Abstract
OBJECTIVES
Changes in postoperative pulmonary function vary among patients after lobectomy. We aimed to define preoperative factors that negatively influence postoperative % vital capacity (%VC) in patients treated by lobectomy.
METHODS
We included 276 patients who had been treated by lobectomy at our institution between 2007 and 2018 and their preoperative and postoperative pulmonary function data were complete. We assigned them to groups based on postoperative pulmonary function defined as better (good) or worse (poor) than predicted %VC, then compared clinicopathological findings between them. Poor postoperative pulmonary function was also assessed using logistic regression analysis.
RESULTS
Interstitial pneumonia (IP) was diagnosed in 37 (13.4%) patients. The preoperative and postoperative %VC values were, respectively, 101.1% (interquartile range, 90.5–110%) and 87.6% (interquartile range, 73.8–99.1%). Logistic regression analysis revealed that IP, advanced age (≥75 years), and induction therapy were independent risk factors for reduced postoperative pulmonary function [odds ratios 3.01 (1.41–6.41), 2.49 (1.35–4.60), and 9.03 (2.43–33.5), P = 0.0044, 0.0035, and 0.001, respectively]. Postoperative %VC worsened with increasing IP severity and advanced age. Six (75%) of 8 patients aged ≥80 years with usual IP or suspected usual IP on preoperative computed tomography images had poor postoperative %VC.
CONCLUSIONS
Surgical indications for lobectomy based on predicted postoperative %VC require careful consideration for elderly patients with IP, particularly those aged ≥80 years.
Keywords: Interstitial pneumonia, Lobectomy, Lung cancer, Pulmonary function
Postoperative pulmonary function is a crucial factor in general status after surgical lung resection.
INTRODUCTION
Postoperative pulmonary function is a crucial factor in general status after surgical lung resection. The feasibility of lung resection including lobectomy, segmentectomy and wedge resection is assessed using several preoperative factors to predict general postoperative status. Among these factors, postoperative pulmonary function is predicted based on preoperative pulmonary function and the number of segments that will be removed. However, postoperative pulmonary function is sometimes worse than predicted. Thus, predictive factors should be identified that would be clinically useful when selecting the most ideal patients for lung resections. Resection mode [1–3], induction chemoradiation therapy [4–6] and surgical approach [7–9] influence postoperative pulmonary function. However, few studies have compared predicted, with actual postoperative pulmonary function, to identify associated risk factors.
Here, we aimed to determine preoperative risk factors for poor postoperative pulmonary function by measuring ratios of % vital capacity (%VC) before, and after surgical lung resection to treat pulmonary diseases. Postoperative pulmonary function is generally determined by the amount of resected lung parenchyma. Lobectomy helps to preserve pulmonary function including forced vital capacity, forced expiratory volume in 1 s (FEV1) and diffusing capacity for carbon monoxide (DLco) compared with pneumonectomy [10, 11]. Segmentectomy is more beneficial than lobectomy for postoperative pulmonary function [1–3]. Thus, we focused on lobectomy, because it is a standard surgical procedure for reducing the influence of the amount resected lung parenchyma in patients with non-small cell lung cancer (NSCLC), and because it affects postoperative pulmonary function more than sublobar resection such as segmentectomy and wedge resection. Our findings generated valuable information with which to determine appropriate treatment strategies for patients who will undergo lung resection.
PATIENTS AND METHODS
Ethics statement
The Ethical Committee for Epidemiology of Hiroshima University approved this retrospective review of a prospective database and waived the requirement for informed consent from individual patients (13 June 2018, E1216) because it was a retrospective study.
Patient population
We initially collected information about 975 consecutive patients who were treated by lobectomy for pulmonary diseases at Hiroshima University Hospital between January 2007 and December 2018. We analysed data from 276 patients who had complete preoperative and postoperative data about %VC (Fig. 1). Definitive or suspected lung cancer at clinical stages 0–III was treated by lobectomy when ground-glass opacity dominant tumours were large (>3 cm) or centrally located, small (≤3 cm) or solid dominant tumours were large (>2 cm), or centrally located, small (≤2 cm). Lobectomy was also applied to infectious diseases with lesions that were not completely resectable by any other means.
Figure 1:

Study flowchart. VC: vital capacity.
