Abstract
The role of follow-up blood cultures (FUBCs) in gram-negative bloodstream infections to improve clinical outcomes remains controversial, especially among immunocompromised patients. Among 139 patients, FUBCs were common (117, 84.2%); however, positive FUBCs were rare (3, 2.6%). Only presence of fever was associated with a positive FUBC.
Keywords: bloodstream infection, gram-negative, immunocompromise, oncology, transplant
To document clearance, routine follow-up blood cultures (FUBCs) are strongly recommended for Staphylococcus aureus and Candida spp. bloodstream infections (BSIs) [1, 2]. Conversely, the importance of FUBC in gram-negative (GN) BSIs remains an area of clinical debate, as GN BSI is often transient and resolves rapidly with appropriate antimicrobial therapy [3]. Previous studies are conflicting in their findings, demonstrating either improved clinical outcomes or limited diagnostic yield and no improvement for patients where FUBCs are obtained [4–8].
A scoping review of blood culture collection practices in immunocompetent adult patients highlighted the lack of guidance on appropriate indications for obtaining blood cultures and the downstream impact of unnecessary FUBCs [9]. Obtaining FUBCs is not benign; not only do they cause patient discomfort, they may result in isolation of skin contaminants, which are often nonpathogenic but lead to unnecessary antibiotic initiation, consequent adverse events, increased length of hospital stay (LOS), and erroneous documentation of central line–associated bloodstream infections (CLABSIs) [6, 10, 11]. With these considerations in mind, the need for diagnostic stewardship interventions for GN BSI is apparent [12].
Patients with risk factors for persistent GN BSI, such as critical illness, persistent fever, infection with multidrug-resistant organisms (MDROs), end-stage renal disease (ESRD), or immunocompromised status, may still benefit from FUBCs [6, 13–15]. FUBCs may be warranted to improve clinical outcomes, but the current body of evidence remains conflicted. Immunocompromised patients are vulnerable to increased infection risk with MDROs and have frequent febrile episodes that result in repeated culturing and antibiotic exposure [16–18]. As such, immunocompromise is one of the most commonly reported risk factors for persistent GN BSI. Our objective was to evaluate the incidence and positivity of FUBCs as well as associated clinical outcomes in immunocompromised patients with GN BSI.
METHODS
This was a retrospective observational cohort of adult (age >18 years), immunocompromised patients treated for confirmed GN BSI between January 2019 and December 2020 at University of Maryland Medical Center (Baltimore, MD, USA). Confirmed GN BSI was defined as at least 1 blood culture positive for at least 1 GN organism on gram stain. Blood cultures with concurrent gram-positive or fungal organisms were excluded. GN organisms were identified and antibiotic susceptibility testing was completed using the Verigene Gram-Negative Blood-Culture Test (Luminex Corporation) and Vitek MS/Vitek2 (bioMerieux, Inc.). The study was approved by the University of Maryland Baltimore Institutional Review Board with a waiver of informed consent.
Immunocompromised status was defined as active hematologic or solid tumor malignancy at the time of BSI diagnosis, or history of hematopoietic stem cell transplantation (HSCT) or solid organ transplantation (SOT). FUBCs were defined as blood cultures drawn between 24 hours and 7 days from index blood culture, within the same hospital encounter. Positive FUBCs was defined as an FUBC with a GN organism identified. MDRO was defined as resistance to >1 antibiotic class. Fever at the time of FUBC was defined as a single measurement of >38.3°C or 38.0°C over 1 hour. Clinical end points included extension of planned antibiotic duration, post-BSI LOS, and inpatient all-cause mortality. The planned antibiotic duration was determined by review of the primary team’s progress and infectious diseases (ID) consult notes. Post-BSI LOS was defined as the length of stay after index positive blood culture. Clinical and microbiological data were extracted from electronic health records and managed in REDCap [19].
