Conspectus

Cyclometalated iridium(III) complexes are frequently employed in organic light emitting diodes, and they are popular photocatalysts for solar energy conversion and synthetic organic chemistry. They luminesce from redox-active excited states that can have high triplet energies and long lifetimes, making them well suited for energy transfer and photoredox catalysis. Homoleptic tris(cyclometalated) iridium(III) complexes are typically very hydrophobic and do not dissolve well in polar solvents, somewhat limiting their application scope. We developed a family of water-soluble sulfonate-decorated variants with tailored redox potentials and excited-state energies to address several key challenges in aqueous photochemistry.
First, we aimed at combining enzyme with photoredox catalysis to synthesize enantioenriched products in a cyclic reaction network. Since the employed biocatalyst operates best in aqueous solution, a water-soluble photocatalyst was needed. A new tris(cyclometalated) iridium(III) complex provided enough reducing power for the photochemical reduction of imines to racemic mixtures of amines and furthermore was compatible with monoamine oxidase (MAO-N-9), which deracemized this mixture through a kinetic resolution of the racemic amine via oxidation to the corresponding imine. This process led to the accumulation of the unreactive amine enantiomer over time. In subsequent studies, we discovered that the same iridium(III) complex photoionizes under intense irradiation to give hydrated electrons as a result of consecutive two-photon excitation. With visible light as energy input, hydrated electrons become available in a catalytic fashion, thereby allowing the comparatively mild reduction of substrates that would typically only be reactive under harsher conditions. Finally, we became interested in photochemical upconversion in aqueous solution, for which it was desirable to obtain water-soluble iridium(III) compounds with very high triplet excited-state energies. This goal was achieved through improved ligand design and ultimately enabled sensitized triplet–triplet annihilation upconversion unusually far into the ultraviolet spectral range.
Studies of photoredox catalysis, energy transfer catalysis, and photochemical upconversion typically rely on the use of organic solvents. Water could potentially be an attractive alternative in many cases, but photocatalyst development lags somewhat behind for aqueous solution compared to organic solvent. The purpose of this Account is to provide an overview of the breadth of new research perspectives that emerged from the development of water-soluble fac-[Ir(ppy)]3 complexes (ppy = 2-phenylpyridine) with sulfonated ligands. We hope to inspire the use of some of these or related coordination compounds in aqueous photochemistry and to stimulate further conceptual developments at the interfaces of coordination chemistry, photophysics, biocatalysis, and sustainable chemistry.
Key References
Guo X.; Okamoto Y.; Schreier M. R.; Ward T. R.; Wenger O. S.. Enantioselective Synthesis of Amines by Combining Photoredox and Enzymatic Catalysis in a Cyclic Reaction Network. Chem. Sci. 2018, 9, 5052–5056.1 A water-soluble iridium(III) complex photocatalyzes the reduction of imines to a racemic mixture of amines, and the enzyme monoamine oxidase MAO-N-9 oxidizes one of the two amine enantiomers back to the initially present imine. The unreactive amine enantiomer accumulates over the course of continuous photoirradiation.
Kerzig C.; Guo X.; Wenger O. S.. Unexpected Hydrated Electron Source for Preparative Visible-Light Driven Photoredox Catalysis. J. Am. Chem. Soc. 2019, 141, 2122–2127.2 An iridium(III) complex is excited by two consecutive laser pulses and thereby photoionizes to yield hydrated electrons. Using a sacrificial electron donor, hydrated electrons can be generated in a catalytic fashion, and photochemical reactions requiring very negative reduction potentials can be driven under continuous irradiation with a laser.
Pfund B.; Steffen D. M.; Schreier M. R.; Bertrams M.-S.; Ye C.; Börjesson K.; Wenger O. S.; Kerzig C.. UV Light Generation and Challenging Photoreactions Enabled by Upconversion in Water. J. Am. Chem. Soc. 2020, 142, 10468–10476.3 Iridium(III) complexes with high triplet energies are developed and used to sensitize the triplet–triplet annihilation upconversion of naphthalene derivatives, resulting in blue-to-ultraviolet light conversion. The UV-emissive excited states of the naphthalenes can be exploited to drive thermodynamically demanding chemical reactions.
Introduction
Many current photophysical and photochemical studies rely on cyclometalated iridium(III) complexes because they exhibit particularly favorable properties. Their excited-state and redox characteristics are tunable through modification of ligands and coordination environments, making them adaptable to many different applications.4,5 For instance, both very oxidizing and strongly reducing variants of cyclometalated iridium complexes are available, whereas others have exceptionally high triplet energies.6 In addition to their initial role as triplet harvesters and luminophores in organic light-emitting diodes,7,8 this class of compounds, therefore, has become useful for solar energy conversion4,9 and synthetic organic photochemistry.10−12
Homoleptic tris(cyclometalated) iridium(III) complexes represent a subgroup within the larger family of cyclometalated iridium(III) complexes, which furthermore includes many heteroleptic compounds typically containing one α-diimine coligand. The prototype of the homoleptic subgroup is fac-[Ir(ppy)3] (ppy = 2-phenylpyridine),13 which luminesces from an excited state with a lifetime of 1.9 μs. Its triplet energy is relatively high (2.50 eV), and with an excited-state oxidation potential of −1.73 V vs SCE, it is a strong photoreductant.14 These combined properties make fac-[Ir(ppy)3] and its congeners (with cyclometalating ligands other than ppy) attractive luminophores and photocatalysts. However, such homoleptic tris(cyclometalated) iridium(III) complexes are typically charge-neutral and as such very lipophilic, which limits their application potential in polar solvents. In water, they are usually completely insoluble, and even the water-solubility of widely used monocationic iridium(III) complexes seems to be too low for applications in homogeneous aqueous photocatalysis.15 However, for some areas of photochemistry, water is an essential solvent or cosolvent, for example, light-driven water splitting, detoxification processes in environmental photochemistry, or the combination of enzymatic and photoredox catalysis. Furthermore, water is one of a rather narrow selection of fluids in which solvated electrons can exist,16 and hydrated electrons are super-reductants that seem yet underexplored in photocatalysis.17
Our Account begins with a brief overview of water-soluble cyclometalated iridium(III) complexes, before delving into our own research on homoleptic tris(cyclometalated) variants and their applications. The core part is structured into three different sections, summarizing our efforts on (i) concurrent photoredox and enzyme catalysis, (ii) the formation and exploitation of hydrated electrons with visible light, and (iii) sensitized triplet–triplet annihilation upconversion for the generation of ultraviolet light in water. At the end, we provide some key conclusions and an outlook.
Previously Known Water-Soluble Cyclometalated Iridium(III) Complexes
Three common strategies to turn lipophilic metal complexes into water-soluble congeners include sulfonation, attachment of poly(ethylene glycol) (PEG) chains, and the introduction of quaternary alkyl ammonium groups. All three strategies have been applied to heteroleptic cyclometalated iridium(III) compounds (Figure 1) by several researchers with different backgrounds and motivations. The Castellano group used bathophenanthroline disulfonate in combination with different cyclometalating ligands (Figure 1a), and they observed strongly concentration-dependent photoluminescence quantum yields and lifetimes due to aggregation of their iridium(III) complexes in aqueous solution.18,19 This phenomenon is reminiscent of aggregation-induced emission but is uncommon for octahedral metal complexes. The group of Lo used PEGylation to obtain water-soluble cyclometalated iridium(III) complexes that can act as biological probes with low cytotoxicity and high cellular uptake efficiency (Figure 1b).20 The incorporation of an aldehyde group on one of the cyclometalating ligands furthermore permitted their covalent attachment to proteins and amine-containing polymers, to result in some of the first PEGylation reagents based on luminescent transition metal complexes. The teams of Francis and Hayne used PEG substituents to obtain water-soluble iridium(III)-based emitters for electrogenerated chemiluminescence (Figure 1c),21 and in related earlier studies furthermore employed a combination of sulfonation and PEGylation strategies for the same purpose.22 In contrast, the Roelfes group introduced quaternary ammonium groups at the 2,2′-bipyridine backbone to obtain a water-soluble iridium(III) complex that acted as photocatalyst for modification of dehydroalanine residues in peptides and proteins (Figure 1d).23 Other polycationic complexes have been developed with tumor imaging and DNA photocleavage applications in mind.24,25 Charge-neutral complexes were investigated by the teams of De Cola and Josel (Figure 1e) in the context of electrochemiluminescence in aqueous buffer solution.26 Coe and co-workers investigated water-soluble iridium(III) complexes with deprotonated N-methylbipyridinium ligands as fluorine-free blue and green emitters (Figure 1f).27,28
Figure 1.

