Abstract
The efficacy of 20(S)-camptothecin (CPT), free and incorporated into sterically stabilized liposomes, has been investigated in vitro against Leishmania donovani promastigotes and in vivo in a murine model of visceral leishmaniasis. Incubation of L. donovani promastigotes with free or liposomal CPT inhibited the growth of parasites in a dose-dependent manner. Tissue distribution studies revealed that the intraperitoneal administration of liposomal CPT was efficient for the delivery of high drug levels to the liver and spleen. Treatment of infected mice with intraperitoneal injections of free and liposomal CPT significantly reduced the parasite loads in the livers by 43 and 55%, respectively, compared with the loads for untreated controls. However, both treatments caused normochromic anemia and neutropenia.
Parasites of the genus Leishmania are intracellular protozoans that infect more than 12 million individuals around the world, with 400,000 new cases every year (3). Various pathologies can develop following infection with Leishmania, depending on the species involved, encompassing cutaneous, mucocutaneous, and visceral leishmaniasis (25). The first line of therapy against visceral leishmaniasis is antimonial derivatives, which have variable efficacies and which produce serious side effects. Amphotericin B and pentamidines, which represent the second line of therapy, also have limited efficacies and high levels of toxicity. The high prevalence of leishmaniasis and the emergence of resistance to conventional drugs demonstrate the need to develop new, less toxic, and more efficient treatments (7, 19, 23).
As liposomes are naturally taken up by macrophages of the liver and spleen, the main reservoirs of parasites in visceral leishmaniasis, the use of liposomes represents a logical strategy for the concentration of drugs within these tissues to more efficiently treat this parasitic infection. Mullen et al. (18) have evaluated the antileishmanial efficacies of different amphotericin B formulations (Fungizone, Ambisome, Abelcet, and Amphocil) in a murine model of visceral leishmaniasis. Their results showed that the liposomal formulations of amphotericin B (Ambisome) and Amphocil were the most active formulations against Leishmania donovani in this animal model of infection. A case of visceral leishmaniasis unresponsive to several courses of treatment with standard drugs was successfully cured by a 21-day course (50 mg/day) of liposomal amphotericin B (10).
20(S)-Camptothecin (CPT), a chemotherapeutic agent used to treat solid tumors and leukemias (13), has been shown to be effective in inhibiting the growth of L. donovani promastigotes in vitro (6, 17) by trapping type I DNA topoisomerase to form a complex which inhibits both cleavage and religation reactions of DNA replication, leading to cell death (14). However, the rapid ring opening of CPT to its less effective hydrolyzed carboxylate form in human plasma and its high degree of toxicity constitute the major drawbacks of this drug. Previous studies showed that the lactone ring of CPT is protected upon incorporation of the drug into a lipid bilayer structure like liposomes (8, 9, 16).
The coupling of polyethylene glycol (PEG) of defined molecular weight to liposomes (sterically stabilized liposomes) of specific lipid composition and size is known to increase the ability of the liposomes to move through the lymph system after subcutaneous injection, thereby reducing their levels of accumulation at the site of injection (1, 2). Our previous studies have demonstrated that sterically stabilized liposomes accumulated more efficiently than PEG-free liposomes in lymph nodes, liver, and spleen after administration of a single subcutaneous dose to mice (5). Liposomes to which PEG was coupled reduced the level of toxicity associated with the administration of a CPT derivative (24). In the present study, the efficacy of CPT, free and incorporated into sterically stabilized liposomes, has been evaluated in vitro against L. donovani promastigotes and in vivo in a murine model of visceral leishmaniasis. The tissue distributions and hematologic profiles of both formulations have also been investigated in mice since it is hypothesized that liposomal CPT might reduce the toxic effects associated with the free drug, as reported for other liposomal drug formulations (4, 11, 15).
CPT was a generous gift of M. C. Wani (Research Triangle Institute, Research Triangle Park, N.C.). Dipalmitoylphosphatidylcholine (DPPC), dipalmitoylphosphatidylglycerol (DPPG), and distearoylphosphatidylethanolamine-N-PEG 2000 (DSPE-PEG) were purchased from Avanti Polar Lipids (Alabaster, Ala.). The parasites (L. donovani donovani Sudanese 1S2D) were grown at room temperature and were transferred twice a week in SDM-79 culture medium supplemented with 10% fetal bovine serum (FBS), as described previously (20, 27).
