Abstract
Ginkgo biloba extract (GBE) contains Ginkgo biloba flavonoids, which are phenolic compounds. These compounds can be introduced into films for their functional properties (such as their antioxidant and antibacterial property), allowing this film to be used as food packaging. Thus, the aim of this study was to introduce the GBE into a gelatin solution to prepare gelatin films and evaluate the influence of the natural extract addition on the physicochemical and antimicrobial properties. The gelatin films were successfully prepared by casting technique, and GBE was incorporated at concentrations of 0, 0.5, 1.5, 2.5, 3, 4, and 5 g/100 g of gelatin. The mechanical properties, film solubility, moisture content, water vapor permeability, infrared spectroscopic characteristics, film microstructure, light barrier property, antioxidant property and antibacterial property of the films were investigated. The incorporation of gelatin films with GBE increased the UV-visible shielding performance of films. The antioxidant ability of the film was improved, which was supposed to be related to the active substances of the GBE. The GBE also exhibited potential antimicrobial activity against tested microorganisms. With the increase in the GBE concentration incorporated into the films, the antimicrobial activity of the gelatin film with GBE was also enhanced.
The incorporation of gelatin films with GBE increased the UV-visible shielding performance of films.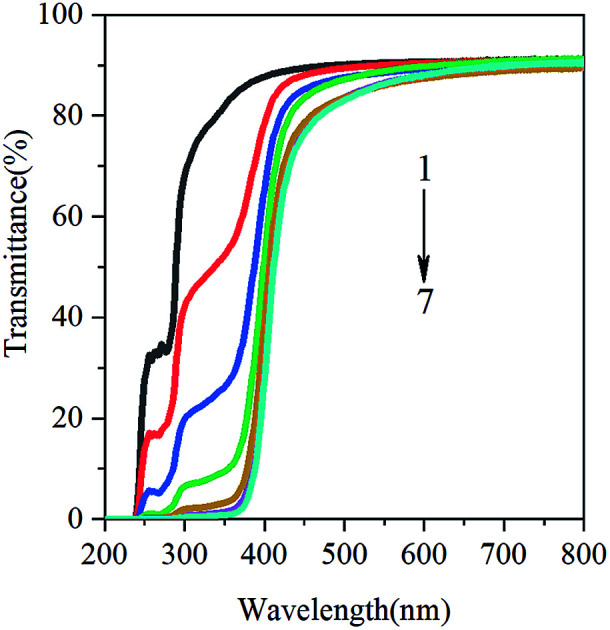
1. Introduction
The usage of biodegradable and sustainable packaging has attracted attention since the past few decades as an effort to reduce packaging waste.1 Accordingly, bio-based active packaging produced with biopolymers from renewable sources has gained much attention. Gelatin is the common material used to prepare the edible protein film, owing to its eco-friendly nature and film-forming properties.2 Food packaging is essential to preserve the food quality and extend the shelf life of perishable agricultural products that are susceptible to oxidative and microbiological deterioration.3 Gelatin possesses excellent film forming and barrier properties against oxygen and light. Recent research on edible films has reported the incorporation of antioxidant and antibacterial compounds to improve the biological activity of films. The field of antioxidant and antimicrobial research is more inclined to develop natural extracts.4 Owing to their rich source and safety, plant extracts are considered as substitutes for traditional chemical reagents. Yu et al. found that active films containing releasable caffeic acid showed 20-fold increase in antioxidant activity and 6-fold increase in antibacterial activity as compared to those of the control film. Besides, the releasable caffeic acid can be continuously released from the films, and the lipid oxidation of the menhaden oil in water emulsion is significantly inhibited.5 Jouki et al. found that quince seed mucilage films containing thyme essential oil increased the percent elongation of films. Moreover, the additivated films presented good antioxidant activity and significant antibacterial activity.6
Ginkgo biloba is the oldest living relict plant with both ecological and medicinal value .7Ginkgo biloba extract (GBE) is obtained by refluxing ginkgo leaves in ethanol. The main chemical components of ginkgo leaves can be divided into two types: flavonoids and terpene lactones. Flavonoids can be divided into three types: flavones, diflavones, and catechins, which are mainly in the form of glucosides. Terpenoid lactones include sesquiterpene lactones, diterpene lactones, and triterpene lactones.8 These chemical components show extensive pharmacological activity.
Previous studies have demonstrated that GBE is known for its antioxidant as well as strong antibacterial properties,9 but there is no report about its application in food packaging. The aim of this work is to use the GBE to prepare gelatin films and evaluate the effects of GBE incorporation on the antioxidant and antimicrobial properties of the gelatin films. Furthermore, the physicochemical properties (such as mechanical properties, surface structure, and UV shielding effect) of the films with the addition of GBE were also evaluated.
