Abstract
Background
Between 2010 and 2016, the proportion of children 12–23 months of age who received full immunization in Malawi decreased from 81% to 76%. Most studies on immunization have mainly focused on the risk factors of vaccination coverage while data on dropouts and equity gaps is very scanty. Thus the aim of the present study was to describe the trend in immunization coverage, dropout rates and effective immunization coverage (EIC) among children ages 12–23 months in Malawi.
Methods
Secondary analyses of the cross-sectional data obtained from the three waves of the Demographic and Health Surveys (2004, 2010 and 2015–16) were conducted. Using bottleneck analysis, outputs were generated based on service coverage, demand/equity (service utilization) and quality (full immunization). The World Health Organization benchmarks were used to assess gaps in the immunization coverage indicators.
Results
The coverage was >90.0% in most of the antigens while full immunization status was estimated at 65%, 84% and 73% in 2004, 2010 and 2015, respectively. The highest coverage was observed in Bacillus Calmette–Guérin (BCG) and lowest in oral polio vaccine 1 (OPV1). OPV1 coverage was <90% in the 2004 cohort year, while pentavalent 3 (Penta3) and measles-containing vaccine 1 (MCV1) coverages were <90% in 2004. Dropout rates of Penta3 and MCV1 were significantly >10% in 2004. The logistic regression analyses showed that children were significantly less likely to be immunized with Penta3 and MCV1 in all cohort years compared with Penta1.
Conclusions
Although immunization coverage was in line with the national and district targets for various antigens, full vaccination coverage (FVC) is still lagging behind. Furthermore, the dropout rates for Penta3 and MCV1 showed upside U-shaped patterns. Thus health education, supervision and orientation of service providers are urgently needed to address disparities that are existing in FVC.
Keywords: bottleneck analysis, dropout rates, equity gaps, immunization coverage, Malawi, under-five children
Background
Since the 20th century, immunization has been an effective tool for controlling and eradicating life-threatening infectious diseases.1 Every year, immunization prevents about 2–3 million child deaths.2 The global efforts to use immunization as a public health intervention began when the World Health Organization (WHO) launched the Expanded Programme on Immunization (EPI) in 1974, where six vaccines (diphtheria, whooping cough, tetanus, measles, poliomyelitis and tuberculosis) were recommended for children 0–24 months of age through routine infant immunization.3 The original six recommended vaccines have been adopted in many countries and new antigens have now been added into the EPI.4 Despite the fact that immunization coverage has shown significant improvements over the last 2 decades, the coverage has remained stagnant at 86%, with no substantial changes over recent years.2 To benefit from the direct and indirect effects of immunization, the WHO came up with the Global Vaccine Action Plan (GVAP), which set country targets of 90% coverage for all antigens at the national level and at least 80% coverage for all antigens in 80% of districts by the year 2020.5
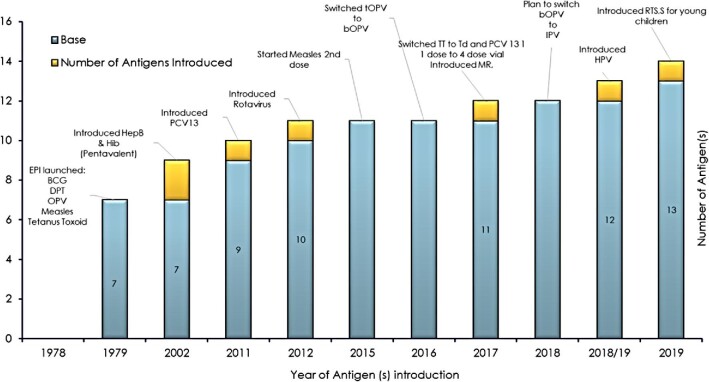
In 1979, the Malawi EPI was officially launched and began to administer vaccines against six diseases: a dose of Bacillus Calmette–Guérin (BCG) vaccine against tuberculosis (TB); three doses of diphtheria, tetanus and pertussis (DPT) vaccine against diphtheria, tetanus and pertussis; at least three doses of oral polio vaccine (OPV) against polio; and one dose of measles-containing vaccine (MCV) against measles.6 However, it was reported that only smallpox vaccinations were administered on a large scale while antigens such as BCG and DPT were delivered in a few health facilities in Malawi in the early 1970s.7 In the year 2012, the Malawi government substituted the DPT vaccine with a pentavalent vaccine that protects against DPT, hepatitis B virus (HBV) and Haemophilus influenzae type b (Hib) in its EPI.8 Additionally, in November 2011 and October 2012, the government of Malawi introduced other new vaccines into its EPI: the pneumococcal conjugate vaccine (PCV13) against bacterial pneumonia and the monovalent human rotavirus vaccine (RV1) against severe diarrhoea caused by rotavirus.9 In 2015 the Malawi government introduced a measles second dose, while in 2016 the trivalent oral polio vaccine (tOPV) was switched to the bivalent oral polio vaccine (bOPV). Similarly, the tetanus toxoid (TT) vaccine was switched to a tetanus–diphtheria (Td) vaccine, PCV13 was switched from one dose to four doses and the measles–rubella (MR) vaccine was introduced. Additionally, in 2018–2019 the Malawi EPI planned to switch the bOPV to an inactivated polio vaccine (IPV) and human papilloma vaccine (HPV) was introduced for girls ≥10 y of age.10 Recently, in May 2019, the Malawi government introduced the malaria vaccine (MV), also known as RTS,S/AS01, into its EPI.11 Figure 1 displays the number of antigens introduced in Malawi's EPI schedule from 1979 to 2019.
Figure 1.
Number of antigens introduced in the EPI schedule since 1979.
