Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is known to play a key role in enhancing multiple immune functions that affect response to infectious pathogens including antigen presentation, complement- and antibody-mediated phagocytosis, microbicidal activity, and neutrophil chemotaxis. Reduced GM-CSF activity and immune response provides a mechanism for increased infection risk associated with autoimmune pulmonary alveolar proteinosis (aPAP) and other disorders involving the presence of GM-CSF autoantibodies. We present a case series of five patients with persistent or unusual pulmonary and central nervous system opportunistic infections (Cryptococcus gattii, Flavobacterium, Nocardia) and elevated GM-CSF autoantibody levels, as well as 27 cases identified on systematic review of the literature.
Keywords: autoantibodies, autoimmune pulmonary alveolar proteinosis, granulocyte-macrophage colony-stimulating factor, infection, sargramostim
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is known to play a key role in enhancing multiple immune functions that affect response to infectious pathogens including antigen presentation, complement- and antibody-mediated phagocytosis, microbicidal activity, and neutrophil chemotaxis [1]. Loss of GM-CSF function can alter lipid homeostasis, causing lipid accumulation within alveolar macrophages and a reduction in surfactant clearance [2]. Excess surfactant may exacerbate inhibition of immune function by preventing effector cells from neutralizing pulmonary infections and removing associated cellular debris, leading to pulmonary disease [3, 4]. In patients with autoimmune pulmonary alveolar proteinosis (aPAP), a rare autoimmune disease, elevated levels of neutralizing autoantibodies to GM-CSF inhibit normal alveolar macrophage and neutrophil immune functions [5]. Reduced GM-CSF activity and immune response provide a mechanism for increased infection risk associated with aPAP and other disorders involving the presence of GM-CSF autoantibodies. Both patients with aPAP and knockout mice deficient in GM-CSF exhibit increased susceptibility to a variety of bacterial and fungal infections [6–10] including systemic and/or central nervous system (CNS) opportunistic infections (OIs) [11–13].
Diagnosis of aPAP can precede, accompany, or follow infections, and fungal infections in particular are associated with a high mortality rate [13, 14]. Numerous case reports document C. gattii or Nocardia infections, often within the CNS, occurring concurrently with aPAP; in other cases, aPAP developed subsequent to an OI [15–18]. In a cohort of Japanese patients with aPAP, 5.7% were diagnosed with intercurrent infections (primarily Aspergillus, atypical mycobacteria, and Mycobacterium tuberculosis), at least half of which were pulmonary in nature [19]. An increased incidence of unusual pulmonary and extrapulmonary fungal infections has been reported in patients with aPAP [20]. Conversely, some patients with infections and GM-CSF autoantibodies may not have clinically apparent aPAP but could be at increased risk for infectious complications [21–24]. Here we present a case series of patients with OIs and elevated GM-CSF autoantibody levels (some with clinical evidence of aPAP, diagnosed either before or after infection). Similar cases reported in the literature are also reviewed.
CASE REPORTS
Case 1
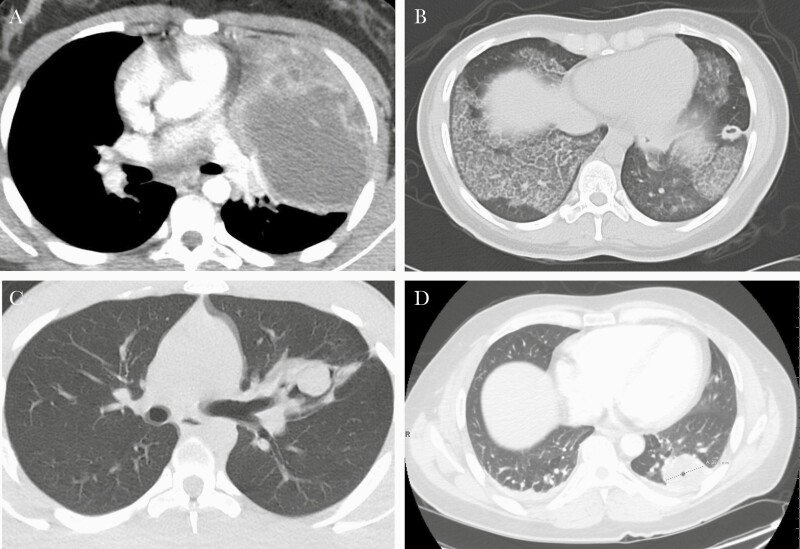
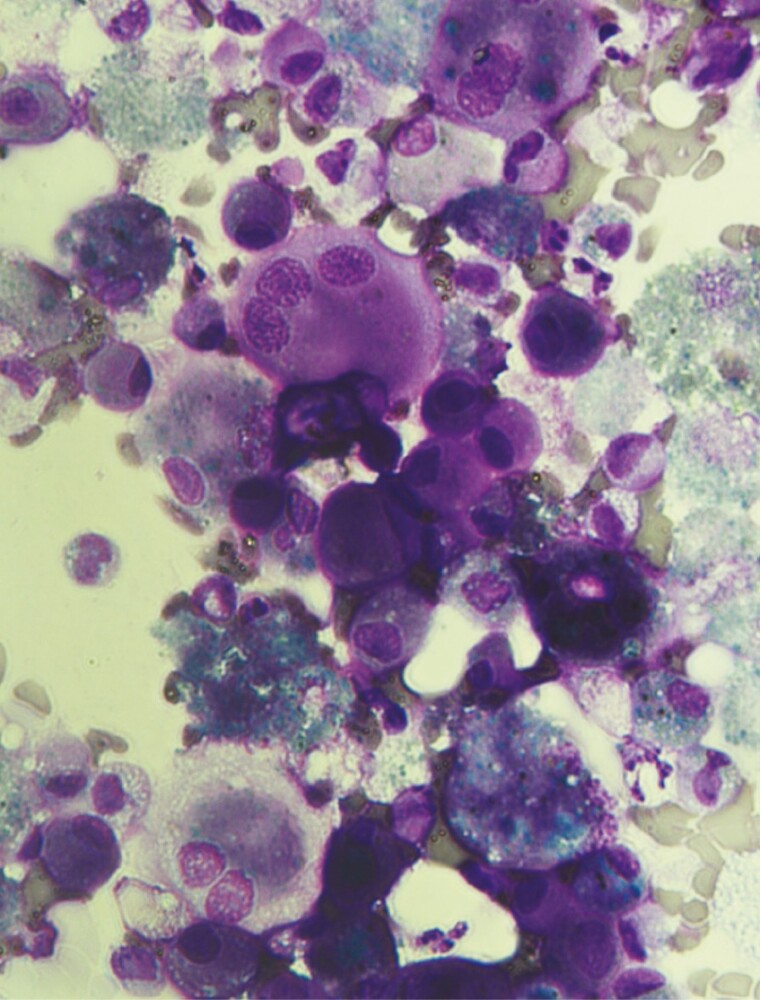
An 11-year-old female with well-controlled asthma presented with worsening cough, dyspnea, tachycardia, and tachypnea (new cases summarized in Table 1). A computed tomography (CT) scan of the chest (Figure 1A) was suggestive of necrotizing pneumonia with left upper lobe abscess and associated left-sided effusion. Cryptococcus gattii was isolated from lung abscess biopsy and pleural fluid cultures and was confirmed as the etiology of pneumonia; other lung parenchyma was normal. Serum cryptococcal antigen titers were elevated (>1:2560) but negative in the cerebrospinal fluid (CSF). She was started on amphotericin and flucytosine (later transitioned to fluconazole prophylaxis). An extensive immunodeficiency workup was unrevealing for underlying immunocompromised conditions. GM-CSF autoantibodies were elevated (476 µg/mL), and STAT5 phosphorylation was abnormal. Repeat lung imaging following recovery from infection was unrevealing, with no evidence of aPAP. Bronchoscopy showed lipid-laden macrophages but no evidence of proteinaceous material (Figure 2). The patient continues to be followed for possible development of clinically evident aPAP.
