Abstract
We previously reported that inactivation of rdxA and/or frxA converted Helicobacter pylori from metronidazole sensitive to metronidazole resistant. To examine the individual roles of rdxA and frxA in the development of metronidazole resistance in H. pylori, we examined the status of rdxA and frxA from 12 pairs of metronidazole-sensitive and -resistant H. pylori isolates obtained following unsuccessful therapy containing metronidazole. Arbitrary primed fingerprinting analyses revealed that the genotypes of 11 sensitive and resistant pairs of strains were essentially identical. Amino acid sequence identities of RdxA and FrxA from the 14 metronidazole-sensitive isolates ranged from 92 to 98% and 95 to 98%, respectively, compared to that of H. pylori J99 (MIC, 1 μg/ml). All strains with high-level metronidazole resistance (MICs, 128 μg/ml) contained premature truncation of both RdxA and FrxA caused by nonsense and/or frameshift mutations. Strains with intermediate resistance to metronidazole (MICs, 32 to 64 μg/ml) contained a single premature truncation and/or altered RdxA and FrxA caused by nonsense, frameshift, and unique missense mutations. The low-level metronidazole-resistant strains (MICs, 8 μg/ml) contained unique missense mutations in FrxA but no specific changes in RdxA. The results demonstrate that alterations in both the rdxA and frxA genes are required for moderate and high-level metronidazole resistance and that metronidazole resistance that develops during anti-H. pylori therapy containing metronidazole is most likely to involve a single sensitive strain infection rather than a coinfection with a metronidazole-resistant strain.
Over 50% of the world population is infected with the important human pathogen Helicobacter pylori. The primary impediments to successful H. pylori treatment are lack of compliance with drug regimens and the presence of antibiotic-resistant H. pylori (10). Metronidazole was a critical ingredient of the first successful therapy for H. pylori and remains a major component of newer proton pump inhibitor-containing quadruple therapies (2, 12). The background prevalence of metronidazole resistance is high in the United States (20 to 45% of isolates) and is increased in those who have used the drug for other indications (23). Effective therapy for H. pylori infection requires two or more antimicrobial agents often given with antisecretory drugs to reduce gastric acidity (2, 12). Metronidazole resistance decreases the effectiveness of metronidazole-containing anti-H. pylori therapies (2, 5).
The antimicrobial action of metronidazole is dependent on reductive activation of metronidazole by the redox system of the target cell. Theoretically, any redox system possessing a reduction potential that is more negative than that of metronidazole in the cell would donate its electrons preferentially to metronidazole and lead to reductive activation (7, 17). Goodwin et al. reported that oxygen-insensitive NADPH nitroreductase (rdxA) was a putative metronidazole nitroreductase-encoding gene involved in metronidazole resistance (9). Several investigators confirmed that observation (3, 14, 20) and recently reported on an additional putative metronidazole nitroreductase-encoding gene (NADPH flavin oxidoreductase; frxA) involved in metronidazole resistance (15, 16, 18, 20). In this report, we examined 12 metronidazole-sensitive and -resistant H. pylori pairs to assess the status of both the rdxA and frxA genes in relation to the presence of mutations and level of metronidazole resistance. Each pair was obtained from individual patients who were initially infected with metronidazole-sensitive H. pylori and had metronidazole-resistant isolates following failed therapy with a metronidazole-containing regimen.
MATERIALS AND METHODS
H. pylori strains and culture conditions.
Paired metronidazole-sensitive and -resistant H. pylori isolates were identified from culture records of individual patients enrolled in clinical trials at the Gastroenterology Section of the Veterans Affairs Medical Center in Houston, Texas. H. pylori strains initially harvested as multiple colonies from individual gastric mucosal biopsy specimens (11) were cultured on brain heart infusion (Difco, Detroit, Mich.) agar plates containing 5% horse blood under microaerobic atmospheric conditions (10% CO2 and 5% O2) at 37°C for 2 to 3 days. Luria-Bertani (24) broth or Luria-Bertani agar plates were used to grow Escherichia coli DH5α (Gibco BRL) for general recombinant DNA manipulation.
Determination of MICs.
MICs were determined by the agar dilution method of the NCCLS (22), with a minor modification. Agar dilution plates were prepared using Mueller-Hinton agar (Difco) containing 5% sheep blood supplemented with twofold serial dilutions of metronidazole (Sigma Chemical Co., St. Louis, Mo.) ranging from 0.25 to 256 μg/ml. Fresh H. pylori isolates (2- to 3-day cultures) were prepared in sterile saline and adjusted to a no. 2 McFarland standard. Using a Steers-type replicating device, 1 to 5 μl of the adjusted inocula was delivered to the agar dilution plates. All plates were incubated under CampyPak Plus conditions (Becton Dickinson BBL, Cockeysville, Md.) for 3 days. The MIC was defined as the lowest concentration of metronidazole that completely inhibited the growth of the inoculum. Metronidazole-resistant H. pylori ATCC 43504 was used as a quality control organism. Any test for which the MIC for the quality control organism was outside the approved range (64 to 256 μg of metronidazole/ml) was discarded, and the test was repeated.
