Abstract
Objective:
To assess disparities in hypoxemia detection by pulse oximetry across self-identified racial groups and associations with clinical outcomes.
Design:
Observational cohort study from May 5, 2018 through December 31, 2020.
Setting:
Three academic medical centers in the United States.
Patients:
Adults ≥18 years who self-identified as White, Black, Asian, or American Indian admitted to the intensive care unit (ICU) or undergoing surgery during inpatient hospitalization with simultaneous measurements of pulse oximetry-estimated oxygen saturation (i.e. SpO2) and arterial blood gas-derived oxygen saturation (i.e. SaO2).
Interventions:
None.
Measurements and Main Results:
Multivariable models were employed to assess the relationships between race, occult hypoxemia (i.e. SaO2 <88% despite SpO2 ≥92%), and clinical outcomes of hospital mortality and hospital-free days. 128,285 paired SpO2-SaO2 measurements were included from 26,603 patients. SpO2 on average overestimated SaO2 by 1.57% (1.54–1.61%). Black, Asian, and American Indian patients were more likely to experience occult hypoxemia during hospitalization (estimated probability 6.2% [5.1–7.6%], 6.6% [4.9–8.8%], and 6.6% [4.4–10.0%], respectively) compared to White patients (3.6% [3.4–3.8%]). Black patients had increased odds of occult hypoxemia compared to White patients after adjustment (OR 1.65 [1.28, 2.14]; p<0.001). Differences in occult hypoxemia between Asian and American Indian patients compared to White patients were not significant after adjustment (OR 1.53 [0.95, 2.47]; p=0.077, and OR 1.31 [0.80, 2.16]; p=0.288, respectively). Occult hypoxemia was associated with increased odds of mortality in surgical (OR 2.96 [1.20, 7.28]; p=0.019) and ICU patients (1.36 [1.03, 1.80]; p=0.033). Occult hypoxemia was associated with fewer hospital-free days in surgical (−2.5 days [−3.9, −1.2]; p<0.001) but not ICU patients (0.4 days [−0.7, 1.4]; p=0.500).
Conclusions:
Occult hypoxemia is more common in Black patients compared to White patients and is associated with increased mortality, suggesting potentially important outcome implications for undetected hypoxemia. It is imperative to validate pulse oximetry with expanded racial inclusion.
Keywords: pulse oximetry, oxygen, hypoxemia, race, disparities
Introduction:
Hypoxemia, or low arterial oxygen tension, is associated with adverse clinical outcomes in hospitalized patients, including those with COVID-19-associated pneumonia.1,2 As such, reliable assessments of arterial oxygen saturation (SaO2) are essential for clinical decisions such as the need for hospitalization, intensive care unit (ICU) admission, and supplemental oxygen therapies, including non-invasive and invasive mechanical ventilation. Pulse oximetry non-invasively estimates peripheral arterial oxygen saturation (SpO2) through spectrophotometry. Given that deoxygenated and oxygenated hemoglobin maximally absorb light at different wavelengths, the ratio of light absorbance from diodes emitting light at different wavelengths is calculated for a given patient and entered into a mathematical algorithm to compare against a set of reference SaO2 measurements to provide the SpO2 value.
Despite ubiquitous utilization, studies in healthy volunteers have demonstrated racial bias in pulse oximetry accuracy for more than a decade,3,4 including SpO2 values which tend to overestimate SaO2 values in those with dark skin pigmentation, particularly at levels of severe hypoxemia. More recent evidence from patients receiving inpatient care has further highlighted racial disparities in pulse oximetry accuracy.5 Specifically, hospitalized Black patients experienced a nearly 3-fold increase in the odds of arterial hypoxemia, as assessed by arterial blood gas-derived SaO2 measurements, despite normal pulse oximetry-derived SpO2 readings (i.e. occult hypoxemia) when compared to White patients. However, this evidence is limited by reliance on SpO2 values separated by up to 10 minutes from arterial blood gas-derived SaO2 measurements, which may introduce substantial bias in hypoxemic patients receiving inpatient care. Additionally, the relationships between occult hypoxemia and clinical outcomes remain undefined such that it is unclear if occult hypoxemia is associated with adverse patient outcomes.
In this large multicenter investigation, we expand upon previous investigations to improve our understanding of race and SpO2-SaO2 relationships in hospitalized patients. Specifically, we address the limitations of previous work by including only simultaneous SpO2 and SaO2 measurements and by assessing the associations between occult hypoxemia and clinical outcomes.
