Abstract
OBJECTIVES
We studied 16 patients after failed alcohol septal ablation who underwent extended septal myectomy to analyse the results of surgical correction and identify technical pitfalls the surgeons may be confronted by.
METHODS
Between October 2017 and March 2019, 16 patients underwent surgical extended septal myectomy with accompanying anomalous secondary chordae resection, papillary muscles mobilization [in 9 (56.3%) patients], and anterior mitral leaflet plication after previously failed alcohol septal ablation. Routine preoperative computed tomography or cardiac magnetic resonance planning and intraoperative transoesophageal echocardiography were performed in each of the studied patients. Major technical features were identified and complemented during septal myectomy of the calcified interventricular septum.
RESULTS
The mean age of the studied patients accounted 50.5 ± 14.6, median—54; males—5 (31.3%). Mean cross-clamp time accounted 52 ± 7.2 min. Calcified basal interventricular septum was identified in 2 (12.5%) patients. No iatrogenic ventricular septal defect (0%) was made during surgical correction. Peak systolic pressure gradient decreased from 86 (interquartile range: 75–104.7) to 20 (16–22) mmHg (P< 0.001). No patients with moderate or severe mitral regurgitation were identified, whereas before the procedure, the number of those accounted 13 (81.2%) individuals. In-hospital and overall mortality after septal myectomy accounted 0%.
CONCLUSIONS
Extended septal myectomy in patients who previously underwent alcohol septal ablation is a safe procedure that affects all pathological manifestations of the disease. Routine preoperative computed tomography or cardiac magnetic resonance provides detailed anatomy of the anomalous left ventricle and subvalvular structures and allows to measure the extension of myectomy preventing the occurrence of iatrogenic ventricular septal defect. Septal myectomy of the calcified interventricular septum requires avoidance of ‘one-piece technique’ since fragmental myectomy allows visually control the adequacy of the left ventricle outflow tract release.
Keywords: Hypertrophic obstructive cardiomyopathy, Alcohol septal ablation, Mitral valve repair
For more than 50 years, transaortic septal myectomy is known to be the gold standard for treatment of hypertrophic obstructive cardiomyopathy (HOCM) [1, 2].
INTRODUCTION
For more than 50 years, transaortic septal myectomy is known to be the gold standard for treatment of hypertrophic obstructive cardiomyopathy (HOCM) [1, 2]. This safe and effective surgical procedure [3–5] allows to affect all pathophysiological mechanisms of the genetically inherited disease. In addition, relief of the obstruction during septal myectomy with concomitant mitral valve (MV) surgery restores the functional capacity and normal life span, and the survival rate of surgical patients is similar to that of the general matched population [6].
With the development of era of catheter-based treatment, septal myectomy has been dramatically eclipsed by less invasive approach—alcohol septal ablation (ASA). Although ASA is performed percutaneously providing better cosmetic effect, the procedural risks of death and morbidity are not lower than the risks of operation [7], and the risk of reintervention after ASA is reported to be much higher compared to septal myectomy [8].
Taking into account that the mechanisms which predispose the left ventricular outflow tract (LVOT) obstruction involve not only basal septal hypertrophy, small size of the left ventricle (LV), hypercontractility of the LV [9], but also the anomalies of the subvalvular apparatus of the MV [10], choosing the less invasive approach for these patients may not always correlate with adequate postoperative haemodynamic result. It has been reported that overall haemodynamic success of the ASA is about 70%, with an approximate 20% incidence of recurrence of severe symptoms [11]. Additionally, the myocardial infarction created by injection of ethanol is associated with higher incidence of early complications compared to surgical correction [8, 12, 13].
In this study, we present the results of treatment of 16 patients with HOCM who underwent surgical septal myectomy with accompanying MV surgery after failed ASA and sought to determine the technical pitfalls the surgeons may be confronted by during the procedure.
MATERIALS AND METHODS
Study population
We studied 16 patients presented at our Institute between October 2017 and March 2019 who previously underwent ASA and had significant residual symptoms of progressive heart failure. All patients were referred to surgical treatment that involved 4 steps of surgical correction: extended/shallow septal myectomy, secondary chordae cutting, papillary muscles (PM) mobilization and anterior mitral leaflet (AML) plication. Three patients underwent ASA at our Institution and 13 patients underwent ablation at other medical facilities. One patient underwent ASA, dual-chamber pacemaker and implantable cardioverter-defibrillator (ICD) implantation. Three patients previously underwent more than 1 ASA procedures.
