Abstract
The regulation of iron storage is crucial to human health, because both excess and deficient iron storage have adverse consequences. Recent studies suggest altered iron storage in adults with obesity, with increased iron accumulation in their liver and skeletal muscle. Exercise training increases iron use for processes such as red blood cell production and can lower whole-body iron stores in humans. However, the effects of exercise training on liver and muscle iron stores in adults with obesity have not been assessed. The aim of this study was to determine the effects of 12 weeks of exercise training on whole-body iron stores, liver iron content and the abundance of ferritin (the key iron storage protein) in skeletal muscle in adults with obesity. Twenty-two inactive adults (11 women and 11 men; age, 31 ± 6 years; body mass index, 33 ± 3 kg/m2) completed 12 weeks (four sessions/week) of either moderate-intensity continuous training (MICT; 45 min at 70% of maximal heart rate; n = 11) or high-intensity interval training (HIIT; 10 × 1 min at 90% of maximal heart rate, interspersed with 1 min active recovery; n = 11). Whole-body iron stores were lower after training, as indicated by decreased plasma concentrations of ferritin (P = 3 × 10−5) and hepcidin (P = 0.02), without any change in C-reactive protein. Hepatic R2*, an index of liver iron content, was 6% lower after training (P = 0.06). Training reduced the skeletal muscle abundance of ferritin by 10% (P = 0.03), suggesting lower muscle iron storage. Interestingly, these adaptations were similar in MICT and HIIT groups. Our findings indicate that exercise training decreased iron storage in adults with obesity, which might have important implications for obese individuals with dysregulated iron homeostasis.
Keywords: exercise training adaptations, iron homeostasis, iron storage
1 |. INTRODUCTION
Iron is an essential micronutrient that must be regulated precisely at the systemic and tissue levels. Inadequate iron bioavailability can lead to the development of iron deficiency anaemia but, because iron is highly reactive, excess iron accumulation can result in oxidative damage (Puntarulo, 2005; Zimmermann & Hurrell, 2007). Accumulating evidence suggests that obesity is associated with dysregulated iron homeostasis, including elevated iron accumulation in several tissues, such as the liver and skeletal muscle (Aigner et al., 2014; Dongiovanni et al., 2011; Moreno-Navarrete et al., 2016). In turn, excess accumulation of iron in these tissues has been linked to oxidative stress and consequent cellular dysfunction (Dongiovanni et al., 2011; Liang et al., 2019; Moreno-Navarrete et al., 2016). Therefore, strategies to lower tissue iron content in obese adults with high iron stores might be warranted in an effort to alleviate excess iron-mediated oxidative stress. A large, sustained reduction in body weight (13% weight loss at 2-year follow-up) was found to lower markers of liver and skeletal muscle iron accumulation (Moreno-Navarrete et al., 2016). Unfortunately, however, many adults with obesity do not successfully achieve long-term weight loss of this magnitude, hence it is important to identify effective alternative strategies.
Exercise training is known to lower circulating ferritin concentration (a biomarker of whole-body iron stores) in healthy adults (Lyle et al., 1992; Shoemaker et al., 1996). This reduction in total body iron stores is driven, at least in part, by increased iron use attributable to augmented erythropoiesis (red blood cell production) that is known to occur with endurance training (Montero & Lundby, 2018). Recent evidence suggests that >80% of this iron used for exercise-mediated erythropoiesis is derived from tissue iron stores (Moretti et al., 2018). Importantly, however, the effects of training (and training intensity) on tissue-specific iron stores is largely unknown. Determining the influence of exercise training on iron storage in obesity might have important implications; for example, training-mediated reductions in tissue iron content might be a valuable adaptation to help alleviate excess iron-mediated oxidative stress in obese individuals with elevated tissue iron stores.
Therefore, the aims of this study were to determine the effects of 12 weeks of exercise training on whole-body iron stores, hepatic iron content and the abundance of ferritin in skeletal muscle in adults with obesity. We hypothesized that exercise training would have the following effects: (i) decrease markers of whole-body iron stores (plasma ferritin and hepcidin concentrations); (ii) reduce hepatic iron content (as assessed by hepatic R2* magnetic resonance imaging); and (iii) reduce skeletal muscle ferritin abundance. Given that many chronic adaptations to moderate-intensity continuous training (MICT) and high-intensity interval training (HIIT) are similar despite large differences in training intensity, exercise duration and energy expenditure (MacInnis & Gibala, 2017), we hypothesized that MICT and HIIT would have similar effects on iron storage.
2 |. METHODS
2.1 |. Ethical approval
This study addresses secondary objectives from a previously published parent study examining the metabolic effects of MICT versus HIIT in adults with obesity (Ryan et al., 2020b). The study was conducted according to the standards in the latest version of the Declaration of Helsinki, registered at clinical trials.gov (NCT02706093) and approved by the University of Michigan Institutional Review Board (HUM00106883). All participants provided written, informed consent.
2.2 |. Subjects
We studied 22 adults (11 women and 11 men) with obesity. All participants were physically inactive, weight stable for ≥6 months, non-smokers, not taking any medications/supplements known to influence their metabolism, and did not have any history of anaemia or cardiometabolic disease. Participants in the larger project who presented with relatively low baseline iron stores and/or haemoglobin concentration (plasma ferritin < 15 ng/ml and/or haemoglobin < 13.0 g/dl for men or < 12.0 g/dl for women) and those with a recent history of blood donation were excluded from the present study. All women were premenopausal and not pregnant or lactating; some were taking contraceptive medication. We did not control the timing of testing relative to menstrual phase.
