Abstract
Background
Trapa L. is a floating-leaved aquatic plant with important economic and ecological values. However, the species identification and phylogenetic relationship within Trapa are still controversial, which necessitates the need for plastid genome information of Trapa. In this study, complete chloroplast genomes of 13 Trapa species/taxa were sequenced and annotated. Combined with released sequences, comparative analyses of chloroplast genomes were performed on the 15 Trapa species/taxa for the first time.
Results
The Trapa chloroplast genomes exhibited typical quadripartite structures with lengths from 155,453 to 155,559 bp. The gene orders and contents within Trapa were conservative, but several changes were found in the microstructure. The intron loss of rpl2, also detected in Lythraceae, was found in all Trapa species/taxa, suggesting close genetic relationship between Lythraceae and Trapaceae. Notably, two small-seed species (T. incisa and T. maximowiczii) showed the smallest genome size with 155,453 and 155,477 bp, respectively. Each cp genome contained the same 130 genes consisting of 85 protein-coding genes, 37 tRNA genes and 8 rRNA genes. Trapa species/taxa showed 37 (T. incisa and T. maximowiczii) to 41 (T. sibirica) long repeats, including forward, palindromic, reversed and complementary repeats. There were 110 (T. quadrispinosa) to 123 (T. incisa and T. maximowiczii) SSR (simple sequence repeat) loci in Trapa chloroplast genomes. Comparative analyses revealed that two hotspot regions (atpA—atpF and rps2—rpoC2) in Trapa chloroplast genomes could be served as potential molecular markers. Three phylogenetic analyses (ML, MP and BI) consistently showed that there were two clusters within Trapa, including large- and small-seed species/taxa, respectively; for the large-seed Trapa, they clustered according to their geographical origin and tubercle morphology on the surface of seeds.
Conclusion
In summary, we have acquired the sequences of 13 Trapa chloroplast genomes, and performed the comparative analyses within Trapa for the first time. The results have helped us better identify the Trapa species/taxa and deepen the understanding of genetic basis and phylogenetic relationship of Trapa, which will facilitate the effective management and utilization of the important genetic resources in the future.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-022-03608-7.
Keywords: Trapa, Complete chloroplast genomes, Comparative analysis, Species identification, Phylogeography
Background
Trapaceae, containing the only genus Trapa, is an annual floating-leaved aquatic herb naturally distributed in tropical, subtropical and temperate regions of Eurasia and Africa, and invading North America and Australia [1]. APG II (The Angiosperm Phylogeny Group) [2] equated Trapaceae with Lythraceae. However, a handful of morphological differences exist between the two families. For example, flowers of Trapaceae are solitary, 4-merous and actinomorphic, with half-inferior and slightly perigynous ovaries; Lythraceae has racemes or cymes, and the flowers are usually 4-, 6- or 8-merous, regular or irregular, with obvious perigynous ovaries. Therefore, Trapaceae is still be used today by some researchers [1]. Trapa has important edible value because of high content of starch in seeds, and it has been widely cultivated as an important aquatic crop in China and India [3]. Trapa seed pericarps were traditional herb medicines in China, and recent studies found that the extract of seed pericarps had bioactive components to restrain cancer, atherosclerosis, inflammation and oxidation [4–8]. Additionally, Trapa plants can be used to purify water bodies due to their excellent performance in absorbing heavy metals and nutrients [9, 10]. Therefore, Trapa has important economic and ecological values. However, many Trapa species are becoming endangered or even locally extinct due to human interferences in Europe and China [11] (Chen et al., field observations). Conversely, Trapa plants were notorious intruders in Canada and the northeastern United States.
The knowledge of species identification and phylogenetic relationship is essential to effectively managing genetic resources [12, 13]. However, the genus Trapa possesses complicated morphological variations, but lacks effective diagnostic criteria. Therefore, researchers have held sharp different opinions on taxonomic classification of Trapa, with 1 or 2 polymorphic species or more than 20, 30 or 70 species within the genus [1, 14–19]. The phylogenetic relationships of Trapa species are still unresolved despite the efforts put in pollen morphology, cytology, quantitative classification and ontogenesis [20–24]. For example, Xiong et al. [25, 26] proposed that T. acornis Nakano and T. bispinosa Roxb were closely related based on the results of quantitative classification; contrastingly, Ding et al. [21] found that T. acornis was closely related to T. quadrispinosa Roxb based on their pollen morphology. Quantitative classification studies showed that the Trapa species/taxa with seeds of similar size evolved closely [24, 27], which was proved by the results of allozymes [28]. Additionally, molecular methods further illustrated that the Trapa species with small seeds, T. incisa and T. maximowiczii, were the primitive species of the genus [29, 30]. For the large-seed Trapa species, a close relationship was found between T. bispinosa and T. quadrispinosa based on RAPDs and AFLPs [29–31]. It is worth noting that there are two diversity centers in Trapa genus, one in the mid-lower reaches of Yangtze River, and the other in the Tumen River and Amur River basins [32, 33]. However, most previous studies involved fewer species/taxa, and the sampling areas were confined to the mid-lower reaches of Yangtze River. Additionally, their experimental methods mostly adopted molecular markers or nuclear genome sequencing [30, 31]. Chloroplast genome studies were rarely carried out [33, 34].
The chloroplast (cp) is a core organelle in plants for photosynthesis [35]. The cp genome of angiosperms is haploid and maternal-inherited. It is shaped into a DNA circle with conserved quadripartite structure, including large single copy (LSC), small single copy (SSC) and two inverted repeat regions (IRs). Additionally, the cp genome has slow evolutionary rate, high copy numbers per cell and compact size with 120-170 kb in length [36]. Those characteristics of the cp genome, combined with the development of high-throughput technology, make sequencing of the complete cp genome an ideal tool for species identification and plant phylogenic evaluation [37–39]. To date, whole sequences of six Trapa chloroplast genomes have been published, comprised of five wild species (T. natans NC_042895, T. quadrispinosa MT941481, T. kozhevnikovirum MW027640, T. incisa MW543307 and T. maximowiczii KY705084) and one cultivated species (T. bicornis MT374084). However, no research involving comparative analysis was conducted for the Trapa cp genomes. For Trapa, more efforts should be made to analyze the interspecific difference and assess the phylogenic relation based on the complete cp sequences.
In this study, we sequenced the whole cp genomes of 13 wild Trapa species/taxa. Among them, two were sequenced for the secondarily time and the rest (11 out of 13) were sequenced for the first time. Given the unresolved taxonomic status and variable morphological characteristics of Trapa, the comprehensive analysis of cp genomes was only be carried out on species with detailed descriptions of taxonomic criteria and seed characteristics. Here, comprehensive analysis was first carried out within Trapa genus based on the 15 cp genomes data, including the 13 generated in this study and two published (T. kozhevnikovirum and T. incisa) Trapa species/taxa. Our specific goals were as follows: (1) to compare the chloroplast structures within Trapa; (2) to detect the highly variable regions as potential DNA barcoding markers for Trapa species identification; (3) to infer the phylogenetic relationships among Trapa species. This study will provide the baseline information for phylogeography within Trapa and facilitate the management and utilization of genetic resources of Trapa.
Results
Basic structure of chloroplast genome
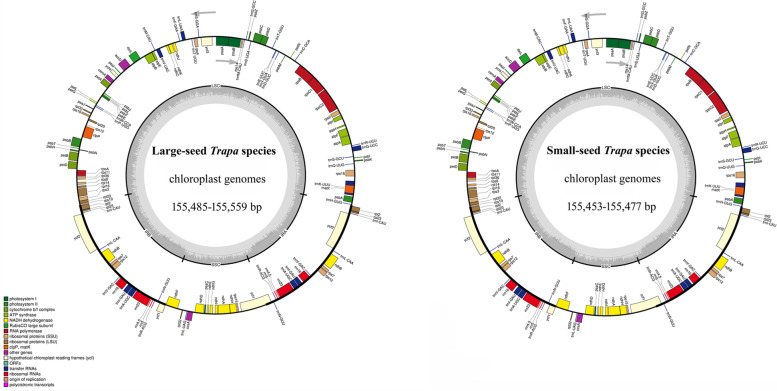
The lengths of the 15 Trapa cp genomes varied from 155,453 to 155,559 bp. The two small-seed species showed the smallest cp genome size with 155,453 for T. incisa and 155,477 for T. maximowiczii. For the 13 Trapa species/taxa with large seeds, the cp genomes of the three species/taxa (T. bispinosa, T. quadrispinosa and T. macropoda var. bispinosa) showed smaller size with 155,485–155,495 bp, and the size of the others varied from 155,535 (T. litwinowii) to 155,559 bp (T. mammillifera and T. baidangensis) (Table 1). There were 130 genes annotated in the cp genomes of the 15 Trapa species/taxa, containing 85 protein-coding genes (PCGs), 37 tRNA genes and 8 rRNA genes. For the 15 Trapa species/taxa, among the unique genes, 44 genes were related to photosynthesis and 59 genes were associated with self-replication (Table 2); same with Lythraceae, all of the 15 cp genomes of Trapa species/taxa consistently showed that the gene rpl2 lost an intron (Fig. 1). Typical quadripartite structure was also found in Trapa, which consisted of a pair of IRs (26,380 – 26,388 bp) separated by the SSC (18,265–18,279 bp) and LSC regions (88,398–88,512 bp) (Table 1).
Table 1.