Liposomes composed of DPPC–DPPG–DSPE-PEG in a molar ratio of 10:3:0.83 (mol:mol) were prepared by the method of thin lipid film hydration as described previously (12). For CPT-containing liposomes, the lipid film was hydrated with a solution of CPT presolubilized (9.5 mg/ml) in dimethyl sulfoxide (DMSO) and diluted in phosphate buffer (pH 6.0) (23.75 μg of CPT/μmol of lipid). A small proportion of [14C]DPPC or [3H]CPT was added as a radioactive tracer. The lipid mixture was sonicated for 2 h by using an ultrasonic bath to generate liposomes with diameters that ranged between 160 and 200 nm. The vesicle size distributions of the small unilamellar vesicles were evaluated with a submicron-size-particle analyzer. Unencapsulated drug was removed by centrifugation of the liposomal preparation through a Sephadex G-50 column, and the efficiency of drug entrapment in the liposomal effluent was determined by radioactivity measurements. In vitro liposomal drug retention was evaluated at 4 and 37°C in phosphate buffer and at 37°C in 80% FBS, as described previously (12). Drug retention was evaluated by measurement of the radioactivity in the eluate in comparison with the value at time zero.
To monitor the efficacies of free and liposomal CPT on the growth of L. donovani, parasites were transferred (6 × 106 log-phase promastigotes) into 3 ml of culture medium in the absence or presence of increasing concentrations of free or liposome-encapsulated CPT (0 to 10 μM) or with the corresponding amount of drug-free liposomes. Parasite growth was monitored over 6 days by measuring the absorbance at 600 nm. For the tissue distribution studies, a single bolus of free or liposomal CPT (5.5 mg of CPT/kg of body weight; 12.9 μCi of [3H]CPT/mg of nonradioactive CPT) was administered subcutaneously in the region of the upper right leg or intraperitoneally to uninfected BALB/c mice (weight, 18 to 20 g) in a volume of 500 μl. At specific time points postinjection, the animals were killed, and selected tissues were collected, washed, and weighed. All samples were treated with tissue solubilizer and decolorized with H2O2. Drug and lipid levels were monitored in all samples, with radioactivity measurements obtained by using a liquid scintillation counter.
For the in vivo efficacy studies, BALB/c mice (weight, 18 to 20 g; n = 6 mice per group) were infected with L. donovani (2 × 107 stationary-phase promastigotes) given intravenously in the tail vein. At 2 weeks postinfection, free CPT and liposomal CPT (2.5 mg of CPT/kg of body weight) were administered intraperitoneally to mice in a volume of 500 μl every 2 days over a period of 2 weeks. Infected mice were also treated with intravenous or subcutaneous administrations of free CPT. Five days after the end of the treatment, the mice were killed and the efficacy was evaluated by using liver impression smears. The parasite load was determined by optical microscopy, and each group was compared with the untreated control group. For study of hematologic toxicity, uninfected BALB/c mice were injected intraperitoneally with 500 μl of free or liposomal CPT (2.5 mg of CPT/kg body weight) every 2 days over a 2-week period. Animals treated with phosphate buffer containing 1% DMSO were used as controls. Blood samples were collected on days 0, 6, and 14 for hematologic toxicity analysis. Uncoagulated blood samples were used to obtain a complete blood evaluation, including total red blood cell, hemoglobin, hematocrit, platelet, total white blood cell, neutrophil, and lymphocyte counts. Statistically significant differences between groups were determined by analysis of variance by the Fisher least-significant-difference tests.
