Abstract
Background
Spasticity and chronic neuropathic pain are common and serious symptoms in people with multiple sclerosis (MS). These symptoms increase with disease progression and lead to worsening disability, impaired activities of daily living and quality of life. Anti‐spasticity medications and analgesics are of limited benefit or poorly tolerated. Cannabinoids may reduce spasticity and pain in people with MS. Demand for symptomatic treatment with cannabinoids is high. A thorough understanding of the current body of evidence regarding benefits and harms of these drugs is required.
Objectives
To assess benefit and harms of cannabinoids, including synthetic, or herbal and plant‐derived cannabinoids, for reducing symptoms for adults with MS.
Search methods
We searched the following databases from inception to December 2021: MEDLINE, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library), CINAHL (EBSCO host), LILACS, the Physiotherapy Evidence Database (PEDro), the World Health Organisation International Clinical Trials Registry Platform, the US National Institutes of Health clinical trial register, the European Union Clinical Trials Register, the International Association for Cannabinoid Medicines databank. We hand searched citation lists of included studies and relevant reviews.
Selection criteria
We included randomised parallel or cross‐over trials (RCTs) evaluating any cannabinoid (including herbal Cannabis, Cannabis flowers, plant‐based cannabinoids, or synthetic cannabinoids) irrespective of dose, route, frequency, or duration of use for adults with MS.
Data collection and analysis
We followed standard Cochrane methodology. To assess bias in included studies, we used the Cochrane Risk of bias 2 tool for parallel RCTs and crossover trials. We rated the certainty of evidence using the GRADE approach for the following outcomes: reduction of 30% in the spasticity Numeric Rating Scale, pain relief of 50% or greater in the Numeric Rating Scale‐Pain Intensity, much or very much improvement in the Patient Global Impression of Change (PGIC), Health‐Related Quality of Life (HRQoL), withdrawals due to adverse events (AEs) (tolerability), serious adverse events (SAEs), nervous system disorders, psychiatric disorders, physical dependence.
Main results
We included 25 RCTs with 3763 participants of whom 2290 received cannabinoids. Age ranged from 18 to 60 years, and between 50% and 88% participants across the studies were female. The included studies were 3 to 48 weeks long and compared nabiximols, an oromucosal spray with a plant derived equal (1:1) combination of tetrahydrocannabinol (THC) and cannabidiol (CBD) (13 studies), synthetic cannabinoids mimicking THC (7 studies), an oral THC extract of Cannabis sativa (2 studies), inhaled herbal Cannabis (1 study) against placebo. One study compared dronabinol, THC extract of Cannabis sativa and placebo, one compared inhaled herbal Cannabis, dronabinol and placebo. We identified eight ongoing studies.
Critical outcomes
• Spasticity: nabiximols probably increases the number of people who report an important reduction of perceived severity of spasticity compared with placebo (odds ratio (OR) 2.51, 95% confidence interval (CI) 1.56 to 4.04; 5 RCTs, 1143 participants; I2 = 67%; moderate‐certainty evidence). The absolute effect was 216 more people (95% CI 99 more to 332 more) per 1000 reporting benefit with cannabinoids than with placebo.
• Chronic neuropathic pain: we found only one small trial that measured the number of participants reporting substantial pain relief with a synthetic cannabinoid compared with placebo (OR 4.23, 95% CI 1.11 to 16.17; 1 study, 48 participants; very low‐certainty evidence). We are uncertain whether cannabinoids reduce chronic neuropathic pain intensity.
• Treatment discontinuation due to AEs: cannabinoids may increase slightly the number of participants who discontinue treatment compared with placebo (OR 2.41, 95% CI 1.51 to 3.84; 21 studies, 3110 participants; I² = 17%; low‐certainty evidence); the absolute effect is 39 more people (95% CI 15 more to 76 more) per 1000 people.
Important outcomes
• PGIC: cannabinoids probably increase the number of people who report 'very much' or 'much' improvement in health status compared with placebo (OR 1.80, 95% CI 1.37 to 2.36; 8 studies, 1215 participants; I² = 0%; moderate‐certainty evidence). The absolute effect is 113 more people (95% CI 57 more to 175 more) per 1000 people reporting improvement.
• HRQoL: cannabinoids may have little to no effect on HRQoL (SMD ‐0.08, 95% CI ‐0.17 to 0.02; 8 studies, 1942 participants; I2 = 0%; low‐certainty evidence);
• SAEs: cannabinoids may result in little to no difference in the number of participants who have SAEs compared with placebo (OR 1.38, 95% CI 0.96 to 1.99; 20 studies, 3124 participants; I² = 0%; low‐certainty evidence);
• AEs of the nervous system: cannabinoids may increase nervous system disorders compared with placebo (OR 2.61, 95% CI 1.53 to 4.44; 7 studies, 1154 participants; I² = 63%; low‐certainty evidence);
• Psychiatric disorders: cannabinoids may increase psychiatric disorders compared with placebo (OR 1.94, 95% CI 1.31 to 2.88; 6 studies, 1122 participants; I² = 0%; low‐certainty evidence);
• Drug tolerance: the evidence is very uncertain about the effect of cannabinoids on drug tolerance (OR 3.07, 95% CI 0.12 to 75.95; 2 studies, 458 participants; very low‐certainty evidence).
Authors' conclusions
Compared with placebo, nabiximols probably reduces the severity of spasticity in the short‐term in people with MS. We are uncertain about the effect on chronic neurological pain and health‐related quality of life. Cannabinoids may increase slightly treatment discontinuation due to AEs, nervous system and psychiatric disorders compared with placebo. We are uncertain about the effect on drug tolerance. The overall certainty of evidence is limited by short‐term duration of the included studies.
Plain language summary
Cannabis and cannabinoids for people with multiple sclerosis
Key messages • Treatment with nabiximols likely results in improvement of spasticity and may not increase serious harmful effects compared with placebo
• Compared with placebo, cannabinoids (nabiximols, Cannabis extract, synthetic cannabinoids) likely improve well‐being when measured with patient‐reported outcomes
• Due to a lack of robust evidence, the benefit of these medicines for treating chronic neuropathic pain is unclear.
What is the issue?
Many people with multiple sclerosis (MS) experience spasticity that causes also pain and impacts on the ability to carry out daily activities. Spasticity is a form of increased muscle tone. Cannabis‐based medicines refer to the use of Cannabis, or its ingredients called cannabinoids, as medical therapies to alleviate spasticity, chronic pain and other symptoms in MS. An international survey found that MS was one of the five medical conditions for which Cannabis was most often used. Another survey conducted in the UK found that more than one in five people with MS reported they had used Cannabis to try to manage their symptoms.
What did we want to find out?
We wanted to find out if cannabinoids were better than placebo in adults with MS to improve:
• spasticity;
• chronic neuropathic pain;
• well‐being,
We also wanted to find out if cannabinoids were associated with:
• treatment discontinuation due to unwanted effects;
• serious harmful effects;
• nervous system disorders or psychiatric disorders;
• drug tolerance defined as a condition that occurs when the body gets used to a medicine so that more medicine is needed.
What did we do? We searched for studies that compared cannabinoids against placebo in adult people with MS. We compared and summarised their results, and rated our confidence in the evidence, based on factors such as study methods and certainty of evidence.
What did we find?
We found 25 studies that involved 3763 people with MS, 2290 of whom received cannabinoids. Fifteen studies were very short term or short‐term studies (two to 12 weeks), seven were intermediate term (12 to 26 weeks), and two were long term (50 and 156 weeks). One study reported results at three days only. The biggest study was conducted in 657 people and the smallest study involved 14 people. Most studies were done in European countries. Thirteen studies evaluated an oral spray (nabiximols) containing two compounds derived from the Cannabis plant. The other studies compared different cannabinoids with placebo. Pharmaceutical companies funded 15 of the studies.
Main results
Compared with placebo, cannabinoids:
• probably increase the number of people who report an important reduction of perceived severity of spasticity for up to 14 weeks (evidence from five studies in 1143 people);
• may increase the number of people who report an important reduction of perceived severity of chronic neuropathic pain, but the evidence is very uncertain (evidence from one study in 48 people).
We are uncertain whether cannabinoids reduce chronic neuropathic pain intensity:
• probably increase the number of people who perceive their well‐being as 'very much' or 'much' improved (evidence from eight studies in 1215 people);
• may increase slightly the number of people who discontinue treatment due to unwanted effects (evidence from 21 studies in 3110 people);
• may result in little to no difference in the number of people who have serious harmful effects (evidence from 20 studies in 3124 people);
• may increase nervous system disorders (evidence from seven studies in 1154 people) or psychiatric disorders (evidence from six studies in 1122 people);
• may have little to no effect on the number of people who have drug tolerance, but the evidence is very uncertain (two studies in 458 people).
What are the limitations of the evidence?
There is no high‐quality evidence.
We are moderately confident that cannabinoids work better versus no cannabinoids to improve severity of spasticity and well‐being in adults with MS. We have little confidence in our results for the effect on chronic neuropathic pain because the available evidence is limited.
There is limited evidence to determine the effects of cannabinoids on serious harmful effects, nervous system or psychiatric disorders, and drug tolerance.
How up to date is the evidence? The evidence is up‐to‐date to December 2021.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Cannabis compared to Placebo for health problem or population.
| Cannabis compared to Placebo for health problem or population | ||||||
| Patient or population: health problem or population Setting: inpatient or outpatient Intervention: Cannabis Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Placebo | Risk with Cannabis | |||||
| Spasticity: number of participants reporting reduction of 30% in the spasticity NRS (follow up 6‐14 weeks) follow‐up: range 6 weeks to 14 weeks | 287 per 1000 | 502 per 1000 (385 to 619) | OR 2.51 (1.56 to 4.04) | 1143 (5 RCTs) | ⊕⊕⊕⊝ Moderatea | Our confidence in this result is moderate, downgraded one level for serious risk of bias. Cannabis likely results in an increase in the number of participants with reduction of spasticity over 6‐14 weeks’ follow‐up, when compared with placebo |
| Pain:number of participants reporting pain relief of 50% or greater in the NRS‐PI (follow up 3 weeks) | 167 per 1000 | 458 per 1000 (182 to 764) | OR 4.23 (1.11 to 16.17) | 48 (1 RCT) | ⊕⊝⊝⊝ Very lowb | Our confidence in this result is very low, downgraded one level for serious risk of bias, two levels for very serious imprecision. The evidence is very uncertain about the effect of cannabis on the number of participants with reduction of pain over 3 weeks’ follow‐up, when compared with placebo. |
| PGIC:number of participants reporting much or very much improvement in the PGIC (follow up 4‐48 weeks) follow‐up: range 4 weeks to 48 weeks | 209 per 1000 | 323 per 1000 (266 to 385) | OR 1.80 (1.37 to 2.36) | 1215 (8 RCTs) | ⊕⊕⊕⊝ Moderatec | Our confidence in this result is moderate, downgraded one level for serious risk of bias. Cannabis likely results in an increase in the number of participants who reported improvement in the PGIC over 4‐48 weeks’ follow‐up, when compared with placebo |
| Health‐related quality of life. Mean change from baseline assessed with: EQ‐5D, SF‐36 PCS, MSIS‐29, Spitzer Quality of Life Index follow‐up: range 3 weeks to 48 weeks | The mean health‐related quality of life. Mean change from baseline was See comments | SMD 0.08 lower (0.17 lower to 0.02 higher) | ‐ | 1942 (8 RCTs) | ⊕⊕⊝⊝ Lowd | Based on Cohen's effect sizes, and SMD of 0.08 represents a small effect. Our confidence in this result is low, downgraded two levels due to very serious risk of bias. We did not downgrade for imprecision given the quite tight confidence intervals and very modest effects at either end of the confidence intervals. |
| Withdrawn due to adverse events (follow up 3‐48 weeks) follow‐up: range 3 weeks to 48 weeks | 30 per 1000 | 69 per 1000 (44 to 106) | OR 2.41 (1.51 to 3.84) | 3110 (21 RCTs) | ⊕⊕⊝⊝ Lowe | Our confidence in this result is low, downgraded one level for serious risk of bias, one level for imprecision. Cannabis may result in an increase in the number of participants who withdrew due to AEs over 3‐48 weeks’ follow‐up, when compared with placebo |
| SAEs: number of participants with SAEs (follow up 3‐48 weeks) follow‐up: range 3 weeks to 48 weeks | 33 per 1000 | 44 per 1000 (31 to 63) | OR 1.38 (0.96 to 1.99) | 3124 (20 RCTs) | ⊕⊕⊝⊝ Lowf | Our confidence in this result is low, downgraded one level for serious risk of bias, one level for imprecision. Cannabis may result in a slight increase in the number of participants who had SAEs over 3‐48 weeks’ follow‐up, when compared with placebo |
| Specific AEs:number of participants reporting nervous system disorders (follow up 4‐48 weeks) follow‐up: range 4 weeks to 48 weeks | 250 per 1000 | 465 per 1000 (338 to 597) | OR 2.61 (1.53 to 4.44) | 1154 (7 RCTs) | ⊕⊕⊝⊝ Lowg | Our confidence in this result is low, downgraded one level for serious risk of bias, one level for inconsistency. Cannabis may result in an increase in the number of participants who had nervous system disorders over 3‐48 weeks’ follow‐up, when compared with placebo |
| Specific AEs: number of participants reporting psychiatric disorders (follow up 4‐48 weeks) follow‐up: range 4 weeks to 48 weeks | 75 per 1000 | 136 per 1000 (96 to 189) | OR 1.94 (1.31 to 2.88) | 1122 (6 RCTs) | ⊕⊕⊝⊝ Lowh | Our confidence in this result is low, downgraded one level for serious risk of bias, one level for imprecision. Cannabis may result in an increase in the number of participants who had psychiatric disorders over 3‐48 weeks’ follow‐up, when compared with placebo |
| Specific AEs: number of participants reporting drug tolerance (follow up 14‐48 weeks) | 0 per 1000 | 0 per 1000 (0 to 0) | OR 3.07 (0.12 to 75.95) | 458 (2 RCTs) | ⊕⊝⊝⊝ Very lowi | Our confidence in this result is very low, downgraded one level for serious risk of bias, two levels for imprecision. The evidence is very uncertain about the effect of cannabis on drug tolerance over 14‐48 weeks' follow up. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_426325342660720441. | ||||||
a Downgraded one level due to study limitations because all the included studies have an overall risk‐of‐bias judgement of some concerns. b Downgraded one level due to study limitations (single study at high risk of attrition bias) and 2 levels for very serious imprecision because of the very small information size (wide confidence intervals). c Downgraded one level due to studies' limitations (high risk of bias for 3 studies or of concerns for 5 studies). d Downgraded 2 levels due to studies' limitations (2 studies due to missing outcome data, one due to deviations from intended interventions and missing outcome data, and one study due to missing outcome data and measurement of the outcome) and one level due to imprecision (wide confidence intervals crossing the line of no effect). e Downgraded one level due to studies' limitations (one study at high risk of bias arising from the randomization process and the other studies with some concerns) and one level for imprecision (small number of events). f Downgraded one level due to studies' limitations (one study at high risk of bias due to deviations from intended interventions, one study at high risk of bias due to randomization process and deviations from intended interventions, the other studies with some concerns) and one level due to imprecision (small number of events and confidence intervals crossed the line of no effect). g Downgraded one level due to studies' limitations (2 studies at high risk of bias due to deviations from intended interventions, one study at high risk of bias due to deviations from intended interventions and in measurement of the outcome) and one level for inconsistency (heterogeneity P = 0.01; I² = 63%). Four studies suggest harm and three studies on either side of the line of no effect. One study reported no nervous system disorders over 4 weeks' follow up. h Downgraded one level due to studies' limitations (one study at high risk of bias due to deviations from intended interventions and in measurement of the outcome, five studies with some concerns) and one level for imprecision (small number of events). i Downgraded one level due to studies' limitations (2 studies with some concerns) and 2 levels due to imprecision (small number of events in one study; the other study reported no drug tolerance disorders).
Background
Description of the condition
Multiple sclerosis (MS) is an immune‐mediated disease of the central nervous system (CNS) that leads to a progressive functional decline. The worldwide prevalence of MS is reported to be 50 to 300 per 100,000 people. About 2.3 million people are estimated to live with MS globally, although this number may be underestimated because data are lacking from large populations, such as populations in India and China (Thompson 2018a). Although the aetiology of MS remains unknown, associations with genetic, environmental, and lifestyle factors have been reported (Thompson 2018a). MS is commonly classified into different forms: relapsing‐remitting (RRMS), secondary progressive (SPMS), and primary progressive (PPMS). Symptoms vary widely from person to person, and include fatigue, muscle spasticity, weakness, chronic neuropathic and musculoskeletal pain, mobility restrictions, visual impairment, depression, anxiety, and bladder and bowel dysfunction (Newsome 2017; Rommer 2018).
People with MS have multiple symptoms; for example, people with spasticity may also have chronic pain resulting from tightening of their muscles. Therefore, it is necessary to consider the overlap of indications people have when use a symptomatic treatment. Spasticity is a form of increased muscle tone and it is a common and serious feature of MS that increases with disease progression and leads to deterioration in disability, weakness, and fatigue. Adaptive features may develop including contractures in muscle, tendons, and joints which can further worsen limb positioning, movement, and function. Spasticity also causes pain, bed sores, instability, and difficulty in maintaining hygiene. Treatment with anti‐spasticity medication is made for different reasons in people with MS. People with severe mobility disability are treated to relieve pain, spasticity and make nursing care easier. Those who are able to walk are treated with the additional aim of improving or preserving mobility (Amatya 2013; Shakespeare 2003). Chronic neuropathic pain occurs in more than half of people with MS and is directly related to MS pathology (Newsome 2017).
Description of the intervention
Cannabis is a plant (Cannabis sativa) that contains over 120 phytocannabinoids. Medical cannabis refers to the use of the plant Cannabis or cannabinoids as a medical therapy to relieve symptoms. The most well‐known phytocannabinoids are: delta‐9‐tetrahydrocannabinol (THC), which produces a variety of effects including altered cognitive and motor functions, psychotropic effects; and cannabidiol (CBD), a non‐euphoric molecule (Hazekamp 2018; Izzo 2009; Morales 2017). Several standardised cannabinoids‐based medicines are currently manufactured. Nabiximols (Sativex®) is made from extracts of the Cannabis sativa plant and contains an equal mix of the cannabinoids THC and CBD. It is taken as an oromucosal spray. Bedrocan® and Bedrobinol® are standardised preparations of Cannabis flowers with a CBD content of less than 1% (in both preparation), 22% and 13.5% THC, respectively. Bediol® (6.3% THC and 8% CBD) and Bedrolite® (less than 1% THC and 9% CBD) are standardised Cannabis flowers both available in granular form. Bedica®, with 14% THC and less than 1% CBD, is a standardised preparation, available in granular form, obtained from the variety indica of Cannabis flowers.
There are several routes of administration for Cannabis, including inhalation, oral, oromucosal, sublingual, transdermal, eye drops, topical and rectal (Russell 2018). In clinical practice, inhalation, oromucosal and oral administration are most commonly used. Depending on the mode of administration, the onset and duration of Cannabis effects may vary. Due to the lipophilicity of cannabinoids, inhalation, i.e. smoking and vaporising, leads to a rapid onset of action (within minutes) and a short duration of action (maximum two hours). Smoking is not used for medicinal purposes due to the inhalation of toxic by‐products (such as carbon monoxide and polycyclic aromatic hydrocarbons) produced by the combustion of cigarettes. Oral and oromucosal administration results in a slow onset of action but a longer duration of action. Following Cannabis use, the bioavailability of cannabinoids after inhalation and oral or oromucosal administration is high and low (due to liver metabolism), respectively. Among the Cannabis products, nabiximols is the most commonly used in clinical trials. A titration phase is required to achieve the optimal dose of nabiximols. The number and timing of sprays vary from patient to patient. The dose is gradually increased by one spray per day, up to a maximum of 12 sprays per day, until optimal symptom relief is achieved. The median dose in clinical trials for people with MS are eight sprays per day. After oromucosal spray administration of nabiximols, plasma levels of THC and other cannabinoids are lower than after smoking or inhaling cannabis at a similar dose.
Several cannabinoids identical in structure to naturally occurring cannabinoids have been synthesised. Dronabinol (Marinol® or Syndros®) and nabilone (Cesamet® or Canemes®) are synthetic delta‐9‐THC analogues. They are administered as oral capsules (both drugs) or oral solutions (dronabinol). According to the literature, elimination of oral cannabinoids from plasma is biphasic with an initial half‐life of about four hours, and the final elimination half‐life is 24 to 36 hours or longer due to slow release from adipose tissue (MHRA 2014).
An international survey found that MS was one of the five medical conditions for which cannabinoids were most often used, with back pain, sleep disorders, depression, and post‐injury pain being the other four conditions (Hazekamp 2013). The UK MS Society conducted a survey of 3994 people with MS from across the UK in September 2014, requesting their attitudes and experiences on Cannabis and Sativex® use. The survey was conducted anonymously through various channels to capture the range of experiences and views that people with MS hold. More than one in five people (22%) reported they had used Cannabis to try to manage their MS symptoms and 7% of those surveyed were still using Cannabis. Most people (56%) currently using Cannabis for medical purposes felt that the benefits outweighed the side effects. Of those currently using Cannabis, 40% were doing so because they were unable to obtain a prescription for a licensed alternative. Use of medical Cannabis was associated with recreational Cannabis use. The symptoms reported by medical Cannabis users to be most effectively relieved were stress, sleep, mood, spasticity, and pain (MS Society 2014). A recent Internet‐based survey in the USA found that 66% of people with MS used Cannabis for symptom treatment (Kindred 2017). A large (2009 participants; response rate of 62%) and comprehensive questionnaire survey on the use of Cannabis in Danish MS patients found that illegal Cannabis use was common among Danes with MS as only 21% of the current Cannabis users received prescribed Cannabis‐based medicine. Current Cannabis users reported high efficacy in relieving pain, spasticity and sleep disturbances. In addition, only mild to moderate severity of adverse effects were reported (Gustavsen 2019). A study from Canada reported that about 50% of people with MS would consider the legal use of Cannabis if evidence of benefit was available (Banwell 2016).
How the intervention might work
Plant‐derived and synthetic cannabinoids exert their biological effects primarily via interaction with the endocannabinoid system which includes cannabinoid receptors (CB1 and CB2), endogenous cannabinoids [endocannabinoid, chiefly anandamide (AEA) and 2‐arachidonoylglycerol (2‐AG)], and the enzymes responsible for the synthesis and degradation of the endocannabinoid (Di Marzo 2018; Kaur 2016; Papaseit 2018). Transient receptor potential (TRP) channels, peroxisome proliferator‐activated receptors (PPARs), glycine receptors, and the orphan G protein‐coupled receptors (GPR55 and GPR18) are also engaged by cannabinoids (Morales 2017). The psychoactive effects of Cannabis are mainly due to the presence of THC. THC binds to the cannabinoid receptors CB1 and CB2, acting as a partial agonist. CB1 receptors are mainly located in the CNS or highly expressed in the CNS (cerebral cortex, hippocampus, basal ganglia, and cerebellum) and are involved in memory processing, motor function, appetite, and sensory perception. CB2 receptors are essentially expressed in immune cells, and they have been attributed a role modulating the immune response.
The endocannabinoid system has been shown to be modulated in MS patients. AEA levels, but not 2‐AG levels, were found to be elevated in the cerebrospinal fluid (CSF) of RRMS patients experiencing current relapse (Centonze 2007). Similarly, Jean‐Gilles and colleagues reported both the presence of higher plasma AEA levels in patients with RRMS or SPMS compared to controls, and a decrease in mRNA expression of fatty acid amide hydrolase (FAAH, an enzyme responsible for endocannabinoids degradation) in SPMS but not in RRMS or PPMS blood (Jean‐Gilles 2009). In contrast, in another study, low levels of endogenous cannabinoids were found in the CSF of patients with MS compared to controls (Di Filippo 2008). However, the authors also reported an increase in AEA levels in the CSF during relapses or in RRMS patients with gadolinium‐enhancing lesions, which were, however, lower than those of control subjects, suggesting a relationship between AEA levels and the number of inflammatory lesions (Di Filippo 2008). Up‐regulation of CB1 and CB2 expression was also found in glial cells within demyelinated plaques from MS patients (Benito 2007) and in blood samples from PPMS patients (Jean‐Gilles 2009). These findings have raised the interesting possibility that drugs targeting the endocannabinoid system (i.e. the use of cannabinoids or inhibitors of FAAH) may represent a potential pharmacological strategy to reduce the symptoms and slow disease progression in MS.
The use of cannabinoids‐containing products has been demonstrated to have the potential to affect both pathogenic mechanisms and symptoms of MS, as they are able to suppress neuro inflammation (via CB2 activation) (Mestre 2018), and exert neuroprotective effects in the CNS (via CB1 activation) (Constantinescu 2018; Gowran 2011; Kaur 2016; Mecha 2019). The effect of cannabinoids on the immune system may also play a role given the autoimmune hypothesis of MS aetiology (Fitzpatrick 2017; Mestre 2018; Oláh 2017), the increased CB1 and CB2 receptors in blood samples from PPMS patients (Jean‐Gilles 2009) and a variety of animal studies demonstrating the immunomodulatory effects of cannabinoids during the inflammatory processes that occur in MS (Gonçalves 2019; Furgiuele 2021). In addition, a recent prospective case‐control study has shown that cannabis use reduces and increases the serum pro‐inflammatory and anti‐inflammatory cytokines levels in MS patients, respectively (Mustafa 2021).
Why it is important to do this review
Results of available surveys show that the demand of people with MS for symptomatic treatment with cannabinoid‐based medicines is high (Banwell 2016; Hazekamp 2013; Kindred 2017; MS Society 2014). Therapies that relieve the disabling symptoms of MS include botulinum toxin injections, baclofen or tizanidine for spasticity, anticonvulsants, antidepressant or analgesics for neuropathic pain, and anticholinergic drugs for bladder dysfunction. However, these symptomatic therapies are of limited efficacy or are poorly tolerated (Newsome 2017). Many patients with MS have a combination of pain and spasticity, and could benefit from cannabinoid‐based medicines that have an overlap of indications.
International guidelines have reached different recommendations on the use of cannabinoids in people with MS. The NICE guidelines did not recommend nabiximols for MS on cost‐effectiveness grounds for the NHS in England, Scotland, and Northern Ireland (NICE 2014). However, nabiximols is considered cost‐effective in Wales. A new review and a guideline scoping document on cannabinoid‐based medicines are in development (NICE 2019). The Association of British Neurologists have advised clinicians to use nabiximols in people with MS who had an unsatisfactory response to conventional anti‐spasticity medications (ABN 2018; RCP 2018). The American Academy of Neurology does not support the legalisation or prescribing of medical marijuana for use in MS, but supports scientific research to investigate the safety and potential benefits (AAN 2018). The Food and Drug Administration (FDA) has not approved any marketing application for cannabinoid‐based medicines for MS, but was recently asked to place this therapy for progressive MS on the fast track (Reston 2019). The European Medicines Agency (EMA) authorised in 2014 the use of nabiximols for the management of moderate to severe spasticity in adults with MS who have not responded to conventional treatment, and showed clear clinical improvement in the initial period with this therapy (EMA 2014). The guidance released in 2018 by the Australian Government Department of Health recommended to use cannabinoid‐based medicines in people with MS who have not responded adequately to other anti‐spasticity medication (Australian Government 2017).
There are differences between countries in the legal authorisation and use of cannabinoid‐based medicines for MS. Nabiximols is approved and available for MS related spasticity in 29 US states including the District of Columbia, in Canada, Israel, and 21 European countries (Abuhasira 2018), and is reimbursed by health insurance companies or state social security systems in 11 European countries (Austria, Belgium, Germany, Israel, Italy, Portugal, San Marino, Spain, Turkey, UK, and Norway) (Krcevski‐Skvarc 2018). Approval of cannabinoid‐based medicines (i.e. the Cannabis flowers Bedrocan®, Bediol®, Bedica®, Bedrobinol®, Bedrolite®) for treatment of chronic neuropathic pain that is refractory to conventional treatment is available in Canada, Croatia, Czech Republic, Denmark, Germany, Israel, Italy, the Netherlands, Norway, Serbia, Slovenia, and Switzerland, but with striking differences in legal and reimbursement rules (Krcevski‐Skvarc 2018). Medical cannabinoids can be prescribed to people with MS under strict controlled conditions, but there are differences between countries on who can and cannot prescribe cannabinoid‐based medicines, e.g. in the UK nabiximols can be prescribed only by specialist doctors with expertise in treating MS.
There is a growing interest into the therapeutic benefit of cannabinoid‐based medicines in the treatment of illness including MS. Following the review of the Chief Medical Advisor to the UK Government, on 1 November 2018, unlicensed cannabinoid‐based products were moved from Schedule 1 to Schedule 2 in the UK. This decision would allow these medicines to be prescribed under controlled conditions by registered practitioners. In addition, moving the whole class of cannabinoids out of Schedule 1, will allow the evidence base on benefits and harms associated with this class of drugs to be improved through research (Davies 2018).
Due to the conflicting conclusions of systematic reviews on benefits and harms of cannabinoids for symptomatic treatment of MS, as well as different recommendations in international guidelines, we see the need for a Cochrane Review undertaken according to rigorous standards.
Objectives
To assess benefit and harms of cannabinoids including synthetic, or herbal and plant‐derived cannabinoids, for symptomatic treatment in MS.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled (parallel or cross‐over) trials (RCTs). We included cross‐over trials irrespective of the length of the washout period.
Types of participants
We included studies in adults, males and females (18 years or older), diagnosed with MS according to the Poser (Poser 1983) or McDonald criteria and its revisions (McDonald 2001; Polman 2005; Polman 2011; Thompson 2018b), and all types of MS such as RRMS, SPMS, PPMS, and progressive‐relapsing MS (PRMS). We included participants regardless of disease duration and degree of disability.
Types of interventions
Any cannabinoids including herbal cannabis (e.g. marijuana), cannabis flowers (Bedrocan®, Bedrobinol®, Bediol®, Bedrolite®, Bedica®), plant‐based cannabinoids (Nabiximols, Cannabidiol), or synthetic cannabinoids (Dronabinol, Nabilone), irrespective of dose, route, frequency, or duration of use. We included as a comparison intervention placebo or any active comparator. We included concomitant interventions if they were used in all the comparison groups.
Types of outcome measures
We included patient‐reported outcomes as critical or important outcomes, because the primary scope and aim of this Cochrane Review is to assess the effects of the intervention on symptoms such as chronic pain and functional limitations due to spasticity. These symptoms are better known to the patients themselves than to clinicians, and the patients' perspective on treatment benefit is a priority. We included short‐ and long‐term outcomes reported in the included trials.
1. Critical outcomes
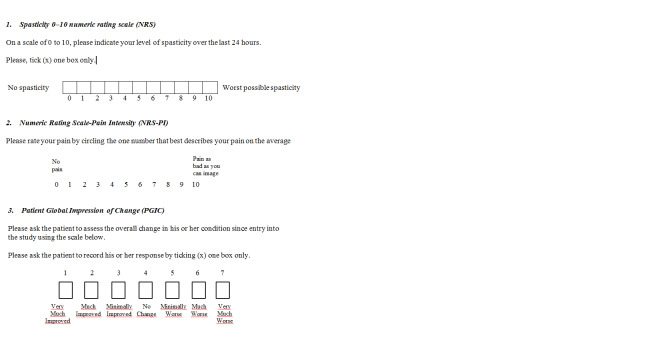
Spasticity: number of participants reporting a reduction of 30% or greater in the spasticity Numeric Rating Scale (NRS), over baseline. This reduction has been identified as a change that represents a clinically important difference (CID) from baseline in participants with MS‐related spasticity (Farrar 2008). NRS is a patient‐rated measure of the perceived severity of spasticity. Scores range from 0 (no spasticity) to 10 (the worst possible spasticity) (Figure 1).
Chronic neuropathic pain: number of participants reporting pain relief of 50% or greater in the Numeric Rating Scale‐Pain Intensity (NRS‐PI), over baseline. NRS‐PI is a 0 to 10 rating scale with scores ranging from 0 ‘no pain' to 10 ‘worst possible pain' (Farrar 2010; Figure 1).
1.
Spasticity and pain scales (NRS); Patient Global Impression of Change (PGIC)
Where studies measure these outcomes as continuous data only, we included them as separate analyses as important outcomes. The raw change CID cutoff points are –1.27 for the spasticity, 0–10 NRS (Farrar 2008) and ‐2.5 for the NRS‐PI (Farrar 2010).
Number of participants withdrawn due to adverse events (AEs) (tolerability).
2. Important outcomes
Patient Global Impression of Change (PGIC): number of participants reporting much or very much improvement in the PGIC. PGIC provides a patient‐reported assessment of overall change in health status on a seven‐point categorical scale with scores ranging from 1 (very much improved) to 7 (very much worse) (Dworkin 2008; Farrar 2008; Guy 1976) (Figure 1). Where studies measured the outcome as continuous data only, we included them as separate analyses as outcomes of limited importance.
Health‐related quality of life (HRQoL), measured with condition‐specific HRQoL as the 54‐item MSQoL (MSQoL‐54) (Vickrey 1995), or generic HRQoL validated measures reported in the included studies, as the 36 item Short Form (SF‐36) (Ware 1992), or Euroqol‐5 dimensions (EQ‐5D) (EuroQol Group 1990).
The total number of serious adverse events (SAEs). If an insufficient number of studies reported the total number of SAEs and person‐years, we used the number of participants with at least one SAE as defined in the study.
Number of participants reporting specific AEs, including nervous system (e.g. cognitive dysfunction, dizziness, somnolence, headache), psychiatric disorders (e.g. confusion state; paranoia, psychosis), and physical dependence effects (e.g. withdrawal and tolerance) according to the Medical Dictionary for Regulatory Activities (MedDRA) (ICH 2019), or as reported in the included studies.
3. Outcomes of limited importance
Reduction in spasticity measured by clinical reported measure, e.g. the Ashworth scale (Ashworth 1964) or the Modified Ashworth scale (MAS) (Ansari 2009), or the Tardieu or Modified Tardieu scale (Ansari 2008).
Participant‐reported pain relief of 30% or greater in a composite neuropathic pain scale or in a single generic pain scale, e.g. the NRS‐PI (0‐10 NRS‐PI).
Improvement of bladder symptoms measured by patient‐reported outcome, e.g. the Overactive Bladder questionnaire (OAB‐q) (Coyne 2005).
Participant‐reported frequency and severity of spasms, e.g. Penn Spasm Frequency Scale (Penn 1989).
Fatigue, measured with the Fatigue Severity scale (FSS) or the Modified‐Fatigue Impact Scale (M‐FIS). FSS is a self‐administered questionnaire with nine questions graded on a seven‐point Likert‐like scale where 1 indicates strong disagreement and 7 strong agreement, and the final score represents the mean value of the nine items questionnaire. M‐FIS is a 21‐item multidimensional questionnaire that measures the physical, cognitive, and psychosocial impact of fatigue using a five‐point ordinal scale (range 0 to 84) (Multiple Sclerosis Council 1998). Higher scores indicate greater impact or severity of fatigue symptoms. A difference of four points on the M‐FIS as been identified as a clinically significant difference in fatigue (Rooney 2019).
Sleep problems, e.g. the NRS (0‐10 NRS).
Improvement of mobility, balance, tremor, and daily functioning, specifically the activities of daily living (ADL), e.g. the Barthel index (BI) which is a 10‐item scale that measures daily function and gives a score out of 20 with higher scores suggesting greater independence (Mahoney 1965) or timed 10‐metre walk test (Kempen 2011).
Depression and anxiety measured by validated scales, e.g. the Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983).
