Abstract
Purpose:
It is well-documented that chronic conditions, such as diabetes, impact quality of life (QoL). QoL assessment is essential when developing and evaluating diabetes self-management education support interventions. The aim of this systematic review was to evaluate the evidence and gaps in the research and the impact of diabetes self-management education on quality of life outcomes in persons with type 1 diabetes mellitus (T1DM).
Methods:
A systematic review of English language studies published between Jan 1, 2007 – March 31, 2020 was conducted using a modified Cochrane review method. Studies were included if they were randomized controlled trials (RCT), participants had T1DM with or without caregivers, a diabetes self-management education (DSME) intervention alone or a component(s) of the ADCES7™ Self-Care Behaviors was described, and QoL was a primary or secondary outcome. A three-tiered review process was utilized for selecting articles. Retained articles were assessed for risk-of-bias.
Results:
Nineteen articles, reporting on 17 RCTs, met inclusion criteria of which seven studies reported QoL as the primary outcome and ten as a secondary outcome. Seven studies detected significant impact of DMSE on QoL outcomes in either the participants or family caregivers, which varied in participant populations, selection of QoL tools (generic verses diabetes-specific), intervention type, intervention length, and type of interventionist.
Conclusion:
DSME has the potential to influence QoL outcomes in people with T1DM. Research using more standardized methods are needed to delineate impact on a broader range of factors that influence QoL for those living with T1DM across the lifespan and their caregivers.
Keywords: type 1 diabetes, quality of life, diabetes care, diabetes education, patient reported outcomes
Background and Introduction
Type 1 diabetes mellitus (T1DM) broadly impacts the lives of individuals and their families, as well as the health care system as a whole. As defined by the Centers for Disease Control (CDC) “quality of life is a broad multidimentional concept that usually includes subjective evaluations of both positive and negative aspects of life.”1 More specifically, health related QoL (HRQoL) involves assessment of factors that effect both physical and mental health parameters including perceptions of mood, perceived health risks, and functional status (CDC same site). For example, HRQoL and work productivity scores are lower in people with T1DM2 particulaly those with 2 or more complications.2 It is recognized that QoL, including HRQoL, are important factors to assess and should be considered when developing and evaluating diabetes self-mangement education and support (DSMES) interventions for persons with T1DM.3–5
Diabetes Education and Support
Diabetes Care and Education Specialists are integral members of the diabetes treatment team. Diabetes Care and Education Specialists represent various health care disciplines and deliver direct patient care to the growing number of persons with or at risk for diabetes and associated cardiometabolic conditions.6 These professional provide person-centered care by establishing partnerships that facilitate decision-making for the 7 core behaviors of diabetes self-management by people with diabetes. This framework, known as the ADCES7 Self-Care Behaviors™, formerly referred to as the AADE7®, is foundational to achieving optimal outcomes.7
The scope of diabetes education broadened in 2012 when the National Standards for Diabetes Self-Management Education (DSME) were transitioned to the National Standards for Diabetes Self-Management Education and Support (DSMES).8 This evolution recognized the need for the continuous support required by most people with diabetes in order to implement and sustain health-related behavior change.8 DSMES plays a pivotal role in successful diabetes care and is critical for an individual’s sustained self-efficacy for and success in managing diabetes.9 The purpose of DSMES is to empower the person with diabetes with the confidence and desire to engage in effective self-care through acquisition of knowledge of what to do, the skills to do it, and problem solving and coping skills to overcome barriers to self-care behaviors.9 Part of this education integrates the ADCES7 Self-Care Behaviors™ which address healthy coping, healthy eating, being active, monitoring, taking medication, problem solving, and reducing risks.7 DSMES is essential at 4 critical times when people with diabetes are most vulnerable to poor outcomes: at time of diagnosis, during annual evaluations, at the onset of new complications, and during transitions of care.9,10
In a 2014 position statement, the American Diabetes Association (ADA) provided a grade B recommendation for individuals with T1DM and their caregivers to receive DSME at the time of the diabetes diagnosis and routinely thereafter.11 This grade B rating was based on evidence found from well conducted cohort and case control studies. Similarly, the National Institute for Health and Care Excellence (NICE) suggested offering a structured education program in the self-management of flexible insulin therapy to all adults with T1DM regardless of duration of diabetes.12 Diabetes Care and Education Specialists provide instrumental and tangible support for effective diabetes self-care.
Impact of DSMES on Patient Reported Outcomes
The 2013 National Quality Forum’s report, Patient Reported Outcomes in Performance Measurement, highlighted the need to integrate information reported from the experiences of patients and families, including QoL assessments, as important outcomes of healthcare delivery.13 A1C, a clinical indicator, remains the most commonly collected outcome measure in diabetes care and research and serves as the provider performance indicator for insurers and government regulatory bodies. Although A1C provides insight into overall glycemic regulation, the measure fails to capture other clinically meaningful outcomes in T1DM management, such as time-in-range, hyperglycemia frequency, hypoglycemia events and incidences of diabetes related ketoacidosis.14 In 2017, a consensus report issued by multiple diabetes care and advocacy organizations, including the American Association of Diabetes Educators (renamed Association of Diabetes Care and Education Specialists [ADCES]), provided definitions for these outcomes and additionally proposed the need to standardize patient reported outcomes measures (PROMs) in care and research, including patient-reported QoL in people with T1DM.14 The ‘Beyond A1C’ Group asserted that regulatory bodies should integrate these revised glycemic measures in clinical and policy decision making.15 The group also concluded that there is a need to standardize, validate, and achieve consensus on PROMs, including those assessing QoL, for use in clinical trials, regulatory and in risk-benefit decision making.15
Impact on Behavior Change, Knowledge, Clinical, Humanistic and Economic Outcomes
The benefits of DSMES on self-management behaviors and other outcomes have been established.16–21 A meta-analysis of 52 studies reported those patients receiving diabetes education had a moderate reduction in A1C from baseline to post-intervention assessment.22 A recent systematic review, primarily focusing on hypoglycemia, reported positive effects associated with diabetes education on different measures of clinical and behavioral outcomes, such as the number of hypoglycemia events, reported symptoms, knowledge gain, and behavior change.23 Similarly, significant positive changes in behavioral and psychosocial outcomes such as diabetes knowledge, understanding, and condition management have also been reported.24 Concerning cost-effectiveness, strong evidence exists in favor of DSMES compared to usual care.25 Research has demonstrated that diabetes education significantly contributes to the reduction of risk for development of diabetes-related complications.26 Along with these positive outcomes, previous research has demonstrated a significant association between improved quality of life (QoL) and DSMES.27,28
Quality of Life (QoL) Measurement in Diabetes Care
There are over 1200 measures of QoL, both generic or general and condition- or disease-specific.29 Several clinical trials reporting the effects of medical or educational interventions for diabetes self-management behaviors have demonstrated positive effects on behavior change and diabetes-related QoL or satisfaction.27, 30–32 The CDC and Healthy People 2030 have identified the more focused concept of HRQoL as an important aspect of clinical and research outcomes related to chronic disease self-management33–35 HRQoL challenges for those with T1DM uniquely encompass physical, psychological, and social well-being factors. In addition, the demands of maintaining glucose levels in the optimal range to prevent serious complications through eating behaviors, medication, and physical activity may impact HRQoL.36
QoL is unique to each person and is dependent on their individual priorities and preferences.29 Various QoL measures, generic and diabetes-specific, have been developed and tested among persons with T1DM. Some of these measures are focused on HRQoL. Rubin and Peyrot4 established the importance of using both generic and diabetes-specific measures in fully understanding person-reported QoL. Table 1 summarizes well-established QoL measures utilized in studies of persons with T1DM, including the characteristics, number of items, and scales. The choice of diabetes-specific QoL measures for patient evaluation or research needs to reflect the purpose of the assessment, concepts under study, and/or research aims in order to provide relevant and actionable data.29
Table 1:
The Most Commonly Used of the Established Quality of Life (QoL) Measures
| Quality of Life (QoL) Measure | Population | Length of Measure | Sub-scales | Scoring direction |
|---|---|---|---|---|
|
| ||||
| Diabetes-specific QoL Measures | ||||
| Diabetes Quality of Life (DQOL)37 | Adolescents and adults | 46 items | Satisfaction, impact, worry, social/vocational worry | Lower scores indicate better QoL |
| Diabetes Quality of Life-Youth scale (DQOL-Y)38 | Children and adolescents | 52 items | Diabetes life satisfaction, disease impact, and disease-related worries | Higher scores indicate better QoL |
| Short form of the Diabetes Quality of Life-Youth scale (DQOLY-SF) 39 | Children and adolescents | 21 items | Impact, worry, and parental concern | Higher scores indicate better QoL |
| Diabetes-Specific Quality of Life Scale (DSQOLS)38 | Persons with T1DM | 64 items | Daily restrictions and burdens, treatment goals, and treatment satisfaction | Higher scores indicate better QoL |
| Audit of Diabetes-Dependent Quality of Life (ADDQOL)40 | Persons with T1DM or T2DM | 19 items | Impact of diabetes on life domains (e.g., work social, family life; enjoyment; worries) | Higher scores indicate less negative impact of diabetes on QoL |
| Pediatric Quality of Life Inventory (PedsQL™) 3.2 Type 1 Diabetes Module5 | 5–18 years old: child self-report 2–18 years old: parent proxy-report |
28 items | Diabetes symptoms, treatment barriers, treatment adherence, worry, and communication |
Higher scores indicate better QoL |
| DQOL Brief Clinical Inventory (DQOL-B)41 | Adults with T1DM or T2DM | 15 items | Satisfaction with diabetes control and self-care behaviors | Higher scores indicate better QoL |
|
Generic QoL Measures | ||||
| Pediatric Quality of Life Inventory (PedsQL™) 4.0 Generic Core Scales42 | 5–18 years old: child self-report 2–18 years old: parent proxy-report |
23 items | Physical functioning and psychosocial functioning (emotional, social, and school functioning) | Higher scores indicate better QoL |
| PedsQL™ Family Impact Module43 | Parent self-report from child’s health | 36 items | Physical, emotional, social, cognitive functioning, communication, worry, family daily activities, and family relationships | Higher scores indicate better QoL |
| Short Form-36 Health Survey Questionnaire (SF-36) 44 | General population | 36 items | Limitations in physical activities because of health problems, limitations in social activities because of physical or emotional problems, limitations in usual role activities because of physical health problems, bodily pain, general mental health, limitations in usual role activities because of emotional problems, vitality, and general health perceptions | Higher scores indicate better QoL |
| European Quality of Life-5 Dimensions (EQ-5D)45 | General population | 5 items | Mobility, self-care, usual activities, pain/discomfort, anxiety/depression | Lower scores indicate no problems (ex: 11221) |
Measurement of QoL is evolving. An initial attempt to measure HRQoL for persons with T1DM occurred in 1988 when the original Diabetes Quality of Life Measure (DQOL) was developed and used in the Diabetes Control and Complications Trial (DCCT).37 By 1998, this scale was known as the Diabetes-Specific Quality of Life Scale (DSQOLS) and focused on assessing specific aspects of diabetes that impact QoL for those living with T1DM (e.g., burden, satisfaction, treatment goal, personal/family relationships, diet, physical symptoms, worries).29,38
In 1991, the adult version of the DQOL was modified for assessing psychosocial impacts of diabetes that were more relevant to children and adolescents (ages 2–18 years).42 This tool, known as the Pediatric Quality of Life Inventory (PedsQL™), integrated both generic and diabetes-specific components. There are 2 versions including PedsQL™ 4.0, a more generic QoL inventory for children with and without chronic conditions, and PedsQL™ 3.2, which measures disease-specific HRQoL for those with T1DM. The PedsQL™ 3.2 allows for use with a wider age range and includes both parent proxy report and child self-report components.5 A separate module of the PedsQL™ series reports impacts of a child’s chronic illness on the parental caregiver and family46
Due to the broad scope and length of the previous DQOL instrument, a brief clinical inventory applicable for people both T1DM or T2DM was developed. The Diabetes Quality of Life Brief Clinical Inventory (DQOL-B) provides a HRQoL score representing the person’s report of diabetes care behaviors and satisfaction with aspects of diabetes treatment.41 The DQOL-B can also identify QoL factors such as fears or concerns over how diabetes can affect work, social, or family situations.41
Quality of life is also a central aim within ADCES’s Vision: “…optimal health and quality of life for persons with, affected by, or at risk for diabetes and its complications.” 47 Although DSMES is a primary strategy for supporting this vision, DSME remains the more common description in the literature. Therefore, the primary focus of this systematic review was to explore and report evidence and gaps in the literature for the impact of DSME on outcomes, particularly QoL, in people with T1DM. Specifically, the aims of this systematic review were to:
Report evidence and gaps for the impact of DSME on QoL as a primary or secondary outcome in adult and pediatric populations with T1DM.
