Abstract
Atrial cardiomyopathy, characterized by abnormalities in atrial structure and function, is associated with increased risk of adverse cardiovascular and neurocognitive outcomes, independent of atrial fibrillation. There exists a critical unmet need for a clinical tool that is cost-effective, easy to use, and that can diagnose atrial cardiomyopathy. P wave parameters (PWPs) reflect underlying atrial structure, size, and electrical activation; alterations in these factors manifest as abnormalities in PWPs that can be readily ascertained from a standard 12-lead electrocardiogram and potentially be used to aid clinical decision making. PWPs include P wave duration, interatrial block, P wave terminal force in V1, P wave axis, P wave voltage, P wave area, and P wave dispersion. PWPs can be combined to yield an index (P wave índex [PWI]), such as the MVP ECG risk score. Abnormal PWPs have been shown in population-based cohort studies to be independently associated with higher risks of atrial fibrillation, ischemic stroke, sudden cardiac death, and dementia. Additionally, PWPs, either individually or in combination (as a PWI), have been reported to enhance prediction of atrial fibrillation or ischemic stroke. To facilitate translation of PWPs to routine clinical practice, additional work is needed to standardize measurement of PWPs (e.g., via semi-automated or automated measurement), confirm their reliability and predictive value, leverage novel approaches (e.g., wavelet analysis of P waves and machine learning algorithms), and finally, define the risk-benefit ratio of specific interventions in high-risk individuals. Our ultimate goal is to repurpose the ubiquitous 12-lead ECG to advance the study, diagnosis, and treatment of atrial cardiomyopathy, thus overcoming critical challenges in prevention of cardiovascular disease and dementia.
INTRODUCTION
Atrial cardiomyopathy is a newly defined entity that encompasses alterations in macro- and micro-structure; reservoir, conduit, and contractile function; and electrical conduction in the atria.1, 2 Compelling evidence has emerged to indicate that atrial cardiomyopathy, which is associated with an increased risk of atrial fibrillation (AF), is also associated with higher incidence of AF-related adverse outcomes such as ischemic stroke, heart failure, cognitive decline, dementia, and death.2, 3 Notably, the associations of atrial cardiomyopathy with cardiovascular and neurocognitive outcomes are independent of AF; hence, atrial cardiomyopathy is a distinct entity that is in and of itself prognostically important. Many methods have been developed and tested to characterize atrial cardiomyopathy; unfortunately, they are varied and inconsistently applied (e.g., cardiac magnetic resonance imaging, 3D-echocardiogram, body surface electrocardiogram (ECG) mapping, electroanatomical mapping, etc.),4–7 inconclusive, and are limited by technical challenges in implementation and interpretation, low acceptability by patients, and high cost. Therefore, to move the field forward, there is a pressing need to identify techniques that can accurately characterize atrial cardiomyopathy, and importantly, be readily translated to the clinical setting.
Fortunately, an age-old clinical tool—the humble 12-lead ECG—may pave the way. The normal sinus impulse depolarizes the atria generating the normal P wave. The P wave can have multiple measured parameters, such as (1) duration, (2) morphology, (3) voltage, (4) spatial axis, and (5) area.8 P wave parameters (PWPs) can can be combined to yield an index (P wave índex [PWI]), such as the morphology-voltage-P-wave duration electrocardiogram (MVP ECG) risk score.9 PWPs can be altered even under various normal physiological conditions, but more so when atrial pathology is present. These changes can be detected on a standard 12-lead ECG and can be readily measured manually or automatically.
Alterations in these parameters, especially in duration and morphology, have been used to diagnose enlargement of atrial chambers and atrial conduction blocks, and have been considered as risk factors for different clinical events, principally AF and ischemic stroke. The recognition of the prognostic value of PWPs is not recent; in fact, in the 1980s, advanced interatrial block (IAB) was already described as a marker of risk of AF or atrial flutter.10 This association (named Bayés syndrome)11, 12 was subsequently demonstrated in several general population cohorts,13, 14 as well as in patients with cardiovascular disease (CVD).15, 16 Additionally, other PWPs—P terminal force in V1 (PTFV1),17, 18 P wave voltage,19 P wave area,20 and more recently, P wave axis21, 22—have been shown to be associated with increased risk of AF, ischemic stroke, dementia, and death.
In 2009, Magnani and colleagues summarized in a review article the status of PWPs for epidemiology, clinical, and research applications.8 In the present review, we summarize advances in this area over the past decade since the original compilation. We will focus on P wave duration, morphology, voltage, axis, and area, and discuss state-of-the-art knowledge on the relationship of these abnormal PWPs to cardiovascular (CV) and neurocognitive outcomes. We will identify limitations and current gaps in knowledge and propose directions for future research. Our ultimate goal is to present a practical way forward to advance the emerging field of atrial cardiomyopathy.
DEFINITIONS OF P WAVE PARAMETERS
P Wave Duration and Morphology
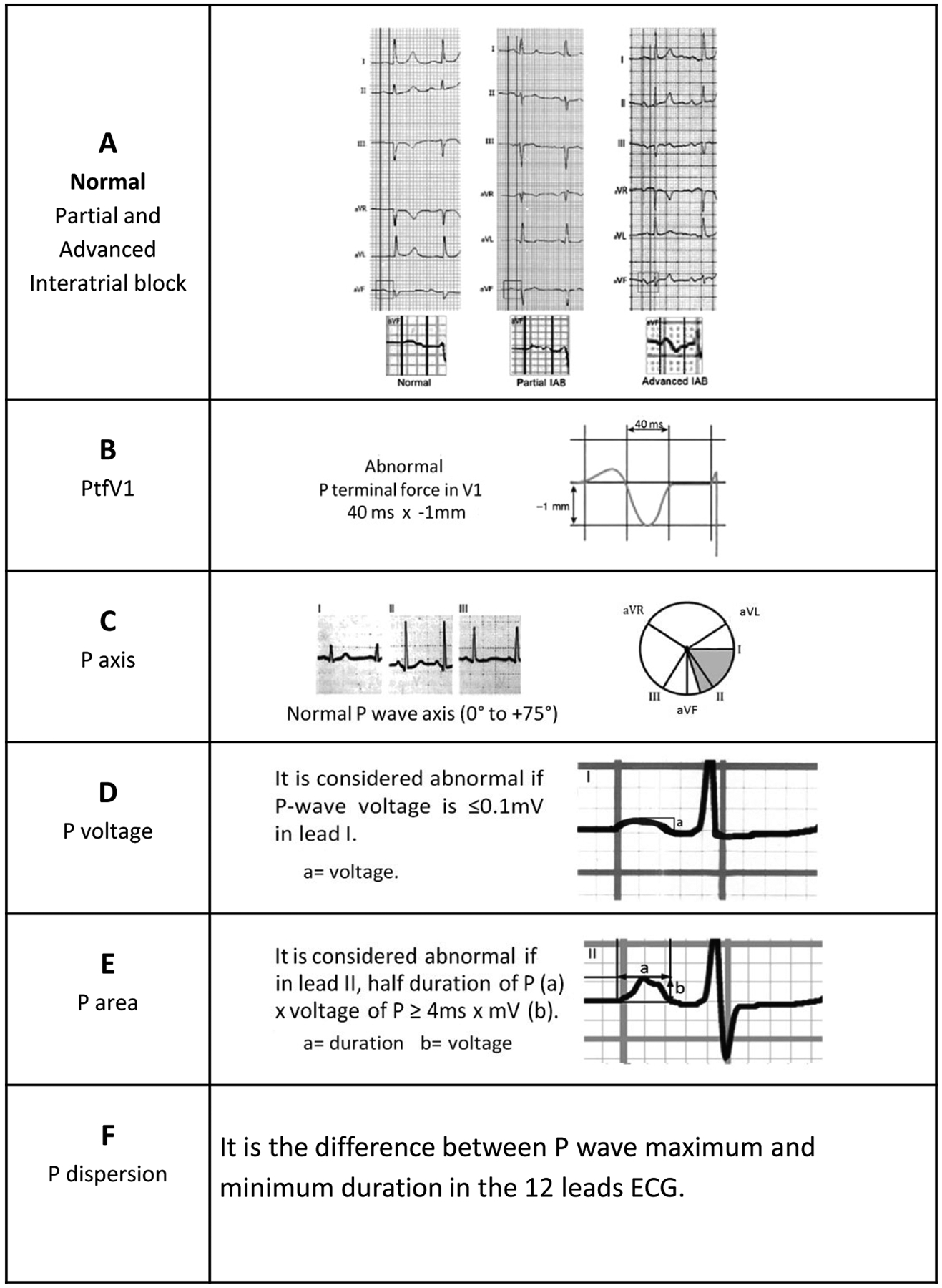
Partial and advanced interatrial block16, 23–26
The normal P wave duration is <120 ms. A P wave duration ≥120 ms is abnormal24, 25 and is a criterion for partial interatrial block (partial IAB), i.e., a conduction delay between right atrium (RA) and left atrium (LA) through the Bachmann’s bundle (Figure 1A).
Figure 1:
Measurement of different P wave parameters
If the block in the Bachmann’s bundle is complete (advanced IAB), the LA is activated retrogradely via muscular bundles located close to the AV junction. Therefore, in advanced IAB, not only is the P wave duration ≥120 ms, but the P wave morphology in leads II, III, or aVF is biphasic (±). Some atypical cases of advanced IAB have been reported such as P wave duration that is slightly shorter than 120 ms or P wave morphology without the typical biphasic pattern in all three inferior leads.26 Nonetheless, in all cases of advanced IAB, since the negative hemifield of aVF starts at 0°, there will be a final negativity in aVF, reflecting retrograde activation of the LA.26
How to measure the P wave duration12, 24
The P wave duration can be measured manually or automatically. In large-scale epidemiological investigation, automatic measurement of the P wave duration is essential and much more practical than manual measurement. In addition, automatic measurements assess P wave duration from its earliest onset to the latest offset in any of the recorded leads.27 However, automatic measurements tend to underestimate the P wave duration compared to manual measurements and are subject to error in cases of noisy tracings.28 Most contemporary ECG machines provide markings indicating fiducial points used for calculation of the reported intervals. In case of doubt, they can help decide whether to accept or reject the automatic measurements. With this in mind, when performing manual measurement of the P wave duration, it is paramount to amplify the digital ECG image as well as use ECG machines that allow the six frontal plane leads to be displayed simultaneously. The specific steps are as follows. First, we should amplify the digital ECG image on the computer screen and no specialized software is needed for the amplification process. Next, we trace two vertical lines on the frontal plane leads: one line for the onset where the P wave first appears in any lead and the other for the offset or the end of the P wave in any lead. Once these two points are defined with vertical lines, the P wave duration can be measured using manual callipers or semi-automatic callipers.
P Wave Terminal Force in V1
The posterior displacement of the LA in left atrial enlargement (LAE) is manifested on the ECG not only with a longer P wave duration, but also with a more pronounced negative component of the P wave in V1. The latter is due to the posterior displacement of the P wave in a vectorcardiography loop, whose final part is located more in the negative hemifield of V1. Morris had originally calculated the PTFV1 by multiplying the duration of the terminal force, measured in seconds, by the amplitude measured in millimeters, and defined a PTFV1 of >0.03 mm*s as being abnormal. Additionally, Morris described that an abnormal PTFV1—terminal negativity of the P wave in V1 of one box in depth (−0.1 mV) and one box in duration (0.04 sec)—yields a 92% specificity and 69% sensitivity for the diagnosis of LAE in patients with valvular heart disease17 (Figure 1B).
P Wave Axis
The P wave axis is measured on the frontal plane. A normal P wave axis is between 0° and +75° (Figure 1C). Of note, the P wave axis cannot be calculated in the presence of advanced IAB due to the ± morphology of the P wave in leads II, III and aVF.
P Wave Voltage
P wave voltage ≤0.1mV in lead I (Figure 1D) is considered abnormal. Park et al. found that P wave amplitude ≤0.1mV in lead I was independently associated with clinical recurrence of AF after radiofrequency ablation.19 Moreover, a score incorporating abnormal P wave voltage was shown to be useful in predicting new onset of AF.9
P Wave Area
P wave area is calculated in lead II using this formula:20
Abnormal P wave area is defined as ≥4ms × mV and has been found to be associated with LAE.20 Of note, modern ECG acquisition and analysis technology allows exact measurement of the área under the P wave on a lead-by-lead basis, which has an advantage over using mathematical formula in not making assumptions about the shape of the P wave.