AP genomic fingerprinting.
Forty nanograms of genomic DNA purified from multiple colony expansion of the 12 paired strains was added to a PCR mixture consisting of 10 mM Tris-Cl (pH 9.0), 50 mM KCl, 0.1% Triton X-100, 3 mM MgCl2, 1 U of Taq polymerase (Promega, Madison, Wis.), and 20 pmol of primer pMAV 17B (5′-CACTCGTCGGGAATGCCCT-3′) in a previously described PCR assay (13). Amplification was carried out in an MJ Research thermocycler (New York, N.Y.) for 40 cycles as follows: 94°C for 30 s, 37°C for 1 min, and 72°C for 1 min. Aliquots of the PCR-amplified product (10 to 20 μl) were resolved in 1% agarose gels containing 0.5× TAE (0.04 M Tris-acetate, 0.001 M EDTA) and stained with ethidium bromide. Arbitrary primed (AP)-PCR amplifications were performed twice to determine the reproducibility of the genomic DNA fingerprinting for each H. pylori isolate. The DNA band patterns were visualized by ethidium bromide staining under a short-wavelength UV light source and were photographed with Polaroid 667 film.
PCR amplification of rdxA and frxA genes.
Genomic DNA from the 12 metronidazole-sensitive and -resistant H. pylori pairs was extracted as described previously (19). Oligonucleotide PCR primers to amplify the rdxA and frxA genes were used as shown in Table 1. Two pairs of oligonucleotide primers for rdxA were used to generate a 427-bp fragment for the NH3 terminus of RdxA (primer pair ERD-1/BRD-3) and a 351-bp fragment for the COOH portion of RdxA (primer pair ERD-2/BRD-4). The two fragments overlap over 41 bp between primers ERD-2 and BRD-3 (in the middle of rdxA), including a full length of rdxA. The primer ERD-1 was designed for positions −54 to −33 upstream of the first rdxA codon and BRD-4 was designed for positions 26 to 45 downstream of the rdxA stop codon. Similarly, two pairs of oligonucleotide primers for frxA were used to generate a 445-bp fragment for the NH3 portion of FrxA (primer pair EFR-1/BFR-3) and a 388-bp fragment for the COOH portion of FrxA (primer pair EFR-2/BFR-4). The two fragments overlap over 42 bp between primers EFR-2 and BFR-3 (in the middle of frxA), including a full length of frxA. The primer EFR-1 was designed for positions −79 to −58 upstream of the first frxA codon and the primer BFR-4 was designed for positions 29 to 48 downstream of the frxA stop codon. The PCR amplification and thermocycler conditions were identical to those described previously (20). The amplified PCR fragments were inserted into the pBluescript SK vector (Stratagene, La Jolla, Calif.) for DNA sequencing analysis.
TABLE 1.
PCR primer pairs used to amplify a portion of H. pylori genes
| Primer pair (forward primer/ reverse primer) | Encoded protein (gene [genome sequence designation]) | Nucleotide sequencea (5′→3′) | Size (bp) of PCR fragment |
|---|---|---|---|
| ERD-1/BRD-3 | Oxygen-insensitive NADPH nitroreductase (rdxA [HP0954]) | GTTAGGGATTTTATTGTATG | 427 |
| ACGCCAAGCATTTGAGCAAA | |||
| ERD-2/BRD-4 | Oxygen-insensitive NADPH nitroreductase (rdxA [HP0954]) | AAAGTTAGAGTGATCCCCTC | 351 |
| TAATTTAGGTTTGATTATAG | |||
| EFR-1/BFR-3 | NADPH flavin oxidoreductase (frxA [HP0642]) | TCTCAAGCGGAAAAATCCGG | 445 |
| AATTTTTGATGATTTGAGCG | |||
| EFR-2/BFR-4 | NADPH flavin oxidoreductase (frxA [HP0642]) | ATTATGACACTAATTCTAGG | 388 |
| CTAACGCCAAGCTTTTTATG |
Nucleotide sequences were obtained from the H. pylori complete genome sequence (26).
DNA sequence determination and analysis.
DNA sequences of the cloned fragments were determined for both strands at the Molecular Genetics Core Facility of Baylor College of Medicine. DNA sequence determinations of the cloned fragments were repeated by SEQWRITE, Houston, Tex., to confirm the identity of the DNA sequences. In parallel, PCR amplification and DNA sequence determination of the known rdxA and frxA genes from H. pylori ATCC 700392 (26) were also performed to ensure PCR amplification fidelity. The resulting DNA sequences were analyzed by the Genetics Computer Group Programs (4), and the similarity search of the deduced protein sequences was performed by the BLASTX algorithm of the National Center for Biotechnology Information.
RESULTS
Identification of metronidazole-sensitive and -resistant pairs of H. pylori.