Report impacts of DSME on QoL and metabolic control as measured by the A1C in adult and pediatric populations with T1DM.
Summarize implications for practice and research for the impact of DSME and QoL in adult and pediatric populations with T1DM.
Methods
Search and Selection
This systematic review used a modified Cochrane review method to address a broad research question using the Cochrane framework48 for search and review; the more broad and scoping research question allows for a report of the ‘state of the science’, or evidence and gaps in the literature evaluating the impact of DSME on QoL in adult and pediatric populations with T1DM. The systematic search employed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guidelines49 and was guided by a health sciences librarian.
Search Terms and Databases
To address the research questions looking at evidence and gaps in the literature for diabetes self-management education as an intervention impacting Quality of Life (QoL) outcomes, the researchers conducted a search pertinent to the terms and their derivations for “diabetes” and “self-management” and “education” and “intervention”, and “quality of life” and ‘outcomes’ in the following 7 databases: CINAHL Plus with Full Text, Medline (EBSCOhost), PsycInfo, Cochrane Database of Systematic Reviews, Cochrane Register of Controlled Trials, and Web of Science. The specific search terms used in this review varied by database in order to account for the differences in controlled vocabulary. “Well-being” was included as a keyword since many articles which emerged in exploratory searches used that term as a synonym for QoL. Specific instruments that focused on QoL measurement were also included as search terms. For a full list of searches performed, see [appendix].
Inclusion/Exclusion Criteria
Articles considered for retention in this review had to specify diabetes education alone as the intervention or as a key component of a combined intervention. Description of a DSME program/intervention was required and had to address at least 1 or more of the ADCES7™ Self-Care Behaviors (healthy coping, healthy eating, being active, taking medication, monitoring, reducing risks, problem-solving)7 to qualify as delivery of diabetes education. Studies describing DSME as a component of care management were also included as were reports of education delivered via technology without a direct human interaction (ie, text-messaging from a practitioner/educator, internet-based education modules), provided they met other inclusion criteria.
In addition, the study had to include an established QoL measure as a primary or secondary outcome. Retained studies had to be published in the English language and in the specified time frame (Jan 1, 2007 – March 31, 2020). This time frame was selected because of consensus that PROMs are essential for assessing quality of diabetes care and delivery. Retained study articles included both United States (US) and international studies, primary original research, and completed studies. Diabetes education could be delivered by a variety of practitioners, to persons across the age spectrum with T1DM and could integrate a caregiver, but could not be delivered to the caregiver alone. Studies were excluded if they did not include an experimental or case-controlled design with at least pre- and post-intervention data collection for the target measures associated with QoL; qualitative studies, opinions/case reports, protocols, conference abstracts, and reviews were excluded. Studies without an abstract were not considered.
Screening Process and Selection
The review process started by importing all records into the EndNote X9 citation management software.50 Duplicates were removed and then uploaded into Covidence screening software where they were evaluated for inclusion and exclusion criteria.51 A three-tiered review process was conducted using a modified Cochrane review methodology.48 The tiers included examination of titles, abstracts, and full text articles. The first two tiers, examined the title and abstracts for inclusion criteria and were conducted independently by teams of at least 2 reviewers (JD, SH, PD, SH, JKD, TH, JC, ML) with a third reviewer (JL) resolving any differences in retain/reject decisions for articles. The research team then met to discuss observations from the first two review tiers. The current review was limited to studies that reported the impact DSME on QoL (both generic and health-related) among persons with T1DM across the age spectrum.
Subsequently, 2 teams comprised of 2 researchers independently conducted the full text review of the preliminarily retained studies (JL and PD; JK and JKD). Each team met to determine consensus for retain/reject decisions. Studies retained after full text review moved to the data extraction phase of the review.
Data Extraction and Study Review
The research team developed a standardized data extraction tool for the purpose of extracting and organizing data from the final retained articles. Two teams (SF and PD; JL and MO) completed the data extraction and met to confirm assessments.
Methodological Quality and Risk of Bias Assessment
The methodological quality of retained studies in a systematic review contributes to the ability to form conclusions from the review results.48 The risk of bias (RoB) assessement was conducted using the revised Cochrane Risk of Bias tool for RCTs (RoB2).52 Four researchers (JL and MO; PD and SF) conducted independent assessments of QoL and A1C outcomes. Both teams of 2 reviewers met to discuss and come to consensus on any differences in the ratings.
Results
Study Design and Description for Retained Studies
Nineteen articles, reporting on 17 RCTs, met inclusion criteria. Figure 1 presents the final result PRISMA for this review focusing on studies addressing QoL outcomes for people with T1DM. One report described a pilot study with a pragmatic parallel group design.53 Three studies (4 reports) were randomized at the cluster level, 54–57 and the number of centers involved in each cluster varied between 6 and 31 sites. With regard to blinding, after randomization of participants, interventionists were not blinded to group membership. However, 4 studies (five reports) indicated blinding of knowledge of group membership of individual participants to assessors or data collectors. 44,45,54,55,58 Table 2 provides a summary of study characteristics.
Figure 1:
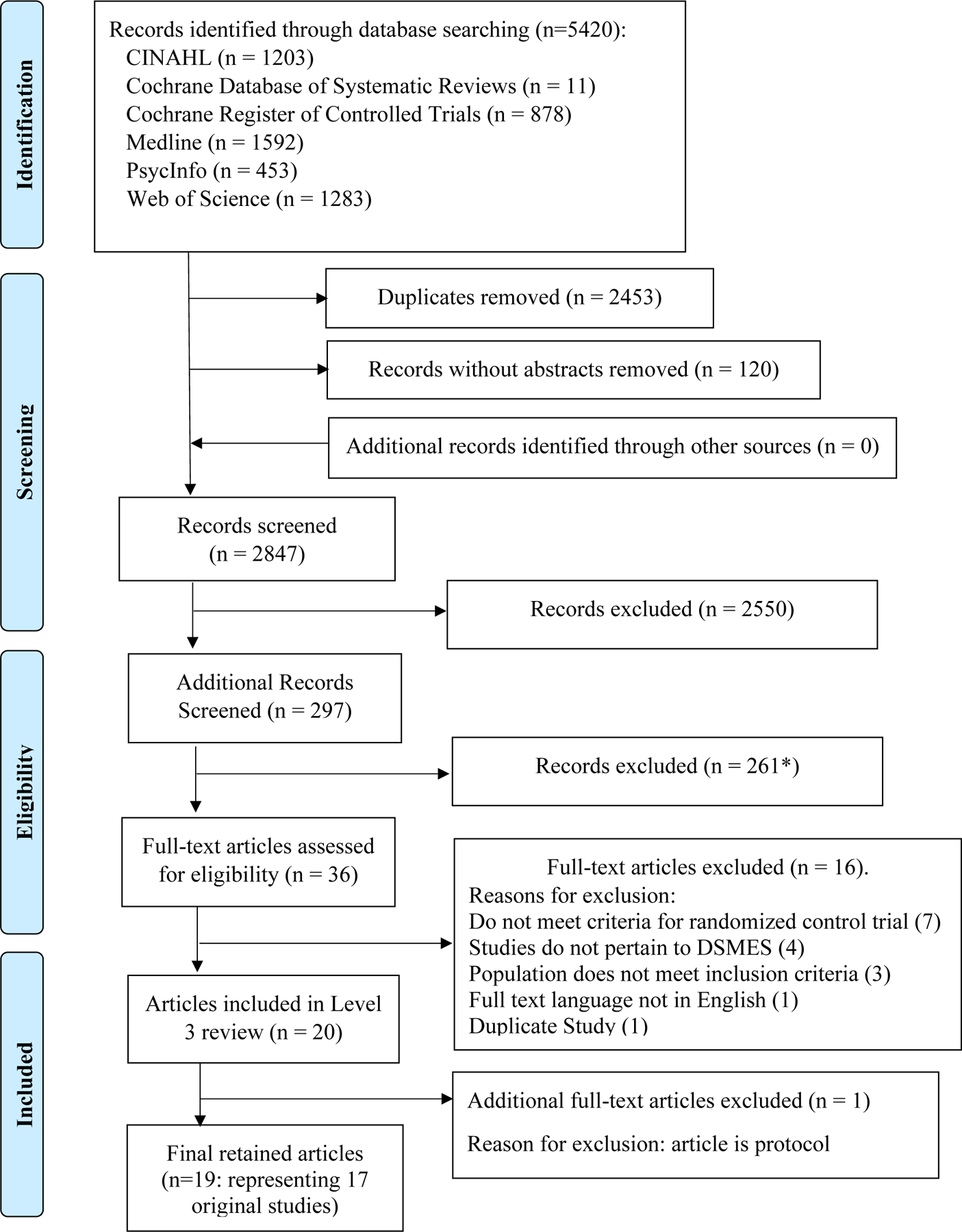
PRISMA49 Flow Diagram
Table 2:
Characteristics of Retained Studies
| Study: Author Year Country Risk of Bias |
Participants: N Age Gender Race/ethnicity (Intervention; Control) |
Study Design | Intervention: Education type Duration Interventionist Recipient of education |
Insulin Delivery Method (Intervention; Control) |
|---|---|---|---|---|
|
| ||||
| Christie et al54 2014 UK High |
159; 168 13.1 ± 2.1; 13.2 ± 2.1 Female: 57.2%; 53.6% White British 83.7%; 76.8% Asian British 3.1%; 8.3% |
Pragmatic, cluster | Manual-based four-module structured education program with psychological approaches vs. usual care 4 months Pediatric diabetes specialist nurse Patient, caregiver |
Not reported |
| Christie et al55 2016 England High |
159; 168 13.1 ± 2.1; 13.2 ± 2.1 Female 57.2%; 53.6% White 86.8%; 79.8% Black 3.1%; 3.6% Asian 3.1%; 8.3% |
Pragmatic, cluster | Manual-based four-module structured education program vs. regular clinic visits 4 months Diabetes specialist, nurse Patient, caregiver |
Not reported |
| Dinneen et al57,59 2009, 2013 Ireland High |
216; 221 40.1 ± 12; 41.5 ± 11.4 Female 50%; 57.5% Not reported |
Cluster | DAFNE course plus booster sessions vs. DAFNE course plus 2, one-to-one visits 12 months MD, nurse, or RD Patient |
MDI or CSII |
| Ellis et al53,55 2019 US High |
23 families; 24 families Patient: 13.46 ± 2.24; 15.02 ± 2.32 Family member: 40.01 ± 6.44; 43.31 ± 8.10 Female 96% White 17%, 17% Black 83%; 75% |
Pragmatic | Structured modules DSME program vs. standard medical care 6 months Community Health Worker Patient, caregiver |
Not reported |
| Fiallo-Scharer et al43 2019 US High |
106 families; 108 families Patient: 8–12 yrs 44.4%, 13–16 yrs 44.3%; 55.6%, 55.7% Family member: 42.0 ± 5.8; 41.7 ± 6.6 Female 54.6%; 43.4% White 85.2%; 82.1% |
Parallel arm clinical trial | Usual care plus group sessions (used results of PRISM surveys to determine type of self-management resource [motivation, understanding and organizing care, and family interactions]) vs. usual care 9 months 12 months (written in another place) Not reported Patient, caregiver |
CSII or MDI |
| Guo et al60 2020 China Low |
50; 52 Patient: 8–12 44%, 13–20 56%; 36%, 64% Female 60%; 52% Not reported |
RCT | 2-day camp and five monthly phone calls (Coping Skills Training) vs. reminders and encouragement to attend quarterly clinic visits for usual care Duration not clearly reported (5 months or 12 months) Nurses, research assistant Patient |
Not reported |
| Ismail et al44 2008 UK High |
Group 1: 117, Group 2: 106; 121 Group 1: 35.7, Group 2: 36.6; 36 Female Group 1: 65%, Group 2: 62.3%; 54.6% Group 1: White 75.2%, Group 2: 79.2%; 86% Group 1: Black 24.8%, Group 2: 20.8%; 14.1% |
RCT (data collector/lab personnel blinded) | Group 1: Usual care with motivation interviewing Group 2: Usual care + MI + Cognitive Behavior Therapy vs. protocol of “minimum standards of diabetes care” based on national guidelines Group 1: 2 months, 4 sessions Group 2: 6 months, 12 sessions Diabetes nurse with training in MI and CBT Patient |
MDI or CSII |
| Ismail et al61 2010 UK High |
MET + CBT: 106 MET: 117; 121 MET + CBT: 37.2 ± 9.9 MET: 35.6 ± 9.6; 36.4 ± 11.3 Female MET + CBT: 62.3%, MET: 65%; 54.6% MET + CBT: White 79.3%, MET: 75.2%; 86% |
Not reported | MET + CBT: motivational enhancement therapy plus cognitive behavioral therapy MET only: motivational enhancement therapy vs. usual care every three months 12 months (not clearly defined) Nurses trained and supervised by clinical psychologists Patient |
Not reported |
| Kirwan et al62 2013 Australia High |
36; 36 35.97 (10.67); 34.42 (10.26) Female 47.2%; 75% Not reported |
RCT | Smart Phone App (Glucose buddy)-weekly text message with educator then usual care every 3 months vs. usual care every 3 months (from data extraction) 6 months – duration 9 months – 3 months STOP Certified Diabetes Educator Patient |
CSII 38% MDI 62% |
| Mayer-Davis63 2018 US Low |
129; 128 14.8(1.1); 14.9 (1.1) Female 45.4%; 53.9% White 78.1%; 76.9% Black 5.4%; 3.1% Hispanic 13.3.%; 12.3% Other 5.5%; 5.4% |
RCT (assessment staff not blinded) | FLEX intervention vs. usual care 18 months Care coach (members of type 1 diabetes care team) trained in MI with guidance of psychologist Patient, caregiver |
