Abstract
The broad effects of bariatric/metabolic surgery on virtually every tissue and organ system remain unexplained. Weight loss, although a major factor, does not fully account for the rapid, full, and durable remission of type 2 diabetes, return of islet function, reduction of the prevalence of cancers, increase in gray matter of the brain, and decrease in all-cause mortality. This review supports the thesis that the metabolic syndrome is not a group of separate diseases but rather multiple expressions of a shared defect in the utilization of carbohydrates and lipids. That error is probably caused by a dysmetabolic signal from the foregut, stimulated by food, that limits entry of 2-carbon fragments into the tricarboxylic acid cycle, the accumulation of lactate and, in turn, increases in glucose and insulin. Surgery limits that signal by reducing contact between food and foregut mucosa. Speciation of that signal(s) may offer a new pathway for drug development.
Keywords: Metabolic, Type 2 diabetes, Gastric bypass, Gastric sleeve, Metabolic syndrome, Bariatric surgery, Lactate
It makes no sense. How could two modest yet sharply different operations on the foregut (Fig. 1A) produce full and durable remission of our most costly diseases, including type 2 diabetes (T2D), severe obesity, and non-alcoholic steatotic hepatitis (NASH); improve cognition and mental health; and even produce a decrease in the prevalence of cancer and all-cause mortality? Further, the mechanisms for these dramatic results remain obscure, with some crediting alterations in gut signaling while others maintain that these changes are not due to the surgery and are only the result of weight loss.
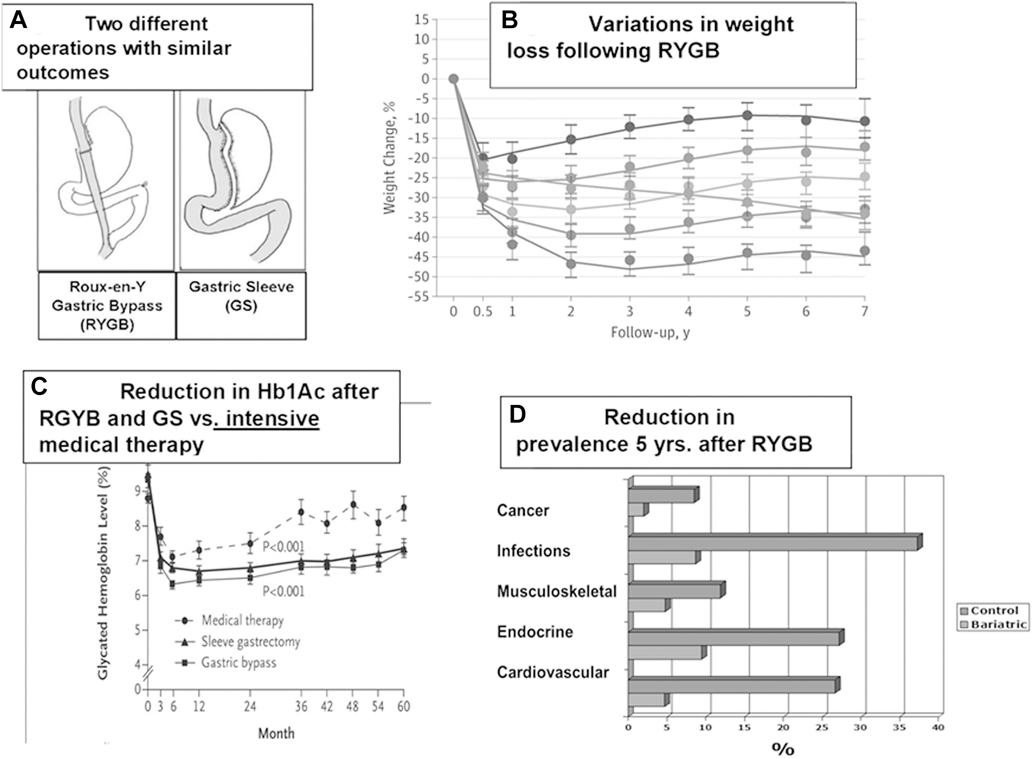
Fig. 1.
Clinical outcomes of metabolic surgery. (A) Configuration of the Roux-en-Y gastric bypass (RYGB) and gastric sleeve surgeries. (B) Weight loss of patients who had RYGB surgery [1]. (C) Remission of diabetes after RYGB surgery [3]. (D) Effects of metabolic surgery on diseases [20].
The purpose of this review is to consider these claims, evaluate these data, and apply these clues to the understanding of T2D and the other expressions of the metabolic syndrome.
What is the evidence?
The evidence is strong. Multiple clinical trials reflect the efficacy of surgery. Metabolic surgery, previously known as bariatric surgery, is the most effective treatment for severe obesity. In the Longitudinal Assessment of Bariatric Surgery (LABS) trial involving 1,738 patients with severe obesity who underwent the Roux-en-Y gastric bypass (RYGB), a mean loss of 28.4% of original weight was maintained for 7 years [1]. None regained their original weight (Fig. 1B).
