SUMMARY.
ONC201 is an oral selective antagonist of the dopamine D2 receptor and direct activator of caseinolytic protease P (ClpP). In a phase 2 study, ONC021 was shown to be well tolerated with notable efficacy in patients with the rare neuroendocrine neoplasms pheochromocytomas-paragangliomas, although biomarkers for activity remain to be elucidated.
COMMENTARY
In this issue of Clinical Cancer Research, Anderson and colleagues report the results from a phase 2 study of ONC201 in patients with rare tumors with a focus on pheochromocytoma and paraganglioma (PC-PG)(1).
Caring for patients with rare, heterogeneous malignancies such as neuroendocrine tumors (NET) is challenging. The tradeoff between efficacy and tolerability is generally considered inevitable in aggressive histologies, but is more nuanced for patients with clinically indolent, less aggressive disease such as the NET subcategory pheochromocytomas-paragangliomas (PC-PG). In these generally slow-growing tumors, treatments can be sequenced over several years; the ideal regimen should be simple, efficacious and tolerable over long periods of therapy. But can one have it all?
ONC201 is an orally administered imipridone heterocyclic small molecule. Initially identified as TRAIL-inducing compound 10, or TIC 10, it was reported to induce TRAIL by inactivating kinases AKT and ERK (2). Binding and reporter assays have also shown that ONC201 is a selective antagonist of the dopamine D2 receptors (DRD2) as highlighted by Anderson and colleagues (see Figure), as well as a direct activator of caseinolytic protease P (ClpP), a serine protease located in the mitochondrial matrix that regulates oxidative phosphorylation (3). ClpP is a beguiling target for anti-cancer therapy given these multiple pathways of activity and since both the inhibition and the activation of ClpP have demonstrated anti-tumor activity through mitochondrial proteolysis leading to apoptotic cell death.
Figure Legend: Proposed mechanisms of anti-tumor activity for ONC201 and potential biomarkers for future study.
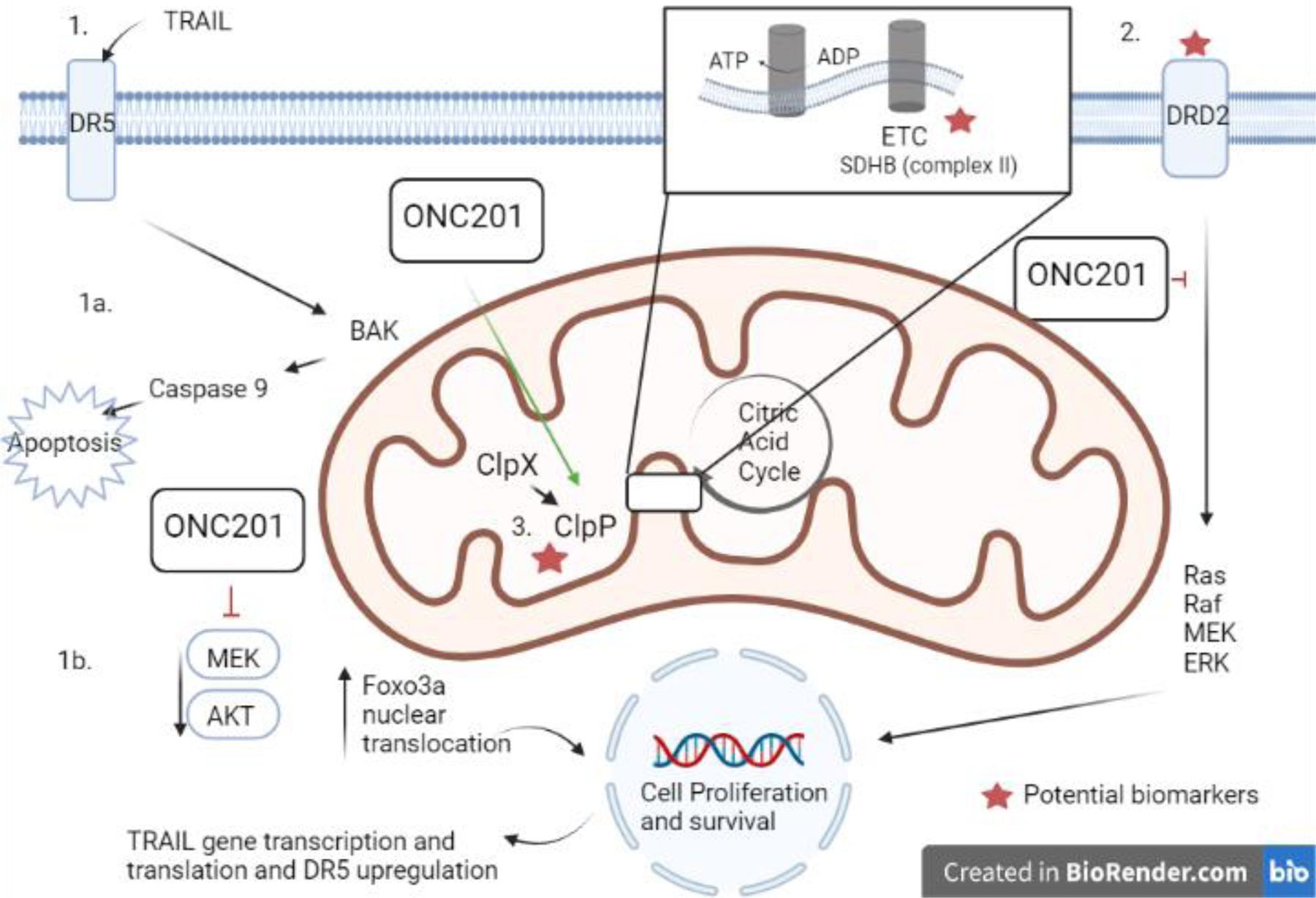
ONC201 is an orally administered imipridone that has demonstrated anti-tumor activity in a number of preclinical models and was found to have clinical efficacy in patients with pheochromocytoma and paraganglioma as reported by Anderson and colleagues in this issue of CCR. (1) Studies utilizing predominantly colon cancer preclinical models identified that ONC201 (originally named TRAIL-inducing compound 10, or TIC 10) induced TRAIL leading to apoptosis (1a) by downregulation of AKT and ERK, and that this anti-tumor activity was dependent on that transcriptional factor Foxo3a which leads to upregulation of TRAIL death receptor 5 (DR5) (1b, Ref 1). (2) ONC201 was also found to be an antagonist of the dopamine D2 receptors (DRD2), a G protein-coupled receptor that acts through ERK and Akt signaling to promotes cancer cell survival. (3) Lastly, ONC201 is also a direct activator of caseinolytic protease P (ClpP), a serine protease located in the mitochondrial matrix that regulates oxidative phosphorylation typically dependent on chaperone protein CLpX. This dependence is disrupted by ONC201 binding, leading to ClpP activation resulting in mitochondrial proteolysis including destruction of components of the electron transport chain. Given the role of succinate dehydrogenase as a bridge between the TCA and the electron transport chain, possibility of tumor suppressor SDH mutations as a potential biomarker for ONC201 could be explored in preclinical models as well as in future clinical studies.
