Abstract
Although schizophrenia is classically thought to involve impaired attentional filtering, people with schizophrenia (PSZ) exhibit a more intense and more exclusive attentional focus than healthy control subjects (HCS) in many tasks. To resolve this contradiction, this functional magnetic resonance imaging study tested the impact of attentional control demands on the modulation of stimulus-induced activation in the fusiform face area and parahippocampal place area when participants (43 PSZ and 43 HCS) were looking for a target face versus house. Stimuli were presented individually, or as face-house overlays that challenged attentional control. Responses were slower for house than face stimuli and when prioritizing houses over faces in overlays, suggesting a difference in salience. Blood-oxygen-level-dependent activity reflected poorer attentional selectivity in PSZ than HCS when attentional control was challenged most, that is, when stimuli were overlaid and the task required detecting the lower-salience house target. By contrast, attentional selectivity was exaggerated in PSZ when control was challenged least, that is, when stimuli were presented sequentially and the task required detecting the higher-salience face target. These findings are consistent with 2 distinct attentional abnormalities in schizophrenia leading to impaired and exaggerated selection under different conditions: attentional control deficits, and hyperfocusing once attention has been directed toward a stimulus.
Keywords: Cognitive, functional magnetic resonance imaging, fusiform face area, parahippocampal place area, psychosis
Introduction
Navigating everyday activities depends on processing the right information at the right time. Selective attention enables our information processing system to select portions of the available sensory input at the expense of other portions. Success depends on whether the right material was selected, and whether the selected material was successfully amplified.
Selective attention in people with schizophrenia (PSZ) has traditionally been described as impaired, with reduced filtering of irrelevant information (McGhie and Chapman 1961; Frith 1979). Experimental evidence for this notion (summarized by Luck et al. 2019b) is primarily based on dichotic listening and other auditory filtering paradigms. Pre-attentional sensory gating deficits are also cited. In contrast, recent studies report that PSZ do not exhibit impaired selective attention and even display a more intense, narrow, and exclusive focus than HCS (reviewed by Luck et al. 2019a). These experiments were conducted with a variety of paradigms in the visual domain, yielding behavioral, eye-tracking, electrophysiological, and functional magnetic resonance imaging (fMRI) evidence that PSZ “hyperfocus” on a narrow spatial window or a small number of locations or representations, irrespective of whether the task requires it.
A potential explanation for this contradiction, aside from different sensory modalities, relates to the distinction between control and implementation of selective attention. Control processes steer the attentional focus to the relevant sources of information, whereas implementation of selective attention refers to the actual amplification of the selected source relative to others (Luck and Gold 2008; Beck and Kastner 2014). Selective attention may be impaired because processing resources are directed toward the wrong input, reflecting control deficits. Impairment in dichotic listening tasks, which challenge attentional control, could be explained in that manner. Findings in the visual domain also suggest attentional control deficits in PSZ (Hepp et al. 1996; Radant et al. 2007; Hahn et al. 2010). However, when control processes are not a limiting factor, stronger implementation of selective attention may result in a more intense and exclusive focus in PSZ. Indeed, paradigms in which PSZ exhibit hyperfocusing are marked by low attentional control demands (Luck et al. 2019a).
The present fMRI study examined the effectiveness of selective attention under high and low attentional control demands using neural measures. We hypothesized that PSZ would exhibit impaired selective attention when control demands were high, but hyperfocusing when control demands were low. To study attentional gain within higher-order visual processing regions, we exploited the finding that separate inferior temporal regions, referred to as fusiform face area (FFA) and parahippocampal place area (PPA), are specialized for face and spatial scene processing, respectively (Kanwisher et al. 1997; Epstein and Kanwisher 1998). When face and house stimuli are presented concomitantly, as semitransparent overlays, directing attention to the face or house component increases the blood-oxygen-level-dependent (BOLD) signal in FFA or PPA, respectively, despite identical visual input (O'Craven et al. 1999; Serences et al. 2004).
The overlaid stimuli create strong competition and challenge attentional control mechanisms. If these mechanisms are impaired in schizophrenia, smaller effects of attention on FFA and PPA activity should be observed in PSZ than HCS. However, when faces and houses are presented as separate sequential stimuli, competition is negligible, minimizing control demands. Indeed, given the lack of competition, implementing selective attention is not essential for successful performance. However, if PSZ overengage this process irrespective of task demands, as prior research suggests (Luck et al. 2019a), larger effects of attention on FFA and PPA activity would be expected in PSZ than HCS for sequential stimuli.
Larger effects of attention on the BOLD response could reflect prolonged engagement of attention (resulting from impaired control) rather than a more intense focus. To disambiguate the interpretation of larger BOLD effects, we carefully examined the time course of the hemodynamic response (HDR). fMRI techniques have advanced to enable sub-second resolution with adequate signal-to-noise ratio, and despite the HDR’s inherent sluggishness, its time course can inform whether gross differences in the length of process engagement could explain differences in response amplitude.
Materials and Methods
Subjects
Forty-eight stably medicated outpatients meeting Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria for schizophrenia (N = 41) or schizoaffective disorder (N = 7), and 45 HCS completed the study. All participants were right-handed. Data from 2 HCS and 5 PSZ were excluded from analyses due to excessive head motion (criteria specified below), resulting in N = 43 per group in the final analyses. Table 1 summarizes the demographic characteristics of these participants. Groups did not differ in age, sex, ethnicity, or parental education (a proxy measure for socioeconomic status), but PSZ had fewer years of education and scored lower than HCS on tests of cognitive functioning, as is expected for this disease.
Table 1.
Participant demographics
| PSZ (N = 43) | HCS (N = 43) | Statistic P-value | |
|---|---|---|---|
| Age | 35.4 ± 11.0 (range 18–55) | 37.1 ± 10.9 (range 19–55) | t(84) = 0.74 P = 0.46 |
| Male: Female | 31: 12 | 29: 14 | χ2 = 0.22 P = 0.64 |
| Afr Am: Asian: Cauc: Other, mixed or unknown | 16: 2: 21: 4 | 14: 3: 23: 3 | χ2 = 0.57 P = 0.90 |
| Education (years)a | 13.7 ± 1.8 | 15.9 ± 2.2 | t(82) = 5.23 P < 0.001 |
| Parental Education (years)b | 14.6 ± 3.2 | 14.0 ± 3.1 | t(77) = 0.85 P = 0.40 |
| Estimated IQc,e | 94.5 ± 13.7 | 108.5 ± 13.6 | t(80) = 4.60 P < 0.001 |
| MCCBd,e | 35.2 ± 14.1 | 52.4 ± 8.5 | t(80) = 6.66 P < 0.001 |
| WRAT 4f | 102.0 ± 14.8 | 108.5 ± 15.1 | t(81) = 1.96 P < 0.054 |
| WTARg | 104.5 ± 17.2 | 111.8 ± 12.2 | t(82) = 2.25 P < 0.027 |
aData missing for 1 PSZ and 1 HCS.
bAverage over maternal and paternal education; data missing for 4 PSZ and 3 HCS.
cBased on the Wechsler Abbreviated Scale of Intelligence—II (Wechsler 2011).
dComposite score on the MATRICS Consensus Cognitive Battery (Nuechterlein and Green 2006).
eData missing for 1 PSZ and 3 HCS.
fWide Range Achievement Test (Wilkinson and Robertson 2006); data missing for 1 PSZ and 2 HCS.
gWechsler Test of Adult Reading (Wechsler 2001); data missing for 1 PSZ and 1 HCS.
Significant P-values are highlighted in bold.
Diagnosis of PSZ was established using a best estimate approach combining information from a Structured Clinical Interview for DSM-IV (SCID; First et al. 1997) with a review of medical records. PSZ averaged a total score of 32.2 ± 7.1 standard deviation (SD) on the Brief Psychiatric Rating Scale (Overall and Gorman 1962, range 22–49), 22.5 ± 11.8 on the Scale for the Assessment of Negative Symptoms (Andreasen 1984, range 0–53), and 22.7 ± 6.1 on the Level of Functioning Scale (Hawk et al. 1975, range 11–34). All PSZ were taking antipsychotic medication: 30 second-generation antipsychotics, 7 first-generation antipsychotics, and 6 both. Twenty-two PSZ additionally took antidepressant medication, 11 mood stabilizers, 13 anxiolytic, and 15 antiparkinsonian medications. Two PSZ were taking prazosin. Medication and dosages had not changed in the 4 weeks preceding the study. Drug or alcohol abuse within the last 6 months was exclusionary for all participants, as verified by targeted screening questions, chart review (if available), the SCID, and urine and breathalyzer tests. Healthy control subjects (HCS) were recruited via online advertising, flyers, and word of mouth. HCS had no Axis 1 or 2 diagnoses as established by a SCID, had no self-reported family history of psychosis, and were not taking any psychotropic medication.
All participants provided informed consent for a protocol approved by the Institutional Review Board of the University of Maryland, Baltimore. Before PSZ signed the consent form, the investigator formally evaluated their understanding in the presence of a witness. Participants were paid for their time.