Table 1.
New Cases of Infection Associated With aPAP or Elevated GM-CSF Autoantibodies
| Case | Age/Sex/Ethnicity | Presenting Signs & Symptoms | Pathogen (Site; Diagnostic) | aPAP Diagnosed | GM-CSF Autoantibody Levels & GM-CSF Functional Testinga | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Cryptococcus | |||||||
| Case 1 | 11/F/African American | Worsening cough, shortness of breath, tachycardia, tachypnea | C. gattii (pulmonary; isolated from biopsy of lung abscess and pleural fluid cultures; Cryptococcal serum Ag titer >1:2560) | No, but elevated GM-CSF autoantibodies and foamy alveolar macrophages detected | 476 µg/mL | Amphotericin and flucytosine ×4 wk, then transitioned to fluconazole prophylaxis ×5 y & ongoing | Complete resolution of infection with no recurrence; at 5-y follow-up, remains on fluconazole prophylaxis and continues to be monitored for possible development of clinically evident aPAP |
| Case 2 | 20/F/African American | Shortness of breath, fever; CT chest with large left lower lobe cavitary lesion and “crazy paving”; later developed progressively worsening hemoptysis |
C. gattii (pulmonary, CNS; cryptococcal serum Ag titer 1:256) | Yes (elevated GM-CSF autoantibody levels and biopsy) | Ranged <3–7 pg/mL over 4-y period (assessed 4 times) following C. gattii Dx; subsequently rose to 120 µg/mL; abnormal JAK/STAT signaling | Amphotericin and flucytosine ×6 wk, then transitioned to fluconazole (800 mg ×1.25 y, 400 mg ×2.75 y, 200 mg ×0.5 y) until MRI improved; serial lumbar punctures to relieve elevated intracranial pressure; due to worsening pulmonary symptoms, patient also received WLL ×4 (last in 2017) | At 5-y follow-up, serial MRI scans indicated stable lesions in L frontal lobe and L cerebellar hemisphere, with residual mild to moderate hydrocephalus; last follow-up 3 y ago |
| Case 3 | 20/M/Caucasian | Progressive occipital headaches, nausea, palpitations | C. gattii (pulmonary, CNS; detected from endobronchial lesion biopsy and resected brain lesion) | No, but elevated GM-CSF autoantibodies detected | Fluorescence intensity 12 428b; inhibition of GM-CSF-induced STAT5 phosphorylationc |
Left upper lobe lobectomy for large cryptococcoma; amphotericin and flucytosine (duration unknown; transferred to another hospital and lost to follow-up) | Patient lost to follow-up after transfer to another hospital |
| Case 4 | 44/M/Hispanic/Latino | Daily headaches, papilledema | C. gattii (pulmonary, CNS; cryptococcal serum Ag titer 1:2048) | Yes (elevated GM-CSF autoantibody levels and abnormal GM-CSF function) | Fluorescence intensity 9958–9939d (1 mo after infection Dx); 15.7 µg/mL (1.5 y after infection Dx) and intermediate STAT5 phosphorylation, but abnormal GM-CSF signaling in EC50 test |
Amphotericin and flucytosine ×9 wk, followed by fluconazole prophylaxis ×1.5 y (ongoing), then prolonged course of high-dose steroids with trimethoprim/sulfamethoxazole prophylaxis | Dyspnea and exertional capacity improved in the 1.5 y after Dx following outpatient rehabilitation; concern for postinfectious inflammatory syndrome 6 mo after Dx; remains on fluconazole prophylaxis (and trimethoprim/sulfamethoxazole while on steroids); continues to be monitored for possible aPAP progression |
| Other pathogens | |||||||
| Case 5 | 50/M/Caucasian | Shortness of breath, abnormal chest imaging | Flavobacterium, Nocardia (CNS; isolated from brain abscess culture) | Yes (VATS R lung biopsy) | 80 µg/mL | Craniotomy for brain abscess; trimethoprim/sulfamethoxazole and amoxicillin/clavulanate ×1.25 y, then trimethoprim/sulfamethoxazole prophylaxis ×9.5 y & ongoing; patient also received WLL ×7 (last in 2015) and sargramostim SC intermittently ×~2.5 y (last in 2016) for aPAP |
Has maintained near-normal pulmonary function for past 9 y, with resolution of abscess/infection and little additional therapy required; remains on trimethoprim/sulfamethoxazole prophylaxis; subsequent MRI showed encephalomalacia and likely scar in prior area of abscess/infection |
Abbreviations: Ag, antigen; aPAP, autoimmune pulmonary alveolar proteinosis; CNS, central nervous system; CT, computed tomography; Dx, diagnosis; EC50, half-maximal effective concentration; GM-CSF, granulocyte-macrophage colony-stimulating factor; MRI, magnetic resonance imaging; rhu; recombinant human; SC, subcutaneously; VATS; video-assisted thoracic surgical; WLL, whole-lung lavage.