ETC – electron transport chain; DRD2 - dopamine D2 receptor; DR5- death receptor 5; TRAIL - TNF-related apoptosis-inducing ligand
Anderson and colleagues conducted an open label, multi-arm phase 2 study of ONC201 in a total of 30 patients across three arms: Arm A included patients with PC-PG, Arm B included patients with other tumors such as adrenocortical cancer (ACC) and desmoplastic small cell round tumor (DSCRT), while Arm C included a dose dense treatment for selected patients who crossed over from Arms A and B. The primary endpoint of this investigator-initiated trial was efficacy of ONC201 measured as radiographic response via RECIST 1.1 with safety, progression free and overall survival as secondary endpoints.
Overall, Anderson and colleagues found that once or twice weekly oral treatment with ONC201 was exceptionally well tolerated. Within the 10-patient pheochromocytoma and paraganglioma cohort, no dose limiting toxicities (DLTs) and no grade 3 or 4 treatment-related adverse events were observed. In fact, no treatment-related serious adverse events (SAEs) were observed at all, and all toxicities were either grade 1 or 2. This is notable especially since several patients received treatment with ONC201 for many months and, in some cases, years. Thus, even in a relatively small study, it is evident that for at least the doses reported here, a clear advantage for ONC201 is its tolerability and ease of administration.
In terms of efficacy, there was only one response in arm B in a patient with DSCRT, although several patients did attain stable disease for over one year. A more interesting signal was seen in patients with PC-PG (arm A), where the response rate was 50% (n=5/10) and three patients remained on treatment for over 30 months. There are a few things to note about the PC-PG cohort – including that only five out of the ten patients had received prior chemotherapy, whereas the other five patients had received locoregional therapy alone such as surgery or radiation. These patients were systemic therapy naïve which may be a consideration when assessing responses. Additionally, in several patients over a decade had passed since the initial diagnosis, indicating potentially a varying natural disease course compared to the patients with a need for systemic therapy closer to their initial diagnosis. Consistent with prior literature, five out of the 10 patients with PC-PG had known mutations in SDHB and, of these, three had a partial response while one had stable disease. Arm B consisted primarily of patients with DSRCT (n=10/16, 62.5%), with a handful for patients with neuroendocrine tumors (NET) including 2 patients with medullary thyroid cancer and one patient with adrenocortical carcinoma. No patients with pancreatic, gastrointestinal or lung NET were included, and other clinical information including differentiation or proliferation markers such as Ki-67 and mitotic index was not presented. Overall, there was evidence of efficacy with disease response mainly in the PC-PG cohort as well as impressive tolerability for ONC-201.
