Abstract
OBJECTIVES
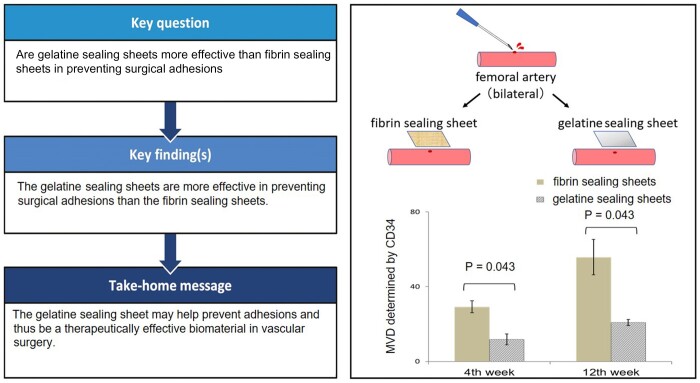
Although reoperation has been increasingly performed in cardiovascular surgery in recent years, preventing surgical adhesions remains an unsolved complication. Therefore, this study aimed to investigate whether gelatine sealing sheets are more effective than fibrin sealing sheets in preventing surgical adhesions.
METHODS
Bilateral femoral arteries of 20 beagle dogs under general anaesthesia were pricked with syringe needles, and gelatine and fibrin sealing sheets were applied on the bleeding points to make canine adhesion models. The femoral artery was harvested after 4 and 12 weeks to evaluate adhesion formations. The adhesive grade was quantified by scoring the area and strength of adhesion tissues. Histological staining was performed to examine the structural features of surgical adhesions.
RESULTS
Significantly fewer macroscopic adhesions were observed with gelatine sealing sheets than those with fibrin sealing sheets at 4 and 12 weeks postoperatively. Microscopically, CD3+ T lymphocytes at 4 and 12 weeks postoperatively in gelatine sealing sheets were significantly lower than those in fibrin sealing sheets. Microvessel density determined by CD34 at 4 and 12 weeks postoperatively in gelatine sealing sheets was also significantly lower than those in fibrin sealing sheets.
CONCLUSIONS
The gelatine sealing sheets are more effective than the fibrin sealing sheets in preventing surgical adhesions. These findings suggest that the gelatine sealing sheet may help prevent adhesions and thus be a therapeutically effective biomaterial in vascular surgery.
Keywords: Gelatine, Fibrin, Haemostasis, Adhesion, Vascular surgery
With ageing patients and increasing performance of cardiovascular surgeries, the number of reoperations has also been increasing [1–3].
INTRODUCTION
With ageing patients and increasing performance of cardiovascular surgeries, the number of reoperations has also been increasing [1–3]. Despite recent reports of good outcomes with transcatheter interventions for patients requiring reoperations, many patients still require reoperations [4–6] primarily due to surgical site adhesions. Debridement is performed at the site of adhesion but may be accompanied by tissue damage, resulting in active bleeding and surgical death. Various anti-adhesion agents have been investigated [7, 8] and developed to solve these problems: nonabsorbable materials, exemplified by the e-polytetrafluoroethylene membrane and absorbable membranes, such as oxidized regenerated cellulose (Interceed; Johnson & Johnson Medical Inc., New Brunswick, NJ) [9] and sodium hyaluronate and carboxymethylcellulose membrane (Seprafilm; Genzyme, Cambridge, MA) [10]. However, no ideal method has yet been established for preventing postoperative adhesion formation because of some complications, such as surgical field bleeding. Recently, the effectiveness of TachoSil (Nycomed Austria GmbH, Linz, Austria) with its haemostatic function in preventing surgical adhesion has been reported. TachoSil appears to not only function as a mechanical barrier between surfaces during the surgical site recovery but also reduce adhesion formation by suppressing plasminogen activator inhibitor-1 levels. Fibrin products are widely used in cardiovascular surgery for haemostatic purposes. However, they are still not being widely used as an anti-adhesion agent in cardiovascular surgery because of plasma derivatives and high cost. Although various other anti-adhesive agents are currently available for use in abdominal surgery, effective anti-adhesive agents are not widespread in cardiovascular surgery because of unsatisfactory clinical outcomes of these materials [10, 11].