Procedure
On a separate day preceding the MR scan, participants provided informed consent, were screened, received task instructions, and performed a 7-min practice version of the attention task on a desktop computer. On the day of the scan, participants were instructed not to consume any caffeine. The scan began with an FFA/PPA functional localizer run consisting of 10 blocks of face stimuli and 10 blocks of house stimuli (details below) for a total run duration of 529 s (678 repetition times [TRs]). This was followed by 6 runs of the attention task (220 s, or 283 TRs, each), the anatomical scan, another 6 runs of the attention task, and another functional localizer run—this time consisting of 5 blocks of face and 5 blocks of house stimuli (269 s, 345 TRs). Neuropsychological testing and psychiatric ratings were completed on a separate day.
Functional Localizer
The localizer scan is described by Vida et al. (2017). It employed 50 pictures of faces and 50 pictures of houses that differed from those in the attention task. Each block contained a train of either 20 face or 20 house images, which were picked randomly without replacement. Each stimulus was presented for 350 ms followed by a 500-ms interstimulus interval (ISI) for a total block length of 17 s. After each block, a fixation cross was presented for 9 s. Face blocks and house blocks were presented in random sequence with the constraint that no more than 2 face or house blocks were presented consecutively.
Attention Task
Task stimuli consisted of 5 face and 5 house images, drawn in grayscale. Face images were obtained from a set of images provided by the Tong lab (Cohen and Tong 2015). House images were obtained from the Internet. Face and house stimuli did not differ in contrast as measured by the SD of luminance across pixels in each image [t(8) = 1.31, P = 0.23]. All stimuli were presented in a circular window (diameter 7.35° visual angle) in the center of a gray screen.
As illustrated in Figure 1, the faces and houses were presented individually in Sequential task blocks. In Overlay blocks, each stimulus consisted of a superimposed face and house image, both rendered semitransparent (opacity = 125/255). In both conditions, participants were presented with blocks of 10 consecutive stimuli and asked to look for a target face or a target house, randomly chosen for each participant out of the 5 face and 5 house images. In Sequential blocks, 5 face and 5 house images were presented in random sequence. In Overlay blocks, 10 face-house overlay images were presented. Each block was preceded by instructions to look for either the target face or the target house. To encourage participants to attend to the relevant dimension, the face target could occur during attend-house blocks, and the house target could occur during the attend-face blocks; participants were instructed that these stimuli should be treated as nontargets.
Figure 1.
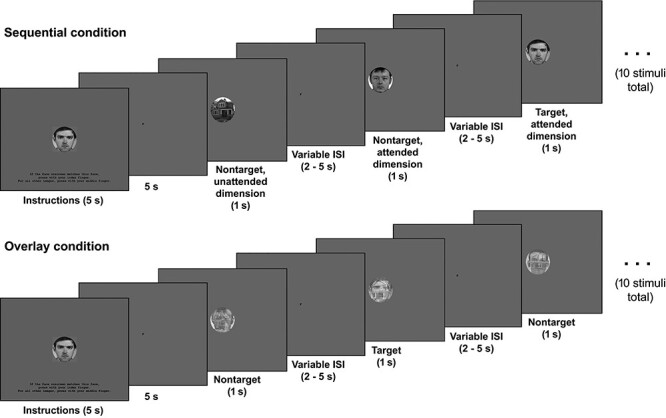
An example of a task block in the Sequential condition (top) and in the Overlay condition (bottom). Stimuli are shown to size relative to the whole screen display. The text on the initial instruction screen reads “If the face [house] onscreen matches this face [house], press with your index finger. For all other images, press with your middle finger.” The screen background of the initial instruction screen was cyan, all other colors are as presented in the real task.
Participants pressed one response button with their index finger when they detected either the target face or the target house (depending on the instruction), and another button with their middle finger for all other stimuli (i.e., nontarget stimuli and the target stimulus of the unattended dimension). In the Sequential condition, each image (including the target) was shown once in 50% of the blocks, twice in 25% of the blocks, and not at all in 25% of the blocks. In the Overlay condition, all possible image combinations were presented an equal number of times, with the exception that the target face and target house were never combined. Stimuli were selected randomly without replacement, but such that each target image was shown once in 50% of the blocks, twice in 25% of the blocks, and not at all in 25% of the blocks.
Each block started with a 5-s instruction screen displaying instruction text and an image of the target of the dimension that was to be attended in this block against a cyan background (Fig. 1). The background of all other screen displays was gray. The instruction screen was followed by a 5-s screen showing a small central F (in attend-face blocks) or H (in attend-house blocks) to serve as a reminder of the to-be-attended stimulus dimension. After that, the 10 images were presented for 1 s each, separated by a variable ISI of 2, 2.5, 3, 3.5, 4, 4.5, or 5 s during which the central F or H was shown. Each block was 55-s long. Four blocks were presented in each scan run: 2 Sequential blocks (one with attend-face and one with attend-house instructions), and 2 Overlay blocks (one with attend-face and one with attend-house instructions). These 4 block conditions were presented in randomized sequence within each of the 12 runs.
Magnetic Resonance Imaging
A 3-Tesla Siemens Prisma scanner (Erlangen) with a 64-channnel head coil acquired whole-brain EPI images for measuring T2*-weighted BOLD effects (72 2-mm axial slices separated by a 2-mm gap, 104 × 104 matrix, FOV = 20.8 × 20.8 cm, TR = 780 ms, echo time [TE] = 34.4 ms, and FA = 52°). A multiband acceleration factor of 8 was used. An axial T1-weighted image (MPRAGE) provided anatomical reference (0.8-mm3 voxels, TR = 2.2 s, TE = 2.81 ms, and FA = 13°).
Data were processed using AFNI (Cox 1996). All 14 scan runs (including functional localizer and attention task) were concatenated into a single time series, and each volume was registered to a base volume. TRs with >0.4-mm displacement or >0.4° rotation relative to the preceding TR were censored out of the time series. Participants with >20% motion-censored TRs were excluded. This applied to 2 HCS and 5 PSZ (see Subjects section). The number of motion-censored TRs did not differ between the remaining 43 HCS and 43 PSZ [t(84) = 0.48, P = 0.63].
The aim was to compare groups on FFA and PPA activity when attending face versus house stimuli. However, participants occasionally attended to the wrong dimension (likely reflecting working memory limitations or attentional lapses). Therefore, we censored out task blocks from BOLD signal analysis in which behavioral performance provided no evidence that the correct stimulus dimension was attended. Specifically, we censored blocks with a false alarm to a target stimulus of the unattended dimension (e.g., a target response to the house target in an attend-face block), and blocks which did not harbor either a target response to the target of the attended dimension, or a nontarget response to the target of the unattended dimension. Although not all blocks had a target of the attended dimension and not all had a target of the unattended dimension, almost all blocks had at least one of these events. The maximum number of censored blocks for any of the 4 block conditions was 6 out of 12. Thus, even the worst-performing participant still retained a sufficient number of events per stimulus condition for event-related analysis. Specifically, the lowest number of included events was 24 nontarget face and 24 nontarget house stimuli in the attend-face and attend-house sequential conditions, each, and 48 nontarget overlay stimuli in the attend-face and attend-house overlay conditions, each. The average number of performance-censored blocks per block condition was 1.76 (SD = 0.83) out of 12 in HCS versus 2.42 (SD = 1.14) in PSZ [t(84) = 3.05, P = 0.003]. The lower number of included task events may somewhat reduce measurement reliability in PSZ relative to HCS, but it does not affect response magnitude.
First-Level Analysis
The localizer and task runs were analyzed in a single voxel-wise multiple regression model—the localizer runs as a block design, and the attention task time series as an event-related design. For the localizer, two 17-s boxcar regressors—corresponding to face and house blocks—were convolved with a model hemodynamic response function (HRF).
For the attention task, a potential confound would be a prolonged engagement with the attended stimulus. Just as differences in the duration of sensory stimulation affect BOLD response amplitude in sensory cortex (e.g., Boynton et al. 1996; Dale and Buckner 1997; Robson et al. 1998), prolonged engagement of any mental process can be expected to augment BOLD response amplitude in the relevant brain regions. Thus, larger effects of attention on stimulus-induced BOLD signal in PSZ, which are hypothesized, could be explained by PSZ continuing to focus on the internal representation of the attended stimulus during the ITI that follows. The predefined temporal parameters of a model HRF do not allow quantifying or controlling for effects resulting from differences in the length of internal stimulus engagement. For this reason, regressors were not convolved with a model HRF but represented by 16 tent functions covering 16 780-ms TRs (12.5 s) from stimulus onset. This convolution-free model simply determines the amount of signal change at each specified timepoint after an event type of interest. This approach is particularly useful to analyze different impulse response function shapes, and it enabled visualizing HRF time courses and analyzing them for group differences.
In the Sequential condition, 4 regressors of interest corresponded to face and house nontarget stimuli when either the face dimension or the house dimension was attended. In the Overlay condition, 2 regressors of interest corresponded to overlays composed of nontargets when either the face dimension or the house dimension was attended. Regressors of no interest corresponded to face and house target stimuli, presented individually or as part of an overlay stimulus (there were too few target events for analysis), to trials in which the participant failed to respond, to task instructions (5-s boxcar regressor convolved with model HRF), and to the 6 motion parameter curves. For each subject, the regression analysis yielded the voxel-wise average amplitude of signal change produced by the face and house localizer blocks relative to periods of passive fixation, and by each task event regressor at each of the 16 TRs. These maps were converted to the Talairach and Tournoux standard coordinate system (Talairach and Tournoux 1988) and spatially blurred using a Gaussian 5-mm root mean square isotropic kernel.