MDI 31.8%; 26.8% CSII 68.2%; 73.2% |
| Mitchell et al64 2018 Scotland Low |
9; 9 12; 12 Female 70%; 50% Not reported |
RCT (data blinded to interventionist) | 4-week individualized, graduated physical activity program vs. waiting list 4 weeks Physical activity specialist Patient, caregiver |
Not reported |
| Murphy et al65 2012 UK High |
158; 147 13.1; 13.1 Female 53%; 51% White 93%; 91% Not specified 7%; 9% |
RCT not blinded | FACTS plus traditional DSMES with family communications training vs. conventional care with 3-monthly outpatient clinic appointments Six, 90-minute sessions held monthly vs. four, 3-month outpatient clinic appointments Multidisciplinary team Adolescent and parent |
Premixed 16%; 20% MDI 77%; 72% CSII 6%; 7% |
| Newton et al66 2013 US High |
25; 25 14; 15 Female 80%; 52% White 92%; 100% Other 8% |
RCT | Teen’s Talk website consisting of blogs, discussion forums, and chat room –topics facilitated discussion about a weekly topic. Allowed teens to discuss difficult psychological problems. Asynchronous. vs standard medical care with no participation in website 7 weeks Not specified Patient Moderator hosted session |
MDI 44%; 36% CSII 56%; 64% |
| Noyes et al45 2020 UK Some concerns |
190; 114 12.4; 12.7 Female 55%; 52% White 94%; 98% Other 6%; 2% |
RCT not blinded; assessors blinded | Standardized but flexible self-management kit known as EPIC vs. usual care 6 months “Clinical team” Patient, caregiver |
MDI 88%; 84% CSII 12%; 16% |
| Price et al56 2016 UK High |
13.71; 13.92 Female 53.8%; 56.8% White 90.5%; 93.4% Other 9.5%; 6.6% |
RCT not blinded | KICK-OFF five-day group intervention focusing on CHO counting and insulin adjustment and acute and chronic complications and scenario based vs. usual education from home care team Five sessions over five straight days Team of nurse and dietitian from program Patient, caregiver |
MDI |
| Pyatak et al58 2018 US High |
31; 30 23.3 ± 3.6; 21.9 ± 3.3 Female 54%; 72% White 7%; 12% Black 7%; 12% Hispanic 85%; 70% |
RCT | Flexibility delivered Seven content modules vs. initial home visit by Healthcare staff providing National Diabetes Education handouts and MyPlate.gov and 11 follow up phone calls 6 months Two licensed occupational therapists with training in motivational interviewing and diabetes self-management education Patient |
Not reported by type Fixed regimen 38% MDI 43% CSII 11% |
| Sanchez-Hernandez et al67 2018 Canary Islands/Spain High |
48; 32 33; 36 Female 48.7%; 50% Not reported |
RCT parallel (not blinded) | 5-day group education intervention with DSMES (ANAIS program – does not spell out) vs. routine care (control participants allowed to access program after 12 months outcomes not reported) 12 months 2 nurses and physician Patient |
Not reported |
| Trento et al68 2011 Italy Some concerns |
27; 29 37.76 ± 12.6; 36.76 ± 7.9 Female 33.3%; 41.4% Not reported |
RCT not blinded | 8-session carbohydrate counting program with usual care by Group Care (session of group education substituted for routine visits – established program x several years) vs. Group Care only 8 sessions every 3 to 4 months Physician, Psychopedagogist, Dietitian, Nurse Patient |
MDI |
| Weinger et al69 2011 US High |
37; 37 (attention) 36 (individual) 46.6 Female 56.4% White 95.5% |
RCT | Structural Behavioral Group: consisted of five, 2-hour sessions of highly structured behavior based activities and information do care on dietary exercise, medication and goal 6 weeks Experienced diabetes nurses and dietitians who were certified diabetes educators Patient |
Not reported |
Abbreviations: CBT, cognitive behavioral therapy; CDE, certified diabetes educator; CGM = continuous glucose monitoring
CSII = continuous subcutaneous insulin infusion (insulin pump)
DAFNE = dose adjustment for normal eating
EPIC = evidence into practice – information counts
FACTS = families, adolescents and children teamwork study
FLEX = Flexible Lifestyle Empowering Changes
KICK-OFF = Kids in Control of Food
MDI = multiple daily injections
MET = motivational enhancement therapy
MI = motivational interviewing
PRISM = Problem Recognition in Illness Self-Management
RCT = randomized controlled trial
US = United States
UK = United Kingdom
Participant Characteristics
Sample sizes ranged from 18 to 437 participants, with an average of 196 participants (SD ± 133) per study. Participants ranged in age from 8 to 51 years-old, with an average age of 22.9 (SD ± 12.4) years. Most study participants were female (54.8%). Eleven trials (12 reports) included children/adolescents,43,45,53–56,60,62–66 with an average age of 13.4 (SD ± 0.92) years. Six trials (7 reports) included adults44,57,58,61,67–69 with an average age of 36.2 (SD ± 6.32) years. Averaging across all studies, participants were 76.1% Caucasian/White, 8.9% African American/Black, 6.9% Hispanic, 0.8% Asian, and 7.3% other. Six international trials did not report the race or ethnicity of participants. 67,60,62,64,68 In addition, two US studies reported higher proportions of African American/Black (79%)68 and Latino (78%)62 participants.
Nine reports did not describe the participants’ insulin plan or insulin delivery modalities. 44,53–55,60,61,64,67,69 Of studies specifying medication plans, 71.9% of participants used multiple daily injections (MDI) while 26.1% reported insulin pump use. One study reported that 20.6% of participants incorporated continuous glucose monitoring (CGM) into diabetes self-management practices.63 Participant co-morbidities, which included retinopathy (n = 2), 44,61,67 neuropathy (n = 2), 44,61,67 chronic kidney disease, 61,67 hypertension (n = 1),67 dyslipidemia (n = 2),67,68 overweight/obesity (n = 1),63 and depression (n = 2), 44,61,67 were reported in only four studies (5 reports).
Study Characteristics
Across all retained reports, the educational interventions incorporated various components of the ADCES7 Self-Care Behaviors™, including blood glucose monitoring, insulin adjustments, medication taking, treatment of hypoglycemia, psychosocial problems, healthy coping, emotional well-being, problem-solving, healthy eating (carbohydrate counting), physical activity, social support, family communication, and goal setting. A detailed summary of the ADCES7 Self-Care Behaviors™ by retained studies is presented in Table 3.
Table 3.
ADCES7™ Self-care Behaviors Addressed by Paper (n = 17 studies and 19 reports)
| First author, Year, Country of Origin | Healthy Eating | Being Active | Monitoring | Taking Medications | Reducing Risk | Problem Solving | Healthy Coping |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Christie, 2014, UK54 | x | x | x | x | x | ||
| Christie, 2016, UK55 | x | x | x | x | x | x | |
| Dinneen, 2009, 2013, Ireland57,59 | x | x | x | x | |||
| Ellis, 2019, US53 | x | x | x | ||||
| Fiallo-Scharer, 2019, US43 | x | x | x | x | x | x | x |
| Guo, 2020, China60 | x | x | x | x | x | x | x |
| Ismail, 2008, UK44 | x | x | x | x | x | ||
| Ismail, 2010, UK61 | x | x | x | x | x | ||
| Kirwan, 2013, Australia62 | x | x | x | x | |||
| Mayer-Davis, 2018, US63 | x | x | x | x | x | ||
| Mitchell, 2018, UK64 | x | x | |||||
| Murphy, 2012, UK65 | x | x | x | x | x | x | x |
| Newton, 2013, US66 | x | x | |||||
| Noyes, 2020, UK45 | x | x | x | x | x | x | x |
| Price, 2016, UK56 | x | x | x | x | x | x | x |
| Pyatak, 2018, US58 | * | x | * | x | x | x | |
| Sanchez-Hernandez, 2018, Spain67 | x | x | x | x | x | x | |
| Trento, 2011, Italy68 | x | x | x | x | x | x | x |
| Weigner, 2011, US69 | x | x | x | x | x | x | x |
Basic self-management
Out of the 19 retained reports (17 studies), most interventions (n = 10) were structured diabetes education programs or courses with multiple sessions/lessons/modules. 53–55,58–60,65–67,69 Out of these, 4 specifically described DSME in their methods, 53,58,65,67 with 1 modeled after the ADCES7™.69 In the remaining 9 reports, 1 study used an intervention derived from the ADA’s education curriculum to address specific barriers within families,43 and another study used a physical activity intervention guided by the Social Cognitive Theory.64 Three interventions were focused on self-management, with one using age-specific education,45 one using motivational interviewing and problem-solving skills training,63 and the other using a diabetes self-management iPhone application to send personalized messages and track blood glucose, insulin dosages, medication taking, healthy eating, and physical activity.62 Another 2 studies had a primary diet-focused intervention based on carbohydrate counting. 56,68 Ismail et al44,61 provided 2 reports of a 3-arm study comparing motivational interviewing to motivational interviewing combined with cognitive behavioral therapy with controls.
The duration of most interventions ranged from 2 to 6 months (n = 9).44,45,53–55,58,62,64–66,69,61 Other intervention durations were 5 days,56 12 months,43,59,67 or more than 1 year.63,68 One study did not clearly define the duration.60 The frequency of encounters varied from daily in course-based education,56,57,67 multiple times per week,53 weekly,62,66 multiple times per month,44,61 monthly,54,55,60,65 and every 3 to 4 months.68 Other interventions utilized only one encounter,64 individualized frequencies,58 decreasing frequencies of participant contact over the intervention period,59 or intervals with unspecified frequencies.43,45,69
The type of encounters included web-based/mHealth (n = 2),62,66 group (n = 10),43,53–56,59,65,67,69,68 or individual (n = 7).44,45,58,60,61,63,64 Two studies combining individual, face-to-face encounters incorporated telehealth within the intervention (i.e., email, text, telephone/video).63,64 The participants in the educational interventions included either participants alone (n =10) 44,58–62,66–69 or participants with their caregivers/parents (n = 9).43,45,53–56,63–65
Participants allocated to control groups in most retained reports received usual or standardized care (n = 14),43–45,53–56,60–62,63,65–67 which typically consisted of routine visits with healthcare providers every 3 months. However, as opposed to usual care, the remaining 5 studies used a waiting list control group (n = 1),64 control groups receiving diabetes education (n = 2),59,68 or attention control groups (n = 2).58,69 In the study by Weinger et al69, the attention control group received the same number of education sessions as the intervention group, and participants in the other control group could meet with diabetes healthcare providers across the entire intervention period. In the study by Pyatak et al 58., the attention control group received an initial home visit with 11 follow-up telephone calls.