The Malawi EPI recommends that the BCG and polio 0 vaccines should be given at birth or within the first 14 days after birth, the Penta, PCV, RV and OPV should be given at approximately 6, 10 and 14 weeks of age. The MCV is recommended to be administered as soon as the child reaches 9 months of age.12 Additionally, the TT vaccine is scheduled to be provided to pregnant women at first contact. Table 1 presents the EPI schedule followed in Malawi. Obstacles to reaching every child with the full complement of vaccines have been identified in some settings. These factors include maternal education, distance to health facilities, inadequate vaccines, poorly trained and motivated human resources and poor quality of services.13 Studies from Malawi on vaccination have reported that women's low education, having one or no antenatal visits, having no immunization card, having an immunization card but not seen, residing in poor households, having a large number of children and living in the central region were the most significant factors associated with decreased odds of achieving vaccination coverage and complete vaccination.7,14 Furthermore, other studies have cited that vaccines being out of stock at the health facility level is a reason for no vaccination.15 Most of these studies have mainly focused on whether the child has received full, under- or non-vaccination coverage.
Table 1.
Schedule of the Malawi EPI
| Vaccine | Description and number of antigens | Schedule | Comments |
|---|---|---|---|
| Primary infant and adolescent vaccination schedule | |||
| BCG | Bacillus Calmette–Guérin (dose) | At birth or first contact | |
| OPV0 | Oral polio vaccine 0 (dose) | At birth to 2 weeks | |
| Rotavirus | Rotavirus vaccine (two doses) | 6 and 10 weeks | |
| Pentavalent | Diphtheria and tetanus and pertussis and Haemophilus influenzae and hepatitis B (three doses) | 6, 10 and 14 weeks | |
| OPV | Oral polio vaccine (three doses) | 6, 10 and 14 weeks | |
| Pneumo_conj | Pneumococcal conjugate vaccine (three doses) | 6, 10 and 14 weeks | |
| MV | Malaria vaccine/RTS,S/AS01 (four doses) | 5, 6, 7 and 22 months | |
| Measles | Measles vaccine (two doses) | 9 and 15 months | |
| MR | Measles and rubella vaccine (two doses) | 9 and 15 months | From July 2017 |
| HPV | Human papillomavirus vaccine (two doses) | 10 y | Girls ages 9–14 y |
| Adult vaccination schedule | |||
| TT | Tetanus toxoid vaccine | First contact; +1 and +6 months; +1 and +1 y | Pregnant women |
Worldwide, about 85% of infants (116 million infants) received a DTP3 and one dose of MCV by the end of 2019, thus protecting them against infectious diseases that can cause serious illness and disability. Meanwhile, 123 countries were reported to have reached at least 90% coverage of DTP3.2 Similarly, the EPI in Malawi has managed to sustain a high coverage of immunization >80% of various antigens for several years. However, recently there has been a significant decline in fully immunized children from 81% in 2010 to 76% in 2016.9 In Malawi, routine data on immunization are collected using two national routine administrative tools, the official WHO/United Nation Children's Fund (UNICEF) Joint Reporting Form (JRF) and the District Vaccination Data Management Tool (DVDMT). Unfortunately, due to data quality issues, these databases usually report inconsistent coverage that is abnormally high as well as high dropout rates. Even though the routine reports provide information on immunization coverage, information may be inaccurate and misleading. Data quality plays a fundamental role in the success or failure of an immunization programme and poor quality immunization data threatens to undermine national and international investments.16
In Malawi, the Malawi Demographic and Health Survey (MDHS) focuses mainly on immunization coverage and completeness of immunization. However, immunization dropouts are not reported. The dropout rate is of great importance, as it indicates whether there is an access problem for parents, i.e. whether they have difficulty in getting to the immunization services for subsequent doses or whether there is a problem for parents in utilizing the health services.17 Therefore coverage surveys can validate routine reports and provide additional information on immunization and identify strategies to improve immunization activities. Accordingly, we aimed to describe the trend in immunization coverage, dropouts, equity gaps and full vaccination coverage (FVC), also known as effective immunization coverage, among children 12–23 months of age in Malawi.
Methods
Study setting
Malawi is part of sub-Saharan Africa, which is located in the southeastern part of the continent. It is bordered to the north and northeast by the United Republic of Tanzania; to the east, south and southwest by the People's Republic of Mozambique; and to the west and northwest by the Republic of Zambia.8,18,19 Malawi is one of the poorest countries in the world, with a gross national income per capita of US$320 and about 85% of the population live in rural areas.20 The economy of Malawi is based primarily on agriculture, which accounts for 30% of the gross domestic product.21 Healthcare services in Malawi are provided through both the public and private sectors.22 The public sector includes all facilities under the Ministry of Health (MoH), Ministry of Local Government and Rural Development, Ministry of Forestry, the police, the prisons and the army. The private sector consists of private for-profit and private not-for-profit providers, mainly the Christian Association of Malawi (CHAM).10,22 The public sector provides services free of charge while the private sector charges user fees for its services. There are currently 977 health facilities in Malawi comprising 113 hospitals, 466 health centres, 48 dispensaries, 327 clinics and 23 health posts. These health facilities are managed by the government (n=472), CHAM (n=163), private organizations (n=283) and non-governmental organizations (n=58). All these institutions provide immunization services in Malawi.5 In the last 2 decades Malawi has seen a 30% decrease in mortality rates in children <5 y of age, from 190 deaths per 1000 live births to 133 per 1000 live births. It was reported that this decline was due in part to neonatal mortality rates that decreased 36%, from 42 per 1000 live births to 27 per 1000 live births.23 The causes of mortality in children <5 y of age in Malawi include malaria, diarrhoea and acute lower respiratory tract infection; all of these conditions are preventable and have vaccines. The prevalence of malaria, diarrhoea and pneumonia were reported at 24%, 22% and 5%, respectively.9,24
Data sources
The current study analysed data obtained from the three waves of the MDHS conducted in 2004, 2010 and 2015–2016. Details on the methodology used in these surveys can be obtained elsewhere.8,9,25 In brief, the surveys employed two-stage sampling designed to produce nationally representative samples. The surveys utilized sampling frames from the Malawi Population and Housing Census (MPHC) conducted in 1998 and 2008. The first stage selected 850, 849 and 522 clusters, known as standard enumeration areas (SEAs), proportional to the population in 2015–2016, 2010 and 2004, respectively. The second stage involved selection of 27 516, 27 307 and 15 041 households from the SEAs with an equal probability systematic selection in 2015–2016, 2010 and 2004, respectively.
