Abstract
Objective
We undertook the study to present a comprehensive overview of COVID-19 related measures, largely centred around the development of vaccination related policies, their implementation and challenges faced in the vaccination drive in India.
Methods
A targeted review of literature was conducted to collect relevant data from official government documents, national as well as international databases, media reports and published research articles. The data were summarized to assess Indian government's vaccination campaign and its outcomes as a response to COVID-19 pandemic.
Results
The five-point strategy adopted by government of India was “COVID appropriate behaviour, test, track, treat and vaccinate”. With respect to vaccination, there have been periodic shifts in the policies in terms of eligible beneficiaries, procurement, and distribution plans, import and export strategy, involvement of private sector and use of technology. The government utilized technology for facilitating vaccination for the beneficiaries and monitoring vaccination coverage.
Conclusion
The monopoly of central government in vaccine procurement resulted in bulk orders at low price rates. However, the implementation of liberalized policy led to differential pricing and delayed achievement of set targets. The population preference for free vaccines and low profit margins for the private sector due to price caps resulted in a limited contribution of the dominant private health sector of the country. A wavering pattern was observed in the vaccination coverage, which was related majorly to vaccine availability and hesitancy. The campaign will require consistent monitoring for timely identification of bottlenecks for the lifesaving initiative.
Keywords: COVID-19, Pandemic, Vaccination, Healthcare policies, India
Introduction
The early instances of coronavirus disease 2019 (COVID-19), were reported as clusters of pneumonia of unknown aetiology from Wuhan city of China in December 2019 [1]. The spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was exceptionally quick with more than 150 countries getting affected by March 2020. This alarming magnitude and severity led to declaration of the disease as a pandemic by World Health Organization on 11th March 2020 [2]. India recorded its first case on 30th January 2020. The initial spread was limited to international travellers and their contacts until March 2020. India witnessed the first peak of COVID-19 in September 2020, after which there was a continuous decline until the end of 2020. Subsequently, India was inflicted with the devastating second wave in March 2021, which had a multi-dimensional effect that exacerbated inequalities in the country.
India is home to over 1.3 billion people, accommodating wide diversities in terms of ethnicity, religious traditions, languages, geographic regions, and social stratifications. Being the second most populous country in the world, it accounts for 18% of the world's population [3]. Around 40% of India's population is below the age of 18 years. The above 60 years cohort makes up 8.6% of population and the age dependency ratio in the country is 49% [4,5]. 65% population is concentrated in the rural areas and the population density is estimated to be 464 people per square kilometre. The population disaggregation by gender values sex ratio at 943 females to 1000 males [3]. The per capita gross domestic product (GDP) in 2020 was US$ 1900.7 [6]. Economic disparities are prevalent with estimated 22% of population (26% in rural India and 14% in urban India) living below poverty line [7].
India had a tradition of centralized planning and policy making and decentralized implementation. But over the years, it has adopted the road of fiscal federalism and decentralized decision making. A mixed healthcare system has been established in India, with involvement of both public and private sector [8]. The Indian public health system has a three-tier structure comprising of sub-centres and primary health centres (more recently known as Health and Wellness centres) for primary healthcare, community health centres for secondary healthcare, and district hospitals, and medical colleges for tertiary healthcare services [9]. The services provided in public health facilities are majorly government funded and due of low investment of 1.28% GDP, there are persistent availability and accessibility issues. The private sector has therefore, emerged as a dominant stakeholder with 62% of Indian health infrastructure, and accounts for 70% of treatment care. Prior to the pandemic, India had a total of 43,486 private hospitals, 1.18 million beds, 59,264 intensive care units (ICUs), and 29,631 ventilators in private sector. On the other side in the public health system, there were 25,778 public hospitals, 713,986 beds, 35,700 ICUs, and 17,850 ventilators [10]. The substantial reliance on private provisioning of care along with underfunded public healthcare system has resulted in high out-of-pocket payments and intensified social and economic inequities.
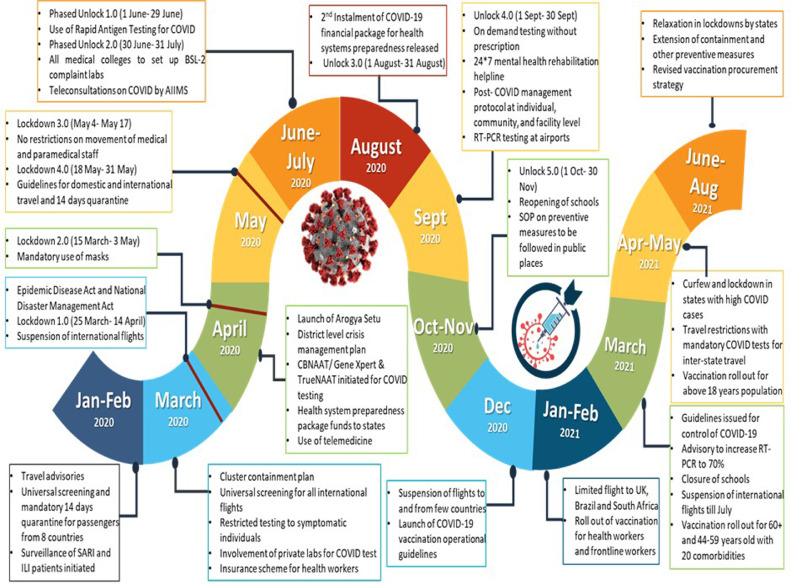
The COVID-19 pandemic necessitated a robust public health strategy and strengthening of weak links in the health system. Accordingly, a series of policy measures were introduced over the year, based on the case load and health system's capacity at different timepoints. The five-point strategy adopted by government of India has had been “COVID-19 appropriate behaviour, test, track, treat and vaccinate”. In the initial months, various travel advisories were generated to regulate international as well as domestic travel, including universal screening of passengers from all international flights followed by mandatory quarantine. [11], [12], [13].
On 25th March, 21 days’ nationwide lockdown was announced by government of India, which inhibited movement completely and led to suspension of nearly all non-essential services. Consecutively, this lockdown was extended until May 2020 and states were ordered to ensure strict enforcement under Disaster Management Act 2005 [14]. Phased reopening of the country started from June 2020. In addition to lockdowns and travel restrictions, measures have been taken to strengthen the health system response in terms of testing, contact tracing, treatment, as well as vaccination. Since the beginning of the pandemic, development of safe and efficacious vaccine against COVID-19 has been a global priority [15]. Under normal circumstances, development of vaccines may take multiple years, but enhanced global cooperation, earmarked funding, existing vaccine technology, accelerated regulatory processes and operational innovation led to launch of vaccines in less than a year [16].
In this paper, we discuss in detail the implementation of various COVID-19 related policies adopted by Indian government with special focus on vaccination drive. Firstly, we provide a comprehensive overview of policy measures adopted by Indian government in order to enhance testing and tracking of infected individuals, ensure access to required treatment, and promote COVID-19 appropriate behaviours. Secondly, we map the course of changing vaccination policy during the course of the pandemic, besides including the development of vaccines, roll out of the program, vaccine coverage and the role of private sector in augmenting the vaccination drive till March 2022. Finally, we discuss the potential factors that influenced the COVID-19 vaccination programme and highlight certain aspects which were crucial to make vaccination campaign effective in India.
Methodology
A chronological investigation was carried out to outline the trajectory of COVID-19 and the policy responses to contain the disease and mitigate its effects in India. The reviewed data sources included press information bureau (PIB) releases from different ministries under the government of India, COVID-19 related dashboards such as COVID Vaccine Intelligence Network (CoWIN) portal, COVID-19 vaccine website of Indian Council of Medical Research (ICMR), and Our World in Data, Ministry of Health and Family Welfare (MoHFW) responses to Parliament questions, government affidavits submitted to the Supreme Court of India, guidelines issued by government of India, and print media reports.
Additionally, we conducted a review of scientific journal articles in PubMed with a targeted search strategy. The search terms included “COVID-19”, “corona virus”, “coronavirus”, “SARS-CoV-2”, “COVID”, “vaccination”, “immunization”, “immunisation”, “vaccination policies”, “vaccination strategies”, “vaccine procurement”, “vaccine pricing policies” and “India” (Supplementary file S1). The search was restricted to articles from 2019 to 2021 and to published literature in English language. A total of 798 articles were screened based on the PICO strategy. Articles on biomedical research on COVID-19 vaccines, and articles representing information on vaccination from countries other than India were excluded. After full text screening, 12 articles which followed the inclusion criteria were analysed. The references from the included articles were also screened to present a comprehensive narrative analysis of COVID-19 vaccination strategies adopted by India.
Findings
Overview of COVID-19 related policy measures taken by the Indian government
The COVID-19 outbreak emerged as an unprecedented challenge for the governments, communities as well as individuals. The primary responses to the pandemic were development of policies based on the recommendations from experts and implementation of standardized measures. The measures taken by our government to facilitate testing, tracking and treatment of COVID-19 cases and ensuring COVID appropriate behaviour have been delineated below:
Testing strategy and upscale
At the start of pandemic, there was only one designated COVID-19 testing laboratory in the country and the testing was restricted to people with history of international travel, symptomatic and asymptomatic high-risk contacts of positive patients, hospitalized patients with severe acute respiratory infection (SARI) and symptomatic health workers [17]. On 21st March 2020, guidelines were formulated for COVID-19 testing at private pathology laboratories with a price capping at ₹ 4500 (US$ 60.75) [18], [19], [20]. In the following months, Cartridge based nucleic acid amplification (CBNAAT) and TrueNat were approved for COVID-19 detection (Fig. 1 ) [21], [22], [23]. A fast-tracked mechanism for validation of diagnostic materials was initiated by ICMR. Initiatives were also taken to increase capacity of government and private medical colleges in testing under mentorship of leading virological/medical institutes [24,25]. The number of laboratories increased consistently from 1 in January to 669 in May, 1614 in September, and 2172 by the end of 2020. During the second wave of COVID-19 in May 2021, there were 2504 testing laboratories across India. In view of COVID cases surge, ICMR issued advisory for optimized use of real-time reverse transcription polymerase chain reaction (RT-PCR) by increasing availability of rapid antigen tests (RAT) to avert shortages [26].
Fig. 1.
COVID-19 related interventions in India.
Surveillance measures (track)
Surveillance of patients with SARI and influenza like illnesses (ILI) begun during the early phase of pandemic as a containment measure. Integrated Disease Surveillance Programme (IDSP) issued an advisory for surveillance of travel related cases and contacts of suspects on 17th January 2020. Cluster containment strategy was delineated in April 2020, which laid down the concept of containment, buffer zones and strict perimeter control [27]. Additionally, areas were also classified as red, orange, and green zones on basis of total active cases and infection rate and accordingly restrictions were implemented in three zones [28]. Arogya setu – a web-based application for public awareness was launched for contact tracing, mapping of likely hotspots and dissemination of information regarding COVID-19 [29]. During the second wave, a decentralised state driven containment frameworks were implemented to deal with the alarming COVID-19 surge [30].
Health system preparedness (treatment and isolation measures)
Government of India announced ₹ 150 billion (US$ 2.02 billion) for “India COVID-19 Emergency Response & Health System Preparedness” in April 2020 [31]. These funds were aimed to create a new three-tier facility arrangement for COVID-19 management, support, train and protect healthcare workforce, expand diagnostic facilities, deploy referral transport, initiate health promotion and risk communication activities, and enhance surveillance [32]. The second instalment of ₹ 8.9 billion (US$ 120 million) with similar objectives was released in August 2020 [33]. In July 2021, an additional funding of ₹ 231.23 billion (US$ 3.12 billion) was announced for enhancing the capacity in terms of availability of beds, liquid medical oxygen tanks, ambulances, creation of paediatric units, strengthening of tertiary care centres, and IT interventions [34].
Advisory for three-tier health facility arrangement was issued for appropriate management of COVID-19 in April 2020. Hostels, schools, hotels, stadiums, railway coaches etc. were turned into COVID care centres (CCC) for management of mild cases. The second category of dedicated COVID health centres (DCHC) for moderate cases were hospitals with oxygen support. Dedicated COVID hospitals (DCH) equipped with ICUs, ventilators, beds with oxygen supply provided services for severe COVID-19 cases. Therefore, along with utilization of existing secondary and tertiary care institutions, new institutions were constructed or acquired and by June 2020, 958 DCHs with 1,67,883 isolation beds, 21,614 ICU beds and 73,469 oxygen supported beds and 2,313 DCHCs with 1,33,037 isolation beds; 10,748 ICU beds and 46,635 oxygen supported beds were operationalised [35].
