Abstract
Background
Pulmonary embolism (PE) is a common life‐threatening cardiovascular condition, with an incidence of 23 to 69 new cases per 100,000 people each year. For selected low‐risk patients with acute PE, outpatient treatment might provide several advantages over traditional inpatient treatment, such as reduction of hospitalisations, substantial cost savings, and improvements in health‐related quality of life. This is an update of an earlier Cochrane Review.
Objectives
To assess the effects of outpatient versus inpatient treatment in low‐risk patients with acute PE.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 31 May 2021.
Selection criteria
We included randomised controlled trials (RCTs) of outpatient versus inpatient treatment of adults (aged 18 years and over) diagnosed with low‐risk acute PE.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were short‐ and long‐term all‐cause mortality. Secondary outcomes were bleeding, adverse effects, recurrence of PE, and patient satisfaction. We used GRADE to assess certainty of evidence for each outcome.
Main results
We did not identify any new studies for this update. We included a total of two RCTs involving 453 participants. Both trials discharged participants randomised to the outpatient group within 36 hours of initial triage, and both followed participants for 90 days. One study compared the same treatment regimens in both outpatient and inpatient groups, and the other study used different treatment regimens. There was no clear difference in treatment effect for the outcomes of mortality at 30 days (risk ratio (RR) 0.33, 95% confidence interval (CI) 0.01 to 7.98; 2 studies, 453 participants; low‐certainty evidence), mortality at 90 days (RR 0.98, 95% CI 0.06 to 15.58; 2 studies, 451 participants; low‐certainty evidence), major bleeding at 14 days (RR 4.91, 95% CI 0.24 to 101.57; 2 studies, 445 participants; low‐certainty evidence) and at 90 days (RR 6.88, 95% CI 0.36 to 132.14; 2 studies, 445 participants; low‐certainty evidence), minor bleeding (RR 1.08, 95% CI 0.07 to 16.79; 1 study, 106 participants; low‐certainty evidence), recurrent PE within 90 days (RR 2.95, 95% CI 0.12 to 71.85; 2 studies, 445 participants; low‐certainty evidence), and patient satisfaction (RR 0.97, 95% CI 0.90 to 1.04; 2 studies, 444 participants; moderate‐certainty evidence). We downgraded the certainty of the evidence because the CIs were wide and included treatment effects in both directions, the sample sizes and numbers of events were small, and it was not possible to determine the effect of missing data or the presence of publication bias. The included studies did not assess PE‐related mortality or adverse effects, such as haemodynamic instability, or adherence to treatment.
Authors' conclusions
Currently, only low‐certainty evidence is available from two published randomised controlled trials on outpatient versus inpatient treatment in low‐risk patients with acute PE. The studies did not provide evidence of any clear difference between the interventions in overall mortality, bleeding, or recurrence of PE.
Plain language summary
Is home or hospital treatment better for people with blood clots in the lungs?
Key message
We are uncertain if treatment in hospital or at home (outpatient) is better for some people with blood clots in the lungs. We did not find clear evidence of an important difference in the number of deaths, bleeding, recurrence of new blood clots, or patient satisfaction, because the results were imprecise and the studies did not report side effects on blood pressure or information on how well people followed medical advice. More well‐designed studies are needed before doctors can make informed practice decisions.
Why is this question important?
A blood clot in the lungs (pulmonary embolism) is a major cause of death and illness. These can occur after a blood clot breaks off from somewhere else in the body and travels in the blood to the lungs. The clot can often travel from the leg, where it is called a deep vein thrombosis. How people are treated depends on whether they are low‐risk or high‐risk, but most people are given blood‐thinning drugs (anticoagulants) in hospital. For some low‐risk cases with sudden onset (acute) pulmonary embolism, treatment at home might be better than keeping them in hospital. Possible benefits include fewer people in hospital, less risk of picking up an infection in hospital, cost savings, and better health‐related quality of life. We wanted to know if there were any differences in risks and benefits between people with blood clots in the lungs who are treated at home compared to those treated in the hospital.
What did we do?
We searched for randomised controlled trials that treated adults with sudden onset, low‐risk, pulmonary embolism at home or in hospital. In randomised controlled trials, the treatments people receive are decided at random, and these types of studies give the most reliable evidence about treatment effects.
What did we find?
We found two studies involving 453 people with sudden onset pulmonary embolism who were treated at home or in hospital. One study used the same treatment for the people treated at home or in hospital, and the second study used different treatments. We did not find any clear differences in the number of deaths, bleeding, new clots, or patient satisfaction between the groups of people who were treated at home or in the hospital.
Neither study provided information on side effects of the treatment or on whether people were able to follow the correct instructions at home.
What are the limitations of the evidence?
We are not very certain about the evidence from the two included studies. This is because there were only small numbers of people in the studies (and small numbers of events) and because we could not be sure that reports of studies where no effect was shown were ever published.
How up to date is this evidence?
This review updates our previous review. The evidence is up to date to May 2021.
Summary of findings
Summary of findings 1. Outpatient versus inpatient treatment for acute pulmonary embolism.
| Outpatient versus inpatient treatment for acute pulmonary embolism | ||||||
|
Patient or population: people with low‐risk acute pulmonary embolism Settings: outpatient and inpatient settings Intervention: outpatient settinga Comparison: inpatient settingb | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (RCTs) | Certainty of the evidence (GRADE) | Comments | |
| Risk with inpatient setting | Risk with outpatient setting | |||||
|
Short‐term all‐cause mortality Follow‐up: 7 to 10 days after randomisation |
See comment | — | 453 (2) | ⊕⊕⊝⊝ Lowc | No deaths occurred in either study within this time period. | |
|
Short‐term all‐cause mortality Follow‐up: to 30 days after randomisation |
Study population | RR 0.33 (0.01 to 7.98) | 453 (2) | ⊕⊕⊝⊝ Lowd | In Aujesky 2011, 1/168 deaths in the inpatient group vs 0/171 deaths in the outpatient group. The death reported by Aujesky 2011 was not PE‐related. No deaths occurred in Peacock 2018. | |
| 4 per 1000 | 1 per 1000 (0 to 35) | |||||
|
Long‐term all‐cause mortality Follow‐up: 90 days after randomisation |
Study population | RR 0.98 (0.06 to 15.58) | 451e (2) | ⊕⊕⊝⊝ Lowd | In Aujesky 2011, 1/168 deaths in the inpatient group vs 1/171 deaths in the outpatient group. The deaths reported by Aujesky 2011 were not PE‐related. No deaths occurred in Peacock 2018. | |
| 4 per 1000 | 4 per 1000 (0 to 68) | |||||
|
Major bleeding Follow‐up: 14 days after randomisation |
Not estimable | RR 4.91 (0.24 to 101.57) | 445 (2) | ⊕⊕⊝⊝ Lowd | In Aujesky 2011, 0/168 major bleeding events in the inpatient group vs 2/171 major bleeding events in the outpatient group. No major bleeding occurred in Peacock 2018. | |
|
Major bleeding Follow‐up: 90 days after randomisation |
Not estimable | RR 6.88 (0.36 to 132.14) | 445 (2) | ⊕⊕⊝⊝ Lowd | In Aujesky 2011, 0/168 major bleeding events in the inpatient group vs 3/171 major bleeding events in the outpatient group. No major bleeding occurred in Peacock 2018. | |
| Minor bleeding | Study population | RR 1.08 (0.07 to 16.79) | 106 (1) | ⊕⊕⊝⊝ Lowd | One participant in each treatment arm reported minor bleeding. | |
| 18 per 1000 | 20 per 1000 (1 to 305) | |||||
|
Recurrent PE Follow‐up: within 90 days |
Not estimable | RR 2.95 (CI 0.12 to 71.85) | 445 (2) | ⊕⊕⊝⊝ Lowd | In Aujesky 2011, 0/168 recurrent PE in inpatient group vs 1/171 recurrent PE in the outpatient group, within 90 days. No recurrent PE occurred in Peacock 2018. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; PE: pulmonary embolism | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of the effect. | ||||||
aOutpatients received subcutaneous enoxaparin twice daily (Aujesky 2011), or rivaroxaban 15 mg orally twice daily for the first 21 days, followed by 20 mg orally once daily for approximately 69 days, for a total treatment duration of 90 days (Peacock 2018). bIn Aujesky 2011, the inpatient group was admitted to hospital and received subcutaneous enoxaparin 1 mg/kg twice daily. In Peacock 2018, the inpatient group was admitted to hospital and received variable pharmacotherapy (standard‐of‐care treatment). cWe downgraded by two levels as the small sample size means it is unlikely that rare events will be seen and because publication bias could not be discounted. dWe downgraded by two levels due to the overall small sample size, small number of events, imprecision in the confidence intervals and the fact that publication bias could not be discounted. eAdditional information was requested from the study authors, but as they were unable to provide it, we used only the available data.
Background
Description of the condition
Pulmonary embolism (PE) is a common life‐threatening cardiovascular illness. It is a potentially fatal disease that, despite adequate treatment, is still associated with high morbidity and mortality; the reported incidence in the USA exceeds one case per 1000 population, and in Europe, annual mortality is more than seven deaths per 100,000 population (Barco 2020). Heit 2005 estimated that there are around 237,000 cases of non‐fatal PE and 294,000 cases of fatal PE each year in the USA. Cohen 2007 reported that of all people admitted to hospitals, 1% die of acute PE, and about 10% of all in‐hospital deaths are PE‐related. In six European countries with a total population of 454.4 million, more than 370,000 deaths were related to venous thromboembolism (VTE) in 2004, as estimated using an epidemiological model (Cohen 2007).
The diagnosis of PE has improved with advances in imaging technology, and PE management has changed with the introduction of treatments such as non‐vitamin K antagonist oral anticoagulants (NOACs) since 2010. In 2011, Pollack and colleagues reported a 5.4% all‐cause mortality rate across 22 medical centres in the USA (Pollack 2011). However, with the progressive use of NOACS, mortality from PE has also changed. The international Computerized Registry of Patients with Venous Thromboembolism (RIETE registry) of over 23,000 patients, Jiménez 2016 noted a 30‐day all‐cause mortality rate of 5.9%, while Vinson 2018 found a 30‐day all‐cause mortality rate of 4.4% across 21 USA medical centres.
The prognosis and treatment of people diagnosed with acute PE are related to initial haemodynamic status. High‐risk PE (massive PE) – defined by the presence of shock or persistent arterial hypotension (systolic blood pressure below 90 mmHg or systolic blood pressure drop by 40 mmHg or more, for over 15 minutes, if not caused by new‐onset arrhythmia, hypovolaemia, or sepsis) – accounts for 5% of all cases of PE and has a poor prognosis, with short‐term mortality of more than 15% (Konstantinides 2014). Conversely, in haemodynamically stable patients, low risk PE (non‐massive PE) accounts for 95% of all cases of PE and has significantly lower short‐term mortality, ranging from under 1% to 15% (Buller 2003; Ibrahim 2008; Konstantinides 2014; Quinlan 2004). The question is whether people with low‐risk PE should be treated as outpatients or inpatients.
Description of the intervention
The traditional initial anticoagulant therapy for acute PE in hospitals is standardised intravenous unfractionated heparin (UFH), subcutaneous low molecular weight heparins (LMWH), or fondaparinux, started together with oral vitamin K antagonists (referred as the overlap treatment period) for at least five days until the prothrombin time yields an international normalised ratio above 2.0 for two consecutive days.
Since the 1990s, the practical advantages of LMWH over UFH have allowed outpatient treatment in patients with VTE in many situations without laboratory anticoagulant monitoring (Othieno 2018). Although most people with acute PE are hospitalised during initial therapy, it is feasible that in selected low‐risk people, outpatient care can safely and effectively be used rather than inpatient care.
The NOACs, including factor Xa inhibitors (e.g. rivaroxaban, apixaban, and edoxaban) and direct thrombin inhibitors (e.g. dabigatran), are available in many countries for treating VTE. In patients with PE who are haemodynamically stable (PE without hypotension), NOACs are efficient and safe (Ghazvinian 2018). The 10th edition of the American College of Chest Physicians (ACCP) guidelines suggests administering initial parenteral anticoagulation before dabigatran and edoxaban, while rivaroxaban and apixaban do not require initial parenteral anticoagulation (Kearon 2016).
Studies comparing inpatient versus outpatient treatment for PE have used early discharge as soon as patients achieve a clinically stable condition (Aujesky 2011; Otero 2010). Outpatient treatment of a substantial number of patients with low‐risk acute PE (even during the traditional period of overlap with heparin and vitamin K antagonists) seems to be a feasible option.
How the intervention might work
Most cases of acute PE are managed within a hospital setting because of the uncertainty in determining whether patients are at high or low risk. In addition, during management of acute PE, some patients require intensive treatment in hospital due to potentially fatal complications such as clinical deterioration. Therefore, when considering management in an outpatient setting, it is important to identify patients who are considered at low risk of major (fatal) complications. In selected low‐risk patients, more than 98% of outpatients have an uncomplicated course (Van der Wall 2018).
Several risk assessment strategies, such as clinical scores, imaging modalities, and laboratory biomarkers are available to identify patients who could potentially be treated safely as outpatients. Agterof 2010 reported that haemodynamically stable patients with low N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels (less than 500 pg/mL) would be a safe group of patients to receive care in an outpatient setting. Furthermore, Lankeit 2011 considered the troponin T (TnT) assay, along with the simplified Pulmonary Embolism Severity Index (sPESI), as a risk assessment tool to identify patients with acute PE who could be safely treated as outpatients.
In addition, there are several scoring systems to select low‐risk patients with acute PE for outpatient management (Jara‐Palomares 2019). The most widely used and validated scores include the Geneva prediction score (GPS), the Pulmonary Embolism Severity Index (PESI), the simplified version of the PESI (sPESI) and Hestia criteria (Aujesky 2005; Den Exter 2016b; Jiménez 2010; Wicki 2000).