Summary of complete chloroplast genomes for 15 Trapa species
| Taxon | chLJ | xlSJ | chQJ | chSL | bdZE | hkDB | tyE | nqG | fGJ | xkGL | qqDB | jxKF | wyXBLY | SJKY | XGY |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total length(bp) | 155,489 | 155,485 | 155,555 | 155,559 | 155,559 | 155,495 | 155,555 | 155,535 | 155,555 | 155,556 | 155,547 | 155,545 | 155,548 | 155,453 | 155,477 |
| GC(%) | 36.41 | 36.41 | 36.40 | 36.40 | 36.40 | 36.41 | 36.40 | 36.40 | 36.40 | 36.40 | 36.41 | 36.41 | 36.40 | 36.41 | 36.41 |
| Length of LSC(bp) | 88,444 | 88,440 | 88,504 | 88,508 | 88,508 | 88,450 | 88,504 | 88,485 | 88,504 | 88,505 | 88,512 | 88,494 | 88,498 | 88,398 | 88,428 |
| GC of LSC(%) | 34.18 | 34.18 | 34.18 | 34.18 | 34.18 | 34.18 | 34.17 | 34.18 | 34.18 | 34.18 | 34.18 | 34.18 | 34.17 | 34.20 | 34.19 |
| Length of SSC(bp) | 18,273 | 18,273 | 18,277 | 18,277 | 18,277 | 18,273 | 18,277 | 18,274 | 18,277 | 18,277 | 18,275 | 18,265 | 18,274 | 18,279 | 18,273 |
| GC of SSC(%) | 30.20 | 30.21 | 30.18 | 30.18 | 30.18 | 30.20 | 30.17 | 30.19 | 30.17 | 30.17 | 30.19 | 30.20 | 30.18 | 30.17 | 30.17 |
| Length of IR(bp) | 24,386 | 24,386 | 24,387 | 24,387 | 24,387 | 24,386 | 24,387 | 24,388 | 24,387 | 24,387 | 24,380 | 24,388 | 24,388 | 24,388 | 24,388 |
| GC of IR(%) | 42.77 | 42.77 | 42.77 | 42.77 | 42.77 | 42.77 | 42.77 | 42.77 | 42.77 | 42.77 | 42.77 | 42.77 | 42.77 | 42.77 | 42.77 |
| Total number of genes | 130 | 130 | 130 | 130 | 130 | 130 | 130 | 130 | 130 | 130 | 130 | 130 | 130 | 130 | 130 |
| Protein-coding gene | 85 | 85 | 85 | 85 | 85 | 85 | 85 | 85 | 85 | 85 | 85 | 85 | 85 | 85 | 85 |
| tRNA | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 | 37 |
| rRNA | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
Table 2.
Genes in the sequenced Trapa chloroplast genome
| Category of genes | Function of genes | Name of genes |
|---|---|---|
| Subunits of ATP synthase | Genes for photosynthesis | atp A, B, E, F, H, I |
| Other genes | accD, ccsA, cemA, clpP, matK | |
| Subunits of NADH dehydrogenase | Genes for photosynthesis | ndh A, B(2), C, D, E, F, G, H, I, K, J |
| Subunits of photosystem I | Psa A, B, C, I, J | |
| Subunits of photosystem II | Genes for photosynthesis | psb A, B, C, E, F, G, H, I, J, K, L, M, N, T, Z |
| Subunits of cytochrome | pet A, B, D, G, L, N | |
| Large subunit of Rubisco | Genes for photosynthesis | rbcL |
| Large subunit of ribosome | Self-replication | rps 2(2), 3, 4, 7(2), 8, 11, 12(2)A, 14, 15, 16, 18, 19 |
| DNA dependent RNA polymerase | Self-replication | rpoA, B, C1, C2 |
| Ribosomal RNA genes | Self-replication | rrn 5(2),4.5(2), 16(2), 23(2) |
| Small subunit of ribosome | Self-replication | rpl2(2), 14, 16, 20, 22, 23(2), 32, 33, 36 |
| Transfer RNA genes | Self-replication | trnA-UGC(2), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnfM-CAU, trnG-GCC, trnG-UCC, trnH-GUG, trnI-CAU(2), trnI-GAU(2), trnK-UUU, trnL-CAA(2), trnL-UAA, trnL-UAG, trnM-CAU, trnN-GUU(2), trnP-UGG, trnQ-UUG, trnR-ACG(2), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC(2), trnV-UAC, trnW-CCA, trnY-GUA |
| Conserved open reading frames | Genes of unknown function | ycf1(2), 2(2), 3, 4 |
(2) indicates the gene duplicated in all species
Fig. 1.
Structural map of the Trapa chloroplast genome. Genes drawn inside the circle are transcribed clockwise, and those outside are counterclockwise. Small single copy (SSC), large single copy (LSC), and inverted repeats (IRa, IRb) are indicated. Genes belonging to different functional groups are color-coded
The 15 Trapa species/taxa showed the identical level of GC content with the total content of 36.40–36.41%. The GC content of rRNA genes reached to 55.48%, which resulted in the highest GC content in the IR regions (42.77%) compared with that of the other two regions (LSC, 34.17–34.20%; SSC, 30.17–30.21%) (Fig. 1; Table 3).
Table 3.
Distribution of genes and intergenic regions for 15 Trapa species
| Species | Protein Coding Genes | rRNA | tRNA | Intergenic Regions | Intron | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Length(bp) | GC(%) | Length(bp) | GC(%) | Length(bp) | GC(%) | Length(bp) | GC(%) | Length(bp) | GC(%) | |
| chLJ | 78,672 | 37.28 | 9034 | 55.48 | 2812 | 53.31 | 46,713 | 30.31 | 18,838 | 36.14 |
| xlSJ | 78,672 | 37.28 | 9034 | 55.48 | 2812 | 53.31 | 46,713 | 30.31 | 18,834 | 36.15 |
| chQJ | 78,672 | 37.27 | 9034 | 55.48 | 2812 | 53.31 | 46,791 | 30.28 | 18,826 | 36.16 |
| chSL | 78,672 | 37.27 | 9034 | 55.48 | 2812 | 53.31 | 46,795 | 30.28 | 18,826 | 36.16 |
| bdZE | 78,672 | 37.27 | 9034 | 55.48 | 2812 | 53.31 | 46,795 | 30.29 | 18,826 | 36.16 |
| hkDB | 78,672 | 37.28 | 9034 | 55.48 | 2812 | 53.31 | 46,723 | 30.30 | 18,834 | 36.15 |
| tyE | 78,672 | 37.27 | 9034 | 55.48 | 2812 | 53.31 | 46,791 | 30.28 | 18,826 | 36.16 |
| nqG | 78,672 | 37.27 | 9034 | 55.48 | 2812 | 53.31 | 46,770 | 30.31 | 18,827 | 36.14 |
| fGJ | 78,672 | 37.27 | 9034 | 55.48 | 2812 | 53.31 | 46,791 | 30.28 | 18,826 | 36.16 |
| xkGL | 78,672 | 37.27 | 9034 | 55.48 | 2812 | 53.31 | 46,792 | 30.28 | 18,826 | 36.16 |
| qqDB | 78,672 | 37.27 | 9034 | 55.48 | 2812 | 53.31 | 46,774 | 30.30 | 18,835 | 36.14 |
| jxKF | 78,627 | 37.24 | 9030 | 55.48 | 2788 | 55.44 | 46,812 | 30.42 | 18,852 | 36.14 |
| wyXBLY | 78,498 | 37.27 | 9034 | 55.48 | 2812 | 53.31 | 46,770 | 30.29 | 18,840 | 36.14 |
| SJKY | 78,510 | 37.24 | 9030 | 55.48 | 2788 | 55.44 | 46,456 | 30.44 | 16,424 | 37.08 |
| XGY | 78,504 | 37.24 | 9030 | 55.48 | 2788 | 55.44 | 46,493 | 30.44 | 16,418 | 37.05 |
Boundaries of IR regions and codon usage
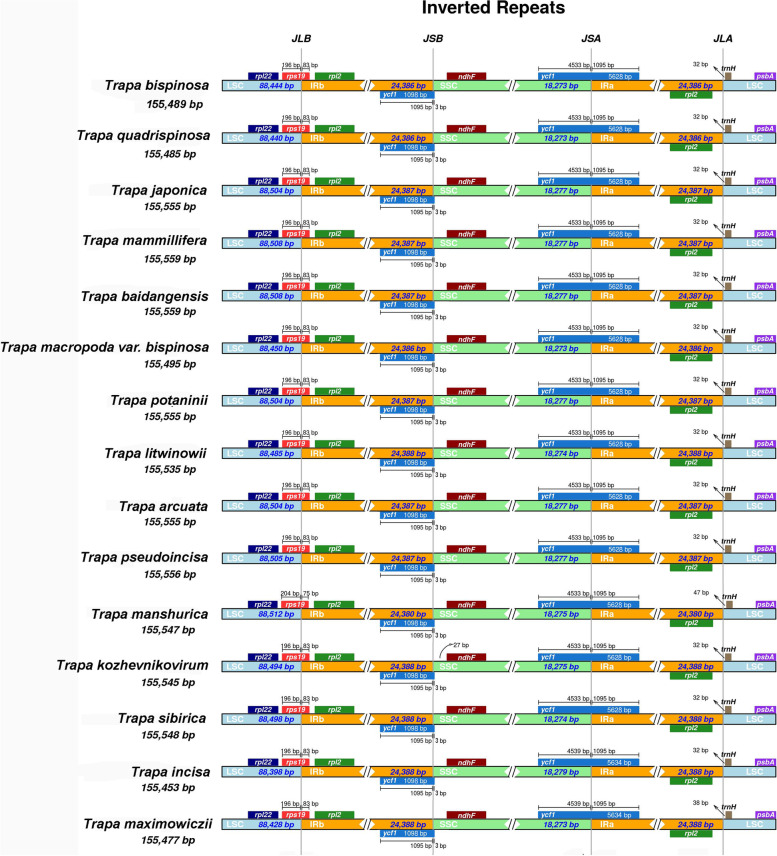
The cp genomes of the 15 Trapa plants showed several minor differences in the boundary regions although the number and order of genes were highly conserved (Fig. 2). The rps19 gene spanned the LSC/IRb border and extended into the IRb region with the length of 75–83 bp (except T. manshurica with 75 bp, the others were 83 bp). The ycf1 gene covered the junction of SSC/IRa showed variable sizes with 5628 or 5634 bp, which extended into IRa by 1095 bp and SSC region by 4533 (for the 13 large-seed Trapa taxa) or 4539 bp (for the two small-seed Trapa species). The gene ycf1 in the border of IRb/SSC showed stable length (1098 bp) extending identical distance into the IRb (1095 bp) and SSC (3 bp) for all the Trapa taxa. The gene trnH was distributed on the right side of the border of IRb/LSC, with an interval of 32–47 bp from the border to the gene.