Ethical statement
This study had been approved by Amosov National Institute of Cardiovascular Surgery Review Board and Ethics Committee (protocol № 6074391 from 12 November 2020) and informed written consent had been acquired from each patient included to the study.
Data availability statement
The data that support the findings in this study are available on request from the corresponding author.
Diagnosis
Following the latest recommendations [1, 2], the diagnosis of HOCM in each patient was established based on the presence of hypertrophied myocardium with non-dilated LV in the absence of other cardiac or systemic diseases which could have explained the hypertrophy. The indications for surgery involved the systolic pressure gradient (SPG) ≥50 mmHg and symptoms refractory to adequate medical treatment. All 16 patients underwent baseline echocardiographic and imaging examinations including transthoracic echocardiography and computed tomography (CT) of the heart or cardiac magnetic resonance (CMR) with late gadolinium enhancement with subsequent calculations of the size of the right ventricle, LV and interventricular septum (IVS) to detect structural abnormalities, degree of fibrotic process and congenital heart defects.
Preoperative planning
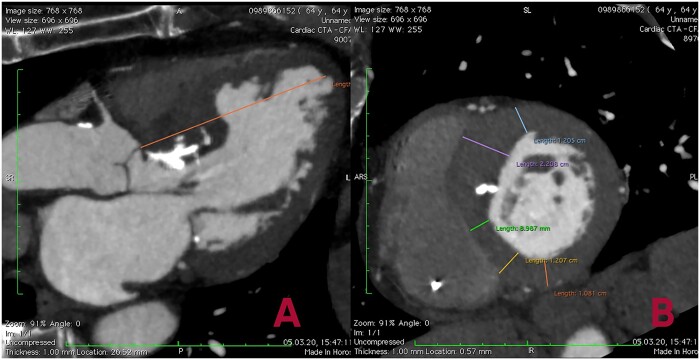
During CT in each patient, the thickness of the anterior, anteroseptal, posteroseptal and posterior segments of the LV was measured in a 2-chamber short-axis view (Fig. 1). The starting point from which the measurement of the thickness of the above-mentioned segments was performed, was determined in a 3-chamber long-axis view at a distance of 8 mm from the aortic valve (AV) ring. Subsequent measurements were performed at every 8 mm beginning from the basal segments of the LV to the PM level (equatorial zone of the LV) to reconstruct the distribution of hypertrophy at the different levels of the LV. The length of the LV was measured as the distance from the AV ring to the LV apex in a 3-chamber long-axis view. The estimated length of resection during septal myectomy was defined as the sum of consecutive hypertrophied LV segments measured at every 8 mm level beginning from the AV ring. During surgery, data obtained from the detailed CT-analysis of IVS thickness were used as a guide for septal myectomy and allowed to avoid iatrogenic ventricular septal defect (VSD) during procedure.
Figure 1:
Preoperative computed tomography planning using long- (A) and short-axis (B) views, where the distance from the aortic valve ring to the left ventricular apex in the 3-chamber view and the thickness of the left ventricle and interventricular septum are measured at a distance of 8 mm from the aortic valve ring at the end of diastole.
Transoesophageal echocardiography
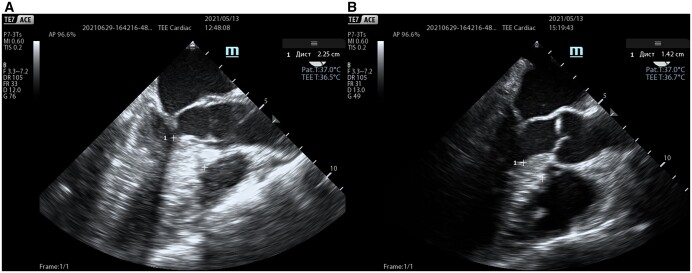
After induction of anaesthesia, intraoperative transoesophageal echocardiography (TEE) was performed to determine the extent of myectomy, as well as to assess the morphology of the MV and the presence of associated primary MV anomalies (Fig. 2). TEE was repeated in the operating room immediately after stopping the cardiopulmonary bypass for the assessment of residual gradient on the LVOT and regurgitation on aortic and MVs, as well as for the detection of possible surgical complications (e.g. iatrogenic VSD). In addition, TEE was used to measure the coaptation depth in mid-oesophageal 4-chamber view and coaptation-to-septal distance in mid-oesophageal AV long-axis view before and after procedure as one of the key predictors of residual systolic anterior motion (SAM) and mitral regurgitation (MR). Coaptation depth was defined as the shortest distance between the coaptation and the annular plane, while coaptation-to-septal distance was defined as the shortest distance between the coaptation point and the IVS at early systole.