Pulmonary function
Pulmonary function was evaluated by spirometry before, and 1 year after lung resection using the same procedures and equipment. The predicted VC was calculated as follows:
The predicted postoperative %VC calculated using the preoperative %VC and the number of segments to be removed was subtracted from the actual postoperative %VC. The numbers of resected segments were right upper (n = 3), right middle (n = 2), right lower (n = 5), left upper (n = 4) and left lower (n = 4) lobes. Calculated values that were <0 or ≥0 were, respectively, considered to indicate poor and good postoperative pulmonary function (Fig. 2).
Figure 2:

Predicted postoperative %VC based on preoperative values subtracted from actual postoperative %VC. Poor and good postoperative pulmonary functions are respectively determined as values <0 and ≥0. VC: vital capacity.
Definition of interstitial pneumonia on high resolution computed tomography images
Interstitial pneumonia was defined as usual interstitial pneumonia (UIP), suspected UIP and inconsistent with UIP according to the 2011 ATS/ERS/JRS/ALAT international guidelines for interstitial pulmonary fibrosis [12]. At least 1 physician and at least 1 blinded independent radiologist interpreted computed tomography images with respect to interstitial pneumonia (IP) and emphysema.
Statistical analyses
Continuous and categorical variables were analysed using nonparametric Mann–Whitney U-tests, Kruskal–Wallis tests and χ2 or Fisher exact tests. Poor postoperative %VC was assessed by multivariable logistic regression analyses with cut-off values in models including gender, age, emphysema, IP and induction therapy that significantly (P ≤ 0.05) differed between patients with good and poor postoperative %VC in univariate analyses. The Brinkman index was excluded from analysis because smoking is a cause of emphysema. Age ≥75 years is defined as elderly and is associated with higher rate of postoperative morbidity [13]. Therefore, we selected 75 years as the cut-off for age. All data were statistically analysed using EZR v. 1.37 (Saitama Medical Centre, Jichi Medical University, Shimotsuke, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 3.4.4) [14]. More precisely, EZR is a modified version of R commander version 2.4-0 that was designed to add statistical functions that are frequently used in biostatistics. Values with P ≤ 0.05 were deemed statistically significant.
RESULTS
Clinicopathological findings of patients with %VC that were poorer than predicted
Table 1 shows the pre- and postoperative clinical characteristics of the 2 groups of patients with complete data regarding preoperative and postoperative pulmonary function as %VC. All of them underwent lobectomy to treat NSCLC, small cell lung cancer, benign pulmonary diseases and other lung pathologies.
Table 1:
Clinical characteristics of patients treated by lobectomy
| Variables | All (n = 276) | |
|---|---|---|
| Median age (year) | 70 (63–75) | |
| Age | <75 years | 203 (73.6%) |
| >75 years | 73 (26.4%) | |
| Gender | Female | 125 (45.3%) |
| Male | 151 (54.7%) | |
| BI | 400 (0–1000) | |
| PS | 0 | 153 (55.4%) |
| 1 | 11 (4.0%) | |
| Unknown | 112 (40.6%) | |
| IP | No | 239 (86.6%) |
| Yes | 37 (13.4%) | |
| Emphysema | No | 190 (68.8%) |
| Yes | 86 (31.2%) | |
| Preoperative VC (ml) (IQR) | 2990 (2495–3572.5) | |
| Preoperative %VC | 101.1 (90.5–110) | |
| Postoperative %VC | 87.6 (73.8–99.1) | |
| Induction therapy | 11 (4.0%) | |
| Diagnosis | NSCLC | 267 (96.7%) |
| SCLC | 3 (1.1%) | |
| Benign | 3 (1.1%) | |
| Others | 3 (1.1%) | |
| Surgical duration (min) | 140.5 (113–175) | |
| Blood loss (g) | 40 (20–85.3) | |
| Surgical approach | Open | 5 (1.8%) |
| VATS | 252 (91.3%) | |
| RATS | 19 (6.9%) | |
| Hospital stay (day) (IQR) | 7 (6–9) | |
| Duration of chest tube placement (day) (IQR) | 3 (2–4) | |
| Postoperative complications | Grade 0 | 222 (80.4%) |
| Grade ≥1 | 54 (19.6%) | |
| Pleurodesis | 20 (7.2%) | |
| Adjuvant therapy | 86 (31.2%) |
Data are shown as medians with IQR in parentheses, or n (%).