The GPS was derived from 296 outpatients confirmed with symptomatic acute PE and identifies six independent predictors (cancer, heart failure, previous deep vein thrombosis (DVT), systolic blood pressure less than 100 mmHg, partial pressure of oxygen dissolved in arterial blood (PaO2) less than 8 kPa, and presence of DVT on ultrasound exam) of an adverse outcome (death, recurrent thromboembolic event or major bleeding) in a three‐month follow‐up period (Wicki 2000, see Appendix 1).
The PESI criteria were derived from 15,531 inpatients discharged with PE and consists of 11 factors independently associated with 30‐day mortality: age, male sex, cancer, heart failure, chronic lung disease, pulse rate 110 beats/minutes or greater, systolic blood pressure less than 100 mmHg, respiratory rate 30 breaths/minute or greater, body temperature less than 36°C, altered mental status, and oxyhaemoglobin saturation less than 90% (Aujesky 2005, see Appendix 2).
The sPESI was developed with six items that may be more useful and practical for routine use in emergency departments. This tool can predict 30‐day mortality after acute PE and has similar prognostic accuracy to the original PESI (Jiménez 2010, see Appendix 3).
The Hestia criteria is specifically designed to identify patients who can be safely treated in an outpatient setting and therefore comprise only contraindications to outpatient treatment. The Hestia criteria were derived from a multicentre prospective cohort study in 297 patients treated as outpatients among 581 patients with PE (Zondag 2011, see Appendix 4). These criteria use 11 practical clinical exclusion rules to select patients for outpatient treatment. Later on, these criteria were validated in 550 patients by combining them with a cutoff of 500 ng/L for NT‐proBNP levels in outpatient treatment (Den Exter 2016b).
Jiménez 2007 compared two models (GPS and PESI) using a distinct set of ambulatory patients with acute symptomatic PE to identify low‐risk patients for anticoagulant therapy in the outpatient setting. In this study, the PESI quantified the prognosis of patients with acute PE significantly better than the GPS. Hence, the PESI can very accurately select and identify low‐risk patients for adverse events within 30 days of anticoagulant therapy in acute PE.
Zondag 2013 compared the performance of the sPESI versus the Hestia criteria for selecting low‐risk patients for anticoagulant therapy in the outpatient setting. This study demonstrated that both the sPESI and the Hestia criteria classified different patients as being suitable for outpatient treatment, suggesting that when the Hestia criteria are applied, they may identify a proportion of patients considered as high risk by sPESI (such as those with malignant diseases, cardiopulmonary comorbidities, or advanced age) to be treated as outpatients.
In a recent direct comparison, the Hestia and sPESI criteria were shown to be equally safe and effective, identifying more than a third of patients with acute PE for outpatient treatment with favourable outcomes (Roy 2021). Haemodynamically stable patients with a PESI score I or II, or negative by the Hestia criteria and who have adequate home support, are eligible for early discharge or outpatient treatment (Barra 2013; Piran 2013).
Why it is important to do this review
The eighth edition of the ACCP guidelines discussed the feasibility of outpatient treatment in acute PE among a substantial proportion of patients, but they provided no formal recommendations (Kearon 2008). In the same way, the task force of the European Society of Cardiology did not clearly recommend early discharge or outpatient management for acute PE in selected patients (Konstantinides 2014). The ninth edition of the ACCP guidelines suggested early discharge over standard discharge (e.g. after the first five days of treatment) for patients with low‐risk PE whose home circumstances were adequate (Grade 2B) (Kearon 2012). However, patients who preferred the security of the hospital to the convenience and comforts of home were likely to choose hospitalisation over outpatient treatment.
The increasing availability of NOACs for treating acute PE allows management without the need for hospitalisation. Hence, the 10th edition of the ACCP guidelines suggested outpatient treatment for acute PE, provided that patients fulfil the following conditions.
Clinically stable with good cardiopulmonary reserve.
No contraindications such as recent haemorrhage, severe kidney or liver disease, or severe thrombocytopenia (i.e. platelets less than 70,000/mm3).
Expected to adhere to treatment.
The patient feels confident to be treated at home.
However, in patients with right ventricular dysfunction or increased cardiac biomarker levels, out‐of‐hospital treatment is not recommended (Kearon 2016).
The 2018 guideline from the British Thoracic Society proposes similar recommendations (Howard 2018). Patients with confirmed PE should be risk‐stratified using a validated clinical risk score: patients in PESI class I/II, sPESI class 0, or those meeting the Hestia criteria should be considered for outpatient management of PE. Where PESI or sPESI is used and indicates a low risk, physicians should consider a set of exclusion criteria for the outpatient management of PE (Appendix 5). Recent NICE recommendations consider outpatient treatment for people with low‐risk PE and stress the importance of using a validated severity score to identify candidates while taking into account individual circumstances (NICE 2020).
Outpatient treatment instead of traditional inpatient treatment in selected low‐risk patients with acute PE can provide several advantages, for example, a reduction in the number of hospitalisations, substantial cost savings, and an improvement in health‐related quality of life (Dasta 2015; Fanikos 2013). This is an update of an earlier Cochrane Review and aims to present evidence to help guide decision‐making on whether outpatient treatment is as safe and effective as inpatient treatment for acute PE in clinically stable, low‐risk patients.
Objectives
To assess the effects of outpatient versus inpatient treatment in low‐risk patients with acute PE.
Methods
Criteria for considering studies for this review
Types of studies
This systematic review included randomised controlled trials (RCTs) as well as quasi‐RCTs (in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods), as we did not anticipate finding many RCTs in this area.
Types of participants
We included adults (18 years and older) diagnosed with low‐risk acute pulmonary embolism (PE), defined as acute onset of dyspnoea or chest pain together with a new contrast‐filling defect on single‐ or multi‐detector computed tomography (CT) pulmonary angiography or pulmonary digital angiography, a new high‐probability ventilation‐perfusion lung scan or documentation of a new proximal deep vein thrombosis (DVT) either by venous ultrasonography or contrast venography.
We considered people to be at low risk if they were classified as such by any validated or non‐validated measurement tool that aimed to classify mortality risk rate related to PE, such as the Geneva prediction score (GPS), the Pulmonary Embolism Severity Index (PESI), the simplified PESI (sPESI), or the Hestia criteria.
Types of interventions
Intervention group: participants allocated to outpatient management for acute PE.
Control group: participants allocated to hospital (inpatient) management for acute PE.
We considered outpatients as people who were discharged within 36 hours after the low‐risk acute PE diagnosis and who then completed treatment as outpatients.
Types of outcome measures
Primary outcomes
Short‐term all‐cause mortality (from the date of randomisation to 10 and to 30 days).
Long‐term all‐cause mortality (from the date of randomisation to 90 days).
Long‐term all‐cause mortality at 90 days included any all‐cause mortality noted from the date of randomisation to 90 days. We considered both all‐cause mortality and PE‐related mortality.
Secondary outcomes
Bleeding (from the date of randomisation to 90 days): we defined major bleeding as fatal or clinically overt bleeding resulting in fall of haemoglobin by 2 g/L or more, or bleeding into critical anatomical sites (subdural haematoma, intraspinal haemorrhage, retroperitoneal, intraocular, pericardial, atraumatic intra‐articular), or leading to transfusion of 2 U or more of blood or red cells (Schulman 2005). We defined minor bleeding as bleeding requiring intervention but not qualifying as a major bleeding, including bleeding precipitating treatment cessation (Schulman 2005).
Adverse effects, such as haemodynamic instability (from the date of randomisation to 90 days).
Recurrence of PE (from the date of randomisation to 90 days).
Patient satisfaction, adherence, or both (from the date of randomisation to 90 days): we accepted methods used by study investigators, including Likert scale questionnaires.
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs and controlled clinical trials, with no restrictions on language, publication year, or publication status.
The Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web, searched on 31 May 2021).
The Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (2021, Issue 4).
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE; searched from 26 March 2018 to 31 May 2021).
Embase Ovid (searched from 26 March 2018 to 31 May 2021).
CINAHL Ebsco (searched from 26 March 2018 to 31 May 2021).
AMED Ovid (searched from 26 March 2018 to 31 May 2021).
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, these were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions,Lefebvre 2021). Search strategies for major databases are provided in Appendix 6.
The Information Specialist searched the following trial registries on 31 May 2021.
The World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch).
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We checked the reference lists of the identified studies for additional citations.
Data collection and analysis
Selection of studies
Two review authors (HHBY, VSNN) independently screened the trials identified by the literature search by title and abstract. Two of a possible four review authors assessed the full text of potentially relevant reports (HHBY, VSNN, PVB, CB). We resolved disagreements by discussion (PJFVB).
Data extraction and management
Two review authors (HHBY, VSNN) independently extracted data, resolving any discrepancies by discussion. We used a standard data extraction form to extract the following information: characteristics of the study (design, randomisation methods), participants, interventions, and outcomes (types of outcome measures, adverse events); and details on study funding and study authors' declarations of interest. We then checked for accuracy before entering the data in Review Manager 5 software (Review Manager 2020).
Assessment of risk of bias in included studies
We assessed methodological quality using Cochrane's 'Risk of bias' tool (Higgins 2011). We used the following six criteria.
Random sequence generation
We considered random sequence generation to be at low risk of bias where the method used was either adequate or unlikely to introduce bias; at unclear risk where there was insufficient information to assess whether the method used was likely to introduce bias; or at high risk when the method used (e.g. quasi‐randomised trials) was improper and likely to introduce bias.
Allocation concealment
We recorded allocation concealment as being at low risk of bias when the method used (e.g. central allocation) was unlikely to introduce bias in the final observed effect; at unclear risk when there was insufficient information to assess whether the method used was likely to introduce bias in the estimate of effect; or at high risk when the method used (e.g. open random allocation schedule) was likely to introduce bias in the final observed effect.
Blinding
For this clinical review, it is not possible to blind participants and investigators to treatment allocation (i.e. inpatient and outpatient), so we did not consider performance bias as part of the risk of bias assessment. We did consider blinding of outcome measures and recorded blinding of assessors as being at low risk of bias if blinding was performed adequately, or the outcome measurement was not likely to be influenced by lack of blinding; at unclear risk if there was insufficient information to assess whether the type of blinding used was likely to introduce bias in the estimate of effect; or at high risk if there was no blinding or incomplete blinding, and the outcome or the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
We recorded incomplete outcome data as being at low risk of bias when the underlying reasons for missing data were unlikely to make treatment effects depart from plausible values, or proper methods were employed to handle missing data. In addition, we considered a withdrawal rate less than 20% in each group to be low risk. We recorded an unclear risk of bias when there was insufficient information to assess whether the missing data mechanism, in combination with the method used to handle missing data, was likely to introduce bias in the estimate of effect; and a high risk of bias when the crude estimate of effects (e.g. complete‐case estimate) was clearly biased due to the underlying reasons for missing data, and the methods used to handle missing data were unsatisfactory.
Selective reporting
We recorded selective reporting as being at low risk of bias when the trial protocol was available and all of the trial's prespecified outcomes that were of interest in the review were reported. We recorded an unclear risk of bias when there was insufficient information to assess whether the magnitude and direction of the observed effect was related to selective outcome reporting; or as being at high risk when not all of the trial's prespecified primary outcomes were reported.
Other bias
We considered aspects of methodology that might have been influenced by vested interests and which may have led directly to a risk of bias as 'other bias'.
Two review authors (VSNN, PJFVB) independently made a judgement as to whether the risk of bias for each criterion was low, unclear, or high. We resolved disagreements by discussion. We considered trials that were classified as being at low risk of bias in sequence generation, allocation concealment, blinding of outcome assessors, incomplete data, and selective outcome reporting as trials that were at overall low risk of bias.
Measures of treatment effect
Binary outcomes
We used risk ratios (RRs) as the effect measure with 95% confidence intervals (CIs) for dichotomous data.
Continuous outcomes
We planned to present the results as mean differences (MDs) with 95% CIs for continuous data. When pooling data across studies, we planned to use MDs if the outcomes were measured in the same way between trials. We planned to use standardised mean differences to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
The unit of analysis was each participant recruited into each study.
Dealing with missing data
In an intention‐to‐treat (ITT) analysis, all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received the intervention or not. For each trial, we reported whether or not the investigators stated if the analysis was performed according to the ITT principle. If participants were excluded after allocation, we reported any details provided in full. Furthermore, we performed the analysis on an ITT basis whenever possible (Newell 1992). Otherwise, we adopted the 'available‐case analysis'. Study authors provided some further information on missing data when requested (Peacock 2018). When this was not possible, we used only available data in the analyses.
Assessment of heterogeneity
We looked for clinical heterogeneity by examining the study details and then tested for statistical heterogeneity between trial results using the Chi2 test and the I2 statistic (Deeks 2011). We are aware that thresholds for interpretation of I2 can be misleading in that the importance of inconsistency depends on several factors. We considered size and direction of effect and interpreted heterogeneity using the following I2 thresholds for interpretation as described in the Cochrane Handbook (Higgins 2021).
0% to 40%: might not be important.
30% to 60%: might represent moderate heterogeneity.
50% to 90%: might represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We planned to assess the likelihood of potential publication bias using funnel plots in addition to assessing the risk of selective outcome reporting, considered under the risk of bias assessment for included studies. When small studies in a meta‐analysis tend to show larger treatment effects, we planned to consider other causes including selection biases, poor methodological quality, heterogeneity, and chance. However, the number of studies included in the review prevented this.
Data synthesis
We used the fixed‐effect model to analyse data. If we identified substantial heterogeneity (e.g. I2 greater 50%), we planned to compute pooled estimates of the treatment effect for each outcome using a random‐effects model (with two or more studies). We undertook quantitative analysis of outcomes on an ITT basis.
