Abstract
Individuals with obesity (OB) prefer immediate rewards of food intake over the delayed reward of healthy well-being achieved through diet management and physical activity, compared with normal-weight controls (NW). This may reflect heightened impulsivity, an important factor contributing to the development and maintenance of obesity. However, the neural mechanisms underlying the greater impulsivity in OB remain unclear. Therefore, the current study employed functional magnetic resonance imaging with a delay discounting (DD) task to examine the association between impulsive choice and altered neural mechanisms in OB. During decision-making in the DD task, OB compared with NW had greater activation in the dorsolateral prefrontal cortex (DLPFC) and posterior parietal cortex, which was associated with greater discounting rate and weaker cognitive control as measured with the Three-Factor Eating Questionnaire (TFEQ). In addition, the association between DLPFC activation and cognitive control (TFEQ) was mediated by discounting rate. Psychophysiological interaction analysis showed decreased connectivity of DLPFC–inferior parietal cortex (within executive control network [ECN]) and angular gyrus–caudate (ECN–reward) in OB relative to NW. These findings reveal that the aberrant function and connectivity in core regions of ECN and striatal brain reward regions underpin the greater impulsivity in OB and contribute to abnormal eating behaviors.
Keywords: delay discounting, cognitive control, fMRI, impulsivity, obesity
Introduction
Obesity, defined as a condition of excessive fat accumulation, is a chronic disease with a large global public health impact (Garrow 1988; Zhang et al. 2014). Obese patients (OB) have higher reward sensitivity to food cues, which is associated with the elevated activity in brain reward circuitry responsible for the encoding of the reward value (Zhang et al. 2020). During food picture visual stimuli and food choice task, OB also exhibit greater activation in frontal regions implicated in self-regulation and control of eating behaviors compared with normal-weight participants (NW), suggesting stronger engagement of executive control to suppress food reinforcers in OB (Davids et al. 2010; Moreno-Padilla et al. 2018). However, OB still prefer the reward of immediate food intake when exposed to external food cues than the delayed reward of health brought about through diet management and physical activity. Thus, sensitization to food cues that predict a food reward may override cognitive control and result in dysfunctional reward-based decision-making, contributing to impulsive maladaptive behaviors that lead to overeating in OB. Therefore, the current study aims to investigate the neural mechanisms underlying impulsive decision-making in OB.
The delay discounting (DD) task, which requires participants to make a series of choices between smaller immediate rewards and larger delayed rewards, is an approach for assessing impulsivity with reward-based decision-making and has been employed to investigate impulsive decision-making behavior in OB (Ainslie 1975; Rachlin et al. 1991; Amlung et al. 2016). Individual differences in choice preference are measured by the discounting rate (K), which represents how quickly the subjective value of a reward decreases as a function of delayed time, with higher K values indicating greater preference for smaller immediate rewards and greater impulsivity (Stoeckel et al. 2013). DD plays an important role in self-regulation of eating behavior, since OB have to repeatedly choose between immediate food rewards and the delayed rewards of improving overall health. Numerous studies that employed DD task found that OB have difficulties in delaying gratification and exhibit higher discounting rate for both monetary and food rewards compared with NW (Manwaring et al. 2011; Lawyer et al. 2015; Schiff et al. 2016). In addition, higher discounting rate correlated with less weight loss in obese children following a 16-week intervention (Best et al. 2012), and lower discounting rate predicted long-term success of weight maintenance following diet initiation (Weygandt et al. 2015). Therefore, steep DD of reward is a robust feature of obesity and appears to play an important role in weight regulation.
Previous studies indicated that the DD task mainly involves 2 neurocognitive processes: rewards evaluation and decision-making (McClure et al. 2004; King et al. 2016). The former process mainly encodes the subjective value of rewards and is associated with the engagement of ventral striatum (VS), ventromedial prefrontal cortex, and orbital frontal cortex (King et al. 2016); the latter process involves the comparison and selection between alternative reward options and is associated with the engagement of the executive control network (ECN) including dorsolateral prefrontal cortex (DLPFC) and posterior parietal cortex (PPC) (McClure et al. 2004; Figner et al. 2010; King et al. 2016). These brain regions are also involved in the encoding of food reward value and in cognitive control when modulating eating behaviors, and OB exhibit aberrant activity and functional connectivity in these regions (Meng et al. 2020; Nakamura et al. 2020). Therefore, the aberrant activation and functional connectivity in regions involved in cognitive control and reward during the DD task may contribute to the impulsive decision-making in OB.
To date, few studies have investigated the neural mechanisms underlying impulsive decision-making during the DD task in OB. In obese women, deactivations in DLPFC and PPC during hard versus easy choices were correlated with greater discounting rate (Stoeckel et al. 2013) and predicted greater weight gain in the subsequent 1–3 years (Kishinevsky et al. 2012), with hard choices defined as those with similar subjective values for immediate and delayed reward options (Stoeckel et al. 2013). Similarly, DLPFC activation was linked with the degree of success in long-term post-dietary weight maintenance (Weygandt et al. 2015). Above studies with a continuous design (i.e., correlation analysis within OB) showed that activations in ECN regions were associated with impulse control, body weight regulation, and maintenance. One recent study employed a case–control design, namely the comparison between OB and NW during a DD task, and found that discounting rate was positively associated with lower activation in left anterior insula in OB compared with NW, but reported no significant difference in discounting rate between 2 groups (Miranda-Olivos et al. 2021). In summary, the aberrant neural mechanisms underlying greater impulsivity in OB remain unclear.