The operations also offer the most effective treatment for T2D. We were startled, in 1982, when glucose values returned to normal within a week after the RYGB, an observation confirmed in our LABS series [2]. Among the 488 subjects with T2D, the rates of full remission at 1, 3, 5, and 7 years were 71.2%, 69.4%, 64.6% and 60.2%, respectively. In a comparison of intensive medical therapy versus the RYGB by Schauer et al. [3], the requirement for antidiabetic medications fell to zero in the surgical group while the level of medications remained the same in the medical cohort (Fig. 1C).
Metabolic surgery has also been shown to improve cardiac function and decrease cardiac adverse events. Aminian et al. [4], in a comparison of 287,438 adult patients with T2D versus 2,287 patients who underwent metabolic surgery, found a significantly lower risk of myocardial infarction, ischemic stroke, and mortality in the operated group. Kindel and Strande [5], in their systematic review, concluded that surgery “significantly improves cardiac geometry, function, and symptoms related to obesity cardiomyopathy. for end-stage heart failure patients, bariatric surgery has been successfully used for weight loss as a bridge to cardiac transplantation.”
Metabolic surgery can also reduce the thickness of atherosclerotic plaques. Marchesi et al. [6] reported significant reduction in carotid intima-media thickness in 22 patients at 3 levels—bulb, common, and internal carotid—with a 1-, 3-, 6-, and 12-month follow-up.
NASH, the most common cause of cirrhosis, is also improved. Lee et al. [7], based on a systematic review and meta-analysis of 32 cohort studies, found that metabolic surgery resulted in a biopsy-confirmed resolution of NASH in 66% of patients, inflammation in 50%, ballooning degeneration in 76%, and fibrosis in 40%.
The efficacy of metabolic surgery in the treatment of polycystic ovary syndrome (PCOS) led the European Society for Endocrinology [8] to recommend that surgery be included as a treatment for PCOS. In their systematic review of 13 studies involving 2,130 patients, the preoperative incidence of PCOS of 45.6% decreased to 6.8% after 12 months. Infertility decreased from 18.2% to 4.3%.
Metabolic surgery is also associated with a decrease in the prevalence of cancer. Adams et al. [9] compared the cancer incidence and mortality data from the Utah Cancer Registry for 6,596 Utah applicants for driver’s licenses who had undergone RYGB and a group of 9,442 persons with severe obesity who had not over 24 years, with a mean of 12.5 years. Total cancer incidence and cancer mortality were significantly lower in the surgical group.
Most remarkable are the anatomic effects on brain structure, as reflected by neuroimaging. A review by Nota et al. [10] noted that the improvement of cognition following metabolic surgery is associated with a recovery from preoperative brain abnormalities, with greater white and gray matter integrity and functional brain changes. Prehn et al. [11] and Bohon and Geliebter [12] also found the improvement of cognitive function following surgery was associated with larger gray matter volumes in frontotemporal brain areas, accompanied by smaller volume in the ventral striatum. Similarly, Hankir et al. [13] found a reduction in hypothalamic inflammation. Handley et al. [14] after a systematic review of 414 publications concluded that “significant and rapid weight loss resulting from bariatric surgery is associated with prompt and sustained improvements in cognitive function including memory, executive function, and cognitive control.”
Early studies also indicate that bariatric surgery affects existing end-organ damage (e.g., diabetic microvascular complications such as nephropathy [15], neuropathy [16], and retinopathy [17]) and improves islet cell function [18].
Finally, surgery is associated with reductions in all-cause, cardiovascular, and cancer-related mortality. As early as 1997, a difference in mortality between the surgical and comparison group, 9% vs. 29%, was reported [19]. That observation has been confirmed in Canada [20], Norway [21], and Sweden [22]. In a study comparing 2,500 surgical patients versus a 7,462 person cohort in the Veterans Administration [23], Adams et al. [24] in an extensive review, concluded that “bariatric surgical patients have: 1) significantly reduced long-term all-cause mortality when compared to severely obese non-bariatric surgical control groups; 2) but still a greater mortality when compared to the general population; 3) reduced cardiovascular-, stroke-, and cancer-caused mortality when compared to severely obese non-operated controls; in spite of 4) increased risk for externally caused death such as suicide.”
Fig. 1D. summarizes the effects of metabolic surgery on cancer, infections, and musculoskeletal, endocrine, and cardiovascular disease. Surgery has also been reported to improve quality of life, especially in those with high psychosocial burdens [25,26].
Ockham’s Razor
So how do we make sense of these observations? In dealing with complex issues, history can be helpful. In the Middle Ages, William of Ockham, an English Franciscan friar and a notable philosopher and theologian (c. 1287–1347), similarly confronted with confusing information, proposed that “simpler solutions are more likely to be correct than complex ones.” That “law of parsimony” is now known as Ockham’s razor. Let’s review the clues.