Methods:
This observational study, conducted under Institutional Review Board approval (Mayo Clinic #21–000073) with employment of Strengthening the Reporting of Observational Studies in Epidemiology guidelines,6 included patients ≥18 years admitted to the ICU or undergoing surgery under general anesthesia or monitored anesthesia care during hospitalization at 3 academic medical centers (Mayo Clinic in Minnesota, Florida, and Arizona) with simultaneous paired measurements of SpO2 and SaO2 by arterial blood gas (i.e. zero minutes of separation) between May 5, 2018 and December 31, 2020 (Supplemental Figure). Patients were included from 4 self-identified racial groups: White, Black (i.e. African, African American, Black), Asian (i.e. Asian, Indian, Cambodian, Chinese, Filipino, Japanese, Korean, Laotian, Pakistani, Taiwanese, Thai, Vietnamese), and American Indian (includes Alaskan Natives).
The primary outcome was the incidence of occult hypoxemia defined as SaO2 <88% despite a normal SpO2 (i.e. ≥92%) as in prior research,5 with modification to include those with SpO2 values of 92–100% rather than 92–96%. Clinical outcomes of interest included hospital-free days and in-hospital mortality. Generalized linear models (GLMs) with robust covariance estimates accounting for multiple observations per subject (generalized estimating equations; [GEE]) estimate the associations between race and outcomes using an autoregressive working correlation, reported with 95% confidence intervals. In the primary analysis, among observations with SpO2 ≥92%, we assessed the association between race and occult hypoxemia through logistic regression fitted using GEE, adjusted for SpO2, age, mean arterial pressure <65 mmHg or the use of continuous infusions of intravenous vasopressors at the time of SaO2 assessment, and presence of chronic obstructive pulmonary disease (COPD) or home oxygen use. Estimated probability of occult hypoxemia after multivariable adjustment for a hypothetical 55-year-old patient was presented graphically as a function of SpO2 by race. The relationship between race and continuous SaO2 was assessed through linear regression fitted with GEE adjusted for SpO2 and the aforementioned covariates. We assessed interactions of race and SpO2. Linear functional form assumptions for age and SpO2 were assessed, and age was flexibly included using restricted cubic splines.
The relationships between occult hypoxemia and hospital outcomes were modeled using multivariable GLMs adjusted for age, sex, COPD or home oxygen use, index location (ICU vs. surgical), and acuity of illness. Index location was defined as the first hospital location where the patient had simultaneous SpO2-SaO2 assessment. Acuity of illness was defined using the American Society of Anesthesiologist’s Physical Status for surgical patients, which serves as a marker for acute and chronic disease severity,7 and the Acute Physiology and Chronic Health Evaluation (APACHE) III score for ICU patients.8 To categorize exposure to occult hypoxemia for outcome assessment, each patient’s first environment during hospitalization and associated SpO2-SaO2 values were included, and data were limited to measurements within 48 hours of index time defined as the start of the surgical procedure or ICU admission. The effect of occult hypoxemia was allowed to differ between ICU and surgical patients (i.e. an interaction term was included). To account for correlated data among patients with ≥1 qualifying admission, GEE was used with a compound symmetric working correlation. Hospital-free days were counted as the number of days alive and out of hospital following the index time through 28 days of follow-up. Results are reported as the estimated increase in mean hospital-free days for those with occult hypoxemia vs. non-hypoxemia. Interactions analyses by race were performed for occult hypoxemia and outcome relationships. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results:
128,285 paired SpO2-SaO2 measurements were included from 26,603 patients (Table 1). SpO2 on average overestimated SaO2 by 1.57% (1.54–1.61%; Figure 1). Asian and American Indian patients had lower estimated SaO2 values compared to White patients at any given SpO2 (mean difference −0.43% [−0.70%, −0.15%], p=0.002; and −0.68% [−0.96%, −0.40%], p<0.001, respectively). Estimated SaO2 values were not significantly different for Black compared to White patients (−0.08% [−0.27%, 0.11%], p=0.392). There was no evidence for interaction between race and SpO2-SaO2 relationships (p=0.217).