In the current study, we employed functional magnetic resonance imaging (fMRI) and DD task to investigate the alterations in brain activation and functional connectivity between OB and matched NW during decision-making in DD task. We hypothesized that OB would exhibit higher discounting rate, and abnormal activation and functional connectivity in regions involved with cognitive control and reward evaluation, and that these alterations would be associated with abnormal eating behavior.
Materials and Methods
Participants
Thirty-five participants with OB were recruited at Xijing Gastrointestinal Hospital affiliated to the Air Force Medical University in Xi’an, China. Patients with psychiatric or neurological diseases, previous intestinal surgery, inflammatory intestinal disease, organ dysfunction, or any current medication that could affect the brain were excluded. Subjects who had a waist circumference greater than the interior diameter of the scanner were also excluded (Li et al. 2018; Zhang et al. 2019). Given these criteria, 5 individuals were disqualified for the magnetic resonance imaging (MRI) scan and 30 OB remained. The control group consisted of 30 NW who were age and gender matched with OB (P > 0.05, Table 1). The experimental protocol was approved by the Institutional Review Board of Xijing Hospital and registered in the Chinese Clinical Trial Registry Center under number: ChiCTR-OOB-15006346 (http://www.chictr.org.cn). The study was conducted in accordance with the Declaration of Helsinki. All participants were informed of the nature of the research and provided written informed consent.
Table 1.
Demographic and clinical information of OB and NW
| Characteristics | OB (n = 30) (mean ± SE) | NW (n = 30) (mean ± SE) | T | P | |
|---|---|---|---|---|---|
| Age (years) | 30.86 ± 1.54 | 28.60 ± 1.17 | 1.174 | 0.245a | |
| Gender | 8 M/22F | 12 M/18F | N/A | 0.273b | |
| BMI (kg/m2) | 35.97 ± 0.87 | 21.12 ± 0.40 | 15.484 | <0.001a | |
| HAMA | 8.97 ± 0.96 | 3.34 ± 0.62 | 4.740 | <0.001a | |
| HAMD | 9.66 ± 1.36 | 4.34 ± 0.84 | 3.312 | 0.002a | |
| TFEQ | Cognitive control | 9.13 ± 0.76 | 8.04 ± 0.68 | 1.050 | 0.298a |
| Disinhibition | 8.52 ± 0.48 | 5.07 ± 0.35 | 5.615 | <0.001a | |
| Hunger | 6.97 ± 0.50 | 2.82 ± 0.39 | 6.337 | <0.001a | |
Abbreviations: OB, obese patients; NW, normal-weight controls; SE, standard error; BMI, body mass index; HAMA, Hamilton Anxiety Rating Scale; HAMD, Hamilton Depression Rating Scale; TFEQ, Three-Factor Eating Questionnaire.
aTwo-tailed 2-sample t-test test.
bChi-square test.
Questionnaires
A trained clinician rated the severity of participant anxiety using the Hamilton Anxiety Rating Scale (HAMA) (Hamilton 1959) and depression using the Hamilton Depression Rating Scale (HAMD) (Hamilton 1960). Subjects were required to complete Three-Factor Eating Questionnaire (TFEQ) (Stunkard and Messick 1985) for assessing eating behaviors.
DD Paradigm
Each trial in the DD task consisted of 2 stages including choice and feedback (Fig. 1). During the choice stage, a smaller immediate reward appeared on a randomly selected side of the screen and a larger delayed reward was shown on the opposite side, and participants were instructed to select their preferred option via a button press within 4 s. After that, a feedback stage started in which the chosen option was presented and lasted for 2 s. Then, a fixation was shown as a baseline for 4–8 s to jitter trial presentation. The DD task consisted of 6 ratios (0, 0.2, 0.4, 0.6, 0.8, and 1) of immediate to delayed monetary rewards magnitudes and used 7 delayed time levels (1 day, 1 week, 2 weeks, 1 month, 3 months, 6 months, and 1 year). Each ratio was paired with each delay level, yielding 42 trials presented to each subject in pseudorandom sequence and a total duration of 8 min 24 s.
Figure 1.
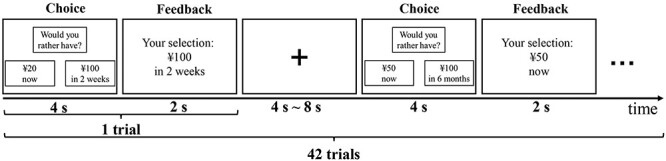
The intertemporal monetary DD task paradigm employed in the current study. Each trial consisted of choice stage and feedback stage and lasted for 6 s in total. A fixation cross was presented as a baseline for 4–8 s to jitter trial presentation.
