Abstract
Worldwide, Verbascum thapsus is used for the treatment of various ailments owing to the presence of bioactives of therapeutic potential. Current study was planned to extract bioactives from V thapsus roots using methanol and water as solvents under stimulated effect of ultrasonic waves and characterize them to evaluate their potential benefits. Proximate analysis explored the presence of significant contents of protein, fats, fiber, organic and inorganic minerals. Fourier transform infrared spectra and high-performance liquid chromatography chromatogram indicated the presence of different phytochemicals having antioxidant potential as evidenced by total phenolic contents, total flavonoids content and 2,2-diphenyl-1-picrylhydrazyl activity of both extracts. Both extracts displayed excellent antimicrobial potential against Staphylococcus aureus (S aureus) and Fusarium Solani (F solani). Aqueous and methanolic extracts exhibited higher inhibition of biofilm made by Bacillus subtilis (B subtilis) as 55.09 and 61.58%, respectively in comparison to biofilm of Escherichia coli (E coli) as 48.11 and 36.51%, respectively. Methanol extract exhibited anti-amylase activity (IC50 5.26 ± .31 μg/mL) with an inhibition rate of 68.11% whereas IC50 of aqueous extract was 6.59 ± .53 μg/mL with an inhibition rate of 63.53%. Inhibitory potential against α-glucosidase (IC50 3.70 ± .94 ppm) was demonstrated by methanol extract in comparison to aqueous extract (IC50 7.58 ± .15). The study concluded that V thapsus roots have significant medicinal potential due to the presence of variety of bioactive molecules and can be used in pharmaceutical preparations.
Keywords: Verbascum thapsus, ultrasound assisted extraction, antioxidants, proximate composition, biofilm inhibition
Introduction
Human beings have depended on plants for food, medicines, and shelter. From the ancient times, medicinal plants continued to play significant role for the improvement of human health and increasingly being used for a variety of ailments. World health organization (WHO) has estimated that 80% of populations worldwide rely on natural medicines. Medicinal plants are adequately known for their beneficial properties in pharmaceuticals as well as in social and economic support system. There are almost 50 000–80 000 flowering plants that are used to cure multiple diseases all over the world. From 6000 species of higher plants, 600–700 species are being practiced as traditional medicine in Pakistan.1-3 Bioactive components obtained from natural origin have significant potential in the life of living beings owing to numerous biological activities. Oxidative stress or overproduction of ROS can result in deleterious effects on biomolecules such as proteins, lipids and DNA and eventually results in cell death. Oxidative stress is critical component of many diseases such as atherosclerosis, aging, AIDS, Alzheimer’s disease, brain disorder, cancers, hypertension, cataracts, diabetes, degenerative diseases, cardiovascular diseases, liver diseases, renal failure, heart failure, myocardial infarction, inflammation, neural disorder, Parkinson’s disease, and hypertension.4,5
Plant derived antioxidants such as polyphenols and flavonoids have been suggested as neuroprotective agents. They act as free radical scavengers, potent antioxidants and metal chelators exhibiting the neuroprotective role. Naturally occurring secondary metabolites play significant role in prohibiting and treating various diseases such as neurodegenerative diseases including Alzheimer’s disease. Naturally occurring plant derived phytochemicals found in various parts of plants are used to improve cognitive function and memory as well as to protect neurodegeneration. These natural compounds manifest anti-Alzheimer activity via targeting β-secretase, β-amyloid, and acetyl cholinesterase.6,7
Verbascum thapsus has large number of bioactive constituents like saponins, flavonoids, proteins, steroids, and amino acids. Different respiratory problems like whooping cough, dry cough and asthma are cured by using bioactives of calyx and corolla of this flora. Extracts of this plant are used as domestic remedy for ailments such as fever, allergies, pneumonia, and anti-inflammatory actions. It has been reported that V thapsus possess various medicinal properties like anti-cancer, anti-inflammatory, cardiovascular disorders, antimicrobial, cytotoxic, and wound healing activities.8-11
V thapsus is a well-known plant in Himalayas, temperate Asia, Europe, and North America. 12 In Pakistan, it is widely distributed in Chitral, Swat, Gilgit, Kashmir, Punjab, Khuram Agency, and Baluchistan. 13 There was a vast range of reports on search of aerial parts (leaves, stem and flowers) of V thapsus while there was deficiency of literature about bioactives and medicinal properties of its roots. Therefore, current research focus was on the ultrasound assisted extraction and characterization of bioactives from V thapsus roots to evaluate their antioxidant as well as medicinal potential.
Materials and Methods
Chemicals and Reagents
Sigma Chemicals Co. (St Louis, MO, USA) were authorized for the provision of gallic acid, 1, 1-diphenylpicrylhydrazyl (DPPH), alpha amylase, alpha glucosidase. Folin-Ciocalteau reagent was procured from Merck Darmstd, Germany. All other chemicals and solvents used were of analytical grade.
Procurement and Pretreatment of Plant Material
V thapsus radix was collected from the desert areas of Sindh, Pakistan during summer and identified by Botany Department (Botanical name: Verbascum thapsus, Family: Scrophulariaceae), Government College University Faisalabad. The root sample was washed thoroughly to remove dust particles and shade dried for 3 weeks. After complete drying, sample was ground to semi powder form using commercial grinder and stored for further analysis (Figure 1).
Figure 1.
Sampling of root (Verbascum thapsus) from d
Proximate Composition
Physiochemical properties give an idea about the purity of crude drug, their mineral composition and soluble components in a given solvent and taken as a tool to regulate and enhance the quality of herbal formulations by minimizing contamination due to adulteration. Evaluation of physiochemical properties were carried out following the protocols as recommended by World Health Organization. 14
Moisture Contents
China dish having powdered plant material of V thapsus (2.00 g) was incubated in oven for drying at temperature 100–105°C for 30 minutes and then cooled. Weight of cooled china dish was calculated by placing it on digital balance. This process was repeated until two successive weighing is constant and moisture contents were calculated by deducting weight of dried cool china dish and empty china dish. 14
where, W1 = container weight along with lid; W2 = container weight with lid and sample before drying; and W3 = container weight with lid and sample after drying.
Crude Fats Content
For crude fats, soxhlet extraction method was performed using n-hexane as solvent. Experimental sample (powdered V thapsus roots) 5.00 g was filled in extraction chamber of soxhlet apparatus fitted on water bath having controlled temperature. During extraction of fats, water bath was thermostated up to 60°C to maintain continuous fall of hexane droplets on the sample in extraction chamber for fixed time period. At the end, solvents with dissolved bioactives were freed from residues and subjected to evaporation on rotary evaporator to concentrate the extract and weighed. The crude fat (%) was calculated by applying the following formula.
Crude fat (%) = (Weight of extract/weight of crude sample) × 100. 14
Nitrogen Contents (Protein) Determination
Micro-Kjeldahl method was authentic to analyze nitrogen contents of protein in sample under investigation. 15 Digestion mixture (5.0 g) was used to digest the experimental sample (2.0 g) in 10 mL of sulphuric acid. Digestion was done in 30 minutes followed by dilution and filtration. Then 10 mL of diluted filtrate along with 10 mL 40% NaOH was taken in distillation chamber of micro-Kjeldhal apparatus. Distillation was carried out with the evolution of fumes of ammonia that was collected in container containing 10 mL of boric acid solution (2% w/v) with methyl orange as indicator. Distilled mixture was titrated against sulphuric acid (.1 N) until yellow color turned into original light pink color as an endpoint.
Then percent of crude protein was calculated by multiplying percent nitrogen with 6.25 factor.
Ash Contents
2.00 g of coarsely ground plant material of V thapsus was taken in tared china dish and incinerated at 550°C for 5 hours until ash became carbon free (turned white). To calculate the weight of ash, the ash obtained was cooled in desiccators and weighed on digital balance. The percentage of total ash contents were estimated with reference to air-dried sample weight using following formula. 16
Crude Fiber
Contents of crude fiber were determined by taking fat free sample (2.0 g) and subjected to heating in sulphuric acid (.1 M) for half an hour. After boiling, the solution was allowed to cool and filtered leaving behind residues. These residues were made free from acidic contents by treating repeatedly with warm H2O until neutral pH was detected by pH paper. The residue was further subjected to basic treatment by taking into 200 mL of 0.1 M NaOH solution for half an hour. The residue was filtered, washed with hot water, dried, weighed, and subjected to ashing by placing the residue in muffle furnace at 600°C for 4 hours. The ash was cooled down to ambient temperature, desiccated and weighed.