Data collection
The primary objective of the MDHS is to provide up-to-date estimates of basic demographic and health indicators. The surveys provide a comprehensive overview of population, maternal and child health issues in Malawi. Specifically, one of the objectives these surveys achieved was the collection of data on the key aspects of family health, such as immunization coverage among children. Respondents were asked to show a health passport or any other document where vaccines were written down. If the respondents could not show a health or immunization card, they were then asked to recall any vaccinations ever received to prevent them from getting diseases, including vaccinations received in health campaigns or immunization or child health days. In particular, respondents were asked to report whether the individual received BCG, polio, pentavalent, rotavirus, pneumococcal and measles vaccines. Regarding polio, pentavalent, rotavirus and pneumococcal vaccines, respondents were further asked to report the number of times the individual received each specific vaccine.8,9,25 In the selected households—11 698 in 2004, 23 020 in 2010 and 24 562 in 2015—women were interviewed, representing 97.7%, 96.9% and 95.7%, respectively. As recommended by the WHO,9 the present study included children 12–23 months of age. Thus the final samples analysed were 2211, 3741 and 3225 children in 2004, 2010 and 2015–2016, respectively.
Inclusion criteria
All children 12–23 months of age prior to each survey, living with their guardians and with information on immunization were included in this study.
Bottleneck analysis (BNA) and framework
BNA is an approach based on the Monitoring of Results for Equity System (MoRES) for planning equity-focused interventions and identifying gaps in their uptake.26 The MoRES was developed in 2010 as part of UNICEF’s refocus on equity to ensure that UNICEF is as effective as possible in the protection and promotion of children's rights.27 The BNA framework is premised on the notion that effective coverage of services is influenced by four main domains: supply, demand, quality and environment. Supply determinants of services are predominantly controlled by the healthcare delivery system and have three important components: commodities, human resources and geographic access. Demand determinants of services are predominantly controlled by the community and have two important components known as initial utilization and continuous utilization of services. Quality determinants of services are predominantly controlled by the healthcare delivery system and relate to the services being able to meet the quality standards set within national guidelines. Lastly, environmental influencers of services are thought to occur across all aspects of the programme and include policy and regulatory frameworks, management, coordination and sociocultural as well as economic-related factors.13 However, in this study we focused on the demand and quality determinants of services since the DHS does not have data on supply.
Variables and operational definitions
The following variables were included to describe the immunization coverage, dropout and equity gaps among children 12–23 months of age in Malawi. Immunization coverage was defined as the proportion of children 12–23 months of age who received the recommended EPI vaccine antigens compared with the total number of infants who survived in the given target population. Partially immunized children were those ages 12–23 months who missed one or more of the scheduled prescribed vaccines considered to protect them against vaccine-preventable diseases. Unimmunized children were those who had not received any of the scheduled EPI vaccinations. Dropout rate was defined as the proportion of vaccination recipients who had begun their schedules but did not complete them. The dropout rate was calculated by comparing the number of infants who were initiated in the vaccination schedule vs those who completed it. Two domains are habitually used to calculate the dropout rate. These measures are the Penta vaccine and MCV1. Specifically, the DPT dropout rate was calculated by dividing the number of children 12–23 months of age who received DPT1 minus the number of children 12–23 months of age who received DPT3 by the number of children 12–23 months of age who received DPT1 (i.e. [Penta1−Penta3]/Penta1×100%). It can also be calculated as the children ages 12–23 months who received Penta1 and MCV1 divide by those who received Penta1 (i.e. [Penta1−measles]/Penta1×100%). The WHO recommends that the coverage of both the Penta1 to Penta3 and Penta1 to MCV1 dropout rates should be <10% so as to have better immunization coverage as well as reduced rates of morbidity and mortality in children <5 y of age.28,29 It should be taken into consideration that a dropout rate of >10% reflects underutilization of immunization services. Initial utilization of an immunization program was defined as the proportion of children who received Penta1 vaccine during the past year in a region/district. The numerator for this indicator is the number of children 12–23 months of age who received either BCG or Penta1 vaccine and the denominator is the number of children 12–23 months of age eligible for Penta1 vaccination. Continuous utilization of an immunization program was defined as the proportion of children 12–23 months of age who received a Penta3 vaccine during the past year in a region/district. This indicator uses the number of children who received Penta 3 vaccine as the numerator and the number of children 12–23 months of age who are eligible for Penta3 vaccination as the denominator. Adequate immunization coverage was defined as the percentage of children ages 12–23 months who were immunized with MR1 during the past year in a region/district. This indicator was calculated by dividing the number of children 12–23 months of age who received an MR1 vaccine by the number of children 12–23 months of age who were eligible for MR1 vaccination. FVC was defined as children ages 12–23 months who received BCG, OPV3, Penta3, PCV3, RV2 and MCV1 vaccines. The number of children fully vaccinated by 12–23 months according to the vaccination calendar timeline is the numerator and the number of children <12 y of age eligible for full vaccination is the denominator.
