Abstract
STAT5 is an essential transcriptional regulator of the sex-biased actions of GH in the liver. Delivery of constitutively active STAT5 (STAT5CA) to male mouse liver using an engineered adeno-associated virus with high tropism for the liver is shown to induce widespread feminization of the liver, with extensive induction of female-biased genes and repression of male-biased genes, largely mimicking results obtained when male mice are given GH as a continuous infusion. Many of the STAT5CA-responding genes were associated with nearby (< 50 kb) sites of STAT5 binding to liver chromatin, supporting the proposed direct role of persistently active STAT5 in continuous GH-induced liver feminization. The feminizing effects of STAT5CA were dose-dependent; moreover, at higher levels, STAT5CA overexpression resulted in some histopathology, including hepatocyte hyperplasia, and increased karyomegaly and multinuclear hepatocytes. These findings establish that the persistent activation of STAT5 by GH that characterizes female liver is by itself sufficient to account for the sex-dependent expression of a majority of hepatic sex-biased genes. Moreover, histological changes seen when STAT5CA is overexpressed highlight the importance of carefully evaluating such effects before considering STAT5 derivatives for therapeutic use in treating liver disease.
Keywords: liver sex differences, STAT5 knockout, pituitary GH secretion profiles, JAK-STAT
Sex differences in pituitary GH secretory patterns control the sex-biased expression of hundreds of genes in mouse liver (1) and contribute to the sexual dimorphism of liver physiology and pathology (2, 3). In males, GH is secreted as a series of intermittent pulses followed by extended periods when circulating GH is undetectable, whereas in females, GH secretion is near continuous (1, 4). Sex differences in liver gene expression are largely abolished when pituitary GH secretion is ablated by hypophysectomy and can be substantially restored when exogenous GH is administered, either as a series of pulses (male circulating GH pattern), which confers a male pattern of expression, or as a continuous infusion (female GH pattern), which results in a female pattern of expression (5, 6).
GH binding to its cell surface receptor activates the receptor-bound tyrosine kinase JAK2, which in turn phosphorylates and thereby activates the latent cytoplasmic transcription factor STAT5 (7, 8). GH receptor/JAK2-activated STAT5 dimerizes and translocates to the nucleus, where it binds to thousands of sites in liver chromatin and induces transcription of many target genes (9, 10). STAT5b, the major liver STAT5 form, plays a crucial role in liver metabolism and is an essential transcriptional regulator of the sex-dependent actions of GH in the liver (11-13). In male liver, repeated pulses of hepatic STAT5 activity, nuclear localization, and transcriptional activity are observed and closely track the successive pulses of male pituitary gland GH release (14-16), whereas in female liver, STAT5 is persistently activated by the near-continuous female plasma GH profile (9, 17). In whole-body STAT5b knockout male mice, 90% of hepatic male-biased genes are repressed and 60% of hepatic female-biased genes are de-repressed (ie, induced) (12). Further, STAT5 binds in a sex-biased manner to several thousand sites in mouse liver chromatin, with the set of male-biased STAT5-binding sites enriched nearby genes showing male-biased expression and the set of female-biased binding sites enriched nearby female-biased genes (9, 18). Recent studies in a hepatocyte-specific STAT5a/STAT5b-KO mouse model (19) confirm these findings (20) and support the proposal that the loss of hepatic STAT5, rather than an indirect feedback response to the loss of STAT5 in the hypothalamus or the pituitary gland, is responsible for the loss of liver sexual dimorphism seen in global STAT5b-KO mice.
Continuous infusion of GH in male mice (cGH treatment) substantially feminizes liver gene expression (21, 22), most likely by a combination of 2 mechanisms. First, cGH overrides the endogenous male pulsatile plasma GH profile. This leads to widespread repression of male-biased genes that are positively regulated by male plasma GH pulses (class I male-biased genes) and to de-repression of female-biased genes that are negatively regulated by male GH pulses (class II female-biased genes) (23). Second, cGH treatment imposes a persistent, female-like pattern of plasma GH stimulation on male liver, which leads to the induction of female-biased genes that are positively regulated by the endogenous female GH pattern (class I female-biased genes) and represses male-biased genes that are negatively regulated by the female GH pattern (class II male-biased genes) (16, 23). GH-activated STAT5 is a strong transcriptional activator and can rapidly induce its well-established direct target genes, such as Igf1 and Socs2 (10, 24). Similarly, STAT5 directly activates at least some class I male-biased genes when it is activated by a plasma GH pulse in male mouse liver (16). However, it is uncertain whether STAT5 directly regulates other classes of sex-biased genes, most notably class I female-biased genes, which require a female plasma GH pattern for expression, and whose female-biased expression could be mediated by other GH-stimulated but STAT5-independent signaling pathways downstream of GH receptor and JAK2 (25-27). The requirement of STAT5 for sex-biased hepatic expression of both class I and class II sex-biased genes (22) does not entirely resolve this question, given the apparent perturbation of feedback mechanisms leading to changes in circulating GH patterns seen in STAT5 knockout mice and in GH receptor-deficient mouse models (19, 28).
Here, we address these questions using AAV8-STAT5CA, a novel adeno-associated virus (AAV) vector that we engineered to deliver STAT5b with a point mutation in its SH2 domain (STAT5b Asn-642 to His) (29). This mutation renders the STAT5 protein constitutively active (“CA”) and capable of supporting robust expression of hepatic Igf1 in the absence of GH (30). AAV8-STAT5CA is based on AAV serotype 8, which has high specificity for infection of hepatocytes (31, 32). Moreover, the STAT5b mutant is expressed from the hepatocyte-specific human thyroxin-binding globulin (TBG; gene name SERPINA7) promoter, which confers additional specificity for persistent expression of the constitutively active STAT5 in hepatocytes. We hypothesized that the persistent expression of active STAT5 protein in male mouse liver will mimic the persistent activation of liver STAT5 that occurs in female liver and will thereby feminize gene expression. Our findings reveal that the delivery of STAT5CA to male mouse liver leads to widespread induction of female-biased genes and repression of male-biased genes. Thus, feminization of the liver by persistent plasma GH stimulation can largely be attributed to the persistent activation of liver STAT5b.
Materials and Methods
Preparation of AAV8-STAT5CA
Wild-type mouse STAT5b expression plasmid with an N-terminal Flag tag was obtained from SinoBiological, Wayne, PA (cat. #MG51116-NF). A point mutation at base 1963 (A to C), which changes Asn642 to His, was introduced into the STAT5b coding sequence by Genscript (Piscataway, NJ). This mutation confers constitutive activity to STAT5b, including the ability to induce Igf1 gene expression in livers of hypophysectomized (GH-deficient) rats (29, 30). The mutated plasmid was sent to Penn Vector Core (University of Pennsylvania, Philadelphia, PA), where the N-Flag-mSTAT5bCA cDNA was cloned into the Penn Vector Core plasmid pENN.AAV.TBG.PI.eGFP.WPRE.bGH, replacing enhance green fluorescent protein by the mutated and tagged STAT5bCA. The resultant plasmid, pENN.AAV.TBG.PI.N-FLAG-mSTAT5bCA.WPRE.bGH, was sequence verified and then packaged in AAV8 virus, which was purified, quantified with respect to genome copy (GC) number, and supplied by Penn Vector as a ready-to-use vector, AAV8-TBGp-mStat5bCA, referred to here as AAV8-STAT5CA. The plasmid pENN.AAV.TBG.PI.N-FLAG-mSTAT5bCA.WPRE.bGH, required to prepare AAV8-STAT5CA, has been deposited and is available through Addgene, plasmid ID 184463 (https://www.addgene.org/).
AAV8-STAT5CA Expression in Mouse Liver
Initial studies to validate AAV8-STAT5CA were performed in male C57Bl/6 mice on a GHRfl/fl background at Jesse Brown VA Medical Center (Chicago, IL) with Institutional Animal Care and Use Committee approval (protocol number 19-05). AAV8-TBGp-Null vector (Penn Vector Core), referred to here as AAV8-Null, was used as a control. Male GHRfl/fl mice (10-12 weeks, and maintained as an in-house breeding colony, originally provided by Dr. John Kopchick, Ohio University (33)) were injected via the lateral tail vein with AAV8-Null or AAV8-STAT5CA (1.5 × 1011 GC per mouse, diluted in sterile PBS). Seven days later, the mice were decapitated and their livers were collected. Nuclear protein was extracted using NE-PER Nuclear Cytoplasmic Extraction Reagent (cat. #78833, Pierce, Rockford, IL). Western blots were performed to detect the Flag-tag sequence using rabbit monoclonal anti-DYKDDDDK Tag antibody (Cell Signaling #14793). To test the efficacy of AAV8-STAT5CA to increase hepatic expression and restore circulating levels of IGF1 to normal levels, we used a mouse model with adult-onset hepatocyte-specific GH receptor knockdown (aHepGHRkd) (34), prepared as follows. Male GHRfl/fl mice (10-12 wk old) were injected via lateral tail vein with 1.5 × 1011 GC/mouse of either AAV8-Null, to obtain GH receptor-intact control mice, or AAV8 expressing Cre recombinase (AAV8-TBGp-Cre, Penn Vector Core), to generate aHepGHRkd mice. The AAV8 injections were done either alone or in combination with AAV8-STAT5CA (at 0.376 or 0.75 × 1011 GC), with the dose of AAV8-Null adjusted to equalize the total GC of AAV8 per mouse across groups. Fourteen days later, blood and liver were collected to assay circulating IGF1 levels (mouse/rat IGF-1 ELISA, 22-IG1MS-E01, ALPCO), and liver IGF1 mRNA levels were assayed by quantitative PCR (qPCR) (34). In other studies, GH receptor-intact male mice (C57Bl/6 strain from Jackson Labs, 10 weeks old) were treated with AAV8-Null or AAV8-STAT5CA (0.75 × 1011 GC per mouse, IV) and 14 days later livers were collected and used for RNA-sequencing (RNA-seq) analysis.
