Abstract
Context
Rare homozygous or biallelic variants in POMC, PCSK1, and LEPR can disrupt signaling through the melanocortin-4 receptor (MC4R) pathway, resulting in hyperphagia and severe early-onset obesity. In pivotal Phase 3 clinical trials, treatment with the MC4R agonist setmelanotide reduced hunger and weight in patients with obesity due to proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency.
Objective
To characterize the historical weight trajectory in these patients.
Methods
This analysis included data from 2 pivotal single-arm, open-label, Phase 3 trials (NCT02896192, NCT03287960). These were multicenter trials. Patients had obesity due to POMC/PCSK1 or LEPR deficiency. During the trial, patients were treated with setmelanotide. Historical data on measured weight and height were obtained during screening.
Results
A total of 17 patients (POMC, n = 8; PCSK1, n = 1; LEPR, n = 8) with historical weight and height data were included in this analysis. Before setmelanotide treatment, patients with obesity due to POMC/PCSK1 or LEPR deficiency were above the 95th percentile for weight throughout childhood, demonstrated continuous weight gain, and did not show long-term weight loss upon interventions (eg, diet, surgery, exercise). Setmelanotide treatment attenuated weight and body mass index trajectories over the observation period of 1 year.
Conclusion
In patients with POMC, PCSK1, or LEPR deficiency, traditional interventions for weight loss had limited impact on the trajectory of severe early-onset obesity. However, setmelanotide treatment attenuated weight and body mass index trajectories and led to weight loss associated with health benefits in most individuals.
Keywords: obesity, MC4R pathway, setmelanotide, POMC, LEPR
While obesity is a multifactorial disease with many contributing biological, environmental, behavioral, and socioeconomic factors, a small subset of individuals with rare variants in genes involved in energy homeostasis develop severe early-onset obesity [1, 2]. The hypothalamic leptin–melanocortin pathway is a central regulator of energy homeostasis [3]. Leptin binds the leptin receptor (LEPR) expressed on proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus, stimulating the expression of the propeptide POMC [4, 5]. POMC is then cleaved by proprotein convertase subtilisin/kexin type 1 (PCSK1) into melanocortin ligands, including α- and β-melanocyte–stimulating hormone [4-6], which bind to and activate the melanocortin-4 receptor (MC4R) expressed on neurons of the paraventricular nucleus, promoting decreased hunger, increased satiety, and increased energy expenditure [1, 4, 6]. Rare homozygous or biallelic variants in POMC, PCSK1, and LEPR can disrupt signaling of the melanocortin pathway and cause insatiable hunger, or hyperphagia, and severe early-onset obesity [1, 7-13]. In individuals with these variants, hyperphagia can be observed as early as the first weeks of life, with severe obesity (eg, >95th percentile) being present within the first few years of life [11, 13-15]. These patients can sometimes go through a lengthy diagnostic process with frustrating therapeutic procedures [16]. However, because these diseases are rare, limited information is available on the natural history of obesity and the effects of various interventions for weight loss in patients with POMC, PCSK1, and LEPR deficiency.
Weight management strategies for these rare genetic diseases of obesity have traditionally been limited to lifestyle modifications and bariatric surgery [17]. In addition, individual pharmacologic treatment approaches including off-label use of methylphenidate to support weight control have been described [18].
These interventions, however, are not sufficiently effective at promoting or maintaining weight loss because they do not address the underlying hyperphagia and specific molecular mechanism, and continued weight gain is often observed [7, 17, 19]. Long-term weight management that reduces obesity-associated comorbidities and mortality [20, 21] and can provide meaningful weight loss and hunger reduction in patients with rare genetic diseases of obesity is needed.
In pivotal clinical trials, treatment with the MC4R agonist setmelanotide led to reductions in weight and hunger in patients with obesity due to POMC, PCSK1, or LEPR deficiency, leading to approval by the US Food and Drug Administration in 2020 for treatment of patients having biallelic variants in POMC, PCSK1, or LEPR [7, 22-25]. The approval of setmelanotide provides a novel treatment option for these patients that may disrupt the natural trajectory of weight gain associated with these diseases.
POMC, PCSK1, and LEPR deficiencies may be underdiagnosed because of a lack of awareness of the disorders [22, 26]. Gaining insight into the natural history of POMC, PCSK1, and LEPR deficiencies may help physicians identify patients for genetic testing. Case studies of patients with POMC, PCSK1, or LEPR deficiency have shown that obesity typically occurs early in life, with continued rapid weight gain into adulthood with minimal or nonsustained success with weight loss interventions [13, 24, 27, 28]. However, data are currently limited. As part of screening in a Phase 3 clinical trial of setmelanotide in patients with POMC, PCSK1, or LEPR deficiency, historical weight and height data were collected, which have not been previously reported. The objective of this analysis is to characterize the historical weight trajectory in patients with obesity due to rare variants in POMC, PCSK1, or LEPR prior to setmelanotide treatment who were enrolled in pivotal Phase 3 clinical trials. These data provide insight into the natural history of obesity in patients with POMC, PCSK1, or LEPR deficiency.