Previously explored water-soluble cyclometalated iridium(III) complexes (left) and the corresponding cyclometalating ligands (right); R = H, CF3, tBu.18−23,25−28
The examples in Figure 1 illustrate the structural diversity and the different motivations for obtaining water-soluble cyclometalated iridium(III) complexes but should not be seen as a comprehensive overview. However, it seems fair to summarize that most efforts so far focused on heteroleptic complexes, comprised of two cyclometalating and one α-diimine ligand. Those types of complexes are cationic and therefore not as strongly lipophilic as fac-[Ir(ppy)3] and structurally analogous (charge-neutral) homoleptic tris(cyclometalated) iridium(III) complexes. However, further chemical modifications are required to obtain sufficient water solubility as illustrated in Figure 1.15
In the course of our research on photoreductions and light-driven multielectron transfer,29 we became interested in hydrophilic variants of fac-[Ir(ppy)3] because this parent complex is known to be particularly reducing and (photo)robust. Sulfonation was our preferred strategy to obtain water-soluble complexes (Figure 2), since long PEG chains surrounding the photoactive core seemed undesirable for allowing productive bimolecular catalyst–substrate encounters, and quaternary ammonium groups can be photochemically fragile. The sulfonation of the lipophilic fac-[Ir(ppy)3] complex and of some fluorinated variants (Figure 2b,c) was performed using trifluoroacetic anhydride in concentrated sulfuric acid.1 In addition, a cyclometalating ligand with a methanesulfonate group was prepared and reacted with the common dichlorotetrakis(2-phenylpyridine)diiridium(III) precursor to yield a tris(cyclometalated) complex with only one hydrophilic sulfonate group (Figure 2d).3 The relatively simple structural variations along the series of complexes in Figure 2 permit a remarkable modulation of oxidation potentials (Eox) and triplet energies (ET), while maintaining excited-state lifetimes (τ0) on the order of 2 μs and good visible light absorption properties (Table 1), which is in line with similar findings made previously for lipophilic congeners.6 The widely differing emission spectra of the four anionic complexes are included in Figure 2 (panel e) to illustrate the triplet energy modulation. Furthermore, some key properties that could facilitate the selection of the proper water-soluble iridium(III) complex for a given application are listed next to the structures.
Figure 2.
New sulfonated variants of the homoleptic fac-[Ir(ppy)3] complex, together with their emission spectra recorded in water at room temperature and selected key properties.
Table 1. Photophysical and Electrochemical Properties of the Water-Soluble Complexes from Figure 2 and the Lipophilic fac-[Ir(ppy)3] Parent Complexa.
| absorption |
emission |
electrochemistry |
||||||
|---|---|---|---|---|---|---|---|---|
| λmaxc (nm) | ε455nm (nm) | λmax (nm) | τ0 (ns) | φd | ETe (eV) | E0oxf (V vs SCE) | E*oxg (V vs SCE) | |
| fac-[Ir(ppy)3]b | 375 | 2800 | 510 | 1900 | 0.38 | 2.50 | 0.77 | –1.73 |
| fac-[Ir(sCH2ppy)(ppy)2]− | 374 | 1900 | 540 | 1560 | 0.13 | 2.50 | 0.56 | –1.94 |
| fac-[Ir(sppy)3]3– | 360 | 900 | 522 | 1625 | 0.73 | 2.65h | 0.76 | –1.89 |
| fac-[Ir(sFppy)3]3– | 338 | 500 | 496 | 2165 | 0.91 | 2.76 | 0.91 | –1.85 |
| fac-[Ir(sdFppy)3]3– | 330 | 230 | 484 | 2110 | 0.84 | 2.81 | 1.05 | –1.76 |
In deaerated aq. NaOH solution (50 mM) at 20 °C.
In CH3CN at room temperature.
Maxima of the lowest energy bands.
Luminescence quantum yields determined relative to fac-[Ir(sppy)3]3–.
Energy of the emissive triplet state estimated from the short-wavelength edge (10% of maximum intensity) of the room-temperature luminescence spectrum.
Ground-state oxidation potential determined by cyclic voltammetry in aqueous phosphate buffer (0.1 M, pH 7).
Oxidation potential in the emissive excited state according to the relationship E*ox = E0ox – ET/e, where e is the elementary charge.
A triplet energy of 2.58 eV was measured in an alcohol mixture at 85 K.30
Fluorination elevates the ground state oxidation potential (Eox), but with the excited-state energy (ET) increasing simultaneously, all four complexes remain very strong photoreductants (featuring excited-state oxidation potentials, E*ox, below −1.7 V vs SCE). Their triplet energies (ET up to 2.81 eV) are among the highest known to date for water-soluble transition metal complexes, yet excitation in the visible region remains possible in all cases (with extinction coefficients at a common LED peak wavelength such as 455 nm (ε455nm) between 230 and 1900 M–1 cm–1). Emission lifetimes remain long (τ0 > 1560 ns) and luminescence quantum yields (φ up to 0.91) are high, despite the fact that H2O is usually an efficient deactivator of electronically excited states due to its high-frequency O–H vibrations.
Application of fac-[Ir(sppy)3]3– in Combined Enzyme and Photoredox Catalysis
In the course of our work on photoinduced multielectron transfer,29 we discovered that reductive amination of carbonyl compounds is possible by photoredox catalysis with [Ru(bpy)3]2+.31,32 In an ensuing collaborative effort with the Ward group, we then targeted the photochemical reduction of imines to enantioenriched amines, using 2-cyclohexyl-1-pyrroline (1a) as a model substrate (Figure 3a).1 The photochemical key step in this overall two-electron process is the reduction of the imine starting material to an α-amino alkyl radical intermediate (Figure 3b). Compared to the reduction of iminium cations achieved earlier with [Ru(bpy)3]2+ in the reductive amination,31,32 the reduction of charge-neutral imines was more challenging. The [Ru(bpy)3]2+ photocatalyst only provided trace amounts of the (racemic) amine photoproduct rac-2a even after 20 h of irradiation (Figure 3a). In a mixture of CD3CN with 10% H2O, the more strongly photoreducing (lipophilic) fac-[Ir(ppy)3] complex catalyzed the reaction to 59% yield after 6 h in the presence of ascorbate as sacrificial reductant. However, this solvent mixture is not at all ideal for the envisioned combination of the photochemical reduction with an enzymatic follow-up reaction. The specific plan was to rely on monoamine oxidase (MAO-N-9) to convert one of the two enantiomers of rac-2a back to the initial imine 1a, to obtain a cyclic reaction network that leads over time to enrichment of the unreactive amine enantiomer. The MAO-N-9 enzyme works best in aqueous solution, and hence a water-soluble variant of fac-[Ir(ppy)3] was needed.
Figure 3.