Table 1 –
Patient demographics, acuity, and hospital outcomes according to race*
| Variable | White (N=24,493) |
Black (N=1,263) |
Asian (N=574) |
American Indian (N=273) |
Overall (N=26,603) |
|---|---|---|---|---|---|
| Age, years | 65 (55, 73) | 56 (44, 66) | 60 (47, 69) | 57 (45, 64) | 64 (54, 73) |
| Male sex | 14,397 (58.8%) | 674 (53.4%) | 309 (53.8%) | 151 (55.3%) | 15,531 (58.4%) |
| BMI, kg/m2 (n=26,434) | 28.3 (24.5, 33.0) | 28.6 (24.5, 34.4) | 25.1 (22.1, 28.5) | 29.8 (25.5, 35.1) | 28.2 (24.4, 32.9) |
| COPD/Home oxygen use | 2,488 (10.2%) | 59 (4.7%) | 29 (5.1%) | 11 (4.0%) | 2,587 (9.7%) |
| Index location | |||||
| ICU | 6,388 (26.1%) | 473 (37.5%) | 182 (31.7%) | 105 (38.5%) | 7,148 (26.9%) |
| Surgery | 18,105 (73.9%) | 790 (62.5%) | 392 (68.3%) | 168 (61.5%) | 19,455 (73.1%) |
| ASA PS (n=19,389/19455) | |||||
| 1–2 | 3,424 (14.0%) | 122 (9.7%) | 99 (17.2%) | 32 (11.7%) | 3,677 (13.8%) |
| 3–4 | 14,461 (59.0%) | 659 (52.2%) | 290 (50.5%) | 135 (49.5%) | 15,545 (58.4%) |
| 5+ | 159 (0.6%) | 4 (0.3%) | 3 (0.5%) | 1 (0.4%) | 167 (0.6%) |
| APACHE III (n=7,148) | 86 (64, 111) | 88 (59, 111) | 86 (61, 112) | 82 (52, 102) | 86 (63, 111) |
| Vasopressor use‡ | 7,030 (28.7%) | 294 (23.3%) | 151 (26.3%) | 72 (26.4%) | 7,547 (28.4%) |
| Respiratory support (n=26,340) ‡ | |||||
| Invasive | 20,729 (85.4%) | 1,001 (80.4%) | 484 (86.0%) | 210 (78.1%) | 22,424 (85.1%) |
| Non-invasive | 3,534 (14.6%) | 244 (19.6%) | 79 (14.0%) | 59 (21.9%) | 3,916 (14.9%) |
| MAP < 65‡ | 11,869 (48.5%) | 474 (37.5%) | 269 (46.9%) | 108 (39.6%) | 12,720 (47.8%) |
| FiO2 (n=22,024)‡ | 50 (41, 60) | 55 (44, 60) | 54 (41, 62) | 53 (44, 60) | 50 (41, 60) |
| Any occult hypoxemia† | 859 (3.5%) | 72 (5.7%) | 38 (6.6%) | 18 (6.6%) | 987 (3.7%) |
| Occult hypoxemia in first 48 hours†† | 353 (1.4%) | 36 (2.9%) | 15 (2.6%) | 3 (1.1%) | 407 (1.5%) |
| Hospital length of stay, days§ | 6 (3, 9) | 6 (3, 12) | 5 (3, 10) | 7 (4, 14) | 6 (3, 9) |
| Hospital mortality | 1,352 (5.5%) | 67 (5.3%) | 38 (6.6%) | 17 (6.2%) | 1,474 (5.5%) |
Categorical variables are reported as number (percentage) and continuous variables are reported as median (25th percentile, 75th percentile). When data are missing, the number of patients with available measurements is reported in parentheses; otherwise, data are complete for the given variable.ASA PS – American Society of Anesthesiologists Physical Status; APACHE – Acute Physiology and Chronic Health Evaluation; FiO2 – fraction of inspired oxygen; MAP – mean arterial pressure
At the time of initial SaO2 measurement
Proportion of patients with any observed episode of occult hypoxemia during hospitalization, which differs slightly from estimated probability of occult hypoxemia accounting for multiple observations, as reported in text.
Any occult hypoxemia during the index location. Data are limited to measurements taken within 48 hours of arrival to index location.
Hospital length of stay following arrival to index location.
Figure 1.