Materials and Methods
Study Design and Overview
Two similarly designed pivotal single-arm, open-label, multicenter, Phase 3 trials investigating the efficacy of setmelanotide in patients with obesity due to POMC (NCT02896192) or LEPR (NCT03287960) deficiency have previously been described [22]. Briefly, patients in each trial were identified by the investigators from existing site databases or genetic obesity registries. The POMC trial included patients ≥6 years old with homozygous or compound heterozygous POMC or PCSK1 deficiency. The LEPR trial included patients ≥6 years old with homozygous or compound heterozygous LEPR deficiency. For inclusion in either trial, obesity was defined as body mass index (BMI) ≥30 kg/m2 (for those ≥18 years old) or body weight ≥95th percentile for age on growth chart assessment (for those <18 years old).
Each patient had their therapeutic dose of setmelanotide established in a variable-duration open-label dose titration phase, during which adult (≥18 years old) and pediatric (<18 years old) patients initially received subcutaneous doses of 1.0 and 0.5 mg, respectively. Doses were uptitrated by 0.5 mg every 2 weeks until the therapeutic dose was established. The final 2 weeks of the dose titration phase were considered the first 2 weeks of the open-label treatment phase, where patients continued open-label active treatment with setmelanotide for an additional 10 weeks. Following the open-label treatment phase, patients who experienced weight loss of ≥5 kg (or ≥5% weight loss for patients with baseline body weight <100 kg) continued to the 8-week double-blind, placebo-controlled withdrawal phase, which included 4 weeks of placebo. Some patients who did not meet this weight loss threshold were allowed to continue into the double-blind, placebo-controlled withdrawal phase, but were not part of the predefined designated use set (DUS). Patients resumed open-label active treatment with setmelanotide for an additional 32 weeks for a total of ~1 year of therapeutic dosing.
The primary endpoint of these trials was the proportion of participants who achieved at least 10% weight loss compared with baseline at ~1 year. Weight measurements were taken in triplicate at each study visit. Height was recorded at screening and at various time points throughout the study.
Both trials were conducted in accordance with the International Council on Harmonisation for Good Clinical Practice and the principals of the Declaration of Helsinki. At all study sites, institutional review board or independent ethics committee approval was obtained. Patients or guardians provided written informed consent.
Historical Data Analysis
During screening, any historical data on measured weight and height since infancy were obtained where available, including any pediatric height and weight information from past pediatricians or other medical providers. Additionally, any prior interventions for weight loss (eg, diet, exercise, surgery) and associated outcomes were documented where available.
Available data were plotted on Centers for Disease Control and Prevention BMI for age, stature for age, and weight for age growth charts for male and female patients aged 2-20 years [29]. Additionally, average annual weight change for each patient within specified age ranges (2-15, >15-20, and >20 years) was calculated using the average annual change from the first available data point to the last within the given time period. For patients in the European Union, because only a birth year was available (ie, not a specific day) due to data protection rules, the date of the first historical weight or height measurement was used as a proxy for the birthdate, if it occurred in their birth year. If no measurement was recorded during the birth year, June 1 was used as a proxy. BMI data at baseline and at the end of the study were determined for patients ≥18 years of age and plotted in terms of obesity classes. Similarly, BMI Z score data, which are adjusted for age and sex, were determined for patients <18 years of age and plotted in terms of obesity categories.
The primary analyses were conducted in pivotal cohorts of patients; however, additional patients were subsequently enrolled in supplemental cohorts for the purpose of collecting supporting data. Patients in the supplemental cohort received setmelanotide treatment in the same manner as those in the pivotal cohort. Patients included in this analysis were those from either the pivotal or supplemental cohorts with ≥2 historical time points (attributable to a specific day or month) recorded as of the November/December 2019 data analyses. Being part of the DUS was not an inclusion criterion for this analysis. One patient with POMC deficiency and a confirmatory genetic test after enrollment demonstrating a benign genetic variant was excluded from this analysis.
Results
A total of 17 patients (POMC, n = 8; PCSK1, n = 1; LEPR, n = 8) had documented historical weight and height data available at ≥2 time points prior to enrolling in the study. Two of these patients (POMC, n = 1; LEPR, n = 1) were enrolled in the supplemental cohort and were not part of the primary analysis. One patient with LEPR deficiency did not experience weight loss of ≥5 kg from baseline within the 12-week open-label setmelanotide treatment period (and thus was not included in the DUS), but had met this threshold by ~19 weeks, received treatment for >52 weeks, and was included in this analysis.