The indigenous manufacturing capacity of personal protective equipment (PPEs) was enhanced by mid-May 2020 [36]. A daily production of three hundred thousand PPE and N95 masks was reported by end of May 2020 [37]. Export restrictions were imposed on certain active pharmaceutical ingredients, masks, and sanitizers from March to June 2020 to ensure local availability [38].
Health promotion (COVID appropriate behaviour)
Awareness campaigns were undertaken consistently to ensure COVID-19 appropriate behaviour. Use of masks outside home was made mandatory in April 2020. Ceilings on social gatherings were imposed to avoid crowding in public places. Niti Aayog launched behaviour change campaign on 25th June 2020 [39]. Short message services and caller tunes were also used through telecommunication service providers to spread awareness regarding appropriate behaviours [40]. A dedicated helpline was introduced to educate the population on COVID-19 related queries on regular basis in mid-March. Around 3.5 million calls were received on the helpline until July 2020 [41]. On 14th October, Jan Andolan– a public movement was started to urge people to follow appropriate behaviours during festive season and winters [42]. During the second wave in May, emphasis was given to reiterate importance of mask use, social distancing, sanitation, and ventilation for containment of disease [43]. Fig. 1 provides a comprehensive view of COVID-19 related strategies adopted in India.
The vaccination drive in India: from development to distribution
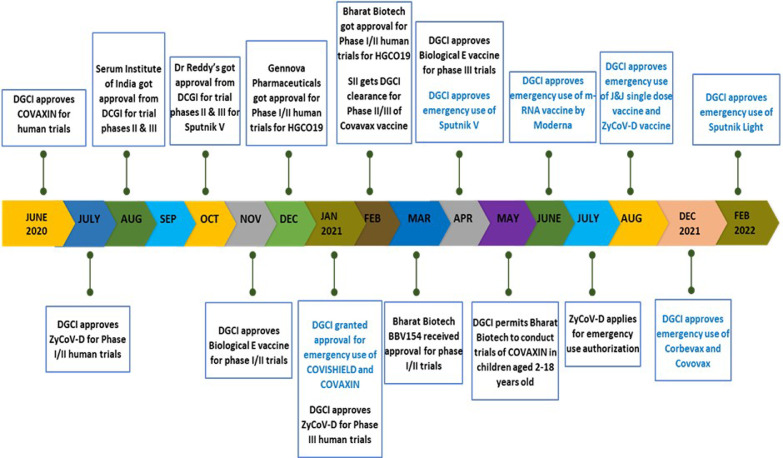
The commencement of efforts for vaccine development overlapped with the first wave of pandemic in the country. A task force for focussed research on COVID-19 vaccine was constituted in April 2020 to promote development of vaccines. Various pharmaceuticals like Bharat Biotech (BB), Zydus Cadila, Serum Institute of India (SII) and Dr Reddy's laboratories began vaccine clinical trials for Covaxin, ZyCoV-D, Covishield and Sputnik V during the course of pandemic. Emergency use authorization for Covishield and Covaxin was granted in January 2021 followed by approval for Sputnik V in April, m-RNA-1273 (Moderna) in last week of June and Johnson and Johnson's single dose vaccine and Zydus Cadila's ZyCoV-D vaccine in August (Fig. 2 ) [44,45]. By the end of August 2021, few vaccines as Covovax (SII), Corbevax (Biological E limited) and BBV154 (BB) were in clinical trial phases and in early 2022, emergency use approvals were granted to Corbevax, Covovax, and Sputnik Light vaccines. The vaccine development in India is detailed in Fig. 2 [46].
Fig. 2.
A glance at vaccine development in India.
Vaccination procurement and pricing policy
National Expert Group on vaccine administration for COVID-19 (NEGVAC) was constituted in August 2020 for preparing blueprint of vaccination roll out [47]. Operational guidelines for COVID-19 vaccination were laid down on 28th December 2020 [48], followed by 10 days dry run. Subsequently, government of India released advisory on COVID-19 vaccination which detailed precautions, contraindications, and comparison of two approved vaccines [49]. The inoculation programme was launched on 16th January across the country in three phases.
The central and state government played important roles in COVID-19 vaccination. The central government was responsible for formulation of policies and guidelines, emergency use approvals to vaccines, provision of financial support to vaccine manufacturers for expansion of production capacity of vaccines, financing, procurement, and distribution of vaccines and monitoring of the vaccination programme. The role of states was to identify vaccination sites, undertake logistic management, train the human resources, update daily vaccination related data. Both the governments organized awareness campaigns to spread right information for uptake of the vaccination drive.
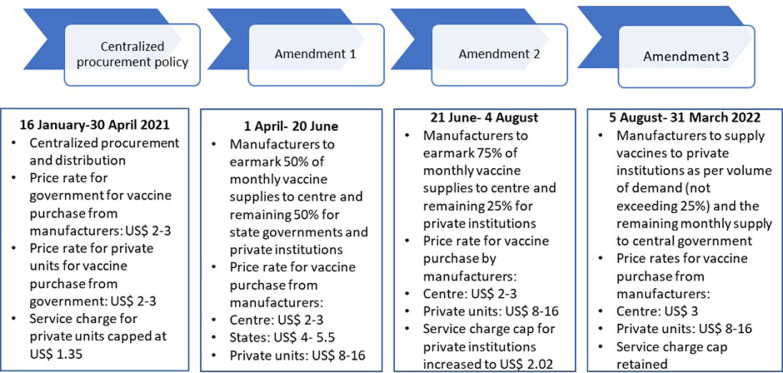
The central government took the sole responsibility of vaccine purchase in first 3.5 months of the initiative. However, in May, there was a shift in procurement policy to make the pricing and procurement flexible and incentivize the manufacturers to scale up production. “Liberalized pricing and accelerated National COVID-19 vaccination strategy” was announced by the central government, outsizing the role of private sector, which mandated the vaccine manufacturers to earmark 50% of their monthly vaccine supplies to government of India and unrestricted the remaining 50% of the doses supply to state governments, private hospitals, and industrial establishments at pre-declared prices (Fig. 3 ) [50]. The government justified the liberalized strategy as a medium to create parallel drives in a decentralized manner, wherein states and private health institutions would focus on procurement of vaccines for 18-44 years old cohorts while the central supply would facilitate vaccination of priority groups (above 45 years old population and frontline workers).
Fig. 3.
Amendments in procurement and pricing policies.
The allocation of vaccine doses to the states/UTs was based on criteria of infection rate, speed of vaccination and extent of wastage of vaccines. First, the infection rate was computed on basis of active case load to give prioritization to states with higher viral load. Second, the parameter evaluating the performance of states in vaccination program was enumerated through seven-day average of vaccine consumption. This would act as an incentive for states to accelerate vaccination drive and promote efficiency. The extent of vaccine wastage was chosen as a unique parameter to minimize vaccine wastage, since, it was noted that by April 11, India had wasted 4.6 million doses, which was a significant number considering the constraints in vaccine availability [51,52]. CoWIN application was used to monitor utilization, wastage, and coverage of COVID-19 vaccination.
Many states reported hurdles in vaccination due to amended policy and the Supreme Court of India also intervened after reports of vaccine scarcity in the country. The government tweaked the policy on 21st June, reverting to a more centralized approach of procurement where-in 75% of vaccines would be procured by the central government and provided to the states at zero cost. The element of 25% procurement of vaccines at pre-declared prices by private sector was retained [53]. However, the low contribution of the commercial sector in the drive led to announcement of supply of vaccines to the private sector as per volume of demand without the restriction to reserve 25% vaccines for the commercial units in the first week of August (Fig. 3) [54].
COVID-19 vaccine financing
The national budget 2021-22 allocated ₹350 billion (US$ 4.7 billion) for COVID-19 vaccination [55]. Government reported spending ₹80.71 billion (US$ 1.08 billion) on purchase of vaccines and ₹16.54 billion (US$ 223 million) on operational costs until July 2021 and ₹196.75 billion (US$ 2.7 billion) by December 2021 [56,57].
Table 1 presents the timeline and volume of vaccine orders placed by the central government [58]. It was noted that a total of 42 million doses (expected 160 million doses) were procured directly by state governments and private institutions from May to mid-July during the enforcement of liberalized COVID-19 vaccination policy [59]. Table 1 also illustrates the first order being placed just 6 days before the commencement of vaccination drive as well as no orders being placed in April and June despite continuous reports of vaccine shortage in the country from March end.
Table 1.
Procurement orders of vaccines by government of India.
| Procurement Period | Source of Payment (Not for Sputnik V) | Orders approved by government of India | Total Procurement Order Delivery Status | ||||
|---|---|---|---|---|---|---|---|
| SII- Covishield | Bharat Biotech Covaxin | Dr Reddy's Sputnik V | Biological E's vaccine (at risk manufacturing) | ||||
| 1. | 10th Jan | PM Cares Fund | 11 million doses @ US$ 2.7/ dose | 5.5 million doses (1.65 million doses free of cost + 3.85 million doses @ US$ 4/dose | - | - | Delivered |
| 2. | 3rd Feb | PM Cares Fund | 10 million doses @ US$ 2.7/dose | 4.5 million doses @ US$ 4/dose | - | - | Delivered |
| 3. | 10th Feb | PM Cares Fund | 15 million doses @ US$ 2.7/dose | - | - | - | Delivered |
| 4. | 24th Feb | PM Cares Fund | 20 million doses @ US$ 2.7/dose | - | - | - | Delivered |
| 5. | Feb | COVAX | 10 million doses | - | - | - | Delivered |
| 6. | 12th March | Ministry of Health | 100 million doses @ US$ 2/ dose | 20 million doses @ US$ 2/ dose | - | - | 81% of the doses received |
| 7. | April |
- | - | - | 250 million doses (Out of which 15-20% to be imported) | - | |
| 8. | 5th May (Order of vaccines for May-July) |
Ministry of Health | 110 million doses @ US$ 2/ dose (Advance payments- US$ 233.8 million) |
50 million doses @ US$ 2/ dose (Advance payments- US$ 106.2) |
- | - | 0.21 million doses of Sputnik V arrived in India |
| 9. | 16th July |
Ministry of Health | 375 million doses @ US$ 2.9/dose (30% advance payment) |
285 million doses @ US$ 3/ dose (30% advance payment) |
- | 300 million doses @ US$ 205.6 million | 38.4 million doses Covaxin remaining to be delivered from May order 3 million doses of Sputnik V arrived in India |
Source: Ministry of Health and Family Welfare, government of India.
The government embarked on various measures to increase the availability of vaccines in the nation. ₹30 billion (US$ 405 million) and ₹15 billion (US$ 202.5) were granted to SII and Bharat Biotech for scale-up of domestic production capacity in April 2021 by government of India [60]. In this context, SII upscaled production of Covishield considerably especially after the upliftment of embargo on raw material by the US in June, but Covaxin's production had been constant majorly due to cited quality issues in first few batches of the vaccine (Table 2 ) [61], [62], [63], [64]. In order to augment the production of Covaxin in the forthcoming months, the Ministry of Science and Technology announced inclusion of 3 public sector companies and subsequently grants were released to facilitate preparedness under Mission COVID Suraksha. [65], [66], [67]. Due to all these measures, there was expansion in the production capacity of Covishield as well as Covaxin by December 2021 [68] (Table 2).
Table 2.
Domestic production of Covishield and Covaxin.
| Year 2021 | Covishield (million doses per month) | Covaxin (million doses per month) |
|---|---|---|
| January | 50 | 10 |
| February | 50 | 10 |
| March | 70 | 25 |
| April | 70 | 25 |
| May | 70 | 25 |
| June | 90 | 25 |
| July | 110 | 25 |
| August | 150 | 25 |
| December | 250-275 | 50-60 |
To supplement domestic vaccines with imports, 10% customs duty was waived off for imported COVID-19 vaccines in April, thus making imports cheaper [69]. In the same month, government of India announced fast tracking of emergency approvals of internationally manufactured vaccines, already authorized for emergency use in the United States (US), United Kingdom (UK), European Union (EU), and those included in World Health Organization (WHO) list of approved vaccines [70]. In addition, these foreign manufactured vaccines were exempted from bridge testing with effect from 1st June 2021 and quality testing norms were relaxed for these vaccines [71,72]. A team was constituted on 11th June 2021, to investigate issues related to procurement of vaccines from foreign manufacturing units including indemnity issues. These strategies are likely to result in quicker import of vaccines [73].