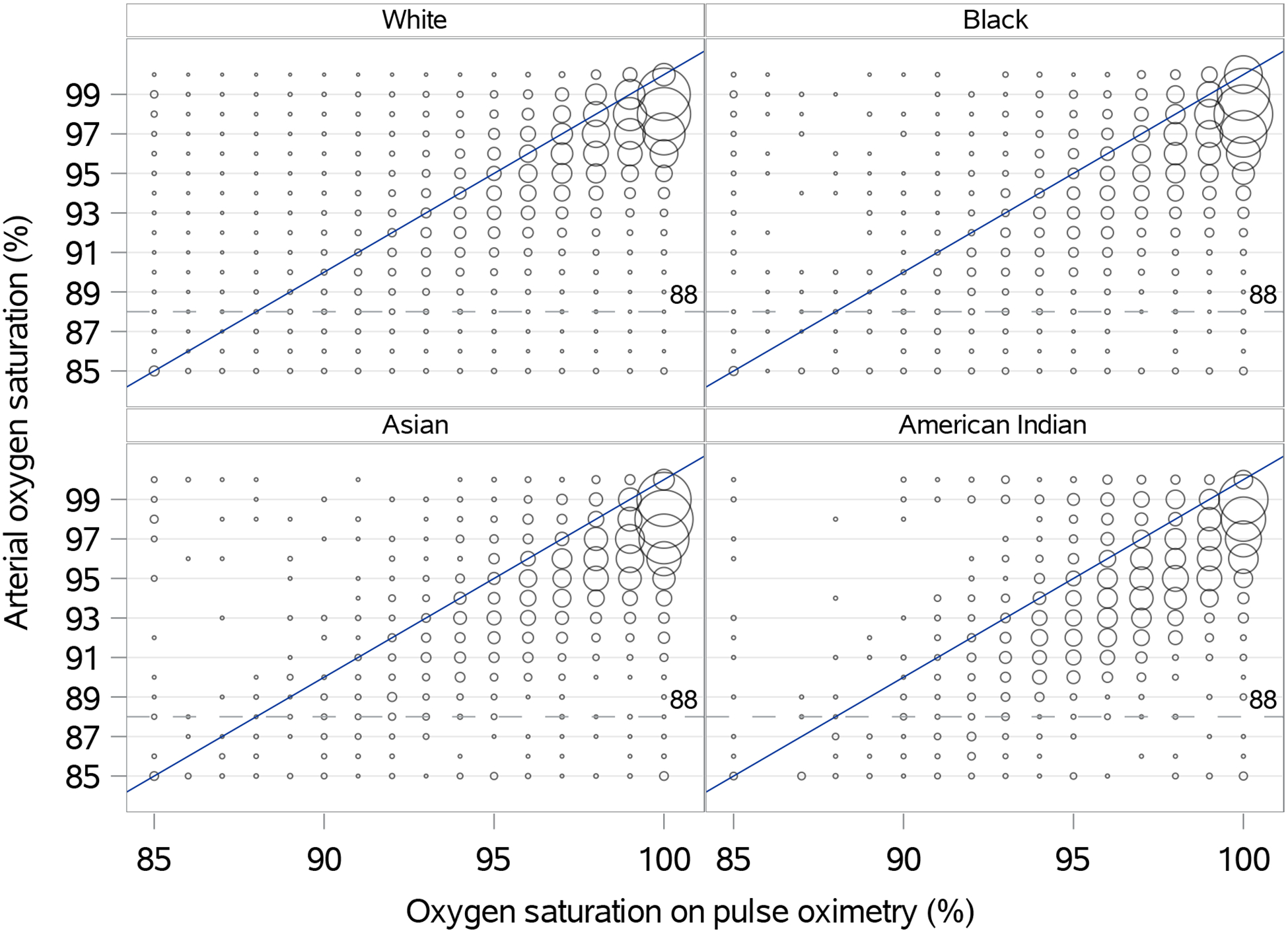
Scatterplot of estimated peripheral oxygen saturation by pulse oximetry and arterial blood gas-derived oxygen saturation across self-identified racial groups
Bubble size is determined by the proportion of the total sample according to race. The dotted lined represents arterial oxygen saturation less than 88% as representation of true arterial hypoxemia.
Occult hypoxemia was identified in 1.29% (1.23–1.35%) of measurements with SpO2 ≥92%, including 1.22% (1.16–1.29%) in White patients, 1.82% (1.51–2.16%) in Black patients, 1.59% (1.20–1.99%) in Asian patients, and 2.02% (1.51–2.54%) in American Indian patients. Accounting for multiple observations, Black, Asian, and American Indian patients were more likely to experience any occult hypoxemia during hospitalization (estimated probability 6.2% [5.1–7.6%], 6.6% [4.9–8.8%], and 6.6% [4.4–10.0%] respectively) compared to White patients (3.6% [3.4–3.8%]). The probability of occult hypoxemia increased with lower SpO2 values, with prominent racial differences (Figure 2). For example, among patients with SpO2 between 92% and 95%, the probability of occult hypoxemia for a given measurement was 7.6% for Black patients compared to 4.7% for White patients (Table 2). Black patients had increased odds of occult hypoxemia compared to White patients after multivariable adjustment (OR 1.65 [1.28, 2.14]; p<0.001), with boxplot comparisons displayed graphically in Figure 3. There were no significant differences in occult hypoxemia between Asian and American Indian patients compared to White patients in adjusted analyses (OR 1.53 [0.95, 2.47]; p=0.077, and OR 1.31 [0.80, 2.16]; p=0.288, respectively).
Figure 2.
