Abstract
Background
Human-induced changes to ecosystems transform the availability of resources to predators, including altering prey populations and increasing access to anthropogenic foods. Opportunistic predators are likely to respond to altered food resources by changing the proportion of food they hunt versus scavenge. These shifts in foraging behavior will affect species interactions through multiple pathways, including by changing other aspects of predator behavior such as boldness, innovation, and social structure.
Methods
To understand how foraging behavior impacts predator behavior, we conducted a controlled experiment to simulate hunting by introducing a prey model to captive coyotes (Canis latrans) and compared their behavior to coyotes that continued to scavenge over one year. We used focal observations to construct behavioral budgets, and conducted novel object, puzzle box, and conspecific tests to evaluate boldness, innovation, and response to conspecifics.
Results
We documented increased time spent resting by hunting coyotes paired with decreased time spent active. Hunting coyotes increased boldness and persistence but there were no changes in innovation. Our results illustrate how foraging behavior can impact other aspects of behavior, with potential ecological consequences to predator ecology, predator-prey dynamics, and human-wildlife conflict; however, the captive nature of our study limits specific conclusions related to wild predators. We conclude that human-induced behavioral changes could have cascading ecological implications that are not fully understood.
Keywords: Anthropogenic food, Foraging ecology, Novel object, Puzzle box, Predator-prey, Canis latrans
Introduction
Predators are strongly impacted by human-induced global change due to their large spatial and resource needs (Ripple et al., 2014). Through large-scale ecological changes, humans have altered the availability of both natural prey and non-prey resources for predators (Coon et al., 2019; Ciucci et al., 2020; Mills & Harris, 2020). Despite a frequent emphasis on the effects of the presence or absence of predator on prey (Salo et al., 2010), minimal research has addressed how the loss of prey may impact predator ecology and behavior (Rodewald, Kearns & Shustack, 2011; Newsome et al., 2015a). Availability of prey has decreased for apex predators globally (Wolf & Ripple, 2016; Vinks et al., 2021), although prey resources have increased in some areas (Prange, Gehrt & Wiggers, 2004). Simultaneously, human activity has altered scavenging opportunities (Yirga et al., 2012; Fey et al., 2015) and increased access to anthropogenic resources (Newsome et al., 2015a; Ciucci et al., 2020). Some predators successfully hunt in disturbed environments, often focusing on small prey that become more abundant with human presence (Prange, Gehrt & Wiggers, 2004; Coon et al., 2019; Rodriguez, Lesmeister & Levi, 2021). However, other predators are likely to shift to using non-prey resources that have become increasingly abundant (Yirga et al., 2012; Newsome et al., 2015a; Cozzi et al., 2016). In some situations, predators have shifted almost exclusively to non-prey resources (Parsons, Newsome & Young, 2022). Changes to resource availability are most evident in globally expanding urban and suburban environments (McDonnell & Hahs, 2015; Robins et al., 2019). However, human influence also alters resource availability in non-urban ecosystems through livestock production, rural landfills, and spillage of food during transportation (Ciucci et al., 2020; Mourier, Claudet & Planes, 2020). These shifts in resource availability may influence predator resource use and ecological interactions (Beckmann & Berger, 2003; Ciucci et al., 2020; Gámez & Harris, 2021).
Changes in resource use could alter predator ecology through multiple pathways. First, the use of alternative resources will affect interactions with prey species and other predators (Manlick & Pauli, 2020). Second, predators incorporating more scavenged foods may alter their behavior (Chilvers & Corkeron, 2001; Cozzi et al., 2016; Parsons, Newsome & Young, 2022), and there is a potential relationship between individual diet specialization and animal personality (Toscano et al., 2016). Animal personality describes consistent differences in behaviors between individuals through time, space, and ecological contexts (Young, Mahe & Breck, 2015; Toscano et al., 2016). However, these consistent differences between individuals do not preclude changes to behavioral traits in response to the environment (Sih, Bell & Johnson, 2004).
Personality traits that are likely related to foraging behavior include boldness, persistence, and innovation (Table S1). Boldness describes an individual’s risk aversion (Brooks, Kays & Hare, 2020) and is linked to foraging decisions including willingness to forage alone and to explore novel foraging opportunities (Michelena et al., 2009; Kurvers et al., 2012; but see Carter et al., 2012). Persistence is defined as the amount of time animals spend interacting with a stimulus, or the rate of interaction, which impacts an animal’s ability to acquire resources from novel sources (Chow, Lea & Leaver, 2016; Jacobson et al., 2021). Innovation describes the ability of animals to display new behaviors or modify present behaviors to solve novel problems (Johnson-Ulrich, Johnson-Ulrich & Holekamp, 2018; Barrett, Stanton & Benson-Amram, 2019); a key trait to acquiring resources in new environments. Bold, persistent, and innovative individuals are more likely to succeed when changing foraging strategies as they will be more likely to interact with and solve new foraging problems (Sol, 2009; Sol, Lapiedra & González-Lagos, 2013). Such behavioral changes could also lead to cascading effects, including increased human-wildlife conflict (Barrett, Stanton & Benson-Amram, 2019; Brooks, Kays & Hare, 2020) and altered predator–prey dynamics (Szopa-Comley et al., 2020). Although abundant research has addressed how prey behavioral traits mediate predator–prey interactions (Blake & Gabor, 2014; Toscano, 2017), knowledge regarding the importance of predator traits is lacking (but see Parsons, Newsome & Young, 2022).
Foraging behavior may also influence social behavior of predators. Foraging behavior relates to group size in coyotes (Canis latrans; Bowen, 1981), cooperative behavior in bottlenose dolphins (Tursiops truncates; Díaz López & Shirai, 2008), and conspecific tolerance in multiple species (Peirce & Van Daele, 2006; Fallows, Gallagher & Hammerschlag, 2013; but see Gilchrist & Otali, 2002). Similarly, the abundance of easily captured food in urban environments may increase conspecific tolerance, reduce territoriality, and increase group sizes for predators exploiting these resources (Bateman & Fleming, 2012). Changes to social structure are complex and could alter the distribution of predators on the landscape as well as the landscape of fear experienced by mesopredators and prey species (Prugh et al., 2009; Kohl et al., 2019).
Opportunistic predators that integrate diverse food items into their diet are most likely to alter resource use and experience behavioral changes (Eddine et al., 2020; Parsons, Newsome & Young, 2022). In North America, coyotes are ubiquitous opportunistic carnivores that have vastly expanded their range over the past century (Hody & Kays, 2018). This expansion includes urban environments where coyotes consume both prey and non-prey resources (Fedriani, Fuller & Sauvajot, 2001; Gehrt, 2007; Murray et al., 2015) and display different behavioral traits than rural coyotes, including increased boldness and exploratory behavior (Breck et al., 2019; Brooks, Kays & Hare, 2020). Coyotes in urban environments differ widely in their use of anthropogenic foods, likely due to the broad spectrum of urban conditions (Schell et al., 2020). Anthropogenic foods constitute 50% or more of coyote diet in some locations (Fedriani, Fuller & Sauvajot, 2001; Murray et al., 2015; Newsome et al., 2015b) and reliance on anthropogenic food may increase the prevalence of human-wildlife conflicts (White & Gehrt, 2009; Schell et al., 2021). Because of their range expansion associated with human development and ability to consume diverse resources, coyotes present a valuable model system for understanding how foraging strategies impact predator behavior and subsequently influence community ecology and human-wildlife conflict.
We used captive coyotes to investigate relationships between foraging behavior and other behavioral traits. Conducting this work with captive animals allowed us to repeatedly observe the same individuals over one year, control their food availability and delivery, and maintain a relatively constant environment; all factors that are infeasible to manage in wild systems where changes to available food resources often occur with changes to the physical environment (Coon et al., 2019; Robins et al., 2019). By working with captive individuals, we were able to isolate the effect of foraging behavior from other factors. However, working with captive animals that were exclusively scavengers, contrary to their wild counterparts, meant we had to change the foraging behavior by introducing hunting opportunities. Captive animals are typically scavengers, with hunting introduced to prepare them for release into the wild (Vargas & Anderson, 1999) and as an enrichment activity (Markowitz & LaForse, 1987). The opposite shift would be expected with wild predators—from hunting to scavenging (e.g., Fedriani, Fuller & Sauvajot, 2001; Murray et al., 2015). Even so, shifts from scavenging to hunting have been observed in wild populations (Whitehead & Reeves, 2005). Identifying the extent of the relationship between foraging behavior and other behavioral traits is valuable because shifts in either direction require behavioral flexibility, a key trait to surviving in dynamic environments (Sol, 2009; Barrett, Stanton & Benson-Amram, 2019), and innovation in solving new foraging tasks to access novel prey or scavenging resources (Griffin & Guez, 2014; Daniels et al., 2019).
We conducted a controlled experiment to assess relationships between predator foraging patterns and individual behavior. We used a population of captive coyotes as a model system that behave similarly to wild coyotes (Shivik et al., 2009). We selected a subset of the captive coyotes to hunt prey—we introduced a prey model that resembled a lagomorph and required pursuit and capture to acquire food. Control coyotes were also given an immobile prey model to control for the novelty of the model. We compared behavioral budgets, innovation, persistence, and boldness between treatment and control coyotes, and modeled trends in each behavior separately with covariates for treatment, month, and interaction to account for seasonal, annual, and other changes not due to our hunting treatment. We hypothesized that hunting coyotes would decrease stereotyping behavior and increase time spent searching for food due to the increased stimulation of pursuing prey. Learning to capture the prey model also required interacting with a novel object, persistence, and problem-solving skills. Coyotes had to be willing to approach the prey model, interact with the model, and successfully knock the model over to acquire food. We expected that hunting coyotes would become bolder, more persistent, and more innovative than control coyotes across the study period. Finally, we expected that hunting coyotes would behave more aggressively towards an unknown conspecific due to the increased effort to acquire food. These hypotheses are based on previous literature (Williams et al., 1996; Kistler et al., 2009; Breck et al., 2019; Newsome, Howden & Wirsing, 2019), but also reflect the unique conditions of our study system because directions of behavioral change will depend both on initial conditions and how resource availability changes. Our results provide insights into how foraging behavior is linked with other behavioral traits. Results will improve our understanding of the role hunting plays on individual behavior, which could impact predator–prey dynamics (Szopa-Comley et al., 2020), human-wildlife conflict (Barrett, Stanton & Benson-Amram, 2019), and predator conservation (Bombieri et al., 2021) in wild populations.