Functional GM-CSF test results shown where performed.
Compared with normal of 718 in this assay.
Value not reported.
Compared with normal of 141.5–122.8 in this assay.
Figure 1.
Chest CT scans of new cases of cryptococcal infection in patients with elevated GM-CSF autoantibody levels. A, Case 1. B, Case 2. C, Case 3. D, Case 4. Abbreviations: CT, computed tomography; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Figure 2.
Photomicrograph of foamy lipid-laden alveolar macrophages obtained by bronchoscopy. No evidence of proteinaceous material was observed.
Case 2
A 20-year-old previously healthy female presented with dyspnea and fever. Chest CT (Figure 1B) revealed a dense left lower lobe consolidation with ground-glass opacities bilaterally and intralobular septal thickening (crazy-paving pattern). She was treated empirically for pneumonia, with resolution in symptoms. Four years later, she presented with 3 months of worsening hemoptysis and was found to have a large left lower lobe cavitary lesion and diffuse patchy ground-glass infiltrates. No immunodeficiency was detected, but infectious evaluation indicated a cryptococcal antigen titer of 1:256. Bronchoalveolar (BAL) fluid culture was positive for C. gattii. Periodic acid-Schiff (PAS) stain was inconclusive. A repeat bronchoscopy with biopsy demonstrated pathology findings consistent with PAP. Lumbar puncture revealed elevated intracranial pressure with positive CSF cryptococcal antigen. Brain magnetic resonance imaging (MRI) was significant for frontal and temporal lobe contrast-enhancing lesions. She was started on amphotericin and flucytosine and underwent serial lumbar punctures with improvement in opening pressures. Upon discharge, she was transitioned to fluconazole. GM-CSF autoantibody level was subsequently found to be elevated (120 µg/mL), confirming the diagnosis of aPAP. She continued treatment for the pulmonary and CNS cryptococcal infections for 4.5 years (discontinued December 2017 after improvement of the brain lesions on serial MRI). During this time, pulmonary symptoms worsened, requiring 4 whole-lung lavages (WLLs), the last in February 2017. As of last follow-up in December 2018 (5.5 years after the infection was diagnosed), her symptoms were well controlled apart from mild dyspnea on exertion, and she had returned to work.
Case 3
A 20-year-old male with well-controlled asthma and childhood eczema presented with a 1-month history of progressive occipital headaches, nausea, and palpitations. He reported casual tobacco and marijuana use. Head CT revealed 2 separate complex cerebellar parenchymal masses with cystic and solid components, with enhancing margins. Chest x-ray and CT (Figure 1C) indicated a left lingular cystic lesion; the remaining lung parenchyma was normal. Bronchoscopy showed an exophytic, friable endobronchial lesion that revealed C. gattii upon biopsy, which was also detected following resection of the brain lesion. He was treated with amphotericin and flucytosine while hospitalized. The patient underwent left upper lobe lobectomy for a large (7.3-cm) cryptococcoma, which showed atelectatic lung with necrotizing and non-necrotizing granulomas containing fungal organisms. The patient was positive for GM-CSF autoantibodies, although extensive workup did not reveal an underlying immunocompromised state or confirm an aPAP diagnosis.
Case 4
A 44-year-old previously healthy male presented with development of daily headaches. He had papilledema on ophthalmology exam and ultimately was hospitalized for cryptococcal meningitis discovered on lumbar puncture, requiring a ventriculoperitoneal shunt. He was also found to have cryptococcal pneumonia with a cavitary lesion caused by C. gattii (Figure 1D). Treatment consisted of amphotericin and flucytosine, followed by a prolonged course of steroids along with fluconazole and trimethoprim/sulfamethoxazole prophylaxis due to MRI-enhancing brain lesions concerning for a postinfectious inflammatory syndrome related to the C. gattii infection. One month after hospitalization, his GM-CSF autoantibody level was 70-fold higher than normal (other immune assessment data not available). About 1 year after Cryptococcus diagnosis, new persistent, bilateral, diffuse ground-glass opacities on serial CT scans were found, raising concerns for aPAP. He underwent transbronchial biopsies that were nondiagnostic, although BAL fluid was PAS positive. A repeat GM-CSF autoantibody level was elevated (15.7 µg/mL), consistent with an aPAP diagnosis. With outpatient rehabilitation, dyspnea and exertional capacity have improved, and he has returned to work. He is currently being weaned off corticosteroids and remains on trimethoprim/sulfamethoxazole prophylaxis. To date, he has not required WLL or GM-CSF therapy, but his symptoms and pulmonary function tests will be monitored for aPAP disease progression.
