Abstract
Objectives:
The 2018–2019 Rwanda Population–based HIV Impact Assessment (RPHIA) was conducted to measure national HIV incidence and prevalence. District-level estimates were modeled to inform resources allocation.
Methods:
RPHIA was a nationally representative cross-sectional household survey. Consenting adults were interviewed and tested for HIV using the national diagnostic algorithm followed by laboratory-based confirmation of HIV status and testing for viral load (VL), limiting antigen (LAg) avidity, and presence of antiretrovirals. Incidence was calculated using normalized optical density ≤ 1·5, VL ≥ 1,000 copies/mL, and undetectable antiretrovirals. Survey and programmatic data were used to model district-level HIV incidence and prevalence.
Results:
Of 31,028 eligible adults, 98·7% participated in RPHIA and 934 tested HIV positive. HIV prevalence among adults in Rwanda was 3·0% (95% CI:2·7–3·3). National HIV incidence was 0·08% (95% CI:0·02–0·14) and 0·11% (95% CI:0·00–0·26) in the City of Kigali (CoK). Based on district-level modeling, HIV incidence was greatest in the 3 CoK districts (0·11% to 0·15%) and varied across other districts (0·03% to 0·10%).
Conclusions:
HIV prevalence among adults in Rwanda is 3.0%; HIV incidence is low at 0.08%. District-level modeling has identified disproportionately affected urban hotspots: areas to focus resources.
Keywords: HIV, Rwanda, Prevalence, Incidence, population-based surveys, Naomi model
Introduction
Over the last decade, Rwanda’s national HIV program has made tremendous achievements in accelerated scale-up of HIV testing, immediate linkage to antiretroviral treatment (ART) for those testing positive (test and treat), and other evidence-based prevention and treatment interventions such as condom availability, targeted HIV testing and index testing, scale up of prevention of mother to child transmission interventions, voluntary medical male circumcision (VMMC), and mass community education with specific focus towards key populations (Rwanda Ministry of Health, 2016, 2018). Collectively, these interventions have resulted in increased ART coverage, (Rwanda Ministry of Health, 2016, 2018, 2020) a decreased proportion of people living with HIV (PLHIV) with unsuppressed viral loads, stable/declining HIV prevalence, and progress towards the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90–90–90 targets (Rwanda Ministry of Health, 2020; National Statistics of Rwanda, 2016; Nsanzimana et al., 2017a).
To measure the current status of the HIV epidemic and the progress of Rwanda’s national HIV response, the Rwanda Population–based HIV Impact Assessment (RPHIA) was conducted in 2018–2019. RPHIA was designed to measure national and City of Kigali (CoK)–specific HIV incidence, national and provincial HIV viral load (VL) suppression, and HIV prevalence. However, RPHIA was not designed to provide HIV incidence, prevalence, and treatment coverage at a district level, which is crucial to better understand localized epidemics and guide the allocation of resources at the district level. To estimate HIV prevalence and incidence at the district level, we used the recently developed UNAIDS Naomi model, which allowed RPHIA data to be incorporated along with routinely collected programmatic data on ART coverage and antenatal HIV testing (UNAIDS, 2021; Eaton et al., 2021).
Here we present sex-specific national, provincial, and district-level estimates of HIV incidence and prevalence in adults aged 15–64 years in Rwanda.
Methods
RPHIA survey methods, sample size, and survey procedures are explained in the survey report.(3) Briefly, RPHIA was a nationally representative, cross-sectional population–based survey of households (HHs) across all 5 provinces in Rwanda. The survey used a 2-stage, stratified cluster sample design involving 375 enumeration areas (EAs) stratified by province using a probability proportional to size sampling approach. Within the sampled EAs, an average of 30 HHs (ranging from 14–60) were randomly selected. Individuals aged 10–64 years who slept in the sampled HH the night before (usual HH members or visitors) were eligible to participate in the survey. The analysis presented here is limited to participants aged 15–64 years.
Participants aged 18–64 years provided written informed consent. Parental or guardian permission and participant assent were required for persons aged 15–17 years. Completed HH and individual questionnaires and field laboratory data were transmitted electronically to a secure cloud server. Laboratory data were cleaned and merged with the final questionnaire data using unique study identification numbers. Anonymized data were used for statistical analyses. Sampling weights were computed to adjust for probability of selection, nonresponse, and noncoverage as previously described (Ministry of Health, 2020).
Laboratory Methods
Consenting participants provided venous blood for household-based HIV testing using the national guidelines, which included 2 tests: the Alere Combo (Alere Determine™ HIV-1/2 Ag/Ab Combo) (Alere Inc., Waltham, Massachusetts, United States) followed by the HIV 1/2 Stat-Pak™ (Chembio Diagnostic Systems, Medford, New York, United States). Blood specimens with a nonreactive result on the first test were classified as HIV negative. Those with a reactive result on both tests were classified as HIV positive. Specimens with a reactive first test result followed by a nonreactive second test result were classified as inconclusive and were excluded from the analysis. Home-based HIV test results were provided to the participants with appropriate counseling and referral to HIV testing and treatment services. All specimens that tested HIV positive during home-based testing were confirmed using the Geenius™ HIV 1/2 Supplemental Assay (Bio-Rad, Hercules, California, United States). A positive Geenius result defined HIV-positive status for the survey.
Plasma or dried blood spots (DBS) samples from individuals with confirmed HIV-positive status were tested to measure VL (HIV RNA copies/mL), using COBAS® TaqMan® Analyser on the COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, v2.0 instrument (Roche Molecular Diagnostics, South Branchburg, New Jersey, United States) for plasma samples. The COBAS AmpliPrep/COBAS TaqMan HIV-1 Test v2.0 free virus elution protocol was used to measure VL from DBS specimens when plasma was insufficient.
Qualitative screening for detectable concentrations of the antiretrovirals (ARVs) efavirenz, tenofovir, nevirapine, and atazanavir was conducted at the University of Cape Town on DBS specimens from HIV-positive participants using high–resolution liquid chromatography coupled with tandem mass spectrometry (Rwanda Ministry of health, 2020). The ARVs were selected based on a review of routine program data on first and second-line ART regimens from a 3-month period before the end of the RPHIA data collection.
A recent infection testing algorithm was used to identify participants with a recent HIV infection. Samples from all confirmed HIV-positive participants were tested using the Maxim HIV-1 Limiting Antigen-Avidity (LAg) enzyme immunoassay (EIA) kit (Maxim Biomedical, Bethesda, Maryland, United States) on DBS and the HIV-1 LAg-Avidity EIA (Sedia Biosciences Corporation, Portland, Oregon, United States) on plasma. Specimens with median normalized optical density (ODn) ≤ 1·5 using plasma or ODn ≤ 1·0 when using DBS, VL ≥ 1,000 copies/mL, and no detectable ARVs were classified as cases of recent HIV infection.