The discounting rate (K) was calculated as the measure of impulsivity based on the hyperbolic function: 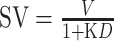 , where SV represents the subjective value, D represents the delayed time and V represents the amount of the larger delayed rewards (Laibson 1997). Because the discounting rate did not follow a normal distribution, the log-transformed K (lg(K)) was calculated (Kobiella et al. 2014). Area under curve (AUC) was further calculated, which is an alternative index of discounting and independent from any theoretical assumptions regarding the form of the discounting function (Myerson et al. 2001).
, where SV represents the subjective value, D represents the delayed time and V represents the amount of the larger delayed rewards (Laibson 1997). Because the discounting rate did not follow a normal distribution, the log-transformed K (lg(K)) was calculated (Kobiella et al. 2014). Area under curve (AUC) was further calculated, which is an alternative index of discounting and independent from any theoretical assumptions regarding the form of the discounting function (Myerson et al. 2001).
MRI Acquisition
Participants were instructed during a brief practice on how to perform the DD task prior to the experiment. The experiment was carried out using a 3.0 T Signa Excite HD (GE, Milwaukee, WI) scanner. A standard head coil was used with foam padding to reduce head motion. First, a high-resolution structural image for each participant was acquired, using a 3D magnetization-prepared rapid acquisition gradient-echo sequence with voxel size of 1 mm3 and with an axial fast spoiled gradient-echo sequence (TR = 7.8 ms, TE = 3.0 ms, matrix size = 256 × 256, field of view = 256 × 256 mm2, slice thickness = 1 mm, and 166 slices). Then, participants performed the DD task within the scanner, whereas a gradient-echo T2*-weighted echo-planar imaging sequence was used for acquiring functional images with the following parameters: TR = 2000 ms, TE = 30 ms, matrix size = 64 × 64, FOV = 256 × 256 mm2, flip angle = 90 degrees, in-plane resolution of 4 mm2, slice thickness = 4 mm, and 32 axial slices. E-Prime software (PST, Pittsburgh, PA) synchronized the stimulus display to the fMRI acquisition and recorded participant responses via an MRI-compatible fiber optic keypad (Sinorad, Shenzhen, China). Stimuli were projected onto a screen located at the back of the bore of the magnet and participants read the choices by looking into a mirror affixed to the top of the head coil.
Image Processing
Imaging data were preprocessed using Statistical Parametric Mapping 12 (SPM12, https://www.fil.ion.ucl.ac.uk/spm/). The first 5 time points were removed to minimize nonequilibrium effects in the fMRI signal. Then, slice-timing and head movement correction were performed using default settings. The echo-planar images of each participant were co-registered with the corresponding T1 structural image and spatially normalized to the stereotactic space of the Montreal Neurological Institute and resampled to a voxel size of 3mm3. An isotropic Gaussian kernel (full-width at half-maximum = 6 mm3) was used to spatially smooth the images.
In the current study, the 20 (~50% of) trials that presented a k-value closest to an individual’s indifference point were categorized as hard-choice trials. These trials had high similarity in the subjective value between immediate and delayed rewards, and all remaining trials were categorized as easy-choice trials. For each participant, a general linear model (GLM) including hard- and easy-choice regressors was constructed. Each regressor was created by convolving the canonical hemodynamic response function with a box-car function corresponding to the duration from the onset of choice presentation till response selection. We excluded trials where the participant failed to respond within 4 s of choice onset. Additionally, 6 realignment parameters were also included in the GLM as covariates. Individual beta images to hard- and easy-choice responses were calculated and submitted to the second level random-effects analysis by calculating a flexible-factorial model with group (OB, NW) and difficulty level (hard, easy) as factors. In addition, a subject factor was included in the model to account for between-subject variance. Activated voxels were determined by means of bi-directed F-contrasts for interactions and directed T-contrasts for main effects of group (Flaisch et al. 2015). Results were corrected for multiple comparisons using family wise error (FWE) corrections at the cluster-level correction approach (PFWE < 0.05) with a minimum cluster size of k = 50 voxels and a cluster defining threshold of P < 0.05 (FWE corrected at the voxel level).
PPI Analysis
To examine functional connectivity during the task, regions of interest (ROIs) with significant group or interaction effects were selected as seed regions, and then whole-brain generalized psychophysiological interaction (gPPI) analysis (McLaren et al. 2012) was performed to investigate alterations in choice difficulty-related functional connectivity between OB and NW (P < 0.001 at the voxel-level and cluster-level correction PFWE < 0.05, k > 50) (see Supplementary Material for detailed information) (Li et al. 2019).
Correlation Analysis
Pearson partial correlation, conducted with age, gender, HAMA, and HAMD as covariates (see Supplementary Material for detailed information), was used to assess the association between task-related brain responses and behavioral measurements, including body mass index (BMI), impulsivity measures (i.e., lg(K) and AUC) and eating behavioral measurements. Similarly, Pearson partial correlation analyses were also performed between PPI values and behavioral measurements (Li et al. 2019). Bonferroni correction was applied for multiple comparisons and level of significance was set at P = 0.003 (0.05/18).