Subgroup analysis and investigation of heterogeneity
In the case of substantial clinical heterogeneity (I2 greater than 50%), we planned to use subgroup analysis to explore the results. We planned to perform the Chi2 test for subgroup differences, set at a P value of 0.05. We planned to carry out analysis of the following subgroups when sufficient data were available.
LMWH (e.g. tinzaparin, enoxaparin, dalteparin) and selective factor Xa inhibitors (e.g. fondaparinux).
Once‐daily versus twice‐daily administration of LMWH and selective factor Xa inhibitors.
Outpatient discharge period (24 hours or less versus more than 24 hours).
Classification criteria (i.e. PESI versus GPS).
Inpatients and outpatients with the same versus different treatment regimens.
Sensitivity analysis
If sufficient numbers of studies were identified for inclusion, we planned to perform a sensitivity analysis to explore causes of heterogeneity and the robustness of the results. We planned to include the following factors in the sensitivity analysis, separating studies according to:
trials at low versus high risk of bias; and
rates of withdrawal for each outcome (less than 20% versus 20% or greater).
As only two studies were included we were unable to do this.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table to present the main results of this review using GRADEpro GDT software (GRADEpro GDT). We used the principles of the GRADE system to assess the certainty of the body of evidence associated with specific primary outcomes (short‐ and long‐term all‐cause mortality), as well as secondary outcomes (bleeding and recurrent PE) that were most clinically relevant to healthcare professionals and patients (Guyatt 2008). The GRADE approach appraises the certainty of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The assessment of the certainty of a body of evidence considers within‐study risk of bias (methodological quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
Results
Description of studies
See Figure 1.
1.
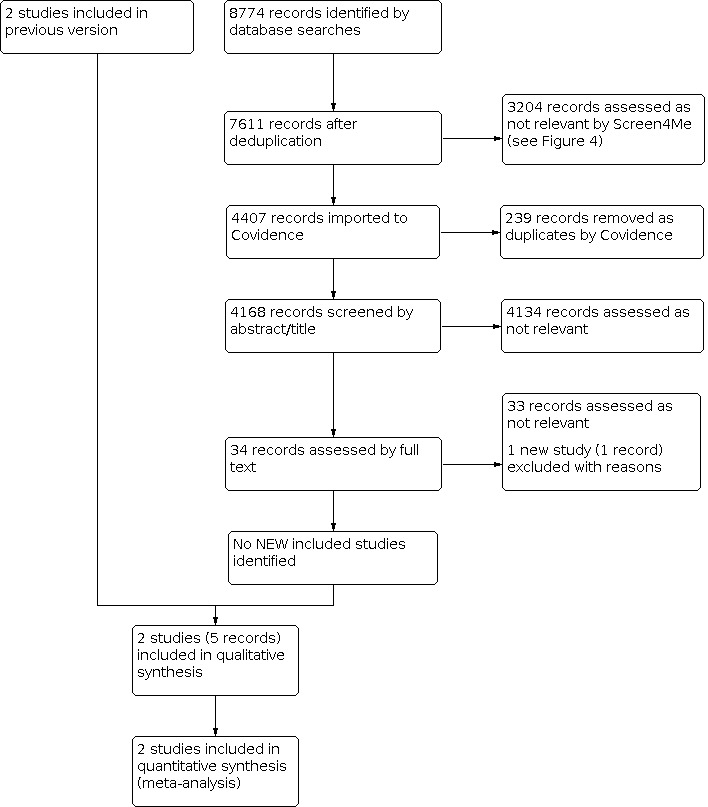
Study flow diagram.
Results of the search
For this update, database searches yielded a total of 8774 records. We used Cochrane's Screen4Me workflow to help identify potential reports of RCTs from the database search records. Results of the Screen4Me assessment process are shown in Figure 2. We then assessed the remaining 4407 'possible' records identified by Screen4Me using Covidence (covidence.org). We did not identify any new studies for inclusion. We assessed one new study as excluded (Medina 2017). See Figure 1.
2.
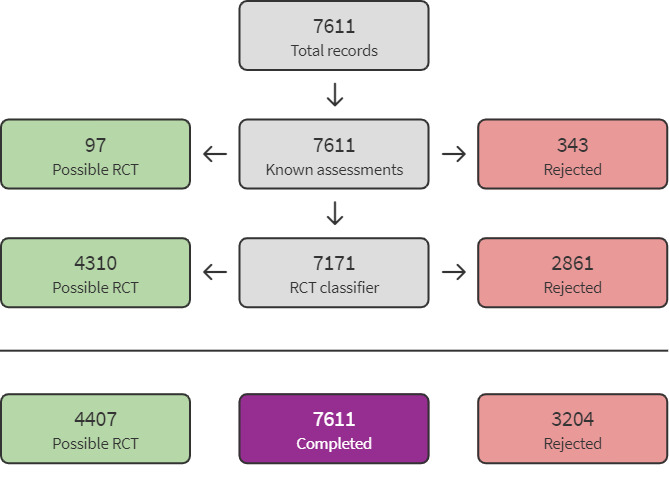
Screen4Me flow diagram
Included studies
We included two studies involving a total of 453 participants that met the inclusion criteria (Aujesky 2011; Peacock 2018). Aujesky 2011 randomised 339 participants and Peacock 2018 randomised 114 participants. See Characteristics of included studies.
Study design
Authors described both trials as international, open‐label, randomised, non‐inferiority trials (Aujesky 2011; Peacock 2018). They evaluated a period of three months. The studies differed both in terms of site of care and pharmacotherapy.
Types of interventions
Aujesky 2011 compared outpatient treatment (171 participants) versus inpatient treatment (168 participants) for acute PE.
Participants assigned to outpatient treatment received subcutaneous enoxaparin 1 mg/kg twice daily and were to be discharged from the emergency department (ED) within 24 hours of randomisation. If self‐injection was not possible, a study nurse either taught a caregiver to inject the enoxaparin or arranged administration by a visiting nurse. Participants assigned to receive inpatient treatment were admitted to hospital and received the same enoxaparin regimen. All participants received vitamin K antagonist therapy.
In Peacock 2018, the outpatient group (51 participants) was discharged home from the ED no later than 24 hours after triage. They received 15 mg oral rivaroxaban twice daily for the first 21 days, followed by 20 mg oral rivaroxaban once daily for approximately 69 days, for a total treatment duration of 90 days. The inpatient comparison group (63 participants) received usual care according to local standard of care protocol and as defined by the medical team caring for the participant, which typically involved intravenous UFH or subcutaneous LMWH and hospitalisation but also included any of the NOACs. About three‐quarters of all participants were initially treated with unfractionated or LMWH but ultimately received NOACs, most commonly rivaroxaban (51%) or apixaban (25%).
Types of outcomes measured
Aujesky 2011 considered the primary outcome of 'recurrence of symptomatic venous thromboembolism', as determined by helical CT or new perfusion defect involving 75% or more of a lung segment by lung scan; or pulmonary angiography; or autopsy; or documentation of a new proximal DVT either by venous ultrasonography or contrast venography (Aujesky 2011).
As a safety measurement, the study assessed bleeding (evaluated at 14 and 90 days) and death. Aujesky 2011 defined major bleeding as fatal bleeding, bleeding at critical sites (i.e. intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular, pericardial or intramuscular with compartment syndrome), bleeding with a reduction of haemoglobin of 20 g/L or more, or resulting in transfusion of two units or more of packed red cells.
Aujesky 2011 also assessed overall satisfaction 14 days after randomisation using a non‐validated five‐point Likert scale questionnaire.
Peacock 2018 evaluated the primary outcomes of duration of initial and subsequent hospitalisations for bleeding or VTE events (or both) within 30 days and 90 days of randomisation. The secondary outcomes of interest were percentage of participants with recurrence of symptomatic venous thromboembolism event (VTE), VTE‐related death (at 7, 14, 30, and 90 days), all‐cause mortality, serious adverse events, percentage of participants with the number of unplanned visits to the hospital or physician office for VTE symptoms or bleeding (at 7, 14, 30 and 90 days), minor bleeding, and mean combined duration of initial and subsequent ED hospitalisation for any reason up to 30 and 90 days. Patient satisfaction was evaluated at day 7 by means of five‐point and three‐point Likert scales, with higher scores indicating greater satisfaction. Satisfaction was further evaluated on day 90 with the Anti‐Clot Treatment Score, which uses two subscales of burdens (12 items) and benefits (3 items), both measured on a five‐point Likert scale, with higher scores indicating greater satisfaction.
Excluded studies
We excluded one new study for this update (Medina 2017), bringing the total number of excluded studies to six (Den Exter 2016a; HOME Study; Kovacs 2003; Medina 2017; Otero 2010; Zondag 2011). Kovacs 2003 was a randomised controlled clinical trial that evaluated different doses of warfarin in outpatients; Zondag 2011 was classified as cohort study; Otero 2010 considered early discharge to be at three to five days from admission, and Den Exter 2016a randomised participants to either outpatient treatment or to management according to N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels. We also excluded HOME Study, which randomised participants to either Hestia or PESI management. Medina 2017 did not randomise participants to either outpatient or inpatient treatment. See Characteristics of excluded studies.
Risk of bias in included studies
3.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
4.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Allocation
We classified Aujesky 2011 as being at low risk of selection bias, as it used a computer programme to generate the allocation to treatment groups in a 1:1 ratio, with a randomised block design. We also classified Peacock 2018 as being at low risk of selection bias because participants were randomly assigned in a 1:1 ratio to discharge or standard care by an interactive web system within 12 hours of diagnosis.
Blinding
Since it is not possible to blind participants and investigators to treatment allocation for this particular clinical question, we did not consider performance bias as part of the risk of bias assessment.
In Aujesky 2011, data analysts were unmasked to treatment group assignment. However, there was a committee unaware of treatment assignment that confirmed all outcomes and classified all deaths as caused (or not) by PE, major bleeding, or another reason. Therefore, we judged the study to be at low risk of detection bias.
In Peacock 2018, analysts were masked to treatment group assignment, and so we considered the study to be at low risk of bias.
Incomplete outcome data
Both studies reported less than 20% of dropouts and withdrawals – two from the outpatient group and five from the inpatient group in Aujesky 2011, and seven from the outpatient group and eight from the inpatient group in Peacock 2018. We classified both as being at unclear risk of attrition bias. In Peacock 2018, despite seven dropouts in the early‐discharge group, the study authors could confirm that all of these participants were alive at the end of the study. However, for the inpatient group they could not confirm whether two participants survived. In Aujesky 2011, the study authors were unable to confirm how many of the individuals who did not complete the study survived.
Selective reporting
There was no evidence of selective reporting in either of the included studies (Aujesky 2011; Peacock 2018).
Other potential sources of bias
There was no evidence of other potential sources of bias in the included studies (Aujesky 2011; Peacock 2018). We considered the imbalance between the two groups in Peacock 2018, as the arms received different pharmacotherapy regimens. However, as 50% of the outpatient group received the same treatment as the inpatient group, we judged both studies as being at low risk of other bias.
Effects of interventions
See: Table 1
Outpatient versus inpatient treatment for acute pulmonary embolism
We identified two trials comparing outpatient (222 participants) versus inpatient (231 participants) treatment for acute PE (Aujesky 2011; Peacock 2018). The studies differed both in terms of site of care and pharmacotherapy. In Aujesky 2011, both groups of participants received subcutaneous enoxaparin 1 mg/kg twice every day. In Peacock 2018, the outpatient group received rivaroxaban, and the inpatient group received standard care (treatment based on local institutional protocols, defined by the medical team caring for the participant, which typically involved bridging therapy and hospitalisation but also any of the NOACs). More than 75% of inpatients ultimately received some type of direct‐acting oral anticoagulant, with 50.8% receiving rivaroxaban. To investigate if the difference in treatment regimens influenced the results, we pooled the data from the studies using subgroup analysis.
Primary outcomes
Short‐term all‐cause mortality (from the date of randomisation to 10 days)
No deaths occurred in either study within 10 days of randomisation (low‐certainty evidence).
Short‐term all‐cause mortality (from the date of randomisation to 30 days)
In Aujesky 2011, one death occurred on day 17 in the inpatient group (1/168), due to pneumonia and cancer, and there were no deaths in the outpatient group (0/171). No deaths occurred in Peacock 2018. There was no clear effect of intervention due to this imprecision (risk ratio (RR) 0.33, 95% confidence interval (CI) 0.01 to 7.98; P = 0.49; 2 studies, 453 participants; low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Outpatient versus inpatient treatment, Outcome 1: Short‐term all‐cause mortality
Long‐term all‐cause mortality (from the date of randomisation to 90 days)
In Aujesky 2011, one death occurred on day 17 in the inpatient group (1/168), due to pneumonia and cancer, and one death occurred on day 34 in the outpatient group (1/171), due to trauma‐related aortic rupture. No deaths occurred in Peacock 2018. There was no clear effect of intervention due to imprecision (RR 0.98, 95% CI 0.06 to 15.58; P = 0.99; 2 studies, 451 participants; low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Outpatient versus inpatient treatment, Outcome 2: Long‐term all‐cause mortality
Short‐term pulmonary embolism‐related mortality (from the date of randomisation to 10 days)
Aujesky 2011 and Peacock 2018 reported no short‐term PE‐related deaths.
Long‐term pulmonary embolism‐related mortality (from the date of randomisation to 90 days)
Aujesky 2011 and Peacock 2018 reported no long‐term PE‐related deaths.