Isolates from 12 duodenal ulcer patients who had pretreatment for metronidazole-sensitive H. pylori and after failing anti-H. pylori therapy containing metronidazole had metronidazole-resistant H. pylori were selected for this study. The 12 patients visited the Veterans Affairs Medical Center between 1994 and 1998 and underwent follow-up endoscopy 1 to 6 months after completing therapy (Table 2). The MICs of metronidazole for the 12 metronidazole-sensitive and -resistant pairs were confirmed by agar dilution MIC measurement and are shown in Table 2. The MICs for all metronidazole-sensitive strains were 0.5 to 2 μg of metronidazole/ml and for the metronidazole-resistant strains that emerged following therapy were 8 to 128 μg of metronidazole/ml.
TABLE 2.
MICs of metronidazole for the 12 pairs of metronidazole-sensitive and -resistant H. pylori isolates from 12 duodenal ulcer patients
| Isolate no.a | H. pylori strain | Metronidazole MIC (μg/ml) | Visit date in hospital (mo-day-yr) |
|---|---|---|---|
| 1a | 5032 | 1 | 3-2-98 |
| 1b | 5413 | 8 | 4-13-98 |
| 2a | 6826 | 1 | 8-26-98 |
| 2b | 6102 | 8 | 10-2-98 |
| 3a | 1769 | 2 | 12-9-94 |
| 3b | 1838 | 32 | 3-17-95 |
| 4a | 6223 | 2 | 2-23-98 |
| 4b | 6413 | 32 | 4-13-98 |
| 5a | 1869 | 1 | 5-11-95 |
| 5b | 1950 | 64 | 7-31-95 |
| 6a | 2024 | 1 | 2-4-98 |
| 6b | 2312 | 64 | 3-12-98 |
| 7a | 2106 | 2 | 1-8-96 |
| 7b | 2228 | 128 | 7-2-96 |
| 8a | 1678 | 1 | 4-28-94 |
| 8b | 1740 | 128 | 9-26-94 |
| 9a | 1365 | 1 | 1-11-94 |
| 9b | 1857 | 128 | 4-25-94 |
| 10a | 5047 | 0.5 | 4-7-98 |
| 10b | 5078 | 128 | 5-13-98 |
| 11a | 2323 | 1 | 3-23-98 |
| 11b | 2511 | 128 | 5-11-98 |
| 12a | 8523 | 1 | 5-22-98 |
| 12b | 8076 | 128 | 7-6-98 |
Isolate numbers with an “a” indicate before and with a “b” indicate after anti-H. pylori therapy containing metronidazole.
Genotype analysis of the metronidazole-sensitive and -resistant H. pylori paired isolates.
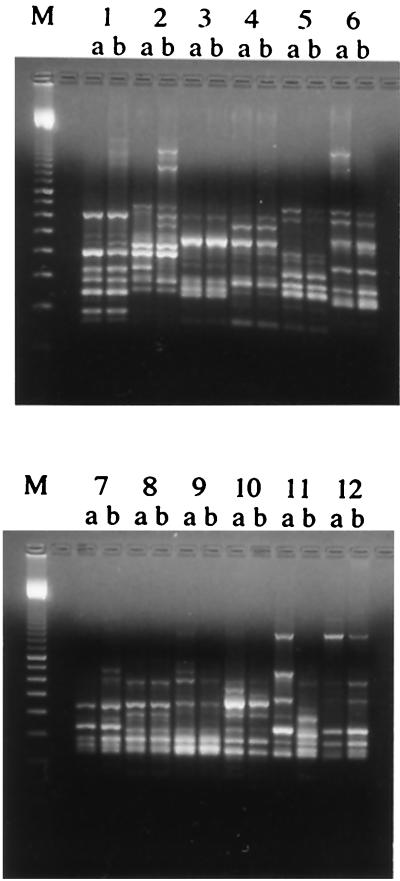
AP-PCR genomic fingerprinting analysis was performed to investigate the genotypes of the 12 paired strains pre- and posttherapy and to assess the genetic relatedness of the strains. Interstrain variation (comparison between individuals) was very high, as expected, while intrastrain variation (pairs from the same individual) was very low, with one exception (Fig. 1). The genotype pairs of patients 2, 6, 7, 9, and 10 showed more intrastrain variation than did the other pairs, although all the major bands were the same. The H. pylori genotype from patient 11 showed a different banding pattern before and after therapy, with the two patterns being as different as the patterns of isolates from other patients and representing completely different strains of H. pylori, suggesting reinfection with a different strain or the emergence of a minor subpopulation.
FIG. 1.
Genomic fingerprinting patterns by AP-PCR. Each pair of H. pylori isolates except that for patient 11 showed similar banding patterns. Isolates 2a and b, 6a and b, 7a and b, 9a and b, and 10a and b showed a higher intrastrain variation than did the other pairs, although the major bands corresponded, which contrasts with isolates 11a and b, for which all the bands were different. M, molecular size marker.
Amino acid sequence comparisons of RdxA and FrxA from the metronidazole-sensitive isolates.
