Abstract
Background:
Cartilage defects result in joint inflammation. The presence of proinflammatory factors has been described to negatively affect cartilage formation.
Purpose:
To evaluate the effect and timing of administration of triamcinolone acetonide (TAA), an anti-inflammatory drug, on cartilage repair using a mouse model.
Study Design:
Controlled laboratory study.
Methods:
A full-thickness cartilage defect was created in the trochlear groove of 10-week-old male DBA/1 mice (N = 80). Mice received an intra-articular injection of TAA or saline on day 1 or 7 after induction of the defect. Mice were euthanized on days 10 and 28 for histological evaluation of cartilage defect repair, synovial inflammation, and synovial membrane thickness.
Results:
Mice injected with TAA had significantly less synovial inflammation at day 10 than saline-injected mice independent of the time of administration. At day 28, the levels of synovitis dropped toward healthy levels; nevertheless, the synovial membrane was thinner in TAA- than in saline-injected mice, reaching statistical significance in animals injected on day 1 (70.1 ± 31.9 µm vs 111.9 ± 30.9 µm, respectively; P = .01) but not in animals injected on day 7 (68.2 ± 21.86 µm vs 90.2 ± 21.29 µm, respectively; P = .26). A thinner synovial membrane was moderately associated with less filling of the defect after 10 and 28 days (r = 0.42, P = .02; r = 0.47, P = .01, respectively). Whereas 10 days after surgery there was no difference in the area of the defect filled and the cell density in the defect area between saline- and TAA-injected knees, filling of the defect at day 28 was lower in TAA- than in saline-injected knees for both injection time points (day 1 injection, P = .04; day 7 injection, P = .01). Moreover, there was less collagen type 2 staining in the filled defect area in TAA- than in saline-injected knees after 28 days, reaching statistical significance in day 1–injected knees (2.6% vs 18.5%, respectively; P = .01) but not in day 7–injected knees (7.4% vs 15.8%, respectively; P = .27).
Conclusion:
Intra-articular injection of TAA reduced synovial inflammation but negatively affected cartilage repair. This implies that inhibition of inflammation may inhibit cartilage repair or that TAA has a direct negative effect on cartilage formation.
Clinical Relevance:
Our findings show that TAA can inhibit cartilage defect repair. Therefore, we suggest not using TAA to reduce inflammation in a cartilage repair setting.
Keywords: cartilage defect, inflammation, corticosteroids, anti-inflammatory agents
Articular cartilage is a highly specialized tissue that serves as a low-friction gliding surface and acts as a shock absorber to minimize peak pressures on the subchondral bone.3,4 Cartilage damage can occur during degenerative joint diseases or as a result of trauma.1,7,18,50 As articular cartilage has a limited capacity for self-repair, untreated cartilage defects have been implicated as a risk factor for the development of early-onset osteoarthritis.6,14 Furthermore, cartilage injuries evoke an inflammatory response that can remain for a long time.19,41
High levels of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin 1 (IL-1), and IL-6, are found in joints with cartilage defects.40,41 The presence of these mediators can negatively affect cartilage formation12,17; however, it is not only the injury itself but also the surgery involved in cartilage repair strategies that will lead to an inflammatory response in the joint. 53 Yet, the role of inflammation in cartilage repair is not completely understood. In wound healing, inflammation is the first step in the repair process, and in fracture repair, the absence of proinflammatory mediators leads to impaired bone healing. 45 On the other hand, prolonged inflammation results in impaired wound healing and increased scar formation. 11 Furthermore, a selective proinflammatory cytokine inhibitor (IL-1Ra) reduced cartilage degeneration and synovitis in an intra-articular fracture model 24 and knee pain and dysfunction in a clinical study in anterior cruciate ligament (ACL) patients. 26 These findings have led to the view that inflammation is initially needed, but then needs to be resolved to achieve optimal tissue repair.
To reduce inflammation, an anti-inflammatory drug can be used. Triamcinolone acetonide (TAA) is a corticosteroid and a potent anti-inflammatory drug. It is often injected intra-articularly to reduce the symptoms of knee osteoarthritis. 32 Also, it is acknowledged that glucocorticoids promote chondrogenic differentiation of human bone Marrow-derived Stromal/stem Cells (MSCs) by enhancing the expression of cartilage extracellular matrix genes. 8 However, there is controversy about its potential catabolic effects on the cartilage, 22 as TAA might increase cartilage loss in situations of knee osteoarthritis, 30 inhibit glycosaminoglycan production,2,21 and be chondrotoxic to chondrocytes.9,42 Moreover, Wernecke et al 49 concluded in their systematic review that corticosteroids seem to have a time- and dose-dependent effect on articular cartilage. They suggested that a beneficial effect on cartilage has been described at a low dose and shorter duration, whereas detrimental effects were found with higher doses and longer duration. This highlights the need to understand clearly under what conditions TAA may be beneficial or harmful in endogenous cartilage defect repair.
In the current study, we investigated the effect of the anti-inflammatory drug TAA on endogenous cartilage repair in a murine cartilage defect model. Moreover, we investigated whether the timing of TAA treatment would influence inflammation and cartilage defect repair.
Methods
Animals
Male DBA/1OlaHsd mice were purchased from Envigo, part of Jackson Laboratory. After transportation, mice were allowed a 7-day acclimatization period. All mice regained normal behavior within 24 hours after transportation. Mice were housed under specific pathogen-free conditions in groups of 3 or 4 per individually ventilated cage. They were maintained under a 12-hour light-dark cycle at 21°C and fed a standard rodent diet with food and water ad libitum.
The study was carried out following the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. Animal protocols and surgical procedures in this study were approved by the Institutional Animal Care and Use Committee (AVD101002016991, AEC 16-691-01; Erasmus MC University Medical Center, the Netherlands).
Experimental Outline
Full-thickness cartilage defects were surgically created as described here on day 0 in the left knee of 10-week-old male DBA/1 mice (N = 80) with a mean weight of 21.3 ± 1.8 g. Young mice were chosen because of their capability for consistent healing of the articular cartilage, whereas cartilage could not be repaired in older mice. 10 Cartilage repair was histologically evaluated after 4 weeks. Injections with TAA or saline (control) were administered either 1 day or 7 days postoperatively to investigate the effect of timing of anti-inflammatory therapy, resulting in 4 experimental groups (Figure 1). To obtain further insight in the repair process, cellularity and early tissue formation were assessed after 10 days. All animals received an intra-articular injection on both days 1 and 7: in the experimental groups, 1 day was injection with TAA, and in the control group, both days with saline. This setup gave a 50% reduction of the number of animals in the control group. Mice were randomly allocated to experimental groups. The number of animals per group was determined using a power calculation based on previous results. 47 The time points for injection at 1 day and 7 days postoperatively were chosen based on previous research. Synovitis levels were found to peak after 7 to 14 days after induction by an inflammatory stimulus. 20 The chosen time points therefore represent 2 phases of inflammation, a very early stage of inflammation (1 day after defect creation) and around the peak (7 days after defect creation).