2. Methods and materials
2.1. Materials
Gelatin and glycerol were purchased from Sinopharm Group Co., Ltd. Ginkgo biloba extract (GBE) was bought from Nanjing Herb Source Bio-Tech Co., Ltd. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) was supplied by TCI (Shanghai) Development Co., and Rose Bengal medium, nutrient broth and nutrition agar were supplied by AOBOXING Bio-Tech Co., Ltd. (Beijing, China).
2.2. Preparation of gelatin film incorporated with GBE
The GBE gelatin films were prepared by the method reported by Li et al. with some modifications.10 Briefly, gelatin was dissolved in distilled water to obtain a protein concentration of 4% (w/v). The mixture was hydrated at room temperature for 60 min, and then heated at 45 °C until it dissolved completely. Based on the gelatin weight, 30% (w/w) glycerol as a plasticizer was added to the film forming solution. In the meantime, concentrations of 0, 0.5, 1.5, 2.5, 3, 4, and 5% (w/w, based on the gelatin content) GBE solubilized with ethanol were added to the gelatin solution. The film forming solutions were poured onto acrylic sheets (10 × 10 cm). The solutions were dried in an oven (FED 115, Binder, Germany) at 25 °C for 3 days.
2.3. Determination of film properties
2.3.1. Mechanical properties
Tensile strength (TS) and elongation at break (EAB) of gelatin films were tested as described by Iwata et al. using an electromechanical universal testing machine (CMT 6104, MTS Systems Co., Ltd, China) equipped with a tensile load cell of 100 N. The sample was cut into 25 mm × 100 mm pieces. The cross-head speed was set at 10 mm min−1.11
2.3.2. Moisture content and film solubility
The moisture content (MC) and film solubility (FS) were measured by the Tammineni et al. method.12 For MC, film portions of 2 × 2 cm2 dimension were weighed and dried in an oven (FED 115, Binder, Germany) at 105 °C for 24 h. The MC was calculated according to the following equation: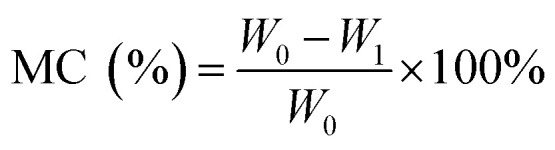 where W0 is the initial dry mass of the sample (g) and W1 is the final dry mass of the sample (g). All tests were carried out in triplicates.
where W0 is the initial dry mass of the sample (g) and W1 is the final dry mass of the sample (g). All tests were carried out in triplicates.
For FS, samples (20–30 mg) were immersed in a centrifuge tube containing 30 mL distilled water for 24 h. The tubes were centrifuged for 15 min at 9000 rpm (centrifuge, Anke TGL-10 C). After this period, the samples were dried in an oven at 105 °C until a constant weight was achieved. The FS was calculated according to the following equation: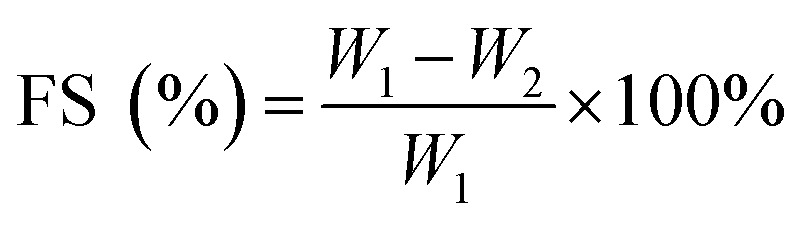 where W1 is the initial film weight and W2 is the weight of undissolved dry matter.
where W1 is the initial film weight and W2 is the weight of undissolved dry matter.
2.3.3. Water vapor permeability
The water vapor permeability (WVP) was determined by the gravimetric method as described by Sobral et al.13 The film was used to cover the mouth of a 50 mL conical flask containing 20 mL of distilled water and sealed tightly using a rubber band. This was placed in a desiccator filled with anhydrous silica gel (relative humidity 0%) at 22 °C. The exposed area of the film was 9.62 cm2. The samples were weighed at 1 h intervals over 12 h periods and the WVP was calculated using the following equation: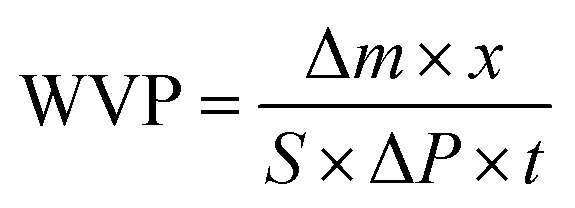 where Δm is the weight gained (mg), x is the average thickness of the film (mm), t is the elapsed time for the weight gained (s), S is the exposed area of the film (m2), and ΔP is the vapor pressure difference across the film (Pa). All tests were carried out in triplicates.
where Δm is the weight gained (mg), x is the average thickness of the film (mm), t is the elapsed time for the weight gained (s), S is the exposed area of the film (m2), and ΔP is the vapor pressure difference across the film (Pa). All tests were carried out in triplicates.