To test for group differences in HDR time courses, we employed the fractional area technique (Hansen and Hillyard 1980) that was originally developed for calculating event-related potential (ERP) latencies. This method determines the timepoint at which a specified percentage of the area under the curve (AUC) has been reached. AUC was the positive area defined by the HDR and the x-axis. We determined the timepoints at which 25%, 50%, and 75% of the AUC had been reached, thus probing the ascending and descending arms of the HDR, as well as its center. This technique was combined with the jackknife-subsample average waveform statistical approach (Miller et al. 1998; Ulrich and Miller 2001), which optimizes the accuracy of latency determinations and the statistical power of latency differences (Kiesel et al. 2008). The 25%, 50%, and 75% AUC time points were compared between groups by independent sample t-tests, with t-values adjusted in accordance with the jackknife method (Miller et al. 1998; Ulrich and Miller 2001). These procedures placed the time point demarcating 50% of the AUC, averaged across task conditions and regions of interest (ROIs), at 5879 ms post stimulus onset (about half-way through TR 7), with no group difference (see Results section for details of group comparisons). Consistent with this and with the literature (Buxton et al. 2004; Drew 2019), the TR associated with the HDR peak in most task conditions was TR 7. Thus, signal from TR 7 and its 4 surrounding TRs (TRs 5–9, corresponding to 3900–7020 ms post stimulus onset) was averaged for analysis of differences in activation amplitude within the FFA and PPA ROIs.
ROI Analysis
ROI coordinates were defined based on the independent functional localizer. To take into account interindividual variation and potential differences between HCS and PSZ in the location of face- and scene-selective visual processing areas (see e.g., McDonald et al. 2000), coordinates were defined on an individual-subject basis using an approach based on Julian et al. (2012). First, we defined search regions on a group level by performing a whole-brain voxel-wise paired t-test contrasting activity induced by house versus face blocks. Voxel-wise α < 1−10 was employed for house-selective search regions (house > face blocks) and α < 0.001 for face-selective search regions (face > house blocks), both with a minimum clustersize threshold of 1000 μL. This yielded 2 search regions in left and 2 in right inferior temporal lobe, ~4000–8000 μL in size (Table 2).
Table 2.
Search regions within which individual subject PPA and FFA ROIs were identified
| Region | Side | Volume (μL) | Center of mass (mm) | |||
|---|---|---|---|---|---|---|
| L-R | P-A | I-S | ||||
| 1 | Parahippocampal place area (PPA) | L | 7392 | −24.9 | −49.1 | −12.4 |
| 2 | Parahippocampal place area (PPA) | R | 8024 | 25.0 | −51.2 | −11.1 |
| 3 | Fusiform face area (FFA) | L | 4072 | −41.8 | −51.0 | −19.7 |
| 4 | Fusiform face area (FFA) | R | 5048 | 41.9 | −51.4 | −17.1 |
L = left; R = right; L-R = left-right; P-A = posterior-anterior; I-S = inferior-superior.
Within the search regions, individual participants’ face- and scene-selective processing regions were localized by subtracting voxel-wise activity induced by face blocks from that induced by house blocks for each participant. In the resulting difference maps, each voxel was set to the average intensity of all voxels within a 5-mm radius to minimize the influence of outlier voxels on the following steps. For each participant, ≥200 μL clusters of all-positive or all-negative voxels were then identified. Most participants had a single positive cluster within both the left and the right house-selective search regions, and a single negative cluster within both the left and right face-selective search regions. No cluster was found for one HCS in the left face-selective search region, for one HCS in the right face-selective search region, and for one PSZ in either the left or right face-selective search region. These 3 subjects were excluded from further analyses. Three participants had 2 separate clusters in one of the search regions. In these cases, the larger cluster was selected. Individual participants’ PPA coordinates were defined as the local maxima within the clusters of positive voxels, and FFA coordinates as the local minima within the clusters of negative voxels. Spheres of 5-mm radius were drawn around these coordinates.
For each participant, event-related activity was averaged across voxels of each ROI at each of the 16 TRs for visualization. For statistical analysis, values were averaged over TRs 5–9, (see above). FFA and PPA responses were thus extracted for nontarget stimuli depicting faces or houses (Sequential condition) or nontarget overlay stimuli (Overlay condition), separately for blocks in which the instruction was to look for the target face or for the target house. In the PPA, an effect of attention would be reflected by a larger response to house or overlay stimuli when participants were attending the house dimension than when attending the face dimension. In the FFA, an effect of attention would be reflected by a larger response to face or overlay stimuli when attending the face dimension than when attending the house dimension.
Statistical Analysis
Behavioral task performance was quantified as the hit rate for the attended target stimulus, false alarm rate for the target stimulus of the unattended dimension, and false alarm rate for nontarget stimuli. These data include all blocks; only the BOLD signal analyses excluded blocks without behavioral evidence that the correct stimulus dimension was attended. Correct response reaction time (RT, average across trials) was also measured for these 3 stimulus types, reflecting RT of hits for the attended target, and RT of correct rejections for the unattended target and nontarget stimuli. Hit rate and RT for the attended target were analyzed by 2-factor analysis of variance (ANOVA) with group (HCS and PSZ) as between-subject factor and attended stimulus dimension (face and house) as within-subject factor. False alarm rate and RT for the target of the unattended dimension and for nontargets were analyzed in the same manner; however, ANOVA of nontarget stimuli in the Sequential condition included the presented stimulus dimension as a third factor.
The FFA BOLD response to face stimuli, the PPA response to house stimuli, and the FFA and PPA response to overlay stimuli were analyzed by separate 3-factor ANOVA, with group as a between-subjects factor, and with attended stimulus dimension and hemisphere as within-subject factors.
Results
Group Comparison of Individual FFA and PPA ROIs Identified by the Functional Localizer
Clusters identified by the face-house contrast in individual subjects were analyzed for volume, maximum contrast intensity, and left-right, posterior-anterior, and inferior-superior coordinates of the maximum intensity location (which defined the center of individual participants’ spherical ROIs) by 2-factor ANOVA (group × hemisphere). Left-right coordinates were analyzed as absolute values.
FFA (see Table 3 for details): Significant main effects of hemisphere on cluster volume (P < 0.001) and maximum intensity (P = 0.003) indicated that FFA clusters tended to be larger and more discriminatory between face and house stimuli on the right than on the left, consistent with the literature (Hemond et al. 2007; Willems et al. 2010). The maximum intensity locus of the face-house contrast was ~ 1 mm more inferior on the left than on the right (main effect of hemisphere: P = 0.023). Importantly, maximum intensity location coordinates displayed no significant main effects of group (all Ps > 0.28) and no group × hemisphere interactions (Ps ≥ 0.48), indicating that ROI locations did not differ between PSZ and HCS.
Table 3.
FFA regions of interest
| HCS mean ± SD | PSZ mean ± SD | Group | Hemisphere | Group × hemisphere | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | F (81) | P | F (81) | P | F (81) | P | |
| MI location L-R (mm) | −41.0 ± 2.9 | 41.7 ± 3.7 | −41.0 ± 3.1 | 41.2 ± 3.5 | 0.28 | 0.60 | 1.25 | 0.27 | 0.35 | 0.55 |
| MI location P-A (mm) | −51.0 ± 7.3 | −50.8 ± 7.9 | −53.0 ± 6.9 | −51.9 ± 8.7 | 1.18 | 0.28 | 0.21 | 0.65 | 0.36 | 0.55 |
| MI location I-S (mm) | −21.0 ± 3.4 | −19.7 ± 2.7 | −20.3 ± 3.0 | −19.7 ± 3.2 | 0.10 | 0.76 | 5.36 | 0.023 | 0.51 | 0.48 |
| MI (face > house) | 1.4 ± 0.8 | 1.6 ± 1.0 | 1.3 ± 1.0 | 1.8 ± 1.0 | 0.08 | 0.77 | 9.15 | 0.003 | 0.85 | 0.36 |
| Volume (μL) | 1983 ± 900 | 2712 ± 1112 | 2102 ± 909 | 2899 ± 953 | 0.42 | 0.52 | 57.8 | < 0.001 | 0.08 | 0.79 |
MI = maximum intensity (percent signal change) of the face-house contrast; L-R = left-right; P-A = posterior-anterior; I-S = inferior-superior.
Significant P-values are highlighted in bold.
PPA (see Table 4 for details): Significant main effects of hemisphere on cluster volume (P < 0.001) and maximum intensity (P = 0.019) again reflected larger and more functionally specialized PPA clusters on the right than on the left. There were significant main effects of group on the posterior-anterior (P = 0.030) and inferior-superior (P = 0.039) coordinates of the maximum-intensity location, which was 5.2 mm more posterior and 1.1 mm more inferior in PSZ than in HCS. There were no significant group × hemisphere interactions (all Ps > 0.42, except maximum intensity: P = 0.139).
Table 4.