Materials & Methods
Study site
We conducted this study at the United States Department of Agriculture National Wildlife Research Center’s (NWRC) Predator Research Facility in Millville, Utah, USA. This facility maintains ∼90 adult coyotes, typically housed as male–female pairs in outdoor enclosures (0.1–1.0 ha).
Study subjects
Our study was conducted on eight coyote pairs, four treatment pairs and four control pairs, ranging from 1.5 to 3.5 years old at the start of the study (details in Supplementary Information). We split coyotes equally among treatment groups based on age. Because our study took place across a year, two pairs of treatment coyotes and two pairs of control coyotes reared pups (April–June) during this study for colony management purposes. All coyotes used in this study were captive-born with minimal human intervention at the facility. Occasionally wild birds and mammals have entered the coyote enclosures and some coyotes have therefore killed live prey (S Brummer, pers. comm., 2019). We observed the coyotes involved in this study and their enclosures on a near-daily basis and monitored for any sign of predation and documented no cases by any of the study coyotes throughout the project.
Ethics statement
All research methods and procedures were approved by NWRC’s Institutional Animal Care and Use Committee (QA-3151).
Feeding treatments
Each coyote was fed 650 g of commercial mink food (Fur Breeders Agricultural Cooperative, Logan, Utah, USA) at least six days per week (i.e., 1,300 g per pair) by animal care staff who enter and scatter the food in each enclosure. We continued this feeding method for our control coyotes and for three days per week with our treatment coyotes.
For the hunting treatment, we created a rabbit prey model from a remote-controlled car (Everest Gen7 Sport RC Crawler, Redcat Racing, Phoenix, AZ, USA). We removed the car body and draped the car chassis with rabbit fur. We used foam to sculpt a rabbit head and attached this to the front of the car (Fig. S1). The prey model was approximately 43 × 18 × 19 cm, similar in size to a snowshoe hare (Lepus americanus; Reid, 2006). We fed treatment coyotes using the prey model three days per week to mimic a wild predator acquiring 50% of its food through hunting and 50% through scavenging. To feed coyotes with the prey model, we placed one portion of food on the prey model and placed the food-laden model in the enclosure. Initially, we left the prey model stationary and scattered additional food around it for coyotes to become comfortable with the prey model and associate it with food. As coyotes became more comfortable with the prey model, we began moving it in small, predictable circles, and slowly increased complexity and speed to an unpredictable path to simulate hunting. Upon capturing the prey model, coyotes were allowed to consume the food. We defined capture as knocking the prey model over so that it could no longer move. This required innovation from study subjects because they had to identify ways to access the mobile food. Then we retrieved the model and scattered the remaining food for the day throughout the enclosure. If coyotes did not successfully hunt on a given day, we removed the prey model after one hour and returned at least two hours later and fed the pair using the standard procedure for the facility. While this treatment does not fully mimic hunting in the wild, it does require pursuit and capture of the prey model, two key components of the hunting process (MacNulty, Mech & Smith, 2007). Control coyote pairs were fed with a stationary prey model three days per week to control for the presence of a novel object during feeding.
Behavioral tests
Feeding and nonfeeding focal sampling
We began this study in October 2019 by collecting baseline measures in a pretrial phase for all coyotes. We first introduced the prey model in December 2019. From January to November 2020, we conducted a suite of behavioral tests (Fig. S2).
We conducted monthly focal observations during both feeding and nonfeeding times to construct behavioral budgets for coyotes (Fig. S2). We modified the ethogram from Shivik et al. (2009; Table 1) and conducted continuous observations for 15 min for each coyote. We conducted shorter observations than Shivik et al. (2009) but the same length as other research with captive coyotes (Leary, Schultz & Young, 2021). Shorter observations facilitated sampling multiple times each month and allowed time for behavioral tests and daily animal care routines to be completed during daylight hours. Each month, we observed each coyote during four nonfeeding times, four feedings without the prey model, and four feedings with the prey model (Table S2), with two nonfeeding observations in the morning (6:00–10:00) and two nonfeeding observations in the afternoon/evening (14:00–21:00; depending on the time of year and day length). We conducted nonfeeding observations using a dedicated observation vehicle as a blind parked >50 m away (Schell et al., 2018). We video-recorded all feeding observations for later behavioral coding. Focal observations provided behavioral budget data and we used the proportion of time coyotes spent interacting with the prey model during prey-model feedings as a measure of persistence while hunting.
Table 1. Ethogram of behaviors used to construct behavioral budgets for coyotes.
Observers coded behavior based on the behavior and descriptions. Behaviors were then condensed into five broad categories for analysis to reduce the number of behaviors and potential for interobserver error. Modified from Shivik et al. (2009).
| Behavior | Description | Broad category |
|---|---|---|
| Resting | Coyote laying or sitting down | Resting |
| Locomotion | Purposeful walking, trotting, or running with head up | Active |
| Standing | Standing still with head raised | NA |
| Foraging | Orientating, stalking, and searching at a slow pace with head lowered | Feeding |
| Eating | Coyote eating with visible jaw movement | Feeding |
| Aggressive | Teeth bared, biting, growling, chasing mate away from food. | Social |
| Play | Playful behavior with mate, tail wagging, non-threatening posture | Social |
| Neutral Social | Howling and other behaviors directed at mate that are neither aggressive, nor playful | Social |
| Stereotyping | Repetitive movement with no apparent goal that is repeated for greater than 2 cycles. | Active |
| Investigating car* | Head and gaze oriented towards prey model from a distance while standing or walking slowly. | Feeding/Car |
| Interacting with car* | Behaviors directed towards prey model such as chasing, pouncing, scratching, or biting. | Feeding/Car |
| Scent marking | Urinating or defecating –point event | NA |
Notes.
These behaviors were only relevant during car feeding observations.
Novel object trials
We conducted a novel object test every three months starting in the pretrial phase to measure coyote boldness (Fig. S2 and Table S2). We placed a novel object near the center of the coyote enclosure and used a video camera to record interactions for one hour. Coyotes were in the enclosure when the novel object was placed and generally retreated to the far end of the enclosure 20–50 m away depending on enclosure size. Observation time started when the researcher closed the gate and exited the enclosure. We used a new novel object each period but kept approximate size consistent between periods; novel objects were the puzzle box during the first puzzle box trial (see below), two car tires attached to form a ball, a traffic cone, a 5-gallon water jug, and a cylindrical wire cage. During the initial presentation of the puzzle box, we did not include food so that we could use it as a novel object. All coyotes received the novel objects in the same order so that any differences between control and treatment coyotes could be attributed to longitudinal trends and not confounded by individuals receiving different objects at different timesteps (Schell, 2015). A trained observer coded videos to extract the latency to approach within 5 m, 1 m, and to touch the novel object.
Multi-access puzzle box tests
We used a multi-access puzzle box to test persistence and repeated innovation every three months starting in the pretrial phase (Fig. S2 and Table S2) (Auersperg et al., 2011; Johnson-Ulrich et al., 2021). The puzzle box was a 45.7 × 45.7 × 45.7 cm cube constructed with three sides of clear PVC sheeting with a door in each and three sides of white PVC sheeting (Fig. S3). One door pushed inwards, one door pulled outwards with a racquetball handle, and the third door swung open if a wooden peg was removed. During each testing period, we presented the coyotes with the puzzle box for 10, two-hour trials. The puzzle box remained in the enclosure throughout each testing period (i.e., between daily trials) to allow coyotes to become and remain familiar with its presence. We introduced the puzzle box with food inside and left all doors open for three days for the coyotes to gain familiarity with the puzzle box before trials began. On the first day of testing, we replaced any remaining food, closed all doors, and filmed interactions with the puzzle box continuously for two hours. We placed small bits of food around the doors and smeared peanut butter on the doors to encourage interaction. At the end of each two-hour test trial, we opened the doors and removed the food to prevent coyotes from learning that they could access the food by waiting for the trial to end. We left the box, with the doors open, to reduce neophobia at the beginning of subsequent days. We repeated this procedure for five consecutive days, then left the box inside the enclosure and open with food inside to give study subjects and observers a reprieve from trials for two days before repeating the test procedure on days 8–12 for a total of 10 test days each period. If a coyote solved one solution to the puzzle box, that solution was locked for the remainder of that test period to encourage interaction with other solutions (Jacobson et al., 2021). During the next trial period, all coyotes started with all solutions available to document whether coyotes used familiar solutions or novel solutions. We did not require an individual to use a solution multiple times before locking the solution (e.g., Johnson-Ulrich, Johnson-Ulrich & Holekamp, 2018) because the lack of interaction with the puzzle box (see Results) made this approach untenable. A trained observer coded puzzle box videos for the proportion of time that coyotes spent within 5 m, 1 m, and interacting with the puzzle box. We defined interaction as touching, pawing, or biting the puzzle box. The proportion of time interacting with the puzzle box was used as a measure of persistence. We also recorded latency to approach, interact with, and touch the puzzle box as a second measure of boldness because the responses of coyotes to the novel object and puzzle box were likely to differ since we presented the puzzle box with food and across repeated trials; thus, providing measures of boldness in novel situations and foraging contexts. Finally, we used the number of solutions a coyote solved as a measure of repeated innovation and flexibility.