Figure 2:
Intraoperative TEE images (mid-oesophageal aortic valve long-axis view) in early systole before (A) and after (B) procedure; elimination of the left ventricular outflow tract obstruction with concomitant procedures on the mitral valve subvalvular apparatus. Septal thickness (1) was measured before and after correction. TEE: transoesophageal echocardiography.
Surgical procedure
Surgical correction included a series of steps: extended myectomy, resection of fibrotic and retracted secondary chordae of the MV, PM mobilization and, in case of necessity, AML plication with reduction of its size and area.
Septal myectomy was performed during cardiopulmonary bypass with mild general hypothermia. Using the exposure through the oblique aortotomy the myectomy was begun from 2 longitudinal incisions in the basal part of the IVS, 2–3 mm below the AV, gradually continuing the resection more distally, to the base of the PM (equatorial zone), creating a trapezoidal muscular band which is wider towards the apex than at the subaortic level. Myectomy was always performed using the data obtained from CT or CMR imaging in order to prevent iatrogenic VSD since the thickness of the IVS on various levels was different due to extensive fibrotic changes of the IVS. In case of the calcified IVS, the myectomy was performed fragmentally by resection of small parts of stiff myocardium in order to achieve better visual control of the LVOT release (Fig. 3). After removal of the cardiac muscle, the cavity of the LV was flushed several times with normal saline in order to remove any small particles of the calcified IVS from the heart and prevent the embolic events.
Figure 3:
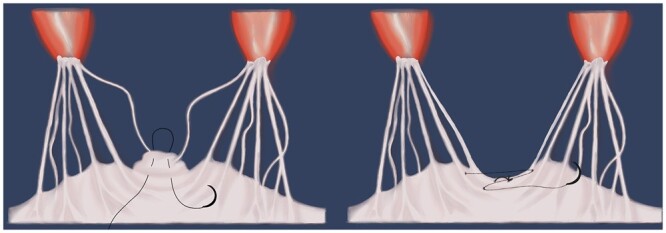
Plication of the anterior mitral leaflet technique. Placing a vertical everting U-suture at the edge of the leaflet in the zone of excess tissue (A). Then, an additional z-suture was placed to approach 2 primary chordae (B).
After septal myectomy, an intervention on the subvalvular mitral apparatus was performed. Fibrous and muscular structures that connect PM with IVS or LV free wall were present practically in all patients with HOCM and were limiting the mobility of the PM. Such structures, which can be identified on CT or, sometimes, only at the time of surgery, were systematically resected in each of the examined patient for the purpose of improvement of the PM mobility (PM mobilization). Fibrotic and retracted chordae (pathological secondary chordae) or fibrous attachments between the AML and PM were found in the majority of patients with HOCM. These structures routinely underwent resection, which decreased the depth of coaptation of the MV leaflets and prevented the phenomenon of SAM in the postoperative period. In case of diastasis between the places of attachment of the primary chordae to the edge of the MV leaflet over 5 mm, this area was plicated. After the area of diastasis and the excess tissue of the AML in between 2 primary chordae was identified, a vertical everting U-suture was placed at the edge of the leaflet. Then, an additional z-suture was placed to approach 2 primary chordae (Fig. 3).
Follow-up
The follow-up was complete and averaged 36 ± 14 months (maximum of 47 months). Follow-up data were collected from medical charts or written correspondents from referring cardiologists of the patients. Vital status assessment was performed via telephone interview directly with patients or their relatives and confirmed by the absence of death certificated that were requested at the medical facilities. The primary endpoints involved cardiac death, advanced heart failure and ICD discharge at follow-up.
Statistical analysis
Categorical variables are expressed as frequencies and percentages, and continuous variables are expressed as mean ± standard deviation or median [interquartile range (IQR)]. Fisher’s exact test was used to compare categorical variables and Wilcoxon rank-sum test was used to compare continuous variables. All reported P-values are 2-sided. The Kaplan–Meier method was used to draw survival curves and calculate survival statistics for long-term outcomes. SPSS statistical software (IBM® SPSS® Statistics 22.0, Chicago, IL, USA) was used for all the calculations.