PPA regions of interest
| HCS mean ± SD | PSZ mean ± SD | Group | Hemisphere | Group × hemisphere | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | F (84) | P | F (84) | P | F (84) | P | |
| MI location L-R (mm) | −25.3 ± 3.5 | 25.7 ± 3.5 | −25.1 ± 3.1 | 24.7 ± 2.9 | 1.10 | 0.30 | 0.00 | 1.00 | 0.67 | 0.42 |
| MI location P-A (mm) | −53.3 ± 13.3 | −52.2 ± 13.7 | −57.3 ± 12.3 | −58.6 ± 13.3 | 4.89 | 0.030 | 0.10 | 0.92 | 0.56 | 0.46 |
| MI location I-S (mm) | −13.4 ± 4.3 | −13.2 ± 3.4 | −14.8 ± 3.3 | −14.1 ± 2.9 | 4.38 | 0.039 | 0.85 | 0.36 | 0.16 | 0.69 |
| MI (house>face) | 1.48 ± 0.8 | 1.54 ± 0.6 | 1.4 ± 0.5 | 1.6 ± 0.7 | 0.04 | 0.84 | 5.74 | 0.019 | 2.23 | 0.14 |
| Volume (μL) | 6020 ± 1651 | 6601 ± 1783 | 6287 ± 1185 | 6948 ± 1302 | 0.97 | 0.33 | 51.3 | < 0.001 | 0.22 | 0.64 |
MI = maximum intensity (percent signal change) of the face-house contrast; L-R = left-right; P-A = posterior-anterior; I-S = inferior-superior.
Significant P-values are highlighted in bold.
FFA and PPA ROI Responses to Face and House Images
In this section, we test whether there were group differences in how the individually defined spherical FFA and PPA ROIs differentiated between face and house stimuli, independent of effects of attention. We assessed this for both the functional localizer stimuli and the main task stimuli. Activation in FFA and PPA ROIs was analyzed by 3-factor ANOVA (group × stimulus dimension × hemisphere).
Functional localizer stimuli: Expectably, FFA ROIs showed greater activation in face than in house blocks, and PPA ROIs showed greater activation in house than face blocks (Fig. 2). Significant main effects of stimulus dimension confirmed this for FFA (F1,81 = 6.13, P = 0.015) and PPA (F1,81 = 7.56, P = 0.007). Importantly, these effects did not differ between HCS and PSZ for either FFA (group × stimulus dimension interaction: F1,81 = 0.47, P = 0.49) or PPA (F1,81 = 0.64, P = 0.43). The effect of stimulus dimension was larger in the right than left hemisphere, as confirmed by a significant interaction of stimulus dimension with hemisphere in FFA (F1,81 = 10.2, P = 0.002) and PPA (F1,81 = 4.52, P = 0.037). There were no significant main effects of group (FFA: F1,81 = 0.26, P = 0.61; PPA: F1,81 = 3.23, P = 0.076) or interactions involving group (all Ps > 0.3).
Figure 2.
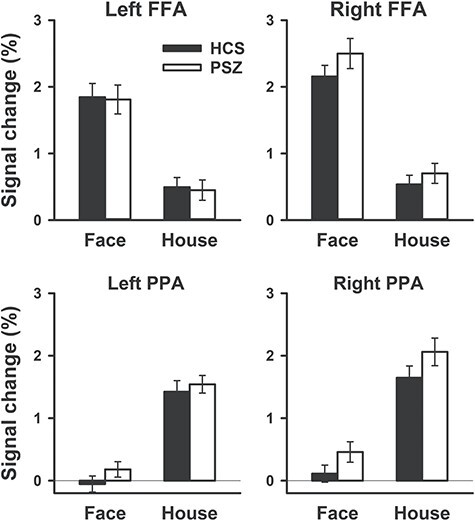
FFA and PPA activation in face blocks and house blocks of the functional localizer. Signal change is relative to passive fixation. Bars represent the mean over 41 HCS and 42 PSZ. Error bars represent the standard error of the mean (SEM).
Task stimuli: To test for group differences in FFA and PPA response to the face and house images that were used in the attention task (which differed from those of the Localizer), we compared responses to face and house stimuli from the Sequential condition averaged over the attend-face and attend-house conditions. Because there were no significant interactions involving hemisphere, Figure 3 shows BOLD responses averaged across hemispheres. The shape and timing of the HDRs were as expected, peaking between 5 and 6-s post stimulus onset with no apparent group differences. Fractional area latency analysis of the HDRs, averaged over ROIs and stimulus dimensions, did not identify any differences between PSZ and HCS in the time to 25% AUC [t(81) = 0.13, P = 0.89], 50% AUC [t(81) = 0.50, P = 0.62], or 75% AUC [t(81) = 0.38, P = 0.70].
Figure 3.
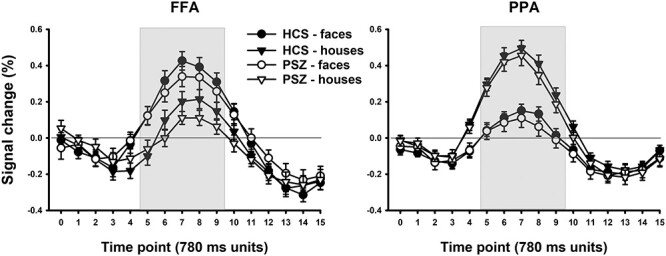
Percentage of BOLD signal change in the FFA and PPA induced by face and house images in the Sequential condition of the attention task, averaged over attended stimulus dimension, at each of 17 measurement time points. A measurement was taken every 780 ms. The graph represents averages (±SEM) over 41 HCS and 42 PSZ.
FFA activation averaged over TRs 5–9 (gray area in Fig. 3) was greater in response to face than house stimuli (main effect of stimulus dimension: F1,81 = 45.3, P < 0.001). Activation of PPA was greater in response to house than face stimuli (F1,81 = 180.3, P < 0.001). Importantly, this effect did not differ between HCS and PSZ in either FFA (group × stimulus dimension interaction: F1,81 = 0.58, P = 0.81) or PPA (F1,81 = 0.34, P = 0.85). Although FFA activation appeared lower in PSZ than HCS overall, that is, for both face and house stimuli, the main effect of group was not significant for FFA (F1,81 = 1.18, P = 0.28) or PPA (F1,81 = 0.46, P = 0.50). As mentioned, there were no interactions involving hemisphere.
Together, the results thus far indicate that the basic operation of the FFA and PPA ROIs was similar in PSZ and HCS. This simplifies the interpretation of the attention effects that are described in the following section.
Effects of Attention—Sequential Condition
Behavioral Task Performance
Relevant (to-be-attended) target stimuli (Fig. 4a): The hit rate was lower overall for PSZ than for HCS (main effect of group F1,84 = 4.18, P = 0.044). However, this effect was driven largely by house stimuli, whereas the hit rate for face stimuli was similar between groups. This pattern led to a significant interaction of group with stimulus dimension (F1,84 = 4.11, P = 0.046). In HCS, hit rate did not differ between face and house stimuli [t(42) = 0.72, P = 0.48], but in PSZ, it was significantly lower for house than for face stimuli [t(42) = 3.02, P = 0.004]. RT reflected a similar pattern. RT was overall slower for PSZ than for HCS (main effect of group F1,84 = 22.3, P < 0.001), but this group difference was more pronounced for house than for face stimuli (group × stimulus dimension interaction: F1,84 = 5.91, P = 0.017). The main effect of stimulus dimension was also significant (F1,84 = 35.8, P < 0.001), reflecting slower responses to the house target across groups. The hit rate and RT effects indicate that the house task was more difficult than the face task, especially for PSZ.
Figure 4.
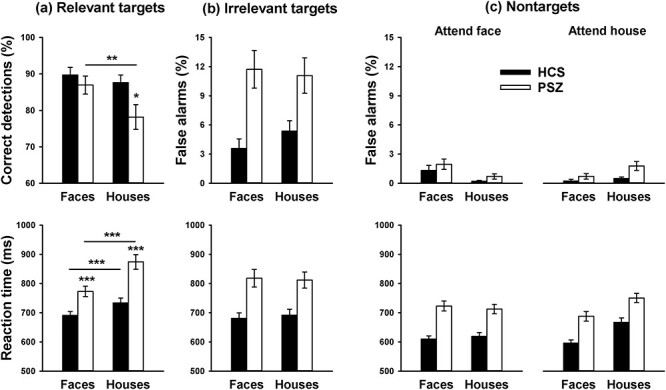
Behavioral task performance in the Sequential condition. (a) Hit rate (% correct detections) and RT of responses to the target stimulus of the to-be-attended dimension. (b) False alarm rate (% incorrect “target” responses) and RT of responses to the irrelevant target stimulus, that is, the target stimulus of the not-to-be-attended dimension. (c) False alarm rate and RT of responses to nontarget stimuli. Bars reflect the mean (±SEM) over 41 HCS and 42 PSZ.
Irrelevant (Not-to-Be-Attended) Target Stimuli (Fig. 4b): The false alarm rate for target stimuli of the not-to-be-attended dimension was higher for PSZ than for HCS, supported by a significant main effect of group (F1,84 = 20.0, P < 0.001). There was no main effect of stimulus dimension (F1,84 = 0.15, P = 0.70) and no interaction of group with stimulus dimension (F1,84 = 0.67, P = 0.42). RT was slower for PSZ than for HCS (F1,84 = 17.3, P = 0.001). Again there was no main effect of stimulus dimension (F1,84 = 0.02, P = 0.89) and no interaction with group (F1,84 = 0.30, P = 0.59). The higher false alarm rate suggests that PSZ were less likely than HCS to hold the to-be-attended stimulus dimension in memory. To prevent this from confounding the BOLD data, blocks in which there was no behavioral performance evidence that the correct stimulus dimension was attended were filtered out for all subjects.