Fig. 2.
Comparison of junctions between the LSC, SSC, and IR regions among 15 species. Distance in the figure is not to scale. LSC, Large single-copy; SSC, Small single-copy; IRa and IRb, inverted repeats. JLB, junction between LSC and IRb; JSB, junction between SSC and IRb; JSA, junction between SSC and IRa; JLA junction between LSC and IRa
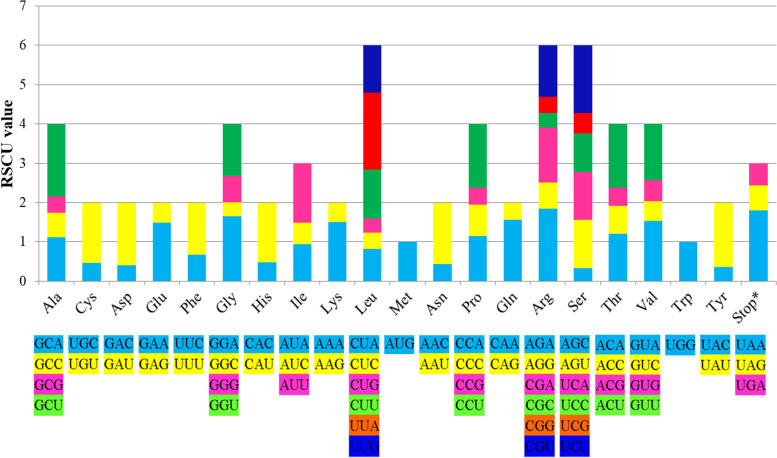
For all the Trapa species/taxa, a total of 64 types of codons encoding 20 amino acids were detected. In all, 85 PCGs within Trapa encoded 26,160 to 26,590 codons. The codons in the 15 cp genomes of Trapa manifested consistent utilization mode. For example, the four codons (GAA, UUU, AUU and AAA) showed a high number of occurrences (> 1000) for all the 15 Trapa species/taxa; additionally, the high-frequency amino acids for all Trapa species/taxa were leucine (2771–2826), isoleucine (2229–2298) and serine (1992–2067), and the low-frequency amino acids were cysteine (288–298) and tryptophan (459–468).
The highest and lowest relative synonymous codon usage (RSCU) values were exhibited in UUA encoding Leucine (1.96) and AGC encoding Serine (approximately 0.34), respectively (Fig. 3). The results also showed that 30 codons were used frequently with RSCU values > 1, and all of them ended with A/U.
Fig. 3.
Codon contents of 20 amino acids and stop codon of coding genes of Trapa chloroplast genome. Color of the histogram corresponds to the color of codons
Hypervariable regions and comparative genomic analysis
Multiple alignments of sequences revealed a high sequence similarity across the 15 Trapa cp genomes, indicating that the genome structure was quite conserved both in gene identity and order (Fig. S1). Some sequence divergences were observed in the LSC and SSC regions. Notably, most of these higher variable regions were observed in conserved non-coding sequences (CNS), and a few existed in PCGs, such as ycf1.
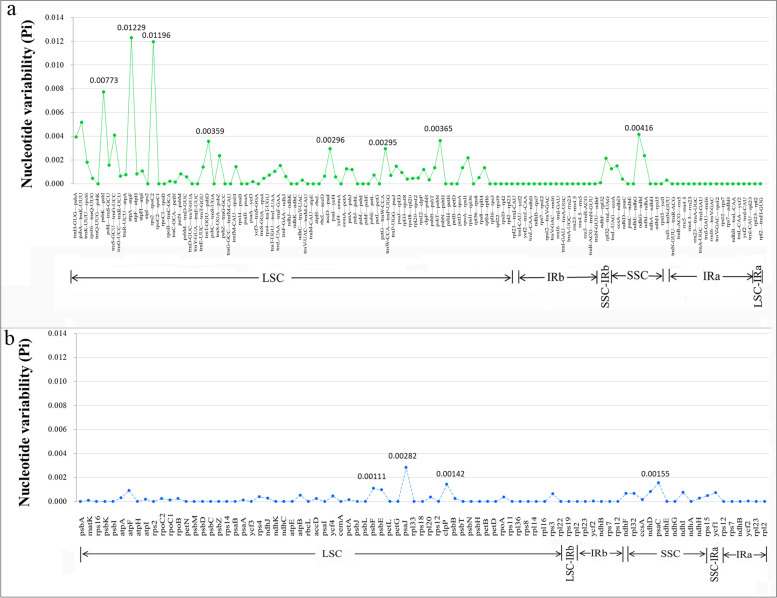
The intergenic regions (IGS) and introns among the 15 Trapa species/taxa ranged from 46,456 to 46,812 bp and from 16,418 to 18,852 bp, respectively (Table 3). The nucleotide diversity (Pi) values ranged from 0 to 0.0123 in the non-coding regions (average 0.000857) and from 0 to 0.00282 in the coding regions (average 0.000217) (Fig. 4), which indicated that the PCGs were more conserved than IGS. IGS regions of LSC possessed two divergence hotspots (atpA—atpF and rps2—rpoC2; Pi > 0.01) and two high variability (psbA—trnK-UUU and psbK—psbI; Pi > 0.005) [41] (Fig. 4).
Fig. 4.
The nucleotide variability (Pi) values in the 15 Trapa chloroplast genomes. a Intergenic regions. b Protein-coding genes. These regions are arranged according to their location in the chloroplast genome
The number of genes containing introns was 16 for all Trapa species. Such genes included 6 tRNA genes (trnK-UUU, trnA-UGC, trnI-GAU, trnG-UCC, trnV-UAC and trnL-UAA) and 8 PCGs (rps16, rpoC1, atpF, ndhB, ndhA, petD, petB and rpl16) with one intron, and two PCGs (ycf3 and clpP) with two introns. For all the Trapa taxa, the position and length of introns in the PCGs were similar (Table 4), suggesting the high conservation of cp genomes within Trapa.
Table 4.
Length of introns and complete gene of intron-contained protein-coding genes
| rps16 | rpoC1 | atpF | ndhB | ndhA | petD | petB | rpl16 | ycf3 | clpP |
|---|---|---|---|---|---|---|---|---|---|
| 866/1133 | 740/2780 | 766/1321 | 684/2217 | 1059/2151 | 742/1225 | 790/1438 | 1012/1420 | 756/770/2033 | 608/818/2017 |
| 866/1133 | 740/2780 | 766/1321 | 684/2217 | 1059/2151 | 742/1225 | 790/1438 | 1012/1420 | 756/766/2029 | 608/818/2017 |
| 864/1131 | 740/2780 | 767/1322 | 684/2217 | 1060/2152 | 742/1225 | 790/1438 | 1003/1411 | 756/766/2029 | 608/819/2018 |
| 864/1131 | 740/2780 | 767/1322 | 684/2217 | 1060/2152 | 742/1225 | 790/1438 | 1003/1411 | 756/766/2029 | 608/819/2018 |
| 864/1131 | 740/2780 | 767/1322 | 684/2217 | 1060/2152 | 742/1225 | 790/1438 | 1003/1411 | 756/766/2029 | 608/819/2018 |
| 866/1133 | 740/2780 | 766/1321 | 684/2217 | 1059/2151 | 742/1225 | 790/1438 | 1012/1420 | 756/766/2029 | 608/818/2017 |
| 864/1131 | 740/2780 | 767/1322 | 684/2217 | 1060/2152 | 742/1225 | 790/1438 | 1003/1411 | 756/766/2029 | 608/819/2018 |
| 865/1132 | 740/2780 | 758/1313 | 684/2217 | 1058/2150 | 742/1225 | 790/1438 | 1012/1420 | 757/766/2030 | 608/820/2019 |
| 864/1131 | 740/2780 | 767/1322 | 684/2217 | 1060/2152 | 742/1225 | 790/1438 | 1003/1411 | 756/766/2029 | 608/819/2018 |
| 864/1131 | 740/2780 | 767/1322 | 684/2217 | 1060/2152 | 742/1225 | 790/1438 | 1003/1411 | 756/766/2029 | 608/819/2018 |
| 865/1132 | 740/2780 | 766/1321 | 684/2217 | 1059/2151 | 742/1225 | 790/1438 | 1011/1419 | 757/766/2030 | 608/820/2019 |
| 869/1136 | 740/2801 | 767/1322 | 684/2163 | 1059/2151 | 742/1225 | 790/1438 | 1012/1420 | 757/766/2030 | 608/820/2019 |
| 865/1132 | 740/2780 | 768/1323 | 684/2217 | 1058/2150 | 742/1225 | 790/1438 | 1013/1421 | 757/768/2032 | 608/820/2019 |
| 865/1132 | 740/2780 | 768/1323 | 685/2218 | 1059/2151 | 751/1234 | 790/1438 | 1011/1419 | 761/766/2034 | 605/819/2015 |
| 865/1132 | 740/2780 | 768/1323 | 685/2218 | 1057/2149 | 751/1234 | 790/1438 | 1014/1422 | 758/766/2031 | 606/820/2017 |
Long repeat and simple sequence repeats (SSRs)
A total of 595 long repeats (30–65 bp) were identified from the 15 Trapa taxa, consisting of 324 forward, 230 palindromic, 25 reverse and 16 complementary repeats (Fig. S2). For the Trapa genus, the size of the top three most frequently shown long repeats was 30 bp, 31 bp and 65 bp occurring 227, 77 and 60 times, respectively. For each species/taxon, the number of long repeats varied from 37 (T. incisa and T. maximowiczii) to 41 (T. sibirica); and the number of forward, palindromic, reversed and complementary repeats were 19–24, 15–16, 0–3 and 1–2, respectively (Fig. S2). Most long repeats were distributed in intergenic areas, and a few existed in shared genes, such as ycf2.