AAV8-STAT5CA Dose-response Studies
Studies to test the ability of AAV-STAT5CA to feminize hepatic gene expression were performed, in compliance with procedures approved by the Boston University Institutional Animal Care and Use Committee (protocol #PROTO201800698), and in compliance with ARRIVE 2.0 Essential 10 guidelines (35), including study design, sample size, randomization, experimental animals and procedures, and statistical methods. Male and female CD1 mice (Crl:CD(ICR), strain code 022), 7 to 8 weeks of age, were purchased from Charles River Laboratories. Mice were housed in a temperature- and humidity-controlled environment with a 12-hour light, 12-hour dark cycle, fed standard rodent chow, and supplied with tap water. AAV8-STAT5CA or AAV8-Luciferase (control) (both produced in-house using methods described below) were administered to male mice by tail vein injection at doses ranging from 0.125 × 1011 to 2 × 1011 GC per mouse. Virus was diluted into PBS + 35 mM NaCl + 5% glycerol (stabilizer) to give a total injection volume of 150 µL. Mice were euthanized 2 to 6 weeks later, as specified in individual figures, by cervical dislocation between 10:30 and 11:30 AM (lights on 7:30 AM-7:30 PM) to minimize the impact of circadian variations of gene expression (36, 37).
AAV8 Production
AAV coding STAT5bCA and Luciferase (control) was prepared and quantified by triple transfection of HEK293FT cells using methods adapted from protocols provided by Addgene (www.addgene.org). HEK293FT is a fast-growing and highly transfectable derivative (cat. #R70007, ThermoFisher Scientific) of the SV40 large T-antigen-expressing HEK293T cell line commonly used for AAV production. On day 0, 80% confluent flasks of HEK293FT cells were split 1:4 into T182.5 flasks containing DMEM culture medium (10% fetal bovine serum [cat. #10437-028, Gibco] and 1% PenStrep [cat. #15140-122, Gibco]) and then grown 1-2 days to achieve ~85% confluence at the time of transfection, which is defined as day 1. T182.5 flasks were each transfected with a total of 80 µg of plasmid DNA in a 1:1:1 (molar ratio) of 3 plasmids: pAAV-Helper helper plasmid, which encodes adenovirus genes E2A and E4 (Cell Biolabs, VA); pAAV2/8 Rep/Cap plasmid (Addgene, plasmid #112864; replication protein derived from AAV-2 serotype, and Capsid protein from AAV-8); and either pENN.AAV.TBG.PI.N-FLAG-tag-mSTAT5bCA.WPRE.bGH or pENN.AAV.TBG.PI.ffLuciferase.RBG (firefly luciferase expressed from TBG promoter; Addgene #105538). Plasmids were mixed in a 3:1 weight ratio of total plasmid DNA:PEI in 4 mL of either OptiMeM (Gibco cat. #31985-062) or serum-free DMEM per flask and then shaken vigorously for 30 seconds. The mixture was incubated for 15 minutes at room temperature in a tissue culture hood, after which 4 mL of the mixture was added to each flask of ~85% confluent HEK293FT cells. After 24 hours, the culture medium was replaced by DMEM containing 2% fetal bovine serum and 1% PenStrep, and the cells were returned to the CO2 incubator for 5 days.
Virus was harvested on day 6 after transfection. A cell scraper was used to detach the cells from the flask, followed by collection of the media with cells in sterile 50-mL conical tubes. The tubes were centrifuged at 1000g for 10 minutes at 4°C, and the supernatant was decanted into a sterile bottle. Pellets were pooled and transferred to smaller tubes with 1× PBS + 200 mM NaCl + 0.001% Pluronic F68 (Poloxamer 188 Solution, cat. #P5556-100ML, Sigma) and pelleted again. Cell pellets were frozen at -80 °C for future use in purifying virus. The supernatant (which contains the majority of virus) was filtered through a 0.2-µM PES filter (VWR, cat. #97066-214). Twenty-five milliliters of 40% PEG8000 (JT Baker, cat. #JTU222-09) was then added for every 100 mL of media supernatant, followed by overnight stirring at 4°C. The next day, the supernatant was incubated for 1 hour at 4 °C without stirring, to allow for full precipitation of virus before spinning at 2818g for 15 minutes at 4 °C. The supernatant was discarded and the PEG pellet, containing concentrated virus, was resuspended in 10 mL of 1× PBS + 0.001% Pluronic F68 + 200 mM NaCl by pipetting back and forth until the pellet was completely in solution.
The following buffers were prepared for iodixanol purification of the PEG-pelleted virus: (1) 1 M NaCl/PBS-MK buffer (5.84 g NaCl, 26.3 mg MgCl2, and 14.91 mg KCl dissolved in 1× PBS to give a final volume of 100 mL), sterile filtered through a 0.2-μm PES filter; and (2) 1× PBS-MK Buffer (26.3 mg MgCl2 and 14.91 mg KCl dissolved in 1× PBS to a final volume of 100 mL) and then filter-sterilized by passage through a 0.2-μm filter. Iodixanol gradients were prepared as follows: 15% Layer (4.5 mL of 60% iodixanol [OptiPrep Density Gradient Medium, cat. #D1556-250ML, Sigma] + 13.5 mL of 1M NaCl/PBS-MK buffer); 25% Layer (5 mL of 60% iodixanol, 7 mL of 1× PBS-MK buffer + 30 µL of Phenol Red [cat. #P0290-100ML, Sigma]); 40% Layer (6.7 mL of iodixanol + 3.3 mL of 1× PBS-MK Buffer; 60% layer [10 mL of 60% iodixanol + 45 µL phenol red]). A 10-mL syringe with an 18-g needle and a glass Pasteur pipet were used to layer the iodixanol gradient in OptiSeal tubes (cat. #362183, Beckman Coulter), from the bottom of the tube, starting with 8 mL of the 15% layer, then 6 mL of the 25% layer, 5 mL of the 40% layer, and 5 mL of the 60% layer (2 tubes per AAV preparation from 10-12 T182.5 flasks). Resuspended virus-PEG pellet solution (5 mL) was added to the top of each gradient and 1× PBS was used to top off each tube before heat sealing. The tubes were spun for 120 minutes at 200,000g in a VTi50 Rotor at 18 °C. The tubes were removed carefully (without disturbing the gradient) and secured to a clamp stand using a clamp. Twenty 1.5-mL Eppendorf tubes were opened and placed in a tube rack beneath the gradient in a biosafety cabinet. The OptiSeal tube was punctured at the 40% to 60% iodixanol interface with an 18-g needle, using the bevel pointing up, and then the top of the tube was punctured with a 16-g needle. Fractions of 1 mL were collected until the 25% to 40% interface was reached. A total of 5 µL was removed from each fraction for analysis by qPCR to locate AAV-rich fractions before storing the remainder of each fraction at 4 °C overnight. Each 5-µL fraction sample was treated with 45 µL DNase I (cat #M6101, Promega) for 1 hour at 37 °C and then diluted 1:20 in 50 µg/mL yeast tRNA (cat #AM7119, Invitrogen). qPCR was performed using primers specific for the TBG promoter sequence: ACCCAGCCTCTGCTTTGTA and CTTCAGCAGGCAGAATAGG. A known concentration of plasmid DNA used to prepare the virus was diluted 1:200 in 50 µg/mL yeast tRNA and processed in parallel. AAV-containing fractions were pooled, typically giving a volume of 4 to 10 mL.
To concentrate the pooled fractions via buffer exchange, 12 mL of 0.1% Pluronic F68 in 1× PBS was added to a new Amicon Ultra-15 100K Centrifugal Filter Unit (cat. #UFC910024, EMD Millipore) and allowed to incubate at room temperature for 10 minutes. The Pluronic F68 in PBS was removed from the filtration unit and 12 mL of 0.01% Pluronic F68 in 1× PBS was added to the filtration unit before spinning at 3000 g for 5 minutes at 4 °C. The flow-through was discarded and 12 mL of 0.001% Pluronic F68 in 1× PBS + 200 mM NaCl was added and spun at 3000 g for 5 minutes at 4 °C. The flow-through was discarded and up to 12 mL of sample was added to the column and spun at 3500 g for 8 minutes at 4°C. The flow-through was discarded and more sample was added up to 12 mL, mixed well with the concentrated remaining sample and spun again. This continued until the sample was concentrated to a total final volume of 0.5 to 1 mL. The sample was washed with 10 mL of 0.001% Pluronic F68 in 1× PBS + 200 mM NaCl, using a pipette to mix thoroughly, and then spun again. The sample was concentrated to about 0.5 mL, collected using a pipette, and the walls washed with a small volume of 0.001% Pluronic F68 in 1× PBS + 200 mM NaCl to ensure all virus was collected. The concentrated virus was stored at 4 °C for up to 1 to 2 weeks, or at -80 °C for long-term storage.