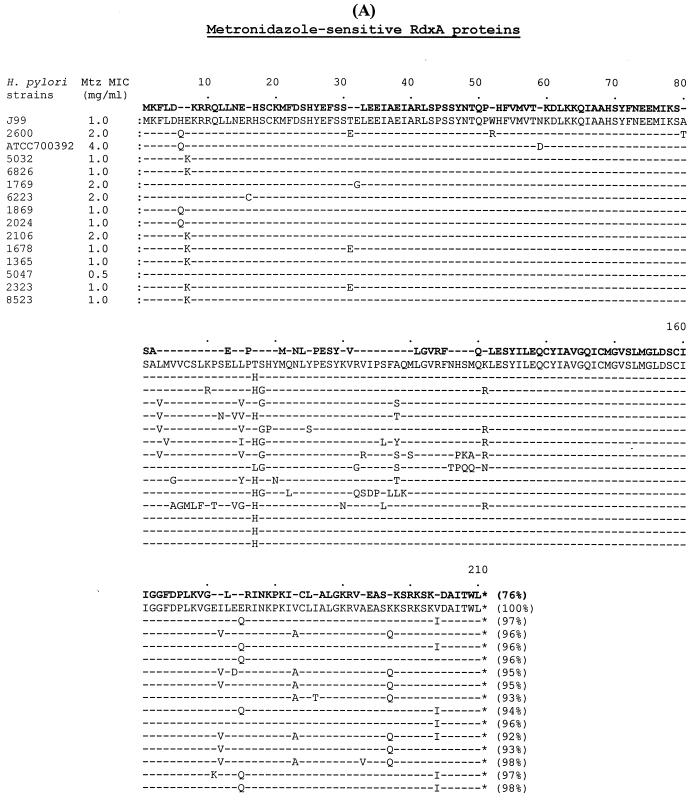
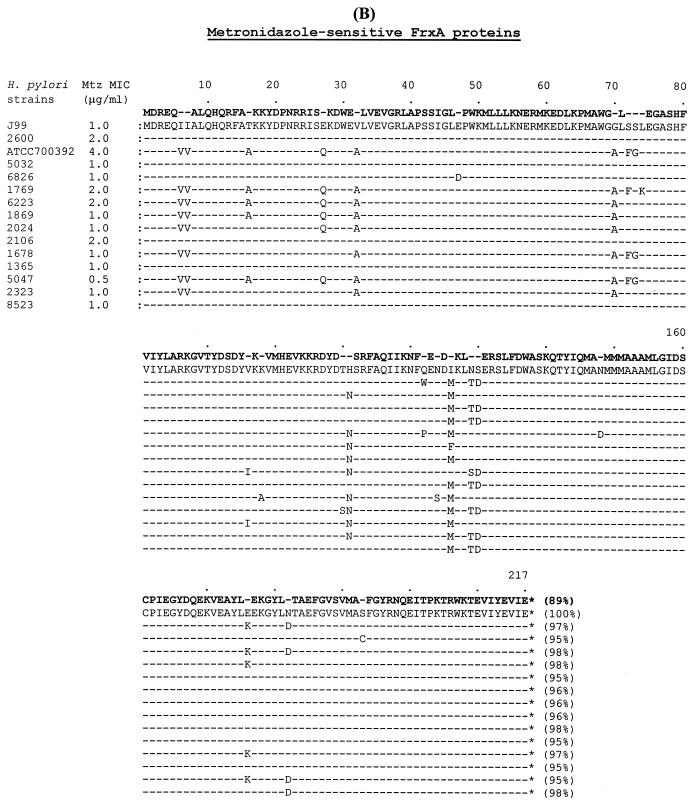
Amino acid sequences from the 12 metronidazole-sensitive isolates were aligned with those from metronidazole-sensitive H. pylori J99 (MIC, 1 μg of metronidazole/ml) (1), ATCC 700392 (MIC, 4 μg/ml) (26), and 2600 (MIC, 2 μg/ml) (20). As shown in Fig. 2A, the amino acid identity among the 14 RdxA proteins ranged from 92 to 98% compared to RdxA of H. pylori J99. However, the variability of amino acid sequences in the RdxA proteins did not relate to the MICs, suggesting that these amino acid changes did not directly relate to the functional activity of the enzyme. One hundred sixty of the 210 amino acids of RdxA (76%) were conserved (100% identity) among the 15 metronidazole-sensitive strains. The amino acid identity among the 14 FrxA proteins ranged from 95 to 98% compared to FrxA of H. pylori J99 (Fig. 2B). The variability of amino acid sequences in the FrxA protein was also not related to the MICs. One hundred ninety-four of the 217 amino acids of FrxA (89%) were conserved (100% identity) among the 15 metronidazole-sensitive strains. FrxA varied less than RdxA did based on the proportion of conserved amino acids, 76% for RdxA and 89% for FrxA.
FIG. 2.
Comparison of RdxA and FrxA amino acid sequences from the 12 metronidazole-sensitive pretreatment H. pylori isolates, including metronidazole-sensitive H. pylori J99 (MIC, 1.0 μg/ml) (1), ATCC 700392 (MIC, 4.0 μg/ml) (26), and 2600 (MIC, 2.0 μg/ml) (20). (A) Amino acid sequence alignment of RdxA proteins. (B) Amino acid sequence alignment of FrxA proteins. The percent identity of the amino acids was compared to the proteins of H. pylori J99. Bold type in the first line shows absolutely conserved amino acids (100% identity) among all compared proteins. Mtz, metronidazole.