Estimating incidence
Incidence estimates were based on the number of HIV infections identified by the recent infection testing algorithm and obtained using the formula recommended by the WHO Incidence Working Group and Consortium for Evaluation and Performance of Incidence Assays. Assay performance characteristics of a mean duration of recent infection = 130 days (95% CI 118, 142), a time cutoff = 1.0 year, and a percentage false recent = 0.00 were used in the incidence calculation (Rwanda Ministry of Health, 2020).
While RPHIA measured HIV incidence at the national level and in the CoK, too few recent HIV infections were observed in any district to derive a robust estimate of incidence. Therefore, to estimate HIV incidence at the district level, we used the Naomi model (UNAIDS, 2021).
Naomi is a Bayesian small-area estimation model intended for the estimation of HIV prevalence, number of PLHIV, ART coverage, and new HIV infections at the district level by sex and 5-year age groups.
The statistical model incorporated district-level data from the following sources: (1) the RPHIA HH survey data on HIV prevalence and ART coverage (based on self-reported and ARV detection data); (2) routine program data about the number receiving ART; (3) the HIV prevalence and ART coverage among pregnant women attending their first antenatal care (ANC) visit (both derived from the national HIV program indicator data reported from health facilities into the nationwide Rwanda Health Management Information System [RHMIS]); and (4) district population estimates from the 2012 census conducted by the National Institute of Statistics of Rwanda. RPHIA survey clusters were assigned to districts based on geo–masked cluster centroid locations and aggregated by district, sex, and 5-year age group using normalized sample weights. The model produced estimates at 3 time points: the year of the RPHIA survey in late 2018, the current period at which the most recent ART and ANC program data are available, and short-term 1-year ahead projections for HIV program planning purposes (UNAIDS, 2021).
Analyses using RPHIA data alone
Two outcome variables were used in our analysis: confirmed HIV-positive status (based on Geenius confirmatory testing) was used to estimate HIV prevalence, and recent HIV infection status (defined by the recent infection testing algorithm described earlier) was used to estimate HIV incidence.
Sex-specific HIV incidence was calculated at the national and provincial level, by urban/rural residence, and by age. Sex-specific HIV prevalence estimates, disaggregated by sociodemographic characteristics, and sexual behaviors were computed. All results are weighted, unless otherwise noted, to account for sample selection probabilities and adjusted for nonresponse and noncoverage (Rwanda Ministry of Health, 2020). Post-stratification to compensate for noncoverage in the sampling process was done by adjusting the weights so that the sum of each set of weights conformed to national population totals by sex and 5-year age groups from the 2018 national population projections from the 2012 national census. Finally, interview and blood weight normalization factors were applied so that the final sum of weights matched the number of respondents to the interview and blood draw, respectively. Variance was estimated using jackknife replicate weights (RPHIA 2018–2019 Sampling and Weighting Technical Report, 2019). All extrapolations made to the population are based on survey weighting. The data were analyzed using SAS 9.4 1 (SAS Institute Inc., Cary, North Carolina, United States).
To transition from the RPHIA-specific analysis of HIV prevalence at the national and provincial level to the district-level estimates from Naomi, the Naomi-derived HIV prevalence estimates and quantile-based 95% Credible Interval at the provincial level have been presented alongside the RPHIA results (HIV prevalence and 95% Confidence Interval), followed by the district-level estimates of prevalence and incidence from the UNAIDS Naomi model stratified by sex (Eaton et al., 2021).
Lastly, a correlation between HIV prevalence and incidence at the district level was estimated, overall (both sexes) and by sex, to assess the relationship between prevalence and incidence at a granular (district) level in Rwanda. We fit a linear model between incidence and prevalence to measure the slope of a linear fit for incidence as a function of HIV prevalence.
Results
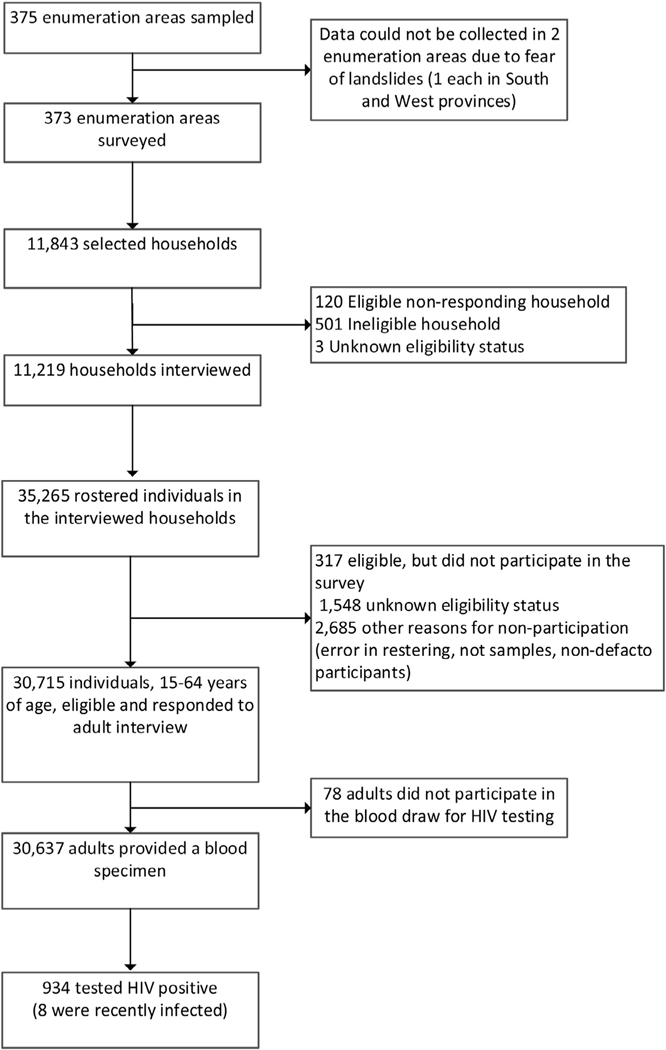
In the 11,219 households which were sampled and responded to the household interview, 35,265 individuals were rostered, 30,715 were eligible for RPHIA participation, and 30,637 provided a blood sample for HIV and other biomarker testing. Of the 30,637 tested for HIV, 934 were confirmed to be HIV positive. (Figure 1).
Figure 1.
