Abstract
Background and Aims:
Opioid agonist therapies (OAT) are highly effective treatments for opioid use disorders (OUD) especially for pregnant women; thus, improving access to OAT is an urgent public policy goal. Our objective was to determine if insurance and pregnancy status were barriers to obtaining access to OAT in four Appalachian states disproportionately impacted by the opioid epidemic.
Methods:
Between April and May 2017, we conducted phone surveys of OAT providers, opioid treatment programs (OTPs) and outpatient buprenorphine providers, in Kentucky, North Carolina, Tennessee, and West Virginia. Survey response rates were 59%. Logistic models for dichotomous outcomes (e.g., patient acceptance) and negative binomial models were created for count variables (e.g., wait time), overall and for pregnant women.
Results:
The majority of OAT providers were accepting new patients; however, providers were less likely to treat pregnant women (91% vs 75%; p<0.01). OTPs were more likely to accept new patients than waivered buprenorphine providers (97% vs 83%; p=0.01); rates of accepting pregnant patients were lower in both (91% and 53%; p<0.01). OTPs and buprenorphine providers accepted cash payments for services at high rates (OTP: 100%; buprenorphine: 89.4%; p<0.01); Medicaid and private insurance were accepted at lower rates. In adjusted models, providers were less likely to accept pregnant women if they took any insurance (aOR 0.15, 95%CI 0.03–0.68) or were a buprenorphine provider (aOR 0.09, 95%CI 0.02–0.37).
Conclusions:
We found that OAT providers frequently did not accept any insurance and frequently did not treat pregnant women in an area of the country disproportionately affected by the opioid epidemic. Policymakers could prioritize improvements in provider training (e.g., training of obstetricians to become buprenorphine prescribers) as a means to enhance access to pregnant women or enhancing reimbursement rates as a means of improving insurance acceptance for OAT.
Keywords: Opioid agonist therapies (OAT), Opioid Use Disorder (OUD), Opioid Treatment Programs (OTPs), buprenorphine, pregnant women, insurance, Appalachia
Introduction
Over the past two decades, the prevalence of opioid use in the U.S. has reached epidemic proportions.1 This substantial increase is reflected in opioid use in pregnancy,2,3 diagnoses of opioid use disorder among pregnant women,4–6 increases in pregnancy related deaths form overdose7 and neonatal complications from in utero opioid exposure in the United States.4,6,8–10 Untreated opioid use disorder among pregnant women leads to poor outcomes for the mother and infant;11 however, treatment with opioid agonist therapy (OAT) is highly effective.11,12 OAT improves treatment retention,13 reduces relapse risk,13–16 reduces HIV-risk,13,17 reduces criminal behavior,15 reduces risk of overdose death18 and improves birth weights.19 Further, ensuring access to OAT before pregnancy decreases the likelihood of illicit drug use during critical times of fetal development in the first trimester. Access to OAT occurs primarily in Opioid Treatment Programs (OTPs) that can provide OAT using either methadone or buprenorphine, or in outpatient medical clinics where providers have obtained DATA (Drug Addiction Treatment Act of 2000) waivers to prescribe buprenorphine. Methadone, a full mu-opioid receptor agonist, typically requires daily outpatient visits to an OTP to receive medication. In contrast, buprenorphine is a partial mu-opioid receptor agonist and kappa-opioid receptor antagonist generally used in the outpatient setting that may require fewer frequent clinic visits. OTPs are more highly regulated, with additional requirements beyond outpatient buprenorphine providers, including mandated counseling.20
As the scope of the opioid epidemic has expanded, understanding and reducing barriers to accessing OAT has emerged as a prominent target for policymakers. In November 2017, The President’s Commission on Combating Drug Addiction and the Opioid Crisis highlighted expanding access to OAT as a chief goal. As The Commission noted, access to OAT, particularly in rural areas disproportionately affected by the opioid epidemic, is particularly challenging.21 Much of the literature evaluating treatment access to OAT in rural areas has focused on the geographic concentration of providers,22 which may not fully characterize access to treatment in these areas. Further, the literature on OAT access for pregnant women concentrates on receipt of OAT while obtaining treatment,23 instead of barriers to treatment. There remains an urgent need to understand barriers to accessing treatment, particularly in areas of the country with high rates of opioid-related complications and for pregnant women in order to craft policy solutions to overcome them. Appalachian states have disproportionately higher rates of opioid use24 and have been more affected by opioid overdose deaths,25 suggesting profound effects on pregnant women in Appalachia, as indicated by high rates of neonatal opioid withdrawal.8,10 However, despite the great need little is known about barriers to treatment access in this region or how insurance or pregnancy status may influence access. Our objective was to determine if insurance and pregnancy status were barriers to obtaining access to OAT in four Appalachian states disproportionately affected by the opioid epidemic.