(a) Photochemical reduction of a cyclic imine to a racemic mixture of amines, using ascorbate (AscH–) as reductant and different photocatalysts. Eox* is the oxidation potential of the emissive 3MLCT excited state of the respective photocatalyst. The value in parentheses is the potential for one-electron oxidation of [Ru(bpy)3]+ in the electronic ground state. (b) Cyclic reaction network, in which the photocatalyst enables imine to amine reduction (via an α-amino alkyl radical intermediate) and the enzyme MAO-N-9 selectively oxidizes the (S)-enantiomer of the amine back to the prochiral imine.
Gratifyingly, with the fac-[Ir(sppy)3]3– complex (Figure 2a), the reaction of 1a to rac-2a in aqueous phosphate buffer at pH 8 is essentially complete after irradiation at 405 nm for 10 h.1 With an excited-state oxidation potential (E*ox) of −1.89 V vs SCE (Table 1), this new complex is considerably more reducing than [Ru(bpy)3]+ (Eox = −1.33 V vs SCE), and consequently the photochemical reduction of imines (coupled to their protonation by H2PO4–) to α-amino alkyl radicals (Figure 3b) becomes efficient. Those radical intermediates are further reduced by hydrogen atom transfer from ascorbate to yield a racemic mixture of the amine (rac-2a). The MAO-N-9 enzyme catalyzes the oxidation of cyclic amines with very high selectivity for the (S)-enantiomer,33 and can thus convert (S)-2a back to the initial imine 1a via aerobic oxidation liberating H2O2 (Figure 3b) while (R)-2a accumulates. Continued photoirradiation of an aqueous solution containing fac-[Ir(sppy)3]3–, MAO-N-9, phosphate buffer to maintain pH = 8, and excess ascorbate therefore leads to the accumulation of (R)-2a. This cyclic deracemization works best when using whole E. coli cells in which MAO-N-9 has been overexpressed, presumably because the E. coli cell membranes prevent direct interaction between the trianionic photocatalyst and the enzyme, thereby limiting mutual deactivation. Enantiomeric excess (ee) values exceeding 99% were achievable in combination with very high substrate specificity. This study represented one of the first cases of combined enzyme and photoredox catalysis for the synthesis of enantioenriched products.34
Hydrated Electrons from Photoionization of fac-[Ir(sppy)3]3– with Visible Light
Hydrated electrons are typically formed by pulse radiolysis or ultraviolet radiation.35 However, Goez and co-workers demonstrated a few years ago that when [Ru(bpy)3]+ in water is excited with green light, hydrated electrons are liberated.36 Since that [Ru(bpy)3]+ species itself can be formed via green excitation of [Ru(bpy)3]2+ in the presence of sacrificial electron donors, the hydrated electrons become accessible via stepwise excitation with two green photons. We adapted this concept to the fac-[Ir(sppy)3]3– complex, but with a mechanistic twist: Instead of photoionizing its one-electron reduced form, we directly excited the long-lived 3MLCT excited state of the fac-[Ir(sppy)3]3– complex and achieved photoionization from an energetically high-lying triplet excited state.2 In pulsed laser experiments, excitation with blue light promoted fac-[Ir(sppy)3]3– to the 3MLCT state (Figure 4a), which has its own characteristic UV–vis absorption profile, notably with an intense band in the green spectral range (spectrum in Figure 4a), where the electronic ground state of fac-[Ir(sppy)3]3– does not absorb. When the sample was further excited into that specific absorption band with a green laser pulse, the fac-[Ir(sppy)3]3– complex was promoted from the 3MLCT state to the above-mentioned high-lying triplet state (Tn in Figure 4d), from which ionization occurs. The iridium(IV) oxidation product is readily detectable in two-pulse UV–vis transient absorption experiments, along with the spectral signature (Figure 4d) of hydrated electrons (eaq•–).
Figure 4.
(a) Energy-level scheme relevant to monophotonic excitation of fac-[Ir(sppy)3]3– along with pertinent properties of its emissive 3MLCT excited state including the triplet energy (ET), redox potential (E1/2, here given vs NHE for better comparison with the hydrated electron potential), and lifetime (τ). Tn denotes an energetically high-lying triplet excited state; 1A1 is the term symbol for the electronic ground state. (b) Catalytic cycle for triplet–triplet energy transfer from the fac-[Ir(sppy)3]3– photocatalyst (PC) to a substrate (S). (c) Catalytic cycle for reductive photocatalysis via an oxidative excited-state quenching step and subsequent photocatalyst regeneration with a sacrificial electron donor (Dsac). (d) Energy-level scheme relevant to biphotonic excitation of fac-[Ir(sppy)3]3– along with pertinent properties of the hydrated electron (eaq•–) resulting from autoionization of the Tn excited state. (e) Catalytic cycle relevant for photoreductions with the solvated electron, following consecutive biphotonic excitation of the photocatalyst to the Tn state (3**PC).
In the presence of excess triethanolamine (Dsac in Figure 4e), the iridium(IV) intermediate (corresponding to PC•+) is spontaneously reduced back to the initial iridium(III) complex, and consequently, hydrated electrons can be generated in a catalytic fashion. With a redox potential of −2.9 V vs NHE, solvated electrons are strongly reducing (Figure 4d), making them attractive reductants for photoredox catalysis. Continuous (rather than pulsed) excitation is desirable for this purpose, and a laser providing an output power of 1 W at a wavelength of 447 nm, where both the electronic ground state and the 3MLCT excited state of fac-[Ir(sppy)3]3– absorb, represented a good choice for several photoreductions.
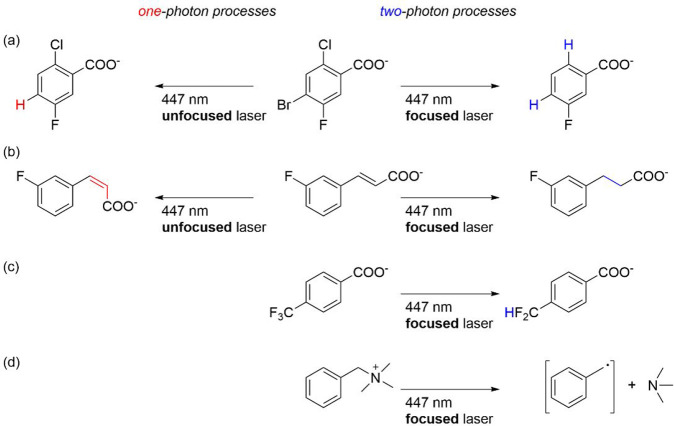
For instance, the photodegradation of a 10 mM solution of the benzyltrimethylammonium cation in D2O (Figure 5d) was readily accomplished with 1 mol % of fac-[Ir(sppy)3]3– and 5 equiv of triethanolamine.2 Quaternary ammonium compounds of this type are applied in many different contexts, such as antimicrobial reagents or fabric softeners, and they tend to accumulate in the environment. In wastewater treatment, they are typically degraded with γ-rays or ultraviolet C light (200–280 nm). Hence, the finding that their photodegradation can also be driven with blue light seems remarkable, even though it is a two-photon process that relies on a comparatively high excitation power density provided by a focused laser beam. However, there is no need for pulsed ultrafast lasers that are typically required for the simultaneous absorption of two photons,37 because our consecutive absorption mechanism has a relatively long-lived intermediate (3MLCT), which can be re-excited with a simpler continuous-wave laser. Under similar conditions, the reduction of trifluoromethylbenzoate to difluoromethylbenzoate (Figure 5c) has been accomplished, albeit with a much lower yield (12%).
Figure 5.