2.3.4. FT-IR spectroscopy
Fourier-transform infrared (FTIR) spectroscopy analyses were performed in a transmission mode using a spectrometer (NicoletTM iSTM10, Thermo Fisher, USA). The spectral range was 4000–750 cm−1. Each spectrum was collected at a resolution of 4 cm−1.
2.3.5. Structural characterization
A scanning electron microscope (SEM) (JSM 6700F, JEOL, Japan) was used to visualize the surface morphology of film samples. The samples were sputtered with gold for 30 seconds. The photographs were taken at an acceleration voltage of 5 kV.
2.3.6. Light transmission
The barrier properties of films against ultraviolet-visible light were performed at selected wavelengths between 200 and 800 nm, using a UV-Vis spectrophotometer (Cary-50, Vivian, Australia).
2.3.7. DPPH free radical-scavenging assay
The film (200 mg) was mixed with 10 mL of 95% ethanol, and then the solution was kept in the dark at 50 °C for 3 h. 1.5 mL of the supernatant obtained was added to 1.5 mL of 0.06 mM DPPH in 95% ethanol solution. After that, the mixture was stirred for 30 min at room temperature in darkness. The absorbance of the mixture solution was measured at 517 nm using a spectrophotometer (Cary-60, Agilent, USA). The control film was prepared in the same manner except that 95% ethanol was used instead of the sample. The test was carried out in triplicate.
2.3.8. Antibacterial activities
The antimicrobial activity of the sample films was assessed against Staphylococcus aureus (S.A.) ATCC 6538 and Candida albicans (B.C.) ATCC 10231 by the agar diffusion method. Microbial strains were inoculated in nutrient broth (NB) at an appropriate temperature for 12 h. Young-type strains (50 μL) were coated on solidified Nutrient agar plates. The film was cut into 9 mm diameter circular discs and placed on the nutrient agar plate's surface. Plates inoculated with Staphylococcus aureus strains were incubated at 37 °C for 24 h, and Candida albicans was incubated at 28 °C for 24 h. The antimicrobial activity of the tested microorganisms was evaluated by measuring the antibacterial inhibition zone. Gelatin films without GBE addition was used as the control group.
2.3.9. Statistical analysis
A statistical test was carried out using the SPSS program (IBM SPSS Statistics 24, USA). One-way analysis of variance (ANOVA) was used for assessing the data. When the value of p < 0.05, the differences were resolved to be statistically significant.
3. Results and discussion
3.1. Mechanical analysis
TS and EAB are the main parameters of the mechanical strength of the edible films, which represent the maximum tensile strength and the flexibility of the films, respectively. Table 1 summarizes the TS and EAB data of the control film and GBE gelatin films. The TS of the control film was 23.1 MPa. When 0.5 g/100 g gelatin of GBE was added, the TS increased to 27.4 MPa. With the increase in the content of GBE, the TS of the composite film reached 38.0 MPa. Wu et al. also reported that the TS of gelatin films increased with the incorporation of green tea extracts into the gelatin film.14
TS, EAB, MC, FS, and WVP of gelatin edible films supplemented with GBE and of the control filma.
| C GBE | TS (MPa) | EAB (%) | MC (%) | FS (%) | WVP (10−11 g s−1 m−1 Pa−1) |
|---|---|---|---|---|---|
| 0 | 23.1 ± 1.46e | 12.8 ± 1.46ab | 13.2 ± 0.53a | 36.1 ± 2.50a | 4.3 ± 0.34a |
| 0.5 | 27.4 ± 1.59d | 14.4 ± 1.59a | 11.9 ± 0.94a | 34.5 ± 2.83a | 4.1 ± 0.15b |
| 1.5 | 31.4 ± 0.89c | 11.4 ± 0.89ab | 10.7 ± 0.68ab | 33.5 ± 3.02a | 3.9 ± 0.41c |
| 2.5 | 33.5 ± 1.16bc | 10.6 ± 1.16b | 10.4 ± 0.57ab | 31.2 ± 1.61b | 3.7 ± 0.09c |
| 3 | 34.5 ± 1.27abc | 10.6 ± 1.27b | 10.0 ± 0.66ab | 30.3 ± 1.13b | 3.4 ± 0.10d |
| 4 | 36.3 ± 1.41ab | 11.5 ± 1.41ab | 9.4 ± 0.22b | 29.9 ± 1.02c | 3.4 ± 0.02d |
| 5 | 38.0 ± 1.53a | 10.3 ± 1.53b | 8.8 ± 0.92b | 28.1 ± 0.19c | 3.3 ± 0.22d |
The superscripted letters represent SPSS single factor analysis.