Figure 1.
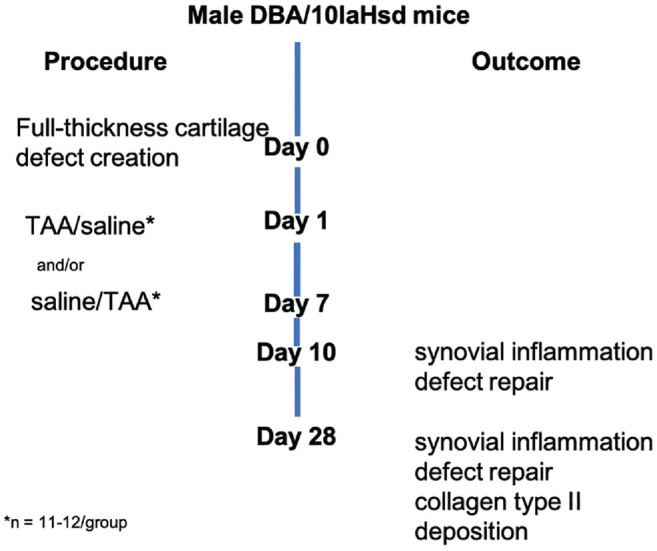
Overview of the experimental setup. TAA, triamcinolone acetonide.
Surgical Procedure
The procedure was performed during daytime in the animal facility operative theater. One hour before surgery, all mice received a subcutaneous injection of buprenorphine hydrochloride 0.05 mg/kg (Temgesic; Indivior Europe Ltd) for analgesia. Mice were anesthetized with an isoflurane/O2 mixture and positioned on a warmed plate, and a full-thickness cartilage defect was surgically created, as previously described, by 1 experienced surgeon (W.W.) who was blinded to the treatment group.10,47 In short, a full-thickness cartilage defect of 250 µm in length and 150 µm in width was made in the intercondylar notch along the patellar groove using a 25-gauge hypodermic needle (BD Bioscience). The procedure was regarded as successful when evident bleeding of the subchondral bone was observed. The joint capsule was closed with 2 resorbable 6-0 Vicryl suture (Ethicon; Johnson & Johnson) and checked for patellar stability during flexion and extension motion. Full weightbearing was allowed after recovery from anesthesia. All mice recovered quickly from the anesthesia.
Intra-articular Injection
Intra-articular injections of 25 µg TAA suspended in sterile 0.9% saline (Kenacort; Bristol Myers Squibb) or sterile 0.9% saline (as vehicle control) were infused into the operated knee 1 day and/or 7 days after surgery. The concentration was based on previous rodent studies.28,35,38 Under isoflurane/O2 anesthesia with the mouse in a supine position, the knee was fully extended and kept in place by surgical forceps. A small skin cut was made to expose the patellar tendon and joint capsule sutures. A 30-µL precision glass syringe (Hamilton Company) with a 30-gauge needle (BD Bioscience) was used to inject 6 µL into the joint space. In each experiment, all injections were performed by 1 person who was blinded to the treatment group (S.C. for the day 28 experiment and M.W. for the day 10 experiment). A second person (N.K.) assisted the injections by preparing all mice and injection fluids for the author performing the injections to ensure blinding of the injecting person.
Tissue Processing
At the experimental endpoint, animals were euthanized under anesthesia by cervical dislocation. Directly after euthanizing and removal of the skin, both hind legs were dissected below the hip and above the ankle. After removal of the subcutaneous fat and muscles, the knees were fixed in 4% formaldehyde for 7 days, followed by 2 weeks of decalcification in 10% ethylenediaminetetraacetic acid (EDTA), pH 7.4. Samples were further processed by dehydration and infiltration with paraffin. The femoral axis was adjusted to be upright against the embedding surface according to the method by Eltawil et al. 10 Subsequently, 6-µm sections were cut using a microtome (Leica RM-2135; Leica Microsystems). Per knee, 6 sections at a distance of 100 µm were used. As a landmark for section level, a cross section of the growth plate in 4 points was used. Each section was stained with hematoxylin and eosin (Sigma-Aldrich) to assess cell morphology and reconstitution of the osteochondral junction. Additionally, thionine staining (0.04%; Sigma-Aldrich) was used to analyze the filling of the defect and matrix staining intensity, representative of the amount and distribution of glycosaminoglycan. Sections from the day 28 endpoint were immunostained with a primary antibody against the cartilage matrix protein collagen type 2 (mouse anti-human, 1:100 dilution, II-II/II6B3; Developmental Studies Hybridoma Bank). In short, antigen retrieval was performed using pronase 1 mg/mL (Sigma-Aldrich) phosphate-buffered saline (PBS; Sigma-Aldrich), followed by hyaluronidase 10 mg/mL PBS (Sigma-Aldrich). To prevent cross-reaction with mouse antigens, the primary collagen type 2 antibody was preincubated overnight with a biotin-SP F(ab)2-labeled goat anti-mouse antibody (No. 115-066-062; Jackson ImmunoResearch Europe). After incubation, an alkaline-phosphatase-avidin–labeled antibody was used (Biogenex Laboratories), which, combined with the Neu Fuchsine substrate, resulted in pink staining. An isotype immunoglobulin G1 monoclonal antibody was used as a negative control. All sections were stained in 1 batch to reduce staining variation between different samples. Slides were imaged using a 40× objective on a slide scanner (NanoZoomer C9600-12; Hamamatsu Photonics) and processed using NanoZoomer Digital Pathology Image software (Hamamatsu Photonics). Knees with a patellar dislocation (determined by histological examination) were excluded from the analysis.