Photoreactions induced with fac-[Ir(sppy)3]3– under low power density (left) and high power density (right) excitation conditions. Reaction conditions: (a, b) fac-[Ir(sppy)3]3– (2–3 mol %), triethanolamine (6 equiv) in H2O;39 (c, d) fac-[Ir(sppy)3]3– (1 mol %), triethanolamine (5 equiv) in H2O and D2O.2
The mechanism of photoionization in fac-[Ir(sppy)3]3– allows for reaction control through the applied excitation power density. As indicated above, photoionization results from the consecutive absorption of two photons (Figure 4d) and as such depends quadratically on the excitation power density,38 while the formation of the 3MLCT-excited state is linearly dependent on the excitation power density. Thus, the introduction (or the removal) of an optical lens in the beam path of a continuous-wave laser operating at constant power can determine whether hydrated electrons or only the 3MLCT-excited fac-[Ir(sppy)3]3– are produced.39 Coincidentally, the hydrated electron and the 3MLCT excited state have very similar kinetic reactivities due to similar lifetimes (1.6 μs vs 1.4 μs), but their thermodynamic reactivities are vastly different, with relevant redox potentials of −2.9 V compared to −1.6 V vs NHE (Figure 4a,d). For substrates such as 4-bromo-2-chloro-5-fluorobenzoate (Figure 5a), in which the different halogen substituents have different thermodynamic reactivities (due to the reduction potentials needed for reductive debromination, dechlorination, and defluorination), the reaction outcome can therefore be determined by the presence or the absence of the focusing lens.39 In its absence, only 3MLCT-excited fac-[Ir(sppy)3]3– is formed, and reductive debromination leads to the hydrodebromination product (leftward arrow in Figure 5a). When the optical lens is inserted into the beam path while keeping all other parameters constant, hydrated electrons are liberated and reductive dechlorination occurs in addition to reductive debromination (rightward arrow in Figure 5a). The reductive dehalogenations likely occur along the reaction mechanisms outlined in Figure 4c,e: For the debromination (Figure 4c), the photocatalyst (PC) is promoted to the 3MLCT excited state (3*PC) through the absorption of a single photon (hν1), and then reduces the substrate (S) to give the oxidized photocatalyst (PC•+) along with one-electron reduced substrate (S•–). The sacrificial electron donor (Dsac) triethanolamine regenerates the photocatalyst in its initial state (PC), and the oxidation product (Dsac•+) undergoes degradation. The activated substrate (S•–) reductively dehalogenates to liberate a halogenide anion, and the resulting aryl radical takes up a hydrogen atom from one of the triethanolamine degradation products. For the dechlorination (Figure 4e), the triplet-excited photocatalyst (3*PC) is further excited by a second photon (hν2) to yield a highly excited triplet state (3**PC in Figure 4e, named, Tn in Figure 4d) from which hydrated electrons are formed and can activate the substrate.
The discrimination between reaction pathways for 4-bromo-2-chloro-5-fluorobenzoate in Figure 5a is based on the different reducing powers of the 3MLCT-excited fac-[Ir(sppy)3]3– complex and the hydrated electron, as well as the fact that reductive debromination requires substantially less reducing power than reductive dechlorination. An alternative mode of operation is exploited with the trans-3-fluorocinnamate substrate in Figure 5b.39 At low excitation power densities, the 3MLCT-excited fac-[Ir(sppy)3]3– complex undergoes triplet–triplet energy transfer (TTET) to the substrate (Figure 4b) and thus sensitizes its photoisomerization from the trans- to the cis-form. At high excitation densities, hydrated electrons cause reduction of the C=C double bond.
Thus, the fac-[Ir(sppy)3]3– complex can be used for the catalytic production of hydrated electrons and furthermore allows reactivity control by the excitation power density,2,39 complementing recent work in which the excitation wavelength dictated the reaction outcome.40,41
Energy Transfer Catalysis and Photochemical Upconversion to the Ultraviolet Region in Water
The cinnamate isomerization discussed in the preceding section relies on the same initial mechanistic step (general mechanism of Figure 4b) as sensitized triplet–triplet annihilation upconversion (sTTA-UC), namely, a triplet–triplet energy transfer (TTET) from the photoexcited iridium(III) complex to an energy acceptor. This isomerization has been investigated with all water-soluble photosensitizers displayed in Figure 2 with seemingly surprising results: The higher the sensitizer’s triplet energy, the lower the yield of the desired cis-isomer (Figure 6). Such counter-thermodynamic alkene isomerizations are increasingly well explored in organic solvents, and the reaction outcome strongly depends on the triplet energy of the sensitizer relative to that of the alkene, in both its cis- and trans-form.11,42 In our case, the TTET reactions between excited fac-[Ir(sppy)3]3–, as well as fac-[Ir(sCH2ppy)(ppy)2]−, and the cis-cinnamate are endergonic (i.e., very slow), whereas all other possible TTET reactions are expected to be exergonic (Figure 6b). Consequently, only the catalyst with the lowest triplet energy efficiently accumulates the thermally less stable cis-isomer in the photostationary state,3 highlighting the importance of tunable triplet energies.
Figure 6.

Sensitized isomerization of trans-3-fluorocinnamate in water (a) with relative triplet energies of both olefin isomers and the four sulfonated iridium(III) photocatalysts (b). The sensitizer’s triplet energy (ET) determines the cis/trans ratio in the photoequilibrium.
Triplet energies are furthermore important in the context of photochemical upconversion. Sensitized triplet–triplet annihilation upconversion is a promising and widely investigated mechanism for the conversion of low-energy input light into higher energy output radiation.43,44 Many of the commonly employed annihilators are polyaromatic hydrocarbon compounds such as anthracene or pyrene derivatives, and thus sTTA-UC is typically investigated in organic solvent or in apolar gels,45 whereas studies in neat water are extremely scarce.46,47 There have been numerous investigations of visible-to-visible upconversion, and while near-infrared-to-visible light conversion is gaining momentum,43,48,49 visible-to-ultraviolet upconversion seems underexplored.50−52 In the course of our research focusing on the generation of highly reactive electronically excited states,53−55 we therefore became interested in blue-to-ultraviolet upconversion in water.
The triplet energy of the sensitizer is a limiting factor for the energy of the achievable upconverted light output, because the upconverted fluorescence cannot be higher than twice the energy of the two annihilated triplet excited states.44 Consequently, for upconversion from the visible to the ultraviolet, it becomes desirable to shift the sensitizer’s triplet energy as high as possible, in contrast to what has been observed for the cinnamate photoisomerization (Figure 6). For lipophilic tris(cyclometalated) complexes of iridium(III), this had been achieved previously through partial fluorination of the ligands,6 and the same strategy is also applicable to our new water-soluble congeners (Figure 2).3 In the fac-[Ir(sppy)3]3– parent compound (Figure 2a), the 3MLCT energy is 2.65 eV (Table 1), and this increases to 2.76 eV in fac-[Ir(sFppy)3]3– where all three cyclometalated ligands are singly fluorinated (Figure 2b). Double fluorination of each ligand increases the triplet energy further to 2.81 eV in fac-[Ir(sdFppy)3]3– (Figure 2c), relatively close to the blue edge of the visible spectrum. Importantly, a molar extinction coefficient of 230 M–1 cm–1 at 455 nm (Table 1) still permits reasonably efficient excitation of this complex with a blue light source.