Patients With Obesity Due to POMC Deficiency
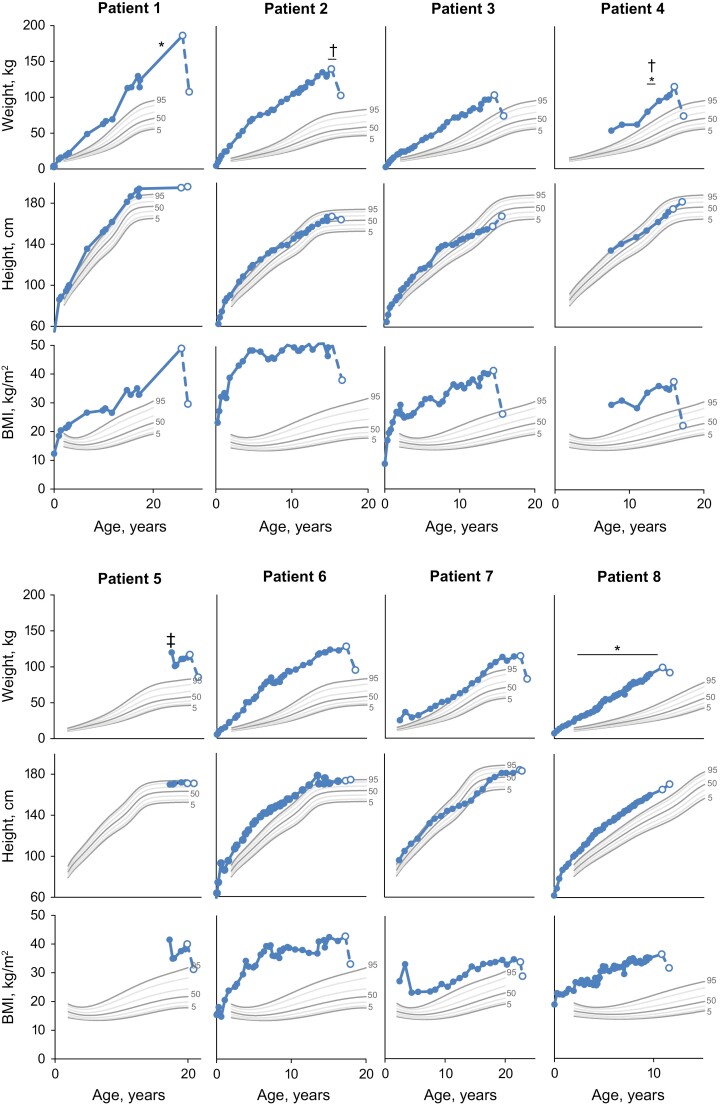
Prior to receiving setmelanotide treatment, weight and BMI in patients with POMC deficiency were generally >95th percentile throughout childhood (Fig. 1). Recorded previous interventions for weight loss in patients with POMC deficiency included diet changes, physical training, outpatient obesity programs, and gastric bypass (Table 1). However, these interventions were largely ineffective because they resulted in either no or short-term weight loss followed by weight regain. These patients gained an average of ~6.9 kg (n = 7) annually from ages 2 to 15 years, ~2.9 kg (n = 4) from ages >15 to 20 years, and ~5.1 kg (n = 1) at >20 years of age over available time points (Table 2).
Figure 1.
Historical weight, height, and BMI in patients with obesity due to POMC deficiency. Solid markers are historical data recorded prior to entry into the trial. Open markers are data at study screening and final study visit for which weight, height, or BMI were recorded after receiving treatment with setmelanotide. Centers for Disease Control and Prevention reference growth charts are shown for the 5th (bold), 10th, 25th, 50th (bold), 75th, 90th, and 95th (bold) percentiles for sex. Patient 8 was enrolled in the supplemental cohort and not reported in primary analysis. *Dietary intervention. †Exercise regimen. ‡Surgery for obesity. BMI, body mass index; POMC, proopiomelanocortin.
Table 1.
Prior interventions for weight loss
| Patient | Gene | Mutation | Sex | Age at intervention, years | Intervention | Outcome |
|---|---|---|---|---|---|---|
| Patient 1 | POMC | c.-11C>A_HOM | M | 22 | Severe diet change | Initial weight loss with regain |
| Patient 2 | POMC | c.304C>T, p.Gln102*_HOM | F | 14-15 | Physical training | Short-term weight loss |
| Patient 3a | POMC | c.225delG, p.Lys76Serfs*82_HOM | M | — | — | — |
| Patient 4 | POMC | c.251G>A, p.Trp84*_HOM | M | 13-14 | Outpatient obesity program | No weight loss |
| Patient 5 | POMC | c.-11C>A_HET; c.403_404dupGG, p.Lys136Alafs*23_HET | F | 17 | Gastric bypass | Initial weight loss with regain |
| Patient 6a | POMC | c.304C>T, p.Gln102*_HOM | F | — | — | — |
| Patient 7a | POMC | c.296delG, p.Gly99Alafs*59_HET; c.151A>T, p.Lys51*_HET | M | — | — | — |
| Patient 8b | POMC | c.296del, p.Gly99Alafs*59_HOM | M | 2-10 | Diet advice | NA |
| Patient 9a | PCSK1 | c.1029C>G, p.Tyr343*_HOM | M | — | — | — |
| Patient 10 | LEPR | c.2051A>C, p.His684Pro_HOM | M | 1-3 | Caloric restriction | No weight loss |
| 9-12 | Methylphenidate | No weight loss | ||||
| Patient 11 | LEPR | c.1874G>A, p.Trp625*_HET; c.2051A>C, p.His684Pro_HET | M | 14-25 | Gastric banding | Initial weight loss with regain |
| Patient 12 | LEPR | c.2598-3_2607del_HET; c.2227T>C, p.Ser743Pro_HET | F | 18 | Gastric sleeve | Initial weight loss with regain |
| Patient 13 | LEPR | c.1604-8A>G, intronic HOM | F | 11-18 | Combined lifestyle intervention | No weight loss |
| 16, ongoing | Metformin | No weight loss | ||||
| 17-19 | Dextroamphetamine | Initial weight loss with regain | ||||
| Patient 14 | LEPR | c.1753-1dup_HET; c.2168C>T, p.S723P_HET | F | 6-9 | Dietitian | Initial weight loss with regain |
| 9-10 | Combined lifestyle intervention | No weight loss | ||||
| Patient 15 | LEPR | c. 2051A>C, p.His684Pro_HET; c.1835G>A, p.Arg612His_HET | F | 1-4 | Dietitian | No weight loss |
| Patient 16c | LEPR | c.2385T>G, p.Tyr795*_HOM | F | 18 | Gastric banding | No weight loss |
| Patient 17a,b | LEPR | Deletion of exons 6, 7 and a portion of 8_HOM | M | — | — | — |
Abbreviations: F, female; LEPR, leptin receptor; M, male; NA, not applicable; PCSK1, proprotein convertase subtilisin/kexin type 1; POMC, proopiomelanocortin.