Using 5 µL of the concentrated virus, purified AAV was treated with DNase I for 30 to 60 minutes at 37 °C to eliminate any contaminating plasmid DNA remaining from the viral purification process. The DNase-treated viral samples were serially diluted 1:20 a total of 5 times in dilution buffer (50 µg/mL Yeast tRNA, 0.05% Pluronic F68) and the reference plasmid (used to prepare the virus) was serially diluted 1:10 a total of 5 times in dilution buffer. qPCR was performed as indicated previously, using each plasmid dilution series to construct a calibration curve used to determine the titer of the virus.
Liver RNA Extraction and qPCR Analysis
Total liver RNA was extracted from frozen mouse liver with TRIzol reagent (Invitrogen Life Technologies Inc., Carlsbad, CA), and nuclear RNA was purified from nuclei isolated from frozen liver tissue and then extracted with TRIzol LS as described elsewhere (38). RNA (1 µg) was treated with DNase I (cat. #M6101, Promega) to remove DNA contamination. cDNA was then synthesized using High-Capacity cDNA Reverse Transcription Kit (cat. #4368814, Applied Biosystems). qPCR was performed using primers specific to each RNA, designed using Primer Express or Primer3 software (http://bioinfo.ut.ee/primer3-0.4.0/), as follows: Sult3a1: 5′-CATTGTCACATATCCAAAGTCTGGT-3′ (forward), 5′-GAAAGGATCTGCTGGGTCCA-3′ (reverse) (oligonucleotides# 5735-5736); A1bg: 5′-GAACCCTCTGAGCCCAGTGA-3′ (forward), 5′-GAGTGGGTGGAGCCTGTGAG-3′ (reverse) (oligonucleotides# 5723-5724); Cux2: 5′-CCTCAAGACGAACACCGTCAT-3′ (forward), 5′-GCGCATCCTGGACCTGTAGT-3′ (reverse) (oligonucleotides# 1382-1383). Quantitative real-time PCR was carried out on a CFX384 Touch Real-Time PCR Detection System (Bio-Rad) using Power SYBR Green PCR Master Mix (ThermoFisher). Normalized linear Ct numbers based on 18S RNA as control were computed to determine the relative expression level of each gene across treatments.
RNA-seq Analysis
Polyadenylated mRNA was isolated from 1 μg of total liver RNA or from 1 μg of liver nuclear RNA from each treatment group (see the following section) using NEBNext Poly(A) mRNA Magnetic Isolation Module (cat. #E7490, New England Biolabs). The resulting polyA-selected RNA was used to prepare RNA-seq libraries using the NEBNext Ultra Directional RNA Library Prep Kit for Illumina (cat. #E7420L, New England Biolabs), NEBNext Multiplex Oligos for Illumina Dual Index Primers (cat. #E7600, New England Biolabs), and AMPure XP Beads (cat. #A63881, Beckman Coulter Inc., Indianapolis, IN). RNA-seq libraries were prepared from 2 independent studies: (1) sample series #G169: livers from 10- to 12-week-old male C57Bl/6 mice treated with 0.75 × 1011 GC of AAV8-STAT5CA or AAV8-Null control then excised 2 weeks later, with 2 sequencing libraries prepared for each treatment group, each derived from polyA-selected total liver RNA pooled from n = 2 or 3 individual livers (biological replicates); and (2) sample series #G186: livers from 7-to 8-week-old male CD1 mice treated with 2 × 1011 GC of AAV8-STAT5CA or control then excised 4 to 6 weeks later, with a total of 9 sequencing libraries prepared, each 1 from an individual liver (n = 5 AAV8-STAT5CA libraries, n = 4 control libraries) and derived from polyA-selected nuclear RNA prepared from nuclei extracted from frozen liver tissue, to increase the sensitivity for detection of the large number of lncRNAs that are nuclear-enriched and tightly bound to chromatin in mouse liver (38). Libraries were multiplexed then sequenced by Novogene, Inc (Sacramento, CA) to an average depth of 18 to 25 million 150 nt paired-end sequence reads each on an Illumina HiSeq instrument. RNA-seq data were analyzed using a modified version of the custom pipeline described earlier (16). Briefly, sequence reads were aligned to mouse genome build mm9 (NCBI 37) using STAR aligner (39) and FeatureCounts (40) was used to count sequence reads mapping to the union of the exonic regions in all isoforms of a given gene (collapsed exon counting) based on an mm9 Gene Transfer Format file comprised of 75 798 mouse genes: n = 20 884 RefSeq protein coding genes, n = 48 360 mouse liver-expressed lncRNA genes (a much more complete listing than the set described previously (41)), n = 2061 RefSeq noncoding genes (NR accession numbers) that do not overlap the set of 48 360 lncRNAs, n = 4490 Ensembl noncoding lncRNAs that do not overlap either the RefSeq NR gene set or the 48 360 lncRNA gene set, and n = 3 other lncRNAs with interesting liver functions (lnc-LFAR1, LeXis, Lnclgr). Gene annotations are provided in Table S1 (42). Raw sequencing files and processed data files are available at GEO (https://www.ncbi.nlm.nih.gov/geo/) under accession numbers GSE196014 and GSE196015.
Differential Expression Analysis
EdgeR (43) was used to determine significant differential expression of RNA-seq data for the following comparisons: males + AAV8-STAT5CA at 2 × 1011 GC versus control males, and males + AAV8-STAT5CA at 0.75 × 1011 GC vs AAV8-null males, with full datasets shown in Tables S1-S3 (42). Further, a set of 475 sex-biased liver-expressed genes was defined as genes with differential expression significant at false discovery rate (FDR) < 0.01 in male liver as compared with female liver (Table S2) (42). A set of 8246 liver-expressed genes whose differential expression is stringently sex-independent was defined based on |fold-change| for sex difference < 1.2 and FDR > 0.1 (Table S3) (42). All analyses reported here for sex-independent genes used this list of 8246 genes. Finally, for female-biased genes, a percent feminization value was calculated based on each gene’s response to the following treatments: AAV8-STAT5CA 2 × 1011 GC, AAV8-STAT5CA 0.75 × 1011 GC, and cGH for 7 or 14 days (21), as follows: % feminization = 100% (FPKM [treated male] – FPKM [control male])/(FPKM [control female] – FPKM [control male]) (Table S2) (42).
Raw RNA-seq data obtained from livers of hypophysectomized mice (16) (GEO accession number GSE66003) were reanalyzed to obtain expression data for the full set of 75 798 mouse genes, including 48 360 liver-expressed lncRNAs, described previously. Two distinct classes of sex-biased genes were identified based on differential expression between livers of control and hypophysectomized male and female mice at FDR < 0.05 (23): class I male-biased genes are genes that show significantly decreased expression in male liver after hypophysectomy because of their dependence on the male plasma GH pulsatile pattern for expression; and class II male-biased genes are genes that are repressed by the female pituitary profile and, consequently, they are significantly induced (de-repressed) in female liver following hypophysectomy. We also identified corresponding sets of class I female-biased genes as those whose expression in female liver decreases significantly after hypophysectomy because of a requirement for the female, near-continuous GH pattern for expression, and class II female-biased genes whose expression is repressed by the male pituitary profile and consequently show significant de-repression in hypophysectomized male liver (see Fig. 1 of (44)). The enrichment of STAT5CA-responsive genes in each set of hypophysectomy-responsive sex-biased genes was calculated compared with that of a background set of the corresponding class of STAT5CA-unresponsive sex-biased genes. Statistical significance was evaluated using Fisher exact test.
Figure 1.
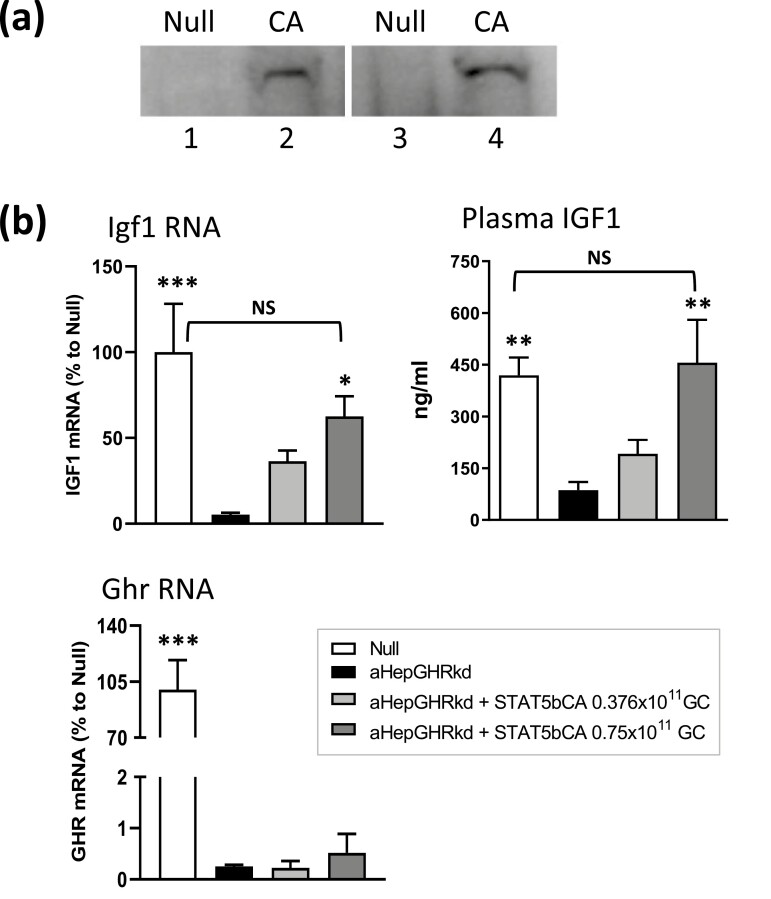
Functional validation of AAV8-STAT5CA in vivo. (A) Nuclear localization of Flag-Tag STAT5CA protein, detected by anti-Flag antibody on a Western blot of nuclear extracts from livers excised from mice 7 days after injection of AAV8-Null (control, lanes 1, 3) or AAV8-STAT5CA (1.5 × 1011 GC, lanes 2, 4). Extracts were prepared from four individual livers. (B) Plasma IGF1 levels and liver Igf1 and GH receptor (Ghr) mRNA levels in male mice, GH receptor floxed control (Null) male mice, or male mice with adult-onset GH receptor knockdown (aHepGHRkd mice), with or without AAV8-STAT5CA treatment at the doses indicated. Values shown are mean ± standard error of the mean for n = 8 mice per group. Significance was evaluated by 1-way ANOVA with Bonferroni multiple comparison correction, implemented in GraphPad Prism. For comparisons to aHepGHRkd (second bar in each set): ***P < 0.001, **P < 0.01, and *P = 0.0595. NS, P > 0.05 for comparisons to Null, indicating restoration of Igf1 RNA and plasma protein.