Nontarget Stimuli (Fig. 4c): The false alarm rate was low overall, but somewhat higher in PSZ than in HCS (main effect of group F1,84 = 4.48, P = 0.037). FAs were higher for nontarget faces than for nontarget houses when participants were looking for the target face, and higher for nontarget houses than for nontarget faces when they were looking for the target house, as reflected by a significant interaction of the attended and presented stimulus dimension (F1,84 = 17.4, P < 0.001). No other main effect or interaction was significant. RT was slower for PSZ than for HCS (F1,84 = 24.9, P < 0.001). There were significant main effects of the attended stimulus dimension (F1,84 = 5.07, P = 0.027) and the presented stimulus dimension (F1,84 = 44.7, P < 0.001). Both effects were driven by slower RT when participants were looking for the target house and a nontarget house was presented, as supported by a significant interaction between the attended and presented stimulus dimension (F1,84 = 75.4, P < 0.001). No interactions involving group were significant.
The finding of slower responses in both groups when looking for the target house and presented with a target or nontarget house suggests that target discrimination was more resource-consuming for house than for face stimuli. Processing house stimuli appeared to be particularly resource-consuming for PSZ because PSZ displayed a lower hit rate and greater response slowing for the house target.
FFA and PPA Activity
Figure 5 shows the FFA BOLD response to nontarget face stimuli (top panels) and the PPA BOLD response to nontarget house stimuli (bottom panels) as a function of whether participants were looking for the target face or the target house. The analyses focused on nontarget stimuli because 1) there were too few target events for analysis and 2) nontarget stimuli of the attended and unattended dimension do not differ in response requirements. There were no significant interactions involving hemisphere; thus, Figure 5 presents the average signal across hemispheres. HDR time courses did not display any apparent group differences. Fractional area analysis was performed on the average over FFA responses to face stimuli and PPA responses to house stimuli. Responses were analyzed separately depending on whether or not the presented stimulus dimension was the attended dimension because we were concerned that attention would lead to longer stimulus engagement, especially in PSZ. However, no differences were identified between PSZ and HCS in the times to 25%, 50%, or 75% AUC when stimuli were attended [all ts(81) < 0.08, all Ps > 0.94] or unattended [all ts(81) < 0.38, all Ps > 0.71].
Figure 5.
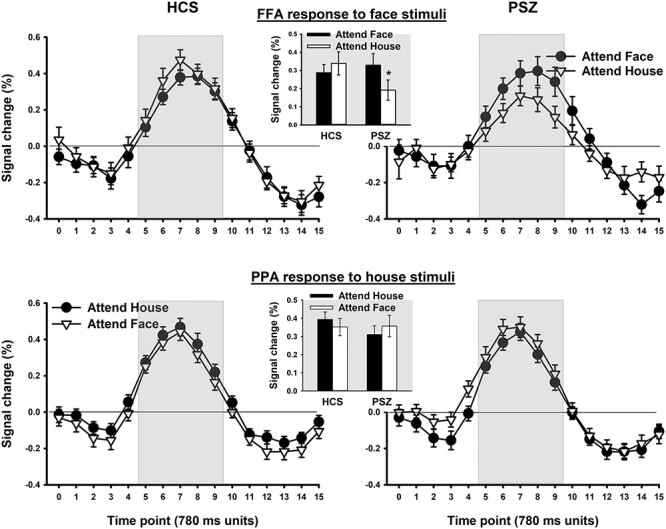
BOLD signal change in the Sequential condition in the FFA in response to face images (top panels) and in the PPA in response to house images (bottom panels) as a function of whether participants were looking for the target face or the target house. A measurement was taken every 780 ms. Measurement timepoints within the gray area entered statistical analysis of response amplitude. Each represented datapoint reflects the mean (±SEM) over 41 HCS and 42 PSZ.
FFA Response to Face Stimuli
Activation averaged over TRs 5–9 (gray area in Fig. 5) was subjected to 3-factor ANOVA. There were no significant main effects of group (F1,81 = 0.61, P = 0.44) or attended stimulus dimension (F1,81 = 0.98, P = 0.33). However, the interaction of group with attended stimulus dimension was significant (F1,81 = 4.54, P = 0.036). PSZ displayed greater FFA activation in response to face stimuli when they were looking for the target face than when looking for the target house [t(41) = 2.02, P < 0.05]. HCS showed no evidence of a larger FFA response to faces in the attend-face than in the attend-house condition [t(40) = 0.90, P = 0.37], consistent with the absence of competition to drive the need for selective focusing. As mentioned, there were no interactions involving hemisphere. The finding of an attention effect for PSZ but not for HCS is consistent with the hypothesis that PSZ exhibit hyperfocusing under conditions of minimal challenge to attentional control.
Because the FFA responses to face and house stimuli independent of attention were not perfectly matched between HCS and PSZ (Fig. 3), with a trend toward overall lower FFA activation in PSZ across stimulus dimensions, it is difficult to determine whether the larger attention effect in PSZ was due to a larger FFA response to faces when the face dimension was attended, or a smaller response to faces when not attended. Either way would reflect a greater implementation of selective attention in the absence of competition.
PPA Response to House Stimuli: In both groups, PPA activity was similar for the attend-face and attend-house conditions. There was no main effect of group (F1,81 = 0.45, P = 0.51) or of the attended stimulus dimension (F1,81 = 0.00, P = 0.99), and no significant group × attended dimension interaction (F1,81 = 2.56, P = 0.11).
Effects of Attention—Overlay Condition
Behavioral Task Performance
Relevant (to-be-attended) target stimuli ( Fig. 6a ): The hit rate for overlay stimuli was overall lower for PSZ than for HCS, as supported by a significant main effect of group (F1,84 = 14.1, P < 0.001). Neither the main effect of attended stimulus dimension (F1,84 = 0.32, P = 0.57) nor its interaction with group (F1,84 = 0.007, P = 0.93) were significant. RT was slower for PSZ than for HCS (F1,84 = 18.6, P < 0.001) and, consistent with results from the Sequential condition, slower when participants were looking for the house than the face target (main effect of attended stimulus dimension: F1,84 = 6.38, P = 0.014). The slowing for house targets was similar in PSZ and HCS, and the interaction of group with attended stimulus dimension was not significant (F1,84 = 1.34, P = 0.25).
Figure 6.
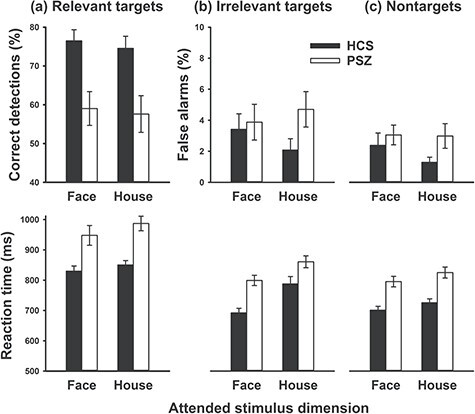
Behavioral task performance in the Overlay condition. (a) Hit rate and RT of responses to stimuli containing the target image of the to-be-attended dimension. (b) False alarm rate (% of incorrect “target” responses) and RT of responses to stimuli containing the irrelevant target stimulus, that is, the target stimulus of the not-to-be-attended dimension. (c) False alarm rate and RT of responses to nontarget stimuli. Bars reflect the mean (±SEM) over 41 HCS and 42 PSZ.
Irrelevant Target Stimuli (Fig. 6b): The false alarm rate for overlay stimuli that included the target of the not-to-be-attended dimension did not differ significantly between groups (F1,84 = 2.53, P = 0.12) or depending on which stimulus dimension was attended (F1,84 = 0.06, P = 0.81). The interaction term was also not significant (F1,84 = 1.03, 0.31). RT was slower for PSZ than for HCS (F1,84 = 14.1, P < 0.001) and slower when participants were looking for the house than the face target (main effect of attended stimulus dimension: F1,84 = 35.1, P < 0.001). The interaction was not significant (F1,84 = 1.66, P = 0.20).
Nontarget Stimuli (Fig. 6c): The false alarm rate for overlay stimuli composed of only nontarget images did not differ significantly between groups (F1,84 = 2.77, P = 0.10) or depending on which stimulus dimension was attended (F1,84 = 0.89, P = 0.35). Although the false alarm rate was numerically greater in PSZ than in HCS for the attend-house condition, the interaction was also not significant (F1,84 = 0.69, 0.41). RT was slower for PSZ than for HCS (F1,84 = 21.0, P < 0.001) and slower when participants were looking for the house than the face target (main effect of attended stimulus dimension: F1,84 = 22.9, P < 0.001). There was no significant interaction (F1,84 = 0.22, P = 0.64).
Overall, the behavioral results from the Overlay condition indicate substantially impaired target detection in PSZ. Furthermore, both groups were slower to respond when attending the house dimension than when attending the face dimension, indicating that prioritizing houses over faces was generally more resource consuming than vice versa.