The addition of GBE also had effects on the EAB of the films, and the EAB values of the gelatin films were between 10.3 and 14.4%. The mechanical properties of the film were primarily related to the distribution and density of intramolecular and intermolecular interactions, depending on the arrangement of the polymer chains in the network.15 Crosslinking between the amino group of the protein and the phenolic group in the extract increases the rigidity of the film, thereby increasing the TS and lowering the EAB of the film.16 Similar results were obtained in the research by Hoque in which the polyphenol could affect the EAB values of the gelatin film incorporated with different plant extracts including cinnamon, clove and star anise.17
3.2. Moisture content and film solubility
Table 1 also shows the moisture content and film solubility of gelatin films. Both MC and FS of the GBE films decreased with the increase in the amount of GBE compared with that of the control film. The MC of the control film was 13.2 ± 0.53%, which is similar to the result obtained by Ciannamea et al.18 It evidently decreased when GBE was incorporated into a gelatin film. The GBE gelatin films possessed lower MC and FS, which may be due to the hydrophobicity of GBE and its strong molecular structure. This might help to extend the quality assurance period of the film.19 Similar results were obtained previously when red cabbage was used as an additive to form gelatin dispersion in film formation.20
3.3. Water vapor permeability
When the food was exposed to high moisture, it was easily susceptible to spoilage. Therefore, it is required to possess a water barrier towards WVP to be used in food packaging. When the concentration of the GBE was increased to 5.0 g/100 g gelatin, the WVP of the composite films decreased significantly (p < 0.05) from 4.3 ± 0.34 × 10−11 g s−1 m−1 Pa−1 to 3.3 ± 0.22 × 10−11 g s−1 m−1 Pa−1 (Table 1). The incorporation of GBE can enhance the hydrophobicity of the films, so the WVP of the films would be limited.21 A gelatin film added with GBE was prepared with a lower WVP. Yao et al. also noticed that the WVP decreased in the films based on fish gelatin–chitosan supplemented with d-limonene possibly because d-limonene was in a hydrophobic oil phase, and hence, it can decrease the water transfer through films.19
3.4. FT-IR analysis
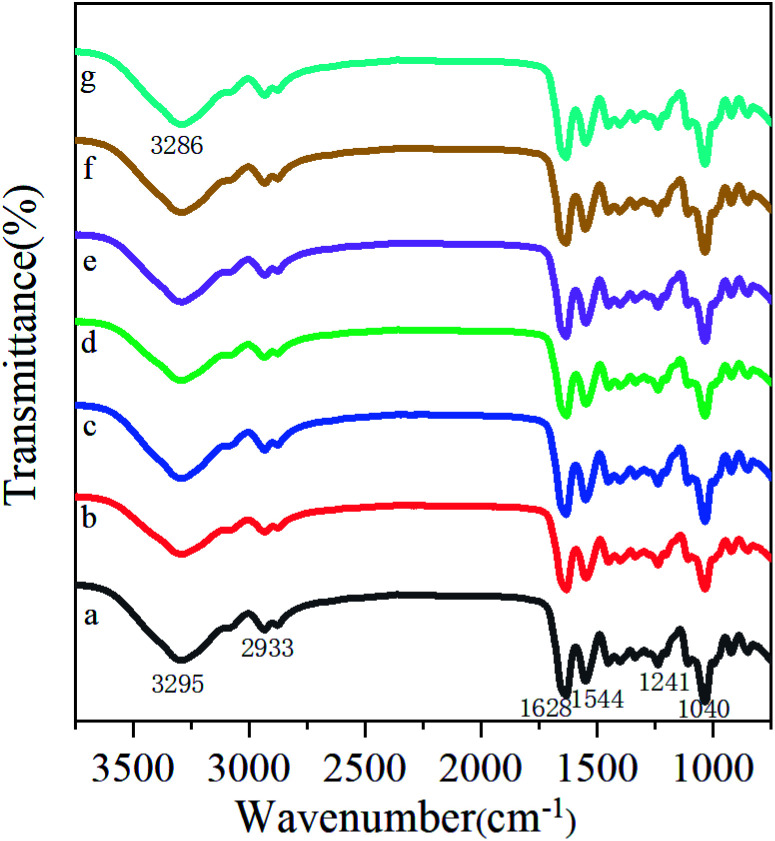
he infrared spectra of the gelatin films containing GBE at different concentrations are exhibited in Fig. 1. The major peaks of the control film (Fig. 1a) are present at 3295 cm−1 (amide-A, N–H stretching), 2933 cm−1 (amide-B, C–H stretching), 1628 cm−1 (amide-I, C O stretching), 1544 cm−1 (amide-II, N–H bending), and 1241 cm−1 (amide-III, C–N and N–H stretching). The addition of GBE to the gelatin film also displayed characteristic peaks in the spectral region, which was similar to that of the control film. As can be seen in Fig. 1, the spectrum of the films incorporated with different concentrations of GBE showed that some of the peaks shifted to lower or higher wavenumbers in comparison with the spectrum of the control film. The amide A peak shifted to lower wavenumbers, which could be attributed to free O–H and N–H groups forming hydrogen bonding with the carbonyl group in the films. Moreover, amide-I could be observed at lower wavenumbers, presumably due to the disordered molecular structure. A band was found at the wavenumber of 1040 cm−1 in the spectra of all films. It was possibly associated with a skeletal stretching vibration of C C bonds. Therefore, the results illustrated that the addition of GBE might alter the intermolecular interactions in the gelatin films.