For each species/taxon, the number of total SSRs was from 110 (T. quadrispinosa) to 123 (T. incisa and T. maximowiczii). Most cp SSRs, with the proportion from 83.48% (T. japonica, T. manshurica and T. litwinowii) to 86.18% (T. maximowiczii) out of the total number of SSRs, were distributed in the LSC regions. Among these SSRs, the mononucleotide A/T repeat units occupied the highest proportion with 78.18–80.49% out of the total number of SSRs, and the second and third highest proportions were dinucleotide repeats (AT/TA and CT/TG) and tetranucleotide repeats (AAAT/AACC/AATA/AGAA/ATAG/ATGT/GGTT/TAAG/TTAA/TTTC) accounting for 9.40–10.00% and 8.13–9.09% out of the total number of SSRs, respectively (Fig. S3). The observed high AT content in cp SSRs was found in the Trapa genus.
Phylogenetic analysis
Based on the whole cp genomes, the three phylogenetic trees, Maximum Likelihood (ML), Maximum Parsimony (MP) and Bayesian Inference (BI), showed similar topologies, and the species from the same family clustered together. The branch with the two Lagerstroemia species (L. calyculata and L. intermedia, Lythraceae) shown as the basal lineage. Sonneratia alba (Sonneratiaceae) showed to be a sister of Trapa species.
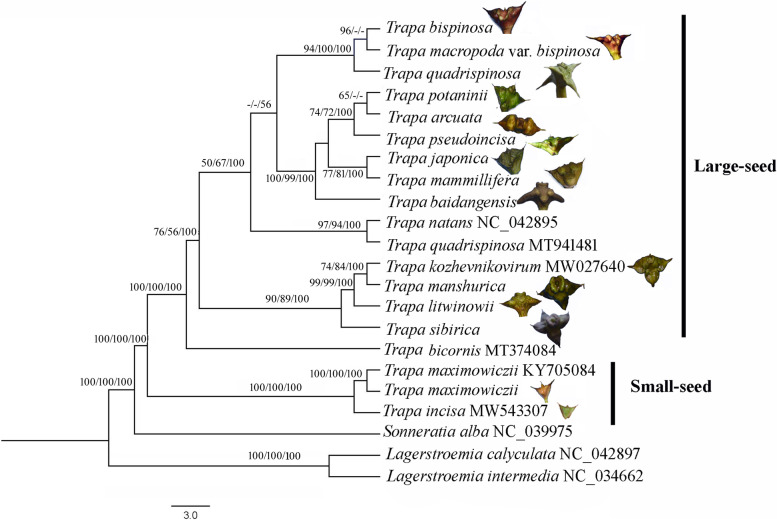
The Trapa species/taxa were divided into two clusters, including small- and large-seed species/taxa (Fig. 5). The two species (T. maximowiczii and T. incisa) with small seeds were separated from the other Trapa taxa with high supports: bootstrap values (BV) of ML and MP trees were 94% and 100%, and posterior probabilities (PP) of BI tree was 100%. Additionally, the two small-seed species showed a sister relationship. In the cluster with large seeds, the cultivated species T. bicornis was the earliest-diverging species. Given that there were no seed picture and identification criteria for further species confirmation of the published species T. quadrispinosa (MT941481) and T. natans (NC_042895), it is difficult to further clarify the phylogenetic relationships of them. Among the 16 large-seed Trapa samples, 13 samples involved in this study were divided into three sub-clusters corresponding to their morphology: (1) the first sub-cluster included three Trapa species (T. quadrispinosa, T. bispinosa and T. macropoda var. bispinosa) with high BV (94% and 100%) and PP (100%); (2) the second sub-cluster contained six species/taxa (T. japonica, T. mammillifera, T. potaninii, T. pseudoincisa, T. arcuata and T. baidangensis), which showed BV of 100%, 99% and PP of 100% supports. The six species/taxa were of the group containing obvious tubercles and thick husks; (3) the third sub-cluster containing four species (T. litwinowii, T. manshurica, T. kozhevnikovirum and T. sibirica) with BV of 90%, 89% and PP of 100%. All of them have smooth and tight seed coats and were collected from the Amur River (Fig. 5).
Fig. 5.
The phylogenetic tree is based on 22 complete chloroplast genome sequences using Maximum likelihood (ML), Maximum parsimony (MP) and Bayesian inference (BI) analyses. The number above the lines indicates bootstrap values (BV) for ML and MP, and posterior probabilities (PP) for BI of the phylogenetic analysis for each clade
Discussion
Structure and comparative analysis
The comparative analysis of cp genomes has been used extensively in many plant taxa, including plants of Lythraceae, a close relative of Trapaceae. However, it is the first time that this analysis is performed in Trapaceae. Previous studies showed that the length of land plant cp genomes ranged from 120-170 kb [36]. In the present study, the 15 Trapa species/taxa (155,453–155,559 bp) have an intermediate cp genome size compared to the plants from Lythraceae (152,049–160,769 bp) [42, 43]. The length of the LSC, SSC and IR regions for the 15 Trapa cp genomes were 88,398–88,512, 18,265–18,279 and 26,380–26,388 bp, respectively. This suggested that the difference in cp genome size of wild Trapa species mainly occurred in LSC region (Table 1). Most previous studies showed that the number of annotated cp genes was stable within the genus, except the two genera Camellia [44] and Aquilaria [45]. For Trapa, the number of genes was stable, with 130 genes including 85 PCGs, 8 rRNA genes, and 37 tRNA genes. An important evolutionary event for Lythraceae was the loss of rpl2 intron in cp genomes, which was presumed to occur after the divergence of Lythraceae from Onagraceae [42, 43, 46, 47]. Similarly, the 15 Trapa species/taxa exhibited the rpl2 intron loss, suggesting a close genetic relationship between Lythraceae and Trapaceae.
Genomes with high GC content are more stable and difficult to mutate [48]. The overall GC content in plastomes typically accounted for 30–40%, which varied greatly among different regions of cp genome and was usually higher in protein-coding regions [49]. In this study, the total GC content in Trapa (36.40- 36.41%) was comparable to that of Lythraceae (36.41–37.72%) [43, 47]. A high level of GC content was generally contained in rRNA genes of IR regions [43]. Similarly, for Trapa, due to the high GC content (55.48%) in rRNA genes, the highest GC content is found in the IR regions (42.77%) compared with that of LSC region (34.17–34.20%) and SSC region (30.17–30.21%).
The high stability of DNA sequences with high GC content resulted in a negative correlation between the GC content and the variability of cp genome sequences [43, 50]. In this study, the most variable regions for Trapa were located in IGS regions with the lowest GC content. Therefore, IGS can be used as DNA barcoding markers, which has been demonstrated in other species [51]. Additionally, the two divergence hotspots (atpA—atpF and rps2—rpoC2; Pi > 0.01) and the two high variability (psbA—trnK-UUU and psbK—psbI; Pi > 0.005), existed in IGS regions, could serve as potential molecular markers for further phylogenetic study (Fig. 4).
When a gene contains several internal stop codons, it tends to be a pseudogene [52, 53]. Alternatively, if the sequence is conserved over broad evolutionary distances and lacks internal stop codons, it tends to be a functional PCG [54]. A previous study about cp genomes for Cardiocrinum found a pseudogene ψycf1 located in the border of IRb/SSC [52]. On the contrary, the gene ycf1 in the border of IRb/SSC of 15 Trapa genomes contained a normal 5' initiation fragment (ATG) and just one stop codon at the border of the IRb/SSC. Thus, the ycf1 covered the junction of IRb/SSC is not pseudogene, and its counterpart ycf1 at the IRa/SSC border remains functional.
Abundant long repeats and cp SSRs
For cp genomes, long repeats play an important role in the sequence rearrangement and recombination [43, 55]. In this study, 37 (T. incisa and T. maximowiczii) to 41 (T. sibirica) repeats were found in each species/taxon (Fig. S2). Like those of previous studies [43, 56], most repeats for Trapa were distributed in intergenic areas.
Due to a high polymorphism rate at the intra- and inter-species level, cp genome SSRs have been viewed as excellent molecular markers in population genetics and phylogenetic research [33, 43]. In this study, we found 110 (T. quadrispinosa) to 123 (T. incisa and T. maximowiczii) SSR loci. More than 80% of SSR loci were distributed in LSC regions of Trapa (Fig. S3), which was higher than that of the genus Lagerstroemia (66.55%) [10] and Myrsinaceae (74.37%) [57]. The mononucleotide (A/T) was of the highest proportion with 78.18–80.49% in the cp genomes of Trapa, which was proved in other genera [41, 58, 59]. Additionally, cp SSRs for Trapa also have high AT content which was positively correlated with variability of cp genome sequences [38].