Enrichment of STAT5 Chromatin Immunoprecipitation-seq Binding Sites at STAT5CA-responsive Genes
Male-enriched, female-enriched, and sex-independent STAT5 binding sites (1765, 1790, and 11 531 sites, respectively) were obtained from our published chromatin immunoprecipitation-(ChIP)-seq data for STAT5 binding in male and female mouse liver (9) (GEO accession number GSE31578). Each STAT5-binding site was mapped to the closest gene using the command bedtools-closest (45). All genes with a STAT5-binding site within 50 kb of the gene body were considered to be a STAT5 target. The enrichment of STAT5CA-responsive genes in each set of STAT5 binding site target genes was calculated by comparison to a background set of STAT5CA-unresponsive genes. Statistical significance was evaluated using Fisher exact test.
Histology
Fresh liver tissue was submerged into 4% paraformaldehyde and snap frozen. A portion of each liver was then placed in PBS with 0.05% NaN3 for frozen tissue sectioning, and the remainder was transferred to 70% ethanol for paraffin embedding and hematoxylin and eosin staining.
Results
Constitutively Active STAT5b Feminizes Liver Gene Expression
GH-activated JAK2 catalyzes tyrosine phosphorylation linked to the activation and nuclear translocation of STAT5, which enables transcriptional activation of STAT5 target genes. GH-activated liver STAT5 is known to be essential for sex-biased liver gene expression; however, GH also activates other, STAT5-independent signaling pathways (25-27), which could contribute to the regulation of sex-biased gene expression. To determine whether the control of sex-biased liver gene expression by plasma GH pulses (male GH pattern) vs persistent GH stimulation (female GH pattern) is due to the downstream effects of pulsatile vs persistent STAT5 activation per se, we expressed a constitutively active form of STAT5 (STAT5CA) in male mouse liver to mimic the pattern of persistent STAT5 activation that occurs in female mouse liver (9). STAT5CA cDNA expressed from the hepatocyte-specific thyroxin-binding globulin promoter (46, 47) was delivered using an AAV serotype 8 vector, which has high intrinsic tropism for the liver (31, 32). We validated the AAV8-STAT5CA viral vector by its ability to target STAT5 protein to the nucleus (Fig. 1A), which is a key feature of activated STAT5, and by its functional ability to increase hepatic Igf1 RNA levels and circulating IGF1 in mice deficient in GH receptor signaling in hepatocytes (Fig. 1B, aHepGHRkd mouse liver).
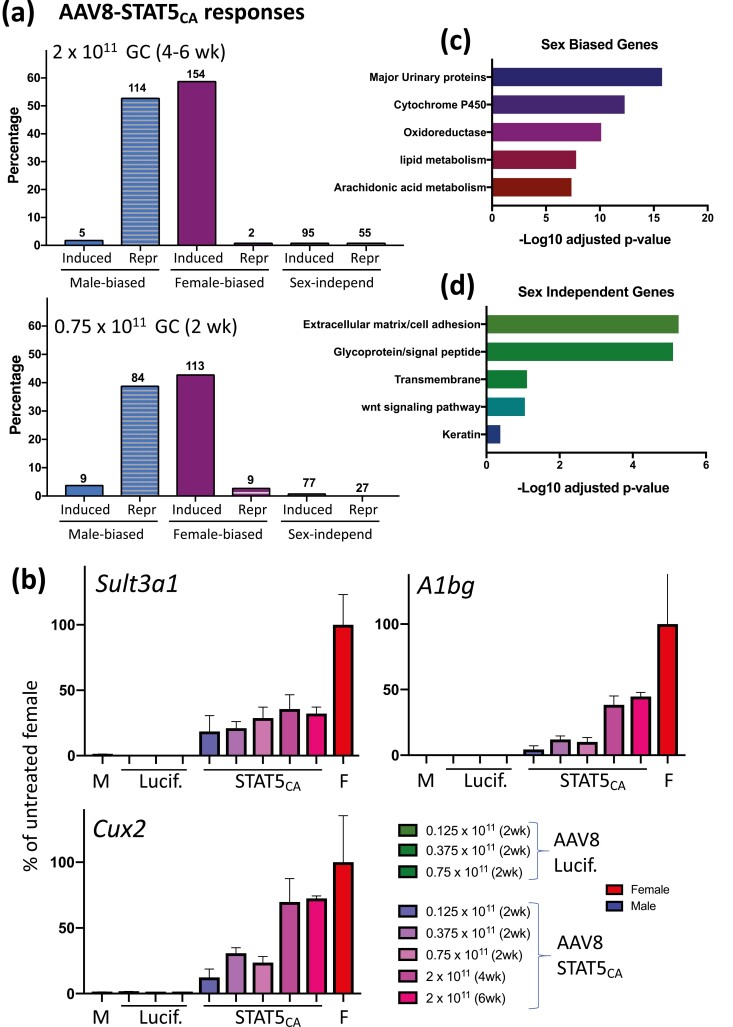
Next, we investigated the impact of STAT5CA on sex-biased gene expression in the liver. We delivered AAV8-STAT5CA (2 × 1011 GC/mouse) or a control virus (AAV8-Luciferase) to young adult male mice and excised the livers 4 to 6 weeks later. Liver gene expression was analyzed by nuclear RNA-seq, which enabled us to determine the effects of STAT5CA on the nuclear transcriptome, including lncRNA genes, which are highly enriched in the nucleus owing to their tight binding to chromatin (38) (Table S1) (42). AAV8-STAT5CA substantially feminized the expression of liver sex-biased genes. Thus, 154 (59%) of 260 female-biased genes were induced and only 2 female-biased genes were repressed by AAV8-STAT5CA in male liver. Furthermore, 114 (53%) of 215 male-biased genes were repressed and only 5 male-biased genes were induced by AAV8-STAT5CA (Fig. 2A, Table S2) (42). For many male-biased genes, > 90% repression was achieved, suggesting AAV8-STAT5CA functionally infects a high fraction of the hepatocytes expressing these genes. In contrast to the widespread responses of sex-biased genes, only 1.1% of stringent sex-independent genes (Table S3) (42) were induced and 0.7% were repressed by AAV8-STAT5CA. These trends, presented for the CD1 mouse liver model used extensively in our studies on liver sex differences (16, 20, 21, 48), were confirmed in an independent study in C57BL6 mice, which were treated with AAV8-STAT5CA at a lower dose and for a shorter duration (0.75 × 1011 GC for 2 weeks) (Fig. 2A, bottom; Table S2) (42). Feminization of the liver transcriptome by AAV8-STAT5CA was dose-dependent, with partial feminization observed at virus doses as low as 0.125 × 1011 GC, as shown for 3 highly female-biased genes (Fig. 2B). Sex-biased genes responsive to AAV8-STAT5CA were enriched for established sex-biased metabolic pathways, whereas the set of sex-independent genes responsive to AAV8-STAT5CA was enriched for extracellular matrix/adhesion and glycoprotein/signal peptide genes (Fig. 2C and 2D).
Figure 2.
AAV8-STAT5CA responsive genes in male liver. (A) Percentage of all genes that are either consistently sex biased at false discovery rate (FDR) < 0.05 (Table S2, n = 475 genes) (42) or stringently sex-independent genes (Table S3, n = 8246 genes) (42) and whose expression in AAV8-STAT5CA-treated liver (as indicated) is significantly induced or repressed compared with control liver (FDR < 0.05). Top: Results for CD-1 male mice; bottom panel: results for C57BL/6 mice. GC, genome copies of AAV8 virus. Number of responding genes is shown above each bar. (B) Dose responses for AAV8-STAT5CA induction of 3 highly female-biased genes. Data based on reverse transcriptase-qPCR analysis of liver RNA, mean ± standard error of the mean for n = 2-6 livers per group, expressed as percentage of gene expression in control (untreated) female (F) mouse liver. M, male group. (C, D) DAVID pathway analysis of the sets of sex-biased genes (C) and sex-independent genes (D) that are responsive to STAT5CA. Shown are the top 5 enriched clusters, with bars indicating -log10 P values (Benjamini-Hochberg corrected) for enrichment.