RdxA and FrxA amino acid sequence comparisons between paired metronidazole-sensitive and -resistant isolates.
Amino acid sequence comparisons of RdxA between the 12 metronidazole-sensitive and -resistant pairs of isolates showed that 8 metronidazole-resistant strains contained prematurely truncated RdxA caused by the presence of nonsense mutations (strains 1838, 6413, 1740, 1857, 2511, and 8076) or frameshift mutations (strains 2228 and 5078). Two resistant strains (1950 and 2312) contained amino acid substitutions (missense mutations) not found in the 12 metronidazole-sensitive strains, strain ATCC 700392 (26), or J99 (1). Two other low-level (8 μg/ml) metronidazole-resistant strains (5413 and 6102) contained unchanged RdxA compared to the sequences of the metronidazole-sensitive partner (Table 3).
TABLE 3.
Pairwise amino acid and nucleotide sequence analysis of RdxA proteins from 11 pairs of metronidazole-sensitive and -resistant H. pylori isolates
| H. pylori strain | MIC (μg/ml) | Genetic alterationsa in:
|
|
|---|---|---|---|
| Amino acid sequenceb | Nucleotide sequencec | ||
| 5413 | 8 | No specific change | No specific changed |
| 6102 | 8 | No specific change | No specific change |
| 1838 | 32 | W at position 209 to stop codon | Nonsense mutation (G to A at position 626) |
| 6413 | 32 | W at position 209 to stop codon | Nonsense mutation (G to A at position 627) |
| 1950 | 64 | D at position 157 to N | Missense mutation (G to A at position 467)e |
| 2312 | 64 | G at position 189 to C; A at position 206 to T | Missense mutations (G to T at position 565; G to A at position 616)e |
| 2228 | 128 | Generated a stop codon at position 15 | Frameshift due to one nucleotide (T) addition at position 17 |
| 1740 | 128 | Q at position 50 to stop codon | Nonsense mutation (C to T at position 148) |
| 1857 | 128 | E at position 35 to stop codon | Nonsense mutation (G to T at position 103) |
| 5078 | 128 | Generated a stop codon at position 98 | Frameshift due to one nucleotide (A) addition at position 228 |
| 8076 | 128 | Q at position 50 to stop codon | Nonsense mutation (C to T at position 148) |
Pairwise amino acid and nucleotide sequence alignment was performed against the metronidazole-sensitive partner strain from the same patient who failed anti-H. pylori therapy containing metronidazole. If the RdxA proteins from metronidazole-resistant strains were not truncated, the amino acid sequences were aligned not only with the partner metronidazole-sensitive strain but also with all other metronidazole-sensitive RdxA amino acid sequences, including H. pylori ATCC 700392 (26) and H. pylori J99 (1).
The initiation amino acid (methionine) was counted as +1.
Nucleotide A from the first codon (ATG) for RdxA was counted as +1.
No specific change, no nucleotide substitution for the specific amino acid change.
Amino acid sequence comparisons of FrxA between the 12 metronidazole-sensitive and -resistant pairs of isolates revealed that the 9 metronidazole-resistant strains contained prematurely truncated FrxA caused by nonsense mutations (strains 2312 and 1857) or frameshift mutations (strains 1838, 1950, 2228, 1740, 5078, 2511, and 8076). The other 3 resistant strains (5413, 6102, and 6413) contained amino acid substitutions (missense mutations) not found in the 12 metronidazole-sensitive strains, strain ATCC 700392 (26), or J99 (1) (Table 4).
TABLE 4.
Pairwise amino acid and nucleotide sequence analysis of FrxA proteins from 11 pairs of metronidazole-sensitive and -resistant H. pylori isolates
| H. pylori strain | MIC (μg/ml) | Genetic alterationsa in:
|
|
|---|---|---|---|
| Amino acid sequenceb | Nucleotide sequencec | ||
| 5413 | 8 | R at position 206 to H | Missense mutation (G to A at position 617)d |
| 6102 | 8 | W at position 137 to R; E at position 164 to G | Missense mutations (T to A at position 409; A to G at position 491)d |
| 1838 | 32 | Generated a stop codon at position 46 | Frameshift due to one nucleotide (G) deletion at position 46 |
| 6413 | 32 | C at position 161 to Y | Missense mutation (G to A at position 482) |
| 1950 | 64 | Generated a stop codon at position 74 | Frameshift due to one nucleotide (G) deletion at position 208 |
| 2312 | 64 | W at position 49 to stop codon | Nonsense mutation (G to A at position 146) |
| 2228 | 128 | Generated a stop codon at position 74 | Frameshift due to one nucleotide (G) deletion at position 208 |
| 1740 | 128 | Generated a stop codon at position 74 | Frameshift due to one nucleotide (G) deletion at position 208 |
| 1857 | 128 | Q at position 168 to stop codon | Nonsense mutation (C to T at position 505) |
| 5078 | 128 | Generated stop codons at positions 22 and 126 | Frameshift due to one nucleotide (A) addition at position 55 and one nucleotide (A) deletion at position 316 |
| 8076 | 128 | Generated a stop codon at position 74 | Frameshift due to one nucleotide (G) deletion at position 208 |
Pairwise amino acid and nucleotide sequence alignment was performed against the metronidazole-sensitive partner strain from the same patient who failed anti-H. pylori therapy containing metronidazole. If the FrxA proteins from metronidazole-resistant strains were not truncated, the amino acid sequences were aligned not only with the partner metronidazole-sensitive strain but also with all other metronidazole-sensitive FrxA amino acid sequences, including H. pylori ATCC 700392 (26) and H. pylori J99 (1).