Participant flowchart in the Rwanda Population-based HIV Impact Assessment (RPHIA) 2018–2019
HIV prevalence
Overall HIV prevalence was 3·0% (95% CI 2·7–3·3) among adults aged 15–64 years and 2·6% (95% CI 2·3–2·9) among adults aged 15–49 years (Table 1). HIV prevalence was highest among men aged 55–59 years (6·5%; 95% CI 4·1–9·0) and women aged 50–54 years (7·4%; 95% CI 5·6–9·2). National HIV prevalence was significantly higher among women than men (3·7%; 95% CI 3·3–4·1 versus 2·2; 95% CI 1·9–2·6) and a similar pattern was observed in every province. The sex disparity in HIV prevalence was greatest among the young adults aged 20–24 years, with HIV prevalence in women 3-times higher than in men. HIV prevalence varied by province, ranging from 2·2% (95% CI 1·8–2·6) in the north to 4·3% (95% CI 3·5–5·1) in the predominantly urban CoK. At the national level, urban areas had higher HIV prevalence (4·8%; 95% CI 4·0–5·7) than rural areas (2·5%; 95% CI 2·2–2·8), and within each province, urban areas had higher HIV prevalence than rural areas. HIV prevalence was lowest among those who had attained higher education, but it did not differ by wealth quintile. Women who reported first sexual intercourse at age <15 years were 3 times more likely to be HIV positive than men who reported first sexual intercourse at age <15 years. HIV prevalence did not differ by the number of sexual partners among men; among women, however, those who reported having more than 2 partners in the last 12 months had a higher HIV prevalence (13·0%) than those who reported having had 1 partner in the past 12 months (3·2%). HIV prevalence was higher among those who reported having used condoms at last sexual intercourse (8·1% (95% CI 6·7–9·4) than those who reported not having used condoms at the last sexual intercourse (2·3%; 95% CI 1·9–2·7); this difference was much larger among women than men.
Table 1.
Prevalence of HIV among persons aged 15–64 years, by sex and selected demographic, sexual behaviour characteristics, RPHIA, 2018–2019.
| Male | Female | Total | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Characteristic | %HIV positive | N | %HIV positive | N | %HIV positive | N |
| Age | ||||||
| 15–19 | 0.4 (0.2–0.6) | 3,071 | 0.8 (0.4–1.1) | 3,347 | 0.6 (0.4–0.8) | 6,418 |
| 20–24 | 0.6 (0.2–0.9) | 2,217 | 1.8 (1.2–2.4) | 2,723 | 1.2 (0.9–1.5) | 4,940 |
| 25–29 | 1.3 (0.6–1.9) | 1,869 | 3.4 (2.5–4.3) | 2,394 | 2.4 (1.8–2.9) | 4,263 |
| 30–34 | 1.4 (0.8–2.0) | 1,777 | 3.7 (2.7–4.7) | 2,120 | 2.6 (1.9–3.2) | 3,897 |
| 35–39 | 2.9 (2.1–3.8) | 1,567 | 4.5 (3.4–5.6) | 1,770 | 3.7 (3.0–4.5) | 3,337 |
| 40–44 | 4.9 (3.4–6.4) | 950 | 7.1 (5.6–8.6) | 1,342 | 6.1 (4.9–7.3) | 2,292 |
| 45–49 | 5.6 (3.9–7.4) | 716 | 7.0 (5.3–8.8) | 963 | 6.4 (5.0–7.8) | 1,679 |
| 50–54 | 6.3 (4.2–8.4) | 594 | 7.4 (5.6–9.2) | 812 | 6.9 (5.5–8.3) | 1,406 |
| 55–59 | 6.5 (4.1–9.0) | 516 | 5.9 (3.8–7.9) | 728 | 6.2 (4.5–7.8) | 1,244 |
| 60–64 | 3.3 (1.7–4.9) | 503 | 4.4 (2.7–6.1) | 658 | 3.9 (2.7–5.2) | 1,161 |
| Residence | ||||||
| Urban | 3.2 (2.4–3.9) | 3,570 | 6.5 (5.3–7.7) | 4,061 | 4.8 (4.0–5.7) | 7,631 |
| Rural | 2.0 (1.6–2.3) | 10,210 | 3.0 (2.7–3.4) | 12,796 | 2.5 (2.2–2.8) | 23,006 |
| Province | ||||||
| City of Kigali | 3.0 (2.4–3.7) | 2,752 | 5.7 (4.5–6.9) | 2,982 | 4.3 (3.5–5.1) | 5,734 |
| Urban | 3.1 (2.4–3.8) | 2,304 | 5.7 (4.4–7.0) | 2,472 | 4.3 (3.5–5.1) | 4,776 |
| Rural | 2.8 (1.1–4.5) | 448 | 5.4 (2.3–8.4) | 510 | 4.1 (2.0–6.1) | 958 |
| South | 2.3 (1.5–3.1) | 2,712 | 3.4 (2.6–4.2) | 3,414 | 2.9 (2.2–3.6) | 6,126 |
| Urban | 3.6 (0.9–6.4) | 340 | 5.5 (4.1–7.1) | 408 | 4.6 (2.7–6.6) | 748 |
| Rural | 2.1 (1.5–2.7) | 2,372 | 3.1 (2.3–4.0) | 3,006 | 2.6 (2.0–3.3) | 5,378 |
| West | 2.4 (1.6–3.2) | 3,225 | 3.6 (2.6–4.6) | 4,251 | 3.0 (2.2–3.9) | 7,476 |
| Urban | 3.7 (1.7–5.7) | 461 | 7.4 (3.8–11.1) | 637 | 5.8 (3.2–8.4) | 1,098 |
| Rural | 2.2 (1.4–3.0) | 2,764 | 2.9 (2.2–3.5) | 3,614 | 2.5 (1.9–3.2) | 6,378 |
| North | 1.5 (1.2–1.9) | 2,586 | 2.8 (2.2–3.3) | 3,323 | 2.2 (1.8–2.6) | 5,909 |