% Crude fiber = weight of residues before ash formation-weight of residues after ash formation x 100/Total weight of crude sample. 16
For the determination of Total carbohydrates, energy and nitrogen free extract, following formulas were proposed by Verma. 17
Extraction and Concentration
Dried powder of V thapsus roots was placed in flask and extraction was carried out separately with distilled water and methanol (1:10, w/v) using ultrasound bath. The fresh plant extracts were obtained by ultrasonication of 150.00 g plant material and 1500 mL of each solvent (water, methanol) for 30 minutes at 25–40°C temperature with ultrasound of 35 KHz. Extraction was carried out three times. The extracts from each sample were separately filtered by filtration of residue through filter paper (Whatmann filter paper no.1) Figure 2A and B. The extracts were dried under reduced pressure by using rotary evaporator at 50°C. The concentrated extracts were weighed accurately for calculating the percentage yield by following equation. 18
Figure 2.
Filtration of (A) methanolic extract, (B) aqueous extract, (C) solidified methanolic extract, and (D) Solidified aqueous extract of Verbascum thapsus roots.
Then the semisolid extract was preserved at 4°C in refrigerator for further analysis (Figure 2C and D).
Characterization of Extracts
Fourier Transform Infrared Analysis
V thapsus powdered plant material was subjected to Fourier transform infrared (FTIR) fingerprinting for qualitative analysis and to detect its functional groups. 1.0 mg of powdered sample was mixed with 300 mg KBr forming the pellet. Scanning of this pellet was carried out in the frequency range of 4000–400 cm−1 with velocity of 16 cm/s at 4 cm−1 resolution to get FTIR spectra. Peaks obtained were compared with standard peaks. 19
High-Performance Liquid Chromatography Analysis
A High Pressure Liquid Chromatographic (HPLC) system (Shimadzu-10A Japan) provided with UV-Vis Detector was used. Separation was done using a Shim-Pack CLC-ODL C-18 column (25 cm × 4.6 mm × 5 µm) at room temperature. A gradient system consisting of solvent A (H2O: CH3COOH, 94:6) and solvent B (CH3COCN) was used at flow rate of 1.0 mL min−1. Using dual pumping system, solvent gradients were formed, by varying the proportion of solvents. The gradient program was started with 15% B at 0–15 min, 45% B at 15–30 min and 100% B at 30–45 minutes. The peak area was calculated by CSW32-DataApex software. For analysis of compounds, the UV-Vis detector was set at 280 nm. The dried extracts were dissolved in 100 mL mobile phases. After filtering through a filter paper, the samples were injected into HPLC. 20
Antioxidant Potential of Extracts
Total Phenolic Contents
Folin-Ciocalteu reagent method was used to evaluate phenolics using 96-well microplate. In short, 1.0 mL of each diluted extract of V thapsus (water, methanol) at concentration of 800 μg/mL was dissolved in 0.5 mL of double deionized water and 1.0 mL of 5% Na2CO3 was added after the addition of Folin-Ciocalteu reagent (500 μL). The mixture was agitated for 60 seconds and incubated at ambient temperature for 90 minutes. The absorbance was measured through microplate reader at 760 nm through spectrophotometer. The contents of phenolic expressed as mg Gallic Acid Equivalents (GAE) per g of plant material and the equation was Y= .0055x + .0987 (R2 = .9988) where Y stand for total phenolic contents, and X is the concentration of standard or sample. It was applied to determine the total polyphenolic contents from the samples. Samples were analyzed thrice and average results were taken. 21
Determination of Flavonoids
Total flavonoid contents were determined by spectrophotometric method. 0.5 mg of each extract (Water/methanol) was dissolved in 1.00 mL of distilled water in volumetric flask separately. Then solution was homogenized after the addition of NaNO2 (.3 mL/5%). NaOH (1 M/2.0 mL) and AlCl3 (10%/0.6 mL) was mixed after 5 minutes. Then reaction mixture was subjected to analysis by spectrophotometer after incubating the sample for 10 minutes. Absorbance was taken at wavelength maximum of 510 nm. Total flavonoid contents were expressed as equivalents of catechin (g/100 g of dry plant matter) and equation was Y= .0038X + .0285 (R2 = .9835). The results were averaged from triplicate measurements. 21
2,2-Diphenyl-1-Picrylhydrazyl Radical Scavenging Assay
DPPH radical scavenging ability assay was performed to examine the antioxidant activity of bioactive components of methanolic and aqueous extract of V thapsus through spectroscopy technique as described by Safdar et al 22 . Samples from each extract of V thapsus (methanol/water) and DPPH solution were mixed in 1:2 proportion, respectively. DPPH solution and stock solution of V thapsus extracts were added in each well of 96-well plate before incubation (30 minutes) in the dark at room temperature. The absorbance at 517 nm was recorded through microplate reader. DPPH solution in methanol was used as negative control. Ascorbic acid was taken as positive control. Three replicates of every sample were recorded. The inhibitory percentage was calculated as follows
Evaluation of Extracts for Medicinal Potential
Bactericidal Potential
Agar well diffusion method was used for antibacterial activity. Two bacterial strains including Escherichia coli and Staphylococcus aureus were used along with ciprofloxacin as standard. Nutrient agar 29.0 g/L was homogenized in distilled water and autoclaved for 15 min at 121°C for sterilization; inoculums (150 μL/mL) were added into medium and transferred to sterilized petri plates to solidify within few minutes. Wells were prepared by sterilized borer and kept the samples on this culture medium (100 μL extract) in these wells. Petri plates were incubated for 24 hours at 37°C. The zones of inhibition (mm) of bacterial growth in each well were measured through zone reader. 23
Fungicidal Potential
Potential of extracts against fungal growth was evaluated using fungal strains of A niger and Fusarium solani and protocol adopted was reported by Tariq et al. 24
Fungal strains were isolated and purified on solid media (Potato Dextrose Agar slant) and further multiplication of microbes was done using liquid media of Sabouraud dextrose broth which was underwent sterilization (121°C) before inoculation and incubation for 3-4 days at 28°C.
For antifungal activity evaluation, well diffusion method was adopted. 3.9 g of Potato Dextrose Agar as growth medium was suspended in boiling water (100 mL) and poured into sterilized Petri plates. Fungal spores were inoculated on petri plates followed by the preparation of sample wells using sterilized borer of 6.0 mm diameter. Sample (Methanol/water extract) as well as standard antifungal agent (Terbinafine 10 mg/mL) was introduced into wells. Fungal growth was taken after 48 hours of incubation at 28°C. Wells with sample extracts showed clear zones of fungal growth. The zones of inhibition were measured in millimeters using zone reader.