FFA and PPA Activity
Figure 7 shows the BOLD response to overlay stimuli in FFA (top panels) and PPA (bottom panels) as a function of whether participants were looking for the target face or the target house. We expected the face-house overlays to produce a stronger response in FFA in the attend-face condition and a stronger response in PPA in the attend-house condition to the extent that attention was successfully focused on the attended dimension. Because there were no significant interactions involving hemisphere in ANOVA, the data are presented averaged across hemispheres.
Figure 7.
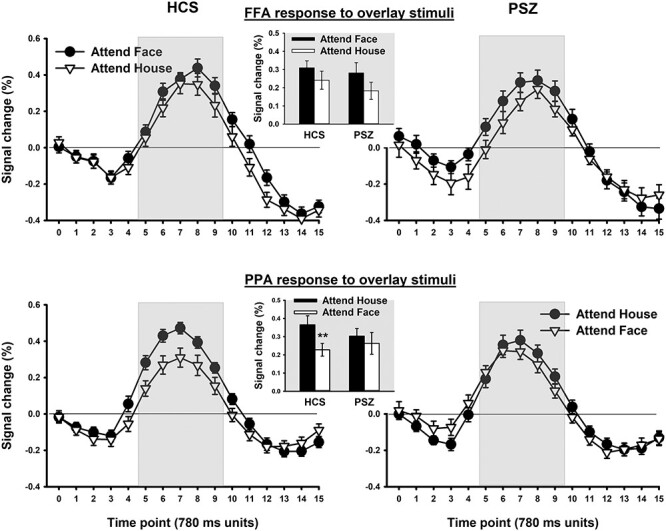
BOLD signal response to overlay stimuli in the FFA (top panels) and in the PPA (bottom panels) as a function of whether participants were looking for the target face or the target house. A measurement was taken every 780 ms. Measurement timepoints within the gray area entered statistical analysis of response amplitude. Each represented datapoint reflects the mean (±SEM) over 41 HCS and 42 PSZ.
In FFA, both groups appeared to exhibit approximately equal effects of attention, with a larger response in the attend-face condition than in the attend-house condition. This led to a significant main effect of attended stimulus dimension (F1,81 = 4.23, P = 0.043) and no significant interaction of group with attended stimulus dimension (F1,81 = 0.14, P = 0.71). The main effect of group was not significant (F1,81 = 0.55, P = 0.46).
In PPA, there was again no main effect of group (F1,81 = 0.17, P = 0.68). As shown in Figure 7 (bottom panels), both groups exhibited a numerically larger PPA response in the attend-house condition than in the attend-face condition, but this effect was larger in HCS than in PSZ. This pattern led to a near-significant main effect of attended stimulus dimension (F1,81 = 3.93, P = 0.051) and a significant group × attended stimulus dimension interaction (F1,81 = 4.12, P = 0.046). HCS displayed greater PPA activation when looking for the target house than when looking for the target face [t(40) = 2.74, P = 0.009], but not PSZ [t(41) = 0.03, P = 0.97]. Thus, PSZ showed reduced attentional selection relative to HCS in the Overlay condition when participants were asked to attend to the house stimuli, which were more difficult to prioritize than face stimuli (as indicated by the behavioral results). In other words, whereas PSZ exhibited increased selectivity relative to HCS in the attend-face, Sequential condition, where control demands were minimal, PSZ exhibited impaired selectivity when control processes of attentional selection were challenged the most.
Relationship of Task Performance with Attentional Modulation of FFA and PPA Activity
These results should be interpreted with caution given that the task was designed to optimize the measurement of attention effects on the BOLD signal—as opposed to measuring individual differences in task performance. Thus, no effort was made to prevent ceiling level performance, and unsurprisingly, a large proportion of participants displayed a 100% hit rate or 0% false alarm rate in several conditions. However, to explore potential associations between attentional modulation of FFA and PPA activity and task performance, we quantified performance using hit rate and false alarm rate (averaged across all nontarget stimuli) to compute the A score, a nonparametric measure of stimulus detection sensitivity that remains defined even when performance is close to or at ceiling (Zhang and Mueller 2005).
A residualized change model assessed the relationship between A scores and the effect of attention on the BOLD signal in the FFA and PPA (increases in FFA response when the face dimension was attended versus unattended, and increases in PPA response when the house dimension was attended versus unattended). In this model, the BOLD response in the attended condition (FFA response when the face dimension was attended, PPA response when the house dimension was attended) was the dependent variable. After controlling for the BOLD response in the unattended condition (FFA response when the house dimension was attended, PPA response when the face dimension was attended) to isolate the attention effect, the predictors of interest were diagnostic group (HCS and PSZ), A scores, and ROI (FFA and PPA).
In the Sequential condition, this analysis revealed a significant positive relationship between behavioral performance (A scores) and the BOLD attention effect, that is, a larger BOLD attention effect was associated with better behavioral performance (F1,69 = 4.90, P = 0.03). This effect did not differ with regard to ROI (F1,69 = 0.10, P = 0.74) or diagnostic group (F1,69 = 0.04, P = 0.84). In the Overlay condition, no significant relationship between behavioral performance and the BOLD attention effect was observed (F1,69 = 3.29, P = 0.07).
Tests for Medication Effects
To test whether antipsychotic medication could explain the observed group differences in BOLD response, we correlated chlorpromazine equivalent dose levels in PSZ with the attention effect for sequentially presented face stimuli in FFA and for overlay stimuli in PPA. The attention effect in FFA was derived by subtracting the response to faces when PSZ were looking for the target house from the response to faces when they were looking for the target face (average over TRs 5–9, as before). This attention effect did not correlate significantly with chlorpromazine equivalents (R = −0.06, P = 0.72). The attention effect in PPA was derived by subtracting the response to overlay stimuli when PSZ were looking for the target face from the response when they were looking for the target house. This effect also did not correlate significantly with chlorpromazine equivalents (R = −0.20, P = 0.21). Finally, there were no significant differences in the attention effect in FFA [t(40) = 0.51, P = 0.61] or PPA [t(40) = 1.04, P = 0.31] between PSZ who were and were not taking an antidepressant. Similarly, no differences therein were detected when comparing PSZ who were and were not taking an anxiolytic [FFA: t(40) = 0.24, P = 0.82; PPA: t(40) = 0.71, P = 0.49].
Discussion
The present findings offer a resolution of the contradiction between the classic idea of impaired filtering in schizophrenia, and more recent evidence for intact selective attention and even hyperfocusing. Specifically, we propose that attentional control processes are impaired in PSZ, making it difficult to select the appropriate information under conditions of strong competition, but that PSZ display stronger implementation of selection (i.e., “hyperfocus”) when competition and attentional control demands are minimal. Thus, we hypothesized that PSZ would exhibit larger effects of attention than HCS when there was no need to boost the relevant or suppress the irrelevant stimulus dimension, and that PSZ would exhibit smaller effects of attention when strong stimulus competition made it difficult to select the relevant over the irrelevant dimension.
The observed pattern of results was consistent with these predictions. Attentional control demands were manipulated directly by comparing sequential and overlaid stimuli, and the behavioral results also indicated that the house task was more demanding than the face task. When stimuli were presented sequentially—well separated in time with no competition between them—we found greater effects of attention in PSZ than HCS for the FFA response to face stimuli, consistent with hyperfocusing. In contrast, when face and house stimuli were presented as overlays—creating competition and attentional control demands—we found reduced effects of attention in PSZ relative to HCS in the PPA, consistent with attentional control deficits.
The finding that greater attention effects for PSZ in the Sequential condition were limited to FFA, and reduced attention effects in the Overlay condition to PPA, was not predicted a priori. However, this pattern follows from our general framework when task difficulty is taken into consideration. Across diagnostic groups, face stimuli appeared to be easier to process and prioritize than house stimuli. This phenomenon may not be specific to the present stimulus set because previous research also found face stimuli to be more salient then house stimuli, even when emotionally neutral (Reinders et al. 2005). Thus, attentional control demands were likely greater for the less salient house stimuli than for the more salient face stimuli. Behavioral performance deficits of PSZ appeared to follow attentional control demands: PSZ exhibited impaired target detection relative to HCS in the Overlay condition, consistent with the high levels of competition present in this condition, but also for house stimuli in the Sequential condition.
BOLD effects consistent with greater implementation of selective attention in PSZ were seen in the FFA response to individually presented face stimuli—the least control-demanding task condition and the one in which stimulus detection of PSZ was unimpaired. Thus, for a salient stimulus to which attention is easily directed, PSZ displayed greater enhancement when attended and/or greater filtering when unattended. It was not surprising that attention effects were absent in HCS in this condition; we expected them to be small, at best. In the absence of stimulus competition and with stimuli being separated by several seconds, enhancing stimuli of the attended dimension or suppressing stimuli of the unattended dimension is not essential to discriminate the target from nontarget stimuli of the same dimension. However, this tendency may help prevent accidental target responses to the wrong stimulus dimension, which may explain the association between attentional modulation of FFA/PPA activity and stimulus detection sensitivity in this condition. In general, hyperfocusing is thought of as a primary schizophrenia-related processing abnormality (Luck et al. 2019a; Hahn et al. 2020), that is, PSZ are thought to display this trait irrespective of whether focused attention is required, and the impact on task performance can be positive or negative depending on the nature of the task (e.g., Cegalis et al. 1977; Cegalis and Deptula 1981; Elahipanah et al. 2010, 2011; Spencer et al. 2011; Hahn et al. 2012a, 2012b; Leonard et al. 2013; Gray et al. 2014; Luck et al. 2014; Hahn et al. 2016; Kreither et al. 2017; Leonard et al. 2017; Sawaki et al. 2017).