The key findings presented in this paper (changes in iron-related outcomes in response to exercise training) are novel. Some of the overall study methods (e.g., exercise training protocols) and results describing the basic responses to training [body mass, peak oxygen uptake, plasma C-reactive protein (CRP) and plasma interleukin-6 (IL-6)] have been reported elsewhere (Ryan et al., 2020b) but are repeated here for convenience. Iron-related data collected before training were included in a cross-sectional report characterizing the relationship between plasma ferritin concentration and skeletal muscle iron proteins in a larger cohort of physically inactive adults with obesity (Ryan et al., 2020a).
2.3 |. Peak oxygen uptake
Peak oxygen uptake was determined before and after training using incremental cycling exercise, as described previously (Ryan et al., 2020b). Briefly, after 4 min at 40 W, the power output was increased by 20 W each minute until volitional fatigue. Oxygen uptake was measured using indirect calorimetery (Max II Physiodyne; AIE Technologies, Pittsburgh, PA, USA), and the highest 30 s average oxygen uptake before volitional fatigue was taken as peak oxygen uptake.
2.4 |. Exercise training protocols
Participants were assigned to either MICT or HIIT in a counter-balanced manner to optimize matching of sex, body weight, body mass index and peak oxygen uptake between groups. The distribution of men and women was similar in MICT (five men and six women) and HIIT (six men and five women). Participants in the MICT group completed four sessions per week involving 45 min at 70% of maximal heart rate. Participants in the HIIT group performed four sessions per week involving 10 × 1 min at 90% of maximal heart rate, with 1 min active recovery between intervals. Exercise was performed using stationary cycling, treadmill, elliptical or rowing ergometers. Training adherence was similarly high (~95%) in MICT and HIIT groups (Ryan et al., 2020b). To study the effects of exercise training independent of weight loss, participants were required to maintain their body weight. Participants were weighed several times each week, and if body mass began to deviate by 1–2% from baseline, our research study dietician provided counselling on adjustments to total caloric intake to facilitate maintenance of body weight near baseline (Ryan et al., 2020b). Participants were required to maintain their habitual dietary pattern throughout training and consulted regularly with the dietician to ensure compliance.
2.5 |. Experimental procedures
Participants were studied before (‘untrained’) and after 12 weeks of exercise training (‘trained’). The measurements in the trained conditions were completed 4 days after the final exercise session. All experiments were conducted in the morning, after an overnight fast. We collected a venous blood sample at ~08.00 h and skeletal muscle biopsy from the vastus lateralis at ~08.30 h. Muscle samples were immediately dissected free of any adipose or connective tissue, rinsed with saline, blotted dry, and flash frozen in liquid nitrogen before storage at −80°C.
2.6 |. Analytical methods
Plasma concentrations of ferritin (S-22; Ramco Laboratories, Stafford, TX, USA), hepcidin (ICE-004; Intrinsic Life Sciences, La Jolla, CA, USA), soluble transferrin receptor (TFC-94; Ramco Laboratories), high-sensitivity C-reactive protein (CR120C; Cal Biotech, El Cajon, CA, USA) and IL-6 (HS600C; R&D Systems, Minneapolis, MN, USA) were assessed using commercially available enzyme-linked immunosorbent assays.
Hepatic R2* (an index of liver iron content; Labranche et al., 2018) was determined using magnetic resonance imaging (Ingenia 3 T MR System; Phillips, The Netherlands) as described in detail elsewhere (Ryan et al. 2020a). Briefly, a multi-echo Dixon sequence was executed during a breath-hold lasting ≥16 s. A blinded investigator defined large regions of interest in three 5-mm-thick axial slices separated by 10 mm in the right lobe of the liver using 3D Slicer v.4.6.2 (https://www.slicer.org). Hepatic R2*, an index directly proportional to hepatic iron content, was calculated as 1/T2* (Labranche et al., 2018; Wood et al., 2005).
Skeletal muscle ferritin, transferrin receptor (TFRC) and myoglobin protein abundances were determined via immunoblotting. Muscle samples (~25 mg) were homogenized in ice-cold radio-immunoprecipitation buffer (#9806; Cell Signaling Technology, Danvers, MA, USA), rotated at 50 r.p.m. for 1 h at 4°C, and centrifuged at 15,000g for 15 min at 4°C. The supernatant was collected, and the protein concentration was measured using the bicinchoninic acid technique (#23225; Thermofisher, Waltham, MA, USA). Equal amounts of protein (15 μg for ferritin/transferrin receptor analysis and 3 μg for myoglobin analysis) were loaded onto gels, separated using SDS-PAGE and transferred onto nitrocellulose membranes. Membranes were stained with Memcode total protein stain (#24580; ThermoFisher) to confirm equal transfer of proteins. After removal of the Memcode stain, membranes were blocked for 2 h in 5% bovine serum albumin in Tris-buffered saline with 0.1% Tween 20 (TBS-T) and incubated overnight at 4°C with appropriate primary antibodies for ferritin (ab75973; RRID:AB_1310222; 1:1000; Abcam, Cambridge, MA, USA), transferrin receptor (ab84036; RRID:AB_10673794; 1:1000; Abcam) and myoglobin (#25919; RRID:AB_2798916; 1:2000; Cell Signaling Technology) diluted in 5% bovine serum albumin in TBS-T. This ferritin antibody recognizes both the heavy and light chains of ferritin. Membranes were then incubated for 90 min with the appropriate secondary antibody (#7074; Cell Signaling Technology; ferritin 1:1000; transferrin receptor 1:3000; myoglobin 1:20000) prepared in 5% bovine serum albumin in TBS-T, treated with enhanced chemiluminescence (#1705061; Bio-Rad, Hercules, CA, USA) and imaged. Protein abundance was normalized to the signal intensity from the Memcode total protein stain as a loading control (Moritz, 2017) and also to an internal standard sample (mixed skeletal muscle lysate from eight obese individuals) that was loaded on each gel in order to reduce gel-to-gel variability.