We have developed a gelatine sealing sheet from gelatine, a proven safe natural polymer, which strongly adheres to biological tissues and shows high haemostaticity while also showing anti-adhesive effects, as previously reported [12]. This thermally crosslinked bilayer gelatine sealing sheet consists of foam and a thin film layer for improved handling of the haemostatic agent and anti-adhesive properties. Our previous studies have shown that the gelatine sealing sheet was a surgical haemostatic agent found to be as effective as the fibrin sealing sheet and more effective in preventing postoperative macroscopic adhesions using a canine model. However, the results about their anti-adhesive effects were based on a study that evaluated a small number of animals and only examined 4 weeks postoperatively. We considered that pathological evaluation, in addition to animal studies in larger numbers over a longer period, is necessary to verify the anti-adhesion effect of this formulation. Therefore, this study aimed to investigate the effectiveness of gelatine sealing sheets as anti-adhesive barriers against the formation of surgical adhesions, including microscopic evaluations in comparison with TachoSil.
MATERIALS AND METHODS
Ethical statement
Animal housing, human care and surgical procedures were performed following the institutional guidelines of the institutional review board of Nara Medical University (institutional review board approval: 12415, date: 31 January 2018). All experiments were performed in accordance with the ARRIVE guidelines.
Materials
Medical grade gelatine extracted from porcine skin with an isoelectric point of 5 (Medigelatin®), fibrin sealing sheets (TachoSil Tissue Sealing Sheet®) and sodium pentobarbital (Somnopentyl®) was purchased from Nippi Co. (Tokyo, Japan), CSL Behring Inc. (Tokyo, Japan) and Kyoritsu Seiyaku (Tokyo, Japan), respectively. All other reagents and surgical materials were purchased from Wakenyaku Co. Ltd (Osaka, Japan). Double-distilled water was used for any preparation. To test the haemostasis and anti-adhesion effects of gelatine sealing sheets, healthy female beagles (aged 11–13 months old; weighed 9–11 kg) were purchased from Shimizu Laboratory Animal Supply Co. (Kyoto, Japan).
Preparation of gelatine sealing sheets
The bilayer gelatine matrix, consisting of a cast film and a foam layer, was prepared. After dissolving the gelatine in distilled water to a final concentration of 3.0 wt%, the solution (13.5 ml) was cast onto a polystyrene dish (corning suspension culture dishes, measuring 150 mm in diameter and 25 mm in height, Cardinal Health Inc., Dublin, Ohio, USA) and allowed to dry overnight in a clean bench with constant air flow at room temperature, yielding a film layer of ∼20 µm in thickness. Then, the upper side of the film layer was exposed to ultraviolet light at a distance of 25 mm from a ultraviolet lamp (GL-15; NEC Lighting, Ltd, Tokyo, Japan) for 5 min. Gelatine solution (1.0%, 70.0 ml) was cast onto the film layer and allowed to remain at room temperature for 10 min to merge the solution into the film layer. Then, these were frozen at −80°C for >10 min using a deep freezer (MDF-394; Panasonic Healthcare Co., Tokyo, Japan) and lyophilized with a freeze-drying vacuum (DRZ 350 WA; ADVANTEC, Tokyo, Japan) for 24 h. The bilayer gelatine matrix was thermally crosslinked using a vacuum oven (DP43; YAMATO, Tokyo, Japan) at 140°C for 4 h unless otherwise indicated. All gelatine sealing sheets used for animal experiments were sterilized using ethylene oxide gas (0.43 g/l for 4 h at 40°C).
Randomized grouping
Twenty dogs were randomly divided into experimental and control groups. One dog died a few days postoperatively. Nineteen dogs were randomly allocated to 2 different sheets, with 2 lesions and 2 sheets per dog. In addition, the location of the surgery was randomized. Each sheet was applied to 19 lesions according to previous studies. No statistical power calculation was carried out. Although observer blinding was not possible due to obvious differences in product appearances during the first operation, the surgeon who graded the adhesion was blinded of the animal groups. The surgeon who performed the first operation was different from the surgeon who graded the adhesion in the second operation.