Role of STAT5 in Liver Feminization Induced by cGH
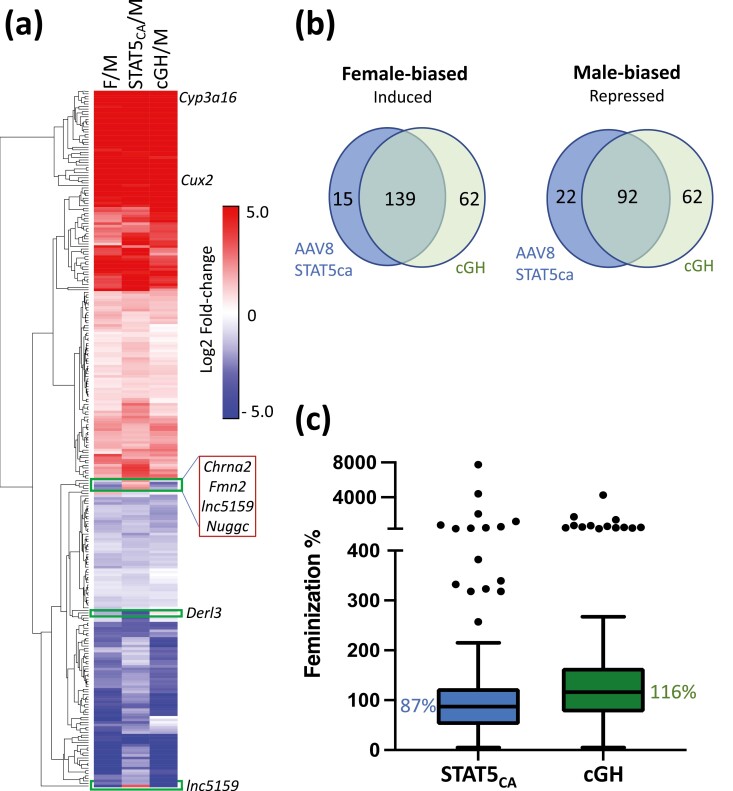
cGH treatment overrides the endogenous male, pulsatile GH secretion pattern and progressively feminizes liver gene expression over a period of days (21). To understand the role of STAT5 in this process, we compared the changes in gene expression following AAV8-STAT5CA treatment (2 × 1011 GC) to the changes seen in livers of male mice given cGH infusion for 7 or 14 days. AAV8-STAT5CA and cGH infusion induced very similar changes in the expression of sex-biased genes in male liver (Fig. 3A, Table S2) (42). Thus, 90% of the female-biased genes induced by AAV8-STAT5CA were also induced by cGH (139/154 genes) and 81% of the male-biased genes repressed by AAV8-STAT5CA were repressed by cGH (92/114 genes) (Fig. 3B). Overall, AAV8-STAT5CA and cGH were both highly effective in feminizing liver gene expression, with median feminization values of 87% and 116%, respectively (Fig. 3C, Table S2) (42). Female-biased genes that are highly induced (> 100-fold) by cGH infusion or by constitutive activation of STAT5 include the cytochrome P450 genes Cyp3a16, Cyp3a41b, Cyp2c69, and Cyp2a4, and other metabolic genes, such as Fmo3 and Hao2. Of note, 62 female-biased genes were induced in male liver by cGH infusion but not by STAT5CA and 62 male-biased genes were repressed in male liver by cGH infusion but not by STAT5CA (Fig. 3B, Table S2) (42). These genes may be regulated by GH signaling pathways independent of STAT5 (26). Finally, 4 male-biased genes that were repressed by cGH were unexpectedly induced by AAV8-STAT5CA (Chrna2, Fmn2, Nuggc, lnc5159; Fig. 3A, Table S2) (42).
Figure 3.
Effects of STAT5CA and cGH infusion on sex-biased gene expression. (A) Heatmap showing impact of STAT5CA or cGH infusion on the expression of 275 responsive sex-biased genes (log2 fold-change increases or decreases) in male mouse liver as indicated. M, male; F, female. (B) Overlap between female-biased genes induced by AAV8-STAT5CA and those induced by cGH after either 7 or 14 days (left), and between male-biased genes repressed by AAV8-STAT5CA and those repressed by cGH after either 7 or 14 days (right). Excluded are 5 male-biased genes induced by AAV8-STAT5CA and 1 female-biased genes repressed by AAV8-STAT5CA. (C) Boxplots showing the distribution of feminization values for 139 female-biased genes induced in male liver in both mouse models (see Table S2) (42). Median value, horizontal line in each box.
STAT5CA Regulates Many Direct STAT5 Gene Targets
We identified an initial list of potential STAT5 target genes, defined as genes that met the following 3 criteria: (1) gene expression shows a significant change upon loss of STAT5, as seen in either male or female STAT5-deficient mouse liver (13, 20); (2) gene expression is induced or repressed in either male or female liver following pituitary hormone ablation by hypophysectomy, which abolishes GH-dependent STAT5 activation (16); and (3) gene expression is restored when hypophysectomized mice are given an exogenous pulse of GH, which restores pulsatile STAT5 signaling in male liver (16). We filtered out genes that showed an inconsistent response to GH treatment or STAT5 deficiency to obtain a final list of 227 putative STAT5 target genes, of which 115 showed sex-biased expression (Table S4) (42). Importantly, 40% (90 of 227) of the predicted STAT5 target genes and 45% (52 of 115) of the sex-biased predicted STAT5 targets were responsive to AAV8-STAT5CA (Table S4, column AC) (42). These STAT5CA-responsive STAT5 targets include both sex-biased genes and well-established, sex-independent STAT5 gene targets, such as Igf1, Socs2, Cish, and Onecut1, whose expression was increased in AAV8-STAT5CA-treated male mouse liver.
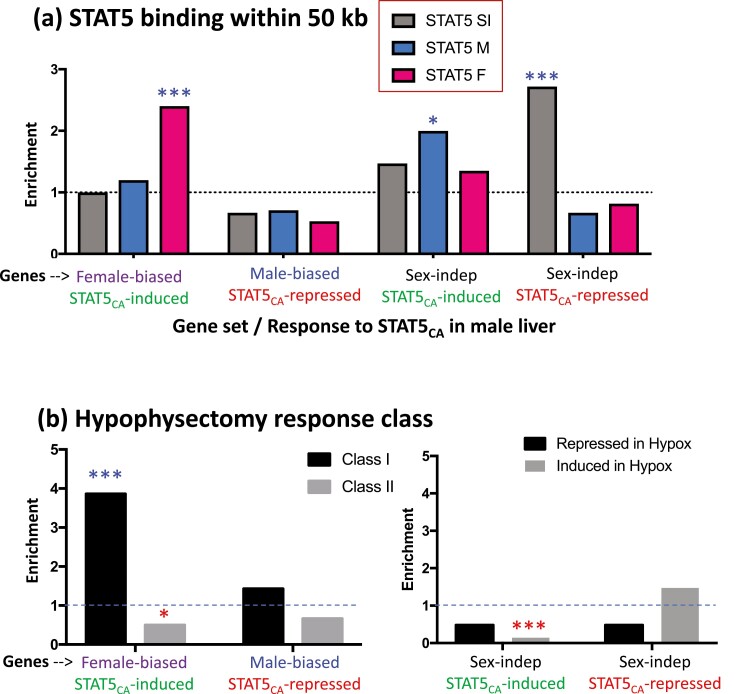
Next, we investigated whether responsiveness to AAV8-STAT5CA is associated with local STAT5 binding to liver chromatin. We mapped liver STAT5 binding sites identified by ChIP-seq (9) to their putative target genes (nearest gene within 50 kb; Table S2, Table S3) (42). These binding sites include many genomic regions where STAT5 binding to liver chromatin is significantly stronger in males than in females, and vice versa (male-enriched and female-enriched STAT5 binding sites, respectively) (9). For each gene class of interest, we determined the enrichment of STAT5 binding for the set of genes responsive to AAV8-STAT5CA compared with the set of genes that were unresponsive to AAV8-STAT5CA. We observed a strong, significant enrichment of female-biased STAT5 binding sites at the set of female-biased genes induced by STAT5CA compared with the corresponding background set of female-biased genes not induced by STAT5CA (Fig. 4A, Table S5, Fig. S1) (42). Moreover, among the set of 227 putative STAT5 target genes discussed previously, female-biased genes induced by STAT5CA showed a much higher range of sex bias, with 17 of 33 genes (52%) showing a sex bias > 3-fold, whereas for the set of female-biased genes not induced by STAT5CA, only 2 of 31 genes (6%) showed a sex bias > 3-fold. Male-biased genes repressed by STAT5CA were depleted of STAT5 binding, but not significantly (Fig. 4A). The strong enrichment of female-biased STAT5 binding at female-biased genes induced by STAT5CA indicates that it is the persistence of STAT5 binding, acting in a positive regulatory manner, that induces these female-biased genes in male liver. By contrast, we observed strong enrichment of sex-independent STAT5 binding at sex-independent genes that are repressed by STAT5CA, but not at sex-independent genes induced by STAT5CA (Fig. 4A). This indicates that, in the genomic context of these sex-independent genes, STAT5 can act as a repressor, consistent with studies in GH-deficient rat liver (49). Finally, sex-independent genes induced by STAT5CA were enriched for male-biased STAT5 binding, consistent with the positive regulatory potential of those sites evident from our earlier work (9).
Figure 4.