Fig. 1. FTIR spectra of the films. (a) CGBE = 0 g, (b) CGBE = 0.5 g/100 g gelatin, (c) CGBE = 1.5 g/100 g gelatin, (d) CGBE = 2.5 g/100 g gelatin, (e) CGBE = 3.0 g/100 g gelatin, (f) CGBE = 4.0 g/100 g gelatin, (g) CGBE = 5.0 g/100 g gelatin.
3.5. Film microstructure
To further understand the internal interactions of the gelatin film, the SEM microstructures of the gelatin edible films were obtained and are displayed in Fig. 2. The control film showed a compact, smooth and homogeneous surface, which was comparable to the previous study.22 After adding GBE, the surface of the gelatin films presented similar characteristics. However, with a higher concentration of GBE added, more pores and cavities appeared in the films. It was evident that there are some aggregates of GBE at the highest concentration of GBE added. As a result, a homogeneous dispersion of GBE would show a better mechanical property. Otherwise, it would induce phase separation and affect some properties. Hanani et al. also reported that the control film prepared by gelatin exhibited more compact internal structures than the films with pomegranate peel powder.23
Fig. 2. SEM micrographs of the surface of the films. (1) CGBE = 0 g, (2) CGBE = 0.5 g/100 g gelatin, (3) CGBE = 1.5 g/100 g gelatin, (4) CGBE = 2.5 g/100 g gelatin, (5) CGBE = 3.0 g/100 g gelatin, (6) CGBE = 4.0 g/100 g gelatin, (7) CGBE = 5.0 g/100 g gelatin.
3.6. Ultraviolet and visible light barrier properties
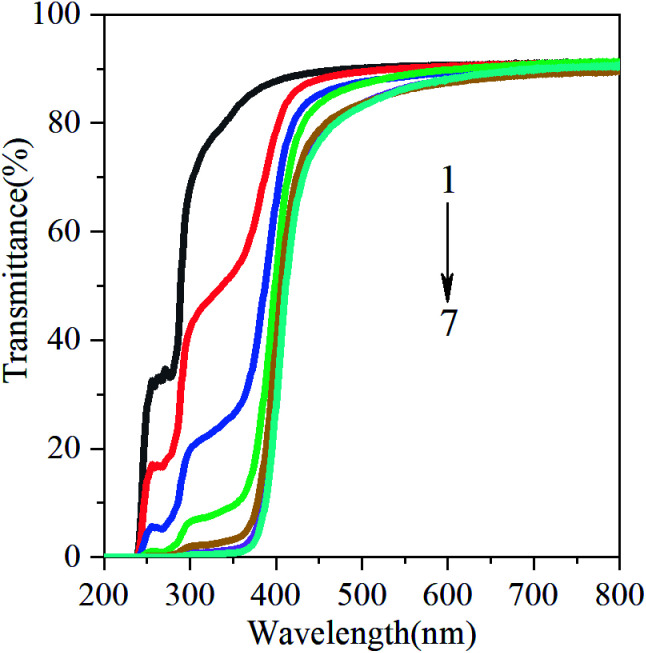
Food oxidation is the main cause of food corruption in production and storage. Nowadays, it has attracted wide attention by adding anti-ultraviolet additives to food packaging films. Bitencourt et al. reported that food packaging with high UV/visible barrier can prevent the lipid oxidation of food.24 Light transmission from 200 to 800 nm in UV and visible ranges of films from gelatin containing glycerol (20%) and various concentrations of GBE are shown in Fig. 3. Regardless of the concentrations of GBE, the transmittance of UV light was very low at 200 and 240 nm for all films, indicating that the GBE gelatin films possess high barrier to UV. This may be related to the chemical structure of GBE. In addition to the aromatic amino acids in gelatin molecules, GBE also exhibits phenolic absorption in the ultraviolet range. Compared with the control film, the addition of GBE reduces the transmittance of the film in the UV-visible region (Fig. 3). In other words, the addition of GBE increases the UV-visible shielding performance of the film. The reduction in light transmission can be attributed to flavonoids and ginkgolides present in the GBE. There are numerous benzene rings, –OH containing polyphenol compounds, C O in flavonoids and ginkgo lactone, which improve the n → π* absorption in the ultraviolet region. This result is similar to the investigation of Dammak et al. They reported that gelatin films incorporated with rutin-loaded nanoemulsions had good barrier property towards the light in the UV region at 200–280 nm.22
Fig. 3. Ultraviolet and visible light barrier properties of films. (1) CGBE = 0 g, (2) CGBE = 0.5 g/100 g gelatin, (3) CGBE = 1.5 g/100 g gelatin, (4) CGBE = 2.5 g/100 g gelatin, (5) CGBE = 3.0 g/100 g gelatin, (6) CGBE = 4.0 g/100 g gelatin, (7) CGBE = 5.0 g/100 g gelatin.