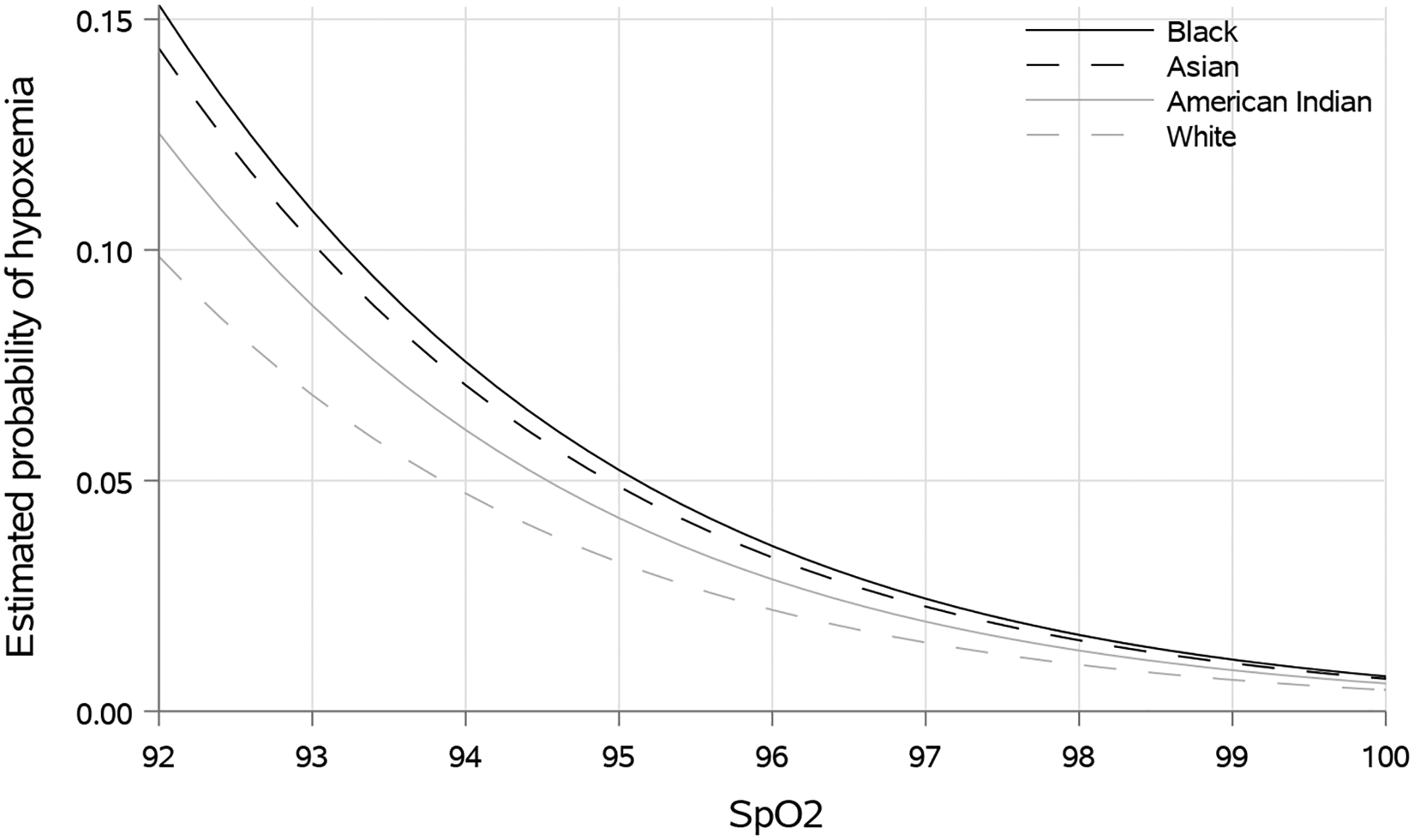
Estimated probability of occult hypoxemia for a 55-year old patient for any given pulse oximetry value across racial groups
Hypoxemia defined as arterial blood gas derived oxygen saturation (SaO2) <88%. SpO2 is the estimated peripheral oxygen saturation as derived from pulse oximetry. Data shown for a hypothetical 55-year old patient without vasopressor requirement or home oxygen use.
Table 2 –
Estimated probability of hypoxemia according to race and SpO2 group*
| SpO2 group | White | Black | Asian | American Indian | Overall |
|---|---|---|---|---|---|
| 88–91 | 24.8% | 36.0% | 32.8% | 39.3% | 25.8% |
| 92–95 | 4.7% | 7.6% | 6.9% | 7.3% | 5.0% |
| 96–98 | 1.2% | 2.1% | 1.2% | 0.7% | 1.2% |
| 99–100 | 0.5% | 0.8% | 0.8% | 0.9% | 0.5% |
Estimates are from the logistic regression model fit with race by SpO2 group category using GEE to account for correlation between multiple assessments. Hypoxemia defined as SaO2 <88%. Reported values are estimated probabilities for hypoxemia for any given SpO2-SaO2 pair within each racial group accounting for multiple observations across hospitalizations; hence, reported values differ slightly from the observed proportion of patients within each racial group experiencing any episode of occult hypoxemia as reported in Table 1.