Caregiver’s global impression of change (CGIC), rating ease of transfer, dressing, and perineal hygiene. CGIC is assessed on a seven‐point Likert‐like scale that used three categories of improvement (slightly improved, much improved, or very much improved), three categories of worsening (slightly worse, much worse, or very much worse), and an option of “no change” (Collin 2010).
Reduced use of other symptomatic treatments (e.g. for spasticity or pain).
Search methods for identification of studies
We did not apply any language restrictions to the search.
Electronic searches
We designed search strategies for electronic databases according to methods suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2019). The Cochrane Multiple Sclerosis and Rare Diseases of the Central Nervous System group's Information Specialist peer‐reviewed them. We searched all potential relevant trials registries in detail to detect ongoing as well as completed studies, but not yet published studies. We searched the following databases and sources, updated on 31 December 2021.
Databases of medical literature
Cochrane Central Register of Controlled Trials (CENTRAL) and the Cochrane Database of Systematic Reviews (CDSR; 2021, Issue 12) in the Cochrane Library (searched 27 December 2021; Appendix 1);
MEDLINE (PubMed) (1966 to 31 December 2021; Appendix 1);
Embase (EMBASE.com) (1974 to 31 December 2021; Appendix 1);
CINAHL (EBSCO host) (Cumulative Index to Nursing and Allied Health Literature; 1981 to 27 December 2021; Appendix 1);
LILACS (Bireme) (Latin American and Caribbean Health Sciences Literature; 1982 to 27 December 2021; Appendix 1);
Physiotherapy Evidence Database (PEDro) (1990 to 27 December 2021; Appendix 1).
Trials registries and registry platforms to identify ongoing studies and results of completed studies
World Health Organisation International Clinical Trials Registry Platform (WHO ICTRP; trialsearch.who.int; searched 20 December 2021; Appendix 1);
US National Library of Medicine ClinicalTrials.Gov study registry (www.ClinicalTrials.gov; searched 29 December 2021; Appendix 1);
European Union Clinical Trials Register (www.clinicaltrialsregister.eu; searched 29 December 2021; Appendix 1);
International Association for Cannabinoid Medicines (IACM) databank (www.cannabis-med.org/studies/study.php; searched 29 December 2021; Appendix 1).
Searching other resources
We reviewed the references of any RCTs identified and relevant reviews. Because of the comprehensive nature of the electronic search and handsearching, we did not contact authors of included studies on information provision for the review. We considered AEs described in included studies only.
Data collection and analysis
Selection of studies
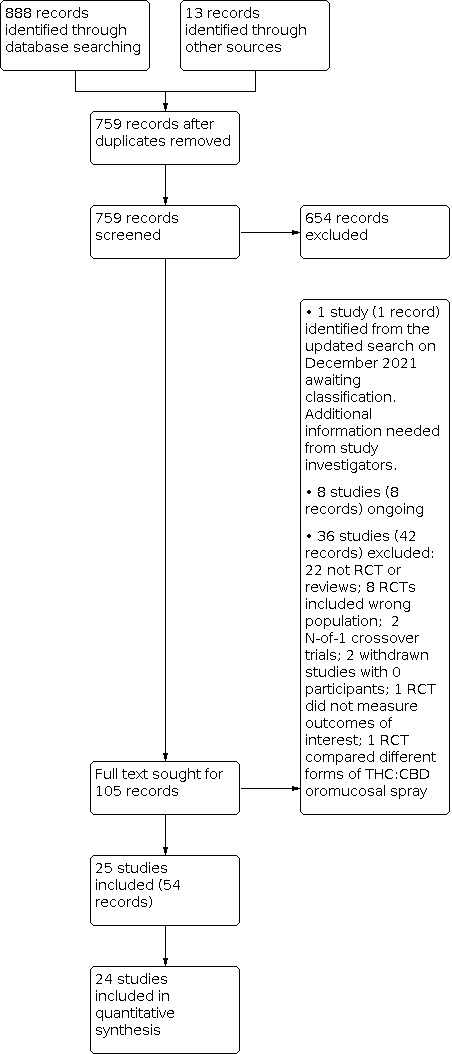
We used the search strategy described in the ‘Search methods for identification of studies' section to obtain titles and abstracts of studies. Two review authors (FB and GF) independently screened the titles and abstracts and discarded studies that were not applicable; however, they initially retained studies and reviews that might include relevant data or information on trials. The two review authors compared multiple reports of the same study and used the most comprehensive report. They linked together multiple publications as companion reports, but excluded true duplicates. FB and GF resolved discrepancies in judgement by discussion and reported excluded studies and their reasons for exclusion in the ‘Characteristics of excluded studies’ table. We include a PRISMA flow chart (Figure 2) reporting the selection process (Moher 2009).
2.
Search updated to December 27, 2021
Data extraction and management
Two teams of two review authors each (FB and GF; SM and MC) independently extracted study characteristics and outcome data from the included parallel trials and cross‐over trials using a predefined data extraction form in an Excel spreadsheet. They resolved any disagreements by discussion all together.
Study characteristics
From each included study we extracted data on the following:
first author or acronym; number of centres; year of publication; years that the study was conducted (recruitment and follow‐up); publication (full‐text publication, abstract publication, unpublished data);
design (parallel or cross‐over); inclusion and exclusion criteria; number of randomised participants; early termination of trial;
length of the washout period in cross‐over trials.
conflict of interests of study authors;
funding of the study.
Outcome data
We extracted the number of participants who had critical and important outcomes and outcomes of limited importance. For the spasticity and pain relief outcomes, we extracted from cross‐over trials the number of participants who:
improved with both treatments;
improved with experimental treatment, deteriorated with control treatment;
improved with control treatment, deteriorated with experimental treatment;
deteriorated with both treatments.
For the AE outcomes, we extracted from cross‐over trials the number of withdrawals due to any AE, and the number of SAEs on each treatment in each treatment period (when possible).
For continuous outcomes we extracted mean and standard deviation (SD) of the comparison groups, where possible, and between‐period correlation in cross‐over studies. To analyse carry‐over, where possible, we extracted also mean and SD by sequence in period I and period II.
We extracted authors’ definition and instruments used to measure spasticity, neuropathic pain, and important outcomes. We extracted arm‐level data when possible, or effect sizes when arm‐level data were not available. We extracted data at the authors' defined timing points.
We noted in the Characteristics of included studies table if outcome data were not reported, or were reported but not in a usable way.
Data on potential effect modifiers
We considered the following potential effect modifiers in each included study:
population: forms of MS; baseline severity and duration of spasticity and pain; prior and actual treatment with anti‐spasticity medications or analgesics; prior use of cannabinoids;
study design: placebo or active control; enriched design; co‐therapies allowed; rescue medication; study duration (less than four weeks; 4 to 12 weeks; 13 to 26 weeks; more than 26 weeks);
intervention: drug, dose, frequency, or duration of treatment.
Assessment of risk of bias in included studies
For the scope of the review, we assessed the effect of the assignment to the intervention (“Intention to treat effect”) for critical and important outcomes. For the total number of SAEs and specific AEs we assessed the effect of adhering to the intervention (‘per protocol effect').
Four review authors (FB, GF, KD, SM) independently assessed the risk of bias of each included study using version 2 of the Cochrane tool for assessing risk of bias in randomised trials (RoB 2) (Higgins 2019) (version 22 August 2019). Review authors did a calibration exercise (i.e. a pilot run of the RoB 2 tool for RCTs, comparison of all the evaluations and agreement on the answers to the signalling questions (SQs) and the final judgments for each domain). On the basis of the results of this exercise, both in terms of the inter‐rater reliability and of the difficulties found in the interpretation and application of the SQs to the specific condition and intervention assessed by the review, all the review authors prepared a detailed implementation document where, for each SQ, explanation was provided on how to interpret the question from a practical point of view (i.e. providing examples) and how to respond considering the issues specific for the condition and the interventions assessed in the review (see Appendix 2 for the implementation document). After the completion of this document, raters reassessed all the included studies (Minozzi 2021).
We assessed critical and important outcomes reported in the Table 1' using RoB 2, which is structured into the following bias:
arising from the randomisation process;
due to deviations from intended interventions;
due to missing outcome data;
in measurement of the outcome;
in selection of the reported result.
Additional considerations for cross‐over trials included (Higgins 2016):
period effect;
carry‐over effect;
selection of the reported results, i.e. selective reporting of first period data on the basis of a test for carry‐over (Freeman 1989).
To implement RoB 2 assessment, we used the Excel tool available at sites.google.com/site/riskofbiastool/welcome/rob‐2‐0‐tool/current‐version‐of‐rob‐2.
We judged each domain as being at low risk of bias, some concerns, or high risk of bias. We reached an overall risk of bias of each included study according to the following criteria:
low risk of bias: low risk of bias for all domains;
some concerns: some concerns in at least one domain, but not at high risk of bias for any domain;
high risk of bias: high risk of bias in at least one domain or some concerns for multiple domains in a way that substantially lowers confidence in the result.
Measures of treatment effect
We calculated dichotomous outcomes as odds ratios (ORs) and 95% confidence intervals (CIs) for parallel and cross‐over trials. For continuous outcomes, we calculated mean difference (MD) or standardised mean difference (SMD) for the same continuous outcome measured with different metric. The SMD is the difference in mean effects in the experimental and control groups divided by the pooled standard deviation of participants’ outcomes.
Unit of analysis issues
Studies with multiple treatment groups
For multi‐arm trials, relevant intervention groups were those that could be included in a pairwise comparison which, if investigated alone, would meet the review inclusion criteria. For example, if we identified a study comparing ‘Nabiximols versus tizanidine versus nabiximols plus tizanidine', only one comparison (‘Nabiximols versus tizanidine') was used since it addressed the review objective. Thus, we would not have used data from the ‘Nabiximols plus tizanidine' treatment as it was not relevant to the review. However, if the study compared ‘Nabiximols versus tizanidine versus baclofen', all three pairwise comparisons of interventions were relevant to the review. In this case we treated the multi‐arm studies as multiple independent two‐arm studies. We converted multi‐arm trials involving the same agent at different doses compared to a control treatment into a single arm by merging of doses and summing the number of participants who had the event and the sample size. For continuous outcomes, we combined means and SDs using methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Cross‐over studies
When possible, we planned to enter mean difference (MD) and standard errors (SEs) from paired data for cross‐over studies with the generic inverse variance (GIV) function in RevMan Web (Review Manager Web). Unfortunately this was mostly not possible due to the way eligible cross‐over trials reported data. These have been included in the meta‐analysis as though data were from parallel trials and footnotes have been included in the forest plot.
Dealing with missing data
We used data that reflected the intention‐to‐treat (ITT) analysis for each included outcome except for safety outcomes, as noted above in Assessment of risk of bias in included studies. In the protocol we had planned to evaluate methods for monitoring and detecting AEs in included studies. This has been removed since we assessed this aspect for SAEs and specific AEs using RoB 2. Different scenarios for assessing the impact of missing data on outcomes were not feasible, and on adverse outcomes is not likely to be plausible (i.e. assuming that participants whose data were missing experienced AEs). For continuous outcomes, where SDs were missing, we calculated them according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
Assessment of clinical heterogeneity within treatment comparisons
To evaluate the presence of clinical heterogeneity, we had planned to assess differences in characteristics of included participants, e.g. MS course, disease duration, baseline severity of spasticity or chronic neuropathic pain across trials using information reported in Characteristics of included studies. However, this was not possible because most studies included grouped data as relapsing and progressive forms of MS, data on disease duration were not available, and studies measured baseline severity of spasticity and pain with different instruments.
Assessment of statistical heterogeneity
We assessed the presence of statistical heterogeneity using the I2 statistic. When the I2 statistic value was greater than 50% (substantial heterogeneity), we considered possible reasons for this.
Assessment of reporting biases
We evaluated the possibility of non‐reporting bias by means of contour‐enhanced funnel plots, if a meta‐analysis included at least 10 studies (Peters 2008).
Data synthesis
We had planned to combine dichotomous outcomes from parallel‐group and cross‐over trials according to the method of Becker 1993. This was not possible because data were not available. We used the Mantel‐Haenszel method in random‐effects meta‐analysis to calculate odds ratios. For continuous outcomes, we calculated MD or SMD, if the outcome was measured on different scales (e.g. pain or quality of life), with 95% CIs. We used a random‐effects model because we assumed that the studies were not all estimating the same intervention effect, and were estimating intervention effects that follow a distribution across studies (DerSimonian 1986). We conducted analyses using RevMan Web (Review Manager Web).
Subgroup analysis and investigation of heterogeneity
We did prespecify subgroup analyses of number of participants reporting spasticity or pain reduction over baseline for study design and duration of follow‐up, baseline severity score, different cannabinoids and co‐therapies, to assess whether treatment effects varied across subgroups. However, we did not conduct subgroup analyses for the following reasons. First, the variation in treatment effect on spasticity and pain tended to be explained by outlying single studies rather than variation across all the studies. Second, less than 10 studies for subgroup analyses as planned were available leading to imbalance in studies when defined by subgroups. Third, there was a predominance of parallel‐group studies and short duration of follow‐up.
Sensitivity analysis
In the protocol we had planned a sensitivity analysis on the exclusion of trials that we judged to be at high risk of bias or to raise some concerns in at least one domain of RoB 2. However, since we judged all included trials at high risk of bias or with some concerns we did not seek to conduct a sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review as summary of findings tables, according to Cochrane guidance (Schünemann 2011). We provided estimates based on the methodology developed from the GRADE Working Group (Atkins 2004).
In the summary of findings tables we included comparison of cannabinoids with placebo and an overall assessment of the evidence for critical and important outcomes and number of participants.
Reporting reduction of 30% in the spasticity Numeric Rating Scale (NRS).
Reporting pain relief of 50% or greater in the NRS‐PI.
Reporting much or very much improvement in the Patient Global Impression of Change (PGIC).
Reporting improvement in quality of life.
Withdrawn due to AEs (tolerability).
Who had at least one SAE.
Reporting specific AEs including nervous system disorders, psychiatric disorders, or physical dependence.
In the summary of findings table, we prioritised long‐term outcomes if they were available, otherwise we included short‐term outcomes.
We assessed the certainty of evidence for each outcome considering risk of bias, indirectness, inconsistency, imprecision of effect estimates, and risk of publication bias. Using GRADEpro GDT software, GRADEpro GDT, we assigned one of four levels of certainty of evidence: high, moderate, low, or very low.
Results
Description of studies
For a full description of studies please see the Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
The results of our searches are detailed in a PRISMA diagram (Figure 2) (Moher 2009). Our electronic searches retrieved 888 records. Our handsearching of other resources produced 13 additional references. After removing 142 duplicate references, we evaluated a total of 759 records, of which we excluded 654 on the basis of title and abstract. From the remaining 105 records, we categorised 1 record (one study identified from the updated search on December 2021) as awaiting classification, because we could not identify the randomisation process and the control intervention, therefore additional information is needed from study investigators. Available details for the study are provided in the Studies awaiting classification table. Eight studies (eight reports) may be eligible as ongoing; further information is in the Ongoing studies table. We excluded 36 studies (42 full‐text reports) (see Figure 2 for details).
Included studies
This review included 25 completed RCTs with 3763 participants of whom 2290 received cannabinoids, (Aragona 2009; Collin 2007; Collin 2010; Corey‐Bloom 2012; Fox 2004; Kavia 2010; Killestein 2002; Langford 2013; Leocani 2015; Markova 2018; NCT00682929; NCT01606176; Notcutt 2012; Novotna 2011; Rog 2005; Schimrigk 2017; Svendsen 2004; Turcotte 2015; Vachova 2014; Van Amerongen 2017; Vaney 2004; Wade 2004; Zajicek 2003_CAMS; ZAJICEK 2012 MUSEC; Zajicek 2013_CUPID). The included studies were published between 2002 and 2018. The table “Characteristics of included studies” provides details of individual studies.
Study design
We included 18 parallel RCTs (Collin 2007; Collin 2010; Kavia 2010; Langford 2013; Markova 2018; NCT00682929; NCT01606176; Notcutt 2012; Novotna 2011; Rog 2005; Schimrigk 2017; Turcotte 2015; Vachova 2014; Van Amerongen 2017; Wade 2004; Zajicek 2003_CAMS; ZAJICEK 2012 MUSEC; Zajicek 2013_CUPID) and seven cross‐over RCTs (Aragona 2009; Corey‐Bloom 2012; Fox 2004; Killestein 2002; Leocani 2015; Svendsen 2004; Vaney 2004). Two studies (Markova 2018; Novotna 2011) used an enriched enrolment two‐phases design, and two studies (Langford 2013; Notcutt 2012) used an enriched enrolment randomised withdrawal design.
Outcome timing
Five studies were very short‐term studies (two to four weeks) (Aragona 2009; Fox 2004; NCT01606176; Svendsen 2004; Vaney 2004), 10 were short‐term studies (four to 12 weeks) (Collin 2007; Kavia 2010; Killestein 2002; Leocani 2015; NCT00682929; Notcutt 2012; Rog 2005; Turcotte 2015; Van Amerongen 2017; Wade 2004), seven were intermediate‐term studies (12 to 26 weeks) (Collin 2010; Langford 2013; Markova 2018; Novotna 2011; Schimrigk 2017; Zajicek 2003_CAMS; ZAJICEK 2012 MUSEC) and two were long‐term studies (50 weeks in Vachova 2014 and 156 weeks in Zajicek 2013_CUPID). Corey‐Bloom 2012 reported outcome at three days.
Study setting
Fifteen studies were multicentre and originated from UK (Fox 2004; NCT01606176; Notcutt 2012; Wade 2004; Zajicek 2003_CAMS; ZAJICEK 2012 MUSEC; Zajicek 2013_CUPID); Germany (Schimrigk 2017); Czech Republic (Vachova 2014); UK and Romania (Collin 2007); UK and Czech Republic (Collin 2010); UK, Belgium and Romania (Kavia 2010); UK, Canada, Spain, France and Czech Republic (Langford 2013); Czech Republic and Austria (Markova 2018); UK, Spain, Poland, Czech Republic and Italy (Novotna 2011). Ten studies were single‐centre and originated from Italy (Aragona 2009; Leocani 2015); the USA (Corey‐Bloom 2012; NCT00682929); the Netherlands (Killestein 2002; Van Amerongen 2017); UK (Rog 2005); Denmark (Svendsen 2004); Canada (Turcotte 2015); and Switzerland (Vaney 2004).
Sample sizes
The sample sizes ranged from 14 (Fox 2004) to 657 (Zajicek 2003_CAMS) participants.
Study funding
Fifteen studies were funded by the manufacturer of the drug (Collin 2007; Collin 2010; Kavia 2010; Langford 2013; Leocani 2015; Markova 2018; NCT01606176; Notcutt 2012; Novotna 2011; Rog 2005; Schimrigk 2017; Turcotte 2015; Vachova 2014; Van Amerongen 2017; Wade 2004), eight studies were founded by public funds (Aragona 2009; Corey‐Bloom 2012; Fox 2004; Killestein 2002; NCT00682929; Vaney 2004; Zajicek 2003_CAMS; Zajicek 2013_CUPID,) and two studies were funded by mixed funds (Svendsen 2004; ZAJICEK 2012 MUSEC)
Participants
Type of MS. Most studies included all types of MS, except the Turcotte 2015 study that included participants with RRMS only and three studies (Leocani 2015; Van Amerongen 2017; Zajicek 2013_CUPID) that included participants with SPMS and PPMS.
Type of symptom. Thirteen studies (Aragona 2009; Collin 2007; Collin 2010; Corey‐Bloom 2012; Killestein 2002; Leocani 2015; Markova 2018; NCT00682929; Notcutt 2012; Novotna 2011; Vachova 2014; Vaney 2004; Zajicek 2003_CAMS) included participants with spasticity, six studies with central neuropathic pain (Langford 2013; NCT01606176; Rog 2005; Schimrigk 2017; Svendsen 2004; Turcotte 2015), two studies (Van Amerongen 2017; ZAJICEK 2012 MUSEC) with spasticity and central neuropathic pain, one study (Fox 2004) with tremor, one study (Kavia 2010) with overactive bladder due to MS, one study (Wade 2004) with multiple symptoms associated with MS, and one study (Zajicek 2013_CUPID) included participants with disease progression in the year preceding randomisation.
Age and gender. Age of the participants ranged from 18 to 60 years. The percentage of females ranged from 50% to 88%.
Inclusion criteria at baseline for spasticity. Four studies (Collin 2010; Markova 2018; Novotna 2011; ZAJICEK 2012 MUSEC) required a score of 4 or above on the spasticity: numeric rating scale (NRS) (moderate to severe spasticity) scale, six studies (Aragona 2009; Collin 2007; Killestein 2002; Van Amerongen 2017; Vaney 2004; Zajicek 2003_CAMS) an Ashworth score of 2 or above (moderate to severe spasticity), one study (Corey‐Bloom 2012) a modified Ashworth score of 3 or above, and one study (Leocani 2015) a modified Ashworth score greater than 1. The remaining studies (NCT00682929; Notcutt 2012; Vachova 2014) did not report on an inclusion criterion of a defined spasticity intensity. Most studies required for inclusion that spasticity was not wholly relieved with current antispastic therapy.
Inclusion criteria at baseline for pain. Four studies (Langford 2013; NCT01606176; Rog 2005; Schimrigk 2017) required a pain score of 4 or above on the NRS‐PI, one study (Svendsen 2004) a score of 3 or above on the NRS‐PI, and one study (Turcotte 2015) a visual analogue score (VAS) pain score of 50 or above. All the included studies stipulated that pain had to be refractory to previous analgesics.
Exclusion criteria. All studies excluded participants with major medical diseases (history of significant psychiatric, renal, hepatic, cardiovascular or convulsive disorders) and with a history of alcohol or substance abuse. Most studies excluded women who were pregnant, breastfeeding, or planning pregnancy during the course of the study.
Previous experience of participants with cannabinoids. Two trials (Markova 2018; Novotna 2011) were with an enriched‐design and one trial (Notcutt 2012) was an enriched enrolment withdrawal study. Ten studies (Collin 2007; Collin 2010; Corey‐Bloom 2012; Fox 2004; Killestein 2002; Langford 2013; Rog 2005; Vachova 2014; Vaney 2004; Wade 2004) reported previous cannabis experience of participants for medical or recreational use. The percentage of participants with previous cannabis experience ranged from 6% to 80%. One study (Aragona 2009) excluded participants with previous experience with cannabinoids.
Interventions
Thirteen RCTs used an oromucosal spray with a plant‐derived combination of tetrahydrocannabinol (THC) and cannabidiol (CBD) (Sativex®) (Aragona 2009; Collin 2007; Collin 2010; Kavia 2010; Langford 2013; Leocani 2015; Markova 2018; NCT01606176; Notcutt 2012; Novotna 2011; Rog 2005; Vachova 2014; Wade 2004). Five studies used oral synthetic cannabinoids mimicking THC (dronabinol: Schimrigk 2017; Svendsen 2004; Zajicek 2013_CUPID; nabilone: Turcotte 2015; namisol: Van Amerongen 2017). Three studies used oral THC extract of Cannabis sativa (Fox 2004; Vaney 2004; ZAJICEK 2012 MUSEC). One study used inhaled herbal Cannabis (Corey‐Bloom 2012). All these studies compared cannabinoids with placebo. Two studies compared dronabinol, THC extract of Cannabis sativa and placebo (Killestein 2002; Zajicek 2003_CAMS), and one compared dronabinol, inhaled herbal Cannabis, and placebo (NCT00682929).
Co‐interventions
Two studies (Langford 2013; Svendsen 2004) allowed paracetamol as rescue medication and one study (Schimrigk 2017) allowed tramadol. Studies including spasticity outcome allowed stable doses of anti‐spasticity medications (e.g. baclofen, tizanidine, benzodiazepines, dantrolene) (Collin 2010; Langford 2013; Markova 2018; Notcutt 2012; Novotna 2011; Van Amerongen 2017; Vaney 2004; ZAJICEK 2012 MUSEC). Studies including pain outcome allowed stable doses of analgesics co‐interventions (e.g. gabapentin) and amitriptyline (Collin 2010; Langford 2013; Markova 2018; Rog 2005; Schimrigk 2017; Van Amerongen 2017).
Critical outcomes
Spasticity. Eight parallel trials (Collin 2007; Collin 2010; Langford 2013; Markova 2018; Notcutt 2012; Novotna 2011; Van Amerongen 2017; ZAJICEK 2012 MUSEC) provided data for the spasticity outcome measured with the NRS 0‐10. We could not include data from one cross‐over study (Leocani 2015) because authors defined benefit as ≥ 20 % improvement in the NRS 0‐10 score that we considered an inappropriate threshold in our protocol. A reduction of 30% or greater in the NRS over baseline is the minimum clinically important difference (MCID) in participants with MS‐related spasticity. We included 11 studies (Collin 2007; Collin 2010; Markova 2018; NCT00682929; Notcutt 2012; Novotna 2011; Vachova 2014; Van Amerongen 2017; Vaney 2004; Wade 2004; Zajicek 2003_CAMS) in the meta‐analysis of spasticity measured with the Ashworth scale or MAS. We could not include data from two cross‐over studies. One (Corey‐Bloom 2012) because authors did not report suitable data accounting for design. Authors of the other study (Killestein 2002) reported the Ashworth scores only in one figure and the numerical data were not available.
Chronic neuropathic pain. We evaluated pain measured with NRS‐PI from nine studies (Collin 2010; Langford 2013; Markova 2018; NCT01606176; Rog 2005; Schimrigk 2017; Svendsen 2004; Van Amerongen 2017; ZAJICEK 2012 MUSEC). We could not include data from two parallel RCTs and one cross‐over trial. One parallel RCT provided data only in one figure demonstrating daily VAS pain trajectories by comparison groups and numerical data were not available (Turcotte 2015). The other parallel RCT (Zajicek 2003_CAMS) provided the number of participants with a clinically relevant response defined as categories 0‐3 of the NRS 0‐10 that we considered an inappropriate threshold in our protocol. The cross‐over study (Leocani 2015)reported data but did not account for design.
Withdrawals due to AEs (tolerability). Data were available from 19 studies that contributed to the analysis of the outcome (Aragona 2009; Collin 2007; Collin 2010; Langford 2013; Leocani 2015; Markova 2018; NCT00682929; NCT01606176; Notcutt 2012; Novotna 2011; Rog 2005; Schimrigk 2017; Turcotte 2015; Vachova 2014; Van Amerongen 2017; Vaney 2004; Wade 2004; Zajicek 2003_CAMS; ZAJICEK 2012 MUSEC).
Important outcomes
PGIC. Eight parallel trials (Collin 2007; Langford 2013; Markova 2018; Novotna 2011; Rog 2005; Turcotte 2015; Vachova 2014; Wade 2004) provided data for the outcome. We could not include data from two trials, one (Notcutt 2012) because the reported outcome did not meet our predefined criteria of much or very much improvement in the PGIC, and one study (Van Amerongen 2017) because it measured the outcome as continuous data only.
HRQoL. There were several HRQoL outcome measures used, including the SF‐36 (Langford 2013; Markova 2018; NCT00682929; Novotna 2011; Schimrigk 2017), EQ‐5D (Collin 2010; Langford 2013; Novotna 2011), and the Spitzer QoL‐index (NCT01606176). Some trials incorporated condition‐specific HRQoL measures such as the 54‐item MSQoL (Collin 2010) and the 29‐item Multiple Sclerosis Impact Scale (MSIS‐29) (ZAJICEK 2012 MUSEC; Zajicek 2013_CUPID). Four studies provided data for each of the eight SF‐36 scales (physical functioning, role physical, bodily pain, general health, vitality, social functioning, role‐emotional, and mental health) (Langford 2013; Markova 2018; NCT00682929; Novotna 2011).
SAEs. Data on SAEs were reported for 20 studies and all of them were included in analysis (Aragona 2009; Collin 2007; Collin 2010; Killestein 2002; Langford 2013; Leocani 2015; Markova 2018; NCT00682929; Notcutt 2012; Novotna 2011; Rog 2005; Schimrigk 2017; Svendsen 2004; Turcotte 2015; Vachova 2014; Van Amerongen 2017; Vaney 2004; Wade 2004; Zajicek 2003_CAMS; ZAJICEK 2012 MUSEC).
Nervous system disorders. Seven trials provided outcome data that were included in analysis (Collin 2010; Killestein 2002; Langford 2013; Notcutt 2012; Novotna 2011; Svendsen 2004; Vachova 2014).
Psychiatric disorders. Six studies contributed to the analysis of the outcome (Collin 2010; Langford 2013; Notcutt 2012; Novotna 2011; Svendsen 2004; Vachova 2014).
Drug tolerance. Information on this outcome was available from two trials only (Collin 2010; Vachova 2014).
Other outcomes of limited importance
One small cross‐over study reported changes on a tremor index, measured using a validated tremor rating scale (Fox 2004). A parallel RCT reported the reduction in daily number of urinary incontinence episodes from baseline to end of treatment (eight weeks) (Kavia 2010).
Ongoing studies
Of the eight ongoing RCTs, three evaluate nabiximols versus placebo (Hansen 2021; NCT04984278; NCT05092191), of which two studies evaluate THC alone, CBD alone, THC and CBD versus placebo: one is expected to be completed in December 2021 with 448 participants (Hansen 2021), and one is expected in March 2025 with 250 participants (NCT05092191). One ongoing RCT is expected to be completed in November 2021 and plan to evaluate 52 participants (NCT04657666), one is expected in November 2022 with 446 participants (NCT04203498), and one is expected in September 2022 with 190 participants (NCT04984278). Recruitment in one study is reported as completed in May 2021 and results are not published yet. This study evaluated dronabinol versus placebo in 397 participants (NCT03756974). Recruitment in one study, which evaluated nabiximols versus placebo in 70 participants, is reported as unknown in the study registry, results are not published yet, and we are waiting for reply from study investigators (NCT03005119). We found one completed study, but with unpublished results, of which we are awaiting information from the authors. This study compared Sativex plus Lokomat training with other anti‐spasticity medications plus Lokomat training in 40 participants (Russo 2017). Please refer to Characteristics of ongoing studies for more detailed information.
Excluded studies
We excluded 36 full‐text articles (42 records) that did not match our inclusion criteria: 22 studies were non randomised trials or reviews; eight included wrong population; one because the aim of the trial was not consistent with this review, and the authors measured no outcomes of interest relevant to this review; two were N‐of‐1 cross‐summary of findings table over trials; two because the studies had been withdrawn, and no participants had been included; and a dose‐comparison trial of nabiximols without a placebo group. Please refer to the Characteristics of excluded studies for more detailed information.
Risk of bias in included studies
For details of the risk of bias judgements for each study, see Characteristics of included studies. A graphical representation of risk of bias for critical and important outcome can be seen in Analysis 1.1; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12.
1.1. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 1: Spasticity: number of participants reporting reduction of 30% in the spasticity NRS (follow up 6‐14 weeks)
1.3. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 3: Pain: number of participants reporting pain relief of 50% or greater in the NRS‐PI (follow up 3 weeks)
1.4. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 4: Pain: NRS‐PI as continuous outcome (follow up 3‐16 weeks)
1.5. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 5: Withdrawn due to adverse events (follow up 3‐48 weeks)
1.6. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 6: PGIC: number of participants reporting much or very much improvement in the PGIC (follow up 4‐48 weeks)
1.7. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 7: Health related quality of life: change score from baseline (follow up 3‐48 weeks)
1.8. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 8: Health related quality of life: change score from baseline for each domain of SF‐36 (follow up 12‐14 weeks)
1.9. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 9: SAEs: number of participants with SAEs (follow up 3‐48 weeks)
1.10. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 10: Specific AEs: number of participants reporting nervous system disorders (follow up 4‐48 weeks)
1.11. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 11: Specific AEs: number of participants reporting psychiatric disorders (follow up 4‐48 weeks)
1.12. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 12: Specific AEs: number of participants reporting drug tolerance (follow up 14‐48 weeks)
For the spasticity outcome (number of participants reporting reduction of 30% in the spasticity NRS), we assumed an overall risk of bias with some concerns, as all the trials (Collin 2007; Collin 2010; Markova 2018; Novotna 2011; ZAJICEK 2012 MUSEC) reporting the outcome were with some concerns (Analysis 1.1)
We also analysed continuous data for spasticity as an important outcome, but we did not include the results in the summary of findings table. We judged all trials as 'some concerns' (Collin 2007; Collin 2010; Langford 2013; Markova 2018; Novotna 2011; Van Amerongen 2017), excluding Notcutt 2012 which we classified as 'high risk' for missing outcome data (Analysis 1.2).
1.2. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 2: Spasticity: NRS as continuous outcome (follow up 2‐14 weeks)
Only Svendsen 2004 reported the pain outcome (number of participants reporting pain relief of 50% or greater in the NRS‐PI) and we judged the study at an overall high risk of bias because outcome data were not available for all randomised participants, and it is likely that missingness in the outcome depended on its true value (Analysis 1.3).
We also analysed continuous data for chronic pain as an important outcome, but we did not include the results in the summary of findings table. We judged all trials as 'some concerns' (Collin 2010; Langford 2013; NCT01606176; Rog 2005; Schimrigk 2017; Van Amerongen 2017; ZAJICEK 2012 MUSEC), excluding Markova 2018 which we classified as 'high risk' for selection of the reported result (Analysis 1.4).
For withdrawals due to AEs, we assumed an overall risk of bias with some concerns for 18 trials reporting outcome data (Aragona 2009; Collin 2007; Collin 2010; Langford 2013; Leocani 2015; Markova 2018; NCT00682929; NCT01606176; Notcutt 2012; Novotna 2011; Rog 2005; Schimrigk 2017; Turcotte 2015; Vachova 2014; Van Amerongen 2017; Wade 2004; Zajicek 2003_CAMS; ZAJICEK 2012 MUSEC). We judged one cross‐over trial (Vaney 2004) at high risk of bias because the method used to conceal the allocation of treatment was not reported and there was no sufficient time for carry‐over effects to have disappeared before outcome assessment in the second period. Moreover, there were different periods by sequence (Analysis 1.5). We explored potential non‐reporting bias by generating a funnel plot (Figure 3) which indicates, although not conclusively, a lack of bias for the outcome.
3.

Funnel plot for withdrawn due to AEs
Important outcomes
We judged PGIC (number of participants reporting much or very much improvement in the PGIC) to be at an overall high risk of bias as three of the studies (Markova 2018; Novotna 2011; Turcotte 2015) contributing 39% to the outcome estimate were at high risk of bias as they did not report any information on missing outcome data, and it is likely that missingness in the outcome depended on its true value. The other five studies (Collin 2007; Langford 2013; Rog 2005; Vachova 2014; Wade 2004) contributing to the effect estimate of the PGIC outcome were with some concerns (Analysis 1.6).
We analysed HRQoL as continuous data. Overall, we rated the risk of bias for the outcome to be high for Markova 2018, NCT01606176, Novotna 2011, and Zajicek 2013_CUPID because in these studies outcome data were not available for all participants. The data were not analysed in accordance with a pre‐specified plan in Markova 2018, and the ascertainment of the outcome could have differed between intervention groups in NCT01606176. We judged risk of bias to be of some concern for Collin 2010, Langford 2013, NCT00682929, Schimrigk 2017, and ZAJICEK 2012 MUSEC; (Analysis 1.7; Analysis 1.8).
Twenty studies reported SAEs (Aragona 2009; Collin 2007; Collin 2010; Killestein 2002; Langford 2013; Leocani 2015; Markova 2018; NCT00682929; NCT01606176; Notcutt 2012; Novotna 2011; Rog 2005; Schimrigk 2017; Svendsen 2004; Turcotte 2015; Vachova 2014; Van Amerongen 2017; Wade 2004; Zajicek 2003_CAMS; ZAJICEK 2012 MUSEC). We assumed an overall risk of bias with some concerns in 18 trials and an overall high risk of bias in two cross‐over trials (Aragona 2009; Vaney 2004). Aragona 2009 did not report information on whether deviations from intended intervention were balanced between sequences and whether an appropriate analysis was used to estimate the effect of adhering to intervention. In Vaney 2004 the method used to conceal the allocation of treatment was unclear, and no information was available on whether failures in implementation and non‐adherence to the assigned intervention could have affected participants’ outcomes (Analysis 1.9).
