Abstract
Protein polymer-based hydrogels have shown potential for tissue engineering applications, but require biocompatibility testing for in vivo use. Enzymatically crosslinked protein polymer-based hydrogels were tested in vitro and in vivo to evaluate their biocompatibility. Endotoxins present in the hydrogel were removed by Trition X-114 phase separation. The reduction of endotoxins decreased TNF-α production by a macrophage cell line in vitro; however, significant inflammatory response was still present compared to collagen control gels. A branched PEG molecule and dexamethasone were added to the hydrogel to reduce the response. In vitro testing showed a decrease in the TNF-α levels with the addition of dexamethasone. In vivo implantations into the epididymal fat pad of C57/BL6 mice, however, indicated a decreased inflammatory mediated immune response with a hydrogel treated with both PEGylation and endotoxin reduction. This study demonstrates the importance of endotoxin testing and removal in determining the biocompatibility of biomaterials.
Keywords: Hydrogel, endotoxins, dexamethasone, polyethylene glycol, biocompatibility
Introduction
Biomaterials play a critical role in regenerative medicine and tissue engineering through the generation of specific biophysical and biochemical environments capable of directing cellular behavior and function.1,2 Both synthetic and natural polymers have been used for cellular encapsulation, but have met varied success. A frequent concern for biomaterials is biocompatibility, which is important for the long-term survival and function of transplanted cells.3,4 Biomaterial implants have the potential to trigger the wound healing process, leading to acute or chronic inflammation, formation of granulation tissue, and development of a fibrous scar or capsule.5 The formation of a fibrous capsule that completely surrounds the biomaterial implant is of particular concern: the capsule creates a diffusion barrier, depriving the transplanted cells of nutrients, oxygen and metabolites while inhibiting the release of factors and waste products from the cells.3 In addition, the capsule can block the formation of blood vessels in and around the cells, precluding revascularization, and ultimately decreasing cell function and survival.
While there are several methods to improve the biocompatibility of a biomaterial, it is of primary importance to ensure the material contains little or no endotoxins (lipopolysaccharides). Endotoxins, which are found in the outer cell membrane of Gram-negative bacteria, activate cells of the innate immune system, such as monocytes, macrophages and neutrophils, evoking the release of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and IL-1.6–8 Although limited in vivo work has been performed to show the exact effects of endotoxin, their presence may complicate biocompatibility studies and should be eliminated.6 Other methods to improve biocompatibility of an implant include the addition of polyethylene glycol (PEG) molecules or anti-inflammatory drugs. PEGylation modulates the immune response by shielding the implant from the immune system and has shown previous success as an immune-isolation barrier to microencapsulated islets.9–12 Additionally, anti-inflammatory drugs, such as dexamethasone, a synthetic glucocorticoid steroid, have proven successful in reducing inflammation and suppressing the immune system when incorporated into biomaterials for tissue engineering.13,14
Our group has previously developed genetically engineered, recombinantly expressed, monodisperse protein polymers from tandem repeat blocks of amino acid sequences.15–18 These polymers have several potential advantages over natural or synthetic polymers for tissue engineering. Since these polymers are genetically engineered, the sequence and structure are controlled, in turn allowing for tunable control over mechanical and chemical properties. These protein polymers may also be less immunogenic than naturally-derived polymers or synthetic materials, which may contain mitogens and show lot-to-lot variability or monomers, catalysts and initiators, respectively.3,19,20 However, it is important to note that the immune system is very specific and the response can vary with protein sequence, material preparation and implantation site.21 Thus, it is critical to further explore the biocompatibility of these protein polymers.
In the present study, the biocompatibility of enzymatically crosslinked protein polymer hydrogels made from lysine- and glutamine-containing protein polymers was investigated. Initially, these protein polymer hydrogels contained a high level of endotoxins, which can interfere with biocompatibility. Thus, a protocol was developed to remove the endotoxins from the hydrogel. Incorporation of a branched PEG and dexamethasone were explored as additional means to decrease the immunogenicity of the endotoxin reduced hydrogel. The hydrogels that caused the lowest cytokine response in vitro were implanted into the epididymal fat pad of mice to investigate the in vivo inflammatory immune response. Through these efforts, the ability to create protein polymer hydrogels with favorable biocompatibility was demonstrated, providing the potential for better survival and function of cells in contact with the material. Additionally, the impact of endotoxins both in vitro and in vivo was explored, demonstrating their effect on biocompatibility.
Methods
Materials
Unless otherwise stated, all materials were purchased from Sigma Aldrich (St. Louis, MO).
Animals
Six- to eight-week-old C57/BL6 male mice were obtained from Jackson Laboratory (Bar Harbor, Maine, USA). All animals were housed in the Northwestern University animal facility and used in compliance with the Institutional Animal Care and Use Committee (IACUC). Animal manipulations were conducted using protocols approved by the IACUC.
Protein polymer synthesis
The DNA sequences and corresponding protein polymers were synthesized as previously described.16 In summary, precisely designed DNA sequences were constructed using controlled cloning methods22 and then inserted into a modified pET-19b plasmid (Novagen, Gibbstown, NJ) followed by transformation into BLR(DE3) cells (Novagen). The modified cells were cultured in shaker flasks with 1 L of terrific broth (Novagen) supplemented with 200 μg/mL ampicillin and 12.5 μg/mL tetracycline (Fisher Scientific, Pittsburgh, PA). Protein expression was induced at OD600 0.6–0.8 utilizing 0.5 mM isopropyl thiogalactoside (U.S. Biologicals, Swampscott, MA) and allowed to continue for 4 h before harvesting via centrifugation. The cell pellets were resuspended in 6 M guanidine hydrochloride (U.S. Biologicals), 20 mM sodium phosphate, 500 mM NaCl, pH 7.8 buffer, lysed by three freeze/thaw cycles and sonication to further break down the cell walls. Centrifugation was used to separate the insoluble portion from the soluble proteins in the supernatant. The protein polymers were purified from the remaining impurities by affinity chromatography using chelating sepharose fast flow nickel-charged resin (GE Healthcare, Piscataway, NJ) under denaturing conditions with competitive elution using imidazole (Fisher Scientific). Elutions containing the protein were identified using SDS-PAGE analysis and then dialyzed and lyophilized to obtain pure protein. The molecular weight was verified using matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) on an Autoflex III Series MALDI-TOF (Bruker Daltonics, Billerica, MA) at Northwestern University’s Integrated Molecular Structure Education and Research Center (IMSERC). A 10 mg/mL sinapinic acid matrix in 50% acetonitrile with 0.1% trifluoroacetic acid (TFA) was used.
Endotoxin reduction and testing
The reduction of endotoxins from the protein polymers was performed by phase separation. The protein polymer was dissolved at 10 mg/mL in endotoxin free water and the pH was adjusted to ~9.5. Triton X-114 was added at 1% and stirred for 30 min at 4°C. The solution was heated to 37°C in a water bath for 10 min and centrifuged at 10,000 g and 37°C for 10 min. The supernatant containing the protein was removed and put in a new conical tube for repeated rounds of phase separation with pH adjustments to ~9.5 every 4 rounds. The protein solution was then placed on degassed Bio-beads SM2 Adsorbents (Bio-rad Laboratories, Hercules, CA) to remove trace amounts of Triton X-114, dialyzed against endotoxin free water, and lyophilized. Endotoxin levels were tested using the QCL-1000 Endpoint Chromogenic LAL assay (Lonza, Walkersville, MD). Endotoxin free water, tubes and glassware were used during endotoxin reduction and any further experiments.