Secondary outcomes
Bleeding
Major bleeding
In Aujesky 2011, two outpatients (2/171) and no inpatients (0/168) had major bleeding within 14 days (one of the events was an intramuscular haematoma on day 3 and one was by insertion of vena cava filter on day 13). Peacock 2018 reported no major bleeding by 90 days; we therefore assumed that no major bleeding had occurred by 14 days. There was no clear effect of intervention within 14 days due to this imprecision (RR 4.91, 95% CI 0.24 to 101.57; P = 0.30; 2 studies, 445 participants; low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Outpatient versus inpatient treatment, Outcome 3: Major bleeding within 14 days
In Aujesky 2011, three outpatients (3/171) and no inpatients (0/168) had major bleeding within 90 days. No major bleeding occurred in Peacock 2018. There was no clear effect of intervention within 90 days due to imprecision (RR 6.88, 95% CI 0.36 to 132.14; P = 0.20; 2 studies, 445 participants; low‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Outpatient versus inpatient treatment, Outcome 4: Major bleeding within 90 days
Minor bleeding
Aujesky 2011 did not report on minor bleeding. In Peacock 2018, two participants reported clinically relevant non‐major bleeding (as defined by the International Society on Thrombosis and Haemostasis), one from each randomisation group, which we considered as being minor bleeding. There was no clear effect of intervention due to imprecision (RR 1.08, 95% CI 0.07 to 16.79; P = 0.96; 1 study, 106 participants; low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Outpatient versus inpatient treatment, Outcome 5: Minor bleeding
Adverse effects such as haemodynamic instability
The trials did not report on adverse effects such as haemodynamic instability.
Recurrence of pulmonary embolism
In Aujesky 2011, one outpatient (1/171) and no inpatients (0/168) had a recurrent PE within 90 days (long term). There were no cases of recurrent pulmonary embolism in Peacock 2018. There was no clear difference between the treatment groups (RR 2.95, 95% CI 0.12 to 71.85; P = 0.51; 2 studies, 445 participants; low‐certainty evidence; Analysis 1.6). There were no events in either group regarding the short‐term recurrence of PE (analysis within 14 days).
1.6. Analysis.

Comparison 1: Outpatient versus inpatient treatment, Outcome 6: Recurrent pulmonary embolism within 90 days
Patient satisfaction, adherence, or both
In Aujesky 2011, 156 of 170 outpatients (92%) and 158 of 167 inpatients (95%) were very satisfied or satisfied with the medical care received. In Peacock 2018, 29 of 48 outpatients (60%) and 37 of 59 inpatients (63%) were very satisfied with assignment to intervention or control group. There was no clear evidence to support a difference between the two interventions regarding patient satisfaction (RR 0.97, 95% CI 0.90 to 1.04; P = 0.97; 2 studies, 444 participants; Analysis 1.7). The certainty of evidence was downgraded to moderate because the effect of missing data and the absence of publication bias could not be verified. The trials did not report on adherence.
1.7. Analysis.

Comparison 1: Outpatient versus inpatient treatment, Outcome 7: Satisfaction questionnaire
Other analyses
As only two studies were included, we were unable to perform sensitivity analyses or assess the risk of publication bias. We had intended to carry out subgroup analyses as described in Subgroup analysis and investigation of heterogeneity. These were also limited by the number of studies included; however, where possible, we performed subgroup analysis according to treatment regimens. For most outcomes, we were unable to check for subgroup differences due to the absence of events in one or more studies. Where data were sufficient, no subgroup differences were seen in the satisfaction levels between treatment regimens (P = 0.97; Analysis 1.7).
Discussion
Outpatient treatment is the usual standard of care for most patients with acute deep venous thrombosis (DVT), whereas hospitalisation is the standard of care for most patients with acute pulmonary embolism (PE) due to different outcomes. Advances in pharmacotherapy have allowed outpatient care in low‐risk acute venous thromboembolism (VTE) (Mansour 2017). Compared to vitamin K antagonists, non‐vitamin K antagonist oral anticoagulants (NOACs) act on only one part of the coagulation cascade, have a prompt onset of action, fixed dosing, and few dietary and drug interactions (Kabrhel 2021).
Although NOACs have gained approval for the management of VTE, their effect on site‐of‐care decision‐making has not yet been fully evaluated for outpatient management of PE (Vinson 2018), with early research suggesting little impact (Kline 2016; Stein 2016). However, the advent of subcutaneous low molecular weight heparins (LMWH), fondaparinux and NOACs has raised the possibility of expanding the traditional in‐hospital treatment of acute PE to early discharge or complete treatment in the outpatient setting (Kearon 2016). Regardless, the proportion of patients with acute PE receiving outpatient treatment is still low in most industrialised countries (Roy 2017).
There are several potential benefits of outpatient treatment of PE over traditional hospital treatment: high patient satisfaction, reduction of hospitalisations, substantial cost savings, improvement in health‐related quality of life (Dasta 2015; Fanikos 2013), and increased physical activity and social functioning. Nevertheless, identifying patients at low risk of mortality who could benefit from outpatient management presents challenges. For many years, the lack of prognostic criteria to identify patients at low risk of mortality precluded safe outpatient management (Vinson 2012; Zondag 2012).
In the RIETE Registry, fatal PE occurred in 12% of patients presenting with massive PE and in 3% of patients with non‐massive PE (Laporte 2008). Therefore, most patients with acute non‐massive PE at presentation have a better prognosis and, as in patients with DVT, it is possible that treatment in a substantial proportion of these individuals can be safely managed completely, or at least partially (early discharge), as outpatients (Othieno 2018; Segal 2007). Although not based on high‐certainty evidence, two retrospective cohort studies, Erkens 2010 and Kovacs 2010, showed that at least 50% of patients presenting with symptomatic PE can be treated safely as outpatients.
Treating acute PE in the outpatient setting in carefully selected patients has been allowed and studied in Europe and Canada more than in the USA. In some hospitals in Canada, about 50% of people with acute PE are managed entirely as outpatients (Baglin 2010; Kovacs 2010). Conversely, based on the report of the Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry (EMPEROR) (Pollack 2011), in the USA only 1.1% of people attending the emergency department were discharged home without hospitalisation. Several factors might explain this geographic discrepancy, such as issues of health insurance compensation and malpractice litigation (Vinson 2012).
The 2014 European Society of Cardiology (ESC) guidelines suggested considering early discharge and outpatient management in patients judged as having low‐risk PE (Konstantinides 2014). Nevertheless, due to insufficient evidence, there is no clear recommendation of whether the Pulmonary Embolism Severity Index (PESI) or the Hestia criteria are more appropriate for determining the safe setting for treatment.
The 2018 guideline from the British Thoracic Society recommended that patients with confirmed PE should be risk‐stratified using a validated clinical risk score (Howard 2018). Patients in PESI class I/II, sPESI class 0, or those meeting the Hestia criteria should be considered for outpatient management of PE. More recently, NICE proposed a similar recommendation considering outpatient treatment in patients with low‐risk PE, using a validated risk‐stratification tool (NICE 2020).
Two prospective studies in the USA, which used the PESI to identify patients eligible for outpatient management, have shown outpatient management to be safe and effective in selected low‐risk patients with PE (Bledsoe 2018; Vinson 2018). Vinson 2018 employed the prediction score flexibly, in that the PESI class was not strictly tied to a site‐of‐care disposition. Rather, the risk class was used to inform clinical judgement, not direct it. This more flexible use of the PESI is endorsed by the American College of Chest Physicians in their recent PE guideline (Kearon 2016).
Whether the prompt echocardiographic assessment of right ventricular dysfunction (RVD) is essential to select PE patients for outpatient treatment is still a matter of debate (Becattini 2020). According to a review and meta‐analysis by Barco 2019, 34% (95% CI 30% to 39%) of patients classified as being as low risk by the PESI, sPESI, or the Hestia criteria were reported to have signs of RVD on echocardiography or computed tomography pulmonary angiogram. The presence of RVD was associated with a four‐fold increased risk of death, but the absolute risk for death was low in patients with versus without RVD on echocardiography: 1.8% (95% CI 0.9% to 3.5%) compared to 0.2% (95% CI 0.03% to 1.7%), respectively (OR 4.19, 95% CI 1.39 to 12.58) (Barco 2019). Becattini 2020 concluded that "right ventricle assessment would preclude about 34% of low‐risk patients from early discharge (95% CI 30% to 39%) to potentially identify 1.8% that will die or 3.5% that will experience a PE‐related adverse outcome."
The 2019 ESC Guidelines recommended considering early discharge of patients with acute PE and continuation of anticoagulant therapy at home if three sets of criteria are met (Konstantinides 2019).
The risk of early PE‐related death or serious complications is low.
There is no serious comorbidity or aggravating condition(s) that would demand hospitalisation.
Proper outpatient care and anticoagulant therapy can be provided, considering the patient’s (anticipated) compliance and the possibilities offered by the healthcare system and social infrastructure.
Similarly, the recently published update of the Chest Guideline and Expert Panel Report recommends outpatient treatment rather than hospitalisation in low‐risk PE patients, provided that access to medication, the ability to access outpatient care, and the home conditions are appropriate (Stevens 2021).
The multidisciplinary consensus panel of the American College of Emergency Physicians provided a robust recommendation to help clinicians safely treat low‐risk PE outpatients (Kabrhel 2021). After confirmed diagnosis of PE, the next step is risk stratifying the patient to assess whether outpatient treatment is suitable. This assessment takes into account the following conditions: risk of bleeding from anticoagulation, access to the medication in a timely manner, and patient's psychosocial condition for early discharge. Then, regular follow‐up is essential, and the patient must also be informed about the warning signs or symptoms that should prompt a return to the emergency department.
In this 2022 Cochrane Review, we included only two randomised controlled trials that compared outpatient versus inpatient management of acute PE (Aujesky 2011; Peacock 2018). Aujesky 2011 used the PESI, a validated risk‐stratification instrument, to select low‐risk patients who were eligible for outpatient treatment. These results suggest a feasible perspective on the safety of PE management in an outpatient setting. Peacock 2018 reported preliminary results using the Hestia criteria to select low‐risk PE patients who can be safely and effectively managed as outpatients with rivaroxaban. The results between outpatient versus inpatient groups for mortality, thromboembolic events and haemorrhagic complications were similarly very low in both groups. No clear differences were detected between the outpatient and inpatient groups for the outcomes of mortality, bleeding, recurrent PE and patient satisfaction. Seemingly both methods for selection of low‐risk PE patients (PESI and Hestia) can be applied with acceptable outcomes. A scientific comparison of the two methods in which patients were randomised to either the sPESI or Hestia criteria was recently completed, showing similar safety and effectiveness for triaging patients with acute PE for outpatient treatment (HOME Study; Roy 2021).
Further large, randomised studies are required to provide information on the selection of low‐risk PE patients for outpatient management. It is currently unclear whether the PESI or the Hestia criteria are more accurate for identifying suitable outpatients, whether troponin levels have to be considered for safe selection of these patients, or whether imaging such as echocardiogram and compression ultrasonography of the leg veins are also necessary.
Summary of main results
This review examined the effects of outpatient versus inpatient treatment in low‐risk patients with acute PE. We included two RCTs, involving 453 participants, that reported our primary outcomes. In Aujesky 2011, inpatients and outpatients received the same treatment regimens (enoxaparin 1 mg/kg twice daily); and in Peacock 2018, inpatients were treated with rivaroxaban 15 mg and outpatients with the local standard of care. No deaths occurred in Peacock 2018, and two deaths occurred in Aujesky 2011, one in each group. For short‐ and long‐term mortality, major and minor bleeding, and recurrent PE there was no clear evidence of a difference between the treatment groups, as there was imprecision in the results (the numbers of events were very small, the confidence intervals were wide and included treatment effects in both directions, and the certainty of the evidence was low). See Table 1. There was no clear evidence to support a difference between the two interventions regarding patient satisfaction: 92% and 60% of outpatients, and 95% and 63% of inpatients, were very satisfied or satisfied with the medical care received (Aujesky 2011 and Peacock 2018, respectively). The included studies did not assess adverse effects such as haemodynamic instability or adherence to treatment.
Overall completeness and applicability of evidence
Because of our comprehensive search strategy and contact with experts in the field, we are confident that we have identified all RCTs and quasi‐RCTs comparing outpatient versus inpatient treatment for acute PE. This review addresses the non‐inferiority hypothesis that outpatient treatment presents the same benefits as inpatient treatment for acute PE. Outpatient treatment can improve health‐related quality of life and reduce hospitalisation rates and costs, although outpatient treatment will incur some health service costs. A key point concerning this clinical question is the appropriate selection of people who are at low risk. More studies are needed to assess the accuracy of the PESI for identifying a population of low‐risk patients who can be safely and effectively treated without hospitalisation. The PESI, as used by Aujesky 2011, consists of 11 routinely available clinical parameters based on signs and symptoms, and it stratifies patients into five risk classes (I to V) at an increasing risk of short‐term mortality (Appendix 2). The validation study of PESI performed by Aujesky 2007 identified low‐risk patients who are potential candidates for outpatient treatment, with very low rates of 90‐day all‐cause mortality (1% or less). Peacock 2018 used the Hestia criteria (see Appendix 4), which consist of 11 practical clinical exclusion rules to select patients for outpatient treatment. The Hestia criteria were later validated in 550 patients by combining them with a 500 ng/L cutoff for NT‐proBNP levels to select patients for outpatient treatment (Den Exter 2016b). Although in Peacock 2018, the inpatients and outpatients received different treatment regimens, local standard of care also involved rivaroxaban. Regardless of the different treatment regimens between groups, there was no difference in results. Neither study reported on all our predefined outcomes, such as haemodynamic instability or adherence. We excluded one RCT from this review because it used a different definition for outpatients (early discharge was considered as discharge after three to five days of admission (Otero 2010), compared with less than 36 hours in Aujesky 2011 and Peacock 2018). In addition, Otero 2010 used a non‐validated clinical prognostic model to identify low‐risk patients.