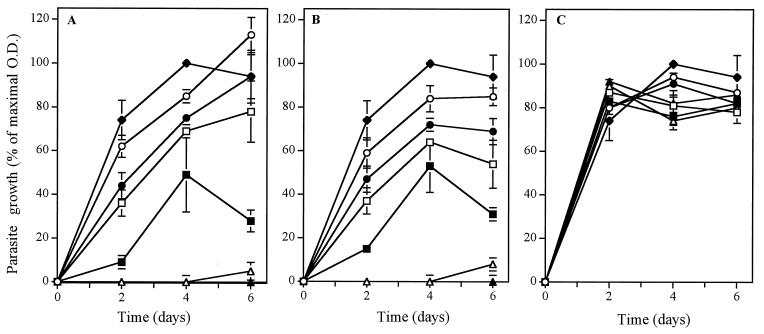
The encapsulation efficiency of CPT in sterically stabilized liposomes was 0.027 ± 0.006 μmol of CPT/μmol of lipid. About 15% of the drug was released from liposomes after a 48-h incubation in phosphate buffer at 4°C, whereas this value reached 30% following incubations of liposomes in phosphate buffer or in 80% FBS at 37°C (data not shown). However, after this initial drug release, complete retention of drug in liposomes was observed for up to at least 14 days. Figure 1 shows the proliferation of L. donovani promastigotes following incubation with different concentrations of free and liposome-encapsulated CPT or with the corresponding amount of drug-free liposomes. Incubation of L. donovani promastigotes with free or liposomal CPT inhibited the growth of parasites in a dose-dependent manner. Complete inhibition of parasite growth was observed following incubation with 10 μM free or liposomal CPT. Drug-free liposomes had no major effect on the proliferation of L. donovani promastigotes.
FIG. 1.
Proliferation of L. donovani promastigotes following incubation with 0.1 μM (○), 0.5 μM (●), 1 μM (□), 2.5 μM (▪), 5 μM (▵), and 10 μM (▴) free CPT (A) or liposome-encapsulated CPT (B) or corresponding drug-free liposomes (C) at 25°C for a 6-day period. Proliferation of L. donovani promastigotes in the absence of any treatment was used as a control (♦). The growth of parasites was evaluated from determination of the optical density (O.D.) at 600 nm on days 0, 2, 4, and 6. The parasite growth is expressed as a percentage of the optical density compared with that for the control. Values represent the mean ± standard error of the mean for three independent experiments.
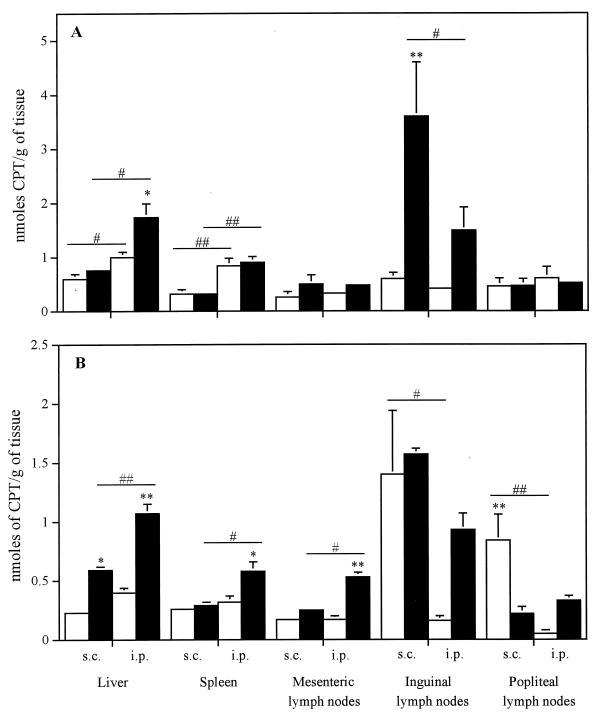
Figure 2 shows the tissue distribution of CPT, free or incorporated into sterically stabilized liposomes, at 6 and 48 h after subcutaneous or intraperitoneal administration of a single bolus to uninfected mice. Overall, except for the concentration observed in popliteal lymph nodes at 48 h after the subcutaneous administration of free drug, the incorporation of CPT into sterically stabilized liposomes resulted in similar or higher levels of drug accumulation in all tissues at all time points. The results show that liposomal drug administered subcutaneously mostly accumulated in inguinal lymph nodes. The intraperitoneal administration of liposomal CPT significantly increased the concentration of drug in the liver, spleen, and mesenteric lymph nodes at 48 h postinjection. Of particular interest, the incorporation of CPT into liposomes increased by 1.8 and 2.7 times the concentration of drug in the spleen and liver, respectively.