Hydrogel formation
Protein polymer hydrogels were formed through enzymatic crosslinking of two protein polymers, K8–30 [GH10SSGHIDDDDKHM(GKAGTGSA)30 G] and Q6 [GH10SSGHIDDDDKHM[(GQQQLGGAGTGSA)2(GAGQGEA)3]6G]. All procedures were performed with aseptic technique in a laminar flow hood to maintain sterility. Tissue transglutaminase (tTG) from guinea pig liver was dissolved at 0.04 units/μL in 2 mM ethylenediaminetetraacetic acid (EDTA), 20 mM dithiothreitol (DTT), pH 7.7. The lysine containing protein, K8–30, was dissolved at 10 wt% in 200 mM 4-Morpholinepropanesulfonic acid (MOPS), 20 mM CaCl2, pH 7.6. The glutamine containing protein, Q6, was resuspended at 15 wt% in 2 mM EDTA, pH 7.3. The three components were combined at a volumetric ratio of 2:3:3 for tTG:K8–30:Q6 solutions followed by incubation at 37°C until gelation occurred.
Collagen gels with a final concentration of 2.97 mg/mL were formed with rat tail collagen Type I (BD Biosciences, San Jose, CA), dissolved on ice at 3.375 mg/mL in a solution of a 1:5 ratio of 1 N cold NaOH and 10× phosphate buffered saline (PBS). The gels were then incubated at 37°C until gelation occurred.
Protein polymer PEGylation
After endotoxin reduction, a 3-armed branched 2420 Da (Methyl-PEG12)3-PEG4-NHS Ester (TMS-PEG) (Fisher Scientific) was chemically conjugated to the K8–30 protein utilizing amide bond formation between the free amines of the lysines on the protein polymer and the NHS ester of the PEG molecules. The branched 2420 Da TMS-PEG was dissolved in dry dimethylformamide (DMF) to a concentration of 250 mM and added to K8–30 dissolved at 2 mg/mL in endotoxin free phosphate buffered saline at 5× and 20× molar excess. Reactions were carried out with 1.5 mL of K8–30 solution and incubated at room temperature for 30 min followed by lyophilization and salt removal with a CENTRI-SEP spin column (Princeton Separations, Freehold, NJ). The branched PEG molecule was chemically conjugated onto the K8–30 protein polymer and formed bonds with ~5 and ~9 of the 31 lysine residues when reacted at 5× and 20× molar excess, respectively. For all samples, gels were formed by mixing K8–30, conjugated K8–30 (K8–30-TMS-PEG), and Q6 with tTG. In all cases, the percentage of K8–30 modified with PEG was varied while keeping the total amount of K8–30 constant. Smaller linear PEG molecules were also conjugated onto the K8–30 protein before endotoxin reduction and included at various concentrations (Supplemental Material). In vivo studies were completed using the K8–30-TMS-PEG at 20x molar excess. The extent of PEGylation was determined through molecular weight determinations of the conjugated products by MALDI-TOF MS.
Dexamethasone incorporation
Dexamethasone (DEX) was incorporated into the protein polymer hydrogels by directly dissolving the steroid in the K8–30 buffer (200 mM MOPS, 20 mM CaCl2, pH 7.6). The compound was first dissolved at 0.62 mg/mL and then further diluted with the MOPS buffer to obtain lower concentrations. The K8–30 protein was then dissolved at 10 wt% utilizing the DEX solution at the desired concentration. Three concentrations of DEX gathered from literature values were used for in vitro studies: 0.1 μM, 1 μM and 10 μM based on the well volume utilizing a 10 μL gel.23,24 Based on hydrogel volume, these concentrations correspond to 5.92 μM, 59.2 μM and 592 μM DEX. All in vivo studies were performed using the highest concentration of DEX.
In vitro testing
The mouse macrophage cell line, RAW 264.7 (American Type Culture Collection, Manassas, VA) was cultured in high glucose Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS) (Gemini Bio-Products, West Sacramento, CA) in a humidified incubator at 37°C and 5% CO2. Cells were plated in 48-well plates at 1.5 × 105 cells/mL and allowed to adhere overnight. 10 μL gels were formed in 0.5 mL microcentrifuge tubes and transferred into wells containing 580 μL media. Aliquots of media were removed at various time points and frozen at –20°C for later analysis.
Mouse TNF-α levels were measured using enzyme linked immunosorbent assay (ELISA) kits (eBiosciences, San Diego, CA) following the manufacturer’s protocol. Samples were read using a M5 Spectramax plate reader (Molecular Probes, Sunnyvale, CA) at the Institute for Bionanotechnology in Medicine (IBNAM) at Northwestern University. To standardize across experiments, TNF-α values were normalized to the highest value in the measured plate.
For the endotoxin reduced, PEGylated and DEX incorporated samples, n ≥ 16, 7 and 10, respectively.
In vitro biocompatibility
Protein polymer hydrogels showing the lowest cytokine response in vitro, were further tested in vivo in the epididymal fat pad of male C57/BL6 mice (n = 5). Hydrogels formed with proteins before endotoxin reduction were implanted as positive controls (n = 3). Collagen gel implants and sham surgery were used as negative controls (n = 3).
Briefly, 6- to 8-week old C57/BL6 mice were anesthetized with 2% isoflurane before administering a 300 μL injection of avertin; through an abdominal incision, the epididymal fat pad was exposed for implantation of the preformed hydrogel, wrapped around the gel, and stitched together with an Ethilon 8–0 suture, before closure of the abdomen wall. The implants were removed 4 days after implantation and slides were prepared for histological evaluation.
Histology
Implants were excised and placed immediately in 10% neutral buffered formalin (Fisher Scientific), processed on a Leica TP 1050 tissue processor (Leica Microsystems, Germany), and paraffin embedded on a Sakura Tissue Tek embedding unit (Sakura Finetek U.S.A., Torrance, CA). Sections, cut with a 5 μm thickness on a Leica 2135 microtome (Leica Microsystems), were stained with hemotoxylin and eosin (H&E), and imaged using a Nikon Eclipse 50i upright microscope (Nikon, Melville, NY) equipped with Spot Advanced software (Diagnostic Instruments, Sterling Heights, MI) at Northwestern University’s IBNAM facility. The thickness of the inflammatory infiltrate surrounding each hydrogel was measured using ImageJ software (National Institutes of Health, Bethesda, MD). For each implant, 4 measurements were taken on each of 2–3 slides, averaged, and recorded. Slides were further evaluated by a pathologist (S.A.M) using standard histological criteria.
Statistical analysis
Statistics were performed using Origin 7 (OriginLab Corporation, Northampton, MA) with a one-tailed, two-sample T-test. A p-value less than 0.01 was considered significant.
Results
Endotoxin testing and reduction
Initial endotoxin testing was performed using a quantitative Limulus Amebocyte Lysate endpoint assay (Lonza, Hopkins, MA). Before endotoxin reduction, Q6 contained ~90 endotoxin units per mg of protein (EU/mg) and K8–30 contained ~7.5 EU/mg. Phase separation with Triton X-114 was used to reduce endotoxins from both protein components based on a protocol described by Liu et al.7 The number of rounds of phase separation, pH and removal of trace amounts of Triton X-114 were all optimized to reduce endotoxin contamination to an acceptable level for transplantation.8,25 Approximately 24 rounds of phase separation for Q6 brought the contamination down to 0.12 EU/mg and 16 rounds for K8–30 brought it down to 0.06 EU/mg. Figure 1(a) shows representative results of EU/mg after every 4 rounds of separation.