Figure 3.
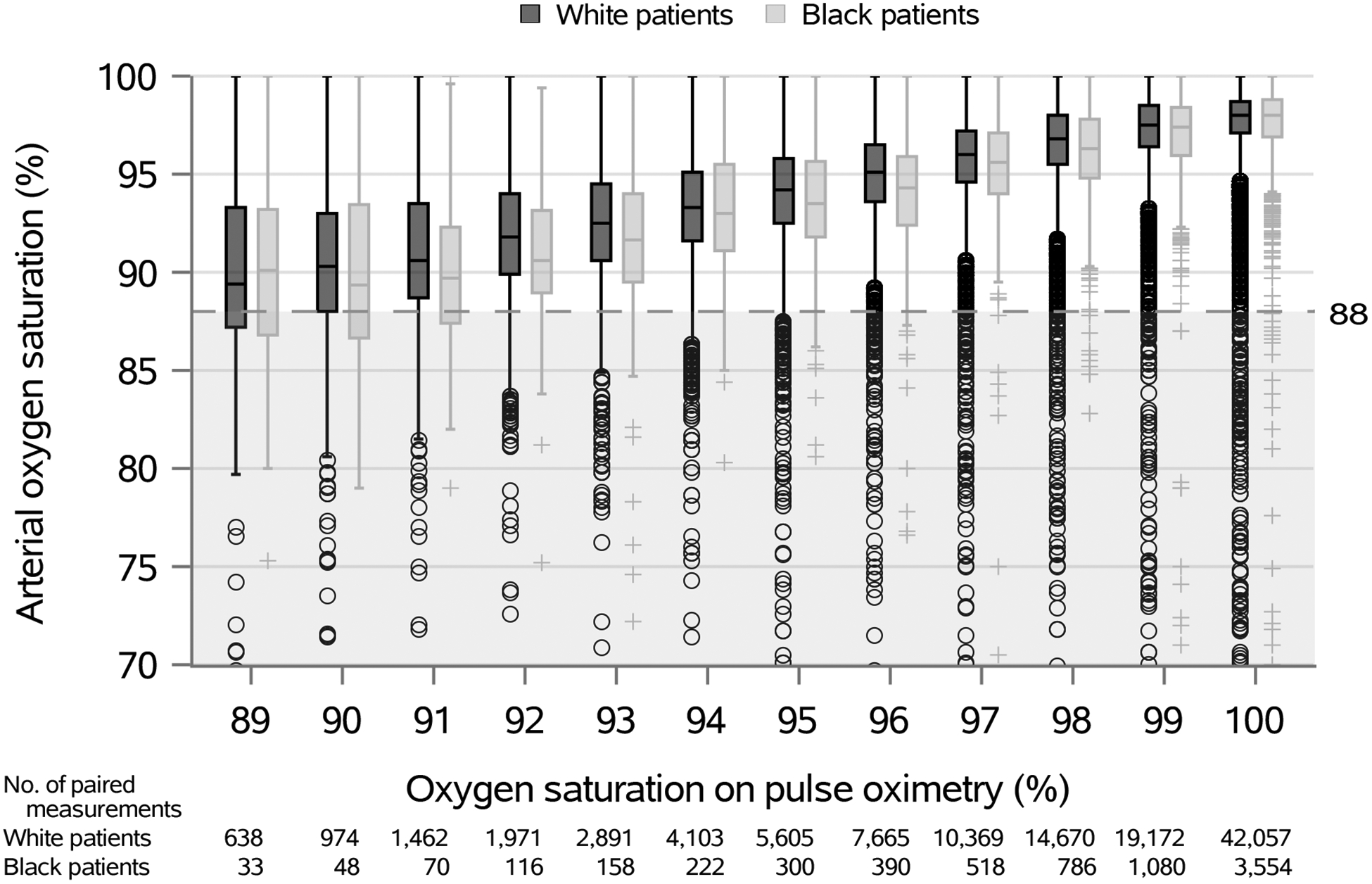
Boxplots of paired oxygen assessments for White and Black patients
The dotted lined represents arterial oxygen saturation less than 88% as representation of true arterial hypoxemia.