Biofilm Inhibition Potential
Biofilms are produced by those bacteria that have good adhesion property to the surface. Micro titre plates were made for given extracts to determine the adhesion power of microbes to the surface. Growth of bacteria (E coli and Bacillus subtilis) was taken in broth media when culture was grown for 24 hours. Then, the cultures were diluted up to 1:100 into fresh medium for biofilm inhibition assay. The diluted solution of 200 μL was introduced into 96-well plate for growth phase. After that, medium was withdrawn from wells with micropipette, followed by rinsing of wells thrice with freshly prepared phosphate buffer saline (PBS) (200 μL). Then 96% ethanol was filled in wells and allowed to evaporate after 15 minutes. Wells of micro titre plates were dried and filled with given extract (200 μL) for five minutes after that washed off with distilled water, and then dried. Finally, absorbance was taken at 540 nm with an ELISA reader after adding 200 μL of glacial acetic acid (33%). Biofilm development by all of the tested strains was compared with negative control. Ciprofloxacin, bacterial strain, and nutrient broth was used as positive control whereas un-inoculated media was used as negative control.22,25
α-amylase inhibition Assay
The 3, 5-dinitrosalicylic acid (DNSA) method was adopted to evaluate the inhibition power of extracted bioactives against α-amylase. 26 The root extracts of V thapsus were homogenized in DMSO (10%) using phosphate buffer (.02 M Na2HPO4/NaH2PO4) and made volume up to 100 μg/mL. α-amylase was added to each extract (1:1) followed by incubation for 10 minutes at ambient temperature. Equal volume of starch solution (1%, w/v) was added and allowed to incubate for 30 min. Reaction termination was done by introducing DNSA reagent (200 μL) and subjected to heat treatment up to 90°C for 10 minutes. The reaction mixture was settled at room temperature by the addition of distilled water. Absorbance at 540 nm was measured through spectrophotometer. Extract free blank was prepared by dissolving enzyme in 200 μL of buffer. Similarly enzyme free blank reactions were prepared using the plant extracts. A carbose was used as positive control. Potential of extracts to inhibit enzyme activity was expressed as percent inhibition and was calculated by formula given below:
α-glucosidase Inhibition
Potential of extract to inhibit α-glucosidase activity was evaluated using p-Nitrophenyl-α-D-glucopyranoside (pNPG) as substrate. 27 Enzyme extract was prepared by taking α-glucosidase (50 μl, 2 units/ml) in phosphate buffer (100 mM). Plant extracts were mixed with 320 μL of 100 mM phosphate buffer and pre incubated for 10 minutes at 37°C and then 50 μL pNPG was incorporated to initiate the reactants and subjected to spectrophotometric analysis by running the sample at 410 nm. A carbose was taken as control (positive). The % inhibition was calculated as follows:
Toxicity Evaluation of Extracts
Hemolytic Activity
Methanolic and aqueous extract of V thapsus root sample was assayed on human erythrocytes.28,29 Three milliliters of human blood cells were collected from healthy volunteers and subjected to centrifugation (10 000 rpm) for 5 minutes. The supernatant was dispensed off and blood suspension was prepared in 2.0 mL chilled PBS for hemolytic activity. A volume of 180 μL of blood suspension was mixed in Eppendorf with 20 μL of each extract (10 mg/mL DMSO) and centrifuged for 5 minutes at 1500 rpm after incubating for 30 minutes. Then 100 μL of supernatants were collected and dilutions were prepared with 900 μL chilled PBS. The liberated hemoglobin in the supernatant was used to measure the absorbance in UV-Vis spectrophotometer at 540 nm. For each assay, two controls were prepared without extract, Triton X-100 was taken as positive hemolytic control with 100% lysis, while PBS as negative control with 0% lysis. Triplicate measurements were taken to calculate average values. The percentage hemolysis for each sample was calculated by following formula:
Statistical Analysis
Data obtained from the present research experimentation were subjected to statistical analysis and the values were presented as mean ± SD for triplicate measurements. Bar charts and graphs were prepared using Microsoft Excel.
Results and Discussion
Proximate Composition
The percentage yield (30.43%) given by V thapsus aqueous root extract was higher than methanolic root extract (22.37%) of this plant as given in Table 1.
Table 1.
Physiochemical Analysis of Verbascum thapsus Roots.
| Parameters | Percentage |
|---|---|
| Yield of aqueous extract | 30.43 |
| Yield of methanolic extract | 22.37 |
| Moisture contents | 17.67 |
| Total ash contents | 8.23 |
| Acid insoluble ash | 5.30 |
| Water insoluble ash | 33.21 |
| Water soluble extractives | 7.81 |
| Alcohol soluble extractives | 24.12 |
| Crude fat contents | .50 |
| Crude proteins | 3.16 |
| Crude fiber | 11.07 |
| Total carbohydrates | 70.44 |
| Energy (kCal/g) | 298.9 |
Available literature reports show different results of extraction yields from this species and these variations might be due to different parts of plant used and different extraction techniques. As per our knowledge, there is no report available about extraction yield for V thapsus root extraction by Ultrasound Assisted Extraction (UAE). The concentration of bioactive molecules in plant extract may vary due to target material, technique applied, solvent system used and agro-climate changes. 30 It has been evaluated that extraction of bioactives was more efficient in aqueous medium as compared to pure methanol through ultrasound assisted extraction method. Improvement in percentage yield of extract can be achieved by application of stronger ultrasonic powers which increases the contact area between solvent and powdered plant material and more disruption of cell walls. 31 Different solvents having different polarities are used for extraction purpose and to liberate the bioactive compounds present in plant sample. Increase in extraction yield is probably due to high diffusion coefficient and molecular movement from plant tissues of V thaspsus to solvent.
In contrary to current research results, reports are available in which comparatively poor yield of ethanolic extract of V thapsus flower was evaluated through hot water extraction and ultrasound assisted extraction. The percent yield of plant sample by hot water extraction and UAE method was 3.60 ± .13 and 5.83 ± .08, respectively. 11 The extraction yield of aerial parts of V thapsus methanol extract by simple maceration method for 24 hours resulted in 8.11% yield. According to Mihailovic et al 32 extraction yield of V thapsus methanolic and water extract by simple maceration was 8.11% and 10.03%, respectively, whereas Mahek et al 33 showed 11.87 and 6.51% w/w yield of ethanol and water soluble bioactives in V thapsus leaf.
The proximate composition of V thapsus root sample is given in Table 1. Moisture content of this plant was 17.6% indicating that plant is susceptible to spoilage and good source of water for body cells. 34 Total ash content was 8.23% indicating the presence of organic and inorganic minerals in the roots which might be incorporated from dissolved minerals of soil. The protein content was 3.16% and fat content was .50%. Acid insoluble ash was 5.30% while water insoluble ash was 33.21%. Water soluble extractives indicated the presence of inorganic compounds, sugars and acids. Alcohol soluble extractives manifested the presence of flavonoids, alkaloids, steroids, glycosides and polyphenolic contents. 35 The crude fiber content was 11.07% which aid in absorption of water from body and stool. Fibers have the ability to soften the stool and therefore prevent constipation. 36 The estimated carbohydrate content in roots of V thapsus was high (70.44%). Carbohydrates are essential nutrients and known to produce energy for body and source of energy to cells such as blood, muscles and brain.37,38
Fewer reports are available for proximate analysis of V thapsus. According to Babamoradi et al 11 chemical composition analysis of flowers of V thapsus showed moisture contents (15–44%), ash (1-8%), fiber (5–32%) and fat (25–30%). According to Mahek et al 33 V thapsus leaf showed 5.57% moisture contents. In contrast to our results, they performed the analysis on leaves of V thapsus and detected higher value of total ash contents (14.32%) whereas acid insoluble contents (4.75%) are in consistent with the present results and water soluble contents of ash was 8.30% w/w.