FIG. 2.
Tissue distribution of free CPT (□) and liposome-encapsulated CPT (▪) at 6 h (A) and 48 h (B) after administration of a single subcutaneous (s.c.) or intraperitoneal (i.p.) dose to uninfected BALB/c mice. Values represent the mean ± standard error of the mean for six animals per group per time point. ∗ and ∗∗, P < 0.05 and P < 0.01 compared with free CPT in the same tissue, respectively. # and ##, P < 0.05 and P < 0.01 compared with mode of administration in the same tissue, respectively.
We next evaluated the effect of the route of administration on the efficacy of free CPT in reducing the parasite loads in the livers of mice infected with L. donovani. As the intraperitoneal injection of liposomal CPT represents the best route of administration tested in the present study to concentrate the drug in the liver, we also evaluated the efficacy of this treatment. The results showed that treatment of mice by subcutaneous or intravenous injection of free CPT did not significantly decrease the parasite loads (L. donovani units) in the livers compared with the loads in the livers of control mice (data not shown). In contrast, treatment of infected animals by intraperitoneal injection of free or liposomal CPT significantly reduced the parasite loads in the livers by 43% ± 12% and 55% ± 17%, respectively, compared with the loads in the livers of untreated control mice.
Table 1 shows the effect of intraperitoneal administration of free and liposome-encapsulated CPT given every 2 days for 14 days to uninfected BALB/c mice on the hematologic parameters on days 6 and 14 of treatment. Both treatments at this dosing regimen caused normochromic anemia, as evidenced by the significant decrease in the proportions of total red blood cells (14 to 24%), hemoglobin (11 to 18%), and hematocrit (13 to 21%) compared with the values for the untreated controls. Treatment of mice with free CPT resulted in 35 and 39% increases in the numbers of platelets on days 6 and 14, respectively, compared with the numbers in the untreated controls. The incorporation of CPT into liposomes to which PEG was coupled had less of an effect on the platelets, increasing their numbers by 14 and 28% on days 6 and 14, respectively, compared with the numbers in the untreated controls. Mice treated with both free and liposomal CPT exhibited neutropenia, with the numbers of neutrophils decreasing by 50 to 67% compared with the numbers in the untreated controls.
TABLE 1.
Hematologic changes on days 6 and 14 after intraperitoneal administration of free or liposomal CPT (2.5 mg/kg) every 2 days for 14 days to uninfected BALB/c micea
| Day and treatment | Red blood cell count (1012/liter) | Hemoglobin concn (g/liter) | Hematocrit | Platelet count (109/liter) | Leukocyte count (109/liter) | Neutrophil count (109/liter) | Lymphocyte count (109/liter) |
|---|---|---|---|---|---|---|---|
| Day 6 | |||||||
| None (control) | 9.38 ± 0.08 | 158 ± 1 | 0.486 ± 0.004 | 1,256 ± 28 | 3.3 ± 0.3 | 0.6 ± 0.0 | 2.6 ± 0.3 |
| Free CPT | 8.05 ± 0.07b | 140 ± 1b | 0.424 ± 0.003b | 1,737 ± 37b | 3.8 ± 0.4 | 0.3 ± 0.1b | 3.5 ± 0.4 |
| Liposomal CPT | 7.82 ± 0.11b | 137 ± 2b | 0.413 ± 0.005b | 1,438 ± 49bc | 5.5 ± 1.5 | 0.3 ± 0.1b | 5.1 ± 1.4b |
| Day 14 | |||||||
| None (control) | 9.62 ± 0.10 | 162 ± 2 | 0.517 ± 0.008 | 1,147 ± 34 | 5.2 ± 0.5 | 0.9 ± 0.1 | 4.2 ± 0.5 |
| Free CPT | 7.33 ± 0.08b | 136 ± 1b | 0.418 ± 0.005b | 1,595 ± 50b | 3.8 ± 1.1 | 0.3 ± 0.1b | 3.4 ± 1.0 |
| Liposomal CPT | 7.27 ± 0.04b | 133 ± 1b | 0.409 ± 0.003b | 1,463 ± 42bc | 5.1 ± 1.0 | 0.4 ± 0.1b | 4.7 ± 0.9 |
Untreated mice were used as controls. Values represent the means ± standard errors of the means for six mice per group.