Descriptive statistics, including frequencies for categorical variables and means (SDs) or medians (interquartile ranges [IQRs]) for continuous data, were used to summarize baseline characteristics. Between-group comparisons were made based on FUBC status (FUBC vs no FUBC) and FUBC outcome (positive FUBC vs negative FUBC). For categorical variables, the chi-square or Fisher exact test was used as appropriate. For continuous variables, the Student t test or Mann Whitney U test was used based on normality. All statistical tests were evaluated at an a priori alpha of .05. Statistical analysis was performed using SPSS, version 25 (IBM Corp., Armonk, NY, USA).
RESULTS
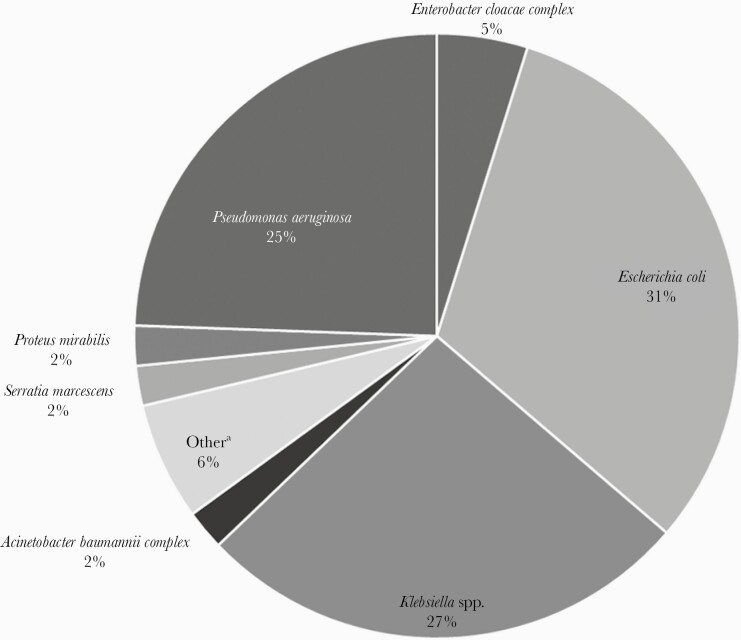
A total of 139 patients with GN BSI were included, with a mean (SD) age of 57 (15) years, and 89 (64%) were male. Patients were divided among SOT (43, 30.9%) and hematologic malignancy (43, 30.9%), followed by HSCT (41, 29.5%), then solid tumor (12, 8.6%). All patients post-HSCT had an underlying hematologic malignancy, except for 1 solid tumor. The most common sources of GN BSI were intraabdominal (ie, abscess, biliary tract infection, enterocolitis, diverticulitis, pancreatitis, peritonitis), followed by genitourinary. The median time to GN BSI onset from hospital admission (IQR) was 9 (0–14) days, and 85 (61.2%) were hospital-acquired. The most common organisms identified included Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae (Figure 1). Among all organisms, 23 (6.5%) were MDRO. All patients had ID consultation at the time of GN BSI. There was a lack of source control in 22 (44%) out of 50 cases where source control could presumably be pursued and/or achieved (ie, catheter/line).
Figure 1.
Microbiologic distribution of index blood cultures. aEnterobacter hormaechei, Citrobacter spp., Klebsiella variicola, Morganella morganii, Pantoea spp., Pluralibacter (Enterobacter) gergoviae, Raoultella (Klebsiella) planticola, Stenotrophomonas maltophilia.
Follow-up blood cultures were collected in 117 (84.2%) patients. There was no difference in frequency of FUBC based on location of care, type of immunosuppression, or source of BSI (Table 1). Factors that were associated with acquisition of FUBC included history of MDRO surveillance culture (30.8% vs 9.1%; P = .038) and absence of central line (70.1% vs 50%; P = .084). The clinical outcomes of those with vs without FUBCs obtained were different. Patients with FUBCs more frequently had antibiotic durations extended beyond the original planned duration (7.8% vs 0%). Those with FUBCs also had a longer median (IQR) post-BSI LOS compared with those without FUBCs (10 [5–17] vs 4.5 [2–12.5] days; P = .01). Inpatient all-cause mortality was not different (6.5% vs 4.5%; P = .732).