Quality of the evidence
The overall certainty of the evidence for the primary outcomes of short‐ and long‐term mortality was low (Table 1). We downgraded our assessments of the certainty of evidence due to the small number of participants and events and imprecision. In addition, we were unable to discount publication bias (Guyatt 2008). It is therefore difficult to draw robust conclusions on the basis of the available evidence.
Potential biases in the review process
One area of potential bias in this review is the weakness of statistical power because of the small number of included studies. Although we performed a well‐designed search strategy to identify all potential studies, we found only two RCTs that met the eligibility criteria, which makes our findings uncertain, as larger trials are needed to detect a difference (or determine there is no meaningful difference) between inpatient and outpatient care.
In Peacock 2018 the outpatient group received rivaroxaban, and the inpatient group received standard of care (treatment based on local institutional protocols, which may include admission, a parenteral anticoagulant and an oral vitamin K antagonist, or any of the NOACs). This is different from Aujesky 2011, which had similar pharmacological treatments in the inpatient and outpatient treatment arms. We decided to include Peacock 2018 because in the inpatient group 50.8% of patients received rivaroxaban, and the results for the primary outcomes between Aujesky 2011 and Peacock 2018 were quite similar (low number of events). We recognise the importance and methodological rigour of Aujesky 2011; however, Peacock 2018 appears to be more pragmatic, so we considered it important to include and to pool the data. In order to highlight any potential differences, we performed a subgroup analysis (inpatients and outpatients with same treatment regimens versus inpatients and outpatients treated with different treatment regimens). Peacock 2018 used the Hestia criteria to classify patients, so we considered that most patients were symptomatic.
Agreements and disagreements with other studies or reviews
We found one systematic review that selected observational studies, including prospective cohort studies that described the outcome of people with PE treated entirely as outpatients (Vinson 2012). The review examined the results of exclusive ambulatory management for people with acute symptomatic PE. However, the authors considered an observational period of less than 24 hours, that is, the review did not consider inpatient stay followed by early discharge. The review also indicated that both treatments had similar effectiveness. Hence, Vinson 2012 recommended that patients with a low risk of adverse clinical outcomes (e.g. mortality, recurrence and bleeding) be treated as outpatients due to the advantages of low cost, avoidance of hospital infections, and high levels of patient satisfaction (Vinson 2012).
Squizzato 2009 also conducted a systematic review that evaluated the effects of outpatient treatment for PE. All the included studies were observational studies, and the authors concluded that patients might be safely treated as outpatients. The authors recommended further studies to confirm or refute these findings.
Three systematic reviews included observational studies and RCTs (including Aujesky 2011, which we also included, and Otero 2010, which we excluded). The reviews evaluated whether outpatient treatment and early discharge were as safe as conventional inpatient treatment in people with acute PE (Piran 2012; Piran 2013; Zondag 2012). Although heterogeneous criteria were used to select participants, the results showed that in a carefully selected group classified as being at low risk, both treatments were equally safe; this conclusion is in agreement with the findings of our systematic review.
Authors' conclusions
Implications for practice.
This review included two published randomised controlled trials comparing outpatient and inpatient treatment for acute pulmonary embolism in low‐risk patients. The evidence was of low certainty; the studies did not provide evidence of any clear difference between the treatment groups in overall mortality, bleeding, or recurrence of pulmonary embolism.
Implications for research.
This review highlights the need for further research into inpatient versus outpatient treatment for acute pulmonary embolism and to determine which validated severity score(s) should be used to identify low‐risk pulmonary embolism. Future trials need to be adequately powered to increase the certainty of detecting a difference or to determine that there is no difference in treatments. Standardised outcome measures with adequate follow‐up, such as mortality, hospitalisation rates, health‐related quality of life, and costs are also required.
Feedback
Aujesky, 2 December 2014
Summary
The systematic review on outpatient versus inpatient treatment of pulmonary embolism (PE) by Yoo, Queluz, and El Dib published in the Cochrane Library in November 14, 2014 noted our randomized non‐inferiority trial1 as the sole study deemed worthy to be included in the systematic review. However, the review authors declare in the abstract that our study quality is “very low” because “blinding of the outcome assessors was not reported”. Furthermore, the authors say in the summary of findings that “it is possible to blind for this clinical question”. We disagree with these statements. While our data analysts knew treatment assignment, we blinded the outcome assessors to treatment arm, as explicitly described in the published manuscript. Yoo, et al. call for “well‐conducted randomized controlled trials (where people are allocated at random to one of two or more treatments groups, one of which is a control (dummy!) treatment)”, we cannot imagine a design differing from ours that would allow participants and study personnel to be blinded for home versus hospital care. Finally, contrary to the assertion in this review, we never described our trial as double‐blind placebo‐controlled clinical trial but as “an international, open‐label, randomised, non‐inferiority trial.” We hope these variances from the trial design will be incorporated into the Cochrane review. The challenge of doing a systematic review that analyses data on a topic where a singular experimental design exists is laid bare in this effort; perhaps this topic – in contrast to the care of deep venous thrombosis absent PE – is not ready for such an analysis. We also look forward to more data that will help guide implementation of care options outside the hospital for those with acute PE. Reference: 1Aujesky D, Roy PM, Verschuren F, et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open‐label, randomised, non‐inferiority trial. Lancet. 2011;378:41‐8.
Reply
We thank you very much for your comments on this review. Please see below our responses:
1. We reviewed the domain related to blinding of personnel and participants and we decided to withdraw this domain from the risk of bias assessment because it is not applicable for this clinical question due to the nature of the interventions.
2. As per your paper on page 42 you quoted that "Data analysers were unmasked to treatment group assignment" but you did not specify that the data analyser was the same as the outcome assessor. As we used the risk of bias assessment according to Higgins 2011, we judged this domain as high risk of bias. However, as per your clarification we have changed this domain to low risk of bias.
3. As per your comment related to "very low quality evidence", this was classified according to the GRADE principles (Guyatt 2008) which recognize the precision of the confidence interval, sample size, publication bias and heterogeneity. However, most of these items were not addressed due to the fact there was only one included study. We have reclassified to 'low quality of evidence' following the reclassification of the risk of detection bias.
4. We revised the description of the trial from 'multicenter randomised double‐blind placebo‐controlled clinical trial' to 'an international, open‐label, randomised, non‐inferiority trial'.
Thank you very much.
Contributors
Feedback: Drahomir Aujesky MD, MSc and Donald M. Yealy MD
Reply: Dr Hugo Yoo, Dr Thais Queluz, and Regina El Dib, PhD
What's new
| Date | Event | Description |
|---|---|---|
| 16 February 2022 | New citation required but conclusions have not changed | Search updated. No new included studies, one new study excluded. Review text amended to reflect current Cochrane recommendations |
| 16 February 2022 | New search has been performed | Search updated. No new included studies, one new study excluded |
History
Protocol first published: Issue 8, 2012 Review first published: Issue 11, 2014
| Date | Event | Description |
|---|---|---|
| 26 March 2018 | New citation required but conclusions have not changed | A new search was run, which resulted in one new included study and two new excluded studies. New authors have joined the review team. There are no changes to the conclusions. |
| 26 March 2018 | New search has been performed | A new search was run, which resulted in one new included study and two new excluded studies. |
| 4 February 2015 | Feedback has been incorporated | The review authors have responded to the feedback submitted December 2014 and revised their review accordingly |
| 2 December 2014 | Feedback has been incorporated | Feedback has been submitted for this review. The review authors have been invited to respond to the feedback |
Acknowledgements
We express our gratitude to the study authors of Peacock 2018 for providing additional data. We express our gratitude to Regina El Dib and Thais Queluz for their important contribution to the first version of this review.
We would like to thank the Cochrane Vascular editorial base and editors for their help and input during the preparation of this review.
Appendices
Appendix 1. Geneva prediction score (GPS)
| Predictors | Point score |
| Cancer | +2 |
| Heart failure | +1 |
| Previous deep vein thrombosis | +1 |
| Systolic blood pressure < 100 mmHg | +2 |
| PaO2 < 8 kPa | +1 |
| Deep vein thrombosis shown by ultrasound | +1 |
| Total score | 0‐8 |
Patients with a total score ≤ 2 were assigned to the low‐risk category, and those with a total score ≥ 3 points to the high‐risk category
kPa: kilopascal PaO2: partial pressure of oxygen
Appendix 2. The Pulmonary Embolism Severity Index (PESI)
| Predictors | Points assigned |
| Age, per year | Age, in years |
| Male sex | +10 |
| History of cancer | +30 |
| History of heart failure | +10 |
| History of chronic lung disease | +10 |
| Pulse rate ≥ 110/minute | +20 |
| Systolic blood pressure < 100 mmHg | +30 |
| Respiratory rate ≥ 30/minutea | +20 |
| Temperature < 36 °C | +20 |
| Altered mental statusb | +60 |
| Arterial oxygen saturation < 90%a | +20 |
A total point score for a given patient is obtained by summing the patient's age in years and the points for each applicable predictor. Points assignments correspond with the following risk classes: ≤ 65 class I; 66‐85 class II; 86‐105 class III; 106‐125 class IV and > 125 class V. Patients in risk classes I and II are defined as low‐risk. aAssessed with or without the administration of supplemental oxygen. bDefined as confusion, disorientation or somnolence.
Appendix 3. The simplified version of the PESI (sPESI)
| Variable | Points assigned |
| Age > 80 years | 1 |
| History of cancer | 1 |
| Chronic cardiopulmonary disease | 1 |
| Pulse rate ≥ 110/minute | 1 |
| Systolic blood pressure < 100 mmHg | 1 |
| Arterial oxygen saturation < 90% | 1 |
A total point score for a given patient is obtained by summing the points. The score corresponds with the following risk classes: 0, low risk; 1 or more, high risk. Empty cells indicate that the variable was not included
Appendix 4. Hestia criteria
| Is the patient haemodynamically unstable?a |
| Is thrombolysis or embolectomy necessary? |
| Active bleeding or high risk of bleeding?b |
| > 24 h of oxygen supply to maintain oxygen saturation > 90%? |
| Is pulmonary embolism diagnosed during anticoagulant treatment? |
| Severe pain needing intravenous pain medication for > 24 h? |
| Medical or social reason for treatment in the hospital for > 24 h (infection, malignancy, no support system)? |
| Does the patient have a creatinine clearance of < 30 mL/min?c |
| Does the patient have severe liver impairment?d |
| Is the patient pregnant? |
| Does the patient have a documented history of heparin‐induced thrombocytopenia? |
Hestia rule interpretation: If the answer to one of the questions is yes, in‐hospital treatment is recommended (Hestia rule positive). If the answer to all the questions is no, outpatient treatment is recommended (Hestia rule negative).
aInclude the following criteria, but leave these to the discretion of the clinician: systolic blood pressure < 100 mmHg with heart rate > 100 beats/min; condition requiring admission to an intensive care unit. bGastrointestinal bleeding in the preceding 14 days, recent stroke (< 4 weeks ago), recent operation (< 2 weeks ago), bleeding disorder or thrombocytopenia (platelet count < 75 x 109/L), uncontrolled hypertension (systolic blood pressure > 180 mmHg or diastolic blood pressure > 110 mmHg). cCalculated creatinine clearance according to the Cockroft–Gault formula. dLeft to the discretion of the physician.
Appendix 5. British Thoracic Society exclusion criteria for outpatients management or early discharge
Haemodynamic instability (heart rate > 110 beats/min; systolic blood pressure < 100 mmHg; requirement for inotropes and critical care; requirement for thrombolysis or embolectomy)
Oxygen saturations < 90% on air
Active bleeding or risk of major bleeding (e.g. recent gastrointestinal bleed or surgery, previous intracranial bleeding, uncontrolled hypertension)
On full‐dose anticoagulation at the time of the pulmonary embolism
Severe pain (e.g. requiring opiates)
Other medical comorbidities requiring hospital admission
Chronic kidney disease stages 4 or 5 (estimated glomerular filtration rate < 30 mL/min) or severe liver disease
Heparin‐induced thrombocytopenia within the last year and where there is no alternative to repeating heparin treatment
Social reasons which may include inability to return home, inadequate care at home, lack of telephone communication, concerns over adherence, etc.
