Abstract
Hypophysiotropic somatostatin (SST) neurons in the periventricular hypothalamic area express growth hormone (GH) receptor (GHR) and are frequently considered as the key neuronal population that mediates the negative feedback loop controlling the hypothalamic–GH axis. Additionally, insulin-like growth factor-1 (IGF-1) may also act at the hypothalamic level to control pituitary GH secretion via long-loop negative feedback. However, to the best of our knowledge, no study so far has tested whether GHR or IGF-1 receptor (IGF1R) signaling specifically in SST neurons is required for the homeostatic control of GH secretion. Here we show that GHR ablation in SST neurons did not impact the negative feedback mechanisms that control pulsatile GH secretion or body growth in male and female mice. The sex difference in hepatic gene expression profile was only mildly affected by GHR ablation in SST neurons. Similarly, IGF1R ablation in SST neurons did not affect pulsatile GH secretion, body growth, or hepatic gene expression. In contrast, simultaneous ablation of both GHR and IGF1R in SST-expressing cells increased mean GH levels and pulse amplitude in male and female mice, and partially disrupted the sex differences in hepatic gene expression. Despite the increased GH secretion in double knockout mice, no alterations in body growth and serum or liver IGF-1 levels were observed. In summary, GHR and IGF1R signaling in SST neurons play a redundant role in the control of GH secretion. Furthermore, our results reveal the importance of GH/IGF-1 negative feedback mechanisms on SST neurons for the establishment of sex differences in hepatic gene expression profile.
Keywords: GH, hypothalamus, neuroendocrinology, sex difference, somatotropic axis
Growth hormone (GH) is produced by the anterior pituitary gland and regulates important physiological functions, including protein synthesis, cell proliferation, tissue and body growth, and different metabolic aspects (1-3). GH secretion occurs in a pulsatile pattern which is controlled by hypothalamic neurons. Although multiple peripheral and central factors regulate GH release by somatotropic cells, it is well accepted that 2 neuronal populations that express either GH-releasing hormone (GHRH) or somatostatin (SST) are critical for the control of pituitary GH secretion (4-8). Neuroendocrine GHRH neurons are mainly located in the arcuate nucleus of the hypothalamus (ARH) and GHRH release into the median eminence stimulates pituitary GH secretion. In contrast, the hypophysiotropic SST neurons are found in the periventricular (PV) and paraventricular (PVH) hypothalamic nuclei (9, 10) and SST release inhibits GH release.
GH secretion is predominantly regulated by negative feedback loops (4, 5, 11, 12). Increases in circulating GH levels inhibit GHRH neurons, whereas SST neurons become activated. In contrast, decreases in GH secretion leads to increased GHRH expression and reduced activity of SST neurons (13-15). However, previous studies have shown that less than 10% of GHRH neurons express the GH receptor (GHR) or are directly responsive to GH (16, 17), suggesting an indirect regulation of GHRH neurons. On the other hand, approximately 70% of PVSST and PVHSST neurons express Ghr mRNA (10). An acute GH injection induces c-fos gene expression in PVSST neurons (18). In addition, Sst mRNA levels are increased in the PV of rats exposed to chronic GH hypersecretion (13, 19), whereas intracerebroventricular (ICV) administration of Ghr mRNA antisense decreases Sst mRNA expression in the PV (20). Furthermore, SST neurons possibly project to ARHGHRH neurons to control their activity (21-24), which allows coordination in the regulation of pituitary GH production. Taken together, these pieces of evidence indicate that PV/PVHSST neurons are likely the dominant negative feedback node in the hypothalamus to control GH secretion (4-8, 11, 12, 25).
Nevertheless, no study so far has tested whether GHR expression specifically in SST neurons is required for the homeostatic control of GH secretion. In addition to GH autofeedback in PV/PVHSST neurons, insulin-like growth factor-1 (IGF-1) may also control pulsatile GH secretion via a long-loop negative feedback, since GH is a key regulator of circulating IGF-1 levels (4, 26). IGF-1 receptor (IGF1R) is abundantly found in the hypothalamus, including in nuclei that contain SST neurons, such as the PVH (27). Furthermore, ICV IGF-1 infusion reduces GH secretion (28, 29), while increases hypothalamic Sst mRNA expression (30), suggesting that IGF-1 may act at the hypothalamic level to control the somatotropic axis.
The present study was designed to investigate whether the GHR or IGF1R signaling in SST neurons is required for the control of pulsatile GH secretion and consequently body growth. For this purpose, we studied male and female mice carrying genetic ablation of Ghr or Igf1r genes specifically in SST neurons. Since redundant mechanisms may compensate the consequences of the ablation of a single receptor, we also generated animals carrying simultaneous ablation of both GHR and IGF1R in SST-expressing cells.
Materials and Methods
Mice
C57BL/6 wild-type mice (Jackson Laboratory, Bar Harbor, ME; stock #000664) were used in the RNAscope experiments. SST-cre mice (Jackson Laboratory; RRID:IMSR_JAX:018973) were crossed with mice carrying loxP-flanked Ghr alleles (31) or loxP-flanked Igf1r alleles (Jackson Laboratory; RRID:IMSR_JAX:012251). SST-specific double knockout (KO) mice (GHR/IGF1R) were also generated. Control groups were composed of Ghrflox/flox, Igf1rflox/flox, or Ghrflox/flox/Igf1rflox/flox littermates that were negative for the Cre transgene. The LoxP-Stop-LoxP (LSL) tdTomato-reporter mouse (RRID:IMSR_JAX:007909) was used to visualize SST neurons in histological and electrophysiological experiments. Mice were produced and maintained in standard conditions of light (12-hour light/dark cycle; lights on at 8:00) with ad libitum access to a regular rodent chow and filtered water. Male and female mice were used in the experiments. The experimental procedures were approved by the Ethics Committee on the Use of Animals of the Institute of Biomedical Sciences at the University of São Paulo, and were performed according to the ethical guidelines adopted by the Brazilian College of Animal Experimentation.
RNAscope
We used the RNAscope® Multiplex Fluorescent Assay v2 from Advanced Cell Diagnostics (ACD) combined with Tyramide Signal Amplification technology (TSA™) and dyes from Akoya Biosciences. Fresh frozen brains collected from wild-type male and female mice were sliced on a cryostat at 16 µm and mounted directly onto superfrost plus slides (Fisher). The tissue was fixed in 10% neutral buffered formalin for 15 minutes and then dehydrated in ethanol. Endogenous peroxidase was blocked with H2O2 for 10 minutes, washed in diethyl pyrocarbonate-treated water and then tissue was gently digested for 30 minutes at room temperature using Protease IV from ACD kit. The tissue was incubated for 2 hours at 40°C using probes targeting Mm-Ghr and Mm-Sst or Mm-Prlr and Mm-Sst, and ACD’s 3-plex RNAscope® Positive Control Probes for mouse tissue. After hybridization, probes were labeled with Akoya Opal fluorophore reagents. Sections were counterstained with 4′, 6-diamidino-2-phenylindole and cover slipped with ProLong Gold antifade mounting medium.
Detection of GH or IGF-1–responsive Neurons
GH-induced phosphorylation of the signal transducer and activator of transcription-5 (pSTAT5) was detected in brain sections of adult mice that received an acute intraperitoneal (IP) injection of saline or porcine pituitary GH (20 µg/g, from Dr. A.F. Parlow, National Institute of Diabetes and Digestive and Kidney Diseases–National Hormone and Pituitary Program [NIDDK-NHPP]) and were perfused approximately 1 hour later (n = 3/group). To identify IGF-1–responsive neurons, via IGF-1 capacity to induce protein kinase B (AKT) phosphorylation (pAKT), as previously demonstrated (32-35), adult mice received an ICV injection of saline or mouse recombinant IGF-1 (1 μg of IGF-1 diluted in 2 μL of saline; ThermoFisher Scientific) and were perfused 20 minutes later (n = 3/group). Mice were anesthetized with isoflurane and perfused transcardially with saline, followed by a 10% buffered formalin solution. Brains were collected and postfixed in the same fixative for 45 minutes and cryoprotected overnight at 4°C in 0.1 M phosphate-buffered saline (PBS) containing 20% sucrose. Brains were cut in 30-µm-thick sections using a freezing microtome. Brain slices were rinsed in 0.02 M potassium PBS, pH 7.4 (KPBS), followed by pretreatment in water solution containing 1% hydrogen peroxide and 1% sodium hydroxide for 20 minutes. After rinsing in KPBS, sections were incubated in 0.3% glycine and 0.03% lauryl sulfate for 10 minutes each. Next, slices were blocked in 3% normal donkey serum for 1 hour, followed by incubation in anti-pSTAT5Tyr694 (1:1000; Cell Signaling Technology; Cat# 9351; RRID:AB_2315225) or anti-pAKTSer473 (1:1000; Cell Signaling Technology; Cat# 3787; RRID:AB_331170) primary antibodies for 40 hours. Subsequently, sections were rinsed in KPBS and incubated for 90 minutes in Alexa Fluor488–conjugated secondary antibody (1:500, Jackson ImmunoResearch). TdTomato-expressing neurons do not require any enhancement of staining to allow their visualization. When double immunofluorescence labeling was required, brain sections were subsequently incubated overnight in anti-SST primary antibody (1:1000; Peninsula Laboratories International; Cat# T4103; RRID: AB_518614), followed by incubation in Alexa Fluor594–conjugated secondary antibody (1:500, Jackson ImmunoResearch). Sections were mounted onto gelatin-coated slides and the slides were covered with Fluoromount G mounting medium (Electron Microscopic Sciences, Hatfield, PA). A Zeiss Axiocam 512 color camera adapted to an Axioimager A1 microscope (Zeiss, Munich, Germany) was used to obtain photomicrographs. ImageJ software (http://rsb.info.nih.gov/ij/) was used to manually count the number of single- or double-labeled neurons in 2 or 3 different rostrocaudal levels of each area of interest. The values obtained at different rostrocaudal levels were averaged, representing the data for each animal.