Our search for suitable annihilators focused largely on naphthalene and p-terphenyl derivatives with sulfonate,56 carboxylate, and ammonium groups, because these two polyaromatic hydrocarbon frameworks have triplet energies close to fac-[Ir(sdFppy)3]3–. Furthermore, their lowest singlet excited states are at high energies with decent fluorescence quantum yields.3 Despite their hydrophilic substituents, solubility issues prevail for many of the investigated naphthalenes and p-terphenyls under upconversion conditions, in some cases due to an inherently low solubility in water and in other cases because of aggregation between the iridium(III) complex and the annihilator as a result of mutual electrostatic attraction. 1,5-Naphthalenedisulfonate (NDS) and naproxen (NPX) emerged as the two best suited choices (Figure 7a) in combination with the sulfonated iridium(III) compounds in Figure 2a–c. Following blue excitation of these sensitizers and ultrafast intersystem crossing into their lowest 3MLCT states (Figure 7b), triplet–triplet energy transfer to NDS is achievable with efficiencies between 84% and 95%, producing a very long-lived (τ0 = 1.1 ms) triplet state on the naphthalene framework (termed 3*Nap in Figure 7b). Upon collisional encounter between two triplet-excited NDS molecules, triplet–triplet annihilation (TTA) can occur to produce one NDS molecule in its fluorescent singlet excited state (1*Nap, Figure 7b) and one in its electronic ground state (1Nap). With NDS in alkaline aqueous solution, triplet–triplet annihilation occurs with a rate constant approaching the diffusion limit, suggesting at first glance that the achievable upconversion efficiency could be high. However, direct measurements comparing the intensity of the upconverted NDS fluorescence to the iridium(III) 3MLCT emission demonstrate that this expectation is not fulfilled. Under high power excitation conditions (near 400 mW), the upconversion quantum yield levels off at roughly 0.035%, far below the theoretical limit of 50% achievable for a two-photon process.57 In part, this is explainable by the moderate fluorescence quantum yield of 0.14 for NDS in alkaline water, but even when considering this, the experimental upconversion efficiency remains about 2 orders of magnitude below the theoretical limit. Evidently, there are important energy-loss mechanisms that would require additional studies to clarify, in addition to the excimer formation issues observed for several water-soluble annihilators.
Figure 7.
(a) Annihilators used for sensitized triplet–triplet annihilation upconversion (sTTA-UC) in water. (b) Energy level scheme relevant for sTTA-UC with the iridium(III) complexes from Figure 2 and the annihilators from panel a; Nap stands for the naphthalene core of the respective annihilator compounds. (c) Photoreactions induced via sTTA-UC; reaction conditions: fac-[Ir(sppy)3]3– (0.1 mM), naproxen (10 mM), isopropanol (100 mM) in H2O (upper); fac-[Ir(sppy)3]3– (0.1 mM), naproxen (20 mM), in H2O (lower).
Gratifyingly, the upconverted NDS fluorescence occurs from a singlet excited state with unusually high energy (3.9 eV), even higher than what was previously the record value in organic solvents (3.6 eV). Furthermore, the achievable pseudo-anti-Stokes shift of −1.1 eV, defined as the energy difference between the excitation wavelength (447 nm, 2.8 eV) and the singlet-excited state energy of 3.9 eV, compares very favorably to most previously investigated upconversion systems in organic solution.43,50,58
While the NDS annihilator was selected with a focus on optimizing the photophysical performance of the overall upconversion system, the NPX annihilator (Figure 7a, bottom) is more readily amenable to photochemical applications. The fluorescent singlet excited state of NPX is at lower energy (3.7 eV) but, at the same time, is substantially more reducing than the singlet excited state of NDS. In that excited singlet state, NPX is oxidized at −2.5 V vs NHE, which is sufficient to induce reductive debromination of 4-bromo-2-chloro-5-fluorobenzoate under upconversion conditions. In the presence of isopropanol as a hydrogen atom donor to the photogenerated aryl radical, the hydrodebromination product is readily obtained (Figure 7c, top). The dimethyl ketyl radical formed after hydrogen atom abstraction from isopropanol can reduce the oxidized NPX annihilator, making the overall process catalytic. The driving-force for reductive debromination of the 4-bromo-2-chloro-5-fluorobenzoate substrate from singlet-excited NPX is substantially higher than that from the emissive excited state of fac-[Ir(sppy)3]3–.
In another experiment, the photo-decomposition of benzyltrimethylammonium was possible under upconversion conditions (Figure 7c, bottom) employing a blue LED as light source, instead of a pulsed laser as for the method in Figure 5d.
The two examples in Figure 7c reveal that sensitized triplet–triplet annihilation upconversion represents a viable mechanism to access classic ultraviolet photochemistry with visible light, complementing the findings regarding the visible-light generated hydrated electrons from the previous section. The key difference is that the formation of the hydrated electrons requires very high excitation power densities (on the order of 1 kW/cm2),2 while the upconversion mechanism operates at substantially lower photon fluxes.3,44 Practical advantages of two-photon based mechanisms can emerge from the fact that direct UV excitation is often harmful and can suffer from selectivity and filter effect issues when compared to visible excitation.38
Conclusions
The development of new photosensitizers and photocatalysts is an integral part of contemporary research in photophysics and photochemistry. With few exceptions,59−62 such research efforts typically focus on organic solvents, whereas the development of new photosensitizers and photocatalysts suitable for applications in water lags behind. In many cases of cationic transition metal complexes, counteranion exchange can suffice to achieve water solubility, but other strategies have to be applied to strongly lipophilic charge-neutral coordination compounds. In the case of homoleptic tris(cyclometalated) iridium(III) complexes, ligand sulfonation leads to 3-fold negatively charged complexes that enable new photoprocesses in water. Specifically, the water solubility of this compound class with comparatively high photoreducing power permitted their combination with the MAO-N-9 enzyme to achieve the enantioselective synthesis of amines from imines. With sufficiently high excitation power densities, photoionization of fac-[Ir(sppy)3]3– via 2-fold consecutive excitation provides hydrated electrons as super-reductants for photoredox catalysis. In combination with suitable annihilators, photochemical upconversion unusually far into the UV spectral range becomes achievable, even in a solvent (water) for which only a handful of sTTA-UC systems have been reported until now. It seems plausible that these complexes can be applied beyond photocatalysis and upconversion, and fac-[Ir(sppy)3]3– has already been shown to be an effective enhancer of [Ru(bpy)3]2+ electrochemiluminescence detection in aqueous solution.30
These applications are possible owing to the high photostability, comparatively strong reducing power, and high energies of the photoactive (triplet) excited states of this family of compounds. All of these properties are maintained when going from the known lipophilic parent complexes to the new water-soluble variants. In particular, their stability under long-term irradiation with both continuous-wave lasers and high-power LEDs, as well as under pulsed excitation with relatively high peak powers, is remarkable. Thus, the inertness of octahedral low-spin d6 complexes with precious iridium(III) represents a significant advantage in comparison to many photoactive complexes based on abundant first-row transition metals.63 It seems plausible to expect that iridium(III) complexes will continue to play important roles in photocatalysis, now including new perspectives for different applications in water.
Acknowledgments
This work was supported by the Swiss National Science Foundation through the NCCR Molecular Systems Engineering and through Grant Number 200021_178760, the German National Academy of Sciences Leopoldina (postdoctoral fellowship to C.K., Grant Number LPDS 2017-11), and the National Research Fund, Luxembourg (Ph.D. Grant 14583224 to B.P.).
Biographies
Mirjam R. Schreier studied chemistry at the University of Basel. After finishing her M.Sc. thesis in the group of Andreas Pfaltz, she joined the group of Oliver S. Wenger for her Ph.D. studies (2016–2020), working on photoactive iridium complexes. Currently, she is a teacher at Kantonsschule Solothurn.
Xingwei Guo received a Ph.D. degree in Chemistry from LMU Munich, Germany (2014), under the supervision of Herbert Mayr. After postdoctoral research experiences at CNRS École Polytechnique with Samir Z. Zard (2014–2015) and at the University of Basel with Oliver S. Wenger (2015–2019), he started his independent career as a principal investigator in the Center of Basic Molecular Science (CBMS), Department of Chemistry, at Tsinghua University, Beijing (2019). The focus of his research is on developing physical organic chemistry tools and advanced technologies to solve fundamental problems in light-induced molecular transformations, particularly in radical kinetics.