Surgery, as noted, leads to rapid remission of T2D and the other co-morbidities of the metabolic syndrome, with a reduction in all-cause mortality. Reversal of diabetes after RYGB occurs very quickly before there is appreciable loss in adiposity [27]. Diabetes remission is durable after RYGB and extends to periods when weight has stabilized and caloric intake equals caloric expenditure [27].
Decreasing contact between food and the foregut, whether by surgical means, intestinal liners [28], ablation of duodenal mucosa [29], or diet [30] reverses the syndrome. In surgery, the extent of diabetes remission depends on the amount of foregut excluded. Weight loss and diabetes resolution were greatest for patients undergoing biliopancreatic diversion/duodenal switch, followed by gastric bypass, and least for banding procedures [31]. In 1998, our research group proposed that diabetes is a “disease of the foregut.” In response to foregut contact with nutrients, individuals with the disease produce a factor (signal) that causes diabetes [32]. When nutrients are withheld (via fasting or very low caloric intake) or when the foregut is bypassed (RYGB), the signal is not generated, with remission of diabetes. The most convincing evidence for an intestinal signal is the recent report by Constantin et al. [33], who found that
compared to 1.1B4 cells incubated with baseline sera (control), cells exposed to sera from LSG-treated participants exhibited (i) increased viability and proliferation (p < 0.05); (ii) diminished levels of reactive oxygen species (ROS) and p53 (p < 0.05); (iii) enhanced protein expression of autophagy-related (Sirtuin 1) SRT1 and p62/SQSTM1 (p < 0.05); (iv) significantly decreased transcript levels of ER stress markers (p, 0.05); and (v) augmented insulin expression (p < 0.05). Conversely, the 6-month conventional therapy appeared not to impact on circulating redox status.
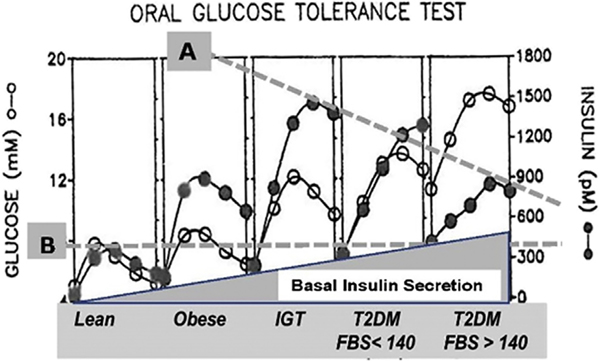
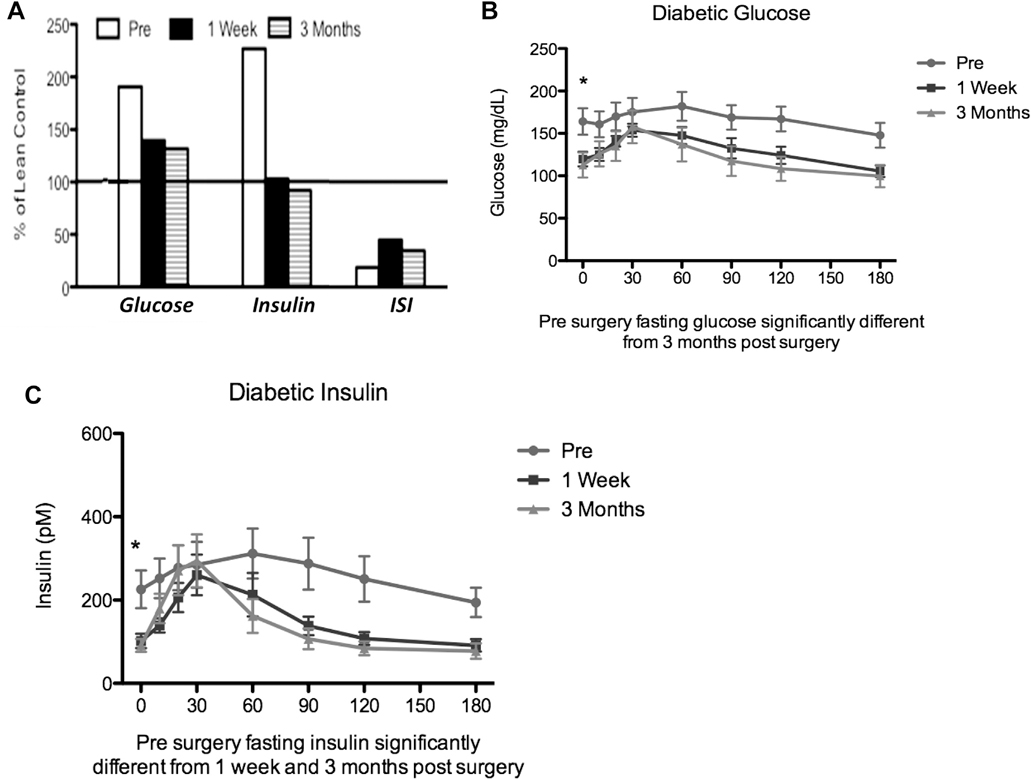
Glucose and insulin, fasting as well as responses to a meal, are normalized after metabolic surgery. The prevailing etiology of diabetes is as follows: 1) Insulin resistance is the underlying cause and first manifestation of the disease; 2) insulin secretion increases to overcome resistance; and 3) hyperglycemia is a result of lower insulin secretion in response to glucose because of beta cell failure. This etiology is clearly demonstrated by the oral glucose tolerance curves of patients as they become obese and then develop diabetes (Fig. 2). Based on this etiology, our original hypothesis for the remission of diabetes was that insulin sensitivity would increase or insulin secretion would increase after metabolic surgery. This was not what we observed. Fasting glucose and insulin concentrations were normalized as early as 1 week after surgery, but insulin resistance remained up to 3 months after surgery (Fig. 3A). The response of glucose to a meal was normalized (Fig. 3B) after surgery, suggesting normal peripheral glucose disposal, but peak insulin concentration was not increased after surgery (Fig. 3C). This normalization occurred in spite of continued insulin resistance (Fig. 3A).