Methods
Participants
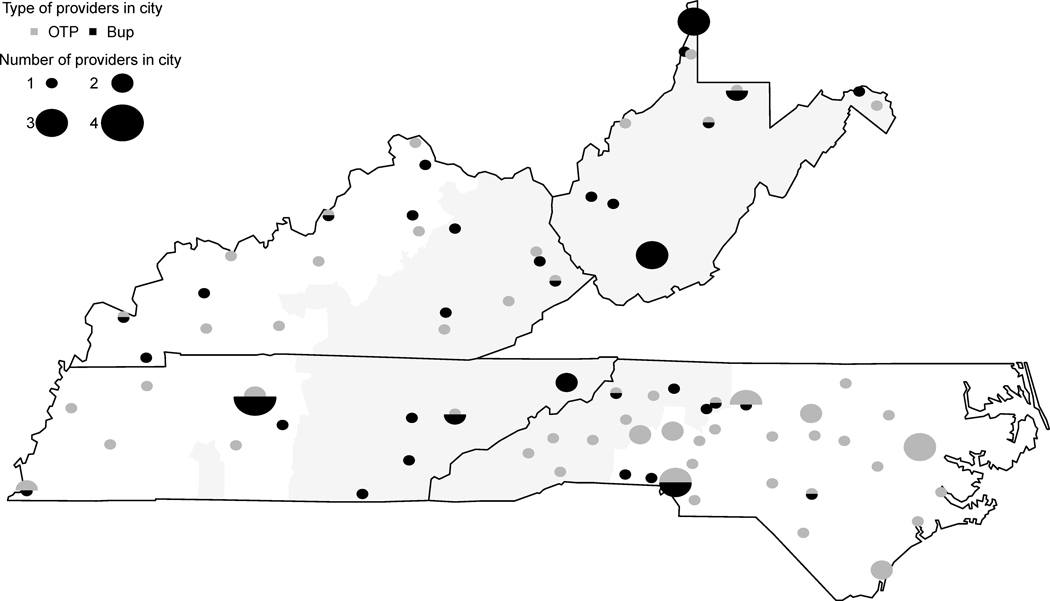
We surveyed all Opioid Treatment Programs (OTPs) and a sample of geographically diverse waivered buprenorphine providers in Kentucky, North Carolina, Tennessee, and West Virginia (Figure 1.). We identified providers from public listings provided by the Substance Abuse and Mental Health Administration (SAMHSA). The University of Chicago Survey Lab administered surveys by phone. This study was deemed exempt from human subject review by the University of Chicago and Vanderbilt University Medical Center institutional review boards.
Figure 1.
Geographic Distribution of Surveyed Providers.
*Appalachian counties shaded in gray.
** OTP = opioid treatment program, Bup = outpatient buprenorphine provider.
Design
Phone surveys were conducted in April and May 2017 using a standardized protocol (Appendix). Prior to conducting the survey, provider information was confirmed to be accurate. We confirmed SAMHSA provider lists accurately identified 96% of providers as OTPs and 77% of buprenorphine providers. Respondents were asked a series of questions to identify if they were currently accepting any patients for OAT or if they accepted pregnant patients for treatment with OAT. Next, several questions were asked to determine differences between Medicaid, private insurance or self-pay acceptance for treatment. Data were then collected for wait times, overall and for pregnant women. Lastly, we collected data on the payment amount required for treatment. Our survey response rate was 59% (75% OTPs, 45% buprenorphine providers).