2.7 |. Statistical analysis
Data were checked for normality using the Kolmogorov–Smirnov test; non-normally distributed variables were log10-transformed before statistical analysis. The muscle protein data from one MICT participant were unavailable for analysis owing to inadequate tissue yield. There were no other occurrences of missing data. Linear mixed models were used to examine the main effects of training status (untrained vs. trained), group (MICT vs. HIIT) and the training status × group interaction. Values of P ≤ 0.05 were considered statistically significant, and trends were noted when 0.05 < P < 0.10. All statistical analyses were performed using SPSS (v.26; IBM, Armonk, NY, USA). Data are presented as mean values ± SD.
3 |. RESULTS
3.1 |. Aerobic capacity, systemic inflammation and haemoglobin concentration
Peak oxygen uptake increased by ~10% in both MICT and HIIT (P = 4 × 10−5), with no difference between groups (Table 1). As designed, body mass did not change with training, and there was also no change in plasma CRP or IL-6 concentrations in either group (Table 1), suggesting that the systemic inflammatory status was not affected by exercise training. Haemoglobin concentration was ~4% lower after training (P = 2 × 10−6), with no difference between MICT and HIIT groups (Table 1).
Table 1.
Subject characteristics before and after exercise training.
| MICT | HIIT | |||
|---|---|---|---|---|
| Age (years) | 30 ± 5 | 31 ± 7 | ||
| Sex | 5 Men, 6 Women | 6 Men, 5 Women | ||
| Race/Ethnicity (n) | ||||
| Non-Hispanic White | 10 | 9 | ||
| Non-Hispanic Black | 0 | 1 | ||
| Hispanic White | 0 | 1 | ||
| Native Hawaiian or other Pacific Islander | 1 | 0 | ||
| Untrained | Trained | Untrained | Trained | |
| Body Mass (kg) | 99.0 ± 13.0 | 98.8 ± 13.3 | 99.6 ± 16.1 | 99.5 ± 15.7 |
| Body Mass Index (kg/m2) | 34 ± 3 | 34 ± 3 | 33 ± 3 | 33 ± 3 |
| Peak Oxygen Uptake (L/min) | 2.5 ± 0.5 | 2.7 ± 0.5* | 2.6 ± 0.6 | 3.0 ± 0.6* |
| Plasma CRP (mg/dL) | 0.8 ± 1.0 | 1.3 ± 1.3 | 0.4 ± 0.3 | 0.4 ± 0.4 |
| Plasma IL-6 (pg/mL) | 2.1 ± 0.7 | 3.2 ± 1.4 | 5.2 ± 8.9 | 5.1 ± 7.8 |
| Hemoglobin Concentration (g/dL) | 13.9 ± 0.8 | 13.3 ± 1.0* | 14.1 ± 1.1 | 13.5 ± 1.2* |
Data are presented as mean ± SD.
Significant main effect of Training Status (Untrained vs. Trained; p<0.0001). There were no significant differences between groups at baseline, and no significant Training Status × Group interactions. CRP: C-Reactive Protein; IL-6: interleukin-6.
3.2 |. Plasma ferritin, hepcidin and soluble transferrin receptor concentrations
The plasma concentration of ferritin, a marker of whole-body iron stores, was lower after training (MICT: untrained, 90 ± 63 ng/ml vs. trained, 45 ± 31 ng/ml; HIIT: untrained, 100 ± 81 ng/ml vs. trained, 55 ± 36 ng/ml; P = 3 × 10−5), with no differences between MICT and HIIT groups (Figure 1a).
FIGURE 1.
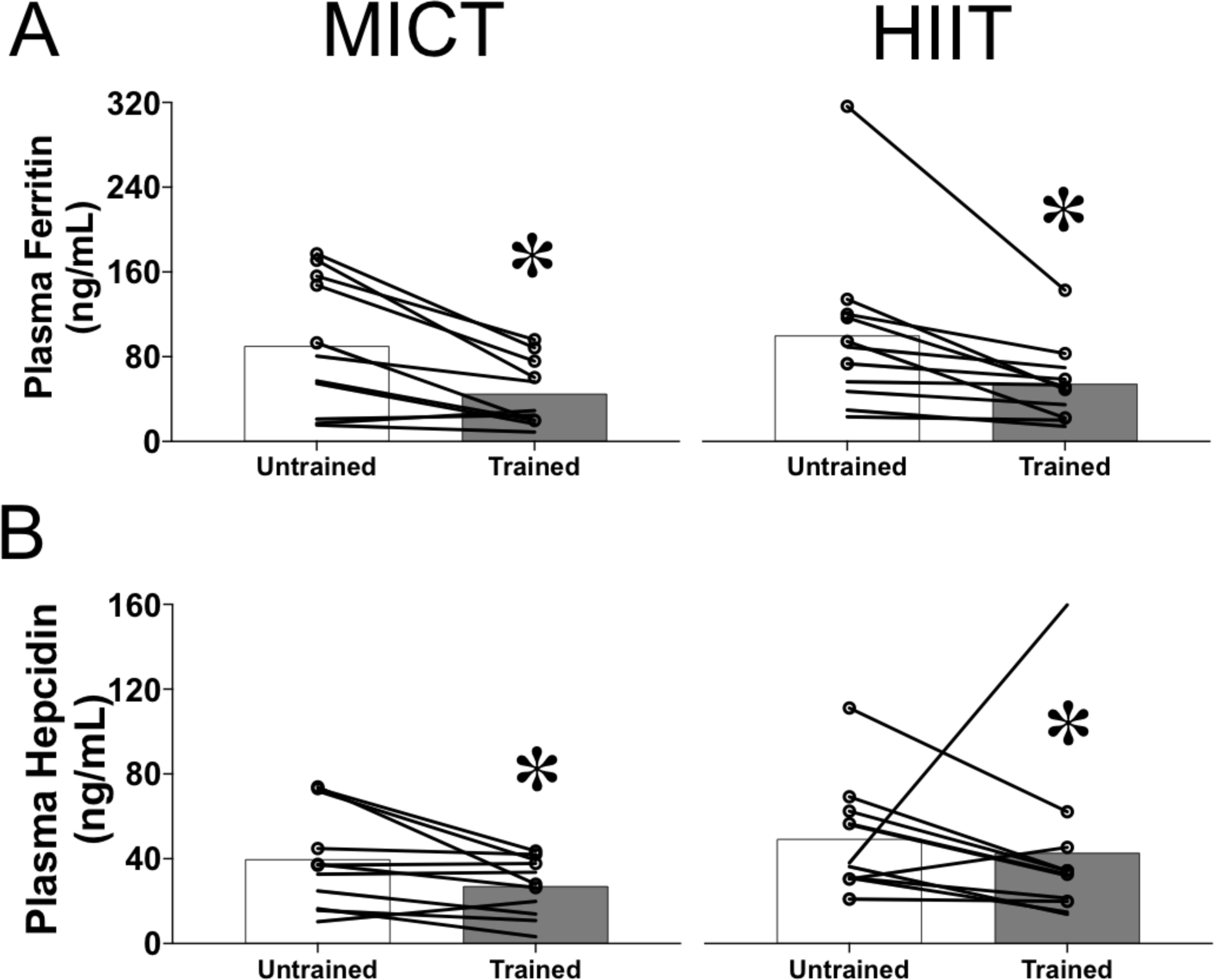