The initiation amino acid (methionine) was counted as +1.
Nucleotide A from the first codon (ATG) for FrxA was counted as +1.
Genetic alterations in both the RdxA and FrxA proteins from the metronidazole-resistant strains, including six high-level metronidazole-resistant strains (MIC, 128 μg/ml) (strains 2228, 1740, 1857, 5078, 2511, and 8076) and one moderate-level (MIC, 32 μg/ml) metronidazole-resistant strain (1838), were in the form of truncated RdxA and FrxA caused by nonsense and frameshift mutations. Three strains with moderately resistant H. pylori (MIC, 32 to 64 μg/ml) (strains 6413, 1950, and 2312) contained a single truncated RdxA or FrxA with correspondingly altered FrxA or RdxA caused by nonsense, frameshift, or unique missense mutations. The two low-level (MIC, 8 μg/ml) metronidazole-resistant strains (5413 and 6102) had untruncated RdxA and FrxA proteins. Both contained unique amino acid substitutions in the FrxA protein with no specific amino acid change in the RdxA protein (Tables 3 and 4).
DISCUSSION
We previously reported that functional inactivation of rdxA and frxA from metronidazole-sensitive H. pylori strains conferred resistance to metronidazole (18, 20). We also previously examined 17 randomly selected metronidazole-resistant H. pylori isolates from North America and found frameshift mutations in rdxA in all isolates associated with metronidazole resistance (21). This study used paired isolates to investigate the roles of both rdxA and frxA in the development of metronidazole resistance. We documented the emergence of posttherapy resistance to metronidazole among patients who had failed therapy with a metronidazole-containing anti-H. pylori regimen. The emergence of posttherapy resistance to metronidazole is most likely due to the mutagenic activity of metronidazole in the cells. The mutagenic activity of metronidazole was proven in H. pylori (25).
Neither rdxA nor frxA is a lethal gene for H. pylori survival (20), and metronidazole has been shown to be mutagenic in H. pylori (25), which is possibly responsible for the observation that clinical use of metronidazole often results in the development of metronidazole resistance. The 12 metronidazole-resistant isolates had metronidazole MICs that ranged from 8 to 128 μg/ml, reflecting the typical distribution seen among clinical H. pylori metronidazole-resistant isolates (6, 27), and the 12 metronidazole-sensitive isolates had an average metronidazole MIC of 1 μg/ml. The results of this study are consistent with the notion that metronidazole susceptibility is dependent on the level of nitroreductase activity produced by rdxA and frxA, and the dual inactivation of these genes conferred high-level resistance, with MICs ranging from 128 to 256 μg of metronidazole/ml (18, 20). It is likely that MICs of less than 128 μg/ml are caused by partial and/or incomplete inactivation of the genes.
The results are also consistent with metronidazole-resistant strains most commonly developing from metronidazole-sensitive strains rather than from coinfection with a sensitive and a resistant strain (i.e., a mixed infection of sensitive and resistant strains). The one individual with different strains pre- and posttherapy (patient 11) could have been reinfected by a different strain of H. pylori between the end of treatment and follow-up, or the patient could have been initially infected with two different strains of H. pylori, with the sensitive strain growing at a higher density than the resistant strain. The frequency of each pathway can be established by performance of further AP-PCR experiments (8) on individual colonies from each isolate of patient 11 because the template DNA used for AP-PCR was purified from multiple colony expansion. The other 11 AP-PCR fingerprinting genotype patterns between the paired isolates before and after therapy were almost identical.