Electrophysiology
Adult (8- to 12-week-old) male and female SST-cre::LSL-tdTomato mice were decapitated, their brains collected and immediately submerged in ice-cold, carbogen-saturated (95% O2 and 5% CO2) artificial cerebrospinal fluid (aCSF; 124 mM NaCl, 2.8 mM KCl, 26 mM NaHCO3, 1.25 mM NaH2PO4, 1.2 mM MgSO4, 5 mM glucose, and 2.5 mM CaCl2). Coronal sections (250 µM thick) from a hypothalamic block were cut with a vibratome (Leica Biosystems, model: VT1000S, Buffalo Grove, IL) and then incubated in oxygenated aCSF at room temperature for at least 1 hour before the recording. Slices were transferred to the recording chamber and allowed to equilibrate for 10 to 20 minutes before the recording. The slices were bathed in oxygenated aCSF (30°C) at a flow rate of 2 mL/minute. In current-clamp mode, neurons were recorded under 0 current injection (I = 0) in whole-cell patch-clamp configuration. The pipette solution was composed of 120 mM K-gluconate, 1 mM NaCl, 10 mM KCl, 10 mM HEPES, 5 mM EGTA, 1 mM CaCl2, 1 mM MgCl2, 3 mM KOH, and 4 mM (Mg)-ATP, pH 7.3. The pipettes had a resistance of 5 to 7 MΩ. The membrane potential was monitored for at least 5 minutes (Basal), followed by the addition of porcine GH (5 µg/mL) to the bath for approximately 5 minutes. The effects of GH were monitored for up to 10 minutes. Only 1 cell was recorded in each brain slice. The membrane potential values were compensated to account for the liquid junction potential (–8 mV).
Evaluation of Body Growth
Body weight of male and female mice were determined weekly from weaning up to 5 months of life. Body fat mass and lean body mass were determined every 2 weeks by time domain nuclear magnetic resonance using the LF50 body composition mice analyzer (Bruker, Germany). At the end of the follow-up period, mice were anesthetized with isoflurane and the naso-anal length was determined.
Evaluation of Pulsatile GH Secretion
To acclimate to the procedure of tail-tip blood sampling and minimize the interference of stress, 4-week-old male and female mice were daily handled for 30 days. Blood collection started at the beginning of the light cycle (approximately 8:00 hours) and 36 sequential tail-tip blood samples of 5 μL were collected from each mouse at 10-minute intervals (17, 36). Immediately before the first sample collection, a small portion of the tail tip (1 mm) was cut with a surgical blade to allow the collection of small drops of blood. During the whole period of the experiment, mice were allowed to move freely in their home cages with ad libitum access to food and water. For each blood collection, mice were placed inside a cardboard tube and quickly held by the base of the tail. Using a 10-μL pipette, a 5-μL sample of whole blood was collected and transferred to 105 μL of PBS with 0.05% tween-20 (PBS-T). After each blood collection, fingertip pressure was gently applied to the tail tip to stop bleeding. Samples were immediately placed on dry ice and stored at –80°C.
Hormone Assessment
To determine GH levels in the blood, a sensitive sandwich enzyme-linked immunosorbent assay was used as previously described (17, 37). A 96-well high-binding plate (9018, Corning, Kennebunk, ME) was coated with 50 µL of monkey antirat GH antibody (NIDDK-NHPP; rGH-IC-1, Cat# AFP411S; RRID:AB_2665564) diluted in PBS at 1:50 000 overnight at 4°C. After decanting the coating antibody, wells were incubated with 200 µL of blocking buffer (5% skim milk powder in PBS-T) for 2 hours at room temperature. The standard curve consisted of a 2-fold serial dilution of mouse recombinant GH (mGH reference preparation; NIDDK-NHPP; Cat# AFP-10783B) in 0.2% bovine serum albumin PBS-T. The wells were incubated with 50 µL of samples at a 1:20 dilution for 24 hours at room temperature. The plate was washed in PBS-T and the wells were incubated with 50 µL of rabbit antirat GH antibody (NIDDK-NHPP; Cat# AFP5672099; RRID:AB_2721132) diluted in blocking buffer at 1:100 000 for 24 hours at 4°C. After washing in PBS-T, wells were incubated with 50 μL of horseradish peroxidase conjugated goat antirabbit IgG antibody (A9169-2ml, Sigma-Aldrich) diluted in 50% PBS, 50% blocking buffer at 1:30 000 for 90 minutes at room temperature. After a final wash in PBS-T, wells were incubated with 100 μL of 2 mg/mL o-phenylenediamine dihydrochloride (P1526, Sigma-Aldrich) in citrate phosphate buffer (pH 5.0) containing 0.02% hydrogen peroxide for 45 minutes at room temperature. The reaction was stopped with 50 μL of 3 M HCl. The absorbance was determined at 490 nm with a microplate reader (Epoch, Biotek) and the wavelength of 650 nm was used for background correction. The standard curve ranged from 0.03 to 7.50 ng/mL and the concentration of GH was calculated by interpolating the optical density of the samples against a nonlinear regression of the standard curve. The lower limit of detection was 0.04 ng/mL, and the intra-assay and interassay coefficients of variation were 2.6% and 9.7%, respectively. GH pulses were detected using default parameters of the DynPeak pulse detection algorithm (38). The number of GH pulses was calculated for a period of 6 hours. The amplitude of each pulse was defined as the difference between peak value and its nadir, and the average levels of the detected pulses were considered for the statistical analysis of pulse amplitude. Mean GH was calculated by averaging all GH values from each mouse. Serum IGF-1 concentration was determined using a commercially available enzyme-linked immunosorbent assay kit (#MG100; R&D Systems).
Quantitative Real-time Polymerase Chain Reaction
The entire hypothalamus and liver samples were collected to determine the gene expression by quantitative real-time polymerase chain reaction (PCR). Initially, total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA), followed by incubation in DNase I RNase-free (Roche Applied Science) and then reverse transcription using 2 µg of total RNA, SuperScript II Reverse Transcriptase (Invitrogen), and random primers p(dN)6 (Roche Applied Science). Real-time PCR was performed using the 7500TM Real-Time PCR System (Applied Biosystems, Warrington, UK), Power SYBR Green Gene Expression PCR Master Mix (Applied Biosystems) and specific primers for target genes (Table 1). Data were normalized to the geometric average of Actb and Ppia. Relative quantification of mRNA was calculated by 2–ΔΔCt.
Table 1.
Primer sequences
| Target gene | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| Abcd2 | tgggatttccctactctcca | gcgactccaacttttgcttt |
| Acot3 | gcacgcatttaccacccaga | gctggtcacaaaggcaacca |
| Actb | gctccggcatgtgcaaag | catcacaccctggtgccta |
| Cyp17a1 | ggactcacctcctcatgctg | cagtgcccagagattgatga |
| Cyp2b13 | tctccacagctggaaggtg | tagcacagaccacagagtgtga |
| Cyp2b9 | ggaaaccaggccattggta | gtggactccagaaatcaaaacag |
| Cyp2d9 | cattctcagcaggccgca | accatagactccagagttgct |
| Cyp7b1 | tgcgtgacgaaattgacagt | tgctggagtatgagcacagc |
| Ghr | atcaatccaagcctggggac | acagctgaatagatcctgggg |
| Ghrh | tatgcccggaaagtgatccag | atccttgggaatccctgcaaga |
| Ghrl | agaggaggagctggagatca | gctggcgcctctttgacc |
| Igf1 | gtacttcctttccttctcctttgc | ccacactgacatgcccaaga |
| Mup1 | acctatccaatgccatggacc | ctggaggcagcgatctgtag |
| Ppia | tatctgcactgccaagactgagt | cttcttgctggtcttgccattcc |
| Prlr | cagtaaatgccaccaacgaa | gaggaggctctggttcaaca |
| Serpina6 | tctgatttcgcagacaccac | aggaagatgaagggcctgtt |
| Slco1a1 | atctacggtgacgcacacac | tcagtagaattgaatgctgtttcag |
| Sst | ctgtcctgccgtctccagt | ctgcagaaactgacggagtct |
Statistical Analysis
The electrophysiological data were analyzed by a paired 2-tailed Student’s t test. Changes in body weight and body composition along time were determined by repeated measures 2-way analysis of variance (ANOVA). Differences between 2 groups were analyzed by the unpaired 2-tailed Student’s t test. When 3 groups were simultaneously analyzed, we used 1-way ANOVA, followed by Newman–Keuls multiple comparisons test. Differences in hepatic gene expression between the sexes and experimental groups were assessed using 2-way ANOVA. Statistical analyses were performed using Prism software (GraphPad, San Diego, CA; RRID:SCR_002798). We considered P < .05 to be statistically significant. All results are expressed as mean ± standard error of the mean. The description of the tests used in each experiment and the sample sizes are found in the figure legends.