Changes in circulating markers of systemic iron homeostasis in response to exercise training. Plasma concentrations of ferritin (a) and hepcidin (b) in obese men and women before (untrained) and after (trained) 12 weeks of either moderate-intensity continuous training (MICT, n = 11, left panels) or high-intensity interval training (HIIT, n = 11, right panels). The group means are indicated by bars, with data for individual subjects connected by lines. Women are indicated by lines only, whereas men are indicated by lines and circular markers. *Significant main effect of training status (untrained vs. trained; P < 0.02)
The plasma concentration of hepcidin, the liver-secreted hormone described as a master regulator of iron homeostasis, was also significantly lower after training (MICT: untrained, 40 ± 24 ng/ml vs. trained, 27 ± 14 ng/ml; HIIT: untrained, 50 ± 26 ng/ml vs. trained, 43 ± 41 ng/ml; P = 0.02), with no differences between MICT and HIIT groups (Figure 1b). One HIIT participant had a large (three-fold) increase in hepcidin concentration after training, which was probably driven by an acute phase reaction, given that plasma IL-6 and CRP concentrations were also elevated after training in this participant. However, plasma ferritin concentration was still 14% lower after training in this participant, and the inclusion/exclusion of data from this participant’s did not alter our statistical findings for any of our outcomes.
Training did not alter the soluble transferrin receptor concentration in plasma (MICT: untrained, 2.6 ± 0.5 mg/l vs. trained, 2.7 ± 0.6 mg/l; HIIT: untrained, 2.8 ± 1.5 mg/l vs. trained, 2.7 ± 1.5 mg/l; P = 0.97), and there were no differences between MICT and HIIT groups.
3.3 |. Liver iron content
In parallel with the evidence for a reduction in whole-body iron stores from circulating biomarkers, we found a trend for hepatic R2* (index of liver iron content) to be lower after exercise training (MICT: untrained, 55.4 ± 12.4 Hz vs. trained, 49.7 ± 13.6 Hz; HIIT: untrained, 49.1 ± 10.2 Hz vs. trained, 48.7 ± 9.1 Hz; P = 0.06; Figure 2). There was a tendency for the reduction in hepatic R2* to be larger in the MICT compared with the HIIT group, but this did not reach statistical significance (training status × group interaction, P = 0.10).
FIGURE 2.
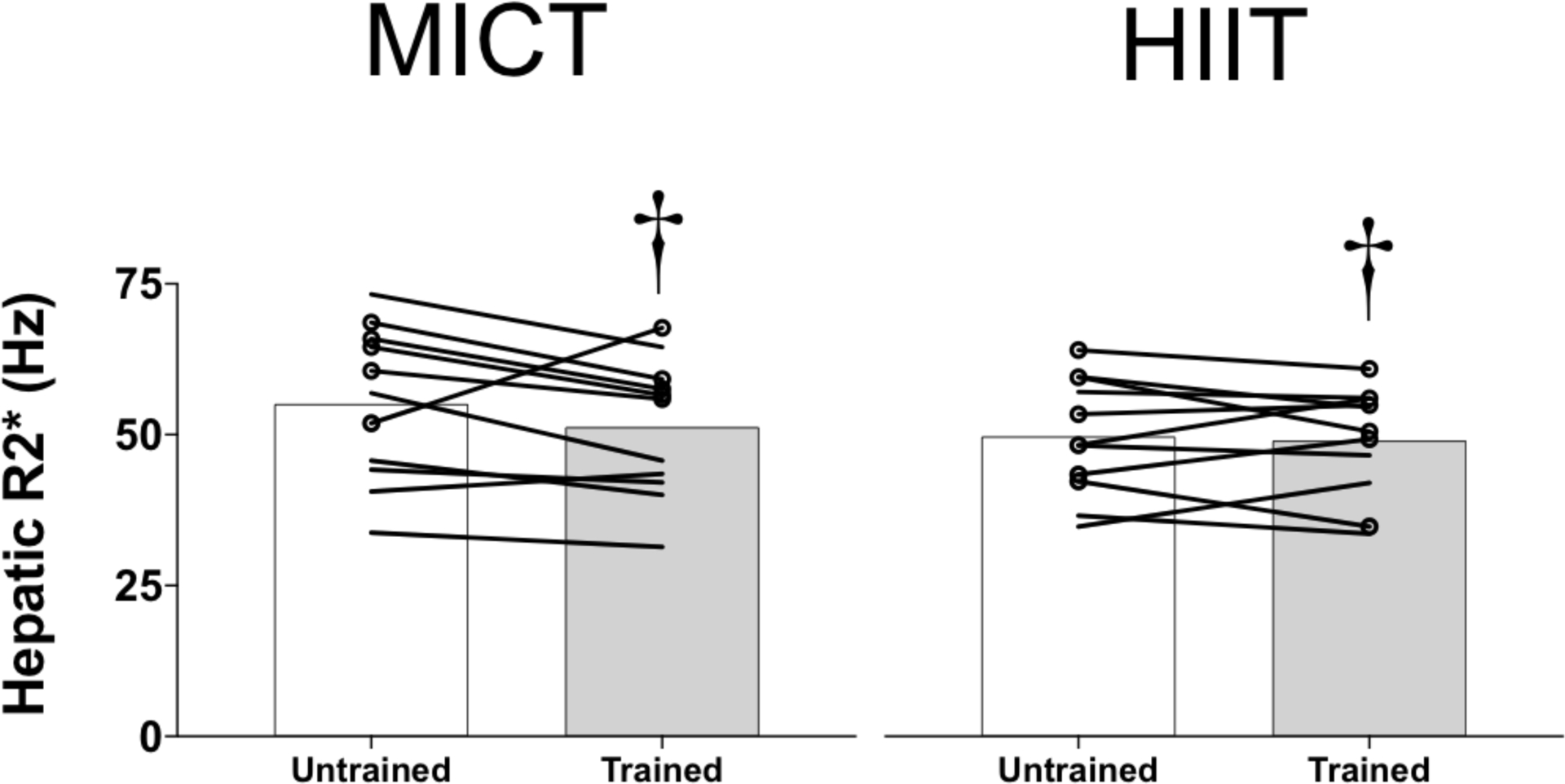
Changes in hepatic R2*, a marker of liver iron content, in obese men and women before (untrained) and after (trained) 12 weeks of either moderate-intensity continuous training (MICT, n = 11, left panels) or high-intensity interval training (HIIT, n = 11, right panels). The group means are indicated by bars, with data for individual subjects connected by lines. Women are indicated by lines only, whereas men are indicated by lines and circular markers. †P = 0.06 for the main effect of training status (untrained vs. trained)
3.4 |. Skeletal muscle ferritin, transferrin receptor and myoglobin protein abundance