Seven trials reported the number of participants who had nervous system AEs (Collin 2010; Killestein 2002; Langford 2013; Notcutt 2012; Novotna 2011; Svendsen 2004; Vachova 2014). We judged Killestein 2002 and Svendsen 2004 at an overall high risk of bias as in these studies co‐interventions were not reported and the method of measuring the outcome was inappropriate because participants, who were aware of the intervention received, used their own words to record AEs in their diaries during each treatment period. We rated the other five studies as some concerns (Analysis 1.10).
Six studies reported the number of participants who had psychiatric disorders (Collin 2010; Langford 2013; Notcutt 2012; Novotna 2011; Svendsen 2004; Vachova 2014). For this outcome, we assumed an overall risk of bias with some concern as five studies were rated as some concerns and one at overall high risk of bias (Svendsen 2004) (Analysis 1.11). Only two studies (Collin 2010; Vachova 2014) reported the number of participants who had drug tolerance and both studies were rated as some concerns (Analysis 1.12).
Effects of interventions
See: Table 1
Critical outcomes
In Table 1 we provided a summary of the effect estimates of cannabinoids for spasticity, pain and withdrawals due to AEs, the certainty of evidence for comparisons with placebo and reasons for downgrading it.
Spasticity: number of participants reporting reduction of 30% over baseline in the spasticity NRS over baseline
I. Data were available from four studies comparing oromucosal spray of nabiximols (Sativex®) with placebo (Collin 2007; Collin 2010; Markova 2018; Novotna 2011) and one study comparing oral THC extract of Cannabis sativa (Cannador®) with placebo (ZAJICEK 2012 MUSEC). Most participants had progressive MS (range from 55% to 100%).
Nabiximols and Cannador® likely increased the number of participants who reported a clinically important reduction of perceived severity of spasticity over the baseline (OR 2.51, 95% CI 1.56 to 4.04; 5 studies, 1143 participants; I2 = 67%; P = 0.02; moderate‐certainty evidence; Analysis 1.1). The absolute effect was 216 more people (95% CI 99 more to 332 more) per 1000 reporting benefit when treated with cannabinoids compared with placebo over a follow‐up range from 6 to 14 weeks.
Despite the high level of heterogeneity of the pooled estimate, we did not downgrade for inconsistency because the direction of effect across the studies consistently favoured cannabinoids compared with placebo. The heterogeneity was almost completely attributable to one small trial (I2 = 36%; Markova 2018) that used an enriched enrolment two‐phases design and reported the largest effect (OR 7.24, 95% CI 3.05 to 17.17; 106 participants). Excluding Markova 2018, heterogeneity decreased substantively and slightly attenuated the average effect (OR 2.04, 95% CI 1.45 to 2.86; 4 studies, 1037participants; I2 = 31%).
II. Seven studies provided data on mean change from baseline in spasticity NRS (Collin 2007; Collin 2010; Markova 2018; Notcutt 2012; Novotna 2011; Van Amerongen 2017; Vaney 2004). Nabiximols likely resulted in a reduction in perceived severity of spasticity compared with placebo (MD ‐0.55, 95% CI ‐0.94 to ‐0.17; 7 studies, 1262 participants; I2 = 68%; moderate‐certainty evidence; Analysis 1.2). Despite the high level of heterogeneity of the pooled estimate, we did not downgrade for inconsistency because of the consistent benefit seen across the studies in favour of cannabinoids compared with placebo. As above, the heterogeneity is almost completely attributable to one small enriched‐design trial (I2 = 53%; Markova 2018). Excluding Markova 2018, heterogeneity decreased substantively (MD ‐0.39, 95% CI ‐0.63 to ‐0.14; 6 studies, 1156 participants; I2 = 24%). Follow‐up ranged from 2 to 14 weeks.
Chronic Pain: number of participants reporting pain relief of 50% or greater, over baseline in the NRS‐PI
I. There was insufficient evidence from one small three‐week trial (Svendsen 2004), that used synthetic THC (dronabinol) to determine the effects of treatment on the number of participants with pain relief of 50% or greater when compared with placebo, over three weeks' follow‐up (OR 4.23, 95% CI 1.11 to 16.17; 48 participants; Analysis 1.3). The certainty evidence was very low (downgraded one level for risk of bias, two levels for very serious imprecision). Participants with progressive MS were 62.5% and those with relapsing MS 37.5%.
II. Eight studies provided data on mean difference change from baseline in pain NRS‐PI over a range from 3 to 16 weeks (Collin 2010; Langford 2013; Markova 2018; NCT01606176; Rog 2005; Schimrigk 2017; Van Amerongen 2017; ZAJICEK 2012 MUSEC). Except for Collin 2010, the direction of effect on pain reduction across the studies favoured nabiximols, Cannabis extract or synthetic THC compared with placebo. There was a high level of statistical heterogeneity (MD ‐0.54, 95% CI ‐0.91 to ‐0.18; 8 studies, 1451 participants; I² = 62%, P = 0.01; Analysis 1.4). The certainty of this evidence was low (downgraded one level for risk of bias, one level for inconsistency). Excluding the enriched‐design study of Markova 2018 led to a moderate heterogeneity (MD ‐0.43, 95% CI ‐0.78 to ‐0.09; 7 studies, 1345 participants; P = 0.05, I2 = 53%).
Number of participants withdrawn due to AEs (tolerability)
Data were available from 19 studies, 12 of which evaluated nabiximols (Sativex®) against placebo. Treatment may have resulted in a slight increase in the number of participsants who withdrew due to AEs over 3 to 48 weeks’ follow‐up, when compared with placebo (OR 2.41, 95% CI 1.51 to 3.84; 3110 participants; I² = 17%, P = 0.25; Analysis 1.5). The absolute effect is 39 more people (95% CI 15 more to 76 more) per 1000 who withdrew due to AEs. The certainty evidence was low (downgraded one level for risk of bias, one level for imprecision). There was no evidence for small‐study effects Figure 3.
Important outcomes
In Table 1 we provided a summary of the effect estimates of cannabinoids for the following important outcomes.
Number of participants reporting much or very much improvement in PGIC
Cannabinoids (nabiximols, Cannabis extract, synthetic THC) likely resulted in an increase in the number of participants who reported improvement in the PGIC over 4 to 48 weeks’ follow‐up, compared with placebo (OR 1.80, 95% CI 1.37 to 2.36; 8 studies, 1215 participants; I² = 0%, P = 0.53; Analysis 1.6); the absolute effect was 113 more people (95% CI 57 more to 175 more) per 1000 having much or very much improvement in PGIC when treated with cannabinoids compared with placebo. The certainty evidence was moderate (downgraded one level for risk of bias).
HRQoL
HRQoL scores were available from eight trials. Three trials reported HRQoL scores from the EQ‐5D (Collin 2010; Langford 2013; Novotna 2011), two reported scores from the SF‐36 physical health component (PCS) (NCT00682929; Schimrigk 2017), one used the Spitzer Quality of Life Index (NCT01606176), and two trials used the MSIS‐29 (ZAJICEK 2012 MUSEC; Zajicek 2013_CUPID). Several trials used more than one of these measures; we only included one trial in each measure. The effect of cannabinoids against placebo on mean change in HRQoL as a SMD was ‐0.08 (95% CI ‐0.17 to 0.02; I2 = 0%, P = 0.65; 8 studies, 1942 participants; Analysis 1.7). The certainty of evidence was low, downgraded two levels for very serious risk of bias. Cannabinoids may have little to no effect on HRQoL compared with placebo over 3 to 48 weeks' follow‐up.
Four trials (Langford 2013; Markova 2018; NCT00682929; Novotna 2011) reported change scores from baseline for each of the SF‐36 domains at 12 to 14 weeks’ follow‐up. All confidence intervals were wide and none showed a difference between cannabinoids and placebo, except the bodily pain domain which improved in the group treated with nabiximols compared with placebo (MD 4.24, 95% CI 0.07 to 8.40; I² = 45%, P = 0.16; 3 studies, 683 participants; Analysis 1.8).
SAEs: number of participants with SAEs
Cannabinoids may have resulted in little to no difference in SAEs compared with placebo (OR 1.38, 95% CI 0.96 to 1.99; 20 studies, 3124 participants; I² = 0%, P = 0.60; Analysis 1.9); the absolute effect is 12 more people per 1000 (95% CI 1 fewer to 30 more) per 1000 having SAEs with cannabinoids compared with placebo. The certainty of evidence was low downgraded one level for risk of bias and one level for imprecision.
Number of participants reporting nervous system AEs
Cannabinoids may have resulted in an increase in the number of participants who had nervous system AEs over 4 to 48 weeks’ follow‐up, when compared with placebo (OR 2.61, 95% CI 1.53 to 4.44; 7 studies, 1154 participants; I² = 64%, P = 0.01; Analysis 1.10); the absolute effect is 210 more people (95% CI 85 more to 341 more) per 1000 having nervous system disorders with cannabinoids compared with placebo. The certainty of evidence was low, downgraded one level for risk of bias, one level for inconsistency. We were unable to find reasons for heterogeneity. Limiting the analysis to studies which evaluated only Sativex did not reduce heterogeneity.
Number of participants reporting psychiatric disorders
Cannabinoids may have resulted in a slight increase in the number of participants who had psychiatric disorders over 4to 48 weeks’ follow‐up, when compared with placebo (OR 1.94, 95% CI 1.31 to 2.88; 6 studies, 1122 participants; I² = 0%, P = 0.42; Analysis 1.11); the absolute effect is 61 more people (95% CI 21 more to 114 more) per 1000 having psychiatric disorders when treated with cannabinoids compared with placebo. The certainty of evidence was low, downgraded one level for risk of bias, one level for imprecision.
Number of participants reporting drug tolerance
The evidence was very uncertain about the effect of cannabinoids on drug tolerance over 14 to 48 weeks' follow‐up (OR 3.07, 95% CI 0.12 to 75.95; 2 studies, 458 participants; very low‐certainty evidence; Analysis 1.12). The certainty of evidence was very low, downgraded one level for risk of bias, two levels for very serious imprecision.
Outcomes of limited importance
Spasticity measured by the Ashworth scale or the MAS
Eleven studies provided usable data on severity of spasticity measured by the Ashworth scale or the MAS. Spasticity was slightly lower at the end of the study period with cannabinoids than with placebo (MD ‐0.23, 95% CI ‐0.44 to ‐0.03; 1777 participants; low‐certainty evidence; Analysis 1.13). Compared with placebo, cannabinoids may have resulted in little to no difference in reduction of spasticity measured with the Ashworth scale or the MAS over 2 to 50 weeks’ follow‐up, when compared to placebo. Our confidence in this result was low, downgraded one level for serious risk of bias, one level for imprecision. We judged all included studies with some concerns, excluding Notcutt 2012 judged at high risk of bias due to missing outcome data.
1.13. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 13: Spasticity: Ashworth or Modified Ashworth (follow up 2‐50 weeks)
Pain relief of 30% or greater
One parallel RCT (Langford 2013) including 339 participants reported the outcome. At 10 weeks' follow‐up, authors reported a treatment difference in favour of nabiximols compared with placebo (OR 1.61, 95 % CI: 1.01 to 2.57, P = 0.046).
Improvement of bladder symptoms
Kavia 2010 evaluated nabiximols as an add‐on therapy in alleviating bladder symptoms in 335 patients with MS and overactive bladder. Authors reported no difference in daily number of urinary incontinence episodes (primary outcome) between nabiximols and placebo at eight weeks. There were significant differences favouring nabiximols against placebo in number of episodes of nocturia, number of voids day and PGIC (secondary outcomes).
Frequency and severity of muscle spasms
Two parallel trials reported the outcome. Wade 2004 reported no difference between nabiximols and placebo in 160 participants. ZAJICEK 2012 MUSEC found that self‐reported spasms' relief was consistently higher with an oral Cannabis extract (Cannador®) than with placebo. The effect increased over time due to an increase in the rate of relief with the Cannabis extract and because of an extremely low responder rate in the placebo group at week 12. Response rates were 30.8% (143 participants) in the Cannabis group and 13.4% (134 participants) in the placebo group (P value < 0.002).
Fatigue
Four parallel‐group trials (Collin 2010; Langford 2013; Van Amerongen 2017; Wade 2004) and one cross‐over trial (Corey‐Bloom 2012) provided data for the analysis of fatigue. Collin 2010 and Langford 2013 used the 0‐10 NRS; Van Amerongen 2017 and Wade 2004 used the Fatigue Severity scale; Corey‐Bloom 2012 used the Modified‐Fatigue Impact Scale. All included studies found no differences between cannabinoids (nabiximols, synthetic THC, smoked Cannabis) and placebo (SMD 0.04, 95% CI ‐0.26 to 0.34; 5 studies, 928 participants; Analysis 1.14).
1.14. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 14: Fatigue as continuous outcome (follow up 4‐14 weeks)
Sleep quality
Seven parallel RCTs provided data of this outcome (Collin 2010; Langford 2013; Markova 2018; Notcutt 2012; Novotna 2011; Rog 2005; Wade 2004). The most commonly reported measure was sleep quality assessed using a 0‐10 NRS. One study (Wade 2004) used a 0‐100 VAS scale. We transformed the 0‐100 VAS results to a 0‐10 scale by dividing by 10 so that these were comparable to the other studies evaluating this outcome. The pooled estimate suggested improvement in sleep quality associated with cannabinoids compared with placebo (MD ‐0.66, 95% CI ‐1.10 to ‐0.22; 7 studies, 1205 participants). There was substantial evidence of heterogeneity (P = 0.001, I2= 73%) (Analysis 1.15). Two studies evaluated sleep quality using a 0‐10 NRS and provided information on the number of participants reporting much or very much improvement in sleep. Both studies reported a significant improvement in sleep quality associated with cannabinoids compared with placebo (OR 1.79, 95% CI 1.30 to 2.46; 2 studies, 756 participants; I2 = 0%; Analysis 1.16).
1.15. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 15: Sleep quality: NRS as continuous outcome (follow up 4‐14 weeks)
1.16. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 16: Sleep quality: number of participants reporting an improvement in the NRS sleep (follow up 6‐14 weeks)
Depression Three parallel RCTs (Novotna 2011; Vachova 2014; Wade 2004) used the BDI scale and suggested no difference between nabiximols and placebo on depression (MD 0.17, 95% CI ‐0.90 to 1.24; 3 studies, 495 participants; I2= 0%; Analysis 1.17). One parallel trial (Rog 2005) used the HADS) and reported no difference between nabiximols and placebo (MD 0.09, CI ‐1.06 to 1.23; 66 participants). In BDI and HADS, higher score indicated more severe depression and thus a negative MD favoured cannabinoids while a positive MD favoured control.
1.17. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 17: Depression: Beck Depression Inventory as continuous outcome
Anxiety
One parallel‐group trial (Rog 2005) evaluated anxiety with the HADS and found no difference between nabiximols and placebo (MD ‐0.64, CI ‐1.75 to 0.46; 66 participants).
ADL
Four parallel‐group trials (Collin 2010; Markova 2018; Wade 2004; Zajicek 2003_CAMS) evaluated ADL using the Barthel Index. The overall effect estimate suggested no difference between cannabinoids (nabiximols, Cannabis extract, synthetic THC) and placebo (MD ‐0.08, 95% CI ‐0.32 to 0.16; 4 studies, 1134 participants; Analysis 1.18). There was no evidence of heterogeneity across studies (I2= 0%, P = 0.49). One small cross‐over study (Corey‐Bloom 2012) evaluated walk time and showed no difference between smoked Cannabis and placebo.
1.18. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 18: Activities of daily living: Barthel index as continuous outcome
Tremor
A small (14 participants) cross‐over study (Fox 2004) assessed the outcome, however available data did not allow quantitative assessment. The study was judged at high risk of bias arising from the randomisation process, deviations from the intended interventions and measurement of the outcome. Authors concluded that an oral Cannabis extract (Cannador®) did not result in a functionally significant improvement in MS‐associated tremor, however the evidence is very uncertain.
CGIC
Four parallel trials (Collin 2010; Notcutt 2012; Novotna 2011; Vachova 2014) measured the CGIC outcome. The main carer was asked to assess the change in the participant's general functional abilities at the end of the study. CGIC was assessed on a 7‐point Likert‐like scale that used three categories of improvement (slightly improved, much improved, or very much improved), three categories of worsening (slightly worse, much worse, or very much worse), and an option of “no change”. The participant’s overall condition improved by at least one category on the CGIC in the nabiximols group as compared with the placebo group (OR 1.66, 95% CI 1.15 to 2.41; 4 studies, 582 participants; I2 = 0%; Analysis 1.19).
1.19. Analysis.

Comparison 1: Cannabis and cannabinoids versus placebo, Outcome 19: Number of caregivers reporting improvement on the CGIC (follow up 4‐48 weeks)
Use of anti‐spasticity medicines
None of the included studies reported the outcome.
Use of analgesics
One parallel‐group trial (Langford 2013) and one cross‐over study (Svendsen 2004) reported that paracetamol was provided for rescue analgesic use during the study and no difference was found between cannabinoids (nabiximols, synthetic THC) and placebo.
Discussion
Summary of main results
This review summarises evidence from 25 studies in people with MS treated with cannabinoid‐based medicines compared with placebo. Cannabinoids include synthetic, herbal, or plant‐derived cannabinoids. Most studies included all types of MS, except one study that included participants with relapsing MS only, and three studies that included participants with SPMS and PPMS. The mean age of the participants ranged from 18 to 60 years. Thirteen trials evaluated nabiximols (Sativex®), five an oral synthetic cannabinoid (dronabinol, nabilone, namisol), three an oral extract of Cannabis sativa, and one trial evaluated inhaled herbal Cannabis. These trials compared cannabinoids against placebo. Two trials compared dronabinol, an oral THC extract of Cannabis sativa and placebo, and one trial compared dronabinol, inhaled herbal Cannabis and placebo. Five studies were of very short duration (two to four weeks), 10 were of short duration (four to 12 weeks), seven studies were of intermediate duration (12 to 26 weeks), and two were long‐term studies (50 weeks and 156 weeks). One trial reported outcome at three days.
We found that nabiximols (Sativex®) probably reduce spasticity severity as perceived by patients at time points up to 14 weeks (moderate‐quality evidence). Nabiximols were likely to increase the number of participants reporting a clinically important reduction of perceived severity of spasticity, and lead to improve average spasticity scores compared with placebo. There was low‐certainty evidence that nabiximols, Cannabis extract or synthetic THC cannabinoids were more effective than placebo in mean change in chronic neuropathic pain relief at time points up to 16 weeks.
For the important outcome of PGIC we found moderate‐certainty evidence of the benefit of nabiximols, Cannabis extract, or synthetic THC cannabinoids over placebo. There was evidence that cannabinoids were likely to increase the number of participants reporting much, or very much improvement in the PGIC at time points up to 48 weeks. We are uncertain about the effect of cannabinoids on HRQoL at time points up to 16 weeks (very low‐certainty evidence).
Cannabinoid‐based medicines may have increased slightly the number of participants who withdrew due to adverse events ( (low‐certainty evidence). We did not find any significant differences between cannabinoids and placebo in terms of serious adverse effects, but this was likely due to the small amount of data available for this outcome (low‐certainty evidence). Cannabinoids may increase nervous system adverse events and psychiatric disorders slightly (low‐certainty evidence). The evidence was very uncertain about the effect on drug tolerance (very low‐certainty evidence).
Overall completeness and applicability of evidence
Eight (32%) of the 25 included studies provided data on the use of cannabinoids for spasticity outcomes. Most participants had a progressive form of MS (range from 55% to 100%) and cannabinoids were added when spasticity was not relieved by current anti‐spasticity medications. Nine (36%) eligible studies provided data on chronic neuropathic pain relief, though most did not report the number of patients with different forms of MS. Cannabinoids were used as an add‐on treatment in participants who had failed to gain adequate pain relief from current analgesics. Our literature search identified a number of ongoing trials which could provide valuable data in addition to that presented in this review; we will include these in future updates.
Several factors limit the applicability of the evidence in our review. First, the baseline level of spasticity or chronic neuropathic pain and their duration varied across participants, and when assessing severity of these symptoms at baseline authors used a number of different instruments. The included studies recruited a mixture of patients with different clinical manifestations of spasticity and chronic neuropathic pain. This led to significant clinical and statistical heterogeneity in the effect estimates that limited the applicability of the evidence to the wider population of people with MS. Second, the proportion of participants with previous or current Cannabis experience varied across the included studies (from 6% to 80%), with only one study excluding participants with previous experience. Benefits andAEs of cannabinoids may differ between Cannabis users and naive users. We do not know if the evidence presently reviewed may be generalisable to Cannabis‐naive participants. Third, the administration of co‐therapies during follow‐up was variable among the included studies, and is another limitation of the evidence. Fourth, the short duration of the studies does not enable us to determine the long ‐term balance between benefits and harms of cannabinoid‐based medicines for people with MS.
The included studies used a large variety of measures to evaluate effects of cannabinoid‐based medicines on spasticity and pain. We prioritised patient‐reported outcomes. This review is therefore not limited by outcomes which are not of primary importance to patients.
Quality of the evidence
The quality of the included studies was difficult to assess, because the majority of the risk of bias judgements were deemed ‘some concerns’. In particular, we judged ‘deviations from intended interventions’ and ‘measurement of outcome’ with some concerns for most included studies. An important bias that may have occurred was in blinding procedures. Given that most participants in the included studies had previous or current Cannabis experience and our outcomes of interest were patient‐reported outcomes, make it likely that participants and personnel could become unblinded during trials.
Half of the cross‐over trials was at high risk of carry‐over effect, as they did not have an adequate washout period or their second period was not long enough for the carry‐over effect to disappear. Furthermore, none of the cross‐over studies considered period effect in the analysis.
We are moderately confident in the effect estimate of an important reduction in spasticity in the cannabinoid group compared with the placebo group. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. With respect to chronic neuropathic pain relief, our confidence in the effect estimate is limited because of the small sample size available from only one small trial that reported the number of participants with pain relief of 50% or greater over baseline. Additional data provided by seven studies showed a reduction of mean chronic neuropathic pain intensity from baseline in cannabinoid‐treated participants compared with placebo, but there was a wide variation in reporting across the included studies. The majority of the evidence was low or very low‐certainty for SAEs, nervous system or psychiatric disorders and drug tolerance, due to most trials having at least one risk of bias domain and some estimates being imprecise.
Potential biases in the review process
To avoid a possible risk of non‐reporting bias, we searched a range of databases and trials registries to identify and include results of unpublished completed studies, and did not apply any language or period restrictions to the search. However, the possible presence of non‐reporting bias could not be totally excluded.
There is a high proportion of risk of bias assessments given as ‘some concerns’ across the studies in our review. The overall risk of bias judgements were deemed 'some concerns' for seven (88%) of the eight included studies available for the spasticity outcome and for seven (78%) of the nine studies for the chronic neuropathic pain outcome. This may well reflect an inadequate reporting of information by the studies. Consequently, we may have overestimated the impact of bias on our findings by downgrading the certainty of evidence of the critical and important outcomes due to risk of bias. We did not account for the crossover design due to inadequate information presented in the studies. This leads to the potential for unit of analysis errors in several analyses of outcomes in our Table 1 where crossover studies provide data (see Analysis 1.3; Analysis 1.5; Analysis 1.9; Analysis 1.10). However, the number of participants recruited to these studies is small, the number of crossover studies included in the analysis is low, and they contribute only small weights to these outcomes. We decided not to attempt adjustment of these effect estimates, and we think it is unlikely that the summary effect estimates will be distorted by their lack of adjustment for crossover design.
The influence of allowed co‐interventions on benefits and harms of cannabinoids was unclear because type and dosage of co‐interventions were not clearly reported or controlled for in the included studies.
Agreements and disagreements with other studies or reviews
Other systematic reviews (Amato 2017; Meza 2017; Torres‐Moreno 2018; Whiting 2015) and one overview (Nielsen 2018) have explored the effects of cannabinoids in the treatment of spasticity and pain among people with MS. See Table 2 for details of these reviews. Comparing these reviews together and with ours highlights challenges inherent in grading the certainty of evidence since the reviews used different criteria for study selection and inclusion, characteristics of participants, assessment of study quality, outcomes measures and different analytic methods. The search strategy of other reviews was not updated (most recent to 7 November 2017 in Mücke 2018), and new studies are available for inclusion in our review. Finally, we assessed risk of bias using the new Revised Cochrane risk‐of‐bias tool for randomised trials (RoB 2).
1. Systematic reviews on cannabis‐based medicines for people with MS.
| Author year country | SR (search) | Included studies | Interventions | Primary outcomes | RoB/quality | Meta‐analysis | Conclusion |
|
Amato 2017 Italy |
SR: yes (updated September 2016) | RCTs (n 15) Parallel and cross‐over |
• Cannabis in any dose, used either as monotherapy or adjunct to conventional drugs • Placebo |
• Spasticity (Ashworth scale* and NRS) |
• Cochrane RoB • GRADE |
Yes | High confidence in the effect estimate in favour of cannabis for spasticity (NRS and VAS, but not the Ashworth scale) and pain. |
|
Meza 2017 Chile |
Epistemonikos database (up to date not reported) |
SRs (n = 25) Spasticity: 4 RCTs Pain: 3 RCTs |
• Cannabinoids • Placebo |
• Pain (VAS or NRS) • Bladder dysfunction (NRS) • Spasticity (Ashworth scale or NRS) • QoL • AEs |
GRADE | Yes | • Cannabinoids do not reduce spasticity and pain (high‐certainty evidence) • AEs were frequent (moderate‐ certainty evidence) |
|
Mücke 2018 Germany |
Cochrane Review (up to 7 November 2017) |
Parallel, cross‐over RCTs Pain: 4 RCTs QoL: 2 RCTs AEs: 3 RCTs Nervous system disorders: 3 RCTs Psychiatric disorders: 3 RCTs |
• Herbal cannabis, plant‐based cannabinoids (dronabinol: nabiximols), or synthetic cannabinoids (e.g. nabilone) • Placebo • Active comparators |
• Pain • QoL • AEs |
• Cochrane RoB • GRADE |
Yes | Confidence in the effect estimate for pain was low. |
|
Nielsen 2018 Australia |
Overview (1980 up to 30 November 2016) |
SR (n = 11) (AMSTAR criteria). RCTs and non‐randomised studies |
• Plant‐based and pharmaceutical cannabinoids • Placebo • Active comparators |
• Pain • Spasticity • Quality of life • AEs |
• SIGN • GRADE |
No | • High‐quality reviews find cannabinoids may have modest effects in MS for pain or spasticity. • AEs were mild to moderate |
|
Torres‐Moreno 2018 Spain |
SR: yes (up to July 26, 2016) | Parallel and cross‐over RCTs (17 studies) | • Medicinal cannabinoids • Placebo |
• Spasticity (Ashworth scale, MAS, or NRS scale) • Pain • Bladder dysfunction • AEs • Withdrawals due to AEs |
• Cochrane RoB | Yes | • Limited efficacy of cannabinoids for spasticity, pain, and bladder
dysfunction • Treatment can be considered as safe. |
|
Whiting 2015 UK |
SR: yes (up to April 2015) | Parallel and cross‐over RCTs Pain: 1 RCT Spasticity: 11 RCTs Non‐randomised studies for AEs |
• Cannabinoids • Usual care, placebo, or no treatment |
• Spasticity (Ashworth scale or NRS) • QoL • AEs |
• Cochrane RoB • GRADE |
Yes | • Moderate quality evidence to support the use of cannabinoids for the treatment of spasticity. • Short‐term AEs relatively common including serious AEs. |
Abbreviations
AEs: adverse events; CI: confidence interval; MAS: modified Ashworth scale; NRS: Numeric Rating Scale; QoL: quality of life; RCTs: randomised controlled trials; RoB: risk of bias; SR: systematic review; VAS: Visual Analogue Scale; WMD: weighted mean difference.
* The Ashworth scale (Ashworth 1964) has been criticised as unreliable, insensitive to therapeutic benefit, and reflective only of passive resistance to movement and not of other features of spasticity (Pandyan 1999; Wade 2010).
Our results agree with and update the findings reported by Amato 2017, Nielsen 2018, and Whiting 2015 who showed that cannabinoid‐based medicines reduced severity of spasticity as perceived by people with MS. However, the certainty of evidence for the treatment effects varied among the reviews. It was judged high by Amato and Nielsen, and moderate by Whiting. We also judged the evidence for cannabis versus placebo as moderate certainty. Our results are not consistent with those reported by Meza 2017. This author included four parallel RCTs in spasticity and concluded that cannabinoids did not reduce spasticity in people with MS. However, the authors interpreted the small treatment effect measured with the Ashworth scale as conferring no effect, which is usually found using the scale. Meza 2017 used GRADE and judged the effect estimate at high‐certainty evidence, but they did not downgrade for risk of bias and imprecision in the results of included trials. Torres‐Moreno 2018 included 17 RCTs and concluded for a limited efficacy of cannabinoids for spasticity in MS, but authors did not assess the certainty of evidence.
We found low‐certainty evidence that nabiximols, Cannabis extract, or synthetic THC were more effective than placebo in terms of chronic neuropathic pain relief measured as the continuous outcome. Our conclusion is consistent with the findings reported in a Cochrane Review by Mücke 2018, and in other published reviews (Nielsen 2018; Torres‐Moreno 2018).
One Cochrane Review (Mücke 2018) found a moderate‐certainty evidence that more people withdrew due to AEs in the cannabinoid group than in the placebo group. Our findings were similar, but our confidence in the effect estimate was limited. In accordance with other systematic reviews (Mücke 2018; Nielsen 2018; Whiting 2015), we found that cannabinoid‐based medicines were associated with a slight increased risk of short‐term AEs, especially nervous system and psychiatric disorders. Different results were found by Amato 2017 who reported that for AEs no differences were observed between cannabinoids and placebo, and by Torres‐Moreno 2018 who concluded that treatment with these drugs can be considered as safe.
Authors' conclusions
Implications for practice.
Overall, this review provides moderate‐certainty evidence for an antispastic effect of nabiximols (as an add‐on therapy to anti‐spasticity medications) compared with placebo in people with MS in spasticity outcomes at 6 to 14 weeks. An important clinical indication for nabiximols in MS would be where spasticity is moderate to severe and other pharmacological and rehabilitation treatments are not effective. Our focus on patient‐reported outcomes gives reasonable grounds to assume that people with MS would value the benefit identified in our review. However, this would need to be traded against their psychotropic effect and the risk of drug intolerance of cannabinoid medicines. In our review, there was limited evidence found on serious adverse events and long‐term adverse events, which does not rule out the possibility of abuse and liability in prescribing these medicines to people with MS in clinical practice. Possible major adverse events from long‐term use of these medicines include cognitive impairment and psychiatric disorders. It is therefore important that both short‐ and long‐term adverse effects are thoroughly evaluated in considering the clinical application of cannabinoids medicines.
Implications for research.
We assessed the certainty of evidence in the present review as low to very low for most critical and important outcomes, excluding spasticity and PGIC (moderate certainty), according to GRADE. In order for robust conclusions to be drawn regarding the antispastic and analgesic effects of cannabinoids‐based medicines for people with MS, we need studies of a high methodological quality, with large sample sizes and longer follow‐up periods. There is also a need for randomised studies which compare these medicines with other active anti‐spasticity medications and analgesics, in order to draw reliable conclusions about comparative efficacy between treatments.
Long‐term adverse effects and drug tolerance of repeated exposure to cannabinoids remain a major concern. The present review did not find definitive evidence on SAEs and other AEs, and therefore we do not know the balance between desirable and undesirable effects of the cannabinoids, particularly the possible increased risk of cognitive impairment in people with MS. Therefore, further research is needed in order to assess the short‐and long‐term adverse effects of these drugs.
In the currently reviewed studies, there is inconsistency regarding the use of co‐therapies. This is something that should be addressed in future studies, owing to the frequent use of disease‐modifying therapies and symptomatic treatments by people with MS in clinical practice. Researchers should ensure that any observed effects cannot be attributed to co‐therapies by monitoring that no deviations from intended intervention arise because of the trial context.
It would be beneficial for future research to assess whether (and how) cannabinoids' effects would differ between relapsing and progressive forms of MS, which was not considered in the trials included in the review.