Evaluation of Cartilage Repair
All knees were scored using the validated semiquantitative histological scoring system for articular cartilage repair developed by Pineda et al. 33 The Pineda score contains the following subdomains: filling of the defect, reconstitution of the osteochondral junction, matrix staining, and cell morphology. A score ranging from 0 to 4 is given to the subdomain filling of the defect, matrix staining, and cell morphology. Reconstitution of the osteochondral junction is scored between 0 and 2, resulting in a total histological score ranging from 0 to 14, where 0 is the best repair and 14 is the worst. Scoring was performed independently by 2 authors (M.A.W., N.K.) blinded to the experimental group. Per knee, 3 different representative sections were scored, resulting in an average score per knee. The average of the 2 observer scores was used per animal. Interobserver reliability for cartilage repair scores based on absolute agreement was excellent (interclass correlation coefficient [ICC], 0.96; 95% CI, 0.92-0.98). The filling of the original defect area was measured using NanoZoomer Digital Pathology Image software. Collagen type 2 deposition was measured by the area stained positive for collagen type 2, divided by the total filled area of the defect. The percentages were averaged as previously described.
Evaluation of Joint Inflammation
Joint inflammation was measured by the synovial membrane thickness and Krenn scores 27 on the lateral side of the patellofemoral joint using NanoZoomer Digital Pathology Image software. The lateral side was chosen to be most representative because of the arthrotomy and sutures on the medial side. Per knee, the synovial thickness was measured in 3 different sections at 3 different positions, and the average of these 9 measurements was calculated. Per mouse, the average of 2 observers was used for further statistical analyses.
Statistical Analysis
Pineda scores were considered nonparametrical data. Differences between groups were assessed using a Kruskal-Wallis test with post hoc Dunn nonparametric comparison analysis. Filling of the defect and synovial thickness data were tested for normality using a Shapiro-Wilk test. All data were normally distributed, described as mean ± SD, and assessed using a 1-way analysis of variance, followed by post hoc Tukey honestly significant difference analysis. Correlation values were calculated using the Pearson rho test in case of normal data distribution. A Spearman rho test was used for nonparametrical data. Interobserver reliability of all measurements is expressed as ICC. A 2-way mixed model based on absolute agreement for single measures was used. An ICC >0.75 is regarded as excellent. 25 All statistical tests were 2-tailed. A P value <.05 was considered statistically significant. SPSS Statistics package for Mac Version 24.0 (IBM Corp) was used for all analyses.
Results
Of 80 animals, 79 reached the endpoint without signs of infection or abnormal behavior. One animal was lethargic and nonresponsive and had ruffled fur 1 day after surgery. Based on these signs and in consultation with the animal board, the animal was euthanized. In some of the operated mice, a limping gait pattern was observed in the first 3 days that resolved in all mice within 7 days. Some mice had a patellar dislocation (determined by histological examination) and were excluded from further histological analyses. Interestingly, the dislocations were not equally distributed over the groups. For the 28-day time point, 6 of 11 mice injected with TAA at day 1 had a patellar dislocation, compared with 1 of 11 mice in the saline control group (P = .02). Also, 6 of 11 mice injected with TAA at day 7 had a patellar dislocation compared with 2 of 11 mice in the saline control group. At the 10-day time point, only 1 mouse (injected with TAA 1 day after creating the defect) had a patellar dislocation, indicating that dislocations most likely developed after this time point.
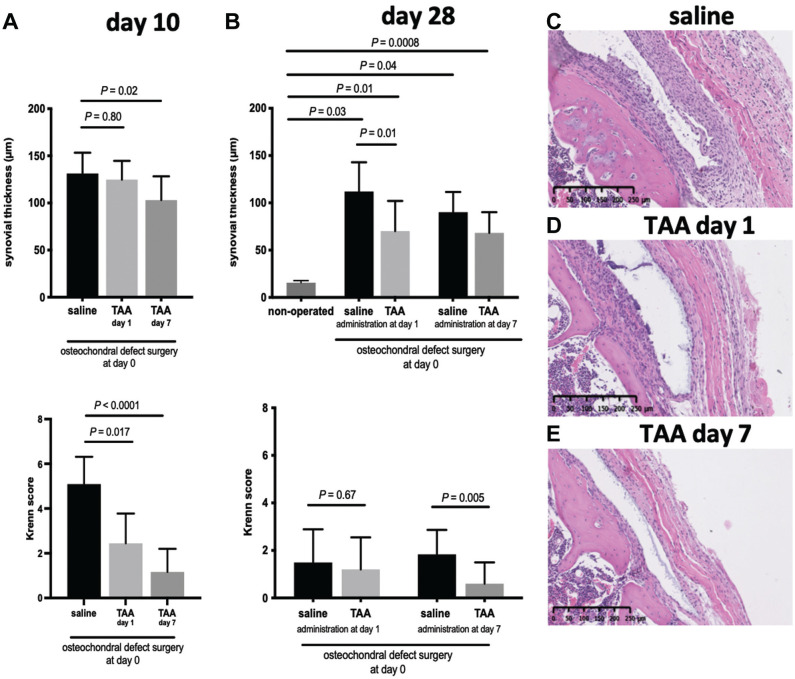
Intra-articular TAA Injection Reduced Synovial Inflammation and Synovial Membrane Thickening
To evaluate the effect of TAA on local inflammation in the joint, the thickness of the synovial membrane and the Krenn scores were evaluated at days 10 and 28 after surgery. The synovium was thicker in knees that underwent full-thickness cartilage defect induction surgery than in nonoperated knees (Figure 2). Mice injected with TAA 1 day after surgery had a significantly thinner synovial membrane than control saline-injected mice at day 28 (70.1 ± 31.9 µm vs 111.9 ± 30.9 µm, respectively; P = .01) (Figure 2), but not at day 10 (124.7 ± 20.0 µm vs 131.3 ± 22.0 µm, respectively; P = .80) (Figure 2). The Krenn scores of the mice injected 1 day after surgery were lower in mice injected with TAA than in saline-injected control mice at day 10 (P = .017), but not at 28 days (P = .67). Mice injected with TAA 7 days after surgery had a thinner synovial membrane than saline-injected mice at day 10 (103.1 ± 25.2 µm vs 131.3 ± 22.0 µm, respectively; P = .02) (Figure 2A) and at day 28 (68.2 ± 21.86 µm vs 90.2 ± 21.29 µm, respectively; P = .26) (Figure 2B), although this did not reach statistical significance. The Krenn scores in the mice injected 7 days after surgery were significantly lower in the TAA-injected than in the saline-injected mice at day 10 (P < .0001) and at day 28 (P = .005).
Figure 2.