Enrichment of STAT5 binding sites (A) and hypophysectomy response class (B) for the sets of genes induced or repressed by STAT5CA. (A) Data are shown for STAT5 binding sites that are male-biased (M; 1765 sites), female-biased (F; 1790 sites), and sex-independent (SI; 11 531 sites), as previously defined by ChIP-seq (9), and mapped to their target genes (nearest gene within 50 kb). Enrichments are expressed as the ratio of STAT5CA-responsive genes compared with STAT5CA-unresponsive genes that are associated with each of the 3 sets of STAT5 binding sites (see box at top). (B) Enrichment of class I or class II sex-biased genes (left) and sex-independent genes (right) in the STAT5CA-responsive gene sets, expressed as the ratio of the number of STAT5CA-responsive genes compared to the number of STAT5CA-unresponsive genes in each class (left) or that are induced or repressed by hypophysectomy (right). Significance was determined by Fisher exact test: *P < 0.05, ***P < 0.001. Asterisks above the dashed line indicate significant enrichment; red asterisks below the dashed line indicate significant depletion (values < 1).
STAT5CA Preferentially Induces Class I Female-biased Genes
In principle, STAT5CA may act by either of 2 distinct mechanisms. By mimicking the actions of a persistent, female-like plasma GH profile, STAT5CA may induce expression of female-biased genes that are positively regulated by the female GH pattern (ie, class I female-biased genes). Alternatively, by eliminating the pulsatile nature of STAT5 signaling that is ongoing in male liver (16), STAT5CA may effectively override the inhibitory action that endogenous male plasma GH pulses can exert on class II female-biased genes and thereby de-repress their expression (23). To distinguish these 2 models, we compared the hypophysectomy response class frequency of the female-biased genes that are induced by STAT5CA to those that are not induced by STAT5CA (Table S2, Table S5) (42). We found that the set of STAT5CA-induced female-biased genes is significantly enriched in class I female-biased genes; furthermore, it is significantly depleted of class II female-biased genes (Fig. 4B, left). No significant hypophysectomy class enrichment was seen for STAT5CA-repressed male-biased genes. Sex-independent genes induced by STAT5CA showed a highly significant 7-fold depletion of genes whose expression increases when GH is ablated by hypophysectomy, whereas sex-independent genes repressed by STAT5CA showed a tendency toward enrichment for genes induced by hypophysectomy (Fig. 4B, right), both consistent with the GH mimic nature of STAT5CA.
Dose-dependent Effects of AAV8-STAT5CA on Liver Histology and Igf1 Expression
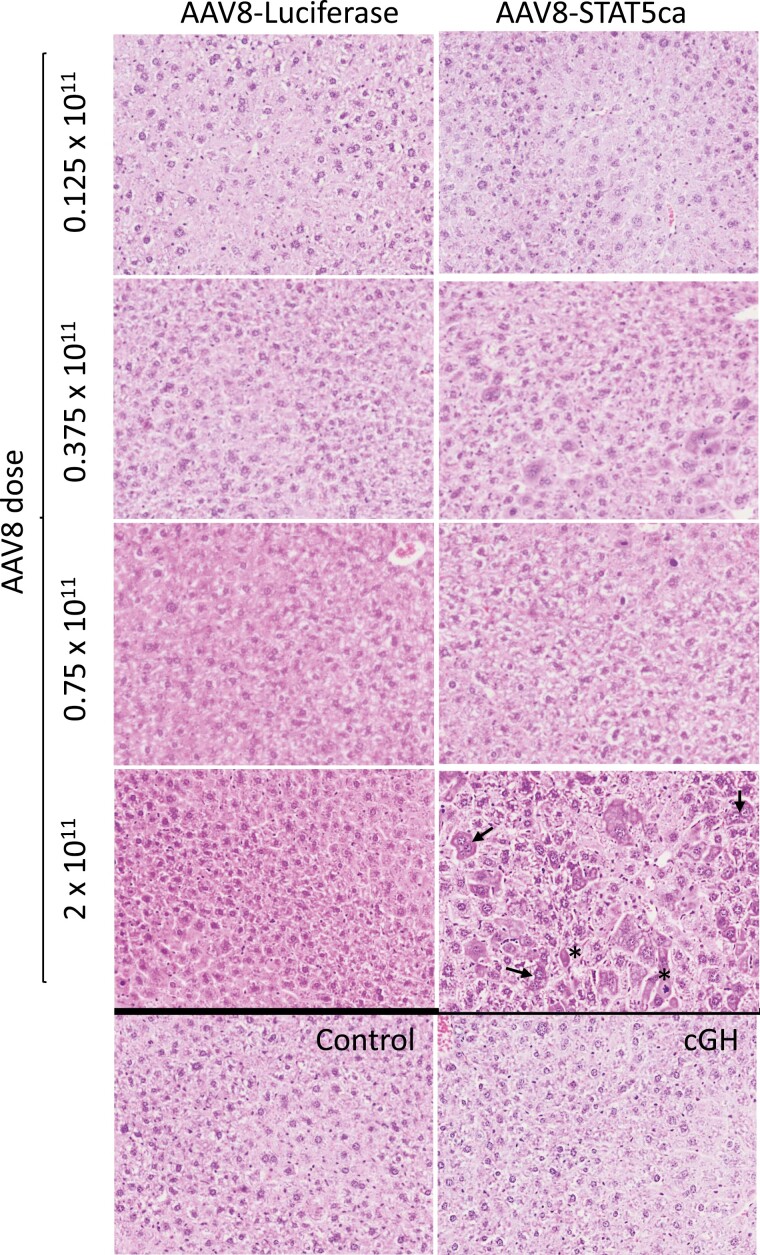
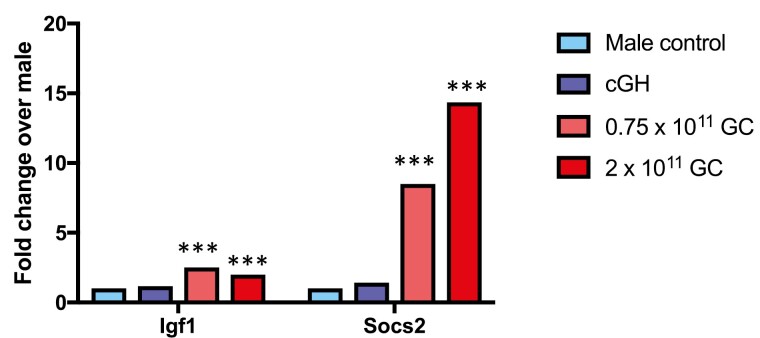
There were no consistent changes in hepatocyte morphology when AAV8-STAT5CA was delivered at doses up to 0.75 × 1011 GC or in livers from male mice given cGH infusion. However, when the dose of AAV8-STAT5CA was increased to 2 × 1011 GC/male mouse, we observed hepatocyte hyperplasia, increased numbers of multinuclear hepatocytes, karyomegaly, and apoptotic cells (Fig. 5). To ascertain whether these responses are associated with STAT5 overexpression, we examined the expression levels of 2 classical STAT5 target genes in liver, Igf1 and Socs2. Whereas AAV8-STAT5CA induced the expression of Igf1 moderately (2- to 2.5-fold increase above control liver levels at both 0.75 × 1011 and 2 × 1011 GC), we observed strong induction of Socs2, which reached 8.5-fold at the lower dose and 14.4-fold above baseline at the higher dose (2 × 1011 GC), consistent with a functional overexpression of STAT5 activity, most notably at the higher AAV8-STAT5CA dose (Fig. 6).
Figure 5.
H&E staining of AAV8-STAT5CA-infected liver. Liver sections from male mice treated with AAV8-STAT5CA or AAV8-Luciferase at the indicated GC dose per mouse, as in Fig. 2B, were stained with H&E. Images reveal a STAT5CA dose-dependent histopathology, which was evident and most consistent at the highest dose of AAV8 dose (2 × 1011 GC), with hepatocyte hyperplasia, increased numbers of multinuclear hepatocytes (arrows), karyomegaly, and apoptosis (asterisks). Also see Fig. S2 (42). No histopathology was evident in mice given cGH infusion for 17 days. Images collected with an Olympus FSX-100 instrument at 16×. H&E, hematoxylin and eosin.
Figure 6.
Expression of direct STAT5 target genes Igf1 and Socs2 in male liver: impact of cGH infusion and AAV8-STAT5CA. Shown are expression level determined by RNA-seq (fold-change values relative to male control), indicating supraphysiological induction of Socs2, and to a lesser extent Igf1, by AAV8-STAT5CA. Significance was determined by edgeR: ***Adjusted P value < E-05.