Appendix 6. Search strategies and databases searched
| Source | Search strategy | Hits retrieved |
| 1. VASCULAR REGISTER IN CRSO | PE treat AND INREGISTER AND INREGISTER AND Outpatient OR Outpatients OR Inpatient OR Inpatients OR Patient Care OR Ambulatory Care OR Home Nursing OR Hospitalization OR Outpatient Clinics | 31 May 2021: 42 |
| 2. CENTRAL via CRSO | #1 MESH DESCRIPTOR Thrombosis 1309 #2 MESH DESCRIPTOR Thromboembolism 953 #3 MESH DESCRIPTOR Venous Thromboembolism 311 #4 MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES 2108 #5 (thrombus* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*):TI,AB,KY 21426 #6 MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES 781 #7 (PE or DVT or VTE):TI,AB,KY 5796 #8 (((vein* or ven*) near thromb*)):TI,AB,KY 7539 #9 (blood near/3 clot* ):TI,AB,KY 0 #10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 25483 #11 MESH DESCRIPTOR Outpatients EXPLODE ALL TREES 1066 #12 MESH DESCRIPTOR Inpatients EXPLODE ALL TREES 782 #13 MESH DESCRIPTOR Patient Care EXPLODE ALL TREES 53193 #14 MESH DESCRIPTOR Ambulatory Care EXPLODE ALL TREES 3410 #15 MESH DESCRIPTOR Home Nursing EXPLODE ALL TREES 273 #16 MESH DESCRIPTOR Hospitalization EXPLODE ALL TREES 11800 #17 MESH DESCRIPTOR Outpatient Clinics, Hospital EXPLODE ALL TREES 626 #18 in‐patient:TI,AB,KY 5836 #19 inpatient:TI,AB,KY 6041 #20 hospitali*:TI,AB,KY 29944 #21 bed‐ridden:TI,AB,KY 22 #22 home:TI,AB,KY 22684 #23 out‐patient:TI,AB,KY 1341 #24 outpatient:TI,AB,KY 17880 #25 ambulatory*:TI,AB,KY 16079 #26 domicil*:TI,AB,KY 418 #27 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 123614 #28 #10 AND #27 3293 #29 01/01/2014 TO 26/03/2018:CD 408317 #30 #28 AND #29 1759 |
31 May 2021: 1970 |
| 3. Clinicaltrials.gov | Outpatient OR Outpatients OR Inpatient OR Inpatients OR Patient Care OR Ambulatory Care OR Home Nursing OR Outpatient Clinics | Pulmonary Embolism OR Acute Pulmonary Embolism OR Pulmonary Thromboembolism | Last update posted from 01/01/2014 to 03/28/2018 | 31 May 2021: 116 |
| 4. ICTRP Search Portal | Pulmonary Embolism OR Acute Pulmonary Embolism OR Pulmonary Thromboembolism OR Pulmonary AND Outpatient OR Outpatients OR Inpatient OR Inpatients OR Patient Care OR Ambulatory Care OR Home Nursing OR Hospitalization OR Outpatient Clinics | 31 May 2021: 29 |
| 5. MEDLINE | 1 THROMBOSIS/ 65220 2 THROMBOEMBOLISM/ 22449 3 Venous Thromboembolism/ 8046 4 exp Venous Thrombosis/ 51000 5 (thrombus* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*).ti,ab. 295531 6 exp Pulmonary Embolism/ 35920 7 (PE or DVT or VTE).ti,ab. 45444 8 ((vein* or ven*) adj thromb*).ti,ab. 60141 9 (blood adj3 clot*).ti,ab. 9925 10 or/1‐9 382689 11 exp OUTPATIENTS/ 13278 12 exp Patient Care/ 849726 13 exp Ambulatory Care/ 49708 14 exp Home Nursing/ 9116 15 exp Outpatient Clinics, Hospital/ 16494 16 in‐patient.ti,ab. 52003 17 inpatient.ti,ab. 63402 18 bed‐ridden.ti,ab. 459 19 exp INPATIENTS/ 17707 20 Home Nursing/ 8361 21 Outpatient Clinics, Hospital/ 15165 22 in‐patient.ti,ab. 52003 23 Inpatient*.ti,ab. 88382 24 bed‐ridden.ti,ab. 459 25 out‐patient.ti,ab. 9890 26 outpatient.ti,ab. 109086 27 ambulatory*.ti,ab. 70535 28 domicil*.ti,ab. 3802 29 or/11‐28 1093389 30 randomized controlled trial.pt. 456087 31 controlled clinical trial.pt. 92247 32 randomized.ab. 405922 33 placebo.ab. 187323 34 drug therapy.fs. 2001767 35 randomly.ab. 287027 36 trial.ab. 421562 37 groups.ab. 1775080 38 or/30‐37 4163193 39 (2017* or 2018*).ed. 1137739 40 10 and 29 and 38 and 39 653 41 from 40 keep 1‐653 653 |
31 May 2021: 2273 |
| 6. Embase | 1 thrombosis/ 93511 2 thromboembolism/ 49292 3 venous thromboembolism/ 30143 4 exp vein thrombosis/ 94140 5 (thrombus* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*).ti,ab. 336866 6 (PE or DVT or VTE).ti,ab. 66507 7 ((vein* or ven*) adj3 thromb*).ti,ab. 84338 8 (blood adj3 clot*).ti,ab. 9231 9 or/1‐8 465829 10 exp outpatient/ 90993 11 exp patient care/ 616173 12 exp ambulatory care/ 31732 13 exp home care/ 48012 14 exp outpatient department/ 45341 15 in‐patient.ti,ab. 79159 16 inpatient.ti,ab. 93577 17 bed‐ridden.ti,ab. 532 18 hospital patient/ 129991 19 exp home care/ 48012 20 exp outpatient department/ 45341 21 in‐patient.ti,ab. 79159 22 Inpatient*.ti,ab. 123955 23 bed‐ridden.ti,ab. 532 24 out‐patient.ti,ab. 12569 25 outpatient.ti,ab. 151131 26 ambulatory*.ti,ab. 73129 27 domicil*.ti,ab. 3148 28 or/10‐27 1066126 29 domicil*.ti,ab. 3148 30 controlled clinical trial/ 411625 31 random$.ti,ab. 1147104 32 randomization/ 69127 33 intermethod comparison/ 222434 34 placebo.ti,ab. 219134 35 (compare or compared or comparison).ti. 329390 36 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1585695 37 (open adj label).ti,ab. 61445 38 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 155643 39 double blind procedure/ 121111 40 parallel group$1.ti,ab. 19247 41 (crossover or cross over).ti,ab. 71025 42 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 244589 43 (assigned or allocated).ti,ab. 285412 44 (controlled adj7 (study or design or trial)).ti,ab. 256876 45 (volunteer or volunteers).ti,ab. 169739 46 trial.ti. 210016 47 or/29‐46 3403612 9 and 28 and 47 |
31 May 2021: 3365 |
| 7. CINAHL | S41 S39 AND S40 154 S40 EM 2017 OR EM 2018 291,195 S39 S25 AND S38 2,520 S38 S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 336,569 S37 (MH "Random Assignment") 37,362 S36 (MH "Single‐Blind Studies") or (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 32,494 S35 (MH "Crossover Design") 11,043 S34 (MH "Factorial Design") 911 S33 (MH "Placebos") 8,331 S32 (MH "Clinical Trials") 92,961 S31 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" (4,394 S30 TX crossover OR "cross‐over" 14,345 S29 AB placebo* 27,892 S28 TX random* 215,310 S27 TX trial* 246,180 S26 TX "latin square" 141 S25 S10 AND S24 12,647 S24 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 700,842 S23 TX "out‐patient" 1,421 S22 TX "in‐patient" 600,886 S21 TX domicil* 1,108 S20 TX ambulatory* 34,561 S19 TX outpatient 65,392 S18 TX bed‐ridden 41 S17 TX inpatient 86,288 S16 (MH "Ambulatory Care Facilities") 4,047 S15 (MH "Home Nursing") 2,937 S14 (MH "Ambulatory Care") 7,125 S13 (MH "Patient Care") 16,853 S12 (MH "Inpatients") 65,637 S11 (MH "Outpatients") 36,503 S10 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 45,072 S9 TX blood n3 clot* 890 S8 TX ((vein* or ven*) n2 thromb*) 11,142 S7 TX PE or DVT or VTE 11,729 S6 (MH "Pulmonary Embolism") 4,685 S5 TX thrombus* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* 35,636 S4 (MH "Venous Thrombosis+") 6,338 S3 (MH "Venous Thromboembolism") 3,034 S2 (MH "Thromboembolism") 3,207 S1 (MH "Thrombosis") 4,590 |
31 May 2021: 974 |
| 8. AMED | 1 THROMBOSIS/ 198 2 THROMBOEMBOLISM/ 72 3 Venous Thromboembolism/ 0 4 exp Venous Thrombosis/ 0 5 (thrombus* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*).ti,ab. 638 6 exp Pulmonary Embolism/ 53 7 (PE or DVT or VTE).ti,ab. 243 8 ((vein* or ven*) adj thromb*).ti,ab. 308 9 (blood adj3 clot*).ti,ab. 34 10 or/1‐9 863 11 exp OUTPATIENTS/ 317 12 exp Patient Care/ 5736 13 exp Ambulatory Care/ 414 14 exp Home Nursing/ 615 15 exp Outpatient Clinics, Hospital/ 87 16 in‐patient.ti,ab. 23780 17 inpatient.ti,ab. 2899 18 bed‐ridden.ti,ab. 7 19 exp INPATIENTS/ 414 20 Home Nursing/ 515 21 Outpatient Clinics, Hospital/ 86 22 in‐patient.ti,ab. 23780 23 Inpatient*.ti,ab. 3501 24 bed‐ridden.ti,ab. 7 25 out‐patient.ti,ab. 23780 26 outpatient.ti,ab. 2926 27 ambulatory*.ti,ab. 1186 28 domicil*.ti,ab. 119 29 or/11‐28 33684 30 10 and 29 246 31 exp CLINICAL TRIALS/ 3711 32 RANDOM ALLOCATION/ 314 33 DOUBLE BLIND METHOD/ 643 34 Clinical trial.pt. 1205 35 (clinic* adj trial*).tw. 5324 36 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 2782 37 PLACEBOS/ 583 38 placebo*.tw. 3070 39 random*.tw. 17240 40 PROSPECTIVE STUDIES/ 1047 41 or/31‐40 22174 42 30 and 41 28 |
31 May 2021: 5 |
| TOTAL before de‐duplication | 8774 | |
Data and analyses
Comparison 1. Outpatient versus inpatient treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Short‐term all‐cause mortality | 2 | 453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.98] |
| 1.1.1 Inpatients and outpatients with same treatment regimens | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.98] |
| 1.1.2 Inpatients and outpatients treated with different treatment regimens | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.2 Long‐term all‐cause mortality | 2 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.58] |
| 1.2.1 Inpatients and outpatients with same treatment regimens | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.58] |
| 1.2.2 Inpatients and outpatients treated with different treatment regimens | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.3 Major bleeding within 14 days | 2 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [0.24, 101.57] |
| 1.3.1 Inpatients and outpatients with same treatment regimens | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [0.24, 101.57] |
| 1.3.2 Inpatients and outpatients treated with different treatment regimens | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4 Major bleeding within 90 days | 2 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.88 [0.36, 132.14] |
| 1.4.1 Inpatients and outpatients with same treatment regimens | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.88 [0.36, 132.14] |
| 1.4.2 Inpatients and outpatients treated with different treatment regimens | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5 Minor bleeding | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.07, 16.79] |
| 1.6 Recurrent pulmonary embolism within 90 days | 2 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.12, 71.85] |
| 1.6.1 Inpatients and outpatients with same treatment regimens | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.12, 71.85] |
| 1.6.2 Inpatients and outpatients treated with different treatment regimens | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.7 Satisfaction questionnaire | 2 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.04] |
| 1.7.1 Inpatients and outpatients treated with same treatment regimens | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.92, 1.03] |
| 1.7.2 Inpatients and outpatients with different treatment regimens | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aujesky 2011.
| Study characteristics | ||
| Methods | Design: international, open‐label, randomised, non‐inferiority trial Multicentre study: 19 EDs in Switzerland, France, Belgium, and USA Period: February 2007 to June 2010 Sample size: justified (160 participants per treatment group would provide 80% power to detect a non‐inferiority margin of 4% using a 1‐sided α of 0.05, assuming a 5% drop‐out rate). Follow‐up: 90 days after randomisation | |
| Participants | 344 eligible participants randomised, but only 339 included in primary analysis; 337 completed follow‐up and 317 were included in per‐protocol analysis. In the outpatient group (N = 171): 84 men, 87 women, mean age 47 years; inpatient group (N = 168): 85 men, 83 women, mean age 49 years Inclusion criteria: aged ≥ 18 years with acute, symptomatic and objectively verified PE who were at low risk of death based on PESI (risk classes I or II) Exclusion criteria: arterial hypoxaemia, SBP < 100 mmHg, chest pain necessitating parenteral opioids, active bleeding, high risk of bleeding, gastrointestinal bleeding, severe renal failure, extreme obesity, history of HIT or allergy to heparins, therapeutic oral anticoagulation at the time of diagnosis of PE, pregnancy, diagnosis of PE > 23 hours before the time of screening |
|
| Interventions | Outpatient (N = 172): subcutaneous enoxaparin 1 mg/kg twice daily and discharged from the ED within 24 hours of randomisation. If self‐injection was not possible, a study nurse either taught a caregiver to give the enoxaparin or arranged administration by a visiting nurse Inpatient (N = 172): subcutaneous enoxaparin 1 mg/kg twice daily and admitted to hospital All participants also received vitamin K antagonist therapy. |
|
| Outcomes | Primary outcome: recurrence of symptomatic confirmed VTE defined as recurrent PE or new or recurrent DVT within 90 days of randomisation Secondary outcomes: overall satisfaction, major bleeding within 14 and 90 days of randomisation, all‐cause mortality within 90 days |
|
| Funding | Quote: "This study was supported by grants from the Swiss National Science Foundation (3200B0‐1121), the Programme Hospitalier de Recherche Clinique 2007 (21‐03), and the US National Heart, Lung, and Blood Institute (1R01HL085565‐01A2). Sanofi ‐Aventis provided free drug supply in the participating European centres." | |
| Declarations of interest | Quote: "DA has received honoraria from Sanofi‐Aventis and Bayer. PMR has received grants, honoraria, consultancy fees, and payments from Glaxo SmithKline, Sanofi‐Aventis, Bayer, Biomérieux, Boehringer Ingelheim, Eli Lilly, and LFB Biomédicaments. PV has received grants, consultancy fees, and payments from Sanofi‐Aventis, Bayer, Boehringer Ingelheim, Daiichi‐Sankyo, ThromboGenics, Leo, and Pfizer. OH has received grants from Pfizer and MSD. HJB has received honoraria from Sanofi‐Aventis, Bayer, Boehringer Ingelheim, Menarini, and GlaxoSmithKline." | |
| Notes | Diagnostic criteria for recurrent PE were: a new intraluminal filling defect on spiral CT or pulmonary angiography, a new perfusion defect of a lung segment with corresponding normal ventilation by lung scan, or confirmation of a new PE on autopsy. Diagnostic criteria for DVT were: the non‐compressibility of a new venous segment or a substantial increase (≥ 4 mm) in the diameter of the thrombus during full compression in a previously abnormal segment on ultrasonography, or a new intraluminal filling defect on contrast venography. Overall satisfaction was assessed by a non‐validated 5‐point Likert scale questionnaire. Participants completed this questionnaire by telephone 14 days after randomisation. Major bleeding was defined as fatal bleeding, bleeding at critical sites (i.e. intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular, pericardial or intramuscular with compartment syndrome), or bleeding with a reduction of haemoglobin of ≥ 20 g/L or resulting in transfusion of ≥ 2 units of packed red cells. Authors of the study declared they received grants, honoraria, consultancy fees, and payments from the pharmaceutical industry that sponsored the study. However, both outpatients and inpatients received the same treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The eligible patients were allocated to outpatient treatment or inpatient treatment groups in a 1:1 ratio with a randomised block design generated from a password protected computer web page. |
| Allocation concealment (selection bias) | Low risk | The patients were stratified by site and using small fixed block sizes (2 or 4). Quote: "To balance recruitment in time and preclude enrolment bias, the blocks varied randomly from two to four patients". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although the paper says that the analysts were unmasked to treatment group assignment, there was a committee unaware of treatment assignment that confirmed all outcomes. Quote: "A committee of three clinical experts from the University Hospital of Lausanne (Switzerland) who were unaware of treatment assignment confirmed all outcomes and classified the cause of all deaths as definitely due to pulmonary embolism, possibly due to pulmonary embolism (e.g., sudden death without obvious cause), due to major bleeding, or due to another cause." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | < 20% in each group: outpatient group had 2 participants who did not complete follow‐up and inpatient group had 5 participants who did not complete follow‐up. The study authors were unable to confirm how many were alive. |
| Selective reporting (reporting bias) | Low risk | There is no evidence of selective reporting. |
| Other bias | Low risk | We did not find aspects of methodology that might be been influenced by vested interests or which could lead directly to a risk of bias. |
Peacock 2018.