RESULTS
There were a total of 16 patients [5 males (31.3%)] who underwent extended/shallow myectomy accompanied by MV surgery after failed ASA. The age of the patients ranged from 11 to 65 years old, and the mean age was 50.5 ± 14.6 (median—54). Three (18.8%) patients previously underwent more than 1 ASA, and other 2 (12.5%) had right bundle branch block after ASA. CT was performed in 13 (81.25%) patients and CMR in 3 (18.75%) patients. Preoperative baseline characteristics are collected in Table 1.
Table 1:
Preoperative baseline characteristics of 16 studied patients
| Variable | Value |
|---|---|
| Age, mean ± SD (median) | 50.5 ± 14.6 (54) |
| Males, n (%) | 5 (31.3) |
| Paroxysmal/persistent AF | 1 (6.3) |
| RBBB, n (%) | 2 (12.5) |
| Preoperative infective endocarditis | 1 (6.3) |
| SCD risk (calculated in 15 patients) | |
| Mild, n (%) | 6 (40) |
| Moderate, n (%) | 7 (46.7) |
| High, n (%) | 2 (13.3) |
| Preoperative ICD implantation | 1 (6.3) |
| Preoperative pacemaker implantation | 1 (6.3) |
| Family history of SCD | 4 (25) |
AF: atrial fibrillation; ICD: implantable cardioverter-defibrillator; RBBB: right bundle branch block; SCD: sudden cardiac death; SD: standard deviation.
Procedural features
The average cross-clamp time was 41.5 ± 3.6 min. Fourteen (87.5%) patients underwent extended septal myectomy and 2 patients (12.5%)—shallow myectomy. Calcified IVS was observed in 12.5% (2) of all the cases and was excised by fragments, providing adequate visual control of the amount of myocardium needed to be removed in order to release the LVOT. In other 14 (87.5%) patients ‘one-piece myectomy’ technique was applied. The average mass of the resected myocardium accounted 5.2 ± 0.2 g. Resection of the pathological secondary chordae was performed in all 16 (100%) studied individuals, while the AML was plicated in 9 (56.3%). The procedure of PM mobilization was performed as follows: the anterior group of PM was mobilized in 3 (18.8%) patients, posterior group—in 1 (6.3%) patient, and both groups were mobilized in 6 patients (3.75%). One (6.3%) patient had a duplication of the anterior group of PM and a fibrotic bundle attaching to A1 segment of the AML which was resected during surgery. One (6.3%) patient underwent MV replacement with bileaflet mechanical prosthesis (St. Jude Medical™, Inc. №29) due to existing preoperative infective endocarditis.
Intraoperatively, coaptation depth and coaptation-to-septal distance were measured using TEE at early systole. According to the data revealed, coaptation depth after MV repair accounted 5.4 ± 1.3 mm vs 9.6 ± 3.1 mm before the surgery (P < 0.001). Coaptation-to-septal distance prior correction was 1.77 ± 0.81 mm, whereas after the procedure it accounted 3.32 ± 0.78 mm (P < 0.001).
No iatrogenic VSD was made during septal myectomy. None of the patients had reoperation or re-exploration for bleeding after the surgery.
Postoperative outcomes
The average in-hospital stay accounted 8.2 ± 2.4 days. All 16 studied patients had significant improvement in their symptoms and haemodynamic parameters after surgery (Table 2). The LVOT release resulted in the dramatic decrease of the SPG from 86 (IQR: 75–104.7) mmHg to 18.8 (IQR: 14.3–26) mmHg at discharge (P < 0.001).