Triamcinolone acetonide (TAA) reduced synovial inflammation. (A) The thickness of the synovium and the Krenn score on the lateral side of the patellofemoral joint at day 10 after full-thickness cartilage defect induction surgery. (B) The thickness of the synovium and the Krenn score on the lateral side of the patellofemoral joint at day 28 after full-thickness cartilage defect induction surgery. (C-E) Representative images of the Krenn scores at day 10 in each group. Mice were injected with TAA or saline 1 and/or 7 days after surgery. Each bar represents the mean ± SD of all defects in the respective group. n = 5-10 mice per group.
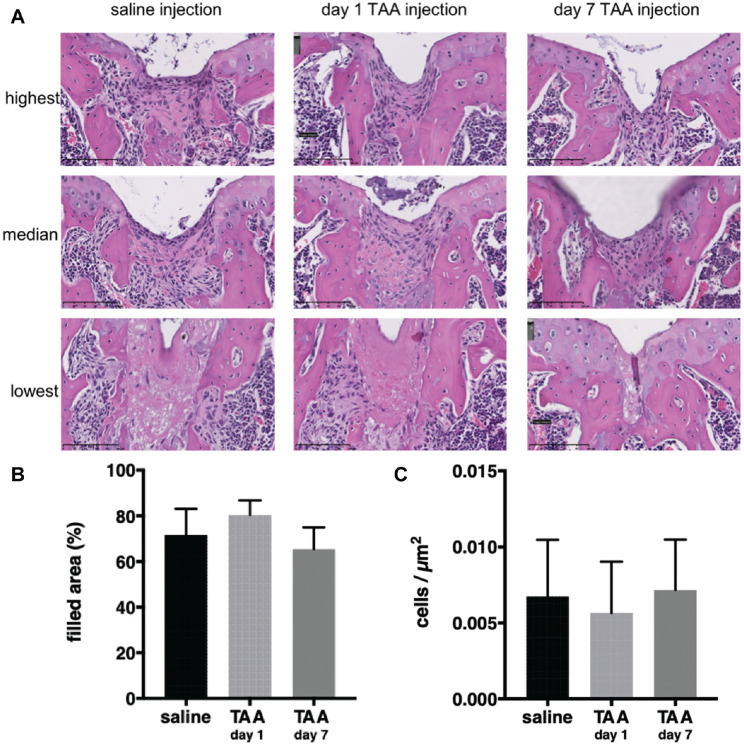
Cell Ingrowth Was Not Inhibited by TAA Administration
At day 10, the defect was filled with undifferentiated cells with a spindle-shaped morphology (Figure 3A). There was no difference in the area of the defect filled between the saline and TAA groups at day 10 (Figure 3B). Also, the timing of TAA injection did not affect the filling of the defect (Figure 3B). In addition, the cell density in the defect area was similar in all groups (Figure 3C). This suggests that TAA did not affect migration and/or proliferation of cells in the defect.
Figure 3.
Cellularity was not affected by triamcinolone acetonide (TAA). (A) Representative images of the highest, median, and lowest cell density in the defect at day 10 stratified per group. (B) Area filled relative to the original size of the defect. (C) Number of cells per µm 2 . Each bar represents the mean ± SD of all defects in the respective group. n = 11-12 mice per group.
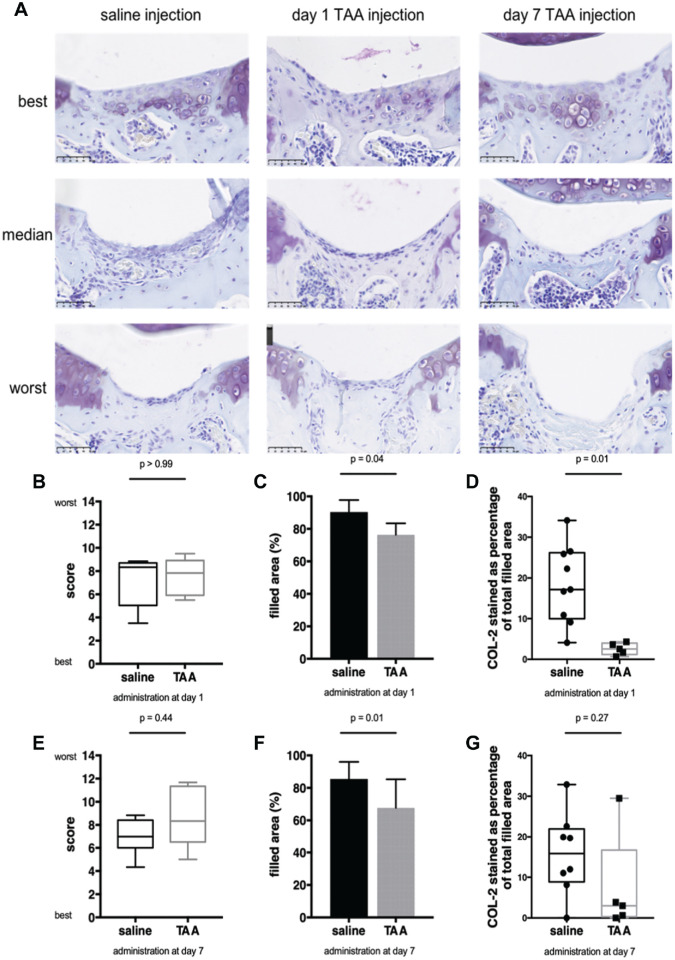
Cartilage Repair Was Inhibited by TAA Treatment
The effect of TAA injection on cartilage repair was histologically evaluated. A total of 28 days after generation of the full-thickness defect, the tissue filling the defect consisted of chondrocyte-like cells surrounded by thionine-stained matrix (Figure 4A). The median Pineda cartilage repair score was similar in mice treated after 1 day with TAA and in control mice that received a saline injection (8.9 [IQR, 5.9-8.9] vs 8.3 [IQR, 5.0-8.7], respectively; P > .99) (Figure 4B). There were no differences between mice injected with saline, TAA at day 1, or TAA at day 7 on subdomains of the Pineda cartilage repair score: restoration of the osteochondral junction, matrix staining intensity, or cell morphology (see Appendix Figure A1, available in the online version of this article). However, a significantly lower percentage of the defect area was filled in mice treated with TAA after 1 day than in control mice injected with saline (76.4% ± 7.0% vs 90.4% ± 7.4%, respectively; P = .04) (Figure 4C). Moreover, a significantly lower percentage of the filled defect area stained positive for collagen type 2 in mice treated with TAA after 1 day than in control mice injected with saline (2.6% ± 1.5% vs 18.5% ± 9.6%, respectively; P = .01) (Figure 4D and Figure 5, A and B). In mice treated with TAA after 7 days, median cartilage repair scores at day 28 were similar to those of control mice injected with saline (8.3 [IQR, 6.5-11.3] vs 7.0 [IQR, 6-8.4], respectively; P = .44) (Figure 4E). The percentage of the defect area that was filled was also significantly lower in TAA-injected than in saline-injected control mice (67.8% ± 17.6% vs 85.55% ± 1.5%, respectively; P = .01) (Figure 4F and Figure 5, A and C). Also, a seemingly lower percentage of the filled defect area stained positive for collagen type 2 in mice treated with TAA after 7 days than in control mice injected with saline (7.4% ± 12.5% vs 15.8% ± 10.1%, respectively; P = .27) (Figure 4G), although this was nonsignificant because of 1 outlier.