Preparation of a canine adhesion model
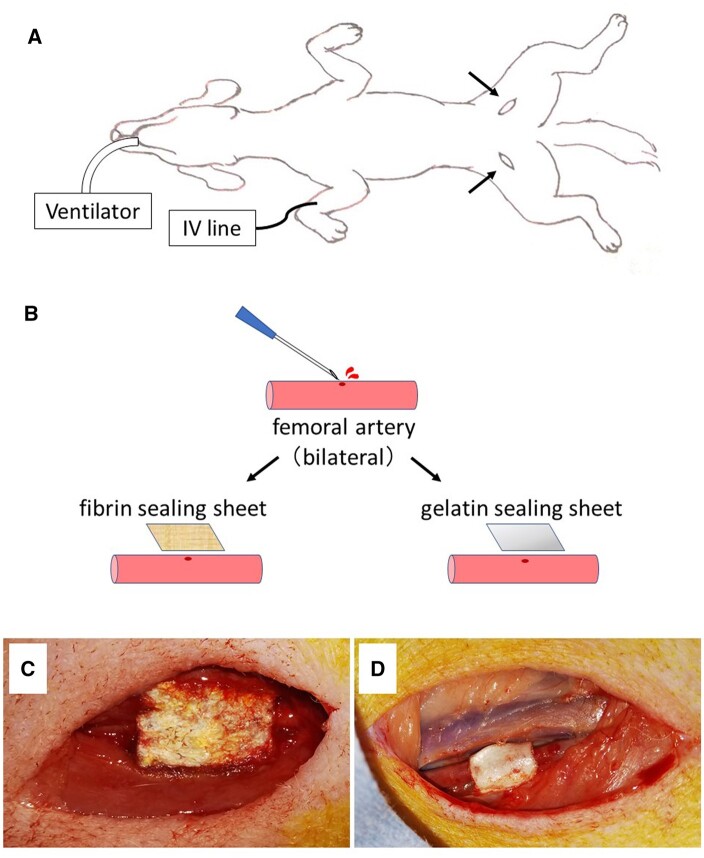
Two surgical sealants were used to perform a randomized trial on dogs: gelatine sealing sheets (i.e. the experimental group, n = 19) and fibrin sealing sheets (i.e. the control group, n = 19) (Fig. 1). Sodium pentobarbital (25 mg/kg) was administered to each dog via intravenous injection for general anaesthesia. After endotracheal intubation and shaving of the groyne area, hibitane solution was used for disinfection. All surgical instruments, gauze and embedding material were sterilized in advance. The operation was performed in an aseptic fashion. An incision was created to expose the femoral artery, and heparin (0.8–0.9 ml) was administered intravenously. Five minutes after the heparin administration, the blood flow was cut-off by clamping 2 places of the femoral artery with haemostatic forceps, and the femoral artery was pricked with a 23-G syringe needle to make a bleeding hole. Then, the blood from the needle hole was carefully wiped off with gauze. For haemostasis, gelatine and fibrin sealing sheets were applied to the experimental and control groups, respectively. The sealing sheets (1.5 × 2 cm2) were wrapped around the artery and compressed with fingers for 5 min. All of the sheets, including the gelatine sealing sheets, effectively stopped the bleeding of the femoral arteries. Thereafter, skin incisions were closed with simple interrupted stitches using a 1–0 nylon suture, leaving gelatine and fibrin sealing sheets on the femoral arteries.
Figure 1:
Establishment of a vascular adhesion model. (A) Schematic representation of an experimental setup with endotracheal intubation and intravenous catheterization. Arrows show skin incision sites. (B) Schematic view of the exposed bilateral femoral arteries punctured with a 23-G syringe needle, with compression haemostasis at fibrin and gelatine sealing sheet, respectively. Gross view of the femoral artery with haemostasis completed with fibrin (C) and gelatine sealing sheet (D).
Macroscopic evaluation of surgical field adhesions
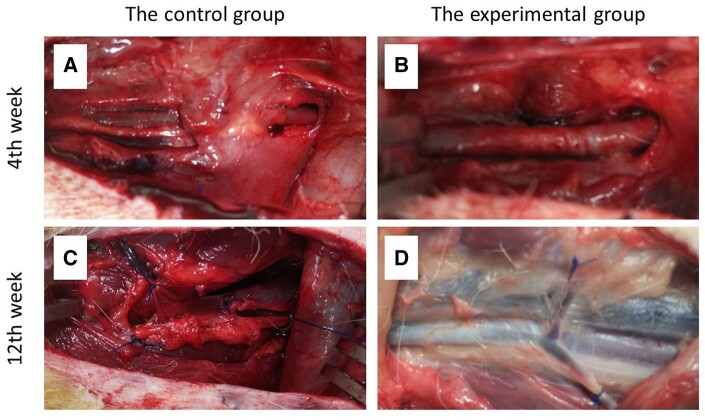
All dogs were euthanized with overdose anaesthesia (pentobarbital 250 mg/kg) at 4 (n = 13 for each group) or 12 weeks (n = 6 for each group) after the first operation, and their femoral arteries and surrounding tissues were examined for postoperative adhesions (Fig. 2). Two independent researchers evaluated the macroscopic adhesions. The adhesion area and force required to separate the adhesion were scored in a blinded manner according to Zuhlke et al.’s method [13, 14].