The skeletal muscle protein abundance of ferritin, the key iron storage protein, was reduced by ~10% after exercise training (MICT: untrained, 2.8 ± 1.4 a.u. vs. trained, 2.6 ± 1.2 a.u.; HIIT: untrained, 1.8 ± 0.8 a.u. vs. trained, 1.5 ± 0.8 a.u.; P = 0.03; Figure 3a). There was a significant group effect for ferritin abundance, but this was attributable to an unanticipated difference between MICT and HIIT at baseline; there was no difference in the training-mediated reduction in muscle ferritin between MICT and HIIT groups (training status × group interaction, P = 0.32).
FIGURE 3.
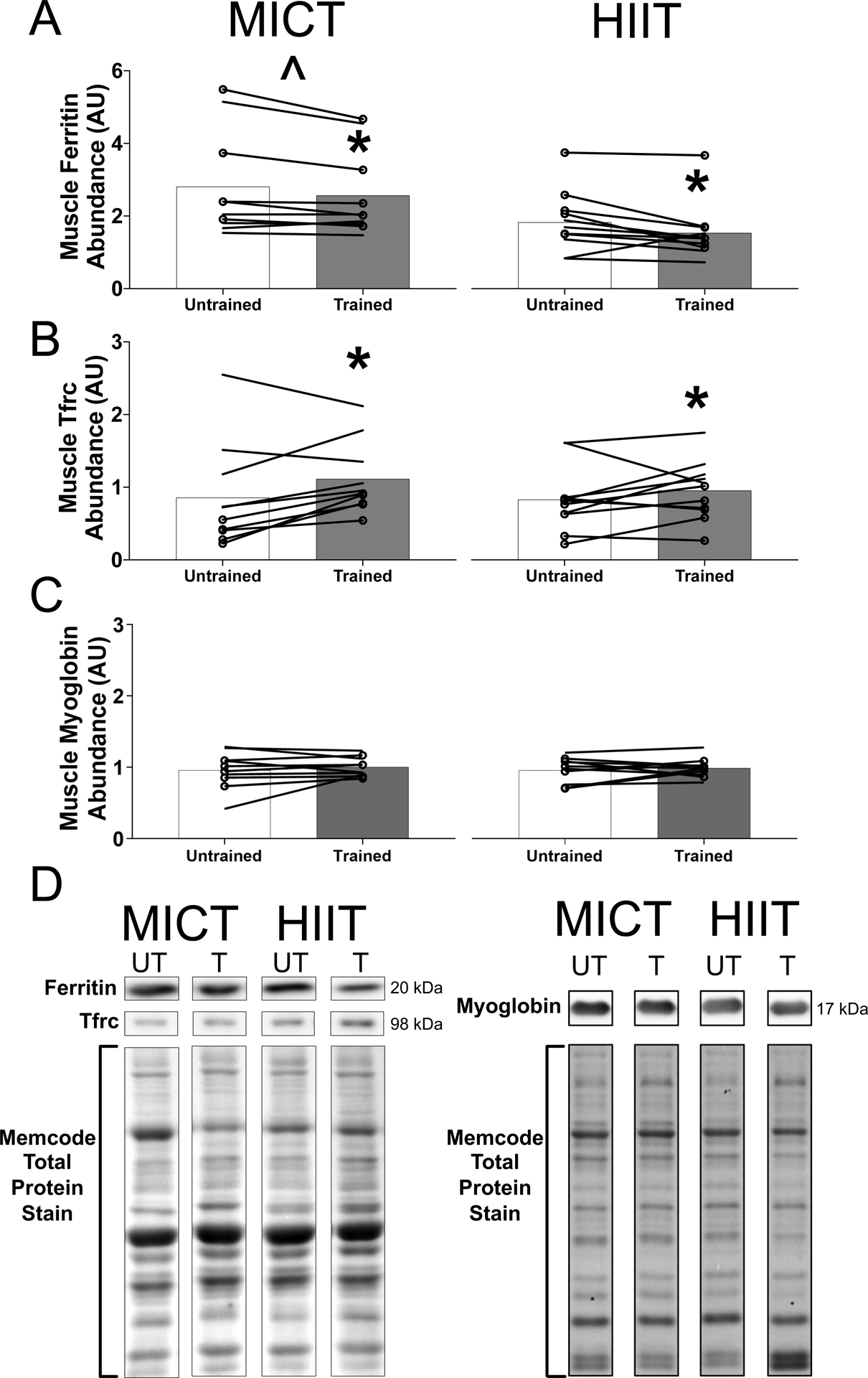
Skeletal muscle iron proteins in response to exercise training. (a–c) Skeletal muscle protein abundance of ferritin (a), transferrin receptor (TFRC; b) and myoglobin (c) in obese men and women before (untrained) and after (trained) 12 weeks of either moderate-intensity continuous training (MICT, n = 10, left panels) or high-intensity interval training (HIIT, n = 11, right panels). The group means are indicated by bars, with data for individual subjects connected by lines. Women are indicated by lines only, whereas men are indicated with lines and circular markers. (d) Representative western blot images from one MICT and one HIIT participant run on the same blots. The images have been cropped, as indicated by the black outline, for clarity. The Memcode total protein stain from these participants is shown below. Abbrevations: T, trained; UT, untrained. *Significant main effect of training status (untrained vs. trained; P < 0.03). ˆSignificant main effect of group (P = 0.02)
The skeletal muscle protein abundance of transferrin receptor, the key iron import protein, was ~20% greater after exercise training (MICT: untrained, 0.9 ± 0.7 a.u. vs. trained, 1.1 ± 0.5 a.u.; HIIT: untrained, 0.8 ± 0.7 a.u. vs. trained, 0.9 ± 0.4 a.u.; P = 0.004), with no difference between HIIT and MICT groups (Figure 3b).
Training did not significantly alter the abundance of myoglobin, the primary functional iron-containing protein in skeletal muscle (MICT: untrained, 1.0 ± 0.3 a.u. vs. trained, 1.0 ± 0.1 a.u.; HIIT: untrained, 1.0 ± 0.2 a.u. vs. trained, 1.0 ± 0.1 a.u.; P = 0.30; Figure 3c), and there was no difference between MICT and HIIT groups.
4 |. DISCUSSION
The principal new findings of this study were that exercise training lowered skeletal muscle ferritin and tended to decrease hepatic iron content in adults with obesity. These adaptations, which indicate reduced tissue iron storage, were similar in response to both of our exercise training programmes (MICT and HIIT). Given that obesity has been linked with elevated liver and muscle iron accumulation (Dongiovanni et al., 2011; Moreno-Navarrete et al., 2016), our findings suggest that exercise training might be a helpful countermeasure to lower tissue iron content in adults with obesity who have high iron stores.