Figure 1.
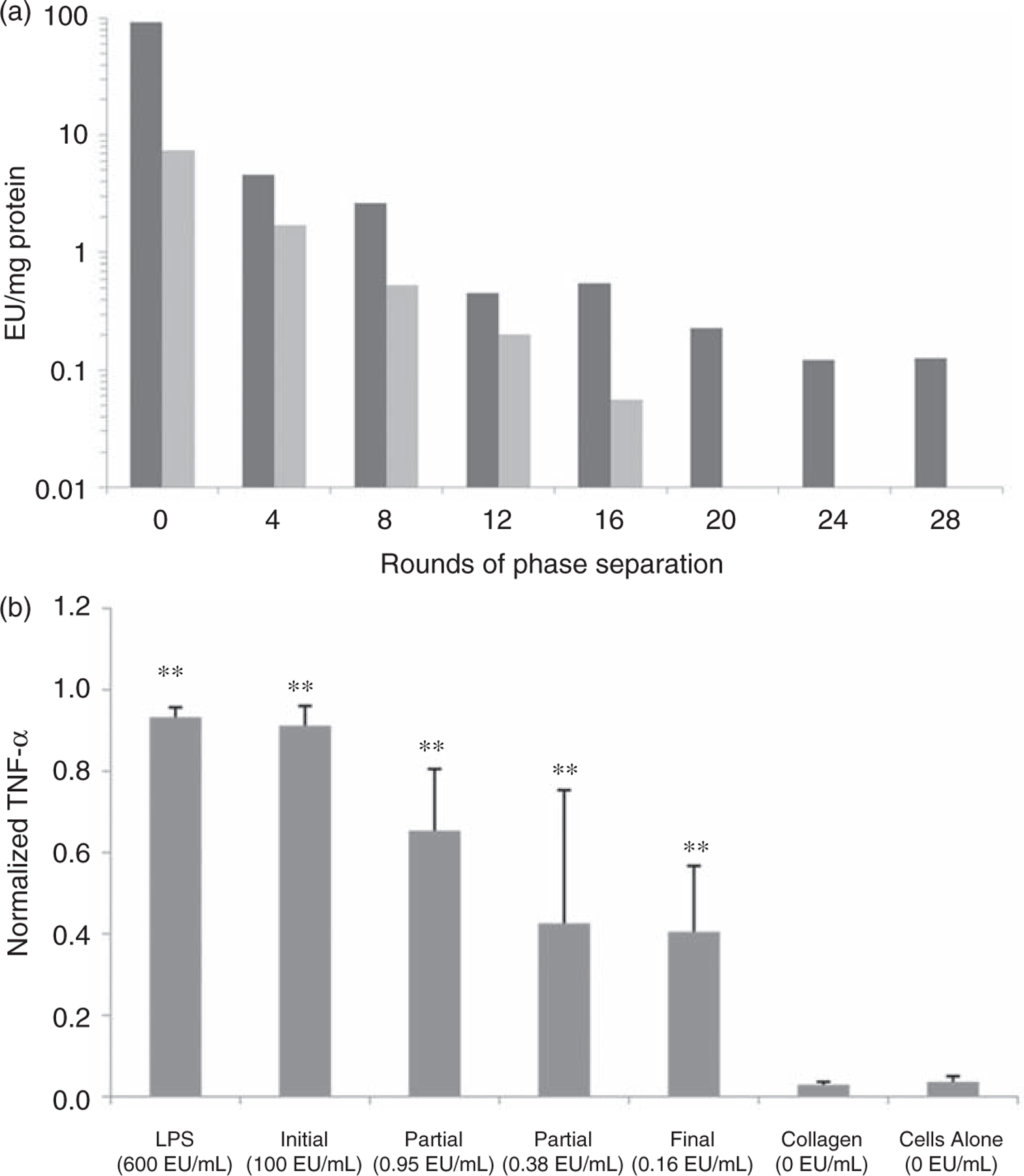
Endotoxin levels after phase separation and inflammatory immune response. (a) Endotoxin levels per mg of protein after every 4 rounds of phase separation. Dark gray represents Q and light gray represents K8–30. (b) TNF-α response of RAW 264.7 cells incubated for 24 h with protein polymer hydrogels before endotoxin removal and with various amounts of endotoxin removal. Lipopolysaccharide (LPS), collagen gels, and cells alone are controls. A statistically significant difference (p < 0.01) is denoted with ** when compared to the ‘‘Cells Alone’’. Error bars in graphs indicate means ± STDEV, n ≥ 16. Endotoxin levels are reported in EU/mL with the media based on 10 μL gels.
In vitro testing after endotoxin reduction
Protein polymer hydrogels of various endotoxin levels were incubated with RAW 264.7 cells to investigate the inflammatory immune response in vitro. Collagen gels and lipopolysaccharide (LPS, the prototypical endotoxin) at 600 EU/mL of media were used as the negative and positive control, respectively. Hydrogel endotoxin levels varied from 100 EU/mL media for non-treated hydrogels to 0.16 EU/mL media for the hydrogels with the fewest endotoxins (600-fold decrease). The lowest level of endotoxin resulted in the lowest level of TNF-α (55% decrease from baseline) (Figure 1(b)); however, it remained ~13 times higher than with collagen gels and cells alone. Following 24 h in vitro incubation, IL-1β was found to be undetectable (Supplemental Material).
In vitro testing after endotoxin reduction and the addition of PEGylated molecules
K8–30-TMS-PEG conjugates with ~5 and ~9 PEG molecules were incorporated into the hydrogel by substituting either 50% or 100% of the K8–30 with the K8–30-TMS-PEG. In vitro testing for macrophage response to PEGylation showed no statistical improvement at any amount of PEGylation when compared to the hydrogel after endotoxin reduction (Figure 2). The 100% PEGylated gel with ~9 grafted PEG molecules, however, did result in a statistically significant decrease in TNF-α level when compared with the other PEG conditions, (~7% decrease) (p < 0.01).
Figure 2.