Mediation Analysis
For each ROI that significantly correlated with an impulsivity measure and eating behavior measurement, we performed exploratory mediation analysis to assess whether the relationship between brain activation and eating behavior could be explained by impulsivity, where there were 6 path models (MacKinnon 2008; Masten et al. 2011). If impulsivity is a full mediator between brain activation and eating behavior, the relationship between brain activation and eating behavior would become insignificant when impulsivity index is controlled. According to standard convention, “a” refers to brain-impulsivity effect, “b” refers to impulsivity-eating behavior effect, and “c’” refers to the direct brain-eating behavior effect, controlling for the mediator impulsivity (Kober et al. 2010; Hu et al. 2021). The product “a × b” tests the significance of the mediator. As is customary, we used a bootstrapping test (5000 repetitions) (Preacher and Hayes 2004) for the statistical significance of the product “a × b.” A significant mediating effect is defined as a 95% confidence interval (CI) that does not include zero. The present mediation analysis was implemented using SPSS macros.
Results
Demographic Characteristics
There were no differences in age and gender between OB and NW groups (P > 0.05; Table 1). Compared with NW, OB showed significant higher BMI (P < 0.001), HAMA (P < 0.001), HAMD (P = 0.002), disinhibition (TFEQ) (P < 0.001), hunger (TFEQ) (P < 0.001) (Table 1).
Behavioral Data Analysis
In line with previous studies, OB presented significant higher impulsivity (mean lg(K) = −1.51, standard error (SE) = 0.16) compared with NW (mean lg(K) = −2.30, SE = 0.17; 2-tailed 2-sample t-test, t = 3.484, P < 0.001). The mean discounting rate value corresponding to K were 0.18 (SE = 0.09) and 0.03 (SE = 0.01) in OB and NW group, respectively. Similarly, OB showed significantly lower AUC (mean AUC = 0.33, SE = 0.04) compared with NW (mean AUC = 0.55, SE = 0.05; 2-tailed 2-sample t-test, t = −3.269, P = 0.002). For response time (RT), OB made choices generally slower than NW (mean ± SE: 2028.75 ± 56.10 vs. 1849.95 ± 61.15 ms; 2-tailed 2-sample t-test, t = 2.154, P = 0.035). While it took significantly longer to make hard relative to easy choices in both group (mean ± SE: 2077.20 ± 50.09 ms [hard] vs. 1813.67 ± 40.78 ms [easy]; 2-tailed 2-sample t-test, t = 4.080, P < 0.001), the differences in RT (hard–easy) were similar between the groups (286.77 ± 44.61 ms [OB] vs. 240.28 ± 42.05 ms [NW]; 2-tailed 2-sample t-test, t = 0.758, P = 0.451). In addition, there were significant negative correlations between lg(K) and cognitive control (TFEQ) (r = −0.518, P = 0.003), between AUC and hunger (TFEQ) (r = −0.370, P = 0.044) and positive correlation between lg(K) and hunger (TFEQ) (r = 0.416, P = 0.022) in OB.
Imaging Results
Main effects of group revealed that OB exhibited greater activation in the right DLPFC (Brodmann area [BA] 9), right angular gyrus [AG], and left inferior parietal lobule (IPL) compared with NW (PFWE < 0.05 at the voxel and cluster level with a minimum cluster size of k = 50 voxels) (Fig. 2A). Post hoc tests showed that NW presented significantly higher activation in the right DLPFC during hard trials than easy trials (P < 0.05, False Discovery Rate corrected), while there were no significant differences in OB. In contrast, NW exhibited higher activation in supplementary motor area postcentral gyrus, superior temporal gyrus, and middle occipital gyrus (Fig. S1). Activations in DLPFC and AG during hard trials were positively correlated with lg(K) and negatively correlated with AUC and cognitive control (TFEQ) in OB (Fig. 2B). There were no significant interaction effects.
Figure 2.

The group differences for brain responses to hard and easy choices and correlation analysis between brain activation and behavior measurements. (A) OB exhibited higher activation than NW in cognitive control-related regions include DLPFC, AG, and IPL (OB > NW) (PFWE < 0.05 at the voxel and cluster level with a minimum cluster size of k = 50 voxels). (B) The activation in DLPFC and AG were correlated with lg(K), AUC, and cognitive control (TFEQ). The dots represent brain activation and behavioral measurements (CA) of subjects. The straight line is the trend line showing the relationship between brain activation and behavioral measurements. CA, covariate adjusted.
gPPI Analysis
In gPPI analysis, OB exhibited decreased connectivity between left IPL and bilateral DLPFC (BA 46), between right AG and right caudate (P < 0.001 at the voxel level and PFWE < 0.05 at the cluster level) (Fig. 3A) in response to hard versus easy choices. In addition, the connectivity between AG and caudate was negatively correlated with lg(K) (r = −0.505, P = 0.006) and positively correlated with AUC (r = 0.585, P = 0.001) in OB (Fig. 3B).
Figure 3.

The gPPI connectivity differences between OB and NW and correlation analysis between PPI connectivity and behavior measures. (A) Lower connectivity between the left IPL and bilateral DLPFC and between right AG and caudate in OB compared with NW (P < 0.001 at the voxel-level and cluster-level correction PFWE < 0.05). (B) The PPI connectivity between AG and caudate was negatively correlated with discounting rate and positively correlated with AUC. The dots represent PPI connectivity and discounting rate (CA) of subjects. The straight line is the trend line showing the relationship between PPI connectivity and discounting rate.