Analysis
Our primary outcomes were provider acceptance of insurance and treatment of pregnant women, our secondary outcome of patient wait time. Our exposures of interest were insurance type and pregnancy status. Descriptive analyses were conducted to determine bivariate associations between patient acceptances and wait times with insurance type and pregnancy status. Qualitative responses were reviewed, standardized and reported for treatment costs. Logistic models for dichotomous outcomes (e.g., patient acceptance) and negative binomial models were created for count variables (e.g., wait time), overall and for pregnant women. Logistic models were not performed for overall patient acceptance due to a high rate of acceptance in the sample. Predicted wait times were calculated applying models overall and for pregnant women. All analyses were completing using Stata 14.1 (College Station, Texas).
Results
We found that overall the majority (91%) of OAT providers were accepting new patients; however, providers were less likely to treat pregnant women (75%; p<0.01). There were differences among OAT providers with more OTPs accepting new patients than buprenorphine providers (97% vs 83%; p=0.01); however, rates of treating pregnant patients were lower in both (91% and 53%, respectively; p<0.01 for both). OTPs reported shorter wait times for treatment (1 day; IQR 0–3) than buprenorphine providers (7 days; IQR 2–14; p<0.01) and wait times were shorter among both OTPs (0 days; IQR 0–1) and buprenorphine providers (3.5 days; IQR1–7; p<0.01) for pregnant women (Table 1).
Table 1.
Patient and Insurance Acceptance, Treatments Offered and Wait Time, Among Opioid Treatment Programs and Buprenorphine Providers in Kentucky, North Carolina, Tennessee and West Virginia.
| Overall | Opioid Treatment Programs | Buprenorphine Providers | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n=113 | n=66 | n=47 | p value | ||||
| n | % | n | % | ||||
| Accepting new patients | 103 | 91.2% | 64 | 97.0% | 39 | 83.0% | 0.01 |
| Accepting pregnant patients | 85 | 75.2% | 60 | 90.9% | 25 | 53.2% | < 0.01 |
| Insurance Acceptance | |||||||
| Medicaid | 60 | 53.1% | 34 | 51.5% | 26 | 55.3% | 0.69 |
| Private insurance | 60 | 53.1% | 32 | 48.5% | 28 | 59.6% | 0.24 |
| Cash Pay | 108 | 95.6% | 66 | 100% | 42 | 89.4% | <0.01 |
|
| |||||||
| Median | IQR | Median | IQR | Median | IQR | ||
|
| |||||||
| Wait time, overall (days) | 2 | (0–7) | 1 | 0–3 | 7 | 2–14 | < 0.01 |
| Wait time, pregnant women (days)* | 1 | (0–2) | 0 | 0–1 | 3.5 | 1–7 | < 0.01 |
Among those accepting pregnant women.