Results
PV/PVHSST Neurons Express Ghr mRNA and Are Responsive to GH
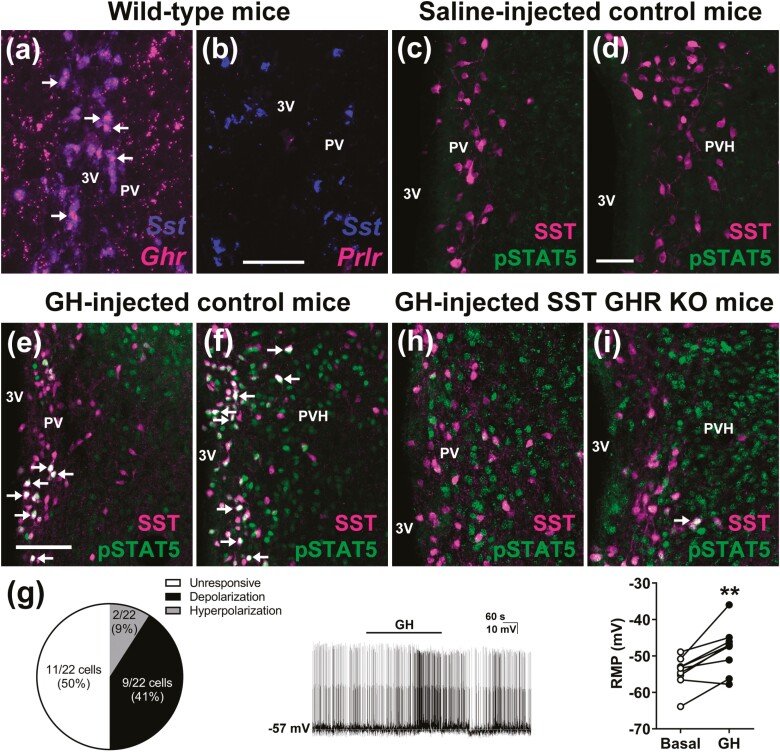
A neuronal population that mediates a negative feedback loop must express the specific hormone receptor, and should also tightly respond to changing hormone levels. Using RNAscope technology, we confirmed the findings of previous studies (10, 20) indicating an elevated percentage (~66%) of PV/PVHSST neurons expressing Ghr mRNA (Fig. 1A). For the purpose of comparison, prolactin receptor mRNA is virtually absent from PV/PVHSST neurons (Fig. 1B). An acute GH injection induces pSTAT5 in different neuronal populations (39, 40). Thus, pSTAT5 immunolabeling has been used as a marker of GH-responsive neurons (3). pSTAT5 was absent in PV/PVHSST neurons of saline-injected mice (Fig. 1C and 1D), indicating that endogenous GH secretion is not sufficient to allow visualization of pSTAT5 in these cells. In contrast, IP injection of a high dose of GH induced pSTAT5 in 64.7 ± 8.6% and 54.7 ± 3.6% of PVSST and PVHSST neurons, respectively (n = 3/group; Fig. 1E and 1F). To further investigate how GH affects periventricular SST neurons, we used whole cell patch-clamp to determine GH-induced changes in the resting membrane potential of PV/PVHSST neurons. The cells were recorded from brain slices of SST-cre::LSL-tdTomato mice. Among the recorded cells (22 neurons from 8 mice), 41% of PV/PVHSST neurons exhibited a significant depolarization a few minutes after GH application to the bath (Fig. 1G), which is in accordance with GH-induced c-fos gene expression observed in PVSST neurons (18). Surprisingly, a small number of SST neurons (2 out 22 cells; 9%) hyperpolarized after GH application (–4.8 ± 0.7 mV change in resting membrane potential), suggesting the existence of distinct subgroups of PV/PVHSST neurons. Thus, PV/PVHSST neurons fulfill the prerequisites necessary to sense and respond to GH, closing a classical negative feedback loop. To determine the neuroendocrine role of SST neurons, we generated mice carrying ablation of GHR specifically in SST-expressing neurons (hereafter named SST GHR KO mice). As expected, a profound reduction in the percentage of cells expressing GH-induced pSTAT5 in PVSST (8.5 ± 0.8%; P = .0002 vs control mice) and PVHSST (20.0 ± 2.5%; P < .0001 vs control mice) neurons was observed in SST GHR KO mice compared with GHR-intact mice (Fig. 1H-1I).
Figure 1.
PV/PVHSST neurons express Ghr mRNA and are responsive to GH. (A,B) Colocalization between Sst mRNA and Grh mRNA, and Sst mRNA and Prlr mRNA in the PV of mice. Scale bar = 100 µm. Abbreviations: 3V, third ventricle. The arrows indicate double-labeled neurons. (C,D) PV/PVHSST neurons exhibit no STAT5 phosphorylation (pSTAT5) in saline-injected mice. Scale bar = 50 µm. (E,F) PV/PVHSST neurons exhibit pSTAT5 after an IP GH injection. (G) Electrophysiological recordings of PV/PVHSST neurons after GH application (22 neurons from 8 mice). Representative recording of a cell that showed significant depolarization a few minutes after GH application to the bath. Changes in the resting membrane potential (RMP) of depolarized neurons (n = 9; **P < .01; paired t-test). (H,I) SST GHR KO mice show only few GH-induced pSTAT5 in PV/PVHSST neurons, demonstrating the efficacy of the cell-specific GHR ablation.
SST GHR KO Mice Exhibit Normal Growth and Pulsatile GH Secretion
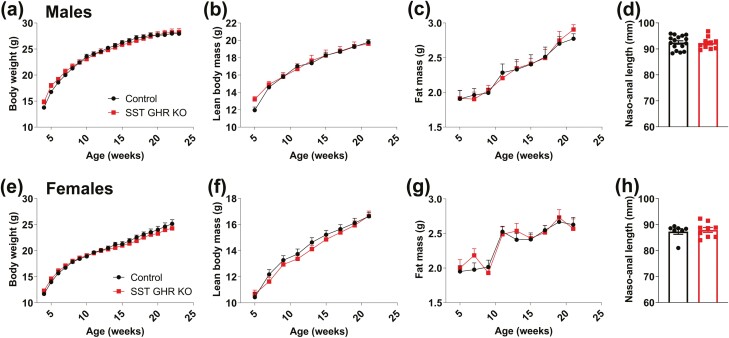
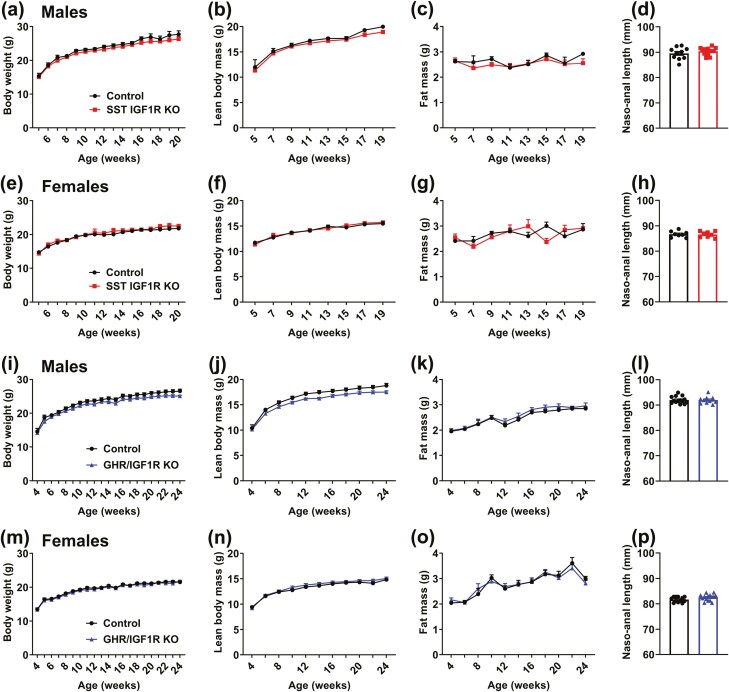
Since alterations in GH secretion can produce profound consequences on growth, the body weight and body composition of SST GHR KO mice and control littermates were determined from weaning up to 5 months of life. We found that GHR ablation in SST neurons did not affect body weight (Fig. 2A and 2E), lean body mass (Fig. 2B and 2F), body fat mass (Fig. 2C and 2G), and naso-anal length (Fig. 2D and 2H) in either male or female mice.