Control variables
The control variables used in the present study included the year in which the survey was carried out and the geographical region. To establish a trend in immunization coverage, a 15-y period was sampled (the years included 2004, 2010 and 2015–2016). The geographical region (northern, central and southern) was used as a proxy for administrative divisions in Malawi. The geographical region was chosen to establish the most underperforming area for policy changes.
Statistical analysis
Data were analysed using SAS software version 9.4 (SAS Institute, Cary, NC, USA) and Stata version 15 (StataCorp, College Station, TX, USA). All analyses were performed separately for 2004, 2010 and 2015–2016. Data were presented as frequency and percentage and, where necessary, data were presented in the form of charts. Using Pearson's χ2, the bivariate analysis was performed to test the differences in distribution between groups (Penta1 vs Penta3, Penta1 vs MCV1, initial utilization [yes/no], continued utilization [yes/no], adequate coverage [yes/no] and FVC [yes/no]). The multivariable analyses were conducted using logistic regression to examine the magnitude of those unimmunized with Penta3 and MCV1. The results of logistic regression were presented as the adjusted odds ratio (aOR) with the corresponding 95% confidence interval (CI). The statistical significance was considered when p-values were <0.05.
Results
Immunization coverage
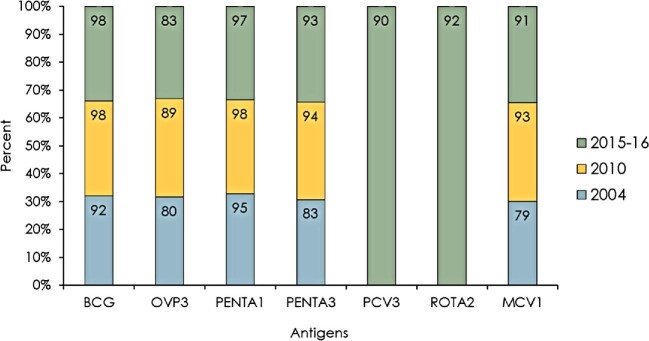
Immunization coverages for various antigens are presented in Figure 2. In all three cohort years, immunization coverage was the highest for BCG antigens (92% in 2004, 98% in 2010 and 98% in 2015–2016) and lowest for OPV antigens (80% in 2004, 89% in 2010 and 83% in 2015–2016). The coverages of Penta3 and MCV1 were <90% in 2004. The other vaccination coverages were 92% for Penta3 in 2010 and 93% for Penta3 in 2015. Furthermore, the coverages for PCV3 and RV2 in 2015 were 90% and 92%, respectively, while the coverages for MCV1 antigen in 2010 and 2015 were 93% and 91%, respectively.
Figure 2.
Trends in immunization coverage between 2004 and 2016.
Penta3 and MCV1 unimmunized children
Figure 3 displays the number of unimmunized children with Penta3 and MCV1 between 2004 and 2015. The highest numbers of unimmunized children were observed in 2004 and lowest in 2010. As seen in the figure, 264 children and 347 children were unimmunized with Penta3 and MCV1 in 2004, respectively. Additionally, 140 and 201 children were unimmunized with Penta3 and MCV1 in 2015, respectively.
Figure 3.
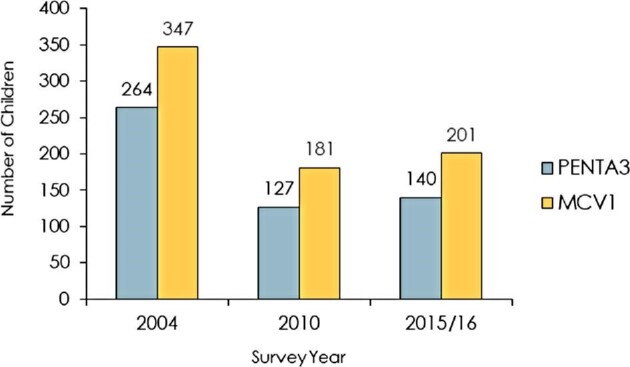
Unvaccinated children with Penta 3 and MCV1.
Penta3 and MCV1 dropout rates
Tables 2 and 3 present dropout rates for Penta3 and MCV1. The dropout rates for both antigens were significantly >10% in 2004, but with a U shape. In 2004 the Penta3 dropout rate was 12.6%, in 2010 the dropout rate decreased significantly to 3.4% and in 2015–2016 the dropout rate increased slightly. Similarly in 2004 the MCV1 dropout rate was 16.5%, however, the dropout rate decreased significantly to about 5% in 2010 and slightly increased in 2015–2016. The dropout rates in 2004 were higher than the 10.0% cut-off point recommended by the WHO.
Table 2.
Dropout rate in immunization coverage using DPT1–Penta1 and DPT3–Penta3
| Year | Infants vaccinated Penta1, n | Infants vaccinated Penta3, n | Difference (Penta1−Penta3), n | Dropout rate (%) | p-Value |
|---|---|---|---|---|---|
| 2004 | 2101 | 1837 | 264 | 12.6 | <0.001 |
| 2010 | 3712 | 3585 | 127 | 3.4 | <0.001 |
| 2015–2016 | 3134 | 2994 | 140 | 4.5 | <0.001 |
Table 3.
Dropout rate in immunization coverage using DPT1–Penta1 and MCV1–MR1
| Year | Infants vaccinated Penta1, n | Infants vaccinated MCV1, n | Difference (Penta1−MCV1), n | Dropout rate (%) | p-Value |
|---|---|---|---|---|---|
| 2004 | 2101 | 1754 | 347 | 16.5 | <0.001 |
| 2010 | 3712 | 3531 | 181 | 4.9 | <0.001 |
| 2015–2016 | 3134 | 2933 | 201 | 6.4 | <0.001 |
Effective immunization coverage
The results for FVC showed an upside-down U-shape pattern in all the indicators (initial utilization, Penta1; continuous utilization, Penta3; adequate coverage, MCV1; and full vaccination coverage) (Figure 4). The coverage for Penta1 was 95% in 2004, 98% in 2010 and 97% in 2015–2016, while FVC was 65% in 2004, 84% in 2010 and 73% in 2015–2016.