Significantly different (P < 0.05) compared with untreated control on the same day.
Significantly different (P < 0.05) compared with free CPT-treated group on the same day.
Our in vitro studies showed that CPT incorporated into 160- to 200-nm-diameter, sterically stabilized liposomes composed of DPPC–DPPG–DSPE-PEG (10:3:0.83 mol:mol) was as effective as the free agent in inhibiting the proliferation of L. donovani promastigotes. These results indicate that the incorporation of CPT into these phospholipid vesicles did not affect its ability to inhibit the proliferation of the parasites. Bodley et al. (6) have previously demonstrated that the 50% inhibitory concentration (IC50) of CPT for L. donovani promastigotes (MHOM/SD/62/1S-CL2D) after a 48-h incubation at 26°C was 2.2 μM. The differences between the IC50s could be due to the different strains and concentrations of L. donovani promastigotes used in the studies.
Our previous studies showed that, as determined by fluorescence microscopy, sterically stabilized liposomes like those used in the present study were mainly localized in macrophage-rich areas such as the subcapsular region of lymph nodes and in the red pulp and marginal zone of the spleen (5). In the present study, tissue distribution studies showed that the intraperitoneal administration of liposome-encapsulated CPT efficiently delivered high levels of drug to the liver and spleen, the main reservoirs of parasites in visceral leishmaniasis. Such targeting of drug to infected tissues should theoretically improve the inhibition of parasite replication. On the other hand, most of the liposome-encapsulated CPT administered subcutaneously accumulated in inguinal lymph nodes. This is in agreement with our previous observations, in which we found that subcutaneously administered sterically stabilized liposomes tend to concentrate in lymph nodes near the injection site instead of other lymph nodes or tissues (5).
The route of administration plays an important role in the efficacy of free CPT in reducing parasite loads in the livers of mice infected with L. donovani. The results showed that both subcutaneous and intravenous injections of free CPT were not effective in reducing the parasite loads in the livers, as determined by comparison of the loads with those in infected untreated controls. In contrast, the intraperitoneal administration of free CPT significantly reduced by 43% the parasite loads in the livers. Incorporation of CPT into sterically stabilized liposomes reduced by 55% the parasite loads in the livers compared with those in the untreated controls. Although significant, the efficacies of both free and liposomal CPT were relatively poor under these experimental conditions. These low levels of efficacy may be associated with the poor ability of CPT to inhibit the intracellular parasite, although it has been shown to have good activity against extracellular parasites in culture medium.
Numerous studies showed that the incorporation of drugs into liposomes reduces the toxic effects associated with their administration (for reviews, see references 4, 11, 15, and 26). For instance, the encapsulation of CPT-11, a hydrophilic derivative of CPT, into liposomes suppressed drug-induced diarrhea, a potentially lethal toxic effect (24). Phillips et al. (21, 22) have shown that the incorporation of zidovudine (AZT) into liposomes abrogated the bone marrow toxicity associated with drug administration. The poor accumulation of liposomes in bone marrow may provide a mechanism for reducing the toxicity of therapeutic agents. In the present study, the administration of CPT induced normochromic anemia and neutropenia. However, in contrast to incorporation of AZT into liposomes, incorporation of CPT into liposomes did not prevent drug hematotoxicity. The poor accumulation of liposomes in the bone marrow may be true for some formulations but not for others. For instance, Mullen et al. (18) showed that liposomal amphotericin B caused significant suppression of parasite burdens in bone marrow in murine visceral leishmaniasis. This suggests that differences in the lipid compositions of liposomes are important contributing factors in the performances of liposomal formulations of drugs.