The more pronounced neural signature of attention in PSZ when selection was easy and the less pronounced effect when selection required control bear resemblance, at first, to the leftward shift of the inverted U-shaped relationship between working memory load and prefrontal cortex (PFC) activity in PSZ (Manoach 2003). This raises the possibility that the larger effect of attention in the Sequential condition might reflect greater engagement in attentional selection because cognitive deficits rendered these processes more effortful and resource consuming for PSZ than for HCS. However, a crucial difference to the PFC relationship with WM load is that FFA and PPA are visual processing regions and not neural mediators of attentional processes. Thus, although greater engagement in attentional selection would likely be associated with greater activation of frontoparietal regions mediating these processes, effects on FFA and PPA activity would be expected to reflect downstream consequences of selective attention processes on sensory processing—whether these processes were implemented with ease or in an effortful manner.
In the Overlay condition, the conclusion that prioritizing houses over faces was more challenging than vice versa was based on significantly slower response times when participants were attending to the house dimension. The stimulus competition inherent in the overlay stimuli, combined with the need to prioritize the less salient over the more salient stimulus dimension, can be assumed to have created the highest attentional control demands among all conditions. HCS displayed the most robust effects of attention in this condition, as reflected by the PPA response when attending houses versus faces, indicating that HCS engaged attentional control to prioritize the relevant stimulus dimension when it was challenging to do so. The absence of this effect in PSZ is consistent with attentional control deficits.
It needs to be acknowledged that performance deficits in the Overlay condition may also reflect perceptual organization deficits in PSZ, that is, difficulty structuring sensory elements into larger units of perceived objects (Uhlhaas and Silverstein 2005; Silverstein and Keane 2011). Impaired perceptual organization would augment attentional control demands when attempting to determine the identity of one of the overlays; thus, effects of the 2 types of deficit are intertwined. However, perceptual organization deficits cannot easily explain why PSZ exhibited normal attention effects in FFA for the Overlay condition, so attentional control deficits seem like the best explanation of the present pattern of results. Furthermore, perceptual organization was minimally challenged when processing individual house stimuli in the Sequential condition, which was also marked by low stimulus detection in PSZ.
Several potential confounds had to be considered in the study design and analysis. First, as for many other regions of the brain, anatomical and functional differences have been reported for FFA and PPA in PSZ as compared with HCS, although results have been inconsistent (McDonald et al. 2000; Yoon et al. 2006; Pinkham et al. 2008; Maher et al. 2016; Kronbichler et al. 2018; Maher et al. 2019). To control for potential neuroanatomical differences, we defined coordinates on an individual-subject basis. PPA ROIs tended to be localized somewhat more posterior and inferior in PSZ than HCS, indicating that this individualized approach was well-advised to avoid potential group differences in functional activation resulting from lack of neuroanatomical correspondence. Importantly, the sensory face-house discrimination of the ROIs thus defined did not differ between groups, for neither localizer nor task stimuli. This speaks against group differences in the basic functioning of these regions and suggests that the ROIs identified equivalent processing areas in HCS and PSZ.
A second potential confound was related to the expectable performance deficits seen in PSZ. If PSZ spent more time “off task,” or more often inadvertently focused on the wrong stimulus dimension, then a group comparison of FFA and PPA activity when instructed to attend to the face or house dimension could pick up on abnormalities in processes other than selective attention. To control for such confounds, we excluded trials from fMRI analysis in which subjects failed to respond, and we included only blocks in which at least one behavioral performance index indicated that the correct stimulus dimension was attended. This ensured that group differences in activation of face- and scene-selective visual regions indeed reflected differences in processes of selective attention.
A third confound to be considered was that larger effects of attention on FFA and PPA BOLD responses as derived with the defined temporal parameters of a model HDR function could be explained by a more intense representation but also by a longer-duration representation. Although the former would be indicative of a more intense attentional focus, the latter could be due to participants holding onto an internal stimulus representation beyond stimulus offset and response, reflecting deficits in the flexible updating and control of the attentional focus. We adopted an analysis strategy that enabled us to compare HDR timing parameters between groups and avoid potential timing-related confounds by focusing on a set time period surrounding the response peak. These analyses, although coarse, suggest that the timing of HDR onset, peak, and decline did not differ between diagnostic groups, favoring an interpretation in terms of a more intense, and not a more prolonged, attentional focus. Furthermore, the fact that we did not find evidence of HDR timing differences that could suggest differences in basic neurovascular mechanisms between PSZ and HCS strengthens confidence in the validity of fMRI studies that compare neural correlates of cognitive processes between these groups.
A limitation was that the present study included only stably medicated chronic outpatients. Although medication analyses provided no evidence that the reported effects were associated with antipsychotic medication dosage or with the use of other psychoactive medications, medication confounds can never be fully excluded in a study of this nature. In addition, the inclusion of first-episode patients would have helped separate disease-intrinsic processing abnormalities from those secondary to living with a debilitating chronic mental health condition. Despite this, the present study provides evidence supporting the hypothesis of 2 distinct attentional abnormalities in schizophrenia, namely an impairment in attentional control (impaired steering of the attentional focus toward the relevant input source when there is competition from other sources), and hyperfocusing (stronger implementation of selective attention once attention has been successfully directed to an input source). The combination of these 2 factors can explain why PSZ appear to exhibit impaired selective attention under some conditions and exaggerated attentional selection under others.
The traditional view of selective attention abnormalities in schizophrenia not been able to account for many observed performance differences between PSZ and HCS. Progress in the basic cognitive neurosciences has improved our ability to conceptualize these abnormalities and enabled us to account for seemingly discrepant observations. A better understanding of the basic functions underlying information processing abnormalities associated with schizophrenia, and ultimately neuronal processes associated with these affected functions, is needed to reduce the spectrum of observable abnormalities to a small number of potentially treatable core deficits.
Funding
National Institutes of Health (grant R01 MH065034 to J.M.G. and S.J.L.).
Notes
We thank Sharon August and Leeka Hubzin for performing neuropsychological assessments. Thanks to all participants for volunteering their time for this study. Conflict of Interest: None declared.
Contributor Information
Britta Hahn, University of Maryland School of Medicine, Maryland Psychiatric Research Center, Baltimore, MD 21228, USA.
Benjamin M Robinson, University of Maryland School of Medicine, Maryland Psychiatric Research Center, Baltimore, MD 21228, USA.
John E Kiat, University of California Davis, Center for Mind and Brain, Davis, CA 95618, USA.
Joy Geng, University of California Davis, Center for Mind and Brain, Davis, CA 95618, USA.
Sonia Bansal, University of Maryland School of Medicine, Maryland Psychiatric Research Center, Baltimore, MD 21228, USA.
Steven J Luck, University of California Davis, Center for Mind and Brain, Davis, CA 95618, USA.
James M Gold, University of Maryland School of Medicine, Maryland Psychiatric Research Center, Baltimore, MD 21228, USA.