Based on recent findings that >80% of the iron for training-mediated increases in erythropoiesis comes from tissue iron stores (Moretti et al., 2018), we propose that the reductions in liver and muscle iron stores we observed facilitated iron provision for increased erythropoiesis. The liver is traditionally viewed as the key site of iron storage in humans (Ganz, 2013). Adequate hepatic iron storage is essential to enable the mobilization of iron in response to increased cellular iron demands, but excessive hepatic iron storage results in oxidative stress that can contribute to adverse liver outcomes, including fibrosis (Anderson & Shah, 2013; Dongiovanni et al., 2011). Elevated hepatic iron content is commonly found in adults with obesity and metabolic syndrome, and therefore, a reduction in liver iron content might be a valuable adaptation to exercise training in these patients (Moreno-Navarrete et al., 2017). To our knowledge, our study is the first to make longitudinal assessments of hepatic iron content in response to exercise training in humans. Although the training-induced reduction in hepatic R2* did not reach statistical significance (P = 0.06), we interpret the strong trend as providing important preliminary support that exercise training might lower liver iron content.
In contrast to the liver, skeletal muscle is not traditionally viewed as an iron storage depot (Ganz, 2013). We recently reported that the abundance of ferritin in skeletal muscle is related tightly and independently to whole-body iron stores in adults with obesity, suggesting that alterations in whole-body iron stores might be accompanied by parallel alterations in muscle iron storage (Ryan et al., 2020a). Our present finding that muscle ferritin abundance decreased in parallel with whole-body iron stores after exercise training provides additional support that skeletal muscle functions as a meaningful iron store. Consistent with the training-mediated reduction in ferritin (the key iron storage protein) in muscle, the protein abundance of transferrin receptor (the key iron import protein) in muscle was increased by exercise training. Our novel findings that muscle ferritin decreased and muscle transferrin receptor increased in response to training might be explained based on the molecular control of these proteins by iron-regulatory proteins, which repress ferritin translation and stabilize transferrin receptor mRNA in low-iron conditions (Torti & Torti, 2002). Our finding that the abundance of myoglobin in skeletal muscle was unaffected by exercise training is in accordance with previous findings in humans (Jacobs et al., 1987; Svedenhag et al., 1983) and indicates that the reduction in skeletal muscle iron storage was not driven by myoglobin expansion. We previously reported that HIIT and MICT increased the abundance of mitochondrial respiratory proteins to a similar extent (Ryan et al., 2020b); several of the mitochondrial respiratory complexes contain functional iron, and it is likely that the decrease in muscle iron storage supports both local (i.e., skeletal muscle) and systemic iron homeostasis. Given that excess iron accumulation in skeletal muscle has been linked with increased oxidative stress and consequent cellular dysfunction (Liang et al., 2019; Reardon & Allen, 2009), training-mediated lowering of muscle iron storage might be beneficial for individuals with obesity and elevated muscle iron stores.
