Abstract
Background
The association of adiposity with prostate cancer specific mortality remains unclear. We examined how adiposity relates to fatal prostate cancer and described the cross-sectional associations of commonly used adiposity measurements with adiposity estimated by imaging in UK Biobank. We also conducted a dose-response meta-analysis to integrate the new data with existing prospective evidence.
Methods
218,237 men from UK Biobank who were free from cancer at baseline were included. Body mass index (BMI), total body fat percentage (using bioimpedance), waist circumference (WC) and waist-to-hip ratio (WHR) were collected at recruitment. Risk of dying from prostate cancer (primary cause) by the different adiposity measurements was estimated using multivariable-adjusted Cox proportional hazards models. Results from this and other prospective cohort studies were combined in a dose-response meta-analysis.
Results
In UK Biobank, 661 men died from prostate cancer over a mean follow-up of 11.6 years. In the subsample of participants with magnetic resonance imaging and dual-energy X-ray absorptiometry, BMI, body fat percentage and WC were strongly associated with imaging estimates of total and central adiposity (e.g. visceral fat, trunk fat). The hazard ratios (HR) for prostate cancer death were 1.07 (95% confidence interval = 0.97–1.17) per 5 kg/m2 higher BMI, 1.00 (0.94–1.08) per 5% increase in total body fat percentage, 1.06 (0.99–1.14) per 10 cm increase in WC and 1.07 (1.01–1.14) per 0.05 increase in WHR. Our meta-analyses of prospective studies included 19,633 prostate cancer deaths for BMI, 670 for body fat percentage, 3181 for WC and 1639 for WHR, and the combined HRs for dying from prostate cancer for the increments above were 1.10 (1.07–1.12), 1.03 (0.96–1.11), 1.07 (1.03–1.11), and 1.06 (1.01–1.10), respectively.
Conclusion
Overall, we found that men with higher total and central adiposity had similarly higher risks of prostate cancer death, which may be biologically driven and/or due to differences in detection. In either case, these findings support the benefit for men of maintaining a healthy body weight.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02336-x.
Keywords: Adiposity, Imaging, Prostate cancer, Mortality, Population-attributable risk
Background
Prostate cancer is the second most common cause of cancer death in men in the UK [1]. Age, black ethnicity, family history of prostate cancer, genetic factors and endogenous hormones are known risk factors for prostate cancer, but apart from hormones none of them is modifiable [2–5]. While many prostate cancer tumours are indolent (slow-growing), others are lethal, and these different tumours may have different risk factors [3]. However, the aetiology of lethal prostate cancer is not well understood, and there is a need to identify risk factors for this most clinically relevant form of the disease.
There is substantial evidence that relates adiposity to prostate cancer risk, but the association appears to vary by tumour behaviour. Previous studies have found an inverse association of obesity with risk of overall prostate cancer and non-aggressive forms of the disease, possibly due to later diagnosis in men with obesity. However, a positive association of greater adiposity with risk for aggressive prostate cancer, including risk of dying from prostate cancer, has been reported [2, 6], although it is unclear whether this positive association may be due to late detection (and thus more advanced tumours with poorer prognosis), a role of excessive adiposity in promoting metabolic and hormonal dysfunction that in turn may stimulate the growth and progression of prostate cancer cells [7], or a combination of both. Moreover, some evidence suggests that fat located within the abdominal cavity may be more aetiologically important for aggressive prostate cancer than total adiposity [6], and the use of “gold standard” methods to determine body fat distribution (e.g. magnetic resonance imaging (MRI)) [8, 9] may help to better understand these associations. However, due to the limited number of prostate cancer deaths in most prospective studies, relatively few studies have investigated whether adiposity or its distribution is related to prostate cancer mortality [2], and more research is needed.
In this report, we describe the results from a prospective analysis using UK Biobank data, and then from a dose-response meta-analysis of findings from all published prospective studies. To inform the interpretation of our findings, we also first describe the cross-sectional associations in UK Biobank of commonly used indices of adiposity with MRI- and dual-energy X-ray absorptiometry (DXA)-derived estimates of adiposity.
Methods
UK Biobank
Study design and population
UK Biobank is a prospective study of ~ 500,000 UK adults aged 40–69 years at recruitment (including 229,000 men) established between 2006 and 2010 to study risk factors for disease. Details of the study protocol and information about data access are available online (https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf) and elsewhere [10]. All individuals provided informed consent to participate and the study was approved by the National Information Governance Board for Health and Social Care and the National Health Service North West Multicentre Research Ethics Committee (reference number 06/MRE08/65). In brief, approximately 9.2 million people living within reasonable travelling distance (∼25 km) of 1 of the 22 assessment centres across England, Wales, and Scotland were identified from National Health Service (NHS) registers and invited to participate in the study, with a participation rate of 5.5% [11].
After excluding 9869 men with prevalent cancer (except C44: non-melanoma skin cancer), 1 man censored on entry day and 999 men with no adiposity measurements, the analyses included a total of 218,237 men (Additional File 1: Figure S1).
Assessment of adiposity and other predictor variables
At recruitment, participants provided detailed information on a range of sociodemographic, physical, lifestyle and health-related factors via self-completed touch-screen questionnaires and a computer assisted personal interview [11]. Anthropometric measurements (standing height, weight, waist and hip circumferences) were taken by trained research clinic staff at the assessment centre, while body mass index (BMI) was calculated and percentage body fat was estimated through bioimpedance measures [12].
UK Biobank imaging sub-cohort
In 2014, the UK Biobank imaging study re-invited a subsample of participants to undergo abdominal MRI and DXA, which has been detailed elsewhere [13, 14]. In brief, participants were scanned in a Siemens MAGNETOM Aera 1.5 T MRI scanner (Siemens Healthineers, Germany) using a 6-min dual-echo Dixon Vibe protocol, providing water-and-fat-separated volumetric information for fat and muscle. Body composition analyses for MRI images were performed using AMRA Profiler Research (AMRA Medical AB, Linköping, Sweden). DXA captures whole-body composition (e.g. bone, fat and lean mass) with no extensive additional processing and analysis. However, it is not possible to obtain direct compartmental volumetric measurements using this method, and therefore, regional volume estimates are obtained indirectly using anatomical models [13, 14]. By December 2021, imaging data on ~ 18,800 men were available. BMI, WC, hip circumference and body fat percentage were also re-assessed at the imaging visit.
Ascertainment of prostate cancer mortality
Our endpoint was prostate cancer as the underlying cause of death recorded on the death certificate (International Classification of Diseases Tenth revision codes: C61 [15]). Men were followed-up until 31 December 2020 for England and Scotland and 19 July 2020 for Wales. Mortality data were provided by NHS Digital for England and Wales and by the NHS Central Register and Information and Statistics Division for Scotland.