| Urban | 1.0 (0.0–2.0) | 271 | 6.0 (3.8–8.3) | 317 | 3.5 (1.6–5.4) | 588 |
| Rural | 1.6 (1.2–2.0) | 2,315 | 2.4 (1.9–2.9) | 3,006 | 2.0 (1.6–2.4) | 5,321 |
| East | 2.0 (1.3–2.7) | 2,505 | 3.9 (2.9–4.9) | 2,887 | 2.9 (2.2–3.7) | 5,392 |
| Urban | 3.9 (1.2–6.6) | 194 | 10.2 (6.4–14.0) | 227 | 7.0 (4.3–9.8) | 421 |
| Rural | 1.8 (1.2–2.4) | 2,311 | 3.3 (2.5–4.1) | 2,660 | 2.6 (1.9–3.2) | 4,971 |
| Marital status | ||||||
| Never married | 0.9 (0.6–1.2) | 6,610 | 2.0 (1.6–2.3) | 6,349 | 1.4 (1.1–1.6) | 12,959 |
| Married or living together | 3.0 (2.5–3.5) | 6,740 | 3.0 (2.5–3.5) | 8,008 | 3.0 (2.6–3.6) | 14,748 |
| Divorced or separated | 7.5 (4.7–10.3) | 351 | 8.0 (6.1–9.9) | 1,328 | 7.9 (6.3–9.5) | 1,679 |
| Widowed | 10.8 (3.3–18.3) | 73 | 12.0 (9.9–14.1) | 1,162 | 11.9 (9.9–14.0) | 1,235 |
| Education | ||||||
| No education | 4.3 (2.9–5.8) | 1,004 | 6.0 (4.8–7.3) | 1,865 | 5.4 (4.4–6.4) | 2,869 |
| Primary | 2.3 (1.9–2.7) | 8,431 | 3.9 (3.4–4.5) | 9,931 | 3.2 (2.7–3.6) | 18,362 |
| Secondary | 1.5 (1.0–1.9) | 3,558 | 2.3 (1.8–2.8) | 4,397 | 1.9 (1.5–2.2) | 7,955 |
| More than secondary | 1.1 (0.3–1.9) | 782 | 2.2 (0.7–3.6) | 648 | 1.5 (0.7–2.4) | 1,430 |
| Wealth quintile | ||||||
| Lowest | 2.2 (1.5–3.0) | 2,166 | 3.1 (2.4–3.9) | 3,123 | 2.7 (2.1–3.4) | 5,289 |
| Second | 1.6 (1.1–2.1) | 2,396 | 2.8 (2.1–3.5) | 3,166 | 2.3 (1.8–2.7) | 5,562 |
| Middle | 2.3 (1.6–2.9) | 2,635 | 3.2 (2.6–3.8) | 3,169 | 2.8 (2.2.3.3) | 5,804 |
| Fourth | 2.4 (1.8–3.0) | 2,864 | 4.7 (3.8–5.6) | 3,230 | 3.6 (2.9–4.2) | 6,094 |
| Highest | 2.5 (1.9–3.1) | 3,707 | 4.7 (3.7–5.6) | 4,162 | 3.6 (2.9–4.2) | 7,869 |
| Pregnancy status | ||||||
| Currently pregnant | NA | NA | 2.3 (1.3–3.2) | 979 | NA | NA |
| Not currently pregnant | NA | NA | 3.8 (3.4–4.3) | 15,729 | NA | NA |
| Age at first sexual intercourse | ||||||
| <15 | 1.7 (0.8–2.6) | 980 | 5.8 (3.8–7.9) | 563 | 3.1 (2.1–4.1) | 1,543 |
| 15–19 | 2.9 (2.3–3.5) | 3,708 | 5.9 (5.0–6.8) | 5,922 | 4.7 (4.0–5.3) | 9,630 |
| 20–24 | 2.9 (2.3–3.5) | 3,552 | 3.2 (2.7–3.8) | 4,801 | 3.1 (2.6–3.5) | 8,353 |
| ≥25 | 2.6 (1.8–3.4) | 2,081 | 3.5 (2.4–4.5) | 1,625 | 3.0 (2.3–3.6) | 3,706 |
| Number of sexual partners in the past 12 months | ||||||
| 0 | 2.4 (1.6–3.2) | 1,611 | 7.2 (6.2–8.3) | 3,007 | 5.4 (4.7–6.2) | 4,618 |
| 1 | 2.6 (2.1–3.0) | 6,944 | 3.2 (2.7–3.7) | 9,313 | 2.9 (2.5–3.3) | 16,257 |
| ≥2 | 3.8 (2.8–4.8) | 1,809 | 13.0 (9.8–16.1) | 631 | 5.9 (4.7–7.0) | 2,440 |
| Condom use at last sexual intercourse in the past 12 months | ||||||
| Used condom | 6.5 (5.0–7.9) | 1,446 | 10.5 (8.2–12.7) | 1,103 | 8.1 (6.7–9.4) | 2,549 |
| Did not use condom | 2.0 (1.6–2.4) | 6,599 | 2.6 (2.2–3.1) | 8,562 | 2.3 (1.9–2.7) | 15,161 |
| No sexual intercourse in the past 12 months | 2.4 (1.6–3.2) | 1,611 | 7.2 (6.2–8.3) | 3,007 | 5.4 (4.7–6.2) | 4,618 |
| Total 15–24 | 0.5 (0.3–0.7) | 5,288 | 1.2 (0.9–1.5) | 6,070 | 0.9 (0.7–1.1) | 11,358 |
| Total 15–49 | 1.8 (1.5–2.1) | 12,167 | 3.3 (2.9–3.8) | 14,659 | 2.6 (2.3–2.9) | 26,826 |
| Total 15–64 | 2.2 (1.9–2.6) | 13,780 | 3.7 (3.3–4.1) | 16,857 | 3.0 (2.7–3.3) | 30,637 |
NOTES:
Weighted figures calculated using final blood test weights.
The sum of the sample sizes for a given classification may be less than the total sample size because of missing responses to the classification variable.
Applying the HIV prevalence measured in RPHIA to the 2018 population projection of people aged 15–64 years based on the 2012 national census, we estimated the number of PLHIV aged 15–64 years in 2018 in Rwanda to be 210,200 (95% CI 186,400–234,000). Distribution of PLHIV in Rwanda by age group was as follows: 20,800 (95% CI 16,100–25,600) in the 15–24 year age-band; 48,100 (95% CI 39,200–57,000) in the 25–34 year age-band; and 88,700 (95% CI 75,700–101,700) in the 35–49 year age-band (not shown in table).
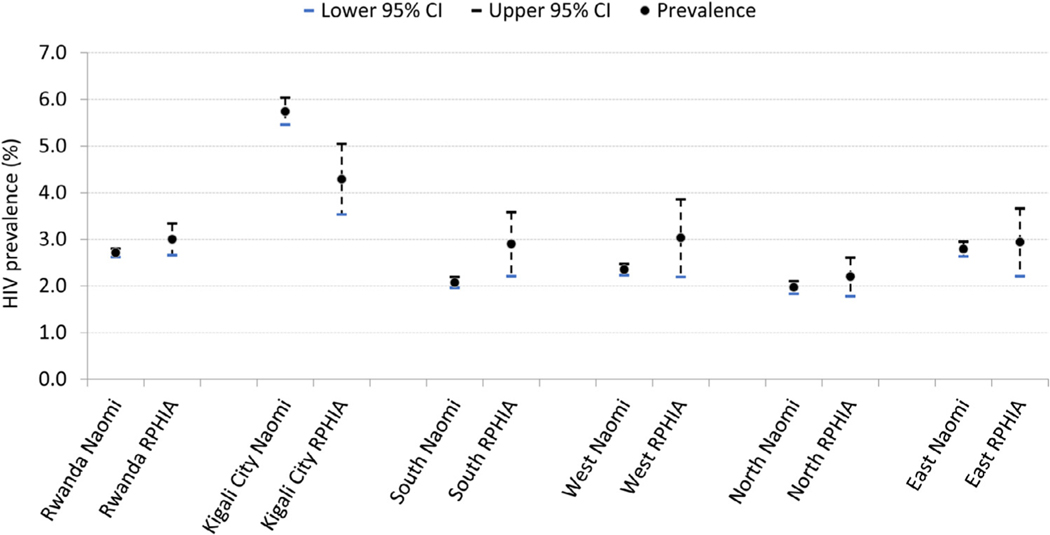
HIV prevalence estimates from RPHIA and the Naomi model at the provincial level are shown in Figure 2.