Conspecific aggression trials
We used a dummy coyote (Lone Howler Coyote Decoy, Flambeau Outdoors, Middlefield, OH, USA; Fig. S4) to test the response of coyotes to an unknown conspecific every three months starting in the pretrial phase (Fig. S2; Table S2). We placed the dummy near the edge of the enclosure and for one hour recorded how coyotes interacted with the dummy. We did not include any scent or auditory stimuli. A trained observer coded videos to record the latency for coyotes to approach within 5 m, 1 m, and interact with the dummy. We documented instances of aggressive or playful behavior directed towards the dummy (Table 1). We conducted the novel object, puzzle box, and conspecific tests every three months to provide longitudinal data without constantly exposing the coyotes to unfamiliar stressors.
Coder reliability
Live focal observations were initially conducted by a single observer before two other observers joined the study in March and August. Before conducting independent observations, new observers conducted at least 10 simultaneous observations with the original observer for training. Training continued until the total time spent in each activity exhibited >90% agreement. The same behavioral categories were used for video-recorded feeding observations, and all three trained observers coded feeding videos after successful training on live observations.
The same three observers coded novel object, puzzle box, and conspecific videos. We trained each coder by watching multiple videos together and at least one video independently to ensure consistency. We conducted a post hoc test of coder reliability with the original coder independently coding one puzzle box video from each of the other coders. The intraclass correlation was >0.95 between all video coders (95% Confidence interval: 0.76–1.00; McGraw & Wong, 1996). We conducted coder reliability on a puzzle box video because it contained all the same behavior definitions as novel object and conspecific videos.
Statistical analyses
We combined behaviors into five categories because of the low proportion of time spent in many activities: resting, active (locomoting and stereotyping), feeding (foraging, eating, investigating prey model, interacting with prey model), social (aggression, play, howling), and prey model (investigating prey model, interacting with prey model). For the feeding trials with the prey model, investigating and interacting with the prey model were included in feeding behavior, but also analyzed separately because of our interest in behaviors directed at the prey model. We did not include standing behavior in the analysis because it made up a small proportion of time and lacked ecological relevance. Initial plotting showed no trends in scent-marking behavior; therefore, we did not formally analyze these data. We used mixed-effects beta regression from the “glmmTMB” package (Brooks et al., 2017) in Program R version 4.1.0 (R Core Team, 2021) to test fixed effects of month and treatment and an interaction between month and treatment. We included random effects for individual coyote ID nested within pair. With this model specification, a significant interaction term indicates that treatment and control coyotes showed different behavioral changes through time, suggesting a treatment effect. The fixed effect of month accounts for seasonal shifts in behavior, and our control group allows us to separate seasonal trends from treatment effects. Our design also accounts for changes in behavior due to breeding since both control and treatment groups had equal numbers of breeding and nonbreeding pairs.
For novel object data, we used mixed-effects cox proportional hazards models from the ‘coxme’ package (Therneau, 2020) to test the effects of time (i.e., month of study), treatment, and their interaction on latency to approach 5 m, 1 m, and touch the novel object. We also included a random effect to control for pair and coyote ID. Each behavioral metric was tested in a separate model for all statistical tests. For the puzzle box data, we used mixed-effects beta regression to model the proportion of trial time that coyotes spent interacting with the puzzle box as a measure of persistence using the same model structure as above. We also used mixed-effects cox proportional hazards models to model the latency to approach 1 m and interact with the puzzle box as a measure of boldness. Because only a single coyote solved the puzzle box, we did not conduct any statistical analyses on solving data. For the conspecific test data, we also used mixed-effects cox proportional hazards models and the same model structure as above to model the latency to approach within 5 m and 1 m of the conspecific model. Due to limited interactions with the conspecific model, we did not conduct statistical analyses with interaction data.
We used principal component analysis (PCA) and permutational multivariate ANOVA (PERMANOVA) to evaluate multivariate trends in coyote behavior. We created a multivariate response matrix that included monthly mean proportions of time resting, feeding, and moving during both feeding and nonfeeding observations, monthly mean proportion of time spent within 1 m of the puzzle box, monthly mean proportion of time interacting with the puzzle box, and the latency to approach within 1 m of the puzzle box and 5 m of the novel object for each coyote. We plotted the first two components of the PCA for each month to visualize the grouping of hunting and nonhunting coyotes in multivariate space. We then used PERMANOVA to statistically evaluate separation between groups (Oksanen et al., 2018). We conducted a separate PERMANOVA for each month and included a fixed effect for foraging treatment. All statistical analyses were conducted in Program R 4.1.0 (R Core Team, 2021). We used the BORIS software to code all videos (Friard & Gamba, 2016).
Results
Feeding and nonfeeding focal sampling
Few coyotes successfully hunted the prey model (Table S3). Ten individual coyotes interacted with the prey model, moving or stationary, at least once. Only six coyotes regularly fed from the prey model, two hunting coyotes and four nonhunting coyotes. The two hunting coyotes were from different pairs, one male and one female, and began regularly feeding from the moving prey model in February and April. The four nonhunting coyotes were from three different pairs, two females and two males, and began regularly feeding from the stationary model in May, June, July, and September (Fig. S2). Despite this lack of hunting, we analyzed the data using the a priori treatment assignments. This approach should lead to conservative estimates of change due to the treatment, as hunting coyotes that did not interact with the prey model should weaken treatment effects.
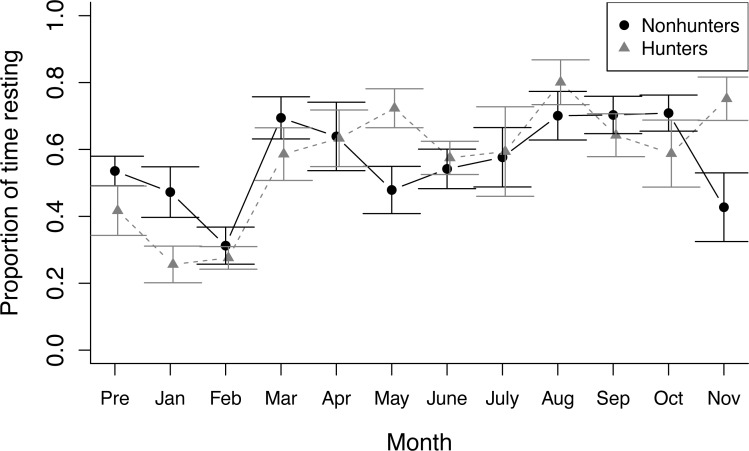
During nonfeeding observations, both hunting and nonhunting coyotes increased the proportion of time spent resting throughout the study (z = 2.284, p = 0.022). This increase was greater for hunting coyotes (z = 2.10, p = 0.036; Fig. 1). The increase in resting behavior was paired with a decrease in time spent active for both groups (temporal term: z = −3.459, p = 0.001; temporal-treatment interaction term: z = −2.302, p = 0.021). Stereotyping behavior decreased in hunting coyotes, but not to a significant degree (z = −0.553, p = 0.580). Contrary to our predictions, there were no significant trends in time spent foraging by either hunting or nonhunting coyotes during nonfeeding times (Table S4).
Figure 1. Nonfeeding behavior.
The proportion of time spent resting by hunting coyotes (grey triangles) and nonhunting coyotes (black circles) during nonfeeding focal observations throughout the study period from November 2019 (Pre) to November 2020. Error bars represent standard error.
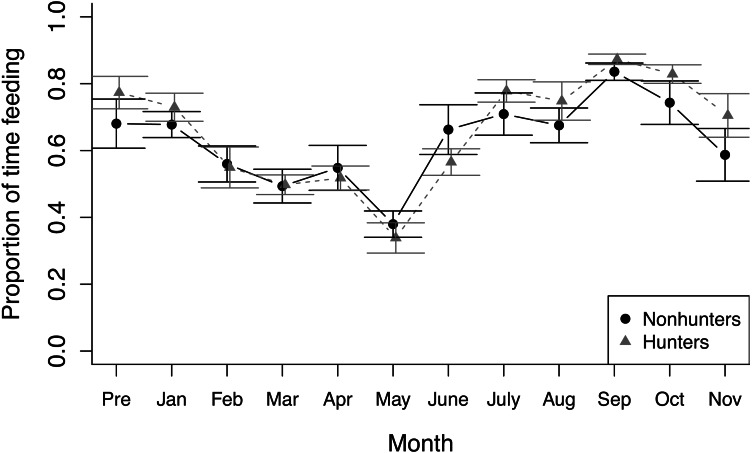
During feeding observations, both hunting and nonhunting coyotes increased the proportion of time they spent feeding as the study progressed (z = 3.025, p = 0.002), which was paired with a decrease in time spent active outside of feeding (z = −8.611, p < 0.001; Fig. 2). We did not observe any changes in other behaviors during feeding observations and there was no evidence of treatment effects on behavioral budgets during feeding observations (Table S5).
Figure 2. Feeding behavior.
The proportion of time that hunting (grey triangles) and nonhunting (black circles) coyotes spent feeding during feeding trials from November 2019 (Pre) through November 2020. Error bars represent standard error.
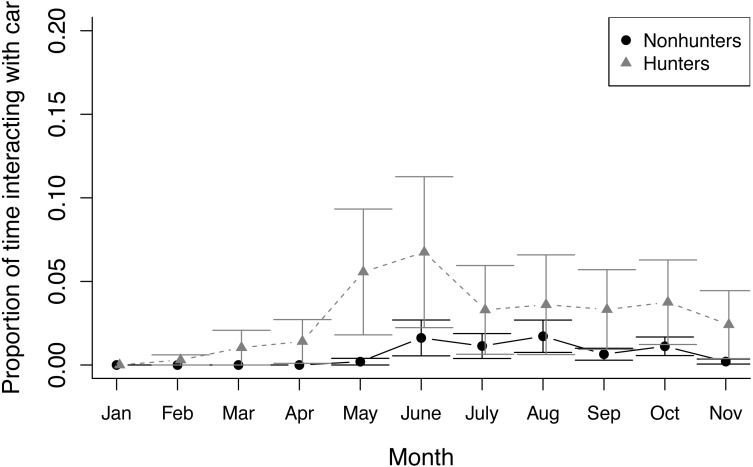
During prey model feeding observations, hunting coyotes decreased the proportion of time spent feeding throughout the study (z = −3.945, p ≤ 0.001) while nonhunting coyotes decreased the proportion of time spent active (z = −7.762, p ≤ 0.001). Both hunting and nonhunting coyotes increased the proportion of time interacting with the prey model (z = 2.394, p = 0.017), and this increase was larger for hunting coyotes (z = 2.522, p = 0.012; Fig. 3; Table S6). The two coyotes that successfully hunted the prey model displayed progressive innovation in solving the foraging task. Initially, they followed the prey model, consuming small bits of food that fell off when the model hit bumps. They then progressed to grabbing mouthfuls of food off the moving model, before ultimately developing strategies of biting or pawing the model to knock it over. While they were able to access some food without innovating, modifying behaviors increased the amount of food available.