Björn Pfund studied chemistry at the University of Applied Sciences in Zurich and the University of Basel (Switzerland). He received his M.Sc. degree under the guidance of Oliver S. Wenger in 2019, working on upconversion in water and its applications to challenging photoreductions. In 2020, he started his doctoral studies in the same group, endowed with a fellowship from the National Research Fund, Luxembourg. Currently, he is focusing on mechanistic photochemistry.
Yasunori Okamoto received his Ph.D. degree in bioinorganic chemistry in 2014 from Osaka University (Japan) under the guidance of T. Hayashi. During and after his Ph.D. studies, he joined D. M. Kurtz’s group at the University of Texas at San Antonio (US) and S. Aono’s group at the National Institute of Natural Science (Japan). He carried out postdoctoral research on artificial metalloenzymes in the group of T. R. Ward at the University of Basel (Switzerland) from 2014 to 2019. He began his independent career in 2019 at Tohoku University (Japan), where he is an Assistant Professor. His current research interests focus on protein engineering for biocatalysis.
Thomas R. Ward received a Ph.D. from the ETHZ in 1991, working under the guidance of Prof. L. M. Venanzi. He then joined the group of Prof. R. Hoffmann (Cornell) to work on modeling the three way catalyst. He returned to Switzerland for a second postdoctoral experience with Prof. C. Floriani (Univ. Lausanne). With the support of an A. Werner Stipend, he initiated his independent career at the University of Berne, where he first met Oliver. He moved to the University of Neuchâtel as full professor of bioinorganic chemistry in 2000. In 2008, he accepted an offer from the University of Basel, where he is currently full professor and director of the National Competence Center in Research “Molecular Systems Engineering”.
Christoph Kerzig studied chemistry at the University of Halle (Germany), where he obtained his Ph.D. degree under the supervision of Martin Goez in 2017. He then joined the group of Oliver S. Wenger at the University of Basel (Switzerland) with a Leopoldina postdoc fellowship. During his research stay in Basel, he was also a visiting scientist in Gothenburg (Sweden) working on upconversion with Karl Börjesson. Christoph started his independent career in 2020 at the Johannes Gutenberg University Mainz as an assistant professor. His research focuses on mechanistic photochemistry, optical spectroscopy, and multiphoton chemistry.
Oliver S. Wenger received a Ph.D. degree from the University of Berne in 2002 after work with Hans U. Güdel, then was a postdoctoral fellow at the California Institute of Technology with Harry B. Gray (2002–2004) and at Université Louis Pasteur in Strasbourg with Jean-Pierre Sauvage (2004–2006). He started his independent career in 2006 at the University of Geneva as an assistant professor funded by the Swiss National Science Foundation. In 2009, he became associate professor at Georg-August-Universität Göttingen, and in 2012, he moved to the University of Basel, where he is currently full professor.
Author Present Address
§ Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing 100084, China
Author Present Address
⊥ Frontier Research Institute for Interdisciplinary Sciences, Tohoku University, 6-3 Aramaki-aza Aoba, Aoba-ku, Sendai 980-8578, Japan.
Author Present Address
# Department of Chemistry, Johannes Gutenberg University Mainz, Duesbergweg 10-14, 55128 Mainz, Germany.
The authors declare no competing financial interest.
References
- Guo X.; Okamoto Y.; Schreier M. R.; Ward T. R.; Wenger O. S. Enantioselective Synthesis of Amines by Combining Photoredox and Enzymatic Catalysis in a Cyclic Reaction Network. Chem. Sci. 2018, 9, 5052–5056. 10.1039/C8SC01561A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerzig C.; Guo X.; Wenger O. S. Unexpected Hydrated Electron Source for Preparative Visible-Light Driven Photoredox Catalysis. J. Am. Chem. Soc. 2019, 141, 2122–2127. 10.1021/jacs.8b12223. [DOI] [PubMed] [Google Scholar]
- Pfund B.; Steffen D. M.; Schreier M. R.; Bertrams M.-S.; Ye C.; Börjesson K.; Wenger O. S.; Kerzig C. UV Light Generation and Challenging Photoreactions Enabled by Upconversion in Water. J. Am. Chem. Soc. 2020, 142, 10468–10476. 10.1021/jacs.0c02835. [DOI] [PubMed] [Google Scholar]
- Mills I. N.; Porras J. A.; Bernhard S. Judicious Design of Cationic, Cyclometalated IrIII Complexes for Photochemical Energy Conversion and Optoelectronics. Acc. Chem. Res. 2018, 51, 352–364. 10.1021/acs.accounts.7b00375. [DOI] [PubMed] [Google Scholar]
- Bevernaegie R.; Wehlin S. A. M.; Elias B.; Troian-Gautier L. A Roadmap Towards Visible Light Mediated Electron Transfer Chemistry with Iridium(III) Complexes. ChemPhotoChem. 2021, 5, 217–234. 10.1002/cptc.202000255. [DOI] [Google Scholar]
- Teegardin K.; Day J. I.; Chan J.; Weaver J. Advances in Photocatalysis: A Microreview of Visible Light Mediated Ruthenium and Iridium Catalyzed Organic Transformations. Org. Process Res. Dev. 2016, 20, 1156–1163. 10.1021/acs.oprd.6b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamansky S.; Djurovich P.; Murphy D.; Abdel-Razzaq F.; Lee H. E.; Adachi C.; Burrows P. E.; Forrest S. R.; Thompson M. E. Highly Phosphorescent Bis-Cyclometalated Iridium Complexes: Synthesis, Photophysical Characterization, and Use in Organic Light Emitting Diodes. J. Am. Chem. Soc. 2001, 123, 4304–4312. 10.1021/ja003693s. [DOI] [PubMed] [Google Scholar]
- Yersin H.; Rausch A. F.; Czerwieniec R.; Hofbeck T.; Fischer T. The Triplet State of Organo-Transition Metal Compounds. Triplet Harvesting and Singlet Harvesting for Efficient OLEDs. Coord. Chem. Rev. 2011, 255, 2622–2652. 10.1016/j.ccr.2011.01.042. [DOI] [Google Scholar]
- Mayo E. I.; Kilsa K.; Tirrell T.; Djurovich P. I.; Tamayo A.; Thompson M. E.; Lewis N. S.; Gray H. B. Cyclometalated iridium(III)-sensitized titanium dioxide solar cells. Photochem. Photobiol. Sci. 2006, 5, 871–873. 10.1039/b608430c. [DOI] [PubMed] [Google Scholar]
- Twilton J.; Le C.; Zhang P.; Shaw M. H.; Evans R. W.; MacMillan D. W. C. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017, 1, 0052. 10.1038/s41570-017-0052. [DOI] [Google Scholar]
- Strieth-Kalthoff F.; James M. J.; Teders M.; Pitzer L.; Glorius F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 2018, 47, 7190–7202. 10.1039/C8CS00054A. [DOI] [PubMed] [Google Scholar]
