. Prevalence of antimicrobial resistance in respiratory tract isolates of Streptococcus pneumoniae: results of a Canadian national surveillance study. Antimicrob Agents Chemother. 1999;43:2504–2509. doi: 10.1128/aac.43.10.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong P, Cao Z, Hammond R, Chen Y, Beyer J, Shortridge V, Phan L, Pratt S, Capobianco J, Reich K, Flamm R, Or Y, Katz L. Induction of ribosome methylation in MLS-resistant Streptococcus pneumoniae by macrolides and ketolides. Microb Drug Resist. 1999;5:183–188. doi: 10.1089/mdr.1999.5.183. [DOI] [PubMed] [Google Scholar]
- 36.Zuckerman J M, Kaye K M. The newer macrolides azithromycin and clarithromycin. Infect Dis Clin N Am. 1995;9:731–745. [PubMed] [Google Scholar]