Abstract
Suppression of endotoxin release and subsequent production of inflammatory cytokines is crucial in the treatment of septic shock. We investigated the effect of clindamycin (CLI) on endotoxic shock induced in mice by Escherichia coli lipopolysaccharide (LPS). Mice were treated with CLI (160 to 600 mg/kg) or saline and then injected with E. coli LPS and d-(+)-galactosamine intraperitoneally 0.5 h after CLI administration. Pretreatment with CLI significantly improved survival in a dose-dependent manner (CLI, at 160, 300, and 440 mg/kg) and significantly lowered the peak concentrations of tumor necrosis factor alpha and interleukin-1β (IL-1β) in serum. However, the peak concentrations of IL-6 in the sera of CLI-treated mice were higher than in control mice. Our findings suggest that CLI alters LPS-induced inflammatory cytokine production and suppresses endotoxin-induced mortality in this murine model.
Septicemia is frequently associated with serious complications, such as disseminated intravascular coagulation and multiple organ failure, and the mortality of patients with septicemia remains high despite recent advances in treatment with antibacterial agents. It has been reported that about 400,000 patients per year suffer from septicemia in the United States and that about 25% of these patients ultimately die of septic shock. Of patients with positive blood cultures, about half showed growth of gram-negative bacilli (11).
Septic shock induced by gram-negative bacilli has been reported to result from overproduction or excessive release of inflammatory cytokines (e.g., tumor necrosis factor [TNF] and interleukin-1 [IL-1]) from immunocytes such as monocytes and macrophages, i.e., cells immunologically activated by endotoxin, which is a structural component (lipopolysaccharide [LPS]) of the cell wall of gram-negative bacilli (20). Shock is induced through a complex cytokine cascade triggered by these cytokines (3, 11). Many attempts have been made to suppress the actions of these inflammatory cytokines (8, 14). However, since the development of septic shock is due to complex interactions, no adequate results have yet been obtained in clinical practice.
Studies have also been conducted using antibacterial agents which have an anticytokine action (1, 10). Quinolones and macrolides have been reported to suppress the production of IL-1 or TNF from LPS-stimulated human monocytes (5, 12). Furthermore, the administration of fosfomycin (FOF) led to a reduction in serum TNF alpha (TNF-α) and IL-1β concentrations in LPS-stimulated mice (9). Clindamycin (CLI), which is effective against gram-positive and anaerobic bacteria, has been demonstrated to induce neutrophil phagocytosis in the host at subMIC levels (18, 19). We have previously reported that CLI significantly suppressed TNF-α release from purified LPS-stimulated human acute monocytic leukemic cell line (THP-1) in vitro (7). In the present study, we extended these early findings by conducting a study in mice, on the assumption that CLI would also suppress inflammatory cytokine release in vivo.
Effect of CLI administration time on survival of mice with endotoxic shock.
An endotoxic shock mouse model using d-(+)-galactosamine (GalN) and purified LPS (derived from Escherichia coli O55:B5; Sigma Chemical Co. St. Louis, Mo.) was used according to the modified method of Galanos et al. (4). Briefly, GalN and purified LPS were diluted with sterilized physiological saline and then 720 mg of GalN solution and 40 μg of purified LPS solution per kg were each administered intraperitoneally to fasted, 10-week-old, specific-pathogen-free, male C3H/HeN mice (Charles River Japan, Inc., Kanagawa, Japan) (6). The survival rates of mice treated intraperitoneally with 440 mg of CLI (Pharmacia & Upjohn, Tokyo, Japan), which is commercially available as Dalacin S Injectable, per kg at various times before and after the administration of GalN and LPS are shown in Table 1. Mice were observed for up to 3 weeks after GalN and LPS administration to document the survival rate. All saline-treated control mice died within 12 h of GalN and LPS injection. In contrast, none of the mice treated with CLI at 0.5 h before administration of GalN and LPS died. Furthermore, the survival rates of mice treated with CLI at 2 or 6 h before administration of GalN and LPS were significantly improved (78.6 and 85.7%, respectively) compared to the control group (P < 0.01). On the other hand, the survival rates of the groups treated with CLI at 0.5 and 1 h after administration of GalN and LPS were significantly decreased (28.6 and 21.4%, respectively; P < 0.01). All mice in the group treated with CLI at 2 h after administration of GalN and LPS died.