The highly conserved IR regions and codon usage
For angiosperms, the boundaries between IR regions and single-copy (SC) regions result in the difference of genome size by expansion or shrinkage [60, 63]. For Trapa, the IR regions had minor expansion or contraction (Fig. 2). The length of the rps19 gene in T. manshurica extending into the IRb was shorter by 8 bp than that of the other species/taxa, and the interval of gene trnH to IRb/LSC border in T. manshurica was longer by 9–15 bp than that of the other species. For the two small-seed species (T. incisa and T. maximowiczii), a 6 bp sequence was inserted into the ycf1 gene spaned SSC/IRa region (Fig. 2) compared with the gene in 13 large-seed species. Therefore, the gene ycf1 was one of the important drivers for the expansion or contraction of the IRs in the small-seed Trapa.
For all the Trapa species/taxa, the 85 PCGs encoded 26,160 to 26,590 codons. The results were comparable to that of the genus Lagerstroemia, with 79 genes encoding 25,068—27,111 codons [43]. The consistent utilization mode in the 15 species suggested the high conservation of the cp genomes in Trapa. Like Lagerstroemia [43], the RSCU value of a single amino acid showed a positive correlation with the number of codons encoding it. Additionally, 30 frequently used codons ended with A/U, which might be associated with the high proportion of A/T in cp genomes [40].
Phylogenetic analysis
Three phylogenetic trees (ML, MP and BI) consistently showed the Sonneratia was a sister genus of Trapa, which was also supported by recent studies [34, 62, 63].
The 15 Trapa species/taxa were divided into two clusters with high bootstrap values (BV of ML and MP trees: 100% and 100%) and posterior probabilities (PP of BI tree: 100%): the small-seed cluster and large-seed one (Fig. 5). The result indicated that the nut size of Trapa species was a diagnostic trait for the identification of genetic relationship within Trapa, which was also proved by the results from allozymic markers for Japanese Trapa [28]. Additionally, the results of two species with small seeds (T. incisa and T. maximowiczii) were the first split from the other Trapa taxa suggested that the two species were the earliest-diverging Trapa species, which was supported by the evidence from nuclear molecular markers [29, 30]. Although their seeds were similar in size and shape, it is the first time that the close genetic relationship between T. incisa and T. maximowiczii was shown by molecular methods. Within the large-seed group, the cultivated species T. bicornis diverged the earliest, which might suggest a complex origin of cultivated Trapa species. The remaining 13 large-seed Trapa taxa in this study were divided into three clusters based on their geographical origin and tubercles morphology on seeds: (1) The first cluster included T. quadrispinosa, T. bispinosa and T. macropoda var. bispinosa with high BV (94% and 100%) and PP (100%). All of them had the tight seed skin and were from the Yangtze River Basin. The intimate relationship between the former two has been proved by many studies [30, 31]. It was the first time to record the genetic data of T. macropoda var. bispinosa, and the only difference between this species and T. bispinosa is the larger seed bottom of this species. (2) The second cluster had six species/taxa (T. japonica, T. mammillifera, T. potaninii, T. pseudoincisa, T. arcuata and T. baidangensis) with BV of 100%, 99% and PP of 100% supports. These six Trapa species/taxa shared protruding tubercles on seed surfaces, and all of them were collected from the basins of the Yangtze River or Amur River. In contrast to this study, the AFLP study showed that T. japonica itself formed a single genetic cluster and didn’t show close relationship with other Trapa taxa [30]. This divergence might be attributed to different molecular markers or discordant patterns of nuclear and plastid DNA sequences. It is the first time for the four species/taxa (T. potaninii, T. pseudoincisa, T. arcuata and T. baidangensis) to be involved in a molecular study. The new species T. baidangensis was collected and described in this study for the first time. The seeds of the taxon have two horns and four tubercles similar to the T. mammillifera. The species was named T. baidangensis based on its collecting location. (3) The four species (T. litwinowii, T. manshurica, T. kozhevnikovirum and T. sibirica) clustered together at high supports with 90% and 89% BV for ML and MP analyses and 100% PP for BI analysis. All of them were from the Amur River, and had strong horns and tight and smooth coats on the seeds. Among them, only T. litwinowii has two horns on the seeds. Trapa litwinowii, T. manshurica and T. sibirica have large and outwardly curled seed crown, while T. kozhevnikovirum has a small seed crown and inconspicuous seed neck. However, the two published species, T. quadrispinosa (MT941481) and T. natans (NC_042895), clustered into a single branch. Given that most previously published Trapa species didn’t provide the identification criteria and seed pictures, and well-known taxonomic confusion in Trapa, we were not sure that the same naming was used in the previous studies.
Conclusions
In summary, the sequences of 13 Trapa chloroplast genomes were acquired. Including the newly released and two previously published genomes, the comparative analyses of complete chloroplast genomes of 15 Trapa species/taxa were the first of their kind to be carried out. The 15 cp genomes are of the similar quadripartite structure with a high degree of the synteny in gene order, suggesting high sequence conservation. Similar to the plants of Lythraceae, the rpl2 intron loss was also found in all Trapa species/taxa, suggesting a close genetic relationship between Lythraceae and Trapaceae. A total of 130 genes were annotated in the 15 Trapa species. Abundant long repeats and SSRs show promise as potential molecular markers for the Trapa population genetics and phylogenetics. Phylogenetic analysis showed that Trapa species separated into two major evolutionary branches: large- and small-seed branches. The small-seed branch, including T. incisa and T. maximowiczii, were shown as basal lineage in the Trapa genus. The 13 large-seed Trapa species involved in this study were divided into three sub-clusters based on their geographical origin and tubercle morphology on seeds. This study provides novel genomic resources that should be useful for species identification and phylogeographic analysis of Trapa, which ultimately will contribute to the effective management and sustainable utilization of the limited conservation funding.
Materials and methods
Plant materials and DNA extraction
In the autumns of 2018 and 2019, 13 Trapa species/taxa were collected from the Yangtze River Basin and Amur River Basin. For the 13 Trapa species/taxa, 10 were recorded in Chinese Flora Republicae Popularis Sinicae (T. bispinosa, T. quadrispinosa, T. japonica, T. mammillifera, T. macropoda var. bispinosa, T. litwinowii, T. arcuata, T. pseudoincisa, T. manshurica, and T. maximowiczii) [64]; two species (T. potaninii and T. sibirica) were first recorded in Floral of USSR [16]. A new Trapa species was collected from Baidang Lake, Anhui province, China. The seeds of the species have two horns with the height from 13.4 to 18.3 mm and the width from 23.4 to 34.8 mm. The horns of the new species were wide and drooping, shaped like a pig's ears. The taxon was named Trapa baidangensis. The formal identification of all Trapa species in this study was undertook by Yuanyuan Chen who learned the Trapa identification following Prof. Wan Wenhao, the writer of the Trapa Genus of the Flora Republicae Popularis Sinicae (Wan, 2000). Because the Trapa species we collected from field were not protected species, no permission was required during the sampling process. All voucher specimens were deposited in the herbarium of Wuhan Botanical Garden (HIB; Table 5).
Table 5.
The GenBank accession numbers of 15 species using in phylogenetic analysis
| No | Abbr | Species | Location | Voucher No | GenBank No |
|---|---|---|---|---|---|
| 1 | chLJ | Trapa bispinosa Roxb | Changhu Lake, Hubei | yychen20180060 | MW579848 |
| 2 | xlSJ | Trapa quadrispinosa Roxb | Xiliang Lake, Hubei | yychen20180055 | MW037838 |
| 3 | chQJ | Trapa japonica Flerow | Changhu Lake, Hubei | yychen20180061 | MW579849 |
| 4 | chSL | Trapa mammillifera Miki | Changhu Lake, Hubei | yychen20180059 | MW579850 |
| 5 | bdZE | Trapa baidangensis | Baidang Lake, Anhui | yychen20180097 | MW784170 |
| 6 | hkDB | Trapa macropoda Miki var. bispinosa W. H. Wan | Haikou Lake, Hubei | yychen20180075 | MW579851 |
| 7 | tyE | Trapa potaninii V. Vassil | Tangyuan, Heilongjiang | yychen20180019 | MW579852 |
| 8 | nqG | Trapa litwinowii V. Vassil | Nongqiao, Heilongjiang | yychen20180034 | MW579853 |
| 9 | fGJ | Trapa arcuata S. H. Li et Y. L. Chang | 856farm, Heilongjiang | yychen20180046 | MW579854 |
| 10 | xkGL | Trapa pseudoincisa Nakai | Xunke, Heilongjiang | yychen20180007 | MW579855 |
| 11 | qqDB | Trapa manshurica Fler | Qiqihaer, Heilongjiang | yychen20180001 | MW579857 |
| 12 | jxKF | Trapa kozhevnikovirum Pshennikova | Jixi, Heilongjiang | yychen20180042 | MW027640 |
| 13 | wyXBLY | Trapa sibirica Fler | Wuyun, Heilongjiang | yychen20180018 | MW579856 |
| 14 | SJKY | Trapa incisa Sieb. et Zucc | Wuhan Botanical Garden, Hubei | yychen20180066 | MW543307 |
| 15 | XGY | Trapa maximowiczii Korsch | Tangyuan, Heilongjiang | yychen20180023 | MW579858 |
The fresh leaves were sampled and dried in silica gel immediately. Genomic DNA was extracted from the dry leaves according to the CTAB protocol [65]. The DNA concentration and quality were quantified by the NanoDrop 2000 microspectrophotometer (Thermo Fisher Scientific).