- Shon J.-H.; Kim D.; Rathnayake M. D.; Sittel S.; Weaver J.; Teets T. S. Photoredox catalysis on unactivated substrates with strongly reducing iridium photosensitizers. Chem. Sci. 2021, 12, 4069–4078. 10.1039/D0SC06306A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M. G.; Brunold T. C.; Riedener T.; Güdel H. U.; Fortsch M.; Bürgi H. B. Facial Tris Cyclometalated Rh3+ and Ir3+ Complexes: Their Synthesis, Structure, and Optical Spectroscopic Properties. Inorg. Chem. 1994, 33, 545–550. 10.1021/ic00081a024. [DOI] [Google Scholar]
- Arias-Rotondo D. M.; McCusker J. K. The photophysics of photoredox catalysis: a roadmap for catalyst design. Chem. Soc. Rev. 2016, 45, 5803–5820. 10.1039/C6CS00526H. [DOI] [PubMed] [Google Scholar]
- Jespersen D.; Keen B.; Day J. I.; Singh A.; Briles J.; Mullins D.; Weaver J. D. Solubility of Iridium and Ruthenium Organometallic Photoredox Catalysts. Org. Process Res. Dev. 2019, 23, 1087–1095. 10.1021/acs.oprd.9b00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefermann K. R.; Liu Y. X.; Lugovoy E.; Link O.; Faubel M.; Buck U.; Winter B.; Abel B. Binding energies, lifetimes and implications of bulk and interface solvated electrons in water. Nat. Chem. 2010, 2, 274–279. 10.1038/nchem.580. [DOI] [PubMed] [Google Scholar]
- Kerzig C.; Goez M. Combining Energy and Electron Transfer in a Supramolecular Environment for the “Green” Generation and Utilization of Hydrated Electrons through Photoredox Catalysis. Chem. Sci. 2016, 7, 3862–3868. 10.1039/C5SC04800A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGoorty M. M.; Khnayzer R. S.; Castellano F. N. Enhanced photophysics from self-assembled cyclometalated Ir(III) complexes in water. Chem. Commun. 2016, 52, 7846–7849. 10.1039/C6CC03932D. [DOI] [PubMed] [Google Scholar]
- McGoorty M. M.; Singh A.; Deaton T. A.; Peterson B.; Taliaferro C. M.; Yingling Y. G.; Castellano F. N. Bathophenanthroline Disulfonate Ligand-Induced Self-Assembly of Ir(III) Complexes in Water: An Intriguing Class of Photoluminescent Soft Materials. ACS Omega 2018, 3, 14027–14038. 10.1021/acsomega.8b02034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. P.-Y.; Liu H.-W.; Zhang K. Y.; Lo K. K.-W. Modification of Luminescent Iridium(III) Polypyridine Complexes with Discrete Poly(ethylene glycol) (PEG) Pendants: Synthesis, Emissive Behavior, Intracellular Uptake, and PEGylation Properties. Chem.—Eur. J. 2010, 16, 8329–8339. 10.1002/chem.201000474. [DOI] [PubMed] [Google Scholar]
- Newman B.; Chen L.; Henderson L. C.; Doeven E. H.; Francis P. S.; Hayne D. J. Water-Soluble Iridium(III) Complexes Containing Tetraethylene-Glycol-Derivatized Bipyridine Ligands for Electrogenerated Chemiluminescence Detection. Front. Chem. 2020, 8, 583631. 10.3389/fchem.2020.583631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr E.; Doeven E. H.; Barbante G. J.; Connell T. U.; Donnelly P. S.; Wilson D. J. D.; Ashton T. D.; Pfeffer F. M.; Francis P. S. Blue Electrogenerated Chemiluminescence from Water-Soluble Iridium Complexes Containing Sulfonated Phenylpyridine or Tetraethylene Glycol Derivatized Triazolylpyridine Ligands. Chem.—Eur. J. 2015, 21, 14987–14995. 10.1002/chem.201502037. [DOI] [PubMed] [Google Scholar]
- van Lier R. C. W.; de Bruijn A. D.; Roelfes G. A Water-Soluble Iridium Photocatalyst for Chemical Modification of Dehydroalanines in Peptides and Proteins. Chem.—Eur. J. 2021, 27, 1430–1437. 10.1002/chem.202002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weynand J.; Moreno-Betancourt A.; Loiseau F.; Berthet N.; Defrancq E.; Elias B. Redox-Active Bis-Cyclometalated Iridium(III) Complex as a DNA Photo-Cleaving Agent. Inorg. Chem. 2020, 59, 2426–2433. 10.1021/acs.inorgchem.9b03312. [DOI] [PubMed] [Google Scholar]
- Yi S.; Lu Z.; Zhang J.; Wang J.; Xie Z.; Hou L. Amphiphilic Gemini Iridium(III) Complex as a Mitochondria-Targeted Theranostic Agent for Tumor Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 15276–15289. 10.1021/acsami.9b01205. [DOI] [PubMed] [Google Scholar]
- Fernandez-Hernandez J. M.; Longhi E.; Cysewski R.; Polo F.; Josel H.-P.; De Cola L. Photophysics and Electrochemiluminescence of Bright Cyclometalated Ir(III) Complexes in Aqueous Solutions. Anal. Chem. 2016, 88, 4174–4178. 10.1021/acs.analchem.6b00312. [DOI] [PubMed] [Google Scholar]
- Coe B. J.; Helliwell M.; Raftery J.; Sánchez S.; Peers M. K.; Scrutton N. S. Cyclometalated Ir(III) complexes of deprotonated N-methylbipyridinium ligands: effects of quaternised N centre position on luminescence. Dalton Trans. 2015, 44, 20392–20405. 10.1039/C5DT03753K. [DOI] [PubMed] [Google Scholar]
- Coe B. J.; Helliwell M.; Sánchez S.; Peers M. K.; Scrutton N. S. Water-soluble Ir(III) complexes of deprotonated N-methylbipyridinium ligands: fluorine-free blue emitters. Dalton Trans. 2015, 44, 15420–15423. 10.1039/C5DT02591E. [DOI] [PubMed] [Google Scholar]
- Pannwitz A.; Wenger O. S. Proton-Coupled Multi-Electron Transfer and its Relevance for Artificial Photosynthesis and Photoredox Catalysis. Chem. Commun. 2019, 55, 4004–4014. 10.1039/C9CC00821G. [DOI] [PubMed] [Google Scholar]
- Kerr E.; Hayne D. J.; Soulsby L. C.; Bawden J. C.; Blom S. J.; Doeven E. H.; Henderson L. C.; Hogan C. F.; Francis P. S. A redox-mediator pathway for enhanced multi-colour electrochemiluminescence in aqueous solution. Chem. Sci. 2022, 13, 469–477. 10.1039/D1SC05609C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.; Wenger O. S. Reductive Amination by Photoredox Catalysis and Polarity-Matched Hydrogen Atom Transfer. Angew. Chem., Int. Ed. 2018, 57, 2469–2473. 10.1002/anie.201711467. [DOI] [PubMed] [Google Scholar]
- Guo X.; Okamoto Y.; Schreier M. R.; Ward T. R.; Wenger O. S. Reductive Amination and Enantioselective Amine Synthesis by Photoredox Catalysis. Eur. J. Org. Chem. 2020, 2020, 1288–1293. 10.1002/ejoc.201900777. [DOI] [Google Scholar]
- Köhler V.; Wilson Y. M.; Dürrenberger M.; Ghislieri D.; Churakova E.; Quinto T.; Knörr L.; Häussinger D.; Hollmann F.; Turner N. J.; Ward T. R. Synthetic cascades are enabled by combining biocatalysts with artificial metalloenzymes. Nat. Chem. 2013, 5, 93–99. 10.1038/nchem.1498. [DOI] [PubMed] [Google Scholar]
- Turner N. J. A new twist on catalytic teamwork. Nature 2018, 560, 310–311. 10.1038/d41586-018-05933-0. [DOI] [PubMed] [Google Scholar]
- Herbert J. M.; Coons M. P. The Hydrated Electron. Annu. Rev. Phys. Chem. 2017, 68, 447–472. 10.1146/annurev-physchem-052516-050816. [DOI] [PubMed] [Google Scholar]