Statistical analysis in UK Biobank
Cross-sectional analyses of adiposity measurements
Pearson correlations between different anthropometric measurements were calculated. A subsample of men had both commonly used anthropometric measures of adiposity (i.e. BMI, body fat percentage, WC and WHR) and adiposity information (MRI and DXA) from the imaging visit. Multivariable linear regression adjusted for categories of age and height was used to estimate the mean differences in each MRI- and DXA-derived measure of body composition per 1-SD difference in the levels of each commonly used anthropometric measure of adiposity. We also used Pearson correlations to assess the associations between adiposity measures. Moreover, men were categorised into tenths of BMI, body fat percentage, WC and WHR, and multivariable linear regressions (adjusted for age and height) were conducted to calculate mean values for MRI and DXA. Moreover,
Prospective analyses
Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for prostate cancer death, using age as the underlying time variable. Person-years were calculated from the date of recruitment to the date of death, loss to follow-up, or the censoring date, whichever occurred first. The proportional hazards assumption was examined using time-varying covariates and Schoenfeld residuals, and this revealed no evidence of deviation. Men were categorised into fourths of adiposity measurements based on the distribution in the cohort. We also modelled HRs per predefined increments and categories of the adiposity measurements: (i) BMI [per 5 kg/m2 increase, and as predefined World Health Organization (WHO) categories [16] (< 25, 25–29.9, and ≥ 30 kg/m2)]; (ii) body fat percentage (per 5% increase); (iii) WC [per 10 cm increase, and as predefined WHO categories [17] (< 94, 94–101.9, ≥ 102 cm)]; and (iv) WHR [per 0.05 unit increase, and as predefined WHO categories [17] (< 0.90, ≥ 0.90)]. Potential nonlinear associations between the anthropometric variables and prostate cancer mortality were evaluated using likelihood ratio tests comparing the model with the anthropometric variable entered as an ordered categorical (ordinal) variable to a model with the categorical variable treated as continuous, and no evidence of non-linearity was observed.
Adjustment covariates were defined a priori based on previous analyses by our group using UK Biobank data [18]. The minimally-adjusted models were stratified by geographical region of recruitment (ten UK regions) and age (< 45, 45–49, 50–54, 55–59, 60–64, ≥ 65 years) at recruitment. The fully adjusted model was further adjusted for Townsend deprivation score (fifths, unknown [0.1%]), ethnic group (white, mixed background, Asian, black, other, and unknown [0.6%]), height (< 170, 170–174.9, 175–179.9, ≥ 180 cm, and unknown [0.2%]), lives with a wife or partner (no, yes), cigarette smoking (never, former, current 1– < 15 cigarettes per day, current ≥ 15 cigarettes per day, current but number of cigarettes per day unknown, and smoking status unknown [0.6%]), physical activity (low [0–9.9 METs/week], moderate [10–49.9 METs/week], and high [≥50 METs/week], unknown [3.6%]), alcohol consumption (non-drinkers, < 1–9.9, 10–19.9, ≥20 g ethanol/day, unknown [0.5%]), diabetes (no, yes, and unknown [0.5%]) and history of PSA testing at recruitment (no, yes, unknown [5%]).
Sensitivity analyses
We also performed the following sensitivity analyses: excluding the first 5 years of follow-up. excluding men with BMI ≥ 27.5 kg/m2, excluding men with BMI ≥ 25 kg/m2, excluding extreme values of exposure variables (percentiles outside 1–99), excluding men < 50 years of age at recruitment, running the statistical analyses per 1 standard deviation (SD) increment, using the BMI-adjusted residuals of WC (or WHR, depending on which one is the exposure of interest) by regressing these variables in a linear model and using the residuals (that are statistically independent of BMI) as the exposures of interest.
Dose-response meta-analysis
We searched on PubMed, Embase, and Web of Science for prospective studies examining the associations of BMI, body fat percentage, WC and WHR with prostate cancer as the underlying cause of death, independently by two researchers up to 15 March 2021; please see details in the Additional File 1: Supplementary Methods [2, 6, 19–38], Tables S1-S3 and Figure S2) [6, 22–38]. We excluded reviews, abstract-only publications or editorials. When the same cohort study published more than one original article looking at these associations, the paper reporting the longest follow-up time was retained.
In the dose-response meta-analysis, we calculated the HR estimates in the studies that reported results for a different increment (e.g. per 1 SD increase) or from the categorical data using generalised least-squares [39] for the increments mentioned above (details in Additional File 1: Supplementary Methods). We then pooled study-specific log HRs to obtain a summarised effect size using a fixed effects model. The I2 statistic was used to assess heterogeneity across studies, and we assessed publication bias with funnel plots and Egger’s test.
Population-attributable risk
The number of prostate cancer deaths attributable to obesity as measured by BMI (population-attributable risk (PAR)) in the UK was calculated using the number of deaths in the UK in 2019, the estimate of relative risk from our dose response meta-analysis and information on the prevalence of obesity in English men aged 55–64 years in 2019 (as a surrogate for the UK; mean BMI 28.9 kg/m2) [40].
All analyses were performed using Stata version 14.1 (Stata Corporation, College Station, TX, USA), and figures were plotted in R version 3.2.3. All tests of significance were two-sided, and P-values < 0.05 were considered statistically significant.
Results
UK Biobank participants’ characteristics
After an average of 11.6 years of follow-up, a total of 661 men died from prostate cancer among the 218,237 men included in the UK Biobank study. The main baseline characteristics of the participants are shown in Table 1, while baseline characteristics of participants according to categories of BMI and WC are reported in Additional File 1: Tables S4 and S5. 12.4% of men reported that they were current cigarette smokers, 43.3% reported drinking ≥ 20 g of alcohol per day and 27.6% of men reported being physically inactive. Men who subsequently died from prostate cancer had higher values of all adiposity measurements at recruitment (Table 1). Moreover, men with higher adiposity at baseline were more likely to be older, drink ≥ 20 g of alcohol per day, be physically inactive and to have hypertension and diabetes than men in the lowest quartiles of BMI and WC (Additional File 1: Tables S4-S6).
Table 1.
Baseline characteristics in all men and in men who died from prostate cancer in men from UK Biobank
| Characteristics at baseline | All men | Men who died from prostate cancer |
|---|---|---|
| No. of men | 218237 | 661 |
| Sociodemographic | ||
| Age at recruitment (years), mean (SD) | 56.5 (8.2) | 63.1 (4.9) |
| Most deprived quintile, n (%) | 44,804 (20.5) | 109 (16.5) |
| No qualifications, n (%) | 29,465 (13.5) | 83 (12.6) |
| Black ethnicity, n (%) | 3,225 (1.5) | 4 (0.6) |
| Not in paid/self-employment, % (n) | 84,578 (38.8) | 410 (62.2) |
| Living with partner, n (%) | 166,378 (76.2) | 493 (74.8) |
| Anthropometric | ||
| Height (cm), mean (SD) | 175.6 (6.8) | 175.4 (7.0) |
| BMI (kg/m2), mean (SD) | 27.8 (4.3) | 28.1 (4.3) |
| Body fat (%), mean (SD) | 25.3 (5.8) | 26.1 (5.8) |
| Waist circumference (cm), mean (SD) | 96.9 (11.4) | 98.9 (11.2) |
| Waist to hip ratio, mean (SD) | 0.936 (0.065) | 0.950 (0.064) |
| Lifestyle | ||
| Current cigarette smokers, n (%) | 27,247 (12.4) | 68 (9.8) |
| Drinking alcohol ≥ 20 g/day, n (%) | 94,407 (43.3) | 299 (45.4) |
| Physically inactive, n (%) | 60,228 (27.6) | 178 (27.0) |
| Health status | ||
| Vasectomy, n (%) | 11,343 (5.2) | 25 (3.8) |
| Hypertension, n (%) | 113,874 (52.2) | 416 (62.0) |
| Diabetes, n (%) | 15,088 (6.9) | 72 (10.9) |
| Prostate specific factors prior recruitment | ||
| PSA test, n (%) | 60,441 (27.7) | 206 (31.3) |
| Enlarged prostate, n (%) | 7,074 (3.2) | 35 (5.3) |
| Family history of prostate cancer, n (%) | 16,383 (7.5) | 65 (9.9) |
Abbreviations: BMI body mass index, PSA prostate specific antigen
Cross-sectional associations in UK Biobank
BMI, body fat percentage and WC were strongly correlated (correlation coefficients (r) = 0.79–0.88), although these measures were less strongly correlated with WHR (r = 0.59–0.79, Additional File 1: Table S7). BMI, body fat percentage and WC were strongly associated with total and central adiposity (e.g. visceral fat, trunk fat) obtained from MRI- and DXA-derived measures of body composition, while the associations for WHR were somewhat smaller (Additional File 1: Tables S7-S12). Muscle fat mass infiltration and liver proton density fat fraction were moderately correlated with the commonly used anthropometric measurements (r = 0.36–0.54) (Additional File 1: Tables S8 & S9).