a No interventions reported.
b Enrolled in supplemental cohort; not reported in primary analysis.
c Not part of the designated use set.
Table 2.
Historical weight changes by age group
| Patient | Gene | Average weight change, kg/year | |
|---|---|---|---|
| 2-15 yearsa | >15-20 yearsa | ||
| Patient 1 | POMC | 7.6 | 5.7 |
| Patient 2 | POMC | 7.1 | NA |
| Patient 3 | POMC | 6.3 | NA |
| Patient 4 | POMC | 6.3 | NA |
| Patient 5 | POMC | NA | −1.2 |
| Patient 6 | POMC | 7.6 | −0.9 |
| Patient 7 | POMC | 4.3 | 7.8 |
| Patient 8b | POMC | 8.4 | NA |
| Patient 9 | PCSK1 | 13.6 | NA |
| Patient 10 | LEPR | 9.1 | NA |
| Patient 11 | LEPR | 10.4 | −4.8 |
| Patient 12 | LEPR | 8.6 | 6.2 |
| Patient 13 | LEPR | 6.0 | 11.4 |
| Patient 14 | LEPR | 8.5 | NA |
| Patient 15 | LEPR | 5.6 | NA |
| Patient 16c | LEPR | 7.9 | 6.0 |
| Patient 17b | LEPR | 7.4 | 9.1 |
Abbreviations: LEPR, leptin receptor; NA, not applicable; PCSK1, proprotein convertase subtilisin/kexin type 1; POMC, proopiomelanocortin.
a Average weight change over available time points in the specified age range.
b Enrolled in supplemental cohort; not reported in primary analysis.
c Not part of the designated use set.
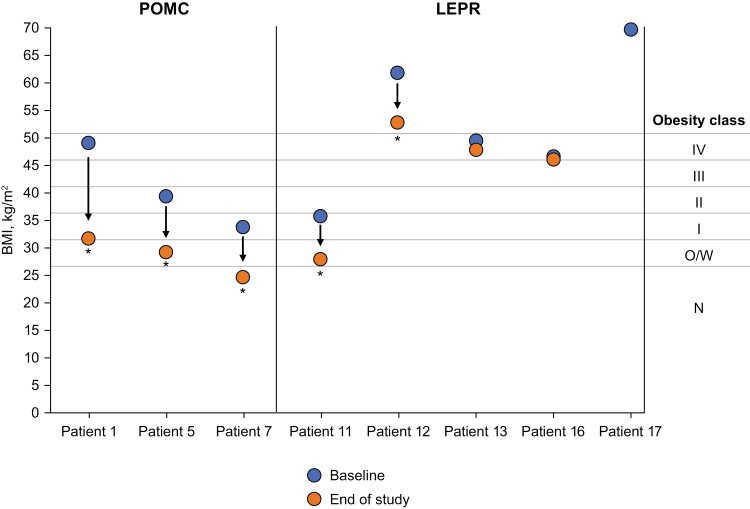
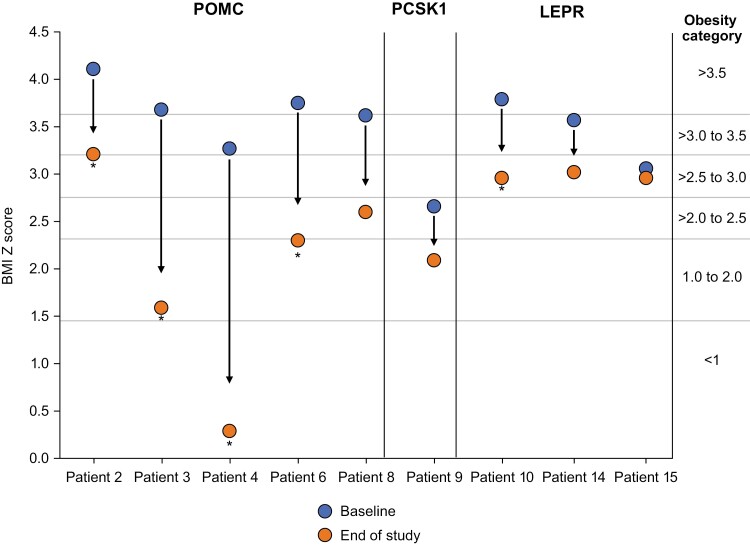
Setmelanotide altered the course of weight gain in patients with obesity due to POMC deficiency, with all patients losing weight and experiencing a reduction in BMI over ~1 year on setmelanotide. All patients who were ≥18 years of age had a decrease in BMI that resulted in a decrease of 1 to 3 obesity classes after 1 year of treatment with setmelanotide (Fig. 2). Similarly, all patients who were <18 years of age had a decrease in BMI Z score that resulted in a decrease of 1 to 4 obesity categories after 1 year of treatment with setmelanotide (Fig. 3). Notably, prepubertal patients had normal height trajectory and puberty progression.