Figure 3. Prey model behavior.
The proportion of time that hunting (grey triangles) and nonhunting (black circles) coyotes spent interacting with the prey model during feedings from January through November 2020. Error bars represent standard error.
Novel object trials
For the novel object, puzzle box, and conspecific tests, results were similar at the different distances observed (5 m, 1 m, touch/interact). We present representative results here, and model results for each distance are in the supplementary material. The initial presentation of the puzzle box, without food, was used as a novel object trial. All subsequent novel objects were different objects.
In general, all coyotes showed an unwillingness to approach novel objects. Only five coyotes approached within 1 m of a novel object, and only one regularly approached novel objects (Table S3). Of 78 recorded trials, coyotes approached the novel object within 5 m 31 times, 1 m eight times, and touched only twice. There were no significant effects of time, treatment, or their interaction on the latency to approach 5 m, 1 m, or touch the novel object (Table S7).
Multi-access puzzle box tests
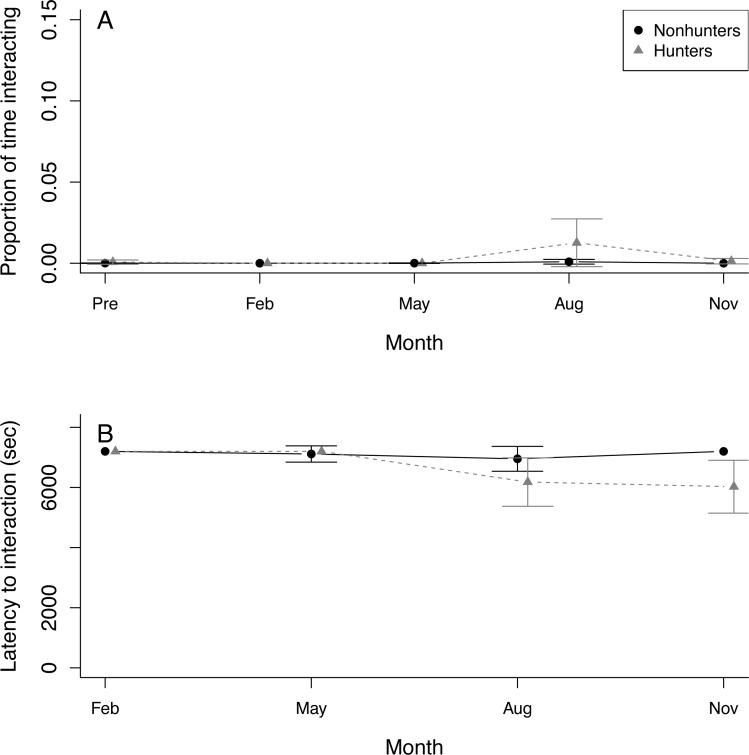
Seven coyotes interacted with the puzzle box at least once, but four of these did not occur until August or November. Only a single coyote successfully solved any of the puzzle box solutions (Table S3). This was a hunting coyote that solved two doors in August and one door in November. Because of this lack of solving, we were unable to use the puzzle box as an indicator of innovation in coyotes. Both hunting and nonhunting coyotes increased the proportion of time they spent within 1 m of the puzzle box throughout the study period (z = 2.292, p = 0.022), and this increase was greater for hunting coyotes (z = 4.271, p < 0.001). Neither group showed any trend in the proportion of time interacting with the puzzle box (Fig. 4A; Table S8). Both hunting and nonhunting coyotes decreased their latency to approach within 1 m of the puzzle box throughout the study (z = 2.78, p = 0.006). Only hunting coyotes decreased their latency to interact with the puzzle box (z = 2.59, p = 0.010; Fig. 4B; Table S9).
Figure 4. Puzzle box behavior.
(A) The proportion of time that hunting coyotes (grey triangles) and nonhunting coyotes (black circles) spent within 1 m of the puzzle box and (B) the latency of coyotes to interact with the puzzle box during puzzle box trials throughout the study period from November 2019 (Pre) through November 2020. Error bars represent standard error.
Conspecific aggression trials
Coyotes showed minimal interest in the coyote dummy used in the conspecific test. Fourteen coyotes approached within 5 m of the conspecific dummy, but only four did so multiple times (Table S3). During the pretrial phase, one coyote pulled the dummy down by the tail, but no other direct interactions occurred throughout the study. Both hunting and nonhunting coyotes slightly decreased their latency to approach within 5 m of the dummy across time (z = 2.00, p = 0.045), but there were no trends in latency to approach within 1 m (z = −1.19, p = 0.23; Table S10).
Combined results
Only a single coyote displayed measurable behaviors in all tests (Table S3). This was a hunting coyote who began regularly capturing the prey model in April, approached within 1 m of four novel objects, approached within 5 m of the conspecific model on four occasions, and solved two doors on the puzzle box in August and one door in November. This was also the only coyote involved in the study that had two wild-born parents.
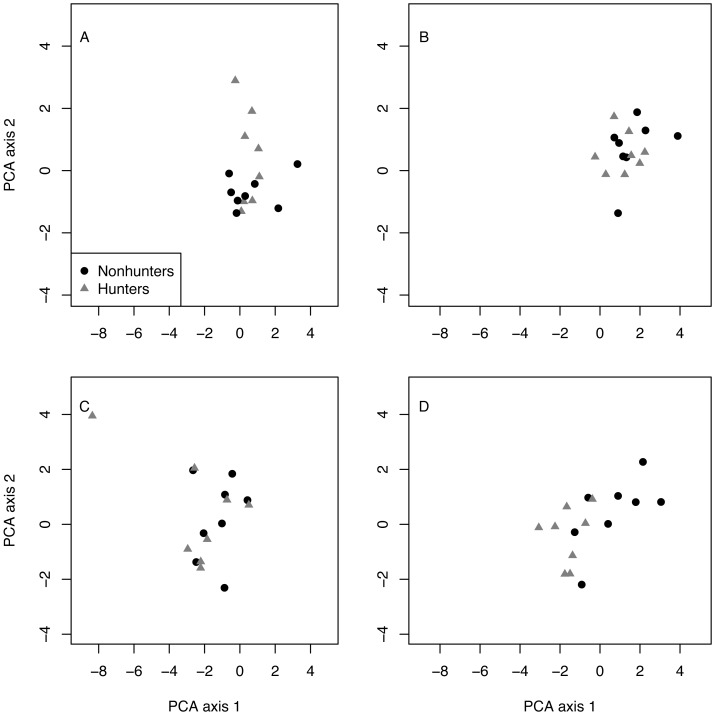
Multivariate analysis revealed that hunting and nonhunting coyotes occupied similar behavioral spaces until the end of the study. The first two components of the PCA explained 69% of the variation in behavioral space (Table 2). Visually, the PCA plots show overlap between the two treatment groups in the pretrial, February, May, and August data. However, the November plot shows some separation between groups. PERMANOVA confirmed that there were significant differences in behavioral space for hunting and nonhunting coyotes in November (F = 4.024, p = 0.018; Fig. 5; Table S11). Based on PCA loadings, this difference was largely due to hunting coyotes spending more time resting during focal observations.
Table 2. Variable loadings and proportion of variance explained by the first two components of the principal component analysis.
The principal component analysis (PCA) loading for each behavioral metric included in the PCA for the first two components. Behavioral metrics followed by (NF) are from nonfeeding observations and metrics followed by (F) are from feeding observations. The final row provides the proportion of variance explained by each component.
| Behavioral metric | PC1 loading | PC2 loading |
|---|---|---|
| Prop time rest (NF) | −0.5466 | 0.2456 |
| Prop time feed (NF) | 0.4099 | −0.0494 |
| Prop time active (NF) | 0.5205 | −0.0989 |
| Prop time rest (F) | 0.0176 | −0.0461 |
| Prop time feed (F) | −0.3777 | 0.0559 |
| Prop time active (F) | 0.2746 | 0.1379 |
| Puzzle box w/in 1 m | −0.1428 | −0.5071 |
| Puzzle box interact | −0.0206 | −0.0456 |
| Novel object latency 5 m | 0.0493 | −0.2328 |
| Puzzle box latency 1 m | 0.1438 | 0.7769 |
| Proportion of Variance Explained | 0.491 | 0.687 |
Figure 5. PCA results.
Plots of the first and second principal component analysis (PCA) axes for hunting (grey triangles) and nonhunting (black circles) coyotes for the pre-treatment data (A), in February (B), August (C), and November (D).
Discussion
We used a controlled experiment to determine how changes in foraging behavior affect individual behavior in captive coyotes. We documented changes in behavioral budgets, boldness, and persistence of coyotes given the opportunity to pursue prey compared to those that remained as scavengers. Consistent with our predictions, hunting coyotes increased boldness and persistence during the puzzle box tests and hunting trials, respectively, and increased time spent resting, which was partially due to decreases in time spent locomoting and stereotyping. Contrary to our hypotheses, we did not observe increases in time spent foraging, innovation, or response to a conspecific. These results indicate that shifts in foraging behavior affect some aspects of predator behavior but not others. Our results are similar to findings from wild black bears (Ursus americanus), which decreased activity and changed temporal activity patterns with changes to foraging behaviors (Beckmann & Berger, 2003). This may have broader consequences, such as shortening hibernation season and altering reproductive patterns of bears (Johnson et al., 2018; Gould et al., 2021). The wild bears shifted from hunting to increased scavenging, while we implemented the opposite shift with coyotes, yet the same pattern of foraging behavior altering other behavioral traits occurred. Thus, this trend could exist across predators and requires further investigation in wild and captive settings.