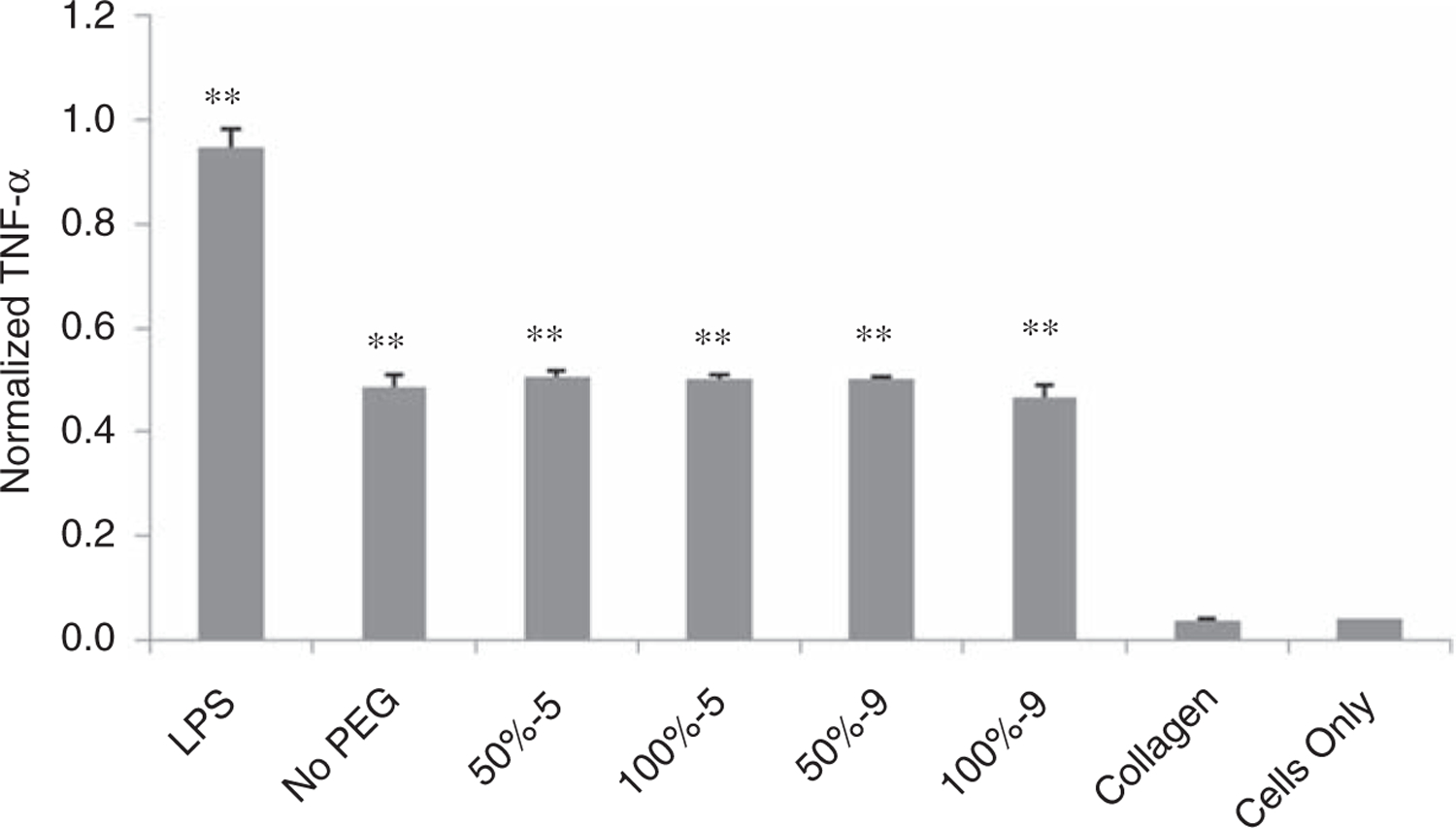
TNF-α response of RAW 264.7 cells incubated for 24 h with protein polymer hydrogel after endotoxin removal (ER) without PEG, or with 50% or 100% K8–30-PEG with either 5 or 9 TMS-PEG molecules per protein molecule. LPS, collagen gels, and cells alone are controls. A statistically significant difference (p < 0.001) is denoted with ** when compared to the ‘‘Cells Only’’. Error bars in graphs indicate means ± STDEV, n ≥ 7.
In vitro testing after endotoxin reduction and the addition of DEX
Three different concentrations of DEX were included in the K8–30 protein polymer hydrogels or with 600 EU/mL LPS. After 48 h incubation, the TNF-α levels of the LPS (LPS: 5.92 μM) and hydrogels with DEX at 5.92 μm (ER: 5.92 μM) were decreased by 39% and 33%, when compared to LPS (LPS: 0 μM) and ER (ER: 0 μM) with no DEX, respectively (Figure 3). TNF-α levels reached a low plateau with DEX concentrations of 59.2 μM or greater (ER: 59.2 μM and ER: 592 μM), reducing the levels by ~65% and ~70% of the LPS (LPS: 0 μM) and hydrogel samples (ER: 0 μM), respectively (Figure 3). A Griess reaction, which provides a measure of NO and is another indication of inflammation, validated these results with similar trends at both 24 and 48 h of incubation (Supplemental Material).
Figure 3.
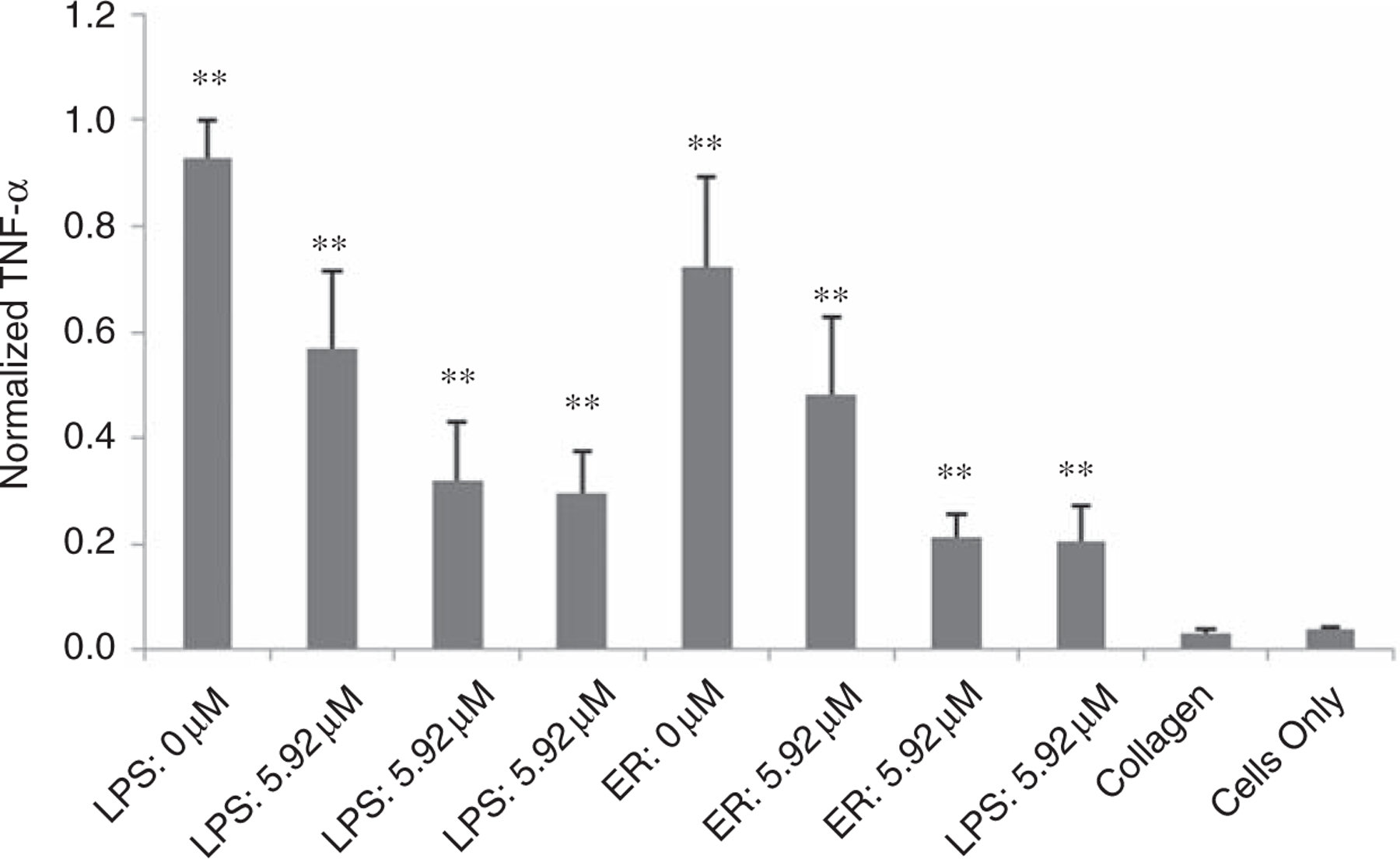
TNF-α response of RAW 264.7 cells incubated for 48 h with protein polymer hydrogels after ER with 0 μM, 5.92 μM, 59.2 μM, or 592 μM dexamethasone. LPS, collagen gels, and cells alone are controls. A statistically significant difference (p < 0.0005) is denoted with ** when compared to the ‘‘Cells Only’’. Error bars in graphs indicate means ±STDEV, n ≥ 10.