Case 5
A 50-year-old previously healthy male presented with dyspnea and abnormal chest imaging, which did not resolve after 2 courses of antibiotics. He subsequently underwent an open lung biopsy 6 months after symptom onset, revealing PAP. GM-CSF autoantibodies were elevated (80 µg/mL), consistent with aPAP (other immune assessment data not available). He underwent bilateral WLL, with marked improvement in symptoms and normalization of pulmonary function testing and oxygenation except for a mild diffusion impairment. The patient resumed working and exercising. Approximately 2 months later and despite the lack of any pulmonary symptom recurrence, he was hospitalized with an acute alteration in mental status related to a large brain abscess requiring emergent craniotomy. Subsequent cultures were positive for Flavobacterium and Nocardia, and he was treated with trimethoprim/sulfamethoxazole and amoxicillin/clavulanate. In the 18 months following infection, he required 4 additional WLLs, 2 in the context of a severe aPAP flare leading to respiratory failure necessitating mechanical ventilation. He was subsequently treated intermittently with sargramostim (yeast-derived recombinant human [rhu] GM-CSF) for ~2.5 years plus chronic trimethoprim/sulfamethoxazole prophylaxis. He has required only 1 additional WLL over the subsequent 9 years and now maintains near-normal pulmonary function.
LITERATURE REVIEW
A systematic literature review was conducted using search terms (title or abstract) combining GM-CSF AND antibodies AND infection, limiting results to English-language publications from January 2001 through November 2021 (Table 2). Of 27 identified cases (including 1 patient with 2 separate infectious cases), 10 (37.0%) patients had only pulmonary infection, 6 (22.2%) had only CNS infection, 10 (37.0%) had both, and 1 (3.7%) had an infection at a different site. Most infections (19 [70.4%]) were due to Cryptococcus, while 4 (14.8%) were from Nocardia and 4 (14.8%) were from other pathogens.
Table 2.
Published Case Reports of Infection With GM-CSF Autoantibodies
| Author & Year | Age/Sex/Ethnicity | Presenting Signs/Symptoms | Infection/Pathogen | Elevated GM-CSF Autoantibodies | Treatment | Duration of Follow-up | Outcome |
|---|---|---|---|---|---|---|---|
| Cryptococcus | |||||||
| Kuo 2021 [16] | 52/M/Asian | Persistent cough | C. gattii (pulmonary, CNS) | Yes | LAmB and flucytosine, then LAmB and fluconazole, then fluconazole (durations not specified) | 2–4 y following C. gattii Dx | Gradual resolution of symptoms; remains on antifungal treatment |
| Kuo 2021 [16] | 61/M/Asian | Progressive cough, intermittent fever, headache | C. gattii (pulmonary, CNS) | Yes | Amphotericin B and flucytosine ×3 wk, then fluconazole ×1 y; 5 mo later, fluconazole reinitiated x1 y | 2–4 y following C. gattii Dx | Recovered; fluconazole reinitiated for additional 1 y (starting 5 mo after initial treatment) for persistent antigenemia and unresolved focal opacity on chest X-ray |
| Kuo 2021 [16] | 71/F/Asian | Mass on dorsal right scapula (history of cancer, diabetes mellitus) | C. gattii (bone) | Yes | Fluconazole ×1 y | 2–4 y following C. gattii Dx | Recovered |
| Kuo 2021 [16] | 39/M/Asian | Chronic dyspnea on exertion | C. gattii (pulmonary) | Yes | Fluconazole and flucytosine, then fluconazole ×1 y | 2–4 y following C. gattii Dx | Recovered |
| Kuo 2021 [16] | 46/M/Asian | Fever, progressive right upper quadrant abdominal pain | C. gattii (pulmonary) | Yes | Amphotericin B and flucytosine, then fluconazole (durations not specified) | 2–4 y following C. gattii Dx | Recovered |
| Stevenson 2019 [38] | 48/M/NS | Hemoptysis, right upper lobe cavitating mass extending to upper bronchus; 1 y later relapsed with acute CNS deficits, cryptococcoma, concomitant mild cryptococcal meningoencephalitis, and aPAP | C. gattii (pulmonary, intracranial) | Yes | Amphotericin B ×2 wk, then fluconazole ×8 mo; 1 y later, LAmB and flucytosine x1 wk, then high dose fluconazole x 4 mo, then fluconazole prophylaxis (ongoing) | 3.5 y | Initial pulmonary symptoms resolved; 1 y later relapsed with CNS infection, now with symptom resolution; remains on long-term fluconazole prophylaxis |
| Stevenson 2019 [38] | 43/M/Asian | Complex right superior cerebellar mass, with small spiculated right upper lobe lesion on chest CT scan | C. gattii (intracranial) | Yes | Occipital craniotomy and resection of mass; amphotericin B and flucytosine ×2 wk, then fluconazole ×3 wk, then voriconazole x4 mo, then posaconazole x20 mo (and ongoing) |
20 mo | Postoperative appearance as expected on brain MRI with no new lesions; patient remains well and continues long-term posaconazole therapy |
| Demir 2018 [15] | 42/M/NS | Fever, headache, weight loss, peripheral facial paralysis | C. gattii (pulmonary, CNS) | Yes | Multiple antifungal regimens (including fluconazole, LAmB, and voriconazole) for nearly 5 y |
6 y | Developed aPAP 3 y after cryptococcal meningitis, at which time BAL microbial cultures were negative; near-complete spontaneous regression of aPAP 3 y later |
| Crum-Cianflone 2017 [39] | 42/M/Caucasian | Acute lower extremity paralysis, chronic cough, progressive constipation |
C. gattii (CNS) | Yes | LAmB and flucytosine ×8 wk, then fluconazole | ~3 mo | Clinical improvement; remains on fluconazole prophylaxis |
| Crum-Cianflone 2017 [39] | 34/M/Hispanic | Slowly enlarging facial lesion, weight loss, night sweats, mild headaches, bilateral visual acuity loss | C. gattii (pulmonary, skin, intracranial) | Yes | Craniotomy with debridement of frontal lesion; LAmB and flucytosine ×10 wk, then fluconazole ×12 mo | ~1 y | Remains on fluconazole prophylaxis |