Figure 4.
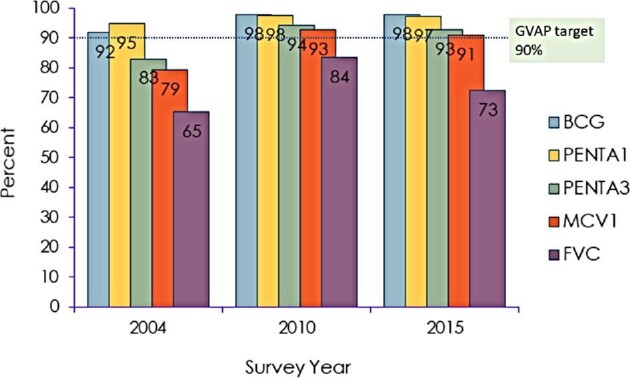
Bottlenecks in the delivery of immunization services.
χ2 results by geographical region
Table 4 shows the results of the bivariate analysis of effective immunization coverage by geographical region. In 2004, significantly high coverage of Penta1 (97.2%), Penta3 (89.7%), MCV1 (83.33%) and FVC (70.6) was observed in the northern region. In 2010, significantly high coverage of Penta1 (98.4%), Penta3 (95.9%), MCV1 (94.51%) and FVC (85.58%) was observed in the northern region. In 2015–2016, significantly high coverage of Penta1 (98.2%) and FVC (75.0%) was observed in the northern region.
Table 4.
Bivariate analysis of the geographical region variation in effective immunization coverage
| Vaccine | Region | 2004 | 2010 | 2015–16 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No, n (%) | Yes, n (%) | p-Value | No, n (%) | Yes, n (%) | p-Value | No, n (%) | Yes, n (%) | p-Value | ||
| Penta1 | <0.0001 | 0.0017 | 0.0016 | |||||||
| North | 7 (2.8) | 245 (97.2) | 14 (2.1) | 667 (97.9) | 11 (1.8) | 588 (98.2) | ||||
| Central | 68 (8.1) | 777 (91.9) | 45 (3.6) | 1217 (96.4) | 21 (1.8) | 1111 (98.2) | ||||
| Southern | 35 (3.1) | 1079 (96.9) | 30 (1.61) | 1828 (98.4) | 59 (3.9) | 1435 (96.1) | ||||
| Penta3 | <0.0001 | <0.001 | 0.5618 | |||||||
| North | 26 (10.2) | 226 (89.8) | 34 (5.0) | 647 (95.0) | 38 (6.3) | 561 (93.7) | ||||
| Central | 190 (22.5) | 655 (77.5) | 105 (8.3) | 1157 (91.7) | 79 (7.0) | 1053 (93.0) | ||||
| Southern | 158 (14.2) | 956 (85.8) | 17 (4.1) | 1781 (95.9) | 114 (7.1) | 1380 (92.9) | ||||
| MCV1 | 0.0002 | <0.001 | 0.7056 | |||||||
| North | 42 (16.7) | 210 (83.3) | 45 (6.6) | 636 (93.4) | 56 (9.4) | 543 (90.6) | ||||
| Central | 213 (25.2) | 632 (74.8) | 123 (9.8) | 1139 (90.2) | 96 (8.5) | 1036 (91.5) | ||||
| Southern | 202 (18.1) | 912 (81.9) | 102 (5.5) | 1756 (94.5) | 140 (9.4) | 1354 (90.6) | ||||
| FVC | <0.0001 | <0.001 | 0.0002 | |||||||
| North | 74 (29.4) | 178 (70.6) | 98 (14.4) | 583 (85.6) | 150 (25.0) | 449 (75.0) | ||||
| Central | 348 (41.2) | 497 (58.8) | 262 (20.8) | 1000 (79.2) | 316 (27.9) | 816 (72.1) | ||||
| Southern | 347 (31.2) | 767 (68.2) | 268 (14.4) | 1590 (85.6) | 418 (27.9) | 1076 (72.1) | ||||
Logistic regression results
Tables 5 and 6 show the magnitude of being unimmunized with Penta3 and MCV1. In Table 5, after adjusting for geographical region, compared with children who were immunized with Penta1, those with Penta3 were 74% (aOR 0.26 [95% CI 0.21 to 0.32]) less likely to be immunized in 2004. Compared with children with Penta1 immunization, those with Penta3 were 60% (aOR 0.40 [95% CI 0.31 to 0.51]) less likely to be immunized in 2010. Additionally, children in 2015–2016 were 62% (aOR 0.29 [95% CI 0.23 to 0.48]) less likely to be immunized with Penta3 compared with Penta1. In Table 6, children were 81% less likely to be immunized with MCV1 (aOR 0.19 [95% 0.16 to 0.24]) in 2004 compared with children who were immunized with Penta1. In 2010, children were 69% (aOR 0.31 [95% CI 0.25 to 0.40]) less likely to be immunized with MCV1 compared with Penta1. Further, in 2015–2016, children were 71% (aOR 0.29 [95% CI 0.23 to 0.37]) less likely to be immunized with MCV1 compared with Penta1.
Table 5.