Interventionists
The interventions were delivered by a variety of healthcare professionals, as well as “other” healthcare and research personnel. Four studies (5 reports) did not describe the specific type of personnel involved.54–56,63,65 Seven of the studies indicated that the intervention was delivered by a multidisciplinary team.45,56,62,63,65,67,68 The healthcare professionals most frequently involved in the delivery of the interventions were nurses, followed by dietitians and physicians. A total of 10 studies (12 reports) indicated that nurses either delivered the intervention or were part of an intervention team.43–45,54–57,60,61,67–69 Five studies (6 reports) included dietitians,54–57,68,69 and 3 studies included physicians as members of the intervention team.45,57,68
Three studies specifically described other healthcare professionals delivering the interventions, including an occupational therapist,58 a pediatric psychologist,43 and a psychopedagogist.68 “Other” healthcare and research personnel involved in the delivery of the interventions were a community health worker,53 a physical activity specialist,64 and a research assistant.60 A Certified Diabetes Care and Education Specialist (CDCES, formerly known as a certified diabetes educator [CDE]) was involved in the delivery of the intervention in 4 of the studies43,62,66,69; one study described the CDCES as providing standard medical care that was provided to all study participants.53
Settings
All of the studies recruited patients and were carried out in clinics or centers specializing in diabetes care.Ten studies (11 reports) were conducted in specialty clinics43,54,55,58,62–66,68,69 and the number of clinics involved varied between 1 and 28. The remaining studies were carried out in hospital outpatient centers.44,45,53,56,57,60,61,67 The the number of sites involved in each study varied from 1 to 31. Six of the studies were conducted in the United States.43,53,58,63,66,69 Nine studies (11 reports) occurred in Europe. Six of these (8 reports) took place in the United Kingdom,54–56,44,45,61,64,65 1 (2 reports) in sites within the Republic of Ireland and Northern Ireland57,59, 1 in Italy,68 and 1 in Spain.67 The 2 remaining studies took place in the Far East and South Pacific, one study in China60 and the other in Australia.62
Diabetes Education Impact on Outcomes
Findings from retained studies for this review’s primary outcome of QoL, A1C, and related psychosocial outcomes are reported in Table 4.
Table 4.
Outcomes in Retained Studies
| First Author, Year | QoL (Measurement Tool) P= Primary S= Secondary |
A1C P= Primary S= Secondary |
Other Psychosocial Outcomes |
|---|---|---|---|
|
| |||
| Christie et al54 2014 | PedsQL™ general and diabetes module child and parental (S) IG vs CG: NS for both Within groups: NR |
S 12 or 24 months IG vs CG: NS |
NR |
| Christie et al55 2016 | PedsQL™ general and diabetes module child and parental (S) IG vs CG: NS for both Within groups: NR |
P 12 or 24 months IG vs CG: NS |
NR |
| Dinneen et al57,59
2009, 2013 |
DSQOL (S) IG vs CG: NS Within groups: NR |
P 18 months IG vs CG: NS |
Anxiety IG vs CG: NS Depression IG vs CG: NS Diabetes Distress IG vs CG:NS Within groups Anxiety ↓ IG and CG: (P<0.001) Depression ↓ IG and CG: (P<0.001) Diabetes Distress ↓ IG and CG: (P<0.001) Burden of Living with Diabetes: NR |
| Ellis et al53 2019 | DQOL-Y (P) IG vs CG: NS Within groups: ↑ IG only: (P=0.001) CG: no change |
P IG vs CG: NS ↓ IG (P=0.05) and ↑ in CG: NS |
NR |
| Fiallo-Scharer et al43
2019 |
PedsQL™- Diabetes-specific PedsQL™- General Parent Family Impact Module (P) Youth IG and CG: NS Within groups: NR Parents (youth ages 8–12 years) IG vs CG: NS IG vs CG at one site (P <0.05) Within groups: NR |
S IG vs CG: NS |
NR |
| Guo et al60 2020 | QoL Scale for Children and Adolescents: Chinese Version (S) School Aged IG vs CG (P= 0.06) Within groups: NR Adolescents IG vs CG: NS Within groups: NR |
S IG vs CG: NS |
School-aged children Positive coping ↑ IG vs CG (P <0.001) Self-efficacy among school-aged children in ↑IG vs CG (P=0.017) Adolescents Positive coping IG vs CG:NS Self-efficacy among IG vs CG: NS |
| Ismail et al44
2008 |
DQOL (S) IG (MET+ CBT) vs CG: NS IG (MET) vs CG: NS Within groups: NR |
P 3 months only ↓ IG (MET+CBT) vs CG (P = 0.007) 12 months ↓ IG (MET) vs CG: NS |
Depression IG vs CG:NS Hypoglycemia Fear IG vs CG: NS |
| Ismail et al61
2010 |
QALYs from SF-36 and EQ-5D (S) IG (MET+ CBT) vs CG: NS IG (MET) vs CG: NS IG (MET) vs CG: NS Within groups: NR |
P 12 months only ↓ IG (MET+CBT) vs CG (P = 0.008) ↓ IG (MET) vs CG: NS ↓ IG (MET+CBT) vs to MET group: NS |
Depression IG (MET+ CBT) vs CG: NS IG (MET) vs CG: NS IG (MET) vs MET: NS Hypoglycemia fear: IG (MET+ CBT) vs CG: NS IG (MET) vs CG: NS IG (MET) vs MET: NS |
| Kirwan et al62 2013 | DQOL (S) IG and CG: NS Within group: NR |
P ↓ IG vs CG (P < 0.001) |
Self-efficacy IG vs CG:NS |
| Mayer-Davis63 2018 | PedsQL™-General (S) Youth ↓ IG vs CG: (P=0.009) Within groups: NR Parental IG vs CG: NS Within groups: NR |
P IG vs CG: NS |
Depression ↓IG vs CG: (P=0.05) Motivation ↑IG vs CG: (P=0.011) Social Problem Solving ↑IG vs CG: (P=0.024) Fear of Hypoglycemia Helplessness Worry Sub-scale ↑IG vs CG: (P=0.036) Parent Maintain High Blood Glucose Sub-scale ↓IG vs CG: (P=0.051) Youth Maintain High Blood Glucose, Youth Worry about Social Consequences, Parent Helplessness, Parent Worry about Social Consequences Subscales IG vs CG: NS Diabetes Family Conflict – Youth IG vs CG: NS Diabetes Family Conflict – Parent ↓IG vs CG: (P=0.001) |
| Mitchell et al64 2018 | PedsQL™ 4.0 Generic Core Scale and PedsQL™ 3.0 T1DM Module (P) Child/teenage and Parental IG vs CG: NS Within groups: NR |
NR | NR |
| Murphy et al65 2012 | DQOLY-SF- 3 sub-scales Impact, Worry and Parental Involvement (S) Impact, Worry, Parental Involvement sub-scales Adolescents and Parents IG vs CG: NS Within groups: NR |
P 3 and 12 months IG vs CG: NS |
Family Responsibility IG vs CG: NS Problem Areas in Diabetes – Parent’s distress IG vs CG: NS |
| Newton et al662013 | DQOL-Y (P) IG vs CG: NS Within groups: NS |
NR | Self-efficacy IG vs CG: NS |
| Noyes et al45
2020 |
PedsQL™-diabetes (P) PedsQL™- general and EQ-5D (S) Child – PedsQL™-General 3 and 6-months follow-up IG vs CG: NS Within groups: ↓ IG: (P=0.024) Control: NS PedsQL™-Diabetes 3 and 6-month follow-up Total score ↓IG vs CG (P=0.020) Worry sub-scale ↓IG vs CG at 3 months (P=0.008) Worry subscale 3 to 6-months ↑IG vs CG (P=0.028) Treatment Barriers subscale 3 to 6 months ↓IG vs CG (P=0.042) Treatment Adherence sub-scale 3 to 6 months ↓IG vs CG (P=0.009) Communication subscale ↓IG vs CG (P=0.008) 3 to 6 months Diabetes Symptoms subscale 3 to 6 months ↓IG vs CG (NS) Parent IG vs CG: NS Within groups: NR |
S 6 months ↓ IG vs CG: NS |
NR |
| Price et al56 2016 | PedsQL™-diabetes PedsQL™-general (P) Adolescent PedsQL™-general ↑ IG vs CG: 6 months (P=0.04); 12 months (P=0.04) Psychosocial Subscale 6 months ↑ IG vs CG: (P=0.049) Within groups: NR 24 months IG vs CG: NS Within groups: NR PedsQL™-diabetes 6, 12, 24 months IG vs CG: NS Within groups: NR Parental PedsQL™-general 6, 12, and 24 months IG vs CG: NS Within groups: NR |
P 6, 12, 24 months ↓ IG vs CG: NS |
Self-efficacy 6 months ↓IG vs CG: (P=0.01) Self-efficacy 12 months ↓IG vs CG: (P=0.02) Fear of hypoglycemia IG vs CG: (NS) |
| Pyatak et al58 2018 | Diabetes-related QoL (ADDQOL) (S) Not reported separately for T1DM |
P ↓ IG vs CG: (P = 0.01) |
Not reported separately for T1DM |
| Sanchez-Hernandez et al67
2018 |
EsDQoL, Spanish (S) IG vs CG: NS Within groups ↑ IG (P<0.001) CG: NS |
P ↓ IG vs CG (unchanged): NS |
Fear of hypoglycemia IG vs CG: NS Depression IG vs CG: NS Anxiety state IG vs CG: NS Anxiety trait IG vs CG: NS |
| Trento et al68
2011 |
DQOL, Italian (P) IG vs CG: NS Within groups ↑ IG (P<0.0001) ↑CG (P<0.0001) |
S 30 months ↓ IG vs CG (P<0.05) |
NR |
| Weigner et al69 2011 | DQOL (S) Not reported separately for Type of Diabetes T1DM x time: NS |
P Not reported separately for T1DM by intervention group |
Diabetes; Specific Self-efficacy; Diabetes Distress; Depression Anxiety; Coping Style Not reported separately for T1DM by intervention group |
Abbreviations: QoL, quality of life; A1C, glycated hemoglobin A1C; PedsQL™, Pediatric Quality of Life Inventory; IG, intervention group; CG, Control Group; NS, not-significant; NR, not reported; DSQOL, Diabetes-specific Quality of Life Scale; DQOL-Y, Diabetes Quality of Life-Youth scale; DQOL, Diabetes-Quality of Life Scale; MET, Motivational Enhancement Therapy; CBT, Cognitive Behavior Therapy; QALY, quality-adjusted life-years; EQ-5D, European Quality of Life-5 Dimensions; ADDQOL, Audit of Diabetes Dependent Quality of Life; T1DM, Type 1 Diabetes Mellitus; EsDQoL, Spanish version of Jacobson’s Diabetes Quality of Life questionnaire
Quality of Life Outcomes
Six studies reported QoL as the primary outcome.43,45,56,64,66,68 Three of these studies demonstrated statistically significant increases in QoL outcomes among either participants or family caregivers.43,56,68 Trento et al68 reported higher QoL scores for adults with T1DM within both groups (intervention P < .001; control P < .001), but statistically significant differences between groups were not noted. Fiallo-Scharer et al43 reported no between-group differences in child QoL but did describe a mean increase in parent-reported QoL by 0.61 points per month (P < .05) in the intervention group at 1 of 2 trial sites.43 Price et al56 reported significant mean between-group increases in self-reported QoL by children at 6 (P = .004) and 12 months (P = .004) but not 24 months following the intervention. In contrast, this study demonstrated no significant increases in parental reports of their child’s QoL.56 Interestingly, Noyes et al45 reported decreased total general (P = .024) and diabetes-specificic QoL (P = 0.020) outcomes in children who received a self-study teaching kit. Additionally differences suggestive of lower QoL in 4 of the 5 subscales assessing worry (P = .028), treatment adherence (P = .009), treatment barriers (P = .042) and communication (P = .008) were observed 6 months after the intervention.