Figure 2:
Gross view. Four weeks postoperatively between the control (A) and experimental groups (B) and 12 weeks postoperatively between the control (C) and experimental groups (D).
Microscopic evaluation of surgical field adhesions
The femoral artery, including the surgical site, in the canine adhesion model, was subsequently harvested and fixed in 10% aqueous formaldehyde solution at 4 weeks (n = 5 for each group) and 12 weeks (n = 5 for each group) after the first operation. The fixed specimens were cross-sectioned and stained with haematoxylin and eosin and immunostained with CD3 and CD34 for histological analysis. Total numbers of lymphocytes (number/mm2) were recorded under original magnification ×400. Quantification of microvessels was performed by the method described by Weidner et al. [15]. The 3 most highly vascularized areas detected by 34 were initially selected under 40× field and 100× field. Then, a 400× field was used to count microvessels in each of these areas. The specimens were evaluated immunohistologically and confirmed by a board-certified pathologist.
Statistical analysis
Continuous variables were expressed as the means and standard deviations or as medians and interquartile ranges. Comparisons were performed using the Wilcoxon signed-rank test for continuous variables. Differences with two-sided P-values <0.05 were statistically significant. JMP software for Windows version 13 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
RESULTS
Macroscopic findings
A comparison of postoperative adhesion scores between the 2 groups in the canine adhesion model is shown in Table 1. The adhesion scores of the adhesion area and strength at 4 weeks postoperatively in the experimental group were significantly lower than those in the control group (P < 0.001, P < 0.001, respectively). The adhesion scores of the adhesion area and strength at 12 weeks postoperatively in the experimental group were also significantly lower than those in the control group (P = 0.041, P = 0.031, respectively).
Table 1:
The adhesion scores postoperatively in the canine adhesion model
| Characteristics | The control group | The experimental group | P-Value |
|---|---|---|---|
| The adhesion 4 weeks postoperatively | (n = 13) | (n = 13) | |
| The adhesion area | <0.001 | ||
| 0 (no adhesions) | 0 | 0 | |
| 1 (adhesions with 1–25% of the SFA) | 0 | 7 | |
| 2 (adhesions with 26–50% of the SFA) | 2 | 6 | |
| 3 (adhesions with 51–75% of the SFA) | 8 | 0 | |
| 4 (adhesions with 76–100% of the SFA) | 2 | 0 | |
| The adhesion strength | <0.001 | ||
| 0 (no adhesions) | 0 | 0 | |
| 1 (mild) | 1 | 12 | |
| 2 (moderate) | 2 | 1 | |
| 3 (severe) | 5 | 0 | |
| 4 (very severe) | 5 | 0 | |
| The adhesion 12 weeks postoperatively | (n = 6) | (n = 6) | |
| The adhesion area | 0.041 | ||
| 0 (no adhesions) | 0 | 0 | |
| 1 (adhesions with 1–25% of the SFA) | 0 | 5 | |
| 2 (adhesions with 26–50% of the SFA) | 3 | 1 | |
| 3 (adhesions with 51–75% of the SFA) | 3 | 0 | |
| 4 (adhesions with 76–100% of the SFA) | 0 | 0 | |
| The adhesion strength | 0.031 | ||
| 0 (no adhesions) | 0 | 0 | |
| 1 (mild) | 0 | 4 | |
| 2 (moderate) | 2 | 2 | |
| 3 (severe) | 2 | 0 | |
| 4 (very severe) | 2 | 0 |
SFA: surgical field area.