Table 2:
The results of septal myectomy in patients with previous ASA
| Variable | Value |
||||
|---|---|---|---|---|---|
| Before surgery | At discharge | 2 years after procedure | P-value | ||
| NYHA functional class | |||||
| I, n (%) | 1 (6.3) | 2 (12.5) | 4 (25) | 0.038 | |
| II, n (%) | 7 (43.8) | 14 (87.5) | 12 (75) | 0.047 | |
| III, n (%) | 8 (50) | 0 (0) | 0 (0) | 0.019 | |
| IV, n (%) | 0 (0) | 0 (0) | 0 (0) | <0.001 | |
| Postoperative echocardiography data | |||||
| Peak SPG on LVOT at rest or on exertion, mmHg; median (IQR) | 86 (75–104.7) | 18.8 (14.3–26) | 20 (16–22) | <0.001 | |
| SAM, n (%) | 15 (93.75) | 0 (0) | 0 (0) | 0.014 | |
| Mitral regurgitation degree, n (%) | |||||
| 0 | 0 (0) | 0 (0) | 1 (6.3) | <0.001 | |
| 1 | 3 (18.8) | 15 (93.7) | 15 (93.7) | 0.016 | |
| 2 | 11 (68.8) | 1 (6.3) | 0 (0) | 0.029 | |
| 3 | 2 (12.5) | 0 (0) | 0 (0) | 0.011 | |
| 4 | 0 (0) | 0 (0) | 0 (0) | <0.001 | |
| LV basal septum, mm; median (IQR) | 22.5 (21–26) | 14 (13–15.3) | 14 (13.3–16) | <0.001 | |
| LV posterior wall, mm; median (IQR) | 13 (12.3–15) | 13 (12.3–14) | 13 (12.3–14) | 0.083 | |
| LA diameter, mm; median (IQR) | 35 (42–48.5) | 42 (40–46.3) | 42 (40–44.8) | <0.001 | |
| EDV, ml; median (IQR) | 87.5 (75.3–97.5) | 91 (87.5–101.5) | 93 (88.3–102) | 0.016 | |
| AML, mm; median (IQR) | 38 (36–40.5) | 34.8 (32.5–36.3) | 33.5 (31–35.8) | <0.001 | |
| EF, %; median (IQR) | 64.5 (61.3–65) | 61.3 (60.5–64.8) | 60.5 (58.3–63) | 0.032 | |
Given P-values are the comparisons between preoperative and late follow-up data.
AML: anterior mitral leaflet; ASA: alcohol septal ablation; EDV: end-diastolic volume; EF: ejection fraction; ICD: implantable cardioverter-defibrillator; LA: left atrium; LV: left ventricle; LVOT: left ventricular outflow tract; NYHA: New York Heart Association; SAM: systolic anterior motion; SCD: sudden cardiac death; SD: standard deviation; SPG: systolic pressure gradient.
Table 3:
Major complications after surgery
| Postoperative LBBB, n (%) | 11 (68.8) |
| Complete AV-block, n (%) | 2 (12,5) |
| Postoperative AF, n (%) | 0 (0) |
| New pacemaker implantation, n (%) | 2 (12.5) |
| New ICD implantation, n (%) | 0 (0) |
| Sudden cardiac death, n (%) | 0 (0) |
| ICD discharge, n (%) | 1 (6.3) |
| Advanced heart failure, n (%) | 0 (0) |
| Cardiac death, n (%) | 0 (0) |
| All-cause death, n (%) | 0 (0) |
AF: atrial fibrillation; AV: aortic valve; ICD: implantable cardioverter-defibrillator; LBBB: left bundle branch block.
In addition, an echocardiographic assessment of the MR was performed and distributed on a scale from 0 to 4 (0—no MR, 1—mild MR, 2—moderate MR, 3—moderate-to-severe MR, 4—severe MR). Each patient had a certain degree of dynamic MR caused by the presence of SAM in 15 (93.75%) of patients. In 1 (6.3%) patient, MR was caused by the presence of the vegetation on the AML which was the indication to MV replacement. In this study, 3 (18.8%) individuals had mild, 11 (68.8%)—moderate, and 2 (12.5%)—moderate-to-severe MR, respectively. No patients with moderate or severe MR were identified after procedure. In addition, the relief of the LVOT and reduction of SAM in all these patients resulted in significant improvement of their New York Heart Association functional class. Four (25%) patients were in New York Heart Association class I, and 12 (75%) patients were in New York Heart Association class II after surgical intervention.
Eventually, 2 (12.5%) patients required pacemaker implantation due to complete AV-block. Kaplan–Meier estimates of survival at 3 years were 100% (95% confidence interval, 100–100) in the studied group. In-hospital and overall mortality after septal myectomy accounted 0%. No cardiovascular events such as sudden cardiac death, cardiac arrest or advanced heart failure were registered during 2-year follow-up. Freedom from reoperation accounted 100% (95% confidence interval, 100–100) at 3-year follow-up. During the same period, 1 (6.3%) patient had an ICD discharge occurred 1.8 years after surgery. Freedom from ICD discharge was 93.7% (95% confidence interval, 51.5–61.3; Fig. 4). Additional major complications after septal myectomy are listed in Table 3.