Figure 2.
Change in obesity class due to setmelanotide treatment in patients aged ≥18 years with obesity caused by POMC or LEPR deficiency. Patient 16 was not part of the designated use set. Patient 17 did not have BMI data available following 1 year of treatment and was enrolled in the supplemental cohort and not reported in primary analysis. *Achieved primary endpoint of ≥10% weight loss after ~1 year of treatment. BMI, body mass index; LEPR, leptin receptor; N, normal; O/W, overweight; POMC, proopiomelanocortin.
Figure 3.
Change in obesity category due to setmelanotide treatment in patients aged <18 years with obesity caused by POMC, PCSK1, or LEPR deficiency. BMI Z score data are adjusted for age and sex. Patient 8 was enrolled in the supplemental cohort and not reported in primary analysis. *Achieved primary endpoint of ≥10% weight loss after ~1 year of treatment. BMI, body mass index; LEPR, leptin receptor; PCSK1, proprotein convertase subtilisin/kexin type 1; POMC, proopiomelanocortin.
Patients With Obesity Due to PCSK1 Deficiency
The 1 patient with PCSK1 deficiency had limited historical data available and had weight at approximately the 90th to 95th percentile from age 10 to 11 years, with weight being >95th percentile at the time of study enrollment. Initially, this patient lost 7.1% of their body weight prior to the placebo-controlled withdrawal period. Upon withdrawal of setmelanotide, the patient regained weight. During the withdrawal phase, the patient was administered risperidone because of depression. Risperidone is known to cause weight gain, and the patient did not lose meaningful weight after restarting setmelanotide treatment, although weight remained below study baseline. The patient stopped taking risperidone at 48 weeks. Following ~52 weeks of treatment with setmelanotide, the patient had lost 2.4% of their body weight from baseline. This equated to a decrease in BMI Z score that resulted in a decrease of 1 obesity category.
Patients With Obesity Due to LEPR Deficiency
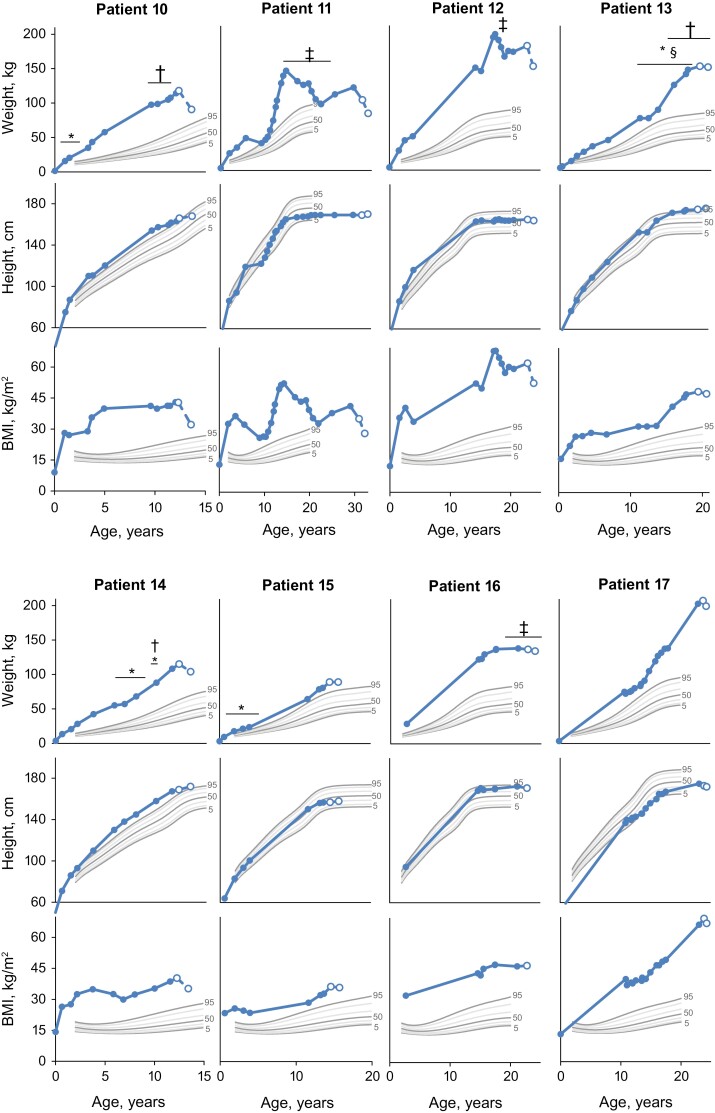
In general, weight and BMI in patients with LEPR deficiency were >95th percentile throughout childhood (Fig. 4). Recorded previous interventions for weight loss in patients with LEPR deficiency included medications (eg, dextroamphetamine, metformin, methylphenidate), diet changes, combined lifestyle interventions, and surgery (eg, gastric banding, gastric sleeve; Table 1). These interventions provided limited long-term benefit, given that they resulted in either no weight loss or initial weight loss followed by weight regain. These patients gained an average of ~7.9 kg (n = 8) annually from ages 2 to 15 years, ~5.6 kg (n = 5) from ages >15 to 20 years, and ~2.1 kg (n = 1) at >20 years of age over available time points.