Orthogonal P Wave Morphology
Of note, orthogonal X, Y, and Z ECG leads can be used to further refine assessment of interatrial electrical activation (Figure 2).29 Based on the P wave morphology in the orthogonal leads, three principal P wave types have been described depending on P wave polarity in leads Y and Z with different risks of AF.29 Type 1, which is overrepresented among young individuals without comorbidities and low risk of AF29 is characterized by upright P waves in all three orthogonal leads, assuming propagation of atrial depolarization wave from sinus node downward, right-to-left via Bachmann’s bundle and/or posterior interatrial connections and forward.30 Type 2, which is characterized by backward propagation of depolarization in the left atrium that occurs when interatrial conduction occurs via Bachmann’s bundle without contribution from other posteriorly located interatrial connections,30 has been shown to be overrepresented among patients with prevalent or incident paroxysmal AF. Type 3 is observed when left atrial depolarization vector is pointed upward as in the case of advanced interatrial block and is associated with the greatest risk of AF.29
Figure 2:
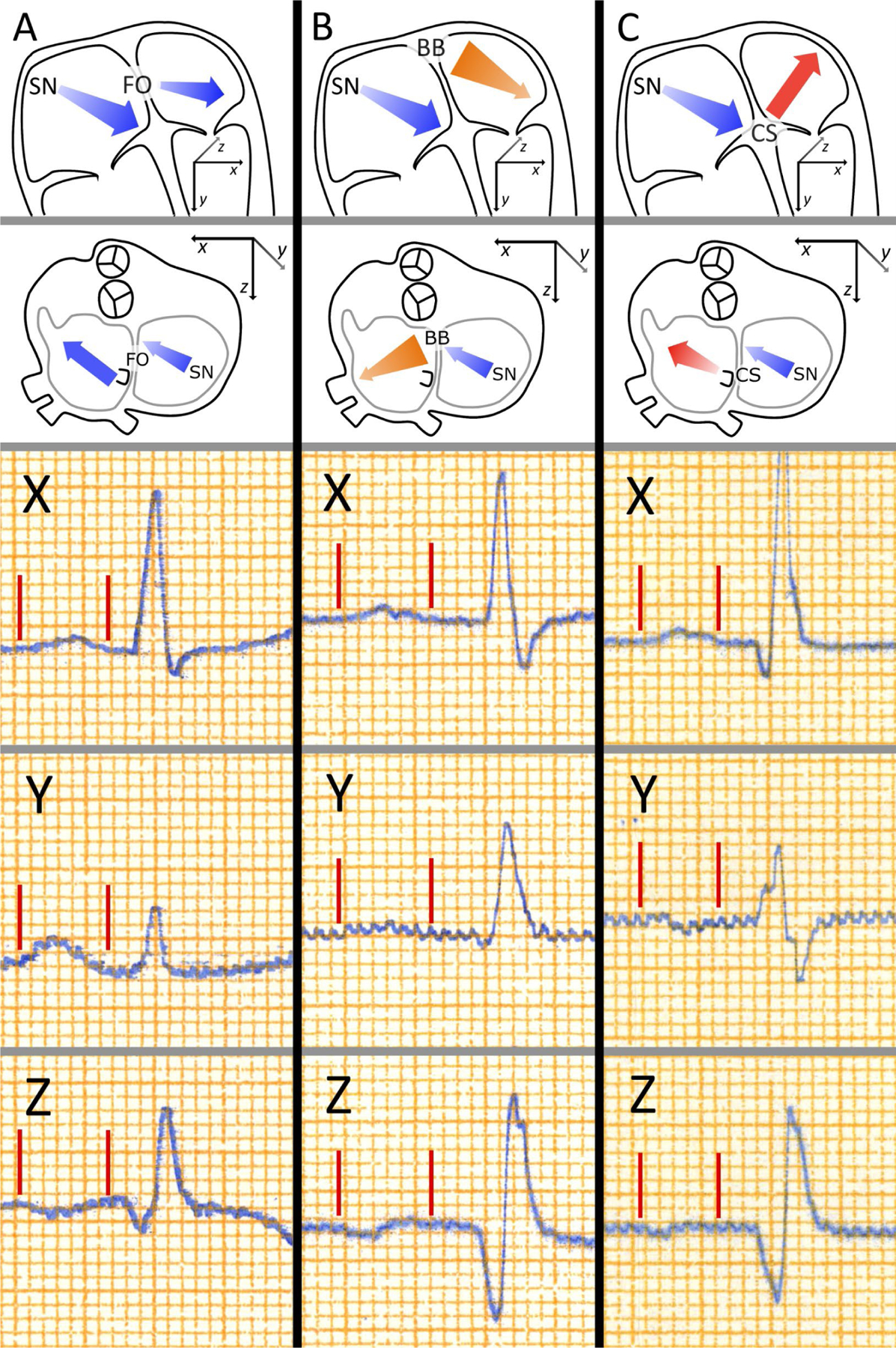
Orthogonal P wave morphology. The main atrial depolarization vectors underlying the three P wave morphologies are presented in schematic illustrations of human atria in the top row (anterior view) and the second row (superior view). The three lowest rows present P waves from leads X, Y, and Z, with P wave onsets and ends marked by red lines. Atrial depolarization begins in the sinus node and the depolarization propagates anteriorly, downwards, and leftwards in the right atrium leading to a positive initial deflection in the P wave in leads X and Y, and a negative initial deflection in lead Z. Type 1 morphology (Column A) is associated with interatrial propagation of activation wavefront through posterior fibres near the fossa ovalis, leading to left atrial depolarization directed forward resulting a negative terminal portion in lead Z. Type 2 morphology (Column B) is associated with the interatrial conduction occurring exclusively through the anteriorly and superiorly located Bachmann’s bundle, which leads to inferiorly and posteriorly directed left atrial depolarization, resulting in a positive terminal portion of the P wave in lead Z. Type 3 morphology (Column C) results from left atrial breakthrough occurring near the coronary sinus, without involvement of the Bachmann’s bundle in the interatrial conduction, as in the case of advanced interatrial block, which results in the left atrial activation directed upwards leading to a negative terminal portion of the P wave in lead Y. Reprinted from reference 29.
P Wave Dispersion
P wave dispersion is defined as the difference between P wave maximum and P wave minimum duration on the12-lead ECG. Studies have shown that greater P wave dispersion is associated with incident AF and AF recurrence after cardioversion31, 32 and severity of coronary artery disease.33 Furthermore, in a study of patients with cryptogenic stroke who received an implantable loop recorder, the only independent predictor of AF was P wave dispersion of 40 ms.34
ANATOMICAL CONSIDERATIONS
Anatomical Determinants of P Wave Morphology
PWPs are to a large extent defined by the trajectory that atrial depolarization wave takes after leaving the sinus node. While the RA component of the sinus P waves demonstrates little variation in regard to the direction of the depolarization vector—which appears as a positive initial component of the sinus P waves in lead II and adjacent leads—LA depolarization is significantly more variable. The course and direction of LA depolarization depends on the location and function of the interatrial connections, relative proximity of the sinus rhythm origin to a specific interatrial bundle, size and shape of the LA, as well as the degree of structural abnormalities of LA myocardium and the presence of fibrosis in the LA walls.
Interatrial Pathway Anatomy and Variability
Interatrial connections in humans exhibit significant interindividual variability and include (1) anterior via the Bachmann’s bundle, (2) posterior via myocardial pathways or bridges connecting the RA and LA at the level of the right pulmonary veins (also described as fossa ovalis [FO] connections), and (3) inferior via myocardial sleeves extending from the coronary sinus ostium and coronary sinus musculature to the inferior portion of the LA wall.
Bachmann’s bundle is the major pathway for the rapid propagation of the depolarization wave from the RA to the LA. It comprises a circumferential muscle bundle located at the anterior wall of the LA and connecting the RA and LA appendages (through the epicardium) in the vast majority of patients. However, based on several anatomical studies, in some patients, Bachmann’s bundle may also be absent or be indistinguishable from the surrounding atrial myocardium while other more posteriorly located interatrial connections would be more developed and provide a substrate for propagation of depolarization waves.35, 36 Predominantly anterior (the most common) and posterior-inferior anatomical variants of interatrial connections have been described,35 which explains that in up to one-third of patients, initial LA breakthrough during sinus rhythm is observed in the FO region, i.e., the area corresponding to the posteriorly located interatrial muscular sleeves.30, 37
Interatrial Pathways and P Wave Parameters
In the majority of healthy people, especially the young, P waves in the right precordial leads have upright positive appearance or have insignificant negative component. P wave positivity in the right precordial leads reflects anterior propagation of the depolarization wave in both the right and left atrium after crossing the interatrial septum in the posterior FO region with or without concomitant breakthrough in the Bachmann’s bundle, as demonstrated by electro-anatomical mapping studies.30
Abnormal PTFV1, characterized by the presence of a prominent negative component in lead V1, reflects predominantly posterior propagation of the depolarization wave across the LA after propagation through the Bachmann’s bundle and LA breakthrough in the superior-anterior part of the LA.30 This LA depolarization pattern assumes the lack of contribution from the posterior interatrial connections, which may be due to either anatomical variation or a result of structural remodeling and fibrotic replacement (Figure 3). Posterior connections are known to be generally thinner compared to the Bachmann’s bundle38 and thus may be more vulnerable to aging or disease processes.
Figure 3:
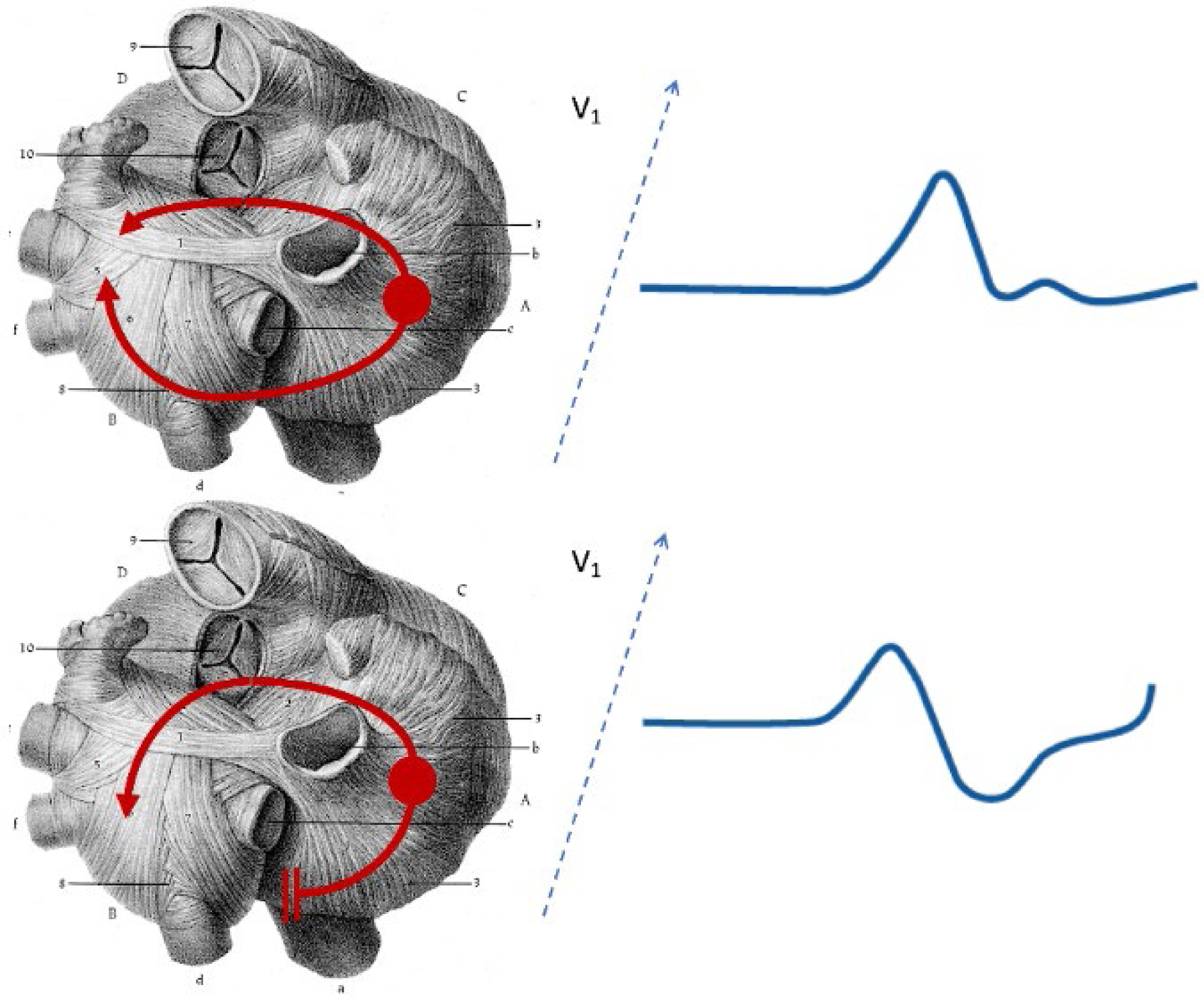
Transverse section of heart showing propagation of sinus node depolarization impulses from the right atrium to the left atrium. The upper panel shows the normal situation whereby atrial impulses conduct from the right atrium to the left atrium both via the Bachmann’s bundle and posteriorly located myocardial connections. The left atrial depolarization vector projection on the lead V1 results in either isoelectric or positive polarity of the terminal P wave component. The lower panel shows interatrial conduction over the Bachmann’s bundle only with no or minimal contribution from the posterior connections with resultant anterior-posterior activation of the left atrium resulting in a negative component in the terminal portion of the P wave in V1. Reprinted from reference 43.