BI: Brinkman index; IP: interstitial pneumonia; IQR: interquartile range; NSCLC: non-small cell lung cancer; PS: performance status; RATS: robot-assisted thoracic surgery; SCLC: small cell lung cancer; VATS: video-assisted thoracic surgery; VC: vital capacity.
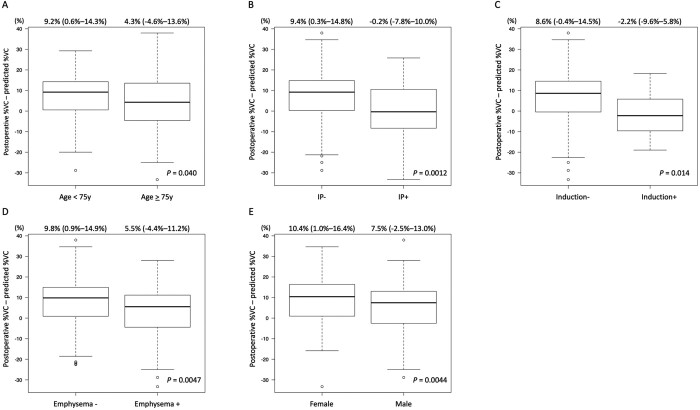
The patients with a postoperative %VC that was worse than predicted were older, more likely to be male, smoke more cigarettes, have a higher rate of IP or emphysema, a poorer preoperative %VC and a higher frequency of induction therapy than those with a good postoperative %VC. However, preoperative VC did not significantly differ between the good and poor groups (Table 2). Male sex, advanced age (≥75 years), induction therapy, IP or emphysema were associated with poorer postoperative %VC (Fig. 3). In terms of intraoperative and postoperative findings, prolonged surgery, a larger volume of intraoperative blood loss, prolonged postoperative hospital stay and prolonged chest tube placement were prevalent among the patients with poor, compared with good postoperative %VC (Table 2). The differences between the predicted and actual postoperative values of FEV1 and %DLco were significantly worse in the group with a poor, than a good %VC (Supplementary Material, Table S1). Supplementary Material, Table S2 shows detailed information about the diagnosis and staging of malignancies.
Table 2:
Clinical characteristics of patients treated by lobectomy based on postoperative %vital capacity
| Variables | Good (n = 199) | Poor (n = 77) | P-value | ||
|---|---|---|---|---|---|
| Age overall | 68 (62.5–74) | 72 (66–76) | 0.001 | ||
| Age, n (%) | <75 years | 157 (78.9%) | 46 (59.7%) | 0.002 | |
| >75 years | 42 (21.1%) | 31 (40.3%) | |||
| Gender, n (%) | Female | 98 (49.2%) | 27 (35.1%) | 0.043 | |
| Male | 101 (50.8%) | 50 (64.9%) | |||
| BI | 170 (0–900) | 620 (0–1000) | 0.027 | ||
| PS, n (%) | 0 | 111 (55.8%) | 42 (54.5%) | 0.15 | |
| 1 | 5 (2.5%) | 6 (7.8%) | |||
| Unknown | 83 (41.7%) | 29 (37.7%) | |||
| IP, n (%) | No | 182 (91.5%) | 57 (74.0%) | <0.001 | |
| Yes | 17 (8.5%) | 20 (26.0%) | |||
| Emphysema, n (%) | No | 147 (73.9%) | 43 (55.8%) | 0.006 | |
| Yes | 52 (26.1%) | 34 (44.2%) | |||
| Preoperative VC | 2970 (2480–3675) | 3020 (2550–3520) | 0.77 | ||
| Preoperative %VC | 102.4 (92.1–110.4) | 94.7 (86.5–107.3) | 0.022 | ||
| Postoperative %VC | 92.2 (83.3–102.2) | 67.8 (61.0–79.2) | <0.001 | ||
| Induction therapy, n (%) | 4 (2.0%) | 7 (9.1%) | 0.013 | ||
| Diagnosis, n (%) | NSCLC | 191 (96.0%) | 76 (98.7%) | 0.70 | |
| SCLC | 3 (1.5%) | 0 (0%) | |||
| Benign | 3 (1.5%) | 0 (0%) | |||
| Other | 2 (1.0%) | 1 (1.3%) | |||
| Surgical duration (min) | 131 (109.5–162) | 162 (137–210) | <0.001 | ||