Prospective analysis in UK Biobank
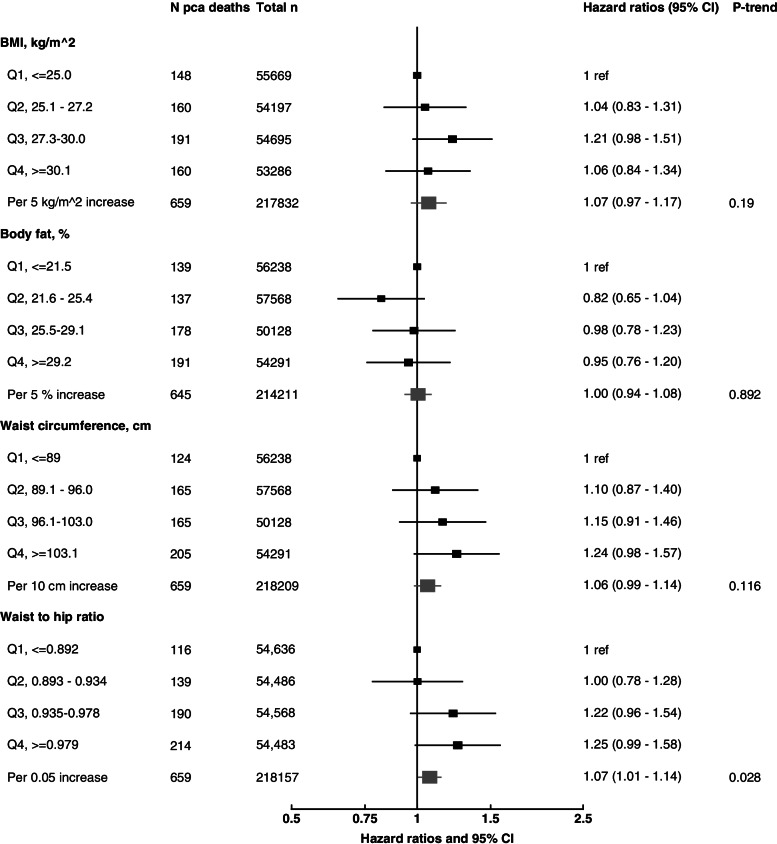
The multivariable-adjusted associations of BMI, body fat percentage, WC and WHR with prostate cancer mortality are shown in Fig. 1 (minimally adjusted associations are shown in Additional File 1: Table S13). Except for the associations of WC and WHR with prostate cancer death, there were no large changes between the minimally- and the multivariable-adjusted models. BMI (HR per 5 kg/m2 = 1.07 (95% CI 0.97–1.17), total body fat percentage (HR per 5% increase = 1.00, 0.94–1.08) and WC (HR per 10 cm increase = 1.06, 0.99–1.14) were not significantly associated with prostate cancer death, whereas WHR (HR per 0.05 increase = 1.07, 1.01–1.14) was significantly associated with risk of dying from prostate cancer; when the highest quartiles were compared to the lowest the HRs were 1.06 (0.84–1.34) for BMI, 0.95 (0.76–1.20) for body fat percentage, 1.24 (0.98–1.57) for WC, and 1.25 (0.99–1.58) for WHR. When BMI, WC and WHR were categorised according to the WHO cutoff points, no significant associations were found (Additional File 1: Table S14).
Fig. 1.
Multivariable-adjusted hazard ratios (95% CI) for prostate cancer death in relation to adiposity measurements at baseline in men from UK Biobank. Abbreviations: BMI, body mass index. Cox regression analyses. All models are stratified by region and age at recruitment and adjusted for age (underlying time variable), Townsend deprivation score, ethnicity, lives with a wife or partner, smoking, physical activity, alcohol consumption, height, diabetes, and history of PSA test. Full details for each covariate are provided in the statistical section
In sensitivity analyses, we found that the associations remained largely unchanged after excluding the first 5 years of follow-up, men with BMI ≥ 25 kg/m2, extreme values and men < 50 years of age (Table 2). When the associations between commonly used anthropometric measurements and prostate cancer death were assessed using a 1 SD increment of each exposure of interest (4.3 kg/m2 for BMI, 5.8% for body fat percentage, 11.4 cm for WC, and 0.065 for WHR), the HRs were 1.06 (0.98–1.15), 1.01 (0.93–1.10), 1.07 (0.99–1.16), and 1.10 (1.01–1.19), respectively for BMI, body fat percentage, WC and WHR (Table 2).
Table 2.