Logistic regression results for Penta
| Year | Infants vaccinated (Penta1) | Infants vaccinated (Penta3) | Logistic regression* | |||
|---|---|---|---|---|---|---|
| No, n (%) | Yes, n (%) | No, n (%) | Yes, n (%) | aOR (95% CI) | p-Value | |
| 2004 | 110 (5.0) | 2101 (95.0) | 374 (16.9) | 1837 (83.1) | 0.26 (0.21 to 0.32) | <0.001 |
| 2010 | 89 (2.3) | 3712 (97.7) | 216 (5.7) | 3585 (94.3) | 0.40 (0.31 to 0.51) | <0.001 |
| 2015–2016 | 91 (2.2) | 3134 (97.8) | 231 (7.2) | 2994 (92.8) | 0.38 (0.29 to 0.48) | <0.001 |
*Adjusted for geographical region.
Table 6.
Logistic regression results for MCV1
| Year | Infants vaccinated (Penta1) | Infants vaccinated (MCV1) | Logistic Regression* | |||
|---|---|---|---|---|---|---|
| No, n (%) | Yes, n (%) | No, n (%) | Yes, n (%) | aOR (95% CI) | p-Value | |
| 2004 | 110 (5.0) | 2101 (95.0) | 475 (20.7) | 1754 (79.3) | 0.19 (0.16 to 0.24) | <0.001 |
| 2010 | 89 (2.3) | 3712 (97.7) | 270 (7.1) | 3531 (92.9) | 0.31 (0.25 to 0.40) | <0.001 |
| 2015–16 | 91 (2.82) | 3134 (97.8) | 292 (9.1) | 2933 (90.9) | 0.29 (0.23 to 0.37) | <0.001 |
*Adjusted for geographical region.
Discussion
Health inequalities occur when health services are not being accessed or utilized by a certain portion of the population (based on socio-economic and demographic characteristics as well as geographical location). These inequalities can result from a lack of resources required to meet the needs of vulnerable populations. Immunization is a process whereby an individual is made immune or resistant to an infectious disease, typically by the administration of a vaccine.30 Immunization can be delivered successfully through outreach clinics and does not require any major lifestyle changes. Hence it is accessible to even the most hard-to-reach and vulnerable populations.30
The overarching aim of the present study was to examine the effective immunization coverage, immunization dropout rate and equity gaps among children ages 12–23 months in Malawi. The current study revealed that immunization coverage was >90.0% for most of the antigens, while the full immunization status was estimated at 65–73% between 2004 and 2015–2016. The highest coverage was observed for BCG and the lowest for OPV1. The BNA revealed that initial utilization of immunization was high (Penta1 coverage) in all cohort years. Regarding continuous utilization, the BNA revealed that over the 15 years the coverage of Penta3 has improved, with 90% coverage in 2010 and 2015–2016. Similar patterns were observed in immunization services for MCV1.
The BNA revealed that despite the high coverages of various antigens, the quality of immunization in Malawi (equity gaps) is lagging. This is reflected in the low prevalence of FVC across the years. This study revealed that across all the cohort years, children were less likely to be immunized with Penta3 and MCV1, which shows that a certain portion of parents have difficulties in getting to (accessing) the immunization services for subsequent doses or parents are not utilizing the health services (underutilization of services). However, Penta1 coverage was high, showing that the availability and initial utilization of immunization services is satisfactory in Malawi.
The dropout rate is assessed as the number of Penta1 and Penta3 or Penta1 and MCV1 vaccines given to a child over 1 y. According to the WHO, a dropout rate of >10% is considered to be undesirable and shows that a certain health facility has utilization constraints.31 In this study, the dropout rates of Penta3 and MCV1 were significantly >10% in 2004 and <10% in 2010 and 2015–2016. A low dropout rate is indicative of good utilization and therefore of good service quality. Similarly, more children were unimmunized with Penta3 and MCV1 in 2004. However, both dropout rates and unimmunized status had U-shaped patterns, indicating that immunization service in Malawi has been deteriorating in recent years. The possible causes of low immunization uptake and high dropout rates vary across countries/regions, but most countries in sub-Saharan Africa share similar root causes. The most common reasons for poor immunization are service organization problems, staffing problems and data collection and reporting problems.32
We could not explicitly determine the reasons for the high dropout rate and poor effective immunization coverage due to the absence of data. However, previous research and information from health facilities has attributed the gaps in immunization to immediate and underlying causes. Immediate causes include cancellation of scheduled immunization sessions, vaccine being out of stock at the health centre, inadequate supportive supervision and performance feedback to the health centre and poor documentation and record keeping for immunization services.33 Also, health surveillance assistants (HSAs) have frequently reported that a lack of clarity about the denominator of the data used creates lots of missing values and that many hard-to-reach communities are underserved by village clinics or outreach services, thus putting women and children at risk of not accessing the service as underlying causes of immunization inequalities.34
In line with previous research,7,14 the current study found that full immunization coverage was below the 90% target set by the WHO. An analysis of the effects of individual and community-level factors on childhood immunization in Malawi indicated that women's education (especially those with no formal education), women with either one antenatal visit or none, women with no immunization card, women from the poorest households, women with three and more children, women from the central region and women from communities where distance to the nearest health facility was perceived as a big problem were reported to have reduced odds of achieving full immunization coverage.7,14 In Mozambique it was reported that accessibility to vaccination sites, no schooling of mothers and children born at home or outside were the reasons for incomplete vaccination.35 Additionally, being born at a health institution, a higher level of maternal education, media exposure, region of residence and residing in communities possessing greater maternal antenatal care utilization were positively associated with full childhood immunization in Ethiopia.36
Limitations
The results of this study should be interpreted with caution. First, a cross-sectional study design cannot be used to infer causality because a temporal sequence cannot be established. Second, we used secondary data whose population estimates were obtained from the 2018 Malawi Population and Housing Census conducted by the National Statistical Office (NSO), thus the denominator obtained from the NSO may differ from the head count collected by the HSAs. Third, information on immunization was collected from vaccination cards, thus our results are subject to recall bias, as the respondents who did not have health cards were asked to recall administered vaccines (2004: vaccine from vaccination card, booklet or other home-based record, 57.43%; vaccine from mother's report, 7.0%; 2010: vaccine from vaccination card, booklet or other home-based record, 74.3%; vaccine from mother's report, 6.6%; 2015–2016: vaccine from vaccination card, booklet or other home-based record, 69%; vaccine from mother's report, 6.8%). Lastly, the datasets did not record any information on unavailability of vaccines due to stockouts, access to services, lack of adequate vaccine supply and inconsistent scheduling of vaccination supply.