| Blood loss (g) (IQR) | 31 (20–69) | 60 (36–115) | <0.001 | ||
| Surgical approach, n (%) | Open | 2 (1.0%) | 3 (3.9%) | 0.061 | |
| VATS | 180 (90.5%) | 72 (93.5%) | |||
| RATS | 17 (8.5%) | 2 (2.6%) | |||
| Hospital stay (days) | 7 (6–8) | 8 (7–10) | <0.001 | ||
| Duration of chest tube placement (days) | 3 (2–3) | 3 (2–5) | <0.001 | ||
| Postoperative complications, n (%) | Grade 0 | 165 (82.9%) | 57 (74.0%) | 0.13 | |
| Grade ≥1 | 34 (17.1%) | 20 (26.0%) | |||
| Pleurodesis, n (%) | 11 (5.5%) | 9 (11.7%) 0.12 | 0.12 | ||
| Adjuvant therapy, n (%) | 66 (33.2%) | 20 (26.0%) 0.31 | 0.31 | ||
BI: Brinkman index; FEV: forced expiratory volume in 1 s; IP: interstitial pneumonia; NSCLC: non-small cell lung cancer; PS: performance status; RATS: robot-assisted thoracic surgery; SCLC: small cell lung cancer; VATS: video-assisted thoracic surgery; VC: vital capacity.
Figure 3:
Predicted postoperative %VC based on preoperative values subtracted from actual postoperative %VC according to several factors. Values are calculated according to (A) age, (B) interstitial pneumonia, (C) induction therapy, (D) emphysema and (E) gender. Data are shown as medians with interquartile ranges. IP, interstitial pneumonia; VC: vital capacity.
Identification of preoperative factors negatively affecting postoperative %VC
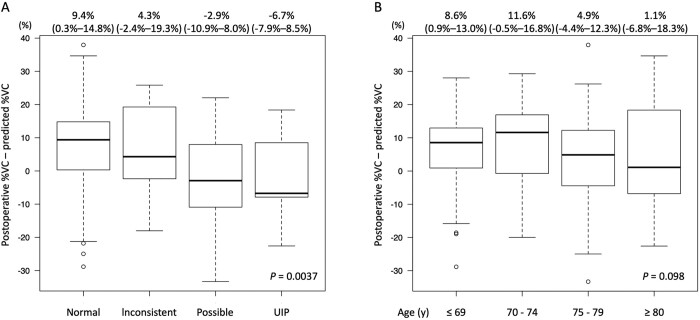
The results of multivariable logistic regression analyses revealed that IP [odds ratio, 3.01 (1.41–6.41); P = 0.0044], age ≥75 years [odds ratio, 2.49 (1.35–4.60); P = 0.0035] and induction therapy [odds ratio, 9.03 (2.43–33.5); P = 0.001] were independent risk factors that negatively affected postoperative %VC in patients who underwent lobectomy (Table 3). The postoperative %VC was poorer among patients with definitive UIP and suspected UIP, than those with normal or inconsistent UIP findings on preoperative high resolution computed tomography images (P = 0.0037; Fig. 4). The median postoperative %VC was worse in elderly patients aged ≥80 years than in younger patients (P = 0.098; Fig. 4), and the frequency of a poor postoperative %VC was 6 (75%) of 8 in this age group with definitive or suspected UIP. The difference between the actual and predicted postoperative %VC was >10% in 3 of 6 patients.
Table 3:
Multivariable logistic regression analysis of poor postoperative %vital capacity
| Variable | OR | P-value | |
|---|---|---|---|
| Gender | Male versus female | 1.38 (0.72–2.65) | 0.33 |
| Age | >75 vs <75 years | 2.49 (1.35–4.60) | 0.0035 |
| IP | Presence versus absence | 3.01 (1.41–6.41) | 0.0044 |
| Emphysema | Yes versus no | 1.75 (0.91–3.36) | 0.095 |
| Induction therapy | Yes versus no | 9.03 (2.43–33.5) | 0.001 |
IP: interstitial pneumonia; OR: odds ratio.