References
- Andreasen NC. 1984. The scale for the assessment of negative symptoms (sans). Iowa City, IA: University of Iowa. [Google Scholar]
- Beck DM, Kastner S. 2014. Neural systems for spatial attention in the human brain: evidence from neuroimaging in the framework of biased competition. In: Nobre AC, Kastner S, editors. Oxford handbook of attention. Oxford, UK: Oxford University Press, pp. 253–288. [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. 1996. Linear systems analysis of functional magnetic resonance imaging in human v1. J Neurosci. 16(13):4207–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. 2004. Modeling the hemodynamic response to brain activation. Neuroimage. 23(Suppl 1):S220–S233. [DOI] [PubMed] [Google Scholar]
- Cegalis JA, Deptula D. 1981. Attention in schizophrenia. Signal detection in the visual periphery. J Nerv Ment Dis. 169(12):751–760. [PubMed] [Google Scholar]
- Cegalis JA, Leen D, Solomon EJ. 1977. Attention in schizophrenia: an analysis of selectivity in the functional visual field. J Abnorm Psychol. 86(5):470–482. [DOI] [PubMed] [Google Scholar]
- Cohen EH, Tong F. 2015. Neural mechanisms of object-based attention. Cereb Cortex. 25(4):1080–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. 1996. Afni: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29(3):162–173. [DOI] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. 1997. Selective averaging of rapidly presented individual trials using fmri. Hum Brain Mapp. 5(5):329–340. [DOI] [PubMed] [Google Scholar]
- Drew PJ. 2019. Vascular and neural basis of the bold signal. Curr Opin Neurobiol. 58:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahipanah A, Christensen BK, Reingold EM. 2010. Visual search performance among persons with schizophrenia as a function of target eccentricity. Neuropsychology. 24(2):192–198. [DOI] [PubMed] [Google Scholar]
- Elahipanah A, Christensen BK, Reingold EM. 2011. Controlling the spotlight of attention: visual span size and flexibility in schizophrenia. Neuropsychologia. 49(12):3370–3376. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. 1998. A cortical representation of the local visual environment. Nature. 392(6676):598–601. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. 1997. User's guide for the structured clinical interview for dsm-iv axis i disorders: clinician version. American Psychiatric Publishing. [Google Scholar]
- Frith CD. 1979. Consciousness, information processing and schizophrenia. Br J Psychiatry. 134:225–235. [DOI] [PubMed] [Google Scholar]
- Gray BE, Hahn B, Robinson B, Harvey A, Leonard CJ, Luck SJ, Gold JM. 2014. Relationships between divided attention and working memory impairment in people with schizophrenia. Schizophr Bull. 40(6):1462–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Bae GY, Robinson BM, Leonard CJ, Luck SJ, Gold JM. 2020. Cortical hyperactivation at low working memory load: a primary processing abnormality in people with schizophrenia? Neuroimage Clin. 26:102270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Harvey AN, Gold JM, Fischer BA, Keller WR, Ross TJ, Stein EA. 2016. Hyperdeactivation of the default mode network in people with schizophrenia when focusing attention in space. Schizophr Bull. 42(5):1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Hollingworth A, Robinson BM, Kaiser ST, Leonard CJ, Beck VM, Kappenman ES, Luck SJ, Gold JM. 2012a. Control of working memory content in schizophrenia. Schizophr Res. 134(1):70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Harvey AN, Kaiser ST, Leonard CJ, Luck SJ, Gold JM. 2012b. Visuospatial attention in schizophrenia: deficits in broad monitoring. J Abnorm Psychol. 121(1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Kaiser ST, Harvey AN, Beck VM, Leonard CJ, Kappenman ES, Luck SJ, Gold JM. 2010. Failure of schizophrenia patients to overcome salient distractors during working memory encoding. Biol Psychiatry. 68(7):603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JC, Hillyard SA. 1980. Endogenous brain potentials associated with selective auditory attention. Electroencephalogr Clin Neurophysiol. 49(3–4):277–290. [DOI] [PubMed] [Google Scholar]
- Hawk AB, Carpenter WT Jr, Strauss JS. 1975. Diagnostic criteria and five-year outcome in schizophrenia. A report from the international pilot study of schizophrenia. Arch Gen Psychiatry. 32(3):343–347. [DOI] [PubMed] [Google Scholar]
- Hemond CC, Kanwisher NG, Op de Beeck HP. 2007. A preference for contralateral stimuli in human object- and face-selective cortex. PLoS One. 2(6):e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp HH, Maier S, Hermle L, Spitzer M. 1996. The stroop effect in schizophrenic patients. Schizophr Res. 22(3):187–195. [DOI] [PubMed] [Google Scholar]
- Julian JB, Fedorenko E, Webster J, Kanwisher N. 2012. An algorithmic method for functionally defining regions of interest in the ventral visual pathway. Neuroimage. 60(4):2357–2364. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. 1997. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 17(11):4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesel A, Miller J, Jolicoeur P, Brisson B. 2008. Measurement of erp latency differences: a comparison of single-participant and jackknife-based scoring methods. Psychophysiology. 45(2):250–274. [DOI] [PubMed] [Google Scholar]
- Kreither J, Lopez-Calderon J, Leonard CJ, Robinson BM, Ruffle A, Hahn B, Gold JM, Luck SJ. 2017. Electrophysiological evidence for hyperfocusing of spatial attention in schizophrenia. J Neurosci. 37(14):3813–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler L, Stelzig-Scholer R, Pearce BG, Tschernegg M, Said-Yurekli S, Reich LA, Weber S, Aichhorn W, Kronbichler M. 2018. Schizophrenia and category-selectivity in the brain: normal for faces but abnormal for houses. Front Psychiatry. 9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CJ, Kaiser ST, Robinson BM, Kappenman ES, Hahn B, Gold JM, Luck SJ. 2013. Toward the neural mechanisms of reduced working memory capacity in schizophrenia. Cereb Cortex. 23(7):1582–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CJ, Robinson BM, Hahn B, Luck SJ, Gold JM. 2017. Altered spatial profile of distraction in people with schizophrenia. J Abnorm Psychol. 126(8):1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. 2008. The construct of attention in schizophrenia. Biol Psychiatry. 64(1):34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Hahn B, Leonard CJ, Gold JM. 2019a. The hyperfocusing hypothesis: a new account of cognitive dysfunction in schizophrenia. Schizophr Bull. 45(5):991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Leonard CJ, Hahn B, Gold JM. 2019b. Is attentional filtering impaired in schizophrenia? Schizophr Bull. 45(5):1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, McClenon C, Beck VM, Hollingworth A, Leonard CJ, Hahn B, Robinson BM, Gold JM. 2014. Hyperfocusing in schizophrenia: evidence from interactions between working memory and eye movements. J Abnorm Psychol. 123(4):783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher S, Ekstrom T, Holt D, Ongur D, Chen Y. 2016. The core brain region for face processing in schizophrenia lacks face selectivity. Schizophr Bull. 42(3):666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher S, Ekstrom T, Ongur D, Levy DL, Norton DJ, Nickerson LD, Chen Y. 2019. Functional disconnection between the visual cortex and right fusiform face area in schizophrenia. Schizophr Res. 209:72–79. [DOI] [PubMed] [Google Scholar]
- Manoach DS. 2003. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 60(2–3):285–298. [DOI] [PubMed] [Google Scholar]
- McDonald B, Highley JR, Walker MA, Herron BM, Cooper SJ, Esiri MM, Crow TJ. 2000. Anomalous asymmetry of fusiform and parahippocampal gyrus gray matter in schizophrenia: a postmortem study. Am J Psychiatry. 157(1):40–47. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J. 1961. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 34:103–116. [DOI] [PubMed] [Google Scholar]
- Miller J, Patterson T, Ulrich R. 1998. Jackknife-based method for measuring lrp onset latency differences. Psychophysiology. 35(1):99–115. [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF. 2006. Matrics consensus cognitive battery, manual. Los Angeles: MATRICS Assessment Inc. [Google Scholar]
- O'Craven KM, Downing PE, Kanwisher N. 1999. Fmri evidence for objects as the units of attentional selection. Nature. 401(6753):584–587. [DOI] [PubMed] [Google Scholar]
- Overall J, Gorman D. 1962. The brief psychiatric rating scale. Psychol Rep. 10:799–812. [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. 2008. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 99(1–3):164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radant AD, Dobie DJ, Calkins ME, Olincy A, Braff DL, Cadenhead KS, Freedman R, Green MF, Greenwood TA, Gur RE, et al. 2007. Successful multi-site measurement of antisaccade performance deficits in schizophrenia. Schizophr Res. 89(1–3):320–329. [DOI] [PubMed] [Google Scholar]
- Reinders AA, den Boer JA, Buchel C. 2005. The robustness of perception. Eur J Neurosci. 22(2):524–530. [DOI] [PubMed] [Google Scholar]
- Robson MD, Dorosz JL, Gore JC. 1998. Measurements of the temporal fmri response of the human auditory cortex to trains of tones. Neuroimage. 7(3):185–198. [DOI] [PubMed] [Google Scholar]
- Sawaki R, Kreither J, Leonard CJ, Kaiser ST, Hahn B, Gold JM, Luck SJ. 2017. Hyperfocusing of attention on goal-related information in schizophrenia: evidence from electrophysiology. J Abnorm Psychol. 126(1):106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Schwarzbach J, Courtney SM, Golay X, Yantis S. 2004. Control of object-based attention in human cortex. Cereb Cortex. 14(12):1346–1357. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP. 2011. Perceptual organization impairment in schizophrenia and associated brain mechanisms: review of research from 2005 to 2010. Schizophr Bull. 37(4):690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Valdman O, Niznikiewicz MA, Shenton ME, McCarley RW. 2011. Enhanced facilitation of spatial attention in schizophrenia. Neuropsychology. 25(1):76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 1988. Co-planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Uhlhaas PJ, Silverstein SM. 2005. Perceptual organization in schizophrenia spectrum disorders: empirical research and theoretical implications. Psychol Bull. 131(4):618–632. [DOI] [PubMed] [Google Scholar]
- Ulrich R, Miller J. 2001. Using the jackknife-based scoring method for measuring lrp onset effects in factorial designs. Psychophysiology. 38(5):816–827. [PubMed] [Google Scholar]
- Vida MD, Nestor A, Plaut DC, Behrmann M. 2017. Spatiotemporal dynamics of similarity-based neural representations of facial identity. Proc Natl Acad Sci U S A. 114(2):388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 2001. Wechsler test of adult reading (wtar). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D. 2011. Wechsler abbreviated scale of intelligence, second edition (wasi-ii). San Antonio, TX: NCS Pearson. [Google Scholar]
- Wilkinson GS, Robertson GJ. 2006. Wide range achievement test (wrat) 4. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Willems RM, Peelen MV, Hagoort P. 2010. Cerebral lateralization of face-selective and body-selective visual areas depends on handedness. Cereb Cortex. 20(7):1719–1725. [DOI] [PubMed] [Google Scholar]
- Yoon JH, D'Esposito M, Carter CS. 2006. Preserved function of the fusiform face area in schizophrenia as revealed by fmri. Psychiatry Res. 148(2–3):205–216. [DOI] [PubMed] [Google Scholar]
- Zhang J, Mueller ST. 2005. A note on roc analysis and non-parametric estimate of sensitivity. Psychometrika. 70(1):203–212. [Google Scholar]