Changes to coyote behavioral budgets could reflect the different mechanisms that facilitate successful foraging by predators who hunt versus scavenge. Hunting predators often pursue prey during times that optimize success and rest otherwise (Kohl et al., 2019), while scavenging predators must cover large areas in search of food and are therefore more active across time (Kane & Kendall, 2017). In our system, scatter feeding likely left small pieces of food more dispersed throughout the enclosure than our hunting treatment. Scavenging coyotes may have been more likely to search for remaining pieces throughout the day and, thus, remained more active. However, we did not observe changes in the proportion of time foraging, which would have provided additional support for this hypothesis. While delivering food unpredictably in space and time increases food searching behavior in captive red foxes (Vulpes vulpes; Kistler et al., 2009), it does not have the same effect on captive coyotes. Instead, captive coyotes change only a few behaviors, such as vocalization and marking, along with the time of day certain behaviors occurred (Gilbert-Norton, Leaver & Shivik, 2009). Thus, adding temporal unpredictability in addition to hunting behavior could alter predator behavior synergistically. Scavenged resources are often more temporally predictable than live prey, potentially impacting behavioral budgets in wild predators (Mourier, Claudet & Planes, 2020). Predator activity patterns that may change to better track novel resource use can have cascading effects on other wildlife by altering the non-consumptive effects of predators (Moll et al., 2017). Prey species and mesopredators often seek refuge in both space and time to minimize predation risk (Kohl et al., 2019; Smith et al., 2020). Altering predator activity budgets could shift when and how frequently prey are released from predation risk.
We also observed seasonal trends in the behavioral data, including changes in resting during nonfeeding observations (Fig. 1) and time spent feeding during feeding observations (Fig. 2). We expected seasonal trends in behavior (Ellington, Muntz & Gehrt, 2020), and some of these trends were likely related to the breeding season. Our treatment-control design allowed us to account for these seasonal trends in analysis. We did not observe any clear differences in the response to the puzzle box or novel objects by coyotes raising pups either during observations or in the data. Because we designed the project to evaluate foraging behavior and due to small sample sizes, we were unable to explicitly examine changes related to breeding season or between breeding and nonbreeding coyotes.
Persistence is likely a key trait for predator success both while hunting and when exploiting scavenged foods. Successfully capturing prey requires identifying, pursuing, and attacking target animals (MacNulty, Mech & Smith, 2007), and cursorial predators may cover multiple kilometers through this process (Hubel et al., 2016). Persistence is required because successful hunts are rare (Cresswell & Quinn, 2010; Hubel et al., 2016). Persistence is also required for scavenging predators due to the risks presented by other predators and humans (Prugh & Sivy, 2020) or having to access novel food resources (Young, Touzot & Brummer, 2019). In our system, hunting coyotes had to learn to pursue and capture a novel prey item. Completing this task required multiple exposures and persistence, and most coyotes did not begin regularly interacting with the prey model until the fifth month of trials (Fig. 2). Hunting coyotes showed a greater increase in time spent interacting with the prey model, indicating higher persistence in the foraging task.
Persistence is also a key trait for scavenging predators learning to access novel food resources, particularly in urban environments where food may be secured in trash bins and containers, behind fences, or inside structures (Beckmann & Berger, 2003; Newsome et al., 2015a; Newsome et al., 2015b; Breck et al., 2019). However, we did not observe changes in persistence during interactions with the puzzle box. Most coyotes approached more quickly and spent more time near the puzzle box as the study progressed but did not spend more time interacting with the puzzle box. This contradiction between persistence during the hunting task and the puzzle box trials could be related to the more visibly accessible food during hunting trials or because coyotes were exposed to the prey model every week instead of every three months thereby increasing habituation. Hunting and scavenging predators are both likely to experience new foraging tasks in the form of novel prey or non-prey foods due to global change. These changes could introduce additional selective pressures on predators for persistence due to links between persistence and problem solving (Chow, Lea & Leaver, 2016; Daniels et al., 2019; Young, Touzot & Brummer, 2019).
We documented increased boldness with respect to the puzzle box in both groups, but not toward the novel objects. This is likely because coyotes associated the puzzle box with a food reward and habituated to its repeated presence compared to the novel objects, and were, therefore, more willing to approach as the study progressed. Previous research has identified boldness as a key trait for urban predators (Breck et al., 2019; Brooks, Kays & Hare, 2020). Bold predators are more likely to approach and interact with novel objects and less likely to be disturbed by human presence, thus increasing the likelihood of discovering new foraging opportunities (Young et al., 2020). However, bold predators may also experience reduced survival because they are more likely to incite human-wildlife conflict (Greenberg & Holekamp, 2017). Therefore, boldness may not only correlate to accessing resources but also human perceptions of wildlife (Young et al., 2020).
The ecological and conservation consequences of persistent and bold predators are complex. Bold predators are more prone to human-wildlife conflict and more likely to be lethally removed due to conflict situations (Bombieri et al., 2021; Schell et al., 2021), and increased predator boldness could hamper predator conservation efforts in wildland-urban interfaces (Nyhus & Tilson, 2004; Brooks, Kays & Hare, 2020). Despite this, the prevalence of increased boldness in predators expanding their range into urban environments indicates boldness is a valuable trait in novel environments (Barrett, Stanton & Benson-Amram, 2019). Boldness also impacts individual foraging decisions. Bold individuals are more likely to forage in dangerous habitats, which may alter available food resources (Toscano et al., 2016). For mesopredators, increased boldness could also increase predation risk (Geffroy et al., 2015), disrupting expected niche partitioning behaviors. Knowledge on the effects of traits like boldness or persistence on hunting success is lacking and should be addressed in the future to better understand how behavioral traits impact predator–prey interactions.
The captive nature of the study subjects allowed necessary control over experimental conditions, yet inherently limits the scope of inference and the transferability to wild predators. Because the subjects were housed as mated pairs in enclosures, they were insulated from the effects of direct intra- and interspecific interactions, dynamic environments, and other aspects of natural systems. They also likely responded to each other during our trials, although we never observed more than one individual within a pair participate in any trials. While we do not think the individuals within a pair prevented the other from participating, and wild coyotes typically maintain pair bonds, further tests of individuals could be warranted to compare with our results. Regardless, supporting the concept of linkages between foraging mode and behavior with captive animals is a valuable first step. Additionally, our approach shifted scavenging predators to become hunting predators, the opposite shift we hypothesize to become more common with global change (Parsons, Newsome & Young, 2022). However, the broad relationship between foraging mode and other behavioral traits we observed likely exists across predators, whether they are captive or wild. This is supported by evidence of behavioral change in urban black bears with access to human refuse (Beckmann & Berger, 2003) and multiple species with access to landfills (Hidalgo-Mihart et al., 2004; Cozzi et al., 2016). Our results indicate that activity budgets, boldness, and persistence may change with altered resource use, but specific changes will be dependent on the context of predator species and resource changes. We encourage future efforts with wild predators in areas with changing resource availability to evaluate behavioral responses.
Many predators successfully adapt to shifts in resource availability (Bateman & Fleming, 2012; Gámez & Harris, 2021). However, certain individuals in a population likely succeed in this adaptation, while others do not (Schell et al., 2021). In this study, only two of eight coyotes in the hunting treatment fully adapted to the foraging task and regularly fed from the prey model, while six remained wary of the prey model and only occasionally knocked it over. Our approach specifically addressed changes in behavior of individual coyotes over a short time. However, the highly variable participation of individual coyotes highlights that each had different baseline behavioral norms. The individuals who were bolder from the beginning were most likely to approach the prey model sooner and learn the new foraging task. Individuals who failed to adjust quickly would likely struggle in response to shifting resources, highlighting that shifts in resource availability can be a strong selection pressure for inherently bold, innovative, persistent, or flexible individuals. The understanding of the heritability of these behavioral traits is still developing, but research has identified genetic correlations with boldness in multiple vertebrate species (Bubac et al., 2021; Stratton, Nolte & Payseur, 2021) and differential heritability among different behaviors in donkeys (Equus asinus; Navas González et al., 2019). Therefore, environmental changes that select for bold, innovative, or persistent individuals paired with the heritability of these traits could result in shifts in behavior at the population level over time. These selection patterns, in addition to resource spatial heterogeneity, could lead to segregation of predators with different traits and the possible development of new ecotypes (Chilvers & Corkeron, 2001).
There were limited interactions between coyotes and the puzzle box and conspecific model. Like previous research, coyotes struggled to solve the puzzle box task (Stanton et al., 2021). Only one coyote solved the puzzle box, indicating that it was possible but that most of the coyotes were unwilling to interact with the puzzle box enough to find solutions. Very few coyotes made significant attempts to access the food inside the puzzle box and were content to eat the small portion scattered around the box even though the quantity was insufficient to reach satiation. Therefore, we were unable to draw conclusions related to innovation. Similarly, few coyotes interacted with the coyote dummy for us to measure conspecific interactions. We observed many coyotes approach the dummy at the first encounter, but few approached within 5 m of or interacted with the dummy. It is likely that as the coyote approached the dummy, they recognized it was inanimate and not a territorial intruder. Adding a scent or auditory cue may have increased interactions, but we elected to keep methods consistent throughout this study. For animal welfare purposes, we could not use a live conspecific coyote; properly documenting interactions with unfamiliar conspecifics would best be observed in the wild.