It is of interest that mutations in both rdxA and frxA associated with metronidazole resistance did not show either a specific type of mutation or a specific position in either gene. For this study, frameshift mutations appeared to be the major cause for the premature truncation of FrxA and nonsense mutations appeared to be the major cause for the premature truncation of RdxA. However, in our previous study frameshift mutations were the major cause for the premature truncation of RdxA (21), suggesting that either type of mutation may be involved for either gene. The analysis of mutations from the 12 metronidazole-resistant isolates showed that there was no specific position at which the substitution, insertion, or deletion typically occurs to produce metronidazole resistance. All six strains that developed high-level metronidazole resistance (MICs of 128 μg/ml) contained premature truncations of both RdxA and FrxA. The four strains with metronidazole MICs between 32 and 64 μg/ml contained a single premature gene truncation and/or unique missense mutations in both RdxA and FrxA. However, low-level metronidazole-resistant strains (MICs, 8 μg/ml) contained unique missense mutations in FrxA with no specific change in RdxA (Table 5). Interestingly, strain 1838 contained premature truncations in both RdxA and FrxA but did not develop high-level metronidazole resistance as was shown for the other six strains. Premature truncation resulted in an RdxA protein that was only one amino acid shorter than the full-length enzyme (i.e., nonsense mutation at amino acid 209 of 210) such that it still retained some metronidazole nitroreductase activity. This isolate was thus more susceptible to metronidazole than isolates having complete RdxA and FrxA truncation. The results presented in this study verify the involvement of FrxA in metronidazole resistance among clinical isolates as shown previously (15, 16, 18, 20). In addition, the results could explain previous observations that some metronidazole-resistant strains containing an intact RdxA demonstrate a wide range of metronidazole MICs (3, 14). In summary, these findings confirm that dual rdxA and frxA inactivation is required for production of high-level metronidazole resistance, whereas a single rdxA or frxA inactivation may result in low- or moderate-level metronidazole resistance (20).
TABLE 5.
Comparison of RdxA and FrxA alterations in metronidazole-resistant H. pylori
| H. pylori strain(s) | Alterationa in:
|
MIC (μg/ml) | |
|---|---|---|---|
| RdxA | FrxA | ||
| 1740, 1857, 2228, 2511, 5078, 8076 | − | − | 128 |
| 1950 | Δ | − | 64 |
| 2312 | Δ | − | 64 |
| 1838 | − | − | 32 |
| 6413 | − | Δ | 32 |
| 5413 | N | Δ | 8 |
| 6102 | N | Δ | 8 |
−, premature truncation; Δ, unique missense mutation; N, no specific change.
ACKNOWLEDGMENTS
This work was supported in part by the Department of Veterans Affairs. K. Hulten was supported by a postdoctoral grant from the Swedish Society for Medical Research.
REFERENCES
- 1.Alm R A, Ling L L, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, Carmel G, Tummino P J, Caruso A, Uria-Nickelsen M, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jinag Q, Taylor D E, Vovis G F, Trust T J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 2.Chiba N, Rao B V, Rademaker J W, Hunt R H. Meta-analysis of the efficacy of antibiotic therapy in eradicating Helicobacter pylori. Am J Gastroenterol. 1992;87:1716–1727. [PubMed] [Google Scholar]
- 3.Debets-Ossenkopp Y J, Pot R G, van Westerloo D J, Goodwin A, Vandenbroucke-Grauls C M, Berg D E, Hoffman P S, Kusters J G. Insertion of mini-IS605 and deletion of adjacent sequences in the nitroreductase (rdxA) gene cause metronidazole resistance in Helicobacter pylori NCTC11637. Antimicrob Agents Chemother. 1999;43:2657–2662. doi: 10.1128/aac.43.11.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dore M P, Leandro G, Realdi G, Sepulveda A R, Graham D Y. Effect of pretreatment antibiotic resistance to metronidazole and clarithromycin on outcome of Helicobacter pylori therapy: a meta-analytical approach. Dig Dis Sci. 2000;45:68–76. doi: 10.1023/a:1005457226341. [DOI] [PubMed] [Google Scholar]
- 6.Dore M P, Osato M S, Kwon D H, Graham D Y, El-Zaatari F A K. Demonstration of unexpected antibiotic resistance of genotypically identical Helicobacter pylori isolates. Clin Infect Dis. 1998;27:84–89. doi: 10.1086/514640. [DOI] [PubMed] [Google Scholar]
- 7.Edwards D I. Nitroimidazole drugs—action and resistance mechanisms. I. Mechanisms of action. J Antimicrob Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 8.Enroth H, Bjorkholm B, Engstrand L. Occurrence of resistance mutation and clonal expansion in Helicobacter pylori multiple strain infection: a potential risk in clarithromycin-based therapy. Clin Infect Dis. 1999;28:1305–1307. doi: 10.1086/514796. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin A, Kersulyte D, Sisson G, Veldhuyzen van Zanten S J O, Berg D E, Hoffman P S. Metronidazole-resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol Microbiol. 1998;28:383–393. doi: 10.1046/j.1365-2958.1998.00806.x. [DOI] [PubMed] [Google Scholar]