Occult hypoxemia was associated with increased odds of hospital mortality in surgical patients (OR 2.96 [1.20, 7.28]; p=0.019) and in ICU patients (1.36 [1.03, 1.80]; p=0.033) compared to those with normal oxygenation. Occult hypoxemia was associated with fewer hospital-free days in surgical patients (estimated −2.5 days [−3.9, −1.2]; p<0.001) but not in ICU patients (0.4 days [−0.7, 1.4]; p=0.500). There were no significant interactions for occult hypoxemia and outcome relationships by race such that the associations between occult hypoxemia and adverse outcomes persisted across races.
Discussion:
In analysis of approximately 130,000 paired SpO2-SaO2 measurements in more than 26,000 patients, occult hypoxemia was encountered in less than 2% of assessments but was substantially more common in Black patients compared to White patients. Occult hypoxemia was associated with increased mortality in both surgical and critically ill patients, suggesting potentially important clinical implications for undetected hypoxemia.
These results build upon previous work highlighting racial disparities in occult hypoxemia detection in hospitalized patients,5 which found adjusted incidences of occult hypoxemia of 11.4% (95% CI, 7.6% to 15.2%) and 3.6% (95% CI, 2.5% to 4.6%) in Black and White patients, respectively. While this work has been foundational in highlighting racial disparities in hypoxemia detection, limitations include the use of SpO2 and SaO2 values separated by up to 10 minutes, incomplete details on patient demographic and clinical characteristics, and lack of clinical outcome assessment. In this investigation, we confirm the presence of racial disparities in hypoxemia detection in a substantially larger patient cohort with the addition of numerous important and novel features, including: 1) the use of high-fidelity pulse oximetry and ABG-derived oxygenation data without time lag between measurements (i.e. zero minutes of separation), which is particularly important for patients in acute care environments who may experience rapid clinical deterioration or prompt escalations in respiratory support and/or oxygen supplementation; 2) incorporation of data from four self-identified racial groups; 3) a focus on acute care environments (i.e. ICU, operating room) that depend most on high-fidelity monitoring of oxygenation; 4) the employment of robust multivariable modeling to adjust for key clinical factors that may confound SpO2, SaO2, and clinical outcome relationships, and most importantly, 5) detailed assessment of the associations between occult hypoxemia and patient outcomes. Additional recent clinical investigations regarding this topic are limited in size and scope, including data from 372 patients prior to extracorporeal membrane oxygenation (ECMO) initiation and 194 patients receiving non-invasive respiratory support for confirmed COVID-19 pneumonitis.9,10 In the former study, Black patients had an approximate 2.5 fold-increase in the risk for occult hypoxemia compared to White patients.10 In the latter, the authors concluded that race did not alter the bias between SpO2 and SaO2 to a clinically significant degree; however, the study included data from only 19 Black patients, yet the incidence of SpO2 values ≥ 94% despite SaO2 values < 90% was substantially greater in Black patients compared to White patients (i.e. 71% vs. 30%).9 Importantly, none of these studies have assessed the relationships between racial disparities in hypoxemia detection and clinical outcomes.
As previously mentioned, a key finding of this study is the confirmation that pulse oximetry accuracy for the detection of hypoxemia is not consistent across self-identified racial groups despite relatively preserved SpO2 and SaO2 relationships. These findings are largely driven by a higher incidence of undetected hypoxemia as SpO2 values fall to 95% or below, most prominently in Black patients. Although occult hypoxemia was generally rare and less frequent than recently reported,5,9 being encountered in approximately 1 in every 82 measurements in White patients, 1 in every 55 measurements in Black patients, 1 in every 63 measurements in Asian patients, and 1 in every 50 measurements in American Indian patients, there are potentially important clinical implications for hypoxemia not detected by pulse oximetry. Most notably, patients with occult hypoxemia had greater mortality regardless of practice environment, a finding which was consistent across racial groups. Additionally, surgical patients with occult hypoxemia experienced fewer hospital free days, which may suggest the presence of adverse downstream clinical consequences related to undetected hypoxemia. While it is unclear why a similar relationship between occult hypoxemia and hospital free days was not observed in ICU patients, the presence of greater mortality in this group suggests adverse outcomes regardless of any perceived effects on hospital duration. Further research into clinical outcomes is warranted, including assessment of granular outcomes most likely to be impacted by undetected hypoxemia (e.g. end-organ dysfunction) and extension into practice settings without continuous management of respiratory status.