On the export front, India started vaccine assistance to other countries under the initiative of “Vaccine Maitri” in the fourth week of January and by end of March, approximately 66 million doses of Covishield and Covaxin had been dispatched to 95 countries in form of gifts, commercial agreements and under COVID-19 Vaccines Global Access Facility (COVAX) [74]. However, the alarming rise in people infected with COVID-19 in March-April led to anticipation of increased domestic demand for vaccination and a temporary halt on export of vaccines in last week of April (Supplementary file S2).
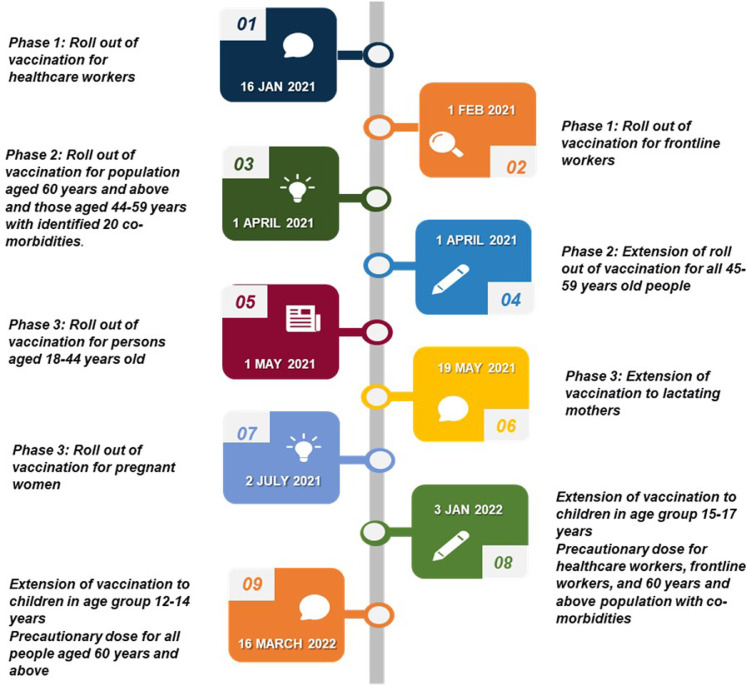
Roll out of COVID-19 vaccination programme (risk-prioritization strategy)
Countries across the globe adopted a prioritization strategy for vaccination, due to limited availability of vaccines as per population needs. NEGVAC, on similar lines, identified three priority groups on basis of potential risk of infection and mortalities and planned a phased vaccination roll out. The first group constituted of pandemic response teams i.e., healthcare workforce and frontline workers including personnel from police department, armed forces, home guards, prison staff, disaster management volunteers, civil defence organisation, municipal workers and revenue officials engaged in surveillance and containment activities [75]. Protecting this group, which was most vulnerable to infection, was important to ensure availability of critical services and curb the spread the disease in the community. It was analysed that during the first COVID wave, 53% of deaths had occurred in above 60 years population and 35% mortalities were recorded in 45-60 years age group [76]. Therefore, the second phase was scheduled for population above 45 years of age. It was preponed from planned window of mid-March to 1st March due to rising COVID-19 infection load in the country and anticipated mortalities in the older age group and population with co-morbidities [77]. The disastrous second wave ascribed to mutated COVID-19 virus, which resulted in higher mortalities in population less than 45 years of age as compared to the first wave, created an alarming situation in the country [78]. Thus, 18-45 years population was identified as third priority group for vaccination in end of April 2021 and consequently vaccination was initiated for the younger population. [79].
Subsequently, the campaign was extended to lactating and pregnant women after a careful investigation and approval by National technical Advisory Group on Immunization (NTAGI). It was approved after advocacy from World Health Organization, recommendations from Federation of Obstetric and Gynaecological Societies of India (FOGSI), and release of results from study conducted by ICMR which concluded higher COVID-19 infection in pregnant and lactating women in the second wave (28.7% v/s 14.2%) and higher fatality rate (5.7% v/s 0.7%) compared to the first wave [80]. Due to global surge of COVID-19 cases credited to the omicron variant in January 2022, vaccination with Covaxin was initiated for 15-17 years age group and a precautionary third dose was recommended for healthcare, frontline workers, and senior citizens of the country with co-morbidities [81]. Later in March 2022, the drive was extended to 12-14 years old cohort with Biological E's Corbevax. The timelines of roll out of different vaccination phases have been outlined in Fig. 4 .
Fig. 4.
Roll out of vaccination programme.
Vaccination coverage
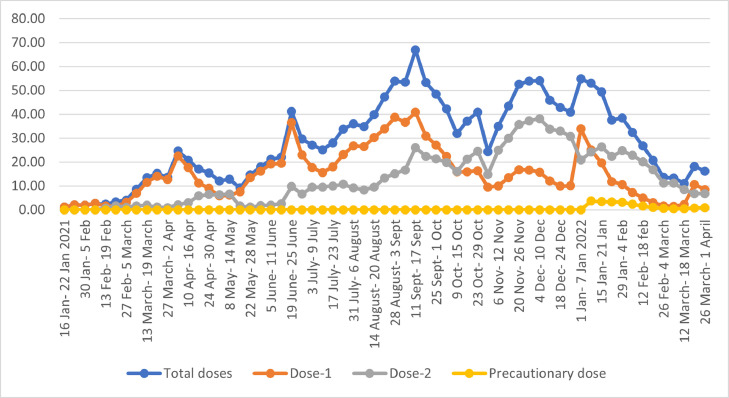
The country witnessed a wavering pattern in vaccination campaign (Fig. 5 ). The vaccination rate was sluggish in the initial phase due to vaccine hesitancy among healthcare and frontline workers [82]. Vaccine hesitancy in the priority group was linked to trust issues in vaccine safety and efficacy, due to quick development of vaccines, early emergency approval to Covaxin before release of results of phase 3 clinical trials and missing data related to adverse effects following immunization [83]. During the pandemic, misinformation through social media fuelled scepticism towards the vaccines among the healthcare and frontline workers and this also had a ripple effect on the general population. Apart from this, high COVID infection rate among the frontline workers in the past and low risk of contracting COVID-19 infection due to its overall decline at population level during the initial phase of vaccination also led to low uptake of the initiative [84].
Fig. 5.
Vaccination coverage by doses.
A rise in vaccination rate was observed from the first week of March to first week of April, when the campaign was extended to general population (Fig. 5). Thereafter in April-May when the pandemic was at its peak, various states flagged issue of availability of vaccines, which led to decline in vaccination rate [85], [86], [87]. Amidst shortage of vaccines and COVID-19 upsurge, the campaign was extended to 18-44 years old with mandatory pre-registration but most of the states deferred inoculations until the second week of May due to supply shortages [88].
The vaccination drive seemed to be marred with vaccine hesitancy in rural areas and demand supply gaps in the initial weeks of July [89,90]. The rate began to increase in third week of July after a consistent decline, and this was attributed to rise in supply of vaccines to the states. The boost in production of Covishield doses, scrapping of reservation of 25% of vaccines for the private sector, advanced visibility of vaccine availability to states for better planning, high risk of infections due to the predicted third wave in September-October, and sustained efforts of community health workers to combat vaccine hesitancy were the likely reasons behind increased accessibility to vaccines in August [91,92].
It can be observed from Fig. 5 that second dose coverage started after 4 weeks in February, as per the protocol of interval of 4-6 weeks between shots. The vaccination rate for second dose plummeted after the second week of May after Ministry of Health and Family Welfare raised the gap between 2 doses of Covishield from 4-8 weeks to 12-16 weeks on 13th May, citing experiences from UK [93]. The rate increased after the second week in June probably due to high number of beneficiaries due for second dose [94]. A rise in first dose can be appreciated in first week of January when the drive was extended to 15-17 aged adolescents in the country. Fig. 5 also depicts the trend of precautionary dose which was started from 10th January 2022.
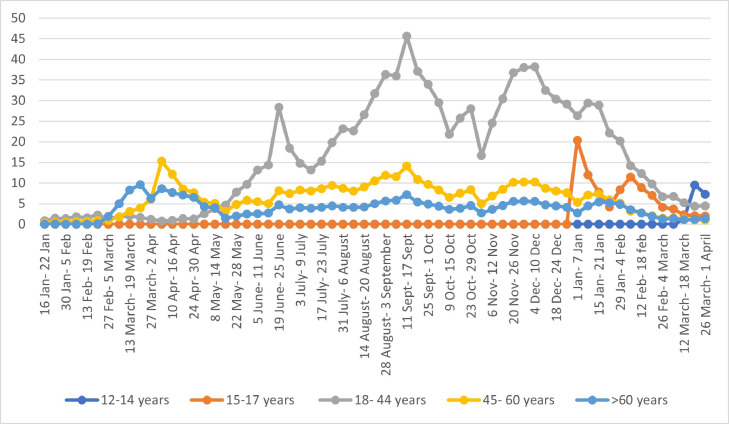
Vaccination analysis for different age groups informed that rates of vaccination in age-appropriate groups were in harmony with timeline of different phases of the campaign and the vaccination rate improved as and when it was extended to new age groups (Fig. 6 ). It was also noted that the extension of vaccination drive to younger population did not undervalue the vaccination of senior citizens.
Fig. 6.
Vaccination coverage by age.
Role of private health sector in vaccine delivery
Government of India ensured participation of private health sector to battle with the pandemic and in the second month of COVID-19 vaccination, advisory was issued by government of India to the states for utilization of all private hospitals including 10,000 empanelled hospitals under Ayushman Bharat and 687 hospitals under the central government health scheme as COVID-19 vaccination centres. The private hospitals were allowed to charge a maximum of ₹250 (US$ 3.3) per person per dose until April 2021 [95]. The price rate was relatively low since according to the then vaccination policy when private hospitals purchased vaccines from the central government at rate of ₹150 (US$ 2) (Fig. 3). After the implementation of liberalized vaccine procurement and pricing policy, the private sector purchased 12.73 million doses directly from the vaccine manufacturers at higher rates (₹600 (US$ 8) for Covishield, ₹1200 (US$ 16) for Covaxin and ₹948 (US$ 12.8) For Sputnik V (Fig. 3). This resulted in exponential rise in prices of the vaccines and 8.31 million doses were administered in the commercialized sector from May to mid-June [96]. Following the revision of COVID-19 pricing policy in June end, the prices capped per dose of Covishield, Covaxin, and Sputnik V in private facilities for the citizens were ₹780 (US$ 10.5), ₹1410 (US$ 19) and ₹1145 (US$ 15.4), respectively [97]. It was analysed that 7% vaccinations were carried out in private vaccination centres from May to mid-July, which was much less compared to the amount of vaccine supply reserved for the sector [98,99].
Government of India mandated order of vaccines through CoWIN portal for private institutions, to keep a check on private vaccine procurement from 1st July. It clarified the private health units about consumption based maximum order quantity, limit of four instalments to place orders, and clause of payments within 3 days of ordering vaccines. This initiative is likely to bring transparency in private procurement of vaccines [100]. The low contribution of the dominant private sector to achieve set vaccination targets, led to government's decision of buying the vaccines not procured by the private sector reserved under its quota, which consequently led to rise in availability of vaccines in August.
Milestones in vaccination campaign
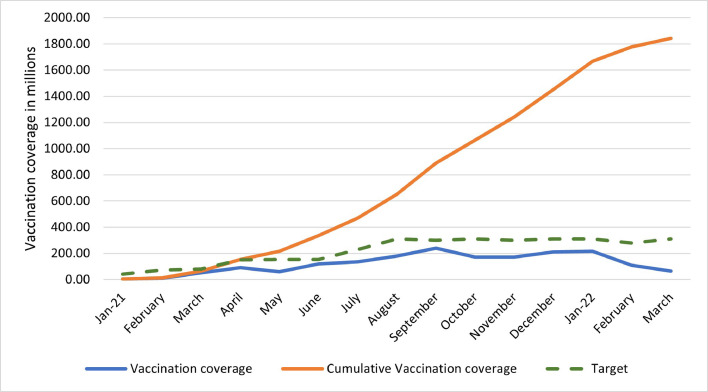
India has set ambitious targets for vaccination and plans to fully vaccinate above 18 years population by the end of 2021. Against an estimated target of 940 million eligible beneficiaries, 10% had been fully vaccinated and 37% had received at least one dose by end of August [101].