History
Protocol first published: Issue 10, 2019
Risk of bias
Risk of bias for analysis 1.1 Spasticity: number of participants reporting reduction of 30% in the spasticity NRS (follow up 6‐14 weeks).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Collin 2007 | Some concerns | There is no information about concealment of the allocation sequence. Any baseline differences observed between intervention groups appear to be compatible with chance. | Some concerns | Participants were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | Low risk of bias | Outcome data were available for nearly all randomized participants. | Some concerns | The method of measuring the outcome was not inappropriate and the ascertainment of the outcome did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received but it is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Some concerns | There is no information on whether the result being assessed is likely to have been selected, on the basis of the results, and from multiple eligible analyses of the data. | Some concerns | The study is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain. |
| Collin 2010 | Some concerns | There is no information about concealment of the allocation sequence and any baseline differences observed between intervention groups appear to be compatible with chance. | Some concerns | Participants were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | Low risk of bias | Outcome data were available for nearly all randomized participants. | Some concerns | The method of measuring the outcome was not inappropriate and the ascertainment of the outcome did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received, but it is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Low risk of bias | The data were analysed in accordance with a pre‐specified plan reported in the Eudract protocol and reported outcome data are unlikely to have been selected, on the basis of the results, from multiple eligible analyses of the data. | Some concerns | The study is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain. |
| Markova 2018 | Some concerns | There is no information to answer any of the signalling questions. | Some concerns | Participants were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | Low risk of bias | Outcome data were available for nearly all randomized participants. | Some concerns | The method of measuring the outcome was not inappropriate and the ascertainment of the outcome likely did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received but it is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Low risk of bias | The data were likely analysed in accordance with a pre‐specified plan and reported outcome data are unlikely to have been selected, on the basis of the results, from multiple eligible analyses of the data. | Some concerns | The study is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain. |
| Novotna 2011 | Some concerns | There is no information to answer any of the signalling questions. | Some concerns | Participants were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | Low risk of bias | Outcome data were available for nearly all randomized participants. | Some concerns | The method of measuring the outcome was not inappropriate and the ascertainment of the outcome likely did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received but it is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Low risk of bias | The data were likely analysed in accordance with a pre‐specified plan and reported outcome data are unlikely to have been selected, on the basis of the results, from multiple eligible analyses of the data. | Some concerns | The study is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain. |
| ZAJICEK 2012 MUSEC | Low risk of bias | The allocation sequence was adequately concealed and any baseline differences observed between intervention groups appear to be compatible with chance. | Some concerns | Participants were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | Low risk of bias | Outcome data were available for nearly all randomized participants. | Some concerns | The method of measuring the outcome was not inappropriate and the ascertainment of the outcome likely did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received but it is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Low risk of bias | All decisions regarding primary outcome data were finalised by a blind data review panel before unblinding. Reported outcome data are unlikely to have been selected, on the basis of the results, from multiple eligible analyses of the data. | Some concerns | The study is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain. |
Risk of bias for analysis 1.2 Spasticity: NRS as continuous outcome (follow up 2‐14 weeks).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Collin 2007 | Some concerns | There is no information about concealment of the allocation sequence. Any baseline differences observed between intervention groups appear to be compatible with chance. | Some concerns | Participants were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | Low risk of bias | Outcome data were available for nearly all randomized participants. | Some concerns | The method of measuring the outcome was not inappropriate and the ascertainment of the outcome did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received but it is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Some concerns | There is no information on whether the result being assessed is likely to have been selected, on the basis of the results, and from multiple eligible analyses of the data. | Some concerns | The study is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain. |
| Collin 2010 | Some concerns | There is no information about concealment of the allocation sequence and any baseline differences observed between intervention groups appear to be compatible with chance. | Some concerns | Participants were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | Low risk of bias | Outcome data were available for nearly all randomized participants. | Some concerns | The method of measuring the outcome was not inappropriate and the ascertainment of the outcome did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received but it is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Low risk of bias | The data were analysed in accordance with a pre‐specified plan reported in the Eudract protocol and reported outcome data are unlikely to have been selected, on the basis of the results, from multiple eligible analyses of the data. | Some concerns | The study is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain. |
| Langford 2013 | Some concerns | There is no information about concealment of the allocation sequence and any baseline differences observed between intervention groups appear to be compatible with chance. | Low risk of bias | Participants were aware of intervention groups during the trial and no deviations from intended intervention arose because of the trial context. An appropriate analysis was probably used to estimate the effect of assignment to intervention. | Low risk of bias | Missingness in the outcome could not depend on its true value. | Some concerns | No information on the method of measuring the outcome. The ascertainment of the outcome probably did not differ between intervention groups. It is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Some concerns | There is no information on whether the result is likely to have been selected, on the basis of the results, and from multiple eligible analyses of the data. | Some concerns | The study is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain |
| Markova 2018 | Some concerns | There is no information to answer any of the signalling questions. | Some concerns | Participants were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | Low risk of bias | Outcome data were available for nearly all randomized participants. | Some concerns | The method of measuring the outcome was not inappropriate and the ascertainment of the outcome likely did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received but it is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Low risk of bias | The data were likely analysed in accordance with a pre‐specified plan and reported outcome data are unlikely to have been selected, on the basis of the results, from multiple eligible analyses of the data. | Some concerns | The study is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain. |
| Notcutt 2012 | Some concerns | There is no information about concealment of the allocation sequence and any baseline differences observed between intervention groups appear to be compatible with chance. | Some concerns | Participants were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | High risk of bias | Outcome data were not available for all randomized participants and there is no evidence that the result was not biased by missing outcome data. It is likely that missingness in the outcome depended on its true value. | Some concerns | The method of measuring the outcome was not inappropriate and the ascertainment of the outcome likely did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received, but it is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Low risk of bias | The data were likely analysed in accordance with a pre‐specified plan and reported outcome data are unlikely to have been selected, on the basis of the results, from multiple eligible analyses of the data. | High risk of bias | The study is judged to be at high risk of bias in at least one domain for this result. |
| Novotna 2011 | Some concerns | There is no information to answer any of the signalling questions. | Some concerns | Participants were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | Low risk of bias | Outcome data were available for nearly all randomized participants. | Some concerns | The method of measuring the outcome was not inappropriate and the ascertainment of the outcome likely did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received but it is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Low risk of bias | The data were likely analysed in accordance with a pre‐specified plan and reported outcome data are unlikely to have been selected, on the basis of the results, from multiple eligible analyses of the data. | Some concerns | The study is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain. |
| Van Amerongen 2017 | Some concerns | There is no information about concealment of the allocation sequence. Any baseline differences observed between intervention groups appear to be compatible with chance. | Some concerns | Participants were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | Low risk of bias | Outcome data were available for nearly all randomized participants. | Some concerns | The method of measuring the outcome was not inappropriate and the ascertainment of the outcome likely did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received but it is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Low risk of bias | The data were likely analysed in accordance with a pre‐specified plan and reported outcome data are unlikely to have been selected, on the basis of the results, from multiple eligible analyses of the data. | Some concerns | The study is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain. |
Risk of bias for analysis 1.7 Health related quality of life: change score from baseline (follow up 3‐48 weeks).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Collin 2010 | Some concerns | There is no information about concealment of the allocation sequence and any baseline differences observed between intervention groups appear to be compatible with chance. | Some concerns | Participants likely were aware of intervention groups during the trial, and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | Low risk of bias | Outcome data were available for nearly all randomized participants. | Some concerns | The method of measuring the outcome was not inappropriate, there is no information on whether the measurement or ascertainment of the outcome could have differed between intervention groups, and the assessment of the outcome could not have been influenced by knowledge of the intervention received. | Low risk of bias | The data likely were analysed in accordance with a pre‐specified plan and reported outcome data are unlikely to have been selected, on the basis of the results, from multiple eligible analyses of the data. | Some concerns | The study is judged to raise some concerns in three domains for this result, but not to be at high risk of bias for any domain. |
| Langford 2013 | Some concerns | There is no information about concealment of the allocation sequence and any baseline differences observed between intervention groups appear to be compatible with chance. | Low risk of bias | Participants were likwly aware of intervention groups during the trial and no deviations from intended intervention likely arose because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | Low risk of bias | Missingness in the outcome could not depend on its true value. | Some concerns | The method of measuring the outcome was not inappropriate and the measurement or ascertainment of the outcome did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received but It is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Some concerns | A pre‐specified analysis plan was not reported and there is no information on whether the result being assessed is likely to have been selected from multiple eligible outcome measurements. | Some concerns | The study is judged to raise some concerns in three domains for this result, but not to be at high risk of bias for any domain. |
| NCT00682929 | Some concerns | There is no information about concealment of the allocation sequence (data available from ClinicalTrials.gov record (NCT00682929). Any baseline differences observed between intervention groups appear to be compatible with chance. | Some concerns | Participants likely were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. No information of the analysis that was used to estimate the effect of assignment to intervention, and the potential impact (on the estimated effect of intervention) of the failure to analyse participants in the group to which they were randomized was not substantial. | Low risk of bias | Missingness in the outcome could not depend on its true value. | Some concerns | The method of measuring the outcome was not inappropriate and the measurement or ascertainment of the outcome did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received but It is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Some concerns | A pre‐specified analysis plan was not reported and there is no information on whether the result being assessed is likely to have been selected from multiple eligible outcome measurements. | Some concerns | The study is judged to raise some concerns in four domains for this result, but not to be at high risk of bias for any domain. |
| NCT00682929 | Some concerns | There is no information about concealment of the allocation sequence (data available from ClinicalTrials.gov record (NCT00682929). Any baseline differences observed between intervention groups appear to be compatible with chance. | Some concerns | Participants likely were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. No information of the analysis that was used to estimate the effect of assignment to intervention, and the potential impact (on the estimated effect of intervention) of the failure to analyse participants in the group to which they were randomized was not substantial. | Low risk of bias | Missingness in the outcome could not depend on its true value. | Some concerns | The method of measuring the outcome was not inappropriate and the measurement or ascertainment of the outcome did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received but It is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Some concerns | A pre‐specified analysis plan was not reported and there is no information on whether the result being assessed is likely to have been selected from multiple eligible outcome measurements. | Some concerns | The study is judged to raise some concerns in four domains for this result, but not to be at high risk of bias for any domain. |
| NCT01606176 | Some concerns | There is no information to answer any of the signalling questions. Data available from clinicaltrials.gov (NCT01606176). | Some concerns | Participants likely were aware of intervention groups during the trial, and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | High risk of bias | Outcome data were not available for all, or nearly all, randomized participants, and there is not evidence that the result was not biased by missing outcome data, and no information on whether missingness in the outcome could depend on its true value. | High risk of bias | It is likely that assessment of the outcome was influenced by knowledge of the intervention received. | Low risk of bias | The data likely were analysed in accordance with a pre‐specified plan and the result being assessed is unlikely to have been selected, and reported outcome data are unlikely to have been selected, on the basis of the results, from multiple eligible analyses of the data. | High risk of bias | The study is judged to be at high risk of bias in two domains for this result. |
| Novotna 2011 | Some concerns | There is no information to answer any of the signalling questions. | Some concerns | Participants likely were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. No information of the analysis that was used to estimate the effect of assignment to intervention, and the potential impact (on the estimated effect of intervention) of the failure to analyse participants in the group to which they were randomized was not substantial. | High risk of bias | Only mean difference of SF36 domains available. Missing outcome data were not clearly documented. Missingness in the outcome could depend on its true value but no information available. | Some concerns | The method of measuring the outcome was not inappropriate and the measurement or ascertainment of the outcome likely did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received but there is no reason to believe that it did. | Some concerns | There is no information on whether the result being assessed is likely to have been selected, on the basis of the results, from multiple eligible analyses of the data. | High risk of bias | The study is judged to be at high risk of bias in one domain for this result. |
| Schimrigk 2017 | Low risk of bias | The allocation sequence was adequately concealed and baseline differences were not observed between intervention groups. | Some concerns | Participants likely were aware of intervention groups during the trial and there is no information on whether there were deviations from intended intervention because of the trial context. No information of the analysis that was used to estimate the effect of assignment to intervention, and the potential impact (on the estimated effect of intervention) of the failure to analyse participants in the group to which they were randomized was not substantial. | Low risk of bias | Missingness in the outcome could not depend on its true value. | Some concerns | The method of measuring the outcome was not inappropriate and the measurement or ascertainment of the outcome did not differ between intervention groups. The assessment of the outcome could have been influenced by knowledge of the intervention received but It is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Some concerns | A pre‐specified analysis plan was not reported and there is no information on whether the result being assessed is likely to have been selected from multiple eligible outcome measurements. | Some concerns | The study is judged to raise some concerns in three domains for this result, but not to be at high risk of bias for any domain. |
| ZAJICEK 2012 MUSEC | Low risk of bias | The allocation sequence was adequately concealed and any baseline differences observed between intervention groups appear to be compatible with chance. | Some concerns | Participants likely were aware of intervention groups during the trial, and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | Low risk of bias | Outcome data were available for nearly all randomized participants. | Some concerns | The method of measuring the outcome was not inappropriate, the measurement or ascertainment of the outcome likely did not differ between intervention groups. Participants likely were aware of the intervention received but it is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Low risk of bias | The data were analysed in accordance with a pre‐specified plan and the result being assessed is unlikely to have been selected, on the basis of the results, from multiple eligible outcome measurements within the outcome domain. Reported outcome data are unlikely to have been selected, on the basis of the results, from multiple eligible analyses of the data. | Some concerns | The study is judged to raise some concerns in two domains for this result, but not to be at high risk of bias for any domain. |
| Zajicek 2013_CUPID | Low risk of bias | The allocation sequence was adequately concealed and any baseline differences observed between intervention groups appear to be compatible with chance | Some concerns | Participants likely were aware of intervention groups during the trial, and there is no information on whether there were deviations from intended intervention because of the trial context. An appropriate analysis was used to estimate the effect of assignment to intervention. | High risk of bias | Outcome data were not available for all randomized participants, and there is not evidence that the result was not biased by missing outcome data. It is likely that missingness in the outcome depended on its true value. | Some concerns | The method of measuring the outcome was not inappropriate, the measurement or ascertainment of the outcome likely did not differ between intervention groups. Participants likely were aware of the intervention received but it is unlikely that assessment of the outcome was influenced by knowledge of intervention received. | Some concerns | There is no information on whether the result being assessed is likely to have been selected, on the basis of the results, from multiple eligible outcome measurements within the outcome domain and from multiple eligible analyses of the data. | High risk of bias | The study is judged to be at high risk of bias in one domain for this result. |
Acknowledgements
This review was published in collaboration with the Cochrane Drugs and Alcohol group. We particularly thank Marina Davoli and Laura Amato (Co‐Editors).
We thank Camerlingo Maria Domenica for developing the search strategy methods used to identify studies and Ben Ridley (Managing Editor) for his support.
We particularly thank Toby Lasserson (Deputy Editor‐in‐Chief, Cochrane Editorial and Methods Department) for his excellent support in developing the review protocol and his valuable comments on the review.
We thank Ella Flemyng (Methods Implementation Coordinator, Cochrane Editorial & Methods Department) and the Cochrane Methods Support Unit team, for helping us to resolve doubts and uncertainties on implementation of RoB 2.
We thank Sarah J Nevitt (Dr Sarah Nevitt, Department of Biostatistics, University of Liverpool UK) and Bernard Le Foll (Translational Addiction Research Laboratory, Campbell Family Mental Health Research Institute, CAMH COMPASS, University of Tortonto, Canada) for their helpful comments and suggestions on the review protocol.
Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group supported the authors in the development of this Review. Dr Graziella Filippini is a member of Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group, but was not involved in the editorial process or decision‐making for this review.
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): [Robert Boyle, Cochrane Senior Editor]
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Anne‐Marie Stephani, Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Central Editorial Service
Copy Editor (copy‐editing and production): [Heather Maxwell, Cochrane Copy‐Edit Support]
Peer‐reviewers (provided comments and recommended an editorial decision): Michelle H. Cameron, Oregon Health & Science University (clinical/content review), Stefan Gustavsen Danish Multiple Sclerosis Center, Copenhagen University Hospital (clinical/content review), Ahmed M Afifi, Baylor College of Medicine (consumer review), Nuala Livingstone, Cochrane Evidence Production & Methods Directorate (methods review), Robin Paynter, Cochrane Fertility Regulation Group](search review).
Appendices
Appendix 1. Draft search strategy
Search strategy for CENTRAL (the Cochrane Library online)
#1 MESH DESCRIPTOR Cannabis
#2 ((cannabi* or hash* or hemp or marijuana or marihuana or ganja or bhang)):TI,AB,KY
#3 MESH DESCRIPTOR Dronabinol
#4 ((dronabinol or marinol or nabilone or cesamet or dexanabinol or tetrahydrocannabinol or sativex or “HU 211”)):TI,AB,KY
#5 #1 OR #2 OR #3 OR #4
#6 MESH DESCRIPTOR Multiple sclerosis EXPLODE ALL TREES
#7 #5 AND #6
Search strategy for MEDLINE (PubMed)
#1"Cannabis"[Mesh]) OR "cannabi*"[Text Word] OR "hash*"[Text Word] OR hemp[Text Word] OR marijuana[Text Word] OR marihuana[Text Word] OR ganja[Text Word] OR bhang[Text Word] OR "Dronabinol"[Mesh] OR dronabinol[Text Word] OR marinol[Text Word] OR nabilone[Text Word] OR cesamet[Text Word] OR cannabidiol[Text Word] OR nabiximols[Text Word] OR dexanabinol[Text Word] OR tetrahydrocannabinol[Text Word] OR sativex[Text Word]
#2"Multiple Sclerosis"[mh] OR "Myelitis, Transverse"[mh:noexp] OR "Demyelinating Diseases"[mh:noexp] OR "Encephalomyelitis, Acute Disseminated"[mh:noexp] OR "Optic Neuritis"[mh] OR "multiple sclerosis" OR "neuromyelitis optica" OR "transverse myelitis" OR encephalomyelitis OR devic OR "optic neuritis" OR "demyelinating disease*" OR "acute disseminated encephalomyelitis"
#3 randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]
#4 NOT (animals[mh] NOT (animals[mh] AND human[mh]))
Search strategy for Embase (EMBASE.com)
#1 'encephalomyelitis'/exp OR 'demyelinating disease'/exp OR 'multiple sclerosis'/exp OR 'myelooptic neuropathy'/exp OR 'multiple sclerosis':ab,ti OR 'neuromyelitis optica':ab,ti OR encephalomyelitis:ab,ti OR devic:ab,ti
#2 'crossover procedure'/exp OR 'double blind procedure'/exp OR 'single blind procedure'/exp OR 'randomized controlled trial'/exp OR random*:ab,ti OR factorial*:ab,ti OR crossover:ab,ti OR (cross:ab,ti AND over:ab,ti) OR placebo*:ab,ti OR 'double blind':ab,ti OR 'single blind':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti
#3 'cannabis'/exp OR hash* OR 'hemp'/exp OR cannabis:ab,ti OR hash*:ab,ti OR hemp:ab,ti OR marijuana:ab,ti OR 'marijuana'/exp OR marihuana:ab,ti OR 'marihuana'/exp OR ganja:ab,ti OR bhang:ab,ti OR 'dronabinol'/exp OR dronabinol:ab,ti OR marinol:ab,ti OR nabilone:ab,ti OR cesamet:ab,ti OR cannabidiol:ab,ti OR nabiximols:ab,ti OR dexanabinol:ab,ti OR tetrahydrocannabinol:ab,ti OR sativex:ab,ti
#4 #1 AND #2 AND #3
Search strategy for CINAHL (EBSCO host)
S1 (encephalomyelitis) OR (demyelinating disease) OR (multiple sclerosis) OR (AB multiple sclerosis) OR (AB neuromyelitis optica) OR (AB encephalomyelitis) OR (devic)
S2 (crossover procedure) OR (double blind procedure) OR (single blind procedure) OR (randomized controlled trial) OR (random*) OR (factorial*) (OR crossover) OR (cross AND over) OR (placebo) OR (double blind) OR (single blind) OR (assign*) OR (allocat*) OR (volunteer*) OR (AB crossover ) OR (AB cross AND AB over ) or (AB placebo* ) OR (AB double blind) OR (AB single blind ) OR (AB assign*) OR (AB allocat*) OR (AB volunteer*)
S3 (cannabis) OR (hash*) OR (hemp) OR (marijuana) OR (marihuana) OR (AB cannabis) OR (AB hash*) OR (AB hemp) OR (AB marijuana) OR (AB marihuana) OR (AB ganja) OR (AB bhang) OR (dronabinol) OR (AB dronabinol) OR (AB marinol) OR (AB nabilone) OR (AB cesamet) OR (AB cannabidiol) OR (AB nabiximols) OR (AB dexanabinol) OR (AB tetrahydrocannabinol) OR (AB sativex)
S4 S1 AND S2 AND S3
Search strategy for LILACS (Bireme)
multiple sclerosis or encephalomyelitis or demyelinating disease or devic [Words] AND cannabis OR hemp OR marijuana OR marihuana OR dronabinol OR marinol OR nabilone OR cesamet OR cannabidiol OR nabiximols OR dexanabinol OR tetrahydrocannabinol OR sativex [Words]
Search strategy for Physiotherapy Evidence Database (PEDro)
Title & Abstract: "multiple sclerosis" Therapy: cannabis; marijuana; marihuana; dronabinol; marinol; nabilone; cesamet; cannabidiol; nabiximols; dexanabinol; tetrahydrocannabinol; sativex Subdiscipline: NA Method: clinical trial
Search strategy for WHO International Clinical Trials Registry Platform (ICTRP)
Basic search: cannabis AND multiple sclerosis OR marijuana AND multiple sclerosis OR marihuana AND multiple sclerosis OR dronabinol AND multiple sclerosis OR marinol AND multiple sclerosis OR nabilone AND multiple sclerosis OR cesameAND multiple sclerosis OR cannabidiol AND multiple sclerosis OR nabiximols AND multiple sclerosis OR dexanabinol AND multiple sclerosis OR tetrahydrocannabinol AND multiple sclerosis OR sativex AND multiple sclerosis
Search strategy for CLINICAL TRIALS.GOV
#1 "multiple sclerosis" OR "encephalomyelitis" OR "demyelinating disease*" OR "neuromyelitis optica" OR devic OR "optic neuritis" OR "transverse myelitis"
#2 cannabis OR hash* OR hemp OR cannabis OR marijuana OR marihuana OR ganja OR bhang OR dronabinol OR marinol OR nabilone OR cesamet OR cannabidiol OR nabiximols OR dexanabinol OR tetrahydrocannabinol OR sativex
Search strategy for European Union Clinical Trials Register
Basic search: cannabis AND multiple sclerosis OR marijuana AND multiple sclerosis OR marihuana AND multiple sclerosis OR dronabinol AND multiple sclerosis OR marinol AND multiple sclerosis OR nabilone AND multiple sclerosis OR cesameAND multiple sclerosis OR cannabidiol AND multiple sclerosis OR nabiximols AND multiple sclerosis OR dexanabinol AND multiple sclerosis OR tetrahydrocannabinol AND multiple sclerosis OR sativex AND multiple sclerosis
Search strategy for International Association for Cannabinoid Medicines (IACM) databank
Multiple sclerosis and controlled study
Appendix 2. Document for implementation of the RoB 2 tool
Pilot review cannabis for people with multiple sclerosis – RoB2 implementation
DOMAIN 1 ‐ the randomisation process
SQ.1.3 (Did baseline differences between intervention groups suggest a problem with the randomisation process?)
if there are imbalance in baseline characteristics do not answer PY if the sample is small (i.e. less than 50 participants in the study) and differences are compatible with chance
DOMAIN 2 – Deviation from the intended intervention ASSIGNMENT
SQ 2.1 (Were participants aware of their assigned intervention during the trial?) and 2.2 (Were carers and people delivering the interventions aware of participants' assigned intervention during the trial?): if the study is stated as double blind but is not reported that drugs and placebo were identical in taste and appearance, answer NI
If you are considering effect of assignment:
SQ 2.1 and 2.2: Answer always PY, independently on what is reported about the blindness of participants and personnel. Epidemiologic data show that the prevalence of cannabis use among the MS population is significantly higher than in the general population [1‐3]. The AEs and the psychotropic effect of cannabis are easily recognisable [4]; for this reason, also if the study is double blind, is very likely that participants will be able to recognise the intervention actually received.
SQ 2.3(If Y/PY/NI to 2.1 or 2.2: Were there deviations from the intended intervention that arose because of the trial context?) answer PN if there were no protocol deviation, if cointerventions were anticipated in the protocol, withdrawals due to AEs is not an issue for assignment. Answer PN if has been specified in the protocol or in the methods section that cannabis has been added to usual practice, so that variation in co‐intervention, as normally happen in usual practice, can happen without protocol. Answer PY if there were cointerventions not anticipated in the protocol, if they were different in frequency between the two groups.
Answer PY if there are the following potential deviations and are not anticipated in the protocol:
Trial personnel (carers or people delivering the interventions) undermine the implementation of the trial protocol in ways that would not happen outside the trial for what concern: rehabilitation treatment, concomitant antispastic medications (e.g. baclofen, tizanidine, dantrolene, benzodiazepines), dose/duration of allowed antispastic medication different from protocol; concomitant analgesic, antiepileptic (gabapentin or pregabalin) or antidepressant (duloxetine, amitriptyline) medications. Rehabilitation and antispastic medication are relevant for spasticity and PGIC, QoL; analgesics, antiepileptics and antidepressants are relevant for pain, PGIC, QoL.
Answer NI if no information is provided concerning the above‐mentioned co‐interventions. In the majority of the studies this information is not reported, so the answer will be often NI
For the outcomes AE, SAEs and withdrawn due to AEs, consider all the concomitant medication listed above but not rehabilitation.
S.Q 2.4(If Y/PY to 2.3: Were these deviations likely to have affected the outcome?) answer PY only if there are the cointervention listed for SQ 2.3., that arose because of the experimental context and that could actually impact the outcome, but not otherwise.
S.Q 2.5(If Y/PY/NI to 2.4: Were these deviations from intended intervention balanced between groups?) consider only the possible deviations listed for SQ 2.3. Answer N/PN if they are not balanced
S.Q 2.6. (Was an appropriate analysis used to estimate the effect of assignment to intervention?) answer PY if: A): the number of participants analysed coincide with the number of randomised participants or to the number of randomised minus the number of participants lost at follow up or for which the outcome measure was not available. B) the method used for ITT is described and it is adequate. The question asks if participants were analysed according to the arm to which they were randomised for what concern the intervention, not the availability of the outcome. So, if participants are excluded from the analysis because they are missing, this does not introduce bias for this question.
Answer PN if only a per protocol analysis was undertaken, where participants were grouped according to the intervention they actually received, instead of to the intervention they were randomised to receive
DOMAIN 2 – Deviation from the intended intervention ADHERING (outcomes SAEs e AEs)
S.Q 2.3 (If Y/PY/NI to 2.1 or 2.2: Were important non‐protocol interventions balanced across intervention groups?), 2.4 (Were there failures in implementing the intervention that could have affected the outcome?),2.5(Was there non‐adherence to the assigned intervention regimen that could have affected participants’ outcomes?) answer to all these questions for adverse events and SAEs
Scenario 1.
SQ 2.1. and SQ 2,2 answer PY If participants (personnel) were aware of their intervention (e.g. they have taken cannabis before randomisation, or all participants took cannabis in the first phase of the trial as in the Markova study)
SQ 2.3 answer PY if any variations in interventions (both if happened because of the experimental context and because of choice of the patients) are balanced between groups; (question focussed on co‐intervention)
Answer PN if has been specified in the protocol or in the methods section that cannabis has been added to usual practice, so that variation in co‐intervention, as normally happen in usual practice, can happen without protocol. Answer PN if important non‐protocol deviations are balanced between groups, e.g., different percentages of participants in the two groups assuming analgesics drugs not accepted in the protocol,
SQ 2.4 answer PN if No additional failures of implementation were found that are likely to affect the outcome; deviation from the protocol in the implementation of the experimental intervention, due to inadequate behaviour of clinician delivering the intervention (question focussed on experimental intervention delivered by clinician)
SQ 2.5 answer PN if non‐adherence to the intervention, including imperfect compliance, cessation of the intervention (>95% completed), and cross over (focussed on patients behaviour) to the other arm that could have affected the outcome, was not found; answer PY if there were cessation or cross to the other intervention
SQ 2.6 (If N/PN/NI to 2.3, or Y/PY/NI to 2.4 or 2.5: Was an appropriate analysis used to estimate the effect of adhering to the intervention?) the analysis of AEs and SAEs are always descriptive, so answer always PY or Y
DOMAIN 3 ‐ Missing outcome data
S.Q 3.1 (Were data for this outcome available for all, or nearly all, participants randomised?) if continuous outcome data are dichotomised, it should be considered as dichotomous data. Therefore, the following rule applies: “If the observed number of events is much greater than the number of participants with missing outcome data, the bias would necessarily be small.
For continuous outcomes, we do not want users to consider 95% as a strict cut off.
Only the overall % of missing should be considered for this SQ. Not the imbalance between groups and reason for missing
For efficacy outcomes we suggest to use the following cut off:
PN: ≤90%
PY: ≥91%
For SAe and AEs answer PY if the safety population coincide with the randomised population
Answer always PY for the outcome withdrawn due to AEs
SQ 3.2(If N/PN/NI to 3.1: Is there evidence that the result was not biased by missing outcome data?) PY if the analysis corrects for missing data. LOCF is not a correct analysis. Baseline Observation Carried Forward (BOCF) is correct‐.
S.Q 3.3. (If N/PN to 3.2: Could missingness in the outcome depend on its true value?) (Couldin theory missing data depend on its true value?) The place where dichotomised continuous outcome data are not treated as dichotomous data (see section 6.1.3 section 3) is for signalling question 3.3.
Answer PY if: for efficacy outcomes missingness could depend on the true value if there are at least 5% missing due to lack of efficacy independently if missing data are unbalanced or balanced between the groups) because our OR come from a dichotomization of continuous outcome. Other reasons for withdrawn (AEs, withdrawn consent, other, should not be considered)
Answer PN if reasons are reported for missingness and all are unrelated to the outcome and to the intervention
Answer NI if reason for missingness is not reported,
S.Q 3.4 (If Y/PY/NI to 3.3: Is it likely that missingness in the outcome depended on its true value?) (is likely that actually missing data depend on its true value?) answer PY if 1) proportion of missing data are unbalanced for lack of efficacy or adverse events; 2) if reasons for missing are reported and depend on the true value (i.e., lack of efficacy); 3) if reasons are different between the groups (e.g., more patients in the cannabis group dropped out for AE and more patients in the placebo group dropped out for lack of efficacy)
DOMAIN 4 ‐ Measurement of the outcome
S.Q 4.1(Was the method of measuring the outcome inappropriate?)
AEs, answer PY if they are recorded on the basis of what patients reported
Answer PN if they information has been achieved by active surveillance (i.e., questionnaire with a list of possible AEs)
Withdrawn due to AE: answer always PN
Spasticity answer PY (Inappropriate) if the following instruments have been used:
The Ashworth scale and the Modified Ashworth scale are not considered ideal scales for assessing the severity of MS spasticity [5,6].
All spasticity outcome measured by electrophysiological tests e.g. (e.g., objective spasticity: the
answer PN (appropriate) if the following measures have been used
1. Reduction of 30% in the spasticity Numeric Rating Scale (NRS), over baseline. Both dichotomic measure (responders) and continuous measure (in our protocol = important outcome). The NRS is a discrete variable describing spasticity level with numbers from 0 to 10 [7].
Please note: Participant‐reported frequency and severity of painful spasms, e.g., Penn Spasm Frequency Scale [8] is an appropriate measure of spasticity, but in our protocol is reported as “Outcome of limited importance”.
Pain: answer PY (inappropriate) if the following instruments have been used
Verbal rating scale (VRS) consisting of a series of verbal pain descriptors, has been shown to lack sensitivity to detect changes in pain intensity when compared with VAS or NRS‐PI [9]
Answer PN (appropriate) if the following instruments have been used
Multidimensional (composite) pain outcome measures, e.g., the McGill Pain Questionnaire (MPQ, SF‐MPQ); the Neuropathic Pain Scale (NPS); the Neuropathic Pain Symptom Inventory (NPSI) [9, 10]
The Multidimensional Pain Inventory (MPI) and the Brief Pain Inventory (BPI) both provide
The Numeric Rating Scale‐Pain Intensity (NRS‐PI) over baseline. Both dichotomic measure (responders 50% reduction) and continuous measure (in our protocol = important outcome). The NRS is a discrete variable describing pain level with numbers from 0 to 10 [9, 10].
The visual analogue scale (VAS), a continuous variable on a 10 cm line representing “no pain” to “worst imaginable pain” [9, 10].
Please note: Pain relief of 30% or greater in a composite neuropathic pain scale or in the 0‐10 NRS‐PI should not be considered inappropriate, but in our protocol is reported as “Outcome of limited importance”.
S.Q 4.2 (Could measurement or ascertainment of the outcome have differed between intervention groups?) this information is very rarely reported in the studies; however, we judged that the answer is PN for all the outcomes
S.Q 4.3 (If N/PN/NI to 4.1 and 4.2: Were outcome assessors aware of the intervention received by study participants?) : answer PY for the all the outcomes also if the study is classified as double blind and it is reported that drug and placebo were identical in taste and appearance. The AEs of cannabis are very specific [4] and make easily recognisable the type of intervention received for provider. Also, participants who have taken cannabis in the past for therapeutic or recreational purpose can easily recognise the AE and the psychotropic effects.
S.Q 4.4 (If Y/PY/NI to 4.3: Could assessment of the outcome have been influenced by knowledge of intervention received? ): answer always PY for the outcomes spasticity, pain, SGIC, QOL, AEs and withdrawn due to AEs. Answer PN for SAEs
S.Q 4.5. (If Y/PY/NI to 4.4: Is it likely that assessment of the outcome was influenced by knowledge of intervention received?) Answer PN for the outcomes: spasticity, pain, SGIC, QOL, AEs and withdrawn due to AEs. This decision was based on the results from the Wright study [11] which reports that there was no evidence that unblinding to Sativex in people with multiple sclerosis led to bias in the assessment of the treatment difference between Sativex and placebo for patient‐reported outcomes or adverse events.
DOMAIN 5 ‐ Selection of the reported result
S.Q 5.1 (Were the data that produced this result analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis?) the question is focussed in knowing whether the analysis was done in accordance to a “prespecified analysis plan; not for what concern the statistical analysis plan, but for what concern the description of the outcome in the protocol, which should be published before the study was completed
Answer PY if the outcome is mentioned in the protocol and the protocol was published before the study is completed; or if the outcome was added after the first protocol registration (i.e., in the amendment) but the reason was reported and it was justified (e.g. a new more valid scale was published)
Answer NI if the prespecified analysis plan is not available
Answer PN if the outcome is not prespecified in the protocol or if it was added after the first registration of the protocol
N.B. in Clinical trial.gov we should look at the date when the protocol (not results) where first registered.
In EUTRACT the date of first registration can be found in the registration number
For AEs, SAEs and withdrawn due to AEs answer always PY also if they are not mentioned in the protocol
S.Q 5.2(Is the numerical result being assessed likely to have been selected, on the basis of the results, from multiple eligible outcome measurements (e.g. scales, definitions, time points) within the outcome domain?)
1) the cut off of 30% and 50% reduction both for spasticity and pain should be considered as two different outcomes, not as two different ways to measure the same outcome. We are interested only in 30% reduction in spasticity and 50% reduction in pain, as reported in our protocol [12]. Answer PN if these outcomes are reported in the protocol and in the results section. No matter if they report in the protocol other cut off level whose results are not shown in the results, because we are not interested in the other cut off. If 30% reduction in spasticity or 50% reduction in pain are described in the protocol but results are not reported in the paper, RoB2 will not be assessed for this outcome. Risk of bias of MA will be covered in the RoB ME tool a tool to assess for missing evidence
2) follow up assessment: we are interested in the longest available FU, not in a particular FU time (e.g. 12 weeks or 16 weeks).
Answer PN if the longest FU period reported in the protocol is the same as the longest FU reported in the paper.
Answer PY if in the longest FU available in the paper is shorter than the longest FU described in the protocol. Unlike the previous case concerning cut off, where we were interest in a specific cut off, here we will extract data on the longest FU available, and we will answer PY (risk of bias) in the case the FU results reported in the paper are shorter that the FU results described in the paper.
3) Quality of life: we give the preference to specific scales; if both specific and generic scales are mentioned in the protocol and, in the study, the specific scale results are reported, answer PN (no matter if the generic scale’s results are not reported) If in the protocol both specific and generic scales are mentioned but, in the study, the generic scale results only are reported, answer PY. We will put the generic scale results in MA but it will be at risk of bias
S.Q 5.3 (Is the numerical result being assessed likely to have been selected, on the basis of the results, from multiple eligible analyses of the data?) answer could be NI because the statistical analysis plan is very rarely reported in the protocol. However, if the study seems to be well done, if authors are known and we trust in them, we can answer PN if there is correspondence between the methods and the results section in the published paper for what concern the type of analysis undertaken
OVERALL JUDGMENT: in case we’ll have many domains with some concern, we will not downgrade the judgment to high risk of bias, but the final judgment will remain some concern.
References
[1] Hazekamp A, Ware MA, Muller‐Vahl KR, Abrams D, Grotenhermen F. The medicinal use of cannabis and cannabinoids‐‐an international cross‐sectional survey on administration forms. Journal of Psychoactive Drugs 2013;45(3):199‐210.
[2] Kindred JH, Li K, Ketelhut NB, Proessl F, Fling BW, Honce JM, et al. Cannabis use in people with Parkinson's disease and Multiple Sclerosis: a web‐based investigation. Complementary Therapies in Medicine 2017; 33:99‐104.
[3] Banwell E, Pavisian B, Lee L, Feinstein A. Attitudes to cannabis and patterns of use among Canadians with multiple sclerosis. Multiple Sclerosis and Related Disorders 2016;10:123‐6.
[4] Killestein J, Hoogervorst EL, Reif M, Kalkers NF, Van Loenen AC, Staats PG, et al. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 2002;58(9):1404–7.
[5] Pandyan AD, Johnson GR, Priec CI, Curless RH, Barnes MP, Rodgers H. A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clinical Rehabilitation 1999;13:373‐83.
[6] Anwar K, Barnes M. A Pilot Study of a Comparison Between a Patient Scored Numeric Rating Scale and Clinician Scored Measures of Spasticity in Multiple Sclerosis. NeuroRehabilitation 2009; 24: 333–340.
[7] Farrar JT, Troxel AB, Stott C, Duncombe P, Jensen MP. Validity, reliability, and clinical importance of change in a 0‐10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double‐blind, placebocontrolled trial. Clinical Therapeutics 2008;30(5):974‐85.
[8] Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B, et al. Intrathecal baclofen for severe spasticity. New England Journal of Medicine 1989;320(23):1517‐21.
[9] European Medicines Agency. EMA/CHMP/970057/2011. Available at https://www.ema.europa.eu/en/clinical-development-medicinal-products-intended-treatment-pain (accessed 15 May 2019).
[10] Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. “Evidence” in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386–9.