Mediation Analysis
DLPFC activation during hard trials, lg(K), and cognitive control (TFEQ) were significantly correlated with each other. Mediation analysis revealed that the relationship between DLPFC activation and cognitive control (TFEQ) (c = −1.5736 ± 0.6869, P = 0.0292) was mediated by the lg(K) (c’ = −0.9488 ± 0.6907, P = 0.1801; a × b: 95% CI [−1.5087, −0.0307]) (Fig. 4A). In addition, the relationship between DLPFC activation and lg(K) (c = 0.3275 ± 0.1456, P = 0.0320) could also be mediated by cognitive control (TFEQ) (c’ = 1925 ± 0.1468, P = 0.2001; a × b: 95% CI [0.0145, 0.4005]) (Fig. 4B). There were no significant mediating effects in the other 4 path models (Fig. S2).
Figure 4.
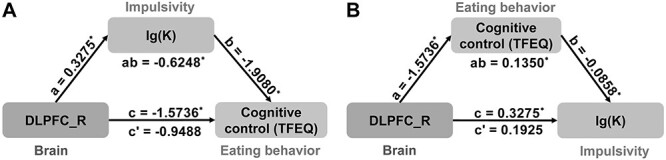
The mediation analysis among DLPFC activation (brain), lg(K) (impulsivity), and cognitive control (TFEQ) (eating behavior). (A) The relationship between DLPFC activation and cognitive control (TFEQ) was mediated by lg(K). (B) The relationship between DLPFC activation and lg(K) was mediated by cognitive control (TFEQ).
Discussion
In the current study, we investigated alterations in brain activation and functional connectivity during decision-making in DD task between OB and NW. As expected, OB exhibited greater discounting rate and impulsivity compared with NW. During the DD task, OB showed enhanced activation in the right DLPFC, right AG, and left IPL. Connectivity within ECN (IPL–bilateral DLPFC) and between ECN and regions involved with reward evaluation (AG–caudate) were decreased in OB. In addition, the relationship between the DLPFC activation and cognitive control (TFEQ) was mediated by impulsivity (lg(K)); likewise, the relationship between the DLPFC activation and impulsivity (lg(K)) was mediated by impulsivity (lg(K)). We discuss these findings in detail below.
Group Differences in Brain Activation
Results showed that OB compared with NW exhibited greater activation on both easy and hard choices in the DLPFC, IPL, and AG, which are core nodes of ECN and are thought to support cognitive control during decision-makings (Hoffman et al. 2008). DLPFC is commonly implicated in various cognitive processes involving reward-related impulse control, decision-making, and planning (Coutlee and Huettel 2012; Stoeckel et al. 2013). The increased activation in DLPFC indicates increased recruitment of neural resources and may reflect the decreased neural efficiency in ECN in processing conflict-based decision-making (King et al. 2016). The positive correlation between DLPFC activation and discounting rate (lg(K)) and the slower decision-making in OB versus NW support this interpretation. Although there were no significant interaction effects, NW exhibited significantly higher activation in the right DLPFC during hard choices than easy choices, whereas there was no significant difference in OB. In general, the neural resources recruited in ECN during easy choices should be small fraction of the recruitment during hard choices (Monterosso et al. 2007; Kishinevsky et al. 2012). Therefore, the similar activity level on both easy and hard trials in OB suggested the high neural expenditure and low neural efficiency in ECN (Hoffman et al. 2008). The PPC (i.e., IPL and AG) is involved in the abstract value comparison of available options during decision-making (Sugrue et al. 2004; Boettiger et al. 2007). The higher activation in PPC may suggest that OB perceived a shorter “distance” between the 2 options and have difficulty comparing, which results in the enhanced activation in PPC (Boettiger et al. 2007). Alternatively, the higher activation may also reflect processing inefficiency of PPC, like DLPFC, as evidenced in the positive association between the AG activation and discounting rate (lg(K)). Taken together, the enhanced activation in regions implicated in cognitive control during decision-making in the DD task indicated lower efficiency in ECN and increased difficulty in comparing alternative choices and may subsequently bias selection for smaller, immediate rewards in OB (Boettiger et al. 2007; Hoffman et al. 2008).
However, we did not observe the altered activation in VS, which is a critical node for assigning value to reward stimuli during decision-making (Hoffman et al. 2008). One plausible reason is that the largely hypothetical monetary rewards in the DD task may not be able to robustly activate VS (Monterosso et al. 2007), and perhaps something more salient like food reward may be necessary to elicit VS activation in OB. In addition, VS activation during decision-making is linked to individual reward sensitivity rather than reward comparison or choice selection (Knutson et al. 2005; Boettiger et al. 2007). Therefore, VS recruitment may not be the main source of the high impulsivity in OB.