Advanced progression of cardiac remodeling secondary to underlying structural heart disease (e.g., heart failure, ischemic heart disease, AF, or atrial cardiomyopathy) may extend to the main interatrial conduction route, i.e., the Bachmann’s bundle, which may become partially or completely replaced by fibrotic tissue.39, 40 The aforementioned process explains the physiopathology of a recently described syndrome, atrial failure, which was defined as any atrial dysfunction (anatomical, mechanical, electrical, and/or rheological, including blood homeostasis) causing impaired heart performance and symptoms, and worsening quality of life or life expectancy, in the absence of significant valvular or ventricular abnormalities.41 Relevant to the foregoing, in patients with type 2 diabetes, the left atrial reservoir strain was significantly lower in those with IAB than in those without.42
The lack of options for interatrial impulse propagation in the upper and posterior parts of the interatrial septum, which may occur when propagation over the Bachmann’s bundle is no longer possible due to advanced remodeling, leaves only the inferior route available, which results in the downward propagation on the RA side and upward activation of the LA (caudal-cranial activation).30, 43 Even partial fibrotic transformation of the Bachmann’s bundle may affect P wave morphology and result in the pattern of partial IAB with prolonged and notched P wave appearance.44 Of note, it has been demonstrated that in the absence of LA enlargement, disruption of the Bachmann’s bundle by icing can induce transient advanced IAB.45 This distortion of normal P wave morphology may challenge interpretation of P or flutter waves of atrial arrhythmias.46
Why inferior connections remain functional when other prominent interatrial bundles cease to conduct is not well understood. It is possible that myocardial fascicles bridging the RA and LA myocardium at the level of coronary sinus are less affected by atrial wall stretch or remodeling process. This explanation is indirectly supported by evidence that the most preserved LA wall thickness is observed in its lowest segment, adjacent to the coronary sinus musculature.47
Whether or not a P wave abnormality in an individual patient is explained primarily by anatomical features of interatrial connections, age- or disease-related structural remodeling, or functional characteristics of atrial substrate is not possible to determine with certainty at this point as data on the subject are still scarce.48 Available mapping data indicate the presence of distinct LA breakthrough sites at the interatrial septum that demonstrate substantial interindividual variability30, 37 corresponding to the location of well characterized and similarly variable interatrial pathways.35, 38 An important functional component affecting P wave morphology is the origin of sinus rhythm (which may be located anywhere from the midseptal region to the junction with the right atrial appendage), its dependence on the heart rate,48 and its relative proximity to specific interatrial connections. In current clinical practice, we evaluate a P wave “phenotype”, i.e., its appearance on the recorded surface ECG lead system without knowing the exact mechanisms underlying its morphology. In the future, it may be possible that improved ability to distinguish anatomical vs. functional factors underpinning specific P wave abnormalities would help to refine ECG-based risk prediction.
Left Atrial Enlargement
Enlargement of atrial chambers may affect P wave morphology and duration. However, ECG parameters proposed as markers of LA enlargement have shown suboptimal test characteristics. Initially, when Morris et al. described negative PTFV1 in patients with mitral and aortic valve disease,17 abnormal PTFV1 was regarded as a reliable ECG surrogate of LAE. However, several years later this concept was challenged by observations made by Josephson et al., who suggested that biphasic P waves in the right precordial leads may represent an interatrial conduction defect and not necessarily linked to LAE.49
Furthermore, in a large study that used computed tomography,50 PTFV1 performed poorly as marker of LAE with sensitivity and specificity of 48% and 51%, respectively. Several reasons can explain the low sensitivity and specificity of abnormal PTFV1 for LAE: (1) the frequent association of LAE with atrial fibrosis, which decreases the amplitude of the P wave, (2) the morphology of PTFV1 depends on the V1 electrode location: the P wave terminal force is more negative if the electrode is located at a higher position than normal (Figure 4).51 This uncertainty and mismatch between ECG and imaging patterns of LAE have led to the introduction of the “LA abnormality” or “atrial cardiomyopathy” terminology that encompasses other atrial alterations such as impaired function and fibrosis. More research is warranted to elucidate the pathophysiological correlates of abnormal PWPs beyond chamber enlargement.
Figure 4:
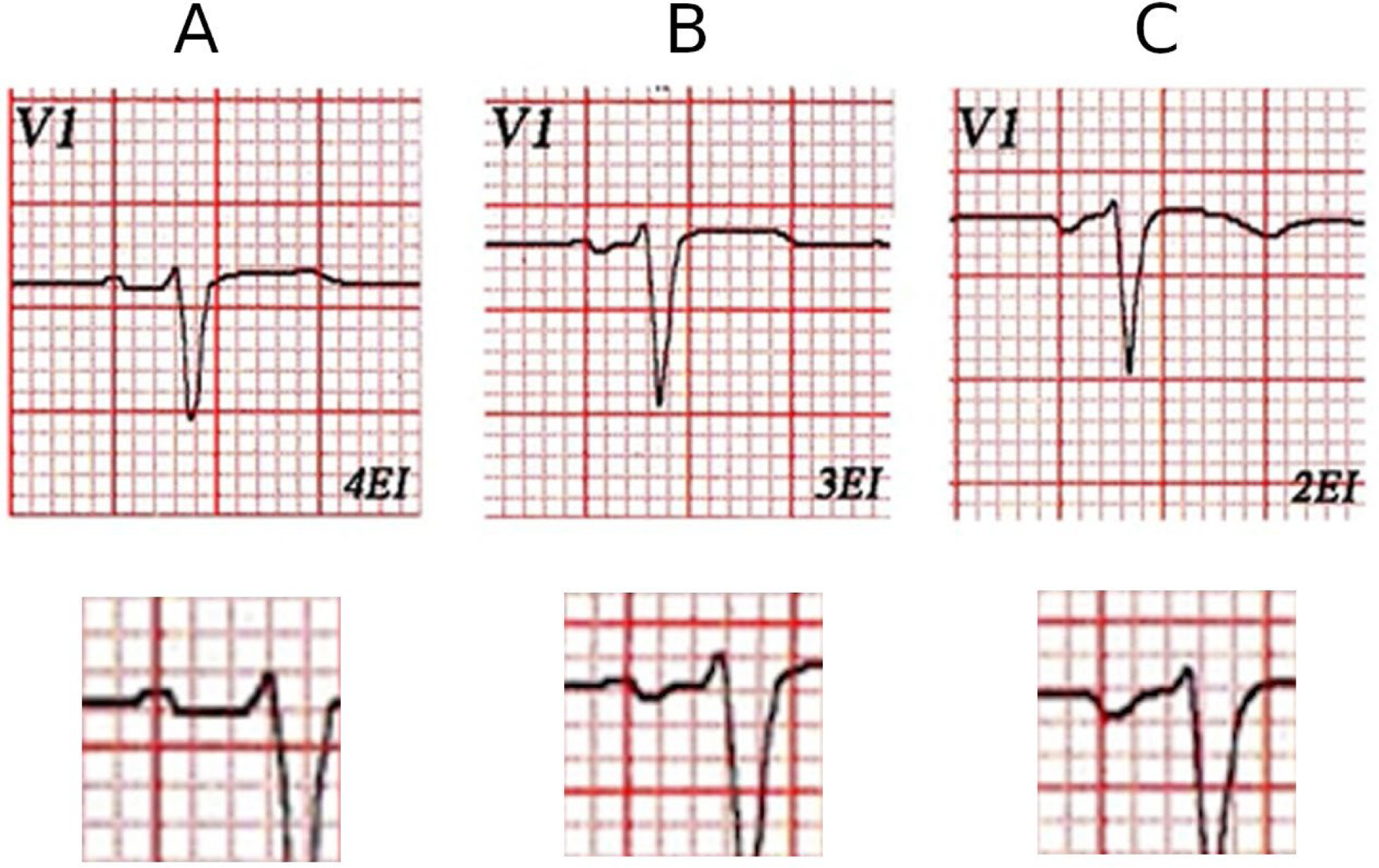
(A) This is V1 lead of a healthy patient with normal left atrial size by echocardiography. V1 electrode placed in normal location (4th ICS) (A), 3rd ICS (B), and in 2nd ICS (C). It is clear that the normal P wave in the 4th ICS becomes progressively more negative (B and C) as the electrode of V1 is placed in higher ICS.
ICS, intercostal space
ASSOCIATION OF P WAVE PARAMETERS WITH CLINICAL OUTCOMES
Despite the inconsistent correlation between abnormal PWPs and LAE, it is increasingly clear that the former are related to poorer health outcomes. In this section, we will summarize new evidence in the past decade that attests to the independent associations of abnormal PWPs with elevated risk of AF, ischemic stroke, sudden cardiac death, poorer prognosis in patients with heart failure, and most recently, cognitive decline and dementia.
Atrial Fibrillation
Abnormal PWPs that have been shown to be associated with higher prevalence or incidence of AF include P wave duration,23, 52, 53 advanced IAB,10, 23, 54 abnormal PTFV1,55, 56 low P wave amplitude in lead I,19 and abnormal P wave axis.22, 57
Advanced IAB was found to be independently associated with an increased risk of AF (hazard ratio [HR], 3.09; 95% CI, 2.51–3.79) in the Atherosclerosis Risk in Communities (ARIC) study23 and in the Copenhagen ECG Study.58 In the latter study, the risk of AF was directly related to the number of inferior leads exhibiting biphasic ± P wave morphology, being the highest in patients with all three inferior leads demonstrating this pattern (HR, 3.38, 95% CI, 2.51–3.79).58 Even in patients with P wave duration less than 120 ms, biphasic P wave morphology in inferior leads, being a variant of atypical advanced IAB,26 was associated with 7-fold increase in the risk of AF.29 Moreover, prolonged P wave duration not meeting criterion for advanced IAB was reported to be associated with elevated AF risk: In a report based on the Framingham Heart Study, the upper 5% of maximum P wave duration had a multivariable-adjusted HR of 2.51 (95% CI, 1.13–5.57, P=0.02) for AF.52 Advanced IAB was also reported to be associated with failure of electrical cardioversion or AF recurrence after cardioversion.59 On the opposite end of the AF spectrum, shorter minimum P wave duration was associated with higher odds of AF in young individuals in the absence of known CVD and AF risk factors.53 In the Copenhagen ECG Study, compared with the reference group (100–105 ms), individuals with very short (≤89 ms; HR, 1.60; 95% CI, 1.41–1.81), intermediate (112–119 ms; HR, 1.22; 95% CI, 1.13–1.31), long (120–129 ms; HR, 1.50; 95% CI, 1.39–1.62), and very long P wave duration (≥130 ms; HR, 2.06; 95% CI, 1.89–2.23) had an increased risk of incident AF.60 Moreover, the different components of P wave duration have varying associations with AF risk: P wave onset to P wave peak duration; HR, 1.57; 95% CI, 1.31–1.88; and P wave peak to P wave end duration; HR, 1.20; 95% CI, 0.99–1.46.61
For each standard deviation increment in PTFV1, the risk of AF increases by 23% (95% CI, 4–46%).56 The upper 5th percentile of PTFV1 is associated with a 1.9-fold increased risk of AF than the lower 95th percentile.56 A simplified ECG metric of abnormal PTFV1—deep terminal negativity of P wave in V1 (DTNPV1)—was found to be independently associated with a 5.02-fold increased risk of AF (95% CI, 3.23–7.80).55
In a retrospective hospital-based cohort study of 525 consecutive patients who underwent radiofrequency catheter ablation for AF, P wave amplitude <0.1 mV in lead I was independently associated with clinical recurrence of AF (adjusted HR, 2.16; 95% CI, 1.30–3.58, P=0.003). Importantly, P wave amplitude in lead I was linearly correlated with LA voltage (β=2.52; 95% CI, 0.61–4.43; P=0.01) and LA conduction velocity (β=1.91; 95% CI, 0.94–2.88; P<0.001).19
Abnormal P wave axis was reported to be associated with increased incidence of AF in community-based cohort studies.22, 57 For example, abnormal P wave axis was independently associated with a 2.34-fold (95% CI, 2.12–2.58) increased risk of AF after adjusting for Cohorts for Heart and Aging Research in Genomic Epidemiology AF (CHARGE-AF) risk score variables.22
More recently, in a study of 366 patients with implantable loop recorders, abnormal PTFV1 (HR, 5.30; 95% CI, 3.25–8.64) and advanced IAB (HR, 5.01; 95% CI, 2.64–9.53) were found to be independently associated with higher risk of AF.62
Ischemic Stroke
In the last few years, several epidemiological studies have further consolidated the role of abnormal PWPs as independent risk factors for ischemic stroke (Table 1).13, 21, 54, 60, 63–67 Some of these studies indicate a stronger association with cardioembolic than thrombotic stroke21 or with non-lacunar than lacunar stroke,21, 64 consistent with the proposition that PWPs are a marker of abnormal atrial structure and function which promote thrombosis and subsequent cardioembolism.