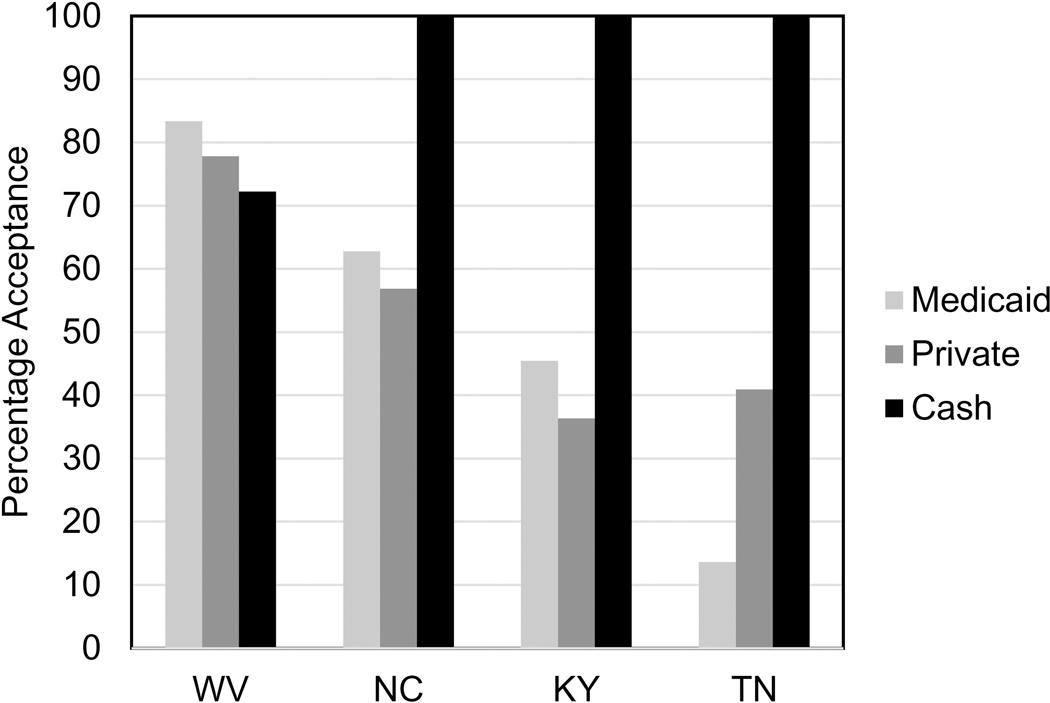
Both OTPs and buprenorphine providers accepted cash payments for services at high rates (OTP: 100%; buprenorphine 89.4%; p<0.01); Medicaid and private insurance were accepted at lower rates (Table 1). Medicaid acceptance ranged from a high of 83.3% in West Virginia to a low of 13.6% in Tennessee (p<0.01; Figure 2). Cash payments varied substantially; fees for treatment intake ranged from $20 to $175, treatment with buprenorphine $35 to $245 per week, and methadone $49 to $160 per week.
Figure 2.
Surveyed providers accepting Medicaid, Private Insurance or Cash Payments for Treatment of Opioid Use Disorder, Kentucky, North Carolina, Tennessee and West Virginia.
*Medicaid: p<0.01; Private Insurance p=0.04; Cash Payments <0.01
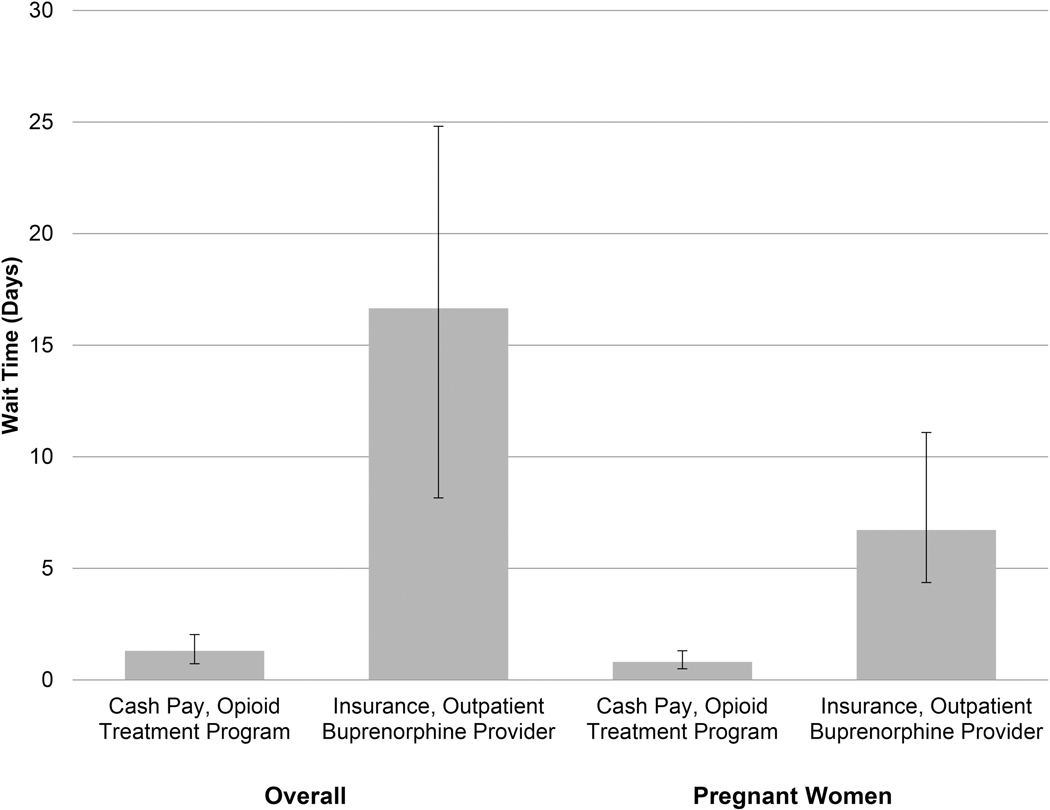
In adjusted models, accounting for insurance, provider type and state, providers were less likely to treat pregnant women if they took any insurance (aOR 0.15, 95%CI 0.03–0.68) or were a buprenorphine provider (aOR 0.09, 95%CI 0.02–0.37). Similarly, wait times were longer overall and for pregnant women with insurance and for buprenorphine providers (Table 2). Applying our models to specific patient scenarios, paying cash at an OTP had a predicted wait time of 1.3 days (95%CI 0.6–2.0); whereas, an individual with insurance (private or Medicaid) at a buprenorphine provider had a predicted wait time of 16.7 days (95%CI 8.5–24.8). Similarly, a pregnant woman paying cash at an OTP had a predicted wait time of 0.8 days (95%CI 0.3–1.3); whereas, a pregnant woman with insurance at a buprenorphine provider had a predicted wait time of 6.7 days (95%CI 2.4–11.1; Figure 3).