Figure 4.
Triamcinolone acetonide (TAA) resulted in less filling and collagen type 2 (COL-2) deposition in the defect. (A) Representative images of the best, median, and worst cartilage repair in the control group treated with saline and groups injected with TAA 1 or 7 days after the creation of a full-thickness defect. The scale bar represents 50 µm. (B) Cartilage repair (Pineda) score at day 28 and injection at day 1. (C) Area filled on day 28 relative to the original size of the defect in knees injected on day 1. (D) Percentage of COL-2 relative to the filled area at day 28 in knees injected on day 1. (E) Cartilage repair (Pineda) score at day 28 and injection at day 7. (F) Area filled at day 28 relative to the original size of the defect in knees injected on day 7. (G) Percentage of COL-2 relative to the filled area at day 28 in knees injected on day 7. Box plots represent the 25th and 75th percentiles with the median, and the whiskers indicate the maximum and minimum. Each bar represents the mean ± SD of all defects in the respective group. n = 5-10 mice per group.
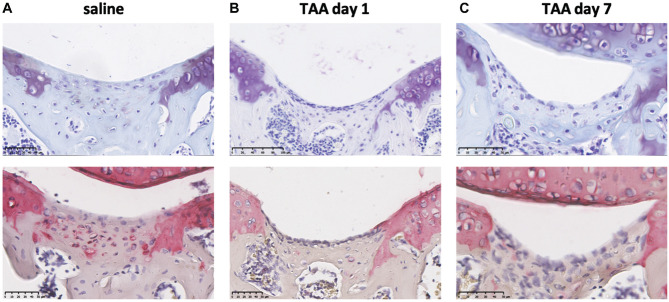
Figure 5.
Triamcinolone acetonide (TAA) resulted in less collagen type 2 deposition in the defect. (A-C) Representative images of thionin (top) and collagen type 2 (bottom) staining at day 28 in the control group treated with saline and groups injected with TAA at day 1 or 7 after the creation of a full-thickness defect. Thionin and collagen type 2 staining on the same sample are shown. The scale bar represents 50 µm.
A Moderate Correlation Was Observed Between Synovial Membrane Thickness and Defect Filling
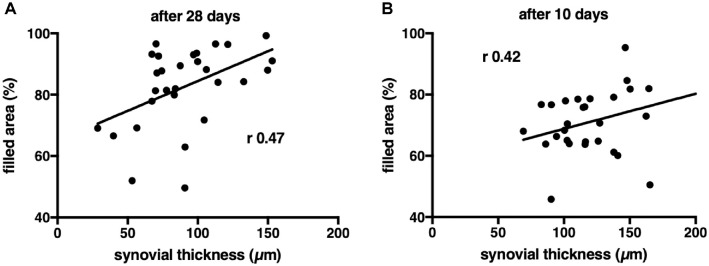
To investigate the relationship between inflammation and cartilage repair, we correlated synovium thickness with defect filling (Figure 6). The thickness of the synovial membrane was moderately associated with the defect filling at day 10 (r = 0.42; P = .02) and day 28 (r = 0.47; P = .01). The thickness of the synovial membrane was not associated with cell density in the filled defect at day 10 (r = −0.08; P = .69). Also, the thickness of the synovial membrane and cartilage repair scores were not associated at day 28 (r = −0.18; P = .35) and day 10 (r = 0.006; P = .97). No associations were found between Krenn score and defect filling, probably because of the limited range and variation of Krenn scores (0-8).
Figure 6.
A thinner synovial membrane was moderately associated with less filling of the defect. (A) Scatterplot showing the correlation between synovial thickness and area of the defect filled at day 28. (B) Scatterplot showing the correlation between synovial thickness and area of the defect filled at day 10. Each dot represents a unique sample.
Discussion
Previous in vitro studies have shown that pro-inflammatory factors inhibit cartilage formation. However, there have only been a few studies exploring the role of anti-inflammatory treatment in cartilage defect repair in vivo. Therefore, the purpose of this study was to evaluate the effect of TAA, an anti-inflammatory drug, on endogenous cartilage defect repair. Our main finding was that intra-articular injection with TAA in a murine model for endogenous cartilage repair reduced synovial inflammation but also inhibited cartilage repair. After 28 days, we observed less filling of the defect in knees injected with TAA. After 10 days, defect filling was not significantly affected by TAA treatment. This observation might suggest that TAA does not affect the influx or proliferation of cells but negatively influences tissue formation in the defect.
To our knowledge, this is the first in vivo study evaluating the role of TAA in a model of endogenous cartilage defect repair. We observed less filling of the defect and less collagen type 2 deposition after 28 days in knees injected with TAA. Although there are no studies on the role of TAA in cartilage defect repair, TAA has been used to study the effect on cartilage in both healthy and osteoarthritic joints, showing conflicting results. One study found dose-dependent degenerative changes in the cartilage of healthy rabbit knees after 2 to 6 weeks of weekly 3-mg TAA injections. 31 Another study found more cartilage degeneration indicated by higher Mankin scores after injection of the extended-release form of TAA in rat knees that had surgical ACL transection and destabilization of the meniscus. 36 These findings can be considered in line with the reduced deposition of collagen type 2 and the reduced filling of the defect we observed. On the other hand, in a collagenase-induced osteoarthritis rat model, injection of TAA as bolus or as an extended-release formulation had an effect on cartilage degeneration. 34 When Frisbie and colleagues 13 administered 2 intra-articular doses of 12 mg TAA 13 and 27 days after surgical induction of osteochondral fractures in equine carpal bones, less cartilage degeneration, indicated by lower Mankin scores, was found. Interestingly, the effects were most pronounced if TAA was injected in the contralateral (uninjured) joint rather than the diseased joint, indicating that beneficial effects were most pronounced at very low concentrations. The extended presence of TAA in the joint and higher concentrations of TAA in the joint can be detrimental for the cartilage, as seen in the study of Rudnik-Jansen et al, 36 although this may depend on the type of pathology present in the joint.