One study by Ellis53 equally evaluated metabolic and QoL parameters. This study reported increase in QoL scores from baseline to follow-up in the intervention group only (3.80 ± 0.56 to 4.00 ± 0.50, P = .001), and did not provide analysis for between-groups comparisons.
In the remaining 10 studies (12 reports), QoL was described as a secondary outcome.44,54,55,57,58,60–63,65,67,69 Only 3 studies60,63,67 described significant improvements in QoL measures for people with T1DM. Pyatek et a.58 did not distinguish QoL outcomes by diabetes type; therefore these results were not included in this portion of the review. Guo et al60 reported significant increases in the QoL outcomes in a school-aged intervention cohort of children with T1DM (P = .016) but did not observe these effects in adolescents. Mayer-Davis et al63, demonstrated greater increases in self-reported QoL by adolescents in the intervention group compared to the control (P = .009). In contrast, this significance was not observed for the family caregivers. The Spanish DAFNE study reported a significant improvement in QoL of adults with T1DM over time only within the intervention group (P < .001).67 The authors of this study acknowledged that the Spanish version of Jacobson’s Diabetes Quality of Life (EsDQoL) questionnaire was not comprehensive enough to fully capture the many aspects of self-management for people with T1DM.
In summary, 7 studies detected significant improved effects of DMSES on QoL outcomes. These studies varied in participant populations, selection of QoL tools (generic verses diabetes-specific), intervention type, intervention length, and type of interventionist (Table 5). In contrast, 1 study (Noyes) reported significant intervention-related descreases in general and diabetes-specific QoL.45
Table 5:
Study Characteristics for Statistically Significant Improvements in Quality of Life
| Study | QoL Measure (generic or diabetes specific): Person reporting | Participants | Educational Intervention | Interventionist Types |
|---|---|---|---|---|
|
| ||||
| Ellis et al53 | Diabetes specific: Adolescents intervention group only (P=0.001) Between group: NR |
Adolescent patients (10–18 years old) and caregivers | 6-month DSME group intervention (10 structured modules) | Community health workers |
| Fiallo-Scharer et al43 | 1. Diabetes specific: Children: NS 2. General: Parents intervention group 1 site only. (P <0.05) Between group: NS |
Pediatric patients (8–16 years old) and caregivers | 9-month self-management intervention (small-group education for patient barriers) | Trained professional facilitators |
| Sanchez-Hernandez et al67 | Diabetes specific: Adult patients intervention group only (P=0.0001) Between group: NS |
Adults (>18 years old) | 12-month DSME intervention (5-day group education with continued access) | Nurses and physicians |
| Mayer-Davis et al63 | 1. General: Adolescents IG vs CG (P=0.009) 2. General: Parents IG vs CG: NS |
Adolescent patients (13–16 years old) and caregivers | 18-month FLEX intervention (self-management, motivational interviewing, problem-solving, and family communication) | Care coach nurse or dietitian |
| Guo et al60 | General: Children and young adults IG vs CG School aged (P=0.016) Adolescent: NS |
Pediatric and young adult patients (8–20 years old) | 5-month follow-up. Coping skills training intervention (2-day group camp and monthly calls) | Nurses |
| Price et al56 | General: Adolescents IG vs CG 6 and 12 months (P=0.04) 24 months: NS Parents: NS Diabetes specific: Adolescents: NS Parents: NS |
Adolescent patients (11–16 years old) and caregivers | 5-day group intervention (carbohydrate counting, insulin adjustment, complications) | Nurse and dietitian team |
| Trento et al68 | Diabetes specific: Adult patients Within IG and CG (P<0.0001) IG vs CG: NS |
Adults (30–50 years old) | 30-month carbohydrate counting intervention (8 group education sessions) | doctor, dietitian, nurse |
Abbreviations: IG, Intervention Group; CG, Control Group; NR, not reported, NS; Not significant; DSME, Diabetes Self-Management Education
Clinical Outcomes
A1C was a targeted outcome in 17 reports (15 studies).43–45,53–56,58,59–62,63,65,67–69 Six studies (7 reports) described statistically significant decreases in A1C among the intervention groups.44,53,58,61,62,68,69 A1C change in intervention groups varied from within-groups, between-groups, and over time. Ellis et al53 reported lower A1C within the intervention group from baseline to follow-up [11.73% ± 2.01 vs. 11.03% ± 2.10, P = .05] and between-groups (P = .001) in a sample of adolescents with elevated baseline A1C values. Ismail et al61 reported a significant reduction in the mean A1C from baseline to 12 months within the intervention group (Motivational Enhancement Therapy [MET]+ Cognitive Behavior Therapy [CBT]) group [mean difference 0.59%, 95% CI 0.31 to 0.87; P < .001]. In addition to within-group A1C decreases over time, Ismail et al44 provided 2 reports showing significant reductions in A1C for the MET+ CBT intervention group as compared to the CBT alone and the control group [−0.46% (95% CI, −0.81% to −0.11%; adjusted mean difference 0.43, 95% CI 0.10 to 0.77 (2010)].61 Additionally, Kirwin et al62 observed a sustained reduction in A1C over time for the intervention group as compared to the controls [F = 28.79, P < .001)] and significant differences at each incremental measure of A1C (3, 6, 9 months) [F = 20.7, P < .001]. Of note, there were significant differences in A1C between intervention (9.08%) and control (8.47%) groups at baseline (P = .01).62 Pyatak et al58 reported a decrease of A1C between intervention and control groups with participants with T1DM (P = .01), but this effect was not observed in participants with T2DM. Trento et al68 reported A1C was lower in the carbohydrate counting intervention group as compared to the control group at 30-month follow up (7.2%±0.9 vs 7.9% in±1.4, P < .05). In addition, Weinger et al69 demonstrated significant reductions in A1C levels, however, the structural behavior group showed greater and sustained reductions as compared to the attention control and general control groups (P = .04 for group x time interaction). These A1C decreases were associated with QoL improvements in participants with the highest baseline A1C values.69 In contrast, Dinneen et al57 reported no significant difference between groups, but approximately one-third of participants had an optimal baseline A1C. Some concerns arose this study based on the randomization process and deviations from intended intervention.
Psychosocial Outcomes with Potential Impacts on QoL
The most frequently measured psychosocial outcomes were depressive symptoms (n =5; 6 reports),44,57,61,63,67,69 and self-efficacy (n = 6).56,60,62,66,69 None of the 5 studies (6 reports) that measured depressive symptoms found signifant changes associated with the DSME intervention.44,57,61,63,67,69 Only Mayer-Davis et al63 evaluated depressive symptom outcomes among children and found that the intervention improved symptoms at 18 months, but this findingwas not statistically significant.63
For other psychosocial outcomes targeted in the retained studies, Dinneen et al57,59 reported no differences in diabetes distress, but significant improvements (P < .001) over time within both groups. Mayer-Davis et al63 also reported additional significant improvements in social problem solving (P = .024) and motivation (P = .011), a decrease in family conflict among parent participants (P < .001), and a decrease in worry and hopelessness regarding hypoglycemia among the children (P = .036). Other target outcomes included were psychosocial well-being57,65 and fear of hypoglycemia.44,61,63,67 Adult studies reporting fear of hypoglycemia showed no significant differences between intervention and controls over time.44,61,67 The one pediatric study evaluating hypoglycemia fear reported significant improvements in several sub-domains for parents and adolescents in the intervention group (P < .05).63 The studies that measured psychosocial well-being did not report significant results.57,63 In a study by Trento et al68 examining carbohydrate counting among adults with T1DM, participants reported significant improvement in problem solving (P < .001), seeking support (P < .005), and coping avoidance (P < .005).
Quality and Risk of Bias
Thirteen out of the 17 reported studies had a high Risk of Bias (RoB) in the QoL outcome assessment (Figure 2). Missing outcome data was the primary reason for this higher RoB. Eight studies43,53,55,57,58,65,66,69 had some to high RoB concerns associated with deviations from the planned intervention, most often related to research team members’ or participants’ awareness of group assignment or failure of participants to fully engage in protocol-described education sessions or targeted interventions. Multiple studies accounted for this bias by including the blinding of those collecting data. One study67 had high RoB due to changes in recruitment protocols during the course of the study, while others reported statistically significant differences between groups or participant drop-out prior to intervention implementation. Although 4 studies had some to high RoB in outcome measurement of QoL findings,58,65,67,68 the remaining studies had low risk in this area. Most studies had low risk for bias in selection of the reported QOL result.
Figure 2:
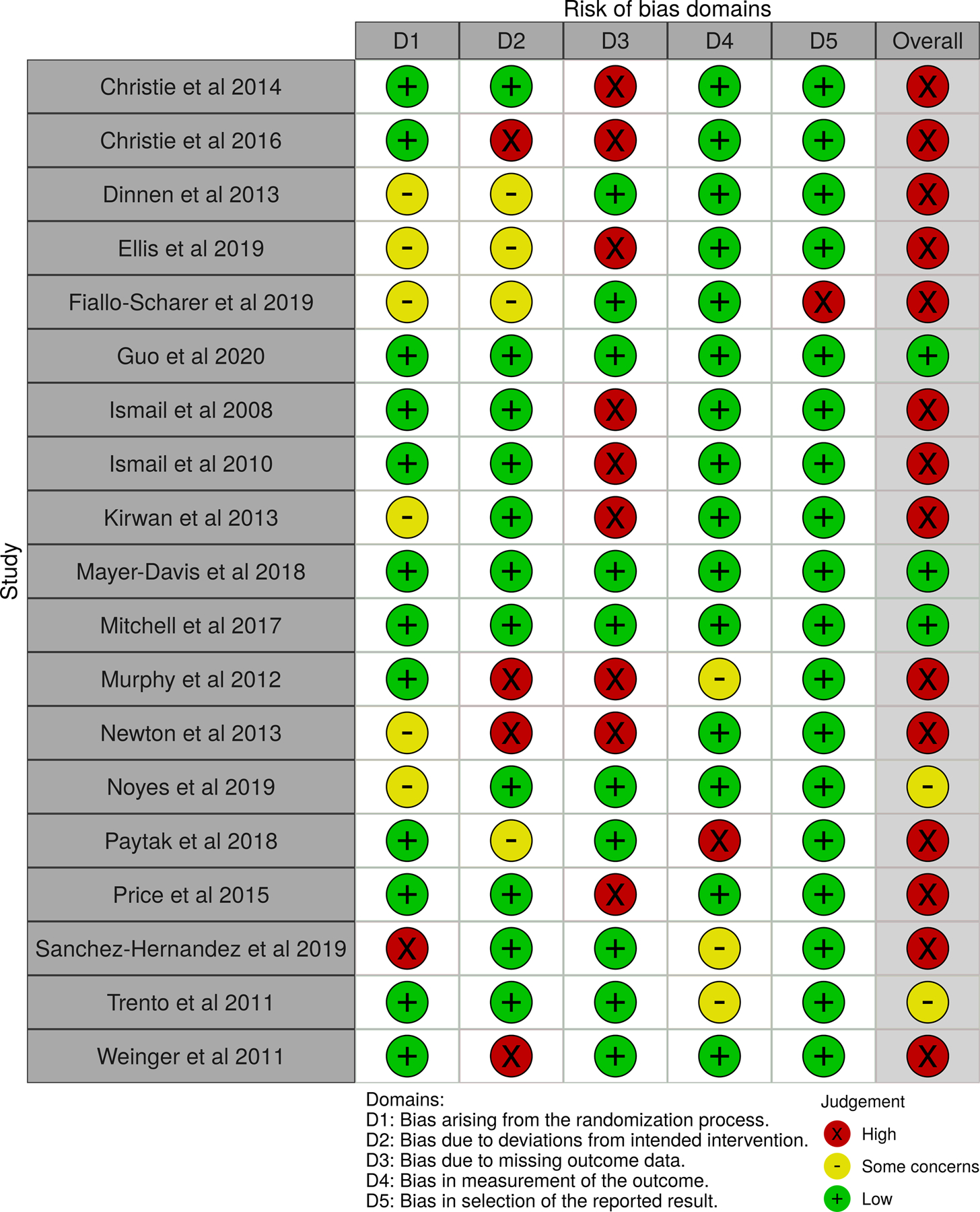
Risk of Bias Data for QoL Outcome Assessment
Similar to RoB assessments for QoL, missing data was a contributor to the higher RoB assessments observed in 14 of the 16 articles reporting the A1C outcome data (Figure 3). Nine of these reports lacked A1C outcome data from at least 95% of study participants across all specified measurement points, primarily due to missed attendance at regular health care visits where these data were most often gathered. RoB concerns for A1C outcome measures in the other domains are comparable to those previously described for QOL outcome measures and included biases related to randomization processes and intervention deviations . Of the 7 articles reporting reductions in A1C,44,53,58,61,62,68,69 5 had higher RoB associated with missing follow-up data.44,53,58,61,62 Weigner et al’s study69 had higher RoB related to deviations from intended interventions.