PPI Connectivity Difference
DD is a complex cognitive behavior mainly consisting of 2 processes including decision-making and reward evaluation. Therefore, the interactions between multiple regions involved in cognitive control and reward evaluation should play an important role during decision-making in DD task. In the current study, PPI connectivity between left IPL and bilateral DLPFC was lower in OB compared with NW. DLPFC and IPL are important nodes of the ECN and are involved in cognitive control when modulating eating behavior in response to food cues. Thus, the weaker connectivity of DLPFC–IPL might reflect dysfunction of ECN and may relate to greater sensitivity to food intake in OB versus NW (Park et al. 2016; Ding et al. 2020; Zhang et al. 2021). Although there was no significant difference in activation in regions associated with reward evaluation between 2 groups, connectivity between right AG and right caudate decreased in OB, which was negatively correlated with lg(K) and positively correlated with AUC. Caudate–PPC connectivity is a fundamental part of corticostriatal circuitry (Wei et al. 2020), which plays an important role in habit-based and goal-directed behavior (Haber 2016), and the co-activation between caudate and PPC is associated with decision-making. The decreased connectivity between AG and caudate suggested an impairment of corticostriatal circuitry associated with impulsive decision-making. This finding was also consistent with prior research that abnormal reward function in this circuit diminishes and redirects cognitive control function towards impulsive behavior (Kohno et al. 2014; Hobkirk et al. 2019).
Mediation Analysis
DLPFC and PPC are involved in cognitive control when modulating eating behavior in response to tempting food (Ding et al. 2020). In the current study, DLPFC activation was correlated with both impulsivity index (lg(K)) and cognitive control (TFEQ), and the impulsivity measure (lg(K)) was negatively correlated with cognitive control (TFEQ). We found that the impulsivity mediated DLPFC activation and cognitive control (TFEQ). In addition, cognitive control (TFEQ) mediated the relationship between DLPFC activation and impulsivity. The above 2 mediation models suggest that the neural inefficiency in ECN may be a primary cause of both decreased cognitive control (TFEQ) and greater impulsivity and the mutually reinforcing relationship between greater impulsivity and decreased cognitive control (TFEQ). That is to say, the greater impulsivity may be both a cause and a result of abnormal eating behavior, similar as in addiction, and so may result in a “vicious cycle” positive feedback loop (Frost and McNaughton 2017). Although there was no direct association between impulsivity measures and BMI, externally cognitive control (TFEQ) was associated with BMI, suggesting an indirect link between impulsivity and BMI (Gerlach et al. 2015).
Limitation
There were several limitations that need to be taken into account. First, due to the strict exclusion criteria, we did not have a larger cohort for both OB and NW groups, which limited the generalizability and statistical power of our observations. Secondly, some correlations between brain activation and discounting rate, eating behavior measurements did not reach significance after multiple comparisons, but certain correlative trends may still be meaningful for delineating the relationship among brain activation, impulsivity, and eating behavior.
Conclusion
In the current study, we investigated the altered neural mechanisms during decision-making in a monetary DD task between OB and NW. OB exhibited significantly greater activation during hard and easy choices in DLPFC, IPL, and AG, indicating neural inefficiency in ECN. PPI connectivity between IPL and bilateral DLPFC and between AG and caudate was lower in OB compared with NW, indicating that aberrant circuit function within ECN and between ECN and reward regions in OB. In addition, the relationship between greater DLPFC activation and abnormal eating behavior was mediated by greater impulsivity, and the association between greater DLPFC activation and greater impulsivity was mediated by abnormal eating behavior. These findings shed light on that the aberrant function of the ECN, and the aberrant interaction between regions involves in cognitive control and reward evaluation underpin the greater impulsivity in OB and may ultimately result in abnormal eating behaviors.
Authors’ Contributions
Y.Z., G.J., and G.J.W.: study design; J.Y., G.C., and Y. Han.: data collection; W.Z., G. Li., Y. Hu., J.W., Y. He., and G. Lv.: data analysis; W.Z.: drafting of the manuscript; K.V., Y.Z., P.M., G.J.W., and N.V.: critical revision of the manuscript; and all authors: critically reviewed the content and approved the final version for publication.
Funding
National Natural Science Foundation of China (grant 8217071870, 61431013, 81730016, 31670828); the Open Funding Project of National Key Laboratory of Human Factors Engineering (grant 6142222190103); National Clinical Research Center for Digestive Diseases, Xi'an, China (grant 2015BAI13B07); Intramural Research Program of the United States National Institute on Alcoholism and Alcohol Abuse (grant Y1AA3009 to P.M., N.D.V., G.J.W.).
Notes
Conflict of Interest: The authors declare no conflict of interest.
Supplementary Material
Contributor Information
Wenchao Zhang, Center for Brain Imaging, School of Life Science and Technology, Xidian University, Xi’an, Shaanxi 710071, China.
Guanya Li, Center for Brain Imaging, School of Life Science and Technology, Xidian University, Xi’an, Shaanxi 710071, China.
Peter Manza, Laboratory of Neuroimaging, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD 20892, USA.
Yang Hu, Center for Brain Imaging, School of Life Science and Technology, Xidian University, Xi’an, Shaanxi 710071, China.
Jia Wang, Center for Brain Imaging, School of Life Science and Technology, Xidian University, Xi’an, Shaanxi 710071, China.
Ganggang Lv, Center for Brain Imaging, School of Life Science and Technology, Xidian University, Xi’an, Shaanxi 710071, China.
Yang He, Center for Brain Imaging, School of Life Science and Technology, Xidian University, Xi’an, Shaanxi 710071, China.