[11] Wright S, Duncombe P, Altman DG. Assessment of blinding to treatment allocation in studies of a cannabis‐based medicine (Sativex®) in people with multiple sclerosis: a new approach. Trials. 2012;13:189.
[12] Filippini G, Lasserson TJ, Dwan K, D'Amico R, Borrelli F, Izzo AA, Minozzi S. Cannabis and cannabinoids for people with multiple sclerosis. Cochrane Database of Systematic Reviews 2019, Issue 10.
Data and analyses
Comparison 1. Cannabis and cannabinoids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Spasticity: number of participants reporting reduction of 30% in the spasticity NRS (follow up 6‐14 weeks) | 5 | 1143 | Odds Ratio (M‐H, Random, 95% CI) | 2.51 [1.56, 4.04] |
| 1.2 Spasticity: NRS as continuous outcome (follow up 2‐14 weeks) | 7 | 1262 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.94, ‐0.17] |
| 1.3 Pain: number of participants reporting pain relief of 50% or greater in the NRS‐PI (follow up 3 weeks) | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.23 [1.11, 16.17] |
| 1.4 Pain: NRS‐PI as continuous outcome (follow up 3‐16 weeks) | 8 | 1451 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.91, ‐0.18] |
| 1.5 Withdrawn due to adverse events (follow up 3‐48 weeks) | 19 | 3110 | Odds Ratio (M‐H, Random, 95% CI) | 2.41 [1.51, 3.84] |
| 1.6 PGIC: number of participants reporting much or very much improvement in the PGIC (follow up 4‐48 weeks) | 8 | 1215 | Odds Ratio (M‐H, Random, 95% CI) | 1.80 [1.37, 2.36] |
| 1.7 Health related quality of life: change score from baseline (follow up 3‐48 weeks) | 8 | 1942 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.17, 0.02] |
| 1.8 Health related quality of life: change score from baseline for each domain of SF‐36 (follow up 12‐14 weeks) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.8.1 Physical functioning | 4 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐2.05, 1.80] | |
| 1.8.2 Role physical | 3 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐3.18, 2.63] | |
| 1.8.3 Bodily pain | 3 | Mean Difference (IV, Random, 95% CI) | 4.24 [0.07, 8.40] | |
| 1.8.4 General health | 3 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐2.53, 2.29] | |
| 1.8.5 Vitality | 3 | Mean Difference (IV, Random, 95% CI) | 1.38 [‐2.85, 5.62] | |
| 1.8.6 Social functioning | 3 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐6.78, 4.01] | |
| 1.8.7 Role emotion | 3 | Mean Difference (IV, Random, 95% CI) | ‐2.09 [‐5.50, 1.32] | |
| 1.8.8 Mental health | 4 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐1.69, 2.50] | |
| 1.9 SAEs: number of participants with SAEs (follow up 3‐48 weeks) | 20 | 3124 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.96, 1.99] |
| 1.10 Specific AEs: number of participants reporting nervous system disorders (follow up 4‐48 weeks) | 7 | 1154 | Odds Ratio (M‐H, Random, 95% CI) | 2.61 [1.53, 4.44] |
| 1.11 Specific AEs: number of participants reporting psychiatric disorders (follow up 4‐48 weeks) | 6 | 1122 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.31, 2.88] |
| 1.12 Specific AEs: number of participants reporting drug tolerance (follow up 14‐48 weeks) | 2 | 458 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.07 [0.12, 75.95] |
| 1.13 Spasticity: Ashworth or Modified Ashworth (follow up 2‐50 weeks) | 11 | 1777 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.44, ‐0.03] |
| 1.14 Fatigue as continuous outcome (follow up 4‐14 weeks) | 5 | 928 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.26, 0.34] |
| 1.15 Sleep quality: NRS as continuous outcome (follow up 4‐14 weeks) | 7 | 1205 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.10, ‐0.22] |
| 1.16 Sleep quality: number of participants reporting an improvement in the NRS sleep (follow up 6‐14 weeks) | 2 | 756 | Odds Ratio (M‐H, Random, 95% CI) | 1.79 [1.30, 2.46] |
| 1.17 Depression: Beck Depression Inventory as continuous outcome | 3 | 495 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.90, 1.24] |
| 1.18 Activities of daily living: Barthel index as continuous outcome | 4 | 1134 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.32, 0.16] |
| 1.19 Number of caregivers reporting improvement on the CGIC (follow up 4‐48 weeks) | 4 | 582 | Odds Ratio (M‐H, Random, 95% CI) | 1.66 [1.15, 2.41] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aragona 2009.
| Study characteristics | ||
| Methods | Design: Randomised cross‐over study Setting: Italy; single‐centre Recruitment: NR Number screened: NR Number randomised: 17 Outcome timing: 3 weeks | |
| Participants |
Psycho pathological and cognitive effects of therapeutic cannabinoids in MS Inclusion criteria: age between 18 and 60 years; right‐handed with normal right‐hand function; a baseline EDSS score from 3.5 to 6.5; a stable disease for at least 30 days before study entry and no systemic corticosteroid therapy within 4 weeks of randomisation; significant spasticity in at least 2 muscle groups; anti spastic and immunomodulatory agents stable, before the study entry, for at least 1 and 6 months, respectively Exclusion criteria: history of epilepsy, alcohol or substance abuse, major medical illnesses; history of psychiatric disorders or cognitive impairment; concomitant therapy with psychoactive drugs; female patient who was pregnant, lactating, or planning pregnancy during the course of the study; previous use of cannabis Randomised: N = 17; % female: 64.7; mean age: 49.8 (SD 6.64) years; % SPMS 100; mean EDSS: 6.1 (SD 0.3); mean duration of MS: 20.76 (SD 8.42) years |
|
| Interventions | Sativex versus placebo. Each actuation delivered 100 µL of spray, containing delta‐9‐THC 2.7 mg and CBD 2.5 mg. Placebo had the appearance, smell, and taste of the active formulation but contained no active components. Study duration: 2 x3 weeks treatment periods. Washout period: 2 weeks | |
| Outcomes | Spasticity: not assessed Pain: not assessed Withdrawal due to AE: N/phase PGIC much or very much improved: not assessed HRQoL. Measure: VAS. Data: mean (SD) difference of the absolute post‐intervention (post placebo vs post Sativex) Serious AEs: N/phase AEs: N/phase Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: NR Dizziness: N/phase Somnolence: N/phase Headache: N/phase Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms severity: not assessed Fatigue. Measure: Fatigue Severity Scale (FSS). Data: mean (SD) difference of the absolute post‐intervention (post placebo vs post Sativex) Sleep quality: not assessed Mobility/ADLs: not assessed Anxiety. Measure: Self‐rating Anxiety Scale (SAS). Data: mean (SD) difference of the absolute post‐intervention (post placebo vs post Sativex) Depression. Measure: Symptom Checklist‐90 Revised (SCL‐90‐R).Data: mean (SD) difference of the absolute post‐intervention (post placebo vs post Sativex) CGIC much or very much improved: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed. | |
| Notes | Funding: public. The study was supported by a grant in the project of University Research year 2004 by the University "Sapienza" of Rome | |
Collin 2007.
| Study characteristics | ||
| Methods | Design: parallel group RCT Setting: multicentre, 8 centres in the UK and 4 centres in Romania Recruitment: April 2002 ‐ March 2004 Number randomised: 189 Outcome timing: 6 weeks | |
| Participants |
Spasticity in MS Inclusion criteria. Age >18 years; diagnosis of MS; stable disease for >3 months; significant spasticity in at least two muscle groups with an Ashworth score ≥2; failed to gain adequate relief using current therapy; stable treatment for at least 30 days before randomization and during the study Exclusion criteria. Psychosis or severe psychiatric disorder other than depression; known alcohol or substance abuse; severe cardiovascular disorder including poorly controlled hypertension; history of seizures; pregnancy or lactation; sensitivity to cannabinoids Treatment group (Nabiximols/Sativex). N: 124; % female: 64.5; mean age: 49.7 (SD 10.2) years; disease severity: NR; mean duration of MS: 13.6 (SD 8.6) years; % previous cannabis use: 41.9 Placebo group. N: 65; % female: 52.3; mean age: 47.8 (SD 9.5) years; disease severity: NR; mean duration of MS: 12.2 (SD 7.7) years; % previous cannabis use: 41.5 |
|
| Interventions | Sativex. Oromucosal spray containing 2.7 mg of delta‐9‐THC and 2.5 mg of CBD per 100 µL spray, max 48 sprays in 24 h Placebo. Oromucosal spray containing peppermint oil, 0.05% (v/v), quinoline yellow, 0.005% (w/v), sunset yellow, 0.0025% (w/v), in ethanol:propylene glycol (50:50) excipients Concomitant medication during the study. NR | |
| Outcomes | Spasticity. Measure: NRS 0‐10. Data: number of participant reporting improvement ≥ 30% (change from baseline in the severity of spasticity based on a daily diary assessment) reported. Mean difference, p value and 95% CI Spasticity. Measure: Ashworth Scale composite score. Data: Mean difference, SE, P value and 95% CI Pain: not assessed Withdrawal due to AE: N / group PGIC. Measure: seven point scale (very much improved to very much worse). Data: number of participants reporting much or very much improved HRQoL: not assessed Serious AE: N / group Specific AE: N / group Nervous system disorders‐related AE: incompletely reported Psychiatric disorders‐related AE: incompletely reported Dizziness: N / group Somnolence: N / group Headache: N / group Confusion‐ disorientation: N / group Paranoia: not assessed Psychosis: not assessed Hallucinations: not assessed Drug tolerance: not assessed Urinary incontinence: not assessed Muscle spasms. Measure: five point spasm frequency score. Data: mean difference, SE, P value and 95% CI Fatigue. Measure: NR. Data: N / group Sleep: not assessed Mobility/ADLs: not assessed Anxiety: not assessed Depression. Measure: NR. Data: N / group CGIC: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed | |
| Notes | Funding: industry ‐ drug manufacturer | |
Collin 2010.
| Study characteristics | ||
| Methods | Design: parallel group RCT Setting: multicentre, 15 centres in the UK and 8 centres in Czech Republic Recruitment: NR Number screened: 388 Number randomised: 337 Outcome timing: 14 weeks | |
| Participants |
Spasticity in MS Inclusion criteria: any MS subtype; ≥6 months duration; ≥ 3 month history of spasticity due to MS not wholly relieved with current therapy; mean daily score of ≥4 on spasticity NRS (moderate spasticity) during the last 6 days of the baseline period; stable anti‐spasticity regimen ≥ 30 days preceding study entry Exclusion criteria: spasticity not due to MS; concurrent history of significant psychiatric, renal, hepatic, cardiovascular or convulsive disorders Treatment group (Nabiximols/Sativex): N = 167; % female: 63.0; mean age: 48.0 (SD 10.06) years; mean EDSS: 6.0 (SD 1.56); mean duration of MS: 14.4 (SD 8.29) years; % previous cannabis use: 20; mean duration of spasticity: 7.5 (SD 5.14) years Placebo group: N = 170; % female: 59.0; mean age: 47.1 (SD 9.15) years; mean EDSS: 6.0 (SD 1.50); mean duration of MS: 16.0 (SD 8.48) years; % previous cannabis use: 28; mean duration of spasticity: 8.0 (SD 5.51) years |
|
| Interventions | Sativex: oromucosal spray: 100 µl containing 2.7 mg of delta‐9‐THC and 2.5 mg of CBD Placebo: oromucosal spray containing peppermint oil flavouring, 0.05%(v/v); quinoline yellow, 0.005% (w/v) and sunset yellow, 0.0025% (w/v) colourants, in ethanol:propylene glycol (50:50) excipients Dose frequency: maximum 24 sprays in any 24 hour period. Daily number of sprays taken during the study: Sativex: mean 8.5 (range: 1–22); Placebo: mean 15.4 (range: 2– 23) Concomitant medication during the study: % Baclofen: Sativex 79; Placebo 81. % Tizanidine: Sativex 41; Placebo 45. % Benzodiazepines: Sativex 26; Placebo 30. % Gabapentin: Sativex 16; Placebo 15. % Dantrolene: Sativex 8; Placebo 6. % Other: Sativex 62; Placebo 59. % No previous or concomitant anti‐spasticity medications: Sativex 4; Placebo 2 | |
| Outcomes |
Spasticity
Pain. Measure: NRS 0‐10. Data: number of participant reporting ≥ 50% improvement not reported. Mean change from baseline without SD reported. Mean treatment difference (P value) reported Withdrawal due to AE: N/group PGIC much or very much improved: not assessed HRQoL
Serious AEs: N/group AEs: N/group Nervous system disorders‐related AE: N/group Psychiatric disorders‐related AE: N/group Dizziness: N/group Somnolence: N/group Headache: N/group Confusion‐ disorientation: N/group Paranoia: N/group Psychosis: NR Hallucinations: NR Drug tolerance: N/group Urinary incontinence: not assessed Muscle spasms severity. Measure: NRS 0‐10. Data: mean change from baseline without SD. Mean treatment difference (P value) Fatigue. Measure: NRS 0‐10. Data: mean change from baseline without SD. Mean treatment difference (P value) Sleep quality. Measure: NRS 0‐10 sleep quality. Data: mean change (SD) from baseline reported. Mean treatment difference (95% CI and P value) reported Mobility/ADLs. Measure: Barthel ADL index score. Data: mean change (SD) from baseline reported. Mean treatment difference (95% CI and P value) reported Anxiety: not assessed Depression: not assessed CGIC much or very much improved. Measure: 7‐point Likert‐type scale with the markers "very much improved, much improved, lightly improved, no change, slightly worse, much worse or very much worse. Data: number of participants reporting "very much improved" or "much improved" reported (OR, 95% CI and p value) Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed |
|
| Notes | Funding: industry ‐ drug manufacturer | |
Corey‐Bloom 2012.
| Study characteristics | ||
| Methods | Design: Randomised cross‐over study. Setting: US, single‐centre Recruitment: NR Number screened: 196 Number randomised: 37 Outcome timing: 3 days | |
| Participants |
Spasticity in MS Inclusion criteria: spasticity and at least moderate increase in tone (score ≥ 3 points on the modified Ashworth scale at the elbow, hip or knee) Exclusion criteria: history of major psychiatric disorder (other than depression) or substance abuse, neurologic disease other than MS (e.g. epilepsy, head trauma) and severe or unstable medical illnesses, known pulmonary disorders (tuberculosis, asthma), patients who used benzodiazepines to control spasticity or high doses of narcotic medications for pain, and women who were pregnant or breastfeeding Randomised: N = 37 participants; % female: 63.0; mean age: 51 (SD 8) years; RRMS 33.0 %; SPMS 67.0 %; mean EDSS: 5.3 (SD 1.5); mean duration of MS: 8.5 (SD 7.4) years; % previous cannabis use: 80; % anti spastics use: 60; % undergoing disease‐modifying therapy: 70 |
|
| Interventions |
Smoked cannabis versus placebo. Cannabis cigarettes contained about 4% delta‐9‐THC by weight. Placebo cigarettes had the same base material but with the delta‐9‐THC removed. Inhalation for 5 seconds, followed by a 10‐second breath‐hold and exhalation, with a 45‐second wait between puffs. Participants completed an average of four puffs per cigarette.
Study duration: 2 x 3 days treatment periods. Washout period: 11 days Concomitant medication during the study: DMDs stable for at least 6 months; antispastics |
|
| Outcomes | Spasticity. Measure: MAS. Data: mean change (95% CI) in the difference (after to before smoking) in the cannabis and placebo phases Pain. Measure: VAS 0‐100. Data: mean change (95% CI) in the difference (after to before smoking) in the cannabis and placebo phases Withdrawal due to AE: not assessed PGIC much or very much improved: not assessed HRQoL. Measure: The Multiple Sclerosis Quality of Life‐Inventory. This outcome was reported in the study protocol but results are missing in the article Serious AEs: N/phase AEs: N/phase Nervous system disorders‐related AE: N/phase Psychiatric disorders‐related AE: N/phase Dizziness: N/phase Somnolence: NR Headache: N/phase Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms severity: not assessed Fatigue. Measure: the modified Fatigue Impact Scale (mFIS). Data: overall differences before and after treatment with placebo and cannabis Sleep quality: not assessed Mobility/ADLs: not assessed Anxiety: not assessed Depression: not assessed CGIC much or very much improved: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed. | |
| Notes | Funding: public. The study was funded by grant number C00‐SD‐103 from the University of California, Centre for Medicinal Cannabis Research (CMCR). | |
Fox 2004.
| Study characteristics | ||
| Methods | Design: Randomised cross‐over study Setting: UK; two centres Recruitment: 3 May to 31 May 2002 Number screened: 27 Number randomised: 14 Outcome timing: 2 weeks | |
| Participants |
Tremor in MS Inclusion criteria: diagnosis of definite MS (Poser 1983), age between 18 and 64 years, and a visible upper limb tremor Exclusion criteria: cognitive impairment; history of ischaemic heart disease or psychotic illness, or unwilling to stop driving for the period of the study Randomised: N = 14; % female: 57.1; mean age: 45 (range: 35 to 56) years; mean EDSS: 6.25 (range: 3.5 to 7.5); previous cannabis use: 1 participant |
|
| Interventions |
Cannador versus placebo. Cannador is an ethanolic extract of cannabis sativa standardised to 2.5 mg of THC per capsule. Identical placebo capsule. In a titration phase the dose was escalated at 3‐day intervals until either the patient reached a maximum dose of 0.125 mg/kg of THC twice a day or they began to experience intolerable side effects, in which case the dose was dropped to the last tolerated dose.
Study duration: 2 x2 weeks treatment periods. No washout Concomitant medication during the study: NR |
|
| Outcomes | Tremor: change on a tremor index, measured using a validated tremor rating scale Spasticity: not assessed Pain: not assessed PGIC: not assed HRQoL: not assessed Serious AEs: NR AEs: N/phase Dizziness: N/phase Somnolence: N/phase | |
| Notes | Funding: public. One author was funded by a grant from the Medical Research Council | |
Kavia 2010.
| Study characteristics | ||
| Methods | Design: parallel group RCT Setting: multicentre, nine centres in the UK, three in Belgium and three in Romania Recruitment: January 2003 ‐ December 2004 Number screened: 168 Number randomised: 135 Outcome timing: 10 weeks | |
| Participants |
Overactive bladder due to MS Inclusion criteria: adults with a diagnosis of MS with symptoms of overactive bladder(OAB) who had failed to respond adequately to first‐line therapies, principally anticholinergics. Stable dose of anticholinergic medication for at least 14 days prior to study entry which remained unchanged throughout the study; at least three incontinence episodes over five consecutive days during the baseline period, as assessed by a self‐report voiding diary, completed daily Exclusion criteria: presence of symptomatic urinary tract infection or any other known cause for detrusor overactivity; performing intermittent self‐catheterisation; use of cannabis or cannabis‐derived medicines within 7 days of study entry; hypersensitivity to cannabinoids or any of the excipients of the medication; history of major psychiatric disorder or severe personality disorder; history of alcohol or substance abuse; severe cardiovascular disorder, epilepsy or significant renal or hepatic impairment; concomitant use of fentanyl, levodopa, or sildenafil citrate Treatment group (Nabiximols/Sativex): N = 67; % female: 77.6; mean age: 48.6 (SD 9.3) years; EDSS: NR; duration of MS: NR; previous cannabis use: NR Placebo group: N = 68; % female: 67.6; mean age: 46.8 (SD 11.2) years; EDSS: NR; duration of MS: NR; previous cannabis use: NR |
|
| Interventions | Sativex: oromucosal spray: 100 µl containing 2.7 mg of delta‐9‐THC and 2.5 mg of CBD Placebo: oromucosal spray containing excipients plus colorants and flavouring Dose frequency: eight sprays in any 3‐hour period, and 48 sprays in any 24‐hours. Daily number of sprays taken during the study: Sativex: mean 8.91 (median 7.19); Placebo: mean 17.5 (median 14.22) Concomitant medication during the study: anticholinergic medication | |
| Outcomes | Urinary incontinence. Measure: participants completed a daily diary for the duration of the study recording the time and frequency of incontinence episodes. Data: mean change from baseline (without SD) and P value Spasticity: not assessed Pain: not assessed Withdrawal due to AE: N/group PGIC much or very much improved. Measure: seven‐point scale (very much improved to very much worse). Data: number of participants reporting improvement HRQoL. Measure: I‐QOL. Data: mean change from baseline (without SD) and P value Serious AEs: N/group AEs: NR Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: NR Dizziness: N/group Somnolence: NR Headache: N/group Confusion‐ disorientation: N/group Paranoia: NR Psychosis: N/group Hallucinations: NR Drug tolerance: NR Urinary incontinence. Measure: participants completed a daily diary for the duration of the study recording the time and frequency of incontinence episodes. Data: mean change from baseline (without SD) and P value | |
| Notes | Funding: industry ‐ drug manufacturer | |
Killestein 2002.
| Study characteristics | ||
| Methods | Design: Randomised twofold cross‐over study Setting: the Netherlands; single‐centre Recruitment: NR Number screened: NR Number randomised: 16 Outcome timing: 4 weeks | |
| Participants |
Spasticity in MS Inclusion criteria: disease duration >1 year, severe spasticity (mean Ashworth spasticity score ≥ 2 in at least one limb and EDSS score between 4 and 7.5 Exclusion criteria: other disease of clinical importance, use of other investigational drug, disease exacerbation, steroid treatment or use of cannabinoids in the 2 months preceding study entry, and history of alcohol or drug abuse, depression, psychosis, or schizophrenia Randomised: N = 16; % female: NR; mean age: 46 (SD 7.9) years; SPMS 62.5 %; PPMS 37.5 %; mean EDSS: 6.2 (SD 1.2); mean duration of MS: 15.0 (SD 10.7) years; % previous cannabis use: 37.5 |
|
| Interventions |
THC capsules (Marinol, Dronabinol) versus Cannabis sativa plant extract (20 to 30% CBD and < 5% other cannabinoids) versus placebo.
Medication was administered in two daily doses of 2.5 mg THC or plant extract, containing the same level of THC. If well‐tolerated, the dose was elevated to 5 mg twice a day for the next 2 weeks.
Study duration: 3 x 4 weeks treatment periods. Washout period: 4 weeks Concomitant medication during the study: NR |
|
| Outcomes | Spasticity. Measure: the Ashworth scale. Data: mean (95% CI) scores at baseline and at the study end in the THC, plant extract and placebo phases (data not available because they were presented only in one figure) Pain. Measure: VAS 0‐100 reported in the Method section of the article. Data: NR Withdrawal due to AE: NR PGIC much or very much improved: VAS “subject’s global impression”. Data: F and P values HRQoL. Measure: the Medical Outcomes Study Short Form 36. Data: F and P values Serious AEs: N/phase AEs: N/phase Nervous system disorders‐related AE: N/phase Psychiatric disorders‐related AE: N/phase Dizziness: N/phase Somnolence: N/phase Headache: N/phase Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms severity: not assessed Fatigue. Measure: the MS specific Fatigue Severity Scale. Data: NR Sleep quality: not assessed Mobility/ADLs: not assessed Anxiety: not assessed Depression: not assessed CGIC much or very much improved: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed | |
| Notes | Funding: public. The study was supported by the Dutch Ministry of Health, Welfare and Sport | |
Langford 2013.
| Study characteristics | ||
| Methods | Design: two phases study. Phase A was a parallel group RCT, placebo controlled, of 1‐week baseline and 14‐week treatment. Phase B was an 18‐week randomised withdrawal study (14‐week, open‐label treatment period plus a double‐blind, 4‐week, randomised‐withdrawal phase) to investigate time to treatment failure and show maintenance of efficacy. Setting: multicentre, UK (12), Canada (5), Spain (5), France (4), Czech Republic (7) Recruitment: NR Number screened: 393 Number randomised: 339 Outcome timing: 14 weeks (phase A) | |
| Participants |
Central neuropathic pain in MS (phase A) Inclusion criteria: chronic neuropathic pain due to MS, ≥ 3 months’ duration, ≥ 24 sum score on pain NRS 0‐10 on the last 6 days during the baseline period. Analgesic regimen stable for ≥ 2 weeks preceding the study entry day Exclusion criteria: severe pain from other concomitant conditions including pain of a nociceptive, musculoskeletal (including spasms), peripheral neuropathic or psychogenic origin, or due to trigeminal neuralgia. Significant psychiatric, renal, hepatic, cardiovascular, or convulsive disorders, or sensitivity to cannabis or cannabinoids Treatment group (Nabiximols/Sativex): N = 167; % female: 68; mean age: 48.42 (SD 10.43) years; RRMS 48%, SPMS 39%, PPMS 11%, PRMS 2%; EDSS: NR; mean duration of MS: 11.42 (SD 8.00) years; % previous cannabis use: 7.0; mean duration of CNP at randomisation: 5.59 (SD 6.12) years; duration of spasticity at randomisation not reported Placebo group: N = 172; % female: 68; mean age: 49.51 (SD 10.50) years; RRMS 45%, SPMS 41%, PPMS 13%, PR 1%; EDSS: NR; mean duration of MS: 12.53 (SD 8.50) years; % previous cannabis use: 6.0; mean duration of CNP at randomisation: 5.33 (SD 4.80) years; duration of spasticity at randomisation not reported |
|
| Interventions | Sativex: oromucosal spray: 100 µl containing 2.7 mg of delta‐9‐THC and 2.5 mg of CBD Placebo: excipients plus colourants Dose frequency: 12 sprays in any 24‐hour period. Daily number of sprays taken during the study: Sativex: mean 8.8 (SD 3.87). Placebo: mean 11.1 (SD 4.6) Rescue medication: paracetamol Allowed co‐therapies: pain medication: stable for at least 2 weeks Concomitant analgesic medication during the study: Sativex 92%; Placebo 97%. Disease‐modifying drugs: Sativex 60%; Placebo 58% | |
| Outcomes |
Phase A (parallel) Spasticity. Measure: NRS 0‐10. Data: number of participant reporting ≥ 30% improvement not reported. Mean change (without SD) from baseline reported. Mean treatment difference and P value reported Pain
Withdrawal due to AE: N/group PGIC much or very much improved: reported (OR, 95% CI and P value) HRQoL
Serious AEs: N/group AEs: N/group Nervous system disorders‐related AE: N/group Psychiatric disorders‐related AE: N/group Dizziness: N/group Somnolence: N/group Headache: N/group Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms. Measure: NRS 0‐10 spasm severity. Data: mean change (without SD) from baseline reported. Mean treatment difference and P value reported Fatigue. Measure: NRS 0‐10. Data: mean change (without SD) from baseline reported. Mean treatment difference and P value reported Sleep disruption due to neuropathic pain. Measure: NRS 0‐10. Data: mean change (SD) from baseline reported. Mean treatment difference (95% CI and P value) reported Mobility/ADLs: not assessed Anxiety: not assessed Depression: not assessed CGIC: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: number of paracetamol tablets taken. Mean change (SD) from baseline reported. Mean treatment difference (95% CI and P value) reported |
|
| Notes | Funding: industry ‐ drug manufacturer | |
Leocani 2015.
| Study characteristics | ||
| Methods | Design: Randomised cross‐over study Setting: Italy; single‐centre Recruitment: April 2012 to June 2013 Number screened: NR Number randomised: 44 Outcome timing: 4 weeks | |
| Participants |
Spasticity in MS Inclusion criteria: males and females aged ≥ 18 years; SPMS or PPMS of at least 12 months’ duration; relapse‐free for at least 3 months prior to screening; EDSS score between 3.0 and 6.5; moderate to severe spasticity at inclusion as defined by a MAS score of at least '1+' in one limb; stable doses of anti spasticity medication for at least 2 months prior to screening Exclusion criteria: any concomitant disease with the potential to cause or interfere with spasticity; botulinum toxin injection for spasticity in the 4 months prior to screening; any known or suspected history of psychotic illness, alcohol or substance abuse; epilepsy or hypersensitivity to cannabinoids; significant cardiac, renal or hepatic disease; females who were pregnant or lactating, or subjects of child‐bearing potential unless willing to use contraception; known contraindications to Sativex. Randomised: N = 44 participants; % female: 46.5; mean age: 48 (SD 8) years; mean EDSS: 5.5 (SD 1.0); mean duration of MS: 17.1 (SD 8.4) years; % previous cannabis use: NR; % anti spastics use: 68; % undergoing disease‐modifying therapy: 64 |
|
| Interventions |
Sativex (THC 2.7 mg and CBD 2.5 mg ) oromucosal spray versus placebo. The maximum permitted dose was 12 sprays over 24 hours.
Study duration: 2 x 4 weeks treatment periods. Washout period: 2 weeks Concomitant medication during the study: antispastic medicines stable for at least 2 months prior to screening. No modifications to DMDs in the 6 months prior to inclusion or during the study period |
|
| Outcomes |
Spasticity.
Pain. Measure: NRS 0‐10. Data: pre‐post treatment change difference between Sativex and placebo Withdrawal due to AE: N/phase PGIC much or very much improved: not assessed HRQoL: not assessed Serious AEs: NR AEs: N/phase Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: NR Dizziness: N/phase Somnolence: N/phase Headache: NR Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms severity. Measure: number of spasms within the last 24 hours. Data: NR Fatigue: Measure: Fatigue Severity Scale. Data: pre‐post treatment change difference between Sativex and placebo Sleep quality: Measure: NRS 0‐10. Data: pre‐post treatment change difference between Sativex and placebo Mobility/ADLs: not assessed Anxiety: not assessed Depression: not assessed CGIC much or very much improved: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed. |
|
| Notes | Funding: private. The study was sponsored by Laboratorios Almirall S.A., Barcelona, Spain | |
Markova 2018.
| Study characteristics | ||
| Methods |
Design: a two‐phase enriched‐design trial. In phase A, eligible patients received add‐on Sativex spray for 4 weeks to identify initial responders (≥ 20% improvement from baseline in spasticity NRS 0‐10 score). Following washout (up to 28 days), eligible initial responders were randomised to receive Sativex or placebo for 12 weeks (Phase B) Setting: multicentre, 14 centres in Czech Republic and 1 centre in Austria Recruitment: NR Number screened: NA Number randomised: 106 Outcome timing: 12 weeks |
|
| Participants |
Spasticity in MS Inclusion criteria: age >18 years; any MS subtype; ≥ 12 months history of spasticity due to MS not wholly relieved with current therapy; score of ≥4 on spasticity NRS (moderate‐to‐severe spasticity); currently receiving optimised treatment with one or more oral anti spasticity drugs (baclofen or tizanidine or both, or dantrolene as monotherapy or in combination therapy) for at least 3 months prior to screening Exclusion criteria: prior administration of THC:CBD spray; current consumption of cannabis herb or other cannabinoid‐based drugs within 30 days prior to study entry; treatment with botulinum toxin injection within the previous 6 months; medical history or family history of major psychiatric disorders other than depression; known or suspected history of a dependence disorder or heavy alcohol consumption; possibility of pregnancy or lactation; history of myocardial infarction or clinically significant cardiac dysfunction, impaired renal or hepatic function. Treatment group (Nabiximols/Sativex): N = 53; % female: NR; age: NR; EDSS: NR; duration of MS: NR; % previous cannabis use: 100 (all participants received Sativex in phase A ); duration of spasticity at randomisation: at least 1 year (study protocol) Placebo group: N = 53; % female: NR; age: NR; EDSS: NR; duration of MS: NR; % previous cannabis use: 100 (all participants received Sativex in phase A); duration of spasticity at randomisation: at least 1 year (study protocol) Most patients had secondary progressive MS (n = 92; 48.2%) or relapsing remitting MS (n = 78; 40.8%). |
|
| Interventions |
Sativex: oromucosal spray: 100 µl containing 2.7 mg of delta‐9‐THC and 2.5 mg of CBD Placebo: NR Dose frequency: maximum 12 sprays in any 24‐hour period. Daily number of sprays taken during the study: Sativex: mean 7.3 (SD 2.7); Placebo: mean 8.5 (SD 3.0) Allowed co‐therapies: optimisation of underlying anti spasticity medications was permitted in both groups across all study periods Concomitant medication during the study: baclofen 84.9%; tizanidine 31.1%; combined therapy 16% |
|
| Outcomes |
Phase B (parallel) Spasticity
Pain. Measure: NRS 0‐10. Data: number of participant reporting ≥ 50% improvement not reported. Mean change (95% CI) from baseline reported. Mean treatment difference (95% CI and P value) reported Withdrawal due to AE: N/group PGIC much or very much improved: reported (OR, 95% CI and P value) HRQoL: Measure: SF36 (eight dimensions). Data: Mean change (95% CI) from baseline reported for the eight dimensions. Mean difference change from baseline (95% CI and p value) between groups for the eight dimensions Serious AEs: N/group AEs: N/group Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: N/group Dizziness: N/group Somnolence: N/group Headache: N/group Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms severity. Measure: 3 levels categorical scale, i.e. mild, moderate, severe. Data: change from baseline reported (least square means and 95% CI). Mean treatment difference (95% CI and P value) reported Fatigue: not assessed Sleep disruption. Measure: NRS 0‐10. Data: mean change (95% CI) from baseline reported. Mean treatment difference (95% CI and P value) reported Mobility/ADLs. Measure: Barthel ADL index score. Data: number of participants reporting an MCID (8.5 points) improvement from baseline reported (OR, 95% CI and P value). Mean change (95% CI) from baseline reported. Mean treatment difference (95% CI and P value) reported Anxiety: not assessed Depression: not assessed CGIC: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed |
|
| Notes | Funding: industry ‐ drug manufacturer | |
NCT00682929.
| Study characteristics | ||
| Methods | Design: parallel group RCT Setting: US, single‐centre Recruitment period began in April 2004 and continued through 2016 Number screened: NR Number randomised: 41 Outcome timing: 7 weeks | |
| Participants | Spasticity in MS Inclusion criteria: diagnosis of clinically definite MS as defined by Poser criteria (Poser 1983); moderate or severe spasticity; age 21 or older; must live close to the Sacramento, CA area Exclusion criteria: pre‐existing pulmonary or cardiac conditions; poorly controlled psychiatric illness or dementia; inability to abstain from tobacco or marijuana smoking, or use of alcohol or sedative or hypnotic medications during the study; history of or currently meets DSM‐IV criteria for dependence on cannabis; use of cannabis, marijuana, or THC in the last four weeks; current use of cyclophosphamide, mitoxantrone, or cladribine; arthritis, bony and soft tissue disorders interfering with spasticity measures; for females of child bearing potential, inability to comply with adequate contraception Treatment group (Marijuana): N = 13; % female: 38.5; age: 18‐65 years; type of MS: NR; EDSS: NR; duration of MS: NR; % previous cannabis use: NR; duration of spasticity at randomisation: NR Treatment group (Dronabinol/Marinol): N = 14; % female: 50.0; age: 18‐64 years (N 13), ≥65 years (N 1); type of MS: NR; EDSS: NR; duration of MS: NR; % previous cannabis use: NR; duration of spasticity at randomisation: NR Placebo group: N = 14; % female: 57.1; age: 18‐64 years (N 12), ≥65 years (N 2); type of MS: NR; EDSS: NR; duration of MS: NR; % previous cannabis use: NR; duration of spasticity at randomisation: NR | |
| Interventions | Marijuana: cannabis cigarette, inhaled (smoked). Participants take their oral medication (two pills of placebo) two and a half hours prior to smoke one cannabis cigarette, daily. Synthetic Delta 9‐ THC (Dronabinol/Marinol): 5 mg tablet. Participants take their oral medication (two 5mg Dronabinol tablets) two and a half hours prior to the inhaled medication (placebo). They take two pills and smoke one cigarette, daily. Placebo: participants take their oral medication (placebo) two and a half hours prior to the inhaled medication (placebo). They take two pills and smoke one cigarette, daily. Concomitant medication during the study: NR | |
| Outcomes | Spasticity. Measure: MAS. Data: mean change (SD) from baseline reported. Pain: not assessed Withdrawal due to AE: n/N PGIC much or very much improved: not assessed HRQOL. Measure: SF3 physical and mental summary domains. Data: mean difference change from baseline (SD) between groups at 7 weeks Serious AEs: n/N AEs: n/N Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: NR Dizziness: n/N Somnolence: n/N Headache: n/N Confusion‐ disorientation: n/N Paranoia: n/N Psychosis: NR Hallucinations: n/N Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms: not assessed Fatigue: not assessed Sleep disturbance: not assessed Mobility/ADLs: not assessed Anxiety: not assessed Depression: not assessed CGIC: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed. | |
| Notes | Unable to complete subject recruitment. The study terminated early due to difficulty with enrolment and logistical issues. Departure of the principal investigator. No data analysis. Funding: public | |
NCT01606176.
| Study characteristics | ||
| Methods | Design: parallel group RCT Setting: multicentre in the UK. Number of centres not reported Recruitment: NR Number screened: NR Number randomised: 70 Outcome timing: 3 weeks | |
| Participants | Chronic pain in MS or other defect of neurological function Inclusion criteria: chronic refractory pain due to MS or other defects of neurological function. Neuropathic pain with a mean severity NRS score at ≥ 4 during last 7 days of the baseline period. Relatively stable neurological condition during the preceding 6 months. Stable medication regimen during the preceding 4 weeks. Had not used cannabis‐based medicines for at least the preceding 7 days and willing to abstain from any use of cannabis‐based medicines during the study Exclusion criteria: history of schizophrenia, other psychotic illness, severe personality disorder or other significant psychiatric disorder other than depression associated with their underlying condition. History of alcohol or substance abuse. Severe cardiovascular disorder, such as ischaemic heart disease, arrhythmias (other than well‐controlled atrial fibrillation), poorly‐controlled hypertension or severe heart failure. History of autonomic dysreflexia. History of epilepsy. Renal and liver problems Treatment group (Nabiximols/Sativex): N = 36; % female: 61.8; mean age: 51.72 (SD 12.11) years, 24 in MS‐subset; type of MS: NR; EDSS: NR; duration of MS: NR; % previous cannabis use: NR; duration of pain at randomisation: NR Placebo group: N = 34; % female: 66.7; mean age: 57.61 (SD 10.28) years, 19 in MS‐subset; type of MS: NR; EDSS: NR; duration of MS: NR; % previous cannabis use: NR; duration of pain at randomisation: NR | |
| Interventions | Sativex: oromucosal spray: 100 µl containing 2.7 mg of delta‐9‐THC and 2.5 mg of CBD Placebo: no active drug, delivered in 100 microlitre oromucosal spray Dose frequency: maximum permitted dose of Sativex 8 sprays in any 3‐hour period (20 mg THC/20 mg CBD) and 48 sprays in any 24 hours period (120 mg THC/120 mg CBD). Placebo same number of sprays possible. Daily number of sprays taken during the study: NR Concomitant medication during the study: NR | |
| Outcomes |
Spasticity: not assessed
Pain.