Chloroplast genome sequencing and assembling
High quality DNA was used to build the genomic libraries. Sequencing was performed using paired end 150 bp (average short-insert about 350 bp) on Illumina NovaSeq 6000 at Beijing Novogene bio Mdt InfoTech Ltd (Beijing, China). To get the high quality clean data, Fastp [39] was run to cut and filter the raw reads with default settings. For the 13 Trapa species/taxa sequenced, 5.22 Gb (T. mammillifera) to 6.06 Gb (T. bispinosa) clean data were generated after removing adapters and low quality reads. De novo assembly was carried out using the assembler GetOrganelle v1.7 [66] with default settings. The software Geneious primer (Biomatters Ltd., Auckland, New Zealand) was employed to align the contigs and determine the order of the newly assembled plastomes, with T. quadrispinosa (MT941481) as reference. All the annotated cp sequences data reported here were deposited in GenBank with accession numbers shown in Table 5.
Annotation and codon usage
We used the genome annotator PGA [67] and GeSeq [68] to annotate PCGs, tRNAs and rRNAs, according to the references of T. quadrispinosa (MT941481). Manual correction was carried out to locate the start and stop codons and the boundaries between the exons and introns. Using tRNAscan-SE v1.21, BLASTN searches were further performed to confirm the tRNA and rRNA genes [69]. The physical maps of cp genomes were generated by OGDRAW [70].
The RSCU was the ratio of the frequency of a particular codon to the expected frequency of that codon, which was obtained by DAMBE v6.04 [37]. When the value of RSCU is larger than 1, the codon is used more often than expected. Otherwise, when the RSCU value < 1, the codon is less used than expected [71].
Comparative genomic analyses
Comparative genomic analyses were carried out among the 15 Trapa species/taxa, which included the 13 species/taxa newly sequenced, and two previously published ones (T. kozhevnikovirum and T. incisa) with the same research team[62, 63]. Notably, among the 15 Trapa species/taxa studied, T. incisa and T. maximowiczii have small size nuts (width, 9–14 mm; height, 9–12 mm), while the other 13 species/taxa are of large size nuts (width, 16–35 mm; height, 13–23 mm).The published cp genomes were downloaded from the National Center for Biotechnology Information (NCBI) organelle genome database (https://www.ncbi.nlm.nih.gov).
The mVISTA program in Shuffle-LAGAN mode was used to compare the 15 Trapa species/taxa complete cp genomes, with the annotation of T. quadrispinosa as a reference (MT941481). After manual multiple alignments using the program MUSCLE [72] in the software MEGA X [73], all regions, including coding and non-coding regions, were extracted to detect the hyper-variable sites. The nucleotide variability (Pi) was computed using DnaSP 5.10 [74].
Analysis of repeat sequences and SSRs
Repeat sequences, including forward, palindromic, reverse and complement repeats, were detected by REPuter [75]. The parameters were set with repeat size of ≥ 30 bp and 90% or greater sequence identity (hamming distance of 3).
Simple sequence repeats (SSRs) were identified using MISA perl script [76], with the threshold number of repeats set as 10, 5, 4, 3, 3 and 3 for mono-, di-, tri-, tetra-, penta- and hexa-nucleotide SSRs, respectively.
Phylogenetic analyses
Phylogenetic analyses were carried out based on 22 complete chloroplast genomes, including 19 Trapa cp genomes and three cp genomes of outgroups (Sonneratia alba and two Lagerstroemia species). Because of the close relationship between Trapaceae and Sonneratiaceae/Lythraceae [34], Sonneratia alba (Sonneratiaceae) and two Lagerstroemia species (L. calyculata and L. intermedia, Lythraceae) were used as outgroups. Except for the 13 Trapa cp genomes which were generated in this study, the other six published Trapa cp genomes and the three outgroup cp genomes were downloaded from Genbank.
The sequences were aligned using program Mafft 7.0 [77] with default parameters. The phylogenetic trees were constructed using three methods: (1) A Maximum Likelihood (ML) tree was performed using PhyML v.3.0 [78] with 5000 bootstrap replicates. The best-fit model of nucleotide substitution JC + I + G was obtained from software Jmodeltest 2 [79]. Previous molecular studies showed close genetic relationships between Trapa and Sonneratia/Lagerstroemia [33, 34]. Thus, Sonneratia alba (Sonneratiaceae) and two Lagerstroemia species (L. calyculata and L. intermedia, Lythraceae) were used as outgroups. The branch leading to two Lagerstroemia species was set as the root of the tree. The result was visible by the software Figtree v1.4 (https://github.com/rambaut/figtree/releases); (2) The Maximum Parsimony (MP) tree was obtained using the Subtree-Pruning-Regrafting (SPR) algorithm in the Mega X [73] with 5000 bootstrap values; (3) Bayesian Inference (BI) tree was built by the MrBayes v. 3.2.6 [80] with 2,000,000 generations and sampling every 5000 generations. The first 25% of all trees were regarded as “burn-in” and discarded, and the Bayesian posterior probabilities (PP) were calculated from the remaining trees.
Supplementary Information
Additional file 1: Figure S1. Sequence alignment of whole chloroplastgenomes using the Shuffle LAGAN alignment algorithm in mVISTA. Trapa quadrispinosa waschosen to be the reference genome. The vertical scale indicates the percentidentity, ranging from 50 to 100%. Figure S2. Number of long repetitive repeats onthe complete chloroplast genome sequence of 15 Trapa species. (a) frequency of the repeats more than 30 bp, (b) frequencyof repeat types. chLJ, Trapa bispinosa;xlSJ, Trapa quadrispinosa; chQJ, Trapa japonica; chSL, Trapa mammillifera; bdZE, Trapa natans var. baidangensis; hkDB, Trapamacropoda var. bispinosa; tyE, Trapa potaninii; nqG, Trapa litwinowii; fGJ, Trapa arcuata; xkGL, Trapa pseudoincisa; qqDB, Trapa manshurica; jxKF, Trapa kozhevnikovirum; wyXBLY, Trapa sibirica; SJKY, Trapa incisa; XGY, Trapa maximowiczii. Figure S3. The comparison of simple sequencerepeats (SSRs) distribution in 15 chloroplast genomes. (a) frequency of commonmotifs; (b) number of different SSR types. chLJ, Trapa bispinosa; xlSJ, Trapaquadrispinosa; chQJ, Trapa japonica;chSL, Trapa mammillifera; bdZE, Trapa natans var. baidangensis; hkDB, Trapamacropoda var. bispinosa; tyE, Trapa potaninii; nqG, Trapa litwinowii; fGJ, Trapa arcuata; xkGL, Trapa pseudoincisa; qqDB, Trapa manshurica; jxKF, Trapa kozhevnikovirum; wyXBLY, Trapa sibirica; SJKY, Trapa incisa; XGY, Trapa maximowiczii.
Acknowledgements
Not applicable.
Abbreviations
- APG
Angiosperm Phylogeny Group
- cp
Chloroplast
- PCGs
Protein-coding genes
- IR(s)
Inverted repeat (regions)
- IRa, IRb
Two IR regions that are identical but in opposite orientations
- LSC
Large single copy; SSC: Small single copy
- SSRs
Simple sequence repeats
- RSCU
Relative synonymous codon usage
- CNS
Conserved non-coding sequences
- IGS
Intergenic regions
- Pi
Nucleotide diversity
- ML
Maximum-likelihood
- MP
Maximum Parsimony
- BI
Bayesian Inference
- USSR
Union of Soviet Socialist Republics
- NCBI
National Center for Biotechnology Information
Authors’ contributions
X.F. collected and identified the species of sample, designed the experiments, analyzed the data and wrote the paper. W.W. performed the experiments, contributed reagents/materials/analysis tools, prepared Figures and/or tables and wrote the paper. Y.C. conceived and designed the experiments, reviewed drafts of the paper. G.K.W. contributed reagents/materials/analysis tools. W.L. and X.L. help conceived and designed the experiments and reviewed the paper. All authors have read and approved the final version of the manuscript.
Funding
This work was supported by the National Scientific Foundation of China (31100247), the Talent Program of Wuhan Botanical Garden of the Chinese Academy of Sciences (Y855291) and the High-level Talent Training Program of Tibet University (2018-GSP-018). The funders were not involved in the design, sample collection, analysis and interpretation of data and in writing the manuscript.
Availability of data and materials
All the annotated cp sequences data reported here were deposited in GenBank (https://www.ncbi.nlm.nih.gov/) with accession numbers shown in Table 5. All voucher specimens were deposited in the herbarium of Wuhan Botanical Garden.
Declarations
Ethics approval and consent to participate
The collecting of all samples in this study followed the Regulations on the Protection of Wild Plants of the People's Republic of China, the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiuling Li, Email: lixiuling@lyu.edu.cn.
Yuanyuan Chen, Email: yychen@wbgcas.cn.
References
- 1.Chen JR, Ding BY, Funston M. Trapaceae. In Flora of China. Beijing & St. Louis: Science Press & Missouri Botanical Garden Press; 2007. p. 290–1 13.