Figure 4.
Historical weight, height, and BMI in patients with obesity due to LEPR deficiency. Solid markers are historical data recorded prior to entry into the trial. Open markers are data at study screening and final study visit for which weight, height, or BMI were recorded after receiving treatment with setmelanotide. Centers for Disease Control and Prevention reference growth charts are shown for the 5th (bold), 10th, 25th, 50th (bold), 75th, 90th, and 95th (bold) percentiles for sex. Patient 16 was not part of the designated use set. Patient 17 was enrolled in the supplemental cohort and not reported in primary analysis. *Dietary intervention. †Medication for obesity. ‡Surgery for obesity. §Exercise regimen. BMI, body mass index; LEPR, leptin receptor.
Setmelanotide altered the course of weight gain in patients with obesity due to LEPR deficiency, and most patients lost weight and had reduced their BMI over ~1 year on setmelanotide. Generally, patients had a decreased or maintained BMI class (≥18 years old) or BMI Z score category (<18 years old) after 1 year of treatment with setmelanotide (Figs. 2 and 3). Notably, height trajectory in children was consistent with expectations based on growth charts.
Discussion
Patients with POMC, PCSK1, and LEPR deficiency are known to have early-onset obesity and insatiable hunger resulting from defects in MC4R pathway signaling [1, 7-9]. Traditional weight loss interventions that are commonly used in patients with general obesity, such as lifestyle modifications and bariatric surgery, have previously shown limited impact in patients with rare genetic diseases of obesity. Continued weight gain in patients with rare genetic diseases of obesity is seen despite traditional weight loss interventions because of the inability of these interventions to address the underlying hyperphagia and MC4R signaling imbalance [7, 17]. Setmelanotide has been shown to significantly reduce body weight in patients with POMC, PCSK1, or LEPR deficiency through targeting the underlying physiologic defects in the MC4R pathway and decreasing the resulting hunger [22, 23].
In this analysis, patients with POMC, PCSK1, and LEPR deficiency were shown to have severe obesity beginning as early as infancy, with a trajectory of yearly weight gain continuing into adulthood. Consistent with the results from other studies of patients with rare genetic diseases of obesity, weight gain and BMI increases are greatest during early childhood [14, 16]. This may be due to defective MC4R signaling and hyperphagia being present from birth in individuals with rare genetic diseases of obesity, as opposed to an accumulation of environmental and social risk factors throughout childhood in individuals with general obesity [30, 31]. In contrast, some individuals with general obesity have BMI and weight increases in early childhood that are more modest, gradually progressing throughout childhood and adolescence [14, 32].
Similar to what has been previously described [13, 27, 28], traditional interventions for weight loss (ie, dietary changes, exercise regimens, medications, surgery) had little long-term impact in patients with POMC and LEPR deficiency. Following treatment with setmelanotide in patients enrolled in the pivotal Phase 3 clinical trials, most patients lost weight over ~1 year on setmelanotide and maintained their weight loss, resulting in an altered course of weight gain. If these patients were left untreated, many might have continued on the trajectory of weight gain that had been observed prior to setmelanotide treatment. Many patients had a notable decrease in obesity class/category following treatment with setmelanotide. These changes may translate to health benefits, which can occur with even modest weight loss (eg, 5%) [33].
In general, obesity is known to have detrimental effects on long-term health. Obesity is associated with an increased risk for long-term complications, including some cancers, respiratory disease, diabetes, and cardiovascular diseases [34, 35]. Additionally, obesity is associated with anxiety, depression, and diminished quality of life [36, 37]. Overall, the presence and severity of obesity is associated with an increased risk of mortality and reduced life expectancy [38-40]. Notably, obesity in childhood or young adulthood is associated with increased mortality later in life, underscoring the importance of early intervention [41, 42]. Thus, the large impact of setmelanotide on the trajectory of weight gain or severity of obesity in patients with rare genetic diseases of obesity may translate to earlier long-term health benefits.