Withdrawal due to AE: NR PGIC much or very much improved: the number of participants reporting "Very Much Improved" or "Much Improved" reported. Comparison between groups (95% CI and Pp value) reported HRQOL. Measure: Spitzer Quality of life index 15‐0. Mean change (SD) from baseline reported. Mean difference change from baseline (95% CI and P value) Serious AEs: NR AEs: NR Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: NR Dizziness: NR Somnolence: NR Headache: NR Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms: not assessed Fatigue: not assessed Sleep disturbance. Measure: NRS 0‐10. Data: Mean change (SD) from baseline reported. Mean treatment difference (95% CI and P value) reported Mobility/ADLs: not assessed Anxiety: not assessed Depression: not assessed CGIC: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics. Measure: the percentage of days on treatment on which analgesic escape medication was used. Mean (SD) reported. Mean treatment difference (95% CI and P value) reported |
|
| Notes | Funding: industry ‐ drug manufacturer | |
Notcutt 2012.
| Study characteristics | ||
| Methods | Design: an enriched enrolment placebo‐controlled parallel randomised withdrawal design. During a 7‐day baseline period, participants continued stable dose with nabiximols at their current effective dose level. At the end of the baseline period, participants were randomised to either nabiximols or placebo. Setting: multicentre, 5 centres in UK Recruitment: NR Number screened: 37 Number randomised: 36 Outcome timing: 4 weeks | |
| Participants |
Spasticity in MS Inclusion criteria: people with MS and receiving Sativex for the relief of spasticity for at least 12 weeks prior to screening, and who were judged to have been receiving benefit from and showing tolerability to Sativex; stable anti spastic medication unchanged ≥3 months Exclusion criteria: concomitant disease or disorder that had spasticity‐like symptoms or that may have influenced the subject’s level of spasticity; use of botulinum toxin or rimonabant, a cannabinoid receptor antagonist, in the 3 months prior to study entry; current or past history of substance or alcohol abuse; significant psychiatric, renal, hepatic, cardiovascular or convulsive disorders Treatment group (Nabiximols/Sativex): N = 18; % female: 50.0; mean age: 59.7 (SD 9.0) years; RRMS 16.7%; SPMS 55.5%; PPMS 27.8%; mean EDSS: 6.75 (median 7.0, range 4.0‐8.5); mean duration of MS: 17.8 (SD 8.5) years; % previous cannabis use: 100 at current effective dose level; mean duration of Sativex use: 4.2 (median 2.0) years; mean spasticity severity (NRS) 3.6 (SD 1.7); mean duration of spasticity 14.38 (SD 9.90) years Placebo group: N = 18; % female: 66.7; mean age: 54.4 (SD 10.4) years; RRMS 22.2%; SPMS 50.0%; PPMS 27.8%; EDSS: mean EDSS: 6.92 (median 7.0, range 5.5‐8.5); mean duration of MS: 15.1 (SD 10.1) years; % previous cannabis use: 100 at current effective dose level; mean duration of Sativex use: 3.0 (median 1.9) years; mean spasticity severity (NRS) 4.1 (SD 2.2); mean duration of spasticity 11.01 (SD 8.25) years |
|
| Interventions | Sativex: oromucosal spray: 100 µl containing 2.7 mg of delta‐9‐THC and 2.5 mg of CBD Placebo: no active drug, delivered in 100 microlitre oromucosal spray Dose frequency: maximum 48 sprays in any 24‐hour period. Daily number of sprays taken during the study Sativex: mean 7.7 (median 6.4); Placebo: mean 9.0 (median 6.0) Concomitant medication during the study. Sativex: DMDs 3 participants, Benzodiazepines 1 participants and Tizanidine or Baclofen 6 participants; Placebo: DMDs 3 participants, Benzodiazepines 4 participants and Tizanidine or Baclofen 9 participants | |
| Outcomes |
Spasticity.
Pain: not assessed Withdrawal due to AE: N/group PGIC much or very much improved: reported (OR, 95% CI and P value) HRQoL: not assessed Serious AEs: N/group AEs: N/group Nervous system disorders‐related AE: N/group Psychiatric disorders‐related AE: N/group Dizziness: N/group Somnolence: N/group Headache: NR Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms severity: not assessed Fatigue: not assessed Sleep quality. Measure: NRS 0‐10 sleep quality. Data: mean baseline score (without SD) reported. Mean (SD) at the outcome timing reported. Mean change (without SD) from baseline reported. Mean treatment difference (90% CI and P value) reported Mobility/ADLs: not assessed Anxiety: not assessed Depression: not assessed CGIC for functional ability much or very much improved. Measure: 7‐point Likert‐type scale with the markers "very much improved, much improved, lightly improved, no change, slightly worse, much worse or very much worse. Data: number of participants reporting "very much improved" or "much improved" (OR, 90% CI and P value) reported Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed |
|
| Notes | Funding: industry ‐ drug manufacturer | |
Novotna 2011.
| Study characteristics | ||
| Methods |
Design: an enriched enrolment two‐phases design. In phase A, eligible patients received add‐on Sativex (Nabiximol) spray for 4 weeks to identify initial responders (≥20% improvement from baseline in spasticity NRS 0‐10 score). Eligible initial responders were randomised to receive Sativex or placebo for 12 weeks (phase B). A washout period between phase A and phase B was not done. Setting: multicentre, UK (18); Spain (11); Poland (10); Czech Republic (8); Italy (5) Recruitment: NR Number screened: NA Number randomised: 241 Outcome timing: 12 weeks |
|
| Participants |
Spasticity in MS Inclusion criteria: age ≥18 years; any MS subtype for ≥6 months; ≥ 3 months history of spasticity due to MS not wholly relieved with current therapy; score of ≥4 on spasticity NRS (moderately severe spasticity); ≥20% reduction in their NRS spasticity score at the end of the first study phase (phase A); no new anti spasticity or disease‐modifying medication and no alterations to dosage of anti spasticity or disease‐modifying medication throughout Phase A; blindness to treatment allocation throughout Phase A. Exclusion criteria: any other medical condition which was expected to influence the participants spasticity; cannabis or cannabinoid‐based medications in the 30‐day period prior to study entry; medical history of psychiatric, renal, hepatic, cardiovascular or convulsive disorders; known or suspected history of a dependence disorder, alcohol or substance abuse; current non‐prescribed use of any prescription drug. Treatment group (Sativex/Nabiximols): N = 124; % female: 58.1; mean age: 49.1 (SD 9.09) years; mean EDSS: 6.5 (SD 1.46); mean duration of MS: 13.3 (SD 8.29) years; % previous cannabis use: 100 (all participants received Sativex in phase A ); mean duration of spasticity: 8.6 (6.89) years Placebo group: N = 117; % female: 62.4; mean age: 48.1 (SD 9.59) years; mean EDSS: 6.0 (SD 1.44); mean duration of MS: 11.8 (SD 7.38) years; % previous cannabis use: 100 (all participants received Sativex in phase A ); mean duration of spasticity: 6.7 (5.40) years |
|
| Interventions |
Sativex: oromucosal spray: 100 µl containing 2.7 mg of delta‐9‐THC and 2.5 mg of CBD Placebo: ethanol:propylene glycol (50:50) excipients, peppermint oil (0.05%) flavouring and colouring Dose frequency: maximum 12 sprays in any 24 hour period. Daily number of sprays taken during the study: Sativex: mean 8.3 (SD 2.43); Placebo: mean 8.9 (SD 2.31) Allowed co‐therapies: the treatment regimen of all medications that might have affected the subjects spasticity was required to remained stable in phase A Concomitant anti spasticity medication during the study: centrally acting agents (Baclofen, Tizanidine, Tolperisone): Sativex 70%, Placebo 77%; Anti‐epileptics: Sativex 29%, Placebo 18%; Benzodiazepine‐related derivatives: Sativex 18%, Placebo 25%; Adamantane derivatives: Sativex 14%, Placebo13%; Others: Sativex 2%, Placebo 0 |
|
| Outcomes |
Phase B (parallel) Spasticity
Pain: not assessed Withdrawal due to AE: N/group PGIC much or very much improved: reported (OR, 95% CI and P value) HRQoL
Serious AEs: N/group AEs: N/group Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: N/group Dizziness: N/group Somnolence: N/group Headache: N/group Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms frequency. Measure: NRS. Data: mean score (SD) at the end of treatment reported. Mean treatment difference (95% CI and P value) reported Fatigue: not assessed Sleep disruption. Measure: NRS 0‐10. Data: mean score (SD) at the outcome timing reported. Mean change from baseline without SD reported. Mean treatment difference (95% CI and P value) reported Mobility/ADLs. Measure: Barthel ADL index score. Data: Number of participants reporting improvement from baseline reported (OR, 95% CI and P value) Anxiety: not assessed Depression. Measure: Beck Depression Inventory ‐ II. Data: mean change (SD) from baseline reported. Mean treatment difference (95% CI and P value) reported CGIC: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed |
|
| Notes | Funding: industry ‐ drug manufacturer | |
Rog 2005.
| Study characteristics | ||
| Methods | Design: parallel group RCT, placebo controlled Setting: UK, single‐centre Recruitment: March 2002 ‐ July 2002 Number screened: 85 Number randomised: 66 Outcome timing: 4 weeks | |
| Participants |
Central neuropathic pain in MS. Inclusion criteria: at least 6 months after MS diagnosis; at least 3 months central pain with unlikely other cause, both with dysaesthetic characteristics or painful spasm; 2 weeks of stable analgesic regimen; no cannabinoid use the last 7 days Exclusion criteria: spasticity‐related pain, visceral pain, headache, acute MS‐related pain; major psychiatric disorder; other than pain‐related depression; severe concomitant illness, seizures; history or suspicion of substance abuse; diabetes mellitus; levodopa use; hypersensitivity to cannabis‐based medicines Treatment group (Nabiximols/Sativex): N = 34; % female: 82; mean age: 50.3 (SD 6.7) years; mean EDSS: 6.0 (SD 1.1); mean duration of MS: 10.4 (SD 7.3) years; % previous cannabis use: 44.0; duration of CNP not reported Placebo group: N = 32; % female: 75; mean age: 48.1 (SD 9.7) years; mean EDSS: 5.8 (SD 1.5); mean duration of MS: 12.8 (SD 8.1) years; % previous cannabis use: 65.6; duration of CNP not reported Combined groups: RRMS 35%, SPMS 50%, PPMS 14%, Benign MS 1% |
|
| Interventions | Sativex: oromucosal spray: 100 µl containing 2.7 mg of delta‐9‐THC and 2.5 mg of CBD Placebo: ethanol:propylene glycol (50:50) excipients Dose frequency: maximum 48 sprays in any 24 hour period. Daily number of sprays taken during the study: Sativex: mean 9.6 (SD 6.1), range: 2–25; Placebo: mean 19.1 (SD 12.9), range: 1–47 Allowed co‐therapies: amitriptyline maximally 75 mg/day Concomitant analgesics medication during the study: Sativex mean 1.8 (SD 1.2) range 0–5; Placebo mean 1.8 (SD 1.3) range 0–4 | |
| Outcomes |
Spasticity: not assessed
Pain
Withdrawal due to AE: N/group PGIC much or very much improved: reported (OR, 95% CI and P value) HRQoL: not assessed Serious AEs: N/group AEs: N/group Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: NR Dizziness: N/group Somnolence: N/group Headache: N/group Confusion‐ disorientation: N/group Paranoia: N/group Psychosis: NR Hallucinations: N/group Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms: not assessed Fatigue: not assessed Sleep disruption due to neuropathic pain: Measure: NRS 0‐10. Data: mean baseline score (95% CI) reported. Mean score (95% CI) at the outcome timing reported. Mean change (SD) from baseline reported. Mean treatment difference (95% CI and P value) reported Mobility/ADLs: not assessed Anxiety: Measure: HADS. Data: mean change (SD) from baseline reported. Mean treatment difference (95% CI and p value) reported Depression: HADS. Data: mean change (SD) from baseline reported. Mean treatment difference (95% CI and p value) reported CGIC: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed |
|
| Notes | Funding: industry ‐ drug manufacturer | |
Schimrigk 2017.
| Study characteristics | ||
| Methods |
Design: parallel group RCT, placebo‐controlled Setting: multicentre, Germany (30 centres) Recruitment: NR Number screened: 260 Number randomised: 240 Outcome timing: 16 weeks |
|
| Participants |
Central neuropathic pain in MS Inclusion criteria: age 18‐70 years; MS according to McDonald criteria (McDonald 2001); stable MS symptoms; ≥ 3 months history of CNP due to MS; score of ≥4 on pain NRS (moderate to severe pain) Exclusion criteria: any peripheral pain syndromes, pre‐existing psychotic disorders, severe cardiac diseases, or known substance abuse Treatment group (Dronabinol): N = 124; % female: 71; mean age: 48.4 (SD 9.6) years; mean EDSS: 5.0 (SD 1.5); mean duration of MS: 10.9 (SD 7.98) years; % previous cannabis use: NR; mean duration of CNP at randomisation: 54.0 (SD 53.8; range 2.0–357.0) months Placebo group: N = 116; % female: 75; mean age: 47.0 (SD 9.7) years; mean EDSS: 4.9 (SD 1.6); mean duration of MS: 11.5 (SD 8.17) years; % previous cannabis use: NR; mean duration of CNP at randomisation: 59,5 (SD 58.1; range 4.0 to 419.0) months |
|
| Interventions |
Synthetic Delta 9‐ THC (Dronabinol): oral solution. Placebo: oral solution. Description NR Treatment duration: 4 weeks’ titration, followed by a 12‐week maintenance phase. 32 weeks open‐label Dose: between 7.5 and 15.0 mg daily. Mean daily dose of Dronabinol taken during the study: 12.7 (SD 2.9) mg Rescue medication: oral intake of tramadol Allowed co‐therapies: amitriptyline and gabapentin, if started at least 3 months earlier with a stable dose Concomitant analgesics medication during the study: Dronabinol 39.5% of participants. Placebo 44.0% of participants |
|
| Outcomes |
Spasticity: not assessed Pain. Measure: NRS 0‐10. Data: number of participant reporting ≥ 50% improvement not reported. Mean score (SD) of patients' retrospective rating of weekly pain intensity reported (baseline score). Mean change (SD) from baseline to mean weekly pain scores within a maximum of 16 weeks reported. P value of the mean treatment difference reported Withdrawal due to AE: N/group PGIC much or very much improved: not assessed HRQoL. Measure: SF3 physical and mental summary domains. Mean change (without SD) at 16 weeks Serious AEs: N/group AEs: N/group Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: NR Dizziness: N/group Somnolence: NR Headache: N/group Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms: not assessed Fatigue: not assessed Sleep disruption: not assessed Mobility/ADLs: not assessed Anxiety: not assessed Depression: not assessed CGIC: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed |
|
| Notes | Funding: industry ‐ drug manufacturer | |
Svendsen 2004.
| Study characteristics | ||
| Methods | Design: Randomised cross‐over study Setting: Denmark; single‐centre Recruitment: 27 February to 21 May 2002 Number screened: 25 Number randomised: 24 Outcome timing: 3 weeks | |
| Participants |
Central neuropathic pain in MS Inclusion criteria: diagnosis of MS (Poser 1983); age 18 to 55 years; CNP intensity score ≥ 3 on a 0‐10 NRS Exclusion criteria: hypersensitivity to cannabinoids or sesame oil; heart disease; mania, depression, or schizophrenia; previous or present alcohol or drug misuse; treatment with tricyclic antidepressants, anticholinergic agents, antihistamine, or central nervous system depressant drugs (with the exception of spasmolytic drugs); use of analgesic drugs except paracetamol; pregnancy or lactation; sexually active women without reliable contraception; patients unable to cooperate or complete the study; participation in other clinical trials within the previous month; use of marihuana within the three months before the study; and unwillingness to abstain from the use of marihuana during the entire period Randomised: N = 24; % female: 58.3; median age: 50 (range 23‐55) years; % RRMS 37.5; % SPMS 37.5 ; % PPMS 25; median EDSS: 6.0 (range 2.5‐6.5); median duration of MS: 7.0 (range 0.3‐25.0) years; median duration of pain: 4.5 (range 0.3‐12.0) years; % previous cannabis use: NR |
|
| Interventions |
THC capsules (Dronabinol) versus placebo. Dose: 2.5 mg daily increased by 2.5 mg every other day to a maximum dose of 10 mg daily. Placebo capsules were administered as identical looking capsules. The active capsules contained dronabinol solution in sesame oil, and the placebo capsules contained pure sesame oil.
Study duration: 2 x 3 weeks treatment periods. Washout period: 3 weeks Allowed co‐therapies: spasmolytic drugs, paracetamol |
|
| Outcomes | Spasticity: not assessed Pain. Measure: NRS 0‐10. Data: number of participant reporting ≥ 50% improvement (end of treatment period) Withdrawal due to AE: number of participants PGIC much or very much improved: not assessed HRQoL. Measure: SF‐36 health survey. Data: medians (25th to 75th centiles and P values active versus placebo) Serious AEs: NR AEs: N/phase Nervous system disorders‐related AE: N/phase Psychiatric disorders‐related AE: N/phase Dizziness: N/phase Somnolence: N/phase Headache: N/phase Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms severity: not assessed Fatigue: not assessed Sleep quality: not assessed Mobility/ADLs: not assessed Anxiety: not assessed Depression: not assessed CGIC much or very much improved: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed. | |
| Notes | Funding: mixed. The study was supported by grants from the Danish MS Society (grant no 2002/71045), and the Warwara Larsen Foundation (grant no 664.28), Denmark. Solvay Pharmaceuticals provided study medication and placebo, labelling, and packaging. In addition, the company provided financial support for study monitoring and data analysis. IPC‐Nordic, Denmark, packaged and labelled the study medication and monitored the study. These companies were not involved in the design or execution of the study or writing the manuscript. | |
Turcotte 2015.
| Study characteristics | ||
| Methods | Design: parallel group RCT, placebo‐controlled Setting: Canada, single‐centre Recruitment: May 2008‐ July 2012 Number screened: 22 Number randomised: 15 Outcome timing: 9 weeks | |
| Participants |
Neuropathic pain in MS. Inclusion criteria: RRMS according to the revised McDonald criteria (Polman 2005). Neuropathic pain defined as a direct consequence of a lesion or disease affecting the somatosensory system, diagnosed by a neurologist and scored 4 as per the Douleur Neuropathique 4 questions (DN4) criteria (Bouhassira 2005); age 18–65 years old; EDSS score <6.5; VAS pain score ≥50; at least 3 months neuropathic pain with unlikely other cause; current pain treatment with Gabapentin not effective at a stabilised dose of 1,800 mg daily Exclusion criteria: past or current non psychotic or psychotic emotional disorders; severe concomitant illness; pregnancy or breastfeeding; history or alcohol of substance abuse; hypersensitivity to nabilone or its derivatives and current reported use of cannabinoids or related products Treatment group (Synthetic Delta 9‐ THC/Nabilone) : N = 8; % female: 88; mean age: 42.12 (SD 11.20) years; RRMS 100%; mean EDSS: 2.56 (SD 0.77); median duration of MS: 5.5 (IQR 4.5‐7.25) years; % previous cannabis use: NR; median duration of NP: 41.5 (IQR 24–64.5)months Placebo group: N = 7; % female: 86; mean age: 50.0 (SD 8.48) years; RRMS 100%; mean EDSS: 3.17 (SD 1.07); median duration of MS: 8.0 (IQR 6.25–9.0) years; % previous cannabis use: NR; median duration of NP: 62 (IQR 1–86) months |
|
| Interventions | Nabilone: synthetic THC 0.5 or 1 mg capsules Placebo: placebo capsules identical in colour, shape, and size to the nabilone capsules Dose frequency: 2 mg/day. Daily number of capsules taken during the study: NR Allowed co‐therapies: Gabapentin 1800 mg daily Concomitant analgesics medication during the study: no additional medications | |
| Outcomes | Spasticity: not assessed Pain. Measure: VAS 0‐100 pain intensity over the previous 24 hours. Data: Mean (SD) baseline scores. RR and p value calculated by imputation method for mean daily neuropathic pain collapsed across all times Withdrawal due to AE: N/group PGIC much or very much improved: number of participants and P value of RR reported HRQoL: not assessed Serious AEs: N/group AEs: NR Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: NR Dizziness: reported for nabilone group Somnolence: NR Headache: reported for nabilone group Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms: not assessed Fatigue: not assessed Sleep disruption due to neuropathic pain: not assessed Mobility/ADLs: not assessed Anxiety: Measure: not assessed Depression: not assessed CGIC: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed | |
| Notes | Funding: industry ‐ drug manufacturer | |
Vachova 2014.
| Study characteristics | ||
| Methods | Design: parallel group RCT Setting: multicentre, 6 centres in Czech Republic Recruitment: NR Number screened: 121 Number randomised: 121 Outcome timing: 50 weeks | |
| Participants |
Long‐term adverse effects on cognitive function or mood in MS Inclusion criteria: any MS subtype; moderate levels of spasticity due to MS not wholly relieved with current therapy; stable in the last three months or four weeks for disease‐modifying or anti spasticity or cognition medications, respectively; be willing to abstain from alternative cannabinoid use for 30 days prior to screening and throughout the study Exclusion criteria: current or past history of drug, alcohol abuse or significant psychiatric illness, hypersensitive to cannabinoids; female and of child bearing potential or male whose partner was of child bearing potential; female and pregnant, lactating or planning pregnancy; received experimental medicinal product within 12 weeks of screening; had any concomitant disorders or abnormalities that could either put the patient at risk, affect the patient’s ability to participate or influence the result of the study Treatment group (Nabiximols/Sativex): N = 62; % female: 48.0; mean age: 49.0 (SD 8.95) years; RRMS 42%, SPMS 39%, PPMS 18%, PR 2%; EDSS: NR; mean duration of MS: 13.9 (SD 8.09) years; % previous cannabis use: 40; mean duration of spasticity: 8.0 (SD 6.08) years Placebo group: N = 59; % female: 48.0; mean age: 48.2 (SD 10.4) years; RRMS 56%, SPMS 32%, PPMS 8%, PR 3%; EDSS: NR; mean duration of MS: 13.9 (SD 9.08) years; % previous cannabis use: 25; mean duration of spasticity: 7.7 (SD 6.57) years |
|
| Interventions | Sativex: oromucosal spray: 100 µl containing 2.7 mg of delta‐9‐THC and 2.5 mg of CBD Placebo: oromucosal spray containing excipients plus colorants Dose frequency: maximum 12 sprays in any 24‐hour period. Daily number of sprays taken during the study: Sativex: first month mean 7.6 (SD 3.1); end of treatment mean 6.4 (SD 3.1). Placebo: first month mean 9.5 (SD 2.4); end of treatment mean 9.5 (SD 2.6) Concomitant medication during the study: Sativex: ≥ 1 anti spastics: 82%; analgesics and antipyretics: 16%. Placebo: ≥ 1 anti spastics: 85%; analgesics and antipyretics: 20% | |
| Outcomes | Spasticity. Measure: MAS. Data: baseline scores (SD) reported. Mean change (SD) from baseline reported. Mean treatment difference (95% CI and P value) reported Pain: not assessed Withdrawal due to AE: N/group PGIC much or very much improved: reported (OR, 95% CI and P value) HRQoL: not assessed Serious AEs: N/group AEs: N/group Nervous system disorders‐related AE: N/group Psychiatric disorders‐related AE: N/group Dizziness: N/group Somnolence: N/group Headache: N/group Confusion‐ disorientation: N/group Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: N/group Urinary incontinence: not assessed Muscle spasms severity: not assessed Fatigue: not assessed Sleep quality: not assessed Mobility/ADLs: not assessed Anxiety: not assessed Depression. Measure: Beck Depression Inventory ‐ II total score. Data: mean change (SD) from baseline reported. Mean treatment difference (97.5% CI one tail and SE) reported CGIC much or very much improved. Measure: 7‐point Likert‐type scale with the markers "very much improved, much improved, lightly improved, no change, slightly worse, much worse or very much worse. Data: number of participants reporting "very much improved" or "much improved" reported (OR, 95% CI and p value) Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed | |
| Notes | Funding: industry ‐ drug manufacturer | |
Van Amerongen 2017.
| Study characteristics | ||
| Methods |
Design: A two phases study. The phase A was designed as a randomised, double‐blind, placebo‐controlled, 2‐way cross‐over design to determine the optimal effective dose of namisol. Following a washout period of 7 to 14 days, patients were randomised to receive namisol or placebo for 4‐weeks (parallel phase B) Setting: one centre in the the Netherlands Recruitment: August 2011 ‐ January 2013 Number screened: 66 Number randomised: 24 Outcome timing: 4 weeks |
|
| Participants |
Spasticity and central neuropathic pain in MS. Inclusion criteria: secondary progressive or primary progressive MS according to the revised McDonald criteria (Polman 2005); > 1 year duration, clinical stable for at least 30 days; baseline score ≥2 on the Ashworth scale and an EDSS score between 4.5 and 7.5 (moderate spasticity) Exclusion criteria: current use of Delta 9‐ THC confirmed per urine drug screen; presence or a significant history of any cardiac or vascular disorder, asthma or other pulmonary disease, major gastrointestinal abnormalities, peptic ulceration, hepatic, psychiatric, haematological (including bleeding disorders), endocrine, renal, or major genitourinary disease or neurological disease other than MS or uses any kind of concomitant medication that ‐ in the opinion of the investigator ‐ may interfere with the study Treatment group (Namisol): N = 12; % female: 66.7; mean age: 57.3 (SD 9.0; range 41‐73) years; mean EDSS: 6.2 (SD 1.2; range 4.5 ‐7.5); mean duration of MS: 10.3 (SD 6.5; range 3.0 ‐ 27.0) years; % previous cannabis use: 100 (all participants received D9‐THC in phase A ); duration of spasticity and pain not reported Placebo group: N = 12; % female: 66.7; mean age: 51.4 (SD 8.0; range 38‐64) years; mean EDSS: 6.3 (SD 0.5; range 5.5 ‐7.5); mean duration of MS: 12.6 (SD 4.9; range 6.0 ‐ 21.0) years; % previous cannabis use: 100 (all participants received D9‐THC in phase A ); duration of spasticity and pain not reported |
|
| Interventions |
Synthetic Delta 9‐ THC (Namisol): oral tablets 1.5 mg and 5 mg Placebo: matching placebo tablets Treatment duration: 4 weeks Dose: 24 mg daily. After 2 weeks of treatment, the daily dose was increased with 4.5 mg in all patients, except 1. For 2 patients, the dose was subsequently decreased to the starting dose (15 mg/dsy and 24 mg/day, respectively) because of adverse events Rescue medication: not reported Allowed co‐therapies: spasmolytic therapy, if started at least 30 days earlier with a stable dose Concomitant medication during the study: not reported |
|
| Outcomes |
Phase B (parallel) Spasticity
Pain. Measure: NRS 0‐10. Data: number of participant reporting ≥ 50% improvement not reported. Mean score (without SD) at the outcome timing reported. Mean change (without SD) from baseline reported. Mean treatment difference (95% CI and P value) reported Withdrawal due to AE: N/group PGIC much or very much improved: mean score (without SD) at the outcome timing reported. Mean change (without SD) from baseline reported. Mean treatment difference (95% CI and P value) reported HRQoL: not assessed Serious AEs: N/group AEs: N/group Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: NR Dizziness: N/group Somnolence: N/group Headache: N/group Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms: not assessed Fatigue. Measure: NRS 0‐10. Data: mean change (without SD) from baseline reported. Mean treatment difference and P value reported Sleep. Measure: PSQI. Data: mean score (without SD) at the outcome timing reported. Mean change (without SD) from baseline reported. Mean treatment difference (95% CI and P value) reported Mobility/ADLs: not assessed Anxiety: not assessed Depression: not assessed CGIC: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed |
|
| Notes | Funding: industry ‐ drug manufacturer | |
Vaney 2004.
| Study characteristics | ||
| Methods | Design: Randomised cross‐over study Setting: Switzerland; single‐centre Recruitment: April 2000 to April 2001 Number screened: NR Number randomised: 57 Outcome timing: 2 weeks for active treatment and 1 week for placebo | |
| Participants |
Spasticity in MS Inclusion criteria: clinically‐confirmed MS and clinically stable spasticity with at least one joint scoring ≥ 2 on the Ashworth scale Exclusion criteria: significant neurological (other than MS), cardiovascular or infectious diseases; clinical disease exacerbation or treatment with steroids during the two months preceding study entry; history of alcohol or drug abuse; depression; history of psychosis; use of cannabinoids during the week prior to inclusion; or significant cognitive impairment Randomised: N = 28 in phase one, N= 29 in phase two; % female: 50.9; mean age: 54.9 (SD 10.0) years; mean EDSS: 7.0 (SD 6.0); mean duration of MS: 17.0 (SD 8.4) years; % previous cannabis use: 58 |
|
| Interventions |
Whole‐plant cannabis extract containing 2.5 mg THC and 0.9 mg CBD versus placebo.
Overall maximum dose was 12 active capsules daily (equivalent to 30 mg THC/day).