Discussion
The sex-dependent patterns of pituitary GH secretion—pulsatile GH release in males and persistent (near-continuous) GH release in females—activate hepatic GH receptor signaling to STAT5 in a manner that mirrors circulating GH profiles (ie, pulsatile STAT5 activation and transcriptional activity in male liver and persistent STAT5 activation in female liver). Here, we show that persistent expression of activated STAT5 in male mouse liver, achieved using a liver-specific AAV8 viral vector to deliver the activated STAT5b mutant STAT5CA to mouse hepatocytes in vivo, has extensive effects on sex-biased gene expression, with 53% of male-biased genes repressed and 59% of female-biased genes induced. These responses to STAT5CA are highly specific for sex-biased genes, insofar as fewer than 2% of genes showing sex-independent expression were responsive to this treatment. Many of the sex-independent gene responses are likely to be specific, given their enrichment for well-defined biological pathways related to extracellular matrix/cell adhesion and Wnt signaling.
cGH treatment mimics the endogenous female pattern of persistent circulating GH and overrides the endogenous pulsatile male secretion pattern, leading to substantial feminization of liver gene expression within 7 days (21, 22). Here, we report a high overlap between gene responses to STAT5CA expression and cGH infusion, providing strong evidence that persistent STAT5 activity alone is sufficient to account for a large fraction of the genes whose expression pattern becomes feminized. Nevertheless, we found that some genes respond in a unique manner to either STAT5CA expression or cGH infusion. Although some of the differences in response to these 2 feminizing treatments may be due to cutoff thresholds, others clearly are not, for example Cyp2b10 and Cyp2c37, whose expression in male liver was induced and substantially feminized, by cGH infusion but that were unresponsive to STAT5CA (Table S2) (42). Differences between these 2 models for feminizing male liver gene expression could in part result from STAT5-independent signaling pathways that are initiated by continuous GH, such as the GH receptor-dependent activation of PI-3 kinase, ERK signaling, and Src family kinases (26).
STAT5CA and cGH infusion both increased the expression in male liver of some female-biased genes to a level that exceeds their expression in untreated female liver. This phenomenon was somewhat more common for cGH infusion (29 genes expressed at > 2-fold the level of female liver after 14 days of cGH infusion vs 18 genes exceeded that level following STAT5CA treatment; Table S2) (42). There are several possible reasons for the excessive feminization of these genes and for the incomplete feminization of many other sex-biased genes. First, levels of STAT5 activity reached in AAV8-STAT5CA-infected hepatocytes may exceed the physiological levels of activated STAT5 in female liver, in particular at the 2 × 1011 GC dose of AAV8-STAT5CA used in our study (see overexpression of Socs2; Fig. 6). Related to this, cGH infusion and STAT5CA treatment both generate signals somewhat different from the physiological signals in female liver, where there are short GH-off and short STAT5 activity-off time periods, albeit not as well defined or as prolonged as the GH-off and STAT5 activity-off interpulse intervals that characterize GH/STAT5 signaling in male liver (14). Second, although cGH and STAT5CA may both mimic female liver with respect to GH stimulation and STAT5 activity, neither treatment recapitulates the female hormonal environment with respect to other factors. One such factor is estrogen, which can also affect sex-biased gene expression in the liver, albeit with direct estrogen receptor-dependent effects on only 4% of liver sex-biased genes (50). Indeed, it is remarkable how effective STAT5CA and cGH are in feminizing male liver, despite the presence of a male gonadal steroid environment. Finally, intrinsic chromosomal sex differences associated with liver-expressed Y-chromosome-encoded epigenetic modifiers, such as Uty and Kdm5d, and partial X-inactivation/incomplete gene dosage compensation (51) remain in place and could also contribute to the observed differences between female liver and STAT5CA-treated or cGH-infused male liver.
Female-biased genes induced by STAT5CA in male liver were significantly enriched for nearby female-biased STAT5 binding sites in liver chromatin compared with a background set of female-biased genes not induced by STAT5CA. This indicates that STAT5 plays a direct, positive role in regulating the STAT5CA-responsive female-biased genes in female liver. Many of the female-biased genes not induced by STAT5CA showed a much weaker sex bias, and could be regulated by other mechanisms, including GH signaling pathways independent of STAT5, as mentioned above. We did not observe a corresponding enrichment of STAT5-binding sites nearby male-biased genes repressed by STAT5CA, suggesting that factors other than differences in STAT5 binding determine whether an individual male-biased gene will be repressed when STAT5CA is expressed in male liver. In contrast, sex-independent genes repressed by STAT5CA showed strong enrichment for local sex-independent STAT5-binding sites, supporting the proposal that STAT5 can repress gene expression by a direct DNA-binding mechanism.
The set of STAT5CA-induced female-biased genes was also significantly enriched for class I female-biased genes, whose expression in female liver is positively regulated by the female plasma GH pattern. This finding lends further support to the hypothesis that in female liver, and in STAT5CA-treated male liver, class I female-biased gene expression is positively regulated by the direct, persistent binding of STAT5 to local STAT5-binding regulatory sites in association with localized, female-biased chromatin opening, which has been linked to female-biased STAT5 binding and gene activation (9, 18). However, any model for the role of STAT5 in regulating the expression of these class I female-biased genes needs to take into account that loss of STAT5 binding in female liver, as occurs in hepatocyte-specific STAT5a/STAT5b-knockout mice, results in minimal decreases in expression of several highly female-biased class I genes, including Sult3a1, A1bg, Fmo3, and Cyp2a4 (20) (Table S4, column X) (42), in distinct contrast to the large increases in the expression of these same genes seen in male liver with STAT5CA treatment.
We observed significant feminization of gene expression at AAV8-STAT5CA doses as low as 0.125 × 1011 GC per mouse (ie, 10-fold or more lower than standard doses of 2 × 1011 GC commonly used for AAV8-induced gene expression in mouse liver) (32). This finding is in line with earlier studies in GH-deficient rats, in which infusion of GH at a dose that restores as little as 3% of the nominal circulating female GH level substantially restored the expression of certain female-biased genes, whereas other genes required higher GH doses and showed only partial induction (52).
A decrease in GH signaling is associated with liver steatosis and development of nonalcoholic steatohepatitis, both in humans and in mouse models, whereas increased GH signaling can improve fatty liver endpoints (53, 54). Here, we found that when GH/STAT5 signaling was increased by treatment of male mice with AAV8-STAT5CA at the highest dose tested (2 × 1011 GC/mouse), certain histopathological changes indicative of liver injury were observed that could not be attributed to AAV8 infection per se. Furthermore, livers from mice given cGH infusion for 17 days did not show these histopathological changes, despite the overall similarity between cGH and AAV8-STAT5CA effects on sex-biased gene expression. Conceivably, the histopathology associated with AAV8-STAT5CA at the 2 × 1011 GC dose could be related to the propensity of this viral vector to integrate into the host genome in the context of liver injury (55, 56) or could be a supraphysiologic effect of STAT5 overexpression. Whether the changes in liver histology seen here after short-term (4-6 weeks) exposure to STAT5CA would ultimately lead to more severe liver dysfunction, including hepatocellular carcinoma, remains to be determined. Of note, there is evidence that STAT5 can play both a hepatoprotective role and an oncogenic role in hepatocellular carcinoma (54, 57). A physiological level of continuous STAT5 activity, as occurs in normal female liver, is associated with a lower prevalence of hepatocellular carcinoma compared with males (2), although cause and effect have not been established. The impact of STAT5CA overexpression in female liver was not tested in this study because our primary aim was to investigate the ability of continuous STAT5 activity to feminize the male liver. Nonetheless, our observation of liver histological changes following AAV8- STAT5CA treatment highlights the importance of carefully evaluating such effects before considering STAT5CA or other STAT5 derivatives for therapeutic use in treating liver disease.
Acknowledgments
The authors thank the following Waxman laboratory members: Kritika Karri for providing annotations for the 48 360 mouse liver-expressed lncRNAs genes examined in this study, Dr. Maxim Pyatkov for RNA-seq analysis pipeline development, and Dr. Ravi Sonkar for assistance with histology imaging.
Glossary
Abbreviations
- AAV
adeno-associated virus
- aHepGHRkd
adult-onset hepatocyte-specific GH receptor knockdown
- cGH
continuous infusion of GH
- ChIP
chromatin immunoprecipitation
- FDR
false discovery rate
- GC
genome copy
- qPCR
quantitative polymerase chain reaction
- RNA-seq
RNA sequencing
- STAT5CA
constitutively active form of STAT5b
Funding
Supported in part by National Institutes of Health grant DK121998 (to D.J.W.) and grants National Institutes of Health DK116878, VA CRS BX005382 and VA merit BX00448 (to R.K.).
Disclosures
The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the associated Supplemental figures and Supplemental tables available at https://figshare.com/ (42) and at the public data repository at GEO (https://www.ncbi.nlm.nih.gov/geo/) under accession numbers GSE196014 and GSE196015.