Undoubtedly, pulse oximeters with continuous estimation of arterial oxygen saturation remain important tools in contemporary ICU and surgical practices. Despite disparities in hypoxemia detection, outright elimination of these devices from clinical practice would be antithetical to our common goals to ensure patient safety and improve outcomes. However, clinicians must recognize limitations and actively pursue opportunities for improvement. Given the physiological and biomechanical underpinnings of pulse oximetry, observed racial differences in hypoxemia detection are most plausibly related to variations in skin pigmentation, which may impact light absorption and scattering in the epidermis, resulting in differential transmission to the photodetector, as previously postulated.3,4 This exposes an issue with a lack of inclusion of persons of color during the validation process from which reference values are derived. It is essential that reference standards are representative across racial groups, which may require the incorporation of quantitative assessment of skin pigmentation to accurately determine anticipated light transmission. It is imperative to improve these processes without delay to ensure equitable, high-quality care delivery for all patients.
Limitations:
There are several notable limitations of this investigation. First, race was determined by patient self-identification in the medical record; however, reported racial groups are broad and are not synonymous with skin pigmentation. Certain racial (e.g. Native Hawaiian or Pacific Islander) and ethnic groups (e.g. Hispanic) did not have sufficient data for inclusion. Additional investigations are necessary to quantify skin pigmentation when evaluating device accuracy. Second, patients were predominantly of White race, limiting external validity. Third, pulse oximeters may vary in measurement accuracy and bias, and these results may not be applicable to all devices. Fourth, SpO2 values may be affected by additional non-captured factors. Finally, clinical outcomes were limited in scope. Future investigations are warranted to evaluate hypoxemia, race, and outcome associations more broadly.
Conclusions:
Pulse oximetry is an essential component of contemporary medical practice, yet there are disparities in the detection of hypoxemia across self-identified racial groups, which may have clinically relevant outcome implications. It is imperative to validate new and existing technologies with expanded racial inclusion.
Supplementary Material
Funding:
NSW is supported by a K23 Grant (1 K23 AG070113-01) from the National Institute on Aging (NIA). MAW is supported by a Clinical and Translational Science Awards (CTSA) KL2 grant (TR002379) from the National Center for the Advancing Translational Science (NCATS) and K23HL153310 from the National Heart Lung and Blood Institute (NHLBI). The remaining authors have no sources of funding to declare for this manuscript. The contents of this manuscript are solely the responsibility of the authors and do not represent the official view of the NIA, NCATS, NHBLI, or the National Institutes of Health (NIH).
References:
- 1.Bowton DL, Scuderi PE, Haponik EF. The incidence and effect on outcome of hypoxemia in hospitalized medical patients. Am J Med. 1994;97(1):38–46. doi: 10.1016/0002-9343(94)90046-9 [DOI] [PubMed] [Google Scholar]
- 2.Xie J, Covassin N, Fan Z, et al. Association Between Hypoxemia and Mortality in Patients With COVID-19. Mayo Clin Proc. 2020;95:1138–1147. doi: 10.1016/j.mayocp.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feiner JR, Severinghaus JW, Bickler PE. Dark skin decreases the accuracy of pulse oximeters at low oxygen saturation: The effects of oximeter probe type and gender. Anesth Analg. 2007;105(SUPPL. 6). doi: 10.1213/01.ane.0000285988.35174.d9 [DOI] [PubMed] [Google Scholar]
- 4.Bickler PE, Feiner JR, Severinghaus JW. Effects of skin pigmentation on pulse oximeter accuracy at low saturation. Anesthesiology. 2005;102(4):715–719. doi: 10.1097/00000542-200504000-00004 [DOI] [PubMed] [Google Scholar]
- 5.Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial Bias in Pulse Oximetry Measurement. N Engl J Med. 2020;383(25):2477–2478. doi: 10.1056/nejmc2029240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann Intern Med. 2007;147(8):573. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 7.Mayhew D, Mendonca V, Murthy BVS. A review of ASA physical status – historical perspectives and modern developments. Anaesthesia. 2019;74(3):373–379. doi: 10.1111/anae.14569 [DOI] [PubMed] [Google Scholar]
- 8.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system: Risk prediction of hospital mortality for critically III hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619 [DOI] [PubMed] [Google Scholar]
- 9.Wiles MD, El-Nayal A, Elton G, et al. The effect of patient ethnicity on the accuracy of peripheral pulse oximetry in patients with COVID-19 pneumonitis: a single-centre, retrospective analysis. Anaesthesia. Published online September 20, 2021. doi: 10.1111/ANAE.15581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valbuena VSM, Barbaro RP, Claar D, et al. Racial Bias in Pulse Oximetry Measurement Among Patients About to Undergo ECMO in 2019–2020, A Retrospective Cohort Study. Chest. Published online September 27, 2021. doi: 10.1016/J.CHEST.2021.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