In our study, mice injected with TAA had less synovial inflammation than saline-injected mice. This is in line with what is known in the literature about rodent arthritis models.29,34 Furthermore, in some cases TAA injection even reduced the inflammation to the healthy nonoperated situation (as demonstrated by a Krenn score of 1), which is in line with other studies that reported less mononuclear cell infiltration and intimal hyperplasia in response to TAA.13,27,39 We found a thicker synovial membrane to be associated with more filling of the defect. This association suggests that inflammation has a positive effect on the filling of the defect. In fracture repair research, knockout studies in mice have shown that the absence of inflammation-related proinflammatory molecules, such as TNF-α and cyclooxygenase 2, leads to a delay in bone healing.15,52 This implies that inflammation is needed to initiate healing. On the contrary, excessive inflammation was shown to inhibit in vitro MSC chondrogenesis in a model with inflammatory factors present from osteoarthritic joints and from traumatically injured joints.17,48 Reducing the inflammation by inhibition of IL-1α, oxozeaenol, or tofacitinib could partially restore this inhibitory effect on cartilage formation.17,44 However, cartilage formation was shown to be inhibited when anti-inflammatory compounds such as a protein kinase inhibitor or TNF-α inhibitor were used at an early stage of chondrogenesis.23,44 This might explain why we observed a decrease in collagen type 2 deposition, as we administered our anti-inflammatory compound at an early stage of new cartilage formation in the defect site. These findings highlight the challenging balance between inflammation and cartilage defect repair. We acknowledge, though, that inflammation is more comprehensive than synovial thickness and mononuclear cell infiltration only. Serological measurements of the synovial fluid and the composition of inflammatory cells inside the synovial membrane over time could help to better evaluate the role of joint inflammation in cartilage repair.
Cartilage repair scores and filling of the defect were slightly worse when the defect was treated after 7 days, albeit nonsignificantly. It is known that the presence of chronic proinflammatory factors can impair chondrogenesis and stimulate degeneration of newly formed cartilage.12,46 Saris et al 37 showed in an in vivo study in goats that cartilage repair scores were worse in surgical defects treated late compared with defects treated immediately after induction. After 7 days, the proinflammatory factors may have dropped to a lower level, reaching a more chronic inflammatory phase. 19 However, it is debatable whether TAA is the right tool to inhibit acute inflammation at these time points. Inflammation is a dynamic process that occurs not only between days 1 and 7, but throughout time. A study in horses that were intra-articularly injected with TAA showed that TAA remained present in the synovial fluid up to 14 days, whereas it was undetectable in serum after 48 hours. 5 This means that TAA administered at day 1 might inhibit inflammation up to 14 days and thereby not only the acute inflammation phase but also the chronic phase. 19 Also, the day 7 injection inhibits this phase, resulting in less inflammation after this time point. Therefore, it remains uncertain what the effect is if only the acute phase would be inhibited. Future studies should explore the role of selectively inhibiting the acute inflammation phase and the effect on cartilage repair. Based on our results, we can conclude that TAA inhibited cartilage repair regardless of the time of administration, and neither early nor late administration is advised.
TAA is known to inhibit both tissue outgrowth from ligaments and collagen synthesis in tenocytes, indicating that TAA can impair wound healing in many respects.43,51 TAA injection in a rat destabilization-induced osteoarthritis model (ACL transection and partial meniscectomy) resulted in increased joint instability and subluxations in the longer term. 36 Although patellar dislocation is relative, frequently occurring after surgical procedures in mice, 47 its frequency was significantly higher in TAA-injected animals (P = .004). Our results indicate that TAA might have a negative effect on wound healing of the arthrotomy site, thus resulting in patellar dislocation. Interestingly, on day 10 only 1 of 24 mice injected with TAA had developed a patellar dislocation, impying that patellar dislocations develop after this time point. For mice in the 10-day group, the endpoint was only 9 or 3 days after TAA injection, and most likely the arthrotomy sutures would still provide some support. Hence, this time point might have been too early to observe effects on wound healing. Another explanation might be that TAA reduced pain in the inflamed joint 1 to 4 weeks after administration. 16 This pain reduction might allow mice in the TAA group to move more freely throughout the study period, causing more patellar dislocations than in the saline-injected group.
For translation of these results to clinical practice, some limitations of our study need to be considered. First, with the model chosen we could only study the effect of reducing inflammation on cartilage repair that relies on the body’s endogenous repair capacity. It is unclear what the effect will be on procedures such as articular chondrocyte implantation (ACI) and/or transplantation strategies, although based on our study it seems that TAA mainly affects the production of extracellular matrix. This indicates that TAA could also negatively influence repair using approaches such as ACI. Moreover, for this study we specifically selected DBA mice at 10 weeks of age, as this strain at this age has been shown to be most optimal to study modulation of the endogenous cartilage repair capacity. It is unclear how the results of these young adult mice will translate to a young or middle-aged patient population with a cartilage defect. Additionally, although the dose regimen we tested was consistent and translated from clinical guidelines, we cannot exclude that another dose, timing, and/or frequency would have generated a different effect. The model furthermore differs from the clinical rehabilitation, as the animals were allowed to move freely after surgery. This could potentially affect the stability of the early repair tissue in the defect. In a clinical situation, the patient would be nonweightbearing for the first few weeks after a cartilage repair procedure. Finally, our focus was mainly on cartilage repair and inflammation of the joint, and we have not measured pain. It would, however, be interesting to see if TAA could play a role in the postoperative care to reduce pain after injuring the articular cartilage.
Conclusion
Intra-articular injection of TAA reduced synovial inflammation but inhibited cartilage repair. This implies that inhibition of inflammation with TAA might not be the right direction to move cartilage repair forward. The thin line between the level of inflammation required and optimal cartilage defect repair underscores the importance of incorporating rational control of inflammation into cartilage repair strategies. The use of TAA to reduce inflammation after cartilage defect treatment to improve cartilage repair is therefore not warranted.