Table 1.
Selected Studies Relating Abnormal P Wave Parameters to Risk of Ischemic Stroke
| Author, Year | P wave Indices | Outcome | Atrial Fibrillation | Adjusted Effect Estimates |
|---|---|---|---|---|
| Soliman, 200956 | PTFV1 P wave area P wave duration |
Incident ischemic stroke | Not excluded not adjusted | PTFV1: HR per 1-SD increase, 1.22; 95% CI, 1.14–1.31. P wave area: HR per 1-SD increase, 1.13; 95% CI, 1.05–1.23. No association with P wave duration. |
| Kamel, 201465 | PTFV1 P wave area P wave duration |
Incident ischemic stroke | Excluded and adjusted | PTFV1: HR per 1-SD increase, 1.21; 95% CI, 1.02–1.44. No associations with P wave area and P wave duration |
| Kamel, 201563 | PTFV1 P wave area P wave duration |
Prevalent and incident brain infarcts on MRI | Excluded and adjusted | PTFV1: Associated with prevalent brain infarcts (RR per SD, 1.09; 95% CI, 1.04–1.16) but not with incident brain infarcts. No associations with P wave area and P wave duration. |
| Kamel, 201564 | PTFV1 | Incident ischemic stroke subtypes | Adjusted | Associated with incident non-lacunar stroke (HR, 1.49; 95% CI: 1.07–2.07) but not with lacunar stroke. |
| Nielsen, 201560 | P wave duration | Incident ischemic stroke | Adjusted | Associated with incident ischemic stroke P wave duration >130 ms: HR, 1.12; 95% CI, 1.02–1.23. |
| O’Neal 201613 | Advanced IAB | Incident ischemic stroke | Adjusted | HR, 1.63; 95% CI, 1.13–2.34. |
| Maheshwari, 201721 | P wave axis | Incident ischemic stroke subtypes | Adjusted | Ischemic stroke: HR, 1.50; 95% CI, 1.22–1.85. Cardioembolic stroke: HR, 2.04; 95% CI, 1.42–2.95. Thrombotic stroke: HR, 1.32; 95% CI, 1.03–1.71. |
| Maheshwari, 201966 | PTFV1 P wave axis P wave duration Advanced IAB |
Incident ischemic stroke | Included | P wave axis: HR, 1.88; 95% CI, 1.36–2.61. Advanced IAB: HR, 2.93; 95% CI, 1.78–4.81. No associations with PTFV1 and P wave duration. Only P wave axis resulted in significant improvement in C-statistic and improvement in risk classification of ischemic stroke compared with CHA2DS2-VASc. |
| García-Talavera 201967 | Advanced IAB | Recurrent ischemic stroke | Prevalent AF excluded | Advanced IAB: HR, 2.3; 95% CI, 1.0–5.5. |
| Martínez-Sellés 202054 | Advanced IAB | Incident ischemic stroke | Prevalent AF excluded | Advanced IAB: HR, 4.89; 95% CI, 1.71–14.05. |
As discussed in the next section, the study by Maheshwari et al.66 not only demonstrated an independent association of abnormal PWPs with ischemic stroke in individuals with AF, but also showed that consideration of PWPs can improve ischemic stroke prediction in individuals with AF, above and beyond the current paradigm, which is the CHA2DS2-VASc (congestive heart failure, hypertension, age, diabetes, stroke, vascular disease, sex) score.68 Therefore, we now have evidence that PWPs are not only associated with AF-related ischemic stroke, but they can also improve risk classification of ischemic stroke in people with AF.
Sudden Cardiac Death
AF is associated with a 2.5-fold increased risk of sudden cardiac death (SCD).69 Risk factors for SCD in people with AF include higher age, higher body mass index, coronary heart disease, hypertension, diabetes, cigarette smoking, left ventricular hypertrophy, higher heart rate, and lower albumin.70 Recent evidence also suggests that PWPs are independently associated with elevated risk of SCD.
Tereshchenko et al. evaluated a simplified ECG metric of abnormal PTFV1—deep terminal negativity of P wave in V1 (DTNPV1)—in relation to SCD in ARIC.55 DTNPV1 was defined from the resting 12-lead ECG as presence of biphasic P wave (positive/negative) in V1 with the amplitude of the terminal negative phase >100 μV. DTNPV1 was associated with an increased risk of SCD after multivariable adjustment including age, sex, coronary heart disease, AF, stroke, and heart failure (HR, 2.49; 95% CI, 1.51–4.10). DTNPV1 also improved reclassification: an additional 3.4% of participants were correctly reclassified into a higher SCD risk group, as compared with traditional coronary heart disease risk factors alone.
In a community-baased study, Maheshwari et al., evaluated the relationship of prolonged P wave duration to SCD.71 The multivariable HR (95% CI) of prolonged P wave duration for SCD was 1.70 (1.31–2.20). This association was attenuated but remained significant after updating covariates (including AF) to the end of follow-up with a HR of 1.35 (1.04–1.76). As an extension to this investigation, Maheshwari et al. evaluated the utility of PWPs—abnormal P wave axis, prolonged P wave duration, and abnormal PTFV1—in improving prediction of SCD benchmarked against the American College of Cardiology/American Heart Association (ACC/AHA) pooled cohort equation72 and an electrical risk score for SCD.73 All three PWPs were independently associated with an increased risk of SCD. Moreover, addition of ECG markers to the foregoing benchmarks improved 10-year model discrimination and risk classification of SCD as measured by C-statistic, net reclassification improvement (NRI) and relative integrated discrimination improvement (IDI).74
Despite the consistent observations above, the mechanisms underlying the relationship of atrial cardiomyopathy to higher SCD risk remain unclear. More research is needed to determine whether the association is explained by shared CV risk factors or that atrial cardiomyopathy inherently increases vulnerability to ventricular tachyarrhyhmias.
Prognosis in Heart Failure
In patients with heart failure who have received cardiac resynchronization therapy (CRT), PWPs provide additional prognostic information. In the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT CRT), patients with normal PTFV1 was associated with lower risk of heart failure or death (HR, 0.55; 95% CI, 0.36–0.84) than those with abnormal PTFV1.75 In another study of patients with heart failure who have received CRT, the presence of IAB (partial or advanced) was associated with 1.9-fold higher risk of AF, death, or heart transplant (95% CI, 1.2–2.9).76
Cognitive Decline and Dementia
Based on the ARIC study, Gutierrez et al. recently reported that abnormal PWPs are associated with greater cognitive decline and higher risk of dementia.18 A total of 13,714 middle-aged participants (mean age, 57 years; 56% women; 23% black) were followed for dementia and change in cognitive function over a mean follow-up of 18 years. Abnormal PTFV1, abnormal P wave axis, prolonged P wave duration, and advanced IAB were determined from study ECGs. All abnormal PWPs except advanced IAB were associated with an increased risk of dementia even after adjustment for incident AF and stroke: multivariable HR of abnormal PTFV1=1.60, 95% CI, 1.41–2.83; abnormal P wave axis, HR=1.36, 95% CI, 1.17–2.57; prolonged P wave duration, HR=1.60, 95% CI, 1.42–1.80. Additionally, abnormal PTFV1 was associated with greater decline in global cognitive function.
Of note, in the foregoing study, there were only 108 participants with advanced IAB; therefore, it is likely that the study was underpowered to detect an association between advanced IAB and dementia. In this regard, Martínez-Sellés et al. evaluated the association of partial and advanced IAB with cognitive impairment in the BAYES registry comprising 332 participants with partial or advanced IAB.77 The investigators found that the prevalence of baseline cognitive impairment was 2.7% in normal P wave, 5.1% in partial IAB, and 10.3% in advanced IAB, P<.001. Advanced IAB was independently associated with baseline cognitive impairment (OR, 4.9; 95% CI, 1.4–16.5). The independent association with cognitive impairment at follow-up existed both for partial IAB (HR, 1.98; 95% CI, 1.18–3.33) and advanced IAB (HR, 2.04; 95% CI, 1.19–3.51).77
In summary, compelling evidence has accumulated in recent years to link abnormal PWPs to a broad range of adverse CV and neurocognitive outcomes. Figure 5 summarizes the key associations discussed in this section. PWPs may soon emerge as a practical method to identify individuals at greater risk of adverse CV and neurocognitive outcomes due to underlying LA abnormality or atrial cardiomyopathy. Before PWPs can change current clinical practice, more work will be needed to confirm their predictive value, and elucidate their biological underpinnings, which will be elaborated in subsequent sections.
Figure 5:
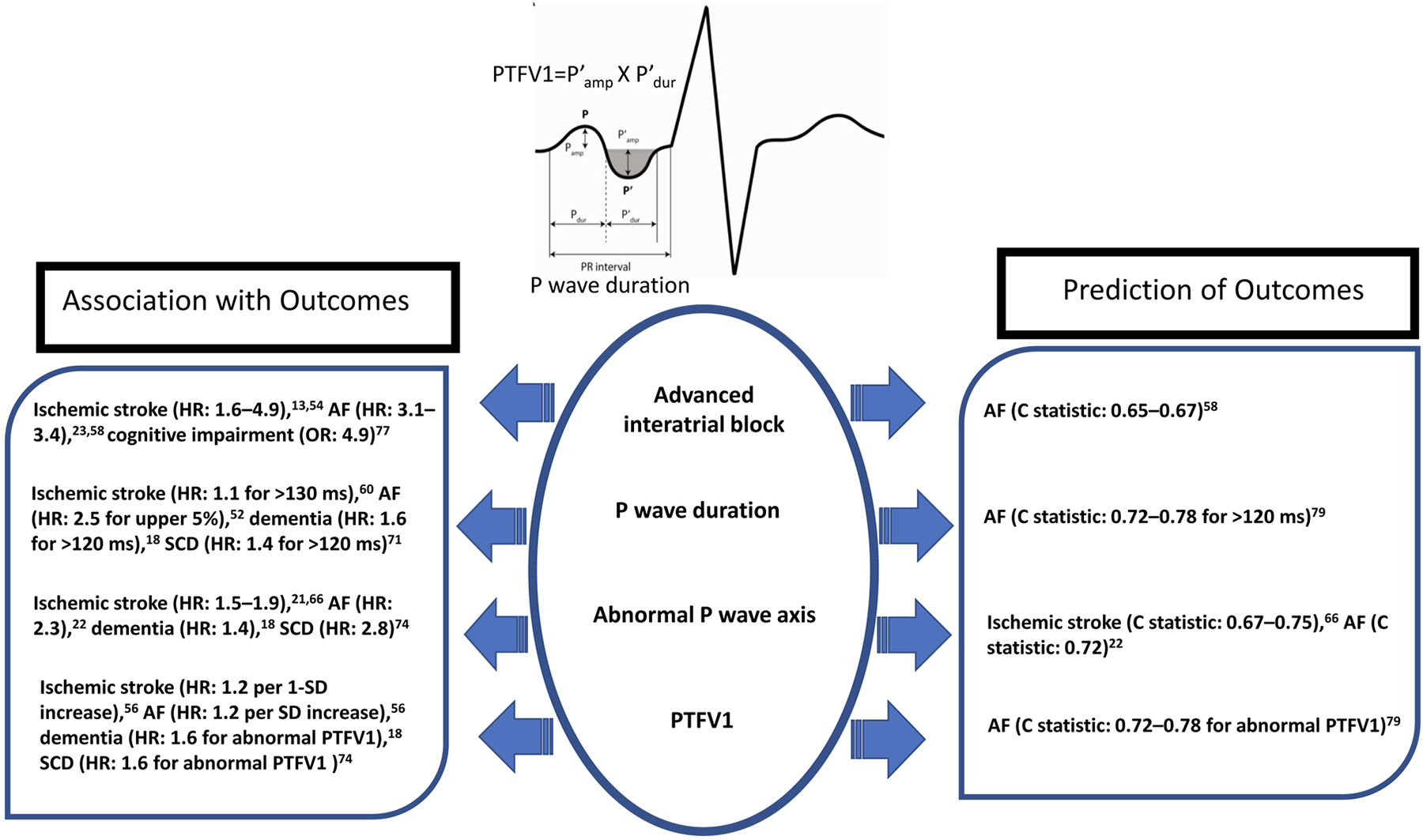
A graphical summary of the key findings from association and prediction studies that link P wave parameters to cardiovascular and dementia outcomes. Hazard ratios and odds ratio are multivariable adjusted. C statistic are based on addition of P wave parameters to conventional benchmarks.