Analytical Characterization
Fourier Transform Infrared analysis
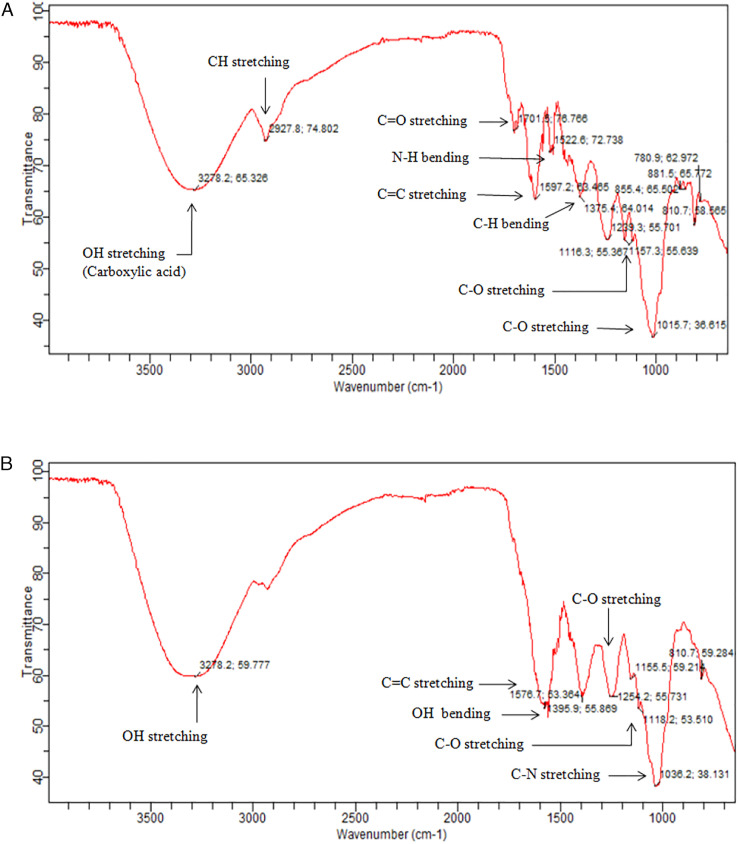
The FTIR spectrum in Figure 3A and B showed the exhibition of biologically potent functional groups in methanol and aqueous extract of V thapsus under the optimal extraction conditions of UAE. FTIR spectra of methanol extract showed the broad peaks at 3278 cm−1 specified the presence of carboxylic acid O-H stretch. 39 Existence of peak at 2927 cm−1 indicates C-H absorption which can be referred to bending and stretching vibrations of methyl (CH3), methylene (CH2) and methine (CH). 40 The absorption peak at 1701 cm−1 indicates C=O stretching suggesting the presence of high quantities of uronic acid in V thapsus methanol extract. 40 The peak at 1597 cm−1 represents presence of C=C stretching in aromatic compounds and peak at 1522 cm−1 indicate amines (N-H bend). The absorption peak at 1375 cm−1 indicates alkanes C-H bend. The absorption peak at 1239 cm−1 is referred to asymmetric stretching of glycosidic bond (C-O-C). 41 The peaks in absorption region of 1157 and 1116 cm−1 show the C-N stretch, C-C-C bend, respectively. It was also demonstrated that absorption peaks at 1239, 1157 and 1116 cm−1 are probably assigned to the presence of C-O stretching vibrations of ester group. 42 Absorption peak at 1015 cm−1 indicates the C-O stretch in alcohol. Absorption peaks at 810–881 cm−1 indicate the C-H out of plane bending in aromatic compound. Whereas, peak at 780 cm−1 show the CH out of plane bending in carbonyl compounds. Overall, fingerprint region between 800 and 1200 cm−1 indicate the area for carbohydrate based biopolymers (Figure 3A).
Figure 3.
(A) FTIR spectra of Verbascum thapsus roots (methanol extract), (B) FTIR spectra of V thapsus roots (aqueous extract). FTIR, Fourier transform infrared.
FTIR spectra of aqueous extract of V thapsus showed several FTIR peaks as shown in Figure 3B. A broad peak at 3278 cm−1 may be attributed to the O-H stretch in alcohols or phenols. Peak at 1576 cm−1 corresponds to the C=C aromatic stretching. Further peaks at 1395 cm−1 and 1254 cm−1 may be attributed to O-H bending in alcohols and C-O stretch in C-O-C bond. Small peaks at 1155 cm−1 and 1118 cm−1 are probably assigned to C-O stretch. Broad peak at 1038 cm−1 may be attributed to C-N stretching in amines. Small peak at 810 cm−1 indicate the CH out of plane bending in aromatic compounds. FTIR spectrum for methanol and water extract of V thapsus root sample showed almost same absorption peaks which confirmed that same phytochemicals are present in both samples. Minor deflections in the spectrum were observed when both spectra were confirmed. The difference in the interaction of phytochemicals with solvent may accounts for the difference in absorption of the phytochemicals present in extracts.
High-Performance Liquid Chromatography Analysis
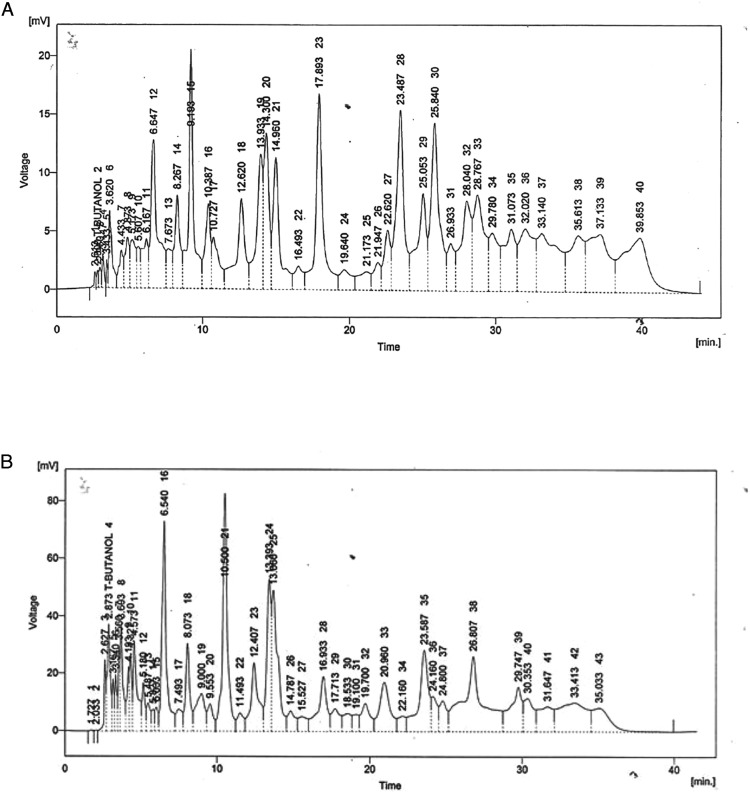
The HPLC-UV chromatogram of methanol and aqueous extract of V thapsus showed the presence of several peaks as clear from chromatogram (Figure 4A and B). The report showed the presence of an array of compounds. Phenolic compounds from methanol and water extract of V thapsus including caffeic acid, cinnamic acid, ferulic acid, quercetin, gallic acid and benzoic acid has been studied. Water extract showed the presence of Vanillic acid, chlorogenic acid, and m-Coumaric acid. Whereas, methanol extract showed the presence of syringic acid and p-Coumaric acid.
Figure 4.
(A) HPLC Chromatogram of methanol extract of Verbascum thapsus roots, (B) HPLC Chromatogram of aqueous extract of V thapsus roots. HPLC, High-performance liquid chromatography.
Evaluation of Extracts for Antioxidant Potential
Total Phenolic Contents
Phenolics are the bioactive, largely found in vegetables, herbs, medicinal plants, and fruits that act as free radical terminators. Polyphenols have antioxidant potential and ability to degrade singlet and triplet oxygen, peroxides, quench free radicals, and prevent the oxidative chain reactions.20,43 Hydroxyl groups in plants are involved in exhibiting free radical scavenging activities. Total phenolics in methanol and aqueous extract of V thapsus were measured through Folin-Ciocalteu reagent method and results are reported in Table 2. The antioxidants play an important role in both exogenous and endogenous defense system and protect cell membranes, lipoproteins and nucleic acids. Exogenous antioxidant defense system includes certain entities which enter the body through diet while endogenous antioxidant defense system has certain enzymes of physiological origin. Antioxidants particularly phenolics from natural sources are important means to balance the oxidants and antioxidants in living organisms.44,45
Table 2.
Antioxidant Potential of Verbascum thapsus Roots Extracts.
| Aqueous Extract (Mean ± SD) | Methanol Extract (Mean ± SD) | |
|---|---|---|
| Total phenolic contents(mg GAE/g extract) | 57.66 ± .64 | 97.72 ± .37 |
| Total flavonoid contents (mg CE/g extract) | 19.92 ± .440 | 62.09 ± .675 |
| % Inhibition of 2,2-diphenyl-1-picrylhydrazyl | 78.35 ± .108 | 94.25 ± .152 |
Folin-Ciocalteu method reported by Ashraf et al 46 was followed to evaluate the total phenolics. TPC was determined by using regression equation (y=.0055x + .0987; R2 = .9988) that was drawn from calibration curve of standard Gallic acid and represented as Gallic acid equivalents (GAE). Phenolic content evaluated with methanol and water extracts of V thapsus root are given below in Table 3. Methanolic extract of V thapsus showed high amount of TPC (mg/g GAE of DW) (97.72) as compared to aqueous extract (57.66). The statistical analysis showed significant difference of TPC among methanolic and aqueous extract of V thapsus root sample. The obtained data reported a positive correlation between phenolic contents and antioxidant potential. The redox potential of phenolics contributed in antioxidant activity and neutralization of free radicals. Morina et al 47 reported the availability of phenolic compounds in root and leaf tissues of V thapsus. According to Esfahani et al 48 amount of phenolic contents in aqueous extract of V thapsus were 96.5 mg GAE/g extract, whereas Mihailovic et al 32 reported total phenolic contents found in aerial parts of V thapsus methanolic and water extract were 85.0 ± 1.4 and 52.6 ± .3 mg GAE/g dry extract, respectively.