Figure 4:
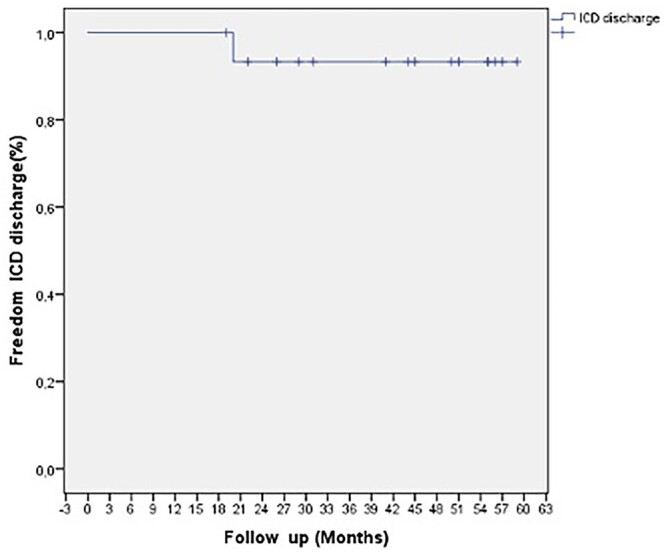
Kaplan–Meier curve showing 93.7% (95% confidence interval, 51.5–61.3) freedom from ICD discharge in patients who underwent septal myectomy after previous alcohol septal ablation. ICD: implantable cardioverter-defibrillator.
DISCUSSION
The results of our study showed that surgical septal myectomy in patients with previously failed ASA are a safe and effective procedure. The surgical intervention allows adequately release the LVOT and manage pathophysiological manifestations of HOCM by resecting anomalous secondary chordae, mobilizing PM and plicating the AML. In addition, the rate of postoperative complications is similar to that in our larger septal myectomy experience [14]. However, this study has shown that these patients are at risk of postoperative AV-block due to already existing right bundle branch block after ASA.
ASA is a mini-invasive catheter-based method of HOCM treatment that involves the injection of ethanol into the septal branch of the left anterior descending artery, thus causing the local myocardial infarction. It results in a certain degree myocardial remodelling and release of the LVOT which contributes to reduction of SPG and relieving obstruction. Despite the attractive features of this procedure, there are still a number of characteristics that limit the routine applicability of ASA in patients with HOCM.
The anomalous anatomy of the MV subvalvular apparatus can be found in the vast majority of patients with HOCM and plays an important role in manifestation of the disease [10, 15]. The structures like anomalous secondary chordae, muscular bands or fibrotic attachments between the AML and PM, IVS or LV free wall can sometimes be observed and managed only intraoperatively and cannot be resolved by ASA. Moreover, several studies report that inconsistency of the septal artery blood supply and hypertrophic obstruction in the ablation target zone may cause recurrent or residual symptomatic LVOT obstruction post-ASA [16, 17]. Another factor that contributes to the SPG recurrence is collateral blood flow that supplies the ischaemic basal septum after ASA.
Recent research conducted by Q. Yang et al. reports that the pathologic features in the ablation zone with interstitial fibrosis, myocardial necrosis and transmural myocardial scaring post-ASA, makes the subsequent septal myectomy more challenging to some extent. Our study also defines the technical difficulties which the surgeon can be confronted by. We observed calcified IVS in 2 (12.5%) out of 16 patients and suggested a slight modification of the standard myectomy which provides optimal visual control during LVOT release. Moreover, our study demonstrates an importance of the preoperative CT/CMR planning in septal myectomy. This method was first described in the work of Spirito et al. [18] According to their study, in 112 consecutive HOCM patients, depth and length of the myectomy planned at CMR were compared with those of the septal muscle excised in a single piece in all patients. Thickness and length of the planned myectomy showed a significant correlation with the excised muscle (R2 = 0.345; P < 0.001; and R2 = 0.358; P < 0.001, respectively). Thus, preoperative planning provides high-resolution images of septal morphology and allows to perform a standardized and apically extended septal excision that was associated with favourable outcome. In our study, applying this approach made myectomy more accessible and as much extended as possible preventing from iatrogenic VSD.