Because of its unique mode of action, CPT could expand the number of available drugs effective against leishmania. Although the results presented in this report show that the encapsulation of CPT in liposomes does not increase the efficacy of this drug in vitro or in vivo or decrease its toxicity profile, modifications of the liposomal formulation might be developed to reduce toxicity and maintain or even improve the efficacy, as already shown in the literature for numerous other liposomal drugs.
Acknowledgments
We thank Pierrette Gourde, Julie Bestman-Smith, Jean-François Gagné, and Rabeea F. Omar for constructive comments and helpful discussions.
M.O. is an FRSQ Junior 2 Scholar and the recipient of a Burroughs Wellcome Fund award in molecular parasitology.
REFERENCES
- 1.Allen T M. Long-circulating (sterically stabilized) liposomes for targeted drug delivery. Trends Pharmacol Res. 1994;15:215–220. doi: 10.1016/0165-6147(94)90314-x. [DOI] [PubMed] [Google Scholar]
- 2.Allen T M, Hansen C B, Guo L S S. Subcutaneous administration of liposomes: a comparison with the intravenous and intraperitoneal routes of injection. Biochim Biophys Acta. 1993;1150:9–16. doi: 10.1016/0005-2736(93)90115-g. [DOI] [PubMed] [Google Scholar]
- 3.Ashford R W, Desjeux P, Raadt P. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitol Today. 1992;8:104–105. doi: 10.1016/0169-4758(92)90249-2. [DOI] [PubMed] [Google Scholar]
- 4.Bakker-Woudenberg I A J M. Liposomes in the treatment of parasitic, viral, fungal and bacterial infections. J Liposome Res. 1995;5:169–191. [Google Scholar]
- 5.Bestman-Smith J, Gourde P, Désormeaux A, Tremblay M J, Bergeron M G. Sterically stabilized liposomes bearing anti-HLA-DR antibodies for targeting the primary cellular reservoirs of HIV-1. Biochim Biophys Acta. 2000;1468:161–174. doi: 10.1016/s0005-2736(00)00254-6. [DOI] [PubMed] [Google Scholar]
- 6.Bodley A L, McGarry M W, Shapiro T A. Drug cytotoxicity assay for African trypanosomes and Leishmania species. J Infect Dis. 1995;172:1157–1159. doi: 10.1093/infdis/172.4.1157. [DOI] [PubMed] [Google Scholar]
- 7.Buates S, Matlashewski G. Treatment of experimental leishmaniasis with the immunomodulators imiquimod and S-28463: efficacy and mode of action. J Infect Dis. 1999;179:1485–1494. doi: 10.1086/314782. [DOI] [PubMed] [Google Scholar]
- 8.Burke T G, Mishra A K, Wani M C, Wall M E. Lipid bilayer partitioning and stability of camptothecin drugs. Biochemistry. 1993;32:5352–5364. doi: 10.1021/bi00071a010. [DOI] [PubMed] [Google Scholar]
- 9.Burke T G, Staubus A E, Mishra A K. Liposomal stabilization of camptothecin's lactone ring. J Am Chem Soc. 1992;114:8318–8319. [Google Scholar]
- 10.Croft S L, Davidson R N, Thornton E A. Liposomal amphotericin B in the treatment of visceral leishmaniasis. J Antimicrob Chemother. 1991;28(Suppl. B):111–118. doi: 10.1093/jac/28.suppl_b.111. [DOI] [PubMed] [Google Scholar]
- 11.Désormeaux A, Bergeron M G. Liposomes as drug delivery system: a strategic approach for the treatment of HIV infection. J Drug Targeting. 1998;6:1–15. doi: 10.3109/10611869808997877. [DOI] [PubMed] [Google Scholar]
- 12.Harvie P, Désormeaux A, Gagné N, Tremblay M, Poulin L, Beauchamp D, Bergeron M G. Lymphoid tissues targeting of liposome-encapsulated 2′,3′-dideoxyinosine (ddI) AIDS. 1995;9:701–707. doi: 10.1097/00002030-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Jones C B, Clements M K, Wasi S, Daoud S S. Sensitivity to camptothecin of human breast carcinoma and normal endothelial cells. Cancer Chemother Pharmacol. 1997;40:475–483. doi: 10.1007/s002800050690. [DOI] [PubMed] [Google Scholar]
- 14.Kjeldsen E, Svejstrup J Q, Gromova I I, Alsner J, Westergaard O. Camptothecin inhibits both the cleavage and religation reactions of eukaryotic DNA topoisomerase I. J Mol Biol. 1992;228:1025–1030. doi: 10.1016/0022-2836(92)90310-g. [DOI] [PubMed] [Google Scholar]
- 15.Lasic D D. Liposomes: from physics to applications. Amsterdam, The Netherlands: Elsevier; 1993. [Google Scholar]
- 16.Lundberg B B. Biologically active camptothecin derivatives for incorporation into liposome bilayers and lipid emulsions. Anticancer Drug Des. 1998;13:453–461. [PubMed] [Google Scholar]
- 17.Marquis, J. F., M. Drolet, and M. Olivier. Inhibition of Leishmania class I DNA topoisomerase by minor groove-binding ligands: effect on parasite growth and cell cycle. Mol. Biochem. Parasitol., in press.