Table 3.
Antimicrobial Activity of Verbascum thapsus Roots Extracts.
| Treatment | Bacterial Growth Inhibition Zone Diameter (mm) | Fungal Growth Inhibition Zone Diameter (mm) | % Biofilm Inhibition | |||
|---|---|---|---|---|---|---|
| E coli | S aureus | A Niger | F solani | E coli | B subtilis | |
| Methanolic extract | 12.01 ± .017 | 20.11 ± .196 | 14.05 ± .016 | 21.14 ± .126 | 48.11 ± .119 | 55.09 ± .416 |
| Aqueous extract | 9.12 ± .115 | 21.66 ± .577 | 15.12 ± .155 | 26.60 ± .077 | 36.51 ± .301 | 61.58 ± .391 |
| Positive control | 44.33 ± .577 | 31.15 ± .256 | 46.27 ± .575 | 33.35 ± .161 | 80.02 ± .370 | 87.06 ± .480 |
Note. Ciprofloxacin (positive control) for antibacterial and % biofilm inhibition activity and Terbinafine (positive control) for antifungal activity.
Total Flavonoid Contents
Total flavonoid contents in methanol and aqueous extract of V thapsus were determined through aluminum chloride colorimetric method and the results are given in Table 2. TFC was determined by using regression equation (y = .0038x + .0285; R2 = .9835) drawn from calibration curve of standard Catechin and represented as Catechin equivalents (CE). Phenolic contents evaluated with methanol and water extracts of V thapsus root are given below in Table 2. Methanolic extract of V thapsus showed high amount of TFC (mg Catechin/g extract) (62.09) whereas aqueous extract displayed TFC up to 19.92. According to Mihailovic et al 32 total flavonoid contents found in aerial parts of V thapsus methanolic and water extract were 43.4 ± .8 and 10.2 ± .2 mg CE/g dry extract.
2,2-Diphenyl-1-Picrylhydrazyl Scavenging Activity
To determine the free radical scavenging and antioxidant ability of plant extract, DPPH is an established assay which was first developed by Blios in 1958. Purple colored solution is formed upon electron transfer in methanol but in antioxidant presence, it becomes colorless. 49 Being a free radical and having odd number of electrons, DPPH is stable at room temperature. DPPH is deep violet color solution because odd number of electrons of DPPH absorbs the visible light (517 nm). In vitro antioxidant activity of V thapsus was determined by DPPH radical scavenging assay and results were reported in terms of % inhibition. The resulting data of %age scavenging ability of both solvents are given in Table 2. Methanol extract of V thapsus showed higher radical scavenging activity of 94.25% while aqueous extract exhibited 78.35% of inhibition of DPPH radical as given in Table 2. Antioxidants present in plant extract act as reducing power agents and donate their electrons to free radicals of DPPH and change in color of solution was quantitatively evaluated by calculating the absorbance at which decolourization occurs. Larger amount of antioxidants in plants resulted in higher decolourization. 50
DPPH is simple, inexpensive, rapid and convenient method to determine antioxidant activity of herbs, plants, fruits and vegetables. There are some disadvantages of this method, that is, only soluble in organic solvents and sensitive towards lewis bases and light.29,49 Few reports are available for free radical scavenging activity of V thapsus. According to Prakash & Sagar, 51 at 100 μg/mL methanol, aqueous, and acetone extract of V thapsus, leaves showed 57%, 41%, and 40% antioxidant activity, respectively. IC50 values for leaf extract with three solvents were 89.25, 118.65, 125.52 μg/mL. According to Mihailovic et al 32 methanolic extract showed better antioxidant activity as compared to water extract. Obtained results showed the IC50 values of methanol (121.5 μg/mL) and water extract (257.0 μg/mL). This powerful antioxidant activity is due to the presence of Verbascoside as major phenolic compound. According to Babamoradi et al, 11 67.66% radical scavenging activity exhibited by aqueous extract of V thapsus flowers. According to Narayana & Balakrishnan, 52 DPPH scavenging activity of ethanolic extract of V thapsus leaves was 94.63% whereas aqueous extract of the plant showed 24% inhibition.
Evaluation of Medicinal Potential
Antibacterial Potential
S aureus and E coli bacterial strains were used for the determination of antibacterial potential. S aureus can cause respiratory and skin infections and food poisoning. E coli can cause urinary tract infections, diarrhea, pneumonia, and respiratory diseases. The antibacterial activity of V thapsus root extract of both solvents are given in Table 3. Results displayed in Table 3 shows the inhibited zones (mm) of microbial growth by extracts. It is clear from results that methanolic extract displayed significant inhibition of E coli (23.02 mm) and S aureus (20.11 mm) growth whereas aqueous extract inhibited significantly the growth of S aureus (21.66 mm) in comparison to growth of E coli (9.12 mm). According to Prakash et al 53 methanolic extract of V thapsus leaves inhibited the bacterial strains more efficiently than acetone leaf extract. Both extract showed maximum inhibition against Gram positive bacterial strains. According to Dulger et al, 54 ethanolic leaf extract of the plant was found effective against microorganisms responsible for urinary tract infections, whereas Weldegebri et al 55 demonstrated that CuO nanoparticles produced by leaf extract of V thapsus showed significant inhibition of two bacterial strains. Still there is no such literature available for antibacterial activity of V thapsus roots extracts.
Traditional plants due to their low toxicity and economic importance are main focus around the medical world. These plants are used for various medicinal purposes and more importantly due to presence of many components which have medicinal or pharmacological activities. 56 Due to their low side effects, antioxidant, and antimicrobial activity, they are safely used as green medicine and more influential as compared to synthetic drugs. Over the last few years, herbal plants are used to synthesize new drugs that can be used to treat infectious diseases and promote resistance against various human pathogens. Due to this reason, scientists are more interested to find out antimicrobial agents from sources like medicinal plants which are the great source of antimicrobial substances. 57
Antifungal Potential
Medicinal plants are precursors of effective and potent drugs might be due to the existence of antiseptic bioactives. Therefore, this can be used as raw materials for drug formulation in the current panorama of the natural world. Roots, leaves and different fruits parts (Peel, pulp, and seed) are rich source of bioactives having nutraceutical potential. 58 In the current research work, both prepared root extracts were tested for antifungal potential against two fungal strains (A niger and F solani) by following the disc diffusion method and inhibited zone was captured by zone reader. Values were comparable with positive control (Terbinafine) and results are reported in Table 3. Both methanol and aqueous extracts optimally inhibited the log phase of F solani in comparison to log phase of A niger. Active phase of F solani was significantly inhibited (26.60 mm) by aqueous extract followed by methanol extract (21.14 mm) that are comparable to positive control (33.35 mm). A niger showed more resistance to both extracts so, inhibition zones calculated were 15.12 & 14.05 mm for aqueous and methanolic extracts, respectively.
Biofilm Inhibition
Biofilms are produced by those bacteria that have good adhesion property to the surface. These biofilms are composed of long chains of extracellular proteins, carbohydrates, cellulose, lipids, glyco peptides and nucleic acids. 59 These bacteria are responsible for causing infectious diseases and destroying seriously the defensive system of host. Bioactive components of methanol and aqueous extracts were evaluated for their potential to inhibit biofilm formation of bacteria. Both extracts exhibited remarkable potential to eradicate biofilm formation of E coli and B subtilis (Table 3). Both extracts (methanol and aqueous) exhibited higher inhibition of biofilm made by B subtilis 55.09 and 61.58%, respectively, in comparison to biofilm of E coli (48.11 and 36.51%, respectively). It indicates that both extracts have displayed promising biofilm inhibition activity and can be used in medicament.