Table 1.
Comparison of Baseline Characteristics by FUBC Status
| FUBC (n = 117) | No FUBC (n = 22) | P Value | |
|---|---|---|---|
| Location of care, No. (%) | |||
| ICU | 17 (14.5) | 2 (9.1) | .738 |
| IMC | 31 (26.5) | 7 (31.8) | .607 |
| Floor | 69 (59.0) | 13 (59.1) | .992 |
| Type of immunosuppression, No. (%) | |||
| Hematologic malignancy | 35 (29.9) | 8 (36.4) | .548 |
| SOT only | 36 (30.8) | 7 (31.8) | .922 |
| HSCT | 37 (31.6) | 4 (18.2) | .205 |
| Solid tumor malignancy | 9 (7.7) | 3 (13.6) | .404 |
| Definitive or probable source of BSI, No. (%) | |||
| Intraabdominal | 67 (57.3) | 12 (54.5) | .692 |
| Genitourinary | 20 (17.1) | 6 (27.3) | .261 |
| Central line–associated | 12 (10.3) | 1 (4.5) | .399 |
| Pulmonary | 8 (6.8) | 1 (4.5) | .689 |
| Bone/joint | 1 (0.9) | 0 (0) | .663 |
| Organism(s) isolated from BSI, No. (%) | |||
| Enterobacterales | 81 (69.2) | 19 (86.3) | .384 |
| Pseudomonas | 31 (26.5) | 3 (13.6) | |
| Othera | 5 (4.3) | 0 (0) | |
| Hospital-acquired GN BSI, No. (%) | 75 (64.1) | 10 (45.5) | .16 |
| Neutropenic, No. (%) | 58 (49.6) | 7 (31.8) | .168 |
| Presence of central line, No. (%) | 82 (70.1) | 11 (50.0) | .066 |
| Prior MDRO surveillance, No. (%) | 36 (30.8) | 2 (9.1) | .038 |
| Prior MDRO infection, No. (%) | 37 (31.6) | 3 (13.6) | .123 |
| Current MDRO BSI, No. (%) | 21 (17.9) | 2 (9.1) | .531 |
| Active empiric antibiotic therapy, No. (%)b | 78 (66.7) | 16 (72.7) | .577 |
Abbreviations: BSI, bloodstream infection; FUBC, follow-up blood culture; GN, gram-negative; HSCT, hematopoietic stem cell transplantation; ICU, intensive care unit; IMC, intermediate care unit; MDRO, multidrug-resistant organism; SOT, solid organ transplantation.
Acinetobacter, Stenotrophomonas maltophilia.
Active empiric antibiotic therapy at time of index blood culture draw.
Among the 117 patients with FUBCs obtained, positive FUBCs occurred in 3 (2.6%) cases. FUBCs were positive with the same GN organism (2 E. coli and 1 K. pneumoniae) and were collected within 24 hours of the index blood cultures. Time to documented culture clearance was 1.5, 3, and 4 days, respectively. Additionally, 1 patient had a BSI caused by vancomycin-resistant enterococci isolated from an FUBC. Compared with patients with negative FUBCs, the 3 patients with positive FUBCs were less likely to have central lines (25.0% vs 71.6%; P = .079) and more likely to be febrile at the time of FUBC collection (66.7% vs 6.1%; P = .006). Inpatient mortality was not significantly different but was numerically higher among those with positive FUBCs (33.3% vs 5.3%; P = .17). Median (IQR) post-BSI LOS did not differ among those with positive vs negative FUBCs (9.3 [4.4–14.8] vs 8.6 [3.5–15.9] days; P = .811).