Sensitivity analyses. Multivariable-adjusted hazard ratios (95 % CI) for prostate cancer death in relation to adiposity measurements at recruitment in 218,237 men from UK Biobank
| BMI | Body fat percentage | Waist circumference | Waist to hip ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n prostate cancer deaths | Per 5 kg/m2 increase | p-trend1 | n cases | Per 5 % increase | p-trend1 | n cases | Per 10 cm increase | p-trend1 | n cases | Per 0.05 unit increase | p-trend1 | |
| Overall | 659 | 1.07 (0.97–1.17) | 0.190 | 645 | 1.00 (0.94–1.08) | 0.892 | 659 | 1.06 (0.99–1.14) | 0.116 | 659 | 1.07 (1.01–1.14) | 0.028 |
| Excluding first 5 years of follow-up | 568 | 1.06 (0.96–1.18) | 0.265 | 559 | 1.01 (0.93–1.09) | 0.802 | 568 | 1.05 (0.98–1.14) | 0.180 | 568 | 1.07 (1.00–1.14) | 0.060 |
| Excluding men with BMI ≥ 25 kg/m2, per 1 SD increment | 144 | 0.93 (0.79–1.10) | 0.404 | 140 | 0.87 (0.74–1.03) | 0.118 | 144 | 1.04 (0.87–1.24) | 0.667 | 144 | 1.13 (0.96–1.33) | 0.144 |
| Excluding extreme values: percentiles 1–99 | 659 | 1.07 (0.97–1.18) | 0.170 | 645 | 1.01 (0.94–1.08) | 0.826 | 659 | 1.06 (0.99–1.14) | 0.101 | 659 | 1.07 (1.01–1.14) | 0.023 |
| Excluding men < 50 years of age | 650 | 1.06 (0.96–1.17) | 0.223 | 636 | 1.00 (0.93–1.08) | 0.950 | 650 | 1.06 (0.98–1.14) | 0.137 | 650 | 1.07 (1.01–1.14) | 0.034 |
| Per 1 SD increment | 659 | 1.06 (0.98–1.15) | 0.170 | 645 | 1.01 (0.93–1.10) | 0.826 | 659 | 1.07 (0.99–1.16) | 0.101 | 659 | 1.10 (1.01–1.19) | 0.023 |
| Residualsa | 659 | 1.08 (0.92–1.27) | 0.325 | 659 | 1.07 (0.99–1.15) | 0.072 | ||||||
Abbreviations: BMI body mass index
Cox regression analyses. All models are stratified by region and age at recruitment and adjusted for age (underlying time variable), Townsend deprivation score, ethnicity, lives with a wife or partner, smoking, physical activity, alcohol consumption, height, diabetes, and history of PSA test. Full details for each covariate are provided in the statistical section
1P-values for trend are obtained by entering the anthropometric variable per increment in the Cox regression model
aHR (95% CI) is from the multiple adjusted model (above) after accounting for the residuals of waist circumference and waist to hip ratio regressed on BMI for analyses of waist circumference and waist to hip ratio as exposures
Dose-response meta-analyses
A total of 19, 2, 6, and 3 prospective studies (including the current report on UK Biobank) were identified that had reported on BMI, body fat percentage, WC and/or WHR, respectively, in relation to prostate cancer-specific mortality (Additional File 1: Tables S1-S3). When these results were combined with UK Biobank, data from a total of 19,633 (for BMI), 670 (for body fat percentage), 3181 (for WC) and 1639 (for WHR) men who died from prostate cancer were available.
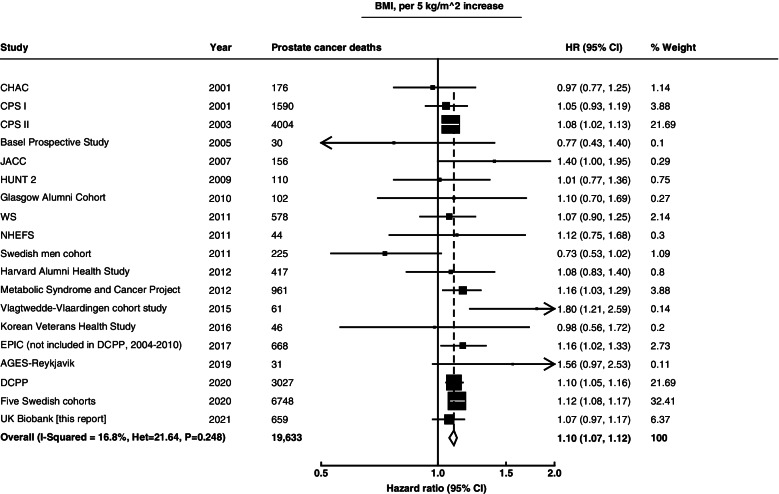
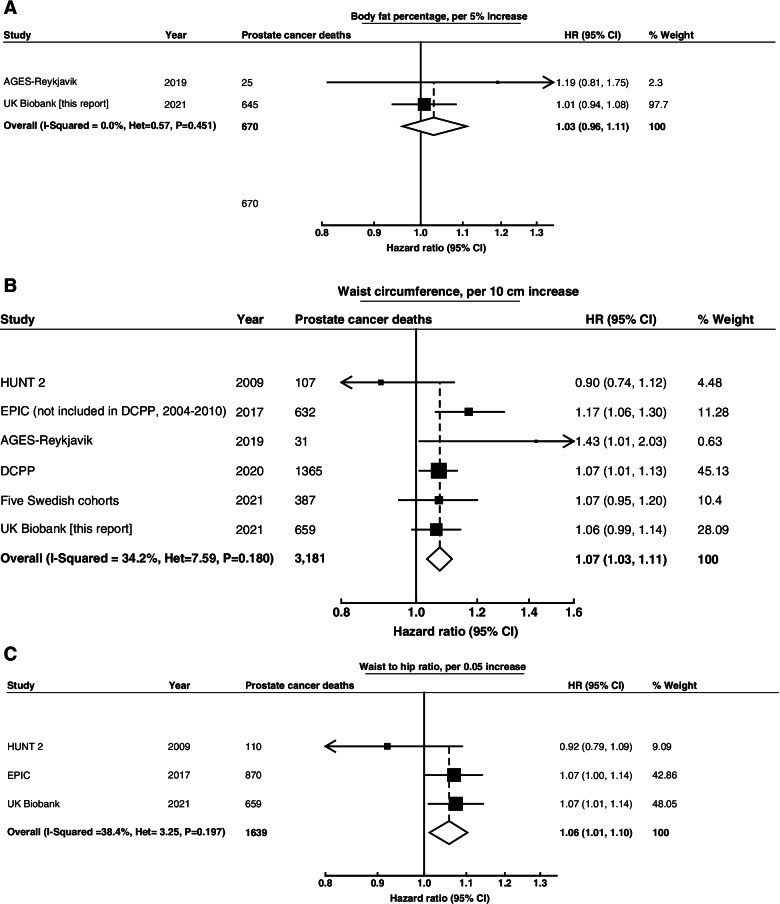
In the dose-response meta-analyses, the weighted average HRs were 1.10 (1.07–1.12) for every 5 kg/m2 increase in BMI, 1.03 (0.96–1.11) for every 5% increase in body fat percentage, 1.07 (1.03–1.11) for every 10 cm increase in WC and 1.06 (1.01–1.10) for every 0.05 increase in WHR. There was no statistically significant heterogeneity between studies for any of these associations (Figs. 2 and 3). When we assessed the associations by region, we observed similar associations in European (1.10 (1.07–1.14)) and USA studies (1.08 (1.04–1.13)) for BMI. The Egger test showed no evidence of publication bias for associations of BMI or waist circumference with prostate cancer death (p value = 0.54 for BMI and p value = 0.71 for WC). On examination of funnel plots (Additional File 1: Figure S3), we observed no evidence of publication bias for the associations of BMI or waist circumference with prostate cancer death. Assessing evidence of possible publication bias via funnel plots for body fat percentage and WHR was not appropriate due to the small number of studies.
Fig. 2.
Meta-analysis of prospective studies on the risk of prostate cancer death in relation to BMI. Study-specific hazard ratios (HR) are represented by squares (with their 95% confidence intervals [CIs] as lines). HRs were combined using inverse-variance-weighted averages of the log HRs in the separate studies, yielding a result and its 95% CI, which is plotted as a diamond. Please see Supplementary Table 1 for further details about each study. Abbreviations: AGES-Reykjavik, Age, Gene/Environment Susceptibility-Reykjavik; CHAC, The Chicago Heart Association; CPS I, Cancer Prevention Study I Nutrition Cohort Study; CPS II, Cancer Prevention Study II Nutrition Cohort Study; DCPP, Diet and Cancer Pooling Project; EPIC, European Prospective Investigation into Cancer and Nutrition; JACC, Japan Collaborative Cohort Study; HUNT 2, Nord-Trøndelag Health Study; NHEFS, Nutrition Examination Survey Epidemiology Follow-Up Study; WS, Whitehall study
Fig. 3.