TABLE 1.
Survival rate of mice treated with CLI (440 mg/kg) before and after GalN+LPS administration in the endotoxin shock murine model
| Time (h) of injection of CLI after GalN+LPS administrationa | No. of survivors/ total no. of mice | Survival rateb (%) |
|---|---|---|
| −24 | 2/14 | 14.3 |
| −6 | 12/14 | 85.7c |
| −2 | 11/14 | 78.6c |
| −0.5 | 14/14 | 100.0c |
| 0 | 11/14 | 78.6c |
| 0.5 | 4/14 | 28.6d |
| 1 | 3/14 | 21.4 |
| 2 | 0/14 | 0 |
| Control | 0/21 | 0 |
A minus sign indicates preinjection of CLI. Control, control group mice were treated with saline only.
Survival was observed at 3 weeks after GalN+LPS administration.
P < 0.01 versus the control (statistical significance as determined by Fisher's exact test).
P < 0.05 versus the control (statistical significance as determined by Fisher's exact test).
Effect of CLI dosage on survival of mice with endotoxic shock.
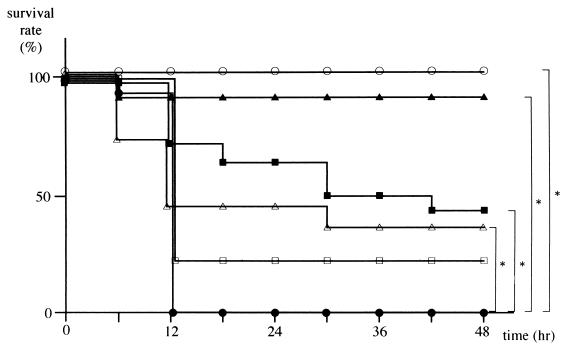
The survival rates of fasted mice treated with CLI (160, 300, 440, 520, or 600 mg/kg) and the control group injected with GalN and LPS at 0.5 h after administration of CLI or saline are shown in Fig. 1. Mice in the control group began to die about 6 h after administration of GalN and LPS, and all mice were dead within 12 h. In contrast, the survival rates in the CLI pretreated groups tended to improve in a dose-dependent fashion, with 100% survival in the group pretreated with a 440-mg/kg dose of CLI. However, survival rates in groups treated with 520- and 600-mg/kg doses of CLI decreased to 92 and 36%, respectively.
FIG. 1.
Effect of CLI on survival rate after LPS and GalN administration. Mice were treated with 160 (□, n = 14), 300 (■, n = 14), 440 (○, n = 14), 520 (▴, n = 12), or 600 (▵, n = 11) mg of CLI per kg or with saline alone (●, n = 21) at 0.5 h before GalN and LPS challenge. Survival rates were scored at 48 h after the GalN+ LPS challenge, and the statistical significance was determined by the Fisher's exact test (∗, P < 0.01 versus the control). No further mortality was observed between day 2 and day 21 of followup.
Effects of CLI pretreatment on cytokine concentrations in serum.