| Rosen 2013 [21] | 31/F/NS | Headache | C. gattii (pulmonary, CNS) | Yes | Amphotericin B and flucytosine x8 d, then fluconazole and flucytosine (durations not specified) | 5 mo | Resolution of cryptococcoma; remains on fluconazole |
| Viola 2021 [24] | 26/M/NS | Ulnar osteolytic lesion, upper lobe lung mass, mediastinal lymphadenopathy | C. neoformans (pulmonary, bone, CNS) | Yes | LAmB and flucytosine ×5 d, then LAmB and fluconazole ×6 wk, then high-dose fluconazole ×6 mo | 3 y | Relapsed within 3 mo, likely due to underlying osseous fungal sequestration |
| Perrineau 2020 [40] | 41/F/NS | Headache, vomiting, confusion, photophobia | C. neoformans (CNS) | Yes | LAmB and flucytosine ×2 wk, then fluconazole ×2 wk | ~6 mo | Meningeal syndrome relapse after 8 wk, with cerebral vasculitis; treated with high-dose fluconazole and corticosteroids |
| Panackal 2017 [22] | 73/M/NS | Fever, headache, myalgia, diplopia | C. neoformans (CNS) | Yes | Antifungals (details not specified) | NS | Responded |
| Rosen 2013 [21] | 20/F/NS | Headache, fever, neck pain, diplopia, confusion | C. neoformans (pulmonary, CNS) | Yes | Amphotericin B and flucytosine, then fluconazole (durations not specified) | 3 y | Recovered; developed aPAP 2 y later (WLL required) |
| Rosen 2013 [21] | 47/M/Hispanic | Cough, weakness, tremors | C. neoformans (pulmonary, CNS, skin, blood) | Yes | Amphotericin B x2 wk, then fluconazole (ongoing) | NS | Recovered; remains on maintenance fluconazole; subsequently diagnosed with aPAP |
| Rosen 2013 [21] | 48/M/Asian | Fever, cough, back pain | C. neoformans (pulmonary, CNS); Mycobacterium tuberculosis (pulmonary) | Yes | Amphotericin B, then fluconazole and antituberculosis therapy x9 mo | 2 y | Recovered |
| Applen Clancey 2019 [23] | 69/M/NS | Headache, clumsiness, vertigo, shuffling gait, memory deficits, worsening motor skills | C. deuterogattii (CNS) | Yes | LAmB and flucytosine ×4 wk, then LAmB and fluconazole ×2 wk, then LAmB and flucytosine ×2 wk, then fluconazole ×12 wk | 10 mo | Responded; remains on maintenance fluconazole |
| Kuo 2021 [16] | 49/M/Asian | Persistant dry cough and chest pain | Cryptococcus (pulmonary) | Yes | Fluconazole (ongoing; duration not specified) | 2–4 y | Antifungal treatment continuing |
| Nocardia | |||||||
| Wu 2021 [41] | 45/M/Asian | Activity-related respiratory exertion, persistent cough (aPAP) |
Nocardia (pulmonary) | Yes | Sulfamethoxazole ×6 mo | 16 mo | Eventual improvement in lung function and chest imaging without need for WLL |
| Berthoux 2020 [12] | 40/M/NS | Subacute left brachiofacial deficit and headaches (parietal cerebral abscess, aPAP) | Nocardia (intracranial) | Yes | Meropenem ×6 wk and high-dose trimethoprim/sulfamethoxazole ×1 y, then trimethoprim/sulfamethoxazole prophylaxis (ongoing) | 18 mo | Clinical improvement, with total neurological recuperation and complete regression of cerebral abscess; for aPAP, sargramostim (SC), with subsequent WLL and rituximab |
| Ekici 2020 [42] | 62/M/NS | Fever, night sweats, chest pain, cavitary nodular infiltrates, “relapsing pneumonias”; multiple pulmonary masses on chest CT (aPAP) | Nocardia brasiliensis (pulmonary) | Yes | Amikacin and trimethoprim/sulfamethoxazole ×6 wk, then moxifloxacin and trimethoprim/sulfamethoxazole ×6 mo | 6 mo | Lung function tests and chest CT normalized, with full resolution of prior pulmonary masses; treated with CyBorD chemotherapy for MGUS; masses remain decreased 1 y following CyBorD therapy |
| Yamaguchi 2010 [43] | 37/M/NS | Persistent cough, sputum (aPAP) | Nocardia (pulmonary) | Yes | Antituberculosis therapy and antibiotics (details not specified) | NS | aPAP rapidly worsened with exacerbation of pulmonary nocardiosis but improved after treating infection |
| Mycobacterium | |||||||
| Shiohira 2021 [44] | 63/M/NS | Exacerbating aPAP | Mycobacterium avium complex (pulmonary) | Yes | Rifampicin, ethambutol, and clarithromycin ×12 mo | 1 y | Resolution of MAC infection with no subsequent recurrence; WLL upon aPAP relapse |
| Price 2006 [45] | 13/F/African Canadian | aPAP, history of cough (6 mo), developmental delay |
Mycobacterium avium
intracellulare (pulmonary) |
Yes | WLL, followed by sargramostim (inhaled) twice daily ×12 mo (decreased to once daily after 4 mo) for aPAP; no anti-infectives administered | 15 mo | Resolution of infection with improvement in lung function and chest imaging; resumption of normal development |
| Aspergillus | |||||||
| Arai 2015 [46] | 59/M/NS | Persistent cough, history of tuberculosis | Aspergillus fumigatus (pulmonary) | Yes | Initially treated with itraconazole ×2 y before aPAP Dx, rhu GM-CSF (SC) ×8 wk following aPAP Dx, then micafungin and amphotericin B |
3 y | Initial reduction of infection followed by relapse; patient died of respiratory failure 4 mo after initiation of aPAP therapy |
Abbreviations: aPAP, autoimmune pulmonary alveolar proteinosis; BAL, bronchoalveolar lavage; CNS, central nervous system; CT, computed tomography; CyBorD, cyclophosphamide, bortezomib, and dexamethasone; Dx, diagnosis; GM-CSF, granulocyte-macrophage colony-stimulating factor; LAmB, liposomal amphotericin B; MGUS, monoclonal gammopathy of undetermined significance; MRI, magnetic resonance imaging; NS, not specified; PAP, pulmonary alveolar proteinosis; rhu, recombinant human; SC, subcutaneous; WLL, whole lung lavage.