In Vitro α-Amylase Inhibitory Study
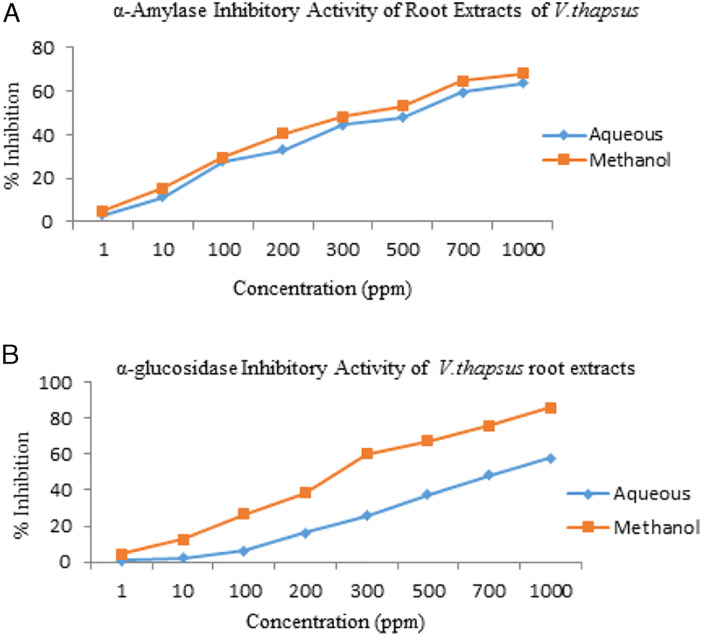
α-amylase is the essential enzyme in digestive system that is used to catalyze the starch hydrolysis to a mixture of low molecular weight oligosaccharides such as malto triose, α (1–4) and α(1–6) oligo-glucans and maltose. α-amylase inhibitors or generally known as starch blockers, have the ability to slow down or inhibit the absorption of starch by blocking the starch hydrolysis into simple sugars. Indeed, medicinal plants are prospective candidates against inhibition of carbohydrate digestive enzymes and widely demonstrated as anti-hyperglycemic agents for the treatment of type 2 diabetes. 6 All the extracts were observed to evaluate the amylase inhibitory potential. The IC50 values were calculated (Table 4) from the graph of % α-amylase inhibition against extract concentrations (Figure 5A). The methanolic extract of V thapsus roots at 1000ppm concentration exhibited the maximum anti-amylase activity with an inhibition rate of 68.11% followed by aqueous extract (63.53%). The methanol extract exhibited the IC50 of 5.26 ± .31 μg/ml and the IC50 of aqueous extract was 6.59 ± .53 μg/ml. The studies performed displayed that the extracts of V thapsus (methanol, aqueous) had significant α-amylase inhibitory potential. The results suggested that α-amylase inhibitory activity of methanolic root extracts is most likely to be due to the presence of polar compounds.
Table 4.
In vitro α-Amylase & α-Glucosidase Inhibitory Activities of Verbascum thapsus Roots Extracts.
| Extract | α-Amylase Inhibitory Activity (IC50 Value) | α-Glucosidase Inhibitory Activity (IC50 Value) |
|---|---|---|
| Aqueous | 6.59±.53 | 7.58 ± .15 |
| Methanol | 5.26±.31 | 3.70 ± .94 |
Figure 5.
Verbascum thapsus root extracts for % inhibition of (A) α-amylase, (B) α-glucosidase.
Other Verbascum species have also been demonstrated to show the inhibition of carbohydrate hydrolyzing enzymes. For example, ethanol, n-butanol, ethyl acetate, and chloroform extracts of V. chinese leaves were found to exhibit the inhibitory activities against α-amylase and α-glucosidase in dose dependent way. 60 Besides methanolic and aqueous extracts of V. bombyciferum have also been reported to show the anti-amylase effects (% inhibition ranging from .09 to .46 mmol ACAE/g). 61 In the comparative study, the aqueous extracts of V oocarpum and V euphraticum were found to be least potent as α-amylase inhibitor. Whereas, hexane extracts of both plants showed maximum inhibition against α-amylase (.53 and .59 mmol ACAE/g, respectively). 62
In Vitro α-Glucosidase Inhibition
α-glucosidase is mainly present in brush border walls of small intestine that execute the breakdown of starch and disaccharides to simple glucose by acting upon the α-(1,4)-glycosidic bonds. The inhibition of these enzymes is therapeutically significant for diminishing the hyperglycemic conditions related to diabetes. 63 The extracts obtained from V thapsus roots were evaluated for their in vitro α-glucosidase inhibitory activities. The % inhibitory effects and IC50 values were given in Table 4 and shown in Figure 5B. Methanol extract exhibited 85.72% inhibition at a concentration of 1000ppm followed by water extract with a value of 57.91%. Methanol extract showed the significant inhibitory activity against α-glucosidase (IC50 3.70±.94 ppm). Whereas, aqueous extract also showed α-glucosidase inhibitory activity (IC50 7.58 ± .15).
A recent report by Angeloni et al 61 reveals that ethyl acetate extracts of V oocarpum and V euphraticum significantly depressed the α-glucosidase activity with the corresponding percent inhibition of .81 and .84 mmol ACAE/g, respectively. In another report, V. kemanenis, displayed more than 50% inhibition of α-glucosidase. 64 In contrast, methanol extract of V sinaiticum at 25ppm concentration exhibited 4% inhibition of α-glucosidase. 65 V thapsus leaves tested for screening experiment using maltose and sucrose as inhibitors for rat intestinal α-glucosidase inhibition resulted in 29.8 and 31.5% inhibition. 66 In this study, it was determined that V thapsus showed similar effects, however, methanol extract was found more effective. Despite the introduction of new therapeutic approaches, diabetes has been a major cause of various diseases. Treatment of diabetes is possible by inhibiting the α-glycosidase enzyme and delaying the absorption of glucose. 67 The tested extracts are rich source of phenolic compounds, which may contribute to its anti-diabetic effects. Investigations are required to explore the possible mechanism of actions and active principles.
Toxicity Evaluation
Hemolytic Activity
Antibacterial preparations from medicinal plants are limited due to high hemolytic activities. Interaction between erythrocyte membrane and peptide antibiotics causes the escape of hemoglobin from cells. So, it is of prime importance to validate the hemolytic activity of newly formulated pharmacological preparations. 68 In this study, hemolytic activity of V thapsus root extracts was screened against normal human blood cells. Both extracts were evaluated for hemolysis against human red blood cells and compared with positive (Triton X-100) and negative (PBS) control. Comparison of outcomes has been reported in Table 5. Percent lysis of human red blood cells were detected below 80% thus both the extracts under investigation exhibited minute toxicity. It is obvious from table that % cytotoxicity was detected maximum but non-significant (4.39%) in aqueous extract in comparison to methanol extract (3.11%) indicating that its nutraceutical consumption is safe and these results are consistent with the findings reported by Sepahi et al 69 . According to them, amount of hemolysis by V thapsus aqueous extract was only 1.2% as compared to positive control (Triton X-100) with 100% hemolysis.
Table 5.
Hemolytic Activity of Verbascum thapsus Against Human Red Blood Cells.
| Test Extracts (μg/mL) | % Hemolysis (Mean ± SD) |
|---|---|
| Aqueous extract | 4.39 ± .126 |
| Methanol extract | 3.11 ± .015 |
| Phosphate buffer saline | .65 ± .0014 |
| Triton-X-100 | 100 ± 2.017 |
Conclusion
V thapsus roots possessed many different bioactive components such as proteins, fibers, and antioxidants. These bioactives not only exhibited the nutritional potential but also have antimicrobial potential. It can be concluded from results that V thapsus roots extracts have significant medicinal potential due to the presence of variety of bioactive molecules and can be used in the preparation of pharmaceutical products for therapeutic purposes. Therefore, V thapsus roots extract can be used for value addition of food supplements as well as medicaments.