Karen M von Deneen, Center for Brain Imaging, School of Life Science and Technology, Xidian University, Xi’an, Shaanxi 710071, China.
Juan Yu, State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, The Air Force Medical University, Xi'an, Shaanxi 710032, China.
Yu Han, Department of Radiology, Tangdu Hospital, The Air Force Medical University, Xi’an, Shaanxi 710038, China.
Guangbin Cui, Department of Radiology, Tangdu Hospital, The Air Force Medical University, Xi’an, Shaanxi 710038, China.
Nora D Volkow, Laboratory of Neuroimaging, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD 20892, USA.
Yongzhan Nie, State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, The Air Force Medical University, Xi'an, Shaanxi 710032, China.
Gang Ji, State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases and Xijing Hospital of Digestive Diseases, The Air Force Medical University, Xi'an, Shaanxi 710032, China.
Gene-Jack Wang, Laboratory of Neuroimaging, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD 20892, USA.
Yi Zhang, Center for Brain Imaging, School of Life Science and Technology, Xidian University, Xi’an, Shaanxi 710071, China.
References
- Ainslie G. 1975. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 82:463–496. [DOI] [PubMed] [Google Scholar]
- Amlung M, Petker T, Jackson J, Balodis I, MacKillop J. 2016. Steep discounting of delayed monetary and food rewards in obesity: a meta-analysis. Psychol Med. 46:2423–2434. [DOI] [PubMed] [Google Scholar]
- Best JR, Theim KR, Gredysa DM, Stein RI, Welch RR, Saelens BE, Perri MG, Schechtman KB, Epstein LH, Wilfley DE. 2012. Behavioral economic predictors of overweight children's weight loss. J Consult Clin Psychol. 80:1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, Fields HL. 2007. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 27:14383–14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutlee CG, Huettel SA. 2012. The functional neuroanatomy of decision making: prefrontal control of thought and action. Brain Res. 1428:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids S, Lauffer H, Thoms K, Jagdhuhn M, Hirschfeld H, Domin M, Hamm A, Lotze M. 2010. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int J Obes (Lond). 34:94–104. [DOI] [PubMed] [Google Scholar]
- Ding Y, Ji G, Li G, Zhang W, Hu Y, Liu L, Wang Y, Hu C, von Deneen KM, Han Y et al. 2020. Altered interactions among resting-state networks in individuals with obesity. Obesity (Silver Spring). 28:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, Weber EU. 2010. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 13:538–539. [DOI] [PubMed] [Google Scholar]
- Flaisch T, Imhof M, Schmalzle R, Wentz KU, Ibach B, Schupp HT. 2015. Implicit and explicit attention to pictures and words: an fMRI-study of concurrent emotional stimulus processing. Front Psychol. 6:1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost R, McNaughton N. 2017. The neural basis of delay discounting: a review and preliminary model. Neurosci Biobehav Rev. 79:48–65. [DOI] [PubMed] [Google Scholar]
- Garrow JS. 1988. Obesity and related diseases. Edinburgh: Churchill Livingstone. [Google Scholar]
- Gerlach G, Herpertz S, Loeber S. 2015. Personality traits and obesity: a systematic review. Obes Rev. 16:32–63. [DOI] [PubMed] [Google Scholar]
- Haber SN. 2016. Corticostriatal circuitry. Dialogues Clin Neurosci. 18:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. 1959. The assessment of anxiety states by rating. Br J Med Psychol. 32:50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M. 1960. A rating scale for depression. J Neurol Neurosurg Psychiatry. 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobkirk AL, Bell RP, Utevsky AV, Huettel S, Meade CS. 2019. Reward and executive control network resting-state functional connectivity is associated with impulsivity during reward-based decision making for cocaine users. Drug Alcohol Depend. 194:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, Mitchell SH. 2008. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology (Berl). 201:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Ji G, Li G, Manza P, Zhang W, Wang J, Lv G, He Y, Zhang Z, Yuan K et al. 2021. Brain connectivity, and hormonal and behavioral correlates of sustained weight loss in obese patients after laparoscopic sleeve gastrectomy. Cereb Cortex. 31:1284–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Geisler D, Bernardoni F, Ritschel F, Bohm I, Seidel M, Mennigen E, Ripke S, Smolka MN, Roessner V et al. 2016. Altered neural efficiency of decision making during temporal reward discounting in anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 55:972–979. [DOI] [PubMed] [Google Scholar]
- Kishinevsky FI, Cox JE, Murdaugh DL, Stoeckel LE, Cook ER, Weller RE. 2012. fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite. 58:582–592. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. 2005. Distributed neural representation of expected value. J Neurosci. 25:4806–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. 2010. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 107:14811–14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobiella A, Ripke S, Kroemer NB, Vollmert C, Vollstadt-Klein S, Ulshofer DE, Smolka MN. 2014. Acute and chronic nicotine effects on behaviour and brain activation during intertemporal decision making. Addict Biol. 19:918–930. [DOI] [PubMed] [Google Scholar]
- Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED. 2014. Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. Jama Psychiat. 71:812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laibson D. 1997. Golden eggs and hyperbolic discounting. Q J Econ. 112:443–478. [Google Scholar]