3.7. Antioxidant activity
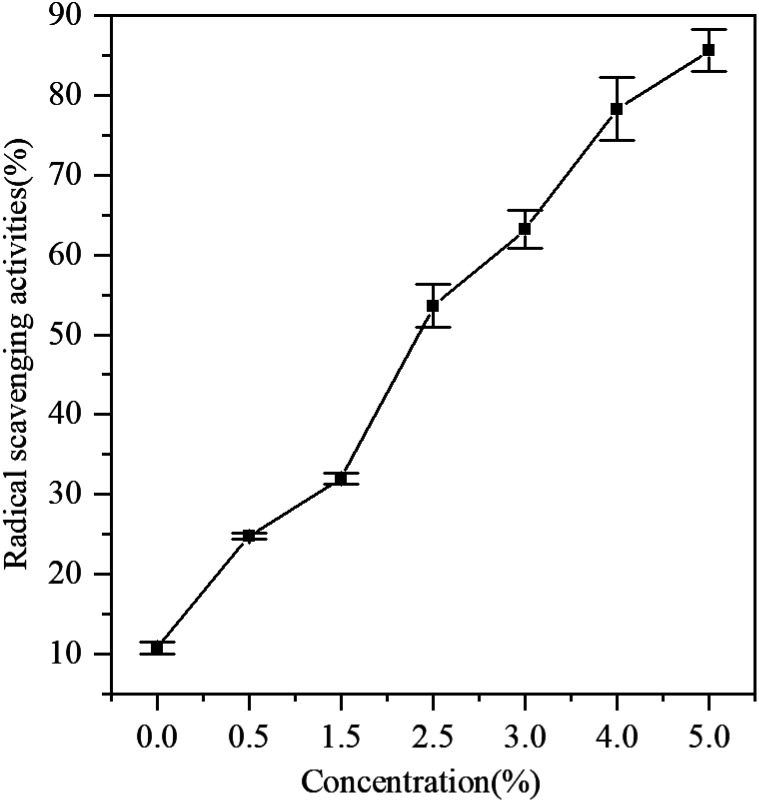
At present, it is generally considered that an important method to determine the antioxidant activity is the scavenging ability of DPPH free radicals. Fig. 4 shows the antioxidant activity of gelatin films with different concentrations of GBE. The films incorporated with GBE showed higher antioxidant activities than the control film. GBE has been reported as a great source of antioxidants.25 Adding antioxidants to active packaging is a promising technique for prolonging the shelf life of food. Furthermore, antioxidants can improve nutritional and aesthetic qualities without affecting food integrity. Compared with the control film, GBE films showed that the radical scavenging activity was 24.7% (CGBE = 0.5 g/100 g gelatin). With the increase in the concentration of GBE, the free radical scavenging effect ranged from 24.7% to 85.6%. The results indicated that the gelatin film containing GBE could be used as the active film with antioxidant activity.
Fig. 4. DPPH radical scavenging activity of films.