Supplemental Material
Supplemental material, sj-pdf-1-ajs-10.1177_03635465221083693 for Intra-articular Administration of Triamcinolone Acetonide in a Murine Cartilage Defect Model Reduces Inflammation but Inhibits Endogenous Cartilage Repair by Marinus A. Wesdorp, Serdar Capar, Yvonne M. Bastiaansen-Jenniskens, Nicole Kops, Laura B. Creemers, Jan A.N. Verhaar, Gerjo J.V.M. Van Osch and Wu Wei in The American Journal of Sports Medicine
Footnotes
Submitted June 3, 2021; accepted January 13, 2022.
One or more of the authors has declared the following potential conflict of interest or source of funding: The research performed in this article received financial support from a grant from the AO Foundation: AO-OCD Consortium TA1711481: Osteochondral Bone Repair with Innovative Tissue Engineering and 3D Bioactive Composite Scaffolds (M.A.W. salary and materials); a grant from the European Union’s Horizon 2020 research and innovation program under Marie Sklodowska Curie grant agreement No. 642414 (S.C. salary and materials). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Supplemental material for this article is available at http://journals.sagepub.com/doi/suppl/10.1177/03635465221083693
References
- 1. Aroen A, Loken S, Heir S, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211-215. [DOI] [PubMed] [Google Scholar]
- 2. Beekhuizen M, Bastiaansen-Jenniskens Y, Koevoet JLM, et al. Osteoarthritic synovial tissue inhibition of proteoglycan production in human osteoarthritic knee cartilage establishment and characterization of a long-term cartilage synovium-coculture. Arthritis Rheum. 2011;63:1918-1927. [DOI] [PubMed] [Google Scholar]
- 3. Buckwalter JA, Mankin HJ, et al. Instructional course lectures, the American Academy of Orthopaedic Surgeons—articular cartilage. Part I: tissue design and chondrocyte-matrix interactions. J Bone Joint Surg Am. 1997;79(4):600-611. [Google Scholar]
- 4. Buckwalter JA, Mankin HJ, et al. Instructional course lectures, the American Academy of Orthopaedic Surgeons—articular cartilage. Part II: degeneration and osteoarthrosis, repair, regeneration, and transplantation. J Bone Joint Surg Am. 1997;79(4):612-632. [Google Scholar]
- 5. Chen CL, Sailor JA, Collier J, Wiegand J, et al. Synovial and serum levels of triamcinolone following intra-articular administration of triamcinolone acetonide in the horse. J Vet Pharmacol Ther. 1992;15(3):240-246. [DOI] [PubMed] [Google Scholar]
- 6. Convery FR, Akeson WH, Keown GH, et al. The repair of large osteochondral defects. An experimental study in horses. Clin Orthop Relat Res. 1972;82:253-262. [PubMed] [Google Scholar]
- 7. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG, et al. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-460. [DOI] [PubMed] [Google Scholar]
- 8. Derfoul A, Perkins GL, Hall DJ, Tuan RS, et al. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells. 2006;24(6):1487-1495. [DOI] [PubMed] [Google Scholar]
- 9. Dragoo JL, Danial CM, Braun HJ, Pouliot MA, Kim HJ, et al. The chondrotoxicity of single-dose corticosteroids. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1809-1814. [DOI] [PubMed] [Google Scholar]
- 10. Eltawil NM, De Bari C, Achan P, Pitzalis C, Dell’accio F, et al. A novel in vivo murine model of cartilage regeneration. Age and strain-dependent outcome after joint surface injury. Osteoarthritis Cartilage. 2009;17(6):695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eming SA, Krieg T, Davidson JM, et al. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514-525. [DOI] [PubMed] [Google Scholar]
- 12. Fahy N, de Vries-van Melle ML, Lehmann J, et al. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthritis Cartilage. 2014;22(8):1167-1175. [DOI] [PubMed] [Google Scholar]
- 13. Frisbie DD, Kawcak CE, Trotter GW, Powers BE, Walton RM, McIlwraith CW, et al. Effects of triamcinolone acetonide on an in vivo equine osteochondral fragment exercise model. Equine Vet J. 1997;29(5):349-359. [DOI] [PubMed] [Google Scholar]
- 14. Fuller JA, Ghadially FN, et al. Ultrastructural observations on surgically produced partial-thickness defects in articular cartilage. Clin Orthop Relat Res. 1972;86:193-205. [DOI] [PubMed] [Google Scholar]
- 15. Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs. 2001;169(3):285-294. [DOI] [PubMed] [Google Scholar]
- 16. Godwin M, Dawes M, et al. Intra-articular steroid injections for painful knees. Systematic review with meta-analysis. Can Fam Physician. 2004;50:241-248. [PMC free article] [PubMed] [Google Scholar]
- 17. Heldens GT, Blaney Davidson EN, Vitters EL, et al. Catabolic factors and osteoarthritis-conditioned medium inhibit chondrogenesis of human mesenchymal stem cells. Tissue Eng Part A. 2012;18(1-2):45-54. [DOI] [PubMed] [Google Scholar]
- 18. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M, et al. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18(7):730-734. [DOI] [PubMed] [Google Scholar]
- 19. Irie K, Uchiyama E, Iwaso H, et al. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee. 2003;10(1):93-96. [DOI] [PubMed] [Google Scholar]
- 20. Jackson MT, Moradi B, Zaki S, et al. Depletion of protease-activated receptor 2 but not protease-activated receptor 1 may confer protection against osteoarthritis in mice through extracartilaginous mechanisms. Arthritis Rheumatol. 2014;66(12):3337-3348. [DOI] [PubMed] [Google Scholar]
- 21. Jansen I, Tellegen A, Tryfonidou M, et al. Brief exposure to triamcinolone acetonide, but not its continous presence, strongly inhibits cartilage regeneration by chondrocytes. Osteoarthritis Cartilage. 2016;24:S337. [Google Scholar]
- 22. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;10:CD005328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawaguchi A, Nakaya H, Okabe T, et al. Blocking of tumor necrosis factor activity promotes natural repair of osteochondral defects in rabbit knee. Acta Orthop. 2009;80(5):606-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kimmerling KA, Furman BD, Mangiapani DS, et al. Sustained intra-articular delivery of IL-1RA from a thermally-responsive elastin-like polypeptide as a therapy for post-traumatic arthritis. Eur Cell Mater. 2015;29:124-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koo TK, Li MY, et al. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropractic Med. 2016;15(2):155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kraus VB, Birmingham J, Stabler TV, et al. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: a randomized controlled pilot trial (NCT00332254). Osteoarthritis Cartilage. 2012;20(4):271-278. [DOI] [PubMed] [Google Scholar]