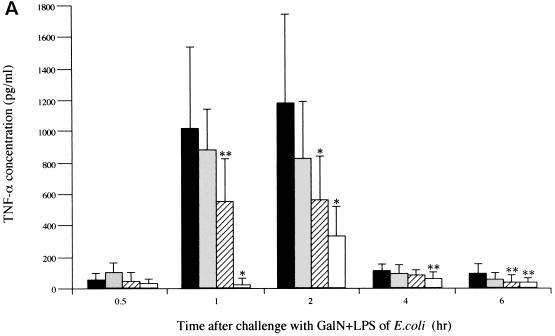
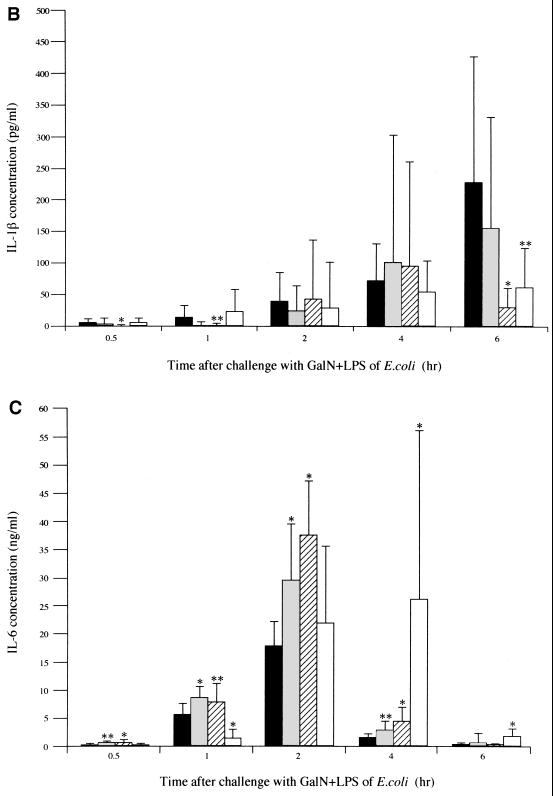
To elucidate the mechanism of improved survival following CLI treatment, we determined the concentrations of cytokines over time in serum. Mice in the CLI-treated groups (CLI at 160, 300, and 440 mg/kg) and the saline-treated control group were used for serum sampling. GalN and LPS solutions were administered intraperitoneally to the fasted mice at 0.5 h after pretreatment with CLI or saline. Mice were then sacrificed at 0.5, 1, 2, 4, and 6 h, and cardiac blood samples were immediately obtained. The blood sample was immediately centrifuged, and serum was stored at −20°C until measurement of cytokine concentrations. Concentrations of TNF-α, IL-1β, and IL-6 in serum were determined by using an enzme-linked immunosorbent assay (ELISA) kit (Cytoscreen; BioSource International, Camarillo, Calif.). Concentrations were determined for two wells in each sample. The TNF-α concentration in serum began to increase 1 h after the administration of GalN and LPS, reached a peak at 2 h (1,183 ± 578 pg/ml), and declined thereafter in the saline-pretreated control group (Fig. 2A). TNF-α concentrations in serum decreased in a dose-dependent manner in all groups pretreated with CLI. Treatment with CLI (300 or 440 mg/kg) reduced TNF-α concentrations in serum to 562 ± 281 pg/ml (P < 0.01 versus the control) and 339 ± 197 pg/ml (P < 0.01 versus the control) at 2 h after administration of GalN and LPS, respectively. These concentrations were significantly lower than those in the control group. In the control group, IL-1β concentrations in serum increased with time following administration of GalN and LPS and reached a peak (228 ± 200 pg/ml) 6 h after administration (Fig. 2B). The IL-1β concentrations in serum at 6 h after the administration of GalN and LPS in mice treated with 300 and 440 mg of CLI per kg were 31 ± 37 and 62 ± 63 pg/ml, respectively, significantly lower than in the control group (P < 0.01 and P < 0.05, respectively). The IL-6 concentration in serum began to rise from 0.5 h after administration of GalN and LPS and reached a peak at 2 h in the control group (Fig. 2C). IL-6 concentrations in serum for groups treated with CLI at 160 and 300 mg/kg reached peak levels at 29.6 ± 10.0 and 37.4 ± 9.8 ng/ml, respectively, at 2 h after the administration of GalN and LPS. These levels were significantly higher than in the control group (P < 0.01 for both groups). The IL-6 concentration in serum in mice treated with a 440-mg/kg dose of CLI reached a peak (26.2 ± 28.9 ng/ml) at 4 h after administration of GalN and LPS, a value significantly higher than that noted in the control group (P < 0.01).
FIG. 2.
Effect of CLI on serum concentration of TNF-α (A), IL-1β (B), or IL-6 (C) in mice. Mice were treated with 160 ( ), 300 (
), 300 ( ), or 440 (□) mg of CLI per kg or with saline alone (■) at 0.5 h before GalN and LPS challenge. TNF-α, IL-1β, and IL-6 concentrations in serum were determined by ELISA at 0.5, 1, 2, 4, and 6 h after GalN and LPS challenge. Each bar represents the mean ± the standard deviation of 12 mice. Significant differences in cytokines concentrations between groups were assessed by using Student's t test (∗, P < 0.01; ∗∗, P < 0.05 versus the control).