Figure 4:
Predicted postoperative %VC based on preoperative values subtracted from actual postoperative %VC stratified by imaging findings of UIP and age. Values were calculated based on (A) CT images of IP, normal, inconsistent with UIP, possible UIP and UIP and (B) age ≤69, 70–74, 75–79 and ≥80 years. IP, interstitial pneumonia; UIP, usual interstitial pneumonia; VC: vital capacity.
DISCUSSION
The present study analysed preoperative factors affecting postoperative %VC as a representative indicator of pulmonary function in patients who underwent lobectomy to treat various lung diseases. We evaluated postoperative pulmonary function by subtracting the postoperative %VC predicted based on preoperative findings from actual postoperative %VC. We found that IP, advanced age (≥75 years), and induction therapy were preoperative factors associated with postoperative pulmonary function indicated by %VC that was worse than predicted.
Pulmonary function can be assessed using VC, %VC, FEV1, FEV1, %FEV1 and DLco. Although DLco is an important variable with which to predict postoperative outcomes especially in patients with IP [15], it was not always assessed, especially during the initial period of this study, and we did not analyse forced vital capacity or VC because they are influenced by age, gender and height. Thus, the absolute values of these factors were inadequate for this study. Postoperative pulmonary function can also be evaluated as %FEV1, but this is affected by pulmonary status, especially the severity of chronic obstructive pulmonary disease including emphysema and chronic bronchitis. Therefore, we evaluated pulmonary function using %VC. Moreover, the differences between the predicted and actual postoperative FEV1 and %DLco supported the notion that %VC can represent pulmonary function.
In terms of lung background, IP and emphysema are typical of pulmonary diseases that might negatively affect pulmonary function. We found that IP negatively affected postoperative %VC, but not emphysema, at 1 year after lobectomy. Because it represents IP severity, %VC is more important for long-term surgical outcomes [16], whereas IP severity affects short-term surgical outcomes [17] more than emphysema in patients with NSCLC and pulmonary fibrosis combined with emphysema. One explanation for this is that IP negatively influences postoperative %VC more than emphysema. In that sense, the significance of the present findings for IP and emphysema are consistent with previous results. Furthermore, postoperative pulmonary function can improve after lobectomy due to a volume reduction effect in patients with moderate to severe emphysema. Relieved airflow obstruction that improves respiratory muscle function, eliminates dead space ventilation in ventilated, but non-perfused areas, and improved cardiovascular haemodynamics might all contribute to such improvement [18–20]. Thus, emphysema usually adversely influences, but does not always negatively affect postoperative pulmonary function after the emphysematous area is resected.
Age ≥75 years was also a crucial factor in reducing %VC after lobectomy. Ageing generally negatively influences pulmonary function due to weakened physical capacity, including respiratory muscles. Thus, the borderline for predicted postoperative function to determine whether patients could tolerate lobectomy should be defined more strictly for patients aged ≥75 years compared with younger patients. The overall survival of patients aged ≥80 years with early-stage NSCLC is comparable between wedge and anatomical resections and others causes of death are less prevalent with wedge resection compared with lobectomy or segmentectomy [21, 22]. One possible interpretation for these findings is that the advantage of postoperative pulmonary function might compensate for the oncological disadvantage of wedge, compared with anatomical resections. Therefore, limited pulmonary resection should be selected to treat lung diseases, especially among elderly patients on the borderline of predicted postoperative pulmonary function in terms of eligibility for lobectomy.
We did not analyse the intraoperative or postoperative factors of surgical duration, blood loss and intraoperative or postoperative complications that affect postoperative %VC because these factors are unknown in the preoperative setting when deciding treatment strategies. In addition, these factors are mainly determined by tumour factors such as location and stage and are thus difficult for surgeons to control. Our multivariable logistic regression analysis included both preoperative and postoperative factors involved in poor postoperative %VC (Supplementary Material, Tables S3 and S4) and selected the same significant risk factors for reduced postoperative %VC as the preoperative findings. Thus, the preoperative findings seemed reliable. However, the degree to which NSCLC has advanced, namely pathological stage, is also an important factor for predicting postoperative %VC.
Induction therapy was also a predictive factor for poor postoperative %VC as described [4–6]. All patients who underwent induction therapy were aged <75 years and did not have IP. Thus, induction therapy presents an elevated risk for patients without a compromised background. In contrast, compromised patients aged ≥80 years and patients with defined and suspected UIP on preoperative high resolution computed tomography, frequently had a poor postoperative %VC. That older patients or those with more severe IP had a worse postoperative %VC also supports the results of the multivariable logistic regression analysis.