3.8. Antibacterial activity
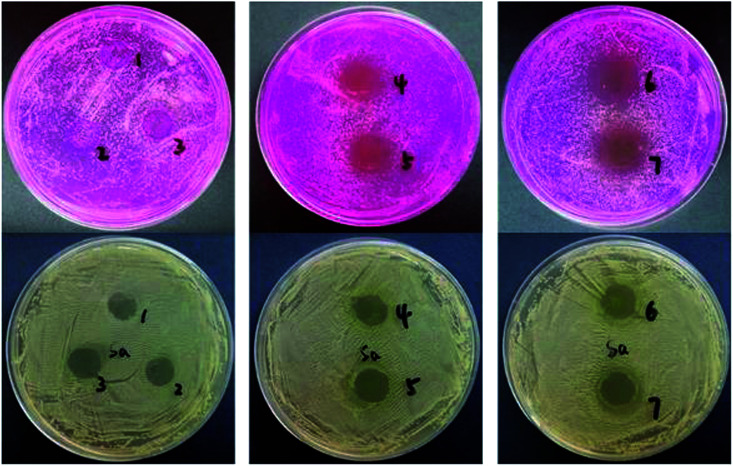
The antibacterial activities of gelatin films containing GBE against Staphylococcus aureus and Candida albicans bacteria are displayed in Fig. 5. Table 2 lists the zone of inhibition. The results presented that the control film did not inhibit the growth of the test microorganisms. As the concentration of GBE increased in the gelatin film, the zone of inhibition enlarged significantly (p < 0.05). Table 2 shows that the zone of inhibition significantly increased from a non-detectable area and 10.8 mm (0.5 g GBE/100 g gelatin) to 20.3 and 13.6 mm (5 g GBE/100 g gelatin) against Candida albicans and Staphylococcus aureus, respectively. Phenolic compounds could destroy the integrity of bacterial walls and cell membranes, leading to the release of intracellular components in microbial cells. It would cause some functional obstacles such as electron transfer, nutrient absorption, nucleotide synthesis and ATP activity in membranes. Therefore, the growth of microorganisms was inhibited. However, the antimicrobial mechanism of GBE should be further explored. Kavoosi et al. noted that the film added with carvacrol had great antibacterial properties. As essential oils possess the ability to destroy the lipid structure of bacterial cell walls, the cell membranes break first, followed by cytoplasmic leakage, cell lysis, and ultimately cell death.26 Liu et al. found that ZnO nanoparticles added to gelatin/chitosan films endows the films with strong antimicrobial activities. The bacteriostatic mechanism was due to the destruction of microbial cells by the highly active free radicals of zinc oxide.27 Overall, the investigation showed that the gelatin films incorporated with GBE had potential application against target microorganisms.
Fig. 5. Photographs of the antibacterial activity on two tested strains. (1) CGBE = 0 g, (2) CGBE = 0.5 g/100 g gelatin, (3) CGBE = 1.5 g/100 g gelatin, (4) CGBE = 2.5 g/100 g gelatin, (5) CGBE = 3.0 g/100 g gelatin, (6) CGBE = 4.0 g/100 g gelatin, (7) CGBE = 5.0 g/100 g gelatin.
Antibacterial activity of edible films with different concentrations of GBE and of the control film.
| C GBE | Inhibition zone for S.A. (mm) | Inhibition zone for B.C. (mm) |
|---|---|---|
| 0 | Not detected | Not detected |
| 0.5 | 10.8 ± 0.08c | Not detected |
| 1.5 | 11.3 ± 0.11c | Not detected |
| 2.5 | 12.6 ± 0.10b | 13.5 ± 0.22b |
| 3 | 13.1 ± 0.05a | 13.8 ± 0.16b |
| 4 | 13.2 ± 0.04a | 19.2 ± 0.46a |
| 5 | 13.6 ± 0.34a | 20.3 ± 0.20a |
4. Conclusions
Edible films with antibacterial and antioxidant activities have been fabricated by incorporating GBE into gelatin. The GBE in the films can interact with the gelatin in the matrix. The interaction affected the mechanical properties, microstructure and barrier properties of the films. Incorporation of the GBE into gelatin films decreased the moisture content and improved the thermal stability and antimicrobial properties, which was a great prospect for its application in food packaging. The antioxidant activities monitored by the DPPH method showed high activities after the incorporation of GBE. Packaging films made from GBE and gelatin provide a new way to inhibit microbial activities, delay lipid oxidation and prolong the shelf-life of foods.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
References
- Dorta E. Lobo M. G. González M. LWT–Food Sci. Technol. 2012;45:261–268. doi: 10.1016/j.lwt.2011.08.016. [DOI] [Google Scholar]
- De Clercq K. Schelfhout C. Bracke M. De Wever O. Van Bockstal M. Ceelen W. Remon J. P. Vervaet C. Biomaterials. 2016;96:33–46. doi: 10.1016/j.biomaterials.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Valdes A. Mellinas A. C. Ramos M. Burgos N. Jimenez A. Garrigos M. C. RSC Adv. 2015;5:40324–40335. doi: 10.1039/C4RA17286H. [DOI] [Google Scholar]