Microscopic findings
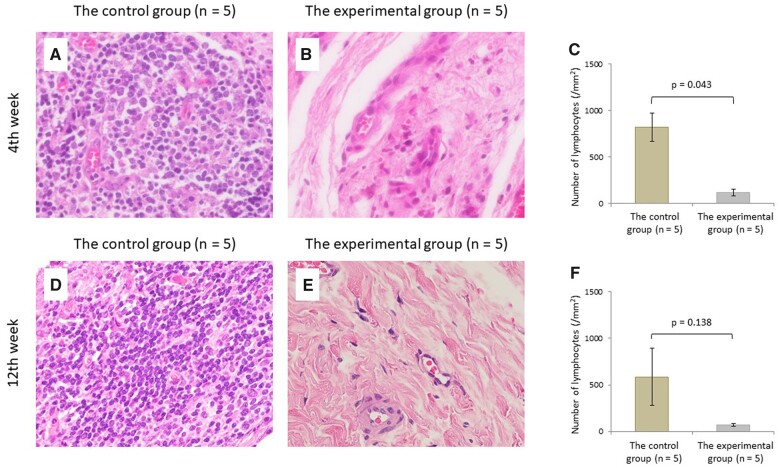
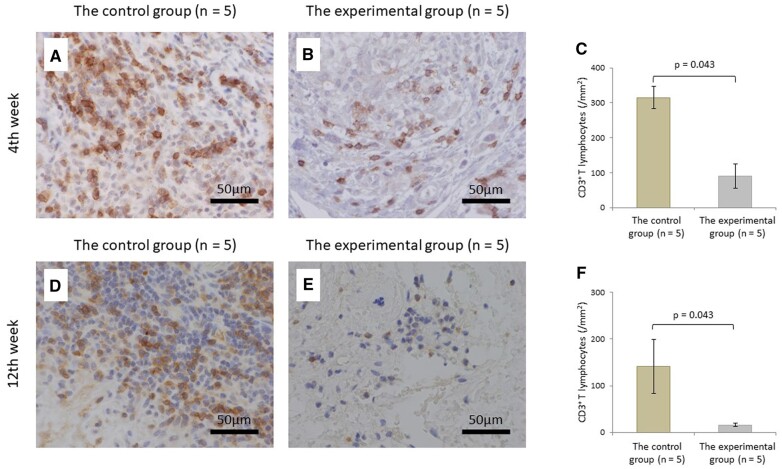
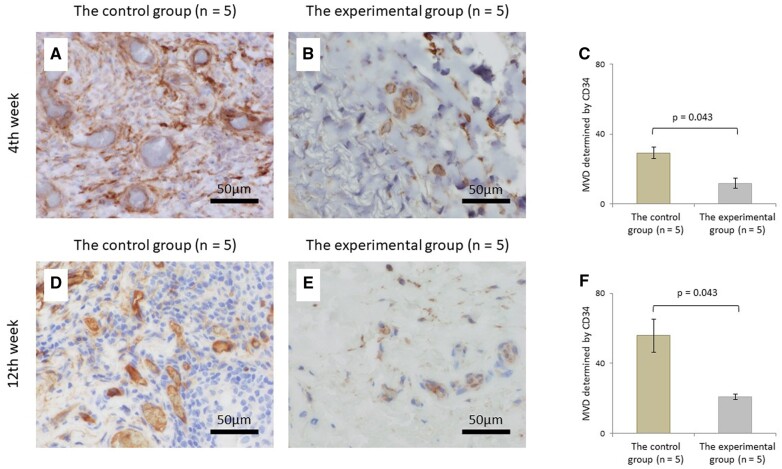
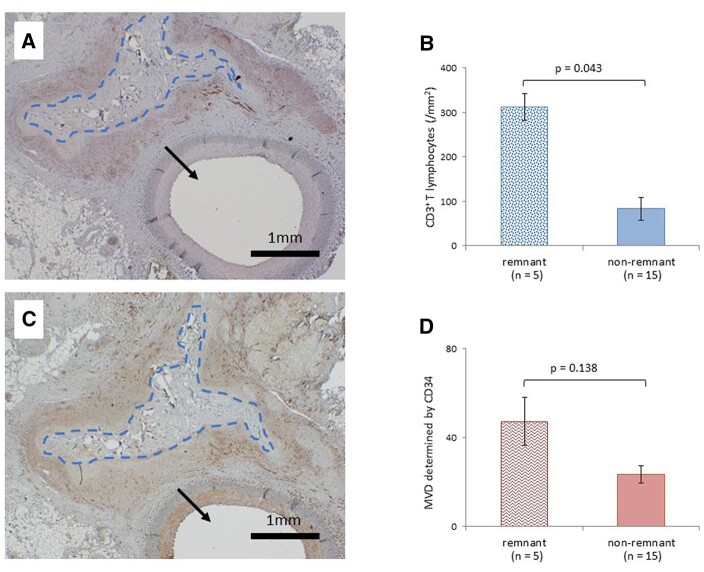
The number of lymphocytes at 4 weeks postoperatively in the experimental group was significantly lower than those in the control group (P = 0.043). Although no significant difference was observed in the number of lymphocytes at 12 weeks postoperatively between the 2 groups (P = 0.138) (Fig. 3). CD3+ T lymphocytes at 4 and 12 weeks postoperatively in the experimental group were significantly lower than those in the control group (P = 0.043, P = 0.043, respectively) (Fig. 4). Microvessel density determined by CD34 at 4 and 12 weeks postoperatively in the experimental group were also significantly lower than those in the control group (P = 0.043, P = 0.043, respectively) (Fig. 5). Of all the samples, the 5 with the highest number of lymphocytes were in the control group, which all showed sheet remnants pathologically. No samples in the experimental group showed sheet remnant. Figure 6 shows that the number of CD3+ T lymphocytes postoperatively in the remnant group was significantly higher than those in the non-remnant group (P = 0.043). Although no significant difference was observed in the number of and microvessel density determined by CD34 postoperatively between the 2 groups (P = 0.138).