Figure 3:
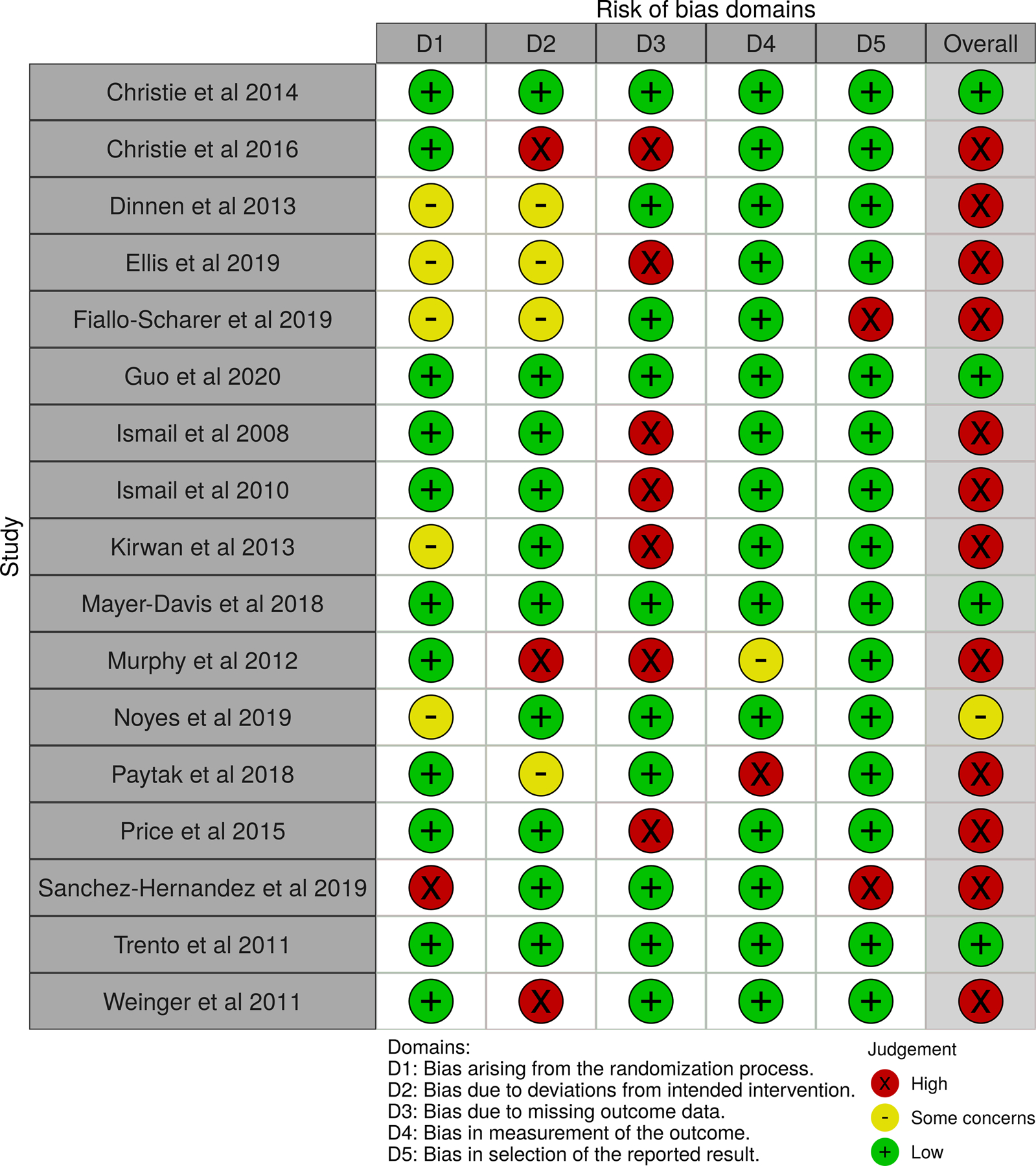
Risk of Bias Data for A1C Outcome Assessment
Discussion
This review found that interventions targeting components of DSME influence QoL outcomes in adults and youth with T1DM. Seven of 17 studies (19 reports) demonstrated positive effects on QoL.43,53,56,60,63,67,68 These studies utilized various components of the ADCES7 Self-Care Behaviors™ and included primarily adolesecents and young adults with T1DM receiving diabetes care in outpatient settings. The discussion summarizes the evidence and gaps for the impact of DSME on QoL, metabolic, and psychosocial outcomes.
Quality of Life Outcomes and Measurement
Only 6 of the retained studies included QoL as a primary outcome,43,45,56,64,66,68 with the majority of the others focusing on metabolic changes as measured by the A1C.44,54,55,57,58,60–63,65,67,69 Three43,45,64 of the 6 studies identifying QoL and 4 of the studies with QoL as an equal or secondary outcome53,58,60,63,67 were conducted following the release of the 2017 Beyond A1C consensus statement.15 The studies in this review that were published after this statement suggest that researchers are beginning to incorporate PROMs, specifically QoL, in current DSME research for people with T1DM. In addition, QoL is becoming more prominent as a secondary outcome.
Studies used general, diabetes-specific, and combined generic/diabetes-specific measures of QoL. Studies with pediatric populations utilized age-specific instruments. Four studies reported significant improvements in QoL through generic tools,43,56,60,63 and the remaining 3 through diabetes-specific measures.53,67,68 Of the studies that reported significant improvements in QoL, 2 targeted adult populations67,68 while 5 included school-age children or adolescents and proxy report of child QoL by a family caregiver.43,53,56,60,63 Both studies that reported improvements in both A1C and QoL53,68 used a diabetes-specific QoL measure. In contrast, studies utilizing generic assessment of QoL did not concurrently demonstrate improvements in both A1C and QoL. This points toward the need for researchers and providers to utilize diabetes-specific tools when assessing for the impact of DSME on QoL outcomes in people with T1DM.
Diabetes Education Interventions and Interventionists
Educational interventions varied by length, structure (group/individual), and content (Table 2). The majority of them used a structured DSME intervention (ADCES 7 or ADA curriculum for adolescents with T1DM43 in US and DAFNE in international settings) across multiple sessions, although some limited the intervention focus to a specific domain of the ADCES7 SelfCare Behaviors™ such as physical activity,64 carbohydrate counting,56,68 or problem-solving strategies including motivational interviewing.44,61 For those with significant changes in QoL outcomes, the intervention was primarily delivered in group settings.43,53,56,60,67,68 In contrast, no studies that utilized technology-enhanced interventions such as web-based66 or text communications62 reported significant changes in QoL outcomes, even when facilitated by a diabetes educator. These findings suggest that direct clinician interaction is an important component to include when developing technology-delivered DSME interventions. Of the studies showing improvements in QoL outcomes, the majority occurred over at least a 6-month period (6 to 30 months) or involved a very intensive dose of DSME over a 2 to 5-day period with follow-up. This finding demonstrates the importance of ongoing self-management support for people with T1DM. This endorses ADCES’s redefinition of diabetes self-management education to include support8 as well as the recently expanded scope of the role and name change of the diabetes educator to the diabetes care and education specialist.6
All studies that reported improved QoL outcomes utilized a team approach, most often with a nurse or dietitian as the primary interventionist (Table 5). Of the entire sample of retained studies, other disciplines including occupational therapists, exercise specialists, or mental health professionals were identified as the lead interventionist. However, no studies, incorporated a pharmacist as a primary interventionist which is considered a gap in the research because pharmacists are the third most common discipline in diabetes care and education.70
Implications for Participant Characteristics and Treatment Mode(s)
Two-thirds of retained studies reported on QoL outcomes of children with T1DM; some addressed parent perceptions of child QoL as well as parental QoL. The participant age range of all studies did not encompass the lifespan. Specifically, the mean age of study participants was 22.9 years, with a mean adult age of 36.2 years. The oldest adult included in retained studies was 51 years-of-age. There were no studies that focused on QoL in older adults with T1DM. The lack of information on mid to older adults with T1DM is an important gap in the research on evaluating the effects of long diabetes duration and comorbid conditions on QoL. As reported in a previous systematic review, there is an urgent need to broaden DSME(S) research on older adults with T1DM.23 Additionally, few studies described participant complication profiles.
Ethnicity profiles of participants were not consistently reported in retained studies, particularly those conducted outside of the United States.57,60,62,64,67,68 Two US studies that were based in large metropolitan areas had disproportionate populations of African American/Black53 and Latino58 individuals and were not congruent with national prevalence rates of T1DM.71 Of studies reporting significant improvements in QoL outcomes, only 3 described ethnic characteristics of participants.43,53,63 All of these studies were conducted in the US and included children or adolescents. This finding suggests there is a gap in specificity of interventions and tools used to ascertain the effects of DSME on QoL in diverse populations of people with T1DM. This finding supports ADCES’ identification of Promoting Person-Centered Care72 and diversity as a core pillar of the organization’s Vision.73 There are research and clinical opportunities to explore avenues that better address individual needs and preferences associated with cultural values and traditions as they relate to DSME(S) and QoL outcomes.
Overall, the retained studies did not focus on participant insulin plan and its impact in the analyses of QoL outcomes in people with T1DM. When reported, participants in international studies primarily utilized MDI treatment plans. In contrast, US studies reported use of continuous subcutaneous insulin infusions (CSII) as a baseline characteristic in at least 50% of the participants. Although utilization CGM is becoming more prevalent in diabetes care, particularly among people with T1DM, only one retained study reporting significant QoL outcomes included data related to CGM use.63 This finding supports the need to better understand the role of the DCES in driving effective implementation of evolving technologies as part of DSME(S) and its subsequent impact on QoL outcomes. This aligns with another of ADCES’s Vision pillars, Leveraging Technology, which strategizes ways to recognize and promote the DCES as an expert in the use of technology in diabetes care.74
Other Psychosocial Outcomes with Potential Impacts on QoL
The majority of studies evaluated at least one psychosocial outcome measure in addition to QoL. Less than 25% of studies assessed behavioral health and self-efficacy outcomes which are considered foundational concepts in effective diabetes self-management.7 Behavioral health outcomes focused exclusively on depressive symptoms. This review found that retained studies in pediatric populations were not concurrently evaluating QoL and depressive symptoms. Similarly, there was limited evaluation of the association between self-efficacy in conjunction with QoL outccomes.
In addition, fear of hypoglycemia was limitedly measured in relation to QoL outcomes. Fischer et al75 and Hessler et al76 asserted that self-efficacy or confidence are integral components for improving self-management and coping with the ongoing tasks associated with T1DM. Overall, only one of the retained articles included measures or discussions of diabetes distress, and these findings were not analyzed with respect to QoL.57 This is an important gap in the research because diabetes distress, persistent worry or fear associated with the daily burdens of diabetes, is a chronic problem in approximately 40% of people with T1DM.77 Diabetes distress adversely impacts disease management and metabolic outcomes.75,76
In the most current revision of the ADCES7 Self-Care Behaviors™, the organization identified healthy coping as the foundational element of diabetes self-management.7 Healthy coping contributes to success in achieving metabolic, clinical, and behavioral health goals associated with the other 6 behaviors described in the framework.7 Similarly, ADCES’s Vision incorporates a Focus on Behavioral Health as a core pillar of DSMES.73 The findings of this review suggest that research incorporating QoL measures currently evaluates QoL or its components in siloes. More comprehensive assessments looking for associations or interaction effects of multiple factors contributing to QoL, including behavioral health concerns, need to be considered in research and practice. This is particularly important as integration of complex technology increases and adds burden to self-management of T1DM.
Strengths and Limitations
A strength of this review is its inclusion of a large sample of RCTs of adequate sample sizes. RCTs are considered the best methodology to answer questions related to patient care and the efficacy of interventions.48 This review utilized robust search and analysis methodologies which incorporated an interdisciplinary perspective, established and valid review processes, and a data management system specific to systematic reviews. Additionally, a research librarian with expertise in search and archiving strategies ensured methodological integrity.