Meta-analysis of prospective studies on the risk of prostate cancer death in relation to body fat percentage (A), waist circumference (B), and waist to hip ratio (C). Study-specific hazard ratios (HR) are represented by squares (with their 95% confidence intervals [CIs] as lines). HRs were combined using inverse-variance-weighted averages of the log HRs in the separate studies, yielding a result and its 95% CI, which is plotted as a diamond. Please see Supplementary Tables 2 and 3 for further details about each study. Abbreviations: AGES-Reykjavik, Age, Gene/Environment Susceptibility-Reykjavik; DCPP, Diet and Cancer Pooling Project; EPIC, European Prospective Investigation into Cancer and Nutrition; HUNT 2, NordTrøndelag Health Study
A total of 11,900 men die from prostate cancer each year (2016-2018 average) in the UK [41]. If it is assumed that this number of annual deaths would otherwise remain stable, that the HR from our meta-analysis is accurate and unbiased, and that the mean BMI in men aged 55–64 years is 28.9 kg/m2 (UK data from 2019), a reduction in the mean BMI of 5 kg/m2 would decrease the mean BMI to within the ideal range and would lead to an estimated 1309 fewer prostate cancer deaths annually in the UK.
Discussion
The dose-response meta-analysis integrating the UK Biobank study and other prospective studies showed positive and similar associations for both total and central adiposity in relation to risk for prostate cancer death. We also report here separately the new analyses from UK Biobank, and they are in line with the results from our meta-analysis.
Our dose-response meta-analysis, which included more than double the number of prostate cancer deaths than previous meta-analyses, suggested that the associations with prostate cancer mortality were similar for total and central adiposity. The totality of the prospective data on central adiposity as assessed by WC and WHR in relation to subsequent prostate cancer death, however, is still relatively limited (e.g. 6 studies with a total of 3181 prostate cancer deaths for WC) [6, 19, 27, 36, 38], and more studies are needed to confirm the magnitude of the association with central adiposity. The latest World Cancer Research Fund meta-analysis on prostate cancer published in 2014 also reported a positive association between overall adiposity (assessed using BMI) and prostate cancer death based on 10,100 prostate cancer deaths in a total of 12 studies; however, it did not have enough data from previous prospective studies to look at the association with central adiposity measurements (i.e. WC and WHR) [2]. A more recent pooled analysis of individual participant data from up to 15 prospective studies that included 3000 prostate cancer deaths for total adiposity and 1300 for central adiposity found a positive association of both total and central adiposity with prostate cancer mortality [19].
Obesity is defined as excessive fat accumulation, but some commonly used measures of adiposity such as BMI do not differentiate reliably between fat and fat-free mass. WC has been proposed as a better marker than BMI of adiposity in middle-aged men [42]; however, in men in UK Biobank, WC and BMI are highly correlated, and they showed similar associations with “gold standard” measurements of adiposity (MRI and DXA) in our cross-sectional analyses. We found that BMI, body fat percentage and WC were strongly positively associated with total and central adiposity (e.g. visceral fat, trunk fat) from the imaging data, with associations smaller for WHR. Previous studies have suggested that visceral fat is more strongly related than subcutaneous fat to metabolic and hormonal dysfunction (e.g. insulin resistance, impaired glucose metabolism, low-grade inflammation) [43, 44] and hence might play a more important role in prostate cancer progression, although the roles of these factors in prostate cancer are not clear. To the best of our knowledge, only one small prospective study (n < 2000 men, 31 prostate cancer deaths) has examined the associations between different fat depots (visceral and subcutaneous fat, and thigh intermuscular and subcutaneous fat) estimated using computed tomography scans and risk of prostate cancer death, finding similar positive associations of these specific fat depots measured by CT, BMI and WC with aggressive and fatal prostate cancer [36]; due to the small sample size of this study and the lack of other prospective studies looking at different fat depots as exposures, more research looking at body fat distribution based on imaging data in relation to risk of prostate cancer mortality is needed before conclusions can be drawn.
Commonly used measures of adiposity also do not assess ectopic fat (fat stored in tissues other than adipose tissue, for example liver proton density fat fraction and muscle mass infiltration) [42]. Correspondingly, in UK Biobank, we found that while correlations of BMI and WC with visceral fat estimates from imaging data were large, the correlations of BMI and WC with liver proton density fat fraction and muscle mass infiltration were weaker. These weaker associations, and also the biological plausibility of associations of liver fat with prostate cancer risk or progression, suggest that there may be additional utility in assessing the associations with risk of prostate cancer mortality using these measures.
Obesity has been associated with a higher risk of being diagnosed with high grade prostate tumours [19], which have poorer prognosis, and several biological mechanisms have been proposed for the association between adiposity and prostate cancer development and progression [7]. However, it does not seem likely that any of the known biological risk factors for prostate cancer may mediate this association. Both IGF-I and free testosterone are positively associated with prostate cancer risk in observational and Mendelian randomisation studies [4, 5]; however, men with obesity have moderately lower concentrations of IGF-I and free testosterone than men with a healthy BMI [45, 46]. Higher BMI is associated with lower concentrations of IGFBP-1 and IGFBP-2 [45], which might lead to higher bioavailability of IGF-I, but evidence on the association of these binding proteins with prostate cancer mortality is very limited. Other biomarkers that are altered in men with obesity include pro-inflammatory cytokines [47], insulin [43], and oxidative stress biomarkers [48], which have been hypothesised to increase prostate cancer risk [49, 50]. Further, some evidence suggests that visceral, periprostatic and pelvic fat might promote proliferation and inhibit apoptosis of prostate cancer cells through paracrine mechanisms, such as the secretion of growth factors and pro-inflammatory cytokines [51–53]. However, although these mechanisms are possible, the current evidence is too limited to suggest that they mediate the positive association between adiposity and prostate cancer mortality and more research is needed. Emerging tools such as metabolomics, proteomics and epigenetics and the integration of this information with the gold standard measures of adiposity have the potential to reveal novel mechanisms through which adiposity may increase prostate cancer development and progression [54].
Although the association between adiposity and prostate cancer mortality may be mediated by metabolic changes, it is likely that differences in detection also play a role. Men with obesity may have a delayed diagnosis of prostate cancer tumours due to their lower prostate-specific antigen (PSA) concentrations (owing to increased blood volume with higher BMI) and to the greater difficulty of performing a thorough digital rectal examination and thus their lower likelihood of undergoing a biopsy [37, 55, 56]. For example, a previous meta-analysis showed that, compared to men with a normal weight, those with obesity have on average 12.9% lower PSA concentrations [55]. Furthermore, men with obesity may have enlarged prostates, which may make cancer detection by biopsy more difficult due to the large size, also resulting in a higher likelihood of the needles missing the cancer [37, 55, 56]. A later detection of a prostate tumour will lead to worse prognosis and a higher risk of dying from the disease. Therefore, further research about how obesity impacts the pathway to prostate cancer diagnosis is needed.