- 2.Group AP An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot J Linnean Soc. 2003;141(4):399–436. doi: 10.1046/j.1095-8339.2003.t01-1-00158.x. [DOI] [Google Scholar]
- 3.Hummel M, Kiviat E. Review of World literature on Water Chestnut with implications for management in North America. J Aquat Plant Manage. 2004;42:17–27. [Google Scholar]
- 4.Akao S, Maeda K, Hosoi Y, Nagare H, Maeda M, Fujiwara T. Cascade utilization of water chestnut: recovery of phenolics, phosphorus, and sugars. Environ Sci Pollut Res. 2013;20:5373–5378. doi: 10.1007/s11356-013-1547-7. [DOI] [PubMed] [Google Scholar]
- 5.Ciou JY, Wang CCR, Chen J, Chiang PY. Total phenolics content and antioxidant activity of extracts from dried water caltrop (Trapa taiwanensis Nakai) hulls. J Food Drug Analysis. 2008;16:41e47. [Google Scholar]
- 6.Yu H, Shen S. Phenolic composition, antioxidant, antimicrobial and antiproliferative activities of water caltrop pericarps extract. LWT-Food Sci Technol. 2015;61:238–243. doi: 10.1016/j.lwt.2014.11.003. [DOI] [Google Scholar]
- 7.Li F, Mao YD, Wang YF, Raza A, Qiu LP, Xu XQ. Optimization of Ultrasonic-Assisted enzymatic extraction conditions for improving total phenolic content, antioxidant and antitumor activities in vitro from Trapa quadrispinosa Roxb. Residues. Molecules. 2017;22(3):396. doi: 10.3390/molecules22030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kauser A, Muhammad S, Shah A, Iqbal N, Riaz M. In vitro antioxidant and cytotoxic potential of methanolic extracts of selected indigenous medicinal plants. Progr Nutr. 2018;20(4):706–712. [Google Scholar]
- 9.Sweta, Bauddh K, Singh R, Singh RP. The suitability of Trapa natans for phytoremediation of inorganic contaminants from the aquatic ecosystems. Ecol Eng. 2015;83:39–42. doi: 10.1016/j.ecoleng.2015.06.003. [DOI] [Google Scholar]
- 10.Xu L, Cheng S, Zhuang P, Xie D, Li S, Liu D, Li Z, Wang F, Xing F. Assessment of the nutrient removal potential of floating native and exotic aquatic macrophytes cultured in Swine Manure Wastewater. Int J Environ Res Public Health. 2020;17:1103. doi: 10.3390/ijerph17031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta AK, Beentje HJ. Trapa natans, The IUCN Red List of Threatened Species. 2017. [Google Scholar]
- 12.Campbell BT, Williams VE, Park W. Using molecular markers and field performance data to characterize the Pee Dee cotton germplasm resources. Euphytica. 2009;169(3):285–301. doi: 10.1007/s10681-009-9917-4. [DOI] [Google Scholar]
- 13.Chorak GM, Dodd LL, Rybicki N, Ingram K, Buyukyoruk M, Kadono Y, Chen YY, Thum RA. Cryptic introduction of water chestnut (Trapa) in the northeastern United States. Aquat Bot. 2019;155:32–37. doi: 10.1016/j.aquabot.2019.02.006. [DOI] [Google Scholar]
- 14.Cook CDK. Aquatic plant book. Amsterdam/New York: SPB Academic Pub; 1996. [Google Scholar]
- 15.Vassiljev V. Species novae Africanicae generis Trapa L. Nov Sist Vyss Rast. 1965;32:175–194. [Google Scholar]
- 16.Vassiljev VN. Water Caltrops-Hydrocaryaceae Raimann. Flora of the USSR. Moscow: Publishing House of As of USSR; 1949. pp. 637–662. [Google Scholar]
- 17.Tutin TG. Flora Europaea. Cambridage: Cambridage University Press; 1968. pp. 303–452. [Google Scholar]
- 18.Yan SZ. Aquatic macrophytes of China. Beijing, China: Science Press; 1983. [Google Scholar]
- 19.Kak AM. Aquatic and wetland vegetation of western Himalayas. J Econ Taxon Bot. 1988;12:447–451. [Google Scholar]
- 20.Diao ZS. The morphogenesis in the ontogeny of the family Trapaceae. J Yuzhou Univ. 1990;3:1–11. [Google Scholar]
- 21.Ding BY, Fang YY. Study on the pollen morphology of Trapa from Zhejiang. Acta phytotaxonomica sinica. 1991;29(3):172–177. [Google Scholar]
- 22.Huang T, Ding BY, Hu RY, et al. Cytotaxonomic studies on the genus Trapa in China. In Research and Application of Life Sciences. Hangzhou: Zhejiang University Press; 1996. pp. 235–239. [Google Scholar]
- 23.Wang LJ, Ding BY. Study on the chromosomes of three Chinese Trapa species. Ningbo Agr Sci Technol. 1997;1:7–9. [Google Scholar]
- 24.Fan XR, Li Z, Chu HJ, Li W, Liu YL, Chen YY. Analysis of morphological plasticity of Trapa L. from China and their taxonomic significance. Plant Sci J. 2016;34(3):340–351. [Google Scholar]
- 25.Xiong Z, Sun X. Numerical taxonomic studies in Trapaceae in Hubei I. Plant Sci J. 1985;3:45–53. [Google Scholar]
- 26.Xiong Z. Numerical taxonomic studies on Trapaceae from Hubei II. Plant Sci J. 1985;3:157–164. [Google Scholar]
- 27.Xiong Z, Huang D, Wang H, Sun X. Numrical taxonomic studies in Trapa in Hubei III. Numerical evaluations of taxonomic characters. Plant Sci J. 1990;8(1):47–52. [Google Scholar]
- 28.Takano A, Kadono Y. Allozyme variations and classification of Trapa (Trapaceae) in Japan. Aquat Bot. 2005;83(2):108–118. doi: 10.1016/j.aquabot.2005.05.008. [DOI] [Google Scholar]
- 29.Jiang WM, Ding BY. Genetic relationship among Trapa species assessed by RAPD markers. J Zhejiang Univ Agr Life Sci. 2004;30:191–196. [Google Scholar]
- 30.Fan XR, Wang WC, Chen L, Li W, Chen YY. Genetic relationship among 12 Trapa species/varietas from Yangtze River Basin revealed by AFLP markers. Aquat Bot. 2021;168:103320. doi: 10.1016/j.aquabot.2020.103320. [DOI] [Google Scholar]
- 31.Li XL, Fan XR, Chu HJ, Li W, Chen YY. Genetic delimitation and population structure of three Trapa taxa from the Yangtze River. China Aquat Bot. 2017;136:61–70. doi: 10.1016/j.aquabot.2016.09.009. [DOI] [Google Scholar]
- 32.Xue JH, Xue ZQ, Wang RX, Rubtsova TA, Pshennikova LM, Guo Y. Didtribution pattren and morphological diversity of Trapa L. in the Heilong and Tumen River Basin. J Plant Sci. 2016;34:506–20.
- 33.Xue ZQ, Xue JH, Victorovna KM, Ma KP. The complete chloroplast DNA sequence of Trapa maximowiczii Korsh. (Trapaceae), and comparative analysis with other Myrtales species. Aquat Bot. 2017;143:54–62. doi: 10.1016/j.aquabot.2017.09.003. [DOI] [Google Scholar]
- 34.Sun F, Yin Y, Xue B, Zhou R, Xu J. The complete chloroplast genome sequence of Trapa bicornis Osbeck (Lythraceae) Mitochondrial DNA Part B. 2020;5(3):2746–2747. doi: 10.1080/23802359.2020.1788432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howe CJ, Barbrook AC, Koumandou VL, Nisbet R, Symington HA, Wightman TF. Evolution of the chloroplast genome. Philos Trans R Soc Lond B Biol. 2003;358(1429):99–107. doi: 10.1098/rstb.2002.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruhlman TA, Jansen RK. The plastid genomes of flowering plants. Methods Mol Biol. 2014;1132:3–38. doi: 10.1007/978-1-62703-995-6_1. [DOI] [PubMed] [Google Scholar]
- 37.Cauz-Santos LA, Munhoz CF, Rodde N, Cauet S, Santos AA, Penha HA, Dornelas MC, Varani AM, Oliveira GCX, Bergès H, et al. The chloroplast genome of Passiflora edulis (Passifloraceae) assembled from long sequence reads: structural organization and phylogenomic studies in malpighiales. Front Plant Sci. 2017;8:334. doi: 10.3389/fpls.2017.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong Z, Wu Z, Zhao K, Yang Z, Zhang N, Guo J, Tembrock LR, Xu D. Comparative analyses of five complete chloroplast genomes from the genus Pterocarpus (Fabacaeae) Int J Mol Sci. 2020;21(11):3758. doi: 10.3390/ijms21113758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics (Oxford, England) 2018;34(17):i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eguiluz M, Rodrigues NF, Guzman F, Yuyama P, Margis R. The chloroplast genome sequence from Eugenia uniflora, a Myrtaceae from Neotropics. Plant Syst Evol. 2017;303:1199–1212. doi: 10.1007/s00606-017-1431-x. [DOI] [Google Scholar]
- 41.Yang Z, Zhao T, Ma Q, Liang L, Wang G. Comparative genomics and phylogenetic analysis revealed the chloroplast genome variation and interspecific relationships of Corylus (Betulaceae) Species. Front Plant Sci. 2018;9:927. doi: 10.3389/fpls.2018.00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu C, Dong W, Li W, Lu Y, Xie X, Jin X, Shi J, He K, Suo Z. Comparative analysis of six Lagerstroemia complete chloroplast. Front Plant Sci. 2017;8:15. doi: 10.3389/fpls.2017.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng G, Wei L, Ma L, Wu Z, Chen K. Comparative analyses of chloroplast genomes from 13 Lagerstroemia (Lythraceae) species: identification of highly divergent regions and inference of phylogenetic relationships. Plant Mol Biol. 2020;102:659–676. doi: 10.1007/s11103-020-00972-6. [DOI] [PubMed] [Google Scholar]