Fig. 2.
Plasma glucose and insulin during oral glucose tolerance tests [27]. Fasting blood insulin levels increase with progression of type 2 diabetes (T2D), but maximum secretion of insulin falls with advancement of the disease in response to a meal (A). However, even in advanced T2D, the response is almost double that of a normal individual (B). The most striking effect, however, of the progression of the disease is the continued increase in fasting basal insulin secretion, a finding that indicates that in advanced T2D, even when these individuals are sleeping and fasting, they secrete insulin at levels that exceed the normal response after meals.
Fig. 3.
Changes in glucose, insulin, and insulin sensitivity index (ISI) after Roux-en-Y gastric bypass (RYGB). (A) Glucose, insulin, and ISI for diabetic patients before surgery (Pre) and after RYGB (1 wk and 3 mo) expressed as a percent of lean control subjects. (B) Time course for changes in glucose after a meal challenge in diabetic patients before surgery (Pre) and after RYGB (1 wk and 3 mo). Data redrawn from Reed et al. [46]. (C) Time course for changes in insulin after a meal challenge in diabetic patients before surgery (Pre) and after RYGB (1 wk and 3 mo). Data redrawn from Reed et al [46].
Could lactate be the answer? Lactate levels, widely used in the assessment of the acutely ill, may be the critical clue to the puzzle of the metabolic syndrome. In a systematic review of the care of acutely ill patients, Vincent et al. [34] documented that a decrease in lactate levels was consistently associated with lower mortality rates in all subgroups. Lazzeri et al. [35] reported that higher admission lactate levels in acute cardiac patients predict high mortality levels and that higher lactate clearance is associated with better outcome. In chronic disease, lactate levels have not found wide application even though there are a number of publications indicating direct relationships between fasting blood lactate and components of the metabolic syndrome [36–39]. In 1993, Chen et al. noted that plasma lactate concentrations are elevated in obesity and yet higher in T2D [40].
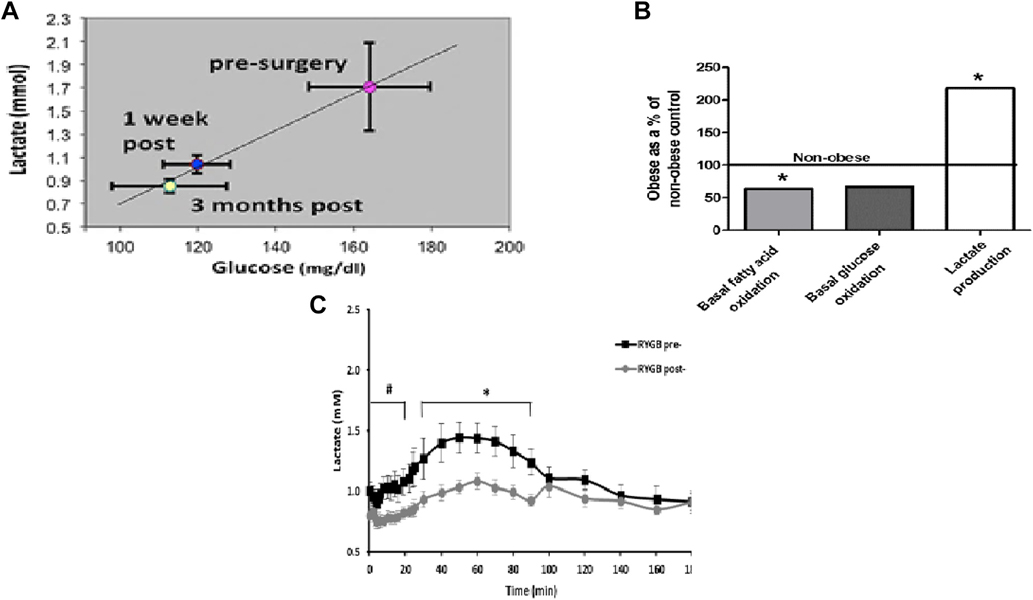
Individuals with severe obesity, with and without T2D, have elevated plasma lactate concentrations that return to levels similar to those in lean individuals after surgery [41]. Fig. 4A shows the correlation between fasting glucose and lactate from diabetic subjects before surgery, 1 week after surgery, and 3 months after surgery [42]. The remarkable correlation between glucose and lactate raises the question as to which is the chicken and which is the egg. Lactate is a major substrate for gluconeogenesis in the liver. If lactate were elevated because of depressed gluconeogenesis, one would predict that glucose production would be reduced, which is not the case. If glucose were elevated because of increased gluconeogenesis, the substrate and lactate would be reduced. This then suggests that elevated lactate may be driving glucose production. Chondronikola et al. [43] demonstrated that the rate of endogenous glucose production in patients with obesity was correlated with fasting plasma lactate, consistent with this hypothesis.