Table 2.
Models evaluating a) likelihood of acceptance of pregnant women, b) overall wait time and c) wait time for pregnant women, accounting for potential confounders.
| Pregnancy Accepted | Wait Time (overall) days | Wait Time (pregnant) Days | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| unadjusted | adjusted | unadjusted | adjusted | unadjusted | adjusted | ||||||||
| OR | 95%CI | aOR* | 95%CI | IRR | 95%CI | aIRR* | 95%CI | IRR | 95%CI | aIRR* | 95%CI | ||
| Cash Pay | reference | ||||||||||||
|
|
|||||||||||||
| Any Insurance | 0.36 | (0.11–1.16) | 0.15 | (0.03–0.68) | 2.14 | (1.08–4.23) | 2.3 | (1.26–4.16) | 1.45 | (0.66–3.17) | 1.64 | (0.80–3.36) | |
|
| |||||||||||||
|
| |||||||||||||
| Opioid Treatment Program | reference | ||||||||||||
|
|
|||||||||||||
| Outpatient Buprenorphine Provider | 0.14 | (0.05–0.42) | 0.09 | (0.02–0.37) | 2.11 | (3.84–3.08) | 5.56 | (3.11–9.96) | 5.37 | (2.66–10.83) | 5.04 | (2.39–10.66) | |
accounting for state, insurance acceptance and provider type
Model interpretation examples: a) Pregnancy accepted: In adjusted models, pregnant women are 85% less likely to be accepted if they have insurance compared to cash pay. B) Wait time (overall): In adjusted models, individuals with any insurance wait 2.3 times as long as an individual paying cash. C) Wait time (pregnant): In adjusted models, pregnant women with insurance wait 1.6 times as long as pregnant women without insurance for an appointment.
Figure 3.
Predicted Wait Time in Days by Insurance and Provider Type.
*Applying models, accounting for insurance, provider type and state.
g for insurance, provider type and state.