), or 440 (□) mg of CLI per kg or with saline alone (■) at 0.5 h before GalN and LPS challenge. TNF-α, IL-1β, and IL-6 concentrations in serum were determined by ELISA at 0.5, 1, 2, 4, and 6 h after GalN and LPS challenge. Each bar represents the mean ± the standard deviation of 12 mice. Significant differences in cytokines concentrations between groups were assessed by using Student's t test (∗, P < 0.01; ∗∗, P < 0.05 versus the control).
In the present study, pretreatment with an intraperitoneal injection of 440 mg of CLI per kg at 0.5 h before challenge with GalN and LPS resulted in an improvement of survival rate to 100%. However, administration of CLI after GalN and LPS challenge resulted in only partial improvement of survival. These findings suggest that it took about 0.5 h to exert CLI effect on cells that release cytokines, such as monocytes. At doses of CLI higher than 440 mg/kg, survival rates tended to be lower. Since the 50% lethal dose of CLI following intraperitoneal administration in mice has been reported to be 997 mg/kg (17), CLI toxicity was suspected to be responsible for this finding.
The results of TNF-α were in concordance with the direct effect of CLI on THP-1 cells that was observed in our previous in vitro study (7) and also with a report by Stevens et al. (15) that CLI suppressed the release of TNF-α from LPS-stimulated human peripheral blood mononuclear cells. It has been assumed that when elevated by LPS stimulation, inflammatory cytokines such as TNF-α and IL-1β serve as mediators of endotoxic death (3, 11). In the endotoxic shock model used in the present study, CLI-mediated reductions in TNF-α and IL-1β concentrations were considered to contribute to the improved survival of mice.
IL-6, a pleiotropic cytokine involved in immunological reaction regulation and hemopoiesis in the acute phase, has been demonstrated to suppress LPS-stimulated production of TNF-α and IL-1β in cultured human monocytes and in mice (13, 16). Barton et al. (2) ascertained that IL-6 increased the survival rate of mice in an LPS-GalN endotoxic shock mouse model. In the present study, the administration of CLI resulted in a rise in IL-6 concentrations and a fall in TNF-α and IL-1β concentrations in serum. We speculate that the elevated IL-6 concentration in serum suppressed inflammatory cytokine release, thereby contributing to improved survival rates, although no detailed mechanism of CLI action on IL-6 has yet been elucidated.
Morikawa et al. (10) demonstrated that treatment with FOF in vitro resulted in the suppression of TNF and IL-1 release from LPS-stimulated human monocytes but an increase in IL-6 release. Furthermore, Matsumoto et al. (9) reported that administration of FOF to LPS-stimulated mice resulted in reductions in TNF-α and IL-1β concentrations in serum and slight increases in IL-6 and IFN-γ levels in serum. These results with FOF resembled our experimental results following the administration of CLI.
Our present study in the endotoxic shock mouse model suggested that CLI suppressed the release of inflammatory cytokines, resulting in a dose-dependent reduction in mortality. The dose of CLI used in the present study was set at a high level, approximately 10 to 20 times the dose suitable for clinical administration to humans. Hence, although immediate clinical application is not feasible, our results suggest that CLI may be a useful antibacterial agent in prevention of septic shock induced by gram-negative bacilli. Further studies are necessary to clarify the mechanism by which CLI exerts its effects.
Acknowledgments
We thank F. G. Issa (Word-Medex, Sydney, Australia) for the careful reading and editing of the manuscript.