- 10.Graham D Y, de Boer W A, Tytgat G N. Choosing the best anti-Helicobacter pylori therapy: effect of antimicrobial resistance. Am J Gastroenterol. 1996;91:1072–1076. [PubMed] [Google Scholar]
- 11.Hachem C Y, Clarridge J E, Evans D G, Graham D Y. Comparison of agar based media for primary isolation of Helicobacter pylori. J Clin Pathol. 1995;48:714–716. doi: 10.1136/jcp.48.8.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houben M H, Beek D V, Hensen E F, Craen A J, Rauws E A, Tytgat G N. A systematic review of Helicobacter pylori eradication therapy—the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999;13:1047–1055. doi: 10.1046/j.1365-2036.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- 13.Hulten K, Gibreel A, Skold O, Engstrand L. Macrolide resistance in Helicobacter pylori: mechanism and stability in strains from clarithromycin-treated patients. Antimicrob Agents Chemother. 1997;41:2550–2553. doi: 10.1128/aac.41.11.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenks P J, Ferrero R L, Labigne A. The role of the rdxA gene in the evolution of metronidazole resistance in Helicobacter pylori. J Antimicrob Chemother. 1999;43:753–758. doi: 10.1093/jac/43.6.753. [DOI] [PubMed] [Google Scholar]
- 15.Jeong J Y, Mukhopadhyay A K, Dailidiene D, Wang Y, Velapatino B, Gilman R H, Parkinson A J, Nair G B, Wong B C, Lam S K, Mistry R, Segal I, Yuan Y, Gao H, Alarcon T, Brea M L, Ito Y, Kersulyte D, Lee H K, Gong Y, Goodwin A, Hoffman P S, Berg D E. Sequential inactivation of rdxA (HP0954) and frxA (HP0642) nitroreductase genes causes moderate and high-level metronidazole resistance in Helicobacter pylori. J Bacteriol. 2000;182:5082–5090. doi: 10.1128/jb.182.18.5082-5090.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong J Y, Berg D E. Mouse-colonizing Helicobacter pylori SS1 is unusually susceptible to metronidazole due to two complementary reductase activities. Antimicrob Agents Chemother. 2000;44:3127–3132. doi: 10.1128/aac.44.11.3127-3132.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kedderis G L, Argenbright L S, Miwa G T. Mechanism of reductive activation of a 5-nitroimidazole by flavoproteins: model studies with dithionite. Arch Biochem Biophys. 1988;262:40–48. doi: 10.1016/0003-9861(88)90166-x. [DOI] [PubMed] [Google Scholar]
- 18.Kwon D H, Kato M, El-Zaatari F A K, Osato M S, Graham D Y. Frame-shift mutations in NADPH flavin oxidoreductase encoding gene (frxA) from metronidazole resistant Helicobacter pylori ATCC43504 and its involvement in metronidazole resistance. FEMS Microbiol Lett. 2000;188:197–202. doi: 10.1111/j.1574-6968.2000.tb09193.x. [DOI] [PubMed] [Google Scholar]
- 19.Kwon D H, El-Zaatari F A K, Woo J S, Perng C L, Graham D Y, Go M F. REP-PCR fragments as biomarkers for differentiating gastroduodenal disease-specific Helicobacter pylori strains. Dig Dis Sci. 1998;43:980–987. doi: 10.1023/a:1018818431828. [DOI] [PubMed] [Google Scholar]
- 20.Kwon D H, El-Zaatari F A K, Kato M, Osato M S, Reddy R, Yamaoka Y, Graham D Y. Analysis of rdxA and involvement of additional genes encoding NADPH flavin oxidoreductase (FrxA) and ferredoxin-like protein (FdxB) in metronidazole resistance of Helicobacter pylori. Antimicrob Agents Chemother. 2000;44:2133–2142. doi: 10.1128/aac.44.8.2133-2142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon D H, Pena J A, Osato M S, Fox J G, Graham D Y, Versalovic J. Frameshift mutations in rdxA and metronidazole resistance in North American Helicobacter pylori isolates. J Antimicrob Chemother. 2000;46:793–796. doi: 10.1093/jac/46.5.793. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard, fifth ed. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 23.Osato M S, Reddy R, Graham D Y. Metronidazole and clarithromycin resistance amongst Helicobacter pylori isolates from a large metropolitan hospital in the United States. Int J Antimicrob Agents. 1999;12:341–347. doi: 10.1016/s0924-8579(99)00079-5. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Sisson G, Jeong J Y, Goodwin A, Bryden L, Rossler N, Lim-Morrison S, Raudonikiene A, Berg D E, Hoffman P S. Metronidazole activation is mutagenic and causes DNA fragmentation in Helicobacter pylori and in Escherichia coli containing a cloned H. pylori RdxA+ (nitroreductase) gene. J Bacteriol. 2000;182:5091–5096. doi: 10.1128/jb.182.18.5091-5096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K A, Klenk H P, Gill S, Dougherty B A, Nelson K, Quackenbush J, Zhou L, Kirkness E F, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H G, Glodek A, McKenny K, Fitzgerald L M, Lee N M, Adams M D, Hickey E K, Berg D E, Gocayne J D, Utterback T R, Peterson J D, Kelley J M, Cotton M D, Weidman J M, Fujii C, Bowman C, Watthey L, Wallin E, Hays W S, Borodovsky M, Karp P D, Smith H O, Fraser C M, Venter J C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 27.Weel J F, van der Hulst R W, Gerrits Y, Tytgat G N, van der Ende A, Dankert J. Heterogeneity in susceptibility to metronidazole among Helicobacter pylori isolates from patients with gastritis or peptic ulcer disease. J Clin Microbiol. 1996;34:2158–2162. doi: 10.1128/jcm.34.9.2158-2162.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]