Figure 2.
HIV prevalence in Rwanda measured in RPHIA and the estimate from Naomi model, at the national and provincial level, 2018–2019.
Footnote: The error bars represent RPHIA 95% confidence intervals for the RPHIA estimates and quantile-based 95% credible intervals for the Naomi estimates
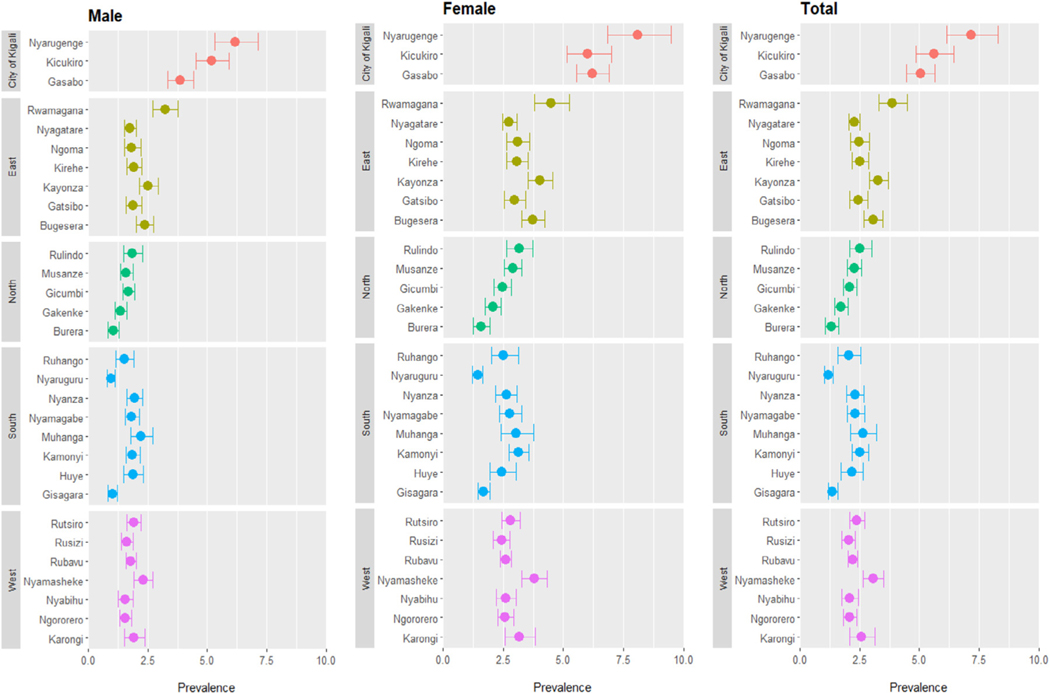
District-level HIV prevalence estimates from the Naomi model were greatest among the 3 districts of the CoK, ranging from 5·1% (95% CI 4·5–5·7) in Gasabo to 7·2% (95% CI 6·1–8·3) in Nyarugenge. There was substantial variation in HIV prevalence across districts, ranging from 1·2% (95% CI 1·0–1·4) in Nyaruguru in the southern province to 7·2% (95% CI 6·1–8·3) in Nyarugenge in the CoK (Figure 3; Supplemental Table 1).
Figure 3.
Naomi model–based estimates of HIV prevalence and 95% credible intervals by district and gender among adults aged 15–64 years, 2018–19.
Footnote: The solid dots represent the point estimates and the error bars represent the quantile-based 95% credible intervals derived from the Naomi model.
HIV Incidence
Eight of the 934 HIV-positive participants in RPHIA were classified as having a recent HIV infection based on the recent infection testing algorithm. Based on these 8 cases, the annual incidence of HIV infection among adults 15–64 years was estimated as 0·08% (95% CI 0·02–0·14) in Rwanda and 0·11% (95% CI 0·00%–0·26%) in the CoK. HIV incidence was 0·09% (95% CI 0·00–0·17) among men and 0·07% (95% CI 0·00–0·15) among women; 0·12% (95% CI 0·00–0·27) in urban areas, and 0·07% (95% CI 0·01–0·13) in rural areas (Table 2).
Table 2.
Annual HIV incidence by residence, province, age, and sex, using the recent infection testing algorithm (using limiting antigen [Lag], viral load [VL], and antiretroviral [ARV] biomarker), RPHIA, 2018–2019.
| Number of estimated HIV positive and HIV recent infections | Annual HIV incidence | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| Characteristic | Male | Female | Total | Male | Female | Total | |||||||||
|
|
|
|
|
|
|
||||||||||
| No. of HIV −ve1 (N) | No. of HIV +ve (P) tested on LAg assay1 (Q) | No. of HIV recent1 (R) | Number HIV −ve1 (N) | No. of HIV +ve (P) tested onLAg assay1(Q) | Number HIV recent1 (R) | No. of HIV −ve1 (N) | No. of HIV +ve (P) tested onLAg assay1 (Q) | No. of HIV recent1 (R) | Percentage annual incidence1 | 95% CI | Percentage annual incidence1 | 95% CI | Percentage annual incidence1 | 95% CI | |
| Residence 2 | |||||||||||||||
| Urban | 3456.7 | 113.3 | 0.8 | 3796.4 | 264.6 | 2.4 | 7261.7 | 369.3 | 3.1 | 0.07 | 0.00–0.21 | 0.18 | 0.00–0.46 | 0.12 | 0.00–0.27 |
| Rural | 10008.8 | 201.2 | 3.3 | 12408.3 | 387.7 | 2.2 | 22424.4 | 581.6 | 5.5 | 0.09 | 0.00–0.20 | 0.05 | 0.00–0.12 | 0.07 | 0.01–0.13 |
| Province 2 | |||||||||||||||
| City of Kigali | 2668.9 | 83.1 | 1.1 | 2812.8 | 169.2 | 1.1 | 5488.0 | 246.0 | 2.2 | 0.11 | 0.00–0.33 | 0.11 | 0.00–0.32 | 0.11 | 0.00–0.26 |
| South | 2649.8 | 62.2 | 0.0 | 3296.8 | 117.2 | 2.0 | 5948.5 | 177.5 | 1.9 | 0.00 | 0.00–0.39 | 0.17 | 0.00–0.42 | 0.09 | 0.00–0.22 |
| West | 3147.9 | 77.1 | 2.0 | 4099.3 | 151.7 | 1.0 | 7249.8 | 226.2 | 3.1 | 0.17 | 0.00–0.42 | 0.07 | 0.00–0.21 | 0.12 | 0.00–0.25 |
| North | 2545.9 | 40.1 | 0.0 | 3231.2 | 91.8 | 0.0 | 5779.2 | 129.8 | 0.0 | 0.00 | 0.00–0.41 | 0.00 | 0.00–0.32 | 0.00 | 0.00–0.18 |
| East | 2455.4 | 49.6 | 1.1 | 2775.1 | 111.9 | 0.0 | 5233.6 | 158.4 | 1.2 | 0.13 | 0.00–0.37 | 0.00 | 0.00–0.37 | 0.06 | 0.00–0.19 |
| Age | |||||||||||||||
| 15–24 | 5263.1 | 24.9 | 0.9 | 5994.7 | 75.3 | 1.3 | 11260.1 | 97.9 | 2.2 | 0.05 | 0.00–0.15 | 0.06 | 0.00–0.20 | 0.06 | 0.00–0.14 |
| 25–34 | 3597.2 | 48.8 | 3.1 | 4355.0 | 159.0 | 0.7 | 7959.3 | 200.7 | 4.0 | 0.24 | 0.00–0.51 | 0.04 | 0.00–0.14 | 0.14 | 0.00–0.28 |
| 35–49 | 3098.8 | 134.2 | 0.0 | 3832.6 | 242.4 | 1.0 | 6935.2 | 372.8 | 1.0 | 0.00 | 0.00–0.33 | 0.08 | 0.00–0.22 | 0.04 | 0.00–0.12 |
| 15–49 | 11952.0 | 215.0 | 4.2 | 14169.1 | 489.9 | 3.0 | 26134.1 | 691.9 | 7.3 | 0.10 | 0.00–0.20 | 0.06 | 0.00–0.13 | 0.08 | 0.02–0.14 |
| 15–64 | 13473.2 | 306.8 | 4.1 | 16232.0 | 625.0 | 4.3 | 29718.6 | 918.4 | 8.4 | 0.09 | 0.00–0.17 | 0.07 | 0.00–0.15 | 0.08 | 0.02–0.14 |
Weighted number
Residence figures are among adults aged 15–64 years.