Setmelanotide has been shown to be well tolerated, with the most common treatment-emergent adverse events being related to injection site reactions and hyperpigmentation [22]. No treatment-related cardiovascular adverse events were reported with setmelanotide, and setmelanotide was not associated with changes in heart rate or blood pressure [22], which have been associated with first-generation MC4R agonists [43, 44]. Hypothalamic POMC deficiency may result in improved glucose tolerance [45]. However, treatment with setmelanotide did not result in significant worsening of glucose metabolism parameters (eg, fasting glucose, glycated hemoglobin) in patients with POMC or LEPR deficiency [22]. In fact, setmelanotide was associated with a significant improvement in fasting glucose levels in patients with POMC deficiency.
Overall, these results showed that patients with POMC, PCSK1, or LEPR deficiency experience weight gain starting early in childhood and continuing into adulthood, with traditional interventions for weight loss having limited impact on the trajectory of weight gain. This leads to a tremendous burden of the disease for patients and caregivers because they fail to stabilize body weight with traditional treatments. As reported previously, setmelanotide can meaningfully and significantly reduce body weight [22] and attenuate weight and BMI trajectories, potentially leading to long-term benefits on morbidities, life expectancy, and long-term health.
Acknowledgments
Editorial assistance was provided under the direction of the authors by Scott Houck, PhD, CMPP, and David Boffa, ELS, MedThink SciCom, and funded by Rhythm Pharmaceuticals, Inc.
Glossary
Abbreviations
- BMI
body mass index
- DUS
designated use set
- LEPR
leptin receptor
- MC4R
melanocortin-4 receptor
- PCSK1
proprotein convertase subtilisin/kexin type 1
- POMC
proopiomelanocortin
Financial Support
This study was supported by Rhythm Pharmaceuticals, Inc.
Disclosures
M.W., S.F., C.E.F., N.B., E.v.d.A., and P.K. have nothing to declare. U.G.M., M.S., and J.G. are employed by Rhythm Pharmaceuticals, Inc.
Data Availability
Some or all data sets generated and/or analyzed during the current study are not publicly available but are available from Rhythm Pharmaceuticals, Inc. for further research on reasonable request.
Clinical Trial Information
POMC/PCSK1 trial: NCT02896192; LEPR trial: NCT03287960; long-term extension study: NCT03651765.
References
- 1. Huvenne H, Dubern B, Clément K, Poitou C. Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obes Facts. 2016;9(3):158-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van der Klaauw AA, Farooqi IS. The hunger genes: pathways to obesity. Cell. 2015;161(1):119-132. [DOI] [PubMed] [Google Scholar]
- 3. Anderson EJ, Çakir I, Carrington SJ, et al. 60 years of POMC: regulation of feeding and energy homeostasis by α-MSH. J Mol Endocrinol. 2016;56(4):T157-T174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeo GSH, Chao DHM, Siegert AM, et al. The melanocortin pathway and energy homeostasis: From discovery to obesity therapy. Mol Metab. 2021;48:101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shen WJ, Yao T, Kong X, Williams KW, Liu T. Melanocortin neurons: multiple routes to regulation of metabolism. Biochim Biophys Acta Mol Basis Dis. 2017;1863(10):2477-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paolacci S, Borrelli A, Stuppia L, et al. Mendelian obesity, molecular pathways and pharmacological therapies: a review. Eur Rev Med Pharmacol Sci. 2019;23(3):1357-1378. [DOI] [PubMed] [Google Scholar]
- 7. Clément K, Mosbah H, Poitou C. Rare genetic forms of obesity: From gene to therapy. Physiol Behav. 2020;227:113134. [DOI] [PubMed] [Google Scholar]
- 8. Kleinendorst L, Abawi O, van der Kamp HJ, et al. Leptin receptor deficiency: a systematic literature review and prevalence estimation based on population genetics. Eur J Endocrinol. 2020;182(1):47-56. [DOI] [PubMed] [Google Scholar]
- 9. Coll AP, Farooqi IS, Challis BG, Yeo GS, O’Rahilly S. Proopiomelanocortin and energy balance: insights from human and murine genetics. J Clin Endocrinol Metab. 2004;89(6):2557-2562. [DOI] [PubMed] [Google Scholar]
- 10. Nunziata A, Funcke JB, Borck G, et al. Functional and phenotypic characteristics of human leptin receptor mutations. J Endocr Soc. 2019;3(1):27-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19(2):155-157. [DOI] [PubMed] [Google Scholar]
- 12. Jackson RS, Creemers JW, Ohagi S, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16(3):303-306. [DOI] [PubMed] [Google Scholar]
- 13. Clément K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398-401. [DOI] [PubMed] [Google Scholar]
- 14. Kohlsdorf K, Nunziata A, Funcke JB, et al. Early childhood BMI trajectories in monogenic obesity due to leptin, leptin receptor, and melanocortin 4 receptor deficiency. Int J Obes (Lond). 2018;42(9):1602-1609. [DOI] [PubMed] [Google Scholar]