Fig. 4.
Changes in plasma lactate and muscle metabolism after Roux-en-Y gastric bypass (RYGB). (A) Relationship of lactate and glucose before surgery and 1 week and 3 months after RYGB [41]. (B) In vitro metabolism of glucose and fatty acids in muscle fiber strips from patients with obesity (data expressed as a percent of lean controls) [50,51]. Plasma lactate from non-diabetic individuals with obesity during an intravenous glucose tolerance test 42.
If lactate is driving gluconeogenesis to cause hyperglycemia, the question arises as to the source of the lactate. Our studies of muscle substrate oxidation using whole body studies, as well as experiments with human muscle fiber strips and human muscle homogenates, are summarized in Fig. 4B [44]. Complete oxidation of fatty acids (CO2 production) and the complete oxidation of glucose are approximately 40% lower in obese muscle than in lean controls. Depressed oxidation of substrates was compensated by an increase in lactate production, indicating a “mitochondrial dysfunction” with production of ATP shifted toward more glycolysis and accumulation of lactate. These findings are in accord with numerous references to the altered mitochondria structure and function in muscle of diabetic patients [45].
If mitochondrial substrate oxidation is impaired, there would, of necessity, be a compensatory increase in glycolysis to lactate; this would be reflected in elevated plasma lactate. Lactate was measured during an intravenous glucose tolerance test in non-diabetic individuals with obesity 1 week before RYGB surgery and again 1 week post surgery (Fig. 4C). Lactate began to rise with the administration of glucose, and the rate of rise was increased after insulin, suggesting uptake of glucose into muscle and incomplete oxidation. A week after surgery, fasting lactate was reduced and there was only a minimal increase with the administration of glucose and insulin, suggesting complete oxidation of glucose. Since insulin sensitivity was not changed a week after surgery, the amount of glucose taken up was the same before and after surgery [46]. Lower lactate suggests that muscle mitochondrial substrate oxidation was enhanced after gastric bypass surgery.
In normal physiology, we usually think about muscle lactate production in relation to intense physical exercise. During a sprint, mitochondrial substrate oxidation is not capable of providing enough ATP, and glycolysis to lactate is increased to maintain energy production at a level to continue exercise. Lactate from muscle goes to the liver, where glucose is produced, and returned to the muscle in a process called the Cori cycle. The same process occurs in the metabolic syndrome, except the muscle is producing lactate in the resting state. Mitochondrial oxidation of substrates is reduced to apoint where ATP production is below that needed to maintain muscle function and a compensatory increase in glycolysis to lactate is required. Lactate produced by muscle causes increased plasma lactate concentration, and this drives liver gluconeogenesis by mass action. We have termed this phenomenon the “vicious Cori cycle” because it occurs during the entire day, rather than just during exercise, as in the original Cori cycle [41]. This is a dangerous metabolic condition because the liver is challenged with an elevated level of substrate and reducing equivalents, which can lead not only to the production of glucose, but also fatty acids, cholesterol, and low density lipoprotein. These synthetic processes are further driven by insulin; thus, hyperinsulinemia compounds symptoms of the metabolic syndrome. As the impairment in mitochondrial substrate oxidation progresses, lactate concentration increases to the point where endogenous glucose production generates hyperglycemia and diabetes.