REFERENCES
- 1.Baldwin G, Alpert G, Caputo G L, Baskin M, Parsonnet J, Gillis Z A, Thompson C, Siber G R, Fleisher G R. Effect of polymyxin B on experimental shock from meningococcal and Escherichia coli endotoxins. J Infect Dis. 1991;164:542–549. doi: 10.1093/infdis/164.3.542. [DOI] [PubMed] [Google Scholar]
- 2.Barton B E, Jackson J V. Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun. 1993;61:1496–1499. doi: 10.1128/iai.61.4.1496-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 4.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iino Y, Toriyama M, Kudo K, Natori Y, Yuo A. Erythromycin inhibition of lipopolysaccharide-stimulated tumor necrosis factor alpha production by human monocytes in vitro. Ann Otol Rhinol Laryngol. 1992;101:16–20. doi: 10.1177/0003489492101s1005. [DOI] [PubMed] [Google Scholar]
- 6.Kirikae T, Kirikae F, Saito S, Tominaga K, Tamura H, Uemura Y, Yokochi T, Nakano M. Biological characterization of endotoxins released from antibiotic-treated Pseudomonas aeruginosa and Escherichia coli. Antimicrob Agents Chemother. 1998;42:1015–1021. doi: 10.1128/aac.42.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kishi K, Hirai K, Hiramatsu K, Yamasaki T, Nasu M. Clindamycin suppresses endotoxin released by ceftazidime-treated Escherichia coli O55:B5 and subsequent production of tumor necrosis factor alpha and interleukin-1β. Antimicrob Agents Chemother. 1999;43:616–622. doi: 10.1128/aac.43.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesslauer W, Tabuchi H, Gentz R, Brockhaus M, Schlaeger E J, Grau G, Piguet P F, Pointaire P, Vassalli P, Loetscher H. Recombinant soluble tumor necrosis factor receptor proteins protect mice from lipopolysaccharide-induced lethality. Eur J Immunol. 1991;21:2883–2886. doi: 10.1002/eji.1830211134. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto T, Tateda K, Miyazaki S, Furuya N, Ohno A, Ishii Y, Hirakata Y, Yamaguchi K. Fosfomycin alters lipopolysaccharide-induced inflammatory cytokine production in mice. Antimicrob Agents Chemother. 1999;43:697–698. doi: 10.1128/aac.43.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morikawa K, Watabe H, Araake M, Morikawa S. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother. 1996;40:1366–1370. doi: 10.1128/aac.40.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parrillo J E. Shock syndromes related to sepsis. In: Bennett J C, Plum F, editors. Cecil textbook of medicine. 20th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1996. pp. 496–501. [Google Scholar]
- 12.Roche Y, Gougerot-Pocidalo M A, Fay M, Etienne D, Forest N, Pocidalo J J. Comparative effects of quinolones on human mononuclear leucocyte functions. J Antimicrob Chemother. 1987;19:781–790. doi: 10.1093/jac/19.6.781. [DOI] [PubMed] [Google Scholar]
- 13.Schindler R, Mancilla J, Endres S, Ghorbani R, Clark S C, Dinarello C A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75:40–47. [PubMed] [Google Scholar]
- 14.Smith A L. Treatment of septic shock with immunotherapy. Pharmacotherapy. 1998;18:565–580. [PubMed] [Google Scholar]
- 15.Stevens D L, Bryant A E, Hackett S P. Antibiotic effects on bacterial viability, toxin production, and host response. Clin Infect Dis. 1995;20(Suppl. 2):S154–S157. doi: 10.1093/clinids/20.supplement_2.s154. [DOI] [PubMed] [Google Scholar]
- 16.Tilg H, Trehu E, Atkins M B, Dinarello C A, Mier J W. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- 17.Toida S, Matsuura S, Sasaki T, Tanihata T, Hidano T, Kudo K. Acute toxicity of clindamycin-2-phosphate. J Med Soc Toho Japan. 1971;18:354–357. [Google Scholar]
- 18.Veringa E M, Lambe D W, Jr, Ferguson D A, Jr, Verhoef J. Enhancement of opsonophagocytosis of Bacteroides spp. by clindamycin in subinhibitory concentrations. J Antimicrob Chemother. 1989;23:577–587. doi: 10.1093/jac/23.4.577. [DOI] [PubMed] [Google Scholar]
- 19.Veringa E M, Verhoef J. Clindamycin at subinhibitory concentrations enhances antibody- and complement-dependent phagocytosis by human polymorphonuclear leukocytes of Staphylococcus aureus. Chemotherapy. 1987;33:243–249. doi: 10.1159/000238502. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Peterson J W, Niesel D W, Klimpel G R. Bacterial lipoprotein and lipopolysaccharide act synergistically to induce lethal shock and proinflammatory cytokine production. J Immunol. 1997;159:4868–4878. [PubMed] [Google Scholar]