DISCUSSION
The cases presented here include patients with persistent or unusual pulmonary and CNS infections, all of whom also exhibited elevated GM-CSF autoantibody levels. Several were diagnosed with aPAP based on active pulmonary findings, while in other cases, signs and symptoms were suggestive of aPAP or its possible future onset.
Deficiencies in GM-CSF due to autoantibodies may play a role in infection with C. gattii or Nocardia, which primarily target immunocompromised patients but also can affect those who are immunocompetent [11, 12, 16, 24, 25]. In patients diagnosed with disseminated or unusual cryptococcosis, 26% were positive for GM-CSF autoantibodies, with higher serum titers in CNS cryptococcosis [16]. A study of 4 cases and 103 archived samples from patients with cryptococcal meningitis found GM-CSF autoantibodies in 7 previously healthy patients; 1 subsequently developed symptomatic PAP, while another exhibited radiographic and cytopathologic changes consistent with PAP but remained asymptomatic [21]. In another report, such autoantibodies were detected in 7 of 30 otherwise immunocompetent patients with meningoencephalitis due to C. gattii and in 1 of 20 healthy subjects [26]. Previously healthy patients, therefore, could have underlying risk factors such as GM-CSF autoantibodies that might predispose them to infection. In a literature review of C. gattii infections, 76% of otherwise healthy, immunocompetent patients had GM-CSF autoantibodies, supporting the idea that these autoantibodies are an unrecognized risk factor for such infections [27]. Infection with Nocardia is also common in patients with aPAP. A review of aPAP cases found 13% to be associated with OIs, 61% of which were due to Nocardia spp. [20]. In 1 study, 5 of 7 otherwise healthy, immunocompetent patients with CNS nocardiosis had elevated GM-CSF autoantibody levels compared with 0 of 14 controls [18]. In patients with C. gattii or Nocardia infection, GM-CSF autoantibodies can inhibit induction of STAT5 phosphorylation in monocytes, thus reducing the normal immune response to these pathogens [18, 28]. Consequently, patients with unexplained pulmonary, disseminated, or extrapulmonary OIs such as Cryptococcus and Nocardia should be evaluated for GM-CSF autoantibodies and monitored for future development of aPAP or other OIs.
In patients with aPAP, studies have demonstrated the benefit of treatment with rhu GM-CSF (eg, yeast-derived sargramostim and bacteria-derived molgramostim)—particularly when administered by inhalation—to increase GM-CSF levels and reduce surfactant accumulation [29–31]. Inhaled rhu GM-CSF may be more beneficial when preceded by WLL as prior lavage can remove excess surfactant that could impede rhu GM-CSF distribution and biological activity [32–34]. In addition to its use in patients diagnosed with aPAP, rhu GM-CSF therapy could be considered for those with GM-CSF autoantibodies who do not respond to standard anti-infectives, even without clinical evidence of frank aPAP. For patients with no confirmed infection but who have significant aPAP disease and thus are more susceptible to OIs, prophylaxis with trimethoprim/sulfamethoxazole can be considered, along with close monitoring for possible infectious complications [35].
In patients with unusual or recurrent infections, diagnosing associated aPAP is critical as it could reduce use of inappropriate treatment and facilitate effective aPAP therapy. Early consideration of an aPAP diagnosis by clinical, radiological, and GM-CSF autoantibody assessments—even in seemingly immunocompetent patients—is, therefore, warranted. Currently, aPAP diagnosis can be delayed an average of 18 months because the nonspecific symptoms are often assumed to be related to other respiratory diseases [10]. An accurate diagnosis is important as inappropriate corticosteroid use, for example, could suppress immune function and worsen aPAP disease severity [36]. As elevated GM-CSF autoantibodies could portend future development of clinical aPAP and because aPAP may be asymptomatic in up to one-third of all patients [19], testing for GM-CSF autoantibodies should be considered for an immunocompetent patient with C. gattii infection (brain, lung, or both) or other unexplained OI such as Nocardia, and in the setting of suspected PAP. Testing is readily available by a simple serum test in the United States through Cincinnati Children’s Hospital Medical Center, National Jewish Health, and the National Institutes of Health, as well as in Japan, Germany, and China [37]. Knowledge of the test is not well known, however, and requires greater community awareness.
Acknowledgments
We thank Pillar Medical Communications and Larry Rosenberg, PhD, for assistance with the preparation of our manuscript.
Financial support. This work was supported by Partner Therapeutics, Inc., which provided professional medical writing assistance and publication fees.