Note: mean duration recent infection = 130 days (95% CI 118–142 days); proportion false recent = 0.00; time cutoff = 1 year
RPHIA was designed to estimate incidence of HIV at the national level and in the City of Kigali.
Although incidence was estimated for the other provinces, these estimates should be interpreted with caution.
Based on these incidence estimates, the extrapolated number of new HIV infections in Rwanda in 2018–2019 was 5,400 (95% CI 1,400–9,400).
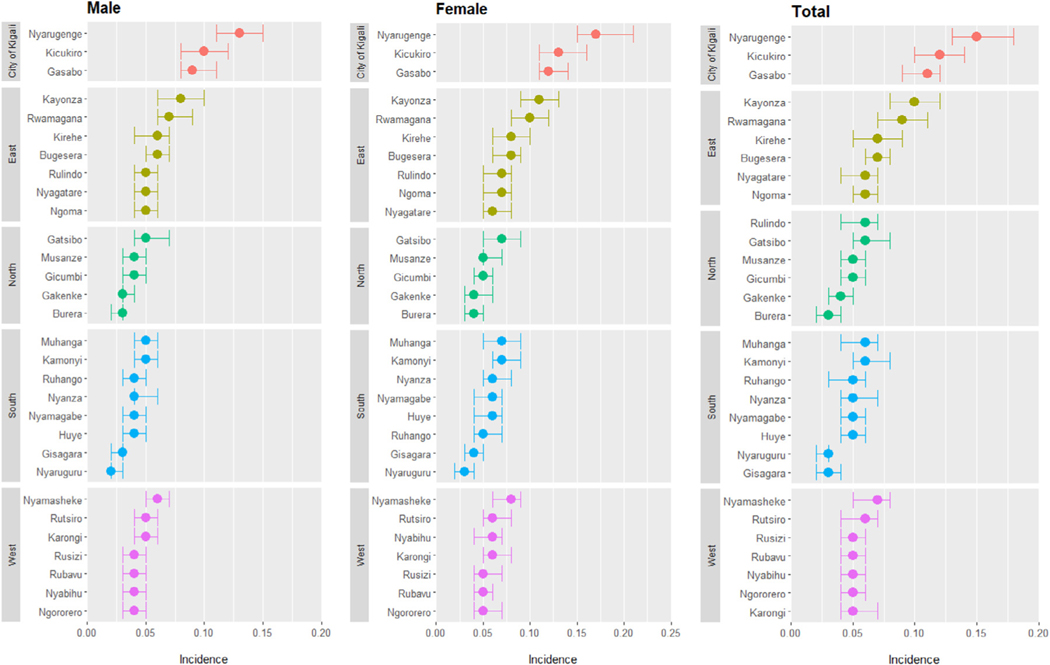
Based on UNAIDS Naomi district-level modeling, HIV incidence was highest in the 3 districts that make up the CoK–0·11% (95% CI 0·09–0·12) in Gasabo, 0·12% (95% CI 0·10–0·14) in Kicukiro, and 0·15% (95% CI 0·13–0·18) in Nyarugenge. HIV incidence varied across the 30 districts of Rwanda; outside of the CoK, HIV incidence ranged from 0·03% (95% CI 0·02–0·04) in Burera to 0·10% (95% CI 0·08–0·12) in Kayonza in the eastern province (Figure 4).
Figure 4.
Naomi model–based estimates of HIV incidence and 95% credible intervals by district and gender among adults aged 15–64 years, 2018–19.
Footnote: The solid dots represent the point estimates and the error bars represent the quantile-based 95% credible intervals derived from the Naomi model.
Correlation between HIV incidence and prevalence at a district level
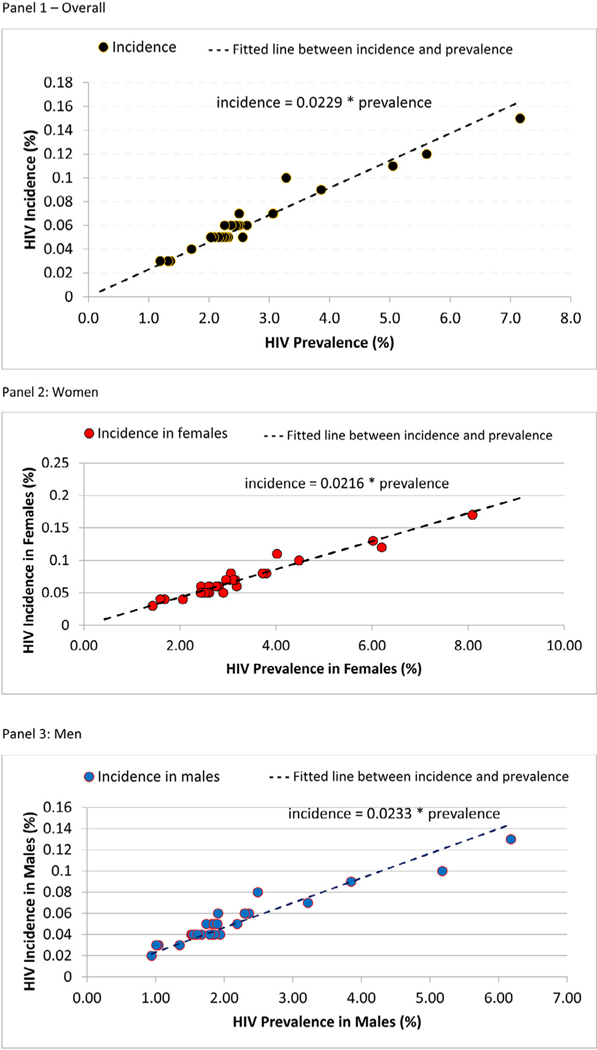
Plotting district-level HIV incidence and prevalence, overall and by sex, using the UNAIDS Naomi model shows a strong correlation between these 2 measures (Figure 5). Using our findings, we can estimate incidence in females using the equation y = 0.0216 ∗ prevalence (females) and in males using the equation y = 0.0233 ∗ prevalence (males).