DISCUSSION
FUBCs were commonly performed up to 7 days after index positive blood cultures but yielded a positive result in only 3 cases. The presence of fever at the time of FUBC was significantly associated with identification of GN organisms on FUBC. This association was not identified in immunocompetent hosts in the scoping review conducted by Fabre et al. [9].
The results of our study parallel those previously reported in a similar population with oncologic malignancies [20]. Although the frequency of FUBCs was higher in our study (84% vs 67%), Clemmons et al. also found FUBCs to be associated with increased LOS and antibiotic durations. Also, fever at the time of FUBC was not associated with positivity; however, neutropenic patients were less likely to have positive FUBCs when controlling for fever. Our definition of FUBCs was more reflective of previous definitions, which included cultures collected between 24 hours and 7 days of index positive culture, while theirs was limited to cultures collected within 72 hours of index culture. Therefore, it is possible that we captured more FUBCs as a result of our definition, as opposed to differing FUBC practices. Notably, FUBC positivity was lower in our study compared with theirs (2.6% vs 9%).
The probable or definitive sources in in our study were among intraabdominal syndromes that vary in complexity and ability to achieve source control. Uncomplicated intraabdominal syndromes (appendicitis, cholecystitis, etc.) are amenable to medical management. Urinary tract infections are also common and may not necessitate FUBCs, a notion that was suggested by Fabre et al. in immunocompetent patients [9]. However, neutropenic patients with mucositis/enterocolitis and ongoing gut translocation are at risk for BSI and may not meet criteria for uncomplicated BSI [21, 22]. Evaluation on a larger scale is needed in order to draw more definitive conclusions.
We were limited by our small sample size and retrospective design. We were unable to assess whether subsequent blood cultures were purely driven by intent to document clearance. As the incidence of fever at the time of FUBC was low, it appears that nursing protocols for neutropenic fever episodes had minimal impact. Of note, we documented fever incidence only among those with FUBCs. Lastly, we did not assess potential harms associated with routine FUBCs in this patient population, which is valuable to discerning diagnostic benefit vs harm.
Although FUBCs were frequently collected in immunocompromised patients with GN BSI, positive FUBCs were uncommon. Previous studies have identified immunocompromised status as a risk factor for persistent GN BSI; however, we found a low FUBC positivity rate. Further work is needed to better ascertain the clinical utility of FUBCs in immunocompromised hosts and to develop diagnostic stewardship interventions to limit unnecessary culturing.
Acknowledgments
Financial support. This work received no external financial support.
Potential conflicts of interest. K.C.C. has served as a speaker for BioFire Diagnostics and received grant funding from Merck & Co. J.K.J. has served as a speaker for BioFire Diagnostics. L.G.B., J.M., J.B., and C.B. have no conflicts to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This study did not necessitate patient consent, and the need for informed consent was waived by the University of Maryland Institutional Review Board.
References
- 1. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52:285–92. [DOI] [PubMed] [Google Scholar]
- 2. Pappas PG, Kauffman CA, Andes DR, et al. . Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62:e1–50. doi: 10.1093/cid/civ933. Epub 2015 Dec 16. PMID: 26679628; PMCID: PMC4725385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harris JA, Cobbs CG.. Persistent gram-negative bacteremia: observations in twenty patients. Am J Surg 1973; 125:705–17. [DOI] [PubMed] [Google Scholar]