AF, atrial fibrillation; HR, hazard ratio; OR, odds ratio; PTFV1, P wave terminal force in V1; SCD, sudden cardiac death; SD, standard deviation
P WAVE PARAMETERS AND INDICES: ENHANCING PREDICTION OF CLINICAL OUTCOMES
Risk prediction studies and association studies differ fundamentally in objectives, measurements, and clinical context. Association studies aim to confirm a hypothesis that a putative biomarker is associated with risk of a disease. The objective is to provide biological insights into the etiology of a disease, which may point to potential prevention or treatment strategy. Nevertheless, all the conclusions are made at the population level, not at an individual clinical decision-making level. On the other hand, risk prediction studies aim to evaluate the usefulness of a particular marker or group of markers or score in aiding specific clinical decisions at the individual level, such as the need for further investigation or prescribing specific preventative therapy. A biomarker with a highly significant association with an outcome in an association study is usually necessary but not a sufficient condition alone for enhancing prediction in a risk prediction study.
This section focuses on the role of PWPs and PWIs in enhancing risk prediction. The discussion is limited to two outcomes, stroke and AF, given their well-established relationships with P wave abnormalities in association studies. In addition, the existence of validated risk prediction scores for stroke and AF enables appropriate examination of the role of PWPs and PWIs in enhancing prediction of these outcomes beyond contemporary paradigms.
Enhancing Risk Prediction of Ischemic Stroke
The CHA2DS2-VASc score is the most commonly utilized method for predicting ischemic stroke and is the recommended tool to determine the need for preventive oral anticoagulation.68 Improving stroke predictive ability of CHA2DS2-VASc score could optimize oral anticoagulation decisions in AF patients. Hence, Maheshwari et al. refined the CHA2DS2-VASc by adding P wave axis to construct the P2-CHA2DS2-VASc score.66 Using data from the ARIC and Multi-Ethnic Study of Atherosclerosis (MESA), Maheshwari et al. found that compared with the CHA2DS2-VASc score, the P2-CHA2DS2-VASc score improved the C-statistic (95% CI) from 0.60 (0.51–0.69) to 0.67 (0.60–0.75) in ARIC and 0.68 (0.52–0.84) to 0.75 (0.60–0.91) in MESA (validation cohort). In ARIC and MESA, the categorical NRI (95% CI) were 0.25 (0.13–0.39) and 0.51 (0.18–0.86), respectively, and the relative integrated discrimination improvement (IDI) (95% CI) were 1.19 (0.96–1.44) and 0.82 (0.36–1.39), respectively (Table 2).
Table 2.
P2-CHA2DS2VASc Score and 1-Year Ischemic Stroke Risk in Atrial Fibrillation: Multi-Ethnic Study of Atherosclerosis and Atherosclerosis Risk in Communities Study
| Study | Score | C-Statistic (95% CI) | NRI (95% CI) * | Relative IDI (95% CI) |
|---|---|---|---|---|
| ARIC | CHA2DS2VASc† | 0.60 (0.51,0.69) | ||
| P2-CHA2DS2VASc‡ | 0.67 (0.60,0.75) | 0.25 (0.13,0.39) | 1.19 (0.96,1.44) | |
| MESA | CHA2DS2VASc† | 0.68 (0.52–0.84) | ||
| P2-CHA2DS2VASc‡ | 0.75 (0.60–0.91) | 0.51 (0.18–0.86) | 0.82 (0.36–1.39) |
IDI indicates integrated discrimination improvement; NRI, net reclassification improvement; AF, Atrial Fibrillation; ARIC, Atherosclerosis Risk in Communities Study; MESA, Multi-Ethic Study of Atherosclerosis Study
For categorical NRI, we used the following categories for stroke risk: <1%, 1% to <2%, and ≥2%.
Age (1 point for >65, 2 points for >75 years), sex (1 point for female), heart failure (1 point), hypertension (1 point), diabetes mellitus (1 point), previous myocardial infarction/peripheral artery disease (1 point), and prevalent stroke/transient ischemic attack (2 points).
CHA2DS2VASc+abnormal P wave axis (2 points)
Adapted from Maheshwari et al Circulation 2019; 139:180–191
Enhancing Risk Prediction of Atrial Fibrillation
Alexander et al. proposed an AF risk score composed of P wave amplitudes and durations.9 The score was developed based on data from 676 patients (mean age, 65 years; 68% male) without previous AF who were scheduled for coronary angiography. Score points (0, 1, or 2) were allocated based on P wave morphology in the inferior leads (non-biphasic <120 ms, non-biphasic ≥120 ms, or biphasic), voltage in I (>0.20 mV, 0.10–20 mV, or <0.10 mV), and P wave duration (<120 ms, 120–140 ms, or >140 ms). Patients with 5–6 score points (high risk) and 3–4 score points (intermediate risk) were more likely to develop AF than those with 0–2 score points (low risk) (OR, 2.4; 95% CI 1.3–4.4; P=0.006, and OR, 2.1; 95% CI, 1.4–3.3; P= 0.009, respectively). The high‐risk group had a significantly shorter mean time to development of AF (258 weeks; 95% CI, 205–310 weeks) compared to the intermediate-risk group (278 weeks; 95% CI, 252–303 weeks) and low-risk group (322 weeks, 95% CI, 307–338 weeks), P=0.005. In another study of 266 consecutive patients who had suffered an acute ischemic stroke, MVP ECG risk score 5–6 group had 13.2 times higher risk of in-hospital AF compared to MVP ECG risk score 0–2 group (referent). For long-term follow-up, MVP ECG risk score 5–6 group had 5.2 times higher risk of long-term AF compared to MVP ECG risk score 0–2 group.78
In a Danish study by Skov et al. that included 152,759 primary care patients free of AF, adding IAB to a conventional risk model for AF significantly increased AUC for 10-year risk prediction of AF in individuals with CVD (difference in AUC, 1.09%; 95% CI, 0.43–1.74%) and without CVD at baseline (difference in AUC, 1.01%; 95% CI, 0.40–1.62%).58
Although Magnani et al. reported significant associations of P wave duration, area, and PTFV1 with incident AF, the contribution of these PWPs toward enhancing AF risk prediction was minimal and heterogeneous (Table 3).79 The analysis included 3,110 participants (mean age, 62.6 years; 56.9% women) from the Framingham Heart Study (FHS) and 8,254 participants (mean age, 62.3 years; 57.3% women) from the ARIC study. The multivariable model (CHARGE-AF risk model) had a C-statistic of 0.78 in FHS (95% CI, 0.75–0.80) and 0.71 in ARIC (95% CI, 0.69–0.73). In neither cohort did the C-statistic improve with the addition of PWPs. The largest NRI was that of P wave duration >120 ms in FHS (2.9%) and PTFV1 >4000 μV∙ms in ARIC (2.0%). PTFV1 showed the largest improvement in IDI, reaching 5.0% (95% CI, 1.5–8.4) in the ARIC study. Notably, ECG recording was conducted in ARIC using standardized methods which involved chest electrode locator, which might have resulted in a more accurate location of chest leads, and subsequently different PTFV1 as compared to FHS. Also, the racial structure of both studies is inherently different, with FHS composed of all White participants and ARIC including over 20% Black participants. The known racial differences in PWPs might have played a role in heterogeneity in the results between the two studies.56
Table 3:
P Wave Parameters and Prediction of Atrial Fibrillation in the Framingham Heart Study and Atherosclerosis Risk in Communities Study
| C-Statistic (95% CI) | NRI | Relative IDI, % (95% CI) | ||||
|---|---|---|---|---|---|---|
| FHS | ARIC | FHS | ARIC | FHS | ARIC | |
| Base model* | 0.78 (0.75,0.80) | 0.71 (0.69,0.73) | - | - | - | - |
| +P duration >120 ms | 0.78 (0.75,0.80) | 0.72 (0.69,0.74) | 2.9% | −0.1% | 2.4 (−0.2,10.8) | 6.1 (2.5,9.8) |
| +P area ≥95th percentile | 0.78 (0.75,0.80) | 0.71 (0.69,0.74) | −0.1% | 1.5% | 0.1 (−0.4,1.7) | 0.8 (−0.4,2.0) |
| +P terminal force >4000 μV∙ms | 0.78 (0.75,0.81) | 0.72 (0.70,0.74) | 0.04% | 2.0% | 0.003 (−0.6,0.6) | 5.0 (1.5,8.4) |
IDI indicates integrated discrimination improvement; NRI, net reclassification improvement; AF, Atrial Fibrillation; ARIC, Atherosclerosis Risk in Communities Study; FHS, Framingham Heart Study; 95 CI, 95% confidence interval
Multivariable-adjusted for age, sex, race (in ARIC), current smoking, height, weight, systolic and diastolic blood pressures, heart rate, total/high-density lipoprotein cholesterol, ECG-based LVH, diabetes, history of MI, and prevalent heart failure. P wave parameters added as dichotomous variables (<95th vs. ≥95th percentile).
Adapted from Magnani et al. Am Heart J 2015;169:53–61.e1
Finally, benchmarked against the CHARGE-AF risk score, consideration of abnormal P wave axis improved the C-statistic for AF prediction from 0.719 (95% CI, 0.702–0.736) to 0.722 (95% CI, 0.705–0.739), which corresponded to an NRI of 0.021 (95% CI, 0.001–0.040) and relative IDI of 0.043 (95% CI, 0.018–0.069).22
In summary, findings from AF prediction studies suggest that PWPs have different predictive abilities, which could also vary across populations based on their racial structure, risk status, and the methods by which ECG was recorded. Figure 5 summarizes the key findings from predictions studies discussed in this section.
CHALLENGES AND FUTURE DIRECTIONS
PWPs may identify a subset of patients with underlying atrial cardiomyopathy and further risk stratify them into groups where specific preventive or therapeutic measures can be employed. However, for PWPs to serve as an everyday practiccal tool that can influence clinical decisions, additional work is warranted to standardize their assessment, confirm their reliability and predictive value, elucidate their biological basis, and ultimately, define the risk-benefit ratio of specific interventions in high-risk individuals.
Technical Challenges
Accurate determination of the P wave beginning and end on the ECG is a key starting point. Traditionally, P wave amplitude and duration have been measured manually on paper or with the use of digital calipers on digitized ECG images, the latter being more accurate. The development of an automated and streamlined process for P wave extraction and analysis is a major advancement towards a time-effective assessment of PWPs. Specific algorithms have been developed aiming to automatically identify the P waves, remove artifacts, and exclude atrial ectopic beats, i.e, factors which could confound the P wave analysis. Signal averaged and beat-to-beat P wave analyses have held promise in the identification of susceptibility to AF by analyzing variations in P wave morphology.80, 81 These methodologies, however, are more technically demanding as they require the analysis of numerous waveforms, and typically utilize automated P wave selection algorithms. Semi-automated processes, where the user can manually evaluate and optimize the outcome of the automated algorithm are also available.