- Lawyer SR, Boomhower SR, Rasmussen EB. 2015. Differential associations between obesity and behavioral measures of impulsivity. Appetite. 95:375–382. [DOI] [PubMed] [Google Scholar]
- Li G, Ji G, Hu Y, Liu L, Jin Q, Zhang W, Liu L, Wang Y, Zhao J, von Deneen KM et al. 2019. Reduced plasma ghrelin concentrations are associated with decreased brain reactivity to food cues after laparoscopic sleeve gastrectomy. Psychoneuroendocrinology. 100:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Ji G, Hu Y, Xu M, Jin Q, Liu L, von Deneen KM, Zhao J, Chen A, Cui G et al. 2018. Bariatric surgery in obese patients reduced resting connectivity of brain regions involved with self-referential processing. Hum Brain Mapp. 39:4755–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP. 2008. Introduction to statistical mediation analysis. New York: Lawrence Erlbaum Associates. [Google Scholar]
- Manwaring JL, Green L, Myerson J, Strube MJ, Wilfley DE. 2011. Discounting of various types of rewards by women with and without binge eating disorder: evidence for general rather than specific differences. Psychol Rec. 61:561–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Morelli SA, Eisenberger NI. 2011. An fMRI investigation of empathy for 'social pain' and subsequent prosocial behavior. Neuroimage. 55:381–388. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. 2004. Separate neural systems value immediate and delayed monetary rewards. Science. 306:503–507. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. 2012. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Huang D, Ao H, Wang X, Gao X. 2020. Food cue recruits increased reward processing and decreased inhibitory control processing in the obese/overweight: an activation likelihood estimation meta-analysis of fMRI studies. Obes Res Clin Pract. 14:127–135. [DOI] [PubMed] [Google Scholar]
- Miranda-Olivos R, Steward T, Martinez-Zalacain I, Mestre-Bach G, Juaneda-Segui A, Jimenez-Murcia S, Fernandez-Formoso JA, Vilarrasa N, Veciana DLHM, Custal N et al. 2021. The neural correlates of delay discounting in obesity and binge eating disorder. J Behav Addict. 10.1556/2006.2021.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. 2007. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 28:383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Padilla M, Verdejo-Roman J, Fernandez-Serrano MJ, Reyes DPG, Verdejo-Garcia A. 2018. Increased food choice-evoked brain activation in adolescents with excess weight: relationship with subjective craving and behavior. Appetite. 131:7–13. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. 2001. Area under the curve as a measure of discounting. J Exp Anal Behav. 76:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Ozawa S, Koike S. 2020. Caudate functional connectivity associated with weight change in adolescents. Front Hum Neurosci. 14:587763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BY, Seo J, Park H. 2016. Functional brain networks associated with eating behaviors in obesity. Sci Rep. 6:23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. 2004. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 36:717–731. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. 1991. Subjective probability and delay. J Exp Anal Behav. 55:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff S, Amodio P, Testa G, Nardi M, Montagnese S, Caregaro L, di Pellegrino G, Sellitto M. 2016. Impulsivity toward food reward is related to BMI: evidence from intertemporal choice in obese and normal-weight individuals. Brain Cogn. 110:112–119. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Murdaugh DL, Cox JE, Cook ER, Weller RE. 2013. Greater impulsivity is associated with decreased brain activation in obese women during a delay discounting task. Brain Imaging Behav. 7:116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. 1985. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 29:71–83. [DOI] [PubMed] [Google Scholar]
- Sugrue LP, Corrado GS, Newsome WT. 2004. Matching behavior and the representation of value in the parietal cortex. Science. 304:1782–1787. [DOI] [PubMed] [Google Scholar]
- Wei SY, Tseng HH, Chang HH, Lu TH, Chang WH, Chiu NT, Yang YK, Chen PS. 2020. Dysregulation of oxytocin and dopamine in the corticostriatal circuitry in bipolar II disorder. Transl Psychiatry. 10:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weygandt M, Mai K, Dommes E, Ritter K, Leupelt V, Spranger J, Haynes JD. 2015. Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity. Neuroimage. 109:318–327. [DOI] [PubMed] [Google Scholar]
- Zhang P, Wu GW, Yu FX, Liu Y, Li MY, Wang Z, Ding HY, Li XS, Wang H, Jin M et al. 2020. Abnormal regional neural activity and reorganized neural network in obesity: evidence from resting-state fMRI. Obesity (Silver Spring). 28:1283–1291. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ji G, Manza P, Li G, Hu Y, Wang J, Lv G, He Y, von Deneen KM, Han Y et al. 2021. Connectome-based prediction of optimal weight loss six months after bariatric surgery. Cereb Cortex. 31:2561–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ji G, Li G, Hu Y, Liu L, Jin Q, Meng Q, Zhao J, Yuan K, Liu J et al. 2019. Ghrelin reductions following bariatric surgery were associated with decreased resting state activity in the hippocampus. Int J Obes (Lond). 43:842–851. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu J, Yao J, Ji G, Qian L, Wang J, Zhang G, Tian J, Nie Y, Zhang YE et al. 2014. Obesity: pathophysiology and intervention. Nutrients. 6:5153–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