Figure 3:
Histopathologic evaluation of lymphocytes by H&E staining. Micrograph of the control (A) and experimental groups (B) and the comparison of lymphocyte count (C) at 4 weeks postoperatively. Micrograph of the control (D) and experimental groups (E) and the comparison of lymphocyte count (F) at 12 weeks postoperatively (original magnification ×400).
Figure 4:
Histopathologic evaluation of T lymphocytes by immunostaining using antibodies against CD3. Micrograph of the control (A) and experimental groups (B) and the comparison of CD3+ T lymphocyte count (C) at 4 weeks postoperatively. Micrograph of the control (D) and experimental groups (E), and the comparison of CD3+ T lymphocyte count (F) at 12 weeks postoperatively (original magnification ×400).
Figure 5:
Histopathologic evaluation of the microvessel by immunostaining using antibodies against CD34. Micrograph of the control (A) and experimental groups (B) and the comparison of the microvessel density (C) at 4 weeks postoperatively. Micrograph of the control (D) and experimental groups (E) and the comparison of microvessel density (F) at 12 weeks postoperatively (original magnification ×400).
Figure 6:
Effects of sheet remnants. (A) Microscopically observed fibrin sheet remnants in specimens 4 weeks postoperatively in the control group stained by CD3. (B) The number of CD3+ T lymphocytes in the remnant and non-remnant groups. (C) Similar viewpoint in the same specimen stained by CD34. (D) Microvessel density determined by CD34 in the remnant and non-remnant groups. Blue dot lines indicate fibrin sheet remnants; arrows show the femoral artery (original magnification ×20).
DISCUSSION
The primary finding of this study is that gelatine sealing sheets more effectively inhibited the inflammatory response in the surgical field than fibrin sealing sheets based on the number of CD3+ T lymphocytes and microvessel density and a lesser adhesion grossly. Although Kuschel et al. [16] showed fibrin sealing sheets effectively prevented surgical adhesion, our results demonstrated more effectiveness of gelatine sheets in terms of preventing adhesion of surgical site than that of fibrin sealing sheets. However, these previous studies only generally evaluated adhesions at 4 weeks postoperatively; therefore, the current study further evaluated adhesions at 12 weeks postoperatively and added additional pathological considerations [12].
CD34 is a marker of vascular endothelial cells, and vascular endothelial growth factor plays a pivotal role in angiogenesis [17]. Angiogenesis is an important pathological process in adhesion formation [18]. Moreover, the inflammatory process plays a vital role in the pathogenesis of postoperative adhesion formation. In addition, studies have shown that transforming growth factor-β is overexpressed in adhesions [19, 20]. In vitro studies have reported that mesothelial cells became fibrotic when cocultured with transforming growth factor-β [21]. Suppression of the fibrinolytic activity of mesothelial cells due to surgical trauma and the presence of blood play an important role in the pathogenesis of adhesion formation [22–25]. Kawai et al. [26] also showed that mesothelial cells grown on gelatine products in vitro showed greater proliferation than on the fibrin glue. In the present study, the number of CD3+ T lymphocytes and microvessel density determined by CD34 at 4 and 12 weeks postoperatively in the gelatine sealing sheet group was significantly lower than those in the fibrin sealing sheet group, with the former showing no evidence of inflammatory cell infiltration in vivo. These results suggest that the gelatine sealing sheet may act as a scaffold for mesothelial-like cell regeneration in vivo, representing a key factor in adhesion prevention.
Regarding sheet remnants, the number of CD3+ lymphocytes in the sheet remnant group was higher than that in the non-remnant group. As a foreign body, sheet remnant caused mild-to-moderate inflammatory reaction. The results indicated the good bioabsorbable effects of gelatine sealing sheets. Interestingly, some fibrin sheets are absorbed and disappeared by the sheet itself at 12 weeks postoperatively, and during the long-term postoperative period, surgical field inflammation may be reduced even with the use of fibrin sheets. Therefore, to prevent surgical field adhesions, the sheet should be developed to act as a barrier to the surrounding tissues during the wound-healing phase, and thereafter, the sheet should be developed to be highly absorbable and to disappear from the surgical field. When sheet remnants remain in the wound for a long period postoperatively, they are recognized as a foreign body and lead to inflammation and tissue adhesion. This gelatine sealing sheet has its high absorbency in addition to haemostatic effect and is expected to be widely applied in the future.