Although this review had several important strengths, there were a number of identified limitations. While this review included the highest level of evidence in research, inclusion of only RCTs may have excluded meaningful studies in real-world settings. Mixed method and qualitative research methods have the potential to capture meaningful patient reported data and an indivdiual’s personal perspectives of QoL or its related components and within the real-life workflow of practice settings beyond an RCT context, particularly when validated and reliable instruments for diabetes-specific QoL are lacking.
Additionally, there are limitations associated with the design and implementation of multiple studies. There was significant heterogeneity in tools that measured QoL, with several only using generic measures of QoL. Overall, the majority of the studies had a high RoB for QoL and metabolic measures for domains related to missing data.
The heterogeneity of interventions also included inconsistent definition of DSME as well as the varied credentials of interventionists in regard to diabetes care expertise. The majority of the studies were international and varied in their health care delivery models, insulin treatment plans, and reporting of demographic data. These differences, including minimal descriptions of ethnic and socioeconomic characteristics of participants, affect the generalizabaility of the outcomes of this review. Another aspect affecting the generalizability of the review findings is the oversampling of females. In addition, the lack of attention to lifespan concerns particularly among adult populations with T1DM over the age of 50 years and limited reporting of comorbid conditions may have impacted the analyses of outcomes.
Implications for Research and Practice
Although the DSME research is moving in the direction of evaluating PROMs, specifically QoL, this investigative focus is in its infancy. The plethora of QoL measures, many of which measure different types of QoL, contribute to difficulty in comparing findings between studies (Table 1). Although many diabetes-specific QoL measures exist, some lack strong validity in specific populations, especially older adults.78 Measures that are validated in specific age-groups are not validated across the lifespan, limiting longitudinal assessment of diabetes-specific QoL. Lastly, these measures are not validated across cultures or countries and may not be easily comparable internationally. Future studies should focus on development of these measures.
These are an important considerations for research and practice of Diabetes Care Education Specialists because diabetes has broad physical and psychosocial impacts on individuals and their families; these need to be considered when assessing QoL outcomes. Some literature2,4 confirms the importance of assessing disease-related burdens for physical, mental, and behavioral impacts, and for a more comprehensive assessment of QoL outcomes. ADCES’s Vision, along with the organization’s support of the 2017 Beyond A1C consensus statement,14 emphasize the importance of Diabetes Care Education Specialists in leading research and practice initiatives that incorporate assessments of components of QoL across the lifespan for people with T1DM.
The findings of this study specifically support the need for research that prompts evidence-based practice for people with T1DM in the following areas: 1) incorporating QoL and its components as a primary research outcome; 2) consistently using validated diabetes-specific measures of QoL in concert with generic instruments; 3) assessing the burden of evolving technologies on general and diabetes-specific QoL; 4) including direct clinician interaction as a component of technological-enabled DSMES interventions; 5) integrating a team approach in DSMES delivery; 6) expanding QoL research to diversify study populations with respect to gender, race, ethnicity, age (older adult), and social determinents of health; 7) connecting study interventions to the defined components of DSMES (ie ADCES7 Self-Care Behaviors™); and 8) including longer duration follow-up in studies evaluating QoL in people with T1DM. Our recommendations can only be implemented if stronger, validated measures for diabetes-related QoL across the lifespan are developed and utilized.
Conclusions
This systematic review demonstrated that DSME has the potential to influence QoL outcomes in people with T1DM. However, due to the mixed findings related to heterogeneity of the tools and DSME used in the retained studies, more standardized methods are needed to delineate impact on a broader range of factors that in turn influence QoL. Future research and practice must address person-specific characteristics, family and environmental factors, and social determinants of health.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge Christina Whitehouse and the Association of Diabetes Care & Education Specialists for their support in completing this study.
Financial Disclosures:
Jan Kavookjian, MBA, PhD, FAPhA, FADCES discloses that she is on the Merck Speakers Bureau for non-product medical education topics including motivational interviewing and others, is a content expert consultant for Merck for person-centered communication and motivational interviewing education materials, and is a consultant for motivational interviewing training for MediMergent, LLC. She is also the president-elect of the Association of Diabetes Care & Education Specialists.
Cassidi C. McDaniel was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR003106. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. McDaniel was also supported by the American Foundation for Pharmaceutical Education (AFPE) under the AFPE Pre-Doctoral Fellowship.
Julia Blanchette is the ADCES/CBDCE Post-Doctoral Fellow in Integrated Diabetes Management.
Kirsten Yehl is on the staff of the Association of Diabetes Care & Education Specialists.
References
- 1.HRQOL concepts. Centers for Disease Control and Prevention. https://www.cdc.gov/hrqol/concept.htm. Published October 31, 2018. Accessed November 18, 2021. https://www.cdc.gov/hrqol/concept.htm [Google Scholar]
- 2.Rydén A, Sörstadius E, Bergenheim K, et al. The humanistic burden of type 1 diabetes mellitus in Europe: examining health outcomes and the role of complications. PLoS One. 2016;11(11):e0164977. doi: 10.1371/journal.pone.0164977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandagriff JL, Marrero DG, Ingersoll GM, Fineberg NS. Parents of children with diabetes: what are they worried about? Diabetes Educ 1992. Jul-Aug;18(4):299–302. doi: 10.1177/014572179201800407. [DOI] [PubMed] [Google Scholar]
- 4.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15(3):205–218. doi: . [DOI] [PubMed] [Google Scholar]
- 5.Varni JW, Delamater AM, Hood KK, et al. PedsQL 3.2 Diabetes Module for Children, Adolescents, and Young Adults: Reliability and Validity in Type 1 Diabetes. Diabetes Care. 2018;41(10):2064–2071. doi: 10.2337/dc17-2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan D, Burke SD, Litchman ML, et al. Competencies for diabetes care and education specialists. Diabetes Educ. 2020;46(4):384–397. doi: 10.1177/0145721720931092. [DOI] [PubMed] [Google Scholar]
- 7.Association of Diabetes Care and Education Specialists, Kolb L. An effective model of diabetes care and education: the ADCES 7 self-care behaviors™. Sci Diabetes Self Manag Care. 2021;47(1):30–53. doi: 10.1177/0145721720978154. [DOI] [PubMed] [Google Scholar]
- 8.Haas L, Maryniuk M, Beck J, et al. National standards for diabetes self-management education and support. Diabetes Educ. 2012;38(5):619–629. doi: 10.1177/0145721712455997. [DOI] [PubMed] [Google Scholar]
- 9.Powers MA, Bardsley JK, Cypress M, et al. Diabetes self-management education and support in adults with type 2 diabetes: a consensus report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. 2020. Diabetes Educ. 2020;46(4):350–369. doi: 10.1177/0145721720930959 [DOI] [PubMed] [Google Scholar]
- 10.Powers MA, Bardsley J, Cypress M, et al. Diabetes self-management education and support in type 2 diabetes. Diabetes Educ. 2017;43(1):40–53. doi: 10.1177/0145721716689694. [DOI] [PubMed] [Google Scholar]
- 11.Chiang JL, Kirkman MS, Laffel LMB, Peters AL. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034–2054. doi: 10.2337/dc14-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amiel SA, Pursey N, Higgins B, Dawoud D. Diagnosis and management of type 1 diabetes in adults: summary of updated NICE guidance. BMJ (Clinical research ed). 2015;351:h4188. doi: 10.1136/bmj.h4188. [DOI] [PubMed] [Google Scholar]
- 13.National Quality Forum. Patient-reported outcomes in performance measurement. National Quality Forum; 2013. Accessed June 17, 2021. https://www.qualityforum.org/Publications/2012/12/Patient-Reported_Outcomes_in_Performance_Measurement.aspx [Google Scholar]
- 14.Agiostratidou G, Anhalt H, Ball D, et al. Standardizing clinically meaningful outcome measures beyond HBA1C for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF international, the Leona M. And Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care. 2017;40(12):1622–1630. doi: 10.2337/dc17-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beyond A1C Writing Group. Need for regulatory change to incorporate beyond A1C glycemic metrics. Diabetes Care. 2018;41(6):e92–e94. doi: 10.2337/dci18-0010. [DOI] [PubMed] [Google Scholar]
- 16.Gagliardino JJ, Chantelot J-M, Domenger C, et al. Impact of diabetes education and self-management on the quality of care for people with type 1 diabetes mellitus in the middle east. Diabetes Res Clin Pract. 2019;147:29–36. doi: 10.1016/j.diabres.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 17.DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ (Clinical research ed). 2002;325(7367):746. doi: 10.1136/bmj.325.7367.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermanns N, Kulzer B, Ehrmann D, Bergis-Jurgan N, Haak T. The effect of a diabetes education programme (primas) for people with type 1 diabetes: results of a randomized trial. Diabetes Res Clin Pract. 2013;102(3):149–157. doi: 10.1016/j.diabres.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Li R, Shrestha SS, Lipman R, Burrows NR, Kolb LE, Rutledge S. Diabetes self-management education and training among privately insured persons with newly diagnosed diabetes--United States, 2011–2012. MMWR. 2014;63(46):1045–1049. [PMC free article] [PubMed] [Google Scholar]
- 20.Sämann A, Mühlhauser I, Bender R, Kloos C, Müller UA. Glycaemic control and severe hypoglycaemia following training in flexible, intensive insulin therapy to enable dietary freedom in people with type 1 diabetes: a prospective implementation study. Diabetologia. 2005;48(10):1965–1970. doi: 10.1007/s00125-005-1905-1. [DOI] [PubMed] [Google Scholar]
- 21.Torres HC, Pace AE, Chaves FF, Velasquez-Melendez G, Reis IA. Evaluation of the effects of a diabetes educational program: a randomized clinical trial. Rev Saude Publica. 2018;52:8. doi: 10.11606/s1518-8787.2018052007132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein HA, Jackson SM, Street K, Whitacre JC, Klein G. Diabetes self-management education: miles to go. Nurs Res Pract. 2013;2013:581012. doi: 10.1155/2013/581012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaManna J, Litchman ML, Dickinson JK, et al. Diabetes education impact on hypoglycemia outcomes: a systematic review of evidence and gaps in the literature. Diabetes Educ. 2019;45(4):349–369. doi: 10.1177/0145721719855931. [DOI] [PubMed] [Google Scholar]
- 24.McCay D, Hill A, Coates V, O’Kane M, McGuigan K. Structured diabetes education outcomes: looking beyond HBA1C. A systematic review. Practical Diabetes. 2019;36(3):86–90h. doi: 10.1002/pdi.2221. [DOI] [Google Scholar]
- 25.Siegel KR, Ali MK, Zhou X, et al. Cost-effectiveness of interventions to manage diabetes: has the evidence changed since 2008? Diabetes Care. 2020;43(7):1557–1592. doi: 10.2337/dci20-0017. [DOI] [PubMed] [Google Scholar]
- 26.Kent D, D’Eramo Melkus G, Stuart PMW, et al. Reducing the risks of diabetes complications through diabetes self-management education and support. Population Health Management. 2013;16(2):74–81. doi: 10.1089/pop.2012.0020. [DOI] [PubMed] [Google Scholar]
- 27.Cochran J, Conn VS. Meta-analysis of quality of life outcomes following diabetes self-management training. Diabetes Educ. 2008;34(5):815–823. doi: 10.1177/0145721708323640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooke D, Bond R, Lawton J, et al. Structured type 1 diabetes education delivered within routine care: impact on glycemic control and diabetes-specific quality of life. Diabetes Care. 2013;36(2):270–272. doi: 10.2337/dc12-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speight J, Holmes-Truscott E, Hendrieckx C, Skovlund S, Cooke D. Assessing the impact of diabetes on quality of life: what have the past 25 years taught us? Diabet Med. 2020;37(3):483–492. doi: 10.1111/dme.14196. [DOI] [PubMed] [Google Scholar]