Discussion
In this study of opioid treatment providers, we found that OAT providers frequently did not accept any insurance and did not treat pregnant women in an area of the country disproportionately affected by the opioid epidemic. We found a six-fold difference in Medicaid acceptance rates based on state; however, Medicaid acceptance rates were still greater than those for private insurance in 3 of the 4 surveyed states. In our study, access to treatment was variable based upon geography, insurance type, type of provider and pregnancy status.
The opioid epidemic has grown in recent years in numbers affected and complexity. While deaths attributed to OPR have recently plateaued, deaths associated with heroin and fentanyl use have grown exponentially.26 Appalachia has been disproportionately impacted by opioid deaths. Notably, West Virginia, a state where all counties are designated to be Appalachian (https://www.arc.gov/counties), leads the nation in many opioid-related complications including, opioid overdose deaths,26 neonatal opioid withdrawal,8 and pregnant women with hepatitis C virus (likely secondary to injection drug use).27 Improving access to OAT in these states could improve relapse risk,13–16 infectious risks related to injection of opioids13,17 risk of overdose death18 and improve birth outcomes.19
Much of the existing literature focuses on supply of providers as a proxy for access to treatment.22 Our findings expand upon the existing literature by focusing on provider acceptance of insurance and pregnant women as well as inclusion of OTPs. While the availability of providers is an important metric, it may not be the only determinant of access to OAT. For example, a recent survey of outpatient buprenorphine providers in Ohio found that only about half of surveyed providers accepted insurance.28 Similarly, we found that only half of surveyed providers accepted any insurance. We also found that Medicaid acceptance varied 6-fold among surveyed states and that Medicaid was likely to be accepted by providers in three of the four surveyed states. Low-income populations and those in Medicaid have been disproportionately affected by the opioid epidemic and Medicaid serves as the primary payer for populations impacted by the epidemic.4,10,29 Our findings suggest that enhancing Medicaid acceptance, perhaps through reimbursement mechanisms, may decrease barriers to OAT.
Pregnant women with OUD are a particularly vulnerable population for whom access to OAT should be prioritized. OAT is the standard of care for pregnant women with OUD,30 and is associated with decreased rates of relapse,12 improved engagement in prenatal care,31 and improved birthweight.11,32 We found that wait times can be long, even several weeks, for pregnant women. A delay in care for this population could endanger mother and fetus. In addition, we found that providers, particularly outpatient buprenorphine providers were less likely to treat pregnant women. This finding is particularly important as data suggests buprenorphine may be superior for that some outcomes. For example, in a recent large randomized-controlled trial, infants exposed to buprenorphine, compared to methadone, had substantially sorter lengths of treatment and required less morphine for neonatal withdrawal.33 Despite its potential benefits, providers lack of willingness to treat pregnant women may be reflective of a lack of experience in caring for this population or may represent a training deficit, or even fear of litigation. Training efforts could focus on two specific domains: 1) training obstetricians to become DATA-waivered or 2) training current DATA-waivered providers to care for pregnant women.
Our findings should also be taken in the context of state policies focused on substance use in pregnancy. Nationwide, states vary substantially in policies related to substance use in pregnancy. For example, 24 states consider substance use in pregnancy to be child abuse, 23 states mandate healthcare providers report substance use in pregnancy and 7 mandate provider toxicology testing if substance use is suspected.34 In contrast, 19 states have created treatment programs specifically targeting pregnant women, 17 states provide priority treatment access to pregnant women in state-funded treatment programs and 10 states explicitly prohibit treatment centers from discriminating against pregnant women.34 States in our sample also vary in their approach to pregnant women. For example, West Virginia requires that programs that accept Medicaid dollars prioritize pregnant women for treatment; whereas, Kentucky and Tennessee have general requirements for prioritization of pregnant women.34 Only Kentucky mandates reporting and testing, and no state in our sample considers substance exposure to be child abuse.34 State policies, like prioritizing pregnant women to treatment, may enhance likelihood of treatment for this vulnerable population; whereas, more punitive policies may deter women from being forthcoming about their substance use.