Our findings that HIIT and MICT lowered whole-body and tissue iron stores to a similar extent suggest comparable increases in iron use, which would be in accordance with a large body of literature indicating that many chronic adaptations to MICT and HIIT are remarkably similar despite large differences in training intensity, exercise duration and energy expenditure (MacInnis & Gibala, 2017). In order to compare two divergent but frequently undertaken training programmes, we did not match the energy expenditure or exercise duration between HIIT and MICT. Therefore, we did not isolate the influence of intensity per se on iron status, but our findings show that both training methods can reduce systemic and tissue iron stores in adults with obesity.
Although reductions in whole-body, liver and skeletal muscle iron stores might be beneficial for adults with obesity and relatively high iron stores, we also recognize that training-mediated reductions in iron stores might challenge iron homeostasis for those with low iron stores. Many adults with obesity have elevated iron stores, but the incidence of iron deficiency is also higher in obese vs. normal-weight populations (Aigner et al., 2014). For adults with obesity and iron deficiency, exercise training-mediated increases in iron use might place an added stress on already limited iron stores and could contribute to the development of iron deficiency anaemia. In particular, premenopausal women with obesity who engage in exercise training have several risk factors for the development of iron deficiency, and routine iron status monitoring might be warranted in this population to identify individuals who might need intervention (i.e., iron supplementation) to maintain adequate iron stores.
Although our study demonstrates important training-induced modifications to iron storage in adults with obesity, there are some potential limitations of our experimental design that warrant consideration. Plasma ferritin and hepcidin concentrations are widely used as biomarkers of iron status, but they can also be influenced by inflammation, and their interpretation is challenging when there are alterations in systemic inflammatory status (Worwood, 2007). Importantly, plasma CRP and IL-6 concentrations were not altered after 12 weeks of either MICT or HIIT, suggesting that the reductions in plasma ferritin and hepcidin we observed reflect lowering of whole-body iron stores and were not the result of reductions in systemic inflammation. We did not assess dietary iron intake throughout the 12 week training period, but participants were required (and were monitored) to maintain their habitual dietary intake pattern throughout the study. Therefore, we contend that a systematic reduction in iron intake is unlikely to explain the reduction in iron stores we observed.
Sex differences in systemic iron homeostasis are well established (Ganz & Nemeth, 2012), and we previously reported large sex differences in tissue iron storage in physically inactive adults with obesity (Ryan et al., 2020a). Although the distribution of men and women was closely matched between MICT and HIIT, we did not have a large enough sample size to determine potential training status × group × sex interactions. Notably, the training-mediated changes in iron storage in our study were qualitatively similar between men and women (see individual data presented in Figures 1–3). We did not control for the timing of testing relative to the menstrual phase, which might have added some variability in circulating markers of iron status. However, previous studies reported that iron status biomarkers remain relatively stable across the menstrual cycle (Belza et al., 2005; Puolakka, 1980), and the magnitude of the decrease in plasma concentrations of ferritin and hepcidin with training in our study dramatically exceeds the variation observed across the menstrual phase. Furthermore, our finding of a small, yet significant decrease in haemoglobin concentration is consistent with the plasma volume expansion commonly reported in response to training (Convertino, 1991), which, in turn, might contribute to reductions in the circulating concentrations of ferritin and hepcidin. Importantly, however, the large (40%) decrease in plasma ferritin in our participants cannot be driven by the small plasma volume expansion that would be associated with the 4% reduction in haemoglobin concentration we observed.
4.1 |. Conclusion
Our findings indicate that exercise training reduced whole-body, liver and skeletal muscle iron stores in adults with obesity and that these adaptations were similar regardless of whether the training was MICT or HIIT. Given that elevated whole-body and tissue iron accumulation is linked with oxidative damage, we suggest that exercise training might be a valuable countermeasure to reduce iron storage in obese individuals with relatively high iron stores.
New Findings.
What is the central question of this study?
Does exercise training modify tissue iron storage in adults with obesity?
What is the main finding and its importance?
Twelve weeks of moderate-intensity exercise or high-intensity interval training lowered whole-body iron stores, decreased the abundance of the key iron storage protein in skeletal muscle (ferritin) and tended to lower hepatic iron content. These findings show that exercise training can reduce tissue iron storage in adults with obesity and might have important implications for obese individuals with dysregulated iron homeostasis.
ACKNOWLEDGEMENTS
The authors thank the participants for their dedicated efforts and Suzette Howton for her contributions as study coordinator. This study was supported by the US National Institutes of Health (R01DK077966, P30DK089503, T32DK007245 and F32DK117522) and the Canadian Institutes of Health Research (338735 and 146190).