In vitro biocompatibility
Prior to endotoxin reduction, protein polymer hydrogels were completely surrounded by a dense infiltrate of acute inflammatory cells, including many neutrophils and some macrophages (Figures 4(a), 5(a) and (b), Table 1). Many of the inflammatory cells were apoptotic or necrotic. There was also a scant infiltrate of inflammatory cells in the surrounding fat (Figure 5(a), ‘f’). The endotoxin reduced gels also induced an acute inflammatory response surrounding the hydrogels (Figures 4(b), 5(c) and (d)), but the mean thickness of the infiltrate decreased by ~35% when compared to the hydrogels before endotoxin reduction (Figures 4(a), 5(a) and (b), Table 1) (p < 0.01). Endotoxin reduction also decreased the infiltrate into the surrounding fat (Figure 5(a) and (c)). The addition of DEX to the hydrogels after endotoxin reduction further decreased the cell infiltrate by ~50% when compared to decreasing endotoxins alone (Figure 4(c), Table 1) (p < 0.01). The addition of 100% K8–30-PEG, or 100% K8–30-PEG and DEX, to the hydrogel after endotoxin reduction led to a significant decrease in the thickness of the surrounding infiltrate, as compared to endotoxin reduction alone (Figures 4(d) and (e), and 5(e) and (f), Table 1). The scant infiltrate surrounding the 100% K8–30-PEG-containing gels was composed mainly of macrophages and was similar in histologic appearance to that of the infiltrate surrounding the collagen control gel (Figures 4(f), 5(g) and (h)). A sham procedure showed very little inflammation, indicating minimal inflammatory response to the procedure itself (Supplemental Material).
Figure 4.
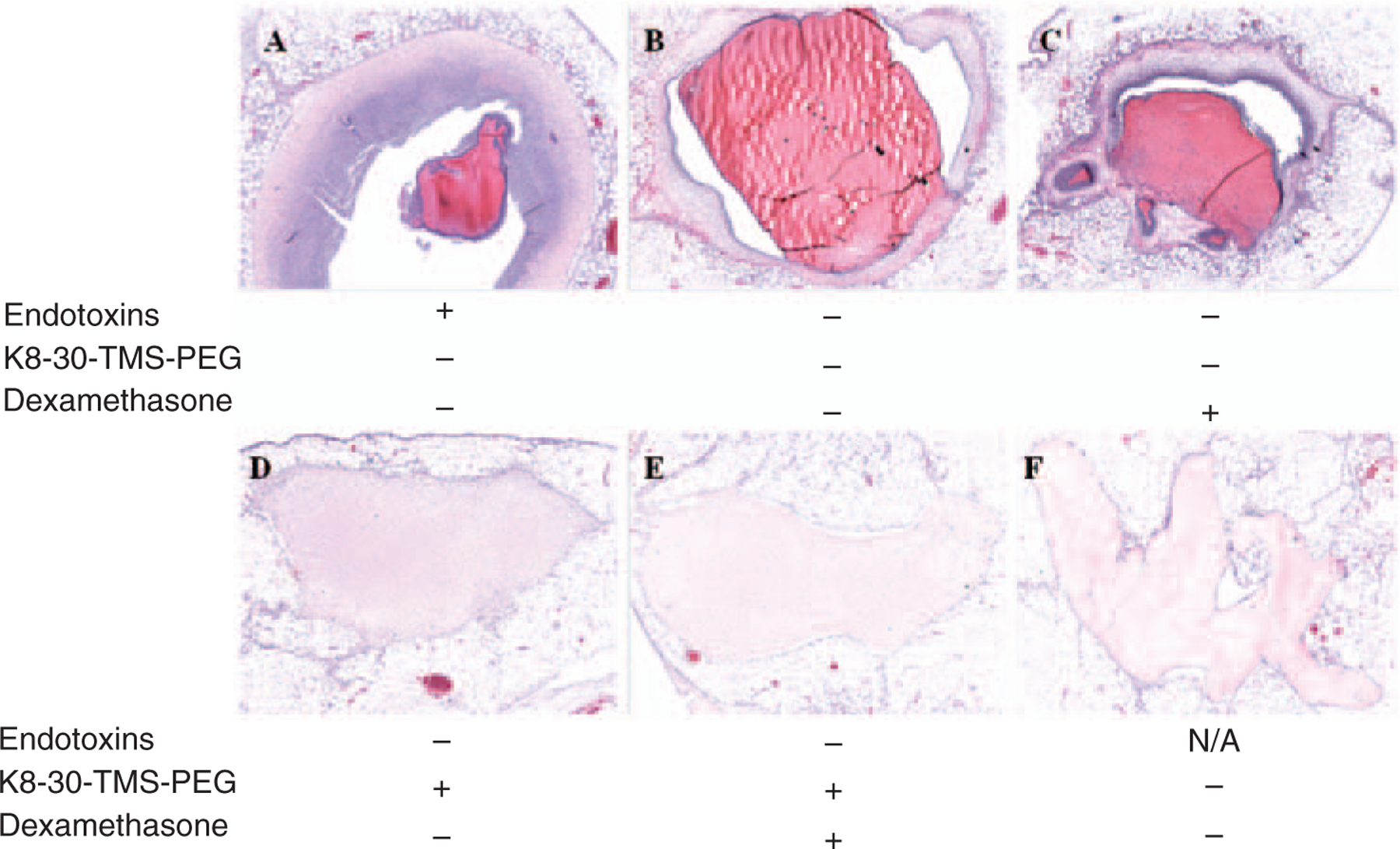
H&E staining of protein polymer hydrogels removed from epididymal fat pad 4 days after implantation. The white round cells are fat, the light pink/red is the hydrogel or collagen, and the purple marked cells denote nuclei. (a) Before endotoxin removal; (b) after endotoxin removal; (c) after endotoxin removal with 592 μM dexamethasone; (d) after endotoxin removal with 100% K8–30-TMS-PEG; (e) after endotoxin removal with 100% K8–30-TMS-PEG and 592 μM dexamethasone; (f) collagen alone. Images are at 4× magnification.
Figure 5.