- 30.Gurková E, Cáp J, Ziaková K. Quality of life and treatment satisfaction in the context of diabetes self-management education. Int J Nurs Pract. 2009;15(2):91–8. doi: 10.1111/j.1440-172X.2009.01733.x. [DOI] [PubMed] [Google Scholar]
- 31.Tang TS, Funnell MM, Noorulla S, Oh M, Brown MB. Sustaining short-term improvements over the long-term: results from a 2-year diabetes self-management support intervention. Diabetes Res Clin Pract. 2012;95(1):85–92. doi: 10.1016/j.diabres.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toobert DJ, Strycker LA, Barrera M, Osuna D, King DK, Glasgow RE. Outcomes from a multiple risk factor diabetes self-management trial for Latinas: ¡Viva bien! Ann Behav Med. 2011;41(3):310–323. doi: 10.1007/s12160-010-9256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Self-management education workshops. Centers for Disease Control and Prevention. Accessed December 20, 2020. https://www.cdc.gov/arthritis/interventions/self_manage.htm [Google Scholar]
- 34.Office of Disease Prevention and Health Promotion. Reduce the proportion of adults with arthritis whose arthritis limits their activities — a‑02. Healthy People 2030. Accessed June 15, 2020. https://health.gov/healthypeople/objectives-and-data/browse-objectives/arthritis/reduce-proportion-adults-arthritis-whose-arthritis-limits-their-activities-02 [Google Scholar]
- 35.Office of Disease Prevention and Health Promotion. Social determinants of health. Healthy People 2030. Accessed June 15, 2020. [Google Scholar]
- 36.Smith-Palmer J, Bae JP, Boye KS, Norrbacka K, Hunt B, Valentine WJ. Evaluating health-related quality of life in type 1 diabetes: a systematic literature review of utilities for adults with type 1 diabetes. Clinicoecon Outcomes Res. 2016;8:559–571. doi: 10.2147/ceor.S114699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DCCT Research Group. Reliability and validity of a diabetes quality-of-life measure for the Diabetes Control and Complications Trial (DCCT). Diabetes Care. 1988;11(9):725–732. doi: 10.2337/diacare.11.9.725. [DOI] [PubMed] [Google Scholar]
- 38.Bott U, Mühlhauser I, Overmann H, Berger M. Validation of a diabetes-specific quality-of-life scale for patients with type 1 diabetes. Diabetes Care. 1998;21(5):757–769. doi: 10.2337/diacare.21.5.757. [DOI] [PubMed] [Google Scholar]
- 39.Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the cross-national Diabetes Attitudes, Wishes and Needs (DAWN) study. Diabet Med. 2005;22(10):1379–1385. doi: 10.1111/j.1464-5491.2005.01644.x. [DOI] [PubMed] [Google Scholar]
- 40.Bradley C, Todd C, Gorton T, Symonds E, Martin A, Plowright R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Qual Life Res. 1999;8(1):79–91. doi: 10.1023/A:1026485130100. [DOI] [PubMed] [Google Scholar]
- 41.Burroughs TE, Desikan R, Waterman BM, Gilin D, McGill J. Development and validation of the Diabetes Quality of Life Brief Clinical Inventory. Diabetes Spectr. 2004;17(1):41–49. doi: 10.2337/diaspect.17.1.41. [DOI] [Google Scholar]
- 42.Ingersoll GM, Marrero DG. A modified quality-of-life measure for youths: psychometric properties. Diabetes Educ. 1991;17(2):114–118. doi: 10.1177/014572179101700219. [DOI] [PubMed] [Google Scholar]
- 43.Fiallo-Scharer R, Palta M, Chewning BA, et al. Impact of family-centered tailoring of pediatric diabetes self-management resources. Pediatr Diabetes. 2019;20(7):1016–1024. doi: 10.1111/pedi.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ismail K, Thomas SM, Maissi E, et al. Motivational enhancement therapy with and without cognitive behavior therapy to treat type 1 diabetes: a randomized trial. Ann Intern Med. 2008;149(10):708–719. doi: 10.7326/0003-4819-149-10-200811180-00005. [DOI] [PubMed] [Google Scholar]
- 45.Noyes J, Allen D, Carter C, et al. Standardised self-management kits for children with type 1 diabetes: pragmatic randomised trial of effectiveness and cost-effectiveness. BMJ Open. 2020;10(3):1–14. doi: 10.1136/bmjopen-2019-032163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varni JW, Sherman SA, Burwinkle TM et al. The PedsQL™ Family Impact Module: Preliminary reliability and validity. Hlth Qual Life Outcomes. 2004;55(2). 10.1186/1477-7525-2-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Association of Diabetes Care and Education Specialists. About ADCES. Accessed June 1, 2021. https://www.diabeteseducator.org/about-adces
- 48.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. Version 6.2. Updated February, 2021. Cochrane; 2021: https://training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Endnote x20 [computer program]. Clarivate Analytics; 2020. [Google Scholar]
- 51.Covidence systematic review software [computer program]. Veritas Health Innovation; 2018. [Google Scholar]
- 52.Sterne JAC, Savović J, Page MJ, et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 53.Ellis DA, Carcone AI, Naar-King S, Rajkumar D, Palmisano G, Moltz K. Adaptation of an evidence-based diabetes management intervention for delivery in community settings: findings from a pilot randomized effectiveness trial. J Pediatr Psychol. 2019;44(1):110–125. doi: 10.1093/jpepsy/jsx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Christie D, Thompson R, Sawtell M, et al. Structured, intensive education maximising engagement, motivation and long-term change for children and young people with diabetes: a cluster randomised controlled trial with integral process and economic evaluation - the CASCADE study. Health Technol Assess. 2014;18(8):1–202. doi: 10.3310/hta18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christie D, Thompson R, Sawtell M, et al. Effectiveness of a structured educational intervention using psychological delivery methods in children and adolescents with poorly controlled type 1 diabetes: a cluster-randomized controlled trial of the CASCADE intervention. BMJ Open Diabetes Res Care. 2016;4(1):1–14. doi: 10.1136/bmjdrc-2015-000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Price KJ, Knowles JA, Fox M, et al. Effectiveness of the Kids in Control of Food (KICk-OFF) structured education course for 11–16 year olds with type 1 diabetes. Diabet Med. 2016;33(2):192–203. doi: 10.1111/dme.12881. [DOI] [PubMed] [Google Scholar]
- 57.Dinneen SF, O’Hara MC, Byrne M, et al. Group follow-up compared to individual clinic visits after structured education for type 1 diabetes: a cluster randomised controlled trial. Diabetes Res Clin Pract. 2013;100(1):29–38. doi: 10.1016/j.diabres.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 58.Pyatak EA, Carandang K, Vigen CLP, et al. Occupational therapy intervention improves glycemic control and quality of life among young adults with diabetes: the Resilient, Empowered, Active Living with Diabetes (REAL Diabetes) randomized controlled trial. Diabetes Care. 2018;41(4):696–704. doi: 10.2337/dc17-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dinneen SF, MC OH, Byrne M, et al. The Irish DAFNE study protocol: a cluster randomised trial of group versus individual follow-up after structured education for type 1 diabetes. Trials. 2009;10:88–88. doi: 10.1186/1745-6215-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo J, Luo J, Yang J, et al. School-aged children with type 1 diabetes benefit more from a coping skills training program than adolescents in China: 12-month outcomes of a randomized clinical trial. Pediatr Diabetes. 2020;21(3):524–532. doi: 10.1111/pedi.12975. [DOI] [PubMed] [Google Scholar]
- 61.Ismail K, Maissi E, Thomas S, et al. A randomised controlled trial of cognitive behaviour therapy and motivational interviewing for people with type 1 diabetes mellitus with persistent sub-optimal glycaemic control: a diabetes and psychological therapies (adapt) study. Health Technol Assess. 2010;14(55):1–218. doi: 10.3310/hta14220. [DOI] [PubMed] [Google Scholar]
- 62.Kirwan M, Vandelanotte C, Fenning A, Duncan MJ. Diabetes self-management smartphone application for adults with type 1 diabetes: randomized controlled trial. J Med Internet Res. 2013;15(11):e235–e235. doi: 10.2196/jmir.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayer-Davis EJ, Maahs DM, Seid M, et al. Efficacy of the flexible lifestyles empowering change intervention on metabolic and psychosocial outcomes in adolescents with type 1 diabetes (flex): A randomised controlled trial. Lancet Child Adolesc Health. 2018;2(9):635–646. doi: 10.1016/S2352-4642(18)30208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitchell F, Wilkie L, Robertson K, Reilly JJ, Kirk A. Feasibility and pilot study of an intervention to support active lifestyles in youth with type 1 diabetes: the ActivPals study. Pediatr Diabetes. 2018(3):443. doi: 10.1111/pedi.12615. [DOI] [PubMed] [Google Scholar]
- 65.Murphy HR, Wadham C, Hassler-Hurst J, Rayman G, Skinner TC. Randomized trial of a diabetes self-management education and family teamwork intervention in adolescents with type1 diabetes. Diabet Med. 2012;29(8):e249–e254. doi: 10.1111/j.1464-5491.2012.03683.x. [DOI] [PubMed] [Google Scholar]
- 66.Newton KT, Ashley A. Pilot study of a web-based intervention for adolescents with type 1 diabetes. J Telemed Telecare. 2013;19(8):443–449. doi: 10.1177/1357633X13512069. [DOI] [PubMed] [Google Scholar]
- 67.Sánchez-Hernández RM, Alvarado-Martel D, López-Plasencia Y, et al. Assessment of Alimentación Normal con Ajuste de Insulina (ANAIS), a Spanish version of the DAFNE programme, in people with type 1 diabetes: a randomized controlled parallel trial. Diabet Med. 2019;36(8):1037–1045. doi: 10.1111/dme.13984. [DOI] [PubMed] [Google Scholar]
- 68.Trento M, Trinetta A, Kucich C, et al. Carbohydrate counting improves coping ability and metabolic control in patients with type 1 diabetes managed by group care. J Endocrinol Invest. 2011;34(2):101–105. doi: 10.3275/7027. [DOI] [PubMed] [Google Scholar]
- 69.Weinger K, Beverly EA, Lee Y, Sitnokov L, Ganda OP, Caballero AE. The effect of a structured behavioral intervention on poorly controlled diabetes: a randomized controlled trial. Arch Intern Med. 2011;171(22):1990–1999. doi: 10.1001/archinternmed.2011.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rinker J, Dickinson JK, Litchman ML, et al. The 2017 diabetes educator and the diabetes self-management education national practice survey. Diabetes Educ. 2018;44(3):260–268. doi: 10.1177/0145721718765446. [DOI] [PubMed] [Google Scholar]
- 71.Centers for Disease Control. National diabetes statistics report 2020: Estimates of diabetes and its burden in the United States. U.S. Dept of Health and Human Services; 2020. Accessed December 19, 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf [Google Scholar]
- 72.Akindana A. Person-centered care: a core pillar of AADE’s vision for the specialty. AADE blog. June 26, 2019. Accessed December 20, 2020. https://www.diabeteseducator.org/news/perspectives/aade-blog-details/adces-perspectives-on-diabetes-care/2019/06/26/person-centered-care-a-core-pillar-of-aade’s-vision-for-the-specialty
- 73.Association of Diabetes Care and Education Specialists. A vision for the future. Accessed May 1, 2021. https://www.diabeteseducator.org/about-adces/For-Diabetes-Care-and-Education-Specialists
- 74.Isaacs D, Cox C, Schwab K, et al. Technology integration: the role of the diabetes care and education specialist in practice. Diabetes Educ. 2020;46(4):323–334. doi: 10.1177/0145721720935123. [DOI] [PubMed] [Google Scholar]
- 75.Fisher L, Hessler DM, Polonsky WH, et al. When is diabetes distress clinically meaningful? establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259–264. doi: 10.2337/dc11-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hessler D, Fisher L, Polonsky W, et al. There is value in treating elevated levels of diabetes distress: the clinical impact of targeted interventions in adults with type 1 diabetes. Diabet Med. 2020;37(1):71–74. doi: 10.1111/dme.14082. [DOI] [PubMed] [Google Scholar]
- 77.Fisher L, Hessler D, Polonsky W, Strycker L, Masharani U, Peters A. Diabetes distress in adults with type 1 diabetes: prevalence, incidence and change over time. J Diabetes Complications. 2016;30(6):1123–1128. doi: 10.1016/j.jdiacomp.2016.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hilliard ME, Minard CG, Marrero DG, et al. Assessing health-related quality of life in children and adolescents with diabetes: development and psychometrics of the Type 1 Diabetes and Life (T1DAL) measures. J Pediatr Psychol. 2020;45(3):328–339. 10.1093/jpepsy/jsz083 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