Figure 5.
Correlation between HIV incidence and prevalence at the district level, overall (Panel 1) and by gender (Panel 2 shows women and Panel 3 shows men), among adults aged 15–64 years old in Rwanda, 2018–2019
Panel 1 – Overall
Panel 2: Women
Panel 3: Men
Note that HIV incidence and prevalence at the district level were estimated from the Naomi model.
Discussion
We described Rwanda’s national HIV prevalence and incidence using empirical data from RPHIA conducted in 2018–2019. HIV incidence in RPHIA, using the recent infection testing detection algorithm, was lower than the previous estimate in 2015 of 0·27% (95% CI 0·13–0·35) (Nsanzimana et al., 2017a; Rwanda Ministry of Health 2020). We also reported on the district-level HIV incidence and prevalence estimates using the Naomi small area estimates model which provides granular level evidence of the status of the epidemic for program planning and resource allocation.
Through the nationally representative RPHIA survey conducted from 2018–2019, a national HIV incidence of 0·08% was estimated in Rwanda. The last empirical measurement of HIV incidence in Rwanda was through the Rwanda AIDS Indicator and HIV Incidence Survey in 2014–2015, which used a prospective cohort design of following up HIV-negative individuals and retesting them after a year of follow-up (Nsanzimana et al., 2017a). This study measured HIV incidence of 0·27% (95% CI 0·13–0·35) but used a different study design and HIV testing methods. The 2019 HIV incidence calculated through the UNAIDS Spectrum model was 0·06% which takes into account HIV program data, demographic information, and other surveillance and survey data. Taken together, HIV incidence in Rwanda is low, but subnational analyses using RPHIA together with other programmatic data have identified areas of higher incidence which are otherwise masked by the national average.
HIV prevalence in Rwanda has stabilized at 3·0% over the last 15 years from 3·0% in 2005, 2010, and 2014–2015 measured in the Rwanda Demographic Health Survey (RDHS) and Rwanda AIDS Indicator and HIV Incidence Survey to 2·6% measured in RPHIA 2018–2019 (National Institute of Statistics Rwanda, 2006, 2012, 2016; Nsanzimana et al., 2017a). HIV prevalence among those aged 15–64 years remained at 3·0%. The shift in HIV prevalence may reflect the cohort effect of aging PLHIV, especially as the peak age of HIV prevalence has been shifting to the older age groups over time and is consistent with declining mortality among PLHIV due to “Treat All” with high coverage of ART and a declining HIV incidence in Rwanda (Nsanzimana et al., 2017a, 2017b; Binagwaho et al., 2014, Nash et al., 2018).
A key finding from RPHIA was the high HIV prevalence seen in urban areas in provinces outside of the CoK, an observation made by other studies in sub–Saharan Africa (Borgdorff et al., 2018; Vandormael et al., 2019; Lesotho, 2019). Compared with rural areas, urban areas in Rwanda have a larger population size of female sex workers, men who have sex with men, and persons who inject drugs (Ingabire et al., 2019; Mutagoma et al., 2015). The point estimates for HIV prevalence in the CoK have decreased from 6·3% to 3·7% in the last 5 years for those aged 15–49 years (Nsanzimana et al., 2017a; Rwanda Ministry of Health 2020). This decline in prevalence in the CoK may be attributed to the reduced number of new infections due to increased ART coverage, substantial numbers of PLHIV aging from the 15–49 age group to the 50+ age group, and population increase in the CoK with more HIV-negative male youths coming from the villages to look for work. It is important to note that there may be methodologic differences that may contribute to these observed differences between the RDHS and RPHIA estimates; DHS did not stratify by age and sex and young men are less likely to participate in a HH survey. Of note, this decrease in HIV prevalence may also be related to the expansion of the CoK boundaries to include more rural areas in the last 5–10 years.
In our study, HIV prevalence was higher among those who reported having used condoms in the last sexual intercourse. Among PLHIV in Rwanda, 83·8% are aware of their HIV-positive status (Rwanda Ministry of Health, 2020), and receive counseling and access to condoms, which might contribute to high condom usage among those who are HIV-positive (Rwanda Ministry of Health, 2016, 2018, 2020). Similar findings were reported in a study in South Africa where men and women who reported condom use at last sexual intercourse were at increased risk of HIV, either due to inconsistent use of condoms or due to social desirability bias in self-reporting condom use (Mabaso et al., 2019). U=U policy was launched in Rwanda in October 2021 and will guide HIV prevention interventions going forward (KT Press, 2021).
District-level modeling for HIV prevalence and incidence has provided a better understanding of the micro-level epidemics in Rwanda. The use of population-based survey data along with other programmatic data provided district-level estimates with greater precision which could not be obtained through RPHIA alone. These modeled estimates have helped identify districts outside of CoK as areas of high incidence and help with resource allocation and planning of interventions. However, further interrogation of the data inputs into Naomi is required.
Data to estimate HIV incidence at the district level are very sparse, and so the district incidence estimates are required to make many assumptions, for example, the sex ratio and age pattern of incidence are assumed to be the same across all districts. We also show that using HIV prevalence and incidence from the Naomi model, we can construct an equation that can be used to determine the incidence of subnational populations given known HIV prevalence for that population and geography. Measuring incidence at small geographical areas is expensive and this correlation between prevalence and incidence would help obtain estimates of incidence without undertaking direct measurements of the same.
Conclusion
HIV prevalence in Rwanda has stabilized at 3·0% over the last 15 years; RPHIA demonstrated a prevalence of 2·6% among those aged 15–49 years and a maintenance of prevalence at 3·0% among those aged 15–64 years, indicating a cohort effect of aging PLHIV. Urban settings other than the CoK have been disproportionately affected and interventions addressing prevention, care, and treatment, and retention in care need to be adapted in these smaller geographies in light of RPHIA and district-level findings. Maintaining the gains made by the national HIV response is critical. Findings from this study will help the national HIV program in Rwanda to streamline its future interventions and priorities to achieve sustained epidemic control.