Despite the promising responses in PC-PG patients, the precise mechanism of action of ONC201 remains elusive. Although the putative anti-tumor activity as explained by Anderson and colleagues was driven by its role as a DRD2 antagonist, preclinically, ONC201 has also been shown to be active in DRD2 low cancers, such as AML (3). Moreover, activity in these studies and other studies was not found to be dependent on TRAIL receptors and occurred despite the lack of DRD2 expression. In the first in human phase 1 trial of ONC201 previously reported in CCR, decreases in tumor volume were primarily in patients with endometrial cancer though no RECIST responses were observed (4). However, the TCGA analysis presented by Anderson demonstrates a lower expression of DRD2 in endometrial tumor compared to normal cells, suggesting that additional biomarkers or pathways of activity must be considered to accurately identify patients who might benefit from ONC201.
One such explanation for the activity of ONC201 in DRD2 low-expressing tumors is its role as a ClpP agonist. In preclinical AML models, Ishizawa et al. clearly demonstrated that the anti-tumor activity of ONC201 was through activation of ClpP, that a single amino acid change in ClpP induced resistance to ONC201, and that expression of ClpP correlated with sensitivity to ONC201 (3). An assessment of ClpP status in the responders of the study reported by Anderson, as well as the previously reported phase 1 trial of ONC201, could help support this as the primary mechanism of action. Given the higher responses seen in the PG-PC cohort, one could explore enhanced efficacy of ONC201in SDH deficient tumors. Recently, another imipridone compound with similar profile was found to downregulate SDH-A and SDH-B (5) in pancreatic cancer preclinical models, pointing to a potential mechanism. Given the role of succinate dehydrogenase as a bridge between the TCA and the electron transfer chain, possibility of tumor suppressor SDH mutations as a potential biomarker for ONC201 could be explored in preclinical models as well as in future clinical studies.
This study contributes important safety and exciting preliminary efficacy data to the literature and suggests a potential role for ONC201 especially in patients with PC-PG. These patients constitute a unique population with a limited number of available regimens and a need for long term treatment strategies. The tolerability of the compound cannot be overstated, and we envision a potential role as maintenance therapy in PC-PG and other slower growing tumors, such as NETs or certain sarcomas. Unfortunately, optimal selection of these patients is not clear based on available data. More work needs to be done to confirm the mechanisms of action and identify biomarkers, including DRD2 expression, ClpP, SDH mutations, or some combination of the above. For now, combining data from completed phase 1 and 2 trials to assess biomarkers as well as rational preclinical studies could be a reasonable next step in order to inform the future TRAIL of ONC201 clinical development.
Funding:
Dr. Owen is supported by the LUNGevity Foundation Career Development Award.
Footnotes
Conflicts of Interest: None.
Contributor Information
Dwight H. Owen, Division of Medical Oncology, Department of Internal Medicine, The Ohio State University Wexner Medical Center and James Comprehensive Cancer Center, 1800 Cannon Drive, Suite 1240, Columbus, OH, USA.
Nikolaos A Trikalinos, Division of Medical Oncology, Department of Internal Medicine, Washington University School of Medicine and Siteman Cancer Center, 4921 Parkview Place, Saint Louis, MO, USA.
References:
- 1.Anderson PM, Trucco MM, Tarapore RS, Zahler S, Thomas S, Gortz J, et al. Phase II Study of ONC201 in Neuroendocrine Tumors including Pheochromocytoma-Paraganglioma and Desmoplastic Small Round Cell Tumor. Clin Cancer Res 2022. doi 10.1158/1078-0432.Ccr-21-4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JE, Krigsfeld G, Mayes PA, Patel L, Dicker DT, Patel AS, et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med 2013;5(171):171ra17 doi 10.1126/scitranslmed.3004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishizawa J, Zarabi SF, Davis RE, Halgas O, Nii T, Jitkova Y, et al. Mitochondrial ClpP-Mediated Proteolysis Induces Selective Cancer Cell Lethality. Cancer Cell 2019;35(5):721–37.e9 doi 10.1016/j.ccell.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein MN, Bertino JR, Kaufman HL, Mayer T, Moss R, Silk A, et al. First-in-Human Clinical Trial of Oral ONC201 in Patients with Refractory Solid Tumors. Clin Cancer Res 2017;23(15):4163–4169. doi: 10.1158/1078-0432.CCR-16-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrarini I, Louie A, Zhou L, El-Deiry WS. ONC212 is a Novel Mitocan Acting Synergistically with Glycolysis Inhibition in Pancreatic Cancer. Mol Cancer Ther 2021;20(9):1572–83 doi 10.1158/1535-7163.Mct-20-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]