Limitations
This study has some limitations. Evidence is weaker in retrospective, single centre, than in prospective and multi-institution studies. Studies of high-quality data from a large registry of patients treated by lobectomy in many centres might be useful for evaluating postoperative pulmonary function. However, such studies usually include patients with good overall status who are likely to have excellent postoperative pulmonary function. Thus, to determine risk factors affecting postoperative %VC could be challenging. The present study included only patients with complete preoperative and postoperative %VC data and excluded those with incomplete data. For instance, severe postoperative complications, that might have worsened postoperative %VC, let patients be unable to take the examination of the pulmonary function. Nevertheless, our findings provided important insights to consider when deciding strategies for treating lung disease. The low rates of 30- and 90-day postoperative mortality, the low rate of death within 1 year after surgical resection and the >90% 1-year overall survival that could affect postoperative %VC were recognized in the excluded patients, and the preoperative findings of age, Brinkman index, IP and emphysema between 699 excluded and 276 included patients did not significantly differ. Therefore, the 276 included patients were representative of the overall population (Supplementary Material, Table S5). Disease status is also a crucial factor for the postoperative pulmonary function. Although stage was more advanced in the group with poor, than good %VC, we excluded stage from the multivariable analysis because the study included patients with benign diseases for an overall main result. We also included only patients with NSCLC in a multivariable analysis with tumour stage. Our database does not include information about whether tumours are centrally or peripherally located, and this is also a limitation of the present study.
CONCLUSIONS
In conclusion, IP, age ≥75 years, and induction therapy negatively influenced %VC after lobectomy for lung diseases. Preoperative findings, especially those of definite and suspected UIP and age ≥80 years, should be carefully considered along with predicted postoperative %VC when determining whether patients can tolerate lobectomy. Further studies of a larger patient cohort after pulmonary resection are needed to confirm the present results.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Supplementary Material
ACKNOWLEDGEMENTS
This manuscript has been professionally proof-checked by a native English speaker with over 25 years of experience in medical and life science editing.
Funding
This study was supported by grants from the Japan Society for the Promotion of Science (JSPS) KAKENHI [grant number 20K09177].
Conflict of interest: none declared.
Data availability statement
The data underlying this article will be shared upon reasonable request to the corresponding author.
Author contributions
Takahiro Mimae: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Validation; Visualization; Writing—original draft; Writing—review & editing. Yoshihiro Miyata: Conceptualization; Supervision; Writing—review & editing. Takashi Kumada: Data curation; Writing—review & editing. Yoshinori Handa: Data curation; Writing—review & editing. Yasuhiro Tsutani: Data curation; Supervision; Writing—review & editing. Morihito Okada: Supervision; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Ilkka Ilonen and the other anonymous reviewers for their contribution to the peer review process of this article.
ABBREVIATIONS
- DLco
Diffusing capacity for carbon monoxide
- FEV1
Forced expiratory volume 1 s
- IP
Interstitial pneumonia
- NSCLC
Non-small cell lung cancer
- UIP
Usual interstitial pneumonia
- VC
Vital capacity
REFERENCES
- 1. Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N.. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769–75. [DOI] [PubMed] [Google Scholar]
- 2. Keenan RJ, Landreneau RJ, Maley RH Jr, Singh D, Macherey R, Bartley S. et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228–33; discussion 228–33. [DOI] [PubMed] [Google Scholar]