The reliability and reproducibility of manual PWP measurements and automated measurements are important considerations. Studies have shown high inter- and intra-observer agreement for manual PWP measurements, a good level of agreement with automated measurements,82 and good reproducibility.83, 84 The short-term repeatability of PWPs had been previously reported: intraclass correlation coefficients were 0.78 for P wave axis, 0.77 for maximum P area, and 0.58 for maximum P duration. Within- and between-visit Kappa for PTFV1 were 0.68 and 0.46, respectively.85
Influence of Physiological Factors
P wave morphology may be affected by various physiological factors. Several PWPs have been shown to exhibit significant circadian variation; therefore, the diurnal temporal frame of signal acquisition may affect the values obtained.86 Optimal duration of ECG recording for the assessment of PWPs has not yet been defined; one would, however, conceivably expect differences between instantaneous ECG snapshots as compared to continuous ECG monitoring of several hours or days. Additionally, the function of the autonomic nervous system affects the P wave duration; specifically, isoproterenol shortens and epinephrine prolongs P wave duration, beta-adrenergic blockade increases and parasympathetic blockade decreases P wave duration.87 Furthermore, autonomic neuropathy is associated with increased P wave duration in patients with diabetes88 and athletes exhibit prolonged P wave duration, possibly due to exercise-induced high vagal tone.89 There are also reports of variation in P wave dispersion90 relating to body position and this may be particularly relevant when analyzing data from ambulatory ECG recordings. Finally, body habitus influences PWPs: greater body mass index has been associated with longer P wave duration.91
Future Directions
Except for P wave axis, none of the other PWPs are routinely reported on ECG printouts. Nevertheless, the internal software of contemporary digital ECG machines depends on the measurements of PWPs to provide automated interpretation of several ECG abnormalities, including LAE and AF. In other words, even though multiple PWPs are calculated in the background as part of the process of providing automatic interpretation of ECG abnormalities by the digital ECG machines and can be retrieved for research purposes, the PWI measurements are not shown for a clinician. Routine reporting, or easy access, of PWPs from digital ECG machines, will probably happen soon. This is because of the already established clinical utility,66 reasonable repeatability,85 and the ongoing effort for standardization and creating reference values for PWPs.92
Although the standard 12-lead ECG is ubiquitous, orthogonal ECG systems, e.g., Frank’s vector ECG system, can yield superior diagnostic information and have been used in clinical studies.29 In a community-based study of Finnish adults, orthogonal P wave Type 3 (positive in lead X and ± biphasic in lead Y) (Figure 2) was found to be independently associated with 3.01-fold higher risk of hospitalization with AF (9F% CI, 1.66–5.45) than those with Type 1 (positive in leads X and Y and negative in lead Z) (Figure 2).29 Importantly, consideration of both P wave duration and orthogonal P wave morphology can identify individuals who are at low risk of AF: In the Finnish study, participants with P wave duration <110 ms and Type 1 morphology had lower risk of hospitalization with AF (HR 0.46; 95% CI, 0.26–0.83) than other participants.29
Signal averaged ECG (SAECG) P wave analysis has been used to calculate filtered P wave duration (FPD), a more precise and reproducible measure of atrial depoloarization duration. FPD was found to be longer in patients with history of AF compared to normal controls.83 Furthermore, prolonged FPD was associated with AF recurrence after pulmonary vein isolation.93
Wavelet analysis of the P waves has introduced novel avenues to characterize atrial function. This non-invasive tool utilizes data derived from any ECG digital recording and can detect and analyze electrical signals coinciding in time and frequency within the cardiac cycle, which would otherwise be masked using time-domain or frequency-domain methods. P wave wavelet analysis can predict recurrences of paroxysmal AF in patients without structural heart disease94 and in patients with hypertrophic cardiomyopathy.95 It should be noted that these methods produce large volumes of data, the processing of which has significantly improved with the advent of artificial intelligence (AI) methods.
In recent years, AI tools applied to ECG tracings have been increasingly used in the diagnosis and risk prediction of CVD.96 AI methods used in ECG research include the support vector machine, the naive Bayesian classifier, the decision tree, the K nearest neighbor, the linear discrimination analysis, and the artificial neural network, but the best results were obtained using deep learning methods,97 including a better accuracy in the diagnosis of AF and other rhythm disorders.98 The most striking advances are in the prediction of a new AF episode based on AI analysis of ECG tracings of patients in sinus rhythm.99 In another recent study, a machine learning method (decision tree) was used to improve the performance of the prediction model of new AF episodes based on P wave and clinical features in patients with mitral stenosis.100
Considerable challenges and gaps in knowledge remain today which hamper the use of PWPs in everyday clinical practice. Reference values from large, community-based studies will assist in correct interpretation of PWPs in different individuals. The combined investigation of PWPs with multi-modality imaging and functional assessment techniques (e.g., magnetic resonance imaging, invasive and non-invasive electro-anatomical mapping, signal-averaged ECG and 3-dimensional echocardiography) is expected to complement our understanding of the complex pathophysiological processes underlying the clinical manifestation of atrial arrhythmias. Ultimately, prospective clinical studies are needed to establish the role of PWPs as a potential novel clinical biomarker. Moreover, the health impact of biomarkers in clinical practice requires embedding biomarker measurement into a clinical strategy, which is compared with alternate strategies in which the biomarker is not measured. The comparison should be made in terms of impact on health outcomes, risk of CV events or death, or quality of life measures. Ultimately, a PWI-guided strategy trial will be required to test the benefit of incorporating PWPs in clinical decision making.
CONCLUSION
In summary, changes in surface P waves may convey significant information about CVD and dementia risk. Notably, the prediction of ischemic stroke is independent of AF and incremental to the CHA2DS2-VASc score, indicating that abnormalities in PWPs reflect pathological processes that are distinct from arrhythmogenesis. The foregoing phenomenon reinforces the importance of atrial cardiomyopathy as a driver of CV outcomes. Moving forward and advancing the field of atrial cardiomyopathy, more research is urgently needed to clarify how PWPs can be integrated into clinical practice for the prevention, prediction, and treatment of CVD and dementia.
ACKNOWLEDGMENTS:
The authors would like to thank Dr. Niki Oldenburg for her assistance in manuscript preparation and submission.
SOURCES OF FUNDING:
LYC is supported by the USA National Institutes of Health (R01 HL141288, R01 HL126637, K24 HL155813); ALPR is supported in part by CNPq (310679/2016-8 and 465518/2014-1) and by FAPEMIG (PPM-00428-17 and RED-00081-16); PGP is supported by The Swedish Heart-Lung Foundation (grant #20200674) and by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (grant #46702); VPV’s work on P-W wavelet analysis was partially supported by a Hellenic Cardiac Society Grant, ABdeLuna is supported by Foundation Jesus Serra (Autonomous University of Barcelona) and Foundation Daniel Bravo; JSS is supported by USA National Institutes of Health (R34 HL133526 and R34 HL153579), Atricure, Inc. and AliveCor, Inc. EZS is supported the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR001420).
NONSTANDARD ABBREVIATIONS AND ACRONYMS
- IAB
Advanced interatrial block
- ACC/AHA
American College of Cardiology/American Heart Association
- AI
Artificial intelligence
- ARIC
Atherosclerosis Risk in Communities
- AF
Atrial fibrillation
- CRT
Cardiac resynchronization therapy
- CV
Cardiovascular
- CVD
Cardiovascular disease
- CHARGE-AF
Cohorts for Heart and Aging Research in Genomic Epidemiology AF
- CHA2DS2-VASc
Congestive heart failure, hypertension, age, diabetes, stroke, vascular disease, sex
- DTNPV1
Deep terminal negativity of P wave in V1
- ECG
Electrocardiogram
- FPD
Filtered P wave duration
- IDI
Integrated discrimination improvement
- LAE
Left atrial enlargement
- LA
Left atrium
- MVP ECG
Morphology-voltage-P-wave duration electrocardiogram
- MADIT CRT
Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy
- MESA
Multi-Ethnic Study of Atherosclerosis
- NRI
Net reclassification improvement
- PTFV1
P terminal force in V1
- PWI
P wave índex
- PWPs
P wave parameters
- partial IAB
Partial interatrial block
- RA
Right atrium
- SAECG
Signal averaged ECG
- SCD
Sudden Cardiac Death
Footnotes
DISCLOSURES: JSS (AliveCor, Inc., Hillrom Holdings, Inc., National Cardiac, Inc., BraveHeart, Inc.), others (None)
REFERENCES:
- 1.Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D’Avila A, Dobrev D, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Heart Rhythm. 2017;14:e3–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberger JJ, Arora R, Green D, Greenland P, Lee DC, Lloyd-Jones DM, Markl M, Ng J, Shah SJ. Evaluating the Atrial Myopathy Underlying Atrial Fibrillation Identifying the Arrhythmogenic and Thrombogenic Substrate. Circ. 2015;132:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamel H, Okin PM, Longstreth WT Jr., Elkind MS, Soliman EZ. Atrial cardiopathy: a broadened concept of left atrial thromboembolism beyond atrial fibrillation. Future Cardiol. 2015;11:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donal E, Lip GY, Galderisi M, Goette A, Shah D, Marwan M, Lederlin M, Mondillo S, Edvardsen T, Sitges M, et al. EACVI/EHRA Expert Consensus Document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2016;17:355–83. [DOI] [PubMed] [Google Scholar]
- 5.Tiffany Win T, Ambale Venkatesh B, Volpe GJ, Mewton N, Rizzi P, Sharma RK, Strauss DG, Lima JA, Tereshchenko LG. Associations of electrocardiographic P-wave characteristics with left atrial function, and diffuse left ventricular fibrosis defined by cardiac magnetic resonance: The PRIMERI Study. Heart Rhythm. 2015;12:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez-Betancor I, Izquierdo-Gomez MM, Garcia-Niebla J, Laynez-Cerdena I, Garcia-Gonzalez MJ, Barragan-Acea A, Irribarren-Sarria JL, Jimenez-Rivera JJ, Lacalzada-Almeida J. Bayes Syndrome and Imaging Techniques. Curr Cardiol Rev. 2017;13:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciuffo L, Bruna V, Martinez-Selles M, de Vasconcellos HD, Tao S, Zghaib T, Nazarian S, Spragg DD, Marine J, Berger RD, et al. Association between interatrial block, left atrial fibrosis, and mechanical dyssynchrony: Electrocardiography-magnetic resonance imaging correlation. J Cardiovasc Electrophysiol. 2020;31:1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnani JW, Williamson MA, Ellinor PT, Monahan KM, Benjamin EJ. P wave indices: current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol. 2009;2:72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander B, Milden J, Hazim B, Haseeb S, Bayes-Genis A, Elosua R, Martinez-Selles M, Yeung C, Hopman W, Bayes de Luna A, et al. New electrocardiographic score for the prediction of atrial fibrillation: The MVP ECG risk score (morphology-voltage-P-wave duration). Ann Noninvasive Electrocardiol. 2019;24:e12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayes de Luna A, Cladellas M, Oter R, Torner P, Guindo J, Marti V, Rivera I, Iturralde P. Interatrial conduction block and retrograde activation of the left atrium and paroxysmal supraventricular tachyarrhythmia. Eur Heart J. 1988;9:1112–8. [DOI] [PubMed] [Google Scholar]