| Study characteristics | ||
| Methods | Design: international, open‐label, randomised, parallel group, multicentre Multicentre study: 35 sites in the USA Sample size: 114 Follow‐up: 90 days after randomisation | |
| Participants | Inclusion criteria: adult patients (age ≥ 18 years) with objectively confirmed PE (with or without symptomatic DVT) deemed to be at low risk for recurrent VTE, major bleeding, or all‐cause mortality based on Hestia criteria, and a life expectancy of at least 6 months. The authors adapted the Hestia criteria by removing the 24‐hour time markers. Exclusion criteria: women of childbearing age with no use of a highly effective birth control method, patients with any Hestia criteria present, any concomitant contraindicated medications, and individuals with contraindications to anticoagulant therapy, allergies to rivaroxaban, or with barriers to treatment adherence or follow‐up |
|
| Interventions | Intervention (N = 51): outpatient treatment with rivaroxaban 15 mg orally twice daily for the first 21 days followed by 20 mg orally once daily for approximately 69 days, for a total treatment duration of 90 days. Comparison (N = 63): local standard of care, participants received local standard of care according to local protocol and defined by the medical team caring for the participant, which typically involves bridging therapy and hospitalisation, but also any of the NOACs. |
|
| Outcomes |
|
|
| Funding | Quote: "Funding for this research was provided by Janssen Pharmaceuticals, Raritan, NJ" | |
| Declarations of interest | Quote: "Consulting for commercial interests, including advisory board work—WFP, CC, DD, and AS have received funding personally from Janssen for consulting. WFP, and CC have received funding personally from Bayer, AG. JK has received grant funding from Janssen for a separate study. Payment for writing independent of grant funding—No author received payment from [sic] for writing any part of this manuscript. Employment—PW and JX are employed by Janssen, which manufactures rivaroxaban. Institutional Grant Receipt—WFP, CC, DD, SF, CKa, CKe, JM, and AS’s institution has received funding from Janssen for this investigator‐initiated research. Miscellaneous—DD has been a member of the SAEM board of directors." | |
| Notes | The authors used the Hestia criteria to classify patients, therefore we considered that most patients were symptomatic. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | They used an interactive web system. Quote: "After obtaining written informed consent, patients were randomly assigned in a 1:1 ratio to ED discharge on open‐label rivaroxaban or standard care (as determined by the attending physician) by an interactive Web system within 12 hours of diagnosis." |
| Allocation concealment (selection bias) | Low risk | They used an interactive web system. Quote: "After obtaining written informed consent, patients were randomly assigned in a 1:1 ratio to ED discharge on open‐label rivaroxaban or standard care (as determined by the attending physician) by an interactive Web system within 12 hours of diagnosis." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The analysers were masked to treatment group assignment. Quote: "Principal investigators and outcome adjudicators were masked to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Less than 20% of dropouts and withdrawals (7 participants in the outpatient group and 8 participants in the inpatient group); however, the authors did not perform intention‐to‐treat analysis. All outpatients completed the study, and authors could confirm that all of them were alive; however, they could not confirm this for two patients in the inpatient group. |
| Selective reporting (reporting bias) | Low risk | There is no evidence of selective reporting. |
| Other bias | Low risk | We did not find aspects of methodology that might be been influenced by vested interests and which may lead directly to a risk of bias. However, comparison of two sites of care (inpatient versus outpatient) was imbalanced by different pharmacotherapy between the arms: the outpatient group received 15 mg oral rivaroxaban twice daily for the first 21 days, followed by 20 mg oral rivaroxaban once daily for approximately 69 days for a total treatment duration of 90 days. The inpatient comparison group received local standard‐of‐care, according to local protocol and defined by the medical team caring for the participant, which typically involved intravenous UFH or subcutaneous LMWH and hospitalisation, but also included any of the NOACs (75% of all patients were initially treated with unfractionated or low‐molecular‐weight heparin but ultimately received NOACs, most commonly rivaroxaban (51%) or apixaban (25%)). |
ACTS: anti‐clot treatment scale; CT: computed tomography; DVT: deep vein thrombosis; ED: emergency department; HIT: heparin‐induced thrombocytopenia; ISTH: International Society on Thrombosis and Haemostasis;LMWH: low molecular weight heparin; NOACs: non‐vitamin K antagonist oral anticoagulants; PE: pulmonary embolism;PESI: Pulmonary Embolism Severity Index; SBP: systolic blood pressure; UFH: unfractionated heparin; VTE: venous thromboembolism.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Den Exter 2016a | Participants were randomised to either outpatient treatment or to management based on NT‐proBNP levels, and not to either outpatient or inpatient management. |
| HOME Study | Participants were randomised to either Hestia or PESI management, and not randomised to either inpatient or outpatient treatment. |
| Kovacs 2003 | RCT which evaluated different doses of warfarin in outpatients and not randomised to outpatient or inpatient treatment |
| Medina 2017 | Not randomised to inpatient or outpatient treatment (post‐hoc analysis) |
| Otero 2010 | RCT considered early discharge to be at 3 to 5 days from admission |
| Zondag 2011 | Cohort study |
NT‐proBNP: N‐terminal pro‐brain natriuretic peptide;PESI: pulmonary embolism severity index; RCT: randomised controlled trial.
Differences between protocol and review
2022 version
In Types of outcome measures, we included the short‐term mortality period 'to 30 days' in addition to '10 days' for completeness.
2019 version
In Types of participants, the measurement tools that aim to classify mortality risk rate was expanded to be as inclusive as possible. The age of participants was amended from > 18 years to ≥ 18 years. In Types of outcome measures, we amended the length of short‐term mortality from 30 days to 7 to 10 days to reflect more accurately the short‐term nature. In Subgroup analysis and investigation of heterogeneity we included inpatients and outpatients with same treatment regimens versus inpatients and outpatients treated with different treatment regimens.
In the single study included in the previous version of this review, both inpatients and outpatients received the same treatment regimen. For this update, the inpatient and outpatient groups in a new included study received different treatment regimens. We have added this subgroup analysis to assess any potential effects.
2014 version
The objective was rephrased according to Cochrane guidelines. In Types of participants, the definition of acute pulmonary embolism was amended to be as inclusive as possible. In Types of interventions, the definition of 'outpatients' was amended to reflect true cases of outpatients only and avoid confusion with 'early discharge' and 'outpatients'.
Contributions of authors
HHBY: conceiving the review, undertaking manual searches, screening search results, organising retrieval of papers, screening retrieved papers against inclusion criteria, abstracting data from papers, writing to authors of papers for additional information, obtaining additional data about papers, obtaining and screening data on unpublished studies, data management for the review, double‐entry of data, interpretation of data, statistical inferences, writing the review, acting as guarantor for the review, reading and checking review before submission VSNN: screening search results, screening retrieved papers against inclusion criteria, appraising the quality of papers, abstracting data from papers, data management for the review, statistical data analysis, double‐entry of data, interpretation of data, statistical inferences, writing the review, reading and checking review before submission PJFVB: screening search results, screening retrieved papers against inclusion criteria, appraising quality of papers, abstracting data from papers, data management for the review, data entry, interpretation of data, statistical inferences, writing the review, reading and checking review before submission CB: co‐ordinating the review, screening retrieved papers against inclusion criteria, writing the review, reading and checking review before submission
Sources of support
Internal sources
-
São Paulo State University‐UNESP, Brazil
RENOVE‐0108/008/13‐PROPe/CDC
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
HHBY: none known. VSNN: none known. PJFVB: reports he has a private healthcare practice. CB: none known. As CB is based within Cochrane Vascular, editorial tasks for this review update were carried out by other members of the Cochrane Vascular editorial team.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aujesky 2011 {published data only}
- Aujesky D, Roy PM, Verschuren F, Righini M, Osterwalder J, Egloff M, et al.An international, randomized non-inferiority trial of outpatient versus inpatient treatment for pulmonary embolism. Journal of General Internal Medicine 2011;26(10):1220. [Google Scholar]
- Aujesky D, Roy PM, Verschuren F, Righini M, Osterwalder J, Egloff M, et al.Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet 2011;378(9785):41-8. [DOI] [PubMed] [Google Scholar]
Peacock 2018 {published data only}
- NCT02584660.A study of rivaroxaban for early discharge of low risk pulmonary embolism from the emergency department (MERCURY PE). clinicaltrials.gov/ct2/show/NCT02584660 (first received 22 October 2015).
- Peacock WF, Coleman CI, Diercks DB, Francis S, Kabrhel C, Keay C, et al.Emergency department discharge of pulmonary embolus patients. Academic Emergency Medicine 2018;25(9):995-1003. [DOI] [PMC free article] [PubMed]
- Singer AJ, Xiang J, Kabrhel C, Merli GJ, Pollack C, Tapson VF, et al.MulticEnter trial of Rivaroxaban for early disCharge of pUlmonaRY embolism from the Emergency Department (MERCURY PE): rationale and design. Academic Emergency Medicine 2016;23(11):1280-6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Den Exter 2016a {published data only}
- Den Exter PL, Zondag W, Klok FA, Brouwer RE, Dolsma J, Eijsvogel M, et al.Efficacy and safety of outpatient treatment based on the Hestia clinical decision rule with or without N-terminal pro–brain natriuretic peptide testing in patients with acute pulmonary embolism. American Journal of Respiratory and Critical Care Medicine 2016;194(8):998-1006. [DOI] [PubMed] [Google Scholar]
HOME Study {published data only}
- Roy PM, Gable B.Hospitalization or Out-treatment ManagEment of Patients with Pulmonary Embolism: a randomized controlled trial (HOME-PE). clinicaltrials.gov/ct2/show/NCT02811237 (first received 23 June 2016).
Kovacs 2003 {published data only}
- Kovacs MJ, Rodger M, Anderson DR, Morrow B, Kells G, Kovacs J, et al.Comparison of 10-mg and 5-mg warfarin initiation nomograms together with low-molecular-weight heparin for outpatient treatment of acute venous thromboembolism. A randomized, double-blind, controlled trial. Annals of Internal Medicine 2003;138(9):714-9. [DOI] [PubMed] [Google Scholar]
Medina 2017 {published data only}
- Medina A, Raskob G, Ageno W, Cohen AT, Brekelmans MP, Chen CZ, et al.Outpatient management in patients with venous thromboembolism with edoxaban: a post hoc analysis of the Hokusai-VTE study. Thrombosis and Haemostasis 2017;117(12):2406-14. [DOI: 10.1160/TH17-05-0523] [DOI] [PMC free article] [PubMed] [Google Scholar]
Otero 2010 {published data only}
- Otero R, Uresandi F, Jiménez D, Cabezudo MA, Oribe M, Nauffal D, et al.Home treatment in pulmonary embolism. Thrombosis Research 2010;126(1):e1-5. [DOI] [PubMed] [Google Scholar]
Zondag 2011 {published data only}
- Zondag W, Mos IC, Creemers-Schild D, Hoogerbrugge AD, Dekkers OM, Dolsma J, et al.Outpatient treatment in patients with acute pulmonary embolism: the Hestia Study. Journal of Thrombosis and Haemostasis 2011;9(8):1500-7. [DOI] [PubMed] [Google Scholar]
Additional references
Agterof 2010
- Agterof MJ, Schutgens RE, Snijder RJ, Epping G, Peltenburg HG, Posthuma EF, et al.Out of hospital treatment of acute pulmonary embolism in patients with a low NT-proBNP level. Journal of Thrombosis and Haemostasis 2010;8(6):1235-41. [DOI] [PubMed] [Google Scholar]
Aujesky 2005
- Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J, et al.Derivation and validation of a prognostic model for pulmonary embolism. American Journal of Respiratory and Critical Care Medicine 2005;172(8):1041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aujesky 2007
- Aujesky D, Perrier A, Roy PM, Stone RA, Cornuz J, Meyer G, et al.Validation of a clinical prognostic model to identify low-risk patients with pulmonary embolism. Journal of Internal Medicine 2007;261(6):597-604. [DOI] [PubMed] [Google Scholar]
Baglin 2010
- Baglin T.Fifty per cent of patients with pulmonary embolism can be treated as outpatients. Journal of Thrombosis and Haemostasis 2010;8(11):2404-5. [DOI] [PubMed] [Google Scholar]
Barco 2019
- Barco S, Mahmoudpour SH, Planquette B, Sanchez O, Konstantinides SV, Meyer G.Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. European Heart Journal 2019;40(11):902-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barco 2020
- Barco S, Mahmoudpour SH, Valerio L, Klok FA, Münzel T, Middeldorp S, et al.Trends in mortality related to pulmonary embolism in the European Region, 2000-15: analysis of vital registration data from the WHO Mortality Database. Lancet Respiratory Medicine 2020;8(3):277-87. [DOI] [PubMed] [Google Scholar]
Barra 2013
- Barra SN, Paiva L, Providência R, Fernandes A, Marques AL.A review on state-of-the-art data regarding safe early discharge following admission for pulmonary embolism: what do we know? Clinical Cardiology 2013;36(9):507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Becattini 2020
- Becattini C, Agnelli G.Acute treatment of venous thromboembolism. Blood 2020;135(5):305-16. [DOI] [PubMed] [Google Scholar]
Bledsoe 2018
- Bledsoe J, Woller S, Stevens S, Aston V, Patten R, Allen T, et al.Management of low-risk pulmonary embolism (LoPE) patients without hospitalization: the LoPE prospective management study. Chest 2018;154(2):249-56. [DOI] [PubMed] [Google Scholar]
Buller 2003
- Buller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, et al.Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. New England Journal of Medicine 2003;349(18):1695-702. [DOI] [PubMed] [Google Scholar]
Cohen 2007
- Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al.Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thrombosis and Haemostasis 2007;98(4):756-64. [DOI] [PubMed] [Google Scholar]
Dasta 2015
- Dasta JF, Pilon D, Mody SH, Lopatto K, Laliberté G, Germain G, et al.Daily hospitalization costs in patients with deep vein thrombosis or pulmonary embolism treated with anticoagulant therapy. Thrombosis Research 2015;135(2):303-10. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG (editors).Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2/chapter-10.