Acknowledgments
The authors highly acknowledged the Higher Education Commission (HEC), Islamabad, Pakistan for the financial assistance under the project pin number NRPU# 4927 and the bioassay section of Medicinal Biochemistry Lab, Department of Biochemistry, University of Agriculture, Faisalabad-Pakistan for providing the lab facilities.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Naheed Akhter https://orcid.org/0000-0003-3933-9944
Muhammad Riaz https://orcid.org/0000-0002-5524-7735
References
- 1.Hussain K, Shahazad A, Zia-ul-Hussnain S. An ethnobotanical survey of important wild medicinal plants of Hattar district Haripur, Pakistan. Ethnobot leafl. 2008;12:29-35. [Google Scholar]
- 2.Shinwari ZK, Khan I, Naz S, Hussain A. Assessment of antibacterial activity of three plants used in Pakistan to cure respiratory diseases. Afr J Biotechnol. 2009;8:7082-7086. [Google Scholar]
- 3.Hilonga S, Otieno JN, Ghorbani A, Pereus D, Kocyan A, de Boer H. Trade of wild-harvested medicinal plant species in local markets of Tanzania and its implications for conservation. S Afr J Bot. 2019;122:214-224. [Google Scholar]
- 4.Riaz M, Mahmood Z, Saeed M, Abbas M, Shahid M. Lipid peroxidation and antioxidant enzymes in relation to semen quality. Oxid Commun. 2016;39:2990-2998. [Google Scholar]
- 5.Riaz M, Mahmood Z, Shahid M, et al. Impact of reactive oxygen species on antioxidant capacity of male reproductive system. Int J Immunopathol Pharmacol. 2016;29:421-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panda SS, Jhanji N. Natural products as potential anti-alzheimer agents. Curr Med Chem. 2020;27:5887-5917. [DOI] [PubMed] [Google Scholar]
- 7.Alam A, Tamkeen N, Imam N, et al. Pharmacokinetic and molecular docking studies of plant-derived natural compounds to exploring potential anti-alzheimer activity. In: Silico Approach for Sustainable Agriculture. New York, NY: Springer; 2018:217-238. [Google Scholar]
- 8.Nykmukanova M, Mukazhanova ZB, Kabdysalym K, Eskalieva B, Beyatli A. Flavonoids from Verbascum marschallianum and V orientale. Chem Nat Compd. 2019;55:937-938. [Google Scholar]
- 9.Dar MA, Bhat MF, Hassan R, Masoodi MH, Mir SR, Mohiuddin R. Extensive phytochemistry, comprehensive traditional uses, and critical pharmacological profile of the great mullein: Verbascum thapsus L. Nat Prod J. 2019;9:158-171. [Google Scholar]
- 10.Riaz M, Rahman N, Zia-Ul-Haq M. Anthelmintic and insecticidal activities of Verbascum thapsus L. Pakistan J Zool. 2013;45:1593-1598. [Google Scholar]
- 11.Babamoradi N, Yousefi S, Ziarati P. Optimization of ultrasound‐assisted extraction of functional polysaccharides from common mullein (Verbascum thapsus L.) flowers. J Food Process Eng. 2018;41:e12851. [Google Scholar]
- 12.Ansari S, Daehler CC. Common Mullein (Verbascum Thapsus): A Literature Review. Honolulu, HI: University of Hawaii at Manoa; 2000. [Google Scholar]
- 13.Shinwari ZK, Gilani SS. Sustainable harvest of medicinal plants at Bulashbar Nullah, Astore (northern Pakistan). J Ethnopharmacol. 2003;84:289-298. [DOI] [PubMed] [Google Scholar]
- 14.Shahid M, Fatima H, Anjum F, Riaz M. Proximate composition, antioxidant activities and fatty acid profiling of selected mushrooms collected from Azad Jammu and Kashmir. Acta Pol Pharm Drug Res. 2020;77:145-153. [Google Scholar]
- 15.Irshad A, Sharif S, Riaz M, Anjum F. An insight into nutritional profile of selected Pleurotus species. J Biol Regul Homeost Agents. 2018;32:107-113. [PubMed] [Google Scholar]
- 16.Sanmugarajah V, Thabrew I, Sivapalan SR. Phyto, physicochemical standardization of medicinal plant Enicostemma littorale, blume. IOSR J Pharm. 2013;3:52-58. [Google Scholar]
- 17.Verma DK, Srivastav PP. Proximate composition, mineral content and fatty acids analyses of aromatic and non-aromatic Indian rice. Rice Sci. 2017;24:21-31. [Google Scholar]
- 18.Zhong K, Wang Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohydr Polym. 2010;80:19-25. [Google Scholar]
- 19.Shahid M, Anjum F, Iqbal Y, Khan SG, Pirzada T. Modification of date palm mucilage and evaluation of their nutraceutical potential. Pakistan J Agric Sci. 2020;57:401-411. [Google Scholar]
- 20.Ayub MA, Hussain AI, Hanif MA, et al. Variation in phenolic profile, β‐carotene and flavonoid contents, biological activities of two tagetes species from Pakistani flora. Chem Biodivers. 2017;14:e1600463. [DOI] [PubMed] [Google Scholar]
- 21.Riaz M, Shahid M, Jamil A, Saqib M. In vitro antioxidant potential of selected aphrodisiac medicinal plants. J Biol Regul Homeost Agents. 2017;31:419-424. [PubMed] [Google Scholar]
- 22.Safdar M, Naqvi SA, Anjum F, et al. Microbial biofilm inhibition, antioxidants, and chemical fingerprints of Afghani pomegranate peel extract documented by gas chromatography–mass spectrometry and Fourier transformation infrared. J Food Process Preserv. 2021;45:e15657. [Google Scholar]
- 23.Rajput M, Yadav A, Mohite S. Synthesis, characterization of benzimidazole derivatives as potent antimicrobial agents. Int J Pharm. 2020;17:279-285. [Google Scholar]
- 24.Tariq M, Gore M, Aruna K. Antibacterial and synergistic activity of ethanolic Ajwain (Trachyspermum ammi) extract on ESBL and MBL producing uropathogens. Int J Pharm Pharm Sci. 2014;6:278-284. [Google Scholar]
- 25.Qasim N, Shahid M, Yousaf F, et al. Therapeutic potential of selected varieties of phoenix Dactylifera L. against microbial biofilm and free radical damage to DNA. Dose-Response. 2020;18:1559325820962609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickramaratne MN, Punchihewa J, Wickramaratne D. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Compl Altern Med. 2016;16:466-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vongsak B, Kongkiatpaiboon S, Jaisamut S, Machana S, Pattarapanich C. In vitro alpha glucosidase inhibition and free-radical scavenging activity of propolis from Thai stingless bees in mangosteen orchard. Rev Bras Farmacogn. 2015;25:445-450. [Google Scholar]
- 28.Gul S, Abbasi MA, Khan KM, et al. Synthesis, antimicrobial evaluation and hemolytic activity of 2-[[5-alkyl/aralkyl substituted-1, 3, 4-oxadiazol-2-yl] thio]-N-[4-(4-morpholinyl) phenyl] acetamide derivatives. J Saudi Chem Soc. 2017;21:S425-S433. [Google Scholar]
- 29.Kauser A, Shah SMA, Iqbal N, et al. In vitro antioxidant and cytotoxic potential of methanolic extracts of selected indigenous medicinal plants. Prog Nutr. 2018;20:706-712. [Google Scholar]
- 30.Izadiyan P, Hemmateenejad B. Multi-response optimization of factors affecting ultrasonic assisted extraction from Iranian basil using central composite design. Food Chem. 2016;190:864-870. [DOI] [PubMed] [Google Scholar]
- 31.Farahani GT, Azari PY. Improving the oil yield of Iranian Jatropha curcas seeds by optimising ultrasound-assisted ethanolic extraction process: a response surface method. Qual Assur Saf Crop Foods. 2016;8:95-104. [Google Scholar]
- 32.Mihailović V, Kreft S, Benković ET, Ivanović N, Stanković MS. Chemical profile, antioxidant activity and stability in stimulated gastrointestinal tract model system of three Verbascum species. Ind Crop Prod. 2016;89:141-151. [Google Scholar]
- 33.Mahek A, Tanveer N, Jayalakshmi S. Pharmacognostical standardisation and physico-chemical valuations of leaves of Verbascum thapsus Linn. Int J Drug Dev Res. 2011;3:334-340. [Google Scholar]
- 34.Okeke C, Izundu A, Uzoechinda E. Phytochemical and proximate study of female pawpaw (Carica papaya Linn.) Caricaceae. J Sci Eng Technol. 2008;15:8207-8216. [Google Scholar]