Conclusions
Changes to resource availability due to global change will continue to alter predator foraging behavior. It is well understood that changes in foraging behavior can impact space use (Hidalgo-Mihart et al., 2004), migration (Cozzi et al., 2016), and social structure (Chilvers & Corkeron, 2001). Individual behavioral changes are less well understood, likely because it is difficult to observe in the wild due to limited ability to repeatedly test or alter available resources for individuals. Working with captive animals allowed us to observe individuals succeed and fail at adapting to a new foraging task and showed how some behavioral traits change in response to altered foraging behavior. Altered activity budgets, persistence, and boldness will affect how predators interact with other wildlife and humans, leading to ecological consequences (Parsons, Newsome & Young, 2022) and increased human-wildlife conflict (Barrett, Stanton & Benson-Amram, 2019). Future research with wild predators should evaluate the relationship between predator behavioral traits and the proportion of natural food in diets to further elucidate links between foraging and behavior and improve our understanding of ecological consequences.
Supplemental Information
Acknowledgments
We thank S Brummer, M Davis, N Floyd, S Keller, A Merical, and J Shultz for assistance with animal care and research logistics. C Dever, T Khvtisiashvili, and K Pelaez helped in coding observation videos.
Funding Statement
This project was funded by the U.S. Department of Agriculture, National Wildlife Research Center (NWRC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Mitchell A. Parsons conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Andrew Garcia performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Julie K. Young conceived and designed the experiments, performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All research methods and procedures were approved by USDA-National Wildlife Research Center’s Institutional Animal Care and Use Committee (QA-3151).
Data Availability
The following information was supplied regarding data availability:
The raw data are available at GitHub: https://github.com/pars2997/scavenging-hunting-coyotes.
References
- Auersperg et al. (2011).Auersperg AMI, Von Bayern AMP, Gajdon GK, Huber L, Kacelnik A. Flexibility in problem solving and tool use of kea and New Caledonian crows in a multi access box paradigm. PLOS ONE. 2011;6:e20231. doi: 10.1371/journal.pone.0020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, Stanton & Benson-Amram (2019).Barrett LP, Stanton LA, Benson-Amram S. The cognition of ‘nuisance’ species. Animal Behaviour. 2019;147:167–177. doi: 10.1016/j.anbehav.2018.05.005. [DOI] [Google Scholar]
- Bateman & Fleming (2012).Bateman PW, Fleming PA. Big city life: carnivores in urban environments. Journal of Zoology. 2012;287:1–23. doi: 10.1111/j.1469-7998.2011.00887.x. [DOI] [Google Scholar]
- Beckmann & Berger (2003).Beckmann JP, Berger J. Rapid ecological and behavioural changes in carnivores: the responses of black bears (Ursus americanus) to altered food. Journal of Zoology. 2003;261:207–212. doi: 10.1017/S0952836903004126. [DOI] [Google Scholar]
- Blake & Gabor (2014).Blake CA, Gabor CR. Effect of prey personality depends on predator species. Behavioral Ecology. 2014;25:871–877. doi: 10.1093/beheco/aru041. [DOI] [Google Scholar]
- Bombieri et al. (2021).Bombieri G, Penteriani V, Delgado M del M, Groff C, Pedrotti L, Jerina K. Towards understanding bold behaviour of large carnivores: the case of brown bears in human-modified landscapes. Animal Conservation. 2021;24:783–797. doi: 10.1111/acv.12680. [DOI] [Google Scholar]
- Bowen (1981).Bowen WD. Variation in coyote social organization: the influence of prey size. Canadian Journal of Zoology. 1981;59:639–652. doi: 10.1139/z81-094. [DOI] [Google Scholar]
- Breck et al. (2019).Breck SW, Poessel SA, Mahoney P, Young JK. The intrepid urban coyote: a comparison of bold and exploratory behavior in coyotes from urban and rural environments. Scientific Reports. 2019;9:1–11. doi: 10.1038/s41598-019-38543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, Kays & Hare (2020).Brooks J, Kays R, Hare B. Coyotes living near cities are bolder: implications for dog evolution and human-wildlife conflict. Behaviour. 2020;157:289–313. doi: 10.1163/1568539x-bja10002. [DOI] [Google Scholar]
- Brooks et al. (2017).Brooks ME, Kristensen K, Van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal. 2017;9:378–400. doi: 10.32614/RJ-2017-066. [DOI] [Google Scholar]
- Bubac et al. (2021).Bubac CM, Cullingham CI, Fox JA, Bowen WD, den Heyer CE, Coltman DW. Genetic association with boldness and maternal performance in a free-ranging population of grey seals (Halichoerus grypus) Heredity. 2021;127:35–51. doi: 10.1038/s41437-021-00439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter et al. (2012).Carter AJ, Marshall HH, Heinsohn R, Cowlishaw G. How not to measure boldness: novel object and antipredator responses are not the same in wild baboons. Animal Behaviour. 2012;84:603–609. doi: 10.1016/j.anbehav.2012.06.015. [DOI] [Google Scholar]
- Chilvers & Corkeron (2001).Chilvers BL, Corkeron PJ. Trawling and bottlenose dolphins’ social structure. Proceedings of the Royal Society B: Biological Sciences. 2001;268:1901–1905. doi: 10.1098/rspb.2001.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, Lea & Leaver (2016).Chow PKY, Lea SEG, Leaver LA. How practice makes perfect: the role of persistence, flexibility and learning in problem-solving efficiency. Animal Behaviour. 2016;112:273–283. doi: 10.1016/j.anbehav.2015.11.014. [DOI] [Google Scholar]
- Ciucci et al. (2020).Ciucci P, Mancinelli S, Boitani L, Gallo O, Grottoli L. Anthropogenic food subsidies hinder the ecological role of wolves: insights for conservation of apex predators in human-modified landscapes. Global Ecology and Conservation. 2020;21:e00841. doi: 10.1016/j.gecco.2019.e00841. [DOI] [Google Scholar]
- Coon et al. (2019).Coon CAC, Nichols BC, McDonald Z, Stoner DC. Effects of land-use change and prey abundance on the body condition of an obligate carnivore at the wildland-urban interface. Landscape and Urban Planning. 2019;192:103648. doi: 10.1016/j.landurbplan.2019.103648. [DOI] [Google Scholar]
- Cozzi et al. (2016).Cozzi G, Chynoweth M, Kusak J, Çoban E, Çoban A, Ozgul A, Şekercioğlu H. Anthropogenic food resources foster the coexistence of distinct life history strategies: year-round sedentary and migratory brown bears. Journal of Zoology. 2016;300:142–150. doi: 10.1111/jzo.12365. [DOI] [Google Scholar]
- Cresswell & Quinn (2010).Cresswell W, Quinn JL. Attack frequency, attack success and choice of prey group size for two predators with contrasting hunting strategies. Animal Behaviour. 2010;80:643–648. doi: 10.1016/j.anbehav.2010.06.024. [DOI] [Google Scholar]
- Daniels et al. (2019).Daniels SE, Fanelli RE, Gilbert A, Benson-Amram S. Behavioral flexibility of a generalist carnivore. Animal Cognition. 2019;22:387–396. doi: 10.1007/s10071-019-01252-7. [DOI] [PubMed] [Google Scholar]
- Díaz López & Shirai (2008).Díaz López B, Shirai JAB. Marine aquaculture and bottlenose dolphins’ (Tursiops truncatus) social structure. Behavioral Ecology and Sociobiology. 2008;62:887–894. doi: 10.1007/s00265-007-0512-1. [DOI] [Google Scholar]
- Eddine et al. (2020).Eddine A, Rocha RG, Mostefai N, Karssene Y, De Smet K, Brito JC, Klees D, Nowak C, Cocchiararo B, Lopes S, Van der Leer P, Godinho R. Demographic expansion of an African opportunistic carnivore during the Neolithic revolution. Biology Letters. 2020;16:20190560. doi: 10.1098/rsbl.2019.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington, Muntz & Gehrt (2020).Ellington EH, Muntz EM, Gehrt SD. Seasonal and daily shifts in behavior and resource selection: how a carnivore navigates costly landscapes. Oecologia. 2020;194:87–100. doi: 10.1007/s00442-020-04754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallows, Gallagher & Hammerschlag (2013).Fallows C, Gallagher AJ, Hammerschlag N. White sharks (Carcharodon carcharias) scavenging on whales and its potential role in further shaping the ecology of an apex predator. PLOS ONE. 2013;8:e60797. doi: 10.1371/journal.pone.0060797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedriani, Fuller & Sauvajot (2001).Fedriani JM, Fuller TK, Sauvajot RM. Does availability of anthropogenic food enhance densities of omnivorous mammals? An example with coyotes in southern California. Ecography. 2001;24:325–331. doi: 10.1111/j.1600-0587.2001.tb00205.x. [DOI] [Google Scholar]
- Fey et al. (2015).Fey SB, Siepielski AM, Nusslé S, Cervantes-Yoshida K, Hwan JL, Huber ER, Fey MJ, Catenazzi A, Carlson SM. Recent shifts in the occurrence, cause, and magnitude of animal mass mortality events. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:1083–1088. doi: 10.1073/pnas.1414894112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friard & Gamba (2016).Friard O, Gamba M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution. 2016;7:1325–1330. doi: 10.1111/2041-210X.12584. [DOI] [Google Scholar]