- 15. Farooqi IS, Wangensteen T, Collins S, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356(3):237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zorn S, von Schnurbein J, Kohlsdorf K, Denzer C, Wabitsch M. Diagnostic and therapeutic odyssey of two patients with compound heterozygous leptin receptor deficiency. Mol Cell Pediatr. 2020;7(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berger C, Klöting N. Leptin receptor compound heterozygosity in humans and animal models. Int J Mol Sci. 2021;22(9):4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brandt S, von Schnurbein J, Lennerz B, et al. Methylphenidate in children with monogenic obesity due to LEPR or MC4R deficiency improves feeling of satiety and reduces BMI-SDS-A case series. Pediatr Obes. 2020;15(1):e12577. [DOI] [PubMed] [Google Scholar]
- 19. Poitou C, Puder L, Dubern B, et al. Long-term outcomes of bariatric surgery in patients with bi-allelic mutations in the POMC, LEPR, and MC4R genes. Surg Obes Relat Dis. 2021;17(8):1449-1456. [DOI] [PubMed] [Google Scholar]
- 20. Ma C, Avenell A, Bolland M, et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017;359:j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ryan DH, Yockey SR. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;6(2):187-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clément K, van den Akker E, Argente J, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8(12):960-970. [DOI] [PubMed] [Google Scholar]
- 23. Markham A. Setmelanotide: first approval. Drugs. 2021;81:397-403. [DOI] [PubMed] [Google Scholar]
- 24. Kühnen P, Clément K, Wiegand S, et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med. 2016;375:240-246. [DOI] [PubMed] [Google Scholar]
- 25. Rhythm Pharmaceuticals. IMCIVREE [package insert]. Rhythm Pharmaceuticals, Inc.; 2020. [Google Scholar]
- 26. Ayers KL, Glicksberg BS, Garfield AS, et al. Melanocortin 4 receptor pathway dysfunction in obesity: patient stratification aimed at MC4R agonist treatment. J Clin Endocrinol Metab. 2018;103(7):2601-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clément K, Biebermann H, Farooqi IS, et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat Med. 2018;24(5):551-555. [DOI] [PubMed] [Google Scholar]
- 28. Le Beyec J, Cugnet-Anceau C, Pépin D, et al. Homozygous leptin receptor mutation due to uniparental disomy of chromosome 1: response to bariatric surgery. J Clin Endocrinol Metab. 2013;98(2):E397-E402. [DOI] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention. Clinical growth charts. Accessed May 5, 2021. https://www.cdc.gov/growthcharts/clinical_charts.htm
- 30. Suglia SF, Duarte CS, Chambers EC, Boynton-Jarrett R. Cumulative social risk and obesity in early childhood. Pediatrics. 2012;129(5):e1173-e1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Campbell MK. Biological, environmental, and social influences on childhood obesity. Pediatr Res. 2016;79:205-211. [DOI] [PubMed] [Google Scholar]
- 32. Lagström H, Hakanen M, Niinikoski H, et al. Growth patterns and obesity development in overweight or normal-weight 13-year-old adolescents: the STRIP study. Pediatrics. 2008;122(4):e876-e883. [DOI] [PubMed] [Google Scholar]
- 33. Williamson DA, Bray GA, Ryan DH. Is 5% weight loss a satisfactory criterion to define clinically significant weight loss? Obesity (Silver Spring). 2015;23(12):2319-2320. [DOI] [PubMed] [Google Scholar]
- 34. Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl):s176-s185. [PubMed] [Google Scholar]
- 35. Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16(1):36-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jia H, Lubetkin EI. The impact of obesity on health-related quality-of-life in the general adult US population. J Public Health (Oxf). 2005;27(2):156-164. [DOI] [PubMed] [Google Scholar]
- 37. Nigatu YT, Reijneveld SA, de Jonge P, van Rossum E, Bültmann U. The combined effects of obesity, abdominal obesity and major depression/anxiety on health-related quality of life: the LifeLines Cohort Study. PLoS One. 2016;11(2):e0148871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6(12):944-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mehta T, Fontaine KR, Keith SW, et al. Obesity and mortality: are the risks declining? Evidence from multiple prospective studies in the United States. Obes Rev. 2014;15(8):619-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Faeh D, Braun J, Tarnutzer S, Bopp M. Obesity but not overweight is associated with increased mortality risk. Eur J Epidemiol. 2011;26(8):647-655. [DOI] [PubMed] [Google Scholar]
- 41. Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond). 2011;35(7):891-898. [DOI] [PubMed] [Google Scholar]
- 42. Hirko KA, Kantor ED, Cohen SS, Blot WJ, Stampfer MJ, Signorello LB. Body mass index in young adulthood, obesity trajectory, and premature mortality. Am J Epidemiol. 2015;182(5): 441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Greenfield JR, Miller JW, Keogh JM, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360(1):44-52. [DOI] [PubMed] [Google Scholar]
- 44. Kuo JJ, da Silva AA, Tallam LS, Hall JE. Role of adrenergic activity in pressor responses to chronic melanocortin receptor activation. Hypertension. 2004;43(2):370-375. [DOI] [PubMed] [Google Scholar]
- 45. Chhabra KH, Adams JM, Fagel B, et al. Hypothalamic POMC deficiency improves glucose tolerance despite insulin resistance by increasing glycosuria. Diabetes. 2016;65(3):660-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated and/or analyzed during the current study are not publicly available but are available from Rhythm Pharmaceuticals, Inc. for further research on reasonable request.