Hypothesis
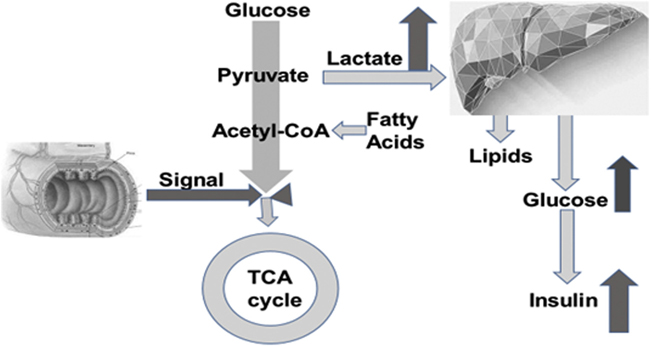
In applying Ockham’s razor, we believe that the simplest explanation for the remission of diabetes after metabolic surgery is the following: In vulnerable individuals, a signal(s) from the foregut, stimulated by contact with food, impairs mitochondrial oxidation of substrates, forcing a greater reliance on glycolytic flux to lactate to maintain energy production. Our data suggest that this may be the case in muscle, but it could also be the situation in other tissues and might explain the pancreatic beta cell impairment that causes fasting hyperinsulinemia and reduced insulin secretion in response to glucose. Further, by a mechanism related to the foregut, RYGB surgery reduces the intestinal signal that impairs mitochondrial substrate oxidation, allowing lactate, glucose, and possibly insulin concentrations to return to normal. This hypothesis is illustrated in Fig. 5.
Fig. 5.
A representation of the “vicious Cori cycle” hypothesis. By this hypothesis, the metabolic syndrome is caused by a signal from the gut that is generated in response to contact with food. This gut signal impairs oxidation of glucose and fatty acids. Glycolysis to lactate is accelerated to supply energy to offset impairment of substrate oxidation. Lactate concentration in the plasma is elevated, and the liver is supplied with excess substrate and reducing equivalents throughout the day. Overproduction of glucose and lipids from lactate results in hyperinsulinemia and symptoms of the metabolic syndrome.
Although we find this a reasonable hypothesis, there are other thoughtful investigators, such as Yoshino et al. [47], who do not agree that intestinal physiology and signaling have any role and conclude, based on their studies, that “the metabolic benefits of gastric bariatric surgery and diet were similar and were apparently related to weight loss itself with no evident clinically important effects independent of weight loss.” There are several clinical observations that counter that view: 1) Glucose and insulin levels normalize within a week after the surgery before there is any significant loss of weight; 2) insulin resistance does not return to normal levels for over 3 months in spite of the resolution of the diabetes, 3) T2D usually does not return when patients regain some weight; 4) 10% of patients who develop T2D are lean; and 5) only one third of those with severe obesity develop T2D. The most convincing evidence, however, of the role of intestinal signaling is the study by Constantin et al. [33] in which serum from bariatric patients produced the metabolic changes of T2D in human islet cultures before surgery but not after the procedures.
Even so, how can we explain the variations in the presentation of this metabolic defect? After all, only one third of those with severe obesity present with T2D, and 10% of patients with T2D are lean. We believe these variations are a reflection of the hosts’ responses, similar to other diseases due to a single cause. COVID-19 is a good example, with its outcomes that vary from no symptoms in one individual to a mortal destruction of lungs, heart, brain, and kidneys in another.
Further, how can we explain how 3 very different approaches (i.e., the gastric bypass, gastric sleeve, and intestinal liner) can have similar effects that only differ in degree? All 3 interventions could include a group of enteroendocrine cells, which respond to food, centered in the fundus of the stomach that diminish in concentration further down the duodenum and upper jejunum. The RYGB bypasses this area, the gastric sleeve removes the largest concentration, and the liner, least effective, reduces distal contact.
The next step in research is to identify the signal and seek approaches either to disable the source or develop a medication to nullify the signal. Ghrelin, as noted by Gu et al. [48], fits many of these characteristics with sharp decreases following the gastric sleeve, but it is difficult to fit that with their findings that it increases after RYGB, underscoring the complexities of this challenge.
Conclusion
The salutary outcomes of bariatric surgery compel a re-evaluation of our concepts about T2D and its comorbidities. The metabolic syndrome is not a group of separate diseases, but rather, multiple expressions of a shared defect in the utilization of carbohydrates and lipids [49–51]. That error is probably caused by a dysmetabolic signal from the foregut, stimulated by food, that limits entry of 2-carbon fragments into the tricarboxylic acid cycle, the accumulation of lactate, and, in turn, increases in glucose and insulin. Speciation of that signal(s) may offer a new pathway for drug development. These changes have been attributed by some solely to weight loss but are far more complex, as indicated by their occurrence before there is any significant change in adiposity.
These observations also indicate that the use of insulin or drugs that increase insulin secretion and/or sensitivity needs careful review. If the “vicious Cori cycle” is the underlying mechanism causing diabetes, treating downstream insulin resistance and insulin secretion only hides the symptom (i.e., hyperglycemia). It would be rather like treating whooping cough with cough syrup; the cough is better, but the disease is unchecked.