Figure 2.
SST GHR KO mice exhibit normal growth. (A-D) Changes along time in body weight, lean body mass, body fat mass, and naso-anal length in control (n = 16) and SST GHR KO (n = 11) male mice. (E-H) Changes along time in body weight, lean body mass, body fat mass, and naso-anal length in control (n = 14) and SST GHR KO (n = 10) female mice.
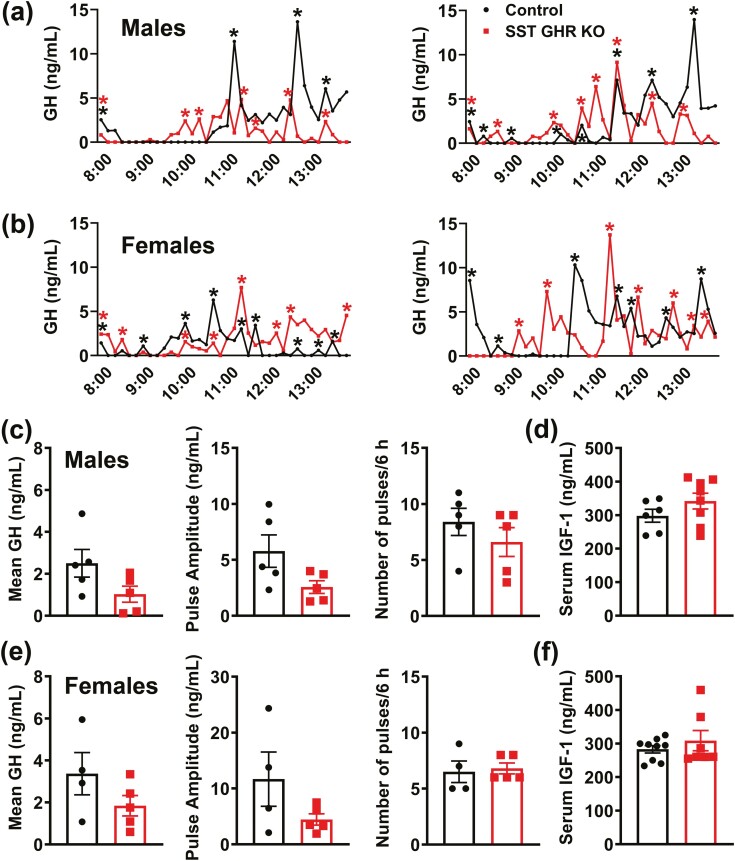
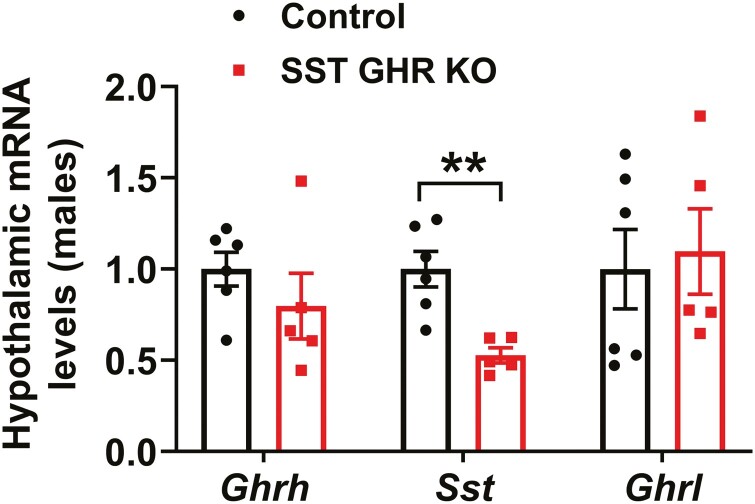
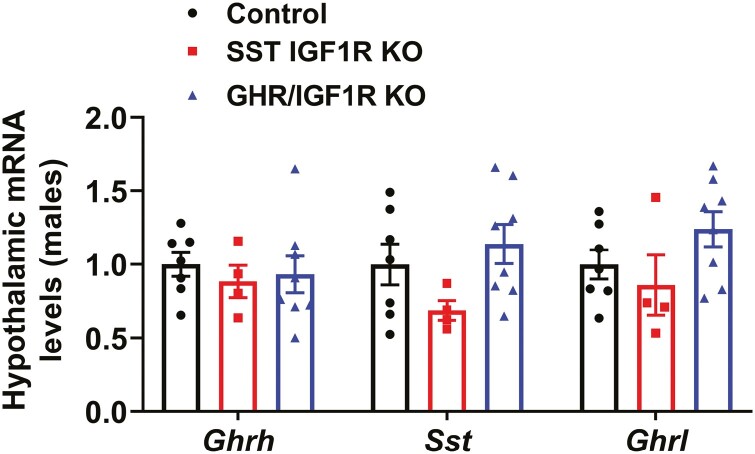
The pulsatile GH secretion pattern was evaluated through 36 serial blood collections in each mouse at 10-minute intervals during the first 6 hours of the light phase (Fig. 3A and 3B). In accordance with the lack of changes in body growth, male and female SST GHR KO mice showed normal mean GH levels, GH pulse amplitude and pulse frequency, compared with control animals (Fig. 3A-3C and 3E). Additionally, serum IGF-1 levels were normal in male and female SST GHR KO mice (Fig. 3D and 3F). No changes between groups were observed in Ghrh mRNA levels in the hypothalamus (Fig. 4). However, Sst mRNA levels were significantly reduced in SST GHR KO mice (Fig. 4). Reduced SST expression has been described in the SST-cre line used in the present study due to an unpredicted consequence of Cre transgene insertion into the 3′ untranslated region of the Sst gene (41). Importantly, in the current study, we only used mice heterozygous for the SST-cre allele since homozygous mice exhibit more pronounced suppression of SST expression (41). Since ghrelin is a powerful GH secretagogue and some studies observed hypothalamic ghrelin expression, we also analyzed Ghrl mRNA levels in the hypothalamus (42-44). However, no difference in the hypothalamic Ghrl mRNA levels was observed between the groups (Fig. 4).
Figure 3.
Normal pulsatile GH secretion pattern in SST GHR KO mice. (A,B) Representative examples of the pulsatile pattern of GH secretion in 8-week-old control and SST GHR KO mice in both males (A) and females (B). The asterisks indicate the identified peaks. (C,D) Mean GH levels (n = 5/group), GH pulse amplitude, GH pulse frequency, and serum IGF-1 levels in 8-week-old control (n = 6) and SST GHR KO (n = 8) male mice. (E,F) Mean GH levels (n = 4-5/group), GH pulse amplitude, GH pulse frequency, and serum IGF-1 levels in 8-week-old control (n = 9) and SST GHR KO (n = 7) female mice.
Figure 4.
Reduced hypothalamic Sst mRNA levels in SST GHR KO mice. Hypothalamic gene expression in 8-week-old control (n = 5-6) and SST GHR KO (n = 4-5) male mice. **P < .01 (unpaired t-test).
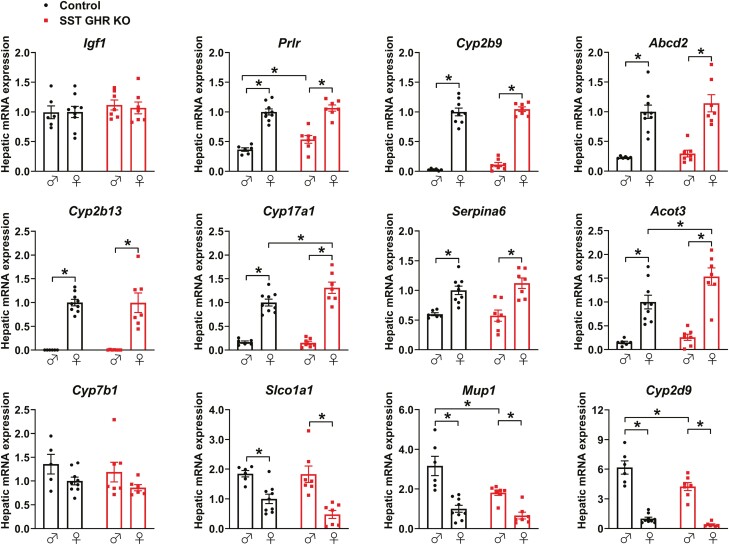
GHR Ablation in SST Neurons Mildly Affects the Hepatic Expression of Genes That Exhibit Marked Sex Differences
SST GHR KO mice showed normal hepatic Igf1 (Fig. 5) and Ghr mRNA levels (data not shown), compared with control mice. SST neurons play a critical role in determining the sexually differentiated hepatic gene expression profile (41, 45, 46). This occurs through the regulation of the pattern of GH secretion, which is different between males and females. Thus, we analyzed the hepatic expression of some genes that exhibit marked sex differences and are regulated by SST (45). Females display higher hepatic expression of Prlr, Cyp2b9, Abcd2, Cyp2b13, Cyp17a1, Serpina6, and Acot3 mRNA levels than males (Fig. 5). In contrast, males showed increased expression of Slco1a1, Mup1, and Cyp2d9 mRNA compared with female mice (Fig. 5). Although the marked sex differences in the expression of these genes were not affected by GHR ablation in SST neurons, SST GHR KO males showed increased Prlr mRNA levels compared with control male mice, whereas SST GHR KO females exhibited higher Cyp17a1 and Acot3 mRNA levels than control female mice (Fig. 5). Additionally, SST GHR KO males displayed reduced expression of Mup1 and Cyp2d9 compared with control males (Fig. 5). Thus, GHR ablation in SST neurons mildly affected the hepatic expression of genes that exhibit marked sex differences.