- 18.Mullen A B, Carter K C, Baillie A J. Comparison of the efficacies of various formulations of amphotericin B against murine visceral leishmaniasis. Antimicrob Agents Chemother. 1997;41:2089–2092. doi: 10.1128/aac.41.10.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olivier M, Romero-Gallo B J, Matte C, Blanchette J, Posner B I, Tremblay M J, Faure R. Modulation of interferon-gamma-induced macrophage activation by phosphotyrosine phosphatases inhibition. Effect on murine leishmaniasis progression. J Biol Chem. 1998;273:13944–13949. doi: 10.1074/jbc.273.22.13944. [DOI] [PubMed] [Google Scholar]
- 20.Olivier M, Tanner C E. Susceptibilities of macrophage populations to infection in vitro by Leishmania donovani. Infect Immun. 1987;55:467–471. doi: 10.1128/iai.55.2.467-471.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips N C, Skamene E, Tsoukas C. Liposomal encapsulation of 3′-azido-3′-deoxythymidine (AZT) results in decreased bone marrow toxicity and enhanced activity against murine AIDS-induced immunosuppression. J Acquir Immune Defic Syndr. 1991;4:959–966. [PubMed] [Google Scholar]
- 22.Phillips N C, Tsoukas C. Liposomal encapsulation of azidothymidine results in decreased hematopoietic toxicity and enhanced activity against murine acquired immunodeficiency syndrome. Blood. 1992;79:1137–1143. [PubMed] [Google Scholar]
- 23.Ray S, Hazra B, Mittra B, Das A, Majumder H K. Diospyrin, a bisnaphthoquinone: a novel inhibitor of type I DNA topoisomerase of Leishmania donovani. Mol Pharmacol. 1998;54:994–999. doi: 10.1124/mol.54.6.994. [DOI] [PubMed] [Google Scholar]
- 24.Sadzuka Y, Hirotsu S, Hirota S. Effect of liposomalization on the antitumor activity, side-effects and tissue distribution of CPT-11. Cancer Lett. 1998;127:99–106. doi: 10.1016/s0304-3835(98)00031-7. [DOI] [PubMed] [Google Scholar]
- 25.Walton B C. American cutaneous and mucocutaneous leishmaniasis. The leishmaniasis in biology and medicine. Vol. 2. London, United Kingdom: Academic Press; 1987. pp. 636–664. [Google Scholar]
- 26.Wasan K M, Lopez-Berenstein G. The past, present, and future uses of liposomes in treating infectious diseases. Immunopharmacol Immunotoxicol. 1995;17:1–15. doi: 10.3109/08923979509052716. [DOI] [PubMed] [Google Scholar]
- 27.White T C, Fase-Fowler F, van Luenen H, Calafat J, Borst P. The H circles of Leishmania tarentolae are a unique amplifiable system of oligomeric DNAs associated with drug resistance. J Biol Chem. 1988;263:16977–16983. [PubMed] [Google Scholar]