- 27. Krenn V, Morawietz L, Burmester GR, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49(4):358-364. [DOI] [PubMed] [Google Scholar]
- 28. Kroin JS, Kc R, Li X, et al. Intraarticular slow-release triamcinolone acetate reduces allodynia in an experimental mouse knee osteoarthritis model. Gene. 2016;591(1):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar A, Bendele AM, Blanks RC, Bodick N, et al. Sustained efficacy of a single intra-articular dose of FX006 in a rat model of repeated localized knee arthritis. Osteoarthritis Cartilage. 2015;23(1):151-160. [DOI] [PubMed] [Google Scholar]
- 30. McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317(19):1967-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moskowitz RW, Davis W, Sammarco J, Mast W, Chase SW, et al. Experimentally induced corticosteroid arthropathy. Arthritis Rheum. 1970;13(3):236-243. [DOI] [PubMed] [Google Scholar]
- 32. Paik J, Duggan ST, Keam SJ, et al. Triamcinolone acetonide extended-release: a review in osteoarthritis pain of the knee. Drugs. 2019;79(4):455-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pineda S, Pollack A, Stevenson S, Goldberg V, Caplan A, et al. A semiquantitative scale for histologic grading of articular cartilage repair. Acta Anat (Basel). 1992;143(4):335-340. [DOI] [PubMed] [Google Scholar]
- 34. Rudnik-Jansen I, Colen S, Berard J, et al. Prolonged inhibition of inflammation in osteoarthritis by triamcinolone acetonide released from a polyester amide microsphere platform. J Control Release. 2017;253:64-72. [DOI] [PubMed] [Google Scholar]
- 35. Rudnik-Jansen I, Schrijver K, Woike N, et al. Intra-articular injection of triamcinolone acetonide releasing biomaterial microspheres inhibits pain and inflammation in an acute arthritis model. Drug Deliv. 2019;26(1):226-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rudnik-Jansen I, Tellegen AR, Pouran B, et al. Local controlled release of corticosteroids extends surgically induced joint instability by inhibiting tissue healing. Br J Pharmacol. 2019;176(20):4050-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saris D, Dhert WJ, Verbout A, et al. Joint homeostasis: the discrepancy between old and fresh defects in cartilage repair. J Bone Joint Surg Br. 2003;85(7):1067-1076. [DOI] [PubMed] [Google Scholar]
- 38. Siebelt M, Korthagen N, Wei W, et al. Triamcinolone acetonide activates an anti-inflammatory and folate receptor-positive macrophage that prevents osteophytosis in vivo. Arthritis Res Ther. 2015;17:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sieker JT, Ayturk UM, Proffen BL, Weissenberger MH, Kiapour AM, Murray MM, et al. Immediate administration of intraarticular triamcinolone acetonide after joint injury modulates molecular outcomes associated with early synovitis. Arthritis Rheumatol. 2016;68(7):1637-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Swärd P, Frobell R, Englund M, Roos H, Struglics A, et al. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis)—a cross-sectional analysis. Osteoarthritis Cartilage. 2012;20(11):1302-1308. [DOI] [PubMed] [Google Scholar]
- 41. Swärd P, Struglics A, Englund M, Roos HP, Frobell RB, et al. Soft tissue knee injury with concomitant osteochondral fracture is associated with higher degree of acute joint inflammation. Am J Sports Med. 2014;42(5):1096-1102. [DOI] [PubMed] [Google Scholar]
- 42. Syed HM, Green L, Bianski B, Jobe CM, Wongworawat MD, et al. Bupivacaine and triamcinolone may be toxic to human chondrocytes: a pilot study. Clin Orthop Relat Res. 2011;469(10):2941-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tempfer H, Gehwolf R, Lehner C, et al. Effects of crystalline glucocorticoid triamcinolone acetonide on cultered human supraspinatus tendon cells. Acta Orthop. 2009;80(3):357-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Beuningen HM, de Vries-van Melle ML, Vitters EL, et al. Inhibition of TAK1 and/or JAK can rescue impaired chondrogenic differentiation of human mesenchymal stem cells in osteoarthritis-like conditions. Tissue Eng Part A. 2014;20(15-16):2243-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van der Kraan PM, et al. The interaction between joint inflammation and cartilage repair. Tissue Eng Regen Med. 2019;16(4):327-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wehling N, Palmer GD, Pilapil C, et al. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009;60(3):801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wei W, Bastiaansen-Jenniskens YM, Suijkerbuijk M, et al. High fat diet accelerates cartilage repair in DBA/1 mice. J Orthop Res. 2017;35(6):1258-1264. [DOI] [PubMed] [Google Scholar]
- 48. Wei W, Rudjito E, Fahy N, et al. The infrapatellar fat pad from diseased joints inhibits chondrogenesis of mesenchymal stem cells. Eur Cell Mater. 2015;30:303-314. [DOI] [PubMed] [Google Scholar]
- 49. Wernecke C, Braun HJ, Dragoo JL, et al. The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop J Sports Med. 2015;3(5):2325967115581163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Widuchowski W, Widuchowski J, Trzaska T, et al. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177-182. [DOI] [PubMed] [Google Scholar]
- 51. Wong MW, Tang YN, Fu SC, Lee KM, Chan KM, et al. Triamcinolone suppresses human tenocyte cellular activity and collagen synthesis. Clin Orthop Relat Res. 2004;421:277-281. [DOI] [PubMed] [Google Scholar]
- 52. Xie C, Ming X, Wang Q, et al. COX-2 from the injury milieu is critical for the initiation of periosteal progenitor cell mediated bone healing. Bone. 2008;43(6):1075-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yang KG, Saris DB, Verbout AJ, Creemers LB, Dhert WJ, et al. The effect of synovial fluid from injured knee joints on in vitro chondrogenesis. Tissue Eng. 2006;12(10):2957-2964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ajs-10.1177_03635465221083693 for Intra-articular Administration of Triamcinolone Acetonide in a Murine Cartilage Defect Model Reduces Inflammation but Inhibits Endogenous Cartilage Repair by Marinus A. Wesdorp, Serdar Capar, Yvonne M. Bastiaansen-Jenniskens, Nicole Kops, Laura B. Creemers, Jan A.N. Verhaar, Gerjo J.V.M. Van Osch and Wu Wei in The American Journal of Sports Medicine