As the opioid epidemic continues to grow in scope and complexity, state and federal policymakers are developing policy solutions and understanding barriers in obtaining OAT may inform future policy decisions. The President’s Commission on Combating Drug Addiction and the Opioid Crisis released their final report in November 2017.21 Among the recommendations from the Commission – expanding access to medication assisted treatment (MAT), including opioid agonist therapies (OAT) methadone and buprenorphine. As our findings suggest, a key to successful implementation of the Commission’s recommendations may be addressing provider (e.g., training) and insurance barriers (e.g., reimbursement). As The Commission’s recommendations are implemented, they could consider reducing wait times for individuals, especially pregnant women, as a goal.
Limitations
Our study has several important limitations. First, our analysis of four key states may limit generalizability to the rest of the US. In these four states our response rate was high among OTPs, but lower among outpatient buprenorphine providers and this may bias our results. Next, our survey may not reflect actual patient experience in obtaining an appointment. Our use of SAMHSA publicly available data for DATA-waivered providers does not represent all providers as providers are permitted to opt out of public listing. In addition, 41% of providers did not respond to our survey. As it is plausible that these providers were more likely to discriminate on the basis of expected revenue, the results reported here may underestimate differences in access due to different payment options. Lastly, we did not ascertain the intensity of care provided by OAT providers (e.g., ASAM Level of Care). Despite these limitations, our results highlight important barriers to OAT in an epicenter of the epidemic.
Conclusion
Improving insurance acceptance and reducing cost barriers, particularly for vulnerable populations such as pregnant women, should be considered as the state and federal governments develop strategies to improve outcomes for individuals with opioid use disorder. Medicaid programs could focus on evaluation of specific barriers, including reimbursement rates or other incentives, as a means to improve access. Improving training among DATA-waivered providers specifically for providers treat treating pregnant women, including obstetricians, may improve ability to access treatment in affected communities.
Acknowledgements:
The authors would like to acknowledge Brad Stein, MD, and the University of Chicago Survey Lab, and Mary White, MPH for their contributions to this paper.
Role of the Sponsor: The sponsor had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript or the decision to submit.
Footnotes
Declarations of competing interest: The authors have no conflicts of interests to disclose. Research reported in this publication was supported by the National Institute On Drug Abuse of the National Institutes of Health under award number K23DA038720. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References:
- 1.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. 2017;167(5):293–301. [DOI] [PubMed] [Google Scholar]
- 2.Epstein RA, Bobo WV, Martin PR, et al. Increasing pregnancy-related use of prescribed opioid analgesics. Ann Epidemiol. 2013;23(8):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123(5):997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307(18):1934–1940. [DOI] [PubMed] [Google Scholar]
- 5.Villapiano NL, Winkelman TN, Kozhimannil KB, Davis MM, Patrick SW. Rural and Urban Differences in Neonatal Abstinence Syndrome and Maternal Opioid Use, 2004 to 2013. JAMA pediatrics. 2017;171(2):194–196. [DOI] [PubMed] [Google Scholar]
- 6.Krans EE, Patrick SW. Opioid Use Disorder in Pregnancy: Health Policy and Practice in the Midst of an Epidemic. Obstet Gynecol. 2016;128(1):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MARYLAND MATERNAL MORTALITY REVIEW 2016 ANNUAL REPORT. Annapolis, Maryland: MARYLAND DEPARTMENT OF HEALTH AND MENTAL HYGIENE PREVENTION AND HEALTH PROMOTION ADMINISTRATION 2016. [Google Scholar]
- 8.Ko JY, Patrick SW, Tong VT, Patel R, Lind JN, Barfield WD. Incidence of Neonatal Abstinence Syndrome - 28 States, 1999–2013. MMWR Morb Mortal Wkly Rep. 2016;65(31):799–802. [DOI] [PubMed] [Google Scholar]
- 9.Tolia VN, Patrick SW, Bennett MM, et al. Increasing Incidence of the Neonatal Abstinence Syndrome in U.S. Neonatal ICUs. N Engl J Med. 2015;372(22):2118–2126. [DOI] [PubMed] [Google Scholar]