Strengths of our analyses in UK Biobank include its prospective design, detailed information on potential confounders and the large cohort size (though with only a moderate number of prostate cancer deaths). Analyses excluding the first 5 years of follow-up did not suggest that the observed associations were influenced by reverse causality, but substantially longer follow-up time is needed to be more confident about this. Adiposity measurements were assessed by trained research clinic staff instead of being self-reported, and we had high-quality body composition data (i.e. DXA- and MRI-derived adiposity measurements) in a subsample, which allowed us to assess the associations of commonly used adiposity measurements with “gold standard” measurements.
Our analyses also have some limitations. UK Biobank includes participants from multiple regions across the UK, including deprived areas; however, it may suffer from selection bias as it is not representative of the whole UK population [11, 57], although the directions of some major risk factor associations in the UK Biobank seem to be generalisable [58]. As in every observational study, residual confounding is possible in both our prospective analysis in UK Biobank and the meta-analysis. Moreover, there may be some misclassification of the underlying cause of death, which could be differential; obese men with prostate cancer are at increased risk of dying from several conditions, and some may die for example from cardiovascular disease but have their cause of death recorded as prostate cancer. Finally, due to the small number of prostate cancer deaths (probably due to the limited follow-up time, as 78% survive prostate cancer after ≥ 10 years [1]), we may have had limited power to find associations with overall adiposity (i.e. BMI and body fat percentage) in UK Biobank; we also had limited data in our meta-analysis for central adiposity and body fat percentage.
Conclusions
In summary, the totality of prospective evidence indicates that men with higher adiposity (both total and central adiposity) have a higher risk of dying from prostate cancer than men with a healthy weight. Prospective studies with more data on stage, grade and clinical information on disease progression, and with high quality measurements of adiposity distribution (e.g. MRI measurements which would allow the study of other characteristics such as ectopic fat), together with better understanding of the biological pathways, are needed to disentangle whether the association is biologically driven or due to differences in detection, but in either case, these findings provide further reason for men to maintain a healthy body weight.
Supplementary Information
Additional file 1: Supplementary Methods. Meta-analyses from prospective studies, literature search, study selection, data extraction, displaying of findings. Figure S1. Flow chart of the study participants in UK Biobank. Figure S2. Flow diagram of literature search and study selection for the meta-analysis. Table S1. Characteristics of prospective studies and previous individual participant data meta-analysis of body mass index and prostate cancer death. Table S2. Characteristics of prospective studies and previous individual participant data meta-analysis of body fat percentage, waist circumference and prostate cancer death. Table S3. Characteristics of prospective studies and previous individual participant data meta-analysis of waist to hip ratio and prostate cancer death. Table S4. Baseline characteristics of participants according to fourths of BMI at recruitment in men from UK Biobank. Table S5. Baseline characteristics of participants according to fourths of waist at recruitment in men from UK Biobank. Table S6. Mean and SD in men from UK Biobank with available imaging data (up to 4800 men). Table S7. Pearson correlation coefficients between main adiposity measurements at baseline in 218,237 men from UK Biobank. Table S8. Pearson correlation coefficients between adiposity measurements (imaging visit) with MRI adiposity measurements from the imagining in up to 11,501 men from UK Biobank. Table S9. Pearson correlation coefficients between adiposity measurements (imaging visit) with DXA adiposity measurements from the imagining in up to 18,827 men from UK Biobank. Table S10. Mean difference in MRI- and DXA-derived body fat compartments per 1 SD higher levels of BMI, body fat percentage, waist circumference, and waist to hip ratio in men from UK Biobank. Table S11. Geometric means of selected MRI measurements by tenths of anthropometric measurements at the imaging visit in up to 11,501 men from UK Biobank. Table S12. Geometric means of selected DXA measurements by tenths of anthropometric measurements at the imaging visit in up to 18,827 men from UK Biobank. Table S13. Minimally- and multivariable-adjusted hazard ratios (95% CI) for prostate cancer death in relation to adiposity measurements at baseline in men from UK Biobank. Table S14. Multivariable-adjusted hazard ratios (95 % CI) for prostate cancer in relation to BMI, waist circumference and WHR using the WHO cut-off points at recruitment in men from UK Biobank.
Acknowledgements
This research has been conducted using the UK Biobank Resource under application numbers 24494. We would like to thank Georgina K. Fensom for her contributions to the preparation and standardisation. We also thank all participants, researchers and support staff who made the study possible.
Abbreviations
- BMI
Body mass index
- CIs
Confidence intervals
- DXA
Dual-energy X-ray absorptiometry
- EPIC
European Prospective Investigation into Cancer and Nutrition
- IARC
International Agency for Research on Cancer
- ICD
International Statistical Classification of Diseases
- IGF-I
Insulin-like growth factor-I
- HRs
Hazard ratios
- MRI
Magnetic resonance imaging
- NHS
National Health Service
- PCa
Prostate cancer
- PSA
Prostate-specific antigen
- UK
United Kingdom
- WC
Waist circumference
- WCRF
World Cancer Research Fund
- WHR
Waist-to-hip ratio
Authors’ contributions
AP-C, TJK and RT conceived and designed the research question; AP-C managed the project, was responsible for acquiring the data, prepared the data for analysis, analysed the data, prepared the figures and tables and wrote the first draft of the manuscript; APC and YD independently screened papers for the meta-analysis. YD, EW, TJK and RT provided input on data analysis and interpretation of results. All authors revised the manuscript critically for important intellectual content and read and approved the final manuscript.
Funding
This research was funded by Cancer Research UK Population Research Fellowship number C60192/A28516 and Cancer Research UK programme grant number C8221/A29017. APC is also supported by the World Cancer Research Fund (WCRF UK), as part of the Word Cancer Research Fund International grant programme (2019/1953). ELW is supported by the NDPH Early Career Research Fellowship.
Availability of data and materials
The datasets generated/and or analysed in the current study will be made available for bona fide researchers who apply to use the UK Biobank data set by registering and applying at http://www.ukbiobank.ac.uk/register-apply.
Declarations
Ethics approval and consent to participate
The study was approved by The National Information Governance Board for Health and Social Care and the National Health Service North West Multicentre Research Ethics Committee (reference number 06/MRE08/65), and participants provided informed consent at baseline and to be followed up using data-linkage. The UK Biobank protocol is available online (http://www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aurora Perez-Cornago, Email: aurora.perez-cornago@ndph.ox.ac.uk.
Yashvee Dunneram, Email: yashvee.dunneram@ndph.ox.ac.uk.
Eleanor L. Watts, Email: elliewatts24@gmail.com
Timothy J. Key, Email: tim.key@ndph.ox.ac.uk
Ruth C. Travis, Email: ruth.travis@ndph.ox.ac.uk
References
- 1.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WCRF/AICR. World Cancer Research Fund International/American Institute for Cancer Research Continuous Update Project Report: Diet, Nutrition, Physical Activity, and Prostate Cancer. Available at: http://www.wcrf.org/sites/default/files/Prostate-Cancer-SLR-2014.pdf. 2014.