Recommendations
There is a need for intensified engagement with communities through the Reaching Every Child approach so that children are fully immunized. There is a need for health education and orientation of service providers. In addition, immunization registers and data quality should be investigated and dealt with accordingly. Further research at health facilities is needed to validate the results of the current study and to determine which health facilities are underperforming. Finally, there is a need to introduce an electronic immunization register in Malawi to facilitate the monitoring of individual immunization schedules and the storage of individual immunization histories. This may help to enhance the performance of the EPI in terms of coverage, efficiency and effectiveness.
Conclusions
Fully immunized status over the 15 y of childhood has been lagging, but immunization coverages for most of antigens are high and the dropout rate over the last 10 y is lower than the WHO's recommended cut-off point. Furthermore, initial utilization of immunization services was satisfactory but continued utilization and adequate coverage of immunization services had irregular patterns. These results should help the Malawi EPI to address disparities that are occurring in immunization services. There is a need for further studies to establish the reasons for the lack of full immunization coverage in Malawi.
Acknowledgements
The authors are sincerely grateful to National Statistical Office of Malawi for data collection. We thank MEASURE DHS for providing us with the population-based dataset through their archives, which can be downloaded from http://dhsprogram.com/data/available-datasets.cfm.
Contributor Information
Kondwani Mmanga, African Field Epidemiology Network, Ministry of Health, Expanded Programme on Immunization, P.O. Box 30377, Lilongwe, Malawi.
Tisungane E Mwenyenkulu, Department of Clinical Sciences, Academy of Medical Sciences, Malawi University of Science and Technology, P.O. Box 5196, Limbe, Malawi.
Owen Nkoka, Institute of Health and Wellbeing, University of Glasgow, Glasgow G12 8QQ, UK.
Peter A M Ntenda, Malaria Alert Centre, College of Medicine, Kamuzu University of Health Sciences, Private Bag 360, Chichiri, Blantyre 3, Malawi.
Authors’ contributions
PAMN, ON, ETM and KM contributed to the conception and design of the study. PAMN acquired data and conducted the analysis. PAMN and KM drafted the manuscript. ON and ETM critically revised the draft for important intellectual content. All authors reviewed and approved the final version of the manuscript.
Funding
This research did not receive a grant from any funding agency in the public, commercial or not-for-profit sectors. Funding for the 2015–2016 MDHS was provided by the government of Malawi, the United States Agency for International Development, UNICEF, the Malawi National AIDS Commission, the United Nations Population Fund, UN WOMEN, Irish Aid and the World Bank.
Conflict of interest
None declared.
Ethics approval
The protocols for the 2004, 2010 and 2015–2016 MDHS were reviewed and approved by the Malawi National Health Sciences Research Committee (approval 15/4/1436), the Institutional Review Board of ICF Macro (project 132989.0.000.DHS 01) and the Centers for Disease Control and Prevention in Atlanta, GA, USA. Data collection was implemented by the National Statistical Office. At the beginning of each interview, informed consent was obtained from the participants. The authors sought permission from the DHS program for the use of the data. The data obtained from respondents were anonymous, as names were not written down, thus ethics approval for this study was not required.
Data availability
The datasets generated and/or analysed during the current study are available in the DHS Program repository (https://dhsprogram.com/data/dataset/Malawi_Standard-DHS_2015.cfm?flag=1).