Supplementary Material
Acknowledgments
The authors acknowledge the commitment and contribution of all members of the RPHIA survey technical working group who worked tirelessly to complete this survey. We also acknowledge all field staff including data collectors, individuals at health centers, and decentralized local government entities for their commitment and rigor in gathering the data presented here. We acknowledge UNAIDs staff and partners who produced the Naomi estimates used for comparison with RPHIA results. The results from this survey were partly presented at the ICASA conference (oral presentation abstract).
Funding
This research has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of the cooperative agreement #U2GGH001226.
Footnotes
Ethical approval statement
Human subjects and ethical approval for the RPHIA survey was granted by the Rwanda National Ethics Committee (Ref: IRB-00001497) and the Institutional Review Boards of the United States Centers for Disease Control and Prevention ([CDC; Atlanta, Georgia, US] [Ref: #6760] and Columbia University Irving Medical Center [Ref. IRB-AAAR8357]).
Disclaimer
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the funding agencies.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.01.032.
References
- Binagwaho A, Farmer PE, Nsanzimana S, Karema C, Gasana M, de Dieu Ngirabega J, et al. Rwanda 20 years on: investing in life. The Lancet 2014;384(9940):371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgdorff MW, Kwaro D, Obor D, Otieno G, Kamire V, Odongo F, Owuor Patrick Jacques Muthusi Mills Lisa A Joseph R, Schmitz EM, Young WP, Zielinski-Gutierrez E, De Cock MK. HIV incidence in western Kenya during scale-up of antiretroviral therapy and voluntary medical male circumcision: a population-based cohort analysis. The Lancet HIV 2018;5(5):e241–e2e9. [DOI] [PubMed] [Google Scholar]
- Eaton JW, Dwyer-Lindgren L, Gutreuter S, O’Driscoll M, Stevens O, Bajaj S, Ashton R, Hill A, Russell E, Esra R, Dolan N, Anifowoshe YO, Woodbridge M, Fellows I, Glaubius R, Haeuser E, Okonek T, Stover J, Thomas ML, Wakefield J, Wolock TM, Berry J, Sabala T, Heard N, Delgado S, Jahn A, Kalua T, Chimpandule T, Auld A, Kim E, Payne D, Johnson LF, FitzJohn RG, Wanyeki I, Mahy MI, Naomi Shiraishi RW. a new modelling tool for estimating HIV epidemic indicators at the district level in sub-Saharan Africa. Journal of the International AIDS Society 2021;24(S5):e25788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingabire R, Parker R, Nyombayire J, Ko JE, Mukamuyango J, Bizimana J, Price AM, Laufer D, Tichacek A, Wall K, Allen S, Karita E. Female sex workers in Kigali, Rwanda: a key population at risk of HIV, sexually transmitted infections, and unplanned pregnancy. International journal of STD & AIDS 2019;30(6):557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesotho PHIA. Lesotho Population-Based Hiv Impact Assessment lePHIA 2016–2017 [Intenet]. 2019. [cited 2021 April 21]. Available from: https://phia.icap.columbia.edu/lephia-final-report/
- Mabaso M, Makola L, Naidoo I, Mlangeni LL, Jooste S, Simbayi L. HIV prevalence in South Africa through gender and racial lenses: results from the 2012 population-based national household survey. Int J Equity Health 2019;18(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutagoma M, Kayitesi C, Gwiza A, Ruton H, Koleros A, Gupta N, Balisanga H, Riedel JD, Nsanzimana S. Estimation of the size of the female sex worker population in Rwanda using three different methods. International journal of STD & AIDS 2015;26(11):810–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash D, Yotebieng M, Sohn AH. Treating all people living with HIV in sub-Saharan Africa: a new era calling for new approaches. J Virus Erad 2018;4(2):1–4 Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of statistics Rwanda (NISR). Rwanda demographic and health Survey 2005 [Internet]. 2006. [cited 2021 Nov 15]. Available from: https://dhsprogram.com/pubs/pdf/FR183/FR183.pdf
- National Institute of statistics Rwanda (NISR). Rwanda demographic and health Survey 2010 [Internet]. 2012. [cited 2021 Nov 15]. Available from: https://dhsprogram.com/publications/publication-fr259-dhs-final-reports.cfm
- National Institute of Statistics Rwanda (NISR). Rwanda Demographic and Health Survey 2014–15 [Internet]. 2016. [cited 2020 Jun 1]. Available from: www.DHSprogram.com.
- Nsanzimana S, Remera E, Kanters S, Mulindabigwi A, Suthar AB, Uwizihiwe JP, Mwumvaneza M, Mills E, Bucher H. Household survey of HIV incidence in Rwanda: a national observational cohort study. The Lancet HIV 2017a;4(10):e457–ee64. [DOI] [PubMed] [Google Scholar]
- Nsanzimana S, Remera E, Ribakare M, Burns T, Dludlu S, Mills EJ, Condo J,HC, Ford N. Phased implementation of spaced clinic visits for stable HIV-positive patients in Rwanda to support Treat All. J Int AIDS Soc. 2017b;20(4):21635 Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press KT. Rwanda Launches WHO Campaign To Improve HIV/AIDS Treatment; 2021. October 1 [press release] https://www.ktpress.rw/2021/10/rwanda-launches-who-campaign-to-improve-hiv-aids-treatment/.
- RPHIA 2018–2019 Sampling and weighting technical Report [Internet]. 2019. [cited 2021 December 12]. Available from: PHIA Data Manager; (columbia.edu). [Google Scholar]
- Rwanda Ministry of Health. Rwanda National HIV and Viral Hepatitis Annual report 2016–2017[Internet]. 2016. [Cited 2021 Feb 10]. Available from: https://rbc.gov.rw/fileadmin/user_upload/report2019/report2019/AnnualReportforHIV2015-2016.pdf.
- Rwanda Ministry of Health. Rwanda National HIV and Viral Hepatitis Annual report 2017–2018 [Internet]. 2018. [cited 2021 Feb 10]. Available from: https://rbc.gov.rw/fileadmin/user_upload/report2019/report2019/AnnualReportforHIVandViralHepatitis2017-2018.pdf
- Rwanda Ministry of Health. Rwanda Population-based HIV Impact Assessment RPHIA 2018–2019 [Internet]. 2020. [cited 2021 April 21]. Available from: https://phia.icap.columbia.edu/rwanda-final-report/.
- UNAIDS 2020. Instructions for using the Naomi model V2.3.1 [Internet]. 2021. [cited 2021 April 20]. Available from: https://naomi.unaids.org/public/resources/Naomi-basic-instructions.pdf
- Vandormael Alain, Akullian Adam, Siedner Mark, de Oliveira Tulio, Bärnighausen Till, Tanser Frank. Declines in HIV incidence among men and women in a South African population-based cohort. Nature Communications 2019;10(1):1–10 5482 (2019). doi: 10.1038/s41467-019-13473-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.