Potential conflicts of interest. E.L.’s institution received NIH-funded grants related to the content of the manuscript. E.L. has served as a consultant to Guidepoint Global and has received travel support from the PAP Foundation and the Rare Lung Disease Consortium. A.A. serves on the Medical and Scientific Advisory Board of the PAP Foundation. T.W.’s institution received a fellowship grant funded by Partner Therapeutics, Inc. T.W. received consultant fees from Partner Therapeutics for research outside of the current work; participated on an Advisory Board for IQVIA; and serves on the Board of Directors, Medical and Scientific Advisory Board, and as Vice President and Clinical Director of the PAP Foundation. C.M. has nothing to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. All authors contributed to co-development of the manuscript including conceptualization, selection of content for inclusion, data abstraction, and review, editing, and revising of content. All authors approved submission for publication and accept accountability for the content.
Patient consent. Due to the retrospective nature of this case series and systematic review, patient consent was not necessitated.
References
- 1. Dranoff G, Crawford AD, Sadelain M, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science 1994; 264:713–6. [DOI] [PubMed] [Google Scholar]
- 2. Thomassen MJ, Barna BP, Malur AG, et al. ABCG1 is deficient in alveolar macrophages of GM-CSF knockout mice and patients with pulmonary alveolar proteinosis. J Lipid Res 2007; 48:2762–8. [DOI] [PubMed] [Google Scholar]
- 3. Ioachimescu OC, Kavuru MS.. Pulmonary alveolar proteinosis. Chron Respir Dis 2006; 3:149–59. [DOI] [PubMed] [Google Scholar]
- 4. Lescoat A, Ballerie A, Augagneur Y, et al. Distinct properties of human M-CSF and GM-CSF monocyte-derived macrophages to simulate pathological lung conditions in vitro: application to systemic and inflammatory disorders with pulmonary involvement. Int J Mol Sci 2018; 19:894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ataya A, Knight V, Carey BC, Lee E, Tarling EJ, Wang T.. The role of GM-CSF autoantibodies in infection and autoimmune pulmonary alveolar proteinosis: a concise review. Front Immunol 2021; 12:752856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. LeVine AM, Reed JA, Kurak KE, Cianciolo E, Whitsett JA.. GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infection. J Clin Invest 1999; 103:563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paine R 3rd, Preston AM, Wilcoxen S, et al. Granulocyte-macrophage colony-stimulating factor in the innate immune response to Pneumocystis carinii pneumonia in mice. J Immunol 2000; 164:2602–9. [DOI] [PubMed] [Google Scholar]
- 8. Seymour JF. Extra-pulmonary aspects of acquired pulmonary alveolar proteinosis as predicted by granulocyte-macrophage colony-stimulating factor-deficient mice. Respirology 2006; 11:S16–22. [DOI] [PubMed] [Google Scholar]
- 9. Ballinger MN, Paine R 3rd, Serezani CH, et al. Role of granulocyte macrophage colony-stimulating factor during gram-negative lung infection with Pseudomonas aeruginosa. Am J Respir Cell Mol Biol 2006; 34:766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trapnell BC, Nakata K, Bonella F, et al. Pulmonary alveolar proteinosis. Nat Rev Dis Primers 2019; 5:16. [DOI] [PubMed] [Google Scholar]
- 11. Carrasco García de León S, González AH, Rivas NV, Gómez JJB.. Brain abscess due to Nocardia infection in an immunocompetent patient with asymptomatic pulmonary alveolar proteinosis. Acta Neurol Belg 2019; 119:281–3. [DOI] [PubMed] [Google Scholar]
- 12. Berthoux C, Mailhe M, Vély F, et al. Granulocyte macrophage colony-stimulating factor-specific autoantibodies and cerebral Nocardia with pulmonary alveolar proteinosis. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khera R, Rao V, Pasam MK, Tagore R, Murthy SS, Sundaram C.. Isolated cerebral Aspergillus abscess as a complication of pulmonary alveolar proteinosis in a child. Chin Neurosurg J 2019; 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Punatar AD, Kusne S, Blair JE, Seville MT, Vikram HR.. Opportunistic infections in patients with pulmonary alveolar proteinosis. J Infect 2012; 65:173–9. [DOI] [PubMed] [Google Scholar]
- 15. Demir S, Chebib N, Thivolet-Bejui F, Cottin V.. Pulmonary alveolar proteinosis following cryptococcal meningitis: a possible cause? BMJ Case Rep 2018; 2018:bcr2017222940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuo PH, Wu UI, Pan YH, et al. Neutralizing anti-GM-CSF autoantibodies in patients with CNS and localized cryptococcosis: a longitudinal follow-up and literature review. Clin Infect Dis. In press. [DOI] [PubMed] [Google Scholar]
- 17. Dennis D, Secasanu V, Magalang U.. Pulmonary alveolar proteinosis presenting as intracerebral nocardiosis. Paper presented at: CHEST Annual Meeting 2018; October 9, San Antonio, TX.