Figure 5.
GHR ablation in SST neurons mildly affects the hepatic expression of genes that exhibit marked sex differences. Hepatic gene expression in 8-week-old control (n = 6-9) and SST GHR KO (n = 7-8) male and female mice. *P < .05 (2-way ANOVA and Newman–Keuls multiple comparisons test).
Ablation of IGF1R or GHR/IGF1R in SST Neurons Does not Affect Body Growth
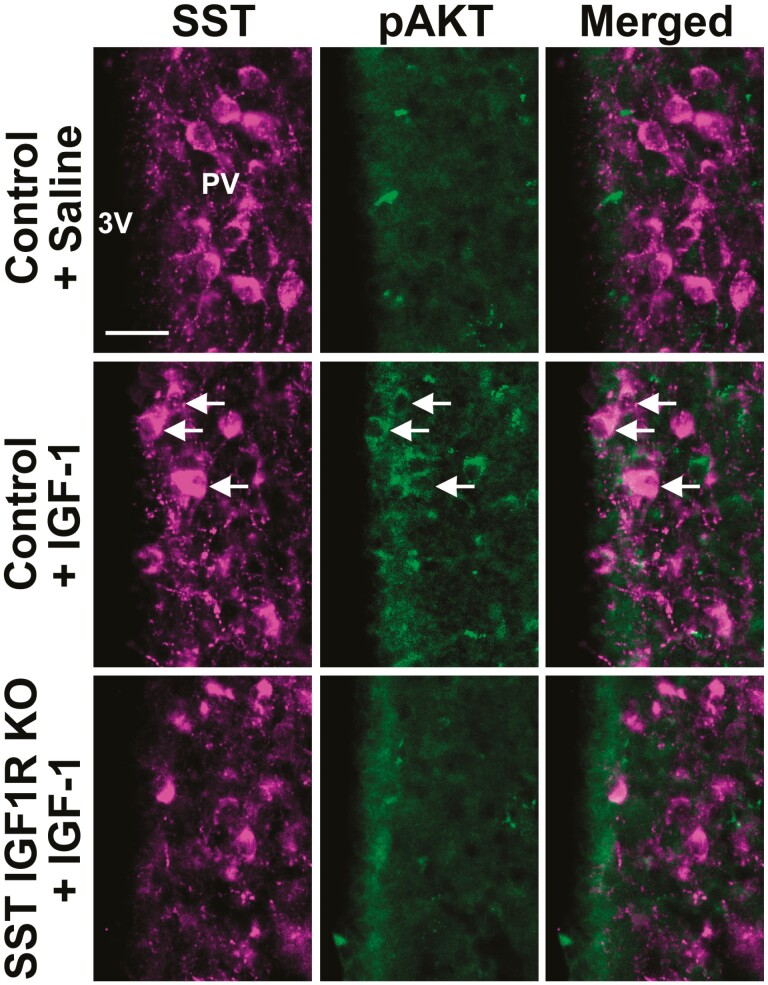
To investigate the long-loop negative feedback mechanisms that control the somatotropic axis, we now determined whether IGF1R signaling regulates body growth and GH secretion via SST neurons. Using the capacity of IGF1R signaling to induce pAKT (32-35), we showed that an ICV IGF-1 injection increased the percentage of PV/PVHSST neurons expressing pAKT (43.4%) compared with saline-injected mice (8.3% of colocalization; P = .0414; Fig. 6). Using SST-specific IGF1R KO mice, we observed that PV/PVHSST neurons were predominantly unresponsive to ICV IGF-1 injection (3.4% of colocalization between SST and pAKT; P = .5969 vs saline-injected mice; n = 3/group), demonstrating the efficacy of the cell-specific deletion (Fig. 6). Initially, possible changes in body weight and growth were investigated in male and female SST IGF1R KO and in double KO mice by simultaneously targeting GHR and IGF1R in SST cells (Fig. 7). Similarly to that observed in SST GHR KO mice, no significant changes in body weight (Fig. 7A and 7E), lean body mass (Fig. 7B and 7F), body fat mass (Fig. 7C and 7G), or naso-anal length (Fig. 7D and 7H) were observed in male or female SST IGF1R KO mice compared with their respective littermate controls. Furthermore, the double deletion of GHR and IGF1R in SST cells did not lead to differences in body weight, composition, or length (Fig. 7I-7P).
Figure 6.
IGF-1 induces AKT phosphorylation in PVSST neurons of control mice but not in SST IGF1R KO mice. Fluorescence photomicrographs showing the colocalization between SST and pAKT in the PV of control mice that received ICV injection of saline or IGF-1, and in SST IGF1R KO mice that received ICV IGF-1 injection. The arrows indicate double-labeled neurons. Abbreviation: 3V, third ventricle. Scale bar = 25 µm.
Figure 7.
SST IGF1R KO and GHR/IGF1R KO mice exhibit normal growth. (A-D) Changes in body weight, lean body mass, body fat mass, and naso-anal length in control (n = 5-10) and SST IGF1R KO (n = 9-14) male mice. (E-H) Changes in body weight, lean body mass, body fat mass, and naso-anal length in control (n = 8-9) and SST IGF1R KO (n = 6-8) female mice. (I-L) Changes in body weight, lean body mass, body fat mass, and naso-anal length in control (n = 14) and GHR/IGF1R KO (n = 10) male mice. (M-P) Changes in body weight, lean body mass, body fat mass, and naso-anal length in control (n = 12) and GHR/IGF1R KO (n = 15) female mice.
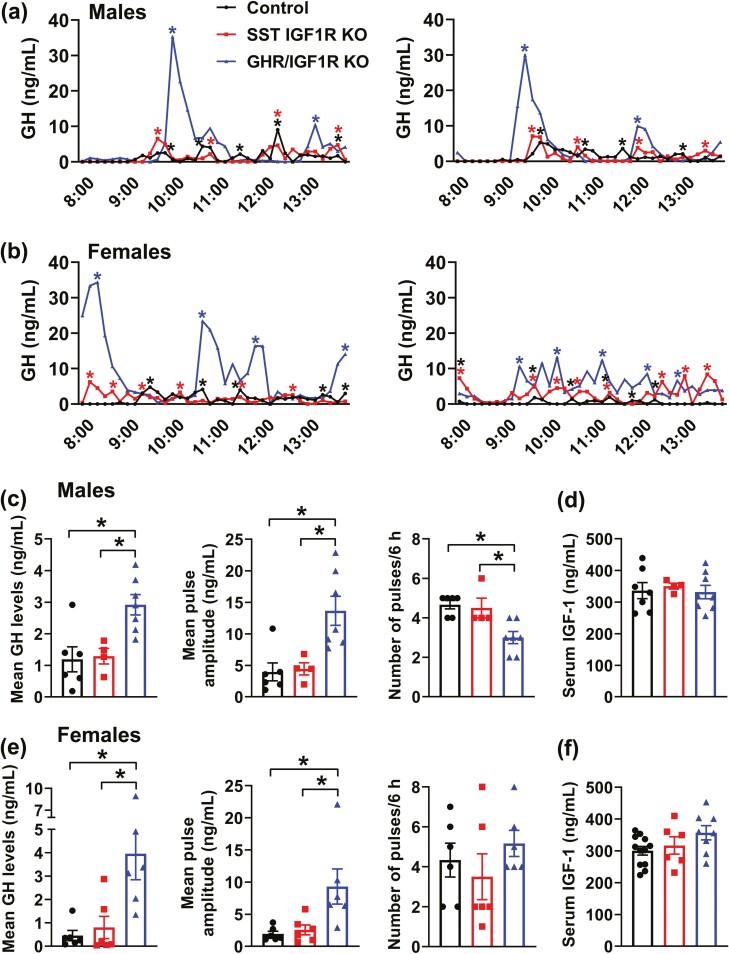
Increased Mean GH Levels and Pulse Amplitude Without Affecting Circulating IGF-1 Concentration in GHR/IGF1R KO mice
Next, possible changes in pulsatile GH secretion were assessed in SST IGF1R KO and GHR/IGF1R KO mice. SST IGF1R KO mice exhibited a normal GH secretion profile and serum IGF-1 levels in male and female mice (Fig. 8A-8F). Notably, GHR/IGF1R KO mice presented a significant increase in mean GH levels and pulse amplitude compared with control and SST IGF1R KO mice (Fig. 8C and 8E). This alteration was observed in both male and female mice. However, while GHR/IGF1R KO females showed normal GH pulse frequency (Fig. 8E), GHR/IGF1R KO male mice displayed a reduction in GH pulse frequency compared with other groups (Fig. 8C). Noteworthy, serum IGF-1 levels remained unaffected in GHR/IGF1R KO mice, despite the alterations in GH secretion (Fig. 8D and 8F). Hypothalamic Ghrh, Sst, and Ghrl mRNA levels were determined in male mice and no significant changes were observed between the groups (Fig. 9). Thus, double deletion of GHR and IGF1R in SST cells causes increased mean GH levels and pulse amplitude without affecting circulating IGF-1 concentration.