Acknowledgments
This study was supported by grants: NIH DK120296, NIH R56HL132961-01A1, and U01DK066526 and Johnson & Johnson #993300014.
Footnotes
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
References
- [1].Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the longitudinal assessment of bariatric surgery (LABS) study. JAMA Surg 2018;153(5):427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222(3):339–50. discussion 350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schauer PR, Bhatt DL, Kirwan JP, et al. , STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 2017;376(7):641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Aminian A, Zajichek A, Arterburn DE, et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA 2019;322(13):1271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kindel TL, Strande JL. Bariatric surgery as a treatment for heart failure: review of the literature and potential mechanisms. Surg Obes Rel Dis 2018;14(1):117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marchesi F, Giacosa R, Reggiani V, et al. Morphological changes in the carotid artery intima after gastric bypass for morbid obesity. Obes Surg 2017;27(2):357–63. [DOI] [PubMed] [Google Scholar]
- [7].Lee Y, Doumouras AG, Yu J, et al. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2019;17(6):1040–1060.E11. [DOI] [PubMed] [Google Scholar]
- [8].Busetto L, Dicker D, Azran C, et al. Obesity management task force of the European association for the study of obesity released “practical recommendations for the post-bariatric surgery medical management.” Obes Surg 2018;28(7):2117–21. [DOI] [PubMed] [Google Scholar]
- [9].Adams TD, Stroup AM, Gress RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring) 2009;17(4):796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nota MHC, Vreeken D, Wiesmann M, Aarts EO, Kiliaan Hazebroek A J. Obesity affects brain structure and function- rescue by bariatric surgery? Neurosci Biobehav Rev 2020. Jan;108:646–57. [DOI] [PubMed] [Google Scholar]
- [11].Prehn K, Profitlich T, Rangus I, et al. Bariatric surgery and brain health-a longitudinal observational study investigating the effect of surgery on cognitive function and gray matter volume. Nutrients 2020;12(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bohon C, Geliebter A. Change in brain volume and cortical thickness after behavioral and surgical weight loss intervention. Neuroimage Clin 2019;21:101640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hankir MK, Seyfried F, Miras AD, Cowley MA. Brain feeding circuits after Roux-en-Y gastric bypass. Trends Endocrinol Metab 2018;29(4):218–37. [DOI] [PubMed] [Google Scholar]
- [14].Handley JD, Williams DM, Caplin S, Stephens JW, Barry J. Changes in cognitive function following bariatric surgery: a systematic review. Obes Surg 2016;26(10):2530–7. [DOI] [PubMed] [Google Scholar]
- [15].Li K, Zou J, Ye Z, et al. Effects of bariatric surgery on renal function in obese patients: a systematic review and meta analysis. PLoS One 2016;11(10):e0163907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Aghili R, Malek M, Tanha K, Mottaghi A. The effect of bariatric surgery on peripheral polyneuropathy: a systematic review and meta-analysis. Obes Surg 2019;29(9):3010–20. [DOI] [PubMed] [Google Scholar]
- [17].Kim YJ, Kim BH, Choi BM, Sun HJ, Lee SJ, Choi KS. Bariatric surgery is associated with less progression of diabetic retinopathy: a systematic review and meta-analysis. Surg Obes Relat Dis 2017;13(2):352–60. [DOI] [PubMed] [Google Scholar]
- [18].Camastra S, Vitali A, Anselmino M, et al. Muscle and adipose tissue morphology, insulin sensitivity and beta-cell function in diabetic and nondiabetic obese patients: effects of bariatric surgery. Sci Rep 2017;7(1):9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].MacDonald KG, Long SD, Swanson MS, et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg 1997;1(3):213–20. discussion 220. [DOI] [PubMed] [Google Scholar]
- [20].Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg 2004;240(3):416–23. discussion 423–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kauppila JH, Tao W, Santoni G, et al. Effects of obesity surgery on overall and disease-specific mortality in a 5-country populationbased study. Gastroenterology 2019;157(1):119–127.e1. [DOI] [PubMed] [Google Scholar]
- [22].Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish Obese Subjects. N Engl J Med 2007;357(8):741–52. [DOI] [PubMed] [Google Scholar]
- [23].Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA 2015;313(1):62–70. [DOI] [PubMed] [Google Scholar]
- [24].Adams TD, Mehta TS, Davidson LE, Hunt SC. All-cause and causespecific mortality associated with bariatric surgery: a review. Curr Atheroscler Rep 2015;17(12):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wolfe BM, Kvach E, Eckel RH. Treatment of obesity: weight loss and bariatric surgery. Circ Res 2016;118(11):1844–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 2017;14(3):160–9. [DOI] [PubMed] [Google Scholar]