References
- 1. Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76(2):215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kur P, Kolasa-Wolosiuk A, Misiakiewicz-Has K, Wiszniewska B. Sex hormone-dependent physiology and diseases of liver. Int J Environ Res Public Health. 2020;17(8):2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lonardo A, Nascimbeni F, Ballestri S, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70(4):1457-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brie B, Ramirez MC, De Winne C, et al. Brain control of sexually dimorphic liver function and disease: the endocrine connection. Cell Mol Neurobiol. 2019;39(2):169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Waxman DJ, Pampori NA, Ram PA, Agrawal AK, Shapiro BH. Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome P450. Proc Natl Acad Sci USA. 1991;88(15):6868-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mode A, Wiersma-Larsson E, Strom A, Zaphiropoulos PG, Gustafsson J, AA. Dual role of growth hormone as a feminizing and masculinizing factor in the control of sex-specific cytochrome P-450 isozymes in rat liver. J Endocrinol. 1989;120(2):311-317. [DOI] [PubMed] [Google Scholar]
- 7. Waters MJ. The growth hormone receptor. Growth Horm IGF Res. 2016;28:6-10. [DOI] [PubMed] [Google Scholar]
- 8. Piwien-Pilipuk G, Huo JS, Schwartz J. Growth hormone signal transduction. J Pediatr Endocrinol Metab. 2002;15(6):771-786. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Laz EV, Waxman DJ. Dynamic, sex-differential STAT5 and BCL6 binding to sex-biased, growth hormone-regulated genes in adult mouse liver. Mol Cell Biol. 2012;32(4):880-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chia DJ. Minireview: mechanisms of growth hormone-mediated gene regulation. Mol Endocrinol. 2014;28(7):1012-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Udy GB, Towers RP, Snell RG, et al. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA. 1997;94(14):7239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol. 2006;20(6):1333-1351. [DOI] [PubMed] [Google Scholar]
- 13. Holloway MG, Cui Y, Laz EV, Hosui A, Hennighausen L, Waxman DJ. Loss of sexually dimorphic liver gene expression upon hepatocyte-specific deletion of Stat5a-Stat5b locus. Endocrinology. 2007;148(5):1977-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi HK, Waxman DJ. Plasma growth hormone pulse activation of hepatic JAK-STAT5 signaling: developmental regulation and role in male-specific liver gene expression. Endocrinology. 2000;141(9):3245-3255. [DOI] [PubMed] [Google Scholar]
- 15. Tannenbaum GS, Choi HK, Gurd W, Waxman DJ. Temporal relationship between the sexually dimorphic spontaneous GH secretory profiles and hepatic STAT5 activity. Endocrinology. 2001;142(11):4599-4606. [DOI] [PubMed] [Google Scholar]
- 16. Connerney J, Lau-Corona D, Rampersaud A, Waxman DJ. Activation of male liver chromatin accessibility and STAT5-dependent gene transcription by plasma growth hormone pulses. Endocrinology. 2017;158(5):1386-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi HK, Waxman DJ. Growth hormone, but not prolactin, maintains, low-level activation of STAT5a and STAT5b in female rat liver. Endocrinology. 1999;140(11):5126-5135. [DOI] [PubMed] [Google Scholar]
- 18. Sugathan A, Waxman DJ. Genome-wide analysis of chromatin states reveals distinct mechanisms of sex-dependent gene regulation in male and female mouse liver. Mol Cell Biol. 2013;33(18):3594-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cui Y, Hosui A, Sun R, et al. Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology. 2007;46(2):504-513. [DOI] [PubMed] [Google Scholar]
- 20. Hao P, Waxman DJ. STAT5 regulation of sex-dependent hepatic CpG methylation at distal regulatory elements mapping to sex-biased genes. Mol Cell Biol. 2021;41(2):e00166-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lau-Corona D, Suvorov A, Waxman DJ. Feminization of male mouse liver by persistent growth hormone stimulation: activation of sex-biased transcriptional networks and dynamic changes in chromatin states. Mol Cell Biol. 2017;37(19):e00301-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holloway MG, Laz EV, Waxman DJ. Codependence of growth hormone-responsive, sexually dimorphic hepatic gene expression on signal transducer and activator of transcription 5b and hepatic nuclear factor 4alpha. Mol Endocrinol. 2006;20(3):647-660. [DOI] [PubMed] [Google Scholar]
- 23. Wauthier V, Sugathan A, Meyer RD, Dombkowski AA, Waxman DJ. Intrinsic sex differences in the early growth hormone responsiveness of sex-specific genes in mouse liver. Mol Endocrinol. 2010;24(3):667-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rotwein P. Regulation of gene expression by growth hormone. Mol Cell Endocrinol. 2020;507:110788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chhabra Y, Lee CMM, Muller AF, Brooks AJ. GHR signalling: receptor activation and degradation mechanisms. Mol Cell Endocrinol. 2021;520:111075. [DOI] [PubMed] [Google Scholar]
- 26. Frank SJ. Classical and novel GH receptor signaling pathways. Mol Cell Endocrinol. 2020;518:110999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carter-Su C, Schwartz J, Argetsinger LS. Growth hormone signaling pathways. Growth Horm IGF Res. 2016;28:11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. List EO, Berryman DE, Funk K, et al. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155(5):1793-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ariyoshi K, Nosaka T, Yamada K, et al. Constitutive activation of STAT5 by a point mutation in the SH2 domain. J Biol Chem. 2000;275(32):24407-24413. [DOI] [PubMed] [Google Scholar]
- 30. Woelfle J, Billiard J, Rotwein P. Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b. J Biol Chem. 2003;278(25):22696-22702. [DOI] [PubMed] [Google Scholar]
- 31. Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16(6):1073-1080. [DOI] [PubMed] [Google Scholar]
- 32. Sands MS. AAV-mediated liver-directed gene therapy. Methods Mol Biol. 2011;807:141-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. List EO, Berryman DE, Funk K, et al. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol. 2013;27(3):524-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cordoba-Chacon J, Majumdar N, List EO, et al. Growth hormone inhibits hepatic de novo lipogenesis in adult mice. Diabetes. 2015;64(9):3093-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Percie du Sert N, Ahluwalia A, Alam S, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18(7):e3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang J, Symul L, Yeung J, et al. Circadian clock-dependent and -independent posttranscriptional regulation underlies temporal mRNA accumulation in mouse liver. Proc Natl Acad Sci USA. 2018;115(8):E1916-E1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Menet JS, Rodriguez J, Abruzzi KC, Rosbash M. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. Elife. 2012;1:e00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goldfarb CN, Waxman DJ. Global analysis of expression, maturation and subcellular localization of mouse liver transcriptome identifies novel sex-biased and TCPOBOP-responsive long non-coding RNAs. BMC Genomics. 2021;22(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923-930. [DOI] [PubMed] [Google Scholar]
- 41. Lodato NJ, Melia T, Rampersaud A, Waxman DJ. Sex-differential responses of tumor promotion-associated genes and dysregulation of novel long noncoding rnas in constitutive androstane receptor-activated mouse liver. Toxicol Sci. 2017;159(1):25-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lau-Corona D, Ma H, Vergato C, Sarmento-Cabral A, del Rio-Moreno M, Kineman RD, Waxman DJ. Data from: Lau-Corona et al (2022) Endocrinology. Constitutively active STAT5b feminizes mouse liver gene expression: Supplemental Figures and Tables.2022. Published online March 25, 2022. doi: 10.6084/m9.figshare.c.5913974 or doi: , https://figshare.com/. [DOI] [PMC free article] [PubMed]
- 43. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Melia T, Waxman DJ. Sex-Biased lncRNAs inversely correlate with sex-opposite gene coexpression networks in diversity outbred mouse liver. Endocrinology. 2019;160(5):989-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26(6):841-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kiourtis C, Wilczynska A, Nixon C, Clark W, May S, Bird TG. Specificity and off-target effects of AAV8-TBG viral vectors for the manipulation of hepatocellular gene expression in mice. Biol Open. 2021;10(9):bio058678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wolf Greenstein A, Majumdar N, Yang P, Subbaiah PV, Kineman RD, Cordoba-Chacon J. Hepatocyte-specific, PPARgamma-regulated mechanisms to promote steatosis in adult mice. J Endocrinol. 2017;232(1):107-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Melia T, Hao P, Yilmaz F, Waxman DJ. Hepatic long intergenic noncoding RNAs: high promoter conservation and dynamic, sex-dependent transcriptional regulation by growth hormone. Mol Cell Biol. 2016;36(1):50-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ono M, Chia DJ, Merino-Martinez R, Flores-Morales A, Unterman TG, Rotwein P. Signal transducer and activator of transcription (Stat) 5b-mediated inhibition of insulin-like growth factor binding protein-1 gene transcription: a mechanism for repression of gene expression by growth hormone. Mol Endocrinol. 2007;21(6):1443-1457. [DOI] [PubMed] [Google Scholar]
- 50. Zheng D, Wang X, Antonson P, Gustafsson JA, Li Z. Genomics of sex hormone receptor signaling in hepatic sexual dimorphism. Mol Cell Endocrinol. 2018;471:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Naqvi S, Godfrey AK, Hughes JF, Goodheart ML, Mitchell RN, Page DC. Conservation, acquisition, and functional impact of sex-biased gene expression in mammals. Science. 2019;365(6450):eaaw7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pampori NA, Shapiro BH. Gender differences in the responsiveness of the sex-dependent isoforms of hepatic P450 to the feminine plasma growth hormone profile. Endocrinology. 1999;140(3):1245-1254. [DOI] [PubMed] [Google Scholar]
- 53. Vazquez-Borrego MC, Del Rio-Moreno M, Kineman RD. Towards understanding the direct and indirect actions of growth hormone in controlling hepatocyte carbohydrate and lipid metabolism. Cells. 2021;10(10):2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kaltenecker D, Themanns M, Mueller KM, et al. Hepatic growth hormone - JAK2 - STAT5 signalling: metabolic function, non-alcoholic fatty liver disease and hepatocellular carcinoma progression. Cytokine. 2019;124:154569. [DOI] [PubMed] [Google Scholar]
- 55. Dalwadi DA, Torrens L, Abril-Fornaguera J, et al. Liver injury increases the incidence of HCC following AAV gene therapy in mice. Mol Ther. 2021;29(2):680-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Deyle DR, Russell DW. Adeno-associated virus vector integration. Curr Opin Mol Ther. 2009;11(4):442-447. [PMC free article] [PubMed] [Google Scholar]
- 57. Hin Tang JJ, Hao Thng DK, Lim JJ, Toh TB. JAK/STAT signaling in hepatocellular carcinoma. Hepat Oncol. 2020;7(1):Hep18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the associated Supplemental figures and Supplemental tables available at https://figshare.com/ (42) and at the public data repository at GEO (https://www.ncbi.nlm.nih.gov/geo/) under accession numbers GSE196014 and GSE196015.