Figure 8.
Increased mean GH levels and pulse amplitude in GHR/IGF1R KO mice. (A,B) Representative examples of the pulsatile pattern of GH secretion in 8-week-old control, SST IGF1R KO, and GHR/IGF1R KO mice in both males (A) and females (B). The asterisks indicate the identified peaks. (C,D). Mean GH levels, GH pulse amplitude, GH pulse frequency, and serum IGF-1 levels in 8-week-old control (n = 6-7), SST IGF1R KO (n = 4), and GHR/IGF1R KO (n = 7-8) male mice. (E,F). Mean GH levels, GH pulse amplitude, GH pulse frequency, and serum IGF-1 levels in 8-week-old control (n = 6-12), SST IGF1R KO (n = 6), and GHR/IGF1R KO (n = 6-8) female mice. *P < 0.05 (1-way ANOVA and Newman–Keuls multiple comparisons test).
Figure 9.
Hypothalamic gene expression in 8-week-old control (n = 7), SST IGF1R KO (n = 4), and GHR/IGF1R KO (n = 8) male mice.
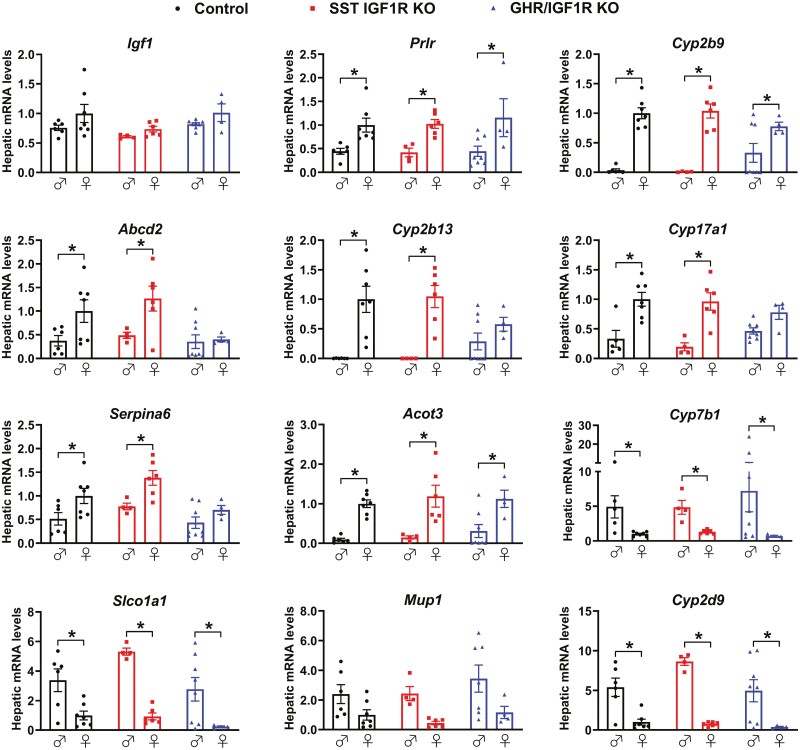
Partial Disruption of the Sex Differences in Hepatic Gene Expression Pattern in GHR/IGF1R KO Mice
Confirming the absence of changes in circulating IGF-1 concentration, hepatic Igf1 mRNA levels did not show significant differences between control, SST IGF1R KO, and GHR/IGF1R KO mice (Fig. 10). Then, the expression of sexually differentiated genes was assessed in the liver. In accordance with previous studies (45) and our former results (Fig. 5), sex differences were observed in hepatic expression of Prlr, Cyp2b9, Abcd2, Cyp2b13, Cyp17a1, Serpina6, Acot3, Cyp7b1, Slco1a1, and Cyp2d9 mRNA in control mice (Fig. 10). The sex differences were not altered in SST IGF1R KO mice. However, GHR/IGF1R KO mice no longer exhibited differences in hepatic expression of Abcd2, Cyp2b13, Cyp17a1, and Serpina6 between males and females (Fig. 10).
Figure 10.
Partial disruption of the sex differences in hepatic gene expression pattern in GHR/IGF1R KO mice. Hepatic gene expression in 8-week-old control (n = 6-7), SST IGF1R KO (n = 4-6), and GHR/IGF1R KO (n = 4-8) male and female mice. *P < .05 (2-way ANOVA and Holm–Sidak’s multiple comparisons test).
Discussion
Currently, it is assumed that PV/PVHSST neurons mediate the short-loop negative feedback that controls GH secretion (4-8, 11, 12). Nevertheless, no study so far has manipulated GHR expression specifically in SST neurons to directly test this concept. Previous studies also suggest that SST neurons are able to sense IGF-1 levels (30), indicating that SST neurons additionally control the hypothalamic–GH axis via long-loop negative feedback. In the present study, we showed that ablation of either GHR or IGF1R in SST neurons is not sufficient to cause a significant impact on pulsatile GH secretion, body growth, or hepatic gene profile. However, simultaneous ablation of both GHR and IGF1R in SST cells led to increased GH secretion and partial disruption of sex differences in the hepatic gene expression pattern, without altering serum IGF-1 levels and body growth.
To manipulate SST-expressing cells, we used an SST-cre mouse model that is known to exhibit reduced SST expression. However, this effect is severe in homozygous and moderate in heterozygous mice (41). Since the current study used heterozygous SST-cre mice and we observed a normal phenotype in SST GHR KO or SST IGF1R KO mice, compared with their respective control animals, we believe that the lower SST expression in mutant mice did not significantly impact our findings.
Surprisingly, GHR ablation in SST neurons did not affect the GH secretion pattern in mice even though this neuronal population presents all prerequisites necessary to sense and respond to variations in GH levels and consequently regulate pituitary GH secretion (10, 13, 20). Although previous studies have shown that PV/PVHSST neurons express markers of neuronal activation after GH administration (eg, c-Fos) (18), whether they show changes in membrane potential was not evident. In this study, we demonstrated that GH depolarizes the membrane of approximately 40% of PV/PVHSST neurons. The role of IGF1R signaling in SST neurons has been much less investigated (4, 30). In contrast, previous studies have already shown that IGF1R signaling in somatotropic cells regulates pituitary GH secretion (47, 48). Similar to GHR/IGF1R KO mice, somatotropic-specific IGF1R KO mice exhibit increased GH secretion without changes in body growth (47), which further demonstrates the existence of multiple redundant and compensatory mechanisms controlling the somatotropic/growth axis.
The role of SST neurons regulating the hypothalamic–GH axis via negative feedback mechanisms only became evident in mice carrying simultaneous ablation of both GHR and IGF1R in SST-expressing cells. Thus, the absence of a single receptor can be compensated by the other, preventing alterations in GH secretion pattern. These findings highlight the importance of taking into consideration possible redundant mechanisms involved in the control of GH secretion. We previously showed that GHR ablation in tyrosine hydroxylase–expressing cells led to increased hypothalamic Ghrh mRNA, GH pulse amplitude, serum IGF-1 levels, and body growth (17). Thus, considering only GH autofeedback, tyrosine hydroxylase–expressing neurons seem to have a more critical importance in controlling GH secretion by short-loop negative feedback than SST neurons. GHRH neurons represent another potential cell population that could regulate GH secretion via negative feedback, possibly compensating the absence of GHR or IGF1R in SST neurons (4). However, previous studies found that only a small percentage of ARHGHRH neurons express Ghr mRNA or are responsive to GH (16, 17). No information exists about whether IGF1R is expressed in ARHGHRH neurons. Thus, the exact importance of GHRH neurons in mediating the negative feedback loops that control the pulsatile pattern of GH secretion it is currently unclear. Nevertheless, it is well known that GHRH and SST act in great coordination to control pituitary GH release (11, 12, 25). Therefore, compensatory mechanisms via GHRH neurons may mask the actual role of SST neurons regulating GH secretion via negative feedback loops.