- Gámez & Harris (2021).Gámez S, Harris NC. Living in the concrete jungle: carnivore spatial ecology in urban parks. Ecological Applications. 2021;31:1–9. doi: 10.1002/eap.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffroy et al. (2015).Geffroy B, Samia DSM, Bessa E, Blumstein DT. How nature-based tourism might increase prey vulnerability to predators. Trends in Ecology and Evolution. 2015;30:755–765. doi: 10.1016/j.tree.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Gehrt (2007).Gehrt S. Ecology of coyotes in urban landscapes. Wildlife damage management conferences.2007. [Google Scholar]
- Gilbert-Norton, Leaver & Shivik (2009).Gilbert-Norton LB, Leaver LA, Shivik JA. The effect of randomly altering the time and location of feeding on the behaviour of captive coyotes (Canis latrans) Applied Animal Behaviour Science. 2009;120:179–185. doi: 10.1016/j.applanim.2009.06.007. [DOI] [Google Scholar]
- Gilchrist & Otali (2002).Gilchrist JS, Otali E. The effects of refuse-feeding on home-range use, group size, and intergroup encounters in the banded mongoose. Canadian Journal of Zoology. 2002;80:1795–1802. doi: 10.1139/z02-113. [DOI] [Google Scholar]
- Gould et al. (2021).Gould NP, Powell R, Olfenbuttel C, De Perno CS. Growth and reproduction by young urban and rural black bears. Journal of Mammalogy. 2021;102:1165–1173. doi: 10.1093/jmammal/gyab066. [DOI] [Google Scholar]
- Greenberg & Holekamp (2017).Greenberg JR, Holekamp KE. Human disturbance affects personality development in a wild carnivore. Animal Behaviour. 2017;132:303–312. doi: 10.1016/j.anbehav.2017.08.023. [DOI] [Google Scholar]
- Griffin & Guez (2014).Griffin AS, Guez D. Innovation and problem solving: a review of common mechanisms. Behavioural Processes. 2014;109:121–134. doi: 10.1016/j.beproc.2014.08.027. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Mihart et al. (2004).Hidalgo-Mihart MG, Cantú-Salazar L, López-González CA, Fernandez EC, González-Romero A. Effect of a landfill on the home range and group size of coyotes (Canis latrans) in a tropical deciduous forest. Journal of Zoology. 2004;263:55–63. doi: 10.1017/S0952836904004868. [DOI] [Google Scholar]
- Hody & Kays (2018).Hody JW, Kays R. Mapping the expansion of coyotes (Canis latrans) across North and Central America. ZooKeys. 2018;2018:81–97. doi: 10.3897/ZOOKEYS.759.15149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel et al. (2016).Hubel TY, Myatt JP, Jordan NR, Dewhirst OP, McNutt JW, Wilson AM. Energy cost and return for hunting in African wild dogs and cheetahs. Nature Communications. 2016;7:1–13. doi: 10.1038/ncomms11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson et al. (2021).Jacobson SL, Puitiza A, Snyder RJ, Sheppard A, Plotnik JM. Persistence is key: investigating innovative problem solving by Asian elephants using a novel multi-access box. Animal Cognition. 2021 doi: 10.1007/s10071-021-01576-3. [DOI] [PubMed] [Google Scholar]
- Johnson et al. (2018).Johnson HE, Lewis DL, Verzuh TL, Wallace CF, Much RM, Willmarth LK, Breck SW. Human development and climate affect hibernation in a large carnivore with implications for human-carnivore conflicts. Journal of Applied Ecology. 2018;55:663–672. doi: 10.1111/1365-2664.13021. [DOI] [Google Scholar]
- Johnson-Ulrich, Johnson-Ulrich & Holekamp (2018).Johnson-Ulrich L, Johnson-Ulrich Z, Holekamp K. Proactive behavior, but not inhibitory control, predicts repeated innovation by spotted hyenas tested with a multi-access box. Animal Cognition. 2018;21:379–392. doi: 10.1007/s10071-018-1174-2. [DOI] [PubMed] [Google Scholar]
- Johnson-Ulrich et al. (2021).Johnson-Ulrich L, Yirga G, Strong RL, Holekamp KE. The effect of urbanization on innovation in spotted hyenas. Animal Cognition. 2021;24:1027–1038. doi: 10.1007/s10071-021-01494-4. [DOI] [PubMed] [Google Scholar]
- Kane & Kendall (2017).Kane A, Kendall CJ. Understanding how mammalian scavengers use information from avian scavengers: cue from above. Journal of Animal Ecology. 2017;86:837–846. doi: 10.1111/1365-2656.12663. [DOI] [PubMed] [Google Scholar]
- Kistler et al. (2009).Kistler C, Hegglin D, Würbel H, König B. Feeding enrichment in an opportunistic carnivore: the red fox. Applied Animal Behaviour Science. 2009;116:260–265. doi: 10.1016/j.applanim.2008.09.004. [DOI] [Google Scholar]
- Kohl et al. (2019).Kohl MT, Ruth TK, Metz MC, Stahler DR, Smith DW, White PJ, MacNulty DR. Do prey select for vacant hunting domains to minimize a multi-predator threat? Ecology Letters. 2019;22:1724–1733. doi: 10.1111/ele.13319. [DOI] [PubMed] [Google Scholar]
- Kurvers et al. (2012).Kurvers RHJM, Nolet BA, Prins HHT, Ydenberg RC, Van Oers K. Boldness affects foraging decisions in barnacle geese: An experimental approach. Behavioral Ecology. 2012;23:1155–1161. doi: 10.1093/beheco/ars091. [DOI] [Google Scholar]
- Leary, Schultz & Young (2021).Leary T, Schultz JT, Young JK. Surgical sterilization impacts on behavior of coyote pairs. Applied Animal Behaviour Science. 2021;241:105382. doi: 10.1016/j.applanim.2021.105382. [DOI] [Google Scholar]
- MacNulty, Mech & Smith (2007).MacNulty DR, Mech LD, Smith DW. A proposed ethogram of large-carnivore predatory behavior, exemplified by the wolf. Journal of Mammalogy. 2007;88:595–605. doi: 10.1644/06-mamm-a-119r1.1. [DOI] [Google Scholar]
- Manlick & Pauli (2020).Manlick PJ, Pauli JN. Human disturbance increases niche overlap in terrestrial carnivore communities. Proceedings of the National Academy of Sciences of the United States of America. 2020;117:26842–26848. doi: 10.1073/pnas.2012774117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz & LaForse (1987).Markowitz H, LaForse S. Artificial prey as behavioral enrichment devices for felines. Applied Animal Behaviour Science. 1987;18:31–43. doi: 10.1016/0168-1591(87)90252-8. [DOI] [Google Scholar]
- McDonnell & Hahs (2015).McDonnell MJ, Hahs AK. Adaptation and adaptedness of organisms to urban environments. Annual Review of Ecology, Evolution, and Systematics. 2015;46:261–280. doi: 10.1146/annurev-ecolsys-112414-054258. [DOI] [Google Scholar]
- McGraw & Wong (1996).McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Physhological Methods. 1996;1:30–46. [Google Scholar]
- Michelena et al. (2009).Michelena P, Sibbald AM, Erhard HW, McLeod JE. Effects of group size and personality on social foraging: the distribution of sheep across patches. Behavioral Ecology. 2009;20:145–152. doi: 10.1093/beheco/arn126. [DOI] [Google Scholar]
- Mills & Harris (2020).Mills K, Harris N. Humans induce differential access to prey for large African carnivores. eLife. 2020;9:e60690. doi: 10.7554/eLife.60690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll et al. (2017).Moll RJ, Redilla KM, Mudumba T, Muneza AB, Gray SM, Abade L, Hayward MW, Millspaugh JJ, Montgomery RA. The many faces of fear: a synthesis of the methodological variation in characterizing predation risk. Journal of Animal Ecology. 2017;86:749–765. doi: 10.1111/1365-2656.12680. [DOI] [PubMed] [Google Scholar]
- Mourier, Claudet & Planes (2020).Mourier J, Claudet J, Planes S. Human-induced shifts in habitat use and behaviour of a marine predator: the effects of bait provisioning in the blacktip reef shark. Animal Conservation. 2020;24:230–238. doi: 10.1111/acv.12630. [DOI] [Google Scholar]
- Murray et al. (2015).Murray M, Edwards MA, Abercrombie B, St. Clair CC. Poor health is associated with use of anthropogenic resources in an urban carnivore. Proceedings of the Royal Society B: Biological Sciences. 2015;282:20150009. doi: 10.1098/rspb.2015.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas González et al. (2019).Navas González FJ, Jordana Vidal J, León Jurado JM, McLean AK, Delgado Bermejo JV. Dumb or smart asses? Donkey’s (Equus asinus) cognitive capabilities share the heritability and variation patterns of human’s (Homo sapiens) cognitive capabilities. Journal of Veterinary Behavior. 2019;33:63–74. doi: 10.1016/j.jveb.2019.06.007. [DOI] [Google Scholar]
- Newsome et al. (2015b).Newsome SD, Garbe HM, Wilson EC, Gehrt SD. Individual variation in anthropogenic resource use in an urban carnivore. Oecologia. 2015b;178:115–128. doi: 10.1007/s00442-014-3205-2. [DOI] [PubMed] [Google Scholar]
- Newsome et al. (2015a).Newsome TM, Dellinger JA, Pavey CR, Ripple WJ, Shores CR, Wirsing AJ, Dickman CR. The ecological effects of providing resource subsidies to predators. Global Ecology and Biogeography. 2015a;24:1–11. doi: 10.1111/geb.12236. [DOI] [Google Scholar]
- Newsome, Howden & Wirsing (2019).Newsome TM, Howden C, Wirsing AJ. Restriction of anthropogenic foods alters a top predator’s diet and intraspecific interactions. Journal of Mammalogy. 2019;100:1522–1532. doi: 10.1093/jmammal/gyz125. [DOI] [Google Scholar]
- Nyhus & Tilson (2004).Nyhus PJ, Tilson R. Characterizing human-tiger conflict in Sumatra, Indonesia: implications for conservation. Oryx. 2004;38:68–74. doi: 10.1017/S0030605304000110. [DOI] [Google Scholar]