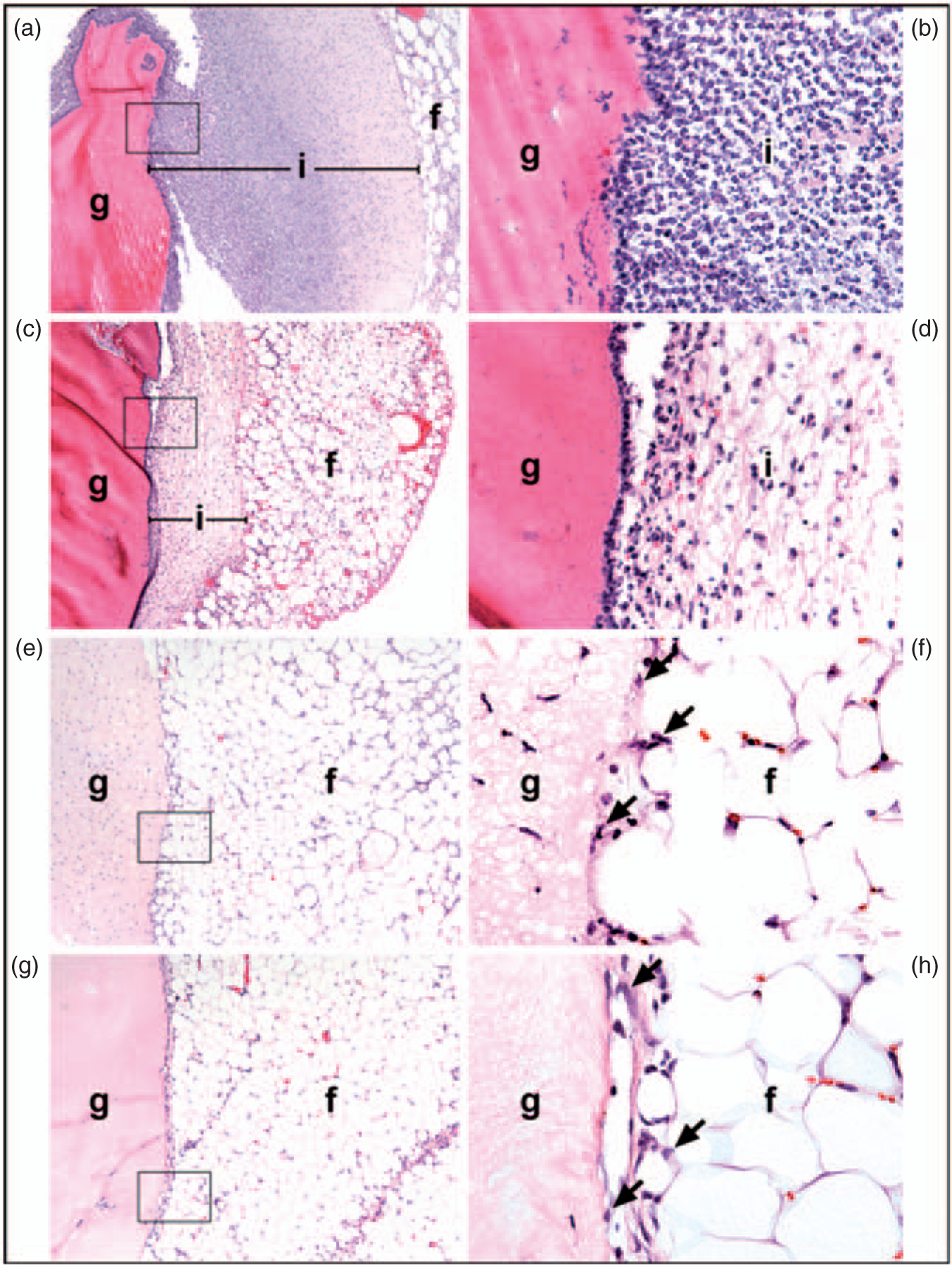
H&E stained images of gels after 4 days implantation of in the epididymal fat pads. (a,b) Gel before endotoxin removal: The gel (g) is surrounded by a dense inflammatory infiltrate (i) composed mainly of neutrophils. Most of the neutrophils in the inner part of the infiltrate (close to the gel) appear viable while those in the outer portion (nearest the fat) are necrotic. The infiltrate extends into the surrounding fat (f ). (c,d) Gel after endotoxin removal: The gel is surrounded by a thin band of inflammatory cells, most of which are necrotic. There is minimal infiltration into the surrounding fat. (e,g) Gel after endotoxin reduction with 100% K8–30-TMS-PEG and (g,h) collagen gel. In both cases, there is minimal inflammation, consisting of a few macrophages at the junction of the gel and the fat; a few macrophages are indicated by arrows (f,h). There are scattered cells within the PEGylated gel. The boxed area (100× magnification) in a, c, e, and g is seen at higher power (600×) in b, d, f, and h, respectively. The spaces between the gel and the infiltrate, most notable in panels A and D, are artifacts due to shrinkage of the tissue during fixation.
Table 1.
Measurement of the inflammatory cell infiltrate and surrounding necrotic tissue in distance from the edge of the gel.
| Samples | Before endotoxin removal | After endotoxin removal | After endotoxin removal+592 μM Dex | After endotoxin removal+100% K8-30-TMS-PEG | After endotoxin removal+100% K8-30-TMS-PEG +592 µM Dex | Collagen |
|---|---|---|---|---|---|---|
| Inflammatory cell infiltrate (μM) | 410.0±69.2 | 49.6±18.2 | 42.3±30.3 | N/Aa | N/Aa | N/Aa |
| Inflammatory cell infiltrate+Necrotic tissue (μM) | 715.6±77.5 | 463.4±113.7 | 228.6±72.5 | 17.3±8.7 | 13.6±1.7 | 19.2±3.7 |
There is a decrease in infiltration between gels before and after endotoxin removal. There is also a decrease in infiltration after the addition of DEX. There is no difference between the endotoxin removed hydrogel with K8–30-TMS-PEG, endotoxin removed hydrogel with K8–30-TMS-PEG and 592 μM DEX and the collagen control gel. N=5 for all except controls. N=3 for collagen control and hydrogel before endotoxin removal.
No necrotic tissue.
The 100% K8–30-PEG (with or without DEX) hydrogel material differed in appearance from the hydrogel material without PEG: specifically, the PEG-containing hydrogels stained weakly with eosin, had a bubbly appearance, and contained small numbers of inflammatory cells, most likely macrophages (Figures 4(d) and (e) and 5(e) and (f)). In contrast, the hydrogels without PEG (Figures 4(b) and (c) and 5(c) and (d)) stained strongly with eosin and contained few, if any, inflammatory cells.
Gels formed with several different PEGylated protein polymers without endotoxin reduction were examined at 3–5 days and 1 month after implantation and showed extensive inflammatory cell infiltrate (Supplemental Material).
Discussion
Endotoxins, which are lipopolysaccharides found in the cell wall of Gram-negative bacteria such as E. coli, are commonly found in recombinantly expressed proteins.26 Endotoxins are composed of monomers of fatty acid chains and negatively charged sugar units that can self assemble into larger complexes. They are known to be potent activators of macrophages, a first responder in the immune cascade, and have proven difficult to remove as they are heat stable and can bind to both hydrophobic and cationic moieties.6 In vivo studies of protein polymers, a relatively new class of materials, do not indicate that endotoxin reduction is necessary,27,28 but most of these focus on elastin-based protein polymers that utilize an inverse transition cycling purification method, which may remove endotoxins in the process.29
Endotoxins are potent activators of the innate immune system. By FDA regulations, endotoxin levels for drugs must be less than 5 EU/kg/h, and for medical devices less than 0.5 EU/mL based on a 40 mL rinse.8,25 In initial testing, high levels of endotoxins were found in the lysine- and glutamine-containing recombinantly expressed protein polymers. During endotoxin testing, the higher endotoxin levels associated with the Q6 protein were unexpected. It was hypothesized that the negatively charged sugars on the LPS would bind more tightly with the positively charged lysines in the K8–30 protein. This finding indicates other binding types may play a significant role in endotoxin association.