Footnotes
COMPETING INTERESTS
None declared.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aigner E, Feldman A, & Datz C (2014). Obesity as an emerging risk factor for iron deficiency. Nutrients, 6, 3587–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ER, & Shah YM (2013). Iron homeostasis in the liver. Comprehensive Physiology, 3, 315–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belza A, Henriksen M, Ersbøll AK, Thilsted SH, & Tetens I (2005). Day-to-day variation in iron-status measures in young iron-deplete women. British Journal of Nutrition, 94, 551–556. [DOI] [PubMed] [Google Scholar]
- Convertino VA (1991). Blood volume: Its adaptation to endurance training. Medicine and Science in Sports and Exercise, 23, 1338–1348. [PubMed] [Google Scholar]
- Dongiovanni P, Fracanzani AL, Fargion S, & Valenti L (2011). Iron in fatty liver and in the metabolic syndrome: A promising therapeutic target. Journal of Hepatology, 55, 920–932. [DOI] [PubMed] [Google Scholar]
- Ganz T (2013). Systemic iron homeostasis. Physiological Reviews, 93, 1721–1741. [DOI] [PubMed] [Google Scholar]
- Ganz T, & Nemeth E (2012). Hepcidin and iron homeostasis. Biochimica et Biophysica Acta, 1823, 1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs I, Esbjörnsson M, Sylvén C, Holm I, & Jansson E (1987). Sprint training effects on muscle myoglobin, enzymes, fiber types, and blood lactate. Medicine and Science in Sports and Exercise, 19, 368–374. [PubMed] [Google Scholar]
- Labranche R, Gilbert G, Cerny M, Vu KN, Soulières D, Olivié D, Billiard J-S, Yokoo T, & Tang A (2018). Liver iron quantification with MR imaging: A primer for radiologists. Radiographics, 38, 392–412. [DOI] [PubMed] [Google Scholar]
- Liang C, Mickey MC, Receno CN, Atalay M, & DeRuisseau KC (2019). Functional and biochemical responses of skeletal muscle following a moderate degree of systemic iron loading in mice. Journal of Applied Physiology, 126, 799–809. [DOI] [PubMed] [Google Scholar]
- Lyle RM, Weaver CM, Sedlock DA, Rajaram S, Martin B, & Melby CL (1992). Iron status in exercising women: The effect of oral iron therapy vs increased consumption of muscle foods. American Journal of Clinical Nutrition, 56, 1049–1055. [DOI] [PubMed] [Google Scholar]
- MacInnis MJ, & Gibala MJ (2017). Physiological adaptations to interval training and the role of exercise intensity. The Journal of Physiology, 595, 2915–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero D, & Lundby C (2018). Regulation of red blood cell volume with exercise training. Comprehensive Physiology, 9, 149–164. [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Blasco G, Xifra G, Karczewska-Kupczewska M, Stefanowicz M, Matulewicz N, Puig J, Ortega F, Ricart W, Straczkowski M, & Fernández-Real JM (2016). Obesity is associated with gene expression and imaging markers of iron accumulation in skeletal muscle. Journal of Clinical Endocrinology and Metabolism, 101, 1282–1289. [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Moreno M, Puig J, Blasco G, Ortega F, Xifra G, Ricart W, & Fernández-Real JM (2017). Hepatic iron content is independently associated with serum hepcidin levels in subjects with obesity. Clinical Nutrition, 36, 1434–1439. [DOI] [PubMed] [Google Scholar]
- Moretti D, Mettler S, Zeder C, Lundby C, Geurts-Moetspot A, Monnard A, Swinkels DW, Brittenham GM, & Zimmermann MB (2018). An intensified training schedule in recreational male runners is associated with increases in erythropoiesis and inflammation and a net reduction in plasma hepcidin. American Journal of Clinical Nutrition, 108, 1324–1333. [DOI] [PubMed] [Google Scholar]
- Moritz CP (2017). Tubulin or not tubulin: Heading toward total protein staining as loading control in western blots. Proteomics, 17, 1600189. [DOI] [PubMed] [Google Scholar]
- Puntarulo S (2005). Iron, oxidative stress and human health. Molecular Aspects of Medicine, 26, 299–312. [DOI] [PubMed] [Google Scholar]
- Puolakka J (1980). Serum ferritin in the evaluation of iron status in young healthy women. Acta Obstetricia et Gynecologica Scandinavica, 59(sup95), 35–41. [DOI] [PubMed] [Google Scholar]
- Reardon TF, & Allen DG (2009). Iron injections in mice increase skeletal muscle iron content, induce oxidative stress and reduce exercise performance. Experimental Physiology, 94, 720–730. [DOI] [PubMed] [Google Scholar]
- Ryan BJ, Foug KL, Gioscia-Ryan RA, Ludzki AC, Ahn C, Schleh MW, Gillen JB, Chenevert TL, & Horowitz JF (2020a). Skeletal muscle ferritin abundance is tightly related to plasma ferritin concentration in adults with obesity. Experimental Physiology, 105, 1808–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BJ, Schleh MW, Ahn C, Ludzki AC, Gillen JB, Varshney P, Van Pelt DW, Pitchford LM, Chenevert TL, Gioscia-Ryan RA, Howton SM, Rode T, Hummel SL, Burant CF, & Horowitz JF (2020b). Moderate-intensity exercise and high-intensity interval training affect insulin sensitivity similarly in obese adults. Journal of Clinical Endocrinology and Metabolism, 105, e2941–e2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JK, Green HJ, Coates J, Ali M, & Grant S (1996). Failure of prolonged exercise training to increase red cell mass in humans. American Journal of Physiology-Heart and Circulatory Physiology, 270, H121–H126. [DOI] [PubMed] [Google Scholar]
- Svedenhag J, Henriksson J, & Sylvén C (1983). Dissociation of training effects on skeletal muscle mitochondrial enzymes and myoglobin in man. Acta Physiologica Scandinavica, 117, 213–218. [DOI] [PubMed] [Google Scholar]
- Torti FM, & Torti SV (2002). Regulation of ferritin genes and protein. Blood, 99, 3505–3516. [DOI] [PubMed] [Google Scholar]
- Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, & Coates TD (2005). MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood, 106, 1460–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worwood M (2007). Indicators of iron status of populations: ferritin. In: Assessing the iron status of populations. (2nd ed., pp. 35–73). World Health Organization. [Google Scholar]
- Zimmermann MB, & Hurrell RF (2007). Nutritional iron deficiency. Lancet, 370(9586), 511–520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