- 18. Rosen LB, Rocha Pereira N, Figueiredo C, et al. Nocardia-induced granulocyte macrophage colony-stimulating factor is neutralized by autoantibodies in disseminated/extrapulmonary nocardiosis. Clin Infect Dis 2015; 60:1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inoue Y, Trapnell BC, Tazawa R, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med 2008; 177:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seymour JF, Presneill JJ.. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med 2002; 166:215–35. [DOI] [PubMed] [Google Scholar]
- 21. Rosen LB, Freeman AF, Yang LM, et al. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol 2013; 190:3959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Panackal AA, Rosen LB, Uzel G, et al. Susceptibility to cryptococcal meningoencephalitis associated with idiopathic CD4(+) lymphopenia and secondary germline or acquired defects. Open Forum Infect Dis 2017; 4:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Applen Clancey S, Ciccone EJ, Coelho MA, et al. Cryptococcus deuterogattii VGIIa infection associated with travel to the Pacific Northwest outbreak region in an anti-granulocyte-macrophage colony-stimulating factor autoantibody-positive patient in the United States. mBio 2019; 10:e02733-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Viola GM, Malek AE, Rosen LB, et al. Disseminated cryptococcosis and anti-granulocyte-macrophage colony-stimulating factor autoantibodies: an underappreciated association. Mycoses 2021; 64:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Browne SK, Holland SM.. Immunodeficiency secondary to anticytokine autoantibodies. Curr Opin Allergy Clin Immunol 2010; 10:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saijo T, Chen J, Chen SC, et al. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio 2014; 5:e00912–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang DH, England MR, Salvator H, et al. Cryptococcus gattii species complex as an opportunistic pathogen: underlying medical conditions associated with the infection. mBio 2021; 12:e0270821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kwon-Chung KJ, Saijo T.. Is Cryptococcus gattii a primary pathogen? J Fungi (Basel) 2015; 1:154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tazawa R, Trapnell BC, Inoue Y, et al. Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis. Am J Respir Crit Care Med 2010; 181:1345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tazawa R, Ueda T, Abe M, et al. Inhaled GM-CSF for pulmonary alveolar proteinosis. N Engl J Med 2019; 381:923–32. [DOI] [PubMed] [Google Scholar]
- 31. Trapnell BC, Inoue Y, Bonella F, et al. Inhaled molgramostim therapy in autoimmune pulmonary alveolar proteinosis. N Engl J Med 2020; 383:1635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohkouchi S, Akasaka K, Ichiwata T, et al. Sequential granulocyte-macrophage colony-stimulating factor inhalation after whole-lung lavage for pulmonary alveolar proteinosis. A report of five intractable cases. Ann Am Thorac Soc 2017; 14:1298–304. [DOI] [PubMed] [Google Scholar]
- 33. Campo I, Mariani F, Paracchini E.. Whole lung lavage followed by inhaled sargramostim as therapy of autoimmune pulmonary alveolar proteinosis. Am J Respir Crit Care Med 2016; 193:A6438. [Google Scholar]
- 34. Campo I, Mariani F, Paracchini E, et al. Inhaled sargramostim and whole lung lavage (WLL) as therapy of autoimmune pulmonary alveolar proteinosis (aPAP). Eur Respir J 2016; 48:PA3870. [Google Scholar]
- 35. Wang T, Lazar CA, Fishbein MC, Lynch JP 3rd. Pulmonary alveolar proteinosis. Semin Respir Crit Care Med 2012; 33:498–508. [DOI] [PubMed] [Google Scholar]
- 36. Akasaka K, Tanaka T, Kitamura N, et al. Outcome of corticosteroid administration in autoimmune pulmonary alveolar proteinosis: a retrospective cohort study. BMC Pulm Med 2015; 15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salvaterra E, Campo I.. Pulmonary alveolar proteinosis: from classification to therapy. Breathe (Sheff) 2020; 16:200018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stevenson B, Bundell C, Mulrennan S, McLean-Tooke A, Murray R, Brusch A.. The significance of anti-granulocyte-macrophage colony-stimulating factor antibodies in cryptococcal infection: case series and review of antibody testing. Intern Med J 2019; 49:1446–50. [DOI] [PubMed] [Google Scholar]
- 39. Crum-Cianflone NF, Lam PV, Ross-Walker S, Rosen LB, Holland SM.. Autoantibodies to granulocyte-macrophage colony-stimulating factor associated with severe and unusual manifestations of Cryptococcus gattii infections. Open Forum Infect Dis 2017; 4:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perrineau S, Guery R, Monnier D, Puel A, Lanternier F.. Anti-GM-CSF autoantibodies and Cryptococcus neoformans var. grubii CNS vasculitis. J Clin Immunol 2020; 40:767–9. [DOI] [PubMed] [Google Scholar]
- 41. Wu XK, Lin Q.. Pulmonary alveolar proteinosis complicated with nocardiosis: a case report and review of the literature. World J Clin Cases 2021; 9:2874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ekici S, Malur A, Thomassen MJ, Murray DL, Wylam ME.. Utilization of LC-MS to determine monoclonal gammopathy-associated granulocyte macrophage colony stimulating factor antibody and novel treatment of pulmonary alveolar proteinosis. J Appl Lab Med 2020; 5:394–400. [DOI] [PubMed] [Google Scholar]
- 43. Yamaguchi S, Takayanagi N, Tokunaga D, Sugita Y, Kawabata Y.. A case of pulmonary alveolar proteinosis which initially deteriorated rapidly with exacerbation of pulmonary nocardiosis, responded promptly to treatment of the pulmonary nocardiosis. Nihon Kokyuki Gakkai Zasshi 2010; 48:580–3. [PubMed] [Google Scholar]
- 44. Shiohira S, Sakayori M, Yoshioka K, et al. Exacerbation of autoimmune pulmonary alveolar proteinosis that improved with lone treatment of complicating nontuberculous mycobacterial infection: a case report. Respir Med Case Rep 2021; 34:101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Price A, Manson D, Cutz E, Dell S.. Pulmonary alveolar proteinosis associated with anti-GM-CSF antibodies in a child: successful treatment with inhaled GM-CSF. Pediatr Pulmonol 2006; 41:367–70. [DOI] [PubMed] [Google Scholar]
- 46. Arai T, Inoue Y, Akira M, Nakata K, Kitaichi M.. Autoimmune pulmonary alveolar proteinosis following pulmonary aspergillosis. Intern Med 2015; 54:3177–80. [DOI] [PubMed] [Google Scholar]