Phase separation with Triton X-114 was found to be the most effective method to reduce endotoxins. An affinity chromatography method with a proteinaceous ligand derived from a bacteriophage to bind endotoxin was abandoned after insufficient endotoxin reduction and low yields. High performance liquid chromatography (HPLC) did effectively reduce endotoxins, however it proved difficult to maintain an endotoxin free environment. Optimization of the phase separation provided consistent endotoxin levels below 0.12 EU/mg protein, while often being much lower.
The macrophage cell line, RAW 264.7, was used to analyze the in vitro effect of endotoxin reduction. Endotoxins stimulate the secretion of pro-inflammatory factors such as TNF-α and IL-1β from leukocytes. Cytokine secretion from macrophage cell lines has been used to analyze inflammatory potential for other biomaterials.30 TNF-α, specifically, is one of the key cytokines involved in macrophage response towards foreign materials.6,31 Endotoxin reduction from the protein polymer hydrogels showed a stepwise decrease in TNF-α levels with continued decrease in endotoxin levels. The TNF-α levels for the hydrogel with the lowest level of endotoxins were less than half of what they were before the phase separation process. However, the hydrogels still showed a TNF-α level ~13 times greater than with collagen, a commonly used protein-based hydrogel, included here as a negative control. The elevated TNF-α levels seen after reducing endotoxins may be due to recognition of the non-natural repetitive amino acid sequence of the protein polymer as foreign. Further studies could be performed to better understand the difference in TNF-α secretion caused by the proteins themselves and that caused by the endotoxins present in the hydrogel. Nevertheless, in vivo endotoxin reduction also led to a decrease in the extent of the inflammatory infiltrate around the protein polymer, confirming the in vitro data.
After reducing the endotoxins, the effects of PEGylation were also evaluated in vitro to determine if it could shield the hydrogel from the immune system and limit the foreign body response. PEG is known to suppress the immune response by decreasing protein adsorption through its hydrophilic nature and its steric barrier from its high bond mobility.32,33 Monodisperse PEG molecules were attached to the K8–30 protein to better help determine the extent of conjugation. While smaller PEG molecules are desired because they will cause the least chemical and mechanical changes to the gel, a high molecular weight, branched TMS-PEG was conjugated here onto the K8–30 protein to effectively block the cellular response of a potentially immunogenic protein polymer. In vitro testing of the K8–30-TMS-PEG at different weight percentage and degrees of conjugation indicated no change in TNF-α levels compared to non-PEGylated hydrogels. In vivo biocompatibility testing, however, showed significantly diminished inflammatory infiltrate, no necrotic tissue, and minimal infiltrate in the surrounding fat. A proposed hypothesis for the difference seen between these studies is that PEG shields the protein polymer in the in vivo studies, such that macrophages are not activated. However, in the in vitro model, the gels are in direct contact with the macrophages, negating the protective effects. In vivo testing of PEGylated gels without endotoxin reduction, however, does not show the same reduction in inflammatory cell infiltration, instead invoking a response similar to hydrogels formed before endotoxin reduction (Supplemental Material). Despite the general application of PEGylation to modulate immune responses, this strategy appears to be successful only after endotoxin reduction. This indicates that both endotoxin reduction and PEGylation are necessary to down regulate the inflammatory response.
A drug-mediated solution was also explored as an alternative method for decreasing the immune response of protein polymer hydrogels. DEX is a well-studied and commonly used glucocorticoid class steroid hormone serving as an anti-inflammatory and immunosuppressant and has been previously used in conjunction with biomaterials.13,14,34,35 It was included at three different concentrations through dissolution in the protein polymer hydrogel. The measurement of both TNF-α and nitric oxide in vitro indicates that the addition of a corticosteroid impacts macrophage response to protein polymer hydrogels. While in vivo testing incorporating the highest concentration of DEX did show decrease in necrosis and in the immune cell infiltrate, compared to controls (hydrogels after endotoxin reduction), it was not to the same extent as the TMS-PEG hydrogel. Furthermore, when added to the TMS-PEG hydrogel, DEX did not produce a significant improvement in vivo. Therefore, PEGylation elicits a down regulation of the inflammatory mediated immune response in vivo, but the addition of DEX is not necessary.
At this time point, the PEGylated protein polymer hydrogel after endotoxin reduction could be considered to have a favorable immune response, with similar cell infiltration to collagen gels in vivo. The histology images of these protein polymer hydrogels with full endotoxin reduction and PEGylation indicate a similar immunogenic response to that of an elastin-based protein polymer hydrogel, presenting with a mild foreign body response,36 but longer time studies would be needed to fully assess the host response to make ultimate determination of its biocompatibility. Lastly, it is important to take into account that the host response to soft tissue injury following any biomaterial implantation initiates a series of events that mimic a foreign body reaction and the resolution of this tissue response may affect the efficacy of the transplanted biomaterial. An appropriate host response is one that allows for the intended function without interference from the immune system.
Conclusions
The biological response of an implanted biomaterial is critical to its in vivo function. The complex foreign body response and wound healing process are difficult to predict and model in vitro. The effect of endotoxins on the immune response both in vitro and in vivo has been shown through the incubation with macrophage cells and implants in the epididymal fat pad of mice. Specifically, endotoxin reduction decreases TNF-α levels in vitro and decreases inflammatory infiltration both in and around the material in vivo. Additionally, PEGylation of endotoxin reduced proteins further decreased cellular infiltration and necrotic tissue
This study shows the importance of properly identifying potential inflammatory triggers such as endotoxins. Here the in vivo effects of endotoxins on the inflammatory response were demonstrated. Without endotoxin reduction, PEGylation does not improve the immune response. Although previous work has shown the importance of endotoxin reduction in vitro,37–39 limited work has been done in vivo.40,41 This study indicates the importance in detecting endotoxins and how their presence can complicate biocompatibility studies.
Supplementary Material
Acknowledgements
We thankfully acknowledge the Institute for Bionanotechnology in Medicine, the Pathology Core Facility and Integrated Molecular Structure Education and Research Center at Northwestern University for the use of their equipment.
Funding
This work was supported by the National Institute of Health/National Institute of Biomedical Imaging and Bioengineering [Grant Number R01EB003806]; and Northwestern University’s National Institute of Health/National Research Service Award Biotechnology Training Grant [Grant Number 2-T32-GM008449].