Den Exter 2016b
- den Exter PL, Zondag W, Klok FA, Brouwer RE, Dolsma J, Eijsvogel M, et al.Efficacy and safety of outpatient treatment based on the Hestia clinical decision rule with or without N-terminal pro–brain natriuretic peptide testing in patients with acute pulmonary embolism. American Journal of Respiratory and Critical Care Medicine 2016;194(8):998-1006. [DOI] [PubMed] [Google Scholar]
Erkens 2010
- Erkens PM, Gandara, Wells P, Shen AY, Bose G, Le Gal G, et al.Safety of outpatient treatment in acute pulmonary embolism. Journal of Thrombosis and Haemostasis 2010;8:2412-7. [DOI] [PubMed] [Google Scholar]
Fanikos 2013
- Fanikos J, Rao A, Seger AC, Carter D, Piazza G, Goldhaber SZ.Hospital costs of acute pulmonary embolism. American Journal of Medicine 2013;126(2):127-32. [DOI] [PubMed] [Google Scholar]
Ghazvinian 2018
- Ghazvinian R, Gottsäter A, Elf JL.Efficacy and safety of outpatient treatment with direct oral anticoagulation in pulmonary embolism. Journal of Thrombosis and Thrombolysis 2018;45:319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT.Version accessed 1 November 2018. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ.What is "quality of evidence" and why is it important to clinicians? BMJ 2008;336:995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heit 2005
- Heit JA, Cohen AT, Anderson FA.VTE Impact Assessment Study Group. Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the US. Blood 2005;106(11):267a. [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JAC.Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Howard 2018
- Howard LS, Barden S, Condliffe R, Connolly V, Davies CW, Donaldson J, et al.British Thoracic Society Guideline for the initial outpatient management of pulmonary embolism (PE). Thorax 2018;73:ii1-ii29. doi:10.1136/thoraxjnl-2018-211539. [DOI] [PubMed] [Google Scholar]
Ibrahim 2008
- Ibrahim SA, Stone RA, Obrosky DS, Geng M, Fine MJ, Aujesky D.Thrombolytic therapy and mortality in patients with acute pulmonary embolism. Archives of Internal Medicine 2008;168(20):2183-90. [DOI] [PubMed] [Google Scholar]
Jara‐Palomares 2019
- Jara-Palomares L, Alfonso M, Maestre A, Jimenez D, Garcia-Bragado F, Font C, et al.Comparison of seven prognostic tools to identify low-risk pulmonary embolism in patients aged <50 years. Scientific Reports 2019;9:20064. [DOI: 10.1038/s41598-019-55213-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiménez 2007
- Jiménez D, Yusen RD, Otero R, Uresandi F, Nauffal D, Laserna E, et al.Prognostic models for selecting patients with acute pulmonary embolism for initial outpatient therapy. Chest 2007;132(1):24-30. [DOI] [PubMed] [Google Scholar]
Jiménez 2010
- Jimenez D, Aujesky D, Moores L, Gomez V, Lobo JL, et al.Simplification of the Pulmonary Embolism Severity Index for prognostication in patients with acute symptomatic pulmonary embolism. Archieves of Internal Medicine 2010;170(15):1383-9. [DOI] [PubMed] [Google Scholar]
Jiménez 2016
- Jiménez D, Miguel-Diez J, Guijarro R, Trujillo-Santos J, Otero R, Barba R, et al.Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE registry. Journal of the American College of Cardiology 2016;67(2):162-70. [DOI] [PubMed] [Google Scholar]
Kabrhel 2021
- Kabrhel C, Vinson DR, Mitchell AM, Rosovsky RP, Chang AM, Hernandez-Nino J, et al.A clinical decision framework to guide the outpatient treatment of emergency department patients diagnosed with acute pulmonary embolism or deep vein thrombosis: results from a multidisciplinary consensus panel. Journal of the American College of Emergency Physicians 2021;2(6):e12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kearon 2008
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ, American College of Chest Physicians.Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133(6 Suppl):454S-545S. [DOI] [PubMed] [Google Scholar]
Kearon 2012
- Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al.Antithrombotic therapy for VTE disease. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guideline. Chest 2012;141(2 Suppl):e419S-94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kearon 2016
- Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al.Antithrombotic therapy for VTE disease and expert panel report (10th edition). Chest 2016;149(2):315-52. [DOI] [PubMed] [Google Scholar]
Kline 2016
- Kline JA, Kahler JP, Beam DM.Outpatient treatment of low-risk venous thromboembolism with monotherapy oral anticoagulation: patient quality of life outcomes and clinician acceptance. Patient Preference and Adherence 2016;10:561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Konstantinides 2014
- Konstantinides S, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al.2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. European Heart Journal 2014;35(43):3033–73. [DOI: 10.1093/eurheartj/ehu283] [DOI] [PubMed] [Google Scholar]
Konstantinides 2019
- Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al.2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). European Respiratory Journal 2019;54(3):1901647. [DOI] [PubMed] [Google Scholar]
Kovacs 2010
- Kovacs MJ, Hawel JD, Rekman JF, Lazo-Langner A.Ambulatory management of pulmonary embolism: a pragmatic evaluation. Journal of Thrombosis and Haemostasis 2010;8(11):2406-11. [DOI] [PubMed] [Google Scholar]
Lankeit 2011
- Lankeit M, Jiménez D, Kostrubiec M, Dellas C, Hasenfuss G, Pruszczyk P, et al.Predictive value of the high-sensitivity troponin T assay and the simplified Pulmonary Embolism Severity Index in hemodynamically stable patients with acute pulmonary embolism: a prospective validation study. Circulation 2011;124(24):2716-24. [DOI] [PubMed] [Google Scholar]
Laporte 2008
- Laporte S, Mismetti P, Décousus H, Uresandi F, Otero R, Lobo JL, et al.Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation 2008;117(13):1711-6. [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al.Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Mansour 2017
- Mansour S, Alotaibi G, Wu C, McMurtry MS.Trends in admission rates and in-hospital stay for venous thromboembolism. Thrombosis Research 2017;156:149-54. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ.Intention-to-treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837-41. [DOI] [PubMed] [Google Scholar]
NICE 2020
- National Institute for Health and Care Excellence (NICE).Venous thromboembolic diseases: diagnosis, management and thrombophilia testing NG158. nice.org.uk/guidance/ng158 (accessed 16 February 2022).
Othieno 2018
- Othieno R, Okpo E, Forster R.Home versus in-patient treatment for deep vein thrombosis. Cochrane Database of Systematic Reviews 2018, Issue 1. Art. No: CD003076. [DOI: 10.1002/14651858.CD003076.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Piran 2012
- Piran S, Gal GL, Wells P, Righini M, Carrier M.Outpatient treatment of symptomatic pulmonary embolism: a systematic review and meta-analysis. Blood (ASH Annual Meeting Abstracts) 2012;120:361. [DOI] [PubMed] [Google Scholar]
Piran 2013
- Piran S, Le Gal G, Wells PS, Gandara E, Righini M, Rodger MA, et al.Outpatient treatment of symptomatic pulmonary embolism: a systematic review and meta-analysis. Thrombosis Research 2013;132(5):515-9. [DOI] [PubMed] [Google Scholar]
Pollack 2011
- Pollack CV, Schreiber D, Goldhaber SZ, Slattery D, Fanikos J, O'Neil BJ, et al.Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry). Journal of the American College of Cardiology 2011;57(6):700-6. [DOI] [PubMed] [Google Scholar]
Quinlan 2004
- Quinlan DJ, McQuillan A, Eikelboom JW.Low-molecular weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: a meta-analysis of randomized, controlled trials. Annals of Internal Medicine 2004;140(3):175-83. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5).Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Roy 2017
- Roy PM, Moumneh T, Penaloza A, Sanchez.Outpatient management of pulmonary embolism. Thrombosis Research 2017;157:92-100. [DOI] [PubMed] [Google Scholar]
Roy 2021
- Roy PM, Penaloza A, Hugli O, Klok FA, Arnoux A, Elias A, et al.Triaging acute pulmonary embolism for home treatment by Hestia or simplified PESI criteria: the HOME-PE randomized trial. European Heart Journal 2021;42(33):3146-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulman 2005
- Schulman S, Kearon C.Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. Journal of Thrombosis and Haemostasis 2005;3(4):692-4. [DOI] [PubMed] [Google Scholar]
Segal 2007
- Segal JB, Streiff MB, Hofmann LV, Thornton K, Bass EB.Management of venous thromboembolism: a systematic review for a practice. Annals of Internal Medicine 2007;146(3):211-22. [DOI] [PubMed] [Google Scholar]
Squizzato 2009
- Squizzato A, Galli M, Dentali F, Ageno W.Outpatient treatment and early discharge of symptomatic pulmonary embolism: a systematic review. European Respiratory Journal 2009;33(5):1148-55. [DOI] [PubMed] [Google Scholar]
Stein 2016
- Stein PD, Matta F, Hughes PG, Hourmouzis ZN, Hourmouzis NP, White RM, et al.Home treatment of pulmonary embolism in the era of novel oral anticoagulants. American Journal of Medicine 2016;129:974-7. [DOI] [PubMed] [Google Scholar]
Stevens 2021
- Stevens SM, Woller SC, Baumann Kreuziger L, Bounameaux H, Doerschug K, Geersing GJ, et al.Executive Summary: antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest 2021;160(6):2247-59. [DOI] [PubMed] [Google Scholar]
Van der Wall 2018
- Wall SJ, Hendriks SV, Huisman MV, Klok FA.Home treatment of acute pulmonary embolism: state of the art in 2018. Current Opinion in Pulmonary Medicine 2018;24(5):425-31. [DOI] [PubMed] [Google Scholar]
Vinson 2012
- Vinson DR, Zehtabchi S, Yealy DM.Can selected patients with newly diagnosed pulmonary embolism be safely treated without hospitalization? A systematic review. Annals of Emergency Medicine 2012;60(5):651-62. [DOI] [PubMed] [Google Scholar]
Vinson 2018
- Vinson DR, Mark DG, Chettipally UK, Huang J, Rauchwerger AS, Reed ME, et al.Increasing safe outpatient management of emergency department patients with pulmonary embolism a controlled pragmatic trial. Annals of Internal Medicine 2018;169(12):855-65. [DOI] [PubMed] [Google Scholar]
Wicki 2000
- Wicki J, Perrier A, Perneger TV, Bounameaux H, Junod AF.Predicting adverse outcome in patients with acute pulmonary embolism: a risk score. Thrombosis and Haemostasis 2000;84(4):548-52. [PubMed] [Google Scholar]
Zondag 2012
- Zondag W, Kooiman J, Klok F, Dekkers O, Huisman M.Outpatient versus inpatient treatment in patients with pulmonary embolism: a meta-analysis. European Respiratory Journal 2012;42(1):134-44. [DOI] [PubMed] [Google Scholar]
Zondag 2013
- Zondag W, den Exter PL, Crobach MJT, Dolsma A, Donker ML, et al.Comparison of two methods for selection of out of hospital treatment in patients with acute pulmonary embolism. Thrombosis and Haemostasis 2013;109:47-52. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Yoo 2012
- Yoo HHB, Queluz THAT, El Dib RP.Outpatient versus inpatient treatment for acute pulmonary embolism. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD010019. [DOI: 10.1002/14651858.CD010019] [DOI] [PubMed] [Google Scholar]
Yoo 2014
- Yoo HHB, Queluz THAT, El Dib R.Outpatient versus inpatient treatment for acute pulmonary embolism. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD010019. [DOI: 10.1002/14651858.CD010019.pub2] [DOI] [PubMed] [Google Scholar]