The month wise announced targets for the nation were reviewed. The first declared target was to fully immunize 300 million people by July-August 2021 [102]. This required 600 million doses in first 7-8 months of the year. Considering the same, the daily target was estimated to be 2.64 million doses for the months of January, February, and March. On 1st April, target to inoculate 5 million people per day was announced by the government [103]. By end of June, chairman of COVID-19 working group declared a target of 10 million vaccinations each day from mid-July [104]. Fig. 7 illustrates that the initiative has not succeeded according to the set targets and requires taking a leap on various fronts to achieve the herculean task of 1.3 billion inoculations.
Fig. 7.
Vaccination trend as per targets.
Table 3 demonstrates the vaccination coverage among different priority groups identified by the government. A positive vaccination trend was analysed for all the priority groups [105], [106], [107].
Table 3.
Vaccination as per priority groups.
| Priority groups | Target population(millions) | Doses | Targets achieved (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2021 | 2022 | |||||||||||||||
| 01- Mar | 01- April | 01- May | 01-June | 01-July | 01-Aug | 01-Sept | 01-Oct | 01-Nov | 01-Dec | 01-Jan | 01-Feb | 01-Mar | 01-April | |||
| Health workers | 10 | 1st | 66.2 | 83.0 | 94.1 | 99.2 | 102.1 | 103.1 | 103.5 | 104 | 104 | 104 | 104 | 104 | 104 | 104 |
| 2nd | 20.3 | 52.8 | 62.4 | 68.2 | 72.4 | 78.4 | 84.1 | 89.0 | 92.2 | 95.0 | 97.1 | 98.7 | 99.7 | 100 | ||
| P* | - | - | - | - | - | - | - | - | - | - | - | 33.9 | 41.8 | 44.7 | ||
| Frontline workers | 20 | 1st | 24.1 | 46.7 | 62.7 | 79.5 | 87.4 | 89.8 | 91.6 | 91.8 | 91.9 | 91.9 | 91.9 | 92.0 | 92.0 | 92.1 |
| 2nd | - | 20.5 | 34.1 | 43.1 | 47.4 | 56.6 | 66.6 | 75.0 | 79.7 | 82.5 | 84.5 | 86.2 | 87.2 | 87.6 | ||
| P* | - | - | - | - | - | - | - | - | - | - | - | 19.8 | 31.2 | 34.4 | ||
| 60 years and above | 138 | 1st | - | 22.9 | 37.9 | 43.2 | 49.2 | 55.1 | 63.7 | 73.5 | 79.6 | 83.9 | 88.0 | 90.5 | 91.6 | 91.8 |
| 2nd | - | 0.2 | 8.0 | 13.7 | 17.4 | 26.4 | 33.0 | 40.7 | 48.3 | 57.5 | 69.1 | 77.5 | 81.4 | 83.7 | ||
| P* | - | - | - | - | - | - | - | - | - | - | - | 3.7 | 7.0 | 8.6 | ||
| 45-59 years old | 207 | 1st | - | 4.7 | 25.4 | 33.1 | 42.6 | 51.3 | 64.3 | 77.1 | 84.5 | 89.3 | 94.0 | 96.8 | 97.7 | 97.9 |
| 2nd | - | 0.02 | 1.8 | 5.3 | 7.6 | 18.9 | 26.9 | 37.1 | 46.6 | 58.5 | 72.9 | 82.9 | 87.1 | 89.5 | ||
| 18-44 years old | 595 | 1st | - | - | - | 4.0 | 15.1 | 26.2 | 43.5 | 60.6 | 70.4 | 77.2 | 84.0 | 91.0 | 92.8 | 93.2 |
| 2nd | - | - | - | 0.01 | 0.35 | 1.46 | 5.01 | 14.0 | 23.9 | 37.9 | 56.1 | 68.3 | 74.7 | 78.3 | ||
| 15-17 years old | 740 | 1st | - | - | - | - | - | - | - | - | - | - | - | 63.0 | 74.2 | 77.3 |
| 2nd | - | - | - | - | - | - | - | - | - | - | - | 0.5 | 37.8 | 51.6 | ||
| 12-14 years old | 711 | 1st | - | - | - | - | - | - | - | - | - | - | - | - | - | 24.3 |
P*- Precautionary dose.
To assess the implementation of the response to COVID-19 in terms of the vaccination strategy, we used the WHO Scientific Advisory Group for emergencies (SAGE) framework for allocation of COVID-19 vaccines and the findings have been presented in Supplementary File: S2.
Discussion
India claimed the milestone of administering more than 1.5 billion vaccine doses to its citizens until January 2022. The vaccine allocation and risk prioritization strategies were devised efficiently since the beginning of the drive. The government announced allocation of vaccine doses to the states/UTs in the first phase of vaccination drive based on the health worker database of the states, thus ensuring equal respect to the contribution of health workers in COVID-19 pandemic (Supplementary Material S2) [108]. Later, for subsequent phases of vaccination, criteria of infection rate, speed of vaccination and extent of wastage of vaccines was announced for vaccine allocation to promote efficiency and reduce wastage [109]. It was debated that the indicators may lead to data manipulations, denial of vaccines to beneficiaries or vaccine utilization beyond the stipulated time [52], however considering the diversity of the vast country, the indicators have been pivotal to guide transparent distribution of vaccines.
Effect of alterations in vaccination policies
The introduction of liberalized vaccination policy was anticipated to facilitate co-operative federalism, decentralize the process, and increase efficiency, while keeping the vaccination of vulnerable groups unobstructed [50]. However, this strategy shifted the onus of vaccination to states and private institutions and was foreseen as an unfair competition between states and of states with the private sector for purchase of vaccines [110]. Consequently, it led to differential procurement pricing and manufacturers indicated higher rates for states and private institutions. It derailed the vaccination drive and led to vaccine scarcity, since the states were not able to procure vaccines due to low expertise in procurement, budgetary considerations, and high level of internal competition for vaccine procurement within the nation. The subsequent alterations in policy reflected the flexible approach of government to alter its decisions, based on evidence. Furthermore, the decisions on financial support for trials and production of vaccines and flexible import policies are likely to improve the availability of vaccines in future.
Operational challenges in implementation of vaccination campaign
The vaccination coverage trends presented a waxing and waning pattern, with unaccomplished targets. The availability of vaccines, hasty extension of roll-out to above 18 years population amidst vaccine shortage and vaccine hesitancy have acted as impediments to the success of the drive. The operational challenges faced in implementation of the nationwide drive were:
a) Late and insufficient orders of vaccines
The availability of vaccines in the nation is dependent on multiple factors as appropriate and timely procurement, strictness of regulatory approvals for vaccine use, proportion of exports, domestic production capacity and import policies. One of the reasons cited for shortage of vaccines have been late and insufficient orders of vaccine in India [111]. (Table 4 ). Other reasons for vaccine insufficiency were associated with natural disaster at SII facility and embargo on raw materials and equipment by US under the Defence Protection Act [112], [113], [114].
Table 4.
Timeline of COVID-19 vaccine procurement orders and vaccination initiation.
| Countries | Timeline of procurement orders | Vaccination initiation | Vaccines | Procurement orders (million doses) | Fully vaccinated as on 01.09.2021 | Partially vaccinated as on 01.09.2021 |
|---|---|---|---|---|---|---|
| UK | May- August 2020 | December 2020 | AstraZeneca, Pfizer-BioNTech, Janssen | 150 | 63% | 8% |
| US | July- August 2020 | December 2020 | Novavax, Pfizer-BioNTech, Janssen, Moderna | 400 | 52% | 9% |
| EU | August- November 2020 | December 2020 | AstraZeneca, Janssen, Pfizer-BioNTech | 800 | 58% | 7% |
| Australia | September 2020 | February 2021 | AstraZeneca | 33.8 | 28% | 20% |
| Japan | July-October 2020 | February 2021 | AstraZeneca, Pfizer-BioNTech, Moderna | 220 | 47% | 11% |
| India | January 2021 | January 2021 | Covishield, Covaxin | 16.5 | 11% | 26% |
Source: Share of people vaccinated against COVID-19, September 1, 2021. Our World in Data.
b) Global commitments
India contributed to ensuring global equity to vaccines through vaccine maitri, COVAX initiative and by presenting the case of temporary waiver of intellectual property rights for COVID-19 vaccines and patents [115]. The cumulative export was higher than domestic inoculation in first three months amid surge in COVID-19 cases, which was cited as an explanation for domestic shortage [116,117]. The export restrictions in April 2021 after the late and insufficient orders of vaccines by the Indian government led to diversion of stock of vaccines reserved for low-and-middle income countries for achievement of domestic vaccination targets, and this resulted in global allegations of injustice (Supplementary material S2) [118].
c) Rural-urban disparity
The states of India have depicted variations in vaccination rates, with western part being more vaccinated compared to the eastern part of the country which include poorer areas. The intra-state variations were seen in form of rural-urban disparity and capital bias, which were reflected as higher rate of inoculations in urban parts and capitals of the states, probably due to misinformation, digital, as well as lingual divide, logistical constraints as infrastructure, supply chain and skilled personnel and late start of vaccination in rural regions [119], [120], [121], [122], [123].
d) Gender gap in vaccination
Vaccination campaign has consistently reflected gender inequity and on 22nd July, vaccination drive constituted of 53.4% males and 46.5% females. On April 10, there was a 2% disparity between vaccinated men and women, which increased to 12% on April 24 [124]. The ratio of per million vaccinated men and women peaked on May 25 at 1.348, after the vaccination drive was extended to 18+ population [125]. The gender gap could be attributed to patriarchal socio-cultural norms, and gender differences in healthcare access including mobility and decision-making capacity issues, gender divide in technological access and digital literacy [126]. Vaccination myths have had additional effect in keeping females away from this public health measure [127]. Government cited late approval of vaccine use in pregnant and lactating women as a reason for the difference.
e) Use of digital systems for registration
The directive of pre-registration in CoWIN system for 18-44 years old population was a source of reluctance due to frequent technical hurdles, first come first serve appointments, delays in receiving one time passwords, issues with captcha submission, lack of availability of slots, privacy policy, access to smartphones, high bandwidth connectivity, digital literacy, and lingual exclusion with its availability only in ten languages [128], [129], [130]. Due to the stated issues, on 21st June, the mandatory registration for 18-44 years old was shifted to optional with additional opportunity of on-site registration for the beneficiaries. Nonetheless, the utilization of CoWIN for procurement, distribution, and monitoring of vaccination has been an ambitious attempt to adopt digital technologies and utilize real-time data for planning of policies. The decision of provision of 25% of vaccines to private sector, which accounted for 4-5% of the total vaccination sites and had a demand of not more than 10% of vaccines, also posed a threat to rational, equitable and ethical distribution [131].
f) Involvement of the private sector
The low performance of the private sector could be attributed to capping of prices which led to low profit margins for the private institutions, and population preference for free vaccines at government institutions. From a private sector perspective, the price capping inhibited the private players to move vaccination to tier 2, tier 3 areas and community-based sites, which would eventually increase vaccination rates, but would lead to higher administrative costs [99]. But a scenario of non-capping of prices would have led to lower vaccination rates due to higher prices to general population, and hence would result in inequity and inefficiency. From people's perspective, a parallel drive with considerably high prices despite the capping expected them to pay as high as two-third of the total price of the vaccination program for 25% vaccination than the government spending for rest of the doses. The underperformance of the private sector indicated that successful vaccination campaign would rather require strengthening of health service system.
g) Vaccine hesitancy
Vaccine hesitancy has been a stumbling block for any vaccination program and there was reported reluctance for COVID-19 vaccines among health workers as well as general population [132], [133], [134]. While there were reports of vaccine refusals from the young population, a survey commissioned by the government of India concluded vaccine hesitancy among 40% of people aged more than 70 years because of safety concerns, mistrust and being too old for vaccination [135]. The reported reasons for hesitancy include lack of trust in safety and efficacy of developed vaccines in less time, fear of side effects such as infertility, effect on menstrual cycles, clotting and death, inconvenience of registration on CoWIN application, loss of productive work due to side effects and perceived low risk of COVID-19 infection [136], [137], [138]. Issues related to travel to the covid vaccination centres and waiting time at the centres (since the vaccinators would open a vial only after required number of beneficiaries present at the centre to avoid wastage of vaccines) also acted as barriers to vaccination [139,140]. A study concluded that vaccine acceptance significantly increased from 38% in mid-January to 77% in the first week of April 2021, after the second COVID-19 wave in the country, however situation did not change considerably in rural areas, and among the marginalized population belonging to lower socio-economic status in various states [141,142].