- Goez M.; Kerzig C.; Naumann R. An “All-Green” Catalytic Cycle of Aqueous Photoionization. Angew. Chem., Int. Ed. 2014, 53, 9914–9916. 10.1002/anie.201405693. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y.; Mutoh K.; Abe J. Stepwise two-photon absorption processes utilizing photochromic reactions. J. Photochem. Photobiol. C 2018, 34, 2–28. 10.1016/j.jphotochemrev.2017.12.006. [DOI] [Google Scholar]
- Glaser F.; Kerzig C.; Wenger O. S. Multi-Photon Excitation in Photoredox Catalysis: Concepts, Applications, Methods. Angew. Chem., Int. Ed. 2020, 59, 10266–10284. 10.1002/anie.201915762. [DOI] [PubMed] [Google Scholar]
- Kerzig C.; Wenger O. S. Reactivity control of a photocatalytic system by changing the light intensity. Chem. Sci. 2019, 10, 11023–11029. 10.1039/C9SC04584H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh I.; König B. Chromoselective Photocatalysis: Controlled Bond Activation through Light-Color Regulation of Redox Potentials. Angew. Chem., Int. Ed. 2016, 55, 7676–7679. 10.1002/anie.201602349. [DOI] [PubMed] [Google Scholar]
- Martínez-Gualda A. M.; Cano R.; Marzo L.; Pérez-Ruiz R.; Luis-Barrera J.; Mas-Ballesté R.; Fraile A.; de la Peña O’Shea V. A.; Alemán J. Chromoselective access to Z- or E- allylated amines and heterocycles by a photocatalytic allylation reaction. Nat. Commun. 2019, 10, 2634. 10.1038/s41467-019-10441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveselý T.; Wienhold M.; Molloy J. J.; Gilmour R. Advances in the E → Z Isomerization of Alkenes Using Small Molecule Photocatalysts. Chem. Rev. 2022, 122, 2650. 10.1021/acs.chemrev.1c00324. [DOI] [PubMed] [Google Scholar]
- Bharmoria P.; Bildirir H.; Moth-Poulsen K. Triplet-triplet annihilation based near infrared to visible molecular photon upconversion. Chem. Soc. Rev. 2020, 49, 6529–6554. 10.1039/D0CS00257G. [DOI] [PubMed] [Google Scholar]
- Singh-Rachford T. N.; Castellano F. N. Photon upconversion based on sensitized triplet-triplet annihilation. Coord. Chem. Rev. 2010, 254, 2560–2573. 10.1016/j.ccr.2010.01.003. [DOI] [Google Scholar]
- Yanai N.; Kimizuka N. Recent emergence of photon upconversion based on triplet energy migration in molecular assemblies. Chem. Commun. 2016, 52, 5354–5370. 10.1039/C6CC00089D. [DOI] [PubMed] [Google Scholar]
- El Roz K. A.; Castellano F. N. Photochemical upconversion in water. Chem. Commun. 2017, 53, 11705–11708. 10.1039/C7CC07188D. [DOI] [PubMed] [Google Scholar]
- Kerzig C.; Wenger O. S. Sensitized triplet-triplet annihilation upconversion in water and its application to photochemical transformations. Chem. Sci. 2018, 9, 6670–6678. 10.1039/C8SC01829D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholizadeh E. M.; Prasad S. K. K.; Teh Z. L.; Ishwara T.; Norman S.; Petty A. J.; Cole J. H.; Cheong S.; Tilley R. D.; Anthony J. E.; Huang S.; Schmidt T. W. Photochemical upconversion of near-infrared light from below the silicon bandgap. Nat. Photonics 2020, 14, 585–590. 10.1038/s41566-020-0664-3. [DOI] [Google Scholar]
- Ravetz B. D.; Pun A. B.; Churchill E. M.; Congreve D. N.; Rovis T.; Campos L. M. Photoredox catalysis using infrared light via triplet fusion upconversion. Nature 2019, 565, 343–346. 10.1038/s41586-018-0835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zähringer T. J. B.; Bertrams M.-S.; Kerzig C. Purely organic Vis-to-UV upconversion with an excited annihilator singlet beyond 4 eV. J. Mater. Chem. C 2022, 10, 4568. 10.1039/D1TC04782E. [DOI] [Google Scholar]
- Harada N.; Sasaki Y.; Hosoyamada M.; Kimizuka N.; Yanai N. Discovery of Key TIPS-Naphthalene for Efficient Visible-to-UV Photon Upconversion under Sunlight and Room Light. Angew. Chem., Int. Ed. 2021, 60, 142–147. 10.1002/anie.202012419. [DOI] [PubMed] [Google Scholar]
- VanOrman Z. A.; Nienhaus L. Feeling blue no more: How TIPS-naphthalene enables efficient visible-to-UV upconversion. Matter 2021, 4, 2625–2626. 10.1016/j.matt.2021.06.024. [DOI] [Google Scholar]
- Herr P.; Glaser F.; Büldt L. A.; Larsen C. B.; Wenger O. S. Long-lived, strongly emissive, and highly reducing excited states in Mo(0) complexes with chelating isocyanides. J. Am. Chem. Soc. 2019, 141, 14394–14402. 10.1021/jacs.9b07373. [DOI] [PubMed] [Google Scholar]
- Glaser F.; Kerzig C.; Wenger O. S. Sensitization-initiated electron transfer via upconversion: mechanism and photocatalytic applications. Chem. Sci. 2021, 12, 9922–9933. 10.1039/D1SC02085D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. R.; Pfund B.; Guo X.; Wenger O. S. Photo-triggered hydrogen atom transfer from an iridium hydride complex to unactivated olefins. Chem. Sci. 2020, 11, 8582–8594. 10.1039/D0SC01820A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrams M.-S.; Kerzig C. Converting p-terphenyl into a novel organo-catalyst for LED-driven energy and electron transfer photoreactions in water. Chem. Commun. 2021, 57, 6752–6755. 10.1039/D1CC01947C. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Castellano F. N.; Schmidt T. W.; Hanson K. On the Quantum Yield of Photon Upconversion via Triplet-Triplet Annihilation. ACS Energy Lett. 2020, 5, 2322–2326. 10.1021/acsenergylett.0c01150. [DOI] [Google Scholar]
- Schmid L.; Glaser F.; Schaer R.; Wenger O. S. High Triplet Energy Iridium(III) Isocyanoborato Complex for Photochemical Upconversion, Photoredox and Energy Transfer Catalysis. J. Am. Chem. Soc. 2022, 144, 963–976. 10.1021/jacs.1c11667. [DOI] [PubMed] [Google Scholar]
- Barata-Vallejo S.; Yerien D. E.; Postigo A. Advances in Photocatalytic Organic Synthetic Transformations in Water and Aqueous Media. ACS Sustainable Chem. Eng. 2021, 9, 10016–10047. 10.1021/acssuschemeng.1c03384. [DOI] [Google Scholar]
- Sun K.; Lv Q.-Y.; Chen X.-L.; Qu L.-B.; Yu B. Recent advances in visible-light-mediated organic transformations in water. Green Chem. 2021, 23, 232–248. 10.1039/D0GC03447A. [DOI] [Google Scholar]
- McCusker C. E.; Castellano F. N. Design of a Long-Lifetime, Earth-Abundant, Aqueous Compatible Cu(I) Photosensitizer Using Cooperative Steric Effects. Inorg. Chem. 2013, 52, 8114–8120. 10.1021/ic401213p. [DOI] [PubMed] [Google Scholar]
- Otto S.; Dorn M.; Förster C.; Bauer M.; Seitz M.; Heinze K. Understanding and Exploiting Long-Lived Near-Infrared Emission of a Molecular Ruby. Coord. Chem. Rev. 2018, 359, 102–111. 10.1016/j.ccr.2018.01.004. [DOI] [Google Scholar]
- Wegeberg C.; Wenger O. S. Luminescent First-Row Transition Metal Complexes. JACS Au 2021, 1, 1860–1876. 10.1021/jacsau.1c00353. [DOI] [PMC free article] [PubMed] [Google Scholar]