- 44.Peng J, Zhao Y, Dong M, Liu S, Xu Z. Exploring the evolutionary characteristics between cultivated tea and its wild relatives using complete chloroplast genomes. BMC Ecol Evo. 2020;21:71. doi: 10.1186/s12862-021-01800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hishamuddin MS, Lee SY, Ng WL, Ramlee SI, Lamasudin DU, Mohamed R. Comparison of eight complete chloroplast genomes of the endangered Aquilaria tree species (Thymelaeaceae) and their phylogenetic relationships. Sci Rep. 2020;10(1):13034. doi: 10.1038/s41598-020-70030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu C, Tembrock LR, Johnson NG, Simmons MP, Wu Z. The complete plastid genome of lagerstroemia fauriei and loss of rpl2 intron from Lagerstroemia (Lythraceae) PLoS ONE. 2016;11(3):e0150752. doi: 10.1371/journal.pone.0150752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu C, Ma L, Wu Z, Chen K, Wang Y. Comparative analyses of chloroplast genomes from 22 Lythraceae species: inferences for phylogenetic relationships and genome evolution within Myrtales. BMC Plant Biol. 2019;19(1):281. doi: 10.1186/s12870-019-1870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terakami S, Matsumura Y, Kurita K, Kanamori H, Katayose Y, Yamamoto T, Katayama H. Complete sequence of the chloroplast genome from pear (Pyrus pyrifolia): genome structure and comparative analysis. Tree Genet Genomes. 2012;8(4):841–854. doi: 10.1007/s11295-012-0469-8. [DOI] [Google Scholar]
- 49.Cai Z, Penaflor C, Kuehl JV, Leebens-Mack J, Carlson JE, dePamphilis CW, Boore JL, Jansen RK. Complete plastid genome sequences of Drimys, Liriodendron, and Piper: implications for the phylogenetic relationships of magnoliids. BMC Evol Biol. 2006;6(1):77. doi: 10.1186/1471-2148-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang CY, Liu T, Mo XL, Huang HR, Yan HF. Comparative analyses of the chloroplast genomes of patchouli plants and their relatives in Pogostemon (Lamiaceae) Plants. 2020;9(11):1497. doi: 10.3390/plants9111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen P, Kim JS, Kim JH. The complete chloroplast genome of colchicine plants (Colchicum autumnale and Gloriosa superba ) and its application for identifying the genus. Planta. 2015;242(1):223–237. doi: 10.1007/s00425-015-2303-7. [DOI] [PubMed] [Google Scholar]
- 52.Lu RS, Li P, Qiu YX. The complete chloroplast genomes of three Cardiocrinum (Liliaceae) species: comparative genomic and phylogenetic analyses. Front Plant Sci. 2017;7:2054. doi: 10.3389/fpls.2016.02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sloan DB, Triant DA, Forrester NJ, Bergner LM, Wu M, Taylor DR. A recurring syndrome of accelerated plastid genome evolution in the angiosperm tribe Sileneae (Caryophyllaceae) Mol Phylogenet Evol. 2014;72:82–89. doi: 10.1016/j.ympev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Raubeson LA, Peery R, Chumley TW, Dziubek C, Fourcade HM, Boore JL, Jansen RK. Comparative chloroplast genomics: analyses including new sequences from the angiosperms Nuphar advena and Ranunculus macranthus. BMC Genomics. 2007;8(1):174. doi: 10.1186/1471-2164-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nazareno AG, Carlsen M, Lohmann LG. Complete chloroplast genome of Tanaecium tetragonolobum: the first Bignoniaceae plastome. PLoS ONE. 2015;10(6):e0129930. doi: 10.1371/journal.pone.0129930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang J, Lucía V, Chen X, Li H, Hao Z, Liu Z, Zhao G. Development of chloroplast and nuclear DNA markers for Chinese Oaks (Quercus subgenus Quercus) and assessment of their utility as DNA barcodes. Front Plant Sci. 2017;8:816. doi: 10.3389/fpls.2017.00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan X, Liu T, Yuan X, Xu Y, Yan H, Hao G. Chloroplast genomes and comparative analyses among thirteen taxa within Myrsinaceae s.str. clade (Myrsinoideae, Primulaceae) Int J Mol Sci. 2019;20(18):4534. doi: 10.3390/ijms20184534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asaf S, Khan AL, Khan AR, Waqas M, Kang SM, Khan MA, Lee SM, Lee IJ. Complete chloroplast genome of Nicotiana otophora and its comparison with related species. Front Plant Sci. 2016;7:843. doi: 10.3389/fpls.2016.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuang DY, Wu H, Wang YL, Gao LM, Lu L. Complete chloroplast genome sequence of Magnolia kwangsiensis (Magnoliaceae): implication for DNA barcoding and population genetics. Genome. 2011;54(8):663–673. doi: 10.1139/g11-026. [DOI] [PubMed] [Google Scholar]
- 60.Kim KJ, Lee HL. Complete chloroplast genome sequences from Korean ginseng (Panax schinseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. DNA res. 2004;11(4):247–261. doi: 10.1093/dnares/11.4.247. [DOI] [PubMed] [Google Scholar]
- 61.Lin CP, Wu CS, Huang YY, Chaw SM. The complete chloroplast genome of Ginkgo biloba reveals the mechanism of inverted repeat contraction. Genome Biol Evol. 2012;4(3):374–381. doi: 10.1093/gbe/evs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wagutu GK, Fan X, Wang W, Li W, Chen Y. The complete chloroplast genome sequence of Trapa kozhevnikoviorum Pshenn. (Lythraceae) Mitochondrial DNA Part B. 2021;6(6):1677–1679. doi: 10.1080/23802359.2021.1927873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W, Fan X, Li X, Chen Y. The complete chloroplast genome sequence of Trapa incisa Sieb. & Zucc. (Lythraceae) Mitochondrial DNA Part B. 2021;6(6):1732–1733. doi: 10.1080/23802359.2021.1930601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wan WH. Trapaceae. Flora Republicae Popularis Sinicae. Beijing: Science Press; 2000. p. 1–26 53.
- 65.Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–15. [Google Scholar]
- 66.Jin JJ, Yu WB, Yang JB, Song Y, dePamphilis CW, Yi TS, Li DZ. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020;21(1):241. doi: 10.1186/s13059-020-02154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qu XJ, Moore MJ, Li DZ, Yi TS. PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods. 2019;15(1):50. doi: 10.1186/s13007-019-0435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S. GeSeq-versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017;45(W1):W6–w11. doi: 10.1093/nar/gkx391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 2005;33:686–689. doi: 10.1093/nar/gki366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lohse M, Drechsel O, Bock R. Organellar Genome DRAW (OGDRAW): a tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr Genet. 2007;52(5–6):267–274. doi: 10.1007/s00294-007-0161-y. [DOI] [PubMed] [Google Scholar]
- 71.Sharp PM, Li WH. The codon adaptation index-a measure of directional synonymous codon usage bias, and its potential applications. Nucl Acids Res. 1987;15(3):1281–1295. doi: 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edgar RC. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucl Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar S, Glen S, Li M, Christina K, Koichiro T. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25(11):1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 75.Kurtz S, Schleiermacher C. REPuter: fast computation of maximal repeats in complete genomes. Bioinformatics. 1999;5:426–427. doi: 10.1093/bioinformatics/15.5.426. [DOI] [PubMed] [Google Scholar]
- 76.Thiel T, Michalek W, Varshney R, Graner A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.) Theor Appl Genet. 2003;106(3):411–422. doi: 10.1007/s00122-002-1031-0. [DOI] [PubMed] [Google Scholar]
- 77.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 79.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9(8):772–772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Sequence alignment of whole chloroplastgenomes using the Shuffle LAGAN alignment algorithm in mVISTA. Trapa quadrispinosa waschosen to be the reference genome. The vertical scale indicates the percentidentity, ranging from 50 to 100%. Figure S2. Number of long repetitive repeats onthe complete chloroplast genome sequence of 15 Trapa species. (a) frequency of the repeats more than 30 bp, (b) frequencyof repeat types. chLJ, Trapa bispinosa;xlSJ, Trapa quadrispinosa; chQJ, Trapa japonica; chSL, Trapa mammillifera; bdZE, Trapa natans var. baidangensis; hkDB, Trapamacropoda var. bispinosa; tyE, Trapa potaninii; nqG, Trapa litwinowii; fGJ, Trapa arcuata; xkGL, Trapa pseudoincisa; qqDB, Trapa manshurica; jxKF, Trapa kozhevnikovirum; wyXBLY, Trapa sibirica; SJKY, Trapa incisa; XGY, Trapa maximowiczii. Figure S3. The comparison of simple sequencerepeats (SSRs) distribution in 15 chloroplast genomes. (a) frequency of commonmotifs; (b) number of different SSR types. chLJ, Trapa bispinosa; xlSJ, Trapaquadrispinosa; chQJ, Trapa japonica;chSL, Trapa mammillifera; bdZE, Trapa natans var. baidangensis; hkDB, Trapamacropoda var. bispinosa; tyE, Trapa potaninii; nqG, Trapa litwinowii; fGJ, Trapa arcuata; xkGL, Trapa pseudoincisa; qqDB, Trapa manshurica; jxKF, Trapa kozhevnikovirum; wyXBLY, Trapa sibirica; SJKY, Trapa incisa; XGY, Trapa maximowiczii.
Data Availability Statement
All the annotated cp sequences data reported here were deposited in GenBank (https://www.ncbi.nlm.nih.gov/) with accession numbers shown in Table 5. All voucher specimens were deposited in the herbarium of Wuhan Botanical Garden.