- 3.Cuzick J, Thorat MA, Andriole G, Brawley OW, Brown PH, Culig Z, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15(11):e484–e492. doi: 10.1016/S1470-2045(14)70211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travis RC, Appleby PN, Martin RM, Holly JM, Albanes D, Black A, et al. A meta-analysis of individual participant data reveals an association between circulating levels of IGF-I and prostate cancer risk. Cancer Res. 2016;76(8):2288–2300. doi: 10.1158/0008-5472.CAN-15-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watts EL, Fensom GK, Smith Byrne K, Perez-Cornago A, Allen NE, Knuppel A, et al. Circulating insulin-like growth factor-I, total and free testosterone concentrations and prostate cancer risk in 200 000 men in UK Biobank. Int J Cancer. 2021;148(9):2274–2288. doi: 10.1002/ijc.33416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Cornago A, Appleby PN, Pischon T, Tsilidis KK, Tjonneland A, Olsen A, et al. Tall height and obesity are associated with an increased risk of aggressive prostate cancer: results from the EPIC cohort study. BMC Med. 2017;15(1):115. doi: 10.1186/s12916-017-0876-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickerman B, Mucci L. Metabolic factors and prostate cancer risk. Clin Chem. 2019;65(1):42–44. doi: 10.1373/clinchem.2018.287243. [DOI] [PubMed] [Google Scholar]
- 8.West J, Dahlqvist Leinhard O, Romu T, Collins R, Garratt S, Bell JD, et al. Feasibility of MR-based body composition analysis in large scale population studies. PLoS One. 2016;11(9):e0163332. doi: 10.1371/journal.pone.0163332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35(1):83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 10.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UK Biobank . UK Biobank anthropometry. 2014. [Google Scholar]
- 13.Linge J, Borga M, West J, Tuthill T, Miller MR, Dumitriu A, et al. Body composition profiling in the UK Biobank imaging study. Obesity (Silver Spring) 2018;26(11):1785–1795. doi: 10.1002/oby.22210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Littlejohns TJ, Holliday J, Gibson LM, Garratt S, Oesingmann N, Alfaro-Almagro F, et al. The UK Biobank imaging enhancement of 100,000 participants: rationale, data collection, management and future directions. Nat Commun. 2020;11(1):2624. doi: 10.1038/s41467-020-15948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO . International statistical classification of diseases and related health problems. 10th revision. 2010. [Google Scholar]
- 16.WHO Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 17.WHO. World Health Organization. Waist circumference and waist-hip ratio: report of a WHO Expert Consultation. Geneva. 2008.
- 18.Perez-Cornago A, Key TJ, Allen NE, Fensom GK, Bradbury KE, Martin RM, et al. Prospective investigation of risk factors for prostate cancer in the UK Biobank cohort study. Br J Cancer. 2017;117(10):1562–1571. doi: 10.1038/bjc.2017.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genkinger JM, Wu K, Wang M, Albanes D, Black A, van den Brandt PA, et al. Measures of body fatness and height in early and mid-to-late adulthood and prostate cancer: risk and mortality in The Pooling Project of Prospective Studies of Diet and Cancer. Ann Oncol. 2020;31(1):103–114. doi: 10.1016/j.annonc.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gapstur SM, Gann PH, Colangelo LA, Barron-Simpson R, Kopp P, Dyer A, et al. Postload plasma glucose concentration and 27-year prostate cancer mortality (United States) Cancer Causes Control. 2001;12(8):763–772. doi: 10.1023/a:1011279907108. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001;10(4):345–353. [PubMed] [Google Scholar]
- 24.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 25.Eichholzer M, Bernasconi F, Jordan P, Stahelin HB. Body mass index and the risk of male cancer mortality of various sites: 17-year follow-up of the Basel cohort study. Swiss Med Wkly. 2005;135(1-2):27–33. doi: 10.4414/smw.2005.10415. [DOI] [PubMed] [Google Scholar]
- 26.Fujino Y. Japan Collaborative Cohort Study for Evaluation of C. Anthropometry, development history and mortality in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC) Asian Pac J Cancer Prev. 2007;8(Suppl):105–112. [PubMed] [Google Scholar]
- 27.Martin RM, Vatten L, Gunnell D, Romundstad P, Nilsen TI. Components of the metabolic syndrome and risk of prostate cancer: the HUNT 2 cohort, Norway. Cancer Causes Control. 2009;20(7):1181–1192. doi: 10.1007/s10552-009-9319-x. [DOI] [PubMed] [Google Scholar]
- 28.Burton A, Martin R, Galobardes B, Davey Smith G, Jeffreys M. Young adulthood body mass index and risk of cancer in later adulthood: historical cohort study. Cancer Causes Control. 2010;21(12):2069–2077. doi: 10.1007/s10552-010-9625-3. [DOI] [PubMed] [Google Scholar]
- 29.Batty GD, Kivimaki M, Clarke R, Davey Smith G, Shipley MJ. Modifiable risk factors for prostate cancer mortality in London: forty years of follow-up in the Whitehall study. Cancer Causes Control. 2011;22(2):311–318. doi: 10.1007/s10552-010-9691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dehal A, Garrett T, Tedders SH, Arroyo C, Afriyie-Gyawu E, Zhang J. Body mass index and death rate of colorectal cancer among a national cohort of U.S. adults. Nutr Cancer. 2011;63(8):1218–1225. doi: 10.1080/01635581.2011.607539. [DOI] [PubMed] [Google Scholar]
- 31.Discacciati A, Orsini N, Andersson SO, Andren O, Johansson JE, Wolk A. Body mass index in early and middle-late adulthood and risk of localised, advanced and fatal prostate cancer: a population-based prospective study. Br J Cancer. 2011;105(7):1061–1068. doi: 10.1038/bjc.2011.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray L, Lee IM, Sesso HD, Batty GD. Association of body mass index in early adulthood and middle age with future site-specific cancer mortality: the Harvard Alumni Health Study. Ann Oncol. 2012;23(3):754–759. doi: 10.1093/annonc/mdr270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haggstrom C, Stocks T, Ulmert D, Bjorge T, Ulmer H, Hallmans G, et al. Prospective study on metabolic factors and risk of prostate cancer. Cancer. 2012;118(24):6199–6206. doi: 10.1002/cncr.27677. [DOI] [PubMed] [Google Scholar]
- 34.Taghizadeh N, Boezen HM, Schouten JP, Schroder CP, Elisabeth de Vries EG, Vonk JM. BMI and lifetime changes in BMI and cancer mortality risk. PLoS One. 2015;10(4):e0125261. doi: 10.1371/journal.pone.0125261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong JS, Yi SW, Yi JJ, Hong S, Ohrr H. Body mass index and cancer mortality among korean older middle-aged men: a prospective cohort study. Medicine. 2016;95(21). 10.1097/MD.0000000000003684 PubMed PMID: WOS:000377777700021. [DOI] [PMC free article] [PubMed]
- 36.Dickerman BA, Torfadottir JE, Valdimarsdottir UA, Giovannucci E, Wilson KM, Aspelund T, et al. Body fat distribution on computed tomography imaging and prostate cancer risk and mortality in the AGES-Reykjavik study. Cancer. 2019;125(16):2877–2885. doi: 10.1002/cncr.32167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jochems SHJ, Stattin P, Haggstrom C, Jarvholm B, Orho-Melander M, Wood AM, et al. Height, body mass index and prostate cancer risk and mortality by way of detection and cancer risk category. Int J Cancer. 2020;147(12):3328–3338. doi: 10.1002/ijc.33150. [DOI] [PubMed] [Google Scholar]
- 38.Jochems SHJ, Wood AM, Haggstrom C, Orho-Melander M, Stattin P, Stocks T. Waist circumference and a body shape index and prostate cancer risk and mortality. Cancer Med. 2021;10(8):2885–2896. doi: 10.1002/cam4.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solans M, Chan DSM, Mitrou P, Norat T, Romaguera D. A systematic review and meta-analysis of the 2007 WCRF/AICR score in relation to cancer-related health outcomes. Ann Oncol. 2020;31(3):352–368. doi: 10.1016/j.annonc.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 40.NHS Digital. Health Survey for England, 2019: Data Tables. NHS Digital, Last Accessed Sept 2021 (https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2019/health-survey-for-england-2019-data-tables).