- 4. Maskarinec SA, Park LP, Ruffin F, et al. Positive follow-up blood cultures identify high mortality risk among patients with gram-negative bacteraemia. Clin Microbiol Infect 2020; 26:904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giannella M, Pascale R, Pancaldi L, et al. Follow-up blood cultures are associated with improved outcome of patients with gram-negative bloodstream infections: retrospective observational cohort study. Clin Microbiol Infect 2020; 26:897–903. [DOI] [PubMed] [Google Scholar]
- 6. Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM.. Follow-up blood cultures in gram-negative bacteremia: are they needed? Clin Infect Dis 2017; 65:1776–9. doi: 10.1093/cid/cix648. PMID: 29020307. [DOI] [PubMed] [Google Scholar]
- 7. Amipara R, Winders HR, Justo JA, et al. Impact of follow up blood cultures on outcomes of patients with community-onset gram-negative bloodstream infection. EClinicalMedicine 2021; 34:100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiggers JB, Daneman N.. The culture of follow-up blood cultures. Clin Microbiol Infect 2020; 26:811–3. [DOI] [PubMed] [Google Scholar]
- 9. Fabre V, Sharara SL, Salinas AB, et al. Does this patient need blood cultures? A scoping review of indications for blood cultures in adult nonneutropenic inpatients. Clin Infect Dis 2020; 71:1339–47. [DOI] [PubMed] [Google Scholar]
- 10. Dempsey C, Skoglund E, Muldrew KL, Garey KW.. Economic health care costs of blood culture contamination: a systematic review. Am J Infect Control 2019; 47:963–7. [DOI] [PubMed] [Google Scholar]
- 11. Bates DW, Goldman L, Lee TH.. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA 1991; 265:365–9. [PubMed] [Google Scholar]
- 12. Fabre V, Carroll KC, Cosgrove SE.. Blood culture utilization in the hospital setting: a call for diagnostic stewardship. J Clin Microbiol 2022; 60:e0100521. doi: 10.1128/JCM.01005-21. Epub 2021 Jul 14. PMID: 34260274; PMCID: PMC8925908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jung J, Song KH, Jun KI, et al. Predictive scoring models for persistent gram-negative bacteremia that reduce the need for follow-up blood cultures: a retrospective observational cohort study. BMC Infect Dis 2020; 20:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitaka H, Gomez T, Lee YI, Perlman DC.. Risk factors for positive follow-up blood cultures in gram-negative bacilli bacteremia: implications for selecting who needs follow-up blood cultures. Open Forum Infect Dis 2020; 7:ofaa110. doi: 10.1093/ofid/ofaa110. PMID: 32328509; PMCID: PMC7166118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cogliati Dezza F, Curtolo A, Volpicelli L, et al. Are follow-up blood cultures useful in the antimicrobial management of gram negative bacteremia? A reappraisal of their role based on current knowledge. Antibiotics (Basel) 2020; 9:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kara Ali R, Surme S, Balkan II, et al. An eleven-year cohort of bloodstream infections in 552 febrile neutropenic patients: resistance profiles of gram-negative bacteria as a predictor of mortality. Ann Hematol 2020; 99:1925–32. [DOI] [PubMed] [Google Scholar]
- 17. Kimura SI, Gomyo A, Hayakawa J, et al. Clinical significance of repeat blood cultures during febrile neutropenia in adult acute myeloid leukaemia patients undergoing intensive chemotherapy. Infect Dis (Lond) 2017; 49:748–57. [DOI] [PubMed] [Google Scholar]
- 18. Wan Q, Ye Q, Huang F.. The bacteremia caused by non-lactose fermenting gram-negative bacilli in solid organ transplant recipients. Surg Infect (Larchmt) 2015; 16:479–89. [DOI] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clemmons AB, Young HN, Bland CM, et al. Incidence and utility of follow-up blood cultures in cancer patients with gram-negative bacteremia. Diagn Microbiol Infect Dis 2021; 101:115444. [DOI] [PubMed] [Google Scholar]
- 21. Viscoli C, Varnier O, Machetti M.. Infections in patients with febrile neutropenia: epidemiology, microbiology, and risk stratification. Clin Infect Dis 2005; 40:S240–5. [DOI] [PubMed] [Google Scholar]
- 22. Heil EL, Bork JT, Abbo LM, et al. . Optimizing the management of uncomplicated gram-negative bloodstream infections: consensus guidance using a modified Delphi process. Open Forum Infect Dis 2021; 8:ofab434. doi: 10.1093/ofid/ofab434. PMID: 34738022; PMCID: PMC8561258. [DOI] [PMC free article] [PubMed] [Google Scholar]