Another important finding is that the presence of fibrosis on the basal septum after ASA is often associated with rhythm disturbances. In some reports, risk of complete heart block may reach 20% after ASA [19]. In our study, only 1 patient had post-ASA pacemaker implantation. However, 2 (12.5%) patients required pacemaker after surgery due to complete AV-block. The explanation for this is that septal myectomy commonly produces a left bundle branch block [5], and thus pre-existing right bundle branch block at the time of surgical myectomy may confer an increased risk of complete heart block. Therefore, clinicians utilizing ASA for the treatment of HOCM should be cautious of conduction system injury, and patient selection should be optimized.
Up to date, only several existing studies report the effectiveness and long-term outcomes of septal myectomy after previous ASA. The largest ones, conducted by E. Quintana et al. [5] and Q. Yang et al. [20], report about the increased rate of complications after surgery in post-ASA patients. Moreover, little is known regarding the risk of late arrhythmias in these patients. Although, a recent study of Balt et al. [21] showed that only 7% of patients experienced malignant tachyarrhythmia (ventricular tachycardia/ventricular fibrillation) in the first post-ASA month, while no ventricular tachycardia/ventricular fibrillation was observed later, no relative data concerning myectomy patients after failed ASA exist up to date. In our studied cohort, only 1 patient had ICD discharge 1.8 years after surgery. However, considering the small number of studied patients and relatively short follow-up period, the risk of late arrhythmias occurrence should be the object of further studies.
Study limitations
This study had several limitations to mention. First, the study was a non-randomized retrospective cohort analysis with a small sample (16 patients). Therefore, the conclusions must be confirmed in a large-sample cohort study. Another limitation was the inability to define the rate of failure of ASA since the procedure was not performed at our Institute. Finally, the relatively short follow-up time does not demonstrate the full spectrum of perioperative outcomes and requires further analysis.
CONCLUSION
Surgical treatment of HOCM in patients who previously underwent ASA requires surgical approach that will provide correction of all pathological manifestations of the disease. Routine preoperative CT or CMR provides detailed anatomy of the anomalous LV and MV structures as well as allows to measure the extension of myectomy preserving from iatrogenic VSD. When performing septal myectomy of calcified IVS, it is recommended to avoid applying 1-piece technique since fragmental myectomy allows visually control the adequacy of LVOT release.
ACKNOWLEDGEMENTS
The authors thank Vitalii Shumar, MD for graphic design and Roman Buriak, MD, PhD for statistical data processing.
Conflict of interest: none declared.
ABBREVIATIONS
- AML
Anterior mitral leaflet
- ASA
Alcohol septal ablation
- AV
Aortic valve
- CMR
Cardiac magnetic resonance
- CT
Computed tomography
- HOCM
Hypertrophic obstructive cardiomyopathy
- ICD
Implantable cardioverter-defibrillator
- IVS
Interventricular septum
- LV
Left ventricle
- LVOT
Left ventricular outflow tract
- MR
Mitral regurgitation
- MV
Mitral valve
- PM
Papillary muscles
- SAM
Systolic anterior motion
- SPG
Systolic pressure gradient
- TEE
Transesophageal echocardiography
- VSD
Ventricular septal defect
Presented at the 35th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 13–16 October 2021.