The increased GH secretion exhibited by GHR/IGF1R KO mice was entirely explained by higher GH pulse amplitude. GH pulse frequency decreased in GHR/IGF1R KO male mice, probably as a compensatory mechanism to limit the impact of GH hypersecretion. Thus, an unknown cell population was probably able to detect the increase in GH pulse amplitude to consequently decrease GH pulse frequency. Future studies are still necessary to better understand the cell populations responsible to mediate changes in GH pulse amplitude and frequency. It is important to note that we did not analyze the GH pulsatile pattern at a specific stage of the estrous cycle of female mice. Considering the well-described influence of estradiol on GH secretion (4, 49-54), variations in the hormonal profile along the estrous cycle may have increased variability in the females.
Our findings using GHR/IGF1R KO mice are mostly in accordance with the results previously observed in mice KO for the Sst gene which show minor alterations in body growth and serum/liver IGF-1 levels (41, 45, 46, 55, 56). Regarding the consequences on GH secretion, SST KO mice have higher random or mean GH levels than wild-type controls (45, 55, 56). Hepatic gene expression is regulated by the GH secretion pattern and exhibits strong sex differences (45). SST neurons are critical to control hepatic gene expression, so sex differences in hepatic expression of several genes are lost in SST KO mice (41, 45, 46, 56). Notably, we observed that hepatic expression of some genes that exhibit a sex difference is regulated by GHR/IGF1R signaling in SST neurons. Therefore, partial disruption in sex differences of the hepatic gene expression pattern was observed in GHR/IGF1R KO mice. These findings reinforce the importance of SST neurons regulating sex differences in the hepatic gene expression profile, and reveal that GH/IGF-1 actions on SST neurons are required for the establishment of sex differences in the hepatic gene expression profile.
In summary, our findings add new and important information to the complex mechanisms involved in the control of the somatotropic axis. Surprisingly, the absence of either GHR or IGF1R alone in SST neurons is insufficient to disrupt GH secretion and its physiological effects. However, simultaneous ablation of both GHR and IGF1R in SST cells led to increased GH secretion and partial disruption in the sexually differentiated hepatic gene expression profile, reproducing a phenotype similar to that observed in mice KO for the Sst gene (41, 45, 46, 55, 56). Taken together, GHR and IGF1R signaling in SST neurons play a redundant control of pituitary GH secretion. Moreover, our study reveals the actual role played by the short- (GH-mediated) and long-loop (IGF-1 mediated) negative feedback mechanisms on SST neurons for the control of the hypothalamic-GH-growth axis.
Acknowledgments
We thank Ana Maria P. Campos (Universidade de Sao Paulo) and Emily Henson from the Michigan Diabetes Research Center (NIH P30 DK020572, MICPC In situ hybridization laboratory) for technical assistance, and Prof. Martin Metzger for critical reading of the article.
Glossary
Abbreviations
- aCFS
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- ARH
arcuate nucleus of the hypothalamus
- GH
growth hormone
- GHR
growth hormone receptor
- GHRH
growth hormone–releasing hormone
- ICV
intracerebroventricular
- IGF
insulin-like growth factor-1
- IGF1R
insulin-like growth-1 receptor
- IP
intraperitoneal
- KPBS
potassium phosphate-buffered saline
- KO
knockout
- LSL
LoxP-Stop-LoxP
- NIDDK-NHPP
National Institute of Diabetes and Digestive and Kidney Diseases–National Hormone and Pituitary Program
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- pSTAT5
phosphorylation of the signal transducer and activator of transcription-5
- PV
periventricular nucleus
- PVH
paraventricular nucleus
- SST
somatostatin
Financial Support
This study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP/Brazil grant numbers: 2016/20897-3 to F.W., 2017/22189-9 to N.S.M. 2017/21854-9 to F.M.C., 2017/25281-3 to P.G.F.Q., 2019/21707-1 to R.F., 2020/10102-9 to M.R.T., 2020/01318-8 to J.D. (Jose Donato Jr.) and 2021/03316-5 to D.O.G.), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG/Brazil grant number: CBB-APQ-03308-16 to RES) and National Institutes of Health (NIA grant number: R01AG059779 to J.J.K. and E.O.L.; NICHD grant number: R01HD069702 to C.F.E.).
Disclosure Summary
The authors have nothing to disclose.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Devesa J, Almenglo C, Devesa P. Multiple effects of growth hormone in the body: is it really the hormone for growth? Clin Med Insights Endocrinol Diabetes 2016;9:47-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basu R, Qian Y, Kopchick JJ. Mechanisms in endocrinology: lessons from growth hormone receptor gene-disrupted mice: are there benefits of endocrine defects? Eur J Endocrinol. 2018;178(5):R155-R181. [DOI] [PubMed] [Google Scholar]
- 3. Donato J Jr., Wasinski F, Furigo IC, Metzger M, Frazao R. Central regulation of metabolism by growth hormone. Cells 2021;10(1):129129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steyn FJ, Tolle V, Chen C, Epelbaum J. Neuroendocrine regulation of growth hormone secretion. Compr Physiol 2016;6(2):687-735. [DOI] [PubMed] [Google Scholar]
- 5. Murray PG, Higham CE, Clayton PE. 60 years of neuroendocrinology: the hypothalamo-GH axis: the past 60 years. J Endocrinol. 2015;226(2):T123-T140. [DOI] [PubMed] [Google Scholar]
- 6. Bertherat J, Bluet-Pajot MT, Epelbaum J. Neuroendocrine regulation of growth hormone. Eur J Endocrinol. 1995;132(1):12-24. [DOI] [PubMed] [Google Scholar]
- 7. Muller EE, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiol Rev. 1999;79(2):511-607. [DOI] [PubMed] [Google Scholar]
- 8. Ranke MB, Wit JM. Growth hormone – past, present and future. Nat Rev Endocrinol. 2018;14(5):285-300. [DOI] [PubMed] [Google Scholar]
- 9. Fodor M, Kordon C, Epelbaum J. Anatomy of the hypophysiotropic somatostatinergic and growth hormone-releasing hormone system minireview. Neurochem Res. 2006;31(2):137-143. [DOI] [PubMed] [Google Scholar]
- 10. Burton KA, Kabigting EB, Clifton DK, Steiner RA. Growth hormone receptor messenger ribonucleic acid distribution in the adult male rat brain and its colocalization in hypothalamic somatostatin neurons. Endocrinology 1992;131(2):958-963. [DOI] [PubMed] [Google Scholar]
- 11. Farhy LS, Veldhuis JD. Joint pituitary-hypothalamic and intrahypothalamic autofeedback construct of pulsatile growth hormone secretion. Am J Physiol Regul Integr Comp Physiol. 2003;285(5):R1240-R1249. [DOI] [PubMed] [Google Scholar]
- 12. Farhy LS, Veldhuis JD. Putative GH pulse renewal: periventricular somatostatinergic control of an arcuate-nuclear somatostatin and GH-releasing hormone oscillator. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R1030-R1042. [DOI] [PubMed] [Google Scholar]
- 13. Bertherat J, Timsit J, Bluet-Pajot MT, et al. Chronic growth hormone (GH) hypersecretion induces reciprocal and reversible changes in mRNA levels from hypothalamic GH-releasing hormone and somatostatin neurons in the rat. J Clin Invest. 1993;91(4):1783-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chomczynski P, Downs TR, Frohman LA. Feedback regulation of growth hormone (GH)-releasing hormone gene expression by GH in rat hypothalamus. Mol Endocrinol. 1988;2(3):236-241. [DOI] [PubMed] [Google Scholar]
- 15. de Gennaro Colonna V, Fidone F, Cocchi D, Muller EE. Feedback effects of growth hormone on growth hormone-releasing hormone and somatostatin are not evident in aged rats. Neurobiol Aging. 1993;14(5):503-507. [DOI] [PubMed] [Google Scholar]
- 16. Burton KA, Kabigting EB, Steiner RA, Clifton DK. Identification of target cells for growth hormone’s action in the arcuate nucleus. Am J Physiol. 1995;269(4 Pt 1):E716-E722. [DOI] [PubMed] [Google Scholar]
- 17. Wasinski F, Pedroso JAB, Dos Santos WO, et al. Tyrosine hydroxylase neurons regulate growth hormone secretion via short-loop negative feedback. J Neurosci. 2020;40(22):4309-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamegai J, Minami S, Sugihara H, Higuchi H, Wakabayashi I. Growth hormone induces expression of the c-fos gene on hypothalamic neuropeptide-Y and somatostatin neurons in hypophysectomized rats. Endocrinology 1994;135(6):2765-2771. [DOI] [PubMed] [Google Scholar]
- 19. Rogers KV, Vician L, Steiner RA, Clifton DK. The effect of hypophysectomy and growth hormone administration on pre-prosomatostatin messenger ribonucleic acid in the periventricular nucleus of the rat hypothalamus. Endocrinology 1988;122(2):586-591. [DOI] [PubMed] [Google Scholar]