To deal with the issue of hesitancy, local authorities, and cultural leaders were engaged to counter the narrative of misinformation and mobilize people to accept vaccines as life saving measure against COVID-19 in various villages of India [143]. Chhattisgarh innovatively utilized folk songs to spread the right information about vaccines, Punjab appointed celebrities as vaccination ambassadors and Jharkhand relied on community-based organizations, who worked with local women and religious leaders to conduct successful vaccination programmes [144], [145], [146]. Few districts in Chhattisgarh also engaged local youth as well as elderly women for community mobilization and vaccine related awareness [147]. Another initiative to tackle the issue was taken through spread of right information by community health workers. A village named Janefal became a model for the nation after achieving 100% vaccination of eligible population in three months. The village administration created a taskforce comprising of medical officer, health workers, police personnel, and village local body members. The taskforce members took the vaccine in front of community members, made advocacy videos, and undertook regular home visits to convince population after mapping the eligible population. After realizing the reason of vaccine hesitancy as rooted in the fear of hospitals, the administration started conducting camps in the village, which led to better uptake of vaccination. Additionally in order to tackle the issue of online registrations for vaccination, the task force collected identity cards of all eligible population and registered them. Thus, a collective effort with bottom-up approach and root cause analysis by the local leadership led to complete vaccination status in the village and had a ripple effect in the neighbouring villages [147,148]. The success story directs towards the need of moving away from hospital-based vaccination to satellite vaccination centres closer to inhabitants of the villages [148]. Moreover, mobile vaccination vans equipped with basic infrastructure, vaccine storage, vaccinator and medical personnel have been deployed in the states of Maharashtra, Telangana, Karnataka, Kerala, and Delhi with help of civil society and private organizations to reach the inaccessible areas [149].
Few regions also tried using disincentives and incentives to address vaccine hesitancy as mandatory requirement of vaccine certificates to acquire job in government schemes, obtain ration from public distribution system and access other government social security schemes [150], [151], [152]. These coercive measures were however unacceptable from equity, rights and social justice perspective and could not fill the gaps in information, rather they led to higher misconceptions that government is trying to complete targets by adopting unfair means. This scepticism in vaccination and in the health system of the country could further lead to fake vaccination certificates and development of illegal markets for provision of such certificates. Therefore, it is essential to work closer to the communities and adopt a bottom-up inclusive approach to tackle hesitancy. While there is no “one size fits all” approach to tackle resistance to vaccination, it is essential to contextualize the successful strategies by addressing the determinants to hesitancy. A faster vaccination drive would require strong context specific information campaigns, financial and non-financial incentives, easy accessibility to vaccines with enhanced focus on rural areas and vaccination for the female gender.
The review presents a comprehensive view of strategies implemented by the Indian government but is limited in its attempt to critically evaluate the effects of COVID-19 outbreak and related interventions as travel restrictions, lockdowns, health systems strengthening strategies etc. Another limitation of the study is that it does not present detailed data on vaccine trials and action of vaccines on human body and is more focussed on vaccination strategies adopted by the Indian government.
Conclusion
The global impact of COVID-19 has made it a priority internationally and all countries have tried to tackle the disastrous situation to the best of their capacities. Vaccination is an important strategy which has the potential to avert hideous consequences of the disease and reduce mortalities. The federal government played an important role in planning, procurement, distribution, and price setting of the vaccines and the state governments focused on implementation of the campaign. The monopoly of central government in vaccine procurement resulted in bulk orders at low price rates. However, the implementation of liberalized policy led to differential pricing and vaccine manufacturers quoted higher rates for the state governments and private health units, while maintaining comparatively low rates for central government. The subsequent revision in vaccination strategy, which enlarged the procurement role of central government accelerated the drive. The dependence on private sector for delivery of vaccines did not contribute significantly to fast track the program.
The risk-based prioritization strategy adopted by the government streamlined the campaign and averted chaotic situation in presence of vaccine scarcity. However sudden extension of the program to 18-44 years old population despite system readiness issues was debated. India was in a privileged situation considering the existing infrastructure and experienced human resources required to deliver successful immunizations since decades. But availability of vaccines to battle COVID-19 waves and vaccine hesitancy among the citizens posed as consistent bottlenecks for achievement of set targets. The government utilized technology for maintaining a database of vaccination in terms of procurement, distribution, utilization, and monitoring of the vaccination coverage. It was also used by the citizens for registrations, location of nearest vaccination centres, and generation of certifications, but this technology leverage needs to be integrated with a strong privacy policy, digital literacy, and linguistic inclusion to promote access to vaccinations.
The goal of inoculating the entire population would require a proactive approach to ensure availability, affordability, and accessibility to vaccines. These initiatives will have to be coupled with constant and targeted awareness campaigns and a bottom-up approach to fade hesitancy and raise demand for vaccines. National as well global studies, along with transparent sharing of captured data on procurement, distribution and vaccination coverage stratified by regions and various socio-demographic factors will help to periodically evaluate the bottlenecks in the programme in nascent stage, design context-based interventions to fortify the campaign and achieve equity and efficiency in the life saving initiative.
Public Interest Summary
The article presents a comprehensive review of COVID-19 related policies with focus on vaccination for containment of the pandemic in India. The five-point strategy adopted by government of India was “COVID appropriate behaviour, test, track, treat and vaccinate”. There have been periodic shifts in the COVID-19 vaccination policies in terms of eligible beneficiaries, procurement, and distribution plans, import and export strategy, involvement of private sector and use of technology. The monopoly of central government in vaccine procurement resulted in bulk orders at low prices. However, the implementation of liberalized policy with division of responsibility of vaccine procurement among national government, state governments and private sector led to differential pricing and delayed achievement of targets. A wavering pattern was observed in the vaccination coverage, which was related majorly to vaccine availability and hesitancy. The campaign will require consistent monitoring for timely identification of bottlenecks for the lifesaving initiative.
Funding
None.
Ethical Approval
Not required
Patient consent
Not required
Declaration of Competing Interest
None declared
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.hlpt.2022.100636.
Appendix. Supplementary materials
References
- 1.National Health Portal. Government of India . 2020. Coronavirus disease 2019 (COVID-19)nhp.gov.in/disease/communicable-disease/novel-coronavirus-2019-ncov [citedJuly 26] Available from. [Google Scholar]
- 2.Human Rights Watch . 2020. Human Rights dimensions of COVID 19 response.https://www.hrw.org/news/2020/03/19/human-rights-dimensions-covid-19-response [citedJuly 26] Available from. [Google Scholar]
- 3.2021. India population.https://www.worldometers.info/world-population/india-population/ [Internet] [citedSeptember 7] Available from. [Google Scholar]
- 4.Ministry of Statistics and Programme Implementation. Government of India . 2016. Elderly in India.http://mospi.nic.in/sites/default/files/publication_reports/ElderlyinIndia_2016.pdf [cited 2021 September 6] Available from. [Google Scholar]
- 5.The World Bank . 2021. Age dependency ratio. (% of working population) – India.https://data.worldbank.org/indicator/SP.POP.DPND?locations=IN [citedSeptember 6] Available from. [Google Scholar]
- 6.The World Bank . 2021. GDP per capita (current US$) -India. 2020.https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=IN [citedSeptember 6] Available from. [Google Scholar]
- 7.Central Bureau of Health Intelligence. Ministry of Health and Family Welfare. Government of India . 2021. National Health Profile. 2019. 14th issue.http://www.cbhidghs.nic.in/showfile.php?lid=1147 [citedSeptember 6] Available from. [Google Scholar]
- 8.Gupta M.D., Rani M. World Bank Policy Research Working Paper; 2004. India's Public Health System. How well does it function at national level?https://openknowledge.worldbank.org/bitstream/handle/10986/14215/WPS3447.pdf?sequence=1&isAllowed=y 3447Available from. [Google Scholar]
- 9.NitiAyog. Public Healthcare system. Chapter VIII. Available from: https://niti.gov.in/planningcommission.gov.in/docs/aboutus/committee/strgrp/stgp_fmlywel/sgfwch8.pdf.
- 10.Jaffrelot C. Jumle V. Private healthcare in India: boons and banes. Institut Montaigne. Available from: https://www.institutmontaigne.org/en/blog/private-healthcare-india-boons-and-banes.
- 11.The Economic Times; 2020. Avoid travel to China: government advisory.https://economictimes.indiatimes.com/news/politics-and-nation/avoid-travel-to-china-government-advisory/articleshow/73752718.cms?from=mdr January 30 [Internet] [cited 2020 July 30]. Available from. [Google Scholar]
- 12.Press Information Bureau. Ministry of Health and Family Welfare. Government of India . 2020. Cabinet Secretary chairs high level review meeting on COVID19.https://pib.gov.in/PressReleasePage.aspx?PRID=1604029 February 22 [Internet] [cited 2021 July 30] Available from. [Google Scholar]
- 13.Ministry of Information and Broadcasting. Government of India . 2020. India's response to COVID outbreak.https://pib.gov.in/PressReleasePage.aspx?PRID=1608727 March 28. [cited on 2021 July 28] Available from. [Google Scholar]
- 14.Ministry of Home Affairs. Government of India . 2020. Consolidated revised guidelines to be taken by ministries/departments of Government of India, states/UT governments, and state/UT authorities for containment of COVID-19 in the country.https://www.mha.gov.in/sites/default/files/MHA%20order%20dt%2015.04.2020%2C%20with%20Revised%20Consolidated%20Guidelines_compressed%20%283%29.pdf April 15 [cited 2021 August 1] Available from. [Google Scholar]
- 15.Rodrigues C.M.C., Plotkin S.A. Impact of vaccines, Health, economic and social perspectives. Front Microbiol. 2020 doi: 10.3389/fmicb.2020.01526. https://www.frontiersin.org/articles/10.3389/fmicb.2020.01526/full#B33 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKinsey and company; 2021. Fast forward: will the spread of COVID 19 vaccine development reset industry norms?https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/fast-forward-will-the-speed-of-covid-19-vaccine-development-reset-industry-norms May 13. Aavailbale from: [Google Scholar]
- 17.Indian Council of Medical Research. Department of Health Research . 2020. Revised strategy of COVID-19 testing in India.https://www.icmr.gov.in/pdf/covid/strategy/2020-03-20_covid19_test_v3.pdf March 20. [cited 2021 July 30] Available from. [Google Scholar]
- 18.Press Trust of India; 2020. Coronavirus: government allows private labs to conduct tests for COVID-19, caps costs at Rs 4500.https://www.indiatoday.in/india/story/coronavirus-govt-allows-private-labs-to-conduct-tests-for-covid-19-caps-cost-at-rs-4-500-1658332-2020-03-22 March 22. [Internet] [cited 2021 July 28] Available from. [Google Scholar]
- 19.Ministry of Health and Family Welfare. Government of India . 2020. Guidelines for COVID-19 testing in private laboratories in India.https://www.mohfw.gov.in/pdf/NotificationofICMguidelinesforCOVID19testinginprivatelaboratoriesiIndia.pdf March 21 [cited 2021 August 1] Available from. [Google Scholar]
- 20.Indian Council of Medical Research. Department of Health Research . 2021. COVID timeline.https://www.icmr.gov.in/COVIDTimeline/cindex.html [citedAugust 1] Available from: [Google Scholar]
- 21.Indian Council of Medical Research. Department of Health Research . 2020. Guidance on the use of Truenat beta CoV.https://www.icmr.gov.in/pdf/covid/labs/Guidance_TrueNat_14042020.pdf April 14. [cited 2021 August 1] Available from. [Google Scholar]
- 22.Indian Council of Medical Research. Department of Health Research . 2020. Augmented plan to fast-track the COVID-19 testing laboratory scale up.https://www.icmr.gov.in/pdf/covid/labs/Centres_of_Excellence_for_Mentoring_Medical_Colleges.pdf April 12. [cited 2021 August 1] Available from. [Google Scholar]
- 23.Indian Council of Medical Research. Department of Health Research . 2020. Advisory on use of rapid antigen detection test for COVID-19.https://www.icmr.gov.in/pdf/covid/strategy/Advisory_for_rapid_antigen_test14062020.pdf June 14. [cited 2021 August 1] Available from. [Google Scholar]
- 24.Indian Council of Medical Research. Department of Health Research . 2020. Advisory on new additional strategies for COVID-19 testing.https://www.icmr.gov.in/pdf/covid/strategy/New_additional_Advisory_23062020_3.pdf June 23. [cited 2021 August 1] Available from. [Google Scholar]
- 25.MedicalCouncil of India . 2020. Inclusion of BSL-2 level laboratory testing facility of infectious pathogens in all medical colleges.https://www.nmc.org.in/MCIRest/open/getDocument?path=/Documents/Public/Portal/LatestNews/Circular-BSL2%20Lab-07.07.2020.pdf July 20 [cited 2021 August 1] Available from. [Google Scholar]
- 26.Indian Council of Medical Research. Department of Health Research . 2021. Advisory for COVID-19 testing during the second wave of the pandemic.https://www.icmr.gov.in/pdf/covid/strategy/Advisory_COVID_Testing_in_Second_Wave_04052021.pdf May 4. [cited 2021 August 1] Available from. [Google Scholar]
- 27.Ministry of Health and Family Welfare. Government of India . Novel Coronavirus disease 2019; 2021. Containment Plan for large outbreaks.https://www.mohfw.gov.in/pdf/3ContainmentPlanforLargeOutbreaksofCOVID19Final.pdf [citedAugust 1] Available from. [Google Scholar]
- 28.Perappadan B.S. The Hindu; 2020. Coronavirus. Health Ministry identifies 130 districts as red zones.https://www.thehindu.com/news/national/coronavirus-india-lists-red-zones-as-it-extends-lockdown-till-may-17/article31478592.ece May 1. [cited 2021 August 1] Available from. [Google Scholar]
- 29.Ministry of Electronics and Information technology. Government of India . 2020. Aarogya setu is now open source.https://pib.gov.in/PressReleasePage.aspx?PRID=1626979 May 26. [cited 2021 August 1] Available from. [Google Scholar]
- 30.Ministry of Home Affairs. Government of India . 2021. Containment framework and implementation framework for community containment/large containment zones.https://www.mha.gov.in/sites/default/files/MHAOrder_29042021.pdf April 29. [cited 2021 August 1] Available from. [Google Scholar]
- 31.Ministry of Health and Family Welfare. Government of India . 2020. Government of India sanctions Rs. 15000 crores for India COVID-19 emergency response and health system preparedness package.https://pib.gov.in/PressReleaseIframePage.aspx?PRID=1612534 April 9 [cited 2021 August 1] Available from. [Google Scholar]
- 32.2021. NHM guidance note on India COVID-19 emergency response and health system preparedness package.http://nhm.gov.in/New_Updates_2018/COVID-19%20ER%26HSP%20PACKAGE/Guidance%20note_COVID%20package.pdf [citedAugust 1] Available from. [Google Scholar]
- 33.Pakrasi S. Centre releases Rs 890 crore as second tranche of package for COVID-19 hindustantimes.com/india-news/centre-releases-rs-890-crore-as-2nd-tranche-of-package-for-covid-19-health-system-preparedness/story-AShKo5gfXmWLDfYi2I1D8L.html.