- 11.Bacharova L, Wagner GS. The time for naming the Interatrial Block Syndrome: Bayes Syndrome. J Electrocardiol. 2015;48:133–4. [DOI] [PubMed] [Google Scholar]
- 12.Baranchuk A, ed. Interatrial block and supraventricular arrhythmias. Clinical implications of Bayés’ Syndrome. Minneapolis, Minnesota, USA: Cardiotext Publishing; 2017. [Google Scholar]
- 13.O’Neal WT, Kamel H, Zhang ZM, Chen LY, Alonso A, Soliman EZ. Advanced interatrial block and ischemic stroke: The Atherosclerosis Risk in Communities Study. Neurol. 2016;87:352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roessel AMV, Escobar-Robledo LA, Degano IR, Grau M, Sala J, Ramos R, Marrugat J, de Luna AB, Elosua R. Analysis of the Association Between Electrocardiographic P-wave Characteristics and Atrial Fibrillation in the REGICOR Study. Rev Esp Cardiol. 2017;70:841–847. [DOI] [PubMed] [Google Scholar]
- 15.Enriquez A, Sarrias A, Villuendas R, Ali FS, Conde D, Hopman WM, Redfearn DP, Michael K, Simpson C, De Luna AB, et al. New-onset atrial fibrillation after cavotricuspid isthmus ablation: identification of advanced interatrial block is key. Europace. 2015;17:1289–1293. [DOI] [PubMed] [Google Scholar]
- 16.Escobar-Robledo LA, Bayes-de-Luna A, Lupon J, Baranchuk A, Moliner P, Martinez-Selles M, Zamora E, de Antonio M, Domingo M, Cediel G, et al. Advanced interatrial block predicts new-onset atrial fibrillation and ischemic stroke in patients with heart failure: The “Bayes’ Syndrome-HF” study. Int J Cardiol. 2018;271:174–180. [DOI] [PubMed] [Google Scholar]
- 17.Morris JJ Jr., Estes EH Jr., Whalen RE, Thompson HK Jr., McIntosh HD. P-Wave Analysis in Valvular Heart Disease. Circ. 1964;29:242–52. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez A, Norby FL, Maheshwari A, Rooney MR, Gottesman RF, Mosley TH, Lutsey PL, Oldenburg N, Soliman EZ, Alonso A, et al. Association of Abnormal P-Wave Indices With Dementia and Cognitive Decline Over 25 Years: ARIC-NCS (The Atherosclerosis Risk in Communities Neurocognitive Study). J Am Heart Assoc. 2019;8:e014553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JK, Park J, Uhm JS, Joung B, Lee MH, Pak HN. Low P-wave amplitude (< 0.1 mV) in lead I is associated with displaced inter-atrial conduction and clinical recurrence of paroxysmal atrial fibrillation after radiofrequency catheter ablation. Europace. 2016;18:384–391. [DOI] [PubMed] [Google Scholar]
- 20.Zeng C, Wei T, Zhao R, Wang C, Chen L, Wang L. Electrocardiographic diagnosis of left atrial enlargement in patients with mitral stenosis: the value of the P-wave area. Acta Cardiol. 2003;58:139–41. [DOI] [PubMed] [Google Scholar]
- 21.Maheshwari A, Norby FL, Soliman EZ, Koene RJ, Rooney MR, O’Neal WT, Alonso A, Chen LY. Abnormal P-Wave Axis and Ischemic Stroke: The ARIC Study (Atherosclerosis Risk In Communities). Stroke. 2017;48:2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maheshwari A, Norby FL, Soliman EZ, Koene R, Rooney M, O’Neal WT, Alonso A, Chen LY. Refining Prediction of Atrial Fibrillation Risk in the General Population With Analysis of P-Wave Axis (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2017;120:1980–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Neal WT, Zhang ZM, Loehr LR, Chen LY, Alonso A, Soliman EZ. Electrocardiographic Advanced Interatrial Block and Atrial Fibrillation Risk in the General Population. Am J Cardiol. 2016;117:1755–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Luna AB, Platonov P, Cosio FG, Cygankiewicz I, Pastore C, Baranowski R, Bayes-Genis A, Guindo J, Vinolas X, Garcia-Niebla J, et al. Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. J Electrocardiol. 2012;45:445–451. [DOI] [PubMed] [Google Scholar]
- 25.Bayés de Luna A, Bayes-Genis A, Fiol M, Baranchuk A. Clinical Electrocardiography. 5th ed: Wiley; 2022. [Google Scholar]
- 26.de Luna AB, Escobar-Robledo LA, Aristizabal D, Restrepo DW, Mendieta G, van Roessel AM, Elosua R, Bayes-Genis A, Martinez-Selles M, Baranchuk A. Atypical advanced interatrial blocks: Definition and electrocardiographic recognition. J Electrocardiol. 2018;51:1091–1093. [DOI] [PubMed] [Google Scholar]
- 27.Kligfield P, Gettes LS, Bailey JJ, Childers R, Deal BJ, Hancock EW, van Herpen G, Kors JA, Macfarlane P, Mirvis DM, et al. Recommendations for the standardization and interpretation of the electrocardiogram: part I: The electrocardiogram and its technology: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circ. 2007;115:1306–24. [DOI] [PubMed] [Google Scholar]
- 28.Burke GM, Wang N, Blease S, Levy D, Magnani JW. Assessment of reproducibility - automated and digital caliper ECG measurement in the Framingham Heart Study. J Electrocardiol. 2014;47:288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eranti A, Carlson J, Kentta T, Holmqvist F, Holkeri A, Haukilahti MA, Kerola T, Aro AL, Rissanen H, Noponen K, et al. Orthogonal P-wave morphology, conventional P-wave indices, and the risk of atrial fibrillation in the general population using data from the Finnish Hospital Discharge Register. Europace. 2020;22:1173–1181. [DOI] [PubMed] [Google Scholar]
- 30.Holmqvist F, Husser D, Tapanainen JM, Carlson J, Jurkko R, Xia YL, Havmoller R, Kongstad O, Toivonen L, Olsson SB, et al. Interatrial conduction can be accurately determined using standard 12-lead electrocardiography: Validation of P-wave morphology using electroanatomic mapping in man. Heart Rhythm. 2008;5:413–418. [DOI] [PubMed] [Google Scholar]
- 31.Dilaveris PE, Gialafos EJ, Andrikopoulos GK, Richter DJ, Papanikolaou V, Poralis K, Gialafos JE. Clinical and electrocardiographic predictors of recurrent atrial fibrillation. Pacing Clin Electrophysiol. 2000;23:352–8. [DOI] [PubMed] [Google Scholar]
- 32.Dilaveris PE, Gialafos EJ, Sideris SK, Theopistou AM, Andrikopoulos GK, Kyriakidis M, Gialafos JE, Toutouzas PK. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J. 1998;135:733–738. [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz R, Demirbag R. P-wave dispersion in patients with stable coronary artery disease and its relationship with severity of the disease. J Electrocardiol. 2005;38:279–84. [DOI] [PubMed] [Google Scholar]
- 34.Marks D, Ho R, Then R, Weinstock JL, Teklemariam E, Kakadia B, Collins J, Andriulli J, Hunter K, Ortman M, et al. Real-world experience with implantable loop recorder monitoring to detect subclinical atrial fibrillation in patients with cryptogenic stroke: The value of p wave dispersion in predicting arrhythmia occurrence. Int J Cardiol. 2021;327:86–92. [DOI] [PubMed] [Google Scholar]
- 35.Platonov PG, Mitrofanova L, Ivanov V, Ho SY. Substrates for intra-atrial and interatrial conduction in the atrial septum: anatomical study on 84 human hearts. Heart Rhythm. 2008;5:1189–95. [DOI] [PubMed] [Google Scholar]
- 36.Ho SY, Anderson RH, Sanchez-Quintana D. Atrial structure and fibres: morphologic bases of atrial conduction. Cardiovasc Res. 2002;54:325–36. [DOI] [PubMed] [Google Scholar]
- 37.Markides V, Schilling RJ, Ho SY, Chow AW, Davies DW, Peters NS. Characterization of left atrial activation in the intact human heart. Circ. 2003;107:733–9. [DOI] [PubMed] [Google Scholar]
- 38.Platonov PG, Mitrofanova LB, Chireikin LV, Olsson SB. Morphology of inter-atrial conduction routes in patients with atrial fibrillation. Europace. 2002;4:183–92. [DOI] [PubMed] [Google Scholar]
- 39.Becker AE. How structurally normal are human atria in patients with atrial fibrillation? Heart Rhythm. 2004;1:627–31. [DOI] [PubMed] [Google Scholar]
- 40.Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol. 2011;58:2225–32. [DOI] [PubMed] [Google Scholar]
- 41.Bisbal F, Baranchuk A, Braunwald E, Bayes de Luna A, Bayes-Genis A. Atrial Failure as a Clinical Entity: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;75:222–232. [DOI] [PubMed] [Google Scholar]
- 42.Dogdus M, Dindas F, Akhan O, Yenercag M, Yildirim A, Abacioglu OO, Kilic S. Impaired left atrial strain in the presence of interatrial block in patients with type 2 diabetes mellitus. Int J Cardiovasc Imaging. 2021;37:2127–2134. [DOI] [PubMed] [Google Scholar]
- 43.Platonov PG. Atrial conduction and atrial fibrillation: what can we learn from surface ECG? Cardiol J. 2008;15:402–7. [PubMed] [Google Scholar]
- 44.Huo Y, Mitrofanova L, Orshanskaya V, Holmberg P, Holmqvist F, Platonov PG. P-wave characteristics and histological atrial abnormality. J Electrocardiol. 2014;47:275–80. [DOI] [PubMed] [Google Scholar]
- 45.Guerra JM, Vilahur G, Bayes de Luna A, Cabrera JA, Martinez-Selles M, Mendieta G, Baranchuk A, Sanchez-Quintana D. Interatrial block can occur in the absence of left atrial enlargement: New experimental model. Pacing Clin Electrophysiol. 2020;43:427–429. [DOI] [PubMed] [Google Scholar]
- 46.Varma N, Suryaprasad A, Cosio FG. Just another case of atrial fibrillation? Pacing Clin Electrophysiol. 2002;25:358–360. [DOI] [PubMed] [Google Scholar]
- 47.Platonov PG, Ivanov V, Ho SY, Mitrofanova L. Left atrial posterior wall thickness in patients with and without atrial fibrillation: data from 298 consecutive autopsies. J Cardiovasc Electrophysiol. 2008;19:689–92. [DOI] [PubMed] [Google Scholar]
- 48.Platonov PG. P-Wave Morphology: Underlying Mechanisms and Clinical Implications. Ann Noninvasive Electrocardiol. 2012;17:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Josephson ME, Kastor JA, Morganroth J. Electrocardiographic left atrial enlargement. Electrophysiologic, echocardiographic and hemodynamic correlates. Am J Cardiol. 1977;39:967–71. [DOI] [PubMed] [Google Scholar]
- 50.Truong QA, Charipar EM, Ptaszek LM, Taylor C, Fontes JD, Kriegel M, Irlbeck T, Mahabadi AA, Blankstein R, Hoffmann U. Usefulness of electrocardiographic parameters as compared with computed tomography measures of left atrial volume enlargement: from the ROMICAT trial. J Electrocardiol. 2011;44:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rasmussen MU, Fabricius-Bjerre A, Kumarathurai P, Larsen BS, Dominguez H, Kanters JK, Sajadieh A. Common source of miscalculation and misclassification of P-wave negativity and P-wave terminal force in lead V1. J Electrocardiol. 2019;53:85–88. [DOI] [PubMed] [Google Scholar]
- 52.Magnani JW, Johnson VM, Sullivan LM, Gorodeski EZ, Schnabel RB, Lubitz SA, Levy D, Ellinor PT, Benjamin EJ. P Wave Duration and Risk of Longitudinal Atrial Fibrillation in Persons >= 60 Years Old (from the Framingham Heart Study). Am J Cardiol. 2011;107:917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang ICY, Austin E, Krishnan B, Benditt DG, Quay CN, Ling LH, Chen LY. Shorter Minimum P-Wave Duration Is Associated with Paroxysmal Lone Atrial Fibrillation. J Electrocardiol. 2014;47:106–112. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Selles M, Elosua R, Ibarrola M, de Andres M, Diez-Villanueva P, Bayes-Genis A, Baranchuk A, Bayes-de-Luna A, Investigators BR. Advanced interatrial block and P-wave duration are associated with atrial fibrillation and stroke in older adults with heart disease: the BAYES registry. Europace. 2020;22:1001–1008. [DOI] [PubMed] [Google Scholar]
- 55.Tereshchenko LG, Henrikson CA, Sotoodehnia N, Arking DE, Agarwal SK, Siscovick DS, Post WS, Solomon SD, Coresh J, Josephson ME, et al. Electrocardiographic deep terminal negativity of the P wave in V(1) and risk of sudden cardiac death: the Atherosclerosis Risk in Communities (ARIC) study. J Am Heart Assoc. 2014;3:e001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC Jr. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rangel MO, O’Neal WT, Soliman EZ. Usefulness of the Electrocardiographic P-Wave Axis as a Predictor of Atrial Fibrillation. Am J Cardiol. 2016;117:100–104. [DOI] [PubMed] [Google Scholar]