Study duration: 14 days cannabis treatment period and 7 days placebo period. Washout period: 3 days Phase one: 13 primary progressive, 14 secondary progressive, 1 relapsing‐remitting. Phase two: 16/12/1 Allowed co‐therapies: rehabilitation and all anti‐spasticity medication |
|
| Outcomes | Spasticity. Measure: the Ashworth scale. Data: mean difference (SD) between treatments of the absolute change from baseline Pain: not assessed Withdrawal due to AE: N/phase PGIC much or very much improved: not assessed HRQoL: not assessed Serious AEs: N/phase AEs: N/phase Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: NR Dizziness: frequency/phase Somnolence: frequency/phase Headache: frequency/phase Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms severity. Measure: spasm‐frequency scales 0‐3. Data: mean difference (SD) between treatments of the absolute change from baseline Fatigue: not assessed Sleep quality: not assessed Mobility/ADLs. Measure: the Rivermead Mobility Index. Data: mean difference (SD) between treatments of the absolute change from baseline Anxiety: not assessed Depression: not assessed CGIC much or very much improved: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed. | |
| Notes | Funding: public. This study was supported by the Swiss Ministry of Health | |
Wade 2004.
| Study characteristics | ||
| Methods | Design: parallel group RCT Setting: multicentre, 3 centres in UK Recruitment: NR Number screened: 217 Number randomised: 160 Outcome timing: 6 weeks | |
| Participants |
Multiple symptoms associated with MS Inclusion criteria: MS clinically stable with no relapse ≤4 weeks; stable regular medication unchanged ≤4 weeks; abstaining from alternative cannabinoid use for 7 days prior to screening and throughout the study; have one of five target symptoms: spasticity, spasms, bladder problems, tremor or pain that was not obviously musculoskeletal. The most troublesome to be identified as the primary symptom Exclusion criteria: primary symptom was rated < 50% of maximal severity; current or past history of drug or alcohol abuse; significant psychiatric illness other than depression associated with MS; serious cardiovascular disorder; significant renal or hepatic impairment or history of epilepsy; specific contraindications to CBME excluded Treatment group (Nabiximols/Sativex): N = 80; % female: 59; mean age: 51.9 (SD 9.4; range 27 ‐ 74) years; any subtypes of MS; EDSS: NR; duration of MS: NR; % previous cannabis use: 37.5; duration of CNP and spasticity at randomization not reported Placebo group: N = 80; % female: 65; mean age: 50.4 (SD 9.3; range 27 ‐ 74) years; any subtypes of MS; EDSS: NR; duration of MS: NR; % previous cannabis use: 40; duration of CNP and spasticity at randomisation not reported |
|
| Interventions | Sativex: oromucosal spray: 100 µl containing 2.7 mg of delta‐9‐THC and 2.5 mg of CBD Placebo: oromucosal spray containing ethanol:propylene glycol (50:50) excipients and peppermint oil (0.05%) flavouring Dose frequency: maximum 48 sprays in any 24‐hour period. Daily number of sprays taken during the study not reported Concomitant medication during the study: not reported | |
| Outcomes | Spasticity. Measure: Ashworth scale. Data: mean change (SD) from baseline reported. Mean treatment difference (95% CI, SE and P value) reported Pain: not assessed Withdrawal due to AE: N/group PGIC much or very much improved: reported (OR, 95% CI and P value) HRQoL: not assessed Serious AEs: N/group AEs: N/group Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: NR Dizziness: N/group Somnolence: N/group Headache: N/group Confusion‐ disorientation: N/group Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms severity. Measure: VAS (0=no problem; 100 = very bad). Data: mean change (SD) from baseline reported. Mean treatment difference (95% CI and P value) reported Fatigue. Measure: Fatigue Severity Scale. Data: mean change (SD) from baseline reported. Mean treatment difference (95% CI, SE and P value) reported Sleep quality:. Measure: VAS. Data: mean change (SD) from baseline reported. Mean treatment difference (95% CI, SE and p value) reported Mobility/ADLs. Measure: Barthel ADL index. Data: mean change (SD) from baseline reported. Mean treatment difference (95% CI, SE and P value) reported Anxiety: not assessed Depression. Measure: Beck Depression Inventory ‐ II total score. Data: mean change (SD) from baseline reported. Mean treatment difference (95% CI, SE and P value) reported CGIC much or very much improved: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed | |
| Notes | Funding: industry ‐ drug manufacturer | |
Zajicek 2003_CAMS.
| Study characteristics | ||
| Methods | Design: parallel group RCT Setting: multicentre, 33 centres in the UK Recruitment: December 2000 ‐ October 2002 Number screened: 821 Number randomised: 657 Outcome timing: 13 weeks | |
| Participants |
Spasticity in MS Inclusion criteria: age 18–64 years; confirmed MS; stable for ≥6 months; spasticity (Ashworth score of ≥2 in ≥ 2 limb muscle groups) Exclusion criteria: ischaemic heart disease; physiotherapy regimen or medication likely to affect spasticity ≤30 days; active infection; illness which could affect spasticity; immunisations associated with foreign travel; unable to avoid driving; fixed‐tendon contractures; severe cognitive impairment; past history of psychotic illness; major illness in another body area; pregnancy; use of Δ9‐THC at any time; use of cannabis ≤30 days Treatment group. Cannador: N = 219; % female: 64.0; mean age: 50.5 (SD 7.6) years; RRMS 3%, SPMS 72.0%, PPMS 25.0%; EDSS scores: 0‐3.5 (0%), 4‐5.5 (3%), 6‐6.5 (49%), 7‐9 (47%); duration of MS: NR; previous cannabis use: NR; duration of spasticity: NR Treatment group. Marinol (Dronabinol): N = 216; % female: 69.4; mean age: 50.2 (SD 8.2) years; RRMS 7%, SPMS 72.0%, PPMS 21.0%; EDSS scores: 0‐3.5 (0.5%), 4‐5.5 (4%), 6‐6.5 (46%), 7‐9 (48%); duration of MS: NR; previous cannabis use: NR; duration of spasticity: NR Placebo group: N = 222; % female: 63.4; mean age: 50.9 (SD 7.6) years; RRMS 6%, SPMS 71.0%, PPMS 23.0%; EDSS scores: 0‐3.5 (1%), 4‐5.5 (4%), 6‐6.5 (47%), 7‐9 (47%); duration of MS: NR; previous cannabis use: NR; duration of spasticity: NR |
|
| Interventions | Cannabis extract (Cannador): soft gelatine capsules (oral) containing Δ9‐THC 2.5 mg, CBD 1.25 mg and less than 5% other cannabinoids per capsule Synthetic Δ9‐THC (Marinol): capsules (oral) Placebo: capsules (oral) contained the respective vegetable oil vehicle Dose frequency: 25 mg /day. Dose of study medication was based on bodyweight, with a maximum possible dose of 25 mg daily Concomitant medication during the study: 34 patients commenced new medication for their spasticity (12 in the Cannador group, 11 in the Marinol group and 11 in the placebo group | |
| Outcomes | Spasticity. Measure: Ashworth scale. Data: mean (SD) baseline score reported. Mean change (SD) from baseline, defined as the mean of two baseline pre‐treatment visits to the end of the 13‐week treatment period, calculated by imputation method Pain. Measure: CRS 0‐10 perceived change in body pain. Categories 0‐3 of the CRS defined a clinical relevant response. Data: N/group Withdrawal due to AE: N/group PGIC much or very much improved: not assessed HRQoL: not assessed Serious AEs: N/group AEs: N/group Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: NR Dizziness: NR Somnolence: NR Headache: NR Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms severity. Measure: CRS 0‐10 perceived change in muscle spasms. Categories 0‐3 of the CRS defined a clinical relevant response. Data: N/group Fatigue: not assessed Sleep quality. Measure: CRS 0‐10 perceived change in sleep quality. Categories 0‐3 of the CRS defined a clinical relevant response. Data: N/group. OR (95%CI) Mobility/ADLs. Measure: Barthel ADL index score. Data: mean change (SD) and p value from baseline reported Anxiety: not assessed Depression. Measure: CRS 0‐10 perceived change in depression. Categories 0‐3 of the CRS defined a clinical relevant response. Data: N/group CGIC much or very much improved: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed | |
| Notes | Funding: public | |
ZAJICEK 2012 MUSEC.
| Study characteristics | ||
| Methods |
Design: parallel group RCT Setting: multicentre, 22 centres in the UK Recruitment: June 2006‐ September 2008 Number screened: 330 Number randomised: 279 Outcome timing: 12 weeks |
|
| Participants |
Spasticity and central neuropathic pain in MS. Inclusion criteria: age between 18 and 64 years; diagnosis of MS according to the McDonald criteria (McDonald 2001); > 1 year duration; clinical stable for the previous 6 months; spasticity for ≥ 3 months and a baseline disability score ≥ 4 on CRS 0‐10 Exclusion criteria: active sources of infection; use of immunomodulatory drugs; fixed tendon contractures; severe cognitive impairment; history of psychosis or major illness; pregnancy; cannabis use in the 30 days before study start Treatment group (Cannador): N = 143; % female: 61.5; age: mean 51.9 (SD 7.7) , median 53.0 (range 32‐64) years; RRMS 9%, SPMS 67%, PPMS 24%; EDSS: NR; duration of MS: mean 14.5 (SD 9.5), median 13.0 (range 0‐40) years; previous cannabis use: NR; duration of spasticity and pain: NR Placebo group: N = 134; % female: 64.9; age: mean 52.0 (SD 7.9), median 54.0 (range 28‐64) years; RRMS 6%, SPMS 70%, PPMS 24%; EDSS: NR; duration of MS: mean 15.1 (SD 8.4), median 14.0 (range 2‐34) years; previous cannabis use: NR; duration of spasticity and pain: NR |
|
| Interventions |
Cannabis extract (Cannador®): oral soft gelatine capsules containing delta‐9‐THC 2.5mg, CBD 0.8 mg‐1.8 mg Placebo: matched placebo oral capsules contained the same partial glyceride vehicle Treatment duration: 12 weeks Dose: 25 mg THC daily. At the end of the titration period, approximately 87% of participants in the placebo group were taking the maximum daily dose of 25 mg. 47% of participants in the Cannador group had up titrated to a maximum daily dose of 25 mg and most of the others were taking daily doses of 10.0 or 15.0 mg Rescue medication: not reported Allowed co‐therapies: physiotherapy regimens or spasmolytic therapy were adjusted, where necessary, before study entry and remained stable in the 30 days before study start Concomitant medication during the study: 59.4% of the participants in the Cannador group used anti spastics and 58% of them used analgesics. 63.4% of the participants in the placebo group used anti spastics and 56.7% of them used analgesics |
|
| Outcomes |
Spasticity. Measure: CRS 0‐10. Categories 0‐3 of the CRS defined a clinical relevant response. Data: OR (95% CI and p value) reported. Mean score (SD) at the outcome timing reported. Mean change (SD and P value) from baseline reported Pain. Measure: CRS 0‐10. Data: rate of relief from body pain (P value). Mean score (SD) at the outcome timing reported. Mean change (SD and P value) from baseline reported Withdrawal due to AE: N/group PGIC much or very much improved: not assessed HRQoL. Measure: MSIS‐29. Data: mean change from baseline (SD) of physical and psychological impact at 12 weeks Serious AEs: N/group AEs: N/group Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: NR Dizziness: N/group Somnolence: NR Headache: N/group Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms. Measure: CRS 0‐10. Data: rate of relief from muscle spasms (P value). Mean score (SD) at the outcome timing reported. Mean change (SD and P value) from baseline reported Fatigue: not assessed Sleep. Measure: CRS 0‐10. Data: rate of improvement in sleep quality (P value). Mean score (SD) at the outcome timing reported. Mean change (SD and P value) from baseline reported Mobility/ADLs: not assessed Anxiety: not assessed Depression: not assessed CGIC: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed |
|
| Notes | Funding: public and industry ‐ drug manufacturer | |
Zajicek 2013_CUPID.
| Study characteristics | ||
| Methods | Design: parallel group RCT Setting: multicentre, 27 centres in the UK Recruitment: May 2006 ‐ July 2008 Number screened: 558 Number randomised: 498 Outcome timing: 36 months | |
| Participants |
Progression in MS Inclusion criteria: age 18–65 years; confirmed MS according to the McDonald 2001 criteria and disease progression in the preceding year; EDSS score of 4.0–6.5 at baseline; abstain from other cannabis use during the trial Exclusion criteria: RRMS; use of DMDs in the previous 12 months; systemic corticosteroid use in the previous 3 months; history of previous psychosis or other serious medical illness; pregnancy; serious cognitive impairment; cannabinoid use within the previous 4 weeks Treatm6ent group. Dronabinol: N =332; % female: 60.0; mean age: 52.29 (SD 7.6) years; SPMS 62.0%, PPMS 38.0%; mean EDSS 5.8 (SD 0.69); duration of MS: NR; previous cannabis use: NR Placebo group: N = 166; % female: 59.0; mean age: 51.97 (SD 8.2) years; SPMS 60.0%, PPMS 40.0%; mean EDSS 5.9 (SD 0.67); duration of MS: NR; previous cannabis use: NR |
|
| Interventions | Synthetic Δ9‐THC (Marinol): capsules (oral) 3.5 mg Placebo: identically matched (in terms of appearance and smell) placebo vegetable oil capsules (oral) Dose frequency: the maximum dose was 28 mg per day Concomitant medication during the study: NR | |
| Outcomes | Spasticity: not assessed Pain: not assessed Withdrawal due to AE: NR PGIC much or very much improved: not assessed HRQoL. Measure: MSIS‐29‐Physical. Data: mean difference change from baseline (95% CI and P value) Serious AEs: N/group AEs: N/group Nervous system disorders‐related AE: NR Psychiatric disorders‐related AE: NR Dizziness: N/group Somnolence: NR Headache: NR Confusion‐ disorientation: NR Paranoia: NR Psychosis: NR Hallucinations: NR Drug tolerance: NR Urinary incontinence: not assessed Muscle spasms severity: not assessed Fatigue: not assessed Sleep quality: not assessed Mobility/ADLs: not assessed Anxiety: not assessed Depression: not assessed CGIC much or very much improved: not assessed Reduced use of anti spastics: not assessed Reduced use of analgesics: not assessed | |
| Notes | Funding: public | |
ADLs: Activities of Daily Living; AE: adverse events; BPI: Brief Pain Inventory; CBD: cannabidiol; CI: confidence interval; CGIC: Caregiver's Global Impression of Change; CNP: central neuropathic pain; CRS: category rating scale; DMDs: disease modifying drugs; EDSS: Expanded Disability Status Scale; EQ‐5D: EuroQol 5‐Dimensions questionnaire; HADS: Hospital Anxiety and Depression Scale; HRQoL: Health Related Quality of Life;I‐QOL: Incontinence Quality of Life; IQR: interquartile range; LOCF: last observation carried forward; MAS: Modified Ashworth scale; MCID: Minimum Clinically Important Difference; µL: microlitre; MS: Multiple Sclerosis; MSQoL‐54: Multiple Sclerosis Quality of Life‐54; N: number; NA: not applicable; NP: neuropathic pain; NPS: Neuropathic Pain Scale; NR: not reported; NRS: Numeric Rating Scale; OR: Odds Ratio; PGIC: Patient Global Impression of Change; PPMS: primary progressive MS; PRMS: progressive relapsing MS; PSQI: Pittsburgh Sleep Quality Index; RCT: Randomized Controlled Trial; RR;relative risk; RRMS: relapsing remitting MS; SD: Standard Deviation; SE: standard error; SF‐36: 36 item Short Form health survey; SPMS: secondary progressive MS; THC: tetrahydrocannabinol; VAS: Visual Analog Scale; v/v: volume/volume; w/v: weight/volume
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alessandria 2020 | A before‐after (pre‐post) study with no control group |
| Banister 2019 | A review |
| Black 2019 | A systematic review |
| Calabrò 2020 | A controlled non randomised clinical trial |
| Centonze 2009 | A before‐after (pre‐post) study with no control group |
| Cristino 2020 | Review of a variety of neurological disorders |
| De Trane 2017 | A before‐after (pre‐post) study with no control group |
| Ergul 2020 | A review |
| Feinstein 2019 | A case‐control study |
| Flachenecker 2014 | A before‐after (pre‐post) study with no control group |
| Frank 2008 | Wrong population: mixed central or peripheral pain of various aetiologies |
| Friedman 2019 | A review |
| Greenberg 1994 | A randomised study comparing 10 patients with MS and 10 normal volunteers |
| Grimaldi 2019 | A before‐after (pre‐post) study with no control group |
| Haleem 2020 | A review |
| Johal 2020 | A systematic review |
| Jones 2020 | A review |
| Karst 2003 | Wrong population: participants with MS not included |
| Katagigiotis 2012 | Non randomised study of the expression of cannabinoid receptors in bladders with neurogenic detrusor overactivity and a possible local bladder effect of oral cannabinoid agonists |
| Lus 2018 | A randomised study comparing sugar‐free chewing gum or a refrigerated bottle or chewing gum and a refrigerated bottle of THC:CBD oromucosal spray to mitigate unpleasant taste and oral mucosal anomalies |
| Mantovani 2020 | A cost‐effectiveness study of Sativex based on the real‐world data of a large registry of Italian patients |
| Martínez‐Rodríguez 2008 | A cross‐sectional study with no control group |
| Martyn 1995 | N‐of‐1 cross‐over trial done in one patient |
| NCT01868048 | Withdrawn. 0 participants enrolled. Last update posted: 11 August 2016 |
| NCT03172741 | Withdrawn. 0 participants enrolled. Last update posted: 18 July 2018 |
| Notcutt 2004 | N‐of‐1 cross‐over trial done in 34 patients with chronic pain |
| Patti 2020 | A before‐after (pre‐post) study with no control group |
| Petro 1981 | A controlled non randomised clinical study |
| Pratt 2019 | An overview |
| Rezapour‐Firouzi 2013 | Outcomes of interest were not measured |
| Trojano 2015 | A before‐after (pre‐post) study with no control group |
| Ungerleider 1987 | The investigators did 3 rerandomisation to increased doses of THC. Quoted: "Of the 13 patients randomised to the study, 12 completed at least two paired trials and five of these completed 3 pairs trials" |
| Wade 2003 | The study included 24 patients with different diseases and separate data for MS patients are not provided |
| Ware 2010 | Wrong participants. Quoted: "Included participants with neuropathic pain of at least three months in duration caused by trauma or surgery, with allodynia or hyperalgesia" |
| Wilsey 2008 | Four (10%) of 38 included participants with MS. Separate data for MS patients are not provided |
| Wilsey 2013 | Three (8%) of 39 included participants with MS. Separate data for MS patients are not provided |
CBD: cannabidol; MS: Multiple sclerosis; RCT: Randomised controlled study; THC: tetrahydrocannabinol
Characteristics of studies awaiting classification [ordered by study ID]
De Blasiis 2021.
| Methods | RCT, parallel‐group, single centre. Sample size: 32 participants. Country: Italy. |
| Participants | multiple sclerosis, relapsing or progressive forms. |
| Interventions | Nabiximols. Control intervention not reported. |
| Outcomes | Primary outcome not defined. Reported outcome measures: EDSS; Modified Tardieu Scale 24 for spasticity; 0‐10 NRSs for patients’ perception of spasticity, 2‐Minutes Walk Test 25 for endurance, 10‐Meter Walking Test 26 for gait speed, Berg Balance Scale 27 for the balance on the feet support surface and Timed Up Go Test (TUG)28 for coordination and speed during standing and walking. MS Spasticity Scale (MSSS‐88)29, MS Walking Scale‐12 (MSws‐12) 30 and Modified Fatigue Impact Scale (MFIS) were also measured. |
| Notes | The randomisation process and control intervention need to be clarified. |
EDSS: Expanded Disability Status Scale;NRS: Numrtic Rating Scale; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Hansen 2021.
| Study name | The effect of cannabis‐based medicine on neuropathic pain and spasticity in patients with multiple sclerosis and spinal cord injury: study protocol of a national multicenter double‐blinded, placebo‐controlled trial |
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary: patient‐reported pain and spasticity on a NRS Secondary: quality of life and sleep, depression and anxiety, relief of pain and spasticity Adverse events |
| Starting date | February 2019 |
| Contact information | Julie Schjødtz Hansen. Department of Neurology, Aarhus University Hospital, DK‐8200 Aarhus N, Denmark. julihans@rm.dk |
| Notes |
|
NCT03005119.
| Study name | Evaluation of the safety, tolerability, and efficacy of orally administered PTL201 in MS patients with spasticity‐related symptoms |
| Methods |
|
| Participants | Definite diagnosis of MS, according to McDonald 2010 criteria at least 6 months prior to enrolment, with MS associated spasticity for at least 3 months prior to enrolment. Age 18‐65 years |
| Interventions | Treatment group: PTL201. Each capsule contains 5 mg THC and 5 mg CBD filled with seamless gelatin matrix green beads Placebo group: each capsule seamless gelatin matrix green beads containing excipients only |
| Outcomes | Primary outcome study
|
| Starting date | Estimated: 1 March 2018 |
| Contact information | Hagit Sacks ‐ hsacks@mmjphytotech.com.au Anat Achiron — Anat.Achiron@sheba.health.gov.il |
| Notes |
|
NCT03756974.
| Study name | A phase III, multi‐centre, randomised, double‐blind, placebo‐controlled, parallel‐group clinical trial to investigate the efficacy and safety of BX‐1 for the symptomatic relief of spasticity in patients with multiple sclerosis (MS) |
| Methods |
|
| Participants | Patients with MS according to 2010 or 2017 revised McDonald criteria. Male or female patients aged 18 to 65 years. Ongoing spasticity for at least 3 months before enrolment |
| Interventions | BX‐1 (dronabinol), oral solution. Placebo of BX‐1, oral solution |
| Outcomes | Primary study outcome: proportion of participants showing improvement in spasticity of 18% or more in average Numerical Rating Scale for Spasticity (NRS‐S) assessment at end of treatment (time frame: 16 weeks) |
| Starting date | 18 February 2019 |
| Contact information | Sabine Mitzenheim (drospas‐1@bionorica.de). Luitgard Spitznagel‐Schminke (drospas‐1@bionorica.de) |
| Notes |
|
NCT04203498.
| Study name | Safety and effectiveness of Nabiximols oromucosal spray as add‐on therapy in participants with spasticity due to multiple sclerosis |
| Methods |
|
| Participants | MS according to the revised 2017 McDonald criteria; aged 18 years or above. Participant is currently receiving optimised treatment with at least 1 oral anti spasticity medication |
| Interventions | Nabiximols compared to placebo |
| Outcomes | Primary study outcome: change from baseline in the average daily spasm count (time frame: baseline to day 84) |
| Starting date | October 1, 2020 |
| Contact information | Medical Enquiries (medinfo@gbio.com) |
| Notes |
|
NCT04657666.
| Study name | Trial to evaluate the effect of Nabiximols oromucosal spray on clinical measures of spasticity in patients with multiple sclerosis (RELEASE MSS1) |
| Methods |
|
| Participants | MS according to the revised 2017 McDonald criteria. Modified Ashworth Scale score of at least 2 in 2 or more of 6 muscle groups. Currently, receiving optimised treatment with at least one oral anti spasticity drug |
| Interventions | Nabiximols oromucosal spray compared to placebo |
| Outcomes | Primary study outcome: change from baseline in lower limb muscle tone to day 51 |
| Starting date | 21 December 2020 |
| Contact information | Medical Enquiries (medinfo@greenwichbiosciences.com) |
| Notes |
|
NCT04984278.
| Study name | A randomised, double‐blind, placebo‐controlled, 2‐way cross‐over trial to evaluate the effect of nabiximols oromucosal spray on clinical measures of spasticity in patients with multiple sclerosis |
| Methods |
|
| Participants | Patients with MS (any subtype), according to the revised 2017 McDonald criteria. Male or female 18 years or older. Spasticity measured with the Modified Ashworth Scale (MAS) untransformed score of at least 2 in 2 or more of 6 muscle groups. A stable dosing regimen of anti‐spasticity therapy for at least 30 days prior to visit 1 (screening). |
| Interventions |
|
| Outcomes | Primary: change in lower limb muscle tone‐6 (LLMT‐6) from day 1 predose to day 21 (treatment period 1) and from day 31 predose to day 51 (treatment period 2) [time frame: predose on days 1 and 31; days 21 and 51]. LLMT‐6 is defined as the average of the 6 individual MAS transformed scores of knee flexors, knee extensors, and plantar flexors on both sides of the body. |
| Starting date | July 2021 |
| Contact information | Medical Enquiries 1‐833‐424‐6724; medinfo@gbio.com; medinfo@gwpharm.com |
| Notes |
|
NCT05092191.
| Study name | Cannabis as a complementary treatment in multiple sclerosis (CAN‐SEP) |
| Methods |
|
| Participants | Patients with MS (any subtype), according to the recent version of the McDonald criteria. Male or female 21 years or older. Spasticity due to MS of at least one‐month duration and not relieved with current therapy, at a level of 4 or more on the numerical rating scale (NRS) |
| Interventions |
|
| Outcomes | Primary
|
| Starting date | 15 December 2021 |
| Contact information | Not reported |
| Notes |
|
Russo 2017.
| Study name | The role of Sativex in robotic rehabilitation in individuals with multiple sclerosis: Rationale, study design, and methodology |
| Methods |
|
| Participants | MS patients affected by spasticity and undergoing a robotic rehabilitation training |
| Interventions | Sativex plus Lokomat training compared with other antispastics plus Lokomat training |
| Outcomes | Primary study outcomes:
|
| Starting date | 28 December 2016 |
| Contact information | Rocco Salvatore Calabrò, IRCCS Centro Neurolesi “Bonino‐Pulejo,” S.S. 113, Contrada Casazza, 98124 Messina, Italy (e‐mail: salbro77@tiscali.it) |
| Notes |
|
CBD: cannabidiol; EDSS: Expanded Disability Status Scala; MS: multip;e sclerosis;NRS: Numeric Rating Scale; RCT: Randomised controlled trial; THC: Δ9‐tetrahydrocannabinol
Differences between protocol and review
1. Title. We changed the review title to "Cannabis and cannabinoids for symptomatic treatment for people with multiple sclerosis" to clearly state that this review is about cannabis and cannabinoids for MS symptoms only.
2. Dealing with missing data. In the protocol we had planned to evaluate methods for monitoring and detecting adverse events in included studies. This has been removed since we assessed this aspect using version 2 of the Cochrane risk of bias tool (RoB2).
3. Dealing with missing data. We did not perform a sensitivity analysis to assess effects of missing data on critical and important outcomes since this bias was assessed as low risk for most studies in this review using version 2 of the Cochrane ‘Risk of bias' tool (RoB2). Moreover, a sensitivity analysis on harms outcomes is unlikely to be plausible (i.e. assuming that participants who contributed to missing outcome data had adverse events).
4. Assessment of clinical heterogeneity. To evaluate the presence of clinical heterogeneity, we had planned to assess differences in characteristics of included participants, e.g. MS course, disease duration, baseline severity of spasticity or chronic neuropathic pain across trials using information reported in the 'Characteristics of included studies' table. However, this was not possible because most studies included grouped data as relapsing and progressive forms of MS, data on disease duration were not available, and studies measured baseline severity of spasticity and pain with different instruments.
5. Data synthesis. We had planned to combined dichotomous outcomes from parallel‐group and cross‐over trials according to the method of Becker 1993 combining lnORs from parallel trials with marginal cross‐over lnORs. This was not possible because data were not available.
6. Subgroup analyses. We did prespecify subgroup analyses of number of participants reporting spasticity or pain reduction over baseline for study design and duration of follow‐up, baseline severity score, different cannabinoids and co‐therapies, to assess whether treatment effects varied across subgroups. However, we did not conduct subgroup analyses for the following reasons. First, the variation in treatment effect on spasticity and pain tended to be explained by outlying single studies rather than variation across all the studies. Second, less than 10 studies for subgroup analyses as planned were available leading to imbalance in studies when defined by subgroups. Third, there was a predominance of parallel group studies and short duration of follow‐up.
7. Sensitivity analysis. In the protocol we had planned a sensitivity analysis on the exclusion of trials that we judged to be at high risk of bias or to raise some concerns in at least one domain of RoB2. However, since we judged all included trials at high risk of bias or with some concerns we did not seek to conduct this sensitivity analysis.
Contributions of authors
GF conceived the review
GF, SM, FB and KD drafted the protocol
FB and GF selected the studies for inclusion
FB, GF, SM and MC extracted data
GF, FB, SM, KD and MC assessed Risk of bias of the included studies
KD performed data analysis
GF drafted the manuscript of the review. All review authors contributed to writing and revising the final report.
Sources of support
Internal sources
-
Fondazione Istituto Neurologico Carlo Besta ‐ Milan, Italy
The Neurological Institute Carlo Besta hosted and supported the Editorial Base of the Multiple Sclerosis and Rare Diseases of the CNS Group up to June 2020
External sources
-
No source of support supplied, Other
No source of support supplied
-
New Source of support, Other
No source of support supplied
Declarations of interest
GF: none
SM: none
FB: She received research grants from GW pharmaceuticals (Cambridge, UK) to perform preclinical studies on phytocannabinoids and intestinal diseases, and patents on phytocannabinoids and colorectal cancer or inflammatory bowel diseases
MC: none
KD: She is employed as statistical editor by Cochrane
New
References
References to studies included in this review
Aragona 2009 {published data only}
- Aragona M, Onesti E, Tomassini V, Conte A, Gupta S, Gilio F, et al.Psychopathological and cognitive effects of therapeutic cannabinoids in multiple sclerosis: a double-blind, placebo controlled, crossover study. Clinical Neuropharmacology 2009;32(1):41-7. [DOI] [PubMed] [Google Scholar]
- Conte A, Bettolo CM, Onesti E, Frasca V, Iacovelli E, Gilio F, et al.Cannabinoid-induced effects on the nociceptive system: a neurophysiological study in patients with secondary progressive multiple sclerosis. European Journal of Pain 2009;13(5):472-7. [DOI] [PubMed] [Google Scholar]
Collin 2007 {published data only}
- Collin C, Davies P, Mutiboko IK, Ratcliffe S for the Sativex Spasticity in MS Study Group.Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. European Journal of Neurology 2007;14(3):290-6. [DOI] [PubMed] [Google Scholar]
- NCT00711646.A study of Sativex® for relief of spasticity in subjects with multiple sclerosis. clinicaltrials.gov/ct2/show/results/NCT00711646 (first posted July 9, 2008).
- Serpell MG, Notcutt W, Collin C.Sativex long-term use: an open-label trial in patients with spasticity due to multiple sclerosis. Journal of Neurology 2013;260(1):285-95. [DOI] [PubMed] [Google Scholar]
Collin 2010 {published data only}
- Collin C, Ehler E, Waberzinek G, Alsindi Z, Davies P, Powell K, et al.A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurological Research 2010;32(5):451-9. [DOI] [PubMed] [Google Scholar]
- NCT01599234.A study to evaluate the efficacy of Sativex in relieving symptoms of spasticity due to multiple sclerosis. clinicaltrials.gov/ct2/show/results/NCT01599234 (first posted May 15, 2012).
Corey‐Bloom 2012 {published data only}
- Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley H, et al.Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. Canadian Medical Association Journal 2012;184:1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2004 {published data only}
- Fox P, Bain PG, Glickman S, Carroll C, Zajicek J.The effect of cannabis on tremor in patients with multiple sclerosis. Neurology 2004;62(7):1105-9. [DOI] [PubMed] [Google Scholar]
Kavia 2010 {published data only}
- Kavia RB, De Ridder D, Constantinescu CS, Stott CG, Fowler CJ.Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2010;16(11):1349–59. [DOI] [PubMed] [Google Scholar]
Killestein 2002 {published data only}
- Killestein J, Hoogervorst EL, Reif M, Blauw B, Smits M, Uitdehaag BM, et al.Immunomodulatory effects of orally administered cannabinoids in multiple sclerosis. Journal of Neuroimmunology 2003;137(1-2):140-3. [DOI] [PubMed] [Google Scholar]
- Killestein J, Hoogervorst EL, Reif M, Kalkers NF, Van Loenen AC, Staats PG, et al.Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 2002;58(9):1404-7. [DOI] [PubMed] [Google Scholar]
Langford 2013 {published data only}
- Langford RM, Mares J, Novotna A, Vachova M, NovakovaI, Notcutt W, et al.A double-blind, randomized, placebo controlled,parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen,in the relief of central neuropathic pain in patients with multiple sclerosis. Journal of Neurology 2013;260:984–97. [DOI] [PubMed] [Google Scholar]
- NCT00391079.Sativex versus placebo when added to existing treatment for central neuropathic pain in MS. clinicaltrials.gov/ct2/show/results/NCT00391079 (first posted October 23, 2006).
Leocani 2015 {published data only}
- Leocani L, Nuara A, Houdayer E, Schiavetti I, Del Carro U, Amadio S, et al.Sativex(®) and clinical-neurophysiological measures of spasticity in progressive multiple sclerosis. Journal of Neurology 2015;262(11):2520-7. [DOI] [PubMed] [Google Scholar]
- NCT01538225.Neurophysiological study of Sativex in Multiple Sclerosis (MS) spasticity (NS-MSS). clinicaltrials.gov/ct2/show/results/NCT01538225 (first posted February 24, 2012).
Markova 2018 {published data only}
- EudraCT Number: 2015-004451-40.The Savant trial. www.clinicaltrialsregister.eu/ctr-search/trial/2015-004451-40/AT/ (first posted 2016, January 14).
- Markovà J, Essner U, Akmaz B, Marinelli M, Trompke C, Lentschat A.Sativex® as Add-on therapy Vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: a double-blind,placebo-controlled randomised clinical trial. International Journal of Neuroscience 2018;129(2):119-28. [DOI] [PubMed] [Google Scholar]
- Meuth SG, Henze T, Essner U, Trompke C, Vila Silván C.Tetrahydrocannabinol and cannabidiol oromucosal spray in resistant multiple sclerosis spasticity: consistency of response across subgroups from the SAVANT randomized clinical trial. International Journal of Neuroscience 2020;130(12):1199-205. [DOI] [PubMed] [Google Scholar]
NCT00682929 {published data only}
- NCT00682929.Cannabis for spasticity in multiple sclerosis. clinicaltrials.gov/ct2/show/results/NCT00682929 (first posted May 23, 2008).
NCT01606176 {unpublished data only}
- NCT01606176.A study to evaluate the effects of Cannabis based medicine in patients with pain of neurological origin. clinicaltrials.gov/ct2/show/results/NCT01606176 (last update posted September 3, 2012).
Notcutt 2012 {published data only}
- NCT00702468.Evaluate the maintenance of effect after long-term treatment with Sativex® in subjects with symptoms of spasticity due to Multiple Sclerosis. clinicaltrials.gov/ct2/show/results/NCT00702468 (first posted: June 20, 2008).
- Notcutt W, Langford R, Davies P, Ratcliffe S, Potts R.A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex® (nabiximols). Multiple Sclerosis Journal 2012;18(2):219–28. [DOI] [PubMed] [Google Scholar]
Novotna 2011 {published data only}
- Haupts M, Vila C, Jonas A, Witte K, Álvarez-Ossorio L.Influence of previous failed antispasticity therapy on the efficacy and iolerability of THC:CBD oromucosal spray for Multiple Sclerosis spasticity. European Neurology 2016;75(5-6):236-43. [DOI] [PubMed] [Google Scholar]
- NCT00681538.A study of the safety and effectiveness of Sativex®, for the relief of symptoms of spasticity in subjects, from phase B, with Multiple Sclerosis (MS). clinicaltrials.gov/ct2/show/results/NCT00681538 (first posted: May 21, 2008).
- Novotna A, Mares J, Ratcliffe S, Novakova I, VachovaM, Zapletalova O, et al.A randomized, double-blind,placebo-controlled, parallel-group, enriched-design study of nabiximols (Sativex(®)), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. European Journal of Neurology 2011;18:1122–31. [DOI] [PubMed] [Google Scholar]
Rog 2005 {published data only}
- NCT01604265.A study of Sativex in the treatment of central neuropathic pain due to multiple sclerosis. clinicaltrials.gov/ct2/show/results/NCT01604265 (first posted: May 23, 2012).
- Rog DJ, Nurmikko TJ, Friede T, Young CA.Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005;65:812–9. [DOI] [PubMed] [Google Scholar]
- Rog DJ, Nurmikko TJ, Young CA.Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. ClinicalTherapeutics 2007;29(9):2068-79. [DOI] [PubMed] [Google Scholar]
Schimrigk 2017 {published data only}
- NCT00959218.Efficacy and safety of the pain relieving effect of Dronabinol in central neuropathic pain related to multiple sclerosis. clinicaltrials.gov/ct2/show/results/NCT00959218 (first posted: August 14, 2009).
- Schimrigk S, Marziniak M, Neubauer C, Kugler EM, Werner G, Abramov-Sommariva D.Dronabinol is a safe long-term treatment option for neuropathic pain patients. European Neurology 2017;78:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Svendsen 2004 {published data only}
- Svendsen KB, Jensen TS, Bach FW.Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ 2004;329(7460):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turcotte 2015 {published data only}
- NCT00480181.Efficacy and safety evaluation of Nabilone as adjunctive therapy to Gabapentin for the management of neuropathic pain in multiple sclerosis. clinicaltrials.gov/ct2/show/results/NCT00480181 (first posted: May 30, 2007).
- Turcotte D, Doupe M, Torabi M, Gomori A, Ethans K, Esfahani F, et al.Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: a randomized controlled trial. Pain Medicine 2015;16:149–59. [DOI] [PubMed] [Google Scholar]
Vachova 2014 {published data only}
- NCT01964547.A randomized study of Sativex on cognitive function and mood: multiple sclerosis patients. clinicaltrials.gov/ct2/show/results/NCT01964547 (first posted: October 17, 2013).
- Vachová M, Novotná A, Mares J, Taláb R, Fiedler J, Lauder H, et al.A multicentre, double-blind, randomised, parallel-group, placebo-controlled study of effect of long-term Sativex® treatment on cognition and mood of patients with spasticity due to multiple sclerosis. Journal of Multiple Sclerosis 2014;1(2):1000122. [Google Scholar]
- Wright S, Vachova MM, Novakova I.The effect of long-term treatment with a prescription cannabis-based THC: CBD oromucosal spray on cognitive function and mood: a 12 month double blind placebo-controlled study in people with spasticity due to multiple sclerosis. Multiple Sclerosis Journal 2013;19(11 Suppl):P1206. [Google Scholar]
Van Amerongen 2017 {published data only}
- EudraCT Number: 2010-022033-28.A two-phased, randomized, double blind, placebo-controlled study of ECP002A (Δ9-THC) to determine safety, tolerability and efficacy in multiple sclerosis patients suffering from spasticity and pain. www.clinicaltrialsregister.eu/ctr-search/trial/2010-022033-28/NL (first posted: October 26, 2010).
- Amerongen G, Kanhai K, Baakman AC, Heuberger J, Klaassen E, Beumer TL, et al.Effects on spasticity and neuropathic pain of an oral formulation of Δ9-tetrahydrocannabinol in patients with progressive multiple sclerosis. Clinical Therapeutics 2017;40(9):1467-82. [DOI] [PubMed] [Google Scholar]
Vaney 2004 {published data only}
- Vaney C, Heinzel-Gutenbrunner M, Jobin P, Tschopp F, Gattlen B, Hagen U, et al.Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study. Multiple Sclerosis (Houndmills, Basingstoke, England) 2004;10(4):417-24. [DOI] [PubMed] [Google Scholar]
Wade 2004 {published data only}
- NCT01610700.An investigation of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in multiple sclerosis patients. clinicaltrials.gov/ct2/show/results/NCT01610700 (first posted June 4, 2012).