- 34.Cabinet . 2021. Cabinet approves India COVID-19 emergency response and health systems preparedness package: phase II at a cost of Rs. 23,123 crores.https://pib.gov.in/PressReleaseIframePage.aspx?PRID=1733841 July 8. [cited 2021 August 1] Available from. [Google Scholar]
- 35.Ministry of Health and Family Welfare. Government of India . 2020. GOM reviews containment measures of COVID-19.https://www.pib.gov.in/PressReleasePage.aspx?PRID=1630445 June 9. [cited 2021 August 1] Available from. [Google Scholar]
- 36.Ministry of Textiles, Government of India . 2020. Export of PPE suits.https://pib.gov.in/PressReleasePage.aspx?PRID=1656231 September 18. [cited 2021 August 1] Available from. [Google Scholar]
- 37.Ministry of Health and Family Welfare. Government of India . 2020. Ensuring quality of PPEs through stringent protocols.https://pib.gov.in/PressReleasePage.aspx?PRID=1626699 May 25. [cited 2021 August 1] Available from. [Google Scholar]
- 38.Reserve Bank of India . 2021. Annual Report Annexure 2.https://rbi.org.in/scripts/AnnualReportPublications.aspx?Id=1327 May 27. [cited 2021 August 1] Available from. [Google Scholar]
- 39.NITI Aayog . 2020. NITI Aayog launches behaviour change campaign, ‘Navigating the new normal’ and website.https://www.pib.gov.in/PressReleasePage.aspx?PRID=1634328 June 25 [cited 2021 August 1] Available from. [Google Scholar]
- 40.RajyaSabha. Parliament of India . One hundred twenty third report on The Outbreak of pandemic COVID-19 and its management. 2020. Department related Parliament standing committee on Health and Family welfare.https://rajyasabha.nic.in/rsnew/Committee_site/Committee_File/ReportFile/14/142/123_2020_11_15.pdf November [cited 2021 August 1] Available from. [Google Scholar]
- 41.Press Trust of India; 2020. Health ministry activates new toll-free number 1075 for queries on COVID-19.https://www.business-standard.com/article/pti-stories/health-ministry-launches-new-toll-free-number-email-id-for-queries-on-covid-19-120031601280_1.html March 16. [cited 2021 August 1] Available from. [Google Scholar]
- 42.Ministry of Health and Family Welfare. Government of India . 2020. Dr Harshvardhan holds a meeting on Jan Andolan and COVID appropriate behaviour with heads of all AIIMS and Central Government hospitals.https://www.pib.gov.in/PressReleasePage.aspx?PRID=1664448 October 14. [cited 2021 August 1] Available from. [Google Scholar]
- 43.Office of Principal Scientific Advisor to Government of India . 2021. Office of the Principal Scientific Advisor to the Government of India releases advisory on “Stop the transmission, crush the pandemic- masks, distance, sanitation and ventilation to prevent the spread of SARS-CoV-2 virus.https://www.pib.gov.in/PressReleasePage.aspx?PRID=1720088 May 20. [cited 2021 August 1] Available from. [Google Scholar]
- 44.Dutta SS. The New Indian Express; 2021. India gets fifth Covid vaccine as Johnson and Johnson's single shot vaccine gets nod.https://www.newindianexpress.com/nation/2021/aug/07/india-gets-fifth-covid-vaccine-as-johnson–johnsons-single-shot-vaccine-gets-nod-2341527.html August 7. [cited 2021 September 07]. Available from. [Google Scholar]
- 45.Financial Express . 2021. Covid-19: Zydus Cadila's ZyCoV-D vaccine gets nod for emergency use in India- here's everything you must know about country's sixth vaccine.https://www.financialexpress.com/lifestyle/health/covid-19-zydus-cadilas-zycov-d-vaccine-gets-nod-for-emergency-use-in-india-heres-everything-you-must-know-about-countrys-sixth-vaccine/2314938/ August 21. [cited 2021 September 07]. Available from. [Google Scholar]
- 46.COVID-19 vaccine tracker . 2021. Vaccine candidates.https://covid19.trackvaccines.org/vaccines/ [citedSeptember 07]. Available from. [Google Scholar]
- 47.Ministry of Health and Family Welfare, Government of India . 2020. COVID-19 vaccine. Operational guidelines.https://www.mohfw.gov.in/pdf/COVID19VaccineOG111Chapter16.pdf Available from. [Google Scholar]
- 48.Ministry of Health and Family Welfare. Government of India . 2021. Precautions and Contraindications of COVID-19 vaccination.https://www.mohfw.gov.in/pdf/LetterfromAddlSecyMoHFWregContraindicationsandFactsheetforCOVID19vaccines.PDF January 14. [cited 2021 August 1] Available from. [Google Scholar]
- 49.Ministry of Health and Family Welfare. Government of India . 2021. Government announces a liberalized and accelerated Phase 3 strategy of COVID-19 vaccination from 1st May.https://pib.gov.in/PressReleasePage.aspx?PRID=1712710 April 19 [cited 2021 August 1] Available from. [Google Scholar]
- 50.SupremeCourt of India. Distribution of essential supplies and services during pandemic. Sou Motu Writ Petition (Civil) No. 3 of 2021. Available from: https://main.sci.gov.in/supremecourt/2021/11001/11001_2021_35_301_27825_Judgement_30-Apr-2021.pdf.
- 51.Gill P. Business Insider; India: 2021. 4.6 million doses of COVID-19 vaccine were wasted in India- enough to vaccinate half of Bangalore.https://www.businessinsider.in/science/health/news/4-6-million-doses-of-covid-19-vaccine-were-wasted-in-india-enough-to-vaccinate-half-of-bangalore/articleshow/82165181.cms April 20. [cited 2021 September 03] Available from. [Google Scholar]
- 52.Nagarajan R. The Times of India; 2021. 1% wastage target could be inimical to vax drive: experts.https://timesofindia.indiatimes.com/india/1-wastage-target-could-be-inimical-to-vax-drive-experts/articleshow/83357352.cms June 9. [cited 2021 September 3]. Available from. [Google Scholar]
- 53.Ministry of Health and Family Welfare. Government of India. Revised guidelines for implementation of National COVID Vaccination program. Available from: https://www.mohfw.gov.in/pdf/RevisedVaccinationGuidelines.pdf.
- 54.Sharma A. News 18; 2021. No need to reserve 25% vaccine for private players, government tells manufacturers as 7-9% lie unused.https://www.news18.com/news/india/no-need-to-reserve-25-vaccine-for-private-players-govt-has-told-manufacturers-as-7-9-doses-lie-unused-4043507.html August 4. [Internet] [cited 2021 September 07]. Available from. [Google Scholar]
- 55.Ministry of Health and Family Welfare, Government of India . 2021. Lok Sabha unstarred question No. 786. to be answered on 23.07. [Google Scholar]
- 56.Sharma N. The Economic Times; 2021. Centre's vaccination bill nears Rs 9725 crore, Lok Sabha told.https://economictimes.indiatimes.com/news/india/centres-vaccination-bill-nears-9725-cr/articleshow/84687337.cms July 23. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 57.Ministry of Health and Family Welfare, Government of India . 2021. Vaccine availability. Lok Sabha, unstarred question number 716. to be answered on 23rd July. [Google Scholar]
- 58.The Economic Times . The Economic Times; 2021. Rs 19,675 cr spent on COVID-19 vaccine procurement: Govt data.https://economictimes.indiatimes.com/news/india/rs-19675-cr-spent-on-covid-19-vaccine-procurement-govt-data/articleshow/88455933.cms?from=mdr December 23. [cited 2022 April 18]. Available from. [Google Scholar]
- 59.Ramakumar R. The Indian Express; 2021. Why India is missing its vaccine targets.https://indianexpress.com/article/opinion/columns/why-india-is-missing-its-covid-vaccine-targets-7410990/ July 19 [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 60.Mint; 2021. Government places vaccine orders till July.https://www.livemint.com/news/india/govt-says-have-orders-for-over-160-million-covid-19-vaccine-doses-till-july-11620036834963.html May 4. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 61.Kaul R. Hindustan Times; 2021. Covaxin output to increase to 58 million doses per month, RS told.https://www.hindustantimes.com/india-news/covaxin-output-to-increase-to-58mn-doses-per-month-rs-told-101626804936682.html July 20. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 62.Sharma K. Nikkei Asia; 2021. India raises COVID jab production amid sharp dip in cases.https://asia.nikkei.com/Spotlight/Coronavirus/India-raises-COVID-jab-production-amid-sharp-dip-in-cases July 7. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 63.Press Trust of India; 2021. Current average monthly production capacity of Covishield 11 crore doses, Covaxin 2.5 crore doses: Government.https://in.news.yahoo.com/current-average-monthly-production-capacity-130634294.html July 20. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 64.Ghosh A. The Print; 2021. At 16.4 crore doses, SII's increased Covishield output helps India cross August vaccination target.https://theprint.in/health/at-16-4-cr-doses-siis-increased-covishield-output-helps-india-cross-aug-vaccination-target/724533/ August 30. [cited 2021 September 3]. Available from. [Google Scholar]
- 65.Shrivastava R. India Today; 2021. Centre approves Rs 3000 crore funds for Serum Institute, Rs. 1500 crore for Bharat Biotech to ramp up vaccine production.https://www.indiatoday.in/coronavirus-outbreak/vaccine-updates/story/centre-to-extend-rs-3000-crore-credit-to-serum-institute-rs-1500-crore-to-bharat-biotech-1792691-2021-04-19 April 20. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 66.Jayan TV, Vora R. Business Line; 2021. Not much Covaxin supply seen from PSUs till early next year.https://www.thehindubusinessline.com/economy/not-much-covaxin-supply-seen-from-psus-till-early-next-year/article35397972.ece July 18 [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 67.Malik F. Hindustan Times; 2021. Haffkine gets Maharashtra government nod, Rs 94 crore grant for Covaxin.https://www.hindustantimes.com/cities/mumbai-news/haffkine-gets-maharashtra-govt-nod-94-crore-grant-for-covaxin-101619638059512.html April 29. [Internet] [cited 2021 August 1]. Available from. [Google Scholar]
- 68.Press Information Bureau. Ministry of Health and Family Welfare. Government of India . 2021. Update on COVID-19 vaccine manufacturing capacity.https://pib.gov.in/PressReleasePage.aspx?PRID=1781267 December 14 [cited 2022 April 18]. Available from. [Google Scholar]
- 69.Business Standard; 2021. Covid: Centre exempts customs duty on vaccines, oxygen for 3 months.https://www.business-standard.com/article/current-affairs/govt-waives-off-customs-duty-health-cess-on-oxygen-related-equipment-121042400490_1.html April 24. [Internet] [cited 2021 August 1]. Available from. [Google Scholar]
- 70.Ministry of Health and Family Welfare. Government of India . 2021. Centre fast tracks emergency approvals for foreign produced COVID-19 vaccines that have been granted EUA in other countries to expand the basket of vaccines for domestic use and hasten the pace and coverage of vaccination.https://pib.gov.in/PressReleasePage.aspx?PRID=1711381 April 13. [cited 2021 August 1] Available from. [Google Scholar]
- 71.Bose J. Hindustan Times; 2021. Foreign approved vaccines no longer need bridging trials in India: DGCI.https://www.hindustantimes.com/india-news/foreignapproved-vaccines-no-longer-need-bridging-trials-in-india-dcgi-101622611412866.html June 2. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 72.Gaon Connection; 2021. Explained: Why the delay in Sputnik V commercial launch in India?https://en.gaonconnection.com/sputnik-v-vaccine-india-russia-commercial-launch-delay-explained-dr-reddys-efficacy-who-europe-coronavirus-vaccination/ July 13. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 73.Ministry of Health and Family Welfare, Government of India . 2021. Indemnity for international vaccines. Lok Sabha, unstarred question number 780. to be answered on 23rd July. [Google Scholar]
- 74.Chandra P. Deccan Herald; 2021. As vaccine maitri flops, China steps in.https://www.deccanherald.com/opinion/in-perspective/as-vaccine-maitri-flops-china-steps-in-992307.html June 1. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 75.Ministry of Health and Family Welfare. Government of India. COVID-19 Vaccination, All you need to know. Healthcare and Frontline Workers Guide. Available from: https://www.mohfw.gov.in/pdf/COVID19VaccinationGuideforHealthcareandFrontlineWorkers.pdf.