- 58.Skov MW, Ghouse J, Kuhl JT, Platonov PG, Graff C, Fuchs A, Rasmussen PV, Pietersen A, Nordestgaard BG, Torp-Pedersen C, et al. Risk Prediction of Atrial Fibrillation Based on Electrocardiographic Interatrial Block. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Relander A, Hellman T, Vasankari T, Nuotio I, Airaksinen JKE, Kiviniemi T. Advanced interatrial block predicts ineffective cardioversion of atrial fibrillation: a FinCV2 cohort study. Ann Med. 2021;53:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nielsen JB, Kuhl JT, Pietersen A, Graff C, Lind B, Struijk JJ, Olesen MS, Sinner MF, Bachmann TN, Haunso S, et al. P-wave duration and the risk of atrial fibrillation: Results from the Copenhagen ECG Study. Heart Rhythm. 2015;12:1887–95. [DOI] [PubMed] [Google Scholar]
- 61.Smith JW, O’Neal WT, Shoemaker MB, Chen LY, Alonso A, Whalen SP, Soliman EZ. PR-Interval Components and Atrial Fibrillation Risk (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2017;119:466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kreimer F, Aweimer A, Pflaumbaum A, Mugge A, Gotzmann M. Impact of P-wave indices in prediction of atrial fibrillation-Insight from loop recorder analysis. Ann Noninvasive Electrocardiol. 2021;26:e12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kamel H, Bartz TM, Longstreth WT, Okin PM, Thacker EL, Patton KK, Stein PK, Gottesman RF, Heckbert SR, Kronmal RA, et al. Association Between Left Atrial Abnormality on ECG and Vascular Brain Injury on MRI in the Cardiovascular Health Study. Stroke. 2015;46:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamel H, O’Neal WT, Okin PM, Loehr LR, Alonso A, Soliman EZ. Electrocardiographic left atrial abnormality and stroke subtype in the atherosclerosis risk in communities study. Ann Neurol. 2015;78:670–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT, Nazarian S, Okin PM. P-Wave Morphology and the Risk of Incident Ischemic Stroke in the Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45:2786–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maheshwari A, Norby FL, Roetker NS, Soliman EZ, Koene RJ, Rooney MR, O’Neal WT, Shah AM, Claggett BL, Solomon SD, et al. Refining Prediction of Atrial Fibrillation-Related Stroke Using the P2-CHA2DS2-VASc Score. Circ. 2019;139:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia-Talavera CS, Acena A, Andres Lopez A, Garcia Torres MA, Olivie Garcia L, de la Cruz Berlanga E, de Los Reyes Oliva Encabo M, Franco-Pelaez J, Tunon J, Rubio JM. Advanced interatrial block: An electrocardiographic marker for stroke recurrence. J Electrocardiol. 2019;57:1–5. [DOI] [PubMed] [Google Scholar]
- 68.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach The Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 69.Chen LY, Sotoodehnia N, Bůžková P, Lopez FL, Yee LM, Heckbert SR, Prineas R, Soliman EZ, Adabag AS, Konety S, et al. Atrial Fibrillation and the Risk of Sudden Cardiac Death: The Atherosclerosis Risk in Communities (ARIC) Study and Cardiovascular Health Study (CHS). JAMA Intern Med. 2013;173:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koene RJ, Norby FL, Maheshwari A, Rooney MR, Soliman EZ, Alonso A, Chen LY. Predictors of sudden cardiac death in atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. PLoS One. 2017;12:e0187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maheshwari A, Norby FL, Soliman EZ, Alraies MC, Adabag S, O’Neal WT, Alonso A, Chen LY. Relation of Prolonged P-Wave Duration to Risk of Sudden Cardiac Death in the General Population (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2017;119:1302–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circ. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 73.Aro AL, Reinier K, Rusinaru C, Uy-Evanado A, Darouian N, Phan D, Mack WJ, Jui J, Soliman EZ, Tereshchenko LG, et al. Electrical risk score beyond the left ventricular ejection fraction: prediction of sudden cardiac death in the Oregon Sudden Unexpected Death Study and the Atherosclerosis Risk in Communities Study. Eur Heart J. 2017;38:3017–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maheshwari A, Norby FL, Soliman EZ, Alonso A, Sotoodehnia N, Chen LY. Association of P-Wave Abnormalities With Sudden Cardiac and Cardiovascular Death: The ARIC Study. Circ Arrhythm Electrophysiol. 2021;14:e009314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baturova MA, Kutyifa V, McNitt S, Polonsky B, Solomon S, Carlson J, Zareba W, Platonov PG. Usefulness of Electrocardiographic Left Atrial Abnormality to Predict Response to Cardiac Resynchronization Therapy in Patients With Mild Heart Failure and Left Bundle Branch Block (a Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy Substudy). Am J Cardiol. 2018;122:268–274. [DOI] [PubMed] [Google Scholar]
- 76.Jacobsson J, Carlson J, Reitan C, Borgquist R, Platonov PG. Interatrial Block Predicts Atrial Fibrillation and Total Mortality in Patients with Cardiac Resynchronization Therapy. Cardiol. 2020;145:720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinez-Selles M, Martinez-Larru ME, Ibarrola M, Santos A, Diez-Villanueva P, Bayes-Genis A, Baranchuk A, Bayes-de-Luna A, Elosua R. Interatrial block and cognitive impairment in the BAYES prospective registry. Int J Cardiol. 2020;321:95–98. [DOI] [PubMed] [Google Scholar]
- 78.Hayiroglu MI, Cinar T, Selcuk M, Cinier G, Alexander B, Dogan S, Cicek V, Kilic S, Atmaca MM, Orhan AL, et al. The significance of the morphology-voltage-P-wave duration (MVP) ECG score for prediction of in-hospital and long-term atrial fibrillation in ischemic stroke. J Electrocardiol. 2021;69:44–50. [DOI] [PubMed] [Google Scholar]
- 79.Magnani JW, Zhu L, Lopez F, Pencina MJ, Agarwal SK, Soliman EZ, Benjamin EJ, Alonso A. P-wave indices and atrial fibrillation: cross-cohort assessments from the Framingham Heart Study (FHS) and Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2015;169:53–61 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Filos D, Chouvarda I, Tachmatzidis D, Vassilikos V, Maglaveras N. Beat-to-beat P-wave morphology as a predictor of paroxysmal atrial fibrillation. Comput Methods Programs Biomed. 2017;151:111–121. [DOI] [PubMed] [Google Scholar]
- 81.Huo Y, Holmqvist F, Carlson J, Gaspar T, Hindricks G, Piorkowski C, Bollmann A, Platonov PG. Variability of P-wave morphology predicts the outcome of circumferential pulmonary vein isolation in patients with recurrent atrial fibrillation. J Electrocardiol. 2015;48:218–25. [DOI] [PubMed] [Google Scholar]
- 82.Rasmussen MU, Kumarathurai P, Fabricius-Bjerre A, Larsen BS, Dominguez H, Davidsen U, Gerds TA, Kanters JK, Sajadieh A. P-wave indices as predictors of atrial fibrillation. Ann Noninvasive Electrocardiol. 2020;25:e12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dhala A, Underwood D, Leman R, Madu E, Baugh D, Ozawa Y, Kasamaki Y, Xue Q, Reddy S, Multicenter P-RS. Signal-averaged P-wave analysis of normal controls and patients with paroxysmal atrial fibrillation: a study in gender differences, age dependence, and reproducibility. Clin Cardiol. 2002;25:525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holmqvist F, Platonov PG, Havmoller R, Carlson J. Signal-averaged P wave analysis for delineation of interatrial conduction - further validation of the method. BMC Cardiovasc Disord. 2007;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Snyder ML, Soliman EZ, Whitsel EA, Gellert KS, Heiss G. Short-term repeatability of electrocardiographic P wave indices and PR interval. J Electrocardiol. 2014;47:257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dilaveris PE, Farbom P, Batchvarov V, Ghuran A, Malik M. Circadian behavior of P-wave duration, P-wave area, and PR interval in healthy subjects. Ann Noninvasive Electrocardiol. 2001;6:92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheema AN, Ahmed MW, Kadish AH, Goldberger JJ. Effects of autonomic stimulation and blockade on signal-averaged P wave duration. JACC. 1995;26:497–502. [DOI] [PubMed] [Google Scholar]
- 88.Bissinger A, Grycewicz T, Grabowicz W, Lubinski A. The effect of diabetic autonomic neuropathy on P-wave duration, dispersion and atrial fibrillation. Arch Med Sci. 2011;7:806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilhelm M, Roten L, Tanner H, Wilhelm I, Schmid JP, Saner H. Atrial remodeling, autonomic tone, and lifetime training hours in nonelite athletes. Am J Cardiol. 2011;108:580–5. [DOI] [PubMed] [Google Scholar]
- 90.Oylumlu M, Dogan A, Ozer O, Yuce M, Ercan S, Davutoglu V. Effects of lying position on P-wave dispersion in patients with heart failure. Med Princ Pract. 2014;23:556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nasir JM, Rubal BJ, Jones SO, Shah AD. The effects of body mass index on surface electrocardiograms in young adults. J Electrocardiol. 2012;45:646–51. [DOI] [PubMed] [Google Scholar]
- 92.Soliman EZ, Alonso A, Misialek JR, Jain A, Watson KE, Lloyd-Jones DM, Lima J, Shea S, Burke GL, Heckbert SR. Reference ranges of PR duration and P-wave indices in individuals free of cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA). J Electrocardiol. 2013;46:702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Okumura Y, Watanabe I, Ohkubo K, Ashino S, Kofune M, Hashimoto K, Shindo A, Sugimura H, Nakai T, Kasamaki Y, et al. Prediction of the efficacy of pulmonary vein isolation for the treatment of atrial fibrillation by the signal-averaged P-wave duration. Pacing Clin Electrophysiol. 2007;30:304–13. [DOI] [PubMed] [Google Scholar]
- 94.Vassilikos V, Dakos G, Chatzizisis YS, Chouvarda I, Karvounis C, Maynard C, Maglaveras N, Paraskevaidis S, Stavropoulos G, Styliadis CI, et al. Novel non-invasive P wave analysis for the prediction of paroxysmal atrial fibrillation recurrences in patients without structural heart disease: a prospective pilot study. Int J Cardiol. 2011;153:165–72. [DOI] [PubMed] [Google Scholar]
- 95.Girasis C, Vassilikos V, Efthimiadis GK, Papadopoulou SL, Dakos G, Dalamaga EG, Chouvarda I, Giannakoulas G, Kamperidis V, Paraskevaidis S, et al. Patients with hypertrophic cardiomyopathy at risk for paroxysmal atrial fibrillation: advanced echocardiographic evaluation of the left atrium combined with non-invasive P-wave analysis. Eur Heart J Cardiovasc Imaging. 2013;14:425–34. [DOI] [PubMed] [Google Scholar]
- 96.Ribeiro ALP, Paixao GMM, Gomes PR, Ribeiro MH, Ribeiro AH, Canazart JA, Oliveira DM, Ferreira MP, Lima EM, Moraes JL, et al. Tele-electrocardiography and bigdata: The CODE (Clinical Outcomes in Digital Electrocardiography) study. J Electrocardiol. 2019;57S:S75–S78. [DOI] [PubMed] [Google Scholar]
- 97.Lim HW, Hau YW, Lim CW, Othman MA. Artificial intelligence classification methods of atrial fibrillation with implementation technology. Computer Assisted Surgery. 2016;21:154–161. [Google Scholar]
- 98.Ribeiro AH, Ribeiro MH, Paixao GMM, Oliveira DM, Gomes PR, Canazart JA, Ferreira MPS, Andersson CR, Macfarlane PW, Meira W Jr., et al. Automatic diagnosis of the 12-lead ECG using a deep neural network. Nat Commun. 2020;11:1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, Carter RE, Yao X, Rabinstein AA, Erickson BJ, et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394:861–867. [DOI] [PubMed] [Google Scholar]
- 100.Tse G, Lakhani I, Zhou J, Li KHC, Lee S, Liu Y, Leung KSK, Liu T, Baranchuk A, Zhang Q. P-Wave Area Predicts New Onset Atrial Fibrillation in Mitral Stenosis: A Machine Learning Approach. Front Bioeng Biotechnol. 2020;8:479. [DOI] [PMC free article] [PubMed] [Google Scholar]