- Shankar S. Jaiswal L. Selvakannan P. R. Ham K. S. Rhim J. W. RSC Adv. 2016;6:67340–67352. doi: 10.1039/C6RA10620J. [DOI] [Google Scholar]
- Yu S. H. Hsieh H. Y. Pang J. C. Tang D. W. Shih C. M. Tsai M. L. Tsai Y. C. Mi F. L. Food Hydrocolloids. 2013;32:9–19. doi: 10.1016/j.foodhyd.2012.11.036. [DOI] [Google Scholar]
- Jouki M. Mortazavi S. A. Yazdi F. T. Koocheki A. Carbohydr. Polym. 2014;99:537–546. doi: 10.1016/j.carbpol.2013.08.077. [DOI] [PubMed] [Google Scholar]
- Gong W. Chen C. Dobes C. Fu C. X. Koch M. A. Mol. Phylogenet. Evol. 2008;48:1094–1105. doi: 10.1016/j.ympev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Ahlemeyer B. Krieglstein J. ACS Symp. Ser. 2003;60:1779–1792. doi: 10.1007/s00018-003-3080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sati P. Dhyani P. Bhatt I. D. Pandey A. J. Tradit. Complement. Med. 2019;9:15–23. doi: 10.1016/j.jtcme.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. H. Miao J. Wu J. L. Chen S. F. Zhang Q. Q. Food Hydrocolloids. 2014;37:166–173. doi: 10.1016/j.foodhyd.2013.10.015. [DOI] [Google Scholar]
- Iwata K. I. Ishizaki S. H. Handa A. K. Tanaka M. U. Fish. Sci. 2000;66:372–378. doi: 10.1046/j.1444-2906.2000.00057.x. [DOI] [Google Scholar]
- Tammineni N. Unlu G. Rasco B. Powers J. Sablani S. Nindo C. J. Food Sci. 2012;77:342–347. doi: 10.1111/j.1750-3841.2012.02964.x. [DOI] [PubMed] [Google Scholar]
- Sobral P. J. A. Menegalli F. C. Hubinger M. D. Roques M. A. Food Hydrocolloids. 2001;15:423–432. doi: 10.1016/S0268-005X(01)00061-3. [DOI] [Google Scholar]
- Wu J. L. Chen S. F. Ge S. Y. Miao J. Li J. H. Zhang Q. Q. Food Hydrocolloids. 2013;32:42–51. doi: 10.1016/j.foodhyd.2012.11.029. [DOI] [Google Scholar]
- Ahmad M. Hani N. M. Nirmal N. P. Fazial F. F. Mohtar N. F. Romli S. R. Prog. Org. Coat. 2015;84:115–127. doi: 10.1016/j.porgcoat.2015.02.016. [DOI] [Google Scholar]
- Maryam Adilah Z. A. Jamilah B. Nur Hanani Z. A. Food Hydrocolloids. 2018;74:207–218. doi: 10.1016/j.foodhyd.2017.08.017. [DOI] [Google Scholar]
- Hoque M. S. Benjakul S. Prodpran T. Food Hydrocolloids. 2011;25:1085–1097. doi: 10.1016/j.foodhyd.2010.10.005. [DOI] [Google Scholar]
- Ciannamea E. M. Stefani P. M. Ruseckaite R. A. LWT–Food Sci. Technol. 2016;74:353–362. doi: 10.1016/j.lwt.2016.07.073. [DOI] [Google Scholar]
- Yao Y. Ding D. Shao H. Peng Q. Huang Y. Int. J. Polym. Sci. 2017;2017:1–9. doi: 10.1155/2017/1837171. [DOI] [Google Scholar]
- Musso Y. S. Salgado P. R. Mauri A. N. Food Hydrocolloids. 2019;89:674–681. doi: 10.1016/j.foodhyd.2018.11.036. [DOI] [Google Scholar]
- Maryam Adilah Z. A. Jamilah B. Nur Hanani Z. A. Food Hydrocolloids. 2018;74:207–218. doi: 10.1016/j.foodhyd.2017.08.017. [DOI] [Google Scholar]
- Dammak I. de Carvalho R. A. Trindade C. S. Lourenço R. V. de Amaral Sobral P. J. Int. J. Biol. Macromol. 2017;98:39–49. doi: 10.1016/j.ijbiomac.2017.01.094. [DOI] [PubMed] [Google Scholar]
- Hanani Z. A. N. Yee F. C. Nor-Khaizura M. A. R. Food Hydrocolloids. 2019;89:253–259. doi: 10.1016/j.foodhyd.2018.10.007. [DOI] [Google Scholar]
- Bitencourt C. M. Fávaro-Trindade C. S. Sobral P. J. A. Carvalho R. A. Food Hydrocolloids. 2014;40:145–152. doi: 10.1016/j.foodhyd.2014.02.014. [DOI] [Google Scholar]
- Han J. Y. Xing D. M. Hong S. Hong L. U. Min L. I. Li-Jun D. U. Chin. Pharmacol. Bull. 2002;18:115–117. [Google Scholar]
- Kavoosi G. Dadfar S. M. M. Purfard A. M. Mehrabi R. J. Food Saf. 2013;33:423–432. doi: 10.1111/jfs.12071. [DOI] [Google Scholar]
- Liu Z. Lv M. Li F. Zeng M. J. Aquat. Food Prod. Technol. 2016;25:1056–1063. doi: 10.1080/10498850.2014.923081. [DOI] [Google Scholar]