References
- 1.Peppas NA and Langer R. New challenges in biomaterials. Science 1994; 263(5154): 1715–1720. [DOI] [PubMed] [Google Scholar]
- 2.Hubbell JA. Biomaterials in tissue engineering. Bio-Technology 1995; 13(6): 565–576. [DOI] [PubMed] [Google Scholar]
- 3.Narang AS and Mahato RI. Biological and biomaterial approaches for improved islet transplantation. Pharmacol Rev 2006; 58(2): 194–243. [DOI] [PubMed] [Google Scholar]
- 4.Schutte RJ, Xie LL, Klitzman B, et al. In vivo cytokine-associated responses to biomaterials. Biomaterials 2009; 30(2): 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JM. Biological responses to materials. Ann Rev Mater Res 2001; 31: 81–110. [Google Scholar]
- 6.Gorbet MB and Sefton MV. Endotoxin: the uninvited guest. Biomaterials 2005; 26(34): 6811–6817. [DOI] [PubMed] [Google Scholar]
- 7.Liu SG, Tobias R, McClure S, et al. Removal of endotoxin from recombinant protein preparations. Clin Biochem 1997; 30(6): 455–463. [DOI] [PubMed] [Google Scholar]
- 8.Magalhaes PO, Lopes AM, Mazzola PG, et al. Methods of endotoxin removal from biological preparations: a review. J Pharm Pharmaceut Sci 2007; 10(3): 388–404. [PubMed] [Google Scholar]
- 9.Cheung CY and Anseth KS. Synthesis of immunoisolation barriers that provide localized immunosuppression for encapsulated pancreatic islets. Bioconj Chem 2006; 17(4): 1036–1042. [DOI] [PubMed] [Google Scholar]
- 10.Cruise GM, Hegre OD, Scharp DS, et al. A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets. Biotechnol Bioeng 1998; 57(6): 655–665. [DOI] [PubMed] [Google Scholar]
- 11.Weber LM, He J, Bradley B, et al. PEG-based hydrogels as an in vitro encapsulation platform for testing controlled beta-cell microenvironments. Acta Biomater 2006; 2(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JT, Cui WX and Chaikof EL. Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett 2008; 8(7): 1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duarte ARC, Mano JF and Reis RL. Dexamethasone-loaded scaffolds prepared by supercritical-assisted phase inversion. Acta Biomater 2009; 5(6): 2054–2062. [DOI] [PubMed] [Google Scholar]
- 14.Na K, Kim S, Sun BK, et al. Bioimaging of dexamethasone and TGF beta-1 and its biological activities of chondrogenic differentiation in hydrogel constructs. J Biomed Mater Res Part A 2008; 87A(2): 283–289. [DOI] [PubMed] [Google Scholar]
- 15.Davis NE, Ding S, Forster RE, et al. Modular enzymatically crosslinked protein polymer hydrogels for in situ gelation. Biomaterials 2010; 31(28): 7288–7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis NE, Karfeld-Sulzer LS, Ding S, et al. Synthesis and characterization of a new class of cationic protein polymers for multivalent display and biomaterial applications. Biomacromolecules 2009; 10(5): 1125–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappello J. The biological production of protein polymers and their use. Trends Biotechnol 1990; 8(11): 309–311. [DOI] [PubMed] [Google Scholar]
- 18.Haider M, Megeed Z and Ghandehari H. Genetically engineered polymers: status and prospects for controlled release. J Control Release 2004; 95(1): 1–26. [DOI] [PubMed] [Google Scholar]
- 19.Omer A, Duvivier-Kali V, Fernandes J, et al. Long-term normoglycemia in rats receiving transplants with encapsulated islets. Transplantation 2005; 79(1): 52–58. [DOI] [PubMed] [Google Scholar]
- 20.Chaikof EL. Engineering and material considerations in islet cell transplantation. Ann Rev Biomed Eng 1999; 1: 103–127. [DOI] [PubMed] [Google Scholar]
- 21.Bakeine GJ, Bertolotti A, Latina M, et al. Surface properties and implantation site affect the capsular fibrotic overgrowth. J Biomed Mater Res Part A 2007; 83A(4): 965–969. [DOI] [PubMed] [Google Scholar]
- 22.Won JI and Barron AE. A new cloning method for the preparation of long repetitive polypeptides without a sequence requirement. Macromolecules 2002; 35(22): 8281–8287. [Google Scholar]
- 23.Gremlich S, Roduit R and Thorens B. Dexamethasone induces posttranslational degradation of GLUT2 and inhibition of insulin secretion in isolated pancreatic beta cells - Comparison with the effects of fatty acids. J Biol Chem 1997; 272(6): 3216–3222. [DOI] [PubMed] [Google Scholar]
- 24.Weinhaus AJ, Bhagroo NV, Brelje TC, et al. Dexamethasone counteracts the effect of prolactin on islet function: Implications for islet regulation in late pregnancy. Endocrinology 2000; 141(4): 1384–1393. [DOI] [PubMed] [Google Scholar]
- 25.Mihardja SS, Gao DW, Sievers RE, et al. Targeted in vivo extracellular matrix formation promotes neovascularization in a rodent model of myocardial infarction. Plos One 2010; 5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen LB, Torp AM, Andersen SB, et al. The biological activity of a recombinantly expressed (His)(6)-tagged peanut allergen (rAra h 1) is unaffected by endotoxin removal. J Immunol Meth 2008; 335(1–2): 116–120. [DOI] [PubMed] [Google Scholar]
- 27.Kempen DHR, Yaszemski MJ, Heijink A, et al. Non-invasive monitoring of BMP-2 retention and bone formation in composites for bone tissue engineering using SPECT/CT and scintillation probes. J Control Release 2009; 134(3): 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parlane NA, Wedlock DN, Buddle BM, et al. Bacterial polyester inclusions engineered to display vaccine candidate antigens for use as a novel class of safe and efficient vaccine delivery agents. Appl Environ Microbiol 2009; 75(24): 7739–7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer DE and Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nature Biotechnol 1999; 17(11): 1112–1115. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia SK, Arthur SD, Chenault HK, et al. Interactions of polysaccharide-based tissue adhesives with clinically relevant fibroblast and macrophage cell lines. Biotechnol Lett 2007; 29(11): 1645–1649. [DOI] [PubMed] [Google Scholar]
- 31.Risbud M, Bhonde M and Bhonde R. Chitosan-polyvinyl pyrrolidone hydrogel does not activate macrophages: Potentials for transplantation applications. Cell Transplant 2001; 10(2): 195–202. [PubMed] [Google Scholar]
- 32.Wilson JT and Chaikof EL. Challenges and emerging technologies in the immunoisolation of cells and tissues. Adv Drug Deliv Rev 2008; 60(2): 124–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DY, Nam JH and Byun Y. Functional and histological evaluation of transplanted pancreatic islets immunoprotected by PEGylation and cyclosporine for 1 year. Biomaterials 2007; 28(11): 1957–1966. [DOI] [PubMed] [Google Scholar]
- 34.Barnes PJ and Adcock I. Antiinflammatory actions of steroids-molecular mechanisms. Trends Pharmacol Sci 1993; 14(12): 436–441. [DOI] [PubMed] [Google Scholar]
- 35.Patil SD, Papadmitrakopoulos F and Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release 2007; 117(1): 68–79. [DOI] [PubMed] [Google Scholar]
- 36.Sallach RE, Cui W, Wen J, et al. Elastin-mimetic protein polymers capable of physical and chemical crosslinking. Biomaterials 2009; 30(3): 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagawa Y, Murai T, Hasegawa C, et al. Endotoxin contamination in wound dressings made of natural biomaterials. J Biomed Mater Res Part B-Appl Biomater 2003; 66B(1): 347–355. [DOI] [PubMed] [Google Scholar]
- 38.Gao BC and Tsan MF. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor a release by murine macrophages. J Biol Chem 2003; 278(1): 174–179. [DOI] [PubMed] [Google Scholar]
- 39.Bi YM, Collier TO, Goldberg VM, et al. Adherent endotoxin mediates biological responses of titanium particles without stimulating their phagocytosis. J Orthopaed Res 2002; 20(4): 696–703. [DOI] [PubMed] [Google Scholar]
- 40.Bi YM, Seabold JM, Kaar SG, et al. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Mineral Res 2001; 16(11): 2082–2091. [DOI] [PubMed] [Google Scholar]
- 41.Skoglund B, Larsson L and Aspenberg PA. Bone-resorptive effects of endotoxin-contaminated high-density polyethylene particles spontaneously eliminated in vivo. J Bone Joint Surg 2002; 84B(5): 767–773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