Author contributions
Kostyantyn V. Rudenko: Investigation; Writing—review & editing. Vasyl V. Lazoryshynets: Funding acquisition; Project administration; Supervision. Lidiia O. Nevmerzhytska: Methodology; Resources. Mariia O. Tregubova: Resources; Visualization. Polina A. Danchenko: Conceptualization; Data curation; Formal analysis; Software; Writing—original draft.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Ulrich Otto von Oppell, Klaus Kaier and the other anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
- 1. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P. et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020;142:e533–57. [DOI] [PubMed] [Google Scholar]
- 2. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA. et al. ; Authors/Task Force members. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–79. [DOI] [PubMed] [Google Scholar]
- 3. Maron BJ. Controversies in cardiovascular medicine. Surgical myectomy remains the primary treatment option for severely symptomatic patients with obstructive hypertrophic cardiomyopathy. Circulation 2007;116:196–206; discussion 206. [DOI] [PubMed] [Google Scholar]
- 4. Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S. et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2005;46:470–6. [DOI] [PubMed] [Google Scholar]
- 5. Quintana E, Bajona P, Arguis MJ, Prat-González S.. Septal myectomy after failed septal alcohol ablation. Ann Cardiothorac Surg 2017;6:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams LK, Rakowski H.. Surgical myectomy for hypertrophic obstructive cardiomyopathy: the cut that heals. Circulation 2013;128:193–7. [DOI] [PubMed] [Google Scholar]
- 7. Nagueh SF, Groves BM, Schwartz L, Smith KM, Wang A, Bach RG. et al. Alcohol septal ablation for the treatment of hypertrophic obstructive cardiomyopathy. A multicenter North American registry. J Am Coll Cardiol 2011;58:2322–8. [DOI] [PubMed] [Google Scholar]
- 8. Bytyçi I, Nistri S, Mörner S, Henein MY.. Alcohol septal ablation versus septal myectomy treatment of obstructive hypertrophic cardiomyopathy: a systematic review and meta-analysis. J Clin Med 2020;9:3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dimitrow PP, Rajtar-Salwa R.. Obstructive form of hypertrophic cardiomyopathy-left ventricular outflow tract gradient: novel methods of provocation, monitoring of biomarkers, and recent advances in the treatment. Biomed Res Int 2016;2016:1575130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Binaco I, Spirito P, Poggio D, Casati V, Grillo M, Ferrazzi P.. Transaortic mitral valve secondary chordal cutting in patients with obstructive hypertrophic cardiomyopathy and mild septal hypertrophy. Ann Cardiothorac Surg 2017;6:426–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sorajja P, Binder J, Nishimura RA, Holmes DR Jr, Rihal CS, Gersh BJ. et al. Predictors of an optimal clinical outcome with alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Cathet Cardiovasc Intervent 2013;81:E58–67. [DOI] [PubMed] [Google Scholar]
- 12. Sorajja P, Ommen SR, Holmes DR, Dearani JA, Rihal CS, Gersh BJ. et al. Survival after alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation 2012;126:2374–80. [DOI] [PubMed] [Google Scholar]
- 13. Maron BJ, Maron MS.. Hypertrophic cardiomyopathy. Lancet 2013;381:242–55. [DOI] [PubMed] [Google Scholar]
- 14. Rudenko KV, Lazoryshynets VV, Trembovetska OM, Nevmerzhytska LO, Tregubova MO, Danchenko PA.. The results of surgical treatment of patients with hypertrophic obstructive cardiomyopathy as a risk factor of sudden cardiac death. World Med Biol 2020;16:109–14. [Google Scholar]
- 15. Raffa GM, Romano G, Turrisi M, Morsolini M, Gentile G, Sciacca S. et al. Pathoanatomic findings and treatment during hypertrophic obstructive cardiomyopathy surgery: the role of mitral valve. Heart Lung Circ 2019;28:477–85. [DOI] [PubMed] [Google Scholar]
- 16. Spirito P, Rossi J, Maron BJ.. Alcohol septal ablation: in which patients and why? Ann Cardiothorac Surg 2017;6:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valeti US, Nishimura RA, Holmes DR, Araoz PA, Glockner JF, Breen JF. et al. Comparison of surgical septal myectomy and alcohol septal ablation with cardiac magnetic resonance imaging in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 2007;49:350–7. [DOI] [PubMed] [Google Scholar]
- 18. Spirito P, Binaco I, Poggio D, Zyrianov A, Grillo M, Pezzoli L. et al. Role of preoperative cardiovascular magnetic resonance in planning ventricular septal myectomy in patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol 2019;123:1517–26. [DOI] [PubMed] [Google Scholar]
- 19. Sorajja P, Valeti U, Nishimura RA, Ommen SR, Rihal CS, Gersh BJ. et al. Outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation 2008;118:131–9. [DOI] [PubMed] [Google Scholar]
- 20. Yang Q, Zhu C, Cui H, Tang B, Wang S, Yu Q. et al. Surgical septal myectomy outcome for obstructive hypertrophic cardiomyopathy after alcohol septal ablation. J Thorac Dis 2021;13:1055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balt JC, Wijffels MCEF, Boersma LVA, Wever EFD, ten Berg JM.. Continuous rhythm monitoring for ventricular arrhythmias after alcohol septal ablation for hypertrophic cardiomyopathy. Heart 2014;100:1865–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings in this study are available on request from the corresponding author.