Limitations
This study had several limitations. First, we only assessed a small number of animals at each time point. Furthermore, a power analysis was not performed before conducting the study because it was a preliminary study and therefore not possible to calculate the appropriate sample size. However, although the current results were limited, previous studies have shown similar results using the same sample size [12]. Thus, we presumed that including additional animals would not greatly change the overall results and would minimize the number of dogs used from the standpoint of animal welfare. Second, observer blinding was not possible due to obvious differences in the product appearances. This might represent a selective bias during the application of the sheet.
CONCLUSION
In conclusion, gelatine sealing sheets more effectively decreased histological inflammation than fibrin sealing sheets. Although long-term studies are required to further investigate the safety of this product for use in clinical setting, these findings suggest that the gelatine sealing sheet may help prevent adhesions and thus be a therapeutically effective biomaterial in vascular surgery.
ACKNOWLEDGEMENT
The authors thank GUNZE Ltd., Kyoto, for providing the gelatine sealing sheet for this study.
Funding
This work was supported by JSPS KAKENHI with Grant Number JP16K10664.
Conflict of interest: none declared.
Data Availability Statement
All relevant data are within the manuscript and its supporting information files.
Author contributions
Kosuke Niwa: Conceptualization; Data curation; Formal analysis; Writing—original draft; Writing—review & editing. Keigo Yamashita: Conceptualization; Data curation; Writing—original draft; Writing—review & editing. Tomoaki Hirose: Conceptualization; Writing—review & editing. Shun Hiraga: Conceptualization; Writing—review & editing. Ryohei Fukuba: Conceptualization; Writing—review & editing. Junichi Takemura: Conceptualization; Writing—review & editing. Hiroshi Nishikawa: Conceptualization; Writing—review & editing. Shigeki Taniguchi: Conceptualization; Data curation; Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Vito Domenico Bruno, Hung-Lung Hsu and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Yang B, Patel HJ, Norton EL, Debenedictus C, Farhat L, Wu X. et al. Aortic valve reoperation after stentless bioprosthesis: short- and long-term outcomes. Ann Thorac Surg 2018;106:521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamashita K, Taniguchi S.. Transcatheter aortic valve implantation for chronic dialysis patients in Japan. Circ J 2015;79:2557–9. [DOI] [PubMed] [Google Scholar]
- 3. Liu RH, Fraser CD 3rd, Zhou X, Beaulieu RJ, Reifsnyder T.. Complete versus partial excision of infected arteriovenous grafts: does remnant graft material impact outcomes? J Vasc Surg 2020;71:174–9. [DOI] [PubMed] [Google Scholar]
- 4. Hirji SA, Percy ED, Zogg CK, Malarczyk A, Harloff Mt Yazdchi F. et al. Comparison of in-hospital outcomes and readmissions for valve-in-valve transcatheter aortic valve replacement vs. reoperative surgical aortic valve replacement: a contemporary assessment of real-world outcomes. Eur Heart J 2020. Aug 1;41(29):2747–2755. https://doi.org/10.1093/eurheartj/ehaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giles KA, Scali ST, Pearce BJ, Huber TS, Berceli SA, Arnaoutakis DJ. et al. Impact of secondary interventions on mortality after fenestrated branched endovascular aortic aneurysm repair. J Vasc Surg 2019;70:1737–46. [DOI] [PubMed] [Google Scholar]
- 6. Tuzcu EM, Kapadia SR, Vemulapalli S, Carroll JD, Holmes DR Jr, Mack MJ. et al. Transcatheter aortic valve replacement of failed surgically implanted bioprostheses: the STS/ACC Registry. J Am Coll Cardiol 2018;72:370–82. [DOI] [PubMed] [Google Scholar]
- 7. Taksaudom N, Ketwong M, Lertprasertsuke N, Kongkaew A.. Postoperative pericardial adhesion prevention using collagen membrane in pigs: a pilot study. Open J Cardiovasc Surg 2017;9. https://doi.org/10.1177/1179065217720909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lescan M, Al-Saidi A, Neumann B, Greiner T-O, Walker T, Hierlemann H. et al. Epicardial adhesion prophylaxis in swine model with a bio-absorbable polymer membrane. J Mater Sci Mater Med 2018;29:157. [DOI] [PubMed] [Google Scholar]