- 41.CRUK. Cancer Research UK. Last accessed: September 2021. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer.
- 42.Thomas EL, Parkinson JR, Frost GS, Goldstone AP, Dore CJ, McCarthy JP, et al. The missing risk: MRI and MRS phenotyping of abdominal adiposity and ectopic fat. Obesity (Silver Spring). 2012;20(1):76–87. doi: 10.1038/oby.2011.142. [DOI] [PubMed] [Google Scholar]
- 43.Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85(1009):1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang YC, Hayashi T, Fujimoto WY, Kahn SE, Leonetti DL, McNeely MJ, et al. Visceral abdominal fat accumulation predicts the conversion of metabolically healthy obese subjects to an unhealthy phenotype. Int J Obes (Lond). 2015;39(9):1365–1370. doi: 10.1038/ijo.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watts EL, Perez-Cornago A, Appleby PN, Albanes D, Ardanaz E, Black A, et al. The associations of anthropometric, behavioural and sociodemographic factors with circulating concentrations of IGF-I, IGF-II, IGFBP-1, IGFBP-2 and IGFBP-3 in a pooled analysis of 16,024 men from 22 studies. Int J Cancer. 2019;145(12):3244–3256. doi: 10.1002/ijc.32276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watts EL, Appleby PN, Albanese D, Black A, Chan JM, Chen C, et al. Circulating sex hormones in relation to anthropometric, sociodemographic and behavioural factors in an international dataset of 12,300 men. Plos One. 2017;12(12). 10.1371/journal.pone.0187741 PubMed PMID: WOS:000419006200002. [DOI] [PMC free article] [PubMed]
- 47.Johnson AR, Milner JJ, Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev. 2012;249(1):218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marseglia L, Manti S, D'Angelo G, Nicotera A, Parisi E, Di Rosa G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2015;16(1):378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paschos A, Pandya R, Duivenvoorden WC, Pinthus JH. Oxidative stress in prostate cancer: changing research concepts towards a novel paradigm for prevention and therapeutics. Prostate Cancer Prostatic Dis. 2013;16(3):217–225. doi: 10.1038/pcan.2013.13. [DOI] [PubMed] [Google Scholar]
- 50.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60(1):199–215. doi: 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dahran N, Szewczyk-Bieda M, Wei C, Vinnicombe S, Nabi G. Normalized periprostatic fat MRI measurements can predict prostate cancer aggressiveness in men undergoing radical prostatectomy for clinically localised disease. Sci Rep-Uk. 2017;7. 10.1038/s41598-017-04951-8 PubMed PMID: WOS:000404846000003. [DOI] [PMC free article] [PubMed]
- 52.Donohoe CL, Doyle SL, Reynolds JV. Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr. 2011;3:12. doi: 10.1186/1758-5996-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seidell JC, Bjorntorp P, Sjostrom L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39(9):897–901. doi: 10.1016/0026-0495(90)90297-P. [DOI] [PubMed] [Google Scholar]
- 54.Dickerman BA, Ebot EM, Healy BC, Wilson KM, Eliassen AH, Ascherio A, et al. A metabolomics analysis of adiposity and advanced prostate cancer risk in the health professionals follow-up study. Metabolites. 2020;10(3). 10.3390/metabo10030099 Epub 2020/03/14. PubMed PMID: 32164144; PubMed Central PMCID: PMCPMC7142752. [DOI] [PMC free article] [PubMed]
- 55.Harrison S, Tilling K, Turner EL, Martin RM, Lennon R, Lane JA, et al. Systematic review and meta-analysis of the associations between body mass index, prostate cancer, advanced prostate cancer, and prostate-specific antigen. Cancer Causes Control. 2020;31(5):431–449. doi: 10.1007/s10552-020-01291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freedland SJ, Platz EA, Presti JC, Jr, Aronson WJ, Amling CL, Kane CJ, et al. Obesity, serum prostate specific antigen and prostate size: implications for prostate cancer detection. J Urol. 2006;175(2):500–504. doi: 10.1016/S0022-5347(05)00162-X. [DOI] [PubMed] [Google Scholar]
- 57.Munafo MR, Tilling K, Taylor AE, Evans DM, Smith GD. Collider scope: when selection bias can substantially influence observed associations. International Journal of Epidemiology. 2018;47(1):226–235. doi: 10.1093/ije/dyx206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Batty GD, Gale CR, Kivimaki M, Deary IJ, Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ. 2020;368:m131. doi: 10.1136/bmj.m131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Methods. Meta-analyses from prospective studies, literature search, study selection, data extraction, displaying of findings. Figure S1. Flow chart of the study participants in UK Biobank. Figure S2. Flow diagram of literature search and study selection for the meta-analysis. Table S1. Characteristics of prospective studies and previous individual participant data meta-analysis of body mass index and prostate cancer death. Table S2. Characteristics of prospective studies and previous individual participant data meta-analysis of body fat percentage, waist circumference and prostate cancer death. Table S3. Characteristics of prospective studies and previous individual participant data meta-analysis of waist to hip ratio and prostate cancer death. Table S4. Baseline characteristics of participants according to fourths of BMI at recruitment in men from UK Biobank. Table S5. Baseline characteristics of participants according to fourths of waist at recruitment in men from UK Biobank. Table S6. Mean and SD in men from UK Biobank with available imaging data (up to 4800 men). Table S7. Pearson correlation coefficients between main adiposity measurements at baseline in 218,237 men from UK Biobank. Table S8. Pearson correlation coefficients between adiposity measurements (imaging visit) with MRI adiposity measurements from the imagining in up to 11,501 men from UK Biobank. Table S9. Pearson correlation coefficients between adiposity measurements (imaging visit) with DXA adiposity measurements from the imagining in up to 18,827 men from UK Biobank. Table S10. Mean difference in MRI- and DXA-derived body fat compartments per 1 SD higher levels of BMI, body fat percentage, waist circumference, and waist to hip ratio in men from UK Biobank. Table S11. Geometric means of selected MRI measurements by tenths of anthropometric measurements at the imaging visit in up to 11,501 men from UK Biobank. Table S12. Geometric means of selected DXA measurements by tenths of anthropometric measurements at the imaging visit in up to 18,827 men from UK Biobank. Table S13. Minimally- and multivariable-adjusted hazard ratios (95% CI) for prostate cancer death in relation to adiposity measurements at baseline in men from UK Biobank. Table S14. Multivariable-adjusted hazard ratios (95 % CI) for prostate cancer in relation to BMI, waist circumference and WHR using the WHO cut-off points at recruitment in men from UK Biobank.
Data Availability Statement
The datasets generated/and or analysed in the current study will be made available for bona fide researchers who apply to use the UK Biobank data set by registering and applying at http://www.ukbiobank.ac.uk/register-apply.