- [27].Pories WJ, MacDonald KG Jr, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr 1992;55(2):582S–5S. [DOI] [PubMed] [Google Scholar]
- [28].van Rijn S, Betzel B, de Jonge C, et al. The effect of 6 and 12 months duodenal-jejunal bypass liner treatment on obesity and type 2 diabetes: a crossover cohort study. Obes Surg 2018;28(5):1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sullivan S, Edmundowicz SA, Thompson CC. Endoscopic bariatric and metabolic therapies: new and emerging technologies. Gastroenterology 2017;152(7):1791–801. [DOI] [PubMed] [Google Scholar]
- [30].Hemmingsen B, Gimenez-Perez G, Mauricio D, Roque I Figuls M, Metzendorf MI, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev 2017;12(12):CD003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122(3):248–256.e5. [DOI] [PubMed] [Google Scholar]
- [32].Hickey MS, Pories WJ, MacDonald KG, et al. A new paradigm for type 2 diabetes mellitus: could it be a disease of the foregut? Ann Surg 1998;227(5):637–43. discussion 643–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Constantin A, Dumitrescu M, Nemecz M, et al. Sera of obese type 2 diabetic patients undergoing metabolic surgery instead of conventional treatment exert beneficial effects on beta cell survival and function: results of a randomized clinical study. Obes Surg 2019;29(5):1485–97. [DOI] [PubMed] [Google Scholar]
- [34].Vincent J, e Silva AQ, Couto L, Taccone FS. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care 2016;20(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lazzeri C, Valente S, Chiostri M, Gensini GF. Clinical significance of lactate in acute cardiac patients. World J Cardiol 2015;7(8):483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Crawford SO, Ambrose MS, Hoogeveen RC, Brancati FL, Ballantyne CM, Young JH. Association of lactate with blood pressure before and after rapid weight loss. Am J Hypertens 2008;21(12):1337–42. [DOI] [PubMed] [Google Scholar]
- [37].Crawford SO, Hoogeveen RC, Brancati FL, et al. Association of blood lactate with type 2 diabetes: the Atherosclerosis Risk in Communities Carotid MRI Study. Int J Epidemiol 2010;39(6):1647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Juraschek SP, Shantha GPS, Chu AY, et al. Lactate and risk of incident diabetes in a case-cohort of the atherosclerosis risk in communities (ARIC) study. PLoS One 2013;8(1):e55113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Juraschek SP, Selvin E, Miller ER, Brancati FL, Young JH. Plasma lactate and diabetes risk in 8045 participants of the atherosclerosis risk in communities study. Ann Epidemiol 2013;23(12):791–796.E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen YD, Varasteh BB, Reaven GM. Plasma lactate concentration in obesity and type 2 diabetes. Diabete Metab 1993;19(4):348–54. [PubMed] [Google Scholar]
- [41].Pories WJ, Dohm GL. Diabetes: have we got it all wrong? Hyperinsulinism as the culprit: surgery provides the evidence. Diabetes Care 2012;35(12):2438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jones TE, Pories WJ, Houmard JA, et al. Plasma lactate as a marker of metabolic health: implications of elevated lactate for impairment of aerobic metabolism in the metabolic syndrome. Surg 2019;166(5):861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chondronikola M, Magkos F, Yoshino J, et al. Effect of progressive weight loss on lactate metabolism: a randomized controlled trial. Obesity 2018;26(4):683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Houmard JA, Pories WJ, Dohm GL. Severe obesity: Evidence for a deranged metabolic program in skeletal muscle? Exerc Sport Sci Rev 2012;40(4):204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 2005;54(1):8–14. [DOI] [PubMed] [Google Scholar]
- [46].Reed MA, Pories WJ, Chapman W, et al. Roux-en-Y gastric bypass corrects hyperinsulinemia implications for the remission of type 2 diabetes. J Clin Endocrinol Metab 2011;96(8):2525–31. [DOI] [PubMed] [Google Scholar]
- [47].Yoshino M, Kayser BD, Yoshino J, et al. Effects of diet versus gastric bypass on metabolic function in diabetes. N Engl J Med 2020;383(8):721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gu L, Lin K, Du N, Ng DM, Lou D, Chen P. Differences in the effect of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass on gut hormones: systematic and meta-analysis. Surg Obes Relat Dis 2021;17(2):444–55. [DOI] [PubMed] [Google Scholar]
- [49].Kelly CT, Mansoor J, Dohm GL, Chapman WH, Pender JR, Pories WJ. Hyperinsulinemic syndrome: the metabolic syndrome is broader than you think. Surgery 2014;156(2):405–11. [DOI] [PubMed] [Google Scholar]
- [50].Friedman JE, Caro J, Pories WJ, Azevedo JL, Dohm GL. Glucose metabolism in incubated human muscle: effect of obesity and noninsulin-dependent diabetes mellitus. Metabolism 1994;43(8):1047–54. [DOI] [PubMed] [Google Scholar]
- [51].Hulver MW, Berggren JR, Cortright RN, et al. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab 2003;284(4):E741–7. [DOI] [PubMed] [Google Scholar]