- 35.Anbarasi G, Bhuvaneshwari S, Tamilarasi G, Selvapriya A, Sowmia C, Kanimozhi M. Preliminary screening of biologically active constituents of salt marsh plants from Vedaranyam cannal of Tamil Nadu. Eur J Pharm Med Res. 2018;5:325-332. [Google Scholar]
- 36.Ayoola P, Adeyeye A. Proximate analysis and nutrient evaluation of some Nigerian pawpaw seeds varieties. Science focus. 2009;14:554-558. [Google Scholar]
- 37.Ejelonu B, Lasisi A, Olaremu A, Ejelonu O. The chemical constituents of calabash (Crescentia cujete). Afr J Biotechnol. 2011;10:19631-19636. [Google Scholar]
- 38.Emebu P, Anyika J. Proximate and mineral composition of kale (Brassica oleracea) grown in Delta State, Nigeria. Pakistan J Nutr. 2011;10(2):190-194. [Google Scholar]
- 39.Zhu W, Xue X, Zhang Z. Ultrasonic-assisted extraction, structure and antitumor activity of polysaccharide from Polygonum multiflorum. Int J Biol Macromol. 2016;91:132-142. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Ai L, Yang Q, Liu Y, Shan L. Isolation and structural characterization of a polysaccharide from fruits of Zizyphus jujuba cv. Junzao. Int J Biol Macromol. 2013;55:83-87. [DOI] [PubMed] [Google Scholar]
- 41.Chang S, Hsu B, Chen B. Structural characterization of polysaccharides from Zizyphus jujuba and evaluation of antioxidant activity. Int J Biol Macromol. 2010;47:445-453. [DOI] [PubMed] [Google Scholar]
- 42.Moharam M, Abbas L. A study on the effect of microwave heating on the properties of edible oils using FTIR spectroscopy. Afr J Microbiol Res. 2010;4:1921-1927. [Google Scholar]
- 43.Shahid M, Riaz M, Talpur MM, Pirzada T. Phytopharmacology of Tribulus terrestris. J Biol Regul Homeost Agents. 2016;30:785-788. [PubMed] [Google Scholar]
- 44.Sultan S, Huma N, Butt MS, Shahid M. Antihypertensive and antioxidative potential of water soluble peptide fraction from different yoghurts. J Food Process Preserv. 2017;41:e12979. [Google Scholar]
- 45.Ahmad M, Prawez S, Manzoor N, et al. Effect of iNOX inhibitor aminoguanidine hemisulfate on amikacin induced consequences on anti-oxidant stress markers in wistar rat. J Anim Res. 2018;8:909-913. [Google Scholar]
- 46.Ashraf A, Sarfraz RA, Rashid MA, Shahid M. Antioxidant, antimicrobial, antitumor, and cytotoxic activities of an important medicinal plant (Euphorbia royleana) from Pakistan. J Food Drug Anal. 2015;23:109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morina F, Jovanovic L, Mojovic M, Vidovic M, Pankovic D, Veljovic Jovanovic S. Zinc‐induced oxidative stress in Verbascum thapsus is caused by an accumulation of reactive oxygen species and quinhydrone in the cell wall. Physiol Plant. 2010;140:209-224. [DOI] [PubMed] [Google Scholar]
- 48.Esfahani RN, Khaghani S, Azizi A, Mortazaeinezhad F, Gomarian M. Facile and eco-friendly synthesis of TiO2 NPs using extracts of Verbascum thapsus plant: An efficient photocatalyst for reduction of Cr (VI) ions in the aqueous solution. J Iran Chem Soc. 2020;17:205-213. [Google Scholar]
- 49.Kedare SB, Singh R. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011;48:412-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hussain AI, Anwar F, Iqbal T, Bhatti IA. Antioxidant attributes of four Lamiaceae essential oils. Pak J Bot. 2011;43:1315-1321. [Google Scholar]
- 51.Prakash V, Sagar A. To investigate leaf extracts of Verbascum thapsus Linn. For their antioxidant potential. J Med Plants. 2021;9:37-40. [Google Scholar]
- 52.Narayanaswamy N, Balakrishnan K. Evaluation of some medicinal plants for their antioxidant properties. Int J Pharm Tech Res. 2011;3:381-385. [Google Scholar]
- 53.Prakash V, Rana S, Sagar A. Studies on antibacterial activity of Verbascum thapsus. J Med Plants Stud. 2016;4:101-113. [Google Scholar]
- 54.Dulger G, Tutenocakli T, Dulger B. Antimicrobial potential of the leaves of common mullein (Verbascum thapsus L., Scrophulariaceae) on microorganisms isolated from urinary tract infections. J Med Plants Stud. 2015;3:86-89. [Google Scholar]
- 55.Weldegebrieal GK. Photocatalytic and antibacterial activityof CuO nanoparticles biosynthesized using Verbascum thapsus leaves extract. Optik. 2020;204:164230. [Google Scholar]
- 56.Manandhar S, Luitel S, Dahal RK. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J Trop Med. 2019;2019:1895340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castronovo LM, Vassallo A, Mengoni A, et al. Medicinal plants and their bacterial microbiota: A review on antimicrobial compounds production for plant and human health. Pathogens. 2021;10:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsuda H, Morikawa T, Ishiwada T, et al. Medicinal flowers. VIII. Radical scavenging constituents from the flowers of Prunus mume: Structure of prunose III. Chemi Pharm Bull. 2003;51:440-443. [DOI] [PubMed] [Google Scholar]
- 59.Shahid M, Naureen I, Riaz M, Anjum F, Fatima H, Rafiq MA. Biofilm inhibition and antibacterial potential of different varieties of garlic (Allium sativum) against sinusitis isolates. Dose-Response. 2021;19:15593258211050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaur V, Pande M, Upadhyaya K. Isolation and characterization of anti-diabetic components (bioactivity guided fractionation) from Verbascum chinense Scrophulariaceae) leaves. Res J Pharmaceut Biol Chem Sci. 2018;9:144-155. [Google Scholar]
- 61.Angeloni S, Zengin G, Sinan KI, et al. An insight into Verbascum bombyciferum extracts: Different extraction methodologies, biological abilities and chemical profiles. Ind Crop Prod. 2021;161:113201. [Google Scholar]
- 62.Zengin G, Mahomoodally MF, Sinan KI, et al. Evaluation of chemical constituents and biological properties of two endemic Verbascum species. Process Biochem. 2021;108:110-120. [Google Scholar]
- 63.Azad SB, Ansari P, Azam S, et al. Anti-hyperglycaemic activity of Moringa oleifera is partly mediated by carbohydrase inhibition and glucose-fibre binding. Biosci Rep. 2017;37:BSR20170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gholam HA, Falah H, Sharififar F, Mirtaj AS. The inhibitory effect of some Iranian plants extracts on the alpha glucosidase. Iran J Basic Med Sci. 2008;11:1-9. [Google Scholar]
- 65.El-Manawaty M, Gohar L. In vitro alpha-glucosidase inhibitory activity of Egyptian plant extracts as an indication for their antidiabetic activity. Asian J Pharm Clin Res. 2018;11:360-367. [Google Scholar]
- 66.Kuroda M, Iwabuchi K, Mimaki Y. Chemical constituents of the aerial parts of Scutellaria lateriflora and their α-glucosidase inhibitory activities. Nat Prod Commun. 2012;7:471-474. [PubMed] [Google Scholar]
- 67.Karakaya S, Gözcü S, Güvenalp Z, et al. The α-amylase and α-glucosidase inhibitory activities of the dichloromethane extracts and constituents of Ferulago bracteata roots. Pharm Biol. 2018;56:18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shahzadi T, Zaib M, Riaz T, Shehzadi S, Abbasi MA, Shahid M. Synthesis of eco-friendly cobalt nanoparticles using Celosia argentea plant extract and their efficacy studies as antioxidant, antibacterial, hemolytic and catalytical agent. Arabian J Sci Eng. 2019;44:6435-6444. [Google Scholar]
- 69.Sepahi S, Ghorani-Azam A, Sepahi S, Asoodeh A, Rostami S. In vitro study to evaluate antibacterial and non-haemolytic activities of four Iranian medicinal plants. W Indian Med J. 2014;63:289-293. [DOI] [PMC free article] [PubMed] [Google Scholar]