- 76.Science the Wire . 2020. Nearly half the people who have died of COVID-19 in India are younger than 60.https://science.thewire.in/health/india-covid-19-mortality-comorbidities-age-health-ministry/ October 14. [Internet] [Cited 2021 September 07]. Available from. [Google Scholar]
- 77.Kaul R, Dutt A. Hindustan Times; 2021. Paid shots from March 1 as India expands COVID-19 vaccine drive.https://www.hindustantimes.com/india-news/paid-shots-from-march-1-as-india-expands-covid-19-vaccine-drive-101614210407582.html February 25. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 78.Bhattacharya S. India Spend; 2021. More younger people without comorbidities died in second wave, Data show.https://www.indiaspend.com/covid-19/covid-19-second-wave-deaths-data-variants-comorbidity-757997 June 28. [Internet] [cited 2021 September 07]. Available from. [Google Scholar]
- 79.Sood R, Kapur K., Kurian O.C. Observer Research Foundation; 2021. India's vaccine rollout: A reality check.https://www.orfonline.org/research/indias-vaccine-rollout-a-reality-check/ May 31. [cited 2021 August 1] Available from. [Google Scholar]
- 80.Mint . 2021. Centre approves COVID-19 vaccination for pregnant women.https://www.livemint.com/news/india/centre-approves-covid-19-vaccination-for-pregnant-women-11624635010798.html June 25. [Internet] [cited 2021 September 07]. Available from. [Google Scholar]
- 81.Ministry of Health and Family Welfare. Government of India . 2022. Guidelines for COVID-19 vaccination of children between 15-18 years and precaution dose to HCWs, FLWs and 60+ populations with co-morbidities.https://www.mohfw.gov.in/pdf/GuidelinesforCOVID19VaccinationofChildrenbetween15to18yearsandPrecautionDosetoHCWsFLWs&60populationwithcomorbidities.pdf [cited 2022 April 18]. Available from. [Google Scholar]
- 82.Sircar S. Science The Wire; 2021. We need to identify and eliminate vaccine hesitancy among healthcare workers.https://science.thewire.in/health/healthcare-workers-vaccine-hesitancy/ April 20. [Internet] [cited 2021 September 07]. Available from. [Google Scholar]
- 83.Dey S. The Times of India; 2021. Only 37% of 3 crore health, frontline workers fully vaccinated.https://timesofindia.indiatimes.com/india/only-37-of-3-crore-health-frontline-workers-fully-vaccinated/articleshow/82135322.cms April 19. [Internet] [cited 2021 September 07]. Available from. [Google Scholar]
- 84.Nayak M. india.com; 2021. Over 70 vaccine centres in Mumbai shut after shortage, Govt calls the Information farce.https://www.india.com/maharashtra/vaccine-shortage-hits-mumbai-as-more-than-70-centres-shut-govt-says-information-not-right-key-points-4570875/ April 9. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 85.Yeung J. CNN; 2021. The world's biggest vaccine producer is running out of COVID-19 vaccines, as second wave accelerates.https://edition.cnn.com/2021/04/17/india/covid-vaccine-shortage-covishield-covaxin-intl-hnk-dst/index.html April 18. [Internet] [Cited 2021 August 1] Available from. [Google Scholar]
- 86.Hindustan Times; 2021. 10 states face severe Covid-19 vaccine shortage. Here's a list.https://www.hindustantimes.com/india-news/10-states-face-severe-covid-19-vaccine-shortage-here-s-a-list-101618047783891.html April 10. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 87.Pakrasi S. Hindustan Times; 2021. These states won't start Phase 3 Covid-19 vaccination drive from today.https://www.hindustantimes.com/india-news/these-states-won-t-start-phase-3-covid-19-vaccination-drive-from-todayseveral-st-101619831661831.html May 1. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 88.NPR; 2021. India is the world's biggest vaccine maker. Yet only 4% of Indians are vaccinated.https://www.npr.org/sections/goatsandsoda/2021/06/29/1011022472/india-is-the-worlds-biggest-vaccine-maker-yet-only-4-of-indians-are-vaccinated June 29. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 89.The Hindu; 2021. Vaccination declines by 60% as States say they have no doses.https://www.thehindu.com/news/national/coronavirus-vaccination-declines-by-60-as-states-say-they-have-no-doses/article35303316.ece July 14. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 90.Press Trust of India; 2021. Vaccine hesitancy in some communities a cause of concern: Amit Shah.https://www.ndtv.com/india-news/vaccine-hesitancy-in-some-communities-a-cause-of-concern-amit-shah-2485113 July 12. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 91.Livemint . 2021. India administers over 1.2 crore vaccine doses, sets new single day record.https://www.livemint.com/news/india/india-administers-over-1-cr-covid-vaccine-doses-in-a-day-for-second-time-50-cr-people-get-first-jab-11630414826384.html August 31. [Internet] [cited 2021 September 07]. Available from. [Google Scholar]
- 92.Singh N. News 18; 2021. August news for India's vaccination as drive picks up amid 3rd wave fear: Government data.https://www.news18.com/news/india/august-brings-in-good-news-for-indias-vaccination-as-drive-picks-up-amid-3rd-wave-fear-data-4126892.html August 25. [Internet] [cited 2021 September 07]. Available from. [Google Scholar]
- 93.Ministry of Health and Family Welfare. Government of India . 2021. Gap between two doses of Covishield vaccine extended from 6-8 weeks to 12-16 weeks based on recommendation of COVID working group.https://pib.gov.in/PressReleasePage.aspx?PRID=1718308 May 13. [cited 2021 August 1] Available from. [Google Scholar]
- 94.Ministry of Health and Family Welfare. Government of India . 2021. SOPs on administration of second dose of Covishield vaccine prior to prescribed time interval to persons intending to undertake international travel for education purpose, for joining employment in foreign countries and for India's contingent to Tokyo Olympics.https://www.mohfw.gov.in/pdf/AdministrationofSecondDoseofCovishieldVaccinePriortoPrescribedTimeInterval.pdf [cited 2021 August 1] Available from. [Google Scholar]
- 95.The Hindu; 2021. Private hospitals can charge up to Rs 250 per dose for COVID-19 vaccine.https://www.thehindu.com/sci-tech/health/covid-19-vaccine-private-hospitals-can-charge-up-to-rs-250-per-dose/article33949740.ece February 27. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 96.Ministry of Health and Family Welfare. Government of India . 2021. COVID 19 vaccine supply to private hospitals. Rajya Sabha unstarred question number 896. to be answered on 27.07. [Google Scholar]
- 97.Singh A. The Republic World; 2021. Private hospitals to charge Rs 150 service charge and 5% GST on COVID vaccine fixed price: MOHFW.https://www.republicworld.com/india-news/general-news/pvt-hospitals-to-charge-rs-150-service-charge-and-5-percent-gst-on-covid-vaccine-fixed-price-mohfw.html June 8. [Internet] [cited 2021 September 07]. Available from. [Google Scholar]
- 98.Najarajan R. The Times of India; 2021. Covid 19: Since May 1, private sector gave 7% of jabs despite 25% quota.https://timesofindia.indiatimes.com/india/covid-19-since-may-1-private-sector-gave-7-of-jabs-despite-25-quota/articleshow/84778026.cms July 27. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 99.Sharma M. 2021. Explained: Why Covid 19 vaccines in private hospitals are lying unused.https://www.indiatoday.in/coronavirus-outbreak/vaccine-updates/story/explained-why-covid-19-vaccines-in-private-hospitals-are-lying-unused-1834299-2021-07-29 July 29. [cited 2022 January 31] Available from. [Google Scholar]
- 100.The Economic Times; 2021. Private hospitals can't directly procure COVID vaccines, need to place orders on CoWIN from July.https://economictimes.indiatimes.com/industry/healthcare/biotech/healthcare/private-hospitals-cant-directly-procure-covid-vaccines-need-to-place-orders-on-cowin-from-july-1/articleshow/83978951.cms June 30. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 101.First post . 2021. India's COVID-19 vaccine milestone: At least 1 jab to 51% adults, August has been best month ever.https://www.firstpost.com/india/indias-covid-19-vaccine-milestone-at-least-1-jab-to-51-adults-august-has-been-best-month-ever-9920091.html August 28. [Internet] [cited 2021 September 07]. Available from. [Google Scholar]
- 102.Kapur M. Quartz India; 2021. Will India meet its target of vaccinating 300 million people by August?https://qz.com/india/2029670/can-india-meet-its-covid-19-vaccination-targets-for-2021/ July 6. [Internet] [cited 2021 August 1]. Available from. [Google Scholar]
- 103.Sharma R. 2021. CoWin upgrade, 50 lakh daily target: What to expect as India vaccinates citizens above 45.https://www.news18.com/news/india/covid-19-vaccination-for-everyone-above-45-years-starts-from-april-1-heres-all-you-need-to-know-3591725.html April 3. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 104.India Today; 2021. Target is to administer 1 crore COVID vaccine doses each day for next 6-8 months: Dr N.K. Arora.https://www.indiatoday.in/coronavirus-outbreak/story/target-is-to-administer-1-crore-covid-vaccines-doses-each-day-for-next-6-8-months-dr-nk-arora-1820011-2021-06-27 June 27. [Internet] [cited 2021 August 1]. Available from. [Google Scholar]
- 105.Press Information Bureau. Government of India. PIBs special webpage on COVID-19. Available from: https://pib.gov.in/newsite/coronaviruss.aspx.