- Wade DT, Makela P, Robson P, House H, Bateman C.Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind,randomized, placebo-controlled study on 160 patients. Multiple Sclerosis (Houndmills, Basingstoke, England) 2004;10:434–41. [DOI] [PubMed] [Google Scholar]
- Wade DT, Makela PM, House H, Bateman C, Robson P.Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2006;12(5):639-45. [DOI] [PubMed] [Google Scholar]
Zajicek 2003_CAMS {published data only}
- Freeman RM, Adekanmi O, Waterfield MR, Waterfield AE, Wright D, Zajicek J.The effect of cannabis on urge incontinence in patients with multiple sclerosis: a multicentre, randomised placebo-controlled trial (CAMS-LUTS). International Urogynecology Journal 2006;17:636–41. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F.Cannabinoids do not reduce objective measurements in muscle spasticity, but people with multiple sclerosis perceive some benefit. Evidence-Based Healthcare 2004;8:159-61. [Google Scholar]
- Katona S, Kaminski E, Sanders H, Zajicek J.Cannabinoid influence on cytokine profile in multiple sclerosis. Clinical and Experimental Immunology 2005;140(3):580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajicek JP, Sanders HP, Wright DE, Vickery PJ, Ingram WM, Reilly SM, et al.Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. Journal of Neurology, Neurosurgery, and Psychiatry 2005;76(12):1664-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, et al.Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomized placebo-controlled trial. Lancet 2003;362:1517–26. [DOI] [PubMed] [Google Scholar]
ZAJICEK 2012 MUSEC {published data only}
- NCT00552604.MUltiple Sclerosis and Extract of Cannabis (MUSEC) study (MUSEC). clinicaltrials.gov/ct2/show/results/NCT00552604 (first posted: November 2, 2007).
- Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG, MUSEC Research Group.Multiple sclerosis and extract of cannabis: results of the MUSEC trial. Journal of Neurology, Neurosurgery and Psychiatry 2012;83:1125–32. [DOI] [PubMed] [Google Scholar]
Zajicek 2013_CUPID {published data only}
- Ball S, Vickery J, Hobart J, Wright D, Green C, Shearer J, et al.The Cannabinoid Use in Progressive Inflammatory brain Disease (CUPID) trial: a randomised double-blind placebo-controlled parallel-group multicentre trial and economic evaluation of cannabinoids to slow progression in multiple sclerosis. Health Technology Assessment (Winchester, England) 2015;19(12:vii-viii, xxv-xxxi):1-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN62942668.The cannabinoid use in progressive inflammatory brain disease (CUPID) trial. doi.org/10.1186/ISRCTN62942668 (first posted: May 3, 2005).
- Zajicek J, Ball S, Wright D, Vickery J, Nunn A, Miller D et al.Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): a randomised, placebo-controlled trial. Lancet Neurology 2013;12(9):857-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alessandria 2020 {published data only}
- Alessandria G, Meli R, Infante MT, Vestito L, Capello E, Bandini F.Long-term assessment of the cognitive effects of nabiximols in patients with multiple sclerosis: a pilot study. Clinical Neurology and Neurosurgery 2020;196:105990. [DOI] [PubMed] [Google Scholar]
Banister 2019 {published data only}
- Banister SD, Arnold JC, Connor M, Glass M, McGregor IS.Dark classics in chemical neuroscience: Δ9-tetrahydrocannabinol. ACS Chemical Neuroscience 2019;10(5):2160-75. [DOI] [PubMed] [Google Scholar]
Black 2019 {published data only}
- Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, et al.Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry 2019;6(12):995-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calabrò 2020 {published data only}
- Calabrò RS, Russo M, Naro A, Ciurleo R, D'Aleo G, Rifici C, et al.Nabiximols plus robotic assisted gait training in improving motor performances in people with multiple sclerosis. Multiple Sclerosis and Related Disorders 2020;43:102177. [DOI] [PubMed] [Google Scholar]
Centonze 2009 {published data only}
- Centonze D, Mori F, Koch G, Buttari F, Codecà C, Rossi S, et al.Lack of effect of cannabis-based treatment on clinical and laboratory measures in multiple sclerosis. Neurological Sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 2009;30(6):531-4. [DOI] [PubMed] [Google Scholar]
Cristino 2020 {published data only}
- Cristino L, Bisogno T, Di Marzo V.Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nature Reviews. Neurology 2020;16(1):9-29. [DOI] [PubMed] [Google Scholar]
De Trane 2017 {published data only}
- De Trane S, Buchanan K, Keenan L, Simeoni S, O’ Brien L, Stevenson V, et al.THC: CBD (Nabiximols) has a beneficial effect on resistant MS related spasticity and reduces the need for Intrathecal baclofen. Multiple Sclerosis Journal 2017;23(3 Supplement 1):1012-3. [Google Scholar]
Ergul 2020 {published data only}
- Ergul M, Nodehi Moghadam A, Soh R.The effectiveness of interventions targeting spasticity on functional clinical outcomes in patients with multiple sclerosis: a systematic review of clinical trials. European Journal of Physiotherapy Feb 23 [Epub ahead of print].
Feinstein 2019 {published data only}
- Feinstein A, Meza C, Stefan C, Staines RW.Coming off cannabis: a cognitive and magnetic resonance imaging study in patients with multiple sclerosis. Brain: a Journal of Neurology 2019;142(9):2800-12. [DOI] [PubMed] [Google Scholar]
Flachenecker 2014 {published data only}
- Flachenecker P, Henze T, Zettl UK.Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice--results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. European Neurology 2014;71(5-6):271-9. [DOI] [PubMed] [Google Scholar]
Frank 2008 {published data only}
- Frank B, Serpell MG, Hughes J, Matthews JN, Kapur D.Comparison of analgesic effects and patient tolerability of nabilone and dihydrocodeine for chronic neuropathic pain: randomised, crossover, double blind study. BMJ 2008;336:199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedman 2019 {published data only}
- Friedman D, French JA, Maccarrone M.Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurology 2019;18(5):504-12. [DOI] [PubMed] [Google Scholar]
Greenberg 1994 {published data only}
- Greenberg HS, Werness SA, Pugh JE, Andrus RO, Anderson DJ, Domino EF.Short-term effects of smoking marijuana on balance in patients with multiple sclerosis and normal volunteers. Clinical Pharmacology & Therapeutics 1994;55(3):324-8. [DOI] [PubMed] [Google Scholar]
Grimaldi 2019 {published data only}
- Grimaldi AE, De Giglio L, Haggiag S, Bianco A, Cortese A, Crisafulli SG, et al.The influence of physiotherapy intervention on patients with multiple sclerosis-related spasticity treated with nabiximols (THC:CBD oromucosal spray). PLOS One 2019;14(7):e0219670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haleem 2020 {published data only}
- Haleem R, Wright R.A scoping review on clinical trials of pain reduction with cannabis administration in adults. Journal of Clinical Medicine Research 2020;12(6):344-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johal 2020 {published data only}
- Johal H, Devji T, Chang Y, Simone J, Vannabouathong C, Bhandari M.Cannabinoids in chronic non-cancer pain: a systematic review and meta-analysis. Clinical Medicine Insights. Arthritis and Musculoskeletal Disorders 2020;13:1179544120906461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2020 {published data only}
- Jones É, Vlachou S.A critical review of the role of the cannabinoid compounds Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) and their combination in multiple sclerosis treatment. Molecules (Basel, Switzerland) 2020;25(21):4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karst 2003 {published data only}
- Karst M, Salim K, Burstein S, Conrad I, Hoy L, Schneider U.Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. JAMA 2003;290:1757–62. [DOI] [PubMed] [Google Scholar]
Katagigiotis 2012 {published data only}
- Katagigiotis S, Kavia R, Gonzales G, Dimitriadis F, Ioannidis E, Fowler CJ, et al.Is there a local bladder effect of oral cannabinoid agonists? In: European Urology Supplements. Vol. 11. Elsevier Inc, 2012:e370- e370a.
Lus 2018 {published data only}
- Lus G, Cantello R, Danni MC, Rini A, Sarchielli P, Tassinari T, et al.Palatability and oral cavity tolerability of THC:CBD oromucosal spray and possible improvement measures in multiple sclerosis patients with resistant spasticity: a pilot study. Neurodegenerative Disease Management 2018;8(2):105-13. [DOI] [PubMed] [Google Scholar]
Mantovani 2020 {published data only}
- Mantovani LG, Cozzolino P, Cortesi PA, Patti F, SAFE study group.Cost-effectiveness analysis of cannabinoid oromucosal spray use for the management of spasticity in subjects with multiple sclerosis. Clinical Drug Investigation 2020;40(4):319-26. [DOI] [PubMed] [Google Scholar]
Martínez‐Rodríguez 2008 {published data only}
- Martínez-Rodríguez JE, Munteis E, Carreño M, Blanco Y, Roquer J, Abanades S, et al.Cannabis use in Spanish patients with multiple sclerosis: fulfilment of patients' expectations? Journal of the Neurological Sciences 2008;273(1-2):103-7. [DOI] [PubMed] [Google Scholar]
Martyn 1995 {published data only}
- Martyn CN, Illis LS, Thom J.Nabilone in the treatment of multiple sclerosis. Lancet 1995;345(8949):579. [DOI] [PubMed] [Google Scholar]
NCT01868048 {published data only}
- NCT01868048.Phase 3, 28-week, randomized, double-blind, placebo-controlled safety and efficacy study of Nabiximols as an add-on therapy in subjects with spasticity due to multiple sclerosis. clinicaltrials.gov/ct2/show/results/NCT01868048 (first posted June 4, 2013).
NCT03172741 {published data only}
- The effects of different medical Marijuana strains on motor and cognitive function in people with multiple sclerosis. clinicaltrials.gov/ct2/show/results/NCT03172741 (first posted June 1, 2017).
Notcutt 2004 {published data only}
- Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, et al.Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 'N of 1' studies. Anaesthesia 2004;59(5):440-52. [DOI] [PubMed] [Google Scholar]
Patti 2020 {published data only}
- Patti F, Chisari CG, Solaro C, Benedetti MD, Berra E, Bianco A, et al.Effects of THC/CBD oromucosal spray on spasticity-related symptoms in people with multiple sclerosis: results from a retrospective multicenter study. Neurological Sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 2020;41(10):2905-13. [DOI] [PubMed] [Google Scholar]
Petro 1981 {published data only}
- Check WA.Marijuana may lessen spasticity of MS. JAMA 1979;241(23):2476. [DOI] [PubMed] [Google Scholar]
- Petro DJ, Ellenberger C.Treatment of human spasticity with Δ9 -tetrahydrocannabinol. Journal of Clinical Pharmacology 1981;21:413S-416S. [DOI] [PubMed] [Google Scholar]
Pratt 2019 {published data only}
- Pratt M, Stevens A, Thuku M, Butler C, Skidmore B, Wieland LS, et al.Benefits and harms of medical cannabis: a scoping review of systematic reviews. Systematic Reviews 2019;8(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rezapour‐Firouzi 2013 {published data only}
- Rezapour-Firouzi S, Arefhosseini SR, Ebrahimi-Mamaghani M, Baradaran B, Sadeghihokmabad E, Mostafaei S, et al.Alteration of delta-6-desaturase (FADS2), secretory phospholipase-A2 (sPLA2) enzymes by hot-nature diet with co-supplemented hemp seed, evening primrose oils intervention in multiple sclerosis patients. Complementary Therapies in Medicine 2015;23(5):652-7. [DOI] [PubMed] [Google Scholar]
- Rezapour-Firouzi S, Arefhosseini SR, Ebrahimi-Mamaghani M, Baradaran B, Sadeghihokmabad E, Torbati M, et al.Activity of liver enzymes in multiple sclerosis patients with hot-nature diet and co-supplemented hemp seed, evening primrose oils intervention. Complementary Therapies in Medicine 2014;22(6):986-93. [DOI] [PubMed] [Google Scholar]
- Rezapour-Firouzi S, Arefhosseini SR, Ebrahimi-Mamaghani M, Farhoudi M, Baradaran B, Ali TM, et al.Erythrocyte membrane fatty acids in multiple sclerosis patients and hot-nature dietary intervention with co-supplemented hemp-seed and evening-primrose oils. African Journal of Traditional, Complementary, and Alternative Medicines: AJTCAM 2013;10(6):519-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezapour-Firouzi S, Arefhosseini SR, Mehdi F, Mehrangiz EM, Baradaran B, Sadeghihokmabad E, et al.Immunomodulatory and therapeutic effects of hot-nature diet and co-supplemented hemp seed, evening primrose oils intervention in multiple sclerosis patients. Complementary Therapies in Medicine 2013;21(5):473-80. [DOI] [PubMed] [Google Scholar]
Trojano 2015 {published data only}
- Trojano M, Vila C.Effectiveness and tolerability of THC/CBD Oromucosal Spray for multiple sclerosis spasticity in Italy: first data from a large observational study. European Neurology 2015;74(3-4):178-85. [DOI] [PubMed] [Google Scholar]
Ungerleider 1987 {published data only}
- Ungerleider JT, Andyrsiak T, Fairbanks L, Ellison GW, Myers LW.Delta-9-THC in the treatment of spasticity associated with multiple sclerosis. Advances in Alcohol & Substance Abuse 1987;7(1):39-50. [DOI] [PubMed] [Google Scholar]
Wade 2003 {published data only}
- Wade DT, Robson P, House H, Makela P, Aram J.A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clinical Rehabilitation 2003;17(1):21-9. [DOI] [PubMed] [Google Scholar]
Ware 2010 {published data only}
- Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, et al.Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ: Canadian Medical Association Journal 2010;182(14):E694-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilsey 2008 {published data only}
- Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, et al.A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. Journal of Pain 2008;9(6):506-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilsey 2013 {published data only}
- Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H.Low-dose vaporized cannabis significantly improves neuropathic pain. Journal of Pain 2013;14(2):136-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsey B, Marcotte TD, Deutsch R, Zhao H, Prasad H, Phan A.Laboratory experiment evaluating vaporized cannabis in the treatment of neuropathic pain from spinal cord injury and disease. Journal of Pain 2016;17(9):982-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
De Blasiis 2021 {published data only}
- De Blasiis P, Siani MF, Fullin A, Sansone M, Melone MA, Sampaolo S, et al.Short and long term effects of Nabiximols on balance and walking assessed by 3D-gait analysis in people with multiple sclerosis and spasticity. Multiple Sclerosis and Related Disorders 2021;51:102805. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Hansen 2021 {published data only}
- Hansen JS, Hansen RM, Petersen T, Gustavsen S, Oturai AB, Sellebjerg F, et al.The effect of cannabis-based medicine on neuropathic pain and spasticity in patients with multiple sclerosis and spinal cord injury: study protocol of a national multicenter double-blinded, placebo-controlled trial. Brain Sciences 2021;11(9):1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03005119 {published data only}
- NCT03005119.Evaluation of the safety, tolerability, and efficacy of orally administered PTL201 in MS patients with spasticity-related symptoms. clinicaltrials.gov/ct2/show/results/NCT03005119 (first posted December 29, 2016).
NCT03756974 {published data only}
- BX-1 in spasticity due to multiple sclerosis. clinicaltrials.gov/ct2/show/results/NCT03756974 (first posted November 28, 2018).
NCT04203498 {published data only}
- NCT04203498.Safety and effectiveness of Nabiximolsoromucosal spray as add-on therapy in participants with spasticity due to multiple sclerosis. clinicaltrials.gov/ct2/show/results/NCT04203498 (first posted December 18, 2019).
NCT04657666 {published data only}
- NCT04657666.Trial to evaluate the effect of Nabiximols oromucosal spray on clinical measures of spasticity in patients with multiple sclerosis (RELEASE MSS1). clinicaltrials.gov/ct2/show/results/NCT04657666 (first posted December 8, 2020).
NCT04984278 {published data only}
- NCT04984278.A randomized, double-blind, placebo-controlled, 2-way crossover trial to evaluate the effect of nabiximols oromucosal spray on clinical measures of spasticity in patients with multiple sclerosis. clinicaltrials.gov/ct2/show/results/NCT04984278 (first posted: July 30, 2021).
NCT05092191 {published data only}
- Cannabis as a complementary treatment in multiple sclerosis (CAN-SEP). clinicaltrials.gov/ct2/show/results/NCT05092191 (first posted: October 25, 2021).
Russo 2017 {published data only}
- Russo M, Dattola V, Logiudice AL, Ciurleo R, Sessa E, De Luca R, et al.The role of Sativex in robotic rehabilitation in individuals with multiple sclerosis: rationale, study design, and methodology. Medicine (Baltimore) 2017;96(46):e8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AAN 2018
- American Academy of Neurology.Position statement: use of medical marijuana for neurologic disorders. February 2018. Available at www.aan.com/policy-and-guidelines/policy/position-statements/medical-marijuana/ (accessed 15 May 2019).
ABN 2018
- Association of British Neurologists.ABN interim guidelines: use of cannabis-based products in neurology. December 2018. Available at https://cdn.ymaws.com/www.theabn.org/resource/collection/6750BAE6-4CBC-4DDB-A684-116E03BFE634/ABN_2018_Guidelines_-_Use_of_cannabis-based_pr.pdf (accessed 18 May 2019).
Abuhasira 2018
- Abuhasira R, Shbiro L, Landschaft Y.Medical use of cannabis and cannabinoids containing products - Regulations in Europe and North America. European Journal of Internal Medicine 2018;49:2-6. [DOI] [PubMed] [Google Scholar]
Amato 2017
- Amato L, Minozzi S, Mitrova Z, Parmelli E, Saulle R, Cruciani F, et al.Systematic review of safeness and therapeutic efficacy of cannabis in patients with multiple sclerosis, neuropathic pain, and in oncological patients treated with chemotherapy [Revisione sistematica sull’efficacia terapeutica e la sicurezza della cannabis per i pazienti affetti da sclerosi multipla, dolore neuropatico cronico e pazienti oncologici che assumono chemioterapie]. Epidemiologia & Prevenzione 2017;41(5-6):279-93. [DOI] [PubMed] [Google Scholar]
Amatya 2013
- Amatya B, Khan F, La Mantia L, Demetrios M, Wade DT.Non pharmacological interventions for spasticity in multiple sclerosis. Cochrane Database of Systematic Reviews 2013, Issue 2. Art. No: CD009974. [DOI: 10.1002/14651858.CD009974.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ansari 2008
- Ansari NN, Naghdi S, Hasson S, Azarsa MH, Azarnia S.The modified Tardieu scale for the measurement of elbow flexor spasticity in adult patients with hemiplegia. Brain Injury 2008;22(13-14):1007-12. [DOI] [PubMed] [Google Scholar]
Ansari 2009
- Ansari NN, Naghdi S, Hasson S, Fakhari Z, Mashayekhi M, Herasi M.Assessing the reliability of the Modified AshworthScale between two physiotherapists in adult patients with hemiplegia. NeuroRehabilitation 2009;25(4):235-40. [DOI] [PubMed] [Google Scholar]
Ashworth 1964
- Ashworth B.Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 1964;192:540-2. [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al, GRADE Working Group.Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Australian Government 2017
- Australian Government, Department of Health, Therapeutic Goods Administration.Guidance for the use of medicinal cannabis in the treatment of multiple sclerosis in Australia. Version 1, December 2017. Available at https://www.tga.gov.au/publication/guidance-use-medicinal-cannabis-treatment-multiple-sclerosis-australia (accessed 15 May 2019).
Banwell 2016
- Banwell E, Pavisian B, Lee L, Feinstein A.Attitudes to cannabis and patterns of use among Canadians with multiple sclerosis. Multiple Sclerosis and Related Disorders 2016;10:123-6. [DOI] [PubMed] [Google Scholar]
Becker 1993
- Becker MP, Balagtas CC.Marginal modeling of binary cross-over data. Biometrics 1993;49(4):997-1009. [PubMed] [Google Scholar]
Benito 2007
- Benito C, Romero JP, Tolón RM, Clemente D, Docagne F, Hillard CJ, et al.Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. Journal of Neuroscience: the Official Journal of the Society for Neuroscience 2007;27(9):2396-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bouhassira 2005
- Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J et al.Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005;114(1-2):29-36. [DOI] [PubMed] [Google Scholar]
Centonze 2007
- Centonze D, Bari M, Rossi S, Prosperetti C, Furlan R, Fezza F, et al.The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain 2007;130(Pt 10):2543-53. [DOI] [PubMed] [Google Scholar]
Collin 2010
- Collin C, Ehler E, Waberzinek G, Alsindi Z, Davies P, Powell K, et al.A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurological Research 2010;32(5):451-9. [DOI] [PubMed] [Google Scholar]
Constantinescu 2018
- Constantinescu CS, Gershkovich P.Therapeutic cannabinoids in multiple sclerosis: immunomodulation revisited. European Journal of Neurology 2018;25(7):905-6. [DOI] [PubMed] [Google Scholar]
Coyne 2005
- Coyne KS, Matza LS, Thompson CL.The responsiveness of the Overactive Bladder Questionnaire (OAB-q). Quality of Life Research 2005;14(3):849-55. [DOI] [PubMed] [Google Scholar]
Davies 2018
- Davies SC.Cannabis scheduling review: part 1. The therapeutic and medicinal benefits of cannabis based products – a review of recent evidence. Published 3 July 2018. Available at www.gov.uk/government/publications/cannabis-scheduling-review-part-1 (accessed prior to 11 September 2019).
DerSimonian 1986
- DerSimonian R, Laird N.Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
Di Filippo 2008
- Di Filippo M, Pini LA, Pelliccioli GP, Calabresi P, Sarchielli P.Abnormalities in the cerebrospinal fluid levels of endocannabinoids in multiple sclerosis. Journal of Neurology, Neurosurgery and Psychiatry 2008;79(11):1224-9. [DOI] [PubMed] [Google Scholar]
Di Marzo 2018
- Di Marzo V.New approaches and challenges to targeting the endocannabinoid system. Nature Reviews. Drug Discovery 2018;17(9):623-39. [DOI] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al.Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105-21. [DOI] [PubMed] [Google Scholar]
EMA 2014
- European Medicines Agency.EMEA-000181-PIP02-13. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/pips/EMEA-000181-PIP02-13/pip_001257.jsp&mid=WC0b01ac058001d129 (accessed 15 May 2019).
EuroQol Group 1990
- EuroQol Group.EuroQol--a new facility for the measurement of health-related quality of life. Health policy (Amsterdam, Netherlands) 1990;16(3):199-208. [DOI] [PubMed] [Google Scholar]
Farrar 2008
- Farrar JT, Troxel AB, Stott C, Duncombe P, Jensen MP.Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clinical Therapeutics 2008;30(5):974-85. [DOI] [PubMed] [Google Scholar]
Farrar 2010
- Farrar JT, Pritchett YL, Robinson M, Prakash A, Chappell A.The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. Journal of Pain 2010;11(2):109-18. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 2017
- Fitzpatrick JK, Downer EJ.Toll-like receptor signalling as a cannabinoid target in multiple sclerosis. Neuropharmacology 2017;113(Pt B):618-26. [DOI] [PubMed] [Google Scholar]
Freeman 1989
- Freeman PR.The performance of the two-stage analysis of two-treatment, two-period cross-over trials. Statistics in Medicine 1989;8(12):1421-32. [DOI] [PubMed] [Google Scholar]
Furgiuele 2021
- Furgiuele A, Cosentino M, Ferrari M, Marino F.Immunomodulatory potential of cannabidiol in multiple sclerosis: a systematic review. Journal of Neuroimmune Pharmacology: the Official Journal of the Society on NeuroImmune Pharmacology 2021;16(2):251-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonçalves 2019
- Gonçalves ED, Dutra RC.Cannabinoid receptors as therapeutic targets for autoimmune diseases: where do we stand? . Drug Discovery Today 2019;24(9):1845-53. [DOI] [PubMed] [Google Scholar]
Gowran 2011
- Gowran A, Noonan J, Campbell VA.The multiplicity of action of cannabinoids:implications for treating neurodegeneration. CNS Neuroscience & Therapeutics 2011;17(6):637-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT.Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 12 June 2019. Available at gradepro.org.
Gustavsen 2019
- Gustavsen S, Søndergaard HB, Andresen SR, Magyari M, Sørensen PS, Sellebjerg F, et al.Illegal cannabis use is common among Danes with multiple sclerosis. Multiple Sclerosis and Related Disorders 2019;33:5-12. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W.ECDEU assessment manual for psychopharmacology (DHEW Publication No. ADM 76-338). https://ia600306.us.archive.org/35/items/ecdeuassessmentm1933guyw/ecdeuassessmentm1933guyw.pdf (accessed prior to 11 September 2019).
Hazekamp 2013
- Hazekamp A, Ware MA, Muller-Vahl KR, Abrams D, Grotenhermen F.The medicinal use of cannabis and cannabinoids--an international cross-sectional survey on administration forms. Journal of Psychoactive Drugs 2013;45(3):199-210. [DOI] [PubMed] [Google Scholar]
Hazekamp 2018
- Hazekamp A.The trouble with CBD oil. Medical Cannabis and Cannabinoids 2018;1:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ (editors).Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. Available from www.cochrane-handbook.org, (updated March 2011). [Google Scholar]
Higgins 2016
- Edited by Julian PT Higgins on behalf of the RoB 2 working group on crossover trials.Revised Cochrane risk of bias tool for randomized trials (RoB 2). Additional considerations for crossover trials. Available at: https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/archive-rob-2-0-cross-over-trials-2016 (accessed 15 June 2019).
Higgins 2019
- Julian PT Higgins, Jelena Savović, Matthew J Page, Jonathan AC Sterne, on behalf of the ROB2 Development Group.Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). In: Cochrane Handbook for Systematic Reviews of Interventions. Chichester: The Cochrane Collaboration, 22 August 2019. Available at: https://drive.google.com/file/d/19R9savfPdCHC8XLz2iiMvL_71lPJERWK/view (accessed 26 August 2019). [Google Scholar]
ICH 2019
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use.MedDRA. Medical Dictionary for Regulatory Activities. www.meddra.org (accessed prior to 11 September 2019).
Izzo 2009
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R.Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends in Pharmacological Sciences 2009;30(10):512-27. [DOI] [PubMed] [Google Scholar]
Jean‐Gilles 2009
- Jean-Gilles L, Feng S, Tench CR, Chapman V, Kendall DA, Barrett DA, et al.Plasma endocannabinoid levels in multiple sclerosis. Journal Of The Neurological Sciences 2009;287(1-2):212-5. [DOI] [PubMed] [Google Scholar]
Kaur 2016
- Kaur R, Ambwani SR, Singh S.Endocannabinoid system: a multi-facet therapeutic target. Current Clinical Pharmacology 2016;11(2):110-7. [DOI] [PubMed] [Google Scholar]
Kempen 2011
- Kempen JC, Groot V, Knol DL, Polman CH, Lankhorst GJ, Beckerman H.Community walking can be assessed using a 10-metre timed walk test. Multiple Sclerosis Journal 2011;17(8):980-90. [DOI] [PubMed] [Google Scholar]
Kindred 2017
- Kindred JH, Li K, Ketelhut NB, Proessl F, Fling BW, Honce JM, et al.Cannabis use in people with Parkinson's disease and Multiple Sclerosis: a web-based investigation. Complementary Therapies in Medicine 2017;33:99-104. [DOI] [PubMed] [Google Scholar]
Krcevski‐Skvarc 2018
- Krcevski-Skvarc N, Wells C, Häuser W.Availability and approval of cannabis-based medicines for chronic pain management and palliative/supportive care in Europe: a survey of the status in the chapters of the European Pain Federation. European Journal of Pain 2018;22(3):440-54. [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- LefebvreC, GlanvilleJ, BriscoeS, LittlewoodA, MarshallC, MetzendorfMI, et al.Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available fromwww.training.cochrane.org/handbook.
Mahoney 1965
- Mahoney FI, Barthel DW.Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:56-61. [PubMed] [Google Scholar]
McDonald 2001
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al.Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50(1):121-7. [DOI] [PubMed] [Google Scholar]
Mecha 2019
- Mecha M, Yanguas-Casás N, Feliú A, Mestre L, Carrillo-Salinas F, Azcoitia I, et al.The endocannabinoid 2-AG enhances spontaneous remyelination by targeting microglia. Brain, Behaviour, and Immunity 2019;77:110-26. [DOI: 10.1016/j.bbi.2018.12.013] [DOI] [PubMed] [Google Scholar]
Mestre 2018
- Mestre L, Carrillo-Salinas FJ, Mecha M, Feliú A, Guaza C.Gut microbiota, cannabinoid system and neuroimmune interactions: new perspectives in multiple sclerosis. Biochemical Pharmacology 2018;157:51-66. [DOI] [PubMed] [Google Scholar]
Meza 2017
- Meza R, Peña J, García K, Corsi O, Rada G .Are cannabinoids effective in multiple sclerosis? Medwave 2017;17(Suppl 1):e6865. [DOI] [PubMed] [Google Scholar]
MHRA 2014
- Medicines and Healthcare Products Regulatory Agency.Sativex oromucosal spray. UK/H/2462/001/DC. www.mhra.gov.uk/home/groups/par/documents/websiteresources/con084961.pdf (accessed prior to 11 September 2019).
Minozzi 2021
- Minozzi S, Dwan K, Borrelli F, Filippini G.Reliability of the revised Cochrane risk-of-bias tool for randomised trials (RoB 2) improves with the use of implementation instruction. Journal of Clinical Epidemiology 2021;Sep 16:S0895-4356(21)00307-3. Epub ahead of print. . [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morales 2017
- Morales P, Hurst DP, Reggio PH.Molecular targets of the phytocannabinoids: a complex picture. Progress in the Chemistry of Organic Natural Products 2017;103:103-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
MS Society 2014
- MS Society.Cannabis and MS. Available at www.mssociety.org.uk/about-ms/treatments-and-therapies/complementary-and-alternative-therapies/cannabis (accessed 23 April 2019).
Mücke 2018
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W.Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2018, Issue 3. Art. No: CD012182. [DOI: 10.1002/14651858.CD012182.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Multiple Sclerosis Council 1998
- Multiple Sclerosis Council for Clinical Practice Guidelines.Fatigue and Multiple Sclerosis: Evidence-Based Management Strategies for Fatigue in Multiple Sclerosis. Washington (DC): Paralyzed Veterans of America, 1998. [Google Scholar]
Mustafa 2021
- Mustafa W, Elgendy N, Salama S, Jawad M, Eltoukhy K.The effect of cannabis on the clinical and cytokine profiles in patients with multiple sclerosis. Multiple Sclerosis International 2021;Feb 5;2021:6611897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newsome 2017
- Newsome SD, Aliotta PJ, Bainbridge J, Bennett SE, Cutter G, Fenton K, et al.A framework of care in multiple sclerosis, part 2: symptomatic care and beyond. International Journal of MS Care 2017;19(1):42-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence.Multiple sclerosis in adults: management. Clinical guideline [CG186]. Published date: October 2014. Last updated: July 2019. Available at www.nice.org.uk/guidance/cg186 (accessed 10 August 2019).
NICE 2019
- National Institute for Health and Care Excellence.Cannabis-based medicinal products. In development [GID-NG10124]. Available at www.nice.org.uk/guidance/indevelopment/gid-ng10124 (accessed 19 May 2019).
Nielsen 2018
- Nielsen S, Germanos R, Weier M, Pollard J, Degenhardt L, Hall W, et al.The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: a systematic review of reviews. Current Neurology and Neuroscience Reports 2018;18(2):8. [DOI] [PubMed] [Google Scholar]
Oláh 2017
- Oláh A, Szekanecz Z, Bíró T.Targeting cannabinoid signaling in the immune system: "high"-ly exciting questions, possibilities, and challenges. Frontiers in Immunology 2017;8:1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandyan 1999
- Pandyan AD, Johnson GR, Priec CI, Curless RH, Barnes MP, Rodgers H.A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clinical Rehabilitation 1999;13:373-83. [DOI] [PubMed] [Google Scholar]
Papaseit 2018
- Papaseit E, Pérez-Mañá C, Pérez-Acevedo AP, Hladun O, Torres-Moreno MC, Muga R, et al.Cannabinoids: from pot to lab. International Journal of Medical Sciences 2018;15(12):1286-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penn 1989
- Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B, et al.Intrathecal baclofen for severe spasticity. New England Journal of Medicine 1989;320(23):1517-21. [DOI] [PubMed] [Google Scholar]
Peters 2008
- Peters J, Sutton A, Jones D, Abrams K, Rushton L.Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991–6. [DOI] [PubMed] [Google Scholar]
Polman 2005
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al.Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Annals of Neurology 2005;58(6):840-6. [DOI] [PubMed] [Google Scholar]
Polman 2011
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al.Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of Neurology 2011;69(2):292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poser 1983
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al.New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(3):227-31. [DOI] [PubMed] [Google Scholar]
RCP 2018
- Royal College of Physicians (RCP).Recommendations on cannabis-based products for medicinal use. October 2018. Available at www.rcplondon.ac.uk/medicinal-use-cannabis-based-products (accessed 16 May 2019).
Reston 2019
- Reston VA.MMJ files FDA fast track approval application for cannabis multiple sclerosis drug [press release]. February 2019. Available at markets.businessinsider.com/news/stocks/mmj-files-fda-fast-track-approval-application-for-cannabis-multiple-sclerosis-drug-1027948379 (accessed 13 March 2019).
Review Manager Web [Computer program]
- Version 2.2.1. The Cochrane Collaboration, 2021 Review Manager Web (RevMan Web).Version 2.2.1. The Cochrane Collaboration, 2021 , Available from revman.cochrane.org.
Rommer 2018
Rooney 2019
- Rooney S, McFadyen DA, Wood DL, Moffat DF, Paul PL.Minimally important difference of the fatigue severity scale and modified fatigue impact scale in people with multiple sclerosis. Multiple Sclerosis and Related Disorders 2019;35:158-63. [DOI] [PubMed] [Google Scholar]
Russell 2018
- Russell C, Rueda S, Room R, Tyndall M, Fischer B.Routes of administration for cannabis use - basic prevalence and related health outcomes: A scoping review and synthesis. International Journal on Drug Policy 2018;52:87-96. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann H, Oxman A, Higgins JP, Vist G, Glasziou P, Guyatt G.Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shakespeare 2003
- Shakespeare D, Boggild M, Young CA.Anti-spasticity agents for multiple sclerosis. Cochrane Database of Systematic Reviews 2003, Issue 4. Art. No: CD001332. [DOI: 10.1002/14651858.CD001332] [DOI] [PubMed] [Google Scholar]
Thompson 2018a
- Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O.Multiple sclerosis. Lancet 2018;391(10130):1622–36. [DOI] [PubMed] [Google Scholar]
Thompson 2018b
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al.Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurology 2018;17(2):162-73. [DOI] [PubMed] [Google Scholar]
Torres‐Moreno 2018
- Torres-Moreno MC, Papaseit E, Torrens M, Farré M.Assessment of efficacy and tolerability of medicinal cannabinoids in patients with multiple sclerosis: a systematic review and meta-analysis. JAMA Network Open 2018;1(6):e183485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vickrey 1995
- Vickrey B, Hays RD, Harooni R, Myers LW, Ellison GW.A health-related quality of life measure for multiple sclerosis. Qualiity of Life Research 1995;4(3):187–206. [DOI] [PubMed] [Google Scholar]
Wade 2010
- Wade DT, Collin C, Stott C and Duncombe P.Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Multiple Sclerosis Journal 2010;16(6):707-14. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD.The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PubMed] [Google Scholar]
Whiting 2015
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al.Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 2015;313(24):2456-73. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP.The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Filippini 2019
- Filippini G, Lasserson TJ, Dwan K, D'Amico R, Borrelli F, Izzo AA, et al.Cannabis and cannabinoids for people with multiple sclerosis. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD013444. [DOI: 10.1002/14651858.CD013444] [DOI] [PMC free article] [PubMed] [Google Scholar]