- Oksanen et al. (2018).Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, Mcglinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E. Vegan: community ecology package. https://cran.r-project.org/web/packages/vegan/vegan.pdf 2018
- Parsons, Newsome & Young (2022).Parsons MA, Newsome TM, Young JK. The consequences of predators without prey. Frontiers in Ecology and the Environment. 2022;20:31–39. doi: 10.1002/fee.2419. [DOI] [Google Scholar]
- Peirce & Van Daele (2006).Peirce KN, Van Daele LJ. Use of garbage dump by brown bears in Dillingham, Alaska. Ursus. 2006;17:165–177. doi: 10.2192/1537-6176(2006)17[165:UOAGDB]2.0.CO;2. [DOI] [Google Scholar]
- Prange, Gehrt & Wiggers (2004).Prange S, Gehrt SD, Wiggers EP. Influences of anthropogenic resources on raccoon (Procyon lotor) movements and spatial distribution. Journal of Mammalogy. 2004;85:483–490. doi: 10.1644/bos-121. [DOI] [Google Scholar]
- Prugh & Sivy (2020).Prugh LR, Sivy KJ. Enemies with benefits: integrating positive and negative interactions among terrestrial carnivores. Ecology Letters. 2020;23:902–918. doi: 10.1111/ele.13489. [DOI] [PubMed] [Google Scholar]
- Prugh et al. (2009).Prugh LR, Stoner CJ, Epps CW, Bean WT, Ripple WJ, Laliberte AS, Brashares JS. The rise of the mesopredator. BioScience. 2009;59:779–791. doi: 10.1525/bio.2009.59.9.9. [DOI] [Google Scholar]
- Reid (2006).Reid FA. Mammals of North America. Houghton Mifflin Company; New York: 2006. [Google Scholar]
- Ripple et al. (2014).Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, Berger J, Elmhagen B, Letnic M, Nelson MP, Schmitz OJ, Smith DW, Wallach AD, Wirsing AJ. Status and ecological effects of the world’s largest carnivores. Science. 2014;343:1241484. doi: 10.1126/science.1241484. [DOI] [PubMed] [Google Scholar]
- Robins et al. (2019).Robins CW, Kertson BN, Faulkner JR, Wirsing AJ. Effects of urbanization on cougar foraging ecology along the wildland-urban gradient of western Washington. Ecosphere. 2019;10:e02605. doi: 10.1002/ecs2.2605. [DOI] [Google Scholar]
- Rodewald, Kearns & Shustack (2011).Rodewald AD, Kearns LJ, Shustack DP. Anthropogenic resource subsidies decouple predator–prey relationships. Ecological Applications. 2011;21:936–943. doi: 10.1890/10-0863.1. [DOI] [PubMed] [Google Scholar]
- Rodriguez, Lesmeister & Levi (2021).Rodriguez JT, Lesmeister DB, Levi T. Mesocarnivore landscape use along a gradient of urban, rural, and forest cover. PeerJ. 2021;9:1–25. doi: 10.7717/peerj.11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo et al. (2010).Salo P, Banks PB, Dickman CR, Korpimäki E. Predator manipulation experiments: impacts on populations of terrestrial vertebrate prey. Ecological Monographs. 2010;80:531–546. doi: 10.1890/09-1260.1. [DOI] [Google Scholar]
- Schell (2015).Schell CJ. Doctor of Philosophy Dissertation. 2015. Differential and long-term impacts of biparental effect on offspring personality and hormones in coyotes (Canis latrans) [Google Scholar]
- Schell et al. (2020).Schell CJ, Dyson K, Fuentes TL, Des Roches S, Harris NC, Miller DS, Woelfle-Erskine CA, Lambert MR. The ecological and evolutionary consequences of systemic racism in urban environments. Science. 2020;369:eaay4497. doi: 10.1126/science.aay4497. [DOI] [PubMed] [Google Scholar]
- Schell et al. (2021).Schell CJ, Stanton LA, Young JK, Angeloni LM, Lambert JE, Breck SW, Murray MH. The evolutionary consequences of human-wildlife conflict in cities. Evolutionary Applications. 2021;14:178–197. doi: 10.1111/eva.13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell et al. (2018).Schell CJ, Young JK, Lonsdorf EV, Mateo JM, Santymire RM. It takes two: evidence for reduced sexual conflict over parental care in a biparental canid. Journal of Mammalogy. 2018;99:75–88. doi: 10.1093/jmammal/gyx150. [DOI] [Google Scholar]
- Shivik et al. (2009).Shivik JA, Palmer GL, Gese EM, Osthaus B. Captive coyotes compared to their counterparts in the wild: does environmental enrichment help? Journal of Applied Animal Welfare Science. 2009;12:223–235. doi: 10.1080/10888700902955989. [DOI] [PubMed] [Google Scholar]
- Sih, Bell & Johnson (2004).Sih A, Bell A, Johnson JC. Behavioral syndromes: an ecological and evolutionary overview. Trends in Ecology and Evolution. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Smith et al. (2020).Smith JA, Donadio E, Bidder OR, Pauli JN, Sheriff MJ, Perrig PL, Middleton AD. Where and when to hunt? Decomposing predation success of an ambush carnivore. Ecology. 2020;0:1–12. doi: 10.1002/ecy.3172. [DOI] [PubMed] [Google Scholar]
- Sol (2009).Sol D. Revisiting the cognitive buffer hypothesis for the evolution of large brains. Biology Letters. 2009;5:130–133. doi: 10.1098/rsbl.2008.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sol, Lapiedra & González-Lagos (2013).Sol D, Lapiedra O, González-Lagos C. Behavioural adjustments for a life in the city. Animal Behaviour. 2013;85:1101–1112. doi: 10.1016/j.anbehav.2013.01.023. [DOI] [Google Scholar]
- Stanton et al. (2021).Stanton LA, Bridge ES, Huizinga J, Johnson SR, Young JK, Benson-Amram S. Variation in reversal learning by three generalist mesocarnivores. Animal Cognition. 2021;24:555–568. doi: 10.1007/s10071-020-01438-4. [DOI] [PubMed] [Google Scholar]
- Stratton, Nolte & Payseur (2021).Stratton JA, Nolte MJ, Payseur BA. Evolution of boldness and exploratory behavior in giant mice from Gough Island. Behavioral Ecology and Sociobiology. 2021;75:65. doi: 10.1007/s00265-021-03003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szopa-Comley et al. (2020).Szopa-Comley AW, Duffield C, Ramnarine IW, Ioannou CC. Predatory behaviour as a personality trait in a wild fish population. Animal Behaviour. 2020;170:51–64. doi: 10.1016/j.anbehav.2020.10.002. [DOI] [Google Scholar]
- R Core Team (2021).R Core Team . Vienna: R Foundation for Statistical Computing; 2021. [Google Scholar]
- Therneau (2020).Therneau TM. coxme: mixed effects Cox models. R package version 2.2-16https://cran.r-project.org/web/packages/coxme/coxme.pdf 2020
- Toscano (2017).Toscano BJ. Prey behavioural reaction norms: response to threat predicts susceptibility to predation. Animal Behaviour. 2017;132:147–153. doi: 10.1016/j.anbehav.2017.08.014. [DOI] [Google Scholar]
- Toscano et al. (2016).Toscano BJ, Gownaris NJ, Heerhartz SM, Monaco CJ. Personality, foraging behavior and specialization: integrating behavioral and food web ecology at the individual level. Oecologia. 2016;182:55–69. doi: 10.1007/s00442-016-3648-8. [DOI] [PubMed] [Google Scholar]
- Vargas & Anderson (1999).Vargas A, Anderson SH. Effects of experience and cage enrichment on predatory skills of black-footed ferrets (Mustela nigripes) Journal of Mammalogy. 1999;80:263–269. doi: 10.2307/1383226. [DOI] [Google Scholar]
- Vinks et al. (2021).Vinks MA, Creel S, Schuette P, Becker MS, Rosenblatt E, Sanguinetti C, Banda K, Goodheart B, Young-Overton K, Stevens X, Chifunte C, Midlane N, Simukonda C. Response of lion demography and dynamics to the loss of preferred larger prey. Ecological Applications. 2021;31:1–12. doi: 10.1002/eap.2298. [DOI] [PubMed] [Google Scholar]
- White & Gehrt (2009).White LA, Gehrt SD. Coyote attacks on humans in the United States and Canada. Human Dimensions of Wildlife. 2009;14:419–432. doi: 10.1080/10871200903055326. [DOI] [Google Scholar]
- Whitehead & Reeves (2005).Whitehead H, Reeves R. Killer whales and whaling: the scavenging hypothesis. Biology Letters. 2005;1:415–418. doi: 10.1098/rsbl.2005.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams et al. (1996).Williams BG, Waran NK, Carruthers J, Young RJ. The effect of a moving bait on the behaviour of captive cheetahs (Acinonyx jubatus) Animal Welfare. 1996;5:271–281. [Google Scholar]
- Wolf & Ripple (2016).Wolf C, Ripple WJ. Prey depletion as a threat to the world’s large carnivores. Royal Society Open Science. 2016;3:160252. doi: 10.1098/rsos.160252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirga et al. (2012).Yirga G, De longh HH, Leirs H, Gebrihiwot K, Deckers J, Bauer H. Adaptability of large carnivores to changing anthropogenic food sources: diet change of spotted hyena (Crocuta crocuta) during Christian fasting period in northern Ethiopia. Journal of Animal Ecology. 2012;81:1052–1055. doi: 10.5061/dryad.pq050620. [DOI] [PubMed] [Google Scholar]
- Young et al. (2020).Young JK, Coppock DL, Baggio JA, Rood KA, Yirga G. Linking human perceptions and spotted hyena behavior in urban areas of Ethiopia. Animals. 2020;10:2400. doi: 10.3390/ani10122400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, Mahe & Breck (2015).Young JK, Mahe M, Breck S. Evaluating behavioral syndromes in coyotes (Canis latrans) Journal of Ethology. 2015;33:137–144. doi: 10.1007/s10164-015-0422-z. [DOI] [Google Scholar]
- Young, Touzot & Brummer (2019).Young JK, Touzot L, Brummer SP. Persistence and conspecific observations improve problem-solving abilities of coyotes. PLOS ONE. 2019;14:e0218778. doi: 10.1371/journal.pone.0218778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available at GitHub: https://github.com/pars2997/scavenging-hunting-coyotes.