- 10.Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35(8):650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070–1076. [DOI] [PubMed] [Google Scholar]
- 12.Fullerton CA, Kim M, Thomas CP, et al. Medication-assisted treatment with methadone: assessing the evidence. Psychiatr Serv. 2014;65(2):146–157. [DOI] [PubMed] [Google Scholar]
- 13.Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. Jama. 2000;283(10):1303–1310. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy JJ, Leamon MH, Parr MS, Anania B. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193(3 Pt 1):606–610. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz RP, Kelly SM, O’Grady KE, Gandhi D, Jaffe JH. Interim methadone treatment compared to standard methadone treatment: 4-month findings. Journal of substance abuse treatment. 2011;41(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz RP, Kelly SM, O’Grady KE, Gandhi D, Jaffe JH. Randomized trial of standard methadone treatment compared to initiating methadone without counseling: 12-month findings. Addiction. 2012;107(5):943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson ME, Schwartz RP, O’Grady KE, Jaffe JH. Impact of interim methadone maintenance on HIV risk behaviors. Journal of urban health : bulletin of the New York Academy of Medicine. 2010;87(4):586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz RP, Gryczynski J, O’Grady KE, et al. Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995–2009. Am J Public Health. 2013;103(5):917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kandall SR, Albin S, Lowinson J, Berle B, Eidelman AI, Gartner LM. Differential effects of maternal heroin and methadone use on birthweight. Pediatrics. 1976;58(5):681–685. [PubMed] [Google Scholar]
- 20.Curda EH. Opioid Addiction: Laws, Regulations and Other Factors can Affect Medication-Assisted Treatment Access. Washington, D.C.: Government Accountability Office;2016. [Google Scholar]
- 21.Crisis TPsCoCDAatO. Final Report. Washington, D.C.: Executive Office of the President, The White House;2017. [Google Scholar]
- 22.Hirchak KA, Murphy SM. Assessing Differences in the Availability of Opioid Addiction Therapy Options: Rural Versus Urban and American Indian Reservation Versus Nonreservation. The Journal of rural health : official journal of the American Rural Health Association and the National Rural Health Care Association. 2017;33(1):102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin CE, Longinaker N, Terplan M. Recent trends in treatment admissions for prescription opioid abuse during pregnancy. Journal of substance abuse treatment. 2015;48(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guy GP Jr., Zhang K, Bohm MK, et al. Vital Signs: Changes in Opioid Prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Number and age-adjusted rates of drug overdose deaths by state, US 2015. 2017; https://www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed June 16, 2017.
- 26.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths--United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–1382. [DOI] [PubMed] [Google Scholar]
- 27.Patrick SW, Bauer AM, Warren MD, Jones TF, Wester C. Hepatitis C Virus Infection Among Women Giving Birth - Tennessee and United States, 2009–2014. MMWR Morb Mortal Wkly Rep. 2017;66(18):470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parran TV, Muller JZ, Chernyak E, et al. Access to and Payment for Office-Based Buprenorphine Treatment in Ohio. Substance abuse : research and treatment. 2017;11:1178221817699247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patrick SW, Dudley J, Martin PR, et al. Prescription opioid epidemic and infant outcomes. Pediatrics. 2015;135(5):842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Committee Opinion No. 711: Opioid Use and Opioid Use Disorder in Pregnancy. Obstet Gynecol. 2017;130(2):e81–e94. [DOI] [PubMed] [Google Scholar]
- 31.Prevention CfDCa. Prescription Opioid Overdose Data. 2016; http://www.cdc.gov/drugoverdose/data/overdose.html. Accessed December 5, 2016.
- 32.Meyer MC, Johnston AM, Crocker AM, Heil SH. Methadone and buprenorphine for opioid dependence during pregnancy: a retrospective cohort study. J Addict Med. 2015;9(2):81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Substance Use During Pregnancy. 2018; https://www.guttmacher.org/state-policy/explore/substance-use-during-pregnancy. Accessed March 8, 2018.