- 3. Harada H, Okada M, Sakamoto T, Matsuoka H, Tsubota N.. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041–5. [DOI] [PubMed] [Google Scholar]
- 4. Funakoshi Y, Takeda S, Sawabata N, Okumura Y, Maeda H.. Long-term pulmonary function after lobectomy for primary lung cancer. Asian Cardiovasc Thorac Ann 2005;13:311–5. [DOI] [PubMed] [Google Scholar]
- 5. Granone P, Cesario A, Margaritora S, Galetta D, Valente S, Corbo GM. et al. Morbidity after induction therapy and surgery in non small cell lung cancer (NSCLC). Focus on pulmonary function. Lung Cancer 2002;36:219–20. [DOI] [PubMed] [Google Scholar]
- 6. Perentes J, Bopp S, Krueger T, Gonzalez M, Jayet PY, Lovis A. et al. Impact of lung function changes after induction radiochemotherapy on resected T4 non-small cell lung cancer outcome. Ann Thorac Surg 2012;94:1815–22. [DOI] [PubMed] [Google Scholar]
- 7. Kaseda S, Aoki T, Hangai N, Shimizu K.. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann Thorac Surg 2000;70:1644–6. [DOI] [PubMed] [Google Scholar]
- 8. Nagahiro I, Andou A, Aoe M, Sano Y, Date H, Shimizu N.. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362–5. [DOI] [PubMed] [Google Scholar]
- 9. Nakata M, Saeki H, Yokoyama N, Kurita A, Takiyama W, Takashima S.. Pulmonary function after lobectomy: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2000;70:938–41. [DOI] [PubMed] [Google Scholar]
- 10. Bolliger CT, Jordan P, Solèr M, Stulz P, Tamm M, Wyser C. et al. Pulmonary function and exercise capacity after lung resection. Eur Respir J 1996;9:415–21. [DOI] [PubMed] [Google Scholar]
- 11. Brunelli A, Xiumé F, Refai M, Salati M, Marasco R, Sciarra V. et al. Evaluation of expiratory volume, diffusion capacity, and exercise tolerance following major lung resection: a prospective follow-up analysis. Chest 2007;131:141–7. [DOI] [PubMed] [Google Scholar]
- 12. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK. et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rueth NM, Parsons HM, Habermann EB, Groth SS, Virnig BA, Tuttle TM. et al. Surgical treatment of lung cancer: predicting postoperative morbidity in the elderly population. J Thorac Cardiovasc Surg 2012;143:1314–23. [DOI] [PubMed] [Google Scholar]
- 14. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohsawa M, Tsutani Y, Fujiwara M, Mimae T, Miyata Y, Okada M.. Predicting severe postoperative complication in patients with lung cancer and interstitial pneumonia. Ann Thorac Surg 2020;109:1054–60. [DOI] [PubMed] [Google Scholar]
- 16. Mimae T, Suzuki K, Tsuboi M, Nagai K, Ikeda N, Mitsudomi T. et al. Surgical outcomes of lung cancer in patients with combined pulmonary fibrosis and emphysema. Ann Surg Oncol 2015;22(Suppl 3):S1371–9. [DOI] [PubMed] [Google Scholar]
- 17. Mimae T, Suzuki K, Tsuboi M, Ikeda N, Takamochi K, Aokage K. et al. Severity of lung fibrosis affects early surgical outcomes of lung cancer among patients with combined pulmonary fibrosis and emphysema. Medicine (Baltimore) 2016;95:e4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jubran A, Laghi F, Mazur M, Parthasarathy S, Garrity ER Jr, Fahey PJ. et al. Partitioning of lung and chest-wall mechanics before and after lung-volume-reduction surgery. Am J Respir Crit Care Med 1998;158:306–10. [DOI] [PubMed] [Google Scholar]
- 19. Laghi F, Jubran A, Topeli A, Fahey PJ, Garrity ER Jr, de Pinto DJ. et al. Effect of lung volume reduction surgery on diaphragmatic neuromechanical coupling at 2 years. Chest 2004;125:2188–95. [DOI] [PubMed] [Google Scholar]
- 20. Sciurba FC, Rogers RM, Keenan RJ, Slivka WA, Gorcsan J 3rd, Ferson PF. et al. Improvement in pulmonary function and elastic recoil after lung-reduction surgery for diffuse emphysema. N Engl J Med 1996;334:1095–9. [DOI] [PubMed] [Google Scholar]
- 21. Mimae T, Miyata Y, Tsutani Y, Imai K, Ito H, Nakayama H. et al. Wedge resection as an alternative treatment for octogenarian and older patients with early-stage non-small-cell lung cancer. Jpn J Clin Oncol 2020;50:1051–7. [DOI] [PubMed] [Google Scholar]
- 22. Mimae T, Saji H, Nakamura H, Okumura N, Tsuchida M, Sonobe M. et al. Survival of octogenarians with early-stage non-small cell lung cancer is comparable between wedge resection and lobectomy/segmentectomy: JACS1303. Ann Surg Oncol 2021;28:7219–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.