- 9. Watanabe J, Ishida F, Ishida H, Fukunaga Y, Watanabe K, Naito M. et al. A prospective multi-center registry concerning the clinical performance of laparoscopic colorectal surgery using an absorbable adhesion barrier (INTERCEED®) made of oxidized regenerated cellulose. Surg Today 2019;49:877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lefort B, El Arid JM, Bouquiaux AL, Soulé N, Chantreuil J, Tavernier E. et al. Is Seprafilm valuable in infant cardiac redo procedures? J Cardiothorac Surg 2015;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kojima A, Sakaue T, Okazaki M, Shikata F, Kurata M, Imai Y. et al. A simple mouse model of pericardial adhesions. J Cardiothorac Surg 2019;14:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu Y, Yamashita K, Tabayashi N, Abe T, Hayata Y, Hirose T. et al. Gelatin sealing sheet for arterial hemostasis and anti-adhesion in vascular surgery: a dog model study. Biomed Mater Eng 2015;25:157–68. [DOI] [PubMed] [Google Scholar]
- 13. Zühlke HV, Lorenz EM, Straub EM, Savvas V.. Pathophysiology and classification of adhesions. Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir 1990;1009–16. [PubMed] [Google Scholar]
- 14. Lo HY, Kuo HT, Huang YY.. Application of polycaprolactone as an anti-adhesion biomaterial film. Artif Organs 2010;34:648–53. [DOI] [PubMed] [Google Scholar]
- 15. Weidner N, Semple JP, Welch WR, Folkman J.. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991;324:1–8. [DOI] [PubMed] [Google Scholar]
- 16. Kuschel TJ, Gruszka A, Hermanns-Sachweh B, Elyakoubi J, Sachweh JS, Vázquez-Jiménez JF. et al. Prevention of postoperative pericardial adhesions with TachoSil. Ann Thorac Surg 2013;95:183–8. [DOI] [PubMed] [Google Scholar]
- 17. Bi J, Zhang S, Du Z, Zhang J, Deng Y, Liu C. et al. Peripheral serotonin regulates postoperative intra-abdominal adhesion formation in mice. Sci Rep 2017;7:10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cahill RA, Wang JH, Soohkai S, Redmond HP.. Mast cells facilitate local VEGF release as an early event in the pathogenesis of postoperative peritoneal adhesions. Surgery 2006;140:108–12. [DOI] [PubMed] [Google Scholar]
- 19. Jin X, Ren S, Macarak E, Rosenbloom J.. Pathobiological mechanisms of peritoneal adhesions: the mesenchymal transition of rat peritoneal mesothelial cells induced by TGF-β1 and IL-6 requires activation of Erk1/2 and Smad2 linker region phosphorylation. Matrix Biol 2016;51:55–64. [DOI] [PubMed] [Google Scholar]
- 20. Wang G, Wu K, Li W, Zhao E, Shi L, Wang J. et al. Role of IL-17 and TGF-β in peritoneal adhesion formation after surgical trauma. Wound Repair Regen 2014;22:631–9. [DOI] [PubMed] [Google Scholar]
- 21. Nasreen N, Mohammed KA, Mubarak KK, Baz MA, Akindipe OA, Fernandez-Bussy S. et al. Pleural mesothelial cell transformation into myofibroblasts and haptotactic migration in response to TGF-beta1 in vitro. Am J Physiol Lung Cell Mol Physiol 2009;297:L115–L124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gabbay S. The need for intensive study of pericardial substitution after open heart surgery. ASAIO Trans 1990;36:789–91. [DOI] [PubMed] [Google Scholar]
- 23. Nkere UU, Whawell SA, Sarraf CE, Schofield JB, Thompson JN, Taylor KM.. Pericardial trauma and adhesions in relation to reoperative cardiac surgery. Thorac Cardiovasc Surg 1995;43:338–46. [DOI] [PubMed] [Google Scholar]
- 24. Snoj M. Pathogenesis and prevention of adhesion formation. Br J Surg 1995;82:1141. [DOI] [PubMed] [Google Scholar]
- 25. Cliff WJ, Grobéty J, Ryan GB.. Postoperative pericardial adhesions. The role of mild serosal injury and spilled blood. J Thorac Cardiovasc Surg 1973;65:744–50. [PubMed] [Google Scholar]
- 26. Kawai N, Suzuki S, Ouji Y, Takeda M, Sakagami M, Tojo T. et al. Effect of covering with cross-linked gelatin glue on tissue regeneration in a rat lung injury model. Interact CardioVasc Thorac Surg 2019;29:1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and its supporting information files.