- 106.Chintan R. The Leaflet; 2021. Vaccination drive in India: A central government botch-up?https://www.theleaflet.in/vaccination-drive-in-india-a-central-government-botch-up/ May 27. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 107.Bhattacharya G. Gulf News; 2021. 300 million target in Phase 1: How will India's COVID-19 vaccination plan work?https://gulfnews.com/world/asia/india/300-million-target-in-phase-i-how-will-indias-covid-19-vaccination-plan-work-1.76205514 January 1. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 108.Ministry of Health and Family Welfare . Ministry of Health and Family Welfare, Government of India; 2021. Initial procurement amount of 1.65 crore doses of Covishield and Covaxin vaccines allocated to all States/UTs. January 14[cited 2022 January 31] Available from https://pib.gov.in/PressReleasePage.aspx?PRID=1688456. [Google Scholar]
- 109.Misra S. The Print; 2021. This is how Modi government will decide Covid vaccine quota for states. April 23. [Internet] [cited 2021 September 07] Available from https://theprint.in/theprint-essential/this-is-how-modi-govt-will-decide-covid-vaccine-quota-for-states/644114/ [Google Scholar]
- 110.Ministry of Health and Family Welfare. Government of India . 2021. Liberalized pricing and accelerated national COVID-19 vaccination strategy.https://www.mohfw.gov.in/pdf/LiberalisedPricingandAcceleratedNationalCovid19VaccinationStrategy2042021.pdf April 21. [cited 2021 August 1] Available from. [Google Scholar]
- 111.Mudur G.S. How we landed in vaccine mess. Telegraph India. [Internet] [cited 2021 August 1] Available from: https://epaper.telegraphindia.com/imageview_359302_24528134_4_undefined_19-04-2021_1_i_1_sf.html.
- 112.Padma T.V. Nature; 2021. India's COVID vaccine woes by the numbers.https://www.nature.com/articles/d41586-021-00996-y April 16. [Internet] [cited 2021 August 1] Available from. [DOI] [PubMed] [Google Scholar]
- 113.Becker I.S. Washington Post; 2021. Biden harnesses Defence Protection Act to speed vaccinations and production of protective equipment.https://www.washingtonpost.com/health/2021/02/05/biden-vaccines-tests-gloves/ February 5. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 114.ANI; 2021. Adar Poonawalla thanks Biden, Jaishankar as US lifts Defence Protection Act ratings on COVID vaccines.https://www.aninews.in/news/world/asia/adar-poonawalla-thanks-biden-jaishankar-as-us-lifts-defence-production-act-ratings-on-covid-vaccines20210604224926/ June 4. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 115.Chattu V.K., Singh B., Kaur J., Jakovljevic M. COVID-19 vaccine, TRIPS, and global health diplomacy: India's role at the WTO platform. Biomed Res Int. 2021 doi: 10.1155/2021/6658070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ministry of External Affairs, Government of India. Vaccine supply. [cited 2021 August 1] Available from: https://www.mea.gov.in/vaccine-supply.html.
- 117.Sundar . YKA; 2021. These two mistakes by the Government caused vaccine shortage in India.https://www.youthkiawaaz.com/2021/05/why-is-india-facing-a-vaccine-shortage/ May 17. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 118.Chintan R. News Click; 2021. COVID-19: How India and COVAX failed the South Asian countries.https://www.newsclick.in/COVID-19-India-COVAX-failed-South-Asian-Countries June 24. [cited 2022 January 31]. Available from. [Google Scholar]
- 119.Singh A. The Times of India; 2021. Rural-urban divide: 15% gap in administration of first dose of COVID-19 vaccination in Indore.https://timesofindia.indiatimes.com/city/indore/rural-urban-divide-15-gap-in-administration-of-first-dose-of-covid-19-vaccination-in-indore/articleshow/83425039.cms June 11. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 120.Madan A. ORF; 2021. Disparity in access to COVID-19 vaccination: The plight of poor vulnerable households.https://www.orfonline.org/expert-speak/disparity-in-access-to-covid-19-vaccination/ June 6. [cited 2021 August 1] Available from. [Google Scholar]
- 121.Iyer R., Maiorano D. ISAS Working Papers; 2021. India's COVID-19 vaccine policy.https://www.isas.nus.edu.sg/papers/indias-covid-19-vaccine-policy/ [cited 2022 January 31] Available from. [Google Scholar]
- 122.Pathak H. Observer Research Foundation; 2021. India's COVID-19 vaccination campaign: a marathon, not a spriny.https://www.orfonline.org/research/indias-covid-19-vaccination-campaign/ ORF Special Report 143[cited 2022 January 31] [Google Scholar]
- 123.Mullick J. Hindustan Times; 2021. The growing urban bias of India's vaccination drive.https://www.hindustantimes.com/india-news/the-growing-urban-bias-of-india-s-vaccination-drive-101623782827156.html June 16. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 124.Deccan Herald; 2021. Worrying gender gap in vaccination.https://www.deccanherald.com/opinion/first-edit/worrying-gender-gap-in-vaccination-997605.html June 15https://www.orfonline.org/research/indias-covid-19-vaccination-campaign/ [Google Scholar]
- 125.Hindustan Times; 2021. Six months into India's vaccination drive: what is right and what is not.https://www.hindustantimes.com/india-news/six-months-into-india-s-vaccination-drive-what-is-right-and-what-is-not-101626986375355.html July 23 [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 126.Madan A. ORF; 2021. Gender parity in the vaccination drive and its underlying causes.https://www.orfonline.org/expert-speak/gender-disparity-in-the-vaccination-drive-and-its-underlying-causes/ July 10. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 127.India's Covid gender gap: women left behind in vaccination drive. The Guardian. [Internet] [cited 2021 August 1] Available from: https://www.theguardian.com/global-development/2021/jun/28/india-covid-gender-gap-women-left-behind-in-vaccination-drive.
- 128.Rai S. Dara Quest; 2021. Citizens highlight CoWIN OTP issue and unavailability of appointments, Indian government responds.https://www.dqindia.com/citizens-highlight-cowin-otp-issue-unavailability-appointments-indian-government-responds/ May 9. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 129.OnCoWIN . The Hindu; 2021. Supreme Court flags digital divide.https://www.thehindu.com/news/national/on-cowin-supreme-court-flags-digital-divide/article34711169.ece June 2. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 130.2021. SFLC.in writes to PMO, MoHFW, and NHA raising concerns related to CoWIN.https://sflc.in/sflcin-writes-pmo-mohfw-nha-raising-concerns-related-cowin May 12. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 131.Rackimuthu S., Hasan M.M., Bardhan M., Essar M.Y. COVID-19 vaccination strategies and policies in India: the need for further re-evaluation is a pressing priority. Int J Health Plann Manage. 2021 doi: 10.1002/hpm.3321. 10.1002/hpm.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.2021. Vaccine hesitancy puts India's gains against coronavirus at risk.https://www.livemint.com/news/india/vaccine-hesitancy-puts-india-s-gains-against-coronavirus-at-risk-11624252466615.html June 21. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 133.Sinha C. BBC News; 2021. COVID India: women in rural Bihar hesitant to take vaccines.https://www.bbc.com/news/world-asia-india-57551345 July 1. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 134.Sarkar J., Das A. 2021. India Inc grapples with vaccine hesitancy.https://timesofindia.indiatimes.com/business/india-business/india-inc-grapples-with-vax-hesitancy/articleshow/84083830.cms July 5. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 135.Sadhu A. The Logical Indian; 2021. Four in ten adults over 70 years show vaccine hesitancy, says study.https://thelogicalindian.com/health/four-in-10-adults-over-70-years-show-vaccine-hesitancy-study-29403 July 6. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 136.The Indian Express; 2021. India has vaccine hesitancy challenge.https://indianexpress.com/article/opinion/india-has-a-vaccine-hesitancy-challenge-7388907/ July 4. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 137.Srinivas S. ORF; 2021. The curious case of vaccine hesitancy in Tamil Nadu.https://www.orfonline.org/expert-speak/the-curious-case-of-vaccine-hesitancy-in-tamil-nadu/ June 15. [cited 2021 August 1] Available from. [Google Scholar]
- 138.Umakanthan S., Patil S., Subramaniam N., Sharma R. COVID-19 vaccine hesitancy and resistance in india explored through a population-based longitudinal survey. Vaccines. 2021;9(10):1064. doi: 10.3390/vaccines9101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chakraborty C., Sharma A.R., Bhattacharya M., Agoramoorthy G., Lee S.S. The current second wave and COVID-19 vaccination status in India. Brain Behav Immun. 2021;96:1–4. doi: 10.1016/j.bbi.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Venugopal V. The Economic Times; 2021. UP awards pradhans, ropes in gurus, imams to tackle vaccine hesitancy.https://economictimes.indiatimes.com/news/india/up-awards-pradhans-ropes-in-gurus-imams-to-tackle-vaccine-hesitancy/articleshow/83164887.cms?from=mdr June 02. [cited 2022 January 31]. Available from. [Google Scholar]
- 141.Pachauli C. The Hindu; 2021. Tackling vaccine hesitancy challenge in rural India.https://www.thehindu.com/sci-tech/science/tackling-vaccine-hesitancy-challenge-in-rural-india/article34994324.ece June 26. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 142.Garari K. Deccan Chronicle; 2021. India's vaccine hesitancy is at high of 74.53 percent.https://www.deccanchronicle.com/nation/current-affairs/160621/indias-vaccine-hesitancy-is-at-a-high-of-7453.html June 16. [Internet] [cited 2021 August 1] Available from. [Google Scholar]
- 143.Changoiwala P. Science; 2021. How a village in India reached 100% vaccination in the face of misinformation and hesitancy.https://www.nationalgeographic.com/science/article/how-a-village-in-india-reached-100-vaccination-in-the-face-of-misinformation-and-hesitancy May 22. [cited 2022 January 31] Available from. [Google Scholar]
- 144.Tarfe A. The Indian Express; 2021. How India's COVID-19 communication strategy is failing to combat vaccine hesitancy.https://indianexpress.com/article/opinion/how-indias-covid-19-communication-strategy-is-failing-to-combat-vaccine-hesitancy-7342890/ June 03. [cited 2022 January 31] Available from. [Google Scholar]
- 145.Trivedi U., Kay C., Makol M.K. Bloomberg Business Week; 2021. India's race to vaccinate its villages meets with rural resistance.https://www.bloomberg.com/news/features/2021-11-09/covid-vaccine-india-races-to-vaccinate-poor-suspicious-citizens-in-villages November 09. [cited 2022 January 31] Available from. [Google Scholar]
- 146.Mandal S., Arinaminpathy N., Bhargava B., Panda S. Responsive and agile vaccination strategies against COVID-19 in India. Lancet Glob Health. 2021;9(9):E1197–E1200. doi: 10.1016/S2214-109X(21)00284-9. https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(21)00284-9/fulltext Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Krishnan M. DW Made for Minds; 2021. COVID: How India is vaccinating isolated tribes in insurgency-hit areas.https://www.dw.com/en/covid-how-india-is-vaccinating-isolated-tribes-in-insurgency-hit-areas/a-59722431 [cited 2022 January 31] Available from. [Google Scholar]
- 148.Venugopal V. The Economic Times; 2021. UP awards pradhans, ropes in gurus, imams to tackle vaccine hesitancy.https://economictimes.indiatimes.com/news/india/up-awards-pradhans-ropes-in-gurus-imams-to-tackle-vaccine-hesitancy/articleshow/83164887.cms?from=mdr June 02. [cited 2022 January 31] Available from. [Google Scholar]
- 149.Vora R.V. Business Line; 2021. Mobile vaccination gains popularity as centre peddle for inoculations.https://www.thehindubusinessline.com/news/mobile-vaccination-gains-popularity-as-centre-presses-peddle-for-inoculations/article38072528.ece December 30. [cited 2022 January 31] Available from. [Google Scholar]
- 150.Nadimpally S. Scroll; 2021. Why vaccine hesitancy should not be tackled through a carrot and stick policy.https://scroll.in/article/997603/why-vaccine-hesitancy-should-not-be-tackled-through-a-carrot-and-stick-policy June 18. [cited 2022 January 31] Available from. [Google Scholar]
- 151.Gupta S. Gaon Connection; 2021. No vaccination, no rations: Madhya Pradesh boosts vaccination campaign?https://en.gaonconnection.com/covid19-vaccination-rations-madhya-pradesh-shivraj-singh-chouhan-health-food-security-right-pds/ November 20. [cited 2022 January 31] Available from. [Google Scholar]
- 152.Gaon Connection . Health Experts Protest; 2021. Show COVID-19 vaccination certificate, get ration under PDS, says IMA.https://en.gaonconnection.com/covid19-vaccination-certificate-ration-indian-medical-health-association-age-bar-vaccine/ April 06. [cited 2022 January 31] Available from. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.