References
- 1. Rodrigues CMC, Plotkin SA.. Impact of vaccines; health, economic and social perspectives. Front Microbiol. 2020;11:1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Vaccines and immunization. Available from: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1 [accessed 24 January 2021]. [Google Scholar]
- 3. World Health Organization . The expanded programme on immunization. Available from: http://www.who.int/immunization/programmes_systems/supply_chain/benefits_of_immunization/en/ [accessed 30 December 2016]. [Google Scholar]
- 4. Centers for Disease Control and Prevention . New and underused vaccines. Available from: https://www.cdc.gov/globalhealth/immunization/sis/vacs_detail.htm [accessed 24 January 2021]. [Google Scholar]
- 5. World Health Organization . Immunization today and in the next decade. Developing together the vision and strategy for immunization 2021–2030. Available from: https://www.who.int/immunization/ia2030_Draft_Zero.pdf?ua=1 [accessed 14 June2021]. [Google Scholar]
- 6. Malawi Ministry of Health . Expanded programme on immunization. Available from: http://www.health.gov.mw/index.php/expanded-programme-on-immunization [accessed 21 December 2017]. [Google Scholar]
- 7. Munthali AC. Determinants of vaccination coverage in Malawi: evidence from the demographic and health surveys. Malawi Med J. 2007;19(2):79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Statistical Office, ICF Macro . Malawi Demographic and Health Survey 2010. Zomba, Malawi and Calverton, MD: National Statistical Office and ICF Macro; 2011. [Google Scholar]
- 9. National Statistical Office, ICF Macro . Malawi Demographic and Health Survey 2015–16. Zomba, Malawi and Rockville, MD: National Statistical Office and ICF Macro; 2017. [Google Scholar]
- 10. Government of Malawi . EPI Comprehensive Multi-Year Plan Malawi. Lilongwe: Government of Malawi; 2016. [Google Scholar]
- 11. Bell GJ, Loop MS, Mvalo Tet al. . Environmental modifiers of RTS,S/AS01 malaria vaccine efficacy in Lilongwe, Malawi. BMC Public Health. 2020;20:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ntenda PAM. Factors associated with non-and under-vaccination among children aged 12–23 months in Malawi. A multinomial analysis of the population-based sample. Pediatrics & Neonatology. 2019;60(6):623–33. [DOI] [PubMed] [Google Scholar]
- 13. Yawson AE, Bonsu G, Senaya LKet al. . Regional disparities in immunization services in Ghana through a bottleneck analysis approach : implications for sustaining national gains in immunization. Arch Public Heal. 2017;75:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ntenda PAM, Chuang K-Y, Tiruneh FNet al. . Analysis of the effects of individual and community level factors on childhood immunization in Malawi. Vaccine. 2017;35(15):1907–17. [DOI] [PubMed] [Google Scholar]
- 15. Tsega A, Hausi H, Chriwa Get al. . Vaccination coverage and timely vaccination with valid doses in Malawi. Vaccine Reports. 2016;6:8–12. [Google Scholar]
- 16. Wetherill O, Lee C, Dietz Vet al. . Root causes of poor immunisation data quality and proven interventions: a systematic literature review. Ann Infect Dis Epidemiol. 2017;2(1):1012. [PMC free article] [PubMed] [Google Scholar]
- 17. Mane AB. Immunization dropout rates: some issues. Ann Med Health Sci Res. 2015;5(2):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The Malawi Project . Geography 2018. Available from: https://www.malawiproject.org/zzz/geography/ [accessed 27 December 2018]. [Google Scholar]
- 19. National Statistical Office . Malawi in figures. Available from: http://www.nsomalawi.mw/index.php?option=com_content&view=article&id=40%3Amalawi-in-figures&catid=2&Itemid=40 [accessed 14 June2021. ]. [Google Scholar]
- 20. World Bank . Malawi. Available from: https://data.worldbank.org/country/malawi [accessed 27 December 2018]. [Google Scholar]
- 21. Government of Malawi . Malawi growth and development strategy II 2011–2016. Available from: https://www.resakss.org/sites/default/files/Malawi%202012%20Malawi%20Growth%20and%20Development%20Strategy%20(MGDS)%20II%202011%20-%202016.pdf [accessed 14 June2021]. [Google Scholar]
- 22. Makwero MT. Delivery of primary health care in Malawi. African J Prim Heal Care Fam Med. 2018;10. doi:10.4102/phcfm.v10i1.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization Partnership for Maternal, Newborn and Child Health. Trends.Available from: https://www.who.int/pmnch/activities/countries/malawi/en/index1.html#:∼:text=Trends&text=During the past fifteen years,133 per 1000 live births [accessed 26 April 2021]. [Google Scholar]
- 24. National Malaria Control Programme, ICF Macro . Malawi Malaria Indicator Survey 2017. Lilongwe, Malawi and Rockville, MD, USA: National Malaria Control Programme and ICF Macro; 2018. [Google Scholar]
- 25. National Statistical Office, ORC Macro . Malawi Demographic and Health Survey 2004. Calverton, MD: ORC Macro; 2005. [Google Scholar]
- 26. Njiraini RCM, Agongo EEA, Awoonor-Williams JKet al. . Adoption and use of the bottleneck analysis approach in Ghana's health sector. In: Maternal, newborn and child health. Working paper. New York: UNICEF; 2015. [Google Scholar]
- 27. UNICEF . Enhanced programming and results through Monitoring Results for Equity Systems (MoRES). Briefing note. New York: UNICEF; 2013. [Google Scholar]
- 28. Open Learn . Health Education and Training (HEAT). Immunization. 10.2.2 How to measure dropout rates. Available from: http://www.open.edu/openlearncreate/mod/oucontent/view.php?id=53371§ion=1.4.2 [accessed 20 January 2019]. [Google Scholar]
- 29. Chinawa J. Immunization dropout rates in Ihe, Awgu Local Government Area, Enugu State, South East Nigeria: a 1 year review. Ann Med Health Sci Res. 2014;4:642–6. doi:10.4103/2141-9248.139360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization . Immunization. Available from: https://www.who.int/topics/immunization/en/ [accessed 24 December 2018]. [Google Scholar]
- 31. World Health Organization . Training for mid-level managers (MLM): module 7: the EPI coverage survey. Available from: https://apps.who.int/iris/handle/10665/337065 [accessed 14 June2021]. [Google Scholar]
- 32. Open Edu . Immunization module: monitoring your immunization programme. Available from: http://www.open.edu/openlearncreate/mod/oucontent/view.php?id=53371&printable=1 [accessed 22 February 2019]. [Google Scholar]
- 33. Zewdie A, Letebo M, Mekonnen T.. Reasons for defaulting from childhood immunization program: a qualitative study from Hadiya zone, Southern Ethiopia. BMC Public Health. 2016;16:1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andermann A. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016;188(17–18):E474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jani JV, De Schacht C, Jani IVet al. . Risk factors for incomplete vaccination and missed opportunity for immunization in rural Mozambique. BMC Public Health. 2008;8:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abadura SA, Lerebo WT, Kulkarni Uet al. . Individual and community level determinants of childhood full immunization in Ethiopia: a multilevel analysis. BMC Public Health. 2015;15:972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the DHS Program repository (https://dhsprogram.com/data/dataset/Malawi_Standard-DHS_2015.cfm?flag=1).