- 20. Pellegrini E, Bluet-Pajot MT, Mounier F, Bennett P, Kordon C, Epelbaum J. Central administration of a growth hormone (GH) receptor mRNA antisense increases GH pulsatility and decreases hypothalamic somatostatin expression in rats. J Neurosci. 1996;16(24):8140-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fodor M, Csaba Z, Kordon C, Epelbaum J. Growth hormone-releasing hormone, somatostatin, galanin and beta-endorphin afferents to the hypothalamic periventricular nucleus. J Chem Neuroanat. 1994;8(1):61-73. [DOI] [PubMed] [Google Scholar]
- 22. Horvath S, Palkovits M, Gorcs T, Arimura A. Electron microscopic immunocytochemical evidence for the existence of bidirectional synaptic connections between growth hormone-releasing hormone- and somatostatin-containing neurons in the hypothalamus of the rat. Brain Res. 1989;481(1):8-15. [DOI] [PubMed] [Google Scholar]
- 23. Liposits Z, Merchenthaler I, Paull WK, Flerko B. Synaptic communication between somatostatinergic axons and growth hormone-releasing factor (GRF) synthesizing neurons in the arcuate nucleus of the rat. Histochemistry 1988;89(3):247-252. [DOI] [PubMed] [Google Scholar]
- 24. Willoughby JO, Brogan M, Kapoor R. Hypothalamic interconnections of somatostatin and growth hormone releasing factor neurons. Neuroendocrinology 1989;50(5):584-591. [DOI] [PubMed] [Google Scholar]
- 25. Wagner C, Caplan SR, Tannenbaum GS. Genesis of the ultradian rhythm of GH secretion: a new model unifying experimental observations in rats. Am J Physiol. 1998;275(6):E1046-E1054. [DOI] [PubMed] [Google Scholar]
- 26. List EO, Berryman DE, Funk K, et al. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology 2014;155(5):1793-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Mendelsohn FA. Localization and characterization of insulin-like growth factor-I receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. A distinct distribution from insulin receptors. J Neuroendocrinol. 1989;1(5):369-377. [DOI] [PubMed] [Google Scholar]
- 28. Abe H, Molitch ME, Van Wyk JJ, Underwood LE. Human growth hormone and somatomedin C suppress the spontaneous release of growth hormone in unanesthetized rats. Endocrinology 1983;113(4):1319-1324. [DOI] [PubMed] [Google Scholar]
- 29. Tannenbaum GS, Guyda HJ, Posner BI. Insulin-like growth factors: a role in growth hormone negative feedback and body weight regulation via brain. Science 1983;220(4592):77-79. [DOI] [PubMed] [Google Scholar]
- 30. Sato M, Frohman LA. Differential effects of central and peripheral administration of growth hormone (GH) and insulin-like growth factor on hypothalamic GH-releasing hormone and somatostatin gene expression in GH-deficient dwarf rats. Endocrinology 1993;133(2):793-799. [DOI] [PubMed] [Google Scholar]
- 31. List EO, Berryman DE, Funk K, et al. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol. 2013;27(3):524-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng WH, Quirion R. Insulin-like growth factor-1 (IGF-1) induces the activation/phosphorylation of Akt kinase and cAMP response element-binding protein (CREB) by activating different signaling pathways in PC12 cells. BMC Neurosci. 2006;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alessi DR, Andjelkovic M, Caudwell B, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15(23):6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 34. Partovian C, Simons M. Regulation of protein kinase B/Akt activity and Ser473 phosphorylation by protein kinase Calpha in endothelial cells. Cell Signal. 2004;16(8):951-957. [DOI] [PubMed] [Google Scholar]
- 35. Stitt TN, Drujan D, Clarke BA, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14(3):395-403. [DOI] [PubMed] [Google Scholar]
- 36. Steyn FJ, Huang L, Ngo ST, et al. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology 2011;152(8):3165-3171. [DOI] [PubMed] [Google Scholar]
- 37. Wasinski F, Barrile F, Pedroso JAB, et al. Ghrelin-induced food intake, but not GH secretion, requires the expression of the GH receptor in the brain of male mice. Endocrinology 2021;162(7):bqab097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vidal A, Zhang Q, Medigue C, Fabre S, Clement F. DynPeak: an algorithm for pulse detection and frequency analysis in hormonal time series. PLoS One. 2012;7(7):e39001e39001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Furigo IC, Metzger M, Teixeira PD, Soares CR, Donato J Jr. Distribution of growth hormone-responsive cells in the mouse brain. Brain Struct Funct. 2017;222(1):341-363. [DOI] [PubMed] [Google Scholar]
- 40. Wasinski F, Klein MO, Bittencourt JC, Metzger M, Donato J Jr. Distribution of growth hormone-responsive cells in the brain of rats and mice. Brain Res. 2021;1751:147189. [DOI] [PubMed] [Google Scholar]
- 41. Viollet C, Simon A, Tolle V, et al. Somatostatin-IRES-Cre mice: between knockout and wild-type? Front Endocrinol (Lausanne) 2017;8:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cowley MA, Smith RG, Diano S, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003;37(4):649-661. [DOI] [PubMed] [Google Scholar]
- 43. Kineman RD, Luque RM. Evidence that ghrelin is as potent as growth hormone (GH)-releasing hormone (GHRH) in releasing GH from primary pituitary cell cultures of a nonhuman primate (Papio anubis), acting through intracellular signaling pathways distinct from GHRH. Endocrinology 2007;148(9):4440-4449. [DOI] [PubMed] [Google Scholar]
- 44. Mozid AM, Tringali G, Forsling ML, et al. Ghrelin is released from rat hypothalamic explants and stimulates corticotrophin-releasing hormone and arginine-vasopressin. Horm Metab Res. 2003;35(8):455-459. [DOI] [PubMed] [Google Scholar]
- 45. Adams JM, Otero-Corchon V, Hammond GL, Veldhuis JD, Qi N, Low MJ. Somatostatin is essential for the sexual dimorphism of GH secretion, corticosteroid-binding globulin production, and corticosterone levels in mice. Endocrinology 2015;156(3):1052-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Low MJ, Otero-Corchon V, Parlow AF, et al. Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest. 2001;107(12):1571-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Romero CJ, Ng Y, Luque RM, et al. Targeted deletion of somatotroph insulin-like growth factor-I signaling in a cell-specific knockout mouse model. Mol Endocrinol. 2010;24(5):1077-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gahete MD, Cordoba-Chacon J, Anadumaka CV, et al. Elevated GH/IGF-I, due to somatotrope-specific loss of both IGF-I and insulin receptors, alters glucose homeostasis and insulin sensitivity in a diet-dependent manner. Endocrinology 2011;152(12):4825-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eriksson E, Modigh K, Jansson JO. Effects of sex steroids on growth hormone responses to clonidine and GHRH in reserpine pretreated rats. J Neural Transm. 1988;71(2):99-113. [DOI] [PubMed] [Google Scholar]
- 50. Norman C, Rollene NL, Erickson D, Miles JM, Bowers CY, Veldhuis JD. Estradiol regulates GH-releasing peptide’s interactions with GH-releasing hormone and somatostatin in postmenopausal women. Eur J Endocrinol. 2014;170(1):121-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shah N, Evans WS, Veldhuis JD. Actions of estrogen on pulsatile, nyctohemeral, and entropic modes of growth hormone secretion. Am J Physiol. 1999;276(5):R1351-R1358. [DOI] [PubMed] [Google Scholar]
- 52. Veldhuis JD. Neuroendocrine control of pulsatile growth hormone release in the human: relationship with gender. Growth Horm IGF Res. 1998;8(Suppl B):49-59. [DOI] [PubMed] [Google Scholar]
- 53. Veldhuis JD, Anderson SM, Kok P, et al. Estradiol supplementation modulates growth hormone (GH) secretory-burst waveform and recombinant human insulin-like growth factor-I-enforced suppression of endogenously driven GH release in postmenopausal women. J Clin Endocrinol Metab. 2004;89(3):1312-1318. [DOI] [PubMed] [Google Scholar]
- 54. Yonezawa T, Mogi K, Li JY, Sako R, Yamanouchi K, Nishihara M. Modulation of growth hormone pulsatility by sex steroids in female goats. Endocrinology 2005;146(6):2736-2743. [DOI] [PubMed] [Google Scholar]
- 55. Zeyda T, Diehl N, Paylor R, Brennan MB, Hochgeschwender U. Impairment in motor learning of somatostatin null mutant mice. Brain Res. 2001;906(1-2):107-114. [DOI] [PubMed] [Google Scholar]
- 56. Luque RM, Kineman RD. Gender-dependent role of endogenous somatostatin in regulating growth hormone-axis function in mice. Endocrinology 2007;148(12):5998-6006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.